Abstract
The interaction between intestinal microecological dysregulation, altered inflammatory factors, and cirrhosis is unclear. The aim of this systematic review and meta-analysis was to synthesize the results of previous studies to assess the efficacy of probiotics in the treatment of cirrhosis and their effect on inflammatory factors, as well as to explore the relationship between gut microecological dysregulation and liver disease to gain a deeper understanding of this interaction. Up to December 2022, eligible studies were identified by searching the following databases: National Knowledge Infrastructure (CNKI), Wanfang Data, Web of Science, PubMed, Embase, Medline, and the Cochrane Library. Statistical analysis was performed using software RevMan Version 5.4. A total of 33 eligible randomized controlled trials were included in the study, and data on probiotic strains, duration of intervention, measures in the control group, and outcomes were extracted and evaluated. Compared to the control group, the experimental group had significant improvements in overall efficacy. The results of the meta-analysis revealed that probiotic use significantly decreased biochemical parameters for liver function, including aspartate transaminase, alanine aminotransferase, and total bilirubin. Similar result was obtained in interleukin-6, tumor necrosis factor-α, and endotoxin. However, probiotic intervention did not significantly affect interleukin-2 and interleukin-10. The current meta-analysis illustrates that probiotic supplementation reduces inflammatory markers and biochemical parameters for liver function in patients with cirrhosis, suggesting that probiotic management may be a novel treatment for cirrhosis. Furthermore, the interaction of the gut microbiota, associated metabolites, and inflammation factors with cirrhosis may provide a promising therapeutic target for the pharmacological and clinical treatment of cirrhosis.
1 Introduction
Liver cirrhosis is widely prevalent and is associated with high morbidity and mortality. With roughly one million annual deaths attributable to the disease [1], it is the 11th most common cause of death worldwide [2]. Cirrhosis is the seventh leading cause of disability-associated life years in the 50–74 age group, the 12th top cause in people aged 25–49 years, and the 15th highest cause in all ages [3]. The most common etiologies of cirrhosis globally are non-alcoholic fatty liver disease, alcoholic liver disease, and chronic hepatitis B and chronic hepatitis C. Regardless of the cause, the complications of cirrhosis are similar, including hepatic encephalopathy (HE) [4]. Cirrhosis has put a strain on global public health; hence, efficient and safe treatment options are urgently needed.
The World Health Organization updated the definition of a probiotic: “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [5]. Probiotics are part of the human microbial community, most of which are beneficial bacteria naturally present in the body. Different strains can be isolated from different parts of the body, including the gastrointestinal tract, mouth, skin, and uterus. Other bacterial genera such as Streptococcus, Enterococcus , Yeast , and Bacillus can also have probiotic properties [6]. While most parts of the body are colonized by microorganisms, the highest number of microorganisms are found in the gut. The most studied species include Lactobacillus and Bifidobacterium [7]. Lactobacillus and Bifidobacterium produce lactic acid, acetic acid, and propionic acid, which lower intestinal pH and inhibit the growth of various pathogenic bacteria, thereby reestablishing the balance of intestinal flora [8,9]. Probiotics have been well investigated in relation to numerous gastrointestinal disorders, leading to a growing consensus that probiotics, the gut microbiota, and both health and disease in the gut are strongly correlated [10,11].
Probiotics are one of the most commonly used food supplements in the health-care industry worldwide [12]. Since approximately 70% of the immune system is located in the intestine [13], it can be further concluded that probiotics typically provide two general benefits: supporting a healthy digestive tract and a healthy immune system. This conclusion was based on a great deal of reliable research, including clinical trials, meta-analyses as well as reviews, suggesting that probiotics can be expected to have effects on diverse disease conditions such as gastrointestinal disease [14,15,16,17], autoimmune disease [18,19,20], diabetes [21,22,23,24], hypertension and hyperlipidemia [25,26], as well as several kinds of liver disease [27,28,29,30]. Probiotics induce their effects primarily due to their role in immune system regulation and anti-inflammatory response [31]. Thus, probiotic administration seems to have a great potential in terms of health, and further research should be conducted. More and more attention has been paid to the health benefits of probiotics, which we believe to be potentially crucial interventions for improving health and well-being.
Probiotic research has rapidly expanded, inspiring scientists to create a wide range of functionally formulated products with validated health advantages. Probiotics, prebiotics, vitamins, and minerals are among the functional food ingredients that can be found in a variety of meals and beverages, including fermented milks, yogurts, and sports drinks [32,33,34]. Customers can benefit nutritionally from probiotics and fermented food, which also strengthen the immune system to fend off infections [35,36]. The demand for probiotic food is expanding quickly on a global scale. Today, 60 to 70 percent of the market for functional food is made up of probiotic products [5,37]. The effectiveness of probiotic is species-, dose-, and disease-specific, and with the selection of appropriate strains, products, and dosages, probiotics may be beneficial in achieving positive effects in different disease areas [38,39]. Probiotics are useful in treating irritable bowel syndrome and ulcerative colitis, according to solid research [40,41]. Importantly, there is strong information about the advantages of probiotics in the prevention and treatment of diarrhea brought on by antibiotic use and Clostridium difficile [42]. Notably, two probiotic medications, Lactasin and Pepcid (Bifidobacterium triplex capsules), have been recommended as typical therapeutic drugs for people with cirrhosis in China, which are suitable for patients who experience abdominal pain, bloating, and diarrhea.
The term “gut–liver axis” was first proposed in 1998 by Marshall [43]. The gut–liver axis refers to the bidirectional relationship between the gut and its microbiota, allowing for the direct transport of intestinal microbes or associated metabolites to the liver via the biliary tract, portal vein, and systemic circulation, where they affect numerous liver functions, as well as a liver-to-gut feedback pathway in which it controls metabolic function and influences microbiota homeostasis and intestinal barrier integrity [44]. Imbalance of the intestinal microbiota will lead to endotoxemia and inflammation, and in the portal circulation, these effects may directly trigger or exacerbate pre-existing liver damage through harmful metabolic consequences, leading step-by-step to hepatic steatosis, chronic hepatitis, fibrosis, and cirrhosis, and the progression of cirrhosis to hepatocellular carcinoma [45]. At present, it is generally believed that inflammation is also the major factor leading to the occurrence and development of liver injury. A great deal of studies on the gut–liver axis suggest that the gut microbiota and associated metabolites may initiate a cytokine cascade that contributes to the maintenance of a poor immune response [45,46]. The adverse inflammatory responses in the liver will release a substantial amount of various serum inflammatory markers, including tumor necrosis factor (TNF), interleukins (ILs), and other general immunity markers. These inflammatory factors may be potential targets for the clinical treatment and prognosis of cirrhosis.
Guidelines advocate lactulose to lower hospitalization and fatality rates in HE in addition to the most widely used microecological treatments in clinical practice today [47,48]. Lactulose can induce the release of immunoglobulin A (IgA) from the intestinal mucosal epithelial zone as a first-line treatment for HE. IgA promotes the growth of Bifidobacterium and Lactobacillus, reduces inflammatory factors, and enhances liver function. IgA can effectively resist microbial invasion by bacteria, viruses, fungi, and other microorganisms, which helps to improve biological resistance to diseases [49]. Adverse reactions such as diarrhea, nausea, and vomiting may occur after taking lactulose oral solution, whereas probiotics have fewer adverse reactions than lactulose because they are naturally present in the body itself.
For the treatment of liver diseases, traditional Chinese medicine (TCM) has a strong theoretical foundation and a wealth of practical experience. TCM has two functions in immunological regulation: immune activation and immune suppression. These functions include the stimulation of immune cells, immune organs, and cytokine synthesis as well as the inhibition of inflammation [50]. TCM has been linked to intestinal flora, cirrhosis, and liver disease in long-term theoretical and practical investigations. This association has a special therapeutic function in the development of CLD [51,52]. Dahuang, Huangqi, and Muxiang are a few specific TCM formulae that have been shown to have anti-hepatic fibrosis benefits in addition to improving endotoxemia, lowering serum inflammatory cytokine levels, and correcting intestinal microecology in cirrhotic patients [53]. Consequently, TCM is a promising therapeutic agent for liver illness, but because of its numerous medicinal components and broad range of targets, treatment is more ambiguous and challenging [53]. The modification of intestinal homeostasis, anticirrhotic activity, and efficacy against inflammatory factors have all been demonstrated for probiotics, lactulose, and herbs. Probiotics have the ability to directly colonize the digestive tract, whereas lactulose and certain specific TCM indirectly encourage the multiplication of beneficial bacteria through medicinal components, regulating the human body.
Several studies have reported the effects of different probiotic strains on host immune factors: Toll-like receptor 2 mediated by Lactobacillus lactis stimulates TNF-α secretion [54], IL-10, and TNF-α secretion stimulated by Bifidobacterium lognum [55]; sortase-dependent pili of Bifidobacterium bifidum evoke the TNF-α response [56]; cell-surface polysaccharides in Lactobacillus longum modulate pro-inflammatory cytokine and T helper cell 17 (TH17) responses [57]; and immunostimulatory cell surface appendages (known as SpaCBA) in Lactobacillus rhamnosus mediate the regulation of TNF-α, IL-6, IL-10, and IL-12 [58]. Various cellular components of gut barrier (GB) (e.g., TNF, interleukins, AMP, defensins, lysozyme) have specific roles in regulating intestinal homeostasis [59,60,61,62]. Ecological imbalance and GB dysfunction are directly related to the development, progression, and appearance of clinical events linked to hepatic and portal hypertension [63]. As a result of their direct interactions with immune cells, probiotics play a significant part in preserving the body’s immunological homeostasis [64]. Probiotics can enhance the body’s immunity by increasing natural killer cell activity and stimulating phagocytosis, inhibiting pro-inflammatory cytokines, and increasing immunoglobulin concentrations, and they have a positive effect on the expression of immune-related genes, inflammatory pathway activity, and the levels of immune markers, including intestinal epithelial cell nuclear factor kappa B, mitogen-activated protein kinase, CRP, IL-6, IL-8, TNF-α, IL-1β, and IFN-γ [65,66]. Clinical outcomes and survival in cirrhotic patients have been demonstrated to be enhanced by microbial manipulation using pre-/probiotics and fecal microbiota transplantation (FMT) [63].
The immunomodulatory activity of different probiotic strains has been well documented in in vivo, in vitro, and animal studies, and specific metabolites such as polyunsaturated fatty acids, polyphenols, flavonoids, and vitamins in probiotic bacteria help to stimulate the growth and survival of beneficial gut microbes [31,67,68]. Specific molecules produced by probiotics have also been shown to be active in their immunomodulatory and anti-inflammatory pleiotropic effects. In addition to inducing the generation of anti-inflammatory cytokines, several postbiotic fractions (supernatants, cell wall fragments) recovered from Bacillus coagulans cultures also encouraged adjuvant T(Th)2-dependent immune responses [69]. Additionally, multiple in vitro studies have demonstrated that supernatants from Bifidobacterium short-term cultures promote DC survival and maturation, which in turn boosts IL-10 secretion and reduces TNF-α secretion [70].
However, there are few meta-analyses on the effect of probiotic interventions on cirrhotic patients in China and abroad, and most of them focus only on the changes in liver function. To the best of our knowledge, no meta-analysis has simultaneously evaluated the effects of probiotic interventions on liver function indicators and a full range of cytokines in patients with cirrhosis and the interaction between changes in gut microbiota and inflammation markers in cirrhosis remains elusive. Therefore, we performed this systematic review and meta-analysis to provide a robust portfolio of evidence to identify and update the association of probiotics with cirrhosis and to explore the potential mechanistic actions and biological effects. We also discuss the results of the interaction of the intestinal microbiota, its related metabolites, and inflammatory factors with liver cirrhosis.
2 Methods
2.1 Protocol registration
The protocol for this systematic review and meta-analysis was registered in advance with PROSPERO (CRD42023386931). The process of systematic evaluation and meta-analysis was recorded in the PRISMA 2020 checklist (Table S1).
2.2 Search strategy
A total of 561 potentially eligible references were identified through electronic and manual searches. Relevant articles published from 2008 to 31 October 2022 with no restriction on English language were searched in the following electronic databases: CNKI, Wanfang Data, Web of Science, PubMed, Embase, Medline, and Cochrane Library. We used the following search strategy: ((((((Liver Cirrhosis[MeSH Terms]) OR (Hepatic Cirrhosis[Title/Abstract])) OR (Cirrhosis, Hepatic[Title/Abstract])) OR (Cirrhosis, Liver[Title/Abstract])) OR (Fibrosis, Liver[Title/Abstract])) OR (Liver Fibrosis[Title/Abstract])) AND ((((((Probiotics[MeSH Terms]) OR (Probiotic[Title/Abstract])) OR (Synbiotics[MeSH Terms])) OR (Synbiotic[Title/Abstract])) OR (Prebiotics[MeSH Terms])) OR (Prebiotic[Title/Abstract])). Moreover, bibliographies of all relevant prior reviews identified by the search strategy were scanned for a comprehensive literature search. Two researchers (Lei and Xia) screened the search outcomes to identify relevant studies for this systematic review and meta-analysis.
2.3 Inclusion and exclusion criteria
The inclusion criteria for selecting eligible studies were as follows: (1) studies provided a measure of inflammatory factors and/or liver function index, (2) studies involved patients with a definite diagnosis of cirrhosis, and (3) study had to be a randomized controlled trial (RCT) comparing the use of probiotics with a placebo or no intervention.
The exclusion criteria were as follows: (1) studies were literature reviews, animal experiments, no control trials, non-RCTs, low-quality literature, and case reports; and (2) patients had the antibiotic before their measurements.
2.4 Data extraction
Data extraction from eligible literature was performed by two investigators (Lei and Xia) following the guidelines laid out in the QUOROM statement [71]. All data were integrated into a standard form and cross-checked independently by two reviewers; in the event of any disagreement, the disputes shall be submitted to a third reviewer for assistance in arbitration. Data related to the following variables were extracted from each targeted study: (1) the last name of first author and year of publication; (2) sample size and duration of study; (3) participants’ baseline information such as gender and age; (4) composition and dose of supplements for the experimental group – the experimental group is based on the control group with the addition of microecological preparations (e.g., Bifidobacterium triplex capsules, Bifidobacterium quadruplex tablets); and (5) treatment of the control group – the control group is usually treated with conventional treatment of cirrhosis (e.g., hepatic protection, diuretic, anti-infective treatment, timely replenishment of albumin and plasma, and symptomatic treatment of patients).
2.5 Statistical analysis
Statistical analysis of data was performed using software RevMan Version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark). For dichotomous variables, the rate difference (RD) and its 95% confidence interval (95% CI) were chosen as effect sizes; for continuous variables, the weighted mean difference was used if the measurement units were consistent, and the standardized mean difference and its 95% CI were used as effect sizes if the measurement units were inconsistent. The chi-squared test was used to assess the presence of statistical heterogeneity, and the I 2 test was used to evaluate the magnitude of heterogeneity. If there was no statistical heterogeneity among the results of the studies (P ≥ 0.05 and I 2 ≤ 50%), a fixed-effects model was used for meta-analysis; if the heterogeneity was large (P < 0.05 and I 2 > 50%), a random-effects model was used and the possible causes were analyzed, and meta-analysis was considered with subgroup analysis and sensitivity analysis. The methodological quality of the included studies was evaluated using the risk of bias assessment tool for RCTs recommended by the Cochrane Handbook for Systematic Reviews of Interventions 6.3, and publication bias was analyzed using a funnel plot (Figures S1–S9). A P-value of <0.05 was considered a statistically significant difference.
2.6 Risk of bias (quality) assessment
Jadad score was used to assess bias of included studies (Figures 1 and 2), based on the following parameters: random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other bias. Two researchers independently evaluated each article that fulfilled the eligibility criteria and resolved disagreements by consensus or by referring to a third investigator.
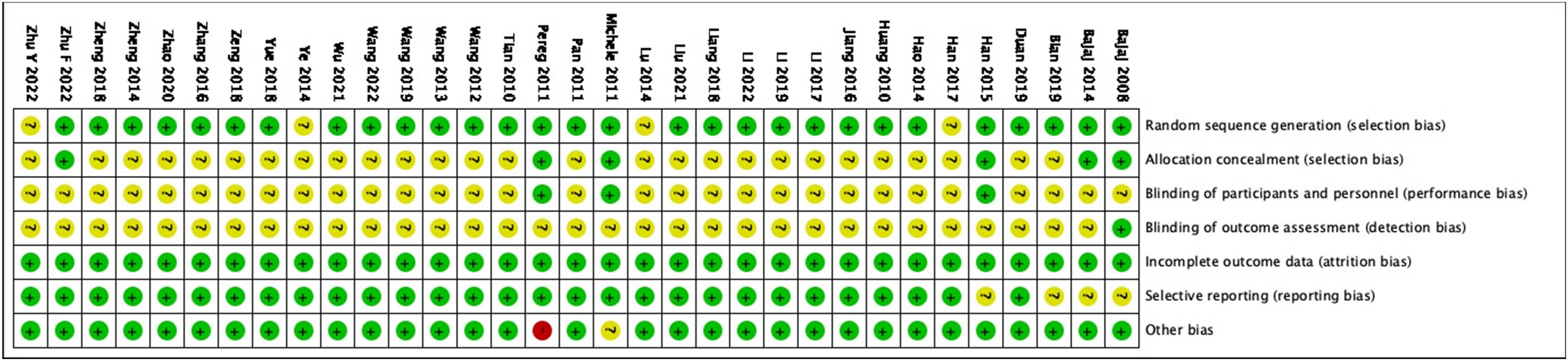
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
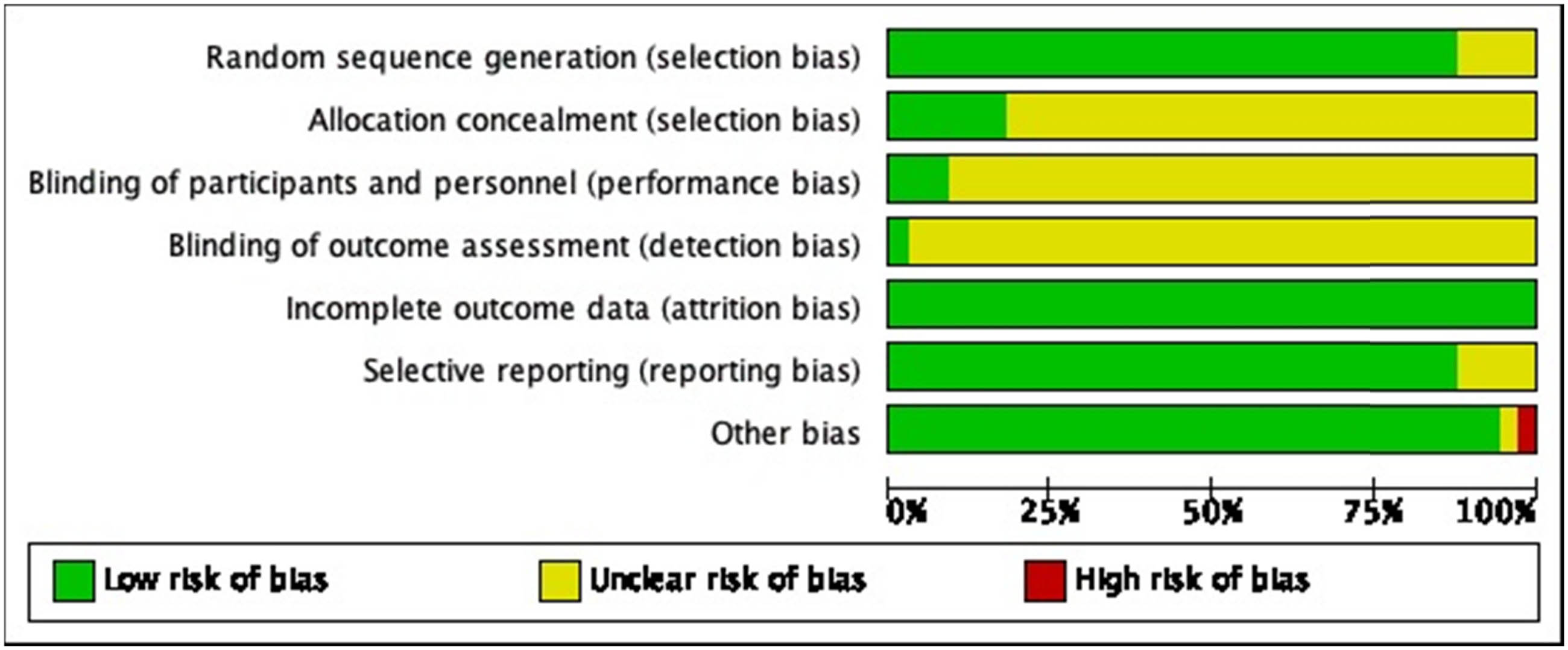
Risk of bias graph: review authors’ judgments about each risk of bias item presented as percentages across all included studies.
3 Results
3.1 Study selection
Figure 3 shows a flow chart for study selection. Among the 1,754 articles identified by the search strategy in the databases, 494 were excluded because of duplication. Title and abstract screening resulted in 548 irrelevant articles removed. The full texts of the remaining 712 articles were reviewed with respect to the study selection criteria, and this process disqualified 679 articles. Eventually, a total of 33 studies were included in this systematic review and meta-analysis.
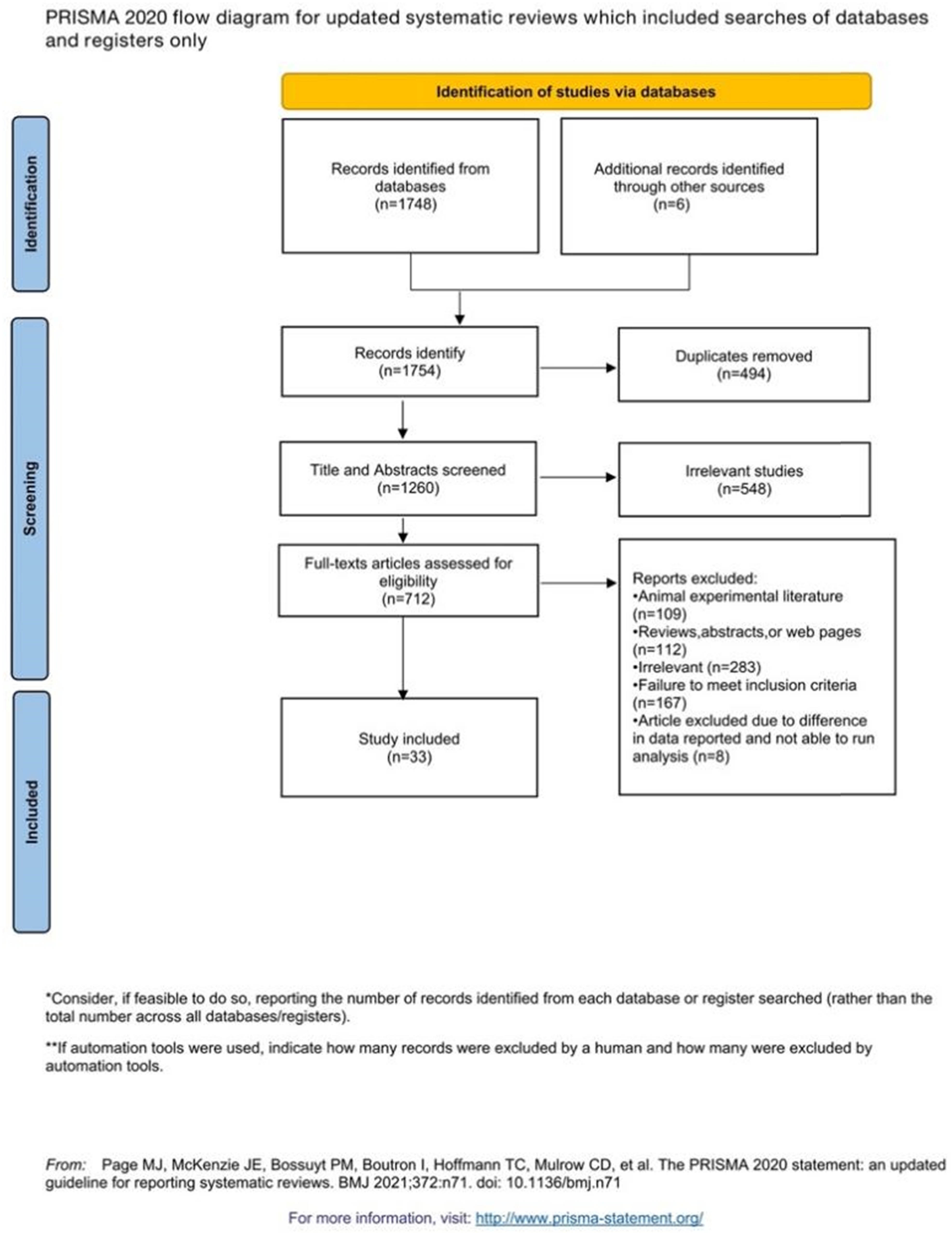
Flow diagram of the study selection procedure.
3.2 Study characteristics
A detailed description of the characteristics (author, year, sample size, age, sex, intervention, duration of trial, main outcomes, and Jaded score) of the 33 eligible studies is summarized in Table 1. We selected 33 RCTs for our final analysis. The control group included 1,367 participants, whereas the experimental group included 1,045 participants. In the literature with relevant data, there were no statistically significant differences between the two groups in terms of age and gender. The trial durations ranged from 1 to 48 weeks. The included studies were published between 2008 and 2022, with sample sizes ranging from 20 to 298.
Main characteristics of studies included in the systematic review and meta-analysis (n = 33)
| Study | Participants | Age (mean ± SD) | Male/female | Intervention | Duration of trail (in weeks) | Outcome | Jadad score | ||
|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | ||||||
| Bajaj et al. (2008) [142] | 14 | 6 | E: 52 ± 8 | Yogurt with L. bulgaricus and S. thermophilus | Placebo | 8 | Ammonia, IL-6, TNF-α | 5 | |
| C: 54 ± 4 | |||||||||
| Pereg et al. (2011) [143] | 18 | 18 | E: 63.2 ± 10.5 | Lactobacillus acidophilus, L. bulgaricus, B. bifidum, and Streptococcus thermophiles | Placebo (non-fermentable fibers) | 24 | Albumin, AST, ALT, ammonia, INR | 6 | |
| C: 65.9 ± 8.4 | |||||||||
| Bajaj et al. (2014) [118] | 14 | 16 | E: 58.4 ± 3.8 | E: 10/4 | Lactobacillus GG | Placebo | 8 | INR, bilirubin, ammonia, albumin, endotoxin, TNF-α, IL-6, IL-2, IL-1β, IL-10, IL-17 | 5 |
| C: 58.5 ± 4.5 | C: 12/4 | ||||||||
| Han et al. (2015) [144] | 60 | 57 | 52.7 ± 11.3 | 75/42 | Lactobacillus subtilis and Streptococcus faecium + conventional therapy | Conventional therapy | 1 | Total protein, albumin, AST, ALT, ALP, yGT, TB, cholesterol, PT | 6 |
| Malaguarnera et al. (2011) [145] | 34 | 32 | E: 46.9 ± 5.4 | E: 18/16 | Bifidobacterium longum + conventional therapy and ofloxacin | Conventional therapy and ofloxacin | 24 | ALT, AST, bilirubin, albumin, TNF-α | 6 |
| C: 46.7 ± 5.7 | C: 15/17 | ||||||||
| Feng et al. (2010) [146] | 13 | 9 | E: 57 ± 12 | E: 10/3 | B. bifidum, L. acidophilus, and E. faecalis + conventional therapy | Conventional therapy | 12 | Endotoxin, IL-2, IL-6, TNF-α | 4 |
| C: 53 ± 11 | C: 7/2 | ||||||||
| Huang et al. (2010) [147] | 15 | 15 | Clostridium butyricum + conventional therapy and ofloxacin | Conventional therapy and ofloxacin | 2 | Endotoxin, TNF-α, IL-6, PAF | 4 | ||
| Pan and Huang (2011) [148] | 34 | 32 | E: 53 ± 7 | E: 20/14 | B. bifidum, L. acidophilus, and E. faecalis + conventional therapy | Conventional therapy | 4 | Endotoxin, ALT, AST, IL-2, IL-6, TNF-α | 4 |
| C: 52 ± 7 | C: 18/14 | ||||||||
| Wang et al. (2012) [149] | 30 | 30 | B. bifidum, L. acidophilus, E. faecalis, and Bacillus cereus + conventional therapy | Conventional therapy | 4 | IL-1α, IL-6, TNF-α | 4 | ||
| Wang and Li (2013) [150] | 32 | 24 | E: 47 ± 8 | E: 19/13 | Bacillus subtilis and E. faecium + antiviral treatment | Antiviral treatment | 4 | Endotoxin, TNF-α | 4 |
| C: 48 ± 4 | C: 16/8 | ||||||||
| Lu (2014) [151] | 17 | 18 | B. subtilis and E. faecium + conventional therapy | Conventional therapy | 4 | Endotoxin, TNF-α | 3 | ||
| Ye and Xiang (2014) [152] | 42 | 42 | E: 68.5 ± 7.1 | E: 23/19 | B. bifidum, L. acidophilus, and E. faecalis + conventional therapy | Conventional therapy | 4 | hs-CRP, TNF-α, efficacy | 3 |
| C: 69.0 ± 7.3 | C: 25/17 | ||||||||
| Zheng et al. (2014) [153] | 45 | 40 | 45.2 ± 13.5 | 62/23 | B. bifidum, L. acidophilus, E. faecalis, and cephalosporin + conventional therapy cephalosporin combined with quinolone | Conventional therapy cephalosporin combined with quinolone | 2 | ALT, AST, albumin, PTA, endotoxin, TNF-α, IL-2, IL-6, IL-10, efficacy | 4 |
| Hao et al. (2014) [154] | 32 | 31 | E: 41.6 ± 6.8 | E: 18/14 | B. bifidum, L. acidophilus, and E. faecalis + conventional therapy | Conventional therapy | 12 | Endotoxin, TNF-α, IL-6 | 4 |
| C: 42.3 ± 5.4 | C: 16/16 | ||||||||
| Zhang (2016) [155] | 52 | 52 | E: 40.7 ± 8.5 | E: 33/19 | B. bifidum, L. acidophilus, and E. faecalis + antiviral treatment | Antiviral treatment | 24 | HBV DNA, ALT, AST, albumin, TBIL, efficacy | 4 |
| C: 40.8 ± 9.1 | C: 34/18 | ||||||||
| Jiang and Xie (2016) [156] | 32 | 32 | E: 54.4 ± 7.12 | E: 21/11 | B. bifidum, L. acidophilus, and E. faecalis + Conventional therapy | Conventional therapy | 2 | DAO, ammonia, endotoxin, ALT, AST, TBIL, albumin, PT | 4 |
| C: 58.2 ± 7.12 | C: 24/8 | ||||||||
| Li et al. (2017) [157] | 40 | 40 | E: 35.8 ± 4.8 | E: 18/22 | L. acidophilus, E. faecalis, B. subtilis + Fluoroquinolone | Fluoroquinolone | 48 | TNF-α, IL-6, ALT, AST, AKP, GGT, albumin, efficacy | 4 |
| C: 36.5 ± 3.5 | C: 17/23 | ||||||||
| Han and Bai (2017) [158] | 49 | 49 | B. bifidum, L. acidophilus, and E. faecalis + conventional therapy | Conventional therapy | 2 | TNF-α, IL-6, IL-10, endotoxin, AST ALT albumin, efficacy | 3 | ||
| Zheng et al. (2018) [159] | 100 | 100 | E: 47.28 ± 7.24 | E: 63/37 | B. bifidum, L. acidophilus, and E. faecalis + Conventional therapy | Conventional therapy | 2 | AST, ALT, albumin, TNF-α, IL-2, IL-6, IL-10, efficacy | 4 |
| C: 46.38 ± 7.04 | C: 60/40 | ||||||||
| Yue and Fang (2018) [160] | 40 | 40 | E: 52.11 ± 9.43 | E: 23/17 | B. bifidum, L. acidophilus, E. faecalis, and B. cereus + Conventional therapy | Conventional therapy | 8 | ALT, AST, albumin, PCT, endotoxin | 4 |
| C: 53.12 ± 10.45 | C: 22/18 | ||||||||
| Zeng et al. (2018) [161] | 80 | 80 | E: 45.06 ± 6.72 | E: 41/39 | B. bifidum, L. acidophilus, and E. faecalis + Conventional therapy | Conventional therapy | 24 | ALT, AST, TBIL, ammonia, endotoxin | 4 |
| C: 43.62 ± 6.29 | C: 45/35 | ||||||||
| Liang and Chen (2018) [162] | 28 | 28 | E: 44.2 ± 6.2 | E: 17/11 | B. bifidum, L. acidophilus, E. faecalis, and B. cereus + Conventional therapy | Conventional therapy | 24 | AST, ALT, TBIL | 4 |
| C: 44.3 ± 6.5 | C: 15/13 | ||||||||
| Wang and Wu (2019) [163] | 149 | 149 | E: 50.93 ± 4.26 | E: 103/46 | B. bifidum, L. acidophilus, E. faecalis + Conventional therapy and entecavir | Conventional therapy and entecavir | 12 | TBIL, ALT, AST, LPS, TNF-α | 4 |
| C: 51.62 ± 3.97 | C: 98/51 | ||||||||
| Li et al. (2019) [164] | 57 | 57 | E: 69.54 ± 3.31 | E: 37/20 | B. bifidum, L. acidophilus, E. faecalis + lamivudine and adefovir dipivoxil | Lamivudine and adefovir dipivoxil | 24 | ALT, AST, TBIL | 4 |
| C: 69.79 ± 3.47 | C: 35/22 | ||||||||
| Duan (2019) [165] | 42 | 42 | E: 45.24 ± 7.38 | E: 26/16 | B. bifidum, L. acidophilus, and E. faecalis + Antiviral treatment | Antiviral treatment | 24 | ALT, AST, TBIL | 4 |
| C: 46.17 ± 6.59 | C: 24/18 | ||||||||
| Bian et al. (2019) [166] | 45 | 45 | E: 57.12 ± 4.02 | E: 26/19 | B. bifidum, L. acidophilus, and E. faecalis + Conventional therapy | Conventional therapy | 2 | ALT, AST, TBIL, IL-10, TNF-α, endotoxin | 4 |
| C: 56.98 ± 4.01 | C: 24/21 | ||||||||
| Zhao and Zhao (2020) [167] | 49 | 49 | E: 48.07 ± 6.98 | E: 33/16 | B. bifidum, L. acidophilus, E. faecalis, and B. cereus + Conventional therapy | Conventional therapy | 1 | IL-6, IL-8, TNF-α | 4 |
| C: 47.33 ± 6.85 | C: 30/19 | ||||||||
| Liu and Li (2021) [168] | 30 | 30 | E: 53.5 ± 11.7 | E: 18/12 | Bacillus licheniformis + Conventional therapy | Conventional therapy | 4 | IL-2, IL-6, TNF-α, TBIL, ALT, AST | 4 |
| C: 52.3 ± 12.1 | C: 16/14 | ||||||||
| Wu et al. (2021) [169] | 50 | 50 | E: 44.32 ± 6.5 | E: 31/19 | B. bifidum, L. acidophilus, and E. faecalis + Conventional therapy | Conventional therapy | 2 | ALT, AST, TBIL, IL-10, TNF-α, endotoxin | 4 |
| C: 43.63 ± 6.82 | C: 32/18 | ||||||||
| Zhu et al. (2022) [170] | 35 | 35 | E: 55.63 ± 2.38 | E: 21/14 | B. bifidum, L. acidophilus, E. faecalis, and B. cereus + Conventional therapy | Conventional therapy | 2 | Efficacy, AST, ALT, TBIL | 5 |
| C: 55.47 ± 2.16 | C: 20/15 | ||||||||
| Li et al. (2022) [171] | 50 | 54 | 51.82 ± 12.46 | Yogurt with B. bifidum, L. rhamnosus, L. acidophilus, L. bulgaricus, and S. thermophilus + Conventional therapy | Conventional therapy | 3 | IL-1β, IL-6, TNF-α, ALT, AST, GGT, ALP | 4 | |
| Zhu et al. (2022) [172] | 42 | 30 | E: 46.12 ± 3.6 | E: 20/22 | B. subtilis, E. faecium + Conventional therapy | Conventional therapy | 4 | Efficacy, AST, ALT, TBIL, albumin | 3 |
| C: 46.41 ± 3.55 | C: 14/16 | ||||||||
| Wang and Jin (2022) [173] | 35 | 35 | 52.38 ± 8.19 | 39/31 | B. bifidum, L. acidophilus, E. faecalis, and B. cereus + Conventional therapy | Conventional therapy | 2 | Efficacy, TBIL, ALT, AST, endotoxin | 4 |
3.3 Main outcomes
3.3.1 Efficacy
A total of eight studies reported efficacy, defining significant and effective as “effective.” The homogeneity among the studies was good (P = 0.11, I 2 = 40%); thus, the fixed-effects model was used. The forest plot showed that the total clinical effective rate of the treatment group was superior to that of the control group, indicating that the probiotic intervention could improve the total clinical efficiency of patients with cirrhosis to some extent (Figure 4: RD = 0.15, 95% CI = 0.11–0.20, P < 0.00001).
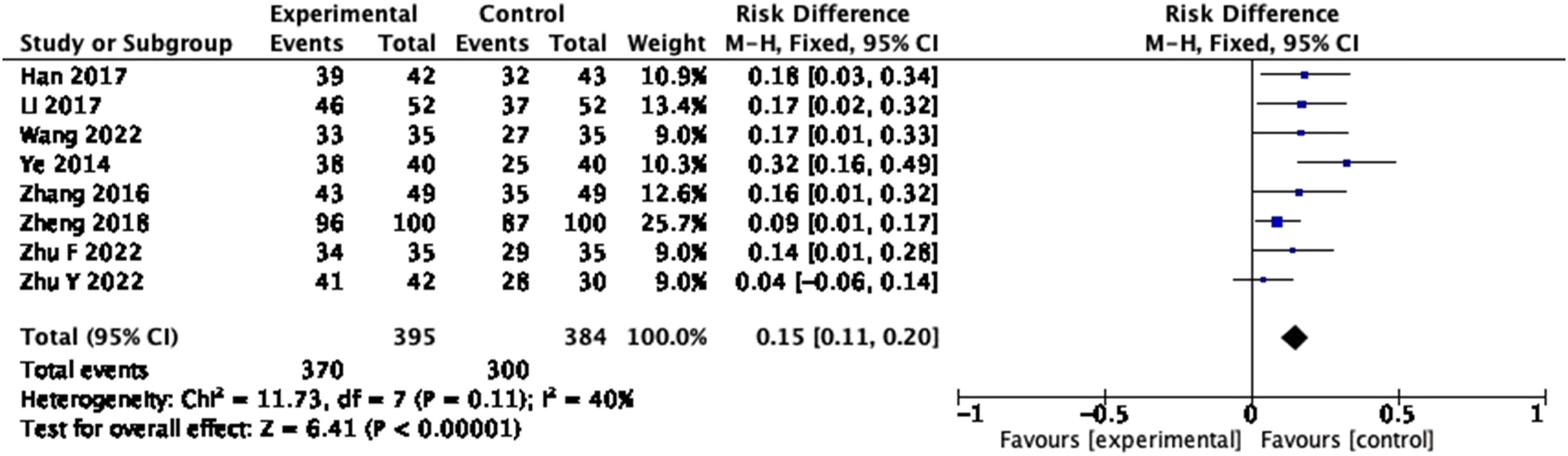
Forest plot of efficacy of probiotics intervention in cirrhosis (fixed-effects model).
3.3.2 Aspartate transaminase (AST)
A total of 22 studies reported the change of AST level, including 2,106 participants. A pooled analysis using the random-effects model showed large statistical differences between the studies (P < 0.001, I 2 = 84%), so the sensitivity analysis was performed, and heterogeneity decreased after exclusion of Zhu (2022). The forest plot proved that the level of AST was significantly lower in the treatment group (Figure 5: mean deviation (MD) = −8.77, 95% CI = −10.50 to −7.04, P < 0.00001), indicating that probiotics supplement was effective in reducing AST in patients with cirrhosis. Further subgroup analysis by duration of trail showed that the homogeneity was best at 1–2 weeks of treatment (P = 0.39, I 2 = 5%).
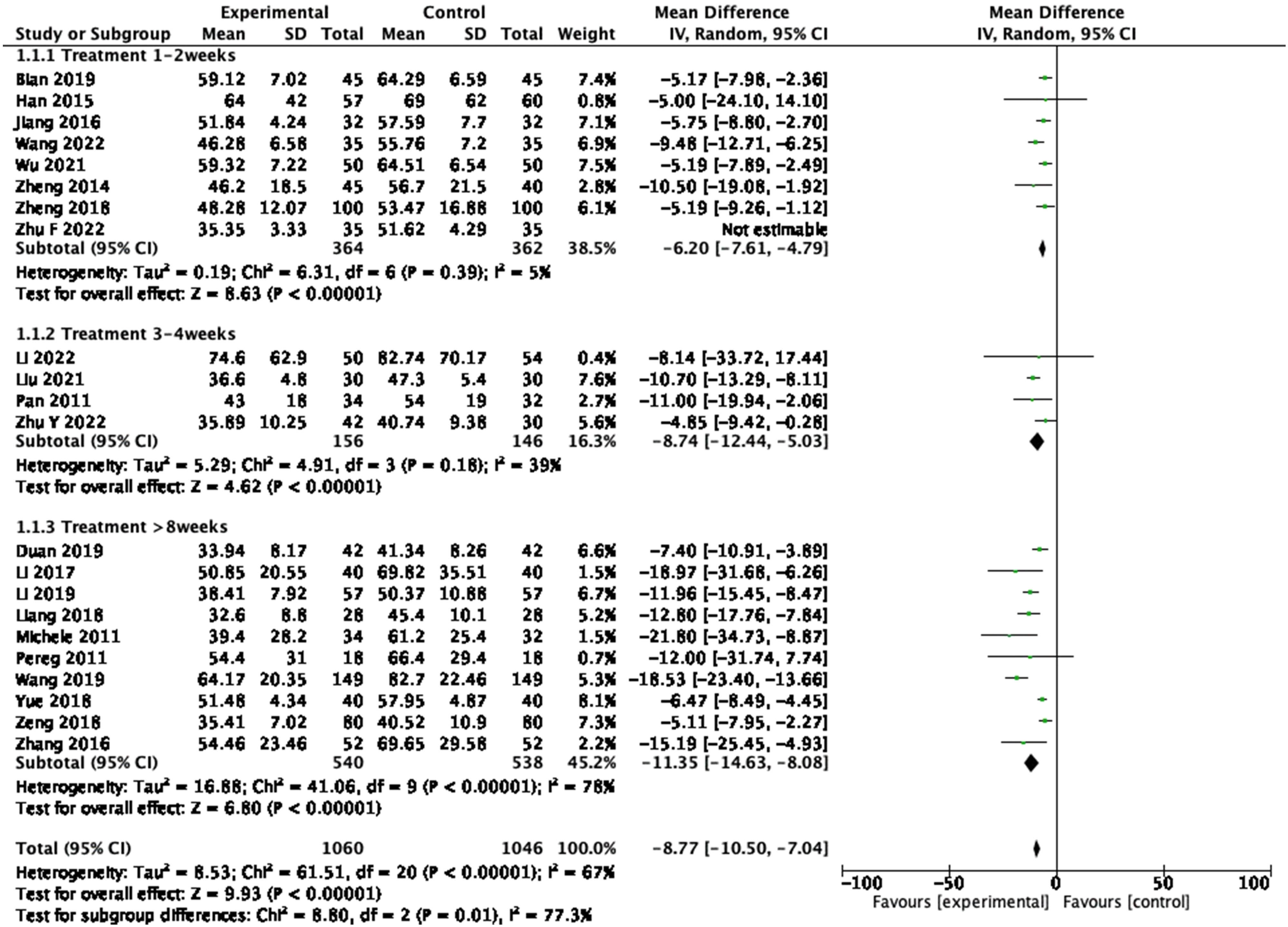
Forest plot of AST between experimental group and control group and subgroup analysis by duration of trail (random-effects model).
3.3.3 ALT
From 23 RCTs reporting on ALT, 1,664 participants were included. In the pooled analysis of studies, a significant effect of probiotic on ALT reduction (Figure 6: MD = −9.13, 95% CI = −10.09 to −8.18, P < 0.00001) was observed with a heterogeneity of 84% (P < 0.001). After the sensitivity analysis was performed, four articles, namely, Jiang (2016), Wang (2019), Yue (2018), and Zhu F (2022) were excluded, and the homogeneity among the study groups was good (P = 0.50, I 2 = 0%).
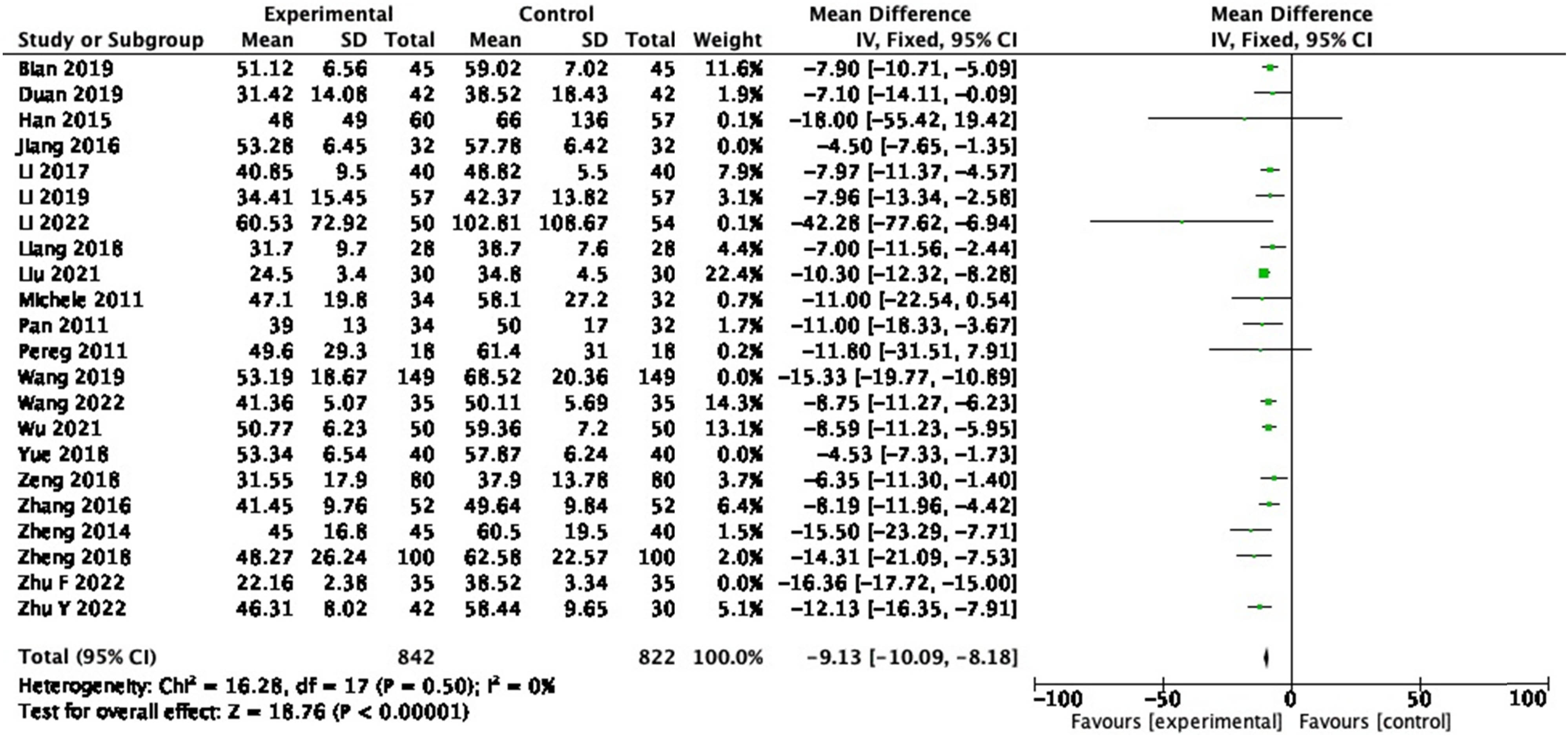
Forest plot of ALT between experimental group and control group (random-effects model).
3.3.4 IL-6
In the 14 RCTs reporting IL-6, 1,016 participants were included. A random-effects meta-analysis of these studies revealed that probiotics decreased IL-6 (Figure 7: MD = −6.12, 95% CI = −9.70 to −2.54, P < 0.001) with a heterogeneity of 96% (P < 0.00001). Sensitivity analysis and subgroup analysis were performed, and no source of heterogeneity could be found.
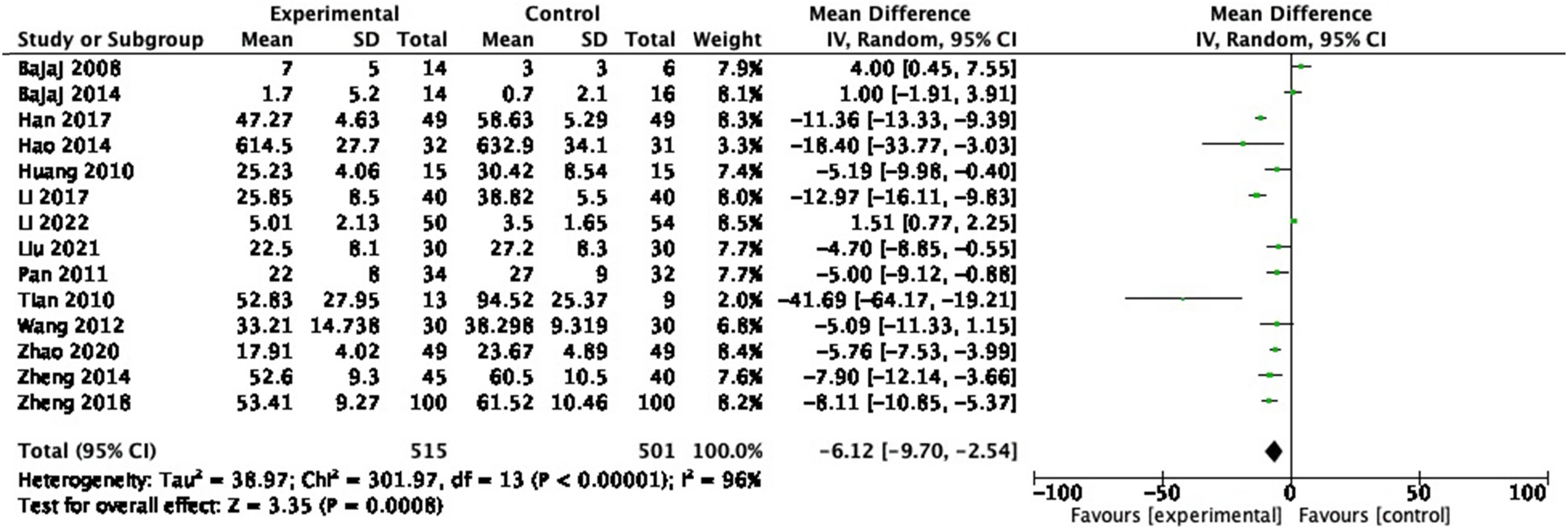
Forest plot of IL-6 between experimental group and control group (random-effects model).
3.3.5 TNF-α
A total of 21 studies reporting the mean change in TNF-α, including 1,745 participants. A random-effects model was used for pooled analysis, and the results demonstrated that the TNF-α levels of patients in the experimental group were lower than those in the control group (Figure 8: MD = −7.76, 95% CI = −10.26 to −5.26, P < 0.00001), with a heterogeneity of 98% (P < 0.00001). Sensitivity analysis and subgroup analysis were performed, and no source of heterogeneity could be found.
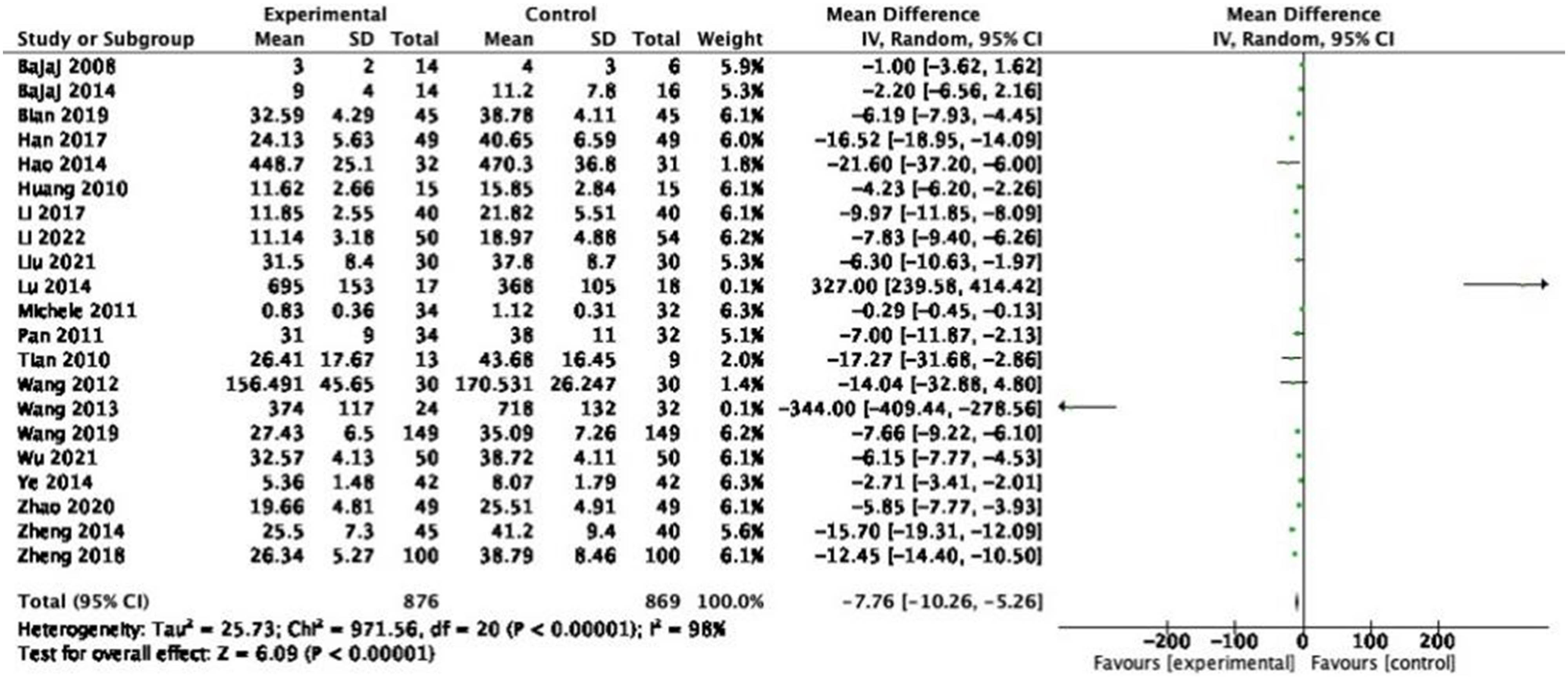
Forest plot of TNF-α between experimental group and control group (random-effects model).
3.3.6 Total bilirubin (TBIL)
In the 13 RCTs reporting TBIL, 1,367 participants were included. A random-effects meta-analysis of these studies illustrated that probiotics decreased TBIL (Figure 9: MD = −6.35, 95% CI = −8.21 to −4.49, P < 0.00001) with a heterogeneity of 84% (P < 0.00001). Sensitivity analysis and subgroup analysis were performed, and no source of heterogeneity could be found.
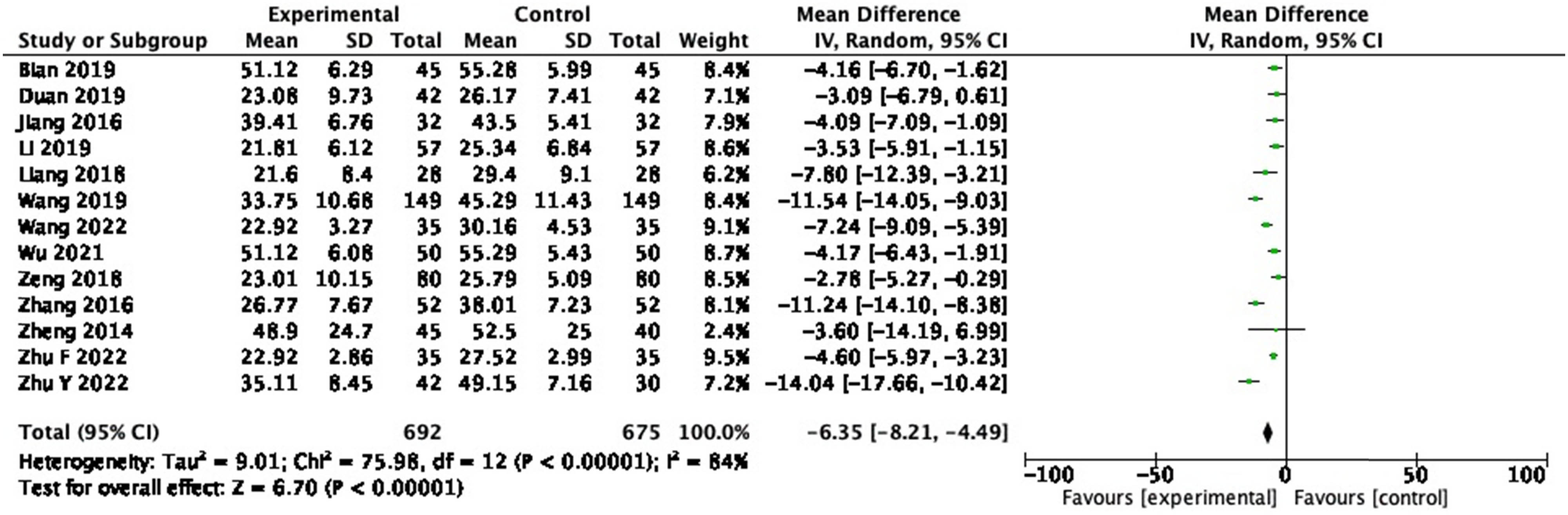
Forest plot of TBIL between experimental group and control group (random-effects model).
3.3.7 Endotoxin
Endotoxin was evaluated in 15 RCTs, and 766 participants were included. In the pooled analysis of studies, a significant effect of probiotic on endotoxin reduction (Figure 10: MD = −0.19, 95% CI = −0.25 to −0.12, P < 0.00001) was observed with a heterogeneity of 99% (P < 0.00001). Sensitivity analysis and subgroup analysis were performed, and no source of heterogeneity could be found.
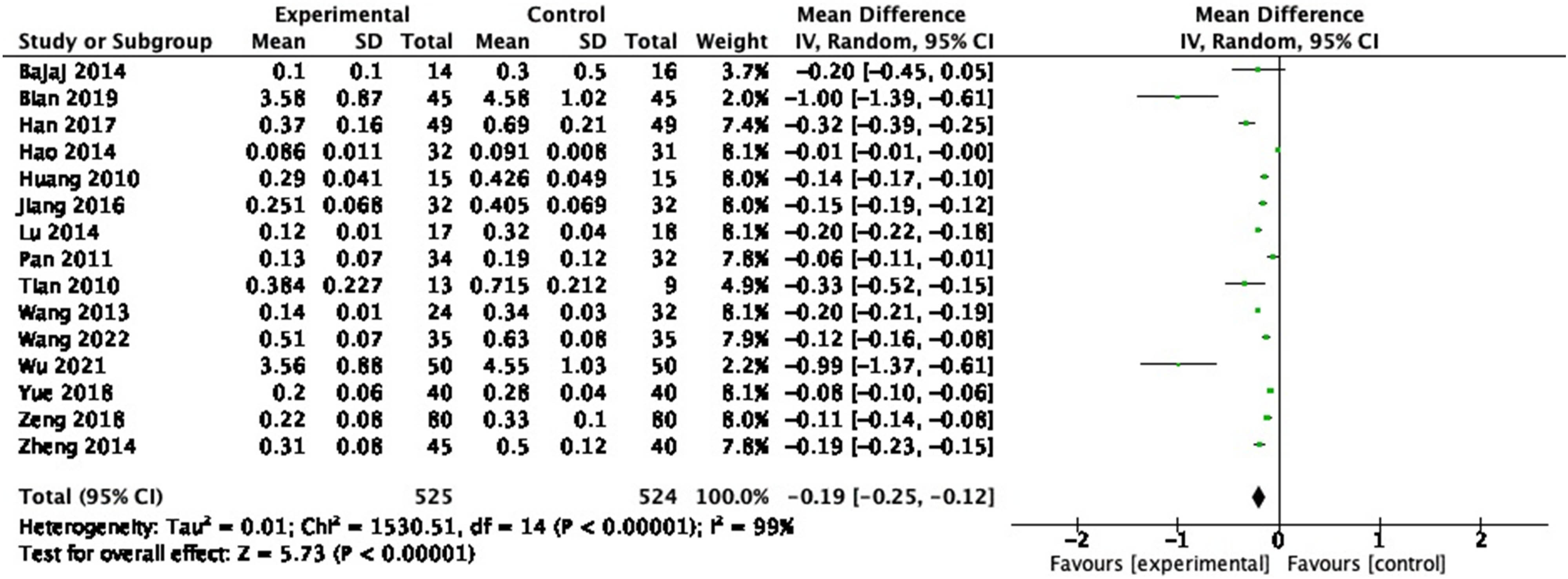
Forest plot of endotoxin between experimental group and control group (random-effects model).
3.3.8 IL-2
In the six RCTs reporting IL-2, 463 participants were included. In the pooled analysis, no significant change in IL-2 (Figure 11: MD = 2.95, 95% CI = −1.40 to 7.30, P = 0.18) was observed with a heterogeneity of 92% (P < 0.00001).
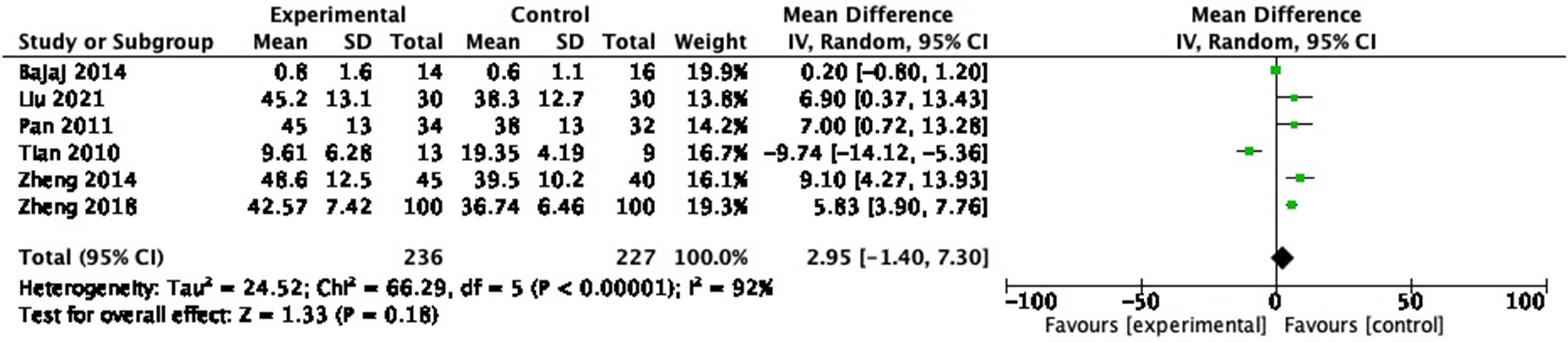
Forest plot of IL-2 between experimental group and control group (random-effects model).
3.3.9 IL-10
Six RCTs measured IL-10, including 629 participants. In the pooled analysis, no significant effect of probiotic on IL-10 (Figure 12: MD = −0.61, 95% CI = −5.63 to 4.41, P = 0.81) was observed with a heterogeneity of 98% (P < 0.00001).
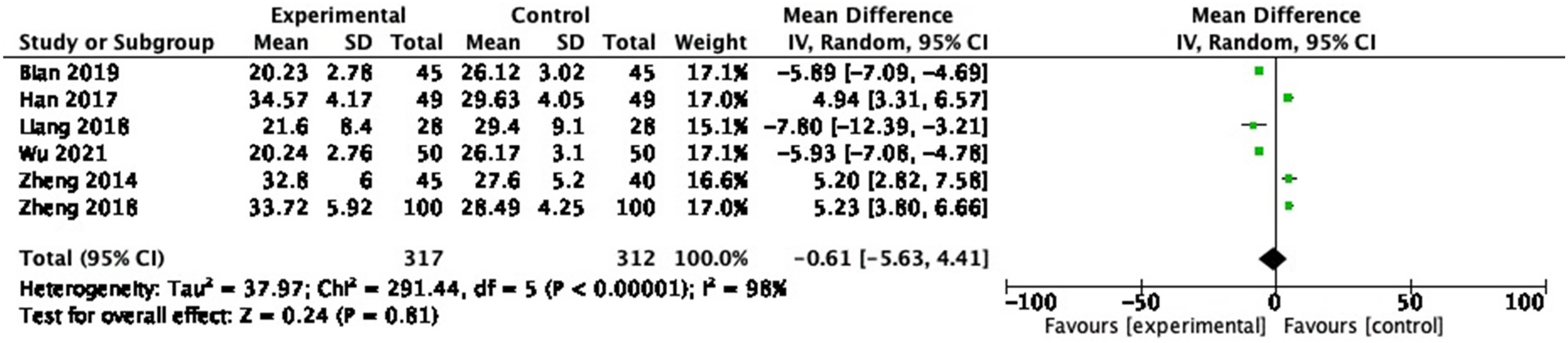
Forest plot of IL-10 between experimental group and control group (random-effects model).
4 Discussion
Cirrhosis is the common end-stage pathological pathway of liver damage arising from a multitude of chronic liver diseases [72], but its potential mechanisms are far from being elucidated. Cirrhosis develops after a prolonged period of inflammation, leading to the replacement of healthy liver parenchyma with regenerative nodules and fibrotic tissue, resulting in portal hypertension. Numerous types of cells, cytokines, and miRNAs might be involved in the initiation and development of cirrhosis.
This study suggests that cirrhosis may interact with microbiota, related metabolites, and inflammatory factors. Furthermore, this study evaluated the efficacy of probiotic therapy in improving liver function and immunity in patients with cirrhosis. As the largest, most complex and most important microecosystem in the human body, the intestinal microecosystem is known as the second human genome. More than 90% of healthy adults have an intestinal microbiota composed of four major components: Bacteroidetes , Firmicutes , Proteobacteria , and Actinobacteria [73]. By contrast, the diversity and proportion of intestinal microorganisms in patients with cirrhosis are different from those in healthy individuals.
A growing amount of recent evidence has elaborated on the altered composition of the gut microbiota in patients with cirrhosis, which is mainly characterized by a shortage of autochthonous non-pathogenic bacteria and an enrichment of potentially pathogenic bacteria [74]. We compiled the relevant literature and found that the intestinal microbiota of patients with cirrhosis contained more Enterobacteriaceae , Streptococcaceae , Fusobacteria , Proteobacteria , Streptococcus, and Veillonella and less Bifidobacteria , Lachnospiraceae , Bacteroidetes, and Firmicutes compared to controls [75,76,77,78,79]. Notably, in order to further explore microbial genes associated with cirrhosis, Qin et al. conducted a large-scale RCT on the macrogenomic characterization of intestinal bioactive metabolites and intestinal microbiota in patients with cirrhosis, bringing new insights from next-generation sequencing [79]. The intestinal microbiota contributes to HE, one of the most devastating and clinically challenging sequelae of cirrhosis, as determined by the analysis of enriched modular microbial function in 98 patients and 83 healthy control individuals.
The concept of “gut–liver axis” was brought up to highlight the close functional and anatomical relationship between two organs. The liver is located near the intestine and is supplied with 75% of its blood by the portal vein. The portal venous flow not only supplies the liver with nutrients but also carries an extremely metabolic microbiota of gastrointestinal origin that provides the liver with broad-spectrum antigens. Most of these are commensal products and harmless dietary factors, but translocated pathogens or bacterial-derived factors also enter the liver continuously via the enterohepatic circulation. Under normal circumstances, a handful of bacteria or bacterial metabolites enter the liver, most of which are eliminated by macrophages in the liver (Kupffer cells), with little or no activation. However, in intestinal microecological dysregulation, the intestinal mucosal barrier is damaged due to inflammation or portal hypertension, and increased intestinal permeability leads to increased pathological bacterial migration [80,81]. A multitude of bacteria could thus enter the liver and activate hepatic stellate cells (HSCs) and Kupffer cells. Once these cells are activated, large amounts of pro-inflammatory cytokines are released and involved in liver injury. Increased intestinal permeability of bacteria and their metabolites, known as “leaky gut,” is pervasive in cirrhosis and is a pivotal pathogenic factor leading to major complications [82]. Overall, the liver may be immensely affected by the composition and abundance of intestinal microorganisms, primarily through the receipt of metabolites from microbes (Figure 13).
![Figure 13
Schematic diagram of the gut–liver axis. Abbreviations: microbial-associated molecular patterns (MAMPs), pathogen-associated molecular patterns (PAMPs), short-chain fatty acids (SCFAs), lipopolysaccharides (LPS), and bile acids (BAs). Three projects that play a prominent role in the relationship between intestinal microbiota and various forms of liver injury are summarized in this study: endotoxin, SCFAs, and BAs [83].](/document/doi/10.1515/biol-2022-0741/asset/graphic/j_biol-2022-0741_fig_013.jpg)
Schematic diagram of the gut–liver axis. Abbreviations: microbial-associated molecular patterns (MAMPs), pathogen-associated molecular patterns (PAMPs), short-chain fatty acids (SCFAs), lipopolysaccharides (LPS), and bile acids (BAs). Three projects that play a prominent role in the relationship between intestinal microbiota and various forms of liver injury are summarized in this study: endotoxin, SCFAs, and BAs [83].
Bacterial translocation (BT) is defined as the passage of viable bacteria from the intestinal lumen through the mesenteric lymph nodes and other sites [83]. There are at least three mechanisms that have an impact on the pathogenesis of BT: increased intestinal permeability, intestinal bacterial overgrowth, and immune alterations in cirrhosis [84]. The concept of BT was later extended to microbial products, including bacterial DNA, peptidoglycans, and endotoxins [85]. MAMPs of intestinal origin, particularly PAMPs, may elicit or exacerbate innate immune responses in the liver. MAMPs are the basic structures of microorganisms, both pathogenic and non-pathogenic. PAMP includes microbial molecular structures, such as gram-negative-associated LPS.
Endotoxins and LPS are active metabolites of gram-negative bacterial envelope components and pathogenic bacterial derivatives and significantly increase (mainly through the activation of the Toll-like receptor [TLR]4 signaling pathway) the synthesis of a range of inflammatory factors, particularly ILs and TNF-α. Inflammatory cells such as mononuclear macrophages, neutrophils, and lymphocytes release proteases, other enzymes, and reactive oxygen metabolites, contributing to the pathological progression of liver injury. Some hepatic non-immune cells, including endothelial cells and HSC, also have TLRs on their surface that recognize bacterial-specific structural components and subsequently release substantial cytokines such as IL-1, IL-6, and TNF-α, further promoting fibrosis and liver inflammation.
The microbiota produces many metabolites, such as SCFAs and BAs, and these key gut microbial-derived metabolites also continue to shape host immunity and metabolism and are critical in liver injury.
SCFAs serve as an additional source of energy, accounting for 3–9% of our daily caloric intake [86]. SCFAs also have a multitude of functions, from immunomodulation and metabolism to mucosal protection, and therefore have direct and indirect effects on our systemic well-being [87]. Wang et al. implemented in vivo and in vitro experiments and confirmed that inulin ameliorates inflammation through M1 inhibition and promotion of M2 Mψ induced by SCFAs, which may contribute to the control of liver disease [88]. Profound changes have taken place in the intestinal microbiota of patients with cirrhosis of liver, along with changes in the content of SCFAs. A controlled study demonstrated the functional impact of disturbances in gut microbiota diversity on the production of SCFAs, with significantly lower SCFAs concentrations in cirrhotic patients than in healthy controls, including propionate, butyric acid, and acetic acid, according to functional and differential classification analyses [89].
One of the possible mechanisms of intestinal ecological dysregulation in patients with cirrhosis is related to the reduced secretion of BAs. BAs are signaling molecules that activate specific nuclear farnesoid X receptors (FXRs) in the intestine and activate various signaling pathways by binding to FXR in intestinal epithelial cells and hepatocytes [90]. Approximately 95% of BAs are reabsorbed in the terminal ileum and transported back to the liver via the enterohepatic circulation [91]. However, a small percentage of BAs reach the colon and are converted by the intestinal microbiota into secondary BAs, which regulate various metabolic processes in the liver, such as glucose, triglyceride, cholesterol metabolism as well as inflammatory responses [92,93].
A recent study conducted an extensive analysis of neutrophil function and serum BA composition in a large number of patients with cirrhosis and found that the therapeutic modulation of serum BAs spectrum to “healthy” compositions can restore impaired neutrophil function in patients with cirrhosis, which in turn can reduce bacterial infection and related morbidity and mortality in cirrhosis [94]. This investigator also proposed that BAs’ spectrum regulation could be achieved by targeting the gut microbiome involved in BA metabolism. In addition, except for being a metabolism regulator and a nutrition supply source, bile acid also has an antibacterial function. BAs exert both direct antimicrobial properties and indirect negative selective pressure on intestinal bacteria through FXR activation in the small intestine, which induces antimicrobial peptide synthesis, and FXR agonists reduce BT through the portal pathway to the liver in cirrhosis [92,95].
The gut–liver axis in hepatitis B virus (HBV) and hepatitis C virus (HCV) infections has received much attention recently, and the close association between alterations in the intestinal microbiota and HBV- and HCV-associated complications, such as fibrosis and cirrhosis, is supported by in vivo and in vitro studies [96,97,98]. Probiotics have an antiviral activity against HBV and HCV infections, and numerous studies have reported mechanisms by which probiotics reduce liver disease by mediating antagonism against HBV and HCV [99]. Lee et al. demonstrated the antiviral activity of Bifidobacterium adolescentis SPM0212 against HBV and that probiotic cellular extracts inhibited the replication of HBV viruses by up-regulating the activation of the MxA protein by STAT1 [100]; El-Adawi et al. showed that treatment with probiotic medium extracts significantly reduced HCV viral load and HepG2 cell death; probiotic supplementation attenuated thioacetamide-induced liver cirrhosis in rats by inhibiting the expression of TLR4, CXCL9, and PREX-2 [101]. Recent studies have also suggested that probiotic-adjuvant therapy reduces the risk of further progression to HCC in cirrhotic patients treated with HBC antiviral drugs [102].
In order to assess the in vitro resistance of common market probiotics to clinically used antibiotics, Feng et al. isolated and cultured 20 strains of probiotics from nine probiotic preparations and subsequently determined the minimum inhibitory concentration (MIC) of probiotics against 16 antibiotics using the concentration gradient test strip method (E-test) [103]. The results showed that the bacterial probiotics were sensitive to some oral antibiotics – among which, Bacillus subtilis, Lactobacillus bulgaricus , Bifidobacterium longum , Bifidobacterium infantis, and Streptococcus thermophilus were sensitive to more than 12 antibiotics, Enterococcus faecalis was sensitive to only 5 antibiotics, and fungal probiotic bacteria, such as Saccharomyces boulardii CNCMI-45, were resistant to all 16 antibiotics. In the clinical prevention and treatment of antibiotic-associated diseases, attention should be paid to the rational use of probiotics and antibiotics, which can increase the dose of probiotics or stagger the time of medication, preferably at intervals of more than 2–3 h, and does not affect the efficacy of the drug [104].
On the other hand, probiotics can be used in antibiotic therapy in order to reduce the risk of drug-related adverse effects (such as diarrhea) and preserve the clinic’s gut flora [105]. Specific probiotic therapy not only increases the likelihood of eradication but also lessens the negative effects brought on by antibiotic treatment [106]. Potential benefits of using probiotics to treat a variety of diseases include their relatively low cost and the fact that they do not increase the incidence of antibiotic resistance [107,108,109]. Probiotic supplementation therapy has been scientifically demonstrated to increase the effectiveness of antibiotics and preserve host gut flora, and experts hypothesize that adding probiotics to antibiotic therapy may be helpful on top of what is already being done [110]. Therefore, due to the widespread nature of antibiotic resistance and the variety of public health issues it creates, the focused use of minimal side effect drugs, such as probiotics, is particularly crucial [111].
Concerns about the safety of probiotics currently center on [112,113]: can the strains used cause potential infections? Can they carry and transmit drug resistance? Is there regulatory confusion about probiotic products on the market? Can harmful metabolites be produced? The majority of probiotic species, such as Lactobacillus, Bifidobacterium, Lactococcus , and Yeasts , fall within the “Generally Recognized as Safe” (GRAS) category [114]. Identification of the origin, antibiotic resistance, purity, potency (amount of live microorganisms given), and final product composition of the strains are among the safety concerns connected with the manufacture of probiotic goods. In order to identify any contaminants, probiotic goods must also be appropriately tested for their intended purpose and shown to be pathogen-free [115]. Numerous extensive systematic studies that looked at hundreds of probiotic trials came to the conclusion that safety problems and adverse events are infrequently documented [116,117]. Probiotics are generally regarded as safe, but caution is advised when using them in immunologically vulnerable populations. Non-industry-sponsored, independent, high-quality, multicenter controlled trials are also required to evaluate the effectiveness and side effects of probiotics in at-risk populations, ideally in conjunction with regulatory agency assessments.
The preceding discussion of this study elucidates the role of the gut microbiota, associated metabolites, and cytokines in the pathophysiology of hepatocyte injury. The results of our meta-analysis showed that the regulation of gut microbiome using probiotics was able to improve the total clinical efficiency of cirrhosis and reduce most of the inflammatory factor indicators. Overall, the enterohepatic circulation, the gut microbiota, and the immune system must establish complex interactions to maintain homeostasis, a disturbance of which can lead to increased intestinal permeability and result in liver disease. It is hoped that this evidence will provide new insights for future research and development of drugs designed to prevent further hepatocyte damage and delay the progression of cirrhosis to hepatocellular carcinoma.
Despite the extensive literature review and rigorous robustness checks, this study comes with a few limitations. First, the heterogeneity of some of the metrics in this meta-analysis is very high, including IL-6, TNF-α, and TBIL. Despite subgroup and sensitivity analyses, unfortunately, the source of heterogeneity could not be found. In view of the inconsistent timeline of the included research and the fact that the participants were from distinct countries and ethnicities, this heterogeneity may be a result of vast individual differences, and therefore, the results in this study for IL-6, TNF-α, and TBIL should be referenced with caution. Second, due to the paucity of relevant literature reporting IL-2 and IL-10, only six studies were included in the analysis of each of these two indicators, and the meta-analysis showed no statistically significant difference. Insufficient attention has been paid to IL-2 and IL-10 in the field of cirrhosis, which indicate potential avenues for further research.
Probiotics not only improve gastrointestinal disorders, but are also beneficial for cirrhosis, as reported in many studies in the literature and confirmed by this meta-analysis [95,118,119,120]. Studies have been conducted to summarize the changes in the gut microbiome of patients with liver disease and discuss the potential role of probiotics in the management of liver disease [83,121]. Alterations in host immune response, host–microbiota metabolism, and intestinal permeability work together in the pathogenesis of cirrhosis, and microbially targeted therapies should focus on increasing potential beneficial taxa and reducing potential harmful taxa.
FMT may shed some light on cirrhosis treatment. As a research hotspot in biomedical and clinical medicine in recent years, FMT is currently the most effective gut microbiota intervention and also an acknowledged treatment for recurrent and refractory Clostridium difficile infection (CDI) [122]. This treatment option has received considerable attention since 2013, and as the clinical response of FMT to substantial diseases has been studied in depth [123], its application has been expanded to include not only gastrointestinal diseases (inflammatory bowel diseases, irritable bowel syndrome, CDI) [124,125,126,127,128], but also extra-gastrointestinal disorders (neurological disorders, diabetes, obesity, HE, metabolic disorders, cancer) [129,130,131,132,133,134]. In two noteworthy RCTs on HE, researchers drew the conclusion that FMT treatment is safe and well tolerated in patients with cirrhosis and that it reduces recurrent hospitalizations and improves ecological dysregulation [135,136].
Although the functions and contributions of probiotics in the pathogenesis and complications of cirrhosis remain to be elucidated, these findings could help develop new therapeutic strategies for cirrhosis by focusing on the gut microbiota. Future research should focus on the development of new bioengineering technology-based therapies for cirrhosis that are more efficient and specific [137]: to illustrate, first, the development of artificial compounds that adsorb or modulate specific intestinal microbiota and their metabolites; second, the use of targeted individual microbiota to replace fecal transplants; and finally, the use of engineered microbiota capable of producing anti-inflammatory or antioxidant molecules as an alternative to antibiotics. Notably, it is important that an individualized approach be developed based on the patient’s baseline bowel pattern, biomarkers of response, and other patient factors, with attention to clinically relevant primary outcomes. Further research in this area should delve into personalized approaches, rigorous experimental design, and microbiome treatment selection [133].
The field of liver disease still needs further work on microbial therapies for cirrhosis, including cross-sectional large-scale cohort studies and longitudinal in-depth mechanistic studies to determine the optimal dosing regimen, ideal donor characteristics, duration of efficacy, and benefits and side effects of probiotics. Demographic characteristics and disease-related factors also need to be controlled and taken into account in the interpretation to ensure patient benefits without negative effects on the body [138].
5 Conclusions
Albeit there is no effective radical cure for cirrhosis, the results of this systematic review and meta-analysis show that the emerging networks of probiotic interventions, gut microbiota, and inflammatory factors currently prove to be possibly profitable therapeutic targets. The use of probiotics in cirrhosis not only reduced liver transaminase levels but also appeared to improve inflammation and decrease pro-inflammatory markers. Previously published systematic reviews and meta-analyses of the same kind also point to a beneficial effect of probiotic/prebiotic use on liver injury [139,140,141]. Probiotics are relatively safe and well tolerated, which makes them advantageous over other treatment regimens for long periods of time. In the future, microbial therapy may become a potential method and component in the treatment of cirrhosis. Further in vitro validation experiments are needed to better understand the mechanism of probiotics in the treatment of cirrhosis. Meanwhile, large-scale population RCTs with homogeneous preparations and the same treatment duration are required to determine whether our results can be generalized to all individuals.
-
Funding information: This research was funded by the Scientific Research and Innovation Fund of Wuhan Asia General Hospital (Project No. 2022KYCX1-A04); 2020 General Planning Fund Project for Humanities and Social Sciences of the Ministry of Education, China (Project No. 20YJA880053); Key Research Project of Philosophy and Social Sciences of Hubei Provincial Department of Education in 2020 (Project No. 20D026); and Hubei Province Key Laboratory of Occupational Hazard Identification and Control, Wuhan University of Science and Technology (Project No. OHIC2022G05).
-
Author contributions: Conceptualization, Y.L. and Q.X.; methodology, Y.L. and Q.X.; formal analysis, Y.L. and Q.X.; investigation, Y.L. J.W and Q.X.; writing – original draft preparation, Y.L. and Q.X.; writing – review and editing, Y.L., Q.W. and Q.X.; supervision, Q.W. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359–76. 10.1016/s0140-6736(21)01374-x. PubMed PMID: 34543610. Epub 2021/09/21.Search in Google Scholar
[2] Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–71. 10.1016/j.jhep.2018.09.014. PubMed PMID: 30266282. Epub 2018/09/30.Search in Google Scholar PubMed
[3] Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;394(10204):1145–58. 10.1016/s0140-6736(19)30427-1. PubMed PMID: 31248666. PubMed Central PMCID: PMCPMC6891889. Epub 2019/06/30.Search in Google Scholar PubMed PubMed Central
[4] Wilson R, Williams DM. Cirrhosis. Med Clin North Am. 2022;106(3):437–46. 10.1016/j.mcna.2021.12.001. PubMed PMID: 35491064. Epub 2022/05/02.Search in Google Scholar PubMed
[5] Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. 10.1038/nrgastro.2014.66. PubMed PMID: 24912386. Epub 2014/06/11.Search in Google Scholar PubMed
[6] Hempel S, Newberry S, Ruelaz A, Wang Z, Miles JN, Suttorp MJ, et al. Safety of probiotics used to reduce risk and prevent or treat disease. Evid Rep Technol Assess (Full Rep). 2011;200:1–645. Epub 2011/04/01 PubMed PMID: 23126627. PubMed Central PMCID: PMCPMC4780970.Search in Google Scholar
[7] Ragan MV, Wala SJ, Goodman SD, Bailey MT, Besner GE. Next-generation probiotic therapy to protect the intestines from injury. Front Cell Infect Microbiol. 2022;12:863949. 10.3389/fcimb.2022.863949. PubMed PMID: 35837474. PubMed Central PMCID: PMCPMC9273849. Epub 2022/07/16.Search in Google Scholar PubMed PubMed Central
[8] Kouhi F, Mirzaei H, Nami Y, Khandaghi J, Javadi A. Potential probiotic and safety characterisation of enterococcus bacteria isolated from indigenous fermented motal cheese. Int Dairy J. 2022;126:105247. 10.1016/j.idairyj.2021.105247.10.1016/j.idairyj.2021.105247Search in Google Scholar
[9] Bintsis T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018;4(4):665–84. 10.3934/microbiol.2018.4.665. PubMed PMID: 31294241. PubMed Central PMCID: PMCPMC6613329. Epub 2019/07/12.Search in Google Scholar PubMed PubMed Central
[10] Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, et al. Role of probiotics in human gut microbiome-associated diseases. J Microbiol Biotechnol. 2019;29(9):1335–40. 10.4014/jmb.1906.06064. PubMed PMID: 31434172. Epub 2019/08/23.Search in Google Scholar PubMed
[11] Haghshenas B, Kiani A, Mansoori S, Mohammadi-Noori E, Nami Y. Probiotic properties and antimicrobial evaluation of silymarin-enriched Lactobacillus bacteria isolated from traditional curd. Sci Rep. 2023;13(1):10916. 10.1038/s41598-023-37350-3. PubMed PMID: 37407617. PubMed Central PMCID: PMCPMC10322947. Epub 2023/07/06.Search in Google Scholar PubMed PubMed Central
[12] Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Rep. 2015;79:1–16. Epub 2015/02/12 PubMed PMID: 25671660. PubMed Central PMCID: PMCPMC4573565.Search in Google Scholar
[13] Jäger R, Mohr AE, Carpenter KC, Kerksick CM, Purpura M, Moussa A, et al. International society of sports nutrition position stand: probiotics. J Int Soc Sports Nutr. 2019;16(1):62. 10.1186/s12970-019-0329-0. PubMed PMID: 31864419. PubMed Central PMCID: PMCPMC6925426. Epub 2019/12/23.Search in Google Scholar PubMed PubMed Central
[14] Ritchie ML, Romanuk TN. A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One. 2012;7(4):e34938. 10.1371/journal.pone.0034938. PubMed PMID: 22529959. PubMed Central PMCID: PMCPMC3329544. Epub 2012/04/25.Search in Google Scholar PubMed PubMed Central
[15] Miller LE, Ouwehand AC, Ibarra A. Effects of probiotic-containing products on stool frequency and intestinal transit in constipated adults: systematic review and meta-analysis of randomized controlled trials. Ann Gastroenterol. 2017;30(6):629–39. 10.20524/aog.2017.0192. PubMed PMID: 29118557. PubMed Central PMCID: PMCPMC5670282. Epub 2017/11/10.Search in Google Scholar PubMed PubMed Central
[16] Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;2010(11):Cd003048. Epub 2010/11/12. 10.1002/14651858.CD003048.pub3. PubMed PMID: 21069673. PubMed Central PMCID: PMCPMC6532699 bifidobacteria provided by Cul tech Ltd, UK, in the prevention of atopic disorders in infants and antibiotic -associated diarrhoea in older people. In previous research, Scientific Hospital Supplies, UK, and Valio Ltd, Finland, have provided L. casei strain GG and also supported his attendance at a training workshop. Elizabeth Martinez is a Medical Manager for United Laboratories Inc., Philippines.Search in Google Scholar PubMed PubMed Central
[17] Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12(12):Cd006095. Epub 2017/12/20. 10.1002/14651858.CD006095.pub4. PubMed PMID: 29257353. PubMed Central PMCID: PMCPMC6486212 known Calvin Ka - Fung Lo: None known Jennifer Beardsley: None known Dominik Mertz: None known Bradley Johnston: None known.Search in Google Scholar PubMed PubMed Central
[18] Liu Y, Alookaran JJ, Rhoads JM. Probiotics in autoimmune and inflammatory disorders. Nutrients. 2018;10(10). 10.3390/nu10101537. PubMed PMID: 30340338. PubMed Central PMCID: PMCPMC6213508. Epub 2018/10/21.Search in Google Scholar PubMed PubMed Central
[19] Jadhav P, Jiang Y, Jarr K, Layton C, Ashouri JF, Sinha SR. Efficacy of dietary supplements in inflammatory bowel disease and related autoimmune diseases. Nutrients. 2020;12(7):2156. 10.3390/nu12072156. PubMed PMID: 32698454. PubMed Central PMCID: PMCPMC7400845. Epub 2020/07/24.Search in Google Scholar PubMed PubMed Central
[20] Aqaeinezhad Rudbane SM, Rahmdel S, Abdollahzadeh SM, Zare M, Bazrafshan A, Mazloomi SM. The efficacy of probiotic supplementation in rheumatoid arthritis: a meta-analysis of randomized, controlled trials. Inflammopharmacology. 2018;26(1):67–76. 10.1007/s10787-017-0436-y. PubMed PMID: 29302905. Epub 2018/01/06.Search in Google Scholar PubMed
[21] Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Medicina (Kaunas). 2016;52(1):28–34. 10.1016/j.medici.2015.11.008. PubMed PMID: 26987497. Epub 2016/03/19.Search in Google Scholar PubMed
[22] Sun Z, Sun X, Li J, Li Z, Hu Q, Li L, et al. Using probiotics for type 2 diabetes mellitus intervention: Advances, questions, and potential. Crit Rev Food Sci Nutr. 2020;60(4):670–83. 10.1080/10408398.2018.1547268. PubMed PMID: 30632770. Epub 2019/01/12.Search in Google Scholar PubMed
[23] Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF. Effects of probiotics on type II diabetes mellitus: a meta-analysis. J Transl Med. 2020;18(1):30. 10.1186/s12967-020-02213-2. PubMed PMID: 31952517. PubMed Central PMCID: PMCPMC6966830. Epub 2020/01/19.Search in Google Scholar PubMed PubMed Central
[24] Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11(1):5015. 10.1038/s41467-020-18414-8. PubMed PMID: 33024120. PubMed Central PMCID: PMCPMC7538905. Epub 2020/10/08.Search in Google Scholar PubMed PubMed Central
[25] Liang T, Wu L, Xi Y, Li Y, Xie X, Fan C, et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit Rev Food Sci Nutr. 2021;61(10):1670–88. 10.1080/10408398.2020.1764488. PubMed PMID: 32436397. Epub 2020/05/22.Search in Google Scholar PubMed
[26] Grylls A, Seidler K, Neil J. Link between microbiota and hypertension: Focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed Pharmacother. 2021;137:111334. 10.1016/j.biopha.2021.111334. PubMed PMID: 33556874. Epub 2021/02/09.Search in Google Scholar PubMed
[27] Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19(40):6911–8. 10.3748/wjg.v19.i40.6911. PubMed PMID: 24187469. PubMed Central PMCID: PMCPMC3812493. Epub 2013/11/05.Search in Google Scholar PubMed PubMed Central
[28] Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49(3):989–97. 10.1002/hep.22711. PubMed PMID: 19115316. PubMed Central PMCID: PMCPMC3756672. Epub 2008/12/31.Search in Google Scholar PubMed PubMed Central
[29] Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20(2):395. 10.3390/ijms20020395. PubMed PMID: 30658519. PubMed Central PMCID: PMCPMC6358912. Epub 2019/01/20.Search in Google Scholar PubMed PubMed Central
[30] Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39(5):1441–9. 10.1002/hep.20194. PubMed PMID: 15122774. Epub 2004/05/04.Search in Google Scholar PubMed
[31] Plaza-Díaz J, Ruiz-Ojeda FJ, Vilchez-Padial LM, Gil A. Evidence of the anti-inflammatory effects of probiotics and synbiotics in intestinal chronic diseases. Nutrients. 2017;9(6):555. 10.3390/nu9060555. PubMed PMID: 28555037. PubMed Central PMCID: PMCPMC5490534. Epub 2017/05/31.Search in Google Scholar PubMed PubMed Central
[32] Khan SH, Ansari FA. Probiotics–the friendly bacteria with market potential in global market. Pak J Pharm Sci. 2007;20(1):76–82. Epub 2007/03/06. PubMed PMID: 17337434.Search in Google Scholar
[33] Nami Y, Kiani A, Elieh-Ali-Komi D, Jafari M, Haghshenas B. Impacts of alginate–basil seed mucilage–prebiotic microencapsulation on the survival rate of the potential probiotic Leuconostoc mesenteroides ABRIINW. N18 in yogurt. Int J Dairy Tec. 2022;76(1):138–48.10.1111/1471-0307.12909Search in Google Scholar
[34] Kiani A, Nami Y, Hedayati S, Elieh Ali Komi D, Goudarzi F, Haghshenas B. Application of Tarkhineh Fermented Product to Produce Potato Chips With Strong Probiotic Properties, High Shelf-Life, and Desirable Sensory Characteristics. Front Microbiol. 2021;12:657579. 10.3389/fmicb.2021.657579. PubMed PMID: 34054754. PubMed Central PMCID: PMCPMC8153181. Epub 2021/06/01.Search in Google Scholar PubMed PubMed Central
[35] Nami Y, Haghshenas B, Haghshenas M, Yari Khosroushahi A. Antimicrobial activity and the presence of virulence factors and bacteriocin structural genes in Enterococcus faecium CM33 isolated from ewe colostrum. Front Microbiol. 2015;6:782. 10.3389/fmicb.2015.00782. PubMed PMID: 26284059. PubMed Central PMCID: PMCPMC4518196. Epub 2015/08/19.Search in Google Scholar PubMed PubMed Central
[36] Ayivi RD, Gyawali R, Krastanov A, Aljaloud SO, Worku M, Tahergorabi R, et al. Lactic acid bacteria: Food Safety and Human Health Applications. Dairy. 2020;1(3):202–32. 10.3390/dairy1030015.Search in Google Scholar
[37] Saarela M, Alakomi HL, Mättö J, Ahonen AM, Puhakka A, Tynkkynen S. Improving the storage stability of Bifidobacterium breve in low pH fruit juice. Int J Food Microbiol. 2011;149(1):106–10. 10.1016/j.ijfoodmicro.2010.12.002. PubMed PMID: 21195496. Epub 2011/01/05.Search in Google Scholar PubMed
[38] Thomas LV, Suzuki K, Zhao J. Probiotics: a proactive approach to health. A symposium report. Br J Nutr. 2015;114(Suppl 1):S1–15. 10.1017/s0007114515004043. PubMed PMID: 26548336. Epub 2015/11/10.Search in Google Scholar PubMed
[39] Kahieshesfandiari M, Nami Y, Lornezhad G, Kiani A, Javanmard A, Jaymand M, et al. Herbal hydrogel-based encapsulated Enterococcus faecium ABRIINW.N7 improves the resistance of red hybrid tilapia against Streptococcus iniae. J Appl Microbiol. 2021;131(5):2516–27. 10.1111/jam.15098. PubMed PMID: 33817937. Epub 2021/04/06.Search in Google Scholar PubMed
[40] Quigley EM. Probiotics in irritable bowel syndrome: The science and the evidence. J Clin Gastroenterol. 2015;49(Suppl 1):S60–4. 10.1097/mcg.0000000000000348. PubMed PMID: 26447967. Epub 2015/10/09.Search in Google Scholar PubMed
[41] Derikx LA, Dieleman LA, Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol. 2016;30(1):55–71. 10.1016/j.bpg.2016.02.005. PubMed PMID: 27048897. Epub 2016/04/07.Search in Google Scholar PubMed
[42] Shan LS, Hou P, Wang ZJ, Liu FR, Chen N, Shu LH, et al. Prevention and treatment of diarrhoea with Saccharomyces boulardii in children with acute lower respiratory tract infections. Benef Microbes. 2013;4(4):329–34. 10.3920/bm2013.0008. PubMed PMID: 24311316. Epub 2013/12/07.Search in Google Scholar PubMed
[43] Lu YX, He CZ, Wang YX, Ai ZS, Liang P, Yang CQ. Effect of entecavir on the intestinal microflora in patients with chronic hepatitis B: A controlled cross-sectional and longitudinal real-world study. Infect Dis Ther. 2021;10(1):241–52. 10.1007/s40121-020-00355-w. PubMed PMID: 33111216. PubMed Central PMCID: PMCPMC7954982. Epub 2020/10/29.Search in Google Scholar PubMed PubMed Central
[44] Costa C, Sampaio-Maia B, Araujo R, Nascimento DS, Ferreira-Gomes J, Pestana M, et al. Gut Microbiome and Organ Fibrosis. Nutrients. 2022;14(2):352. 10.3390/nu14020352. PubMed PMID: 35057530. PubMed Central PMCID: PMCPMC8781069. Epub 2022/01/22.Search in Google Scholar PubMed PubMed Central
[45] Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. 10.1038/s41575-018-0011-z. PubMed PMID: 29748586. PubMed Central PMCID: PMCPMC6319369. Epub 2018/05/12.Search in Google Scholar PubMed PubMed Central
[46] Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72(3):558–77. 10.1016/j.jhep.2019.10.003. PubMed PMID: 31622696. Epub 2019/10/18.Search in Google Scholar PubMed
[47] Rahimi RS, Brown KA, Flamm SL, Brown Jr RS. Overt Hepatic Encephalopathy: Current Pharmacologic Treatments and Improving Clinical Outcomes. Am J Med. 2021;134(11):1330–8. 10.1016/j.amjmed.2021.06.007. PubMed PMID: 34242619. Epub 2021/07/10.Search in Google Scholar PubMed
[48] de Wit K, Schaapman JJ, Nevens F, Verbeek J, Coenen S, Cuperus FJC, et al. Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterol. 2020;7(1):e000531. 10.1136/bmjgast-2020-000531. PubMed PMID: 33372103. PubMed Central PMCID: PMCPMC7783616. Epub 2020/12/30.Search in Google Scholar PubMed PubMed Central
[49] Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: A Review. Jama. 2023;329(18):1589–602. 10.1001/jama.2023.5997. PubMed PMID: 37159031. Epub 2023/05/09.Search in Google Scholar PubMed
[50] Ma HD, Deng YR, Tian Z, Lian ZX. Traditional Chinese medicine and immune regulation. Clin Rev Allergy Immunol. 2013;44(3):229–41. 10.1007/s12016-012-8332-0. PubMed PMID: 22826112. Epub 2012/07/25.Search in Google Scholar PubMed
[51] Xie Z, Qiang J, Pi X, Wang J, Chen Y, Yu Q, et al. Favorable outcome of adjunctive traditional Chinese medicine therapy in liver cirrhosis: A large cohort study in Southwest China. Complement Ther Med. 2020;51:102446. 10.1016/j.ctim.2020.102446. PubMed PMID: 32507445. Epub 2020/06/09.Search in Google Scholar PubMed
[52] Che Q, Luo T, Shi J, He Y, Xu DL. Mechanisms by which traditional Chinese medicines influence the intestinal flora and intestinal barrier. Front Cell Infect Microbiol. 2022;12:863779. 10.3389/fcimb.2022.863779. PubMed PMID: 35573786. PubMed Central PMCID: PMCPMC9097517. Epub 2022/05/17.Search in Google Scholar PubMed PubMed Central
[53] Li H. Advances in anti hepatic fibrotic therapy with traditional Chinese medicine herbal formula. J Ethnopharmacol. 2020;251:112442. 10.1016/j.jep.2019.112442. PubMed PMID: 31891799. Epub 2020/01/01.Search in Google Scholar PubMed
[54] Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, et al. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol. 2003;10(2):259–66. 10.1128/cdli.10.2.259-266.2003. PubMed PMID: 12626452. PubMed Central PMCID: PMCPMC150522. Epub 2003/03/11.Search in Google Scholar PubMed PubMed Central
[55] Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. 2007;150(3):531–8. 10.1111/j.1365-2249.2007.03522.x. PubMed PMID: 17956582. PubMed Central PMCID: PMCPMC2219384. Epub 2007/10/25.Search in Google Scholar PubMed PubMed Central
[56] Turroni F, Serafini F, Foroni E, Duranti S, O’Connell Motherway M, Taverniti V, et al. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium-host interactions. Proc Natl Acad Sci U S A. 2013;110(27):11151–6. 10.1073/pnas.1303897110. PubMed PMID: 23776216. PubMed Central PMCID: PMCPMC3703987. Epub 2013/06/19.Search in Google Scholar PubMed PubMed Central
[57] Schiavi E, Gleinser M, Molloy E, Groeger D, Frei R, Ferstl R, et al. The surface-associated exopolysaccharide of bifidobacterium longum 35624 plays an essential role in dampening host proinflammatory responses and repressing local TH17 Responses. Appl Env Microbiol. 2016;82(24):7185–96. 10.1128/aem.02238-16. PubMed PMID: 27736791. PubMed Central PMCID: PMCPMC5118929. Epub 2016/11/01.Search in Google Scholar
[58] von Ossowski I, Pietilä TE, Rintahaka J, Nummenmaa E, Mäkinen VM, Reunanen J, et al. Using recombinant Lactococci as an approach to dissect the immunomodulating capacity of surface piliation in probiotic Lactobacillus rhamnosus GG. PLoS One. 2013;8(5):e64416. 10.1371/journal.pone.0064416. PubMed PMID: 23691212. PubMed Central PMCID: PMCPMC3653913. Epub 2013/05/22.Search in Google Scholar PubMed PubMed Central
[59] Allam-Ndoul B, Castonguay-Paradis S, Veilleux A. Gut microbiota and intestinal trans-epithelial permeability. Int J Mol Sci. 2020;21(17):6402. 10.3390/ijms21176402. PubMed PMID: 32899147. PubMed Central PMCID: PMCPMC7503654. Epub 2020/09/10.Search in Google Scholar PubMed PubMed Central
[60] Shi N, Li N, Duan X, Niu H. Interaction between the gut microbiome and mucosal immune system. Mil Med Res. 2017;4:14. 10.1186/s40779-017-0122-9. PubMed PMID: 28465831. PubMed Central PMCID: PMCPMC5408367. Epub 2017/05/04.Search in Google Scholar PubMed PubMed Central
[61] Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18(5):479–97. 10.1046/j.1440-1746.2003.03032.xPubMed PMID: 12702039. Epub 2003/04/19.Search in Google Scholar PubMed
[62] Stange EF. Improvement of a ‘Leaky’ intestinal barrier. Dig Dis. 2017;35(1–2):21–4. 10.1159/000449078. PubMed PMID: 28147355. Epub 2017/02/02.Search in Google Scholar PubMed
[63] Philips CA, Augustine P. Gut barrier and microbiota in Cirrhosis. J Clin Exp Hepatol. 2022;12(2):625–38. 10.1016/j.jceh.2021.08.027. PubMed PMID: 35535069. PubMed Central PMCID: PMCPMC9077238. Epub 2022/05/11.Search in Google Scholar PubMed PubMed Central
[64] Nami Y, Haghshenas B, Javanmard A, Samari M, Mohammadi N, Oroojalian F, et al. A critical review of the recent concept of artificial mechanical uterus design in relation to the maternal microbiome: An Update to past researches. J Reprod Immunol. 2023;156:103828. 10.1016/j.jri.2023.103828. PubMed PMID: 36796148. Epub 2023/02/17.Search in Google Scholar PubMed
[65] Dickinson B, Surawicz CM. Infectious diarrhea: an overview. Curr Gastroenterol Rep. 2014;16(8):399. 10.1007/s11894-014-0399-8. PubMed PMID: 25064318. Epub 2014/07/30.Search in Google Scholar PubMed
[66] Ricci A, Tagliacarne SC, Valsecchi C, Boggini T, Cattaneo F, Licari A, et al. Probiotics and inflammatory bowel diseases. J Biol Regul Homeost Agents. 2015;29(2 Suppl 1):96–113. Epub 2015/12/05. PubMed PMID: 26634595.Search in Google Scholar
[67] Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol. 2021;12:578386. 10.3389/fimmu.2021.578386. PubMed PMID: 33717063. PubMed Central PMCID: PMCPMC7953067. Epub 2021/03/16.Search in Google Scholar PubMed PubMed Central
[68] Peng M, Tabashsum Z, Anderson M, Truong A, Houser AK, Padilla J, et al. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr Rev Food Sci Food Saf. 2020;19(4):1908–33. 10.1111/1541-4337.12565. PubMed PMID: 33337097. Epub 2020/12/19.Search in Google Scholar PubMed
[69] Jensen GS, Benson KF, Carter SG, Endres JR. GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 2010;11:15. 10.1186/1471-2172-11-15. PubMed PMID: 20331905. PubMed Central PMCID: PMCPMC2858026. Epub 2010/03/25.Search in Google Scholar PubMed PubMed Central
[70] Hoarau C, Martin L, Faugaret D, Baron C, Dauba A, Aubert-Jacquin C, et al. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One. 2008;3(7):e2753. 10.1371/journal.pone.0002753. PubMed PMID: 18648505. PubMed Central PMCID: PMCPMC2447180. Epub 2008/07/24.Search in Google Scholar PubMed PubMed Central
[71] Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354(9193):1896–900. 10.1016/s0140-6736(99)04149-5. PubMed PMID: 10584742. Epub 1999/12/10.Search in Google Scholar PubMed
[72] Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–61. 10.1016/s0140-6736(14)60121-5. PubMed PMID: 24480518. Epub 2014/02/01.Search in Google Scholar
[73] Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, et al. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019;7(1):14. 10.3390/microorganisms7010014. PubMed PMID: 30634578. PubMed Central PMCID: PMCPMC6351938. Epub 2019/01/13.Search in Google Scholar PubMed PubMed Central
[74] Fukui H. Gut microbiome-based therapeutics in liver cirrhosis: basic consideration for the next step. J Clin Transl Hepatol. 2017;5(3):249–60. 10.14218/jcth.2017.00008. PubMed PMID: 28936406. PubMed Central PMCID: PMCPMC5606971. Epub 2017/09/25.Search in Google Scholar
[75] Chen Y, Yang F, Lu H, Wang B, Chen Y, Lei D, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54(2):562–72. 10.1002/hep.24423. PubMed PMID: 21574172. Epub 2011/05/17.Search in Google Scholar PubMed
[76] Lu H, Wu Z, Xu W, Yang J, Chen Y, Li L. Intestinal microbiota was assessed in cirrhotic patients with hepatitis B virus infection. Intestinal microbiota of HBV cirrhotic patients. Microb Ecol. 2011;61(3):693–703. 10.1007/s00248-010-9801-8. PubMed PMID: 21286703. Epub 2011/02/03.Search in Google Scholar PubMed
[77] Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci Rep. 2017;7(1):2594. 10.1038/s41598-017-02995-4. PubMed PMID: 28572676. PubMed Central PMCID: PMCPMC5453967. Epub 2017/06/03.Search in Google Scholar PubMed PubMed Central
[78] Xu M, Wang B, Fu Y, Chen Y, Yang F, Lu H, et al. Changes of fecal Bifidobacterium species in adult patients with hepatitis B virus-induced chronic liver disease. Microb Ecol. 2012;63(2):304–13. 10.1007/s00248-011-9925-5. PubMed PMID: 21814872. Epub 2011/08/05.Search in Google Scholar PubMed
[79] Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. 10.1038/nature13568PubMed PMID: 25079328. Epub 2014/08/01.Search in Google Scholar PubMed
[80] Chen P, Stärkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61(3):883–94. 10.1002/hep.27489PubMed PMID: 25251280. PubMed Central PMCID: PMCPMC4340725. Epub 2014/09/25.Search in Google Scholar PubMed PubMed Central
[81] Quigley EM, Stanton C, Murphy EF. The gut microbiota and the liver. Pathophysiological and clinical implications. J Hepatol. 2013;58(5):1020–7. 10.1016/j.jhep.2012.11.023PubMed PMID: 23183530. Epub 2012/11/28.Search in Google Scholar PubMed
[82] Fukui H. Leaky gut and gut-liver axis in liver cirrhosis: clinical studies update. Gut Liver. 2021;15(5):666–76. 10.5009/gnl20032. PubMed PMID: 33071239. PubMed Central PMCID: PMCPMC8444108. Epub 2020/10/20.Search in Google Scholar PubMed PubMed Central
[83] Fukui H. Role of gut dysbiosis in liver diseases: what have we learned so far? Diseases. 2019;7(4):31–9. 10.3390/diseases7040058. PubMed PMID: 31726747. PubMed Central PMCID: PMCPMC6956030. Epub 2019/11/16.Search in Google Scholar PubMed PubMed Central
[84] Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33(1):31–9. 10.1111/liv.12021. PubMed PMID: 23121656. Epub 2012/11/06.Search in Google Scholar PubMed
[85] Fukui H. Gut-liver axis in liver cirrhosis: How to manage leaky gut and endotoxemia. World J Hepatol. 2015;7(3):425–42. 10.4254/wjh.v7.i3.425. PubMed PMID: 25848468. PubMed Central PMCID: PMCPMC4381167. Epub 2015/04/08.Search in Google Scholar PubMed PubMed Central
[86] Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obes (Silver Spring). 2010;18(1):190–5. 10.1038/oby.2009.167. PubMed PMID: 19498350. Epub 2009/06/06.Search in Google Scholar PubMed
[87] Ohira H, Tsutsui W, Fujioka Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J Atheroscler Thromb. 2017;24(7):660–72. 10.5551/jat.RV17006. PubMed PMID: 28552897. PubMed Central PMCID: PMCPMC5517538. Epub 2017/05/30.Search in Google Scholar PubMed PubMed Central
[88] Wang Z, Zhang X, Zhu L, Yang X, He F, Wang T, et al. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int Immunopharmacol. 2020;78:106062. 10.1016/j.intimp.2019.106062. PubMed PMID: 31830621. Epub 2019/12/13.Search in Google Scholar PubMed
[89] Baltazar-Díaz TA, González-Hernández LA, Aldana-Ledesma JM, Peña-Rodríguez M, Vega-Magaña AN, Zepeda-Morales ASM, et al. Escherichia/Shigella, SCFAs, and Metabolic Pathways-The Triad That Orchestrates Intestinal Dysbiosis in Patients with Decompensated Alcoholic Cirrhosis from Western Mexico. Microorganisms. 2022;10(6):1231. 10.3390/microorganisms10061231. PubMed PMID: 35744749. PubMed Central PMCID: PMCPMC9229093. Epub 2022/06/25.Search in Google Scholar PubMed PubMed Central
[90] Stanimirov B, Stankov K, Mikov M. Bile acid signaling through farnesoid X and TGR5 receptors in hepatobiliary and intestinal diseases. Hepatobiliary Pancreat Dis Int. 2015;14(1):18–33. 10.1016/s1499-3872(14)60307-6. PubMed PMID: 25655287. Epub 2015/02/07.Search in Google Scholar PubMed
[91] Park JW, Kim SE, Lee NY, Kim JH, Jung JH, Jang MK, et al. Role of microbiota-derived metabolites in alcoholic and non-alcoholic fatty liver diseases. Int J Mol Sci. 2021;23(1):426. 10.3390/ijms23010426. PubMed PMID: 35008852. PubMed Central PMCID: PMCPMC8745242. Epub 2022/01/12.Search in Google Scholar PubMed PubMed Central
[92] Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut. Dig Dis. 2015;33(3):338–45. 10.1159/000371678. PubMed PMID: 26045267. PubMed Central PMCID: PMCPMC4470395. Epub 2015/06/06.Search in Google Scholar PubMed PubMed Central
[93] Chiang JYL, Ferrell JM. Bile acids as metabolic regulators and nutrient sensors. Annu Rev Nutr. 2019;39:175–200. 10.1146/annurev-nutr-082018-124344. PubMed PMID: 31018107. PubMed Central PMCID: PMCPMC6996089. Epub 2019/04/25.Search in Google Scholar PubMed PubMed Central
[94] Balazs I, Horvath A, Leber B, Feldbacher N, Sattler W, Rainer F, et al. Serum bile acids in liver cirrhosis promote neutrophil dysfunction. Clin Transl Med. 2022;12(2):e735. 10.1002/ctm2.735. PubMed PMID: 35220689. PubMed Central PMCID: PMCPMC8882235. Epub 2022/02/28.Search in Google Scholar PubMed PubMed Central
[95] Sorribas M, Jakob MO, Yilmaz B, Li H, Stutz D, Noser Y, et al. FXR modulates the gut-vascular barrier by regulating the entry sites for bacterial translocation in experimental cirrhosis. J Hepatol. 2019;71(6):1126–40. 10.1016/j.jhep.2019.06.017. PubMed PMID: 31295531. Epub 2019/07/12.Search in Google Scholar PubMed
[96] Ali RO, Quinn GM, Umarova R, Haddad JA, Zhang GY, Townsend EC, et al. Longitudinal multi-omics analyses of the gut-liver axis reveals metabolic dysregulation in hepatitis C infection and cirrhosis. Nat Microbiol. 2023;8(1):12–27. 10.1038/s41564-022-01273-y. PubMed PMID: 36522461. Epub 2022/12/16.Search in Google Scholar PubMed
[97] Newman KL, Kamada N. The gut-liver axis in HCV infection. Nat Microbiol. 2023;8(1):6–7. 10.1038/s41564-022-01277-8. PubMed PMID: 36522460. Epub 2022/12/16.Search in Google Scholar PubMed
[98] Li YG, Yu ZJ, Li A, Ren ZG. Gut microbiota alteration and modulation in hepatitis B virus-related fibrosis and complications: Molecular mechanisms and therapeutic inventions. World J Gastroenterol. 2022;28(28):3555–72. 10.3748/wjg.v28.i28.3555. PubMed PMID: 36161048. PubMed Central PMCID: PMCPMC9372803. Epub 2022/09/27.Search in Google Scholar PubMed PubMed Central
[99] Thilakarathna W, Rupasinghe HPV, Ridgway ND. Mechanisms by which probiotic bacteria attenuate the risk of hepatocellular carcinoma. Int J Mol Sci. 2021;22(5):2606. 10.3390/ijms22052606. PubMed PMID: 33807605. PubMed Central PMCID: PMCPMC7961993. Epub 2021/04/04.Search in Google Scholar PubMed PubMed Central
[100] Lee DK, Kang JY, Shin HS, Park IH, Ha NJ. Antiviral activity of Bifidobacterium adolescentis SPM0212 against Hepatitis B virus. Arch Pharm Res. 2013;36(12):1525–32. 10.1007/s12272-013-0141-3. PubMed PMID: 23657805. Epub 2013/05/10.Search in Google Scholar PubMed
[101] El-Adawi H, Nour I, Fattouh FA, Deeb NME. Investigation of the antiviral bioactivity of lactobacillus bulgaricus 761N extracellular extract against hepatitis C virus (HCV). Int J Pharmacology. 2015;11:19–26.10.3923/ijp.2015.19.26Search in Google Scholar
[102] Shi K, Zhang Q, Zhang Y, Bi Y, Zeng X, Wang X. Association between probiotic therapy and the risk of hepatocellular carcinoma in patients with hepatitis B-related cirrhosis. Front Cell Infect Microbiol. 2022;12:1104399. 10.3389/fcimb.2022.1104399. PubMed PMID: 36710968. PubMed Central PMCID: PMCPMC9880196. Epub 2023/01/31.Search in Google Scholar PubMed PubMed Central
[103] Feng DH, Zheng ZL, Lin Y. Analysis of resistance of probiotics to 16 kinds of antibiotics. Chin J Microecology. 2021;33:1176. 10.13381/j.cnki.cjm.202110011.Search in Google Scholar
[104] Zheng YJ, Huang ZH, Liu ZY, Wang WJ, Cheng Q, editors. Expert Consensus on pediatric Application of microecological agents (October 2010). The academic conference of the Microecology Professional Committee of Henan Provincial Preventive Medical Association and the founding conference of the microecology Professional Committee of Henan Provincial Microbiology Society; 2011.Search in Google Scholar
[105] Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. Jama. 2012;307(18):1959–69. 10.1001/jama.2012.3507. PubMed PMID: 22570464. Epub 2012/05/10.Search in Google Scholar PubMed
[106] Quigley EMM. Prebiotics and probiotics in digestive health clin gastroenterol hepatol. 2019;17(2):333–44. 10.1016/j.cgh.2018.09.028. PubMed PMID: 30267869. Epub 2018/09/30.Search in Google Scholar PubMed
[107] Romano-Keeler J, Weitkamp JH. Maternal influences on fetal microbial colonization and immune development. Pediatr Res. 2015;77(1-2):189–95. 10.1038/pr.2014.163. PubMed PMID: 25310759. PubMed Central PMCID: PMCPMC4289016. Epub 2014/10/14.Search in Google Scholar PubMed PubMed Central
[108] Lukasik J, Dierikx T, Besseling-van der Vaart I, de Meij T, Szajewska H. Multispecies probiotic for the prevention of antibiotic-associated diarrhea in children: a randomized clinical trial. JAMA Pediatr. 2022;176(9):860–6. 10.1001/jamapediatrics.2022.1973. PubMed PMID: 35727573. PubMed Central PMCID: PMCPMC9214631 Winclove Probiotics B.V. Dr Dierikx reported grants and nonfinancial support from Winclove Probiotics B.V. Dr de Meij reported grants and nonfinancial support from Winclove B.V. Dr Szajewska reported nonfinancial support from Winclove B.V. Epub 2022/06/22.Search in Google Scholar PubMed PubMed Central
[109] Mekonnen SA, Merenstein D, Fraser CM, Marco ML. Molecular mechanisms of probiotic prevention of antibiotic-associated diarrhea. Curr Opin Biotechnol. 2020;61:226–34. 10.1016/j.copbio.2020.01.005. PubMed PMID: 32087535. PubMed Central PMCID: PMCPMC7096272. Epub 2020/02/23.Search in Google Scholar PubMed PubMed Central
[110] Ji J, Yang H. Using probiotics as supplementation for helicobacter pylori antibiotic therapy. Int J Mol Sci. 2020;21(3):1136. 10.3390/ijms21031136. PubMed PMID: 32046317. PubMed Central PMCID: PMCPMC7037652. Epub 2020/02/13.Search in Google Scholar PubMed PubMed Central
[111] Aghamohammad S, Rohani M. Antibiotic resistance and the alternatives to conventional antibiotics: The role of probiotics and microbiota in combating antimicrobial resistance. Microbiol Res. 2023;267:127275. 10.1016/j.micres.2022.127275. PubMed PMID: 36493661. Epub 2022/12/10.Search in Google Scholar PubMed
[112] Cohen PA. Probiotic safety-no guarantees. JAMA Intern Med. 2018;178(12):1577–8. 10.1001/jamainternmed.2018.5403. PubMed PMID: 30242393. Epub 2018/09/23.Search in Google Scholar PubMed
[113] Freedman SB, Schnadower D, Tarr PI. The probiotic conundrum: regulatory confusion, conflicting studies, and safety concerns. Jama. 2020;323(9):823–4. 10.1001/jama.2019.22268PubMed PMID: 32125410. Epub 2020/03/04.Search in Google Scholar PubMed
[114] Zawistowska-Rojek A, Tyski S. Are probiotic really safe for humans? Pol J Microbiol. 2018;67(3):251–8. 10.21307/pjm-2018-044. PubMed PMID: 30451441. PubMed Central PMCID: PMCPMC7256845. Epub 2018/11/20.Search in Google Scholar PubMed PubMed Central
[115] Roe AL, Boyte ME, Elkins CA, Goldman VS, Heimbach J, Madden E, et al. Considerations for determining safety of probiotics: A USP perspective. Regul Toxicol Pharmacol. 2022;136:105266. 10.1016/j.yrtph.2022.105266. PubMed PMID: 36206977. PubMed Central PMCID: PMCPMC10292223. Epub 2022/10/08.Search in Google Scholar PubMed PubMed Central
[116] Zoppi G, Cinquetti M, Benini A, Bonamini E, Minelli EB. Modulation of the intestinal ecosystem by probiotics and lactulose in children during treatment with ceftriaxone. Curr Therapeutic Res. 2001;62:418–35.10.1016/S0011-393X(01)89006-8Search in Google Scholar
[117] Wang ZJ, Chen XF, Zhang ZX, Li YC, Deng J, Tu J, et al. Effects of anti-Helicobacter pylori concomitant therapy and probiotic supplementation on the throat and gut microbiota in humans. Microb Pathog. 2017;109:156–61. 10.1016/j.micpath.2017.05.035. PubMed PMID: 28552806. Epub 2017/05/30.Search in Google Scholar PubMed
[118] Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, Puri P, Sterling RK, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther. 2014;39(10):1113–25. 10.1111/apt.12695PubMed PMID: 24628464. PubMed Central PMCID: PMCPMC3989370. Epub 2014/03/19.Search in Google Scholar PubMed PubMed Central
[119] Dhiman RK, Rana B, Agrawal S, Garg A, Chopra M, Thumburu KK, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology. 2014;147(6):1327–37 e3. 10.1053/j.gastro.2014.08.031. PubMed PMID: 25450083. Epub 2014/12/03.Search in Google Scholar PubMed
[120] Shi D, Lv L, Fang D, Wu W, Hu C, Xu L, et al. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 prevents CCl(4)-induced liver cirrhosis by protecting the intestinal barrier in rats. Sci Rep. 2017;7(1):6927. 10.1038/s41598-017-07091-1. PubMed PMID: 28761060. PubMed Central PMCID: PMCPMC5537250. Epub 2017/08/02.Search in Google Scholar PubMed PubMed Central
[121] Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015;21(6):1691–702. 10.3748/wjg.v21.i6.1691. PubMed PMID: 25684933. PubMed Central PMCID: PMCPMC4323444. Epub 2015/02/17.Search in Google Scholar PubMed PubMed Central
[122] Wang JW, Kuo CH, Kuo FC, Wang YK, Hsu WH, Yu FJ, et al. Fecal microbiota transplantation: Review and update. J Formos Med Assoc. 2019;118(Suppl 1):S23–31. Epub 2018/09/06. 10.1016/j.jfma.2018.08.011. PubMed PMID: 30181015.Search in Google Scholar PubMed
[123] Zhang F, Cui B, He X, Nie Y, Wu K, Fan D. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9(5):462–73. 10.1007/s13238-018-0541-8. PubMed PMID: 29691757. PubMed Central PMCID: PMCPMC5960466. Epub 2018/04/25.Search in Google Scholar PubMed PubMed Central
[124] Wu J, Wei Z, Cheng P, Qian C, Xu F, Yang Y, et al. Rhein modulates host purine metabolism in intestine through gut microbiota and ameliorates experimental colitis. Theranostics. 2020;10(23):10665–79. 10.7150/thno.43528. PubMed PMID: 32929373. PubMed Central PMCID: PMCPMC7482825. Epub 2020/09/16.Search in Google Scholar PubMed PubMed Central
[125] Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes. 2017;8(3):238–52. 10.1080/19490976.2017.1290757. PubMed PMID: 28609251. PubMed Central PMCID: PMCPMC5479396. Epub 2017/06/14.Search in Google Scholar PubMed PubMed Central
[126] Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, et al. Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology. 2021;160(1):145–57 e8. 10.1053/j.gastro.2020.07.013. PubMed PMID: 32681922. Epub 2020/07/19.Search in Google Scholar PubMed
[127] Hvas CL, Dahl Jørgensen SM, Jørgensen SP, Storgaard M, Lemming L, Hansen MM, et al. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent clostridium difficile infection. Gastroenterology. 2019;156(5):1324–32.e3. 10.1053/j.gastro.2018.12.019. PubMed PMID: 30610862. Epub 2019/01/06.Search in Google Scholar PubMed
[128] van Prehn J, Reigadas E, Vogelzang EH, Bouza E, Hristea A, Guery B, et al. European society of clinical microbiology and infectious diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. 2021;27(Suppl 2):S1–21. 10.1016/j.cmi.2021.09.038. PubMed PMID: 34678515. Epub 2021/10/23.Search in Google Scholar PubMed
[129] Vendrik KEW, Ooijevaar RE, de Jong PRC, Laman JD, van Oosten BW, van Hilten JJ, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. 10.3389/fcimb.2020.00098. PubMed PMID: 32266160. PubMed Central PMCID: PMCPMC7105733. Epub 2020/04/09.Search in Google Scholar PubMed PubMed Central
[130] de Groot P, Nikolic T, Pellegrini S, Sordi V, Imangaliyev S, Rampanelli E, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut. 2021;70(1):92–105. Epub 2020/10/28. 10.1136/gutjnl-2020-322630. PubMed PMID: 33106354. PubMed Central PMCID: PMCPMC7788262.Search in Google Scholar PubMed PubMed Central
[131] Zhou Z, Sun B, Yu D, Zhu C. Gut microbiota: an important player in type 2 diabetes mellitus. Front Cell Infect Microbiol. 2022;12:834485. 10.3389/fcimb.2022.834485. PubMed PMID: 35242721. PubMed Central PMCID: PMCPMC8886906. Epub 2022/03/05.Search in Google Scholar PubMed PubMed Central
[132] Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Torres Soto M, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17(3):e1003051. 10.1371/journal.pmed.1003051. PubMed PMID: 32150549. PubMed Central PMCID: PMCPMC7062239. Epub 2020/03/10.Search in Google Scholar PubMed PubMed Central
[133] Bloom PP, Tapper EB, Young VB, Lok AS. Microbiome therapeutics for hepatic encephalopathy. J Hepatol. 2021;75(6):1452–64. 10.1016/j.jhep.2021.08.004. PubMed PMID: 34453966. Epub 2021/08/29.Search in Google Scholar PubMed PubMed Central
[134] Antushevich H. Fecal microbiota transplantation in disease therapy. Clin Chim Acta. 2020;503:90–8. 10.1016/j.cca.2019.12.010. PubMed PMID: 31968211. Epub 2020/01/23.Search in Google Scholar PubMed
[135] Bajaj JS, Kassam Z, Fagan A, Gavis EA, Liu E, Cox IJ, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology. 2017;66(6):1727–38. 10.1002/hep.29306. PubMed PMID: 28586116. PubMed Central PMCID: PMCPMC6102730. Epub 2017/06/07.Search in Google Scholar PubMed PubMed Central
[136] Bajaj JS, Salzman NH, Acharya C, Sterling RK, White MB, Gavis EA, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. 2019;70(5):1690–703. 10.1002/hep.30690. PubMed PMID: 31038755. PubMed Central PMCID: PMCPMC6819208. Epub 2019/05/01.Search in Google Scholar PubMed PubMed Central
[137] Pan X, Wen SW, Kaminga AC, Liu A. Gut metabolites and inflammation factors in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Sci Rep. 2020;10(1):8848. 10.1038/s41598-020-65051-8. PubMed PMID: 32483129. PubMed Central PMCID: PMCPMC7264254. Epub 2020/06/03.Search in Google Scholar PubMed PubMed Central
[138] Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol. 2021;75(Suppl 1):S67–81. 10.1016/j.jhep.2020.11.013. PubMed PMID: 34039493. PubMed Central PMCID: PMCPMC8973011. Epub 2021/05/28.Search in Google Scholar PubMed PubMed Central
[139] Holte K, Krag A, Gluud LL. Systematic review and meta-analysis of randomized trials on probiotics for hepatic encephalopathy. Hepatol Res. 2012;42(10):1008–15. 10.1111/j.1872-034X.2012.01015.x. PubMed PMID: 22548675. Epub 2012/05/03.Search in Google Scholar PubMed
[140] Zhao LN, Yu T, Lan SY, Hou JT, Zhang ZZ, Wang SS, et al. Probiotics can improve the clinical outcomes of hepatic encephalopathy: An update meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39(6):674–82. 10.1016/j.clinre.2015.03.008. PubMed PMID: 25956487. Epub 2015/05/10.Search in Google Scholar PubMed
[141] Khan MY, Mihali AB, Rawala MS, Aslam A, Siddiqui WJ. The promising role of probiotic and synbiotic therapy in aminotransferase levels and inflammatory markers in patients with nonalcoholic fatty liver disease – a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2019;31(6):703–15. 10.1097/meg.0000000000001371. PubMed PMID: 31009401. Epub 2019/04/23.Search in Google Scholar
[142] Bajaj JS, Saeian K, Christensen KM, Hafeezullah M, Varma RR, Franco J, et al. Probiotic yogurt for the treatment of minimal hepatic encephalopathy. Am J Gastroenterology. 2008;103(7):1707–15. 10.1111/j.1572-0241.2008.01861.x. PubMed PMID: WOS:000257693900018.Search in Google Scholar PubMed
[143] Pereg D, Kotliroff A, Gadoth N, Hadary R, Lishner M, Kitay-Cohen Y. Probiotics for patients with compensated liver cirrhosis: a double-blind placebo-controlled study. Nutr (Burbank, Los Angeles County, Calif). 2011;27(2):177–81. 10.1016/j.nut.2010.01.006. PubMed PMID: CN-00781168.Search in Google Scholar PubMed
[144] Han SH, Suk KT, Kim DJ, Kim MY, Baik SK, Kim YD, et al. Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur J Gastroenterol Hepatol. 2015;27(11):1300–6. 10.1097/meg.0000000000000458. PubMed PMID: WOS:000369597500010.Search in Google Scholar PubMed
[145] Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57(2):545–53. 10.1007/s10620-011-1887-4. PubMed PMID: 21901256. Epub 2011/09/09.Search in Google Scholar PubMed
[146] Feng T, Liang S, Cai S, Cai Y, Li W. Clinical study on the application value of microecological preparation combined with Mosapride in the prevention of bleeding from esophageal and gastric varices in cirrhosis. Guide China Med. 2010;22(5):297–9 + 304.Search in Google Scholar
[147] Huang Y, Zhou L, Zhang Y. Effect of Fluoroquinolones Combined with Microecological Agent on Plasma Endotoxin and Levels of Some Cytokines in Liver Cirrohsis Patients. J Guizhou Med Univ. 2010;35(3):278–9 + 85. 10.19367/j.cnki.1000-2707.2010.03.021.Search in Google Scholar
[148] Pan X, Huang Y. Effects of bifidobacterium capsules on plasma endotoxin and inflammatory factors in patients with cirrhosis. Chinese Remedies &. Clinics. 2011;11(9):1056–7.Search in Google Scholar
[149] Wang S, Lin L, Guo M, Deng J. Effect of four-probiotics on the serum cytokines in peripheral blood of pa tients with liver cirrhosis. China Mod Dr. 2012;50(14):67–8.Search in Google Scholar
[150] Wang M, Li J. The effect of compound glutamine and probiotics on intestinal barrier in decompensated hepatitic cirrhosis patients. Chin J Clin Gastroenterol. 2013;25(1):39–41.Search in Google Scholar
[151] Lu Q. The effect of compound glutamine and probiotics on intestinal barrier in decompensated hepatitic cirrhosis patients. J Henan Med Coll. 2014;26(02):169–70.Search in Google Scholar
[152] Ye Y, Xiang Y. Influence and curative effect observation of bifid triple viable capsules on plasma inflammatory cytokines of patients with liver cirrhosis spontaneous bacterial peritonitis (SBP). China Mod Dr. 2014;52(31):40–2.Search in Google Scholar
[153] Zheng S, Wang C, Zhan G. Efficacy of microbioecologics in hepatic cirrhosis patients with spontaneous bacterial peritonitis. Chin J Microecol. 2014;26(1):62–5. 10.13381/j.cnki.cjm.201401016.Search in Google Scholar
[154] Hao H, Chen M, Ding L. Effect of probiotics combined with compound glutamine on the prevention of spontaneous bacterial peritonitis in patients with decompensated cirrhosis. J Med Res. 2014;43(11):25–8.Search in Google Scholar
[155] Zhang S. Curative effects of probiotics combined with adefovir dipivoxil in treatment of HBV-related cirrhosis and the effects on immune function. Chin J Liver Diseases (Electronic Version). 2016;8(02):44–7.Search in Google Scholar
[156] Jiang Q, Xie Q. Clinical effect of assistant intake of probiotics on patients with decompensation stage of HBV-related hepatic cirrhosis. China Med Her. 2016;13(20):111–4.Search in Google Scholar
[157] Li Z, Xu D, Han X. Effect of microecological preparation combined with fluoroquinolone on improving immune function in patients with posthepatitis B cirrhosis. International Journal of Digestive Diseases. 2017;37(4):246–8.Search in Google Scholar
[158] Han Y, Bai Y. Effects of microecological preparations on serum inflammatory factors, intestinal barrier function and liver function in patients with cirrhosis and spontaneous peritonitis. Shaanxi Med J. 2017;46(4):537–8.Search in Google Scholar
[159] Zheng D, Luo Y, Jiang K, Ma Y, Yao Q. Clinical efficacy of piperacillin/tazobatam combined with probiotics in treatment of cirrhosis complicated with spontaneous bacterial peritonitics and its effects on liver function and inflammatory cytokines. Chin J Nosocomiology. 2018;28(23):3544–8.Search in Google Scholar
[160] Yue G, Fang H. Effect of intestinal flora regulation on plasma endotoxin and liver function in patients with cirrhosis. Chin Hepatology. 2018;23(12):1096–9. 10.14000/j.cnki.issn.1008-1704.2018.12.016.Search in Google Scholar
[161] Zeng C, Hu A, Hu Y, Yuan G, Zhu D, Shi X. Clinical efficacy of Entecavir combined with microecological preparation in the treatment of Hepatitis B cirrhosis. Chin J Clin Pharmacol Ther. 2018;23(7):796–801.Search in Google Scholar
[162] Liang R, Chen Y. Analysis of Entecavir combined with microecological preparation in the treatment of hepatitis B cirrhosis. Heilongjiang Med J. 2018;42(1):61–2.Search in Google Scholar
[163] Wang X, Wu S. Clinical study on the treatment of decompensated hepatitis B cirrhosis with microecological preparation combined with nucleoside (acid) analogues. J Bethune Med Sci. 2019;17(04):339–40. 10.16485/j.issn.2095-7858.2019.04.010.Search in Google Scholar
[164] Li Y, Li Y, Liu P, Li B, Chang Z. Effect of adefovir dipivoxil, lamivudine combined with microecological preparations on hepatitis B cirrhosis and its effect on serum sICAM-1 and plasma PKM2 levels. Hainan Med J. 2019;30(20):2612–5.Search in Google Scholar
[165] Duan H. Effects of Microecologics combined with Antiviral Therapy on Immune Function and Liver Fibrosis Indexes in Patients with Hepatitis B Cirrhosis. Hebei Med. 2019;25(11):1861–5.Search in Google Scholar
[166] Bian J, Wang L, Wan X, Weng H, Lin H, Chen S. Effect of probiotics on intestinal flora and immune function in patients with hepatitis B associated cirrhosis complicated with liver failure secondary infection. Chin J Control Endemic Dis. 2019;34(3):335–7.Search in Google Scholar
[167] Zhao W, Zhao T. Clinical study of probiotics in adjuvant treatment of bacterial peritonitis secondary to cirrhosis. China Mod Dr. 2020;58(4):101–3.Search in Google Scholar
[168] Liu S, Li X. Effects of probiotics on intestinal flora and intestinal barrier in patients with cirrhosis. Chin J Microecology. 2021;33(3):326–30. 10.13381/j.cnki.cjm.202103015.Search in Google Scholar
[169] Wu C, Guo F, Su G. Influence of probiotics on the immune function and intestinal flora of pa- tients with chronic hepatitis B cirrhosis. J North Sichuan Med Coll. 2021;36(1):83–6.Search in Google Scholar
[170] Zhu F, Chen M, Li M. Clinical efficacy of probiotics combined with glutamine in the treatment of cirrhosis and its effect on liver function. Chin J Clin Ration Drug Use. 2022;15(18):103–5. 10.15887/j.cnki.13-1389/r.2022.18.031.Search in Google Scholar
[171] Li Q, Liu J, Sun J, Fan M, Yao J. Effects of probiotics yogurt combined with enteral nutrition as adjuvant treatment in patients with liver fibrosis. Chin J Microecol. 2022;34(4):434–9. 10.13381/j.cnki.cjm.202204011.Search in Google Scholar
[172] Zhu Y, Yang F, Meng L, Yang L, Li W, Guo X, et al. Effect Observation of Live Combined Bacillus Subtilis and Entercoccus Faecium Enteric-Coated Capsules on Liver Cirrhosis Complicated with Upper Gastrointestinal Bleeding. Chin J Ration Drug Use. 2022;19(7):69–73.Search in Google Scholar
[173] Wang J, Jin X. Efficacy of microecological preparation on hepatocirrhosis with encephalopathy and its effect on intestinal flora. Chin J Microecol. 2022;34(1):74–7 + 90. 10.13381/j.cnki.cjm.202201014.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
- Immunosuppressive treatment for idiopathic membranous nephropathy: An updated network meta-analysis
- Identifying two pathogenic variants in a patient with pigmented paravenous retinochoroidal atrophy
- Effects of phytoestrogens combined with cold stress on sperm parameters and testicular proteomics in rats
- A case of pulmonary embolism with bad warfarin anticoagulant effects caused by E. coli infection
- Neutrophilia with subclinical Cushing’s disease: A case report and literature review
- Isoimperatorin alleviates lipopolysaccharide-induced periodontitis by downregulating ERK1/2 and NF-κB pathways
- Immunoregulation of synovial macrophages for the treatment of osteoarthritis
- Novel CPLANE1 c.8948dupT (p.P2984Tfs*7) variant in a child patient with Joubert syndrome
- Antiphospholipid antibodies and the risk of thrombosis in myeloproliferative neoplasms
- Immunological responses of septic rats to combination therapy with thymosin α1 and vitamin C
- High glucose and high lipid induced mitochondrial dysfunction in JEG-3 cells through oxidative stress
- Pharmacological inhibition of the ubiquitin-specific protease 8 effectively suppresses glioblastoma cell growth
- Levocarnitine regulates the growth of angiotensin II-induced myocardial fibrosis cells via TIMP-1
- Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study
- Single-cell transcription analysis reveals the tumor origin and heterogeneity of human bilateral renal clear cell carcinoma
- Identification of iron metabolism-related genes as diagnostic signatures in sepsis by blood transcriptomic analysis
- Long noncoding RNA ACART knockdown decreases 3T3-L1 preadipocyte proliferation and differentiation
- Surgery, adjuvant immunotherapy plus chemotherapy and radiotherapy for primary malignant melanoma of the parotid gland (PGMM): A case report
- Dosimetry comparison with helical tomotherapy, volumetric modulated arc therapy, and intensity-modulated radiotherapy for grade II gliomas: A single‑institution case series
- Soy isoflavone reduces LPS-induced acute lung injury via increasing aquaporin 1 and aquaporin 5 in rats
- Refractory hypokalemia with sexual dysplasia and infertility caused by 17α-hydroxylase deficiency and triple X syndrome: A case report
- Meta-analysis of cancer risk among end stage renal disease undergoing maintenance dialysis
- 6-Phosphogluconate dehydrogenase inhibition arrests growth and induces apoptosis in gastric cancer via AMPK activation and oxidative stress
- Experimental study on the optimization of ANM33 release in foam cells
- Primary retroperitoneal angiosarcoma: A case report
- Metabolomic analysis-identified 2-hydroxybutyric acid might be a key metabolite of severe preeclampsia
- Malignant pleural effusion diagnosis and therapy
- Effect of spaceflight on the phenotype and proteome of Escherichia coli
- Comparison of immunotherapy combined with stereotactic radiotherapy and targeted therapy for patients with brain metastases: A systemic review and meta-analysis
- Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation
- Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy
- The role of IL-31 and IL-34 in the diagnosis and treatment of chronic periodontitis
- Triple-negative mouse breast cancer initiating cells show high expression of beta1 integrin and increased malignant features
- mNGS facilitates the accurate diagnosis and antibiotic treatment of suspicious critical CNS infection in real practice: A retrospective study
- The apatinib and pemetrexed combination has antitumor and antiangiogenic effects against NSCLC
- Radiotherapy for primary thyroid adenoid cystic carcinoma
- Design and functional preliminary investigation of recombinant antigen EgG1Y162–EgG1Y162 against Echinococcus granulosus
- Effects of losartan in patients with NAFLD: A meta-analysis of randomized controlled trial
- Bibliometric analysis of METTL3: Current perspectives, highlights, and trending topics
- Performance comparison of three scaling algorithms in NMR-based metabolomics analysis
- PI3K/AKT/mTOR pathway and its related molecules participate in PROK1 silence-induced anti-tumor effects on pancreatic cancer
- The altered expression of cytoskeletal and synaptic remodeling proteins during epilepsy
- Effects of pegylated recombinant human granulocyte colony-stimulating factor on lymphocytes and white blood cells of patients with malignant tumor
- Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report
- NUDT21 relieves sevoflurane-induced neurological damage in rats by down-regulating LIMK2
- Association of interleukin-10 rs1800896, rs1800872, and interleukin-6 rs1800795 polymorphisms with squamous cell carcinoma risk: A meta-analysis
- Exosomal HBV-DNA for diagnosis and treatment monitoring of chronic hepatitis B
- Shear stress leads to the dysfunction of endothelial cells through the Cav-1-mediated KLF2/eNOS/ERK signaling pathway under physiological conditions
- Interaction between the PI3K/AKT pathway and mitochondrial autophagy in macrophages and the leukocyte count in rats with LPS-induced pulmonary infection
- Meta-analysis of the rs231775 locus polymorphism in the CTLA-4 gene and the susceptibility to Graves’ disease in children
- Cloning, subcellular localization and expression of phosphate transporter gene HvPT6 of hulless barley
- Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome
- Significant elevated CXCL14 and decreased IL-39 levels in patients with tuberculosis
- Whole-exome sequencing applications in prenatal diagnosis of fetal bowel dilatation
- Gemella morbillorum infective endocarditis: A case report and literature review
- An unusual ectopic thymoma clonal evolution analysis: A case report
- Severe cumulative skin toxicity during toripalimab combined with vemurafenib following toripalimab alone
- Detection of V. vulnificus septic shock with ARDS using mNGS
- Novel rare genetic variants of familial and sporadic pulmonary atresia identified by whole-exome sequencing
- The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology
- Novel compound heterozygous mutations in TELO2 in an infant with You-Hoover-Fong syndrome: A case report and literature review
- ctDNA as a prognostic biomarker in resectable CLM: Systematic review and meta-analysis
- Diagnosis of primary amoebic meningoencephalitis by metagenomic next-generation sequencing: A case report
- Phylogenetic analysis of promoter regions of human Dolichol kinase (DOLK) and orthologous genes using bioinformatics tools
- Collagen changes in rabbit conjunctiva after conjunctival crosslinking
- Effects of NM23 transfection of human gastric carcinoma cells in mice
- Oral nifedipine and phytosterol, intravenous nicardipine, and oral nifedipine only: Three-arm, retrospective, cohort study for management of severe preeclampsia
- Case report of hepatic retiform hemangioendothelioma: A rare tumor treated with ultrasound-guided microwave ablation
- Curcumin induces apoptosis in human hepatocellular carcinoma cells by decreasing the expression of STAT3/VEGF/HIF-1α signaling
- Rare presentation of double-clonal Waldenström macroglobulinemia with pulmonary embolism: A case report
- Giant duplication of the transverse colon in an adult: A case report and literature review
- Ectopic thyroid tissue in the breast: A case report
- SDR16C5 promotes proliferation and migration and inhibits apoptosis in pancreatic cancer
- Vaginal metastasis from breast cancer: A case report
- Screening of the best time window for MSC transplantation to treat acute myocardial infarction with SDF-1α antibody-loaded targeted ultrasonic microbubbles: An in vivo study in miniswine
- Inhibition of TAZ impairs the migration ability of melanoma cells
- Molecular complexity analysis of the diagnosis of Gitelman syndrome in China
- Effects of maternal calcium and protein intake on the development and bone metabolism of offspring mice
- Identification of winter wheat pests and diseases based on improved convolutional neural network
- Ultra-multiplex PCR technique to guide treatment of Aspergillus-infected aortic valve prostheses
- Virtual high-throughput screening: Potential inhibitors targeting aminopeptidase N (CD13) and PIKfyve for SARS-CoV-2
- Immune checkpoint inhibitors in cancer patients with COVID-19
- Utility of methylene blue mixed with autologous blood in preoperative localization of pulmonary nodules and masses
- Integrated analysis of the microbiome and transcriptome in stomach adenocarcinoma
- Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy
- DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway
- Lung abscess by Fusobacterium nucleatum and Streptococcus spp. co-infection by mNGS: A case series
- Genetic alterations of KRAS and TP53 in intrahepatic cholangiocarcinoma associated with poor prognosis
- Granulomatous polyangiitis involving the fourth ventricle: Report of a rare case and a literature review
- Studying infant mortality: A demographic analysis based on data mining models
- Metaplastic breast carcinoma with osseous differentiation: A report of a rare case and literature review
- Protein Z modulates the metastasis of lung adenocarcinoma cells
- Inhibition of pyroptosis and apoptosis by capsaicin protects against LPS-induced acute kidney injury through TRPV1/UCP2 axis in vitro
- TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats
- Primary mediastinum Ewing’s sarcoma with pleural effusion: A case report and literature review
- Association of ADRB2 gene polymorphisms and intestinal microbiota in Chinese Han adolescents
- Tanshinone IIA alleviates chondrocyte apoptosis and extracellular matrix degeneration by inhibiting ferroptosis
- Study on the cytokines related to SARS-Cov-2 in testicular cells and the interaction network between cells based on scRNA-seq data
- Effect of periostin on bone metabolic and autophagy factors during tooth eruption in mice
- HP1 induces ferroptosis of renal tubular epithelial cells through NRF2 pathway in diabetic nephropathy
- Intravaginal estrogen management in postmenopausal patients with vaginal squamous intraepithelial lesions along with CO2 laser ablation: A retrospective study
- Hepatocellular carcinoma cell differentiation trajectory predicts immunotherapy, potential therapeutic drugs, and prognosis of patients
- Effects of physical exercise on biomarkers of oxidative stress in healthy subjects: A meta-analysis of randomized controlled trials
- Identification of lysosome-related genes in connection with prognosis and immune cell infiltration for drug candidates in head and neck cancer
- Development of an instrument-free and low-cost ELISA dot-blot test to detect antibodies against SARS-CoV-2
- Research progress on gas signal molecular therapy for Parkinson’s disease
- Adiponectin inhibits TGF-β1-induced skin fibroblast proliferation and phenotype transformation via the p38 MAPK signaling pathway
- The G protein-coupled receptor-related gene signatures for predicting prognosis and immunotherapy response in bladder urothelial carcinoma
- α-Fetoprotein contributes to the malignant biological properties of AFP-producing gastric cancer
- CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa
- Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis
- Significance of sTREM-1 and sST2 combined diagnosis for sepsis detection and prognosis prediction
- Diagnostic value of serum neuroactive substances in the acute exacerbation of chronic obstructive pulmonary disease complicated with depression
- Research progress of AMP-activated protein kinase and cardiac aging
- TRIM29 knockdown prevented the colon cancer progression through decreasing the ubiquitination levels of KRT5
- Cross-talk between gut microbiota and liver steatosis: Complications and therapeutic target
- Metastasis from small cell lung cancer to ovary: A case report
- The early diagnosis and pathogenic mechanisms of sepsis-related acute kidney injury
- The effect of NK cell therapy on sepsis secondary to lung cancer: A case report
- Erianin alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation
- Loss of ACOX1 in clear cell renal cell carcinoma and its correlation with clinical features
- Signalling pathways in the osteogenic differentiation of periodontal ligament stem cells
- Crosstalk between lactic acid and immune regulation and its value in the diagnosis and treatment of liver failure
- Clinicopathological features and differential diagnosis of gastric pleomorphic giant cell carcinoma
- Traumatic brain injury and rTMS-ERPs: Case report and literature review
- Extracellular fibrin promotes non-small cell lung cancer progression through integrin β1/PTEN/AKT signaling
- Knockdown of DLK4 inhibits non-small cell lung cancer tumor growth by downregulating CKS2
- The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles
- Inflammation-related signaling pathways in tendinopathy
- CD4+ T cell count in HIV/TB co-infection and co-occurrence with HL: Case report and literature review
- Clinical analysis of severe Chlamydia psittaci pneumonia: Case series study
- Bioinformatics analysis to identify potential biomarkers for the pulmonary artery hypertension associated with the basement membrane
- Influence of MTHFR polymorphism, alone or in combination with smoking and alcohol consumption, on cancer susceptibility
- Catharanthus roseus (L.) G. Don counteracts the ampicillin resistance in multiple antibiotic-resistant Staphylococcus aureus by downregulation of PBP2a synthesis
- Combination of a bronchogenic cyst in the thoracic spinal canal with chronic myelocytic leukemia
- Bacterial lipoprotein plays an important role in the macrophage autophagy and apoptosis induced by Salmonella typhimurium and Staphylococcus aureus
- TCL1A+ B cells predict prognosis in triple-negative breast cancer through integrative analysis of single-cell and bulk transcriptomic data
- Ezrin promotes esophageal squamous cell carcinoma progression via the Hippo signaling pathway
- Ferroptosis: A potential target of macrophages in plaque vulnerability
- Predicting pediatric Crohn's disease based on six mRNA-constructed risk signature using comprehensive bioinformatic approaches
- Applications of genetic code expansion and photosensitive UAAs in studying membrane proteins
- HK2 contributes to the proliferation, migration, and invasion of diffuse large B-cell lymphoma cells by enhancing the ERK1/2 signaling pathway
- IL-17 in osteoarthritis: A narrative review
- Circadian cycle and neuroinflammation
- Probiotic management and inflammatory factors as a novel treatment in cirrhosis: A systematic review and meta-analysis
- Hemorrhagic meningioma with pulmonary metastasis: Case report and literature review
- SPOP regulates the expression profiles and alternative splicing events in human hepatocytes
- Knockdown of SETD5 inhibited glycolysis and tumor growth in gastric cancer cells by down-regulating Akt signaling pathway
- PTX3 promotes IVIG resistance-induced endothelial injury in Kawasaki disease by regulating the NF-κB pathway
- Pancreatic ectopic thyroid tissue: A case report and analysis of literature
- The prognostic impact of body mass index on female breast cancer patients in underdeveloped regions of northern China differs by menopause status and tumor molecular subtype
- Report on a case of liver-originating malignant melanoma of unknown primary
- Case report: Herbal treatment of neutropenic enterocolitis after chemotherapy for breast cancer
- The fibroblast growth factor–Klotho axis at molecular level
- Characterization of amiodarone action on currents in hERG-T618 gain-of-function mutations
- A case report of diagnosis and dynamic monitoring of Listeria monocytogenes meningitis with NGS
- Effect of autologous platelet-rich plasma on new bone formation and viability of a Marburg bone graft
- Small breast epithelial mucin as a useful prognostic marker for breast cancer patients
- Continuous non-adherent culture promotes transdifferentiation of human adipose-derived stem cells into retinal lineage
- Nrf3 alleviates oxidative stress and promotes the survival of colon cancer cells by activating AKT/BCL-2 signal pathway
- Favorable response to surufatinib in a patient with necrolytic migratory erythema: A case report
- Case report of atypical undernutrition of hypoproteinemia type
- Down-regulation of COL1A1 inhibits tumor-associated fibroblast activation and mediates matrix remodeling in the tumor microenvironment of breast cancer
- Sarcoma protein kinase inhibition alleviates liver fibrosis by promoting hepatic stellate cells ferroptosis
- Research progress of serum eosinophil in chronic obstructive pulmonary disease and asthma
- Clinicopathological characteristics of co-existing or mixed colorectal cancer and neuroendocrine tumor: Report of five cases
- Role of menopausal hormone therapy in the prevention of postmenopausal osteoporosis
- Precisional detection of lymph node metastasis using tFCM in colorectal cancer
- Advances in diagnosis and treatment of perimenopausal syndrome
- A study of forensic genetics: ITO index distribution and kinship judgment between two individuals
- Acute lupus pneumonitis resembling miliary tuberculosis: A case-based review
- Plasma levels of CD36 and glutathione as biomarkers for ruptured intracranial aneurysm
- Fractalkine modulates pulmonary angiogenesis and tube formation by modulating CX3CR1 and growth factors in PVECs
- Novel risk prediction models for deep vein thrombosis after thoracotomy and thoracoscopic lung cancer resections, involving coagulation and immune function
- Exploring the diagnostic markers of essential tremor: A study based on machine learning algorithms
- Evaluation of effects of small-incision approach treatment on proximal tibia fracture by deep learning algorithm-based magnetic resonance imaging
- An online diagnosis method for cancer lesions based on intelligent imaging analysis
- Medical imaging in rheumatoid arthritis: A review on deep learning approach
- Predictive analytics in smart healthcare for child mortality prediction using a machine learning approach
- Utility of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in predicting acute-on-chronic liver failure survival
- A biomedical decision support system for meta-analysis of bilateral upper-limb training in stroke patients with hemiplegia
- TNF-α and IL-8 levels are positively correlated with hypobaric hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats
- Stochastic gradient descent optimisation for convolutional neural network for medical image segmentation
- Comparison of the prognostic value of four different critical illness scores in patients with sepsis-induced coagulopathy
- Application and teaching of computer molecular simulation embedded technology and artificial intelligence in drug research and development
- Hepatobiliary surgery based on intelligent image segmentation technology
- Value of brain injury-related indicators based on neural network in the diagnosis of neonatal hypoxic-ischemic encephalopathy
- Analysis of early diagnosis methods for asymmetric dementia in brain MR images based on genetic medical technology
- Early diagnosis for the onset of peri-implantitis based on artificial neural network
- Clinical significance of the detection of serum IgG4 and IgG4/IgG ratio in patients with thyroid-associated ophthalmopathy
- Forecast of pain degree of lumbar disc herniation based on back propagation neural network
- SPA-UNet: A liver tumor segmentation network based on fused multi-scale features
- Systematic evaluation of clinical efficacy of CYP1B1 gene polymorphism in EGFR mutant non-small cell lung cancer observed by medical image
- Rehabilitation effect of intelligent rehabilitation training system on hemiplegic limb spasms after stroke
- A novel approach for minimising anti-aliasing effects in EEG data acquisition
- ErbB4 promotes M2 activation of macrophages in idiopathic pulmonary fibrosis
- Clinical role of CYP1B1 gene polymorphism in prediction of postoperative chemotherapy efficacy in NSCLC based on individualized health model
- Lung nodule segmentation via semi-residual multi-resolution neural networks
- Evaluation of brain nerve function in ICU patients with Delirium by deep learning algorithm-based resting state MRI
- A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis
- Markov model combined with MR diffusion tensor imaging for predicting the onset of Alzheimer’s disease
- Effectiveness of the treatment of depression associated with cancer and neuroimaging changes in depression-related brain regions in patients treated with the mediator-deuterium acupuncture method
- Molecular mechanism of colorectal cancer and screening of molecular markers based on bioinformatics analysis
- Monitoring and evaluation of anesthesia depth status data based on neuroscience
- Exploring the conformational dynamics and thermodynamics of EGFR S768I and G719X + S768I mutations in non-small cell lung cancer: An in silico approaches
- Optimised feature selection-driven convolutional neural network using gray level co-occurrence matrix for detection of cervical cancer
- Incidence of different pressure patterns of spinal cerebellar ataxia and analysis of imaging and genetic diagnosis
- Pathogenic bacteria and treatment resistance in older cardiovascular disease patients with lung infection and risk prediction model
- Adoption value of support vector machine algorithm-based computed tomography imaging in the diagnosis of secondary pulmonary fungal infections in patients with malignant hematological disorders
- From slides to insights: Harnessing deep learning for prognostic survival prediction in human colorectal cancer histology
- Ecology and Environmental Science
- Monitoring of hourly carbon dioxide concentration under different land use types in arid ecosystem
- Comparing the differences of prokaryotic microbial community between pit walls and bottom from Chinese liquor revealed by 16S rRNA gene sequencing
- Effects of cadmium stress on fruits germination and growth of two herbage species
- Bamboo charcoal affects soil properties and bacterial community in tea plantations
- Optimization of biogas potential using kinetic models, response surface methodology, and instrumental evidence for biodegradation of tannery fleshings during anaerobic digestion
- Understory vegetation diversity patterns of Platycladus orientalis and Pinus elliottii communities in Central and Southern China
- Studies on macrofungi diversity and discovery of new species of Abortiporus from Baotianman World Biosphere Reserve
- Food Science
- Effect of berrycactus fruit (Myrtillocactus geometrizans) on glutamate, glutamine, and GABA levels in the frontal cortex of rats fed with a high-fat diet
- Guesstimate of thymoquinone diversity in Nigella sativa L. genotypes and elite varieties collected from Indian states using HPTLC technique
- Analysis of bacterial community structure of Fuzhuan tea with different processing techniques
- Untargeted metabolomics reveals sour jujube kernel benefiting the nutritional value and flavor of Morchella esculenta
- Mycobiota in Slovak wine grapes: A case study from the small Carpathians wine region
- Elemental analysis of Fadogia ancylantha leaves used as a nutraceutical in Mashonaland West Province, Zimbabwe
- Microbiological transglutaminase: Biotechnological application in the food industry
- Influence of solvent-free extraction of fish oil from catfish (Clarias magur) heads using a Taguchi orthogonal array design: A qualitative and quantitative approach
- Chromatographic analysis of the chemical composition and anticancer activities of Curcuma longa extract cultivated in Palestine
- The potential for the use of leghemoglobin and plant ferritin as sources of iron
- Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM
- Bioengineering and Biotechnology
- Biocompatibility and osteointegration capability of β-TCP manufactured by stereolithography 3D printing: In vitro study
- Clinical characteristics and the prognosis of diabetic foot in Tibet: A single center, retrospective study
- Agriculture
- Biofertilizer and NPSB fertilizer application effects on nodulation and productivity of common bean (Phaseolus vulgaris L.) at Sodo Zuria, Southern Ethiopia
- On correlation between canopy vegetation and growth indexes of maize varieties with different nitrogen efficiencies
- Exopolysaccharides from Pseudomonas tolaasii inhibit the growth of Pleurotus ostreatus mycelia
- A transcriptomic evaluation of the mechanism of programmed cell death of the replaceable bud in Chinese chestnut
- Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis
- Effects of plant density on alfalfa (Medicago sativa L.) seed yield in western Heilongjiang areas
- Identification of rice leaf diseases and deficiency disorders using a novel DeepBatch technique
- Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture
- Animal Sciences
- Effect of ketogenic diet on exercise tolerance and transcriptome of gastrocnemius in mice
- Combined analysis of mRNA–miRNA from testis tissue in Tibetan sheep with different FecB genotypes
- Isolation, identification, and drug resistance of a partially isolated bacterium from the gill of Siniperca chuatsi
- Tracking behavioral changes of confined sows from the first mating to the third parity
- The sequencing of the key genes and end products in the TLR4 signaling pathway from the kidney of Rana dybowskii exposed to Aeromonas hydrophila
- Development of a new candidate vaccine against piglet diarrhea caused by Escherichia coli
- Plant Sciences
- Crown and diameter structure of pure Pinus massoniana Lamb. forest in Hunan province, China
- Genetic evaluation and germplasm identification analysis on ITS2, trnL-F, and psbA-trnH of alfalfa varieties germplasm resources
- Tissue culture and rapid propagation technology for Gentiana rhodantha
- Effects of cadmium on the synthesis of active ingredients in Salvia miltiorrhiza
- Cloning and expression analysis of VrNAC13 gene in mung bean
- Chlorate-induced molecular floral transition revealed by transcriptomes
- Effects of warming and drought on growth and development of soybean in Hailun region
- Effects of different light conditions on transient expression and biomass in Nicotiana benthamiana leaves
- Comparative analysis of the rhizosphere microbiome and medicinally active ingredients of Atractylodes lancea from different geographical origins
- Distinguish Dianthus species or varieties based on chloroplast genomes
- Comparative transcriptomes reveal molecular mechanisms of apple blossoms of different tolerance genotypes to chilling injury
- Study on fresh processing key technology and quality influence of Cut Ophiopogonis Radix based on multi-index evaluation
- An advanced approach for fig leaf disease detection and classification: Leveraging image processing and enhanced support vector machine methodology
- Erratum
- Erratum to “Protein Z modulates the metastasis of lung adenocarcinoma cells”
- Erratum to “BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells”
- Retraction
- Retraction to “Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats”
Articles in the same Issue
- Biomedical Sciences
- Systemic investigation of inetetamab in combination with small molecules to treat HER2-overexpressing breast and gastric cancers
- Immunosuppressive treatment for idiopathic membranous nephropathy: An updated network meta-analysis
- Identifying two pathogenic variants in a patient with pigmented paravenous retinochoroidal atrophy
- Effects of phytoestrogens combined with cold stress on sperm parameters and testicular proteomics in rats
- A case of pulmonary embolism with bad warfarin anticoagulant effects caused by E. coli infection
- Neutrophilia with subclinical Cushing’s disease: A case report and literature review
- Isoimperatorin alleviates lipopolysaccharide-induced periodontitis by downregulating ERK1/2 and NF-κB pathways
- Immunoregulation of synovial macrophages for the treatment of osteoarthritis
- Novel CPLANE1 c.8948dupT (p.P2984Tfs*7) variant in a child patient with Joubert syndrome
- Antiphospholipid antibodies and the risk of thrombosis in myeloproliferative neoplasms
- Immunological responses of septic rats to combination therapy with thymosin α1 and vitamin C
- High glucose and high lipid induced mitochondrial dysfunction in JEG-3 cells through oxidative stress
- Pharmacological inhibition of the ubiquitin-specific protease 8 effectively suppresses glioblastoma cell growth
- Levocarnitine regulates the growth of angiotensin II-induced myocardial fibrosis cells via TIMP-1
- Age-related changes in peripheral T-cell subpopulations in elderly individuals: An observational study
- Single-cell transcription analysis reveals the tumor origin and heterogeneity of human bilateral renal clear cell carcinoma
- Identification of iron metabolism-related genes as diagnostic signatures in sepsis by blood transcriptomic analysis
- Long noncoding RNA ACART knockdown decreases 3T3-L1 preadipocyte proliferation and differentiation
- Surgery, adjuvant immunotherapy plus chemotherapy and radiotherapy for primary malignant melanoma of the parotid gland (PGMM): A case report
- Dosimetry comparison with helical tomotherapy, volumetric modulated arc therapy, and intensity-modulated radiotherapy for grade II gliomas: A single‑institution case series
- Soy isoflavone reduces LPS-induced acute lung injury via increasing aquaporin 1 and aquaporin 5 in rats
- Refractory hypokalemia with sexual dysplasia and infertility caused by 17α-hydroxylase deficiency and triple X syndrome: A case report
- Meta-analysis of cancer risk among end stage renal disease undergoing maintenance dialysis
- 6-Phosphogluconate dehydrogenase inhibition arrests growth and induces apoptosis in gastric cancer via AMPK activation and oxidative stress
- Experimental study on the optimization of ANM33 release in foam cells
- Primary retroperitoneal angiosarcoma: A case report
- Metabolomic analysis-identified 2-hydroxybutyric acid might be a key metabolite of severe preeclampsia
- Malignant pleural effusion diagnosis and therapy
- Effect of spaceflight on the phenotype and proteome of Escherichia coli
- Comparison of immunotherapy combined with stereotactic radiotherapy and targeted therapy for patients with brain metastases: A systemic review and meta-analysis
- Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation
- Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy
- The role of IL-31 and IL-34 in the diagnosis and treatment of chronic periodontitis
- Triple-negative mouse breast cancer initiating cells show high expression of beta1 integrin and increased malignant features
- mNGS facilitates the accurate diagnosis and antibiotic treatment of suspicious critical CNS infection in real practice: A retrospective study
- The apatinib and pemetrexed combination has antitumor and antiangiogenic effects against NSCLC
- Radiotherapy for primary thyroid adenoid cystic carcinoma
- Design and functional preliminary investigation of recombinant antigen EgG1Y162–EgG1Y162 against Echinococcus granulosus
- Effects of losartan in patients with NAFLD: A meta-analysis of randomized controlled trial
- Bibliometric analysis of METTL3: Current perspectives, highlights, and trending topics
- Performance comparison of three scaling algorithms in NMR-based metabolomics analysis
- PI3K/AKT/mTOR pathway and its related molecules participate in PROK1 silence-induced anti-tumor effects on pancreatic cancer
- The altered expression of cytoskeletal and synaptic remodeling proteins during epilepsy
- Effects of pegylated recombinant human granulocyte colony-stimulating factor on lymphocytes and white blood cells of patients with malignant tumor
- Prostatitis as initial manifestation of Chlamydia psittaci pneumonia diagnosed by metagenome next-generation sequencing: A case report
- NUDT21 relieves sevoflurane-induced neurological damage in rats by down-regulating LIMK2
- Association of interleukin-10 rs1800896, rs1800872, and interleukin-6 rs1800795 polymorphisms with squamous cell carcinoma risk: A meta-analysis
- Exosomal HBV-DNA for diagnosis and treatment monitoring of chronic hepatitis B
- Shear stress leads to the dysfunction of endothelial cells through the Cav-1-mediated KLF2/eNOS/ERK signaling pathway under physiological conditions
- Interaction between the PI3K/AKT pathway and mitochondrial autophagy in macrophages and the leukocyte count in rats with LPS-induced pulmonary infection
- Meta-analysis of the rs231775 locus polymorphism in the CTLA-4 gene and the susceptibility to Graves’ disease in children
- Cloning, subcellular localization and expression of phosphate transporter gene HvPT6 of hulless barley
- Coptisine mitigates diabetic nephropathy via repressing the NRLP3 inflammasome
- Significant elevated CXCL14 and decreased IL-39 levels in patients with tuberculosis
- Whole-exome sequencing applications in prenatal diagnosis of fetal bowel dilatation
- Gemella morbillorum infective endocarditis: A case report and literature review
- An unusual ectopic thymoma clonal evolution analysis: A case report
- Severe cumulative skin toxicity during toripalimab combined with vemurafenib following toripalimab alone
- Detection of V. vulnificus septic shock with ARDS using mNGS
- Novel rare genetic variants of familial and sporadic pulmonary atresia identified by whole-exome sequencing
- The influence and mechanistic action of sperm DNA fragmentation index on the outcomes of assisted reproduction technology
- Novel compound heterozygous mutations in TELO2 in an infant with You-Hoover-Fong syndrome: A case report and literature review
- ctDNA as a prognostic biomarker in resectable CLM: Systematic review and meta-analysis
- Diagnosis of primary amoebic meningoencephalitis by metagenomic next-generation sequencing: A case report
- Phylogenetic analysis of promoter regions of human Dolichol kinase (DOLK) and orthologous genes using bioinformatics tools
- Collagen changes in rabbit conjunctiva after conjunctival crosslinking
- Effects of NM23 transfection of human gastric carcinoma cells in mice
- Oral nifedipine and phytosterol, intravenous nicardipine, and oral nifedipine only: Three-arm, retrospective, cohort study for management of severe preeclampsia
- Case report of hepatic retiform hemangioendothelioma: A rare tumor treated with ultrasound-guided microwave ablation
- Curcumin induces apoptosis in human hepatocellular carcinoma cells by decreasing the expression of STAT3/VEGF/HIF-1α signaling
- Rare presentation of double-clonal Waldenström macroglobulinemia with pulmonary embolism: A case report
- Giant duplication of the transverse colon in an adult: A case report and literature review
- Ectopic thyroid tissue in the breast: A case report
- SDR16C5 promotes proliferation and migration and inhibits apoptosis in pancreatic cancer
- Vaginal metastasis from breast cancer: A case report
- Screening of the best time window for MSC transplantation to treat acute myocardial infarction with SDF-1α antibody-loaded targeted ultrasonic microbubbles: An in vivo study in miniswine
- Inhibition of TAZ impairs the migration ability of melanoma cells
- Molecular complexity analysis of the diagnosis of Gitelman syndrome in China
- Effects of maternal calcium and protein intake on the development and bone metabolism of offspring mice
- Identification of winter wheat pests and diseases based on improved convolutional neural network
- Ultra-multiplex PCR technique to guide treatment of Aspergillus-infected aortic valve prostheses
- Virtual high-throughput screening: Potential inhibitors targeting aminopeptidase N (CD13) and PIKfyve for SARS-CoV-2
- Immune checkpoint inhibitors in cancer patients with COVID-19
- Utility of methylene blue mixed with autologous blood in preoperative localization of pulmonary nodules and masses
- Integrated analysis of the microbiome and transcriptome in stomach adenocarcinoma
- Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy
- DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway
- Lung abscess by Fusobacterium nucleatum and Streptococcus spp. co-infection by mNGS: A case series
- Genetic alterations of KRAS and TP53 in intrahepatic cholangiocarcinoma associated with poor prognosis
- Granulomatous polyangiitis involving the fourth ventricle: Report of a rare case and a literature review
- Studying infant mortality: A demographic analysis based on data mining models
- Metaplastic breast carcinoma with osseous differentiation: A report of a rare case and literature review
- Protein Z modulates the metastasis of lung adenocarcinoma cells
- Inhibition of pyroptosis and apoptosis by capsaicin protects against LPS-induced acute kidney injury through TRPV1/UCP2 axis in vitro
- TAK-242, a toll-like receptor 4 antagonist, against brain injury by alleviates autophagy and inflammation in rats
- Primary mediastinum Ewing’s sarcoma with pleural effusion: A case report and literature review
- Association of ADRB2 gene polymorphisms and intestinal microbiota in Chinese Han adolescents
- Tanshinone IIA alleviates chondrocyte apoptosis and extracellular matrix degeneration by inhibiting ferroptosis
- Study on the cytokines related to SARS-Cov-2 in testicular cells and the interaction network between cells based on scRNA-seq data
- Effect of periostin on bone metabolic and autophagy factors during tooth eruption in mice
- HP1 induces ferroptosis of renal tubular epithelial cells through NRF2 pathway in diabetic nephropathy
- Intravaginal estrogen management in postmenopausal patients with vaginal squamous intraepithelial lesions along with CO2 laser ablation: A retrospective study
- Hepatocellular carcinoma cell differentiation trajectory predicts immunotherapy, potential therapeutic drugs, and prognosis of patients
- Effects of physical exercise on biomarkers of oxidative stress in healthy subjects: A meta-analysis of randomized controlled trials
- Identification of lysosome-related genes in connection with prognosis and immune cell infiltration for drug candidates in head and neck cancer
- Development of an instrument-free and low-cost ELISA dot-blot test to detect antibodies against SARS-CoV-2
- Research progress on gas signal molecular therapy for Parkinson’s disease
- Adiponectin inhibits TGF-β1-induced skin fibroblast proliferation and phenotype transformation via the p38 MAPK signaling pathway
- The G protein-coupled receptor-related gene signatures for predicting prognosis and immunotherapy response in bladder urothelial carcinoma
- α-Fetoprotein contributes to the malignant biological properties of AFP-producing gastric cancer
- CXCL12/CXCR4/CXCR7 axis in placenta tissues of patients with placenta previa
- Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis
- Significance of sTREM-1 and sST2 combined diagnosis for sepsis detection and prognosis prediction
- Diagnostic value of serum neuroactive substances in the acute exacerbation of chronic obstructive pulmonary disease complicated with depression
- Research progress of AMP-activated protein kinase and cardiac aging
- TRIM29 knockdown prevented the colon cancer progression through decreasing the ubiquitination levels of KRT5
- Cross-talk between gut microbiota and liver steatosis: Complications and therapeutic target
- Metastasis from small cell lung cancer to ovary: A case report
- The early diagnosis and pathogenic mechanisms of sepsis-related acute kidney injury
- The effect of NK cell therapy on sepsis secondary to lung cancer: A case report
- Erianin alleviates collagen-induced arthritis in mice by inhibiting Th17 cell differentiation
- Loss of ACOX1 in clear cell renal cell carcinoma and its correlation with clinical features
- Signalling pathways in the osteogenic differentiation of periodontal ligament stem cells
- Crosstalk between lactic acid and immune regulation and its value in the diagnosis and treatment of liver failure
- Clinicopathological features and differential diagnosis of gastric pleomorphic giant cell carcinoma
- Traumatic brain injury and rTMS-ERPs: Case report and literature review
- Extracellular fibrin promotes non-small cell lung cancer progression through integrin β1/PTEN/AKT signaling
- Knockdown of DLK4 inhibits non-small cell lung cancer tumor growth by downregulating CKS2
- The co-expression pattern of VEGFR-2 with indicators related to proliferation, apoptosis, and differentiation of anagen hair follicles
- Inflammation-related signaling pathways in tendinopathy
- CD4+ T cell count in HIV/TB co-infection and co-occurrence with HL: Case report and literature review
- Clinical analysis of severe Chlamydia psittaci pneumonia: Case series study
- Bioinformatics analysis to identify potential biomarkers for the pulmonary artery hypertension associated with the basement membrane
- Influence of MTHFR polymorphism, alone or in combination with smoking and alcohol consumption, on cancer susceptibility
- Catharanthus roseus (L.) G. Don counteracts the ampicillin resistance in multiple antibiotic-resistant Staphylococcus aureus by downregulation of PBP2a synthesis
- Combination of a bronchogenic cyst in the thoracic spinal canal with chronic myelocytic leukemia
- Bacterial lipoprotein plays an important role in the macrophage autophagy and apoptosis induced by Salmonella typhimurium and Staphylococcus aureus
- TCL1A+ B cells predict prognosis in triple-negative breast cancer through integrative analysis of single-cell and bulk transcriptomic data
- Ezrin promotes esophageal squamous cell carcinoma progression via the Hippo signaling pathway
- Ferroptosis: A potential target of macrophages in plaque vulnerability
- Predicting pediatric Crohn's disease based on six mRNA-constructed risk signature using comprehensive bioinformatic approaches
- Applications of genetic code expansion and photosensitive UAAs in studying membrane proteins
- HK2 contributes to the proliferation, migration, and invasion of diffuse large B-cell lymphoma cells by enhancing the ERK1/2 signaling pathway
- IL-17 in osteoarthritis: A narrative review
- Circadian cycle and neuroinflammation
- Probiotic management and inflammatory factors as a novel treatment in cirrhosis: A systematic review and meta-analysis
- Hemorrhagic meningioma with pulmonary metastasis: Case report and literature review
- SPOP regulates the expression profiles and alternative splicing events in human hepatocytes
- Knockdown of SETD5 inhibited glycolysis and tumor growth in gastric cancer cells by down-regulating Akt signaling pathway
- PTX3 promotes IVIG resistance-induced endothelial injury in Kawasaki disease by regulating the NF-κB pathway
- Pancreatic ectopic thyroid tissue: A case report and analysis of literature
- The prognostic impact of body mass index on female breast cancer patients in underdeveloped regions of northern China differs by menopause status and tumor molecular subtype
- Report on a case of liver-originating malignant melanoma of unknown primary
- Case report: Herbal treatment of neutropenic enterocolitis after chemotherapy for breast cancer
- The fibroblast growth factor–Klotho axis at molecular level
- Characterization of amiodarone action on currents in hERG-T618 gain-of-function mutations
- A case report of diagnosis and dynamic monitoring of Listeria monocytogenes meningitis with NGS
- Effect of autologous platelet-rich plasma on new bone formation and viability of a Marburg bone graft
- Small breast epithelial mucin as a useful prognostic marker for breast cancer patients
- Continuous non-adherent culture promotes transdifferentiation of human adipose-derived stem cells into retinal lineage
- Nrf3 alleviates oxidative stress and promotes the survival of colon cancer cells by activating AKT/BCL-2 signal pathway
- Favorable response to surufatinib in a patient with necrolytic migratory erythema: A case report
- Case report of atypical undernutrition of hypoproteinemia type
- Down-regulation of COL1A1 inhibits tumor-associated fibroblast activation and mediates matrix remodeling in the tumor microenvironment of breast cancer
- Sarcoma protein kinase inhibition alleviates liver fibrosis by promoting hepatic stellate cells ferroptosis
- Research progress of serum eosinophil in chronic obstructive pulmonary disease and asthma
- Clinicopathological characteristics of co-existing or mixed colorectal cancer and neuroendocrine tumor: Report of five cases
- Role of menopausal hormone therapy in the prevention of postmenopausal osteoporosis
- Precisional detection of lymph node metastasis using tFCM in colorectal cancer
- Advances in diagnosis and treatment of perimenopausal syndrome
- A study of forensic genetics: ITO index distribution and kinship judgment between two individuals
- Acute lupus pneumonitis resembling miliary tuberculosis: A case-based review
- Plasma levels of CD36 and glutathione as biomarkers for ruptured intracranial aneurysm
- Fractalkine modulates pulmonary angiogenesis and tube formation by modulating CX3CR1 and growth factors in PVECs
- Novel risk prediction models for deep vein thrombosis after thoracotomy and thoracoscopic lung cancer resections, involving coagulation and immune function
- Exploring the diagnostic markers of essential tremor: A study based on machine learning algorithms
- Evaluation of effects of small-incision approach treatment on proximal tibia fracture by deep learning algorithm-based magnetic resonance imaging
- An online diagnosis method for cancer lesions based on intelligent imaging analysis
- Medical imaging in rheumatoid arthritis: A review on deep learning approach
- Predictive analytics in smart healthcare for child mortality prediction using a machine learning approach
- Utility of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in predicting acute-on-chronic liver failure survival
- A biomedical decision support system for meta-analysis of bilateral upper-limb training in stroke patients with hemiplegia
- TNF-α and IL-8 levels are positively correlated with hypobaric hypoxic pulmonary hypertension and pulmonary vascular remodeling in rats
- Stochastic gradient descent optimisation for convolutional neural network for medical image segmentation
- Comparison of the prognostic value of four different critical illness scores in patients with sepsis-induced coagulopathy
- Application and teaching of computer molecular simulation embedded technology and artificial intelligence in drug research and development
- Hepatobiliary surgery based on intelligent image segmentation technology
- Value of brain injury-related indicators based on neural network in the diagnosis of neonatal hypoxic-ischemic encephalopathy
- Analysis of early diagnosis methods for asymmetric dementia in brain MR images based on genetic medical technology
- Early diagnosis for the onset of peri-implantitis based on artificial neural network
- Clinical significance of the detection of serum IgG4 and IgG4/IgG ratio in patients with thyroid-associated ophthalmopathy
- Forecast of pain degree of lumbar disc herniation based on back propagation neural network
- SPA-UNet: A liver tumor segmentation network based on fused multi-scale features
- Systematic evaluation of clinical efficacy of CYP1B1 gene polymorphism in EGFR mutant non-small cell lung cancer observed by medical image
- Rehabilitation effect of intelligent rehabilitation training system on hemiplegic limb spasms after stroke
- A novel approach for minimising anti-aliasing effects in EEG data acquisition
- ErbB4 promotes M2 activation of macrophages in idiopathic pulmonary fibrosis
- Clinical role of CYP1B1 gene polymorphism in prediction of postoperative chemotherapy efficacy in NSCLC based on individualized health model
- Lung nodule segmentation via semi-residual multi-resolution neural networks
- Evaluation of brain nerve function in ICU patients with Delirium by deep learning algorithm-based resting state MRI
- A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis
- Markov model combined with MR diffusion tensor imaging for predicting the onset of Alzheimer’s disease
- Effectiveness of the treatment of depression associated with cancer and neuroimaging changes in depression-related brain regions in patients treated with the mediator-deuterium acupuncture method
- Molecular mechanism of colorectal cancer and screening of molecular markers based on bioinformatics analysis
- Monitoring and evaluation of anesthesia depth status data based on neuroscience
- Exploring the conformational dynamics and thermodynamics of EGFR S768I and G719X + S768I mutations in non-small cell lung cancer: An in silico approaches
- Optimised feature selection-driven convolutional neural network using gray level co-occurrence matrix for detection of cervical cancer
- Incidence of different pressure patterns of spinal cerebellar ataxia and analysis of imaging and genetic diagnosis
- Pathogenic bacteria and treatment resistance in older cardiovascular disease patients with lung infection and risk prediction model
- Adoption value of support vector machine algorithm-based computed tomography imaging in the diagnosis of secondary pulmonary fungal infections in patients with malignant hematological disorders
- From slides to insights: Harnessing deep learning for prognostic survival prediction in human colorectal cancer histology
- Ecology and Environmental Science
- Monitoring of hourly carbon dioxide concentration under different land use types in arid ecosystem
- Comparing the differences of prokaryotic microbial community between pit walls and bottom from Chinese liquor revealed by 16S rRNA gene sequencing
- Effects of cadmium stress on fruits germination and growth of two herbage species
- Bamboo charcoal affects soil properties and bacterial community in tea plantations
- Optimization of biogas potential using kinetic models, response surface methodology, and instrumental evidence for biodegradation of tannery fleshings during anaerobic digestion
- Understory vegetation diversity patterns of Platycladus orientalis and Pinus elliottii communities in Central and Southern China
- Studies on macrofungi diversity and discovery of new species of Abortiporus from Baotianman World Biosphere Reserve
- Food Science
- Effect of berrycactus fruit (Myrtillocactus geometrizans) on glutamate, glutamine, and GABA levels in the frontal cortex of rats fed with a high-fat diet
- Guesstimate of thymoquinone diversity in Nigella sativa L. genotypes and elite varieties collected from Indian states using HPTLC technique
- Analysis of bacterial community structure of Fuzhuan tea with different processing techniques
- Untargeted metabolomics reveals sour jujube kernel benefiting the nutritional value and flavor of Morchella esculenta
- Mycobiota in Slovak wine grapes: A case study from the small Carpathians wine region
- Elemental analysis of Fadogia ancylantha leaves used as a nutraceutical in Mashonaland West Province, Zimbabwe
- Microbiological transglutaminase: Biotechnological application in the food industry
- Influence of solvent-free extraction of fish oil from catfish (Clarias magur) heads using a Taguchi orthogonal array design: A qualitative and quantitative approach
- Chromatographic analysis of the chemical composition and anticancer activities of Curcuma longa extract cultivated in Palestine
- The potential for the use of leghemoglobin and plant ferritin as sources of iron
- Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM
- Bioengineering and Biotechnology
- Biocompatibility and osteointegration capability of β-TCP manufactured by stereolithography 3D printing: In vitro study
- Clinical characteristics and the prognosis of diabetic foot in Tibet: A single center, retrospective study
- Agriculture
- Biofertilizer and NPSB fertilizer application effects on nodulation and productivity of common bean (Phaseolus vulgaris L.) at Sodo Zuria, Southern Ethiopia
- On correlation between canopy vegetation and growth indexes of maize varieties with different nitrogen efficiencies
- Exopolysaccharides from Pseudomonas tolaasii inhibit the growth of Pleurotus ostreatus mycelia
- A transcriptomic evaluation of the mechanism of programmed cell death of the replaceable bud in Chinese chestnut
- Melatonin enhances salt tolerance in sorghum by modulating photosynthetic performance, osmoregulation, antioxidant defense, and ion homeostasis
- Effects of plant density on alfalfa (Medicago sativa L.) seed yield in western Heilongjiang areas
- Identification of rice leaf diseases and deficiency disorders using a novel DeepBatch technique
- Artificial intelligence and internet of things oriented sustainable precision farming: Towards modern agriculture
- Animal Sciences
- Effect of ketogenic diet on exercise tolerance and transcriptome of gastrocnemius in mice
- Combined analysis of mRNA–miRNA from testis tissue in Tibetan sheep with different FecB genotypes
- Isolation, identification, and drug resistance of a partially isolated bacterium from the gill of Siniperca chuatsi
- Tracking behavioral changes of confined sows from the first mating to the third parity
- The sequencing of the key genes and end products in the TLR4 signaling pathway from the kidney of Rana dybowskii exposed to Aeromonas hydrophila
- Development of a new candidate vaccine against piglet diarrhea caused by Escherichia coli
- Plant Sciences
- Crown and diameter structure of pure Pinus massoniana Lamb. forest in Hunan province, China
- Genetic evaluation and germplasm identification analysis on ITS2, trnL-F, and psbA-trnH of alfalfa varieties germplasm resources
- Tissue culture and rapid propagation technology for Gentiana rhodantha
- Effects of cadmium on the synthesis of active ingredients in Salvia miltiorrhiza
- Cloning and expression analysis of VrNAC13 gene in mung bean
- Chlorate-induced molecular floral transition revealed by transcriptomes
- Effects of warming and drought on growth and development of soybean in Hailun region
- Effects of different light conditions on transient expression and biomass in Nicotiana benthamiana leaves
- Comparative analysis of the rhizosphere microbiome and medicinally active ingredients of Atractylodes lancea from different geographical origins
- Distinguish Dianthus species or varieties based on chloroplast genomes
- Comparative transcriptomes reveal molecular mechanisms of apple blossoms of different tolerance genotypes to chilling injury
- Study on fresh processing key technology and quality influence of Cut Ophiopogonis Radix based on multi-index evaluation
- An advanced approach for fig leaf disease detection and classification: Leveraging image processing and enhanced support vector machine methodology
- Erratum
- Erratum to “Protein Z modulates the metastasis of lung adenocarcinoma cells”
- Erratum to “BRCA1 subcellular localization regulated by PI3K signaling pathway in triple-negative breast cancer MDA-MB-231 cells and hormone-sensitive T47D cells”
- Retraction
- Retraction to “Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats”

