Abstract
Neuroinflammation represents a critical pathway in the brain for the clearance of foreign bodies and the maintenance of homeostasis. When the neuroinflammatory process is dysregulate, such as the over-activation of microglia, which results in the excessive accumulation of free oxygen and inflammatory factors in the brain, among other factors, it can lead to an imbalance in homeostasis and the development of various diseases. Recent research has indicated that the development of numerous neurodegenerative diseases is closely associated with neuroinflammation. The pathogenesis of neuroinflammation in the brain is intricate, involving alterations in numerous genes and proteins, as well as the activation and inhibition of signaling pathways. Furthermore, excessive inflammation can result in neuronal cell apoptosis, which can further exacerbate the extent of the disease. This article presents a summary of recent studies on the relationship between neuronal apoptosis caused by excessive neuroinflammation and neurodegenerative diseases. The aim is to identify the link between the two and to provide new ideas and targets for exploring the pathogenesis, as well as the prevention and treatment of neurodegenerative diseases.
Graphical abstract

1 Introduction
Neurodegenerative diseases are defined as conditions characterized by the gradual loss or deterioration of neurons and myelin sheaths, leading to impaired function over time. A number of neurodegenerative diseases are commonly encountered in clinical practice, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) and amyotrophic lateral sclerosis (ALS), among others [1]. Neurodegenerative diseases constitute a substantial threat to human health and quality of life. For example, AD, the most prevalent neurodegenerative disease globally, is estimated to affect approximately 55 million individuals worldwide, with over 10 million cases in China [2,3]. The incidence rate is 5–6% at the age of 65, 10% at the age of 70, and 48% at the age of 90. The age of onset is earlier. It is projected that by 2050, the global prevalence of patients will reach 152 million [4]. As indicated by the World Health Organization, neurodegenerative diseases may become the second leading cause of mortality in humans by 2040 [5]. Neurodegenerative diseases not only inflict significant suffering on patients but also place considerable economic and psychological burdens on families and society. Currently, however, there is no effective treatment for neurodegenerative diseases. The pathogenesis of these diseases is complex and still controversial, and an in-depth understanding of the pathogenesis of neurodegenerative diseases will help to discover new therapeutic targets and drugs, providing new treatment ideas and theoretical foundations for the diagnosis and treatment of neurodegenerative diseases.
It is becoming increasingly clear that neuroinflammation may play an important role in the pathogenesis of neurodegenerative diseases that cannot be ignored. In the brain, the occurrence of inflammatory reactions can result in oxidative stress and damage to the antioxidant defense system of nerve cells, thereby accelerating the progression of neurodegenerative diseases [6]. Concurrently, neurodegenerative diseases can also result in aberrant protein accumulation and the release of inflammatory mediators, thereby establishing a vicious cycle. Additionally, inflammatory cells are capable of secreting neurotoxins such as glutamate, which can result in neuronal overexcitement and subsequent damage. Moreover, inflammatory factors can also stimulate neuronal apoptosis signaling pathways, ultimately leading to neuronal death. Consequently, the inflammatory response is intimately associated with the progression of neurodegenerative diseases, manifesting not only in the initial stages of the disease but also progressively worsening as the disease progresses. Inflammation is a protective mechanism of the body that maintains the internal environment of the brain in a balanced state by repairing, regenerating, and removing damaged histiocytes or infectious agents, toxins from the body [7–10]. Nevertheless, the role of inflammation in the process of organismal aging is also significant. It is a concomitant response to cellular senescence and organismal aging [11–13]. During the aging process, the functionality of the immune system is disrupted due to a deterioration of the body’s immunological defenses. These defenses play a pivotal role in the eradication of pathogens. Consequently, when they weaken, both the innate and acquired immunity systems of the organism become compromised. This results in an imbalance in immune system functioning, which in turn affects the ability of the immune system to clear pathogens, damaged tissues, and senescent cells. Consequently, there is increased expression of pro-inflammatory cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukin [IL]-1β, IL-6, IL-8, reactive oxygen species [ROS]) and C–C chemokine ligand-regulated factors (CCL-2 and CCL-5), which contribute to the inflammatory response [14,15] (Table 1). The chronic stimulation of these factors not only results in a chronic, low-grade, microinflammatory senescent state of the organism, but also induces neuroinflammation and leads to neuronal damage, ultimately resulting in age-related neurodegeneration (Table 1) [16–19].
Neurologic diseases and neuroinflammatory factors associated with neuroinflammation
| Disease | Inflammatory factors | References |
|---|---|---|
| AD | TNF-α, IL-1β, IL-6, Tau, NFT | [14,15,22–30,76–82] |
| PD | TNF-α, IL-1β, IL-6, NLRP3, Iba-1, GFAP, iNOS, COX-2 | [14,15,22–28,31–35,116–118,178,179] |
| HD | TNF-α, IL-1β, IL-6, HTT | [14,15,22–28,36–39,121–136] |
| Multiple sclerosis (MS) | IL-1β, IL-6, CXC1, CCL2, CCL3, CCL4 | [14,15,40–42] |
| Traumatic brain injury (TBI) | TNF-α, IL-1β, IL-6, IL-8, IL-10, ROS, GFAP | [14,15,43–46] |
| Gulf war diseases (GWI) | IL-1β, IL-6, IL-2, IL-10, IFN-γ, IL-4, IL-5, IL-17A, IL-33, TSPO | [14,15,47–51] |
| ALS | G-CSF, IL2, IL15, IL17, MCP-1, MIP1α, TNF-α, VEGF | [14,15,52–55] |
Neuroinflammation is defined as an inflammatory response within the central nervous system (CNS) that involves intricate interactions between a multitude of immune cells, factors, and receptors, both in its occurrence and in subsequent development. The principal immune cells involved are microglia, astrocytes, macrophages, T cells, and B cells. The principal factors involved are cytokines, chemokines, ROS, nitric oxide (NO), and prostaglandins. Furthermore, the receptors involved in the inflammatory response include pattern recognition receptors (PRRs) and chemokine receptors (CCR).
Microglia are resident immune cells within the CNS. In physiological conditions, microglia facilitate brain development, repair cellular damage, and promote neuronal survival, thereby maintaining the internal environment of the brain in a state of homeostasis. In pathological conditions, microglia are overactivated by disease factors, which results in excessive inflammatory responses within the brain. This results in the release of inflammatory cytokines and the inhibition of nerve regeneration, which collectively exert neurotoxic effects [20,21]. As individuals age, the misfolded proteins, cellular debris, and other inflammatory stimuli accumulated in the brain lead to the continued stimulation of microglia, thereby accelerating the aging process. Furthermore, elevated levels of organismal senescence result in a reduction in the phagocytic capacity and monitoring abilities of microglia, thereby initiating a self-perpetuating cycle that stimulates the production of inflammatory substances detrimental to neuronal health and facilitates the development of neurodegenerative diseases [22–24]. Consequently, neurodegeneration resulting from neuroinflammation plays a role in the progression of neurodegenerative diseases. The role of microglia-mediated neuroinflammation as a hallmark of several CNS diseases, including AD, PD, and HD, is now well-established (Table 1) [25–28].
A search for inflammatory factors related to several neurodegenerative diseases with high incidence rates or significant adverse effects on human health revealed commonalities in early neuritis. As illustrated in Table 1, an increase in the expression of inflammatory factors, including TNF-α, IL-1β, IL-6, Tau, and NFT was observed in AD [29,30]. A noteworthy increase in inflammatory factors, including TNF-α, IL-1β, IL-6, NLRP3, Iba-1, glial fibrillary acidic protein (GFAP), iNOS, and COX-2, was observed in PD [31–35]. In HD, it has been demonstrated that the expression of inflammatory factors such as TNF-α, IL-1β, IL-6, and HTT exhibited a notable increase [36–39]. In MS, there is a significant increase in the levels of IL-1β, IL-6, CXCL1, CCL2, CCL3, CCL4, and other factors [40–42]. A number of inflammatory factors, including TNF-α, IL-1β, IL-6, IL-8, IL-10, ROS, and GFAP, have been demonstrated to be significantly elevated in individuals with TBI [43–46].GulfWar illness (GWI) has been linked to notable elevations in the levels of various cytokines, including IL-1β, IL-6, IL-2, IL-10, IFN-γ, IL-4, IL-5, IL-17A, IL-33, TSPO, and others, which have been demonstrated to exhibit significant increases in a number of cases [47–51]. A number of factors, including G-CSF, IL-2, IL-15, IL-17, MCP-1, MIP-1α, TNF-α, VEGF, and other factors have been identified in the context of ALS research [52–55].
Neuroinflammation is a critical element in the pathogenesis and progression of neurodegenerative disorders. In recent years, there have been significant advancements in research on the factors, pathways, and cell fate associated with neuroinflammation in neurodegenerative diseases. This article will discuss and summarize the research progress of neuroinflammation in typical neurodegenerative diseases from several perspectives, including neuroinflammation, neuroinflammatory signaling pathways, and changes in cell fate caused by neuroinflammation. The objective is to provide an understanding of the molecular mechanisms of inflammation that contribute to the occurrence and development of neurodegenerative diseases, and to offer insights into potential molecular targets and strategies for the treatment of neurodegenerative diseases at the neuroinflammatory level.
2 Neuroinflammation in the most prevalent neurodegenerative diseases
2.1 AD
AD, the most prevalent neurodegenerative disease worldwide, which affects over 44 million individuals. Its pathogenesis is complex and plays a pivotal role in the development of dementia. The primary pathological processes of AD include the deposition of amyloid-β (Aβ) protein, hyperphosphorylation of Tau protein, and the production of neurofibrillary tangles (NFTs) [56–60]. The recent discovery of AD-related inflammatory markers, as well as the finding that some innate immune-related genes are also associated with the pathogenesis of AD, suggests that neuroinflammation also plays an important role in the pathogenesis of AD [61–63]. The principal immune cells in the brain are astrocytes and microglia. Neuroinflammation in AD is also mainly related to these cells. Microglia play a pivotal role in the brain, with the capacity to be activated in order to regulate homeostatic balance within the brain when stimulated [64–66]. Some studies have indicated that microglia are abnormally activated in the brains of patients with AD, and this phenomenon may be related to the pathogenesis and development of AD [67]. The development of this state is influenced by a variety of factors, including brain injury, infection, or other stimuli. Upon stimulation, microglia can be divided into two main phenotypes: anti-inflammatory and pro-inflammatory [68–72].
Some studies have indicated that in the context of aging, microglia exhibit a proclivity toward a pro-inflammatory phenotype [73,74]. Upon activation to the anti-inflammatory phenotype, microglia release anti-inflammatory factors, including IL-10, IL-13, and others to counteract the inflammatory response in the brain [75]. When microglia are activated to the pro-inflammatory phenotype, their capacity to remove toxic substances and waste products is diminished, resulting in the accumulation of neurotoxins such as Aβ protein in the brain. Concurrently, the discharge of inflammatory mediators such as TNF-α, IL-1, and IL-6 is enhanced, which in turn facilitates the occurrence of neuroinflammation, accelerates neurodegeneration, and inflicts damage upon neurons within the brain. The diminished clearance capacity of pro-inflammatory microglia results in the accumulation of Aβ protein and NFTs, which are formed by hyperphosphorylated Tau protein, in the brains of AD patients [76–81]. The accumulation of Aβ protein and NFTs may act as a stimulus to continue to activate microglia, thereby creating a vicious cycle and accelerating the development of AD [80,81].
Astrocytes and microglia exhibit analogous functions and are capable of recognizing Aβ, thereby undergoing activation and alterations in morphology and function. Both cells are capable of regulating synapse formation, but the interaction between astrocytes and neurons is bidirectional. Astrocytes and oligodendrocytes are interconnected in the brain, forming a large syncytial glial network comprising hundreds of cells. This occurs through the formation of a tripartite synapse, which involves the connection with neurons. After activation, astrocytes can be classified into two distinct phenotypes, A1 and A2. The A1 type is mainly induced by TNF-α and IL-1 α, while A1 type astrocytes lose their normal morphology and function, can secrete neurotoxins, and have components such as C3 that mediate synaptic elimination, leading to synaptic reduction and inducing neuronal apoptosis [82]. Synapses are associated with memory processes, and a reduction in synapse number or function may contribute to memory deficits associated with AD. IL-4 and IL-10 have been demonstrated to induce the production of A2 type astrocytes, which retain their phagocytic function and capacity to secrete neuroprotective substances such as TGF-β. This has been shown to exert neuroprotective effects on neurons. Additionally, it has been demonstrated to facilitate cell proliferation, promote synapse formation, and inhibit cell apoptosis. Studies have shown that estrogen can promote the transformation of A2 phenotype and reduce the transformation of A1 phenotype, thereby protecting synapses and neurons. Both phenotypes of astrocytes show an increase in GFAP expression, and the transformation of A1 and A2 glial cells may not be independent, but a continuous process. Their proportion is related to pathological changes and the degree of cognitive impairment.
2.2 PD
PD is a neurodegenerative disease that is caused by the death of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). It is the second most prevalent neurodegenerative disease worldwide, with a prevalence rate only surpassed by AD [83,84]. However, the precise pathogenesis of PD remains unclear. The extant literature and reports have demonstrated that PD is characterized by a number of factors, including oxidative stress [85–87], calcium homeostatic imbalance [88–90], abnormal accumulation of alpha synuclein [91,92], impaired mitochondrial function [93–95], endoplasmic reticulum stress [96–98], intestinal flora dysbiosis [99,100], intestinal flora dysregulation, and many other factors. Recent studies have demonstrated that neuroinflammation plays a pivotal role in the pathogenesis of PD. In the absence of neuroinflammation, the brain is capable of removing toxins through a process known as the glymphatic system. However, excessive levels of neuroinflammation can lead to the sustained degeneration and apoptosis of dopaminergic neurons [101,102].
The brain contains a considerable number of glial cells, which have an immune effect and can produce oxidative stress and inflammation. In normal conditions, oxidative stress and inflammation have a protective effect on brain tissue. Nevertheless, aberrant activation of glial cells can result in the generation of a considerable number of free radicals and inflammatory factors, which in turn can cause severe inflammation and oxidative stress, ultimately leading to damage of brain tissue [103–111]. The high concentration of microglia in the substantia nigra region renders the substantia nigra region more susceptible to inflammation. Microglia initiate the neuroinflammatory response following the recognition of lipopolysaccharide (LPS), heat shock protein, and other stimuli, releasing inflammatory factors that initially affect DAergic neurons, resulting in neuronal stress and oxidative damage. Concurrently, the activation of astrocytes through TLR2 receptors intensifies the inflammatory response, thereby exacerbating neuroinflammation in PD [112,113]. It has recently been demonstrated that neuroinflammation, defined as the activation of microglia and astrocytes in the brain, can result in the induction of a pro-inflammatory programmed cell death pathway. This pathway is induced by caspase family proteins and is termed necroptosis. It is a necrotic and inflammatory programmed apoptotic cell death pathway [114,115]. This pathway is closely associated with neuroinflammation, a process whereby glial cells in the brain respond to inflammatory factors secreted by immune cells. When glial cells detect these factors, they regulate the inflammatory response in the CNS, secrete proinflammatory factors, increase the expression of Iba-1 and GFAP in the brain, exacerbate oxidative damage, and induce necrotic apoptosis in DAergic neurons [116–118].
The pathological features of PD are primarily characterized by the formation of Lewy bodies, which are intracellular inclusions resulting from the aberrant aggregation of α-synuclein [119]. In addition, there may be a loss of dopaminergic neurons and neurotransmitters in the SNpc and striatum of the midbrain. The substantia nigra of the midbrain is the core area of pathological changes in PD, and the degeneration and loss of dopaminergic neurons are key to the occurrence of PD [120]. The striatum is a crucial region that receives projections from dopaminergic neurons in the substantia nigra, and a decrease in dopamine directly affects the function of the striatum, leading to symptoms such as motor disorders. Moreover, the basal ganglia are the part of the brain responsible for coordinating body movements and controlling muscles. PD can cause dysfunction of the basal ganglia, which in turn affects the patient’s motor abilities.
2.3 HD
HD is an autosomal dominant, progressive neurodegenerative disorder with a distinctive phenotype. The primary pathological feature of HD is the production of mutant Huntington proteins, which result from the mis-expression of polynucleotide repeat sequences on the Huntingtin (Htt) gene on the patient’s chromosome 4 [121,122]. It has been demonstrated that the normal Htt protein performs multiple functions in neurons, including the maintenance of primitive neural stem cell lineage potential. In contrast, studies have demonstrated that Htt protein variants are responsible for the observed dysfunctions [123–125]. The high expression of Htt protects cells of other CNS origins from lethal injury. However, if overexpressed Htt accumulates in nerve cells to form aberrant Huntington proteins, it can lead to the development of HD, affecting the ability of nerve cells to function properly. In patients, mutant proteins typically result in damage to predominantly striatal neurons [126–132].
The pathogenesis of HD is closely related to neuroinflammation. Impairment of Htt clearance in the brain results in the accumulation of abnormalities, which in turn leads to overactivation of microglia. This leads to the clearance of the abnormal accumulation of proteins by microglia, which in turn causes neuroinflammation and dysfunction of the ubiquitin protease system and autophagy system in the brain [133,134]. Additionally, the overactivation of microglia can also result in neuroinflammation by activating NLRP3 inflammatory vesicles and secreting substantial quantities of inflammatory factors, which can cause damage to mHTT nerve cells and neurodegeneration. Furthermore, the overactivation of microglia results in the dysfunction of the ubiquitin-proteasome and autophagy systems, which in turn accelerates the progression of HD [135,136].
3 Neuroinflammation pathways
3.1 NF-κB signaling pathway
The NF-κB signaling pathway represents a prototypical inflammatory signaling pathway. The NF-κB family comprises five members: p65 (RelA), RelB, c-Rel, p50 (NFκB1), and p52 (NFκB2) [137]. The NF-κB signaling pathway is regulated by a homology domain, RHD, which binds to form a dimer and is involved in the regulation of the NF-κB signaling pathway [138,139]. The activation of the NF-κB pathway has been demonstrated to play a role in a number of biological processes, including inflammatory responses [140], cell proliferation [141], cell differentiation [142], and immune response [143]. The activation of this pathway can be classified into two categories: classical and non-classical. The classical activation pathway is associated with functions related to inflammation [144]. From a physiological perspective, NF-κB is repressed by IκB binding and is predominantly localized to the cytoplasm. Upon stimulation by bacteria, inflammation, or other stimuli, the protein kinase TAK1 is activated by pathogen-associated molecular pattern (PAMP) or damage-associated molecular pattern (DAMP) occurring in PRR-expressing immune cells. Activation of TAK1 results in phosphorylation of IKK, which subsequently releases the inhibitory effect of IκB on NF-κB, thereby promoting its activation. Activated NF-κB then transfers to the nucleus and binds to specific DNA binding sites, regulating the inflammation, apoptosis, and other responses [145,146]. The overexpression of inflammatory factors can also result in the loss of synaptic connections between neurons, impairing neuronal signal transduction and synaptic plasticity, ultimately leading to a decline in cognitive function. This process is especially evident in neurodegenerative diseases such as AD.
Some studies have demonstrated that microglia pretreated with IL-10 and subsequently stimulated with LPS exhibit a reduction in IL-6 levels. This evidence suggests that IL-10 may prevent the nuclear translocation of NF-κB, thereby reducing the transcriptional initiation of IL-6 by NF-κB. Consequently, the quantity of IL-6 is diminished, thereby attenuating the inflammatory response [147,148]. peroxisome proliferator-activated receptor alpha (PPARα) ligands have also been demonstrated to inhibit radiation-induced inflammatory responses in microglia by negatively regulating the NF-κB and AP-1 pathways [149]. These findings suggest that the interference with the nuclear translocation of NF-κB plays an important role in the attenuation of microglia inflammatory responses in neurodegenerative diseases.
3.2 TOLL-like receptor (TLR) signaling pathway
The MyD88 and TRIF pathways are the two pathways present in TLRs. All TLRs except TLR3 can use the MyD88 pathway, and TLR3 and TLR4 can use the TRIF pathway [150]. Activation of the TLR4 can result in the recruitment of TIR–TIRAP–MyD88 complexes, which in turn can interact with the death domain of MyD88 (Figure 1). This interaction can then lead to the recruitment of IL-1 receptor-associated kinase 4 (IRAK4) [151]. Notably, the two proteins can interact with IRAK4 also acting as an agonist to activate other proteins of the IRAK family, such as IRAK-1 [152]. This leads to the activation of TRAF6, which is activated in conjunction with E2 ubiquitin-protein ligase to activate a complex consisting of TGF-β-activated kinase 1 (TAK1), TAK1 assembly protein 1 (TAB1), TAB2, and TAB3. Ultimately the mitogen-activated protein kinase (MAPK) and NF-κB pathways are initiated by the activation of the TAK1/TAB complex [153]. The extracellular portion of TLR3 contains a horseshoe-shaped structure that facilitates the recognition of dsRNA and plays an essential role in antiviral immunity [154]. This structure then recruits the junction protein TRIF to the dsRNA. Further activation of TBK1 and RIP1 kinase forms a complex that mediates the phosphorylation process of IRF3. This translocates from the cytoplasm to the nucleus and regulates the synthesis process of type I interferon. Additionally, the activation of RIP1 also leads to ubiquitination and activation of TAK1, which in turn leads to NF-κB transcription [155]. In addition, TLR7 and TLR9 also induce type I interferon production, but rely on MyD88 activation rather than IRF3 [156]. In AD, Aβ as an endogenous messenger can activate TLR4 and other receptors, causing neuroinflammation and neuronal damage [157]. Neuroinflammation promotes the deposition of Aβ and further neuronal damage, creating a vicious cycle that exacerbates the pathological changes of AD [158]. In PD, α-synuclein aggregation and oxidative stress may also activate the TLR signaling pathway, induce microglial activation and neuroinflammation, and then exacerbate the damage and death of dopaminergic neurons in PD [159]. In MS, the TLR signaling pathway plays a key role in autoimmune inflammation. The TLR pathway can activate the infiltration and activation of immune cells such as T cells and B cells, which can lead to neuroinflammation and demyelinating lesions, thus exacerbating the pathological changes of MS [160].

Using the String database to analyze protein interactions. TLR4 can regulate apoptosis-related proteins such as Bcl-2 and Bax through MYD88. Additionally, the P53 gene can directly regulate the TLR4 signaling pathway, as well as regulate Bcl-2 and Bax.
3.3 MAPK signaling pathway
MAPK plays a prominent role as a major signaling mechanism that responds rapidly to a wide range of environmental changes and influences a variety of physiological mechanisms. The MAPK family comprises four major members: p38, extracellular signal-regulated protein kinase (ERK), c-Jun N-terminal kinase (JNK), and ERK5 [161]. The MAPK signaling pathway plays a variety of roles in various biological processes, including growth and development, oxidative stress, anti-inflammatory responses, and endoplasmic reticulum stress [162]. MAPK signaling pathways play a multitude of roles in diverse biological processes, including growth and development, oxidative stress, anti-inflammatory responses, and endoplasmic reticulum stress. The JNK–p38MAPK signaling pathway is primarily implicated in apoptosis and stress response, whereas the ERK–MAPK signaling pathway is associated with cell proliferation and differentiation. It is also inextricably linked to the cellular signaling network [163].
In vivo, oxidative stress stimulates the generation of ROS, which can induce the activation of ASK1, an upstream regulator of MAPK. Activated ASK1 then activates MEK4/MEK7 and MEK3/MEK6, which in turn induce the activation of JUN and P38. This activates the MAPK signaling pathway, which regulates the production of inflammatory factors and the inflammatory response in vivo. The activated MAPK signaling pathway is capable of regulating the production of inflammatory factors and the inflammatory response in the body. Additionally, it exerts anti-inflammatory and antioxidant effects on TLR receptors and macrophages [164]. The MAPK signaling pathway has been demonstrated to regulate the production of inflammatory factors in the body. In addition, in neurodegenerative diseases such as AD, the activated MAPK signaling pathway can also act on the NF-κB signaling pathway, promoting the release of TNF-α and IL-1β inflammatory factors to regulate the inflammatory response and further exacerbate neuronal damage in neurodegenerative diseases [165]. JNK is a critical component of the MAPK pathway, which can suppress the expression of c-Jun transcription factors and influence the genetic balance between c-Jun and AP-1. This makes it an important player in the pathway. The P38 pathway is considered to be the foundation of MAPK signaling, which is capable of responding to a multitude of environmental stimuli, and thus plays a pivotal role in the diverse functions and behaviors of the cell. ERK5 can be stimulated by a variety of external stimuli. Research has shown that ERK5 is effective in increasing insulin levels in neurons, thereby improving cell viability. It may therefore be an effective means of treating chronic degenerative brain diseases [166]. Some studies have demonstrated that ERK5 is an effective means of increasing insulin levels in neurons, thereby improving cell viability. This may have implications for the treatment of chronic degenerative brain diseases.
In neurodegenerative diseases, extracellular stimuli (such as inflammatory factors, oxidative stress products, etc.) can activate the MAPK pathway. For example, in AD, abnormal metabolism of amyloid precursor protein (APP) leads to the formation and accumulation of Aβ, which in turn triggers inflammatory responses and oxidative stress, leading to abnormal activation of the MAPK pathway [167]. The activated MAPK pathway further activates its downstream substrates, such as transcription factors and protein kinases, through phosphorylation, and then regulates the expression of inflammation-related genes [168]. In addition, the MAPK pathway can also regulate the expression of inflammation-related factors such as cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and TNF [168]. In neurodegenerative diseases, activated MAPK signaling may participate in the pathological process of the disease by promoting neuronal apoptosis or necrosis. Abnormal activation of the MAPK pathway can lead to apoptosis and necrosis of dopaminergic neurons, thereby accelerating the progression of the PD [169]. Given the important role of the MAPK pathway in neuroinflammation and neurodegenerative diseases, intervention strategies targeting this pathway provide new ideas for disease treatment.
3.4 PPAR signaling pathway
PPAR is a group of nuclear receptors in the nuclear receptor family in vivo, including PPARα, PPARβ/α, and PPARγ. PPAR is a transcription factor that plays a pivotal role in the inflammatory response and immune regulation by regulating the metabolic and anti-inflammatory effects of transcription factors. It functions by inhibiting the release of inflammatory cytokines, adhesion molecules, and extracellular matrix proteins. Additionally, it exerts a protective effect on nerves by releasing anti-inflammatory factors that are neuroprotective. It is therefore of great importance for the recovery of cognitive function in neurodegenerative diseases. It has been demonstrated that the administration of the PPARα receptor agonist GW7647 prior to the induction of an inflammatory response in microglia results in a reduction in the phosphorylation of the AP-1C-JUN subunit, which subsequently leads to a decline in nuclear NF-kB activity [170,171]. This process slows down the inflammatory response of microglia and consequently reduces neuronal damage. PPAR-γ receptor agonists have been demonstrated to inhibit the expression of surface antigens, enhance the synthesis of NO, and decrease the secretion of prostaglandins, inflammatory factors, chemokines, and ROS. Consequently, the inhibition of PPARs expression exerts an inhibitory effect on both oxidative stress and inflammatory responses in microglia [172]. In addition, in neurodegenerative diseases such as AD, abnormal accumulation of proteins (such as Aβ) is the main cause of neuronal damage. The activation of PPARs signaling pathway may help to regulate the metabolism and clearance of these proteins to alleviate disease progression.
3.5 Notch signaling pathway
The Notch signaling pathway plays a pivotal role in the growth and development of astrocytes, oligodendrocytes, and dopaminergic neural precursors. Notch receptors bind to a variety of ligands and regulate the differentiation and development of cells, tissues, and organs. Activation of the Notch signaling receptor induces an inflammatory response in microglia, which is mediated by binding to the ligand Jagged1. This process ultimately leads to the production of pro-inflammatory factors by microglia, which can cause neuronal damage [173]. The activated Notch signaling pathway has been demonstrated to increase macrophage sensitivity to γ-interferon, promote inflammatory responses, and promote nuclear translocation of NF-kB, thereby exacerbating inflammatory responses. The inflammatory mediators produced can also activate the Notch signaling pathway. The activation of the Notch signaling pathway and the subsequent response involves a variety of aspects, and thus may be a potential protocol for studying early-onset neuroinflammation in neurodegenerative diseases [174]. It has been demonstrated that in neurodegenerative diseases such as AD, abnormalities in the Notch signaling pathway may contribute to pathological changes in neurons, including the formation of NFTs and plaques [175]. These changes may subsequently influence neuronal proliferation, differentiation, and apoptosis, thereby affecting neuronal survival and number. Furthermore, the Notch signaling pathway plays a role in neurodegenerative diseases, where pathological changes are exacerbated by reduced synaptic plasticity and disruption of neuronal networks [175]. This, in turn, leads to cognitive dysfunction and behavioral abnormalities.
3.6 PI3K/Akt signaling pathway
PI3K is a critical anti-apoptotic regulator that has been classified into three distinct types (I–III) based on its structural and regulatory characteristics. Among these, type I has been the subject of the most extensive research, and it is present in all cells, where it participates in the transduction of various signaling pathways. After the activation of PI3K, it can bind to the PH domain of downstream Akt, thereby exerting anti-apoptotic and regulatory functions on cell growth [176]. Akt is a serine/threonine protein kinase that mediates the anti-apoptotic effects of growth factor regulation and inactivates downstream apoptotic factors. Previous studies have demonstrated that neuroinflammation in the brain induces microglia to secrete inflammatory factors. Furthermore, the activation of the PI3K/Akt pathway can promote the expression of anti-inflammatory factors, such as IL-4 and IL-10, and inhibit the expression of pro-inflammatory factors, such as IL-6 and IL-1β. This is achieved by inhibiting the nuclear translocation of NF-κB, thus exerting its anti-inflammatory function [177,178].
Activation of the PI3K/Akt pathway has been demonstrated to inhibit the pro-apoptotic effect of BAD and other pro-apoptotic proteins by phosphorylating them, thereby protecting neurons from damage and death [179]. Meanwhile, the PI3K/Akt pathway can provide neurons with the necessary energy and nutrients by regulating metabolic pathways, including glycogen synthesis and fatty acid synthesis, thereby promoting the recovery of neuronal function in neurodegenerative diseases [179,180]. Reduced activity of the PI3K/Akt signaling pathway has been observed to result in pathological changes, including reduced neuronal viability, impaired synaptic plasticity, and metabolic abnormalities in AD [181]. Conversely, activation of the PI3K/Akt signaling pathway has been demonstrated to protect neurons from damage and death, thereby slowing the progression of AD [181]. Abnormalities in the PI3K/Akt signaling pathway have been linked to the death of dopaminergic neurons in PD [182]. The PI3K/Akt pathway plays a critical role in regulating neuronal apoptosis and protecting dopaminergic neurons from injury and death [182]. Therefore, it can be proposed that the PI3K/Akt signaling pathway may be a potential therapeutic target for neurodegenerative diseases.
A thorough examination of the inflammation-related pathway factors revealed that numerous inflammation-related pathways interact with p53 and apoptosis-related proteins, including Bax, BID, BIK, Bak, and Bcl-2 (Figure 1). These interactions are exemplified by the well-studied iNOS or TLR family of receptors (Figure 2), which can regulate apoptosis-related proteins expression by regulating the interaction of downstream MyD88 with P53 or by directly interacting with p53 (Figure 1). In addition to the interactions between PI3K/Akt and p53, other pathways, such as NF-κB, can also influence the expression of Bax and other proteins. This is consistent with the fact that inflammation has been shown to promote neuronal apoptosis in the pathological process of neurodegenerative diseases, a phenomenon that has been the subject of increasing research in recent years.

Using the String database to analyze protein interactions. The NF-κB pathway is regulated by NOS2 (iNOS), which in turn regulates TNF, Bax, and the Bcl family. It is also possible that p53 may play a role in the NF-kB pathway.
3.7 Autophagy and inflammation in nervous system diseases
Autophagy represents a pivotal mechanism for cells to maintain intracellular stability and respond to diverse stress stimuli [183], with a particularly pronounced impact on nerve cells. This mechanism not only facilitates the clearance of damaged organelles and misfolded proteins, but also the removal of pathological protein aggregation [184], thereby ensuring the optimal functioning of neurons.
In neurodegenerative diseases, a defect in autophagy function is frequently a significant contributor to the accumulation of pathological proteins and the subsequent decline in neuronal function. For example, in AD and PD, the abnormal accumulation of Aβ and α-syn is closely associated with the deficiency of autophagy function [185–187]. The aggregation of these proteins not only directly damages neuronal cells, but also may trigger an inflammatory response, thereby exacerbating the damage to neurons.
In the context of neuroinflammation, activated microglia can impede the autophagy of neurons by releasing pro-inflammatory cytokines such as TNF-α and IL-1β [188]. This inhibition not only exacerbates the accumulation of pathological proteins but may also play a pivotal role in the pathogenesis of neurodegenerative diseases. Concurrently, autophagy exerts a certain anti-inflammatory effect, which can mitigate the neuroinflammatory response by eliminating inflammatory mediators in glial cells. It has been demonstrated that a deficiency in autophagy may result in the excessive activation of microglia, thereby forming a vicious circle [189]. This is to say that the inflammation that occurs in response to the initial insult exacerbates the inhibition of autophagy, which in turn further promotes inflammation.
In addition to its role in nervous system diseases, autophagy also plays an important part in the etiology of other chronic diseases. In cardiovascular diseases, autophagy is of particular importance for the survival of myocardial cells and endothelial cells [190]. This is especially the case in myocardial ischemia–reperfusion injury, where autophagy can remove damaged organelles and deal with metabolic pressure, thus protecting cardiac function. However, in contrast to its role in the nervous system, autophagy’s involvement in cardiovascular disease is more narrowly focused on cell protection and repair, with a relatively limited influence at the local cellular level [191].
In respiratory diseases, autophagy is also involved in the clearance of harmful substances, antiviral agents, and the maintenance of cellular homeostasis. Although autophagy plays an important role in lung health, the respiratory system is less dependent on autophagy than the nervous system [192,193]. In chronic lung inflammation, autophagy dysfunction may contribute to an inflammatory response and apoptosis. However, this does not directly result in neuronal degeneration, which differs from the effects observed in neurodegenerative diseases [194,195].
In conclusion, autophagy plays a complex and pivotal role in neuroinflammation and degenerative diseases. A comprehensive investigation into the regulatory mechanisms of autophagy and its specific role in various diseases is anticipated to yield novel insights and strategies for the treatment of neurodegenerative diseases and other chronic illnesses.
3.8 Mitosis and inflammatory in nervous system diseases
As the core mechanism of cell division, mitosis is of great importance in maintaining the homeostasis of various tissue functions. In the nervous system, this mechanism is observed to exhibit a distinctive degree of complexity. Neurons, as highly specialized cells, typically lack the capacity for division. However, glial cells, particularly astrocytes, demonstrate substantial proliferation in response to nerve injury and disease. This aberrant hyperplasia may not only exacerbate neuroinflammation but also accelerate the pathological process of neurodegenerative diseases [196].
Neuroinflammation is a pivotal mechanism in the pathogenesis of neurodegenerative diseases, whereby microglia and astrocytes are activated. Among these, astrocytes may play a pro-inflammatory role in neuritis, thereby exacerbating the inflammatory response [197]. In certain neurodegenerative diseases, the uncontrolled proliferation of glial cells (i.e., mitosis) results in an exacerbation of the inflammatory response, which directly endangers the survival of neurons [198]. The long-term proliferation of glial cells is accompanied by the release of pro-inflammatory factors, which presents a significant challenge to the function and survival of neurons. Ultimately, this may result in the death or functional decline of neurons.
It is important to highlight that the capacity for nerve regeneration in neurodegenerative diseases is also significantly influenced by the mitotic mechanism [199]. It has been demonstrated that neuritis can impede the proliferation of neural stem cells, constrain nerve regeneration, and accelerate the progression of the disease [200]. In particular, following a brain injury, this limitation of regenerative capacity is of significant consequence [201].
The proliferation of glial cells in AD and PD exhibits distinct pathological characteristics, contingent on the specific disease process. In AD, the excessive activation of microglia promotes the release of inflammatory factors, which in turn exacerbates the accumulation of Aβ, thereby forming a vicious cycle [202]. In PD, the proliferation of astrocytes may exacerbate the inflammatory response and impact the survival of neurons [25].
In contrast, cell proliferation in cardiovascular diseases (such as smooth muscle cells) and airway smooth muscle and fibroblast proliferation in respiratory diseases (such as COPD) are also involved in the process of cell proliferation. However, their primary role is tissue repair and remodeling, which does not directly result in the loss of neural function [203]. For example, in atherosclerosis, the proliferation of smooth muscle cells is essential for vascular repair; however, excessive proliferation may result in the thickening of the vascular wall, further obstructing blood flow, and ultimately leading to vascular sclerosis [204]. In COPD, the proliferation of airway smooth muscle and fibroblasts may result in airway remodeling and an increase in lung injury. However, these proliferation reactions are more closely associated with tissue repair [205,206].
The neuroinflammation and functional loss caused by glial cell proliferation in neurodegenerative diseases are more persistent and destructive, and are difficult to reverse [8]. This not only elucidates the pivotal role of glial cells in neuroinflammation and neurodegenerative diseases but also identifies a crucial target for future treatment strategies. A comprehensive investigation of the regulatory mechanisms governing glial cell proliferation may facilitate the development of novel therapeutic strategies for neurodegenerative disorders (Table 2).
Summary of pharmacology related to inflammatory signaling pathways in neurodegenerative diseases
| Signaling pathways | Related neurodegenerative diseases | Primary mechanism of action | Pharmacological intervention strategies | Known drugs or treatments | References |
|---|---|---|---|---|---|
| Notch signaling pathway | AD, HD | Involved in neuronal differentiation and neural stem cell renewal, regulating neural development and synaptic function | Inhibits Notch signaling pathway to regulate the course of neurodegenerative diseases | Gamma-secretase inhibitors (e.g., DAPT) | [207–209] |
| mTOR signaling pathway | AD, PD, HD | Involved in neuronal metabolism and clearance by controlling processes such as protein synthesis, cell growth, and survival | Promotes autophagy and removal of damaged proteins by inhibiting the mTOR signaling pathway | Rapamycin | [210–212] |
| JAK/STAT signaling pathway | AD, PD | Involved in immune and neuroinflammatory responses that may exacerbate neurodegenerative pathologies | Inhibit JAK/STAT pathway to reduce neuroinflammation | JAK inhibitors (e.g., Tofacitinib) | [213,214] |
| NF-κB signaling pathway | AD, PD, HD | Modulation of inflammatory response, involved in neuroinflammation and apoptosis | Inhibit NF-κB signaling pathway to reduce neuroinflammatory response | Curcumin | [215–217] |
| PI3K/Akt/mTOR pathway | AD, PD | Influences neuronal survival and stress response by regulating processes such as cell survival, proliferation, and metabolism | Activate PI3K/Akt pathway to enhance neuroprotection and anti-stress ability | Lysophosphatidic acid | [218,219] |
4 Immune cells, receptors, and factors related to neuroinflammation
Neuroinflammation is an important pathological process in neurodegenerative diseases, with various factors, including immune cells, cytokines, chemokines, and receptor factors, playing a pivotal role in its mediation. These inflammatory mediators interact with each other to jointly promote neuronal cell damage, functional disorders, and even apoptosis, thereby driving the occurrence and development of neurodegenerative diseases.
4.1 Immune cells
Microglia are resident immune cells in the CNS, constituting approximately 10% of CNS cells and 20% of glial cells in the brain [232,233]. In their resting state, microglia assist in the detection of subtle alterations in pathogens and the microenvironments, acting as sentinels to recognize a multitude of molecular patterns through surface receptors. Upon the perception of damage or PAMPs and DAMPs, microglia undergo a rapid activation process, transitioning from a quiescent state to an activated state and subsequently releasing a variety of inflammatory mediators [234,235]. Astrocytes also play a significant role in neuroinflammation, as they are capable of releasing inflammatory mediators and participating in the regulation of neuroinflammation by altering cell morphology and function [236]. Furthermore, activated astrocytes can form glial scars, which to some extent limit the spread of inflammation [236]. However, excessive glial scar formation may also hinder nerve regeneration. Macrophages have the capacity to migrate from the peripheral blood to the nervous system, participate in inflammatory responses, release inflammatory mediators, and engulf pathogens or damaged cells [237]. In certain neuroinflammatory diseases, such as MS, T cells and B cells contribute to the occurrence and development of neuroinflammation by recognizing self-antigens or foreign antigens, thereby activating immune responses [238].
4.2 Cytokines
As crucial signaling molecules in the neuroinflammatory system, cytokines include a variety of types, the most representative of which include TNF-α, IL-1β, and IL-6 [239]. These cytokines play a pivotal role in the complex immune network, mainly released by activated immune cells such as microglia, macrophages, and certain T cells and B cells [240,241]. In the pathophysiology of neuroinflammation, they are not only the key mediators of early responses, but also profoundly affect the subsequent developmental trajectory of inflammation. Specifically, TNF-α, as a potent pro-inflammatory cytokine, can trigger a series of cascade reactions, promote the activation of vascular endothelial cells, increase vascular permeability, and allow more immune cells and inflammatory mediators to enter the site of inflammation [242]. At the same time, TNF-α can also activate the expression of other cytokines and chemokines, further aggravating the neuroinflammatory response [243]. IL-1β mainly activates transcription factors such as NF-κB, upregulates the expression of adhesion molecules and pro-inflammatory mediators, and promotes the adhesion and migration of inflammatory cells [244]. It plays a particularly significant role in neuroinflammation, inducing the activation of neurons and glial cells, triggering excitotoxicity in neurons, and even leading to neuronal death [244]. IL-6 exhibits more complex functions. On the one hand, it can serve as an acute-phase protein and participate in the body’s defense response; on the other hand, in persistent or excessive inflammatory responses, the overproduction of IL-6 may be closely related to the pathogenesis of various autoimmune diseases and neurodegenerative diseases [245]. It can finely regulate the immune response by regulating the proliferation and differentiation of T cells and B cells, as well as promoting the synthesis of acute-phase proteins by liver cells [245]. These cytokines play a key role in the initiation and maintenance of neuroinflammation by promoting the activation and recruitment of inflammatory cells and enhancing the immune response.
4.3 Chemokines
As a class of cytokines that can specifically guide immune cells (such as leukocytes, monocytes, macrophages, etc.) to migrate to the site of inflammation, chemokines play an indispensable role in the process of neuroinflammation. They bind to CCRs on the surface of cells, triggering intracellular signaling pathways, thereby guiding the directional movement of cells. Monocyte chemoattractant protein-1 (MCP-1), as an important member of the chemokine family, is particularly noteworthy [246]. During the onset of neuroinflammation, the expression of MCP-1 is significantly upregulated, attracting monocytes to cross the blood–brain barrier and enter the CNS, further promoting the exacerbation of inflammatory reactions [221]. The receptor factors associated with neuroinflammation mainly include PRRs and CCRs [246]. Among them, PRRs mainly include TLRs, purinergic receptors, etc., which can recognize PAMPs or DAMPs to trigger immune responses [247]. CCRs such as CCR2 can bind to chemokines and direct immune cells to the site of inflammation [248].
4.4 Oxidative stress and other factors
ROS, as a product of oxidative stress, also play a critical regulatory role in neuroinflammation. The excessive production of ROS can disrupt the redox balance within cells, leading to oxidative damage to neurons and glial cells, which in turn can cause cellular dysfunction and even death [249]. This oxidative stress state not only directly exacerbates the severity of neuroinflammation, but also may indirectly promote the expression of chemokines and the migration of immune cells by activating related signaling pathways. On the other hand, NO and prostaglandins, as important signaling molecules in the body, also participate in the regulation of neuroinflammation [250]. NO, through its free radical properties, can affect various physiological and pathological processes such as vascular permeability, cell proliferation, and apoptosis, thereby influencing the occurrence and development of neuroinflammation [250]. Prostaglandins are a class of lipid mediators with a wide range of biological activities [250]. They have a profound effect on the neuroinflammatory process by regulating vascular relaxation, pain perception, and recruitment of inflammatory cells in inflammatory responses.
5 Neuroinflammation-induced apoptosis
Neuroinflammation in neurodegenerative diseases leads to apoptosis of neurons by producing inflammatory mediators, exacerbating oxidative stress, increasing the concentration of glutamate in the intercellular space, regulating the cell death mode, the direct effect of immune cells, and destroying the blood–brain barrier. Apoptosis is a genetically controlled process of programmed cell death. It is a process of cell death carried out by the organism to maintain the stability of the internal environment. Morphologically, the principal manifestations are the crumpling of the nucleus, as well as the degradation of the chromosomal DNA of the cell, the rupture of the nucleolus, the formation of vesicles in the cytosolic membrane, and the gradual division of the cell into several apoptotic vesicles, which are ultimately absorbed by phagocytes [251,252]. These different pathways of neuroinflammation interact to form a complex network that leads to the neuronal apoptosis and then promotes the onset and development of neurodegenerative diseases (Figure 3 and Table 3).
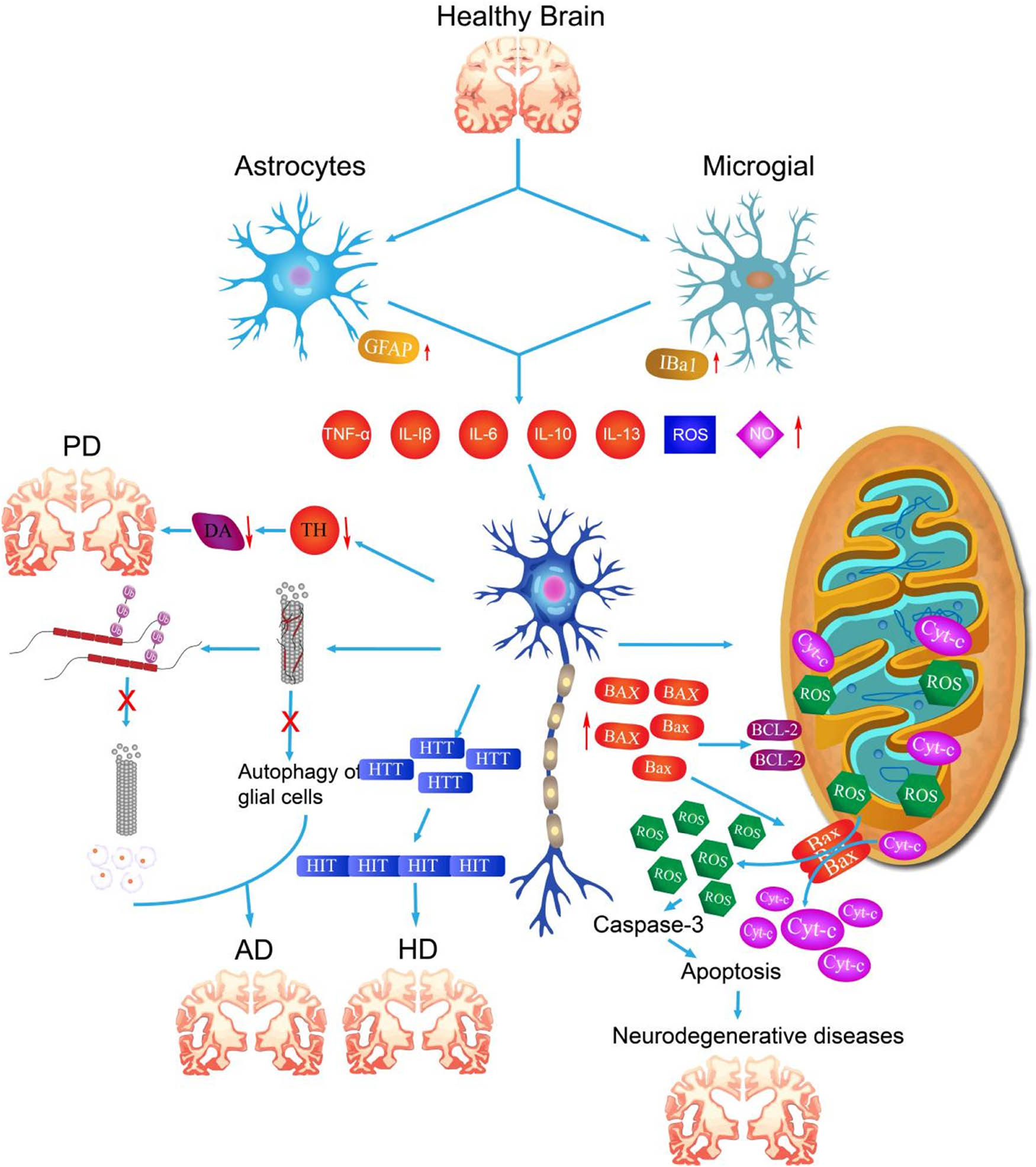
Occurrence and development of neurodegenerative diseases induced by neuroinflammation. Due to the influence of environmental stimuli, aging or genetic factors, neuroinflammation in the brain is induced, and further immune cells release excessive inflammatory factors, which stimulate the changes in the levels of various degenerative markers of neuron cells, abnormal mitochondrial metabolism, and htt gene mutation, leading to the occurrence of PD, AD, HD, and other neurodegenerative diseases.
Relationship between neuroinflammation regulated by apoptosis pathway and neurodegenerative diseases
| Apoptosis pathway | Neurodegenerative diseases | Degree of association | Associations | References |
|---|---|---|---|---|
| Mitochondrial pathway | AD | High | Impaired mitochondrial function in the brain of AD patients leads to the activation of apoptosis-related proteins (e.g., Caspase-9), which accelerates neuronal death. | [220] |
| PD | High | In PD, mitochondrial dysfunction promotes dopaminergic neuronal death through oxidative stress and caspase-dependent pathways. | [221] | |
| HD | Medium | In HD, mitochondrial damage exacerbates intracellular Ca²⁺ accumulation, which in turn activates apoptotic pathways. | [222] | |
| Fas/FasL pathway | AD | Medium | Hyperactivation of the Fas/FasL signaling pathway in the brains of AD patients promotes neuronal death. | [223] |
| ALS | Medium | The Fas/FasL pathway in ALS plays an important role in the apoptotic process of motor neurons. | [224] | |
| HD | Medium | In HD, over-activation of Fas receptors is closely associated with nerve damage. | [225] | |
| Notch pathway | AD | Medium | Aberrant activation of the Notch signaling pathway is associated with neuronal injury and amyloid deposition. Notch interacts with APP proteins and may exacerbate the aberrant processing of APPs, which promotes the formation of amyloid plaques and affects neuronal cell survival. | [226] |
| PD | Medium | The Notch pathway may have an impact on neurodegenerative processes by regulating the production of neuroprotective factors and their ROS. Studies have shown that proper regulation of Notch signaling helps protect dopaminergic neurons from oxidative stress-induced injury. | [227] | |
| HD | low | Evidence suggests that Notch may have a slight effect on disease course by regulating intracellular Htt protein development and neuronal survival signaling. | [228] | |
| p53 pathway | AD | High | p53 plays an important role in AD by promoting neuronal apoptosis, and its expression correlates with AD progression. | [229] |
| PD | High | p53 promotes disease progression by inducing apoptosis and oxidative stress in neuronal cells in PD models. | [230] | |
| HD | Medium | The p53 pathway acts in HD by regulating neuronal death and mitochondrial functional response. | [231] |
5.1 Mitochondrial pathway apoptosis
The mitochondrial pathway is one of the important pathways of neuronal apoptosis induced by neuroinflammation. The regulation of mitochondrial apoptosis is primarily attributed to the Bcl-2 family, which is divided into two major classes based on its function. These classes include anti-apoptotic and pro-apoptotic proteins, which work in concert to maintain the stability of mitochondria [253]. The pro-apoptotic proteins contain the BH3 domain and are categorized as Bak, Bax, Bok, Bim, Bad, Bid, Bik, and Bmf. Antiapoptotic proteins include Bcl-2, Bcl-xl, Bcl-W, and others, which contain the BH4 domain [254]. These anti-apoptotic proteins mainly distributed in the mitochondrial membrane, and can stabilize the mitochondrial membrane potential, inhibit the activity of pro-apoptotic proteins, and maintain the normal functions of mitochondria [255,256].
In neuroinflammatory cells associated with neurodegenerative diseases, upon receiving apoptotic signals, apoptotic proteins, such as Bak and Bax, undergo a transition from inhibition to activation, translocation, and localization to the mitochondrial membrane. They bind to apoptotic proteins such as Bcl-2 and inhibit its function. This results in the formation of transmembrane pores on the surface of the mitochondrial membrane, a reduction in the mitochondrial membrane potential, and the destruction of mitochondrial stability [257,258]. A reduction in the mitochondrial membrane potential and an imbalance in stability results in the release of mitochondrial contents, such as Cytc, ROS, and other mitochondrial contents, into the cytoplasm, activating intracytoplasmic caspase-3 to initiate the apoptotic process of neurons in an inflammatory state in neurodegenerative diseases such as PD (Figure 4) [259,260]. P53, which is highly related to mitochondrial metabolism, can be transferred to the surface of mitochondria and bind to Bcl-2, inhibiting its anti-apoptotic activity (Figure 5). It can also interact with Bax protein, resulting in increased expression of both, and promoting the apoptotic response [261,262].

Schematic diagram of the signaling pathway of LPS-induced apoptosis. LPS, as well as inflammatory factors, activate MYD88 by acting on the membrane receptor TLR4, thus mediating the activation of the NF-κB signaling pathway. Activated NF-κB moves to the nucleus and exerts the role of a transcription factor to regulate the expression of genes. It is also involved in regulating the expression of genes such as Bax, Bcl-2, etc., and up-regulating the expression of apoptosis-associated proteins such as Bax and down-regulating the expression level of anti-apoptotic proteins such as Bcl-2. Bax and Bak form a pore-like structure at the mitochondrial surface, which leads to the loss of structural integrity of the mitochondrial membrane and the release of contents. This results in the activation of the intracellular caspase family and the apoptosis of the cellular mitochondrial pathway.

Using the String database to analyze protein interactions. A search of the String website revealed that P53 has robust interactions with apoptosis-related proteins, including Bax, Bcl-2, and Bak.
5.2 Pyroptosis pathway
In this article, apoptosis-related proteins such as Bax, Bcl-2, and P53 were found to be closely related to the caspase protein family associated with cellular pyroptosis by searching the STRING protein interactions website (Figure 6). Pyroptosis is a caspase-1-dependent programmed cell death pathway that results in the rupture of cell membranes and the subsequent release of large amounts of inflammatory factors. In 2005, Prof. Shao Feng’s team was the first to identify the cellular pyroptosis pathway. The main mechanism is that caspase-1/4/5/11 induces cellular pyroptosis by cleaving GSDMs [263]. GSDMs are a family of proteins with perforation effects, consisting of six types of genes. Of these, GSDMD and GSDME are the key molecules in the development of cellular pyroptosis [264–267]. The current study identified two distinct pathways for the activation of the cellular pyroptosis process: the classical and non-classical pathways.

Using the String database to analyze protein interactions. P53 interacts with a number of proteins involved in apoptosis, including Bax and Bcl-2. In addition, caspase-6, caspase-7, and caspase-9, among others in the caspase family, interact with proteins involved in apoptosis, including Bax and Bcl-2.
The classical activation pathway is initiated by a class of protein complexes, designated as inflammatory vesicles, which are composed of PRRs, ASCs, and caspase-1. Activation of caspase-1 results in the maturation of pro-IL-1β and pro-IL-18, as well as cleavage of GSDMD into the small molecule proteins NT and CT. NT can bind to lipid molecules on the surface of the cell membrane, forming pores in the membrane, which results in the release of IL-1β. TNF-α and HMGB1 are released from the cell through the pores, and at the same time, water molecules enter the cell through the pore, leading to cell rupture. This further increases the release of inflammatory factors and exacerbates apoptosis and inflammatory responses [268,269].
The nonclassical pathway, also known as noncaspase-1-dependent nonclassical activation, involves direct stimulation of caspase-4/5/11 by LPS, which promotes the cleavage of GSDMD and the maturation and release of inflammatory factors [270,271]. Alternatively, the non-inflammatory apoptosis of cells can be converted to cellular pyroptosis by the activation of caspase-3, which cleaves GSDME. Furthermore, the activation of caspase-8 cleaves GSDMD, thereby switching cells from the apoptotic pathway to pyroptosis [272,273].
In the process of neuroinflammation, the pro-inflammatory factors released by activated immune cells can trigger the process of cell death. Pyrosis further exacerbates the inflammatory response, creating a vicious cycle that continuously damages neurons in neurodegenerative diseases [274]. On the other hand, cell death leads directly to the death of neurons through the rupture of the cell membrane and the release of cell contents [274]. This is an important mechanism of neuronal loss in neurodegenerative diseases. The onset and development of neurodegenerative diseases are often accompanied by neuroinflammation and cell death. For example, in AD, the abnormal accumulation of β-amyloid protein can cause an inflammatory response and then trigger cell death, leading to neuronal death. Similar inflammatory responses and cell death may occur in PD, ALS, and other neurodegenerative diseases.
Recent studies on neuronal apoptosis have provided additional insights beyond the two pathways mentioned above. For example, it has been demonstrated that this process can influence the occurrence of neuronal apoptosis by triggering the activation of NLRP3 inflammatory vesicles. Moreover, several studies have demonstrated that GSDMD and GSDME are also involved in the process of necrotic apoptosis [275,276]. In addition to apoptosis, the ubiquitin metabolism system within neurons, the autophagy system, epitope modification of DNA, and abnormal levels of intracellular lactate have been proposed as potential contributors to the development of neurodegenerative diseases [277,278]. The current comprehensive investigation of neuronal damage and apoptotic pathways offers a substantial theoretical foundation for the pursuit of neuronal apoptosis-associated neurodegenerative disease mechanisms. This will facilitate the identification of the most pivotal pathways or biomarkers influencing neurodegenerative disease progression and the development of therapeutic strategies for neurodegenerative diseases.
6 Perspective
As life expectancy continues to increase, aging-related neurodegenerative diseases such as PD and AD will become one of the most significant threats to the quality of healthy life in old age. Therefore, there is an urgent need to investigate the pathogenesis of such diseases and their potential possible pathogenic factors, and to explore drugs and medical treatments that can effectively treat or delay the development of the disease, so as to help patients improve their quality of life. This study reviews the characteristics and specific pathways of neuroinflammation in the early stages of neurodegenerative diseases. Nevertheless, the relationship between long-term inflammatory infiltration and neuronal cell activity, as well as the association between neuronal damage and loss and long-term neuroinflammation, remains poorly understood. These areas warrant further investigation by neuroscientists. It remains unclear whether the cellular pyroptosis pathway, which has been identified in recent years, is also involved in the pathogenesis of neurodegenerative diseases. Consequently, the relationship between long-term inflammation and neuronal damage and loss, as well as the potential role of cellular pyroptosis in neuronal loss, will likely be a significant area of investigation in the field of neurodegenerative diseases and neuroinflammation.
Acknowledgements
The authors sincerely apologize to all their colleagues whose important work could not be cited in this review owing to space limitations, especially many prominent and pioneer works in the neurodegenerative diseases and neuroinflammation field.
-
Funding information: This work was supported by the Key Research Foundation of Wannan Medical College (WK2021Z06), The Anhui Province College Student Innovation and Entrepreneurship Training Program Project (S202310368017), The Natural Science Foundation of Guangdong Province (2020A1515010113), The National Natural Science Foundation of China (82072890 and 31701288), and Anhui Province Outstanding Youth Research Program in Colleges and Universities (2024AH020014).
-
Author contributions: Shi Huang drafted the manuscript and prepared the figures. Yaxin Lu, Wanzhen Fang, and Yanjiao Huang polished and modified the content. Qiang Li and Zhiliang Xu developed the concept and revised the manuscript. All authors read and approved the final version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Wilson DM, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell. 2023;186(4):693–714.10.1016/j.cell.2022.12.032Search in Google Scholar PubMed
[2] World Alzheimer Report. Alzheimer’s disease international (ADI) [Internet]. [cited 2025 Jan 10]; 2019. https://www.alzint.org/resource/world-alzheimer-report-2019/.Search in Google Scholar
[3] World Alzheimer Report. Alzheimer’s disease international (ADI) [Internet]. [cited 2025 Jan 10]; 2018, https://www.alzint.org/resource/world-alzheimer-report-2018/.Search in Google Scholar
[4] Mecca AP, van Dyck CH. Alzheimer’s & dementia: the journal of the alzheimer’s association. Alzheimers Dement. 2021 Feb;17(2):316–7.10.1002/alz.12190Search in Google Scholar PubMed PubMed Central
[5] Gammon K. Neurodegenerative disease: brain windfall. Nature. 2014 Nov;515(7526):299–300.10.1038/nj7526-299aSearch in Google Scholar PubMed
[6] Gambini J, Stromsnes K. Oxidative stress and inflammation: from mechanisms to therapeutic approaches. Biomedicines. 2022 Mar;10(4):753.10.3390/biomedicines10040753Search in Google Scholar PubMed PubMed Central
[7] Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease – a double-edged sword. Neuron. 2002 Aug;35(3):419–32.10.1016/S0896-6273(02)00794-8Search in Google Scholar
[8] Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020 Nov;9(1):42.10.1186/s40035-020-00221-2Search in Google Scholar PubMed PubMed Central
[9] Kempuraj D, Thangavel R, Natteru P, Selvakumar G, Saeed D, Zahoor H, et al. Neuroinflammation induces neurodegeneration. J Neurol Neurosurg Spine. 2016;1(1):1003.Search in Google Scholar
[10] Levite M. Neuro faces of beneficial T cells: essential in brain, impaired in aging and neurological diseases, and activated functionally by neurotransmitters and neuropeptides. Neural Regen Res. 2022 Oct;18(6):1165–78.10.4103/1673-5374.357903Search in Google Scholar PubMed PubMed Central
[11] Vizioli MG, Liu T, Miller KN, Robertson NA, Gilroy K, Lagnado AB, et al. Mitochondria-to-nucleus retrograde signaling drives formation of cytoplasmic chromatin and inflammation in senescence. Genes Dev. 2020 Mar;34(5–6):428–45.10.1101/gad.331272.119Search in Google Scholar PubMed PubMed Central
[12] Paul BD, Snyder SH, Bohr VA. Signaling by cGAS-STING in neurodegeneration, neuroinflammation and aging. Trends Neurosci. 2021 Feb;44(2):83–96.10.1016/j.tins.2020.10.008Search in Google Scholar PubMed PubMed Central
[13] Teissier T, Boulanger E, Cox LS. Interconnections between inflammageing and immunosenescence during ageing. Cells. 2022 Jan;11(3):359.10.3390/cells11030359Search in Google Scholar PubMed PubMed Central
[14] Kölliker-Frers R, Udovin L, Otero-Losada M, Kobiec T, Herrera MI, Palacios J, et al. Neuroinflammation: an integrating overview of reactive-neuroimmune cell interactions in health and disease. Mediators Inflamm. 2021 May;2021:9999146.10.1155/2021/9999146Search in Google Scholar PubMed PubMed Central
[15] Shabab T, Khanabdali R, Moghadamtousi SZ, Kadir HA, Mohan G. Neuroinflammation pathways: a general review. Int J Neurosci. 2017 Jul;127(7):624–33.10.1080/00207454.2016.1212854Search in Google Scholar PubMed
[16] Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. 2010 Feb;12(1):39–45.10.1007/s11920-009-0082-1Search in Google Scholar PubMed
[17] Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30(6):791–807.10.1016/j.neubiorev.2006.06.005Search in Google Scholar PubMed
[18] Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Arch Intern Med. 2007 Apr;167(7):635–41.10.1001/archinte.167.7.635Search in Google Scholar PubMed
[19] Qiu J, Guo J, Liu L, Liu X, Sun X, Chen H. Vav1 promotes inflammation and neuronal apoptosis in cerebral ischemia/reperfusion injury by upregulating microglial and NLRP3 inflammasome activation. Neural Regen Res. 2023 Mar;18(11):2436–42.10.4103/1673-5374.371368Search in Google Scholar PubMed PubMed Central
[20] Singh D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J Neuroinflammation. 2022 Aug;19(1):206.10.1186/s12974-022-02565-0Search in Google Scholar PubMed PubMed Central
[21] Lee JA, Kwon YW, Kim HR, Shin N, Son HJ, Cheong CS, et al. A novel pyrazolo [3,4-d]pyrimidine induces heme oxygenase-1 and exerts anti-inflammatory and neuroprotective effects. Mol Cell. 2022 Mar;45(3):134–47.10.14348/molcells.2021.0074Search in Google Scholar PubMed PubMed Central
[22] Lau V, Ramer L, Tremblay MÈ. An aging, pathology burden, and glial senescence build-up hypothesis for late onset Alzheimer’s disease. Nat Commun. 2023 Mar;14(1):1670.10.1038/s41467-023-37304-3Search in Google Scholar PubMed PubMed Central
[23] Verkhratsky A, Zorec R, Rodriguez-Arellano JJ, Parpura V. Neuroglia in ageing. Adv Exp Med Biol. 2019;1175:181–97.10.1007/978-981-13-9913-8_8Search in Google Scholar PubMed PubMed Central
[24] Onyango IG, Jauregui GV, Čarná M, Bennett JP, Stokin GB. Neuroinflammation in Alzheimer’s disease. Biomedicines. 2021 May;9(5):524.10.3390/biomedicines9050524Search in Google Scholar PubMed PubMed Central
[25] Miyazaki I, Asanuma M. Neuron–astrocyte interactions in Parkinson’s disease. Cells. 2020 Dec;9(12):2623.10.3390/cells9122623Search in Google Scholar PubMed PubMed Central
[26] Wilton DK, Stevens B. The contribution of glial cells to Huntington’s disease pathogenesis. Neurobiol Dis. 2020 Sep;143:104963.10.1016/j.nbd.2020.104963Search in Google Scholar PubMed PubMed Central
[27] Han T, Xu Y, Sun L, Hashimoto M, Wei J. Microglial response to aging and neuroinflammation in the development of neurodegenerative diseases. Neural Regen Res. 2024 Jun;19(6):1241–8.10.4103/1673-5374.385845Search in Google Scholar PubMed PubMed Central
[28] Twarowski B, Herbet M. Inflammatory processes in Alzheimer’s disease-pathomechanism, diagnosis and treatment: a review. Int J Mol Sci. 2023 Mar;24(7):6518.10.3390/ijms24076518Search in Google Scholar PubMed PubMed Central
[29] Newcombe EA, Camats-Perna J, Silva ML, Valmas N, Huat TJ, Medeiros R. Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J Neuroinflammation. 2018 Sep;15(1):276.10.1186/s12974-018-1313-3Search in Google Scholar PubMed PubMed Central
[30] Wu J, Han Y, Xu H, Sun H, Wang R, Ren H, et al. Deficient chaperone-mediated autophagy facilitates LPS-induced microglial activation via regulation of the p300/NF-κB/NLRP3 pathway. Sci Adv. 2023 Oct;9(40):eadi8343.10.1126/sciadv.adi8343Search in Google Scholar PubMed PubMed Central
[31] Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in parkinson disease. Nat Rev Immunol. 2022 Nov;22(11):657–73.10.1038/s41577-022-00684-6Search in Google Scholar PubMed PubMed Central
[32] Asanuma M, Nishibayashi-Asanuma S, Miyazaki I, Kohno M, Ogawa N. Neuroprotective effects of non-steroidal anti-inflammatory drugs by direct scavenging of nitric oxide radicals. J Neurochem. 2001 Mar;76(6):1895–904.10.1046/j.1471-4159.2001.00205.xSearch in Google Scholar PubMed
[33] Yan Y, Jiang W, Liu L, Wang X, Ding C, Tian Z, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015 Jan;160(1–2):62–73.10.1016/j.cell.2014.11.047Search in Google Scholar PubMed
[34] Zhao Z, Ning J, Bao XQ, Shang M, Ma J, Li G, et al. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota–gut–brain axis. Microbiome. 2021 Nov;9(1):226.10.1186/s40168-021-01107-9Search in Google Scholar PubMed PubMed Central
[35] Crotti A, Glass CK. The choreography of neuroinflammation in Huntington’s disease. Trends Immunol. 2015 Jun;36(6):364–73.10.1016/j.it.2015.04.007Search in Google Scholar PubMed PubMed Central
[36] Saba J, Couselo FL, Bruno J, Carniglia L, Durand D, Lasaga M, et al. Neuroinflammation in Huntington’s disease: a starring role for astrocyte and microglia. Curr Neuropharmacol. 2022;20(6):1116–43.10.2174/1570159X19666211201094608Search in Google Scholar PubMed PubMed Central
[37] Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, et al. A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med. 2008 Aug;205(8):1869–77.10.1084/jem.20080178Search in Google Scholar PubMed PubMed Central
[38] Silvestroni A, Faull RLM, Strand AD, Möller T. Distinct neuroinflammatory profile in post-mortem human Huntington’s disease. Neuroreport. 2009 Aug;20(12):1098–103.10.1097/WNR.0b013e32832e34eeSearch in Google Scholar PubMed
[39] Zelic M, Pontarelli F, Woodworth L, Zhu C, Mahan A, Ren Y, et al. RIPK1 activation mediates neuroinflammation and disease progression in multiple sclerosis. Cell Rep. 2021 May;35(6):109112.10.1016/j.celrep.2021.109112Search in Google Scholar PubMed PubMed Central
[40] Khaibullin T, Ivanova V, Martynova E, Cherepnev G, Khabirov F, Granatov E, et al. Elevated levels of proinflammatory cytokines in cerebrospinal fluid of multiple sclerosis patients. Front Immunol. 2017;8:531.10.3389/fimmu.2017.00531Search in Google Scholar PubMed PubMed Central
[41] Burman J, Svensson E, Fransson M, Loskog ASI, Zetterberg H, Raininko R, et al. The cerebrospinal fluid cytokine signature of multiple sclerosis: a homogenous response that does not conform to the Th1/Th2/Th17 convention. J Neuroimmunol. 2014 Dec;277(1–2):153–9.10.1016/j.jneuroim.2014.10.005Search in Google Scholar PubMed
[42] Hanscom M, Loane DJ, Shea-Donohue T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J Clin Invest. 2021 Jun;131(12):e143777.10.1172/JCI143777Search in Google Scholar PubMed PubMed Central
[43] Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab. 2009 Sep;29(9):1503–16.10.1038/jcbfm.2009.71Search in Google Scholar PubMed PubMed Central
[44] Karve IP, Taylor JM, Crack PJ. The contribution of astrocytes and microglia to traumatic brain injury. Br J Pharmacol. 2016 Feb;173(4):692–702.10.1111/bph.13125Search in Google Scholar PubMed PubMed Central
[45] Shi K, Zhang J, Dong JF, Shi FD. Dissemination of brain inflammation in traumatic brain injury. Cell Mol Immunol. 2019 Jun;16(6):523–30.10.1038/s41423-019-0213-5Search in Google Scholar PubMed PubMed Central
[46] Cohen J, Mathew A, Dourvetakis KD, Sanchez-Guerrero E, Pangeni RP, Gurusamy N, et al. Recent research trends in neuroinflammatory and neurodegenerative disorders. Cells. 2024 Mar;13(6):511.10.3390/cells13060511Search in Google Scholar PubMed PubMed Central
[47] Alshelh Z, Albrecht DS, Bergan C, Akeju O, Clauw DJ, Conboy L, et al. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav Immun. 2020 Jul;87:498–507.10.1016/j.bbi.2020.01.020Search in Google Scholar PubMed PubMed Central
[48] Nkiliza A, Joshi U, Evans JE, Ait-Ghezala G, Parks M, Crawford F, et al. Adaptive immune responses associated with the central nervous system pathology of Gulf War illness. Neurosci Insights. 2021;16:26331055211018458.10.1177/26331055211018458Search in Google Scholar PubMed PubMed Central
[49] Dickey B, Madhu LN, Shetty AK. Gulf War illness: mechanisms underlying brain dysfunction and promising therapeutic strategies. Pharmacol Ther. 2021 Apr;220:107716.10.1016/j.pharmthera.2020.107716Search in Google Scholar PubMed PubMed Central
[50] Saha P, Skidmore PT, Holland LA, Mondal A, Bose D, Seth RK, et al. Andrographolide attenuates gut–brain–axis associated pathology in Gulf War illness by modulating bacteriome-virome associated inflammation and microglia-neuron proinflammatory crosstalk. Brain Sci. 2021 Jul;11(7):905.10.3390/brainsci11070905Search in Google Scholar PubMed PubMed Central
[51] Obrador E, Salvador R, López-Blanch R, Jihad-Jebbar A, Vallés SL, Estrela JM. Oxidative stress, neuroinflammation and mitochondria in the pathophysiology of amyotrophic lateral sclerosis. Antioxidants (Basel). 2020 Sep;9(9):901.10.3390/antiox9090901Search in Google Scholar PubMed PubMed Central
[52] Chen X, Hu Y, Cao Z, Liu Q, Cheng Y. Cerebrospinal fluid inflammatory cytokine aberrations in Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis: a systematic review and meta-analysis. Front Immunol. 2018;9:2122.10.3389/fimmu.2018.02122Search in Google Scholar PubMed PubMed Central
[53] Wilms H, Sievers J, Dengler R, Bufler J, Deuschl G, Lucius R. Intrathecal synthesis of monocyte chemoattractant protein-1 (MCP-1) in amyotrophic lateral sclerosis: further evidence for microglial activation in neurodegeneration. J Neuroimmunol. 2003 Nov;144(1–2):139–42.10.1016/j.jneuroim.2003.08.042Search in Google Scholar PubMed
[54] Hu Y, Cao C, Qin XY, Yu Y, Yuan J, Zhao Y, et al. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci Rep. 2017 Aug;7(1):9094.10.1038/s41598-017-09097-1Search in Google Scholar PubMed PubMed Central
[55] Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021 Apr;397(10284):1577–90.10.1016/S0140-6736(20)32205-4Search in Google Scholar PubMed PubMed Central
[56] Weller J, Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research. 2018;7:F1000 Faculty Rev-1161.10.12688/f1000research.14506.1Search in Google Scholar PubMed PubMed Central
[57] Harrison TM, La Joie R, Maass A, Baker SL, Swinnerton K, Fenton L, et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann Neurol. 2019 Feb;85(2):229–40.10.1002/ana.25406Search in Google Scholar PubMed PubMed Central
[58] Ossenkoppele R, van der Kant R, Hansson O. Tau biomarkers in Alzheimer’s disease: towards implementation in clinical practice and trials. Lancet Neurol. 2022 Aug;21(8):726–34.10.1016/S1474-4422(22)00168-5Search in Google Scholar PubMed
[59] Trejo-Lopez JA, Yachnis AT, Prokop S. Neuropathology of Alzheimer’s disease. Neurotherapeutics. 2022 Jan;19(1):173–85.10.1007/s13311-021-01146-ySearch in Google Scholar PubMed PubMed Central
[60] Boon BDC, Hoozemans JJM, Lopuhaä B, Eigenhuis KN, Scheltens P, Kamphorst W, et al. Neuroinflammation is increased in the parietal cortex of atypical Alzheimer’s disease. J Neuroinflammation. 2018 May;15(1):170.10.1186/s12974-018-1180-ySearch in Google Scholar PubMed PubMed Central
[61] Armstrong AR. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019;57(2):87–105.10.5114/fn.2019.85929Search in Google Scholar PubMed
[62] Jorfi M, Maaser-Hecker A, Tanzi RE. The neuroimmune axis of Alzheimer’s disease. Genome Med. 2023 Jan;15(1):6.10.1186/s13073-023-01155-wSearch in Google Scholar PubMed PubMed Central
[63] Al-Ghraiybah NF, Wang J, Alkhalifa AE, Roberts AB, Raj R, Yang E, et al. Glial cell-mediated neuroinflammation in Alzheimer’s disease. Int J Mol Sci. 2022 Sep;23(18):10572.10.3390/ijms231810572Search in Google Scholar PubMed PubMed Central
[64] Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017 Apr;35:441–68.10.1146/annurev-immunol-051116-052358Search in Google Scholar PubMed PubMed Central
[65] Zhang G, Wang Z, Hu H, Zhao M, Sun L. Microglia in Alzheimer’s disease: a target for therapeutic intervention. Front Cell Neurosci. 2021;15:749587.10.3389/fncel.2021.749587Search in Google Scholar PubMed PubMed Central
[66] Jiang CT, Wu WF, Deng YH, Ge JW. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol Med Rep. 2020 May;21(5):2006–18.10.3892/mmr.2020.11003Search in Google Scholar PubMed PubMed Central
[67] Liu M, Wang W, Zhang Y, Xu Z. Effects of combined electroacupuncture and medication therapy on the RhoA/ROCK-2 signaling pathway in the striatal region of rats afflicted by cerebral ischemia. Brain Res Bull. 2023 Dec;205:110828.10.1016/j.brainresbull.2023.110828Search in Google Scholar PubMed
[68] Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018 Sep;105:518–25.10.1016/j.biopha.2018.05.143Search in Google Scholar PubMed
[69] Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, et al. Interleukin-4 Is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke. 2016 Feb;47(2):498–504.10.1161/STROKEAHA.115.012079Search in Google Scholar PubMed PubMed Central
[70] Feng Y, He X, Luo S, Chen X, Long S, Liang F, et al. Chronic colitis induces meninges traffic of gut-derived T cells, unbalances M1 and M2 microglia/macrophage and increases ischemic brain injury in mice. Brain Res. 2019 Mar;1707:8–17.10.1016/j.brainres.2018.11.019Search in Google Scholar PubMed
[71] Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015 Jan;11(1):56–64.10.1038/nrneurol.2014.207Search in Google Scholar PubMed PubMed Central
[72] Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol. 2013 Feb;39(1):19–34.10.1111/j.1365-2990.2012.01306.xSearch in Google Scholar PubMed PubMed Central
[73] Michels M, Sonai B, Dal-Pizzol F. Polarization of microglia and its role in bacterial sepsis. J Neuroimmunol. 2017 Feb;303:90–8.10.1016/j.jneuroim.2016.12.015Search in Google Scholar PubMed
[74] Thu VTA, Hoang TX, Kim JY. 1,25-Dihydroxy vitamin D3 facilitates the M2 polarization and β-amyloid hptake by human microglia in a TREM2-dependent manner. Biomed Res Int. 2023;2023:3483411.10.1155/2023/3483411Search in Google Scholar PubMed PubMed Central
[75] Babalola JA, Lang M, George M, Stracke A, Tam-Amersdorfer C, Itxaso I, et al. Astaxanthin enhances autophagy, amyloid beta clearance and exerts anti-inflammatory effects in in vitro models of Alzheimer’s disease-related blood brain barrier dysfunction and inflammation. Brain Res. 2023 Nov;1819:148518.10.1016/j.brainres.2023.148518Search in Google Scholar PubMed
[76] Lue LF, Kuo YM, Beach T, Walker DG. Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Mol Neurobiol. 2010 Jun;41(2–3):115–28.10.1007/s12035-010-8106-8Search in Google Scholar PubMed PubMed Central
[77] Mudher A, Lovestone S. Alzheimer’s disease-do tauists and baptists finally shake hands? Trends Neurosci. 2002 Jan;25(1):22–6.10.1016/S0166-2236(00)02031-2Search in Google Scholar PubMed
[78] Iqbal K, Grundke-Iqbal I. Alzheimer neurofibrillary degeneration: significance, etiopathogenesis, therapeutics and prevention. J Cell Mol Med. 2008;12(1):38–55.10.1111/j.1582-4934.2008.00225.xSearch in Google Scholar PubMed PubMed Central
[79] Shackleton B, Crawford F, Bachmeier C. Inhibition of ADAM10 promotes the clearance of Aβ across the BBB by reducing LRP1 ectodomain shedding. Fluids Barriers CNS. 2016 Aug;13(1):14.10.1186/s12987-016-0038-xSearch in Google Scholar PubMed PubMed Central
[80] Heckmann BL, Teubner BJW, Tummers B, Boada-Romero E, Harris L, Yang M, et al. LC3-associated endocytosis facilitates β-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell. 2019 Jul;178(3):536–51.e14.10.1016/j.cell.2019.05.056Search in Google Scholar PubMed PubMed Central
[81] Morales I, Guzmán-Martínez L, Cerda-Troncoso C, Farías GA, Maccioni RB. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front Cell Neurosci. 2014;8:112.10.3389/fncel.2014.00112Search in Google Scholar PubMed PubMed Central
[82] Fan YY, Huo J. A1/A2 astrocytes in central nervous system injuries and diseases: angels or devils? Neurochem Int. 2021 Sep;148:105080.10.1016/j.neuint.2021.105080Search in Google Scholar PubMed
[83] Park JM, Rahmati M, Lee SC, Shin JI, Kim YW. Effects of mesenchymal stem cell on dopaminergic neurons, motor and memory functions in animal models of Parkinson’s disease: a systematic review and meta-analysis. Neural Regen Res. 2024 Jul;19(7):1584–92.10.4103/1673-5374.387976Search in Google Scholar PubMed PubMed Central
[84] Burchill E, Watson CJ, Fanshawe JB, Badenoch JB, Rengasamy E, Ghanem DA, et al. The impact of psychiatric comorbidity on Parkinson’s disease outcomes: a systematic review and meta-analysis. Lancet Reg Health Eur. 2024 Apr;39:100870.10.1016/j.lanepe.2024.100870Search in Google Scholar PubMed PubMed Central
[85] González-May CA, Barradas-Castillo MDR, Perera-Rios JH, Gallegos-Tintoré S, Pérez-Izquierdo O. Aranda-González II. Dietary flavonoids may have a protective and therapeutic effect in Parkinson disease: a systematic review. Nutr Res. 2024 Jan;121:39–50.10.1016/j.nutres.2023.10.004Search in Google Scholar PubMed
[86] Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, et al. Oxidative stress in neurodegenerative diseases. Mol Neurobiol. 2016 Aug;53(6):4094–125.10.1007/s12035-015-9337-5Search in Google Scholar PubMed PubMed Central
[87] Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis. 2018 Jan;109(Pt B):249–57.10.1016/j.nbd.2017.04.004Search in Google Scholar PubMed
[88] Wang J, Zhao J, Zhao K, Wu S, Chen X, Hu W. The role of calcium and iron homeostasis in Parkinson’s disease. Brain Sci. 2024 Jan;14(1):88.10.3390/brainsci14010088Search in Google Scholar PubMed PubMed Central
[89] Kadala A, Verdier D, Morquette P, Kolta A. Ion homeostasis in rhythmogenesis: the interplay between neurons and astroglia. Physiol (Bethesda). 2015 Sep;30(5):371–88.10.1152/physiol.00023.2014Search in Google Scholar PubMed
[90] Ureshino RP, Erustes AG, Bassani TB, Wachilewski P, Guarache GC, Nascimento AC, et al. The tnterplay between Ca2+ signaling pathways and neurodegeneration. Int J Mol Sci. 2019 Nov;20(23):6004.10.3390/ijms20236004Search in Google Scholar PubMed PubMed Central
[91] Idowu OK, Oremosu AA, Dosumu OO, Mohammed AA. Ribose-cysteine and levodopa abrogate Parkinsonism via the regulation of neurochemical and redox activities in alpha-synuclein transgenic Drosophila melanogaster models. Fly (Austin). 2024 Dec;18(1):2306687.10.1080/19336934.2024.2306687Search in Google Scholar PubMed PubMed Central
[92] Schaffrath A, Schleyken S, Seger A, Jergas H, Özdüzenciler P, Pils M, et al. Patients with isolated REM-sleep behavior disorder have elevated levels of alpha-synuclein aggregates in stool. NPJ Parkinsons Dis. 2023 Feb;9(1):14.10.1038/s41531-023-00458-4Search in Google Scholar PubMed PubMed Central
[93] Yang K, Yan Y, Yu A, Zhang R, Zhang Y, Qiu Z, et al. Mitophagy in neurodegenerative disease pathogenesis. Neural Regen Res. 2024 May;19(5):998–1005.10.4103/1673-5374.385281Search in Google Scholar PubMed PubMed Central
[94] Marder KS, Tang MX, Mejia-Santana H, Rosado L, Louis ED, Comella CL, et al. Predictors of parkin mutations in early-onset Parkinson disease: the consortium on risk for early-onset Parkinson disease study. Arch Neurol. 2010 Jun;67(6):731–8.10.1001/archneurol.2010.95Search in Google Scholar PubMed PubMed Central
[95] Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, et al. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015 Aug;524(7565):309–14.10.1038/nature14893Search in Google Scholar PubMed PubMed Central
[96] Zhang Y, Yin S, Song R, Lai X, Shen M, Wu J, et al. A novel mechanism of PHB2-mediated mitophagy participating in the development of Parkinson’s disease. Neural Regen Res. 2024 Aug;19(8):1828–34.10.4103/1673-5374.389356Search in Google Scholar PubMed PubMed Central
[97] Rodríguez-Arribas M, Yakhine-Diop SMS, Pedro JMBS, Gómez-Suaga P, Gómez-Sánchez R, Martínez-Chacón G, et al. Mitochondria-associated membranes (MAMs): overview and its role in Parkinson’s disease. Mol Neurobiol. 2017 Oct;54(8):6287–303.10.1007/s12035-016-0140-8Search in Google Scholar PubMed
[98] Rozzi SJ, Avdoshina V, Fields JA, Mocchetti I. Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov. 2018 Dec;4:8.10.1038/s41420-017-0013-6Search in Google Scholar PubMed PubMed Central
[99] Bhattarai Y, Si J, Pu M, Ross OA, McLean PJ, Till L, et al. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson’s disease. Gut Microbes. 2021 Jan;13(1):1866974.10.1080/19490976.2020.1866974Search in Google Scholar PubMed PubMed Central
[100] Bhattarai Y, Kashyap PC. Parkinson’s disease: are gut microbes involved? Am J Physiol Gastrointest Liver Physiol. 2020 Nov;319(5):G529–40.10.1152/ajpgi.00058.2020Search in Google Scholar PubMed PubMed Central
[101] Sharma M, Malim FM, Goswami A, Sharma N, Juvvalapalli SS, Chatterjee S, et al. Neuroprotective effect of Swertiamarin in a Rotenone model of Parkinson’s disease: role of neuroinflammation and alpha-synuclein accumulation. ACS Pharmacol Transl Sci. 2023 Jan;6(1):40–51.10.1021/acsptsci.2c00120Search in Google Scholar PubMed PubMed Central
[102] Domingues AV, Pereira IM, Vilaça-Faria H, Salgado AJ, Rodrigues AJ, Teixeira FG. Glial cells in Parkinson’s disease: protective or deleterious? Cell Mol Life Sci. 2020 Dec;77(24):5171–88.10.1007/s00018-020-03584-xSearch in Google Scholar PubMed PubMed Central
[103] Boonpraman N, Yi SS. NADPH oxidase 4 (NOX4) as a biomarker and therapeutic target in neurodegenerative diseases. Neural Regen Res. 2024 Sep;19(9):1961–6.10.4103/1673-5374.390973Search in Google Scholar PubMed PubMed Central
[104] Dong H, Zhang X, Duan Y, He Y, Zhao J, Wang Z, et al. Hypoxia inducible factor-1α regulates microglial innate immune memory and the pathology of Parkinson’s disease. J Neuroinflammation. 2024 Mar;21(1):80.10.1186/s12974-024-03070-2Search in Google Scholar PubMed PubMed Central
[105] Ransohoff RM. How neuroinflammation contributes to neurodegeneration. Science. 2016 Aug;353(6301):777–83.10.1126/science.aag2590Search in Google Scholar PubMed
[106] Companys-Alemany J, Turcu AL, Vázquez S, Pallàs M, Griñán-Ferré C. Glial cell reactivity and oxidative stress prevention in Alzheimer’s disease mice model by an optimized NMDA receptor antagonist. Sci Rep. 2022 Oct;12(1):17908.10.1038/s41598-022-22963-xSearch in Google Scholar PubMed PubMed Central
[107] Finnie JW. Neuroinflammation: beneficial and detrimental effects after traumatic brain injury. Inflammopharmacology. 2013 Aug;21(4):309–20.10.1007/s10787-012-0164-2Search in Google Scholar PubMed
[108] Wee Yong V. Inflammation in neurological disorders: a help or a hindrance? Neuroscientist. 2010 Aug;16(4):408–20.10.1177/1073858410371379Search in Google Scholar PubMed
[109] Yong HYF, Rawji KS, Ghorbani S, Xue M, Yong VW. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol. 2019 Jun;16(6):540–6.10.1038/s41423-019-0223-3Search in Google Scholar PubMed PubMed Central
[110] Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014 Jul;14(7):463–77.10.1038/nri3705Search in Google Scholar PubMed
[111] Novellino F, Saccà V, Donato A, Zaffino P, Spadea MF, Vismara M, et al. Innate immunity: a common denominator between neurodegenerative and neuropsychiatric diseases. Int J Mol Sci. 2020 Feb;21(3):1115.10.3390/ijms21031115Search in Google Scholar PubMed PubMed Central
[112] guirre A, Maturana CJ, Harcha PA, Sáez JC. Possible involvement of TLRs and hemichannels in stress-induced CNS dysfunction via mastocytes, and glia activation. Mediators Inflamm. 2013;2013:893521.10.1155/2013/893521Search in Google Scholar PubMed PubMed Central
[113] Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, et al. Author correction: Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep. 2023 Jan;13(1):826.10.1038/s41598-022-26917-1Search in Google Scholar PubMed PubMed Central
[114] Peng L, Zhu X, Wang C, Jiang Q, Yu S, Song G, et al. Indole-3-carbinol (I3C) reduces apoptosis and improves neurological function after cerebral ischemia-reperfusion injury by modulating microglia inflammation. Sci Rep. 2024 Feb;14(1):3145.10.1038/s41598-024-53636-6Search in Google Scholar PubMed PubMed Central
[115] McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2013 Apr;5(4):a008656.10.1101/cshperspect.a008656Search in Google Scholar PubMed PubMed Central
[116] Qin Y, Qiu J, Wang P, Liu J, Zhao Y, Jiang F, et al. Impaired autophagy in microglia aggravates dopaminergic neurodegeneration by regulating NLRP3 inflammasome activation in experimental models of Parkinson’s disease. Brain Behav Immun. 2021 Jan;91:324–38.10.1016/j.bbi.2020.10.010Search in Google Scholar PubMed
[117] Booth HDE, Hirst WD, Wade-Martins R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017 Jun;40(6):358–70.10.1016/j.tins.2017.04.001Search in Google Scholar PubMed PubMed Central
[118] Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016 Jul;142:23–44.10.1016/j.pneurobio.2016.05.001Search in Google Scholar PubMed
[119] Mahul-Mellier AL, Burtscher J, Maharjan N, Weerens L, Croisier M, Kuttler F, et al. The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci USA. 2020 Mar;117(9):4971–82.10.1073/pnas.1913904117Search in Google Scholar PubMed PubMed Central
[120] Kamath T, Abdulraouf A, Burris SJ, Langlieb J, Gazestani V, Nadaf NM, et al. Single-cell genomic profiling of human dopamine neurons identifies a population that selectively degenerates in Parkinson’s disease. Nat Neurosci. 2022 May;25(5):588–95.10.1038/s41593-022-01061-1Search in Google Scholar PubMed PubMed Central
[121] D’Egidio F, Castelli V, Lombardozzi G, Ammannito F, Cimini A, d’Angelo M. Therapeutic advances in neural regeneration for Huntington’s disease. Neural Regen Res. 2024 Sep;19(9):1991–7.10.4103/1673-5374.390969Search in Google Scholar PubMed PubMed Central
[122] Andhale R, Shrivastava D. Huntington’s disease: a clinical review. Cureus. 2022 Aug;14(8):e28484.10.7759/cureus.28484Search in Google Scholar PubMed PubMed Central
[123] Jimenez-Sanchez M, Licitra F, Underwood BR, Rubinsztein DC. Huntington’s disease: mechanisms of pathogenesis and therapeutic strategies. Cold Spring Harb Perspect Med. 2017 Jul;7(7):a024240.10.1101/cshperspect.a024240Search in Google Scholar PubMed PubMed Central
[124] Nguyen GD, Gokhan S, Molero AE, Mehler MF. Selective roles of normal and mutant huntingtin in neural induction and early neurogenesis. PLoS One. 2013 May;8(5):e64368.10.1371/journal.pone.0064368Search in Google Scholar PubMed PubMed Central
[125] Zuccato C, Tartari M, Crotti A, Goffredo D, Valenza M, Conti L, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003 Sep;35(1):76–83.10.1038/ng1219Search in Google Scholar PubMed
[126] Woda JM, Calzonetti T, Hilditch-Maguire P, Duyao MP, Conlon RA, MacDonald ME. Inactivation of the Huntington’s disease gene (Hdh) impairs anterior streak formation and early patterning of the mouse embryo. BMC Dev Biol. 2005 Aug;5:17.10.1186/1471-213X-5-17Search in Google Scholar PubMed PubMed Central
[127] DiFiglia M, Sapp E, Chase K, Schwarz C, Meloni A, Young C, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995 May;14(5):1075–81.10.1016/0896-6273(95)90346-1Search in Google Scholar PubMed
[128] Fusco FR, Chen Q, Lamoreaux WJ, Figueredo-Cardenas G, Jiao Y, Coffman JA, et al. Cellular localization of huntingtin in striatal and cortical neurons in rats: lack of correlation with neuronal vulnerability in Huntington’s disease. J Neurosci. 1999 Feb;19(4):1189–202.10.1523/JNEUROSCI.19-04-01189.1999Search in Google Scholar PubMed PubMed Central
[129] Becher MW, Kotzuk JA, Sharp AH, Davies SW, Bates GP, Price DL, et al. Intranuclear neuronal inclusions in Huntington’s disease and dentatorubral and pallidoluysian atrophy: correlation between the density of inclusions and IT15 CAG triplet repeat length. Neurobiol Dis. 1998 Apr;4(6):387–97.10.1006/nbdi.1998.0168Search in Google Scholar PubMed
[130] Perutz MF, Windle AH. Cause of neural death in neurodegenerative diseases attributable to expansion of glutamine repeats. Nature. 2001 Jul;412(6843):143–4.10.1038/35084141Search in Google Scholar PubMed
[131] Martindale D, Hackam A, Wieczorek A, Ellerby L, Wellington C, McCutcheon K, et al. Length of huntingtin and its polyglutamine tract influences localization and frequency of intracellular aggregates. Nat Genet. 1998 Feb;18(2):150–4.10.1038/ng0298-150Search in Google Scholar PubMed
[132] Lajoie P, Snapp EL. Detecting soluble polyQ oligomers and investigating their impact on living cells using split-GFP. Methods Mol Biol. 2013;1017:229–39.10.1007/978-1-62703-438-8_17Search in Google Scholar PubMed PubMed Central
[133] Holmberg CI, Staniszewski KE, Mensah KN, Matouschek A, Morimoto RI. Inefficient degradation of truncated polyglutamine proteins by the proteasome. EMBO J. 2004 Oct;23(21):4307–18.10.1038/sj.emboj.7600426Search in Google Scholar PubMed PubMed Central
[134] Bradford J, Shin JY, Roberts M, Wang CE, Sheng G, Li S, et al. Mutant huntingtin in glial cells exacerbates neurological symptoms of Huntington disease mice. J Biol Chem. 2010 Apr;285(14):10653–61.10.1074/jbc.M109.083287Search in Google Scholar PubMed PubMed Central
[135] de Oliveira Furlam T, Roque IG, Machado da Silva EW, Vianna PP, Costa Valadão PA, Guatimosim C, et al. Inflammasome activation and assembly in Huntington’s disease. Mol Immunol. 2022 Nov;151:134–42.10.1016/j.molimm.2022.09.002Search in Google Scholar PubMed
[136] Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, et al. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell. 2001 May;12(5):1393–407.10.1091/mbc.12.5.1393Search in Google Scholar PubMed PubMed Central
[137] Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008 Feb;132(3):344–62.10.1016/j.cell.2008.01.020Search in Google Scholar PubMed
[138] Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733.10.1146/annurev.immunol.021908.132641Search in Google Scholar PubMed
[139] Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017 Sep;17(9):545–58.10.1038/nri.2017.52Search in Google Scholar PubMed PubMed Central
[140] Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009 Dec;1(6):a001651.10.1101/cshperspect.a001651Search in Google Scholar PubMed PubMed Central
[141] Zinatizadeh MR, Schock B, Chalbatani GM, Zarandi PK, Jalali SA, Miri SR. The nuclear factor kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2020 Jul;8(3):287–97.10.1016/j.gendis.2020.06.005Search in Google Scholar PubMed PubMed Central
[142] Ulivi V, Giannoni P, Gentili C, Cancedda R, Descalzi F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J Cell Biochem. 2008 Jul;104(4):1393–406.10.1002/jcb.21717Search in Google Scholar PubMed
[143] Barnabei L, Laplantine E, Mbongo W, Rieux-Laucat F, Weil R. NF-κB: at the borders of autoimmunity and inflammation. Front Immunol. 2021 Aug;12:716469.10.3389/fimmu.2021.716469Search in Google Scholar PubMed PubMed Central
[144] Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020 Sep;5(1):209.10.1038/s41392-020-00312-6Search in Google Scholar PubMed PubMed Central
[145] Anilkumar S, Wright-Jin E. NF-κB as an inducible regulator of inflammation in the central nervous system. Cells. 2024 Mar;13(6):485.10.3390/cells13060485Search in Google Scholar PubMed PubMed Central
[146] Mussbacher M, Derler M, Basílio J, Schmid JA. NF-κB in monocytes and macrophages – an inflammatory master regulator in multitalented immune cells. Front Immunol. 2023 Feb;14:1134661.10.3389/fimmu.2023.1134661Search in Google Scholar PubMed PubMed Central
[147] Heyen JR, Ye SM, Brian NF, Johnson RW. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-κB. Mol Brain Res. 2000 Apr;77:138–47.10.1016/S0169-328X(00)00042-5Search in Google Scholar
[148] Liu M, Gong R, Ding L, Zhao Y, Yan X, Shi L, et al. Gastrodin combined with electroacupuncture prevents the development of cerebral ischemia via rebalance of brain-derived neurotrophic factor and interleukin-6 in stroke model rats. Neuroreport. 2024 Jul;35(10):664–72.10.1097/WNR.0000000000002050Search in Google Scholar PubMed PubMed Central
[149] Ramanan S, Kooshki M, Zhao W, Hsu FC, Robbins ME. PPARα ligands inhibit irradiation-induced microglial inflammatory responses by negatively regulating NF-κ B and AP-1 pathways. Free Radic Biol Med. 2008 Dec;45:1695–704.10.1016/j.freeradbiomed.2008.09.002Search in Google Scholar PubMed PubMed Central
[150] Owen AM, Luan L, Burelbach KR, McBride MA, Stothers CL, Boykin OA, et al. MyD88-dependent signaling drives toll-like receptor-induced trained immunity in macrophages. Front Immunol. 2022 Nov;13:1044662.10.3389/fimmu.2022.1044662Search in Google Scholar PubMed PubMed Central
[151] Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006 Jun;125(5):943–55.10.1016/j.cell.2006.03.047Search in Google Scholar PubMed
[152] Li L, Cousart S, Hu J, McCall CE. Characterization of interleukin-1 receptor-associated kinase in normal and endotoxin-tolerant cells. J Biol Chem. 2000 Jul;275(30):23340–5.10.1074/jbc.M001950200Search in Google Scholar PubMed
[153] Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011 Apr;90:417–27.10.1177/0022034510381264Search in Google Scholar PubMed PubMed Central
[154] Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006 Feb;124(4):783–801.10.1016/j.cell.2006.02.015Search in Google Scholar PubMed
[155] Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010 May;11:373–84.10.1038/ni.1863Search in Google Scholar PubMed
[156] Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9- mediated interferon-{alpha} induction. J Exp Med. 2005 Mar;201(6):915–23.10.1084/jem.20042372Search in Google Scholar PubMed PubMed Central
[157] Hughes C, Choi ML, Yi JH, Kim SC, Drews A, George-Hyslop PS, et al. Beta amyloid aggregates induce sensitised TLR4 signalling causing long-term potentiation deficit and rat neuronal cell death. Commun Biol. 2020 Feb;3(1):79.10.1038/s42003-020-0792-9Search in Google Scholar PubMed PubMed Central
[158] Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023 Jul;8(1):267.10.1038/s41392-023-01486-5Search in Google Scholar PubMed PubMed Central
[159] Calabresi P, Mechelli A, Natale G, Volpicelli-Daley L, Di Lazzaro G, Ghiglieri V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: from overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023 Mar;14(3):176.10.1038/s41419-023-05672-9Search in Google Scholar PubMed PubMed Central
[160] Attfield KE, Jensen LT, Kaufmann M, Friese MA, Fugger L. The immunology of multiple sclerosis. Nat Rev Immunol. 2022 Dec;22(12):734–50.10.1038/s41577-022-00718-zSearch in Google Scholar PubMed
[161] Zhang W, Xu H, Li C, Han B, Zhang Y. Exploring Chinese herbal medicine for ischemic stroke: insights into microglia and signaling pathways. Front Pharmacol. 2024 Jan;15:1333006.10.3389/fphar.2024.1333006Search in Google Scholar PubMed PubMed Central
[162] Yang Z, Wang S, Liu H, Xu S. MAPK/iNOS pathway is involved in swine kidney necrosis caused by cadmium exposure. Environ Pollut. 2021 Apr;274:116497.10.1016/j.envpol.2021.116497Search in Google Scholar PubMed
[163] Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020 Mar;19(3):1997–2007.10.3892/etm.2020.8454Search in Google Scholar PubMed PubMed Central
[164] Behl T, Rana T, Alotaibi GH, Shamsuzzaman M, Naqvi M, Sehgal A, et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed Pharmacother. 2022 Feb;146:112545.10.1016/j.biopha.2021.112545Search in Google Scholar PubMed
[165] Li H, Qian J, Wang Y, Wang J, Mi X, Qu L, Song N, et al. Potential convergence of olfactory dysfunction in Parkinson’s disease and COVID-19: the role of neuroinflammation. Ageing Res Rev. 2024 Jun;97:102288.10.1016/j.arr.2024.102288Search in Google Scholar PubMed
[166] Liu TT, Zhang SP, Qin XY. Progress in the study of MAPK signaling pathway and nerve injury. China Public Health. 2016;32(2):248–54.Search in Google Scholar
[167] Zhang J, Zhang Y, Wang J, Xia Y, Zhang J, Chen L. Recent advances in Alzheimer’s disease: mechanisms, clinical trials and new drug development strategies. Signal Transduct Target Ther. 2024 Aug;9(1):211.10.1038/s41392-024-01911-3Search in Google Scholar PubMed PubMed Central
[168] Wang F, Wen H, Liu L, Aisa HA, Xin X. A pair of epimers of lignan alleviate neuroinflammatory effects by modulating iNOS/COX-2 and MAPK/NF-κB signaling pathways. Inflammation. 2024 Jun;1–11.10.1007/s10753-024-02080-9Search in Google Scholar PubMed
[169] Iba M, Kim C, Kwon S, Szabo M, Horan-Portelance L, Peer CJ, et al. Inhibition of p38α MAPK restores neuronal p38γ MAPK and ameliorates synaptic degeneration in a mouse model of DLB/PD. Sci Transl Med. 2023 May;15(695):eabq6089.10.1126/scitranslmed.abq6089Search in Google Scholar PubMed
[170] Ju Z, Su M, Hong J, Kim E, Jung JH. Anti-inflammatory effects of an optimized PPAR-γ agonist via NF-κB pathway inhibition. Bioorg Chem. 2020 Mar;96:103611.10.1016/j.bioorg.2020.103611Search in Google Scholar PubMed
[171] Qu XX, He JH, Cui ZQ, Yang T, Sun XH. PPAR-α agonist GW7647 protects against oxidative stress and iron deposit via GPx4 in a transgenic mouse model of Alzheimer’s diseases. ACS Chem Neurosci. 2022 Jan;13(2):207–16.10.1021/acschemneuro.1c00516Search in Google Scholar PubMed
[172] Schnegg CI, Kooshki M, Hsu FC, Sui G, Robbins ME. PPARδ prevents radiation-induced proinflammatory responses in microglia via transcriptional repression of NF-κB and inhibition of the PKCα/MEK1/2/ERK1/2/AP-1 pathway. Free Rad Biol Med. 2012 May;52(9):1734–43.10.1016/j.freeradbiomed.2012.02.032Search in Google Scholar PubMed PubMed Central
[173] Grandbarbe L, Michelucci A, Heurtaux T, Hemmer K, Morga E, Heuschling P. Notch signaling modulates the activation of microglial cells. Glia. 2007 Nov;55(15):1519–30.10.1002/glia.20553Search in Google Scholar PubMed
[174] Wei Z, Chigurupati S, Arumugam TV, Jo DG, Li H, Chan SL. Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke. 2011 Sep;42(9):2589–94.10.1161/STROKEAHA.111.614834Search in Google Scholar PubMed
[175] Perna A, Marathe S, Dreos R, Falquet L, Akarsu Egger H, Auber LA. Revealing NOTCH-dependencies in synaptic targets associated with Alzheimer’s disease. Mol Cell Neurosci. 2021 Sep;115:103657.10.1016/j.mcn.2021.103657Search in Google Scholar PubMed
[176] Acosta-Martinez M, Cabail MZ. The PI3K/Akt pathway in meta-inflammation. Int J Mol Sci. 2022 Dec;23(23):15330.10.3390/ijms232315330Search in Google Scholar PubMed PubMed Central
[177] Zheng Y, Zhang X, Zhang R, Wang Z, Gan J, Gao Q, et al. Inflammatory signaling pathways in the treatment of Alzheimer’s disease with inhibitors, natural products and metabolites (Review). Int J Mol Med. 2023 Nov;52(5):111.10.3892/ijmm.2023.5314Search in Google Scholar PubMed PubMed Central
[178] Xia T, Fu S, Yang R, Yang K, Lei W, Yang Y, et al. Advances in the study of macrophage polarization in inflammatory immune skin diseases. J Inflamm (Lond). 2023 Oct;20(1):33.10.1186/s12950-023-00360-zSearch in Google Scholar PubMed PubMed Central
[179] Ramasubbu K, Devi Rajeswari V. Impairment of insulin signaling pathway PI3K/Akt/mTOR and insulin resistance induced AGEs on diabetes mellitus and neurodegenerative diseases: a perspective review. Mol Cell Biochem. 2023 Jun;478(6):1307–24.10.1007/s11010-022-04587-xSearch in Google Scholar PubMed
[180] Chu E, Mychasiuk R, Hibbs ML, Semple BD. Dysregulated phosphoinositide 3-kinase signaling in microglia: shaping chronic neuroinflammation. J Neuroinflammation. 2021 Nov;18(1):276.10.1186/s12974-021-02325-6Search in Google Scholar PubMed PubMed Central
[181] Kumar M, Bansal N. Implications of phosphoinositide 3-kinase-Akt (PI3K-Akt) pathway in the pathogenesis of Alzheimer’s disease. Mol Neurobiol. 2022 Jan;59(1):354–85.10.1007/s12035-021-02611-7Search in Google Scholar PubMed
[182] Long HZ, Cheng Y, Zhou ZW, Luo HY, Wen DD, Gao LC. PI3K/AKT signal pathway: a target of natural products in the prevention and treatment of Alzheimer’s disease and Parkinson’s disease. Front Pharmacol. 2021 Apr;12:648636.10.3389/fphar.2021.648636Search in Google Scholar PubMed PubMed Central
[183] Shang Y, Wang H, Jia P, Zhao H, Liu C, Liu W, et al. Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy. 2016 Sep;12(9):1575–92.10.1080/15548627.2016.1192750Search in Google Scholar PubMed PubMed Central
[184] Liu C, Wang H, Shang Y, Liu W, Song Z, Zhao H, et al. Autophagy is required for ectoplasmic specialization assembly in sertoli cells. Autophagy. 2016 May;12(5):814–32.10.1080/15548627.2016.1159377Search in Google Scholar PubMed PubMed Central
[185] Eshraghi M, Adlimoghaddam A, Mahmoodzadeh A, Sharifzad F, Yasavoli-Sharahi H, Lorzadeh S, et al. Alzheimer’s disease pathogenesis: Role of autophagy and mitophagy focusing in microglia. Int J Mol Sci. 2021 Mar;22(7):3330.10.3390/ijms22073330Search in Google Scholar PubMed PubMed Central
[186] Lizama BN, Chu CT. Neuronal autophagy and mitophagy in Parkinson’s disease. Mol Asp Med. 2021 Dec;82:100972.10.1016/j.mam.2021.100972Search in Google Scholar PubMed PubMed Central
[187] Limanaqi F, Biagioni F, Gambardella S, Familiari P, Frati A, Fornai F. Promiscuous roles of autophagy and proteasome in neurodegenerative proteinopathies. Int J Mol Sci. 2020 Apr;21(8):3028.10.3390/ijms21083028Search in Google Scholar PubMed PubMed Central
[188] Ma Z, Wu Y, Xu J, Cao H, Du M, Jiang H, et al. Sodium tanshinone IIA sulfonate ameliorates oxygen-glucose deprivation/reoxygenation-induced neuronal injury via protection of mitochondria and promotion of autophagy. Neurochem Res. 2023 Nov;48(11):3378–90.10.1007/s11064-023-03985-xSearch in Google Scholar PubMed
[189] Choi I, Wang M, Yoo S, Xu P, Seegobin SP, Li X, et al. Autophagy enables microglia to engage amyloid plaques and prevents microglial senescence. Nat Cell Biol. 2023 Jul;25(7):963–74.10.1038/s41556-023-01158-0Search in Google Scholar PubMed PubMed Central
[190] Gatica D, Chiong M, Lavandero S, Klionsky DJ. The role of autophagy in cardiovascular pathology. Cardiovasc Res. 2022 Mar;118(4):934–50.10.1093/cvr/cvab158Search in Google Scholar PubMed PubMed Central
[191] Peng Y, Tao Y, Liu L, Zhang J, Wei B. Crosstalk among reactive oxygen species, autophagy and metabolism in myocardial ischemia and reperfusion stages. Aging Dis. 2024 May;15(3):1075–107.10.14336/AD.2023.0823-4Search in Google Scholar PubMed PubMed Central
[192] Martelli A, Omrani M, Zarghooni M, Citi V, Brogi S, Calderone V, et al. New visions on natural products and cancer therapy: autophagy and related regulatory pathways. Cancers (Basel). 2022 Nov;14(23):5839.10.3390/cancers14235839Search in Google Scholar PubMed PubMed Central
[193] Zhang Y, Zhang J, Fu Z. Role of autophagy in lung diseases and ageing. Eur Respir Rev. 2022 Jan;31(166):220134.10.1183/16000617.0134-2022Search in Google Scholar PubMed PubMed Central
[194] Barnes PJ, Baker J, Donnelly LE. Autophagy in asthma and chronic obstructive pulmonary disease. Clin Sci (Lond). 2022 May;136(10):733–46.10.1042/CS20210900Search in Google Scholar PubMed PubMed Central
[195] Li YY, Qin ZH, Sheng R. The multiple roles of autophagy in neural function and diseases. Neurosci Bull. 2023 Oct;40(3):363–82.10.1007/s12264-023-01120-ySearch in Google Scholar PubMed PubMed Central
[196] Jiwaji Z, Hardingham GE. Good, bad, and neglectful: astrocyte changes in neurodegenerative disease. Free Radic Biol Med. 2022 Mar;182:93–9.10.1016/j.freeradbiomed.2022.02.020Search in Google Scholar PubMed PubMed Central
[197] Zimmer TS, Orr AL, Orr AG. Astrocytes in selective vulnerability to neurodegenerative disease. Trends Neurosci. 2024 Apr;47(4):289–302.10.1016/j.tins.2024.02.008Search in Google Scholar PubMed PubMed Central
[198] Sosunov A, Wu X, McGovern R, Mikell C, McKhann GM, Goldman JE. Abnormal mitosis in reactive astrocytes. Acta Neuropathol Commun. 2020 Apr;8(1):47.10.1186/s40478-020-00919-4Search in Google Scholar PubMed PubMed Central
[199] Joseph C, Mangani AS, Gupta V, Chitranshi N, Shen T, Dheer Y, et al. Cell cycle deficits in neurodegenerative disorders: uncovering molecular mechanisms to drive innovative therapeutic development. Aging Dis. 2020 Jul;11(4):946–66.10.14336/AD.2019.0923Search in Google Scholar PubMed PubMed Central
[200] Liu Z, Wang X, Jiang K, Ji X, Zhang YA, Chen Z. TNFα-induced up-regulation of Ascl2 affects the differentiation and proliferation of neural stem cells. Aging Dis. 2019 Dec;10(6):1207–20.10.14336/AD.2018.1028Search in Google Scholar PubMed PubMed Central
[201] He N, Mao XJ, Ding YM, Zuo T, Chen YY, Wang LL. New insights into the biological roles of immune cells in neural stem cells in post-traumatic injury of the central nervous system. Neural Regen Res. 2023 Sep;18(9):1908–16.Search in Google Scholar
[202] Mandrekar-Colucci S, Landreth GE. Microglia and inflammation in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2010 Apr;9(2):156–67.10.2174/187152710791012071Search in Google Scholar PubMed PubMed Central
[203] Ciminieri C, Woest ME, Reynaert NL, Heijink IH, Wardenaar R, Spierings DCJ, et al. IL-1β induces a proinflammatory fibroblast microenvironment that impairs lung progenitors’ function. Am J Respir Cell Mol Biol. 2023 Apr;68(4):444–55.10.1165/rcmb.2022-0209OCSearch in Google Scholar PubMed
[204] Francis GA. The greatly under-represented role of smooth muscle cells in atherosclerosis. Curr Atheroscler Rep. 2023 Oct;25(10):741–49.10.1007/s11883-023-01145-8Search in Google Scholar PubMed PubMed Central
[205] Kume H, Yamada R, Sato Y, Togawa R. Airway smooth muscle regulated by oxidative stress in COPD. Antioxidants (Basel). 2023 Jan;12(1):142.10.3390/antiox12010142Search in Google Scholar PubMed PubMed Central
[206] Yang Q, Miao Q, Chen H, Li D, Luo Y, Chiu J, et al. Myocd regulates airway smooth muscle cell remodeling in response to chronic asthmatic injury. J Pathol. 2023 Mar;259(3):331–41.10.1002/path.6044Search in Google Scholar PubMed PubMed Central
[207] Coronel R, Bernabeu-Zornoza A, Palmer C, González-Sastre R, Rosca A, Mateos-Martínez P, et al. Amyloid precursor protein (APP) regulates gliogenesis and neurogenesis of human neural stem cells by several signaling pathways. Int J Mol Sci. 2023 Aug;24(16):12964.10.3390/ijms241612964Search in Google Scholar PubMed PubMed Central
[208] Wang J, Ye Z, Zheng S, Chen L, Wan Y, Deng Y, et al. Lingo-1 shRNA and Notch signaling inhibitor DAPT promote differentiation of neural stem/progenitor cells into neurons. Brain Res. 2016 Mar;1634:34–44.10.1016/j.brainres.2015.11.029Search in Google Scholar PubMed
[209] Salazar JL, Yang SA, Yamamoto S. Post-developmental roles of notch signaling in the nervous system. Biomolecules. 2020 Jul;10(7):985.10.3390/biom10070985Search in Google Scholar PubMed PubMed Central
[210] Subramanian A, Tamilanban T, Alsayari A, Ramachawolran G, Wong LS, Sekar M, et al. Trilateral association of autophagy, mTOR and Alzheimer’s disease: potential pathway in the development for Alzheimer’s disease therapy. Front Pharmacol. 2022 Dec;13:1094351.10.3389/fphar.2022.1094351Search in Google Scholar PubMed PubMed Central
[211] Ramalingam M, Huh YJ, Lee YI. The impairments of α-Synuclein and mechanistic target of rapamycin in rotenone-induced SH-SY5Y cells and mice model of Parkinson’s disease. Front Neurosci. 2019 Sep;13:1028.10.3389/fnins.2019.01028Search in Google Scholar PubMed PubMed Central
[212] Querfurth H, Lee HK. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener. 2021 Jul;16(1):44.10.1186/s13024-021-00428-5Search in Google Scholar PubMed PubMed Central
[213] Yan M, Sun Z, Zhang S, Yang G, Jiang X, Wang G, et al. SOCS modulates JAK-STAT pathway as a novel target to mediate the occurrence of neuroinflammation: molecular details and treatment options. Brain Res Bull. 2024 Jul;213:110988.10.1016/j.brainresbull.2024.110988Search in Google Scholar PubMed
[214] Faquetti ML, Slappendel L, Bigonne H, Grisoni F, Schneider P, Aichinger G, et al. Baricitinib and tofacitinib off-target profile, with a focus on Alzheimer’s disease. Alzheimers Dement (N Y). 2024 Jan;10(1):e12445.10.1002/trc2.12445Search in Google Scholar PubMed PubMed Central
[215] Kaltschmidt B, Helweg LP, Greiner JFW, Kaltschmidt C. NF-κB in neurodegenerative diseases: recent evidence from human genetics. Front Mol Neurosci. 2022 Aug;15:954541.10.3389/fnmol.2022.954541Search in Google Scholar PubMed PubMed Central
[216] Genchi G, Lauria G, Catalano A, Carocci A, Sinicropi MS. Neuroprotective effects of curcumin in neurodegenerative diseases. Foods. 2024 Jun;13(11):1774.10.3390/foods13111774Search in Google Scholar PubMed PubMed Central
[217] Kwakowsky A, Palpagama TH. Neuroinflammation as a therapeutic target in Huntington’s disease. Neural Regen Res. 2025 Mar;20(3):817–18.10.4103/NRR.NRR-D-24-00195Search in Google Scholar PubMed PubMed Central
[218] Goyal A, Agrawal A, Verma A, Dubey N. The PI3K-AKT pathway: a plausible therapeutic target in Parkinson’s disease. Exp Mol Pathol. 2023 Feb;129:104846.10.1016/j.yexmp.2022.104846Search in Google Scholar PubMed
[219] Kim JH, Lee RM, Oh HB, Kim TY, Rhim H, Choi YK, et al. Atypical formations of gintonin lysophosphatidic acids as new materials and their beneficial effects on degenerative diseases. J Ginseng Res. 2024 Jan;48(1):1–11.10.1016/j.jgr.2023.02.004Search in Google Scholar PubMed PubMed Central
[220] Litwiniuk A, Kalisz M, Domańska A, Chmielowska M, Martyńska L, Baranowska-Bik A, et al. Nicotinic acid attenuates amyloid β1-42-induced mitochondrial dysfunction and inhibits the mitochondrial pathway of apoptosis in differentiated SH-SY5Y cells. Neurochem Int. 2024 Sep;178:105772.10.1016/j.neuint.2024.105772Search in Google Scholar PubMed
[221] Liang X, Wen Y, Feng C, Xu L, Xian Y, Xie H, et al. Neuroglobin protects dopaminergic neurons in a Parkinson’s cell model by interacting with mitochondrial complex NDUFA10. Neuroscience. 2024 Dec;562:43–53.10.1016/j.neuroscience.2024.10.033Search in Google Scholar PubMed
[222] Dai Y, Wang H, Lian A, Li J, Zhao G, Hu S, et al. A comprehensive perspective of Huntington’s disease and mitochondrial dysfunction. Mitochondrion. 2023 May;70:8–19.10.1016/j.mito.2023.03.001Search in Google Scholar PubMed
[223] Huo J, Xu S, Lam KP. FAIM: an antagonist of Fas-killing and beyond. Cells. 2019 Jun;8(6):541.10.3390/cells8060541Search in Google Scholar PubMed PubMed Central
[224] Setter DO, Runge EM, Schartz ND, Kennedy FM, Brown BL, McMillan KP, et al. Impact of peripheral immune status on central molecular responses to facial nerve axotomy. Brain Behav Immun. 2018 Feb;68:98–110.10.1016/j.bbi.2017.10.005Search in Google Scholar PubMed PubMed Central
[225] Wu BT, Chiang MC, Tasi CY, Kuo CH, Shyu WC, Kao CL, et al. Cardiac fas-dependent and mitochondria-dependent apoptotic pathways in a transgenic mouse model of Huntington’s disease. Cardiovasc Toxicol. 2016 Apr;16(2):111–21.10.1007/s12012-015-9318-ySearch in Google Scholar PubMed
[226] Arber C, Lovejoy C, Harris L, Willumsen N, Alatza A, Casey JM, et al. Familial Alzheimer’s disease mutations in PSEN1 lead to premature human stem cell neurogenesis. Cell Rep. 2021 Jan;34(2):108615.10.1016/j.celrep.2020.108615Search in Google Scholar PubMed PubMed Central
[227] Liang SQ, Li PH, Hu YY, Zhao JL, Shao FZ, Kuang F, et al. Myeloid-specific blockade of notch signaling alleviates dopaminergic neurodegeneration in Parkinson’s disease by dominantly regulating resident microglia activation through NF-κB signaling. Front Immunol. 2023 Aug;14:1193081.10.3389/fimmu.2023.1193081Search in Google Scholar PubMed PubMed Central
[228] Nguyen GD, Molero AE, Gokhan S, Mehler MF. Functions of huntingtin in germ layer specification and organogenesis. PLoS One. 2013 Aug;8(8):e72698.10.1371/journal.pone.0072698Search in Google Scholar PubMed PubMed Central
[229] Talebi M, Talebi M, Kakouri E, Farkhondeh T, Pourbagher-Shahri AM, Tarantilis PA, et al. Tantalizing role of p53 molecular pathways and its coherent medications in neurodegenerative diseases. Int J Biol Macromol. 2021 Mar;172:93–103.10.1016/j.ijbiomac.2021.01.042Search in Google Scholar PubMed
[230] Wolfrum P, Fietz A, Schnichels S, Hurst J. The function of p53 and its role in Alzheimer’s and Parkinson’s disease compared to age-related macular degeneration. Front Neurosci. 2022 Dec;16:1029473.10.3389/fnins.2022.1029473Search in Google Scholar PubMed PubMed Central
[231] Intihar TA, Martinez EA, Gomez-Pastor R. Mitochondrial dysfunction in Huntington’s disease; interplay between HSF1, p53 and PGC-1α transcription factors. Front Cell Neurosci. 2019 Mar;13:103.10.3389/fncel.2019.00103Search in Google Scholar PubMed PubMed Central
[232] Sousa C, Biber K, Michelucci A. Cellular and molecular characterization of microglia: a unique immune cell population. Front Immunol. 2017 Mar;8:198.10.3389/fimmu.2017.00198Search in Google Scholar PubMed PubMed Central
[233] Augusto-Oliveira M, Arrifano GP, Lopes-Araújo A, Santos-Sacramento L, Takeda PY, Anthony DC, et al. What do microglia really do in healthy adult brain? Cells. 2019 Oct;8(10):1293.10.3390/cells8101293Search in Google Scholar PubMed PubMed Central
[234] Liu X, Jiang N, Zhou W. Various energetic metabolism of microglia in response to different stimulations. Molecules. 2023 Jun;28(11):4501.10.3390/molecules28114501Search in Google Scholar PubMed PubMed Central
[235] Lin MM, Liu N, Qin ZH, Wang Y. Mitochondrial-derived damage-associated molecular patterns amplify neuroinflammation in neurodegenerative diseases. Acta Pharmacol Sin. 2022 Oct;43(10):2439–47.10.1038/s41401-022-00879-6Search in Google Scholar PubMed PubMed Central
[236] Cieri MB, Ramos AJ. Astrocytes, reactive astrogliosis, and glial scar formation in traumatic brain injury. Neural Regen Res. 2025 Apr;20(4):973–89.10.4103/NRR.NRR-D-23-02091Search in Google Scholar PubMed PubMed Central
[237] Chiot A, Zaïdi S, Iltis C, Ribon M, Berriat F, Schiaffino L, et al. Modifying macrophages at the periphery has the capacity to change microglial reactivity and to extend ALS survival. Nat Neurosci. 2020 Nov;23(11):1339–51.10.1038/s41593-020-00718-zSearch in Google Scholar PubMed
[238] van Langelaar J, Rijvers L, Smolders J, van Luijn MM. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers. Front Immunol. 2020 May;11:760.10.3389/fimmu.2020.00760Search in Google Scholar PubMed PubMed Central
[239] Pamies D, Sartori C, Schvartz D, González-Ruiz V, Pellerin L, Nunes C, et al. Neuroinflammatory response to TNFα and IL1β cytokines is accompanied by an increase in glycolysis in human astrocytes in vitro. Int J Mol Sci. 2021 Apr;22(8):4065.10.3390/ijms22084065Search in Google Scholar PubMed PubMed Central
[240] Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017 Jan;17(1):49–59.10.1038/nri.2016.123Search in Google Scholar PubMed
[241] Becher B, Derfuss T, Liblau R. Targeting cytokine networks in neuroinflammatory diseases. Nat Rev Drug Discov. 2024 Nov;23(11):862–79.10.1038/s41573-024-01026-ySearch in Google Scholar PubMed
[242] Gonzalez Caldito N. Role of tumor necrosis factor-alpha in the central nervous system: a focus on autoimmune disorders. Front Immunol. 2023 Jul;14:1213448.10.3389/fimmu.2023.1213448Search in Google Scholar PubMed PubMed Central
[243] Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021 Mar;22(5):2719.10.3390/ijms22052719Search in Google Scholar PubMed PubMed Central
[244] Fetsko AR, Sebo DJ, Budzynski LB, Scharbarth A, Taylor MR. IL-1β disrupts blood–brain barrier development by inhibiting endothelial Wnt/β-catenin signaling. . bioRxiv [Preprint]; 2024 Feb2023.12.04.569943 .10.1101/2023.12.04.569943Search in Google Scholar PubMed PubMed Central
[245] Shan C, Zhang C, Zhang C. The role of IL-6 in neurodegenerative disorders. Neurochem Res. 2024 Apr;49(4):834–46.10.1007/s11064-023-04085-6Search in Google Scholar PubMed
[246] Quaranta DV, Weaver RR, Baumann KK, Fujimoto T, Williams LM, Kim HC, et al. Transport of the proinflammatory chemokines C-C motif chemokine ligand 2 (MCP-1) and C-C motif chemokine ligand 5 (RANTES) across the intact mouse blood-brain barrier is inhibited by heparin and eprodisate and increased with systemic inflammation. J Pharmacol Exp Ther. 2023 Jan;384(1):205–23.10.1124/jpet.122.001380Search in Google Scholar PubMed PubMed Central
[247] Jin M, Fang J, Wang JJ, Shao X, Xu SW, Liu PQ, et al. Regulation of toll-like receptor (TLR) signaling pathways in atherosclerosis: from mechanisms to targeted therapeutics. Acta Pharmacol Sin. 2023 Dec;44(12):2358–75.10.1038/s41401-023-01123-5Search in Google Scholar PubMed PubMed Central
[248] Shroka TM, Kufareva I, Salanga CL, Handel TM. The dual-function chemokine receptor CCR2 drives migration and chemokine scavenging through distinct mechanisms. Sci Signal. 2023 Jan;16(770):eabo4314.10.1126/scisignal.abo4314Search in Google Scholar PubMed PubMed Central
[249] Lee KH, Cha M, Lee BH. Crosstalk between neuron and glial cells in oxidative injury and neuroprotection. Int J Mol Sci. 2021 Dec;22(24):13315.10.3390/ijms222413315Search in Google Scholar PubMed PubMed Central
[250] Kim SF. The nitric oxide-mediated regulation of prostaglandin signaling in medicine. Vitam Horm. 2014;96:211–45.10.1016/B978-0-12-800254-4.00009-XSearch in Google Scholar PubMed
[251] Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007 Jun;35(4):495–516.10.1080/01926230701320337Search in Google Scholar PubMed PubMed Central
[252] Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020 Jul;17(7):395–417.10.1038/s41571-020-0341-ySearch in Google Scholar PubMed PubMed Central
[253] Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019 Feb;10(3):177.10.1038/s41419-019-1407-6Search in Google Scholar PubMed PubMed Central
[254] Vucicevic K, Jakovljevic V, Colovic N, Tosic N, Kostic T, Glumac I, et al. Association of Bax expression and Bcl2/Bax ratio with clinical and molecular prognostic markers in chronic lymphocytic leukemia. J Med Biochem. 2016 Apr;35(2):150–7.10.1515/jomb-2015-0017Search in Google Scholar PubMed PubMed Central
[255] Ahsan N, Shariq M, Surolia A, Raj R, Khan MF, Kumar P. Multipronged regulation of autophagy and apoptosis: emerging role of TRIM proteins. Cell Mol Biol Lett. 2024 Jan;29(1):13.10.1186/s11658-023-00528-8Search in Google Scholar PubMed PubMed Central
[256] Kaloni D, Diepstraten ST, Strasser A, Kelly GL. BCL-2 protein family: attractive targets for cancer therapy. Apoptosis. 2023 Feb;28(1–2):20–38.10.1007/s10495-022-01780-7Search in Google Scholar PubMed PubMed Central
[257] Schweighofer SV, Jans DC, Keller-Findeisen J, Folmeg A, Ilgen P, Bates M, et al. Endogenous BAX and BAK form mosaic rings of variable size and composition on apoptotic mitochondria. Cell Death Differ. 2024 Apr;31(4):469–78.10.1038/s41418-024-01273-xSearch in Google Scholar PubMed PubMed Central
[258] Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106.10.1146/annurev.biochem.73.011303.073706Search in Google Scholar PubMed
[259] Abate M, Festa A, Falco M, Lombardi A, Luce A, Grimaldi A, et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin Cell Dev Biol. 2020 Feb;98:139–53.10.1016/j.semcdb.2019.05.022Search in Google Scholar PubMed
[260] Robichaux DJ, Harata M, Murphy E, Karch J. Mitochondrial permeability transition pore-dependent necrosis. J Mol Cell Cardiol. 2023 Jan;174:47–55.10.1016/j.yjmcc.2022.11.003Search in Google Scholar PubMed PubMed Central
[261] Wang H, Yu W, Wang Y, Wu R, Dai Y, Deng Y, et al. p53 contributes to cardiovascular diseases via mitochondria dysfunction: a new paradigm. Free Radic Biol Med. 2023 Nov;208:846–58.10.1016/j.freeradbiomed.2023.09.036Search in Google Scholar PubMed
[262] Follis AV, Llambi F, Merritt P, Chipuk JE, Green DR, Kriwacki RW. Pin1-induced proline isomerization in cytosolic p53 mediates BAX activation and apoptosis. Mol Cell. 2015 Aug;59(4):677–84.10.1016/j.molcel.2015.06.029Search in Google Scholar PubMed PubMed Central
[263] Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015 Oct;526(7575):660–5.10.1038/nature15514Search in Google Scholar PubMed
[264] Tamura M, Tanaka S, Fujii T, Aoki A, Komiyama H, Ezawa K, et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics. 2007 May;89(5):618–29.10.1016/j.ygeno.2007.01.003Search in Google Scholar PubMed
[265] Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017 Jan;8:14128.10.1038/ncomms14128Search in Google Scholar PubMed PubMed Central
[266] Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017 Jul;547(7661):99–103.10.1038/nature22393Search in Google Scholar PubMed
[267] He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015 Dec;25(12):1285–98.10.1038/cr.2015.139Search in Google Scholar PubMed PubMed Central
[268] Liu X, Lieberman J. Knocking ‘em dead: pore-forming proteins in immune defense. Annu Rev Immunol. 2020 Apr;38:455–85.10.1146/annurev-immunol-111319-023800Search in Google Scholar PubMed PubMed Central
[269] Liu X, Lieberman J. A mechanistic understanding of pyroptosis: the fiery death triggered by invasive infection. Adv Immunol. 2017;135:81–117.10.1016/bs.ai.2017.02.002Search in Google Scholar PubMed PubMed Central
[270] Yang D, He Y, Muñoz-Planillo R, Liu Q, Núñez G. Caspase-11 requires the pannexin-1 channel and the purinergic P2X7 pore to mediate pyroptosis and endotoxic shock. Immunity. 2015 Nov;43(5):923–32.10.1016/j.immuni.2015.10.009Search in Google Scholar PubMed PubMed Central
[271] Broz P, Pelegrín P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020 Mar;20(3):143–57.10.1038/s41577-019-0228-2Search in Google Scholar PubMed
[272] Zheng Z, Deng W, Bai Y, Miao R, Mei S, Zhang Z, et al. The lysosomal rag-ragulator complex licenses RIPK1 and caspase-8-mediated pyroptosis by yersinia. Science. 2021 Jun;372(6549):eabg0269.10.1126/science.abg0269Search in Google Scholar PubMed PubMed Central
[273] Hughes SA, Lin M, Weir A, Huang B, Xiong L, Chua NK, et al. Caspase-8-driven apoptotic and pyroptotic crosstalk causes cell death and IL-1β release in X-linked inhibitor of apoptosis (XIAP) deficiency. EMBO J. 2023 Mar;42(5):e110468.10.15252/embj.2021110468Search in Google Scholar PubMed PubMed Central
[274] Li Y, Li YJ, Zhu ZQ. To re-examine the intersection of microglial activation and neuroinflammation in neurodegenerative diseases from the perspective of pyroptosis. Front Aging Neurosci. 2023 Nov;15:1284214.10.3389/fnagi.2023.1284214Search in Google Scholar PubMed PubMed Central
[275] Miao R, Jiang C, Chang WY, Zhang H, An J, Ho F, et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity. 2023 Nov;56(11):2523–41.e8.10.1016/j.immuni.2023.10.004Search in Google Scholar PubMed PubMed Central
[276] Vasudevan SO, Behl B, Rathinam VA. Pyroptosis-induced inflammation and tissue damage. Semin Immunol. 2023 Sep;69:101781.10.1016/j.smim.2023.101781Search in Google Scholar PubMed PubMed Central
[277] Yang C, Pan RY, Guan F, Yuan Z. Lactate metabolism in neurodegenerative diseases. Neural Regen Res. 2024 Jan;19(1):69–74.10.4103/1673-5374.374142Search in Google Scholar PubMed PubMed Central
[278] Li Q, Yuan Y, Huang S, Di G, Chen H, Zhuang Y, et al. Excess Ub-K48 induces neuronal apoptosis in Alzheimer’s disease. J Integr Neurosci. 2024 Dec;23(12):223.10.31083/j.jin2312223Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

