Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
-
Murni Handayani
, Hendrik
, Rahmat Mulyawan
, Charline Tiara Rehuellah Pingak
Abstract
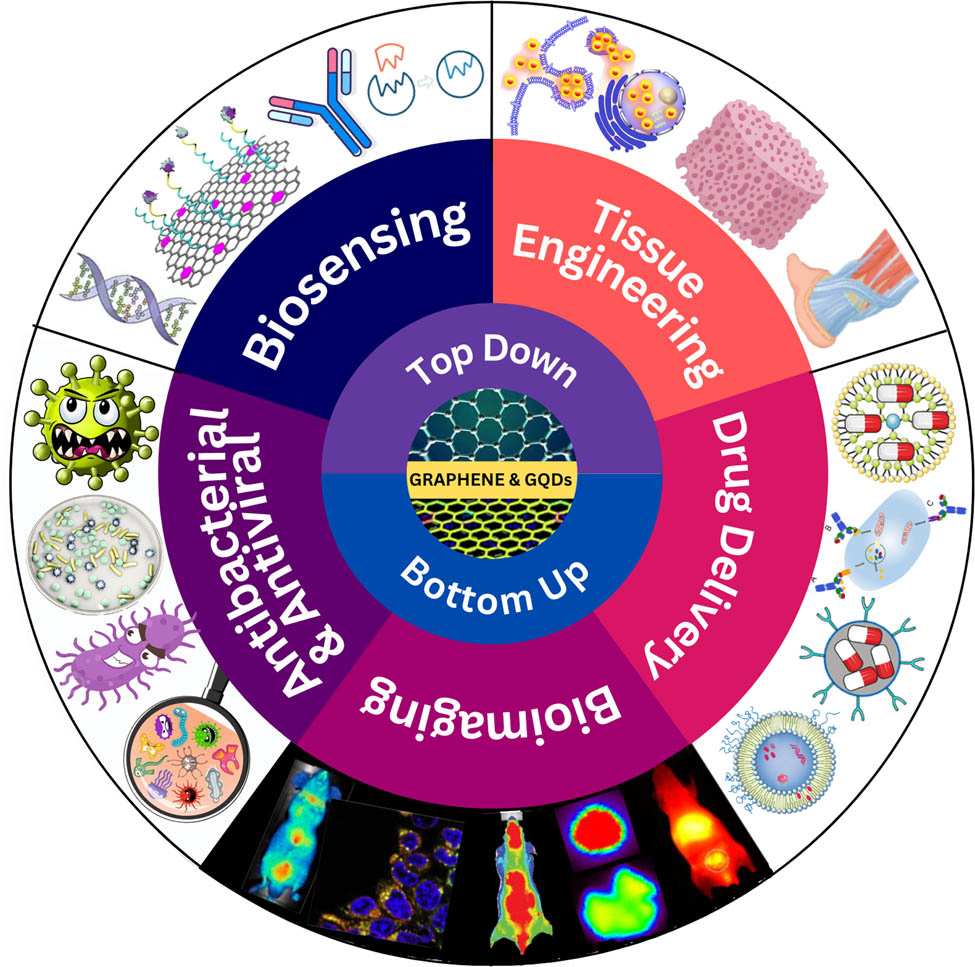
Research on the application of graphene (G) and graphene quantum dots (GQDs) for biomedical engineering has attracted much attention over the last decade. Graphene and its derivatives have shown great biocompatibility, solubility, selectivity, large surface area, high purity, biofunctionalization, high drug loading capacity, and cell membrane penetration capability potential to be applied in biomedical engineering areas. The unique physical and chemical properties of GQDs, including small size, chemical inertness, high photoluminescence stability, low cytotoxicity, and good biocompatibility, made them a promising candidate for biomedical engineering applications. The recent progress related to the development of G and GQDs toward biomedical engineering applications is presented in this work. This study reviews and discusses the development of G and GQDs, both top-down and bottom-up synthesis methods, for biomedical engineering applications, such as biosensing, tissue engineering, drug delivery, bioimaging, antibacterial, and antiviral.
Graphical abstract
1 Introduction
Graphene (G) and graphene quantum dots (GQDs) are two intriguing nanomaterials that have garnered significant interest in the field of biomedical engineering [1]. Graphene is an extremely thin layer composed of carbon atoms, with a thickness of just one atom. Graphene exhibits exceptional mechanical strength, flexibility, high thermal and electrical conductivity, a large surface area, high resistance to corrosion, and excellent biocompatibility [2]. These properties make G suitable for a wide range of biomedical applications. On the other hand, GQDs are nanoscale particles made from G [3]. GQDs possess distinct structural and electronic properties. Due to their incredibly small size, they exhibit unique optical properties and the capability to selectively release electrons. This makes GQDs highly promising for biomedical applications. The distinct characteristics of both materials make them highly promising candidates for applications in the biomedical field [4]. As a result, an upsurge of research based on G and nanocomposite materials has accelerated, especially after the discovery of single-layer G by Novoselov et al. in 2004 [5].
Generally, researchers observed that G possesses dual properties that make new fields of its properties go beyond G and depend on the material the G is composited. For example, intrinsic G has a zero band-gap semiconductor in which electron mobility (200,000 cm2 V−1 s−1) is very promising for sensor applications because its conduction and valence bands meet at Dirac points [6]. Furthermore, its transport characteristics and thermal conductivity (5,000 W m−1 K−1) can be tuned by electrostatic or magnetostatic gating via chemical doping.
Besides the electronic properties, G has excellent mechanical properties: elastic modulus (elastic to a maximum of 20%), substantially lighter than paper, and a high surface area (2,630 m2 g−1) that exceeds known steel but is difficult to cut into a precise dimension [7]. One of the most popular approaches that may provide hugely positive environmental impacts is using the flash Joule heating process to turn almost any carbon-based rubbish – from banana skins to car tires – into G flakes due to lower production costs. Graphene can be produced by heating waste products up to 3,000 K (2,727°C) that breaks the carbon bonds inside the target material and is reconstructed as G in 10 ms [8], similar to the process that researchers used previously in forming metal nanoparticles. In contrast, the G generated is inexpensive and can be used in more places, for instance, as reinforcement in concrete that can reduce greenhouses gases that waste food would have emitted in landfills [9]. It also applies to other renewable precursors and may open a new avenue for the low-cost synthesis of G films [10].
More recently, some researchers have attempted different classes of material that can be placed on G. Some examples of G metal nanocomposite include Mg [11], Boron Nitride [12], Si/Cu [13], and ion metals [14], such as lithium and noble metals [15]. The metal oxide-based nanocomposite, including a semiconducting metal oxide, could be strengthened by G [16,17,18,19,20]. Polymer-based nanocomposite could also be reinforced by G [21]. These nanocomposites have properties distinct from 2-Dimensional (2D) and conventional 3-Dimensional (3D) materials, leading to the development of large-scale practical applications, such as electric cars and mobile devices. Coupled graphene oxide (GO) possesses beneficial properties for biomedical applications, particularly when used in conjunction with hybrid metallic nanoparticles as electrochemical biosensors for the precise detection of ascorbic acid in blood [22]. In addition, integrating G with Au nanostars, represents a significant advancement toward the direct detection of IgG antibodies of SARS-CoV-2 in blood [23].
2D G has opened new perspectives in studying some basic quantum relativistic phenomena compared to zero-dimensional (0D) fullerenes and one-dimensional carbon nanotubes (CNTs). It has a large surface area, high intrinsic carrier mobility, excellent mechanical properties, and superior flexibility [24,25,26,27]. Recently, GQDs have emerged as a new type of 0D G material [28]. It is defined as graphene sheets (GSs) with a plane size of less than 100 nm and a thickness of fewer than ten layers. The GQDs emerge as superior and universal fluorophores because of their unique physical and chemical properties, including small size, chemical inertness, high photoluminescence stability, low cytotoxicity, and good biocompatibility [28]. Owing to their unique size-dependent optical and physicochemical properties, GQDs find promising biomedical applications in the selective and sensitive sensing of various analytes. Doping plays a pivotal role in enhancing the properties of GQDs. For example, sulfur-doped GQDs (S-GQDs) exhibit excellent water solubility and display stronger fluorescence compared to undoped GQDs. Additionally, they possess a significantly higher quantum yield (QY) of 57.44%, making them promising candidates for a wide range of applications, including biomedical uses [29]. In addition, boron-sulfur GQDs have demonstrated exceptional efficiency in the detection of dopamine, showcasing their potential for highly sensitive and accurate biomedical sensing applications [30].
This review discusses the recent G and GQDs development for biomedical engineering applications. We provide an overview of G derivatives, synthesis methods of G and GQDs, and their applications in the field of biomedicine. Specifically, we demonstrate five representative types of biomedical applications based on G and GQDs for biosensing, tissue engineering, drug delivery, bioimaging, and antibacterial and antiviral. This review provides essential insights for researchers and practitioners in the field, enabling them to explore the potential of G-based materials for advancing biomedical engineering applications. It also addresses the challenges and proposes solutions to optimize the use of G and GQDs in biomedical applications.
2 G and its derivatives
2.1 Pristine G
Graphene is a monolayer of carbon atoms arranged in a 2D honeycomb lattice with sp2 hybridization with a C–C bond length of 0.142 nm (Figure 1). It possesses remarkable properties, including a large surface area (2,630 m2), high thermal conductivity (5,000 W m−1 K−1), high electrical conductivity (106 S cm−1), high mechanical strength (∼40 N m−1), great optical transmittance (∼97.7%), high modulus of elasticity (1 TPa), and high electron intrinsic mobility (250,000 cm2 V−1 s−1) [31]. These extraordinary characteristics have attracted significant attention from researchers, opening up new avenues for advanced materials research. Graphene has gained popularity in both academic and industrial domains due to its exceptional properties, driving its demand in research and the market [32]. Initially, the mechanical exfoliation technique was employed for making pristine G, pioneered by Nobel Prize winners Geim and Novoselov [33]. This method involves the peeling off of layers from highly oriented pyrolytic graphite (HOPG) sheets using scotch tape. Pure G holds significant potential for biomedical applications, such as the development of a G-based femtogram-level sensitive molecularly imprinted polymer of SARS-CoV-2 [34]. Furthermore, in the field of energy storage, computational studies have explored the interaction between G and hydrogen for hydrogen storage, offering valuable insights into G’s potential as a medium for hydrogen storage [35]. Furthermore, when G is employed in the form of nanographene, it exhibits additional fascinating properties arising from quantum confinement effects, making it even more promising for various applications [36].
![Figure 1
The structure of G and its derivative. (a) G, (b) GO, (c) reduced graphene oxide (rGO), and (d) GQD. Reproduced from [37].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_001.jpg)
The structure of G and its derivative. (a) G, (b) GO, (c) reduced graphene oxide (rGO), and (d) GQD. Reproduced from [37].
2.2 GO
One derivative of G materials is GO, which is the oxidized form of G containing oxygen-functional groups such as epoxides, hydroxyls on the basal plane, and carboxyl groups at the edges (Figure 1) [38]. Recently, GO has acquired increasing interest due to its excellent attributes. The abundance of oxygen-containing groups, including –OH and –COOH, makes GO exhibit strong hydrophilic properties [39]. Therefore, it can be dispersed in water or other solvents to form a stable suspension owing to the oxygen-functional groups. Furthermore, GO offers the advantage of facile combination with other molecules through covalent or noncovalent interactions.
The commonly used method for producing GO is the Hummers’ method [40,41]. In the synthesis process of GO, graphite oxide is first prepared from graphite by forming hydroxyl or carboxyl groups, covalently bound to a graphite planar carbon network. The material is then treated with oxidizing agents, such as sulfuric acid, nitric acid, and potassium permanganate. The resulting layers of GO are thicker than pristine GSs due to the displacement of sp3 hybridizations. The ability of GO to form stable suspensions in water has positioned it as a prominent product segment in the G market. GO exhibits great potential for biomedical applications, and its utility can be enhanced by compositing it with other materials, as demonstrated by Hashemi et al. They successfully developed an ultrasensitive biomolecule-less nanosensor by decorating GO with β-Cyclodextrin/Quinoline, enabling prompt and distinguishable detection of corona and influenza viruses [42].
2.3 rGO
rGO is a form of GO that is produced using thermal, chemical, and other techniques to reduce the content of oxygen functional groups in GO (Figure 1). rGO sheets exhibit higher conductivity compared to GO due to the restoration of the conjugated network within the sheets. rGO possesses oxygen-containing functional groups, which contribute to its exceptionally high specific surface area, superior electronic conductivity, and excellent mechanical behavior [43]. The chemical reduction of GO sheets can be achieved using various reducing agents such as hydrazine, sodium borohydride, and ascorbic acid [44]. Coupling GO with hybrid metallic nanoparticles shows promising potential for biomedical applications, as demonstrated by Hashemi et al. They reported the utilization of coupled GO with hybrid metallic nanoparticles as electrochemical biosensors for precise detection of ascorbic acid in blood [22]. Another application is in the field of energy storage systems, where the development of composite materials, such as rGO/chitosan/zinc oxide, has shown remarkable potential. These composites exhibit superior performance as supercapacitor electrodes, offering enhanced electrochemical properties [45].
2.4 GQDs
GQDs belong to a class of nanomaterials that possess unique and fascinating properties attributed to their size and G-like structure (Figure 1). GQDs typically exhibit a nanoscale size, ranging from a few nanometers to a few hundred nanometers. They consist of sp2-hybridized carbon atoms arranged in a two-dimensional lattice, similar to G. However, GQDs differ in that they possess finite dimensions and often have irregular edges or a non-hexagonal structure, resulting in quantum confinement effects [46]. Significant progress has been made in synthesizing and controlling the size and structure of GQDs [47]. Various methods, such as chemical oxidation, laser ablation, and hydrothermal/solvothermal processes, have been developed to precisely control the dimensions of GQDs. Techniques like size-selective precipitation, size sorting, and template-assisted synthesis have facilitated the production of GQDs with uniform size distributions and customized structures [48].
GQDs exhibit fascinating optical properties, making them highly appealing for a wide range of applications [49]. They have size-dependent optical absorption and emission phenomena, commonly known as quantum confinement. As the size of GQDs decreases, their bandgap increases, allowing for tunable absorption and emission wavelengths across the ultraviolet (UV), visible, and near-infrared (NIR) regions [50]. This tunability proves advantageous for optoelectronic applications such as photodetection, imaging, and sensing [51]. Extensive research has been conducted on the tunable optical properties of GQDs. Researchers have demonstrated the size-dependent absorption and emission spectra of GQDs across various wavelengths. By precisely controlling synthesis parameters, such as reaction temperature and time, it becomes possible to obtain GQDs with desired absorption and emission characteristics [52]. Furthermore, surface functionalization and doping techniques have been employed to further customize the optical properties of GQDs [53].
GQDs exhibit strong and stable photoluminescence, which refers to the emission of light upon excitation by a light source. The fluorescence emission of GQDs can be adjusted by modifying their size, surface functionalization, or surrounding environment [54]. Numerous efforts have been dedicated to enhancing the fluorescence properties of GQDs. Surface passivation, chemical modification, and ligand exchange techniques have been explored to improve the QY, photostability, and emission color of GQDs [55]. Moreover, doped-GQDs can be integrated into materials to achieve tunable photoluminescence. For instance, a recent study conducted by Kumar et al. demonstrated the incorporation of sulfur-doped GQDs for achieving tunable photoluminescence in quasi-2D CH3NH3PbBr3 [56]. Furthermore, strategies such as bandgap engineering and energy transfer mechanisms have been employed to enhance the emission efficiency of GQDs, enabling their applications in bioimaging and optoelectronics [57,58].
GQDs possess unique electronic properties due to the quantum confinement effects. As the size of GQDs decreases, the energy levels become discrete due to the confinement of the charge carriers. This leads to a size-dependent bandgap, and GQDs can exhibit either direct or indirect bandgap characteristics. The tunable bandgap of GQDs makes them suitable for electronic devices, including field-effect transistors, light-emitting diodes, and solar cells [59,60]. Extensive research has been conducted on the electronic properties of GQDs. The size-dependent bandgap of GQDs has been characterized using various spectroscopic and electrochemical techniques. Techniques such as doping and edge functionalization have been employed to modify the electronic structure of GQDs, allowing for control over their conductivity, carrier mobility, and band alignment. GQDs have been successfully integrated into electronic devices, demonstrating their potential for advanced electronic applications [61,62,63,64].
The surface of GQDs can be easily functionalized by introducing various functional groups or doping them with heteroatoms [65]. Functionalization enhances the dispersibility, stability, and compatibility of GQDs in different solvents or matrices. It also provides a means to tailor the properties and interactions of GQDs with other materials, facilitating their integration into composite structures and enabling applications in energy storage, catalysis, and biomedicine [58]. Surface functionalization plays a crucial role in improving the dispersibility, stability, and compatibility of GQDs in diverse environments. Various functionalization methods, including covalent functionalization, noncovalent interactions, and surface modification with polymers or biomolecules, have been developed. These approaches have enabled the introduction of desired functional groups, tailoring the surface charge, and enhancement of interactions with target materials, thus expanding the application potential of GQDs [66,67].
GQDs exhibit excellent biocompatibility, low cytotoxicity, and minimal long-term toxicity, making them highly suitable for biomedical applications [68]. Extensive research has been conducted on their use in bioimaging, drug delivery, biosensing, and photothermal therapy, leveraging their unique optical properties and biocompatibility. GQDs can be functionalized with targeting ligands or encapsulated within biocompatible matrices, enabling selective targeting and controlled release of therapeutics [69]. GQDs have demonstrated good biocompatibility and have been extensively studied for biomedical applications, as reported by Kalkal et al. By integrating the quantum confinement and edge effects of carbon dots with the G structure, GQDs have emerged as a remarkable material with remarkable biocompatibility [70]. Research efforts have focused on optimizing synthesis methods to produce biocompatible GQDs with low cytotoxicity. Surface functionalization with biocompatible polymers or targeting ligands has enabled selective targeting, cellular uptake, and controlled release of GQDs for drug delivery applications. Moreover, GQDs have found applications in bioimaging, biosensing, and photothermal therapy, showcasing their potential in various biomedical fields [71,72].
GQDs exhibit high electrical conductivity, thermal conductivity, and charge carrier mobility, making them promising candidates for electronic and thermal management applications [73]. Their exceptional electrical conductivity arises from the intrinsic G-like structure and the high crystallinity of GQDs. Additionally, GQDs can be integrated into polymer composites or used as conductive ink for printed electronics, enabling the development of flexible and wearable electronic devices [49]. Techniques such as chemical doping, surface functionalization, and heteroatom incorporation have been employed to enhance the electrical conductivity of GQDs for improved biomedical applications. In a study conducted by Chatterjee et al., they demonstrated the use of fluorescent Boron and Sulfur co-doped GQDs for efficient dopamine detection, thus opening up the possibility of designing a low-cost biosensor [30]. Integration of GQDs into conductive matrices, such as polymers or G-based materials, has facilitated their utilization in flexible and printable electronics. Moreover, GQDs have shown promise as fillers in composite materials to enhance thermal conductivity and mechanical strength [74].
GQDs possess remarkable mechanical properties, including high strength and flexibility. These properties stem from their sp2 carbon framework and the absence of defects or impurities. However, research progress specifically focused on their mechanical properties is relatively limited compared to other aspects. Nonetheless, GQDs can be integrated into composite materials to enhance their mechanical strength or used as reinforcing agents in polymers [75]. For instance, the incorporation of GQDs into polymers or carbon-based matrices has demonstrated improved mechanical properties, opening opportunities for applications in flexible electronics and structural materials. Additionally, GQDs have been explored for use in energy storage devices, such as supercapacitors, due to their mechanical robustness [76,77].
Finally, GQDs exhibit a diverse range of properties, including size-dependent optical and electronic characteristics, excellent photoluminescence, biocompatibility, high electrical and thermal conductivity, and remarkable mechanical strength. These properties make GQDs as promising candidates for various applications, including optoelectronics, biomedicine, energy storage, and electronics [78].
3 Synthesis methods
Synthesis of G is referred to as any procedure for producing or extracting graphene based on the required size, purity, and efflorescence of the result [79]. Various synthesis processes can yield G materials with varying surface area sizes and number of layers, as well as introduce defects that impact the chemical and physical properties of graphene. These factors ultimately influence the suitability of G for applications in the biomedical field [80,81,82,83]. Furthermore, the impact of functionalizing G [84] and GQDs [85], as well as doping them with other materials [86], is significant in terms of influencing the characteristics of these materials. Another synthesis approach of G provides variable numbers of wrinkles in the G surface, which are correlated with the chemical flexibility of G materials [87,88]. The increased chemical flexibility exhibited by G enables a broader spectrum of analyte components to be efficiently attached onto its surface. Furthermore, the increased surface area of G material increases its susceptibility to chemical and biological agents, making it an important component in the use of biosensors. Thus, it is critical to understand G and GQDs synthesis because it will provide insight into its applicability in biomedical disciplines.
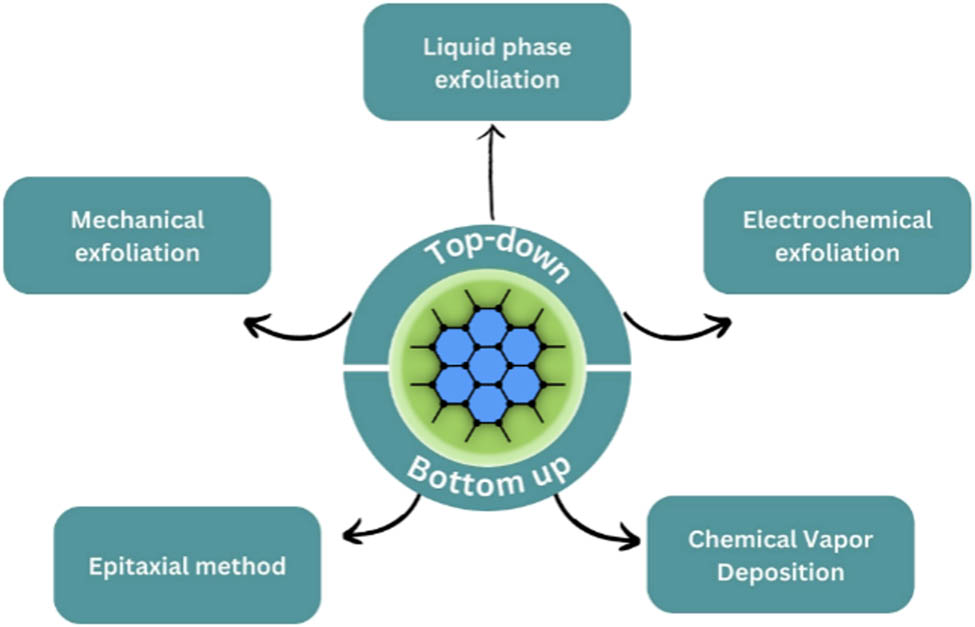
Various techniques have been exploited to synthesize G and GQDs resulting in top-down and bottom-up routes. The top-down approach focuses on separating G precursor (graphite) layers or exfoliating the bulk graphite material to produce G. Whereas the bottom-up approach focuses on implementing carbon molecules from alternative sources as building blocks, also it is described as small molecular growth from carbon precursors. On that note, synthesis methods for G and GQDs could be categorized as shown in Figures 2 and 3. These synthesis methods mentioned in the figures will be discussed in this section.
Graphene-based nanocomposites synthesis flowchart.
GQDs-based nanocomposites synthesis.
3.1 Synthesis methods of G
Numerous chemical synthesis routes have been developed by researchers to synthesize G. Chemical vapor deposition (CVD) is one example of chemical synthesis methods involving precise control over synthesis parameters: temperature, pressure, deposition time, and precursor type. Despite its complexity, it remains an appealing method for producing high-quality G. Other methods based on physical routes, such as mechanical exfoliation, pyrolysis, drop-casting, and high-current arc evaporation, are also developed a lot. The approach to synthesizing G and GO through chemical and physical synthesis has shown great prospects depending on the availability of simple industrial-scale synthesis. These considerations make the approaches on using the green synthesis of graphene and GO an interesting topic due to its continuous development in environmental applications. For G and G-based materials to have specific properties, their synthesis must be carefully controlled. As is well known, G can be synthesized in two ways, which are bottom-up and top-down. Bottom-up methods involve the synthesis of G from alternative carbon sources, whereas top-down methods involve separating stacked graphite layers to produce single GSs.
3.1.1 Top-down synthesis
3.1.1.1 Mechanical exfoliation
In 2004, a team of scientists, headed by Geim and Novoselov, published a study detailing a mechanical exfoliation method for the synthesis of single-layer G [89]. Graphene layers were produced through the process of mechanical exfoliation of HOPG with adhesive tape. The initial step involved the preparation of graphite on particles through the utilization of dry etching in an oxygen plasma environment. Subsequently, the prepared graphite was pressed onto a layer of photoresist that had been applied onto a glass substrate. The graphite mesas become affixed to the photoresist layer upon the application of heat. The adhesive tape was attached onto the graphite surface and subsequently removed, therefore causing the separation of the G flakes from the mesas. Following that, the G flakes were dissolved in acetone and then transferred onto a clean SiO2/Si substrate, resulting in the generation of flakes exhibiting diverse dimensions in terms of both size and thickness [90]. This technique is commonly known as the Scotch tape or peel-off technique. Despite the ability of this approach to generate G monolayers of high-quality efficiently and affordably, its reproducible results and yield are significantly low. Furthermore, the size of G exhibits non-uniformity. Ball milling is another method employed for the mechanical exfoliation of G layers from bulk graphite. The milling process involves the participation of both normal and lateral forces in the exfoliation of GSs. However, the crystalline structure of GSs may be compromised during the milling process [91].
3.1.1.2 Liquid-phase exfoliation
Another commonly used method for producing G is liquid phase exfoliation, which involves three steps: dispersion in a solvent or surfactant, exfoliation, and purification to separate the exfoliated material from the non-exfoliated and, if supplied as a powder, completely remove any solvent traces [92]. Sonication duration is critical since longer sonication times can generate greater G concentrations at the trade-off of increased energy consumption. Following the sonication process, the material comprises thicker flakes that should then be extracted by ultracentrifugation. Higher centrifugation rates produce thinner flakes with tiny lateral sizes, which are unsuitable for applications such as composites. For G dispersion, a range of liquids, including aqueous surfactants, can be utilized. The yield of single-layer G percentage, defined as the ratio of the number of single-layer flakes to the total number of graphitic flakes in the dispersion, estimates this technique’s output [93].
Paton et al. demonstrated that large shear forces, rather than ultrasonic cavitation, may be utilized to exfoliate G on a 100 L scale. The essential shear rate for G exfoliation was discovered to be 104 s−1, which is achievable even with standard kitchen blenders. Following centrifugation, the average number of layers was fewer than 10, with typical lateral diameters of the nanosheets ranging from 300 to 800 nm. However, it should be emphasized that the yield obtained was rather low, and the choice of starting material and rotor optimization might significantly impact exfoliation efficiency [94]. Furthermore, Dimiev et al. prepared graphene nanoplatelets (GNPs) at room temperature for 3–4 h, and the conversion yield from graphite to GNPs was nearly 100%. Due to current industry expertise and equipment, liquid exfoliation may be the most practical approach for upscaling G synthesis [95].
3.1.1.3 Electrochemical exfoliation
The specific technique involves employing a liquid solution (electrolyte) and an electrical current to consume a graphite electrode. The graphite-based electrode undergoes anodic oxidation or cathodic reaction during this procedure. Cathodic reaction techniques are better suited for producing high-quality, few-layer conductive G for energy and optical applications [96]. Furthermore, anodic oxidation has received greater attention in the scientific literature. In contrast to pure monolayer G, the resultant anodic substance is composed of many G layers, has a limited yield, and mimics GO in an oxidation state [97]. Figure 4 shows a schematic illustration of (a) the electrochemical exfoliation of graphite and (b) the mechanism of electrochemical exfoliation. Figure 4a shows natural graphite flakes and platinum wires partially immersed in H2SO4 while the other end is connected to a 10 V voltage source. The bias voltage applied results in water oxidation and produces hydroxyl (OH˙) and oxygen radicals (O˙), which trigger oxidation or hydroxylation of the graphite electrode (step 1), as shown in Figure 4b. The graphite electrode defective sites caused by oxidation facilitate intercalation by anionic
![Figure 4
Schematic illustration of (a) electrochemical exfoliation of graphite and (b) mechanism of electrochemical exfoliation. Reproduced with permission from Ref. [98].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_004.jpg)
Schematic illustration of (a) electrochemical exfoliation of graphite and (b) mechanism of electrochemical exfoliation. Reproduced with permission from Ref. [98].
The benefit of electrochemical exfoliation over other methods is that it occurs in a single step, making it easier to run, and it occurs within minutes/hours, as opposed to most procedures, which need longer timeframes for preparation and stability of the final material. The lateral size of the flakes produced in nanocomposites is an essential characteristic that depends on the graphite supply and the intercalation-exfoliation process parameters. Intercalation products with nonoxidative salts can have lateral dimensions of 50 μm and a layer thickness of 2–3 layers [99].
3.1.2 Bottom-up synthesis
3.1.2.1 Epitaxial method
The epitaxial method yields epitaxial G, and the size of G flakes is determined by the size of wafers, e.g., SiC. According to studies, the surface of SiC influences the thickness, mobility, and carrier density of G generated in this system [100]. Unlike exfoliated G, G generated by this procedure has mild anti-localization. SiC-epitaxial G, on the other hand, has extremely large, temperature-independent mobility, similar to G generated by drawing or peeling off, but not as high as exfoliated G. Graphene may be epitaxially grown on SiC substrates, making it excellent for usage in transistors and circuits due to the thin GSs obtained (>50 m). Graphene is produced using this approach by heating a silicon carbide (SiC) to 1,100°C [101].
The weak van der Waals forces responsible for multilayer cohesion in multi-layered epitaxial G do not always affect the electrical characteristics of individual sheets within a stack. This effect is connected to interlayer interaction symmetry [102]. In other circumstances, such as bulk graphite, this behavior does not occur, and electrical characteristics are altered. A 2 in SiC wafer may provide cut-off frequencies of up to 100 GHz [103]. This technique produces high-quality G at a high cost due to the high cost of the SiC substrate and the limited yield produced. As a result, this approach is unsuitable for industrial manufacturing.
3.1.2.2 CVD
CVD is the chemical process of depositing material as a thin layer onto surfaces from vapor species, a popular bottom-up method for producing multi-layer and single-layer G films. Many complicated elements influence the process and types of chemical reactions in a CVD reactor, including system setup, reactor layout, gas feedstock, gas ratios, reactor and partial gas pressures, reaction temperature, growth time, and temperature. A schematic diagram of the CVD reaction for G from methane and hydrogen is shown in Figure 5 [104]. The CVD reaction begins with the reactants’ convective transport in a gas stream (step 1), followed by their thermal activation (step 2). The reactants are then transported by gaseous diffusion from the main gas stream through the stationary boundary layer (step 3). The reactants are adsorbed on the substrate surface (step 4) and diffused into the substrate bulk (step 5), depending on the carbon’s solubility and the substrate's physical properties. Reactive species catalytic decomposition occurs in addition to surface migration to the attachment site and other heterogeneous reactions (step 6). After the film growth, by-products are desorbed from the substrate (step 7). The by-products are subsequently diffused through the boundary layer into the main gas stream (step 8) to be carried by convection forces to the exhaust system (step 9).
![Figure 5
Schematic diagram of the CVD reaction for G from methane and hydrogen. Reproduced with permission from Ref. [104].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_005.jpg)
Schematic diagram of the CVD reaction for G from methane and hydrogen. Reproduced with permission from Ref. [104].
Thermal CVD on metals (such as Ru, Ir, Pt, Co, Pd, and Re) was initially used to create highly crystalline graphite films on Nickel (Ni) substrates in 1966 [104]. Ni and copper (Cu) are less expensive, have greater control over G layers, and are easier to transfer G. As a result, they are commonly employed as CVD substrates. The CVD development of G was accomplished using cold-wall and hot-wall reaction chambers [97]. The development of G in this approach is quick, has excellent quality, and requires little power. There is also an improvement in charge carrier mobility. (Table 1).
Synthesis methods of graphene
| Type of synthesis | Method | Process | Advantage | Ref. |
|---|---|---|---|---|
| Top-down | Liquid phase exfoliation | The method consists of three steps: dispersion in a solvent or surfactant, exfoliation, and purification. | Scalable process; suitable for large-scale production. | [92,105] |
| Electrochemical exfoliation | The method employs an electrolyte and an electric current to consume graphite electrodes. The graphite electrode undergoes anodic oxidation or cathodic reaction during the process. | Straightforward process (only takes one step of synthesis). | [96,106] | |
| Bottom-up | Epitaxial method | Graphene is grown epitaxially on a silicon carbide (SiC) substrate, by heating (thermal decomposition). | Produce high quality G; suitable for electronic application. | [103,107] |
| CVD | The CVD technique involves exposing the substrate to a volatile precursor, then chemically reacting and decomposing the precursor on the surface of the substrate to form G coatings. | Produce high quality of G layers; potential to be upscaled. | [104] |
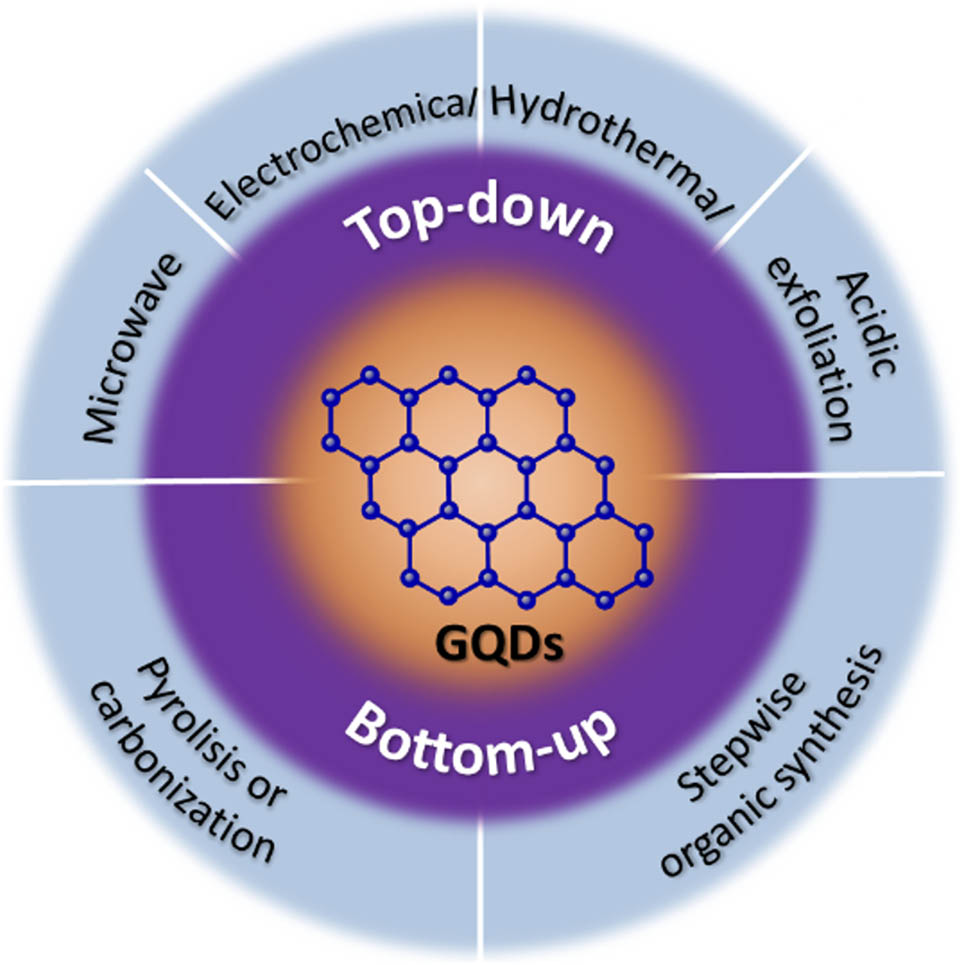
3.2 Synthesis method of GQDs
GQDs can be synthesized top-down or bottom-up, with varying structures and characteristics. The top-down approach is obtained by cutting the carbons into small-sized GQDs using chemical or physical procedures such as oxidative cleavage, hydrothermal methods, electrochemical oxidation, acidic exfoliation and oxidation, and microwave-assisted processes that use carbon materials, including fullerenes, GO, carbon fibers, carbon black, or graphite into small-sized GQDs [108,109]. Alternatively, GQDs could be synthesized from small organic compounds using pyrolysis, carbonization, stepwise organic synthesis, and cage-opening of C60. Various approaches are shown to analyze their properties and effects on the GQD [110].
3.2.1 Top-down synthesis
3.2.1.1 Hydrothermal method
Hydrothermal synthesis is a widely utilized and facile technique for synthesizing GQDs. Additionally, it also affects the structure and particle size distribution of GQDs. There are several techniques used in the synthesis process. In 2010, Pan et al. published the first report on a hydrothermal method for cutting GSs into surface-functionalized GQDs [28]. The GSs were first oxidized in concentrated H2SO4 and HNO3. However, treating GO with acidic oxidizing agents (e.g., H2SO4 and HNO3) introduced numerous strong oxidizing acids in a comprehensive operation and was time-consuming, requiring 10–24 h [111]. In addition, it is challenging for rGO oxidation while making GQDs with specified optical properties. Because of this, Yang et al. described an easier and more efficient way to make GQDs from rGO utilizing an ozonation pre-oxide technique [112]. Ozone system pH can be modified to influence GQD fluorescence. Ozone exposure changed the emission peaks of the GQDs. Comparatively, ozonation processing is easy to manage, efficient, and low-cost [112]. Another technique reported by Tetsuka et al. is an amino-hydrothermal method to synthesize amino-functionalized GQDs with distinct molecular weights and edges [113]. The amino-hydrothermal method [111] allows tuning the PL of GQDs by altering the hydrothermal treatment temperature and initial ammonia percentage.
Figure 6 shows the process of crystalline GQDs from paddy straw waste using hydrothermal method. The process started by grinding the paddy straw into powder. The powder was then cleaned with 0.15 M HCl and heated at 50°C for 3 h. 100 mg cleaned powder was then dissolved in 25 mL of water and kept in an autoclave hydrothermal at a temperature of 160°C for 6 h. After the hydrothermal finished, the solution was then filtered and centrifugated to obtain GQDs [114].
![Figure 6
Synthesis process of crystalline GQDs from paddy straw waste using hydrothermal method. Reproduced with permission from Ref. [115].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_006.jpg)
Synthesis process of crystalline GQDs from paddy straw waste using hydrothermal method. Reproduced with permission from Ref. [115].
3.2.1.2 Acidic exfoliation and oxidation
Acid exfoliation and oxidation of carbon sources were early GQD preparation processes. These researchers improved the previous method by adding fluorescence to the reduction of the graphite oxide (hydrazine hydrate) [116]. In the previous experiment, GO was oxidized by HNO3 for 24 h to cut into small GO sheets [110]. However, the large tracts of GO had to be removed, which required an ultrasonic cell crusher. Moreover, GO is commonly generated by oxidizing bulk graphite particles over several days using a large number of chemical reagents. In another case, a facile one-step method uses three different types of coal: anthracite, bituminous coal, and coke to synthesize GQDs. According to Dong et al., an efficient and less expensive method for producing GQDs uses a new facile method by chemically oxidizing a commonly used carbon source, CX-72 carbon black [117].
3.2.1.3 Microwave-assisted synthesis
The utilization of microwave-assisted technologies to produce G materials is gaining prominence. Nguyen et al. announced complete breakthroughs in refining the production procedure of GQDs and nitrogen-doped GQDs (N-GQDs) from citric acid (CA) and urea [118]. Microwaves speed up GQD production. It was discovered using Raman scattering spectra of the typical C–C G vibration mode (G-peak), and GQD defects detected these GQDs (D-peak) [118]. Other reports use highly acidic or alkaline conditions, sonication, or thermal reactions to create GQD. However, these operations take a long time to complete [111]. Luo et al. reported a simple microwave-assisted two-color GQD synthesis technique using a two-step hydrothermal technique in acidic conditions [119]. Surprisingly, they created white-light-emitting graphene quantum dots (WGQDs) by exfoliating oxidized graphite with ultrasonication and microwave irradiation. The collected WGQDs had a consistent size of 2–5 nm and a 1.25–2.75 nm thickness during microwave irradiation. As a result, the microwave-assisted approach may minimize reaction time and boost product yield, but it requires specialized equipment.
Figure 7 shows the procedures to synthesis GQDs from spent tea using a microwave-assisted technique. The process started with pyrolysis of the spent tea to produce carbon-rich precursor. The carbon-rich precursor was then converted into GQDs via microwave-assisted technique. The study was carried out at a microwave power range of 100–900 W with 15–180 min duration. It is found from the experiment, the optimum condition of power and duration for the synthesis of high quality GQDs with excellent optical properties derived from spent tea is 500 W and 120 min [118].
![Figure 7
Illustration of the synthesis procedure for GQDs derived from spent tea using microwave assisted treatment. Reproduced with permission from Ref. [118].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_007.jpg)
Illustration of the synthesis procedure for GQDs derived from spent tea using microwave assisted treatment. Reproduced with permission from Ref. [118].
3.2.1.4 Electrochemical oxidation
The electrochemical method may be worth considering for sizing GQDs. It is possible to obtain GQDs by electrochemical selective oxidation and reduction. Shinde and Pillai synthesized GQDs electrochemically from MWCNTs [120]. However, because of the high temperature and prolonged oxidation duration, the overall reaction time is relatively long [120,121]. Electrochemical synthesis at room temperature for a short time may be a viable alternative by using big electrodes for facilitating bulk synthesis. Figure 8a shows hydroxyl and oxygen radicals attacking graphite edge planes during exfoliation [122]. Li et al. used an electrochemical approach to create functional GQDs with green fluorescence that are stable in water for months [111]. Zhang et al. demonstrated a simple electrochemical approach for manufacturing GQDs with a 14% QY [123]. As illustrated in Figure 8b, Ananthanarayanan et al. reported a simple electrochemical approach to exfoliate GQDs from 3D G [124].
![Figure 8
Schematic representation of the preparation route for green-luminescent GQDs and blue-luminescent GQDs. (a) Exfoliation process showing the attack on the graphite edge planes by hydroxyl and oxygen radicals, and intercalation of BF4 anion [122]. (b) Schematic illustration of GQD synthesis from 3D G [124]. Adapted with permission from Refs [122 and 124].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_008.jpg)
Schematic representation of the preparation route for green-luminescent GQDs and blue-luminescent GQDs. (a) Exfoliation process showing the attack on the graphite edge planes by hydroxyl and oxygen radicals, and intercalation of BF4 anion [122]. (b) Schematic illustration of GQD synthesis from 3D G [124]. Adapted with permission from Refs [122 and 124].
3.2.2 Bottom-up methods
3.2.2.1 Pyrolysis or carbonization
The bottom-up approaches involve complex reaction processes, and specialized organic ingredients make optimizing it challenging. GQDs can be produced by pyrolysis or the carbonization of organic precursors. Precursors used in pyrolysis or carbonization include CA, l-glutamic acid, 1,3,5-triamino-2,4,6-trinitrobenzene, and glucose [125]. To overcome this, Dong et al. produced blue fluorescent GQDs and GO from CA, carbonized to varying degrees [126]. The GQDs were made by pyrolyzing CA. Figure 9a shows that Li et al. used TATB as the only precursor that underwent a single-layered intermolecular carbonization process [127]. Figure 9b depicts a bottom-up synthesis of massive GQDs with 132 carbon atoms from 3-iodo-4-bromoaniline [128].
![Figure 9
(a) Illustration of the proposed formation mechanism of N-GQDs from single-layered TATB intermolecular condensation (reproduced with permission from Ref. [51], Copyright 2016, Springer) [127]. (b) Bottom-up synthesis of large GQDs containing 132 carbon atoms from 3-iodo-4-bromoaniline via stepwise organic chemistry [128].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_009.jpg)
(a) Illustration of the proposed formation mechanism of N-GQDs from single-layered TATB intermolecular condensation (reproduced with permission from Ref. [51], Copyright 2016, Springer) [127]. (b) Bottom-up synthesis of large GQDs containing 132 carbon atoms from 3-iodo-4-bromoaniline via stepwise organic chemistry [128].
3.2.3 Electron beam irradiation (EBI) method
The EBI approach is not frequently utilized since it needs expensive expert equipment and exposes users to radiation [129]. Wang et al. reported that single-crystal fluorescent GQDs were produced at ambient temperature [130]. 1,3,6-trinitropyrene was dissolved in a solution of hydrazine hydrate to produce the desired results. The mixture was sealed in a plastic bag after being stirred and exposed to ionizing radiation through a titanium window in a dynamitron electron accelerator. To get GQDs with 32% QY, the sample was dialyzed for 2 days using a 0.22 mm microporous membrane filter and a dialysis bag. Precursors for GQD synthesis include 1-Nitropyrene, urea, and CA [129]. Possible reaction formation mechanism pathway of GQDs in hydrazine hydrate solution from 1,3,6-trinitropyrene molecules is shown in Figure 10. (Table 2).
![Figure 10
Formation mechanism of GQDs (reproduced with permission from Ref. [130]).](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_010.jpg)
Formation mechanism of GQDs (reproduced with permission from Ref. [130]).
Synthesis methods of GQDs
| Type of synthesis | Method | Process | Advantage | Ref. |
|---|---|---|---|---|
| Top-down | Hydrothermal | This approach employs an aqueous solution as the reaction system in a closed reaction vessel, such as a Teflon-lined autoclave | Simple and inexpensive | [111,131] |
| — | Acid exfoliation and oxidation | Strong acid such as HNO3 is used for this method to exfoliate GQDs from carbon-based materials | Suitable for large scale production | [111,132] |
| — | Microwave-assisted synthesis | This method uses microwave irradiation to escalate the reaction | Short reaction time; uniform GQDs size distribution | [118,133] |
| — | Electrochemical oxidation | An electric potential is given to the precursor to push charged ions to the graphitic layers of carbon material and break carbon–carbon bonds to form GQDs | Produce uniform sized GQDs; tunable properties of GQDs | [122,134] |
| Bottom-up | Pyrolysis or carbonization | The carbon-containing materials is carbonized under high temperature and often under non-oxidizing atmosphere | Inexpensive; short reaction time; high yield; suitable for large production | [127,135] |
| — | EBI method | This method uses high-energy electrons to break the carbon–carbon bond in the graphitic layers in the carbon materials | Produce uniform sized GQDs | [136] |
4 Applications of G and GQDs
4.1 Graphene for biomedical applications
4.1.1 Biosensing application
Sensors comprise a receptor and a transducer (Figure 11). The receptor is the organic or inorganic substance that specifically interacts with the target molecule. Organic, inorganic, or even complete cells might be used as the target molecule. The transducer is the sensor component that turns chemical information into a quantifiable signal. Graphene-based nanomaterials (GBNs) are excellent biosensor transducers, translating the interactions between the receptor and the target molecules into observable readings [136].
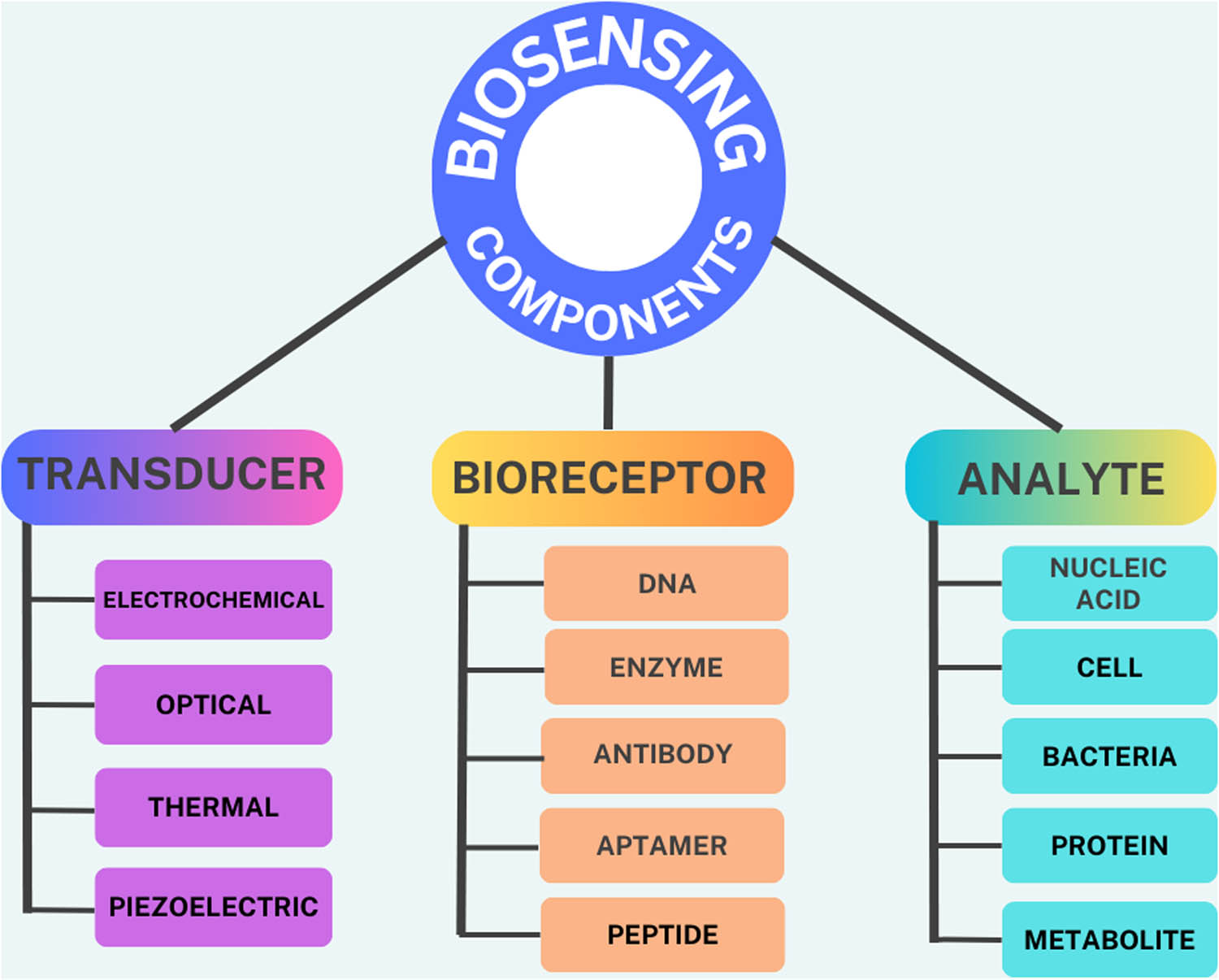
Biosensors and components on a biosensing platform.
The bioreceptor (molecules such as antibodies, ssDNA, and enzymes) must be linked to the transducer surface for this to happen. EDC/NHS/1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE) chemistry is the most often used attachment approach for antibodies and ssDNA immobilization onto G and its derivatives (GO, rGO). In contrast, physisorption is the most regularly used method for enzyme immobilization. Seo et al. succeeded in developing a COVID-19 field effect transistor (FET) sensor based on integration of SARS-CoV-2 spike antibody with G [137]. The developed platforms use PBASE for immobilization of the SARS-CoV-2 spike antibody and have a limit of detection (LOD) SARS-CoV-2 antigen protein of 1 fg/mL (Figure 12).
![Figure 12
Schematic diagram of COVID-19 FET sensor operation procedure based on integration of SARS-CoV-2 spike antibody with G. Reprinted with permission from [137].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_012.jpg)
Schematic diagram of COVID-19 FET sensor operation procedure based on integration of SARS-CoV-2 spike antibody with G. Reprinted with permission from [137].
Because of its huge surface area, electrical conductivity, rapid electron transfer rate, and ability to immobilize diverse compounds, G has been used in the creation of several biosensors of various transduction modalities [138]. In addition, G’s connected structure can enhance electron flow between the bioreceptor and transducer, resulting in high signal sensitivity for electrochemical sensors [139]. Additionally, GBNs can operate as a quencher in the transducer to produce fluorescent biosensors. G, GO, and rGO have been shown in studies to have very high efficiency in fluorescent quenching [140].
Li et al. developed a dual-channel biosensor based on fluorescence and surface-enhanced Raman spectroscopy [141]. Upconversion G was combined with Au and Ag nanoparticles resulting in Au@Ag-G upconversion. These nanohybrids were then conjugated to complementary DNA and immobilized into polymethacrylic acid magnetite-magnetic colloidal nanocrystal clusters which were previously conjugated with aptamers. The fluorescence of the sample solution was quenched if no Hg2+ was detected. The detection limit of this dual sensor is also sufficient to be used as a qualified sensor which is 0.33 and 1 ppb [141]. Wong et al. functionalized rGO with folic acid through covalent interaction [142]. This FA-rGO is then mixed with bovine serum albumin-templated AuNCs (BSA/AuNCs) so that it can be used as a fluorescence biosensor for glutathione. Fluorescence quenching was generated by the strong connection between glutathione and FA-rGO-BSA/AuNCs, which was primarily mediated by van der Waals interaction and hydrogen bonding. Glutathione also has a tendency to bind with Au nuclei and BSA/AuNCs ligands. These interactions can cause Au cluster aggregation and alterations in electron transport, resulting in fluorescence interference. This is where FA-rGO comes in to assist in the stabilization of BSA/AuNCs [142]. In addition to glutathione sensors, rGO has been claimed to have the potential to be employed as a virus sensor for Ebola [143], SARS-CoV-2 [143], and Hepatitis C [144].
4.1.2 Tissue engineering (implant)
Due to its ability to react with other biomolecules such as DNA, enzymes, proteins, and peptides for regenerative medicine, G nanomaterial is now widely used in the medical and tissue engineering fields. Graphene and its by-products have recently gained new interest in the development and application of biocompatible systems. Nayak et al. investigated the effect of G on stem cell growth and discovered that G-based films do not inhibit the proliferation of human mesenchymal stem cells (hMSCs). Instead, it controls their specific differentiation into bone cells by using growth factors and osteogenic inducers, implying the potential use of stem cells for proliferation and transplantation and their specific differentiation into muscles, bones, and cartilage for bone regeneration therapy [154]. A further recent study discovered that G could improve the biological characteristics used in bone regeneration therapy. They concluded that G nanocomposites could pave the way to construct scaffolds for specific organ/tissue targets [155].
Mohammadi et al. used the electrospinning method and successfully fabricated uniform and bead-free GO-reinforced polycaprolactone (GO-PCL) fibrous scaffolds [156]. The total porosity of the samples was greater than 95% during the porosity measurement test, which appears to be the ideal solution for tissue regeneration and fibers that adsorb more protein while incorporating GO. Cell-surface attachment and cell spreading patterns were also evaluated, and it shows that cells cultivated on GO-PCL fibrous scaffolds reached higher confluency and spread out over larger areas, indicating that GO-PCL provides excellent cell attachment. Furthermore, low-magnification micrographs revealed that GO-PCL (2%) had the highest cell viability and proliferation rate, implying that GO nanosheets (GONS) in fibers may enhance cell attachment and growth. It concluded that adding GONS to MG-63 cells significantly improved the adhesion and proliferation. Compared to bulk PCL, GO-PCL biocomposites increased physicomechanical properties and significantly enhanced biological features in bone tissue engineering via electrospun fibers [157].
Also, regarding in vitro cell study, Kalbacova et al. found that G substrate created by CVD is biocompatible with human osteoblasts and hMSCs, with higher cell proliferation than SiO2 substrate and stimulates cell growth and differentiation [158]. In a further study by Li et al., CVD-grown G film was examined as a substrate for neurites, the essential structures for neural functions during development in a mouse hippocampal culture model. The average length of neurites was substantially increased on G film than on tissue culture polystyrene (TCPS) during the first 2–7 days following cell seeding. GAP-43 expression was also much higher in the G group compared to the TCPS group, most likely due to an increase in neurite sprouting and outgrowth, suggesting that pristine G could be used as a novel material for neural interfacing [159]. In another study, Ahmed et al. reported that GO was applied to nanofiber scaffolds made of cellulose acetate and polyvinyl alcohol (PVA) that had been modified with Fe3O4 nanoparticles for use in wound healing. The result showed that the incorporation of GO induced a significant variation in cell growth, where cells seem to spread over the GO surfaces. In addition, GO also contributed to the mechanical stability of nanofiber [160].
4.1.3 Drug delivery
Nanomaterial-based drug delivery systems (DDS) have been widely researched for cancer treatment during the last decade, aiming to improve therapeutic efficacy while reducing hazardous side effects. Many organizations have begun to investigate G-based medication delivery methods since 2008. The surface area of G (2,600 m2 g−1 ) is higher, which makes researchers to explore them for drug delivery [168]. A G monolayer, in essence, offers an extreme scenario in which every atom is exposed on the surface, allowing for a substantially larger drug-loading capacity. Chemical modification by electrostatic contact and binding to the aromatic molecule via p–p stacking interaction are the two most prevalent alterations described in the literature for drug delivery utilizing GBNs. Another advantage of drug delivery through GBNs is adjusting the release rate for long-term medication release [169].
Figure 13 shows the schematic design of the cellular protease-mediated G-based co-delivery system. The main component of the nanocomposites is composed of DOX-loaded GO, polyethylene glycol (PEG) linker, and TRAIL-conjugated furin-cleavable peptide as can be seen in Figure 13a. The delivery process of TRAIL/DOX-fGO, from vessel administration to drug release in the cell nucleus is shown in Figure 13b. (i) The process started by intravenous administration of GO, (ii) accumulation of GO at the tumor site through passive and active targeting effects, (iii) TRAIL binding on the death receptor and degradation of peptide linker by furin on the cell membrane, (iv) activation of caspase-mediated apoptosis, (v) induction of cell death, (vi) endocytosis of GO by the tumor cells, (vii) acid-promoted DOX release in endosome, (viii) accumulation of released DOX into nucleus, and (ix) induction of DNA damage-mediated apoptosis and cytotoxicity.
![Figure 13
Schematic design of the cellular protease-mediated G-based co-delivery system. (a) Main components of TRAIL/DOX-fGO, (b) site-specific delivery of TRAIL to cell membrane and DOX to nuclei for enhanced synergistic cancer treatment. Reproduced with permission from Ref. [153].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_013.jpg)
Schematic design of the cellular protease-mediated G-based co-delivery system. (a) Main components of TRAIL/DOX-fGO, (b) site-specific delivery of TRAIL to cell membrane and DOX to nuclei for enhanced synergistic cancer treatment. Reproduced with permission from Ref. [153].
4.1.4 Bioimaging
In bioimaging, GO is used in many ways, such as optical imaging. Non-invasive optical imaging combines visible light and the unique characteristics of photons to provide comprehensive pictures of organs and tissues, as well as tiny objects such as cells and molecules [179]. It offers several benefits over other imaging modalities, including low cost, high sensitivity (109–1012 mol/L), nonionizing radiation, real-time imaging, a rapid acquisition time, and multiplexing capacity. This modality, however, has low tissue penetration (0–2 cm), substantial photon scattering in the visible light area (395–600 nm), and considerable background due to tissue autofluorescence and light absorption by proteins (257–280 nm), heme groups (absorbance maximum at 560 nm), and even water (above 900 nm). To address these challenges, NIR window (NIR, 650–900 nm) and second NIR window (NIR-II, 1,000–1,700 nm) imaging modalities with reduced autofluorescence, lower tissue scattering, and better depth of penetration for in vivo imaging have been investigated [180]. The bioimaging application of nanocomposite consisting of GO was reported by Nunez et al. They covalently attached mono-iodinated boron-cluster derivatives into GO. In vitro cytotoxicity experiments with HeLa cells for up to 48 h revealed negligible cytotoxicity of the nanocomposite, as shown by cell mortality of less than 10%. Furthermore, in vivo testing indicates a similar outcome to in vitro tests, in which Caenorhabditis elegans was used to prove that nanocomposite could be ingested by the worms, with no substantial harm and very low toxicity [181].
4.1.5 Antibacterial and antiviral property
Graphene and its by-products were found to be a superior antibacterial agent against various bacteria because of their sharp edges and induction of oxidative stress. Akhavan and Ghaderi discovered that using GO and G to treat Escherichia coli and Staphylococcus aureus results in bacterial RNA efflux. The result shows that the bacterial cell membrane is harshly pierced by the direct sinking of sharp ends of nanosheets into the bacterial cell membrane. According to their study, some GO sheets are almost perpendicular to the surface of bacterial cells. These perpendicular sheets have incredibly sharp edges that damage bacterial cell membranes and cause RNA efflux into the solution [190].
The study by Liu et al. found that the antibacterial activities of GO and rGO are time and concentration dependent. A significant bacterial inactivation occurs during the first hour of incubation, and the cell death rate constantly increases as material concentration increases. The increase in GO concentration from 5 to 80 g/mL resulted in the loss of E. coli viability from
Liu et al. examined wound recovery and infection control using GO quaternary ammonium nanocomposites (GO-QAS). The GO-QAS nanocomposite demonstrates outstanding biocompatibility and synergistic antibacterial efficacy against multi-drug bacteria resistance to mechanical membrane disruption and oxidative stress induction. Specifically, GO-QAS has the potential effectiveness of re-epithelialization and enhanced granulation tissue creation in treating wound infections and injuries and improving recovery. GO-QAS nanocomposite might undeniably be manufactured as an antimicrobial agent for wound treatment and antibacterial wound dressing [192]. In a further study, Valentini et al. used functionalized G to research antibacterial and cytotoxicity factors. Functionalized G was used to create GO via the chemical and electrochemical
![Figure 14
Scanning electron microscopy of Escherichia coli cell treated (a) without nanocomposite and (b) with GO suspensions. Reproduced with permission from references [192].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_014.jpg)
Scanning electron microscopy of Escherichia coli cell treated (a) without nanocomposite and (b) with GO suspensions. Reproduced with permission from references [192].
Figure 15 shows the application of G and its derivatives as oral bacteria inhibitors, such as S. mutans (S.m), E. faecalis (E.f), P. gingivalis (P.g), A. actinomycetemcomitans (A.a), F. nucleatum (F.n), and P. intermedia (P.i). He et al. reported that the number of bacterial cells decreased in GO-exposed groups compared to the control group [194]. They found that GO nanosheets caused cell membrane and cell wall integrity loss. The intracellular densities of S.m, F.n, and P.g decreased when surrounded by GONS, indicating that they lost some intracellular substance. They also compared the activity of P.g, S.m, and F.n treated with different concentrations of GO and found that bacterial activity decreased with the increase in GO concentration.
![Figure 15
An illustration of the G-based materials as oral bacteria inhibitors. Graphene-based materials can inhibit the growth of cariogenic bacteria S.m, control dental pulp infection by effectively reducing the biovolumes of E.f, and suppress periodontal pathogens, such as P.g, A.a, F.n, and P.i. Reproduced from Ref. [194].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_015.jpg)
An illustration of the G-based materials as oral bacteria inhibitors. Graphene-based materials can inhibit the growth of cariogenic bacteria S.m, control dental pulp infection by effectively reducing the biovolumes of E.f, and suppress periodontal pathogens, such as P.g, A.a, F.n, and P.i. Reproduced from Ref. [194].
The antibacterial properties of GO and rGO sheets led researchers to suggest that these nanomaterials could have antiviral properties. According to a study by Ye et al., GO and rGO exhibit comparable antiviral activity, indicating that the oxygen-containing group is not essential for antiviral activity. At a noncytotoxic concentration (6 g/mL), they demonstrated that GO and rGO have broad-spectrum antiviral activity against both DNA viruses (PRV) and RNA viruses (Porcine epidemic diarrhea virus; PEDV). GO was found to have significant antiviral properties even at low concentrations (1.5 g/mL). It has been observed that GO can inactivate viruses even before they enter the cell due to physical disruption of the structure caused by direct contact with the sharp edge of the GO layers. The antiviral activity depended on concentration and incubation time and was effective against DNA and RNA viruses [195].
Furthermore, the antiviral activity of GO may also be attributed to the negative charge of GO, which promotes electrostatic interaction with positively charged viruses. The higher the interactions, the more the virus is destroyed and inactivated. Sametband et al. investigated the antiviral activity of GO layers and partially reduced sulfonated GO. The result showed that both the nanomaterials have a negative charge due to carbonyl and sulfonate surface groups and can block herpes simplex virus type 1 (HSV-1) infections [196]. Deokar et al. designed and synthesized sulfonated magnetic nanoparticles functionalized with rGO (SMRGO) to capture and photothermally kill HSV-1 [197]. The light absorbance of G can be utilized to eliminate virus particles once they have been captured. SMRGO were successfully used to capture and photothermally destroy HSV-1 using NIR light. These findings show that G composites could aid in treating viral diseases, including but not limited to HSV-1 [197].
The biomedical applications of G and its derivatives have enormous specific and unique characteristics. Through the compilation of Tables 3–7, we present a reader-friendly guide that highlights how G is potentially used in various biomedical fields. Each table offers a snapshot of G and its derivatives’ contributions, making it easy to explore their role in enhancing biomedical applications.
Graphene material for biosensor
| Precursor materials | Synthesis method of graphene materials | Sensor type | Target molecules | LOD | Ref. |
|---|---|---|---|---|---|
| GO-Ag-Fe3O4 | Electrochemical oxidation | Electrochemical impedance spectroscopy (EIS) | Ascorbic acid | 74 nM | [22] |
| NiO/GO | Oxidation of graphite using Hummer’s method | Cyclic voltammetry (CV) and differential pulse voltammetry (DPV) | Ascorbic acid, dopamine, and uric acid | 0.14, 0.10, and 5.50 μM | [145] |
| N-rGO | Oxidation of graphite using Hummer’s method | CV and DPV | Ascorbic acid, dopamine, and uric acid | 9.6, 0.1, and 0.2 μM | [146] |
| Graphene wall/Cu2O | CVD | EIS | Glucose | 0.21 μM | [147] |
| MoS2-G | CVD | Surface plasmon resonance) | Glucose | — | [148] |
| GO-Pep-FAM | Oxidation of graphite using Hummer’s method | Fluorescence detection | HIV-1 protease | 1.18 ng mL−1 | [149] |
| GO-Au nanostar | Oxidation of graphite using Hummer’s method | CV and EIS | SARS-CoV-2 | 0.18 × 10−19%V/V | [23] |
| rGO-Au NPs | Oxidation of graphite using Hummer’s method | CV, square wave voltammetry, and EIS | Hepatitis B | 0.0014 fg/mL | [150] |
| rGO | Chemical reduction | FET based immunoassay | Inactivated Ebola virus | 2.4 pg·mL−1 | [151] |
| GO-AuNPs | Oxidation of graphite | Surface-enhanced Raman scattering | DNA | 10 fM | [152] |
| Thionine functionalized rGO | Ultrasonication exfoliation | CV and EIS | DNA | 4.28 × 10−19 M | [153] |
Graphene-based scaffold for tissue engineering
| Materials | Preparation method | Type of tissue or cells | Improvement in mechanical and physical properties | Key result | Ref. |
|---|---|---|---|---|---|
| Pd/polypyrrole (PPy)/rGO nanocomposite | Oxidative polymerization | Bone tissue | rGO and PPy enhance mechanical properties of scaffolds | Pd/PPy/rGO has 91.90% of cell viability corresponding to 10 μg mL−1 of Pd/PPy/rGO NC for Saos-2 osteo cells | [161] |
| rGO/PPy/casein phosphopeptide (3D rGO/PPY/CPP) | Electrostatic layer by layer assembly | Bone tissue | Hydrophilic and good water uptake performance | rGO/PPY/CPP facilitated the accelerated development of hydroxyapatite (HA) in a solution with a strength of 1.5 times more than that of simulated body fluid in an in vitro setting | [162] |
| HA/GO/Chitosan (CS) ternary composite hydrogel | 3D molding | Bone tissue | Having compact microstructure and high mechanical strength | High porosity (84.37%) and large pores (average pore size of 122 m) provide benefits to osteoblast proliferation and differentiation | [163] |
| Ferric ion crosslinking-GO | 3D printing and freeze-drying | Liver tissue | Having controllable porous structure | Low cytotoxicity, good viability, and good attachment behaviour (the immobilized cells were approximately 3.06 × 106 cells/cm3 in the scaffold) | [164] |
| PCL/gelatin/G nanofibrous | Electrospinning | Nerve tissue | Hydrophilic; G has preserved the structural integrity of the scaffold | Higher hydrophilicity is suitable for cell adhesion, attachment, and proliferation; no cytotoxicity detected | [165] |
| Polyvinylidene fluoride (PVDF)/GO | Non-solvent induced phase separation | Nerve tissue | Adding 3 wt% GO to PVDF scaffolds enhanced the tensile modulus and strength | On the first day of culture, the GO-PVDF scaffold (3 wt% GO) exhibited significantly greater cell viability owing to its increased hydrophilicity. | [166] |
| GO aerogel/gelatin | Thermal-induced phase separation | Nerve tissue | Mechanical strength was increased after addition of GO aerogels (22.6 MPa) | In vitro analysis revealed an increase in metabolic activity, which resulted in the differentiation of P19 cells on the scaffold surface | [167] |
Graphene material for drug delivery
| Materials | Type of drugs | Experimental model for drug release | Key result | Ref. |
|---|---|---|---|---|
| CS/tripolyphosphate/GO hydrogel | Sumatriptan succinate | In vitro | The addition of GO increased the swelling degree (100–200%) and a decrease in drug release rates (20–45%) | [170] |
| Metronidazole (MTD)-Chi/GO bionanocomposite | MTD | In vitro | MTD-Chi/GO, particularly MCG12, demonstrated more effective drug release patterns than pure MTD drug (90.34% at pH 7.4 at 24h and 9.50% at pH 1.2 at 12 h) | [171] |
| Polyvinylpyrrolidone-functionalized GO (GO-PVP) | Quercetin and gefitinib | In vitro | GO-PVPs loaded with anticancer drugs showed higher cumulative release and cytotoxicity against PA-1 ovarian cancer cells | [172] |
| CS derivatives/rGO/alginate | Fluorescein sodium | In vitro | Quaternized carboxymethyl chitosan (QCMC)-rGO showed a optimum drug-loading rate of 82.8%. In vitro release rate of fluorescein sodium from QCMC-rGO/alginate showed ∼95% at pH 7.4 and 1.7 | [173] |
| GO with 3-aminopropyltrimethoxysilane | Doxorubicin | In vitro | After 30 min, the values for DOX release at pH 5.4 and 7.4 were 87.1 and 11.3%, respectively. At pH 5.4, DOX release was defined as 99.3–100.0% after 6.0–7.0 h, but at pH 7.4, DOX release was 94.3% after 72.0 h. | [174] |
| Gold nanorods/GO@polydopamine | Doxorubicin | In vitro | Drug release at pH 4.5 was 49.84% with laser irradiation of 4 W cm−2 for 12 h. DOX release amount in PBS at pH 7.4 was 9.51%. | [175] |
| Polyethylenimine-PEG-rGO (mBPEI-PEG-rGO) | Doxorubicin | In vitro and in vivo | In low pH, the drug loading was 81%, and the release was greater than 50%. mBPEI-PEG-rGO increased the cell uptake efficiency and cytotoxicity of DOX in cancer cells in both in vitro and in vivo tests | [176] |
| rGO encapsulated CS/alginate | Doxycycline | In vitro | At pH 7.4, 90% of DXC was released after 8 h. | [177] |
| GO@CoFe2O4@Ag | Ciprofloxacin | In vitro | Drug release efficiency of ciprofloxacin was higher at pH 4 with ∼50% release | [178] |
Graphene material for bioimaging
| Materials | Type of imaging technique | PL emission | Key result | Ref. |
|---|---|---|---|---|
| GO-modified luminescent lanthanide (Ln3+-NCs@GO) | Fluorescence imaging | NCs emit luminescence at 540, 650, and 1,525 nm | NCs@GO demonstrated strong dispersibility in a variety of solvents, easy surface modification, improved cell uptake effectiveness and cytocompatibility, and multicolor imaging | [182] |
| Silver sulfide quantum dot@mesoporous silica/GO/folic acid (QD@Si-D/GO–FA) | Fluorescence imaging dual-modal | ∼1,120 nm | Based on in vivo and in vitro investigations, the NCs demonstrated recognition of FA receptors present in tumor cells, therefore enabling chemo-photothermal treatment | [183] |
| rGO@AuNS | Photothermal imaging dual-modal | — | FA crosslinking on the surface of rGO@AuNS-lipid allows binding when FA receptors on the surface of cancer cells are recognized. Through receptor-mediated endocytosis, this binding process improves the effectiveness of imaging diagnostics | [184] |
| Graphene/folic acid-zinc oxide | Fluorescence imaging | High intensity peak at 485 and low intensity peak at 538 nm | Fast tumor tissue absorption was shown by a robust and distinct fluorescent signal in mice following nanocomposite injection | [185] |
| AgInZnS–GO (AIZS–GO) | Fluorescence imaging | 530–680 nm under excitation of UV (365 nm) | The photoluminescence intensity of AIZS-GO does not diminish during irradiation, demonstrating strong biocompatibility and long-term fluorescent label imaging. NCs also exhibit multicolor bioimaging | [186] |
| GO nanoparticles (nGOs) | Fluorescence imaging | PL emission at 455 nm | nGOs showed bright blue-green emission in the range of 430–510 nm and had photostability in cells. | [187] |
| Triphenylamine-derivative (DNDT)-modified nanographene oxide | Fluorescence imaging | PL emission 364–410 nm | GO-KH550-DNDT showed blue emission in the range of 364–410 nm in the nucleus cells and stability. | [188] |
| Gadolinium-decorated rGO (Gd-rGO) | Magnetic resonance imaging | — | Gd-rGONSs have the potential to be a viable MRI T1 contrast agent for magnetically induced imaging. At 1.5 T magnetic field, Gd-rGONSs has a 4-fold relaxivity value (r1). | [189] |
Graphene material with antibacterial and antiviral properties
| Materials | Synthesis method of graphene materials | Bacteria/virus | Mechanism | Inhibition | Ref. |
|---|---|---|---|---|---|
| GO nanoparticles | Oxidation of graphite using Hummer’s method | Pseudomonas putida | Membrane damage | ∼90–100% for biofilm 48h | [198] |
| GO and rGO | Oxidation of graphite using Tour’s method | Staphylococcus aureus and Pseudomonas aeruginosa | Membrane damage | GO = 48.6 and 93.7% | [199] |
| rGO = 93.3 and 67.7%. | |||||
| GO–AgNPs | Oxidation of graphite using Hummer’s method | Escherichia coli and the Staphylococcus aureus | Disruption of membrane integrity and inhibition of cell division | Severe inhibition | [200] |
| rGO films | Chemical reduction | Staphylococcus aureus and Pseudomonas aeruginosa | Inhibition of cell division | 81–84% and 50–62% | [201] |
| Magnetic GO–TiO2 | Oxidation of graphite using Hummer’s method | Escherichia coli | Membrane damage | 100% | [202] |
| rGO-ZnO | Oxidation of graphite using Hummer’s method | Escherichia coli and Staphylococcus aureus | Membrane damage by •OH radicals’ generation | ∼100% | [203] |
| Dialdehyde cellulose (DAC)/GO/ Cysteine (Cys) and DAC/GO/Methionine (Meth) | Oxidation of graphite using Hummer’s method | E. coli, P. aeruginosa, B. subtilis, S. aureus, C. albicans, and C. neoformans | Membrane damage by amino residue | DAC/GO/Cys = 19 ± 1.01, 27 ± 0.95, 17 ± 1.27, 11 ± 0.69, 23 ± 0.87, and 32 ± 0.93 | [204] |
| DAC/GO/Meth = 1 ± 1.00, 13 ± 0.61, 15 ± 0.32, 12 ± 0.72, and 18 ± 0.55 mm | |||||
| GO-PVP | Ultrasonication | PEDV | Inhibits virus entry into host cells | ∼90% | [205] |
| β-cyclodextrin (CD) functionalized GO | Ultrasonication | Respiratory syncytial virus | Virus inactivation and viral attachment inhibition | 5.00 g/mL materials could stop the virus from being able to spread | [206] |
4.2 GQDs for biomedical applications
4.2.1 Biosensing applications
GQDs have excellent PL performance with certain functional groups, allowing them to bond with target analytes via electrostatic interactions, π–π conjugation, or electron transfer, resulting in GQD PL turn-on or turn-off [207]. Hai et al. reported that GQDs could also be coated with additional compounds, resulting in moderate optical characteristics with specific recognition or dual emissions [207]. GQDs outperform organic dyes and semiconductor quantum dot probes for biosensing devices in terms of sensitivity, selectivity, stability, and security.
4.2.1.1 Ion sensing
In biological systems, ions need regulation and transportation at a cellular level, and their acute toxicity implies precise measurement with high sensitivity and selectivity [208,209]. Biosensors are developed based on the affinity of different functional groups on GQDs to detect a specific ion. A large number of studies have been presented for sensing different types of ions, such as Cu2+ [210], Pb2+ [211], Hg2+ [212], Ag+ [213], Fe3+ [135], and others [110,214]. One of the major studies used ethylenediamine-modified GQDs with a QY of 83% to detect Ni2+ with a detection limit of 30 nM. The PL of GQDs was substantially quenched upon the addition of Ni2+. In vitro sensing was demonstrated by treating rat adipocyte-derived stem cells with GQDs, and the PL was quenched upon adding Ni2+ to the cell [215].
In a recent study, biomass-derived GQDs, inherently functionalized with hydroxyl/carboxyl groups, were utilized as a photoluminescence detection probe for sensing ferric ions (Fe3+) [135]. The selectivity of the GQDs-based biosensor was assessed by evaluating the photoluminescence quenching efficiency of Fe3+ compared to numerous other metal ions. Moreover, the sensitivity was evaluated at a range of 1–50 μm, and the detection limit was reported to be as low as 2.5 μM, highlighting the practical applicability of the sensor (Figure 16). The high selectivity and sensitivity of the sensor were attributed to the strong affinity of biomass-derived GQDs toward Fe3+.
![Figure 16
The biomass-derived GQDs-based sensor for selective and sensitive detection of ferric ions (Fe3+). (a) Comparison of the photoluminescence intensities of GQDs solution in the presence of 100 µM of different metal ions, (b) the assessment of the different metal ion’s affinity toward GQD, (c) the photoluminescence spectra of GQDs at various concentrations of Fe3+
, and (d) equivalent liner regression plot [135].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_016.jpg)
The biomass-derived GQDs-based sensor for selective and sensitive detection of ferric ions (Fe3+). (a) Comparison of the photoluminescence intensities of GQDs solution in the presence of 100 µM of different metal ions, (b) the assessment of the different metal ion’s affinity toward GQD, (c) the photoluminescence spectra of GQDs at various concentrations of Fe3+ , and (d) equivalent liner regression plot [135].
4.2.1.2 In vitro sensing of small molecules
GQDs have been widely employed in biosensing based on their optical properties. Biosensors based on GQDs utilize the affinity of specific functional groups within GQDs toward analyte molecules. When the affinity of a functional group is higher toward an analyte molecule, binding between GQDs and analyte molecules occurs, resulting in different electronic states of GQDs. The GQD’s electronic state variation changes photoluminescence and helps detect analytes [110,214]. GQDs have been used to detect DNA and other analytes [214]. In general, sensitivity, selectivity, and simplicity are the main requirements for an efficient sensor, and GQDs provide an excellent platform due to their good photostability and fast response.
GQDs-based sensors have also been developed for in vitro sensing of small molecules. For example, GQDs functionalized with (2,4-dinitrophenyl) tyrosine (DNPTYR) were utilized to develop a turn-on sensor for indicating the H2S attack. Different types of diseases, including cancer and Alzheimer’s, are related to an unusual concentration of H2S in the cells. The mechanism involved in this novel sensor is the photoinduced electron transfer between the DNPTYR functional group and GQDs. The photoluminescence of GQDs was quenched due to the covalent conjugation of DNPTYR. However, after adding H2S, photoluminescence recovered due to the cleavage of the dinitrophenyl group by H2S. Figure 17 demonstrates the schematic illustration of the H2S biosensor. This novel biosensor could potentially detect the in vitro levels of H2S. MCF-7 cells were incubated with GQDs-DNPTYR until the particles were interred in the cells. The photoluminescence of GQDs was enhanced by adding H2S, as indicated by small specs of green in confocal microscope images (Figure 17b). Finally, phorbol myristate acetate (PMA) was added to the cells to reduce the H2S concentration, which resulted in the photoluminescence quenching again. The detection limit of the biosensor was calculated to be 2 nM, signifying the system's viability [216]. The outstanding biocompatibility, good photostability and excellent water solubility are several obvious advantages of GQDs for biosensing over other systems, such as organic dyes and semiconductor QDs.
![Figure 17
GQDs-DNPTYR-based turn-on fluorescence probe for H2S biosensing. (a) Schematic representation of synthesis mechanism and quenching of GQDs by DNPTYR and (b) confocal microscope image of MCF-7 cells (first left), after 1h incubation with GQDs-DNPTYR (second left), 25 min after the addition of H2S (third left) and in the presence of PMA and H2S (rightmost) [216].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_017.jpg)
GQDs-DNPTYR-based turn-on fluorescence probe for H2S biosensing. (a) Schematic representation of synthesis mechanism and quenching of GQDs by DNPTYR and (b) confocal microscope image of MCF-7 cells (first left), after 1h incubation with GQDs-DNPTYR (second left), 25 min after the addition of H2S (third left) and in the presence of PMA and H2S (rightmost) [216].
Most of the GQDs-based sensing systems are based on the interaction of the analyte with the molecules attached to GQDs, resulting in the recovery of photoluminescence through an irreversible process. While in other GQDs-based sensors, analyte interaction occurs directly with GQDs, usually modified with specific functional groups. The affinity between the functional groups and analytes is vital in these biosensing probes. For example, amine-functionalized N-GQDs were utilized to sense 2,4,6-trinitrophenol (TNP) [217]. To evaluate the selectivity of N-GQDs toward TNP, the level of photoluminescence quenching in the presence of TNP was compared to the presence of other aromatic compounds similar to TNP and various metal ions. The results indicated that considerable photoluminescence quenching only occurred in the presence of TNP. This selectivity was attributed to the overlap between the emission spectrum of N-GQDs and the absorbance spectrum of TNP, leading to effective resonance electron transfer.
4.2.1.3 In vitro sensing
In addition to in vitro investigations where GQDs are utilized to detect analytes in a cellular environment, in vivo sensing could bring the GQDs nearer to clinical research. Recent advancements in the GQDs field indicate that in vivo biosensing is also possible through GQDs. For example, GQDs with NIR-II windows for photoluminescence emission has been used for deeper tissue penetration. Although good biocompatibility and water solubility suggest that these GQDs can be potentially used for in vivo biosensing, only a few studies have been conducted on GQDs based on in vivo biosensing. CA and neutral red-derived carbon quantum dots, usually called CDs, were used for in vivo biosensing noble metal ions, such as Au3+, Pt2+, and Pd2+ in zebrafish [218]. CDs’ optical and physicochemical properties were similar to those of GQDs, especially synthesized by the hydrothermal method, highlighting the potential of GQDs for in vivo sensing. In order to evaluate in vivo sensing ability of CDs, the zebrafish were placed in a CDs solution for 4 h after feeding with different concentrations of Pt2+. Subsequently, fluorescence imaging of the zebrafish was performed, and the photoluminescence intensity of CDs was measured against the Pt2+ concentration. The results indicated a good relationship between the CDs photoluminescence intensity and Pt2+ concentration [218]. Although several barriers exist for translation to mammalian models, such as high signal absorbance by the tissues and immunogenicity of GQDs, this study indicates that GQDs-based biosensors can be used in living systems.
4.2.1.4 Hydrogen peroxide (H2O2) sensing
H2O2 is an essential chemical widely used as an oxidant in industries. However, the toxic nature of H2O2 implies that its precise measurement is substantially important [219]. Several methods have been developed for its detection and measurement, among which electrochemical sensing is advantageous owing to its high accuracy, fast response, and ease of handling [220]. As an alternative to usually employed enzymatic sensing, GQDs have recently emerged as a non-enzymatic sensing platform for H2O2 [221]. Recently, GQDs and ZnO nanofiber composite platforms were developed for intracellularly sensing H2O2 after anticancer drug treatment [222]. Excellent response and catalytic activity were observed due to the high surface area and synergetic effect of GQDs and ZnO nanofibers. In another study, GQDs/MWCNTs composite on glassy carbon electrodes was utilized to sense H2O2 by reduction [223]. N-GQDs were used as a calorimetric indicator of glucose and H2O2 and as a catalyst for reducing H2O2. The kinetics of N-GQDs for reducing H2O2 were compared with different substrates. The results showed that the kinetics of N-GQDs were much higher than other substrates, showing a high affinity of N-GQDs toward aromatic compounds [224].
4.2.2 Tissue engineering (implant)
GQDs-based materials possess a highly beneficial physicochemical property that has been used in tissue engineering, from tissue scaffolds, high-strength hydrogels, wound healing agents, to bone regeneration agents. Qiu et al. investigated the effects of GQDs on MSCs osteogenic differentiation and evaluate the effect of GQDs exposure on MSC differentiation after incubating MSCs with different GQDs concentrations for 7 and 14 days. The result showed that MSCs incubated with GQDs had higher alkaline phosphatase (ALP) activity than the control group, indicating that GQDs enhance the osteogenic differentiation of MSCs. To further confirm the findings, they used quantitative polymerase chain reaction to measure the expression of phenotypically related genes associated with osteogenic differentiation at the mRNA level. The expression of bone extracellular matrix proteins such as osteopontin (OPN) and osteocalcin (OCN) was also investigated. The result after 14 days of incubation showed Runt-related transcription factor 2 (Runx2) mRNA expression in MSCs cultured with GQDs at a concentration of 50 μg/mL was up-regulated by 13.9-folds compared to control cells, indicating that Runx2 mRNA expression in MSCs cultured with GQDs significantly increased in a concentration- and time-dependent manner. The OPN expression was also increased in a concentration- and time-dependent manner up to 10 days of exposure, whereas OCN expression remained unchanged after 7 days of GQD exposure but increased in a concentration-dependent manner on days 10 and 14. These findings confirmed that GQDs could be potentially used in applying stem cell and musculoskeletal tissue engineering [196]. A more recent study investigated the effect of graphene oxide quantom dots (GOQDs) on the osteogenic differentiation of SHEDs, stem cells derived from human exfoliated deciduous teeth, which recently emerged as one of the most promising MSCs in bone tissue engineering. The resulting study showed that GOQDs promoted SHED proliferation significantly, and GOQDs performed better in promoting SHEDs osteogenesis than GO. Furthermore, GOQDs were dispersed uniformly throughout the cytoplasm of SHEDs due to their photoluminescence characteristics [232]. These studies suggest that GQDs-based nanomaterials are promising for tissue engineering advancement.
4.2.3 Drug delivery
Drug loading, targeting, and efficacy have been improved with nanoparticle-based DDS [236]. Recent research shows that GQDs are less poisonous, more hydrophobic, and have stronger fluorescence than graphene [237]. Furthermore, GQDs have been proven to improve the chemotherapeutic efficacy of anticancer medications that are unsatisfactory due to drug resistance [20]. Several studies have used DFT or MD simulations to understand GQDs properties better, for example, the 5-fluorouracil (FU) interaction with undoped/doped GQDs [238]. The findings suggest AlN and AlP-doped GQDs as FU drug carriers in nanomedicine. These nanoparticles have a low toxicity, a large surface/volume ratio, and a wide range of surface functionalization options compared to other nanoparticles. In addition, GQDs hold great biological prospects. Nitrogen-doped modified GQDs have also been shown to have drug-carrying potential. N-GQD has been reported to be employed as the DDS of methotrexate, an anticancer medication, and in vitro cytotoxicity studies showed that N-GQD is highly biocompatible [239]. The position of nitrogen doping on GQDs has varied implications on its potential to be used as a drug carrier of gemcitabine as reported by Vantaparast et al. By using DFT calculations, they revealed that the binding energy values for each nitrogen doping location were negative, indicating that gemcitabine adsorption happened spontaneously on both GQDs and N-GQDS. However, the binding energy values of pure GQD and edge-NGQD were lower than those of the central N-GQD, indicating lower performance as a drug carrier [238].
4.2.4 Bioimaging
Along with small-molecule sensing, GQDs have been successfully implemented in bioimaging acute diseases such as cancer. The metabolic and nutritional environment of tumors is quite different from healthy tissues. Particularly, the pH in tumors is considerably low due to the anaerobic hydrolysis of ATP and the generation of lactic acid. Moreover, energy-deficient conditions are found in tumors [245]. Such an abnormal behavior of tumors has been used for efficient cancer diagnostics [246]. Numerous studies have demonstrated that the photoluminescence of GQDs varies with a change in the pH of the solution. Recently, nitrogen and sulfur co-doped GQDs (pRF-GQDs) demonstrated blue photoluminescence above pH 6.8 and transition to green photoluminescence below pH 6.8. In order to realize the practical application of the pH-based diagnostic system, pRF-GQDs were injected into mice bearing HeLa tumors, and fluorescence measurements were performed after 24 h. The results showed that green photoluminescence was observed in tumors.
Moreover, after imaging various tissues under identical conditions, a relatively high concentration of pRF-GQDs was found in tumor tissues due to enhanced penetration, permeation, and retention of GQDs. The pRF-GQDs system was demonstrated for fluorescence imaging of different tumors in mice, such as HepG2, PANC-1, U87MG, A549, and HeLa tumors [247]. The spatial resolution of tumors is relatively higher than the required value for early-stage cancer diagnostics. Thus, these results indicate the high potential of GQDs as an advanced platform for cancer diagnosis.
Intracellular multicolor imaging using N-GQDs, boron/nitrogen (BN-GQDs), and nitrogen/sulfur-doped GQDs (NS-GQDs) as fully biocompatible multifunctional platforms for multicolor visible/near-IR imaging and cancer-sensing has been presented by Campbell et al. [248]. The GQDs were derived from a single biocompatible glucosamine precursor. The experiment shows high-yield intrinsic fluorescence emitted in blue/green color and NIR are applicable for multicolor in vitro imaging on their own or in combination with other fluorophores as seen in Figure 18. Furthermore, the materials offer the capabilities for in vivo near-IR fluorescence tracking. Each type of quantum dot is imaged at various excitation and emission wavelengths and shows emission when internalized into a HeLa cell in blue (450 nm), green (535 nm), and NIR (750 nm).
![Figure 18
Multicolor imaging of N-GQDs, NS-GQDs, and BN-GQDs in blue, green, and near-IR. Reproduced with permission from Ref. [248].](/document/doi/10.1515/ntrev-2023-0168/asset/graphic/j_ntrev-2023-0168_fig_018.jpg)
Multicolor imaging of N-GQDs, NS-GQDs, and BN-GQDs in blue, green, and near-IR. Reproduced with permission from Ref. [248].
4.2.5 Antibacterial and antiviral property
GQDs with biocompatible, photo-stable, enhanced surface grafting and superior thermal, electrical, and mechanical properties inherited from graphene are well suited for use in the biological field, including the antibacterial and antiviral fields. Sun et al. designed an antibacterial system by combining GQDs with a low dose of a standard medical reagent, H2O2. Gram-positive bacteria E. coli and gram-negative bacteria S. aureus were used as models to investigate the antibacterial activities of the designed system. The results suggest that GQDs could act as a catalyst to enhance the antibacterial activity of H2O2 in this system. It is shown that the antibacterial ability of H2O2 has been significantly improved with the assistance of GQDs (100 g/mL). H2O2 significantly reduced the viability of both E. coli and S. aureus cells in a concentration-dependent manner. The enhanced antibacterial activity of H2O2 could also inhibit the growth of E. coli and S. aureus bacteria [255].
Furthermore, the GQD-Band-Aids, with the assistance of H2O2 at low doses, were prepared to examine the antibacterial efficacy of the designed system in actual wound disinfection. Kunming mice with a wound on their back were used as models to test the antibacterial efficacy of the designed system for wound disinfection in vivo. The wounds of mice were treated with H2O2+GQD/Band-Aid. The result showed that bacteria in the H2O2+GQD/Band-Aid treated group were nearly four orders lower than those in the saline-treated group, which implied that the combination of H2O2 and GQD/Band-Aid could kill bacteria during the wound treatment most effectively. These results indicate that GQD-Band-Aid has potential use for wound disinfection since GQD-Band-Aid showed an excellent antibacterial property in vivo with a low concentration of H2O2 [255].
Zeng et al. reported that GO quantum dots covalently functionalized poly (vinylidene fluoride) (GOQDs-PVDF) membrane exhibits significantly improved hydrophilic, antibacterial, and anti-biofouling properties while maintaining the permeation properties of the pristine PVDF membrane [256]. The presence of the GOQD coating layer effectively inhibits bacterial cell growth and prevents biofilm formation on the membrane surface, resulting in a higher bacterial inactivation efficiency. The GOQD loading on the membrane could be adjusted to obtain an optimal functionalized membrane with improved water permeability, antibacterial activity, and biofouling resistance. This finding shows the potential application of GOQDs-PVDF membrane as an antimicrobial agent and anti-biofouling membrane [256].
Chowdhury et al. studied the electrochemical detection of Hepatitis E virus (HEV) by a graphene-based nanocomposite NS-GQDs and gold-embedded polyaniline nanowires) [257]. The study found that gold nanoparticles loaded polyaniline nanowire improves electron transport and provides a large surface area for loading monoclonal antibody-conjugated GQDs. Whereas the latter may serve as active sites for the target HEV. Compared to other traditional electrochemical sensors, introducing an external electrical pulse during the virus accumulation step boosts the sensitivity of the antiviral drug toward HEV. In addition, the external electrical pulse in this study could give insight into GQDs (with sizes of around 500 nm) to attach to the virus effectively [257].
Studies on the antiviral performance of GQDs based nanocomposites are still in small numbers compared to antibacterial performance. The significant differences in the size of the virus (2–300 nm) and bacteria (500–5,000 nm) make viral studies more challenging to conduct. Nevertheless, graphene and GQDs-based nanocomposites show promising applications in antiviral field for such nanomaterials.
Beyond graphene, another fascinating material is GQDs. These structures, with their remarkable properties, showcase diverse applications, ranging from biosensors to antibacterial and antiviral agents, as depicted in the data across Tables 8–12. These tables provide a comprehensive overview, ensuring that readers can effortlessly grasp the potential uses of GQDs in biomedical applications.
GQDs material for biosensor
| Precursor materials | Synthesis method | PL emission | Sensor type | Target molecules | LOD | Ref. |
|---|---|---|---|---|---|---|
| B-doped GQDs | Hydrothermal | 480 and 520 nm | Direct fluorescence | Fe3+ | 31.2 nM | [225] |
| Layered double hydroxide GQD composite | Hydrothermal | 350 nm | Fluorescence | Ascorbic acid | 1.73 μmol L−1 | [226] |
| GQDs/CoNiAl-layered double-hydroxide | Pyrolysis | 380 nm | Amperometry | Glucose | 6 μM | [227] |
| Aniline functionalized GQDs | Hydrothermal | 470 nm | Fluorescence probe | Glucose | 2.1 μM | [228] |
| AuNPs/N-GQDs-P-MOF | Hydrothermal | — | Amperometry | Glucose | 0.7 μM | [229] |
| PEDOT:PSS/Ti3C2/GQD | Hydrothermal | — | EIS and DPV | Glucose | 65 µM | [230] |
| GQDs and ionic liquid (IL) modified screen-printed carbon electrode | Carbonization | 460 nm | CV and EIS | Ascorbic acid, dopamine and uric acid | 6.64, 0.06, and 0.03 μM | [231] |
GQDs material for tissue engineering
| Materials | Preparation | PL emission | Type of tissue or cells | Improvement in mechanical and physical properties | Key result | Ref. |
|---|---|---|---|---|---|---|
| GQD gelatin methacrylamide hydrogel | Lyophilization and UV irradiation | 480, 540, and 520 | Bone tissue | Good mechanical properties | In vitro and in vivo analysis showed that negatively charged GQD‾ may boost bone regeneration | [225] |
| GO quantum dots (GOQDs) | Electrospinning | 570 nm | Nerve tissue | The addition of GOQD increased mechanical strength to 5.27 ± 0.16 MPa | The application of GOQD in the scaffold promoted motor and sensory recovery | [233] |
| PCL/PVA-TCP-CD nanofibers | Electrospinning | 463 ± 5 nm | Bone tissue | The material increased mechanical propertis | Nanofibers has proliferated and differentiated osteogenically | [234] |
| Oxidized alginate/gelatin-nitrogen-GQDs | Freeze drying | — | Cartilage tissue | Pore diameters are smaller (102 m), water absorption is lower (813%), and mechanical strength are improved | The nanocomposite hydrogels tested in this study are injectable, making them a promising candidate for use in the treatment of degenerative cartilage | [235] |
GQDs material for drug delivery
| Materials | Type of drugs | Experimental model for drug release | Key result | Ref. |
|---|---|---|---|---|
| CS/GQDs/Cytarabine (Cyt) and GQDs/Cyt | Cyt | In vitro | The cumulative release of Cyt from CS/GQDs/Cyt and GQDs/Cyt at pH 5.8 were 75.6 and 90.8% | [240] |
| GQDs@Bio-MOF(Cu) | Naproxen | In vitro | The drug release was 71.58% at pH 6.8 and pH 7.4. | [171] |
| Arginine-glycine-aspartic acid-conjugated GQDs | Doxorubicin | In vitro | Drug loading capacity was about 54.6%. Doxorubicin drug release is about 40.1% at pH 5.0 and 9.2% at pH 7.4 | [241] |
| GQDs-CoFe2O4@SiO2 /Folic Acid | Doxorubicin | In vitro | Drug loading capacity was 65.4 wt%. Doxorubicin drug release was 71% at pH 5.5 and 30% at pH 7.4 after 48 h. | [242] |
| MiRGD-GQDs peptideticles | Doxorubicin and curcumin | In vitro | Drug release were 80 and 33.8% for Doxorubicin and Curcumin at acidic condition | [243] |
| Carboxymethyl cellulose-GQDs | Doxorubicin | In vitro | Drug release was ∼60% at pH 4.5 | [244] |
GQDs material for bioimaging
| Materials | Type of imaging technique | PL emission | Key result | Ref. |
|---|---|---|---|---|
| N-GQDs | Fluorescence imaging | Yellow emission at 562 nm | After 24 h in N-GQDs solution at 37°C, fibroblast cell viability was not affected by concentrations of N-GQDs up to 300 g/mL. This demonstrates the high biocompatibility and minimal cytotoxicity of N-GQDs in vitro. | [249] |
| NGQDs | Fluorescence imaging | Green and blue emission at 500–550 nm | NGQD-d can be served as fluorescent nanoagent for in vivo bioimaging in zebrafish | [250] |
| GQDs | Fluorescence imaging | Deep red at 610 nm | GQDs can penetrate to the cells through endocytosis process and the PL stable for 48 h. | [251] |
| GQDs | Fluorescence imaging | Red emission at 650 and 750 nm | GQDs showed emission in the range of 650–750 nm (NIR region). Cells containing mGQDs showed NIR emission at 561 and 637 nm | [252] |
| Nitrogen–sulfur doped GQDs (NS-GQDs) | Fluorescence imaging | Blue emission at 480 nm | The addition of Cu2+ in the cells containingd NS-GQDs did not interfere the fluorescence imaging, showing the stability of NS-GQDs | [253] |
| Boron-doped GQDs (B-GQDs) and phosphorus-doped GQDs (P-GQDs) | Fluorescence imaging | Blue and yellow emission at 460 and 630 nm | The cells viability was not decreased after addition of both B-GQDS and P-GQDs showing high biocompatibility of the materials | [254] |
GQDs material with antibacterial and antiviral property
| Materials | Synthesis method of GQDs materials | Bacteria/virus | Mechanism | Inhibition | Ref. |
|---|---|---|---|---|---|
| N-doped GQDs | Microwave-assisted synthesis | Methicillin-resistant Staphylococcus aureus | Generation of hyperthermia by N-GQDs plus a NIR-II laser | — | [198] |
| Crystalline GQDs | Hydrothermal | Pseudomonas aeruginosa | Membrane damage | Crystalline GQDs could inhibit the growth of bacteria | [258] |
| GQDs/SnO2 | Hydrothermal | Pseudomonas aeruginosa | Membrane disruption and leakage | GQDs/SnO2 nanocomposite had larger zone of inhibition means good antibacterial properties | [259] |
| TiO2/Sb2S3/GQDs | Hydrothermal | E. coli and Staphylococcus aureus | Membrane damage caused by strong electrostatic interactions | MIC value for E. coli and Staphylococcus aureus were 0.03 and 0.1, showing high antibacterial activity | [260] |
| Cotton/Ag/GQDs | Hydrothermal | E. coli and Staphylococcus aureus | Membrane damage by reactive oxidative species | MIC value for E. coli and Staphylococcus aureus were 0.01 and 0.09, showing high antibacterial activity | [261] |
| GQDs@AgNPs | Hydrothermal | E. coli and Staphylococcus aureus | Change the membrane permeability; DNA damage; decrease dehydrogenase activity | MIC value for E. coli and Staphylococcus aureus were 12 ± 0.4 and 25 ± 0.2, showing high antibacterial activity | [262] |
5 Challenge and its possible solution
In recent years, there has been significant interest in the utilization of graphene and GQDs in biomedical applications. However, their implementation in the biomedical application also poses notable challenges that require attention. In this study, we aim to identify the specific issues and challenges associated with the use of graphene and GQDs in biomedical applications, as well as explore potential directions for addressing these challenges.
5.1 Synthesis, cost-effectiveness, and waste management
Large-scale synthesis methodologies and cost-effectiveness remain significant concerns that demand serious attention and require resolution [263]. In order to overcome the limitations in large-scale and affordable synthesis methods, it is advisable to optimize existing techniques. This can be achieved by researching different parameters such as precursor materials, reaction conditions, and catalysts, which can effectively reduce production costs [264]. Furthermore, the exploration and development of alternative synthesis processes that can be feasibly scaled up at reasonable costs are highly encouraged. In addition to the aforementioned synthesis and production cost issues, there are other challenges arising from the synthesis process. The waste generated from the use of graphene and GQDs can contaminate water sources. Nano-sized particles from these materials can be released into the environment, posing a potential threat to aquatic ecosystems and the organisms within them. We suggest to develop environmentally friendly synthesis methods that generate less waste. This can be achieved by using more environmentally friendly materials, such as renewable precursors and environmentally friendly solvents [265]. By implementing these strategies, the synthesis process can effectively reduce its environmental impact. Moreover, it is also advisable to implement an effective waste management system to prevent the generated waste from contaminating water sources [266]. Thus, appropriate and efficient waste treatment procedures are required to achieve this.
5.2 Biocompatibility and toxicity
Graphene and GQDs are appealing for biomedical applications due to their unique features. However, in addition to their potential, the compatibility of these materials with living tissues and organisms needs to be considered. Graphene and GQDs can undergo degradation or agglomeration over time [267], which may affect their biocompatibility and performance in biomedical applications. Additionally, biocompatibility and toxicity remain key concerns for these applications. Investigations of the biocompatibility and the toxicity of graphene and its derivatives have been reported through in vivo animal tests and in vitro cell cultures. In vivo biodistribution of graphene functionalized with PEG in mice has been investigated [268]. The result indicates that PEGylated graphene (PEG-GO) does not cause appreciable toxicity at a tested dose of 20 mg/kg for 3 months. PEG-GO was mainly accumulated in the liver and spleen, and it was removed from these organs by renal and fecal excretion [268].
Chang et al. have reported in vitro toxicity of GO on A 549 cells by examining the effect of size and dose on biocompatibility of GO exposed to A549 cells [269]. The investigation shows that GO does not enter A549 cell and has no obvious cytotoxicity. However, GO arouses oxidative stress, and induces the slight decrease in the cell viability at high GO dose. Therefore, the effect of GO on A549 cells is dose and size related. GOs with sizes of 780 ± 410 nm (l-GO), 430 ± 300 nm (m-GO), and 160 ± 90 nm (s-GO) were used in their work. The cells maintained a high level of viability (over 80%), even at high GO concentration of 200 µg/mL. However, viability loss was observed for s-GO at 200 µg/mL. At this dosage, cell viability was 67% for 24 h incubation [269].
Wang et al. studied the effect of GO on human fibroblast cells and mice to investigate the biocompatibility of GO. The GO was synthesized by the Hummer method. The result exhibits that GOs with dose less than 20 μg/mL does not show toxicity to human fibroblast cells, and the dose of more than 50 μg/mL shows obvious cytotoxicity such as decreasing cell adhesion, inducing cell apoptosis, entering into lysosomes, mitochondrion, endoplasm, and cell nucleus. For the investigation on 30 mice, GOs under low dose (0.1 mg) and middle dose (0.25 mg) do not show obvious toxicity to mice, but under high dose (0.4 mg) shows chronic toxicity, such as 4/9 mice death and lung granuloma formation, mainly located in lung, liver, spleen, and kidney, almost could not be cleaned by kidney [270].
A study on the exposure of 1 and 10 mg/L GO on eastern oysters (Crassostrea virginica) showed an elevated lipid peroxidation and a reduction in total protein levels in tissues of the digestive glands [271]. Another study showed a negative regenerative effect of GO concentration at 0.01, 0.10, and 1.00 mg/L on Diopatra neapolitana [272]. The effect of oxidative stress when GO and carboxyl graphene were introduced to the fish cell at different concentrations also has been reported in the literature [273].
Hu et al. reported highly biocompatible GQDs (HGQDs) with narrow size distribution range from 1.2 to 3.2 nm which have been successfully synthesized only by glucose in aqueous solution via one-step hydrothermal method. Importantly, no acute toxicity or morphological changes were noted from in vitro cytotoxicity and fluorescence imaging studies of the prepared HGQDs as determined by CCK-8 assay, flow cytometric analysis and confocal microscopy imaging. The more interesting results are that the cancer cells, normal cells, and gram-negative bacteria can be imaged without being destroyed. It is proved that the biocompatibility of HGQDs is better than that of conventional GQDs. The ex vivo fluorescence imaging of isolated organs demonstrated that the HGQDs accumulated in the liver, kidney, and brain at 24 h after intravenous injection of HGQDs. No inflammation was observed in the heart, liver, spleen, lung, and kidney at 20 days after the administration of the prepared HGQDs [274].
Therefore, to enhance their compatibility, research efforts focusing on surface functionalization, such as attaching functional groups or coatings, can modify their surface properties and improve biocompatibility [275]. Coating the materials with a biocompatible layer, such as biomolecules or polymers or encapsulating them within nanocarriers which can improve their stability, preventing unwanted interactions and maintaining their properties over an extended period [276]. Thus, systematic research on surface modification and coating techniques to enhance biocompatibility should be systematically conducted. In the development of biomedical technology, G and GQDs have demonstrated tremendous potential as new materials for numerous biomedical applications. However, the effect of G and GQD concentrations on toxicity must be carefully studied [277]. Thus, it is crucial to systematically research on the challenges and issues associated with the toxicity of G and GQDs at various concentration levels in a thorough and systematic manner. This approach is necessary to minimize the potential risks of toxicity.
6 Conclusion and future prospects
There are various approaches for synthesizing G and GQDs-based nanocomposites and preparing G nanocomposite material concerning its biomedical engineering applications. Numerous studies in the synthesis of graphene and GQDs-based nanocomposites research are being undertaken to explore graphene for its biomedical application. G/GO is continually discussed. More research is needed on G’s ecotoxicity and non-recyclability. Graphene’s interaction with the environment is still being studied. Dual-property G could produce a new realm with exceptional qualities that exceed G’s and are governed by its nanomaterial. Graphene derivatives are continuously being studied for environmental sensing applications. This research will be useful when synthesizing G and GQDs and developing improved methodologies for innovative functional G and GQD nanomaterials for further biomedical applications. However, it is difficult to find the application of Gin biomedicine, especially in implants and antivirals, due to the level of G and GQDs toxicity that can be tolerated and achieved by the human body or deck cells. Thus, in the future, scientists should be able to minimize the toxicity of graphene to make it safe for human use.
The synthesis of graphene and GQDs for biomedical engineering applications is an active research area in which studies are being conducted to investigate graphene's potential in various biomedical applications. However, important issues such as large-scale synthesis, cost-effectiveness, waste management, biocompatibility, and toxicity must be addressed in order to use G and GQDs for biomedical applications. Thus, it is advisable to optimize existing procedures, investigate alternative synthesis methods, implement ecofriendly approaches, adopt effective waste management systems, improve biocompatibility properties, and address toxicity concerns. We can unlock the promise of G and GQDs for new and safe biomedical applications by overcoming these hurdles.
Acknowledgments
The authors acknowledge School of Electrical Engineering and Informatics, Bandung Institute of Technology for the support. The authors also acknowledge the support of the National Research and Innovation Agency (BRIN) through characterization facilities of the E-Layanan Sains (ELSA).
-
Funding information: The authors acknowledge research funding from Bandung Institute of Technology through Program Riset Internasional LPPM ITB 2022 [Grant number 4949/IT1.B07.1/TA.00/2022]. Murni Handayani acknowledges the Indonesia Toray Science Foundation (ITSF), Science and Technology Research Grant 2022, No. 1210/XII/ITSF/SEK/2022.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Iannazzo D, Celesti C, Espro C. Recent advances on graphene quantum dots as multifunctional nanoplatforms for cancer treatment. Biotechnol J. 2021;16:1900422.10.1002/biot.201900422Search in Google Scholar PubMed
[2] Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Controlled Release. 2014;173:75–88.10.1016/j.jconrel.2013.10.017Search in Google Scholar PubMed
[3] Haque E, Kim J, Malgras V, Reddy KR, Ward AC, You J, et al. Recent advances in graphene quantum dots: synthesis, properties, and applications. Small Methods. 2018;2:1800050.10.1002/smtd.201800050Search in Google Scholar
[4] Younis MR, He G, Lin J, Huang P. Recent advances on graphene quantum dots for bioimaging applications. Front Chem. 2020;8:424.10.3389/fchem.2020.00424Search in Google Scholar PubMed PubMed Central
[5] Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306:666–9.Search in Google Scholar
[6] Kalita H, Palaparthy VS, Baghini MS, Aslam M. Electrochemical synthesis of graphene quantum dots from graphene oxide at room temperature and its soil moisture sensing properties. Carbon N Y. 2020;165:9–17.10.1016/j.carbon.2020.04.021Search in Google Scholar
[7] Late DJ, Rout CS, Chakravarty D, Ratha S. Emerging energy applications of two-dimensional layered materials. Can Chem Trans. 2015;3:118–57.10.13179/canchemtrans.2015.03.02.0174Search in Google Scholar
[8] Luong DX, Bets KV, Algozeeb WA, Stanford MG, Kittrell C, Chen W, et al. Gram-scale bottom-up flash graphene synthesis. Nature. 2020;577:647–51.10.1038/s41586-020-1938-0Search in Google Scholar PubMed
[9] Luo J, Chen S, Li Q, Liu C, Gao S, Zhang J, et al. Influence of graphene oxide on the mechanical properties, fracture toughness, and microhardness of recycled concrete. Nanomaterials 9:325. Epub ahead of print 2019. 10.3390/nano9030325.Search in Google Scholar PubMed PubMed Central
[10] Seo DH, Pineda S, Fang J, Gozukara Y, Yick S, Bendavid A, et al. Single-step ambient-air synthesis of graphene from renewable precursors as electrochemical genosensor. Nat Commun. 2017;8:1–9.10.1038/ncomms14217Search in Google Scholar PubMed PubMed Central
[11] Wan LF, Cho ES, Marangoni T, Shea P, Kang S, Rogers C, et al. Edge-functionalized graphene nanoribbon encapsulation to enhance stability and control kinetics of hydrogen storage materials. Chem Mater. 2019;31:2960–70.10.1021/acs.chemmater.9b00494Search in Google Scholar
[12] Yu ZG, Zhang YW. Band gap engineering of graphene with inter-layer embedded BN: From first principles calculations. Diam Relat Mater. 2015;54:103–8.10.1016/j.diamond.2014.11.014Search in Google Scholar
[13] Yang Y, Yang HX, Wu YQ, Pu H, Meng WJ, Gao RZ, et al. Graphene caging core-shell Si@Cu nanoparticles anchored on graphene sheets for lithium-ion battery anode with enhanced reversible capacity and cyclic performance. Electrochim Acta. 2020;341:136037.10.1016/j.electacta.2020.136037Search in Google Scholar
[14] Bediako DK, Rezaee M, Yoo H, Larson DT, Zhao SYF, Taniguchi T, et al. Heterointerface effects in the electrointercalation of van der Waals heterostructures. Nature. 2018;558:425–9.10.1038/s41586-018-0205-0Search in Google Scholar PubMed
[15] Liu J, Ma Q, Huang Z, Liu G, Zhang H. Recent progress in graphene-based noble-metal nanocomposites for electrocatalytic applications. Adv Mater. 2019;31:1–20.10.1002/adma.201800696Search in Google Scholar PubMed
[16] Zholnin AG, Klyatskina EA, Grigoryev EG, Salvador MD, Misochenko AA, Dobrokhotov PL, et al. Spark-plasma sintering of Al2O3–graphene nanocomposite. Inorg Mater: Appl Res. 2018;9:498–503.10.1134/S2075113318030334Search in Google Scholar
[17] Khan M, Tahir MN, Adil SF, Khan HU, Siddiqui MRH, Al-warthan AA, et al. Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J Mater Chem A Mater. 2015;3:18753–808.10.1039/C5TA02240ASearch in Google Scholar
[18] Anwar AW, Majeed A, Iqbal N, Ullah W, Shuaib A, Ilyas U, et al. Specific capacitance and cyclic stability of graphene based metal/metal oxide nanocomposites: A review. J Mater Sci Technol. 2015;31:699–707.10.1016/j.jmst.2014.12.012Search in Google Scholar
[19] Xue F, Shu R, Xie Y. Controlling synthesis and gas-sensing properties of ordered mesoporous In2O3-reduced graphene oxide (rGO) nanocomposite. Sci Bull (Beijing). 2015;60:1348–54.10.1007/s11434-015-0852-6Search in Google Scholar
[20] Wang C, Zhang L, Huang H, Xi R, Jiang DP, Zhang SH, et al. A nanocomposite consisting of ZnO decorated graphene oxide nanoribbons for resistive sensing of NO2 gas at room temperature. Microchim Acta 186:554. Epub ahead of print 2019. 10.1007/s00604-019-3628-x.Search in Google Scholar PubMed
[21] Noamani S, Niroomand S, Rastgar M, Sadrzadeh M. Carbon-based polymer nanocomposite membranes for oily wastewater treatment. NPJ Clean Water. 2019;2:1–14.10.1038/s41545-019-0044-zSearch in Google Scholar
[22] Hashemi SA, Mousavi SM, Bahrani S, Ramakrishna S, Babapoor A, Chiang WH. Coupled graphene oxide with hybrid metallic nanoparticles as potential electrochemical biosensors for precise detection of ascorbic acid within blood. Anal Chim Acta. 2020;1107:183–92.10.1016/j.aca.2020.02.018Search in Google Scholar PubMed
[23] Alireza Hashemi S, Bahrani S, Mojtaba Mousavi S, Omidifar N, Ghaleh Golab Behbahan N, Arjmand M, et al. Ultra-precise label-free nanosensor based on integrated graphene with Au nanostars toward direct detection of IgG antibodies of SARS-CoV-2 in blood. J Electroanal Chem. 2021;894:115341.10.1016/j.jelechem.2021.115341Search in Google Scholar PubMed PubMed Central
[24] Geng D, Yang HY. Recent advances in growth of novel 2D materials: beyond graphene and transition metal dichalcogenides. Adv Mater. 2018;30:1800865.10.1002/adma.201800865Search in Google Scholar PubMed
[25] Derakhshi M, Daemi S, Shahini P, Habibzadeh A, Mostafavi E, Ashkarran AA. Two-dimensional nanomaterials beyond graphene for biomedical applications. J Funct Biomater. 2022;13:27.10.3390/jfb13010027Search in Google Scholar PubMed PubMed Central
[26] Mir SH, Yadav VK, Singh JK. Recent advances in the carrier mobility of two-dimensional materials: a theoretical perspective. ACS Omega. 2020;5:14203–11.10.1021/acsomega.0c01676Search in Google Scholar PubMed PubMed Central
[27] Shi L-B, Cao S, Yang M. Strain behavior and carrier mobility for novel two-dimensional semiconductor of GeP: First principles calculations. Phys E Low Dimens Syst Nanostruct. 2019;107:124–30.10.1016/j.physe.2018.11.024Search in Google Scholar
[28] Pan D, Zhang J, Li Z, Wu M. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv Mater. 2010;22:734–8.10.1002/adma.200902825Search in Google Scholar PubMed
[29] Kadian S, Manik G, Ashish K, Singh M, Chauhan RP. Effect of sulfur doping on fluorescence and quantum yield of graphene quantum dots: an experimental and theoretical investigation. Nanotechnology. 2019;30:435704.10.1088/1361-6528/ab3566Search in Google Scholar PubMed
[30] Chatterjee M, Nath P, Kadian S, Kumar A, Kumar V, Roy P, et al. Highly sensitive and selective detection of dopamine with boron and sulfur co-doped graphene quantum dots. Sci Rep. 2022;12:9061.10.1038/s41598-022-13016-4Search in Google Scholar PubMed PubMed Central
[31] Liao L, Peng H, Liu Z. Chemistry makes graphene beyond graphene. J Am Chem Soc. 2014;136:12194–200.10.1021/ja5048297Search in Google Scholar PubMed
[32] Mohanta Z, Gaonkar SK, Kumar M, Saini J, Tiwari V, Srivastava C, et al. Graphene research and their outputs: Status and prospect. J Sci: Adv Mater Devices. 2020;5:10–29.10.1016/j.jsamd.2020.01.006Search in Google Scholar
[33] Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6:183–91.10.1038/nmat1849Search in Google Scholar PubMed
[34] Hashemi SA, Bahrani S, Mousavi SM, Omidifar N, Behbahan N, Arjmand M, et al. Graphene‐based femtogram‐level sensitive molecularly imprinted polymer of SARS‐CoV‐2. Adv Mater Interfaces. 2021;8:2101466.10.1002/admi.202101466Search in Google Scholar PubMed PubMed Central
[35] Sunnardianto GK, Maruyama I, Kusakabe K. Systematic study of the effect of H adsorption on the electron-transfer rate in graphene. J Comput Theor Nanosci. 2016;13:4883–7.10.1166/jctn.2016.5361Search in Google Scholar
[36] Morishita N, Sunnardianto GK, Miyao S, Kusakabe K. Theoretical analysis of pseudodegenerate zero-energy modes in vacancy-centered hexagonal armchair nanographene. J Phys Soc Jpn. 2016;85:084703.10.7566/JPSJ.85.084703Search in Google Scholar
[37] Suvarnaphaet P, Pechprasarn S. Graphene-based materials for biosensors: A review. Sensors. 2017;17:2161.10.3390/s17102161Search in Google Scholar PubMed PubMed Central
[38] Zhu Y, Murali S, Cai W, Li X, Suk JW, Potts JR, et al. Graphene and graphene oxide: Synthesis, properties, and applications. Adv Mater. 2010;22:3906–24.10.1002/adma.201001068Search in Google Scholar PubMed
[39] Wang X, Zhao Y, Tian E, Li J, Ren Y. Graphene oxide‐based polymeric membranes for water treatment. Adv Mater Interfaces. 2018;5:1701427.10.1002/admi.201701427Search in Google Scholar
[40] Febriana E, Handayani M, Susilo DNA, Yahya MS, Ganta M, Sunnardianto GK. A simple approach of synthesis of graphene oxide from pure graphite: Time stirring duration variation. In AIP Conference Proceedings. AIP Publishing; 2021.10.1063/5.0060626Search in Google Scholar
[41] Handayani M, Ganta M, Susilo DNA, Yahya S, Sunnardianto GK, Darsono N. Synthesis of graphene oxide from used electrode graphite with controlled oxidation process. In IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2019. p. 012032.10.1088/1757-899X/541/1/012032Search in Google Scholar
[42] Hashemi SA, Bahrani S, Mousavi SM, Omidifar N, Arjmand M, Behbahan NGG, et al. Ultrasensitive biomolecule‐less nanosensor based on β‐cyclodextrin/quinoline decorated graphene oxide toward prompt and differentiable detection of corona and influenza viruses. Adv Mater Technol. 2021;6:2100341.10.1002/admt.202100341Search in Google Scholar
[43] Delbari SA, Ghadimi LS, Hadi R, Farhoudian S, Nedaei M, Babapoor A, et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J Alloy Compd. 2021;857:158281.10.1016/j.jallcom.2020.158281Search in Google Scholar
[44] Agarwal V, Zetterlund PB. Strategies for reduction of graphene oxide–A comprehensive review. Chem Eng J. 2021;405:127018.10.1016/j.cej.2020.127018Search in Google Scholar
[45] Handayani M, Mulyaningsih Y, Aulia Anggoro M, Abbas A, Setiawan I, Triawan F, et al. One-pot synthesis of reduced graphene oxide/chitosan/zinc oxide ternary nanocomposites for supercapacitor electrodes with enhanced electrochemical properties. Mater Lett. 2022;314:131846.10.1016/j.matlet.2022.131846Search in Google Scholar
[46] Ponomarenko LA, Schedin F, Katsnelson MI, Yang R, Hill EW, Novoselov KS, et al. Chaotic Dirac billiard in graphene quantum dots. Science (1979). 2008;320:356–8.10.1126/science.1154663Search in Google Scholar PubMed
[47] Zhang F, Liu F, Wang C, Xin X, Liu J, Guo S, et al. Effect of lateral size of graphene quantum dots on their properties and application. ACS Appl Mater Interfaces. 2016;8:2104–10.10.1021/acsami.5b10602Search in Google Scholar PubMed
[48] Mohanty N, Moore D, Xu Z, Sreeprasad TS, Nagaraja A, Rodriguez AA, et al. Nanotomy-based production of transferable and dispersible graphene nanostructures of controlled shape and size. Nat Commun. 2012;3:844.10.1038/ncomms1834Search in Google Scholar PubMed
[49] Yan J, Pan G, Lin W, Tang Z, Zhang J, Li J, et al. Multi-responsive graphene quantum dots hybrid self-healing structural color hydrogel for information encoding and encryption. Chem Eng J. 2023;451:138922.10.1016/j.cej.2022.138922Search in Google Scholar
[50] Hu Y, Neumann C, Scholtz L, Turchanin A, Resch-Genger U, Eigler S. Polarity, intramolecular charge transfer, and hydrogen bond co-mediated solvent effects on the optical properties of graphene quantum dots. Nano Res. 2023;16:45–52.10.1007/s12274-022-4752-1Search in Google Scholar
[51] Liu K, Li H, Wang F, Su Y. Recent advancement in graphene quantum dots based fluorescent sensor: Design, construction and bio-medical applications. Coord Chem Rev. 2023;478:214966.10.1016/j.ccr.2022.214966Search in Google Scholar
[52] Abbas A, Rubab S, Rehman A, Irfan S, Sharif HMA, Liang Q, et al. One-step green synthesis of biomass-derived graphene quantum dots as a highly selective optical sensing probe. Mater Today Chem. 2023;30:101555.10.1016/j.mtchem.2023.101555Search in Google Scholar
[53] Abbas A, Liang Q, Abbas S, Liaqat M, Rubab S, Tabish TA. Eco-friendly sustainable synthesis of graphene quantum dots from biowaste as a highly selective sensor. Nanomaterials. 2022;12:3696.10.3390/nano12203696Search in Google Scholar PubMed PubMed Central
[54] Zhu S, Song Y, Wang J, Wan H, Zhang Y, Ning Y, et al. Photoluminescence mechanism in graphene quantum dots: Quantum confinement effect and surface/edge state. Nano Today. 2017;13:10–4.10.1016/j.nantod.2016.12.006Search in Google Scholar
[55] Rajender G, Goswami U, Giri PK. Solvent dependent synthesis of edge-controlled graphene quantum dots with high photoluminescence quantum yield and their application in confocal imaging of cancer cells. J Colloid Interface Sci. 2019;541:387–98.10.1016/j.jcis.2019.01.099Search in Google Scholar PubMed
[56] Kumar R, Kumar J, Kadian S, Srivastava P, Manik G, Bag M. Tunable ionic conductivity and photoluminescence in quasi-2D CH3NH3PbBr3 thin films incorporating sulphur doped graphene quantum dots. Phys Chem Chem Phys. 2021;23:22733–42.10.1039/D1CP03621ASearch in Google Scholar
[57] Ye R, Peng Z, Metzger A, Lin J, Mann JA, Huang K, et al. Bandgap engineering of coal-derived graphene quantum dots. ACS Appl Mater Interfaces. 2015;7:7041–8.10.1021/acsami.5b01419Search in Google Scholar PubMed
[58] Yan Y, Chen J, Li N, Tian J, Li K, Jiang J, et al. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and CO2 reduction. ACS Nano. 2018;12:3523–32.10.1021/acsnano.8b00498Search in Google Scholar PubMed
[59] Choi S-H. Unique properties of graphene quantum dots and their applications in photonic/electronic devices. J Phys D Appl Phys. 2017;50:103002.10.1088/1361-6463/aa5244Search in Google Scholar
[60] Wu J, Lin H, Moss DJ, Loh KP, Jia B. Graphene oxide for photonics, electronics and optoelectronics. Nat Rev Chem. 2023;7:1–22.10.1038/s41570-022-00458-7Search in Google Scholar PubMed
[61] Ghosh D, Sarkar K, Devi P, Kim KH, Kumar P. Current and future perspectives of carbon and graphene quantum dots: From synthesis to strategy for building optoelectronic and energy devices. Renew Sustain Energy Rev. 2021;135:110391.10.1016/j.rser.2020.110391Search in Google Scholar
[62] Hasan MT, Gonzalez‐Rodriguez R, Ryan C, Faerber N, Coffer JL, Naumov AV. Photo‐and electroluminescence from nitrogen‐doped and nitrogen–sulfur codoped graphene quantum dots. Adv Funct Mater. 2018;28:1804337.10.1002/adfm.201804337Search in Google Scholar
[63] Zhao J, Tang L, Xiang J, Ji R, Hu Y, Yuan J, et al. Fabrication and properties of a high-performance chlorine doped graphene quantum dot based photovoltaic detector. RSC Adv. 2015;5:29222–9.10.1039/C5RA02358KSearch in Google Scholar
[64] Wang X, Sun G, Routh P, Kim DH, Huang W, Chen P. Heteroatom-doped graphene materials: syntheses, properties and applications. Chem Soc Rev. 2014;43:7067–98.10.1039/C4CS00141ASearch in Google Scholar
[65] Rani P, Dalal R, Srivastava S. Effect of surface modification on optical and electronic properties of graphene quantum dots. Appl Surf Sci. 2023;609:155379.10.1016/j.apsusc.2022.155379Search in Google Scholar
[66] Georgakilas V, Tiwari JN, Kemp KC, Perman JA, Bourlinos AB, Kim KS, et al. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev. 2016;116:5464–519.10.1021/acs.chemrev.5b00620Search in Google Scholar PubMed
[67] Miao X, Qu D, Yang D, Nie B, Zhao Y, Fan H, et al. Synthesis of carbon dots with multiple color emission by controlled graphitization and surface functionalization. Adv Mater. 2018;30:1704740.10.1002/adma.201704740Search in Google Scholar PubMed
[68] Tabish TA, Scotton CJ, Ferguson DCJ, Lin L, der Veen AV, Lowry S, et al. Biocompatibility and toxicity of graphene quantum dots for potential application in photodynamic therapy. Nanomedicine. 2018;13:1923–37.10.2217/nnm-2018-0018Search in Google Scholar PubMed
[69] Geng B, Hu J, Li Y, Feng S, Pan D, Feng L, et al. Near-infrared phosphorescent carbon dots for sonodynamic precision tumor therapy. Nat Commun. 2022;13:5735.10.1038/s41467-022-33474-8Search in Google Scholar PubMed PubMed Central
[70] Kalkal A, Kadian S, Pradhan R, Manik G, Packirisamy G. Recent advances in graphene quantum dot-based optical and electrochemical (bio) analytical sensors. Mater Adv. 2021;2:5513–41.10.1039/D1MA00251ASearch in Google Scholar
[71] Ni P, Li Q, Xu C, Lai H, Bai Y, Chen T. Optical properties of nitrogen and sulfur co-doped carbon dots and their applicability as fluorescent probes for living cell imaging. Appl Surf Sci. 2019;494:377–83.10.1016/j.apsusc.2019.07.196Search in Google Scholar
[72] Mondal MK, Mukherjee S, Joardar N, Roy D, Chowdhury P, Sinha Babu SP. Synthesis of smart graphene quantum dots: A benign biomaterial for prominent intracellular imaging and improvement of drug efficacy. Appl Surf Sci. 2019;495:143562.10.1016/j.apsusc.2019.143562Search in Google Scholar
[73] Xu X, He G, Wang L, Wang W, Jiang S, Fang Z. Optimization of electrical performance and stability of fully solution-driven α-InGaZnO thin-film transistors by graphene quantum dots. J Mater Sci Technol. 2023;141:100–9.10.1016/j.jmst.2022.09.016Search in Google Scholar
[74] Du F-P, Zhang H, Yao J-A, Chen SY, Xiao JK, Fu P, et al. Enhanced thermoelectric performance by constructing PEDOT: PSS/graphene quantum dots/single-walled carbon nanotube multilayer films. J Alloy Compd. 2022;911:164998.10.1016/j.jallcom.2022.164998Search in Google Scholar
[75] Mousavi SM, Hashemi SA, Kalashgrani MY, Omidifar N, Bahrani S, Vijayakameswara Rao N, et al. Bioactive graphene quantum dots based polymer composite for biomedical applications. Polym (Basel). 2022;14:617.10.3390/polym14030617Search in Google Scholar PubMed PubMed Central
[76] Wilczewska P, Breczko J, Bobrowska DM, Wysocka-Żołopa M, Goclon J, Basa A, et al. Enhancement of polypyrrole electrochemical performance with graphene quantum dots in polypyrrole nanoparticle/graphene quantum dot composites. J Electroanal Chem. 2022;923:116767.10.1016/j.jelechem.2022.116767Search in Google Scholar
[77] Zhu H, Li L, Shi M, Xiao P, Liu Y, Yan X. Coupling of graphene quantum dots with MnO2 nanosheets for boosting capacitive storage in ionic liquid electrolyte. Chem Eng J. 2022;437:135301.10.1016/j.cej.2022.135301Search in Google Scholar
[78] Kumar P, Dhand C, Dwivedi N, Singh S, Khan R, Verma S, et al. Graphene quantum dots: A contemporary perspective on scope, opportunities, and sustainability. Renew Sustain Energy Rev. 2022;157:111993.10.1016/j.rser.2021.111993Search in Google Scholar
[79] Bhuyan MSA, Uddin MN, Islam MM, Bipasha FA, Hossain SS. Synthesis of graphene. Int Nano Lett. 2016;6:65–83.10.1007/s40089-015-0176-1Search in Google Scholar
[80] Eissa S, N'diaye J, Brisebois P, Izquierdo R, Tavares AC, Siaj M. Probing the influence of graphene oxide sheets size on the performance of label-free electrochemical biosensors. Sci Rep. 2020;10:13612.10.1038/s41598-020-70384-5Search in Google Scholar PubMed PubMed Central
[81] Krishnan SK, Singh E, Singh P, Meyyappan M, Nalwa HS. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019;9:8778–881.10.1039/C8RA09577ASearch in Google Scholar
[82] Tian W, Li W, Yu W, Liu X. A review on lattice defects in graphene: types, generation, effects and regulation. Micromachines (Basel). 2017;8:163.10.3390/mi8050163Search in Google Scholar
[83] Alhourani A, Førde J-L, Eichacker LA, Herfindal L, Hagland HR. Improved pH-responsive release of phenformin from low-defect graphene compared to graphene oxide. ACS Omega. 2021;6:24619–29.10.1021/acsomega.1c03283Search in Google Scholar PubMed PubMed Central
[84] Najafi rad Z, Farzad F, Razavi L. Surface functionalization of graphene nanosheet with poly (l-histidine) and its application in drug delivery: Covalent vs non-covalent approaches. Sci Rep. 2022;12:19046.10.1038/s41598-022-21619-0Search in Google Scholar PubMed PubMed Central
[85] Lakshmanakumar M, Nesakumar N, Sethuraman S, Rajan KS, Krishnan UM, Rayappan J. Functionalized graphene quantum dot interfaced electrochemical detection of cardiac troponin I: an antibody free approach. Sci Rep. 2019;9:17348.10.1038/s41598-019-53979-5Search in Google Scholar PubMed PubMed Central
[86] Esfahani S, Akbari J, Soleimani-Amiri S, Mirzaei M, Ghasemi Gol A. Assessing the drug delivery of ibuprofen by the assistance of metal-doped graphenes: insights from density functional theory. Diam Relat Mater. 2023;135:109893.10.1016/j.diamond.2023.109893Search in Google Scholar
[87] Deng S, Gao E, Wang Y, Sen S, Sreenivasan ST, Behura S, et al. Confined, oriented, and electrically anisotropic graphene wrinkles on bacteria. ACS Nano. 2016;10:8403–12.10.1021/acsnano.6b03214Search in Google Scholar PubMed
[88] Hu KM, Liu YQ, Zhou LW, Xue ZY, Peng B, Yan H, et al. Delamination‐Free Functional Graphene Surface by Multiscale, Conformal Wrinkling. Adv Funct Mater. 2020;30:2003273.10.1002/adfm.202003273Search in Google Scholar
[89] Geim A, Novoselov K. Graphene calling. Nat Mater. 2007;6:169.10.1038/nmat1858Search in Google Scholar PubMed
[90] Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, et al. Electric field effect in atomically thin carbon films. Science (1979). 2004;306:666–9.10.1126/science.1102896Search in Google Scholar PubMed
[91] Zhu H, Cao Y, Zhang J, Zhang W, Xu Y, Guo J, et al. One-step preparation of graphene nanosheets via ball milling of graphite and the application in lithium-ion batteries. J Mater Sci. 2016;51:3675–83.10.1007/s10853-015-9655-zSearch in Google Scholar
[92] Papageorgiou DG, Kinloch IA, Young RJ. Mechanical properties of graphene and graphene-based nanocomposites. Prog Mater Sci. 2017;90:75–127.10.1016/j.pmatsci.2017.07.004Search in Google Scholar
[93] Emtsev KV, Bostwick A, Horn K, Jobst J, Kellogg GL, Ley L, et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nat Mater. 2009;8:203–7.10.1038/nmat2382Search in Google Scholar PubMed
[94] Paton KR, Varrla E, Backes C, Smith RJ, Khan U, O'Neill A, et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat Mater. 2014;13:624–30.10.1038/nmat3944Search in Google Scholar PubMed
[95] Dimiev AM, Ceriotti G, Metzger A, Kim ND, Tour JM. Chemical mass production of graphene nanoplatelets in ∼100% yield. ACS Nano. 2016;10:274–9.10.1021/acsnano.5b06840Search in Google Scholar PubMed
[96] Raccichini R, Varzi A, Passerini S, Scrosati B. The role of graphene for electrochemical energy storage. Nat Mater. 2015;14:271–9.10.1038/nmat4170Search in Google Scholar PubMed
[97] Abdelkader AM, Cooper AJ, Dryfe RA, Kinloch IA. How to get between the sheets: A review of recent works on the electrochemical exfoliation of graphene materials from bulk graphite. Nanoscale. 2015;7:6944–56.10.1039/C4NR06942KSearch in Google Scholar PubMed
[98] Parvez K, Li R, Puniredd SR, Hernandez Y, Hinkel F, Wang S, et al. Electrochemically exfoliated graphene as solution-processable, highly conductive electrodes for organic electronics. ACS Nano. 2013;7:3598–606.10.1021/nn400576vSearch in Google Scholar PubMed
[99] Shih CJ, Vijayaraghavan A, Krishnan R, Sharma R, Han JH, Ham MH, et al. Bi- and trilayer graphene solutions. Nat Nanotechnol. 2011;6:439–45.10.1038/nnano.2011.94Search in Google Scholar PubMed
[100] Ohta T, Bostwick A, McChesney JL, Seyller T, Horn K, Rotenberg E. Interlayer interaction and electronic screening in multilayer graphene. 2006;2:2–5.10.1140/epjst/e2007-00220-xSearch in Google Scholar
[101] Han D, Wang X, Zhao Y, Chen Y, Tang M, Zhao Z. High-quality graphene synthesis on amorphous SiC through a rapid thermal treatment. Carbon. 2017;124:105–10.10.1016/j.carbon.2017.06.002Search in Google Scholar
[102] Morozov SV, Novoselov KS, Katsnelson MI, Schedin F, Ponomarenko LA, Jiang D, et al. Strong suppression of weak localization in graphene. Phys Rev Lett. 2006;97(1):016801.Search in Google Scholar
[103] Giusca CE, Spencer SJ, Shard AG, Yakimova R, Kazakova O. Exploring graphene formation on the C-terminated face of SiC by structural, chemical and electrical methods. Carbon N Y. 2014;69:221–9.10.1016/j.carbon.2013.12.018Search in Google Scholar
[104] Saeed M, Alshammari Y, Majeed SA, Al-Nasrallah E. Chemical vapour deposition of graphene synthesis, characterisation, and application: A review. Molecules. 2020;25:2–62.10.3390/molecules25173856Search in Google Scholar PubMed PubMed Central
[105] Morton JA, Kaur A, Khavari M, Tyurnina AV, Priyadarshi A, Eskin DG, et al. An eco-friendly solution for liquid phase exfoliation of graphite under optimised ultrasonication conditions. Carbon N Y. 2023;204:434–46.10.1016/j.carbon.2022.12.070Search in Google Scholar
[106] Lee H, Choi JI, Park J, Jang SS, Lee SW. Role of anions on electrochemical exfoliation of graphite into graphene in aqueous acids. Carbon N Y. 2020;167:816–25.10.1016/j.carbon.2020.06.044Search in Google Scholar
[107] Chen Y, Zhao Y, Han D, Fu D, Chen Y, Zhou D, et al. A graphite enclosure assisted synthesis of high-quality patterned graphene on 6H–SiC by ion implantation. Carbon N Y. 2021;172:353–9.10.1016/j.carbon.2020.09.083Search in Google Scholar
[108] Tian P, Tang L, Teng KS, Lau SP. Graphene quantum dots from chemistry to applications. Mater Today Chem. 2018;10:221–58.10.1016/j.mtchem.2018.09.007Search in Google Scholar
[109] Singh I, Arora R, Dhiman H, Pahwa R. Carbon quantum dots: Synthesis, characterization and biomedical applications. Turk J Pharm Sci. 2018;15:219–30.10.4274/tjps.63497Search in Google Scholar PubMed PubMed Central
[110] Li M, Chen T, Gooding JJ, Liu J. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019;4:1732–48.10.1021/acssensors.9b00514Search in Google Scholar PubMed
[111] Li K, Liu W, Ni Y, Li D, Lin D, Su Z, et al. Technical synthesis and biomedical applications of graphene quantum dots. J Mater Chem B. 2017;5:4811–26.10.1039/C7TB01073GSearch in Google Scholar
[112] Yang F, Zhao M, Zheng B, Xiao D, Wu L, Guo Y. Influence of pH on the fluorescence properties of graphene quantum dots using ozonation pre-oxide hydrothermal synthesis. J Mater Chem. 2012;22:25471–9.10.1039/c2jm35471cSearch in Google Scholar
[113] Tetsuka H, Asahi R, Nagoya A, Okamoto K, Tajima I, Ohta R, et al. Optically tunable amino-functionalized graphene quantum dots. Adv Mater. 2012;24:5333–8.10.1002/adma.201201930Search in Google Scholar PubMed
[114] Kumar Chaturvedi A, Pappu A, Kumar Srivastava A, Kumar Gupta M. Synthesis, dielectric and mechanical properties of paddy straw derived graphene quantum dots-stone waste nanocomposite. Mater Lett. 2021;301:130323.10.1016/j.matlet.2021.130323Search in Google Scholar
[115] Srivastava A, Badatya S, Chaturvedi AK, Kashyap DK, Srivastava AK, Gupta MK. Paddy-Straw-Derived graphene quantum dots reinforced vertical aligned zinc oxide nanosheet-based flexible triboelectric nanogenerator for self-powered UV sensors and tribotronics application. ACS Appl Mater Interfaces. 2023;15:24724–35.10.1021/acsami.3c02036Search in Google Scholar PubMed
[116] Shen J, Zhu Y, Yang X, Zong J, Zhang J, Li C. One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. N J Chem. 2012;36:97–101.10.1039/C1NJ20658CSearch in Google Scholar
[117] Dong Y, Chen C, Zheng X, Gao L, Cui Z, Yang H, et al. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J Mater Chem. 2012;22:8764–6.Search in Google Scholar
[118] Nguyen HY, Le XH, Dao NT, Pham NT, Vu THH, Nguyen NH, et al. Microwave-assisted synthesis of graphene quantum dots and nitrogen-doped graphene quantum dots: Raman characterization and their optical properties. Adv Nat Sci: Nanosci Nanotechnol. 2019;10:025005.10.1088/2043-6254/ab1b73Search in Google Scholar
[119] Luo Z, Qi G, Chen K, Zou M, Yuwen L, Zhang X, et al. Microwave-assisted preparation of white fluorescent graphene quantum dots as a novel phosphor for enhanced white-light-emitting diodes. Adv Funct Mater. 2016;26:2739–44.10.1002/adfm.201505044Search in Google Scholar
[120] Shinde DB, Pillai VK. Electrochemical preparation of luminescent graphene quantum dots from multiwalled carbon nanotubes. Chem - A Eur J. 2012;18:12522–8.10.1002/chem.201201043Search in Google Scholar PubMed
[121] Kalita H, Harikrishnan V, Shinde DB, Pillai VK, Aslam M. Hysteresis and charge trapping in graphene quantum dots. Appl Phys Lett. 2013;102:1–5.10.1063/1.4800236Search in Google Scholar
[122] Lu J, Yang JX, Wang J, Lim A, Wang S, Loh KP. One-pot synthesis of fluorescent carbon nanoribbons, nanoparticles, and graphene by the exfoliation of graphite in ionic liquids. ACS Nano. 2009;3:2367–75.10.1021/nn900546bSearch in Google Scholar PubMed
[123] Zhang M, Bai L, Shang W, Xie W, Ma H, Fu Y, et al. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J Mater Chem. 2012;22:7461–7.10.1039/c2jm16835aSearch in Google Scholar
[124] Ananthanarayanan A, Wang X, Routh P, Sana B, Lim S, Kim DH, et al. Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing. Adv Funct Mater. 2014;24:3021–6.10.1002/adfm.201303441Search in Google Scholar
[125] Liu R, Wu D, Feng X, Müllen K. Bottom-up fabrication of photoluminescent graphene quantum dots with uniform morphology. J Am Chem Soc. 2011;133:15221–3.10.1021/ja204953kSearch in Google Scholar PubMed
[126] Dong Y, Shao J, Chen C, Li H, Wang R, Chi Y, et al. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon N Y. 2012;50:4738–43.10.1016/j.carbon.2012.06.002Search in Google Scholar
[127] Li R, Liu Y, Li Z, Shen J, Yang Y, Cui X, et al. Bottom-up fabrication of single-layered nitrogen-doped graphene quantum dots through intermolecular carbonization arrayed in a 2D plane. Chem - A Eur J. 2016;22:272–8.10.1002/chem.201503191Search in Google Scholar PubMed
[128] Yan X, Cui X, Li LS. Synthesis of large, stable colloidal graphene quantum dots with tunable size. J Am Chem Soc. 2010;132:5944–5.10.1021/ja1009376Search in Google Scholar PubMed
[129] Zhao C, Song X, Liu Y, Fu Y, Ye L, Wang N, et al. Synthesis of graphene quantum dots and their applications in drug delivery. J Nanobiotechnol. 2020;18:142. Epub ahead of print 2020. 10.1186/s12951-020-00698-z.Search in Google Scholar PubMed PubMed Central
[130] Wang L, Li W, Wu B, Li Z, Pan D, Wu M. Room-temperature synthesis of graphene quantum dots via electron-beam irradiation and their application in cell imaging. Chem Eng J. 2017;309:374–80.10.1016/j.cej.2016.10.022Search in Google Scholar
[131] Facure MHM, Schneider R, Mercante LA, Correa DS. Rational hydrothermal synthesis of graphene quantum dots with optimized luminescent properties for sensing applications. Mater Today Chem. 2022;23:100755.10.1016/j.mtchem.2021.100755Search in Google Scholar
[132] Dong Y, Chen C, Zheng X, Gao L, Cui Z, Yang H, et al. One-step and high yield simultaneous preparation of single- and multi-layer graphene quantum dots from CX-72 carbon black. J Mater Chem. 2012;22:8764–6.10.1039/c2jm30658aSearch in Google Scholar
[133] Li W, Li M, Liu Y, Pan D, Li Z, Wang L, et al. Three minute ultrarapid microwave-assisted synthesis of bright fluorescent graphene quantum dots for live cell staining and white LEDs. ACS Appl Nano Mater. 2018;1:1623–30.10.1021/acsanm.8b00114Search in Google Scholar
[134] Ghaffarkhah A, Hosseini E, Kamkar M, Sehat AA, Dordanihaghighi S, Allahbakhsh A, et al. Synthesis, applications, and prospects of graphene quantum dots: A comprehensive review. Small. 2022;18:2102683.10.1002/smll.202102683Search in Google Scholar PubMed
[135] Abbas A, Tabish TA, Bull SJ, Lim TM, Phan AN. High yield synthesis of graphene quantum dots from biomass waste as a highly selective probe for Fe3+ sensing. Sci Rep. 2020;10:1–16.10.1038/s41598-020-78070-2Search in Google Scholar PubMed PubMed Central
[136] Pumera M. Graphene in biosensing. Mater Today. 2011;14:308–15.10.1016/S1369-7021(11)70160-2Search in Google Scholar
[137] Seo G, Lee G, Kim MJ, Baek SH, Choi M, Ku KB, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–42.10.1021/acsnano.0c02823Search in Google Scholar PubMed
[138] Morozov SV, Novoselov KS, Katsnelson MI, Schedin F, Ponomarenko LA, Jiang D, et al. Strong suppression of weak localization in graphene. Phys Rev Lett. 2006;97:7–10.10.1103/PhysRevLett.97.016801Search in Google Scholar PubMed
[139] Peña-Bahamonde J, Nguyen HN, Fanourakis SK, Rodrigues DF. Recent advances in graphene-based biosensor technology with applications in life sciences. J Nanobiotechnology. 2018;16:1–17.10.1186/s12951-018-0400-zSearch in Google Scholar PubMed PubMed Central
[140] Wu X, Xing Y, Zeng K, Huber K, Zhao JX. Study of fluorescence quenching ability of graphene oxide with a layer of rigid and tunable silica spacer. Langmuir. 2018;34:603–11.10.1021/acs.langmuir.7b03465Search in Google Scholar PubMed
[141] Li H, Huang X, Mehedi Hassan M, Zuo M, Wu X, Chen Y, et al. Dual-channel biosensor for Hg2+ sensing in food using Au@ Ag/graphene-upconversion nanohybrids as metal-enhanced fluorescence and SERS indicators. Microchem J. 2020;154:104563.10.1016/j.microc.2019.104563Search in Google Scholar
[142] Wong XY, Quesada-González D, Manickam S, New SY, Muthoosamy K, Merkoçi A. Integrating gold nanoclusters, folic acid and reduced graphene oxide for nanosensing of glutathione based on “turn-off” fluorescence. Sci Rep. 2021;11:2375.10.1038/s41598-021-81677-8Search in Google Scholar PubMed PubMed Central
[143] Kadadou D, Tizani L, Wadi VS, Banat F, Alsafar H, Yousef AF, et al. Detection of SARS-CoV-2 in clinical and environmental samples using highly sensitive reduced graphene oxide (rGO)-based biosensor. Chem Eng J. 2023;453:139750.10.1016/j.cej.2022.139750Search in Google Scholar PubMed PubMed Central
[144] Fan J, Yuan L, Liu Q, Tong C, Wang W, Xiao F, et al. An ultrasensitive and simple assay for the Hepatitis C virus using a reduced graphene oxide-assisted hybridization chain reaction. Analyst. 2019;144:3972–9.10.1039/C9AN00179DSearch in Google Scholar PubMed
[145] Gao J, He P, Yang T, Zhou L, Wang X, Chen S, et al. Electrodeposited NiO/graphene oxide nanocomposite: an enhanced voltammetric sensing platform for highly sensitive detection of uric acid, dopamine and ascorbic acid. J Electroanal Chem. 2019;852:113516.10.1016/j.jelechem.2019.113516Search in Google Scholar
[146] Zhang H, Liu S. Electrochemical sensors based on nitrogen-doped reduced graphene oxide for the simultaneous detection of ascorbic acid, dopamine and uric acid. J Alloy Compd. 2020;842:155873.10.1016/j.jallcom.2020.155873Search in Google Scholar
[147] Yang H, Bao J, Qi Y, Zhao J, Hu Y, Wu W, et al. A disposable and sensitive non-enzymatic glucose sensor based on 3D graphene/Cu2O modified carbon paper electrode. Anal Chim Acta. 2020;1135:12–9.10.1016/j.aca.2020.08.010Search in Google Scholar PubMed
[148] Yu H, Chong Y, Zhang P, Ma J, Li D. A D-shaped fiber SPR sensor with a composite nanostructure of MoS2-graphene for glucose detection. Talanta. 2020;219:121324.10.1016/j.talanta.2020.121324Search in Google Scholar PubMed
[149] Zhang Y, Chen X, Roozbahani GM, Guan X. Graphene oxide-based biosensing platform for rapid and sensitive detection of HIV-1 protease. Anal Bioanal Chem. 2018;410:6177–85.10.1007/s00216-018-1224-2Search in Google Scholar PubMed PubMed Central
[150] Mohsin DH, Mashkour MS, Fatemi F. Design of aptamer-based sensing platform using gold nanoparticles functionalized reduced graphene oxide for ultrasensitive detection of Hepatitis B virus. Chem Pap. 2021;75:279–95.10.1007/s11696-020-01292-1Search in Google Scholar
[151] Jin X, Zhang H, Li Y-T, Xiao MM, Zhang ZL, Pang DW, et al. A field effect transistor modified with reduced graphene oxide for immunodetection of Ebola virus. Microchim Acta. 2019;186:1–9.10.1007/s00604-019-3256-5Search in Google Scholar PubMed
[152] Khalil I, Yehye WA, Julkapli NM, Rahmati S, Sina AA, Basirun WJ, et al. Graphene oxide and gold nanoparticle based dual platform with short DNA probe for the PCR free DNA biosensing using surface-enhanced Raman scattering. Biosens Bioelectron. 2019;131:214–23.10.1016/j.bios.2019.02.028Search in Google Scholar PubMed
[153] Ye Y, Xie J, Ye Y, Cao X, Zheng H, Xu X, et al. A label-free electrochemical DNA biosensor based on thionine functionalized reduced graphene oxide. Carbon N Y. 2018;129:730–7.10.1016/j.carbon.2017.12.060Search in Google Scholar
[154] Nayak TR, Andersen H, Makam VS, Khaw C, Bae S, Xu X, et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano. 2011;5:4670–8.10.1021/nn200500hSearch in Google Scholar PubMed
[155] Shadjou N, Hasanzadeh M, Khalilzadeh B. Graphene based scaffolds on bone tissue engineering. Bioengineered. 2018;9:38–47.10.1080/21655979.2017.1373539Search in Google Scholar PubMed PubMed Central
[156] Mohammadi S, Shafiei SS, Asadi-Eydivand M, Ardeshir M, Solati-Hashjin M. Graphene oxide-enriched poly (ε-caprolactone) electrospun nanocomposite scaffold for bone tissue engineering applications. J Bioact Compat Polym. 2017;32:325–42.Search in Google Scholar
[157] Mohammadi S, Shafiei SS, Asadi-Eydivand M, Ardeshir M, Solati-Hashjin M. Graphene oxide-enriched poly(ϵ-caprolactone) electrospun nanocomposite scaffold for bone tissue engineering applications. J Bioact Compat Polym. 2017;32:325–42.10.1177/0883911516668666Search in Google Scholar
[158] Kalbacova M, Broz A, Kong J, Kalbac M. Graphene substrates promote adherence of human osteoblasts and mesenchymal stromal cells. Carbon N Y. 2010;48:4323–9.10.1016/j.carbon.2010.07.045Search in Google Scholar
[159] Li N, Zhang X, Song Q, Su R, Zhang Q, Kong T, et al. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials. 2011;32:9374–82.10.1016/j.biomaterials.2011.08.065Search in Google Scholar PubMed
[160] Ahmed MK, Menazea AA, Mansour SF, Al-Wafi R. Differentiation between cellulose acetate and polyvinyl alcohol nanofibrous scaffolds containing magnetite nanoparticles/graphene oxide via pulsed laser ablation technique for tissue engineering applications. J Mater Res Technol. 2020;9:11629–40.10.1016/j.jmrt.2020.08.041Search in Google Scholar
[161] Murugesan B, Pandiyan N, Arumugam M, Sonamuthu J, Samayanan S, Yurong C, et al. Fabrication of palladium nanoparticles anchored polypyrrole functionalized reduced graphene oxide nanocomposite for antibiofilm associated orthopedic tissue engineering. Appl Surf Sci. 2020;510:145403.10.1016/j.apsusc.2020.145403Search in Google Scholar
[162] Jie W, Song F, Li X, Li W, Wang R, Jiang Y, et al. Enhancing the proliferation of MC3T3-E1 cells on casein phosphopeptide-biofunctionalized 3D reduced-graphene oxide/polypyrrole scaffolds. RSC Adv. 2017;7:34415–24.10.1039/C7RA02146ASearch in Google Scholar
[163] Yu P, Bao R-Y, Shi X-J, Yang W, Yang MB. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr Polym. 2017;155:507–15.10.1016/j.carbpol.2016.09.001Search in Google Scholar PubMed
[164] Lu R, Zhang W, He Y, Zhang S, Fu Q, Pang Y, et al. Ferric ion crosslinking‐based 3D printing of a graphene oxide hydrogel and its evaluation as a bio‐scaffold in tissue engineering. Biotechnol Bioeng. 2021;118:1006–12.10.1002/bit.27592Search in Google Scholar PubMed
[165] Heidari M, Bahrami SH, Ranjbar-Mohammadi M, Milan PB. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater Sci Eng: C. 2019;103:109768.10.1016/j.msec.2019.109768Search in Google Scholar PubMed
[166] Abzan N, Kharaziha M, Labbaf S. Development of three-dimensional piezoelectric polyvinylidene fluoride-graphene oxide scaffold by non-solvent induced phase separation method for nerve tissue engineering. Mater Des. 2019;167:107636.10.1016/j.matdes.2019.107636Search in Google Scholar
[167] Zeinali K, Khorasani MT, Rashidi A, Daliri Joupari M. Preparation and characterization of graphene oxide aerogel/gelatin as a hybrid scaffold for application in nerve tissue engineering. Int J Polym Mater Polym Biomater. 2021;70:674–83.10.1080/00914037.2020.1760269Search in Google Scholar
[168] Dasari Shareena TP, McShan D, Dasmahapatra AK, Tchounwou PB. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nanomicro Lett. 2018;10:1–34.10.1007/s40820-018-0206-4Search in Google Scholar PubMed PubMed Central
[169] Lage T, Rodrigues RO, Catarino S, Gallo J, Bañobre-López M, Minas G. Graphene-based magnetic nanoparticles for theranostics: An overview for their potential in clinical application. Nanomaterials. 2021;11(5):1073. 10.3390/nano11051073.Search in Google Scholar PubMed PubMed Central
[170] Jafari Z, Rad AS, Baharfar R, Asghari S, Esfahani MR. Synthesis and application of chitosan/tripolyphosphate/graphene oxide hydrogel as a new drug delivery system for Sumatriptan Succinate. J Mol Liq. 2020;315:113835.10.1016/j.molliq.2020.113835Search in Google Scholar
[171] Kumar G, Chaudhary K, Mogha NK, Kant A, Masram DT. Extended release of metronidazole drug using chitosan/graphene oxide bionanocomposite beads as the drug carrier. ACS Omega. 2021;6:20433–44.10.1021/acsomega.1c02422Search in Google Scholar PubMed PubMed Central
[172] Tiwari H, Karki N, Pal M, Basak S, Verma RK, Bal R, et al. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf B Biointerfaces. 2019;178:452–9.10.1016/j.colsurfb.2019.03.037Search in Google Scholar PubMed
[173] Chen K, Ling Y, Cao C, Li X, Chen X, Wang X. Chitosan derivatives/reduced graphene oxide/alginate beads for small-molecule drug delivery. Mater Sci Eng: C. 2016;69:1222–8.10.1016/j.msec.2016.08.036Search in Google Scholar PubMed
[174] Mahmoud ME, Attia AA, Helmy MW, Hemdan IH, Abouelanwar ME. Doxorubicin drug release behavior from amino-silanated graphene oxide nanocarrier. Diam Relat Mater. 2023;131:109569.10.1016/j.diamond.2022.109569Search in Google Scholar
[175] Qi Z, Shi J, Zhu B, Li J, Cao S. Gold nanorods/graphene oxide nanosheets immobilized by polydopamine for efficient remotely triggered drug delivery. J Mater Sci. 2020;55:14530–43.10.1007/s10853-020-05050-2Search in Google Scholar
[176] Tong C, Zhang X, Fan J, Li B, Liu B, Daniyal M, et al. PEGylated mBPEI-rGO nanocomposites facilitate hepotocarcinoma treatment combining photothermal therapy and chemotherapy. Sci Bull (Beijing). 2018;63:935–46.10.1016/j.scib.2018.06.003Search in Google Scholar PubMed
[177] Singh G, Nenavathu BP. Development of rGO encapsulated polymeric beads as drug delivery system for improved loading and controlled release of doxycycline drug. Drug Dev Ind Pharm. 2020;46:462–70.10.1080/03639045.2020.1724137Search in Google Scholar PubMed
[178] Kooti M, Sedeh AN, Motamedi H, Rezatofighi SE. Magnetic graphene oxide inlaid with silver nanoparticles as antibacterial and drug delivery composite. Appl Microbiol Biotechnol. 2018;102:3607–21.10.1007/s00253-018-8880-1Search in Google Scholar PubMed
[179] Horgan CC, Bergholt MS, Nagelkerke A, Thin MZ, Pence IJ, Kauscher U, et al. Integrated photodynamic Raman theranostic system for cancer diagnosis, treatment, and post-treatment molecular monitoring. Theranostics. 2021;11:2006–19.10.7150/thno.53031Search in Google Scholar PubMed PubMed Central
[180] Lin J, Chen X, Huang P. Graphene-based nanomaterials for bioimaging. Adv Drug Deliv Rev. 2016;105:242–54.10.1016/j.addr.2016.05.013Search in Google Scholar PubMed PubMed Central
[181] Ferrer-Ugalde A, Sandoval S, Pulagam KR, Muñoz-Juan A, Laromaine A, Llop J, et al. Radiolabeled Cobaltabis (dicarbollide) Anion–Graphene Oxide Nanocomposites for In Vivo Bioimaging and Boron Delivery. ACS Appl Nano Mater. 2021;4:1613–25.10.1021/acsanm.0c03079Search in Google Scholar
[182] Song X, Li S, Guo H, You W, Shang X, Li R, et al. Graphene‐oxide‐modified lanthanide nanoprobes for tumor‐targeted visible/NIR‐II luminescence imaging. Angew Chem Int Ed. 2019;58:18981–6.10.1002/anie.201909416Search in Google Scholar PubMed
[183] Song Y-Y, Li C, Yang X-Q, An J, Cheng K, Xuan Y, et al. Graphene oxide coating core–shell silver sulfide@ mesoporous silica for active targeted dual-mode imaging and chemo-photothermal synergistic therapy against tumors. J Mater Chem B. 2018;6:4808–20.10.1039/C8TB00940FSearch in Google Scholar PubMed
[184] Jia X, Xu W, Ye Z, Wang Y, Dong Q, Wang E, et al. Functionalized graphene@ gold nanostar/lipid for pancreatic cancer gene and photothermal synergistic therapy under photoacoustic/photothermal imaging dual‐modal guidance. Small. 2020;16:2003707.10.1002/smll.202003707Search in Google Scholar PubMed
[185] Wanas W, Abd El-Kaream SA, Ebrahim S, Soliman M, Karim M. Cancer bioimaging using dual mode luminescence of graphene/FA-ZnO nanocomposite based on novel green technique. Sci Rep. 2023;13:27.10.1038/s41598-022-27111-zSearch in Google Scholar PubMed PubMed Central
[186] Zang Z, Zeng X, Wang M, Hu W, Liu C, Tang X. Tunable photoluminescence of water-soluble AgInZnS–graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sens Actuators B Chem. 2017;252:1179–86.10.1016/j.snb.2017.07.144Search in Google Scholar
[187] Yogesh GK, Shuaib EP, Roopmani P, Gumpu MB, Krishnan UM, Sastikumar D. Synthesis, characterization and bioimaging application of laser-ablated graphene-oxide nanoparticles (nGOs). Diam Relat Mater. 2020;104:107733.10.1016/j.diamond.2020.107733Search in Google Scholar
[188] Lei L, Ma H, Yang M, Qin Y, Ma Y, Wang T, et al. Fluorophore-functionalized graphene oxide with application in cell imaging. N J Chem. 2017;41:12375–9.10.1039/C7NJ02416ASearch in Google Scholar
[189] Chawda N, Basu M, Majumdar D, Poddar R, Mahapatra SK, Banerjee I. Engineering of gadolinium-decorated graphene oxide nanosheets for multimodal bioimaging and drug delivery. ACS Omega. 2019;4:12470–9.10.1021/acsomega.9b00883Search in Google Scholar PubMed PubMed Central
[190] Akhavan O, Ghaderi E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano. 2010;4:5731–6.10.1021/nn101390xSearch in Google Scholar PubMed
[191] Liu S, Zeng TH, Hofmann M, Burcombe E, Wei J, Jiang R, et al. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano. 2011;5:6971–80.10.1021/nn202451xSearch in Google Scholar PubMed
[192] Liu T, Liu Y, Liu M, Wang Y, He W, Shi G, et al. Synthesis of graphene oxide-quaternary ammonium nanocomposite with synergistic antibacterial activity to promote infected wound healing. Burn Trauma. 2018;6:1–23.10.1186/s41038-018-0115-2Search in Google Scholar PubMed PubMed Central
[193] Valentini F, Calcaterra A, Ruggiero V, Pichichero E, Martino A, Iosi F, et al. Functionalized graphene derivatives: Antibacterial properties and cytotoxicity. J Nanomater. 2019;2019:1–14. 10.1155/2019/2752539.Search in Google Scholar
[194] He J, Zhu X, Qi Z, Wang C, Mao X, Zhu C, et al. Killing dental pathogens using antibacterial graphene oxide. ACS Appl Mater Interfaces. 2015;7:5605–11.10.1021/acsami.5b01069Search in Google Scholar PubMed
[195] Ye S, Shao K, Li Z, Guo N, Zuo Y, Li Q, et al. Antiviral activity of graphene oxide: How sharp edged structure and charge matter. ACS Appl Mater Interfaces. 2015;7:21578–9.10.1021/acsami.5b06876Search in Google Scholar PubMed
[196] Sametband M, Kalt I, Gedanken A, Sarid R. Herpes simplex virus type-1 attachment inhibition by functionalized graphene oxide. ACS Appl Mater Interfaces. 2014;6:1228–35.10.1021/am405040zSearch in Google Scholar PubMed
[197] Deokar AR, Nagvenkar AP, Kalt I, Shani L, Yeshurun Y, Gedanken A, et al. Graphene-based ‘hot Plate’ for the capture and destruction of the herpes simplex virus type 1. Bioconjugate Chem. 2017;28:1115–22.10.1021/acs.bioconjchem.7b00030Search in Google Scholar PubMed
[198] Fallatah H, Elhaneid M, Ali-Boucetta H, Overton TW, El Kadri H, Gkatzionis K. Antibacterial effect of graphene oxide (GO) nano-particles against Pseudomonas putida biofilm of variable age. Environ Sci Pollut Res. 2019;26:25057–70.10.1007/s11356-019-05688-9Search in Google Scholar PubMed PubMed Central
[199] Sengupta I, Bhattacharya P, Talukdar M, Neogi S, Pal SK, Chakraborty S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloid Interface Sci Commun. 2019;28:60–8.10.1016/j.colcom.2018.12.001Search in Google Scholar
[200] Tang J, Chen Q, Xu L, Zhang S, Feng L, Cheng L, et al. Graphene oxide–silver nanocomposite as a highly effective antibacterial agent with species-specific mechanisms. ACS Appl Mater Interfaces. 2013;5:3867–74.10.1021/am4005495Search in Google Scholar PubMed
[201] Ali NH, Amin MCIM, Ng S-F. Sodium carboxymethyl cellulose hydrogels containing reduced graphene oxide (rGO) as a functional antibiofilm wound dressing. J Biomater Sci Polym Ed. 2019;30:629–45.10.1080/09205063.2019.1595892Search in Google Scholar PubMed
[202] Chang Y-N, Ou X-M, Zeng G-M, Gong JL, Deng CH, Jiang Y, et al. Synthesis of magnetic graphene oxide–TiO2 and their antibacterial properties under solar irradiation. Appl Surf Sci. 2015;343:1–10.10.1016/j.apsusc.2015.03.082Search in Google Scholar
[203] Dhandapani P, AlSalhi MS, Karthick R, Chen F, Devanesan S, Kim W, et al. Biological mediated synthesis of RGO-ZnO composites with enhanced photocatalytic and antibacterial activity. J Hazard Mater. 2021;409:124661.10.1016/j.jhazmat.2020.124661Search in Google Scholar PubMed
[204] Hashem AH, Hasanin M, Kamel S, Dacrory S. A new approach for antimicrobial and antiviral activities of biocompatible nanocomposite based on cellulose, amino acid and graphene oxide. Colloids Surf B Biointerfaces. 2022;209:112172.10.1016/j.colsurfb.2021.112172Search in Google Scholar PubMed
[205] Du T, Lu J, Liu L, Dong N, Fang L, Xiao S, et al. Antiviral activity of graphene oxide–silver nanocomposites by preventing viral entry and activation of the antiviral innate immune response. ACS Appl Bio Mater. 2018;1:1286–93.10.1021/acsabm.8b00154Search in Google Scholar PubMed
[206] Yang XX, Li CM, Li YF, Wang J, Huang CZ. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–92.10.1039/C7NR06520ESearch in Google Scholar
[207] Hai X, Feng J, Chen X, Wang J. Tuning the optical properties of graphene quantum dots for biosensing and bioimaging. J Mater Chem B. 2018;6:3219–34.10.1039/C8TB00428ESearch in Google Scholar PubMed
[208] Xu JJ, Zhao WW, Song S, Fan C, Chen HY. Functional nanoprobes for ultrasensitive detection of biomolecules: An update. Chem Soc Rev. 2014;43:1601–11.10.1039/C3CS60277JSearch in Google Scholar
[209] Zhang J, Cheng F, Li J, Zhu JJ, Lu Y. Fluorescent nanoprobes for sensing and imaging of metal ions: Recent advances and future perspectives. Nano Today. 2016;11:309–29.10.1016/j.nantod.2016.05.010Search in Google Scholar PubMed PubMed Central
[210] Wang F, Gu Z, Lei W, Wang W, Xia X, Hao Q. Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II) ions. Sens Actuators B Chem. 2014;190:516–22.10.1016/j.snb.2013.09.009Search in Google Scholar
[211] Niu X, Zhong Y, Chen R, Wang F, Liu Y, Luo D. A “turn-on” fluorescence sensor for Pb2+ detection based on graphene quantum dots and gold nanoparticles. Sens Actuators B Chem. 2018;255:1577–81.10.1016/j.snb.2017.08.167Search in Google Scholar
[212] Anh NTN, Chowdhury AD, Doong RA. Highly sensitive and selective detection of mercury ions using N, S-codoped graphene quantum dots and its paper strip based sensing application in wastewater. Sens Actuators B Chem. 2017;252:1169–78. 10.1016/j.snb.2017.07.177Search in Google Scholar
[213] Ran X, Sun H, Pu F, Ren J, Qu X. Ag nanoparticle-decorated graphene quantum dots for label-free, rapid and sensitive detection of Ag+ and biothiols. Chem Commun. 2013;49:1079–81.10.1039/c2cc38403eSearch in Google Scholar PubMed
[214] Chung S, Revia RA, Zhang M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater. 2021;33:1–26.10.1002/adma.201904362Search in Google Scholar PubMed PubMed Central
[215] Xu A, He P, Huang T, Li J, Hu X, Xiang P, et al. Selective supramolecular interaction of ethylenediamine functionalized graphene quantum dots: Ultra-sensitive photoluminescence detection for nickel ion in vitro. Synth Met. 2018;244:106–12.10.1016/j.synthmet.2018.05.013Search in Google Scholar
[216] Li N, Than A, Chen J, Xi F, Liu J, Chen P. Graphene quantum dots based fluorescence turn-on nanoprobe for highly sensitive and selective imaging of hydrogen sulfide in living cells. Biomater Sci. 2018;6:779–84.10.1039/C7BM00818JSearch in Google Scholar
[217] Lin L, Rong M, Lu S, Song X, Zhong Y, Yan J, et al. A facile synthesis of highly luminescent nitrogen-doped graphene quantum dots for the detection of 2, 4, 6-trinitrophenol in aqueous solution. Nanoscale. 2015;7:1872–8.10.1039/C4NR06365ASearch in Google Scholar
[218] Gao W, Song H, Wang X, Liu X, Pang X, Zhou Y, et al. Carbon dots with red emission for sensing of Pt2+, Au3+, and Pd2+ and their bioapplications in vitro and in vivo. ACS Appl Mater Interfaces. 2018;10:1147–54.10.1021/acsami.7b16991Search in Google Scholar PubMed
[219] Su Y, Zhou X, Long Y, Li W. Immobilization of horseradish peroxidase on amino-functionalized carbon dots for the sensitive detection of hydrogen peroxide. Microchim Acta. 2018;185:1–8.10.1007/s00604-017-2629-xSearch in Google Scholar PubMed
[220] Hassanvand Z, Jalali F, Nazari M, Parnianchi F, Santoro C. Carbon nanodots in electrochemical sensors and biosensors: A review. ChemElectroChem. 2021;8:15–35.10.1002/celc.202001229Search in Google Scholar
[221] Mollarasouli F, Asadpour-Zeynali K, Campuzano S, Yáñez-Sedeño P, Pingarrón JM. Non-enzymatic hydrogen peroxide sensor based on graphene quantum dots-chitosan/methylene blue hybrid nanostructures. Electrochim Acta. 2017;246:303–14.10.1016/j.electacta.2017.06.003Search in Google Scholar
[222] Yang C, Hu LW, Zhu HY, Ling Y, Tao JH, Xu CX. RGO quantum dots/ZnO hybrid nanofibers fabricated using electrospun polymer templates and applications in drug screening involving an intracellular H2O2 sensor. J Mater Chem B. 2015;3:2651–9.10.1039/C4TB02134GSearch in Google Scholar PubMed
[223] Bai J, Sun C, Jiang X. Carbon dots-decorated multiwalled carbon nanotubes nanocomposites as a high-performance electrochemical sensor for detection of H2O2 in living cells. Anal Bioanal Chem. 2016;408:4705–14.10.1007/s00216-016-9554-4Search in Google Scholar PubMed
[224] Lin L, Song X, Chen Y, Rong M, Zhao T, Wang Y, et al. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal Chim Acta. 2015;869:89–95.10.1016/j.aca.2015.02.024Search in Google Scholar PubMed
[225] Ge S, He J, Ma C, Liu J, Xi F, Dong X. One-step synthesis of boron-doped graphene quantum dots for fluorescent sensors and biosensor. Talanta. 2019;199:581–9.10.1016/j.talanta.2019.02.098Search in Google Scholar PubMed
[226] Shi H, Chen L, Niu N. An off-on fluorescent probe based on graphene quantum dots intercalated hydrotalcite for determination of ascorbic acid and phytase. Sens Actuators B Chem. 2021;345:130353.10.1016/j.snb.2021.130353Search in Google Scholar
[227] Samuei S, Fakkar J, Rezvani Z, Shomali A, Habibi B. Synthesis and characterization of graphene quantum dots/CoNiAl-layered double-hydroxide nanocomposite: Application as a glucose sensor. Anal Biochem. 2017;521:31–9.10.1016/j.ab.2017.01.005Search in Google Scholar PubMed
[228] Tam TV, Hur SH, Chung JS, Choi WM. Novel paper- and fiber optic-based fluorescent sensor for glucose detection using aniline-functionalized graphene quantum dots. Sens Actuators B Chem. 2021;329:129250.10.1016/j.snb.2020.129250Search in Google Scholar
[229] Zhang Y, Wei X, Gu Q, Zhang J, Ding Y, Xue L, et al. Cascade amplification based on PEI-functionalized metal–organic framework supported gold nanoparticles/nitrogen–doped graphene quantum dots for amperometric biosensing applications. Electrochim Acta. 2022;405:139803.10.1016/j.electacta.2021.139803Search in Google Scholar
[230] Nashruddin SNA, Abdullah J, Mohammad Haniff MAS, Mat Zaid MH, Choon OP, Mohd Razip Wee MF. Label free glucose electrochemical biosensor based on poly (3, 4-ethylenedioxy thiophene): Polystyrene sulfonate/titanium carbide/graphene quantum dots. Biosensors (Basel). 2021;11:267.10.3390/bios11080267Search in Google Scholar PubMed PubMed Central
[231] Kunpatee K, Traipop S, Chailapakul O, Chuanuwatanakul S. Simultaneous determination of ascorbic acid, dopamine, and uric acid using graphene quantum dots/ionic liquid modified screen-printed carbon electrode. Sens Actuators B Chem. 2020;314:128059.10.1016/j.snb.2020.128059Search in Google Scholar
[232] Qiu J, Li D, Mou X, Li J, Guo W, Wang S, et al. Effects of graphene quantum dots on the self-renewal and differentiation of mesenchymal stem cells. Adv Healthc Mater. 2016;5:702–10.10.1002/adhm.201500770Search in Google Scholar PubMed
[233] Yan Z, Ye T, Yang L, Jiang H, Chen C, Chen S, et al. Nanobiology dependent therapeutic convergence between biocompatibility and bioeffectiveness of graphene oxide quantum dot scaffold for immuno‐inductive angiogenesis and nerve regeneration. Adv Funct Mater. 2023;33:2211709.10.1002/adfm.202211709Search in Google Scholar
[234] Shafiei S, Omidi M, Nasehi F, Golzar H, Mohammadrezaei D, Rezai Rad M, et al. Egg shell-derived calcium phosphate/carbon dot nanofibrous scaffolds for bone tissue engineering: Fabrication and characterization. Mater Sci Eng: C. 2019;100:564–75.10.1016/j.msec.2019.03.003Search in Google Scholar PubMed
[235] Ghanbari M, Salavati-Niasari M, Mohandes F. Thermosensitive alginate–gelatin–nitrogen-doped carbon dots scaffolds as potential injectable hydrogels for cartilage tissue engineering applications. RSC Adv. 2021;11:18423–31.10.1039/D1RA01496JSearch in Google Scholar PubMed PubMed Central
[236] Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6:1306–23.10.7150/thno.14858Search in Google Scholar PubMed PubMed Central
[237] Zhu S, Meng Q, Wang L, Zhang J, Song Y, Jin H, et al. Highly photoluminescent carbon dots for multicolor patterning, sensors, and bioimaging. Angew Chem - Int Ed. 2013;52:3953–7.10.1002/anie.201300519Search in Google Scholar PubMed
[238] Vatanparast M, Shariatinia Z. AlN and AlP doped graphene quantum dots as novel drug delivery systems for 5-fluorouracil drug: Theoretical studies. J Fluor Chem. 2018;211:81–93.10.1016/j.jfluchem.2018.04.003Search in Google Scholar
[239] Khodadadei F, Safarian S, Ghanbari N. Methotrexate-loaded nitrogen-doped graphene quantum dots nanocarriers as an efficient anticancer drug delivery system. Mater Sci Eng: C. 2017;79:280–5.10.1016/j.msec.2017.05.049Search in Google Scholar PubMed
[240] Sheng Y, Dai W, Gao J, Li H, Tan W, Wang J, et al. pH-sensitive drug delivery based on chitosan wrapped graphene quantum dots with enhanced fluorescent stability. Mater Sci Eng: C. 2020;112:110888.10.1016/j.msec.2020.110888Search in Google Scholar PubMed
[241] Dong J, Wang K, Sun L, Sun B, Yang M, Chen H, et al. Application of graphene quantum dots for simultaneous fluorescence imaging and tumor-targeted drug delivery. Sens Actuators B Chem. 2018;256:616–23.10.1016/j.snb.2017.09.200Search in Google Scholar
[242] Teng Y, Yuan S, Shi J, Pong PWT. A multifunctional nanoplatform based on graphene quantum dots‐cobalt ferrite for monitoring of drug delivery and fluorescence/magnetic resonance bimodal cellular imaging. Adv Nanobiomed Res. 2022;2:2200044.10.1002/anbr.202200044Search in Google Scholar
[243] Moasses Ghafary S, Rahimjazi E, Hamzehil H, Modarres Mousavi SM, Nikkhah M, Hosseinkhani S. Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of doxorubicin/curcumin and graphene quantum dots. Nanomedicine. 2022;42:102544.10.1016/j.nano.2022.102544Search in Google Scholar PubMed
[244] Javanbakht S, Namazi H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater Sci Eng: C. 2018;87:50–9.10.1016/j.msec.2018.02.010Search in Google Scholar PubMed
[245] Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: Potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–8.Search in Google Scholar
[246] Tannock IF, Rotin D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989;49:4373–84.Search in Google Scholar
[247] Fan Z, Zhou S, Garcia C, Fan L, Zhou J. PH-Responsive fluorescent graphene quantum dots for fluorescence-guided cancer surgery and diagnosis. Nanoscale. 2017;9:4928–33.10.1039/C7NR00888KSearch in Google Scholar
[248] Campbell E, Hasan MT, Gonzalez Rodriguez R, Akkaraju GR, Naumov AV. Doped graphene quantum dots for intracellular multicolor imaging and cancer detection. ACS Biomater Sci Eng. 2019;5:4671–82.10.1021/acsbiomaterials.9b00603Search in Google Scholar PubMed
[249] Zhao N, Li B, Chen D, Ahmad R, Zhu Y, Li G, et al. Yellow emissive nitrogen-doped graphene quantum dots as a label-free fluorescent probe for Fe3+ sensing and bioimaging. Diam Relat Mater. 2020;104:107749.10.1016/j.diamond.2020.107749Search in Google Scholar
[250] Shah H, Xie W, Wang Y, Jia X, Nawaz A, Xin Q, et al. Preparation of blue-and green-emissive nitrogen-doped graphene quantum dots from graphite and their application in bioimaging. Mater Sci Eng: C. 2021;119:111642.10.1016/j.msec.2020.111642Search in Google Scholar PubMed
[251] Narasimhan AK, Lakshmi B S, Santra TS, Rao MSR, Krishnamurthi G. Oxygenated graphene quantum dots (GQDs) synthesized using laser ablation for long-term real-time tracking and imaging. RSC Adv. 2017;7:53822–9.10.1039/C7RA10702ASearch in Google Scholar
[252] Kumawat MK, Thakur M, Gurung RB, Srivastava R. Graphene quantum dots from mangifera indica: application in near-infrared bioimaging and intracellular nanothermometry. ACS Sustain Chem Eng. 2017;5:1382–91.10.1021/acssuschemeng.6b01893Search in Google Scholar
[253] Schroer ZS, Wu Y, Xing Y, Wu X, Liu X, Wang X, et al. Nitrogen–sulfur-doped graphene quantum dots with metal ion-resistance for bioimaging. ACS Appl Nano Mater. 2019;2:6858–65.10.1021/acsanm.9b01309Search in Google Scholar
[254] Wang G, He P, Xu A, Guo Q, Li J, Wang Z, et al. Promising fast energy transfer system between graphene quantum dots and the application in fluorescent bioimaging. Langmuir. 2018;35:760–6.10.1021/acs.langmuir.8b03739Search in Google Scholar PubMed
[255] Sun H, Gao N, Dong K, Ren J, Qu X. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano. 2014;8:6202–10.10.1021/nn501640qSearch in Google Scholar PubMed
[256] Zeng Z, Yu D, He Z, Liu J, Xiao FX, Zhang Y, et al. Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly-enhanced bactericidal and antibiofouling performances. Sci Rep. 2016;6:20142.10.1038/srep20142Search in Google Scholar PubMed PubMed Central
[257] Chowdhury AD, Takemura K, Li T-C, Suzuki T, Park EY. Electrical pulse-induced electrochemical biosensor for hepatitis E virus detection. Nat Commun. 2019;10:3737.10.1038/s41467-019-11644-5Search in Google Scholar PubMed PubMed Central
[258] Raju L, Jacob MS, Rajkumar E. Don’t dust off the dust!–A facile synthesis of graphene quantum dots derived from indoor dust towards their cytotoxicity and antibacterial activity. N J Chem. 2022;46:14859–66.10.1039/D2NJ02876JSearch in Google Scholar
[259] Mohan AN, Manoj B. Biowaste derived graphene quantum dots interlaced with SnO 2 nanoparticles–a dynamic disinfection agent against Pseudomonas aeruginosa. N J Chem. 2019;43:13681–9.10.1039/C9NJ00379GSearch in Google Scholar
[260] Teymourinia H, Salavati-Niasari M, Amiri O, Yazdian F. Application of green synthesized TiO2/Sb2S3/GQDs nanocomposite as high efficient antibacterial agent against E. coli and Staphylococcus aureus. Mater Sci Eng: C. 2019;99:296–303.10.1016/j.msec.2019.01.094Search in Google Scholar PubMed
[261] Teymourinia H, Amiri O, Salavati-Niasari M. Synthesis and characterization of cotton-silver-graphene quantum dots (cotton/Ag/GQDs) nanocomposite as a new antibacterial nanopad. Chemosphere. 2021;267:129293.10.1016/j.chemosphere.2020.129293Search in Google Scholar PubMed
[262] Zhang L, Liu L, Wang J, Niu M, Zhang C, Yu S, et al. Functionalized silver nanoparticles with graphene quantum dots shell layer for effective antibacterial action. J Nanopart Res. 2020;22:1–12.10.1007/s11051-020-04845-3Search in Google Scholar
[263] Choi SH, Yun SJ, Won YS, Oh CS, Kim SM, Kim KK, et al. Large-scale synthesis of graphene and other 2D materials towards industrialization. Nat Commun. 2022;13:1484.10.1038/s41467-022-29182-ySearch in Google Scholar PubMed PubMed Central
[264] Munuera J, Britnell L, Santoro C, Cuéllar-Franca R, Casiraghi C. A review on sustainable production of graphene and related life cycle assessment. 2d Mater. 2021;9:012002.10.1088/2053-1583/ac3f23Search in Google Scholar
[265] Torres FG, Troncoso OP, Rodriguez L, De-la-Torre GE. Sustainable synthesis, reduction and applications of graphene obtained from renewable resources. Sustain Mater Technol. 2021;29:e00310.10.1016/j.susmat.2021.e00310Search in Google Scholar
[266] Lü M, Li J, Yang X, Zhang C, Yang J, Hu H, et al. Applications of graphene-based materials in environmental protection and detection. Chin Sci Bull. 2013;58:2698–710.10.1007/s11434-013-5887-ySearch in Google Scholar
[267] Kuo W-S, Tai N-H, Chang T-W. Deformation and fracture in graphene nanosheets. Compos Part A Appl Sci Manuf. 2013;51:56–61.10.1016/j.compositesa.2013.03.020Search in Google Scholar
[268] Yang K, Wan J, Zhang S, Zhang Y, Lee ST, Liu Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano. 2011;5:516–22.10.1021/nn1024303Search in Google Scholar PubMed
[269] Chang Y, Yang S-T, Liu J-H, Dong E, Wang Y, Cao A, et al. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol Lett. 2011;200:201–10.10.1016/j.toxlet.2010.11.016Search in Google Scholar PubMed
[270] Wang K, Ruan J, Song H, Zhang J, Wo Y, Guo S, et al. Biocompatibility of graphene oxide. Nanoscale Res Lett. 2011;6:1–8.10.1007/s11671-010-9751-6Search in Google Scholar PubMed PubMed Central
[271] Khan B, Adeleye AS, Burgess RM, Smolowitz R, Russo SM, Ho KT. A 72‐h exposure study with eastern oysters (Crassostrea virginica) and the nanomaterial graphene oxide. Environ Toxicol Chem. 2019;38:820–30.10.1002/etc.4367Search in Google Scholar PubMed PubMed Central
[272] De Marchi L, Neto V, Pretti C, Figueira E, Brambilla L, Rodriguez-Douton MJ, et al. Physiological and biochemical impacts of graphene oxide in polychaetes: The case of Diopatra neapolitana. Comp Biochem Physiol Part C: Toxicol Pharmacol. 2017;193:50–60.10.1016/j.cbpc.2017.01.005Search in Google Scholar PubMed
[273] Lammel T, Boisseaux P, Navas JM. Potentiating effect of graphene nanomaterials on aromatic environmental pollutant‐induced cytochrome P450 1A expression in the topminnow fish hepatoma cell line PLHC‐1. Environ Toxicol. 2015;30:1192–204.10.1002/tox.21991Search in Google Scholar PubMed
[274] Yan C, Hu X, Guan P, Hou T, Chen P, Wan D, et al. Highly biocompatible graphene quantum dots: green synthesis, toxicity comparison and fluorescence imaging. J Mater Sci. 2020;55:1198–215.10.1007/s10853-019-04079-2Search in Google Scholar
[275] Guo Z, Chakraborty S, Monikh FA, Varsou DD, Chetwynd AJ, Afantitis A, et al. Surface functionalization of graphene‐based materials: Biological behavior, toxicology, and safe‐by‐design aspects. Adv Biol. 2021;5:2100637.10.1002/adbi.202100637Search in Google Scholar PubMed
[276] Shi S, Chen F, Ehlerding EB, Cai W. Surface engineering of graphene-based nanomaterials for biomedical applications. Bioconjugate Chem. 2014;25:1609–19.10.1021/bc500332cSearch in Google Scholar PubMed PubMed Central
[277] Chiticaru EA, Ioniţă M. Graphene toxicity and future perspectives in healthcare and biomedicine. FlatChem. 2022;35:100417.10.1016/j.flatc.2022.100417Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

