Abstract
The process of aging is marked by a gradual deterioration in the physiological functions and functional reserves of various tissues and organs, leading to an increased susceptibility to diseases and even death. Aging manifests in a tissue- and organ-specific manner, and is characterized by varying rates and direct and indirect interactions among different tissues and organs. Cardiovascular disease (CVD) is the leading cause of death globally, with older adults (aged >70 years) accounting for approximately two-thirds of CVD-related deaths. The prevalence of CVD increases exponentially with an individual’s age. Aging is a critical independent risk factor for the development of CVD. AMP-activated protein kinase (AMPK) activation exerts cardioprotective effects in the heart and restores cellular metabolic functions by modulating gene expression and regulating protein levels through its interaction with multiple target proteins. Additionally, AMPK enhances mitochondrial function and cellular energy status by facilitating the utilization of energy substrates. This review focuses on the role of AMPK in the process of cardiac aging and maintaining normal metabolic levels and redox homeostasis in the heart, particularly in the presence of oxidative stress and the invasion of inflammatory factors.
1 Introduction
Cardiovascular disease (CVD) has long been one of the leading causes of death worldwide, as a non-communicable disease causing over 17.3 million deaths per year. Models suggest that the number of deaths could increase to over 23.6 million per year by 2030 [1]. Aging is an independent risk factor for the development of CVD [2]. Cardiac aging is closely associated with time-dependent alterations in cellular metabolism, cardiomyocyte dysfunction (or senescence), and increased occurrence of tissue scarring (fibrosis). Ultimately these events can induce cardiac remodeling stress and potentially initiate heart failure [3–6].
Senescent cardiomyocytes exhibit various characteristic features, including DNA damage, endoplasmic reticulum stress, mitochondrial dysfunction, contractile dysfunction, and the expression of a senescence-associated secretory phenotype (SASP) [7]. Increased cardiac metabolic demand can exacerbate energy production imbalances and oxidative damage [8]. Previous studies have shown that AMP-activated protein kinase (AMPK) regulates mitochondrial biogenesis through the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1α) signaling pathway. This regulation enhances oxidative mitochondrial metabolism and serves as an important regulator of cardiac metabolism, functioning in both normal and ischemic conditions [9–12]. In addition, AMPK activation leads to substantial inhibition of the mTOR signaling pathway, which effectively reduces apoptosis [13,14]. AMPK also increases autophagy levels via Unc-51 like autophagy activating kinase 1 (ULK1) and reduces tissue fibrosis by inhibiting transforming growth factor beta (TGF-β) signaling [15]. Thus, AMPK activation is thought to play a significant cardioprotective role against cardiotoxicity and is closely associated with the cardiac remodeling process. Furthermore, proteins such as Humanin and SIRT1 are implicated in the cardioprotective effects of AMPK, indicating their participation in AMPK-mediated processes [16–18]. A substantial body of evidence supports the notion that AMPK serves as a metabolic hub, contributing to the improvement of heart health. However, as a heterologous protein complex found in numerous cells and organs, the precise role of AMPK is still being further elucidated [19]. The information compiled in this review will serve as a valuable reference for researchers studying AMPK and is anticipated to contribute to future experimental investigations and advancements in therapeutics targeting age-related CVDs.
1.1 AMPK basic mechanism and function
AMPK can regulates cellular energy status by promoting the adenosine triphosphate (ATP) production pathway and inhibiting the ATP utilization pathway when the body state is altered or stimulated by external factors [20]. As a highly conserved master regulator of metabolism, AMPK maintains energy homeostasis at both cellular and physiological levels during metabolic stress [21]. AMPK is commonly recognized as a precise energy sensor due to its crucial role in regulating the pathways of energy production and consumption in organisms, ensuring a dynamic equilibrium between them [22,23]. Under conditions of oxidative stress and DNA damage, AMPK regulates various cellular processes, including the inhibition of protein synthesis and cell proliferation, promotion of autophagy and DNA repair [24,25]. AMPK is a member of the serine/threonine (Ser/Thr) kinase group and is widely distributed in various cells [26,27]. It plays diverse regulatory roles in different tissues and organs. As a heterologous structured protein kinase, AMPK exerts protective effects on the heart by regulating energy homeostasis. In the brain, AMPK functions as an important endogenous defense molecule that responds promptly to harmful stimuli, such as cerebral ischemia, cerebral hemorrhage, and neurodegenerative diseases [28–30]. In the liver, it regulates glucose and fat metabolism and promotes fat oxidation to reduce fat accumulation [31,32]. In muscles, AMPK promotes glucose and fat oxidation, thereby improving muscle energy metabolism [33,34]. In adipose tissue, it promotes fat oxidation and decomposition, thereby reducing fat accumulation [35,36]. In pancreatic islets, it promotes insulin secretion and insulin receptor sensitivity and regulates blood glucose metabolism [37,38]. In the intestine, it regulates food absorption and metabolism, thereby affecting digestive tract function [39,40]. In the lungs, it promotes oxygen uptake and utilization, and improves oxygenation [41–43]. In summary, the distinctive regulatory roles of AMPK in diverse tissues and organs are crucial for maintaining normal physiological function and overall health (Figure 1).
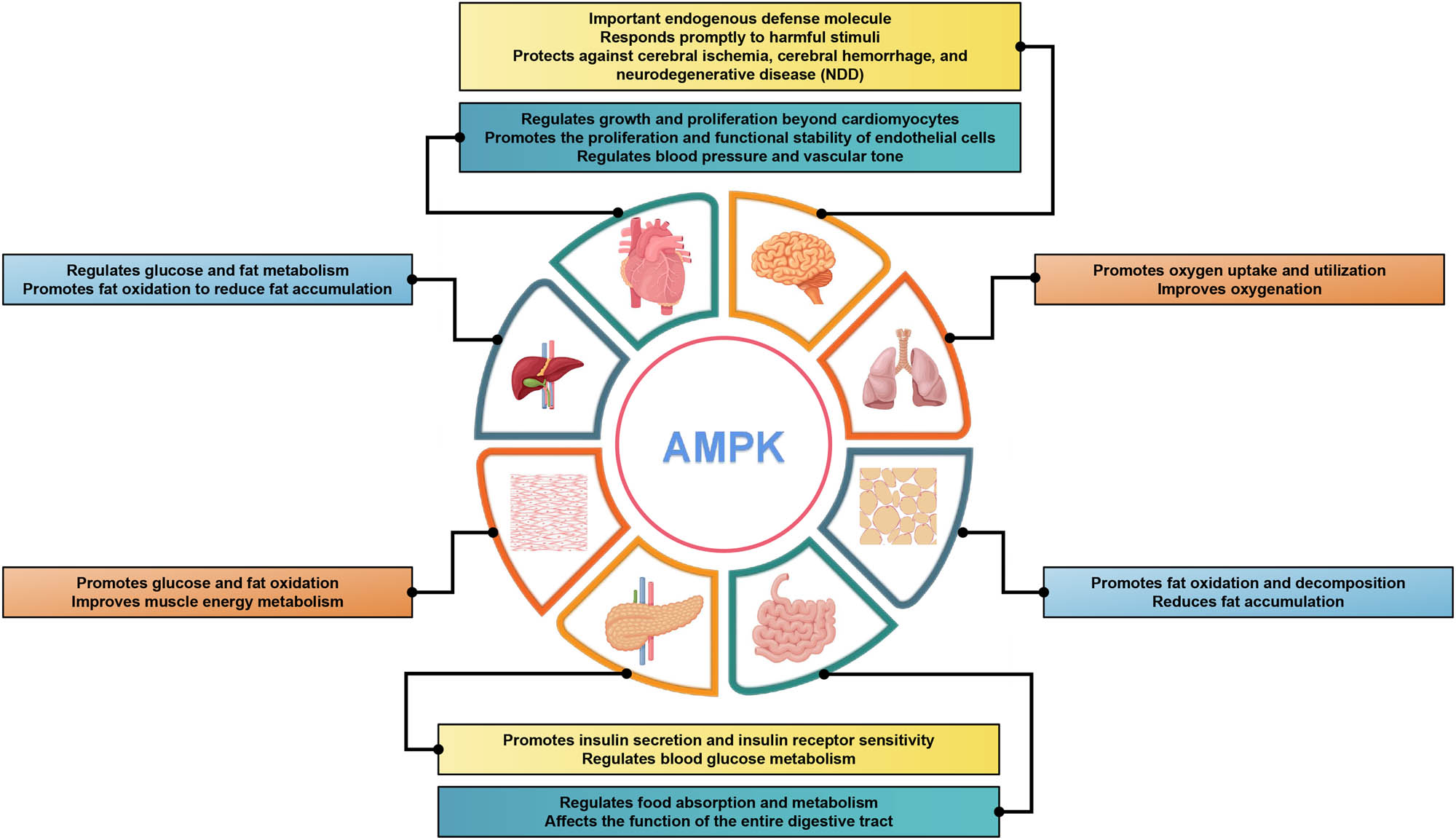
AMPK regulation in different tissues and organs. Diagram of a human body with different organs highlighted and labeled with the specific regulatory roles of AMPK in each organ. Overall, this comprehensive depiction of AMPK’s distinctive regulatory roles across various tissues and organs emphasizes its paramount importance in maintaining optimal physiological function and overall well-being. Understanding the multifaceted functions of AMPK holds potential for targeted therapeutic approaches in metabolic disorders, CVDs, neurological conditions, and respiratory ailments.
The varying roles of AMPK in different tissues can be primarily attributed to its complex structural composition, which consists of different subunits forming distinct structures. AMPK complexes in the body consist of three subunits: α, β, and γ. Each subunit has multiple isoforms, including α1, α2, β1, β2, γ1, γ2, and γ3, which are encoded by different genes (α1 and α2 are encoded by PRKAA1 and RKAA2 genes, β1 and β2 by PRKAB1 and PRKAB2 genes, and the three γ subunits, γ1, γ2, and γ3, are encoded by PRKAG1, PRKAG2, and PRKAB3 genes, respectively) [44]. The α subunit serves as the catalytic subunit and possesses a protein kinase structural domain. When there is an elevation in free adenosine monophosphate (AMP) and adenosine diphosphate (ADP) levels within an organism, these molecules bind to the γ subunit, inducing conformational changes in the AMPK complex. This, in turn, facilitates the phosphorylation of the α subunit threonine residue site 172 (Thr172), which represents a crucial activation pathway of AMPK [45,46]. The extent of phosphorylation at conserved threonine residues (Thr172) significantly affects the AMPK kinase activity [47]. The β-subunit is a scaffolding subunit necessary for the formation of the AMPK heterotrimer complexes. In addition, the β subunit of AMPK possesses a glycogen-binding domain, which plays a role in regulating AMPK activity thereby impacting glycogen levels within the body. β subunits are closely associated with adipogenesis, potentially due to the interaction between the β subunit of AMPK and the cystathionine-beta-synthase 2 (CBS2) structural domain of the AMPKγ subunit [48]. Interestingly, the AMPKβ subunits selectively activate AMPK complexes. For instance, specific drugs like A769622 and salicylates can selectively activate AMPK complexes containing the β1 subunit, while SUMOlation (SUMO) influences only AMPK complexes containing β2 subunit. This selective activation paves the way for the potential design of specific drugs [49–52]. These features make the AMPKβ subunit a critical factor in regulating the activity of the AMPK complex. The AMPKγ subunit has four CBS domains that are important for binding to AMP or ADP [20]. When the body has excess energy, higher levels of ATP in the body will displace AMP and bind to the AMPKγ subunit at CBS3, weakening the conformational activity of the catalytic structural domain and allowing upstream kinases and protein phosphatases to easily approach it for phosphorylation or dephosphorylation reactions. In conclusion, the AMPK phosphorylation level is an important indicator of AMPK activity [53]. Figure 2 provides a general description of AMPK structure and activity.

Structure of AMPK and its activation mode in the heart. There are two mechanisms through which AMPK can be activated. (1) Phosphorylation: this mechanism involves the addition of a phosphate group to the AMPK enzyme, which activates it. AMPK can be phosphorylated by several upstream kinases, including LKB1 and CaMKKβ. Once phosphorylated, AMPK becomes fully active and can regulate various cellular processes. (2) AMP/ADP binding: another mechanism of AMPK activation is through the binding of AMP or ADP molecules. When cellular energy levels drop, AMP accumulates, leading to increased binding with AMPK. This allosteric binding causes conformational changes in the enzyme, resulting in its activation. Both phosphorylation and nucleotide binding work together to activate AMPK, allowing it to modulate cellular metabolism and maintain energy homeostasis.
1.2 Activation of AMPK in the heart
AMPK activation is a complex process that is primarily achieved through two complementary mechanisms. First, AMPKα subunits are activated after the phosphorylation of Thr172 residues in the kinase structural domain via specific upstream kinases (liver kinase B1 [LKB1] and calcium/calmodulin-dependent protein kinase kinase beta [CaMKKβ]). However, the LKB1 pathway appears to selectively activate the complex containing the AMPKα2 subunit (but not AMPKα1) [54,55]. Mice deficient in LKB1 in skeletal and cardiac muscles exhibited loss of activity of their AMPK complex, particularly the complex containing the AMPKα2 subunit. This observation provides compelling evidence indicating that the α2 subtype plays a crucial role in protecting the myocardium against injury during ischemia [56,57]. Related studies have also elucidated the role of other important upstream kinases CaMKKβ, where an increase in intracellular Ca2+ concentration causes an increase in CaMKKβ activity, ultimately leading to the activation of TGF-β via transforming growth factor kinase 1 (TAK1), which ultimately culminates in the activation of AMPK through phosphorylation of Thr172. Furthermore, AMPK can be activated by heterodimerization through the binding of AMP to the AMPKγ subunit [58,59]. However, it should be noted that in cardiac tissues, the currently known expression isoforms of AMPK are α1/2, β1/2, γ1, and γ2, whereas γ3 is not expressed in the heart [60,61]. This indicates that there are several classes of AMPK variants in the heart, despite all being characterized by the presence of α, β, and γ subunits [62,63]. It has been shown that the binding of AMP to the AMPKγ subunit promotes the phosphorylation of Thr172 residues. This conformational change also protects Thr172 from dephosphorylation by protein phosphatases, including protein phosphatase 2A (PP2A), and ensures a 10-fold increase in activity [64]. AMPK activation in the heart has multiple physiological effects, including promotion of glucose uptake and oxidation, improvement of energy metabolism efficiency, enhancement of antioxidant enzyme expression and activity, reduction of oxidative stress, protection of cardiomyocytes against apoptosis, promotion of autophagy, and reduction of inflammation [65–67]. For instance, in lipopolysaccharide (LPS)-induced adipocytes, the TBK1-AMPK signaling pathway is activated and regulates the expression of omentin, which is a protein secreted by adipocytes that has multiple biological functions. Studies have shown that omentin regulates adipocyte metabolic function, promotes glucose uptake and utilization, and lowers blood glucose levels. Additionally, omentin inhibits inflammation and oxidative stress, reduces endothelial cell damage, and protects cardiovascular health [68]. Moreover, certain exogenous drugs like empagliflozin have demonstrated the ability to activate the AMPK/glycogen synthase kinase 3 beta (GSK3β) signaling pathway in diabetic cardiomyopathy. In animal experiments, this drug has shown a protective effect against cardiac injury by preventing excessive autophagy-induced cardiomyocyte death [69]. AMPK activation also regulates cardiovascular function by modulating cardiac contractility and heart rate and promoting endothelial cell function. Beyond its impact on cardiomyocytes, AMPK plays a regulatory role in the growth and proliferation of various cell types within the heart. It stimulates angiogenesis, promotes the proliferation and functional stability of endothelial cells, and regulates blood pressure and vascular tone. These functions are important for maintaining the normal physiological state of the heart [70–72]. These findings suggest that AMPK activation plays a crucial role in maintaining cardiac health and preventing CVD.
1.3 AMPK plays a protective role in the aging heart by regulating metabolism
Aging often leads to a progressive deterioration of cardiac geometry and systolic function; however, the exact mechanisms remain elusive [73,74]. More specifically, these changes in the heart involve a progressive decline in cardiac function and an inadequate cardiac reserve [75]. They are accompanied by myocardial hypertrophy and interstitial fibrosis, which compromise cardiac health during ventricular remodeling [6]. To date, many hypotheses have been proposed regarding the pathogenesis of cardiac aging, including inflammation, lipotoxicity, oxidative stress, apoptosis, mitochondrial damage, autophagy dysregulation, and intracellular Ca2+ disorders [8,76–81]. Nevertheless, given the complexity of cardiac tissues and the specific non-renewable nature of cardiomyocytes, the precise mechanisms and targets of intervention in the cardiac aging process are still in the exploratory stage [82,83]. Two signaling molecules that have been shown to be strongly regulated in age-related heart disease are protein kinase b (AKT) and AMPK, which are involved in energy metabolism [84–86]. Previously, in studies targeting obese fatty liver, it was demonstrated that AKT2 and AMPKα2, as isoforms of AKT, have an influence on obesity and hepatic steatosis induced by a high-fat diet. This suggests that Akt2 and AMPKα2 subunits have synergistic effects and may be promising therapeutic targets [87]. Contrastingly, this synergistic effect was mainly manifested by hyperactivated AKT, which promoted AMPK-Ser485 site phosphorylation and interfered with AMPK (Thr172) phosphorylation [88,89]. The synergistic effect of AKT2 and AMPKα2 subunits was also observed in the heart [90]. In particular, middle-aged mice (12 months old) with double knockout of AKT2 and AMPK showed significant changes in heart size, cardiomyocyte cross-sectional area, and interstitial fibrosis. Interestingly, this knockout did not affect Kaplan–Meier survival or the expression of senescence markers such as p16 and p21. This suggests that AKT2–AMPK knockdown does not significantly alter the biological course of cardiac senescence, but leads to a senescence-like phenotype. However, the age-related changes in myocardial contractile function caused by AKT2–AMPK knockout can be attributed to mitochondrial dysfunction. These changes can be indicated by alterations in mitochondrial structural genes (UCP2, PGC-1α, and electron microscopic ultrastructure), autophagic genes (Beclin-1, LC3B, Atg5, Atg7, and p62), phagocytic genes (PINK1, Parkin, Fundc1, and BNIP3), and lysosomal biogenesis related gene (TFEB). These findings suggest a critical role for AMPK–AKT-mediated autophagy in altered cardiac geometry and function [91,92]. Furthermore, AKT2–AMPK knockdown fails to alter the intracellular processing of Ca2+ levels at a young age, which eventually manifests as defective intracellular Ca2+ processing in cardiac myocytes with the progression in age leading to myocardial contractile dysfunction [93]. In addition, the downregulation of autophagy mediated by the senescence-associated AMPK-S-phase kinase associated protein 2 (SKP2)-coactivator associated arginine methyltransferase 1 (CARM1) pathway also leads to cardiomyocyte dysfunction [94,95]. Much has been reported regarding the role of autophagy in the regulation of cardiac function; however, there are dramatic differences between young and old hearts. For instance, light fasting affects the autophagic flux differently in young and old hearts [96,97]. CARM1 stability is significantly reduced in the aging heart, leading to impairment of the nuclear TFEB–CARM1 complex and autophagic flux [92]. With a reduction in AMPK–FOXO3 activity in the nucleus, it is unable to inhibit SKP2-E3 ubiquitin ligase. However, this failure of inhibition can be restored by the activation of AMPK. Nevertheless, excessive activation of the AMPK–SKP2–CARM1 pathway may also lead to cardiomyocyte hypertrophy [98]. As a macroscopic means of regulating metabolism, calorie restriction can extend the average and maximum life span and has beneficial effects on age-related diseases [99–101]. Although calorie restriction may lead to different intervention outcomes in younger hearts, calorie restriction in middle-aged or older populations has been effective in preventing CVDs associated with aging [102,103]. A study has shown that starting a calorie-restricted diet for 3 months (40% less than ad libitum) in 12- and 19-month-old mice significantly reversed aging-related markers, including p16 and p21, and significantly improved markers of cardiac remodeling (cardiac hypertrophy and myocardial fibrosis), inflammation, mitochondrial damage, and telomere shortening [104]. The analysis of related miRNAs and corresponding target genes revealed that this result was likely due to a significant increase in the phosphorylation level of AMPK (Thr172) in the heart under caloric restriction, which regulates the expression of FOXO transcription factors and, subsequently, the expression of autophagy-related genes to achieve cardioprotection [105,106]. Autophagy plays an important role in the pathogenesis of atherosclerosis and other age-related diseases. In the heart, C1q/TNF-related protein 9 (CTRP9) has anti-aging and anti-atherosclerotic effects that highly resemble lipocalin in structure, while AMPK plays a positive role in cardioprotection mediated by CTRP9 [107,108]. Activated AMPK is involved in LC3 conversion and reduces the levels of p62 induced by CTRP9. Conversely, CTRP9 restores autophagy and autophagic flux via AMPK activation, thereby inhibiting endothelial senescence produced by palmitic acid [109]. Similarly, the role of AMPK in LPS-induced myocardial dysfunction is similarly age-related [110]. The AMPK/mTOR (mammalian target of rapamycin, mTOR) pathway serves as a potential mechanism for regulating autophagic function in mice of different ages, and its impairment or deficiency leads to significant alterations in the mouse heart, including echocardiography, pathology, contractility, and intracellular Ca2+ levels [111]. However, A769662, an AMPK agonist, appeared to have a better regulatory function in aged mice [112]. When the expression of the AMPK upstream regulators PP2A and PP2Cα was significantly increased, AMPK activity was inhibited, mTOR was activated, and autophagy was inhibited. In contrast, the addition of the AMPK activator A769662 significantly decreased the expression of p-mTOR and p-S6, increased the levels of autophagy markers Atg5 and p62, and the LC3-II/LC3-I ratio, ultimately improving cardiac function and upregulating cardiac autophagy levels under LPS in aged mice [113]. In addition, it has been shown that increased CD36 in aged male mice may reduce AMPK activity, leading to activation of the mTOR-p70S6K pathway and causing myocardial hypertrophy [114].
1.4 AMPK and sirtuin family co-regulate cardiac function and aging
A common feature of heart disease and aging is the alterations in metabolic organs, which ultimately lead to changes in circulating metabolite levels [115]. AMPK, an important regulator of energy homeostasis, emerges as a crucial player in aging-induced metabolic dysregulation. For instance, a study has shown that NADPH pretreatment of neonatal rat cardiomyocytes significantly increased AMPK phosphorylation while downregulating mTOR phosphorylation and effectively inhibiting hypoglycemic hypoxia/reoxygenation OGD/R(oxygen-glucose deprivation/reoxygenation)-induced apoptosis [116]. Conversely, NADPH-induced AMPK phosphorylation and cardioprotection were blocked when AMPK, which inhibits mitochondrial damage and cardiomyocyte apoptosis, was inhibited by compound C (Dorsomorphin) [117].
The Nmrk2 gene is a co-responsive gene for AMPK and PPARA; additionally, in isolated rat cardiomyocytes, energy stress and high NAD+ depletion will activate the Nmrk2 gene [118]. Moreover, promoting the synthesis of NAD+ can effectively stimulate glycolysis in cardiomyocytes, increase AMPK activity, and improve or reduce the development of heart failure in mice [119]. Of note, NAD is involved in cellular metabolism and DNA repair through its role as a sensing or consumable molecule for the enzymes poly (ADP-ribose) polymerase 1 (PARP1) and sirtuin protein deacetylases, and these deacetylations directly or indirectly regulate cellular aging and inflammatory responses [120,121].
Aging, as an important risk factor for left ventricular hypertrophy and CVD development, can be effectively mitigated by targeting AMPK. The sirtuin family of nicotinamide adenine dinucleotide-dependent deacetylases (SIRT1-7) plays a significant role in improving cardiac metabolism and maintaining essential cardiac functions [122]. Most sirtuin family proteins delay aging to some extent when their expression is upregulated in the heart. Age-related changes in SIRT1, AMPK, and SIRT3 are associated with mitochondrial biogenesis, antioxidant defense, and cardiac inflammation. Among them, AMPK and SIRT1 are partner proteins that coordinate multiple intracellular processes, including cellular resistance to oxidative stress, general metabolism, inflammation, and mitochondrial biogenesis and function, while overexpression of SIRT3 activates the AMPK pathway and improves mitochondrial biogenesis, which is required to maintain mitochondrial redox homeostasis, sustain mitochondrial respiration, and inhibit mitochondrial apoptosis [123,124]. SIRT2 promotes downstream AMPK activation by deacetylating LKB1, a kinase upstream of AMPK [125]. However, the physiological and pathological roles of SIRT4 in cardiac aging are unknown. In response to DOX-induced cardiotoxicity, SIRT3 and SIRT4 increase autophagy through the AMPK/mTOR signaling pathway, while activation of the FOXO and P53 pathways to reduce apoptosis may be a joint action of SIRT3 and SIRT4 as well as AMPK [126]. The absence or abnormality of SIRT5, a key enzyme regulating mitochondrial function in the heart, leads to the inhibition of mitochondrial NADH oxidation and ATP synthase activity. Interestingly, when SIRT5 is blocked, with reduced ATP levels and an increased AMP/ATP ratio, AMPK is activated to a great extent. In a SIRT5 knockout mouse model, this was accompanied by elevated phosphorylation AMPK (Thr172), which alleviates left atrial dilation, a structural change in the aging heart [127,128]. Moreover, unlike the conventional perception of reduced expression of plasminogen activator inhibitor-1 (PAI-1) mediated by Jun N-terminal kinase (JNK) and p38, the inhibition or silencing of SIRT5 inhibits the expression of PAI-1 genes and proteins during thrombosis by increasing AMPK activation and reducing the phosphorylation of mitogen-activated protein kinase and extracellular signal-regulated kinase 1/2 (ERK 1/2 kinase), ultimately achieving a response to TNF-α and reducing thromboembolic episodes [129]. SIRT6, a crucial member of the sirtuin family, plays an essential role in regulating DNA repair, telomere maintenance, and glucose and lipid metabolism [130]. In the heart, the melatonin membrane receptor-mediated SIRT6–AMPK–PGC-1α–AKT axis may be a potentially effective strategy to attenuate dilated cardiomyopathy and reduce the myocardial response to ischemia/reperfusion injury (I/RI) in patients with diabetes [131]. With the activation of SIRT6, it significantly increases nocturnal circulating melatonin and cardiac melatonin levels, while elevated melatonin levels in the heart activate the downstream AMPK–PGC-1α–AKT axis, ultimately improving the outcome of I/RI [132]. In in vitro experiments, sustained activation of AMPK increased the mRNA and protein expression of Troponin T type 2 (TNNT2) and Troponin I type 3 (TNNI3), maintained the stability of myocardial muscle contraction, and enhanced the activity of SIRT1 and SIRT6 by decreasing histone acetylation [133]. SIRT7 is a histone H3K18-specific deacetylase that epigenetically controls mitochondrial biogenesis, ribosomal biosynthesis, and DNA repair [134]. There is a relative lack of research on the role of SIRT7 in cardiac aging, and previous studies that have been conducted have presented different conclusions. A study has shown that SIRT7 deficiency protects against aging-associated glucose intolerance and extends lifespan in male mice [135]. However, other studies have shown that SIRT7-deficient mice exhibit several signs of aging, including degenerative cardiac hypertrophy, kyphosis, reduced subcutaneous fat, and poor stress resistance [136,137]. Interestingly, proteasome activator subunit 3 (REGγ) has been reported to promote SIRT7 degradation in an AMPK phosphorylation-dependent manner [138]. However, fasting coordinates AMPK and GSK3β activity to ensure the stabilization of SIRT7. More precisely, AMPK phosphorylates SIRT7 at T263 to prime subsequent phosphorylation at T255/S259 by GSK3β, decoupling SIRT7 from UBR5 E3 ligase, and thereby preventing K48-linked polyubiquitination and proteasomal degradation of SIRT7 [139].
1.5 AMPK inhibits SASP transmission between cells and resists damage to the heart from excessive reactive oxygen species (ROS)
Cellular senescence is closely related to the SASP of inflammatory and secretory proteins. The close transfer of SASP between cells is a major trigger for cellular senescence, in other words, SASP secretion inevitably increases with age, and inhibiting or eliminating SASP secretion and activity is one of the important ways to combat aging [140,141]. Cardiovascular smooth muscle cells (VSMCs) in the heart are the main cells that express SASP, which promotes chronic vascular inflammation, loss of vascular function, and the development of age-related heart diseases. Prednisolone inhibits p-NF-κB via the SIRT1 and p-AMPK (Ser485) pathways, ultimately resisting VSMC aging and inflammatory responses [142]. Another drug, metformin, has a potential resistance to aging-related injuries and can improve mitochondrial function to mitigate ischemia–reperfusion damage to the heart and effectively resist myocardial necrosis [143]. As an known AMPK receptor agonist, it inhibits LPS-induced chemokine expression via the AMPK and NF-κB signaling pathways, including CCL2, CXCL10, and CXCL11, which are all chemokines in the SASP [144,145]. Recent in vitro experiments have revealed that Licochalcone D (Lico-D)-mediated autophagy activation through the upregulation of AMPK may reduce H2O2-induced oxidative stress-induced senescence [24]. Figure 3 shows several molecules/drugs that interact with AMPK. In vivo experiments showed that the antioxidant, anti-aging, and cardioprotective effects of Lico-D may arise through the activation of AMPK and autophagy and ameliorate oxidative stress-induced aging. Along with AMPK activation, the expression levels of senescence markers (such as p53 and p21) were also significantly downregulated [146]. Excess mitochondrial production of ROS free radicals is directly and causally linked to aging of the heart and other organs and plays a deleterious role in several types of age-related cardiac diseases, including I/RI and heart failure, which occur in a high proportion of elderly patients [147]. With the gradual refinement of the ROS theory of oxidative stress, ROS production is also considered a fundamental mitochondrial function that coordinates several signaling pathways to exert beneficial effects, some of which are protective in the heart [148,149]. However, aging cardiomyocytes undergo cytoarchitectural and physiological changes as a result of their timely response to exercise, stress, injury, and their own reduced adaptive reserve capacity. At this time, the disturbed redox state may synergistically promote the production of mitochondrial ROS and exacerbate cardiomyocyte death in the elderly heart. As the heart ages, leading to elevated levels of ROS in the body, a feedback mechanism is triggered, and the expression of antioxidant enzymes is stimulated, which is inherent to the heart. The main player in this process is the transcriptional coactivator, PPARγ coactivator 1α (PGC-1α), which is a regulator of mitochondrial biogenesis and one of the important inducers of antioxidant gene expression during oxidative stress; however, PGC-1α levels are low in aging cardiac myocytes. AMPK can directly phosphorylate PGC-1α in skeletal muscle and, using positive feedback triggering its own transcriptional activation. It can be argued that when normal homeostatic metabolic mechanisms are disrupted, AMPK is activated and stimulates the expression of antioxidant enzymes to limit the production of ROS [150]. Previous reports have proposed that AMPK activity is affected by ROS, mainly through the action of ROS on redox-sensitive cysteine residues (Cys-299/Cys-304) on the AMPKα subunit, which increases AMPK activity [151]. However, it has also been suggested that altered AMPK activity in response to redox changes is not due to the regulation of AMPK per se, but is a secondary result of redox effects on other processes, such as mitochondrial ATP production [152]. In cardiomyocytes, most ROS are generated by electron leakage from the mitochondrial electron transfer chain (ETC) [153]. Age-related changes in mitochondrial function and decreased ETC complex activity lead to higher ROS production rates associated with oxidative stress, ultimately leading to cardiac aging. NOX, an NADPH oxidase, is a major contributor to ROS production in the cardiovascular system [154]. The reason for the production of ROS by nicotinamide adenine dinucleotide phosphate oxidase (NOX) lies in its specific function in the transmembrane electron transport of superoxide anions produced by immune cells [155]. All seven isoforms of NOX are expressed in the vascular smooth muscle, with Nox1, Nox2, Nox4, and Nox5 being the most abundant in relative terms [156]. NOX plays a crucial role in physiological and pathological processes, as it physiologically produces ROS necessary to maintain cardiovascular homeostasis; however, the abnormal production of ROS often originates from this and is accompanied by the onset of accelerated aging. In fact, there is considerable controversy regarding the harmful or non-harmful functions of NOX. When AMPK knockdown increases Nox2 protein expression, and conversely, AMPK agonists decrease Nox2 expression, AMPK can directly or indirectly resist Nox2-associated oxidative stress, leading to I/RI exacerbation [117,157]. In addition, in a pressure overload model, Nox2 activation led to cardiac systolic dysfunction and interstitial fibrosis formation [158]. However, elevated endothelial Nox4-derived ROS promote endothelial cell migration and angiogenesis in an endothelial nitric oxide synthase (eNOS)-dependent manner during ischemia and can protect the heart to some extent [159]. Additionally, endogenous coenzyme Q10 can improve the functionality of endothelial precursor cells by increasing eNOS activity and nitric oxide production. Through CAMKK activation of AMPK, it promotes the expression of eNOS and Heme Oxygenase-1, thus improving cellular apoptosis induced by high glucose and mitochondrial membrane potential imbalance [160,161]. Interestingly, there is also evidence that metformin activates AMPK and inhibits NOX4 expression, leading to reduced myocardial oxidative damage and apoptosis, thereby attenuating reperfusion injury [162,163]. Overall, although the effects of NOX and its isoforms and their corresponding derivatives on the heart are still controversial, the effects of AMPK against NOX are mostly favorable for survival.
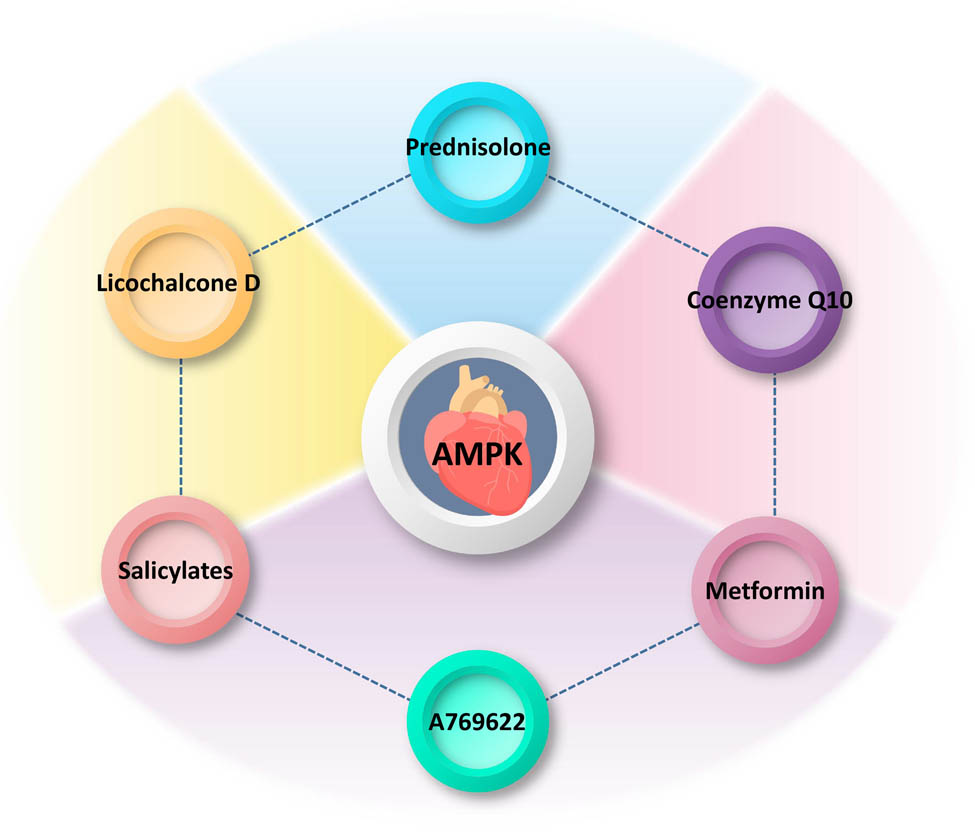
Interactions of molecules/drugs with AMPK in the heart. The diagram illustrates several molecules/drugs that interact with AMPK specifically in the heart, a crucial organ involved in cardiovascular health. Prednisolone: a synthetic corticosteroid commonly used as an anti-inflammatory and immunosuppressant agent. Prednisolone may modulate the activity of AMPK in the heart, potentially influencing downstream signaling pathways involved in cardiac metabolism, inflammation, and oxidative stress. Licochalcone D: a natural flavonoid found in licorice root, known for its antioxidant and anti-inflammatory properties. Licochalcone D has been reported to activate AMPK in the heart, suggesting its potential cardioprotective effects by enhancing cellular energy regulation, reducing oxidative damage, and modulating inflammatory responses. Salicylates: salicylates have been shown to activate AMPK in the heart, indicating their potential in ameliorating cardiac dysfunction through effects on glucose metabolism, mitochondrial function, and oxidative stress. Coenzyme Q10: a naturally occurring compound involved in cellular energy production. Coenzyme Q10 has been reported to regulate AMPK activity in the heart, potentially improving myocardial energy metabolism, protecting against oxidative damage, and promoting overall cardiac health. Metformin: a widely prescribed drug for the treatment of type 2 diabetes. Metformin activates AMPK in the heart, leading to beneficial effects on glucose metabolism, mitochondrial function, calcium handling, and overall cardiac performance. A769622: an experimental small molecule compound targeting AMPK activation. A769622 has been studied for its potential cardioprotective effects by enhancing cardiac energy metabolism, attenuating hypertrophy, improving contractility, and reducing cardiac ischemic injury.
2 Conclusions
Globally, it is estimated that the number of individuals with CVD will reach 1.9 billion, and there is a significant correlation between age and morbidity/mortality. Therefore, it is crucial to understand the molecular mechanisms of cardiac aging and the important pathways that can influence cardiac function during aging to develop interventions that target these mechanisms. AMPK is required for embryonic development, growth, and maintenance of the physiological functions of several organs, including the heart. The pathophysiological functions of the AMPK pathway as a central energy regulator in aging, particularly cardiac aging, have been extensively studied. When activated in response to nutritional signals, AMPK increases the body’s energy reserves, promotes cell growth, and regulates autophagy to a certain extent. Conversely, AMPK which is overexpressed or affected by inhibitors, inhibits the overall protein translation and reduces autophagic flux, ultimately affecting protein quality. The role of AMPK in the heart varies across different life stages. In the aged heart, AMPK exhibits the ability to effectively block the in vivo transmission of SASP signals emanating from senescent cells. Moreover, AMPK activates autoimmune cells to eliminate senescent cells, thus preventing contact-induced aging. These beneficial effects involve mechanisms like autophagy. Furthermore, the reciprocal regulation with the sirtuin family and enhanced resistance to ROS are likely to promote an overall extension of lifespan. Although the regulation of AMPK is stable in vivo and is hardly overexpressed, studies on AMPK isoforms in different tissue sites of the heart need to be improved, and the advantages and disadvantages of various modes of AMPK activation also need to be investigated. The molecular pathways initiated or influenced by AMPK and associated with cardiac aging are summarized in Figure 4. In conclusion, AMPK holds significant potential as a therapeutic target for addressing age-related cardiac diseases and combating the aging process. However, further research is warranted to investigate ways to activate AMPK, specifically in cardiac tissues, and to investigate the development of specific agonists for this purpose.
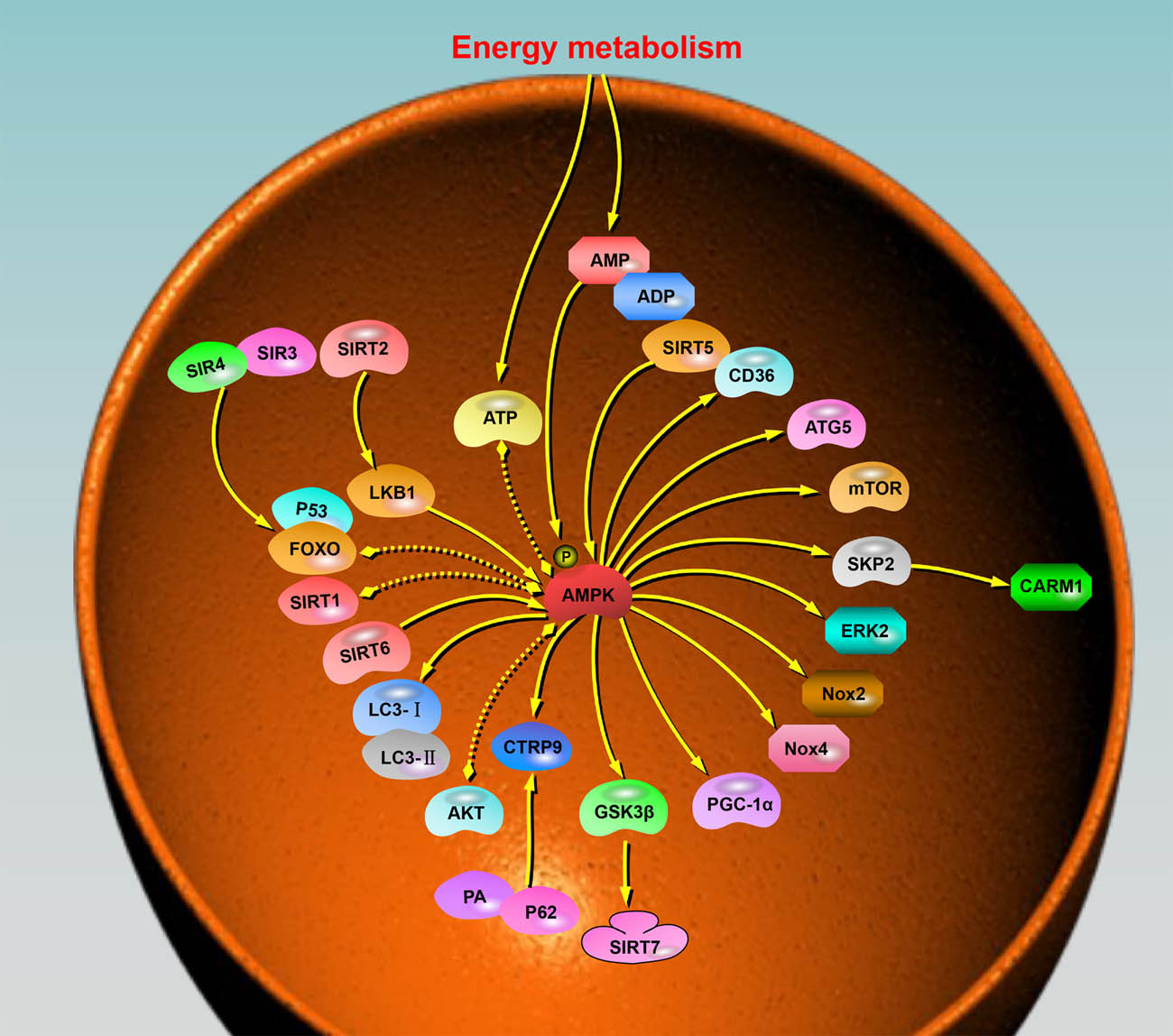
Molecular pathways triggered by or associated with AMPK during cardiac aging. AMPK is crucial in regulating energy metabolism, autophagy, and protein synthesis in the heart. The diagram illustrates the interactions of multiple molecules with AMPK. The arrows within the diagram indicate the general direction of action or influence between AMPK and the respective molecules. These arrows represent the regulatory effects exerted by AMPK on the molecules or vice versa. Furthermore, the presence of dotted double arrows in the diagram signifies the existence of mutual regulation between AMPK and the associated molecules. This mutual regulation suggests a bidirectional influence, where AMPK affects the activity or function of the molecules, while the molecules, in turn, impact AMPK signaling or activity.
Acknowledgements
The authors would like to express their sincere gratitude to Dr Jia Liu for her invaluable support and insightful suggestions throughout the course of this article.
-
Funding information: Authors state no funding involved.
-
Author contributions: Z.Q. and Y.L. drafted the manuscript. Y.F. and Y.Y. reviewed and made modifications to the manuscript. All authors read and approved the final manuscript. The authors applied the SDC approach for the sequence of authors.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Balakumar P, Maung-U K, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res. 2016;113:600–9.10.1016/j.phrs.2016.09.040Search in Google Scholar PubMed
[2] Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19.10.3390/jcdd6020019Search in Google Scholar PubMed PubMed Central
[3] Werbner B, Tavakoli-Rouzbehani OM, Fatahian AN, Boudina S. The dynamic interplay between cardiac mitochondrial health and myocardial structural remodeling in metabolic heart disease, aging, and heart failure. J Cardiovasc Aging. 2023;3(1):9.10.20517/jca.2022.42Search in Google Scholar PubMed PubMed Central
[4] Yu Y, Sun Q, Li T, Ren X, Lin L, Sun M, et al. Adverse outcome pathway of fine particulate matter leading to increased cardiovascular morbidity and mortality: an integrated perspective from toxicology and epidemiology. J Hazard Mater. 2022;430:128368.10.1016/j.jhazmat.2022.128368Search in Google Scholar PubMed
[5] Ren L-L, Miao H, Wang Y-N, Liu F, Li P, Zhao Y-Y. TGF-β as a master regulator of aging-associated tissue fibrosis. Aging Dis. 2023;10.14336/AD.2023.0222.10.14336/AD.2023.0222Search in Google Scholar PubMed
[6] Mehdizadeh M, Aguilar M, Thorin E, Ferbeyre G, Nattel S. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat Rev Cardiol. 2022;19(4):250–64.10.1038/s41569-021-00624-2Search in Google Scholar PubMed
[7] Chen MS, Lee RT, Garbern JC. Senescence mechanisms and targets in the heart. Cardiovasc Res. 2022;118(5):1173–87.10.1093/cvr/cvab161Search in Google Scholar PubMed PubMed Central
[8] Pagan LU, Gomes MJ, Gatto M, Mota GA, Okoshi K, Okoshi MP. The role of oxidative stress in the aging heart. Antioxidants. 2022;11(2):336.10.3390/antiox11020336Search in Google Scholar PubMed PubMed Central
[9] Yu L, Gong B, Duan W, Fan C, Zhang J, Li Z, et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: role of AMPK-PGC-1α-SIRT3 signaling. Sci Rep. 2017;7(1):1–13.10.1038/srep41337Search in Google Scholar PubMed PubMed Central
[10] Bairwa SC, Parajuli N, Dyck JR. The role of AMPK in cardiomyocyte health and survival. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2016;1862(12):2199–210.10.1016/j.bbadis.2016.07.001Search in Google Scholar PubMed
[11] Yu S, Qian H, Tian D, Yang M, Li D, Xu H, et al. Linggui zhugan decoction activates the SIRT1-AMPK-PGC1α signaling pathway to improve mitochondrial and oxidative damage in rats with chronic heart failure caused by myocardial infarction. Front Pharmacol. 2023;14:1074837.10.3389/fphar.2023.1074837Search in Google Scholar PubMed PubMed Central
[12] Qi X, Wang J. Melatonin improves mitochondrial biogenesis through the AMPK/PGC1α pathway to attenuate ischemia/reperfusion-induced myocardial damage. Aging. 2020;12(8):7299–312.10.18632/aging.103078Search in Google Scholar PubMed PubMed Central
[13] Li SX, Li C, Pang XR, Zhang J, Yu GC, Yeo AJ, et al. Metformin attenuates silica-induced pulmonary fibrosis by activating autophagy via the AMPK-mTOR signaling pathway. Front Pharmacol. 2021;12:719589.10.3389/fphar.2021.719589Search in Google Scholar PubMed PubMed Central
[14] Mei R, Lou P, You G, Jiang T, Yu X, Guo L. 17β-estradiol induces mitophagy upregulation to protect chondrocytes via the SIRT1-mediated AMPK/mTOR signaling pathway. Front Endocrinol (Lausanne). 2020;11:615250.10.3389/fendo.2020.615250Search in Google Scholar PubMed PubMed Central
[15] Timm KN, Tyler DJ. The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc Drugs Ther. 2020;34(2):255–69.10.1007/s10557-020-06941-xSearch in Google Scholar PubMed PubMed Central
[16] Wang L, Quan N, Sun W, Chen X, Cates C, Rousselle T, et al. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc Res. 2018;114(6):805–21.10.1093/cvr/cvy033Search in Google Scholar PubMed PubMed Central
[17] Lu G, Wang Y, Shi Y, Zhang Z, Huang C, He W, et al. Autophagy in health and disease: from molecular mechanisms to therapeutic target. MedComm. 2022;3(3):e150.10.1002/mco2.150Search in Google Scholar PubMed PubMed Central
[18] Cai H, Liu Y, Men H, Zheng Y. Protective mechanism of humanin against oxidative stress in aging-related cardiovascular diseases. Front Endocrinol (Lausanne). 2021;12:683151.10.3389/fendo.2021.683151Search in Google Scholar PubMed PubMed Central
[19] Feng Y, Zhang Y, Xiao H. AMPK and cardiac remodelling. Sci China Life Sci. 2018;61(1):14–23.10.1007/s11427-017-9197-5Search in Google Scholar PubMed
[20] Trefts E, Shaw RJ. AMPK: restoring metabolic homeostasis over space and time. Mol Cell. 2021;81(18):3677–90.10.1016/j.molcel.2021.08.015Search in Google Scholar PubMed PubMed Central
[21] González A, Hall MN, Lin S-C, Hardie DG. AMPK and TOR: the Yin and Yang of cellular nutrient sensing and growth control. Cell Metab. 2020;31(3):472–92.10.1016/j.cmet.2020.01.015Search in Google Scholar PubMed
[22] Hardie DG, Schaffer BE, Brunet A. AMPK: an energy-sensing pathway with multiple inputs and outputs. Trends Cell Biol. 2016;26(3):190–201.10.1016/j.tcb.2015.10.013Search in Google Scholar PubMed PubMed Central
[23] Steinberg GR, Hardie DG. New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol. 2023;24(4):255–72.10.1038/s41580-022-00547-xSearch in Google Scholar PubMed
[24] Maharajan N, Ganesan CD, Moon C, Jang C-H, Oh W-K, Cho G-W. Licochalcone D ameliorates oxidative stress-induced senescence via AMPK activation. Int J Mol Sci. 2021;22(14):7324.10.3390/ijms22147324Search in Google Scholar PubMed PubMed Central
[25] Szewczuk M, Boguszewska K, Kaźmierczak-Barańska J, Karwowski BT. The role of AMPK in metabolism and its influence on DNA damage repair. Mol Biol Rep. 2020;47(11):9075–86.10.1007/s11033-020-05900-xSearch in Google Scholar PubMed PubMed Central
[26] Sharma A, Anand SK, Singh N, Dwivedi UN, Kakkar P. AMP-activated protein kinase: an energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp Cell Res. 2023;428(1):113614.10.1016/j.yexcr.2023.113614Search in Google Scholar PubMed
[27] Rodríguez C, Muñoz M, Contreras C, Prieto D. AMPK, metabolism, and vascular function. FEBS J. 2021;288(12):3746–71.10.1111/febs.15863Search in Google Scholar PubMed
[28] Hu Y, Dong Y-D, Wu Y-C, Wang Q-X, Nan X, Wang D-L. AMPK inhibitor BML-275 induces neuroprotection through decreasing cyt c and AIF expression after transient brain ischemia. Bioorg Med Chem. 2021;52:116522.10.1016/j.bmc.2021.116522Search in Google Scholar PubMed
[29] Wu X, Liu X, Yang L, Wang Y. Berberine protects against neurological impairments and blood–brain barrier injury in mouse model of intracerebral hemorrhage. Neuroimmunomodulation. 2022;29(4):317–26.10.1159/000520747Search in Google Scholar PubMed
[30] Neumann NR, Thompson DC, Vasiliou V. AMPK activators for the prevention and treatment of neurodegenerative diseases. Expert Opin Drug Metab Toxicol. 2021;17(10):1199–210.10.1080/17425255.2021.1991308Search in Google Scholar PubMed
[31] Xin C, Liu J, Zhang J, Zhu D, Wang H, Xiong L, et al. Irisin improves fatty acid oxidation and glucose utilization in type 2 diabetes by regulating the AMPK signaling pathway. Int J Obes. 2016;40(3):443–51.10.1038/ijo.2015.199Search in Google Scholar PubMed
[32] Kong Y, Zhao C, Tan P, Liu S, Huang Y, Zeng F, et al. FGF21 reduces lipid accumulation in bovine hepatocytes by enhancing lipid oxidation and reducing lipogenesis via AMPK signaling. Animals. 2022;12(7):939.10.3390/ani12070939Search in Google Scholar PubMed PubMed Central
[33] Pirkmajer S, Kulkarni SS, Tom RZ, Ross FA, Hawley SA, Hardie DG, et al. Methotrexate promotes glucose uptake and lipid oxidation in skeletal muscle via AMPK activation. Diabetes. 2015;64(2):360–9.10.2337/db14-0508Search in Google Scholar PubMed PubMed Central
[34] Spaulding HR, Yan Z. AMPK and the adaptation to exercise. Annu Rev Physiol. 2022;84:209–7.10.1146/annurev-physiol-060721-095517Search in Google Scholar PubMed PubMed Central
[35] Xu W, Luo Y, Yin J, Luo F. Targeting AMPK signaling by polyphenols: a novel strategy for tackling aging. Food Funct. 2023;14(1):56–73.10.1039/D2FO02688KSearch in Google Scholar
[36] Li J, Wu K, Zhong Y, Kuang J, Huang N, Guo X, et al. Si–Ni-SAN ameliorates obesity through AKT/AMPK/HSL pathway-mediated lipolysis: network pharmacology and experimental validation. J Ethnopharmacol. 2023;302:115892.10.1016/j.jep.2022.115892Search in Google Scholar PubMed
[37] Abdou HM, Hamaad FA, Ali EY, Ghoneum MH. Antidiabetic efficacy of Trifolium alexandrinum extracts hesperetin and quercetin in ameliorating carbohydrate metabolism and activating IR and AMPK signaling in the pancreatic tissues of diabetic rats. Biomed Pharmacother. 2022;149:112838.10.1016/j.biopha.2022.112838Search in Google Scholar PubMed
[38] Fu A, Eberhard CE, Screaton RA. Role of AMPK in pancreatic beta cell function. Mol Cell Endocrinol. 2013;366(2):127–34.10.1016/j.mce.2012.06.020Search in Google Scholar PubMed
[39] Li Q, Chen H, Zhang M, Wu T, Liu R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food Funct. 2019;10(11):7174–87.10.1039/C9FO01465ASearch in Google Scholar PubMed
[40] Xu J, Li T, Xia X, Fu C, Wang X, Zhao Y. Dietary ginsenoside T19 supplementation regulates glucose and lipid metabolism via AMPK and PI3K pathways and its effect on intestinal microbiota. J Agric Food Chem. 2020;68(49):14452–62.10.1021/acs.jafc.0c04429Search in Google Scholar PubMed
[41] Xu W, Zhao T, Xiao H. The implication of oxidative stress and AMPK-Nrf2 antioxidative signaling in pneumonia pathogenesis. Front Endocrinol. 2020;11:400.10.3389/fendo.2020.00400Search in Google Scholar PubMed PubMed Central
[42] Yadav A, Rana U, Michalkiewicz T, Teng RJ, Konduri GG. Decreased AMP‐activated protein kinase (AMPK) function and protective effect of metformin in neonatal rat pups exposed to hyperoxia lung injury. Physiol Rep. 2020;8(18):e14587.10.14814/phy2.14587Search in Google Scholar PubMed PubMed Central
[43] Radhakrishnan J, Baetiong A, Kaufman H, Huynh M, Leschinsky A, Fresquez A, et al. Improved exercise capacity in cyclophilin-D knockout mice associated with enhanced oxygen utilization efficiency and augmented glucose uptake via AMPK-TBC1D1 signaling nexus. FASEB J: Off Publ Fed Am Soc Exp Biol. 2019;33(10):11443–57.10.1096/fj.201802238RSearch in Google Scholar PubMed
[44] Stapleton D, Woollatt E, Mitchelhill KI, Nicholl JK, Fernandez CS, Michell BJ, et al. AMP‐activated protein kinase isoenzyme family: subunit structure and chromosomal location. FEBS Lett. 1997;409(3):452–6.10.1016/S0014-5793(97)00569-3Search in Google Scholar PubMed
[45] Voss CM, Andersen JV, Jakobsen E, Siamka O, Karaca M, Maechler P, et al. AMP‐activated protein kinase (AMPK) regulates astrocyte oxidative metabolism by balancing TCA cycle dynamics. Glia. 2020;68(9):1824–39.10.1002/glia.23808Search in Google Scholar PubMed
[46] Ladli M, Richard C, Aguilar LC, Ducamp S, Bondu S, Sujobert P, et al. Finely-tuned regulation of AMP-activated protein kinase is crucial for human adult erythropoiesis. Haematologica. 2019;104(5):907.10.3324/haematol.2018.191403Search in Google Scholar PubMed PubMed Central
[47] Heidorn-Czarna M, Heidorn H-M, Fernando S, Sanislav O, Jarmuszkiewicz W, Mutzel R, et al. Chronic activation of AMPK induces mitochondrial biogenesis through differential phosphorylation and abundance of mitochondrial proteins in Dictyostelium discoideum. Int J Mol Sci. 2021;22(21):11675.10.3390/ijms222111675Search in Google Scholar PubMed PubMed Central
[48] Katwan OJ, Alghamdi F, Almabrouk TA, Mancini SJ, Kennedy S, Oakhill JS, et al. AMP-activated protein kinase complexes containing the β2 regulatory subunit are up-regulated during and contribute to adipogenesis. Biochem J. 2019;476(12):1725–40.10.1042/BCJ20180714Search in Google Scholar PubMed PubMed Central
[49] Sanz P, Rubio T, Garcia-Gimeno MA. AMPK beta subunits: more than just a scaffold in the formation of AMPK complex. FEBS J. 2013;280(16):3723–33.10.1111/febs.12364Search in Google Scholar PubMed
[50] Banskota S, Wang H, Kwon YH, Gautam J, Gurung P, Haq S, et al. Salicylates ameliorate intestinal inflammation by activating macrophage AMPK. Inflamm Bowel Dis. 2021;27(6):914–26.10.1093/ibd/izaa305Search in Google Scholar PubMed PubMed Central
[51] Li Y, Yang P, Zhao L, Chen Y, Zhang X, Zeng S, et al. CD36 plays a negative role in the regulation of lipophagy in hepatocytes through an AMPK-dependent pathway [S]. J Lipid Res. 2019;60(4):844–55.10.1194/jlr.M090969Search in Google Scholar PubMed PubMed Central
[52] Dou X, Zhou W-Y, Ding M, Ma Y-J, Yang Q-Q, Qian S-W, et al. The protease SENP2 controls hepatic gluconeogenesis by regulating the SUMOylation of the fuel sensor AMPKα. J Biol Chem. 2022;298(2):101544.10.1016/j.jbc.2021.101544Search in Google Scholar PubMed PubMed Central
[53] Yan Y, Mukherjee S, Harikumar KG, Strutzenberg TS, Zhou XE, Suino-Powell K, et al. Structure of an AMPK complex in an inactive, ATP-bound state. Science. 2021;373(6553):413–9.10.1126/science.abe7565Search in Google Scholar PubMed PubMed Central
[54] Ren Y, Chen J, Chen P, Hao Q, Cheong L-K, Tang M, et al. Oxidative stress-mediated AMPK inactivation determines the high susceptibility of LKB1-mutant NSCLC cells to glucose starvation. Free Radic Biol Med. 2021;166:128–39.10.1016/j.freeradbiomed.2021.02.018Search in Google Scholar PubMed
[55] MacDonald AF, Bettaieb A, Donohoe DR, Alani DS, Han A, Zhao Y, et al. Concurrent regulation of LKB1 and CaMKK2 in the activation of AMPK in castrate-resistant prostate cancer by a well-defined polyherbal mixture with anticancer properties. BMC Complement Altern Med. 2018;18(1):1–13.10.1186/s12906-018-2255-0Search in Google Scholar PubMed PubMed Central
[56] Jessen N, Koh H-J, Folmes CD, Wagg C, Fujii N, Løfgren B, et al. Ablation of LKB1 in the heart leads to energy deprivation and impaired cardiac function. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2010;1802(7-8):593–600.10.1016/j.bbadis.2010.04.008Search in Google Scholar PubMed PubMed Central
[57] Jeppesen J, Maarbjerg SJ, Jordy AB, Fritzen AM, Pehmøller C, Sylow L, et al. LKB1 regulates lipid oxidation during exercise independently of AMPK. Diabetes. 2013;62(5):1490–9.10.2337/db12-1160Search in Google Scholar PubMed PubMed Central
[58] Vargas R, Ortega Y, Bozo V, Andrade M, Minuzzi G, Cornejo P, et al. Thyroid hormone activates rat liver adenosine 5,-monophosphate-activated protein kinase: relation to CaMKKb, TAK1 and LKB1 expression and energy status. J Biol Regul Homeost Agents. 2013;27(4):989–99.Search in Google Scholar
[59] Zhang M, Yang D, Gong X, Ge P, Dai J, Lin L, et al. Protective benefits of AMP-activated protein kinase in hepatic ischemia-reperfusion injury. Am J Transl Res. 2017;9(3):823.Search in Google Scholar
[60] Kim M, Shen M, Ngoy S, Karamanlidis G, Liao R, Tian R. AMPK isoform expression in the normal and failing hearts. J Mol Cell Cardiol. 2012;52(5):1066–73.10.1016/j.yjmcc.2012.01.016Search in Google Scholar PubMed PubMed Central
[61] Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S. AMPK in cardiac fibrosis and repair: actions beyond metabolic regulation. J Mol Cell cardiology. 2016;91:188–200.10.1016/j.yjmcc.2016.01.001Search in Google Scholar PubMed
[62] Ross FA, MacKintosh C, Hardie DG. AMP-activated protein kinase: a cellular energy sensor that comes in 12 flavours. FEBS J. 2016;283(16):2987–3001.10.1111/febs.13698Search in Google Scholar PubMed PubMed Central
[63] Kim M, Tian R. Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol. 2011;51(4):548–53.10.1016/j.yjmcc.2010.12.004Search in Google Scholar PubMed PubMed Central
[64] Kundu A, Shelar S, Ghosh AP, Ballestas M, Kirkman R, Nam H, et al. 14-3-3 proteins protect AMPK-phosphorylated ten-eleven translocation-2 (TET2) from PP2A-mediated dephosphorylation. J Biol Chem. 2020;295(6):1754–66.10.1074/jbc.RA119.011089Search in Google Scholar PubMed PubMed Central
[65] Cai J, Chen X, Liu X, Li Z, Shi A, Tang X, et al. AMPK: the key to ischemia–reperfusion injury. J Cell Physiol. 2022;237(11):4079–96.10.1002/jcp.30875Search in Google Scholar PubMed
[66] Zhang F, Liu L, Xie Y, Wang J, Chen X, Zheng S, et al. Cardiac contractility modulation ameliorates myocardial metabolic remodeling in a rabbit model of chronic heart failure through activation of AMPK and PPAR-α pathway. Open Med. 2022;17(1):365–74.10.1515/med-2022-0415Search in Google Scholar PubMed PubMed Central
[67] Pasini E, Corsetti G, Dioguardi FS. Nutritional supplementation and exercise as essential allies in the treatment of chronic heart failure: the metabolic and molecular bases. Nutrients. 2023;15(10):2337.10.3390/nu15102337Search in Google Scholar PubMed PubMed Central
[68] Wan S, Cui Z, Wu L, Zhang F, Liu T, Hu J, et al. Ginsenoside Rd promotes omentin secretion in adipose through TBK1-AMPK to improve mitochondrial biogenesis via WNT5A/Ca(2+) pathways in heart failure. Redox Biol. 2023;60:102610.10.1016/j.redox.2023.102610Search in Google Scholar PubMed PubMed Central
[69] Madonna R, Moscato S, Cufaro MC, Pieragostino D, Mattii L, Del Boccio P, et al. Empagliflozin inhibits excessive autophagy through the AMPK/GSK3β signalling pathway in diabetic cardiomyopathy. Cardiovasc Res. 2023;119(5):1175–89.10.1093/cvr/cvad009Search in Google Scholar PubMed
[70] Bu Y, Peng M, Tang X, Xu X, Wu Y, Chen AF, et al. Protective effects of metformin in various cardiovascular diseases: clinical evidence and AMPK‐dependent mechanisms. J Cell Mol Med. 2022;26(19):4886–903.10.1111/jcmm.17519Search in Google Scholar PubMed PubMed Central
[71] Ge Y, Zhou M, Chen C, Wu X, Wang X. Role of AMPK mediated pathways in autophagy and aging. Biochimie. 2022;195:100–3.10.1016/j.biochi.2021.11.008Search in Google Scholar PubMed
[72] Gao J, Pan X, Li G, Chatterjee E, Xiao J. Physical exercise protects against endothelial dysfunction in cardiovascular and metabolic diseases. J Cardiovasc Transl Res. 2022;15(3):604–20.10.1007/s12265-021-10171-3Search in Google Scholar PubMed PubMed Central
[73] Peverill RE. Changes in left ventricular size, geometry, pump function and left heart pressures during healthy aging. Rev Cardiovasc Med. 2021;22(3):717–29.10.31083/j.rcm2203079Search in Google Scholar PubMed
[74] Yan M, Sun S, Xu K, Huang X, Dou L, Pang J, et al. Cardiac aging: from basic research to therapeutics. Oxid Med Cell Longev. 2021;2021:9570325.10.1155/2021/9570325Search in Google Scholar PubMed PubMed Central
[75] Abdellatif M, Rainer PP, Sedej S, Kroemer G. Hallmarks of cardiovascular ageing. Nat Rev Cardiol. 2023;10.1038/s41569-023-00881-3.10.1038/s41569-023-00881-3Search in Google Scholar PubMed
[76] Liberale L, Badimon L, Montecucco F, Lüscher TF, Libby P, Camici GG. Inflammation, aging, and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. 2022;79(8):837–47.10.1016/j.jacc.2021.12.017Search in Google Scholar PubMed PubMed Central
[77] Cole LK, Mejia EM, Sparagna GC, Vandel M, Xiang B, Han X, et al. Cardiolipin deficiency elevates susceptibility to a lipotoxic hypertrophic cardiomyopathy. J Mol Cell Cardiol. 2020;144:24–34.10.1016/j.yjmcc.2020.05.001Search in Google Scholar PubMed
[78] Deryabin PI, Shatrova AN, Borodkina AV. Apoptosis resistance of senescent cells is an intrinsic barrier for senolysis induced by cardiac glycosides. Cell Mol Life Sci. 2021;78(23):7757–76.10.1007/s00018-021-03980-xSearch in Google Scholar PubMed PubMed Central
[79] Wei X, Wu Y, Wang W, Zhang S, Liu D, Liu H. Decreased dynamin-related protein 1-related mitophagy induces myocardial apoptosis in the aging heart. Acta Biochim Biophys Sin. 2021;53(10):1354–66.10.1093/abbs/gmab112Search in Google Scholar PubMed
[80] Picca A, Calvani R, Coelho-Júnior HJ, Marzetti E. Mitophagy: at the heart of mitochondrial quality control in cardiac aging and frailty. Exp Gerontol. 2021;153:111508.10.1016/j.exger.2021.111508Search in Google Scholar PubMed
[81] Hamilton S, Terentyev D. Altered intracellular calcium homeostasis and arrhythmogenesis in the aged heart. Int J Mol Sci. 2019;20(10):2386.10.3390/ijms20102386Search in Google Scholar PubMed PubMed Central
[82] Cianflone E, Torella M, Biamonte F, De Angelis A, Urbanek K, Costanzo FS, et al. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells. 2020;9(6):1558.10.3390/cells9061558Search in Google Scholar PubMed PubMed Central
[83] Shimizu I. Exploration of new therapies for heart failure targeting age-related mechanisms. Circ J: Off J Jpn Circ Soc. 2023;10.1253/circj.CJ-23-0419.10.1253/circj.CJ-23-0419Search in Google Scholar PubMed
[84] Li L, Aslam M, Siegler BH, Niemann B, Rohrbach S. Comparative analysis of CTRP-mediated effects on cardiomyocyte glucose metabolism: cross talk between AMPK and Akt signaling pathway. Cells. 2021;10(4):905.10.3390/cells10040905Search in Google Scholar PubMed PubMed Central
[85] Xie X, Shu R, Yu C, Fu Z, Li Z. Mammalian AKT, the emerging roles on mitochondrial function in diseases. Aging Dis. 2022;13(1):157–74.10.14336/AD.2021.0729Search in Google Scholar PubMed PubMed Central
[86] Guo Z, Wang M, Ying X, Yuan J, Wang C, Zhang W, et al. Caloric restriction increases the resistance of aged heart to myocardial ischemia/reperfusion injury via modulating AMPK-SIRT(1)-PGC(1a) energy metabolism pathway. Sci Rep. 2023;13(1):2045.10.1038/s41598-023-27611-6Search in Google Scholar PubMed PubMed Central
[87] Sohn JY, Kwak HJ, Rhim JH, Yeo EJ. AMP-activated protein kinase-dependent nuclear localization of glyceraldehyde 3-phosphate dehydrogenase in senescent human diploid fibroblasts. Aging. 2022;14(1):4–27.10.18632/aging.203825Search in Google Scholar PubMed PubMed Central
[88] Hu C, Zhang X, Teng T, Ma ZG, Tang QZ. Cellular senescence in cardiovascular diseases: a systematic review. Aging Dis. 2022;13(1):103–28.10.14336/AD.2021.0927Search in Google Scholar PubMed PubMed Central
[89] Sung JY, Kim SG, Kang YJ, Choi HC. Metformin mitigates stress-induced premature senescence by upregulating AMPKα at Ser485 phosphorylation induced SIRT3 expression and inactivating mitochondrial oxidants. Mech Ageing Dev. 2022;206:111708.10.1016/j.mad.2022.111708Search in Google Scholar PubMed
[90] Wan X, Tian J, Hao P, Zhang J, Zhou Y, Ge C, et al. The cGAS-STING pathway: a ubiquitous checkpoint perturbing myocardial attributes. Curr Vasc Pharmacol. 2023;21(3):152–62.10.2174/1570161121666230501201756Search in Google Scholar PubMed
[91] Gong Y, Li G, Tao J, Wu NN, Kandadi MR, Bi Y, et al. Double knockout of Akt2 and AMPK accentuates high fat diet-induced cardiac anomalies through a cGAS-STING-mediated mechanism. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2020;1866(10):165855.10.1016/j.bbadis.2020.165855Search in Google Scholar PubMed
[92] Wang S, Tao J, Chen H, Kandadi MR, Sun M, Xu H, et al. Ablation of Akt2 and AMPKα2 rescues high fat diet-induced obesity and hepatic steatosis through Parkin-mediated mitophagy. Acta Pharm Sin B. 2021;11(11):3508–26.10.1016/j.apsb.2021.07.006Search in Google Scholar PubMed PubMed Central
[93] Wang S, Kandadi MR, Ren J. Double knockout of Akt2 and AMPK predisposes cardiac aging without affecting lifespan: role of autophagy and mitophagy. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2019;1865(7):1865–75.10.1016/j.bbadis.2018.08.011Search in Google Scholar PubMed PubMed Central
[94] Zhai J, Pan Y, Hao C, Wang X, Sun J. Caloric restriction induced epigenetic effects on aging. Front Cell Dev Biol. 2023;10:1079920.10.3389/fcell.2022.1079920Search in Google Scholar PubMed PubMed Central
[95] Li C, Yu L, Xue H, Yang Z, Yin Y, Zhang B, et al. Nuclear AMPK regulated CARM1 stabilization impacts autophagy in aged heart. Biochem Biophys Res Commun. 2017;486(2):398–405.10.1016/j.bbrc.2017.03.053Search in Google Scholar PubMed
[96] Shen W, Chen J, Zhou J, Martin CK, Ravussin E, Redman LM. Effect of 2-year caloric restriction on organ and tissue size in nonobese 21- to 50-year-old adults in a randomized clinical trial: the CALERIE study. Am J Clin Nutr. 2021;114(4):1295–303.10.1093/ajcn/nqab205Search in Google Scholar PubMed PubMed Central
[97] Caristia S, De Vito M, Sarro A, Leone A, Pecere A, Zibetti A, et al. Is caloric restriction associated with better healthy aging outcomes? A systematic review and meta-analysis of randomized controlled trials. Nutrients. 2020;12(8):2290.10.3390/nu12082290Search in Google Scholar PubMed PubMed Central
[98] Sheng Z, Xu J, Li F, Yuan Y, Peng X, Chen S, et al. The RING-domain E3 ubiquitin ligase RNF146 promotes cardiac hypertrophy by suppressing the LKB1/AMPK signaling pathway. Exp Cell Res. 2022;410(1):112954.10.1016/j.yexcr.2021.112954Search in Google Scholar PubMed
[99] Waziry R, Ryan CP, Corcoran DL, Huffman KM, Kobor MS, Kothari M, et al. Effect of long-term caloric restriction on DNA methylation measures of biological aging in healthy adults from the CALERIE trial. Nat Aging. 2023;3(3):248–57.10.1038/s43587-022-00357-ySearch in Google Scholar PubMed PubMed Central
[100] Wei M, Brandhorst S, Shelehchi M, Mirzaei H, Cheng CW, Budniak J, et al. Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017;9(377):eaai8700.10.1126/scitranslmed.aai8700Search in Google Scholar PubMed PubMed Central
[101] Di Giosia P, Stamerra CA, Giorgini P, Jamialahamdi T, Butler AE, Sahebkar A. The role of nutrition in inflammaging. Ageing Res Rev. 2022;77:101596.10.1016/j.arr.2022.101596Search in Google Scholar PubMed
[102] Eckel-Mahan K. The importance of “when” in calorie restriction-induced lifespan extension. J Cardiovasc Aging. 2023;3(1):5.10.20517/jca.2022.40Search in Google Scholar PubMed PubMed Central
[103] Perry CA, Gadde KM. The role of calorie restriction in the prevention of cardiovascular disease. Curr Atheroscler Rep. 2022;24(4):235–42.10.1007/s11883-022-00999-8Search in Google Scholar PubMed
[104] Vega-Martín E, Gonzalez-Blazquez R, Manzano-Lista FJ, Martín-Ramos M, Garcia-Prieto CF, Viana M, et al. Impact of caloric restriction on AMPK and endoplasmic reticulum stress in peripheral tissues and circulating peripheral blood mononuclear cells from Zucker rats. J Nutr Biochem. 2020;78:108342.10.1016/j.jnutbio.2020.108342Search in Google Scholar PubMed
[105] Sheng Y, Lv S, Huang M, Lv Y, Yu J, Liu J, et al. Opposing effects on cardiac function by calorie restriction in different-aged mice. Aging Cell. 2017;16(5):1155–67.10.1111/acel.12652Search in Google Scholar PubMed PubMed Central
[106] Niemann B, Pan R, Issa H, Simm A, Schulz R, Rohrbach S. AMPK activation is indispensable for the protective effects of caloric restriction on left ventricular function in postinfarct myocardium. Biology. 2022;11(3):448.10.3390/biology11030448Search in Google Scholar PubMed PubMed Central
[107] Gao C, Zhao S, Lian K, Mi B, Si R, Tan Z, et al. C1q/TNF-related protein 3 (CTRP3) and 9 (CTRP9) concentrations are decreased in patients with heart failure and are associated with increased morbidity and mortality. BMC Cardiovasc Disord. 2019;19(1):139.10.1186/s12872-019-1117-0Search in Google Scholar PubMed PubMed Central
[108] Zuo A, Zhao X, Li T, Li J, Lei S, Chen J, et al. CTRP9 knockout exaggerates lipotoxicity in cardiac myocytes and high-fat diet-induced cardiac hypertrophy through inhibiting the LKB1/AMPK pathway. J Cell Mol Med. 2020;24(4):2635–47.10.1111/jcmm.14982Search in Google Scholar PubMed PubMed Central
[109] Lee J, Yoo JH, Kim HS, Cho YK, La Lee Y, Lee WJ, et al. C1q/TNF-related protein-9 attenuates palmitic acid-induced endothelial cell senescence via increasing autophagy. Mol Cell Endocrinol. 2021;521:111114.10.1016/j.mce.2020.111114Search in Google Scholar PubMed
[110] Di S, Wang Z, Hu W, Yan X, Ma Z, Li X, et al. The protective effects of melatonin against LPS-induced septic myocardial injury: a potential role of AMPK-mediated autophagy. Front Endocrinol. 2020;11:162.10.3389/fendo.2020.00162Search in Google Scholar PubMed PubMed Central
[111] Zhao Q, Chen Y, Wang J, Small DS. Powerful three-sample genome-wide design and robust statistical inference in summary-data Mendelian randomization. Int J Epidemiol. 2019;48(5):1478–92.10.1093/ije/dyz142Search in Google Scholar PubMed
[112] van Vliet T, Varela-Eirin M, Wang B, Borghesan M, Brandenburg SM, Franzin R, et al. Physiological hypoxia restrains the senescence-associated secretory phenotype via AMPK-mediated mTOR suppression. Mol Cell. 2021;81(9):2041–52.e6.10.1016/j.molcel.2021.03.018Search in Google Scholar PubMed
[113] André E, De Pauw A, Verdoy R, Brusa D, Bouzin C, Timmermans A, et al. Changes of metabolic phenotype of cardiac progenitor cells during differentiation: neutral effect of stimulation of AMP-activated protein kinase. Stem Cell Dev. 2019;28(22):1498–513.10.1089/scd.2019.0129Search in Google Scholar PubMed
[114] Chen YP, Kuo WW, Baskaran R, Day CH, Chen RJ, Wen SY, et al. Acute hypoxic preconditioning prevents palmitic acid-induced cardiomyocyte apoptosis via switching metabolic GLUT4-glucose pathway back to CD36-fatty acid dependent. J Cell Biochem. 2018;119(4):3363–72.10.1002/jcb.26501Search in Google Scholar PubMed
[115] de Lucia C, Piedepalumbo M, Wang L, Carnevale Neto F, Raftery D, Gao E, et al. Effects of myocardial ischemia/reperfusion injury on plasma metabolomic profile during aging. Aging Cell. 2021;20(1):e13284.10.1111/acel.13284Search in Google Scholar PubMed PubMed Central
[116] Zhu J, Wang Y-F, Chai X-M, Qian K, Zhang L-W, Peng P, et al. Exogenous NADPH ameliorates myocardial ischemia–reperfusion injury in rats through activating AMPK/mTOR pathway. Acta Pharmacol Sin. 2020;41(4):535–45.10.1038/s41401-019-0301-1Search in Google Scholar PubMed PubMed Central
[117] Wang C, Zhu L, Yuan W, Sun L, Xia Z, Zhang Z, et al. Diabetes aggravates myocardial ischaemia reperfusion injury via activating Nox2-related programmed cell death in an AMPK‐dependent manner. J Cell Mol Med. 2020;24(12):6670–9.10.1111/jcmm.15318Search in Google Scholar PubMed PubMed Central
[118] Diguet N, Trammell SA, Tannous C, Deloux R, Piquereau J, Mougenot N, et al. Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation. 2018;137(21):2256–73.10.1161/CIRCULATIONAHA.116.026099Search in Google Scholar PubMed PubMed Central
[119] Lai Y, Wang L, Liu W. Nicotinamide pretreatment alleviates mitochondrial stress and protects hypoxic myocardial cells via AMPK pathway. Eur Rev Med Pharmacol Sci. 2019;23(4):1797–806.Search in Google Scholar
[120] Lin Q, Zuo W, Liu Y, Wu K, Liu Q. NAD(+) and cardiovascular diseases. Clin Chim Acta; Int J Clin Chem. 2021;515:104–10.10.1016/j.cca.2021.01.012Search in Google Scholar PubMed
[121] Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19(2):121–35.10.1038/nrm.2017.95Search in Google Scholar PubMed PubMed Central
[122] Wang Y-J, Paneni F, Stein S, Matter CM. Modulating sirtuin biology and nicotinamide adenine diphosphate metabolism in cardiovascular disease—from bench to bedside. Front Physiol. 2021;12:755060.10.3389/fphys.2021.755060Search in Google Scholar PubMed PubMed Central
[123] Barcena ML, Pozdniakova S, Haritonow N, Breiter P, Kühl AA, Milting H, et al. Dilated cardiomyopathy impairs mitochondrial biogenesis and promotes inflammation in an age- and sex-dependent manner. Aging (Albany NY). 2020;12(23):24117.10.18632/aging.202283Search in Google Scholar PubMed PubMed Central
[124] Pereira AS, Gouveia AM, Tomada N, Rodrigues AR, Neves D. Cumulative effect of cardiovascular risk factors on regulation of AMPK/SIRT1-PGC-1α-SIRT3 pathway in the human erectile tissue. Oxid Med Cell Longev. 2020;2020:1525949.10.1155/2020/1525949Search in Google Scholar PubMed PubMed Central
[125] Tang X, Chen X-F, Wang N-Y, Wang X-M, Liang S-T, Zheng W, et al. SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation. 2017;136(21):2051–67.10.1161/CIRCULATIONAHA.117.028728Search in Google Scholar PubMed PubMed Central
[126] He L, Liu F, Li J. Mitochondrial sirtuins and doxorubicin-induced cardiotoxicity. Cardiovasc Toxicol. 2021;21(3):179–91.10.1007/s12012-020-09626-xSearch in Google Scholar PubMed
[127] Buler M, Aatsinki SM, Izzi V, Uusimaa J, Hakkola J. SIRT5 is under the control of PGC-1α and AMPK and is involved in regulation of mitochondrial energy metabolism. FASEB J. 2014;28(7):3225–37.10.1096/fj.13-245241Search in Google Scholar PubMed
[128] Jackson CW, Escobar I, Xu J, Perez-Pinzon MA. Effects of ischemic preconditioning on mitochondrial and metabolic neruoprotection: 5’adenosine monophosphate-activated protein kinase and sirtuins. Brain Circ. 2018;4(2):54.10.4103/bc.bc_7_18Search in Google Scholar PubMed PubMed Central
[129] Liberale L, Akhmedov A, Vlachogiannis NI, Bonetti NR, Nageswaran V, Miranda MX, et al. Sirtuin 5 promotes arterial thrombosis by blunting the fibrinolytic system. Cardiovasc Res. 2021;117(10):2275–88.10.1093/cvr/cvaa268Search in Google Scholar PubMed
[130] Fiorentino F, Mai A, Rotili D. Emerging therapeutic potential of SIRT6 modulators. J Med Chem. 2021;64(14):9732–58.10.1021/acs.jmedchem.1c00601Search in Google Scholar PubMed PubMed Central
[131] Pillai VB, Samant S, Hund S, Gupta M, Gupta MP. The nuclear sirtuin SIRT6 protects the heart from developing aging-associated myocyte senescence and cardiac hypertrophy. Aging (Albany NY). 2021;13(9):12334.10.18632/aging.203027Search in Google Scholar PubMed PubMed Central
[132] Yu LM, Dong X, Xue XD, Xu S, Zhang X, Xu YL, et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: role of SIRT6. J Pineal Res. 2021;70(1):e12698.10.1111/jpi.12698Search in Google Scholar PubMed
[133] Sarikhani M, Garbern JC, Ma S, Sereda R, Conde J, Krähenbühl G, et al. Sustained activation of AMPK enhances differentiation of human iPSC-derived cardiomyocytes via sirtuin activation. Stem Cell Rep. 2020;15(2):498–514.10.1016/j.stemcr.2020.06.012Search in Google Scholar PubMed PubMed Central
[134] Tang M, Tang H, Tu B, Zhu W-G. SIRT7: a sentinel of genome stability. Open Biol. 2021;11(6):210047.10.1098/rsob.210047Search in Google Scholar PubMed PubMed Central
[135] Mizumoto T, Yoshizawa T, Sato Y, Ito T, Tsuyama T, Satoh A, et al. SIRT7 deficiency protects against aging-associated glucose intolerance and extends lifespan in male mice. Cells. 2022;11(22):3609.10.3390/cells11223609Search in Google Scholar PubMed PubMed Central
[136] Vakhrusheva O, Smolka C, Gajawada P, Kostin S, Boettger T, Kubin T, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102(6):703–10.10.1161/CIRCRESAHA.107.164558Search in Google Scholar PubMed
[137] Fukuda M, Yoshizawa T, Karim MF, Sobuz SU, Korogi W, Kobayasi D, et al. SIRT7 has a critical role in bone formation by regulating lysine acylation of SP7/Osterix. Nat Commun. 2018;9(1):2833.10.1038/s41467-018-05187-4Search in Google Scholar PubMed PubMed Central
[138] Lagunas-Rangel FA. SIRT7 in the aging process. Cell Mol Life Sci. 2022;79(6):297.10.1007/s00018-022-04342-xSearch in Google Scholar PubMed PubMed Central
[139] Tang X, Li G, Shi L, Su F, Qian M, Liu Z, et al. Combined intermittent fasting and ERK inhibition enhance the anti-tumor effects of chemotherapy via the GSK3β-SIRT7 axis. Nat Commun. 2021;12(1):5058.10.1038/s41467-021-25274-3Search in Google Scholar PubMed PubMed Central
[140] Takasugi M, Yoshida Y, Hara E, Ohtani N. The role of cellular senescence and SASP in tumour microenvironment. FEBS J. 2023;290(5):1348–61.10.1111/febs.16381Search in Google Scholar PubMed
[141] Wang TW, Johmura Y, Suzuki N, Omori S, Migita T, Yamaguchi K, et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature. 2022;611(7935):358–64.10.1038/s41586-022-05388-4Search in Google Scholar PubMed
[142] Sung JY, Kim SG, Kim J-R, Choi HC. Prednisolone suppresses adriamycin-induced vascular smooth muscle cell senescence and inflammatory response via the SIRT1-AMPK signaling pathway. PLoS One. 2020;15(9):e0239976.10.1371/journal.pone.0239976Search in Google Scholar PubMed PubMed Central
[143] Mohammed I, Hollenberg MD, Ding H, Triggle CR. A critical review of the evidence that metformin is a putative anti-aging drug that enhances healthspan and extends lifespan. Front Endocrinol. 2021;12:718942.10.3389/fendo.2021.718942Search in Google Scholar PubMed PubMed Central
[144] Feng YY, Wang Z, Pang H. Role of metformin in inflammation. Mol Biol Rep. 2023;50(1):789–98.10.1007/s11033-022-07954-5Search in Google Scholar PubMed
[145] Feng X, Chen W, Ni X, Little PJ, Xu S, Tang L, et al. Metformin, macrophage dysfunction and atherosclerosis. Front Immunol. 2021;12:682853.10.3389/fimmu.2021.682853Search in Google Scholar PubMed PubMed Central
[146] Hou J, Chen J, Fan J, Tang Z, Zhou W, Lin H. Inhibition of NF-κB signaling-mediated crosstalk between macrophages and preosteoblasts by metformin alleviates trauma-induced heterotopic ossification. Inflammation. 2023;46(4):1414–29.10.1007/s10753-023-01817-2Search in Google Scholar PubMed
[147] Rizvi F, Preston CC, Emelyanova L, Yousufuddin M, Viqar M, Dakwar O, et al. Effects of aging on cardiac oxidative stress and transcriptional changes in pathways of reactive oxygen species generation and clearance. J Am Heart Assoc. 2021;10(16):e019948.10.1161/JAHA.120.019948Search in Google Scholar PubMed PubMed Central
[148] Chen J, Li L, Bai X, Xiao L, Shangguan J, Zhang W, et al. Inhibition of autophagy prevents Panax notoginseng saponins (PNS) protection on cardiac myocytes against endoplasmic reticulum (ER) stress-induced mitochondrial injury, 2+homeostasis and associated apoptosis. Front Pharmacol. 2021;12:620812.10.3389/fphar.2021.620812Search in Google Scholar PubMed PubMed Central
[149] Brandt EB, Li X, Nelson TJ. Activation of P53 via nutlin-3a reveals role for P53 in ROS signaling during cardiac differentiation of hiPSCs. J Stem Cell Rep. 2021;3(1).Search in Google Scholar
[150] Zhang J, Wei C, Wang H, Tang S, Jia Z, Wang L, et al. Protective effect of qiliqiangxin capsule on energy metabolism and myocardial mitochondria in pressure overload heart failure rats. Evid-Based Complement Altern Med. 2013;2013:378298.10.1155/2013/378298Search in Google Scholar PubMed PubMed Central
[151] Hinchy EC, Gruszczyk AV, Willows R, Navaratnam N, Hall AR, Bates G, et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J Biol Chem. 2018;293(44):17208–17.10.1074/jbc.RA118.002579Search in Google Scholar PubMed PubMed Central
[152] Zhang L, Tian J, Diao S, Zhang G, Xiao M, Chang D. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction. Chem-Biol Interact. 2020;332:109252.10.1016/j.cbi.2020.109252Search in Google Scholar PubMed
[153] Marino A, Hausenloy DJ, Andreadou I, Horman S, Bertrand L, Beauloye C. AMP-activated protein kinase: a remarkable contributor to preserve a healthy heart against ROS injury. Free Radic Biol Med. 2021;166:238–54.10.1016/j.freeradbiomed.2021.02.047Search in Google Scholar PubMed
[154] Park J-M, Do VQ, Seo Y-S, Kim HJ, Nam JH, Yin MZ, et al. NADPH oxidase 1 mediates acute blood pressure response to angiotensin II by contributing to calcium influx in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2022;42(5):e117–e30.10.1161/ATVBAHA.121.317239Search in Google Scholar PubMed
[155] Visnagri A, Oexner RR, Kmiotek-Wasylewska K, Zhang M, Zoccarato A, Shah AM. Nicotinamide adenosine dinucleotide phosphate oxidase-mediated signaling in cardiac remodeling. Antioxid Redox Signal. 2023;38(4):371–87.10.1089/ars.2022.0176Search in Google Scholar PubMed
[156] Qian X, Wu W, Hu H, Yu X, Wang S, Zhu J, et al. The role of reactive oxygen species derived from different NADPH oxidase isoforms and mitochondria in oxalate-induced oxidative stress and cell injury. Urolithiasis. 2022;50(2):149–58.10.1007/s00240-022-01309-2Search in Google Scholar PubMed PubMed Central
[157] Dambrova M, Zuurbier CJ, Borutaite V, Liepinsh E, Makrecka-Kuka M. Energy substrate metabolism and mitochondrial oxidative stress in cardiac ischemia/reperfusion injury. Free Radic Biol Med. 2021;165:24–37.10.1016/j.freeradbiomed.2021.01.036Search in Google Scholar PubMed
[158] Mighiu AS, Recalde A, Ziberna K, Carnicer R, Tomek J, Bub G, et al. Inducibility, but not stability, of atrial fibrillation is increased by NOX2 overexpression in mice. Cardiovasc Res. 2021;117(11):2354–64.10.1093/cvr/cvab019Search in Google Scholar PubMed PubMed Central
[159] Matsushima S, Sadoshima J. Yin and Yang of NADPH oxidases in myocardial ischemia-reperfusion. Antioxidants. 2022;11(6):1069.10.3390/antiox11061069Search in Google Scholar PubMed PubMed Central
[160] Yu Q, Zhao G, Liu J, Peng Y, Xu X, Zhao F, et al. The role of histone deacetylases in cardiac energy metabolism in heart diseases. Metab: Clin Exp. 2023;142:155532.10.1016/j.metabol.2023.155532Search in Google Scholar PubMed
[161] Barbagallo I, Galvano F, Frigiola A, Cappello F, Riccioni G, Murabito P, et al. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid Redox Signal. 2013;18(5):507–21.10.1089/ars.2011.4360Search in Google Scholar PubMed
[162] Shi Y, Hou S-A. Protective effects of metformin against myocardial ischemia‑reperfusion injury via AMPK‑dependent suppression of NOX4. Mol Med Rep. 2021;24(4):1–10.10.3892/mmr.2021.12351Search in Google Scholar PubMed PubMed Central
[163] Kandula N, Kumar S, Mandlem VKK, Siddabathuni A, Singh S, Kosuru R. Role of AMPK in myocardial ischemia-reperfusion injury-induced cell death in the presence and absence of diabetes. Oxid Med Cell Longev. 2022;2022:7346699.10.1155/2022/7346699Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
- Immunosuppressive treatment for idiopathic membranous nephropathy: An updated network meta-analysis
- Identifying two pathogenic variants in a patient with pigmented paravenous retinochoroidal atrophy
- Effects of phytoestrogens combined with cold stress on sperm parameters and testicular proteomics in rats
- A case of pulmonary embolism with bad warfarin anticoagulant effects caused by E. coli infection
- Neutrophilia with subclinical Cushing’s disease: A case report and literature review
- Isoimperatorin alleviates lipopolysaccharide-induced periodontitis by downregulating ERK1/2 and NF-κB pathways
- Immunoregulation of synovial macrophages for the treatment of osteoarthritis
- Novel CPLANE1 c.8948dupT (p.P2984Tfs*7) variant in a child patient with Joubert syndrome
- Antiphospholipid antibodies and the risk of thrombosis in myeloproliferative neoplasms
- Immunological responses of septic rats to combination therapy with thymosin α1 and vitamin C
- High glucose and high lipid induced mitochondrial dysfunction in JEG-3 cells through oxidative stress
- Pharmacological inhibition of the ubiquitin-specific protease 8 effectively suppresses glioblastoma cell growth
- Levocarnitine regulates the growth of angiotensin II-induced myocardial fibrosis cells via TIMP-1
- Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study
- Single-cell transcription analysis reveals the tumor origin and heterogeneity of human bilateral renal clear cell carcinoma
- Identification of iron metabolism-related genes as diagnostic signatures in sepsis by blood transcriptomic analysis
- Long noncoding RNA ACART knockdown decreases 3T3-L1 preadipocyte proliferation and differentiation
- Surgery, adjuvant immunotherapy plus chemotherapy and radiotherapy for primary malignant melanoma of the parotid gland (PGMM): A case report
- Dosimetry comparison with helical tomotherapy, volumetric modulated arc therapy, and intensity-modulated radiotherapy for grade II gliomas: A single‑institution case series
- Soy isoflavone reduces LPS-induced acute lung injury via increasing aquaporin 1 and aquaporin 5 in rats
- Refractory hypokalemia with sexual dysplasia and infertility caused by 17α-hydroxylase deficiency and triple X syndrome: A case report
- Meta-analysis of cancer risk among end stage renal disease undergoing maintenance dialysis
- 6-Phosphogluconate dehydrogenase inhibition arrests growth and induces apoptosis in gastric cancer via AMPK activation and oxidative stress
- Experimental study on the optimization of ANM33 release in foam cells
- Primary retroperitoneal angiosarcoma: A case report
- Metabolomic analysis-identified 2-hydroxybutyric acid might be a key metabolite of severe preeclampsia
- Malignant pleural effusion diagnosis and therapy
- Effect of spaceflight on the phenotype and proteome of Escherichia coli
- Comparison of immunotherapy combined with stereotactic radiotherapy and targeted therapy for patients with brain metastases: A systemic review and meta-analysis
- Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation
- Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy
- The role of IL-31 and IL-34 in the diagnosis and treatment of chronic periodontitis
- Triple-negative mouse breast cancer initiating cells show high expression of beta1 integrin and increased malignant features
- mNGS facilitates the accurate diagnosis and antibiotic treatment of suspicious critical CNS infection in real practice: A retrospective study
- The apatinib and pemetrexed combination has antitumor and antiangiogenic effects against NSCLC
- Radiotherapy for primary thyroid adenoid cystic carcinoma
- Design and functional preliminary investigation of recombinant antigen EgG1Y162–EgG1Y162 against Echinococcus granulosus
- Effects of losartan in patients with NAFLD: A meta-analysis of randomized controlled trial
- Bibliometric analysis of METTL3: Current perspectives, highlights, and trending topics
- Performance comparison of three scaling algorithms in NMR-based metabolomics analysis
- PI3K/AKT/mTOR pathway and its related molecules participate in PROK1 silence-induced anti-tumor effects on pancreatic cancer
- The altered expression of cytoskeletal and synaptic remodeling proteins during epilepsy
- Effects of pegylated recombinant human granulocyte colony-stimulating factor on lymphocytes and white blood cells of patients with malignant tumor
- Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report
- NUDT21 relieves sevoflurane-induced neurological damage in rats by down-regulating LIMK2
- Association of interleukin-10 rs1800896, rs1800872, and interleukin-6 rs1800795 polymorphisms with squamous cell carcinoma risk: A meta-analysis
- Exosomal HBV-DNA for diagnosis and treatment monitoring of chronic hepatitis B
- Shear stress leads to the dysfunction of endothelial cells through the Cav-1-mediated KLF2/eNOS/ERK signaling pathway under physiological conditions
- Interaction between the PI3K/AKT pathway and mitochondrial autophagy in macrophages and the leukocyte count in rats with LPS-induced pulmonary infection
- Meta-analysis of the rs231775 locus polymorphism in the CTLA-4 gene and the susceptibility to Graves’ disease in children
- Cloning, subcellular localization and expression of phosphate transporter gene HvPT6 of hulless barley
- Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome
- Significant elevated CXCL14 and decreased IL-39 levels in patients with tuberculosis
- Whole-exome sequencing applications in prenatal diagnosis of fetal bowel dilatation
- Gemella morbillorum infective endocarditis: A case report and literature review
- An unusual ectopic thymoma clonal evolution analysis: A case report
- Severe cumulative skin toxicity during toripalimab combined with vemurafenib following toripalimab alone
- Detection of V. vulnificus septic shock with ARDS using mNGS
- Novel rare genetic variants of familial and sporadic pulmonary atresia identified by whole-exome sequencing
- The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology
- Novel compound heterozygous mutations in TELO2 in an infant with You-Hoover-Fong syndrome: A case report and literature review
- ctDNA as a prognostic biomarker in resectable CLM: Systematic review and meta-analysis
- Diagnosis of primary amoebic meningoencephalitis by metagenomic next-generation sequencing: A case report
- Phylogenetic analysis of promoter regions of human Dolichol kinase (DOLK) and orthologous genes using bioinformatics tools
- Collagen changes in rabbit conjunctiva after conjunctival crosslinking
- Effects of NM23 transfection of human gastric carcinoma cells in mice
- Oral nifedipine and phytosterol, intravenous nicardipine, and oral nifedipine only: Three-arm, retrospective, cohort study for management of severe preeclampsia
- Case report of hepatic retiform hemangioendothelioma: A rare tumor treated with ultrasound-guided microwave ablation
- Curcumin induces apoptosis in human hepatocellular carcinoma cells by decreasing the expression of STAT3/VEGF/HIF-1α signaling
- Rare presentation of double-clonal Waldenström macroglobulinemia with pulmonary embolism: A case report
- Giant duplication of the transverse colon in an adult: A case report and literature review
- Ectopic thyroid tissue in the breast: A case report
- SDR16C5 promotes proliferation and migration and inhibits apoptosis in pancreatic cancer
- Vaginal metastasis from breast cancer: A case report
- Screening of the best time window for MSC transplantation to treat acute myocardial infarction with SDF-1α antibody-loaded targeted ultrasonic microbubbles: An in vivo study in miniswine
- Inhibition of TAZ impairs the migration ability of melanoma cells
- Molecular complexity analysis of the diagnosis of Gitelman syndrome in China
- Effects of maternal calcium and protein intake on the development and bone metabolism of offspring mice
- Identification of winter wheat pests and diseases based on improved convolutional neural network
- Ultra-multiplex PCR technique to guide treatment of Aspergillus-infected aortic valve prostheses
- Virtual high-throughput screening: Potential inhibitors targeting aminopeptidase N (CD13) and PIKfyve for SARS-CoV-2
- Immune checkpoint inhibitors in cancer patients with COVID-19
- Utility of methylene blue mixed with autologous blood in preoperative localization of pulmonary nodules and masses
- Integrated analysis of the microbiome and transcriptome in stomach adenocarcinoma
- Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy
- DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway
- Lung abscess by Fusobacterium nucleatum and Streptococcus spp. co-infection by mNGS: A case series
- Genetic alterations of KRAS and TP53 in intrahepatic cholangiocarcinoma associated with poor prognosis
- Granulomatous polyangiitis involving the fourth ventricle: Report of a rare case and a literature review
- Studying infant mortality: A demographic analysis based on data mining models
- Metaplastic breast carcinoma with osseous differentiation: A report of a rare case and literature review
- Protein Z modulates the metastasis of lung adenocarcinoma cells
- Inhibition of pyroptosis and apoptosis by capsaicin protects against LPS-induced acute kidney injury through TRPV1/UCP2 axis in vitro
- TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats
- Primary mediastinum Ewing’s sarcoma with pleural effusion: A case report and literature review
- Association of ADRB2 gene polymorphisms and intestinal microbiota in Chinese Han adolescents
- Tanshinone IIA alleviates chondrocyte apoptosis and extracellular matrix degeneration by inhibiting ferroptosis
- Study on the cytokines related to SARS-Cov-2 in testicular cells and the interaction network between cells based on scRNA-seq data
- Effect of periostin on bone metabolic and autophagy factors during tooth eruption in mice
- HP1 induces ferroptosis of renal tubular epithelial cells through NRF2 pathway in diabetic nephropathy
- Intravaginal estrogen management in postmenopausal patients with vaginal squamous intraepithelial lesions along with CO2 laser ablation: A retrospective study
- Hepatocellular carcinoma cell differentiation trajectory predicts immunotherapy, potential therapeutic drugs, and prognosis of patients
- Effects of physical exercise on biomarkers of oxidative stress in healthy subjects: A meta-analysis of randomized controlled trials
- Identification of lysosome-related genes in connection with prognosis and immune cell infiltration for drug candidates in head and neck cancer
- Development of an instrument-free and low-cost ELISA dot-blot test to detect antibodies against SARS-CoV-2
- Research progress on gas signal molecular therapy for Parkinson’s disease
- Adiponectin inhibits TGF-β1-induced skin fibroblast proliferation and phenotype transformation via the p38 MAPK signaling pathway
- The G protein-coupled receptor-related gene signatures for predicting prognosis and immunotherapy response in bladder urothelial carcinoma
- α-Fetoprotein contributes to the malignant biological properties of AFP-producing gastric cancer
- CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa
- Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis
- Significance of sTREM-1 and sST2 combined diagnosis for sepsis detection and prognosis prediction
- Diagnostic value of serum neuroactive substances in the acute exacerbation of chronic obstructive pulmonary disease complicated with depression
- Research progress of AMP-activated protein kinase and cardiac aging
- TRIM29 knockdown prevented the colon cancer progression through decreasing the ubiquitination levels of KRT5
- Cross-talk between gut microbiota and liver steatosis: Complications and therapeutic target
- Metastasis from small cell lung cancer to ovary: A case report
- The early diagnosis and pathogenic mechanisms of sepsis-related acute kidney injury
- The effect of NK cell therapy on sepsis secondary to lung cancer: A case report
- Erianin alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation
- Loss of ACOX1 in clear cell renal cell carcinoma and its correlation with clinical features
- Signalling pathways in the osteogenic differentiation of periodontal ligament stem cells
- Crosstalk between lactic acid and immune regulation and its value in the diagnosis and treatment of liver failure
- Clinicopathological features and differential diagnosis of gastric pleomorphic giant cell carcinoma
- Traumatic brain injury and rTMS-ERPs: Case report and literature review
- Extracellular fibrin promotes non-small cell lung cancer progression through integrin β1/PTEN/AKT signaling
- Knockdown of DLK4 inhibits non-small cell lung cancer tumor growth by downregulating CKS2
- The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles
- Inflammation-related signaling pathways in tendinopathy
- CD4+ T cell count in HIV/TB co-infection and co-occurrence with HL: Case report and literature review
- Clinical analysis of severe Chlamydia psittaci pneumonia: Case series study
- Bioinformatics analysis to identify potential biomarkers for the pulmonary artery hypertension associated with the basement membrane
- Influence of MTHFR polymorphism, alone or in combination with smoking and alcohol consumption, on cancer susceptibility
- Catharanthus roseus (L.) G. Don counteracts the ampicillin resistance in multiple antibiotic-resistant Staphylococcus aureus by downregulation of PBP2a synthesis
- Combination of a bronchogenic cyst in the thoracic spinal canal with chronic myelocytic leukemia
- Bacterial lipoprotein plays an important role in the macrophage autophagy and apoptosis induced by Salmonella typhimurium and Staphylococcus aureus
- TCL1A+ B cells predict prognosis in triple-negative breast cancer through integrative analysis of single-cell and bulk transcriptomic data
- Ezrin promotes esophageal squamous cell carcinoma progression via the Hippo signaling pathway
- Ferroptosis: A potential target of macrophages in plaque vulnerability
- Predicting pediatric Crohn's disease based on six mRNA-constructed risk signature using comprehensive bioinformatic approaches
- Applications of genetic code expansion and photosensitive UAAs in studying membrane proteins
- HK2 contributes to the proliferation, migration, and invasion of diffuse large B-cell lymphoma cells by enhancing the ERK1/2 signaling pathway
- IL-17 in osteoarthritis: A narrative review
- Circadian cycle and neuroinflammation
- Probiotic management and inflammatory factors as a novel treatment in cirrhosis: A systematic review and meta-analysis
- Hemorrhagic meningioma with pulmonary metastasis: Case report and literature review
- SPOP regulates the expression profiles and alternative splicing events in human hepatocytes
- Knockdown of SETD5 inhibited glycolysis and tumor growth in gastric cancer cells by down-regulating Akt signaling pathway
- PTX3 promotes IVIG resistance-induced endothelial injury in Kawasaki disease by regulating the NF-κB pathway
- Pancreatic ectopic thyroid tissue: A case report and analysis of literature
- The prognostic impact of body mass index on female breast cancer patients in underdeveloped regions of northern China differs by menopause status and tumor molecular subtype
- Report on a case of liver-originating malignant melanoma of unknown primary
- Case report: Herbal treatment of neutropenic enterocolitis after chemotherapy for breast cancer
- The fibroblast growth factor–Klotho axis at molecular level
- Characterization of amiodarone action on currents in hERG-T618 gain-of-function mutations
- A case report of diagnosis and dynamic monitoring of Listeria monocytogenes meningitis with NGS
- Effect of autologous platelet-rich plasma on new bone formation and viability of a Marburg bone graft
- Small breast epithelial mucin as a useful prognostic marker for breast cancer patients
- Continuous non-adherent culture promotes transdifferentiation of human adipose-derived stem cells into retinal lineage
- Nrf3 alleviates oxidative stress and promotes the survival of colon cancer cells by activating AKT/BCL-2 signal pathway
- Favorable response to surufatinib in a patient with necrolytic migratory erythema: A case report
- Case report of atypical undernutrition of hypoproteinemia type
- Down-regulation of COL1A1 inhibits tumor-associated fibroblast activation and mediates matrix remodeling in the tumor microenvironment of breast cancer
- Sarcoma protein kinase inhibition alleviates liver fibrosis by promoting hepatic stellate cells ferroptosis
- Research progress of serum eosinophil in chronic obstructive pulmonary disease and asthma
- Clinicopathological characteristics of co-existing or mixed colorectal cancer and neuroendocrine tumor: Report of five cases
- Role of menopausal hormone therapy in the prevention of postmenopausal osteoporosis
- Precisional detection of lymph node metastasis using tFCM in colorectal cancer
- Advances in diagnosis and treatment of perimenopausal syndrome
- A study of forensic genetics: ITO index distribution and kinship judgment between two individuals
- Acute lupus pneumonitis resembling miliary tuberculosis: A case-based review
- Plasma levels of CD36 and glutathione as biomarkers for ruptured intracranial aneurysm
- Fractalkine modulates pulmonary angiogenesis and tube formation by modulating CX3CR1 and growth factors in PVECs
- Novel risk prediction models for deep vein thrombosis after thoracotomy and thoracoscopic lung cancer resections, involving coagulation and immune function
- Exploring the diagnostic markers of essential tremor: A study based on machine learning algorithms
- Evaluation of effects of small-incision approach treatment on proximal tibia fracture by deep learning algorithm-based magnetic resonance imaging
- An online diagnosis method for cancer lesions based on intelligent imaging analysis
- Medical imaging in rheumatoid arthritis: A review on deep learning approach
- Predictive analytics in smart healthcare for child mortality prediction using a machine learning approach
- Utility of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in predicting acute-on-chronic liver failure survival
- A biomedical decision support system for meta-analysis of bilateral upper-limb training in stroke patients with hemiplegia
- TNF-α and IL-8 levels are positively correlated with hypobaric hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats
- Stochastic gradient descent optimisation for convolutional neural network for medical image segmentation
- Comparison of the prognostic value of four different critical illness scores in patients with sepsis-induced coagulopathy
- Application and teaching of computer molecular simulation embedded technology and artificial intelligence in drug research and development
- Hepatobiliary surgery based on intelligent image segmentation technology
- Value of brain injury-related indicators based on neural network in the diagnosis of neonatal hypoxic-ischemic encephalopathy
- Analysis of early diagnosis methods for asymmetric dementia in brain MR images based on genetic medical technology
- Early diagnosis for the onset of peri-implantitis based on artificial neural network
- Clinical significance of the detection of serum IgG4 and IgG4/IgG ratio in patients with thyroid-associated ophthalmopathy
- Forecast of pain degree of lumbar disc herniation based on back propagation neural network
- SPA-UNet: A liver tumor segmentation network based on fused multi-scale features
- Systematic evaluation of clinical efficacy of CYP1B1 gene polymorphism in EGFR mutant non-small cell lung cancer observed by medical image
- Rehabilitation effect of intelligent rehabilitation training system on hemiplegic limb spasms after stroke
- A novel approach for minimising anti-aliasing effects in EEG data acquisition
- ErbB4 promotes M2 activation of macrophages in idiopathic pulmonary fibrosis
- Clinical role of CYP1B1 gene polymorphism in prediction of postoperative chemotherapy efficacy in NSCLC based on individualized health model
- Lung nodule segmentation via semi-residual multi-resolution neural networks
- Evaluation of brain nerve function in ICU patients with Delirium by deep learning algorithm-based resting state MRI
- A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis
- Markov model combined with MR diffusion tensor imaging for predicting the onset of Alzheimer’s disease
- Effectiveness of the treatment of depression associated with cancer and neuroimaging changes in depression-related brain regions in patients treated with the mediator-deuterium acupuncture method
- Molecular mechanism of colorectal cancer and screening of molecular markers based on bioinformatics analysis
- Monitoring and evaluation of anesthesia depth status data based on neuroscience
- Exploring the conformational dynamics and thermodynamics of EGFR S768I and G719X + S768I mutations in non-small cell lung cancer: An in silico approaches
- Optimised feature selection-driven convolutional neural network using gray level co-occurrence matrix for detection of cervical cancer
- Incidence of different pressure patterns of spinal cerebellar ataxia and analysis of imaging and genetic diagnosis
- Pathogenic bacteria and treatment resistance in older cardiovascular disease patients with lung infection and risk prediction model
- Adoption value of support vector machine algorithm-based computed tomography imaging in the diagnosis of secondary pulmonary fungal infections in patients with malignant hematological disorders
- From slides to insights: Harnessing deep learning for prognostic survival prediction in human colorectal cancer histology
- Ecology and Environmental Science
- Monitoring of hourly carbon dioxide concentration under different land use types in arid ecosystem
- Comparing the differences of prokaryotic microbial community between pit walls and bottom from Chinese liquor revealed by 16S rRNA gene sequencing
- Effects of cadmium stress on fruits germination and growth of two herbage species
- Bamboo charcoal affects soil properties and bacterial community in tea plantations
- Optimization of biogas potential using kinetic models, response surface methodology, and instrumental evidence for biodegradation of tannery fleshings during anaerobic digestion
- Understory vegetation diversity patterns of Platycladus orientalis and Pinus elliottii communities in Central and Southern China
- Studies on macrofungi diversity and discovery of new species of Abortiporus from Baotianman World Biosphere Reserve
- Food Science
- Effect of berrycactus fruit (Myrtillocactus geometrizans) on glutamate, glutamine, and GABA levels in the frontal cortex of rats fed with a high-fat diet
- Guesstimate of thymoquinone diversity in Nigella sativa L. genotypes and elite varieties collected from Indian states using HPTLC technique
- Analysis of bacterial community structure of Fuzhuan tea with different processing techniques
- Untargeted metabolomics reveals sour jujube kernel benefiting the nutritional value and flavor of Morchella esculenta
- Mycobiota in Slovak wine grapes: A case study from the small Carpathians wine region
- Elemental analysis of Fadogia ancylantha leaves used as a nutraceutical in Mashonaland West Province, Zimbabwe
- Microbiological transglutaminase: Biotechnological application in the food industry
- Influence of solvent-free extraction of fish oil from catfish (Clarias magur) heads using a Taguchi orthogonal array design: A qualitative and quantitative approach
- Chromatographic analysis of the chemical composition and anticancer activities of Curcuma longa extract cultivated in Palestine
- The potential for the use of leghemoglobin and plant ferritin as sources of iron
- Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM
- Bioengineering and Biotechnology
- Biocompatibility and osteointegration capability of β-TCP manufactured by stereolithography 3D printing: In vitro study
- Clinical characteristics and the prognosis of diabetic foot in Tibet: A single center, retrospective study
- Agriculture
- Biofertilizer and NPSB fertilizer application effects on nodulation and productivity of common bean (Phaseolus vulgaris L.) at Sodo Zuria, Southern Ethiopia
- On correlation between canopy vegetation and growth indexes of maize varieties with different nitrogen efficiencies
- Exopolysaccharides from Pseudomonas tolaasii inhibit the growth of Pleurotus ostreatus mycelia
- A transcriptomic evaluation of the mechanism of programmed cell death of the replaceable bud in Chinese chestnut
- Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis
- Effects of plant density on alfalfa (Medicago sativa L.) seed yield in western Heilongjiang areas
- Identification of rice leaf diseases and deficiency disorders using a novel DeepBatch technique
- Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture
- Animal Sciences
- Effect of ketogenic diet on exercise tolerance and transcriptome of gastrocnemius in mice
- Combined analysis of mRNA–miRNA from testis tissue in Tibetan sheep with different FecB genotypes
- Isolation, identification, and drug resistance of a partially isolated bacterium from the gill of Siniperca chuatsi
- Tracking behavioral changes of confined sows from the first mating to the third parity
- The sequencing of the key genes and end products in the TLR4 signaling pathway from the kidney of Rana dybowskii exposed to Aeromonas hydrophila
- Development of a new candidate vaccine against piglet diarrhea caused by Escherichia coli
- Plant Sciences
- Crown and diameter structure of pure Pinus massoniana Lamb. forest in Hunan province, China
- Genetic evaluation and germplasm identification analysis on ITS2, trnL-F, and psbA-trnH of alfalfa varieties germplasm resources
- Tissue culture and rapid propagation technology for Gentiana rhodantha
- Effects of cadmium on the synthesis of active ingredients in Salvia miltiorrhiza
- Cloning and expression analysis of VrNAC13 gene in mung bean
- Chlorate-induced molecular floral transition revealed by transcriptomes
- Effects of warming and drought on growth and development of soybean in Hailun region
- Effects of different light conditions on transient expression and biomass in Nicotiana benthamiana leaves
- Comparative analysis of the rhizosphere microbiome and medicinally active ingredients of Atractylodes lancea from different geographical origins
- Distinguish Dianthus species or varieties based on chloroplast genomes
- Comparative transcriptomes reveal molecular mechanisms of apple blossoms of different tolerance genotypes to chilling injury
- Study on fresh processing key technology and quality influence of Cut Ophiopogonis Radix based on multi-index evaluation
- An advanced approach for fig leaf disease detection and classification: Leveraging image processing and enhanced support vector machine methodology
- Erratum
- Erratum to “Protein Z modulates the metastasis of lung adenocarcinoma cells”
- Erratum to “BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells”
- Retraction
- Retraction to “Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats”
Articles in the same Issue
- Biomedical Sciences
- Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
- Immunosuppressive treatment for idiopathic membranous nephropathy: An updated network meta-analysis
- Identifying two pathogenic variants in a patient with pigmented paravenous retinochoroidal atrophy
- Effects of phytoestrogens combined with cold stress on sperm parameters and testicular proteomics in rats
- A case of pulmonary embolism with bad warfarin anticoagulant effects caused by E. coli infection
- Neutrophilia with subclinical Cushing’s disease: A case report and literature review
- Isoimperatorin alleviates lipopolysaccharide-induced periodontitis by downregulating ERK1/2 and NF-κB pathways
- Immunoregulation of synovial macrophages for the treatment of osteoarthritis
- Novel CPLANE1 c.8948dupT (p.P2984Tfs*7) variant in a child patient with Joubert syndrome
- Antiphospholipid antibodies and the risk of thrombosis in myeloproliferative neoplasms
- Immunological responses of septic rats to combination therapy with thymosin α1 and vitamin C
- High glucose and high lipid induced mitochondrial dysfunction in JEG-3 cells through oxidative stress
- Pharmacological inhibition of the ubiquitin-specific protease 8 effectively suppresses glioblastoma cell growth
- Levocarnitine regulates the growth of angiotensin II-induced myocardial fibrosis cells via TIMP-1
- Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study
- Single-cell transcription analysis reveals the tumor origin and heterogeneity of human bilateral renal clear cell carcinoma
- Identification of iron metabolism-related genes as diagnostic signatures in sepsis by blood transcriptomic analysis
- Long noncoding RNA ACART knockdown decreases 3T3-L1 preadipocyte proliferation and differentiation
- Surgery, adjuvant immunotherapy plus chemotherapy and radiotherapy for primary malignant melanoma of the parotid gland (PGMM): A case report
- Dosimetry comparison with helical tomotherapy, volumetric modulated arc therapy, and intensity-modulated radiotherapy for grade II gliomas: A single‑institution case series
- Soy isoflavone reduces LPS-induced acute lung injury via increasing aquaporin 1 and aquaporin 5 in rats
- Refractory hypokalemia with sexual dysplasia and infertility caused by 17α-hydroxylase deficiency and triple X syndrome: A case report
- Meta-analysis of cancer risk among end stage renal disease undergoing maintenance dialysis
- 6-Phosphogluconate dehydrogenase inhibition arrests growth and induces apoptosis in gastric cancer via AMPK activation and oxidative stress
- Experimental study on the optimization of ANM33 release in foam cells
- Primary retroperitoneal angiosarcoma: A case report
- Metabolomic analysis-identified 2-hydroxybutyric acid might be a key metabolite of severe preeclampsia
- Malignant pleural effusion diagnosis and therapy
- Effect of spaceflight on the phenotype and proteome of Escherichia coli
- Comparison of immunotherapy combined with stereotactic radiotherapy and targeted therapy for patients with brain metastases: A systemic review and meta-analysis
- Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation
- Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy
- The role of IL-31 and IL-34 in the diagnosis and treatment of chronic periodontitis
- Triple-negative mouse breast cancer initiating cells show high expression of beta1 integrin and increased malignant features
- mNGS facilitates the accurate diagnosis and antibiotic treatment of suspicious critical CNS infection in real practice: A retrospective study
- The apatinib and pemetrexed combination has antitumor and antiangiogenic effects against NSCLC
- Radiotherapy for primary thyroid adenoid cystic carcinoma
- Design and functional preliminary investigation of recombinant antigen EgG1Y162–EgG1Y162 against Echinococcus granulosus
- Effects of losartan in patients with NAFLD: A meta-analysis of randomized controlled trial
- Bibliometric analysis of METTL3: Current perspectives, highlights, and trending topics
- Performance comparison of three scaling algorithms in NMR-based metabolomics analysis
- PI3K/AKT/mTOR pathway and its related molecules participate in PROK1 silence-induced anti-tumor effects on pancreatic cancer
- The altered expression of cytoskeletal and synaptic remodeling proteins during epilepsy
- Effects of pegylated recombinant human granulocyte colony-stimulating factor on lymphocytes and white blood cells of patients with malignant tumor
- Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report
- NUDT21 relieves sevoflurane-induced neurological damage in rats by down-regulating LIMK2
- Association of interleukin-10 rs1800896, rs1800872, and interleukin-6 rs1800795 polymorphisms with squamous cell carcinoma risk: A meta-analysis
- Exosomal HBV-DNA for diagnosis and treatment monitoring of chronic hepatitis B
- Shear stress leads to the dysfunction of endothelial cells through the Cav-1-mediated KLF2/eNOS/ERK signaling pathway under physiological conditions
- Interaction between the PI3K/AKT pathway and mitochondrial autophagy in macrophages and the leukocyte count in rats with LPS-induced pulmonary infection
- Meta-analysis of the rs231775 locus polymorphism in the CTLA-4 gene and the susceptibility to Graves’ disease in children
- Cloning, subcellular localization and expression of phosphate transporter gene HvPT6 of hulless barley
- Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome
- Significant elevated CXCL14 and decreased IL-39 levels in patients with tuberculosis
- Whole-exome sequencing applications in prenatal diagnosis of fetal bowel dilatation
- Gemella morbillorum infective endocarditis: A case report and literature review
- An unusual ectopic thymoma clonal evolution analysis: A case report
- Severe cumulative skin toxicity during toripalimab combined with vemurafenib following toripalimab alone
- Detection of V. vulnificus septic shock with ARDS using mNGS
- Novel rare genetic variants of familial and sporadic pulmonary atresia identified by whole-exome sequencing
- The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology
- Novel compound heterozygous mutations in TELO2 in an infant with You-Hoover-Fong syndrome: A case report and literature review
- ctDNA as a prognostic biomarker in resectable CLM: Systematic review and meta-analysis
- Diagnosis of primary amoebic meningoencephalitis by metagenomic next-generation sequencing: A case report
- Phylogenetic analysis of promoter regions of human Dolichol kinase (DOLK) and orthologous genes using bioinformatics tools
- Collagen changes in rabbit conjunctiva after conjunctival crosslinking
- Effects of NM23 transfection of human gastric carcinoma cells in mice
- Oral nifedipine and phytosterol, intravenous nicardipine, and oral nifedipine only: Three-arm, retrospective, cohort study for management of severe preeclampsia
- Case report of hepatic retiform hemangioendothelioma: A rare tumor treated with ultrasound-guided microwave ablation
- Curcumin induces apoptosis in human hepatocellular carcinoma cells by decreasing the expression of STAT3/VEGF/HIF-1α signaling
- Rare presentation of double-clonal Waldenström macroglobulinemia with pulmonary embolism: A case report
- Giant duplication of the transverse colon in an adult: A case report and literature review
- Ectopic thyroid tissue in the breast: A case report
- SDR16C5 promotes proliferation and migration and inhibits apoptosis in pancreatic cancer
- Vaginal metastasis from breast cancer: A case report
- Screening of the best time window for MSC transplantation to treat acute myocardial infarction with SDF-1α antibody-loaded targeted ultrasonic microbubbles: An in vivo study in miniswine
- Inhibition of TAZ impairs the migration ability of melanoma cells
- Molecular complexity analysis of the diagnosis of Gitelman syndrome in China
- Effects of maternal calcium and protein intake on the development and bone metabolism of offspring mice
- Identification of winter wheat pests and diseases based on improved convolutional neural network
- Ultra-multiplex PCR technique to guide treatment of Aspergillus-infected aortic valve prostheses
- Virtual high-throughput screening: Potential inhibitors targeting aminopeptidase N (CD13) and PIKfyve for SARS-CoV-2
- Immune checkpoint inhibitors in cancer patients with COVID-19
- Utility of methylene blue mixed with autologous blood in preoperative localization of pulmonary nodules and masses
- Integrated analysis of the microbiome and transcriptome in stomach adenocarcinoma
- Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy
- DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway
- Lung abscess by Fusobacterium nucleatum and Streptococcus spp. co-infection by mNGS: A case series
- Genetic alterations of KRAS and TP53 in intrahepatic cholangiocarcinoma associated with poor prognosis
- Granulomatous polyangiitis involving the fourth ventricle: Report of a rare case and a literature review
- Studying infant mortality: A demographic analysis based on data mining models
- Metaplastic breast carcinoma with osseous differentiation: A report of a rare case and literature review
- Protein Z modulates the metastasis of lung adenocarcinoma cells
- Inhibition of pyroptosis and apoptosis by capsaicin protects against LPS-induced acute kidney injury through TRPV1/UCP2 axis in vitro
- TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats
- Primary mediastinum Ewing’s sarcoma with pleural effusion: A case report and literature review
- Association of ADRB2 gene polymorphisms and intestinal microbiota in Chinese Han adolescents
- Tanshinone IIA alleviates chondrocyte apoptosis and extracellular matrix degeneration by inhibiting ferroptosis
- Study on the cytokines related to SARS-Cov-2 in testicular cells and the interaction network between cells based on scRNA-seq data
- Effect of periostin on bone metabolic and autophagy factors during tooth eruption in mice
- HP1 induces ferroptosis of renal tubular epithelial cells through NRF2 pathway in diabetic nephropathy
- Intravaginal estrogen management in postmenopausal patients with vaginal squamous intraepithelial lesions along with CO2 laser ablation: A retrospective study
- Hepatocellular carcinoma cell differentiation trajectory predicts immunotherapy, potential therapeutic drugs, and prognosis of patients
- Effects of physical exercise on biomarkers of oxidative stress in healthy subjects: A meta-analysis of randomized controlled trials
- Identification of lysosome-related genes in connection with prognosis and immune cell infiltration for drug candidates in head and neck cancer
- Development of an instrument-free and low-cost ELISA dot-blot test to detect antibodies against SARS-CoV-2
- Research progress on gas signal molecular therapy for Parkinson’s disease
- Adiponectin inhibits TGF-β1-induced skin fibroblast proliferation and phenotype transformation via the p38 MAPK signaling pathway
- The G protein-coupled receptor-related gene signatures for predicting prognosis and immunotherapy response in bladder urothelial carcinoma
- α-Fetoprotein contributes to the malignant biological properties of AFP-producing gastric cancer
- CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa
- Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis
- Significance of sTREM-1 and sST2 combined diagnosis for sepsis detection and prognosis prediction
- Diagnostic value of serum neuroactive substances in the acute exacerbation of chronic obstructive pulmonary disease complicated with depression
- Research progress of AMP-activated protein kinase and cardiac aging
- TRIM29 knockdown prevented the colon cancer progression through decreasing the ubiquitination levels of KRT5
- Cross-talk between gut microbiota and liver steatosis: Complications and therapeutic target
- Metastasis from small cell lung cancer to ovary: A case report
- The early diagnosis and pathogenic mechanisms of sepsis-related acute kidney injury
- The effect of NK cell therapy on sepsis secondary to lung cancer: A case report
- Erianin alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation
- Loss of ACOX1 in clear cell renal cell carcinoma and its correlation with clinical features
- Signalling pathways in the osteogenic differentiation of periodontal ligament stem cells
- Crosstalk between lactic acid and immune regulation and its value in the diagnosis and treatment of liver failure
- Clinicopathological features and differential diagnosis of gastric pleomorphic giant cell carcinoma
- Traumatic brain injury and rTMS-ERPs: Case report and literature review
- Extracellular fibrin promotes non-small cell lung cancer progression through integrin β1/PTEN/AKT signaling
- Knockdown of DLK4 inhibits non-small cell lung cancer tumor growth by downregulating CKS2
- The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles
- Inflammation-related signaling pathways in tendinopathy
- CD4+ T cell count in HIV/TB co-infection and co-occurrence with HL: Case report and literature review
- Clinical analysis of severe Chlamydia psittaci pneumonia: Case series study
- Bioinformatics analysis to identify potential biomarkers for the pulmonary artery hypertension associated with the basement membrane
- Influence of MTHFR polymorphism, alone or in combination with smoking and alcohol consumption, on cancer susceptibility
- Catharanthus roseus (L.) G. Don counteracts the ampicillin resistance in multiple antibiotic-resistant Staphylococcus aureus by downregulation of PBP2a synthesis
- Combination of a bronchogenic cyst in the thoracic spinal canal with chronic myelocytic leukemia
- Bacterial lipoprotein plays an important role in the macrophage autophagy and apoptosis induced by Salmonella typhimurium and Staphylococcus aureus
- TCL1A+ B cells predict prognosis in triple-negative breast cancer through integrative analysis of single-cell and bulk transcriptomic data
- Ezrin promotes esophageal squamous cell carcinoma progression via the Hippo signaling pathway
- Ferroptosis: A potential target of macrophages in plaque vulnerability
- Predicting pediatric Crohn's disease based on six mRNA-constructed risk signature using comprehensive bioinformatic approaches
- Applications of genetic code expansion and photosensitive UAAs in studying membrane proteins
- HK2 contributes to the proliferation, migration, and invasion of diffuse large B-cell lymphoma cells by enhancing the ERK1/2 signaling pathway
- IL-17 in osteoarthritis: A narrative review
- Circadian cycle and neuroinflammation
- Probiotic management and inflammatory factors as a novel treatment in cirrhosis: A systematic review and meta-analysis
- Hemorrhagic meningioma with pulmonary metastasis: Case report and literature review
- SPOP regulates the expression profiles and alternative splicing events in human hepatocytes
- Knockdown of SETD5 inhibited glycolysis and tumor growth in gastric cancer cells by down-regulating Akt signaling pathway
- PTX3 promotes IVIG resistance-induced endothelial injury in Kawasaki disease by regulating the NF-κB pathway
- Pancreatic ectopic thyroid tissue: A case report and analysis of literature
- The prognostic impact of body mass index on female breast cancer patients in underdeveloped regions of northern China differs by menopause status and tumor molecular subtype
- Report on a case of liver-originating malignant melanoma of unknown primary
- Case report: Herbal treatment of neutropenic enterocolitis after chemotherapy for breast cancer
- The fibroblast growth factor–Klotho axis at molecular level
- Characterization of amiodarone action on currents in hERG-T618 gain-of-function mutations
- A case report of diagnosis and dynamic monitoring of Listeria monocytogenes meningitis with NGS
- Effect of autologous platelet-rich plasma on new bone formation and viability of a Marburg bone graft
- Small breast epithelial mucin as a useful prognostic marker for breast cancer patients
- Continuous non-adherent culture promotes transdifferentiation of human adipose-derived stem cells into retinal lineage
- Nrf3 alleviates oxidative stress and promotes the survival of colon cancer cells by activating AKT/BCL-2 signal pathway
- Favorable response to surufatinib in a patient with necrolytic migratory erythema: A case report
- Case report of atypical undernutrition of hypoproteinemia type
- Down-regulation of COL1A1 inhibits tumor-associated fibroblast activation and mediates matrix remodeling in the tumor microenvironment of breast cancer
- Sarcoma protein kinase inhibition alleviates liver fibrosis by promoting hepatic stellate cells ferroptosis
- Research progress of serum eosinophil in chronic obstructive pulmonary disease and asthma
- Clinicopathological characteristics of co-existing or mixed colorectal cancer and neuroendocrine tumor: Report of five cases
- Role of menopausal hormone therapy in the prevention of postmenopausal osteoporosis
- Precisional detection of lymph node metastasis using tFCM in colorectal cancer
- Advances in diagnosis and treatment of perimenopausal syndrome
- A study of forensic genetics: ITO index distribution and kinship judgment between two individuals
- Acute lupus pneumonitis resembling miliary tuberculosis: A case-based review
- Plasma levels of CD36 and glutathione as biomarkers for ruptured intracranial aneurysm
- Fractalkine modulates pulmonary angiogenesis and tube formation by modulating CX3CR1 and growth factors in PVECs
- Novel risk prediction models for deep vein thrombosis after thoracotomy and thoracoscopic lung cancer resections, involving coagulation and immune function
- Exploring the diagnostic markers of essential tremor: A study based on machine learning algorithms
- Evaluation of effects of small-incision approach treatment on proximal tibia fracture by deep learning algorithm-based magnetic resonance imaging
- An online diagnosis method for cancer lesions based on intelligent imaging analysis
- Medical imaging in rheumatoid arthritis: A review on deep learning approach
- Predictive analytics in smart healthcare for child mortality prediction using a machine learning approach
- Utility of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in predicting acute-on-chronic liver failure survival
- A biomedical decision support system for meta-analysis of bilateral upper-limb training in stroke patients with hemiplegia
- TNF-α and IL-8 levels are positively correlated with hypobaric hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats
- Stochastic gradient descent optimisation for convolutional neural network for medical image segmentation
- Comparison of the prognostic value of four different critical illness scores in patients with sepsis-induced coagulopathy
- Application and teaching of computer molecular simulation embedded technology and artificial intelligence in drug research and development
- Hepatobiliary surgery based on intelligent image segmentation technology
- Value of brain injury-related indicators based on neural network in the diagnosis of neonatal hypoxic-ischemic encephalopathy
- Analysis of early diagnosis methods for asymmetric dementia in brain MR images based on genetic medical technology
- Early diagnosis for the onset of peri-implantitis based on artificial neural network
- Clinical significance of the detection of serum IgG4 and IgG4/IgG ratio in patients with thyroid-associated ophthalmopathy
- Forecast of pain degree of lumbar disc herniation based on back propagation neural network
- SPA-UNet: A liver tumor segmentation network based on fused multi-scale features
- Systematic evaluation of clinical efficacy of CYP1B1 gene polymorphism in EGFR mutant non-small cell lung cancer observed by medical image
- Rehabilitation effect of intelligent rehabilitation training system on hemiplegic limb spasms after stroke
- A novel approach for minimising anti-aliasing effects in EEG data acquisition
- ErbB4 promotes M2 activation of macrophages in idiopathic pulmonary fibrosis
- Clinical role of CYP1B1 gene polymorphism in prediction of postoperative chemotherapy efficacy in NSCLC based on individualized health model
- Lung nodule segmentation via semi-residual multi-resolution neural networks
- Evaluation of brain nerve function in ICU patients with Delirium by deep learning algorithm-based resting state MRI
- A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis
- Markov model combined with MR diffusion tensor imaging for predicting the onset of Alzheimer’s disease
- Effectiveness of the treatment of depression associated with cancer and neuroimaging changes in depression-related brain regions in patients treated with the mediator-deuterium acupuncture method
- Molecular mechanism of colorectal cancer and screening of molecular markers based on bioinformatics analysis
- Monitoring and evaluation of anesthesia depth status data based on neuroscience
- Exploring the conformational dynamics and thermodynamics of EGFR S768I and G719X + S768I mutations in non-small cell lung cancer: An in silico approaches
- Optimised feature selection-driven convolutional neural network using gray level co-occurrence matrix for detection of cervical cancer
- Incidence of different pressure patterns of spinal cerebellar ataxia and analysis of imaging and genetic diagnosis
- Pathogenic bacteria and treatment resistance in older cardiovascular disease patients with lung infection and risk prediction model
- Adoption value of support vector machine algorithm-based computed tomography imaging in the diagnosis of secondary pulmonary fungal infections in patients with malignant hematological disorders
- From slides to insights: Harnessing deep learning for prognostic survival prediction in human colorectal cancer histology
- Ecology and Environmental Science
- Monitoring of hourly carbon dioxide concentration under different land use types in arid ecosystem
- Comparing the differences of prokaryotic microbial community between pit walls and bottom from Chinese liquor revealed by 16S rRNA gene sequencing
- Effects of cadmium stress on fruits germination and growth of two herbage species
- Bamboo charcoal affects soil properties and bacterial community in tea plantations
- Optimization of biogas potential using kinetic models, response surface methodology, and instrumental evidence for biodegradation of tannery fleshings during anaerobic digestion
- Understory vegetation diversity patterns of Platycladus orientalis and Pinus elliottii communities in Central and Southern China
- Studies on macrofungi diversity and discovery of new species of Abortiporus from Baotianman World Biosphere Reserve
- Food Science
- Effect of berrycactus fruit (Myrtillocactus geometrizans) on glutamate, glutamine, and GABA levels in the frontal cortex of rats fed with a high-fat diet
- Guesstimate of thymoquinone diversity in Nigella sativa L. genotypes and elite varieties collected from Indian states using HPTLC technique
- Analysis of bacterial community structure of Fuzhuan tea with different processing techniques
- Untargeted metabolomics reveals sour jujube kernel benefiting the nutritional value and flavor of Morchella esculenta
- Mycobiota in Slovak wine grapes: A case study from the small Carpathians wine region
- Elemental analysis of Fadogia ancylantha leaves used as a nutraceutical in Mashonaland West Province, Zimbabwe
- Microbiological transglutaminase: Biotechnological application in the food industry
- Influence of solvent-free extraction of fish oil from catfish (Clarias magur) heads using a Taguchi orthogonal array design: A qualitative and quantitative approach
- Chromatographic analysis of the chemical composition and anticancer activities of Curcuma longa extract cultivated in Palestine
- The potential for the use of leghemoglobin and plant ferritin as sources of iron
- Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM
- Bioengineering and Biotechnology
- Biocompatibility and osteointegration capability of β-TCP manufactured by stereolithography 3D printing: In vitro study
- Clinical characteristics and the prognosis of diabetic foot in Tibet: A single center, retrospective study
- Agriculture
- Biofertilizer and NPSB fertilizer application effects on nodulation and productivity of common bean (Phaseolus vulgaris L.) at Sodo Zuria, Southern Ethiopia
- On correlation between canopy vegetation and growth indexes of maize varieties with different nitrogen efficiencies
- Exopolysaccharides from Pseudomonas tolaasii inhibit the growth of Pleurotus ostreatus mycelia
- A transcriptomic evaluation of the mechanism of programmed cell death of the replaceable bud in Chinese chestnut
- Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis
- Effects of plant density on alfalfa (Medicago sativa L.) seed yield in western Heilongjiang areas
- Identification of rice leaf diseases and deficiency disorders using a novel DeepBatch technique
- Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture
- Animal Sciences
- Effect of ketogenic diet on exercise tolerance and transcriptome of gastrocnemius in mice
- Combined analysis of mRNA–miRNA from testis tissue in Tibetan sheep with different FecB genotypes
- Isolation, identification, and drug resistance of a partially isolated bacterium from the gill of Siniperca chuatsi
- Tracking behavioral changes of confined sows from the first mating to the third parity
- The sequencing of the key genes and end products in the TLR4 signaling pathway from the kidney of Rana dybowskii exposed to Aeromonas hydrophila
- Development of a new candidate vaccine against piglet diarrhea caused by Escherichia coli
- Plant Sciences
- Crown and diameter structure of pure Pinus massoniana Lamb. forest in Hunan province, China
- Genetic evaluation and germplasm identification analysis on ITS2, trnL-F, and psbA-trnH of alfalfa varieties germplasm resources
- Tissue culture and rapid propagation technology for Gentiana rhodantha
- Effects of cadmium on the synthesis of active ingredients in Salvia miltiorrhiza
- Cloning and expression analysis of VrNAC13 gene in mung bean
- Chlorate-induced molecular floral transition revealed by transcriptomes
- Effects of warming and drought on growth and development of soybean in Hailun region
- Effects of different light conditions on transient expression and biomass in Nicotiana benthamiana leaves
- Comparative analysis of the rhizosphere microbiome and medicinally active ingredients of Atractylodes lancea from different geographical origins
- Distinguish Dianthus species or varieties based on chloroplast genomes
- Comparative transcriptomes reveal molecular mechanisms of apple blossoms of different tolerance genotypes to chilling injury
- Study on fresh processing key technology and quality influence of Cut Ophiopogonis Radix based on multi-index evaluation
- An advanced approach for fig leaf disease detection and classification: Leveraging image processing and enhanced support vector machine methodology
- Erratum
- Erratum to “Protein Z modulates the metastasis of lung adenocarcinoma cells”
- Erratum to “BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells”
- Retraction
- Retraction to “Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats”

