Abstract
In the process of CO2 reduction with dielectric barrier discharge (DBD)-coupled catalysis, the existing material presents unsatisfactory synergy, such as high cost, complicated preparation processes, and low conversion rates. An inexpensive and environmentally friendly mesoporous SiO2 with different morphologies by gel–sol method was synthesized and then introduced for synergistic conversion of CO2 with DBD. The physicochemical properties of the synthesized mesoporous SiO2 materials were analyzed using X-ray diffraction, thermogravimetric analysis, scanning electron microscopy and Brunauer-Emmett-Teller method, indicated the prepared mesoporous materials manifested large specific surface areas, ordered pore channels and pore size, and good stability. The CO2 reduction performance, CO selectivity, and energy efficiency of DBD alone and DBD-coupled mesoporous SiO2 were investigated at different input powers. The SiO2 prepared with 1.05 g cetyltrimethylammonium bromide addition had the highest activity, in which the conversion of CO2, CO yield and energy efficiency were increased by 56.73, 68.41, and 122.31%, respectively, compared with DBD alone. The primary CO2 conversion mechanism of the mesoporous SiO2-coupled DBD was analyzed. It is shown that the suitable pore capacity structure, the large specific surface area, and the presence of filament discharge within the pore size of suitable mesoporous material can promote the decomposition of CO2 on its surface.
1 Introduction
With the rapid advancement of human civilization, global energy resources are becoming increasingly scarce, leading to an increased reliance on fossil fuels and a consequent rise in fossil combustion year after year. According to the World Energy Outlook 2022, global energy demand is projected to increase by about 0.8% annually until 2030, and burning fossil fuels caused by energy consumption has resulted in significant CO2 emissions. The US National Oceanic and Atmospheric Administration has reported that atmospheric CO2 levels have risen from 280 ppm in the industrial era to 405 ppm in 2017 and continue to rise [1]. A significant catastrophe has resulted from excessive CO2 emissions, which have upset nature’s natural carbon balance and caused ecological harm [2], sea level rise [3], global warming [4], and other problems. Therefore, reducing CO2 emissions has become an essential strategic concern for all nations across the globe. Today’s CO2 problem is usually solved by carbon capture and storage (CCS) and carbon capture and utilization (CCU), which can be captured from point sources in industrial processes such as power plants. In CO2 storage, environmentally friendly chemical additives have recently been found to accelerate the process of repeated recycling, such as hydrate-based CO2 sequestration [5]. Unlike CCS, CCU uses captured CO2 to convert it into valuable chemicals [6], such as syngas [7], methane [8], and methanol [9]. Carbon monoxide (CO) is an important chemical raw material that has been used in many reactions, such as feedstock for Fischer–Tropsch synthesis and hydrocarbons, as well as a primary feedstock for fuel synthesis and other chemicals. Utilizing CO2 as a carbon source and selectively converting it into CO is a promising way to reduce CO2 and generate new clean energy sources.
The stability of CO2 as the final form of carbon and oxygen compounds makes it difficult to activate. Thermodynamic analysis shows that CO2 is difficult to decompose into O2 and CO at reaction temperatures below 2,000°C, making conventional methods such as thermocatalytic decomposition of CO2 difficult and uneconomical, limiting their large-scale industrial applications [10]. To overcome the disadvantages of these traditional methods, various new technologies have been developed, such as photocatalysis [11], electrocatalysis [12], photoelectric reduction, and plasma technology [13]. Photocatalysis and electrocatalysis have a low selectivity of the products, unstable reactions, and high electrical potential required, and they usually have low energy efficiency. In contrast, plasma technology can overcome thermodynamic barriers at room temperature and pressure and is characterized by mild operating conditions, compactness, fast reaction rates and reaction times, relatively low cost, and environmental friendliness [14]. Dielectric barrier discharge (DBD) is a standard gas discharge method with a more consistent and stable discharge performance, and the discharge area can produce a large number of high-energy electrons (1–10 eV) that can effectively excite molecules to chemical reactions [15]. Coaxial cylindrical DBD reactors are often used as reaction devices for the treatment of gases due to the advantages of uniform gas distribution, good confinement, and easy adjustment of the reaction process [16], and they can produce a uniform and stable low-temperature plasma [17]. As a result, it is a promising technology for large-scale CO2 conversion at atmospheric pressure. Materials are required to create a plasma-catalytic synergy to boost the conversion and selectivity of CO2 due to the low conversion and energy efficiency of a single DBD.
The material filled in the DBD is a critical factor in the conversion of CO2, and the addition of a material is an effective way to regulate and optimize the plasma discharge process to improve the conversion and energy efficiency of CO2. The material in the discharge zone alters the plasma discharge pattern, increasing the local electric field at the contact point and the average electric field within the reactor, among other relevant chemical properties. Michielsen et al. [18] found that adding BaTiO3 to a DBD-filled bed reactor promoted the conversion of CO2. Mei and Tu [19] found that adding Ni/γ-Al2O3 to a BaTiO3-filled bed reactor increased CO2 conversion. Li et al. [20] found that adding a mixed ZrO2–CeO2 filler to a coaxial reactor promoted CO2 conversion due to oxygen vacancies. Wang et al. [21] investigated the effect of various media materials on CO2 discharge performance. The highest conversion rate of 23.6% was found for reactors with filled MgO.
Nowadays, the reported materials are expensive, difficult to prepare and have low conversion rates, necessitating the development of more cost-effective, eco-friendly, and highly efficient materials to expand the use of CCU. SiO2 is an attractive non-toxic, odorless, non-polluting non-metallic material, often used as a new catalyst carrier, selective adsorbent, etc. [22]. It is also economically efficient and easy to prepare the material. Cho et al. [22] investigated SiO2-based adsorbents with high CO2 capture capacity and regeneration performance. Shawabkeh et al. [23] found that the adsorption of CO2 by SiO2 at different Cu loadings. Its morphological regularity and pore size can be tuned, where both parameters, material size, and morphology, greatly influence the performance effect of the material. The effect of the addition of filler material depends to a large extent on the type of material used and the corresponding size and gap. Uytdenhouwen et al. [15] reported that in SiO2, ZrO2, and Al2O3 microspheres as well as glass wool reactors, SiO2 and glass wool were the best in terms of conversion. The available data suggest that porous material can generate plasma within the pores, increase gas residence time through their adsorption properties, and react synergistically in catalytic plasma reactions. The mesoporous SiO2 has microporous discharge, surface defects, and property modifications such as CO2 adsorption that favor the plasma system. Therefore, mesoporous SiO2 also has potential applications in plasma-catalyzed CO2 conversion. Mesoporous SiO2 has been studied in many different fields, yet people have yet to investigate the mechanism of action of a single SiO2 in CO2 conversion in plasma.
In this study, large specific surface area mesoporous SiO2 material with ordered pore channels and pore sizes were prepared by a simple and economical method using cetyltrimethylammonium bromide (CTAB) as a template and ethyl orthosilicate (TEOS) as a silicon source. Different CTAB additions were adjusted to change the morphology, pore size, and other structural characteristics of the material and then characterized by X-ray diffraction (XRD), TGA, scanning electron microscope (SEM), and Brunauer-Emmett-Teller method. The performance of DBD alone and DBD-coupled mesoporous SiO2 for CO2 reduction, CO selectivity, and energy efficiency were investigated at different input powers, and the related mechanisms involved in the reaction were also analyzed.
2 Experimental
2.1 Material preparation
Weigh different masses (0.65, 0.85, 1.05, and 1.25 g) of CTAB (purchased from Sinopharm Chemical Reagent Co., Ltd., China) and mix with 200 mL of deionized water, stirring at 50°C in a magnetic mixer until the solution is clarified. After clarification, 15 mL of ammonia solution (provided by Shanghai Ling Feng Chemical Reagent Co., Ltd.) is added, stirring for 30 min at room temperature, 5 mL of n-ethyl silicate (TEOS, purchased from Macklin) dropwise is then slowly (1 mL/min) added, and then the reaction is allowed to stand for 3 h at 85°C. The sample is washed by centrifuging (at 6000 rpm for 5 min) and three times with deionized water and ethanol, dry at 80°C and calcine at 600°C (heating rate 5℃/min) for 8 h to obtain a white powder, which is ground and pressed, and the sample is taken between 40 and 60 mesh. The samples are labeled as Si-a, Si-b, Si-c, and Si-d according to the content of CTAB.
2.2 Characterization
The prepared materials were characterized by XRD performed on an X pert Powder with Cu Kα radiation (30 mA, 40 kV) in a 2θ range from 10° to 80° with a scanning speed of 10° per minute and the step size is 0.02°. The surface morphologies of the material were analyzed using an electron microscope (SEM, Sigma 300, Germany), and energy-dispersive X-ray spectroscopy (EDS) tests (Xplore, Oxford) were performed to determine the elemental distribution of the products. Specific surface area analysis of the samples was obtained from N2 physisorption isotherms at −196℃, using Quantachrome Instruments, and the specific surface area and the average pore size of the adsorbent were determined; the pressure range is Relative pressure P/P 0 = 0–1. Thermal analysis was conducted with a NETZSCH STA 2,500 thermal analyzer at N2 flow from 20 to 900℃ with 10℃/min. Fourier-transform infrared spectroscopy (FTIR) analysis was done in the range of 500–4,000 cm−1, with a resolution of 4 cm−1 using TENSOR 27, the scanning mode and number of scans are KBr compression test, 32 times. Measurements of X-ray photoelectron spectroscopy (XPS) were recorded on Thermo Scientific K-Alpha using Al Kα (hv = 1486.6 eV) radiation, with charge correction by C 1s (284.8 eV) done for the obtained spectra.
2.3 Experimental methods
2.3.1 Reaction procedure
The experiments are carried out at atmospheric pressure. The reaction gas during the experiments is high-purity CO2, supplied from a cylinder, which is fed into the plasma reactor at a controlled gas flow rate of 30 mL/min by means of a mass flow meter. The material is filled to a volume of 4 mL. The products of the reaction are determined analytically using a meteorological chromatograph (GC-9860-5C-NJ) equipped with a TCD detector and a double FID detector.
The plasma reactor used in the experiments is a cooled coaxial DBD reactor with the structure shown in Figure 1(a). The material used is stainless steel 316 as the inner electrode (high voltage electrode) of the reactor. The outer electrode is a quartz tube (120 mm long) with an outer diameter of 25 mm and an inner diameter of 20 mm, with a one-sided discharge gap of 3 mm. To investigate the effect of different materials on CO2 conversion, the material is filled uniformly and flatly in the discharge gap, and the lower end is fixed with quartz wool. The unit is equipped with a circulating water cooling device to control the temperature of the reaction system. The principle of the cooling device is shown in Figure 1(b).

(a) The detailed structure of the coaxial DBD reactor; (b) the schematic diagram of the cooling device.
2.3.2 Calculation of discharge parameters
The oscilloscope (TBS1102C) can detect and display the current and voltage waveforms during the experiment. The Lissajous graph method [24] can be used to calculate discharge power and other discharge characteristics. The discharge power (P) is calculated in the following manner:
where
The low voltage signal at both ends of the capacitor and the voltage measured by the high voltage probe at the high voltage end of the reactor is input into the CH1 and CH2 channels of the oscilloscope, and a closed curve, namely, the Lissajous graph, is obtained as shown in Figure 2. The discharge charge Q d, peak-to-peak charge Q pk-pk, and transferred charge Q trans in one cycle can be obtained from the Lissajous graph.

Lissajous figure.
2.3.3 Calculation of reaction parameters
Gas chromatography was used to determine the content of various gases before and after the reaction, as well as to analyze the CO2 conversion (
Of which, the reaction enthalpy
3 Results and discussion
3.1 Material characterization
3.1.1 XRD
The XRD results (Figure 3a) show that all four samples obtained by adding different quantities of CTAB show a broad diffraction peak at 2θ = 20°–30°, which belongs to the quartz crystalline phase [25], and the high width and low intensity of this peak confirm the amorphous structure of the formed phase. The addition of CTAB caused a change in the pore size and structure of the samples, resulting in a slight shift in the characteristic diffraction peaks in the range of 0.5°–1°, which may be related to the varying pore size and morphology of the samples. In addition, no other peaks were observed in this range, which is proof of the high purity of the synthesized samples.

(a) XRD patterns, (b) TGA spectra.
3.1.2 TGA
Figure 3b shows the results of the thermogravimetric analysis of material Si-a, Si-b, Si-c, and Si-d. The weight loss below 200℃ is attributed to the removal of physically adsorbed water and interlayer water, and the weight loss of the four materials is different, indicating that SiO2 particles of different morphologies adsorb different amounts of water molecules. After 200℃, with the increase in temperature, the four SiO2 materials of different morphologies all experience gradual weight loss, mainly due to dehydroxylation, and the weight loss is not significant [26].
3.1.3 SEM
Based on the principle of synergistic self-assembly, it is known that CTAB plays a controlling role in the synthesis of silica samples. During the reaction, the silicate anion and CTAB cation interact with each other, which can cause a change in the charge density of SiO3 2− [26]. When the additional amount of CTAB was 0.65 g (as in Figure 4a), the silica nanoparticles basically showed irregular patterns; when the additional amount of CTAB was 0.85 g (as in Figure 4b), the silica nanoparticles gradually formed rods with a size of 20–30 nm; when the addition amount of CTAB was 1.05 and 1.25 g, the silicon dioxide nanoparticles appear in the shape of rods with small uneven spheres on top. This is because the excessive addition of CTAB causes part of the CTAB to be wrapped in the skeleton of SiO2 without reaction, and part of it cannot be removed by high-temperature calcination. The energy spectrum analysis graph (Figure 4e and d) shows that the sample was synthesized as homogeneous SiO2 particles of high purity. Figure S1 shows the histograms of the particle size distribution for all samples (Si-a, Si-b, Si-c, and Si-d), and Table S1 shows the particle sizes of the synthesized materials.

(a–d) SEM Images of SiO2 with different morphologies, (e and f) EDS mapping of SiO2-c.
3.1.4 BET
Figure 5 shows the physical adsorption/desorption isotherms and pore size distribution of N2 for the four material Si-a, Si-b, Si-c, and Si-d. The specific data of the specific surface area, pore size, and pore capacity are shown in Table 1, in which it can be seen that the specific surface area of SiO2 increases with the addition of CTAB. The addition of different amounts of CTAB resulted in the formation of pores of varying degrees of order in the SiO2 particles, with a uniform distribution of pore size between 2 and 3 nm. Figure 5a shows the N2 adsorption–desorption profiles for SiO2 added with different amounts of CTAB, which is a typical type IV adsorption–desorption profile, indicating the presence of an excellent mesoporous structure of the material. Due to the relatively small cavity pore size and pore capacity on the particle surface, the isotherm has almost no hysteresis loop [27]. Moreover, due to gaps between the SiO2 particles, the isotherm rises rapidly under high-pressure conditions. Figure 5b shows the pore size distributions of the four types of SiO2 added with different amounts of CTAB, confirming the uniformity of the pore distribution. At the same time, material Si-c (Table 1) has the largest pore capacity.

(a) BET isotherms, (b) porosity distribution.
Specific surface area, pore size, and pore capacity
| Sample | S BET (m2/g) | Pore volume (cc/g) | Pore diameter (nm) |
|---|---|---|---|
| Si-a | 741.0 | 0.86 | 2.47 |
| Si-b | 798.7 | 0.86 | 2.733 |
| Si-c | 859.7 | 1.10 | 2.73 |
| Si-d | 878.6 | 0.87 | 2.46 |
3.2 Catalytic performance analysis
The CO2 conversion performance, CO yield, and energy efficiency of four mesoporous SiO2 with different CTAB additions in the DBD plasma system were investigated, as shown in Figure 6. All other reaction conditions being the same, the CO2 conversion of the mesoporous SiO2 material filled with different CTAB additions showed an increase compared to unfilled. The CO2 conversion and CO yield gradually increased as the input power increased from 20 to 60 W. This is likely due to the fact that as the input power increases, the intensity of the discharge region increases, the density of activated particles and energetic electrons increase, and the energetic electrons in the discharge region bombard CO2 more readily, generating excess CO2 + and O2 −, thereby increasing the CO2 conversion rate [18]. However, increasing the input power increases the energy in the reaction region, but the magnitude of the energy input is greater than the increase in CO2 conversion, resulting in a decrease in energy efficiency as the input power increases.

(a) CO2 conversion rate, (b) yield of CO, and (c) energy efficiency with different packing materials.
Figure 6a shows the CO2 conversions of samples Si-a, Si-b, Si-c, and Si-d in the DBD plasma reactor and without filling. In this case, the best CO2 conversion without filling was 9.57% at an input power of 60 W. Under the same conditions, samples Si-a, Si-b, Si-c, and Si-d improved by 35.37, 12.88, 56.73, and 36.81%, respectively.
Figure 6b shows the CO yields of the four samples Si-a, Si-b, Si-c, and Si-d in the plasma reactor, with the best CO yield of 7.8% for unfilled and an increase of 65.38, 42.3, 68.41 and 52% for filled samples Si-a, Si-b, Si-c, and Si-d, respectively. The previous results demonstrate the expected CO2 conversion reaction activity of the mesoporous SiO2 synergistic DBD reactor in which the mesoporous SiO2 is all activated by the plasma.
It was also found that the material filling contributed to the energy use efficiency of the reaction process (Figure 6c). At an input power of 20 W, the material was all filled with higher energy efficiency than unfilled, with filled samples Si-a, Si-b, Si-c, and Si-d improving the energy efficiency by 70.81, 72.98, 122.31, and 88.84% compared to unfilled (2.33%). This leads to the conclusion that sample c shows the best conversion of CO2 in collaboration with DBD. The material Si-c was put through a performance stability test, as shown in Figure S2, and was found to have good performance stability, with no tendency for the CO2 conversion to decrease under long periods of discharge (4 h). The low initial CO2 conversion is due to the fact that the plasma power supply has just been switched on, and there has not been sufficient time for the CO2 to react with the plasma. Table 2 summarizes the recent literature on energy efficiency with DBD-assisted CO2 decomposition. It can be seen that the material Si-c has a high CO selectivity and energy efficiency (mmol/KJ). Although the performance in terms of CO2 conversion is not as good, this is only the performance of a single silicon-based material, and it is believed that the performance will be even better with the subsequent addition of active components. The next step will be to focus on the reaction mechanism of CO2 conversion brought about by sample Si-c in terms of FTIR and XPS.
Comparison of DBD plasma-assisted conversion of CO2
| Plasma | Flowrate (mL/min) | Power (W) | Diluent gas | Packed material |
|
|
|
Energy efficiency (mmol/kJ) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| DBD | 50 | 50 | — | BaTiO3 | 28 | 96 | — | 0.254 | [28] |
| DBD | 39 | 30 | — | SiO2@TiO2-0.25 M with LUDO | 15 | — | — | — | [29] |
| DBD | 55 | 150 | — | Molecular sieves 5 A | 25 | 63 | — | — | [30] |
| DBD | 30 | 2.4 | Ar | Glass beads | 19.5 | 86 | 16.8 | 0.6 | [31] |
| DBD | 30 | 2.4 | — | 15% CuO/CeAl | 13.5 | 59 | 7.8 | 1.373 | [32] |
| DBD | 30 | 2.4 | — | 5% ZnO + g-C3N4 | 12 | 70 | — | 1.1 | [33] |
| DBD | 30 | 60 | — | Si-c | 15 | 87 | 13.13 | 0.697 | This work |
3.3 FTIR analysis of material
Figure 7 shows the FTIR spectra of sample Si-c before and after the reaction. The IR spectrogram of mesoporous SiO2 before the reaction shows that the peak at 467 cm−1 appears due to the bending vibration of the Si–O–Si bond [34]; the symmetric stretching vibration peak of the Si–O–Si bond appears at 802 cm−1. The complete vibration of the Si–OH bond causes an absorption peak at 962 cm−1, which is relatively weak. The antisymmetric stretching vibration peak of Si−O−Si appears around 1,090 cm−1 [35,36]. The peak at 3450 cm−1 in the spectrum is the antisymmetric stretching vibration peak of the –OH group with a broad absorption band. The absorption peaks at 2,860 and 2,930 cm−1 are due to the presence of the –CH2− bond in CTAB [25], which also supports the elaboration of sample c in the SEM, where there is too much CTAB to allow complete removal of CTAB. Comparing the FTIR plots of sample Si-c before and after the reaction, it can be seen that the peak intensity of the sample after the plasma experiment has become more prominent, likely due to the fact that high-energy electrons in the plasma bombard the sample, thus exposing more of the active components in the sample.

FTIR Spectrum of Si-c before and after the reaction.
3.4 XPS analysis
XPS was used to further understand the changes in mesoporous SiO2 before and after the experimental reaction and to provide a mechanism for plasma enhancement. Figure 8 shows the XPS spectral profile of sample Si-c before and after the reaction, the XPS fine spectrum of Si 2p, and the fine spectrum of O 1s. XPS analysis (Figure 8a) shows that material Si-c contains C 1s, O 1s, Si 2p, and Si 2s peaks. The characteristic peak of Si 2p was observed at 103.38 eV, which also indicates that sample Si-c is in the form of SiO2 as shown in Figure 8b, Si 2p1/2 and Si2p3/2 in the Si 2s spectrum correspond to the Si–C and Si–O bonds, respectively [37]. A broad peak of O 1s can be observed at 532.88 eV (Figure 8c). We can see that the Si 2p spectrum and O 1s spectrum of sample Si-c after the reaction, where the higher binding energy (BE) of the nanoparticles before the reaction shifts to a lower BE, undergo a slight shift (not significant), which could be due to the fact that the plasma discharge contains many reactive species that lead to the formation of free radicals, and the complexation of free radicals reduces the surface charging effect of the sample, such that the reduction leads to a shift to a lower BE [38].
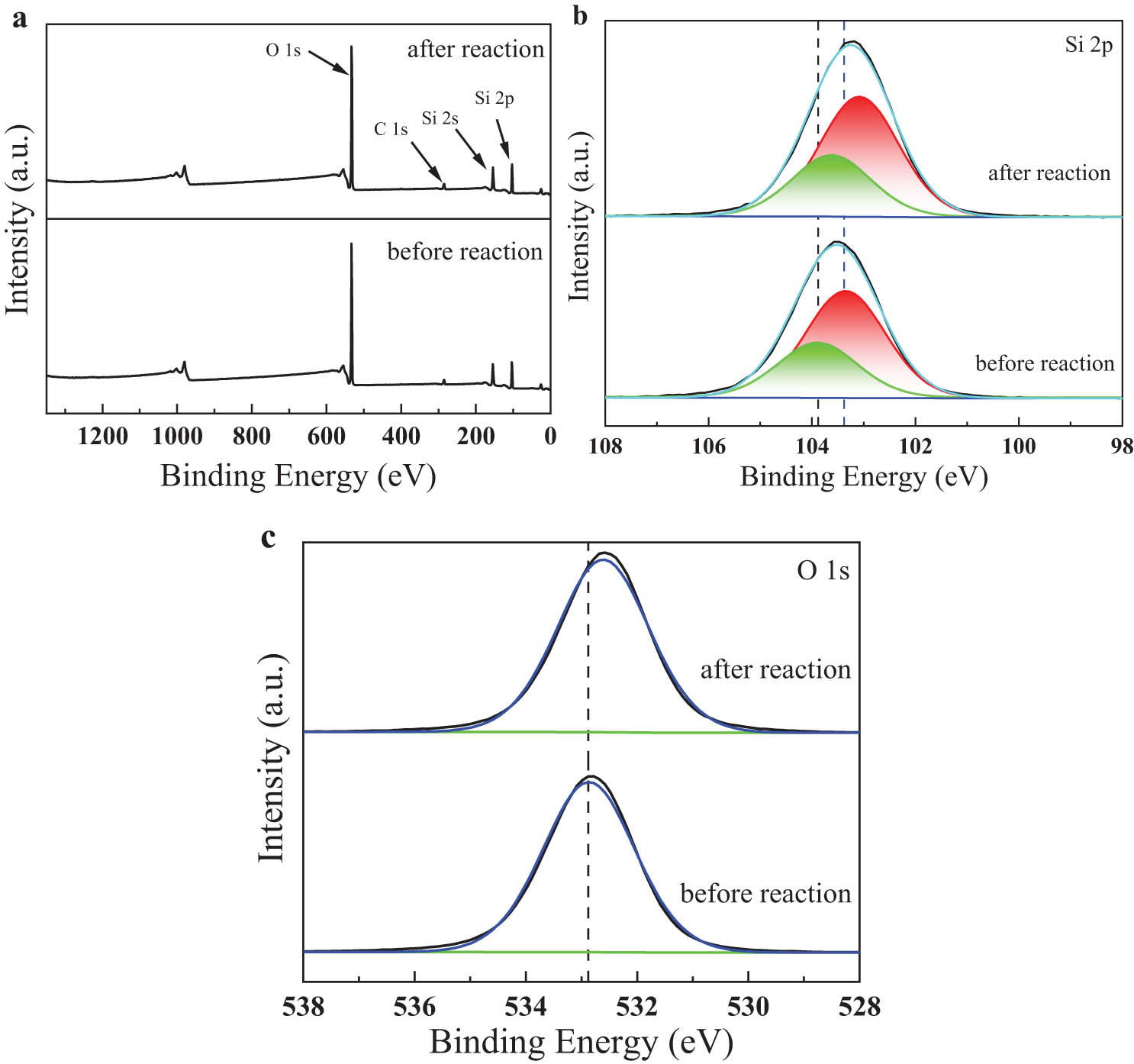
XPS Spectrum of Si-c before and after the reaction: (a) XPS survey spectra; (b) Si 2p; (c) O 1s.
3.5 Discharge characteristics
In the material-filled DBD plasma reactor, the discharge frequency, input power, and CO2 flow rate were 9.0 kHz, 60 W, and 30 mL/min. Compared to the unfilled DBD plasma, the material-filled DBD had a more vigorous discharge intensity and produced more active components, such as high-energy electrons, to carry out the reaction. As can be seen in Figure 9, due to the filling of the material, the Lissajous diagram of DBD is a parallelogram with an increased area than when it is not filled, which indicates a change in the discharge state. The increase in discharge charge (Q d = 0.5181) for the filled material compared to the unfilled discharge charge (Q d = 0.4903) indicates that the DBD of the filled material facilitates the generation of more discharge charge, thus increasing the reactivity of the discharge region. The DBD filled with material also increases the peak-to-peak charge during the discharge process from Q pk-pk = 0.6429–0.7076, which shows that the introduction of dielectric materials can effectively promote charge transfer in the discharge region and facilitate the physical and chemical reaction changes in the DBD [39].

Lissajous graphs of discharge system with or without material packing (material selection: Si-c, circulating water temperature = 20℃, input power = 60 W).
3.6 Mechanism analysis
In the case of no material filling, DBD only showed filamentary discharge distributed in the discharge area and whistling noise, while with material filling, DBD showed surface discharge and faint filamentary discharge. As shown in Table 1, the specific surface area increased with the addition of CTAB; the average pore size of all samples was in the mesopore range of 2–3 nm, while the pore volume of sample Si-c was larger than that of the other samples, reaching 1.095 cc/g. The larger specific surface area and more mesopore distribution may provide more surface active centers, which is favorable for CO2 adsorption on the material surface, thus further accelerating the conversion of CO2. The adsorption capacity of SiO2 for CO2 is weak compared with most materials. Therefore, we investigate some new studies on the properties of adsorption factor and specific surface area. In terms of adsorption factors, the sample Si-c-doped Al [40] was selected to study the effect of its enhanced CO2 adsorption capacity on the CO2 reduction performance of DBD, as can be found in Figure S3, the addition of Al reduces the performance activity of CO2 reduction; this is probably because the addition of Al disrupts the characteristic pore structure of the material Si-c. On the other hand, it also proves that the CO2 adsorption effect brought by Al is not sufficient for the change of properties brought by the pore structure of the material. In terms of specific surface area, the reported conventional silicas [34] was chosen. The specific surface area of this material is 345.93 m2/g, and the pore volume is 1.128 cm3/g, which is similar to the Si-c material in this article. After performance testing, Figure S4 reveals that the performance of conventional SiO2 is much inferior to that of Si-c, indicating that the specific surface area has a significant effect on the performance effect of DBD synergistic materials. The depth of plasma penetration into the pore is influenced by a variety of factors, such as the size of the pore volume, the shape of the pore, and the density of the plasma. Suitable pores have a relatively large specific surface area, and the deposition of electrons on the dielectric surface creates a strong regional electric field at the bottom of the pore, resulting in ionization occurring mainly near or inside the pore [41,42]. The mechanism present in the DBD synergistic mesoporous SiO2 reduction of CO2 is as follows: the conversion of CO2 in DBD plasma (Figure 10) is mainly excited by high-energy electrons, which are the main active particles in the decomposition of CO2 in DBD plasmas, where CO2 is decomposed into CO and O2 by electron collisional dissociation reactions. After the DBD is filled with a dielectric material, the discharge mode changes from filament discharge to a combination of surface discharge and filament discharge, the intensity of the discharge area is enhanced, and the discharge is uniform and stable, which is conducive to the conversion of CO2. In addition, the appropriate pore volume and specific surface area may have formed an intra-pore discharge, which further changes the regional electric field increasing the effective ionization volume and changing the ionization rate, which enhances the conversion reaction of CO2.

Schematic diagram of mesoporous SiO2 with DBD to decompose CO2.
4 Conclusion
The synthesis of mesoporous SiO2 with different morphologies by gel–solution method and construction of a material-assisted fixed-bed DBD system to study the mesoporous SiO2 in DBD for the conversion of CO2 was carried out. Characterization by XRD, FTIR, XPS, and SEM revealed that, as predicted, SiO2 with mesoporous characteristics contributed to the conversion of CO2. Among the SiO2 prepared with different additions of CTAB, the addition of 1.05 g of SiO2 had the highest conversion performance, and compared to the unfilled system, the conversion of CO2, energy efficiency, and CO yield were increased by 56.73, 68.41, and 122.31%, respectively. The filling of mesoporous SiO2 changed the discharge form and intensity of the discharge region, and the richer surface structure promoted the decomposition of CO2 on its surface and the presence of filament discharge with suitable mesoporous material pore size, all three of which contributed to the performance of CO2 conversion in DBD. This study provides an experimental basis for further understanding the mechanism of material synergy for CO2 conversion in DBD. Although there is currently no capability to investigate the phenomenon of plasma discharge in pores in mesoporous materials, the synergistic reduction of CO2 by mesoporous materials and DBD proves that it is feasible to probe the direction of mesoporous materials. This preliminary study provides a basis for selecting an additional material carrier for plasma catalytic technology in CO2 conversion applications and, furthermore, for realizing the industrial application of plasma material on a large scale with cheap and active carriers and accelerating the use of CCU.
Acknowledgments
The authors acknowledge the support from the Natural Science Foundation of Jiangsu Province (BK20210983, BK20221405), the Changzhou Science and Technology Bureau (CQ20210103, CM20223017), the Zhongwu Scientific Research Air Pollution Detection Team (11830512201), the Talent Introduction Program of Jiangsu University of Technology (KYY19022, KYY19040), and Shuangchuang Ph.D award (from World Prestigious Universities).
-
Funding information: This study was supported by the Natural Science Foundation of Jiangsu Province (BK20210983, BK20221405), the Changzhou Science and Technology Bureau (CQ20210103, CM20223017), the Zhongwu Scientific Research Air Pollution Detection Team (11830512201), the Talent Introduction Program of Jiangsu University of Technology (KYY19022, KYY19040), and Shuangchuang Ph.D award (from World Prestigious Universities).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Supporting information: Catalyst preparation, Fig S1-S4.
-
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article.
References
[1] Del Toro FJ, Choi KS, Rakhshandehroo F, Aguilar E, Tenllado F, Canto T. Ambient conditions of elevated temperature and CO2 levels are detrimental to the probabilities of transmission by insects of a Potato virus Y isolate and to its simulated prevalence in the environment. Virology. 2019;530:1–10.10.1016/j.virol.2019.02.001Search in Google Scholar PubMed
[2] Büntgen U, Krusic PJ, Piermattei A, Coomes DA, Esper J, Myglan VS, et al. Limited capacity of tree growth to mitigate the global greenhouse effect under predicted warming. Nat Commun. 2019;10(1):1–6.10.1038/s41467-019-10174-4Search in Google Scholar PubMed PubMed Central
[3] Cordier C, Guyomard K, Stavrakakis C, Sauvade P, Coelho F, Moulin P. Culture of microalgae with ultrafiltered seawater: a feasibility study. Sci Med J. 2020;2(2):56–62.10.28991/SciMedJ-2020-0202-2Search in Google Scholar
[4] D'Amato G, Akdis C. Global warming, climate change, air pollution and allergies. Authorea Preprints; 2020.10.22541/au.159526736.69654469Search in Google Scholar
[5] Liu X, Ren J, Chen D, Yin Z. Comparison of SDS and l-Methionine in promoting CO2 hydrate kinetics: Implication for hydrate-based CO2 storage. Chem Eng J. 2022;438:135504.10.1016/j.cej.2022.135504Search in Google Scholar
[6] Salehizadeh H, Yan N, Farnood R. Recent advances in microbial CO2 fixation and conversion to value-added products. Chem Eng J. 2020;390:124584.10.1016/j.cej.2020.124584Search in Google Scholar
[7] Xia Y, Lu N, Li J, Jiang N, Shang K, Wu Y. Combined steam and CO2 reforming of CH4 for syngas production in a gliding arc discharge plasma. J CO2 Util. 2020;37:248–59.10.1016/j.jcou.2019.12.016Search in Google Scholar
[8] Ahmad F, Lovell EC, Masood H, Cullen PJ, Ostrikov KK, Scott JA, et al. Low-temperature CO2 methanation: synergistic effects in plasma-Ni hybrid catalytic system. ACS Sustain Chem Eng. 2020;8(4):1888–98.10.1021/acssuschemeng.9b06180Search in Google Scholar
[9] Wang L, Yi Y, Guo H, Tu X. Atmospheric pressure and room temperature synthesis of methanol through plasma-catalytic hydrogenation of CO2. ACS Catal. 2018;8(1):90–100.10.1021/acscatal.7b02733Search in Google Scholar
[10] Snoeckx R, Bogaerts A. Plasma technology–a novel solution for CO2 conversion. Chem Soc Rev. 2017;46(19):5805–63.10.1039/C6CS00066ESearch in Google Scholar
[11] Xu L, Xiu Y, Liu F, Liang Y, Wang S. Research progress in conversion of CO2 to valuable Fuels. Molecules. 2020;25(16):3653.10.3390/molecules25163653Search in Google Scholar PubMed PubMed Central
[12] Zhang Y, Guo SX, Zhang X, Bond AM, Zhang J. Mechanistic understanding of the electrocatalytic CO2 reduction reaction – New developments based on advanced instrumental techniques. Nano Today. 2020;31:100835.10.1016/j.nantod.2019.100835Search in Google Scholar
[13] Mittal D, Ahlawat M, Govind Rao V. Recent progress and challenges in plasmon‐mediated reduction of CO2 to chemicals and fuels. Adv Mater Interfaces. 2022;9(12):2102383.10.1002/admi.202102383Search in Google Scholar
[14] George A, Shen B, Craven M, Wang Y, Kang D, Wu C, et al. A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renew Sustain Energy Rev. 2021;135:109702.10.1016/j.rser.2020.109702Search in Google Scholar
[15] Uytdenhouwen Y, Van Alphen S, Michielsen I, Meynen V, Cool P, Bogaerts A. A packed-bed DBD micro plasma reactor for CO2 dissociation: does size matter. Chem Eng J. 2018;348:557–68.10.1016/j.cej.2018.04.210Search in Google Scholar
[16] Mei D, Tu X. Conversion of CO2 in a cylindrical dielectric barrier discharge reactor: Effects of plasma processing parameters and reactor design. J CO2 Util. 2017;19:68–78.10.1016/j.jcou.2017.02.015Search in Google Scholar
[17] Lu N, Liu N, Zhang C, Su Y, Shang K, Jiang N, et al. CO2 conversion promoted by potassium intercalated g-C3N4 catalyst in DBD plasma system. Chem Eng J. 2021;417:129283.10.1016/j.cej.2021.129283Search in Google Scholar
[18] Michielsen I, Uytdenhouwen Y, Pype J, Michielsen B, Mertens J, Reniers F, et al. CO2 dissociation in a packed bed DBD reactor: First steps towards a better understanding of plasma catalysis. Chem Eng J. 2017;326:477–88.10.1016/j.cej.2017.05.177Search in Google Scholar
[19] Mei D, Tu X. Atmospheric pressure non-thermal plasma activation of CO2 in a packed-bed dielectric barrier discharge reactor. Chemphyschem. 2017;18(22):3253–9.10.1002/cphc.201700752Search in Google Scholar PubMed
[20] Li J, Zhu S, Lu K, Ma C, Yang D, Yu F. CO2 conversion in a coaxial dielectric barrier discharge plasma reactor in the presence of mixed ZrO2-CeO2. J Environ Chem Eng. 2021;9(1):104654.10.1016/j.jece.2020.104654Search in Google Scholar
[21] Wang B, Li X, Wang X, Zhang B. Effect of filling materials on CO2 conversion with a dielectric barrier discharge reactor. J Environ Chem Eng. 2021;9(6):106370.10.1016/j.jece.2021.106370Search in Google Scholar
[22] Cho MS, Lee SC, Chae HJ, Kwon YM, Kim HJ, Ryu MY, et al. Optimum design and characteristics of potassium-based sorbents using SiO2 for post-combustion CO2 capture. Renew Energy. 2019;144:107–15.10.1016/j.renene.2018.10.057Search in Google Scholar
[23] Shawabkeh RA, Faqir NM, Rawajfeh KM, Hussein IA, Hamza A. Adsorption of CO2 on Cu/SiO2 nano-catalyst: Experimental and theoretical study. Appl Surf Sci. 2022;586:152726.10.1016/j.apsusc.2022.152726Search in Google Scholar
[24] Lu N, Bao X, Jiang N, Shang K, Li J, Wu Y. Non-thermal plasma-assisted catalytic dry reforming of methane and carbon dioxide over g-C3N4 based catalyst. Top Catal. 2017;60(12–14):855–68.10.1007/s11244-017-0750-zSearch in Google Scholar
[25] Khoeini M, Najafi A, Rastegar H, Amani M. Improvement of hollow mesoporous silica nanoparticles synthesis by hard-templating method via CTAB surfactant. Ceram Int. 2019;45(10):12700–7.10.1016/j.ceramint.2019.03.125Search in Google Scholar
[26] Gundanna SK, Mitra A, Bhatta LK, Bhatta UM. Effect of thermal treatment on the surface/interfacial behaviour of Au@SiO2 nanoparticles in the presence of CTAB surfactant molecules. Powder Technol. 2021;38:503–8.10.1016/j.powtec.2020.12.010Search in Google Scholar
[27] Arooj A, Tahir K, Khan AU, Khan A, Jevtovic V, El-Zahhar AA, et al. One-step fabrication of surfactant mediated Pd/SiO2, A prospect toward therapeutic and photocatalytic applications. Inorg Chem Commun. 2022;142:109692.10.1016/j.inoche.2022.109692Search in Google Scholar
[28] Mei D, Zhu X, He YL, Yan JD, Tu X. Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: understanding the effect of packing materials. Plasma Sources Sci Technol. 2014;24(1):015011.10.1088/0963-0252/24/1/015011Search in Google Scholar
[29] Kaliyappan P, Paulus A, D’Haen J, Samyn P, Uytdenhouwen Y, Hafezkhiabani N, et al. Probing the impact of material properties of core-shell SiO2@TiO2 spheres on the plasma-catalytic CO2 dissociation using a packed bed DBD plasma reactor. J CO2 Util. 2021;46:101468.10.1016/j.jcou.2021.101468Search in Google Scholar
[30] Wang T, Liu H, Xiong X, Feng X. Conversion of carbon dioxide to carbon monoxide by pulse dielectric barrier discharge plasma. IOP Conf Ser Earth Environ Sci. 2017;52(1):012100.10.1088/1742-6596/52/1/012100Search in Google Scholar
[31] Ray D, Saha R, Ch S. DBD plasma assisted CO2 decomposition: influence of diluent gases. Catalysts. 2017;7(9):244.10.3390/catal7090244Search in Google Scholar
[32] Ray D, Chawdhury P, Bhargavi KV, Thatikonda S, Lingaiah N, Subrahmanyam C. Ni and Cu oxide supported γ-Al2O3 packed DBD plasma reactor for CO2 activation. J CO2 Util. 2021;44:101400.10.1016/j.jcou.2020.101400Search in Google Scholar
[33] Ray D, Chawdhury P, Subrahmanyam C. A facile method to decompose CO2 using a g-C3N4-assisted DBD plasma reactor. Environ Res. 2020;183:109286.10.1016/j.envres.2020.109286Search in Google Scholar PubMed
[34] Wu Y, Du X, Kou Y, Wang Y, Teng F. Mesoporous SiO2 nanostructure: light-induced adsorption enhancement and its application in photocatalytic degradation of organic dye. Ceram Int. 2019;45(18):24594–600.10.1016/j.ceramint.2019.08.188Search in Google Scholar
[35] Fang N, Ding Y, Liu C, Chen Z. Role of SiO2 in synthesis of SiO2-supported CeO2 composites. Ceram Int. 2018;44(11):12363–9.10.1016/j.ceramint.2018.04.024Search in Google Scholar
[36] Ahkam QM, Khan EU, Iqbal J, Murtaza A, Khan MT. Synthesis and characterization of cobalt-doped SiO2 nanoparticles. Phys B: Condens Matter. 2019;572:161–7.10.1016/j.physb.2019.07.044Search in Google Scholar
[37] Mutuma BK, Mathebula X, Nongwe I, Mtolo BP, Matsoso BJ, Erasmus R, et al. Unravelling the interfacial interaction in mesoporous SiO2@nickel phyllosilicate/TiO2 core-shell nanostructures for photocatalytic activity. Beilstein J Nanotechnol. 2020;11:1834–46.10.3762/bjnano.11.165Search in Google Scholar PubMed PubMed Central
[38] Musa FN, Bashir N, Ahmad MH, Buntat Z, Piah MA. Investigating the influence of plasma-treated SiO2 nanofillers on the electrical treeing performance of silicone-rubber. Appl Sci. 2016;6(11):348.10.3390/app6110348Search in Google Scholar
[39] Alliati M, Mei D, Tu X. Plasma activation of CO2 in a dielectric barrier discharge: A chemical kinetic model from the microdischarge to the reactor scales. J CO2 Util. 2018;27:308–19.10.1016/j.jcou.2018.07.018Search in Google Scholar
[40] Wilson SM, Tezel FH. CO2 and CO adsorption equilibrium on ZSM-5 for different SiO2/Al2O3 ratios. Sep Sci Technol. 2019;54(5):722–30.10.1080/01496395.2018.1518332Search in Google Scholar
[41] Zhang YR, Neyts EC, Bogaerts A. Enhancement of plasma generation in catalyst pores with different shapes. Plasma Sources Sci Technol. 2018;27(5):055008.10.1088/1361-6595/aac0e4Search in Google Scholar
[42] Zhang Y, Wang HY, Zhang YR, Bogaerts A. Formation of microdischarges inside a mesoporous catalyst in dielectric barrier discharge plasmas. Plasma Sources Sci Technol. 2017;26(5):054002.10.1088/1361-6595/aa66beSearch in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

