Abstract
Cardiovascular diseases (CVD) are the leading cause of death worldwide. Chemokines are a class of proteins that possess characteristics of both chemoattractants and cytokines. They play a pivotal role in CVD by regulating the recruitment of immune cells and suppressing inflammatory responses. These proteins are crucial for maintaining cardiac function and for managing myocardial cell damage under various stress conditions. Autophagy, a vital intracellular degradation mechanism, is essential for clearing misfolded proteins and damaged organelles, thus promoting cell survival during starvation and other stress conditions. A substantial body of research indicates that chemokines can modulate the development of CVD by influencing the autophagy process. Research has shown that targeting pathways such as CXCR4 and CXCL12, defective CXCL16/CXCR6, and inhibiting CX3CL1 can regulate autophagy and impact CVD. The protective role of chemokines in CVD through the modulation of autophagy may offer new perspectives for treatment.
1 Introduction
Cardiovascular diseases (CVD) continue to be a major cause of death globally, with atherosclerotic CVD accounting for the majority of cardiovascular mortality [1]. Extensive research has revealed a close connection between chemokines and CVD. Chemokines can influence CVD through regulation of the recruitment of immune cells to migrate to the site of injury, thereby suppressing inflammation, carrying out immune defense, tissue reconstruction, and adjusting pathological responses in disease states. In the global response to CVD, primary prevention strategies are also particularly important. Smoking, unhealthy eating habits, a lack of physical exercise, excessive alcohol consumption, and hypertension are considered the main preventable risk factors for CVD [2,3].
Autophagy is a critical intracellular degradation mechanism that primarily involves the clearance of proteins and damaged organelles due to aging or misfolding, thus facilitating cell survival under starvation and stress conditions [4]. Under both homeostatic and stress conditions, autophagy is crucial in cardiomyocytes and has emerged as a potential therapeutic target for preventing heart disease. Disruptions in myocardial cell autophagy, particularly its impairment, are central to the pathogenesis of heart failure, hypertrophic cardiomyopathy, dilated cardiomyopathy, cardiac aging, diabetic cardiomyopathy, and ischemia/reperfusion (I/R) injury [5,6].
The upstream signals of autophagy are transmitted mainly through mTOR-dependent and mTOR-independent pathways, including the AMPK, PI3K, Ras-MAPK, p53, and PTEN pathways, and through endoplasmic reticulum stress. Recent studies have shown that chemokines can influence CVD pathogenesis and progression by affecting autophagy, such as by blocking CXCR4 combined with its specific receptor CXCL12 through the PI3K/AKT/mTOR pathway to increase macrophage autophagy, alleviate atherosclerosis, and improve myocardial structure. Defective CXCL16/CXCR6 may protect the heart by inhibiting monocyte infiltration and IFN-γ production, thereby inducing cardiac autophagy. Inhibition of CX3CL1 can reverse the therapeutic effects of NPAS2 on myocardial injury [7,8,9]. This review summarizes recent literature on the impact of chemokines on CVD through autophagy, aiming to provide new insights into the mechanisms of chemokines in CVD and identify new drug targets for treatment.
2 Chemokines
Chemokines are a class of cytokines with a molecular weight of approximately 8–12 kDa [10] that play a role in coordinating immune responses during acute and chronic tissue damage in mammalian organs [11]. They are characterized by four conserved cysteine residues that form two disulfide bonds, with the first and third as well as the second and fourth cysteines paired [12]. A single chemokine can bind to multiple chemokine receptors, and a single chemokine receptor can have multiple ligands. To date, 47 chemokines and 20 chemokine receptors have been identified in humans [7]. Chemokines can affect monocyte survival, leukocyte activation, foam cell development, smooth muscle cell proliferation, and angiogenesis. Thus, chemokines induce endothelial dysfunction, atherosclerosis formation, and cardiac hypertrophy by mediating leukocyte chemotaxis, vascular cell migration, and proliferation. Changes in chemokines and their receptors play a significant role in the pathophysiology of CVD [10].
2.1 Classification of chemokine ligands
Chemokines are classified into CC, CXC, CX3C, and C families on the basis of the position of the cysteine residues [13]. Both the CC and CXC families contain four cysteines. The CC family includes 28 members, ranging from CCL1 to CCL28. The CXC family includes 17 members, ranging from CXCL1 to CXCL17.
2.1.1 CCL chemokine ligands
The CC family is the largest group of chemokines. CCL1 is primarily a T-cell product that is secreted by macrophages, dendritic cells, mast cells, and tumor cells. It is an effective chemoattractant for monocytes and lymphocytes and is expressed in endothelial cells, macrophages, and atherosclerotic plaques, stimulating vascular smooth muscle cell migration and endothelial cell activation [14,15]. CCL2, also known as monocyte chemoattractant protein-1, facilitates monocyte attraction, influences monocyte and lymphocyte phenotypes, and contributes to fibrous tissue accumulation, blood vessel formation, and atherosclerotic plaque development. It affects the migration of leukocytes to the arterial wall by controlling the movement of inflammatory cells and is significantly induced in the infarcted myocardium [16,17]. CCL3 is derived primarily from macrophages and induces chemotaxis of different leukocyte subsets, including monocytes/macrophages and T lymphocytes, through CCR1, CCR4, or CCR5. Activated platelets, neutrophils, and mast cells can release this chemokine, and neutrophils stimulated by tumor necrosis factor-alpha respond to CCL3, contributing to atherosclerosis overall by upregulating the integrins CD11b and CD18 [18,19,20]. CCL5 is one of the most widely used chemokines and acts on three chemokine receptors, namely, CCR1, CCR3, and CCR5. It is highly induced in many cells and is stored in large amounts in platelet alpha granules and cytoplasmic granules of memory or effector CD8+ T cells. For example, CCL5 facilitates the recruitment and penetration of immune cells into atherosclerotic plaques, thus playing a pivotal role in orchestrating the homing of T cells in atherosclerotic lesions [20,21]. CCL14 and CCL15 are present in high concentrations in serum, have an extended N-terminus, and are converted into high-affinity ligands through N-terminal cleavage. CCL23 also has a long N-terminus and is closely related to CCL15. CCL17 is expressed mainly by a subset of myeloid dendritic cells and promotes atherosclerosis by mediating T-cell chemotactic activity through its receptor CCR4 [22,23]. Increased levels of CCL19 and CCL21 in atherosclerosis can lead to changes in T-cell and macrophage responses by regulating dysfunction, thus reducing plaque stability and contributing to atherosclerosis development [24]. CCL20 is produced by cells associated with inflammation and autoimmune responses, including endothelial cells, neutrophils, NK cells, TH17 cells, and B cells. CCL20 selectively acts on its receptor CCR6, chemoattracting dendritic cells, effector/memory T lymphocytes, and naive B cells, with significantly elevated levels in atherosclerotic lesions [25,26]. CCL24 is primarily produced by activated immune cells (especially M2-type macrophages) and epithelial cells. It plays an important role in various inflammatory responses, fibrotic processes, and vascular-related biological activities. In addition, elevated levels of CCL24 are closely associated with increased risk of CVD attacks [27,28]. Most members of the CC family are involved in the development of CVD, particularly the pathogenesis of atherosclerosis. Their role in regulating immune cell recruitment, inflammation, and plaque formation highlights their potential as targets for therapeutic intervention and prevention strategies for CVD.
2.1.2 CXCL chemokine ligands
CXC chemokines are the second-largest group of chemokines and are characterized by a nonconserved single amino acid between the N-terminal cysteine residues. CXCL1 is found primarily in cells such as neutrophils, macrophages, and epithelial cells. It promotes the recruitment of neutrophils and monocytes/macrophages to the injured heart and arterial walls through its receptor CXCR2, inducing myocardial infarction, I/R injury, atherosclerosis, and hypertension [29]. CXCL1 is also involved in the fibrosis of various organs, such as cardiac and liver fibrosis. Endothelial cells release CXCL1 when induced by oxLDL, and high levels of CXCL1 can lead to leukocyte recruitment and migration to the carotid bifurcation, accelerating the progression of atherosclerosis [30]. CXCL4 is a chemokine that is stored in large amounts in platelets; during injury and atherosclerosis, it affects the function of vascular smooth muscle cells by regulating proliferation, migration, gene expression, and cytokine release through vascular wall transport. CXCL4L1 is a nonallelic variant of CXCL4 [31]. CXCL4, CXCL4L1, CXCL7, and CCRL5 comprise a group of chemokines that can be highly concentrated in platelet alpha granules and rapidly released upon platelet activation, thus playing important roles as inflammatory mediators of vascular injury, with CXCL4 being one of the oldest members of the chemokine superfamily [32,33]. CXCL8, named neutrophil-activating factor, monocyte-derived neutrophil-activating peptide, and monocyte-derived neutrophil chemotactic factor, is produced by various cell types. CXCL10, an inducer of smooth muscle cell migration, also known as interferon-gamma-induced protein 10, increases vascular wall endothelial permeability and is highly induced in endothelial cells, fibroblasts, keratinocytes, monocytes, T lymphocytes, and macrophages by interferon [10,34]. CXCL8 and CXCL10 increase their expression in atherosclerotic lesions by mediating chemotaxis and mitogenic effects on neutrophils, T cells, and vascular smooth muscle cells [32]. CXCL12, also known as stromal cell-derived factor 1α, plays a key role in the migration and differentiation of leukocytes, hematopoietic stem cells, and progenitor cells, and has an important function in the repair process of ischemic tissue. CXCL12 derived from endothelial cells affects the progression of atherosclerosis, and the source of its release has a crucial impact on its role in vascular inflammation [32,35]. CXCL16 belongs to the α-chemokine subfamily and contains a mucin stalk, transmembrane, and cytoplasmic domains. It exists in two forms, membrane-bound and soluble, and is expressed on activated T cells and natural killer T cells. Soluble CXCL16 has a proatherosclerotic effect [36,37]. The majority of CXC family members influence CVD onset and progression, particularly myocardial infarction, I/R injury, atherosclerosis, and hypertension, and hold potential as targets for CVD treatment and prevention. They are also implicated in fibrotic processes affecting various organs, including cardiac fibrosis and liver fibrosis. They hold potential value for the treatment and prevention of CVD; however, specific therapeutic and preventive approaches require further research and support from clinical observations.
2.1.3 CX3CL chemokine ligands
CX3CL1 is the only known membrane-bound chemokine with both chemoattractant and adhesive functions [38]. CX3CL1 is expressed on activated endothelial cells, smooth muscle cells, macrophages, and platelets and is involved in the development of inflammatory pathologies such as atherosclerosis. It mediates chemotaxis through the CX3CR1 receptor and can act as a medium for smooth muscle cell migration [10]. On the surfaces of endothelial and epithelial cells, membrane-bound CX3CL1 is involved mainly in adhesion and plays a role in capturing CX3CR1-positive neutrophils, whereas the soluble form of CX3CL1 acts primarily as a chemokine, affecting the chemotaxis of monocytes, natural killer cells, dendritic cells, and T cells [20,38].
2.2 Classification of chemokine receptors
Chemokine receptors are class A G protein-coupled receptors (GPCRs) associated with heterotrimeric Gai class G proteins. The primary chemokine ligands are divided into four subfamilies, namely, CCR, CXCR, CX3CR, and XCR, with 10 CC chemokine receptors, 6 CXC chemokine receptors, 1 CX3C chemokine receptor, and 1 XC chemokine receptor [39]. Most chemokine receptors can interact with many different ligands within the same class, and ligands and receptors may also form heterodimers.
2.2.1 CC chemokine receptors
CCR1 is expressed on monocytes, neutrophils, and T cells, and is involved in various inflammatory responses, including ischemia-reperfusion injury and immune reactions. It binds several chemokine ligands, including CCL5, CCL3, and CCL4, and its role in several inflammatory diseases, such as ischemia-reperfusion injury, is well characterized [39,40]. CCR2, a GPCR, mediates the recruitment of monocytes and is an effective promoter during the inflammatory phase of neutrophil recruitment. The CCL2-CCR2 axis, formed by the binding of CCR2 with CCL2, is associated with the progression of various diseases, such as atherosclerosis, diabetes, and cancer. Therefore, the CCL2-CCR2 axis is considered a potential therapeutic target for these diseases [41,42]. CCR5 is expressed mainly on activated T cells and monocytes/macrophages and mediates the activity of the chemokines CCL3, CCL4, and CCL5. It has an atherogenic phenotype, and the expression of CCR5 is significantly increased during inflammatory infiltration in various diseases, indicating that this receptor plays a role in transporting cells to inflammatory sites [21,43]. CCR1, CCR2, and CCR5 play major roles in the firm adhesion of leukocytes under ischemic conditions, helping to recruit leukocytes to reperfused tissues. CCR7 and its two ligands, CCL19 and CCL21, are crucial for the homing of lymphocytes and dendritic cells to secondary lymphoid tissues. Dendritic cells equipped with CCR7 can accumulate in the T-cell zone of the lymph node, thereby initiating the process of antigen presentation and eliciting T-cell reactions. Abnormal expression of CCR7 in dendritic cells can cause a series of inflammatory diseases due to disordered dendritic cell-derived transport [44]. CCR7 plays a role in regulating the migration of dendritic cell exosomes, thereby improving cardiac function after myocardial infarction [45]. Most CCR8+ cells are lymphocytes, especially CD4+ αβ T cells, as well as a few γδ T cells and NK cells. Disruption of the binding of the CCL1-CCR8 axis can impair the recruitment and function of vascular Treg cells, promoting atherosclerosis [14,46].
2.2.2 CXC chemokine receptors
CXCR1 and CXCR2 are expressed across multiple cell types, such as white blood cells, fibroblasts, endothelial cells, and smooth muscle cells. Their main role is to stimulate the directional movement of leukocytes, especially neutrophils, which is essential for guiding T and NK cells to areas of inflammation. They are also involved in the mobilization of neutrophils and the early formation of atherosclerotic plaques. CXCR2 may mediate leukocyte recruitment that leads to cell death during ischemia-reperfusion, as well as direct cell protection that leads to survival, with the recruitment of damaging inflammatory cells being stronger than the direct protective effect of resident cells on the myocardium [47]. CXCR3 is a transmembrane G protein-coupled receptor that selectively binds to its functional ligands CXCL9, CXCL10, and CXCL11. Predominantly expressed on immune cells, CXCR3 is expressed on activated T lymphocytes, natural killer cells, and monocytes/macrophages. Therefore, it plays a crucial role in immune processes, such as regulating the direction of effector cells to the site of infection and clearing pathogens [48]. Furthermore, it is also expressed in a variety of cell types within the cardiovascular system and is associated with several systemic diseases, such as atherosclerosis, chronic Chagas cardiomyopathy, and hypertension [49]. CXCR4 is a key immune response regulatory factor that is mediated by macrophages. The infiltration of CXCR4+ macrophages in the heart exacerbates hypertension-induced diastolic dysfunction by promoting the production of proinflammatory cytokines [50]. CXCR4 is expressed in stem cells, peripheral blood leukocytes, endothelial cells, smooth muscle cells, and myocardial cells. Together with its ligand CXCL12, it coordinates rapid vascular reconstruction of damaged, ischemic, and regenerative tissues and plays a key role in ischemic responses. CXCR7 has a tenfold greater affinity for CXCL12 than CXCR4 and is generally considered a negative regulatory factor for the expression and function of CXCL12. Upon binding of their ligand CXCL12, CXCR4 and CXCR7 play significant roles in cardiovascular development. Additionally, CXCR7 has crucial functions in the morphogenesis and remodeling of cardiac valves following myocardial infarction [51]. CXCR6 plays a proatherosclerotic role in atherosclerosis and regulates the recruitment of proinflammatory IL-17A-producing T cells to atherosclerotic aortas [36].
2.2.3 CX3C chemokine receptor
CX3CR1 is a G protein-coupled receptor with seven transmembrane domains that can guide cells to closely adhere to the fixed form of CX3CL1 under in vitro flow conditions. Vascular smooth muscle cells express the CX3CR1 receptor and play a role in the formation of platelet‒monocyte complexes [20,38] (Table 1).
Chemokines potentially associated with CVD
| Chemokine | Expression location/source | Main function |
|---|---|---|
| Classification of chemokine ligands | ||
| CCL1 | Pancreatic duct epithelium, peribiliary glands, vascular endothelial cells, CCR8+ lymphocytes | — |
| CCL2 | Monocytes, lymphocytes, platelets, neutrophils, mast cells | Regulates monocyte and lymphocyte phenotypes, promotes atherosclerosis formation [16,17] |
| CCL3 | Macrophages, activated platelets, neutrophils, mast cells | Induces chemotaxis of different leukocyte subsets through CCR1, CCR4, or CCR5, leading to atherosclerosis [18,19,20] |
| CCL5 | Platelet alpha granules, cytoplasmic granules of CD8+ T cells, various cells | Mediates immune cell recruitment and infiltration into atherosclerotic plaques [20,21] |
| CCL14 | High concentration in serum | — |
| CCL15 | High concentration in serum | — |
| CCL17 | Subset of myeloid dendritic cells | Mediates T-cell chemotactic activity through CCR4, promotes atherosclerosis [22,23] |
| CCL19 | — | Changes in T-cell and macrophage responses due to dysfunction, reducing plaque stability, contributes to atherosclerosis [24] |
| CCL20 | Endothelial cells, neutrophils, NK cells, TH17 cells, and B cells | Chemoattracts dendritic cells, effector/memory T lymphocytes, and naive B cells, elevated levels in atherosclerotic lesions [25,26] |
| CCL21 | — | Changes in T-cell and macrophage responses due to dysfunction, reducing plaque stability, contributes to atherosclerosis [24] |
| CCL24 | Activated immune cells (especially M2-type macrophages) and epithelial cells | Involved in inflammatory response, fibrosis process, and CVD attack [27,28]. |
| CXCL1 | Neutrophils, macrophages, epithelial cells | Promotes recruitment of neutrophils and monocytes/macrophages, induces myocardial infarction, atherosclerosis, and participates in organ fibrosis [29] |
| CXCL4/CXCL4L1/CXCL7 | Platelet alpha granules | Involved in vascular injury inflammation, may act as an inflammatory mediator for vascular injury, promotes atherosclerosis [32,33] |
| CXCL8 | — | Enhances chemotaxis and mitogenic effects on neutrophils, T cells, and vascular smooth muscle cells [10] |
| CXCL10 | Endothelial cells, fibroblasts, keratinocytes, monocytes, T lymphocytes, macrophages | Enhances chemotaxis and mitogenic effects on neutrophils, T cells, and vascular smooth muscle cells [10] |
| CXCL12 | Endothelial cells | Involved in the migration and differentiation of leukocytes, hematopoietic stem cells, and progenitor cells, plays a role in vascular inflammation [35] |
| CXCL16 | Activated T cells, natural killer T cells | Promotes atherosclerosis [36,37] |
| CX3CL1 | Endothelial cells, smooth muscle cells, macrophages, platelets | Participates in the development of inflammatory pathology such as atherosclerosis, mediates smooth muscle cell migration, has dual functions of chemotaxis and adhesion [20,38] |
| Chemokine receptors | ||
| CCR1 | Monocytes, neutrophils, T cells | Involved in inflammatory responses, promotes leukocyte recruitment, acts on chemokines CCL5, CCL3, CCL4 [39,40] |
| CCR2 | — | Promotes neutrophil recruitment, may be involved in inflammatory responses [41,42] |
| CCR5 | Monocytes, neutrophils, T cells | Involved in the activity of chemokines CCL3, CCL4, CCL5, atherosclerosis, regulates inflammatory infiltration [21,43] |
| CCR7 | Lymphocytes, dendritic cells | Plays a role in the homing of lymphocytes and dendritic cells to secondary lymphoid tissues, regulates the migration of dendritic cell exosomes, helps improve cardiac function after myocardial infarction [44,45] |
| CCR8 | CD4+αβ T cells, γδ T cells, NK cells | Recruits vascular Treg cells [46] |
| CXCR1, CXCR2 | Leukocytes, fibroblasts, endothelial cells, smooth muscle cells | Participates in the mobilization of neutrophils and the early formation of atherosclerosis [47] |
| CXCR3 | Immune cells, cardiovascular system cells | Regulates effector cell direction to infection sites during immune processes, associated with systemic diseases such as atherosclerosis, chronic Chagas cardiomyopathy, and hypertension [48,49] |
| CXCR4 | Stem cells, peripheral blood leukocytes, endothelial cells, smooth muscle cells, myocardial cells | Coordinates rapid vascular reconstruction of damaged, ischemic, and regenerative tissues, plays a key role in ischemic responses [51] |
| CXCR6 | — | Plays a pro-atherosclerotic role, regulates the production of pro-inflammatory IL-17A and T cell recruitment to atherosclerotic aortas [36] |
| CXCR7 | — | Plays an important role in cardiovascular development, involved in the morphogenesis and remodeling of cardiac valves after myocardial infarction [51] |
| CX3CR1 | Vascular smooth muscle cells, platelet-monocyte complexes | Guides cells to closely adhere to CX3CL1 under ex vivo flow conditions, involved in the formation of platelet-monocyte complexes [20,38] |
3 Autophagy
3.1 Definition of autophagy
The term “autophagy” was coined by Christian de Duve, who discovered lysosomes. Autophagy is an essential intracellular degradation mechanism whose primary purpose is to eliminate proteins and damaged organelles caused by misfolding, thereby promoting cell survival under conditions of starvation and other stresses [4]. This mechanism is currently unique to eukaryotic organisms. Autophagy activity is relatively low under physiological conditions, is used to maintain homeostasis within the cell, and is activated to ensure normal functioning under complex and variable pathological conditions. Autophagy can take several forms, such as macroautophagy, microautophagy, and chaperone-mediated autophagy, with macroautophagy being the most extensively studied [52] and henceforth, referred to in this review simply as autophagy.
3.2 Autophagy signaling pathways
The pathways activated by autophagy include the mammalian target of rapamycin (mTOR)-dependent pathway and the mTOR-independent pathway. The mTOR-independent pathways involve various mechanisms, such as AMP-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K), protein kinase A (PKA), phosphatase and tensin homolog (PTEN), and tumor protein p53 (p53) [4].
3.2.1 mTOR-dependent pathway
mTOR exists in two distinct multiprotein complexes: mTORC1 and mTORC2. mTORC1 is composed of mTOR, RAPTOR, MLST8, PRAS40, and DEPTOR, and it is markedly sensitive to the drug rapamycin [53]. mTORC1 maintains autophagy at a basal level by binding to and phosphorylating the autophagy kinase complex ULK1/2, inhibiting the formation of autophagosomes. When mTORC1 is inactivated, it dissociates from the ULK1/2 complex, inducing autophagy, and ULK1 can activate the VPS34 complex by phosphorylating Beclin 1 to induce autophagy [54]. Although mTORC2 is less sensitive to rapamycin, its function can also be indirectly inhibited by long-term treatment with rapamycin, which contributes to the regulation of autophagy [53].
3.2.2 mTOR-independent pathways
When there is insufficient energy within the cell, AMPK is activated and stimulates autophagy. AMPK directly promotes autophagy by phosphorylating ULK1, thereby inhibiting the binding of mTORC1 and enhancing the turnover of autophagy after glucose deprivation. It can also directly promote autophagy by phosphorylating autophagy-related proteins in the mTORC1, PIK3C3/VPS34 complex, or indirectly promote autophagy by regulating the expression of downstream autophagy-related genes through transcription factors such as FOXO3, TFEB, and BRD4 [55]. The RAS/RAF/MEK/ERK signaling pathway is one of the most important pathways in the MAPK system and is involved primarily in regulating cell proliferation, differentiation, and apoptosis. This pathway begins with the RAS protein, a monomeric small GTPase encoded by the NRAS, HRAS, and KRAS genes. Upon activation by growth factors or other signaling molecules, RAS is activated to form a complex of Src homology collagen (SHC)/growth factor receptor-bound protein 2 (GRB2)/son of sevenless (SOS), which stimulates SOS to replace GTP with RAS, generating active RAS-GTP. Activated RAS recruits RAF kinase to the plasma membrane, and the activation of RAF requires specific phosphorylation and dephosphorylation processes. RAF kinase phosphorylates downstream MEK1/2 and forms a tetramer composed of RAF-MEK dimers, further promoting the activation of MEK. Ultimately, ERK1/2, the terminal kinase of the cascade reaction, is phosphorylated and activated by MEK1/2 and then translocates to the cell nucleus or remains in the cytoplasm, where it regulates a variety of substrates [56]. PI3K, a member of the phosphoinositide kinase family, possesses both PI3k activity and serine/threonine kinase activity. It is capable of phosphorylating PI to produce phosphatidylinositol 3-phosphate (PI3P). Specifically, class III PI3K (PI3KC3) can phosphorylate PI to generate PI3P, which functions downstream of the ULK complex as an important protein that regulates autophagy. PI3KC3 has two complexes: PI3KC3-C1 and PI3KC3-C2, with PI3KC3-C1 acting in the early stages of autophagy and PI3KC3-C2 acting mainly in the later stages [57]. The PTEN protein is a negative regulator of the PI3K pathway and has proautophagy activity. PKA is a negative regulator of autophagy that mainly acts on Atg1, Atg8, and Atg13 [58]. The Tp53 gene encodes the transcription factor p53, which is involved in DNA repair, aging, cell cycle control, autophagy, and apoptosis and can regulate various targeted genes to activate AMPK or inhibit mTOR, promoting autophagy [59] (Figure 1).
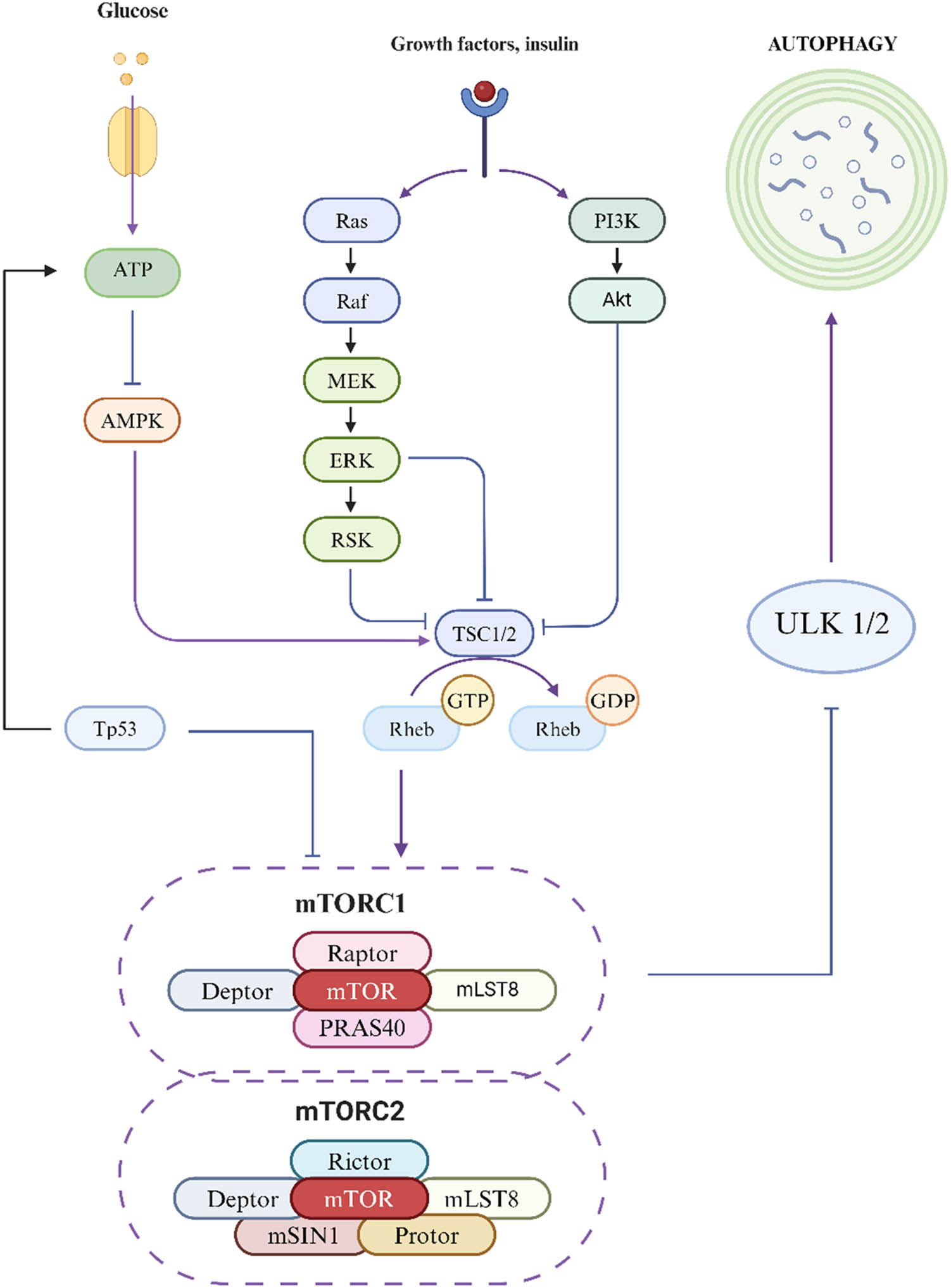
Autophagy signaling pathway diagram.
mTOR exists in two distinct multiprotein complexes: mTORC1 and mTORC2. mTORC1 is composed of mTOR, Raptor, mLST8, PRAS40, and Deptor. It is highly sensitive to rapamycin, a known inhibitor. mTORC1 inhibits autophagy by binding to and phosphorylating the autophagy kinase complex ULK1/2, maintaining autophagy at a baseline level. Inactivation of mTORC1 leads to the dissociation of the ULK 1/2 complex, triggering autophagy. mTORC2 consists of mTOR, Rictor, mLST8, mMSIN1, Protor, and Deptor. It is less sensitive to rapamycin, but its function can be indirectly inhibited by long-term treatment with the drug, thus participating in the regulation of autophagy. When cellular energy is scarce, Tp53 activates AMPK, which inhibits the binding of mTORC1 and promotes autophagy. Upon activation by growth factors or other signaling molecules, the RAS protein is first activated, promoting the translocation of RAF kinase to the cell membrane, which triggers a series of phosphorylation events, successively activating MEK and ERK. Ultimately, the ERK proteins are translocated to the cell nucleus or function within the cytoplasm. Concurrently, PI3K phosphorylates phospholipids to generate PI3P, promoting the activation of Akt, which in turn inhibits TSC1/2 and Rheb, promoting autophagy.
4 Research progress on the impact of chemokines on autophagy in CVD
Research on how chemokines affect autophagy and thereby alleviate CVD is increasing. To date, chemokines such as CXCR4/CXCL12, CXCL16/CXCR6, and CX3CL1 have been found to protect the heart by inducing autophagy when inhibited.
CXCR4 is one of the specific receptors for CXCL12, and its activation upon binding with CXCL12 plays a role in signal transduction related to inflammation, chemotaxis, survival, and proliferation, with high expression of CXCR4 being closely related to the stability of atherosclerosis [60]. Research has shown that CXCL12-CXCR4 can regulate blood lipids, activate vascular inflammatory responses, promote tumor endothelial proliferation, stimulate angiogenesis, and exacerbate insulin resistance. Autophagy plays a key role in the biological functions of vascular endothelial cells, and its regulation through the PI3K/AKT/mTOR pathway is also crucial for autophagy. Studies have shown that after the specific knockout (KO) of CXCR4, the activation of the PI3K/AKT/mTOR signaling pathway is inhibited. This leads to an increase in the expression of the autophagy-related proteins LC3 and Beclin1 and a decrease in the level of the autophagy substrate p62, indicating increased autophagy activity. Further experimental results have indicated that blocking CXCR4 can promote autophagy in macrophages through the PI3K/AKT/mTOR pathway. This mechanism can not only reduce the degree of atherosclerosis but also improve myocardial structure, thereby reducing the severity of coronary artery disease. These findings reveal the important role of the CXCL12-CXCR4 signaling pathway in CVD and provide potential targets for the development of new therapeutic strategies. By regulating this pathway, especially by promoting macrophage autophagy, new directions may be provided for the prevention and treatment of atherosclerosis and related CVD [7]. Another study showed that blocking TLR2/CXCR4 can significantly delay or perhaps even reverse atherosclerosis induced by Chlamydia pneumoniae infection [61].
CXCL16 belongs to the CXC chemokine family and exists in both membrane-bound and soluble forms, with soluble CXCL16 in the serum having a proatherosclerotic effect. CXCR6 is the specific receptor for CXCL16, and the CXCL16/CXCR6 axis plays an important role in pathological mechanisms following I/R injury in cardiac remodeling and heart failure development. IFN-γ plays a crucial role in immune regulation, as it can modulate immune defense mechanisms by activating autophagosomes. These autophagosomes are essential for inducing the death of activated T lymphocytes and macrophages. Although autophagy induced by IFN-γ may help alleviate excessive inflammatory responses during I/R injury in some cases, an increasing number of studies suggest that autophagy may actually have detrimental effects on ischemic heart disease during the reperfusion phase. In one study, a CXCR6 KO mouse model was used to induce myocardial injury through myocardial I/R surgery and to assess myocardial function and infarct size. The results showed that after 6 h of reperfusion, the serum IFN-γ level significantly increased, accompanied by an increase in the level of the autophagy-related protein Beclin1. Notably, CXCR6-deficient mice presented smaller myocardial infarct areas and better cardiac function than control mice did, indicating that CXCR6 deficiency inhibited monocyte infiltration and IFN-γ production, thereby alleviating myocardial I/R injury. The mechanism may be that I/R triggers the infiltration of monocytes into the myocardium and activates IFN-γ-dependent autophagy signaling pathways through CXCL16/CXCR6. Therefore, disrupting the CXCL16/CXCR6 signaling cascade has a cardioprotective effect on I/R injury. These findings suggest that the absence of CXCR6 may protect the heart from severe damage during reperfusion by blocking the secretion of IFN-γ and thereby inhibiting IFN-γ-dependent autophagy. This mechanism provides new insights into the role of CXCR6 in I/R injury and opens new directions for potential therapeutic strategies [8].
CX3CL1 is a unique membrane-bound chemokine with both chemoattractant and adhesive functions. The serum level of CX3CL1 is associated with CVD, such as carotid artery stenosis, unstable angina, and systolic heart failure. NPAS2 has been demonstrated to regulate the autophagy process through the CX3CL1/AKT pathway, with high expression levels of NPAS2 capable of inhibiting autophagy and apoptosis in cardiomyocytes. Studies have indicated that AKT/mTOR-mediated autophagy is closely associated with myocardial I/R injury. However, other studies have shown that Beclin1-mediated autophagy is also involved in the process of myocardial I/R injury. The inhibition of Beclin1 expression in cardiomyocytes can reduce the degree of autophagy and apoptosis induced by I/R. The primary mechanism involves the upregulation of Beclin1 induced by reactive oxygen species (ROS), leading to defective autophagosome maturation and increased cell death. Experimental results have revealed that NPAS2 can promote the transcription of CX3CL1. In H9C2 cells, NPAS2 binds to the promoter region of CX3CL1. NPAS2 overexpression promotes the expression of CX3CL1, whereas NPAS2 suppression reduces the expression of CX3CL1. CX3CL1 has been confirmed as a direct transcriptional target of NPAS2 in both breast cancer and cardiomyocytes. NPAS2 overexpression increases the level of CX3CL1, thereby promoting the phosphorylation of AKT and mTOR and participating in the regulation of autophagy. Notably, the inhibition of CX3CL1 can reverse the protective effect of NPAS2 on myocardial injury, indicating that the NPAS2/CX3CL1 pathway plays a significant role in myocardial protection. This discovery provides a new potential therapeutic target for myocardial protection [9] (Figure 2) (Table 2).

Chemokines regulate autophagy to improve cardiac injury.
Chemokines that protect the heart by modulating autophagy
| Chemokine ligand | Chemokine receptor | Autophagy regulation role | Related research/mechanism of action |
|---|---|---|---|
| CXCR4 | CXCL12 | Regulates autophagy through the PI3K/AKT/mTOR pathway, alleviating atherosclerosis, and improving myocardial structure. | Blocking CXCR4 can promote autophagy in macrophages, alleviate atherosclerosis, improve myocardial structure, reduce the severity of coronary artery disease, and reverse atherosclerosis induced by Chlamydia pneumoniae infection by blocking TLR2/CXCR4 [7]. |
| CXCL16 | CXCR6 | Has a pro-atherosclerotic effect; CXCR6 deficiency inhibits monocyte infiltration and IFN-γ production, reducing myocardial I/R injury | CXCR6 deficiency reduces monocyte infiltration and IFN-γ production, mitigating myocardial I/R injury, possibly through CXCL16/CXCR6-dependent paracrine IFN-γ inducing cardiac autophagy [8]. |
| CX3CL1 | CX3CR1 | Has dual functions of chemotaxis and adhesion; NPAS2/CX3CL1 is involved in the regulation of autophagy. | NPAS2 promotes the transcription of CX3CL1, and the NPAS2/CX3CL1 axis is involved in the regulation of autophagy by promoting the phosphorylation of AKT and mTOR, playing a role in myocardial protection [9]. |
Blockade of CXCR4 can inhibit the activation of CXCR4 after binding with CXCL12 and regulate autophagy through the PI3K/AKT/mTOR pathway to improve and reduce atherosclerosis and improve cardiac structure. CXCR6 deficiency can inhibit monocyte infiltration and the production of IFN-γ, thereby reducing myocardial I/R injury. NPAS2 promotes the transcription of CX3CL1 and is involved in the regulation of autophagy to protect the heart.
5 Conclusion and expectations
Autophagy is crucial for maintaining cellular homeostasis and plays a vital role in cardiomyocytes under both basal and stress conditions, helping to sustain cardiac function. Chemokines, a group of inflammatory mediators rapidly released upon platelet activation, also play a significant role in the development of CVD such as atherosclerosis. Therefore, modulating chemokines to regulate autophagy may have an impact on CVD. For example, the binding of CXCR4 to CXCL12 promotes macrophage autophagy through the PI3K/AKT/mTOR pathway, which alleviates atherosclerosis and improves myocardial structure. Additionally, blocking TLR2/CXCR4 can significantly delay or even nearly reverse atherosclerosis induced by Chlamydia pneumoniae infection. Soluble CXCL16 in the serum, when bound to CXCR6, plays an important role in pathological mechanisms following I/R injury in cardiac remodeling and heart failure development. CXCR6 deficiency activates the IFN-γ-dependent autophagy signaling pathway, inhibiting monocyte infiltration and mitigating myocardial I/R injury. CX3CL1, with its chemotactic and adhesive functions, is upregulated by NPAS2, which promotes the phosphorylation of AKT and mTOR and is involved in the regulation of autophagy. The NPAS2/CX3CL1 axis plays a role in myocardial protection, and the therapeutic effect of NPAS2 on myocardial injury can be reversed by inhibiting CX3CL1.
Research has shown the feasibility of using chemokines to regulate autophagy for cardiac protection. Therefore, in addition to the known chemokines CXCR4, CXCL12, CXCR6, and CXCL16, it is also important to investigate the regulatory mechanisms and mechanisms of action of other chemokines associated with CVD to verify whether they can also prevent or treat CVD by regulating autophagy. Furthermore, on the basis of the interaction between chemokines and the autophagy pathway, new molecular targets can be identified, and targeted drugs can be developed to provide more effective treatment plans. For example, drugs that can specifically block or activate chemokine receptors to regulate autophagy and improve the prognosis of CVD patients may be designed. By detecting the levels of chemokines and their receptors in the blood, early diagnostic tools can be developed to predict the risk of CVD, achieve early intervention and treatment, and improve patient survival rates and quality of life. Large-scale clinical trials should be conducted to ensure the actual effectiveness and safety of the regulation of autophagy by chemokines in the treatment of CVD and to determine the best treatment plans and medication strategies.
In summary, in-depth research on the interaction between chemokines and autophagy in CVD will not only help reveal the mechanisms of diseases but also provide new ideas and strategies for the diagnosis and treatment of CVD. It is also important to explore whether chemokines can contribute to the primary prevention of CVD. Such research will promote the innovation and application of related treatment methods and ultimately improve the health and well-being of patients.
Acknowledgments
Thanks to Li Dongping for granting the usage license for the illustrations created with Biorender.
-
Funding information: This work was supported by the Foundation of Hunan Province Health Commission Research Project (202104010694), Hunan Province Natural Science Foundation Joint Project with Health (2023JJ60369) and Natural Science Foundation of Hunan Province (2022JJ80028).
-
Author contributions: Jingfeng Ma wrote the manuscript. Juling Feng provided resources. Xiaotong Tan, Zhihui Li, Shuo Tan, and Boling Li consulted relevant literature. Lei Zhao designed the framework of the manuscript and participated in the revision. All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Goldsborough E, Osuji N, Blaha MJ. Assessment of cardiovascular disease risk. Endocrinol Metab Clin North Am. 2022;51:483–509. 10.1016/j.ecl.2022.02.005.Search in Google Scholar PubMed
[2] Tekkeşin Aİ, Hayıroğlu Mİ, Çinier G, Özdemir YS, İnan D, Yüksel G, et al. Lifestyle intervention using mobile technology and smart devices in patients with high cardiovascular risk: A pragmatic randomised clinical trial. Atherosclerosis. 2021;319:21–7. 10.1016/j.atherosclerosis.2020.12.020.Search in Google Scholar PubMed
[3] Hayıroğlu Mİ, Çınar T, Çinier G, Karakaya A, Yıldırım M, Güney BÇ, et al. The effect of 1-year mean step count on the change in the atherosclerotic cardiovascular disease risk calculation in patients with high cardiovascular risk: a sub-study of the LIGHT randomized clinical trial. Kardiol Pol. 2021;79:1140–2. 10.33963/KP.a2021.0108.Search in Google Scholar PubMed
[4] Cao W, Li J, Yang K, Cao D. An overview of autophagy: Mechanism, regulation and research progress. Bull Cancer (Paris). 2021;108:304–22. 10.1016/j.bulcan.2020.11.004.Search in Google Scholar PubMed
[5] Wu X, Liu Z, Yu X, Xu S, Luo J. Autophagy and cardiac diseases: Therapeutic potential of natural products. Med Res Rev. 2021;41:314–41. 10.1002/med.21733.Search in Google Scholar PubMed
[6] Klionsky DJ, Petroni G, Amaravadi RK, Baehrecke EH, Ballabio A, Boya P, et al. Autophagy in major human diseases. EMBO J. 2021;40:e108863. 10.15252/embj.2021108863.Search in Google Scholar PubMed PubMed Central
[7] Li F, Peng J, Lu Y, Zhou M, Liang J, Le C, et al. Blockade of CXCR4 promotes macrophage autophagy through the PI3K/AKT/mTOR pathway to alleviate coronary heart disease. Int J Cardiol. 2023;392:131303. 10.1016/j.ijcard.2023.131303.Search in Google Scholar PubMed
[8] Zhao G, Wang S, Wang Z, Sun A, Yang X, Qiu Z, et al. CXCR6 deficiency ameliorated myocardial ischemia/reperfusion injury by inhibiting infiltration of monocytes and IFN-γ-dependent autophagy. Int J Cardiol. 2013;168:853–62. 10.1016/j.ijcard.2012.10.022.Search in Google Scholar PubMed
[9] Huang J, Qing W, Pan Y. NPAS2 ameliorates myocardial ischaemia/reperfusion injury in rats via CX3CL1 pathways and regulating autophagy. Aging. 2021;13:20569–84. 10.18632/aging.203445.Search in Google Scholar PubMed PubMed Central
[10] Hedayati-Moghadam M, Hosseinian S, Paseban M, Shabgah AG, Gholizadeh J, Jamialahmadi T, et al. The role of chemokines in cardiovascular diseases and the therapeutic effect of curcumin on CXCL8 and CCL2 as pathological chemokines in atherosclerosis. In: Sahebkar A, Sathyapalan T, editors. Natural products and human diseases. Vol. 1328, Cham: Springer International Publishing; 2021. p. 155–70. 10.1007/978-3-030-73234-9_11.Search in Google Scholar PubMed
[11] Hammerich L, Tacke F. Hepatic inflammatory responses in liver fibrosis. Nat Rev Gastroenterol Hepatol. 2023;20:633–46. 10.1038/s41575-023-00807-x.Search in Google Scholar PubMed
[12] Cecchinato V, Martini V, Pirani E, Ghovehoud E, Uguccioni M. The chemokine landscape: One system multiple shades. Front Immunol. 2023;14:1176619. 10.3389/fimmu.2023.1176619.Search in Google Scholar PubMed PubMed Central
[13] Bogacka J, Pawlik K, Ciapała K, Ciechanowska A, Mika J. CC chemokine receptor 4 (CCR4) as a possible new target for therapy. Int J Mol Sci. 2022;23:15638. 10.3390/ijms232415638.Search in Google Scholar PubMed PubMed Central
[14] Vila-Caballer M, González-Granado JM, Zorita V, Abu Nabah YN, Silvestre-Roig C, Del Monte-Monge A, et al. Disruption of the CCL1-CCR8 axis inhibits vascular treg recruitment and function and promotes atherosclerosis in mice. J Mol Cell Cardiol. 2019;132:154–63. 10.1016/j.yjmcc.2019.05.009.Search in Google Scholar PubMed
[15] Liu S, Liu C, Lv X, Cui B, Yan J, Li Y, et al. The chemokine CCL1 triggers an AMFR-SPRY1 pathway that promotes differentiation of lung fibroblasts into myofibroblasts and drives pulmonary fibrosis. Immunity. 2021;54:2042–2056.e8. 10.1016/j.immuni.2021.06.008.Search in Google Scholar PubMed
[16] Lin Z, Shi J-L, Chen M, Zheng Z-M, Li M-Q, Shao J. CCL2: An important cytokine in normal and pathological pregnancies: A review. Front Immunol. 2023;13:1053457. 10.3389/fimmu.2022.1053457.Search in Google Scholar PubMed PubMed Central
[17] Zhang H, Yang K, Chen F, Liu Q, Ni J, Cao W, et al. Role of the CCL2-CCR2 axis in cardiovascular disease: Pathogenesis and clinical implications. Front Immunol. 2022;13:975367. 10.3389/fimmu.2022.975367.Search in Google Scholar PubMed PubMed Central
[18] Yang Y-L, Li X-F, Song B, Wu S, Wu Y-Y, Huang C, et al. The role of CCL3 in the pathogenesis of rheumatoid arthritis. Rheumatol Ther. 2023;10:793–808. 10.1007/s40744-023-00554-0.Search in Google Scholar PubMed PubMed Central
[19] Komissarov A, Potashnikova D, Freeman ML, Gontarenko V, Maytesyan D, Lederman MM, et al. Driving T cells to human atherosclerotic plaques: CCL3/CCR5 and CX3CL1/CX3CR1 migration axes. Eur J Immunol. 2021;51:1857–9. 10.1002/eji.202049004.Search in Google Scholar PubMed
[20] Potashnikova D, Maryukhnich E, Vorobyeva D, Rusakovich G, Komissarov A, Tvorogova A, et al. Cytokine profiling of plasma and atherosclerotic plaques in patients undergoing carotid endarterectomy. Int J Mol Sci. 2024;25:1030. 10.3390/ijms25021030.Search in Google Scholar PubMed PubMed Central
[21] Costa RM, Cerqueira DM, Bruder-Nascimento A, Alves JV, Awata WMC, Singh S, et al. Role of the CCL5 and its receptor, CCR5, in the genesis of aldosterone-induced hypertension, vascular dysfunction, and end-organ damage. Hypertension. 2024;81:776–86. 10.1161/HYPERTENSIONAHA.123.21888.Search in Google Scholar PubMed PubMed Central
[22] Lupancu TJ, Eivazitork M, Hamilton JA, Achuthan AA, Lee KM. CCL17/TARC in autoimmunity and inflammation—not just a T-cell chemokine. Immunol Cell Biol. 2023;101:600–9. 10.1111/imcb.12644.Search in Google Scholar PubMed
[23] Feng G, Bajpai G, Ma P, Koenig A, Bredemeyer A, Lokshina I, et al. CCL17 aggravates myocardial injury by suppressing recruitment of regulatory T cells. Circulation. 2022;145:765–82. 10.1161/CIRCULATIONAHA.121.055888.Search in Google Scholar PubMed PubMed Central
[24] Katra P, Hennings V, Nilsson J, Engström G, Engelbertsen D, Bengtsson E, et al. Plasma levels of CCL21, but not CCL19, independently predict future coronary events in a prospective population-based cohort. Atherosclerosis. 2023;366:1–7. 10.1016/j.atherosclerosis.2023.01.004.Search in Google Scholar PubMed
[25] Won EJ, Kim H-J, Lee YJ, Kim M-J, Lee H-I, Jang HH, et al. CCL20 inhibition for treating inflammation in ankylosing spondylitis. Rheumatology. 2023;62:4000–5. 10.1093/rheumatology/kead268.Search in Google Scholar PubMed
[26] Elnabawi YA, Garshick MS, Tawil M, Barrett TJ, Fisher EA, Lo Sicco K, et al. CCL20 in psoriasis: A potential biomarker of disease severity, inflammation, and impaired vascular health. J Am Acad Dermatol. 2021;84:913–20. 10.1016/j.jaad.2020.10.094.Search in Google Scholar PubMed PubMed Central
[27] R&D, Chemomab Ltd, Tel Aviv, Israel, Levy H, Gluschnaider U, R&D, Chemomab Ltd, Tel Aviv, Israel, Balbir-Gurman A, Rheumatology Institute, Rambam Health Care Campus, Haifa, Israel, et al. The role of CCL24 in systemic sclerosis. Rambam Maimonides Med J. 2023;14:e0016. 10.5041/RMMJ.10504.Search in Google Scholar PubMed PubMed Central
[28] Burger F, Baptista D, Roth A, Brandt KJ, Da Silva RF, Montecucco F, et al. Single-cell RNA-seq reveals a crosstalk between hyaluronan receptor LYVE-1-expressing macrophages and vascular smooth muscle cells. Cells. 2022;11:411. 10.3390/cells11030411.Search in Google Scholar PubMed PubMed Central
[29] Korbecki J, Maruszewska A, Bosiacki M, Chlubek D, Baranowska-Bosiacka I. The potential importance of CXCL1 in the physiological state and in noncancer diseases of the cardiovascular system, respiratory system and skin. Int J Mol Sci. 2022;24:205. 10.3390/ijms24010205.Search in Google Scholar PubMed PubMed Central
[30] Van Der Vorst EPC, Maas SL, Theodorou K, Peters LJF, Jin H, Rademakers T, et al. Endothelial ADAM10 controls cellular response to oxLDL and its deficiency exacerbates atherosclerosis with intraplaque hemorrhage and neovascularization in mice. Front Cardiovasc Med. 2023;10:974918. 10.3389/fcvm.2023.974918.Search in Google Scholar PubMed PubMed Central
[31] Kaczor DM, Kramann R, Hackeng TM, Schurgers LJ, Koenen RR. Differential effects of platelet factor 4 (CXCL4) and its non-allelic variant (CXCL4L1) on cultured human vascular smooth muscle cells. Int J Mol Sci. 2022;23:580. 10.3390/ijms23020580.Search in Google Scholar PubMed PubMed Central
[32] Korbecki J, Kojder K, Kapczuk P, Kupnicka P, Gawrońska-Szklarz B, Gutowska I, et al. The effect of hypoxia on the expression of CXC chemokines and CXC chemokine receptors—a review of literature. Int J Mol Sci. 2021;22:843. 10.3390/ijms22020843.Search in Google Scholar PubMed PubMed Central
[33] Hoeft K, Schaefer GJL, Kim H, Schumacher D, Bleckwehl T, Long Q, et al. Platelet-instructed SPP1 + macrophages drive myofibroblast activation in fibrosis in a CXCL4-dependent manner. Cell Rep. 2023;42:112131. 10.1016/j.celrep.2023.112131.Search in Google Scholar PubMed PubMed Central
[34] Munjal A, Khandia R. Atherosclerosis: Orchestrating cells and biomolecules involved in its activation and inhibition. Advances in protein chemistry and structural biology. Vol. 120, Elsevier; 2020. p. 85–122. 10.1016/bs.apcsb.2019.11.002.Search in Google Scholar PubMed
[35] Chen B, Frangogiannis NG. Chemokines in myocardial infarction. J Cardiovasc Transl Res. 2021;14:35–52. 10.1007/s12265-020-10006-7.Search in Google Scholar PubMed
[36] Korbecki J, Bajdak-Rusinek K, Kupnicka P, Kapczuk P, Simińska D, Chlubek D, et al. The role of CXCL16 in the pathogenesis of cancer and other diseases. Int J Mol Sci. 2021;22:3490. 10.3390/ijms22073490.Search in Google Scholar PubMed PubMed Central
[37] Schielke L, Zimmermann N, Hobelsberger S, Steininger J, Strunk A, Blau K, et al. Metabolic syndrome in psoriasis is associated with upregulation of CXCL16 on monocytes and a dysbalance in innate lymphoid cells. Front Immunol. 2022;13:916701. 10.3389/fimmu.2022.916701.Search in Google Scholar PubMed PubMed Central
[38] Szukiewicz D. CX3CL1 (fractalkine)-CX3CR1 axis in inflammation-induced angiogenesis and tumorigenesis. Int J Mol Sci. 2024;25:4679. 10.3390/ijms25094679.Search in Google Scholar PubMed PubMed Central
[39] Kraus S, Kolman T, Yeung A, Deming D. Chemokine receptor antagonists: Role in oncology. Curr Oncol Rep. 2021;23:131. 10.1007/s11912-021-01117-8.Search in Google Scholar PubMed
[40] Liang W-L, Liao H-L, Liang B. Immune landscape and regulatory mechanisms in human atherosclerotic coronary plaques: Evidence from single-cell and bulk transcriptomics. Heliyon. 2023;9:e19392. 10.1016/j.heliyon.2023.e19392.Search in Google Scholar PubMed PubMed Central
[41] Xu M, Wang Y, Xia R, Wei Y, Wei X. Role of the CCL2‐CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021;54:e13115. 10.1111/cpr.13115.Search in Google Scholar PubMed PubMed Central
[42] Živković L, Asare Y, Bernhagen J, Dichgans M, Georgakis MK. Pharmacological targeting of the CCL2/CCR2 axis for atheroprotection: A meta-analysis of preclinical studies. Arterioscler Thromb Vasc Biol. 2022;42. 10.1161/ATVBAHA.122.317492.Search in Google Scholar PubMed
[43] Miao H, Li X, Zhou C, Liang Y, Li D, Ji Q. NR4A2 alleviates cardiomyocyte loss and myocardial injury in rats by transcriptionally suppressing CCR5 and inducing M2 polarization of macrophages. Microvasc Res. 2022;140:104279. 10.1016/j.mvr.2021.104279.Search in Google Scholar PubMed
[44] Hong W, Yang B, He Q, Wang J, Weng Q. New insights of CCR7 signaling in dendritic cell migration and inflammatory diseases. Front Pharmacol. 2022;13. 10.3389/fphar.2022.841687.Search in Google Scholar PubMed PubMed Central
[45] Zhang Y, Gao W, Yuan J, Zhong X, Yao K, Luo R, et al. CCR7 mediates dendritic-cell-derived exosome migration and improves cardiac function after myocardial infarction. Pharmaceutics. 2023;15:461. 10.3390/pharmaceutics15020461.Search in Google Scholar PubMed PubMed Central
[46] Moser B. Chemokine receptor-targeted therapies: Special case for CCR8. Cancers. 2022;14:511. 10.3390/cancers14030511.Search in Google Scholar PubMed PubMed Central
[47] Dhayni K, Zibara K, Issa H, Kamel S, Bennis Y. Targeting CXCR1 and CXCR2 receptors in cardiovascular diseases. Pharmacol Ther. 2022;237:108257. 10.1016/j.pharmthera.2022.108257.Search in Google Scholar PubMed
[48] Moreno Ayala MA, Campbell TF, Zhang C, Dahan N, Bockman A, Prakash V, et al. CXCR3 expression in regulatory T cells drives interactions with type I dendritic cells in tumors to restrict CD8 + T cell antitumor immunity. Immunity. 2023;56:1613–1630.e5. 10.1016/j.immuni.2023.06.003.Search in Google Scholar PubMed PubMed Central
[49] Satarkar D, Patra C. Evolution, expression and functional analysis of CXCR3 in neuronal and cardiovascular diseases: A narrative review. Front Cell Dev Biol. 2022;10:882017. 10.3389/fcell.2022.882017.Search in Google Scholar PubMed PubMed Central
[50] Zhang N, Ma Q, You Y, Xia X, Xie C, Huang Y, et al. CXCR4-dependent macrophage-to-fibroblast signaling contributes to cardiac diastolic dysfunction in heart failure with preserved ejection fraction. Int J Biol Sci. 2022;18:1271–87. 10.7150/ijbs.65802.Search in Google Scholar PubMed PubMed Central
[51] Murad HAS, Rafeeq MM, Alqurashi TMA. Role and implications of the CXCL12/CXCR4/CXCR7 axis in atherosclerosis: Still a debate. Ann Med. 2021;53:1598–612. 10.1080/07853890.2021.1974084.Search in Google Scholar PubMed PubMed Central
[52] Liu S, Yao S, Yang H, Liu S, Wang Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023;14:648. 10.1038/s41419-023-06154-8.Search in Google Scholar PubMed PubMed Central
[53] Deleyto-Seldas N, Efeyan A. The mTOR–autophagy axis and the control of metabolism. Front Cell Dev Biol. 2021;9:655731. 10.3389/fcell.2021.655731.Search in Google Scholar PubMed PubMed Central
[54] Debnath J, Gammoh N, Ryan KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol. 2023;24:560–75. 10.1038/s41580-023-00585-z.Search in Google Scholar PubMed PubMed Central
[55] Ge Y, Zhou M, Chen C, Wu X, Wang X. Role of AMPK mediated pathways in autophagy and aging. Biochimie. 2022;195:100–13. 10.1016/j.biochi.2021.11.008.Search in Google Scholar PubMed
[56] Huang Y, Zhen Y, Chen Y, Sui S, Zhang L. Unraveling the interplay between RAS/RAF/MEK/ERK signaling pathway and autophagy in cancer: From molecular mechanisms to targeted therapy. Biochem Pharmacol. 2023;217:115842. 10.1016/j.bcp.2023.115842.Search in Google Scholar PubMed
[57] Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/akt/mTOR pathway and its role in cancer therapeutics: Are we making headway? Front Oncol. 2022;12:819128. 10.3389/fonc.2022.819128.Search in Google Scholar PubMed PubMed Central
[58] Grisan F, Iannucci LF, Surdo NC, Gerbino A, Zanin S, Di Benedetto G, et al. PKA compartmentalization links cAMP signaling and autophagy. Cell Death Differ. 2021;28:2436–49. 10.1038/s41418-021-00761-8.Search in Google Scholar PubMed PubMed Central
[59] Voskarides K, Giannopoulou N. The role of TP53 in adaptation and evolution. Cells. 2023;12:512. 10.3390/cells12030512.Search in Google Scholar PubMed PubMed Central
[60] Kircher M, Tran-Gia J, Kemmer L, Zhang X, Schirbel A, Werner RA, et al. Imaging Inflammation in Atherosclerosis with CXCR4-Directed 68 Ga-Pentixafor PET/CT: Correlation with 18 F-FDG PET/CT. J Nucl Med. 2020;61:751–6. 10.2967/jnumed.119.234484.Search in Google Scholar PubMed
[61] Miao G, Zhao X, Wang B, Zhang L, Wang G, Zheng N, et al. TLR2/CXCR4 coassociation facilitates Chlamydia pneumoniae infection-induced atherosclerosis. Am J Physiol-Heart Circ Physiol. 2020;318:H1420–35. 10.1152/ajpheart.00011.2020.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Safety assessment and modulation of hepatic CYP3A4 and UGT enzymes by Glycyrrhiza glabra aqueous extract in female Sprague–Dawley rats
- Adult-onset Still’s disease with hemophagocytic lymphohistiocytosis and minimal change disease
- Role of DZ2002 in reducing corneal graft rejection in rats by influencing Th17 activation via inhibition of the PI3K/AKT pathway and downregulation of TRAF1
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- YAP1 inhibition protects retinal vascular endothelial cells under high glucose by inhibiting autophagy
- Identification of secretory protein related biomarkers for primary biliary cholangitis based on machine learning and experimental validation
- Integrated genomic and clinical modeling for prognostic assessment of radiotherapy response in rectal neoplasms
- Stem cell-based approaches for glaucoma treatment: a mini review
- Bacteriophage titering by optical density means: KOTE assays
- Neutrophil-related signature characterizes immune landscape and predicts prognosis of esophageal squamous cell carcinoma
- Integrated bioinformatic analysis and machine learning strategies to identify new potential immune biomarkers for Alzheimer’s disease and their targeting prediction with geniposide
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- Histone modification and non-coding RNAs in skin aging: emerging therapeutic avenues
- A multiplicative behavioral model of DNA replication initiation in cells
- Biogenic gold nanoparticles synthesized from Pergularia daemia leaves: a novel approach for nasopharyngeal carcinoma therapy
- Creutzfeldt-Jakob disease mimicking Hashimoto’s encephalopathy: steroid response followed by decline
- Impact of semaphorin, Sema3F, on the gene transcription and protein expression of CREB and its binding protein CREBBP in primary hippocampal neurons of rats
- Iron overloaded M0 macrophages regulate hematopoietic stem cell proliferation and senescence via the Nrf2/Keap1/HO-1 pathway
- Revisiting the link between NADPH oxidase p22phox C242T polymorphism and ischemic stroke risk: an updated meta-analysis
- Exercise training preferentially modulates α1D-adrenergic receptor expression in peripheral arteries of hypertensive rats
- Overexpression of HE4/WFDC2 gene in mice leads to keratitis and corneal opacity
- Tumoral calcinosis complicating CKD-MBD in hemodialysis: a case report
- Mechanism of KLF4 Inhibition of epithelial-mesenchymal transition in gastric cancer cells
- Dissecting the molecular mechanisms of T cell infiltration in psoriatic lesions via cell-cell communication and regulatory network analysis
- Circadian rhythm-based prognostic features predict immune infiltration and tumor microenvironment in molecular subtypes of hepatocellular carcinoma
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Molecular identification and control studies on Coridius sp. (Hemiptera: Dinidoridae) in Al-Khamra, south of Jeddah, Saudi Arabia
- 10.1515/biol-2025-1218
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Response of hybrid grapes (Vitis spp.) to two biotic stress factors and their seedlessness status
- Metabolomic profiling reveals systemic metabolic reprogramming in Alternaria alternata under salt stress
- Effects of mixed salinity and alkali stress on photosynthetic characteristics and PEPC gene expression of vegetable soybean seedlings
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Causal effects of trace elements on congenital foot deformities and their subtypes: a Mendelian randomization study with gut microbiota mediation
- Honey meets acidity: a novel biopreservative approach against foodborne pathogens
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Fluorescent detection of sialic acid–binding lectins using functionalized quantum dots in ELISA format
- Smart tectorigenin-loaded ZnO hydrogel nanocomposites for targeted wound healing: synthesis, characterization, and biological evaluation
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Safety assessment and modulation of hepatic CYP3A4 and UGT enzymes by Glycyrrhiza glabra aqueous extract in female Sprague–Dawley rats
- Adult-onset Still’s disease with hemophagocytic lymphohistiocytosis and minimal change disease
- Role of DZ2002 in reducing corneal graft rejection in rats by influencing Th17 activation via inhibition of the PI3K/AKT pathway and downregulation of TRAF1
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- YAP1 inhibition protects retinal vascular endothelial cells under high glucose by inhibiting autophagy
- Identification of secretory protein related biomarkers for primary biliary cholangitis based on machine learning and experimental validation
- Integrated genomic and clinical modeling for prognostic assessment of radiotherapy response in rectal neoplasms
- Stem cell-based approaches for glaucoma treatment: a mini review
- Bacteriophage titering by optical density means: KOTE assays
- Neutrophil-related signature characterizes immune landscape and predicts prognosis of esophageal squamous cell carcinoma
- Integrated bioinformatic analysis and machine learning strategies to identify new potential immune biomarkers for Alzheimer’s disease and their targeting prediction with geniposide
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- Histone modification and non-coding RNAs in skin aging: emerging therapeutic avenues
- A multiplicative behavioral model of DNA replication initiation in cells
- Biogenic gold nanoparticles synthesized from Pergularia daemia leaves: a novel approach for nasopharyngeal carcinoma therapy
- Creutzfeldt-Jakob disease mimicking Hashimoto’s encephalopathy: steroid response followed by decline
- Impact of semaphorin, Sema3F, on the gene transcription and protein expression of CREB and its binding protein CREBBP in primary hippocampal neurons of rats
- Iron overloaded M0 macrophages regulate hematopoietic stem cell proliferation and senescence via the Nrf2/Keap1/HO-1 pathway
- Revisiting the link between NADPH oxidase p22phox C242T polymorphism and ischemic stroke risk: an updated meta-analysis
- Exercise training preferentially modulates α1D-adrenergic receptor expression in peripheral arteries of hypertensive rats
- Overexpression of HE4/WFDC2 gene in mice leads to keratitis and corneal opacity
- Tumoral calcinosis complicating CKD-MBD in hemodialysis: a case report
- Mechanism of KLF4 Inhibition of epithelial-mesenchymal transition in gastric cancer cells
- Dissecting the molecular mechanisms of T cell infiltration in psoriatic lesions via cell-cell communication and regulatory network analysis
- Circadian rhythm-based prognostic features predict immune infiltration and tumor microenvironment in molecular subtypes of hepatocellular carcinoma
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Molecular identification and control studies on Coridius sp. (Hemiptera: Dinidoridae) in Al-Khamra, south of Jeddah, Saudi Arabia
- 10.1515/biol-2025-1218
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Response of hybrid grapes (Vitis spp.) to two biotic stress factors and their seedlessness status
- Metabolomic profiling reveals systemic metabolic reprogramming in Alternaria alternata under salt stress
- Effects of mixed salinity and alkali stress on photosynthetic characteristics and PEPC gene expression of vegetable soybean seedlings
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Causal effects of trace elements on congenital foot deformities and their subtypes: a Mendelian randomization study with gut microbiota mediation
- Honey meets acidity: a novel biopreservative approach against foodborne pathogens
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Fluorescent detection of sialic acid–binding lectins using functionalized quantum dots in ELISA format
- Smart tectorigenin-loaded ZnO hydrogel nanocomposites for targeted wound healing: synthesis, characterization, and biological evaluation
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

