Abstract
This investigation explores the impact of hesperidin and its zinc(ii) complex on osteoblast differentiation and subsequent bone formation. The biocompatibility of synthesized complexes (0–20 μg/mL) was assessed in vitro using mouse mesenchymal stem cells, while in vivo toxicity was evaluated using a chick embryo model. Both hesperidin and its zinc(ii) complex were found to be non-toxic at a concentration of 10 μg/mL. Notably, these compounds significantly increased alkaline phosphatase activity and enhanced calcium deposition. Molecular analyses revealed upregulation of Runx2 and type 1 collagen mRNA expression, along with increased levels of osteonectin and osteocalcin proteins, while negative regulators of osteoblast differentiation (Smad7, Smurf1, HDAC7) were downregulated. A new aspect of this study is demonstrating that the zinc(ii) complex of hesperidin uniquely enhances osteogenic activity compared to hesperidin alone, highlighting its potential to improve bone formation significantly. Additionally, we elucidated the role of miR-143-3p in mediating these effects, achieved through HDAC7 suppression and enhanced Runx2 expression, assessed using the pmirGLO dual luciferase reporter system. Zebrafish studies further demonstrated the complexes’ effects on bone formation, revealing increased osteoblastic activity and improved calcium-to-phosphorus ratios in regenerated scales. These findings underscore the potential of hesperidin–Zn(ii) as a promising therapeutic agent for bone tissue engineering.
1 Introduction
Hesperidin, a natural bioflavonoid compound commonly found in citrus fruits such as oranges, lemons, tangerines, and grapefruits [1] possesses a wide range of biological properties, including anti-inflammatory, antioxidant, anti-aging, anticancer, and antibacterial effects [2,3,4]. Recent research indicates that flavonoid compounds, like hesperidin, play a crucial role in improving skeletal health and addressing conditions such as osteoporosis. These compounds act through various pathways to enhance bone density, such as by reducing oxidative stress and inflammation, thus promoting osteoblast formation and osteoclast differentiation [5,6,7]. Numerous studies have demonstrated hesperidin’s potential in preventing bone loss and facilitating bone defect regeneration. Additionally, the literature suggests that hesperidin may stimulate osteogenesis [6], which investigated its effects on preosteoblast cell function, osteogenesis, and collagen matrix quality. Their findings suggest that hesperidin regulates mineralized tissue formation by modulating osteoblast differentiation and controlling the ratio between matrix tissue and mineral.
Metal ions play indispensable roles in life’s metabolic processes. Among various compounds, flavonoids are recognized as efficient chelators of several metal ions, including zinc, iron, and copper. Flavonoid metal complexes have emerged as potential contributors to bone formation, presenting promising avenues for bone tissue regeneration. Research suggests that these complexes exhibit enhanced activity compared to their individual flavonoid counterparts. For instance, zinc/copper–silibinin complexes have demonstrated superior potential in promoting angiogenesis and osteogenesis relative to natural silibinin activity. Similarly, the quercetin–copper combination has shown enhanced angiogenesis and osteogenesis compared to native quercetin. Furthermore, the Kaempferol–zinc(ii) combination has been observed to enhance bone development in zebrafish, surpassing the stimulation achieved by Kaempferol alone [8]. Zinc (Zn), a prevalent trace element, forms complexes with flavonoids, displaying robust biological activities in cellular signaling pathways, particularly those involved in bone growth. Additionally, copper is a critical trace mineral vital for maintaining optimal bone health. Animal studies have demonstrated that a copper-deficient diet leads to reduced bone mineral content and strength [9]. Therefore, this study aims to synthesize a hesperidin–zinc(ii) complex to enhance the osteogenic activity of hesperidin. We hypothesize that this complex will improve the osteogenic properties of hesperidin, promoting increased osteoblast differentiation and mineralization. Moreover, it may exhibit superior effects on bone formation compared to hesperidin alone by modulating key cellular pathways and enhancing bioavailability.
2 Materials and methods
2.1 Synthesis of hesperidin–zinc(ii) complex
To synthesize the hesperidin–zinc(ii) complex, 1.00 × 10−3 mol/L of hesperidin (purchased from Sigma Aldrich) was mixed with an equal concentration of ZnCl2 in a ratio of 4:1 [10]. The resulting blended liquid, totaling 500 mL, was transferred into a round-bottom flask and subjected to heating, stirring, and refluxing at 70°C for 3 h until complete dissolution was achieved. Subsequently, the pH of the mixture was adjusted to 10.50 using 0.1 mol/L NaOH, and after allowing the solution to stand for 5 min, it was concentrated to 50 mL using a rotary evaporator. The residue obtained was washed multiple times with absolute methanol to eliminate impurities. Finally, the complex was dried under a vacuum for 12 h and utilized as a sample for infrared analysis.
2.2 Cell culture and MTT assay
For cell culture and MTT assay, immortalized mouse mesenchymal stem cells (MSCs), specifically C3H10T1/2, were acquired from NCCS, Pune, India, and maintained in DMEM supplemented with 10% FBS under standard conditions at 37°C with 5% CO2. The MTT assay was conducted following established protocols. Briefly, cells were seeded at a density of 2 × 104 cells per well in 96-well plates and allowed to adhere for 12 h. Subsequently, the cells were treated with varying concentrations (ranging from 0 to 20 μg/mL) of either hesperidin or the hesperidin–zinc(ii) complex for up to 48 h. Following the incubation period, MTT solution at a concentration of 5 mg/mL was added to each well, and the plates were further incubated at 37°C for 4 h. Absorbance readings were taken at 570 nm. After treatment, the cells were washed and incubated with Fluorescein diacetate (FDA), a live cell staining solution at a concentration of 30 μg/mL for 5 min. The cellular morphology was then examined under a fluorescent microscope.
2.3 Alkaline phosphatase (ALP) activity assay
On day 14 of post-osteogenic induction, the assessment of ALP activity was conducted. ALP activity was determined utilizing an ALP activity kit following the manufacturer’s instructions provided by Beyotime. The absorbance of the samples was measured at 405 nm wavelength.
2.4 Alizarin red staining
After the treatment, the cell samples underwent a thorough washing procedure with phosphate-buffered saline (PBS), aimed at removing any extraneous debris or residual media. Subsequently, the cells were fixed by immersion in 95% ethanol for a duration of 10 min, ensuring the preservation of their structural integrity. Following fixation, the samples underwent another round of washing with PBS, which served to eliminate any excess ethanol and prepare the cells for staining. The cell cultures were then covered with a 0.1% solution of Alizarin red and allowed to incubate for 10 min, facilitating the specific staining of calcium deposits within the nodules. Upon completion of the incubation period, the samples were rinsed meticulously with PBS to remove any unbound dye molecules. Finally, the stained cell cultures were subjected to observation under an inverted light microscope to visualize and analyze the presence and distribution of calcified nodules.
2.5 Real-time RT-PCR analysis
Total RNA extraction was conducted utilizing TRIzol reagent (Invitrogen), following the manufacturer’s protocol to ensure efficient isolation. Subsequently, complementary DNA (cDNA) synthesis was performed using the RevertAid First Strand cDNA Synthesis Kit (K1622, Thermo Fermentas), employing the isolated RNA as the template. For quantitative PCR amplification, iTaq Universal SYBR Green Supermix (Bio-Rad) was utilized according to the manufacturer’s instructions. To ensure accuracy and reliability, GAPDH or U6 served as internal reference controls for normalization purposes. The primer sequence used is listed in Table S1 [11,12]. The gene expression levels were quantified using the 2−ΔΔCt method, which allows for the comparative analysis of gene expression between different samples while considering variations in PCR efficiency and RNA input.
2.6 ELISA quantification of osteonectin (ON) and osteocalcin (OC)
Following treatment with hesperidin or hesperidin–zinc(ii) complex, the conditioned medium containing secreted proteins was collected from the cells. The levels of OC and ON were quantified using commercially available ELISA Kits (Elabscience) according to the manufacturer’s instructions. Briefly, the conditioned medium samples were added to the ELISA plate pre-coated with specific antibodies for OC and ON. After an appropriate incubation period allowing for binding of the target proteins, the unbound substances were washed away. Subsequently, enzyme-conjugated secondary antibodies were added to facilitate detection. Following another washing step to remove excess conjugates, a substrate solution was added to induce a colorimetric reaction. The intensity of the resulting color was measured spectrophotometrically at a specific wavelength corresponding to the detection of OC and ON. The concentrations of OC and ON in the samples were determined by comparing the absorbance values to standard curves generated using known concentrations of OC and ON provided with the ELISA Kits. This method allowed for accurate quantification of OC and ON levels in the conditioned medium, providing valuable insights into the effects of hesperidin or hesperidin–zinc(ii) complex on the secretion of these osteogenic markers.
2.7 Toxicity assessment of hesperidin and hesperidin–zinc(ii) complex on chick embryo development: a morphological evaluation
Fertilized leghorn chicken eggs procured from a local authorized dealer were utilized to investigate the toxicity of hesperidin or hesperidin–zinc(ii) complex following established protocols [13]. Injection of various concentrations of hesperidin or hesperidin–zinc(ii) complex was performed on chick embryos at the HH-12 stage (day 2) of development, with double-distilled water serving as the control. The experiment encompassed multiple replicates (n = 30) to ensure statistical robustness. Subsequent to treatment, embryos were dissected at the HH-37 stage (11th day) to assess morphological alterations, enabling a thorough evaluation of hesperidin and hesperidin–zinc(ii) complex toxicity on chick embryo development and yielding insights into their safety profiles.
2.8 Assessment of bone formation effects of hesperidin and hesperidin–zinc(ii) complex using the zebrafish scale model
The zebrafish (Danio rerio) scale model was employed to examine the effects of hesperidin and hesperidin–zinc(ii) complex on bone formation. Adult zebrafish, aged 3 months, were exposed to 10 μg/mL of hesperidin and hesperidin–zinc(ii) complex for a period of 14 days. Scales were carefully excised from each fish at day zero, totaling 30 scales per fish, and the fish were then maintained under the designated treatment conditions. After 14 days, the regenerating scales were collected for further analysis. The removed scales were fixed in 4% paraformaldehyde and subjected to von Kossa staining to assess calcium deposition, utilizing a procedure involving silver nitrate staining under ultraviolet light, which selectively stains calcium deposits black. Mineral analysis of the scales was performed using inductively coupled plasma mass spectrometry (ICP-MS). The scales were dissolved in 65% nitric acid and diluted 100 times with MilliQ water for subsequent ICP-MS analysis. Total phosphorus, calcium, and magnesium contents were quantified, and the calcium/phosphorus molar ratio was calculated to evaluate mineral composition. This integrated approach facilitated the comprehensive assessment of bone mineralization and composition in response to hesperidin and hesperidin–zinc(ii) complex in the zebrafish scale model.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and has been approved by the institutional animal ethics committee.
2.9 Assessment of microRNA (miRNA) regulation on HDAC7 3′UTR constructs using a luciferase reporter assay
The wild-type or mutant 3′UTR sequences of HDAC7 were chemically synthesized. The sequences were cloned between the PmeI and XbaI restriction sites in the pmirGLO dual-luciferase miRNA target expression vector (Promega), incorporating an internal NotI site. The resulting constructs were screened for the presence of the HDAC7 3′UTR region using the NotI restriction enzyme. MG63 cells were transiently transfected with either wild-type or mutant HDAC7 3′UTR constructs (200 ng) along with control miRNA or miR-143-3p mimic (50 nM) using Lipofectamine-2000 transfection reagent (Invitrogen). After 24 h, luciferase activity was measured using a dual-luciferase reporter assay kit (Promega). Firefly luciferase activity was normalized to Renilla luciferase activity according to established protocols [14]: 3′UTR of HDAC7 wild (sense 5′ AAAC TA GCGGCCGC TAGT ATGGTGCCAAACCCTTCATCTC T 3′ and antisense 3′ TTTG AT CGCCGGCG ATCA TACCACGGTTTGGGAAGTAGAG AGATC 5′) and 3′UTR of HDAC7 mutant (sense 5′ AAAC TA GCGGCCGC TAGT ATGGTGCCAAACCCTTCTATTC T 3′ and antisense 3′ TTTG AT CGCCGGCG ATCA TACCACGGTTTGGGAAGATAAG AGATC 5′).
2.10 Statistical analysis
All experimental results underwent validation through a minimum of three independent experiments, ensuring reliability. Statistical analyses were carried out employing SPSS 20.0 software to ascertain significance. The outcomes were presented as mean values accompanied by standard deviations to depict data dispersion accurately. Quantitative data were subjected to comparison utilizing one-way analysis of variance and Student’s t-test, facilitating the assessment of intergroup differences. A significance threshold of p < 0.05 was established for all statistical evaluations, ensuring robustness and reliability in the interpretation of results.
3 Results and discussion
3.1 Comprehensive evaluation of the biocompatibility of hesperidin and its zinc(ii) complex
The characterization of the hesperidin–zinc(ii) complex has been extensively reported in the previous manuscript. Various techniques were employed to study the structure of the complex, including scanning electron microscopy (SEM)–energy-dispersive X-ray spectroscopy for morphological analysis, X-ray diffraction for crystallographic information, Fourier-transform infrared spectroscopy for functional group identification, thermogravimetric analysis for thermal stability, and differential scanning calorimetry for phase transitions. These methods collectively provide a comprehensive understanding of the properties and behavior of the hesperidin–zinc(ii) complex [15,16,17,18]. The biocompatibility of hesperidin and its zinc(ii) complex was thoroughly evaluated using both in vitro and in vivo investigations, with the goal of understanding their effects on osteoblast development and bone formation. At first, mouse MSCs were tested using the MTT assay to measure their cellular reaction to different doses (ranging from 0 to 20 μg/mL) of hesperidin and hesperidin–Zn(ii) complex during a 48-h timeframe. Figure 1a demonstrates the metabolic activity of the cells after treatment, showing a significant increase in cellular activity, especially at a concentration of 10 μg/mL of the hesperidin–Zn(ii) complex. Surprisingly, there were no indications of toxicity seen at concentrations of up to 10 μg/mL for both hesperidin and its zinc(ii) complex. In addition, the impact of a concentration of 10 μg/mL of hesperidin and hesperidin–Zn(ii) complex on the shape and structure of osteoblasts was assessed by FDA staining, as shown in Figure 1b. The acquired pictures showed well-dispersed osteoblasts with distinct cytoplasmic extensions, indicating favorable circumstances for cell development. Furthermore, the biocompatibility of hesperidin and its complex was evaluated in vivo using a chick embryo model. Table 1 displays the examination of physical characteristics after treatment, indicating that there were no noticeable changes in chick embryo growth up to a concentration of 10 μg/mL for both substances. Moreover, zebrafish embryos that were subjected to hesperidin and its zinc(ii) complex at a concentration of up to 10 μg/mL did not display any harmful or negative effects (unpublished data). Based on the outcomes of biocompatibility tests conducted in a laboratory setting and on living organisms, it was determined that a concentration of 10 μg/mL for both hesperidin and its zinc(ii) complex is the most suitable for further investigations.
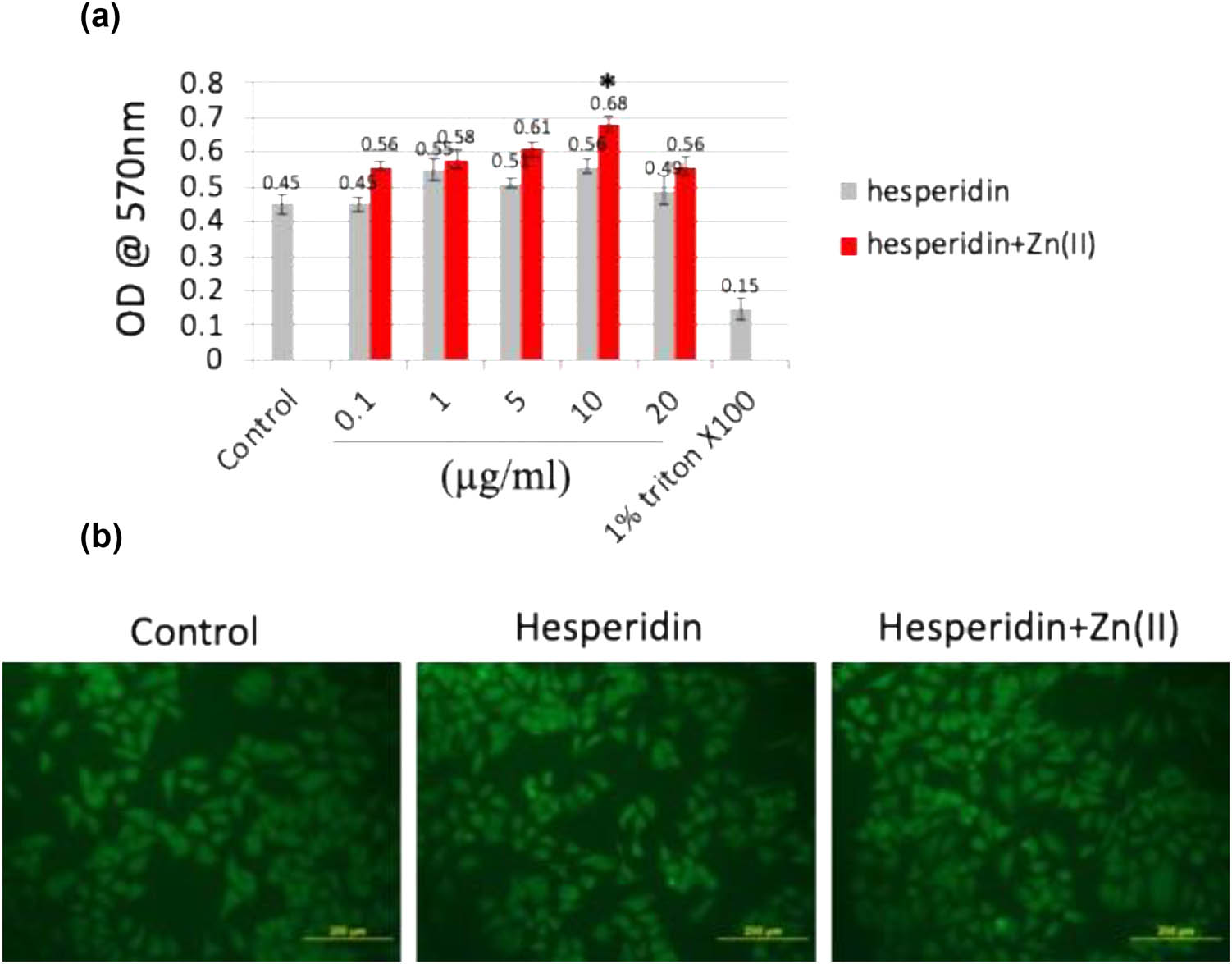
(a) The MTT assay was conducted to assess the cellular metabolic activity of mouse MSC cells exposed to various concentrations (0–20 μg/mL) of hesperidin and hesperidin–Zn(ii) complexes for up to 48 h. (b) FDA staining was performed to visualize cell morphology and viability after treatment with 10 μg/mL of hesperidin and hesperidin–Zn(ii) complexes for 24 h, followed by observation under a fluorescent microscope. Scale bar: 200 μm. * indicates a statistically significant increase compared to control.
The results of toxicity studies conducted on hesperidin and hesperidin–Zn complexes using a chick embryo model
| Group | Animal condition | Heart rate (Bpm) | Weight of body (g) | Weight of heart (mg) | Length of animal (cm) | Twisted neck (%) | Hemorrhage (rupture blood vessels) (%) | Microphthalmia (abnormal eyes) (%) | Polydactyly (extra finger) (%) | Micromelia (smallness of one limb) (%) | Excencephaly (brain outside skull) (%) | Omphalocele (organ develop outside) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | Live | 56.6 ± 5.2 | 4.1 ± 1.4 | 62 ± 9 | 5.3 ± 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hesperidin (10 µM) | Live | 56.6 ± 4.3 | 4.1 ± 1.2 | 61 ± 12 | 5.3 ± 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hesperidin (20 µM) | Live | 56.7 ± 4.5 | 4.2 ± 1.3 | 61 ± 10 | 5.2 ± 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hesperidin–Zn(ii) (10 µM) | Live | 56.2 ± 3.4 | 4.2 ± 1.4 | 61 ± 12 | 5.2 ± 1.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hesperidin–Zn(ii) (20 µM) | Live | 56.3 ± 3.3 | 4.3 ± 1.5 | 61 ± 11 | 5.4 ± 1.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The percentage mean values for each group were obtained from three independent experiments, with the SEM indicated as ±. Each treatment group consisted of 30 eggs.
3.2 Hesperidin and its zinc(ii) complex enhance the differentiation of mouse MSCs into osteoblasts
Exposing mouse MSCs to varying concentrations (0–20 μg/mL) of hesperidin and hesperidin–zinc(ii) complex enabled the assessment of osteoblast differentiation at both cellular and molecular levels. Initially, after 7 days of treatment, ALP activity was measured (Figure 2a), where 10 μg/mL of both hesperidin and hesperidin–Zn(ii) complex exhibited notably higher activity compared to other concentrations. ALP, produced by osteoblasts, plays a crucial role in bone matrix calcification and serves as a precursor marker for osteoblastic cells, under the influence of Runx2, a critical bone transcription factor [8]. Remarkably elevated ALP activity was observed at 10 μg/mL concentration of both hesperidin and hesperidin–Zn(ii) complex. Furthermore, to elucidate the role of these compounds in calcium deposition, mouse MSCs were treated with 10 μg/mL hesperidin and the hesperidin–Zn(ii) complex for up to 14 days (Figure 2b and c). Post-treatment, cells were stained with Alizarin red (Figure 3b), and calcium deposition was quantified (Figure 2c). Results revealed that hesperidin increased calcium deposition compared to the control, with further enhancement observed with the hesperidin–Zn(ii) complex. The cellular-level effects of hesperidin and hesperidin–Zn(ii) complex positively regulated osteoblast differentiation, with the 10 μg/mL concentration deemed optimal for osteoblastic stimulation.

Hesperidin and hesperidin–Zn(ii) complex promoted mouse MSC differentiation toward osteoblasts at the cellular level. (a) Mouse MSCs were treated with different concentrations (5–20 μg/mL) of hesperidin and hesperidin–Zn(ii) complexes for up to 7 days, and ALP activity was measured. (b) Cells treated with 10 μg/mL of hesperidin and hesperidin–Zn(ii) complexes for 7 days were subjected to Alizarin red staining to assess calcium deposition. (c) Quantification of calcium deposition. * indicates a significant increase compared to the control, while # indicates a significant increase compared to hesperidin.

Hesperidin and hesperidin–Zn(ii) complexes increased osteoblast marker gene expression in mouse MSCs. Mouse MSCs were treated with 10 μg/mL of hesperidin and hesperidin–Zn(ii) complexes for up to 14 days. Total RNA was isolated, and the expression of Runx2 mRNA (a) and type 1 collagen mRNA (b) was analyzed by real-time RT-PCR. * indicates a significant increase compared to the control, while # indicates a significant increase compared to hesperidin.
To clarify the effects of hesperidin and its zinc(ii) complex on the process of osteoblast differentiation, we exposed mouse MSCs to different concentrations (ranging from 0 to 20 μM) of hesperidin and hesperidin–Zn(ii) complex. We then assessed the levels of osteoblast differentiation at both the cellular and molecular levels. After 7 days of treatment, the activity of ALP was evaluated (Figure 2a). Both hesperidin and its zinc(ii) complex, when present at a concentration of 10 μg/mL, showed a notably increased ALP activity compared to other concentrations. ALP is an essential signal produced by osteoblasts that play a critical role in the calcification of the bone matrix. Its regulation is controlled by Runx2, a key transcription factor involved in bone development [8]. The significant rise in ALP activity observed at a concentration of 10 μg/mL of hesperidin and hesperidin–Zn(ii) combination indicates their strong ability to stimulate osteoblasts.
To investigate the impact of hesperidin and its complex on calcium deposition, mouse MSCs were exposed to a concentration of 10 μg/mL hesperidin and the hesperidin–Zn(ii) combination for a duration of 14 days (Figure 2b and c). After the treatment, Alizarin red staining was performed to observe the deposition of calcium (Figure 3b), and then the amount of calcium deposition was measured (Figure 2c). The findings demonstrated that the administration of hesperidin alone resulted in an increase in calcium deposition compared to the control group. Additionally, the hesperidin–Zn(ii) combination further enhanced this effect.
In summary, the impact of hesperidin and its zinc(ii) complex on osteoblast development is clearly demonstrated at the cellular level, highlighting their ability to enhance this process. The most effective concentration for promoting osteoblastic activity seems to be 10 μg/mL for both hesperidin and hesperidin–Zn(ii) combination. The results emphasize the potential of hesperidin and its zinc(ii) complex as effective agents for enhancing the differentiation of osteoblasts and the creation of bone. This suggests that more research should be conducted to explore their potential applications in bone tissue engineering.
To investigate the molecular processes involved in osteoblast differentiation, we treated mouse MSCs with 10 μg/mL concentrations of hesperidin and hesperidin–Zn(ii) complex for a duration of 14 days. Subsequently, we evaluated the expression of genes that serve as markers for osteoblasts. Runx2, a crucial controller, coordinates the process of osteoblast development by stimulating the production of certain indicators such as ALP, type 1 collagen (Col1), OC, and ON [19].
At first, the levels of Runx2 expression (Figure 3a) and type 1 collagen mRNA (Figure 3b) were evaluated. The findings demonstrated that administering 10 μg/mL hesperidin notably increased the expression of Runx2 and type 1 collagen mRNAs on both day 7 and day 14, in comparison to the control group. In addition, the levels of expression were increased even more by the treatment with the 10 μg/mL hesperidin–Zn(ii) complex, compared to both the control and hesperidin alone.
In addition, ALP activity was quantified on day 7 (Figure 2a), while the secretion levels of OC and ON were evaluated on both day 7 and day 14 after treatment with 10 μg/mL hesperidin and hesperidin–Zn(ii) complex (Figure 4). The results demonstrated that hesperidin significantly increased ALP activity, OC, and ON levels compared to the control group at both time points, which is consistent with the expression patterns of osteoblast marker genes Runx2 and type 1 collagen. Furthermore, the administration of the hesperidin–Zn(ii) complex enhanced these effects much more when compared to hesperidin alone. The molecular study showed that 10 μg/mL hesperidin significantly increased the expression of Runx2 and type 1 collagen mRNAs, as well as ALP activity, OC, and ON secretion at both day 7 and day 14 compared to the control group. Significantly, the presence of zinc(ii) in hesperidin increased the observed effects, indicating a synergistic improvement in osteoblast differentiation when hesperidin is combined with zinc(ii) compared to hesperidin alone.

Hesperidin and hesperidin–Zn(ii) complexes increased OC and ON secretion levels in mouse MSCs during osteoblast differentiation. Mouse MSCs were treated with 10 μg/mL of hesperidin and hesperidin–Zn(ii) complexes for up to 14 days. The levels of OC and ON in the conditioned medium were measured. * indicates a significant increase compared to the control, while # indicates a significant increase compared to hesperidin.
3.3 Modulation of Runx2 co-repressors by hesperidin and its zinc(ii) complex in osteoblast differentiation
The results depicted in Figures 1–4 clearly establish the ability of hesperidin and its zinc(ii) complex to enhance the development of osteoblasts. Figure 2 illustrates that both hesperidin and hesperidin–Zn(ii) complex greatly enhanced the expression of Runx2, an essential transcription factor involved in the development of osteoblasts. Expanding on this result, we performed additional investigation on the levels of expression of Runx2 co-repressors, specifically Smad7, Smurf1, and HDAC7, as depicted in Figure 5. Smad7, a type of Smad that inhibits the activity of other Smads, has a negative effect on the signaling pathways of BMP and TGF-β. It does this by blocking the transmission of signals from regulatory Smads. As a result, the expression of Runx2 is reduced and bone formation is inhibited [14]. Smurf1, an E3 ubiquitin ligase with an HECT domain, plays a role in breaking down important proteins that are involved in the process of osteoblast differentiation. This includes proteins like Runx2 and BMP receptors. As a result, Smurf1 has a negative regulatory effect on this process. HDAC7, a histone deacetylase, has been linked to the suppression of osteoblast development by reducing the activity of Runx2 through many methods [20]. Our findings from Figure 5 demonstrate that both hesperidin and its zinc(ii) complex effectively reduced the expression of Smad7, Smurf1, and HDAC7 in comparison to the control. This suggests that they have the potential to alleviate the inhibitory effects on osteoblast differentiation caused by these regulatory proteins.

Hesperidin and hesperidin–Zn(ii) complexes reduced the expression of negative regulator genes associated with osteoblast differentiation. Mouse MSCs were treated with 10 μg/mL of hesperidin and hesperidin–Zn(ii) complexes for up to 14 days. Total RNA was isolated, and real-time RT-PCR analysis was performed to measure the expression levels of smad7, Smurf1, and HDAC7 mRNAs. ** indicates a significant decrease compared to the control.
3.4 Regulation of osteoblast differentiation by hesperidin and its zinc(ii) complex via miR-143-3p/HDAC7 signaling pathway
The role of miRNAs in controlling osteoblast differentiation through post-transcriptional regulation of gene expression is well-established. These small, non-coding RNA molecules have been shown to interact with the 3′UTR of specific mRNAs, resulting in their suppression [14]. Figure 5 clearly demonstrates that both hesperidin and its zinc(ii) complex significantly suppress the expression of HDAC7 during osteoblast differentiation. Recent literature suggests that miR-143 is expressed in osteoblasts and directly targets HDAC7 to promote osteoblast differentiation [20]. To investigate whether hesperidin and its zinc(ii) complex regulate miR-143 to control HDAC7 expression, mouse MSCs were treated with these compounds for up to 14 days, and total RNA was isolated to analyze precursor miR-143 expression via real-time RT-PCR (Figure 6a). As expected, the expression of miR-143 was increased by both hesperidin and its zinc(ii) complex. Furthermore, we predicted the putative target region of miR-143-3p within the 3’UTR of HDAC7 using TargetScan (http://www.targetscan.org/vert_72/) (Figure 6b). To confirm HDAC7 as a target of miR-143-3p, MG63 cells were transiently transfected with control miRNA or miR-143-3p mimic, followed by real-time RT-PCR analysis of HDAC7 expression (Figure 6c). Consistent with our hypothesis, the mRNA level of HDAC7 expression was decreased by miR-143-3p mimic compared to control miRNA transfection. To further confirm HDAC7 as a direct target of miR-143-3p, a dual-luciferase reporter assay was employed. Wild-type and mutant HDAC7 3′UTR sequences were synthesized and cloned into the pmirGLO vector. Co-transfection of wild-type HDAC7 3′UTR-containing pmirGLO vector with the miR-143-3p mimic resulted in significantly reduced luciferase activity compared to co-transfection with control miRNA (Figure 6d). However, co-transfection with mutant HDAC7 3′UTR-containing pmirGLO vector had no significant effect on luciferase activity. These findings provide experimental evidence that miR-143-3p directly targets the 3′UTR of the HDAC7 gene, highlighting the regulatory role of hesperidin and its zinc(ii) complex in osteoblast differentiation through the activation of the miR-143-3p/HDAC7 signaling pathway. The miRNA-143 specifically targeted the HDAC7 inhibitor, and reducing the expression of HDAC7 was seen to restore the function impaired by the deficit of miRNA-143. Therefore, miR-143 enhances the process of angiogenesis and osteoblast development by specifically targeting HDAC7. This suggests that HDAC7 could be a promising target for treating illnesses related to both angiogenesis and osteogenesis [20].

Hesperidin and hesperidin–Zn(ii) complexes modulate mir-143 expression in mouse MSCs. Mouse MSCs were treated with 10 µg/mL of hesperidin and hesperidin–Zn(ii) complexes for up to 14 days. (a) Total RNA was isolated, and mir-143 expression was analyzed by real-time RT-PCR. (b) HDAC7 was identified as the predicted target gene for miR-143-3p. (c) Transfection of MG63 cells with miR-143-3p mimic resulted in decreased HDAC7 mRNA and protein expression. (d) Luciferase reporter assay showing direct targeting of HDAC7 3′UTR by miR-143-3p. Wild-type or mutant HDAC7 3′UTR pmirGLO vectors were co-transfected with control miRNA or miR-143-3p mimic in MG63 cells, and luciferase activity was measured after 24 h. ** indicates a significant decrease compared to the control. */# indicates a significant increase compared to the control.
3.5 Enhancement of osteoblast differentiation by hesperidin and its zinc(ii) complex in a zebrafish scale model
During our study on the impact of hesperidin and its zinc(ii) complex on bone formation, we administered hesperidin and hesperidin–Zn(ii) complex (at a concentration of 10 μg/mL) to adult zebrafish for 14 days in a controlled laboratory environment. After the treatment, the scales were removed and analyzed using several methods to evaluate calcium deposition, mineral content, and the expression of osteoblast markers. The unique qualities of zebrafish scales, such as their clarity, lack of epidermal coating, and ability to regenerate, make them an excellent model for researching osteoblast characteristics. The initial von Kossa (Figure 7a) and Alizarin red (Figure 7b) staining showed higher levels of calcium deposition in scales treated with hesperidin compared to the control. Additionally, scales treated with the hesperidin–Zn(ii) complex exhibited even greater augmentation of calcium deposition quantification (Figure 7c and d). ICP-MS analysis revealed increased concentrations of calcium, phosphorus, and magnesium in scales treated with hesperidin. Notably, scales treated with hesperidin–Zn(ii) complex showed a significant boost in these mineral levels (Figure 8a). The calculation of the calcium/phosphorus molar ratio provided additional confirmation of the stimulating effect of hesperidin and its zinc(ii) complex on mineralization. A considerable increase was detected in comparison to the control. In addition, examination of the expression of genes that indicate the presence of osteoblasts, such as runx2a MASNA isoform, collagen 1α, OC, and ON, showed an increase in hesperidin-treated scales compared to the control. Furthermore, there was a greater increase in scales treated with the hesperidin–Zn(ii) complex (Figure 8b). The current study aligns with existing literature highlighting the role of hesperidin in osteoblast differentiation and bone formation [21,22,23]. Moreover, our findings are consistent with research indicating that the complexation of metals enhances the activity of flavonoids for bone formation, as documented previously [8]. This suggests that the combination of hesperidin with zinc(ii) in our study may have contributed to its efficacy in promoting osteoblast differentiation and bone formation. These collective insights reinforce the potential of hesperidin and its metal complexes as promising candidates for bone health interventions, warranting further exploration in future studies. Overall, these discoveries emphasize the ability of hesperidin to stimulate the growth of bones. This effect is even stronger when hesperidin is combined with zinc(ii), which suggests that hesperidin has great potential for encouraging the development of bone cells and the production of bones in living organisms.

Hesperidin and hesperidin–Zn(ii) complexes enhance bone formation in the zebrafish scale model. Adult zebrafish were treated with 10 µg/mL of hesperidin and hesperidin–Zn(ii) complexes for up to 14 days. (a) Scales were stained with von Kossa staining to visualize calcium deposition. (b) Alizarin red staining was performed to assess mineralization. Quantification of calcium deposition was carried out based on von Kossa staining (c) and Alizarin red staining (d). * indicates a significant increase compared to the control, while # indicates a significant increase compared to hesperidin.

After 14 days of treatment, mineral analysis of the scales was conducted using ICP-MS, and the calcium-to-phosphorus (Ca:P) molar ratio was determined (a). The mRNA expression levels of osteoblast marker genes, including runx2a MASNA isoform, collagen1α, OC, and ON, were analyzed by real-time RT-PCR (b). * denotes a significant increase compared to the control group, while # indicates a significant increase compared to hesperidin treatment.
4 Conclusion
Our work concludes that the synthesized hesperidin–Zn(ii) combination shows great promise in enhancing the differentiation of osteoblasts and the creation of bone. By conducting thorough investigations both in laboratory conditions (in vitro) and in living organisms (in vivo), we have confirmed that the hesperidin–Zn(ii) combination is compatible with MG63 cells and chick embryos. Additionally, we have observed that this complex has the ability to increase ALP activity. At an optimum dose of 10 μg/mL, both hesperidin and its zinc(ii) complex showed significant impacts on osteoblast development in mouse MSCs, both at the cellular and molecular levels. More precisely, hesperidin enhanced the process of calcium deposition, increased the activity of ALP, and promoted the expression of important osteoblast markers like Runx2 and type 1 collagen. In addition, the hesperidin–Zn(ii) combination exhibited a higher level of effectiveness in promoting osteoblast development when compared to hesperidin alone. The mechanism of action of the hesperidin–Zn(ii) combination involves the activation of the miR-143-3p/HDAC7 signaling pathway, resulting in elevated miR-143 expression and reduced HDAC7 levels. Furthermore, our results from the zebrafish scale model provide evidence for the ability of hesperidin and its complex to promote bone formation. This is demonstrated by an increase in calcium deposition, a higher Ca:P molar ratio, and an upregulation of genes associated with osteoblasts. Our findings indicate that the hesperidin–Zn(ii) complex has potential as an exceptional biomaterial for bone regeneration compared to hesperidin alone. This presents new opportunities for advancing treatment strategies focused on enhancing bone health and facilitating repair.
-
Funding information: Authors state no funding involved.
-
Author contributions: Pan Li and Jing Wang performed the experiments and initiated the manuscript preparation. Huan Wang and Songchun Liu performed the experiments. Qibin Zhang designed the work and approved the final version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Abdelgawad AAM, El Bassossy TAI, Ahmed FA. Synthesis, chemical characterization, and biological investigation of naturally isolated hesperidin and its metal complexes. Bull Chem Soc Ethiop. 2024;38(2):385–95. 10.4314/bcse.v38i2.8.Search in Google Scholar
[2] Binkowska I. Hesperidin: synthesis and characterization of bioflavonoid complex. SN Appl Sci. 2020;2:445. 10.1007/s42452-020-2256-8.Search in Google Scholar
[3] Chen B, Zhu S. Spectroscopic and structural study of the zinc(II)-hesperidin complexes and its analysis application. J Chem Soc Pak. 2018;40(4):682–9.Search in Google Scholar
[4] Chiba H, Uehara M, Wu J, Wang X, Masuyama R, Suzuki K, et al. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr. 2003;133(6):1892–7. 10.1093/jn/133.6.1892.Search in Google Scholar PubMed
[5] Choi SS, Lee SH, Lee KA. A comparative study of hesperetin, hesperidin and hesperidin glucoside: antioxidant, anti-inflammatory, and antibacterial activities in vitro. Antioxidants. 2022;11(8):1618. 10.3390/antiox11081618.Search in Google Scholar PubMed PubMed Central
[6] Daoud S, Afifi FU, Al-Bakri AG, Kasabri V. Hamdan II. Preparation, physicochemical characterization and biological evaluation of some hesperidin metal complexes. Iran J Pharm Res. 2014;13(3):909–18. PMID: 25276191; PM CID: PMC4177651.Search in Google Scholar
[7] Gonçalves VP, Musskopf ML, Rivera-Concepcion A, Yu C, Wong SW, Tuin SA, et al. Systemic dietary hesperidin modulation of osteoclastogenesis, bone homeostasis, and periodontal disease in mice. Int J Mol Sci. 2022;23(13):7100. 10.3390/ijms23137100.Search in Google Scholar PubMed PubMed Central
[8] Horcajada MN, Habauzit V, Trzeciakiewicz A, Morand C, Gil-Izquierdo A, Mardon J, et al. Hesperidin inhibits ovariectomy-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J Appl Physiol. 2008;104(3):648–54. 10.1152/japplphysiol.00441.2007.Search in Google Scholar PubMed
[9] Hu HY, Zhang ZZ, Jiang XY, Duan TH, Feng W, Wang XG. Hesperidin anti-osteoporosis by regulating estrogen signaling pathways. Molecules. 2023;28(19):6987. 10.3390/molecules28196987.Search in Google Scholar PubMed PubMed Central
[10] Kalinowska M, Piekut J, Bruss A, Follet C, Sienkiewicz-Gromiuk J, Świsłocka R, et al. Spectroscopic (FT-IR, FT-Raman, ¹H, ¹³C NMR, UV/Vis), thermogravimetric and antimicrobial studies of Ca(II), Mn(II), Cu(II), Zn(II), and Cd(II) complexes of ferulic acid. Spectrochim Acta Part A. 2014;122:631–8. 10.1016/j.saa.2013.11.089.Search in Google Scholar PubMed
[11] Kuo PJ, Fu E, Lin CY, Ku CT, Chiang CY, Fu MM, et al. Ameliorative effect of hesperidin on ligation-induced periodontitis in rats. J Periodontol. 2019;90(3):271–80. 10.1002/JPER.16-0708.Search in Google Scholar PubMed
[12] Lapmanee S, Charoenphandhu N, Aeimlapa R, Suntornsaratoon P, Wongdee K, Tiyasatkulkovit W, et al. High dietary cholesterol masks type 2 diabetes-induced osteopenia and changes in bone microstructure in rats. Lipids. 2014;49(10):975–86. 10.1007/s11745-014-3950-3.Search in Google Scholar PubMed
[13] Li Y, Yang ZY, Wang MF. Synthesis, characterization, DNA binding properties, fluorescence studies, and antioxidant activity of transition metal complexes with hesperetin-2-hydroxy benzoyl hydrazone. J Fluoresc. 2010;20(4):891–905. 10.1007/s10895-010-0635-z.Search in Google Scholar PubMed
[14] Miguez PA, de Paiva Gonçalves V, Musskopf ML, Rivera-Concepcion A, McGaughey S, Yu C, et al. Mitigation of BMP-induced inflammation in craniofacial bone regeneration and improvement of bone parameters by dietary hesperidin. Sci Rep. 2024;14(1):2602. 10.1038/s41598-024-52566-7.Search in Google Scholar PubMed PubMed Central
[15] Miguez PA, Tuin SA, Robinson AG, Belcher J, Jongwattanapisan P, Perley K, et al. Hesperidin promotes osteogenesis and modulates collagen matrix organization and mineralization in vitro and in vivo. Int J Mol Sci. 2021;22(6):3223. 10.3390/ijms22063223.Search in Google Scholar PubMed PubMed Central
[16] Ortiz AC, Fideles SOM, Reis CHB, Bellini MZ, Pereira ESB, Pilon JPG, et al. Therapeutic effects of citrus flavonoids neohesperidin, hesperidin, and its aglycone, hesperetin, on bone health. Biomolecules. 2022;12(5):626. 10.3390/biom12050626.Search in Google Scholar PubMed PubMed Central
[17] Pepa GD, Brandi ML. Microelements for bone boost: The last but not the least. Clin Cases Miner Bone Metab. 2016;13(3):181–5. 10.11138/ccmbm/2016.13.3.181.Search in Google Scholar PubMed PubMed Central
[18] Veeriah V, Saran U, Swaminathan A, Balaguru UM, Thangaraj P, Nagarajan S, et al. Cadmium-induced embryopathy: Nitric oxide rescues teratogenic effects of cadmium. Toxicol Sci. 2015;144(1):90–104. 10.1093/toxsci/kfu258.Search in Google Scholar PubMed
[19] Vimalraj S, Arumugam B, Miranda PJ, Selvamurugan N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int J Biol Macromol. 2015;78:202–8. 10.1016/j.ijbiomac.2015.04.008.Search in Google Scholar PubMed
[20] Vimalraj S, Rajalakshmi S, Raj Preeth D, Kumar S, Deepak T, Gopinath V, et al. Mixed-ligand copper(II) complex of quercetin regulates osteogenesis and angiogenesis. Mater Sci Eng C Mater Biol Appl. 2018;83:187–94. 10.1016/j.msec.2017.09.005.Search in Google Scholar PubMed
[21] Vimalraj S, Rajalakshmi S, Saravanan S, Raj Preeth D, Vasanthi RL, Shairam M, et al. Synthesis and characterization of zinc-silibinin complexes: A potential bioactive compound with angiogenic and antibacterial activity for bone tissue engineering. Colloids Surf, B. 2018;167:134–43. 10.1016/j.colsurfb.2018.04.007.Search in Google Scholar PubMed
[22] Vimalraj S, Saravanan S, Hariprabu G, Yuvashree R, Ajieth SK, Sujoy K, et al. Kaempferol-zinc (II) complex synthesis and evaluation of bone formation using zebrafish model. Life Sci. 2020;256:117993. 10.1016/j.lfs.2020.117993.Search in Google Scholar PubMed
[23] Vishal M, Vimalraj S, Ajeetha R, Gokulnath M, Keerthana R, He Z, et al. MicroRNA-590-5p stabilizes Runx2 by targeting Smad7 during osteoblast differentiation. J Cell Physiol. 2017;232(2):371–80. 10.1002/jcp.25434.Search in Google Scholar PubMed
[24] Wang R, Zhang H, Ding W, Fan Z, Ji B, Ding C, et al. miR-143 promotes angiogenesis and osteoblast differentiation by targeting HDAC7. Cell Death Dis. 2020;11(3):179. 10.1038/s41419-020-2377-4.Search in Google Scholar PubMed PubMed Central
[25] Zhao M, Qiao M, Harris SE, Oyajobi BO, Mundy GR, Chen D. Smurf1 inhibits osteoblast differentiation and bone formation in vitro and in vivo. J Biol Chem. 2004;279(13):12854–9. 10.1074/jbc.M313294200.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

