Abstract
Pancreatic cancer (PAAD) is usually found when it is already in its advanced stage, which has limited options available for treatment and poor overall survival. The SDR16C5 gene is necessary for embryonic and adult tissue differentiation, development, and apoptosis, and it also participates in immune response and regulates energy metabolism. However, the role of SDR16C5 in PAAD remains unclear. In this study, we find that SDR16C5 was highly expressed in multiple tumors including PAAD. Furthermore, higher expression of SDR16C5 was significantly associated with poorer survival. We also find that the knockdown of SDR16C5 can inhibit PAAD cell proliferation and promote cell apoptosis by repressing Bcl-2, cleaved caspase 3, and cleaved caspase 9 protein expression. Moreover, silencing SDR16C5 inhibits the migration of PANC-1 and SW1990 cells by interrupting epithelial–mesenchymal transition. KEGG pathway analysis and immunofluorescence staining indicate that SDR16C5 is associated with immunity and may also participate in the development of PAAD through the IL-17 signaling pathway. Collectively, our findings provide evidence that SDR16C5 is overexpressed in PAAD patients and promotes its proliferation, migration, invasion, and apoptosis-inhibition of PAAD cells. Thus, SDR16C5 may be a potential prognostic and therapeutic target.
Graphical abstract
1 Introduction
Pancreatic cancer (PAAD) is the fourth most common malignancy in the United States, with a 5-year survival rate of approximately 6% [1]. Furthermore, mutations of the KRAS oncogene, which represent one of the most prevalent genetic alterations in cancer, occur in over 90% of PAAD patients. In addition, mutations in KRAS, TP53, SMAD4, and CDKN2A also commonly contribute to PAAD progression [2]. However, the specific mechanism of this action remains unclear. Unfortunately, for many patients, the low detection rate of PAAD in its early stage is significantly related to poor survival prognosis. Therefore, in this study, we explore new oncogenes to help to understand the occurrence and development of PAAD and to evaluate whether they can become biomarkers for earlier diagnosis and correspondingly better prognoses in PAAD.
Gene expression profiling based on microarray technology can be used to simultaneously study the expression changes of thousands of genes in limited tumor samples, which is helpful for the discovery of new drug targets and molecular-based diagnosis and prognosis. Indeed, the quantification and identification of differentially expressed transcripts in cells and tissues have become an important method for deciphering cellular molecular physiology [3]. Therefore, in this article, we make full use of tumor data sources by downloading mRNA chip data sets from TCGA (The Cancer Genome Atlas), GEO (Gene Expression Omnibus), and other databases for comprehensive analysis, screening out genes that have an impact on the prognosis of PAAD, and validating them in multiple PAAD cell lines.
The short-chain dehydrogenase/reductase family 16C member 5 (SDR16C5) protein encodes a member of the short-chain alcohol dehydrogenase/reductase superfamily of proteins; it was also known as epidermal retinol dehydrogenase 2. The SDR16C5 protein is located in the endoplasmic reticulum [4] and is active in both oxidation and reduction directions [5]. It participates in the retinol metabolism pathway, which is a cofactor part of the overall metabolism that is related to tumor formation [6,7]. SDR16C5 is also closely related to the growth and development of embryos and responsible for the increase of both height and weight. Previous studies revealed that SDR16C5 was related to the development and progression of tumor cells; it was highly expressed in PAAD [8], laryngeal carcinoma [9], and colorectal cancer [10] tissues, suggesting that it might serve as a vital diagnostic biomarker in cancers. However, the regulatory mechanism of SDR16C5 in PAAD is still unknown. Hence, in this study, we analyze the possible biological functions of SDR16C5 in PAAD based on bioinformatics analysis and in vitro experiments.
2 Methods
2.1 UCSC Xena database analysis
The UCSC Xena database (https://xenabrowser.net/) [11] is a powerful genomic online tool that provides visualization and integration heat maps for analyzing publicly accessible datasets. For this study, we used UCSC Xena to download clinical data and gene expression information about Pan-Cancer studies. We extracted the expression data for SDR16C5 and used Wilcox tests to analyze differential gene expression. Survival analysis of Pan-Cancer patients was conducted for disease-related survival, disease-free interval (DFI), and progression-free interval (PFI) using the Kaplan–Meier (KM) method.
2.2 TCGA database analysis
We obtained the mRNA sequencing data (178 PAAD samples and four pancreatic normal tissues) of PAAD patients as well as the corresponding clinical information from TCGA (https://cancergenome.nih.gov/). KM curves with log-rank tests were used for survival analysis, and time ROC analysis was performed to measure the predictive power and risk scores of SDR16C5 for PAAD. All analysis was performed using R software (v4.0.3), and we considered P < 0.05 to indicate a statistically significant test result.
2.3 GEPIA2 database analysis
To validate our results further, we utilized the online GEPIA2 database based on the TCGA dataset to investigate SDR16C5 expression levels and overall survival in various cancers besides PAAD. GEPIA (http://gepia2.cancer-pku.cn/) [12] is an online database that incorporates gene expression data from TCGA and GTEx, bringing together 9,736 tumor samples and 8,587 normal controls. In this study, we analyzed SDR16C5 expression in TCGA-cancer tissues and matched normal tissues with boxplots. To do this, we set a cutoff of |log2 FC| = 1, P < 0.001, and a Jitter size >0.7. We used log2(TPM + 1) for the log-scale.
2.4 GEO database analysis
For analysis of differential gene expression between normal tissues and cancer tissues, we utilized GSE15471, GSE16515, and GSE28735 datasets obtained from the GEO. The GEO (https://www.ncbi.nlm.nih.gov/geo/) [13] is an international public repository for high-throughput microarray and next-generation sequence functional genomic datasets submitted by the research community. The GSE15471 dataset has a total of 72 samples, containing 36 PAAD samples and 36 noncancerous samples, which are based on the GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 plus 2.0 Array. GSE16515 consists of 36 PAAD samples and 16 normal samples and lives on the same platform as GSE15471. The GSE28735 dataset has 45 matching pairs of pancreatic tumor and adjacent nontumor tissues from 45 patients and is based on the GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array [transcript (gene) version]. Data processing and analysis were all performed using R software (v4.0.3) and P < 0.05 as the threshold of statistical significance once again.
2.5 CPTAC database analysis
We downloaded the protein expression profile of PAAD from CPTAC, which contains 77 normal samples and 141 PAAD samples. The CPTAC data portal is a centralized repository for the public dissemination of proteomic sequences, along with the corresponding genomic sequence datasets (https://cptac-data-portal.georgetown.edu/cptacPublic/). SDR16C5 protein expression levels were analyzed based on CPTAC data and visualized using R software (v4.0.3).
2.6 CCLE database and gene enrichment analysis
For further exploration of the biological processes and signaling pathways in which SDR16C5 could be involved and also in an attempt to provide a theoretical foundation for future studies, we also carried out functional analysis for SDR16C5. To accomplish this, we downloaded gene expression data for PAAD cell lines from the CCLE database (https://portals.broadinstitute.org/ccle). Genes with P-values <0.001, and correlation coefficients >0.5 or <−0.5 were selected for further analysis. Gene ontology (GO) and KEGG analyses were then performed for differentially expressed SDR16C5-associated immune genes.
2.7 Correlation between gene expression and immune cells
To explore the correlation between SDR16C5 expression and immune cell subset abundance, we used the immunedeconv R package to carry out immune infiltration estimations. According to the PAAD sequencing data from TCGA, we divided the overall sample into a high SDR16C5 expression group and a low SDR16C5 expression group. According to this, we used differential analysis to identify the immune cells that showed differential abundance between the two groups. All the results from the above analysis were processed using the “ggplot2” and “pheatmap” packages in R software (v4.0.3).
2.8 Cells and tissues
We purchased human PAAD cell lines (Panc-1, BxPC, SW1990, ASPC, and MiaCaPa-2) and immortalized human pancreatic ductal epithelial cells (HPDE6) from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The HPDE6, Panc-1, SW1990, and MiaCaPa-2 cells were cultured in Dulbecco's modified Eagle medium (DMEM-H) (Gibco), and the BxPC and ASPC cells were cultured in RPMI-1640 (Gibco) medium that contained 10% fetal bovine serum (FBS, Sijiqing, Hangzhou, China) in a humidified atmosphere of 5% CO2 at 37°C. PAAD cells transfected with siRNA-SDR16C5 were cultured for further experiments. PAAD tissues and corresponding paracancerous tissues (approximately 5 cm from cancerous tissues) were taken from 20 patients undergoing surgery for PAAD at the Renmin Hospital of Wuhan University (Wuhan, China) between 2020 and 2022. Patients received no prior treatment before surgery. In all cases, pathological assessment and diagnosis were conducted by two expert pathologists. The diagnosis and treatment standard of PAAD was used as a reference for staging PAAD [1]. The research related to human use has complied with all the relevant national regulations and institutional policies, and in accordance with the tenets of the Helsinki Declaration, and has been approved by the Medical Ethics Committee of the Renmin Hospital of Wuhan University (No. WDRY2019-K070). Tissues collected for immunofluorescence staining were fixed in formalin and embedded in paraffin, and tissues were immediately snap-frozen in liquid nitrogen and stored at −80°C until further qRT-PCR experimentation.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: The research related to human use has complied with all the relevant national regulations, institutional policies, and in accordance with the tenets of the Helsinki Declaration, and has been approved by the Medical Ethics Committee of the Renmin Hospital of Wuhan University (No. WDRY2019-K070).
2.9 Cell transfection
Panc-1 and SW1990 cells were seeded overnight to grow to 50% confluence and were then transfected with siRNA-SDR16C5. siRNAs that target the human SDR16C5 gene (siRNA-SDR16C5) and nontargeting negative control siRNAs (siRNA-NCs) were purchased from Suzhou GenePharma Co., Ltd. (Shanghai, China). The siRNA sequences were as follows. si-RNA1-SDR16C5: sense, 5-GCUAAUGACCAUGGACAUUTT-3, antisense, 5-AAUGUCCAUGGUCAUUAGCTT-3; si-RNA2-SDR16C5: sense, 5-GCCUUUGGGUUUGCUGAAUTT-3, antisense, 5-AUUCAGCAAACCCAAAGGCTT-3; si-RNA3-SDR16C5: sense,5-GCACAGGAUGGGUCAGAAUTT-3, antisense, 5-AUUCUGACCCAUCCUGUGCTT-3. SiRNA-NC: sense, 5-UUCUCCGAACGUACGUTT-3, antisense, 5-ACGUGACACGUUCGGAGAATT-3. Lipid-based transient transfections were performed using Lipofectamine 6000 Transfection Reagent (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions, and the culture medium was replaced 6 h later. After 48 to 72 h of transfection, quantitative reverse transcription (qRT) PCR and western blotting were used to assess transfection efficiency.
2.10 CCK8 experiment
We used the CCK8 assay to detect the viability of cells in accordance with the manufacturer’s protocol. Cells transfected with Si-SDR16C5-2 and siRNA-NC were seeded at a density of 5,000 cells/well in 96-well plates. Then, 10 μl of CCK8 solution (CCK-8; Biosharp, Shanghai, China) was added to the cells after 0, 24, 48, 72, and 96 h, and the cells were incubated for another 1 h at 37°C. After 1 h incubation, absorbance was measured at 450 nm with a microplate reader (BD Biosciences, USA). These data were analyzed and visualized using Prism software.
2.11 Scratch-wound experiment
We determined cell migration by performing a scratch-wound healing assay. To achieve this, the cells were planted in 6-well plates, and the scratch test was performed when the wells were nearly full. Specifically, scratches were made with 200 µl sterile pipette tips, and the cells were incubated in the corresponding medium without FBS. We took pictures of the cells under a microscope at 0 and 24 h and compared the photos to calculate the migration rate. In total, we performed three of these experiments independently.
2.12 Clone formation experiment
In order to analyze the effect of SDR16C5 on cell proliferation, we conducted a clone formation assay. For the clone formation assay, we transfected PAAD cells with siRNA-SDR16C5 or siRNA-NC and 24 h later seeded them at a density of 200 cells/per well in 6-well plates and cultured them for 2 weeks. Each culture was terminated when clones in the 6-well plates became visible to the naked eye. The cells were then fixed with 4% paraformaldehyde for 15 min and stained with crystal violet for 5 min. After this, the colonies in each group were washed before counting. We used ImageJ software (ImageJ 1.52a, United States) to analyze and quantify the number of colonies.
2.13 Transwell migration experiments
To assess cell migration, we performed a transwell migration assay. Here, transfected cell concentration was adjusted to 2 × 105 cells/ml, and a 200 μl/well cell suspension was placed in the upper chamber of a 24-well plate. In the lower chamber, 500 μl of DMEM with 15% FBS was added as a chemo-attractant. After 24 h, cells from the top of the membrane were carefully removed, and the cells that had migrated to the lower side were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The migrated cells were then observed, photographed, and quantified under three random microscopic fields.
2.14 Cell apoptosis experiment
To study cellular apoptosis, Panc-1 and SW1990 cells were transfected as described in Section 2.9 and cultured for another 24 h to perform apoptosis analysis. Apoptosis was evaluated by Annexin V assay (Beyotime Biotechnology, China) following the manufacturer’s protocol. A total of 5 to 10 × 106 Panc-1 and SW1990 cells were centrifuged at 1,000 × g for 5 min, and after the supernatant was discarded, 195 µl of Annexin V-FITC solution was added to resuspend the cells gently. After this, 5 μl of Annexin V-FITC and 10 μl of propidium iodide (PI) solution were added. Apoptotic cells were then detected using a flow cytometer (BD Biosciences).
2.15 Western blotting
In order to analyze the effect of SDR16C5 on downstream pathway proteins, cells and tissues were lysed with RIPA lysis buffer containing 1% PMSF. The lysates were then centrifuged, and the supernatant was collected. The quantified protein supernatant was supplemented with 4× protein loading buffer proportionally, boiled for 10 min to denature the protein, and stored at −80°C. Proteins were then separated by 10% SDS-PAGE and electrophoretically transferred onto polyvinylidene fluoride membranes where they were blocked with 5% skim milk and incubated with anti-SDR16C5 (1:5,000, #PA5-31421; Invitrogen, USA), anti-E-cadherin (1:200, #K1815; Santa Cruz, USA), anti-vimentin (1:1,000, #5741T; CST, USA), anti-Bcl-2 (1:500, #T40056; Abmart, China), Bax (1:1,000, # 2772T; CST, USA), cleaved caspase 3 (1:500, #T40044; Abmart, China), and cleaved caspase 9 (1:500, #T40046; Abmart, China) overnight at 4°C. Next, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG. Antigen–antibody complexes were then detected with enhanced chemiluminescence reagent. The resulting images were processed and analyzed using ImageJ software.
2.16 qRT-PCR
To carry out our qRT-PCR analysis, cells were harvested and dissolved in Trizol reagent, and then 200 μl of chloroform solution was added. Extracts were then centrifuged at 12,000 × g for 15 min. An equal volume of isopropanol precooled at 4°C was added to the supernatant and then centrifuged again at 12,000 × g for 10 min. Finally, the supernatant was purified using ethanol, and we detected the concentration of RNA. Using a PrimeScript RT Master Mix kit (RR047A; Takara, Japan) for reverse transcription, the expression of genes was determined using SYBR Green PCR Mix (RR420A; Takara, Japan) as follows. SDR16C5: F-CAGCCTTTGGGTTTGCTGA, R-GGTTCCAGAATTGGCAACAGA; GAPDH: F-GGAGCGAGATCCCTCCAAAAT and R-GGCTGTTGTCATACTTCTCATGG. All experiments were performed in triplicate and analyzed by the 2−ΔΔCT method using GAPDH as the reference gene [14].
2.17 Immunofluorescence staining
We performed immunofluorescence staining to determine the co-expression of SDR16C5 and IL-17. First, paraffin-embedded tissue sections were dewaxed and rehydrated stepwise, and then repair antigen was performed. Next, endogenous peroxidase was blocked with 3% H2O2, and primary antibodies were diluted to appropriate concentrations in 3% bovine serum albumin, and incubated overnight at 4°C. After washing the tissue sections with PBS, the secondary antibody of horseradish peroxidase-associated goat anti-rabbit was added and the samples were incubated at room temperature for 1 h. The nuclei were then visualized with 4′,6-diamidino-2-phenylindole, (DAPI) and images were obtained using a fluorescence microscope.
2.18 Statistical analysis
All statistical analyses were conducted using R software (v4.0.3) and GraphPad Prism 8.0 software (La Jolla, CA, USA). Data are expressed as mean ± SD. Correlation analysis was performed using Spearman’s correlation analyses. The differences in quantitative data between the two groups were compared using the unpaired Student’s scale t-test, Mann–Whitney U-tests, or Dunnett’s t-tests, as appropriate. When more than two groups of data were compared, one-way ANOVA was used. The log-rank test was used to compare differences in survival between two groups. The timeROC (v 0.4) analysis was used to compare the predictive accuracy of SDR16C5 mRNA. For Kaplan–Meier curves, P-values and hazard ratio (HR) with 95% confidence interval (CI) were generated by log-rank tests and univariate Cox proportional hazards regression. P value <0.05 was considered statistically significant.
3 Results
3.1 SDR16C5 is highly expressed in many cancers
This study was designed to investigate the role of SDR16C5 in PAAD, and the process of construction and validation is shown in Figure 1. From our analysis of TCGA transcriptome data, we found that SDR16C5 has high expression in breast invasive carcinoma (BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), PAAD and thyroid carcinoma (THCA), and other tumors and low expression in glioblastoma multiforme (GBM), head and Neck squamous cell carcinoma (HNSC), kidney chromophobe (KICH), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), prostate adenocarcinoma (PRAD). (Figure 2a). The GEPIA database also allowed us to analyze the differential expression level of SDR16C5 in tumor tissues and normal tissues further in a large sample database, and these results suggest that SDR16C5 has high expression in BRCA, CESC, PAAD, colon adenocarcinoma (COAD), stomach adenocarcinoma (STAD) and rectum adenocarcinoma (READ), and low expression in LUSC, rain lower grade glioma (LGG), GBM, esophageal carcinoma (ESCA) and skin cutaneous melanoma (SKCM) (Figure 2b).

Flowchart for generating and validating the role of SDR16C5 in PAAD.
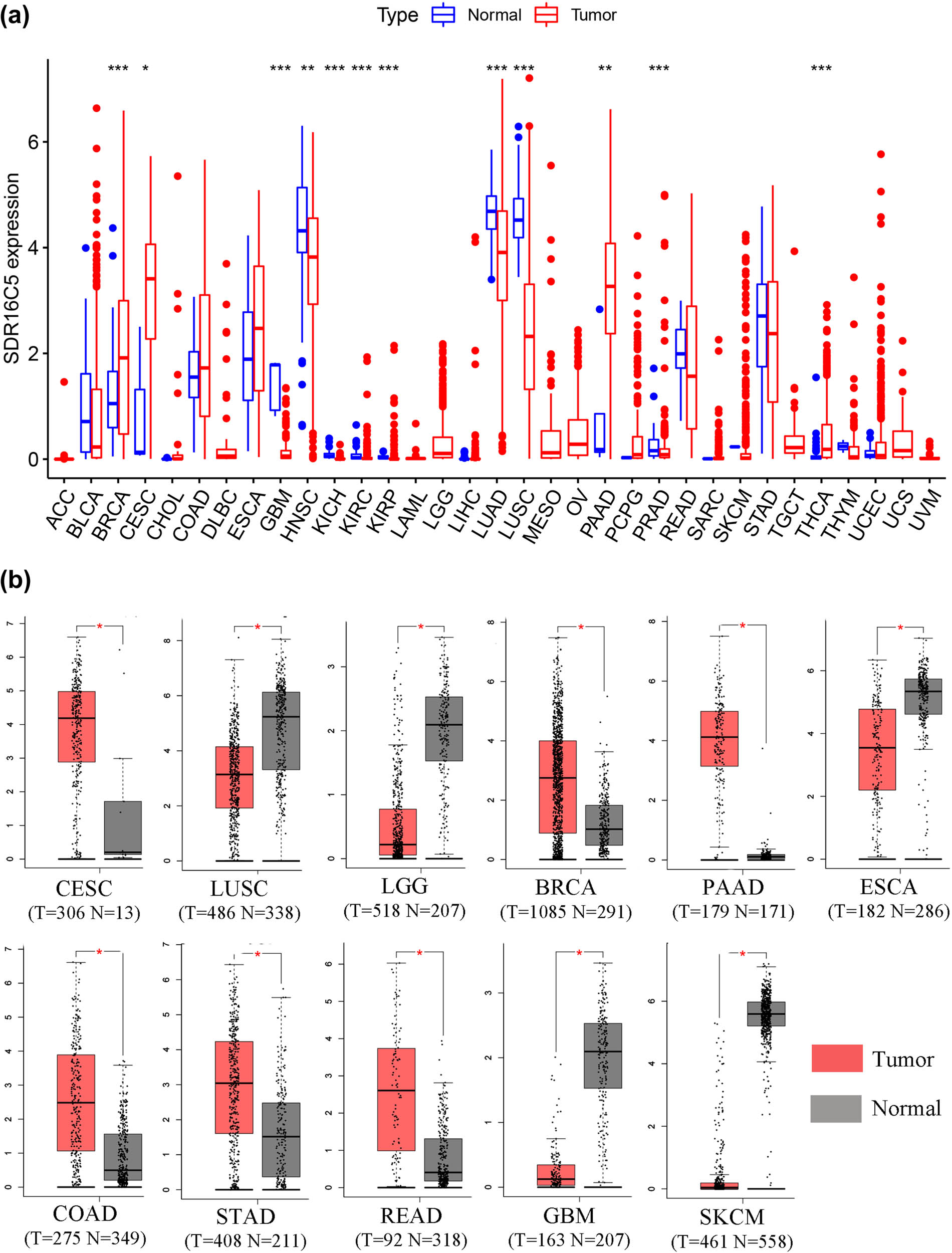
SDR16C5 expression is upregulated in many types of cancers. (a) The expression of SDR16C5 in 33 types of human cancer based on UCSC Xena cancer and normal data. (b) SDR16C5 expression in TCGA and GTEx normal tissues. *P < 0.05; **P < 0.01; ***P < 0.001.
3.2 Overexpression of SDR16C5 predicts poorer prognoses in many cancers
By analyzing the impact of SDR16C5 expression in multiple cancers on overall survival, we found that high expression of SDR16C5 is significantly correlated with poor prognosis in KIRC (P = 0.002), PAAD (P = 0.005), SKCM (P = 0.002), and UCEC (P = 0.036) (Figure 3). Since there may have been nontumor death factors during follow-up, we analyzed the relationship between the expression of SDR16C5 and the prognosis of disease-specific survival (DSS) separately. These results were very similar to the above: the higher the expression of SDR16C5, the worse the prognosis of KIRC (P < 0.001), PAAD (P = 0.029), SKCM (P = 0.024), and UCEC (P = 0.032). Furthermore, the higher the expression of SDR16C5, the worse the DFI of ACC (P = 0.005), CHOL (P = 0.025), KIRC (P = 0.048), PAAD (P = 0.041), and PCPG (P = 0.044), and worse the PFI of CHOL (P = 0.016), PAAD (P = 0.038), and UCEC (P = 0.026).

Overexpression of SDR16C5 predicts poor survival in various human cancers as determined by TCGA. Red represents high expression, and blue represents low expression. The abscissa represents survival time, and the ordinate represents survival rate.
3.3 Overexpression of SDR16C5 predicts poorer prognoses in PAAD
Analysis of many types of cancers showed that SDR16C5 was significantly associated with all the analyzed survival values (OS, DSS, DFI, and PFI). Therefore, we further analyzed the prognostic efficacy of SDR16C5 in PAAD specifically. As shown in Figure 4a, the predictive power of the SDR16C5 gene on PAAD prognosis was statistically significant (log-rank P < 0.05), and the higher the expression, the worse the prognosis. The figures show how the samples ranked according to SDR16C5 expression from low to high (top A), and the corresponding middle scatter dot plots showed more and more patients dying and shorter survival time from left to right.
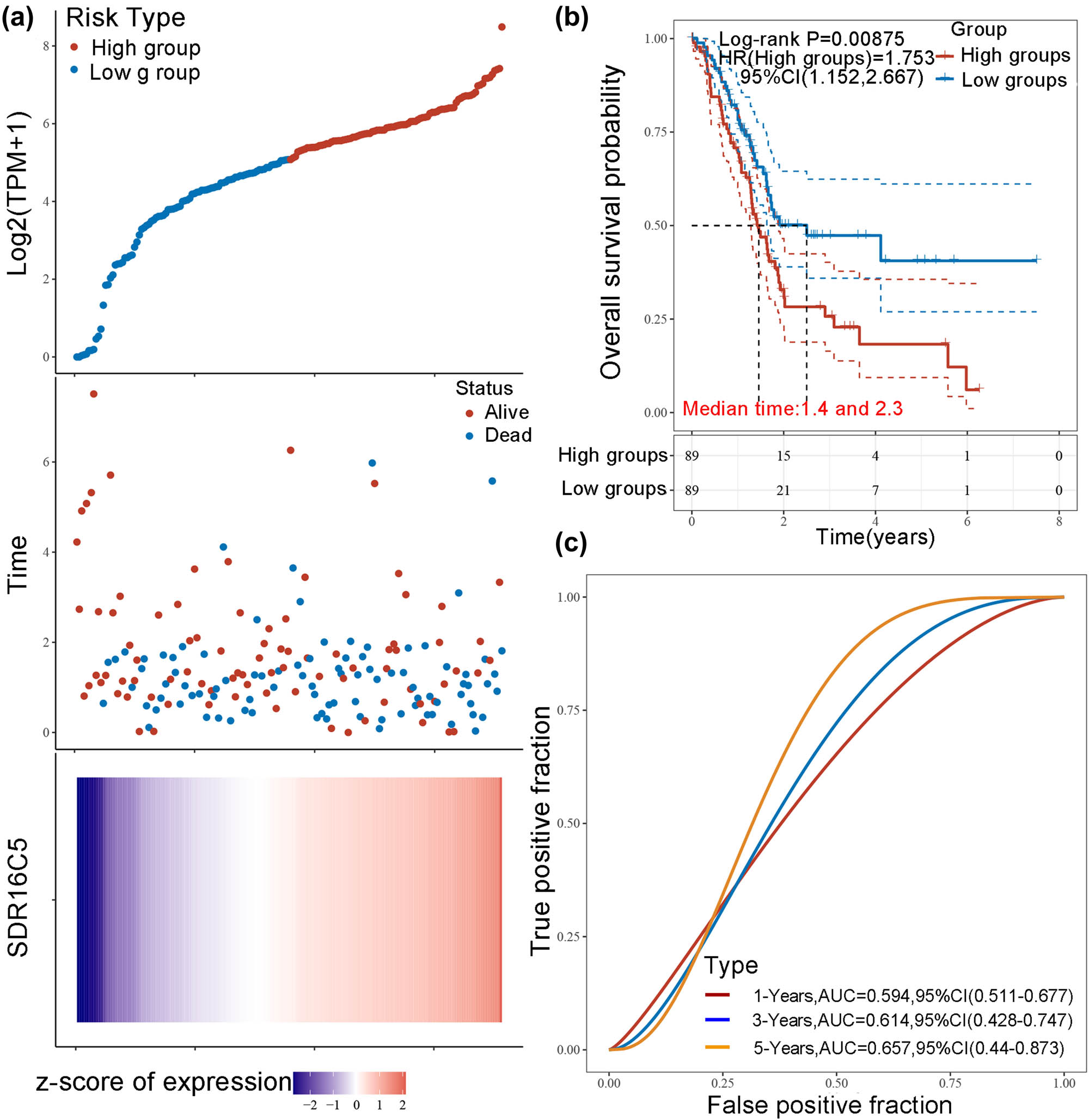
Overexpression of SDR16C5 predicts poorer prognoses in PAAD. (a) The relationship between the expression of SDR16C5 and patient survival time and status in TCGA data. The top image represents the scatter plot of gene expression from low to high, and different colors represent different expression groups. The middle image represents the distribution of survival time and survival status corresponding to the gene expression of different samples. The bottom image represents the expression heat map of the SDR16C5 gene. (b) The KM survival curve distribution of the SDR16C5 gene in the TCGA data set. (c) The AUC curve of the model evaluation index. The higher the P-value, the better the predictive effects of the model.
The heat map in Figure 4a represents relative gene expression (bottom a). Here, we can clearly see how the SDR16C5 gene was identified as a risk factor according to the risk score and gene expression trend after the rankings. Figure 4b shows the KM survival curve of SDR16C5 in TCGA data, where the risk coefficient HR = 1.753 and the 95% HR confidence interval was 1.152–2.667. Additionally, the median survival times of the high and low-expression groups were 1.4 and 2.3 years, respectively. To measure the efficacy of the SDR16C5 gene as a prognostic biomarker even further, we performed the ROC analyses. The AUC of the model evaluation index is shown in Figure 4c. The AUC of 1 year is 0.594, of 2 years is 0.614, and of 3 years is 0.657, indicating that SDR16C5 does have predictive effects on PAAD prognosis.
3.4 SDR16C5 expression was related to immune cell infiltration and immune-associated pathways in PAAD
In recent years, the clinical success of tumor immunotherapy has made it necessary to study the interaction between malignant cells and the host immune system, and immune cells in the tumor microenvironment profoundly influence the success of immunotherapy. Correlation analysis may be able to provide key clues for studying the function and mechanism of SDR16C5, and we therefore evaluated the correlation of SDR16C5 expression with immune cell infiltration.
As presented in Figure 5, SDR16C5 expression was significantly associated with B cells (CD38 r = −0.21, P = 0.0044; CD79A r = −0.16, P = 0.036), CD8 + T cells (CD8A r = −0.27, P = 0.00025; CD8B r = −0.25, P = 0.00079), T cell follicular helper (ICOS, r = −0.21, P = 0.0057) and dendritic cells (HLA-DPB1 r = −0.26, P = 0.00043; HLA-DRA r = −0.16, P = 0.031; HLA-DPA1 r = −0.16, P = 0.027; CD1C r = −0.2, P = 0.0074) in PAAD. Further correlation analysis also showed that SDR16C5 was related to immune cell markers as well (Table 1). Screening 549 genes significantly related to SDR16C5 through the CCLE database for GO and KEGG analysis; we found that in BP, the terms were mostly related to extracellular matrix organization, extracellular structure organization, and external encapsulating structure organization. In CC, they were related to collagen-containing extracellular matrix and endoplasmic reticulum lumen, and in MF, they were related to extracellular matrix structural constituent, glycosaminoglycan binding, and growth factor activity (Figure 6a). The KEGG pathway analysis showed that these SDR16C5-related genes are most significantly enriched in the IL-17 and TNF signaling pathways (Figure 6b).
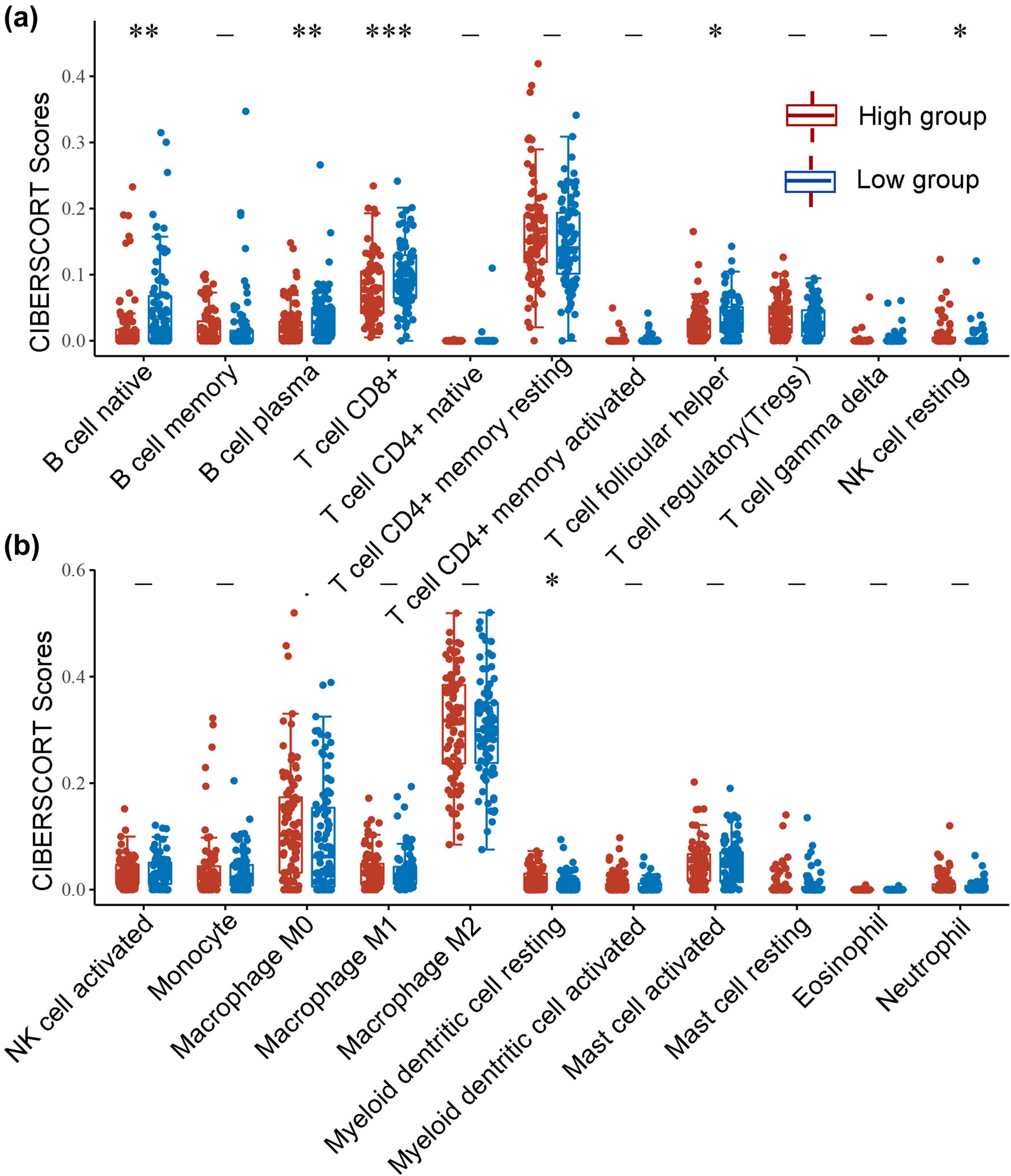
(a and b) SDR16C5 expression might correlate with immune cell infiltration in PAAD. The horizontal axis represents the type of immune cell, and the vertical axis represents the expression of SDR16C5. The statistical difference between the high SDR16C5 and low SDR16C5 groups was compared using the Wilcox test. *P < 0.05, **P < 0.01, ***P < 0.001.
Correlation analysis between SDR16C5 and biomarkers of immune cells in PAAD
| Immune cell | Biomarker | Cor | P-value |
|---|---|---|---|
| B cell | CD19 | −0.14 | 0.061 |
| CD38 | −0.21 | 0.0044** | |
| CD79A | −0.16 | 0.036* | |
| CD8+ T cell | CD8A | −0.27 | 0.00025*** |
| CD8B | −0.25 | 0.00079*** | |
| Tfh | CXCR5 | −0.087 | 0.25 |
| ICOS | −0.21 | 0.0057** | |
| BCL-6 | 0.054 | 0.47 | |
| Dendritic cell | HLA-DPB1 | −0.26 | 0.00043*** |
| HLA-DQB1 | −0.023 | 0.76 | |
| HLA-DRA | −0.16 | 0.031* | |
| HLA-DPA1 | −0.16 | 0.027* | |
| CD1C | −0.2 | 0.0074** | |
| NRP1 | −0.099 | 0.19 | |
| ITGAX | −0.13 | 0.073 |
Note. Pearson correlation analysis *P < 0.05; **P < 0.01; ***P < 0.001.

SDR16C5-related protein function enrichment analysis. (a and b) A total of 549 genes were screened as significant from GO and KEGG analysis. These genes may be enriched in immune-associated pathways.
3.5 SDR16C5 was overexpressed in PAAD tissues and cells
To study the expression of SDR16C5 in PAAD, we analyzed the mRNA expression levels of SDR16C5 in the GEO datasets (GSE15471, GSE16515, and GSE2873). SDR16C5 mRNA expression was significantly upregulated in tumor tissues, compared to para-carcinoma tissues (P < 0.001) (Figure 7a). Subsequently, we utilized the CPTAC database to assess the protein expression of SDR16C5 genes and found that there is a high expression of SDR16C5 in PAAD (Figure 7b). In addition, qRT-PCR analysis of SDR16C5 expression in HPDE6 and five PAAD cell lines showed that the mRNA expression of SDR16C5 was markedly enhanced in the PDAC cell lines (SW1990, PANC-1, MIA PaCa-2, ASPC, and BXPC) compared to HPDE6 (Figure 7c). Moreover, the results of the western blotting analysis were consistent with the qRT-PCR results (Figure 7d). Western blotting revealed a positive expression of SDR16C5 in PAAD tissues (Figure 7e), and that SDR16C5 was upregulated in PAAD.
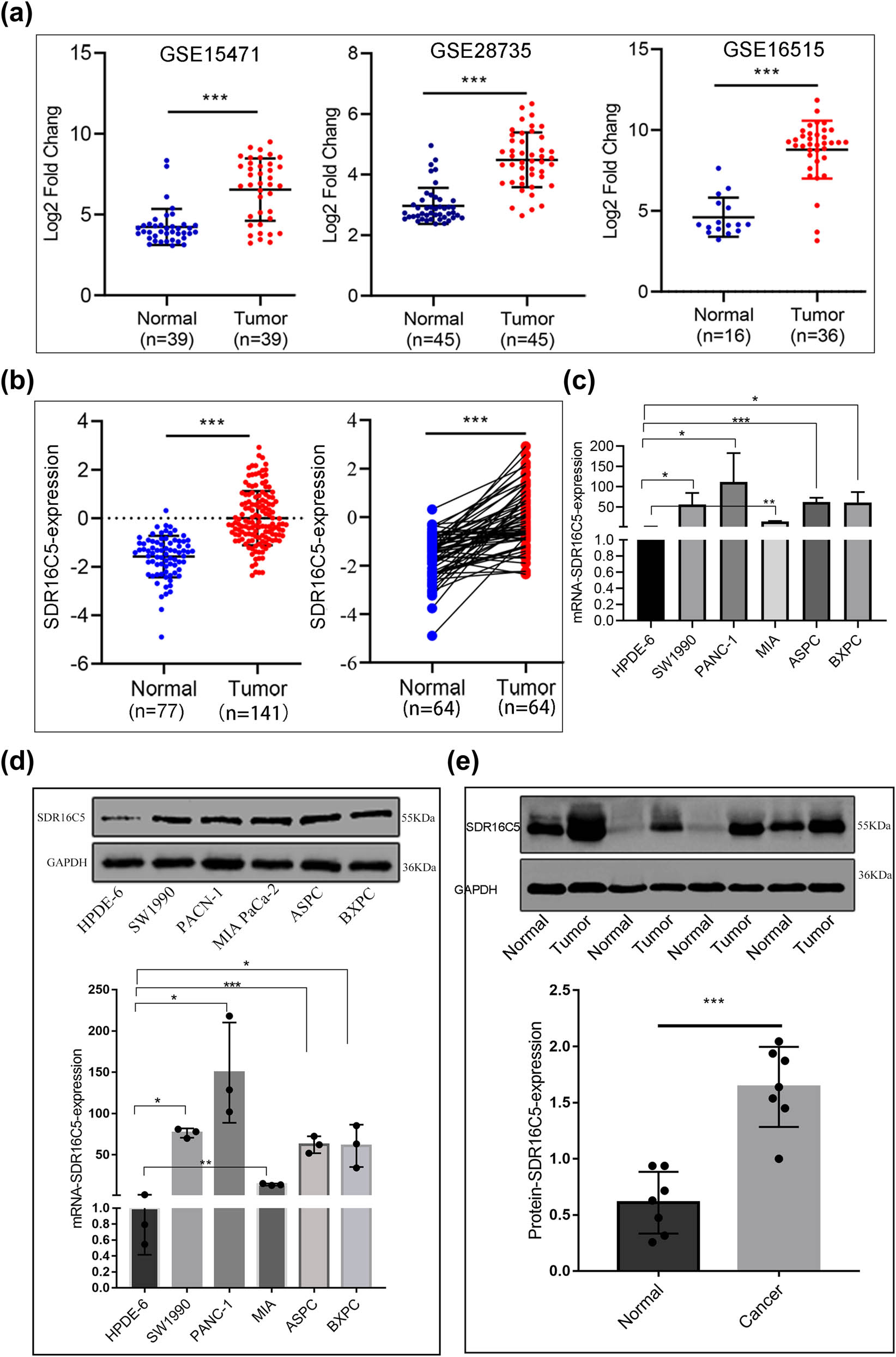
SDR16C5 was highly upregulated in PAAD tissues and cell lines. (a) The expression of SDR16C5 in the GEO data: GSE15471 (N = 36, T = 36), GSE28735 (N = 45, T = 45), GSE16515 (N = 16, T = 36). (b) SDR16C5 protein expression in PAAD: samples containing cancerous tissues and paired adjacent noncancerous samples were screened out in CPTAC data. (c) qRT-PCR analysis of SDR16C5 in different cell lines of PAAD. (d) Western blot analysis of the expression of SDR16C5 in different cell lines of PAAD. (e) Western blot analysis of SDR16C5 in PAAD tissues and adjacent tissues. Experiments were repeated three times with three replicates of each sample. ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001.
3.6 Knockdown of SDR16C5 inhibited PAAD cell proliferation
To explore whether SDR16C5 affects the development of PAAD cells, human PAAD cell lines PANC-1 and SW1990 were transfected with SDR16C5 siRNA. qRT-PCR assay was then applied to validate the transfection efficiency, and SDR16C5 siRNA2 (S2) was chosen for subsequent experiments (Figure 8a). Both qRT-PCR and western blotting showed that SDR16C5 was lower in SDR16C5-knockdown cells compared to NC-transfected cells (Figure 8b and c). Additionally, CCK-8 results showed that OD values in cells transfected with si-SDR16C5 gradually decreased after cell culture times of 24, 48, 72, and 96 h (Figure 8d). We also observed the formation of clones after transfection and found that SDR16C5 downregulation dramatically decreased clone formation in PANC-1 and SW1990 cells (Figure 8e).
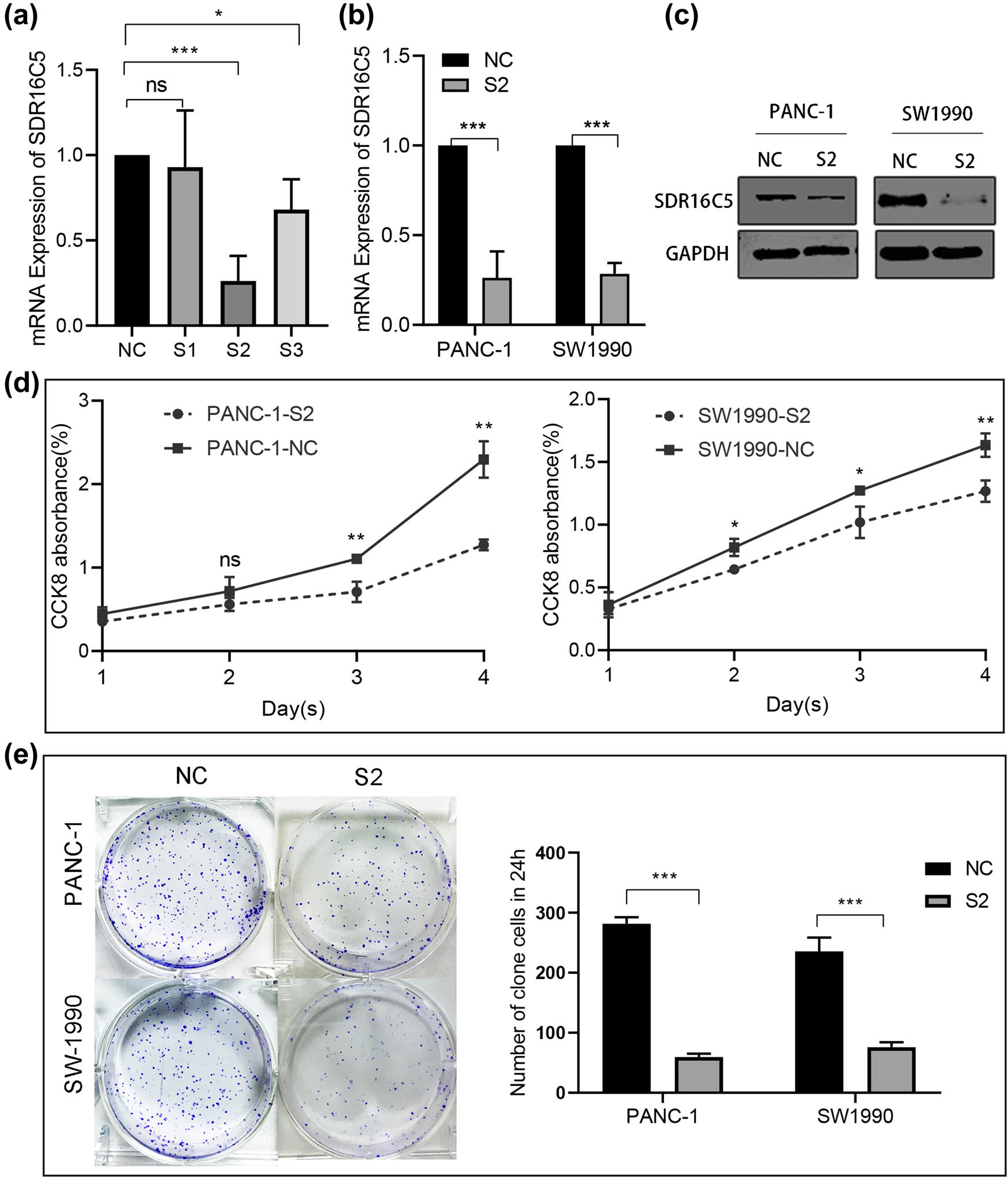
Knockdown of SDR16C5 can inhibit cell proliferation of PANC-1 and SW1990. (a) The expression of SDR16C5 was determined by qRT-PCR after transfection of SDR16C5 siRNA. (b and c) The successful transfection of SDR16C5 was validated using RT-qPCR and WB. (d) Cell proliferation experiment. (e) Clone formation experiment for PANC-1 and SW1990 cells. Experiments were repeated three times with three replicates of each sample.
3.7 Reduction of SDR16C5 repressed PAAD cell migration and induced apoptosis
To explore cell migration ability after SDR16C5 silencing, we next performed transwell assay and wound-healing scratch experiments. We observed that the scratches continued to heal with time, compared to the control group, and that SDR16C5 knockdown reduced the ability of the cells to migrate to the scratched area (Figure 9a). Additionally, the transwell assays showed that SDR16C5-silenced PANC-1 and SW1990 cells significantly decreased on the lower chamber’s membrane, compared to controls (Figure 9b). Western blotting indicated that the knockdown of SDR16C5 in PAAD cells repressed the expression of the migration-associated protein vimentin while upregulating E-cadherin expression (Figure 9c).
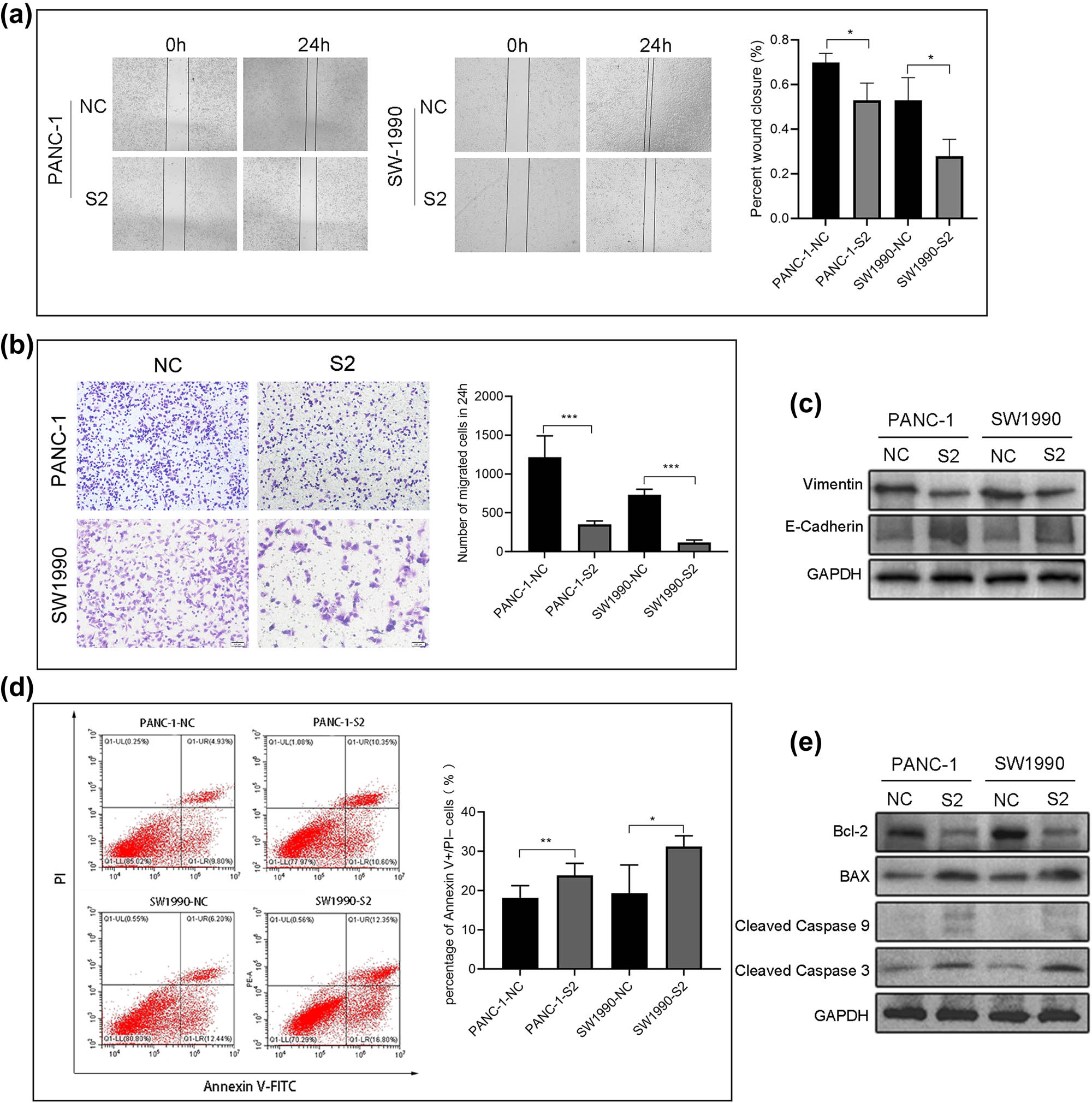
Reduction of SDR16C5 repressed PANC-1 and SW1990 cell migration and induced apoptosis. (a) Inhibiting the expression of SDR16C5 reduced the ability of cells to migrate to the scratched area. (b) The number of PANC-1 and SW1990 cells that traversed the transwell membrane decreased after inhibiting SDR16C5. (c) Western blot was used to detect the migration-associated proteins vimentin and E-cadherin in SDR16C5-silenced PAAD cells. (d) Downregulating the expression of SDR16C5 decreased the percentage of apoptotic PANC-1 and SW1990 cells. (e) Western blot assay was used to examine the expression of the anti-apoptotic proteins Bcl-2, cleaved caspase 3, and cleaved caspase 9 and the apoptotic protein BAX in SDR16C5-silenced PAAD cells. Experiments were repeated three times with three replicates of each sample.
Additionally, we measured the apoptosis rate of PANC-1 and SW1990 cells via flow cytometry. The percentage of apoptotic cells was increased compared to controls in PANC-1 and SW1990 cells following the SDR16C5 knockdown (Figure 9d). Knockdown of SDR16C5 can promote PAAD apoptosis by inhibiting Bcl-2, cleaved-caspase 3, and cleaved-caspase 9 protein expression as well as increasing Bax protein expression (Figure 9e). These results indicate that overexpression of SDR16C5 increased the migration and inhibited the apoptosis of PAAD cells.
3.8 SDR16C5 may affect the IL-17 signaling pathway in PAAD cells
According to the results of our KEGG analysis, we performed the immunofluorescence assay to determine whether SDR16C5 affects IL-17 expression. Our results suggest that both SDR16C5 and IL-17 proteins had higher expression in PAAD tissues compared to normal tissues. In addition, SDR16C5 and IL-17 proteins are located in the cytoplasm, and IL-17 protein expression is positively correlated with that of SDR16C5 (Figure 10a–d). This suggests that SDR16C5 might affect PAAD progression by regulating the IL-17 signaling pathway.
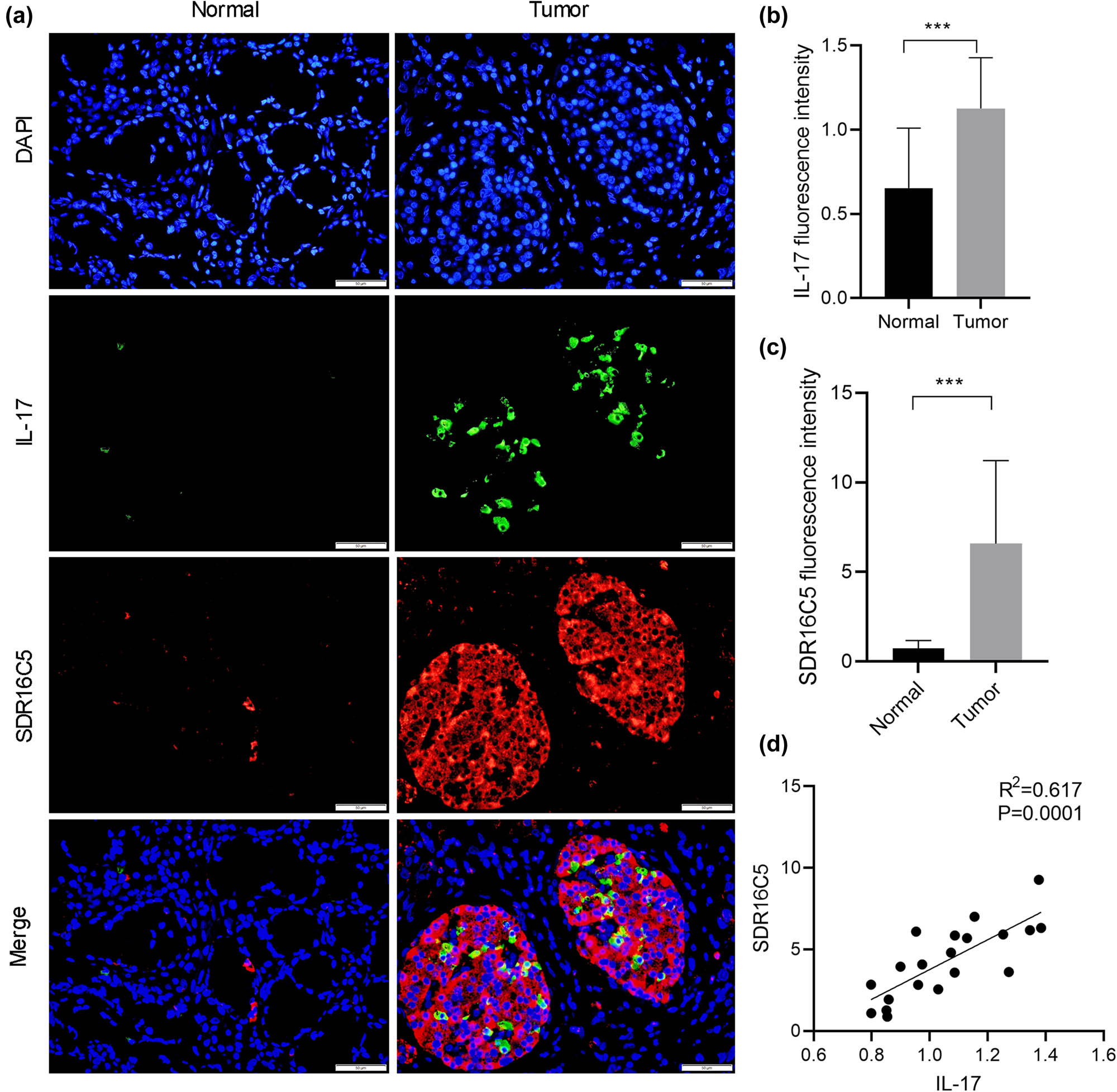
SDR16C5 may regulate the IL-17 pathway in PAAD. Co-expression of SDR16C5 and IL-17 was measured by immunofluorescence. (a) Representative pictures of multiplex IF staining showing SDR16C5, IL-17, and DAPI (top panels) staining in PAAD tissues. Scale bars represent 50 µm. (b and c) Quantification analysis of SDR16C5 and IL-17 measured in terms of mean fluorescence density. (d) Correlation analysis between SDR16C5 and IL-17. Experiments were repeated three times with three replicates of each sample. *P < 0.05, **P < 0.01, ***P < 0.001.
4 Discussion
PAAD is the seventh leading cause of cancer death worldwide primarily because it is often not detected until it is in its advanced stage [15]. Furthermore, its incidence increases with age [16], making surgery and treatment even more difficult. Hence, early identification of premalignant lesions is crucial for earlier diagnoses and the development of effective prevention strategies [1]. Although many advancements have been achieved in the treatment of cancer, such as laparoscopic surgical techniques, neoadjuvant chemoradiotherapy, immunotherapy, and targeted therapy, the outcomes of PAAD have remained poor, and diagnosis and treatment have not improved at all in the past several decades [17]. In the era of precision medicine, personalized treatment of PAAD clearly lags behind.
In an effort to bring PAAD treatment up to speed, this study explored the possible regulatory mechanism of SDR16C5 in PAAD through bioinformatics analysis and in vitro experiments. We observed that high expression of SDR16C5 in a variety of tumors including KIRC, PAAD, SKCM, and UCEC is associated with poor prognoses. We similarly found that SDR16C5 is highly expressed in PAAD. Moreover, the upregulation of SDR16C5 is associated with poor prognosis of PAAD patients, which was consistent with previous research [8]. In addition, high expression of SDR16C5 is related to various notions of survival rate in PAAD patients (OS, DSS, DFI, and PFI) and has good predictive power for their prognoses as well.
By the analysis of GEO and CPTAC databases, we found that SDR16C5 has a higher expression in PAAD tissues from the gene to protein level than in normal tissues. In addition, we found that both the mRNA and protein expression levels of SDR16C5 were highly expressed in our PAAD samples and cell lines, suggesting that SDR16C5 may be an oncogene in PAAD. The latest research shows that SDR16C5 is associated with the occurrence of PAAD [8]; we then performed functional experiments to determine the role of SDR16C5 in the development and progression of PAAD. From these experiments, we found that SDR16C5 downregulation inhibited the proliferation capacity of PAAD cells (using CCK8 and colony formation assays), which indicates that SDR16C5 may play a vital role in PAAD cell growth. Proliferation and apoptosis are two important cellular processes involved in the development and spread of cancer [18]; thus, we conducted cell flow cytometry experiments and found that the knockdown of SDR16C5 can promote the apoptosis of PAAD cells. Moreover, the Bcl2 protein family can regulate the mitochondrial-mediated intrinsic pathway and includes pro-apoptotic proteins and anti-apoptotic proteins such as Bax and Bcl-2 [19]. Hence, we performed a western blotting assay and found that downregulation of SDR16C5 decreased Bcl-2, cleaved-caspase 3, and cleaved-caspase 9 protein expression and increase Bax expression, suggesting that SDR16C5 is responsible for the inhibition of apoptosis in PAAD. Epithelial–mesenchymal transition is a dynamic process in cancer development, which leads to functional changes in cell migration and invasion [20]. Additionally, we found that silencing SDR16C5 reduced vimentin expression and increased E-cadherin expression, indicating that SDR16C5 might regulate PAAD cell migration (a crucial step in metastasis) by means of epithelial–mesenchymal transition.
The tumor microenvironment comprises a variety of immune cells, among which T cells exert a significant role in tumor development and progression or anti-tumor responses in PAAD patients. This study also indicated that the differential expression of SDR16C5 is closely related to tumor immune cells and their corresponding immune markers including CD8 + T cell and T-cell follicular helper; however, further research is needed to confirm. High or low levels of anti-inflammatory cytokines, such as interleukin-10, in the absence or presence of proinflammatory cytokines, such as interleukin-17, delineate the fate of T cells (regulatory T or T-helper 17 cells), which subsequently affect the progression of cancer [21]. Our KEGG analysis showed that SDR16C5 may participate in the occurrence of PAAD through the IL-17 pathway and TNF signaling pathways. For TNF-R1/TNF signaling, the predominant pathway in most models investigated so far is the activation of proliferative signaling [22]. Interleukin-17 (IL-17) family cytokines are potent drivers of inflammatory responses [21]; however, recent studies also revealed IL-17 as an immune marker in patients with bladder cancer [23], breast cancer [24], non-small cell lung cancer [25], renal cell carcinoma [26], and colorectal cancer [27]. Thus, we conducted immunofluorescence staining to detect the co-expression of SDR16C5 and IL-17. As a result, we found that SDR16C5 and IL-17 proteins were both highly expressed in PAAD tissues compared to normal tissues. Moreover, SDR16C5 and IL-17 proteins are both located in the cytoplasm, and IL-17 protein expression is positively correlated with that of SDR16C5, suggesting that there may be an interaction between them. The specific mechanism that governs this relationship is a topic for future work.
5 Conclusions
In this study, we found that SDR16C5 is related to the occurrence and development of multiple types of tumors and poor prognoses. More specifically, SDR16C5 is significantly upregulated in PAAD tissues and cell lines. The knockdown of SDR16C5 inhibited PAAD cell proliferation, induced PAAD cell apoptosis, and repressed cell migration by affecting epithelial–mesenchymal transition. SDR16C5 may also be involved in the occurrence of PAAD through the IL-17 signaling pathway. Finally, SDR16C5 may become a novel diagnostic, prognostic marker, and therapeutic target for PAAD.
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
-
Funding information: This research was supported by the National Natural Science Foundation of China (81770638) and the Science and Technology Fund of the Guizhou Provincial Health Commission (gzwkj2022-049).
-
Author contributions: K.Q.H. designed the project and wrote the manuscript. Q.Y. processed the datasets and revised the manuscript. H.S.Y. and J.W.Z. analyzed the data. B.P.Y. oversaw the project design. All authors read and approved the final manuscript. The authors applied the SDC approach for the sequence of authors.
-
Conflict of interest: Authors state no conflicts of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Wood LD, Canto MI, Jaffee EM, Simeone DM. Pancreatic cancer: Pathogenesis, screening, diagnosis, and treatment. Gastroenterology. 2022;163(2):386–402.e1.10.1053/j.gastro.2022.03.056Search in Google Scholar PubMed PubMed Central
[2] Klatte DCF, Wallace MB, Löhr M, Bruno MJ, van Leerdam ME. Hereditary pancreatic cancer. Best Pract Res Clin Gastroenterol. 2022;58–59:101783.10.1016/j.bpg.2021.101783Search in Google Scholar PubMed
[3] Ji F, Sadreyev RI. RNA-seq: Basic bioinformatics analysis. Curr Protoc Mol Biol. 2018;124(1):e68.10.1002/cpmb.68Search in Google Scholar PubMed PubMed Central
[4] Chen H, Fang X, Zhu H, Li S, He J, Gu P, et al. Gene expression profile analysis for different idiopathic interstitial pneumonias subtypes. Exp Lung Res. 2014;40(8):367–79.10.3109/01902148.2014.933985Search in Google Scholar PubMed
[5] Adams MK, Lee SA, Belyaeva OV, Wu L, Kedishvili NY. Characterization of human short chain dehydrogenase/reductase SDR16C family members related to retinol dehydrogenase 10. Chem Biol Interact. 2017;276:88–94.10.1016/j.cbi.2016.10.019Search in Google Scholar PubMed PubMed Central
[6] Ashmore JH, Luo S, Watson CJW, Lazarus P. Carbonyl reduction of NNK by recombinant human lung enzymes: Identification of HSD17β12 as the reductase important in (R)-NNAL formation in human lung. Carcinogenesis. 2018;39(8):1079–88.10.1093/carcin/bgy065Search in Google Scholar PubMed PubMed Central
[7] Pareek CS, Błaszczyk P, Dziuba P, Czarnik U, Fraser L, Sobiech P, et al. Single nucleotide polymorphism discovery in bovine liver using RNA-seq technology. PLoS One. 2017;12(2):e0172687.10.1371/journal.pone.0172687Search in Google Scholar PubMed PubMed Central
[8] Ye H, Li T, Wang H, Wu J, Yi C, Shi J, et al. TSPAN1, TMPRSS4, SDR16C5, and CTSE as novel panel for pancreatic cancer: A bioinformatics analysis and experiments validation. Front Immunol. 2021;12:649551.10.3389/fimmu.2021.649551Search in Google Scholar PubMed PubMed Central
[9] Zhang J, Hu X, Liu S, He Y, Lyu L, Jiang L. Clinical value screening, prognostic significance, and key gene identification of TrkB in laryngeal carcinoma. Dis Markers. 2022;2022:1354005.10.1155/2022/1354005Search in Google Scholar PubMed PubMed Central
[10] Jing Z, Ziwang F, Yinhang W, Yani Z, Jian C, Jingwen W, et al. Novel acetylation-related gene signatures for predicting the prognosis of patients with colorectal cancer. Hum Cell. 2022;35(4):1159–73.10.1007/s13577-022-00720-6Search in Google Scholar PubMed
[11] Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–8.10.1038/s41587-020-0546-8Search in Google Scholar PubMed PubMed Central
[12] Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–w102.10.1093/nar/gkx247Search in Google Scholar PubMed PubMed Central
[13] Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: Archive for functional genomics data sets–update. Nucleic Acids Res. 2013;41(Database issue):D991–5.10.1093/nar/gks1193Search in Google Scholar PubMed PubMed Central
[14] Yang Q, Tian S, Liu Z, Dong W. Knockdown of RIPK2 inhibits proliferation and migration, and induces apoptosis via the NF-kappaB signaling pathway in gastric cancer. Front Genet. 2021;12:627464.10.3389/fgene.2021.627464Search in Google Scholar PubMed PubMed Central
[15] Aziz H, Acher AW, Krishna SG, Cloyd JM, Pawlik TM. Comparison of society guidelines for the management and surveillance of pancreatic cysts: A review. JAMA Surg. 2022;157(8):723–30.10.1001/jamasurg.2022.2232Search in Google Scholar PubMed
[16] Chen X, Yi B, Liu Z, Zou H, Zhou J, Zhang Z, et al. Global, regional and national burden of pancreatic cancer, 1990 to 2017: Results from the Global Burden of Disease Study 2017. Pancreatol Off J Int Assoc Pancreatol [et al]. 2020;20(3):462–9.10.1016/j.pan.2020.02.011Search in Google Scholar PubMed
[17] Zhang Z, Liu X, Huang R, Liu X, Liang Z, Liu T. Upregulation of nucleoprotein AHNAK is associated with poor outcome of pancreatic ductal adenocarcinoma prognosis via mediating epithelial-mesenchymal transition. J Cancer. 2019;10(16):3860–70.10.7150/jca.31291Search in Google Scholar PubMed PubMed Central
[18] Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–74.10.1016/j.cell.2011.02.013Search in Google Scholar PubMed
[19] Green DR. The mitochondrial pathway of apoptosis part II: The BCL-2 protein family. Cold Spring Harb Perspect Biol. 2022;14(6):a041046.10.1101/cshperspect.a041046Search in Google Scholar PubMed PubMed Central
[20] Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. Author Correction: Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2021;22(12):834.10.1038/s41580-021-00428-9Search in Google Scholar PubMed PubMed Central
[21] Meehan EV, Wang K. Interleukin-17 family cytokines in metabolic disorders and cancer. Genes (Basel). 2022;13(9):1643.10.3390/genes13091643Search in Google Scholar PubMed PubMed Central
[22] Fritsch J, Särchen V, Schneider-Brachert W. Regulation of death receptor signaling by S-palmitoylation and detergent-resistant membrane micro domains-greasing the gears of extrinsic cell death induction, survival, and inflammation. Cancers. 2021;13(11):2513.10.3390/cancers13112513Search in Google Scholar PubMed PubMed Central
[23] Mousa FA, Jasim HA, Shakir F. A prognostic impact of interleukin 17 (IL-17) as an immune-marker in patients with bladder cancer. Arch Razi Inst. 2022;77(3):1059–65.Search in Google Scholar
[24] Seif F, Torki Z, Zalpoor H, Habibi M, Pornour M. Breast cancer tumor microenvironment affects Treg/IL-17-producing Treg/Th17 cell axis: Molecular and therapeutic perspectives. Mol Ther Oncolytics. 2023;28:132–57.10.1016/j.omto.2023.01.001Search in Google Scholar PubMed PubMed Central
[25] Xu R, Ke X, Shang W, Liu S, Fu X, Wang T, et al. Distribution and clinical significance of IL-17A in tumor-infiltrating lymphocytes of non-small cell lung cancer patients. Pathol Oncol Res. 2022;28:1610384.10.3389/pore.2022.1610384Search in Google Scholar PubMed PubMed Central
[26] Jarocki M, Karska J, Kowalski S, Kiełb P, Nowak Ł, Krajewski W, et al. Interleukin 17 and its involvement in renal cell carcinoma. J Clin Med. 2022;11(17):4973.10.3390/jcm11174973Search in Google Scholar PubMed PubMed Central
[27] Li S, Na R, Li X, Zhang Y, Zheng T. Targeting interleukin-17 enhances tumor response to immune checkpoint inhibitors in colorectal cancer. Biochim Biophys Acta Rev cancer. 2022;1877(4):188758.10.1016/j.bbcan.2022.188758Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
- Immunosuppressive treatment for idiopathic membranous nephropathy: An updated network meta-analysis
- Identifying two pathogenic variants in a patient with pigmented paravenous retinochoroidal atrophy
- Effects of phytoestrogens combined with cold stress on sperm parameters and testicular proteomics in rats
- A case of pulmonary embolism with bad warfarin anticoagulant effects caused by E. coli infection
- Neutrophilia with subclinical Cushing’s disease: A case report and literature review
- Isoimperatorin alleviates lipopolysaccharide-induced periodontitis by downregulating ERK1/2 and NF-κB pathways
- Immunoregulation of synovial macrophages for the treatment of osteoarthritis
- Novel CPLANE1 c.8948dupT (p.P2984Tfs*7) variant in a child patient with Joubert syndrome
- Antiphospholipid antibodies and the risk of thrombosis in myeloproliferative neoplasms
- Immunological responses of septic rats to combination therapy with thymosin α1 and vitamin C
- High glucose and high lipid induced mitochondrial dysfunction in JEG-3 cells through oxidative stress
- Pharmacological inhibition of the ubiquitin-specific protease 8 effectively suppresses glioblastoma cell growth
- Levocarnitine regulates the growth of angiotensin II-induced myocardial fibrosis cells via TIMP-1
- Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study
- Single-cell transcription analysis reveals the tumor origin and heterogeneity of human bilateral renal clear cell carcinoma
- Identification of iron metabolism-related genes as diagnostic signatures in sepsis by blood transcriptomic analysis
- Long noncoding RNA ACART knockdown decreases 3T3-L1 preadipocyte proliferation and differentiation
- Surgery, adjuvant immunotherapy plus chemotherapy and radiotherapy for primary malignant melanoma of the parotid gland (PGMM): A case report
- Dosimetry comparison with helical tomotherapy, volumetric modulated arc therapy, and intensity-modulated radiotherapy for grade II gliomas: A single‑institution case series
- Soy isoflavone reduces LPS-induced acute lung injury via increasing aquaporin 1 and aquaporin 5 in rats
- Refractory hypokalemia with sexual dysplasia and infertility caused by 17α-hydroxylase deficiency and triple X syndrome: A case report
- Meta-analysis of cancer risk among end stage renal disease undergoing maintenance dialysis
- 6-Phosphogluconate dehydrogenase inhibition arrests growth and induces apoptosis in gastric cancer via AMPK activation and oxidative stress
- Experimental study on the optimization of ANM33 release in foam cells
- Primary retroperitoneal angiosarcoma: A case report
- Metabolomic analysis-identified 2-hydroxybutyric acid might be a key metabolite of severe preeclampsia
- Malignant pleural effusion diagnosis and therapy
- Effect of spaceflight on the phenotype and proteome of Escherichia coli
- Comparison of immunotherapy combined with stereotactic radiotherapy and targeted therapy for patients with brain metastases: A systemic review and meta-analysis
- Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation
- Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy
- The role of IL-31 and IL-34 in the diagnosis and treatment of chronic periodontitis
- Triple-negative mouse breast cancer initiating cells show high expression of beta1 integrin and increased malignant features
- mNGS facilitates the accurate diagnosis and antibiotic treatment of suspicious critical CNS infection in real practice: A retrospective study
- The apatinib and pemetrexed combination has antitumor and antiangiogenic effects against NSCLC
- Radiotherapy for primary thyroid adenoid cystic carcinoma
- Design and functional preliminary investigation of recombinant antigen EgG1Y162–EgG1Y162 against Echinococcus granulosus
- Effects of losartan in patients with NAFLD: A meta-analysis of randomized controlled trial
- Bibliometric analysis of METTL3: Current perspectives, highlights, and trending topics
- Performance comparison of three scaling algorithms in NMR-based metabolomics analysis
- PI3K/AKT/mTOR pathway and its related molecules participate in PROK1 silence-induced anti-tumor effects on pancreatic cancer
- The altered expression of cytoskeletal and synaptic remodeling proteins during epilepsy
- Effects of pegylated recombinant human granulocyte colony-stimulating factor on lymphocytes and white blood cells of patients with malignant tumor
- Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report
- NUDT21 relieves sevoflurane-induced neurological damage in rats by down-regulating LIMK2
- Association of interleukin-10 rs1800896, rs1800872, and interleukin-6 rs1800795 polymorphisms with squamous cell carcinoma risk: A meta-analysis
- Exosomal HBV-DNA for diagnosis and treatment monitoring of chronic hepatitis B
- Shear stress leads to the dysfunction of endothelial cells through the Cav-1-mediated KLF2/eNOS/ERK signaling pathway under physiological conditions
- Interaction between the PI3K/AKT pathway and mitochondrial autophagy in macrophages and the leukocyte count in rats with LPS-induced pulmonary infection
- Meta-analysis of the rs231775 locus polymorphism in the CTLA-4 gene and the susceptibility to Graves’ disease in children
- Cloning, subcellular localization and expression of phosphate transporter gene HvPT6 of hulless barley
- Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome
- Significant elevated CXCL14 and decreased IL-39 levels in patients with tuberculosis
- Whole-exome sequencing applications in prenatal diagnosis of fetal bowel dilatation
- Gemella morbillorum infective endocarditis: A case report and literature review
- An unusual ectopic thymoma clonal evolution analysis: A case report
- Severe cumulative skin toxicity during toripalimab combined with vemurafenib following toripalimab alone
- Detection of V. vulnificus septic shock with ARDS using mNGS
- Novel rare genetic variants of familial and sporadic pulmonary atresia identified by whole-exome sequencing
- The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology
- Novel compound heterozygous mutations in TELO2 in an infant with You-Hoover-Fong syndrome: A case report and literature review
- ctDNA as a prognostic biomarker in resectable CLM: Systematic review and meta-analysis
- Diagnosis of primary amoebic meningoencephalitis by metagenomic next-generation sequencing: A case report
- Phylogenetic analysis of promoter regions of human Dolichol kinase (DOLK) and orthologous genes using bioinformatics tools
- Collagen changes in rabbit conjunctiva after conjunctival crosslinking
- Effects of NM23 transfection of human gastric carcinoma cells in mice
- Oral nifedipine and phytosterol, intravenous nicardipine, and oral nifedipine only: Three-arm, retrospective, cohort study for management of severe preeclampsia
- Case report of hepatic retiform hemangioendothelioma: A rare tumor treated with ultrasound-guided microwave ablation
- Curcumin induces apoptosis in human hepatocellular carcinoma cells by decreasing the expression of STAT3/VEGF/HIF-1α signaling
- Rare presentation of double-clonal Waldenström macroglobulinemia with pulmonary embolism: A case report
- Giant duplication of the transverse colon in an adult: A case report and literature review
- Ectopic thyroid tissue in the breast: A case report
- SDR16C5 promotes proliferation and migration and inhibits apoptosis in pancreatic cancer
- Vaginal metastasis from breast cancer: A case report
- Screening of the best time window for MSC transplantation to treat acute myocardial infarction with SDF-1α antibody-loaded targeted ultrasonic microbubbles: An in vivo study in miniswine
- Inhibition of TAZ impairs the migration ability of melanoma cells
- Molecular complexity analysis of the diagnosis of Gitelman syndrome in China
- Effects of maternal calcium and protein intake on the development and bone metabolism of offspring mice
- Identification of winter wheat pests and diseases based on improved convolutional neural network
- Ultra-multiplex PCR technique to guide treatment of Aspergillus-infected aortic valve prostheses
- Virtual high-throughput screening: Potential inhibitors targeting aminopeptidase N (CD13) and PIKfyve for SARS-CoV-2
- Immune checkpoint inhibitors in cancer patients with COVID-19
- Utility of methylene blue mixed with autologous blood in preoperative localization of pulmonary nodules and masses
- Integrated analysis of the microbiome and transcriptome in stomach adenocarcinoma
- Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy
- DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway
- Lung abscess by Fusobacterium nucleatum and Streptococcus spp. co-infection by mNGS: A case series
- Genetic alterations of KRAS and TP53 in intrahepatic cholangiocarcinoma associated with poor prognosis
- Granulomatous polyangiitis involving the fourth ventricle: Report of a rare case and a literature review
- Studying infant mortality: A demographic analysis based on data mining models
- Metaplastic breast carcinoma with osseous differentiation: A report of a rare case and literature review
- Protein Z modulates the metastasis of lung adenocarcinoma cells
- Inhibition of pyroptosis and apoptosis by capsaicin protects against LPS-induced acute kidney injury through TRPV1/UCP2 axis in vitro
- TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats
- Primary mediastinum Ewing’s sarcoma with pleural effusion: A case report and literature review
- Association of ADRB2 gene polymorphisms and intestinal microbiota in Chinese Han adolescents
- Tanshinone IIA alleviates chondrocyte apoptosis and extracellular matrix degeneration by inhibiting ferroptosis
- Study on the cytokines related to SARS-Cov-2 in testicular cells and the interaction network between cells based on scRNA-seq data
- Effect of periostin on bone metabolic and autophagy factors during tooth eruption in mice
- HP1 induces ferroptosis of renal tubular epithelial cells through NRF2 pathway in diabetic nephropathy
- Intravaginal estrogen management in postmenopausal patients with vaginal squamous intraepithelial lesions along with CO2 laser ablation: A retrospective study
- Hepatocellular carcinoma cell differentiation trajectory predicts immunotherapy, potential therapeutic drugs, and prognosis of patients
- Effects of physical exercise on biomarkers of oxidative stress in healthy subjects: A meta-analysis of randomized controlled trials
- Identification of lysosome-related genes in connection with prognosis and immune cell infiltration for drug candidates in head and neck cancer
- Development of an instrument-free and low-cost ELISA dot-blot test to detect antibodies against SARS-CoV-2
- Research progress on gas signal molecular therapy for Parkinson’s disease
- Adiponectin inhibits TGF-β1-induced skin fibroblast proliferation and phenotype transformation via the p38 MAPK signaling pathway
- The G protein-coupled receptor-related gene signatures for predicting prognosis and immunotherapy response in bladder urothelial carcinoma
- α-Fetoprotein contributes to the malignant biological properties of AFP-producing gastric cancer
- CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa
- Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis
- Significance of sTREM-1 and sST2 combined diagnosis for sepsis detection and prognosis prediction
- Diagnostic value of serum neuroactive substances in the acute exacerbation of chronic obstructive pulmonary disease complicated with depression
- Research progress of AMP-activated protein kinase and cardiac aging
- TRIM29 knockdown prevented the colon cancer progression through decreasing the ubiquitination levels of KRT5
- Cross-talk between gut microbiota and liver steatosis: Complications and therapeutic target
- Metastasis from small cell lung cancer to ovary: A case report
- The early diagnosis and pathogenic mechanisms of sepsis-related acute kidney injury
- The effect of NK cell therapy on sepsis secondary to lung cancer: A case report
- Erianin alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation
- Loss of ACOX1 in clear cell renal cell carcinoma and its correlation with clinical features
- Signalling pathways in the osteogenic differentiation of periodontal ligament stem cells
- Crosstalk between lactic acid and immune regulation and its value in the diagnosis and treatment of liver failure
- Clinicopathological features and differential diagnosis of gastric pleomorphic giant cell carcinoma
- Traumatic brain injury and rTMS-ERPs: Case report and literature review
- Extracellular fibrin promotes non-small cell lung cancer progression through integrin β1/PTEN/AKT signaling
- Knockdown of DLK4 inhibits non-small cell lung cancer tumor growth by downregulating CKS2
- The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles
- Inflammation-related signaling pathways in tendinopathy
- CD4+ T cell count in HIV/TB co-infection and co-occurrence with HL: Case report and literature review
- Clinical analysis of severe Chlamydia psittaci pneumonia: Case series study
- Bioinformatics analysis to identify potential biomarkers for the pulmonary artery hypertension associated with the basement membrane
- Influence of MTHFR polymorphism, alone or in combination with smoking and alcohol consumption, on cancer susceptibility
- Catharanthus roseus (L.) G. Don counteracts the ampicillin resistance in multiple antibiotic-resistant Staphylococcus aureus by downregulation of PBP2a synthesis
- Combination of a bronchogenic cyst in the thoracic spinal canal with chronic myelocytic leukemia
- Bacterial lipoprotein plays an important role in the macrophage autophagy and apoptosis induced by Salmonella typhimurium and Staphylococcus aureus
- TCL1A+ B cells predict prognosis in triple-negative breast cancer through integrative analysis of single-cell and bulk transcriptomic data
- Ezrin promotes esophageal squamous cell carcinoma progression via the Hippo signaling pathway
- Ferroptosis: A potential target of macrophages in plaque vulnerability
- Predicting pediatric Crohn's disease based on six mRNA-constructed risk signature using comprehensive bioinformatic approaches
- Applications of genetic code expansion and photosensitive UAAs in studying membrane proteins
- HK2 contributes to the proliferation, migration, and invasion of diffuse large B-cell lymphoma cells by enhancing the ERK1/2 signaling pathway
- IL-17 in osteoarthritis: A narrative review
- Circadian cycle and neuroinflammation
- Probiotic management and inflammatory factors as a novel treatment in cirrhosis: A systematic review and meta-analysis
- Hemorrhagic meningioma with pulmonary metastasis: Case report and literature review
- SPOP regulates the expression profiles and alternative splicing events in human hepatocytes
- Knockdown of SETD5 inhibited glycolysis and tumor growth in gastric cancer cells by down-regulating Akt signaling pathway
- PTX3 promotes IVIG resistance-induced endothelial injury in Kawasaki disease by regulating the NF-κB pathway
- Pancreatic ectopic thyroid tissue: A case report and analysis of literature
- The prognostic impact of body mass index on female breast cancer patients in underdeveloped regions of northern China differs by menopause status and tumor molecular subtype
- Report on a case of liver-originating malignant melanoma of unknown primary
- Case report: Herbal treatment of neutropenic enterocolitis after chemotherapy for breast cancer
- The fibroblast growth factor–Klotho axis at molecular level
- Characterization of amiodarone action on currents in hERG-T618 gain-of-function mutations
- A case report of diagnosis and dynamic monitoring of Listeria monocytogenes meningitis with NGS
- Effect of autologous platelet-rich plasma on new bone formation and viability of a Marburg bone graft
- Small breast epithelial mucin as a useful prognostic marker for breast cancer patients
- Continuous non-adherent culture promotes transdifferentiation of human adipose-derived stem cells into retinal lineage
- Nrf3 alleviates oxidative stress and promotes the survival of colon cancer cells by activating AKT/BCL-2 signal pathway
- Favorable response to surufatinib in a patient with necrolytic migratory erythema: A case report
- Case report of atypical undernutrition of hypoproteinemia type
- Down-regulation of COL1A1 inhibits tumor-associated fibroblast activation and mediates matrix remodeling in the tumor microenvironment of breast cancer
- Sarcoma protein kinase inhibition alleviates liver fibrosis by promoting hepatic stellate cells ferroptosis
- Research progress of serum eosinophil in chronic obstructive pulmonary disease and asthma
- Clinicopathological characteristics of co-existing or mixed colorectal cancer and neuroendocrine tumor: Report of five cases
- Role of menopausal hormone therapy in the prevention of postmenopausal osteoporosis
- Precisional detection of lymph node metastasis using tFCM in colorectal cancer
- Advances in diagnosis and treatment of perimenopausal syndrome
- A study of forensic genetics: ITO index distribution and kinship judgment between two individuals
- Acute lupus pneumonitis resembling miliary tuberculosis: A case-based review
- Plasma levels of CD36 and glutathione as biomarkers for ruptured intracranial aneurysm
- Fractalkine modulates pulmonary angiogenesis and tube formation by modulating CX3CR1 and growth factors in PVECs
- Novel risk prediction models for deep vein thrombosis after thoracotomy and thoracoscopic lung cancer resections, involving coagulation and immune function
- Exploring the diagnostic markers of essential tremor: A study based on machine learning algorithms
- Evaluation of effects of small-incision approach treatment on proximal tibia fracture by deep learning algorithm-based magnetic resonance imaging
- An online diagnosis method for cancer lesions based on intelligent imaging analysis
- Medical imaging in rheumatoid arthritis: A review on deep learning approach
- Predictive analytics in smart healthcare for child mortality prediction using a machine learning approach
- Utility of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in predicting acute-on-chronic liver failure survival
- A biomedical decision support system for meta-analysis of bilateral upper-limb training in stroke patients with hemiplegia
- TNF-α and IL-8 levels are positively correlated with hypobaric hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats
- Stochastic gradient descent optimisation for convolutional neural network for medical image segmentation
- Comparison of the prognostic value of four different critical illness scores in patients with sepsis-induced coagulopathy
- Application and teaching of computer molecular simulation embedded technology and artificial intelligence in drug research and development
- Hepatobiliary surgery based on intelligent image segmentation technology
- Value of brain injury-related indicators based on neural network in the diagnosis of neonatal hypoxic-ischemic encephalopathy
- Analysis of early diagnosis methods for asymmetric dementia in brain MR images based on genetic medical technology
- Early diagnosis for the onset of peri-implantitis based on artificial neural network
- Clinical significance of the detection of serum IgG4 and IgG4/IgG ratio in patients with thyroid-associated ophthalmopathy
- Forecast of pain degree of lumbar disc herniation based on back propagation neural network
- SPA-UNet: A liver tumor segmentation network based on fused multi-scale features
- Systematic evaluation of clinical efficacy of CYP1B1 gene polymorphism in EGFR mutant non-small cell lung cancer observed by medical image
- Rehabilitation effect of intelligent rehabilitation training system on hemiplegic limb spasms after stroke
- A novel approach for minimising anti-aliasing effects in EEG data acquisition
- ErbB4 promotes M2 activation of macrophages in idiopathic pulmonary fibrosis
- Clinical role of CYP1B1 gene polymorphism in prediction of postoperative chemotherapy efficacy in NSCLC based on individualized health model
- Lung nodule segmentation via semi-residual multi-resolution neural networks
- Evaluation of brain nerve function in ICU patients with Delirium by deep learning algorithm-based resting state MRI
- A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis
- Markov model combined with MR diffusion tensor imaging for predicting the onset of Alzheimer’s disease
- Effectiveness of the treatment of depression associated with cancer and neuroimaging changes in depression-related brain regions in patients treated with the mediator-deuterium acupuncture method
- Molecular mechanism of colorectal cancer and screening of molecular markers based on bioinformatics analysis
- Monitoring and evaluation of anesthesia depth status data based on neuroscience
- Exploring the conformational dynamics and thermodynamics of EGFR S768I and G719X + S768I mutations in non-small cell lung cancer: An in silico approaches
- Optimised feature selection-driven convolutional neural network using gray level co-occurrence matrix for detection of cervical cancer
- Incidence of different pressure patterns of spinal cerebellar ataxia and analysis of imaging and genetic diagnosis
- Pathogenic bacteria and treatment resistance in older cardiovascular disease patients with lung infection and risk prediction model
- Adoption value of support vector machine algorithm-based computed tomography imaging in the diagnosis of secondary pulmonary fungal infections in patients with malignant hematological disorders
- From slides to insights: Harnessing deep learning for prognostic survival prediction in human colorectal cancer histology
- Ecology and Environmental Science
- Monitoring of hourly carbon dioxide concentration under different land use types in arid ecosystem
- Comparing the differences of prokaryotic microbial community between pit walls and bottom from Chinese liquor revealed by 16S rRNA gene sequencing
- Effects of cadmium stress on fruits germination and growth of two herbage species
- Bamboo charcoal affects soil properties and bacterial community in tea plantations
- Optimization of biogas potential using kinetic models, response surface methodology, and instrumental evidence for biodegradation of tannery fleshings during anaerobic digestion
- Understory vegetation diversity patterns of Platycladus orientalis and Pinus elliottii communities in Central and Southern China
- Studies on macrofungi diversity and discovery of new species of Abortiporus from Baotianman World Biosphere Reserve
- Food Science
- Effect of berrycactus fruit (Myrtillocactus geometrizans) on glutamate, glutamine, and GABA levels in the frontal cortex of rats fed with a high-fat diet
- Guesstimate of thymoquinone diversity in Nigella sativa L. genotypes and elite varieties collected from Indian states using HPTLC technique
- Analysis of bacterial community structure of Fuzhuan tea with different processing techniques
- Untargeted metabolomics reveals sour jujube kernel benefiting the nutritional value and flavor of Morchella esculenta
- Mycobiota in Slovak wine grapes: A case study from the small Carpathians wine region
- Elemental analysis of Fadogia ancylantha leaves used as a nutraceutical in Mashonaland West Province, Zimbabwe
- Microbiological transglutaminase: Biotechnological application in the food industry
- Influence of solvent-free extraction of fish oil from catfish (Clarias magur) heads using a Taguchi orthogonal array design: A qualitative and quantitative approach
- Chromatographic analysis of the chemical composition and anticancer activities of Curcuma longa extract cultivated in Palestine
- The potential for the use of leghemoglobin and plant ferritin as sources of iron
- Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM
- Bioengineering and Biotechnology
- Biocompatibility and osteointegration capability of β-TCP manufactured by stereolithography 3D printing: In vitro study
- Clinical characteristics and the prognosis of diabetic foot in Tibet: A single center, retrospective study
- Agriculture
- Biofertilizer and NPSB fertilizer application effects on nodulation and productivity of common bean (Phaseolus vulgaris L.) at Sodo Zuria, Southern Ethiopia
- On correlation between canopy vegetation and growth indexes of maize varieties with different nitrogen efficiencies
- Exopolysaccharides from Pseudomonas tolaasii inhibit the growth of Pleurotus ostreatus mycelia
- A transcriptomic evaluation of the mechanism of programmed cell death of the replaceable bud in Chinese chestnut
- Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis
- Effects of plant density on alfalfa (Medicago sativa L.) seed yield in western Heilongjiang areas
- Identification of rice leaf diseases and deficiency disorders using a novel DeepBatch technique
- Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture
- Animal Sciences
- Effect of ketogenic diet on exercise tolerance and transcriptome of gastrocnemius in mice
- Combined analysis of mRNA–miRNA from testis tissue in Tibetan sheep with different FecB genotypes
- Isolation, identification, and drug resistance of a partially isolated bacterium from the gill of Siniperca chuatsi
- Tracking behavioral changes of confined sows from the first mating to the third parity
- The sequencing of the key genes and end products in the TLR4 signaling pathway from the kidney of Rana dybowskii exposed to Aeromonas hydrophila
- Development of a new candidate vaccine against piglet diarrhea caused by Escherichia coli
- Plant Sciences
- Crown and diameter structure of pure Pinus massoniana Lamb. forest in Hunan province, China
- Genetic evaluation and germplasm identification analysis on ITS2, trnL-F, and psbA-trnH of alfalfa varieties germplasm resources
- Tissue culture and rapid propagation technology for Gentiana rhodantha
- Effects of cadmium on the synthesis of active ingredients in Salvia miltiorrhiza
- Cloning and expression analysis of VrNAC13 gene in mung bean
- Chlorate-induced molecular floral transition revealed by transcriptomes
- Effects of warming and drought on growth and development of soybean in Hailun region
- Effects of different light conditions on transient expression and biomass in Nicotiana benthamiana leaves
- Comparative analysis of the rhizosphere microbiome and medicinally active ingredients of Atractylodes lancea from different geographical origins
- Distinguish Dianthus species or varieties based on chloroplast genomes
- Comparative transcriptomes reveal molecular mechanisms of apple blossoms of different tolerance genotypes to chilling injury
- Study on fresh processing key technology and quality influence of Cut Ophiopogonis Radix based on multi-index evaluation
- An advanced approach for fig leaf disease detection and classification: Leveraging image processing and enhanced support vector machine methodology
- Erratum
- Erratum to “Protein Z modulates the metastasis of lung adenocarcinoma cells”
- Erratum to “BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells”
- Retraction
- Retraction to “Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats”
Articles in the same Issue
- Biomedical Sciences
- Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
- Immunosuppressive treatment for idiopathic membranous nephropathy: An updated network meta-analysis
- Identifying two pathogenic variants in a patient with pigmented paravenous retinochoroidal atrophy
- Effects of phytoestrogens combined with cold stress on sperm parameters and testicular proteomics in rats
- A case of pulmonary embolism with bad warfarin anticoagulant effects caused by E. coli infection
- Neutrophilia with subclinical Cushing’s disease: A case report and literature review
- Isoimperatorin alleviates lipopolysaccharide-induced periodontitis by downregulating ERK1/2 and NF-κB pathways
- Immunoregulation of synovial macrophages for the treatment of osteoarthritis
- Novel CPLANE1 c.8948dupT (p.P2984Tfs*7) variant in a child patient with Joubert syndrome
- Antiphospholipid antibodies and the risk of thrombosis in myeloproliferative neoplasms
- Immunological responses of septic rats to combination therapy with thymosin α1 and vitamin C
- High glucose and high lipid induced mitochondrial dysfunction in JEG-3 cells through oxidative stress
- Pharmacological inhibition of the ubiquitin-specific protease 8 effectively suppresses glioblastoma cell growth
- Levocarnitine regulates the growth of angiotensin II-induced myocardial fibrosis cells via TIMP-1
- Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study
- Single-cell transcription analysis reveals the tumor origin and heterogeneity of human bilateral renal clear cell carcinoma
- Identification of iron metabolism-related genes as diagnostic signatures in sepsis by blood transcriptomic analysis
- Long noncoding RNA ACART knockdown decreases 3T3-L1 preadipocyte proliferation and differentiation
- Surgery, adjuvant immunotherapy plus chemotherapy and radiotherapy for primary malignant melanoma of the parotid gland (PGMM): A case report
- Dosimetry comparison with helical tomotherapy, volumetric modulated arc therapy, and intensity-modulated radiotherapy for grade II gliomas: A single‑institution case series
- Soy isoflavone reduces LPS-induced acute lung injury via increasing aquaporin 1 and aquaporin 5 in rats
- Refractory hypokalemia with sexual dysplasia and infertility caused by 17α-hydroxylase deficiency and triple X syndrome: A case report
- Meta-analysis of cancer risk among end stage renal disease undergoing maintenance dialysis
- 6-Phosphogluconate dehydrogenase inhibition arrests growth and induces apoptosis in gastric cancer via AMPK activation and oxidative stress
- Experimental study on the optimization of ANM33 release in foam cells
- Primary retroperitoneal angiosarcoma: A case report
- Metabolomic analysis-identified 2-hydroxybutyric acid might be a key metabolite of severe preeclampsia
- Malignant pleural effusion diagnosis and therapy
- Effect of spaceflight on the phenotype and proteome of Escherichia coli
- Comparison of immunotherapy combined with stereotactic radiotherapy and targeted therapy for patients with brain metastases: A systemic review and meta-analysis
- Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation
- Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy
- The role of IL-31 and IL-34 in the diagnosis and treatment of chronic periodontitis
- Triple-negative mouse breast cancer initiating cells show high expression of beta1 integrin and increased malignant features
- mNGS facilitates the accurate diagnosis and antibiotic treatment of suspicious critical CNS infection in real practice: A retrospective study
- The apatinib and pemetrexed combination has antitumor and antiangiogenic effects against NSCLC
- Radiotherapy for primary thyroid adenoid cystic carcinoma
- Design and functional preliminary investigation of recombinant antigen EgG1Y162–EgG1Y162 against Echinococcus granulosus
- Effects of losartan in patients with NAFLD: A meta-analysis of randomized controlled trial
- Bibliometric analysis of METTL3: Current perspectives, highlights, and trending topics
- Performance comparison of three scaling algorithms in NMR-based metabolomics analysis
- PI3K/AKT/mTOR pathway and its related molecules participate in PROK1 silence-induced anti-tumor effects on pancreatic cancer
- The altered expression of cytoskeletal and synaptic remodeling proteins during epilepsy
- Effects of pegylated recombinant human granulocyte colony-stimulating factor on lymphocytes and white blood cells of patients with malignant tumor
- Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report
- NUDT21 relieves sevoflurane-induced neurological damage in rats by down-regulating LIMK2
- Association of interleukin-10 rs1800896, rs1800872, and interleukin-6 rs1800795 polymorphisms with squamous cell carcinoma risk: A meta-analysis
- Exosomal HBV-DNA for diagnosis and treatment monitoring of chronic hepatitis B
- Shear stress leads to the dysfunction of endothelial cells through the Cav-1-mediated KLF2/eNOS/ERK signaling pathway under physiological conditions
- Interaction between the PI3K/AKT pathway and mitochondrial autophagy in macrophages and the leukocyte count in rats with LPS-induced pulmonary infection
- Meta-analysis of the rs231775 locus polymorphism in the CTLA-4 gene and the susceptibility to Graves’ disease in children
- Cloning, subcellular localization and expression of phosphate transporter gene HvPT6 of hulless barley
- Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome
- Significant elevated CXCL14 and decreased IL-39 levels in patients with tuberculosis
- Whole-exome sequencing applications in prenatal diagnosis of fetal bowel dilatation
- Gemella morbillorum infective endocarditis: A case report and literature review
- An unusual ectopic thymoma clonal evolution analysis: A case report
- Severe cumulative skin toxicity during toripalimab combined with vemurafenib following toripalimab alone
- Detection of V. vulnificus septic shock with ARDS using mNGS
- Novel rare genetic variants of familial and sporadic pulmonary atresia identified by whole-exome sequencing
- The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology
- Novel compound heterozygous mutations in TELO2 in an infant with You-Hoover-Fong syndrome: A case report and literature review
- ctDNA as a prognostic biomarker in resectable CLM: Systematic review and meta-analysis
- Diagnosis of primary amoebic meningoencephalitis by metagenomic next-generation sequencing: A case report
- Phylogenetic analysis of promoter regions of human Dolichol kinase (DOLK) and orthologous genes using bioinformatics tools
- Collagen changes in rabbit conjunctiva after conjunctival crosslinking
- Effects of NM23 transfection of human gastric carcinoma cells in mice
- Oral nifedipine and phytosterol, intravenous nicardipine, and oral nifedipine only: Three-arm, retrospective, cohort study for management of severe preeclampsia
- Case report of hepatic retiform hemangioendothelioma: A rare tumor treated with ultrasound-guided microwave ablation
- Curcumin induces apoptosis in human hepatocellular carcinoma cells by decreasing the expression of STAT3/VEGF/HIF-1α signaling
- Rare presentation of double-clonal Waldenström macroglobulinemia with pulmonary embolism: A case report
- Giant duplication of the transverse colon in an adult: A case report and literature review
- Ectopic thyroid tissue in the breast: A case report
- SDR16C5 promotes proliferation and migration and inhibits apoptosis in pancreatic cancer
- Vaginal metastasis from breast cancer: A case report
- Screening of the best time window for MSC transplantation to treat acute myocardial infarction with SDF-1α antibody-loaded targeted ultrasonic microbubbles: An in vivo study in miniswine
- Inhibition of TAZ impairs the migration ability of melanoma cells
- Molecular complexity analysis of the diagnosis of Gitelman syndrome in China
- Effects of maternal calcium and protein intake on the development and bone metabolism of offspring mice
- Identification of winter wheat pests and diseases based on improved convolutional neural network
- Ultra-multiplex PCR technique to guide treatment of Aspergillus-infected aortic valve prostheses
- Virtual high-throughput screening: Potential inhibitors targeting aminopeptidase N (CD13) and PIKfyve for SARS-CoV-2
- Immune checkpoint inhibitors in cancer patients with COVID-19
- Utility of methylene blue mixed with autologous blood in preoperative localization of pulmonary nodules and masses
- Integrated analysis of the microbiome and transcriptome in stomach adenocarcinoma
- Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy
- DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway
- Lung abscess by Fusobacterium nucleatum and Streptococcus spp. co-infection by mNGS: A case series
- Genetic alterations of KRAS and TP53 in intrahepatic cholangiocarcinoma associated with poor prognosis
- Granulomatous polyangiitis involving the fourth ventricle: Report of a rare case and a literature review
- Studying infant mortality: A demographic analysis based on data mining models
- Metaplastic breast carcinoma with osseous differentiation: A report of a rare case and literature review
- Protein Z modulates the metastasis of lung adenocarcinoma cells
- Inhibition of pyroptosis and apoptosis by capsaicin protects against LPS-induced acute kidney injury through TRPV1/UCP2 axis in vitro
- TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats
- Primary mediastinum Ewing’s sarcoma with pleural effusion: A case report and literature review
- Association of ADRB2 gene polymorphisms and intestinal microbiota in Chinese Han adolescents
- Tanshinone IIA alleviates chondrocyte apoptosis and extracellular matrix degeneration by inhibiting ferroptosis
- Study on the cytokines related to SARS-Cov-2 in testicular cells and the interaction network between cells based on scRNA-seq data
- Effect of periostin on bone metabolic and autophagy factors during tooth eruption in mice
- HP1 induces ferroptosis of renal tubular epithelial cells through NRF2 pathway in diabetic nephropathy
- Intravaginal estrogen management in postmenopausal patients with vaginal squamous intraepithelial lesions along with CO2 laser ablation: A retrospective study
- Hepatocellular carcinoma cell differentiation trajectory predicts immunotherapy, potential therapeutic drugs, and prognosis of patients
- Effects of physical exercise on biomarkers of oxidative stress in healthy subjects: A meta-analysis of randomized controlled trials
- Identification of lysosome-related genes in connection with prognosis and immune cell infiltration for drug candidates in head and neck cancer
- Development of an instrument-free and low-cost ELISA dot-blot test to detect antibodies against SARS-CoV-2
- Research progress on gas signal molecular therapy for Parkinson’s disease
- Adiponectin inhibits TGF-β1-induced skin fibroblast proliferation and phenotype transformation via the p38 MAPK signaling pathway
- The G protein-coupled receptor-related gene signatures for predicting prognosis and immunotherapy response in bladder urothelial carcinoma
- α-Fetoprotein contributes to the malignant biological properties of AFP-producing gastric cancer
- CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa
- Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis
- Significance of sTREM-1 and sST2 combined diagnosis for sepsis detection and prognosis prediction
- Diagnostic value of serum neuroactive substances in the acute exacerbation of chronic obstructive pulmonary disease complicated with depression
- Research progress of AMP-activated protein kinase and cardiac aging
- TRIM29 knockdown prevented the colon cancer progression through decreasing the ubiquitination levels of KRT5
- Cross-talk between gut microbiota and liver steatosis: Complications and therapeutic target
- Metastasis from small cell lung cancer to ovary: A case report
- The early diagnosis and pathogenic mechanisms of sepsis-related acute kidney injury
- The effect of NK cell therapy on sepsis secondary to lung cancer: A case report
- Erianin alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation
- Loss of ACOX1 in clear cell renal cell carcinoma and its correlation with clinical features
- Signalling pathways in the osteogenic differentiation of periodontal ligament stem cells
- Crosstalk between lactic acid and immune regulation and its value in the diagnosis and treatment of liver failure
- Clinicopathological features and differential diagnosis of gastric pleomorphic giant cell carcinoma
- Traumatic brain injury and rTMS-ERPs: Case report and literature review
- Extracellular fibrin promotes non-small cell lung cancer progression through integrin β1/PTEN/AKT signaling
- Knockdown of DLK4 inhibits non-small cell lung cancer tumor growth by downregulating CKS2
- The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles
- Inflammation-related signaling pathways in tendinopathy
- CD4+ T cell count in HIV/TB co-infection and co-occurrence with HL: Case report and literature review
- Clinical analysis of severe Chlamydia psittaci pneumonia: Case series study
- Bioinformatics analysis to identify potential biomarkers for the pulmonary artery hypertension associated with the basement membrane
- Influence of MTHFR polymorphism, alone or in combination with smoking and alcohol consumption, on cancer susceptibility
- Catharanthus roseus (L.) G. Don counteracts the ampicillin resistance in multiple antibiotic-resistant Staphylococcus aureus by downregulation of PBP2a synthesis
- Combination of a bronchogenic cyst in the thoracic spinal canal with chronic myelocytic leukemia
- Bacterial lipoprotein plays an important role in the macrophage autophagy and apoptosis induced by Salmonella typhimurium and Staphylococcus aureus
- TCL1A+ B cells predict prognosis in triple-negative breast cancer through integrative analysis of single-cell and bulk transcriptomic data
- Ezrin promotes esophageal squamous cell carcinoma progression via the Hippo signaling pathway
- Ferroptosis: A potential target of macrophages in plaque vulnerability
- Predicting pediatric Crohn's disease based on six mRNA-constructed risk signature using comprehensive bioinformatic approaches
- Applications of genetic code expansion and photosensitive UAAs in studying membrane proteins
- HK2 contributes to the proliferation, migration, and invasion of diffuse large B-cell lymphoma cells by enhancing the ERK1/2 signaling pathway
- IL-17 in osteoarthritis: A narrative review
- Circadian cycle and neuroinflammation
- Probiotic management and inflammatory factors as a novel treatment in cirrhosis: A systematic review and meta-analysis
- Hemorrhagic meningioma with pulmonary metastasis: Case report and literature review
- SPOP regulates the expression profiles and alternative splicing events in human hepatocytes
- Knockdown of SETD5 inhibited glycolysis and tumor growth in gastric cancer cells by down-regulating Akt signaling pathway
- PTX3 promotes IVIG resistance-induced endothelial injury in Kawasaki disease by regulating the NF-κB pathway
- Pancreatic ectopic thyroid tissue: A case report and analysis of literature
- The prognostic impact of body mass index on female breast cancer patients in underdeveloped regions of northern China differs by menopause status and tumor molecular subtype
- Report on a case of liver-originating malignant melanoma of unknown primary
- Case report: Herbal treatment of neutropenic enterocolitis after chemotherapy for breast cancer
- The fibroblast growth factor–Klotho axis at molecular level
- Characterization of amiodarone action on currents in hERG-T618 gain-of-function mutations
- A case report of diagnosis and dynamic monitoring of Listeria monocytogenes meningitis with NGS
- Effect of autologous platelet-rich plasma on new bone formation and viability of a Marburg bone graft
- Small breast epithelial mucin as a useful prognostic marker for breast cancer patients
- Continuous non-adherent culture promotes transdifferentiation of human adipose-derived stem cells into retinal lineage
- Nrf3 alleviates oxidative stress and promotes the survival of colon cancer cells by activating AKT/BCL-2 signal pathway
- Favorable response to surufatinib in a patient with necrolytic migratory erythema: A case report
- Case report of atypical undernutrition of hypoproteinemia type
- Down-regulation of COL1A1 inhibits tumor-associated fibroblast activation and mediates matrix remodeling in the tumor microenvironment of breast cancer
- Sarcoma protein kinase inhibition alleviates liver fibrosis by promoting hepatic stellate cells ferroptosis
- Research progress of serum eosinophil in chronic obstructive pulmonary disease and asthma
- Clinicopathological characteristics of co-existing or mixed colorectal cancer and neuroendocrine tumor: Report of five cases
- Role of menopausal hormone therapy in the prevention of postmenopausal osteoporosis
- Precisional detection of lymph node metastasis using tFCM in colorectal cancer
- Advances in diagnosis and treatment of perimenopausal syndrome
- A study of forensic genetics: ITO index distribution and kinship judgment between two individuals
- Acute lupus pneumonitis resembling miliary tuberculosis: A case-based review
- Plasma levels of CD36 and glutathione as biomarkers for ruptured intracranial aneurysm
- Fractalkine modulates pulmonary angiogenesis and tube formation by modulating CX3CR1 and growth factors in PVECs
- Novel risk prediction models for deep vein thrombosis after thoracotomy and thoracoscopic lung cancer resections, involving coagulation and immune function
- Exploring the diagnostic markers of essential tremor: A study based on machine learning algorithms
- Evaluation of effects of small-incision approach treatment on proximal tibia fracture by deep learning algorithm-based magnetic resonance imaging
- An online diagnosis method for cancer lesions based on intelligent imaging analysis
- Medical imaging in rheumatoid arthritis: A review on deep learning approach
- Predictive analytics in smart healthcare for child mortality prediction using a machine learning approach
- Utility of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in predicting acute-on-chronic liver failure survival
- A biomedical decision support system for meta-analysis of bilateral upper-limb training in stroke patients with hemiplegia
- TNF-α and IL-8 levels are positively correlated with hypobaric hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats
- Stochastic gradient descent optimisation for convolutional neural network for medical image segmentation
- Comparison of the prognostic value of four different critical illness scores in patients with sepsis-induced coagulopathy
- Application and teaching of computer molecular simulation embedded technology and artificial intelligence in drug research and development
- Hepatobiliary surgery based on intelligent image segmentation technology
- Value of brain injury-related indicators based on neural network in the diagnosis of neonatal hypoxic-ischemic encephalopathy
- Analysis of early diagnosis methods for asymmetric dementia in brain MR images based on genetic medical technology
- Early diagnosis for the onset of peri-implantitis based on artificial neural network
- Clinical significance of the detection of serum IgG4 and IgG4/IgG ratio in patients with thyroid-associated ophthalmopathy
- Forecast of pain degree of lumbar disc herniation based on back propagation neural network
- SPA-UNet: A liver tumor segmentation network based on fused multi-scale features
- Systematic evaluation of clinical efficacy of CYP1B1 gene polymorphism in EGFR mutant non-small cell lung cancer observed by medical image
- Rehabilitation effect of intelligent rehabilitation training system on hemiplegic limb spasms after stroke
- A novel approach for minimising anti-aliasing effects in EEG data acquisition
- ErbB4 promotes M2 activation of macrophages in idiopathic pulmonary fibrosis
- Clinical role of CYP1B1 gene polymorphism in prediction of postoperative chemotherapy efficacy in NSCLC based on individualized health model
- Lung nodule segmentation via semi-residual multi-resolution neural networks
- Evaluation of brain nerve function in ICU patients with Delirium by deep learning algorithm-based resting state MRI
- A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis
- Markov model combined with MR diffusion tensor imaging for predicting the onset of Alzheimer’s disease
- Effectiveness of the treatment of depression associated with cancer and neuroimaging changes in depression-related brain regions in patients treated with the mediator-deuterium acupuncture method
- Molecular mechanism of colorectal cancer and screening of molecular markers based on bioinformatics analysis
- Monitoring and evaluation of anesthesia depth status data based on neuroscience
- Exploring the conformational dynamics and thermodynamics of EGFR S768I and G719X + S768I mutations in non-small cell lung cancer: An in silico approaches
- Optimised feature selection-driven convolutional neural network using gray level co-occurrence matrix for detection of cervical cancer
- Incidence of different pressure patterns of spinal cerebellar ataxia and analysis of imaging and genetic diagnosis
- Pathogenic bacteria and treatment resistance in older cardiovascular disease patients with lung infection and risk prediction model
- Adoption value of support vector machine algorithm-based computed tomography imaging in the diagnosis of secondary pulmonary fungal infections in patients with malignant hematological disorders
- From slides to insights: Harnessing deep learning for prognostic survival prediction in human colorectal cancer histology
- Ecology and Environmental Science
- Monitoring of hourly carbon dioxide concentration under different land use types in arid ecosystem
- Comparing the differences of prokaryotic microbial community between pit walls and bottom from Chinese liquor revealed by 16S rRNA gene sequencing
- Effects of cadmium stress on fruits germination and growth of two herbage species
- Bamboo charcoal affects soil properties and bacterial community in tea plantations
- Optimization of biogas potential using kinetic models, response surface methodology, and instrumental evidence for biodegradation of tannery fleshings during anaerobic digestion
- Understory vegetation diversity patterns of Platycladus orientalis and Pinus elliottii communities in Central and Southern China
- Studies on macrofungi diversity and discovery of new species of Abortiporus from Baotianman World Biosphere Reserve
- Food Science
- Effect of berrycactus fruit (Myrtillocactus geometrizans) on glutamate, glutamine, and GABA levels in the frontal cortex of rats fed with a high-fat diet
- Guesstimate of thymoquinone diversity in Nigella sativa L. genotypes and elite varieties collected from Indian states using HPTLC technique
- Analysis of bacterial community structure of Fuzhuan tea with different processing techniques
- Untargeted metabolomics reveals sour jujube kernel benefiting the nutritional value and flavor of Morchella esculenta
- Mycobiota in Slovak wine grapes: A case study from the small Carpathians wine region
- Elemental analysis of Fadogia ancylantha leaves used as a nutraceutical in Mashonaland West Province, Zimbabwe
- Microbiological transglutaminase: Biotechnological application in the food industry
- Influence of solvent-free extraction of fish oil from catfish (Clarias magur) heads using a Taguchi orthogonal array design: A qualitative and quantitative approach
- Chromatographic analysis of the chemical composition and anticancer activities of Curcuma longa extract cultivated in Palestine
- The potential for the use of leghemoglobin and plant ferritin as sources of iron
- Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM
- Bioengineering and Biotechnology
- Biocompatibility and osteointegration capability of β-TCP manufactured by stereolithography 3D printing: In vitro study
- Clinical characteristics and the prognosis of diabetic foot in Tibet: A single center, retrospective study
- Agriculture
- Biofertilizer and NPSB fertilizer application effects on nodulation and productivity of common bean (Phaseolus vulgaris L.) at Sodo Zuria, Southern Ethiopia
- On correlation between canopy vegetation and growth indexes of maize varieties with different nitrogen efficiencies
- Exopolysaccharides from Pseudomonas tolaasii inhibit the growth of Pleurotus ostreatus mycelia
- A transcriptomic evaluation of the mechanism of programmed cell death of the replaceable bud in Chinese chestnut
- Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis
- Effects of plant density on alfalfa (Medicago sativa L.) seed yield in western Heilongjiang areas
- Identification of rice leaf diseases and deficiency disorders using a novel DeepBatch technique
- Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture
- Animal Sciences
- Effect of ketogenic diet on exercise tolerance and transcriptome of gastrocnemius in mice
- Combined analysis of mRNA–miRNA from testis tissue in Tibetan sheep with different FecB genotypes
- Isolation, identification, and drug resistance of a partially isolated bacterium from the gill of Siniperca chuatsi
- Tracking behavioral changes of confined sows from the first mating to the third parity
- The sequencing of the key genes and end products in the TLR4 signaling pathway from the kidney of Rana dybowskii exposed to Aeromonas hydrophila
- Development of a new candidate vaccine against piglet diarrhea caused by Escherichia coli
- Plant Sciences
- Crown and diameter structure of pure Pinus massoniana Lamb. forest in Hunan province, China
- Genetic evaluation and germplasm identification analysis on ITS2, trnL-F, and psbA-trnH of alfalfa varieties germplasm resources
- Tissue culture and rapid propagation technology for Gentiana rhodantha
- Effects of cadmium on the synthesis of active ingredients in Salvia miltiorrhiza
- Cloning and expression analysis of VrNAC13 gene in mung bean
- Chlorate-induced molecular floral transition revealed by transcriptomes
- Effects of warming and drought on growth and development of soybean in Hailun region
- Effects of different light conditions on transient expression and biomass in Nicotiana benthamiana leaves
- Comparative analysis of the rhizosphere microbiome and medicinally active ingredients of Atractylodes lancea from different geographical origins
- Distinguish Dianthus species or varieties based on chloroplast genomes
- Comparative transcriptomes reveal molecular mechanisms of apple blossoms of different tolerance genotypes to chilling injury
- Study on fresh processing key technology and quality influence of Cut Ophiopogonis Radix based on multi-index evaluation
- An advanced approach for fig leaf disease detection and classification: Leveraging image processing and enhanced support vector machine methodology
- Erratum
- Erratum to “Protein Z modulates the metastasis of lung adenocarcinoma cells”
- Erratum to “BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells”
- Retraction
- Retraction to “Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats”

