Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
-
Tunde Jurikova
Abstract
Although cornelian cherry is an underutilized fruit species, its fruits have a high biological value due to valuable biologically active substances, especially polyphenols. The total content of polyphenols accounts for 37% of all the bioactive substances examined. Flavonols, anthocyanins, flavan-3-ols, and phenolic acids represent the main groups of phenolic compounds present and thanks to these compounds, cornelian cherry fruits possess mainly antioxidant, anti-inflammatory, antimicrobial, metabolic, antidiabetic, cardioprotective, and anticancer effects. This review summarizes new research aimed at popularizing this lesser-known species not only for direct consumption but also for further processing.
1 Introduction
Among 65 species belonging to the genus Cornus, only two, Cornus mas L. and Cornus officinalis Sieb. et Zucc. (Cornaceae), have been traditionally used since ancient times. C. mas (cornelian cherry) is native to southern Europe and southwest Asia, whereas C. officinalis (Asiatic dogwood, cornel dogwood) is a deciduous tree found in eastern Asia, primarily in China, as well as Korea and Japan [1,2]. C. mas L. (cornelian cherry) has been popular in garden cultivation for 4,000 years [2]. Although the fruit is one of the lesser-known and valued species, an interest in growing this crop has been increasing [3,4].
Oval or pear-shaped fruits of C. mas L. ranging in color from red to purple [5], represented a valuable source of bioactive substances, including polyphenols (anthocyanins, phenolic acids, flavonoids) [6,7], catechins (CATs) and tannins (37.36%), monoterpenes (26.3%), organic acids (25.9%), vitamin C (10.7%), and lipophilic compounds such as carotenoids [8,9].
90% of the polyphenolic extract obtained from cornelian cherry fruit was represented by loganoic acid, cornuside, and anthocyanins – cyanidin 3-galactoside and pelargonidin 3-galactoside [10,11]. Despite its high biological value, the fruit is classified as underutilized and forgotten fruit species [12].
Among the group of polyphenols are the most abundant phenolic acids (i.e., benzoic acid derivatives and cinnamic acid derivatives) and second flavonols, especially quercetin derivatives [13]. Irridois represent also valuable part of fruits – especially loganoic acid and cornuside [2,14]. To sum up all bioactive compounds, Przybylska et al. [15] highlighted and determined 37 bioactive compounds, which included various gallotannins (11), monomeric ellagitannins (7), dimeric ellagitannins (10), and trimeric ellagitannins (7).
The fruits of cornelian cherry have been valued in folk medicine for years [2].
The fruits can be consumed fresh or dried as a decoction [16] and they are also widely used in the food industry to produce various beverages, syrups, gels, jams, and compotes [17,18] and for the liquor and wine as well [10,19]. Farmers from Bosnia and Herzegovina produce a special alcoholic beverage “rakija” [12]. The fruits are also valuable in folk medicine to prevent and treat diarrhea, hemorrhoids, diabetes, sore throat, indigestion, measles, chickenpox, anemia, rickets, liver, and kidney diseases [20].
Furthermore, they can be used as a flavoring ingredient in functional ice creams, desserts, and cakes [21,22].
In general, studies about biologically active compounds in cornelian cherry have been published, but only a few of them have focused on the main group of bioactive substances – polyphenolic compounds, which are responsible for their antioxidant activity (AA) and health benefits. Moreover, the review discusses the influence of locality, climatic conditions, and chemical extraction methodology. Therefore, the main aim of the present review is to provide an overview of the main polyphenolic compounds contained in fruits including their health benefits.
Databases WOS, Scopus, and PubMed were used to summarize information for this review.
2 Total polyphenol content (TPC) of C. mas fruit
According to De Biaggi et al. [5], the TPC reached up to 37% of all bioactive compounds presented (polyphenols, monoterpenes, organic acids, and vitamin C) in fruit fresh weight (FW). Therefore, it is very important to focus on this group of compound, particularly with regard to its variability.
The polyphenol content exhibited wide variability despite the identical cultivation origin. For example, the TPC of C. mas fruit from Ukraine determined by the Folin–Ciocalteu reagent by Klymenko et al. [2] showed a wide range of values from 100.71 (“Koralovyi”) to 924.65 mg GAE·100 g−1 FW (“Uholok”). On the other hand, in a comparative study of the interspecific hybrids C. mas × C. officinalis contained higher concentration of polyphenolic compounds than C. officinalis and C. mas. In another study provided by Klymenko et al. [23], 20 different cultivars collected in the M.M. Gryshko National Botanical Garden of NAS of Ukraine were analyzed for TPC. The study found that TPC was genotype dependent, with values ranging from 91.34 (“Kozerog”) to 289.79 (“Bolgarskyj”) mg GAE·100 g−1 FW. These results demonstrated that TPC is dependent on cultivar and genotype.
Six genotypes of cornelian cherry, selected from spontaneous flora in different areas in Romania, were studied for TPC as well. Cosmulescu et al. [24] reported TPC ranging from 163.69 (S1) to 359.28 (H2) mg GAE·100 g−1 FW. Genotypes H2 and H3 had the highest TPC (359.28 and 343.50 mg GAE·100 g−1 FW, respectively) [24]. Skender et al. [12] studied the TPC of 22 promising local cornelian cherry (C. mas L.) genotypes from Bosnia and Herzegovina. The results showed that the TPC was quite variable among studied genotypes and ranged from 1,240 to 6,958 mg GAE·100 g−1 FW. They found statistically significant differences among genotypes in terms of bioactive compounds content at the 0.05 level of significance. Additionally, Kucharska et al. [25] investigated cultivars of Polish breeding in relation to fruit morphological parameters. According to their findings, the Szafer cultivar was the richest source of polyphenols (464 mg·100 g−1 FW), with a fruit length of 1.72 cm (second longest) and displayed the highest fruit mass max (4.41 g). In contrast, the cultivar Juliusz had the lowest polyphenol content (262 mg GAE·100 g−1 FW), the smallest fruit length (1.10 cm), and the lowest fruit mass 3.24 g.
Table 1 shows that the polyphenol content in cornelian cherry fruit is influenced by genetic factors as well as the fruit’s origin and cultivation environment [26]. The TPC in C. mas of Greek cultivars was 1 from 592 mg GAE; in cultivars from Azerbaijan 1,097–2,695 mg GAE 100 g–1 DW, and in Turkish cultivars, 2,659–7,483 mg GAE 100 g–1 DW [26,27].
TPC in relation to place of cultivation of C. mas fruit samples (mg GAE·100 g−1 FW/DW)
| Place of cultivation | TPC (mg GAE·100 g−1 FW) | Source |
|---|---|---|
| Ukraine | 100.71–924.65 FW | Klymenko et al. [2] |
| 91.34–289.79 FW | Klymenko et al. [23] | |
| Czech Republic | 61–253 FW | Cetkovská et al. [27] |
| 261–811 FW | Rop et al. [17] | |
| Bosnia and Herzegovina | 1240–6958 FW | Skender et al. [12] |
| 38.98–103.37 FW | Drkenda et al. [33] | |
| Poland | 262–464 FW | Kucharska et al. [25] |
| Turkey | 653–1009 FW | Sengul et al. [32] |
| Serbia | 47.60–116.38 FW | Bijelić et al. [28] |
| Montenegro | 47.60–116.38 FW | Martinović and Cavoski [30] |
| 32.54–157.06 FW | Jaćimović et al. [31] |
Cultivars cultivated in the Czech Republic displayed TPC content between 61 and 253 mg GAE·100 g−1 FW. Based on TPC and AA, Ekotišnovský, Fruchtal, and Ruzyňský cultivars were recommended for breeding programs [17]. Rop et al. [17] reported a significant difference between the Czech cultivar Devin, which had the lowest TPC at 261 mg GAE·100 g−1 FW, and the Ukranian cultivar Vydubieckii, which exhibited the highest TPC (811 mg GAE·100 g−1 FW).
From the territory of Serbia, Bijelić et al. [28] reported 47.60–116.38 mg GAE·100 g−1 FW and Milenković-Andjelković et al. [29] 9.89 ± 0.45 to 117.34 ± 1.40 mg of gallic acid equivalents GAE·100 g−1 extract dry matter DW. According to experiment of Martinović and Cavoski the content of TPC was reported 47.60–116.38 mg GAE·100 g−1 fresh weight FW for samples from Jaćimović et al. [31] reported 32.54–157.06 mg GAE·100 g−1 FW). Similarly, Sengul et al. [32] examined five different varieties from Turkey and determined TPC ranging from 6.53 to 10.09 μg GAE mg−1 FW. Drkenda et al. [33] reported TPC in cornelian cherry samples from Bosnia and Herzegovina ranging from 38.98 to 103.37 mg GAE·100 g−1 FW.
Total yield efficiency was negatively correlated with TPC, as demonstrated by Gunduz et al. [35]. TPC is also associated with fruit color, which is linked to anthocyanin content [24,28]. The above-mentioned variety “Szafer” with the most intense purple color thus showed the highest TPC of all varieties tested [25,34].
TPC, which correlates with anthocyanin content (and thus fruit color), is also influenced by the fruit’s maturity stage, as shown in a study by Gunduz et al. [35]. In the early stages of ripeness, yellow-colored fruits showed the lowest concentration of polyphenols (4.162 mg GAE·100 g−1 DW), while at the end of ripeness, red fruits contained the highest concentration of polyphenols (8.206 mg GAE·100 g−1 DW).
Dupak et al. [36] brought attention to the fact that polyphenolic compounds vary in different parts of the fruit. TPC in the pulp showed significantly (p < 0.001) lower concentration compared to the stone. The value of TPC in the stone was 29.608 mg GAE·100 g−1 DW, while the pulp contained only 10.204 mg GAE·100 g−1 DW.
Polyphenolic content is also affected by storage conditions. Moldovan et al. [37] observed the effect of storage temperature on the stability of polyphenolic compounds in cornelian cherry (C. mas L) in the dark at four different temperatures – 2, 22, 55, and 75°C. The results showed that the polyphenolic substances stored at 75°C had the lowest stability. According to the results of the experiments, they recommended storage for at least 2 months at room temperature, when no significant loss of bioactive substances, including polyphenols, was observed.
Tarko et al. [38] studied the effect of the extraction method on TPC. The results of the experiment showed that the best result was obtained by 80% methanol solution – TPC was 16.36 ± 0.37 mg·g−1 FW compared to 10.33 ± 0.38 mg·g−1 FW in 80% ethanol solution, 4.63 ± 0.47 mg·g−1 FW in aqueous solution and 0.07 ± 0.00 mg·g−1 FW in methylene chloride solution. Szczepaniak et al. [39] compared the TPC in ethanol and aqueous extracts of C. mas fruits. The observed values ranged from 25.98 ± 4.76 to 5.50 ± 0.66 mg GAE·g−1 DW and 7.75 ± 0.13 to 2.90 ± 0.01 mg GAE·g−1 DW, respectively.
Processing also has a significant impact on TPC. Tarko et al. [40] demonstrated that the differences in TPC in fresh fruit were 6.11 ± 0.08 mg·g−1 FW CAT equivalent, 0.75 ± 0.12 mg·ml−1 CAT equivalent in CM in wine and 1.54 ± 0.15 mg·ml−1 CAT equivalent in CM (C. mas) liqueur. De Biaggii et al. [5] presented four major polyphenolic compounds such as ellagic acid, epicatechin, CAT, and chlorogenic acid.
According to Milenković-Andjelković et al. [29], TPC is also influenced by the year of cultivation, which is shaped by climatic conditions of growing season [17].
3 Polyphenolic spectrum of C. mas fruit
The main groups of phenolic compounds found in fruits include flavonols, anthocyanins, flavan-3-ols, and phenolic acids [29].
The total phenolic content (359.28 and 343.50 mg GAE 100 g−1 FW, respectively) and flavonoid content of cornelian cherry represented (54.26 and 64.48 mg QE 100 g−1 FW, respectively) with antioxidant capacity (2.39 and 2.71 mmol Trolox 100 g−1 FW, respectively) were genotype and cultivar dependent [24].
The fresh berries from the group of polyphenols were predominated – especially ellagic acid, epicatechin, CAT, and chlorogenic acid. Cinnamic acid derivatives – ferulic acid, coumaric acid, and caffeic acid – were represented in smaller amounts [5]. Moldovan et al. [41] determined quercetin-3-O glucuronide (471 mg·100 g−1 body weight [BW]), kaempferol-3-O-galactoside (366.88 mg·100 g−1 BW), and ellagic acid as the most abundant polyphenolic compounds.
3.1 Phenolic acids
Different authors have determined different amounts of phenolic acids in cornelian cherry, ranging from 2.81 to 5.79 mg·g−1 ml in methanol extract FW [42] to 29.76–74.83 mg GAE·g−1 (gallic acid equivalent) DW [26].
Cosmulescu et al. [24] identified and quantified 10 phenolic acids (gallic, vanillic, chlorogenic, caffeic, syringic, p-coumaric, ferulic, sinapic, salicylic, ellagic, and trans-cinnamic). Ellagic acid exhibited the highest concentration among phenolic acids in amount 2.59 mg·100 g−1 FW (C. mas), which represented a significantly lower content compared to the highest source of ellagic acid studied (Hippophae rhamnoides) with a value of 15.14 mg·100 g−1 FW. The analysis also revealed high concentrations of caffeic acid in C. mas fruits (1.26 mg·100 g−1 FW), followed by Prunus padus (0.99 mg·100 g−1 FW). The less abundant cinnamic acid derivatives were represented by ferulic, coumaric, caffeic, and vescalaginic acids (about 2, 4, 1, and 5 mg·100 g−1 FW, respectively) [5].
When comparing the amount of phenolic acids in pulp and pit, a significant difference was established. The pulp contained significantly lower values (p < 0.001) compared to the pit. Cornelian cherry pulp had 6.659 mg·CAE g−1, whereas pit only had 0.621 mg CAE g−1 FW [36].
The structure of phenolic acids included gallic acid [24,43], ellagic acid [24,37,44], chlorogenic acid [33,45], neochlorogenic acid [46], 3-O-caffeoylquinic acid [44,47], p-coumaric acid [24], caffeic acid [24], protocatechuic acid [48], cinnamic acid [24,48], ferulic acid [48,24], sinapic acid [24], salicylic acid [24,49], syringic acid [24], vanillic acid [24,49], and rosmarinic acid [3,48].
Dzydzan et al. [47] demonstrated that the amount of phenolic acids is related to the variety and color of the fruit. They determined a total phenolic acid content of 21.57 mg·g−1 DW for yellow CM fruits and 12.86 mg·g−1 DW for red CM fruits.
According to Sochor et al. [45], “Devin,” “Vydubeckij,” and “Titus” were the most valuable source of chlorogenic acid (135.6, 110.9, and 115.1 mg 100 g–1 FW, respectively).
3.2 Flavonoids
In a recent study by Cosmulescu et al. [24], the total flavonoid content found in C. mas fruits was 17.27 mg QE·100 g−1 FW (quercetin equivalent). Compared to another less explored species, the order of total flavonoid content was as follows: P. padus > H. rhamnoides > Prunus spinosa > R. fruticosus > R. canina > C. monogyna > C. mas. When comparing the species, the total flavonoid content of P. padus was found to be higher compared to the other species, e.g., 9.58 times higher than the content recorded in C. mas.
Stanković et al. [50] evaluated the total flavonoid content of C. mas fruits from the Pčinja River Gorge area in southern Serbia. They found out the influence of different extraction procedures. Water extracts of C. mas fruits contained 3.53 mg·g−1 of flavonoids, while ethyl acetate extracts yielded a higher concentration of 41.49 mg·g−1 FW.
Flavonoid content is related to the timing of fruit collection, different content was investigated in early, mid, and late-ripening cultivars [17]. Levon and Klymenko [51] evaluated C. mas varieties grown in Ukraine in terms of total flavonoid content with respect to ripening time. The results of the experiment showed that among the early-ripening C. mas cultivars, the most promising were the cultivars “Pervenets” and “Volodimirskii,” among the mid-ripening C. mas cultivars the cultivars “Shajdarovoi,” “Vydubetskyi,” and “Titus,” and among the late-ripening C. mas cultivars the cultivar “Sokoline.” The flavonol content of the fruits of the early-ripening C. mas cultivars was 34–176 mg·l−1, the medium-ripening cultivars in the range 27–165 mg·100 g−1 DW, and the late-ripening cultivars in the range 36–88 mg·100 g−1 DW.
In summary, the most abundant flavonoids were quercetin [45], quercetin 3-O-robinobioside [33], quercetin 3-O-glucuronide [52,47], quercetin-3-O-xyloside [33], quercetin-3-O-rhamnoside [33,45], quercetin-3-O-glucoside [48,53], quercetin-3-O-galactoside [33,53,54], kaempferol 3-O-galactoside [53], kaempferol 3-glucoside [54,33,29], myricetin [24], myricetin 3-galactoside [48], and aromadendrin [3,53].
The following compounds predominated among flavonols: procyanidin B1 [33,54], procyanidin B2 [33], (+) CAT [29], and (−) epicatechin [37]. Using the high-performance thin-layer chromatography method, Moldovan et al. [41] reported the average amount of flavanols to be 0.67 mg g–1 FW. The flavonoid content was higher in the pulp than in the pit and anthocyanins were found only in the pulp [36]. All predominated flavonoids are summed up in Figure 1.
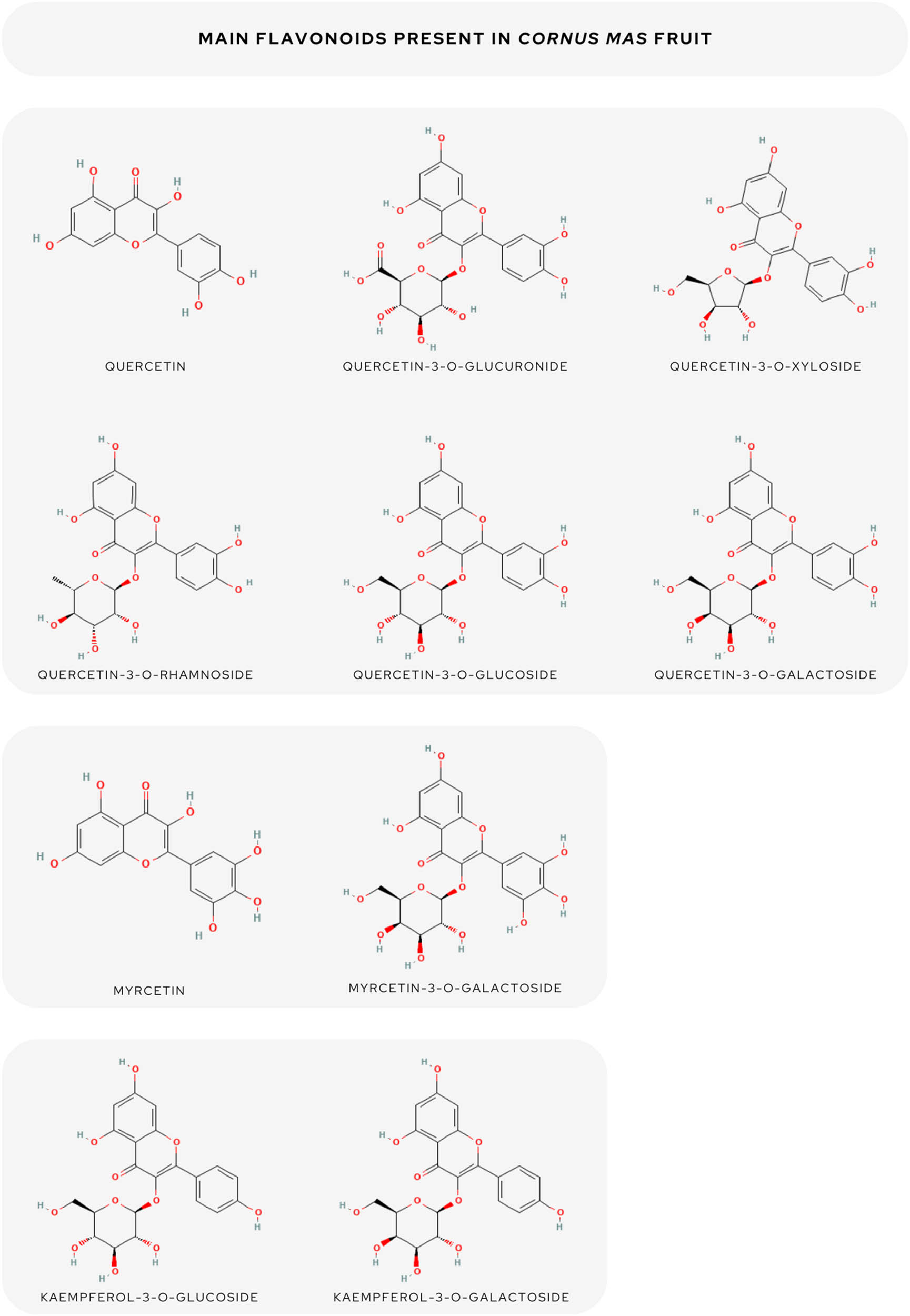
The overview of the main flavonoids of C. mas fruit.
The total flavonoid content was dependent on the extraction method. Karaaslan et al. [55] demonstrated that the acetone extract of CM fruits has the highest values of total flavonoid content. Mesgari Abbasi et al. [56] found that the mean total flavonoids are 0.07 ± 0.01% DW in 70% methanol extract of CM fruit.
According to Sochor et al. [45], “Devin,” “Vydubeckij,” and “Titus” cultivars were the most valuable source of quercetin (24.9, 25.2, and 24.2 mg·100 g−1 FW, respectively).
3.3 Anthocyanins
Total anthocyanin content in cornelian cherry has been estimated by several authors in the range of 810–2,920 mg·kg−1 FW [25,28,29,57].
The total anthocyanin content in different varieties of C. mas fruits from the National Botanical Garden of M.M. Gryshko in Ukraine ranged from 477.1 to 850.0 mg% in the skin and 7.8–190.6 mg% in the flesh FW [58]. Similarly, Milenković-Andjelković et al. [29] determined the total anthocyanin content in C. mas fruits from the Vlasina region (Serbia) by high-performance liquid chromatography with 1,383.2 mg·kg−1 FW.
Anthocyanin content varies among different fruit parts. The anthocyanin levels determined in the pulp represented 0.54 g from 2 g of total polyphenols in cornelian cherry [36].
Levon and Klymenko [51] observed that anthocyanin content is related to ripening time. The content of total anthocyanins in C. mas fruits with early ripening period ranged from 9 up to 259 mg·100 g−1 DW, with middle ripening period from 10 to 166 mg·100 g−1 DW, and with late ripening period from 33 to 96 mg·100 g−1 DW. They found a very strong correlation between the anthocyanin and flavonol content of C. mas plant fruits with the early ripening period of the fruits (r = 0.955).
Anthocyanin content also depends on the genotype. Skender et al. [12] studied the TPC in 22 local genotypes of cornelian cherry (C. mas L.) from Bosnia and Herzegovina. The results demonstrated that anthocyanin content was highly variable among genotypes and ranged from 5.57 to 205.6 mg cyanide-3-glucoside equivalents 100 g–1 FW, respectively. Similarly, Sengul et al. [32] analyzed five different genotypes of cornelian cherry grown in Turkey. The genotypes differed significantly in total anthocyanin content. The highest total anthocyanin content was recorded in red genotype 1 (342 mg·100 ml), while yellow genotypes 2, 3, 4, and 5 reached 276, 271, 239, and 262 mg·100 ml−1. The influence of cultivar on anthocyanin content was reported by Kucharska et al. [8]. According to the study, the “Czarny” variety exhibited the highest amount of anthocyanin of all varieties tested (341.18 mg 100 g−1 w/w). On the other hand, the “Jantarnyj variety” contained no detectable concentration of anthocyanin.
Moreover, anthocyanin content is influenced by origin. In samples from Turkey, anthocyanin content ranged from 0.12 to 4.2 mg·g−1 [42,26], while in Serbia, the values ranged from 0.36 to 1.27 mg·g−1 [28]. In the Czech Republic, the range was from 0.061 ± 0.007 to 0.347 ± 0.004 mg·g−1 FW [27], and in Poland, values ranging from 0.05 to 3.42 mg·g−1 FW were reported [8].
Anthocyanin content depends on the chemical composition of the fruit, particularly the organic acids. The differences in anthocyanin and organic acid concentrations between the tested varieties and genotypes were related to pH. High temperatures have a negative effect on the anthocyanin degradation process. The degradation rate of anthocyanin isolated from CM extracts was 1.8 times faster at 2°C, while the process was 172 times faster at 75°C [59].
The prevalent anthocyanidins present in cornelian cherry fruits were determined as follows: 3-O-galactosides of delphinidin (162 mg·100 g−1), cyanidin (166 mg·100 g−1), and pelargonidin; cyanidin-3-O-galactoside (3.82 mg 100 g−1 FW); as well as pelargonidin 3-O-glucoside (58.62 mg·100 g−1 FW) and 3-O-rutinoside (33.8 mg·100 g−1 FW) [1,8,41].
All identified anthocyanidins in the fruits of cornelian cherry were cyanidin 3-O-glucoside [6,48], cyanidin 3-O-robinobioside [8,47,60], cyanidin 3-O-rutinoside [32,42,57], pelargonidin 3-O-galactoside [8,61,62,63,64] pelargonidin 3-O-glucoside [6,42,48], pelargonidin 3-O-robinobioside [8,47,48], pelargonidin 3-O-rutinoside [53], peonidin-3-O-glucoside [32,33], delphinidin-3-O-galactoside [8,61], delphinidin 3-O-β-glucoside [6], and petunidin 3-glucoside [48].
The overview of anthocyanins present in C. mas fruit is given in Figure 2.

The overview of the main anthocyanidins in C. mas fruit.
4 AA
Flavonoids from the fruits of C. mas may possess beneficial health effects, especially by acting as powerful antioxidants [65].
In most of the studies, the AA of the fruits was determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical method. According to the results of Milenković-Andjelković et al. [29], 100 ml of cornelian cherry fruit extract scavenged 94–109 mg of DPPH radicals. Tural and Koca [42] observed that the methanol extracts of cornelian cherry fruit showed EC50 (mg·ml−1) values (DPPH reduction) as 0.52. Dragović-Uzelac et al. [66] found that DPPH values in two different species of cornelian cherry were as high as 33.41 and 39.89 mmol Trolox equivalent·kg−1 FW.
The final value of AA is influenced by the method of detection used, as different techniques may yield varying results. Additionally, AA is also cultivar and genotype dependent. Klymenko et al. [23] assessed AA in 20 cultivars of cornelian cherry cultivated in Ukraine by DPPH, ABTS (ABTS assay is a colorimetric assay in which the ABTS radical suffers a color decrease in the presence of antioxidants), and fluorescence recovery after photobleaching (FRAP) assays. The values AA (μmol Trolox·g−1) determined by DPPH method showed values ranging from 5.94 (“Kozerog”) to 16.56 (“Kostia”), in ABTS method varied from 13.560 (“Koralovyj Marka”) to 33.96 (“Semen”), in FRAP method ranged from 8.45 (“Koralovyj”) to 22.49 (“Kostia”). Kucharska et al. [8] studied the AA of Polish breeding varieties and determined the highest activity in the variety “Dublany” (20.72 μmol Trolox·g−1) and the lowest in the variety “Juliusz” (10.85 μmol Trolox·g−1).
The color of fruit is closely related to its chemical composition, which determines also its antioxidant properties. The cultivar “Uholok” with almost black fruits showed the highest antioxidant potential, while cultivars with pink fruits displayed the lowest potential [2]. The antioxidant capacity is genotype dependent, as demonstrated in the study of Cosmulescu et al. [24] (2.39 and 2.71 mmol Trolox 100 g−1 FW, respectively).
A study conducted by Yilmaz et al. [26] showed considerable variability in FRAP values, with a minimum of 73 and a maximum of 114 μmol of AA equivalent g−1 DW. Pantelidis et al. [67] confirmed that the FRAP value is approximately 84 μmol AA·g−1 DW.
The bioactive compounds in fruit exhibit a synergistic effect, which is reflected in the relationship between AA and chemical composition of fruit [17].
Hassanpour et al. [68] stated that the DPPH radical scavenging efficiency depends on the total concentration of polyphenolic compounds, total flavonoids, and AA (Pearson coefficients: 0.54, 0.60, and 0.47).
Radical scavenging activity (DPPH) significantly correlated with total phenolic content (R 2 = 0.9832) [29]. TPC positively correlated with FRAP (r = 0.845, F < 0.05) and DPPH assays (r = 0.706, p < 0.05). A very weak correlation (r = 0.102) was found between TPC and ABTS [23]. A positive correlation between AA and polyphenols has been demonstrated in studies by Ersoy et al. [69] and Milenković-Andjelković et al. [29]. C. mas fruit extracts exhibited strong AA, which correlated positively with the total phenolic content and phenolic compounds such as anthocyanins and flavonols, but did not correlate with the iridoid content. In C. mas cultivars, AA was dependent on fruit color; thus, the cultivar Uholok with black fruits showed the highest antioxidant potential [2].
According to Cetkovská et al. [27], the AA determined by electron paramagnetic resonance and DPPH radical scavenging assay ranged from 29.5 to 67.2%. They studied different cultivars grown in the conditions of the Czech Republic.
The antioxidant behavior significantly depends on the cultivar of the species and its genotype [13].
AA is also affected by processing and storage conditions. Storage of fruit at low temperature (freezing), the AA of fruit, liqueurs prepared from frozen fruit had a higher antioxidant capacity and dry weight than liqueurs produced in the traditional way [70]. The antioxidant capacity values of stored CM fruits were temperature dependent. Moldovan et al. [71] observed a 10% decrease in the antioxidant capacity of CM fruits stored at 2°C for 60 days. Higher losses were observed when stored at 75°C, with an average 29% reduction in total vitamin C capacity after 10 days.
5 Biological effect of fruit
The fruit of the cornelian cherry has been valued for medical properties [2]. Among frequently reported health-promoting effects, antidiabetic, antiatherogenic, anti-inflammatory, anticancer, and neuroprotective effects can be mentioned [9,18,39,72]. The health-promoting activity of the fruit summarized in Table 2 is provided by polyphenolic compounds which displayed the highest AA [13,73].
Main biological effects of C. mas L. fruits
| Biological effect | Mechanism of action | Source |
|---|---|---|
| Anti-inflammatory | Suppression of IL-1β and IL-13 | Moldovan et al. [41] |
| Reduction of IL-6 and TNF-α concentrations | Sozański et al. [74], Wójciak et al. [75] | |
| Increase in TNF-α and IL-1β secretion | Czerwińska et al. [76] | |
| Immunomodulatory activity – regulations of the Th17/Treg | Szandruk-Bender et al. [77] | |
| Decrease in C-reactive protein | Aryaeian et al. [78] | |
| Decrease in the release of NO, IL-12, and TNF | Crisan et al. [79] | |
| Antimicrobial | Supression of the propagation of Bacillus, Escherichia coli, Staphyloccocus aureus, Pseudomonas aeruginosa | Bayram and Ozturkcanm [3], Aurori et al. [7] |
| Inhibition of Staphylococcus aureus, Candida sp., and Aspergillus fumigatus (methanolic extract) | Krisch et al. [80], Krzyściak et al. [81], Turker et al. [82] | |
| Metabolic and antidiabetic effect | Inhibitory effect on α-amylase and α-glucosidase | Shishehbor et al. [83], Blagojević et al. [11], Okan et al. [43], Sip et al. [84] |
| Decrease in leptin and increase in adiponectin reduction of triglycerides | Danielewski et al. [85] | |
| Regulating insulin sensitivity in adipocytes | Małodobra et al. [14] | |
| Stimulation of the secretion of insulin by pancreatic β cells | Jayaprakasam [86] | |
| Inhibition of pancreatic lipase activity by pelargonidin 3-O-galactoside | Świerczewska et al. [64] | |
| Cardioprotective effect | Reduction in MMP-1, IL-6, and NOX mRNA expression in the aorta | Danielewski et al. [87] |
| Decrease in VCAM-1, ICAM-1, PON-1, MCP-1, and PCT serum level | ||
| Decrease in triglycerides (p < 0.001) | Moldovan et al. [88] | |
| C-reactive protein | ||
| Antiplatelet effects and reduction of platelet hyper-reactivity | Lietava et al. [89] | |
| Anticancer effect | Inhibition of breast adenocarcinoma cell line growth | Odzakovic et al. [90] |
| Cytotoxic, antiproliferative effect | Šavikin et al. [91]; Rezaei et al. [92]; Hosseini et al. [93]; Tiptiri-Kourpeti et al. [94]; Lewandowski et al. [95] | |
| Downregulation of Bcl-2 | Ji et al. [96] |
Abbreviations: TNF-α, tumor necrosis factor; IL-1β IL-13, IL-12, IL-6, interleukins; NO, nitric oxide; MMP-1, metalloproteinase-1; NOX, nitric oxidases; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1; PON-1, paraoxonase-1; MCP-1, monocyte chemotactic protein-1; PCT, procalcitonin; Bcl-2, gene – apoptosis regulator.
5.1 Anti-inflammatory effect
Extracts from cornelian cherry have been found to be potential anti-inflammatory agents in both in vitro and in vivo studies, even in situations where cornelian cherries have been processed into functional foods or traditional dishes [39,89].
The anti-inflammatory activity of cornelian cherry fruit extract was demonstrated in various models of Wister rats. The ability of the extract to suppress the levels of interleukin (IL)-1β and IL-13 [37,41] or to reduce the concentration of IL-6 and tumor necrosis factor (TNF)-α, [74] was studied. Czerwińska et al. [76] observed the effect C. mas fruit extract on reactive oxygen species generation in human neutrophils as well as cytokines secretion both in neutrophils (TNF-α, IL-8, IL-1β) and in human colon adenocarcinoma cell line Caco-2 (IL-8). The results of the experiment showed that the aqueous-ethanolic extract of C. mas fruit had a tendency to increase TNF-α and IL-1β secretion. On the other hand, the modulatory activity of C. mas extracts was observed in the case of IL-8 secretion in Caco-2 cells. Similarly, a study by Wójciak et al. [75] indicated that cornelian cherry extract suppressed the production of IL-6, IL-8, and TNF-α in human skin keratinocytes and fibroblasts. Loganoic acid and cornuside were identified as the primary compounds responsible for this effect.
Szandruk-Bender et al. [77] studied the effect of cornelian cherry (C. mas L.) extract, rich in iridoids and polyphenols, at two doses (20 or 100 mg kg−1 FW) on the crucial factors for Th17/Treg-cell differentiation in the course of experimental colitis. They demonstrated a beneficial effect of cornelian cherry extract on experimental colitis which they explained by immunomodulatory activity – regulations of the Th17/Treg developmental pathway molecules due to the high polyphenolic content in the extract. Th17/Treg promotes the development of chronic inflammatory disorders and induces autoimmune diseases.
Regular consumption of C. mas extract resulted in a decrease in highly sensitive C-reactive protein in postmenopausal women [78].
Crisan et al. [79] assessed that the effect of polyphenolic extract from cornelian cherry fruit on psoriasis displayed anti-inflammatory activity. They described the mechanism of action of silver and gold nanoparticles (GNPs) complexed with C. mas (Ag-NPs-CM, Au-NPs-CM) in psoriasis at the cellular and molecular levels. The experimental results showed that incubation of pro-inflammatory macrophages with the nanoparticles significantly reduced the release of NO (nitric oxide), IL-12, and TNF. NPs-CM (nanoparticles) appear to repress NF-B (nuclear factor-B) activation in macrophages, inhibiting the production of pro-inflammatory factors with a causal role in psoriasis.
Another clinical study on the influence of CM (C. mas) fruit extract on ameliorating lipid profile and vascular inflammation in 40 dyslipidemic children and adolescents aged 9–16 years was conducted by Asgary et al. [97]. The results demonstrated a trend toward improvement in lipid profile and vascular inflammation after adding CM to the daily diet of dyslipidemic children and adolescents.
5.2 Antimicrobial activity
The fruits are well known as an important antimicrobial agent displaying significant activity against numerous bacteria and fungi including Bacillus, Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. The majority of studies have identified methanol, aqueous, and ethanol extracts of fruits as potent antimicrobial agents [3]. Methanol extract of CM seeds inhibited S. aureus, fungi Candida, and Aspergillus fumigatus, whereas ethanol extract of CM seeds inhibited S. aureus and Candida albicans. Methanol extract of CM fruits displayed a moderate effect on S. aureus, E. coli, and P. aeruginosa, while ethanol extract of CM fruits displayed a moderate effect on E. coli and P. aeruginosa [80,81].
The 50°C ethanol extract of CM fruits has a strong antibacterial activity against S. aureus, Staphylococcus epidermidis, and Streptococcus pyogenes [82].
Significant antimicrobial activity against Gram-positive, followed by Gram-negative strains and yeasts, was observed for all tested extracts [29].
Aurori et al. [7] used the ethanolic extract of C. mas fruit to determine the antimicrobial and cytoprotective effects in vitro on renal cells stressed with gentamicin. The antimicrobial activity was investigated by agar well diffusion and broth microdilution methods, with excellent results for P. aeruginosa.
The aim of the study by Savas et al. [98] was to determine the antimicrobial effects of cornelian cherry marmalade “Garagurt.” The antimicrobial activity of the sample was evaluated by disc diffusion method at a minimum inhibitory concentration against Listeria monocytogenes, Bacillus cereus, S. aureus, E. coli, E. coli O157:H7, Salmonella typhimurium, Pseudomonas fluorescens, and Yersinia enterocolitica. The results of the study showed that the sample provided antimicrobial and AA (195 ± 6.35 mg GAE·100 g−1) in aqueous methanol extract of Garagurt. This product could be, therefore, used for its antimicrobial effect to increase the shelf life of various foods [98].
5.3 Metabolic and antidiabetic effects
The fruit of cornelian cherry can be widely used in prevention of metabolic disorders, including diabetes [99].
Shishehbor et al. [83] studied the inhibitory effects of hydroalcoholic extracts from cornelian cherry (C. mas L.) fruit extracts against pancreatic α-amylase and intestinal α-glucosidase. The results indicated that cornelian cherry was an effective inhibitor of α-glucosidase with the IC50 values of 6.87 mg·ml−1. The fruit extract was recommended for use in controlling postprandial hyperglycemia.
Capcarova et al. [100] studied the effect of orally administered C. mas fruit on the development of diabetes mellitus symptoms in ZDF rats (the Zucker fatty rat). In the experiment, male ZDF rats (fa/fa) and their age-matched non-diabetic lean controls (fa/+) were utilized. Male ZDF rats were administered two doses (500 and 1,000 mg kg−1 BW) for 10 weeks. The control group received only distilled water. The control and experimental rats were fed with normal chow – KKZ-P/M (complete feed mixture for rats and mouse, reg. no 6147, Dobra Voda, Slovak Republic) on an ad libitum basis. They found a significant decrease in glucose levels after oral administration of C. mas at a dose of 1,000 mg kg−1 BW in the prediabetic state of the animals (up to the 7th week of the experiment) and a significant reduction in water intake in both C. mas groups compared to the control. It was concluded that a higher dose of cornelian cherry could be beneficial and useful in preventing diabetic symptoms consumed regularly in young animals [100].
Cornelian cherry (C. mas L.) has been recommended as an alternative treatment for type 2 diabetes mellitus (T2DM). The effect of a polyphenolic extract from the pulp and pit of cornelian cherry was studied in ZDF (the Zucker fatty rat) rats. ZDF rats received cornelian cherry in three different doses (1,500, 2,000 mg·kg−1 BW of the pulp, and 250 mg·kg−1 BW of the stone) for 4 months. Blood glucose, insulin, AA, and compounds in cornelian cherry were monitored and measured. The experimental results revealed that cornelian cherry pit significantly reduced the affected blood glucose level in ZDF rats compared to the control group. The content of total polyphenols and phenolic acids was significantly higher in the pit of cornelian cherry than in the pulp. At the same time, high concentrations of anthocyanins were determined in the pulp [36].
Fruits of C. mas L. are valued not only for their hypoglycemic effects but also in the treatment of a very important secondary complication of T2DM – diabetic bone disease. Omelka et al. [101] studied the effect of C. mas pulp on lipid profile and bone quality parameters in Zucker diabetic rats (ZDF). They discovered that cornelian cherry pulp could be used as a potential therapeutic agent to alleviate T2DM of reduced bone quality and impaired bone health. In addition, the lipid-lowering properties of this fruit have also been identified.
Cornelian cherry fruit extract positively influences metabolism – lowering leptin levels and increasing adiponectin concentrations and lowering triglycerides (TGs), as opposed to total cholesterol (TC) and LDL in rats fed a high-fat diet [85].
Most recent literature emphasizes antidiabetic properties of C. mas fruit and products [76,102]. C. mas L. is one of the most valuable fruit plants with hypoglycemic potential. One proven mechanism of effect is based on inhibition of digestive enzymes or glucose uptake [64]. The fruits display an inhibitory effect on exocrine enzymes responsible for breaking down complex carbohydrates (α-amylase and α-glucosidase) into easily digestible simple sugars [11,43]. Sip et al. [84] offered a new approach to diabetes therapy based on α-glucosidase inhibition and the antioxidant effect resulting from the activity of the plant extract used, combined with the prebiotic effect of inulin. A study by Małodobra et al. [14] proved that cornelian cherry fruit extract can reverse insulin resistance by expression of genes involved in the transmission of the insulin signal or regulating insulin sensitivity in adipocytes.
7-O-Galloyl-d-sedoheptulose decreased the levels of advanced glycation end products-related proteins as well as some inflammation-related protein expressions in hepatic tissues [103,104]. In addition to reducing BW and hepatic lipids in rats, anthocyanins of cornelian cherry correctly alleviate the course of diabetes [86]. Moreover, anthocyanins stimulate β cells in the rodent pancreas to secrete insulin and improve glucose tolerance and insulin resistance [105]. Furthermore, anthocyanins isolated from CM fruits at a dose of 500 mg·kg−1 BW per day for 8 weeks reduced serum total TG and TC, resulting in a 24% reduction in BW [86].
The high AA of cornelian cherry fruit extract can contribute to the prevention of changes in blood cell structure that can occur in diabetes. Ethanolic extract of red and yellow CM fruits (20 mg·kg−1) administered by intraperitoneal injection for 14 days reduced FBG (area under the glycemic curve index) and increased glucose intolerance [47].
As mentioned, the fruits of C. mas possess anti-inflammatory activity, which is essential in the treatment of diabetes [106].
Świerczewska et al. [64] demonstrated that pelargonidin 3-O-galactoside isolated from CM fruits (7.5 mg·ml−1) significantly inhibited pancreatic lipase activity by 28.3 ± 1.5%. They recommended the fruits as a food for the prevention of hyperlipidemia-related diseases.
5.4 Skin
C. mas L. fruit extracts may be an effective strategy to prevent skin cell damage caused by free radicals. The aqueous glycerin extract of dried cornelian cherry has proven to be a rich source of antioxidant compounds, such as phenols and flavonoids, and is capable of scavenging free radicals in a dose-dependent manner, as demonstrated by Wójciak et al. [75]. Novel nanoparticle-based biomaterials carrying polyphenol-rich extracts (C. mas) have recently shown promising anti-inflammatory activity in psoriasis [75].
5.5 Cardioprotective effect
Sozański et al. [107] reported the cardioprotective effect of loganoic acid and anthocyanins isolated from the fruits of C. mas L. on diet-induced atherosclerosis in rabbits. Danielewski et al. [87] applied a C. mas L. fruit extract rich in iridoids and anthocyanins (at doses of 10 or 50 mg kg−1 BW) to a rabbit model of a cholesterol-rich diet. The application resulted in a significant reduction in the mRNA expression of metalloproteinase-1, IL-6, and NOX (oxidases) in the aorta and a decrease in serum levels of vascular cell adhesion molecule-1, intercellular adhesion molecule-1, paraoxonase-1, monocyte chemotactic protein-1, and procalcitonin. The best results were obtained at a dose of 50 mg kg−1 BW. They concluded that cornelian cherry fruit extract may be useful in atherogenesis-related cardiovascular diseases such as atherosclerosis or metabolic syndrome.
Moldovan et al. [88] applied GNPs functionalized with bioactive compounds extracted from C. mas L. fruits (AuNPsCM) in an experimental model of a high-fat diet and evaluated the effects of the extract on the aortic wall, but also in serum, compared to different administration of C. mas fruits (CM). The results of the study showed that AuNPsCM exhibited better effects on lipid peroxidation (p < 0.01) and TNF-α (p < 0.001) in aortic homogenates compared to the natural extract (CM). CM and AuNPsCM exhibited hypolipidemic TG (p < 0.001) and C-reactive protein (CM, p < 0.01; AuNPsCM, p < 0.001) lowering effects.
The fruits of C. mas L. show high antioxidant potential, reduce inflammatory markers, have a significant effect on the lipid spectrum (comparable to statins), reduce glycemia, and increase insulin levels. The polyphenols of cherry cornelian exhibit both direct antiplatelet effects and a reduction in platelet hyperreactivity. Clinical studies on the administration of polyphenol extract of C. mas L. fruits have shown clinically relevant reductions in TC and low-density lipoprotein, triacylglycerols, lipoproteins, improvement in inflammatory activity, and improvement in insulin secretion. C. mas fruit extract also reduced inflammatory markers – the treatment showed beneficial effects on lipid spectrum (comparable to statins), reduction in glycemia, and increase in insulin. According to the results of studies by Lietava et al. [89], fruit consumption is recommended as an effective tool for the treatment of atherosclerosis.
5.6 Anticancer activity
Consumption of wild cornelian cherry extract can have a significant anti-cancer potential, which has been suggested in samples from Macedonia. The first CC3 sample from Drinic with the highest anthocyanin content (1.40 mg CyGE·g−1 FW) inhibited free radicals (IC50DPPH = 262.19 mg·ml−1; IC50ABTS = 76.78 mg·ml−1; IC50OH center dot = 102.31 mg·ml−1) and inhibited the growth of a breast adenocarcinoma cell line (IC50MCF-7 = 1.37 mg·ml−1). The second sample CC4 from Drvar showed the highest content of total polyphenols (55.92 mg GAE·g−1 DW) and vitamin C (88.74 mg·g−1 FW) and significantly inhibited the growth of cervical epithelioid carcinoma (IC50HeLa = 0.62 mg·ml−1) and lung adenocarcinoma cell line (IC50A549 = 0.48 mg·ml−1) [90].
Recently, the cytotoxic, antiproliferative, and anticancer properties of fruits have received attention from various researchers [91,92,93].
Perde-Schrepler et al. [108] studied two different cell lines, normal keratinocytes and A431, epidermoid carcinoma GNPs (spherical between 2 and 24 nm in size) with the addition of CM extract. The experimental results recommend GNPs-CM for further testing and possible dermatological applications.
Tiptiri-Kourpeti et al. [94] demonstrated that cornelian cherry has significant AA against the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and moderate antiproliferative potential against four human cancer cell lines and one mouse cell line: MCF-7 mammary adenocarcinoma, HepG2 hepatocellular carcinoma, and Caco2, HT-29 colon adenocarcinomas, as well as CT26 murine colon carcinoma. Cell viability decreased by 40–50% after incubation with the highest concentration of juice.
Cornelian cherry fruits are characterized by high antioxidant capacity and antiproliferative activity on colon cancer cells (HT29) [11].
Lewandowski et al. [95] studied the cytotoxic effect of fruit extract isolated from C. mas L. – red-stained fruit (cultivar “Podolski”) and yellow-stained C. mas L. (“Yantarnyi” and “Flava”) on two melanoma cell lines (A375 and MeWo). Cytotoxicity was analyzed using sulforhodamine B assay and a colorimetric assay for assessing cell metabolic activity methods (sulforhodamine B assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). The results showed that both extracts from C. mas L. induced cytotoxicity in A375 and MeWo cell lines.
Ji et al. [96] determined the antitumor potential of cornelian fruit extract on major regulatory genes in renal carcinogenesis. They observed down-regulation of Bcl-2 (apoptosis regulator) expression as an anti-apoptotic gene by 4.34-fold due to the addition of the extract. In addition, messenger RNA expression of the Her2 oncogene was inhibited by a concentration of 250 μg ml−1 similar to 10-fold CME.
Aside from the health-promoting effect of polyphenols, it is also important to consider their side effects, particularly when consumed in supplement form rather than in their natural state. Using supplement form represents an uncontrolled method of treating diseases, as it often contains high doses of pure polyphenols [109].
Except for medicinal values, the cornelian cherry is valuable because it is undemanding in cultivation and able to grow in an extreme environment. Due to early ripeness, it represents a valuable source of vitamin C and another bioactive compound. It has an important landscape-forming as well as an aesthetic, rehabilitation, isolation, and cultural function [110].
6 Conclusions
Recently, C. mas has been considered a minor, forgotten, and underutilized fruit species in the modern world. However, the fruit of C. mas has been widely used in folk medicine and for the production of various drinks, syrups, gels, jams and compotes, liqueurs, and wines. Polyphenols, including flavonols, anthocyanins, flavan-3-ols, and phenolic acids, along with iridoids, largely contribute to its AA and the associated health-promoting effects such as anti-inflammatory, antidiabetic, cardioprotective, and anti-cancer properties. Research has shown that the content of polyphenolic compounds varies significantly and depends on factors such as the origin (cultivation territory), genotype, method of sample extraction, detection methodology, and processing techniques. Nowadays, there has been a growing interest in cultivating new varieties of C. mas that are rich in biologically active compounds, including polyphenols, that can have a wide range of potential applications in medicine and as functional foods. Further studies are needed to explore the mechanism of action of polyphenolic compounds of fruit as well as studies focused on their bioavailability.
-
Funding information: This article was supported by the Internal Grant Agency of Tomas Bata University in Zlin (No. IGA/FT/2025/003).
-
Author contributions: Conceptualization, investigation, resources, formal analysis, and software, S.E., L.D., and A.A.; writing – original draft preparation, T.J. and J.M.; and writing – review and editing, N.S., M.Z., J.O., and K.F.S.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Czerwińska ME, Melzig MF. Cornus mas and Cornus officinalis—analogies and differences of two medicinal plants traditionally used. Front Pharmacol. 2018;9:894.10.3389/fphar.2018.00894Search in Google Scholar PubMed PubMed Central
[2] Klymenko S, Kucharska AZ, Łętowska AS, Piórecki N, Przybylska D, Grygorieva O. Iridoids, flavonoids, and antioxidant capacity of Cornus mas, C. officinalis, and C. mas × C. officinalis fruits. Biomolecules. 2021;11:776.10.3390/biom11060776Search in Google Scholar PubMed PubMed Central
[3] Bayram HM, Ozturkcan SA. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J Funct Foods. 2020;75:104252.10.1016/j.jff.2020.104252Search in Google Scholar
[4] Jurecková Z, Divis P, Cetkovská J, Vespalcová M, Porízka J, Rezníček V. Fruit characteristics of different varieties of cornelian cherry (Cornus mas L.) cultivated in the Czech Republic. Erwerbs-Obstbau. 2021;63:143–9.10.1007/s10341-021-00551-zSearch in Google Scholar
[5] De Biaggi M, Donno D, Mellano MG, Riondato I, Rakotoniaina EN, Beccaro GL. Cornus mas (L.) fruit as a potential source of natural health-promoting compounds: physico-chemical characterisation of bioactive components. Plant Foods Hum Nutr. 2018;73:89–94.10.1007/s11130-018-0663-4Search in Google Scholar PubMed
[6] Seeram NP, Schutzki R, Chandra A, Nair MG. Characterization, quantification, and bioactivities of anthocyanins in Cornus species. J Agric Food Chem. 2002;50:2519–23.10.1021/jf0115903Search in Google Scholar PubMed
[7] Aurori M, Niculae M, Hanganu D, Pall E, Cenariu M, Vodnar DC, et al. Phytochemical profile, antioxidant, antimicrobial and cytoprotective effects of cornelian cherry (Cornus mas L.) fruit extracts. Pharmaceuticals (Basel). 2023;16:420.10.3390/ph16030420Search in Google Scholar PubMed PubMed Central
[8] Kucharska AZ, Szumny A, Sokół-Łętowska A, Piórecki N, Klymenko SV. Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J Food Comp Anal. 2015;40:95–102.10.1016/j.jfca.2014.12.016Search in Google Scholar
[9] Šimora V, Ďúranová H, Brindza J, Moncada M, Ivanišová E, Joanidis P, et al. Cornelian cherry (Cornus mas) powder as a functional ingredient for the formulation of bread loaves: physical properties, nutritional value, phytochemical composition, and sensory attributes. Foods. 2023;12:593.10.3390/foods12030593Search in Google Scholar PubMed PubMed Central
[10] Adamenko K, Kawa-Rygielska J, Kucharska AZ, Piórecki N. Characteristics of biologically active compounds in cornelian cherry meads. Molecules. 2018;23:2024.10.3390/molecules23082024Search in Google Scholar PubMed PubMed Central
[11] Blagojević B, Agić D, Serra AT, Matić S, Matovina M, Bijelić S, et al. An in vitro and in silico evaluation of bioactive potential of cornelian cherry (Cornus mas L.) extracts rich in polyphenols and iridoids. Food Chem. 2021;335:127619.10.1016/j.foodchem.2020.127619Search in Google Scholar PubMed
[12] Skender A, Hadžiabulić S, Ercisli S, Hasanbegović J, Dedić S, Almeer R, et al. Morphological and biochemical properties in fruits of naturally grown cornelian cherry (Cornus mas L.) genotypes in northwest Bosnia and Herzegovina. Sustainability. 2022;14:4579.10.3390/su14084579Search in Google Scholar
[13] Szczepaniak OM, Kobus-Cisowska J, Kusek W, Przeor M. Functional properties of cornelian cherry (Cornus mas L.): a comprehensive review. Eur Food Res Technol. 2019;245:2071–87.10.1007/s00217-019-03313-0Search in Google Scholar
[14] Małodobra M, Cierzniak A, Ryba M, Sozański T, Piórecki N, Kucharska AZ. Cornus mas L. increases glucose uptake and the expression of PPARG in insulin-resistant adipocytes. Nutrients. 2022;14:2307.10.3390/nu14112307Search in Google Scholar PubMed PubMed Central
[15] Przybylska D, Kucharska AZ, Cybulska I, Sozański T, Piorecki N, Fecka I. Cornus mas L. stones: a valuable by-product as an ellagitannin source with high antioxidant potential. Molecules. 2020;25:4646.10.3390/molecules25204646Search in Google Scholar PubMed PubMed Central
[16] Petkova NT, Ognyanov MH. Phytochemical characteristics and in vitro antioxidant activity of fresh, dried and processed fruits of cornelian cherries (Cornus mas L.). Bulg Chem Commun. 2018;50:302–7.Search in Google Scholar
[17] Rop O, Mlcek J, Kramarova D, Jurikova T. Selected cultivars of cornelian cherry (Cornus mas L.) as a new food source for human nutrition. Afr J Biotechnol. 2010;9:205–10.10.5897/AJB09.1722Search in Google Scholar
[18] Dinda B, Kyriakopoulos AM, Dinda S, Zoumpourlis V, Thomaidis NS, Velegraki A, et al. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J Ethnopharmacol. 2016;193:670–90.10.1016/j.jep.2016.09.042Search in Google Scholar PubMed
[19] Nawirska-Olszańska A, Biesiada A, Kucharska AZ, Sokół-Łętowska A. Effect of chokeberry, strawberry, and raspberry added to cornelian cherry puree on its physical and chemical composition. Zywn Nauka Technologia Jakosc. 2011;3:168–78.Search in Google Scholar
[20] Isik F, Celik I, Yilmaz Y. Effect of cornelian cherry use on physical and chemical properties of tarhana. Acad Food J. 2014;12:34–40.Search in Google Scholar
[21] Topdaş EF, Çakmakçi S, Akiroğlu K. The antioxidant activity, vitamin C contents, physical, chemical and sensory properties of ice cream supplemented with cornelian cherry (Cornus mas L.) paste. Kafkas Univ Vet Fak Derg. 2017;23:691–7.Search in Google Scholar
[22] Haghani S, Hadidi M, Pouramin S, Adinepour F, Hasiri Z, Moreno A, et al. Application of cornelian cherry (Cornus mas L.) peel in probiotic ice cream: functionality and viability during storage. Antioxidants. 2021;10:1777.10.3390/antiox10111777Search in Google Scholar PubMed PubMed Central
[23] Klymenko S, Kucharska AZ, Łętowska AS, Piórecki N. Antioxidant activities and phenolic compounds in fruits of cultivars of cornelian cherry (Cornus mas L.). Agrobiodiversity. 2019;3:484–99.Search in Google Scholar
[24] Cosmulescu S, Trandafir I, Cornescu F. Antioxidant capacity, total phenols, total flavonoids and color component of cornelian cherry (Cornus mas L.) wild genotypes. Not Bot Horti Agrob. 2019;47:390–4.10.15835/nbha47111375Search in Google Scholar
[25] Kucharska AZ, Sokół-Łętowska A, Piórecki N. Morphological, physical & chemical, and antioxidant profiles of Polish varieties of cornelian cherry fruit (Cornus mas L.). Zywn Nauka Technol Jakosc. 2011;3:78–89.10.15193/zntj/2011/76/078-089Search in Google Scholar
[26] Yilmaz KU, Ercisli S, Zengin Y, Sengul M, Kafkas EY. Preliminary characterization of cornelian cherry (Cornus mas L.) genotypes for their physicochemical properties. Food Chem. 2009;114:408–12.10.1016/j.foodchem.2008.09.055Search in Google Scholar
[27] Cetkovská J, Diviš P, Vespalcová M, Porízka J, Rezníček V. Basic nutritional properties of cornelian cherry (Cornus mas L.) cultivars grown in the Czech Republic. Acta Aliment. 2015;44:357–64.10.1556/AAlim.2014.0013Search in Google Scholar
[28] Bijelić SM, Golosin BR, Ninić Todorović JI, Cerović SB, Popović BM. Physicochemical fruit characteristics of cornelian cherry (Cornus mas L.) genotypes from Serbia. Hortic Sci. 2011;46:849–53.10.21273/HORTSCI.46.6.849Search in Google Scholar
[29] Milenković-Andjelković AS, Andjelković MZ, Radovanovic AN, Radovanovic BC, Nikolic V. Phenol composition, DPPH radical scavenging and antimicrobial activity of cornelian cherry (Cornus mas) fruit and leaf extracts. Hem Ind. 2015;69:331–7.10.2298/HEMIND140216046MSearch in Google Scholar
[30] Martinović A, Cavoski I. The exploitation of cornelian cherry (Cornus mas L.) cultivars and genotypes from Montenegro as a source of natural bioactive compounds. Food Chem. 2020;318:126549.10.1016/j.foodchem.2020.126549Search in Google Scholar PubMed
[31] Jaćimović V, Božović D, Ercisli S, Bosančić B, Necas T. Sustainable cornelian cherry production in Montenegro: importance of local genetic resources. Sustainability. 2020;12:8651.10.3390/su12208651Search in Google Scholar
[32] Sengul M, Eser Z, Ercisli S. Chemical properties and antioxidant capacity of cornelian cherry genotypes grown in Coruh Valley of Turkey. Acta Sci Pol Hortorum Cultus. 2014;13:73–82.Search in Google Scholar
[33] Drkenda P, Spahić A, Begić–Akagić A, Gaši F, Vranac A, Hudina M, et al. Pomological characteristics of some autochthonous genotypes of cornelian cherry (Cornus mas L.) in Bosnia and Herzegovina. Erwerbs-Obstbau. 2014;56:59–66.10.1007/s10341-014-0203-9Search in Google Scholar
[34] Kucharska A, Brindza J, Piórecki N, Grygorieva O. Biochemical features and medical properties of cornelian cherry (Cornus mas L.). In Scientific Proceedings of the International Network AgroBioNet “Biodiversity after the Chernobyl Accident”, Slovak University of Agriculture in Nitra. Nitra, Slovakia; 2016. p. 111–8.Search in Google Scholar
[35] Gunduz K, Saracoglu O, Ozgen M, Serce S. Antioxidant, physical and chemical characteristics of cornelian cherry fruits (Cornus mas L.) at different stages of ripeness. Acta Sci Pol Hortorum Cultus. 2013;12:59–66.Search in Google Scholar
[36] Dupak R, Jaszcza K, Kalafova A, Schneidgenova M, Ivanisova E, Tokarova K, et al. Characterization of compounds in cornelian cherry (Cornus mas L.) and its effect on interior milieu in ZD. Emir J Food Agric. 2020;32:368–75.10.9755/ejfa.2020.v32.i5.2106Search in Google Scholar
[37] Moldovan B, Popa A, David L. Effects of storage temperature on the total phenolic content of cornelian cherry (Cornus mas L.) fruits extracts. J Appl Bot Food Qual. 2016;89:208–11.Search in Google Scholar
[38] Tarko T, Duda-Chodak A, Satora P, Sroka P, Pogoń P, Machalica J. Application of principal component analysis for optimization of polyphenol extraction from alternative plant sources. J Food Nutr Res. 2017;56:61–72.Search in Google Scholar
[39] Szczepaniak OM, Ligaj M, Kobus-Cisowska J, Maciejewska P, Tichoniuk M, Szulc P. Application for novel electrochemical screening of antioxidant potential and phytochemicals in Cornus mas extracts. CYTA J Food. 2019;17:781–9.10.1080/19476337.2019.1653378Search in Google Scholar
[40] Tarko T, Duda-Chodak A, Satora P, Sroka P, Pogoń P, Machalica J. Chaenomeles japonica, Cornus mas, Morus nigra fruits characteristics and their processing potential. J Food Sci Technol. 2014;51:3934–41.10.1007/s13197-013-0963-5Search in Google Scholar PubMed PubMed Central
[41] Moldovan B, Filip A, Clichici S, Suharoschi R, Bolfa P, David L. Antioxidant activity of cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J Funct Foods. 2016;26:77–87.10.1016/j.jff.2016.07.004Search in Google Scholar
[42] Tural S, Koca I. Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci Hortic. 2008;116:362–6.10.1016/j.scienta.2008.02.003Search in Google Scholar
[43] Okan OT, Serencam H, Baltaş N, Can Z. Some edible forest fruits, their in vitro antioxidant activities, phenolic compounds and some enzyme inhibition effects. Fresenius Env Bull. 2019;28:6090–8.Search in Google Scholar
[44] Natić M, Pavlović A, Bosco FL, Stanisavljević N, Zagorac DD, Akšić MF, et al. Nutraceutical properties and phytochemical characterization of wild Serbian fruits. Eur Food Res Technol. 2019;245:469–78.10.1007/s00217-018-3178-1Search in Google Scholar
[45] Sochor J, Jurikova T, Ercisli S, Mlcek J, Baron M, Balla S, et al. Characterization of cornelian cherry (Cornus mas L.) genotypes—genetic resources for food production in Czech Republic. Genetika (Belgrade). 2014;46:915–24.10.2298/GENSR1403915SSearch in Google Scholar
[46] Bajić-Ljubičić J, Popović Z, Matić R, Bojović S. Selected phenolic compounds in fruits of wild growing Cornus mas L. Indian J Tradit Knowl. 2018;17:91–6.Search in Google Scholar
[47] Dzydzan O, Bila I, Kucharska AZ, Brodyak I, Sybirna N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019;10:6459–72.10.1039/C9FO00515CSearch in Google Scholar
[48] Antolak H, Czyżowska A, Sakač M, Mišan A, Đuragić O, Kregiel D. Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria Asaia spp. Molecules. 2017;22:1256.10.3390/molecules22081256Search in Google Scholar PubMed PubMed Central
[49] Krivoruchko EV. Carboxylic acids from Cornus mas. Chem Nat Compd. 2014;50:112–3.10.1007/s10600-014-0879-ySearch in Google Scholar
[50] Stanković MS, Zia-ul-Haq M, Bojović BM, Topuzović MD. Total phenolics, flavonoid content and antioxidant power of leaf, flower, and fruits from cornelian cherry (Cornus mas L.). Bulg J Agric Sci. 2014;20:358–63.Search in Google Scholar
[51] Levon V, Klymenko S. Content of anthocyanins and flavonols in the fruits of Cornus spp. Agrobiodivers. 2021;5:7–19.10.15414/ainhlq.2021.0002Search in Google Scholar
[52] Popović Z, Matić R, Bajić-Ljubičić J, Tešević V, Bojović S. Geographic variability of selected phenolic compounds in fresh berries of two Cornus species. Trees. 2018;32:203–14.10.1007/s00468-017-1624-5Search in Google Scholar
[53] Pawlowska AM, Camangi F, Braca A. Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem. 2010;119:1257–61.10.1016/j.foodchem.2009.07.063Search in Google Scholar
[54] Begić-Akagić A, Drkenda P, Vranac A, Orazem P, Hudina M. Influence of growing region and storage time on phenolic profile of cornelian cherry jam and fruit. Eur J Hortic Sci. 2013;78:30–9.10.1079/ejhs.2013/3687717Search in Google Scholar
[55] Karaaslan MG, Karaaslan NM, Ates B. Investigation of mineral components and antioxidant properties of a healthy red fruit: Cornelian cherry (Cornus mas L.). J Turk Chem Soc Sect A: Chem. 2018;5:1319–26.10.18596/jotcsa.449593Search in Google Scholar
[56] Mesgari Abbasi M, Hassanalilou T, Khordadmehr M, Mohammadzadeh Vardin A, Behroozi Kohlan A, Khalili L. Effects of Cornus mas fruit hydro-methanolic extract on liver antioxidants and histopathologic changes induced by cisplatin in rats. Indian J Clin Biochem. 2020;35:218–24.10.1007/s12291-018-0809-zSearch in Google Scholar PubMed PubMed Central
[57] Capanoglu E, Boyacioglu D, De Vos RCH, Hall RD, Beekwilder J. Procyanidins in fruit from sour cherry (Prunus cerasus) differ strongly in chain length from those in laurel cherry (Prunus laurocerasus) and cornelian cherry (Cornus mas). J Berry Res. 2011;1:137–46.10.3233/BR-2011-015Search in Google Scholar
[58] Klymenko SV. Malorasprostranennye plodovye rasteniya kak lekarstvennye [Sparsely distributed fruit plants as medicinal plants]. Introdukciia Rosl. 2001;3–4:37–44.Search in Google Scholar
[59] Moldovan B, David L. Influence of temperature and preserving agents on the stability of cornelian cherries anthocyanins. Molecules. 2014;19:8177–88.10.3390/molecules19068177Search in Google Scholar PubMed PubMed Central
[60] Szumny D, Sozański T, Kucharska AZ, Dziewiszek W, Piórecki N, Magdalan J, et al. Application of cornelian cherry iridoid-polyphenolic fraction and loganic acid to reduce intraocular pressure. Evid Based Complement Altern Med. 2015;2015:939402.10.1155/2015/939402Search in Google Scholar PubMed PubMed Central
[61] Sozański T, Kucharska AZ, Szumny A, Magdalan J, Bielska K, Merwid-Lad A, et al. The protective effect of the Cornus mas fruits (cornelian cherry) on hypertriglyceridemia and atherosclerosis through PPARα activation in hypercholesterolemic rabbits. Phytomedicine. 2014;21:1774–84.10.1016/j.phymed.2014.09.005Search in Google Scholar PubMed
[62] Perova IB, Zhogova AA, Poliakov AV, Eller KI, Ramenskaia GV, Samylina IA. Biologically active substances of cornelian cherry fruits (Cornus mas L.). Voprosy Pitaniia. 2014;83:86–94.Search in Google Scholar
[63] Ochmian I, Oszmiański J, Lachowicz S, Krupa-Małkiewicz M. Rootstock effect on physico-chemical properties and content of bioactive compounds of four cultivars cornelian cherry fruits. Sci Hortic. 2019;256:108588.10.1016/j.scienta.2019.108588Search in Google Scholar
[64] Świerczewska A, Buchholz T, Melzig MF, Czerwińska ME. In vitro α-amylase and pancreatic lipase inhibitory activity of Cornus mas L. and Cornus alba L. fruit extracts. J Food Drug Anal. 2019;27:249–58.10.1016/j.jfda.2018.06.005Search in Google Scholar PubMed PubMed Central
[65] Moldovan B, David L. Bioactive flavonoids from Cornus mas L. fruits. Mini-Rev Org Chem. 2017;14:489–95.10.2174/1573398X13666170426102809Search in Google Scholar
[66] Dragović-Uzelac V, Levaj B, Bursa D, Pedis S, Radojičić R, Biško A, et al. Total phenolics and antioxidant capacity assays of selected fruits. Agri Conspec Sci. 2007;72:279–84.Search in Google Scholar
[67] Pantelidis GE, Vasilakakis M, Manganaris GA, Diamantidis GR. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and cornelian cherries. Food Chem. 2007;102:777–83.10.1016/j.foodchem.2006.06.021Search in Google Scholar
[68] Hassanpour H, Yousef H, Jafar H, Mohammad A. Antioxidant capacity and phytochemical properties of cornelian cherry (Cornus mas L.) genotypes in Iran. Sci Hortic. 2011;129:459–63.10.1016/j.scienta.2011.04.017Search in Google Scholar
[69] Ersoy N, Bagci Y, Gok V. Antioxidant properties of 12 cornelian cherry fruit types (Cornus mas L.) selected from Turkey. Sci Res Essays. 2011;6:98–102.Search in Google Scholar
[70] Szczepaniak OM, Kobus-Cisowska J, Kmiecik D. Freezing enhances the phytocompound content in cornelian cherry (Cornus mas L.) liqueur. Czech J Food Sci. 2020;38:259–63.10.17221/13/2020-CJFSSearch in Google Scholar
[71] Moldovan B, David L, Man SC. Impact of thermal treatment on the antioxidant activity of cornelian cherries extract. Studia Chem. 2017;62:311–7.10.24193/subbchem.2017.2.24Search in Google Scholar
[72] Rosseini FS, Karimabad MN, Hajizadeh MR, Khoshdel A, Falahati-Pour SK, Mirzaei MR, et al. Evaluating of induction of apoptosis by Cornus mas L. extract in the gastric carcinoma cell line (AGS). Asian Pac J Cancer Prev. 2019;20:123.Search in Google Scholar
[73] Kazimierski M, Regula J, Molska M. Cornelian cherry (Cornus mas L.) - characteristics, nutritional and pro-health properties. Acta Sci Pol Technol Aliment. 2019;18:5–12.10.17306/J.AFS.2019.0628Search in Google Scholar
[74] Sozański T, Kucharska AZ, Rapak A, Szumny D, Trocha M, Merwid-Lad A, et al. Iridoid-loganic acid versus anthocyanins from the Cornus mas fruits (Cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis. 2016;254:151–60.10.1016/j.atherosclerosis.2016.10.001Search in Google Scholar PubMed
[75] Wójciak M, Zagórska-Dziok M, Nizioł-Łukaszewska Z, Ziemlewska A, Furman-Toczek D, Szczepanek D, et al. In vitro evaluation of anti-inflammatory and protective potential of an extract from Cornus mas L. fruit against H₂O₂-induced oxidative stress in human skin keratinocytes and fibroblasts. Int J Mol Sci. 2022;23:13755.10.3390/ijms232213755Search in Google Scholar PubMed PubMed Central
[76] Czerwińska ME, Bobińska A, Cichocka K, Buchholz T, Woliński K, Melzig MF. Cornus mas and Cornus officinalis - A comparison of antioxidant and immunomodulatory activities of standardized fruit extracts in human neutrophils and Caco-2 models. Plants. 2021;10:2347.10.3390/plants10112347Search in Google Scholar PubMed PubMed Central
[77] Szandruk-Bender M, Nowak B, Merwid-Ląd A, Kucharska AZ, Krzystek-Korpacka M, Bednarz-Misa I, et al. Cornus mas L. extract targets the specific molecules of the Th17/Treg developmental pathway in TNBS-induced experimental colitis in rats. Molecules. 2023;28:3034.10.3390/molecules28073034Search in Google Scholar PubMed PubMed Central
[78] Aryaeian N, Amiri F, Rahideh ST, Abolghasemi J, Jazayeri S, Gholamrezayi A, et al. The effect of Cornus mas extract consumption on bone biomarkers and inflammation in postmenopausal women: A randomized clinical trial. Phytother Res. 2021;35:4425–32.10.1002/ptr.7143Search in Google Scholar PubMed
[79] Crisan D, Scharffetter-Kochanek K, Crisan M, Schatz S, Hainzl A, et al. Topical silver and gold nanoparticles complexed with Cornus mas suppress inflammation in human psoriasis plaques by inhibiting NF-κB activity. Exp Dermatol. 2018;27:1166–9.10.1111/exd.13707Search in Google Scholar PubMed
[80] Krisch J, Galgóczy L, Tölgyesi M, Papp T, Vágvölgyi C. Effect of fruit juices and pomace extracts on the growth of Gram-positive and Gram-negative bacteria. Acta Biol Szeged. 2008;52:267–70.Search in Google Scholar
[81] Krzyściak P, Krośniak M, Gąstoł M, Ochońska D, Krzyściak W. Antimicrobial activity of Cornelian cherry (Cornus mas L.). Postępy Fitoter. 2011;4:227–31.Search in Google Scholar
[82] Turker AU, Yildirim AB, Karakas FP. Antibacterial and antitumor activities of some wild fruits grown in Turkey. Biotechnol Biotechnol Equip. 2012;26:2765–72.10.5504/BBEQ.2011.0156Search in Google Scholar
[83] Shishehbor F, Azemi ME, Zameni D, Saki A. Inhibitory effect of hydroalcoholic extracts of barberry, sour cherry and Cornelian cherry on α-amylase and α-glucosidase activities. Int J Pharm Res Allied Sci. 2016;5:423–8.Search in Google Scholar
[84] Sip S, Szymanowska D, Chanaj-Kaczmarek J, Skalicka-Woźniak K, Budzyńska B, Wronikowska-Denysiuk O, et al. Potential for prebiotic stabilized Cornus mas L. lyophilized extract in the prophylaxis of diabetes mellitus in streptozotocin diabetic rats. Antioxidants. 2022;11:380.10.3390/antiox11020380Search in Google Scholar PubMed PubMed Central
[85] Danielewski M, Kucharska AZ, Matuszewska A, Rapak A, Gomułkiewicz A, Dzimira S, et al. Cornelian cherry (Cornus mas L.) iridoid and anthocyanin extract enhances PPAR-α, PPAR-γ expression and reduces I/M ratio in aorta, increases LXR-α expression and alters adipokines and triglycerides levels in cholesterol-rich diet rabbit model. Nutrients. 2020;13:3621.10.3390/nu13103621Search in Google Scholar PubMed PubMed Central
[86] Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J Agric Food Chem. 2006;54:243–8.10.1021/jf0520342Search in Google Scholar PubMed
[87] Danielewski M, Gomułkiewicz A, Kucharska AZ, Matuszewska A, Nowak B, Piórecki N, et al. Cornelian cherry (Cornus mas L.) Iridoid and anthocyanin-rich extract reduces various oxidation, inflammation, and adhesion markers in a cholesterol-rich diet rabbit model. Int J Mol Sci. 2020;24:3890.10.3390/ijms24043890Search in Google Scholar PubMed PubMed Central
[88] Moldovan R, Mitrea DR, Florea A, Chiş IC, Suciu Ş, David L, et al. Effects of gold nanoparticles functionalized with bioactive compounds from Cornus mas fruit on aorta ultrastructural and biochemical changes in rats on a hyperlipid diet—A preliminary study. Antioxidants. 2022;11:1343.10.3390/antiox11071343Search in Google Scholar PubMed PubMed Central
[89] Lietava J, Beerova N, Klymenko SV, Panghyova E, Varga I, Pechanova O. Effects of Cornelian cherry on atherosclerosis and its risk factors. Oxid Med Cell Longev. 2019;2515270.10.1155/2019/2515270Search in Google Scholar PubMed PubMed Central
[90] Odzakovic B, Sailovic P, Bodroza D, Kojic V, Jakimov D, Kukric Z. Bioactive components and antioxidant, antiproliferative, and antihyperglycemic activities of wild Cornelian cherry (Cornus mas L.). Maced J Chem Chem Eng. 2021;40:221–30.10.20450/mjcce.2021.2417Search in Google Scholar
[91] Šavikin K, Zdunić G, Janković T, Stanojković T, Juranić Z, Menković N, et al. In vitro cytotoxic and antioxidative activity of Cornus mas and Cotinus coggygria. Nat Prod Res. 2009;23:1731–9.10.1080/14786410802267650Search in Google Scholar PubMed
[92] Rezaei F, Shokrzadeh M, Majd A, Nezhadsattari T. Cytotoxic effect of hydroalcoholic extract of Cornus mas L. fruit on MCF7, HepG2 and CHO cell line by MTT assay. J Maz Univ Med Sci. 2014;24:130–8.Search in Google Scholar
[93] Hosseini FS, Noroozi Karimabad M, Hajizadeh MR, Khoshdel A, Khanamani Falahati-Pour S, Reza Mirzaei M, et al. Evaluating of induction of apoptosis by Cornus mas L. extract in the gastric carcinoma cell line (AGS). Asian Pac J Cancer Prev. 2019;20(1):123–30.10.31557/APJCP.2019.20.1.123Search in Google Scholar PubMed PubMed Central
[94] Tiptiri-Kourpeti A, Fitsiou E, Spyridopoulou K, Vasileiadis S, Iliopoulos C, Galanis A, et al. Evaluation of antioxidant and antiproliferative properties of Cornus mas L. fruit juice. Antioxidants. 2019;8:377.10.3390/antiox8090377Search in Google Scholar PubMed PubMed Central
[95] Lewandowski Ł, Bednarz-Misa I, Kucharska AZ, Kubiak A, Kasprzyk P, Sozański T, et al. Cornelian cherry (Cornus mas L.) extracts exert cytotoxicity in two selected melanoma cell lines—A factorial analysis of time-dependent alterations in values obtained with SRB and MTT assays. Molecules. 2022;27:4193.10.3390/molecules27134193Search in Google Scholar PubMed PubMed Central
[96] Ji J, Hou SF, Gao Y, Feng XL, Li S. Transcriptional expression of Bcl-2, Her2, VEGF, and hTERT in Caki-1 human renal cancer cells modulated by Cornus mas extract. Iran Red Crescent Med J. 2021;22.Search in Google Scholar
[97] Asgary S, Kelishadi R, Rafieian-Kopaei M, Najafi S, Najafi M, Sahebkar A. Investigation of the lipid-modifying and anti-inflammatory effects of Cornus mas L. supplementation on dyslipidemic children and adolescents. Pediatr Cardiol. 2013;34:1729–35.10.1007/s00246-013-0693-5Search in Google Scholar PubMed
[98] Savas E, Tavsanli H, Çatalkaya G, Çapanoglu E, Tamer CE. The antimicrobial and antioxidant properties of garagurt: traditional Cornelian cherry (Cornus mas) marmalade. Qual Assur Saf Crop Foods. 2020;12:12–23.10.15586/qas.v12i2.627Search in Google Scholar
[99] Olędzka A, Cichocka K, Woliński K, Melzig MF, Czerwińska ME. Potentially bio-accessible metabolites from an extract of Cornus mas fruit after gastrointestinal digestion in vitro and gut microbiota ex vivo treatment. Nutrients. 2022;14:2287.10.3390/nu14112287Search in Google Scholar PubMed PubMed Central
[100] Capcarova M, Kalafova A, Schwarzova M, Schneidgenova M, Svik K, Prnova MS, et al. Cornelian cherry fruit improves glycaemia and manifestations of diabetes in obese Zucker diabetic fatty rats. Res Vet Sci. 2019;126:118–23.10.1016/j.rvsc.2019.08.024Search in Google Scholar PubMed
[101] Omelka R, Blahova J, Kovacova V, Babikova M, Mondockova V, Kalafova A, et al. Cornelian cherry pulp has beneficial impact on dyslipidemia and reduced bone quality in Zucker diabetic fatty rats. Animals. 2020;10(12):2435.10.3390/ani10122435Search in Google Scholar PubMed PubMed Central
[102] Popović BM, Štajner D, Karaman S, Boza S. Antioxidant capacity of Cornelian cherry (Cornus mas L.)—comparison between permanganate reducing antioxidant capacity and other antioxidant methods. Food Chem. 2012;134:734–41.10.1016/j.foodchem.2012.02.170Search in Google Scholar PubMed
[103] Yokozawa T, Kang KS, Park CH, Noh JS, Yamabe N, Shibahara N, et al. Bioactive constituents of Corni Fructus: The therapeutic use of morroniside, loganin, and 7-O-galloyl-D-sedoheptulose as renoprotective agents in type 2 diabetes. Drug Discov Ther. 2010;4:223–34.Search in Google Scholar
[104] Park CH, Noh JS, Tanaka T, Roh SS, Lee JC, Yokozawa T. Polyphenol isolated from Corni Fructus, 7-O-galloyl-D-sedoheptulose, modulates advanced glycation endproduct-related pathway in type 2 diabetic db/db mice. Arch Pharm Res. 2015;38:1270–80.10.1007/s12272-014-0457-7Search in Google Scholar PubMed
[105] Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28–31.10.1021/jf049018+Search in Google Scholar PubMed
[106] Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, et al. The role of inflammation in diabetes: Current concepts and future perspectives. Eur Cardiol. 2019;14:50–9.10.15420/ecr.2018.33.1Search in Google Scholar PubMed PubMed Central
[107] Sozański T, Kucharska AZ, Dzimira S, Magdalan J, Szumny D, Matuszewska A, et al. Loganic acid and anthocyanins from Cornelian cherry (Cornus mas L.) fruits modulate diet-induced atherosclerosis and redox status in rabbits. Adv Clin Exp Med. 2018;27:1505–13.10.17219/acem/74638Search in Google Scholar PubMed
[108] Perde-Schrepler M, David L, Olenic L, Potara M, Fischer-Fodor E, Virag P, et al. Gold nanoparticles synthesized with a polyphenols-rich extract from Cornelian cherry (Cornus mas) fruits: Effects on human skin cells. J Nanomater. 2016;6986370.10.1155/2016/6986370Search in Google Scholar
[109] Duda-Chodak A, Tarko T. Possible side effects of polyphenols and their interactions with medicines. Molecules. 2023;28(6):2536.10.3390/molecules28062536Search in Google Scholar PubMed PubMed Central
[110] Dokoupil L, Řezníček V. Production and use of the Cornelian cherry – Cornus mas L. Acta Univ Agric Silv Mendel Brun. 2012;60(8):49–58.10.11118/actaun201260080049Search in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”
Articles in the same Issue
- Biomedical Sciences
- Mechanism of triptolide regulating proliferation and apoptosis of hepatoma cells by inhibiting JAK/STAT pathway
- Maslinic acid improves mitochondrial function and inhibits oxidative stress and autophagy in human gastric smooth muscle cells
- Comparative analysis of inflammatory biomarkers for the diagnosis of neonatal sepsis: IL-6, IL-8, SAA, CRP, and PCT
- Post-pandemic insights on COVID-19 and premature ovarian insufficiency
- Proteome differences of dental stem cells between permanent and deciduous teeth by data-independent acquisition proteomics
- Optimizing a modified cetyltrimethylammonium bromide protocol for fungal DNA extraction: Insights from multilocus gene amplification
- Preliminary analysis of the role of small hepatitis B surface proteins mutations in the pathogenesis of occult hepatitis B infection via the endoplasmic reticulum stress-induced UPR-ERAD pathway
- Efficacy of alginate-coated gold nanoparticles against antibiotics-resistant Staphylococcus and Streptococcus pathogens of acne origins
- Battling COVID-19 leveraging nanobiotechnology: Gold and silver nanoparticle–B-escin conjugates as SARS-CoV-2 inhibitors
- Neurodegenerative diseases and neuroinflammation-induced apoptosis
- Impact of fracture fixation surgery on cognitive function and the gut microbiota in mice with a history of stroke
- COLEC10: A potential tumor suppressor and prognostic biomarker in hepatocellular carcinoma through modulation of EMT and PI3K-AKT pathways
- High-temperature requirement serine protease A2 inhibitor UCF-101 ameliorates damaged neurons in traumatic brain-injured rats by the AMPK/NF-κB pathway
- SIK1 inhibits IL-1β-stimulated cartilage apoptosis and inflammation in vitro through the CRTC2/CREB1 signaling
- Rutin–chitooligosaccharide complex: Comprehensive evaluation of its anti-inflammatory and analgesic properties in vitro and in vivo
- Knockdown of Aurora kinase B alleviates high glucose-triggered trophoblast cells damage and inflammation during gestational diabetes
- Calcium-sensing receptors promoted Homer1 expression and osteogenic differentiation in bone marrow mesenchymal stem cells
- ABI3BP can inhibit the proliferation, invasion, and epithelial–mesenchymal transition of non-small-cell lung cancer cells
- Changes in blood glucose and metabolism in hyperuricemia mice
- Rapid detection of the GJB2 c.235delC mutation based on CRISPR-Cas13a combined with lateral flow dipstick
- IL-11 promotes Ang II-induced autophagy inhibition and mitochondrial dysfunction in atrial fibroblasts
- Short-chain fatty acid attenuates intestinal inflammation by regulation of gut microbial composition in antibiotic-associated diarrhea
- Application of metagenomic next-generation sequencing in the diagnosis of pathogens in patients with diabetes complicated by community-acquired pneumonia
- NAT10 promotes radiotherapy resistance in non-small cell lung cancer by regulating KPNB1-mediated PD-L1 nuclear translocation
- Phytol-mixed micelles alleviate dexamethasone-induced osteoporosis in zebrafish: Activation of the MMP3–OPN–MAPK pathway-mediating bone remodeling
- Association between TGF-β1 and β-catenin expression in the vaginal wall of patients with pelvic organ prolapse
- Primary pleomorphic liposarcoma involving bilateral ovaries: Case report and literature review
- Effects of de novo donor-specific Class I and II antibodies on graft outcomes after liver transplantation: A pilot cohort study
- Sleep architecture in Alzheimer’s disease continuum: The deep sleep question
- Ephedra fragilis plant extract: A groundbreaking corrosion inhibitor for mild steel in acidic environments – electrochemical, EDX, DFT, and Monte Carlo studies
- Langerhans cell histiocytosis in an adult patient with upper jaw and pulmonary involvement: A case report
- Inhibition of mast cell activation by Jaranol-targeted Pirin ameliorates allergic responses in mouse allergic rhinitis
- Aeromonas veronii-induced septic arthritis of the hip in a child with acute lymphoblastic leukemia
- Clusterin activates the heat shock response via the PI3K/Akt pathway to protect cardiomyocytes from high-temperature-induced apoptosis
- Research progress on fecal microbiota transplantation in tumor prevention and treatment
- Low-pressure exposure influences the development of HAPE
- Stigmasterol alleviates endplate chondrocyte degeneration through inducing mitophagy by enhancing PINK1 mRNA acetylation via the ESR1/NAT10 axis
- AKAP12, mediated by transcription factor 21, inhibits cell proliferation, metastasis, and glycolysis in lung squamous cell carcinoma
- Association between PAX9 or MSX1 gene polymorphism and tooth agenesis risk: A meta-analysis
- A case of bloodstream infection caused by Neisseria gonorrhoeae
- Case of nasopharyngeal tuberculosis complicated with cervical lymph node and pulmonary tuberculosis
- p-Cymene inhibits pro-fibrotic and inflammatory mediators to prevent hepatic dysfunction
- GFPT2 promotes paclitaxel resistance in epithelial ovarian cancer cells via activating NF-κB signaling pathway
- Transfer RNA-derived fragment tRF-36 modulates varicose vein progression via human vascular smooth muscle cell Notch signaling
- RTA-408 attenuates the hepatic ischemia reperfusion injury in mice possibly by activating the Nrf2/HO-1 signaling pathway
- Decreased serum TIMP4 levels in patients with rheumatoid arthritis
- Sirt1 protects lupus nephritis by inhibiting the NLRP3 signaling pathway in human glomerular mesangial cells
- Sodium butyrate aids brain injury repair in neonatal rats
- Interaction of MTHFR polymorphism with PAX1 methylation in cervical cancer
- Convallatoxin inhibits proliferation and angiogenesis of glioma cells via regulating JAK/STAT3 pathway
- The effect of the PKR inhibitor, 2-aminopurine, on the replication of influenza A virus, and segment 8 mRNA splicing
- Effects of Ire1 gene on virulence and pathogenicity of Candida albicans
- Small cell lung cancer with small intestinal metastasis: Case report and literature review
- GRB14: A prognostic biomarker driving tumor progression in gastric cancer through the PI3K/AKT signaling pathway by interacting with COBLL1
- 15-Lipoxygenase-2 deficiency induces foam cell formation that can be restored by salidroside through the inhibition of arachidonic acid effects
- FTO alleviated the diabetic nephropathy progression by regulating the N6-methyladenosine levels of DACT1
- Clinical relevance of inflammatory markers in the evaluation of severity of ulcerative colitis: A retrospective study
- Zinc valproic acid complex promotes osteoblast differentiation and exhibits anti-osteoporotic potential
- Primary pulmonary synovial sarcoma in the bronchial cavity: A case report
- Metagenomic next-generation sequencing of alveolar lavage fluid improves the detection of pulmonary infection
- Uterine tumor resembling ovarian sex cord tumor with extensive rhabdoid differentiation: A case report
- Genomic analysis of a novel ST11(PR34365) Clostridioides difficile strain isolated from the human fecal of a CDI patient in Guizhou, China
- Effects of tiered cardiac rehabilitation on CRP, TNF-α, and physical endurance in older adults with coronary heart disease
- Changes in T-lymphocyte subpopulations in patients with colorectal cancer before and after acupoint catgut embedding acupuncture observation
- Modulating the tumor microenvironment: The role of traditional Chinese medicine in improving lung cancer treatment
- Alterations of metabolites related to microbiota–gut–brain axis in plasma of colon cancer, esophageal cancer, stomach cancer, and lung cancer patients
- Research on individualized drug sensitivity detection technology based on bio-3D printing technology for precision treatment of gastrointestinal stromal tumors
- CEBPB promotes ulcerative colitis-associated colorectal cancer by stimulating tumor growth and activating the NF-κB/STAT3 signaling pathway
- Oncolytic bacteria: A revolutionary approach to cancer therapy
- A de novo meningioma with rapid growth: A possible malignancy imposter?
- Diagnosis of secondary tuberculosis infection in an asymptomatic elderly with cancer using next-generation sequencing: Case report
- Hesperidin and its zinc(ii) complex enhance osteoblast differentiation and bone formation: In vitro and in vivo evaluations
- Research progress on the regulation of autophagy in cardiovascular diseases by chemokines
- Anti-arthritic, immunomodulatory, and inflammatory regulation by the benzimidazole derivative BMZ-AD: Insights from an FCA-induced rat model
- Immunoassay for pyruvate kinase M1/2 as an Alzheimer’s biomarker in CSF
- The role of HDAC11 in age-related hearing loss: Mechanisms and therapeutic implications
- Evaluation and application analysis of animal models of PIPNP based on data mining
- Therapeutic approaches for liver fibrosis/cirrhosis by targeting pyroptosis
- Fabrication of zinc oxide nanoparticles using Ruellia tuberosa leaf extract induces apoptosis through P53 and STAT3 signalling pathways in prostate cancer cells
- Haplo-hematopoietic stem cell transplantation and immunoradiotherapy for severe aplastic anemia complicated with nasopharyngeal carcinoma: A case report
- Modulation of the KEAP1-NRF2 pathway by Erianin: A novel approach to reduce psoriasiform inflammation and inflammatory signaling
- The expression of epidermal growth factor receptor 2 and its relationship with tumor-infiltrating lymphocytes and clinical pathological features in breast cancer patients
- Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights
- BAP1 complexes with YY1 and RBBP7 and its downstream targets in ccRCC cells
- Hypereosinophilic syndrome with elevated IgG4 and T-cell clonality: A report of two cases
- Electroacupuncture alleviates sciatic nerve injury in sciatica rats by regulating BDNF and NGF levels, myelin sheath degradation, and autophagy
- Polydatin prevents cholesterol gallstone formation by regulating cholesterol metabolism via PPAR-γ signaling
- RNF144A and RNF144B: Important molecules for health
- Analysis of the detection rate and related factors of thyroid nodules in the healthy population
- Artesunate inhibits hepatocellular carcinoma cell migration and invasion through OGA-mediated O-GlcNAcylation of ZEB1
- Endovascular management of post-pancreatectomy hemorrhage caused by a hepatic artery pseudoaneurysm: Case report and review of the literature
- Efficacy and safety of anti-PD-1/PD-L1 antibodies in patients with relapsed refractory diffuse large B-cell lymphoma: A meta-analysis
- SATB2 promotes humeral fracture healing in rats by activating the PI3K/AKT pathway
- Overexpression of the ferroptosis-related gene, NFS1, corresponds to gastric cancer growth and tumor immune infiltration
- Understanding risk factors and prognosis in diabetic foot ulcers
- Atractylenolide I alleviates the experimental allergic response in mice by suppressing TLR4/NF-kB/NLRP3 signalling
- FBXO31 inhibits the stemness characteristics of CD147 (+) melanoma stem cells
- Immune molecule diagnostics in colorectal cancer: CCL2 and CXCL11
- Inhibiting CXCR6 promotes senescence of activated hepatic stellate cells with limited proinflammatory SASP to attenuate hepatic fibrosis
- Cadmium toxicity, health risk and its remediation using low-cost biochar adsorbents
- Pulmonary cryptococcosis with headache as the first presentation: A case report
- Solitary pulmonary metastasis with cystic airspaces in colon cancer: A rare case report
- RUNX1 promotes denervation-induced muscle atrophy by activating the JUNB/NF-κB pathway and driving M1 macrophage polarization
- Morphometric analysis and immunobiological investigation of Indigofera oblongifolia on the infected lung with Plasmodium chabaudi
- The NuA4/TIP60 histone-modifying complex and Hr78 modulate the Lobe2 mutant eye phenotype
- Experimental study on salmon demineralized bone matrix loaded with recombinant human bone morphogenetic protein-2: In vitro and in vivo study
- A case of IgA nephropathy treated with a combination of telitacicept and half-dose glucocorticoids
- Analgesic and toxicological evaluation of cannabidiol-rich Moroccan Cannabis sativa L. (Khardala variety) extract: Evidence from an in vivo and in silico study
- Wound healing and signaling pathways
- Combination of immunotherapy and whole-brain radiotherapy on prognosis of patients with multiple brain metastases: A retrospective cohort study
- To explore the relationship between endometrial hyperemia and polycystic ovary syndrome
- Research progress on the impact of curcumin on immune responses in breast cancer
- Biogenic Cu/Ni nanotherapeutics from Descurainia sophia (L.) Webb ex Prantl seeds for the treatment of lung cancer
- Dapagliflozin attenuates atrial fibrosis via the HMGB1/RAGE pathway in atrial fibrillation rats
- Glycitein alleviates inflammation and apoptosis in keratinocytes via ROS-associated PI3K–Akt signalling pathway
- ADH5 inhibits proliferation but promotes EMT in non-small cell lung cancer cell through activating Smad2/Smad3
- Apoptotic efficacies of AgNPs formulated by Syzygium aromaticum leaf extract on 32D-FLT3-ITD human leukemia cell line with PI3K/AKT/mTOR signaling pathway
- Novel cuproptosis-related genes C1QBP and PFKP identified as prognostic and therapeutic targets in lung adenocarcinoma
- Bee venom promotes exosome secretion and alters miRNA cargo in T cells
- Treatment of pure red cell aplasia in a chronic kidney disease patient with roxadustat: A case report
- Comparative bioinformatics analysis of the Wnt pathway in breast cancer: Selection of novel biomarker panels associated with ER status
- Kynurenine facilitates renal cell carcinoma progression by suppressing M2 macrophage pyroptosis through inhibition of CASP1 cleavage
- RFX5 promotes the growth, motility, and inhibits apoptosis of gastric adenocarcinoma cells through the SIRT1/AMPK axis
- ALKBH5 exacerbates early cardiac damage after radiotherapy for breast cancer via m6A demethylation of TLR4
- Phytochemicals of Roman chamomile: Antioxidant, anti-aging, and whitening activities of distillation residues
- Circadian gene Cry1 inhibits the tumorigenicity of hepatocellular carcinoma by the BAX/BCL2-mediated apoptosis pathway
- The TNFR-RIPK1/RIPK3 signalling pathway mediates the effect of lanthanum on necroptosis of nerve cells
- Longitudinal monitoring of autoantibody dynamics in patients with early-stage non-small-cell lung cancer undergoing surgery
- The potential role of rutin, a flavonoid, in the management of cancer through modulation of cell signaling pathways
- Construction of pectinase gene engineering microbe and its application in tobacco sheets
- Construction of a microbial abundance prognostic scoring model based on intratumoral microbial data for predicting the prognosis of lung squamous cell carcinoma
- Sepsis complicated by haemophagocytic lymphohistiocytosis triggered by methicillin-resistant Staphylococcus aureus and human herpesvirus 8 in an immunocompromised elderly patient: A case report
- Sarcopenia in liver transplantation: A comprehensive bibliometric study of current research trends and future directions
- Advances in cancer immunotherapy and future directions in personalized medicine
- Can coronavirus disease 2019 affect male fertility or cause spontaneous abortion? A two-sample Mendelian randomization analysis
- Heat stroke associated with novel leukaemia inhibitory factor receptor gene variant in a Chinese infant
- PSME2 exacerbates ulcerative colitis by disrupting intestinal barrier function and promoting autophagy-dependent inflammation
- Hyperosmolar hyperglycemic state with severe hypernatremia coexisting with central diabetes insipidus: A case report and literature review
- Efficacy and mechanism of escin in improving the tissue microenvironment of blood vessel walls via anti-inflammatory and anticoagulant effects: Implications for clinical practice
- Merkel cell carcinoma: Clinicopathological analysis of three patients and literature review
- Genetic variants in VWF exon 26 and their implications for type 1 Von Willebrand disease in a Saudi Arabian population
- Lipoxin A4 improves myocardial ischemia/reperfusion injury through the Notch1-Nrf2 signaling pathway
- High levels of EPHB2 expression predict a poor prognosis and promote tumor progression in endometrial cancer
- Knockdown of SHP-2 delays renal tubular epithelial cell injury in diabetic nephropathy by inhibiting NLRP3 inflammasome-mediated pyroptosis
- Exploring the toxicity mechanisms and detoxification methods of Rhizoma Paridis
- Concomitant gastric carcinoma and primary hepatic angiosarcoma in a patient: A case report
- Ecology and Environmental Science
- Optimization and comparative study of Bacillus consortia for cellulolytic potential and cellulase enzyme activity
- The complete mitochondrial genome analysis of Haemaphysalis hystricis Supino, 1897 (Ixodida: Ixodidae) and its phylogenetic implications
- Epidemiological characteristics and risk factors analysis of multidrug-resistant tuberculosis among tuberculosis population in Huzhou City, Eastern China
- Indices of human impacts on landscapes: How do they reflect the proportions of natural habitats?
- Genetic analysis of the Siberian flying squirrel population in the northern Changbai Mountains, Northeast China: Insights into population status and conservation
- Diversity and environmental drivers of Suillus communities in Pinus sylvestris var. mongolica forests of Inner Mongolia
- Global assessment of the fate of nitrogen deposition in forest ecosystems: Insights from 15N tracer studies
- Fungal and bacterial pathogenic co-infections mainly lead to the assembly of microbial community in tobacco stems
- Influencing of coal industry related airborne particulate matter on ocular surface tear film injury and inflammatory factor expression in Sprague-Dawley rats
- Temperature-dependent development, predation, and life table of Sphaerophoria macrogaster (Thomson) (Diptera: Syrphidae) feeding on Myzus persicae (Sulzer) (Homoptera: Aphididae)
- Eleonora’s falcon trophic interactions with insects within its breeding range: A systematic review
- Agriculture
- Integrated analysis of transcriptome, sRNAome, and degradome involved in the drought-response of maize Zhengdan958
- Variation in flower frost tolerance among seven apple cultivars and transcriptome response patterns in two contrastingly frost-tolerant selected cultivars
- Heritability of durable resistance to stripe rust in bread wheat (Triticum aestivum L.)
- Molecular mechanism of follicular development in laying hens based on the regulation of water metabolism
- Animal Science
- Effect of sex ratio on the life history traits of an important invasive species, Spodoptera frugiperda
- Plant Sciences
- Hairpin in a haystack: In silico identification and characterization of plant-conserved microRNA in Rafflesiaceae
- Widely targeted metabolomics of different tissues in Rubus corchorifolius
- The complete chloroplast genome of Gerbera piloselloides (L.) Cass., 1820 (Carduoideae, Asteraceae) and its phylogenetic analysis
- Field trial to correlate mineral solubilization activity of Pseudomonas aeruginosa and biochemical content of groundnut plants
- Correlation analysis between semen routine parameters and sperm DNA fragmentation index in patients with semen non-liquefaction: A retrospective study
- Plasticity of the anatomical traits of Rhododendron L. (Ericaceae) leaves and its implications in adaptation to the plateau environment
- Effects of Piriformospora indica and arbuscular mycorrhizal fungus on growth and physiology of Moringa oleifera under low-temperature stress
- Effects of different sources of potassium fertiliser on yield, fruit quality and nutrient absorption in “Harward” kiwifruit (Actinidia deliciosa)
- Comparative efficiency and residue levels of spraying programs against powdery mildew in grape varieties
- The DREB7 transcription factor enhances salt tolerance in soybean plants under salt stress
- Using plant electrical signals of water hyacinth (Eichhornia crassipes) for water pollution monitoring
- Food Science
- Phytochemical analysis of Stachys iva: Discovering the optimal extract conditions and its bioactive compounds
- Review on role of honey in disease prevention and treatment through modulation of biological activities
- Computational analysis of polymorphic residues in maltose and maltotriose transporters of a wild Saccharomyces cerevisiae strain
- Optimization of phenolic compound extraction from Tunisian squash by-products: A sustainable approach for antioxidant and antibacterial applications
- Liupao tea aqueous extract alleviates dextran sulfate sodium-induced ulcerative colitis in rats by modulating the gut microbiota
- Toxicological qualities and detoxification trends of fruit by-products for valorization: A review
- Polyphenolic spectrum of cornelian cherry fruits and their health-promoting effect
- Optimizing the encapsulation of the refined extract of squash peels for functional food applications: A sustainable approach to reduce food waste
- Advancements in curcuminoid formulations: An update on bioavailability enhancement strategies curcuminoid bioavailability and formulations
- Impact of saline sprouting on antioxidant properties and bioactive compounds in chia seeds
- The dilemma of food genetics and improvement
- Bioengineering and Biotechnology
- Impact of hyaluronic acid-modified hafnium metalorganic frameworks containing rhynchophylline on Alzheimer’s disease
- Emerging patterns in nanoparticle-based therapeutic approaches for rheumatoid arthritis: A comprehensive bibliometric and visual analysis spanning two decades
- Application of CRISPR/Cas gene editing for infectious disease control in poultry
- Preparation of hafnium nitride-coated titanium implants by magnetron sputtering technology and evaluation of their antibacterial properties and biocompatibility
- Preparation and characterization of lemongrass oil nanoemulsion: Antimicrobial, antibiofilm, antioxidant, and anticancer activities
- Corrigendum
- Corrigendum to “Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells”
- Corrigendum to “Effects of Ire1 gene on virulence and pathogenicity of Candida albicans”
- Retraction
- Retraction of “Down-regulation of miR-539 indicates poor prognosis in patients with pancreatic cancer”

