Abstract
In this study, we conducted the hydrothermal synthesis of cobalt (Co)–doped NiWO4, resulting in the formation of Co–NiWO4 nanoparticles (NPs), followed by calcination at 550℃ for 12 h. Comprehensive analyses were performed to characterize the composition, structure, and morphology of the synthesized material. X-ray diffraction results confirmed the successful inclusion of Co in the NiWO4 lattice, with the presence of characteristic peaks of CoWO4. The crystallite size, determined using the Scherrer equation, was measured to be 22 nm. Using UV-Vis spectroscopy and Tauc’s equation, we calculated the band gap energy (E g) to be 3.75 eV for NiWO4 and 1.75 eV for Co–NiWO4. The potential application of the synthesized material as a photocatalyst was investigated for the degradation of the diazo dye Congo red (CR). Under optimized reaction conditions, Co–NiWO4 NPs demonstrated outstanding efficiency, degrading a total of 95% of CR. The degradation kinetics were well-described by the Langmuir–Hinshelwood (L–H) kinetic model, indicating that photoabsorption played a crucial role in the rate-controlling step. These encouraging results suggest that Co–NiWO4 NPs hold promise as a viable option for addressing other pollutants in various applications.
1 Introduction
In the current context of robust globalization and industrial growth, the decline in clean water quality is evident, primarily due to the introduction of hazardous pollutants like heavy metals and dyes from direct and indirect sources [1,2]. Azo dyes, a group of synthetic compounds containing one or more azo groups (–N…N–) as chromophores, are particularly noteworthy among these pollutants [3,4]. Their distinctive colors, ease of synthesis, high solubility, and excellent fastness rating make them favored colorants in industries such as food, pharmaceuticals, and textiles [5,6]. However, despite their advantageous properties, azo dyes are known to be toxic, allergenic to human skin, and carcinogenic, leading to strict regulations governing their use in many countries [7,8]. One specific azo dye of concern is Congo red (CR), an anionic bis azo dye containing benzidine salt, which poses a significant health risk due to its tendency to bioaccumulate in the human body and contribute to various neurological and respiratory ailments [1,9]. Therefore, there is a pressing need to develop efficient and environmentally friendly methods and materials capable of effectively degrading the CR dye in wastewater before its release into water bodies or the environment.
The literature presents various methods for CR removal from wastewater, including biodegradation, ultrafiltration, adsorption, and chemical oxidation. However, the production of secondary-level sludge, the generation of pollutants, and complex operational procedures have limited their practical application [10,11]. To address these challenges, a promising approach is the photocatalysis-based advanced oxidation process. This method harnesses solar light to interact with a catalyst material, leading to the generation of electron–hole (e‒/h+) pairs. These active e−/h+ pairs effectively attack CR molecules, breaking them into smaller, non-toxic compounds such as CO2 and H2O [12,13]. Designing materials with the ability to efficiently generate and stabilize these e‒/h+ pairs is crucial for the success of this eco-friendly and sustainable process.
Various semiconductor-based nanomaterials have been synthesized and used as photocatalysts for the degradation of CR such as V2O5–TiO2, ZnO, plasmonic metal nanoparticles (NPs) (Ag, Au, Pd, and Pt), ZnO–ZnS–CdO–CdS, ZnS:Fe, Fe2(MoO4)3, etc. [14,15,16,17,18,19]. Among all these metal oxide and metal sulfide NPs, a high rate of charge recombination and wide band gap hinders the efficiency of TiO2, the aqueous medium instability of ZnO NPs at variational pH values, toxic sulfide release under illumination rules out the utilization of sulfide catalyst water decontamination [14,17,18]. These discrepancies between metal oxide and sulfide NPs draw the attention of scientists and researchers toward the utilization of multi-metal component-based photocatalytic materials. The materials, such as SrTiO3 [20], NaTaO3 [21], BaTi4O9 [22], CaIn2O4 [23], and La1–X Cr x FeO3 [24], have been reported to decontaminate wastewater
One of the classes of multi-metal-based oxide attracting the attention of the scientific community is tungstate-based nanomaterials with the empirical formula MWO4 (M = Co, Ni, Cu, and Fe) [25,26]. They have been proven to be a promising agent for environmental decontamination and solar water splitting due to narrow band gaps with extraordinary light absorption quality [27,28]. They are widely applied in various industrial processes such as scintillation, microwave technology, fiber optics, catalysis, and photoluminescence [29,30]. Among all these, NiWO4 with an energy band gap value of 2.6 eV is one of the important tungstate family members having very high demands in fields like laser hosts, humidity sensors, catalysis, and microwave application [31]. Appreciable work has been reported in the literature corresponding to the utilization of ZnWO4 as a photocatalyst toward the degradation of toxic dyes and amplification in photocatalytic efficiency through hetero atom doping [32,33,34,35]. The photocatalytic behavior of other tungstate materials such as PbWO4 and Bi2WO4 was also explored in the literature [36,37]. However, no studies are reported in the literature regarding the utilization of Co-doped NiWO4 NPs toward photocatalytic degradation of CR. Therefore, this research gap provides the opportunity to elaborate the information regarding variations in the structural, morphological, optical, and photocatalytic properties of Co-doped NiWO4. Although pristine NiWO4 exhibits very good photocatalytic performances for the mineralization of organic contaminants, however, because of its high purity, the high e‒/h+ recombination rate limits its photocatalytic efficiency [30]. To address these issues and improve the overall catalytic performance of NiWO4, various chemical techniques such as semiconductor coupling, [31], doping [29], noble metal deposition [35], and morphology control [30] were considered. In the present study, the method of doping with a suitable metal atom in the pure crystal lattice of NiWO4 was chosen to reflect changes in the structural matrix to achieve the required optical properties. From the literature, various studies have been reported on the metal doping of NiWO4 for multidisciplinary applications such as Cu doping, Mn doping, Bi doping, etc. [38,39,40]. In this study, cobalt (Co2+) was specifically chosen as a dopant due to its similar ionic radius and strong magnetic moment (μCo = 1.8 μB d7 low spin configuration) [41,42]. By introducing Co2+ into the NiWO4 solid matrix, we aimed to inhibit e‒/h+ recombination and enhance photocatalytic activities via Ni d‒d transitions and Co‒W metal charge transfer mechanisms [43]. Several methods have been reported in the literature for synthesizing NiWO4 and mixed metal-based tungstate nanomaterials, including sol‒gel processing [44], solid-state reaction [45], a hydrothermal method [46], and polymerized complex method [47]. These methods have shown enhanced stability, improved electron transport mechanisms, and higher energy density in the resulting nanostructures. In this study, we employed a hydrothermal route to synthesize pure phase (Co, Ni, Cu, Zn)-tungstates with a uniform particle size distribution, and we explored their potential as a photocatalyst for degrading the diazo dye CR. Additionally, we investigated the impact of various reaction parameters, such as irradiation time (minutes), pH, catalyst dose, visible light intensity, temperature, and leaching experiments, on the photocatalytic efficiency of Co–NiWO4 NPs in degrading CR. These investigations aimed to understand the factors influencing the photocatalytic process and optimize the performance of Co–NiWO4 NPs as an effective photocatalyst for CR degradation.
2 Materials and methods
2.1 Chemicals
Sodium tungstate dehydrate (Na2WO4·2H2O, 98%) was purchased from Loba Chemie. Nickel nitrate hexahydrate (Ni (NO3)2·6H2O, 98%) and cobalt nitrate hexahydrate (Co (NO3)2·6H₂O, 98%) were purchased from Merck. CR (99%) and ammonia solution (25%) were purchased from Otto Chemie. Double distilled water was used throughout the experiments, and the chemicals received were used as such without any further purification (Figure 1).

Chemical structure of the CR dye.
2.2 Preparation of the Co–NiWO4 nanocomposite
The pristine NiWO4 and Co–NiWO4 NPs were synthesized using the hydrothermal method as described in a previous study [46]. To prepare the NPs, a solution containing equimolar (1:1) amounts of Ni (NO3)2·6H2O and Co (NO3)2·6H2O (both 5 mmol) was prepared in 25 mL of deionized (DI) water and stirred for 1 h at 25℃ until a clear solution was obtained. This solution was labeled as Solution A. Next, Solution A was added dropwise to a 10 mmol solution of Na2WO4·2H2O (Solution B). The resulting mixture was stirred on a magnetic stirrer until homogeneity was achieved. To maintain the pH ∼8–10, 10 mL of a 25% ammonia solution was added to the mixture (Solution A + Solution B). After 30 min of stirring, the mixture was transferred to a 100 mL Teflon-lined autoclave and placed in a digital oven at 190℃ for 12 h for the hydrothermal treatment. Subsequently, the material was obtained by centrifugation and washed multiple times with DI water until the effluents exhibited a neutral pH. The washed material was then dehydrated in an oven at 100℃ for 6 h and subsequently calcined at 500℃ for 4 h under an N2 atmosphere.
2.3 Material characterization
Various analytical techniques were employed to characterize the synthesized material and confirm the successful formation of the desired Co–NiWO4 nanocomposite. Fourier transform infrared spectroscopy (FTIR) was used to investigate the bond formation between Co and WO4 2− and the peak shifting upon Co inclusion in the NiWO4 matrix, with measurements taken in the range of 4,000–400 cm−1 using a Perkin Elmer Spectrum 2 ATR spectrometer. The X-ray diffraction (XRD) method, conducted with a Rigaku Ultima 1 V X-ray diffractometer, provided insights into changes in the lattice, crystallite size, and interplanar distance resulting from Co doping in the NiWO4 crystal lattice. Scanning electron microscopy (SEM) in conjunction with energy X-ray diffraction (EDX) and mapping (JEOL GSM 6510LV, Japan) were utilized to study the surface morphology and elemental composition of the synthesized material. Transmission electron microscopy (TEM) using a JEM 2100 microscope (Japan) allowed the observation of variations in the crystallite size and crystal structure after solid-state reactions. To analyze the electron shift occurring upon Co doping and to assess the concentration of CR remaining after the photocatalytic experiment, ultraviolet-visible spectroscopy (UV-Vis) was employed with a Shimadzu UV-1900 spectrophotometer. These comprehensive characterizations contributed to a thorough understanding of the material’s properties and its potential application as an efficient photocatalyst for CR degradation.
2.4 Photocatalytic activity
The photocatalytic performance of the as-synthesized nanocomposite material as a catalyst was evaluated by studying the degradation of CR (30, 50, and 70 ppm) under visible solar radiation. An aliquot of 10 mL of 20 ppm CR dye was treated with 10 mg of Co–NiWO4 NPs sonicated for 3 min and then placed under dark for 1 h to maintain absorption–desorption equilibrium. Then, the mixture was placed under visible light radiation (350 W, Xe lamp with 100 mW cm–2 intensity, λ > 420 nm) for an optimum amount of time, and the concentration of CR post-reaction was assessed by taking 3 mL of aliquot using UV–Vis spectrophotometer at λ max = 494 nm. All the experiments were repeated three times to get better precision and suppress maximum error in the data. The photocatalytic efficiency of Co–NiWO4 NPs toward the CR dye was evaluated by the following equation:
where C o and C e are the concentration of CR at the initial state and each time interval, respectively.
3 Results and discussion
3.1 Material characterization
Figure 2(a) displays the FTIR spectra of NiWO4, CoWO4, and Co–NiWO4 NPs in which pristine NiWO4 shows the characteristic peaks at 470 cm–1 (stretching vibrations of Ni–O bond), 535 cm–1 (W─O bonds), 620 and 706 cm–1 (bending and stretching vibrations of W–O bond in WO6 6– octahedron), and 821 and 886 cm–1 (bending and stretching vibration of the WO2 entity associated with W2O8 groups) [48]. In addition, the peaks at 3,430 and 1,629 cm−1 (stretching and bending vibrations of –OH groups) suggest the presence of a notable amount of surface-adsorbed water. The FTIR spectra of Co–NiWO4 NPs reveal all the peaks like pristine NiWO4 with some shifted values due to doping of Co in the solid lattice. The observed absorption bands are in good agreement with IR data on NiWO4 with the wolframite structure [43,49].

(a) FTIR of NiWO4 (black line), CoWO4 (red line), and Co–NiWO4 NPs (red line). (b) XRD spectra of NiWO4 (red line) and Co–NiWO4 NPs (blue line).
Figure 2(b) shows the XRD spectra of as-synthesized Co–NiWO4 NPs, which exhibit the characteristic peaks at 2θ values of 15.62, 19.45, 24.03, 25.05, 27.86, 30.92, 32.96, 36.45, 41.75, 44.92, 46.71, 52.45, 54.62, 58.81, 62.39, 65.95, and 72.84° corresponding to Miller indices values of (010), (001), (011), (110), (200), (111), (020), (002), (102), (112), (211), (130), (132), and (302) (JCPDS No. 15-0755), respectively. From the literature, the Miller indices values for pure NiWO4 have been reported as (010), (100), (011), (110), (111), (021), (200), (121), (112), (211), (022), (220), (130), (202), (113), (311), and (041) with wolframite monoclinic structure associated with JCPDS No. 72-0480. Based on the Miller indices data, it was observed that XRD spectra of Co–NiWO4 represented the maximum of Miller indices values from NiWO4, except (002) and (310), which belong to the hkl planes associated with CoWO4 (JCPDS No. 15-0867). The obtained values of Miller indices suggested that the synthesized material is a solid solution instead of the mixture of CoWO4 and NiWO4, implying that Co2+ ions have successfully taken the lattice position in NiWO4 due to the closeness of ionic radii of both Co2+ and Ni2+ ions [42]. The XRD spectra of Co–NiWO4 NPs were composed of sharp peaks suggesting a crystalline nature with a wolframite monoclinic phase [50]. Further, the crystallite size and interplanar distance of Co–NiWO4 were determined by the Scherer equation given by the following equations [51]:
where D is the size of the crystal, λ is the wavelength used (i.e., 1.5 Å), β is the half-width of the intense peak, and θ is the diffraction angle. Using the XRD data in equation (2), the average crystallite size (D) was found to be 22 ± 0.05 nm with the interplanar distance d 111 = 0.21 Å, which is found to be lesser than reported in the literature.
The morphology and topography of NiWO4 and Co–NiWO4 NPs were investigated using scanning electron microscopy (SEM) and their images are displayed in Figure 3(a) and (b). The pristine NiWO4 (Figure 3a) appeared as a porous aggregation of small particles while Co–NiWO4 NPs also appeared porous with the corrugated surface with some flakes with voids (Figure 3b). Therefore, SEM results supported the Co doping in the NiWO4, which was further verified by elemental composition analysis using EDX (Figure 3(c) and (d)). The EDX spectra of NiWO4 in Figure 3(c) revealed the elemental composition as O (70.03%), Ni (14.75%), and W (15.22%); while the EDX spectra of Co–NiWO4 NPs (Figure 3d) have the elemental composition as O (74.63%), Co (4.09%), Ni (5.88%), and W (15.40%). The EDX results revealed the presence of elements in stoichiometry and purity of the pristine and doped material. The uniformity of elemental distribution in the solid lattice was checked by elemental mapping analysis given in Figure S1, which suggests that all the elements are uniformly distributed over a range of space selected by the SEM image.

SEM–EDX image of (a, c) NiWO4 and (b, d) Co–NiWO4 NPs.
Further information about the shape and distribution of particles in the nanocomposite material upon doping of Co was assessed by TEM analysis, and the results are given in Figure 4(a) and (b). Figure 4(a) represents the TEM image of Co–NiWO4 NPs at 100 nm magnification bar showing the distribution of nanorods of size 24 ± 0.35 nm. Further magnification at 20 nm bar TEM image ( Figure 4(b)) suggested a mitigated hexagonal type of geometry of the particles. The obtained value of particle size by TEM analysis is also approximately close to the Scherrer crystallite size.

TEM images of Co–NiWO4 NC: (a) 100 nm and (b) 20 nm magnification range.
The change in optical properties of the material upon Co doping was investigated by UV–Vis spectroscopy and the results are given in Figure 5(a). The UV–Vis profile of NiWO4 (black line) has been given in the range of 200–600 nm and the presence of a small peak at 268 nm suggested the material to be UV light active. The type of transition could be attributed to the excitation of electrons from W and O within the WO6 matrix [51]. The UV-Vis spectra of CoWO4 given in Figure 5(a) (red line) exhibited a low-intensity broad spectrum at 588 nm, suggesting the material is visible light active. The UV spectra of Co–NiWO4 NPs exhibited two broad spectra, one in the range from 200 to 600 nm with absorption maxima at 354 nm and another at 667 nm with a low intensity suggesting the material to be both UV and visible light active. These bands could be attributed to the electron transition from 3A2g to 1Eg and 3A2g to 1T2g, respectively, in the Ni2+ O6 matrix [52]. The change in optical properties after doping of Co and the appearance of a broad spectrum is a direct reflectance of contribution from the Co–W metal charge transfer and Ni d–d transition [53,54]. Furthermore, the energy band gap (E g) value of the pristine and doped material was calculated by the following equation [55]:
where h is the Planck constant, α is the absorption coefficient, A is a constant, ν is the frequency of radiation, and n is the constant of transition variation corresponding to n = 2 as indirect transitions and n = 1/2 belonging to direct transitions. The energy band gap value was obtained by extrapolating the linear part of the curve which cut the hν (eV) axis as illustrated in Figure 5(b). The values of E g from Tauc’s plot were obtained as 3.54 eV for NiWO4, 3.05 eV for CoWO4, and 1.58 eV for Co–NiWO4 NC. From the literature, the band gap values of NiWO4 have been reported as 2.53, 3.7, and 3.6 eV depending upon the synthesis protocols [43,56]. Therefore, the calculated value in the present study is in good agreement with the reported values. The outcomes suggested that the Co doping in NiWO4 created intermediate impurity level formation inside the band gap structure of NiWO4, which reduced its band gap and turned it into a promising catalyst for photocatalytic applications in visible light [57]. The UV–Vis analysis suggested that the optical properties of NiWO4 can be tuned by Co doping, which results in greater light absorption capacity due to low band gap values.
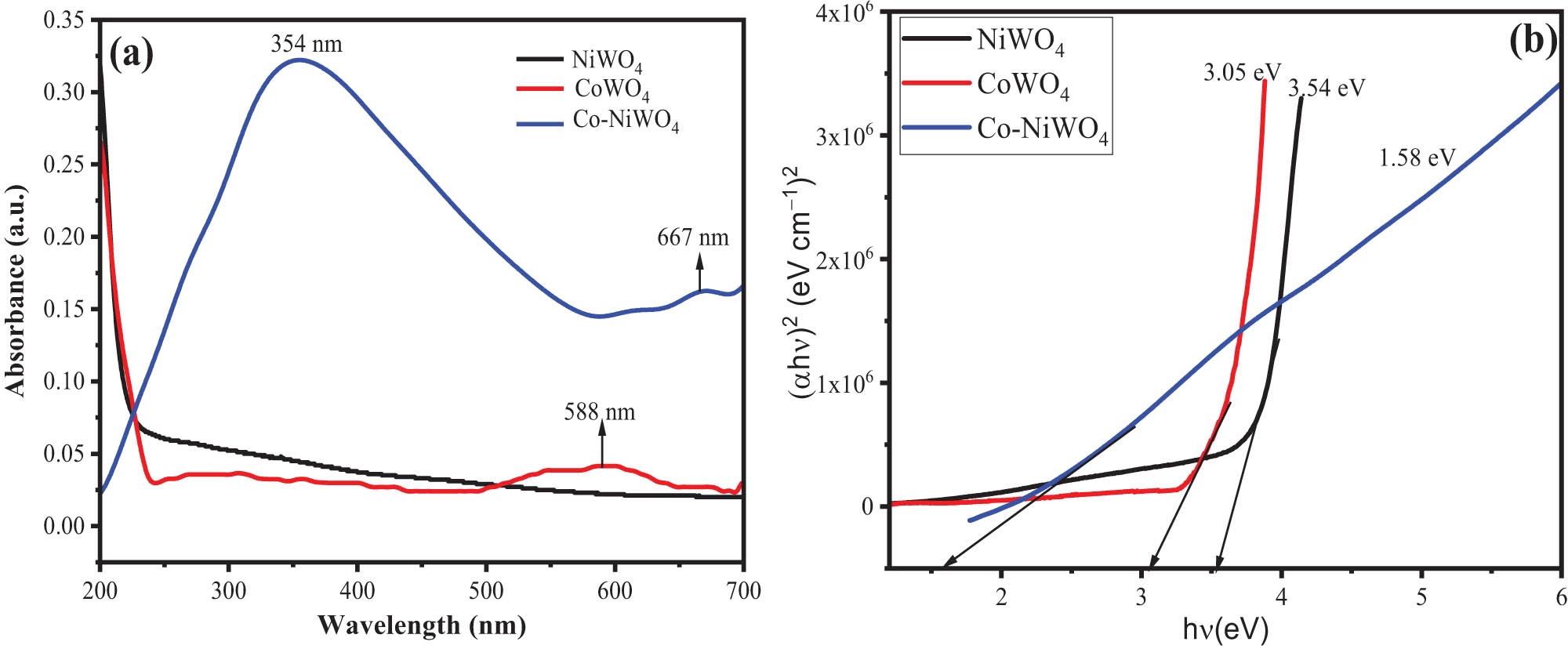
(a) UV–Vis spectra and (b) Tauc’s plot for energy band gap for NiWO4, CoWO4, and Co–NiWO4 NPs.
Thermogravimetric analysis (TGA) was carried out to evaluate the thermal stability of the synthesized material Co–NiWO4 NPs. Figure 6 shows the TGA profiles for NiWO4, CoWO4, and Co–NiWO4. The initial weight loss at a temperature of 200℃ is due to the loss of water molecules and adsorbed gases. Further weight loss in the temperature range between 248.63 and 489.86℃ is due to the loss of metal hydroxides or breakage in M–O linkages. The final weight loss beyond 490℃ is due to the formation of intermediate products [58,59]. NiWO4 shows a total weight loss of 43.28%, CoWO4 shows 44.23%, and Co–NiWO4 shows 45.69%.

Thermogram profiles for NiWO4 (black line), CoWO4 (red line), and Co–NiWO4 (blue line).
3.2 Photocatalytic applications
3.2.1 Selectivity test
To evaluate the photocatalytic efficiency of pristine NiWO4 and synthesized Co–NiWO4 NC, experiments were conducted using 10 mg of the catalyst with 10 mL of 50 ppm organic pollutant solution such as bromophenol (BP), methyl orange (MO), CR, malachite green (MG), crystal violet (CV), and methylene blue (MB) under visible solar radiation. From the results shown in Figure S2(a–c), it was found that the synthesized material was most effective toward the degradation of the CR dye (88.20%) as compared to pristine NiWO4 (38.13%) under the given conditions as compared to other organic pollutants. Therefore, Co doping in NiWO4 has tuned the photocatalytic activity of Co–NiWO4 NPs toward the CR dye. Hence, further photocatalytic experiments were designed for CR degradation by Co–NiWO4 NC such as the effect of irradiation time, pH of the media, catalyst dose, and kinetics of the photocatalytic reaction with the mechanism.
3.2.2 Effect of dye concentration and irradiation time
Figure 7(a–c) represents the UV-Vis spectra having variation of CR concerning irradiation time. With an increase in the irradiation time from 5 to 90 min, the absorbance values of the dye decrease indicating an increase in the photocatalytic degradation of the dye. A similar effect was observed with various dye concentrations of 30, 50, and 70 ppm having photocatalytic efficiencies of 91.45, 95.72, and 89.35% (Figure 7d). As can be seen, increasing the dye concentration from 30 to 50 ppm results in an increase in the photocatalytic efficiency from 91.45 to 95.72%, and with 70 ppm, it decreases to 89.35% [60]. Therefore, 50 ppm CR concentration was chosen as the optimized concentration for further photocatalytic experiments. The decrease in photocatalytic efficiency at high pollutant concentrations is due to the formation of an active layer of dye molecules above the surface of the catalyst acting as a screen. This screening effect hinders the path of light toward the surface of the catalyst and thus inhibits the generation of reactive oxidant species (ROS) such as •OH or •O2 ─ radicals to degrade the dye molecule [61].
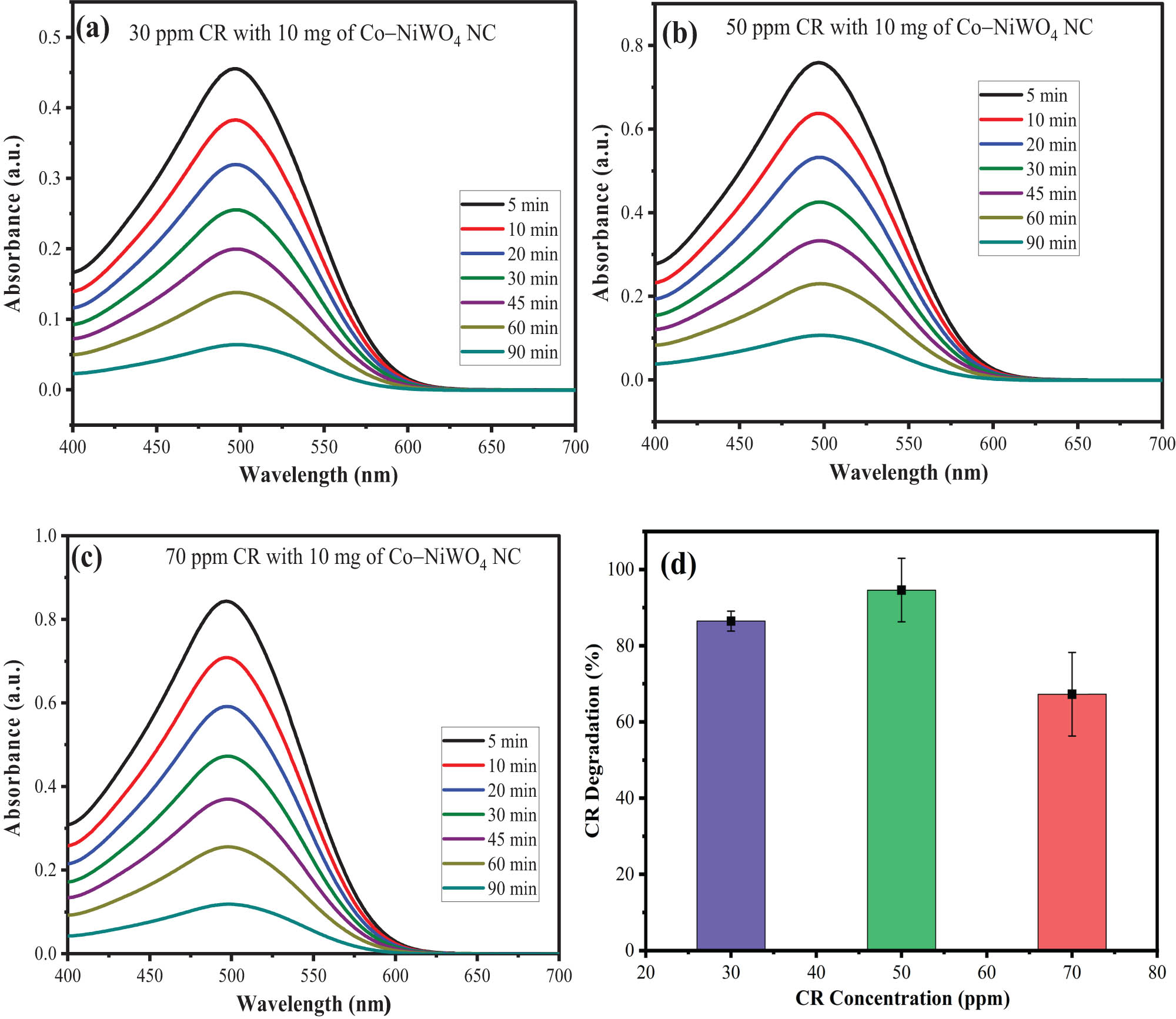
Time-dependent UV–Vis spectra of (a) 30 ppm CR, (b) 50 ppm CR, and (c) 70 ppm CR, and (d) the effect of concentration on the photocatalytic activity of Co–NiWO4 NPs.
3.2.3 Effect of catalyst dose
The amount of catalyst to be utilized during the photocatalytic reaction is a very important factor in verifying the efficiency of the synthesized material. Photocatalytic experiments were carried out using 50 ppm CR concentration with 5, 10, 15, and 20 mg of Co–NiWO4 NPs under visible radiations. The results obtained are shown in Figure 8(a and b), suggesting the 10 mg catalyst dose is optimal and exhibits a maximum photocatalytic efficiency of 91.81% under the given experimental conditions. Initially, as the catalyst dose increases from 5 to 10 mg, the photocatalytic efficiency increases from 77.81 to 91.81% due to a drastic increase in the number of active sites on the surface of the catalyst accommodating a greater number of dye molecules. As the catalyst dose increases from 10 to 20 mg, the degree of agglomeration also increases, which reduces the number of surface-active sites of the catalyst, thereby lowering the photocatalytic efficiency observed [62].

(a) UV–Vis absorption spectra for the effect of variable catalyst doses on CR degradation, and (b) bar graph representing the % CR degradation vs catalyst dose (mg).
3.2.4 Effect of pH
The pH of the solution plays an important role in the degradation of dyes on the surface of the catalyst. To evaluate the optimized value of pH at which maximum photocatalytic activity can be achieved, experiments were performed by varying the solution pH from 2 to 8 under optimized reaction conditions. The results obtained are shown in Figure 9(a) and (b), which suggests that maximum photocatalytic efficiency was achieved at pH 4 associated with 96.25% of CR degradation. Further increase in the pH value beyond 4 results in a decrease in photocatalytic efficiency. The trend can be explained based on the point of zero charges (pHpzc), which is equal to 5.4 as shown in Figure S3. At pH < 5.4, the surface of the catalyst is positive and susceptible to the negatively charged CR molecules via electrostatic forces of attraction, and thereby under the radiation degrades them using •OH radicals. At pH > 5.4, the surface of the catalyst is negative, which would exert a negative repulsive force between the negatively charged CR molecules and the surface of the catalyst. So, fewer molecules of CR will adhere on the surface of the catalyst, and thereby a lower value of photocatalytic efficiency is obtained [63]. Hence, pH 4 was chosen as the optimized pH for further photocatalytic experiments.

(a) UV–Vis absorption spectra for the effect of various pH on CR degradation, and (b) bar graph representing the % CR degradation vs pH of the medium.
3.2.5 Effect of temperature
Photocatalytic experiments were conducted at temperatures from 298 to 328 K under the optimized reaction conditions. The results obtained are shown in Figure 10(a and b), which suggests that an increase in the temperature of the system from 298 to 328 K reflects the increase in photocatalytic efficiency of Co–NiWO4 NC to 86.63, 90.38, 93.14%, and finally 94.31%. The trend can be explained based on the fact that as the temperature increases, molecules gain more thermal energy, which results in greater collision frequency at high temperatures between the CR molecules and the surface of the catalyst. Moreover, the molecules can gain sufficient energy, which enables them to cross the activation energy barrier and thus high photocatalytic activity is obtained [64].
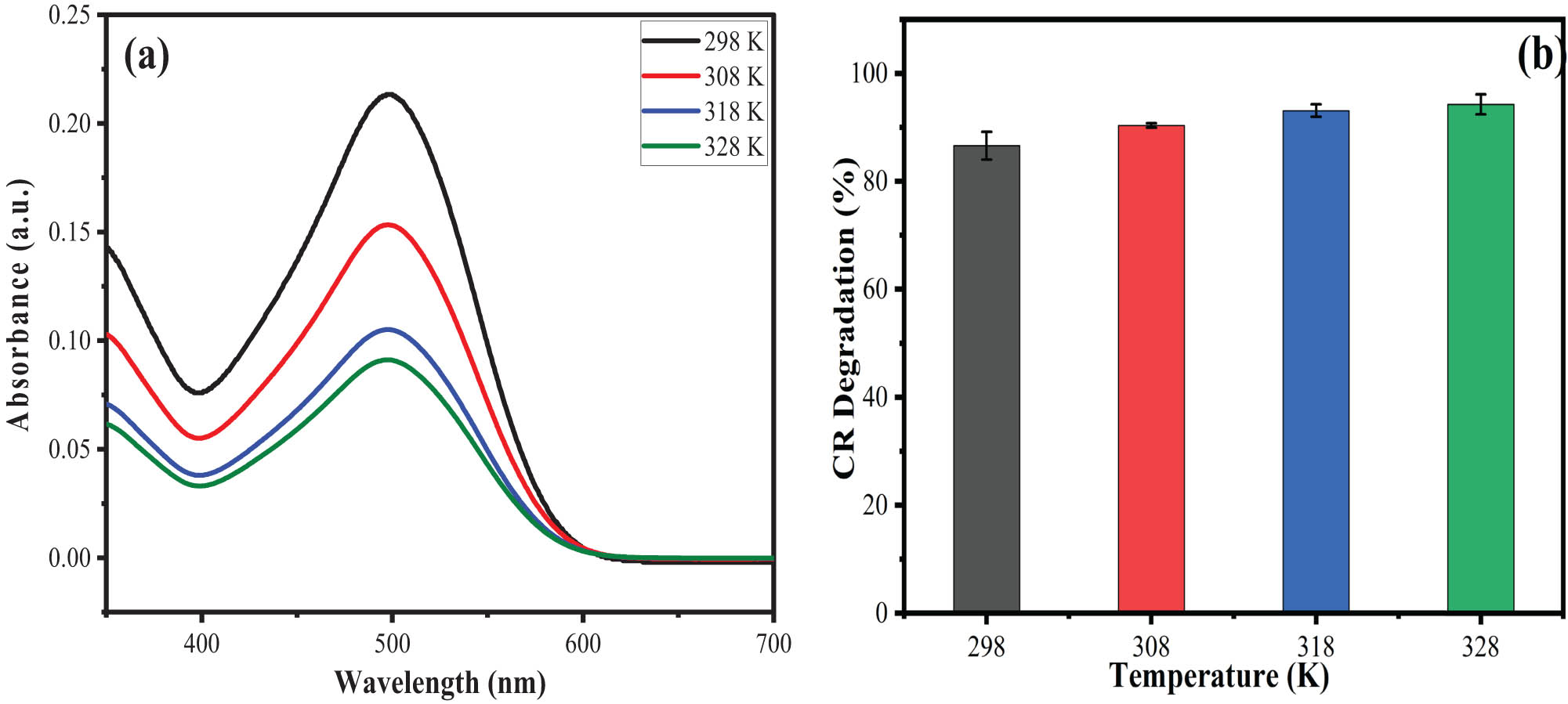
(a) UV–Vis absorption spectra for the effect of various temperatures (298–329 K) on CR degradation, and (b) bar graph representing the % CR degradation vs temperature.
3.2.6 Effect of visible light intensity
The intensity of the radiation source (λ > 420 nm) greatly influences the decolorization reaction, and to observe this photocatalytic experiments were performed under the optimized reaction conditions for various light intensities such as 26.4, 45.5, 78.3, and 92.5 mW cm–2. The results obtained are shown in Figure 11(a and b). It can be seen from the results that as the light intensity increases, the photocatalytic efficiency of the synthesized material also increases achieving an optimized value of 95.29% CR degradation at 92.5 mW cm–2. The trend can be explained based on the fact that an increase in light intensity causes an increase of more amount of light on the catalyst surface and thus a high rate of photoabsorption, which produces a greater number of hydroxyl radicals resulting in high photocatalytic activity [65].

(a) UV–Vis absorption spectra for the effect of various visible light power intensities on CR degradation, and (b) bar graph representing the % CR degradation vs power intensity.
3.3 Kinetics of the reaction
The kinetic data obtained from photocatalytic experiments were adjusted to L–H first-order kinetic model, as given by the following equations [66,67]:
where C 0 and C e are the concentrations of CR at the initial state (t = 0) and each time interval, k app is the rate constant, k s (L/mg) is the adsorption constant, and k r (mg/L min) is the reaction rate. Figure 12(a) represents a plot of –ln(C e/C 0) versus irradiation time for all studied concentrations of CR, which gives the apparent constant (k app) values as listed in Table 1 with correlation coefficients (R 2). The value of k app (0.034 min−1 for 30 ppm, 0.028 for 40 ppm, and 0.025 min−1 for 50 ppm CR) decreases with an increase in the CR concentration. The value of photocatalytic half-life value (t 1/2 = ln2/k app) was evaluated as 20.38 min for 30 ppm, 24.75 min for 40 ppm, and 27.72 min for 50 ppm CR concentration. The values of k app are very significant in correlating the characteristics of the photocatalytic reaction with the L–H model at low dye concentrations. Figure 12(b) represents the graph (1/k app) versus CR concentration, which appears as linear, suggesting the validity of the L–H model with photocatalytic data of CR degradation. Using equation (7), the value of the adsorption constant (k s) and the reaction rate (k r) were found to be 0.42 L/mg and 0.76 mg/L min, respectively. The value of k r was found to be greater than the value of k s which suggests that photoabsorption is the rate-determining step, i.e., the photocatalytic reaction starts with the absorption of light by the surface of the catalyst [68].

(a) –ln(C e/C 0) versus irradiation time for all studied concentrations of the CR plot. (b) L–H plot for (1/k app) versus CR concentration.
L–H first-order kinetic parameters for photocatalytic degradation of CR by Co–NiWO4 NPs
| CR concentration (ppm) | k app | Error | t 1/2 | R 2 | k r | k s |
|---|---|---|---|---|---|---|
| 30 | 0.034 | 2.07 × 10−4 | 20.98 | 0.999 | 0.76 | 0.42 |
| 40 | 0.028 | 1.66 × 10−4 | 24.75 | 0.999 | ||
| 50 | 0.025 | 2.66 × 10−4 | 27.72 | 0.999 |
3.4 Scavenger experiment and mechanism of photodegradation
To evaluate the type of ROS involved in CR degradation, scavenger experiments were performed by taking 10 mg of Co–NiWO4 NPs with 30 ppm of CR with 1.5 mmol each of isopropyl alcohol (IPA) for •OH radical, benzoquinone (BQ) for •O2 − radical, acrylamide (AA) for e– CB, and potassium iodide (KI) for h+ vb under visible solar radiation for 90 min [56,69]. The obtained results after the reaction are shown in Figure 13 which suggested that it is •OH radicals that act as primary ROS in CR degradation as, in the presence of IPA, maximum suppression of photocatalytic efficiency (63.34%) occurs.

Effect of scavengers on the photocatalytic efficiency of Co–NiWO4 NPs under optimized conditions.
The hypothetical reaction mechanism of CR degradation is given as [3,4,5] follows:
As the solar radiation falls on the surface of the catalyst, the photoabsorption process occurs in which the excitation of an electron from the valence band (VB) to the conduction band (CB) occurs, thus creating a hole (h+) in VB and photogenerated e– in CB. The holes interact with water molecules of the media and thus oxidize them to highly excited •OH radicals. Similarly, photogenerated e– in CB interacts with the surface adsorbed oxygen on Co–NiWO4 NPs and thus transforms them to highly reactive •O2 – radicals, which further combine with protons (H+ ions) to form •OH2 radicals. These •OH2 radicals further unite to form H2O2, which is further attacked by photogenerated e– and thus form •OH radicals, which are responsible for the mineralization of CR [60,70].
3.5 Effect of leaching experiments
Various radiation processes such as photolysis (CR solution under radiation without catalyst), adsorption (CR solution with the catalyst in the dark), and CR solution with catalyst under UV and visible light were tested under optimized conditions. The results obtained are shown in Figure S4(a and b), which suggest that under visible light radiation CR is degraded to a maximum (94.96%). The effect of photolysis (2.13%) was negligible while adsorption plays a significant role by adsorbing 64.23% of the CR dye and UV light resulted in 86.19% of CR degradation. Therefore, for the best possible results, visible light as radiation was chosen for further photocatalytic experiments.
3.6 Reusability and TOC test
The stability and reusability of the synthesized material are important aspects of experimental studies and to evaluate this, photocatalytic experiments were performed under optimized reaction conditions in a cyclic mode. In cycle 1, 10 mg of Co–NiWO4 NPs was dispersed in 20 mL of 50 ppm CR solution at pH 4, a temperature of 328 K, visible light power intensity of 92.5 mW cm–2 for 90 min. After the completion of the reaction, the material was separated by centrifugation and the supernatant was utilized under a UV–Vis spectrophotometer for the remaining concentration of CR after degradation. The collected material was washed with distilled water and ethanol and dried in a hot air oven at 50℃ for 2 h. The material was again utilized for cycle 2 of CR degradation and regeneration. This procedure was repeated till four cycles of regeneration and the obtained results are shown in Figure 14(a), which suggests even after four consecutive cycles of reuse, the photocatalytic efficiency of the material decreased from 96 to 92%. The reusability experiments suggested that the synthesized material is highly stable toward photocatalytic degradation of CR dye.

(a) Reusability test for the synthesized Co–NiWO4 NPs. (b) TOC analysis during degradation of CR.
The mineralization of CR by Co–NiWO4 NPs was followed by total organic carbon (TOC) analysis using a TOC analyzer (Matelar Toledo). The results obtained are shown in Figure 14(b), which suggest that as the irradiation time increases, the TOC removal (%) increases continuously and finally the material achieves a total of 72% TOC removal. The TOC (%) was calculated using the following equation:
3.7 Comparison with the literature
To check the add-on information concluded by the present study to the literature, the reaction conditions with maximum efficiency toward organic pollutants were compared and are given in Table 2.
Comparison with the literature
| Materials | Light source used | Dye used | Irradiation time (min) | %CR degradation | Ref. |
|---|---|---|---|---|---|
| Ag/ZnO | UV light | CR | 180 | 81.60 | [61] |
| Graphene−TiO2 | Sunlight | CR | 60 | 90.00 | [71] |
| MnFe2O4/TA/ZnO | Xenon lamp | CR | 90 | 84.20 | [72] |
| P−ZrO2CeO2ZnO | LED light | CR | 250 | 85.85 | [73] |
| Doped ZnO-Gd | Visible light | CR | 120 | 68.02 | [74] |
| CS-BiOCl/ZnO | UV light | CR | 40 | 93 | [75] |
| Co–NiWO4 | Visible light | CR | 90 | 95.00 | Present study |
4 Conclusion
The current study focuses on the hydrothermal synthesis of Co-doped NiWO4 NPs at 180℃, utilizing a Teflon-lined autoclave. The results revealed significant morphological, crystallographic, and spectroscopic changes induced by Co doping in the nanocrystalline solid. The XRD spectra of Co–NiWO4 NPs displayed sharp peaks, indicating a crystalline nature with a wolframite monoclinic phase. The UV spectra of Co–NiWO4 NPs exhibited two broad bands at 354 and 667 nm, suggesting that the material is active in both UV and visible light regions. EDX analysis confirmed the presence of elements in stoichiometric proportions, ensuring the purity of the pristine and doped materials. The synergistic effect of dopants led to alterations in various parameters such as d spacing, atomic positions, orientations, average crystallite size, surface morphology, and thermal stability. Notably, the energy band gap reduced from 3.75 (for NiWO4) to 1.75 eV in Co–NiWO4 NPs, resulting in enhanced photocatalytic activity toward CR degradation. The photocatalytic efficiency increased from 38.13% with pristine NiWO4 to an impressive 88.20% with Co–NiWO4 NPs, reaching 95% after optimizing the reaction conditions. The optimized parameters included 50 ppm CR concentration, 10 mg catalyst dose, pH 4, an irradiation time of 90 min, a temperature of 328 K, and a light intensity of 92.5 mW cm–2. The photocatalytic data fit well with the L–H pseudo-first-order model, with k app values of 0.034 min–1 for 30 ppm, 0.028 min–1 for 40 ppm, and 0.025 min–1 for 50 ppm CR, displaying a correlation coefficient of 0.99. Trapping experiments revealed that •OH radicals primarily acted as ROS in CR degradation. Moreover, reusability experiments demonstrated the high stability of the synthesized Co–NiWO4 NPs for photocatalytic CR degradation. The findings strongly support the outstanding efficiency of Co–NiWO4 NPs in treating organic pollutants in wastewater. As prospects, we intend to explore the photocatalytic water splitting and hydrogen production potential of these multi-metal tungstate NPs.
Acknowledgments
The authors extend their thanks to Researchers Supporting Project (Ref: RSPD2023R670), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: This research was funded by The Researchers Supporting Project (Ref: RSPD2023R670), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data that support the findings of this study are available on reasonable request from the corresponding author.
References
[1] Vattikuti SVP, Byon C, Ngo IL. Highly crystalline multi-layered WO3 Sheets for photodegradation of congo red under visible light irradiation. Mater Res Bull. 2016;84:288–97. 10.1016/J.MATERRESBULL.2016.08.008.Search in Google Scholar
[2] Mayoufi A, Nsib MF, Houas A. Doping level effect on visible-light irradiation W-Doped TiO2–anatase photocatalysts for congo red photodegradation. Comptes Rendus Chimie. 2014;17:818–23. 10.1016/J.CRCI.2014.01.019.Search in Google Scholar
[3] Wang W, Lu T, Chen Y, Tian G, Sharma VK, Zhu Y, et al. Mesoporous silicate/carbon composites derived from dye-loaded palygorskite clay waste for efficient removal of organic contaminants. Sci Total Environ. 2019;696:133955.10.1016/j.scitotenv.2019.133955Search in Google Scholar PubMed
[4] Wang Y, Pan Y, Zhu T, Wang A, Lu Y, Lv L, et al. enhanced performance and microbial community analysis of bioelectrochemical system integrated with bio-contact oxidation reactor for treatment of wastewater containing azo dye. Sci Total Environ. 2018;634:616–27.10.1016/j.scitotenv.2018.03.346Search in Google Scholar PubMed
[5] Guo K, Gao B, Tian X, Yue Q, Zhang P, Shen X, et al. Synthesis of Polyaluminium Chloride/Papermaking Sludge-Based Organic Polymer Composites for Removal of Disperse Yellow and Reactive Blue by Flocculation. Chemosphere. 2019;231:337–48.10.1016/j.chemosphere.2019.05.138Search in Google Scholar PubMed
[6] Amanulla B, Sannasi S, Abubakar AKM, Ramaraj SK. A Magnetically Recoverable Bimetallic Au-FeNPs Decorated on g-C3N4 for Efficient Photocatalytic Degradation of Organic Contaminants. J Mol Liq. 2018;249:754–63.10.1016/j.molliq.2017.11.103Search in Google Scholar
[7] Nasirian M, Mehrvar M. Photocatalytic degradation of aqueous methyl orange using nitrogen-doped TiO2 photocatalyst prepared by novel method of ultraviolet-assisted thermal synthesis. J Environ Sci. 2018;66:81–93.10.1016/j.jes.2017.05.032Search in Google Scholar PubMed
[8] Aziz A, Ali N, Khan A, Bilal M, Malik S, Ali N, et al. Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for Azo Dyes. Int J Biol Macromol. 2020;153:502–12.10.1016/j.ijbiomac.2020.02.310Search in Google Scholar PubMed
[9] Aboutaleb WA, El-Salamony RA. Effect of Fe2O3-CeO2 nanocomposite synthesis method on the congo red dye photodegradation under visible light irradiation. Mater Chem Phys. 2019;236:121724. 10.1016/J.MATCHEMPHYS.2019.121724.Search in Google Scholar
[10] Boudiaf S, Nasrallah N, Mellal M, Belhamdi B, Belabed C, Djilali MA, et al. Kinetic studies of congo red photodegradation on the hetero-system CoAl2O4/ZnO with a stirred reactor under solar light. J Environ Chem Eng. 2021;9:105572. 10.1016/j.jece.2021.105572.Search in Google Scholar
[11] Said M, Rizki WT, Asri WR, Desnelli D, Rachmat A, Hariani PL. SnO2–Fe3O4 nanocomposites for the photodegradation of the congo red dye. Heliyon. 2022;8:e09204. 10.1016/j.heliyon.2022.e09204.Search in Google Scholar PubMed PubMed Central
[12] Hao M, Meng X, Miao Y. Synthesis of NiWO 4 powder crystals of polyhedron for photocatalytic degradation of rhodamine. Solid State Sci. 2017;72:103–8. 10.1016/j.solidstatesciences.2017.08.018.Search in Google Scholar
[13] Shee NK, Kim H-J. Sn(IV) porphyrin-based ionic self-assembled nanostructures and their application in visible light photo-degradation of malachite green. Catalysts. 2022;12:799. 10.3390/catal12070799.Search in Google Scholar
[14] Idriss H, Ibrahem MA, Modwi A. Photocatalytic degradation of congo red pigment by V2O5-TiO2nanohybrid. Z Naturforschung - Sect A J Phys Sci. 2023;78(1):67–76.10.1515/zna-2022-0166Search in Google Scholar
[15] Jamal N, Radhakrishnan A, Raghavan R, Bhaskaran B. Efficient photocatalytic degradation of organic dye from aqueous solutions over zinc oxide incorporated nanocellulose under visible light irradiation. Main Group Met Chem. 2020;43(1):84–91.10.1515/mgmc-2020-0009Search in Google Scholar
[16] Kora AJ. Gum tragacanth-mediated synthesis of metal nanoparticles, characterization, and their applications as a bactericide, catalyst, antioxidant, and peroxidase mimic. Green Process Synth. 2023;12(1):20228138.10.1515/gps-2022-8138Search in Google Scholar
[17] Khan Y, Sharafat U, Gul S, Khan MI, Ismail M, Khan MA, et al. Novel in situ synthesis of quaternary core–shell metallic sulfide nanocomposites for degradation of organic dyes and hydrogen production. Green Process Synth. 2023;12(1):20228128.10.1515/gps-2022-8128Search in Google Scholar
[18] Pouretedal HR, Narimany S, Keshavarza MH. Nanoparticles of ZnS Doped with Iron as Photocatalyst under UV and Sunlight Irradiation. Int J Mater Res. 2010;101(8):1046–51.10.3139/146.110371Search in Google Scholar
[19] Nisar J, Hassan S, Khan MI, Iqbal M, Nazir A, Sharif A, et al. Hetero-Structured Iron Molybdate Nanoparticles: Synthesis, Characterization and Photocatalytic Application. Int J Chem React Eng. 2020;18(2):20190123.10.1515/ijcre-2019-0123Search in Google Scholar
[20] Aravinthkumar K, Praveen E, Jacquline Regina Mary A, Raja Mohan C. Investigation on SrTiO3 Nanoparticles as a Photocatalyst for Enhanced Photocatalytic Activity and Photovoltaic Applications. Inorg Chem Commun. 2022;140:109451. 10.1016/J.INOCHE.2022.109451.Search in Google Scholar
[21] Puga F, Navío JA, Hidalgo MC. Boosting the Photocatalytic Properties of NaTaO3 by Coupling with AgBr. Photochem Photobiol Sci. 2022;22(3):549–66. 10.1007/S43630-022-00334-9/TABLES/3.Search in Google Scholar
[22] Anzai A, Yamamoto A, Yoshida H. BaTi4O9 Photocatalysts with Variously Loaded Ag Cocatalyst for Highly Selective Photocatalytic CO2 Reduction with Water. Catal Lett. 2022;152(8):2498–506. 10.1007/S10562-021-03831-1/METRICS.Search in Google Scholar
[23] Zhang Y, Selvaraj R, Sillanpää M, Kim Y, Tai CW. Coprecipitates Synthesis of CaIn2O4 and Its Photocatalytic Degradation of Methylene Blue by Visible Light Irradiation. Ind Eng Chem Res. 2014;53(29):11720–6. 10.1021/IE403401Y.Search in Google Scholar
[24] Abbas S, Bibi I, Majid F, Ata S, Ibrahim SM, Kamal S, et al. Micro-emulsion synthesis of La1-XCrxFeO3nanoparticles: Effect of Cr doping on ferroelectric, dielectric and photocatalytic properties. Int J Chem React Eng. 2020;18(10–11):20190201. 10.1515/IJCRE-2019-0201/ASSET/GRAPHIC/J_IJCRE-2019-0201_FIG_013.JPG.Search in Google Scholar
[25] Thanh Truc NT, Pham TD, Van Thuan D, Son LT, Tran DT, Nguyen MV, et al. Superior activity of Cu-NiWO4/g-C3N4 Z direct system for photocatalytic decomposition of VOCs in aerosol under visible light. J Alloy Compd. 2019;798:12–8.10.1016/j.jallcom.2019.05.236Search in Google Scholar
[26] Mousavi M, Habibi-Yangjeh A. Decoration of Fe3O4 and CoWO4 nanoparticles over graphitic carbon nitride: Novel visible-light-responsive photocatalysts with exceptional photocatalytic performances. Mater Res Bull. 2018;105:159–71.10.1016/j.materresbull.2018.04.052Search in Google Scholar
[27] Mohamed Jaffer Sadiq M, Krishna Bhat D. Novel NiWO4-ZnO-NRGO ternary nanocomposites with enhanced photocatalytic activity. Mater Today Proc. 2018;5:22412–20. 10.1016/j.matpr.2018.06.610.Search in Google Scholar
[28] Lakshmi Prabavathi S, Muthuraj V. Superior visible light driven photocatalytic degradation of fluoroquinolone drug norfloxacin over novel NiWO4 nanorods anchored on G-C3N4 nanosheets. Colloids Surf A Physicochem Eng Asp. 2019;567:43–54. 10.1016/j.colsurfa.2019.01.040.Search in Google Scholar
[29] Liaquat H, Imran M, Latif S, Iqbal S, Hussain N, Bilal M. Citric acid-capped NiWO4/Bi2S3 and RGO-Doped NiWO4/Bi2S3 nanoarchitectures for photocatalytic decontamination of emerging pollutants from the aqueous environment. Environ Res. 2022;212:113276. 10.1016/j.envres.2022.113276.Search in Google Scholar PubMed
[30] Habibi-Yangjeh A, Feizpoor S. Combination of NiWO4 and polyaniline with TiO2: fabrication of ternary photocatalysts with highly visible-light-induced photocatalytic performances. J Iran Chem Soc. 2020;17(2):351–65.10.1007/s13738-019-01771-7Search in Google Scholar
[31] Kamaraj E, Lee YR, Balasubramani K. Fabrication of a visible-light-driven p-Type NiWO4/n-Type SnO2 heterojunction with efficient photocatalytic activity for degradation of amaranth. J Chin Chem Soc. 2022;69(7):1020–31. 10.1002/JCCS.202200009.Search in Google Scholar
[32] Faka V, Griniezaki M, Kiriakidis G, Grilla E, Mantzavinos D, Mao S, et al. Solar light induced photocatalytic degradation of sulfamethoxazole by ZnWO4/CNNs nanocomposites. J Photochem Photobiol A Chem. 2022;432:114108. 10.1016/j.jphotochem.2022.114108.Search in Google Scholar
[33] Zhang C, Zhang H, Zhang K, Li X, Leng Q, Hu C. Photocatalytic Activity of ZnWO4: Band Structure, Morphology and Surface Modification. ACS Appl Mater Interfaces. 2014;6(16):14423–32. 10.1021/AM503696B/ASSET/IMAGES/MEDIUM/AM-2014-03696B_0008.GIF.Search in Google Scholar
[34] Faka V, Tsoumachidou S, Moschogiannaki M, Kiriakidis G, Poulios I, Binas V. ZnWO4 nanoparticles as efficient photocatalyst for degradation of para-aminobenzoic acid: impact of annealing temperature on photocatalytic performance. J Photochem Photobiol A Chem. 2021;406:113002. 10.1016/J.JPHOTOCHEM.2020.113002.Search in Google Scholar
[35] Nie J, Hassan QU, Jia Y, Gao J, Peng J, Lu J, et al. La-Doped ZnWO4 Nanorods with Enhanced Photocatalytic Activity for NO Removal: Effects of La Doping and Oxygen Vacancies. Inorg Chem Front. 2020;7(2):356–68. 10.1039/C9QI01152H.Search in Google Scholar
[36] Huang C, Chen L, Li H, Mu Y, Yang Z. Synthesis and Application of Bi2WO6 for the Photocatalytic Degradation of Two Typical Fluoroquinolones under Visible Light Irradiation. RSC Adv. 2019;9(48):27768–79. 10.1039/C9RA04445K.Search in Google Scholar
[37] Saraf R, Shivakumara C, Behera S, Nagabhushana H, Dhananjaya N. Facile Synthesis of PbWO4: Applications in Photoluminescence and Photocatalytic Degradation of Organic Dyes under Visible Light. Spectrochim Acta A Mol Biomol Spectrosc. 2015;136(PB):348–55. 10.1016/J.SAA.2014.09.038.Search in Google Scholar PubMed
[38] Tri NL, Duc DS, Van Thuan D, Al Tahtamouni T, Pham TD, Tran DT, et al. Superior Photocatalytic Activity of Cu Doped NiWO4 for Efficient Degradation of Benzene in Air Even under Visible Radiation. Chem Phys. 2019;525:110411. 10.1016/j.chemphys.2019.110411.Search in Google Scholar
[39] Chatterjee M, Saha S, Chatterjee T, Das S, Pradhan SK. Mn-Doped NiWO4 quantum dots with superior electrochemical and conductivity performance for energy storage application. J Energy Storage. 2022;56:105946. 10.1016/j.est.2022.105946.Search in Google Scholar
[40] H H, John M, Jose A, Kuriakose S, Varghese T. Influence of Bi3 + Doping on Structural, Optical and Photocatalytic Degradation Properties of NiWO4 Nanocrystals. J Solid State Chem. 2021;295:121892. 10.1016/j.jssc.2020.121892.Search in Google Scholar
[41] Flihh SM, Ammar SH. Fabrication and photocatalytic degradation activity of Core/Shell ZIF-67@CoWO4@CoS heterostructure photocatalysts under visible light. Env Nanotechnol Monit Manag. 2021;16:100595. 10.1016/j.enmm.2021.100595.Search in Google Scholar
[42] Wahba MA, Yakout SM, Khaled R. Interface engineered efficient visible light photocatalytic activity of MWCNTs/Co doped ZnO nanocomposites: morphological, optical, electrical and magnetic properties. Opt Mater (Amst). 2021;115:111039. 10.1016/j.optmat.2021.111039.Search in Google Scholar
[43] Alam U, Verma N. Direct Z-scheme-based novel cobalt nickel tungstate/graphitic carbon nitride composite: Enhanced photocatalytic degradation of organic pollutants and oxidation of benzyl alcohol. Colloids Surf A Physicochem Eng Asp. 2021;630:127606. 10.1016/j.colsurfa.2021.127606.Search in Google Scholar
[44] Dridi R, Dridi D, Hammami S, Dimassi W, Chtourou R, Amlouk M. Growth and Physical Investigations on NiWO4 Thin Films as a Potential for NO2 Sensing. Opt (Stuttg). 2023;273:170330. 10.1016/J.IJLEO.2022.170330.Search in Google Scholar
[45] Niu L, Li Z, Xu Y, Sun J, Hong W, Liu X, et al. Simple Synthesis of Amorphous NiWO4 Nanostructure and Its Application as a Novel Cathode Material for Asymmetric Supercapacitors. ACS Appl Mater Interfaces. 2013;5(16):8044–52. 10.1021/AM402127U/SUPPL_FILE/AM402127U_SI_001.PDF.Search in Google Scholar
[46] Mani S, Vediyappan V, Chen SM, Madhu R, Pitchaimani V, Chang JY, et al. Hydrothermal synthesis of NiWO4 crystals for high performance non-enzymatic glucose biosensors. Sci Rep. 2016;6(1):1–8. 10.1038/srep24128.Search in Google Scholar PubMed PubMed Central
[47] Oliveira YL, Costa MJS, Jucá ACS, Silva LKR, Longo E, Arul NS, Cavalcante LS. Structural characterization, morphology, optical and colorimetric properties of NiWO4 crystals synthesized by the co-precipitation and polymeric precursor methods. J Mol Struct. 2020;1221:128774. 10.1016/J.MOLSTRUC.2020.128774.Search in Google Scholar
[48] Rosal FJO, Gouveia AF, Sczancoski JC, Lemos PS, Longo E, Zhang B, et al. Electronic structure, growth mechanism, and sonophotocatalytic properties of sphere-like self-assembled NiWO4 nanocrystals. Inorg Chem Commun. 2018;98:34–40. 10.1016/j.inoche.2018.10.001.Search in Google Scholar
[49] Abdul Kader HD, Sh. Mohammed I, Ammar SH. Synthesis of recyclable core/shell CoFe2O4@CoWO4 photocatalysts for efficient visible-light photocatalytic degradation of environmental pollutants. Environ Nanotechnol Monit Manag. 2022;17:100664. 10.1016/j.enmm.2022.100664.Search in Google Scholar
[50] Karthiga R, Kavitha B, Rajarajan M, Suganthi A. Photocatalytic and antimicrobial activity of NiWO4 nanoparticles stabilized by the plant extract. Mater Sci Semicond Process. 2015;40:123–9. 10.1016/j.mssp.2015.05.037.Search in Google Scholar
[51] Scherrer P. Bestimmung der Größe und der inneren Struktur von Kolloidteilchen mittels Röntgenstrahlen. Nachrichten von der Ges der Wissenschaften zu Göttingen, Mathematisch-Physikalische Kl. 1918;26:98–100.Search in Google Scholar
[52] Kuzmin A, Kalinko A, Evarestov R. First-principles LCAO study of phonons in NiWO4. Open Phys. 2011;9:502–9. 10.2478/s11534-010-0091-z.Search in Google Scholar
[53] Azizi S, Asadpour‐Zeynali K. Electrochemical synthesis of tungstate bimetallic nanoparticles with application in electrocatalytic determination of paracetamol. ChemistrySelect. 2022;7:e202104548. 10.1002/slct.202104548.Search in Google Scholar
[54] Leeladevi K, Arunpandian M, Vinoth Kumar J, Chellapandi T, Mathumitha G, Lee J-W, et al. CoWO4 decorated ZnO nanocomposite: efficient visible-light-activated photocatalyst for mitigation of noxious pollutants. Phys B Condens Matter. 2022;626:413493. 10.1016/j.physb.2021.413493.Search in Google Scholar
[55] Tauc J. Optical Properties of Solids. In: Ables A, editor. North- Holland, Amsterdam; 1970.Search in Google Scholar
[56] Finčur NL, Krstić JB, Šibul FS, Šojić Dv, Despotović VN, Banić ND, et al. Removal of alprazolam from aqueous solutions by heterogeneous photocatalysis: Influencing Factors, intermediates, and products. Chem Eng J. 2017;307:1105–15. 10.1016/j.cej.2016.09.008.Search in Google Scholar
[57] Shekofteh-Gohari M, Habibi-Yangjeh A. Combination of CoWO 4 and Ag 3 VO 4 with Fe 3 O 4/ZnO nanocomposites: Magnetic photocatalysts with enhanced activity through p-n-n heterojunctions under visible light. Solid State Sci. 2017;74:24–36. 10.1016/j.solidstatesciences.2017.10.001.Search in Google Scholar
[58] de Oliveira ALM, Ferreira JM, Silva MRS, de Souza SC, Vieira FTG, Longo E, et al. Influence of the Thermal Treatment in the Crystallization of NiWO4 and ZnWO4. J Therm Anal Calorim. 2009;97:167–72. 10.1007/s10973-009-0244-8.Search in Google Scholar
[59] Pandey PK, Bhave NS, Kharat RB. Structural, Optical, Electrical and Photovoltaic Electrochemical Characterization of Spray Deposited NiWO4 Thin Films. Electrochim Acta. 2006;51:4659–64. 10.1016/J.ELECTACTA.2005.12.042.Search in Google Scholar
[60] Murcia MD, Gómez M, Gómez E, Gómez JL, Christofi N. Photodegradation of Congo Red Using XeBr, KrCl and Cl2 Barrier Discharge Excilamps: A Kinetics Study. Desalination. 2011;281(1):364–71. 10.1016/J.DESAL.2011.08.011.Search in Google Scholar
[61] Güy N, Özacar M. The Influence of Noble Metals on Photocatalytic Activity of ZnO for Congo Red Degradation. Int J Hydrog Energy. 2016;41:20100–12. 10.1016/j.ijhydene.2016.07.063.Search in Google Scholar
[62] Ahmed Y, Yaakob Z, Akhtar P. Degradation and mineralization of methylene blue using a heterogeneous photo-fenton catalyst under visible and solar light irradiation. Catal Sci Technol. 2016;6:1222–32. 10.1039/C5CY01494H.Search in Google Scholar
[63] Botsa SM, Jagadeesh Babu M, Suresh P, Kalyani P, Venkateswararao B, Muralikrishna R. Spherical NiWO4-reduced graphene oxide nanocomposite for effective visible light driven photocatalytic activity for the decolourisation of organic pollutants. Arab J Chem. 2020;13(11):8489–97. 10.1016/J.ARABJC.2020.09.017.Search in Google Scholar
[64] Pirhashemi M, Habibi-Yangjeh A. ZnO/NiWO4/Ag2CrO4 nanocomposites with p-n-n heterojunctions: Highly improved activity for degradations of water contaminants under visible light. Sep Purif Technol. 2018;193:69–80. 10.1016/J.SEPPUR.2017.11.007.Search in Google Scholar
[65] Habibi-Yangjeh A, Shekofteh-Gohari M. Novel magnetic Fe3O4/ZnO/NiWO4 nanocomposites: Enhanced visible-light photocatalytic performance through p-n heterojunctions. Sep Purif Technol. 2017;184:334–46. 10.1016/J.SEPPUR.2017.05.007.Search in Google Scholar
[66] Prabavathi SL, Govindan K, Saravanakumar K, Jang A, Muthuraj V. construction of heterostructure CoWO4/g-C3N4 nanocomposite as an efficient visible-light photocatalyst for norfloxacin degradation. J Ind Eng Chem. 2019;80:558–67. 10.1016/j.jiec.2019.08.035.Search in Google Scholar
[67] Mohan S, Subramanian B. A strategy to fabricate bismuth ferrite (BiFeO3) nanotubes from electrospun nanofibers and their solar light-driven photocatalytic properties. RSC Adv. 2013;3:23737. 10.1039/c3ra44085k.Search in Google Scholar
[68] Aazam ES. Degradation of methylene blue dye under visible light using silver/calcium hydroxide nanospheres. Desalin Water Treat. 2018;104:330–7. 10.5004/DWT.2018.21906.Search in Google Scholar
[69] Frias Batista LM, Meader VK, Romero K, Kunzler K, Kabir F, Bullock A, et al. Kinetic Control of [AuCl4]- photochemical reduction and gold nanoparticle size with hydroxyl radical scavengers. J Phys Chem B. 2019;123(33):7204–13.10.1021/acs.jpcb.9b04643Search in Google Scholar PubMed
[70] Bhagwat UO, Wu JJ, Asiri AM, Anandan S. Photocatalytic degradation of congo red using PbTiO 3 nanorods synthesized via a sonochemical approach. ChemistrySelect. 2018;3:11851–8. 10.1002/slct.201802303.Search in Google Scholar
[71] Alamelu K, Raja V, Shiamala L, Jaffar Ali BM. Biphasic TiO 2 nanoparticles decorated graphene nanosheets for visible light driven photocatalytic degradation of organic dyes. Appl Surf Sci. 2018;430:145–54. 10.1016/j.apsusc.2017.05.054.Search in Google Scholar
[72] Boutra B, Güy N, Özacar M, Trari M. Magnetically separable MnFe2O4/TA/ZnO nanocomposites for photocatalytic degradation of congo red under visible light. J Magn Magn Mater. 2020;497:165994. 10.1016/j.jmmm.2019.165994.Search in Google Scholar
[73] Hokonya N, Mahamadi C, Mukaratirwa-Muchanyereyi N, Gutu T, Zvinowanda C. Green synthesis of P − ZrO2CeO2ZnO nanoparticles using leaf extracts of flacourtia indica and their application for the photocatalytic degradation of a model toxic dye, congo red. Heliyon. 2022;8:e10277. 10.1016/j.heliyon.2022.e10277.Search in Google Scholar PubMed PubMed Central
[74] Sadek Kadari A, Khane Y, Nebatti Ech-Chergui A, Popa A, Guezzoul M, Silipas D, et al. Growth, properties and photocatalytic degradation of congo red using Gd:ZnO thin films under visible light. Inorg Chem Commun. 2022;142:109626. 10.1016/j.inoche.2022.109626.Search in Google Scholar
[75] Ranjithkumar R, van Nguyen C, Wong LS, Thiruvengadam Nandagopal JG, Djearamane S, Palanisamy G, et al. Chitosan functionalized bismuth oxychloride/zinc oxide nanocomposite for enhanced photocatalytic degradation of congo red. Int J Biol Macromol. 2023;225:103–11. 10.1016/j.ijbiomac.2022.11.302.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

