Abstract
Cancer treatment continues to face challenges due to adverse effects, drug resistance linked with conventional therapies, and high costs. As increasing interest in safer and cost-effective alternatives drugs, natural products such as flavonoids have been explored for treating cancer. Rutin, a dietary flavonoid, exhibits diverse pharmacological activities that may contribute to cancer prevention and treatment. It interferes with cancer progression by inducing apoptosis and autophagy, promoting cell cycle arrest, regulating oxidative stress, activating tumor suppressor gene, and modulating various signaling cascades. Recent studies also suggest that combining rutin with other therapeutic agents or employing nanoformulations may enhance its bioavailability and anticancer efficacy. This review critically examines anticancer mechanisms across various cancer types and highlights novel strategies to explored their therapeutic potential. The comprehensive clinical trials and mechanistic studies are needed to validate its safety, bioavailability, and efficacy in cancer management.
1 Introduction
With rising incidence and fatality rates in recent years, cancer has become a primary global health concern. Despite advancements in many therapeutic modalities, cancer remains a leading cause of mortality globally [1,2]. Although current cancer therapies offer excellent benefits, these medicines are associated with severe side effects, including extreme nausea and vomiting [3]. Thus, there is a need for more effective, cost-effective, and safer treatment approaches to manage cancer. Numerous herbs are being studied for their tumoricidal properties against various types of cancer. Many of these herbs have undergone clinical trials. New strategies are being developed that combine molecular targeting of cancer pathways with cytotoxic therapies [4]. Continuous research and development have been aimed at discovering new cytotoxic compounds from medicinal plants with anti-proliferative activity [5]. Numerous medicinal plants have been demonstrated to aid in the prevention and treatment of a variety of ailments. People have utilized plants as remedies to treat a wide range of diseases throughout history. This is because a number of bioactive compounds found in plants can be used to treat a wide range of diseases [6]. Numerous therapeutically important substances for the development of new drugs have been found in natural products and secondary metabolites [7]. They are also reasonably non-toxic, affordable, and available in an ingestible form [8]. These naturally occurring substances have been demonstrated to meaningfully contribute to the prevention as well as treatment of disease in various pathologies, especially cancer [9,10,11,12].
Additionally, plants have a variety of flavonoids, which are excellent sources of antioxidants and can suppress the pathogenesis of numerous diseases. Moreover, several plant-based chemotherapy medicines have demonstrated appreciable enhancements in the anti-cancer activity of several chemotherapeutic agents [13,14,15]. Rutin (3′,4′,5,7-tetrahydroxy-flavone-3-rutinoside) (Figure 1) is found in fruits, vegetables, and medicinal plants (Figure 2) and is an important nutritional component of many foodstuffs [16,17]. Rutin is best known for its antioxidant and anti-inflammatory effects. These potential benefits of rutin are essential factors implicated in the prevention of numerous pathogeneses. Previously, rutin exerts several health-promoting effects by modulating key molecular pathways [18].

Chemical structure of rutin (drawn by ChemDraw Professional 15.0).

Dietary sources of rutin. Rutin is a plant-derived flavonoid abundantly found in a variety of natural food sources such as citrus fruits, apples, asparagus, peaches, buckwheat, onions, tea, plums, berries, etc.
Numerous investigations have revealed that rutin modulates several cell signaling molecules, such as those involved in apoptosis, the cell cycle, angiogenesis, and autophagy, and so plays a significant role in managing cancer [19,20,21]. Therefore, this review explores rutin’s potential as an anticancer agent through a detailed analysis of these modulatory effects.
2 Search strategy
This review article discusses the role of rutin in various types of cancer. The review gathered a wide-ranging literature search based on English-language publications using PubMed, Google Scholar, and Scopus databases. They used keywords such as “rutin and cancer,” “rutin and inflammation,” “rutin and oxidative stress,” “rutin and angiogenesis,” “rutin and STAT-3,” “rutin and Akt/PI3K,” “rutin and breast cancer,” “rutin and lung cancer,” “rutin and cervix cancer,” “rutin and liver cancer,” “rutin and gastric cancer,” and “rutin and leukemia.” The review also explores the synergistic effects of rutin with other compounds.
3 Major mechanisms of rutin involved in cancer management
Natural products and their active compounds have been reported for their anti-inflammatory potential that helps prevent or mitigate the progression of different disease pathogeneses [22,23,24,25]. Rutin has demonstrated its anticancer properties across diverse types of cancer by modulating essential molecular and cellular pathways, as evidenced in both in vivo and in vitro studies (Table 1). The following are some of the main approaches through which rutin manages cancer.
Mechanism of action of rutin in cancer
| Action on | Cancer | Study types | Doses | Findings of the study | Refs. |
|---|---|---|---|---|---|
| Inflammation | Breast cancer | In vivo | 100 mg/kg | Decreased iNOS and NF-kB, increased antioxidant levels, inhibited inflammatory cytokines | [30] |
| Brain cancer | In vitro | 30 µM | Modulated inflammatory mediators | [32] | |
| Endoplasmic reticulum | Breast cancer | In vitro | 40 µM | Induced apoptosis via ER stress with doxorubicin | [37] |
| P53 | Brain cancer | In vitro | 0–20 M | Apoptosis via upregulation of P53; Apoptosis was reversed by P53 knockdown | [41] |
| Prostate cancer | In vitro | 700 µM | Increased expression of the p53 gene | [42] | |
| In vitro | 100 and 150 µM | Increased expression of p53 and Bax | [44] | ||
| Oxidative stress | Breast cancer | In vitro | 100 mM | Reversed ROS increase | [57] |
| Lung and colon cancer | In vitro | 5–100 mM | Dose-dependent reduction of superoxide anion | [58] | |
| Breast cancer | In vivo | 20 mg/kg | Increased GSH and decreased MDA level content | [59] | |
| Apoptosis | Brain cancer | In vitro | 0, 1, 5, and 10 µM | Induced apoptosis | [41] |
| In vitro | 25, 50, 100 µM | Downregulated BCL2/BAX ratios, Induced apoptosis | [65] | ||
| Pancreatic cancer | In vitro | 5 and 10 μg/mL | Induced apoptosis, and apoptosis-related protein expression | [66] | |
| Cell cycle | Breast cancer | In vitro | 20 μM and 50 μM | Increased the rate of cell death. | [71] |
| Brain cancer | In vitro | 50–100 μM | Inhibited cell viability and proliferation, reduced ERK1/2 phosphorylation, G2 arrest | [73] | |
| Brain cancer | In vitro | 0, 25, 50, and 100 μM | Cells in the G2/M phase accumulation | [65] | |
| Angiogenesis | Human promyelocytic leukemia | In vitro | 10 and 30 μM | Decreased VEGF secretion | [80] |
| Breast cancer | In vitro | 25, 50, and 100 µM | Decreased Bcl2 and VEGF in a dose-dependent manner via vanadium-rutin treatment | [44] | |
| Colon cancer | In vitro | 1, 10, 20 mg/kg | Decreased VEGF level | [83] | |
| Breast cancer | In vitro | 200 µM | Induced angiogenesis by downregulating the anti-angiogenic marker | [85] | |
| AP-1 | Human promyelocytic leukemia | In vitro | 10 and 30 µM | Rutin and VE together inhibited the binding actions of AP | [80] |
| Autophagy | Liver cancer | In vitro | 75 μM | Reduced autophagy and BANCR expression | [95] |
| Oral & lung cancer | In vitro | 0–40 μM | Stimulated autophagy | [96] | |
| Brain cancer | In vitro | 100 or 200 μM | TMZ increased the expression of LC3II, whereas rutin treatment reversed this effect | [97] | |
| PI3K/AKT/mTOR | Ehrlich ascites carcinoma | In vivo | 25 mg/kg | Reduced expressions of PI3K, AKT, mTOR | [100] |
3.1 Anti-inflammatory effects
Chronic inflammation is an important driving force behind tumor initiation and progression [26,27,28]. Rutin has demonstrated potent anti-inflammatory properties through the modulation of several signaling pathways implicated in cancer development. In the lipopolysaccharide-induced mastitis mouse model, rutin was shown to alleviate inflammation by inhibiting the nuclear factor kappa B (NF-κB) signaling pathway and attenuating endoplasmic reticulum (ER) stress, revealing its ability to disrupt pro-inflammatory responses [29]. Another study using a 7,12-di-methylbenz(a)anthracene (DMBA)-induced breast cancer model of female rats, highlighted rutin’s impact on inflammatory signaling by downregulating interleukin 6/NF-κB, and modulating SRC1/HSP90, and ER-α signaling pathways, ultimately suppressing tumor biomarkers, including Carcinoma Antigen 15-3, proto-oncogene Tyrosine-Protein Kinase Src1 and Inducible Nitric Oxide Synthase (iNOS) [30]. Importantly, this work links anti-inflammatory modulation directly with tumor suppression.
In mice with the human papillomavirus type 16 (HPV16) transgene, rutin reduced cyclooxygenase-2 (COX2) expression in both epidermis and dermis. Co-treatment with rutin and curcumin decreased tumor-associated inflammation, suggesting that these nutritional substances could regulate COX2 expression in HPV16-induced lesions by limiting leukocytic infiltration [31]. In glioblastoma (GBM) microenvironment, rutin indirectly modulated inflammatory signaling by altering microglial miRNA-125b and STAT3 expression in co-culture with cells of GBM. Rutin significantly reduced the viability of GL15 cells [32]. The K14-HPV16 mouse model, commonly used to study the wasting syndrome associated with HPV-induced cancers, demonstrated that rutin exerted strong muscle-protective effects through NF-κB downregulation [33].
In summary, the anti-inflammatory potential of rutin is a major mechanism in the suppression of cancer, particularly through targeting NF-κB, signal transducer and activator of transcription 3 (STAT-3), and COX-2 pathways in tumor-related inflammatory environments.
3.2 Modulation of ER stress
Tumor cells often encounter microenvironmental stress, leading to the disruption of the functions of the ER. ER stress response has been reported to disturb intracellular homeostasis and impair normal cell function [34]. ER stress may help tumor cells return to homeostasis and foster an environment conducive to tumor survival and growth [35]. Cancer cells are known for their capacity to spread locally or metastasize into neighboring tissues. Unfavorable circumstances in these novel settings, such as low glucose, hypoxia, growth factor deprivation, oxidative stress, lactic acidosis, and amino acid starvation, can impair the endoplasmic reticulum’s ability to fold proteins correctly [36]. One finding focused on the role of ER stress in apoptotic cell death. The disruption of ER activities plays a critical role in triggering apoptosis. The study shows that combinational treatment with rutin and doxorubicin significantly enhances ER stress-mediated apoptotic cell death in triple-negative breast cancer cells [37]. Recently, the effects of rutin at different concentrations (400–700 mM/mL) on the cell cycle, metabolism, and apoptosis of human colon cancer cells (SW480) were investigated. The enrichment analysis of the miRNAs–lncRNAs–mRNAs–TFs network in these cells suggested that rutin may modulate ER stress [38]. Collectively, modulation of ER stress by rutin contributes significantly to apoptosis and growth inhibition in cancer cells, making it a valuable component of combinational approaches for cancer management.
3.3 Activation of p53 gene
The gene p53 protects the genome and suppresses tumors. It is essential in deciding how cells react to stimuli that cause DNA damage [39]. This well-known tumor suppressor gene induces apoptosis and arrest of the cell cycle [40]. Through boosting p53 activity, several natural compounds have been shown to combat cancer. In glioma CHME cells, rutin induced the marked cytotoxicity and cell death by p53-mediated apoptosis. It upregulated BAX, promoted cytochrome c release, activated caspases 3 and 9, and downregulated BCL. Moreover, apoptosis induced by rutin was reversed in a concentration-specific way when p53 was knocked down, confirming a p53-dependent mechanism [41].
The effects of rutin and 5-fluorouracil (5-FU) combination were investigated on prostate cancer cells. The combined administration of 5-FU and rutin increased both apoptosis and expression of p53 genes in PC3 cells, a cell line used to model prostate cancer. Moreover, it was concluded that the synergistic potential of the 5-FU/rutin combination on this cancer cell line improved the expression of the p53 gene, downregulation of Bcl-2 protein, and apoptosis compared to control separate applications [42]. In contrast to tamoxifen, in MCF-7 cells, genes associated with the cell cycle (CDK1 and p21) and both tumor suppressors (p53 and PTEN) were upregulated by rutin; using a p53-dependent mechanism, this compound caused G2/M arrest and apoptosis of the cells of MCF-7 [43].
A study expertly focused on many apoptotic pathways in human breast cancer cell lines to investigate the vanadium–rutin complex’s involvement in curing cancer. The complex’s chemotherapeutic properties were evidenced by activating p-53-dependent intrinsic apoptosis and regulating the vascular endothelial growth factor (VEGF) cascades. The complex effectively began apoptosis by activating the p53-dependent intrinsic apoptotic route in both kinds of cells [44]. Thus, the ability of rutin to modulate p53-dependent intrinsic apoptosis pathways underscores its potential as an effective anticancer compound targeting genomic integrity regulators.
3.4 Antioxidant potential
The accumulation of reactive oxygen species (ROS), including superoxide ions, is a sign of oxidative stress. ROS are signaling molecules that regulate vascular smooth muscle cell growth, contraction, and relaxation [45]. Oxidative stress is a critical factor implicated in several pathologies [46], including diabetes mellitus and neurodegenerative diseases, and it plays a dual role in cancer, contributing to genetic mutations and tumorigenesis at moderate levels. Cancer cells show abnormal redox homeostasis, whereas ROS are pro-tumorigenic, and higher levels of ROS are cytotoxic [47,48]. The management of ROS concentrations has been considered an auspicious therapeutic approach, mainly focusing on the anticancer role of natural products [49]. Additionally, numerous epidemiological studies showed a negative correlation between a diet rich in fruit/vegetables and cancer occurrence or progression [50,51]. Flavonoids protect plants against microbial infections, ultraviolet radiation, and oxidative stress [52].
Dietary nutrients can enhance the body’s natural antioxidant defenses by scavenging ROS [53]. Rutin has been demonstrated to act as a neuroprotectant in ischemic animal models and reperfusion-induced cerebral injury [54,55,56]. In one study, olmesartan exhibited cytotoxic activity against HeLa and MCF-7 cell lines when administered following rutin pretreatment. The observed decrease in ROS levels suggested decreased viability of cells treated with olmesartan [57]. Another study examined the effects of rutin on human lung (A549) and colon (HT29 and Caco-2) cancer cell lines, focusing on migration, viability, adherence, and superoxide anion formation. As per the outcomes, rutin treatment prevented the viability of malignant cells and reduced the formation of superoxide in HT29 cells. Furthermore, rutin impaired the migration and adherence of HT29 and A549 cells [58]. In a separate study, both rutin and orlistat showed anticancer efficacy in two breast carcinoma models (in vivo EAC and in vitro MCF7) as well as in pancreatic cancer (PANC-1) cell lines. Lowering of tumor volume, fatty acid synthase expression, cholesterol, carcinoembryonic antigen (CEA) level, along with increased antioxidant capacity (elevated GSH and lowered malondialdehyde (MDA) content), and histological improvements supported the anticancer effect [59]. Therefore, by restoring redox balance and inhibiting oxidative stress, rutin exhibits strong protective effects against cancer initiation and progression.
3.5 Induction of apoptosis
Apoptosis is a tightly regulated cellular process characterized by condensation of chromatin, shrinkage of cells, fragmentation of chromosomal DNA, blebbing of the cell membrane, and fragmentation of the nucleus [60]. Various diseases, including cancer, can arise from the disruption of the balance among homeostasis and cell growth and apoptosis. Unchecked apoptosis is a key mechanism underlying tumorigenesis [61]. There are two mechanisms, including extrinsic and intrinsic signaling mechanisms, for apoptosis [62]. Despite diverse origins, all types of cancer share common characteristics, such as unchecked growth, angiogenesis, and evasion of apoptosis [63,64]. Therefore, therapies based on natural compounds have shown promise in modulating these cancer hallmarks. In 2018, rutin and orlistat exhibited cytotoxicity against MCF-7 and Panc-1 cell lines by inducing apoptosis [59]. Rutin was also shown to induce cytotoxicity in CHME cells and cause cell death by inducing apoptosis through the upregulation of p53. Rutin caused nuclear condensation, membrane blebbing, and fragmentation, and increased oxidative stress. It further promoted apoptosis by stimulating the release of cytochrome c, downregulating BCL, upregulating BAX, and activating both caspase 9 and caspase 3 [41]. Through inducing cell death and G2/M arrest in the cell cycle progression, as well as controlling the expression of genes linked to apoptosis, it also showed notable anti-neuroblastoma actions in human neuroblastoma cell lines. These findings suggested that rutin has significant anti-neuroblastoma properties by boosting cell death, triggering G2/M arrest in cell cycle progression, and modulating the expression of genes linked to apoptosis [65]. Additionally, rutin promoted apoptosis in pancreatic cancer cells by elevating the production of miR-877-3p, which in turn suppressed Bcl-2 transcription. It is concluded that miR-877-3p mimics and rutin may increase the production of apoptotic proteins [66]. Rutin-induced apoptosis has been linked to caspase-3 activation and a lowering in mitochondrial membrane potential (MMP) [67]. Overall, rutin effectively induces apoptosis via mitochondrial dysfunction and caspase activation, positioning it as a potent natural apoptotic inducer in cancer therapy.
3.6 Cell cycle arrest
Disruption of the cell cycle checkpoints is central to tumor progression, as cell cycle alterations contribute to uncontrolled cell proliferation and cancer growth [68,69]. Additionally, inducing cell cycle arrest at various checkpoints has been reported to be a promising anticancer strategy [70]. Rutin exhibits its anticancer potential through cell cycle arrest, and studies prove it. In MCF-7 cells, it upregulated the expression of cell cycle-related regulators like p21 and CDK1, as well as key tumor suppressors, including p53 and PTEN, in contrast to tamoxifen. This compound induced G2/M arrest and apoptosis in MCF-7 cells through a p53-dependent pathway [43].
Interestingly, rutin has been reported to increase the sensitivity of cancer cells to cyclophosphamide and methotrexate, while also reversing multidrug resistance. It also induces cell cycle arrest at both G2/M and G0/G1 phases, thereby promoting apoptotic cell death [71]. In another study focused on lung cancer, the combination of rutin and cisplatin significantly increased the number of apoptotic cells as compared to the untreated control group. The results suggest that rutin promotes the tumor necrosis factor-α (TNF-α)-induced apoptosis of A549 human lung carcinoma cells [72]. Rutin has been found to have pro-apoptotic and antiproliferative effects on human GBM cell lines (GL-15). In addition, it arrests the cell cycle at the G2 stage and promotes the differentiation of GL-15 cells into an astroglial phenotype [73]. In 2013, research was published on the anti-tumor effect of rutin on human neuroblastoma cell lines. Rutin reduced LAN-5 cell proliferation and chemotactic potential. It caused cell death and G2/M arrest in the cell cycle progression, as shown by flow cytometry analysis [65]. Rutin was reported to show anticancer activity in HPV-C33A cervical cancer cells through G0/G1 cell cycle arrest and induction of apoptosis [67]. Therefore, rutin’s ability to modulate cell cycle progression contributes significantly to its anti-proliferative and cytostatic effects against diverse cancers.
3.7 Inhibition of angiogenesis
The over-expression of pro-angiogenic factors and the suppression of anti-angiogenic factors lead to aberrant and excessive angiogenesis in various non-neoplastic angiogenic diseases [74,75,76]. The “angiogenic switch” process in tumors marks the onset of the creation of blood vessels, which is triggered when pro-angiogenic signaling dominates over anti-angiogenic control [77]. Additionally, angiogenesis aids in advancing several malignant cancers [78,79].
It has been demonstrated that natural components can help inhibit cancer progression through limiting angiogenesis. According to Chuang et al., a combination of vitamin E and rutin suppresses VEGF expression in HL-60 cells by the downregulation of insulin-like growth factor 1 receptor (IGF1-R)/insulin receptor substrate-1 (IRS-1) protein production, mediated by reduced activator protein 1 (AP-1) interaction activity [80]. Similarly, the flavonoids identified in Murraya koenigii leaf extract, including quercetin, rutin, kaempferol, and apigenin, reduced the activity of the endogenous 26S proteasome in MDA-MB-231 cells in a dose-dependent way. The lower expression of angiogenic and anti-apoptotic gene markers in the leaf extract-treated tumors indicates that angiogenesis is inhibited and apoptosis is promoted [81]. The rutin-vanadium compound efficiently caused cell death by interfering with Bax, p53, and Bcl-2 and lowering VEGF expression in cancer cells [44].
Furthermore, rutin has also been demonstrated to suppress VEGF expression and angiogenesis in MDA-MB-231 carcinoma cells [82]. In the colon cancer model, rutin exerted the most potent cytotoxic activity on SW480 cells. These effects declined with increasing doses. In comparison to untreated animals, treatment with 20 mg/kg rutin reduced VEGF serum levels by 55%, extended lifespan by 50 days, and showed no adverse effects on body weight (BW) or relative organ weight in mice [83].
By decreasing the levels of VEGF and transforming growth factor (TGF)-β1, rutin inhibited angiogenesis of GL-15 cells [84]. In breast cancer (MCF-7 and MDA-MB-231) cell lines, rutin modulated proliferation, metastasis, and angiogenesis. Additionally, in MDA-MB-231 and MCF-7 cells, rutin modulated angiogenesis and metastasis-related genes by downregulating THBS1 and CDH1 genes, and upregulating FN1, MKI67, CDH2, VIM, and VEGFA genes [85]. In conclusion, rutin exerts anti-angiogenic effects by attenuating VEGF signaling and associated pro-angiogenic factors, thereby inhibiting tumor vascularization and progression.
3.8 Suppression of AP-1 transcription factor
AP-1 is a transcription factor complex involved in the modulation of gene expression linked to proliferation, angiogenesis, and survival. Its dysregulation drives tumor progression by promoting the expression of cancer-related genes that trigger mitogenic, pro-angiogenic, and anti-apoptotic signaling pathways [86,87,88,89]. Natural products or their active molecules have been shown to exert anticancer effects by inhibiting AP-1. A study on anti-metastatic actions of Phyllanthus urinaria L extracts (PUE) provided evidence suggesting that several polyphenols, including methyl gallate, gallic acid, epicatechin, gallocatechin-3-gallate, rutin, and naringin, contribute to the anticancer effects of this plant. PUE significantly inhibited the migration and invasion of metastatic A549 and Lewis lung cancer cells. Furthermore, PUE demonstrated the ability to block NF-κB and AP-1 nuclear translocation as well as their DNA-binding activity [90].
A different study using the human promyelocytic leukemia (HL-60) cell line found that cotreatment with rutin and vitamin E (VE) significantly decreased the expression of the c-Jun protein and diminished the binding affinity of nuclear factor-activator protein-1 (AP-1) to the VEGF gene promoter. This is accompanied by reduced VEGF expression. This inhibitory potential was attributed to downregulation of insulin-like growth factor 1 receptor (IGFR) and IRS-1, resulting in reduced AP-1 transcriptional activity [80].
Collectively, rutin’s suppression of AP-1 contributes to its anticancer activity by inhibiting the expression of key oncogenic mediators.
3.9 Modulation of autophagy
Autophagy was first introduced by Christian de Duve in 1963 as a lysosome-mediated disposal process [91]. Autophagy is a lysosome-dependent degradation process that maintains cellular homeostasis. Autophagy is an intracellularly regulated process necessary for preserving cellular homeostasis by eliminating misfolded and undesirable proteins [92]. Autophagy has both tumor-promoting and tumor-suppressive properties, making it a two-edged sword [93]. Autophagy controls pro-survival or pro-death signaling pathways in many cancer types and is impacted by environmental factors such as stress and the kind and stage of malignancies [94]. Rutin modulates autophagy, which contributes to its involvement in cancer control. A recent study investigated the function of rutin in hepatocellular carcinoma (HCC) autophagy and chemoresistance produced by sorafenib (SO). The results showed that rutin treatment decreases the number of autophagosomes formed following rutin-treated HCCLM3/SO and HepG2/SO cells, as well as BRAF-activated non-protein coding RNA (BANCR) expression and autophagy in SO-resistant cells. Furthermore, rutin inhibits autophagy via the BANCR/miRNA-590-5P/OLR1 axis, according to an in vivo investigation [95]. BANCR, miRNA-590-5p, and OLR1 are molecules involved in various cellular processes, particularly in cancer and other diseases. BANCR is a long non-coding RNA, while miRNA-590-5p is a microRNA, and OLR1 encodes for the protein LOX-1 (Lectin-like oxidized low-density lipoprotein receptor 1). HCCLM3/SO and HepG2/SO cells are derived from HCC.
Park et al. showed that rutin-activated autophagy in lung (A549), oral (CA9-22), and leukemia (THP-1) cell lines. The inhibition of rutin-induced autophagy increased TNF-α, an important regulator of inflammation. Together, these results revealed that rutin increased autophagy, which decreased TNF-α production [96]. Rutin inhibits JNK-mediated autophagy in GBM multiforme, thereby increasing the therapeutic efficacy of temozolomide (TMZ) in both in vitro and in vivo. This was demonstrated by immunoblotting analysis, which showed that TMZ activated JNK signaling to induce protective autophagy [97]. Hence, modulation of autophagy by rutin may offer a strategic pathway to target the mechanisms underlying cancer cell survival and resistance to treatment.
3.10 Inhibition of PI3K/AKT/mTOR pathway
In eukaryotic cells, the PI3K/AKT/mTOR (PAM) signaling pathway is a highly conserved signal transduction network that supports cell growth, survival, and cell cycle progression. Phosphorylinositol 4,5 bisphosphate (PIP2) is phosphorylated by the PI3Ks to Phosphorylinositol 3,4,5-triphosphate (PIP3), leading to the activation of serine/threonine kinase. Activated Akt to become phosphorylated, affecting cancer cells’ proliferation, survival, and cycling [98]. The tumor suppressor phospholipase and tensin homolog deleted on chromosome ten (PTEN) counteracts this pathway by dephosphorylating PIP3 back to PIP2 [99].
A new study used the argyrophilic nucleolar regulatory area and the mTOR-signaling pathway to investigate the anti-tumor activity of rutin at various doses. In the mice with solid tumors, rutin (25 and 50 mg/kg) was administered intraperitoneally, while in the experimental groups, EAC cells were induced subcutaneously. Using immunohistochemistry, it was shown that there was a significant decrease in the expression of AKT, PI3K, mTOR, and F8, especially in the groups that received rutin (25 mg) as opposed to the control group. The average AgNOR number and AgNOR area/nuclear area (TAA/NA) were calculated, and statistically significant differences were found between the TAA/NA ratio groups. Significant statistical variations were seen in the mRNA expression of the mTOR, PI3K, and AKT1 genes. Rutin had anti-tumor properties overall, both in vitro and in vivo, against the growth of solid tumors produced by EAC cells [100]. Therefore, the inhibition of the PI3K/AKT/mTOR pathway by rutin represents a key molecular mechanism underlying the antitumor potential of rutin.
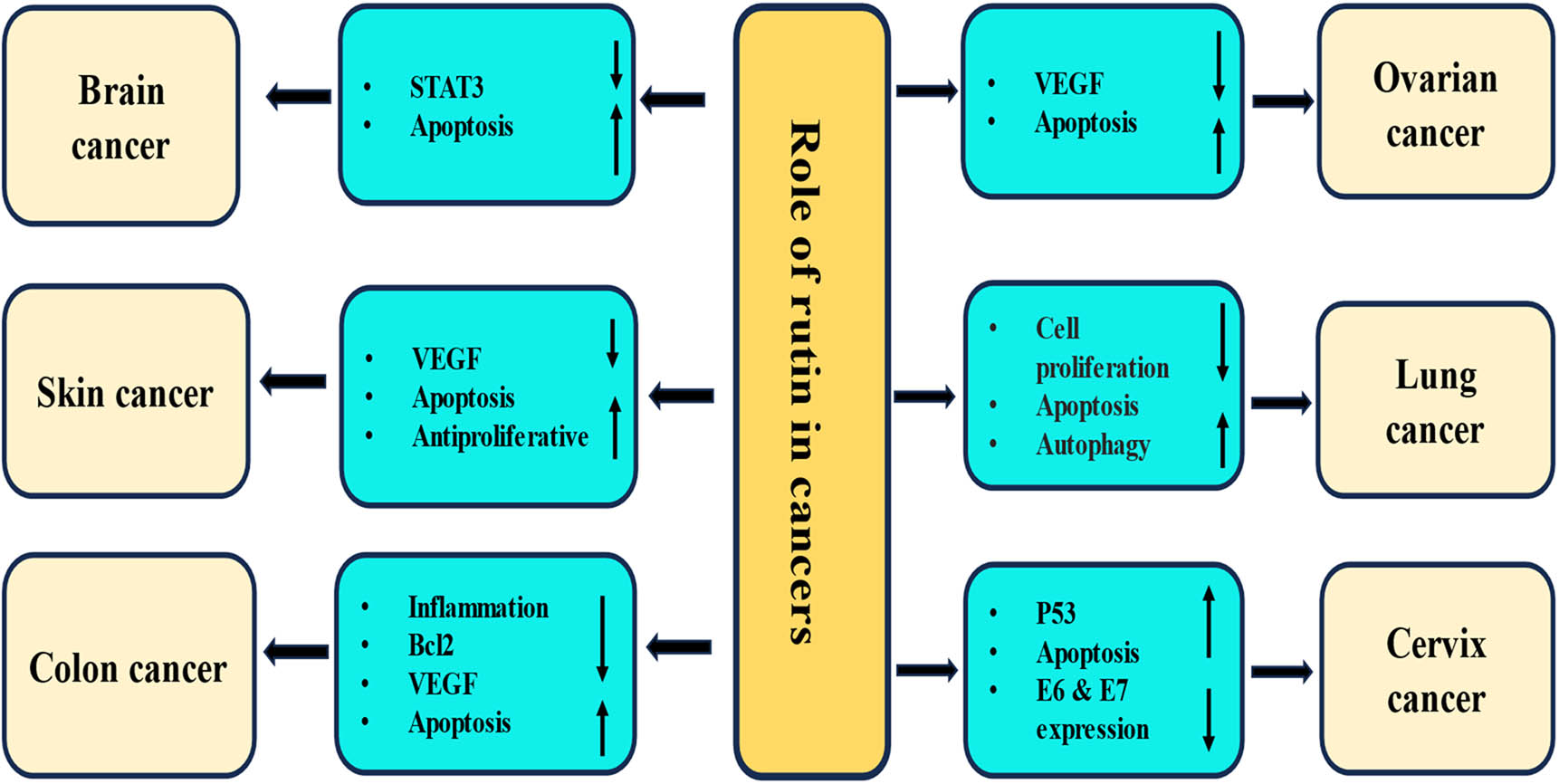
4 Role of rutin in different cancers
Rutin has been discovered to combat multiple types of malignancies. Numerous molecular processes, both in vivo and in vitro, demonstrate rutin’s involvement in cancer (Table 2). The role of rutin in different cancers is described as:
Role of rutin in management of different cancers
| Cancer types | Study types | Model type | Outcomes after rutin treatment | Refs |
|---|---|---|---|---|
| Lung cancer | In vitro | Human lung epithelial cancer | Inhibited proliferation, migration, and induced apoptosis | [105] |
| In vitro | Human lung cancer cells | Induces apoptosis, enhances the expression of GSK-3β and TNF-α | [72] | |
| In vivo, C57BL/6 mice | Lung metastasis in C57BL/6 mice | The lung tumor nodule formation inhibited, and life span was increased | [107] | |
| In vitro, A549 | Human lung cancer cells | Induced autophagy, inhibited the production of TNFα, and inhibited NF-κB signaling | [96] | |
| Brain cancer | In vitro, GL-15 | Human GBM cell line | Induced apoptosis, delayed cell migration and upregulated the expression of metalloproteinase | [109] |
| In vitro, GL-15 | Human GBM cell line | Reduced the viability of cancer cells, downregulated the expression of miRNA-125b and STAT3 | [32] | |
| In vitro, CHME | Human glioma cells | Induced cytotoxicity and apoptosis via P53 upregulation; promoted nuclear condensation, membrane blebbing, and fragmentation was caused by treatment | [41] | |
| In vitro (D54-MG, U87-MG and U251-MG) & in vivo (BALB/c athymic mice) | Human GBM cell line & male nude mice tumor | Rutin with TMZ enhanced the cytotoxicity; cotreatment reduced tumor volumes; reduced autophagy | [97] | |
| Breast cancer | In vitro, MDA-MB-231& MCF-7 | Breast cancer cells | Modulated the proliferation of cancer and the migration of the cells; Downregulated THBS1 and CDH1 genes and upregulated FN1, MKI67, CDH2, VIM, and VEGFA in cancer cells | [85] |
| In vivo, female albino rats | Breast carcinogenesis induced by DMBA | Ameliorated the carcinogenic effect and liver function enzymes; Increased antioxidant enzymes and reduced the inflammatory markers | [30] | |
| In vitro, 4T1 | Breast cancer cell line | Suppressed cell proliferation; Increased the expression of miR‐129‐1‐3p; Confined the growth of mouse breast cancer cells | [115] | |
| Cervix cancer | In vitro, SiHa | Cervical cancer cells | Modulated tumor suppressor genes and oncogenes; Induced apoptosis and activation of Caspase | [119] |
| In vitro, C33A | Human cervical cancer | Induced ROS production, reduced cell viability and nuclear condensation, provoked apoptosis by activating caspase-3 and reducing MMP | [67] | |
| Ovarian cancer | In vitro, OVCAR-3 | Human ovarian cancer cells | Exhibited different levels of inhibition in VEGF expression | [124] |
| Prostate cancer | In vitro, PC3 | Prostate cancer cell line | 5-FU/rutin combination enhanced apoptosis and p53 gene expression, downregulated Bcl-2 protein | [42] |
| Colon cancer | In vitro, HT-29 | Colon cancer cell line | Showed anticancer effects, induced apoptosis, regulated the expressions of genes linked to apoptosis and inflammation | [142] |
| In vitro, HT-29 | Human colon adenocarcinoma cells | Reduced cell proliferation and increased apoptotis, modulated MMP in the group treated with Gy and rutin. | [144] | |
| In vivo, rat model | Colon cancer model induced by azoxymethane | Reduced the number of aberrant crypt foci, promoted apoptosis | [150] | |
| Liver cancer | In vivo, male wistar rats | HCC | Decreased liver weight and tumor marker enzymes and liver marker enzymes | [156] |
| In vivo, male albino rats | HCC induced by chemicals | Decreased enzyme activities, decreased VEGF levels Rutin improved hepatocellular architecture with minor hepatic changes | [110] | |
| In vitro, HepG2 | Human hepatoma cells | Decreased ROS and MDA concentration | [158] | |
| Gastric cancer | In vitro, SGC-7901 cells | Human gastric adenocarcinoma | Inhibited the growth of cancer cells, and induced cell cycle arrest at G0/G1, increased the expression of Caspase, reduced the Bcl-2/Bax ratio | [163] |
| In vitro, AGS and MGC803 | Gastric cancer cells | Inhibited the proliferation, migration, as well as invasion, induced apoptosis, and suppressed the EMT process | [164] | |
| Leukemia | In vitro, U937, KG1, HL-60 and KG1a | Human leukemic cells | Induced apoptosis in adherent leukemic cells, inhibitory effect on the survival of adherent leukemic progenitors | [170] |
| In vivo, BALB/c mice | Animal model leukemia | Decreased the percentage of Mac-3 markers, reduced weights of the spleen and liver | [171] | |
| In vitro, HL-60 cells | Human promyelocytic leukemia | VEGF expression attenuated by rutin with vitamin E | [80] | |
| Bone cancer | In vitro, SAOS2 | Osteosarcoma cell lines | Reduced the proliferation, migration, of cancer cells and induced apoptosis | [179] |
| Skin cancer | In vitro, RPMI-7951 and SK-MEL-28 | Human melanoma cell lines | Reduced the viability, exerted a senescence-inducing potential | [183] |
| In vitro, B164A5 | Murine melanoma cell line | It demonstrated to be an active pro-apoptotic and antiproliferative compound against B164A5 cells | [186] | |
| Renal cancer | In vitro, 786-O | Renal cancer cell | Reduced the cell viability, induced a substantial increase in sub-G1 population, decreased G0/G1 population | [190] |
| Pancreatic cancer | In vitro, PANC-1 | Human pancreatic cancer cell line | Reduced growth in a dose-dependent manner, upregulated miRNA-877-3p expression | [66] |
| Multiple myeloma | In vitro, RPMI8226 | Multiple myeloma cells | Demonstrated antioxidant action and cytotoxicity | [202] |
| In vitro, ARH-77 | Multiple myeloma cells | Demonstrated cytotoxic effects | [203] |
4.1 Lung cancer
Lung cancer is one of the leading causes of cancer-related morbidity and mortality rates worldwide, with non-small cell lung cancer (NSCLC) accounting for over 85% of all cases [101]. Currently, the primary clinical treatments for NSCLC are surgical resection, chemotherapy, and radiotherapy. For early-stage NSCLC, timely surgery can lead to a curative outcome [102,103]. Although the significance of immunotherapy in treating NSCLC has grown in recent years, the response rate remains below 40% in specific populations [104]. Rutin-loaded liquid crystalline nanoparticles (LCNs) exhibited promising anti-proliferative and anti-migratory properties [105]. An experiment was performed to evaluate the expression of NF-κB and p38 under the intervention of rutin in lung cancer. It was reported that treatment with rutin as well as cisplatin led to an increase in the subpopulation of sub-G1 phase cells and a decrease in the cells in the G2 phase, indicating its ability to regulate the growth of cells. Furthermore, rutin reduced the expression of P38 and NF-κB, suggesting that rutin exerts its anticancer effects through modulation of signaling pathways [106]. The genoprotective effects of β-carotene and different flavonoids (naringin, quercetin, or rutin), both individually and in combination, were explored against NNK-induced DNA damage. Human lung cancer cells were pre-treated with β-carotene, a flavonoid, or a combination of both for 1 h before being exposed to NNK for 4 h. The study found that β-carotene increased NNK-induced DNA strand breaks and 7-mGua levels. In contrast, when cells were treated with naringin, quercetin, or rutin, there was a significant reduction in NNK-induced DNA strand breaks, with quercetin showing the most substantial effect, followed by naringin and rutin [107]. The rutin and cisplatin groups exhibited significantly higher TNF-α expression than the control group. Furthermore, DAPI staining indicated more apoptotic cells in the rutin and cisplatin groups than in the control group. Immunofluorescence analysis revealed that GSK-3β expression was markedly higher in the cisplatin and rutin groups compared to the control group. In conclusion, rutin enhances TNF-α-induced apoptosis of A549 human lung carcinoma cells [72]. When administered orally at a concentration of 200 nmol/kg BW, rutin markedly inhibited lung tumor nodules by 63.59% and extended the life span [108]. A study was made to analyze the effect of rutin on autophagy and inflammation in cancer cell lines. Remarkably, rutin induced autophagy in lung, leukemia, and oral cancer cell lines, which is linked with suppressed TNF-α production [96]. In summary, rutin exhibits significant anticancer potential against lung cancer by inducing apoptosis, inhibiting NF-kB and p38 signaling, reducing DNA damage, suppressing inflammation, promoting autophagy, and enhancing the efficacy of chemotherapeutic drugs.
4.2 Brain cancer
Flavonoids, including rutin, have demonstrated significant anticancer effects in GBM cell models by reducing mitochondrial metabolism and cell viability, inducing apoptosis through damage to the rough endoplasmic reticulum and mitochondria. Flavonoids also caused a delay in and impaired cell migration via downregulation of metalloproteinase (MMP-2) and a reduction in filopodia-like structures on the cell surface. It further enhanced intracellular expression of laminin and intra-and extracellular expression of fibronectin [109]. The use of rutin in cancer management, including brain cancer, has been shown to play a significant role in the modulation of various cell signaling molecules.
Another study demonstrated that rutin reduced the viability of GL15 cells and the expression of miRNA-125b, underscoring the flavonoid’s anti-glioma properties. This highlights its potential as a promising adjunctive therapy for GBM [32]. In CHME cells, rutin-induced cytotoxicity was linked to apoptosis via p53 upregulation, evidenced by nuclear condensation, membrane blebbing, and fragmentation, as evidenced by DAPI staining. The release of cytochrome c further confirmed the apoptotic effect, the activation of caspase 9 and caspase 3, upregulation of BAX, and downregulation of BCL expression [41].
A study explored the synergistic effects of rutin and TMZ in GBM multiforme. The in vitro cell viability assay showed that rutin by itself generally had a low cytotoxic effect, but it did enhance the effectiveness of TMZ in a manner that depended on the dosage. Subcutaneous and orthotopic xenograft studies further indicated that mice receiving combined TMZ/Rutin treatment exhibited significantly lower tumor volumes than those treated with TMZ or rutin alone. Additionally, immunoblotting analysis revealed that TMZ stimulated JNK activity to trigger a protective autophagic response, which rutin inhibited, leading to reduced autophagy and increased apoptosis [97]. Treatment with rutin at 50–100 μM concentrations resulted in decreased proliferation and viability of GL-15 cells. This was accompanied by a reduction in ERK1/2 phosphorylation (P-ERK1/2) levels and an accumulation of cells in the G2 phase of the cell cycle. Additionally, exposure to 100 μM rutin led to apoptosis in 87.4% of GL-15 cells [73].
4.3 Breast cancer
Breast cancer is the most common form of cancer in females, with around 2.3 million new cases diagnosed each year, accounting for 11.7% of all cancer diagnoses [110,111]. Globally, breast cancer represents 25% of cancer cases and 16.7% of cancer-related deaths in most countries [111]. The current mode of treatment for this pathogenesis is effective, but it also causes various side effects. Clinicians need alternative therapies that can effectively cure breast cancer without adverse effects, so it is essential to supplement current chemotherapeutic drugs with drugs based on natural compounds/medicinal plants. In this regard, previous studies have demonstrated the anticancer potential of natural compounds in the protection and management of breast cancer [112]. Rutin in breast cancer prevention is explained through modulation of various cell signaling pathways (Figure 3).
Rutin exerts its anticancer effects through inhibition of inflammation, cell proliferation, angiogenesis, signal transducer and activator of transcription, induction of apoptosis, and activation of tumor suppressor genes. The downward-pointing arrow shows downregulation, whereas the upward arrow represents upregulation.
The use of rutin in cancer management, including breast cancer, has shown a significant role in the modulation of various cell signaling pathways. A recent study evaluated the role of rutin on the proliferation, angiogenesis, and metastasis of breast cancer cell lines. Dose-dependent treatment with rutin upregulated genes, including FN1, MKI67, CDH2, VIM, and VEGFA, while downregulating THBS1 and CDH1 genes [85].
A nanohybrid formulation of rutin was developed by loading it onto 4-carboxyphenylboronic acid (PBA) linked and amine-functionalized MgO nanoparticles (MgO-NH-PBA). This MgO-NH-PBA-rutin nanohybrid showed significant anticancer activity against MDA-MB-231 cells through intracellular ROS-mediated apoptosis and inhibited the migration of cancer cells [113]. The growth of the TNBC MDA-MB-231/GFP orthotopic xenograft in the nude mice model was considerably inhibited by an intraperitoneal injection of rutin at a dose of 30 mg/kg, three times per week. These findings unequivocally identify rutin, a functional dietary flavonoid, as a promising treatment and preventative option for c-Met-dependent breast cancers [114].
Rutin suppresses cell proliferation and increases the expression of miR‐129‐1‐3p in 4T1 cells. miR‐129‐1‐3p overexpression inhibits the proliferation, invasion, migration, and calcium overload of mouse breast cancer cells and increases apoptosis. Knockdown of miR‐129‐1‐3p reversed these effects, suggesting that rutin exerts its anticancer effects against mouse breast cancer cells through regulation of miR‐129‐1‐3p/Ca2+ signaling pathway [115]. Glycosylated flavonoids such as rutin (found in cranberries) and naringin (a constituent of citrus fruits) demonstrated potent anticancer activity, even at the lowest concentration in human breast cancer cells [82].
4.4 Cervical cancer
Cervical cancer is one of the most common gynecological malignancies globally, ranking fourth among female malignancies and even taking the top spot in some developing countries [116]. The 5-year survival rates by histologic type are 75.8% for adenocarcinoma and 81.4% for squamous cell carcinoma, with adenocarcinoma having a worse prognosis than squamous cell carcinoma [117]. Chemotherapy, surgery, and radiotherapy are treatment modalities for cervical cancer. However, these treatments also cause side effects. Therefore, it is essential to explore natural compound-based therapies to mitigate these complications [118].
Furthermore, the FITC-Annexin V/PI double staining method confirmed the induction of apoptosis [119]. The anticancer effect of rutin was explored using key biomarkers, including nuclear condensation and apoptosis, ROS, and changes in MMP. It was reported that rutin treatment reduced cell viability and induced a dose-dependent increase in ROS production and nuclear condensation [67]. Rutin treatment showed decreased cell viability with increased cell accumulation in the G0/G1 cell cycle phase in HeLa cell lines. Moreover, rutin treatment has led to the downregulation of E6 and E7 expression linked with increased p53 and pRB levels [120].
By causing apoptosis, G0/G1 phase arrest, and downregulating the levels of Notch-1 and Hes-1 of the Notch signaling pathway, rutin demonstrated strong anticancer effects in human cervical carcinoma Caski cells [121]. In the G0/G1 phase of the cell cycle, HeLa cell lines treated with rutin showed decreased cell viability and increased cell accumulation. Furthermore, rutin therapy has been linked to an increase in p53 and pRB levels, accompanied by a downregulation of E6 and E7 expression. This has also led to increased expression of Bax and decreased expression of Bcl-2, which releases cytochrome c into the cytoplasm and activates the caspase cascade by cleaving caspase-3, caspase-8, and caspase-9. Thus, by focusing on two important oncoproteins linked to viral progression, rutin is suggested as one of the most effective medication candidates for treating cervical cancer [120]. Rutin was documented to inhibit the growth of Caski cervical cancer cells in a dose-dependent manner. Through the activation of caspase-3/9, the production of ROS, and changes in the expression of Bax/Bcl2 mRNA, rutin caused notable apoptotic effects. Rutin administration reduces the expression of Notch-1 and Hes-1 mRNA. Through inducing apoptosis, causing G0/G1 phase arrest, and downregulating the levels of Notch-1 and Hes-1 in the Notch signaling pathway, these findings collectively demonstrate that rutin has strong anticancer effects in human cervical carcinoma Caski cells [67]. An innovative phytotherapy for cervical cancer based on the rutin–fucoidan complex was studied in order to increase bioavailability and induce cancer cell death. A novel flavonoid-polysaccharide combination called rutin–fucoidan (Ru–Fu) was created. In HeLa cells, the Ru–Fu combination effectively contributes to anti-proliferation, S-phase cell cycle arrest, and the induction of cell death associated with apoptosis [122].
4.5 Ovarian cancer
Rutin-loaded PCL-PEG was made, and its anti-cancer effects against human ovarian cancer were tested. Cytotoxicity testing demonstrated that RUT-loaded PCL-PEG improved cytotoxicity in a dose- and time-dependent way. In treated MDA-MB-231 cells with RUT-loaded PCL-PEG, a significant upregulation of Bax genes and caspase-8, caspase-9, and caspase-3 was observed compared to cells treated with free rutin [123]. The results of another study revealed that all the tested flavonoids exhibited different levels of inhibition in VEGF expression. The inhibitory strength of VEGF protein secretion was found to be in the following order: genistein > kaempferol > apigenin > quercetin > tocopherol > luteolin > cisplatin > rutin > naringin > taxifolin [124]. Ru-NLC has a significant effect on SK-OV-3 cell metabolism, which in turn increases the levels of apoptosis-related proteins. The cell’s ability to proliferate is reduced as a result of these changes, and intrinsic apoptosis finally causes it to die. The Western blotting experiment’s findings indicate that Ru-NLC induces cancer cells to undergo increased apoptosis [125]. Another study showed that rutin could directly stimulate ovarian cell steroidogenesis while also inhibiting the cells’ survival, proliferation, and apoptosis, as well as their response to FSH’s stimulatory activity. It is essential to consider the potential adverse effects of rutin on ovarian cells when consuming foods and using medications that contain rutin and its metabolites [126]. Rutin’s protective effect against acrylamide-induced ovarian inflammation, oxidative stress, DNA damage, and hormonal changes was assessed. The findings demonstrated that RU provided significant protection against oxidative stress, DNA damage, and inflammation induced by AA, likely due to its antioxidant properties [127].
4.6 Prostate cancer
Prostate cancer stands as the second most prevalent cancer in men and bears the fourth highest mortality rate among men worldwide. The anticipated surge in prostate cancer cases from 1.41 million in 2020 to 2.43 million in 2040 underscores its substantial socioeconomic burden [128]. The current mode of treatment for prostate cancer is expensive and also shows adverse effects. In this regard, natural products have proven their role in prostate cancer management. Evidence from the literature suggests that various natural products can selectively target multiple molecules and signaling pathways associated with the development and advancement of tumors [129,130,131,132,133]. The use of rutin in cancer management, including prostate cancer, has been shown to play a significant role in modulating various cell signaling molecules. The study examined the combined effects of rutin with the widely used anticancer drug, 5-fluorouracil (5-FU), on the prostate cancer cell line (PC3). When the cells were treated separately with rutin and 5-FU, the 50% inhibitory concentrations (IC50) were reported as 900 μM and 3 mM, respectively. However, the combination index of the combined 5-FU/rutin application indicated synergistic effects [42].
An extensive in-silico investigation into rutin compounds as potential inhibitors of prostate cancer signaling pathways revealed that the AKT, EGFR, and ERK signaling pathways were targeted by rutin derivatives, which could prevent prostate cancer from spreading [134]. WST-1, Annexin V ELISA, DAPI, and Acridine Orange staining were used to assess the anticancer efficacy of rutin in prostate cancer cells (PC-3), and the Scratch Assay test was used to assess rutin’s anticancer and anti-metastatic qualities. It has been established that rutin can both promote apoptosis and prevent the epithelial–mesenchymal transition [135]. In rats, rutin naturally protected the prostate cells from damage caused by zinc nanoparticles [136]. Rutin can increase the effectiveness of chemotherapy and is a potent senomorphic drug that targets senescent cells. The malignant phenotypes of prostate cancer cells, such as in vitro proliferation, migration, invasion, and most importantly chemoresistance, were improved by conditioned media generated by senescent stromal cells; however, rutin markedly inhibited these gain-of-function effects. Combining a chemotherapeutic medication with rutin significantly improved the treatment success, even when traditional chemotherapy decreased tumor development [137].
4.7 Colon cancer
Colorectal cancer remains the primary cause of mortality, with the rise in its occurrence in Western countries possibly linked to lifestyle changes. Specifically, alterations in dietary patterns could account for this trend [138,139]. About one-third of metastatic colorectal cancer (mCRC) patients have localized liver metastases and may be eligible for potentially curative surgery following systemic therapy [140]. Regrettably, despite the progress in existing treatments, the 5-year survival rate for metastatic CRC patients remains low, at less than 15% [141]. Consequently, there is a clear need for new strategies and agents to enhance the prevention and treatment of CRC. Rutin, as well as Silibinin, exhibited anticancer potential through the induction of apoptosis and the regulation of gene expressions associated with apoptosis, inflammation, and mitogen-activated protein kinase (MAPK) pathway proteins more efficiently in combination than separately [142]. Treating colon cancer cells HT-29 with hyperoside and rutin decreased cell viability dose dependently. The simultaneous activation of the mitochondria-dependent apoptotic pathway by hyperoside and rutin occurred via modulation of B-cell lymphoma 2 (Bcl-2) expression and Bcl-2-associated X protein (Bax), resulting in the activation of cleaved caspases-3, -8, and -9, as well as cleaved poly-(ADP-ribose) polymerase [143].
The cytotoxicity study revealed that exposing colon cancer HT-29 cells to varying concentrations of rutin resulted in a dose- and time-dependent decrease in cell proliferation. Furthermore, after irradiation, it was noted that the combined impact of 4 Gy radiation and rutin at a concentration of 80 µM resulted in reduced cell viability compared to cells treated with rutin alone and those exposed to 4 Gy radiation alone [144]. Diets containing quercetin, curcumin, silymarin, ginseng, and rutin decreased the number of aberrant crypt foci compared to the control group. Analysis of the colon mucosa revealed that all the herbal supplements, except silymarin, stimulated apoptosis, with quercetin demonstrating the most potent effect [145]. Rutin exhibited the most significant cytotoxic effects against SW480 cells compared to other cancer cell lines. It also reduced the tumor volume, and it increased the mean survival time by 50 days, while lowering VEGF serum levels by 55% compared to untreated mice [83]. By triggering apoptosis through cell cycle arrest and caspase protein activation, rutin prevents colon cancer cells from proliferating. Furthermore, rutin administration markedly increased the expression of β-actin and caspase-3 in HCT116 [146]. To ascertain rutin’s effectiveness in treating A549 lung cancer cells and colon cancer cells, the cytotoxic effect of rutin in the preemulsion was examined using in vitro tests. The findings showed that rutin in the prenanoemulsion effectively inhibited colon and lung cancer cells [147]. The growth of Caco-2 cells is inhibited more effectively by rutin and anticancer drugs (5-FU and/or oxaliplatin) when taken together than when taken separately. Lower dosages of 5-FU and oxaliplatin, which have comparable efficacy, may also be helpful in minimizing any potential side effects of these drugs [148]. The in vitro cytotoxicity of rutin, rutin-PVP K30 SD, frankincense, and a combination of rutin-PVP K30 SD and frankincense was evaluated against human colon cancer (HCT-116) cell lines. The combination of frankincense and rutin-PVP K30 SD was more successful than either frankincense or rutin alone at stopping the growth of cancer cells [149]. CF1 female mice were given dietary quercetin and rutin for 50 weeks in order to evaluate their capacity to prevent colonic neoplasia caused by azoxymethanol (AOM). Quercetin and rutin show notable efficacy in lowering the incidence of FAD and the hyperproliferation of colonic epithelial cells brought on by AOM [150].
4.8 Liver cancer
The world’s sixth most frequently diagnosed cancer is primary liver cancer, with HCC being the most common histological type [111,151]. Chromosomal alterations, genetic mutations, hormonal changes, and changes in signaling pathways are the chief players in this pathogenesis [152,153]. Effective therapeutic options are available for patients with HCC, significantly improving survival rates. Nevertheless, a considerable portion of patients do not have a positive response to locoregional or systemic treatment and ultimately succumb to their illness [154,155]. The use of rutin in cancer management, including liver cancer, has been shown to play a significant role in modulating various cell signaling molecules.
Zhou et al. experimented to explore the molecular mechanism of rutin in SO-induced autophagy and chemoresistance in HCC. Results indicated that rutin treatment reduces autophagy and BANCR expression in SO-resistant cells. Transmission electron microscopy revealed a significantly reduced number of autophagosomes in rutin-treated HepG2/SO and HCCLM3/SO cells [95]. A study was conducted to examine the effects of rutin on liver carcinoma. Results reported a substantial increase in relative liver weight and tumor marker enzymes; liver marker enzymes and abnormalities were noticed in membrane-bound enzymes as well as electrolytes, while such alterations were meaningfully restored in the rutin-treated Group as compared to the treated Group [156].
A study evaluated the potential of chitosan/poly (acrylic acid) nanogel (CAN) in enhancing the anticancer effects of rutin. The formulation was tested for its ability to combat liver cancer in rats. The results showed that levels of liver function enzymes and total bilirubin were significantly higher in the Group with liver tumors induced by DENA/carbon tetrachloride (CCl4) as compared to the control group. In the HCC Group, VEGF levels were higher than in the control Group. However, rutin administration notably decreased VEGF levels in both the free and loaded Groups compared to the HCC group. The serum AFP level also increased in the HCC group compared to the control Group, indicating tumor cell proliferation and HCC progression in the DENA/CCl4-treated rats. However, in the rutin Group, histopathological examination of the liver sections revealed improved hepatocellular architecture with minor hepatic changes such as hepatocyte swelling and vacuolations [110]. The research aimed to create poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) containing rutin (RT) for treating HCC. RT-PLGA-NPs demonstrated considerable enhancements in hematologic, renal biochemical, and hepatic parameters when evaluated preclinically for anticancer effects through oral administration. Particularly notable improvements were observed in the regulation of inflammatory markers, oxidative stress, cytokines, and antioxidant enzymes associated with liver tissue damage [108]. The role of rutin isolated from the Morinda citrifolia plant against hepatic carcinoma (HepG2) cell lines was explored. It was reported that cell viability was decreased with an increase in the concentration of rutin [157]. Another study sought to determine if quercetin and rutin could have chemoprotective effects on the growth, viability, and antioxidant defense system response of a human hepatoma cell line (HepG2). The findings show that both natural antioxidants cause beneficial alterations in the cultured HepG2’s antioxidant defense system, which stop or postpone circumstances that encourage cellular oxidative stress [158].
4.9 Gastric cancer
Gastric cancer (GC) ranks among the top causes of cancer-related mortality, and its incidence exhibits significant geographic variability [159]. Gastric cancer remains an important global health concern with a dismal prognosis. A considerable number of patients are diagnosed with advanced-stage gastric cancer, leading to minimal life spans due to delayed diagnosis [160]. Patients with metastatic disease typically have a survival of just over 1 year, even with the use of targeted therapy [161,162]. Rutin has been found to inhibit the growth of human gastric adenocarcinoma SGC-7901 cells, leading to cell cycle arrest at the G0/G1 phase, a shift in the Bcl-2/Bax ratio, and an increase in the expression of caspase-3, caspase-7, and caspase-9. When rutin is administered in combination with oxaliplatin, it demonstrates a synergistic anticancer impact, enabling a reduction in the oxaliplatin dosage and subsequently reducing its toxicity [163].
In 2024, Huang et al. reported that rutin significantly inhibited the proliferation, migration, and invasion abilities of gastric cancer cells, inducing apoptosis. Further experiments proved that rutin achieved this effect by suppressing the activation of the Wnt/β-catenin pathway, thereby inhibiting the biological behavior of these cancer cells [164]. Whey protein isolate WPI and rutin together dramatically increased in vitro cell migration in L929 monolayers, suggesting that they can heal wounds and treat stomach ulcers [165]. Furthermore, the apoptotic processes of rutin were linked to caspase-mediated signaling; however, the combination of rutin with the chemotherapeutic drug Oxaliplatin increased the death of gastric cancer cells SGC-7901, as evidenced by a lowered BCL-2/Bax ratio [163]. The protective effect of rutin against acute gastric mucosal lesions caused by ischemia-reperfusion (I/R) was investigated in a study. Rutin at doses of 50, 100, and 200 mg/kg significantly inhibited the rise in the stomach mucosal injury index induced by gastric I/R compared to the I/R group [166].
Rutin significantly increased the rates of apoptosis in AGS and MGC803 cells. This suggests that Rutin may partially mediate its antiproliferative effect on gastric cancer cells by inducing apoptosis [164].
3: Rutin exerts its anticancer effects through inhibition of inflammation, cell proliferation, angiogenesis, signal transducer and activator of transcription, induction of apoptosis, and activation of tumor suppressor genes. The downward-pointing arrow shows downregulation, whereas the upward arrow represents upregulation.
4.10 Leukemia
Leukemia is a malignant disorder characterized by the uncontrolled clonal proliferation of leukocytes in the bone marrow and peripheral blood [167,168]. In 2020, the World Health Organization (WHO) reported 474,519 new cases of leukemia worldwide, representing 2.5% of all cancer cases, and 311,594 new leukemia deaths, accounting for 3.1% of all malignancy deaths [111]. The current mode of treatment is causing several side effects. A recent study reported that the natural compound plays a role in anti-leukemic activity [169]. However, a safe and non-toxic treatment with no side effects is required to control this disease and overcome the adverse effects of chemotherapeutic drugs.
The flavonoidic fraction of Hammada scoparia, as well as its compound rutin, has been shown to induce apoptosis in adherent leukemic cells and eliminate CAM-DR. Additionally, rutin has demonstrated an inhibitory effect on the survival of adherent leukemic progenitors (CD34+ 38−123+). The pro-apoptotic actions of rutin have been associated with a reduction in active GSK3β [170]. The effects of rutin on WEHI-3 in BALB/c mice in vivo were also studied, and the outcomes indicated that rutin decreased the percentage of Mac-3 markers. The weights of the spleen and liver were reduced in rutin-treated groups, and the results showed that rutin reduced the weight of these organs [171]. After receiving a dose of 120 mg/kg of rutin, the tumors in mice were smaller and lighter than those in the control group. The tumors in the mice treated with 120 mg/kg of rutin were significantly smaller than those in the untreated control group [172]. Additionally, rutin, in combination with vitamin E, was found to attenuate VEGF expression in HL-60 cells [80]. In a combination study, the antiproliferative action of AraC was synergistically improved by isorhamnetin, kaempferol, and myricetin when combined with AraC. On the other hand, rutin and AraC were antagonistic (CIs > 1). According to the cell cycle study, the synergism of flavonoids (10 µM) with AraC (0.5 µM) results from notable alterations in the L1210 cells’ cell cycle profile. Cells in the G2/M stages were blocked when treated with a flavonoid and AraC combination [173]. A work demonstrated ZnO-Rutin NPs’ effective antitumor potential against CML cells. The proportion of early apoptosis rose marginally after exposure to ZnO-Rutin NPs, while the percentage of late apoptosis increased significantly. The downregulation of the Bcl-2 gene highlighted the mechanism by which ZnO-Rutin NPs promote apoptosis, resulting in a 0.33-fold reduction and a 1.98-fold elevation of the Bax mRNA level [174].
4.11 Bone cancer
Osteosarcoma, a type of bone cancer, primarily affects children and teenagers aged 10 to 14, accounting for 3–5% of childhood cancers [175,176]. Osteosarcoma has an incidence of approximately 3.5 cases per million worldwide [177]. A combination of surgery and adjuvant chemotherapy is currently the standard therapeutic approach for patients with osteosarcoma [178]. Soosai et al. experimented to explore the seeds of buckwheat (Fagopyrum sp.) for the isolation of rutin and its anticancer properties against Osteosarcoma. The study findings reported that SAOS2 cells exposed to standard rutin at 10 g/mL and rutin fraction at 20 g/mL demonstrated substantial cell shrinkage, decreased cell density, and morphological alterations, which indicate apoptotic cells. Rutin-fraction significantly increased the expression of pro-apoptotic protein Bad, resulting in the negative regulation of Bcl-2’s expression [179]. ALP activity, Runx2 protein expression, and osteoblast MC3T3-E1 development were all shown to be impacted by rutin. Alizarin red staining results demonstrated that calcified nodules developed in all groups and that the area of calcified nodules in rutin-treated groups was larger than that in the control group; the larger the area, the higher the concentration. Osteoblastic differentiation can be aided by rutin, and its effectiveness increases with concentration [180]. In rats given rutin, the protein FNDC1, which contains the fibronectin type III domain, may be a novel factor related to bone metabolism that is reduced. Through the Akt/mTOR signaling pathway, rutin may control autophagy and FNCD1 levels [181]. In a study, rutin was added to human osteosarcoma MG63 and U2OS cells at 10, 20, and 40 μmol/L. Rutin treatment at 20 and 40 μmol/L significantly reduced the rate of proliferation, increased the rate of apoptosis, decreased the ability to migrate and invade, significantly downregulated the Ki67 protein, and significantly increased the Bax/Bcl-2 ratio in MG63 and U2OS cells. All of these changes were statistically significant. Additionally, rutin decreased the expression of VEGF and Ki67 in tumor tissues, increased cell death, and dramatically suppressed the development of osteosarcoma cells in vivo [182].
4.12 Melanoma
A critical study evaluates the cytotoxic effects of rutin against two different human melanoma cell lines. The outcomes show a dose-dependent reduction in the viability of both cell lines, accompanied by a visible decrease in the cell confluency and apoptotic characteristics within the cell nuclei [183]. The antimetastatic potential of the flavonoids, including rutin, was examined, and the rutin group exhibited decreases in the growth index compared with the ethanol group [184]. In an animal model, rutin has antiangiogenic effects against melanoma. Additionally, it has been found to downregulate the expression of VEGF and IL-1β. These findings suggest that rutin’s antiangiogenic activity may be due to its modulation of these cytokines as well as growth factors [185]. Rutin showed antioxidant activity, and complexation enhances this property. Further incubation with rutin (100 µM) demonstrated that it is an active pro-apoptotic and antiproliferative compound against B164A5 cells [186]. In mouse B16F1 melanoma, the leaf extract of Mallotus japonicus and its main active ingredient, rutin, inhibited the production of melanin [187]. To assess the fisetin and rutin, and their synergistic treatment, A431 and A375 cells were utilized. Following a 24-h treatment period, cell morphology was examined, and cell viability was evaluated using the MTT test. According to the findings, the combined effect of these agents is more effective than their separate treatment, resulting in a dose-dependent reduction in cell viability and an enhancement of cell dimorphologies as concentration increases [188]. The preparation and assessment of rutin-loaded solid-lipid nanoparticles (Ru-SLNs) gel for the treatment of melanoma cells was the goal of a study. A gel based on Ru-SLNs showed encouraging promise and successfully targeted melanoma cells to the skin’s epidermal layer [189].
4.13 Renal cancer
The potential anticancer effects of rutin against human renal cancer cell lines were evaluated. While considering its safety in Vero kidney cells, the possible anticancer effects of rutin on a human renal cancer cell line (786-O) were evaluated, along with the suitability of ionic liquids (ILs) for enhancing drug transport. In the cancer cells, a concentration-dependent reduction of cell viability was noticed. The outcomes presented that rutin induced a notable decrease in cell viability compared to non-treated control cells. Additionally, rutin (50 µM) induced a substantial increase of approximately 30% in the sub-G1 population, a decrease in the G0/G1 population, and an increase in the S population cells. At 50 µM, however, a notable reduction in cell viability was already seen in 786-O cells. Furthermore, rutin exposure led to a noteworthy rise in the sub-G1 population of 786-O cells, supporting the biomolecule’s potential anticancer properties [190].
Additionally, rutin may decrease the expression of TGF-β1, fibronectin, and collagen IV in kidney tissues. Through Western blot analysis, it was discovered that rutin could lower the expression of TGF-β1 and p-smad2 in rat kidney tissues. In 5/6-nephrectomized rats, rutin reduces renal fibrosis and proteinuria by preventing oxidative damage and blocking TGFβ1-smad signaling activation [191]. In Wistar rats, rutin inhibits cisplatin-induced kidney inflammation and death by reducing the expression of NF-κB, TNF-α, and caspase-3 [192]. In rats with obstructive nephropathy, rutin reduces kidney interstitial fibrosis. In obstructive nephropathy, rutin’s renoprotective benefits are likely attributed to its anti-inflammatory properties and suppression of TGF-β1/Smad3 signaling [193].
4.14 Pancreatic cancer
With over 300,000 deaths annually, pancreatic cancer ranks as the seventh most common cause of cancer-related deaths worldwide. Pancreatic ductal adenocarcinoma, an infiltrating neoplasm with glandular differentiation originating from the pancreatic ductal tree, is the most prevalent tumor form among pancreatic malignancies [194] and progresses without precise symptoms, making early diagnosis challenging. The current treatment approach for this type of malignancy involves surgery, radiotherapy, adjuvant therapy, and chemotherapy. While certain chemotherapeutic drugs and immunotherapy have been used for these patients, their effectiveness is somewhat restricted [195,196,197]. Here is a rising interest in utilizing natural compounds for chemotherapy to improve the therapeutic efficacy of specific anti-cancer medications [198].
A study on pancreatic cancer was conducted to evaluate the potent anti-proliferative, pro-apoptotic, and anti-migration potential of rutin. The results revealed that rutin effectively slowed down the growth of PANC-1 cells in a dose-dependent manner, with 5 μg/mL of rutin having a significant inhibitory effect on cell growth. Additionally, using a wound-healing assay, we observed that the migration of PANC-1 cells was markedly reduced after the administration of rutin and a decrease in the expression of the migration-related protein MMP-9. After the rutin treatment, an increase in cell apoptosis was observed compared to the untreated cells [66]. By preventing acinar to ductal metaplasia, rutin shields the pancreas from inflammatory damage and oncogene-driven carcinogenesis. It is thought that one possible way that rutin inhibits Kras-driven carcinogenesis is through AMPK activation. By blocking ADM, rutin prevented oncogenic Kras-driven carcinogenesis and AP-induced pancreatic damage [199].
In cell lines under investigation, rutin increases the quantity of caspases 3/7 and has an anti-proliferative effect. In conclusion, the normal cell lines HPDE, BxPC-3, Huh-7, MiaPaCa-2, Suit-2, and HepG-2 cells displayed the highest cellular growth inhibition. Additionally, rutin showed dose-dependent suppression of glutathione-S-transferase activity and the cytochrome P450 enzyme (CYP450 3A4). Moreover, rutin’s anti-inflammatory properties were reinforced by the inhibition of PGE2 synthesis in BxPC-3 cells (high COX-2 expression) [200].
4.15 Multiple myeloma
Multiple myeloma (MM) comprises nearly 10% of all blood cancers and exhibits significant variations in treatment response [201]. Natural products have been reported to play a role in the management of MM [201]. Rutin-zinc(II) has demonstrated antioxidant activity, as evidenced by its cytotoxic effects against leukemia cell lines, such as multiple myeloma (RPMI8226), in vitro [202]. A research study was conducted to examine the cytotoxic impact of rutin on ARH-77 cells. Following a 24-h incubation period, rutin demonstrated cytotoxic effects, particularly at concentrations of 50, 100, and 200 µM [203].
5 Approaches to improve the bioavailability and efficacies of rutin
It has been discovered that rutin can control cancer by modifying different cell signaling pathways. Furthermore, its function in both in vitro and in vivo cancer has been reported. Rutin’s therapeutic potential may be limited by its rapid elimination, low absorption, poor solubility, metabolism, and unstable nature, despite its numerous health benefits, particularly in cancer prevention. These factors restrict its ability to reach valuable quantities in tumor tissues. When these circumstances come together, they can greatly reduce the compound’s bioavailability, preventing the body from using it to its full potential for promoting health [204,205].
Rutin’s solubility, bioavailability, and activity are increased by various nanotechnology-based methods (Figure 4 and Table 3). Tablets containing rutin nanocrystals that had been lyophilized were assembled, and the tablets’ dissolving characteristics were investigated. The tablet loaded with rutin nanocrystals had a faster disintegration rate than the tablet that was loaded with rutin nanocrystals. Rutin was liberated and dissolved in water from the nanocrystal tablets after 30 min. However, only 71 and 55%, respectively, of the total amount of rutin was dissolved from the marketed tablet and the microcrystal tablets. The body’s bioavailability of the insoluble rutin is anticipated to increase due to the tablet’s increased solubility, which contains rutin nanocrystals [206].

Various approaches based on nanotechnology are used to increase activities. This figure illustrates various nanotechnology-driven strategies, such as nanohybrids, chitosan nanoparticles, nanoemulsions, and fucoidan complexes, that enhance their anticancer efficacy by promoting apoptosis, increasing ROS production, inducing cell cycle arrest, and improving cellular targeting. These advanced delivery systems significantly contribute to the inhibition of cancer pathogenesis across multiple tumor types. The upward arrow represents upregulation.
Nanoformulation and its effects on cancer through in vivo and in vitro analysis
| Nanoformulation | Cancer types | Study types | Findings of the study | Refs. |
|---|---|---|---|---|
| MgO-NH-PBA-Rutin nanohybrids | Breast | In vitro (MDA-MB-231) | Induced apoptosis and migration in cancer cells via generating intracellular ROS and demonstrated the most anticancer effects | [113] |
| Chitosan nanoparticles (CNPs) loaded with rutin | Liver | In vitro (Hep3B cells) | Showed significant lethality to cancer cells and excellent hemocompatibility | [209] |
| Rutin-loaded PLGA NPs | Liver | In vivo (albino male rats) | Prevented the formation of nodules in the hepatic tissue, reduced total bilirubin, increased albumin and total protein, and displayed a lower frequency of hepatic nodules, decreased NF-κB level | [210] |
| Rutin-chitosan nanoconjugates | Breast | In vitro (MDA-MB-231) | Enhanced activity against cancer cells, cell cycle arrest at DNA synthesis phase | [211] |
| Chitosan/poly (acrylic acid) nanogel) | Liver | In vivo (Male albino rat) | Improved anti-proliferative, apoptotic, and anti-angiogenic effects | [212] |
| Chitosan/copper oxide (CS–CuO) nanocomposite using rutin | Lung | In vitro (A549 cells) | Nanocomposite exhibited concentration-dependent anti-proliferative potential, induced apoptosis | [213] |
| Encapsulated FA conjugated keratin NPs | Breast | In vitro (MCF-7 cells) | Increased ROS levels, cell death | [214] |
| Rutin–fucoidan (Ru–Fu) complex | Prostate | In vitro (PC3cells) | Disrupted cell cycle regulation, induced cellular apoptosis | [133] |
| Rutin nanoemulsion | Prostate | In vitro (PC3 cells) | Showed significant efficiency against cancer cells | [215] |
An earlier investigation was conducted to design and assess a solid self-emulsifying drug delivery system (SSEDDS) as a potential rutin carrier. Liquid SEDDS was created following the screening of several carriers (oils, surfactants, and co-surfactants) and selecting those with superior drug solubilizing power. Neusilin® US2: S6 (1:2) in the SSEDDS (SS4) produced the best drug release characteristics. In summary, the optimal liquisolid dosage form of rutin demonstrated good flowability and rapid drug release properties, making it a potential candidate for oral administration systems [207]. In order to create rutin-containing nanomedicines, Almeida and colleagues produced rutin-loaded nanocapsules and nanoemulsions as aqueous intermediates or final systems. Improved rutin photostability and extended in vitro antioxidant activity were demonstrated by both formulations [208].
Different types of nanoformulation are used to evaluate their effects on cancer (Table 3). To create MgO-NH-PBA-Rutinnanohybrids, new PBA-tagged MgO nanoparticles were engineered to target human breast cancer cells. Rutin was then loaded into the nanoparticles. In addition to inhibiting MDA-MB-231 cell migration and death, the MgO-NH-PBA-Rutin nanohybrid exhibited significant anticancer potential against MDA-MB-231 cells by inducing intracellular ROS production. Furthermore, in vivo investigations demonstrated the nanohybrid’s more substantial anticancer effects in mice with tumors. However, when compared to both MgO-NH-PBA and free rutin, this formulation had the most anticancer effects in both circumstances [113].
Rutin-loaded chitosan nanoparticles (rCNPs) were synthesized and confirmed to have a cytotoxic effect on human hepatoma Hep3B cells. It was observed that the particles exhibited significant hemocompatibility and were highly cytotoxic to Hep3B cells compared to normal cells. Additionally, variations in the potential of the mitochondrial membrane (MMP) and elevated generation of ROS were observed [209]. After creation, the Rutin-loaded PLGA NPs (RT-PLGA-NPs) underwent an activity assessment. With RT-PLGA-NP treatment, it was observed that this formulation demonstrated a significant downregulation of proinflammatory cytokines and a decreased incidence of hepatic nodules, inflammatory cell infiltration, necrosis development, cell swelling, and blood vessel inflammation [210].
The rutin–chitosan nano-conjugates were made, and these exhibit remarkable anti-breast cancer cell activity. Furthermore, it was observed that after apoptotic cell death, cell cycle arrest occurs at the DNA synthesis phase. The results of this study confirm that designed nano-conjugates could effectively trigger the apoptotic process in triple-negative breast cancer cells [211]. Activity was assessed after producing chitosan/poly (acrylic acid) nanogel. The developed formulation exhibited significantly improved anti-proliferative, apoptotic, and anti-angiogenic effects compared to free rutin. This suggests that the formulation may offer a novel therapeutic approach as a promising agent for treating hepatocellular cancer [212].
Another study used rutin to generate an eco-friendly, bioinspired chitosan/copper oxide (CS–CuO) nanocomposite. The produced CS–CuO nanocomposite exhibited concentration-dependent anti-proliferative activity against A549 cancer cells, as indicated by the results. Furthermore, formulation causes treated cancer cells to undergo apoptosis [213]. After rutin-encapsulated FA-conjugated keratin NPs (FA@Ker NPs) were created, 50% of MCF-7 cancer cells were killed in the formulation. Additionally, the rutin absorption in MCF-7 cells was enhanced by this formulation, and it was noted that the apoptotic potential of deformed membrane bodies and condensed nuclei was present. Additionally, formulation infiltrated the MCF-7 cells’ mitochondria and may have raised the concentration of ROS, which resulted in cell death [214].
The compound known as rutin-fucoidan, or Ru–Fu, is prepared and assessed. The outcome demonstrated that the complex’s formulation may disrupt the control of the cell cycle and cause cellular death by causing nuclear fragmentation, loss of mitochondrial potential, and the production of ROS. Additionally, the complex demonstrates biocompatibility with normal cells [113,122]. In PC3 cancer cells, the IC50 value of the improved nanoemulsion formulation was 11.8 μM. Significant ROS induction was shown by fluorescent microscopic examination and intracellular ROS formation, which may start the apoptotic process [215].
6 Synergistic effect of rutin with other compounds in cancer management
Rutin has been widely investigated for its potential to enhance the efficacy of conventional anticancer agents through synergistic interactions with chemicals and anticancer medications (Figure 5 and Table 4). Combination therapy involving rutin aims to reduce drug resistance, minimizes toxicity, and boosts therapeutic efficacy. For example, in the colon cancer model, the combination of silibinin and rutin was shown to induce apoptosis more effectively by modulating the expression of genes associated with inflammation, apoptosis, and MAPK pathway proteins [142]. In prostate cancer cells (PC3 cells), rutin with 5-fluorouracil upregulated p53 gene expression and promoted apoptosis. Further, this combination decreased the formation of PC3 cell colonies and suppressed Bcl-2 signaling protein [42].

Synergistic effects of rutin with compounds and cancer drugs. Synergistic effects of rutin with various anticancer agents enhance therapeutic outcomes by induction of apoptosis, activation of tumor suppressor genes, cell cycle arrest, inhibition of inflammation, and angiogenesis. These combinations demonstrate rutin’s potential as an adjunct compound in cancer treatment.
Synergistic effect of rutin with other compounds in cancer
| Cancer types | Other compounds | Study types | Cell lines | Outcome | Refs. |
|---|---|---|---|---|---|
| Colon cancer | Silibinin | In vitro | HT-29 | The apoptosis induction and regulation of the expressions of genes associated with inflammation are more efficient in combination than individually | [142] |
| Prostate cancer | 5-fluorouracil | In vitro | PC3 | Combination of 5-fluorouracil/rutin increased p53 gene expression and apoptosis | [42] |
| Breast cancer | Tamoxifen | In vitro | MCF-7 | The rutin synergistically enhanced tamoxifen’s anti-proliferative potential | [43] |
| Leukemia | Vitamin E | In vitro | HL-60 | The rutin plus vitamin E synergistically decreases IRS-1 protein expression | [80] |
| When vitamin E is combined with rutin, VEGF expression is decreased | |||||
| Gastric cancer | Oxaliplatin | In vitro | SGC-7901 | Apoptosis was significant after combination treatment | [163] |
| The greatest notable alteration was produced by the combination therapy |
Similarly, rutin combined with tamoxifen exhibited antiproliferative effects in breast cancer MCF-7 cells. Meanwhile, rutin protects non-cancerous breast MCF-10A cells against tamoxifen toxicity [43]. In human promyelocytic leukemia (HL-60) cells, rutin and vitamin E jointly reduced the expression of the IRS-1 protein, implicating redox-independent mechanisms in their anticancer activity [80].
Another study in gastric cancer SGC-7901 cells demonstrated that cotreatment with rutin and oxaliplatin promoted apoptosis in the cells. Additionally, the cell cycle progression of gastric cancer SGC-7901 cells was evaluated after this combination treatment. The cell cycle arrest at the G0/G1 phase was increased compared to the control cells to variable degrees [116,163].
Despite these promising findings, it has been documented that not all interactions between rutin and chemotherapeutic drugs are beneficial. For example, rutin exhibited antagonism with cytarabine in human leukemia cells, in contrast to other flavonoids like isorhamnetin and kaempferol, which showed synergy. This antagonism was associated with a lack of G2/M phase arrest and may result from rutin’s redox-modulating effects, highlighting the importance of carefully selecting flavonoid combinations based on their molecular interactions [173].
Therefore, while rutin shows considerable promise in enhancing the therapeutic index of anticancer drugs, its use in combination regimens should be guided by careful pharmacodynamic and mechanistic studies. Evaluating both synergistic and antagonistic interactions is essential to ensure efficacy and avoid unintended compromise in treatment outcomes.
7 Pharmacokinetics, safety profile, and limitations of rutin in cancer
Rutin is absorbed in the intestine and distributed as quercetin. 35± Because dietary rutin is metabolized by the microbes in the gastrointestinal tract and transformed into other chemicals that can be absorbed, it is rarely or never absorbed in its original form. Rutin is broken down enzymatically by the microbes in the lower gastrointestinal system into its aglycone form, quercetin, and the sugar residue. The epithelial cells that line the small intestine subsequently absorb these resultant metabolites. Rats and rabbits given rutin orally have metabolic changes that produce three rutin metabolites: 3,4-dihydroxyphenylacetic acid, 3-methoxy-4-hydroxyphenylacetic acid, and 3-hydroxyphenylacetic acid. Urine excretion is the next step in the body’s removal of these metabolites [216]. Rats given 328 μmol/kg orally showed quercetin sulfates and glucuronides as the primary metabolites, although rutin was eliminated from the bloodstream [204]. Additional rutin metabolites were also documented, including quercetin, m-hydroxyphenyl-acetic acid, 3,4-dihydroxyphenyl-acetic acid, homovanillic acid, and 3,4-dihydroxytoluene. Roughly 10% of rutin is excreted in urine, with the remaining 10% ending up in feces [217].
In order to verify a sample’s safety during the drug discovery process, toxicity assessment is a crucial prerequisite. Rutin can be deemed safe for additional clinical usage based on the results of previous investigations [218]. Based on earlier research, rutin can be considered safe for ongoing therapeutic use. Rats, guinea pigs, and rabbits were used to test the acute and long-term toxicity of rutin; none of the test animals showed any negative symptoms. After 400 days of this kind of diet, histological examination of the tissues showed no evidence of damage that could be directly attributed to the administration of rutin, and even 1% of rutin in the diet did not slow down the growth rate of albino rats. The organ weights of the test animals fell within typical bounds. S. Rats given a 1% rutin diet reproduced well, produced offspring that looked healthy, and had an estrous cycle that was the same length as control animals. By the standards employed, rutin poses no immediate or long-term risks. Furthermore, up to 50% of the 75 mg of rutin that had been consumed was due to microbial metabolites discovered in human urine [217].
Intestinal enzymes are unable to effectively break down rutin after oral ingestion. However, the gut microbiota can break down rutin through the enzymes β-rutinosidase and α-rhamnosidase. These enzymes convert therutin to aglycones, or genins, which the intestinal epithelial cells can absorb to a certain degree. The gut microbiota greatly enhances rutin metabolism, but intestinal epithelial layers still only absorb a small amount of it. Because of its hydrophobic molecular structure, rutin’s use as a bioactive molecule in biomedical applications is limited. Reduced absorption and, as a result, lower bioavailability of this substance are caused by insufficient solubility in aqueous systems. Other drawbacks of rutin include its short half-life, limited thermal stability, and quick metabolism, all of which can lessen its pharmacological benefits [219].
8 Conclusion and future direction
The primary treatment modalities for cancer, including surgery and chemotherapy, often lead to unwanted side effects and drug resistance. Therefore, it is essential to explore safe and effective cancer treatment drugs. Previous investigations support the effectiveness of rutin in preventing and managing various types of cancers. However, rutin faces challenges in clinical development due to its low solubility and limited oral bioavailability. Nano-formulations of rutin have been shown to improve bioavailability and increase efficacy in various cancer cell lines.
Currently, there is no large-scale clinical trial dealing with rutin as a standalone anticancer agent in humans. Most of the evidence for rutin’s anticancer potential comes from in vitro studies and animal models, where it has been shown to induce apoptosis, anti-angiogenesis potential, and anti-metastasis activity across various cancer types. Pharmacological limitations and a lack of clinical trials hinder its clinical translation. Advances in nanotechnology-based delivery systems and better clinical trial design could help bridge this gap in the future.
Future research should focus on comprehensive human studies, mechanistic exploration in diverse tumor microenvironments, and the development of advanced targeted nanoformulations. Furthermore, additional research is needed to investigate the mechanism of action and safety of rutin. Additionally, combination therapy involving rutin and existing chemotherapeutic drugs may offer synergistic effects while reducing side effects and toxicity.
Acknowledgments
The Researchers would like to thank the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025).
-
Funding information: Funding was provided by the Deanship of Graduate Studies and Scientific Research at Qassim University for financial support (QU-APC-2025).
-
Author contributions: Conceptualization, A.H.R. and H.O.A.A. writing – original draft preparation, A.H.R. and S.A. writing – review and editing, A.H.R., H.O.A.A., and S.A.; data curation, A.H.R. and S.A.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Zhang CL, Huang T, Wu BL, He WX, Liu D. Stem cells in cancer therapy: opportunities and challenges. Oncotarget. 2017;8(43):75756.10.18632/oncotarget.20798Search in Google Scholar PubMed PubMed Central
[2] Ayob AZ, Ramasamy TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci. 2018;25:1–8.10.1186/s12929-018-0426-4Search in Google Scholar PubMed PubMed Central
[3] Adel N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am J Manag Care. 2017;23(14 Suppl):S259–65.Search in Google Scholar
[4] Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, et al. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008;9(7):581–91.10.2174/138920008785821657Search in Google Scholar PubMed PubMed Central
[5] Madhuri S, Pandey G. Some anticancer medicinal plants of foreign origin. Curr Sci. 2009;96:779–83.Search in Google Scholar
[6] Alrumaihi F, Raut R, Yahia EA, Kumar V, Anwar S. A review on risk factors, diagnostic innovations, and plant based therapies for the management of erectile dysfunction. Uro. 2024;4(2):60–88. 10.3390/uro4020006.Search in Google Scholar
[7] Anwar S, Kumar V, Kanwal B, Yahia EA. A review on utilization of ashoka plant in oxidative stress induced women reproductive health complications. IJCRT. 2023;11(9):f249–58.Search in Google Scholar
[8] Younus H, Anwar S. Prevention of non-enzymatic glycosylation (glycation): Implication in the treatment of diabetic complication. Int J Health Sci (Qassim). 2016;10(2):261–77.10.12816/0048818Search in Google Scholar
[9] Rahmani AH, Khan AA, Babiker AY, Khan MA, Alzohairy MA. Therapeutic implications of ajwa dates (Phoenix dactylifera) in the inhibition of liver tissue alterations through the modulation of vascular endothelial growth factor and phosphatase, and tensin homolog gene. Pharmacogn Res. 2018;10(2):151–5.10.4103/pr.pr_119_17Search in Google Scholar
[10] Almatroodi SA, Khan AA, Aloliqi AA, Syed MA, Rahmani AH. Therapeutic potential of Tamarix aphylla in the prevention of lung injury through the regulation of inflammation, oxidative stress and cell-signaling molecules. Appl Sci. 2022;12(19):9925.10.3390/app12199925Search in Google Scholar
[11] Almatroodi SA, Alsahli MA, Rahmani AH. Berberine: An important emphasis on its anticancer effects through modulation of various cell signaling pathways. Molecules. 2022;27(18):5889.10.3390/molecules27185889Search in Google Scholar PubMed PubMed Central
[12] Almatroodi SA, Syed MA, Rahmani AH. Potential therapeutic targets of Curcumin, most abundant active compound of turmeric spice: Role in the management of various types of cancer. Recent Pat Anticancer Drug Discov. 2021;16(1):3–29.10.2174/1574892815999201102214602Search in Google Scholar PubMed
[13] Mahato M, Patra S, Gogoi M. Herbal Nanocarriers for cancer therapy. Nanopharmaceuticals: Princ Appl. 2021;2:41–75.10.1007/978-3-030-44921-6_2Search in Google Scholar
[14] Najafi M, Mortezaee K, Rahimifard M, Farhood B, Haghi-Aminjan H. The role of curcumin/curcuminoids during gastric cancer chemotherapy: a systematic review of non-clinical study. Life Sci. 2020;257:118051.10.1016/j.lfs.2020.118051Search in Google Scholar PubMed
[15] Bishayee A, Sethi G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin Cancer Biol. 2016;40:1–3.10.1016/j.semcancer.2016.08.006Search in Google Scholar PubMed
[16] Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharm J. 2017;25(2):149–64.10.1016/j.jsps.2016.04.025Search in Google Scholar PubMed PubMed Central
[17] Harborne JB. Nature, distribution and function of plant flavonoids. Prog Clin Biol Res. 1986;213:15–24.Search in Google Scholar
[18] Chen LY, Huang CN, Liao CK, Chang HM, Kuan YH, Tseng TJ, et al. Effects of rutin on wound healing in hyperglycemic rats. Antioxidants. 2020;9(11):1122.10.3390/antiox9111122Search in Google Scholar PubMed PubMed Central
[19] Nouri Z, Fakhri S, Nouri K, Wallace CE, Farzaei MH, Bishayee A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers. 2020;12(8):2276.10.3390/cancers12082276Search in Google Scholar PubMed PubMed Central
[20] Pandey P, Khan F, Qari HA, Oves M. Rutin (Bioflavonoid) as cell signaling pathway modulator: Prospects in treatment and chemoprevention. Pharmaceuticals. 2021;14(11):1069.10.3390/ph14111069Search in Google Scholar PubMed PubMed Central
[21] Perk AA, Shatynska-Mytsyk I, Gerçek YC, Boztaş K, Yazgan M, Fayyaz S, et al. Rutin mediated targeting of signaling machinery in cancer cells. Cancer Cell Int. 2014;14:1–5.10.1186/s12935-014-0124-6Search in Google Scholar PubMed PubMed Central
[22] Alsulaim AK, Almutaz TH, Albati AA, Rahmani AH. Therapeutic potential of curcumin, a bioactive compound of turmeric, in prevention of streptozotocin-induced diabetes through the modulation of oxidative stress and inflammation. Molecules. 2023;29(1):128.10.3390/molecules29010128Search in Google Scholar PubMed PubMed Central
[23] Almatroodi SA, Khan AA, Aloliqi AA, Ali Syed M, Rahmani AH. Therapeutic potential of ajwa dates (Phoenix dactylifera) extract in prevention of benzo (a) pyrene-induced lung injury through the modulation of oxidative stress, inflammation, and cell signalling molecules. Appl Sci. 2022;12(13):6784.10.3390/app12136784Search in Google Scholar
[24] Almatroodi SA, Almatroudi A, Alsahli MA, Khan AA, Rahmani AH. Thymoquinone, an active compound of Nigella sativa: role in prevention and treatment of cancer. Curr Pharm Biotechnol. 2020 Sep 1;21(11):1028–41.10.2174/1389201021666200416092743Search in Google Scholar PubMed
[25] Rahmani AH, Babiker AY, Anwar S. Hesperidin, a bioflavonoid in cancer therapy: a review for a mechanism of action through the modulation of cell signaling pathways. Molecules. 2023;28(13):5152.10.3390/molecules28135152Search in Google Scholar PubMed PubMed Central
[26] Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–49.10.1146/annurev-pathol-012615-044359Search in Google Scholar PubMed
[27] Niccolai E, Boem F, Emmi G, Amedei A. The link “Cancer and autoimmune diseases” in the light of microbiota: Evidence of a potential culprit. Immunol Lett. 2020;222:12–28.10.1016/j.imlet.2020.03.001Search in Google Scholar PubMed
[28] Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22(1):33–40.10.1016/j.semcancer.2011.12.005Search in Google Scholar PubMed
[29] Su S, Li X, Li S, Ming P, Huang Y, Dong Y, et al. Rutin protects against lipopolysaccharide-induced mastitis by inhibiting the activation of the NF-κB signaling pathway and attenuating endoplasmic reticulum stress. Inflammopharmacology. 2019;27:77–88.10.1007/s10787-018-0521-xSearch in Google Scholar PubMed
[30] Youssef SS, Ibrahim NK, El-Sonbaty SM, El-Din Ezz MK. Rutin suppresses DMBA carcinogenesis in the breast through modulating IL-6/NF-κB, SRC1/HSP90 and ER-α. Nat Prod Commun. 2022;17(9):1934578X221118213.10.1177/1934578X221118213Search in Google Scholar
[31] Moutinho MS, Aragao S, Carmo D, Casaca F, Silva S, Ribeiro J, et al. Curcumin and rutin down-regulate cyclooxygenase-2 and reduce tumor-associated inflammation in HPV16-transgenic mice. Anticancer Res. 2018;38(3):1461–6.10.21873/anticanres.12371Search in Google Scholar PubMed
[32] Lima IS, Soares ÉN, Nonaka CK, Souza BS, Dos Santos BL, Costa SL. Flavonoid rutin presented anti-glioblastoma activity related to the modulation of onco miRNA-125b expression and STAT3 signaling and impact on microglia inflammatory profile. Brain Sci. 2024;14(1):90.10.3390/brainsci14010090Search in Google Scholar PubMed PubMed Central
[33] da Costa RM, Aragao S, Moutinho M, Alvarado A, Carmo D, Casaca F, et al. HPV16 induces a wasting syndrome in transgenic mice: Amelioration by dietary polyphenols via NF-κB inhibition. Life Sci. 2017;169:11–9.10.1016/j.lfs.2016.10.031Search in Google Scholar PubMed
[34] Avril T, Vauléon E, Chevet E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis. 2017;6:e373.10.1038/oncsis.2017.72Search in Google Scholar PubMed PubMed Central
[35] Martinon F. Targeting endoplasmic reticulum signaling pathways in cancer. Acta Oncol. 2012;51:822–30.10.3109/0284186X.2012.689113Search in Google Scholar PubMed
[36] Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67:10631–4.10.1158/0008-5472.CAN-07-1705Search in Google Scholar PubMed
[37] Suganya K, Poornima A, Sumathi S, Chigurupati S, Alyamani NM, Felemban SG, et al. Rutin induces endoplasmic reticulum stress-associated apoptosis in human triple-negative breast carcinoma MDA-MB-231 cells–in vitro and in silico docking studies. Arab J Chem. 2022;15(9):104021.10.1016/j.arabjc.2022.104021Search in Google Scholar
[38] Nasri Nasrabadi P, Zareian S, Nayeri Z, Salmanipour R, Parsafar S, Gharib E, et al. A detailed image of rutin underlying intracellular signaling pathways in human SW480 colorectal cancer cells based on miRNAs-lncRNAs-mRNAs-TFs interactions. J Cell Physiol. 2019;234(9):15570–80.10.1002/jcp.28204Search in Google Scholar PubMed
[39] Williams AB, Schumacher B. p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med. 2016;6(5):a026070.10.1101/cshperspect.a026070Search in Google Scholar PubMed PubMed Central
[40] Chen J. The cell-cycle arrest and apoptotic functions of p53 in tumor initiation and progression. Cold Spring Harb Perspect Med. 2016;6(3):a026104.10.1101/cshperspect.a026104Search in Google Scholar PubMed PubMed Central
[41] Yan X, Hao Y, Chen S, Jia G, Guo Y, Zhang G, et al. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl Cancer Res. 2019;8(5):2005.10.21037/tcr.2019.09.07Search in Google Scholar PubMed PubMed Central
[42] Satari A, Amini SA, Raeisi E, Lemoigne Y, Heidarian E. Synergetic impact of combined 5-fluorouracil and rutin on apoptosis in pc3 cancer cells through the modulation of p53 gene expression. Adv Pharm Bull. 2019;9(3):462.10.15171/apb.2019.055Search in Google Scholar PubMed PubMed Central
[43] Hasani NA, Amin IM, Kamaludin R, Rosdyd NM, Ibahim MJ, Kadir SH. P53 and cyclin B1 mediate apoptotic effects of apigenin and rutin in ERï¡ + -breast cancer MCF-7 Cells. J Teknol. 2018;80(1):133–40.10.11113/jt.v80.10704Search in Google Scholar
[44] Haifeng Z, Yinghou W, Dan L, Xiuqing S, Furong W. Vanadium rutin complex sensitizes breast cancer cells via modulation of p53/Bax/Bcl2/VEGF correlated with apoptotic events. Acta Pol Pharm. 2020;77(1):89–98.10.32383/appdr/115158Search in Google Scholar
[45] Anwar S, Raut R, Alhumaydhi FA. A comprehensive investigation on alleviating oxidative stress and inflammation in hyperglycaemic conditions through in vitro experiments and computational analysis. Saudi J Biol Sci. 2024;31(7):104003.10.1016/j.sjbs.2024.104003Search in Google Scholar PubMed PubMed Central
[46] Anwar S, Raut R, Kanwal B, Yahia E, Kumar V. In vitro investigation of antiinflammatory and antioxidant activities of Curcuma longa rhizome methanol extract. Int J Creat Res Thoughts (IJCRT). 2022;10:c559–81.Search in Google Scholar
[47] Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–3.10.1016/j.redox.2015.01.002Search in Google Scholar PubMed PubMed Central
[48] Reczek CR, Birsoy K, Kong H, Martínez-Reyes I, Wang T, Gao P, et al. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nat Chem Biol. 2017;13(12):1274–9.10.1038/nchembio.2499Search in Google Scholar PubMed PubMed Central
[49] Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen YM, et al. Natural polyphenols for prevention and treatment of cancer. Nutrients. 2016;8(8):515.10.3390/nu8080515Search in Google Scholar PubMed PubMed Central
[50] Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality – a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56.10.1093/ije/dyw319Search in Google Scholar PubMed PubMed Central
[51] Li Y, Li S, Meng X, Gan RY, Zhang JJ, Li HB. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9:728.10.3390/nu9070728Search in Google Scholar PubMed PubMed Central
[52] Kozlowska A, Szostak-Wegierek D. Flavonoids-food sources and health benefits. Rocz Panstw Zakl Hig. 2014;65(2):79–85.Search in Google Scholar
[53] Nosrati N, Bakovic M, Paliyath G. Molecular mechanisms and pathways as targets for cancer prevention and progression with dietary compounds. Int J Mol Sci. 2017;18(10):2050.10.3390/ijms18102050Search in Google Scholar PubMed PubMed Central
[54] Pyrzanowska J, Piechal A, Blecharz-Klin K, Joniec-Maciejak I, Zobel A, Widy-Tyszkiewicz E. Influence of long-term administration of rutin on spatial memory as well as the concentration of brain neurotransmitters in aged rats. Pharmacol Rep. 2012;64(4):808–16.10.1016/S1734-1140(12)70876-9Search in Google Scholar PubMed
[55] Cho JY, Kim IS, Jang YH, Kim AR, Lee SR. Protective effect of quercetin, a natural flavonoid against neuronal damage after transient global cerebral ischemia. Neurosci Lett. 2006;404(3):330–5.10.1016/j.neulet.2006.06.010Search in Google Scholar PubMed
[56] Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K, et al. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007;104(4):329–34.10.1254/jphs.FP0070247Search in Google Scholar PubMed
[57] Bakhtiari E, Hosseini A, Mousavi SH. The role of ROS and NF-κB pathway in olmesartan induced-toxicity in HeLa and mcf-7 cell lines. Biomed Pharmacother. 2017;93:429–34.10.1016/j.biopha.2017.06.074Search in Google Scholar PubMed
[58] ben Sghaier M, Pagano A, Mousslim M, Ammari Y, Kovacic H, Luis J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed Pharmacother. 2016;84:1972–8.10.1016/j.biopha.2016.11.001Search in Google Scholar PubMed
[59] Saleh A, ElFayoumi HM, Youns M, Barakat W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:165–75.10.1007/s00210-018-1579-0Search in Google Scholar PubMed
[60] Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: A matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–52.10.1038/s41575-018-0065-ySearch in Google Scholar PubMed PubMed Central
[61] Yang JY, Sung B, Kim ND. Role of induced programmed cell death in the chemopreventive potential of apigenin. Int J Mol Sci. 2022;23:3757.10.3390/ijms23073757Search in Google Scholar PubMed PubMed Central
[62] Verbrugge I, Johnstone RW, Smyth MJ. SnapShot: extrinsic apoptosis pathways. Cell. 2010;143(7):1192.10.1016/j.cell.2010.12.004Search in Google Scholar PubMed
[63] Arbiser JL, Bonner MY, Gilbert LC. Targeting the duality of cancer. NPJ Precis Oncol. 2017;1(1):23.10.1038/s41698-017-0026-xSearch in Google Scholar PubMed PubMed Central
[64] Xu W, Jing L, Wang Q, Lin CC, Chen X, Diao J, et al. Bax-PGAM5L-Drp1 complex is required for intrinsic apoptosis execution. Oncotarget. 2015;6(30):30017.10.18632/oncotarget.5013Search in Google Scholar PubMed PubMed Central
[65] Chen H, Miao Q, Geng M, Liu J, Hu Y, Tian L, et al. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci World J. 2013;2013:269165.10.1155/2013/269165Search in Google Scholar PubMed PubMed Central
[66] Huo M, Xia A, Cheng W, Zhou M, Wang J, Shi T, et al. Rutin promotes pancreatic cancer cell apoptosis by upregulating miRNA-877-3p expression. Molecules. 2022;27(7):2293.10.3390/molecules27072293Search in Google Scholar PubMed PubMed Central
[67] Khan F, Pandey P, Upadhyay TK, Jafri A, Jha NK, Mishra R, et al. Anti-cancerous effect of rutin against HPV-C33A cervical cancer cells via G0/G1 cell cycle arrest and apoptotic induction. Endocr Metab Immune Disord Drug Targets. 2020;20(3):409–18.10.2174/1871530319666190806122257Search in Google Scholar PubMed
[68] Matera R, Saif MW. New therapeutic directions for advanced pancreatic cancer: cell cycle inhibitors, stromal modifiers and conjugated therapies. Expert Opin Emerg Drugs. 2017;22(3):223–33.10.1080/14728214.2017.1362388Search in Google Scholar PubMed
[69] Jahagirdar D, Gore CR, Patel H, Maria K, Tandon I, Sharma NK. Induction of apoptotic death and cell cycle arrest in HeLa cells by extracellular factors of breast cancer cells. Asian Pac J Cancer Prev. 2018;19(12):3307–16.10.31557/APJCP.2018.19.12.3307Search in Google Scholar PubMed PubMed Central
[70] Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: Antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15(2):196–212.10.1080/15384101.2015.1120925Search in Google Scholar PubMed PubMed Central
[71] Iriti M, Kubina R, Cochis A, Sorrentino R, Varoni EM, Kabała‐Dzik A, et al. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother Res. 2017;31(10):1529–38.10.1002/ptr.5878Search in Google Scholar PubMed
[72] Wu F, Chen J, Fan LM, Liu K, Zhang N, Li SW, et al. Analysis of the effect of rutin on GSK-3β and TNF-α expression in lung cancer. Exp Ther Med. 2017;14(1):127–30.10.3892/etm.2017.4494Search in Google Scholar PubMed PubMed Central
[73] Santos BL, Silva AR, Pitanga BP, Sousa CS, Grangeiro MS, Fragomeni BO, et al. Antiproliferative, proapoptotic and morphogenic effects of the flavonoid rutin on human glioblastoma cells. Food Chem. 2011;127(2):404–11.10.1016/j.foodchem.2010.12.131Search in Google Scholar PubMed
[74] Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–66.10.1007/s10456-007-9074-0Search in Google Scholar PubMed
[75] Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–74.10.1016/S0140-6736(07)60784-3Search in Google Scholar PubMed
[76] Caldwell RB, Bartoli M, Behzadian MA, El‐Remessy AE, Al‐Shabrawey M, Platt DH, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19(6):442–55.10.1002/dmrr.415Search in Google Scholar PubMed
[77] Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64.10.1016/S0092-8674(00)80108-7Search in Google Scholar
[78] Zimna A, Kurpisz M. Hypoxia‐inducible factor‐1 in physiological and pathophysiological angiogenesis: applications and therapies. BioMed Res Int. 2015;2015(1):549412.10.1155/2015/549412Search in Google Scholar PubMed PubMed Central
[79] Khosravi Shahi P, Soria Lovelle A, Pérez Manga G. Tumoral angiogenesis and breast cancer. Clin Transl Oncol. 2009;11:138–42.10.1007/S12094-009-0329-7Search in Google Scholar
[80] Chuang CH, Huang CS, Hu ML. Vitamin E and rutin synergistically inhibit expression of vascular endothelial growth factor through down-regulation of binding activity of activator protein-1 in human promyelocytic leukemia (HL-60) cells. Chem Biol Interact. 2010;183(3):434–41.10.1016/j.cbi.2009.12.007Search in Google Scholar PubMed
[81] Noolu B, Gogulothu R, Bhat M, Qadri SS, Reddy VS, Reddy GB, et al. In vivo inhibition of proteasome activity and tumour growth by Murraya koenigii leaf extract in breast cancer xenografts and by its active flavonoids in breast cancer cells. Anticancer Agents Med Chem. 2016;16(12):1605–14.10.2174/1871520616666160520112210Search in Google Scholar PubMed
[82] Schindler R, Mentlein R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J Nutr. 2006;136:1477–82.10.1093/jn/136.6.1477Search in Google Scholar PubMed
[83] Alonso-Castro AJ, Domínguez F, García-Carrancá A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch Med Res. 2013;44(5):346–51.10.1016/j.arcmed.2013.06.002Search in Google Scholar PubMed
[84] Freitas S, Costa S, Azevedo C, Carvalho G, Freire S, Barbosa P, et al. Flavonoids inhibit angiogenic cytokine production by human glioma cells. Phytother Res. 2011;25(6):916–21.10.1002/ptr.3338Search in Google Scholar PubMed
[85] Hajimehdipoor H, Tahmasvand Z, Nejad FG, Maresca M, Rajabi S. Rutin promotes proliferation and orchestrates epithelial–mesenchymal transition and angiogenesis in MCF-7 and MDA-MB-231 breast cancer cells. Nutrients. 2023;15(13):2884.10.3390/nu15132884Search in Google Scholar PubMed PubMed Central
[86] Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859–68.10.1038/nrc1209Search in Google Scholar PubMed
[87] Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68(2):320–44.10.1128/MMBR.68.2.320-344.2004Search in Google Scholar PubMed PubMed Central
[88] Lopez-Bergami P, Lau E, Ronai ZE. Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer. 2010;10(1):65–76.10.1038/nrc2681Search in Google Scholar PubMed PubMed Central
[89] Arnott CH, Scott KA, Moore RJ, Hewer A, Phillips DH, Parker P, et al. Tumour necrosis factor-α mediates tumour promotion via a PKCα-and AP-1-dependent pathway. Oncogene. 2002;21(31):4728–38.10.1038/sj.onc.1205588Search in Google Scholar PubMed
[90] Tseng HH, Chen PN, Kuo WH, Wang JW, Chu SC, Hsieh YS. Antimetastatic potentials of Phyllanthus urinaria L on A549 and Lewis lung carcinoma cells via repression of matrix-degrading proteases. Integr Cancer Ther. 2012;11(3):267–78.10.1177/1534735411417128Search in Google Scholar PubMed
[91] De Duve C. The lysosome. Sci Am. 1963;208:64–72.10.1038/scientificamerican0563-64Search in Google Scholar PubMed
[92] Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci. 2018;19(11):3466.10.3390/ijms19113466Search in Google Scholar PubMed PubMed Central
[93] Rao S, Tortola L, Perlot T, Wirnsberger G, Novatchkova M, Nitsch R, et al. A dual role for autophagy in a murine model of lung cancer. Nat Commun. 2014;5(1):3056.10.1038/ncomms4056Search in Google Scholar PubMed
[94] Singh SS, Vats S, Chia AY, Tan TZ, Deng S, Ong MS, et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37(9):1142–58.10.1038/s41388-017-0046-6Search in Google Scholar PubMed
[95] Zhou M, Zhang G, Hu J, Zhu Y, Lan H, Shen X, et al. Rutin attenuates sorafenib-induced chemoresistance and autophagy in hepatocellular carcinoma by regulating BANCR/miRNA-590-5P/OLR1 axis. Int J Biol Sci. 2021;17(13):3595.10.7150/ijbs.62471Search in Google Scholar PubMed PubMed Central
[96] Park MH, Kim S, Song YR, Kim S, Kim HJ, Na HS, et al. Rutin induces autophagy in cancer cells. Int J Oral Biol. 2016;41(1):45–51.10.11620/IJOB.2016.41.1.045Search in Google Scholar
[97] Zhang P, Sun S, Li N, Ho AS, Kiang KM, Zhang X, et al. Rutin increases the cytotoxicity of temozolomide in glioblastoma via autophagy inhibition. J Neurooncol. 2017;132:393–400.10.1007/s11060-017-2387-ySearch in Google Scholar PubMed
[98] Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27(41):5486–96.10.1038/onc.2008.244Search in Google Scholar PubMed PubMed Central
[99] Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3, 4, 5-trisphosphate. J Biol Chem. 1998;273(22):13375–8.10.1074/jbc.273.22.13375Search in Google Scholar PubMed
[100] Yılmaz S, Doğanyiğit Z, Oflamaz AO, Ateş Ş, Söylemez ES, Nisari M, et al. Determination of Rutin’s antitumoral effect on EAC solid tumor by AgNOR count and PI3K/AKT/mTOR signaling pathway. Med Oncol. 2023;40(5):131.10.1007/s12032-023-01999-7Search in Google Scholar PubMed
[101] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.10.3322/caac.21551Search in Google Scholar PubMed
[102] Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–46.10.1038/nrc3775Search in Google Scholar PubMed PubMed Central
[103] Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–9.10.1200/JCO.2007.13.9030Search in Google Scholar PubMed
[104] Prelaj A, Tay R, Ferrara R, Chaput N, Besse B, Califano R. Predictive biomarkers of response for immune checkpoint inhibitors in non-small-cell lung cancer. Eur J Cancer. 2019;106:144–59.10.1016/j.ejca.2018.11.002Search in Google Scholar PubMed
[105] Paudel KR, Wadhwa R, Tew XN, Lau NJX, Madheswaran T, Panneerselvam J, et al. Rutin loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Life Sci. 2021;276:119436.10.1016/j.lfs.2021.119436Search in Google Scholar PubMed
[106] Li XH, Liu ZY, Gu Y, Lv Z, Chen Y, Gao HC. Expression of NF-kappaB and p38 under intervention of rutin in lung cancer therapy. Biomed Res. 2017;14:2344–7.Search in Google Scholar
[107] Yeh SL, Wang WY, Huang CS, Hu ML. Flavonoids suppresses the enhancing effect of beta-carotene on DNA damage induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A549 cells. Chem Biol Interact. 2006;160:175–82.10.1016/j.cbi.2006.01.006Search in Google Scholar PubMed
[108] Menon LG, Kuttan R, Kuttan G. Inhibition of lung metastasis in mice induced by B16F10 melanoma cells by polyphenolic compounds. Cancer Lett. 1995;95:221–5.10.1016/0304-3835(95)03887-3Search in Google Scholar PubMed
[109] Santos BL, Oliveira MN, Coelho PL, Pitanga BP, da Silva AB, Adelita T, et al. Flavonoids suppress human glioblastoma cell growth by inhibiting cell metabolism, migration, and by regulating extracellular matrix proteins and metalloproteinases expression. Chem Biol Interact. 2015;242:123–38.10.1016/j.cbi.2015.07.014Search in Google Scholar PubMed
[110] Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23.10.1016/j.breast.2022.08.010Search in Google Scholar PubMed PubMed Central
[111] Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.10.3322/caac.21660Search in Google Scholar PubMed
[112] Islam MR, Islam F, Nafady MH, Akter M, Mitra S, Das R, et al. Natural small molecules in breast cancer treatment: understandings from a therapeutic viewpoint. Molecules. 2022;27(7):2165.10.3390/molecules27072165Search in Google Scholar PubMed PubMed Central
[113] Singh TA, Sadhukhan P, Ghosh N, Thakur N, Sharma A, Tejwan N, et al. Targeted delivery of rutin into breast cancer cells via using phenylboronic acid functionalized MgO nanoparticles. Mater Sci Eng B. 2023;296:116623.10.1016/j.mseb.2023.116623Search in Google Scholar
[114] Elsayed HE, Ebrahim HY, Mohyeldin MM, Siddique AB, Kamal AM, Haggag EG, et al. Rutin as a novel c-Met inhibitory lead for the control of triple negative breast malignancies. Nutr Cancer. 2017;69(8):1256–71. 10.1080/01635581.2017.1367936.Search in Google Scholar PubMed PubMed Central
[115] Li Q, Xu D, Gu Z, Li T, Huang P, Ren L. Rutin restrains the growth and metastasis of mouse breast cancer cells by regulating the microRNA‐129‐1‐3p‐mediated calcium signaling pathway. J Biochem Mol Toxicol. 2021;35(7):e22794.10.1002/jbt.22794Search in Google Scholar PubMed
[116] Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203.10.1016/S2214-109X(19)30482-6Search in Google Scholar PubMed PubMed Central
[117] Nagase S, Ohta T, Takahashi F, Yamagami W, Yaegashi N. Board Members of the 2020 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual report of the committee on gynecologic oncology, the Japan society of obstetrics and gynecology: annual patient report for 2018 and annual treatment report for 2013. J Obstet Gynaecol Res. 2022;48:541–52.10.1111/jog.15134Search in Google Scholar PubMed
[118] Zhou ZW, Long HZ, Xu SG, Li FJ, Cheng Y, Luo HY, et al. Therapeutic effects of natural products on cervical cancer: based on inflammatory pathways. Front Pharmacol. 2022;13:899208. 10.3389/fphar.2022.899208.Search in Google Scholar PubMed PubMed Central
[119] Pandey P, Khan F, Alzahrani FA, Qari HA, Oves M. A novel approach to unraveling the apoptotic potential of rutin (Bioflavonoid) via targeting Jab1 in cervical cancer cells. Molecules. 2021;26(18):5529.10.3390/molecules26185529Search in Google Scholar PubMed PubMed Central
[120] Pandey P, Khan F, Farhan M, Jafri A. Elucidation of rutin’s role in inducing caspase-dependent apoptosis via HPV-E6 and E7 down-regulation in cervical cancer HeLa cells. Biosci Rep. 2021;41(6):BSR20210670.10.1042/BSR20210670Search in Google Scholar PubMed PubMed Central
[121] Khan F, Pandey P, Jha NK, Khalid M, Ojha S. Rutin mediated apoptotic cell death in Caski cervical cancer cells via Notch-1 and Hes-1 downregulation. Life (Basel). 2021;11(8):761. 10.3390/life11080761.Search in Google Scholar PubMed PubMed Central
[122] Deepika MS, Thangam R, Sheena TS, Sasirekha R, Sivasubramanian S, Babu MD, et al. A novel rutin-fucoidan complex based phytotherapy for cervical cancer through achieving enhanced bioavailability and cancer cell apoptosis. Biomed Pharmacother. 2019;109:1181–95. 10.1016/j.biopha.2018.10.178.Search in Google Scholar PubMed
[123] Firouzai-Amandi A, Tarahomi M, Rahmani Youshanlouie H, Mosaddeghi Heris R, Jafari-Gharabaghlou D, Zarghami N, et al. Development, characterization, and in vitro evaluation of cytotoxic activity of rutin loaded PCL-PEG nanoparticles against Skov3 ovarian cancer cell. Asian Pac J Cancer Prev. 2022;23(6):1951–7.10.31557/APJCP.2022.23.6.1951Search in Google Scholar PubMed PubMed Central
[124] Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60(6):800–9.10.1080/01635580802100851Search in Google Scholar PubMed
[125] Li Y. The utilization of Rutin-loaded lipid nanoparticles as promise carriers to enhance the therapeutic efficacy in ovarian cancer: A computational and experimental study. Inorg Chem Commun. 2025;172:113516. 10.1016/j.inoche.2024.113516 Search in Google Scholar
[126] Sirotkin AV, Pelleova B, Fabova Z, Makovicky P, Alwasel S, Harrath AH. Rutin directly affects stimulatory action of FSH on the ovarian cell, Pharma. Nutrition. 2021;15:100247.10.1016/j.phanu.2020.100247Search in Google Scholar
[127] Gupta D, Shrivastava S, Kumar S, Bhardwaj G, Jain C, Kumar S, et al. Protective effect of rutin on acrylamide induced ovarian inflammation, oxidative stress, DNA damage, and hormonal changes: Based on in silico and in vivo study. J Biochem Mol Toxicol. 2024;38(8):e23784. 10.1002/jbt.23784. PMID: 39095945.Search in Google Scholar PubMed
[128] Ferlay J, Laversanne M, Ervik M, Lam F, Colombet M, Mery L. Global cancer observatory: Cancer tomorrow [online]. Lyon, France: International agency for research on cancer; 2020. https://gco.iarc.fr/tomorrow (Accessed 04 12, 2022 2022).Search in Google Scholar
[129] Rahmani AH, Aly SM, Ali H, Babiker AY, Srikar S, Khan AA. Therapeutic effects of date fruits (Phoenix dactylifera) in the prevention of diseases via modulation of anti-inflammatory, anti-oxidant and anti-tumour activity. Int J Clin Exp Med. 2014;7(3):483–91.Search in Google Scholar
[130] Rahmani AH, Shabrmi FM, Aly SM. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol Pathophysiol Pharmacol. 2014;6(2):125–36.Search in Google Scholar
[131] Rahmani AH, Aldebasi YH, Srikar S, Khan AA, Aly SM. Aloe vera: Potential candidate in health management via modulation of biological activities. Pharmacogn Rev. 2015;9(18):120–6.10.4103/0973-7847.162118Search in Google Scholar PubMed PubMed Central
[132] Rahmani AH, Al Shabrmi FM, Allemailem KS, Aly SM, Khan MA. Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. BioMed Res Int. 2015;2015(1):925640.10.1155/2015/925640Search in Google Scholar PubMed PubMed Central
[133] Rahmani AH, Al Zohairy MA, Aly SM, Khan MA. Curcumin: a potential candidate in prevention of cancer via modulation of molecular pathways. BioMed Res Inter. 2014;2014(1):761608.10.1155/2014/761608Search in Google Scholar PubMed PubMed Central
[134] Modanwal S, Mishra A, Mishra N. Exploration of rutin derivatives as potential inhibitors of prostate cancer signaling pathways: A comprehensive in-silico study. Biochem Biophys Res Commun. 2025;746:151279.10.1016/j.bbrc.2024.151279Search in Google Scholar PubMed
[135] Bal E, Deveci Özkan A, Betts Z. Determination of the effect of rutin on epithhelial and mesenchimal transition in prostate cancer cells. Acta Med Nicomedia. 2023;6(1):131–6. 10.53446/actamednicomedia.1171654.Search in Google Scholar
[136] Hashem H, Amin MA. The effect of zinc nanoparticles on adult rat prostate gland and the possible protective role of rutin: Histological and biochemical study. Egypt J Histology. 2022;45(2):372–85.Search in Google Scholar
[137] Liu H, Xu Q, Wufuer H, Li Z, Sun R, Jiang Z, et al. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell. 2024;23(1):e13921.10.1111/acel.13921Search in Google Scholar PubMed PubMed Central
[138] Castelló A, Amiano P, Fernández de Larrea N, Martín V, Alonso MH, et al. Low adherence to the western and high adherence to the mediterranean dietary patterns could prevent colorectal cancer. Eur J Nutr. 2019;58(4):1495–505. 10.1007/s00394-018-1674-5.Search in Google Scholar PubMed
[139] Barone M, Lofano K, De Tullio N, Licinio R, Albano F, Di Leo A. Dietary, endocrine, and metabolic factors in the development of colorectal cancer. J Gastrointest Cancer. 2012;43(1):13–9. 10.1007/s12029-011-9332-7.Search in Google Scholar PubMed
[140] De Falco V, Napolitano S, Rosello S, Huerta M, Cervantes A, Ciardiello F, et al. How we treat metastatic colorectal cancer. ESMO Open. 2020;4(Suppl 2):e000813.10.1136/esmoopen-2020-000813Search in Google Scholar PubMed PubMed Central
[141] Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med. 2020;9:361–73.10.1002/cam4.2673Search in Google Scholar PubMed PubMed Central
[142] Nafees S, Mehdi SH, Zafaryab M, Zeya B, Sarwar T, Rizvi MA. Synergistic interaction of rutin and silibinin on human colon cancer cell line. Arch Med Res. 2018;49(4):226–34.10.1016/j.arcmed.2018.09.008Search in Google Scholar PubMed
[143] Guon TE, Chung HS. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol Lett. 2016;11(4):2463–70.10.3892/ol.2016.4247Search in Google Scholar PubMed PubMed Central
[144] Vijay M, Sivagami G, Thayalan K, Nalini N. Radiosensitizing potential of rutin against human colon adenocarcinoma HT-29 cells. Bratisl Lek Listy. 2016;117(3):171–8.10.4149/BLL_2016_033Search in Google Scholar
[145] Volate SR, Davenport DM, Muga SJ, Wargovich MJ. Modulation of aberrant crypt foci and apoptosis by dietary herbal supplements (quercetin, curcumin, silymarin, ginseng and rutin). Carcinogenesis. 2005;26(8):1450–6.10.1093/carcin/bgi089Search in Google Scholar PubMed
[146] Jayameena P, Sivakumari K, Ashok K, Rajesh S. Rutin: A potential anticancer drug against human colon cancer (HCT116) cells. Int J Biol Pharm Allied Sci. 2018;7(9):1731–45.10.31032/IJBPAS/2018/7.9.4532Search in Google Scholar
[147] Hoai TT, Yen PT, Dao TT, Long LH, Anh DX, Minh LH, et al. Evaluation of the cytotoxic effect of rutin prenanoemulsion in lung and colon cancer cell lines. J Nanomater. 2020;2020(1):8867669.10.1155/2020/8867669Search in Google Scholar
[148] Nasiri F, Kismali G, Alpay M, Kosova F, Cakir DU, Sel T. Rutin enhances the antiproliferative effect of 5-FU and oxaliplatin in colon cancer cells. Cancer Res. 2016;76(14_Supplement):2177.10.1158/1538-7445.AM2016-2177Search in Google Scholar
[149] Ismail A, El-Biyally E, Sakran W. An innovative approach for formulation of rutin tablets targeted for colon cancer treatment. AAPS PharmSciTech. 2023;24(2):68. 10.1208/s12249-023-02518-7.Search in Google Scholar PubMed
[150] Deschner EE, Ruperto J, Wong G, Newmark HL. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991;12:1193–6.10.1093/carcin/12.7.1193Search in Google Scholar PubMed
[151] Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, et al. A changing paradigm for the treatment of intermediate-stage hepatocellular carcinoma: Asia-Pacific primary liver cancer expert consensus statements. Liver Cancer. 2020;9(3):245–60.10.1159/000507370Search in Google Scholar PubMed PubMed Central
[152] Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13(2):125–37. 10.1007/s12072-018-9919-1.Search in Google Scholar PubMed
[153] Moon H, Cho K, Shin S, Kim DY, Han KH, Ro SW. High risk of hepatocellular carcinoma development in fibrotic liver: Role of the hippo-YAP/TAZ signaling pathway. Int J Mol Sci. 2019;20(3):581. 10.3390/ijms20030581.Search in Google Scholar PubMed PubMed Central
[154] Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14.10.1016/S0140-6736(18)30010-2Search in Google Scholar PubMed
[155] Luo XY, Wu KM, He XX. Advances in drug development for hepatocellular carcinoma: Clinical trials and potential therapeutic targets. J Exp Clin Cancer Res. 2021;40:1722021.10.1186/s13046-021-01968-wSearch in Google Scholar PubMed PubMed Central
[156] Chandra YP, Viswanathswamy A. Chemopreventive effect of Rutin against N-nitrosodiethylamine-induced and phenobarbital-promoted hepatocellular carcinoma in Wistar rats. Indian J Pharm. Educ Res. 2018;52:78–86.10.5530/ijper.52.1.9Search in Google Scholar
[157] Labh AK, Priya VV, Gayathri R. Cytotoxic action of rutin isolated from Morinda citrifolia against hepatic carcinoma cell lines. Drug Invent Today. 2019;12:1904–7.Search in Google Scholar
[158] Alía M, Mateos R, Ramos S, Lecumberri E, Bravo L, Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2). Eur J Nutr. 2006;45(1):19–28.10.1007/s00394-005-0558-7Search in Google Scholar PubMed
[159] Guan WL, He Y, Xu RH. Gastric Cancer Treatment: Recent Progress and Future Perspectives. J Hematol Oncol. 2023;16:57.10.1186/s13045-023-01451-3Search in Google Scholar PubMed PubMed Central
[160] Li H, Shen M, Wang S. Current therapies and progress in the treatment of advanced gastric cancer. Front Oncol. 2024;14:1327055.10.3389/fonc.2024.1327055Search in Google Scholar PubMed PubMed Central
[161] Yu Y, Ma X, Zhang Y, Zhang Y, Ying J, Zhang W, et al. Changes in expression of multiple checkpoint molecules and infiltration of tumor immune cells after neoadjuvant chemotherapy in gastric cancer. J Cancer. 2019;10:2754–63.10.7150/jca.31755Search in Google Scholar PubMed PubMed Central
[162] Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.10.1016/S0140-6736(10)61121-XSearch in Google Scholar PubMed
[163] Li Q, Ren L, Zhang Y, Gu Z, Tan Q, Zhang T, et al. P38 signal transduction pathway has more cofactors on apoptosis of SGC-7901 gastric cancer cells induced by combination of rutin and oxaliplatin. Biomed Res Int. 2019;2019:6407210.10.1155/2019/6407210Search in Google Scholar PubMed PubMed Central
[164] Huang H, Shi J, Chen W, Liu L. Rutin suppresses the malignant biological behavior of gastric cancer cells through the Wnt/β-catenin pathway. Discov Oncol. 2024;15(1):407.10.1007/s12672-024-01281-wSearch in Google Scholar PubMed PubMed Central
[165] Pumjan S, Praparatana R, Issarachot O, Wiwattanapatapee R. Rutin-loaded raft-forming systems developed from alginate and whey protein isolate for the treatment of gastric ulcers. J Drug Delivery Sci Technol. 2025;107:106842.10.1016/j.jddst.2025.106842Search in Google Scholar
[166] Liu Y, Gou L, Fu X, Li S, Lan N, Yin X. Protective effect of rutin against acute gastric mucosal lesions induced by ischemia-reperfusion. Pharm Biol. 2013;51(7):914–9. 10.3109/13880209.2013.771375.Search in Google Scholar PubMed
[167] Mao J, Li S, Zhao H, Zhu Y, Hong M, Zhu H, et al. Effects of chidamide and its combination with decitabine on proliferation and apoptosis of leukemia cell lines. Am J Transl Res. 2018;10(8):2567.Search in Google Scholar
[168] Rahman A, Naheed NH, Raka SC, Qais N, Momen AR. Ligand-based virtual screening, consensus molecular docking, multi-target analysis and comprehensive ADMET profiling and MD stimulation to find out noteworthy tyrosine kinase inhibitor with better efficacy and accuracy. Adv Tradit Med. 2020;20:645–61.10.1007/s13596-019-00406-9Search in Google Scholar
[169] Cotoraci C, Ciceu A, Sasu A, Miutescu E, Hermenean A. The anti-leukemic activity of natural compounds. Molecules. 2021;26(9):2709.10.3390/molecules26092709Search in Google Scholar PubMed PubMed Central
[170] Bourogaa E, Bertrand J, Despeaux M, Jarraya R, Fabre N, Payrastre L, et al. Hammada scoparia flavonoids and rutin kill adherent and chemoresistant leukemic cells. Leuk Res. 2011;35:1093–101.10.1016/j.leukres.2010.12.011Search in Google Scholar PubMed
[171] Lin JP, Yang JS, Lu CC, Chiang JH, Wu CL, Lin JJ, et al. Rutin inhibits the proliferation of murine leukemia WEHI-3 cells in vivo and promotes immune response in vivo. Leuk Res. 2009;33(6):823–8.10.1016/j.leukres.2008.09.032Search in Google Scholar PubMed
[172] Lin JP, Yang JS, Lin JJ, Lai KC, Lu HF, Ma CY, et al. Rutin inhibits human leukemia tumor growth in a murine xenograft model in vivo. Env Toxicol. 2012;27(8):480–4.10.1002/tox.20662Search in Google Scholar PubMed
[173] Nadova S, Miadokova E, Cipak L. Flavonoids potentiate the efficacy of cytarabine through modulation of drug-induced apoptosis. Neoplasma. 2007;54(3):202–6.Search in Google Scholar
[174] Alidoust FA, Zahmatkesh H, Rasti B, Zamani H, Mirpour M, Mirzaie A. Zinc oxide fabricated by rutin as potent anti-leukemia nanostructure. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(6):7005–15. 10.1007/s00210-024-03724-1.Search in Google Scholar PubMed
[175] Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13.10.1007/978-1-4419-0284-9_1Search in Google Scholar PubMed
[176] Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–34.10.1002/ijc.24320Search in Google Scholar PubMed PubMed Central
[177] Denduluri SK, Wang Z, Yan Z, Wang J, Wei Q, Mohammed MK, et al. Molecular pathogenesis and therapeutic strategies of human osteosarcoma. J Biomed Res. 2016;30(1):5.10.7555/JBR.29.20150075Search in Google Scholar PubMed
[178] Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J. 2018;4:12.10.1051/sicotj/2017028Search in Google Scholar PubMed PubMed Central
[179] Soosai D, Ramalingam R, Perumal E, Veeramani K, Pancras C, Almutairi MH, et al. Anticancer effects of rutin from Fagopyrum tataricum (tartary buckwheat) against osteosarcoma cell line. Mol Biol Rep. 2024;51(1):312.10.1007/s11033-024-09218-wSearch in Google Scholar PubMed
[180] Liu XW, Ma B, Zi Y, Xiang LB, Han TY. Effects of rutin on osteoblast MC3T3-E1 differentiation, ALP activity and Runx2 protein expression. Eur J Histochem. 2021;65(1):3195. 10.4081/ejh.2021.3195.Search in Google Scholar PubMed PubMed Central
[181] Xiao Y, Wei R, Yuan Z, Lan X, Kuang J, Hu D, et al. Rutin suppresses FNDC1 expression in bone marrow mesenchymal stem cells to inhibit postmenopausal osteoporosis. Am J Transl Res. 2019;11(10):6680–90.Search in Google Scholar
[182] Xiang LI, Ming WEI, Wenxi WU, Xiaoqin LUO, Biao YAO, Siyu WU. Inhibitory effect of rutin on the growth and metastasis of osteosarcoma in vitro and in vivo. J Shanghai Jiao Tong Univ (Med Sci). 2025;45(1):20–8.Search in Google Scholar
[183] Pinzaru I, Chioibas R, Marcovici I, Coricovac D, Susan R, Predut D, et al. Rutin exerts cytotoxic and senescence-inducing properties in human melanoma cells. Toxics. 2021;9(9):226.10.3390/toxics9090226Search in Google Scholar PubMed PubMed Central
[184] Martínez Conesa C, Vicente Ortega V, Yáñez Gascón MJ, Alcaraz Baños M, Canteras Jordana M, Benavente-García O, et al. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J Agric Food Chem. 2005;53(17):6791–7.10.1021/jf058050gSearch in Google Scholar PubMed
[185] Guruvayoorappan C, Kuttan G. Antiangiogenic effect of rutin and its regulatory effect on the production of VEGF, IL-1β and TNF-α in tumor-associated macrophages. J Biol Sci. 2007;7:1511–9.10.3923/jbs.2007.1511.1519Search in Google Scholar
[186] Corina D, Florina B, Iulia P, Cristina D, Rita A, Alexandra P, et al. Rutin and its cyclodextrin inclusion complexes: Physico-chemical evaluation and in vitro activity on B164A5 murine melanoma cell line. Curr Pharm Biotechnol. 2017;18(13):1067–77.10.2174/1389201019666180209165523Search in Google Scholar PubMed
[187] Taira J, Tsuchida E, Uehara M, Ohhama N, Ohmine W, Ogi T. The leaf extract of Mallotus japonicus and its major active constituent, rutin, suppressed on melanin production in murine B16F1 melanoma. Asian Pac J Trop Biomed. 2015;5(10):819–23.10.1016/j.apjtb.2015.05.017Search in Google Scholar
[188] Dumitrescu C, Neagu M, Smeu A, Cristea AM, Boia E, Briciu DM, et al. The association between fisetin and rutin triggers an enhanced cytotoxicity in A431 and A375 skin cancer cells. Med Evolution. 2025;31(1):97–105.10.70921/medev.v31i1.1277Search in Google Scholar
[189] Dewangan HK, Shah K, Vadaga AK, Veer M, Alam P. Optimisation and evaluation of long circulating Ru-SLNs carrier for targeting melanoma cells. J Microencapsul. 2025;42(2):107–19. 10.1080/02652048.2024.2443436.Search in Google Scholar PubMed
[190] Caparica R, Júlio A, Araújo MEM, Baby AR, Fonte P, Costa JG, et al. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules. 2020;10(2):233.10.3390/biom10020233Search in Google Scholar PubMed PubMed Central
[191] Han Y, Lu JS, Xu Y, Zhang L, Hong BF. Rutin ameliorates renal fibrosis and proteinuria in 5/6-nephrectomized rats by anti-oxidation and inhibiting activation of TGFβ1-smad signaling. Int J Clin Exp Pathol. 2015;8(5):4725–34.Search in Google Scholar
[192] Arjumand W, Seth A, Sultana S. Rutin attenuates cisplatin induced renal inflammation and apoptosis by reducing NFκB, TNF-α and caspase-3 expression in wistar rats. Food Chem Toxicol. 2011;49(9):2013–21.10.1016/j.fct.2011.05.012Search in Google Scholar PubMed
[193] Wang B, Liu D, Zhu QH, Li M, Chen H, Guo Y, et al. Rutin ameliorates kidney interstitial fibrosis in rats with obstructive nephropathy. Int Immunopharmacol. 2016;35:77–84.10.1016/j.intimp.2016.03.029Search in Google Scholar PubMed
[194] Luchini C, Capelli P, Scarpa A. Pancreatic ductal adenocarcinoma and its variants. Surg Pathol Clin. 2016;9(4):547–60. 10.1016/j.path.2016.05.003.Search in Google Scholar PubMed
[195] Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–74.10.1016/j.cell.2011.02.013Search in Google Scholar PubMed
[196] Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: An evolving paradigm. Nat Rev Cancer. 2013;13(10):714–26.10.1038/nrc3599Search in Google Scholar PubMed
[197] Wang Y, Wang Y, Ren Y, Zhang Q, Yi P, Cheng C. Metabolic modulation of immune checkpoints and novel therapeutic strategies in cancer. Semin Cancer Biol. 2022;86:542–65.10.1016/j.semcancer.2022.02.010Search in Google Scholar PubMed
[198] Turek M, Krzyczmonik M, Balczewski P. New hopes in cancer battle-a review of new molecules and treatment strategies. Med Chem. 2016;12(8):700–19.10.2174/1573406412666160502153700Search in Google Scholar PubMed
[199] Wang X, Gong M, Zhu Z, Zhang B, Han L, Li W, et al. Rutin protects the pancreas from inflammatory injury and oncogene-driven tumorigenesis by inhibiting acinar to ductal metaplasia. Eur J Pharmacol. 2025;998:177536.10.1016/j.ejphar.2025.177536Search in Google Scholar PubMed
[200] Alyami BA, Zaki M, Youns M, Amin B, Abdou R, Dawoud M, et al. Rutin inhibits hepatic and pancreatic Cancer cell proliferation by inhibiting CYP3A4 and GST. Ind J Pharm Edu Res. 2023;57(2):1–8.10.5530/ijper.57.2s.48Search in Google Scholar
[201] Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from international Myeloma working group. J Clin Oncol. 2015;33:2863–9.10.1200/JCO.2015.61.2267Search in Google Scholar PubMed PubMed Central
[202] Ikeda NE, Novak EM, Maria DA, Velosa AS, Pereira RM. Synthesis, characterization and biological evaluation of Rutin-zinc(II) flavonoid-metal complex. Chem Biol Interact. 2015;239:184–91.10.1016/j.cbi.2015.06.011Search in Google Scholar PubMed
[203] Canturk Z, Dikmen M, Artagan O, Ozarda MG, Ozturk N. Cytotoxic effects of resveratrol, rutin and rosmarinic acid on ARH-77 human (Multiple Myeloma) cell line. Nat Prod Commun. 2016;11(10):1441–4.10.1177/1934578X1601101007Search in Google Scholar
[204] Yang CY, Hsiu SL, Wen KC, Lin SP, Tsai SY, et al. Bioavailability and metabolic pharmacokinetics of rutin and quercetin in rats. J Food Drug Anal. 2005;13(3):5.10.38212/2224-6614.2517Search in Google Scholar
[205] Miyake K, Arima H, Hirayama F, Yamamoto M, Horikawa T, Sumiyoshi H, et al. Improvement of solubility and oral bioavailability of rutin by complexation with 2-hydroxypropyl-β-cyclodextrin. Pharm Dev Technol. 2000;5(3):399–407.10.1081/PDT-100100556Search in Google Scholar
[206] Mauludin R, Müller RH, Keck CM. Development of an oral rutin nanocrystal formulation. Int J Pharm. 2009;370(1–2):202–9.10.1016/j.ijpharm.2008.11.029Search in Google Scholar PubMed
[207] Kamel R, Basha M. Preparation and in vitro evaluation of rutin nanostructured liquisolid delivery system. Bull Fac Pharm Cairo Univ. 2013;51(2):261–72.10.1016/j.bfopcu.2013.08.002Search in Google Scholar
[208] Almeida JS, Lima F, Ros SD, Bulhoes LO, de Carvalho LM, Beck RC. Nanostructured systems containing rutin: in vitro antioxidant activity and photostability studies. Nanoscale Res Lett. 2010;5:1603–10.10.1007/s11671-010-9683-1Search in Google Scholar PubMed PubMed Central
[209] Wu P, Wang X, Yin M, Zhu W, Chen Z, Zhang Y, et al. ULK1 mediated autophagy-promoting effects of rutin-loaded chitosan nanoparticles contribute to the activation of NF-κB signaling besides inhibiting EMT in Hep3B hepatoma cells. Int J Nanomed. 2024;19:4465–93.10.2147/IJN.S443117Search in Google Scholar PubMed PubMed Central
[210] Pandey P, Rahman M, Bhatt PC, Beg S, Paul B, Hafeez A, et al. Implication of nano-antioxidant therapy for treatment of hepatocellular carcinoma using PLGA nanoparticles of rutin. Nanomedicine. 2018;13(8):849–70.10.2217/nnm-2017-0306Search in Google Scholar PubMed
[211] Chang C, Zhang L, Miao Y, Fang B, Yang Z. Anticancer and apoptotic-inducing effects of rutin-chitosan nanoconjugates in triple-negative breast cancer cells. J Clust Sci. 2021;32(2):331–40.10.1007/s10876-020-01792-wSearch in Google Scholar
[212] Radwan RR, Ali HE. Radiation-synthesis of chitosan/poly (acrylic acid) nanogel for improving the antitumor potential of rutin in hepatocellular carcinoma. Drug Deliv Transl Res. 2021;11:261–78.10.1007/s13346-020-00792-7Search in Google Scholar PubMed
[213] Bharathi D, Ranjithkumar R, Chandarshekar B, Bhuvaneshwari V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. Int J Biol Macromol. 2019;141:476–83.10.1016/j.ijbiomac.2019.08.235Search in Google Scholar PubMed
[214] Kunjiappan S, Panneerselvam T, Govindaraj S, Parasuraman P, Baskararaj S, Sankaranarayanan M, et al. Design, in silico modelling, and functionality theory of novel folate receptor-targeted rutin encapsulated folic acid-conjugated keratin nanoparticles for effective cancer treatment. Anti-Cancer Agents Med Chem. 2019;19(16):1966–82.10.2174/1871520619666190702145609Search in Google Scholar PubMed
[215] Ahmad M, Akhtar J, Hussain A, Arshad M, Mishra A. Development of a new rutin nanoemulsion and its application on prostate carcinoma PC3 cell line. Excli J. 2017;16:81.Search in Google Scholar
[216] Akash SR, Tabassum A, Aditee LM, Rahman A, Hossain MI, Hannan MA, et al. Pharmacological insight of rutin as a potential candidate against peptic ulcer. Biomed Pharmacother. 2024;177:116961. 10.1016/j.biopha.2024.116961.Search in Google Scholar PubMed
[217] Madkour DA, Ahmed MM, Elkirdasy AF, Orabi SH, Mousa AA. Rutin: Chemical properties, Pharmacokinetic properties and Biological activities. MJVM. 2024;4(2):26–34. 10.21608/mjvm.2024.341806.Search in Google Scholar
[218] Semwal R, Joshi SK, Semwal RB, Semwal DK. Health benefits and limitations of rutin-A natural flavonoid with high nutraceutical value. Phytochem Lett. 2021;46:119–28.10.1016/j.phytol.2021.10.006Search in Google Scholar
[219] Malekpour M, Ebrahiminezhad A, Karimi Z, Saadi MI, Berenjian A. Current strategies for rutin nano-formulation; a promising bioactive compound with increased efficacy. Bioprocess Biosyst Eng. 2025;48(6):877–98. 10.1007/s00449-025-03156-y.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

