Abstract
Green synthesis of zinc oxide (ZnO) nanoparticles (NPs) using various plant extracts as reducing and capping agents has gained attention in recent research. The green synthesis of ZnO NPs offers several advantages such as being simple, eco-friendly, safe, cost-effective, and reproducible approach with high stability. Hence, this article provides an overview of zinc metal and ZnO compounds, and traditional chemical and physical synthesis of ZnO NPs with primary focuses on the green synthesis of ZnO NPs. This study discusses various plant extracts used and the proposed mechanisms in the green synthesis of ZnO NPs. Additionally, it explores the cytotoxic mechanisms of the green-synthesized ZnO NPs and addresses the various biomedical applications of ZnO NPs, including antibacterial, anticancer, antidiabetic, antioxidant, antifungal, antiviral, antiparasitic, anti-inflammatory, and wound healing. Moreover, the review critically discusses the toxicity of ZnO NPs and emphasizes the need for more toxicological studies to ensure the safety and facilitate the risk assessments and risk management of ZnO NPs. Furthermore, this review underlines the challenges associated with the translation process of ZnO NPs from bench to market, including the complex and time-consuming regulatory approval process for ZnO NPs, which requires a multidisciplinary approach involving scientists, regulators, and manufacturers.
1 Introduction
Nanotechnology is the science of design, synthesis, characterization, and development of nanomaterials [1,2]. Nanoparticles (NPs) are objects that range in size from 1 to 100 nm [3,4]. They are used to target drugs to a specific site in the body by passive or active targeting strategies, which helps reduce the side effects of drugs [5]. Presently, metal oxide NPs (MONPs) are synthesized and are used in many biomedical applications [6,7]. These MONPs include iron oxide NPs (Fe2O3 NPs and Fe3O4 NPs), magnesium oxide NPs, zinc oxide NPs (ZnO NPs), titanium dioxide NPs, and copper oxide NPs [8,9,10]. MONPs are being used in diverse fields, including medical treatments, industries like solar and oxide fuel batteries for energy storage, cosmetics and sunscreens, and in textiles [11,12].
Among other MONPs, ZnO NPs have received significant attention due to their distinct physicochemical properties and potential biomedical applications such as antibacterial, anticancer, antidiabetic, antioxidant, antifungal, antiviral, antiparasitic, anti-inflammatory, and in wound healing [13,14,15,16,17]. The characteristic properties of ZnO NPs including size, crystallinity, morphology, and chemical composition showed great potential in biomedical applications [18,19]. The nanoscale size of ZnO NPs can modify their mechanical, chemical, structural, morphological, electrical, medicinal, and optical properties [19].
Recently, the green synthesis approach has been adopted to provide biocompatible and biodegradable ZnO NPs, making them suitable for various biomedical applications [15,20]. The green synthesis approach avoids the production of unwanted or harmful by-products, offering a reliable, sustainable, and eco-friendly synthesis approach [21,22]. This approach minimizes the waste and environmental pollution resulting from toxic substances generated during the chemical synthesis process. In addition, it reduces the use of toxic solvents or chemical agents required for chemical synthesis, forming biocompatible and environmentally friendly NPs [23]. Moreover, green synthesis emerges as an inexpensive and simple approach where no sophisticated pieces of equipment are required [24]. Finally, reducing and capping agents, essential for the stabilization of the green-synthesized ZnO NPs, are obtained from natural compounds available in the plant extracts, limiting the use of toxic and expensive reagents [25,26].
The growing interest in the green synthesis of ZnO NPs, its potential applications in various fields, including biomedicine, with the intrinsic properties of the environmentally friendly nature and cost-effectiveness makes it an attractive choice to be reviewed. Therefore, this review aims to provide deep insight information of plant-mediated green synthesis of ZnO NPs recently described in the literature to clarify the unique properties of ZnO NPs that can achieve their optimal bioactivities. This article provides a comprehensive review of the synthesis methods of ZnO NPs, with a particular emphasis on green synthesis using plant extracts. The authors explore the mechanisms involved in the green synthesis of ZnO NPs, the cytotoxic mechanisms underlying their bioactivities, and a thorough discussion of their potential biomedical applications. Additionally, a concise discussion is made on the toxicological studies of green-synthesized ZnO NPs, emphasizing the need for safety, risk assessment, and risk management strategies. Moreover, the authors address the challenges associated with the translation process of ZnO NPs from basic research to successful clinical applications and commercial products, where a multidisciplinary collaboration involving scientists, regulators, and manufacturers is needed.
2 An overview of zinc and ZnO
Zinc is an essential mineral for the body and is present in the brain, bone, muscles, skin, hair, nails, and immune system [27]. Additionally, Zn is a cofactor of >300 enzymes in the body that help in the synthesis of proteins and nucleic acids, maintaining DNA replication and repair, and cell cycle progression and apoptosis [28]. Moreover, Zn plays an important role in host defense against the initiation and progression of cancer [28]. Furthermore, Zn exhibits an antioxidant activity via the catalytic action of copper/Zn peroxide dismutase, stabilizes membrane structure by competing with iron and copper which are redox active metals and protects proteins from oxidation by binding to sulfhydryl groups. Further, Zn upregulates the metal-binding protein metallothionein expression which is very rich in cysteine and is an excellent scavenger of the hydroxy free radical (·OH) [29,30,31]. Finally, Zn acts as an anti-inflammatory agent by regulating the tumor nuclear factor-kappa B (NF-κβ) transcription via the anti-inflammatory protein A20 and the receptor signaling pathway activated by peroxisome proliferator-α [31,32,33]. Deficiency in Zn may lead to the release of vitamin A from the liver due to the reduced synthesis of plasma retinol-binding protein [33,34].
ZnO has been listed by the US Food and Drug Administration as “generally recognized as safe (GRAS)” [35]. Hence, it can be used as a food additive, where Zn is considered an essential trace element that plays a vital role in the growth and development of humans and animals [35,36]. Additionally, ZnO is a II–VI semiconductor, where Zn and O are found in groups 2 and 6 on the periodic table, respectively [37]. ZnO has distinctive properties including chemical, sensing, optical, semiconducting, and electrical conductivity [38]. Moreover, ZnO is characterized by a direct wide band gap of 3.3 eV in the near UV spectrum, high exciton binding energy of 60 meV at room temperature, and a natural n-type electrical conductivity [39]. These characteristic properties make ZnO a potential material to be used for a broad range of applications in electronics, optoelectronic devices, and photocatalysis [40].
Although ZnO has a slight covalent characteristic, it has a strong ionic interaction between Zn and O with higher selectivity and heat resistance, compared with other organic/inorganic compounds. The small size of ZnO improves the Zn2+ dissolution rate, making it more biocompatible with normal body cells and a potential antibacterial, anti-inflammatory, and anti-tumor agent [41].
3 Chemical and physical methods for the synthesis of ZnO NPs
The multitudinous applications of ZnO NPs in various fields have raised their production. It has been reported that the global production of ZnO NPs was 570 tons/year in 2010 [42] and between 32,000 and 36,000 tons/year in 2014 [43]. Additionally, it was expected that the production would be between 1,600 and 58,000 tons/year by 2020 [42]. Yashni et al. [44] estimated the production cost of ZnO NPs (<100 nm) synthesized from orange peel extract to be 20.25 USD per kg, which was considered a cost-effective method.
Traditional chemical and physical methods have been employed in the synthesis of ZnO NPs [16,45,46]. Chemically, ZnO NPs can be synthesized by sol–gel [47,48], solvothermal [49], vapor deposition [50], co-precipitation [51], hydrothermal [52,53], thermal decomposition [54], and electrochemical reduction methods [55]. Whereas physically, ZnO NPs can be synthesized by laser ablation [56], ultrasonication [57], photoirradiation [58], ball milling [59], evaporation condensation [60], microwave-assisted combustion [61], sonochemical [62], and mechanochemical methods [63] (Figure 1). These methods have many disadvantages such as high cost, high impurities, instability, and limitations in reproducibility and reliability [64,65]. In addition, they require toxic chemicals that are hazard to the environment and medical applications [66]. Therefore, to avoid these disadvantages, alternative eco-friendly and cost-effective green methods have recently been employed to synthesize ZnO NPs [67,68,69].
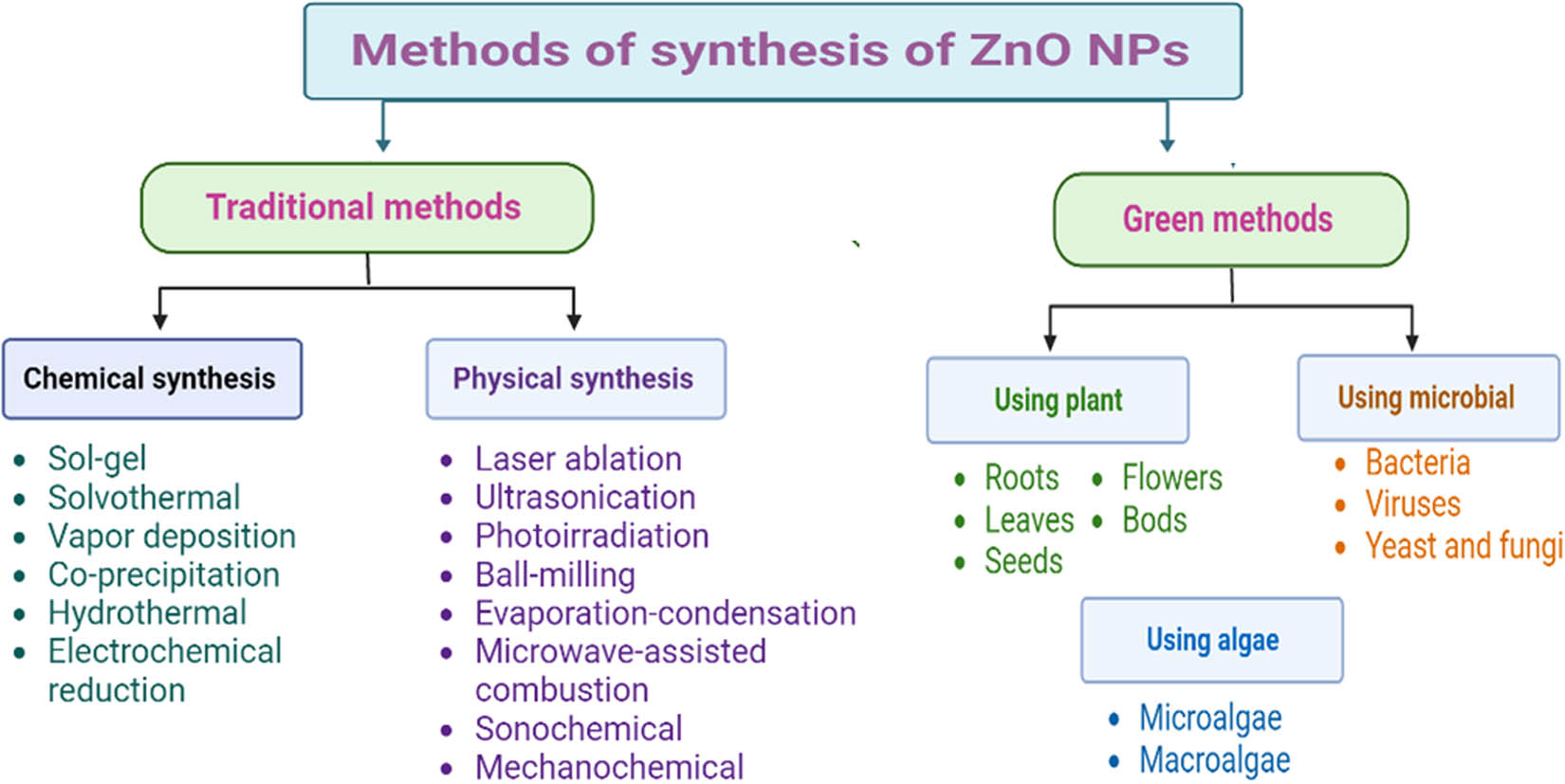
Methods employed in the synthesis of ZnO NPs.
4 Green synthesis of ZnO NPs
The green synthesis of ZnO NPs exhibited many advantages like simplicity, eco-friendly, and biologically safe [70,71,72]. The green synthesis method reduces the need of using toxic chemicals and increases the limit to produce pure NPs [73]. By using plants or unicellular microorganisms (algae, bacteria, fungi, yeasts, and viruses) for NP synthesis (Figure 1), the addition of stabilizing agents can be reduced [74]. This is because plant phytochemicals (e.g., alkaloids, flavonoids, and polyphenols) and proteins secreted by microorganisms act as reducing and stabilizing agents which provide colloidal stability and prevent agglomeration of NPs [75,76,77]. It has been shown that plant leaves are the best source for the synthesis of metal and MO NPs. This is because the phytochemicals, especially those present in plant leaf extracts, play a dual role in reducing the metal ions and stabilizing the NPs [20,21]. Additionally, plant waste products or by-products are safe and eco-friendly because they are composed of the leftovers of natural plant extracts [21,78]. Moreover, other green resources such as egg albumin, L-alanine, and starch have been used to synthesize ZnO NPs [45]. Ijaz et al. [79] reported that the best method for the synthesis of NPs is one that is not harmful to the environment. Thus, the green method is the most preferable method. Table 1 summarizes the advantages and limitations of the chemical, physical, and green synthesis of ZnO NPs.
Advantages and limitations of the chemical, physical, and green synthesis methods of ZnO NPs
| Method | Advantages | Limitations |
|---|---|---|
| Chemical synthesis [80,81,82] | – Controlled size | – Use of toxic chemicals |
| – High purity and quality | – Use of organic solvents | |
| – NPs are considerably stable | – Hazard | |
| – Low purity | ||
| – Capping agents and stabilizers are required to control the size and avoid agglomeration | ||
| – Some of the capping and stabilizing agents might be toxic | ||
| – Irreversible pollution of the environment | ||
| Physical synthesis [82,83] | – No use of toxic ingredients | – High cost |
| – High-speed method | – High energy | |
| – High production rates | – Low stability | |
| – High purity and quality | – Irreversible pollution of the environment | |
| – Uniform size and shape | ||
| Green synthesis [81,84,85] | – Safe and toxic free | – Difficult to control the particle size and shape |
| – Simple and cost-effective | – NPs are relatively less stable | |
| – No expensive equipment | ||
| – No toxic chemicals | ||
| – Eco-friendly | ||
| – Biocompatible | ||
| – Energy efficient |
The use of plant extracts was found to be more valuable than other biological organisms since it reduces the duration of the reactions from days to hours and prevents the risk and complicated process of preserving cell cultures [86]. The green synthesis using plant extracts provides ZnO NPs with an antibacterial effect that cannot be achieved when using physical and chemical methods. This is because the green synthesis coats the surface of the NPs with many pharmacologically active biomolecules such as flavonoids, organic acids, ketones, aldehydes, amides, quinones, or polysaccharides which enhance the ligand-based conjugation of NPs to the bacterial membrane receptor [72,87,88].
In plant-mediated-green synthesis, the use of “ideal solvents” and plant extracts (as natural resources) is essential to achieve biocompatible NPs, where the utilization of plant extracts is a rather simple and easy process to produce NPs at a large scale relative to bacteria- and/or fungi-mediated synthesis [21,89]. The “ideal solvents” such as water, carbon dioxide (CO2), and ionic liquids such as hexafluorophosphate (PF6) or tetrafluoroborate (BF4) are employed in the green synthesis of MONPs [21,90]. Water is always considered an “ideal solvent” as it is the cheapest, non-toxic, non-explosive, environmentally friendly, and most available solvent [21,91]; whereas, CO2 is a universal solvent that is available as liquid or supercritical solvent (fluid at temperature and pressure above critical point) [90]. Ionic liquids, which are composed of ions with melting points <100°C, are among the best solvents used in green synthesis. This is because ionic liquids act as reducing and protective agents, making the NP synthesis process simpler [21,90].
Although the green synthesis of ZnO NPs, using plant extracts, has attracted attention due to their broad range of applications, there has been a concern regarding their reproducibility and repeatability. This is attributed to the variation in the chemical composition of the phytochemicals of plant extracts of the same species owing to their collection from different parts of the world. This might significantly affect the reproducibility and repeatability of ZnO NPs [92]. Therefore, identifying the phytochemicals present in the plant extracts is crucial to enhance the reproducibility and repeatability of ZnO NPs. Sharma et al. [93] used ZnO NPs synthesized greenly from the seed extract of Carica papaya (C. papaya) to investigate the electrochemical sensing of silymarin molecules which decrease serum transaminases in viral hepatitis patients. The reproducibility of nanocomposite ZnO NPs was tested using three electrochemical sensors independently in 0.2 mM silymarin solution. The nanocomposite ZnO NPs showed promising reproducibility of a relative standard deviation (RSD) of 1.92% (n = 6). In addition, a repeatability of RSD of 2.31% (n = 6) was reported when the nanocomposite ZnO NPs were investigated for 0.1 mg l−1 silymarin. Moreover, Muthuchamy et al. [94] fabricated glucose biosensors immobilized into ZnO NP-embedded nitrogen-doped carbon sheets for monitoring glucose. The ZnO NPs were synthesized using the peach fruit extract. The reproducibility and repeatability were determined by measuring the amperometric response against 3 mM glucose in phosphate buffer saline (PBS, pH 7). The ZnO NP biosensor showed good reproducibility (RSD = 2.99%, n = 5) and repeatability (RSD = 2.86%, n = 5).
Several factors may impact the green synthesis of ZnO NPs including temperature, pH, mixing speed, reaction time, plant extract/chemical precursor ratio, calcination temperature, and precursor concentration. These parameters may ultimately affect the shape, size, and phase of ZnO NPs [95]. It has been shown that the higher the temperature of the reaction, the smaller the size of ZnO NPs [96]. Naseer et al. [96] used a relatively high temperature of 70°C to greenly synthesize ZnO NPs from the leaf extracts of Cassia fistula (C. fistula) and Melia azedarach (M. azedarach). The average nano-size was between 68.1 and 3.62 nm, respectively. In a recent study, Jayachandran et al. [97] prepared ZnO NPs using a leaf extract of Cayratia pedata (C. pedata) at different reaction temperatures (55, 65, and 75°C). A reaction temperature of 65°C confirmed the production of ZnO NPs of 52.2 nm. Hassan Basri et al. [98] investigated the effect of the reaction temperature on the size and shape of ZnO NPs greenly synthesized using the peel extract of pineapple. When the reaction temperature was maintained at 28°C, the size of ZnO NPs was between 8 and 45 nm with a mixture of spherical and rod shapes. However, when the reaction was increased to 60°C, the size of ZnO NPs was between 73 and 123 nm with a flower rod shape.
Padalia et al. [99] demonstrated the effect of pH on the size and morphology of ZnO NPs synthesized using the leaf extract of Salvadora oleoides (S. oleoides). Based on the transmission electron microscope (TEM) analysis, ZnO NPs synthesized at pH 5 were spherical in shape with an average size of 26.6 nm, whereas, at pH 8, irregular shape ZnO NPs with an average size of 38.6 nm were formed. Additionally, ZnO NPs synthesized at pH 5 showed higher antibacterial activity against Gram-positive and Gram-negative bacteria, compared to pH 8, indicating the importance of pH on the biological activity of ZnO NPs. In another study, the effect of pH (8–14) on the green synthesis of ZnO NPs prepared from the leaf extract of Raphanus sativus var. Longipinnatus was investigated [100]. The authors showed that no UV-vis absorption peaks were observed at pH 8–10 and pH 14. At pH 12, an absorption peak was observed at 369 nm, indicating the formation of ZnO NPs with an average size of 209 nm. Mohammadi and Ghasemi [101] used different pH values (4–10), temperatures (25, 60, and 90°C), and precursor concentrations of zinc nitrate (0.005, 0.02, 0.05, and 0.3 M) to greenly synthesize ZnO NPs using the cherry leaf extract. The results showed that the size of ZnO NPs increased from 87.5 to 116 nm as the temperature increased. Additionally, the optimum synthesis conditions of pH 8, temperature 25°C, zinc nitrate concentration of 0.005 M, and incubation time of 12 h confirmed the formation of ZnO NPs with an average size of 20.2 nm and spherical morphology.
All physical features of ZnO NPs like size, morphology, and surface properties can be assessed using various characterization tools such as UV-vis spectroscopy, X-ray powder diffraction (XRPD), Fourier transform infrared (FTIR), energy-dispersive X-ray analysis, TEM, scanning electron microscopy (SEM), and dynamic light scattering [102]. Figure 2 shows the various characterization analyses of green-synthesized ZnO NPs.
![Figure 2
Characterization analyses of green-synthesized ZnO NPs including particle size distribution, differential scanning calorimetry, field emission scanning electron microscopy, X-ray diffraction (XRD), UV-vis spectroscopy (UV-vis), and FTIR [103].](/document/doi/10.1515/ntrev-2023-0112/asset/graphic/j_ntrev-2023-0112_fig_002.jpg)
Characterization analyses of green-synthesized ZnO NPs including particle size distribution, differential scanning calorimetry, field emission scanning electron microscopy, X-ray diffraction (XRD), UV-vis spectroscopy (UV-vis), and FTIR [103].
5 Mechanism of green synthesis of ZnO NPs
The proposed mechanism of the green synthesis of ZnO NPs is related to the presence of phytochemicals, particularly polyphenols such as flavonoids, tannins, anthocyanidins, and phenolic acids, which act as reducing/capping agents [92,104]. The phenolic rings rich in hydroxyl groups act as chelating agents, forming complexes with Zn2+. A hydrolysis reaction transforms the intermediate chelated compound into zinc hydroxide Zn(OH)2, which is then transformed into ZnO during calcination. Finally, ZnO undergoes a growth phase by electrostatic interaction, forming ZnO NPs [104,105]. Another proposed mechanism has been reported in the literature which involves the formation of ZnO NPs upon the reduction of Zn2+ ions, by various phytochemicals in the aqueous extract, to nanoscale zinc particles (Zn0) which react with dissolved oxygen to form ZnO, followed by capping with active phytochemicals to prevent agglomeration and increase the stability of ZnO NPs [105].
The redox potential of the phytochemicals is very important. It determines the extent of reduction that can occur. In the case of ZnO NP synthesis, the redox potential is usually not high enough to completely reduce Zn2+ ions to zerovalent Zn, resulting in the formation of ZnO NPs. The redox potential of the phytochemicals can be influenced by various factors such as the presence of functional groups, the presence of electron-donating or electron-withdrawing substituents, and the overall chemical reaction conditions (reaction time, temperature, pH, types of phytochemicals, and plant extract and metal salt concentrations) [106,107]. The other attribute is related to the stability and complexation of Zn2+ complexes [107]. Phytochemicals often form stable complexes with metal ions. In the case of ZnO NP synthesis, these complexes help in the stabilization of ZnO NPs by preventing their aggregation and facilitating controlled growth. The presence of functional groups in phytochemicals, such as hydroxyl (–OH), carboxyl (–COOH), or amino (–NH2) groups, allows for the binding and complexation with Zn2+ ions, leading to the formation of ZnO NPs. Furthermore, the pH of the reaction medium plays a significant role in the formation of ZnO NPs. Most green synthesis methods involve alkaline conditions (pH > 7), which promote the formation of ZnO. Under these alkaline conditions, Zn(OH)2 is formed, which subsequently reacts to produce ZnO NPs [108].
Alamdari et al. [109] describe the mechanism of the green synthesis of ZnO NPs using the Sambucus ebulus (S. ebulus) leaf extract (Figure 2). The authors proposed a reaction between flavonoid/phenolic molecules with Zn2+ ions via the donor–acceptor mechanism, resulting in Zn2+ complexes and the formation of ZnO NPs (Figure 3).
![Figure 3
A schematic diagram representing the experimental work and the possible mechanism of the green synthesis of ZnO NPs using the leaf extract of S. ebulus [109].](/document/doi/10.1515/ntrev-2023-0112/asset/graphic/j_ntrev-2023-0112_fig_003.jpg)
A schematic diagram representing the experimental work and the possible mechanism of the green synthesis of ZnO NPs using the leaf extract of S. ebulus [109].
6 Cytotoxic mechanisms of ZnO NPs
ZnO NPs are one of the most commonly used MONPs in biomedical applications as antibacterial and anticancer agents and in the cellular imaging fields [110,111,112]. The cytotoxic effect of ZnO NPs is mainly concerned with changing the cytoskeleton and nucleoskeleton of proteins and/or generating reactive oxygen species (ROS) in cells exposed to ZnO NPs [113]. Ultimately, three mechanisms are involved simultaneously in the cytotoxicity of ZnO NPs including the release of Zn2+, production of ROS, and direct interaction between ZnO NPs and cells [28].
6.1 Release of Zn2+ ions from ZnO NPs
One of the main mechanisms of ZnO NP cytotoxicity is that these NPs dissolve into free Zn2+ in the lysosomal acidic media, causing lysosomal destabilization, mitochondrial dysfunction, and disruption of cellular zinc homeostasis, leading to cell death [114]. The release of Zn2+ into the cells enables the dynamic transport of membrane, and mitochondrial and DNA damage [115]. The dissolved Zn2+ induced lactate dehydrogenase (LDH) leakage into the cell culture medium, which indicates cell membrane damage. Additionally, it was found that there is a significant relationship among the Zn2+ content, cell viability, and the LDH level [115]. Murali et al. [15] discussed the potential cytotoxicity of ZnO NPs that stems from the release of Zn2+ and hence their role in different biomedical applications including antibacterial, antidiabetic, antifungal, anticancer, anti-inflammatory, and antioxidant. It has been reported that Zn2+ is released due to the dissolution of ZnO NPs which may occur either extracellularly or intracellularly [116]. In the extracellular dissolution of ZnO NPs and due to their small size, Zn2+ can enter the cytoplasm via the cell membrane, inducing the production of ROS that leads to oxidative stress in many cells. However, the intracellular dissolution of ZnO NPs involves the release of Zn2+ due to the acidic environment of lysosomes after they were internalized by endocytosis, inducing oxidative stress and apoptosis [117]. Additionally, Zn2+ is one of the main contributors to the antibacterial activity of ZnO NPs, where Zn2+ interacts with proteins in bacteria and oxidize their amino acids, leading to protein denaturation and loss of enzyme activity. This results in the blockage of the metabolic and growth functions [118]. To summarize, the released Zn2+ in high levels contributed to the cytotoxicity of ZnO NPs.
6.2 Production of ROS
The second mechanism of ZnO NP cytotoxicity involves the production of ROS. When ZnO NPs enter the cells, the defense mechanism begins inside the cells, generating ROS. If the generation of ROS exceeds the limit of the antioxidant capacity of cells, ROS produces pro-inflammatory cytokines, which initiate inflammation. Inflammation induces mitochondrial disturbance that leads to an impairment in the cellular membrane, cellular components, and DNA, in addition to an increase in LDH from necrosis or apoptosis, leading to cell death [119,120]. Additionally, the generation of the intracellular ROS and the entrance of the anticancer agent into cancer cells are the main causes of destroying the electron transport chain [121]. A high amount of ROS leads to mitochondrial damage and loss in protein activity, leading to cell apoptosis [122,123,124].
In 2018, Attia et al. [125] studied the neurotoxicity of ZnO NPs when given orally to rats in different doses. They reported that ZnO NPs may reach the brain when administered orally. In addition, the exposure of cells to ZnO NPs decreases the concentration of antioxidants such as glutathione (GSH), catalase, and superoxide dismutase, where these antioxidants generally prevent or repair the damage caused by ROS and regulate the redox-sensitive signaling pathways. Thus, exposure to ZnO NPs resulted in DNA damage confirmed by increasing the percentage of DNA tail, length, and intensity. This indicates that ZnO NPs deteriorate and damage the antioxidant system via developing ROS in the brain, elevating the inflammatory response, cytokines, DNA fragmentation, and apoptosis [125]. Therefore, the green-synthesized ZnO NPs displayed oxidative stress-mediated cytotoxic effects.
6.3 Direct interaction between ZnO NPs and cells
It has been shown that there is a direct interaction between ZnO NPs and cells, resulting in a change in the cell morphology, disruption of the membrane, damage of mitochondria, and spillage of intracellular structure [126,127]. In addition, exposure to ZnO NPs changed the cellular distribution of lipid biosynthetic enzymes and the structure of the endoplasmic reticulum (ER), mitochondria, and ER–mitochondria encounter structure complex, leading to cell death [126]. Altogether, the interaction of ZnO NPs with cells caused cell death by affecting the integrity of the cell wall, homeostasis of ER, and the generation of ROS and saturated free fatty acids [126]. Yu et al. [127] showed that exposure to ZnO NPs leads to cell death via the accumulation of autophagic vacuoles and the damage of the mitochondria in normal skin cells via inducing the generation of ROS. Figure 4 illustrates the three mechanisms involved in the cytotoxicity of ZnO NPs.
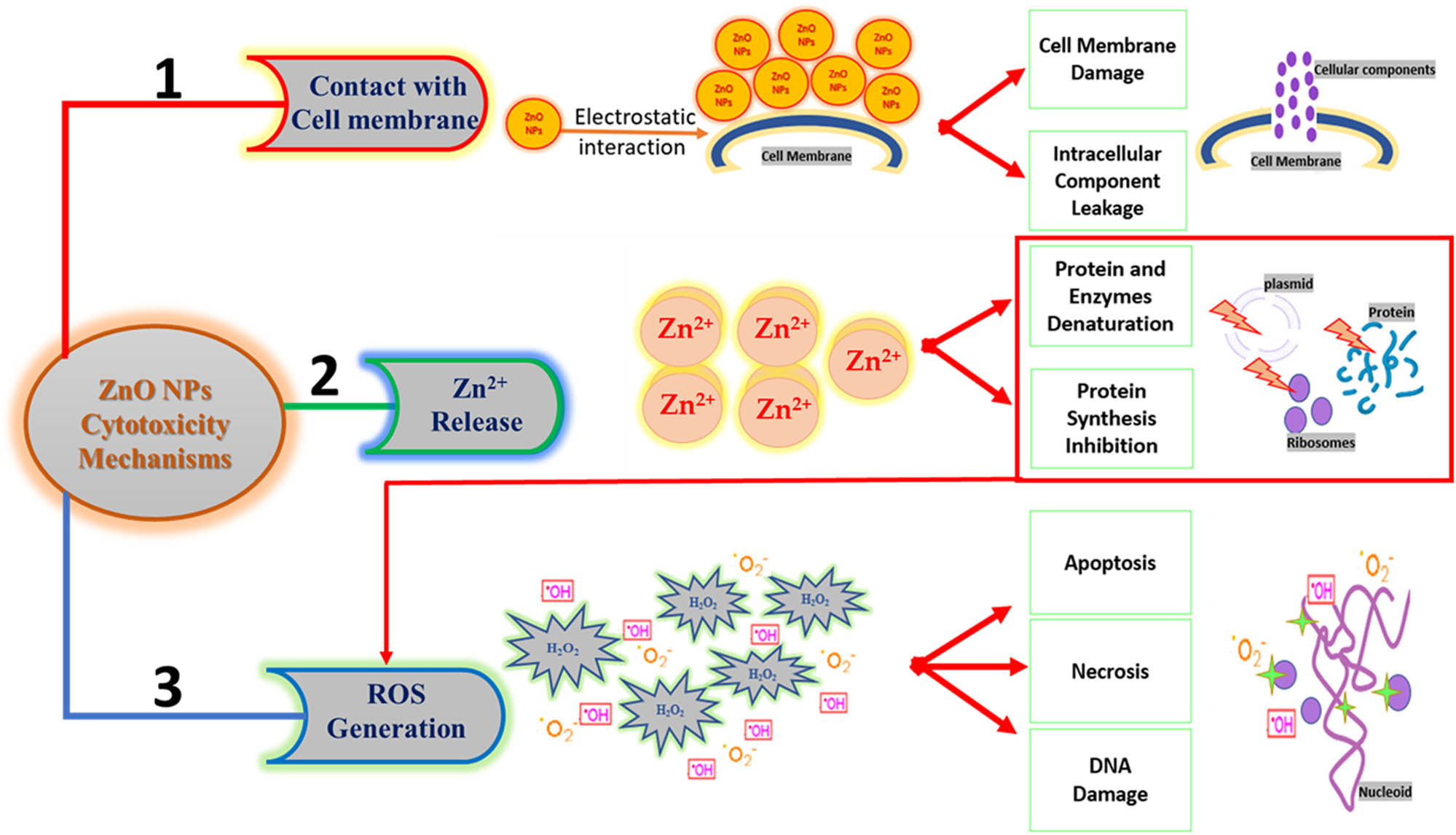
The three mechanisms involved in the cytotoxicity of ZnO NPs: (1) direct contact with the cell membrane, (2) release of Zn2+, and (3) generation of ROS.
7 Biomedical applications of ZnO NPs
The use of eco-friendly, safe, inexpensive, and dexterous ways for the green synthesis of ZnO NPs has offered a revolution in various biomedical fields [111,128,129]. ZnO NPs have been employed in many life applications including drug delivery, cosmetics, medical installations (as antibacterial paints in hospitals), dentistry (for blocking microbial leakage), and orthopedics (as a reinforcing material) [35]. Recently, ZnO NPs showed promising biomedical applications such as antibacterial, anticancer, antidiabetic, antioxidant, antifungal, antiviral, and anti-inflammatory, and in wound healing [13,14,15,16]. Biomedically, several studies have proven the nontoxic effect of the surface-modified ZnO NPs, using hyaluronan, PEG, and Triton X-100, on normal human cells, without affecting the anticancer effects of ZnO NPs [111].
7.1 Antibacterial activity of ZnO NPs
The green-synthesized ZnO NPs exhibited a substantial antibacterial activity for wide-spectrum bacteria, whereby interacting with the bacterial cell surface and its core, ZnO NPs can enter inside the cells and display their bactericidal effect [130,131,132]. Therefore, the antibacterial activity of ZnO NPs revealed a promising potential to be linked with antibiotic functions, overcoming antimicrobial resistance [36]. In addition, the toxic interaction between ZnO and bacteria has proved as an antibacterial agent in the food industry [102]. This is attributed to their high specific surface area and high activity to block a wide range of pathogenic bacteria [14,133]. Recently, the antibacterial activity of ZnO NPs was proved by their ability to generate ROS such as hydroxyl radicals (·OH), hydrogen peroxide (H2O2), superoxide anions (O2 · −), and singlet oxygen (1O2). The four ROS displayed different levels of antibacterial activity. It has been shown that O2˙− and H2O2 exhibited lower antibacterial activity than ˙OH and 1O2. This is because O2˙− and H2O2 are less reactive and can be detoxified by endogenous antioxidants that are induced by oxidative stress. However, no enzyme can detoxify ˙OH or 1O2, making them more toxic [134]. Additionally, it has been reported that the negative charge of hydroxyl radicals (˙OH) and superoxide (O2˙−) prevents their penetration into the bacterial cell wall. However, the direct contact between these ROS and bacterial membranes can cause bacterial death. Whereas, hydrogen peroxide (H2O2) can penetrate through the bacterial cell wall, getting internalized in the bacterial cell, causing cell death [104,135,136]. Additionally, the antibacterial activity involves the accumulation of ZnO NPs in the outer membrane or in the cytoplasm of the bacterial cells which triggers the release of Zn2+, causing disintegration of the cell membrane, damaging the membrane proteins, and instability of bacterial genomics, resulting in bacterial cell death [137,138]. Because ZnO has an amphoteric nature, it can react with acids and bases, releasing Zn2+ which binds to the bacterial biomolecules [139]. Figure 5 shows a schematic diagram of the antibacterial mechanisms of ZnO NPs.
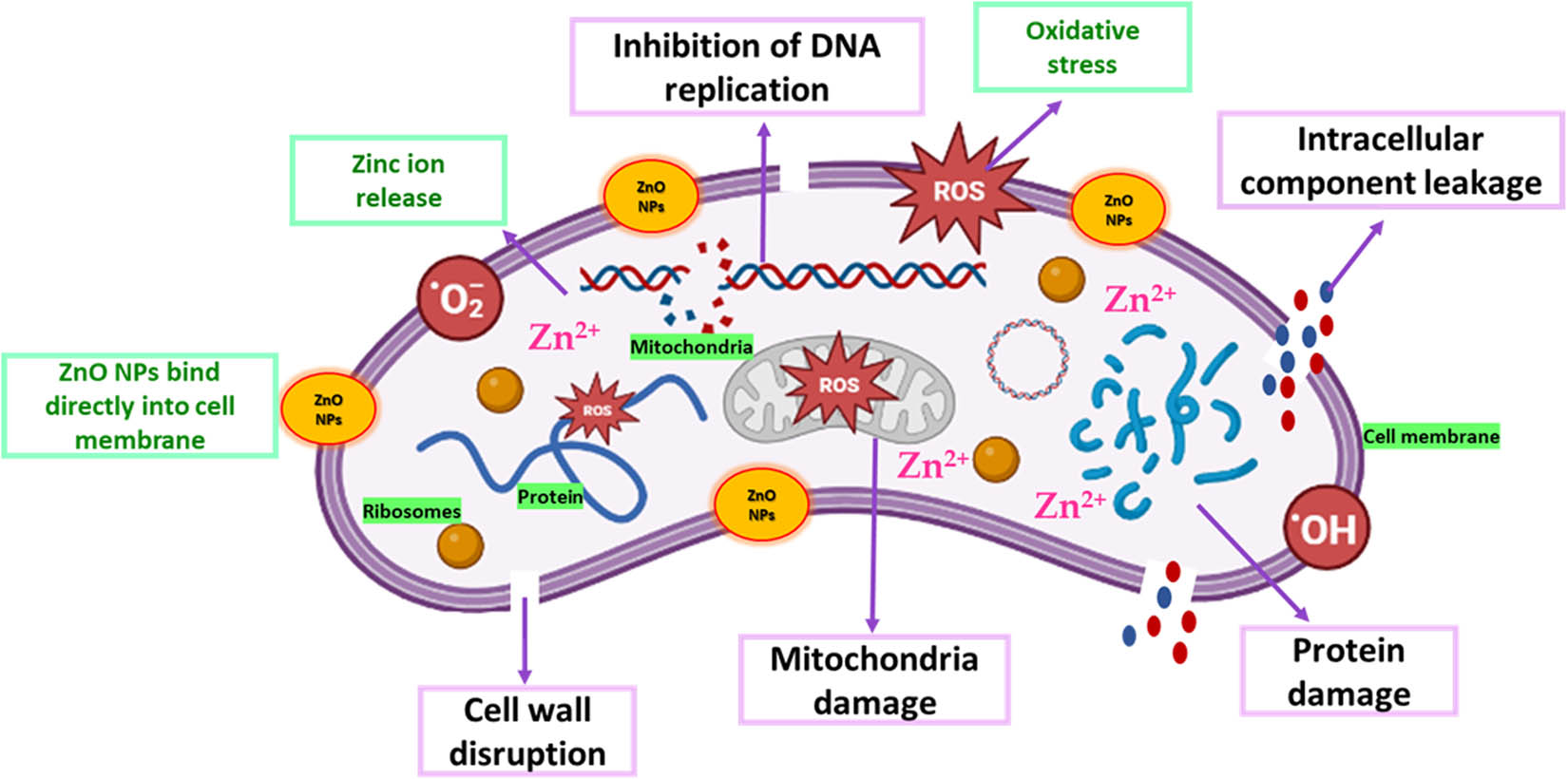
Schematic diagram representing the antibacterial mechanisms of ZnO NPs.
Although ZnO NPs showed antibacterial activity against both Gram-positive and Gram-negative bacteria [135], the antibacterial activity of ZnO NPs depends on the sensitivity of the microorganism and the difference in the cell wall structure of Gram-positive and Gram-negative bacteria [140]. The membrane of Gram-positive bacteria is composed of a thick peptidoglycan layer (20–80 nm) that is covalently attached to teichoic, lipoteichoic acids, and surface proteins [135]. This thick layer acts as a physical barrier that protects the cells from the exterior environment, whereas the membrane of the Gram-negative bacteria is composed of a thin peptidoglycan layer (∼8 nm) and a thick lipopolysaccharide external layer (1–3 µm) [135,136].
Reddy et al. [141] reported that the minimum inhibitory concentration (MIC) of ZnO NPs (13 nm) in the Gram-positive (Staphylococcus aureus, S. aureus) and Gram-negative (Escherichia coli, E. coli) was 1 and 3.4 mg ml−1, respectively. This indicates that the inhibition of Gram-positive bacteria required a lower concentration of ZnO NPs than the Gram-negative bacteria. This was attributed to the fact that the peptidoglycan layer of S. aureus can promote the attack of ZnO NPs inside the cell, whereas the cell wall components of E. coli, particularly lipopolysaccharides, can impede this attack [135]. Similar results were reported by Tayel et al. [142], who studied the antibacterial activity of ZnO powder and ZnO NPs against nine bacterial strains. The study showed that ZnO NPs were more efficient as antibacterial agents than the powder and that Gram-positive bacteria were more sensitive to ZnO NPs than Gram-negative bacteria. Contrarily, Shinde et al. [140] showed that the antibacterial activity of ZnO microspheres against S. aureus was lower than E. coli. Researchers suggested that the difference in the cell wall structure of Gram-positive and Gram-negative bacteria is responsible for this outcome, where the thick peptidoglycan layer of S. aureus acts as a physical barrier, preventing the penetration of ZnO NPs through the cell wall. However, the thinner peptidoglycan layer of E. coli allows the penetration of ZnO NPs, rupturing the cell wall [140].
Moreover, d’Agua et al. [143] evaluated the antibacterial activity of ZnO NPs containing cotton fibers against Gram-positive bacteria (S. aureus, S. epidermidis, and Propionibacterium acnes [P. acnes]) and Gram-negative bacteria (E. coli and Pseudomonas aeruginosa [P. aeruginosa]). The agar diffusion and absorption methods were used in the study. The results showed that ZnO NPs containing cotton fibers exhibited higher antibacterial activity against Gram-positive bacteria than Gram-negative bacteria. The lower sensitivity of ZnO NPs toward Gram-negative bacteria can be attributed to the complex structure of the bacterial cell wall, where the thick outer lipopolysaccharide membrane surrounds the thin peptidoglycan layer and acts as a barrier, protecting against ZnO NPs. The authors reported that the antimicrobial studies of ZnO NPs against Gram-positive and Gram-negative bacteria showed contradictory results, where some studies have shown a stronger antibacterial effect of ZnO NPs on Gram-positive bacteria, whereas other studies have shown that Gram-negative bacteria are more sensitive to ZnO NPs [143].
Jiang et al. [144] studied the interaction between ZnO NPs and the Gram-negative bacteria E. coli to examine the antibacterial mechanism of ZnO NPs. It was found that using ZnO NPs (30 nm) and the direct contact between ZnO NPs and the phospholipids bilayer of E. coli membrane led to a disruption in the E. coli membrane, measured by quantifying the leakage of the intracellular K+ from the bacterial membrane, causing cell death. Although mannitol, vitamin E, and GSH were used as radical scavengers to quench the release of ROS generated from ZnO NPs and thereby suppress their antibacterial activity, the antibacterial activity of ZnO NPs against E. coli indicates the generation of ROS, particularly the (·OH) radicals.
Agarwal et al. [102] reviewed the mechanism of the antibacterial activity of the green-synthesized ZnO NPs. The results showed that ZnO NPs, due to their large surface area to volume ratio, can bind to the bacterial cell surface via a large number of ligand binding sites like proteins, lipids, phospholipids, and lipoteichoic acid. Additionally, the authors reported that Gram-negative bacteria have less resistance to ZnO NPs due to their thin layer of peptidoglycan, making ZnO NPs more toxic to this type of bacterium. The antibacterial mechanisms of ZnO NPs involve the disruption of cell membranes, release of Zn2+ resulting in ROS generation, DNA disruption, protein oxidation, lipid peroxidation, and metabolic enzyme inhibition which blocks food or respiratory pathways, causing cell death.
The cytotoxicity of ZnO NPs depends on different parameters of NPs such as size, shape, surface charge, and concentration. In addition, the pH and temperature of the culture medium impact the antibacterial activity of ZnO NPs, where the acidic pH from 4 to 5 and high temperatures increased the antibacterial activity of ZnO NPs. This is because acidic media enhanced the dissolution rate of ZnO NPs, hence increasing the concentration of Zn2+ in the media and improving the activity against the microorganisms [102,145]; however, high temperatures enhanced the production of ROS [146,147].
Several studies have confirmed the relationship between the size and morphology of ZnO NPs and their antibacterial activities [104,118,148]. Recently, Sharma et al. [148] studied the antibacterial activity of different sizes and shapes of ZnO NPs, prepared from the plant extract of Aloe vera. The size of ZnO NPs ranged between 40 and 180 nm with hexagonal, spherical, cylindrical, and cuboidal shapes. The cuboidal ZnO NPs exhibited higher antibacterial activity against S. aureus, B. subtilis, and E. coli, compared to the spherical and hexagonal ZnO NPs. Another study by Álvarez-Chimal et al. [118] investigated the effect of the size of the green-synthesized ZnO NPs (7–130 nm), prepared from the Dysphania ambrosioides (D. ambrosioides) extract, on the antibacterial activity against various pathogenic bacteria. It was found that the smaller the size of ZnO NPs (4–10 nm), the higher the antibacterial potential, particularly against S. aureus and E. coli. A very recent study by Fouda et al. [104] demonstrated the high antimicrobial potential of spherical ZnO NPs (10–45 nm), prepared from the peel aqueous extract of Punica granatum (P. granatum), against Gram-positive and Gram-negative bacteria, and unicellular fungi with an MIC of 12.5–6.25 µg ml−1. The authors attributed the high antimicrobial potential to the high generation of ROS when pathogenic microbes are treated with smaller sizes of ZnO NPs.
Additionally, it has been shown that the interaction between NPs and bacterial membranes is influenced by the shape of NPs, where the triangular-shaped NPs showed better interaction with cell membranes than the rod or spherical ones, enhancing the antibacterial effect [102]. Moreover, the shape of ZnO NPs affects the release of Zn2+, where the shape influences the surface area of NPs and hence their solubility [102]. For instance, it has been shown that the spherical-shaped ZnO NPs showed better solubility and higher Zn2+ release than the rod-shaped ones [149].
The surface charge of ZnO NPs is one of the important parameters that determine their antibacterial activity, where a correlation was established between the zeta potential of NPs, the surface charge of bacterial cells, and the antibacterial activity [150,151]. For instance, Mendes et al. [152] stated that the positively charged ZnO NPs showed better binding toward the negatively charged surface cell membrane via electrostatic interactions, improving the toxicity of NPs against Gram-positive and Gram-negative bacteria. Teichoic acid in the peptidoglycan layer and lipoteichoic acid in the membrane, responsible for the negative charge of the bacterial cell membrane, act as chelating agents for Zn2+ which is carried by passive diffusion across membrane proteins [152].
Thi et al. [153] reported the synthesis of spherical ZnO NPs (20 nm) from the orange fruit peel extract and zinc acetate dihydrate which were used as a reducing agent and a chemical precursor, respectively. The antibacterial activity of ZnO NPs was studied against E. coli and S. aureus. The results showed a strong bactericidal effect of >99.9% against E. coli and 89.4–98.1% against S. aureus at an NP concentration of 0.025 mg ml−1 and 8 h of incubation. Additionally, the mechanism of the bactericidal activity of ZnO NPs was based on the generation of ROS and their interaction with cell membranes, resulting in DNA and cell wall damage. In the same year, Umavathi et al. [154] synthesized green spherical ZnO NPs from the Parthenium hysterophorus (P. hysterophorus) leaf extract by a single-step process. ZnO NPs were characterized by UV-Vis spectroscopy and FTIR to confirm the presence of flavonoids, phenolics, tannins, phytic acid, and ZnO, whereas the TEM and SEM micrographs revealed the spherical shape and the nanosized range of 10 nm of ZnO NPs. The XRPD results confirmed the formation of the hexagonal wurtzite structure. ZnO NPs exhibited strong antibacterial activity against Gram-positive and Gram-negative organisms and fungal strains with a concentration-dependent effect. For instance, it was found that 10 mg of ZnO NPs was sufficient to exhibit a maximum inhibitory concentration against E. coli. Moreover, Safavinia et al. [155] used Daphne oleoides (D. oleoides) leaf extract, which contains five polyphenolic compounds, to prepare ZnO NPs and embed them into silica gel (SG) matrix, forming ZnO/SG nanocomposites. The antibacterial activity of ZnO/SG nanocomposites was evaluated against pathogenic bacteria and compared with the unembedded ZnO NPs. Data showed that ZnO NPs were stabilized by the SG matrix which prevents their agglomeration. Additionally, the surface area of ZnO/SG nanocomposites was higher than the unembedded ZnO NPs, demonstrating greater antibacterial activity against pathogens than the unembedded ZnO NPs.
The antibacterial activity of green-synthesized ZnO NPs was compared to their chemical counterparts. Gunalan et al. [133] found that the antimicrobial activity of green-synthesized ZnO NPs (40 nm), prepared from aloe leaf extract, was higher than that of the chemical ZnO NPs (25 nm). The antimicrobial activity was tested against different types of bacteria and fungi at various concentrations. In another study [156], the antibacterial activity of ZnO NPs, prepared from the leaf extract of Sesbania grandiflora (S. grandiflora), was compared to that of the chemical ZnO NPs, prepared by the co-precipitation method. The study showed that the green-synthesized ZnO NPs exhibited higher antibacterial activity against Gram-positive (S. aureus) and Gram-negative (P. aeruginosa) bacteria, in agreement with Gunalan et al. [133]. Similarly, a recent study by Bekele et al. [157] compared the antibacterial activity of the green (11–21 nm) and chemically synthesized (30–40 nm) ZnO NPs against Bacillus subtilis (B. subtilis), S. aureus, and Salmonella typhimurium (S. typhimurium). The green and chemical ZnO NPs showed a strong antibacterial activity with zones of inhibition of 15–24 and 7–15 mm, respectively, suggesting that the green-synthesized ZnO NPs showed promising antibacterial activity.
7.2 Anticancer activity of ZnO NPs
Scientists are putting a lot of effort to develop effective treatments to kill cancer cells with minimum side effects. Nowadays, numerous studies have been performed to advance the use of green-synthesized ZnO NPs to treat different types of cancers [158,159,160]. The role of ZnO NPs involves the anticancer activity on different types of cancers and targeted drug delivery by using ZnO NPs as nanocarriers for chemotherapeutic drugs.
7.2.1 Role of ZnO NPs in anticancer activity
ZnO NPs cause oxidative stress in cancer cells, leading to their damage, due to the rapid dissolution of ZnO NPs into Zn2+ at acidic pH [70]. The low endosomal pH of 6.3, 5.5, and 4.7 in the early endosome, late endosome, and endosome, respectively, induce the release of soluble Zn2+, causing lysosomal destabilization and cell death [28]. The higher selectivity of ZnO NPs toward cancer cells, compared to normal cells, is attributed to the electrostatic interaction between the positively charged ZnO NPs, under physiological conditions, and the anionic phospholipids on the outer membrane of cancer cells which are present at high concentration. This interaction increases the cellular uptake of ZnO NPs by cancer cells and hence their cytotoxicity [161]. Hanley et al. [162] showed that ZnO NPs have 28–35 times more selective cytotoxicity against cancer cells compared to normal cells. Additionally, they reported that altering the surface properties of ZnO NPs may further enhance their cytotoxicity against cancer cells [162]. Moreover, cancer cells possess higher levels of ROS, owing to their rapid metabolism, when compared to normal cells. Hence, when cancer cells are treated with ZnO NPs, more ROS are produced, resulting in severe oxidative stress that promotes cell death [28,161]. Finally, the enhanced permeation and retention effect in cancer cells to ZnO NPs, due to their small size and surface properties, enhances the cytotoxicity of ZnO NPs against cancer cells compared to normal cells [28].
Sharmila et al. [163] used the Tecoma castanifolia (T. castanifolia) leaf extract for the green synthesis of spherical ZnO NPs (70–75 nm) to evaluate their anticancer activity against lung cancer. The results demonstrated that ZnO NPs exhibited antibacterial, antioxidant, and anticancer activities. Additionally, it was found that an increase in the ZnO NP concentration proportionally increases the radical scavenging activity. ZnO NPs exhibited an anticancer activity with half-maximal inhibitory concentration (IC50) around 65 µg ml−1, confirmed by the cytotoxic effect on the proliferation of human lung carcinoma (A549) cells, tested by 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Thus, ZnO NPs can be used as nano-delivery systems in various biomedical applications. Ruangtong et al. [164] used banana peel crude extract as a reducing and capping agent and zinc acetate as a chemical precursor to synthesize ZnO NPs with rod-like or sheet-like structure, where the shape of NPs was varied based on the crude extract concentration used in the reaction. The ZnO NPs and nanosheets were investigated for their antibacterial and anticancer activities against skin, colorectal, and liver cancers. The ZnO nanosheets inhibited the growth of Gram-negative (E. coli) and Gram-positive B. subtilis and Staphylococcus epidermidis (S. epidermidis) bacteria. In addition, ZnO nanosheets demonstrated potent anticancer activity against colorectal (SW620), skin (A431), and liver cancer cells (HepG2) without affecting the normal cells. Similarly, Jevapatarakul et al. [165] synthesized green ZnO NPs (200 nm) and nanosheets (500 nm) from the crude extract of Cratoxylum formosum (C. formosum) leaves to assess their anticancer activity against non-melanoma skin cancer cells. The shape of ZnO NPs varied based on the concentration of the crude extract and the synthesis process. The cytotoxic activity of ZnO NPs nanosheets was evaluated by testing the cell viability on epidermoid carcinoma cell line (A431), normal kidney fibroblasts (Vero), and liver cancer cells (HepG2). ZnO NPs at 120 µg ml−1 concentration significantly inhibited the cell viability of A431 cells without affecting Vero cells. Contrarily, a higher concentration of ZnO NPs (300 µg ml−1) showed a weak cytotoxic activity when applied to the HepG2 liver cell line. The ZnO NPs nanosheets showed greater cytotoxicity against A431 cells when compared to the spherical ZnO NPs (∼200 nm). Additionally, the spherical ZnO NP nanosheets inhibited the growth of E. coli, B. subtilis, and S. epidermidis. Moreover, ZnO NP nanosheets altered several transcript pathways responding to the generation of ROS and hydrogen peroxides that are involved in the cytotoxic effect on A431, causing cell death due to oxidative stress and inflammatory response [165].
Naser et al. [72] synthesized ZnO NPs using the root hair extract of Phoenix dactylifera (P. dactylifera) and 0.6 M zinc acetate dihydrate to assess their anticancer activity against lung and breast cancers. The spherical ZnO NPs (31–48 nm) were 45% more cytotoxic than doxorubicin (DOX) hydrochloride alone. The ZnO NPs proved their cytotoxic efficacy by reducing the cell viability of the triple negative breast cancer cell line (TNBC) to 9.0% and showed 82.3% cytotoxic efficacy against lung cancer cell line (A549), confirming that ZnO NPs exhibited a potent anticancer activity. Moreover, ZnO NPs showed higher antibacterial activity against various microorganisms than those of penicillin, gentamycin, and tetracycline. A follow-up study conducted by the same research group evaluated the loading of these ZnO NPs into transdermal patches for breast cancer therapy [71]. Patches were prepared from the film-forming polymer Carbopol, backing layer ethylene-vinyl acetate, and ZnO NPs (88 nm) with a zeta potential of −16.63 mV. Patches were clear, transparent, and flexible with uniform thickness and weight of 0.25 mm and 0.42 g, respectively, and a surface pH of 6.25. The content of ZnO NPs in the patches was 91.7–94.4%. In addition, ZnO NPs from patches exhibited a sustained release profile over 25 h. Moreover, the cytotoxic effect of ZnO NPs against the TNBC cell line was dose-dependent ranging from 0.16 to 2.5 µg ml−1 with IC50 of 0.42 µg ml−1, significantly lower than the IC50 of DOX (4.58 µg ml−1) (p < 0.05). Whereas the IC50 on human dermal fibroblast (HDF) was 1.61 µg ml−1, demonstrating a stronger effect of ZnO NPs in inhibiting the growth of cancerous cells, compared to that of DOX. Thus, ZnO NP-loaded patches may offer a potential transdermal delivery platform for breast cancer treatment by overcoming the limitations of invasive chemotherapy delivery.
In 2020, Duan et al. [166] synthesized ZnO NPs from Cardiospermum halicacabum (C. halicacabum) using an eco-friendly green method. The spherical shape of ZnO NPs (10–20 nm) was determined by TEM and evaluated against skin melanoma. The antitumor activity and apoptosis for the prepared ZnO NPs were explored using a human melanoma cell line (A375). The ZnO NPs exhibited cytotoxic activity against cancer cells. In addition, the exposure to ZnO NPs resulted in an elevation in the ROS level and apoptotic markers (caspases 3, 8, and 9) and stimulation of apoptotic cell necrosis in the tumor cells. Furthermore, Selim et al. [167] synthesized ZnO NPs from the aerial part of Deverra tortuosa (D. tortuosa) to examine their anticancer activity against colorectal and lung cancers that are associated with the greatest mortality. The plant was collected from a natural ecosystem in Egypt. The resulting ZnO NPs (15.2 nm) showed selective cytotoxic activity against two cancer cell lines, the human lung cancer cell line (A594) and the human colon cancer cell line (Caco-2), providing a safer alternative platform to conventional cancer therapy.
The research on the green synthesis of ZnO NPs has been continued. Recently, Vakayil et al. [168] synthesized ZnO NPs from rhizomes of the Acorus calamus (A. calamus) to examine their cytotoxic effect against skin cancer. The ZnO NPs were then coated with cotton fabrics to reduce skin infections. The cotton-coated ZnO NPs showed a cytotoxic and antiproliferative activity against the SK-MEL-3 skin cancer cell line which was proved by the morphological changes in cells such as shrinkage, rounding, irregular shape, and detachment in cancer cells. In addition, the MTT assay test showed a suppression in cell viability against the SK-MEL-3 cell line with an IC50 value of 17.50 µg ml−1 of the coated ZnO NPs. Finally, the cotton-coated ZnO NPs efficiently inhibited the growth of bacteria, demonstrating a potential antibacterial activity. Thus, the cotton-coated ZnO NPs could be utilized in the medical field in the future.
A recent study by Chandrasekaran et al. [169] compared the anticancer activity of ZnO NPs, prepared by green and chemical methods, against the MCF-7 breast cancer cell line. The green-synthesized ZnO NPs were prepared using the Vinca rosea (V. rosea) leaf extract, whereas chemical ZnO NPs were prepared by the precipitation method. The results showed that the anticancer activity of the green-synthesized ZnO NPs (16–41 nm) with spherical shape was higher compared to chemically synthesized counterparts [169].
7.2.2 Role of ZnO NPs in targeted drug delivery
The combination between ZnO NPs and the chemotherapeutic agents demonstrated a potential effect in treating cancer. George et al. [170] studied the therapeutic effect of quercetin (QE), extracted from onion peel waste and formulated as chitosan hydrogel. Using melon seeds, the QE hydrogel matrix was loaded with green-synthesized ZnO NPs forming QE-loaded nanohybrid hydrogels. The release of QE from the hydrogels was the highest in an acidic environment (pH 5), thus suitable for anticancer applications. The growth of S. aureus and Trichophyton rubrum (T. rubrum) strains was inhibited by the commercial QE and QE-loaded nanohybrid hydrogels. The cytotoxicity and anticancer activity of QE-loaded nanohybrid hydrogels were studied against skin cancer using normal murine fibroblast cells (L929) and human dermal carcinoma cell lines (A431), respectively. It was found that the QE-loaded nanohybrid hydrogels were biocompatible toward healthy cells and exhibited enhanced anticancer activity toward A431, compared to commercial QE. The kinetic drug release mechanism followed Fickian diffusion. This suggests that the combination of ZnO NPs and QE in the QE-loaded nanohybrid hydrogels showed synergistic antibacterial and anticancer activities via enhancing the cellular uptake of QE, highlighting their potential applications in the biomedical fields. Recently, Chelladurai et al. [171] synthesized amine-functionalized ZnO NPs that were covalently linked to mupirocin using an organosilane linker. Mupirocin is a crotonic acid derivative extracted from Pseudomonas fluorescens (P. fluorescens) used to treat superficial skin infections like impetigo and microbial infections caused by Gram-positive and Gram-negative bacteria. The ZnO NPs were green synthesized from the Alpinia calcarata (A. calcarata) rhizome extract. The effect of mupirocin-loaded ZnO NPs on skin cancer and skin infection was investigated. The green spherical ZnO NPs (19 nm) exhibited a crystallite grain size of 24.75 nm. The conjugated NPs showed a strong antiproliferative effect of 63% on human epidermoid carcinoma cells (A431), providing a new nanotechnology platform for skin cancer therapy. Moreover, mupirocin-loaded ZnO NPs demonstrated potency as antioxidant agents and significantly inhibited the growth of Vibrio cholerae (V. cholerae), Enterococcus faecalis (E. faecalis), and Listeria monocytogenes (L. monocytogenes).
Additionally, it has been shown that the biocompatibility and biomedical activity of coated and embedded ZnO NPs were further improved compared to their uncoated and unembedded counterparts [155,172]. For instance, Batool et al. [172] greenly synthesized ZnO NPs (20–40 nm) using the Aloe barbadensis (A. barbadensis) leaf extract. The ZnO NPs were loaded with the chemotherapeutic agents, doxorubicin (DOX) and gemcitabine (GEM), and were studied for their antitumor cytotoxic activity against breast cancer (the leading cause of cancer death among females), surface-PEGylation, and drug loading capacity. PEGylated ZnO NPs (PEG-ZnO NPs) and their non-PEGylated counterparts (ZnO NPs) showed higher DOX loading capacity and encapsulation efficiency compared to GEM. In addition, DOX/PEG-ZnO NPs and DOX/ZnO NPs exhibited significant anticancer activity against TNBC, with DOX/PEG-ZnO NPs showing greater anticancer activity. The loading of DOX into green-synthesized ZnO NPs has been investigated by Vimala et al. [173], who used the palm fruit extract Borassus flabellifer (B. flabellifer) to treat breast and colon carcinoma. The results proved that DOX/ZnO NPs exhibited high efficacy against breast and colon cancer, and thus are considered a promising drug delivery system. Therefore, plant-mediated green-synthesized ZnO NPs could have huge applications in cancer treatment and thus become a major area of research.
Finally, the anticancer potential of different parts of a plant might vary based on the specific plant species and the phytochemicals present in those parts. Because it is difficult to make a general statement about which part of plants consistently shows the best anticancer potential, different parts of plants have been studied extensively for their anticancer activity. Leaves, roots, stems, flowers, and even certain fruits of various plants have demonstrated anticancer activity [174]. It is important to note that the presence and concentration of phytochemicals might vary among different plant parts. Additionally, the extraction methods used to obtain these compounds can affect their potency. In some cases, leaves have been found to possess high levels of phytochemicals such as polyphenols, flavonoids, and alkaloids, which exhibit anticancer activity [175]. Leaves are often rich in phytochemicals due to their primary role in photosynthesis [176]. However, this does not necessarily mean that leaves always show the best anticancer potential compared to other plant parts. Contrarily, the roots of certain plants have also been extensively studied for their anticancer activity [177,178]. Roots may contain a range of phytochemicals such as alkaloids, terpenoids, and polysaccharides, which contribute to their therapeutic potential [179]. Ultimately, the choice of the plant part to be investigated for its anticancer potential depends on the specific plant species, desired phytochemicals, and traditional or scientific knowledge about the plant’s medicinal properties. Further research is still necessary to compare the effectiveness of different plant parts and their phytochemicals in combating cancer cells.
In the context of anticancer applications, the association of phytochemicals with ZnO NPs can enhance their cytotoxic activity against cancer cells [20,180]. Phytochemicals, such as polyphenols and flavonoids, have shown anticancer activity on their own [181]. When attached to the surface of ZnO NPs, these compounds can potentially exert synergistic effects, leading to increased cytotoxicity against cancer cells [182]. For example, a study by Gobinath et al. [183] demonstrated that the metabolites present in the leaf extract of Calotropis gigantea (C. gigantea) enhanced the cytotoxicity of ZnO NPs against human breast, cervical, and hepatic cancer cells.
7.3 Antidiabetic activity of ZnO NPs
Zinc is a vital key factor in the glucose metabolic process which improves hepatic glycogenesis [184]. In addition, Zn reduces gluconeogenesis and glycogenolysis processes by inhibiting glucagon excretion [185]. Rajakumar et al. [186] used the leaf extract of Andrographis paniculata (A. paniculata) as a reducing and capping agent to synthesize ZnO NPs and study their antioxidant, antidiabetic, and anti-inflammatory activities. The antidiabetic activity was investigated by evaluating the α-amylase inhibitor activity. The proteins, polyphenols, alkaloids carboxylic acids, and flavonoids in the leaf extract, which contained free amino acids and carboxylic groups in their structures, influenced the formation of ZnO NPs by interacting with Zn2+. The FTIR results demonstrated the role of the phenolics, terpenoids, and proteins of the A. paniculata leaf extract in the synthesis and stabilization of ZnO NPs. The XRPD pattern revealed that ZnO NPs were in the form of nanocrystals. The ZnO NPs were spherical (96–115 nm) and hexagonal (57 ± 0.3 nm) in shape, as corroborated by SEM and TEM studies, respectively. The results of the antioxidant, antidiabetic, and anti-inflammatory studies showed that ZnO NPs can decrease the sugar level and inflammations, possessing strong biological activities which could be utilized in pharmaceutical and biomedical applications.
Argade et al. [187] used the Albizzia lebbeck (A. lebbeck) aqueous bark extract for the synthesis of ZnO NPs. The ZnO NPs were evaluated for antidiabetic activity in vitro using the α-amylase inhibitor activity and glucose uptake by yeast cells. The α-amylase inhibitory activities by the A. lebbeck bark extract, ZnO NPs, and the antidiabetic drug Acarbose (used as a control) were found to be 73.3, 54.7, and 46.7%, respectively. The IC50 of the α-amylase activity of the A. lebbeck bark extract, ZnO NPs, and Acarbose were 4.9, 9.6, and 3.9 µg ml−1, respectively. The data demonstrated that Acarbose exhibited effective antidiabetic activity compared with the plant bark extract and ZnO NPs. In addition, the glucose uptake by yeast cells showed a dose-dependent manner which was directly proportional to the sample concentration. The ZnO NPs at 1 µg ml−1 concentration enhanced the glucose uptake by 89.2%, thus higher than that of the plant bark extract and the antidiabetic drug metformin (used as a control) of 62.5 and 94.2%, respectively, at the same concentration. Additionally, ZnO NPs were evaluated in vitro for their antioxidant activity using the reducing power ability and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay tests. The ZnO NPs showed powerful DPPH scavenging activity of 66.7% and IC50 of 7.0 µg ml−1. Moreover, the results revealed that ascorbic acid (used as a control) exhibited higher reducing power ability compared to the bark extract and ZnO NPs. Taking together, the results of these studies demonstrated that the green-synthesized ZnO NPs acted as a potent antidiabetic agent as evidenced by the decreasing glucose level.
7.4 Antioxidant activity of ZnO NPs
One of the promising biomedical applications for ZnO NPs is their antioxidant activity [15]. The antioxidant activity of ZnO NPs can be determined by the DPPH assay method. This method is associated with a change in the color of the DPPH methanolic solution from deep violet to a stable pale yellow, upon the addition of ZnO NPs. This indicates the scavenging activity of ZnO NPs by donating an electron from the oxygen atom to the odd electron of the nitrogen atom, resulting in the formation of a stable DPPH molecule [15,188].
Suresh et al. [189] synthesized ZnO NPs using Artocarpus gomezianus (A. gomezianus). ZnO NPs showed substantial antioxidant activity against DPPH free radicals with IC50 of 10.8 mg ml−1. The same research group synthesized green ZnO NPs using the aqueous plant extract of C. fistula, employing the solution combustion method [190]. The results showed that ZnO NPs exhibited a potential antioxidant activity with IC50 of 2.8 mg ml−1 via scavenging DPPH free radicals [190]. Nagajyothi et al. [191] evaluated the antioxidant activity of ZnO NPs, prepared from the root extract of Polygala tenuifolia (P. tenuifolia), using DPPH free radical assay. The results showed that ZnO NPs established moderate antioxidant activity by scavenging 45.5% DPPH at 1 mg ml−1. Siripireddy et al. [192] used the Eucalyptus globulus (E. globulus) leaf extract to synthesize spherical ZnO NPs (12 nm) under ambient conditions. ZnO NPs possessed antioxidant activity of 82% scavenging potential against DPPH, compared to ascorbic acid (used as a reference) with IC50 of 46.6 µg ml−1. In another study [85], ZnO NPs were green-synthesized using the aqueous stem extract of Ruta graveolens (L.) (R. graveolens L). The results showed that ZnO NPs (28 nm) possessed DPPH free radical scavenging activity with IC50 of 9.3 mg ml−1. Additionally, the antioxidant activity of ZnO NPs, prepared from the leaf extract of Ceropegia candelabrum (C. candelabrum), showed DPPH free radical scavenging activity ranging from 0 to 55% with IC50 of 95.1 µg ml−1, compared to 75% inhibition by ascorbic acid (used a positive control) at 50 µg ml−1 [193]. Moreover, it was found that the antioxidant activity increased with an increase in the ZnO NP concentration. Khan et al. [194] investigated the antioxidant activity of ZnO NPs, prepared from the Trianthema portulacastrum (T. portulacastrum), using the DPPH assay method. The authors reported that the antioxidant activity of ZnO NPs is attributed to the small size, large surface area, surface charge density, and capping materials present on the surface of ZnO NPs. Additionally, it was found that the free radical inhibition of ZnO NPs was concentration-dependent [194]. Alamdari et al. [109] revealed that ZnO NPs (17 nm), green-synthesized from the leaf extract of S. ebulus, exhibited H2O2 free radical scavenging activity with IC50 of 43 µg ml−1. The researchers reported that the presence of Zn2+ could enhance the antioxidant activity of ZnO NPs. Recently, Faisal et al. [195] green-synthesized ZnO NPs (66 nm) from the aqueous fruit extract of Myristica fragrans (M. fragrans). The antioxidant activity of ZnO NPs was proved using four assay methods (total antioxidant capacity, total reduction power, 2,2′-azino-bis(3-ethylbenzothiazoline 6-sulfonic acid), and DPPH free radical scavenging assay. In another recent study, Abdelbaky et al. [26] demonstrated the antioxidant activity of ZnO NPs, prepared from Pelargonium odoratissimum (L.) (P. odoratissimum L.) with IC50 of 28.1 µg ml−1. Therefore, the results of these studies revealed that the green-synthesized ZnO NPs possessed antioxidant activity by scavenging DPPH free radicals.
7.5 Antifungal activity of ZnO NPs
In addition to the aforementioned biomedical applications, the green-synthesized ZnO NPs demonstrated a potential antifungal activity toward several types of fungi [15,74,135]. The antifungal mechanism was described by the ability of ZnO NPs to enter the fungal membrane by diffusion and endocytosis. In the cytoplasm, ZnO NPs interfere with the mitochondrial function, initiating the production of ROS and the release of Zn2+. The overproduction of ROS and Zn2+ resulted in irreversible DNA damage and cell death [15,135]. Padalia and Chanda [196] reported the green synthesis of ZnO NPs (17.3 nm) using the leaf extract of Ziziphus nummularia (Z. nummularia). ZnO NPs showed higher antifungal activity against Candida albicans (C. albicans) than the azole antifungal agents. Miri et al. [197] used Prosopis farcta (P. farcta) to synthesize ZnO NPs (40–80 nm) and investigated their antifungal activity against C. albicans with MIC of 128 µg ml−1 and minimal fungicidal concentration (MFC) of 256 µg ml−1. Recently, Yassin et al. [198] studied the antifungal activity of ZnO NPs (22.8 nm) synthesized greenly from the peel extract of pomegranate. The antifungal activity of ZnO NPs was tested against three types of candida strains, C. albicans, C. tropicalis, and C. glabrata. A 100 µg of ZnO NPs per disk demonstrated antifungal activity against C. albicans, C. tropicalis, and C. glabrata, with zones of inhibition of 24.18, 20.17, and 26.35 mm, respectively. The MIC and MFC of ZnO NPs against C. tropicalis were 10 and 20 μg ml−1, respectively. Further, ZnO NPs demonstrated a synergistic efficiency against C. albicans with the antifungal drugs fluconazole, nystatin, and clotrimazole; whereas terbinafine, nystatin, and itraconazole showed a potential synergism against C. glabrata with ZnO NPs.
Additionally, the green-synthesized ZnO NPs exhibit antifungal activity against plant pathogenic fungi [199,200]. Jamdagni et al. [201] synthesized ZnO NPs using the Nyctanthes arbortristis (N. arbortristis) flower extract. The ZnO NPs (12–32 nm) were tested against five phytopathogens Alternaria alternata (A. alternata), Fusarium oxysporum (F. oxysporum), Aspergillus niger (A. niger), Penicillium expansum (P. expansum), and Botrytis cinerea (B. cinerea), which showed MICs of 64, 64, 16, 128, 128 µg ml−1, respectively. Zhu et al. [202] synthesized ZnO NPs from Cinnamomum camphora (L.) (C. camphora L.) at pH values 7, 8, and 9 with corresponding sizes of 13.9, 15.2, and 21.1 nm, respectively. The antifungal activity was evaluated against A. alternata. The MIC of ZnO NPs was 20 mg l−1 at pH 7, which showed the best antifungal effect. It was found that ZnO NPs induced excessive accumulation of malondialdehyde in A. alternata, causing damage to the cell membrane and thereby leakage of protein and nucleic acid. Despite that ZnO NPs exhibited antimicrobial activity, it was noticed that their sensitivity against fungi was lower compared to bacteria. This is attributed to the ability of fungi to form resistant spores when they grow under aggressive conditions [203].
7.6 Antiviral activity of ZnO NPs
The green synthesis of ZnO NPs demonstrated antiviral activity against many viruses. Melk et al. [204] synthesized ZnO NPs (32.58 ± 7.98 nm) from the alcoholic extract of Plumbago indica (P. indica), a valuable source of alkaloids, phenolics, and saponins. The results showed that ZnO NPs and the plant extract exhibited inhibitory effects against herpes simplex virus type 1 (HSV-1), which could be considered a promising adjuvant to improve the efficacy of HSV-1 drugs. In another study, Melk et al. [205] synthesized ZnO NPs (38.29 ± 6.88 nm) from the alcoholic extract of the flowering aerial parts of Plumbago auriculata Lam (P. auriculata Lam). The ZnO NPs and the plant extract possessed a significant antiviral activity against avian metapneumovirus subtype B, which would be beneficial for controlling the spreading of the virus in infected birds. Moreover, the green synthesis nanocomposites of ZnO NPs/activated carbon (ZnO NPs/AC) (30–70 nm) were prepared using the water hyacinth (Pontederia crassipes) [206]. In addition to the antiviral activity against the HSV1 virus, the ZnO NPs/AC nanocomposites showed antibacterial and antifungal activities. Studies revealed that the mechanism of the antiviral activity of ZnO NPs is attributed to the damage of the lipid membrane and RNA, thus inactivating the virus [207]. Hamdi et al. [208] conducted a computational analysis to investigate the interaction between ZnO NPs and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) targets. It was found that the hexagonal and spherical ZnO NPs with a crystallite size of ∼11 nm and positive zeta potential exhibited the highest antiviral activity. Recently, Alrabayah et al. [209] used the alcoholic leaf extract of Cestrum diurnum L. (C. diurnum L.) and zinc acetate to prepare green-synthesized ZnO NPs (3.4–4.9 nm) to evaluate their antiviral activity against human corona-229E (HCOV-229E). The C. diurnum L. leaf extract contained catechin, ferulic acid, chlorogenic acid, and syringic acid. Both ZnO NPs and the C. diurnum L. extract showed antiviral activity against HCOV-229E with IC50 of 7.01 and 61.15 µg ml−1; however, their combination showed higher activity than individually with IC50 of 2.41 µg ml−1.
7.7 Antiparasitic activity of ZnO NPs
The antiparasitic activity of the green-synthesized ZnO NPs has been reported in the literature [17]. The antiparasitic mechanism of ZnO NPs involves the disruption of the parasite membrane, damaging the parasite DNA, inhibition of protein synthesis, and formation of free radicals [17]. Dorostkar et al. [210] tested the anthelmintic activity of ZnO NPs and FeO NPs against Toxocara vitulorum (T. vitulorum) using different concentrations of NPs (0.004, 0.008, and 0.012% w/v). The results showed that both NPs increased the mortality rate of worms and increased the levels of malondialdehyde (MDA) and nitric oxide (NO). The levels of MDA and NO increased with an increase in the exposure time and concentration of NPs. The anthelmintic activity of both NPs was attributed to the induction of oxidative/nitrosative stress, resulting in the generation of ROS. Saleh et al. [211] studied the antiprotozoal effects of the metallic NPs (gold, silver, and ZnO) against Ichthyophthirius multifiliis (I. multifiliis), a ciliated protozoan ectoparasite of fish. The results showed that silver and ZnO NPs at 10 and 5 ng m−1 killed 100 and 97% of theronts, respectively, and inhibited the production of tomonts after 2 h of exposure. However, the antiparasitic effect of gold NPs was lower compared to that of silver and ZnO NPs, killing 80 and 78% of tomonts and theronts, respectively, at 20 ng ml−1 after 2 h of exposure. Recently, spherical and elliptical ZnO NPs (34.2 nm), greenly synthesized using the aqueous extract of Himalayan Columbine (Aquilegia pubiflora), were evaluated for their anti-leishmanial activity. The results showed a dose-dependent cytotoxic effect of ZnO NPs against Leishmania tropica (KWH23) with IC50 of 48 and 51 µg ml−1 for promastigote and amastigote, respectively [212]. Likewise, Najoom et al. [213] green-synthesized ZnO NPs (70–90 nm) using the leaf extract of Rhazya stricta (R. stricta) as capping and reducing agents. ZnO NPs showed antiplasmodial activity against plasmodium parasites with IC50 of 3.41 µg ml−1. Similarly, spherical- or elliptical-shaped ZnO NPs (66 nm) were prepared from the aqueous fruit extracts of Myristica fragrans (M. fragrans) and evaluated for their antiparasitic activity [195]. The results showed potential leishmanicidal activity against two forms of parasites (promastigote and amastigote) with a mortality rate of 71.5 and 61.4%, respectively. Hence, these studies demonstrated that the green-synthesized ZnO NPs could be effective against parasites.
7.8 Anti-inflammatory activity of ZnO NPs
The green-synthesized ZnO NPs showed excellent activity toward inflammation [15]. The anti-inflammatory mechanism involves the dissolution of ZnO NPs, releasing Zn2+ ions. The release of Zn2+ can be involved in several pathways, such as suppressing the release of the pro-inflammatory cytokines (interleukin-1 (IL)-1), IL-1β, IL-13, and tumor necrosis factor-α (TNF-α)) in mast cells. Additionally, Zn2+ release can suppress the expression of the lipopolysaccharide (LPS)-induced cyclooxygenase-2 (COX-2) gene in macrophages, inducible nitric oxide synthase (iNOS), myeloperoxidase, and NF-κβ. Moreover, Zn2+ release may block the caspase-1 enzyme in activated mast cells [15].
Nagajyothi et al. [191] green-synthesized ZnO NPs using the root extract of P. tenuifolia. The anti-inflammatory activity of ZnO NPs was evaluated in LPS-stimulated RAW 264.7 murine macrophages. The results showed a dose-dependent suppression effect of ZnO NPs on mRNA and protein expression of iNOS and pro-inflammatory cytokines. Rajakumar et al. [186] green-synthesized ZnO NPs (57–115 nm) from the leaf extract of Andrographis paniculata (A. paniculata). By using heat to induce albumin denaturation, the results showed an inhibition in the protein denaturation after exposure to ZnO NPs. Recently, the green-synthesized ZnO NPs, prepared from the leaf extract of Kalanchoe pinnata (K. pinnata), showed anti-inflammatory activity by inhibiting the production and release of the pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and COX-2 [214]. Additionally, Mohammad et al. [215] evaluated the anti-inflammatory activity of spherical ZnO NPs, prepared from the Hyssops officinalis L. (H. officinalis L.) extract, using the mouse paw edema test. The results showed a decrease in edema when treated with a dose of 5 mg/kg ZnO NPs. Similarly, Liu et al. [216] proved the anti-inflammatory activity of ZnO NPs, greenly synthesized from the leaf extract of Vernonia amygdalina (V. amygdalina), against inflammation-induced mice model by reducing the inflammatory response and pro-inflammatory cytokines level in mice. Recently, Abdelbaky et al. [26] proved the anti-inflammatory activity of ZnO NPs (34.1 nm), synthesized by employing the aqueous leaf extract of P. odoratissimum L. as a reducing agent. The anti-inflammatory effect of ZnO NPs was evaluated via an in vitro model of human red blood cells (RBCs) which involves hypotonicity-induced hemolysis. ZnO NPs were able to maximally stabilize the membrane by 95.6% at a dose of 1,000 µg ml−1, compared with the standard indomethacin. Finally, a very recent study by Lopez-Miranda et al. [217] evaluated the anti-inflammatory activity of ZnO NPs, prepared from the sargassum extract. The results revealed that ZnO NPs could inhibit protein denaturation and exhibited higher anti-inflammatory activity than the reference drug diclofenac. Thus, these studies suggested that the green-synthesized ZnO NPs showed promising anti-inflammatory activity.
7.9 Wound healing of ZnO NPs
ZnO NPs showed wound-healing activity [46,218]. Mahdavi et al. [219] investigated the cutaneous wound-healing activity of ZnO NPs (32 nm) prepared using the Ziziphora clinopodioides (Z. clinopodioides) leaf extract. The wound healing of ZnO NPs was confirmed by increasing the level of fibrocytes/fibroblasts, hydroxyl proline, hexosamine, hexuronic acid, and fibrocytes, and decreasing the wound area, total cells, and lymphocytes compared to control groups in rats. Additionally, green and chemical ZnO NPs, loaded into gels, were investigated for wound healing [220]. ZnO NPs were prepared by green synthesis using the Lawsonia inermis (L. inermis) leaf extract. The green and chemical ZnO NP-loaded gels showed promising wound-healing properties with reduced healing time in treated groups when compared with the control group and the green ZnO NPs gels were more effective than the chemical ones. Moreover, Shao et al. [221] reported that the green-synthesized ZnO NPs using the Barleria gibsoni (B. gibsoni) leaf extract and loaded into Carbopol gels showed wound-healing properties due to burns in rats.
Furthermore, the literature reported the effective wound-healing activity of ZnO NPs prepared using aqueous leaf extracts of Coleus amboinicus (C. amboinicus) [222], A. barbadensis [223], and Azadirachta indica (A. indica) neem leaves [224] as reducing, capping, and stabilizing agents. In addition, the literature reported an improved treatment of infected wounds caused by resistant bacteria by the embedding of green ZnO NPs prepared using the Alternanthera sessilis (A. sessilis) leaf extract into cotton fabrics [225], incorporation of ZnO NPs prepared using Ilex paraguariensis (I. paraguariensis) leaves into polymeric electrospun fibers of polyacrylic acid and polyallylamine hydrochloride [226], and gel formulations loaded with green synthesis silver NPs and ZnO NPs, prepared from the Annona squamosa (A. squamosa) leaf extract [227]. Altogether, these studies highlighted more promising biomedical applications of the green-synthesized ZnO NPs. Plants, part of the plants, chemical precursors, and size and biological activity of the green-synthesized ZnO NPs reported in Sections 7.1–7.9 are summarized in Table 2.
Plants, part of plants, chemical precursors, size, and biological activity of green-synthesized ZnO NPs
| Plant | Part of the plant | Chemical precursor | Size (nm) | Biological activity | Ref. |
|---|---|---|---|---|---|
| Aloe vera | Leaf extract | Zinc acetate | 40–180 | Antibacterial | [148] |
| Dysphania ambrosioides (D. ambrosioides) | Leaf extract | Zinc nitrate hexahydrate | 7–130 | Antibacterial | [118] |
| Punica granatum (P. granatum) | Peel extract | Zinc acetate | 10–45 | Antibacterial | [104] |
| Orange fruit | Peel extract | Zinc acetate | 20 | Antibacterial | [153] |
| Parthenium hysterophorus (P. hysterophorus) | Leaf extract | Zinc nitrate | 10 | Antibacterial | [228] |
| Daphne oleoides (D. oleoides) | Leaf extract | Zinc nitrate hexahydrate | 30–44 | Antibacterial | [155] |
| Aloe vera | Leaf extract | Zinc nitrate | 40 | Antibacterial | [133] |
| Sesbania grandiflora (S. grandiflora) | Leaf extract | Zinc acetate | 45 | Antibacterial | [156] |
| Avocado, papaya, and mango fruit | Fruit extract | Zinc nitrate hexahydrate | 11–21 | Antibacterial | [157] |
| Tecoma castanifolia (T. castanifolia) | Leaf extract | Zinc sulfate | 70–75 | Anticancer | [163] |
| Banana fruit | Fruit extract | Zinc acetate | NR* | Antibacterial and anticancer | [164] |
| Cratoxylum formosum (C. formosum) | Leaf extract | Zinc acetate | 200–500 | Anticancer | [165] |
| Phoenix dactylifera (P. dactylifera) | Root hair extract | Zinc acetate | 31–48 | Anticancer | [72] |
| Cardiospermum halicacabum (C. halicacabum) | Leaf extract | Zinc acetate | 10–20 | Anticancer | [166] |
| Deverra tortuosa (D. tortuosa) | Aerial extract | Zinc nitrate hexahydrate | 9–31 | Anticancer (lung and colon cancer) | [167] |
| Acorus calamus (A. calamus) | Rhizomes extract | Zinc acetate | 50–100 | Anticancer and antibacterial | [168] |
| Vinca rosea (V. rosea) | Leaf extract | Zinc nitrate hexahydrate | 16–41 | Anticancer, antidiabetic, and antimicrobial | [169] |
| Onion peel | Waste extract | Zinc acetate | NR* | Anticancer and antimicrobial | [170] |
| Alpinia calcarata (A. calcarata) | Rhizomes extract | NR* | 19 | Anticancer | [171] |
| Aloe barbadensis (A. barbadensis) | Leaf extract | Zinc nitrate | 20–40 | Anticancer and wound healing | [172] |
| Borassus flabellifer (B. flabellifer) | Palm fruit extract | Zinc nitrate | 55 | Anticancer | [173] |
| Ilex paraguariensis (I. paraguariensis) | Leaf extract | Zinc acetate and zinc nitrate | 18–389 | Anticancer | [229] |
| Andrographis paniculata (A. paniculata) | Leaf extract | Zinc nitrate hexahydrate | 57–115 | Antidiabetic, antioxidant, and anti-inflammatory | [186] |
| Albizzia lebbeck (A. lebbeck) | Bark extract | Zinc acetate | NR* | Antidiabetic and antioxidant | [187] |
| Artocarpus gomezianus (A. gomezianus) | Fruit extract | Zinc nitrate hexahydrate | NR* | Antioxidant | [189] |
| Cassia fistula (C. fistula) | Leaf extract | Zinc nitrate hexahydrate | 5–15 | Antioxidant and antibacterial | [190] |
| Polygala tenuifolia (P. tenuifolia) | Root extract | Zinc nitrate hexahydrate | NR* | Antioxidant and anti-inflammatory | [191] |
| Eucalyptus globulus (E. globulus) | Leaf extract | Zinc nitrate hexahydrate | 12 | Antioxidant | [192] |
| Ruta graveolens (L.) (R. graveolens L.) | Stem extract | Zinc nitrate | 20–30 | Antioxidant and antibacterial | [85] |
| Ceropegia candelabrum (C. candelabrum) | Leaf extract | Zinc nitrate hexahydrate | 12–35 | Antioxidant and antibacterial | [193] |
| Trianthema portulacastrum (T. portulacastrum) | Fruit extract | Zinc sulfate | 2–8 | Antioxidant | [194] |
| Sambucus ebulus (S. ebulus) | Leaf extract | Zinc acetate | 17 | Antioxidant and antibacterial | [109] |
| Myristica fragrans (M. fragrans) | Fruit extract | Zinc acetate | 66 | Antioxidant, antibacterial, and antidiabetic | [195] |
| Pelargonium odoratissimum (L.) (P. odoratissimum L.) | Leaf extract | Zinc acetate | 76 | Antioxidant antibacterial antiparasitic anti-inflammatory | [26] |
| Prosopis farcta (P. farcta) | Aerial extract | Zinc sulfate | 40–80 | Antifungal | [197] |
| Pomegranate | Peel extract | Zinc nitrate hexahydrate | 22.8 | Antifungal | [198] |
| Nyctanthes arbortristis (N. arbortristis) | Flower extract | Zinc acetate | 12–32 | Antifungal | [201] |
| Cinnamomum camphora (L.) (C. camphora L.) | Leaf extract | Zinc acetate | 14–21 | Antifungal | [202] |
| Plumbago indica (P. indica) | Aerial extract | Zinc acetate | 33 | Antiviral | [204] |
| Plumbago auriculata Lam (P. auriculata Lam) | Aerial extract | Zinc acetate | 38 | Antiviral | [205] |
| Water hyacinth (Pontederia crassipes) | Raw water hyacinth | Zinc chloride | 30–70 | Antiviral antimicrobial | [206] |
| Cestrum diurnum L. (C. diurnum L.) | Leaf extract | Zinc acetate | 3–5 | Antiviral | [209] |
| Aquilegia pubiflora (A. pubiflora) | Leaf extract | Zinc acetate | 34 | Antiparasitic | [212] |
| Rhazya stricta (R. stricta) | Leaf extract | Zinc chloride | 70–90 | Antiparasitic | [213] |
| Kalanchoe pinnata (K. pinnata) | Leaf extract | Zinc acetate | 24 | Anti-inflammatory | [214] |
| Hyssops officinalis L. (H. officinalis L.) | Leaf and flower extract | Zinc acetate | 20–40 | Anticancer anti-angiogenesis, and anti-inflammatory | [215] |
| Vernonia amygdalina (V. amygdalina) | Leaf extract | Zinc acetate | 20–40 | Anti-inflammatory | [216] |
| Sargassum | Plant extract | Zinc acetate | 20–200 | Anti-inflammatory | [217] |
| Ziziphora clinopodioides (Z. clinopodioides) | Leaf extract | Zinc nitrate hexahydrate | 32 | Wound healing | [219] |
| Lawsonia inermis (L. inermis) | Leaf extract | Zinc nitrate hexahydrate | NR* | Wound healing | [220] |
| Barleria gibsoni (B. gibsoni) | Leaf extract | Zinc nitrate hexahydrate | 30–80 | Wound healing | [221] |
| Coleus amboinicus (C. amboinicus) | Leaf extract | Zinc nitrate | 15–20 | Wound healing | [222] |
| Azadirachta indica (A. indica) | Leaf extract | Zinc acetate | 52 | Wound healing | [224] |
| Alternanthera sessilis (A. sessilis) | Leaf extract | Zinc acetate | NR* | Wound healing | [225] |
| Ilex paraguariensis (I. paraguariensis) | Leaf extract | Zinc acetate and zinc nitrate | 18–389 | Wound healing and anticancer | [226] |
| Annona squamosal (A. squamosa) | Leaf extract | NR* | NR* | Wound healing | [227] |
| Calotropis gigantea (C. gigantean) | Leaf extract | Zinc nitrate | 16–27 | Anticancer | [183] |
| Salvadora oleoides (S. oleoides). | Leaf extract | Zinc nitrate | 12–27 | Anticancer and antifungal | [99] |
| Azadirachta indica (L.) (A. Indica) | Leaf extract | Zinc nitrate hexahydrate | NR* | Antibacterial | [230] |
| Aloe barbadensis miller (Aloe vera) | Leaf extract | Zinc nitrate | 25–40 | NR* | [67] |
| Hibiscus subdariffa (H. subdariffa) | Leaf extract | Zinc acetate | 16–60 | Antibacterial and antidiabetic | [231] |
| Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae | Leaf extract | Zinc nitrate | 50 | NR* | [232] |
| – Beta vulgaris (B. vulgaris) | Whole plant extract | Zinc nitrate | 20 | Antibacterial antifungal | [87] |
| – Cinnamomum tamala (C. tamala) | |||||
| – Cinnamomum verum (C. verum) | |||||
| – Brassica oleracea var. Italica (B. oleracea) | |||||
| Atalantia monophylla (A. monophylla) | Leaf extract | Zinc acetate dihydrate | 33 | Antibacterial | [233] |
| Thymbra spicata L. (T. spicata L.) | Leaf extract | Zinc acetate dihydrate | 6.5–7.5 | Antibacterial, antioxidant protection against DNA damage | [234] |
| Stevia | Leaf extract | Zinc acetate | 10–90 | Antibacterial | [235] |
| Cassia auriculata (C. auriculata) | Leaf extract | Zinc acetate | 20–30 | Antibacterial | [236] |
| Phoenix dactylifera (P. dactylifera) | Date pulp waste extract | Zinc hexahydrate | 30 | Antibacterial | [88] |
| Chamomile flower (Matricaria chamomilla L, M. chamomilla L), olive leaves (Olea europaea, O. europaea), and red tomato fruit (Lycopersicon esculentum M, L. esculentum M) | Flower, leaf, and fruit extract | ZnO | 48 | Antibacterial | [237] |
| Beta vulgaris (B. vulgaris) | Fruit extract | Zinc nitrate | 20 | Anticancer | [238] |
| Punica granatum (P. granatum) (pomegranate) | Leaf and flower extract | Zinc nitrate hexahydrate | NR* | Antibacterial | [239] |
| Justicia adhatoda (J. adhatoda) | Leaf extract | Zinc acetate, zinc sulfate, and zinc nitrate | 15–20 | Antibacterial | [240] |
| Mussaenda frondosa L. (M. frondosa L.) | Leaf, stem, and callus extracts | Zinc nitrate hexahydrate | 5–20 | Antibacterial, anti-inflammatory, anticancer, antidiabetic and antioxidant | [241] |
| Parthenium hysterophorus (P. hysterophorus) | Leaf extract | NR* | NR* | Antibacterial | [130] |
| Raphanus sativus var. Longipinnatus (R. Sativus) | Leaf extract | Zinc acetate dihydrate | 209 | Anticancer | [100] |
| Citrus sinensis (C. sinensis) | Peel extract | Zinc nitrate hexahydrate | NA | Antibacterial | [242] |
| Eucalyptus globules (E. globules) | Leaf extract | Zinc nitrate hexahydrate | 52–70 | Antifungal | [243] |
| Salvia officinalis (S. officinalis) | Leaf extract | NR* | NR* | Antifungal | [128] |
| Ziziphus nummularia (Z. nummularia) | Leaf extract | Zinc nitrate | 17 | Antifungal and anticancer | [196] |
| Syzygium cumini (S. cumini) | Leaf extract | Zinc acetate | 30 | Antioxidant and anticancer | [92] |
| Cissus quadrangularis (C. quadrangularis) | Stem extract | Zinc acetate dihydrate | 75–90 | Antibacterial and anticancer | [244] |
| Sabal blackburniana (S. blackburniana) | Leaf, fruit, and pollen grains extracts | Zinc acetate dihydrate | 2–50 | Acetylcholinesterase inhibitory activity and anti-Alzheimer | [245] |
| Amygdalus scoparia (A. scoparia) | Stem bark extract | Zinc acetate dihydrate | 15–40 | Anticancer, antidiabetic, and antibacterial | [246] |
| Cnidoscolus aconitifolius (C. aconitifolius) | Leaf extract | Zinc acetate | 100 | Antioxidant, anti-inflammatory, and antibacterial | [247] |
| Solanum rantonnetii (S. rantonnetii) | Leaf extract | NR* | 12 | Antifungal activity | [248] |
NR*: not reported.
8 Toxicity of ZnO NPs
Although bulk ZnO has been GRAS [35], there is still doubt regarding the safety of ZnO NPs on human health that warrants further investigation [249]. Because ZnO NPs are commonly used in broad biomedical applications, the advantages of these types of NPs must be wisely weighed against possible toxic effects [250]. It has been shown that the concentration and exposure time of NPs and cell type play a vital role in posing toxic effects [251]. Additionally, the toxicity of NPs is determined by their physicochemical properties such as size, shape, composition, and surface properties [252].
Moatamed et al. [253] compared the impact of ZnO NPs (39 nm) and bulk ZnO (5 µm) on the liver function of Wistar rats. Three groups of rats received three dose levels of ZnO NPs according to body weight (bw) (25, 50, and 100 mg/kg bw). It was found that the group that received 100 mg/kg bw ZnO NPs showed the most significant changes in liver enzymes, histopathological structure, and oxidative stress markers, indicating a dose-dependent hepatic toxicity of ZnO NPs. However, the group that received 100 mg/kg bw of bulk ZnO showed no significant effects on liver function. This can be explained by the increase in the Zn2+ concentration in the hepatic tissues of rats treated with ZnO NPs, while no significant change in Zn2+ concentration was found in rats that received the same dose of bulk ZnO. A study by Salami et al. [254] determined the hemolytic toxic effects of ZnO NPs and their bulk counterparts on isolated RBCs. A concentration range of 0.01–1 mM of ZnO (bulk and NPs) was used in this study. The results demonstrated that ZnO NPs caused toxic hemolytic effects due to the generation of ROS, lipid peroxidation, and GSH depletion, whereas bulk ZnO did not show any toxic hemolytic effects. Additionally, Roy et al. [255] assessed the impact of ZnO NPs versus bulk ZnO on the activity of murine macrophages. The study revealed that ZnO NPs were more potent in activating the proinflammatory cytokine production by macrophages, whereas the small size of ZnO NPs may help in the evasion of the macrophage response.
The toxicity studies of green-synthesized ZnO NPs are limited. Saranya et al. [256] assessed the in vitro cytotoxicity of three green-synthesized NPs including ZnO NPs, FeO NPs, and copper NPs (Cu NPs), prepared using the aqueous leaf extract of Musa ornate (M. Ornate) and Zea mays (Z. mays). The cytotoxicity of NPs was assessed in three cell lines, the African green monkey kidney cell line (Vero), pig kidney cell line (PK15), and Madin Darby bovine kidney (MDBK) at 24 and 48 h. The results showed that the cytotoxic effect of green NPs varied based on the concentration and exposure time of NPs and cell type. Additionally, the green ZnO NPs exhibited higher cell viability at lower concentrations (10 µg/100 µL) when compared to green FeO NPs and Cu NPs in all cell types [256]. Additionally, the toxicity of the ZnO NPs, prepared from the aqueous extract of Amaranthus caudatus (A. caudatus), was evaluated using zebrafish embryos [257]. The toxicity of ZnO NPs showed a concentration-dependent pattern, where the ZnO NP concentration of ≤10 mg ml−1 showed no toxic effect, whereas at 50–100 mg ml−1 high levels of mortalities were observed [257].
The toxicity of chemically synthesized ZnO NPs was evaluated. Earlier, Najim et al. [258] investigated the cytotoxicity of ZnO NPs of different sizes in both normal and cancer cell lines. The results showed that ZnO NPs of 85.7 and 190 nm displayed cytotoxic effects on all cell lines derived from the lung tissue (Hs888Lu), neuron-phenotypic cells (SH-SY5Y), neuroblastoma (SH-SY5Y), human histiocytic lymphoma (U937), with no effect on lung cancer (A549). Additionally, it was found that the toxicity of ZnO NPs depends on the particle size, concentration, and exposure time. Khan et al. [259] assessed the toxic effect of ZnO NPs at different concentrations (50, 100, 250, and 500 ppm) on human erythrocytes. ZnO NPs showed a concentration-dependent hemolytic activity in red blood cells. Additionally, the genotoxic potential of ZnO NPs, investigated by the in vitro alkaline comet assay, showed concentration-dependent DNA damage at 250 and 500 ppm concentrations. The toxic effects of ZnO NPs were attributed to the generation of ROS that resulted from oxidative stress. Moreover, the in vitro cytotoxicity of ZnO NPs (25 nm) on an in vitro model cell line, obtained from the gill tissue of Wallago attu (WAG), was evaluated by MTT, neutral red uptake, and LDH assay tests [260]. Researchers found that ZnO NPs induced acute toxicity to WAG cells with IC50 of 5.7 ± 0.1, 3.1 ± 0.1, and 5.6 ± 0.12 mg l−1, respectively. Uzar et al. [261] studied the nephrotoxic potential of ZnO NPs (10–50 nm) against rat kidney epithelial cells (NRK-52E) at 25–100 µg ml−1 exposure concentration. The IC50 on NRK-52E was 73 µg ml−1. Comet assay, used to assess the genotoxic effect of ZnO NPs at 12.5–50 µg ml−1, showed significant DNA damage in NRK-52E cells. Additionally, Saranya et al. [256] assessed the in vitro cytotoxicity of three green-synthesized NPs including ZnO NPs, FeO NPs, and Cu NPs, prepared using the aqueous leaf extract of Musa ornate (M. Ornate) and Zea mays (Z. mays). The cytotoxicity of NPs was assessed in three cell lines, the African green monkey kidney cell line (Vero), pig kidney cell line (PK15), and MDBK at 24 and 48 h. The results showed that the cytotoxic effect of green NPs varied based on the concentration and exposure time of NPs and cell type. Additionally, the green ZnO NPs exhibited higher cell viability at lower concentrations (10 µg/100 µL) when compared to green FeO NPs and Cu NPs in all cell types. Furthermore, Yousef et al. [262] confirmed the hepatic and renal toxicity of ZnO NPs in male Wistar rats, when ZnO NPs were daily administered orally for 75 days. Despite these studies, there is no standard methodology for assessing the toxicity of ZnO NPs [263]. Therefore, more toxicological studies are still needed to provide information on the safety, risk assessments, and risk management of ZnO NPs.
9 Challenges associated with the translation process of ZnO NPs from bench to market
The translation process of ZnO NPs from bench to market is facing several potential bottlenecks and challenges. The green synthesis of ZnO NPs may suffer from batch-to-batch reproducibility and large-scale production including plant selection, synthesis conditions, NPs’ size, and applications [24,264]. For instance, plants used for the green synthesis of NPs are commonly selected based on their local availability and abundancy [264]. This, in turn, makes it difficult to green-synthesize NPs globally, using specific plant extracts. Additionally, the time of plant collection hinders their use, where plants are either collected during flowering or fruiting seasons [265]. Moreover, the green synthesis of NPs requires extremely high or low temperatures, long reaction and extraction times, inert conditions (i.e., prepared under an inert atmosphere of argon or nitrogen), and the use of chemical precursors (such as zinc nitrate and zinc acetate) [264]. In addition, the specific reaction mechanism of the green synthesis of NPs is still difficult to elucidate, and the mass balance and stoichiometric ratio that scale up the process is a major challenge. Furthermore, the size of NPs, synthesized from different plant extracts, is heterogeneous with irregular shapes [264].
The safety profile of ZnO NPs needs to be thoroughly investigated and assessed before their use. It was found that ZnO NPs may cause hepatic, nephron, neuronal, pulmonary, and reproductive toxicity [70]. ZnO NPs can enter the biological systems through three primary routes: dermal, inhalation, and ingestion, followed by circulating in the bloodstream [266]. The way zinc interacts with proteins, nucleic acids, and cells plays a crucial role in determining the application of ZnO NPs. Additionally, factors such as size, physicochemical properties, dose, and duration of exposure are important considerations in assessing the toxicity of ZnO NPs [251,252].
The regulatory approval process for novel NPs can be complex and time-consuming. This is because meeting the regulatory requirements for safety, efficacy, and quality can be a challenging and costly process, often leading to a delay in market entry. Moreover, moving from laboratory-scale production to large-scale manufacturing can be a significant hurdle, where ensuring efficient and cost-effective production methods while maintaining product integrity can be a challenge. For example, NPs can be susceptible to changes in the physicochemical properties over time, such as aggregation, agglomeration, or degradation. Hence, ensuring long-term stability and adequate shelf-life for NP-based products is crucial for commercial viability [267]. Therefore, addressing these challenges requires a multidisciplinary approach involving scientists, regulators, and manufacturers. Additionally, thorough research, robust safety assessments, adherence to regulatory guidelines, and efficient manufacturing processes are essential for the successful translation and commercialization of ZnO NPs.
10 Conclusion and future perspectives
Scientists are actively dedicating their efforts to enhance the green synthesis of ZnO NPs for various biomedical applications. The green-synthesized ZnO NPs have gained significant importance due to their eco-friendly, biocompatibility, and cost-effectiveness than those prepared by chemical and physical methods. The variety of plant extracts with their phytochemicals such as flavonoids, terpenoids, polyphenols, tannins, and alkaloids can be considered a potential source for the green synthesis of ZnO NPs that contribute to the stability and discovery of new physicochemical properties for ZnO NPs. Research studies have shown that the green-synthesized ZnO NPs exhibited superior bioactivities due to the presence of various phytochemicals on their surfaces. The antibacterial activity of ZnO NPs holds promise as a potential solution for the treatment of infections caused by multidrug-resistant bacteria and as an alternative to antibiotics. Moreover, as an anticancer agent, ZnO NPs localize in cancer cells through enhanced permeability and retention effects and electrostatic interactions, leading to cell death through the release of Zn2+ ions and/or generation of ROS. This suggests that ZnO NPs are a promising anticancer agent in targeting drug delivery. The green-synthesized ZnO NPs may offer a remedy for diabetes as an antidiabetic agent and an increase in the radical scavenging activity as an antioxidant agent. Therefore, ZnO NPs hold vast potential for pharmaceutical and biomedical applications, making them a major area of research. Furthermore, ZnO NPs exhibited antifungal, antiviral, antiparasitic, and anti-inflammatory activities, expanding their use in health care products such as sunscreen lotions for skin acne, blemishes, and UV/visible light protection. However, it is crucial to address the limitations of ZnO NP toxicity toward biological systems and the need for evidence-based research exploring the bioactivities of ZnO NPs, which require further investigation. Currently, to our knowledge, the green-synthesized ZnO NPs have not been introduced into the market, and no clinical trials have been reported. Hence, additional in vivo studies on the biomedical applications of green-synthesized ZnO NPs are necessary for future clinical use.
Acknowledgments
The authors are thankful to their respective departments/universities for the successful completion of this review.
-
Funding information: The authors state no funding involved.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Contera S, Bernardino de la Serna J, Tetley TD. Biotechnology, nanotechnology and medicine. Emerg Top Life Sci. 2020;4(6):551–4.10.1042/ETLS20200350Suche in Google Scholar PubMed PubMed Central
[2] Garg R, Rani P, Garg R, Khan MA, Khan NA, Khan AH, et al. Biomedical and catalytic applications of agri-based biosynthesized silver nanoparticles. Environ Pollut. 2022;310:119830.10.1016/j.envpol.2022.119830Suche in Google Scholar PubMed
[3] Saxena SK, Nyodu R, Kumar S, Maurya VK. Current advances in nanotechnology and medicine. NanoBioMedicine. United Kingdom: Springer; 2020. p. 3–16.10.1007/978-981-32-9898-9_1Suche in Google Scholar
[4] Fouda A, Eid AM, Guibal E, Hamza MF, Hassan SE-D, Alkhalifah DHM, et al. Green synthesis of gold nanoparticles by aqueous extract of zingiber officinale: Characterization and insight into antimicrobial, antioxidant, and in vitro cytotoxic activities. Appl Sci. 2022;12(24):12879.10.3390/app122412879Suche in Google Scholar
[5] Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arab J Chem. 2019;12(7):908–31.10.1016/j.arabjc.2017.05.011Suche in Google Scholar
[6] Mohamed AA, Fouda A, Abdel-Rahman MA, Hassan SE-D, El-Gamal MS, Salem SS, et al. Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatal Agric Biotechnol. 2019;19:101103.10.1016/j.bcab.2019.101103Suche in Google Scholar
[7] Abu-Huwaij R, Al-Assaf SF, Mousli F, Kutkut MS, Al-Bashtawi A. Perceptive review on properties of iron oxide nanoparticles and their antimicrobial and anticancer activity. Sys Rev Pharm. 2020;11(8):418–31.Suche in Google Scholar
[8] Nikolova MP, Chavali MS. Metal oxide nanoparticles as biomedical materials. Biomimetics. 2020;5(2):27.10.3390/biomimetics5020027Suche in Google Scholar PubMed PubMed Central
[9] Fouda A, Hassan SE-D, Eid AM, Awad MA, Althumayri K, Badr NF, et al. Endophytic bacterial strain, Brevibacillus brevis-mediated green synthesis of copper oxide nanoparticles, characterization, antifungal, in vitro cytotoxicity, and larvicidal activity. Green Process Synth. 2022;11(1):931–50.10.1515/gps-2022-0080Suche in Google Scholar
[10] Fouda A, Eid AM, Abdel-Rahman MA, El-Belely EF, Awad MA, Hassan SE-D, et al. Enhanced antimicrobial, cytotoxicity, larvicidal, and repellence activities of brown algae, Cystoseira crinita-mediated green synthesis of magnesium oxide nanoparticles. Front Bioeng Biotechnol. 2022;10:84992.10.3389/fbioe.2022.849921Suche in Google Scholar PubMed PubMed Central
[11] Ealia SAM, Saravanakumar M, editors. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP conference series: materials science and engineering. IOP Publishing; 2017.Suche in Google Scholar
[12] Abu Hajleh MN, Abu‐Huwaij R, AL‐Samydai A, Al‐Halaseh LK, Al‐Dujaili EA. The revolution of cosmeceuticals delivery by using nanotechnology: A narrative review of advantages and side effects. J Cosmet Dermatol. 2021;20(12):3818–28.10.1111/jocd.14441Suche in Google Scholar PubMed
[13] Jiang J, Pi J, Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl. 2018;2018:1062562.10.1155/2018/1062562Suche in Google Scholar PubMed PubMed Central
[14] Al-Shalabi R, Abu-Huwaij R, Hamed R, Abbas MM. The antimicrobial and the antiproliferative effect of human triple negative breast cancer cells using the greenly synthesized iron oxide nanoparticles. J Drug Deliv Sci Technol. 2022;75:103642.10.1016/j.jddst.2022.103642Suche in Google Scholar
[15] Murali M, Kalegowda N, Gowtham HG, Ansari MA, Alomary MN, Alghamdi S, et al. Plant-mediated zinc oxide nanoparticles: advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharmaceutics. 2021;13(10):1662.10.3390/pharmaceutics13101662Suche in Google Scholar PubMed PubMed Central
[16] Alhujaily M, Albukhaty S, Yusuf M, Mohammed MK, Sulaiman GM, Al-Karagoly H, et al. Recent advances in plant-mediated zinc oxide nanoparticles with their significant biomedical properties. Bioengineering. 2022;9(10):541.10.3390/bioengineering9100541Suche in Google Scholar PubMed PubMed Central
[17] Bajwa HUR, Khan MK, Abbas Z, Riaz R, Rehman TU, Abbas RZ, et al. Nanoparticles: Synthesis and their role as potential drug candidates for the treatment of parasitic diseases. Life Sci Rep. 2022;12(5):750.10.3390/life12050750Suche in Google Scholar PubMed PubMed Central
[18] Babayevska N, Przysiecka Ł, Iatsunskyi I, Nowaczyk G, Jarek M, Janiszewska E, et al. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci Rep. 2022;12(1):8148.10.1038/s41598-022-12134-3Suche in Google Scholar PubMed PubMed Central
[19] Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015;7(3):219–42.10.1007/s40820-015-0040-xSuche in Google Scholar PubMed PubMed Central
[20] Marslin G, Siram K, Maqbool Q, Selvakesavan RK, Kruszka D, Kachlicki P, et al. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials. 2018;11(6):940.10.3390/ma11060940Suche in Google Scholar PubMed PubMed Central
[21] Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumar P. Green synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnology. 2018;16(1):1–24.10.1186/s12951-018-0408-4Suche in Google Scholar PubMed PubMed Central
[22] Rana A, Yadav K, Jagadevan S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J Clean Prod. 2020;272:122880.10.1016/j.jclepro.2020.122880Suche in Google Scholar
[23] Ren J, editor. Research on Green Synthetic Iron Nanoparticles. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2019.10.1088/1757-899X/612/2/022025Suche in Google Scholar
[24] Kaur K, Sidhu AK. Green synthesis: An eco-friendly route for the synthesis of iron oxide nanoparticles. Front Nanosci. 2021;3:655062.10.3389/fnano.2021.655062Suche in Google Scholar
[25] Naiel B, Fawzy M, Halmy MWA, Mahmoud AED. Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci Rep. 2022;12(1):20370.10.1038/s41598-022-24805-2Suche in Google Scholar PubMed PubMed Central
[26] Abdelbaky AS, Abd El-Mageed TA, Babalghith AO, Selim S, Mohamed AM. Green synthesis and characterization of ZnO nanoparticles using Pelargonium odoratissimum (L.) aqueous leaf extract and their antioxidant, antibacterial and anti-inflammatory activities. Antioxidants. 2022;11(8):1444.10.3390/antiox11081444Suche in Google Scholar PubMed PubMed Central
[27] Hara T, Takeda TA, Takagishi T, Fukue K, Kambe T, Fukada T. Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiol Sci. 2017;67(2):283–301.10.1007/s12576-017-0521-4Suche in Google Scholar PubMed
[28] Bisht G, Rayamajhi S. ZnO nanoparticles: a promising anticancer agent. Nanobiomedicine. 2016;3(9):3–9.10.5772/63437Suche in Google Scholar PubMed PubMed Central
[29] Tubek S. Zinc supplementation or regulation of its homeostasis: advantages and threats. Biol Trace Elem Res. 2007;119(1):1–9.10.1007/s12011-007-0043-7Suche in Google Scholar PubMed
[30] Lee SR. Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxid Med Cell Longev. 2018;2018:9156285.10.1155/2018/9156285Suche in Google Scholar PubMed PubMed Central
[31] Prasad AS. Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr. 2014;1:14.10.3389/fnut.2014.00014Suche in Google Scholar PubMed PubMed Central
[32] Marreiro DDN, Cruz KJC, Morais JBS, Beserra JB, Severo JS, De Oliveira ARS. Zinc and oxidative stress: current mechanisms. Antioxidants. 2017;6(2):24.10.3390/antiox6020024Suche in Google Scholar PubMed PubMed Central
[33] Prasad AS. Zinc: an overview. Nutrition (Burbank, Los Angeles County, Calif). 1995;11(1 Suppl):93–9.Suche in Google Scholar
[34] Boron B, Hupert J, Barch DH, Fox CC, Friedman H, Layden TJ, et al. Effect of zinc deficiency on hepatic enzymes regulating vitamin A status. J Nutr. 1988;118(8):995–1001.10.1093/jn/118.8.995Suche in Google Scholar PubMed
[35] Espitia P, Otoni C, Soares N. Zinc oxide nanoparticles for food packaging applications. Antimicrobial food packaging. Amsterdam, The Netherlands: Elsevier; 2016. p. 425–3110.1016/B978-0-12-800723-5.00034-6Suche in Google Scholar
[36] Jin S-E, Jin H-E. Antimicrobial activity of zinc oxide nano/microparticles and their combinations against pathogenic microorganisms for biomedical applications: From physicochemical characteristics to pharmacological aspects. Nanomaterials. 2021;11(2):263.10.3390/nano11020263Suche in Google Scholar PubMed PubMed Central
[37] Miao J, Liu B. II–VI semiconductor nanowires: ZnO. Semiconductor Nanowires. Amsterdam, The Netherlands: Elsevier; 2015. p. 3–28.10.1016/B978-1-78242-253-2.00001-3Suche in Google Scholar
[38] Kołodziejczak-Radzimska A, Jesionowski T. Zinc oxide—from synthesis to application: a review. Materials. 2014;7(4):2833–81.10.3390/ma7042833Suche in Google Scholar PubMed PubMed Central
[39] Wang L, Giles N. Temperature dependence of the free-exciton transition energy in zinc oxide by photoluminescence excitation spectroscopy. J Appl Phys. 2003;94(2):973–8.10.1063/1.1586977Suche in Google Scholar
[40] Capper P, Kasap SO, Willoughby A. Zinc oxide materials for electronic and optoelectronic device applications. Hoboken, New Jersey, US: John Wiley & Sons; 2011.Suche in Google Scholar
[41] Mirzaei H, Darroudi M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram Int. 2017;43(1):907–14.10.1016/j.ceramint.2016.10.051Suche in Google Scholar
[42] Dai H, Sun T, Han T, Guo Z, Wang X, Chen Y. Aggregation behavior of zinc oxide nanoparticles and their biotoxicity to Daphnia magna: Influence of humic acid and sodium alginate. Environ Res. 2020;191:110086.10.1016/j.envres.2020.110086Suche in Google Scholar PubMed
[43] Pulit-Prociak J, Banach M. Silver nanoparticles–a material of the future…? Open Chem. 2016;14(1):76–91.10.1515/chem-2016-0005Suche in Google Scholar
[44] Yashni G, Al-Gheethi A, Mohamed RMSR, Dai-Viet NV, Al-Kahtani AA, Al-Sahari M, et al. Bio-inspired ZnO NPs synthesized from Citrus sinensis peels extract for Congo red removal from textile wastewater via photocatalysis: Optimization, mechanisms, techno-economic analysis. Chemosphere. 2021;281:130661.10.1016/j.chemosphere.2021.130661Suche in Google Scholar PubMed
[45] Islam F, Shohag S, Uddin MJ, Islam MR, Nafady MH, Akter A, et al. Exploring the journey of zinc oxide nanoparticles (ZnO-NPs) toward biomedical applications. Materials. 2022;15(6):2160.10.3390/ma15062160Suche in Google Scholar PubMed PubMed Central
[46] Sadhasivam S, Shanmugam M, Umamaheswaran PD, Venkattappan A, Shanmugam A. Zinc oxide nanoparticles: green synthesis and biomedical applications. J Clust Sci. 2021;32(6):1441–55.10.1007/s10876-020-01918-0Suche in Google Scholar
[47] Ismail A, Menazea A, Kabary HA, El-Sherbiny A, Samy A. The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by Sol–Gel method. J Mol Struct. 2019;1196:332–7.10.1016/j.molstruc.2019.06.084Suche in Google Scholar
[48] Chung YT, Ba-Abbad MM, Mohammad AW, Hairom NHH, Benamor A. Synthesis of minimal-size ZnO nanoparticles through sol–gel method: Taguchi design optimisation. Mater Des. 2015;87:780–7.10.1016/j.matdes.2015.07.040Suche in Google Scholar
[49] Ghoshal T, Biswas S, Paul M, De S. Synthesis of ZnO nanoparticles by solvothermal method and their ammonia sensing properties. J Nanosci Nanotechnol. 2009;9(10):5973–80.10.1166/jnn.2009.1290Suche in Google Scholar PubMed
[50] Reuge N, Bacsa R, Serp P, Caussat B. Chemical vapor synthesis of zinc oxide nanoparticles: experimental and preliminary modeling studies. J Phys Chem C. 2009;113(46):19845–52.10.1021/jp9070955Suche in Google Scholar
[51] Goorabjavari SVM, Golmohamadi F, Haririmonfared S, Ahmadi H, Golisani S, Yari H, et al. Thermodynamic and anticancer properties of inorganic zinc oxide nanoparticles synthesized through co-precipitation method. J Mol Liq. 2021;330:115602.10.1016/j.molliq.2021.115602Suche in Google Scholar
[52] Ramimoghadam D, Bin Hussein MZ, Taufiq-Yap YH. Hydrothermal synthesis of zinc oxide nanoparticles using rice as soft biotemplate. Chem Cent J. 2013;7(1):1–10.10.1186/1752-153X-7-136Suche in Google Scholar PubMed PubMed Central
[53] Madathil ANP, Vanaja K, Jayaraj M, editors. Synthesis of ZnO nanoparticles by hydrothermal method. Nanophotonic materials IV. United States: SPIE; 2007.Suche in Google Scholar
[54] Shamsipur M, Pourmortazavi SM, Hajimirsadeghi SS, Zahedi MM, Rahimi-Nasrabadi M. Facile synthesis of zinc carbonate and zinc oxide nanoparticles via direct carbonation and thermal decomposition. Ceram Int. 2013;39(1):819–27.10.1016/j.ceramint.2012.07.003Suche in Google Scholar
[55] Anand V, Srivastava VC. Zinc oxide nanoparticles synthesis by electrochemical method: Optimization of parameters for maximization of productivity and characterization. J Alloys Compd. 2015;636:288–92.10.1016/j.jallcom.2015.02.189Suche in Google Scholar
[56] El-Gendy AO, Nawaf KT, Ahmed E, Samir A, Hamblin MR, Hassan M, et al. Preparation of zinc oxide nanoparticles using laser-ablation technique: Retinal epithelial cell (ARPE-19) biocompatibility and antimicrobial activity when activated with femtosecond laser. J Photochem Photobiol B, Biol. 2022;234:112540.10.1016/j.jphotobiol.2022.112540Suche in Google Scholar PubMed
[57] Xiong HM, Shchukin DG, Möhwald H, Xu Y, Xia YY. Sonochemical synthesis of highly luminescent zinc oxide nanoparticles doped with magnesium (II). Angew Chem Int Ed. 2009;48(15):2727–31.10.1002/anie.200805590Suche in Google Scholar PubMed
[58] Sajjad A, Bhatti SH, Ali Z, Jaffari GH, Khan NA, Rizvi ZF, et al. Photoinduced fabrication of zinc oxide nanoparticles: Transformation of morphological and biological response on light irradiance. ACS Omega. 2021;6(17):11783–93.10.1021/acsomega.1c01512Suche in Google Scholar PubMed PubMed Central
[59] Salah N, Habib SS, Khan ZH, Memic A, Azam A, Alarfaj E, et al. High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int J Nanomed. 2011;6:863.10.2147/IJN.S18267Suche in Google Scholar PubMed PubMed Central
[60] Mandal AK, Katuwal S, Tettey F, Gupta A, Bhattarai S, Jaisi S, et al. Current research on zinc oxide nanoparticles: Synthesis, characterization, and biomedical applications. Nanomaterials. 2022;12(17):3066.10.3390/nano12173066Suche in Google Scholar PubMed PubMed Central
[61] Köseoğlu Y. A simple microwave-assisted combustion synthesis and structural, optical and magnetic characterization of ZnO nanoplatelets. Ceram Int. 2014;40(3):4673–9.10.1016/j.ceramint.2013.09.008Suche in Google Scholar
[62] Kandjani AE, Tabriz MF, Pourabbas B. Sonochemical synthesis of ZnO nanoparticles: The effect of temperature and sonication power. Mater Res Bull. 2008;43(3):645–54.10.1016/j.materresbull.2007.04.005Suche in Google Scholar
[63] Tsuzuki T, McCormick PG. ZnO nanoparticles synthesised by mechanochemical processing. Scr Mater. 2001;44(8–9):1731–4.10.1016/S1359-6462(01)00793-XSuche in Google Scholar
[64] Chandrasekaran R, Gnanasekar S, Seetharaman P, Keppanan R, Arockiaswamy W, Sivaperumal S. Formulation of Carica papaya latex-functionalized silver nanoparticles for its improved antibacterial and anticancer applications. J Mol Liq. 2016;219:232–8.10.1016/j.molliq.2016.03.038Suche in Google Scholar
[65] Fouda A, Eid AM, Abdelkareem A, Said HA, El-Belely EF, Alkhalifah DHM, et al. Phyco-synthesized zinc oxide nanoparticles using marine macroalgae, Ulva fasciata Delile, characterization, antibacterial activity, photocatalysis, and tanning wastewater treatment. Catalysts. 2022;12(7):756.10.3390/catal12070756Suche in Google Scholar
[66] Dhandapani P, Siddarth AS, Kamalasekaran S, Maruthamuthu S, Rajagopal G. Bio-approach: ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr Polym. 2014;103:448–55.10.1016/j.carbpol.2013.12.074Suche in Google Scholar PubMed
[67] Sangeetha G, Rajeshwari S, Venckatesh R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater Res Bull. 2011;46(12):2560–6.10.1016/j.materresbull.2011.07.046Suche in Google Scholar
[68] Sohail MF, Rehman M, Hussain SZ, Huma Z-E, Shahnaz G, Qureshi OS, et al. Green synthesis of zinc oxide nanoparticles by Neem extract as multi-facet therapeutic agents. J Drug Deliv Sci Technol. 2020;59:101911.10.1016/j.jddst.2020.101911Suche in Google Scholar
[69] Agarwal H, Kumar SV, Rajeshkumar S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resource-Efficient Technologies. 2017;3(4):406–13.10.1016/j.reffit.2017.03.002Suche in Google Scholar
[70] Singh TA, Das J, Sil PC. Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interface Sci. 2020;286:102317.10.1016/j.cis.2020.102317Suche in Google Scholar PubMed
[71] Abu-Huwaij R, Abbas MM, Al-Shalabi R, Almasri FN. Synthesis of transdermal patches loaded with greenly synthesized zinc oxide nanoparticles and their cytotoxic activity against triple negative breast cancer. Appl Nanosci. 2022;12(1):69–78.10.1007/s13204-021-02166-ySuche in Google Scholar
[72] Naser R, Abu-Huwaij R, Al-khateeb I, Abbas MM, Atoom AM. Green synthesis of zinc oxide nanoparticles using the root hair extract of Phoenix dactylifera: Antimicrobial and anticancer activity. Appl Nanosci. 2021;11(5):1747–57.10.1007/s13204-021-01837-0Suche in Google Scholar
[73] Fakhari S, Jamzad M, Kabiri Fard H. Green synthesis of zinc oxide nanoparticles: a comparison. Green Chem Lett Rev. 2019;12(1):19–24.10.1080/17518253.2018.1547925Suche in Google Scholar
[74] Salem SS, Fouda A. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res. 2021;199:344–70.10.1007/s12011-020-02138-3Suche in Google Scholar PubMed
[75] Zong Y, Li Z, Wang X, Ma J, Men Y. Synthesis and high photocatalytic activity of Eu-doped ZnO nanoparticles. Ceram Int. 2014;40(7):10375–82.10.1016/j.ceramint.2014.02.123Suche in Google Scholar
[76] Ramesh M, Anbuvannan M, Viruthagiri G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136:864–70.10.1016/j.saa.2014.09.105Suche in Google Scholar PubMed
[77] Bahrulolum H, Nooraei S, Javanshir N, Tarrahimofrad H, Mirbagheri VS, Easton AJ, et al. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J Nanobiotechnol. 2021;19(1):1–26.10.1186/s12951-021-00834-3Suche in Google Scholar PubMed PubMed Central
[78] Dhanemozhi AC, Rajeswari V, Sathyajothi S. Green synthesis of zinc oxide nanoparticle using green tea leaf extract for supercapacitor application. Mater Today: Proc. 2017;4(2):660–7.10.1016/j.matpr.2017.01.070Suche in Google Scholar
[79] Ijaz I, Gilani E, Nazir A, Bukhari A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem Lett Rev. 2020;13(3):223–45.10.1080/17518253.2020.1802517Suche in Google Scholar
[80] Hasnidawani J, Azlina H, Norita H, Bonnia N, Ratim S, Ali E. Synthesis of ZnO nanostructures using sol-gel method. Procedia Chem. 2016;19:211–6.10.1016/j.proche.2016.03.095Suche in Google Scholar
[81] Naveed Ul Haq A, Nadhman A, Ullah I, Mustafa G, Yasinzai M, Khan I. Synthesis approaches of zinc oxide nanoparticles: the dilemma of ecotoxicity. J Nanomater. 2017;2017:8510342.10.1155/2017/8510342Suche in Google Scholar
[82] Xu J, Huang Y, Zhu S, Abbes N, Jing X, Zhang L. A review of the green synthesis of ZnO nanoparticles using plant extracts and their prospects for application in antibacterial textiles. J Eng Fibers Fabr. 2021;16:15589250211046242.10.1177/15589250211046242Suche in Google Scholar
[83] Krstulović N, Salamon K, Budimlija O, Kovač J, Dasović J, Umek P, et al. Parameters optimization for synthesis of Al-doped ZnO nanoparticles by laser ablation in water. Appl Surf Sci. 2018;440:916–25.10.1016/j.apsusc.2018.01.295Suche in Google Scholar
[84] Sundrarajan M, Ambika S, Bharathi K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv Powder Technol. 2015;26(5):1294–9.10.1016/j.apt.2015.07.001Suche in Google Scholar
[85] Lingaraju K, Raja Naika H, Manjunath K, Basavaraj R, Nagabhushana H, Nagaraju G, et al. Biogenic synthesis of zinc oxide nanoparticles using Ruta graveolens (L.) and their antibacterial and antioxidant activities. Appl Nanosci. 2016;6:703–10.10.1007/s13204-015-0487-6Suche in Google Scholar
[86] da Silva LAL, Pezzini BR, Soares L. Spectrophotometric determination of the total flavonoid content in Ocimum basilicum L.(Lamiaceae) leaves. Pharmacogn Mag. 2015;11(41):96.10.4103/0973-1296.149721Suche in Google Scholar PubMed PubMed Central
[87] Pillai AM, Sivasankarapillai VS, Rahdar A, Joseph J, Sadeghfar F, Rajesh K, et al. Green synthesis and characterization of zinc oxide nanoparticles with antibacterial and antifungal activity. J Mol Struct. 2020;1211:128107.10.1016/j.molstruc.2020.128107Suche in Google Scholar
[88] Rambabu K, Bharath G, Banat F, Show PL. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater. 2021;402:123560.10.1016/j.jhazmat.2020.123560Suche in Google Scholar PubMed
[89] Alsmadi MM, Al-Nemrawi NK, Obaidat R, Alkahsi AEA, Koeshed KM, Lahlouh IK. The mapping between the green synthesis conditions of zinc oxide nanoparticles and their toxicokinetics. Nanomedicine. 2022;17(18):1281–303.10.2217/nnm-2022-0092Suche in Google Scholar PubMed
[90] Soltys L, Olkhovyy O, Tatarchuk T, Naushad M. Green synthesis of metal and metal oxide nanoparticles: Principles of green chemistry and raw materials. Magnetochemistry. 2021;7(11):145.10.3390/magnetochemistry7110145Suche in Google Scholar
[91] Nasrollahzadeh M, Sajadi SM. Pd nanoparticles synthesized in situ with the use of Euphorbia granulate leaf extract: Catalytic properties of the resulting particles. J Colloid Interface Sci. 2016;462:243–51.10.1016/j.jcis.2015.09.065Suche in Google Scholar PubMed
[92] Arumugam M, Manikandan DB, Dhandapani E, Sridhar A, Balakrishnan K, Markandan M, et al. Green synthesis of zinc oxide nanoparticles (ZnO NPs) using Syzygium cumini: Potential multifaceted applications on antioxidants, cytotoxic and as nanonutrient for the growth of Sesamum indicum. Environ Technol Innov. 2021;23:101653.10.1016/j.eti.2021.101653Suche in Google Scholar
[93] Sharma D, Sabela MI, Kanchi S, Bisetty K, Skelton AA, Honarparvar B. Green synthesis, characterization and electrochemical sensing of silymarin by ZnO nanoparticles: Experimental and DFT studies. J Electroanal Chem. 2018;808:160–72.10.1016/j.jelechem.2017.11.039Suche in Google Scholar
[94] Muthuchamy N, Atchudan R, Edison TNJI, Perumal S, Lee YR. High-performance glucose biosensor based on green synthesized zinc oxide nanoparticle embedded nitrogen-doped carbon sheet. J Electroanal Chem. 2018;816:195–204.10.1016/j.jelechem.2018.03.059Suche in Google Scholar
[95] Shaba EY, Jacob JO, Tijani JO, Suleiman MAT. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl Water Sci. 2021;11:1–41.10.1007/s13201-021-01370-zSuche in Google Scholar
[96] Naseer M, Aslam U, Khalid B, Chen B. Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci Rep. 2020;10(1):1–10.10.1038/s41598-020-65949-3Suche in Google Scholar PubMed PubMed Central
[97] Jayachandran A, Aswathy T, Nair AS. Green synthesis and characterization of zinc oxide nanoparticles using Cayratia pedata leaf extract. Biochem Biophys Rep. 2021;26:100995.10.1016/j.bbrep.2021.100995Suche in Google Scholar PubMed PubMed Central
[98] Hassan Basri H, Talib RA, Sukor R, Othman SH, Ariffin H. Effect of synthesis temperature on the size of ZnO nanoparticles derived from pineapple peel extract and antibacterial activity of ZnO–starch nanocomposite films. Nanomaterials. 2020;10(6):1061.10.3390/nano10061061Suche in Google Scholar PubMed PubMed Central
[99] Padalia H, Baluja S, Chanda S. Effect of pH on size and antibacterial activity of Salvadora oleoides leaf extract-mediated synthesis of zinc oxide nanoparticles. Bionanoscience. 2017;7:40–9.10.1007/s12668-016-0387-6Suche in Google Scholar
[100] Umamaheswari A, Prabu SL, John SA, Puratchikody A. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol Rep. 2021;29:e00595.10.1016/j.btre.2021.e00595Suche in Google Scholar PubMed PubMed Central
[101] Mohammadi FM, Ghasemi N. Influence of temperature and concentration on biosynthesis and characterization of zinc oxide nanoparticles using cherry extract. J Nanostructure Chem. 2018;8:93–102.10.1007/s40097-018-0257-6Suche in Google Scholar
[102] Agarwal H, Menon S, Kumar SV, Rajeshkumar S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem Biol Interact. 2018;286:60–70.10.1016/j.cbi.2018.03.008Suche in Google Scholar PubMed
[103] Barzinjy AA, Azeez HH. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globulus Labill. leaf extract and zinc nitrate hexahydrate salt. SN Appl Sci. 2020;2(5):991.10.1007/s42452-020-2813-1Suche in Google Scholar
[104] Fouda A, Saied E, Eid AM, Kouadri F, Alemam AM, Hamza MF, et al. Green synthesis of zinc oxide nanoparticles using an aqueous extract of punica granatum for antimicrobial and catalytic activity. J Funct Biomater. 2023;14(4):205.10.3390/jfb14040205Suche in Google Scholar PubMed PubMed Central
[105] Aldeen TS, Mohamed HEA, Maaza M. ZnO nanoparticles prepared via a green synthesis approach: Physical properties, photocatalytic and antibacterial activity. J Phys Chem Solids. 2022;160:110313.10.1016/j.jpcs.2021.110313Suche in Google Scholar
[106] Happy A, Soumya M, Kumar SV, Rajeshkumar S, Sheba RD, Lakshmi T, et al. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem Biophys Rep. 2019;17:208–11.10.1016/j.bbrep.2019.01.002Suche in Google Scholar PubMed PubMed Central
[107] Khan MF, Khan MA. Plant-derived metal nanoparticles (PDMNPs): Synthesis, characterization, and oxidative stress-mediated therapeutic actions. Future Pharmacol. 2023;3(1):252–95.10.3390/futurepharmacol3010018Suche in Google Scholar
[108] Vijayaraghavan K, Ashokkumar T. Plant-mediated biosynthesis of metallic nanoparticles: A review of literature, factors affecting synthesis, characterization techniques and applications. J Environ Chem Eng. 2017;5(5):4866–83.10.1016/j.jece.2017.09.026Suche in Google Scholar
[109] Alamdari S, Sasani Ghamsari M, Lee C, Han W, Park H-H, Tafreshi MJ, et al. Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl Sci. 2020;10(10):3620.10.3390/app10103620Suche in Google Scholar
[110] Sabir S, Arshad M, Chaudhari SK. Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J. 2014;2014:925494.10.1155/2014/925494Suche in Google Scholar PubMed PubMed Central
[111] Mishra PK, Mishra H, Ekielski A, Talegaonkar S, Vaidya B. Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today. 2017;22(12):1825–34.10.1016/j.drudis.2017.08.006Suche in Google Scholar PubMed
[112] El-Belely EF, Farag MM, Said HA, Amin AS, Azab E, Gobouri AA, et al. Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (Class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials. 2021;11(1):95.10.3390/nano11010095Suche in Google Scholar PubMed PubMed Central
[113] Pinho AR, Martins F, Costa MEV, Senos AM, da Cruz e Silva, Pereira MdL OA, et al. In vitro cytotoxicity effects of zinc oxide nanoparticles on spermatogonia cells. Cells. 2020;9(5):1081.10.3390/cells9051081Suche in Google Scholar PubMed PubMed Central
[114] Aydin Sevinç B, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. J Biomed Mater Res Part B Appl Biomater. 2010;94(1):22–31.10.1002/jbm.b.31620Suche in Google Scholar PubMed PubMed Central
[115] Song W, Zhang J, Guo J, Zhang J, Ding F, Li L, et al. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol Lett. 2010;199(3):389–97.10.1016/j.toxlet.2010.10.003Suche in Google Scholar PubMed
[116] Shen C, James SA, de Jonge MD, Turney TW, Wright PF, Feltis BN. Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle–exposed human immune cells. Toxicol Sci. 2013;136(1):120–30.10.1093/toxsci/kft187Suche in Google Scholar PubMed
[117] Liao C, Jin Y, Li Y, Tjong SC. Interactions of zinc oxide nanostructures with mammalian cells: cytotoxicity and photocatalytic toxicity. Int J Mol Sci. 2020;21(17):6305.10.3390/ijms21176305Suche in Google Scholar PubMed PubMed Central
[118] Álvarez-Chimal R, García-Pérez VI, Álvarez-Pérez MA, Tavera-Hernández R, Reyes-Carmona L, Martínez-Hernández M, et al. Influence of the particle size on the antibacterial activity of green synthesized zinc oxide nanoparticles using Dysphania ambrosioides extract, supported by molecular docking analysis. Arab J Chem. 2022;15(6):103804.10.1016/j.arabjc.2022.103804Suche in Google Scholar
[119] Preedia Babu E, Subastri A, Suyavaran A, Premkumar K, Sujatha V, Aristatile B, et al. Size dependent uptake and hemolytic effect of zinc oxide nanoparticles on erythrocytes and biomedical potential of ZnO-ferulic acid conjugates. Sci Rep. 2017;7(1):1–12.10.1038/s41598-017-04440-ySuche in Google Scholar PubMed PubMed Central
[120] Wahab R, Mishra A, Yun S-I, Kim Y-S, Shin H-S. Antibacterial activity of ZnO nanoparticles prepared via non-hydrolytic solution route. Appl Microbiol Biotechnol. 2010;87(5):1917–25.10.1007/s00253-010-2692-2Suche in Google Scholar PubMed
[121] Gupta J, Bhargava P, Bahadur D. Fluorescent ZnO for imaging and induction of DNA fragmentation and ROS-mediated apoptosis in cancer cells. J Mater Chem B. 2015;3(9):1968–78.10.1039/C4TB01661KSuche in Google Scholar
[122] Choudhury SR, Ordaz J, Lo C-L, Damayanti NP, Zhou F, Irudayaraj J. From the cover: zinc oxide nanoparticles-induced reactive oxygen species promotes multimodal cyto-and epigenetic toxicity. Toxicol Sci. 2017;156(1):261–74.Suche in Google Scholar
[123] Hajjo R, Sabbah DA, Al, Bawab AQ. Unlocking the Potential of the Human Microbiome for Identifying Disease Diagnostic Biomarkers. Diagnostics. 2022;12(7):1742.10.3390/diagnostics12071742Suche in Google Scholar PubMed PubMed Central
[124] Aljanabi R, Alsous L, Sabbah DA, Gul HI, Gul M, Bardaweel SK. Monoamine oxidase (MAO) as a potential target for anticancer drug design and development. Molecules. 2021;26(19):6019.10.3390/molecules26196019Suche in Google Scholar PubMed PubMed Central
[125] Attia H, Nounou H, Shalaby M. Zinc oxide nanoparticles induced oxidative DNA damage, inflammation and apoptosis in rat’s brain after oral exposure. Toxics. 2018;6(2):29.10.3390/toxics6020029Suche in Google Scholar PubMed PubMed Central
[126] Babele PK, Thakre PK, Kumawat R, Tomar RS. Zinc oxide nanoparticles induce toxicity by affecting cell wall integrity pathway, mitochondrial function and lipid homeostasis in Saccharomyces cerevisiae. Chemosphere. 2018;213:65–75.10.1016/j.chemosphere.2018.09.028Suche in Google Scholar PubMed
[127] Yu K-N, Yoon T-J, Minai-Tehrani A, Kim J-E, Park SJ, Jeong MS, et al. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol In Vitro. 2013;27(4):1187–95.10.1016/j.tiv.2013.02.010Suche in Google Scholar PubMed
[128] Abomuti MA, Danish EY, Firoz A, Hasan N, Malik MA. Green synthesis of zinc oxide nanoparticles using salvia officinalis leaf extract and their photocatalytic and antifungal activities. Biology. 2021;10(11):1075.10.3390/biology10111075Suche in Google Scholar PubMed PubMed Central
[129] Hameed S, Khalil AT, Ali M, Numan M, Khamlich S, Shinwari ZK, et al. Greener synthesis of ZnO and Ag–ZnO nanoparticles using Silybum marianum for diverse biomedical applications. Nanomedicine. 2019;14(6):655–73.10.2217/nnm-2018-0279Suche in Google Scholar PubMed
[130] Datta A, Patra C, Bharadwaj H, Kaur S, Dimri N, Khajuria R. Green synthesis of zinc oxide nanoparticles using parthenium hysterophorus leaf extract and evaluation of their antibacterial properties. J Biotechnol Biomater. 2017;7(3):271–6.10.4172/2155-952X.1000271Suche in Google Scholar
[131] Nair S, Sasidharan A, Divya Rani V, Menon D, Nair S, Manzoor K, et al. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J Mater Sci Mater Med. 2009;20(1):235–41.10.1007/s10856-008-3548-5Suche in Google Scholar PubMed
[132] Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27(7):4020–8.10.1021/la104825uSuche in Google Scholar PubMed
[133] Gunalan S, Sivaraj R, Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog Nat Sci: Mater Int. 2012;22(6):693–700.10.1016/j.pnsc.2012.11.015Suche in Google Scholar
[134] Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, et al. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev. 2013;37(6):955–89.10.1111/1574-6976.12026Suche in Google Scholar PubMed PubMed Central
[135] Lallo da Silva B, Abuçafy MP, Berbel Manaia E, Oshiro Junior JA, Chiari-Andréo BG, Pietro RCR, et al. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int J Nanomed. 2019;9395–410.10.2147/IJN.S216204Suche in Google Scholar PubMed PubMed Central
[136] Slavin YN, Asnis J, Hńfeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. 2017;15:1–20.10.1186/s12951-017-0308-zSuche in Google Scholar PubMed PubMed Central
[137] Shi L-E, Li Z-H, Zheng W, Zhao Y-F, Jin Y-F, Tang Z-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food Addit Contam Part A. 2014;31(2):173–86.10.1080/19440049.2013.865147Suche in Google Scholar PubMed
[138] Joe A, Park S-H, Shim K-D, Kim D-J, Jhee K-H, Lee H-W, et al. Antibacterial mechanism of ZnO nanoparticles under dark conditions. J Ind Eng Chem. 2017;45:430–9.10.1016/j.jiec.2016.10.013Suche in Google Scholar
[139] Siddiqi KS, Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res Lett. 2018;13(1):1–13.10.1186/s11671-018-2532-3Suche in Google Scholar PubMed PubMed Central
[140] Shinde VV, Dalavi DS, Mali SS, Hong CK, Kim JH, Patil PS. Surfactant free microwave assisted synthesis of ZnO microspheres: Study of their antibacterial activity. Appl Surf Sci. 2014;307:495–502.10.1016/j.apsusc.2014.04.064Suche in Google Scholar
[141] Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett. 2007;90(21):213902.10.1063/1.2742324Suche in Google Scholar PubMed PubMed Central
[142] Tayel AA, EL-TRAS WF, Moussa S, EL-BAZ AF, Mahrous H, Salem MF, et al. Antibacterial action of zinc oxide nanoparticles against foodborne pathogens. J Food Saf. 2011;31(2):211–8.10.1111/j.1745-4565.2010.00287.xSuche in Google Scholar
[143] d’Água RB, Branquinho R, Duarte MP, Maurício E, Fernando AL, Martins R, et al. Efficient coverage of ZnO nanoparticles on cotton fibres for antibacterial finishing using a rapid and low cost in situ synthesis. New J Chem. 2018;42(2):1052–60.10.1039/C7NJ03418KSuche in Google Scholar
[144] Jiang Y, Zhang L, Wen D, Ding Y. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater Sci Eng C. 2016;69:1361–6.10.1016/j.msec.2016.08.044Suche in Google Scholar PubMed
[145] Saliani M, Jalal R, Goharshadi EK. Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and Staphylococcus aureus. Jundishapur J Microbiol. 2015;8(2).10.5812/jjm.17115Suche in Google Scholar PubMed PubMed Central
[146] Moreau JW, Weber PK, Martin MC, Gilbert B, Hutcheon ID, Banfield JF. Extracellular proteins limit the dispersal of biogenic nanoparticles. Science. 2007;316(5831):1600–3.10.1126/science.1141064Suche in Google Scholar PubMed
[147] Miao AJ, Zhang XY, Luo Z, Chen CS, Chin WC, Santschi PH, et al. Zinc oxide–engineered nanoparticles: dissolution and toxicity to marine phytoplankton. Environ Toxicol Chem. 2010;29(12):2814–22.10.1002/etc.340Suche in Google Scholar PubMed
[148] Sharma S, Kumar K, Thakur N, Chauhan S, Chauhan M. The effect of shape and size of ZnO nanoparticles on their antimicrobial and photocatalytic activities: a green approach. Bull Mater Sci. 2020;43:1–10.10.1007/s12034-019-1986-ySuche in Google Scholar
[149] Peng X, Palma S, Fisher NS, Wong SS. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat Toxicol. 2011;102(3–4):186–96.10.1016/j.aquatox.2011.01.014Suche in Google Scholar PubMed
[150] Arakha M, Saleem M, Mallick BC, Jha S. The effects of interfacial potential on antimicrobial propensity of ZnO nanoparticle. Sci Rep. 2015;5(1):1–10.10.1038/srep09578Suche in Google Scholar PubMed PubMed Central
[151] Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater. 2018;7(13):1701503.10.1002/adhm.201701503Suche in Google Scholar PubMed
[152] Mendes CR, Dilarri G, Forsan CF, Sapata VdMRLopes, de Moraes PRM, PB, et al. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci Rep. 2022;12(1):2658.10.1038/s41598-022-06657-ySuche in Google Scholar PubMed PubMed Central
[153] Thi TUD, Nguyen TT, Thi YD, Thi KHT, Phan BT, Pham KN. Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv. 2020;10(40):23899–907.10.1039/D0RA04926CSuche in Google Scholar
[154] Umavathi S, Mahboob S, Govindarajan M, Al-Ghanim KA, Ahmed Z, Virik P, et al. Green synthesis of ZnO nanoparticles for antimicrobial and vegetative growth applications: A novel approach for advancing efficient high quality health care to human wellbeing. Saudi J Biol Sci. 2021;28(3):1808–15.Suche in Google Scholar
[155] Safavinia L, Akhgar MR, Tahamipour B, Ahmadi SA. Green synthesis of highly dispersed zinc oxide nanoparticles supported on silica gel matrix by Daphne oleoides extract and their antibacterial activity. Iran J Biotechnol. 2021;19(1):e2598.Suche in Google Scholar
[156] S M NH, P.P, V. In vitro biocompatibility and antimicrobial activities of zinc oxide nanoparticles (ZnO NPs) prepared by chemical and green synthetic route— A comparative study. BioNanoScience. 2020;10(1):112–21.10.1007/s12668-019-00698-wSuche in Google Scholar
[157] Bekele B, Degefa A, Tesgera F, Jule LT, Shanmugam R, Priyanka Dwarampudi L, et al. Green versus chemical precipitation methods of preparing zinc oxide nanoparticles and investigation of antimicrobial properties. J Nanomater. 2021;2021:1–10.10.1155/2021/9210817Suche in Google Scholar
[158] Hussein BY, Mohammed AM. Green synthesis of ZnO nanoparticles in grape extract: Their application as anti-cancer and anti-bacterial. Mater Today: Proc. 2021;42:A18–26.10.1016/j.matpr.2021.03.729Suche in Google Scholar
[159] Nilavukkarasi M, Vijayakumar S, Prathipkumar S. Capparis zeylanica mediated bio-synthesized ZnO nanoparticles as antimicrobial, photocatalytic and anti-cancer applications. Mater Sci Energy Technol. 2020;3:335–43.10.1016/j.mset.2019.12.004Suche in Google Scholar
[160] Ahamed M, Akhtar MJ, Khan MM, Alhadlaq HA. Facile green synthesis of ZnO-RGO nanocomposites with enhanced anticancer efficacy. Methods. 2022;199:28–36.10.1016/j.ymeth.2021.04.020Suche in Google Scholar PubMed
[161] Ahmadi Shadmehri A, Namvar F. A review on green synthesis, cytotoxicity mechanism and antibacterial activity of Zno-NPs. Int J Appl Basic Med Res. 2020;6(1):23–31.Suche in Google Scholar
[162] Hanley C, Layne J, Punnoose A, Reddy K, Coombs I, Coombs A, et al. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 2008;19(29):295103.10.1088/0957-4484/19/29/295103Suche in Google Scholar PubMed PubMed Central
[163] Sharmila G, Thirumarimurugan M, Muthukumaran C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem J. 2019;145:578–87.10.1016/j.microc.2018.11.022Suche in Google Scholar
[164] Ruangtong J, Jiraroj T, T-Thienprasert NP. Green synthesized ZnO nanosheets from banana peel extract possess anti-bacterial activity and anti-cancer activity. Mater Today Commun. 2020;24:101224.10.1016/j.mtcomm.2020.101224Suche in Google Scholar
[165] Jevapatarakul D, Jiraroj T, Payungporn S, Chavalit T, Khamwut A, T-Thienprasert NP. Utilization of Cratoxylum formosum crude extract for synthesis of ZnO nanosheets: Characterization, biological activities and effects on gene expression of nonmelanoma skin cancer cell. Biomed Pharmacother. 2020;130:110552.10.1016/j.biopha.2020.110552Suche in Google Scholar PubMed
[166] Duan X, Liao Y, Liu T, Yang H, Liu Y, Chen Y, et al. Zinc oxide nanoparticles synthesized from Cardiospermum halicacabum and its anticancer activity in human melanoma cells (A375) through the modulation of apoptosis pathway. J Photochem Photobiol B, Biol. 2020;202:111718.10.1016/j.jphotobiol.2019.111718Suche in Google Scholar PubMed
[167] Selim YA, Azb MA, Ragab I, Abd El-Azim HM, M. Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverra tortuosa and their cytotoxic activities. Sci Rep. 2020;10(1):1–9.10.1038/s41598-020-60541-1Suche in Google Scholar PubMed PubMed Central
[168] Vakayil R, Muruganantham S, Kabeerdass N, Rajendran M, Ramasamy S, Alahmadi TA, et al. Acorus calamus-zinc oxide nanoparticle coated cotton fabrics shows antimicrobial and cytotoxic activities against skin cancer cells. Process Biochem. 2021;111:1–8.10.1016/j.procbio.2021.08.024Suche in Google Scholar
[169] Chandrasekaran S, Anusuya S, Anbazhagan V. Anticancer, anti-diabetic, antimicrobial activity of zinc oxide nanoparticles: A comparative analysis. J Mol Struct. 2022;1263:133139.10.1016/j.molstruc.2022.133139Suche in Google Scholar
[170] George D, Maheswari PU, Begum KMS. Synergic formulation of onion peel quercetin loaded chitosan-cellulose hydrogel with green zinc oxide nanoparticles towards controlled release, biocompatibility, antimicrobial and anticancer activity. Int J Biol Macromol. 2019;132:784–94.10.1016/j.ijbiomac.2019.04.008Suche in Google Scholar PubMed
[171] Chelladurai M, Margavelu G, Vijayakumar S, González-Sánchez ZI, Vijayan K, Sahadevan R. Preparation and characterization of amine-functionalized mupirocin-loaded zinc oxide nanoparticles: A potent drug delivery agent in targeting human epidermoid carcinoma (A431) cells. J Drug Deliv Sci Technol. 2022;70:103244.10.1016/j.jddst.2022.103244Suche in Google Scholar
[172] Batool M, Khurshid S, Daoush WM, Siddique SA, Nadeem T. Green synthesis and biomedical applications of ZnO nanoparticles: Role of PEGylated-ZnO nanoparticles as doxorubicin drug carrier against MDA-MB-231 (TNBC) cells line. Crystals. 2021;11(4):344.10.3390/cryst11040344Suche in Google Scholar
[173] Vimala K, Sundarraj S, Paulpandi M, Vengatesan S, Kannan S. Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem. 2014;49(1):160–72.10.1016/j.procbio.2013.10.007Suche in Google Scholar
[174] Morovati MR, Ghanbari-Movahed M, Barton EM, Farzaei MH, Bishayee A. A systematic review on potential anticancer activities of Ficus carica L. with focus on cellular and molecular mechanisms. Phytomedicine. 2022;154333.10.1016/j.phymed.2022.154333Suche in Google Scholar PubMed
[175] Shrihastini V, Muthuramalingam P, Adarshan S, Sujitha M, Chen J-T, Shin H, et al. Plant derived bioactive compounds, their anti-cancer effects and in silico approaches as an alternative target treatment strategy for breast cancer: An updated overview. Cancers (Basel). 2021;13(24):6222.10.3390/cancers13246222Suche in Google Scholar PubMed PubMed Central
[176] Ibrahim MH, Jaafar HZ, Karimi E, Ghasemzadeh A. Primary, secondary metabolites, photosynthetic capacity and antioxidant activity of the Malaysian Herb Kacip Fatimah (Labisia Pumila Benth) exposed to potassium fertilization under greenhouse conditions. Int J Mol Sci. 2012;13(11):15321–42.10.3390/ijms131115321Suche in Google Scholar PubMed PubMed Central
[177] Yadav B, Bajaj A, Saxena M, Saxena A. In vitro anticancer activity of the root, stem and leaves of Withania somnifera against various human cancer cell lines. Indian J Pharm Sci. 2010;72(5):659.10.4103/0250-474X.78543Suche in Google Scholar PubMed PubMed Central
[178] Akindele AJ, Wani ZA, Sharma S, Mahajan G, Satti NK, Adeyemi OO, et al. In vitro and in vivo anticancer activity of root extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evid Based Complement Alternat Med. 2015;2015:560404.10.1155/2015/560404Suche in Google Scholar PubMed PubMed Central
[179] Ardalani H, Hejazi Amiri F, Hadipanah A, Kongstad KT. Potential antidiabetic phytochemicals in plant roots: A review of in vivo studies. J Diabetes Metab Disord. 2021;20:1–18.10.1007/s40200-021-00853-9Suche in Google Scholar PubMed PubMed Central
[180] Mutukwa D, Taziwa R, Khotseng LE. A review of the green synthesis of ZnO nanoparticles utilising Southern African Indigenous medicinal plants. Nanomaterials. 2022;12(19):3456.10.3390/nano12193456Suche in Google Scholar PubMed PubMed Central
[181] Roszkowski S. Application of Polyphenols and Flavonoids in Oncological Therapy. Molecules. 2023;28(10):4080.10.3390/molecules28104080Suche in Google Scholar PubMed PubMed Central
[182] Koklesova L, Jakubikova J, Cholujova D, Samec M, Mazurakova A, Šudomová M, et al. Phytochemical-based nanodrugs going beyond the state-of-the-art in cancer management—Targeting cancer stem cells in the framework of predictive, preventive, personalized medicine. Front Pharmacol. 2023;14:1121950.10.3389/fphar.2023.1121950Suche in Google Scholar PubMed PubMed Central
[183] Gobinath P, Packialakshmi P, Hatamleh AA, Al-Dosary MA, Al-Wasel YA, Balasubramani R, et al. Calotropis gigantea assisted synthesis of zinc oxide nanoparticle catalysis: synthesis of novel 3-amino thymoquinone connected 1, 4-dihyropyridine derivatives and their cytotoxic activity. J Nanomater. 2022;2022:9697057.10.1155/2022/9697057Suche in Google Scholar
[184] Jansen J, Karges W, Rink L. Zinc and diabetes—clinical links and molecular mechanisms. J Nutr Biochem. 2009;20(6):399–417.10.1016/j.jnutbio.2009.01.009Suche in Google Scholar PubMed
[185] Egefjord L, Petersen AB, Bak AM, Rungby J. Zinc, alpha cells and glucagon secretion. Curr Diabetes Rev. 2010;6(1):52–7.10.2174/157339910790442655Suche in Google Scholar PubMed
[186] Rajakumar G, Thiruvengadam M, Mydhili G, Gomathi T, Chung I-M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst Eng. 2018;41(1):21–30.10.1007/s00449-017-1840-9Suche in Google Scholar PubMed
[187] Argade PA, Bhutkar MA, Magdum CS. Albizzia lebbeck extract mediated synthesis of Zinc Oxide Nanoparticles and study of its In-vitro Anti-diabetic and Anti-oxidant activity. Asian J Pharm Technol. 2019;9(2):93–8.10.5958/2231-5713.2019.00016.3Suche in Google Scholar
[188] Rahman MM, Islam MB, Biswas M, Khurshid, Alam A. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res Notes. 2015;8(1):1–9.10.1186/s13104-015-1618-6Suche in Google Scholar PubMed PubMed Central
[189] Suresh D, Shobharani R, Nethravathi P, Kumar MP, Nagabhushana H, Sharma S. Artocarpus gomezianus aided green synthesis of ZnO nanoparticles: luminescence, photocatalytic and antioxidant properties. Spectrochim Acta A Mol Biomol Spectrosc. 2015;141:128–34.10.1016/j.saa.2015.01.048Suche in Google Scholar PubMed
[190] Suresh D, Nethravathi P, Rajanaika H, Nagabhushana H, Sharma S. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater Sci Semicond Process. 2015;31:446–54.10.1016/j.mssp.2014.12.023Suche in Google Scholar
[191] Nagajyothi P, Cha SJ, Yang IJ, Sreekanth T, Kim KJ, Shin HM. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B Biol. 2015;146:10–7.10.1016/j.jphotobiol.2015.02.008Suche in Google Scholar PubMed
[192] Siripireddy B, Mandal BK. Facile green synthesis of zinc oxide nanoparticles by Eucalyptus globulus and their photocatalytic and antioxidant activity. Adv Powder Technol. 2017;28(3):785–97.10.1016/j.apt.2016.11.026Suche in Google Scholar
[193] Murali M, Mahendra C, Rajashekar N, Sudarshana M, Raveesha K, Amruthesh K. Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L.–an endemic species. Spectrochim Acta A Mol Biomol Spectrosc. 2017;179:104–9.10.1016/j.saa.2017.02.027Suche in Google Scholar PubMed
[194] Khan ZUH, Sadiq HM, Shah NS, Khan AU, Muhammad N, Hassan SU, et al. Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J Photochem Photobiol B Biol. 2019;192:147–57.10.1016/j.jphotobiol.2019.01.013Suche in Google Scholar PubMed
[195] Faisal S, Jan H, Shah SA, Shah S, Khan A, Akbar MT, et al. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: their characterizations and biological and environmental applications. ACS Omega. 2021;6(14):9709–22.10.1021/acsomega.1c00310Suche in Google Scholar PubMed PubMed Central
[196] Padalia H, Chanda S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol. 2017;45(8):1751–61.10.1080/21691401.2017.1282868Suche in Google Scholar PubMed
[197] Miri A, Mahdinejad N, Ebrahimy O, Khatami M, Sarani M. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater Sci Eng C. 2019;104:109981.10.1016/j.msec.2019.109981Suche in Google Scholar PubMed
[198] Yassin MT, Mostafa AA-F, Al-Askar AA, Al-Otibi FO. Facile green synthesis of zinc oxide nanoparticles with potential synergistic activity with common antifungal agents against multidrug-resistant candidal strains. Crystals. 2022;12(6):774.10.3390/cryst12060774Suche in Google Scholar
[199] T-Thienprasert NP, T-Thienprasert J, Ruangtong J, Jaithon T, Srifah Huehne P, Piasai O. Large scale synthesis of green synthesized zinc oxide nanoparticles from banana peel extracts and their inhibitory effects against Colletotrichum sp., isolate KUFC 021, causal agent of anthracnose on dendrobium orchid. J Nanomater. 2021;2021:1–10.10.1155/2021/5625199Suche in Google Scholar
[200] Dhiman S, Varma A, Prasad R, Goel A. Mechanistic Insight of the Antifungal Potential of Green Synthesized Zinc Oxide Nanoparticles against Alternaria brassicae. J Nanomater. 2022;2022:713884.10.1155/2022/7138843Suche in Google Scholar
[201] Jamdagni P, Khatri P, Rana J. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J King Saud Univ Sci. 2018;30(2):168–75.10.1016/j.jksus.2016.10.002Suche in Google Scholar
[202] Zhu W, Hu C, Ren Y, Lu Y, Song Y, Ji Y, et al. Green synthesis of zinc oxide nanoparticles using Cinnamomum camphora (L.) Presl leaf extracts and its antifungal activity. J Environ Chem Eng. 2021;9(6):106659.10.1016/j.jece.2021.106659Suche in Google Scholar
[203] Pasquet J, Chevalier Y, Couval E, Bouvier D, Noizet G, Morlière C, et al. Antimicrobial activity of zinc oxide particles on five micro-organisms of the Challenge Tests related to their physicochemical properties. Int J Pharm. 2014;460(1–2):92–100.10.1016/j.ijpharm.2013.10.031Suche in Google Scholar PubMed
[204] Melk MM, El-Hawary SS, Melek FR, Saleh DO, Ali OM, El Raey MA, et al. Antiviral activity of zinc oxide nanoparticles mediated by plumbago indica L. Extract against herpes simplex virus type 1 (HSV-1). Int J Nanomed. 2021;16:8221.10.2147/IJN.S339404Suche in Google Scholar PubMed PubMed Central
[205] Melk MM, El-Hawary SS, Melek FR, Saleh DO, Ali OM, El Raey MA, et al. Nano zinc oxide green-synthesized from plumbago auriculata lam. alcoholic extract. Plants. 2021;10(11):2447.10.3390/plants10112447Suche in Google Scholar PubMed PubMed Central
[206] Hassan HS, Abol-Fotouh D, Salama E, Elkady MF. Assessment of antimicrobial, cytotoxicity, and antiviral impact of a green zinc oxide/activated carbon nanocomposite. Sci Rep. 2022;12(1):8774.10.1038/s41598-022-12648-wSuche in Google Scholar PubMed PubMed Central
[207] Rodelo CG, Salinas RA, JaimeArmenta EA, Armenta S, Galdámez-Martínez A, Castillo-Blum SE, et al. Zinc associated nanomaterials and their intervention in emerging respiratory viruses: Journey to the field of biomedicine and biomaterials. Coord Chem Rev. 2022;457:214402.10.1016/j.ccr.2021.214402Suche in Google Scholar PubMed PubMed Central
[208] Hamdi M, Abdel-Bar HM, Elmowafy E, El-Khouly A, Mansour M, Awad GA. Investigating the internalization and COVID-19 antiviral computational analysis of optimized nanoscale zinc oxide. ACS Omega. 2021;6(10):6848–60.10.1021/acsomega.0c06046Suche in Google Scholar PubMed PubMed Central
[209] Alrabayah IN, Elhawary SS, Kandil ZA, El-Kadder EMA, Moemen YS, Saleh AM, et al. Green Synthesized Zinc Oxide Nanoparticles Based on Cestrum diurnum L. of Potential Antiviral Activity against Human Corona 229-E Virus. Molecules. 2022;28(1):266.10.3390/molecules28010266Suche in Google Scholar PubMed PubMed Central
[210] Dorostkar R, Ghalavand M, Nazarizadeh A, Tat M, Hashemzadeh MS. Anthelmintic effects of zinc oxide and iron oxide nanoparticles against Toxocara vitulorum. Int Nano Lett. 2017;7:157–64.10.1007/s40089-016-0198-3Suche in Google Scholar
[211] Saleh M, Abdel-Baki A-A, Dkhil MA, El-Matbouli M, Al-Quraishy S. Antiprotozoal effects of metal nanoparticles against Ichthyophthirius multifiliis. Parasitology. 2017;144(13):1802–10.10.1017/S0031182017001184Suche in Google Scholar PubMed
[212] Jan H, Shah M, Usman H, Khan MA, Zia M, Hano C, et al. Biogenic synthesis and characterization of antimicrobial and antiparasitic zinc oxide (ZnO) nanoparticles using aqueous extracts of the Himalayan Columbine (Aquilegia pubiflora). Front Mater. 2020;7:249.10.3389/fmats.2020.00249Suche in Google Scholar
[213] Najoom S, Fozia F, Ahmad I, Wahab A, Ahmad N, Ullah R, et al. Effective antiplasmodial and cytotoxic activities of synthesized zinc oxide nanoparticles using Rhazya stricta leaf extract. Evid-based Complement Altern Med. 2021;2021:558674.10.1155/2021/5586740Suche in Google Scholar PubMed PubMed Central
[214] Agarwal H, Shanmugam VK. Synthesis and optimization of zinc oxide nanoparticles using Kalanchoe pinnata towards the evaluation of its anti-inflammatory activity. J Drug Deliv Sci Technol. 2019;54:101291.10.1016/j.jddst.2019.101291Suche in Google Scholar
[215] Rahimi Kalateh Shah Mohammad G, Homayouni Tabrizi M, Ardalan T, Yadamani S, Safavi E. Green synthesis of zinc oxide nanoparticles and evaluation of anti-angiogenesis, anti-inflammatory and cytotoxicity properties. J Biosci. 2019;44(2):1–9.10.1007/s12038-019-9845-ySuche in Google Scholar
[216] Liu H, Kang P, Liu Y, An Y, Hu Y, Jin X, et al. Zinc oxide nanoparticles synthesised from the Vernonia amygdalina shows the anti-inflammatory and antinociceptive activities in the mice model. Artif Cells Nanomed Biotechnol. 2020;48(1):1068–78.10.1080/21691401.2020.1809440Suche in Google Scholar PubMed
[217] Lopez-Miranda JL, Molina GA, González-Reyna MA, España-Sánchez BL, Esparza R, Silva R, et al. Antibacterial and anti-inflammatory properties of ZnO nanoparticles synthesized by a green method using sargassum extracts. Int J Mol Sci. 2023;24(2):1474.10.3390/ijms24021474Suche in Google Scholar PubMed PubMed Central
[218] Anjum S, Hashim M, Malik SA, Khan M, Lorenzo JM, Abbasi BH, et al. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers (Basel). 2021;13(18):4570.10.3390/cancers13184570Suche in Google Scholar PubMed PubMed Central
[219] Mahdavi B, Saneei S, Qorbani M, Zhaleh M, Zangeneh A, Zangeneh MM, et al. Ziziphora clinopodioides Lam leaves aqueous extract mediated synthesis of zinc nanoparticles and their antibacterial, antifungal, cytotoxicity, antioxidant, and cutaneous wound healing properties under in vitro and in vivo conditions. Appl Organomet Chem. 2019;33(11):e5164.10.1002/aoc.5164Suche in Google Scholar
[220] Metwally AA, Abdel-Hady A-NA, Haridy MA, Ebnalwaled K, Saied AA, Soliman AS. Wound healing properties of green (using Lawsonia inermis leaf extract) and chemically synthesized ZnO nanoparticles in albino rats. Environ Sci Pollut Res. 2021;28:1–13.10.21203/rs.3.rs-639379/v1Suche in Google Scholar
[221] Shao F, Yang A, Yu DM, Wang J, Gong X, Tian HX. Bio-synthesis of Barleria gibsoni leaf extract mediated zinc oxide nanoparticles and their formulation gel for wound therapy in nursing care of infants and children. J Photochem Photobiol B: Biol. 2018;189:267–73.10.1016/j.jphotobiol.2018.10.014Suche in Google Scholar PubMed
[222] Wang P, Jiang L, Han R. Biosynthesis of zinc oxide nanoparticles and their application for antimicrobial treatment of burn wound infections. Mater Res Express. 2020;7(9):095010.10.1088/2053-1591/abb150Suche in Google Scholar
[223] Batool M, Khurshid S, Qureshi Z, Daoush WM. Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem Pap. 2021;75:893–907.10.1007/s11696-020-01343-7Suche in Google Scholar
[224] Iqbal Y, Malik AR, Iqbal T, Aziz MH, Ahmed F, Abolaban FA, et al. Green synthesis of ZnO and Ag-doped ZnO nanoparticles using Azadirachta indica leaves: Characterization and their potential antibacterial, antidiabetic, and wound-healing activities. Mater Lett. 2021;305:130671.10.1016/j.matlet.2021.130671Suche in Google Scholar
[225] Kabeerdass N, Thangasamy S, Murugesan K, Arumugam N, Almansour AI, Suresh Kumar R, et al. Embedding green synthesized zinc oxide nanoparticles in cotton fabrics and assessment of their antibacterial wound healing and cytotoxic properties: An eco-friendly approach. Green Process Synth. 2022;11(1):875–85.10.1515/gps-2022-0072Suche in Google Scholar
[226] Bandeira M, Chee BS, Frassini R, Nugent M, Giovanela M, Roesch-Ely M, et al. Antimicrobial PAA/PAH electrospun fiber containing green synthesized zinc oxide nanoparticles for wound healing. Materials. 2021;14(11):2889.10.3390/ma14112889Suche in Google Scholar PubMed PubMed Central
[227] Kalyani R, Kumar KS, Swamy P, Sekhar KC, Manish S, Murthy KR, et al. Synergetic potential of green synthesized nano-antibiotic combinational therapy for wound healing. Durham, North Carolina: Research Square; 2021.10.21203/rs.3.rs-1204154/v1Suche in Google Scholar
[228] Umavathi S, Mahboob S, Govindarajan M, Al-Ghanim KA, Ahmed Z, Virik P, et al. Green synthesis of ZnO nanoparticles for antimicrobial and vegetative growth applications: A novel approach for advancing efficient high quality health care to human wellbeing. Saudi J Biol Sci. 2021;28(3):1808–15.10.1016/j.sjbs.2020.12.025Suche in Google Scholar PubMed PubMed Central
[229] Bandeira M, Possan AL, Pavin SS, Raota CS, Vebber MC, Giovanela M, et al. Mechanism of formation, characterization and cytotoxicity of green synthesized zinc oxide nanoparticles obtained from Ilex paraguariensis leaves extract. Nano-Struct Nano-Objects. 2020;24:100532.10.1016/j.nanoso.2020.100532Suche in Google Scholar
[230] Elumalai K, Velmurugan S. Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl Surf Sci. 2015;345:329–36.10.1016/j.apsusc.2015.03.176Suche in Google Scholar
[231] Bala N, Saha S, Chakraborty M, Maiti M, Das S, Basu R, et al. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015;5(7):4993–5003.10.1039/C4RA12784FSuche in Google Scholar
[232] Salam HA, Sivaraj R, Venckatesh R. Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae leaf extract. Mater Lett. 2014;131:16–8.10.1016/j.matlet.2014.05.033Suche in Google Scholar
[233] Vijayakumar S, Mahadevan S, Arulmozhi P, Sriram S, Praseetha P. Green synthesis of zinc oxide nanoparticles using Atalantia monophylla leaf extracts: Characterization and antimicrobial analysis. Mater Sci Semicond Process. 2018;82:39–45.10.1016/j.mssp.2018.03.017Suche in Google Scholar
[234] Gur T, Meydan I, Seckin H, Bekmezci M, Sen F. Green synthesis, characterization and bioactivity of biogenic zinc oxide nanoparticles. Environ Res. 2022;204:111897.10.1016/j.envres.2021.111897Suche in Google Scholar PubMed
[235] Khatami M, Alijani HQ, Heli H, Sharifi I. Rectangular shaped zinc oxide nanoparticles: Green synthesis by Stevia and its biomedical efficiency. Ceram Int. 2018;44(13):15596–602.10.1016/j.ceramint.2018.05.224Suche in Google Scholar
[236] Ramesh P, Saravanan K, Manogar P, Johnson J, Vinoth E, Mayakannan M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sens Bio-Sens Res. 2021;31:100399.10.1016/j.sbsr.2021.100399Suche in Google Scholar
[237] Ogunyemi SO, Abdallah Y, Zhang M, Fouad H, Hong X, Ibrahim E, et al. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif Cells Nanomed Biotechnol. 2019;47(1):341–52.10.1080/21691401.2018.1557671Suche in Google Scholar PubMed
[238] Patrón-Romero L, Luque P, Soto-Robles C, Nava O, Vilchis-Nestor A, Barajas-Carrillo V, et al. Synthesis, characterization and cytotoxicity of zinc oxide nanoparticles by green synthesis method. J Drug Deliv Sci Technol. 2020;60:101925.10.1016/j.jddst.2020.101925Suche in Google Scholar
[239] Ifeanyichukwu UL, Fayemi OE, Ateba CN. Green synthesis of zinc oxide nanoparticles from pomegranate (Punica granatum) extracts and characterization of their antibacterial activity. Molecules. 2020;25(19):4521.10.3390/molecules25194521Suche in Google Scholar PubMed PubMed Central
[240] Pachaiappan R, Rajendran S, Ramalingam G, Vo D-VN, Priya PM, Soto-Moscoso M. Green synthesis of zinc oxide nanoparticles by Justicia adhatoda leaves and their antimicrobial activity. Chem Eng Technol. 2021;44(3):551–8.10.1002/ceat.202000470Suche in Google Scholar
[241] Jayappa MD, Ramaiah CK, Kumar MAP, Suresh D, Prabhu A, Devasya RP, et al. Green synthesis of zinc oxide nanoparticles from the leaf, stem and in vitro grown callus of Mussaenda frondosa L.: characterization and their applications. Appl Nanosci. 2020;10(8):3057–74.10.1007/s13204-020-01382-2Suche in Google Scholar PubMed PubMed Central
[242] Gao Y, Xu D, Ren D, Zeng K, Wu X. Green synthesis of zinc oxide nanoparticles using Citrus sinensis peel extract and application to strawberry preservation: A comparison study. LWT. 2020;126:109297.10.1016/j.lwt.2020.109297Suche in Google Scholar
[243] Ahmad H, Venugopal K, Rajagopal K, De Britto S, Nandini B, Pushpalatha HG, et al. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules. 2020;10(3):425.10.3390/biom10030425Suche in Google Scholar PubMed PubMed Central
[244] Sathappan S, Kirubakaran N, Gunasekaran D, Gupta PK, Verma RS, Sundaram J. Green synthesis of zinc oxide nanoparticles (ZnO NPs) using cissus quadrangularis: Characterization, antimicrobial and anticancer studies. Proc Natl Acad Sci India Sect B Biol Sci. 2021;91(2):289–96.10.1007/s40011-020-01215-wSuche in Google Scholar
[245] El-Hawwary SS, Abd Almaksoud HM, Saber FR, Elimam H, Sayed AM, El Raey MA, et al. Green-synthesized zinc oxide nanoparticles, anti-Alzheimer potential and the metabolic profiling of Sabal blackburniana grown in Egypt supported by molecular modelling. RSC Adv. 2021;11(29):18009–25.10.1039/D1RA01725JSuche in Google Scholar PubMed PubMed Central
[246] Jobie FN, Ranjbar M, Moghaddam AH, Kiani M. Green synthesis of zinc oxide nanoparticles using Amygdalus scoparia Spach stem bark extract and their applications as an alternative antimicrobial, anticancer, and anti-diabetic agent. Adv Powder Technol. 2021;32(6):2043–52.10.1016/j.apt.2021.04.014Suche in Google Scholar
[247] Dangana RS, George RC, Agboola FK. The biosynthesis of zinc oxide nanoparticles using aqueous leaf extracts of Cnidoscolus aconitifolius and their biological activities. Green Chem Lett Rev. 2023;16(1):2169591.10.1080/17518253.2023.2169591Suche in Google Scholar
[248] Salem NM, Awwad AM. Green synthesis and characterization of ZnO nanoparticles using Solanum rantonnetii leaves aqueous extract and antifungal activity evaluation. Chem Int. 2022;8(1):12–7.Suche in Google Scholar
[249] Garg R, Rani P, Garg R, Eddy NO. Study on potential applications and toxicity analysis of green synthesized nanoparticles. Turk J Chem. 2021;45(6):1690–706.10.3906/kim-2106-59Suche in Google Scholar
[250] Sharma R, Garg R, Kumari A. A review on biogenic synthesis, applications and toxicity aspects of zinc oxide nanoparticles. EXCLI J. 2020;19:1325.Suche in Google Scholar
[251] Verma R, Pathak S, Srivastava AK, Prawer S, Tomljenovic-Hanic S. ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications. J Alloys Compd. 2021;876:160175.10.1016/j.jallcom.2021.160175Suche in Google Scholar
[252] Cheng T-M, Chu H-Y, Huang H-M, Li Z-L, Chen C-Y, Shih Y-J, et al. Toxicologic Concerns with Current Medical Nanoparticles. Int J Mol Sci. 2022;23(14):7597.10.3390/ijms23147597Suche in Google Scholar PubMed PubMed Central
[253] Moatamed ER, Hussein AA, El-Desoky MM, Khayat ZE. Comparative study of zinc oxide nanoparticles and its bulk form on liver function of Wistar rat. Toxicol Ind Health. 2019;35(10):627–37.10.1177/0748233719878970Suche in Google Scholar PubMed
[254] Salami M, Khosravi M, Zarei MH. Comparative toxic effect of bulk zinc oxide (ZnO) and ZnO nanoparticles on human red blood cells. Main Group Met Chem. 2022;45(1):219–24.10.1515/mgmc-2022-0024Suche in Google Scholar
[255] Roy R, Tripathi A, Das M, Dwivedi PD. Cytotoxicity and uptake of zinc oxide nanoparticles leading to enhanced inflammatory cytokines levels in murine macrophages: comparison with bulk zinc oxide. J Biomed Nanotech. 2011;7(1):110–1.10.1166/jbn.2011.1226Suche in Google Scholar PubMed
[256] Saranya S, Vijayaranai K, Pavithra S, Raihana N, Kumanan K. In vitro cytotoxicity of zinc oxide, iron oxide and copper nanopowders prepared by green synthesis. Toxicol Rep. 2017;4:427–30.10.1016/j.toxrep.2017.07.005Suche in Google Scholar PubMed PubMed Central
[257] Jeyabharathi S, Kalishwaralal K, Sundar K, Muthukumaran A. Synthesis of zinc oxide nanoparticles (ZnONPs) by aqueous extract of Amaranthus caudatus and evaluation of their toxicity and antimicrobial activity. Mater Lett. 2017;209:295–8.10.1016/j.matlet.2017.08.030Suche in Google Scholar
[258] Najim N, Rusdi R, Hamzah AS, Shaameri Z, Zain MM, Kamarulzaman N. Effects of the absorption behaviour of ZnO nanoparticles on cytotoxicity measurements. J Nanomater. 2014;2014:19.10.1155/2014/694737Suche in Google Scholar
[259] Khan M, Naqvi AH, Ahmad M. Comparative study of the cytotoxic and genotoxic potentials of zinc oxide and titanium dioxide nanoparticles. Toxicol Rep. 2015;2:765–74.10.1016/j.toxrep.2015.02.004Suche in Google Scholar PubMed PubMed Central
[260] Dubey A, Goswami M, Yadav K, Chaudhary D. Oxidative stress and nano-toxicity induced by TiO2 and ZnO on WAG cell line. PLoS One. 2015;10(5):e0127493.10.1371/journal.pone.0127493Suche in Google Scholar PubMed PubMed Central
[261] Uzar NK, Abudayyak M, Akcay N, Algun G, Özhan G. Zinc oxide nanoparticles induced cyto-and genotoxicity in kidney epithelial cells. Toxicol Mech Methods. 2015;25(4):334–9.10.3109/15376516.2015.1045654Suche in Google Scholar PubMed
[262] Yousef MI, Mutar TF, Kamel MAE-N. Hepato-renal toxicity of oral sub-chronic exposure to aluminum oxide and/or zinc oxide nanoparticles in rats. Toxicol Rep. 2019;6:336–46.10.1016/j.toxrep.2019.04.003Suche in Google Scholar PubMed PubMed Central
[263] Nazir S, Rabbani A, Mehmood K, Maqbool F, Shah GM, Khan MF, et al. Antileishmanial activity and cytotoxicity of ZnO-based nano-formulations. Int J Nanomed. 2019;14:7809–22.10.2147/IJN.S203351Suche in Google Scholar PubMed PubMed Central
[264] Guan Z, Ying S, Ofoegbu PC, Clubb P, Rico C, He F, et al. Green synthesis of nanoparticles: Current developments and limitations. Environ Technol Innov. 2022;26:102336.10.1016/j.eti.2022.102336Suche in Google Scholar
[265] Sana SS, Dogiparthi LK. Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater Lett. 2018;226:47–51.10.1016/j.matlet.2018.05.009Suche in Google Scholar
[266] Scherzad A, Meyer T, Kleinsasser N, Hackenberg S. Molecular mechanisms of zinc oxide nanoparticle-induced genotoxicity. Materials. 2017;10(12):1427.10.3390/ma10121427Suche in Google Scholar PubMed PubMed Central
[267] Martínez R, Poupard MFN, Álvarez A, Soprano E, Migliavacca M, Carrillo-Carrión C, et al. Nanoparticle behavior and stability in biological environments. Nanoparticles for Biomedical Applications. Amsterdam, The Netherlands: Elsevier; 2020. p. 5–18.10.1016/B978-0-12-816662-8.00002-3Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Artikel in diesem Heft
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

