Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
-
Sunitha Kumari Krishnan Kutty
, Padma Devi Skandasamy Natchimuthu
, Lai-Hock Tey
Abstract
The excessive use of phosphorus (P) fertilizers increases crop production but can lead to P-induced zinc (Zn) deficiencies, making both nutrients unavailable to plants. Plant–microbe interactions, such as with Pseudomonas aeruginosa, can alleviate these constraints by solubilizing Zn and P in soil. A soil incubation study revealed that applying P. aeruginosa with farmyard manure (FYM) significantly increased Zn and P solubilization (6.86 mg/l; 14.83 mg/l) compared to control (3.15 mg/l; 13.67 mg/l). A field experiment evaluated the effects of P. aeruginosa on the biochemical composition of groundnut plants under five treatments. The T2, T3, and T4 treatments had the highest protein, carbohydrate, and chlorophyll levels, likely due to the heterogeneous activity of FYM and the mineral solubilizing ability of P. aeruginosa. Groundnut seeds from T3 (combined liquid inoculant and FYM) had the highest iodine (88.47 mg KOH/g), saponification value (195.56 mg KOH/g), and free fatty acid content (2.23 g oleic acid). The pH of the T3 soil decreased from 8.3 to 7.5, and significant increases were observed in electrical conductivity (from 2.88 to 0.30 dS/m), calcium carbonate (2.53–1.7%), organic carbon (0.39–1.91%), nitrogen (273.75–788.25 kg/ha), P (20.1–59.65 kg/ha), potassium (182.25–346.5 kg/ha), and Zn (1.53–7.24 mg/kg). The study suggests that the combined application of liquid formulants of P. aeruginosa with FYM is advantageous, as FYM supports microbial growth by providing essential nutrients for mineralization. Moreover, liquid inoculants formulated with polyvinylpyrrolidone as an osmo protectant demonstrated enhanced shelf-life and mineral solubilization, contributing to improved biochemical properties in groundnut plants.
1 Introduction
The presence of fourteen mineral elements in the soil is essential for plant growth as they regulate plant metabolic processes and produce crops with excellent quality and yield. They can be categorized into macronutrients like nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) and micronutrients like chlorine (Cl), boron (B), iron (Fe), manganese (Mn), copper (Cu), zinc (Zn), nickel (Ni), and molybdenum (Mo) [1]. Farmers focus more on macronutrients since they are critical for plant development and growth, ranging from structural elements to redox-sensing agents [2]. Despite being crucial for crop quality and productivity, providing inherent protection against disease and adverse conditions, micronutrients have received less attention. They also act as co-factors for several enzymes involved in metabolizing various organic compounds, including proteins, lipids, carbohydrates, and nucleic acids [3,4,5]. Micronutrient deficiencies in the soil are a universal phenomenon these days due to the crop’s inability to absorb micronutrients, as well as positive or negative interactions with macronutrients accumulating in the soil as a result of excessive application of chemical fertilizers by the agrarian communities [6,7,8,9]. It is estimated that 49, 31, 15, 14, 10, and 3% of agricultural soils worldwide are deficient in Zn, B, Mo, Cu, Mn, and Fe, respectively [10,11]. Of the micronutrients, only Zn is directly linked in the food chain such that deficiency is extensive in humans, food crops, and soil. The Zn deficiency is therefore the highest priority among micronutrients for agriculture to address [12].
Plants require Zn as a micronutrient because it is vital to many of their bio-physicochemical processes, including protein synthesis, gene regulation and activation, carbohydrate metabolisms, morphological and anatomical participation in bio-membranes, and numerous other critical cellular processes like ion homeostasis, metabolic and physiological processes, and enzyme activation [13,14,15]. Zn must therefore be properly absorbed, transported, and distributed throughout plant tissues, cells, and intracellular spaces to sustain optimal growth and development [16]. Stunted growth and development, chlorosis, necrosis, membrane breakdown, poor metabolism, nutritional imbalance, and increased vulnerability to biotic and abiotic stresses are the symptoms of Zn deficiency in plants [17]. Some of the factors that affect the quantity of Zn available to plants are soil type, soil pH, and the presence of other minerals that compete with Zn absorption, one such interaction is between Zn and P [18]. The Zn-induced P deficiencies are uncommon because farmers usually use more P fertilizer than Zn, which could prevent Zn from being absorbed [19]. According to Sharma et al. [20], precipitation on Fe, Al, and Ca complexes causes 75–90% of the applied P fertilizer to accumulate in the soil. The formation of H+ ions by phosphate salts causes the soil’s phosphate content to rise, which in turn prevents Zn from being absorbed by plants due to their co-precipitation as Zn phosphate (Zn3(PO4)2) in both alkaline soils (submerged conditions) and acidic soils. This causes Zn an inaccessible soil element to be absorbed by plants [21,22].
The Zn and P are essential for the metabolism of carbohydrates in plants, to catalyze the enzymes such as carbonic anhydrase, fructose-1,6-bisphosphate, and aldolase. The rapid drop-in activity of these three enzymes slows the metabolism of carbohydrates in plants [21,22,23,24,25]. Nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate (NADP), the crucial nutrients involved in plant glucose metabolism, are made up exclusively of P [26]. The ribosome, a location for protein synthesis, disintegrates without Zn because it is part of its structural makeup. Protein synthesis and protein levels are severely reduced in Zn-deficient plants, which results in the accumulation of free amino acids, as Zn is essential for synthesizing numerous groups of amino acids into enzymes and proteins [25,27,28,29]. Consequently, a deficiency in Zn prevents the plant from producing specific enzymes and proteins. P, which is a crucial component of adenosine triphosphate (ATP) and aids in the activation of amino acids for protein synthesis, operates similarly [26].
The availability of mineral nutrients to plants also affects the production of chlorophyll. The dynamics of leaf surface development and area are greatly influenced by mineral nutrition, which is reflected in leaf surface area, photosynthetic capability, and net photosynthetic productivity. Among the minerals necessary for photosynthesis, Zn and P are essential for the production of chlorophyll in plants. Zn is a key enzyme co-factor necessary for the regular functioning of the photosynthetic apparatus and plays a significant role in the control of chlorophyll production in plants. In addition, P contributes to the stability of the chlorophyll molecule as a crucial component [30].
For millions of people around the world, groundnuts (Arachis hypogaea L.) are a significant source of cooking oil. As integrated food components [31] and oleo chemicals [32], they give food products a distinct flavor and texture. In seeds, Zn plays a significant role in the production of proteins and lipids [33]. Groundnut-growing soils in India have significant yield loss owing to a 50% Zn shortfall [34,35]. Zn deficiency in groundnuts results in interveinal yellow-vein chlorosis and uneven mottle on upper leaves. The entire leaflet turned chlorotic in conditions of severe deficit [36].
The Zn fertilization boosts nodulation, chlorophyll content, and pod yield in soils with low levels of Zn. According to ILZRO [37], a deficit impaired pegging but had no overt leaf symptoms. Numerous enzymes are activated by Zn [38,39], and this can directly affect developmental processes that result in noticeable variations in seed oil quality. The P is also necessary for the generation of high-quality seeds because it is a coenzyme in reactions that transfer energy; in photosynthesis, energy is used in the form of ATP and NADP. Following that, this energy is utilized in the photosynthetic fixation of CO2 and the production of lipids and other vital organic molecules [26].
Thus, employing Zn and P becomes crucial for plant development as they are necessary for controlling the biochemical activities in plants. Even though they accumulate in the soil, they are often found in insoluble forms as a result of their antagonistic interactions. The relationship between the plant and microbes is self-sustaining; the former uses the latter to extract soluble nutrients from the soil, which increases soil fertility. In the rhizosphere, microorganisms that can convert a variety of unavailable metal forms into available ones can be isolated and used to supply plants with Zn and P in their stable forms.
Pseudomonas aeruginosa is a rhizobacterium that stimulates plant development by secreting phytohormones, siderophores, hydrogen cyanide, and lytic enzymes, solubilizing P, K, and Zn, and so gaining great attention in the field of Biofertilization [40]. Therefore, in the current investigation, the impact of P. aeruginosa’s mineral solubilizing activity on the biochemical content of the groundnut plants cultivated under various treatments at regular 30-day intervals was examined.
2 Materials and methods
Laboratory assessment of the Zn-solubilizing ability of P. aeruginosa was carried out qualitatively and quantitatively under different cultural conditions [41,42]. Field experiments were also conducted to test its influence on the growth and yield of A. hypogaea [43].
The primary goal of the present investigation was to evaluate the impact of P. aeruginosa’s mineral (Zn and P)-solubilizing activity on the biochemical characterization of A. hypogaea L. A field study was performed on an agricultural farm at Kangeyam, Tirupur District, Tamil Nadu. The P. aeruginosa was formulated in a variety of ways, including solid forms employing lignite at a rate of 100 ml (109 CFU/ml) per 200 g and liquid forms using 3% poly vinyl pyrrolidone (PVP), which has a cell load of 109 CFU/ml. Before sowing, 100 seeds were treated with 5 g of solid-based inoculant with a cell load of 109 CFU/ml and also 5 ml of liquid inoculum with a cell load of 109 CFU/ml per 100 seeds and incubated for 6 h at room temperature.
The experimental field was set up using a randomized complete block design with five treatments (T1: control [uninoculated seeds]; T2: seeds treated with liquid inoculant; T3: seeds treated with liquid inoculant and the soil amended with farmyard manure [FYM]; T4: soil amended with FYM alone; T5: seeds treated with lignite [solid] based bioinoculant) replicated four times on a 3 m by 3 m plot size. At regular intervals of 30 days, P. aeruginosa’s effects on the biochemical components of the randomly chosen plants were investigated.
2.1 The efficiency of P. aeruginosa in solubilizing minerals (Zn and P) present in the soil reserve (soil incubation study) [44]
The P. aeruginosa’s effects on the release of Zn and P from the soil reserve were investigated through a soil incubation study using field soil that was taken from an agricultural farm in Kangeyam, Tirupur District, Tamil Nadu. A 1 kg of field soil was taken in a series of pots. FYM was applied to one set of pots at a rate of 5%, while another set was left untreated. The test bacterial culture was centrifuged, and the supernatant solution was discarded after the bacteria were cultured in nutrient broth for 48 h. The bacterial pellets were rinsed with sterile distilled water, and 10 ml (109 CFU/ml) of cell suspension per 1 kg of soil was used to inoculate the pots. Additionally, an uninoculated control was also maintained. Throughout the incubation phase, the soil’s moisture content was maintained. Minerals were extracted and their Zn and P availabilities were evaluated after 10 days of incubation.
2.1.1 Estimation of soil’s available Zn [45]
Diethylene triamine penta acetic acid (DTPA) solution (0.005 M) in a 1:2 ratio was poured into 20 ml of a 100 ml Erlenmeyer flask containing 10 g of soil, and the mixture was then agitated for 2 h. The extract was obtained after the contents were filtered via the Whatman No. 42 filter paper. The amount of available Zn in the soil was measured using an atomic absorption spectrometer.
2.1.1.1 Preparation of DTPA extraction solution
To make the DTPA extraction solution, 200 ml of distilled water was used to dissolve 149.2 g of 0.1 M triethanolamine, 19.67 g of 0.005 M DTPA, and 14.7 g of 1 M CaCl2·2H2O. This solution was then concentrated up to 10 l. 1 N HCl was used to bring the pH down to 7.3 ± 0.05.
2.1.2 Estimation of available P in soil
The available P in soil was estimated by Olsen’s method [46].
2.1.2.1 Reagents preparation
Sodium bicarbonate (0.5 M),
Activated carbon, and
5 N sulfuric acid.
Conc. H2SO4, 137 ml was added to 1 l of distilled water
2.1.2.2 Reagent A
Ammonium molybdate (12 g) was dissolved in distilled water (250 ml).
Antimony potassium tartrate (0.291 g) was dissolved in distilled water (100 ml).
100 ml of 5 N H2SO4 was prepared by dissolving 137 ml of conc. H2SO4 in 1 l of distilled water.
The three reagents were mixed as described above and the volume was increased up to 2 l with distilled water.
2.1.2.3 Reagent B
Ascorbic acid (1.056 g) was dissolved in reagent A (200 ml).
2.1.2.4 Procedure
One teaspoon of activated carbon and 50 ml of 0.5 M sodium bicarbonate was added to a 100 ml Erlenmeyer flask containing 5 g of soil. The mixture was filtered through Whatman No. 40 filter paper after being agitated for 30 min in an orbital shaker. To get a clear filtrate, additional activated carbon may have to be added. Using 5 N H2SO4, 5 ml of filtrate was pipetted into a 25 ml volumetric flask and acidified to pH 5.0. After the mixture was diluted to 20 ml, 4 ml of freshly prepared reagent B was added, and the volume was then increased to 25 ml with distilled water. The flask was then thoroughly shaken and let to stand for 10 min. In a Vis spectrophotometer, the resulting blue color’s absorbance was detected at 660 nm. Distilled water was used as blank. The available P is represented in mg/l, and the unknowns were determined using the standard graph.
2.2 Biochemical characterization of the groundnut plants
The biochemical contents of the fresh leaves and seeds of groundnut plants such as total carbohydrate, soluble protein, total chlorophyll content of the leaves, oil percentage, iodine value, saponification value, and free fatty acids content of the oil extracted from the groundnut seeds were assessed at regular 30-day intervals in triplicates.
2.2.1 Total carbohydrate
The total carbohydrate content of groundnut plant leaves and seeds was evaluated by the Hedge and Hofreiter method [47]. A 1 g of the fresh sample (leaves as well as seeds of groundnut plants) was hydrolyzed individually in a water bath for 3 h with 2.5 N HCl (5 ml), and after cooling to room temperature, it was neutralized with Na2CO3. 4 ml of Anthrone was added to 0.1 ml of individual extracts in test tubes after they had been prepared to a volume of 1 ml with distilled water. Color intensity at 630 nm was measured after the tubes had been kept boiling for 5 min. The glucose standard graph was used to compute the total carbohydrate concentration of the samples.
2.2.2 Soluble protein
By using Bradford’s method [48], the total soluble protein in fresh leaves and seeds was determined. The samples were weighed at 0.1 g, homogenized in 2 ml of 0.1 M phosphate buffer pH 6.8 in a mortar and pestle that had been pre-chilled while homogenization was taking place, maintained on ice, then centrifuged at 5,000 rpm for 10 min at 4°C. Equal parts of 20% trichloroacetic acid that had already been cooled were added to the supernatant before being centrifuged once more at 5,000 rpm. The particle was rinsed with acetone and then dissolved in 1 ml of 0.1 N NaOH after the supernatant was discarded. For optimal color development, tubes were covered with aluminum foil and kept in the dark, and 5 ml of Bradford reagent was added to a 1 ml aliquot. The amount of soluble protein was estimated and represented as mg/g using the standard bovine serum albumin (Sigma-Aldrich) as a reference.
2.2.3 Chlorophyll content of the leaves [49]
2.2.3.1 Extraction of chlorophyll
A 20 ml of 80% acetone was used to grind one gram of fresh leaves. It was then centrifuged for 5 min at 5,000 rpm. The process was repeated until the residue was colorless after the supernatant was transferred. At 645 and 663 nm, the solution’s absorbance was read in comparison to a solvent (acetone) blank.
2.2.3.2 Estimation of chlorophyll content
The following equation was used to determine the levels of chlorophyll a (1), chlorophyll b (2), and total chlorophyll (3):
2.2.4 Extraction of groundnut oil
Groundnut seeds were powdered using a laboratory Ball Mill. Using petroleum ether (60°C) for 6 h, oil was extracted using the Soxhlet technique [50].
2.2.4.1 Determination of percentage yield
The percentage (%) yield was calculated using [51]
2.2.4.2 Iodine value
According to the AOAC method [51], the iodine value of oils was determined. A conical flask containing 0.25 g of oil was filled with a 10 ml solution of CCl4. Similarly, 30 ml of the Hanus solution was added, and the mixture was left to stand for 45 min while being occasionally shaken. Any free iodine on the stopper was rinsed down with 10 ml of 10% KI solution and 100 ml of distilled water. The iodine was titrated using a previously standardized Na2S2O3 solution, which was added gradually while being constantly shaken until the yellow solution became nearly colorless. The addition of a few drops of the starch indicator was followed by a series of titrations until the blue color completely vanished. Iodine that was still present in the CCl4 solution was vigorously shaken out of the bottle so that it could be absorbed by the KI solution. The amount of Na2S2O3 solution needed for the experiment was recorded. Along with the sample, a control experiment was conducted.
The following calculation was used to determine the percent weight of iodine that the oil sample had absorbed:
1 ml 1 N Na2S2O3 = 0.127 g; B = ml of 0.1 N Na2S2O3 required by blank; A = ml of 0.1 N Na2S2O3 required by oil sample; N = normality of Na2S2O3; and W = weight of oil in g.
2.2.4.3 Saponification value
To calculate the saponification value, 1.0 g of oil was placed in a conical flask together with 25 ml of 0.5 N alcoholic KOH, which was then heated under a reserved condenser for 30–40 min to ensure that the sample was completely dissolved. Phenolphthalein was added and titrated with 0.2 N HCl after chilling the sample until a pink endpoint was obtained [52].
The saponification value was calculated by the following formula:
S = saponification value; B = ml of HCl required by blank; T = ml of HCl required by oil sample; N = normality of HCl; W = weight of oil in g; and 56.1 = equivalent weight of KOH.
2.2.4.4 Free fatty acids content of the oil
Around 2 ml of 1% phenolphthalein solution was added to 20 ml of ethanol–diethyl ether (1:1 v/v) mixture, and the mixture was neutralized with 0.10 M NaOH solution. The neutralized mixture was then combined with 5 g of each oil sample, and the combination was titrated against a 0.10 M NaOH solution while being constantly shaken until a pink color appeared and lasted for 15 min. The free fatty acid value was calculated using the titer values [53].
2.3 Analysis of the characteristics of soil samples subjected to different treatments
The different parameters of the soil samples subjected to different treatments such as pH, electrical conductivity (EC), CaCO3, organic carbon, N, P, K, and Zn were analyzed before and after the cultivation of the groundnut
2.3.1 pH
In a conical flask, 10 g of soil and 100 ml of distilled water were combined, and the mixture was shaken vigorously for 30 min. It was filtered through muslin cloth after 30 min, and the filtrate was used to calculate the pH using an Elico pH meter.
2.3.2 EC
The soil water ratio of 1:10 was used to determine the EC of the soil samples, and the results are expressed in dS/m, according to the method of Jackson [54].
2.3.3 CaCO3
The soil samples were crushed and sieved through a 2 mm sieve after being air-dried. As stated by Dreleimanis [55], the equivalent amount of calcium carbonate (E-CaCO3) was measured using 0.5 M HCl for dissolving CaCO3 and determined between the two titrations of the surplus acid by using 0.2 M NaOH.
2.3.4 Organic carbon
The titration method, recommended by Walkley and Black [56], was used to determine the amount of organic carbon in the soil samples.
2.3.5 N content
The Kjeldahl method was used to determine the soil sample’s total N content, and the calculation was made per Vogel’s [57] recommendations.
2.3.6 K content
By using the titration method recommended by Jackson [54], the K content of the soil sample was calculated.
2.3.7 Zn
By using the method outlined in Section 2.1.1, the available Zn present in the soil samples was determined.
2.3.8 P
By using the method outlined in Section 2.1.2, the available P present in the soil samples was determined.
2.4 Statistical analysis
A randomized complete block design with four replicates of each treatment was used to set up plots with various treatments in field trials. Analysis of variance was used to analyze the data, and Duncan’s multiple range test was used to compare means at p ≤ 0.05. The SPSS software (2019 version) was used for all analyses.
3 Results and discussion
3.1 Efficiency of P. Aeruginosa in solubilizing minerals (Zn and P) present in the soil reserve (soil incubation study)
This investigation was carried out to ascertain the impact of P. aeruginosa inoculation to solubilize Zn and P present in the soil. The results showed no significant difference in the mineral solubilizing ability of bacteria that is applied alone versus applied along with FYM. But the efficacy of the organism to solubilize Zn was little enhanced when it was applied along with FYM (6.86 mg/l) compared to mineral solubilizing bacteria alone (6.02 mg/l) and control (3.15 mg/l) (Table 1). By providing a nutrient-rich environment that supports the growth and activity of this beneficial microbe (P. aeruginosa), and by enhancing their effectiveness in producing organic acid, which may have increased Zn availability in the soil, FYM application has the potential to increase available Zn concentrations beyond the deficiency threshold limits. Several forms of Zn, including ZnO, ZnCO3, and ZnS, that are present in the soil can be dissolved by the Zn-solubilizing bacterial strains producing organic acids mainly 2-ketogluconic acid and gluconic acid [58,59]. Furthermore, FYM has a significant role in regulating temperature, retaining moisture, increasing soil organic carbon (SOC) availability, and improving nutrient availability and soil pH [60,61,62]. These actions and conditions generate an environment that is conducive to microbial growth and activity [63,64]. Since certain Zn-solubilizing microorganism (ZSM) species are copiotrophic which favor habitats that are high in carbon and nutrients, the addition of FYM may increase the availability of carbon and nutrients in the soil, which will in turn encourage the activity of copiotrophic ZSM species in soil [65,66]. Applying FYM and Zn-solubilizing bacteria raised the soil’s DTPA-Zn content, as reported by Senthil Kumar et al. [67]. ZSMs are therefore viable substitutes for increasing Zn bioavailability and supplementing plants in an economical, environmentally responsible, and sustainable way [68,69].
Release of minerals in the soil inoculated with P. aeruginosa
| Treatments | Amount of minerals (mg/l) | |
|---|---|---|
| Zn | P | |
| Control | 3.15 ± 0.50b | 13.67 ± 0.58b |
| MSB | 6.02 ± 0.18ab | 14.33 ± 0.29ab |
| MSB + FYM | 6.86 ± 0.03a | 14.83 ± 0.29a |
| SD CD (p < 0.05) | 0.2511 | 0.3333 |
| 0.6145 | 0.8157 | |
Values are mean ± SD of three samples; MSB: mineral-solubilizing bacteria; FYM: farmyard manure.
Regarding the amount of P that was readily available, inoculating P. aeruginosa with FYM led to the highest concentration (14.83 mg/l), surpassing both the control (13.67 mg/l) and the organism alone (14.33 mg/l) (Table 1). By encouraging microbial growth in soils, FYM enables organic matter to be mineralized by microorganisms, which releases nutrients into the crop gradually [44]. Primary P minerals, such as apatite, and secondary clay minerals, such as calcium, iron, and aluminum phosphates, are sources of P in soil and play a crucial role in preserving P availability and building via desorption and dissolution processes [70]. Applying FYM and biofertilizer causes the manure to break down and release macro and micronutrients, including P. It also dissolves the type of P that is not available by releasing organic acids throughout the breakdown process [70]. Because of the mineralization, solubilization, and translocation action of P-solubilizing bacteria through the generation of organic acid and proton extrusion, the use of biofertilizers has also increased the availability of P to plants [71,72]. Nahas [73] attributed the increase in solubilization of insoluble phosphate due to organic acids produced by microbes to the drop in soil pH, soil cations chelating, and competition with phosphate for adsorption sites in the soil solution. Kaur and Reddy [74] and Shahzad et al. [75] also observed improved soil P status with inoculation with phosphate-solubilizing bacteria resulting from higher alkaline phosphatase activity. Thus, the addition of FYM not only adds up to nutrient pools but also creates a favorable root zone environment for better root activity and nutrient uptake.
3.2 Effect of different treatments on the total carbohydrate and soluble protein contents of the leaves and seeds of groundnut plants
Mineral-solubilizing bacteria positively influence the biochemical content of plants by enhancing Zn and P uptake, which in turn improves chlorophyll synthesis, protein content, carbohydrate metabolism, antioxidant activity, phytohormone levels, nutrient use efficiency, and secondary metabolite production. This results in healthier plants with better growth, higher stress resistance, and improved overall productivity [76]. Therefore, the ability of the mineral-solubilizing bacterium P. aeruginosa to raise the carbohydrate and soluble protein content of the leaves of groundnut plants grown under diverse circumstances was evaluated at regular intervals of 30 days. The findings showed that the soluble protein and carbohydrate content of the leaves of T2, T3, and T4 plants recorded maximum values in comparison to the control (Table 2). On the 120th day of harvest, estimates of the groundnut plants’ seeds’ carbohydrate and soluble protein contents were also made. Additionally, the results revealed that T2, T3, and T4 plants’ groundnut seeds had the highest value when compared to the control (Table 3). This could be because of the common factor of all the three treatments “P. aeruginosa and FYM” provided vital nutrients for controlling biochemical activity in plants along with as nearly all of the enzymes and coenzymes involved in biochemical activity are activated by Zn [23] and P [77,78], respectively, through its mineral solubilizing ability. Studies have shown that MSB can increase the carbohydrate content in plants. For instance, a study on wheat, rice, and maize found that MSB increased the carbohydrate content in these crops [79,80]. This is likely since Zn and P play a crucial role in carbohydrate metabolism, and increased Zn and P availability can enhance photosynthesis and carbohydrate production. MSB has also been found to increase the protein content in plants. A study on groundnut plants found that zinc solubilizing bacteria (ZSB) increased the protein content in the plants, likely due to the increased availability of Zn, which is essential for protein synthesis [43,81]. Additionally, Zn-mobilizing plant growth-promoting rhizobacteria (PGPR) have been found to increase protein content in rice [82]. Additionally, threonine, an amino acid, has been shown to promote bacterial solubilization and plant uptake of nutrients, including P, which can lead to increased protein production [83]. According to Yousefi and Sadeghi [84], the application of FYM and vermicompost significantly increased the carbohydrate and protein content of wheat. This is likely due to the improved soil fertility and nutrient availability provided by FYM, which promotes healthy plant growth and development [85]. A similar finding was made by Prathiba and Siddalingeshwara [86], who investigated the impact of plant growth-promoting activity of Bacillus subtilis and Pseudomonas fluorescence as Rhizobacteria on seed quality of sorghum and observed that both strains were effective in enhancing seed quality, including seed germination, vigor index, and nutritional quality, including protein and carbohydrate contents. The favorable impact of P. fluorescens strain Psd on wheat production and nutritional quality was also evaluated by Sirohi et al. [87], who reported that seeds from plants collected from the strain Psd-infected soil had higher carbohydrate and protein contents than seeds from controls. Furthermore, Mathivanan et al. [88] evaluated the impact of PGPR (Rhizobium + Pseudomonas + Bacillus) on the nutritional value of groundnut (A. hypogaea L.) seedlings. Their findings showed that high biochemical content was observed in the groundnut seedlings grown using PGPR compared to uninoculated control. The current study thus shows the ability of P. aeruginosa to promote plant growth with effected the biochemical composition of the plants in conjunction with organic manure, which acted as a nutrient reservoir for its microbial activity.
Effect of different treatments on the total carbohydrate and protein contents of the leaves of groundnut plants
| Treatments | Total carbohydrate content of the plants (mg/g) | Protein Content of the Plants (mg/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| 30th | 60th | 90th | 120th | 30th | 60th | 90th | 120th | |
| T1 | 0.20 ± 0.03c | 0.21 ± 0.02c | 1.64 ± 0.11c | 2.52 ± 0.19b | 2.17 ± 0.11b | 3.44 ± 0.22a | 3.68 ± 0.17c | 3.93 ± 0.28b |
| T2 | 0.32 ± 0.02ab | 0.42 ± 0.04a | 2.24 ± 0.16b | 2.96 ± 0.21ab | 2.25 ± 0.16b | 3.85 ± 0.19a | 3.94 ± 0.60c | 4.09 ± 0.21b |
| T3 | 0.41 ± 0.09a | 0.49 ± 0.05a | 2.68 ± 0.24a | 3.53 ± 0.31a | 3.25 ± 0.34a | 4.05 ± 0.71a | 4.86 ± 0.21a | 5.06 ± 0.30a |
| T4 | 0.32 ± 0.04ab | 0.46 ± 0.07a | 2.34 ± 0.17ab | 3.08 ± 0.74ab | 2.5 ± 0.38b | 3.93 ± 0.64a | 4.05 ± 0.77ab | 4.88 ± 0.29a |
| T5 | 0.3 ± 0.06b | 0.31 ± 0.08b | 1.73 ± 0.36c | 2.56 ± 0.39b | 2.16 ± 0.18b | 3.68 ± 0.37a | 3.81 ± 0.74c | 4.15 ± 0.44b |
| Treatments: 17.393***Days: 568.473*** | Treatments: 14.139***Days: 81.132*** | |||||||
| Treatments × days: 2.652*** | Treatments × days: 0.829*** | |||||||
Values are mean ± SD of four replication samples in each group; *** indicates p < 0.001; ** indicates p < 0.01; and * indicates p < 0.05 versus control.
T1: control; T2: seeds treated with liquid inoculant; T3: seeds treated with liquid inoculant and the soil amended with FYM; T4: soil amended with FYM alone; T5: seeds treated with lignite (solid) based bioinoculant.
Effect of different treatments on the carbohydrate and protein contents of the seeds
| Treatments | Total carbohydrate (mg/g) | Soluble protein (mg/g) |
|---|---|---|
| T1 | 1.68 ± 0.27c | 7.13 ± 0.77c |
| T2 | 2.22 ± 0.30ab | 8.82 ± 0.16b |
| T3 | 2.56 ± 0.45a | 10.1 ± 0.68a |
| T4 | 2.41 ± 0.19ab | 8.88 ± 0.13b |
| T5 | 1.96 ± 0.44bc | 8.08 ± 0.53b |
Values are mean ± SD of four replication samples.
T1: control; T2: seeds treated with liquid inoculant; T3: seeds treated with liquid inoculant and the soil amended with FYM; T4: soil amended with FYM alone; and T5: seeds treated with lignite (solid)-based bioinoculant.
3.3 Effect of different treatments on the total chlorophyll content of the leaves of groundnut plants
The ability of P. aeruginosa to promote plant growth by increasing the chlorophyll content (chlorophyll “a,” chlorophyll “b,” and total chlorophyll) of the leaves of groundnut plants raised under different treatments was assessed in the current experimental study at regular intervals of 30 days. The results demonstrated that, in comparison to the control, the groundnut leaves of T3 plants showed a significant (p < 0.05) increase in the levels of chlorophyll (chlorophyll “a,” chlorophyll “b,” and total chlorophyll) (Figure 1). Plants with low levels of Zn have less efficient photosynthesis [89]. It causes a 50–70% reduction in net photosynthesis in many plant species [90,91]. This decline in net photosynthesis in plants is ascribed to a reduction in Hill reaction activity, which may be brought on by a drop in chlorophyll concentration [92] and a disruption in the structure of the chloroplasts [25] in Zn-deficient plants. Under Zn-deficient conditions, Sharma et al. [92] reported that cauliflower leaves had significantly lower stomatal conductance, which resulted in a drop in CO2 supply, which in turn caused a considerable decrease in photosynthesis. In addition, Zn controls the synthesis of carotenoids and chlorophyll in plants, which is crucial for the efficient operation of the photosynthetic apparatus [93]. The application of MSB along with FYM can have a positive impact on the chlorophyll contents of plants. The chlorophyll content of plants has also been found to be influenced by the application of FYM and bioinoculants. Mean chlorophyll content values were higher with the application of 9.6 ton/ha of FYM [94]. Additionally, the use of bioinoculant has been found to promote the growth of beneficial microorganisms in the soil, which can help to increase the availability of nutrients, including sulfur, which is essential for chlorophyll formation [95]. Zn plays a crucial role in the synthesis of chlorophyll, and its deficiency can lead to reduced chlorophyll content and impaired photosynthesis [43]. Studies have demonstrated that the application of ZSB along with FYM can result in increased chlorophyll content, leading to improved photosynthesis and plant productivity [96,97]. The exact mechanisms behind this interaction are not fully understood, but it is believed that the ZSB’s ability to solubilize Zn and make it available to plants, combined with the nutrient-rich properties of FYM, contributes to the observed effects [79]. Research has shown that ZSB can increase the availability of Zn in the soil, which is an essential micronutrient for plant growth and development [98]. Studies have demonstrated that the application of phosphorus solubilizing bacteria (PSB) along with FYM can lead to a significant increase in chlorophyll content in plants. For instance, a study on okra plants found that the integrated use of PSB and FYM resulted in a 62% increase in chlorophyll content [99]. Similarly, another study on tomato plants showed that the inoculation of PSB strains, either singly or in combination, significantly increased the chlorophyll content of leaves [100]. The increased chlorophyll content in plants can be attributed to the ability of PSB to solubilize P in the soil, making it available to plants. The P is an essential nutrient for plant growth and development, and its availability can limit plant productivity. By solubilizing P, PSB can enhance plant growth and development, leading to increased chlorophyll content [101].
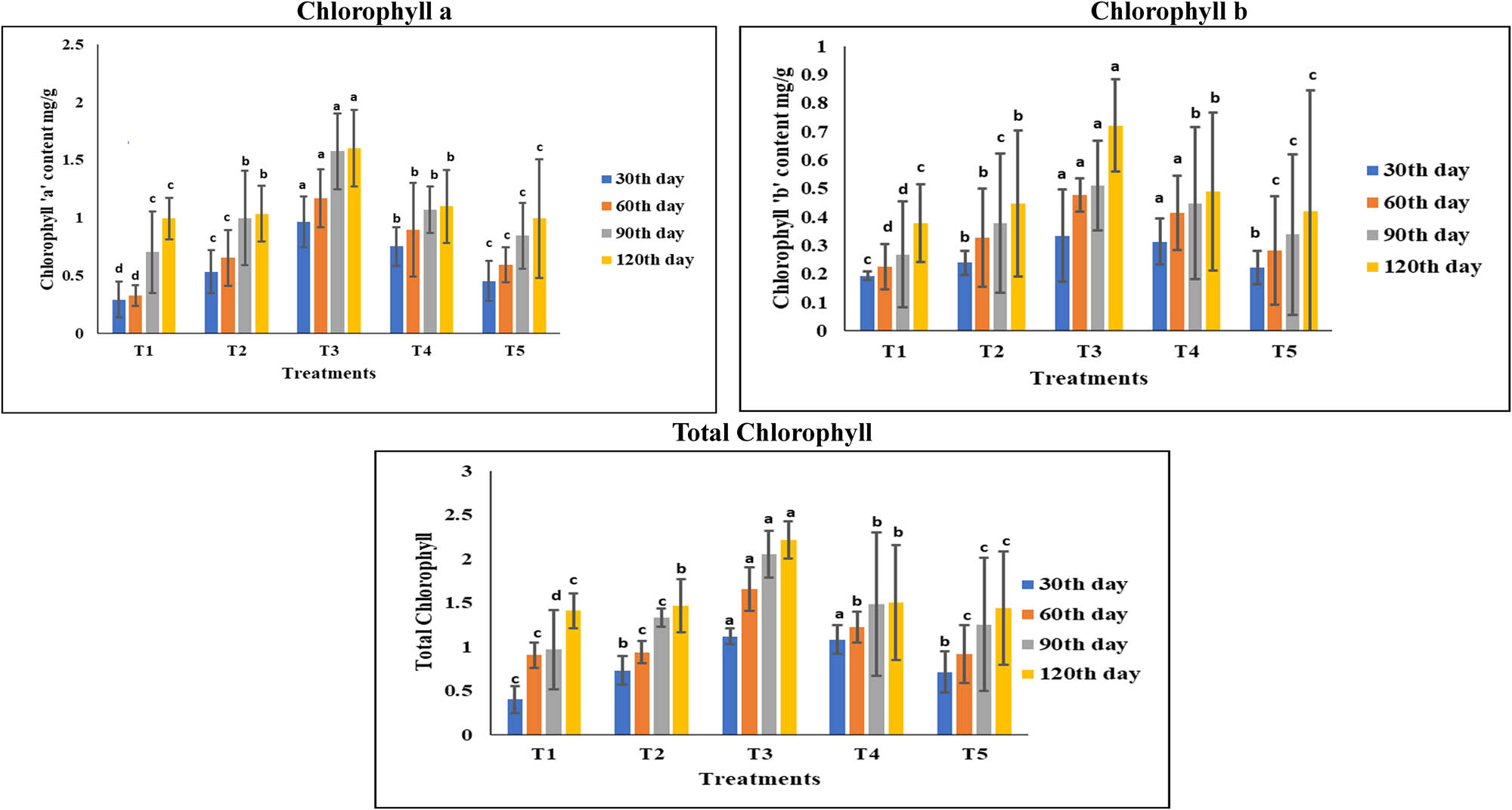
Effect of different treatments on the chlorophyll content of the leaves of groundnut plant. Values are mean ± SD of four replication samples. T1: control; T2: seeds treated with liquid inoculant; T3: seeds treated with liquid inoculant and the soil amended with FYM; T4: soil amended with FYM alone; and T5: seeds treated with lignite (solid)-based bioinoculant.
3.4 Analysis of percentage of oil content and the chemical characteristics of the oil extracted from the groundnut seeds harvested on the 120th day
Groundnut oil is an organic substance made from groundnut seeds that is known to taste and smell like its parent legume. In Table 4, the oil content and chemical properties of the oil extracted from the peanut seeds of the plants through various treatments are shown. It was a light-yellow tint and liquid at ambient temperature (Figure 2). The amount of crude oil in the peanut seeds of five different treatments ranged from 54.13 to 44.03% of the dry weight of the seeds (Table 4). The crude oil content varied significantly (p ≤ 0.05) among the seeds of the five different treatments. This may be attributable to the additive effects of liquid inoculant (P. aeruginosa) and organic manure, which would have enhanced seed oil content.
Effect of different treatments on the oil percentage and the chemical characteristics of the oil extracted from the groundnut seeds
| Treatments | Oil (%) | Iodine (mg KOH/g oil) | Saponification (mg KOH/g oil) | Free fatty acid (mg KOH/g oil) |
|---|---|---|---|---|
| T1 | 44.03 ± 1.34c | 84.88 ± 0.77c | 191.01 ± 0.91d | 3.62 ± 0.51a |
| T2 | 46.05 ± 1.14c | 86.92 ± 0.09b | 194.36 ± 0.99ab | 2.73 ± 0.38bc |
| T3 | 54.13 ± 1.54a | 88.47 ± 0.44a | 195.56 ± 1.05a | 2.23 ± 0.53c |
| T4 | 50.08 ± 1.39b | 87.17 ± 0.51b | 192.85 ± 0.75c | 3.12 ± 0.48ab |
| T5 | 45.1 ± 1.29c | 86.24 ± 0.85b | 193.06 ± 0.77bc | 2.97 ± 0.30ab |
Values are mean ± SD of four replication samples in each group.
Oil from the seeds of the following treatments: T1: control; T2: seeds treated with liquid formulation; T3: seeds treated with liquid formulation and the soil amended with FYM; T4: soil amended with FYM alone; and T5: seeds treated with lignite (solid)-based bioinoculant.

Oil extracted from the seeds of groundnut plants harvested on the 120th day. Oil from the seeds of the following treatments: T1: control; T2: seeds treated with liquid inoculant; T3: seeds treated with liquid inoculant and the soil amended with FYM; T4: soil amended with FYM alone; and T5: seeds treated with lignite (solid)-based bioinoculant.
The degree of oil unsaturation can be gauged by the iodine value. For certain kinds of oil or fat, it remains constant. The iodine value is a valuable metric for analyzing the oxidative rancidity of oils since the higher the unsaturation, the higher the probability of the oils becoming rancid [102]. The findings of the iodine value of oil that was extracted from groundnut seeds under various treatments are shown in Table 4. The control had the least iodine content, at 84.88 mg KOH/g oil. A decrease in iodine value is a sign of lipid oxidation, and this could be because metallic ions were present and accelerated or allowed oxidation after hydroperoxide formed [103–107]. Oil from plant seeds grown using a combination of organic manure and liquid inoculant (T3) had the highest iodine content (88.47 mg KOH/g oil) when compared to organic manure alone (87.17 mg KOH/g oil). The iodine content of the oil extracted from the seeds of T2 and T5 plants yielded identical results.
The total quantity of milligrams of potassium hydroxide that reacts with one gram of sample is the saponification value, which is used to assess the amount of alkali reactive groups in fats and oils [101]. In comparison to the control (191.01 mg KOH/g), the oil from the seeds of T3 plants had a greater saponification value (195.56 mg KOH/g). Similar outcomes were seen in the seeds of the T2 and T5 plants. Higher saponification values indicate a higher percentage of lower fatty acids since they are inversely related to the average molecular weight or chain length of the fatty acids [108]. Due to the oil’s high saponification value, its potential use in the saponification sector may be suggested.
The acid number or acid value refers to the quantity of free fatty acids. It specifies the oil’s functional characteristics, shelf life, nutritional value, and flavors [109]. So, the amount of free fatty acids determines the oil’s quality. The results for the oil’s free fatty acid content are displayed in Table 4. Compared to control plants (3.62 g oleic acid), the oil recovered from the seeds of T3 plants contained the least quantity of free fatty acid (2.23 g). In comparison to other treatments, the oil extracted from the seeds of the T2 and T5 plants produced comparable results. The stability of the products is shown by the low free fatty acid content of the oil [110]. If oil is stored for a long time, its unpleasant flavor and odor are caused by the existence of free fatty acids and other fatty components [111]. Because the sample of oil obtained from various treatments had a pleasant smell. This can be caused by the low levels of free fatty acids present. The oil’s suitability for human ingestion increases with a decrease in free fatty acid content [112].
As shown by the results, the application of liquid inoculant along with organic manure (T3) had a significant impact on the oil yield and its chemical characteristics, which may be attributable to the availability of vital nutrients like Zn and P due to P. aeruginosa’s activity in mineral solubilizing. Similar results were observed by Radwan and Awad [113] who discovered that applying biofertilizer (Azospirillum sp. and Pseudomonas sp.) together with organic amendments significantly increased the oil yield and quality of peanut seeds in comparison to chemical fertilizer treatment. Safwat and Badran [114] additionally observed that the cumin plants’ essential oil production was increased when organic amendments mixed with Azotobacter and Bacillus megaterium were applied. Mekki et al. [115] also stated that soybean plants fertilized with biofertilizers in addition to organic manure had higher soybean seed oil levels. The largest amount of essential oil was produced when fennel plants were fertilized with organic manure and biofertilizers (Azotobacter and Azospirillum), according to Azzaz et al. [116]. As a result, in the current study, liquid inoculants and organic manure improved the oil content of the seeds of groundnut plants by making vital nutrients available to the plants through their action to promote plant growth.
3.5 Analysis of characteristics of soil before and after treatments
The addition of mineral-solubilizing bacteria to field soil in the form of a liquid bioinoculant would alter the soil’s characteristics because of the microorganisms’ ability to promote plant growth. Therefore, both before and after treatments, the parameters of the soil, such as pH, EC, CaCO3, organic carbon, and mineral content, including NPK and Zn, were investigated in this study. The results showed that all treatments significantly improved the soil’s properties in comparison to the control (Table 5).
Characteristics of the soil before and after treatments
| Treatments | pH | EC (dS/m) | N (kg/ha) | P (kg/ha) | K (kg/ha) | Zn (mg/kg) | CaCO3 (%) | SOC (%) |
|---|---|---|---|---|---|---|---|---|
| Before treatment | 8.3 ± 0.17a | 2.88 ± 0.32a | 273.75 ± 2.06f | 20.1 ± 2.12e | 182.25 ± 4.5e | 1.53 ± 0.10d | 2.53 ± 0.20a | 0.39 ± 0.03d |
| T1 | 8.08 ± 0.15ab | 1.35 ± 0.15b | 417.00 ± 2.94e | 29.6 ± 0.81d | 204.75 ± 4.27d | 2.09 ± 0.78cd | 1.13 ± 0.28c | 0.75 ± 0.02c |
| T2 | 7.8 ± 0.25bc | 0.37 ± 0.09d | 478.75 ± 1.70c | 37.33 ± 1.58c | 218.00 ± 4.89c | 5.38 ± 0.97b | 1.3 ± 0.24c | 0.84 ± 0.04ab |
| T3 | 7.5 ± 0.31c | 0.30 ± 0.05d | 788.25 ± 1.25a | 59.65 ± 1.71a | 346.5 ± 4.72a | 7.24 ± 0.83a | 1.7 ± 0.14b | 1.91 ± 0.05a |
| T4 | 7.9 ± 0.22b | 0.35 ± 0.10d | 483.75 ± 2.62b | 47.83 ± 1.22b | 231.5 ± 2.51b | 6.79 ± 1.26a | 1.3 ± 0.40c | 0.89 ± 0.06a |
| T5 | 8 ± 0.18ab | 0.93 ± 0.14c | 432.00 ± 2.82d | 33.15 ± 1.49d | 207.25 ± 4.11d | 3.22 ± 0.99c | 1.23 ± 0.09c | 0.81 ± 0.08bc |
Values are mean ± SD of four replication samples.
T1: control; T2: seeds treated with liquid formulation; T3: seeds treated with liquid formulation and the soil amended with FYM; T4: soil amended with FYM alone; and T5: seeds treated with lignite (solid)-based bioinoculant.
The pH of the soil, which represents the degree of acidity in the soil, has a big impact on microbial activity, plant nutrition availability, and even soil aggregate durability. The pH of the soil in the T3 plot was 7.5, which is lower than in the other treatments. This suggests that the addition of FYM and liquid bioinoculant affected the physical, chemical, and biological processes as well as the characteristics of the soil [117,118]. The pH of all the treated soils slightly decreased after the last groundnut plant was harvested. This could be because the bioinoculant P. aeruginosa produced organic acids, which led to a drop in pH, which is thought to be the main mechanism of metal solubilization. Because of the soil’s natural ability to act as a buffer in this experiment, it was discovered that soil pH decline was far lower than in the culture medium. Similar to this, Son et al. [119] showed that the main source of mineral (such as Zn and P) solubilization was the acidification of the culture by bacteria; however, a high level of metal solubilization compared to in vitro may not be possible in soil because most soils have a great pH buffering capacity. Lakshmi et al. [120] also observed similar results, i.e., the highest reduction in soil pH with the combined application of biofertilizers.
Soil EC has a direct impact on plants growing in the soil, it is a valuable indication for controlling agricultural systems [121] as the quantity of moisture that soil particles can hold varies with soil EC. Good soil health is often indicated by an EC range of 0–1 dS/m [122]. According to the current study, all of the inoculant-treated soil had an EC range of 0.3–1.3, which is acceptable and demonstrates the soil’s fertility [123].
The T3 plot showed a drop in EC contents, which is consistent with findings from Sushila et al. [124], who proposed that applying biofertilizers to saline soil would reduce soil salinity because the biofertilizers activate microorganisms in soil and increase the production of the enzyme dehydrogenase led to decrease the soil salinity compared with control. Additionally, Shaban and Attia [125] demonstrated that the values of EC dropped with an increase in mineral fertilizer in combination with biofertilizer, as compared to mineral fertilizers alone.
SOC plays a significant role in improving soil quality by improving ion exchange capacity, supporting biological component function, reducing bulk density, increasing water holding capacity, and increasing macro- and micronutrient availability [126,127]. SOC concentration greater than 1.7% indicates fertile soil [128]. In the current study, a T3 plot with a SOC concentration of 1.9% demonstrated that biofertilizers both directly and indirectly promote SOC accrual through microbial biomass turnover, the secretion of organic extracellular polymeric substances, or promoting plant growth through nutrient solubilization, associations, hormonal changes, etc. [129].
The physical and chemical properties of soil are impacted by the presence of CaCO3. Excessive lime concentration might hinder root penetration and water circulation. Within the soil’s fertile range, all the plots had CaCO3 concentrations ranging between 1 and 2.5% [123]. As a result, all of the inoculant-treated soil has optimal pH, EC, SOC, and CaCO3 content. Additionally, plant nutrients including N, P, K, and Zn were found to be within the fertile range of >480, >56, >280, and >0.6 kg/ha [123].
In terms of soil characteristics, the incorporation of mineral solubilizing bacteria with organic manure (T3) performed better than the other treatments, although there is no significant difference in the properties of the soil treated with different treatments when compared to liquid inoculant (T2), organic manure (T4), and control (T1) (Table 5). This could be a result of the advantageous effects of organic manures on nutrient availability. They accomplish this by directly or indirectly influencing the chemical processes that alter soil microbial activity and nutrients. These findings also correspond with the study by Mengel et al. [130], who stated that adding organic manure and biofertilizer promoted the synthesis of chemicals that control growth, enhancing the physico-chemical characteristics, microbial activity, and soil’s ability to retain water. Mahapatra et al. [131] also observed that bacterial biofertilizers are crucial for boosting root accessibility to soil components including N, P, K, and Zn, which promotes rhizosphere biocontrol. Thus, the current study observed that the liquid inoculant (P. aeruginosa) and organic manure had a positive impact on the availability of N, P, K, and Zn as well as other soil parameters, demonstrating the essential role of this organism in the transformation reaction of vital nutrients in the soil through its numerous plant growth-promoting activities, such as mineral solubilization and phytohormone synthesis. Regenerative agriculture practices involving the incorporation of organic matter (FYM application and residue retention) among others [132] can strongly influence the soil physicochemical parameters and biological properties and enhance soil fertility by stimulating microbial abundance and nutrient cycling and regulating SOC availability [99]. The application of organic matter (i.e., FYM) has been shown to improve soil physiochemical conditions [60] alongside stimulating a positive microbial link between nutrient availability [133], the regulation of SOC, and moisture.
4 Conclusion
The current study reveals that P. aeruginosa can effectively improve A. hypogaea L.’s biochemical profile by providing essential minerals (Zn and P) through the solubilization process. The incorporation of PVP proved to be a potential ingredient in P. aeruginosa liquid formulations as it protects bacteria by reducing their mortality and prolonging their shelf life. Liquid formulant supplementation in conjunction with FYM is also very beneficial because it provides the bioinoculant with a carbon and energy source, which improves nutrient availability, prolongs nutrient retention in the soil, and increases soil carbon sequestration, which reduces climate change by absorbing and storing atmospheric carbon dioxide. Thus, the study implies that crops cultivated using liquid bioinoculants will be more nutritious, leading to healthier products for consumers, as they boost nutrient availability and uptake. This kind of bioinoculant has the potential to replace chemical fertilizers, thereby reducing their adverse ecological impacts and leading to more sustainable agricultural practices. This is consistent with customers’ growing demands for environmentally friendly and sustainable food production methods. Overall, bioinoculants generally aid in the advancement of a more sustainable agricultural system that is advantageous to both food producers and consumers.
Acknowledgments
The authors acknowledge the Department of Biotechnology (DBT), India, for the support through DBT-BUILDER (No. BT/INF/22/SP45369/2022) scheme and DST FIST (Sanction No. SR/FST/COLLEGE-/2022/1293), New Delhi for the infrastructure provided. The project was also funded by Researchers Supporting Project number (RSP2025R143), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: The project was funded by Researchers Supporting Project number (RSP2025R143), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Sunitha kumari Krishnan Kutty, Padma Devi Skandasamy Natchimuthu, and Rajamani Ranjithkumar: performed the experiments and analyzed and interpreted the data and writing manuscript; Sinouvassane Djearamane, Lai-Hock Tey, Ling Shing Wong, and Saminathan Kayarohanam: methodology, writing and editing the manuscript; Natarajan Arumugam, Abdulrahman I. Almansour: investigation of the whole project; and Sakkarapalayam M. Mahalingam: writing and editing the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Mengel K, Kirkby EA. Principles of plant nutrition. Ann Bot. 2004;93(4):479–80.10.1093/aob/mch063Search in Google Scholar
[2] Monib AW, Alimyar O, Mohammad MU, Akhundzada MS, Niazi P. Macronutrients for plants growth and human health. J Res Appl Sci Biotech. 2023;2:268–9.10.55544/jrasb.2.2.38Search in Google Scholar
[3] Jan AU, Hadi F, Ditta A, Suleman M, Ullah M. Zn-induced anti-oxidative defense and osmotic adjustments to enhance drought stress tolerance in sunflower (Helianthus annuus L.). Env Exp Bot. 2022;193:104682.10.1016/j.envexpbot.2021.104682Search in Google Scholar
[4] Johnson VJ, Mirza A. Role of macro and micronutrients in the growth and development of plants. Int J Curr Microbiol Appl Sci. 2020;9:576–87.10.20546/ijcmas.2020.911.071Search in Google Scholar
[5] Barker AV, Pilbeam DJ, (Eds.). Handbook of plant nutrition. Boca Raton: CRC Press; 2015.10.1201/b18458Search in Google Scholar
[6] Voortman RL, Bindraban PS. Beyond N and P: Toward a land resource ecology perspective and impactful fertilizer interventions in sub-Saharan Africa. VFRC Report 2015/1. Washington, DC: Virtual Fertilizer Research Center; 2015.Search in Google Scholar
[7] Monreal CM, DeRosa M, Mallubhotla SC, Bindraban PS, Dimkpa CO. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol Fertil Soils. 2016;52(3):423–37.10.1007/s00374-015-1073-5Search in Google Scholar
[8] Fageria NK, Baligar VC, Li YC. The role of nutrient efficient plants in improving crop yields in the twenty first Century. J Plant Nutr. 2008;31:1121–57.10.1080/01904160802116068Search in Google Scholar
[9] Baligar VC, Fageria NK, He ZL. Nutrient use efficiency in plants. Commun Soil Sci Plant Anal. 2001;32:921–50.10.1081/CSS-100104098Search in Google Scholar
[10] Alloway BJ. Zn in soils and crop nutrition. International Zn Association, Brussels, Belgium; 2008.Search in Google Scholar
[11] Otieno EO, Mucheru-Muna MW, Kifuko-Koech MN, Kamau CN, Ndung’u-Magiroi KW H, Mogaka H, et al. Strategic research in the domain of secondary nutrients, micronutrients, liming and 4R stewardship in sub-Saharan Africa: Review. Environ Chall. 2024;16:100960.10.1016/j.envc.2024.100960Search in Google Scholar
[12] Graham RD. Micronutrient deficiencies in crops and their global significance. In: Alloway BJ, editors. Micronutrient deficiencies in global crop production. Dordrecht: Springer; 2008.10.1007/978-1-4020-6860-7_2Search in Google Scholar
[13] Zaheer IE, Ali S, Saleem MH, Ali M, Riaz M, Javed S, et al. Interactive role of Zn and iron lysine on Spinacia oleracea L. growth, photosynthesis and antioxidant capacity irrigated with tannery wastewater. Physiol Mol Biol. 2020a;26(12):2435–52.10.1007/s12298-020-00912-0Search in Google Scholar PubMed PubMed Central
[14] Yang M, Li Y, Liu Z, Tian J, Liang L, Qiu Y, et al. A high activity Zn transporter OsZIP9 mediates Zn uptake in rice. Plant J. 2020;103:1695–709.10.1111/tpj.14855Search in Google Scholar PubMed
[15] Alsafran M, Usman K, Ahmed B, Rizwan M, Saleem MH, Al Jabri H. Understanding the phytoremediation mechanisms of potentially toxic elements: A proteomic overview of recent advances. Front Plant Sci. 2022;13:881242.10.3389/fpls.2022.881242Search in Google Scholar PubMed PubMed Central
[16] Zlobin I. Current understanding of plant Zn homeostasis regulation mechanisms. Plant Physiol Biochem. 2021;162:327–35.10.1016/j.plaphy.2021.03.003Search in Google Scholar PubMed
[17] Hamzah Saleem M, Usman K, Rizwan M, Al Jabri H, Alsafran M. Functions and strategies for enhancing Zn availability in plants for sustainable agriculture. Front Plant Sci. 2022;13:1033092.10.3389/fpls.2022.1033092Search in Google Scholar PubMed PubMed Central
[18] Nandal V, Solanki M. Zn as a vital micronutrient in plants. J Microbiol Biotechnol Food Sci. 2021;11:e4026.10.15414/jmbfs.4026Search in Google Scholar
[19] Souvik N, Manoranjan O, Tapu Raihan G, Partha G, Melepurath D. Dendrite growth inhibition in a V6O13 nanorods based non-aqueous Zn-ion battery by a scalable polycarbazole at Carbon nanotubes overlayer. Compos B Eng. 2023;252:110516.10.1016/j.compositesb.2023.110516Search in Google Scholar
[20] Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA. Phosphate solubilizing microbes: sustainable approach for managing P deficiency in agricultural soils. SpringerPlus. 2013;2:587.10.1186/2193-1801-2-587Search in Google Scholar PubMed PubMed Central
[21] Marschner H, Cakmak J. High light intensity enhances chlorosis and necrosis inleaves of Zn, potassium, and magnesium deficient bean (Phaseolus vulgaris L.) plants. J Plant Physiol. 1989;134:924–34.10.1016/S0176-1617(89)80248-2Search in Google Scholar
[22] Faisal N, Sundas A, Faiza W, Najeeb A, Rashid M, Sadia B, et al. P (P) and Zn (Zn) nutrition constraints: A perspective of linking soil application with plant regulations. Env Exp Bot. 2024;226:105875.10.1016/j.envexpbot.2024.105875Search in Google Scholar
[23] Mousavi SR. Zn in crop production and interaction with P. Aust J Basic Appl Sci. 2011;5(9):1503–9.Search in Google Scholar
[24] Taheri N, Heidari H, Yousefi K, Mousavi SR. Effect of organic manure with P and Zn on yield of seed potato. Aust J Basic Appl Sci. 2011;5(8):775–80.Search in Google Scholar
[25] Brown PH, Cakmak IQ, Zhang Q. Form and function of Zn in plants. In: Robson AD, editors. Zn in soils and plants. Dordrecht, Boston, London: Kluwer Academic Publishers; 1993. p. 90–106.10.1007/978-94-011-0878-2_7Search in Google Scholar
[26] Taiz L, Zeiger E. Plant physiology: mineral nutrition. Redwood City: The Benjamin Cummings Publishing Co.; 1991. pp. 100–19.Search in Google Scholar
[27] Marschner M. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. p. 200–55.Search in Google Scholar
[28] Outten CE, O’Halloran TV. Femtomolar sensitivity of metallo regulatory protein controlling Zn homeostasis. Science. 2001;292:2488–92.10.1126/science.1060331Search in Google Scholar PubMed
[29] Pandey N, Pathak GC, Sharma CP. Zn is critically required for pollen function and fertilization in lentils. J Trace Elem Med Biol. 2006;20:89–96.10.1016/j.jtemb.2005.09.006Search in Google Scholar PubMed
[30] Bojovic B, Stojanovic J. Chlorophyll and carotenoid content in wheat cultivars as a function of mineral nutrition. Arch Biol Sci. 2005;57(4):283–90.10.2298/ABS0504283BSearch in Google Scholar
[31] Odoemelam SA. Proximate composition and selected physicochemical properties of the seeds of African Oil Bean (Pentaclethra marcrophylla). Pak J Nutr. 2005;4:382–3.10.3923/pjn.2005.382.383Search in Google Scholar
[32] Morrison WH, Hamilton RJ, Kalu C. Sunflower seed oil. In: Hamilton RJ, editor. Developments in oils and fats. Glasgow: Blackie Academic and Professional; 1995. p. 132–52.10.1007/978-1-4615-2183-9_5Search in Google Scholar
[33] Omidian A, Seyadat SA, Naseri R, Moradi M. Effects of Zn-sulfate foliar onyield, oil content and seed protein of four cultivars of canola. Iranian. J Agric Sci. 2012;14:26–8.Search in Google Scholar
[34] Singh AL. Mineral nutrition of groundnut. In: Helantaranjan A, editor. Advances in plant physiology. Jodhpur, India: Scientific Publishers; 1999. p. 161–200.Search in Google Scholar
[35] Singh AL, Basu MS, Singh NB. Mineral disorders of groundnut, National Research centre for groundnut. Junagadh, India: National Research centre for groundnut (ICAR); 2004. p. 85.Search in Google Scholar
[36] Alloway BJ. Zn in soils and plant nutrition. 2nd edn. Brussels, Belgium and Paris, France: International Zn Association and International Fertilizer Industry Association; 2008. p. 139.Search in Google Scholar
[37] ILZRO. Zn in crop nutrition. International Lead Zn Research Organisation Inc, Research Triangle Park, Durham, North Carolina; 1975. p. 54.Search in Google Scholar
[38] Klug A, Rhodes D. “Zn fingers”: A novel protein motif for nucleic acid recognition. Trends Biochem Sci. 1987;12:464–9.10.1016/0968-0004(87)90231-3Search in Google Scholar
[39] Romheld V, Marschner H. Micronutrients in agriculture. Soil Science Society of America Book Series, No 4, Published by: Soil Science Society of America, Inc., Madison, Wisconsin, USA. 2nd edn. 1991. p. 297–28.Search in Google Scholar
[40] Kumar P, Kaushal N, Dubey RC. Isolation and identification of plant growth promoting rhizobacteria (Pseudomonas sp.) and their effect on growth promotion of Lycopersicon esculentum L. Academ. Arena. 2015;7(5):44–51.Search in Google Scholar
[41] Sunithakumari K, PadmaDevi S, Vasandha S. Zn solubilizing bacterial isolates from the agricultural fields of Coimbatore, Tamil Nadu, India. Curr Sci. 2016;110:196–205.10.18520/cs/v110/i2/196-205Search in Google Scholar
[42] Devi SNP, Kumari KS, Vasandha S. Assessment of competence of the Pseudomonas aeruginosa to solubilize insoluble form of Zn under various cultural parameters. Arab J Sci Eng. 2016;41:2117–21.10.1007/s13369-015-1907-3Search in Google Scholar
[43] Devi SP, Ranjithkumar R, Djearamane S, Tey LH, Wong LS, Kayarohanam S, et al. Organic Remobilization of Zn and P availability to plants by application of mineral solubilizing bacteria Pseudomonas aeruginosa. Heliyon. 2023;9(11):e22128.10.1016/j.heliyon.2023.e22128Search in Google Scholar PubMed PubMed Central
[44] Nithya K. Studies on solubilization of minerals by soil microorganisms (B.Sc., Agri., thesis) Department of Agricultural Microbiology, Agricultural College and research institute, Tamil Nadu Agricultural University; 2008.Search in Google Scholar
[45] Lindsay L, Norvell WA. Development of DTPA soil test for Zn, iron, manganese and copper. Soil Sci Am J. 1978;42:421–8.10.2136/sssaj1978.03615995004200030009xSearch in Google Scholar
[46] Olsen SR, Cole CV, Watanabe S, Dean LA. Estimation of available P in soils by extraction with sodium bicarbonate. US Dept Agr Circ. 1954;939:19.Search in Google Scholar
[47] Hedge JE, Hofreiter BT. Methods in carbohydrate chemistry. In: Whistler RL, Be Miller JN, editor. Vol. 17, New York: Academic Press; 1962. p. 420.Search in Google Scholar
[48] Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.10.1006/abio.1976.9999Search in Google Scholar
[49] Arnon DI. Copper enzymes in isolated chloroplast. Plant Physiol. 1949;24:1.10.1104/pp.24.1.1Search in Google Scholar PubMed PubMed Central
[50] Anjani MK. Extraction and characterization of crude and refined groundnut (Arachis hypoagae, L.) oil. Int J Chem stud. 2017;5:2031–3.Search in Google Scholar
[51] AOAC (Association of Official Analytical Chemist), Official method of Analysis. Washington D.C.; 1984.Search in Google Scholar
[52] Sabir H, Rownok J, Ashraful A, Khodeza K, Sharif MA. Study on physic-chemical properties of edible oils available in Bangaladesh local market. Arch Curr Res Int. 2016;6:1–6.10.9734/ACRI/2016/29464Search in Google Scholar
[53] Birnin-Yauri UA, Garba S. Comparative studies of some physico-chemical properties of Baobab, Vegetables, Peanut and Palm oils. Niger J Basic Appl Sci. 2011;19:64–7.10.4314/njbas.v19i1.69345Search in Google Scholar
[54] Jackson ML. Soil chemical analysis. Prentice Hall of India, New Delhi, India; 1973.Search in Google Scholar
[55] Dreleimanis A. Quantities gasometric determination of calcite and dolomite by using Chittick apparatus. J Sediment Pet. 1962;32(3):20–9.10.1306/74D70D08-2B21-11D7-8648000102C1865DSearch in Google Scholar
[56] Walkley A, Black IA. An examination of Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–37.10.1097/00010694-193401000-00003Search in Google Scholar
[57] Vogel AI. A textbook of quantitative inorganic analysis. Chapter X. New York: Longman Inc.; 1961. p. 312.Search in Google Scholar
[58] Mahendra S, Ruhal DS, Narendra S. Zn availability in sodic soils reclaimed partially to different ESP levels. J Indian Soil Soc. 1993;41(2):256–60.Search in Google Scholar
[59] Fasim F, Ahmed N, Parsons R, Gadd GM. Solubilization of Zn salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol Lett. 2002;213(1):1–6.10.1111/j.1574-6968.2002.tb11277.xSearch in Google Scholar PubMed
[60] Mucheru-Muna M, Mugendi D, Pypers P, Mugwe J, James K, Vanlauwe B, et al. Enhancing maize productivity and profitability using organic inputs and mineral fertilizer in central Kenya small-hold farms. Exp Agric. 2014;50:250–69.10.1017/S0014479713000525Search in Google Scholar
[61] Mugwe J, Mugendi D, Mucheru-Muna M, Odee D, Mairura F. Effect of selected organic materials and inorganic fertilizer on the soil fertility of a humic nitisol in the central highlands of Kenya. Soil Use Manag. 2009;25:434.10.1111/j.1475-2743.2009.00244.xSearch in Google Scholar
[62] Kihanda FM, Warren GP, Micheni AN. Effects of manure application on crop yield and soil chemical properties in a long-term field trial in semi-arid Kenya. In: Bationo A, Waswa B, Kihara J, Kimetu J, editors. Advances in integrated soil fertility management in Sub-Saharan Africa: Challenges and opportunities. Dordrecht, The Netherlands: Springer; 2007. p. 471–86.10.1007/978-1-4020-5760-1_44Search in Google Scholar
[63] Gautam A, Sekaran U, Guzman J, Kovács P, Hernandez JLG, Kumar S. Responses of soil microbial community structure and enzymatic activities to long-term application of mineral fertilizer and beef manure. Env Sustain Indic. 2020;8:100073.10.1016/j.indic.2020.100073Search in Google Scholar
[64] Tang H, Li C, Xiao X, Shi L, Cheng K, Wen L, et al. Effects of short-term manure nitrogen input on soil microbial community structure and diversity in a double-cropping paddy field of Southern China. Sci Rep. 2020;10:13540.10.1038/s41598-020-70612-ySearch in Google Scholar PubMed PubMed Central
[65] Babin D, Deubel A, Jacquiod S, Sørensen SJ, Geistlinger J, Grosch R, et al. Impact of long-term agricultural management practices on soil prokaryotic communities. Soil Biol Biochem. 2019;129:17–28.10.1016/j.soilbio.2018.11.002Search in Google Scholar
[66] Lladó S, Baldrian P. Community-level physiological profiling analyses show potential to identify the copiotrophic bacteria present in soil environments. PLoS ONE. 2017;12:e0171638.10.1371/journal.pone.0171638Search in Google Scholar PubMed PubMed Central
[67] Senthil Kumar PS, Aruna Geetha S, Savithri P, Jagadeeswaran R, Ragunath KP. Effect of Zn enriched organic manures and Zn solubilizer application on the yield, curcumin content and nutrient status of soil under turmeric cultivation. J Appl Hortic. 2004;6(2):82–6.10.37855/jah.2004.v06i02.18Search in Google Scholar
[68] Kushwaha P, Kashyap PL, Pandiyan K, Bhardwaj AK. Zn-solubilizing microbes for sustainable crop production: current understanding, opportunities, and challenges. In: Solanki MK, Kashyap PL, Kumari B, editors. Phytobiomes: Current insights and future vistas. Singapore: Springer; 2020. p. 281–98.10.1007/978-981-15-3151-4_11Search in Google Scholar
[69] Nitu R, Rajinder K, Sukhminderjit K. Zn solubilizing bacteria to augment soil fertility – A comprehensive review. Int J Agric Sci Vet Med. 2020;8:38–44.Search in Google Scholar
[70] Mitran T, Mani PK. Effect of organic amendments on rice yield trend, P use efficiency, uptake, and apparent balance in soil under long-term rice-wheat rotation. J Plant Nutr. 2017;40(9):1312–22.10.1080/01904167.2016.1267205Search in Google Scholar
[71] Marra LM, de Oliveira SM, Soares CRFS, de Souza Moreira FM. Solubilisation of inorganic phosphates by inoculants strains from tropical legumes. Sci Agricola. 2011;68:603–9.10.1590/S0103-90162011000500015Search in Google Scholar
[72] Gupta G, Panwar J, Jha P. Natural occurrence of Pseudomonas aeruginosa, a dominant cultivable Diazotrophic endophytic bacterium colonizing Pennisetum glaucum (L) R. Br. Appl Soil Ecol. 2013;64:252–61.10.1016/j.apsoil.2012.12.016Search in Google Scholar
[73] Nahas E. Factors determining rock phosphate solubilization by microorganism isolated from soil. World J Microbiol Biotechnol. 1996;12:18–23.10.1007/BF00327716Search in Google Scholar PubMed
[74] Kaur G, Reddy MS. Influence of P-solubilizing bacteria on crop yield and soil fertility at multi locational sites. Euro J Soil Biol. 2014;61:35–40.10.1016/j.ejsobi.2013.12.009Search in Google Scholar
[75] Shahzad SM, Khalid A, Arif MS, Riaz M, Ashraf M, Iqbal Z. Co-inoculation integrated with P-enriched compost improved nodulation and growth of chickpea (Cicer arietinum L.) under irrigated and rainfed farming systems. Biol Fertil Soils. 2014;50:1–12.10.1007/s00374-013-0826-2Search in Google Scholar
[76] Andrade DLA, Santos CHB, Frezarin ET, Sales LR, Rigobelo EC. Plant growth-promoting rhizobacteria for sustainable agricultural production. Microorganisms. 2023 Apr;11(4):1088.10.3390/microorganisms11041088Search in Google Scholar PubMed PubMed Central
[77] Bisson P, Cretenet M, Jallas E. Nitrogen, P and potassium availability in the soil physiology of the assimilation and use of these nutrients by the plant. In: Constable GA, Forrester NW, editros. Challenging the Future: Proceedings of the World Cotton Research Conference-1, Brisbane Australia, February 14–17. Melbourne: CSIRO; 1994. p. 15–124.Search in Google Scholar
[78] Rodriguez D, Zubillaga MM, Ploschuck E, Keltjens W, Goudriaan J, Lavado R. Leaf area expansion and assimilate prediction in sunflower growing under low P conditions. Plant Soil. 1998;202:133–47.10.1023/A:1004348702697Search in Google Scholar
[79] Upadhayay VK, Singh AV, Khan A. Cross talk between Zn-solubilizing bacteria and plants: a short tale of bacterial-assisted Zn biofortification. Front Soil Sci. 2022;1:788170.10.3389/fsoil.2021.788170Search in Google Scholar
[80] Silva LI, Pereira MC, Carvalho AM, Buttrós VH, Pasqual M, Dória J. Phosphorus-solubilizing microorganisms: a key to sustainable agriculture. Agriculture. 2023;13(2):462.10.3390/agriculture13020462Search in Google Scholar
[81] Timofeeva AM, Galyamova MR, Sedykh SE. Bacterial siderophores: classification, biosynthesis, perspectives of use in agriculture. Plants. 2022;11(22):3065.10.3390/plants11223065Search in Google Scholar PubMed PubMed Central
[82] Singh S, Chhabra R, Sharma A, Bisht A. Harnessing the power of Zn-solubilizing bacteria: a catalyst for a sustainable agrosystem. Bacteria. 2024;3(1):15–29.10.3390/bacteria3010002Search in Google Scholar
[83] Pantigoso HA, Manter DK, Fonte SJ, et al. Root exudate-derived compounds stimulate the P solubilizing ability of bacteria. Sci Rep. 2023;13:4050.10.1038/s41598-023-30915-2Search in Google Scholar PubMed PubMed Central
[84] Yousefi AA, Sadeghi M. Effect of vermicompost and urea chemical fertilizers on yield and yield components of wheat (Triticum aestivum) in the field condition. Intl J Agri Crop Sci. 2014;7(12):1227–30.Search in Google Scholar
[85] Agrawal SB, Anoop Singh AS, Gaurav Dwivedi GD. Effect of vermicompost, farm yard manure and chemical fertilizers on growth and yield of wheat (Triticum aestivum L. var. HD 2643). Plant Arch. 2003;3:9–14.Search in Google Scholar
[86] Prathibha KS, Siddalingeshwara KG. Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescence as rhizobacteria on seed quality of sorghum. Int J Curr Microbiol App Sci. 2013;2(3):11–8.Search in Google Scholar
[87] Sirohi G, Upadhyay A, Srivastava PS, Srivastava S. PGPR mediated Zn biofertilization of soil and its impact on growth and productivity of wheat. J Soil Sci Plant Nutr. 2015;15:202–16.10.4067/S0718-95162015005000017Search in Google Scholar
[88] Mathivanan S, Chidambaram ALA, Sundaramoorthy P, Baskaran L, Kalaikandhan R. The effect of plant growth promoting rhizobacteria on groundnut (Arachis hypogaea L.) seed germination and biochemical constituents. Int J Curr Res Aca Rev. 2014;2(9):187–94.Search in Google Scholar
[89] Shrotri CK, Rathore VS, Mohanty P. Studies on photosynthetic electron transport, photophosphorylation and CO2 fixation in Zn deficient leaf cells of Zea mays L. J Plant Nutr. 1981;3:945–54.10.1080/01904168109362890Search in Google Scholar
[90] Pearson JN Z, Rengel Z. Genotypic differences in the production and partitioning of carbohydrates between roots and shoots of wheat grown under Zn or manganese deficiency. Ann Bot. 1997;80:803–8.10.1006/anbo.1997.0523Search in Google Scholar
[91] Alloway BJ. Zn in soils and crop nutrition. International Zn Association (IZA)Publications, Brussels: Belgium. 2004. p. 1–116.Search in Google Scholar
[92] Sharma PN, Kumar N, SBisht SS. Effect of Zn deficiency on chlorophyll content, photosynthesis and water relations of cauliflower plants. Photosynthetica. 1994;30:353–9.Search in Google Scholar
[93] Aravind P, Prasad MNV. Zn protects chloroplasts and associated photochemical functions in cadmium-exposed Ceratophyllum demersum L., a freshwater macrophyte. Plant Sci. 2004;166:1321–27.10.1016/j.plantsci.2004.01.011Search in Google Scholar
[94] Farid H, Saied El S, Amany Abdel Mohsen R, Doaa MA. The combined effects of farm yard manure and boron application on growth, and oil quality of Canola grown under newly reclaimed soils. Oil Crop Sci. 2024;9(1):53–9.10.1016/j.ocsci.2023.12.007Search in Google Scholar
[95] Chaudhary P, Singh S, Chaudhary A, Sharma A, Kumar G. Overview of biofertilizers in crop production and stress management for sustainable agriculture. Front Plant Sci. 2022;23(13):930340.10.3389/fpls.2022.930340Search in Google Scholar PubMed PubMed Central
[96] Kamran S, Shahid I, Baig DN, Rizwan M, Malik KA, Mehnaz S. Contribution of Zn solubilizing bacteria in growth promotion and Zn content of wheat. Front Microbiol. 2017;21(8):2593.10.3389/fmicb.2017.02593Search in Google Scholar PubMed PubMed Central
[97] Chanchal AK, Singh M, Pradhan AK, Kumar S, Beura K, Singh P. Changes in soil Zn fractions upon inoculation of Zn solubilizing bacteria (ZnSB) under rice rhizospheric soil. Commun Soil Sci Plant Anal. 2024;55(15):2322–38.10.1080/00103624.2024.2356170Search in Google Scholar
[98] Bolo P, Mucheru-Muna MW, Mwirichia RK, Kinyua M, Ayaga G, Kihara J. Influence of farmyard manure application on potential Zn solubilizing microbial species abundance in a ferralsol of Western Kenya. Agriculture. 2023;13(12):2217.10.3390/agriculture13122217Search in Google Scholar
[99] Rahim N, Yasin Z, Tahir M, Majeed A, Yaqub A, Mahmood B. Rock phosphate and manures with P solubilizing bacteria increases the growth, yield and P uptake of okra (Abelmoschus esculentus). J Appl Res Plant Sci. 2023;5:111–8.10.38211/joarps.2024.05.226Search in Google Scholar
[100] Wu F, Li J, Chen Y, Zhang L, Zhang Y, Wang S, et al. Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera Abel. Forests. 2019;10(4):348.10.3390/f10040348Search in Google Scholar
[101] Pyone A, Pechrada P, Saowanuch T. Effect of P solubilizing bacteria on soil available P and growth and yield of sugarcane. Walailak J Sci Tech (WJST). 2021;18(12):1–9.10.48048/wjst.2021.10754Search in Google Scholar
[102] Sadasivam S, Manickam A. Biochemical methods. 3rd edn. New Delhi, India: New Age International Publishers; 2008.Search in Google Scholar
[103] Joseph AF. Measuring flavour deterioration of fats, oils and foods. New York: General Food Cooperation Technical Center; 1977. p. 1–7.Search in Google Scholar
[104] Tanlor SL, Beg CM, Shoptangh NH, Traisman E. Mutagive formation in deep fat fried foods as a function of frying conditions. JAOCS. 1983;60:576–80.10.1007/BF02679790Search in Google Scholar
[105] Rossel JB. Vegetable oils and fats. 1st ed. London: C.M.E. Casterberg; 1984. p. 263–65.Search in Google Scholar
[106] Chan HWS, Cotton DT. The mechanism of autoxidation. Orland: Academic Press, Inc; 1987. p. 49.Search in Google Scholar
[107] Ruiz MC, Margaret TM, Dobarganea MC. Quantitative and distribution of altered fatty acids in frying fats. J Am Oil Chem Soc. 1995;72(10):1171–76.10.1007/BF02540984Search in Google Scholar
[108] Muhammad N, Bamishaiye E, Bamishaiye O, Usman L, Salawu M, Nafiu M, et al. Physicochemical properties and fatty acid composition of Cyperus esculentus (Tiger Nut) Tuber Oil. Biores Bull. 2011;5:51–4.Search in Google Scholar
[109] Lea CH. The oxidative deterioration of food lipids. In: Schultz HW, Day EA, Sinnhuber RO, editors. Symposium on food: lipids and their oxidation. Westport, Connecticut: The AVI Publishing Company, Inc; 1962. p. 3–28.Search in Google Scholar
[110] Olaposi AR, Adunni AO. Nutritional and physico-chemical properties of Bombax glabrum Seeds. Pak J Nutr. 2010;9(9):856–57.10.3923/pjn.2010.856.857Search in Google Scholar
[111] Aluyor EO, Aluyor P, Ozigagu CE. Effect of refining on the quality and composition of groundnut oil. Afr J Food Sci. 2009;3(8):201–5.Search in Google Scholar
[112] Kirk RS, Sawyer R. Pearson’s composition and analysis of foods. 9th edn. England: Addison Wesley Longman Ltd; 1991. p. 9–29, 608–40.Search in Google Scholar
[113] Radwan SMA, Awad NM. Effect of soil Amendment with various organic wastes with multi-biofertilizer on yield of peanut plants in sandy soil. J Agric Sci Mansoura Univ. 2002;27(5):3129–38.10.21608/jacb.2002.254783Search in Google Scholar
[114] Safwat MSA, Badran FS. The efficiency of organic and bio-fertilizers, in comparison with chemical fertilization, on growth, yield, and essential oil of cumin plants. The10th Conference Medicinal and Aromatic Plants in Arab Countries, Sustainable Development; 14–16 December, 2002. p. 11–9.Search in Google Scholar
[115] Mekki BB, Amal G, Ahmed G. Growth, yield, and seed quality of soybean (Glycine max L.) as affected by organic, biofertilizer, and yeast application. Res J Agric Biol Sci. 2005;1(4):320–4.Search in Google Scholar
[116] Azzaz NA, Hassan EA, Hamad EH. The chemical constituent and vegetative and yielding characteristics of fennel plants treated with organic and bio-fertilizer instead of mineral fertilizer. Aust J Basic Appl Sci. 2009;3(2):579–87.Search in Google Scholar
[117] Brady NC, Weil RR. The nature and properties of soil. 14th edn. New Jersey: Prentice Hall; 2008.Search in Google Scholar
[118] Parikh SJ, James BR. Soil: The foundation of agriculture. Nat Educ Knowl. 2012;3(10):2.Search in Google Scholar
[119] Son HJ, Park GT, Cha M, Hea MS. Solubilization of insoluble inorganic P by a novel salt and pH tolerant Pantoea agglomerans R- 42 Isolated from soybean rhizosphere. Biores Technol. 2006;97:204–10.10.1016/j.biortech.2005.02.021Search in Google Scholar PubMed
[120] Lakshmi CS, Sreelatha T, Rani T, Rao SRK, Naidu NV. Effect of organic manures on soil fertility and productivity of sugarcane in North Coastal Zone of Andhra Pradesh. Indian J Anim Res. 2011;45:307–13.Search in Google Scholar
[121] Arnold SL, Doran JW, Schepers J, Wienhold B, Ginting D, Amos B, et al. Portable probes to measure electrical conductivity and soil quality in the field. Commun Soil Sci Plant Anal. 2005;36:2271–87.10.1080/00103620500196689Search in Google Scholar
[122] Smith JL, Doran JW. Measurement and use of pH and electrical conductivity for soil quality analysis. In: Doran JW, Jones AJ, editors. Methods for assessing soil quality. Vol. 49, SSSA, Madison: Soil Science Society of America Journal; 1996. p. 169–85.10.2136/sssaspecpub49.c10Search in Google Scholar
[123] Mandali R, Subbaiah V. Available nutrient status in rice growing soils of various Mandals in Krishna District. Int J Chem Stud. 2020;8(4):2745–8. 10.22271/chemi.2020.v8.i4af.10061Search in Google Scholar
[124] Sushila A, Yadav BL, Bhuli DG, Jitendra SB. Effect of soil salinity, P, and biofertilizers on physical properties of soil, yield attributes and yield of cowpea Vigna unguiculata (L.) Wilczek. J Pharmacogn Phytochem. 2017;6(4):169–1695.Search in Google Scholar
[125] Shaban Kh A, Attia AM. Evaluation of bio- and chemical fertilizers applied to corn grown on a saline sandy soil. Minufiya J Agric Res. 2009;34(3):1311–26.Search in Google Scholar
[126] Carter MR, Gregorich EG, (Eds.). Soil sampling and methods of analysis. Boca Raton, FL, USA: CRC Press, Inc; 2007. p. 607–35.10.1201/9781420005271Search in Google Scholar
[127] Carter MR, Stewart BA, (Eds). Structure and Organic Matter Storage in Agricultural Soils (Advances in Soil Science). 1st edn. Boca Raton: CRC Press; 1995. p. 15–41.Search in Google Scholar
[128] Musinguzi P, Ebanyat P, Tenywa JS, Basamba TA, Tenywa MM, Mubiru DN. Critical soil organic carbon range for optimal crop response to mineral fertiliser nitrogen on a ferralsol. Expe Agric. 2016;52(4):635–53.10.1017/S0014479715000307Search in Google Scholar
[129] Kohli I, Mohapatra S, Kumar P, Goel A, Varma A, Joshi NC. Role of bacterial endophytes in the promotion of plant growth. In: Singh AK, Tripathi V, Shukla AK, Kumar P, editors. Bacterial endophytes for sustainable agriculture and environmental management. Singapore: Springer; 2022.10.1007/978-981-16-4497-9_12Search in Google Scholar
[130] Mengel K, Kirkby EA, Kosegarten H, Appel T. Principles of plant nutrition. Dordrecht: Kluwer Academic Publishing Co., Inc. Redwood City, CA; 2001. p. 100–19.10.1007/978-94-010-1009-2Search in Google Scholar
[131] Mahapatra DM, Satapathy KC, Panda B. Biofertilizers and nanofertilizers for sustainable agriculture: Phycoprospects and challenges. Sci Total Env. 2022;803:149990.10.1016/j.scitotenv.2021.149990Search in Google Scholar PubMed
[132] Giller KE, Hijbeek R, Andersson JA, Sumberg J. Regenerative agriculture: an agronomic perspective. Outlook Agric. 2021;50:13–25.10.1177/0030727021998063Search in Google Scholar PubMed PubMed Central
[133] Ayaga G, Todd A, Brookes PC. Enhanced biological cycling of P increases its availability to crops in low-input sub-saharan farming systems. Soil Biol Biochem. 2006;38:81–90.10.1016/j.soilbio.2005.04.019Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Safety assessment and modulation of hepatic CYP3A4 and UGT enzymes by Glycyrrhiza glabra aqueous extract in female Sprague–Dawley rats
- Adult-onset Still’s disease with hemophagocytic lymphohistiocytosis and minimal change disease
- Role of DZ2002 in reducing corneal graft rejection in rats by influencing Th17 activation via inhibition of the PI3K/AKT pathway and downregulation of TRAF1
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- YAP1 inhibition protects retinal vascular endothelial cells under high glucose by inhibiting autophagy
- Identification of secretory protein related biomarkers for primary biliary cholangitis based on machine learning and experimental validation
- Integrated genomic and clinical modeling for prognostic assessment of radiotherapy response in rectal neoplasms
- Stem cell-based approaches for glaucoma treatment: a mini review
- Bacteriophage titering by optical density means: KOTE assays
- Neutrophil-related signature characterizes immune landscape and predicts prognosis of esophageal squamous cell carcinoma
- Integrated bioinformatic analysis and machine learning strategies to identify new potential immune biomarkers for Alzheimer’s disease and their targeting prediction with geniposide
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- Histone modification and non-coding RNAs in skin aging: emerging therapeutic avenues
- A multiplicative behavioral model of DNA replication initiation in cells
- Biogenic gold nanoparticles synthesized from Pergularia daemia leaves: a novel approach for nasopharyngeal carcinoma therapy
- Creutzfeldt-Jakob disease mimicking Hashimoto’s encephalopathy: steroid response followed by decline
- Impact of semaphorin, Sema3F, on the gene transcription and protein expression of CREB and its binding protein CREBBP in primary hippocampal neurons of rats
- Iron overloaded M0 macrophages regulate hematopoietic stem cell proliferation and senescence via the Nrf2/Keap1/HO-1 pathway
- Revisiting the link between NADPH oxidase p22phox C242T polymorphism and ischemic stroke risk: an updated meta-analysis
- Exercise training preferentially modulates α1D-adrenergic receptor expression in peripheral arteries of hypertensive rats
- Overexpression of HE4/WFDC2 gene in mice leads to keratitis and corneal opacity
- Tumoral calcinosis complicating CKD-MBD in hemodialysis: a case report
- Mechanism of KLF4 Inhibition of epithelial-mesenchymal transition in gastric cancer cells
- Dissecting the molecular mechanisms of T cell infiltration in psoriatic lesions via cell-cell communication and regulatory network analysis
- Circadian rhythm-based prognostic features predict immune infiltration and tumor microenvironment in molecular subtypes of hepatocellular carcinoma
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Molecular identification and control studies on Coridius sp. (Hemiptera: Dinidoridae) in Al-Khamra, south of Jeddah, Saudi Arabia
- 10.1515/biol-2025-1218
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Response of hybrid grapes (Vitis spp.) to two biotic stress factors and their seedlessness status
- Metabolomic profiling reveals systemic metabolic reprogramming in Alternaria alternata under salt stress
- Effects of mixed salinity and alkali stress on photosynthetic characteristics and PEPC gene expression of vegetable soybean seedlings
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Causal effects of trace elements on congenital foot deformities and their subtypes: a Mendelian randomization study with gut microbiota mediation
- Honey meets acidity: a novel biopreservative approach against foodborne pathogens
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Fluorescent detection of sialic acid–binding lectins using functionalized quantum dots in ELISA format
- Smart tectorigenin-loaded ZnO hydrogel nanocomposites for targeted wound healing: synthesis, characterization, and biological evaluation
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Safety assessment and modulation of hepatic CYP3A4 and UGT enzymes by Glycyrrhiza glabra aqueous extract in female Sprague–Dawley rats
- Adult-onset Still’s disease with hemophagocytic lymphohistiocytosis and minimal change disease
- Role of DZ2002 in reducing corneal graft rejection in rats by influencing Th17 activation via inhibition of the PI3K/AKT pathway and downregulation of TRAF1
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- YAP1 inhibition protects retinal vascular endothelial cells under high glucose by inhibiting autophagy
- Identification of secretory protein related biomarkers for primary biliary cholangitis based on machine learning and experimental validation
- Integrated genomic and clinical modeling for prognostic assessment of radiotherapy response in rectal neoplasms
- Stem cell-based approaches for glaucoma treatment: a mini review
- Bacteriophage titering by optical density means: KOTE assays
- Neutrophil-related signature characterizes immune landscape and predicts prognosis of esophageal squamous cell carcinoma
- Integrated bioinformatic analysis and machine learning strategies to identify new potential immune biomarkers for Alzheimer’s disease and their targeting prediction with geniposide
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- TRIM21 accelerates ferroptosis in intervertebral disc degeneration by promoting SLC7A11 ubiquitination and degradation
- Histone modification and non-coding RNAs in skin aging: emerging therapeutic avenues
- A multiplicative behavioral model of DNA replication initiation in cells
- Biogenic gold nanoparticles synthesized from Pergularia daemia leaves: a novel approach for nasopharyngeal carcinoma therapy
- Creutzfeldt-Jakob disease mimicking Hashimoto’s encephalopathy: steroid response followed by decline
- Impact of semaphorin, Sema3F, on the gene transcription and protein expression of CREB and its binding protein CREBBP in primary hippocampal neurons of rats
- Iron overloaded M0 macrophages regulate hematopoietic stem cell proliferation and senescence via the Nrf2/Keap1/HO-1 pathway
- Revisiting the link between NADPH oxidase p22phox C242T polymorphism and ischemic stroke risk: an updated meta-analysis
- Exercise training preferentially modulates α1D-adrenergic receptor expression in peripheral arteries of hypertensive rats
- Overexpression of HE4/WFDC2 gene in mice leads to keratitis and corneal opacity
- Tumoral calcinosis complicating CKD-MBD in hemodialysis: a case report
- Mechanism of KLF4 Inhibition of epithelial-mesenchymal transition in gastric cancer cells
- Dissecting the molecular mechanisms of T cell infiltration in psoriatic lesions via cell-cell communication and regulatory network analysis
- Circadian rhythm-based prognostic features predict immune infiltration and tumor microenvironment in molecular subtypes of hepatocellular carcinoma
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Molecular identification and control studies on Coridius sp. (Hemiptera: Dinidoridae) in Al-Khamra, south of Jeddah, Saudi Arabia
- 10.1515/biol-2025-1218
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Response of hybrid grapes (Vitis spp.) to two biotic stress factors and their seedlessness status
- Metabolomic profiling reveals systemic metabolic reprogramming in Alternaria alternata under salt stress
- Effects of mixed salinity and alkali stress on photosynthetic characteristics and PEPC gene expression of vegetable soybean seedlings
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Causal effects of trace elements on congenital foot deformities and their subtypes: a Mendelian randomization study with gut microbiota mediation
- Honey meets acidity: a novel biopreservative approach against foodborne pathogens
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Fluorescent detection of sialic acid–binding lectins using functionalized quantum dots in ELISA format
- Smart tectorigenin-loaded ZnO hydrogel nanocomposites for targeted wound healing: synthesis, characterization, and biological evaluation
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

