Abstract
The Lamiaceae family is one of the widest plant families among Greek flora, consisting of a great variety of species, with the genus Stachys being one of its largest representatives, spread to most continents. The genus Stachys is also known for its beneficial properties and has been used for years as a traditional remedy for healing various health conditions. Stachys iva, an endemic plant in the Kozani Regional unit, has also been consumed as an infusion by locals and is reported to relieve common cold symptoms, have antimicrobial properties, and contribute to normalizing blood glucose levels. The present study aimed to identify the chemical compounds (such as phenolic acids, flavonoids, and phenylethanoid glycosides) responsible for the herb’s pharmacological properties and determine the optimal extraction conditions to gather an extract with high therapeutic value without solvent and energy waste. Experiments conducted proved that extracting by simple stirring with deionized water for 75 min at 80°C is the best option. In contrast, the extract’s total polyphenol content was determined, and the compounds were identified by high-performance liquid chromatography analysis. In addition, other methods were utilized (e.g., ferric-reducing antioxidant power assay and 2,2-diphenyl-1-picrylhydrazyl antiradical activity assay) to reveal potent antioxidant, anti-hydrogen peroxide, and anti-inflammatory activity, while the correlation between these properties and extraction conditions was also examined.
Graphical Abstract

1 Introduction
Genera of the Lamiaceae family are widely known for their therapeutic properties and the variety of traditional uses as remedies over the ages. Since ancient times, different Lamiaceae species, conditioned as herbal infusions, edible greens, and extracts, have been mostly consumed as antioxidant, anti-inflammatory, antimicrobial, and analgesic herbal medicines [1,2]. Specific examples of genera from the Lamiaceae family known for their therapeutic properties include Lavandula (lavender) for its calming effects, Mentha (mint) for digestive health, and Salvia (sage) for its anti-inflammatory properties. All the above properties are also common among the species of genus Stachys, one of the largest representatives of the Lamiaceae family as it includes more than 300 species, spread around the Mediterranean area, Asia, America, and southern Africa [3]. The phytochemical analysis conducted on several Stachys species has scientifically verified the empirical use of these herbs as a treatment for different conditions. The polyphenolic compounds which have been isolated and identified are related to the inhibition of lipid peroxidation and result in antioxidant and anti-inflammatory activity [4,5,6]. In addition, flavonoids, iridoids, and glucosides seem to enhance the anti-inflammatory activity. Fatty acids, alkaloids, triterpenes, lignans, and secondary metabolites have also been isolated from different Stachys species [2,7,8]. The numerous pharmacological activities of the latter are also significant as – except for those mentioned above – some species have been efficiently used as antibacterial agents [9,10,11,12], as well as treatment for polycystic ovary syndrome and as potent hepatoprotective, as well as neuroprotective agents against Alzheimer’s disease. Extracts have been additionally examined for their anti-proliferative cytotoxic and antidiabetic properties, proving once again the plethora of pharmacological activities as a potent alternative approach to diabetes and cancer treatment [2,3,5,8,10,13,14].
Despite the plethora of Stachys’ beneficial properties, the potential side effects or even the possibility of interaction among its extracts and other medications should be considered before consuming its infusions. Even though there is not adequate information about Stachys iva toxicity or contraindications – as it has not been thoroughly studied before – some Stachys species are mentioned to be emmenagogue and uterotonic while others cause hypotension [15] or even are toxic damaging liver and renal tissue [16]. In any case, as the above data are derived from experiments in rats, pregnant women and individuals with chronic health issues consuming medicaments should not better consume any herbal remedies without a specialist’s advice due to lack of evidence about potential adverse effects.
Polyphenolic compounds found in abundance in Stachys extracts are more likely to be responsible for their therapeutic properties. These compounds can be found in many fruits, vegetables, and plants, being associated with their beneficial properties and some of their characteristics such as their color, flavor, and odor [17]. Chemically, they contain phenolic rings combined with various structures which are responsible for their classification as well as for their properties. Phenolic acids, flavonoids, lignans, and stilbenes are the main groups of chemical compounds classified as polyphenols [18].
Several studies have been conducted to determine how polyphenol consumption improves our health [19,20]. Primarily, polyphenols are considered to be a natural “weapon” against inflammation. Even inflammation is a natural response of our immune system after tissue damage or infection as an attempt to restore the primer healthy condition, it is characterized by unpleasant symptoms such as redness, pain, swelling, and heat leading, if not suppressed on time, to loss of tissues or even organ’s function. Since the pathophysiology of inflammation is complicated, involving immune cell responses (mainly by cytokines) and a plethora of biochemical molecules that trigger the procedure (such as prostaglandins, cyclooxygenase, and TNFa), its suppression requires a kind of intervention in this “mechanism” [21].
Polyphenolic compounds are also characterized as natural antioxidants, providing oxidative stability to the plant and scavenging free radicals when consumed [17,20]. Even though reactive oxygen species (ROS) are necessary for signal transmission involved in crucial biological functions, their excessive production results in bonding with macromolecules, causing DNA damage, and mutations and affecting normal functions in general [22]. As a result, chronic exposure to oxidative stress due to free radical formation is the main “suspect” for causing cancer, inflammation, as well as degenerative and circulatory system conditions [23,24,25].
Summarizing, polyphenols seem to have a protective role in inflammatory (chronic) conditions, neurodegenerative diseases, and many other consequences of aging in general, whereas several epidemiological studies have co-related the polyphenolic compounds consumption with the reduction of coronary artery disease risk and glucose levels in the blood as well [26]. Even though Stachys species have been thoroughly studied, some endemic species such as Stachys iva are still “unexplored.” S. iva, known as “fluffy mountain tea,” grows in Northern Greece, especially in the mountains of Kozani Regional unit (Western Macedonia), at an altitude of approximately 600 m [27]. It is a perennial herb with a hairy four-angled stem and opposite narrow leaves. Flowers occur in summer, and the corollas are yellow, sympetalous, and two-lipped [28]. A typical S. iva plant with its leaves is illustrated in Figure 1.

A typical Stachys iva plant.
As S. iva has been traditionally used as a herbal infusion instead of Sideritis spp. due to its antibacterial properties as well as a remedy for the relief of common cold symptoms, the present study aims to identify the chemical compounds that could be responsible for these therapeutic properties. Regarding the identification of the active compounds, high-performance liquid chromatography (HPLC) has been utilized, combined with other conventional experimental procedures. Specifically, Folin–Ciocalteu and ferric-reducing antioxidant power (FRAP) assay, in addition to 2,2-diphenyl-1-picrylhydrazyl (DPPH) antiradical activity assay and hydrogen peroxide scavenging assay, were conducted for the determination of the total phenolic compounds and antioxidant/antiradical capacity of the extract. The anti-inflammatory activity of the S. iva extract has also been assessed in vitro as an attempt to experimentally justify some of the plant’s pharmacological properties.
An additional purpose of this study is to detect the optimal extraction conditions of S. iva in order to investigate which temperature–duration combination leads to the most enriched, in bioactive compounds, extract. The optimization also has an environmentally friendly aspect, as it aims to the reduction of energy and solvent consumption. Moreover, using water as solvent through the process does not involve harmful organic solvents which are harmful to the environment and may provide extracts unsuitable for consumption by humans. To the best of our knowledge, there are no other comprehensive phytochemical analyses of S. iva available in the literature. Therefore, our study represents a novel contribution to the understanding of this species’ bioactive compounds.
2 Materials and methods
2.1 Chemicals and reagents
All the experiments were conducted by using mainly deionized water as solvent except for solvents used in chromatography (water, formic acid, and acetonitrile) that were HPLC grade. Neochlorogenic acid, chlorogenic acid, rutin, verbascoside, narirutin, myricetin, and rosmarinic acid were purchased from Sigma-Aldrich (Darmstadt, Germany) as chemical standards and used in HPLC for determination of polyphenolic compounds. Deionized water was chosen as the extraction solvent due to its safety, cost-effectiveness, and ability to extract a wide range of polyphenolic compounds.
Hydrochloric acid, phosphate buffer solution, methanol, l-ascorbic acid, trichloroacetic acid, aluminum chloride, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were also purchased from Sigma-Aldrich (Darmstadt, Germany). The Folin–Ciocalteu reagent, ethanol, and gallic acid were obtained from Panreac Co. (Barcelona, Spain). Hydrogen peroxide (35% v/v) was purchased from Chemco (Malsch, Germany), iron(iii) chloride from Merck (Darmstadt, Germany), and anhydrous sodium carbonate from Penta (Prague, Czech Republic). Egg albumin used for the detection of anti-inflammatory action was provided by eggs purchased from the local market.
2.2 Plant material
Plant materials (leaves, stems, and flowers) were collected in the blooming stage in June 2023 from Polyrrachos village (at 40°08′23.0″N and 21°57'12.0″E, based on Google Earth version 9.185.0.0) in Kozani, Municipality of Western Macedonia Greece, at about 630 m altitude. The microclimate of the area is characterized by warm summers, cool autumns and springs, and cold winters, wetter than other regions of the municipality affected by the Polyphytos Lake nearby.
After collection, the aerial parts of S. iva were weighed and dried for 30 days at room temperature in a shaded room without excessed humidity until their weight stabilized, indicating complete loss of water, and were subjected to extraction processes. The aerial parts were ground after drying, and the particle size of the dried plant material was determined.
2.3 Plant extraction with response surface methodology (RSM)
In order to maximize the efficiency of extracting polyphenols and assessing antioxidant and anti-inflammatory activity from medicinal plant extracts, the RSM technique was utilized. First, the optimal temperature and extraction time were studied. To do this, 0.4 g of the S. iva plant was accurately weighed (Kern PLS 3100-2F, Kern & Sohn GmbH, Balingen, Germany) using 20 mL of deionized water. As shown in Table 1, the experiments were conducted in a temperature range of 20–80°C and the time of extraction ranged from 30 to 90 min. The experiment that used a Main Effects Screening design with a major impact screening arrangement served as the basis for the optimization procedure. There were 10 design points in the experiment. The experimental design resulted in the creation of five levels of process variables. Analysis of variance (ANOVA) and summary-of-fit tests were used to evaluate the overall model significance, as indicated by the R 2 and p-values, and the significance of the model coefficient, as indicated by the equation, with a minimum level of 95% confidence. After the 500-rpm stirring extraction process was finished, the samples were centrifuged at 10,000 × g for 10 min at room temperature using a NEYA 16R Remi Elektrotechnik Ltd. (Palghar, India). The supernatants were then gathered and kept in storage at −40°C.
Actual and coded levels of the independent variables were used to optimize the extraction process using the Main Effects Screening design
| Independent variables | Code units | Coded variable level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Temperature (T, °C) | X 1 | 20 | 35 | 50 | 65 | 80 |
| Time (t, min) | X 2 | 30 | 45 | 60 | 75 | 90 |
Additionally, a quadratic (second-order) polynomial model was used to predict the response variable as a function of the investigated independent components, as shown by equation (1):
The predicted response variable is denoted as Y k , while the independent variables are X i and X j . The intercept and regression coefficients for the linear, quadratic, and interaction terms of the model are denoted as β 0, β i , β ii , and β ij , respectively.
The RSM was used to identify the largest peak region and evaluate the impact of a significant independent variable on the response [29]. To visually depict the model equation, the creation of a three-dimensional surface response graph took place.
2.4 Other extraction techniques
In order to confirm that water is the optimal extraction solvent, other solvents were used for extraction purposes, such as ethanol as an alternative solvent. Specifically, 2 g of the dried S. iva sample were extracted in 100 mL ethanol as well as in ethanol with water mixture (60:40 v/v) at 50°C for 30 min. In contrast, two more plant samples of the same weight were extracted in deionized water at 100°C for 5 min and at 50°C for 30 min in order to collect comparable results.
2.5 Polyphenol determination
2.5.1 Total polyphenol content (TPC)
A previously established methodology [30] was applied to determine TPC. Briefly, 100 μL of the properly diluted sample extract was mixed with 100 μL of Folin–Ciocalteu reagent, and after 2 min, 800 μL of 5% w/v aqueous sodium carbonate solution was added. The mixture was incubated at 40°C for 20 min and the absorbance was recorded at 740 nm in a Shimadzu UV-1700 PharmaSpec Spectrophotometer (Kyoto, Japan). The TPC (C TP) was calculated from a gallic acid calibration curve (10–80 mg/L). TPC was determined as mg gallic acid equivalents (GAE) per g of dry weight (dw), using the following equation:
where the volume of the extraction medium is indicated with V (expressed in L) and the dry weight of the sample as w (expressed in g).
2.5.2 HPLC quantification of polyphenolic compounds
HPLC was used to detect and quantify individual polyphenols from the sample extracts, as established in our previous research [30]. A Shimadzu CBM-20A liquid chromatograph and a Shimadzu SPD-M20A diode array detector (DAD) (both purchased by Shimadzu Europa GmbH, Duisburg, Germany) were employed for the analysis of medicinal plant extracts. The compounds were separated into a Phenomenex Luna C18(2) column from Phenomenex Inc. (Torrance, CA, USA), kept at 40°C (100 Å, 5 μm, 4.6 mm × 250 mm). The mobile phase included 0.5% aqueous formic acid (A) and 0.5% formic acid in acetonitrile/water (3:2) (B). The gradient program required: initially from 0 to 40% B, then to 50% B in 10 min, to 70% B in another 10 min, and then constant for 10 min. The flow rate of the mobile phase was set at 1 mL/min. The compounds were identified at 320 nm by comparing the absorbance spectrum and retention time to those of pure standards and then quantified through calibration curves (0–50 μg/mL).
2.6 Antioxidant capacity of the extracts
2.6.1 FRAP assay
An established technique by Shehata et al. [31] was used for the evaluation of FRAP. In a 1.5-mL Eppendorf tube, 50 μL of the properly diluted sample extract was mixed with 50 μL of FeCl3 solution (4 mM in 0.05 M HCl). The mixture was incubated for 30 min at 37°C, with 900 μL of TPTZ solution (1 mM in 0.05 M HCl) being immediately added right after, and the absorbance was measured after 5 min at 620 nm. The ferric-reducing power (P R) was calculated using an ascorbic acid calibration curve (C AA) in 0.05 M HCl with ranging values (50–500 μM). The P R was calculated as μmol of ascorbic acid equivalents (AAE) per g of dw, using equation (3):
where V is represented (in L) as the entire volume of the extraction medium and w (in g) represents the dried weight of the material.
2.6.2 DPPH• antiradical activity assay
The extracted polyphenols from the dried material were evaluated for their antiradical activity (A AR) using a slightly modified DPPH˙ method, as previously established by Shehata et al. [31]. In brief, 25 μL of the properly diluted sample extract was mixed with a quantity of 975 μL of a 100 μM DPPH˙ solution in methanol, with the solution being kept at room temperature for 30 min in the dark right after. The absorbance was measured at 515 nm. Moreover, a blank sample was used instead of the sample, including DPPH˙ solution and methanol, with the absorbance immediately being measured. To calculate the percentage of scavenging, equation (4) was employed:
An ascorbic acid calibration curve (C AA, 100–1,000 μmol/L in methanol) in equation (5) was used to evaluate antiradical activity (A AR), which was expressed as μmol AAE per g of dw:
where V is represented (in L) as the entire volume of the extraction medium and w (in g) represents the dried weight of the material.
2.6.3 Hydrogen peroxide (H2O2) scavenging assay
A previous method [32] was applied for the H2O2 scavenging assay. A quantity of 400 μL of the properly diluted sample extract and 600 μL of an H2O2 solution (40 mM, made in phosphate buffer, pH 7.4) was added into an Eppendorf tube. The absorbance was recorded right after 10 min at 230 nm. The scavenging capacity of the H2O2 was expressed as follows:
where the absorbances of the blank solution, the extract solution in the absence of hydrogen peroxide, and the sample are denoted by A 0, A c, and A, respectively.
The concentration of ascorbic acid ranged in the calibration curve (C AA, 50–500 μmol/L in 0.05 M HCl) and the following equation (7) was used to determine the anti-hydrogen peroxide activity (A AHP) as μmol AAE per g of dw:
where V denotes the volume of the extraction medium (in L), and w is the dry weight of the sample.
2.7 Assessment of in vitro anti-inflammatory activity
The in vitro evaluation of the anti-inflammatory properties of the S. iva extracts was conducted using the albumin denaturation assay [33]. Briefly, a mixture containing egg albumin and PBS (pH = 6.4) with a 0.1:1.4 mL ratio, or 6.67% v/v (mixture A), respectively, was prepared. Then, 400 μL of the properly diluted sample extract or standard were mixed with 600 μL of mixture A in a 1.5 mL Eppendorf tube, and then, the tubes were incubated at 37°C for 15 min. Afterwards, the mixture was heated at 70°C for 5 min. The absorbance was then recorded at 660 nm. This wavelength was chosen to minimize interference from the extract’s color or turbidity. To determine the % inhibition of protein denaturation, the following equation (8) was used:
By measuring the absorbance of the control (without extract) against that of the sample (with extract), we can assess the degree to which the extract prevents protein denaturation.
2.8 Statistical analysis
The statistical analysis was applied to the RSM through Main Effects Screening design and distribution analysis, using the JMP® Pro 16 program (SAS, Cary, NC, USA). The extraction processes were carried out at least twice for every batch of plant extract, and the quantitative analysis was carried out in triplicate. The Kolmogorov–Smirnov test was employed to verify data normality. To detect statistically significant differences, a one-way ANOVA was conducted, succeeded by a post hoc Tukey HSD (honestly significant difference) test using the Tukey–Kramer method. The means and standard deviations of the results are used to demonstrate them. Using JMP® Pro 16 software, Pareto plot analysis, principal component analysis (PCA), and multivariate correlation analysis (MCA) were carried out.
3 Results and discussion
3.1 Optimization of extraction conditions
Even though extraction is considered to be a common laboratory technique, it may result in being energy consuming, requiring also a significant amount of solvents, as each plant has a divergent abundance of bioactive compounds with different chemical properties. Supposing that cost-effectiveness is not taken into consideration, the majority of the organic solvents being used are harmful to the environment. Moreover, it may be necessary for the plant material to be extracted with many different solvents in order to conclude which of them and under which conditions is able to extract as many substances as possible, providing the researcher with an extraction that may lead to significant conclusions about the plant’s potential properties [34]. Additionally, the extraction duration and temperature are crucial parameters that define the energy consumption during the procedure. Obviously, the detection of optimal extraction conditions is necessary as it enhances the efficiency of the procedure, and utilizing water as solvent seems to be the most suitable choice, as it is approachable, affordable, and without environmental impact [35]. Investigating the effect of temperature and duration on the extraction process has improved our understanding of optimizing the yield and efficacy of polyphenolic compounds. This knowledge is crucial for devising more effective extraction techniques that ensure the preservation and maximization of bioactive compounds.
Taking all the above into consideration, as well as the predicted antioxidant activity and TPC of the extract, we came up with a correlation between the extraction conditions and the extract’s properties using the RSM approach, as presented in Tables 2 and 3, as an attempt to investigate the optimal design point. Obviously, extracting the plant material for 75 min at 80°C (design point-DP 5) seems to provide us with an extract rich in polyphenolic compounds and with enhanced antioxidant potency, as it is demonstrated in Figure 2, where the predicted response referring to parameters of DP 5 presenting as statistically significant. The optimal temperature was quite anticipated, as there are references documenting that the highest polyphenolic substance recovery is conducted under 50–80°C [36]. Also, the three-dimensional plot for TPC can be seen in Figure 3.
Experimental findings for the two independent variables under investigation and the dependent variable’s response to TPC
| Design point | Independent variables | Response TPC (mg GAE/g dw) | ||
|---|---|---|---|---|
| X 1 (T, °C) | X 2 (t, min) | Actual | Predicted | |
| 1 | 1 (20) | 2 (45) | 9.02 ± 0.62 | 10.27 |
| 2 | 2 (35) | 2 (45) | 7.69 ± 0.43 | 7.76 |
| 3 | 3 (50) | 3 (60) | 11.33 ± 0.56 | 10.39 |
| 4 | 4 (65) | 4 (75) | 15.75 ± 1.04 | 15.46 |
| 5 | 5 (80) | 4 (75) | 31.13 ± 2.18 | 29.52 |
| 6 | 1 (20) | 1 (30) | 8.96 ± 0.34 | 7.58 |
| 7 | 2 (35) | 1 (30) | 10.92 ± 0.29 | 11.57 |
| 8 | 3 (50) | 5 (90) | 18.12 ± 1.20 | 17.70 |
| 9 | 4 (65) | 3 (60) | 19.72 ± 0.67 | 21.10 |
| 10 | 5 (80) | 5 (90) | 21.67 ± 1.13 | 22.97 |
Coded values of the two independent variables under investigation and the actual values of antioxidant and anti-inflammatory activity assays
| Design point | Independent variables | Responses | ||||
|---|---|---|---|---|---|---|
| X 1 (T, °C) | X 2 (t, min) | FRAP (μmol AAE/g dw) | DPPH (μmol AAE/g dw) | Anti-hydrogen peroxide activity (μmol AAE/g dw) | Anti-inflammatory activity (%) | |
| 1 | 1 (20) | 2 (45) | 47.02 ± 3.53 | 17.81 ± 1.26 | 129.78 ± 5.84 | 42.53 ± 2.64 |
| 2 | 2 (35) | 2 (45) | 41.55 ± 2.99 | 14.03 ± 0.86 | 76.86 ± 1.77 | 42.30 ± 3.13 |
| 3 | 3 (50) | 3 (60) | 80.05 ± 1.68 | 32.92 ± 1.35 | 192.42 ± 14.24 | 44.50 ± 2.18 |
| 4 | 4 (65) | 4 (75) | 94.69 ± 5.40 | 59.18 ± 3.08 | 301.86 ± 14.79 | 56.02 ± 3.53 |
| 5 | 5 (80) | 4 (75) | 182.85 ± 11.15 | 95.83 ± 5.17 | 459.36 ± 31.70 | 72.53 ± 4.71 |
| 6 | 1 (20) | 1 (30) | 51.04 ± 3.67 | 16.29 ± 0.46 | 82.44 ± 3.38 | 47.61 ± 3.52 |
| 7 | 2 (35) | 1 (30) | 60.73 ± 3.16 | 22.41 ± 1.43 | 127.80 ± 6.77 | 55.69 ± 1.28 |
| 8 | 3 (50) | 5 (90) | 98.50 ± 3.25 | 47.72 ± 2.72 | 270.18 ± 7.57 | 55.09 ± 3.97 |
| 9 | 4 (65) | 3 (60) | 107.31 ± 3.97 | 49.69 ± 1.94 | 280.98 ± 16.02 | 60.83 ± 3.35 |
| 10 | 5 (80) | 5 (90) | 131.41 ± 6.44 | 58.88 ± 3.36 | 348.84 ± 10.12 | 64.17 ± 4.17 |

Plot (a) displays the actual versus the predicted response (TPC, mg GAE/g dw) for the optimization of extraction of S. iva plant performed with water solutions. The inset tables provide statistics related to the evaluation of the resulting model. Values with color and asterisk are statistically significant. The desirability function for the optimization of extraction of S. iva performed with water solutions is displayed in Plot (b).

Three-dimensional graph depicting the covariation of X 1 (T, °C) and X 2 (t, min) and the effect of the process variables considered on the response (TPC, mg GAE/g dw) for the optimization of extraction of S. iva performed in water solutions.
The desirability function was used in the optimization process to combine multiple response variables into a single composite response. Each response variable was transformed into a desirability value ranging from 0 (completely undesirable) to 1 (fully desirable). The overall desirability was calculated as the geometric mean of the individual desirability values, providing a single metric that reflects the optimal conditions for all response variables. This approach allows for the simultaneous optimization of multiple criteria, ensuring a balanced and comprehensive evaluation of the extraction conditions.
The TPC was expressed in milligrams of gallic acid equivalents per gram of dry weight (mg GAE/g dw). This unit standardizes the phenolic content based on a gallic acid calibration curve, allowing for comparison across different studies and samples. The antioxidant capacity measured by assays such as FRAP and DPPH was expressed in micromoles of ascorbic acid equivalents per gram of dry weight (µmol AAE/g dw). This unit standardizes the antioxidant activity based on an ascorbic acid calibration curve.
The ferric-reducing antioxidant power (FRAP) assay, commonly utilized to assess the antioxidant capacity of different samples, presents multiple limitations. Its high sensitivity to pH fluctuations, especially it is performed at an acidic pH of approximately 3.6, may not provide an accurate measure of antioxidant capacity at physiological pH. Moreover, temperature changes can affect the outcomes, with elevated temperatures possibly causing an overestimation and reduced temperatures an underestimation. The FRAP assay is designed to measure the reducing power of antioxidants, which may not reflect all antioxidant activities, such as radical scavenging or metal chelation. This limitation restricts its capacity for a complete assessment. Additionally, the assay can be affected by other reducing agents present in the sample, potentially leading to erroneous outcomes. Nevertheless, the FRAP assay is valued for its simplicity, rapidity, cost-efficiency, and consistent reproducibility when standard conditions are applied. Ascorbic acid is often employed as a benchmark for its notable antioxidant properties; however, it might not adequately reflect the intricate antioxidant activities of plant extracts. Employing alternative methods, such as a blend of standards like Trolox, or integrating the FRAP assay with other antioxidant evaluations (for instance, DPPH, ABTS, ORAC) could offer a more comprehensive evaluation of antioxidant potential by encompassing various mechanisms of action.
The second-order polynomial equations obtained for the responses were expressed as follows:
Depending on ANOVA analysis results, we may also evaluate the responses of the extract, as an attempt to assess its potent properties. Apparently, all the responses of the conducted experiments seem to be statistically significant, indicating the pharmacological properties of S. iva plant extract. Moreover, according to the RMSE, total phenolic content and anti-inflammatory activity appear to be closer to the actual values, so we anticipated these properties to be experimentally proved, as demonstrated in Table 4.
ANOVA analysis of the responses
| Responses | F-ratio | p-value | R 2 | Adjusted R 2 | RMSE |
|---|---|---|---|---|---|
| TPC | 34.24 | 0.0022 | 0.9772 | 0.9486 | 1.67 |
| FRAP | 15.65 | 0.0099 | 0.9514 | 0.8906 | 14.54 |
| DPPH | 8.57 | 0.0292 | 0.9146 | 0.8078 | 11.38 |
| Anti-hydrogen peroxide activity | 22.03 | 0.0052 | 0.9650 | 0.9212 | 35.41 |
| Anti-inflammatory activity | 46.11 | 0.0013 | 0.9829 | 0.9616 | 1.96 |
RMSE means root mean square error.
3.2 Impact of extraction parameters on assays through Pareto plot analysis
In Pareto plot analysis, factors are chosen for their contribution to total variance, with the most influential ones accounting for the largest percentage of variance. In order to examine the positive or negative impact of extraction parameters on the extract properties, we conducted a Pareto plot analysis including results from all the experimental assays that took place. Observing the plots (Figure 4), we may conclude to the fact that the combination of temperature and time of extraction does not affect either the TPC of the extract or its antioxidant and anti-inflammatory properties, as it is demonstrated to adopt negative or very low values. On the other hand, the temperature itself (factor X 1) mainly contributes to eventually collecting the optimal extract. The temperature not only affects the extract’s quality but also overcomes the critical value’s threshold (gold line) indicating statistical significance. Pareto plot analysis may occasionally simplify the complex interplay between factors too much, which could lead to a misinterpretation of the results.
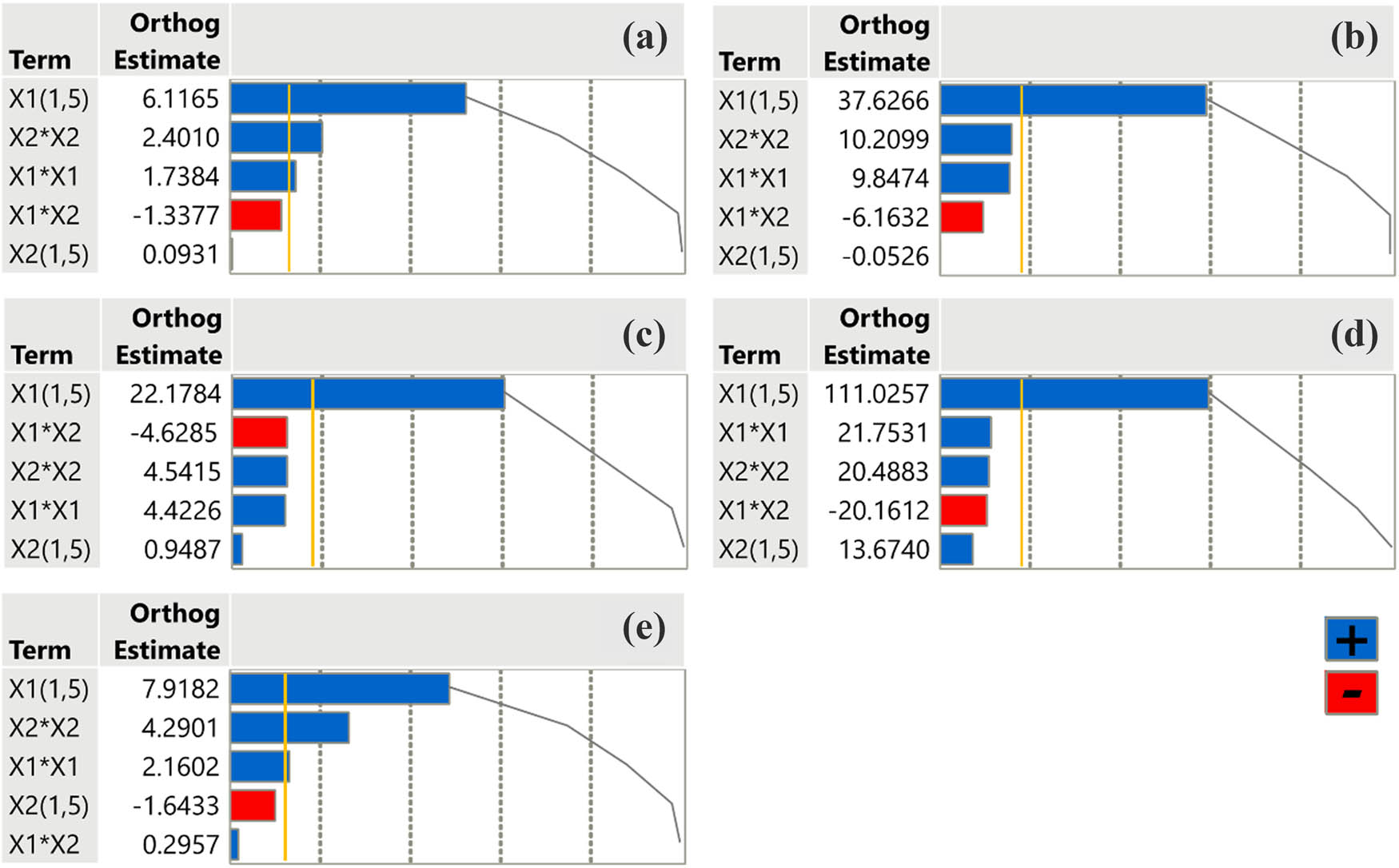
Pareto plots of transformed estimates for TPC (a), FRAP (b), DPPH (c), anti-hydrogen peroxide activity (d), and anti-inflammatory activity (e) assays. A reference gold line is drawn on the plot to indicate the level of significance (p < 0.05).
3.3 Optimal extraction conditions
Attempting to optimize the extraction conditions furthermore, partial least squares (PLS) analysis was used for the assessment of the extraction parameters impact, as depicted in Figure 5. Taking into consideration that desirability equals 0.8496, variables estimated over this point seem to have a significant contribution. As demonstrated in the plot, temperature (X 1 variable) affects the quality of extract, the quantity of polyphenols (TPC), and its antioxidant and anti-inflammatory properties. Specifically, the DP5 (80°C) seems to provide the optimum results. Examining the impact of extraction duration (X 2 variable), it can be noticed that this parameter solely does not determine the extract’s content at the same level comparatively with the temperature. However, extracting S. iva at 80°C for 75 min (DP5) is predicted to be the ideal parameter combination.

Maximum predicted responses and optimum extraction conditions for the dependent variables.
3.4 PCA and MCA
To obtain a more objective aspect of how the temperature and the duration of the extraction are correlated to the TPC, the antioxidant (FRAP, DPPH, H2O2), and anti-inflammatory assays, a PCA was conducted. In PCA, the selection criteria focus on identifying the principal components that account for the majority of the variance in the data, those with eigenvalues >1. As the parameter’s sign determines whether or not two or more variables are associated, we may conclude once again, according to Figure 6, that the extraction’s temperature positively affects the extract’s properties and its concentration of polyphenolic compounds. Additionally, all the variables were well discriminated, and it was revealed that TPC and FRAP assay, are also positively correlated. On the contrary, the negative sign of extraction’s duration, reveals, as anticipated, that this parameter does not noticeably determine the extract’s composition or its experimental assays measurements. PCA is beneficial for dimensionality reduction but may not consistently capture the non-linear relationships between variables.

PCA for the measured variables. Each X variable is presented with a blue color.
Multiple correspondence analysis was utilized to discern correlations among various variables, with the most significant factors identified by the highest correlation coefficients. Moreover, taking into consideration the data displayed in Table 5, the high association among all the parameters is also confirmed as they all have positive signs and correlation >0.89. A low correlation, compared to other measured variable pairs, was shown between anti-inflammatory activity and anti-hydrogen peroxide activity (0.8866), in contrast with the highest correlation that appears among the latter and DPPH (0.9858). MCA may be constrained by multicollinearity and the possibility of spurious correlations.
Multivariate correlation analysis of measured variables
| Responses | TPC | FRAP | DPPH | Anti-hydrogen peroxide activity | Anti-inflammatory activity |
|---|---|---|---|---|---|
| TPC | — | 0.9691 | 0.9411 | 0.9583 | 0.9419 |
| FRAP | — | 0.9755 | 0.9740 | 0.9177 | |
| DPPH | — | 0.9858 | 0.8924 | ||
| Anti-hydrogen peroxide activity | — | 0.8866 | |||
| Anti-inflammatory activity | — |
3.5 Optimal extract and comparison with other extraction techniques
The correlation between the values generated by the second-order polynomial model and those acquired through experimental analysis is determined to be 0.9973, and they show no deviations with the p-value being 0.0002. Comparing data from Table 6, the optimal extract’s responses are similar to the maximum predicted ones and mainly seem more increased than the latter, concluding that extracting at 80°C for 75 min is the ideal condition for S. iva. Extracting in boiling water for 5 min and in water:ethanol mixture (40:60 v/v) at 50°C for 30 min, leads to quite similar and pretty significant experimental responses, except the TPC, but lower than the expecting ones. This mixture was chosen based on its ability to extract a broad range of polyphenolic compounds. The less interesting results that depict significantly decreased compared to the optimal extract are derived if we extract the plant material with pure ethanol or deionized water at 50°C for 30 min. Ethanol was chosen as an alternative solvent due to its effectiveness in extracting polyphenolic compounds and its safety for use in food and pharmaceutical applications.
Maximum predicted responses, optimal extract under optimal extraction conditions, and comparison with other extraction techniques
| Extracts | Responses | ||||
|---|---|---|---|---|---|
| TPC (mg GAE/g) | FRAP (μmol AAE/g) | DPPH (μmol AAE/g) | Anti-hydrogen peroxide activity (μmol AAE/g) | Anti-inflammatory activity (%) | |
| Maximum predicted response | 29.52 ± 3.83a | 169.44 ± 33.30a | 86.30 ± 26.05a | 439.27 ± 81.08a | 72.12 ± 4.49a |
| Optimal extract | 31.55 ± 0.79a | 185.78 ± 5.57a | 95.40 ± 3.91a | 410.42 ± 16.42a | 70.06 ± 1.47a,b |
| Boiling water | 12.50 ± 0.89c | 92.92 ± 2.23b | 73.56 ± 4.56a,b | 229.14 ± 16.50b | 61.74 ± 4.01b |
| Ethanol 60% v/v | 19.99 ± 0.54b | 92.88 ± 3.81b | 51.74 ± 3.88b | 231.84 ± 4.64b | na |
| Ethanol | 2.52 ± 0.07d | 11.33 ± 0.69c | 3.46 ± 0.13c | 26.64 ± 0.80c | na |
| Water | 10.99 ± 0.31c | 32.93 ± 1.75c | 19.80 ± 1.05c | 63.36 ± 1.96c | 49.57 ± 3.32c |
Within each column, statistically significant differences (p < 0.05) are denoted with lowercase letters (e.g., a–d); na means not analyzed.
3.6 Analysis of the optimal extract
The experimental assays we conducted, indicated that S. iva extract is abundant in polyphenolic compounds, as we anticipated due to statistical predictions and bibliographic references to other Stachys species. Especially the optimal extract has the ultimate TPC, 31.55 ± 0.79 mg GAE/g dw (actual) and 29.52 ± 3.83 mg GAE/g dw (predicted response), compared to others. The polyphenol compounds as we mentioned are mainly responsible for the plant’s beneficial properties, affecting its antioxidant and anti-inflammatory activity. This is also proved by the fact that all these variables correlate and their values are associated, as results from our data analysis.
The main polyphenolic compounds of the optimal extract were identified and also quantified, data demonstrated in Table 7, by using HPLC-DAD. A representative chromatogram is illustrated in Figure 7, where according to the retention time of each compound and their UV spectrum, phenolic acids such as rosmarinic, neochlorogenic and chlorogenic acid, and flavonoids (rutin, verbascoside, narirutin, myricetin) were identified.
Polyphenolic compounds analysis of optimal extract under optimal extraction conditions
| Polyphenolic compound | Optimal extract (mg/g dw) |
|---|---|
| Neochlorogenic acid | 0.55 ± 0.03 |
| Chlorogenic acid | 5.02 ± 0.21 |
| Rutin | 0.45 ± 0.02 |
| Verbascoside | 0.87 ± 0.06 |
| Narirutin | 0.03 ± 0 |
| Myricetin | 0.46 ± 0.01 |
| Rosmarinic acid | 1.4 ± 0.04 |
| Total identified | 8.79 ± 0.38 |

Representative HPLC chromatogram at 320 nm of optimal extract, demonstrating polyphenolic compounds that were identified. 1: Neochlorogenic acid; 2: chlorogenic acid; 3: rutin; 4: verbascoside; 5: narirutin; 6: myricetin; 7: rosmarinic acid.
Examining further each compound’s properties we may better understand the traditional use of “fluffy mountain tea’s” extract as a remedy for curing a wide range of health conditions. Initially, referring to rosmarinic acid (RA), many studies have been conducted both in vivo and in vitro, trying to suggest its mechanisms of action against inflammation and oxidative stress. RA’s chemical structure allows the compound to be lipophilic enough to penetrate the cell membrane and protect the cell by scavenging free radicals. Its antioxidant properties protect different organs such as liver and heart enhancing also its potent use as a neuroprotective and anticancer agent. Furthermore, studies are reporting RA’s action against different types of bacteria and fungi as well as against inflammatory conditions, possibly by inhibiting the synthesis of cytokines and suppresses the activation of cyclooxygenase and even of T-leukocytes in autoimmune diseases [37,38,39,40].
Another well-known phenolic acid that is pretty ubiquitous in plant extracts, especially in coffee and tea infusions, and with the highest concentration in our extract is chlorogenic acid (CGA). Despite its action against free radicals and inflammation due to its chemical properties, CGA seems to affect glucose absorption decreasing its concentration in blood circulation. Thus, fat burning increases as an alternative source of energy, leading to both antidiabetic and anti-obesity action. Additionally, clinical trials proved that CGA consumption is associated with attenuation of blood pressure, probably because of the induction of vessel relaxation, antimicrobial activity has also been reported [41,42,43]. As for neochlorogenic acid, that is CGA’s isomer and has similar properties. However, we may highlight that its anti-inflammatory activity derives from the inhibition of lipopolysaccharide (LPS). The latter acts like a stimulant that activates microglia (type of macrophages), causing neuroinflammation and triggering the onset of neurodegenerative diseases [44,45].
Even though phenolic acids concentration in the extract was 6.97 ± 0.28 mg/g dw out of the 8.79 ± 0.38 mg/g dw of totally identified polyphenolic compounds, many flavonoids contribute to S. iva properties and verbascoside (VERB) is the dominant one (0.85 ± 0.06 mg/g dw). VERB is a phenylethanoid glycoside, initially isolated from Verbascum sp. (mullein) but distributed in plenty of other plant species that have also been used as traditional remedies. Studies report that VERB combines anti-inflammatory, antibacterial, and even anti-androgen properties being a potent alternative approach for acne vulgaris treatment. Moreover, preventing lipoprotein oxidation protects cells from oxidative stress while repairs DNA damage caused by the latter. It may also be used as an anti-depressant, acting by triggering monoamine neurotransmitters’ production and increasing their levels and availability, while it seems to appear a protective role against atherosclerosis [46,47,48].
Rutin and myricetin are detected in approximately equal concentrations. They both possess diverse beneficial properties acting in vitro, as the majority of polyphenols, against lipid peroxidation, inflammation, and as radical scavengers [49]. Furthermore, rutin appears to have vasoprotective, anti-hypercholesterolemic, anti-hypertensive, and antiplatelet aggregation effects in vitro and after being administered to rats, while also promoting cancer cell apoptosis as indicated in experiments conducted using cell lines from various cancer types [50,51,52,53]. Highlighting some distinct properties of myricetin, we should mention that there are pieces of evidence about its intervention in diabetes and obesity management in rats [54,55].
Last but not least, even in minimum concentration, the flavonoid narirutin, which is mainly found in citrus fruit, has a plethora of beneficial properties similar to the ones documented for the rest of the extract’s flavonoids [56,57,58,59].
4 Conclusions
The present study aimed to identify the ideal extraction solvent (deionized water and ethanol) and optimal extraction parameters (temperature and duration) to obtain the most enriched extract of Stachys iva, with high therapeutical value. S. iva has been traditionally used as a remedy with diverse pharmacological properties, which this study attempted to validate experimentally. RSM was utilized to identify the optimal extraction conditions, combined with Pareto plot analysis. Additionally, PCA and MCA detected positive correlations between experimental assay responses and extraction conditions, as well as the association among these responses.
Each extract underwent experimental procedures to determine its TPC, antioxidant capacity (FRAP, DPPH, anti-hydrogen peroxide activity), and anti-inflammatory activity. The collected data were compared with predicted values. HPLC analysis identified and quantified polyphenolic compounds, with a total concentration of 8.79 ± 0.38 mg/g dw. Chlorogenic acid was the dominant compound (5.02 ± 0.21 mg/g dw), followed by verbascoside (0.87 ± 0.06 mg/g dw), neochlorogenic acid, rutin, narirutin, myricetin, and rosmarinic acid.
Considering evidence from in vitro/in vivo studies and clinical trials referring to these compounds’ beneficial properties, the traditional use of S. iva extracts as antioxidant, anti-inflammatory, antimicrobial, and antidiabetic agents can be experimentally validated. These compounds also show potential pharmacological properties against neurodegenerative and other diseases, leading to further research on S. iva with promising results.
Future research will explore the use of alternative solvents, such as ethanol, methanol, and acetone, which may enhance the extraction efficiency of bioactive compounds from S. iva. The selection of solvents will be based on their polarity, safety, and environmental impact. Additional response variables such as flavonoid content, tannin content, and specific antioxidant activities (e.g., superoxide dismutase activity) will be investigated to provide a more comprehensive understanding of the bioactive profile of S. iva extracts. Advanced extraction techniques such as supercritical fluid extraction, microwave-assisted extraction, and ultrasound-assisted extraction will be explored to optimize the yield and quality of bioactive compounds. These techniques offer potential benefits in terms of efficiency, selectivity, and environmental sustainability.
Moreover, the investigation of S. iva’s composition of bioactive compounds and the experimental justification of its traditional uses may lead to a well-established use of this herb in products of food, cosmetic, and pharmaceutical industries. S. iva infusions may replace water or other extracts in lotions and creams, providing them with an aqueous phase rich in polyphenols and antioxidants enhancing the skin barrier against free radicals. Due to its abundance in polyphenolic substances, S. iva extract may be added to beverages as flavoring and perhaps as a natural preservative due to its antimicrobial potency, resulting in the production of a functional drink. As for the pharmaceutical industry, even though S. iva has many potential pharmacological properties (antidiabetic, antiproliferative) as mentioned, more research is required in order to ensure the lack of toxicity, severe adverse effects, or even interaction with medications. However, its extract could be included in supplements against the common cold only if the sufficiency of safety data is proven.
Acknowledgments
The authors would like to thank Mr. Christos Papagiannis for collecting Stachys iva and providing us with plant material, as well as for capturing the photograph of the plant in its natural habitat. Also, the authors are grateful for the reviewers’ valuable comments that improved the manuscript.
-
Funding information: Authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results, and approved the final version of the manuscript. Conceptualization was carried out by A.V. and P.M.; methodology was developed by V.A. and S.I.L.; experimentation was conducted by A.V., I.M., and V.A.; data analysis and figures preparation were performed by A.V., I.M., and V.A.; writing – original draft preparation was done by A.V. and V.A.; writing – review and editing were contributed by A.V., I.M., V.A., S.I.L., and P.M.; and supervision was provided by S.I.L. and P.M.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Kanjevac M, Zlatić N, Bojović B, Stanković M. Pharmaceutical and biological properties of Stachys species: A review. Braz J Pharm Sci. 2022;58:e20211.10.1590/s2175-97902022e20211Search in Google Scholar
[2] Tomou EM, Barda C, Skaltsa H. Genus stachys: A review of traditional uses, phytochemistry and bioactivity. Medicines. 2020 Sep;7(10):63.10.3390/medicines7100063Search in Google Scholar PubMed PubMed Central
[3] Benedec D, Oniga I, Hanganu D, Tiperciuc B, Nistor A, Vlase AM, et al. Stachys species: Comparative evaluation of phenolic profile and antimicrobial and antioxidant potential. Antibiotics. 2023 Nov;12(11):1644.10.3390/antibiotics12111644Search in Google Scholar PubMed PubMed Central
[4] Bahadori MB, Zengin G, Dinparast L, Eskandani M. The health benefits of three Hedgenettle herbal teas (Stachys byzantina, Stachys inflata, and Stachys lavandulifolia) - profiling phenolic and antioxidant activities. Eur J Integr Med. 2020 Jun;36:101134.10.1016/j.eujim.2020.101134Search in Google Scholar
[5] Slimani W, Maioli M, Cruciani S, Zerizer S, Santaniello S, Kabouche Z, et al. Antioxidant, anti-inflammatory and anti-proliferative properties of Stachys circinata on HepG2 and MCF7 cells. Plants. 2023 Jun;12(12):2272.10.3390/plants12122272Search in Google Scholar PubMed PubMed Central
[6] Mansourian M, Mirzaei A, Azarmehr N, Vakilpour H, Kokhdan EP, Doustimotlagh AH. Hepatoprotective and antioxidant activity of hydroalcoholic extract of Stachys pilifera. Benth on acetaminophen-induced liver toxicity in male rats. Heliyon. 2019 Dec;5(12):e03029.10.1016/j.heliyon.2019.e03029Search in Google Scholar PubMed PubMed Central
[7] Mantovska DI, Zhiponova MK, Georgiev MI, Alipieva K, Tsacheva I, Simova S, et al. Biological activity and NMR-fingerprinting of balkan endemic species Stachys thracica Davidov. Metabolites. 2022 Mar;12(3):251.10.3390/metabo12030251Search in Google Scholar PubMed PubMed Central
[8] Anagnostou M, Tomou E, Goya‐Jorge E, Chatzopoulou P, Giner RM, Skaltsa H. Phytochemical study of Stachys iva Griseb. and in vitro evaluation of AhR transcriptional activity. Chem Biodivers. 2024 Nov;21(11):e202400457.10.1002/cbdv.202400457Search in Google Scholar PubMed
[9] Farjam MH. Biological activity of the n-butanolic extract of Stachys pilifera. Afr J Microbiol Res. 2011 Nov;5(28):5115–9.10.5897/AJMR11.1066Search in Google Scholar
[10] Stegăruș DI, Lengyel E, Apostolescu GF, Botoran OR, Tanase C. Phytochemical analysis and biological activity of three Stachys species (Lamiaceae) from Romania. Plants. 2021 Dec;10(12):2710.10.3390/plants10122710Search in Google Scholar PubMed PubMed Central
[11] Napolitano A, Di Napoli M, Castagliuolo G, Badalamenti N, Cicio A, Bruno M, et al. The chemical composition of the aerial parts of Stachys spreitzenhoferi (Lamiaceae) growing in Kythira Island (Greece), and their antioxidant, antimicrobial, and antiproliferative properties. Phytochemistry. 2022 Nov;203:113373.10.1016/j.phytochem.2022.113373Search in Google Scholar PubMed
[12] Jaradat N, Hawash M, Al-Maharik N, Qadi M, Issa L, Sobuh S, et al. Characterization of volatile compounds and evaluation of antibacterial, antifungal, and cytotoxic properties of Stachys palaestina from Palestine. Arab J Sci Eng. 2024 Jul. [cited 2024 Dec 6] https://link.springer.com/10.1007/s13369-024-09306-w.10.1007/s13369-024-09306-wSearch in Google Scholar
[13] Pashova S, Karcheva-Bahchevanska D, Ivanov K, Ivanova S. Genus Stachys – phytochemistry, traditional medicinal uses, and future perspectives. Molecules. 2024 Nov;29(22):5345.10.3390/molecules29225345Search in Google Scholar PubMed PubMed Central
[14] Jassbi AR, Miri R, Asadollahi M, Javanmardi N, Firuzi O. Cytotoxic, antioxidant and antimicrobial effects of nine species of woundwort (Stachys) plants. Pharm Biol. 2014 Jan;52(1):62–7.10.3109/13880209.2013.810650Search in Google Scholar PubMed
[15] Duke JA, Bogenshcutz-Godwin MJ, duCellier J, Duke PAK. Handbook of medicinal herbs. 2nd edn. Boca Raton (FL): CRC Press; 2002.10.1201/9781420040463Search in Google Scholar
[16] Modarresi M, Hosseinzadeh L, Nematy N, Siavash-Haghighi ZM, Ghanbari K. Acute and subchronic toxicological evaluation of Stachys lavandulifolia aqueous extract in Wistar rats. Res Pharm Sci. 2014;9(3):165–72.Search in Google Scholar
[17] Aljerf L, Aljerf N. Food products quality and nutrition in relation to public. Balancing health and disease: Food safety & hygiene promotion. Prog Nutr. 2023 Mar;25(1):e2023024.Search in Google Scholar
[18] Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009 Jan;2(5):270–8.10.4161/oxim.2.5.9498Search in Google Scholar PubMed PubMed Central
[19] Onydinma UP, Aljerf L, Obike A, Onah OE, Caleb NJ. Evaluation of physicochemical characteristics and health risk of polycyclic aromatic hydrocarbons in borehole waters around automobile workshops in Southeastern Nigeria. Groundw Sustain Dev. 2021 Aug;14:100615.10.1016/j.gsd.2021.100615Search in Google Scholar
[20] Di Lorenzo C, Colombo F, Biella S, Stockley C, Restani P. Polyphenols and human health: The role of bioavailability. Nutrients. 2021 Jan;13(1):273.10.3390/nu13010273Search in Google Scholar PubMed PubMed Central
[21] Xu Z, Chenli Z. Inflammation. In: Chen K, Liang L, Li M, Pan Y, editors. Textbook of pathologic anatomy. Singapore: Springer Nature Singapore; 2024. p. 75–105. [cited 2024 Dec 5]. https://link.springer.com/10.1007/978-981-99-8445-9_4.10.1007/978-981-99-8445-9_4Search in Google Scholar
[22] Aljerf L, Williams M, Ajong AB, Onydinma UP, Dehmchi F, Pham VT, et al. Comparative study of the biochemical response behavior of some highly toxic minerals on selenosis in rats. Rev Chim. 2021 May;72(2):9–18.10.37358/RC.21.2.8415Search in Google Scholar
[23] Bai R, Guo J, Ye XY, Xie Y, Xie T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev. 2022 May;77:101619.10.1016/j.arr.2022.101619Search in Google Scholar PubMed
[24] Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020 Aug;38(2):167–97.10.1016/j.ccell.2020.06.001Search in Google Scholar PubMed PubMed Central
[25] Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch Toxicol. 2023 Oct;97(10):2499–574.10.1007/s00204-023-03562-9Search in Google Scholar PubMed PubMed Central
[26] Rana A, Samtiya M, Dhewa T, Mishra V, Aluko RE. Health benefits of polyphenols: A concise review. J Food Biochem. 2022 Oct;46(10):e14264.10.1111/jfbc.14264Search in Google Scholar PubMed
[27] Pritsas A, Tomou EM, Tsitsigianni E, Papaemmanouil CD, Diamantis DA, Chatzopoulou P, et al. Valorisation of stachysetin from cultivated Stachys iva Griseb. as anti-diabetic agent: A multi-spectroscopic and molecular docking approach. J Biomol Struct Dyn. 2021 Nov;39(17):6452–66.10.1080/07391102.2020.1799864Search in Google Scholar PubMed
[28] Kokkini S, Karousou R, Hanlidou E. HERBS | Herbs of the labiatae. In: Caballero B, editor. Encyclopedia of food sciences and nutrition. 2nd ed. London: Academic Press; 2003. p. 3082–90.10.1016/B0-12-227055-X/00593-9Search in Google Scholar
[29] Aljerf L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J Environ Manag. 2018 Nov;225:120–32.10.1016/j.jenvman.2018.07.048Search in Google Scholar PubMed
[30] Chatzimitakos T, Athanasiadis V, Makrygiannis I, Kalompatsios D, Bozinou E, Lalas SI. An investigation into Crithmum maritimum L. leaves as a source of antioxidant polyphenols. Compounds. 2023 Dec;3(4):532–51.10.3390/compounds3040038Search in Google Scholar
[31] Shehata E, Grigorakis S, Loupassaki S, Makris DP. Extraction optimisation using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Sep Purif Technol. 2015 Jul;149:462–9.10.1016/j.seppur.2015.06.017Search in Google Scholar
[32] Chatzimitakos T, Athanasiadis V, Kotsou K, Bozinou E, Lalas SI. Response surface optimization for the enhancement of the extraction of bioactive compounds from Citrus limon peel. Antioxidants. 2023 Aug;12(8):1605.10.3390/antiox12081605Search in Google Scholar PubMed PubMed Central
[33] Kasouni AI, Chatzimitakos TG, Stalikas CD, Trangas T, Papoudou-Bai A, Troganis AN. The unexplored wound healing activity of Urtica dioica L. extract: An in vitro and in vivo study. Molecules. 2021 Oct;26(20):6248.10.3390/molecules26206248Search in Google Scholar PubMed PubMed Central
[34] Dirar AI, Alsaadi DHM, Wada M, Mohamed MA, Watanabe T, Devkota HP. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. South Afr J Botany. 2019 Jan;120:261–7.10.1016/j.sajb.2018.07.003Search in Google Scholar
[35] Thong-on W, Pathomwichaiwat T, Boonsith S, Koo-amornpattana W, Prathanturarug S. Green extraction optimization of triterpenoid glycoside-enriched extract from Centella asiatica (L.) Urban using response surface methodology (RSM). Sci Rep. 2021 Nov;11(1):22026.10.1038/s41598-021-01602-xSearch in Google Scholar PubMed PubMed Central
[36] Osorio-Tobón JF. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J Food Sci Technol. 2020 Dec;57(12):4299–315.10.1007/s13197-020-04433-2Search in Google Scholar PubMed PubMed Central
[37] Luo C, Zou L, Sun H, Peng J, Gao C, Bao L, et al. A review of the anti-inflammatory effects of rosmarinic acid on inflammatory diseases. Front Pharmacol. 2020 Feb;11:153.10.3389/fphar.2020.00153Search in Google Scholar PubMed PubMed Central
[38] Guan H, Luo W, Bao B, Cao Y, Cheng F, Yu S, et al. A comprehensive review of rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules. 2022 May;27(10):3292.10.3390/molecules27103292Search in Google Scholar PubMed PubMed Central
[39] Dahchour A. Anxiolytic and antidepressive potentials of rosmarinic acid: A review with a focus on antioxidant and anti-inflammatory effects. Pharmacol Res. 2022 Oct;184:106421.10.1016/j.phrs.2022.106421Search in Google Scholar PubMed
[40] Kernou ON, Azzouz Z, Madani K, Rijo P. Application of rosmarinic acid with its derivatives in the treatment of microbial pathogens. Molecules. 2023 May;28(10):4243.10.3390/molecules28104243Search in Google Scholar PubMed PubMed Central
[41] Miao M, Xiang L. Pharmacological action and potential targets of chlorogenic acid. In Advances in pharmacology. London: Academic Press; 2020. p. 71–88.10.1016/bs.apha.2019.12.002Search in Google Scholar PubMed
[42] Nguyen V, Taine EG, Meng D, Cui T, Tan W. Chlorogenic acid: A systematic review on the biological functions, mechanistic actions, and therapeutic potentials. Nutrients. 2024 Mar;16(7):924.10.3390/nu16070924Search in Google Scholar PubMed PubMed Central
[43] Huang J, Xie M, He L, Song X, Cao T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front Pharmacol. 2023 Sep;14:1218015.10.3389/fphar.2023.1218015Search in Google Scholar PubMed PubMed Central
[44] Park SY, Jin ML, Yi EH, Kim Y, Park G. Neochlorogenic acid inhibits against LPS-activated inflammatory responses through up-regulation of Nrf2/HO-1 and involving AMPK pathway. Environ Toxicol Pharmacology. 2018 Sep;62:1–10.10.1016/j.etap.2018.06.001Search in Google Scholar PubMed
[45] Gao XH, Zhang SD, Wang LT, Yu L, Zhao XL, Ni HY, et al. Anti-inflammatory effects of neochlorogenic acid extract from mulberry leaf (Morus alba L.) against LPS-stimulated inflammatory response through mediating the AMPK/Nrf2 signaling pathway in A549 cells. Molecules. 2020 Mar;25(6):1385.10.3390/molecules25061385Search in Google Scholar PubMed PubMed Central
[46] Zhao Y, Wang S, Pan J, Ma K. Verbascoside: A neuroprotective phenylethanoid glycosides with anti-depressive properties. Phytomedicine. 2023 Nov;120:155027.10.1016/j.phymed.2023.155027Search in Google Scholar PubMed
[47] Pongkitwitoon B, Putalun W, Triwitayakorn K, Kitisripanya T, Kanchanapoom T, Boonsnongcheep P. Anti-inflammatory activity of verbascoside- and isoverbascoside-rich Lamiales medicinal plants. Heliyon. 2024 Jan;10(1):e23644.10.1016/j.heliyon.2023.e23644Search in Google Scholar PubMed PubMed Central
[48] Lei P, Lü J, Yao T, Zhang P, Chai X, Wang Y, et al. Verbascoside exerts an anti-atherosclerotic effect by regulating liver glycerophospholipid metabolism. Food Sci Hum Wellness. 2023 Nov;12(6):2314–23.10.1016/j.fshw.2023.03.035Search in Google Scholar
[49] Kumar S, Swamy N, Tuli HS, Rani S, Garg A, Mishra D, et al. Myricetin: A potential plant-derived anticancer bioactive compound – an updated overview. Naunyn-Schmiedeberg’s Arch Pharmacol. 2023 Oct;396(10):2179–96.10.1007/s00210-023-02479-5Search in Google Scholar PubMed
[50] Agraharam G, Girigoswami A, Girigoswami K. Myricetin: A multifunctional flavonol in biomedicine. Curr Pharmacol Rep. 2022 Feb;8(1):48–61.10.1007/s40495-021-00269-2Search in Google Scholar PubMed PubMed Central
[51] Imani A, Maleki N, Bohlouli S, Kouhsoltani M, Sharifi S, Maleki Dizaj S. Molecular mechanisms of anticancer effect of rutin. Phytother Res. 2021 May;35(5):2500–13.10.1002/ptr.6977Search in Google Scholar PubMed
[52] Farha AK, Gan RY, Li HB, Wu DT, Atanasov AG, Gul K, et al. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit Rev Food Sci Nutr. 2022 Jan;62(3):832–59.10.1080/10408398.2020.1829541Search in Google Scholar PubMed
[53] Choi SS, Park HR, Lee KA. A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants. 2021 Oct;10(11):1696.10.3390/antiox10111696Search in Google Scholar PubMed PubMed Central
[54] Taheri Y, Suleria HAR, Martins N, Sytar O, Beyatli A, Yeskaliyeva B, et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement Med Ther. 2020 Dec;20(1):241.10.1186/s12906-020-03033-zSearch in Google Scholar PubMed PubMed Central
[55] Imran M, Saeed F, Hussain G, Imran A, Mehmood Z, Gondal TA, et al. Myricetin: A comprehensive review on its biological potentials. Food Sci & Nutr. 2021 Oct;9(10):5854–68.10.1002/fsn3.2513Search in Google Scholar PubMed PubMed Central
[56] Mitra S, Lami MS, Uddin TM, Das R, Islam F, Anjum J, et al. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomed Pharmacother. 2022 Jun;150:112932.10.1016/j.biopha.2022.112932Search in Google Scholar PubMed
[57] Pandey P, Khan F, Ramniwas S, Saeed M, Ahmad I. A mechanistic review of the pharmacological potential of narirutin: A dietary flavonoid. Naunyn-Schmiedeberg’s Arch Pharmacol. 2024 Aug;397(8):5449–61.10.1007/s00210-024-03022-wSearch in Google Scholar PubMed
[58] Ri MH, Li MY, Xing Y, Zuo HX, Li G, Li C, et al. Narirutin exerts anti‐inflammatory activity by inhibiting NLRP3 inflammasome activation in macrophages. Phytotherapy Res. 2023 Apr;37(4):1293–308.10.1002/ptr.7686Search in Google Scholar PubMed
[59] Qurtam AA, Mechchate H, Es-safi I, Al-zharani M, Nasr FA, Noman OM, et al. Citrus flavanone narirutin, in vitro and in silico mechanistic antidiabetic potential. Pharmaceutics. 2021 Oct;13(11):1818.10.3390/pharmaceutics13111818Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

