Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
-
Haseeb Ahmad
, Arham Shabbir
, Amira Metouekel
and Mohammed Bourhia
Abstract
Rheumatoid arthritis (RA) is an autoimmune disease causing joint inflammation, deformity, cartilage deterioration, and pain. Benzimidazole derivatives exhibit various pharmacological properties. This study evaluated the antiarthritic, immunomodulatory, and anti-inflammatory potential of a benzimidazole derivative 2-(2-(benzylthio)-1H-benzo[d]imidazol-1-yl)-N′-(4-nitrobenzylidiene) acetohydrazide (BMZ-AD) in a Freund’s complete adjuvant (FCA)-induced arthritic rat model. FCA was administered on day 0, and treatment with BMZ-AD (25 mg/kg, 50 mg/kg, and 75 mg/kg) and piroxicam (10 mg/kg) began on day 7 and continued up to 28 days. Rats were sacrificed on day 28. Arthritis was assessed using an arthritic scoring index, and paw edema was measured with a digital water plethysmometer. Biochemical and hematological parameters were analyzed, reverse transcription polymerase chain reaction measured the mRNA expression of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6), and molecular docking evaluated BMZ-AD interactions with these proteins. Enzyme-linked immunosorbent assay determined prostaglandin E2 levels. BMZ-AD treatment reduced inflammation, pannus formation, and pro-inflammatory cytokines (TNF-α and IL-6) and decreased PGE2 levels, comparable to piroxicam. Blood profiles improved with significant reductions in white blood cells and platelets in treatment groups. BMZ-AD demonstrated antiarthritic, anti-inflammatory, and immunomodulatory properties, suggesting that it could be a potential drug for RA treatment with fewer side effects.
1 Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by joint cartilage erosions, bone remodeling, and synovium inflammation. Around 1% of the world population has been afflicted by the chronic inflammatory joint disease RA and the resultant disability [1]. Environmental and genetic factors contribute to the disease pathology. It is gender specific and four times more prevalent in females than in males. The cardinal features of RA include joint stiffness, severe pain, and functional disability which worsen in early morning [2].
RA develops with the inflammation of the synovial membrane, known as synovitis, which leads to joint swelling and pain during movement. A high number of immune cells move to synovial fluids in response to joint inflammation. Later on, the inflammation extends to the synovial tissue, joint cavity spaces, and cartilages. Pannus formation in synovium culminates in loss of joint cartilage. The condition aggravates further with fibrous tissue formation and fusion of bones [3].
Macrophages, T and B lymphocytes, fibroblasts, and a myriad of pro-inflammatory cytokines, notably interleukin-6 (IL-6) and TNF-α (tumor necrosis factor alpha), in conjunction with prostaglandin E2 (PGE2), are pivotal actors in the intricate pathogenesis of RA, contributing to the hyperplasia of articular cartilage. As a primary inducer of the production of acute-phase proteins, IL-6 orchestrates acute inflammatory responses, amplifying the cascade of immune-mediated processes characteristic of RA [4].
The five classes of drugs currently used in treatment include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, disease-modifying anti-rheumatoid drugs, and anti-cytokine therapies [5]. However, these therapies are associated with several undesirable effects such as irritation of stomach lining, gastrointestinal bleeding, weight gain, hypertension, increased blood sugar level, and loss of bone density. Since inflammation is the main culprit in the pathology of RA, agents having anti-inflammatory and antioxidant potential could be beneficial in RA therapy. A plethora of studies corroborate the anti-inflammatory potential of benzimidazole derivatives in various diseases [6,7,8,9].
The benzimidazole nucleus, often hailed as the “Master Key” in the realm of medicinal compounds, holds a pivotal and multifaceted role in drug development. By altering the benzimidazole structure at various positions, researchers can notably enhance the physicochemical, metabolic, and pharmacokinetic properties of the synthesized compounds. A host of benzimidazole-containing drugs have garnered FDA approval, including omeprazole, a proton pump inhibitor, and albendazole, an antifungal agent [10].
Benzimidazole is remarkable for its broad spectrum of biological activities. It showcases antifungal [11], anthelmintic [12], and anti-asthmatic properties [13]. Moreover, it acts as an anti-diabetic [14], antihypertensive [15], and antiparasitic agent [16]. Its antihistaminic [17] and gastroprotective capabilities [18] are also noteworthy, alongside its antibacterial [19], anticoagulant [20], and anti-obesity effects [21]. Furthermore, benzimidazole exhibits significant antioxidant [22], antitumor [23], and anti-inflammatory activities [24].
The versatility and breadth of action underscore the importance of the benzimidazole nucleus in medicinal chemistry, making it a cornerstone in the development of new therapeutic agents. On the basis of its significant anti-inflammatory potential, this study was designed to investigate its immunomodulatory role in arthritis. In this research study, we evaluated the mechanistic effects of a benzimidazole derivative [2-(2-(benzylthio)-1H-benzo[d]imidazol-1-yl)-N′-(4-nitrobenzylidiene) acetohydrazide (BMZ-AD) in a Freund’s complete adjuvant (FCA)-induced arthritic rat model. The results revealed that the benzimidazole derivative attenuated joint inflammation, bone erosion, and cartilage erosion. Moreover, its immunomodulatory role was attributed to the downregulation of pro-inflammatory markers such as TNF-α, IL-6, and PGE2 levels. Thus, this study provides valuable insights into discovering a benzimidazole derivative as a novel therapeutic drug for this challenging autoimmune disorder.
2 Materials and methods
2.1 Animals
Thirty-six healthy albino male rats weighing 250–350 g were procured from the Central Animal House of University of Lahore and divided into six equal groups, each containing six rats. Animals were kept in propylene cages having a stainless steel cover lid, under the conditions of ambience temperature of 22–25°C and a 12-h light/dark cycle, with free access to food and clean water. The rats were acclimatized to the laboratory conditions for 1 day before the experiment. The study protocol was approved by the Institutional Research Ethics Committee (IREC) at the University of Lahore prior to the commencement of the experiments, under Trial Registry No: IREC-PHM-21-46 [23].
-
Ethical approval: The research related to animal use complied with all the relevant national regulations and institutional policies for the care and use of animals and was approved by the Institutional Research Ethics Committee (IREC) at the Faculty of Pharmacy, the University of Lahore, Pakistan (No: IREC-PHM-2021-46).
2.2 Induction and assessment of arthritic progression
Rats were divided into six groups each containing six rats. Group I – vehicle control administered with 1% Tween 80 and 2% DMSO in distilled water. Group II – disease group administered with FCA (0.15 ml), Group III – reference group that received piroxicam (10 mg/kg b.w., p.o.), Group-IV – treatment group that received the test drug (BMZ-AD) 25 mg/kg b.w., p.o., Group-V – treatment group that received BMZ-AD 50 mg/kg b.w., p.o., and Group-VI – treatment group that received BMZ-AD 75 mg/kg b.w., p.o. At day 0, FCA was injected into the sub-plantar region of right hind paws of rats of all groups except control. From day 7 to day 28, BMZ-AD was administered to all test drug treatment groups (Group-IV, Group-V, and Group-VI), and piroxicam was administered to Group III [25,26]. Inflammation, redness, and erythema were noted on days 7, 14, 21, and 28. Macroscopic observations of paw inflammation, redness, and swelling were used to determine the arthritic score. Redness, erythema, and inflammation of the paws were graded on a scale of 0, 1, 2, 3, and 4, which indicated normal, minimal, mild, moderate, and severe, respectively [27]. A digital water displacement plethysmometer was used to measure the paw edema of all groups at days 0, 8, 15, 22, and 28 [28].
2.3 Investigations of histopathology
Albino rats were sacrificed on day 28 and ankle joints were dissected. They were cut into ihalf lengthwise and preserved in 10% formalin and were subjected to decalcification by methanoic acid. After fixing in paraffin blocks, tissue slides of bisected paws were prepared [29]. The prepared slides were deparaffinized using xylene, rehydrated using gradient alcohol concentration (95–70%), and finally with distilled water. These slides were then stained with the hematoxylin and eosin (H&E) dyes and blindly evaluated by a histo-pathologist.
2.4 Analysis of mRNA expression levels of TNF-α and IL-6
The mRNA expression levels of cytokines involved in the development of RA were evaluated using the conventional polymerase chain reaction methodology. Initially, blood samples were collected through intracardiac puncture after sacrificing the rats in order to extract RNA, using TRIzol reagent in accordance with the established technique. For homogenization, 200 µl blood was mixed with 600 µl TRIzol to extract mRNA. Quantification of the isolated RNA samples was performed using a NanoDrop spectrophotometer. The complementary DNA was synthesized in accordance with the methodology provided by the kit manufacturer (Thermo Scientific, America). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as the reference. A sequence of primers for genes such as IL-6 and TNF-α was selected from previously published studies [30,31].
| Genes | Forward primer | Reverse primer |
|---|---|---|
| IL-6 | 5′-CCCACCAAGAACGATAGTCA-3′ | 5′-CTCCGACTTGTGAAGTGGTA-3′ |
| TNF-α | 5′-AGTCCGGGCAGGTCTACTTT-3′ | 5′-GGAAATTCTGAGCCCGGAGT-3′ |
2.5 Assessment of PGE2 through ELISA
PGE2 levels were detected from serum using Elabscience’s enzyme-linked immunosorbent assay (ELISA) kit (E-EL-003 96T). Absorbance was measured using an ELISA reader (BioTek Instruments, Inc., ELx808IU, USA) at 450 nm wavelength [32].
2.6 Evaluation of hematological parameters
A cardiac puncture was used to collect blood samples, which were then placed in a Vacutainer (Lab Vac) with EDTA. Hematological parameters, such as hemoglobin (Hb), red blood cells (RBCs), platelets (PLT), and white blood cells (WBCs), were analyzed with the help of an automated hematology analyzer (Sysmex XT-1800i) [33].
2.7 Evaluation of biochemical markers
Serum was separated from blood and was used for evaluation of biochemical parameters. Different biochemical parameters such as urea, creatinine, aspartate aminotransferase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP) were analyzed using an automated chemistry analyzer (HumaLyzer 3500) by following kit manufacturer’s protocols [30,34].
2.8 In silico studies
2.8.1 Preparation of target proteins
The 3D crystal structures of target protein molecules were retrieved from protein data bank, PDB ID: 1N26 (IL-6), having a resolution of 2.40 Å, and PDB ID: 2AZ5 (TNF-α) having a resolution of 2.10 Å (Figure 1). The active binding sites of IL-6 and TNF-α were identified through literature review [35,36]. Processing of target protein was done using Discovery Studio Visualizer. Any co-crystallized ligands were removed and polar hydrogen atoms were added and saved in pdb format [37].
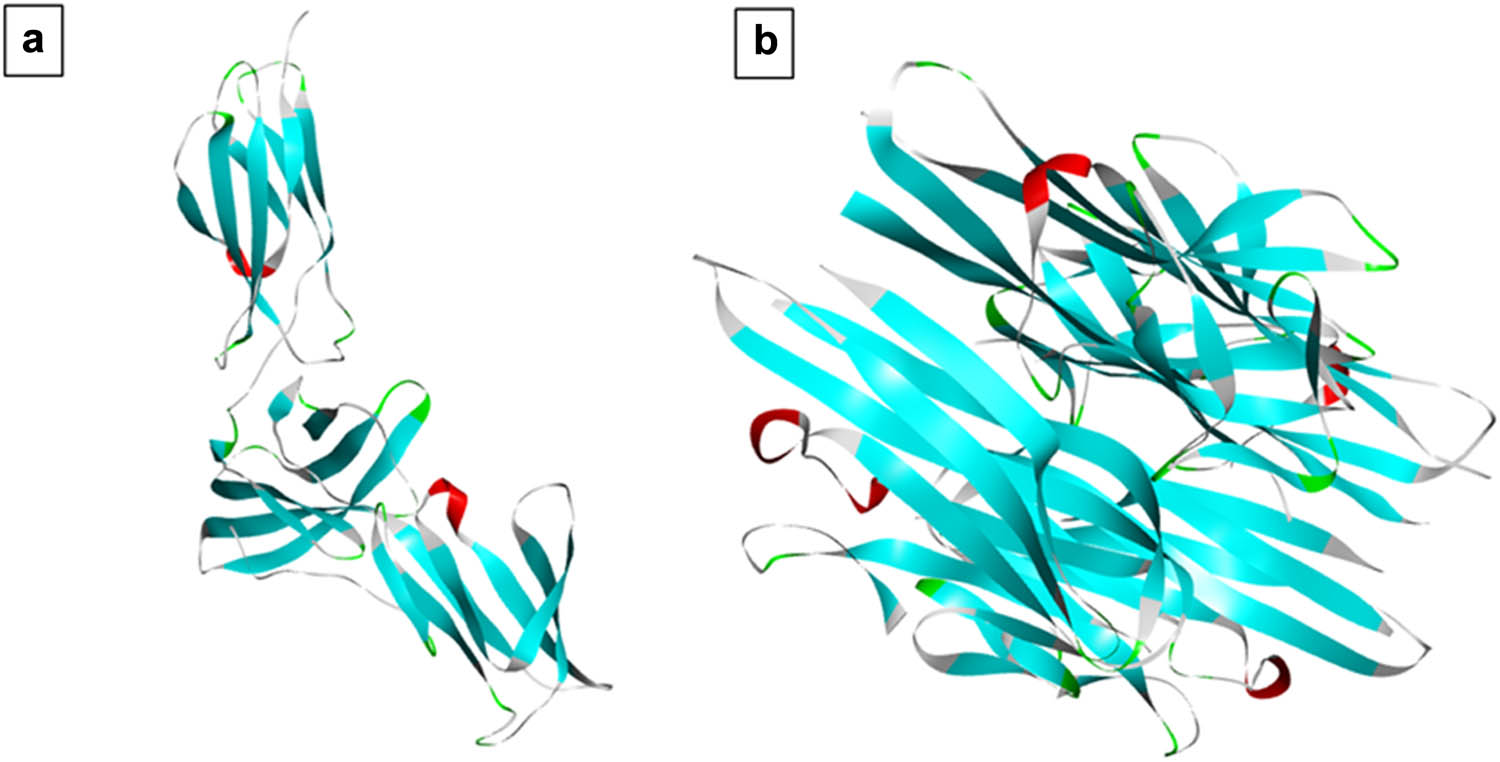
Three-dimensional (3D) X-ray crystal structures of (a) IL-6 (PDB ID: 1N26) and (b) TNF-α (PDB ID: 2AZ5).
2.8.2 Preparation of ligands
The 3D structure of piroxicam was downloaded from the PubChem database in sdf format, while the 2D structure of BMZ-AD was drawn in ChemSketch (Figure 2) and converted to pdb format using Discovery Studio Visualizer. Energy optimization and conversion to pdbqt format were done using Open Babel in PyRx software [38].

2D structure of BMZ-AD.
2.8.3 Molecular docking
Molecular docking studies were executed using AutoDock Vina 4.2 suite of PyRx software. The prepared proteins and ligands were used as inputs in PyRx. The number of runs was set to 100 for each docking. The grid boxes were created around the amino acids of IL-6 (Glu144, Asn226, Asn110, and Gln158) and TNF-α (Leu57, Tyr59, Ser60, Gln61, Tyr119, Leu120, Gly121, Gly122, and Tyr151). The output docking scores were defined as the binding affinity (kcal/mol). To visualize the interaction of target amino acid residues with selected ligands, Discovery Studio Visualizer was utilized. The results obtained were expressed as the number of conventional hydrogen bonds with amino acid residues and binding affinity as kcal/mol [8,39].
2.9 Statistical analysis
For data interpretation, GraphPad Prism (v 6.0) was used. Values were presented as mean and standard error of mean (SEM). The results were analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc comparison test. P < 0.05 was set as the significance level.
3 Results
3.1 Effect of BMZ-AD on arthritic score
On day 7, no significant effect on arthritic score was observed between piroxicam (3.221 ± 0.087), BMZ-AD 25 mg/kg (3.326 ± 0.084), BMZ-AD 50 mg/kg (3.306 ± 0.085), and BMZ-AD 75 mg/kg (3.281 ± 0.089) treatment groups and the disease group (Figure 3) (Table 1). After 28 days, substantial inhibition in arthritic score was observed with piroxicam (2.191 ± 0.048), BMZ-AD 25 mg/kg (2.370 ± 0.055), BMZ-AD 50 mg/kg (2.281 ± 0.049), and BMZ-AD 75 mg/kg (2.224 ± 0.044) treatment groups as compared to the disease group (3.822 ± 0.071).

Effect of treatment on arthritic score with piroxicam 10 mg/kg and BMZ-AD (25, 50, and 75 mg/kg) on (a) 7th day, (b) 14th day, (c) 21st day, and (d) 28th day. The data are expressed as mean ± SEM, *** and ### indicate p < 0.001 and ** indicates p < 0.01. ###p < 0.001 vs vehicle control, while ***p < 0.001 and **p < 0.01 vs disease group.
BMZ-AD significantly decreased arthritic development
| Days | Disease | Piroxicam (10 mg/kg) | BMZ-AD (25 mg/kg) | BMZ-AD (50 mg/kg) | BMZ-AD (75 mg/kg) |
|---|---|---|---|---|---|
| Mean ± SEM (ml) | |||||
| Day 7 | 3.35 ± 0.079 | 3.22 ± 0.087 | 3.32 ± 0.084 | 3.30 ± 0.085 | 3.28 ± 0.089 |
| Day 14 | 3.41 ± 0.069 | 2.36 ± 0.067*** | 2.58 ± 0.054*** | 2.44 ± 0.064*** | 2.40 ± 0.073*** |
| Day 21 | 3.53 ± 0.067 | 2.32 ± 0.062*** | 2.50 ± 0.071*** | 2.43 ± 0.067*** | 2.36 ± 0.064*** |
| Day 28 | 3.82 ± 0.071 | 2.19 ± 0.048*** | 2.37 ± 0.055*** | 2.28 ± 0.049*** | 2.22 ± 0.044*** |
In contrast to the disease group, BMZ-AD showed a significant difference of ***p < 0.001.
3.2 Effect of BMZ-AD on histopathological evaluation
3.2.1 Inflammation was significantly reduced by BMZ-AD
In contrast to the disease group (2.567 ± 0.080), there was a substantial reduction in inflammation in piroxicam 10 mg/kg (1.489 ± 0.093; P < 0.001), BMZ-AD 25 mg/kg (1.930 ± 0.028; P < 0.001), BMZ-AD 50 mg/kg (1.836 ± 0.029; P < 0.001), and BMZ-AD 75 mg/kg (1.634 ± 0.030; P < 0.001) treatment groups (Figure 4 and Table 2).

Infiltration of inflammatory cells with piroxicam 10 mg/kg and BMZ-AD (25, 50, and 75 mg/kg). The data are expressed as mean ± SEM, ***P < 0.001 vs disease group. Vehicle group data are shown as 0.
BMZ-AD effect on histopathological parameters
| Parameters | Disease | Piroxicam (10 mg/kg) | BMZ-AD (25 mg/kg) | BMZ-AD (50 mg/kg) | BMZ-AD (75 mg/kg) |
|---|---|---|---|---|---|
| Mean ± SEM | |||||
| Infiltration of inflammatory cells | 2.56 ± 0.080 | 1.48 ± 0.093*** | 1.93 ± 0.028*** | 1.83 ± 0.029*** | 1.63 ± 0.030*** |
| Pannus formation | 3.61 ± 0.068 | 2.37 ± 0.094*** | 2.63 ± 0.02*** | 2.61 ± 0.024*** | 2.57 ± 0.011*** |
| Bone erosion | 2.65 ± 0.070 | 2.16 ± 0.063*** | 2.17 ± 0.066*** | 2.16 ± 0. 066*** | 2.16 ± 0.058*** |
BMZ-AD were shown to have a ***P < 0.001 difference in contrast to the disease group.
3.2.2 Effect of BMZ-AD on pannus formation
Pannus formation was significantly augmented in the disease group as compared to the vehicle group. In contrast to the disease group (3.611 ± 0.068), piroxicam 10 mg/kg (2.374 ± 0.094), BMZ-AD 25 mg/kg (2.584 ± 0.083), BMZ-AD 50 mg/kg (2.665 ± 0.105), and BMZ-AD 75 mg/kg (2.581 ± 0.083) treatment groups showed a reduction (P < 0.001) in pannus development (Figure 5 and Table 2).

Effect of BMZ-AD on pannus formation after treatment with piroxicam 10 mg/kg and BMZ-AD (25, 50, and 75 mg/kg) on day 28. ###P < 0.001 vs vehicle group and ***P < 0.001 vs disease group.
3.2.3 BMZ-AD significantly suppressed bone erosion
In contrast to the disease group, the piroxicam 10 mg/kg (2.164 ± 0.063; P < 0.001), BMZ-AD 25 mg/kg (2.177 ± 0.066; P < 0.001), BMZ-AD 50 mg/kg (2.166 ± 0.066; P < 0.001), and BMZ-AD 75 mg/kg (2.169 ± 0.058; P < 0.001) treatment groups showed a significant decrease in bone erosion (Figure 6) (Table 2).

BMZ-AD reduced bone erosion after treatment with piroxicam 10 mg/kg and BMZ-AD (25, 50, and 75 mg/kg) on day 28. The data are expressed as mean ± SEM, ###P < 0.001 vs vehicle group and ***P < 0.001 vs disease group. Normal group data are shown as zero.
3.3 Effect of treatment on histopathology
After treatment with BMZ-AD and piroxicam, obvious significant reduction in inflammation was observed through reduction in infiltration of inflammatory cells, pannus formation, and bone erosion (Figure 7(a)–(f)).

(a) Vehicle control: no pannus formation and erosion of cartilage. (b) Positive control: severe inflammation, pannus formation, and bone erosion. Inflammed synovial membrane (SM) and significant bone erosion (BE). (c) Piroxicam: moderate infiltration of inflammatory cells and pannus formation. (d) BMZ-AD 25 mg/kg: SM is surrounded by focal, dispersed inflammatory cells. (e) BMZ-AD 50 mg/kg: no inflammatory cells and pannus development. (f) BMZ-AD 75 mg/kg: no tissue changes, sloughing at the joint surface, with no infiltration of inflammatory cells and pannus formation. Scale bar is mentioned in the figure.
3.4 Effect of BMZ-AD on mRNA expression levels of pro-inflammatory markers
The disease group (50.21 ± 1.978) had substantially increased expression levels of TNF-α (P < 0.001) as compared to the vehicle group (33.35 ± 1.784). After treatment with piroxicam 10 mg/kg (32.86 ± 1.034), BMZ-AD 25 mg/kg (34.39 ± 0.965), BMZ-AD 50 mg/kg (33.65 ± 1.029), and BMZ-AD 75 mg/kg (32.98 ± 1.057), TNF-α levels were significantly reduced (P < 0.001). Nonetheless, piroxicam (32.08 ± 1.152; P < 0.001), BMZ-AD 25 mg/kg (33.01 ± 1.230; P < 0.001), BMZ-AD 50 mg/kg (32.82 ± 1.393; P < 0.001), and BMZ-AD 75 mg/kg (32.42 ± 1.255; P < 0.001) treatment also showed significant downregulation of IL-6 (Figure 8 and Table 3).

Effect of treatment with BMZ-AD on inflammatory markers (a) TNF-α and (b) IL-6. The data are expressed as mean ± SEM, while the symbol *** or ### indicates significant difference at p < 0.001, while ## indicates significant difference at p < 0.01. ### vs vehicle control, while *** vs disease group.
BMZ-AD reduced pro-inflammatory marker mRNA expression
| Biomarkers | Vehicle control | Disease | Piroxicam (10 mg/kg) | BMZ-AD (25 mg/kg) | BMZ-AD (50 mg/kg) | BMZ-AD (75 mg/kg) |
|---|---|---|---|---|---|---|
| Mean ± SEM | ||||||
| TNF-α | 33.35 ± 1.784 | 50.21 ± 1.978### | 32.86 ± 1.034*** | 34.39 ± 0.965*** | 33.65 ± 1.029*** | 32.98 ± 1.057*** |
| IL-6 | 33.71 ± 1.750 | 40.69 ± 1.189## | 32.08 ± 1.152*** | 33.01 ± 1.230*** | 32.82 ± 1.393*** | 32.42 ± 1.255*** |
In comparison with the disease group BMZ-AD ***P < 0.001, while ### represents the comparison between disease and vehicle control groups.
3.5 BMZ-AD considerably lowered PGE2 levels
The PGE2 value was greater (P < 0.001) in the disease group (0.98 ± 0.027) in contrast to the vehicle group (0.490 ± 0.040). Piroxicam (0.61 ± 0.021), BMZ-AD 25 mg/kg (0.70 ±0.030), BMZ-AD 50 mg/kg (0.665 ± 0.013), and BMZ-AD 75 mg/kg (0.631 ± 0.020) treatment groups had significantly decreased PGE2 levels, P < 0.001 (Figure 9).

Effect of treatment with BMZ-AD on PGE2 levels. The data are expressed as mean ± SEM. The symbol *** or ### indicates significant difference at p < 0.001. ### vs vehicle control, while *** vs disease group.
3.6 Effect of BMZ-AD treatment on hematological parameters
The RBC count in the disease group (5.638 ± 0.366) was considerably decreased (P < 0.001) when compared to the vehicle control group (8.289 ± 0.314). RBC count was less affected (P < 0.01) in piroxicam (7.480 ± 0.345), BMZ-AD 25 mg/kg (7.451 ± 0.276), BMZ-AD 50 mg/kg (7.480 ± 0.273), and BMZ-AD 75 mg/kg (7.546 ± 0.256) treatment groups (Figure 10 and Table 4).

Effect of treatment with piroxicam and BMZ-AD on hematological parameters: (a) RBC count, (b) Hb content, (c) WBC count, and (d) PLT count. The data are expressed as mean ± SEM; *** and ### indicate p < 0.001 and ** indicates p < 0.01. ###p < 0.001 vs vehicle, while ***p < 0.001 and **p < 0.01 vs disease group.
BMZ-AD treatment effect on hematological parameters
| Parameters | Vehicle control | Disease | Piroxicam (10 mg/kg) | BMZ-AD (25 mg/kg) | BMZ-AD (50 mg/kg) | BMZ-AD (75 mg/kg) |
|---|---|---|---|---|---|---|
| Mean ± SEM | ||||||
| RBC (106/Ul) | 8.28 ± 0.314 | 5.63 ± 0.366### | 7.480 ± 0.345** | 7.451 ± 0.276** | 7.480 ± 0.273** | 7.546 ± 0.256** |
| Hb (g/dl) | 14.44 ± 0.150 | 11.66 ± 0.213### | 13.51 ± 0.291** | 13.58 ± 0.337** | 13.51 ± 0.336** | 13.68 ± 0.359*** |
| WBC (103/Ul) | 0.90 ± 0.381 | 15.08 ± 0.292### | 12.45 ± 0.323*** | 12.47 ± 0.163*** | 12.60 ± 0.180*** | 12.75 ± 0.133*** |
| PLT (103/Ul) | 798.83 ± 27.20 | 1.447 ± 15.98### | 1.045 ± 35.17*** | 940.22 ± 17.07*** | 923.31 ± 17.11*** | 911.23 ± 19.06*** |
###p < 0.001 vs vehicle control; **p < 0.01 and ***p < 0.001 vs disease group.
Moreover, the Hb content in the disease group (11.66 ± 0.213) was considerably lower (P < 0.001) than in the vehicle group (14.44 ± 0.150). Piroxicam (13.51 ± 0.291; P < 0.01), BMZ-AD 25 mg/kg (13.58 ± 0.337; P < 0.01), BMZ-AD 50 mg/kg (13.5 ± 0.336; P < 0.01), and BMZ-AD 75 mg/kg (13.68± 0.359; P < 0.001) treatment groups all showed statistically remarkable increases in Hb content in contrast to the disease group (Figure 10 and Table 4).
The data showed that the WBC count decreased in treatment groups, piroxicam (12.45 ± 0.323 P < 0.001), BMZ-AD 25 mg/kg (12.47 ± 0.163; P < 0.001), BMZ-AD 50 mg/kg (12.60 ± 0.180; P < 0.001), and BMZ-AD 75 mg/kg (12.75 ± 0.133; P < 0.001), when compared to the disease group (15.08 ± 0.292) (Figure 10 and Table 4).
PLT count was practically normalized after treatment with BMZ-AD. The disease group had a higher PLT count (1,447 ± 15.98) than the vehicle control group (798.83 ± 27.20). Piroxicam (1,045 ± 35.17), BMZ-AD 25 mg/kg (940.22 ± 17.07), BMZ-AD 50 mg/kg (923.31 ± 17.11), and BMZ-AD 75 mg/kg (911.23 ± 19.06) treatment groups all demonstrated a drop in PLT count (P < 0.001) in contrast to the disease group (Figure 10 and Table 4).
3.7 Effects of BMZ-AD on biochemical parameters
3.7.1 Effects of BMZ-AD on urea levels
In contrast to the disease group (27.11 ± 0.670), there were no variations in urea levels with piroxicam (26.14 ± 0.615), BMZ-AD 25 mg/kg (26.52 ± 0.665), BMZ-AD 50 mg/kg (26.46 ± 0.644), and BMZ-AD 75 mg/kg (26.39 ± 0.668) treatment (Figure 11 and Table 5).

Effect of treatment with BMZ-AD on biochemical parameters: (a) urea, (b) creatinine, (c) ALT, and (d) AST levels.
Biochemical parameters were not affected by BMZ-AD
| Biochemical parameters | Vehicle control | Disease | Piroxicam (10 mg/kg) | BMZ-AD (25 mg/kg) | BMZ-AD (50 mg/kg) | BMZ-AD (75 mg/kg) |
|---|---|---|---|---|---|---|
| Mean ± SEM | ||||||
| Urea (mg/dl) | 26.50 ± 0.670 | 27.10 ± 0.67 | 26.12 ± 0.615 | 26.55 ± 0.665 | 26.44 ± 0.664 | 26.38 ± 0.668 |
| Creatinine (mg/dl) | 0.81 ± 0.213 | 0.88 ± 0.00 | 0.83 ± 0.020 | 0.86 ± 0.017 | 0.84 ± 0.018 | 0.83 ± 0.022 |
| AST (IU/L) | 92.50 ± 0.763 | 101.0 ± 0.258 | 94.17 ± 0.749 | 94.33 ± 1.02 | 94.50 ± 0.885 | 95.83 ± 1.046 |
| ALT (IU/L) | 31.17 ± 0.477 | 32.00 ± 0.365 | 32.33 ± 0.494 | 32.17 ± 0.307 | 32.50 ± 0.223 | 33.0 ± 0.516 |
3.7.2 BMZ-AD effect on creatinine levels
There were no variations in creatinine levels between the disease group (0.883 ± 0.008) and the piroxicam (0.836 ± 0.020), BMZ-AD 25 mg/kg (0.866 ± 0.017), BMZ-AD 50 mg/kg (0.844 ± 0.018), and BMZ-AD 75 mg/kg (0.837 ± 0.022) treatment groups (Figure 11 and Table 5).
3.7.3 Effects of BMZ-AD on ALT levels
When the vehicle control (31.19 ± 0.477), disease group (32.02 ± 0.365), and piroxicam (32.34 ± 0.494), BMZ-AD 25 mg/kg (32.19 ± 0.307), BMZ-AD 50 mg/kg (32.53 ± 0.223), and BMZ-AD 75 mg/kg (33.02 ± 0.516) treatment groups were examined; there was no significant difference in ALT levels (Figure 11 and Table 5).
3.7.4 Effects of BMZ-AD on AST levels
When AST levels were assessed, there were no significant changes between the vehicle control (92.52 ± 0.763), disease group (101.03 ± 0.258), and piroxicam (94.19 ± 0.749), BMZ-AD25 mg/kg (94.35 ± 1.022), BMZ-AD 50 mg/kg (94.52 ± 0.885), and BMZ-AD 75 mg/kg (95.85 ± 1.046) treatment groups (Figure 11 and Table 5).
3.8 Molecular docking
The best poses obtained from molecular docking were visualized for hydrogen bonds using Discovery Studio Visualizer. The key residues of IL-6 found to interact with BMZ-AD through hydrogen bonds included Gln158, Ser156, Asn110, Ser109, and Phe103 with a binding affinity of −6.7 (Figure 12 and Table 6).

2D and 3D interactions of (a) BMZ-AD and (b) piroxicam with IL-6.
Number of hydrogen bonds with amino acid residues of IL-6 and TNF-α and binding affinity
| Protein | Ligand | Binding affinity (kcal/mol) | Hydrogen bonds |
|---|---|---|---|
| IL-6 | BMZ-AD | 6.7 | Gln158, Ser156, Asn110, Ser109, and Phe103 |
| IL-6 | Piroxicam | 7.2 | Lys126 |
| TNF-α | BMZ-AD | 7.1 | Phe64, Glu116, Asp143, Gln61, Tyr119, and Tyr151 |
| TNF-α | Piroxicam | 8.2 | Tyr151 and Leu120 |
Moreover, BMZ-AD formed hydrogen bonds with Phe64, Glu116, Asp143, Gln61, Tyr119, and Tyr151 residues of target protein TNF-α with a binding affinity of −7.1 (Table 6). When compared to the standard drug piroxicam, our drug showed better interaction and binding affinity with the target protein molecules IL-6 and TNF-α. Both bonded and non-bonded interactions between BMZ-AD and the standard drug piroxicam with the targeted proteins IL-6 and TNF-α are shown in Figures 12 and 13.

2D and 3D interactions of (a) BMZ-AD and (b) piroxicam with TNF-α.
4 Discussion
Chronic autoimmune disease RA mainly affects the bone joints [40] and synovial lining, leading to cartilage destruction, bone erosion, disability, and increased mortality. Arthralgia, swelling, and redness are some of the clinical manifestations of RA [41]. Inflammatory cytokines are the main contributors to RA pathophysiology [42] that produce systemic effects such as acute-phase protein (CRP) formation, anemia, cardiovascular disorders, and osteoporosis [43]. The goal of current RA therapy is pain relief, inflammatory regulation, and remission of disease for all patients. However, the therapy has adverse consequences such as increased risk of heart attack and stroke after prolonged use [44], gastrointestinal disorders, dermatitis, pneumonitis, hematologic disorders, infections, nephrotoxicity, and hepatic dysregulations. Mild to severe side effects may need treatment discontinuation [44,45]. Therefore, new safer drugs are urgently required.
The FCA arthritic rat model recapitulates the immune response illicit by RA in humans. Induction of disease requires subcutaneous or intradermal administration of FCA-emulsified antigen to produce RA-like inflammation in rats [46,47]. This model mimics clinical arthritis with synovial proliferation, inflammation, and cartilage degradation [48]. In this study, we used an FCA-induced arthritic rat model to determine the anti-arthritic potential of the benzimidazole derivative BMZ-AD.
Swollen inflamed and painful joint counts are critical for RA patients to assess clinical progression of the disease and identify treatment targets [49]. Treatment with the benzimidazole derivative significantly reduced FCA-induced paw edema in rats, as compared to the disease group, after 28 days. BMD-AD 75 mg/kg produced anti-inflammatory effects comparable to our standard drug piroxicam [50]. Additionally, histopathological assessment showed a reduction in inflammatory cell infiltration after treatment with the benzimidazole derivative BMZ-AD. Pannus formation due to synovial hyperproliferation leads to cell activities that subsequently promote inflammation and disease progression [51]. BMZ-AD suppressed pannus formation significantly. The results obtained are closer to those obtained for the anti-arthritic agent piroxicam.
IL-6 and TNF-α are the primary inflammatory mediators involved in the pathogenesis of RA. High levels of IL-6 are found in the synovial fluid and serum of RA patients, which correlate with disease activity and joint degeneration [52]. As demonstrated by various studies, treatment with TNFα and IL-6 inhibitors impede the progression of joint impairment and bone erosion [53–55]. In our study, mRNA expression levels of pro-inflammatory markers IL-6 and TNFα were significantly reduced after treatment with BMZ-AD. These results were also corroborated by in silico studies where BMZ-AD bound strongly with IL-6 and TNF-α inhibitor binding sites. BMZ-AD also reduced bone erosion and inflammation aggravated by the production of PGE2-inducible enzymes [56] through suppressing the PGE2 level [57].
Chronic inflammatory processes in RA also tend to increase WBC and PLT counts because of infection and tissue damage. In this study, BMZ-AD treatment significantly reduced WBC and PLT counts, indicating its ability to affect inflammation. Additionally, BMZ-AD was helpful in increasing the RBC count and Hb concentration, indicating that it reduced the inflammatory stress that caused anemia [58]. Both Hb and RBC were found to have an inverse relationship with disease activity in RA patients [59]. In this study, BMZ-AD and the standard drug piroxicam significantly replenished the RBC count and Hb levels and reduced the WBC and PLT counts simultaneously.
Liver function impairment is prevalent in RA patients and is mostly the consequence of prolonged use of anti-rheumatic drugs. Up to 22% of the RA patients on anti-rheumatoid therapy experience abnormal LFTs, with a higher risk in alcohol consumers or having hepatic disorder [60]. AST, ALT, and ALP are reliable markers of liver as well as kidney impairment [61]. To establish the safety of BMZ-AD, we measured serum levels of ALT, AST, creatinine, and urea in all groups. The results showed no difference among all groups, suggesting that BMZ-AD does not possess hepatotoxic and nephrotoxic effects and could be marked as a safe drug.
The use of the novel benzimidazole derivative in RA treatment has its own advantages over the traditional NSAID piroxicam. Piroxicam is a non-selective COX inhibitor, and its action is only able to reduce inflammation. Literature review showed that docking results of benzimidazole derivative claimed that they can be used as COX inhibitors. The benzimidazole–NSAID conjugate showed lesser chance of side effects, especially gastrointestinal and renal complications, which are common with most NSAIDs, hence making it a safer option for patients with long-term use [62]. Moreover, benzimidazole derivatives target inflammatory cytokines, such as TNF-α and IL-6. It has been shown that such a dual effect may be able to control the disease better than piroxicam. On the basis of previous research studies which are in line with our current study, it can be concluded that BMZ-AD possesses combined anti-inflammatory and immunomodulatory properties.
Moreover, the molecular docking analysis indicates that the benzimidazole derivative BMZ-AD can form strong binding with the key proteins IL-6 and TNF-α in RA. BMZ-AD had a binding affinity of −6.7 kcal/mol toward IL-6, which involved an interaction with some important residues such as Gln158, Ser156, Asn110, Ser109, and Phe103. For TNF-α, BMZ-AD showed more pronounced binding energy of −7.1 kcal/mol, which interacted with Phe64, Glu116, Asp143, Gln61, Tyr119, and Tyr151. The results showed that BMZ-AD could target and antagonize the effect of IL-6 and TNF-α, which are vital in the RA inflammatory process. BMZ-AD had a lower affinity to bind IL-6 but had stronger interaction with TNF-α, as compared to piroxicam, which may give it a better potential for treating RA. Generally, BMZ-AD suggests it as a promising new candidate for RA treatment with significant anti-inflammatory and antiarthritic immunomodulatory potential.
5 Conclusions
RA presents a cascade of pathological events, such as inflammation, bone erosion, synovial hypertrophy, and loss of joint, that culminate in physical disability. The current study validates the use of a benzimidazole derivative BMZ-AD against RA using an FCA-induced arthritic model. Our data highlighted that the benzimidazole derivative possessed significant immunomodulatory and anti-inflammatory properties through attenuation of joint inflammation, bone erosion, and cartilage erosion. It might be ascribed to the downregulation of pro-inflammatory markers (TNF-α and IL-6). PGE2 levels were downregulated after treatment with the benzimidazole derivatives, while no adverse effect on renal and hepatic parameters was observed. Therefore, our findings suggest that our drug BMZ-AD can be further screened in-depth for further development in RA treatment.
Acknowledgments
The authors extend their appreciation to the Ongoing Research Funding Program (ORF-2025-457), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: This work is financially supported by the Ongoing Research Funding Program (ORF-2025-457), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Conceptualization, writing of the original draft, formal analysis, investigations, data curation, software: Haseeb Ahmad, Halima Usman, and Aisha Mobashar. Investigations, resources, project administration, reviewing, and editing: Arham Shabbir, Yousef A. Bin Jardan, Musaab Dauelbait, Amira Metouekel, and Mohammed Bourhia.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Xu Y, Wu Q. Prevalence trend and disparities in rheumatoid arthritis among US Adults, 2005-2018. J Clin Med. 2021;10(15):3289.10.3390/jcm10153289Search in Google Scholar PubMed PubMed Central
[2] Sierakowski S, Cutolo M. Morning symptoms in rheumatoid arthritis: a defining characteristic and marker of active disease. Scand J Rheumatol. 2011;40(sup125):1–5.10.3109/03009742.2011.566433Search in Google Scholar PubMed
[3] Al-Rubaye AF, Kadhim MJ, Hameed IH. Rheumatoid arthritis: history, stages, epidemiology, pathogenesis, diagnosis and treatment. Int J Toxicological Pharmacol Res. 2017;9(2):145–55.10.25258/ijtpr.v9i02.9052Search in Google Scholar
[4] Sattar S, Shabbir A, Shahzad M, Akhtar T, Ahmad A, Alnasser SM, et al. Eichhornia crassipes ameliorated rheumatoid arthritis by modulating inflammatory cytokines and metalloproteinase enzymes in a rat model. Medicina. 2023;59(9):1594.10.3390/medicina59091594Search in Google Scholar PubMed PubMed Central
[5] Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–48.10.1016/S0140-6736(17)31491-5Search in Google Scholar PubMed
[6] Rathore A, Sudhakar R, Ahsan MJ, Ali A, Subbarao N, Jadav SS, et al. In vivo anti-inflammatory activity and docking study of newly synthesized benzimidazole derivatives bearing oxadiazole and morpholine rings. Bioorg Chem. 2017;70:107–17.10.1016/j.bioorg.2016.11.014Search in Google Scholar PubMed
[7] Brishty SR, Hossain MJ, Khandaker MU, Faruque MRI, Osman H, Rahman SMA. A Comprehensive account on recent progress in pharmacological activities of benzimidazole derivatives. Front Pharmacol. 2021;12:762807.10.3389/fphar.2021.762807Search in Google Scholar PubMed PubMed Central
[8] Usman H, Tan Z, Gul M, Rashid S, Ali T, Shah FA, et al. Identification of novel and potential PPARγ stimulators as repurposed drugs for MCAO associated brain degeneration. Toxicol Appl pharmacol. 2022;446:116055.10.1016/j.taap.2022.116055Search in Google Scholar PubMed
[9] Goebel M, Wolber G, Markt P, Staels B, Unger T, Kintscher U, et al. Characterization of new PPARγ agonists: Benzimidazole derivatives - Importance of positions 5 and 6, and computational studies on the binding mode. Bioorg Med Chem. 2010;18:5885–95.10.1016/j.bmc.2010.06.102Search in Google Scholar PubMed
[10] Bano S, Nadeem H, Zulfiqar I, Shahzadi T, Anwar T, Bukhari A, et al. Synthesis and anti-inflammatory activity of benzimidazole derivatives; an In vitro, in vivo and in silico approach. Heliyon. 2024;10(9):e30102.10.1016/j.heliyon.2024.e30102Search in Google Scholar PubMed PubMed Central
[11] Chauhan M, Gupta S, Paliwal S, Nain S. Synthesis, characterization and biological evaluation of novel benzimidazole derivatives against candida albicans. Pharm Chem J. 2024;57(12):1934–40.10.1007/s11094-024-03099-wSearch in Google Scholar
[12] Davie T, Serrat X, Imhof L, Snider J, Štagljar I, Keiser J, et al. Identification of a family of species-selective complex I inhibitors as potential anthelmintics. Nat Commun. 2024;15(1):3367.10.1038/s41467-024-47331-3Search in Google Scholar PubMed PubMed Central
[13] Ali A, Vasanthi R, Tiwari Y, Ahmad MTDS, Zambare YB, Deokule TA, et al. Synthesis of some novel benzimidazole derivatives as potential therapeutic agents. Eur Chem Bull. 2023;12(si6):4235–46Search in Google Scholar
[14] Patagar DN, Batakurki SR, Kusanur R, Patra SM, Saravanakumar S, Ghate M. Synthesis, antioxidant and anti-diabetic potential of novel benzimidazole substituted coumarin-3-carboxamides. J Mol Struct. 2023;1274:134589.10.1016/j.molstruc.2022.134589Search in Google Scholar
[15] Sharma S, Gupta M, Sahu J. Significance of Benzimidazole analogues for the creation of novel molecules in drug discovery. Curr Chem Lett. 2023;12(1):27–44.10.5267/j.ccl.2022.9.008Search in Google Scholar
[16] Francesconi V, Rizzo M, Schenone S, Carbone A, Tonelli M. State-of-the-art review on the antiparasitic activity of benzimidazole-based derivatives: facing malaria, leishmaniasis, and trypanosomiasis. Curr Med Chem. 2024;31(15):1955.10.2174/0929867331666230915093928Search in Google Scholar PubMed PubMed Central
[17] Aljameel SS, Fataftah HM, EL-Rahman SN, Asma ME, Hafiane A, Kamoun M. Ultrasound synthesis of benzimidazolo-1, 3, 5-triazine derivatives and their anti-histamine and anti-diabetic activities. Orient J Chem. 2019;35(4):1368.10.13005/ojc/350417Search in Google Scholar
[18] Ezgallaey HM, Abuskhuna SM, Dugani AM. Gastroprotective effects of 1-Hydroxy-2-phenylbenzimidazole in ethanol-induced gastric ulcers in rats. Libyan Int Med Univ J. 2016;1(2):82–92.10.21502/limuj.009.01.2016Search in Google Scholar
[19] Sur VP, Sen MK, Mazumdar A. Redefining antibacterial strategies with computational screening of benzimidazole ligands against vanz protein for alternatives of antibiotic. Biological and Medicinal Chemistry; 2024.10.26434/chemrxiv-2024-0hzg8Search in Google Scholar
[20] El Alami A, Sdassi H, Bouzikri S. Review of synthesis process of benzimidazole-heterocycle hybrid compounds. Synth Commun. 2024;54(8):613–35.10.1080/00397911.2024.2316718Search in Google Scholar
[21] Abouzied AS, Break MKB, Huwaimel B, Hussein W, Alafnan A, Younes KM, et al. Discovery of a novel synthetic thiazole-benzimidazole conjugate that acts as a potent pancreatic lipase inhibitor using in silico and in vitro approaches. Indian J Pharm Educ Res. 2023;57(1):218–27.10.5530/001954642131Search in Google Scholar
[22] Almansour AI, Arumugam N, Soliman SM, Viswanathan E, Dege N, Karuppiah P, et al. Eco-friendly synthesis, structural elucidation, computational investigation and in vitro antioxidant activity of a new N-tosylated benzimidazole derivative. J Mol Struct. 2024;1296:136825.10.1016/j.molstruc.2023.136825Search in Google Scholar
[23] Wang X-J, Hou X, Zhang L-Y, Wang B-Y, Wu M-y, Chen H-J, et al. Design, synthesis, and antitumor activity of benzimidazole derivatives as CDK4/6 inhibitors. J Mol Struct. 2024;1309:138189.10.1016/j.molstruc.2024.138189Search in Google Scholar
[24] Jahan J, Barik S, Chagaleti BK, Kathiravan MK, Deivasigamani P. The synthetic approach of benzimidazole derivatives as anti-inflammatory agents. Inflammation. 2023;14:19.10.55522/jmpas.V12I5.4826Search in Google Scholar
[25] Carter JL, Lubahn C, Lorton D, Osredkar T, Der TC, Schaller J, et al. Adjuvant-induced arthritis induces c-Fos chronically in neurons in the hippocampus. J Neuroimmunol. 2011;230(1–2):85–94.10.1016/j.jneuroim.2010.09.005Search in Google Scholar PubMed
[26] Ebenezer O, Oyetunde-Joshua F, Omotoso OD, Shapi M. Benzimidazole and its derivatives: Recent Advances (2020-2022). Results Chem. 2023;5:100925.10.1016/j.rechem.2023.100925Search in Google Scholar
[27] Singh VS, Dhawale SC, Shakeel F, Faiyazuddin M, Alshehri S. Antiarthritic potential of calotropis procera leaf fractions in fca-induced arthritic rats: involvement of cellular inflammatory mediators and other biomarkers. Agriculture. 2021;11(1):68.10.3390/agriculture11010068Search in Google Scholar
[28] Mobashar A, Shabbir A, Shahzad M. Evaluation of immunomodulatory and antiarthritic potential of Trigonella gharuensis extracts. Evidence-based Complementary Altern Med. 2020;2020:1–7.Search in Google Scholar
[29] Gurram S, Anchi P, Panda B, Tekalkar SS, Mahajan RB, Godugu C. Amelioration of experimentally induced inflammatory arthritis by intra-articular injection of visnagin. Curr Res Pharmacol Drug Discovery. 2022;3:100114.10.1016/j.crphar.2022.100114Search in Google Scholar PubMed PubMed Central
[30] Mobashar A, Shabbir A, Shahzad M, Saeed Ul H. Evaluation of immunomodulatory and antiarthritic potential of trigonella gharuensis extracts. Evidence-based Complementary Altern Med. 2020;2020:8836080.10.1155/2020/8836080Search in Google Scholar PubMed PubMed Central
[31] Dar E, Mobashar A, Shabbir A, Mushtaq MN, Anjum I Z, Gaafar A-R, et al. Mechanistic evaluation of antiarthritic effects of citronellol in CFA-induced arthritic rats. ACS omega. 2023;8(47):44955–63.10.1021/acsomega.3c06374Search in Google Scholar PubMed PubMed Central
[32] Sattar S, Shabbir A, Shahzad M, Akhtar T, Anjum SM, Bourhia M, et al. Evaluation of anti-inflammatory and immunomodulatory potential of Lawsone (2-hydroxy-1, 4-naphthoquinone) using pre-clinical rodent model of rheumatoid arthritis. Front Pharmacol. 2023;14:1279215.10.3389/fphar.2023.1279215Search in Google Scholar PubMed PubMed Central
[33] Mashaal K, Shabbir A, Khan MA, Hameed H, Shahzad M, Irfan A, et al. Anti-arthritic and immunomodulatory potential of methanolic, n-hexane, and ethyl acetate fractions of bark of Acacia modesta on complete Freund’s adjuvant-induced arthritis in rats. Pharmaceutics. 2023;15(9):2228.10.3390/pharmaceutics15092228Search in Google Scholar PubMed PubMed Central
[34] Mashaal K, Shabbir A, Shahzad M, Mobashar A, Akhtar T, Fatima T, et al. Amelioration of rheumatoid arthritis by fragaria nubicola (wild strawberry) via attenuation of inflammatory mediators in sprague dawley rats. Medicina. 2023;59(11):1917.10.3390/medicina59111917Search in Google Scholar PubMed PubMed Central
[35] Agarwal V, Kaushik AS, Rehman M, Chaudhary R, Jawaid T, Kamal M, et al. Interleukin-6 expression and its modulation by diacerein in a rat model of chronic stress induced cardiac dysfunction. Heliyon. 2021;7(12):e08522.10.1016/j.heliyon.2021.e08522Search in Google Scholar PubMed PubMed Central
[36] Zia K, Ashraf S, Jabeen A, Saeed M, Nur-e-Alam M, Ahmed S, et al. Identification of potential TNF-α inhibitors: from in silico to in vitro studies. Sci Rep. 2020;10(1):20974.10.1038/s41598-020-77750-3Search in Google Scholar PubMed PubMed Central
[37] Adeniji SE, Shallangwa GA, Arthur DE, Abdullahi M, Mahmoud A, Haruna A. Quantum modelling and molecular docking evaluation of some selected quinoline derivatives as anti-tubercular agents. Heliyon. 2020;6(3).10.1016/j.heliyon.2020.e03639Search in Google Scholar PubMed PubMed Central
[38] Chandel V, Raj S, Rathi B, Kumar D. In silico identification of potent COVID-19 main protease inhibitors from fda approved antiviral compounds and active phytochemicals through molecular docking: a drug repurposing approach. Preprints: Preprints; 2020.10.20944/preprints202003.0349.v1Search in Google Scholar
[39] Oraibi AI, Karav S, Khallouki F. Exploration of rosmarinic acid as anti-esophageal cancer potential by use of network pharmacology and molecular docking approaches. Atl J Life Sci. 2025;2025(1):11. 10.71005/b5jben88.Search in Google Scholar
[40] Derksen VFAM, Huizinga TWJ, van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. SemImmunopathology. 2017;39(4):437–6.10.1007/s00281-017-0627-zSearch in Google Scholar PubMed PubMed Central
[41] Guo Q, Wang Y, Xu D, Nossent J, Pavlos NJ, Xu J. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(1):15.10.1038/s41413-018-0016-9Search in Google Scholar PubMed PubMed Central
[42] Choy E. Understanding the dynamics: pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology. 2012;51(suppl_5):v3–11.10.1093/rheumatology/kes113Search in Google Scholar PubMed
[43] Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: a systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–19.10.1016/j.bbi.2017.01.011Search in Google Scholar PubMed PubMed Central
[44] Wang W, Zhou H, Liu L. Side effects of methotrexate therapy for rheumatoid arthritis: A systematic review. Eur J Medicinal Chem. 2018;158:502–16.10.1016/j.ejmech.2018.09.027Search in Google Scholar PubMed
[45] George MD, Baker JF, Winthrop K, Hsu JY, Wu Q, Chen L, et al. Risk for serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis: a cohort study. Ann Intern Med. 2020;173(11):870–8.10.7326/M20-1594Search in Google Scholar PubMed PubMed Central
[46] Fontes JA, Barin JG, Talor MV, Stickel N, Schaub J, Rose NR, et al. Complete Freund’s adjuvant induces experimental autoimmune myocarditis by enhancing IL-6 production during initiation of the immune response. Immun Inflammation Dis. 2017;5(2):163–76.10.1002/iid3.155Search in Google Scholar PubMed PubMed Central
[47] Xie X, Li H, Wang Y, Wan Z, Luo S, Zhao Z, et al. Therapeutic effects of gentiopicroside on adjuvant-induced arthritis by inhibiting inflammation and oxidative stress in rats. Int Immunopharmacol. 2019;76:105840.10.1016/j.intimp.2019.105840Search in Google Scholar PubMed
[48] Patel R, Kadri S, Gohil P, Deshpande S, Shah G. Amelioration of complete Freund’s adjuvant-induced arthritis by Calotropis procera latex in rats. Future J Pharm Sci. 2021;7(1):213.10.1186/s43094-021-00361-wSearch in Google Scholar
[49] Sun X, Deng X, Xie W, Wang L, Wang Y, Zhang Z. The agreement between ultrasound-determined joint inflammation and clinical signs in patients with rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):100.10.1186/s13075-019-1892-0Search in Google Scholar PubMed PubMed Central
[50] Huskisson EC.Treatment of osteoarthritis with piroxicam. Eur J Rheumatol Inflamm. 1983;6(1):73–83.Search in Google Scholar
[51] Cajas LJ, Casallas A, Medina YF, Quintana G, Rondón F. Pannus and rheumatoid arthritis: Historic and pathophysiological evolution. Rev Colomb Reumatol. 2019;26(2):118–28.10.1016/j.rcreue.2018.10.005Search in Google Scholar
[52] Srirangan S, Choy EH. The role of interleukin 6 in the pathophysiology of rheumatoid arthritis. Ther Adv Musculoskeletal Dis. 2010;2(5):247–56.10.1177/1759720X10378372Search in Google Scholar PubMed PubMed Central
[53] Hayashi S, Matsubara T, Fukuda K, Funahashi K, Hashimoto M, Maeda T, et al. Predictive factors for effective selection of Interleukin-6 inhibitor and tumor necrosis factor inhibitor in the treatment of rheumatoid arthritis. Sci Rep. 2020;10(1):16645.10.1038/s41598-020-73968-3Search in Google Scholar PubMed PubMed Central
[54] Kistner TM, Pedersen BK, Lieberman DE. Interleukin 6 as an energy allocator in muscle tissue. Nat Metab. 2022;4(2):170–9.10.1038/s42255-022-00538-4Search in Google Scholar PubMed
[55] Kim GW, Lee NR, Pi RH, Lim YS, Lee YM, Lee JM, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharmacal Res. 2015;38(5):575–84.10.1007/s12272-015-0569-8Search in Google Scholar PubMed
[56] McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110(5):651–8.10.1172/JCI15528Search in Google Scholar PubMed PubMed Central
[57] Nazir S, Ahmad I, Mobashar A, Sharif A, Shabbir A, Chaudhary WA. Mechanistic evaluation of antiarthritic and anti-inflammatory effect of campesterol ester derivatives in complete Freund’s adjuvant-induced arthritic rats. Front Pharmacol. 2024;14:1346054.10.3389/fphar.2023.1346054Search in Google Scholar PubMed PubMed Central
[58] Saleem MU, Muhammad F, Sharif A, Arshad MI, Akhtar K, Javed Y, et al. Methotrexate-loaded biodegradable nanoparticles exert anti-arthritic effect by downregulating pro-inflammatory cytokines in Freund’s complete adjuvant-induced arthritic rats. Inflammopharmacology. 2022;30(3):1079–91.10.1007/s10787-022-00977-1Search in Google Scholar PubMed
[59] Dechanuwong P, Phuan-Udom R. Hematological parameters as a predictor of disease remission in patients with rheumatoid arthritis. Ann Med Surg. 2012;72:103085.10.1016/j.amsu.2021.103085Search in Google Scholar PubMed PubMed Central
[60] Fahmy A, MacDonald G, Evans A. Abnormal liver function tests in inflammatory arthritis: think beyond the DMARDs. Oxf Med case Rep. 2018;2018(9);omy058.10.1093/omcr/omy058Search in Google Scholar PubMed PubMed Central
[61] Nana WY, Kwenteh EAH, Ateufack G, Tsafack EG, Nguemnang SFD, Njoya ZLF, et al. Anti-arthritic effect of Distemonanthus benthamianus extracts against rheumatoid arthritis in rats. Asian Pac J Trop Biomed. 2022;12(10):411.10.4103/2221-1691.357740Search in Google Scholar
[62] Elrayess R, Elrayess R, Ghareb N. Synthesis, molecular docking, and anti-inflammatory activities of some novel benzimidazole derivatives as potential cyclo-oxygenase-2 inhibitors. Egypt J Chem. 2024;67(1):255–66.Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

