Abstract
This research investigates the therapeutic efficacy of phytol-mixed micelles in mitigating dexamethasone (Dex)-induced osteoporosis in zebrafish, with a particular focus on scale regeneration. Osteoporosis was induced in zebrafish through exposure to Dex, and the effects of phytol-mixed micelles were evaluated in this model. Following phytol therapy, bone mineralization was assessed using calcium, phosphorus, and alizarin red staining tests. Additionally, commercially available kits quantified the levels of tartrate-resistant acid phosphatase (TRAP), hydroxyproline (HP), and alkaline phosphatase (ALP). The mRNA expression levels of MMP3, osteopontin (OPN), and mitogen-activated protein kinase (MAPK) were examined using reverse transcription polymerase chain reaction (RT-PCR). The findings indicated that phytol significantly increased calcium and phosphorus concentrations. Phytol-mixed micelle therapy led to increased calcium deposition and enhanced bone formation, as evidenced by alizarin red staining. Moreover, phytol administration resulted in increased HP content and upregulated ALP and TRAP activities in zebrafish. RT-PCR tests demonstrated that phytol plays a role in the restoration of the MMP3–OPN–MAPK pathway. In summary, this research highlights the potential of phytol-mixed micelles in ameliorating Dex-induced osteoporosis in zebrafish. Clarifying phytol’s mechanism, particularly its stimulation of the MMP3–OPN–MAPK pathway, provides insight into its role in facilitating bone remodeling.
Graphical abstract
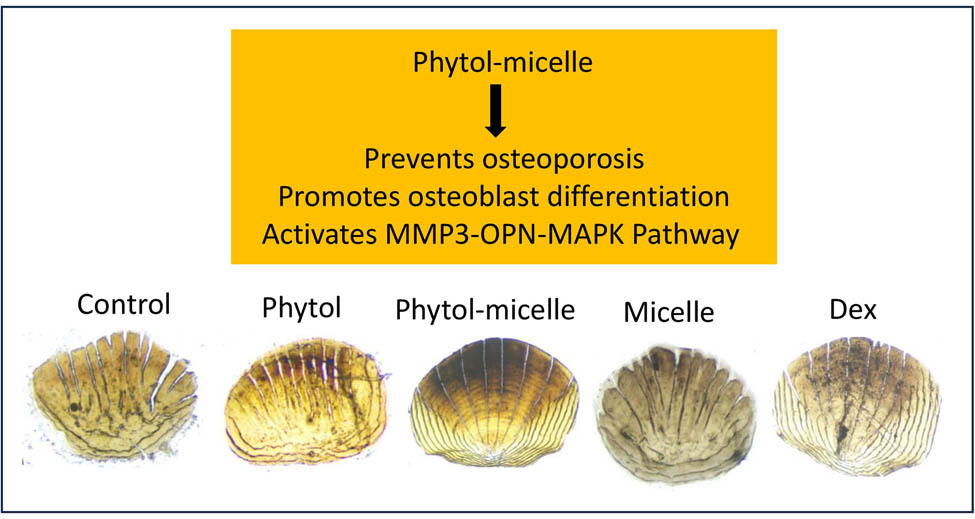
1 Introduction
Osteoporosis, a pervasive chronic bone condition characterized by decreased bone density and structural integrity, is a serious public health concern that affects the quality of life for millions of people worldwide [1]. The onset of osteoporosis is influenced by a wide range of intrinsic and extrinsic factors, including aging, hormonal fluctuations, a sedentary lifestyle, inadequate intake of essential nutrients such as calcium and vitamin D, exposure to certain medications like glucocorticosteroids, and genetic predispositions [2].
The preservation of bone mass and integrity depends on the delicate balance between bone creation and bone resorption, regulated by osteoclasts, osteoblasts, and osteocytes [3]. In the process of bone remodeling, osteoclasts, responsible for bone resorption, and osteoblasts, engaged in bone production, play vital roles, ensuring that skeletal tissue is continuously renewed and corrected. The development and progression of osteoporosis are marked by disruptions in this delicate equilibrium, occurring when bone resorption exceeds bone production [3]. Conversely, reduced osteoclastic activity can result in bone mass accumulation, leading to disorders such as osteosclerosis. To effectively manage bone disorders, it is essential to understand the complex processes regulating the physiological and pathological functions of osteoclasts.
One of the most important mediators of osteoclast development and activation is the receptor activator of nuclear factor-κB ligand (RANKL), a member of the tumor necrosis factor family of ligands. The binding of RANKL to its receptor RANK on osteoclasts activates signaling cascades that enhance osteoclast maturation and activity, facilitating bone resorption [1,2,3,4]. Osteoprotegerin (OPG), a decoy receptor for RANKL, acts as an antagonist to this process by preventing the interaction between RANKL and RANK, thereby limiting osteoclastogenesis and bone resorption. The RANKL/RANK/OPG pathway is thus crucial in the control of bone remodeling and presents potential therapeutic intervention targets [5].
Recent research has highlighted the therapeutic potential of targeting the RANKL pathway in the treatment of osteoclast-mediated bone loss diseases. Clinical trials have shown that RANKL inhibitors, such as denosumab, are effective in reducing bone remodeling, increasing bone mineral density, and lowering the incidence of fractures in postmenopausal women with osteoporosis [6,7]. Additionally, substances like melatonin and OPG have shown potential in preventing bone loss by inhibiting the RANKL pathway [8,9]. Estrogen, known to modulate bone resorption by increasing OPG levels, also holds promise as a potential therapeutic agent [10].
Despite progress in osteoporosis treatment, there remains a need for safer and more effective therapeutic methods. Most current medications aim to prevent bone resorption by decreasing osteoclast activity; bisphosphonates are among the most commonly prescribed. However, long-term use of bisphosphonates is associated with several adverse effects, including gastrointestinal issues and jaw bone necrosis, necessitating the exploration of alternative treatments [11,12,13].
Investigating the therapeutic potential of natural compounds like phytol, which possesses various pharmacological properties such as antioxidant and anti-inflammatory actions, offers promise for developing novel osteoporosis treatments [12]. The potential of natural compounds to improve bone health and reduce bone loss has made them attractive choices for treating osteoporosis. Through their modulation of bone remodeling processes, compounds including genistein, resveratrol, and curcumin have proven anti-osteoporotic properties. For example, studies have shown that curcumin inhibits osteoclastogenesis and increases osteoblast activity, whilst resveratrol improves bone mineral density and lowers inflammation. It has been noted that the phytoestrogen genistein enhances bone strength and density. It is necessary to do more studies and clinical trials to confirm the safety and effectiveness of these natural chemicals, which provide a less harmful, more comprehensive approach to controlling osteoporosis [12,13,14]. However, there is a paucity of research on the effects of phytol on bone health, underscoring the need for further studies in this area. Elucidating the molecular mechanisms regulating osteoclasts, particularly the RANKL/RANK/OPG pathway, is essential for expanding our understanding of osteoporosis pathogenesis and identifying novel treatment targets. By leveraging insights from these pathways and exploring new therapeutic approaches, we aim to address the urgent need for more effective and safer osteoporosis treatments.
2 Materials and methods
2.1 Phytol mixing in micelles
To begin the production of phytol-mixed micelles, 1 g of phytol was dissolved in 10 mL of chloroform. Next, 0.2 mL of this solution was added to 3.8 mL of chloroform containing various concentrations of lipids (EPC and DSPE-PEG 2000; 50:50). The solvent was then evaporated under vacuum at a temperature of 60°C for 20 min. Concurrently, 110 mg of glycocholic acid hydrate (0.24 mmol) was dissolved in 8 mL of 0.067 M phosphate buffer at 60°C. This solution was applied to the lipid film made of EPC, DSPE-PEG 2000, and phytol to hydrate it. After magnetic stirring for a minimum of 4 h at room temperature, a clear dispersion was obtained. This dispersion was further processed by being extruded three times through a syringe filter.
To investigate the phytol-loading capacity of the DSPE-PEG/EPC 50/50-mixed micelles, DSPE-PEG (288 mg, 0.10 mmol) and EPC (80 mg, 0.10 mmol) were dissolved in 4 mL of chloroform in a round-bottom flask. Subsequently, either 0.4 or 0.8 mL of phytol (100 mg/mL in chloroform) was mixed, and the process was repeated as described until a clear dispersion was achieved. To prevent filter occlusion, the micellar dispersions were diluted tenfold with a 0.067 M phosphate buffer before filtration.
2.2 Cell culture
The MG63 immortalized human osteoblast cell line was cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum under standard conditions (37°C and 5% CO2) to ensure optimal growth and viability. Cellular activity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, following a previously established protocol [15]. Cells were seeded at a density of 2 × 104 cells per well in 96-well plates and allowed to adhere and proliferate for up to 12 h. Subsequently, the cells were exposed to varying concentrations of phytol-micelles (0–50 μg) for up to 48 h. After the specified exposure time, an MTT solution (5 mg/mL) was added to each well, and the plates were incubated at 37°C for an additional 4 h, allowing viable cells to convert the yellow MTT dye into purple formazan crystals via mitochondrial activity. The formazan crystals were then solubilized using an appropriate solvent, and the absorbance of the resulting solution was measured at 570 nm using a microplate reader. The absorbance readings provided a quantitative measure of cellular activity. Additionally, alkaline phosphatase (ALP) activity and alizarin red staining assays were conducted as outlined in a previous study [15], providing further insights into the osteogenic potential and mineralization capacity of the treated cells. These complementary assays offered comprehensive information regarding the effects of phytol-micelles on osteoblast cellular activity and bone formation processes.
2.3 Quantification of osteonectin (ON) and osteocalcin (OC)
To quantify ON and OC levels, an enzyme-linked immunosorbent assay (ELISA) was conducted using an ELISA kit from Elabscience following the manufacturer’s instructions. A conditioned medium was obtained following the treatment of cells with phytol-micelles. The ELISA procedure began by adding specific volumes of the conditioned medium, along with appropriate diluents or reagents, to the wells of a microplate pre-coated with antibodies specific to ON and OC. After an incubation period allowing ON and OC in the conditioned medium to bind to the immobilized antibodies, the microplate was washed to remove any unbound substances. Next, enzyme-conjugated secondary antibodies were added to the wells, specifically binding to the captured ON and OC. Following another washing step to eliminate excess secondary antibodies, a substrate solution was added, triggering a colorimetric reaction. The intensity of the resulting color was measured spectrophotometrically at a specific wavelength, and the absorbance values were recorded. These absorbance values were then used to determine the concentrations of ON and OC in the conditioned medium by comparing them to a standard curve generated using known concentrations of ON and OC standards provided in the kit. This process facilitated the accurate quantification of ON and OC levels in the conditioned medium, thereby enabling the assessment of the effects of phytol-micelles on the secretion of these osteogenic markers by the cells.
2.4 Animal maintenance and scale regeneration model
The experiment involved maintaining 6-month-old zebrafish at 28°C with a light cycle of 14 h and a dark cycle of 10 h in an aquarium with regular fresh water exchange. The fish were fed live brine shrimp and commercial flake food three times daily. To induce osteoporosis, 20 adult zebrafish were treated with dexamethasone (Dex) at a concentration of 10 μM. Following osteoporosis induction, the fish were subjected to various doses of phytol-micelles, ranging from 0 to 50 μM, to evaluate its impact on bone production and scale regeneration. Thirty scales were surgically removed from each fish on the first day after Dex treatment, taken from below the operculum to the center of the first dorsal fin. The fish were then maintained under the specified treatment conditions for 14 days. At the end of the treatment period, the regenerated scales were carefully collected for further analysis. The experimental protocol followed established in-house procedures and techniques previously published [15].
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals.
2.5 Calcium and phosphorus ratio analysis by inductively coupled plasma optical emission spectrophotometry (ICP-OES)
The mineral content, specifically calcium and phosphorus, in the scale samples was tested following a series of steps. Initially, and the scales were freeze-dried until completely dehydrated. The dried materials were then dissolved in 70% nitric acid (Sigma-Aldrich) to disintegrate the organic matrix and extract the mineral components. The dissolved samples were diluted 100-fold with demineralized water for analysis. The diluted samples were analyzed using ICP-OES, an analytical method that accurately measures calcium and phosphorus levels by analyzing their emission spectra. This provided precise and reliable data on the mineral composition of the scale samples.
2.6 Hydroxyproline (HP) assay
Scale samples were collected and thoroughly washed with saline to remove external contaminants and then dried at approximately 80°C for 48 h until a constant weight was achieved. The dried samples were incinerated in a silica crucible at 800°C to remove any remaining organic material, leaving behind only the mineral components. The weight of the pulverized samples was accurately recorded. The samples were then hydrolyzed using 6 mol/L hydrochloric acid (HCl) to break down collagen fibers into their constituent amino acids, including HP. The concentration of HP was determined using a standardized protocol provided by the assay kit manufacturer, likely involving colorimetric or spectrophotometric methods. The absorbance of the chromophore produced during the reaction between HP and specific reagents was measured, and the absorbance values were correlated with known HP concentrations to quantify its presence in the samples. This assay provided valuable information about the collagen content in the scale samples, essential for assessing bone health and integrity.
2.7 ALP activity
To determine ALP activity, the following procedure was followed. Fixed scales were carefully cut and pretreated with an alkaline buffer solution consisting of 100 mM Tris–HCl (pH 9.5), 1 mM MgCl2, and 0.1 mM ZnCl2. The scales were incubated in this alkaline buffer for 30 min for proper conditioning. After pretreatment, the scales were incubated with 150 μL of alkaline buffer containing 20 mM 4-nitrophenyl phosphate disodium salt hexahydrate (pNPP, Sigma) for 1 h. pNPP serves as a substrate for ALP, and its hydrolysis results in the formation of a yellow-colored product. Following the incubation period, the enzymatic reaction was halted by adding a solution containing 3 N NaOH and 20 mM ethylenediaminetetraacetic acid, effectively stopping further enzymatic activity and stabilizing the reaction mixture. The ALP activity was quantified using a spectrophotometric method at a wavelength of 405 nm. The absorbance of the reaction mixture was measured, with the intensity of the yellow color formed being directly proportional to the ALP activity present in the sample. This procedure accurately determined the ALP activity in the scales, providing valuable insights into the bone metabolism and health of the specimens.
2.8 Tartrate-resistant acid phosphatase (TRAP) activity
To assess TRAP activity, the protocol outlined in Persson et al. [16] was followed with slight modifications. Initially, fixed scales were immersed in a solution containing 0.1 M sodium acetate buffer supplemented with 20 mM tartrate and incubated for 1 h. Subsequently, the solution was replaced with a fresh one consisting of 20 mM tartrate, 0.1 M sodium acetate buffer, and 20 mM pNPP, allowing the enzymatic reaction to proceed and facilitating the hydrolysis of pNPP. After incubation, the reaction was halted by adding 50 μL of 2 N NaOH, ensuring no further hydrolysis occurred. The amount of para-nitrophenol (pNP) produced, indicative of TRAP activity, was quantified by measuring the absorbance at 405 nm using a spectrophotometer. These absorbance data were then used to calculate the concentration of pNP produced, employing a standard curve specifically generated for pNP.
2.9 Alizarin red staining
For Alizarin red staining, zebrafish scales were first collected and fixed overnight in a 4% paraformaldehyde solution. Following fixation, the scales were washed with 10% glycerol/0.5% KOH solution to remove excess fixative. Subsequently, the fish larvae were stained overnight with 0.02% Alizarin red stain/10% glycerol/0.5% KOH solution to visualize the formed bone. After staining, the scales were destained using 50% glycerol/0.5% KOH solution to remove any excess stain and enhance clarity. A bleaching step was then performed using 3% H2O2/0.5% KOH solution to remove background interference. Finally, digital photographs of the stained scales were acquired using a fluorescence stereomicroscope for further analysis and documentation.
2.10 Real-time RT-PCR analysis
Total RNA was extracted from the scales using TRIzol reagent (Invitrogen, USA) following the manufacturer’s protocol. Immediately after extraction, RNA samples were assessed for purity and concentration using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). To prevent RNA degradation, samples were aliquoted and stored at −80°C until further analysis. For sample preparation, care was taken to work quickly and on ice to minimize RNA degradation during processing. RNA was reverse transcribed into complementary DNA using a reverse transcriptase kit (Takara Biotech, Japan), ensuring that all reagents and equipment were pre-cooled. Quantitative real-time PCR (qRT-PCR) analysis was performed using ChamQ SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., China) and CFX Manager software (Bio-Rad Laboratories Inc.). GAPDH was used as an internal control to standardize expression levels in each sample. The primer sequences used in this study are detailed in Table 1. Relative expression levels were determined using the 2−ΔΔCt method.
Primers used in this study
| Gene | 5′→3′ Sequence | |
|---|---|---|
| Runx2 | Forward | CAGTTCCCAAGCATTTCATC |
| Reverse | TCAATATGGTCGCCAAACAG | |
| Type-1 collagen | Forward | TAACCCCCTCCCCAGCCACAAA |
| Reverse | TTCCTCTTGGCCGTGCGTCA | |
| GAPDH | Forward | TTGATGTCATCATACTTGGCAGGT |
| Reverse | CAG TCAAGGCTGAGAATGGGA | |
| Runx2a masns-isoform | Forward | CTCCCGCTTTAGGACTTCGA |
| Reverse | GGAGTCACCGAGCTGAAAAGACT | |
| Collagen 1α2 | Forward | GGAAACCTGAAGAAGGCTGTGT |
| Reverse | TGAAAGTGAAGCGGCTGTTG | |
| OC | Forward | TGGCCTCTATCATCATGAGACAGA |
| Reverse | CTCTCGAGCTGAAATGGAGTCA | |
| Osteopontin (OPN) | Forward | CGCTCAGCAAGCAGTTCAGA |
| Reverse | AGAATAGGAGGTGGCCGTTGA | |
| β-actin | Forward | CAACAGGGAAAAGATGACACAGAT |
| Reverse | CAGCCTGGATGGCAACGT | |
| MMP3 | Forward | CTCCCCACCTTGAATGAAGA |
| Reverse | ACTGGGTCGCTTCTCTTGAA | |
| OPN | Forward | AACCCAGACACAAGCATTCC |
| Reverse | GCCTTTGAGGTTTTTGGTCA | |
| Mitogen-activated protein kinase (MAPK) | Forward | ATTGTCAGCAATGCATCCTG |
| Reverse | ATGGACTGTGGTCATGAGCC | |
| GAPDH | Forward | AGCAATGCTTGTCAATCCTG |
| Reverse | ATTGGTCGGACTGATGAGCC |
2.11 Statistical analysis
For statistical analysis, all quantitative data were presented as mean values with standard deviations. Initially, the Student’s t-test was used to compare significant differences between the two experimental groups. For comparisons involving more than two groups, data were subjected to one-way analysis of variance (ANOVA) to evaluate overall group differences. To identify specific differences between groups following the ANOVA, post hoc analysis was performed using Tukey’s multiple comparison test. Statistical significance was set at P < 0.05.
3 Results
3.1 Biocompatibility nature of phytol-micelles: in vitro and in vivo studies
To investigate the biocompatibility of phytol-micelles, MG63 cells underwent MTT assay after exposure to various concentrations of phytol-micelles (0–50 μg) for up to 48 h. The results, illustrated in Figure 1a and b, demonstrated a concentration-dependent increase in cellular metabolic activity. Particularly, at a concentration of 25 μg, phytol-micelles significantly enhanced cellular activity compared to the control group. Importantly, no cytotoxic effects were observed even at the highest tested concentration of 50 μM. Furthermore, the in vivo biocompatibility of phytol-micelles was assessed using a zebrafish larvae model. Following treatment, the live percentage of larvae was determined, revealing no significant toxicity in zebrafish larvae even at the highest concentration of 50 μg phytol-micelles (Figure 1c). These findings collectively indicate the favorable biocompatibility profile of phytol-micelles both in vitro and in vivo, supporting its potential utility in osteoblast differentiation and bone formation studies.
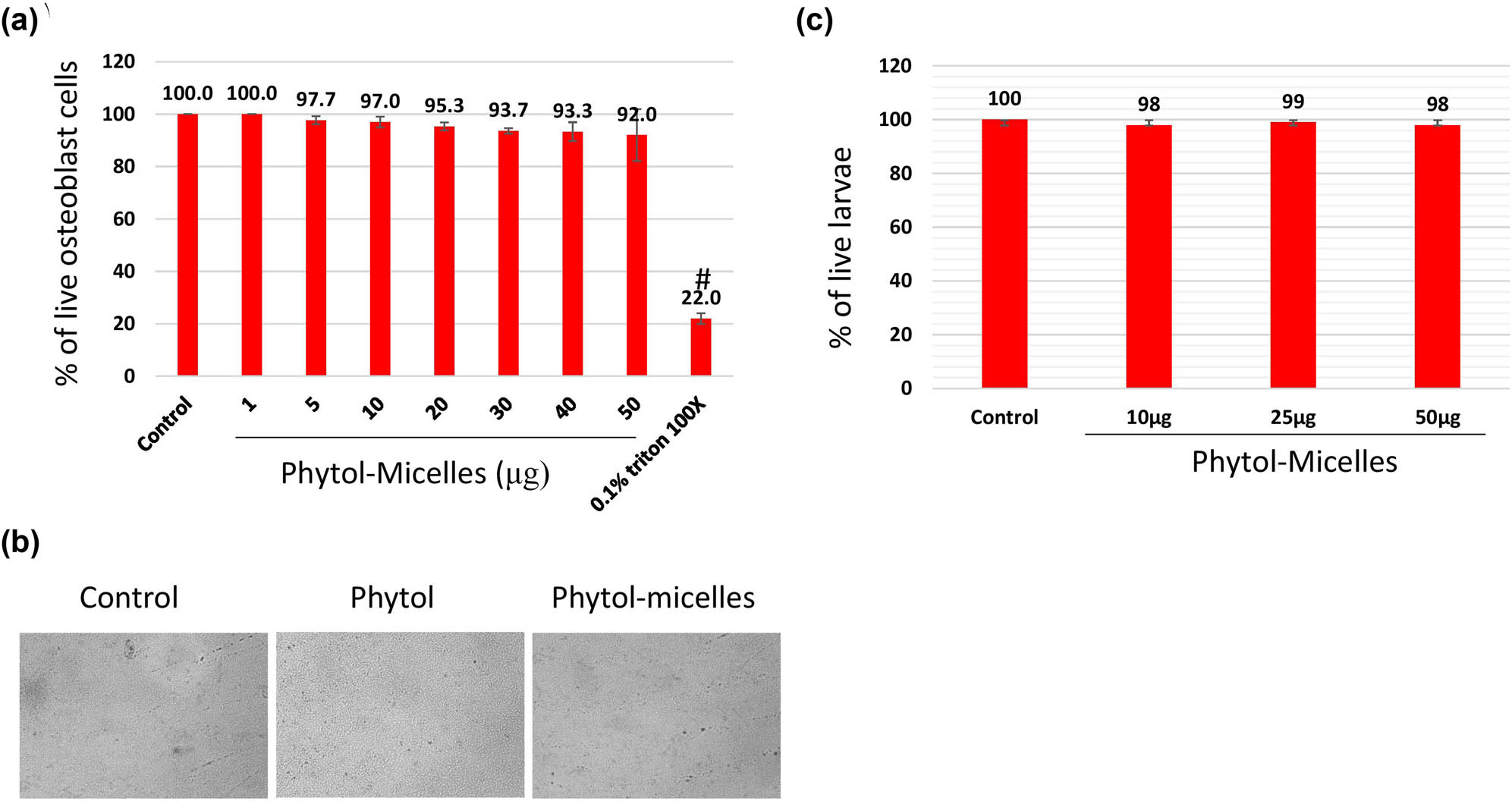
Biocompatibility assessment of phytol-micelles. MG63 cells treated with varying phytol-micelles concentrations (0–50 μg) for 48 h showed no toxicity (a) representative cell image following exposure to 50 μg of phytol-micelles for 48 h. The image shows the cellular morphology and there are no any notable changes induced by the treatment (b). Zebrafish larvae treated with phytol-micelles (50 μg) showed no toxicity (c). # indicates a significant decrease.
3.2 Phytol-micelles promote osteoblast differentiation at the cellular level
In this study, we investigated the effects of phytol-micelles on osteoblast differentiation using MG63 cells exposed to varying concentrations (5–50 μM) of phytol-micelles in osteogenic medium. After 7 days of treatment, ALP activity was assessed, revealing that 25 μg of phytol-micelles exhibited notably higher ALP activity compared to other concentrations (Figure 2a). To further elucidate the impact of phytol-micelles on osteoblast mineralization, MG63 cells were treated with 25 μg of phytol-micelles for a period of up to 14 days. Following treatment, the cells were subjected to alizarin staining to evaluate calcium deposition qualitatively (Figure 2b) and quantitatively (Figure 2c). These findings suggest a potential role for phytol-micelles in enhancing osteoblast differentiation and mineralization, particularly at the concentration of 25 μg.

Effects of phytol-micelles on osteoblast differentiation and mineralization. MG63 cells treated with varying phytol-micelles concentrations (5–50 μM) for 7 days showed increased ALP activity, notably higher at 25 μg (a). Treatment with 25 μg phytol-micelles for 14 days enhanced calcium deposition, indicating the potential for osteoblast differentiation and mineralization (b) and quantification (c). * indicates a significant increase.
3.3 Phytol-micelles promote osteoblast differentiation at the molecular level
To elucidate the molecular mechanisms underlying the effects of phytol-micelles, MG63 cells were treated with 25 μg of phytol-micelles in osteogenic medium for 7 days, and the expression of osteoblast marker genes was analyzed. Initially, the mRNA expression levels of Runx2 (Figure 3a) and type 1 collagen (Figure 3b) were assessed. The results demonstrated a significant increase in the expression of both genes at day 7 compared to the control group, with a further enhancement observed in cells treated with 25 μg of phytol-micelles. Additionally, ALP activity was measured at day 7 (Figure 3c and d), revealing a notable increase in activity following treatment with 25 μg of phytol-micelles. Furthermore, the levels of OC and ON were assessed after 7 days of phytol-micelles treatment (Figure 4). Consistent with the upregulation of osteoblast marker genes and ALP activity, the secretion of OC and ON was significantly enhanced in cells treated with 25 μg of phytol-micelles compared to the control group. These findings collectively indicate that phytol-micelles, particularly at a concentration of 25 μg, exert a stimulatory effect on osteoblast differentiation and molecular markers associated with bone formation.
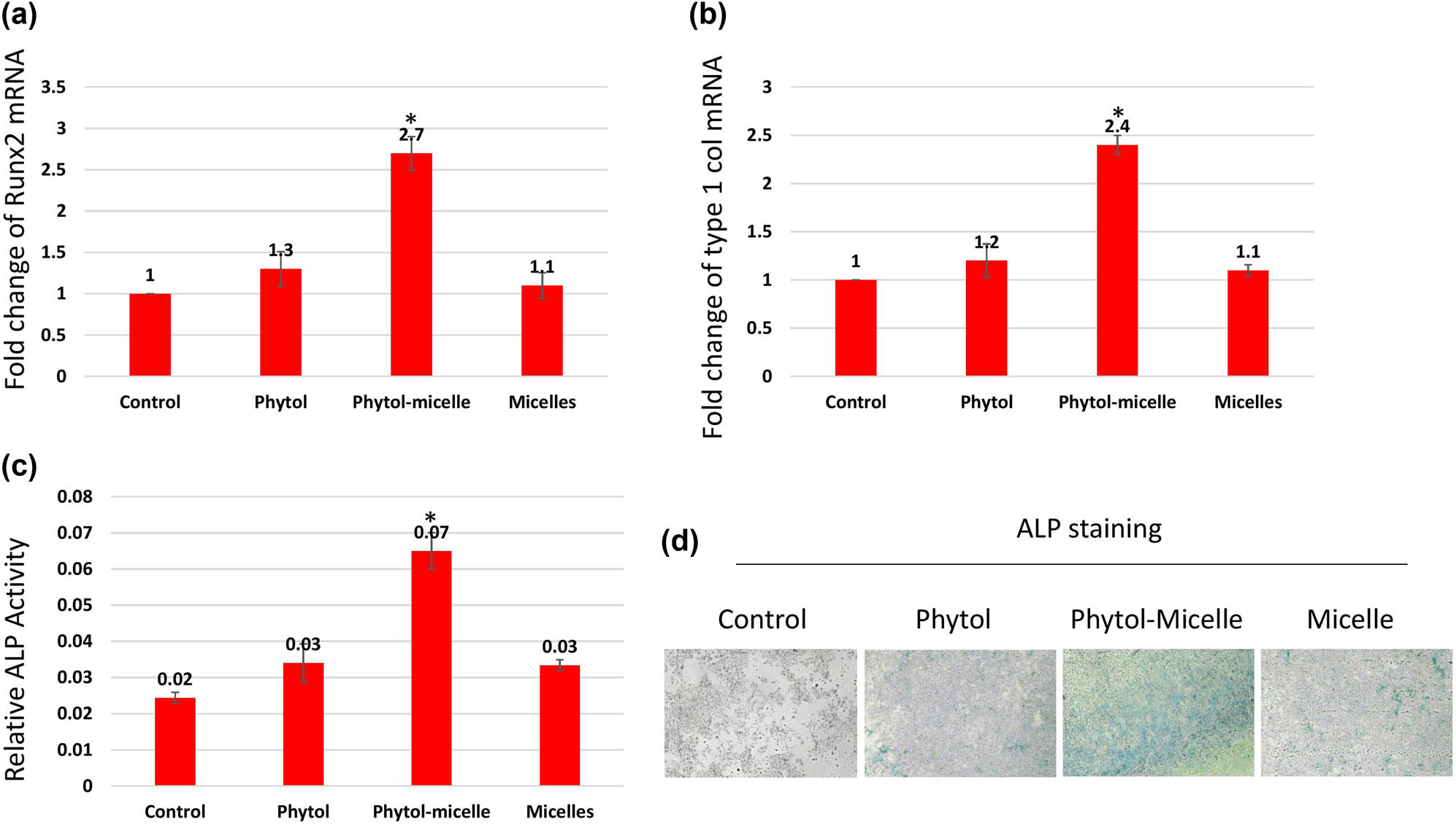
Molecular mechanisms underlying phytol-micelles effects on osteoblast differentiation. MG63 cells treated with 25 μg phytol-micelles in an osteogenic medium for 7 days showed increased mRNA expression of Runx2 (a) and type 1 collagen (b), along with elevated ALP activity (c) and ALP staining (d). * indicates a significant increase.
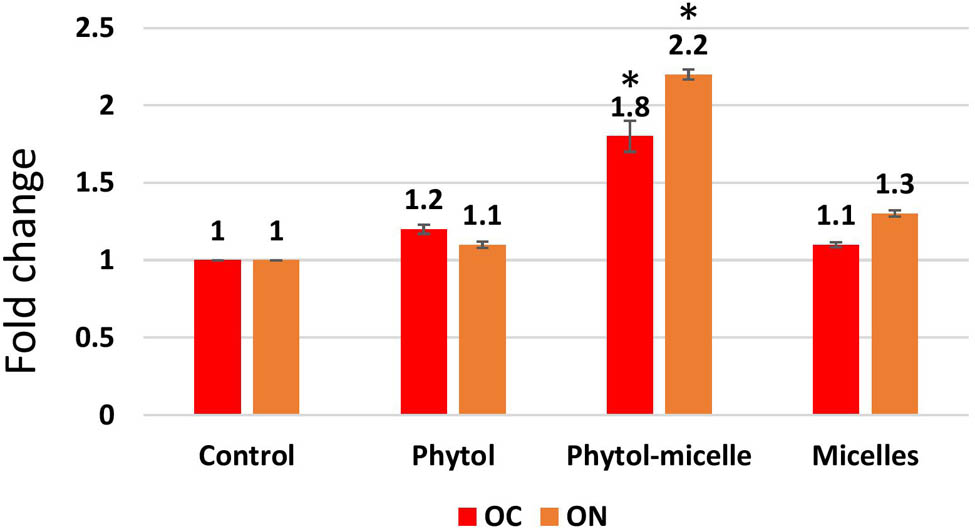
The enhanced secretion of OC and ON was observed. The data illustrate the enhanced levels of these bone matrix proteins, indicating improved bone formation and mineralization. * indicates a significant increase.
3.4 Effect of phytol-micelles on calcium and phosphorus
The influence of phytol-micelles on calcium and phosphorus levels was meticulously examined in this study. Initially, the impact of Dex treatment on calcium and phosphorus content was assessed, revealing a significant reduction in both minerals compared to untreated, normal zebrafish, as visually depicted in Figure 5. This decline underscores the deleterious effect of Dex on calcium and phosphorus homeostasis in zebrafish scales. Conversely, upon treatment with phytol-micelles, a remarkable contrast was observed in the calcium and phosphorus levels of regenerated zebrafish scales (Figure 5a and b). Phytol-micelles treatment led to a substantial increase in both calcium and phosphorus content compared to the Dex-treated group, as well as the untreated, normal zebrafish group. This notable enhancement in mineral levels suggests a potential protective and regenerative effect of phytol-micelles on calcium and phosphorus metabolism in zebrafish scales. These findings underscore the promising therapeutic implications of phytol-micelles in mitigating Dex-induced mineral depletion and promoting mineral restoration in zebrafish scales.
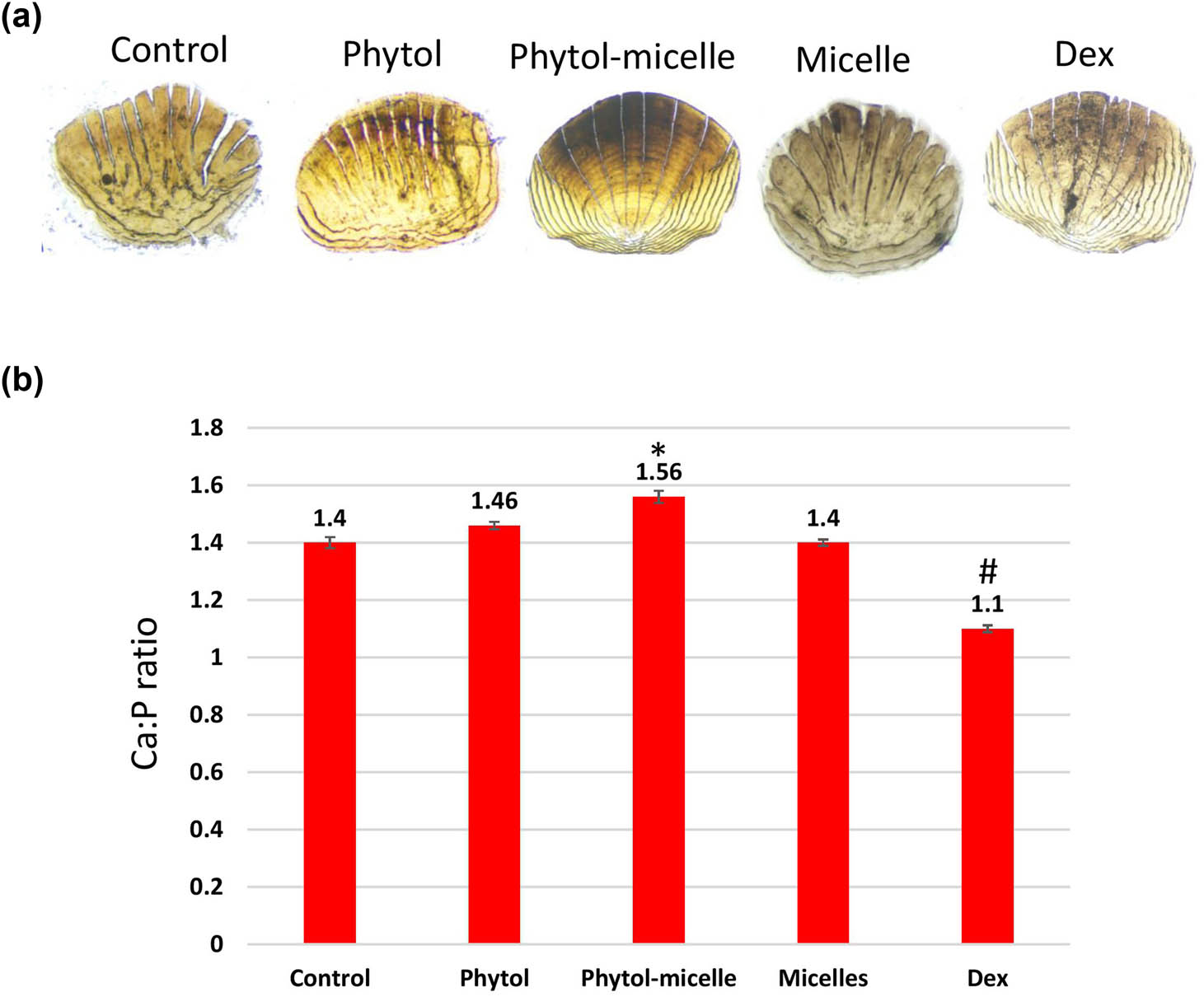
Phytol-micelles influence on calcium and phosphorus levels in zebrafish scales. (a) vonKossa staining of scales and (b) calcium and phosphorus content. * indicates a significant increase. # indicates a significant decrease.
3.5 Effect of phytol-micelles on HP
The impact of phytol-micelles on HP levels was investigated in this study. As illustrated in Figure 6, treatment with Dex resulted in a significant downregulation of HP levels compared to control zebrafish scales. This decrease highlights the suppressive effect of Dex on HP content in zebrafish scales. Conversely, treatment with phytol-micelles yielded a starkly different outcome, with a significant increase observed in HP levels compared to the Dex-treated group. This elevation underscores the stimulatory effect of phytol-micelles on HP production in zebrafish scales. These findings indicate the contrasting effects of Dex and phytol-micelles on HP levels, with Dex exhibiting a suppressive effect whilst phytol-micelles demonstrates a stimulatory effect (Figure 6). Such observations shed light on the potential therapeutic benefits of phytol-micelles in counteracting Dex-induced suppression of HP and promoting its augmentation in zebrafish scales.
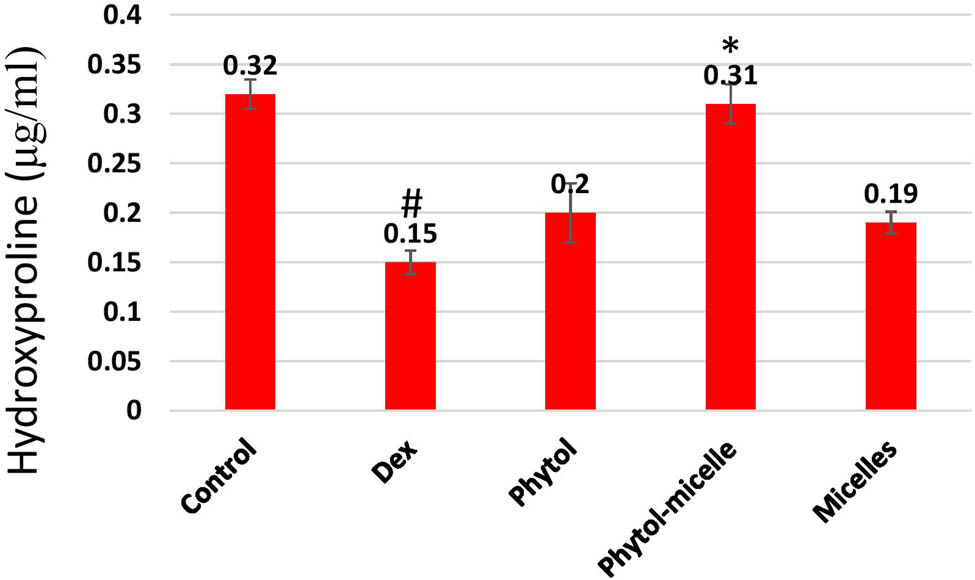
Dex treatment reduces HP levels in zebrafish scales, contrasting with phytol-micelles’ stimulatory effect, as depicted. * indicates a significant increase. # indicates a significant decrease.
3.6 Effect of phytol-micelles on ALP and TRAP activities
The impact of phytol-micelles on ALP and TRAP activities was assessed in this study. As depicted in Figure 7, treatment with Dex led to a significant decrease in ALP activity compared to the control zebrafish scale. This reduction highlights the inhibitory effect of Dex on ALP activity in zebrafish scales. Conversely, Dex treatment resulted in a notable increase in TRAP activity relative to the control group. This elevation underscores the stimulatory effect of Dex on TRAP activity in zebrafish scales. In contrast, treatment with phytol-micelles yielded opposing outcomes. Phytol-micelles significantly increased ALP activity compared to the Dex-treated group, indicating its stimulatory effect on ALP activity in zebrafish scales. Additionally, phytol-micelles treatment resulted in a significant decrease in TRAP activity relative to the Dex-treated group, highlighting its inhibitory effect on TRAP activity in zebrafish scales. These findings elucidate the differential effects of Dex and phytol-micelles on ALP and TRAP activities, with Dex exerting suppressive effects on ALP activity while promoting TRAP activity, and phytol-micelles exhibiting stimulatory effects on ALP activity while inhibiting TRAP activity in zebrafish scales. Such insights underscore the potential therapeutic benefits of phytol-micelles in modulating bone metabolism parameters and mitigating the adverse effects associated with Dex treatment.

Dex treatment decreases ALP, runx2a mansn-soform, and collagen1a2 mRNAs but increases TRAP activity in zebrafish scales, contrasting with phytol-micelles’ stimulatory effect on ALP and inhibitory effect on TRAP activities, as depicted. * indicates a significant increase. # indicates a significant decrease.
3.7 Effect of phytol-micelles on alizarin red staining
The impact of phytol-micelles on alizarin red staining, indicative of calcium mineralization, was investigated in this study. As illustrated in Figure 8, treatment with Dex led to a significant reduction in alizarin red staining intensity compared to the normal scales. This decrease suggests the inhibitory effect of Dex on calcium mineralization in zebrafish scales. Conversely, treatment with phytol-micelles resulted in a notable increase in alizarin red staining intensity relative to the Dex-treated group. This elevation highlights the stimulatory effect of phytol-micelles on calcium mineralization in zebrafish scales. These findings underscore the differential effects of Dex and phytol-micelles on calcium mineralization, with Dex exerting suppressive effects while phytol-micelles exhibit stimulatory effects in zebrafish scales. Such insights provide valuable implications for the potential therapeutic use of phytol-micelles in promoting bone mineralization and counteracting the detrimental effects associated with Dex treatment.
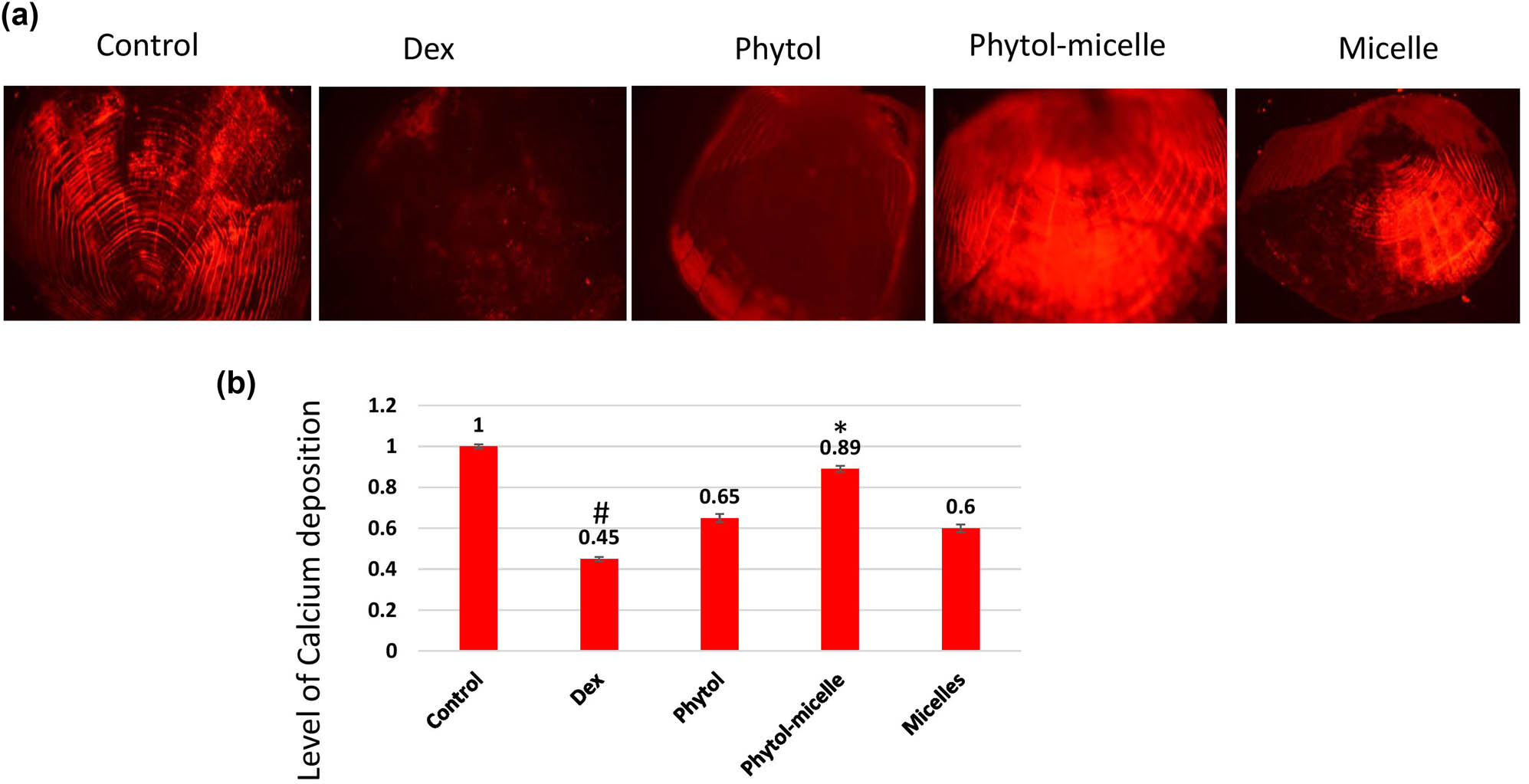
Dex treatment decreases alizarin red staining intensity (a), indicating reduced calcium mineralization (b) in zebrafish scales, contrasting with phytol-micelles’ stimulatory effect on alizarin red staining, suggesting enhanced calcium mineralization. * indicates a significant increase. # indicates a significant decrease.
3.8 Effect of phytol-micelles on MMP3, OPN, and MAPK mRNA levels
The influence of phytol-micelles on the mRNA levels of MMP3, OPN, and MAPKp38 was examined to elucidate its molecular effects in this study. As depicted in Figure 9, treatment with Dex resulted in a significant decrease in the mRNA levels of OPN and MAPKp38, while MMP3 mRNA levels were notably increased compared to the control group. These observations indicate the suppressive effect of Dex on OPN and MAPKp38 expression, accompanied by an upregulation of MMP3 expression in zebrafish scales. In contrast, treatment with phytol-micelles led to a substantial increase in the mRNA levels of OPN and MAPKp38, while MMP3 mRNA levels were significantly reduced relative to the Dex-treated group. These findings underscore the stimulatory impact of phytol-micelles on OPN and MAPKp38 expression, along with its suppressive effect on MMP3 expression in zebrafish scales. Overall, the results suggest that phytol-micelles exerts differential effects on the mRNA levels of MMP3, OPN, and MAPKp38 compared to Dex treatment. Such molecular alterations provide valuable insights into the potential mechanisms underlying the therapeutic effects of phytol-micelles in modulating bone metabolism and remodeling processes.
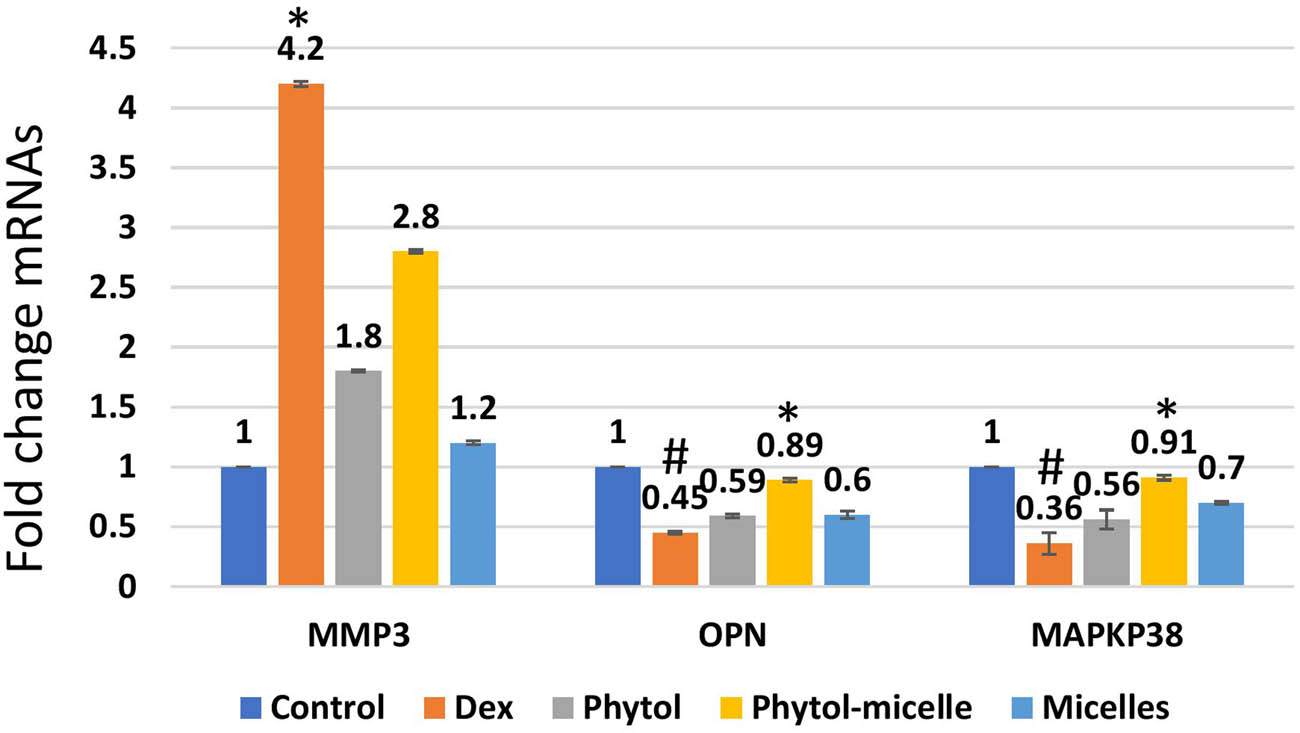
Dex treatment alters mRNA levels, reducing OPN and MAPKp38 while increasing MMP3 in zebrafish scales, indicating suppressive effects. Conversely, phytol-micelles significantly increase OPN and MAPKp38 mRNA levels while reducing MMP3, suggesting stimulatory effects. These molecular changes elucidate phytol-micelles’ potential mechanisms in bone metabolism and remodeling. * indicates a significant increase. # indicates a significant decrease.
4 Discussion
Osteoporosis is a prevalent systemic bone disorder characterized by diminished bone mass and deterioration of bone tissue microarchitecture, leading to increased bone fragility and fracture susceptibility [16]. With the aging population and escalating incidence of osteoporosis, it poses a significant threat to human health [17]. In this investigation, we utilized Dex to induce an osteoporosis model in zebrafish to assess the pharmacological effects and underlying mechanisms of phytol-micelles against osteoporosis. Bone remodeling, essential for maintaining bone strength and integrity, is a dynamic process influenced by the opposing actions of osteoblasts, responsible for bone formation, and osteoclasts, responsible for bone resorption [18]. Mixed micelles, first proposed by Hoffman and Borgstrom, are formed by a mixture of amphiphilic biliary and dietary components, specifically phospholipids and bile salts. The hydrophobic tails of EPC molecules are positioned in the micellar core to reduce interaction with water molecules, while their polar head groups are located near the water interface. Phospholipids in water typically form bilayered structures; however, the addition of bile salts causes the development of mixed micelles. The micellar structures can dissolve poorly water-soluble medicines in their inner core [19,20,21,22,23,24,25,26,27]. Mixed micelles demonstrate thermodynamic stability, usually retaining sizes ranging from 5 to 60 nm [28]. Small diameters are essential for delivering phytol-micelles to the enterocyte surface and retaining mixed micelles at the base of microvilli. The equilibrium between bone formation and resorption is crucial for bone metabolism, as an imbalance may lead to osteoporosis and increased fracture risk [19]. According to the findings of our research, phytol-micelles demonstrated a significant increase in mineralization inside osteoblastic cells. This was observed at the cellular level. The development and mineralization of osteoblasts are processes that are highly regulated and choreographed by several genes. These genes include ALP, collagen type 1, OC, and ON, and they are controlled by the bone-specific transcription factor Runx2. The treatment with phytol was shown to be related to a considerable increase in the mRNA expression of Runx2 and Type 1 collagen in MG63 cells, according to the findings of our experiment. Furthermore, the levels of OC and ON secretion were significantly increased as a result of the phytol-micelles therapy, with the ON levels exhibiting a particularly marked rise in comparison to the levels associated with OC and the control groups. These data together imply that phytol can enhance osteoblast development and mineralization in vitro, possibly through its impact on critical regulatory genes and proteins involved in bone formation. Phytol-micelles has been shown to have this ability.
To evaluate bone metabolism, we measured TRAP activity as a marker of osteoclast activity and ALP activity as a measure of osteoblast differentiation. Our results indicated that Dex significantly decreased ALP and increased TRAP activity compared to the control group, signifying an imbalance favoring bone resorption. Conversely, phytol-micelles treatment significantly increased ALP and decreased TRAP activity, suggesting a restoration of bone formation/resorption equilibrium closer to the control level. HP is an essential amino acid involved in bone remodeling, predominantly present in collagen, a significant constituent of the bone matrix. It contributes significantly to the stabilization of the collagen triple helix structure, which is crucial for maintaining bone strength and integrity. Collagen is produced by osteoblasts and broken down by osteoclasts during bone remodeling, resulting in the release of HP into the circulation. Elevated concentrations of HP in the blood or urine can suggest heightened bone resorption. Therefore, the measurement of HP levels functions as a biomarker for bone turnover and aids in evaluating the efficacy of therapies for bone-related diseases, such as osteoporosis. Its function in preserving the integrity of the bone matrix highlights its significance in bone health [29,30].
Matrix metalloproteinases (MMPs) have emerged as crucial regulators of osteoclast-mediated bone resorption [20]. Among the MMP family, MMP3 plays a pivotal role in degrading type I collagen, facilitating osteoclast activation [21]. Our findings revealed that Dex treatment significantly upregulated MMP3 expression, whereas phytol-micelles treatment markedly downregulated MMP3 expression in zebrafish scales. These results suggest that phytol-micelles may mitigate osteoclast-mediated bone resorption, potentially through MMP3 regulation. OPN, secreted by osteoblasts, plays a vital role in bone mineralization and matrix adhesion [22]. Reduced OPN expression is associated with impaired bone mineralization and osteoporosis [23]. In our study, Dex treatment led to a significant decrease in OPN expression, while phytol-micelles treatment substantially increased OPN expression in zebrafish scales. These findings suggest that phytol-micelles may enhance bone mineralization and osteoblast function by upregulating OPN expression.
The MAPK signaling pathway is intricately involved in regulating osteoblast proliferation, differentiation, and apoptosis [24]. Activation of the MMP3-OPN-MAPK pathway has been implicated in enhancing osteoblast activity and proliferation [25]. Our results demonstrated that Dex treatment significantly decreased MAPK expression, whereas phytol-micelles treatment markedly increased MAPK expression in zebrafish scales. These findings indicate that phytol-micelles may promote osteoblast activity and bone formation by activating the MMP3–OPN–MAPK signaling pathway. This study suggests that phytol-micelles may mitigate osteoporosis by restoring the balance of bone formation and resorption and activating the MMP3–OPN–MAPK pathway. In osteoporosis, dysregulation of the MMP3–OPN–MAPK pathway can lead to increased bone resorption and decreased bone formation. Further investigations are warranted to elucidate the therapeutic potential of phytol-micelles for osteoporosis treatment [31].
These findings highlight the therapeutic potential of phytol-micelles in mitigating Dex-induced osteoporosis in zebrafish. Through a series of in vitro and in vivo experiments, phytol-micelles demonstrated a significant impact on bone mineralization, osteoblast differentiation, and molecular pathways involved in bone remodeling. Phytol-micelles treatment resulted in elevated calcium and phosphorus levels, increased bone mineralization, and enhanced osteoblast differentiation, as evidenced by ALP activity and alizarin red staining. Moreover, phytol-micelles exhibited a favorable biocompatibility profile both in vitro and in vivo, further supporting its potential utility in osteoporosis management. At the molecular level, phytol-micelles treatment was associated with the restoration of the MMP3–OPN–MAPK pathway, which plays a crucial role in regulating osteoblast activity and bone formation. By modulating key genes and proteins involved in bone remodeling, phytol-micelles may help restore the balance between bone formation and resorption, thereby mitigating osteoporosis progression. Overall, these findings provide valuable insights into the pharmacological effects and underlying mechanisms of phytol-micelles in osteoporosis treatment. Further research is warranted to explore the translational potential of phytol-micelles-based therapies for human osteoporosis and to elucidate its long-term safety and efficacy profiles. With continued investigation, phytol-micelles holds promise as a potential therapeutic agent for addressing the growing burden of osteoporosis and improving bone health worldwide.
Although zebrafish are a useful model for investigating osteoporosis, there are several significant drawbacks. Their metabolism and bone structure are quite different from those of mammals, which might have an impact on how discoveries are directly applied. Zebrafish, for example, have a distinct scale-based system instead of a genuine bone structure, which could not accurately mimic the mechanisms involved in human bone remodeling. Zebrafish also have distinct metabolic and regulatory processes, which may affect the outcome. By bridging the gap between preclinical findings and clinical relevance, this comparison directs future directions in research. Furthermore, it facilitates the recognition of possible obstacles and modifications required during the transfer of therapeutic approaches from zebrafish to mammals, ultimately leading to more precise and efficacious interventions for osteoporosis in humans [32,33]. To translate these results into clinical applications, future research should focus on conducting clinical trials to evaluate the efficacy and safety of phytol in human subjects. Additionally, long-term safety studies are necessary to assess any potential adverse effects and confirm the sustained benefits of phytol treatment. This comprehensive approach will help establish phytol’s viability as a therapeutic option for osteoporosis.
-
Funding information: Authors state no funding involved.
-
Author contributions: Bo Liu and Peng Wang: writing – review and editing, methodology, and funding acquisition. Xiangyang Lv: writing – review and editing, writing – original draft, methodology, funding acquisition, formal analysis, data curation, and conceptualization.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Cornelius C, Koverech G, Crupi R, Di Paola R, Koverech A, Lodato F, et al. Osteoporosis and Alzheimer pathology: Role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Front Pharmacol. 2014;5:120. 10.3389/fphar.2014.00120.Search in Google Scholar PubMed PubMed Central
[2] Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med. 2024;177(1), ITC1–16. 10.7326/AITC202401160.Search in Google Scholar PubMed
[3] Sato AY, Cregor M, Delgado-Calle J, Condon KW, Allen MR, Peacock M, et al. Protection from glucocorticoid-induced osteoporosis by anti-catabolic signaling in the absence of sost/sclerostin. J Bone Min Res. 2016;31(10):1791–802. 10.1002/jbmr.2869.Search in Google Scholar PubMed PubMed Central
[4] Maria S, Samsonraj RM, Munmun F, Glas J, Silvestros M, Kotlarczyk MP, et al. Biological effects of melatonin on osteoblast/osteoclast cocultures, bone, and quality of life: Implications of a role for MT2 melatonin receptors, MEK1/2, and MEK5 in melatonin-mediated osteoblastogenesis. J Pineal Res. 2018;64(3):e12465. 10.1111/jpi.12465.Search in Google Scholar PubMed PubMed Central
[5] Jang JS, Hong SJ, Mo S, Kim MK, Kim YG, Lee Y, et al. PINK1 restrains periodontitis-induced bone loss by preventing osteoclast mitophagy impairment. Redox Biol. 2024;69:103023. 10.1016/j.redox.2023.103023.Search in Google Scholar PubMed PubMed Central
[6] Wu M, Wu S, Chen W, Li YP. The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis, and disease. Cell Res. 2024;34(2):101–23. 10.1038/s41422-023-00918-9.Search in Google Scholar PubMed PubMed Central
[7] Selvaraj V, Sekaran S, Dhanasekaran A, Warrier S. Type 1 collagen: Synthesis, structure, and key functions in bone mineralization. Differentiation. 2024;136:100757. 10.1016/j.diff.2024.100757.Search in Google Scholar PubMed
[8] Li T, Jiang S, Lu C, Yang W, Yang Z, Hu W, et al. Melatonin: Another avenue for treating osteoporosis? J Pineal Res. 2019;66(2):e12548. 10.1111/jpi.12548.Search in Google Scholar PubMed
[9] Przerwa F, Uzar I, Bogacz A, Kotrych K, Sulikowski T, Wolek M, et al. Osteoprotegerin gene as a biomarker in the development of osteoporosis in postmenopausal women. Biomedicines. 2023;11(12):3218. 10.3390/biomedicines11123218.Search in Google Scholar PubMed PubMed Central
[10] Park SH, Kim JY, Cheon YH, Baek JM, Ahn SJ, Yoon KH, et al. Protocatechuic acid attenuates osteoclastogenesis by downregulating JNK/c-Fos/NFATc1 signaling and prevents inflammatory bone loss in mice. Phytother Res. 2016;30(4):604–12. 10.1002/ptr.5565.Search in Google Scholar PubMed
[11] Qu B, Xia X, Yan M, Gong K, Deng S, Huang G, et al. miR-218 is involved in the negative regulation of osteoclastogenesis and bone resorption by partial suppression of p38MAPK-c-Fos-NFATc1 signaling: Potential role for osteopenic diseases. Exp Cell Res. 2015;338(1):89–96. 10.1016/j.yexcr.2015.07.023.Search in Google Scholar PubMed
[12] Marcucci G, Domazetovic V, Nediani C, Ruzzolini J, Favre C, Brandi ML. Oxidative stress and natural antioxidants in osteoporosis: Novel preventive and therapeutic approaches. Antioxidants (Basel). 2023;12(2):373. 10.3390/antiox12020373.Search in Google Scholar PubMed PubMed Central
[13] Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J. Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2014;25(Suppl 3):iii124–37. 10.1093/annonc/mdu103.Search in Google Scholar PubMed
[14] Yang S, Sun Y, Kapilevich L, Zhang X, Huang Y. Protective effects of curcumin against osteoporosis and its molecular mechanisms: A recent review in preclinical trials. Front Pharmacol. 2023;14:1249418. 10.3389/fphar.2023.1249418.Search in Google Scholar PubMed PubMed Central
[15] Vimalraj S, Saravanan S, Subramanian R. Rutin-Zn(II) complex promotes bone formation: A concise assessment in human dental pulp stem cells and zebrafish. Chem Biol Interact. 2021;349:109674. 10.1016/j.cbi.2021.109674.Search in Google Scholar PubMed
[16] Persson P, Björnsson BT, Takagi Y. Characterization of morphology and physiological actions of scale osteoclasts in the rainbow trout. J Fish Biol. 1999;54(3):669–84. 10.1006111/jfbi.1998.tb00645.x.Search in Google Scholar
[17] Govindarajan D, Saravanan S, Sudhakar S, Vimalraj S. Graphene: A multifaceted carbon-based material for bone tissue engineering applications. ACS Omega. 2023;9(1):67–80. 10.1021/acsomega.3c07062.Search in Google Scholar PubMed PubMed Central
[18] Zhai S, Liu C, Vimalraj S, Subramanian R, Abullais SS, Arora S, et al. Glucagon-like peptide-1 receptor promotes osteoblast differentiation of dental pulp stem cells and bone formation in a zebrafish scale regeneration model. Peptides. 2023;163:170974. 10.1016/j.peptides.2023.170974.Search in Google Scholar PubMed
[19] Park WY, Han S, Choi BS, Park CW, Yang CW, Kim YS, et al. Progression of osteoporosis after kidney transplantation in patients with end-stage renal disease. Transpl Proc. 2017;49(5):1033–7. 10.1016/j.transproceed.2017.03.038.Search in Google Scholar PubMed
[20] Phan TC, Xu J, Zheng MH. Interaction between osteoblast and osteoclast: Impact in bone disease. Histol Histopathol. 2004;19(4):1325–44. 10.14670/HH-19.1325.Search in Google Scholar PubMed
[21] Crockett JC, Rogers MJ, Coxon FP, Hocking LJ, Helfrich MH. Bone remodelling at a glance. J Cell Sci. 2011;124(7):991–8. 10.1242/jcs.063032.Search in Google Scholar PubMed
[22] Yang Y, Nian H, Tang X, Wang X, Liu R. Effects of the combined Herba Epimedii and Fructus Ligustri Lucidi on bone turnover and TGF-β1/Smads pathway in GIOP rats. J Ethnopharmacol. 2017;201:91–9. 10.1016/j.jep.2017.02.033.Search in Google Scholar PubMed
[23] Vimalraj S. Alkaline phosphatase: Structure, expression, and its function in bone mineralization. Gene. 2020;754:144855. 10.1016/j.gene.2020.144855.Search in Google Scholar PubMed
[24] Xu W, Angelis K, Danielpour D, Haddad MM, Bischof O, Campisi J, et al. Ski acts as a co-repressor with Smad2 and Smad3 to regulate the response to type β transforming growth factor. Proc Natl Acad Sci U S A. 2000;97(11):5924–9. 10.1073/pnas.090097797.Search in Google Scholar PubMed PubMed Central
[25] Haghwerdi F, Khozaei Ravari M, Taghiyar L, Shamekhi MA, Jahangir S, Haririan I, et al. Application of bone and cartilage extracellular matrices in articular cartilage regeneration. Biomed Mater. 2021;16(4):e14557. 10.1088/1748-605X/ac094b.Search in Google Scholar PubMed
[26] Li Y, Selvaraj V, Saravanan S, Abullais SS, Wankhade V. Exploring the osteogenic potential of chitosan-quercetin bio-conjugate: In vitro and in vivo investigations in osteoporosis models. Int J Biol Macromol. 2024;274(Pt 2):133492. 10.1016/j.ijbiomac.2024.133492.Search in Google Scholar PubMed
[27] Pereira-Silva M, Miranda-Pastoriza D, Diaz-Gomez L, Sotelo E, Paiva-Santos AC, Veiga F, et al. Gemcitabine-vitamin E prodrug-loaded micelles for pancreatic cancer therapy. Pharmaceutics. 2024;16(1):95. 10.3390/pharmaceutics16010095.Search in Google Scholar PubMed PubMed Central
[28] Haghighatseir N, Mozafari N, Shadvand E, Ashrafi H, Daneshamouz S, Azadi A. Mixed-micelle in situ gel as a candidate for oral inflammatory ulcerative diseases. AAPS PharmSciTech. 2024;25(6):144. 10.1208/s12249-024-02862-2.Search in Google Scholar PubMed
[29] Schini M, Vilaca T, Gossiel F, Salam S, Eastell R. Bone turnover markers: Basic biology to clinical applications. Endocr Rev. 2023;44(3):417–73. 10.1210/endrev/bnac031.Search in Google Scholar PubMed PubMed Central
[30] Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark Res. 2017;5:18. 10.1186/s40364-017-0097-4.Search in Google Scholar PubMed PubMed Central
[31] Yin H, Wang J, Wu M, Ma Y, Wang S, Su Q. Preventive effects of evodiamine on dexamethasone-induced osteoporosis in zebrafish. Biomed Res Int. 2019;2019:5859641. 10.1155/2019/5859641.Search in Google Scholar PubMed PubMed Central
[32] Bergen DJM, Kague E, Hammond CL. Zebrafish as an emerging model for osteoporosis: A primary testing platform for screening new osteo-active compounds. Front Endocrinol (Lausanne). 2019;10:6. 10.3389/fendo.2019.00006.Search in Google Scholar PubMed PubMed Central
[33] Adhish M, Manjubala I. Effectiveness of zebrafish models in understanding human diseases: A review of models. Heliyon. 2023;9(3):e14557. 10.1016/j.heliyon.2023.e14557.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

