Abstract
The pollution of silver ion (Ag(i)) has become a serious environmental threat and hazard to ecosystem and human health. Thus, the nanoscale zero valent iron (nZVI)-doped polypyrrole-based carbon nanotube (nZVI/CNT) composites were synthesized by a facile method to remove Ag(i) from wastewater due to the toxicity and scarcity nature of Ag(i). In this process, Fe3+ initiated the self-assembly of polypyrrole tubes in the presence of methyl orange, while it also served as an iron source generated nZVI/CNTs by carbothermal reduction method. The nZVI/CNT composites exhibited a homogeneous tubular structure, and the nZVI formed were uniformly dispersed in the nZVI/CNT composites. The nZVI/CNT composites were used as an adsorbent for the removal of Ag(i) and showed a higher adsorption capacity compared to nZVI and CNTs, with a maximum adsorption capacity of 522.41 mg g−1. Ag(i) was adsorbed on nZVI/CNT composites by ion exchange and chelation, where Ag(i) was reduced to non-toxic Ag due to the redox reaction among pyrrolic-N, nZVI, and Ag(i). The adsorption process of Ag(i) on nZVI/CNT composites was dominated by monolayer adsorption. According to our results, nZVI/CNT composites can be used as economical treatment for wastewater containing Ag(i).
1 Introduction
As one of the valuable metals, silver shows extensive applications in the fields of catalysis, electronics, photography, and antibiotics because of its brilliant photosensitivity, electrical and thermal conductivity, ductility, and antimicrobial properties [1]. The extensive use of silver has led to an increase in its release into the environment, particularly in water sources. Silver ions (Ag(i)) are second only to mercury among metal ions in terms of toxicity to aquatic organisms and cause serious pollution to the environment [2]. Through the food chain, Ag(i) can tend to bioaccumulate in the human body, causing several health problems [3] for humans. On the other hand, silver is a scarce and non-renewable resource [4]. Therefore, the removal and recovery of silver from water is essential from an economic and environmental protection point of view.
In recent decades, many approaches have been employed to reduce/remove Ag(i) from aqueous environment, including ion exchange, chemical precipitation, membrane filtration, adsorption method, etc. [5,6]. In these approaches, adsorption method has attracted great attention due to its simple operating procedures, low cost, and friendly environment [7].
Nanoscale zero valent iron (nZVI) particles are ideal candidates to remove toxic Ag(i). Despite micron- and millimeter-sized ZVIs have been proven very useful for removing contaminants [8], the smaller size of nZVI has gained more attention because it shows higher reaction rates and better removal rate of heavy metal ions [9]. nZVI has been shown to enjoy considerably effective abilities in the treatment of Ag(i) because of its high specific surface area and noteworthy characteristics for the reduction of contaminants. Despite the efficacy of ZVI as adsorption materials for the removal of Ag(i), nZVI particles are more susceptible to such problems as aggregation [10], passivation [11], poor transportability [12], and instability [13]. To overcome these problems, various methods have been reported to obtain more stable and efficient nZVI, such as employing a cheap short-abundant surfactant [14], immobilization of nZVI onto support materials [15], and impregnation of a second non-toxic inert metal (which can enhance the production of free radicals) [16]. However, these techniques may additionally inhibit the response of nZVI with pollutants or entrust nZVI with undesirable properties for environmental remediation. For instance, the inhibitory of surfactants are probably connected with blockage of reactive sites, scavenging of reactive free radicals, reduction in diffusion channels, partitioning of contaminants to the surfactants, and inhibition of electron transfer from the nZVI core to contaminants [17]. The usage of bimetallic particles might result in faster corrosion, which brings about shorter lifetime, and might introduce extra environmental concerns associated with the second metal [18]. nZVI is immobilized in some porous materials such as silica [19], resin [20], activated carbon [21], and biochar [22] which can provide sufficient loading sites for the stable dispersion of nZVI, and prevent it from oxidizing and agglomerating [14].
Carbon nanotubes (CNTs) have a lot of oxygen-containing functional groups like carboxyl and hydroxyl groups, and have gotten increasing attention in the fields of wastewater treatment because of their unusual hollow tube structure and exceptional physicochemical qualities [23]. Many studies have been widely reported on nZVI/CNTs due to excellent magnetic properties, high adsorption capacities, and large specific surface areas. It was reported that Xu et al. [24] synthesized nZVI-multiwalled carbon nanotube (MWCNT) nanocomposites by depositing nZVI particles onto MWCNTs via in situ reduction of ferrous sulfate, then they used the nanocomposites to remove Cr(vi) from wastewater. It was shown that nZVI-MWCNT nanocomposites were about 36% more efficient in the removal of Cr(vi) than bare nZVI or nZVI-activated carbon composites, which indicated that nZVI/CNT could significantly improve the reduction of Cr(vi) to Cr(iii).
Other researchers have prepared nZVI/CNTs using comparable methods, and the CNTs played the role of substrate in composites, then nZVI was immobilized on CNTs by NaBH4 reduction method. The composites were employed as nano-adsorbents for the removal of hexavalent, Se, Co [25], Pb [26], Te [27], etc. The composites exhibited the highest affinity for metal ions and were effective in removing heavy metal ions from aqueous solution. As a result, introducing CNTs into the nZVI system can not only solve the agglomeration of nZVI but also combine the superior reduction ability of nZVI with the high adsorption capacity of CNTs [25].
However, the full-scale application of CNT-supported nZVI in water treatment generally requires overcoming some technical difficulties, such as the low adsorption capacity for heavy metal ions, tedious synthesis processes, and lack of adsorption mechanism of nZVI in composites. Hence, it is vital to exploit accessible technologies to make nZVI/CNT composites with outstanding adsorption property. The introduction of nitrogen-containing functional groups is an effective way. Nitrogen-containing functional groups can effectively bind heavy metal ions, which were conducive to improving the adsorption capacity of CNTs. Polypyrrole (PPy), a conducting polymer containing pyrrolic-N and imino group [28,29], was a good precursor to prepare nZVI/CNTs. And most of the processes for preparing nZVI/CNT composites were time consuming and expensive. Thus, the development of accessible technology to create magnetic CNT composites with good hydrophilicity and high adsorption capacity was still a significant challenge.
In this study, CNT composites doped with nZVI were synthesized by in situ carbon thermal reduction method. First, the polymerization of pyrrole (Py) monomer into PPy nanotubes was initiated by Fe3+ in the presence of methyl orange (MO). Second, Py was pyrolyzed to CNTs and provided a matrix with a large specific surface area and abundant active sites, which benefits the uptake and enrichment of Ag(i). Meanwhile, Fe3+ was used as an iron source to synthesize nZVI/CNT composites by a one-step carbothermal reductive method. The morphologies and properties of nZVI/CNT composites were characterized and analyzed by Transmission Electron Microscope (TEM), Diffraction of X-Ray (XRD), Fourier Transform Infrared Spectrometer (FT-IR), and Thermal Gravimetric Analyzer (TGA). The adsorption capacity of nZVI/CNT composites for Ag(i) in aqueous solution was investigated, and the impact of various adsorption conditions (contact time and initial concentration of Ag(i)) on adsorption capacity was analyzed. Moreover, kinetic models and isothermal models of Ag(i) onto nZVI/CNT composites were studied.
2 Materials and methods
2.1 Materials
Py (AR, Aladdin Chemistry Co. Ltd) was distilled under reduced pressure, and MO was provided by Tianjin Tianxin Fine Chemical Co. Ltd, FeCl3·6H2O and AgNO3 were purchased from Sinopharm Group Chemical Reagent Co., Ltd. All trials were conducted with deionized water.
2.2 Synthesis of nZVI/CNT composites
We developed a novel method to synthesize nZVI/CNT composites through a one-pot route (Figure 1). In a typical synthesis process, 0.98 g MO and certain amount of FeCl3·6H2O were dissolved in 480 mL of deionized water, and the mixture was stirred slowly for 30 min at room temperature until completely dissolved. Then, after adding 0.7 mL of Py to the above mixture, the new mixture was allowed to react for 24 h at room temperature with continuous slow stirring. Finally, the collected product was dried at 120°C for 8 h. The product was carbonized for 5 h at 850°C with a heating rate of 3 °C/min in a tubular furnace under nitrogen atmosphere. In order to understand how FeCl3·6H2O affects the morphologies and properties of nZVI/CNT composites, we set the molar ratios of Py to Fe3+ at 0.1, 0.2, 0.3, 0.4, 0.5, and 0.6, corresponding to the masses of FeCl3·6H2O as from 27.00, 13.50, 9.00, 6.75, 5.40, and 4.50 g. Therefore, the resulting composites were named as S6, S5, S4, S3, S2, and S1, respectively.

Schematic representation of synthesis procedure of nZVI/CNT composites.
For comparison purposes, PPy-based CNTs were prepared by mixing MO and FeCl3·6H2O. Then, Py was progressively added to the mixture and it was slowly stirred for 24 h at room temperature. The obtained PPy nanotubes were washed thoroughly with deionized water and anhydrous ethanol to remove the excess Fe3+. Finally, PPy nanotubes were carbonized in a tubular furnace under nitrogen atmosphere, and the obtained product was named CNTs.
2.3 Characterization of nZVI/CNTs composites
The morphology of nZVI/CNT composites were examined using TEM, scanning electron microscope (SEM) image with energy dispersive spectroscope (EDS) spectra, and selected area electron diffraction (SAED). The crystalline structures of nZVI/CNT composites were determined by XRD. The surface functional groups of the nZVI/CNT composites were analyzed using FT-IR. The magnetic properties of nZVI/CNT composites were confirmed with vibrating sample magnetometer. The thermostability of the nZVI/CNT composites were evaluated by TGA.
2.4 Adsorption experiments
The stock solutions of Ag(i) (1,000 mg L−1) were prepared by dissolving an appropriate amount of AgNO3 in 1,000 mL of deionized water. Various concentrations of Ag(i) were freshly prepared by diluting the stock solution with ultrapure water. The standard calibration curve of Ag(i) was generated at 365 nm by measuring absorbance of different concentrations of Ag(i) standard solution with UV-Visible spectrophotometers.
A batch approach was carried out for the adsorption experiments. nZVI/CNT composites (10 mg) were added to 50 mL of Ag(i) solution and ultrasonicated for 30 min to obtain a dispersion. Then, the dispersion was agitated at 120 rpm, 25°C for 24 h using a constant-temperature oscillator. Effects of the initial concentration of Ag(i) and contact time on adsorption capacity were explored. The initial concentration of Ag(i) solution was varied from 20 to 200 mg L−1, and the reaction time was fixed from 0 to 24 h. The removal rate of Ag(i) (adsorption efficiency) and equilibrium adsorption capacity were determined using equations (1) and (2) as follows:
where R is the removal rate of Ag(i) (mg g−1), Q e is the equilibrium adsorption capacity of Ag(i) (mg g−1), C e and C o are the equilibrium and initial concentrations (mg L−1) of Ag(i), respectively, V is the experimental solution volume (L), and m is the adsorbent mass (g).
The effect of reaction time on the adsorption capacity of Ag(i) with nZVI/CNT composites was investigated by adjusting the reaction time from 0 to 24 h while the initial concentration of Ag(i) was 180 mg L−1. The adsorption capacity was determined using equation (3) as follows:
where Q t was the adsorption capacity (mg L−1) of Ag(i) at t and C t was the concentration (mg L−1) of Ag(i) at t.
3 Results and discussion
3.1 Morphology and structure of nZVI/CNT composites
The crystalline structure of the adsorbent was characterized by XRD as shown in Figure 2. A broad peak appeared in the range of 15–25° was shown in CNTs (Figure 2(a)) and various nZVI/CNT composites (Figure 2(b)–(g)), this peak was mainly for amorphous carbon due to carbonization of PPy [30,31]. However, a decrease in peak intensity was observed in various nZVI/CNT composites, which was mainly due to the formation of nZVI by carbothermal reduction. After loading nZVI, various nZVI/CNT composites (Figure 2(b)–(g)) show diffraction peaks at 44.8°, 65.02°, and 82.45°, indexed as (110), (200), and (211) reflections of nZVI, respectively (Figure 2(h)), and these peaks are consistent with standard profile JCPDS (No. 06-0696) and also reveal that the core of the nanocomposites is nZVI.

XRD pattern of CNTs (a), S1 (b), S2 (c), S3 (d), S4 (e), S5 (f), S6 (g), and nZVI (h).
Figure 3 shows the TEM and the SAED images of CNTs and various nZVI/CNT composites. The TEM images (Figure 3(a)) of CNTs show uniform tubular structures with an average diameter of about 130 nm. In Figure 3(b)–(f), the diameters of various nZVI/CNT composites decrease with the increase in the concentration of Fe3+, because the increasing concentration of Fe3+ will partially solubilize the MO–FeCl3 micelle template resulting in the formation of CNTs having smaller diameters [32]. As shown in (Figure 3(b)–(f)), with the increase in the concentration of Fe3+, S1, S2, and S3 composites (Figure 3(b)–(d)) still keep intact the tube structures, but the tube structures of S4 and S5 were broken. The breaking of tube is caused by excessive Fe3+ content. The formation process of PPy nanotubes is a coating growth process, and the Fe3+ content directly affects the integrity and diameter of PPy nanotubes. The increase in Fe3+ content is conducive to the polymerization of pyrrole monomer and improves the structural integrity of the tube. However, Fe3+ content makes pyrrole monomer polymerize too fast when the molar ratios of Py to Fe3+ is lower than 0.4, which leads to smaller diameter of PPy nanotubes and even broken tube structure. And mass nanoparticles were dispersed homogeneously on CNTs, and the SAED pattern of S3 (Figure 3(g)) revealed diffraction rings of nZVI, which was consistent with the XRD pattern (Figure 2).

TEM images of CNTs (a), S1 (b), S2 (c), S3 (d), S4 (e), S5 (f), SAED and SEM images (g), distribution of S3 Fe (h), and distribution of S3 N (i).
On the other hand, elemental mapping has been carried out to verify the doping of N and Fe in the CNT. The nitrogen content was about 3.9%, which was slightly higher than the other nitrogen-doped materials reported in the literature [33]. Furthermore, the mapping images (Figure 3(h)–(i)) showed that the nitrogen, and iron atoms are overlapped, confirming the uniform distribution of N and Fe in the S3 composite.
The FT-IR spectra of Fe (a), CNTs (b), and S3 (c) composites are shown in Figure 4. In the nZVI spectrum (Figure 4(a)), the weak absorption peak at 521 cm−1 corresponds to the stretching vibration of Fe–O [33]. In CNTs spectrum (b), 3,420 cm−1 arises from the stretching vibration of N–H. The peak of 1,584, 1,390 cm−1 correspond to the C═C symmetric stretching vibrations and C–N stretching vibration of the pyrrole ring. In-plane and out-of-plane C–H, as well as N–H bending vibrations generate the wide peaks at 1,240 and 1,170 cm−1. Compared with these peaks in CNTs, the Fe–O bond at 532 cm−1 was observed for S3 composites. It is worthy to note that the peak corresponds to Fe–O stretching vibration blue-shifts to high frequency, indicating that the magnetic and nonmagnetic components are strongly combined rather than blended.

FT-IR spectra of nZVI (a), CNTs (b), and S3 (c).
To test the thermal properties of CNTs, S3 composites, and nZVI, TGA-DSC was carried out at a heating rate of 10°C/min from 50 to 80°C in an air atmosphere (Figure 5). The TGA curves of the CNTs show a two-step weight loss during the heating processes, and the first step between 50 and 250°C was mainly due to the desorption of CO2, the loss of bound water, and residual moisture. In the temperature range of 350–600°C, CNTs exhibited a sharp weight loss corresponding to the exothermic process, which was attributed to the continuous decomposition of the residue carbon–nitrogen complex. The S3 composites showed a similar trend to that of CNTs, but in the temperature range of 300–800°C, the weight loss of the S3 composites was 23.68%, which was lower than that of the CNTs (87.91%). For nZVI, the weight loss of 7.23% between 50 and 250°C was primarily due to the loss of adsorption water and bound water. Whereas, in the temperature ranges of 450–800°C, the weight of nZVI gradually increased and the weight gain was about 6.2%. The DSC curves of the nZVI exhibited a clear exothermic peak at 548.3°C, which was mainly due to the oxidation of nZVI into FeO and Fe2O3. Only 34.7% of weight loss was observed for S3 composites and it had a weak exothermic peak at around 500°C in DSC, which was due to the loading of nZVI on S3 composites. nZVI content in S3 composites was about 66.2%, indicating the high nZVI content in S3 composites.

TGA (a) and DSC (b) curves of CNTs, S3 composites, and nZVI in air.
3.2 Magnetic behavior of nZVI/CNT composites
The magnetic hysteresis loops of the nZVI (a) and S3 (b) composites are shown in Figure 6, and the magnetic parameters of the nZVI and S3 composites are listed in Table 1. In Figure 6, the S3 composites exhibited a saturation magnetization of 64.85 emu g−1, which indicates that S3 composites have excellent magnetic properties. S3 composites with high saturation magnetization quickly responded to the external magnetic fields, which will provide an easy and efficient avenue for separating nZVI and S3 composites from solution, which is easily recycled and avoids secondary pollution.

Hysteresis loops of nZVI (a), S3 (b), and local magnification of S3 (c).
Magnetic properties of nZVI (a) and S3 (b)
| Sample | M s (emu g−1) | M r (emu g−1) | M r/M s | H c/O e |
|---|---|---|---|---|
| nZVI | 142.31 | 10.51 | 0.073 | 61.74 |
| S3 | 64.85 | 1.45 | 0.022 | 11.52 |
3.3 Removal effect of Ag(i)
The ratio of Py to iron affects the morphology of nZVI/CNTs, thereby affecting the adsorption of Ag(i). Therefore, we investigated the adsorption capacity and removal rate of Ag(i) for CNTs and various nZVI/CNTs (Figure 7).

Adsorption capacity of CNTs (S0) and various nZVI/CNTs for Ag(i).
The adsorption capacity of nZVI/CNTs increased with the decrease in the molar ratio of Py to Fe3+. The increase in adsorption capacity gradually slows down when the molar ratio of Py to Fe3+ is lower than 0.4:1(S3) as shown in Figure 7. At the same time, S3 has complete tubular morphology. Based on the above considerations, S3 is specifically chosen to treat wastewater containing Ag(i).
The effect of different initial concentrations of Ag(i) on the adsorption capacity of CNTs, nZVI, and S3 for Ag(i) is depicted in Figure 8. As can be noticed that the adsorption capacity of the adsorbents increased with the increase in the initial concentration and then tended to a dynamic equilibrium. The maximum adsorption capacities of the CNTs, nZVI, and S3 for Ag(i) were 238.49, 401.49, 522.41 mg g−1. The adsorption capacity of S3 composites for Ag(i) was higher than that of CNTs and nZVI, because nZVI-doped CNTs can improve the agglomeration and oxidation of nZVI, and CNTs have pyrrole-N and imino functional groups, which can react with Ag(i). Therefore, the S3 composites showed good adsorption capacity for Ag(i).
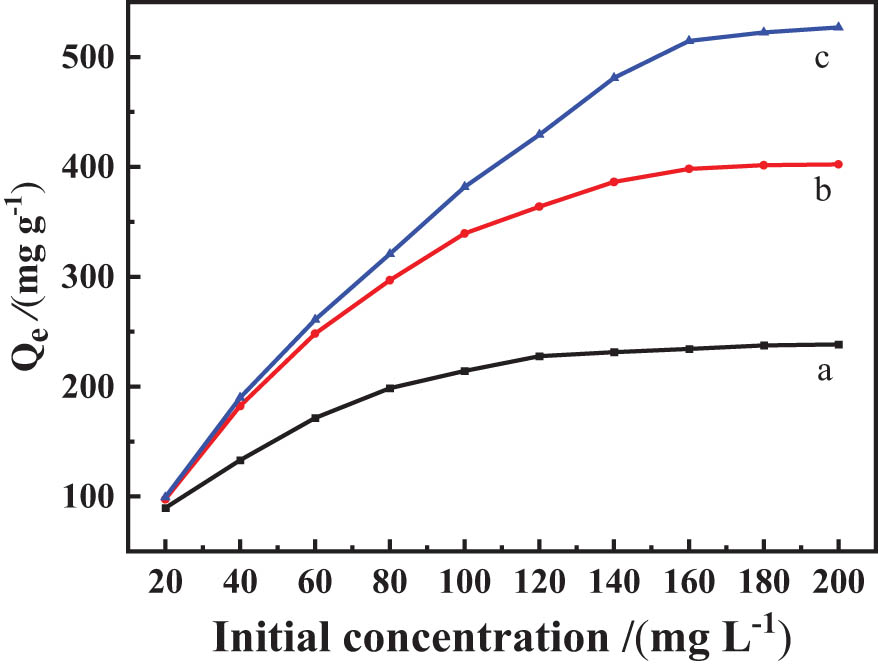
Effects of different initial concentrations on adsorption capacity of CNTs (a), nZVI (b), and S3 (c) for Ag(i).
The effect of varying contact time of Ag(i) on the adsorption capacity of CNTs, nZVI, and S3 for Ag(i) is depicted in Figure 9. The adsorption capacities had a sharp increase in the first 30 min, and then adsorption capacities increase slowly until an adsorption equilibrium was reached at about 4 h. This rapid adsorption of Ag(i) process could be explained by the large number of active sites on the nZVI/CNTs during the initial stage. However, in the second stage, when the remaining active sites on the free surface sites were not facilely occupied, the adsorption process slowed down and the adsorption rates were limited by increasing the repulsive force between the solute ions in the adsorbent and those in the liquid phase [34].

Effects of different adsorption times on adsorption capacity of CNTs (a), nZVI (b), and S3 (c) for Ag(i).
In order to understand the porosity of nZVI/CNT microsphere, N2 adsorption–desorption isotherms were performed. As Figure 10 shown, the specific surface area of the nZVI/CNT composite was 712.56 m² g−1, nZVI/CNT had type IV adsorption isotherm defined by the International Union of Pure Chemistry and Applications, and an H4 type hysteresis loop appeared between the relative pressure P/P 0 = 0.4–1.0, and the pore size distribution showed that the pore size of nZVI/CNT composite was mainly mesopores and microporous.

N2 adsorption and desorption curves of nZVI/CNT, with the pore size distribution curve as inset.
3.4 Adsorption kinetics
In order to explore the adsorption process and mechanism of Ag(i) on the nZVI/CNT composites, the pseudo-first-order, pseudo-second-order, and intraparticle diffusion kinetic models were employed to fit the experimental data under different adsorption times to explore the adsorption kinetics. The pseudo-first-order, pseudo-second-order, and intraparticle diffusion rate equations were expressed as follows [35,36,37]:
where Q e1 and Q e2 (mg g−1) are the adsorption capacities of Ag(i) at equilibrium on the nZVI/CNTs composites, k 1 is the equilibrium rate constant (min−1) of Pseudo-first-order, k 2 is the equilibrium rate constant (min−1) of the pseudo-second-order model, k i is the intraparticle diffusion rate constant (mg g−1 min−0.5), and C is the intercept in intraparticle diffusion.
The kinetic curves were obtained by further fitting the experimental data to pseudo-first-order, pseudo-second-order, and intraparticle diffusion kinetic models (Figure 11). The corresponding adsorption kinetic model linear correlation coefficients (R 2) and rate constants are listed in Table 2. The correlation coefficients of the pseudo-second-order equation (R 2 = 0.9999) were obviously larger than those of the pseudo-first-order equation (R 2 = 0.9456), and the Q e calculated by the pseudo-second-order equation was quite near to the experimental value. Therefore, the adsorption process of Ag(i) on CNTs, nZVI, and S3 composites was well described by the pseudo-second-order kinetic model, which revealed that the adsorption process was controlled by a chemisorption mechanism involving electron transfer or electron transfer between the adsorbent and Ag(i).
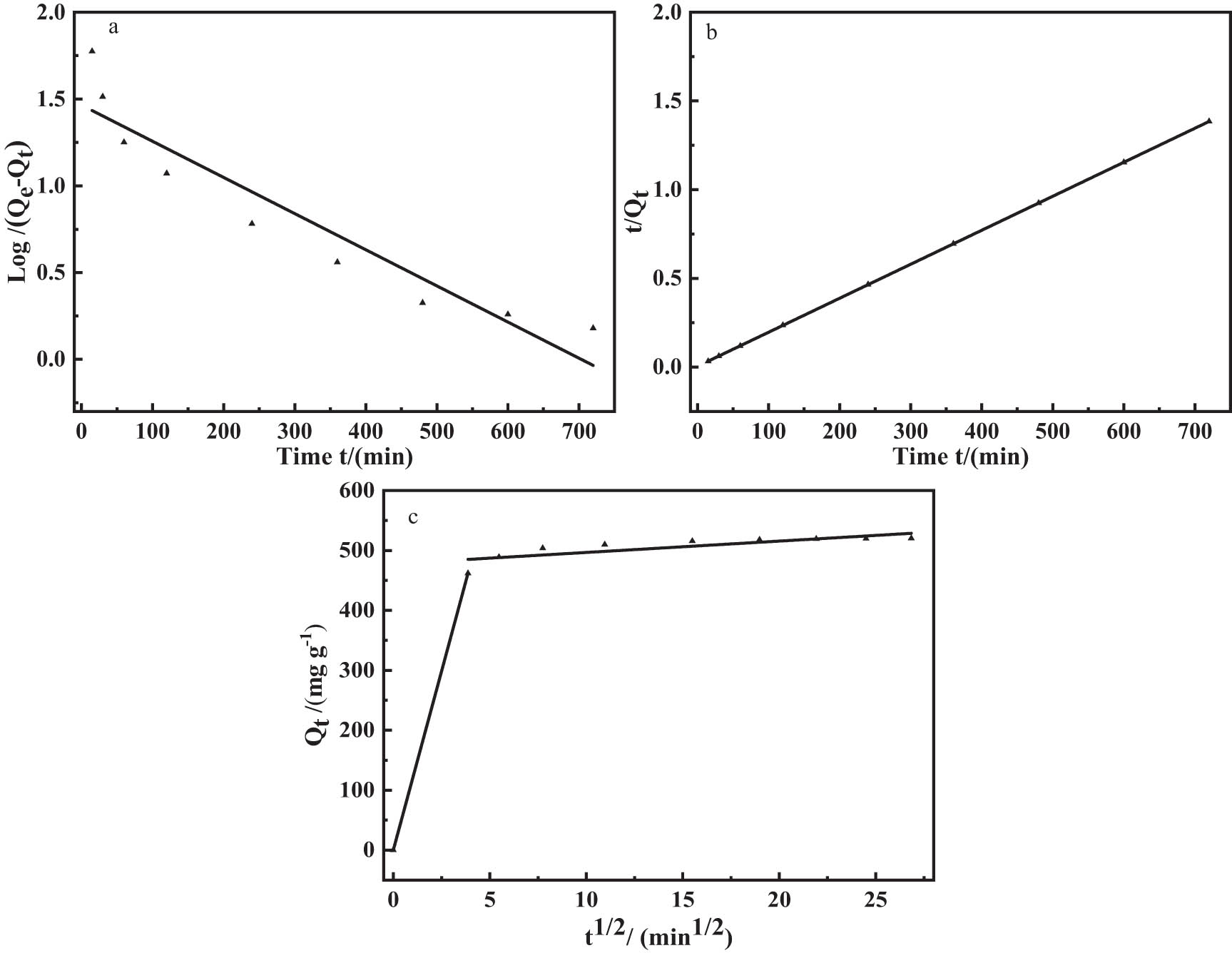
Experimental data fitted using pseudo-first-order (a), pseudo-second-order (b), and intraparticle diffusion (c) models for Ag(i) adsorption on S3.
Parameters of adsorption kinetics model for adsorption of Ag(i) by S3 (C 0 = 180 mg L−1, T = 298 K)
| Pseudo-first-order | ||||
|
|
K 1 (min−1) | Q t (mg g−1) | Q e (mg g−1) | Equation |
| 0.9456 | 0.0021 | 525.47 | 552.11 | log(Q e−Q t) = 1.4652−0.0021t |
| Pseudo-second-order | ||||
|
|
K 2 (min−1) | Q t (mg g−1) | Q e (mg g−1) | Equation |
| 0.9999 | 0.0008 | 525.47 | 526.31 | t/Q t = 0.0019t + 0.0044 |
| Intraparticle diffusion | ||||
| Rate constant | value | R² | Equation | |
| k i1 (mg g−1 min−0.5) | 119.2749 | 1 | Q t = 119.2749t 1/2 + 4.0194 × 10−14 | |
| k i2 (mg g−1 min−0.5) | 1.8965 | 0.8257 | Q t = 1.8965t 1/2 + 477.6804 | |
With the intraparticle diffusion model, the adsorption process was investigated to confirm the primarily rate-controlling phase. From Figure 11(c), we can see that the Ag(i) was adsorbed on the adsorbent in two steps as follows: a rapid step and a subsequent slow step. In the first rapid step, Ag(i) moved to the surface of the adsorbent by surface or film diffusion. In this stage, the rate of adsorption was proportional to the number of active sites present on the surface of the adsorbent, which indicated physical adsorption. In the other stage, because a large amount of adsorbed Ag(i) on the adsorbent needs to be further diffused into the adsorbent, Ag(i) was combined with the adsorption active sites of the inner surface, and gradually be absorbed by inner surface of adsorbent [38], which involves both physical and chemical adsorption. The results indicate that the adsorption rate may be mainly controlled by the intraparticle diffusion step in the integrated adsorption process of Ag(i).
3.5 Adsorption isotherms
Adsorption isotherm experiments were conducted to study the adsorption behavior of Ag(i) on the adsorbent at equilibrium, and the adsorption data are presented in Figure 11. The Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms adsorption models given in equations (7)–(16) were used to describe the experiment results [39,40].
where C e is the equilibrium concentration (mg L−1), Q m is the equilibrium adsorption capacity (mg L−1), K L is the equilibrium constant (mg L−1) of Langmuir, K F is the equilibrium constant (mg L−1) of Freundlich, K T is the equilibrium binding constant (L g−1), T is the reaction temperature (K), and the value of R is 8.314.
In addition, the kinetic curve was obtained by fitting the isothermal adsorption model of Langmuir, Freundlich, Temkin and Dubinin Radushkevich to experimental data (Figure 12) [39,40,41], the constants and value parameters calculated from the above models are listed in Table 3. From the R 2 value (0.9957, 0.9915, 0.9895, and 0.9047), we could see that the Langmuir isotherm adsorption model was significantly higher than the other three models in the concentration range studied. These findings revealed that the adsorption isotherms of Ag(i) onto adsorbents was more compatible with the Langmuir isotherm adsorption model, revealing that the adsorption process was single layer adsorption on a homogeneous surface, and the adsorption sites provided by adsorbents had the same adsorption energies. Moreover, the values of R L determined for all considered initial concentrations of Ag(i) (20–200 mg L−1) were also in the range of 0–1, implying the favorable adsorption of Ag(i).

Ag(i) adsorption on samples described by: Langmuir (a), Freundlich (b), Temkin (c), and Dubinin-Radushkevich (d) isotherm adsorption models.
Adsorption isotherm model parameters for adsorption of Ag(i) on S3 (t = 24 h, T = 298 K)
| Models | Constants | Value | R 2 | Equation |
|---|---|---|---|---|
| Langmuir | Q max (mg g−1) | 555.56 | 0.9957 | C e/Q e = 0.1728 + 0.0018C e |
| K L (L mg−1) | 0.0104 | |||
| Freundlich | K F (mg g−1) | 12.1813 | 0.9915 | ln Q e = 2.4999 + 0.7353ln C e |
| n (mg L−1) | 1.3599 | |||
| Temkin | K T (L g−1) | 0.0689 | 0.9895 | Q e = 203.5599ln C e − 544.5347 |
| b (J mol−1) | 12.1712 | |||
| Dubinin−Radushkevich | Q max (mg g−1) | 430.69 | 0.9047 | ln Q e = 6.0654 − 110.1037 ε 2 |
| β (mol2 kJ−2) | 110.103 | |||
| E (kJ mol−1) | 0.06739 |
3.6 Adsorption mechanisms
XPS was employed to study the element states to further understand the adsorption mechanism of Ag(i) on the surface of nZVI/CNTs composites. As depicted in Figure 13(a), the XPS survey indicates that C, O, N, and Fe are the main elements in the nZVI/CNT composites before adsorption, and Ag3d peak was detected after adsorption, which shows that the nZVI/CNT composites successfully adsorbed Ag. Ag0 (3d5/2) and Ag0 (3d3/2) signals appear at 368.28 and 374.43 eV in the spectrum of Ag3d (Figure 13(b)), respectively. The 3d doublet split at 6.0 eV shows that the deposited Ag on the adsorbent surface was mainly in the Ag0 state [37]. At the same time, the binding energies of 367.13 eV (3d5/2) and 373.17 eV (3d3/2) correspond to Ag(i), implying that the Ag(i) in solution was chelated with the nZVI/CNT composites. Those results suggested that the adsorption process involves chemical adsorption, which is consistent with the kinetic results. Figure 13(c) and (d) reveals three major energy peaks of the O1s atom located at 530.13, 531.74, and 533.52 eV, corresponding to the oxygen of oxide (O2−), −C−O, and O−C═O, respectively. The oxide (O2−) group at 529.88 eV corresponds to the Fe–O group in the oxidized Fe3O4 crystal structure of nZVI/CNTs composites [42], which is consistent with the XRD characterization results [43]. The Fe2p peaks observed before and after adsorption are shown in Figure 13(e and f), there are two energy bands before adsorption at 708.11 eV (2p3/2) and 720.61 eV (2p1/2), which corresponds to the Fe0(15.89%). The binding energies of 710.2 eV (2p3/2) and 723.02 eV (2p1/2) correspond to Fe(ii) (26.94%), 711.98 eV (2p3/2) and 725.23 eV (2p1/2) correspond to Fe(iii) (57.17%). The peaks corresponding to Fe0 after adsorption were visibly different. Due to the high reactivity of nZVI, when the adsorbent is present in a solution containing Ag(i), a considerable number of electrons can be released to reduce Ag(i) to Ag0, and the resulting Ag0 will adhere to the surface of nZVI. The layer of Ag0 with a few nanometers thick will have a certain shielding effect on X-rays, which caused the peak intensity of Fe0 to be greatly lowered. On the other hand, Fe(ii) (32.17%), Fe(iii) (67.83%), and the O2− groups in O1s showed an obvious increase in area, which was caused by partial Fe0 being oxidized to Fe3O4 during reduction. The deconvolution of N1s spectrum before adsorption produced three peaks at 398.65, 401.11, and 402.89 eV, ascribed to the quinoid imine (═N−), amino group (−NH−), and positively charged nitrogen (−NH+), respectively, as shown in Figure 13(g). The area of the peak had decreased to 399.64 and 402.88 eV after adsorption (Figure 13(h)). The chelation might occur between Ag(i) and the amino group (−NH−) and amino group (−NH2), and redox adsorption could occur between Ag(i) and nitrogen with free −NH2, resulting in partially reducing Ag(i) to Ag0 [41].

XPS spectra (a) of nZVI/CNT composites before and after adsorption of Ag(i), high-resolution spectra of Ag3d (b) after adsorption of Ag(i). XPS spectra of O1s (c) and (d), Fe2p (e) and (f), and N1s (g) and (h) for nZVI/CNT composites before and after adsorption of Ag(i).
The comparison of this work and similar works reported (the Q m, initial concentration of Ag(i) and separation methods of different sorbents) in literature is depicted in Table 4 [41,44–49]. These results indicated that nZVI/CNT composites prepared in this study could be a promising adsorbent for the selective adsorption of Ag(i). The adsorption of Ag(i) on nZVI/CNT composites has various mechanisms including ion exchange and chelation. Among them, Ag(i) was partially reduced to Ag because of the existence of nZVI and –NH– in the nZVI/CNT composites. Compared to other adsorbents, the nZVI/CNT composites exhibited much higher adsorption efficiency on Ag(i) due to the large specific surface areas and more active adsorption sites in CNTs preventing the agglomeration of the nZVI (Figure 14). In addition, the nZVI/CNT composites can be easily separated from the wastewater and quickly recovered by applying an external magnetic field to achieve its reuse. The excellent properties of nZVI/CNT composites provide a good prospect for cost-effective application with the adsorption of Ag(i) in wastewater treatment.
Comparison of Q max of different sorbents for Ag(i)
| Adsorbents | Q max (mg g−1) | Initial concentration of Ag(i) (mg L−1) | Separation | Ref. |
|---|---|---|---|---|
| nZVI/HPAC | 350 | 200 | Magnetic | [40] |
| Biochar-supported ZVI | 600 | 1,000 | Magnetic | [37] |
| nZVI/BBH | 534.5 | 300 | Magnetic | [41] |
| PPy/Fe3O4 nanocomposite | 169.41 | 180 | Magnetic | [42] |
| PPy/Fe3O4 nanocomposite | 230.17 | 180 | Magnetic | [43] |
| PPy/γ-Fe2O3 | 209 | 180 | Magnetic | [44] |
| nZVI/CNTs | 522.41 | 180 | Magnetic | (Our work) |
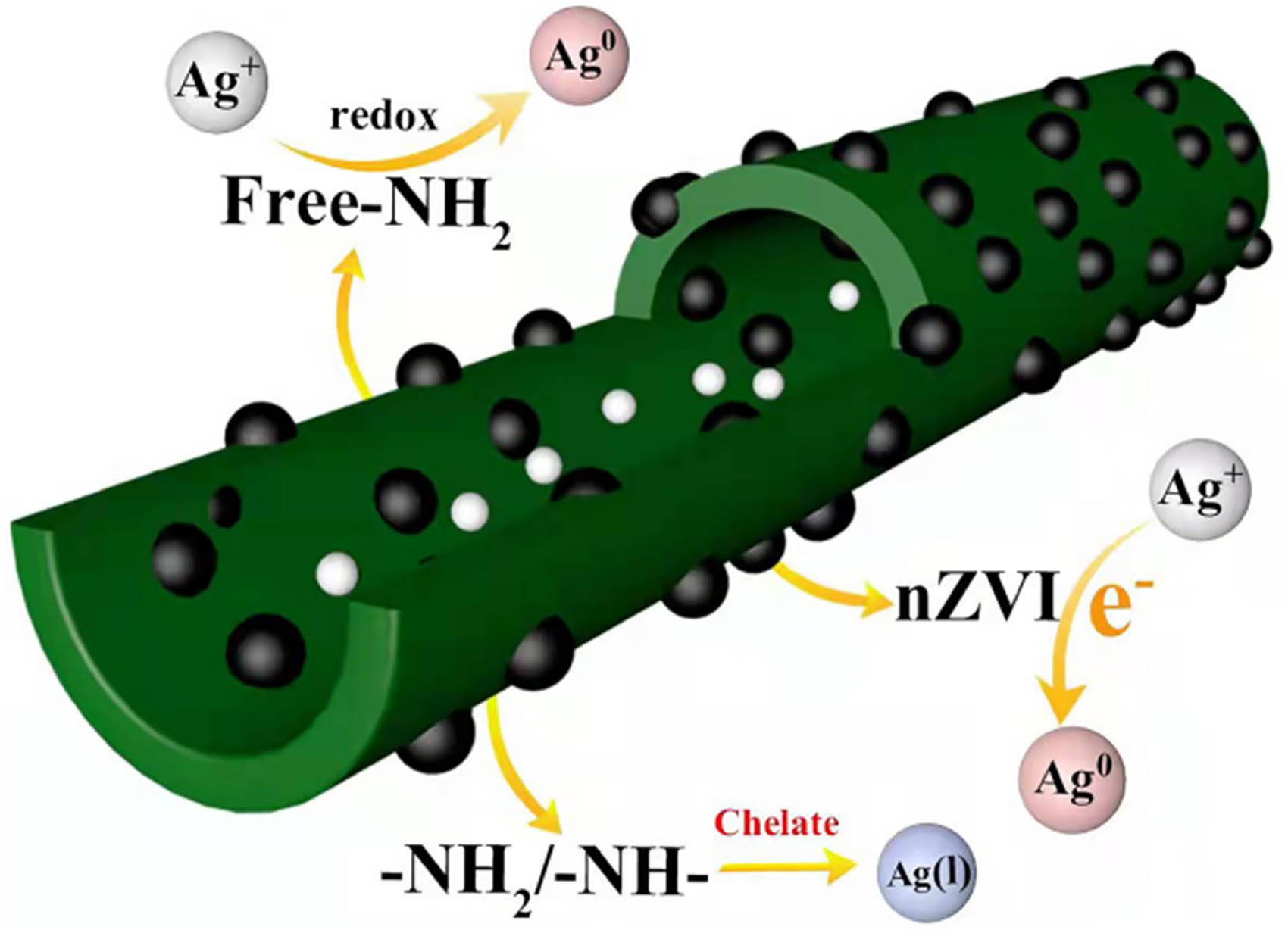
The process of adsorption for Ag(i) by nZVI/CNT composites.
3.7 Adsorption reusability
The reusability of adsorbent is very important factors in industrial application of wastewater treatment. The reusability ability of nZVI/CNT composite was investigated by consecutive sorption–desorption cycles for five times as shown as in Figure 15. After 5 cycles, the adsorption capacity of the composite to Ag(i) can reach 60% of the initial property, indicating that the nZVI/CNT composite has good reusability.

Adsorption capacity on Ag(i) for five consecutive adsorption–desorption cycles.
4 Conclusion
nZVI-doped nZVI/CNT composites were synthesized by in situ carbothermic reduction method in one-pot using nitrogen-rich PPy as a precursor, FeCl3·6H2O as an initiator, and MO as a template. The diameter of nZVI/CNTs composites decreased with the increase in the concentration of Fe3+, and the nZVI/CNT composites formed a uniform tubular structure when n(Py:Fe3+) was 0.4, and the nZVI formed by in situ carbothermal reduction were dispersed in the composites. The saturation magnetization of the composites was 64.85 emu/g when n(Py:Fe3+) was 0.4 and showed excellent magnetic property. Furthermore, when the initial concentration of Ag(i) was 180 mg L−1, the maximum adsorption capacities of nZVI/CNT composites for Ag(i) was up to 522.41 mg g−1. The adsorption process of Ag(i) on nZVI/CNT composites can be well described by the pseudo-second-order kinetic model and the Langmuir isotherm model, which revealed that the adsorption process was controlled by a monolayer chemical adsorption and a favorable process. And intraparticle diffusion was the main rate-controlling step during the adsorption process of Ag(i).
-
Funding information: The authors kindly thank the National Natural Science Foundation of China (52262013, 51703088); Key laboratory of polymer materials opening fund project in 2018 (KF-18-03); Hongliu Youth Fund of Lanzhou University of Technology (061805); the Natural Science Foundation of Gansu Province (21JR7RA259); Gansu Province for young doctor (2021QB-048).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Suber L, Campi G. Hierarchic self-assembling of silver nanoparticles in solution. Nanotechnol Rev. 2012;1(1):57–78. 10.1515/ntrev-2011-0004.Search in Google Scholar
[2] Ghobashy MM, Elkodous MA, Shabaka SH, Younis SA, Alshangiti DM, et al. An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment. Nanotechnol Rev. 2021;10(1):954–77. 10.1515/ntrev-2021-0066.Search in Google Scholar
[3] Tanweer MS, Iqbal Z, Alam M. Experimental insights into mesoporous polyaniline-based nanocomposites for anionic and cationic dye removal. Langmuir: ACS J Surf Colloids. 2022;38(29):8837–53. 10.1021/acs.langmuir.2c00889.Search in Google Scholar PubMed
[4] Ma YX, Xing D, Ruan YX, Du XY, La PQ. Fabrication of amino-functionalized magnetic graphene oxide nanocomposites for adsorption of Ag(I) from aqueous solution. Env Eng Sci. 2018;35(3):219–30. 10.1089/ees.2016.0483.Search in Google Scholar
[5] Yang T, Hodson ME. Investigating the potential of synthetic humic-like acid to remove metal ions from contaminated water. Sci Total Env. 2018;635(1):1036–46. 10.1016/j.scitotenv.2018.04.176.Search in Google Scholar PubMed
[6] Wu J, Weng XL, Owens G, Chen ZL. Enhanced activity of Fe/Mn nanoparticles using a response surface methodology and mechanism for removing oxytetracycline and copper ion. Chemosphere. 2023;319:138057. 10.1016/j.chemosphere.2023.138057.Search in Google Scholar PubMed
[7] Lin Y, Xu J, Sudhakar S, Gu J, Hong R. Preparation of spherical aminopropyl-functionalized MCM-41 and its application in removal of Pb(II) ion from aqueous solution. Nanotechnol Rev. 2019;8(1):275–84. 10.1515/ntrev-2019-0026.Search in Google Scholar
[8] Wang S, Zhao M, Zhou M, Li YC, Wang J, Gao B, et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review. J Hazard Mater. 2019;76:197–214. 10.1016/j.jhazmat.2019.03.080.Search in Google Scholar PubMed
[9] Yu SH, Li H, Yao QZ, Fu SQ, Zhou GT. Microwave-assisted preparation of sepiolite-supported magnetite nanoparticles and their ability to remove low concentrations of Cr(VI). RSC Adv. 2015;5(103):84471–82. 10.1039/c5ra14130c.Search in Google Scholar
[10] Yang JZ, Tia PR, He JH. 3D porous carbon-embedded nZVI@Fe2O3 nanoarchitectures enable prominent performance and recyclability in antibiotic removal. Chemosphere. 2023;331:138716. 10.1016/j.chemosphere.2023.138716.Search in Google Scholar PubMed
[11] Wei AL, Ma J, Chen JJ, Zhang Y, Song JX, Yu XY. Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero valent iron. Chem Eng J. 2018;353:595–605. 10.1016/j.cej.2018.07.127.Search in Google Scholar
[12] Su H, Fang Z, Tsang PE, Fang J, Zhao D. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in-situ remediation of hexavalent chromium in soil. Environ Pollut. 2016;214:94. 10.1016/j.envpol.2016.03.072.Search in Google Scholar PubMed
[13] Oh SY, Seo YD, Ryu KS, Park DJ, Lee SH. Redox and catalytic properties of biochar-coated zero-valent iron for the removal of nitro explosives and halogenated phenols. Environ Pollut. 2017;19(5):711–9. 10.1039/c7em00035a.Search in Google Scholar PubMed
[14] Wu YW, Yue QY, Ren ZF, Gao BY. Immobilization of nanoscale zero-valent iron particles (nZVI) with synthesized activated carbon for the adsorption and degradation of chloramphenicol (CAP). J Mol Liq. 2018;262:19–28. 10.1016/j.molliq.2018.04.032.Search in Google Scholar
[15] Zhang LB, Shen SY. Adsorption and catalytic degradation of sulfamethazine by mesoporous carbon loaded nano zero valent iron. J Ind Eng Chem. 2020;83:123–35. 10.1016/j.jiec.2019.11.020.Search in Google Scholar
[16] Dong HR, Jiang Z, Deng JM, Zhang C, Cheng YJ, Hou KJ, et al. Physicochemical transformation of Fe/Ni bimetallic nanoparticles during aging in simulated groundwater and the consequent effect on contaminant removal. Water Res. 2018;129:51–7. 10.1016/j.watres.2017.11.002.Search in Google Scholar PubMed
[17] Wang X, Yang J, Zhu M, Taiwan J. Effects of PMMA/anisole hybrid coatings on discoloration performance of nano zero valent iron toward organic dyes. Inst Chem E. 2014;45(3):937–46. 10.1016/j.jtice.2013.08.019.Search in Google Scholar
[18] O’Carroll D, Sleep B, Krol M, Boparai H, Kocur C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour. 2013;51:104–22. 10.1016/j.advwatres.2012.02.005.Search in Google Scholar
[19] Xiang MH, Huang MF, Li H, Wang W, Huang Y, Lu Z, et al. Nanoscale zero-valent iron/cobalt@mesoporous hydrated silica core-shell particles as a highly active heterogeneous Fenton catalyst for the degradation of tetrabromobisphenol A. Chem Eng J. 2021;417:129208. 10.1016/j.cej.2021.129208.Search in Google Scholar
[20] Gao W, Zhong DJ, Xu YL, Han L, Zeng SJ. Nano zero-valent iron supported by macroporous styrene ion exchange resin for enhanced Cr(VI) removal from aqueous solution. J Disper Sci Technol. 2020;43:1197–207. 10.1080/01932691.2020.1848583.Search in Google Scholar
[21] Chen WF, Pan L, Chen LF, Wang Q, Yan CC. Dechlorination of hexachlorobenzene by nano zero-valent iron/activated carbon composite: iron loading, kinetics and pathway. RSC Adv. 2014;4:46689–96. 10.1039/c4ra06760f.Search in Google Scholar
[22] Nistico R, Carlos L. High yield of nano zero-valent iron (nZVI) from carbothermal synthesis using lignin-derived substances from municipal biowaste. J Anal Appl Pyrolysis. 2019;140:239–44. 10.1016/j.jaap.2019.03.022.Search in Google Scholar
[23] Yien JL, Mubarak NM, Juey YM, Sie YL, Bing CH, Mohammad K, et al. An overview of functionalised carbon nanomaterial for organic pollutant removal. J Ind Eng Chem. 2018;67:175–86. 10.1016/j.jiec.2018.06.028.Search in Google Scholar
[24] Lv XS, Xu, Jiang G, Xu X. Removal of chromium(VI) from wastewater by nanoscale zero-valent iron particles supported on multiwalled carbon nanotubes. Chemosphere. 2011;85(7):1204–9. 10.1016/j.chemosphere.2011.09.005.Search in Google Scholar PubMed
[25] Vilardi G, Mpouras T, Dermatas D, Verdone N, Polydera A, Di Palma L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zero-valent iron and carbon nanotubes. Competitive adsorption non-linear modeling. Chemosphere. 2018;201:716–29. 10.1016/j.chemosphere.2018.03.032.Search in Google Scholar PubMed
[26] Sheng G, Alsaedi A, Shammakh W, Monaquel S, Sheng J, Wang X, et al. Enhanced sequestration of selenite in water by nanoscale zero valent iron immobilization on carbon nanotubes by a combined batch, XPS and XAFS investigation. Carbon. 2016;99:123–30. 10.1016/j.carbon.2015.12.013.Search in Google Scholar
[27] Yu HQ, Zhang T, Jing ZF, Xu JC, Qiu FX, Yang DY, et al. In situ fabrication of dynamic nano zero-valent iron/activated carbon nanotubes membranes for tellurium separation. Chem Eng Sci. 2019;205:278. 10.1016/j.ces.2019.05.012.Search in Google Scholar
[28] Debnath A, Deb K, Sarkar K, Saha B. Low interfacial energy barrier and improved thermoelectric performance in Te-incorporated polypyrrole. J Phys Chem C. 2020;125(1):168–77. 10.1021/acs.jpcc.0c09100.Search in Google Scholar
[29] Debnath A, Deb K, Bhowmik KL, Saha B. Reduced hopping barrier potential in NiO nanoparticle-incorporated, polypyrrole-coated graphene with enhanced thermoelectric properties. ACS Appl Energy Mater. 2020;3(8):7772–81. 10.1021/acsaem.0c01174.Search in Google Scholar
[30] Wang A, Ren J, Shi B, Lu G, Wang Y. A facile one-pot synthesis of mesoporous graphite-like carbon through the organic-organic co-assembly. Microporous Mesoporous Mater. 2012;151:287–92. 10.1016/j.micromeso.2011.10.022.Search in Google Scholar
[31] Ulfa M, Pertiwi YE, Saraswati TE, Bahruji H, Holilah H. Synthesis of iron triad metals-modified graphitic mesoporous carbon for methylene blue photodegradation. S Afr J Chem Eng. 2023;45:149–61. 10.1016/j.sajce.2023.05.008.Search in Google Scholar
[32] Sun XH, Zheng M, Zhang FX, Yang YL. Size-controlled synthesis of magnetite (Fe3O4) nanoparticles coated with glucose and gluconic acid from a single Fe(III) precursor by a sucrose bifunctional hydrothermal method. J Phys Chem C. 2009;113(36):16002–08. 10.1021/jp9038682.Search in Google Scholar
[33] Li K, Zhou Y, Zhang QF, Cen YQ, Li XN. Preparation of doped carbon materials and their application in catalytic hydrogenation loaded with noble metals. J Chem Eng Chin Uni. 2019;33(3):516. http://en.cnki.com.cn/Article_en/CJFDTotal-GXHX201903002.Search in Google Scholar
[34] Ma YX, Kou YL, Du XY. Synthesis of magnetic graphene oxide grafted polymaleicamide dendrimer nanohybrids for adsorption of Pb(II) in aqueous solution. J Hazard Mater. 2017;340:407–16. 10.1016/j.jhazmat.2017.07.026.Search in Google Scholar PubMed
[35] Zhang WJ, Zhang BB, Du XY, Fei YL. Synthesis of magnetic N-doped mesoporous carbon composite for adsorption of Ag(I) in aqueous solution. J N N. 2021;21(3):1462–73. 10.1166/jnn.2021.18893.Search in Google Scholar PubMed
[36] Tan Y, Chen M, Hao Y. High efficient removal of Pb(II) by amino-functionalized Fe3O4 magnetic nano-particles. Chem Eng J. 2012;191:104–11. 10.1016/j.cej.2012.02.075.Search in Google Scholar
[37] Ozmen M, Can K, Akin I, Arslan G, Tor A, Cengeloglu Y. Surface modification of glass beads with glutaraldehyde: Characterization and their adsorption property for metal ions. J Hazard Mater. 2009;171(1–3):594–600. 10.1016/j.jhazmat.2009.06.045.Search in Google Scholar PubMed
[38] Wang D, Liu L, Jiang X, Yu J, Chen X. Adsorption and removal of malachite green from aqueous solution using magnetic β-cyclodextrin-graphene oxide nanocomposites as adsorbents. Colloids Surf A: Physicochem Eng Aspects. 2015;466:166–73. 10.1016/j.colsurfa.2014.11.021.Search in Google Scholar
[39] Langmuir I. The constitution and fundamental properties of solids and liquids. J Am Chem Soc. 1916;184(5):102–5. 10.1016/S0016-0032(17)90088-2.Search in Google Scholar
[40] Richuan R, Huang YH, Ling Q, Hu CM, Dong XZ, Xiang J, et al. A facile pyrolysis synthesis of Ni doped Ce2O3@CeO2/CN composites for adsorption removal of Congo red: Activation of carbon nitride structure. Sep Purif Technol. 2022;305:122505. 10.1016/j.seppur.2022.122505.Search in Google Scholar
[41] Zhou Y, Gao B, Zimmerman AR, Cao XD. Biochar-supported zerovalent iron reclaims silver from aqueous solution to form antimicrobial nanocomposite. Chemosphere. 2014;117(1):801–5. 10.1016/j.chemosphere.2014.10.057.Search in Google Scholar PubMed
[42] Zhao XC, Su DS. Reactivity of mesoporous carbon against water - An in-situ XPS study. Carbon. 2014;77:175–83. 10.1016/j.carbon.2014.05.019.Search in Google Scholar
[43] Zhang WJ, Ran MY, Wang YL, Feng QC, Zhang BB, Wu XY, et al. One-pot synthesis of magnetic N-doped mesporous carbons as an efficient adsorbent for Ag(I) removal. Mater Chem Phys. 2023;305:127846. 10.1016/j.matchemphys.2023.127846.Search in Google Scholar
[44] Sun WH, Zhang WB, Li HL. Insight into the synergistic effect on adsorption for Cr (VI) by a polypyrrole-based composite. RSC Adv. 2020;10(15):8790–9. 10.1039/c9ra08756g.Search in Google Scholar PubMed PubMed Central
[45] Wang J, Zhang W, Kang X, Zhang C. Rapid and efficient recovery of silver with nanoscale zerovalent iron supported on high performance activated carbon derived from straw biomass. Env Pollut. 2019;255:113043. 10.1016/j.envpol.2019.113043.Search in Google Scholar PubMed
[46] Wang SS, Zhao MY, Zhao YT, Wang N, Bai J, Feng K, et al. Pyrogenic temperature affects the particle size of biochar-supported nanoscaled zero valent iron (nZVI) and its silver removal capacity. Chem Speciat Bioavailab. 2017;29:179. 10.1080/09542299.2017.1395712.Search in Google Scholar
[47] Mao GY, Bu FX, Jiang DM, Zhao ZJ, Zhang QH, Jiang JS. Synthesis characterization and adsorption properties of magnetic γ-Fe2O3/C nanocomposite. J Nanosci Nanotechnol. 2015;15(8):5924–32. 10.1166/jnn.2015.10281.Search in Google Scholar PubMed
[48] Bhaumik M, Setshedi K, Maity A, Onyango MS. Chromium (VI) removal from water using fixed bed column of polypyrrole/Fe3O4 nanocomposite. Sep Purif Technol. 2013;110:11–9. 10.1016/j.seppur.2013.02.037.Search in Google Scholar
[49] Chávez-Guajardo AE, Maqueira L, Medina-Llamas JC, Alcaraz-Espinoza J, Araújo TL, Vinhas GM, et al. Use of magnetic and fluorescent polystyrene/tetraphenyl porphyrin/maghemite nanocomposites for the photoinactivation of pathogenic bacteria. React Funct Polym. 2015;96:39–43. 10.1016/j.reactfunctpolym.2015.08.012.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

