Abstract
Cadmium induces toxicity to both flora and fauna, even when it is present in trace amounts. Electroplating, pigments, smelting, mining, alloy production, plastic, cadmium–nickel batteries, fertilizers, pesticides, paint, synthesis of dye, textile operations, and refining sectors all release cadmium into the aquatic environment. “Solvent extraction, adsorption, ion exchange, and precipitation” are a few strategies for removing cadmium. Biochar is an inexpensive and sustainable adsorbent that has proven to be an efficacious adsorbent for the recovery of Cd(ii) from water. This study discusses the toxicity of cadmium as well as some recent developments of pristine biochar and modified biochar for the elimination of cadmium (Cd) from aqueous solution.
Graphical abstract
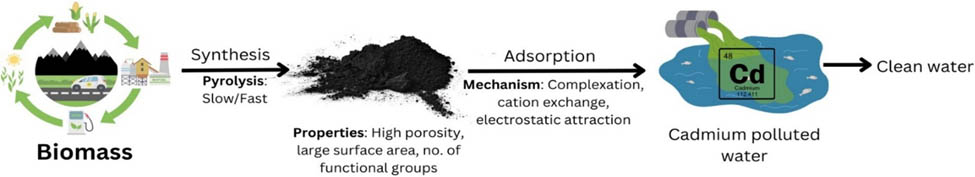
1 Introduction
Heavy metal contamination in water has become a major concern for the community, industries, and government agencies [1]. Cadmium (Cd) is released into the environment by a variety of anthropogenic and geological processes, including the burning of fossil fuels, the production of batteries, pigments, and fertilisers, the making of iron, steel, and cement, mining, and the stabilisation of PVC products. Geogenic processes include rock weathering, soil particles, volcanic eruptions, forest fires, and sea salt [2]. Because exposure to it through contaminated water can result in kidney and liver dysfunction, damage to the haematopoietic system, bone, testicles, and adrenal glands, neurological disorders, pulmonary oedema, and chronic itai-itai disease, it is categorised as a hazardous and priority pollutant. Additionally, the International Agency for Research on Cancer has classified it as a human carcinogen (group I) [3]. Consequently, a cadmium level limit of 0.005 mg/L has been set for drinking water by the Argentine Food Code and the US Environmental Protection Agency [4]. One of the difficulties humanity faces in ensuring sustainable growth and environmental safety is protecting the environment and human health. Membrane separation, ion exchange, and chemical precipitation are the top three standard methods for extracting or recovering cadmium from wastewater. Regretfully, these procedures can be costly to run and materially expensive, and they can generate a large amount of sludge [3]. Adsorption is a surface phenomenon in which mass is transferred by weak physical forces, such as van der Waals forces, or strong chemical interactions, such as covalent bonds, between a liquid (or gas) phase containing the adsorbate and a solid phase containing active adsorption sites [5]. The best adsorbent one that is inexpensive, has a large surface area, a high adsorption capacity, mechanical stability, and is easily regenerable has not yet been discovered, despite adsorption’s recent promotion as an economical, effective, and user-friendly technology. The interaction between the adsorbent and the contaminant, and consequently, the efficacy of the adsorption process, is influenced by various factors, including pH, temperature, ionic strength, contact time, initial adsorbate concentration, and adsorbent dose [6].
Activated carbon is used as an adsorbent in many industries, but its primary drawback is the high cost of manufacture and regeneration [7]. A large amount of solid waste is composed of agro-industrial waste, and because effective treatment is expensive, managing this garbage can be difficult in developing nations. These wastes gain economic value from their reuse, which thus acts as a motivator for the agro-industrial sector [8]. These wastes, which include banana peels [9], lentil husks [10], eggshell powder [11], and peanut shells [12], have been explored as Cd(ii) adsorbents since they are abundant, affordable, and readily available in the area. The affinity and selectivity of these materials for cadmium and other heavy metals are facilitated by the presence of binding groups, such as ether, carbonyl, and hydroxyl, on their surfaces.
The production of biochar, a carbonised form of biomass generated under oxygen-limited conditions and moderate temperatures (700°C), is another option for the reuse or management of agro-industrial waste [13]. Because of its high efficacy and lack of need for sophisticated activation techniques, this material has recently been suggested as an adsorbent for the removal of heavy metals [14]. Biochar’s structure differs from activated carbon in that it lacks activation and contains both carbonised and noncarbonised fractions with micro-, meso-, and macropores, a large surface area, and oxygen functional groups and minerals. These characteristics improve biochar’s adsorbent properties compared to its source biomass.
On the basis of a literature survey, it was found that only a few review articles have been published previously on the application of biochar for the removal of cadmium from water [15,16]. However, there are several reviews on the role of biochar in the removal of heavy metals from water [17,18,19,20,21,22,23,24]. The recently published review focused on the application of the modified biochar for the remediation of cadmium from the water. To the best of our knowledge, limited information is available on the critical evaluation of biochar for the adsorptive removal of Cd(ii) from water and wastewater. Additionally, the relative performance of pristine biochar versus modified/composite biochar for heavy metals (or cadmium) removal from aquatic systems was reported with limited information. Critical discussion on the sustainability of the adsorptive removal of heavy metals (or cadmium) from water and wastewater, potential regeneration methods to enhance biochar lifecycle, and management of spent biochar was not considered in earlier reviews. Further, the previous reviews did not critically discuss the toxicity caused due to the exposure of Cd(ii).
Therefore, the main objective of this review paper is to critically analyse the recent developments in the application of pristine and engineered biochar for the removal of Cd(ii) from water. The technologies used to produce biochar and the characterisation of biochar using both conventional and cutting-edge technologies for desired environmental remediation applications are presented. The influence of various factors such as water chemistry, adsorbate concentrations, the presence of competing ions, adsorbent (biochar) characteristics, and environmental parameters (e.g. temperature) on the Cd(ii) adsorption performance is discussed. Insights into potential adsorption mechanisms, sorption isotherms, kinetic behaviour, and thermodynamics of the adsorption phenomenon are provided. This review identifies key knowledge gaps and points out research directions that should be considered in the future since these recommendations would be helpful for the scale-up of the biochar-based adsorption technology for optimum decontamination of Cd(ii) contaminated water and wastewater systems.
2 Toxicity of cadmium on human health
Chronic accumulation of cadmium results in multiorgan toxicity, primarily targeting the kidney, skeleton, liver, and nervous system [25]. Among these, cadmium poisoning is especially dangerous for the neurological system. According to Viaene et al. [26], cadmium can raise the risk of peripheral neuropathy, disturbed balance, and subpar visuomotor ability. According to Li et al. [27], exposure to cadmium is linked to decreased concentration, worse cognitive function in the elderly, and worse learning outcomes in children. Additionally, neurodegenerative disease abnormalities seen in amyotrophic lateral sclerosis, Parkinson’s disease, and Alzheimer’s disease have been linked to cadmium exposure [28]. There are several ways that cadmium causes neurotoxicity [29]. The toxic influence of cadmium on the health of humans and its mechanism are shown in Figures 1(a), (b), and 2.
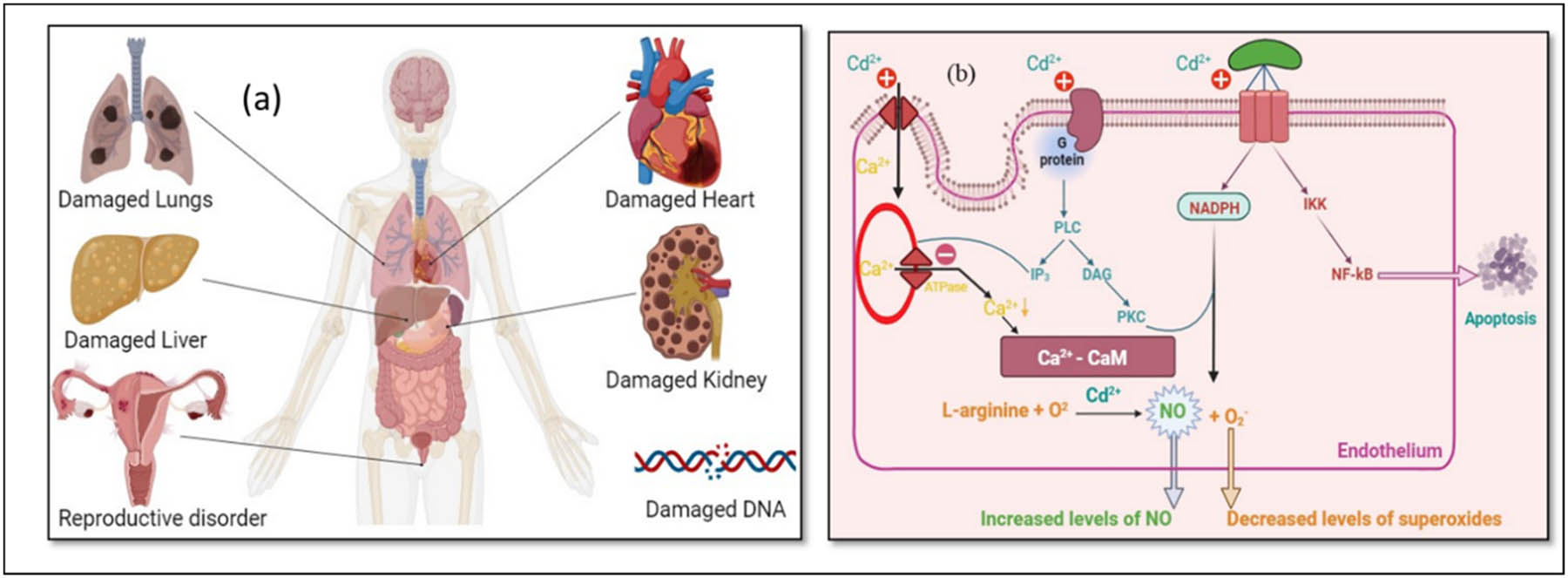
(a) and (b) Toxic effects of cadmium on human health and mechanism.
Mechanism of toxicity of cadmium in the human body.
2.1 Kidney damage
The primarily targeted organ for cadmium toxicity is the kidney. Cadmium accumulates in the proximal tubule’s SI section, where it causes oxidative injuries in the mitochondria and transports proteins that cause tubular cell death [30]. The cadmium accumulation in the tubule area of the kidney is almost 30% of the body, and the injuries in the tubule are linked to the amount of cadmium that does not bind to metallothionein (MT) [31].
The toxicity of cadmium-engendered defects in the metabolism of vitamin D in the kidney leads to venomous effects on the bones. This effect is directly related to cadmium mutilation of calcium absorption in the gut as well as disruption of collagen metabolism, both of which lead to osteoporosis and osteomalacia [32].
The degree of kidney damage caused by cadmium is contingent upon the accumulation level of Cd–MT in organs, primarily affecting the primary tubules and showing proteinuria symptoms. Long-term exposure to cadmium is indicated by detectable concentrations of cadmium in urine (Cd-U), generally estimated in accordance with creatinine, signifying kidney burden. Symptoms may affect multiple organs, with kidneys being the most impacted. In the renal cortex, cadmium critical concentration can reach up to 200 mg/kg in exposed individuals, while urinary levels range from 0.889 mol/L (10 g/g creatinine) to 1.333 mol/L (15 g/g creatinine). The biologically permissible level of Cd in urine is 0.0445 mol/L (5 g/g creatinine). The estimated prevalence of elevated urine cadmium range (>2 g/g creatinine) is 2.3% of the US population. Proteinuria may persist as a prominent symptom, followed by glycosuria, aminoaciduria, elevated urinary excretion of calcium and phosphorus as well as elevated creatinine level. Often, exposure only results in Cd-induced toxic nephropathy [33].
Significant threats are also associated with the slow excretion of cadmium from the body, which mostly occurs through sweat, saliva, faeces, and urine. Urinary cadmium excretion occurs in three stages: (1) involves Cd accumulation at the renal cortex, with urinary excretion proportional to kidney content, reflecting past exposure; (2) marked by large exposure and MT depletion, incorporates exposure from the past and the present; and (3) characterised by renal tubular damage, results in significant urinary cadmium excretion, reflecting ongoing exposure and kidney elimination [34,35,36].
2.2 Cadmium nephrotoxicity
Once Cd enters the bloodstream through absorption from the lungs and/or intestine and conjugates with the blood protein albumin, it is carried to the liver, where it has a detrimental effect on the heart, kidneys, and liver, among other prominent organs. Cd and MT mix in hepatic cells to create Cd–MT complexes, which are filtered by the kidneys after traveling there via the bloodstream [37]. The primary organs in which chromium build-up damages glomeruli and proximal tubules are the kidneys. Renal Cd is indicated by the presence of Cd in urine, which is a biomarker for renal Cd accumulation and chronic exposure [38]. Urinary creatinine of 0.67 µg/g signifies injury to the tubules, while 0.8 µg/g implies damage to the glomerulus [39].
There are numerous main pathways through which cadmium may damage the neurological system. First, entering the body by eating, cadmium eventually finds its way into the bloodstream and the central nervous system through the blood–brain barrier (BBB). Alternatively, cadmium can be inhaled and build up in the olfactory bulb. Because cadmium weakens the BBB, there is a greater chance of more cadmium entry. The transporters for calcium and zinc can then allow cadmium to enter cells and cellular compartments. Intracellular cadmium has the potential to significantly impair mitochondrial function, change neurotransmitter signalling, and seriously impair glycogen metabolism, all of which raise the risk of neurodegenerative consequences. Cadmium causes neurotoxicity in several ways [40,41].
2.3 Cancer
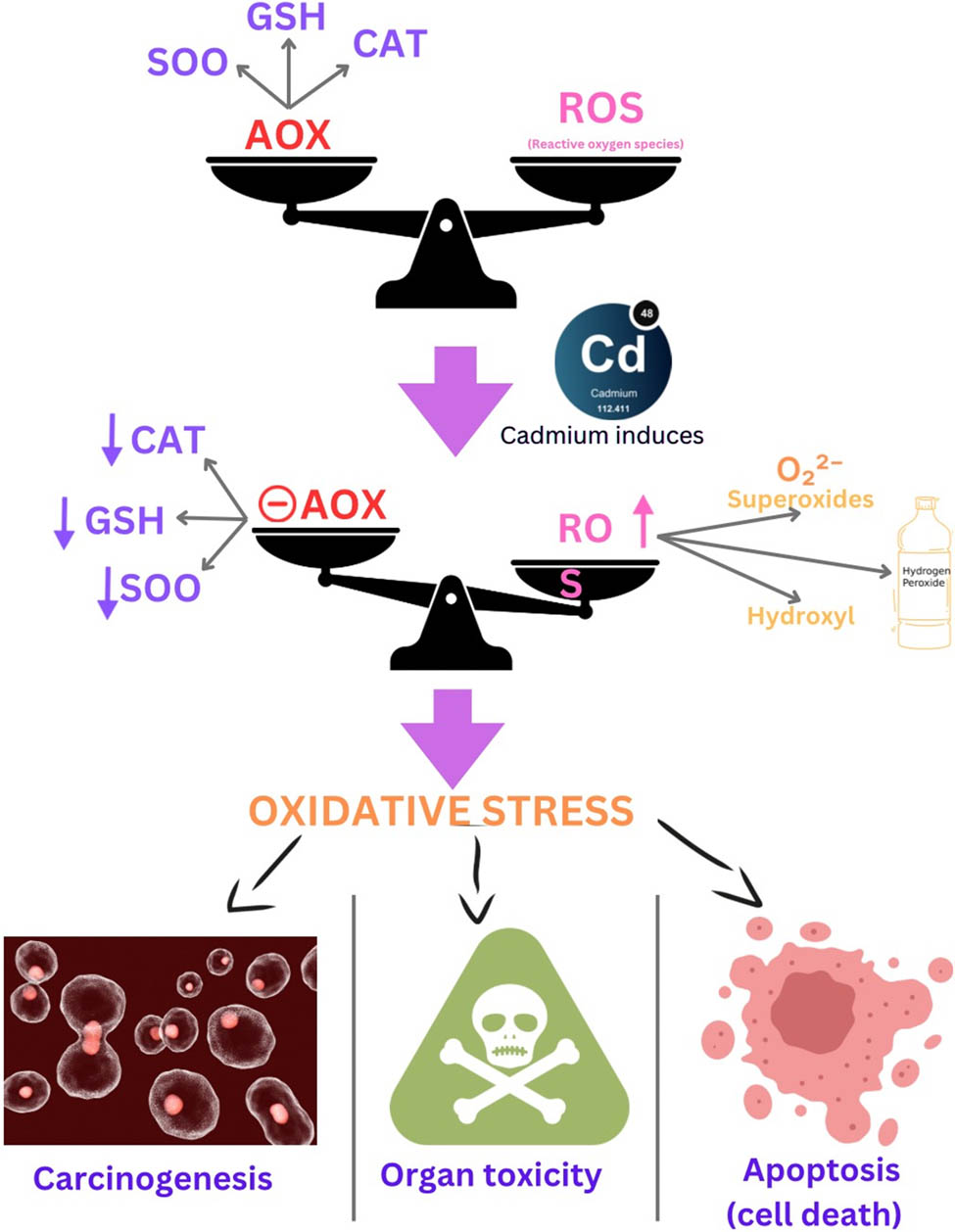
According to Chen et al. [42], mercury is recognised as a human carcinogen that can lead to cancer in a number of organs, such as the kidneys, breast, prostate, and lungs. In addition, it produces reactive oxygen species (ROS) like superoxide, nascent oxygen, hydrogen peroxide, and hydroxyl free radicals, which can be genotoxic and cytotoxic [23]. ROS causes severe damage to DNA, which leads to mutations if repairs are not made. Replication-born errors are caused by the incorrect base being incorporated and the proofreading system malfunctioning. ROS have the ability to damage proteins, and if these proteins are involved in DNA replication, replication-born mistakes may ensue. This oxidative stress caused by metals is also harmful to various human organs and tissues [43].
Several studies have determined that cadmium plays a major role in the development of a variety of cancers. Evidence connecting occupational Cd exposure to lung cancer provides significant support for this view. The lungs are one of the most vulnerable organs to cadmium exposure in humans. Furthermore, a number of studies have found a link between exposure to cadmium and the emergence of kidney and prostate cancers. There is compelling evidence of cadmium’s direct effect on the human prostatic epithelium since recent research indicates that exposure to cadmium in vitro can cause cancer transformation of prostate epithelial cells. Furthermore, two recent investigations have brought attention to the connection between occupational Cd exposure and renal cell carcinoma. A more conclusive link between cadmium exposure and the emergence of prostatic or renal cancer may be possible with the use of genomic fingerprinting, which has been used to find signatures for a variety of hepatotoxic substances. A small number of studies have linked occupational cadmium exposure to cancers of different organs, such as the stomach, liver, haematopoietic system, and bladder. Given the extremely deadly nature of pancreatic cancer, the latest studies claimed that cadmium exposure might be connected to the development of pancreatic tumours in humans is both persuasive and significant [44,45,46].
2.4 Cardiovascular disorder
It was detected from outcomes of the in vitro investigations that cadmium toxicity was involved in the disturbance in the function of endothelial and carotid intima-media thickness. Although in vivo atherosclerotic plaque formation has been supported [47], subsequent cadmium “intoxication,” endothelial ailment at the start of “cardiovascular ailment,” loss of endothelial cell structure that led to the death of cells, and some minor thrombogenicity were observed. These outcomes supported the assumption that cadmium toxicity was associated with cardiovascular disorders as well as heart attack [48]. Furthermore, different epidemiologic studies support the risk of hypertension associated with cadmium toxicity. The endothelial cell injury caused by this promotes the production of cytokines. It could enhance the rate of cardiovascular mortality.
Cardiovascular muscles get injured by cadmium before the liver and kidneys. Heart muscles need a constant supply of ATP, which is produced by the oxidative degradation of glucose in mitochondria, in order to operate properly. According to Azevedo et al. [49], cadmium stress has been shown in rats to obstruct the pyruvate-malate route of mitochondrial respiration, which results in the death of heart cells or a variety of cardiovascular diseases. As of now, no particular treatment has been suggested for Cd toxicity [50]. As a result, we must act quickly to protect and preserve our environment by reducing the levels of heavy metal contamination, or else the situation will quickly be out of control. Cadmium inhibits the formation of “endothelial nitric oxide synthase” as well as acetylcholine-induced vascular relaxation induced in hypertension [51]. It would stimulate the generation of cytokines and cause damage to endothelial. These mechanisms could induce atherogenesis, and longer-term exposure would elevate the occurrence of peripheral arterial syndrome [52]. The rate of death due to cardiovascular disorders induced by exposure to cadmium is very high.
2.5 Reproductive disorder
In rats, cadmium seems to obstruct the ovarian steroidogenic pathway. Piasek and Laskey [53] assessed the direct effects of cadmium exposure in vitro on rat ovarian steroidogenesis. Progesterone and testosterone production was the most impacted. It has been observed that ovarian progesterone production is stimulated by low dosages of cadmium and inhibited by high dosages. Low birth weight and an increase in spontaneous abortions are linked to maternal exposure to cadmium [54]. There is also some evidence that, both in vivo and in vitro, cadmium is a strong nonsteroidal oestrogen. Research conducted on rats revealed that cadmium causes increased uterine weight and accelerated mammary development [55].
2.6 Itai-Itai disorder
A number of investigations conducted in the twentieth century, such as those involving workers exposed to dust and fumes contaminated with cadmium, demonstrated a link between cadmium poisoning and bone injury [56]. Additionally, it may be demonstrated that cadmium is linked to Itai-Itai cases. Patients with this condition exhibit a variety of symptoms, including low bone mineralisation, a high fracture rate, an increased risk of osteoporosis, and excruciating pain related to the bones. In the 1940s, an epidemic of the Itai-Itai sickness was noted in the Jinzu River basin in Japan. In this instance, a study revealed that patients exhibited the typical symptoms following consumption of rice cultivated on heavily cadmium-polluted irrigation fields. Severe bone decalcification and pseudofractures indicative of osteomalacia were also detected. The bulk of the patient collection consisted of postmenopausal women, which raised criticisms for this study [57].
Honda et al. [58] established additional evidence that cadmium poisoning is causally related to bone ailments. They might explain a negative relationship between urine cadmium levels and the STIFF index, an ultrasonography technique for determining bone density [59]. Similar results were obtained in the OSCAR Study, which involved 1,021 participants from southern Sweden. Here, it was found that there was a strong negative link between poor bone mineral density and urine cadmium content, particularly in people 60 years of age and older. Furthermore, it was discovered that people exposed to cadmium had a higher incidence of forearm fractures. Participants in this research were either employees of the battery company or locals living in the town near the plant. A group of individuals who had not been exposed is included as a reference group.
3 Remediation techniques
As demonstrated in Figure 3, numerous procedures were applied to efficiently remove Cd(ii) from aquatic environments; for instance, “ion exchange, flotation, electrodialysis, co-precipitation, membrane filtration, coagulation, and adsorptions” [60].
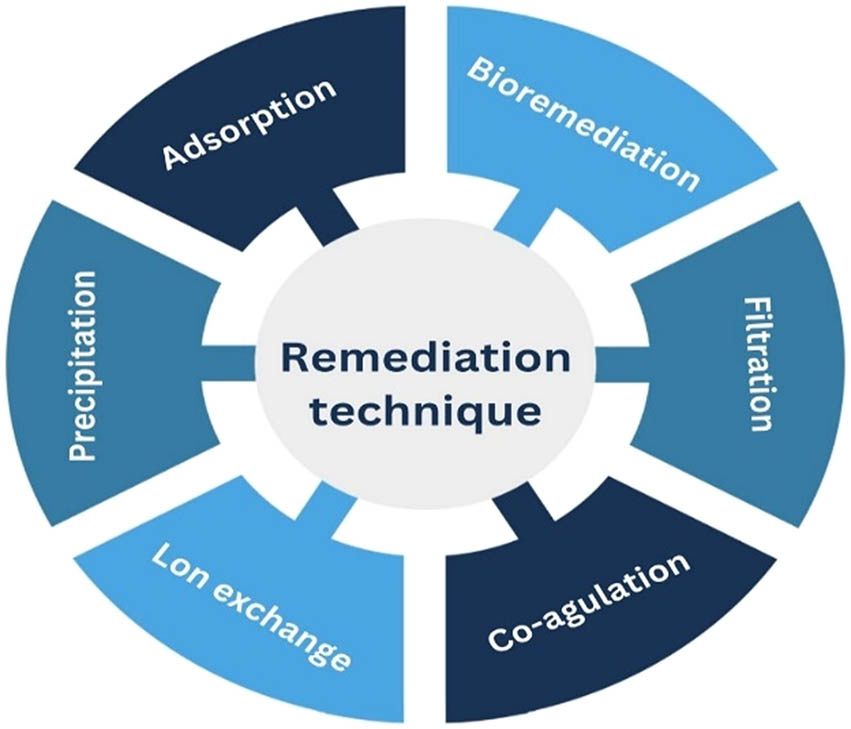
Methods utilised for the recovery of cadmium from waste water.
Heavy metal removal from wastewater has been accomplished by the widespread use of flotation. The primary flotation methods for removing metal ions from solution include foam flotation, dissolved air flotation, ion flotation, precipitation flotation, and adsorbing colloid flotation [60]. The process of creating foam in foam flotation involves using a surfactant to make a non-surface-active substance surface-active. This produces a product, which is then removed by bubbling a gas through the bulk solution. Foam flotation has various unique benefits when dealing with diluted wastewaters that contain heavy metals in parts per billion or parts per million ranges.
Ion exchange methods are widely used in heavy metal removal due to their major advantages, which include high treatment capacity, high removal efficiency, and quick kinetics, as reported by Kang et al. [61]. We can selectively remove a particular metal ion from a solution or remove all metal ions from it using ion-exchange [62]. Synthetic resins are the most favoured material among those employed in ion-exchange procedures because they are very good at eliminating heavy metal ions from solution. In the ion exchange technique, the volume of sludge produced is low due to the selective recovery of metal ions [63]. However, it has many disadvantages, like its high cost and time consumption.
Heavy metals are also removed from wastewater by coagulation and flocculation, which are followed by sedimentation and filtration. Destabilising colloids by eliminating the forces holding them apart is known as coagulation. In conventional wastewater treatment processes, a variety of coagulants are commonly employed, including aluminium, ferrous sulphate, and ferric chloride. These coagulants effectively remove wastewater particulates and impurities by neutralising particle charge and entangling impurities in the precipitates that form, which are amorphous metal hydroxides [60].
There are many advantages of “membrane filtration” compared to other methods, like high removal efficiency, being environment friendly, and saving energy, but the cost of this method is very high [64]. The precipitation technique is widely used for the recovery of the cadmium ion from the “water,” and it is carried out in a column for pilot-scale experiments. This technique is simple to use and less expensive.
Chemical precipitation is by far the most popular method for removing heavy metals from industrial wastewater; roughly 75% of electroplating facilities use precipitation treatment treating their wastewater with hydroxide, carbonate, or sulphide precipitation treatment, or various combinations of these treatments [65]. Chemicals and heavy metal ions combine during precipitation processes to create insoluble precipitates. Sedimentation or filtration can be used to remove the precipitates from the water. After treatment, the water is appropriately released or recycled [60].
Electrodialysis is a commonly used technique to remediate cadmium ions from water. This technique has several merits, including elevated efficiency, simple operation, along selective removal of heavy metals, although it also suffers from many shortcomings, like being expensive and producing highly hazardous waste. Further, bioremediation is another effective approach to recovering heavy metals from wastewater, and Cd(ii) “remediation” has been accomplished via the interaction of microorganisms with heavy metal ions. Although it is lower expenditure, environmentally friendly, requires less technology, and produces no waste, it has some limitations, such as complex operation and high specificity, which necessitates suitable environmental conditions and proper contaminated nutrition [66].
Adsorption is the most favourable of these approaches owing to its easy operation, lower cost, and ability to produce fewer toxic wastes. As the nature of most of the adsorption process is reversible, the adsorbent could be recovered only via an appropriate process of desorption, which could be utilised several times [67]. Furthermore, the desorption methods are more efficient, have a simpler operation, and are less expensive. As a result, adsorption has emerged as the superior method for extracting Cd(ii) from aquatic systems.
Adsorption is the most effective way for recovering Cd(ii)-contaminated water. Adsorption is the way of transferring a “substance from the liquid phase to the solid surface and substance linked by physical or chemical bonding” [68]. For the recovery of Cd(ii) from the aqueous phase, a variety of adsorbents are used, including activated carbon, nanoparticles, macromolecules, and biomass. The primary drawbacks of these adsorbents include their high cost, poor selectivity, and limited efficiency. Researchers are currently searching for adsorbents that are inexpensive, highly effective, and environmentally favourable. The researchers were drawn to biochar because of its strong Cd(ii) adsorption capacity. Agro-waste and industrial wastes would be used for making biochar [69].
4 Biochar
By heating biomass, “particularly waste from agricultural and industrial” in an air-restricted environment (pyrolysis) generates a solid and carbon-rich by-product, which is known as biochar. First, it was thought to be a substance that could store carbon and so help in reducing global warming [70]. Owing to its distinct surface chemistry, which includes higher aromaticity, multiple functional groups, higher surface area, and high alkalinity [71], biochar was subsequently known to have higher immobilisation abilities for heavy metals in water. The availability of biochar in water treatment has enormous potential due to its many additional advantages, such as waste reuse, low carbon footprint, and affordability [72]. According to Lehmann and Joseph, “a high carbon-content product was generated when biomass, for example, leaves, manure, and wood, had been heated in the presence of an inadequate supply of oxygen.” Biochar is defined as “a porous carbonaceous solid substance with physical and chemical features that make it an acceptable for sustained safe supply of carbon via the thermochemical operation in the exhausting environment” [73].
According to Lehmann and Joseph [70], biochar differs from charcoal in the way it is utilised. Biochar may be used for controlling environmental pollution, and charcoal is used as a source of energy and fuel. Biochar and hydrochar are strongly associated with each other, but hydrochar differs in the way of production by the hydrothermal process [74].
4.1 Derivation of biochar
Biochar is characterised by physical and chemical parameters, as shown in Figure 4. It is observed that the alteration in the physical characteristics of the biochar is clearly seen when biochar is in a pelleted or granular arrangement. The melting rate of fine biochar is greater than that of pellets. The uniform addition of pellet form of biochar is quite difficult compared to amending soil as well as biochar of improved quality. The life span of biochar having greater amounts of ash is lesser in the natural system of soil due to a greater rate of deterioration [75].
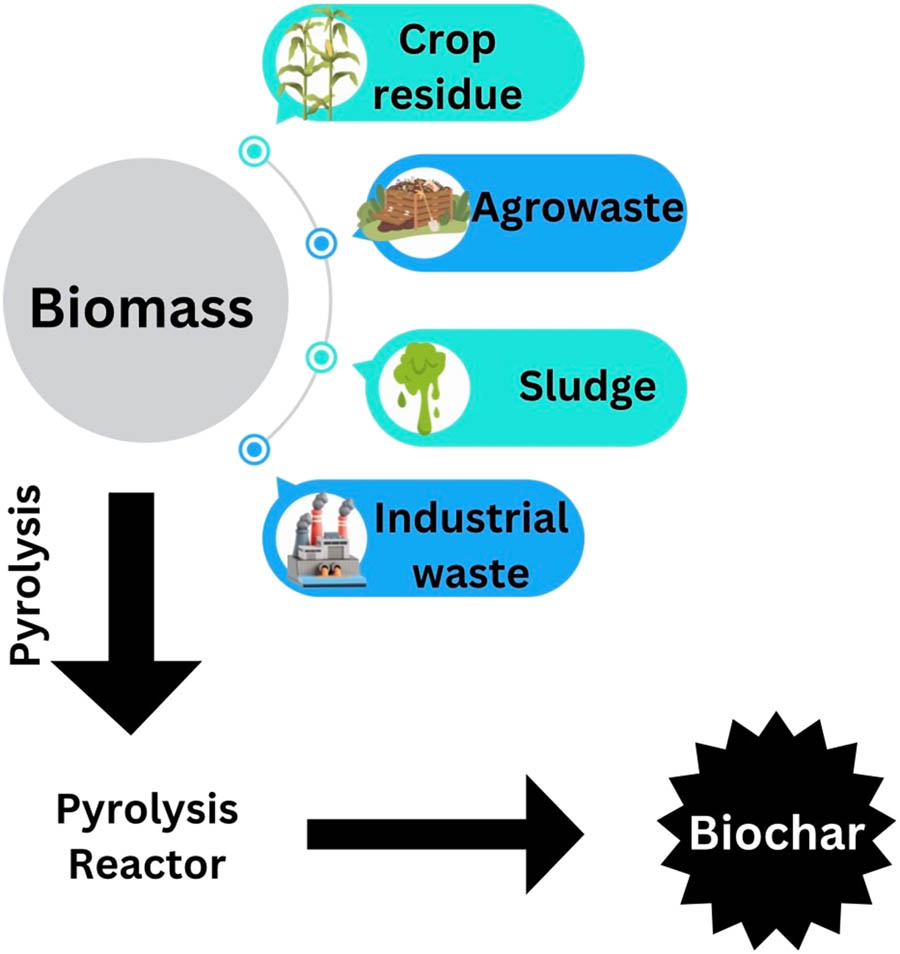
Synthesis of biochar by the pyrolysis method.
4.2 Pyrolysis
As shown in Figure 4, the most familiar procedure for producing biochar was pyrolysis. Pyrolysis is divided into two classes, slow and fast, according to the residence time and heating rate. By heating the biomass at a lower rate for a longer residual period, biochar is produced during slow pyrolysis (conventional carbonisation). Since ancient times, this process has been employed to make charcoal. Fast pyrolysis generated charcoal at a faster rate and for a minuscule period of time. The yields of biochar and bio-oil produced by these two processes are different; slower pyrolysis produces greater yields of biochar [76]. Based on process criteria like residence time, type of feedstock, rate of heating, and temperature, biochar produced by slow pyrolysis has certain physicochemical characteristics and yields [77].
4.3 Characteristics of biochar
Biochar is characterised by physical and chemical parameters. The characterisation of techniques for biochar is shown in Figure 5. It is observed that the alteration in the physical characteristics of the biochar is clearly seen when the biochar is in a pelleted or granular arrangement. The melting rate of fine biochar is higher than that of pellets. The uniform addition of the pellet form biochar is quite difficult as compared to amending soil, as well as biochar of improved quality. The life span of biochar with greater amounts of ash is shorter in the natural system of soil due to a greater rate of deterioration [75].
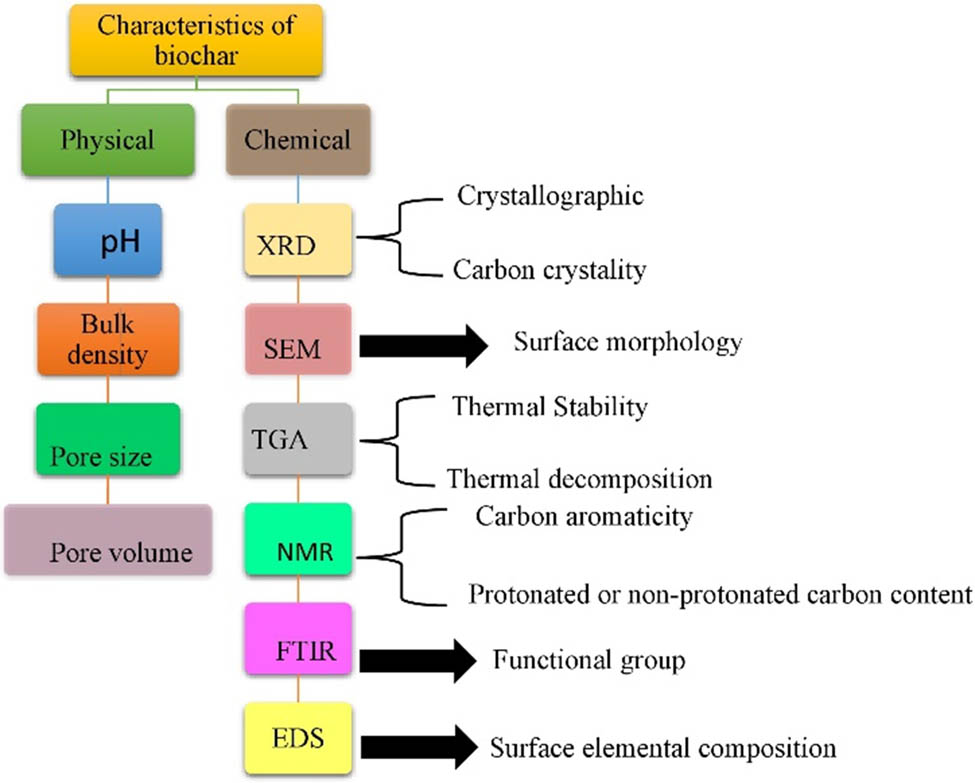
Characterisation techniques for biochar.
The extraction of biochar at a lower pyrolysis temperature gives a higher yield and has a greater amount of volatile matter compared to biochar generated at elevated temperatures. With the increase in temperature, the yield of biochar, as well as the amount of volatile matter, decreased. Additionally, the yields of the biochar and the value of volatile matter are changed with the “nature” of the feedstock. For example, non-woody biochar shows lesser alteration in the amount of volatile value than woody biochar when pyrolysis temperature increases from 400 to 800°C [78]. Woody biochar consists of a higher amount of volatile matter at lower temperatures because woody feedstock contains lignin that would hinder the pyrolytic breakdown at a pyrolysis temperature of 400°C, although it cannot hinder the process when the temperature is higher than 950°C for the assessment of the amount of ash. However, biochar obtained from the rice material has a higher amount of ash matter at almost all temperature ranges, and it might result in partial alteration in the constitution enhanced by the interaction between inorganic and organic components by the process of feedstock to synthesise biochar, which has an ash content greater than 20% [78].
Chemical characterisation of individualistic feedstock variety and, consequently, biochar obtained from the feedstock have played a vital role spatially as well as temporarily [79]. The manufacturing of biochar is frequently evaluated by means of alteration that would take place in the elemental concentration of carbon, hydrogen, nitrogen, oxygen, and sulphur and related ratios [80]. When a particle sample is carbonised, fixed carbon remains as a solid-combustible residue, and volatile components are removed. Therefore, it is utilised for the estimation of carbonaceous substances, which are produced from the solid sample. To determine the maturation and the degree of aromaticity, generally, O/C and H/C (hydrogen/carbon ratio) ratios are utilised. Yu et al. [81] employed biochar as a catalyst in the transesterification of “canola oil.” They observed that during the elemental analysis of biochar with elevation in the pyrolysis temperature, the “ratios” of H/C and O/C would decline. It was believed that the amount of pyrolysis and the degree of oxidative modification in water and soil are assessed using the elemental ratios of “O/C, O/H, and C/H” [81]. Biochar synthesised by the “pyrolysis” method is characterised by elemental analysis, zeta potential, CEC analysis, and pH measurement of colloids of biochar by various processes [69]. As apparent in earlier reports, the biochar prepared by fast pyrolysis has a fine structure because of quick modification in a fast pyrolysis reactor as well as holding a high grade of thermal mutation with lower carbon and hydrogen ratio compared to gasification or slow biochar [82]. Other characteristics are surface area [70] and pH.
Biochar with a high porosity and larger surface area would often have better sorption properties. When there is an increase in water loss during the dehydration process, the pyrolysis process forms the porous surface in biochar. The International Union of Pure and Applied Chemistry states that there are three possible types of pores in biochar: micro (<2 nm), meso (2−50 nm), and macro (>50 nm). Regardless of the pesticide molecules’ charges or polarity, the biochar with smaller pores is unable to adsorb them. Scanning electron microscopy (SEM) was used to characterise the size of the pores in biochar. Temperature has a significant impact on the creation of biochar, although surface area is the primary factor in determining biochar sorption capacity. Raw materials that have been treated or not may have different surface areas. Activated carbon has a larger surface area in the market. Lacking an activating mechanism, the biochar generated has a lower surface area and is less porous [83]. Therefore, the activation procedure is used during the manufacturing of biochar to improve the surface area and porosity of the material. The process of activation may entail both chemical and physical activation.
The economics of biochar systems and their effects on energy and climate change were estimated using life cycle assessment (LCA). In contrast, gasification technology has superior economic qualities compared to the other two technologies (pyrolysis and torrefaction), LCA. The study revealed that process and feedstock have a major impact on the characteristics and pace of production of biochar. Applications of biochar in agriculture have the advantageous effect of lowering emissions and sequestering carbon, particularly when it comes to minimising the carbon footprint of farms. Nonetheless, the intricacy of the manufactured feedstock’s composition and the discrepancy between the characteristics of biochar and the intended use are seen as possible dangers.
4.4 Environmental impacts
LCA has been widely used to evaluate these effects, with the majority of research concentrating on the possible decrease in greenhouse gas emissions, which is one of the biochar’s main benefits. Furthermore, recent studies have conducted comparisons among metal-based catalysts, activated carbon, and biochar [84,85]. Gallego-Ramírez et al. [86] discovered that the environmental effects of gasification-based production of raw and Fe-modified biochars are mostly influenced by the energy source and the gasification process.
LCA is an essential instrument for examining the possible environmental effects of goods, procedures, or activities. LCA offers thorough insights into the ecological effects of a system or product by assessing the full life cycle, from the procurement, production, and use of raw materials to disposal or recycling [87,88]. To properly conduct an LCA, comprehensive information on energy, materials, and environmental outputs, including the effects on soil, water, air, and waste, must be gathered. From the procurement of raw materials to the end of life, these data are examined using certain approaches to evaluate environmental consequences and pinpoint areas in need of improvement.
4.5 Sustainability
The parameters determining the sustainability of the biochar are technical considerations, environmental fate, economic considerations, and social considerations, as shown in Figure 6.
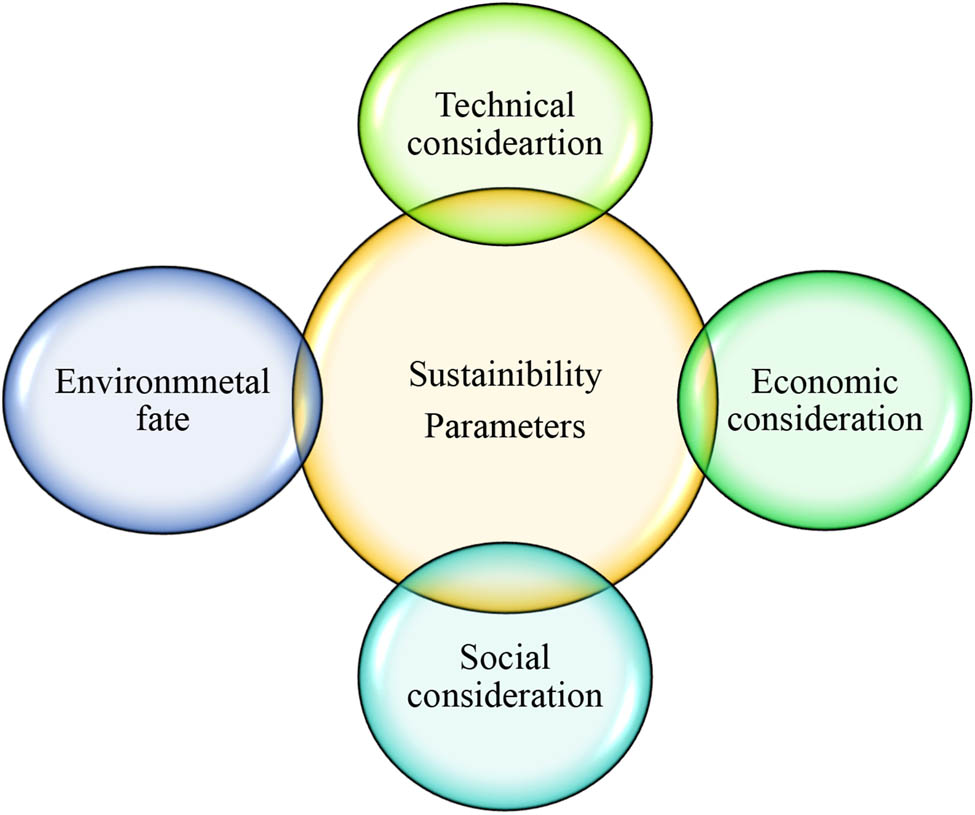
Parameters determining the sustainability of the biochar.
5 Application of different types of biochar to remove cadmium
5.1 Pristine biochar
Xu et al. [89] derived a biochar from dairy manure and rice husk to extract “Cu, Zn, Cd, and Pb” from water. All four metals are removed more efficiently by dairy manure biochar in comparison to “rice husk biochar.” In dairy manure biochar, heavy metals are adsorbed on the ionised hydroxyl-O- groups and also formed precipitate by reacting with PO4 3− or CO3 2−; on the other hand, in the case of rice husk biochar, only adsorption occurred on ionised phenolic O– groups. Therefore, this study indicated that minerals, for example, PO4 3− and CO3 2− and “functional groups,” play an amazing role in the adsorptive elimination of these “heavy metals” (Cha et al., 2016). Kim et al. (2015) have prepared a biochar from giant Miscanthus by slow pyrolysis in the temperature range of 300–600°C within a 100°C interval. At temperature >500°, the surface area and pH of the biochar increased, which led to an increase in the adsorptive potential of Cd(ii). The detailed summary of pristine biochar applied for the remediation of cadmium and the aqueous phase is presented in Table 1.
Adsorption capacities of pristine biochar for the adsorption of Cd(ii)
| Biochar as adsorbent | Pyrolysis temperature (K) | Initial concentration (mg/L) | Adsorbent dose (g/L) | pH | Adsorption capacity (mg/g) | Isotherm model | Kinetic models | Contact time (min) | Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Pine bark | 673 | 60 | 10 | 4–5 | 0.34 | Freundlich | First | 1,440 | Ion exchange | [96] |
| Oak wood | 0.37 | |||||||||
| Oak bark | 5.4 | Langmuir | First | |||||||
| Carbon F-400 | 8 | |||||||||
| MBCG | 773 | 0.3 | 20 | 5 | 84.76 | Langmuir | Pseudo- second | 4,320 | Electrostatic | [97] |
| Rape straw | 873 | 20 | 1.25 | 5.5 | 81.10 | Langmuir | Pseudo-second | 720 | Chemosorption | [98] |
| Banana peel | 873 | 200 | 0.5 | 103.22 | Freundlich | Pseudo-second | 120 | Electrostatic | [99] | |
| Langmuir | ||||||||||
| Cauliflower | 873 | 200 | 3–8 | 70.83 | Freundlich | 120 | ||||
| Langmuir | ||||||||||
| W-BC | 873 | 5 | 2 | 5 | 18.06 | Langmuir | Pseudo-second | 40 | Complexation | [100] |
| C- BC | 18.8 | |||||||||
| B- BC | 19.12 | |||||||||
| APBC | <473 | — | 0.02 | — | 23.54 | Langmuir | Pseudo-second | — | Complexation | [101] |
| OPBC | — | — | 19.04 | — | ||||||
| HMO-BC | <973 | 3 | 3–7 | 22.3 | Freundlich | Pseudo-second | 120 | Complexation | [102] | |
| BWBC | 973 | 50 | 1 | 5 | 11.63 | Langmuir | Pseudo-second | 240 | Complexation | [91] |
| LDH-Kiwi biochar | 773 | 50 | 0.02 | 6.5 | 30.96 | Langmuir | Pseudo-second | 1,440 | Complexation, isomorphic substitution, and chelation | [92] |
| CSB | 773 | 50 | 1 | 7 | 52.1 | Langmuir | Pseudo-second | The formation of chemical bonds between Cd ion and the adsorbent functional groups) | [103] | |
| PSB | 44.44 | |||||||||
| PWB | 8.07 | |||||||||
| Mg-BC | 723 | 50 | 0.01 | 5 | 178.97 | Freundlich | Intraparticle diffusion | 1,440 | Precipitation, cation π-binding, complexation, and ion exchange | [104] |
Regmi et al. [90] synthesised biochar from switchgrass by the hydrothermal carbonisation method. The authors analysed the influence of adsorbate dose, pH, and equilibrium time. The maximum adsorption capacity obtained was 34 mg/g. Further, Yakkala et al. [91] derived biochar from buffalo weed by the pyrolysis method. The adsorption data were expressed via the “pseudo-second-order kinetic model.” The maximum adsorption capacity was 11.63 mg/g for Cd(ii). The principle mechanism behind the destruction of cadmium was “ion exchange and complexation.”
Tan et al. [92] examined the adsorption of Cd(ii) on the kiwi biochar functionalised with Fe–Mg-layered double hydroxide. They studied the impact of coexisting ions, pH, adsorbate dose, and contact time in the adsorption batch experiment. It was demonstrated that the adsorption capacity of this biochar was excellent. The maximum cadmium elimination capacity reached 25.6 mg/g.
Saeed et al. [93] extracted biochar from “rice husk” and examined the adsorption behaviour for Cd(ii). It was demonstrated that yield, along with the adsorption potential of biochar for Cd(ii) removal, was influenced by the time and temperature of pyrolysis. The authors have revealed that the optimised pyrolysis temperature was 4,580°C, reaction time of 2 h, and NaOH impregnation ratio of 3, leading to biochar yields of about 34.5 and 72% for the removal of cadmium and cadmium adsorption capacity of 17.8 mg/g.
Hussain et al. [94] created magnetically modified biochar out of used coffee grounds. This adsorbent had a very high capacity for Cd(ii) adsorption (10.42 mg/g). The characterisation of the biochar and modified biochar was performed by various approaches like XRD, BET, FTIR spectroscopy, and SEM. The “pseudo-second-order kinetic model” adequately explained the adsorption experiment and demonstrated that Cd(ii) adsorption occurred via chemisorption. “Langmuir and the Freundlich model” were applied to study the adsorption isotherm, and the data were well expressed using the “Langmuir model.” Adsorption of Cd(ii) via magnetic biochar was demonstrated to occur in a monolayer over a uniform surface. The thermodynamics study also revealed that the adsorption occurred spontaneously because the negative values of ΔG ο and positive values of H revealed that the adsorption process is endothermic.
Luo et al. [95] synthesised modified biochar by using corncob as a biowaste and the modification of the biochar was conducted by treating the corncob biochar with acrylonitrile. The modified biochar was shown to have an adsorption capacity of >85.65 mg/g for cadmium.
The roughness of the surface increases with increasing pyrolysis temperatures (BC350, BC450, and BC550), as shown by the SEM image of the sample. The MCB350 had a larger pore size, surface area, and pore volume, which were responsible for the “grafting reaction” in the “cellulose” of biochar and provided more Cd(ii) adsorption sites. While the rough surface vanished in BC450 and BC550, the pore size increased after the modification. The functional groups lying over the biochar were determined by FTIR spectroscopy. MBC350 has shown a broad band at 3,430 cm−1, indicating the presence of the O–H bond, a vibration band near about 1,614 and 1,583 cm−1 confirming the presence of C═C and C═O groups (aromatic nature), and a peak at 2,249 cm−1, revealing the presence of the C≡N group. Further, the authors have revealed the impact of the pH on the adsorption capacity of the different biochars. The elimination efficiency of Cd(ii) on the different biochars quickly increased from 2 to 4 and became constant at pH 5–7. MBC350 demonstrated greater than 90% removal efficiency for Cd(ii) at pH 3 compared to the other biochars; this may be due to the presence of cyano groups in MBC350 and the alkalinity of MBC350 after modification. An isotherm study was performed to explore the mechanism, and the data well fitted the “Langmuir isotherm” model, revealing that Cd(ii) adsorption was primarily caused by the ion exchange mechanism. The experimental data are well explained by the “pseudo-second-order kinetic model.”
5.2 Modified biochar
Khan et al. [105] created a “magnetic biochar composite” to recover Cd(ii) from water. The magnetic adsorption capacity was 46.90 mg/g (pH 6), and equilibrium was reached within 360 min. The authors also used the sorption isotherm model to determine the mechanism behind the adsorption of cadmium on magnetic biochar. The experimental data are well suited to the Langmuir isotherm model, which is associated with the adsorption process as a monolayer. The thermodynamic investigation shows that the process is “endothermic,” as ΔG° for the adsorption process is positive. Because precipitation is the primary reaction, the adsorption capacity increased with an increase in pH and decreased by >6.
Burk et al. [106] examined the adsorption of “cadmium” on the chitosan-coated gasifier biochar (CGBC) obtained from pine wood. The CGBC was synthesised by activation with aqueous CH3COOH and then treated with NaOH. It showed an adsorption capacity of 85.5 mg/g. The adsorption data fitted the Langmuir isotherm model and revealed that chitosan modification increased the adsorption capacity by producing amine coordination sites, which increased Cd(ii) adsorption. “Pseudo second-order” kinetic data well explained the adsorption data.
Jing et al. [107] created a clay–biochar composite from “Alternanthera philoxeroides (APBC)” and investigated its behaviour in the recovery of Cd(ii) from the aqueous phase. The maximum adsorption capacity was 44.3 mg/g (pH 6), and equilibrium was accomplished within 24 h. The isotherm study was conducted to explore the mechanism of Cd(ii) adsorption on APBC, and the data were well interpreted using the Freundlich isotherm model. The experiment was well expressed by the “pseudo-second-order kinetic model.” The thermodynamic analysis of the adsorption revealed that the process occurred spontaneously because of the negative value of the Gibbs free energy and occurred via physisorption.
Water supplies, human health, and the production of food are all at risk due to the frequent occurrence of Cd and Zn contamination in water. For the treatment of Cd2+ and Zn2+ in solutions, MnFeB, a new absorbent biochar modified with KMnO4 and haematite, was used. MnFeB has a higher adsorption capacity for Cd2+ and Zn2+ due to its rough surface structure, large specific surface area, larger total pore volume, huge functional groups, and abundant iron oxide. The maximal Cd2+ and Zn2+ adsorption capabilities of MnFeB in single metal systems were 1.88 and 1.79 times greater than those of unmodified biochar (CSB), respectively. In the binary metal system, the maximal Cd2+ and Zn2+ adsorption capabilities of MnFeB were 2.73 and 2.65 times greater than those of CSBs. The electrostatic contact, co-precipitation, π–π interaction, complexation, and ion exchange were important adsorption processes of Cd2+ and Zn2+ by MnFeB. Thus, water contaminated with Cd and Zn can be treated using MnFeB, a new absorbent [108].
In order to remove cadmium (Cd) from the contaminated water, this study used one-step and two-step methods to create rice straw biochar (BC), chitosan-modified rice straw biochar (CT-BC), and thiol-grafted chitosan-modified biochar (TH@CT-BC). Here, a two-step combination of chitosan and thiol was added to improve the biochar’s adsorption capacity. According to our findings, TH@CT-BC had the highest Cd adsorption at pH 5.5 (261.47 mg g−1), followed by CT-BC (103.14 mg/g) and BC (29.64 mg/g). In contrast to the Freundlich and Temkin models (0.949 and 0.925, respectively), the Langmuir and pseudo-second-order kinetic models (with R 2 values of 0.997 for TH@CT-BC) are best suited to the experimental data. In river water that had been tainted with 30 mg/L Cd, TH@CT-BC was still able to remove up to 89% Cd. Surface complexation and electrostatic interactions were shown to be the main underlying mechanisms for Cd elimination by TH@CT-BC in experimental studies and data computations. Furthermore, the vast volume of rice straw that was created must be used to create new materials that will aid in turning the trash into products that can be used to clean up the environment. As a result, our study shows that rice straw may be used to produce TH@CT-BC, an efficient Cd adsorbent from aqueous systems that may be investigated as a possible option for real-world wastewater treatment applications [109].
This study used a liquid-phase reduction approach to create a nano-zero-valent iron-loaded biochar–zeolite composite material (nZVI-BCZo) utilising biochar, zeolite, and FeSO₄·7 H₂O as precursors in order to remove Cd(ii) and As(iii) from water. The adsorption properties and performance of nZVI-BCZo for Cd(ii) and As(iii) were assessed by batch adsorption tests. Cd(ii) and As(iii) were found to have maximum adsorption capacities of 28.09 and 186.99 mg/g, respectively. The removal efficiency was more affected by pH than by temperature, and nZVI-BCZo had a greater affinity for As(iii) than for Cd(ii). Kinetic studies demonstrated that the adsorption process follows a monolayer adsorption mechanism and is mainly regulated by chemical adsorption. With adsorption efficiencies of 67.78% for Cd(ii) and 53.04% for As(iii), regeneration experiments showed that nZVI-BCZo maintained a good adsorption capacity after three cycles, suggesting its potential for recurrent use in water treatment applications. According to the economic analysis, the processing cost of nZVI-BCZo is more economical. Consequently, the study showed that nZVI-BCZo is a cost-effective, reusable, and effective adsorbent for treating water that contains heavy metals [110].
The effectiveness of pure biochar (BC) and silicate-modified biochar (SiBC) in Cd2 + adsorption was examined in this work after they were made from sawdust by pyrolysis at 500°C. With a maximum Cd2+ adsorption capacity ranging from 87.19 to 179.85 mg/g, SiBC demonstrated competitive adsorption capability than BC. This is a significant increase over 4.13 mg/g recorded for BC. The batch experiment findings showed that a 1:1 ratio of Na₂SiO₃ to sawdust produced the best Cd2+ adsorption. The Freundlich isotherm model best explained the adsorption behaviour of Cd2+ on both BC and SiBC, whereas the pseudo-second-order kinetic model provided a good fit to the adsorption kinetics, indicating a combination of chemical and physical adsorption mechanisms.
Characterisation by XPS, FTIR spectroscopy, and XRD proved that complexation, ο-electron interactions, mineral precipitation, and cation exchange were involved in Cd2+ adsorption by SiBC. In addition to increasing the amount of silicate in biochar, silicate modification encouraged the development of alkaline groups (such as CO3 2− and −OH). According to quantitative studies, the main adsorption mechanisms were precipitation and cation exchange, which accounted for 33.7–55.4% and 42.1–52.9% of the total Cd2+ adsorption for BC and SiBC, respectively. This study provides insights into sustainable environmental applications and highlights the possibility of modified sawdust-derived biochar for efficient heavy metal remediation [111]. Table 2 depicts the various biochar composites used for Cd(ii) recovery [112,113,114,115,116].
Adsorption capacity of the biochar composite for Cd(ii)
| Biochar as adsorbent | Pyrolysis temperature (K) | Initial concentration. (mg/L) | Adsorbent dose (g/L) | pH | Adsorption capacity (mg/g) | Isotherm model | Kinetic models | Contact time (min) | Mechanism | References |
|---|---|---|---|---|---|---|---|---|---|---|
| APB | 573 | 30 | 0.5 | 6 | 44.3 | Freundlich model | Pseudo-second | 1,440 | Physiosorption | [112] |
| MNPs/EDB/SS a | 673 | 100 | 1 | 8 | 117.38 | Freundlich model | Pseudo-second | 120 | Electrostatic binding | [113] |
| MBC | 1,123 | 4–6 | Langmuir | Pseudo-second | [114] | |||||
| MgO–BCR | 623 | 100 | 1 | 18.1 | Langmuir | Pseudo-second | Surface complexation and electrostatic attraction | [115] | ||
| Mn/SA-BC@Nzvi | 898 | 20 | 120 | Langmuir | Pseudo-second | 45 | Surface complexation and precipitation | [116] |
5.3 Mechanism for cadmium removal
The mechanisms controlling the “adsorption” of Cd(ii) via biochar are complexation, electrostatic interaction, and cation exchange. Harvey et al. [117] studied an adsorption mechanism responsible for “Cd(ii)” on a “biochar” extracted from loblolly pine, cordgrass, and honey mesquite. Biochar derived from these plants is categorised into two categories based on their capacity: higher and lower. The biochar derived from the plants is classified into two groups: one with a lower capacity and another with a higher capacity. The cation exchange mechanism was used to adsorb Cd(ii) by high-capacity group biochar. A flow calorimetric experiment was performed to investigate the behaviour of K and Cd(ii) to exchange Na-saturated biochar. According to flow calorimetry, the shape and duration of the cadmium heat signal for Na were the same as for K exchange Na; thus, “cation exchange” is the primary mechanism for Cd(ii) adsorption. Zhang et al. [118] demonstrated that the discharge of cations like K, Ca, Na, and Mg was similar to the degree of Cd(ii) adsorbed. The FTIR analysis of biochar after and before Cd(ii) adsorption revealed a negligible shift in carboxyl peaks, indicating that complexes with carboxyl groups were formed during Cd(ii) adsorption, which was an insignificant change.
6 Future scope and conclusion
Biochar is a sustainable adsorbent for the remediation of pollutants from wastewater, soil, and air. Another specific way to increase the use of biochar for eliminating specific contaminants is by activating it. Additional study is required to discover novel activation techniques as well as the adsorption and desorption mechanisms of different contaminants. A life-cycle analysis of biochar is required to assess its benefits to the environment and economy. New methodology breakthroughs have led to advancements in biochar characterisation methodologies. To achieve optimal efficiency, biochar characteristics and activation must be optimised. New technique adoption is influenced by accessibility and economic viability. Standard characterisation techniques must be used in light of the emergence of biochar as a substitute source in order to better understand its properties.
The level of heavy metal water contamination has risen to an alarming level. Cd(ii) is a highly toxic heavy metal that is harmful to plants and animals. As a result, recovering Cd(ii) from wastewater has become an urgent need for the entire universe. To recover Cd(ii) from water, various treatment methods like “floatation, filtration, ion exchange, and adsorption” have been used. Among these procedures, adsorption is very effective for remediating Cd(ii)-contaminated aqueous solutions. Further, multiple adsorbents were applied for the adsorption of Cd(ii), but there is a need for a sustainable adsorbent for the adsorption of Cd(ii),” and in this regard, biochar is the best adsorbent. This study has critically discussed the exploitation of biochar in the recovery of Cd(ii) from the aqueous phase.
Cadmium pollution is a major problem faced worldwide, and adsorption is one of the promising processes for its removal from wastewater. Activated carbon is used by many industries, but the cost is a major drawback of this adsorbent. Therefore, the increase in the use of bioadsorbent for the removal of heavy metals could be observed. According to the literature reviewed, bioadsorbents represent a promising green technology and can potentially be applied to full-scale wastewater treatment. The main objective of this review is to study toxicity due to cadmium exposure and to bring awareness to the public about the detrimental effects of cadmium on flora and fauna.
Future research would be directed towards the use of biochar in the treatment of real-world water. Moreover, research should be focused on the effect of other ions’ existence in the aqueous solution on the “capacity” of the biochar for the elimination of cadmium.
Acknowledgments
The authors express their gratitude to Chitkara University, Punjab, for providing the required lab facilities.
-
Funding information: Authors state no funding involved.
-
Author contributions: LR: drafting of the review; JK: editing and final revision; AR: editing and supervision; CP: final compilation; HJ: editing; and SA: resources.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
References
[1] Saraeian A, Hadi A, Raji F, Ghassemi A, Johnson M. Cadmium removal from aqueous solution by low-cost native and surface modified Sorghum x drummondii (Sudangrass). J Environ Chem Eng. 2018 Apr;6(2):3322–31. Satarug, S. and Moore, M.R., Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environmental health perspectives, 2004, 112(10), 1099–1103.10.1016/j.jece.2018.05.018Search in Google Scholar
[2] Kubier A, Wilkin RT, Pichler T. Cadmium in soils and groundwater: A review. Appl Geochem. 2019 Sep;108:104388.10.1016/j.apgeochem.2019.104388Search in Google Scholar PubMed PubMed Central
[3] Kumar A, Subrahmanyam G, Mondal R, Cabral-Pinto MM, Shabnam AA, Jigyasu DK, et al. Bio-remediation approaches for alleviation of cadmium contamination in natural resources. Chemosphere. 2021 Apr;268:128855.10.1016/j.chemosphere.2020.128855Search in Google Scholar PubMed
[4] United states environmental protection agency (USEPA). National Primary Drinking Water Regulations. Washington, DC, USA: USEPA; 2019.Search in Google Scholar
[5] Vardhan KH, Kumar PS, Panda RC. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J Mol Liq. 2019 Sep;290:111197.10.1016/j.molliq.2019.111197Search in Google Scholar
[6] Pyrzynska K. Removal of cadmium from wastewaters with low-cost adsorbents. J Environ Chem Eng. 2019 Feb;7(1):102795.10.1016/j.jece.2018.11.040Search in Google Scholar
[7] Acharya J, Kumar U, Rafi PM. Removal of heavy metal ions from wastewater by chemically modified agricultural waste material as potential adsorbent-a review. Int J Curr Eng Technol. 2018 May;8(3):526–30.10.14741/ijcet/v.8.3.6Search in Google Scholar
[8] Yousef R, Qiblawey H, El-Naas MH. Adsorption as a process for produced water treatment: A review. Processes. 2020 Dec;8(12):1657.10.3390/pr8121657Search in Google Scholar
[9] Deshmukh PD, Khadse GK, Shinde VM, Labhasetwar P. Cadmium removal from aqueous solutions using dried banana peels as an adsorbent: kinetics and equilibrium modeling. J Biorem Biodegrad. 2017 Apr;8(3):395.10.4172/2155-6199.1000395Search in Google Scholar
[10] Basu M, Guha AK, Ray L. Adsorption behavior of cadmium on husk of lentil. Process Saf Environ Prot. 2017 Feb;106:11–22.10.1016/j.psep.2016.11.025Search in Google Scholar
[11] Abatan OG, Alaba PA, Oni BA, Akpojevwe K, Efeovbokhan V, Abnisa F. Performance of eggshells powder as an adsorbent for adsorption of hexavalent chromium and cadmium from wastewater. SN Appl Sci. 2020 Dec;2:1–3.10.1007/s42452-020-03866-wSearch in Google Scholar
[12] Santos VH, Do Nascimento GE, Sales DS, Dos Santos JH, Rodríguez-Díaz JM, Duarte MM. Preparation of adsorbents from agro-industrial wastes and their application in the removal of Cd2+ and Pb2+ ions from a binary mixture: Evaluation of ionic competition. Chem Eng Res Des. 2022 Aug;184:152–64.10.1016/j.cherd.2022.05.043Search in Google Scholar
[13] Mallarino-Miranda L, Venner-Gonzalez J, Tejeda-Benitez L. Heavy metal adsorption using biocarbón from agricultural and agro-industrial waste for decontamination of soils and water sources. Chem Eng Trans. 2022 Jun;92:709–14.Search in Google Scholar
[14] Liu T, Lawluvy Y, Shi Y, Ighalo JO, He Y, Zhang Y, et al. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J Environ Chem Eng. 2022 Feb;10(1):106502.10.1016/j.jece.2021.106502Search in Google Scholar
[15] Ahmed MB, Zhou JL, Ngo HH, Guo W, Chen M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour Technol. 2016 Aug;214:836–51.10.1016/j.biortech.2016.05.057Search in Google Scholar PubMed
[16] Pathania D, Srivastava AK. Advances in nanoparticles tailored lignocellulosic biochars for removal of heavy metals with special reference to cadmium (II) and chromium (VI). Environ Sustainability. 2021 Jun;4:201–14.10.1007/s42398-020-00142-wSearch in Google Scholar
[17] Inyang MI, Gao B, Yao Y, Xue Y, Zimmerman A, Mosa A, et al. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit Rev Environ Sci Technol. 2016 Feb;46(4):406–33.10.1080/10643389.2015.1096880Search in Google Scholar
[18] Li H, Dong X, da Silva EB, de Oliveira LM, Chen Y, Ma LQ. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere. 2017 Jul;178:466–78.10.1016/j.chemosphere.2017.03.072Search in Google Scholar PubMed
[19] Shakoor MB, Ali S, Rizwan M, Abbas F, Bibi I, Riaz M, et al. A review of biochar-based sorbents for separation of heavy metals from water. Int J Phytorem. 2020 Jan;22(2):111–26.10.1080/15226514.2019.1647405Search in Google Scholar PubMed
[20] Li Y, Gao L, Lu Z, Wang Y, Wang Y, Wan S. Enhanced removal of heavy metals from water by hydrous ferric oxide-modified biochar. ACS Omega. 2020 Oct;5(44):28702–11.10.1021/acsomega.0c03893Search in Google Scholar PubMed PubMed Central
[21] Lee HS, Shin HS. Competitive adsorption of heavy metals onto modified biochars: Comparison of biochar properties and modification methods. J Environ Manag. 2021 Dec;299:113651.10.1016/j.jenvman.2021.113651Search in Google Scholar PubMed
[22] Liu C, Zhang HX. Modified-biochar adsorbents (MBAs) for heavy-metal ions adsorption: A critical review. J Environ Chem Eng. 2022 Apr;10(2):107393.10.1016/j.jece.2022.107393Search in Google Scholar
[23] Biswal BK, Balasubramanian R. Use of biochar as a low-cost adsorbent for removal of heavy metals from water and wastewater: A review. J Environ Chem Eng. 2023 Oct;11(5):110986.10.1016/j.jece.2023.110986Search in Google Scholar
[24] Bayar J, Ali N, Dong Y, Ahmad U, Anjum MM, Khan GR, et al. Biochar-based adsorption for heavy metal removal in water: A sustainable and cost-effective approach. Environ Geochem Health. 2024 Nov;46(11):428.10.1007/s10653-024-02214-wSearch in Google Scholar PubMed
[25] Ma Y, Su Q, Yue C, Zou H, Zhu J, Zhao H, et al. The effect of oxidative stress-induced autophagy by cadmium exposure in kidney, liver, and bone damage, and neurotoxicity. Int J Mol Sci. 2022 Nov;23(21):13491.10.3390/ijms232113491Search in Google Scholar PubMed PubMed Central
[26] Viaene MK, Masschelein R, Leenders J, De Groof M, Swerts LJ, Roels HA. Neurobehavioural effects of occupational exposure to cadmium: a cross sectional epidemiological study. Occup Environ Med. 2000 Jan;57(1):19–27.10.1136/oem.57.1.19Search in Google Scholar PubMed PubMed Central
[27] Li H, Wang Z, Fu Z, Yan M, Wu N, Wu H, et al. Associations between blood cadmium levels and cognitive function in a cross-sectional study of US adults aged 60 years or older. BMJ open. 2018;8(4):e020533.10.1136/bmjopen-2017-020533Search in Google Scholar PubMed PubMed Central
[28] Raj K, Kaur P, Gupta GD, Singh S. Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci Lett. 2021 May;753:135873.10.1016/j.neulet.2021.135873Search in Google Scholar PubMed
[29] Witkowska D, Słowik J, Chilicka K. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules. 2021 Oct;26(19):6060.10.3390/molecules26196060Search in Google Scholar PubMed PubMed Central
[30] Fujiwara Y, Lee JY, Tokumoto M, Satoh M. Cadmium renal toxicity via apoptotic pathways. Biol Pharm Bull. 2012;35(11):1892–7. 10.1248/bpb.b212014.Search in Google Scholar PubMed
[31] Rinaldi M, Micali A, Marini H, Adamo EB, Puzzolo D, Pisani A, et al. Cadmium, organ toxicity and therapeutic approaches: a review on brain, kidney and testis damage. Curr Med Chem. 2017 Oct;24(35):3879–93. 10.2174/0929867324666170801101448.Search in Google Scholar PubMed
[32] Åkesson A, Barregard L, Bergdahl IA, Nordberg GF, Nordberg M, Skerfving S. Non-renal effects and the risk assessment of environmental cadmium exposure. Environ Health Perspect. 2014 May;122(5):431–8. 10.1289/ehp.1307110.Search in Google Scholar PubMed PubMed Central
[33] Samuel MS, Datta S, Khandge RS, Selvarajan E. A state of the art review on characterization of heavy metal binding metallothioneins proteins and their widespread applications. Sci Total Environ. 2021 Jun;775:145829.10.1016/j.scitotenv.2021.145829Search in Google Scholar
[34] Feng X, Zhou R, Jiang Q, Wang Y, Yu C. Analysis of cadmium accumulation in community adults and its correlation with low-grade albuminuria. Sci Total Environ. 2022 Aug;834:155210.10.1016/j.scitotenv.2022.155210Search in Google Scholar PubMed
[35] Siquier-Coll J, Bartolomé I, Pérez-Quintero M, Muñoz D, Robles MC, Maynar-Mariño M. Effect of exposure to high temperatures in the excretion of cadmium and lead. J Therm Biol. 2020;89:102545.10.1016/j.jtherbio.2020.102545Search in Google Scholar PubMed
[36] Yimthiang S, Vesey DA, Pouyfung P, Khamphaya T, Gobe GC, et al. Chronic kidney disease induced by cadmium and diabetes: A quantitative case-control study. Int J Mol Sci. 2023 May;24(10):9050.10.3390/ijms24109050Search in Google Scholar PubMed PubMed Central
[37] Ferraro PM, Costanzi S, Naticchia A, Sturniolo A, Gambaro G. Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999-2006. BMC Public Health. 2010 Dec;10:1–8.10.1186/1471-2458-10-304Search in Google Scholar PubMed PubMed Central
[38] Rabl C, Campos GM. The impact of bariatric surgery on nonalcoholic steatohepatitis. InSeminars in liver disease. Vol. 32, No. 1, New York, NY, USA: Thieme Medical Publishers; 2012 Feb. p. 080–91.10.1055/s-0032-1306428Search in Google Scholar PubMed
[39] Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Cienc saude coletiva. 2011 May;16(5):2587–602.10.1590/S1413-81232011000500029Search in Google Scholar
[40] Bautista CJ, Arango N, Plata C, Mitre-Aguilar IB, Trujillo J, Ramírez V. Mechanism of cadmium-induced nephrotoxicity. Toxicology. 2024 Feb;502:153726.10.1016/j.tox.2024.153726Search in Google Scholar PubMed
[41] Laib I, Ali BD, Alsalme A, Cornu D, Bechelany M, Barhoum A. Therapeutic efficacy of Helianthemum lippii extract and silver nanoparticles synthesized from the extract against cadmium-induced renal nephrotoxicity in wistar rats. Pharmaceuticals. 2024 Jul;17(8):982.10.3390/ph17080982Search in Google Scholar PubMed PubMed Central
[42] Chen D, Yao Y, Shi X, Li X, Cui W, Xu S. Cadmium exposure causes mitochondrial fission and fusion disorder in the pig hypothalamus via the PI3K/AKT pathway. Ecotoxicol Environ Saf. 2022 Sep;242:113880.10.1016/j.ecoenv.2022.113880Search in Google Scholar PubMed
[43] Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev. 2017;2017(1):8416763.10.1155/2017/8416763Search in Google Scholar PubMed PubMed Central
[44] Lee J, Shin DY, Jang Y, Han JP, Cho EM, Seo YR. Cadmium-induced carcinogenesis in respiratory organs and the prostate: Insights from three perspectives on toxicogenomic approach. J Cancer Prev. 2023 Dec;28(4):150.10.15430/JCP.2023.28.4.150Search in Google Scholar PubMed PubMed Central
[45] Tyagi A, Chandrasekaran B, Navin AK, Shukla V, Baby BV, Ankem MK, et al. Molecular interplay between NOX1 and autophagy in cadmium-induced prostate carcinogenesis. Free Radical Biol Med. 2023 Apr;199:44–55.10.1016/j.freeradbiomed.2023.02.007Search in Google Scholar PubMed
[46] Zhao L, Islam R, Wang Y, Zhang X, Liu LZ. Epigenetic regulation in chromium-, nickel-and cadmium-induced carcinogenesis. Cancers. 2022 Nov;14(23):5768.10.3390/cancers14235768Search in Google Scholar PubMed PubMed Central
[47] Ren XM, Wang GG, Xu DQ, Luo K, Liu YX, Zhong YH, et al. The protection of selenium on cadmium-induced inhibition of spermatogenesis via activating testosterone synthesis in mice. Food Chem Toxicol. 2012 Oct;50(10):3521–9. 10.1016/j.fct.2012.07.021.Search in Google Scholar PubMed
[48] Rahimzadeh MR, Rahimzadeh MR, Kazemi S, Moghadamnia AA. Cadmium toxicity and treatment: An update. Casp J Intern Med. 2017;8(3):135. 10.22088%2Fcjim.8.3.135.Search in Google Scholar
[49] Azevedo R, Gennaro D, Duro M, Pinto E, Almeida A. Further evidence on trace element imbalances in haemodialysis patients—Paired analysis of blood and serum samples. Nutrients. 2023 Apr;15(8):1912.10.3390/nu15081912Search in Google Scholar PubMed PubMed Central
[50] Zhai Q, Yin R, Yu L, Wang G, Tian F, Yu R, et al. Screening of lactic acid bacteria with potential protective effects against cadmium toxicity. Food Control. 2015 Aug;54:23–30.10.1016/j.foodcont.2015.01.037Search in Google Scholar
[51] Chowdhury S, Mazumder MJ, Al-Attas O, Husain T. Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ. 2016 Nov;569:476–88. 10.1016/j.scitotenv.2016.06.166.Search in Google Scholar PubMed
[52] Chowdhury S, Mazumder MJ, Al-Attas O, Husain T. Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ. 2016 Nov;569:476–88. 10.1016/j.scitotenv.2016.06.166.Search in Google Scholar
[53] Piasek M, Laskey JW. Acute cadmium exposure and ovarian steroidogenesis in cycling and pregnant rats. Reprod Toxicol. 1994 Nov;8(6):495–507.10.1016/0890-6238(94)90032-9Search in Google Scholar PubMed
[54] Shiverick KT, Salafia C. Cigarette smoking and pregnancy I: ovarian, uterine and placental effects. Placenta. 1999 May;20(4):265–72.10.1053/plac.1998.0377Search in Google Scholar PubMed
[55] Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003 Aug;9(8):1081–4.10.1038/nm902Search in Google Scholar PubMed
[56] Taha MM, Mahdy-Abdallah H, Shahy EM, Ibrahim KS, Elserougy S. Impact of occupational cadmium exposure on bone in sewage workers. Int J Occup Environ health. 2018 Oct;24(3-4):101–8.10.1080/10773525.2018.1518745Search in Google Scholar PubMed PubMed Central
[57] Nogawa K, Kobayashi E, Okubo Y, Suwazono Y. Environmental cadmium exposure, adverse effects and preventive measures in Japan. Biometals. 2004 Oct;17:581–7.10.1023/B:BIOM.0000045742.81440.9cSearch in Google Scholar
[58] Honda H, Matsumoto T, Sakamoto M, Kobayashi A, Chang HC, Kato M. Integration of alkyl-substituted bipyridyl benzenedithiolato platinum (II) complexes with cadmium (II) ion via selective dative bond formation. Inorg Chem. 2013 Apr;52(8):4324–34.10.1021/ic302352kSearch in Google Scholar PubMed
[59] Frery N, Nessmann C, Girard F, Lafond J, Moreau T, Blot P, et al. Environmental exposure to cadmium and human birthweight. Toxicology. 1993;79(2):109–18.10.1016/0300-483X(93)90124-BSearch in Google Scholar
[60] Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review. J Environ Manag. 2011 Mar;92(3):407–18.10.1016/j.jenvman.2010.11.011Search in Google Scholar PubMed
[61] Kang SY, Lee JU, Moon SH, Kim KW. Competitive adsorption characteristics of Co2 +, Ni2 +, and Cr3 + by IRN-77 cation exchange resin in synthesized wastewater. Chemosphere. 2004 Jul;56(2):141–7.10.1016/j.chemosphere.2004.02.004Search in Google Scholar PubMed
[62] Wang W, Fthenakis V. Kinetics study on separation of cadmium from tellurium in acidic solution media using ion-exchange resins. J Hazard Mater. 2005 Oct;125(1-3):80–8.10.1016/j.jhazmat.2005.02.013Search in Google Scholar PubMed
[63] Zewail TM, Yousef NS. Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alex Eng J. 2015 Mar;54(1):83–90. 10.1016/j.aej.2014.11.008.Search in Google Scholar
[64] Zhu Y, Wang D, Jiang L, Jin J. Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater. 2014 May;6(5):e101. 10.1038/am.2014.23.Search in Google Scholar
[65] Peters RW, Ku Y, Bhattacharyya D. Evaluation of recent treatment techniques for removal of heavy metals from industrial wastewaters. InAICHE symposium series. Vol. 81, No. 243, Citeseer; 1985 Sep. p. 165–203.Search in Google Scholar
[66] Martín-González A, Díaz S, Borniquel S, Gallego A, Gutiérrez JC. Cytotoxicity and bioaccumulation of heavy metals by ciliated protozoa isolated from urban wastewater treatment plants. Res Microbiol. 2006 Mar;157(2):108–18.10.1016/j.resmic.2005.06.005Search in Google Scholar PubMed
[67] Pan B, Pan B, Zhang W, Lv L, Zhang Q, Zheng S. Development of polymeric and polymer-based hybrid adsorbents for pollutants removal from waters. Chem Eng J. 2009 Aug;151(1–3):19–29. 10.1016/j.cej.2009.02.036.Search in Google Scholar
[68] Babel S, Kurniawan TA. Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater. 2003 Feb;97(1–3):219–43.10.1016/S0304-3894(02)00263-7Search in Google Scholar
[69] Zhao B, Nartey OD. Characterization and evaluation of biochars derived from agricultural waste biomasses from Gansu, China. In Proceedings of the World Congress on Advances in Civil, Environmental, and Materials Research. Vol. 30, No. 1, Busan, Republic of Korea: 2014 Aug.Search in Google Scholar
[70] Lehmann J, Joseph S. Biochar systems. In Biochar for environmental management. Routledge; 2012 May. p. 179–200.10.4324/9781849770552Search in Google Scholar
[71] Tomczyk A, Sokołowska Z, Boguta P. Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Bio Technol. 2020 Mar;19(1):191–215.10.1007/s11157-020-09523-3Search in Google Scholar
[72] Gupta M, Savla N, Pandit C, Pandit S, Gupta PK, Pant M, et al. Use of biomass-derived biochar in wastewater treatment and power production: A promising solution for a sustainable environment. Sci Total Environ. 2022 Jun;825:153892.10.1016/j.scitotenv.2022.153892Search in Google Scholar PubMed
[73] Shackley S, Carter S, Knowles T, Middelink E, Haefele S, Sohi S, et al. Sustainable gasification–biochar systems? A case-study of rice-husk gasification in Cambodia, Part I: Context, chemical properties, environmental and health and safety issues. Energy Policy. 2012 Mar;42:49–58. 10.1016/j.enpol.2011.11.026.Search in Google Scholar
[74] Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol. 2009 Aug;238(3):272–9.10.1016/j.taap.2009.01.011Search in Google Scholar PubMed
[75] Huisman DJ, Braadbaart F, van Wijk IM, van Os BJ. Ashes to ashes, charcoal to dust: micromorphological evidence for ash-induced disintegration of charcoal in Early Neolithic (LBK) soil features in Elsloo (The Netherlands). J Archaeol Sci. 2012 Apr;39(4):994–1004. 10.1016/j.jas.2011.11.019.Search in Google Scholar
[76] Qian K, Kumar A. Reforming of lignin-derived tars over char-based catalyst using Py-GC/MS. Fuel. 2015 Dec;162:47–54. 10.1016/j.fuel.2015.08.064.Search in Google Scholar
[77] Dhar SA, Sakib TU, Hilary LN. Effects of pyrolysis temperature on production and physicochemical characterization of biochar derived from coconut fiber biomass through slow pyrolysis process. Biomass Convers Biorefinery. 2022 Jul;12(7):2631–47.10.1007/s13399-020-01116-ySearch in Google Scholar
[78] Enders A, Hanley K, Whitman T, Joseph S, Lehmann J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour Technol. 2012 Jun;114:644–53. 10.1016/j.biortech.2012.03.022.Search in Google Scholar PubMed
[79] Zhang M, Gao B, Varnoosfaderani S, Hebard A, Yao Y, Inyang M. Preparation and characterization of a novel magnetic biochar for arsenic removal. Bioresour Technol. 2013 Feb;130:457–62. 10.1016/j.biortech.2012.11.132.Search in Google Scholar PubMed
[80] Jindo K, Mizumoto H, Sawada Y, Sanchez-Monedero MA, Sonoki T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences. 2014 Dec;11(23):6613–21.10.5194/bg-11-6613-2014Search in Google Scholar
[81] Yu JT, Dehkhoda AM, Ellis N. Development of biochar-based catalyst for transesterification of canola oil. Energy Fuels. 2011 Jan;25(1):337–44. 10.1021/ef100977d.Search in Google Scholar
[82] Yargicoglu EN, Sadasivam BY, Reddy KR, Spokas K. Physical and chemical characterization of waste wood derived biochars. Waste Manage. 2015 Feb;36:256–68. 10.1016/j.wasman.2014.10.029.Search in Google Scholar PubMed
[83] Kim KH, Kim JY, Cho TS, Choi JW. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour Technol. 2012 Aug;118:158–62.10.1016/j.biortech.2012.04.094Search in Google Scholar PubMed
[84] Carvalho J, Nascimento L, Soares M, Valério N, Ribeiro A, Faria L, et al. Life cycle assessment (LCA) of biochar production from a circular economy perspective. Processes. 2022 Dec;10(12):2684.10.3390/pr10122684Search in Google Scholar
[85] Rajabi Hamedani S, Kuppens T, Malina R, Bocci E, Colantoni A, Villarini M. Life cycle assessment and environmental valuation of biochar production: Two case studies in Belgium. Energies. 2019 Jun;12(11):2166.10.3390/en12112166Search in Google Scholar
[86] Gallego-Ramírez C, Chica E, Rubio-Clemente A. Life cycle assessment of raw and Fe-modified biochars: Contributing to circular economy. Materials. 2023 Sep;16(17):6059.10.3390/ma16176059Search in Google Scholar PubMed PubMed Central
[87] Kamali M, Appels L, Kwon EE, Aminabhavi TM, Dewil R. Biochar in water and wastewater treatment-a sustainability assessment. Chem Eng J. 2021 Sep;420:129946.10.1016/j.cej.2021.129946Search in Google Scholar
[88] Uddin MM, Wright MM. Life cycle analysis of biochar use in water treatment plants. In Sustainable biochar for water and wastewater treatment. Elsevier; 2022 Jan. p. 705–35.10.1016/B978-0-12-822225-6.00012-9Search in Google Scholar
[89] Xu X, Cao X, Zhao L, Wang H, Yu H, Gao B. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ Sci Pollut Res. 2013 Jan;20:358–68. 10.1007/s11356-012-0873-5.Search in Google Scholar PubMed
[90] Regmi P, Moscoso JL, Kumar S, Cao X, Mao J, Schafran G. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process. J Environ Manag. 2012 Oct;109:61–9. 10.1016/j.jenvman.2012.04.047.Search in Google Scholar PubMed
[91] Yakkala K, Yu MR, Roh H, Yang JK, Chang YY. Buffalo weed (Ambrosia trifida L. var. trifida) biochar for cadmium (II) and lead (II) adsorption in single and mixed system. Desalin Water Treat. 2013 Dec;51(40–42):7732–45. 10.1080/19443994.2013.792546.Search in Google Scholar
[92] Tan Y, Yin X, Wang C, Sun H, Ma A, Zhang G, et al. Sorption of cadmium onto Mg-Fe layered double hydroxide (LDH)-Kiwi branch biochar. Environ Pollut Bioavailab. 2019 Jan;31(1):189–97. 10.1080/26395940.2019.1604165.Search in Google Scholar
[93] Saeed AA, Harun NY, Sufian S, Ghaleb AA, Jagaba AH, Mohammed HG, et al. Removal of cadmium (II) from aqueous solution by rice husk waste. In 2021 International Congress of Advanced Technology and Engineering (ICOTEN). IEEE; 2021 Jul. p. 1–6. 10.1109/ICOTEN52080.2021.9493473.Search in Google Scholar
[94] Hussain N, Chantrapromma S, Suwunwong T, Phoungthong K. Cadmium (II) removal from aqueous solution using magnetic spent coffee ground biochar: Kinetics, isotherm and thermodynamic adsorption. Mater Res Express. 2020 Aug;7(8):085503.10.1088/2053-1591/abae27Search in Google Scholar
[95] Luo M, Lin H, Li B, Dong Y, He Y, Wang L. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour Technol. 2018 Jul;259:312–8.10.1016/j.biortech.2018.03.075Search in Google Scholar PubMed
[96] Mohan D, Pittman Jr CU, Bricka M, Smith F, Yancey B, Mohammad J, et al. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci. 2007 Jun;310(1):57–73. 10.1016/j.jcis.2007.01.020.Search in Google Scholar PubMed
[97] Wu J, Ren D, Zhang X, Chen Z, Zhang S, Li S, et al. The adsorption properties of biochar derived from woody plants or bamboo for cadmium in aqueous solution. Desalin Water Treat. 2019 Aug;160:268–75. 10.1007/s13762-013-0291-3.Search in Google Scholar
[98] Li B, Yang L, Wang CQ, Zhang QP, Liu QC, Li YD, et al. Adsorption of Cd (II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere. 2017 May;175:332–40. 10.1016/j.chemosphere.2017.02.061.Search in Google Scholar PubMed
[99] Ahmad Z, Gao B, Mosa A, Yu H, Yin X, Bashir A, et al. Removal of Cu (II), Cd (II) and Pb (II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J Clean Prod. 2018 Apr;180:437–49. 10.1016/j.jclepro.2018.01.133.Search in Google Scholar
[100] Wu J, Ren D, Zhang X, Chen Z, Zhang S, Li S, et al. The adsorption properties of biochar derived from woody plants or bamboo for cadmium in aqueous solution. Desalin Water Treat. 2019 Aug;160:268–75.10.5004/dwt.2019.24174Search in Google Scholar
[101] Zhu L, Tong L, Zhao N, Wang X, Yang X, Lv Y. Key factors and microscopic mechanisms controlling adsorption of cadmium by surface oxidized and aminated biochars. J Hazard Mater. 2020 Jan;382:121002. 10.1016/j.jhazmat.2019.121002.Search in Google Scholar PubMed
[102] Wan S, Wu J, Zhou S, Wang R, Gao B, He F. Enhanced lead and cadmium removal using biochar-supported hydrated manganese oxide (HMO) nanoparticles: Behavior and mechanism. Sci Total Environ. 2018 Mar;616:1298–306. 10.1016/j.scitotenv.2017.10.188.Search in Google Scholar PubMed
[103] Ding J, Chen W, Zhang Z, Qin F, Jiang J, He A, et al. Enhanced removal of cadmium from wastewater with coupled biochar and Bacillus subtilis. Water Sci Technol. 2021 May;83(9):2075–86. 10.2166/wst.2021.138.Search in Google Scholar PubMed
[104] Deng Y, Li X, Ni F, Liu Q, Yang Y, Wang M, et al. Synthesis of magnesium modified biochar for removing copper, lead and cadmium in single and binary systems from aqueous solutions: adsorption mechanism. Water. 2021 Feb;13(5):599. 10.3390/w13050599.Search in Google Scholar
[105] Khan ZH, Gao M, Qiu W, Islam MS, Song Z. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution. Chemosphere. 2020 May;246:125701. 10.1016/j.chemosphere.2019.125701.Search in Google Scholar PubMed
[106] Burk GA, Herath A, Crisler GB, Bridges D, Patel S, Pittman Jr CU, et al. Cadmium and copper removal from aqueous solutions using chitosan-coated gasifier biochar. Front Environ Sci. 2020 Nov;8:541203. 10.3389/fenvs.2020.541203.Search in Google Scholar
[107] Jing Y, Wang X, Wang D, Yang Q. Optimization and modelling of Cd (II) removal from aqueous solution with composite adsorbent prepared from Alternanthera philoxeroides biochar and bentonite by response surface methodology. Bioresources. 2020 Nov;15(4):9413.Search in Google Scholar
[108] Kang X, Geng N, Li Y, Li X, Yu J, Gao S, et al. Treatment of cadmium and zinc-contaminated water systems using modified biochar: Contaminant uptake, adsorption ability, and mechanism. Bioresour Technol. 2022 Nov;363:127817.10.1016/j.biortech.2022.127817Search in Google Scholar PubMed
[109] Hamid Y, Liu L, Haris M, Usman M, Lin Q, Chen Y, et al. Novel thiol-grafted composite of chitosan and rice straw biochar (TH@ CT-BC): A two-step fabrication for highly selective adsorption of cadmium from contaminated water. J Environ Chem Eng. 2023 Oct;11(5):110527.10.1016/j.jece.2023.110527Search in Google Scholar
[110] Wu M, Wu L, Zhang W, Zhong X, Guo R, Cui Z, et al. Efficient removal of cadmium (II) and arsenic (III) from water by nano-zero-valent iron modified biochar-zeolite composite. Ecotoxicol Environ Saf. 2025 May;296:118178.10.1016/j.ecoenv.2025.118178Search in Google Scholar PubMed
[111] Zhou Q, Huang Y, Liu L, Li Z, Xiao Y, Li Z, et al. Enhanced cadmium adsorption by silicate-modified biochar derived from sawdust: Mechanisms and performance analysis. Surf Interfaces. 2025 May;65:106430.10.1016/j.surfin.2025.106430Search in Google Scholar
[112] Jing Y, Wang X, Wang D, Yang Q. Optimization and modelling of Cd (II) removal from aqueous solution with composite adsorbent prepared from Alternanthera philoxeroides biochar and bentonite by response surface methodology. Bioresources. 2020 Nov;15(4):9413.10.15376/biores.15.4.9413-9428Search in Google Scholar
[113] Imran M, Khan ZU, Iqbal MM, Iqbal J, Shah NS, Munawar S, et al. Effect of biochar modified with magnetite nanoparticles and HNO3 for efficient removal of Cr (VI) from contaminated water: a batch and column scale study. Environ Pollut. 2020 Jun;261:114231. 10.1016/j.envpol.2020.114231.Search in Google Scholar PubMed
[114] Reddy DH, Lee SM. Magnetic biochar composite: facile synthesis, characterization, and application for heavy metal removal. Colloids Surf, A. 2014 Jul;454:96–103. 10.1016/j.colsurfa.2014.03.105.Search in Google Scholar
[115] Xiang J, Lin Q, Cheng S, Guo J, Yao X, Liu Q, et al. Enhanced adsorption of Cd (II) from aqueous solution by a magnesium oxide–rice husk biochar composite. Environ Sci Pollut Res. 2018 May;25:14032–42.10.1007/s11356-018-1594-1Search in Google Scholar PubMed
[116] Mao W, Zhang L, Zhang Y, Guan Y. Simultaneous removal of arsenite and cadmium by a manganese-crosslinking sodium alginate modified biochar and zerovalent iron composite from aqueous solutions. Environ Sci. 2022;9(1):214–28. 10.1039/D1EN00722J.Search in Google Scholar
[117] Harvey OR, Herbert BE, Rhue RD, Kuo LJ. Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol. 2011 Jul;45(13):5550–6. 10.1021/es104401h.Search in Google Scholar PubMed
[118] Zhang F, Wang X, Yin D, Peng B, Tan C, Liu Y, et al. Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes). J Environ Manag. 2015 Apr;153:68–73. 10.1016/j.jenvman.2015.01.043.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Safety assessment and modulation of hepatic CYP3A4 and UGT enzymes by Glycyrrhiza glabra aqueous extract in female Sprague–Dawley rats
- Adult-onset Still’s disease with hemophagocytic lymphohistiocytosis and minimal change disease
- Role of DZ2002 in reducing corneal graft rejection in rats by influencing Th17 activation via inhibition of the PI3K/AKT pathway and downregulation of TRAF1
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- YAP1 inhibition protects retinal vascular endothelial cells under high glucose by inhibiting autophagy
- Identification of secretory protein related biomarkers for primary biliary cholangitis based on machine learning and experimental validation
- Integrated genomic and clinical modeling for prognostic assessment of radiotherapy response in rectal neoplasms
- Stem cell-based approaches for glaucoma treatment: a mini review
- Bacteriophage titering by optical density means: KOTE assays
- Neutrophil-related signature characterizes immune landscape and predicts prognosis of esophageal squamous cell carcinoma
- Integrated bioinformatic analysis and machine learning strategies to identify new potential immune biomarkers for Alzheimer’s disease and their targeting prediction with geniposide
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- Histone modification and non-coding RNAs in skin aging: emerging therapeutic avenues
- A multiplicative behavioral model of DNA replication initiation in cells
- Biogenic gold nanoparticles synthesized from Pergularia daemia leaves: a novel approach for nasopharyngeal carcinoma therapy
- Creutzfeldt-Jakob disease mimicking Hashimoto’s encephalopathy: steroid response followed by decline
- Impact of semaphorin, Sema3F, on the gene transcription and protein expression of CREB and its binding protein CREBBP in primary hippocampal neurons of rats
- Iron overloaded M0 macrophages regulate hematopoietic stem cell proliferation and senescence via the Nrf2/Keap1/HO-1 pathway
- Revisiting the link between NADPH oxidase p22phox C242T polymorphism and ischemic stroke risk: an updated meta-analysis
- Exercise training preferentially modulates α1D-adrenergic receptor expression in peripheral arteries of hypertensive rats
- Overexpression of HE4/WFDC2 gene in mice leads to keratitis and corneal opacity
- Tumoral calcinosis complicating CKD-MBD in hemodialysis: a case report
- Mechanism of KLF4 Inhibition of epithelial-mesenchymal transition in gastric cancer cells
- Dissecting the molecular mechanisms of T cell infiltration in psoriatic lesions via cell-cell communication and regulatory network analysis
- Circadian rhythm-based prognostic features predict immune infiltration and tumor microenvironment in molecular subtypes of hepatocellular carcinoma
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Molecular identification and control studies on Coridius sp. (Hemiptera: Dinidoridae) in Al-Khamra, south of Jeddah, Saudi Arabia
- 10.1515/biol-2025-1218
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Response of hybrid grapes (Vitis spp.) to two biotic stress factors and their seedlessness status
- Metabolomic profiling reveals systemic metabolic reprogramming in Alternaria alternata under salt stress
- Effects of mixed salinity and alkali stress on photosynthetic characteristics and PEPC gene expression of vegetable soybean seedlings
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Causal effects of trace elements on congenital foot deformities and their subtypes: a Mendelian randomization study with gut microbiota mediation
- Honey meets acidity: a novel biopreservative approach against foodborne pathogens
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Fluorescent detection of sialic acid–binding lectins using functionalized quantum dots in ELISA format
- Smart tectorigenin-loaded ZnO hydrogel nanocomposites for targeted wound healing: synthesis, characterization, and biological evaluation
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Safety assessment and modulation of hepatic CYP3A4 and UGT enzymes by Glycyrrhiza glabra aqueous extract in female Sprague–Dawley rats
- Adult-onset Still’s disease with hemophagocytic lymphohistiocytosis and minimal change disease
- Role of DZ2002 in reducing corneal graft rejection in rats by influencing Th17 activation via inhibition of the PI3K/AKT pathway and downregulation of TRAF1
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- YAP1 inhibition protects retinal vascular endothelial cells under high glucose by inhibiting autophagy
- Identification of secretory protein related biomarkers for primary biliary cholangitis based on machine learning and experimental validation
- Integrated genomic and clinical modeling for prognostic assessment of radiotherapy response in rectal neoplasms
- Stem cell-based approaches for glaucoma treatment: a mini review
- Bacteriophage titering by optical density means: KOTE assays
- Neutrophil-related signature characterizes immune landscape and predicts prognosis of esophageal squamous cell carcinoma
- Integrated bioinformatic analysis and machine learning strategies to identify new potential immune biomarkers for Alzheimer’s disease and their targeting prediction with geniposide
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- Histone modification and non-coding RNAs in skin aging: emerging therapeutic avenues
- A multiplicative behavioral model of DNA replication initiation in cells
- Biogenic gold nanoparticles synthesized from Pergularia daemia leaves: a novel approach for nasopharyngeal carcinoma therapy
- Creutzfeldt-Jakob disease mimicking Hashimoto’s encephalopathy: steroid response followed by decline
- Impact of semaphorin, Sema3F, on the gene transcription and protein expression of CREB and its binding protein CREBBP in primary hippocampal neurons of rats
- Iron overloaded M0 macrophages regulate hematopoietic stem cell proliferation and senescence via the Nrf2/Keap1/HO-1 pathway
- Revisiting the link between NADPH oxidase p22phox C242T polymorphism and ischemic stroke risk: an updated meta-analysis
- Exercise training preferentially modulates α1D-adrenergic receptor expression in peripheral arteries of hypertensive rats
- Overexpression of HE4/WFDC2 gene in mice leads to keratitis and corneal opacity
- Tumoral calcinosis complicating CKD-MBD in hemodialysis: a case report
- Mechanism of KLF4 Inhibition of epithelial-mesenchymal transition in gastric cancer cells
- Dissecting the molecular mechanisms of T cell infiltration in psoriatic lesions via cell-cell communication and regulatory network analysis
- Circadian rhythm-based prognostic features predict immune infiltration and tumor microenvironment in molecular subtypes of hepatocellular carcinoma
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Molecular identification and control studies on Coridius sp. (Hemiptera: Dinidoridae) in Al-Khamra, south of Jeddah, Saudi Arabia
- 10.1515/biol-2025-1218
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Response of hybrid grapes (Vitis spp.) to two biotic stress factors and their seedlessness status
- Metabolomic profiling reveals systemic metabolic reprogramming in Alternaria alternata under salt stress
- Effects of mixed salinity and alkali stress on photosynthetic characteristics and PEPC gene expression of vegetable soybean seedlings
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Causal effects of trace elements on congenital foot deformities and their subtypes: a Mendelian randomization study with gut microbiota mediation
- Honey meets acidity: a novel biopreservative approach against foodborne pathogens
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Fluorescent detection of sialic acid–binding lectins using functionalized quantum dots in ELISA format
- Smart tectorigenin-loaded ZnO hydrogel nanocomposites for targeted wound healing: synthesis, characterization, and biological evaluation
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

