Abstract
Alzheimer’s disease (AD) is a multifactorial neurodegenerative central system disease with a high prevalence among the elderly and is the most common form of dementia. Oxidative stress is crucial on AD pathogenesis and leads to deposition of neurofibrillary tangles and Aβ plaques; therefore, the use of natural antioxidants or ROS scavengers could help avoid the formation of these aggregates. Similarly, Aβ-degrading/anti-aggregating molecules could help arrest AD progression. Otherwise, traditional anti-Alzheimer drugs such as acetylcholinesterase inhibitors help improve memory and attention deficits. Nevertheless, all these drugs are extensively metabolized, have low plasma concentration, and cannot cross the blood–brain barrier freely. This review discusses different strategies for nanocarrier conjugation of these drugs for brain targeting and delivery, and new approaches on AD treatment according to the most accepted hypotheses of AD pathogenesis. Although none of the existent compounds or drugs can completely arrest the disease’s progression, nanocarrier development of anti-Alzheimer drugs could help delaying the initial or late stages of neurodegeneration. The discovery of new and more complex nanosystems with multiple approaches in AD treatment is needed and will be the next step in AD treatment in the near future.
Abbreviations
- Ach
-
acetylcholine
- AChEI
-
acetylcholinesterase inhibitors
- AD
-
Alzheimer’s disease
- AGEs
-
advanced glycation end products
- APOE4
-
ε4 allele of apolipoprotein E
- APP
-
amyloid precursor protein
- Aβ
-
amyloid-beta
- BACE1
-
β-site amyloid precursor protein cleaving enzyme 1
- BBB
-
blood–brain barrier
- BChE
-
butyrylcholinesterase
- CaN
-
calcineurin
- CuGly
-
copper glycinate
- MAPK
-
mitogen-activated protein kinase
- Myd88
-
myeloid differentiation primary response 88
- NFATc
-
nuclear factor of activated T cell c
- NFT
-
neurofibrillary tangles
- NMDAR
-
N-methyl-d-aspartate receptor
- NPs
-
nanoparticles
- NSAIDs
-
nonsteroidal anti-inflammatory drugs
- ORAI 1–3
-
Ca2+ release-activated Ca2+ channel protein 1–3
- PEG
-
poly(ethylene-glycol)
- PKC
-
protein kinase C
- PLGA
-
poly(lactic-co-glycolic acid)
- PSEN1/2
-
presenilin ½
- RAGE
-
receptor for advanced glycation end products
- RNS
-
reactive nitrogen species
- ROS
-
reactive oxygen species
- SLN
-
solid lipid nanoparticles
- STIM1
-
stromal interaction molecule 1
- TAO1/2
-
thousand and one amino acid protein kinase ½
- TLR
-
toll-like receptor
1 Introduction
Alzheimer’s disease (AD) is a neurodegenerative central system disease with a high prevalence among the older people and is the most common form of dementia. In 2005, 24.2 million people had dementia worldwide, and approximately 70% of the cases were associated with AD [1]. In 2018, Patterson reported that this number increased to 50 million worldwide, and it is expected to reach 152 million in 2050 [2]. AD affects memory, thought, and language processes; in the early phase, short- and medium-term memory is affected, and patients have problems executing complex activities; in the middle phase, the patient is unable to remember for specific periods, impairing their communication, and they experience disorientation on the familiar environment; on the late phase, memory, language, and thought processes are impaired, and AD patients are unable to execute basic activities [3].
The neurobiological mechanism of AD has been extensively discussed over the years [4]; the main hypotheses that explain this pathology are (i) amyloid-beta (Aβ) deposition, (ii) neurofibrillary tangles (NFT) formation, (iii) Ca2+ homeostasis dysregulation, (iv) cholinergic axis dysfunction, and (v) oxidative stress-related Aβ and NFT deposition. AD is considered a mixed proteinopathy with the deposition of amyloid and Tau protein [5]. Aβ deposition is one of the more accepted hypotheses that explain familial AD, mutations in the genes encoding amyloid precursor protein (APP), ε4 allele of apolipoprotein E (APOE4), and presenilin 1/2 (PSEN1/2) result in aberrant Aβ monomer and Aβ fibril production [6,7,8] and as a result, Aβ aggregation, leading to neuronal death and dementia. PSEN mutation increases the levels of Aβ42 or Aβ43, in addition to Aβ40, amyloid fibrils, and predisposes patients to AD onset [9,10,11]. Tau proteins stabilize the microtubule under normal conditions; hyperphosphorylation and cleavage of these proteins lead to their accumulation, aggregation, and neuronal toxicity [12].
Multiple factors contribute to Ca2+ homeostasis dysregulation. Ca2+ release from the endoplasmic reticulum (through InsP3 and ryanodine) is caused by mutations on presenilin; the increased cytosolic Ca2+ levels activate APP protein phosphorylation and, in consequence, increased production of Aβ42 and Aβ oligomers, which leads to the release of Ca2+ from the endoplasmic reticulum and memory impairment [13]. It has been observed that AD patients have a significant loss of STIM1 in hippocampal neurons, which explains cytosolic Ca2+ upregulation [14].
Cholinergic neurons within the nucleus basalis and septal diagonal band complex are important in memory and attentional function [15]. A severe loss of cholinergic neurons of the basal forebrain in the late stages of AD has been observed; this atrophy is associated with normal aging and is aggravated in patients with mild cognitive impairment and AD [16]. Aβ deposition is strongly correlated with the loss of cholinergic neurons on the basal forebrain; Aβ plaques were observed in postmortem studies of cognitively unimpaired patients [17]. In the early stages of AD, basal forebrain dysfunction could be explained due to dystrophic shrinkage and dysfunction of cholinergic neurons rather than cholinergic neuron loss [18].
Patients with AD and mild cognitive impairment show higher levels of lipid peroxidation metabolites in the brain and reduced levels of glutathione peroxidase and superoxide dismutase [19]. Reactive oxygen species (ROS) production damages the cell and organelle membranes and forms intermediates with proteins by reacting with metals, N− and C− [20]. Oxidative stress on neurons can increase the production of Aβ by increasing APP levels and modulating the activity of BACE-1 and γ-secretase (protease that cleaves APP to produce Aβ), the elevated levels of Aβ oligomers induce Tau protein phosphorylation and NFT formation [21].
AD treatment needs drugs that can cross the blood–brain barrier (BBB), which is not easy. More than 98% of the small lipophilic molecules do not cross the BBB via lipid-mediated free diffusion; these molecules have to weigh less than 400 Da and have less than 8–10 hydrogen bonds [22]. Traditional drugs used on AD have low bioavailability, limited transport, and high brain clearance [23]. The International Organization for Standardization defines nanoparticles (NPs) as particles in the nanometric scale of 1–100 nm [24]; they can be different materials such as polymeric, lipidic, and inorganic and can be used as drug carriers. These nanocarriers can target the brain and surpass the BBB due to their size, surface potential, surface coatings, and conjugation with proteins or antibodies [25] and can cross the BBB by receptor-mediated transcytosis, carrier-mediated transcytosis, adsorptive-mediated transcytosis, or paracellular diffusion [26,27]. This review discusses different strategies for nanocarrier conjugation for brain targeting and delivery of traditional and non-traditional therapies for AD according to the principal hypotheses of AD pathogenesis.
2 NPs across the BBB
The BBB is a semi-permeable barrier formed by microvascular endothelial cells, supported by astrocytes, pericytes, neurons, and the basement membrane [28]. It is responsible for selectively controlling the transport to the brain through the prevention of paracellular diffusion, efflux of hydrophobic substances and drugs, regulating the active transport of nutrients, and transendothelial migration [29]. The NP-assisted delivery of drugs across the BBB must overcome these mechanisms to efficiently accumulate in the brain.
NPs can cross the BBB by active and passive transport that depends on size, charge, and surface modification (Figure 1). Ultra-small NPs (<3 nm) can cross the BBB by paracellular diffusion, while larger NPs (<200 nm) are transported by transcytosis [30,31]. Organic NPs (such as liposomes, PLGA, PLA, and nano-lipids, among others) can be modified for BBB receptor targeting. For example, lactoferrin, insulin, and transferrin receptors are overexpressed on the BBB endothelial cells, and surface modification with their ligands has been successfully used for drug delivery to the brain in vitro [32,33,34]. Another strategy for NP transport across the BBB is targeting carrier proteins. GLUT1 is highly expressed on the BBB endothelial cells, and so mannose and glucose surface modification can be used as targeting ligands [35,36].

Schematic diagram of the NP-mediated anti-Alzheimer drug delivery across the BBB. From left to right, the first NP represents the coating with the receptor (IR, TfR, or LDLR) ligand, the second one represents a glucose- or mannose-coated NP, the third one represents a positively charged gold NP, and the last one represents an ultra-small gold NP. RMT: receptor-mediated transcytosis; CMT: carrier-mediated transcytosis; AMT: adsorptive-mediated transcytosis; IR: insulin receptor, TfR: transferrin receptor; LDLR: low-density lipoprotein receptor; GLUT1; glucose transporter 1.
The luminal side of the BBB is highly negatively charged due to the endothelial cells having a rich phospholipid membrane covered by a glycocalyx formed by negatively charged heparan sulfate proteoglycans [37]. Positively charged NPs interact with the negatively charged surface of the endothelial cells of the BBB, triggering their internalization. The conjugation of NPs with cationic proteins and polymers, such as cationic bovine serum albumin, avidin, and wheat germen agglutinin, among others, has shown evidence of vesicular transcytosis in vitro and in vivo [38,39,40].
3 Applications of nanotechnology on AD
3.1 Antioxidant molecules for neuroprotection in AD
Oxidative stress is one of the primary mechanisms whereby AD produces and perpetuates neuronal damage; antioxidant molecules play a crucial role in the prevention, possible treatment, and arresting AD progression. The activation of stress triggers the protein kinase pathway SAPK/JNK and enables Aβ deposition, lipid peroxidation, and upregulates BACE-1 overexpression, which increases Aβ peptide levels and neuronal death (Figure 2) [41,42,43,44]. At the same time, Aβ deposition activates the JNK/p38 pathway, consequently, mitogen-activated protein kinase (MAPK), which leads to tau hyperphosphorylation and NFT accumulation, causing neuronal damage (Figure 2) [45,46].
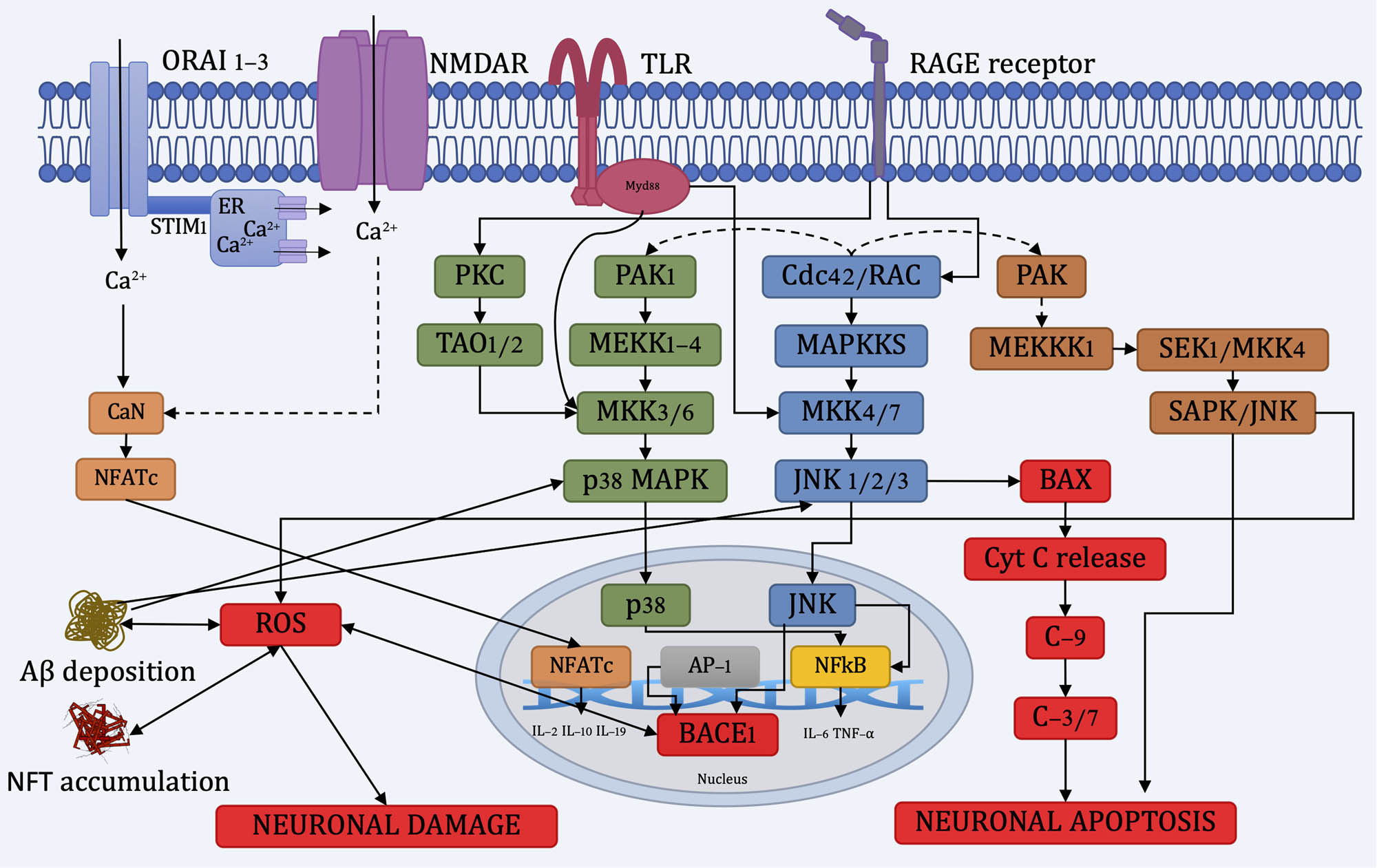
Diagram of the neuronal damage and neuronal apoptosis pathways. ORAI 1-3: Ca2+ release-activated Ca2+ Channel Protein 1-3, STIM1: stromal interaction molecule 1, NMDAR: N-methyl-d-aspartate receptor, TLR: toll-like receptor, Myd88: myeloid differentiation primary response 88, RAGE: receptor for AGEs, CaN: calcineurin, NFATc: nuclear factor of activated T cell c, PKC: protein kinase C, TAO1/2: thousand and one amino acid protein kinase 1/2.
Advanced glycation end products (AGEs) are glycated proteins or lipids produced by pathologies like diabetes and brain aging [47]. AGEs interact with the receptor for AGEs (RAGE) and activate JNK/p38 pathway, enhancing the pro-inflammatory response [48], oxidative stress, and, consequently, Aβ deposition [49,50,51].
Aβ plaque deposition can seize Ca2+ in the cytosol, and its polarity change decreases the endogenous levels of GSH over accumulating ROS inside the neurons, causing long-term oxidative stress [52]. Aβ oligomers activate N-methyl-d-aspartate-type glutamate receptors (NMDARs), which increase the Ca2+ influx, boosting the accumulation of ROS and reactive nitrogen species [53,54]. Mitochondrial dysfunction can also alter Ca2+ homeostasis, ROS deregulation, decreased ATP production, and stress signaling [52]. The change in the cytosolic Ca2+ levels activates the enzyme calcineurin [55], which activates Bcl-2 associated death promoter (BAD), releasing cytochrome C from the mitochondria and promoting caspase activation leading to proapoptotic signaling [56,57].
Due to Aβ deposition, glial cells produce pro-inflammatory cytokines, cyclooxygenase, chemokines, and increasing ROS levels and cellular toxicity [58]. Aβ peptides can activate the NADPH oxidase of glia, producing free radicals like superoxide radicals and hydrogen peroxide [59].
3.1.1 Molecular mechanism of natural antioxidants in neurodegeneration
Natural antioxidants exert neuroprotective activity by reducing ROS stress, acting as scavengers of divalent metal ions (Fe2+, Zn2+, Cu2+), disrupting Aβ plaques, and reducing tau tangle formation [60]. These compounds also present anti-neuroinflammatory properties by inhibiting NF-kB and MAPK signaling pathways (Figure 2) [61]. In addition, these molecules modulate the Nrf2/ARE pathway (Figure 3) and inhibit the Keap1 involved in the partial and selective autophagy, reducing oxidative stress and neuroinflammation [62]. Keap1 targets Nrf2 for ubiquitination and proteasome degradation under normal conditions; the oxidative stress-induced environment inactivates Keap1, and Nrf2 binds to ARE, which induces the expression of heme oxygenase-1 (HO-1) gene, superoxide dismutase, and catalase [63,64].

Diagram showing the Nrf2/Keap1/ARE pathway involved on the prevention of AD neurodegeneration by natural antioxidant molecules carried by NPs. ARE: antioxidant response element; GCR: G protein-coupled receptors; Keap1: kelch-like ECH-associated protein-1; Nrf2: nuclear factor erythroid related factor 2; RTK: receptor tyrosine kinase.
The pharmacological use of natural antioxidants for AD prevention and treatment is an attractive alternative to conventional drugs due to their relative safety and efficacy. However, their low absorption, poor bioavailability, rapid metabolism, and excretion limited their use. Orally administered polyphenols are extensively metabolized into glucuronide and sulfoglucuronide in the intestine and liver; these are the main metabolites in plasma [65]. These molecules have low brain uptake ranging from approximately 8 to 166 ng/g [66,67,68,69]. Nanoencapsulation of natural antioxidants is a strategy used to surpass these problems, protecting them from enzymatic metabolization on the gastrointestinal tract, liver uptake, and plasma concentration, and enabling brain targeting.
3.1.2 Nanoencapsulation of natural antioxidant molecules
Natural antioxidants have been widely discussed in recent years for neuroprotection of AD due to their lipophilicity and capacity to transverse the BBB in vitro. Langasco et al. [70] synthesized transfersomes loaded with genistein, a highly lipophilic flavonoid (Table 1), to improve the delivery of genistein by the nose-to-brain route. Transfersomes have many properties, including deformability, size, and in vitro permeation, making them suitable for intranasal administration. This formulation attenuated the ROS overproduction induced by oxidative stress compared to genistein alone and improved the cellular internalization of genistein. Thus, this formulation could be an efficient delivery system of genistein and other highly lipophilic flavonoids by the olfactory nerve pathway.
Summary of research on natural antioxidant molecules used for AD
| Class | Antioxidant | Chemical structure | Log P | Obtained | Ref. |
|---|---|---|---|---|---|
| Flavonoids | Genistein |

|
3.04 | Soy | [54] |
| Quercetin |

|
1.81 | Capparis spinosa | [57] | |
| Hesperetin |

|
2.60 | Oranges | [58] | |
| Curcumin |

|
3.62 | Curcuma longa | [61] | |
| [62] | |||||
| Fatty acid | Punicic acid |

|
6.4 | Pomegranate seed oil | [66] |
| Stilbene | Resveratrol |

|
2.57 | Grapes | [69] |
Other natural flavonoids with lower lipophilicity, such as quercetin, have shown functional neuroprotective potential, although they cannot cross the BBB freely. One strategy of nanoencapsulation for flavonoids delivery is the PEGylation of the surface of the NPs, avoiding non-specific binding to proteins and macrophages and, at the same time, increasing their systemic circulation time by evading their aggregation opsonization and phagocytosis [71].
In late stages of AD, the neurotoxic role of glial cells is not clear; overactivation of microglia by cytokines release promotes Aβ accumulation in the brain; at the same time, Aβ activates astrocytes that trigger neuronal death through ROS production [72]. Nday et al. [73] synthesized quercetin-loaded PEGylated silica NPs and compared them to quercetin-loaded cetyltrimethylammonium bromide (CTAB) silica NPs for enhanced quercetin delivery to the brain. The PEGylated NPs in concentrations of 30 or 150 μM improved glial cell viability against 10 or 50 μM copper glycinate (CuGly) on in vitro primary hippocampal cultures; the CTAB NPs did not protect glial cells efficiently from CuGly in any concentration. Avoiding glial ROS production could help reduce the inflammatory response and neurotoxic effect of Aβ accumulation on the brain.
Another strategy to deliver flavonoids is reducing powder particle size to the nanometric scale; this could improve the BBB’s penetration due to the natural lipophilicity of flavonoids. Kheradmand et al. [74] synthesized hesperetin nanocrystals; nano-hesperetin showed better results than hesperetin at 10 and 20 mg/kg, administered by oral gavage on the streptozotocin-induced AD rats in vivo model. The rats treated with nano-hesperetin showed improved memory and learning; decreased lipid peroxidation of the hippocampal area; and increased GSH level, glutathione peroxidase, superoxide dismutase, and catalase activity.
Curcumin is one of the leading natural antioxidant molecules studied in recent years for AD due to its antioxidant, anti-inflammatory, anti-amyloid, and anti-Tau hyperphosphorylation neuroprotective capabilities [75,76]. Despite these findings, curcumin has low gastrointestinal absorption and has the smallest brain uptake of all flavonoids in mice (8.2 ± 1.8 ng/g) [68]. Huo et al. [77] synthesized selenium NP-loaded curcumin/PLGA composites using transgenic 5XFAD mice to exhibit plaques; this material can cross the BBB entirely and bounded to amyloid Aβ plaques.
Kuo and Lin [78] synthesized liposomes with cardiolipin (CL) and wheat germ agglutinin (WGA) loaded with curcumin and nerve growth factor (NGF), CL was used to stabilize the bilayer structure in liposomes and favored the ligand binding of liposomes to Aβ1–42 in HBMEC-cells and human astrocyte culture cells, observing an increase in permeability of the nanosystem compared to curcumin alone. Also, an increased neuroprotective effect on SK-N-MC cells compared to curcumin alone was shown. The authors also assessed the antiphosphorylation activity on SK-N-MC cells [79]; a reduction in phosphorylated p38 and phosphorylated JNK levels and decreased phosphorylated tau expression protein were observed.
Polyunsaturated fatty acids, such as punicic acid, have shown antioxidant and neuroprotective attributes due to free radical scavenging properties [80,81]. Mizrahi et al. [82] synthesized water-soluble pomegranate seed oil nanoemulsions, containing punicic acid as an antioxidant molecule flavonoids and anthocyanins. In TgMHu2ME199K mice, a model for Creutzfeldt–Jakob disease, these nanoemulsions reduced neuronal death, lipid oxidation, and increased neurogenesis.
Other natural polyphenols, for example, stilbenes, have also shown antioxidant and neuroprotective properties for potential use on AD [83,84]. Loureiro et al. [85] synthesized solid lipid nanoparticles (SLN) containing resveratrol and grape seed and grape skin extracts. They were functionalized with OX26, a monoclonal antibody that binds to cells expressing the transferrin receptors in BBB, mediating their internalization. The SLN containing resveratrol and extracts of grape skin inhibit Aβ(1–42) (a more neurotoxic residue) [86] fibril formation in 26 and 31%, respectively, when compared to the control. Additionally, the SLN functionalized with the OX26 antibody increases the SLN uptake by BBB cells compared with the LB509 antibody, which recognizes α-synuclein, a protein that is non-specific to the BBB (2-fold higher) or without antibody (fourfold higher). On the other hand, Sun et al. [87] synthesized mesoporous nano-selenium based on the borneol target, β-cyclodextrin nanovalves with loaded resveratrol. Borneol has a transient and rapid BBB-opening effect that could enhance the penetration of nanocarriers [88]. This nanosystem had an encapsulation efficiency of >70% and found that resveratrol release was H2O2 concentration dependent. SHSY5Y and bend3 cell lines showed a cell viability of >90% when incubated with 200 µg/mL of the nanosystem. Also, a reduction in ROS and NO levels on SHSY5Y cells treated with Aβ was observed. Finally, the nanosystem could penetrate and release resveratrol on an in vitro BBB model formed by bEnd.3 and SHSY5Y-cells.
Natural antioxidant molecules are being extensively studied nowadays due to their neuroprotective and neurorestorative properties. Due to its lipophilicity and chemical structure, reducing the particle size of highly lipophilic flavonoids could enhance their BBB uptake. Conventional nanocarriers (e.g., SLN, nanoemulsion, nanoethosomes, polymeric NPs) are also alternatives. However, the selection of polymers and materials is restricted by the physiological conditions of BBB uptake and chemical compatibility. These natural compounds have a hormetic biphasic dose–response characterized by low-dose stimulation and high-dose inhibition [89,90]. It is necessary to study this low dose-dependent effect associated with the microdosing of natural antioxidants encapsulated with NPs according to the modulation of the Nrf2/ARE/Keap1 pathway.
3.1.3 ROS/metal scavengers
Metal dyshomeostasis is another hypothesis that explains AD pathogenesis; this theory involves metal ion dysregulation (Zn(ii), Cu(i)/(ii), and Fe(ii)/(iii)), which interacts with Aβ monomers and, in consequence, cause Aβ aggregation [91,92]. Copper and iron can also induce ROS generation through the Fenton (equations (1) and (2)) and Harber–Weiss (equation (3)) reactions [93,94], causing neuronal damage and apoptosis (Figure 2).
Chelation of these metal ions and ROS products could help avoid Aβ aggregation, Kwon et al. [95] synthesized 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino (polyethylene glycol)-2000] ceria (TPP-ceria) (CeO2) NPs. TPP-ceria NPs can scavenge mitochondrial ROS, reducing oxidative stress and mitigating the reactive gliosis suppressing cytotoxicity shown in the 5XFAD mouse model. On the other hand, Liu et al. [96] synthesized a prototype NP-chelator conjugate (Nano-N2PY); these polystyrene NPs were functionalized with 2-methyl-N-(2′-aminoethyl)-3-hydroxyl-4-pyridinone. Nano-N2PY showed cellular protection from Aβ cytotoxicity on HCN-1A cells compared with cells treated with Aβ alone. Also, nano-N2PY inhibits Aβ aggregates’ formation in vitro due to their ability to chelate metal ions that formed the aggregates. Notwithstanding the chelation of metal ions and ROS products could help avoid Aβ aggregation, p38/JNK, and SAPK/JNK pathways and, consequently, the pro-inflammatory response cascade remains “activated” causing neuronal damage (Figure 2), the use of nanochelators as carriers of natural antioxidants could help improve the response observed in vitro and in vivo studies shown in this section.
3.2 Aβ degrading/anti-aggregating molecules
Another strategy for treating AD is using anti-Aβ molecules for tagging or with amyloid degrading properties. Agyare et al. [97] developed chitosan polymeric core nanocarriers labeled with a polyamine-modified F(‘ab’) portion of IgG4.1, an anti-amyloid antibody for Aβ tagging in vivo. This formulation accumulated over time in the brain of wild-type (B6/SJL) mice. The uptake of the NPs was higher than the controls on bovine brain microvascular endothelial cells in vitro assay, suggesting the transcytosis of the NPs at the BBB.
Nattokinase is an enzyme capable of degrading amyloid fibrils, suppressing β amyloid and BACE-1 activity; it has shown a recovery of impaired learning and memory capability in vivo and in vitro [98]. Bhatt et al. [99] formulated the PLGA NP-delivery system of the Tet1-conjugated nattokinase enzyme. Tet1 can retrograde transport to neuronal cells and interact with motor neurons. A complete degradation of Aβ40 amyloid plaques was observed after 24 h of digestion.
Another strategy for reducing these aggregates’ interaction is changing the surface charge of Aβ amyloid fibrils with other proteins and inhibiting their toxicity. Klementieva et al. [100] developed poly(propylene imine) glycodendrimers. An inhibition of Aβ1–40 aggregation and a complete reduction of Aβ1–42 toxicity were observed on the SHSY5Y cell line compared to the control. APP/PS1 transgenic mice treated intranasally for 1 month did not improve memory impairment. Metallic NPs with negative surface charge can also interfere with Aβ aggregation; Liao et al. [101] synthesized carboxyl conjugated gold NPs. These NPs interfered with Aβ fibrillation in a concentration-dependent manner and affected the preformed Aβ fibrils. It was observed that BE-2-C cells treated with 1.36 nM gold NPs reduced Aβ fibrils’ toxicity to 16%.
Other molecules could inhibit the formation of Aβ by interfering with the synthetic pathways of the fibrils. Sun et al. [102] synthesized immunogenic epitopes (Aβ1–6, (Aβ1–6)2, and Aβ1–15) on the Aβ C-terminal coupled to protein keyhole limpet hemocyanin or MAP4. Aβ1–6-MAP4 showed the strongest immunogenicity and immunogenic response and reduced the Aβ fiber production by inhibiting the fiber’s formation, and it could depolymerize the previously formed fiber. Also, it was observed that the monoclonal antibody 1H7 specifically recognizes the Aβ1−42.
Even though anti-Aβ use seems a viable alternative for AD treatment, it does not address the main problem, which is the formation of Aβ amyloid fibrils and their accumulation on neurons, causing neuronal damage and eventually neuronal apoptosis. It has been reported that Aβ has a biphasic hormetic dose–response, improving the GABAergic connectivity at 20 nM and at 800 nM have the opposite effect on mouse hippocampal neurons [103]. Modulating the hormetic response of the anti-Aβ and antioxidant drugs could be an excellent alternative to regulate the accumulation of sub-toxic concentrations of Aβ and their protective cellular response.
3.3 Acetylcholinesterase inhibitors (AChEI)
AChEI is the primary medication used nowadays for treating dementia associated with AD. Acetylcholinesterase (AChE) is an enzyme that hydrolyzed acetylcholine (ACh) neurotransmitters into acetate and choline; in AD, the loss of neurons is one of the significant symptoms; the loss of neurons on the basal forebrain nuclei results in a reduction of ACh levels [104]. Although in AD patients the ACh receptor system remains unaltered, restoring normal ACh levels could improve neuropsychiatric symptoms [105], which is difficult to achieve because these molecules present low BBB penetration and poor tolerability due to peripheral cholinergic effects. Therefore, nanovehiculization of these drugs could help overcome these problems.
Tacrine was the first AChEI drug approved for the treatment of AD; it was discontinued in 2013 due to safety issues associated with a high incidence of cholinergic peripheral adverse effects and hepatotoxicity; although it is a potent AChE and butyrylcholinesterase (BChE) inhibitor, the use of nanocarriers could help avoid unwanted peripheral effects and reduce hepatotoxicity [106]. Luppi et al. [107] synthesized bovine serum albumin NPs carrying β-cyclodextrins loaded with tacrine. A 100% permeability and a low release rate of tacrine were observed on sheep intranasal mucosa ex vivo assay. It was a suitable option for intranasal delivery of the AChEI drug to the brain, avoiding systemic liberation of tacrine and consumption outside the brain. Joe and Kumar [108] developed PLGA NPs loaded with tacrine. The NPs showed an entrapment efficiency of 81.32% and an in vitro release of about 80% at 24 h on phosphate buffer 7.4. An improvement in cell viability was observed in SHSY5Y cells treated with Aβ at 100 µg/mL.
Galantamine is a slowly reversible dual inhibitor of AChE and BChE (a non-specific esterase) and has acted as an allosteric modulator of nicotinic ACh receptors, which downregulates the central expression of ACh [109]. Galantamine is the best-tolerated AChEI drug with a low incidence of peripheral cholinergic adverse effects [110] compared to rivastigmine and donepezil. However, due to its poor lipophilicity, it is unable to transverse the BBB efficiently. Fornaguera et al. [111] synthesized galantamine-loaded PLGA NPs; this formulation inhibited 80% AChE activity and was not toxic at 0.3 mg/mL on SH-SY5Y cells. In another investigation, Woo et al. [112] synthesized galantamine hydrobromide-loaded Carbopol gel drug reservoirs as a transdermal patch delivery system, showing a sustained galantamine release, reaching almost 69% (maximum concentration of 29 mg/mL, approximately) after 8 h, being higher than the recommended concentration at a dose of 5 mg/kg in rabbits.
Donepezil is a highly specific AChE inhibitor with similar clinical efficacy and limitations of galantamine, such as low BBB penetration, peripheral cholinergic adverse effects, and gastrointestinal intolerance [113]. AnjiReddy and Karpagam [114] developed donepezil-loaded chitosan nanofibers films for oral disintegration. The drug association was about 99%, and about 80% of the drug was released to the medium after 6 min in the in vitro dissolution assay. Almost 100% was released after 60 min in the in vivo drug plasma release assay. A 44.1% A549 cell viability was observed when incubated with 150 µg/mL of the formulation. Other authors, like Jakki et al. [115], synthesized mango gum/chitosan loaded with donepezil NPs and oil/water mango gum loaded with donepezil nanoemulsion. The formulations were nontoxic on SK-N-SK cells at 10–80 µg/mL. Biodistribution studies were performed on Wistar rats; a higher concentration of drugs was observed in the brain, 102.34 ng/mL for NPs and 312.09 ng/mL for the nanoemulsion compared to the pure drug 25.97 ng/mL. Concluding that mango gum extract could be used as a suitable anionic polymer for brain delivery due to its nontoxic and nonirritant properties.
Huperzine A is a highly specific reversible AChEI; Meng et al. [116] synthesized lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA loaded with huperzine A for intranasal delivery. The NPs were not cytotoxic on 16HBE cells at concentrations lesser than 10 mg/mL and were taken up into the cytoplasm of 16HBE and SH-SY5Y cells in vivo. Kunming mice study showed that the nanosystem was highly distributed in the brain.
In an alternative approach, Roumiani and Dorosti [117] postulated that organophosphates and phosphoramides display inhibitory activities against AChE and BChE; the authors synthesized nanodandelion tin(iv) complex with carbacylamidophosphate (Sn(CH3)2Cl2{NC5H4C(O)NHP(O)[NHC6H11]2}2). An inhibition constant (IC50) of 326.59 µg/mL was determined, although it was 1.1–1.5-fold higher on BChE than AChE.
AChEI drugs are the primary treatment for AD patients nowadays; these drugs present higher doses tolerance problems, mainly due to peripheral cholinergic adverse effects (i.e., nausea, vomiting, drowsiness, and shakiness) and low BBB penetration. The use of nanocarriers could help improve these problems. However, clearance by the spleen and the liver should be considered, especially on the drugs with reported hepatotoxicity.
3.4 New drugs for AD treatment
New drugs have been assessed for potential AD treatment; for example, Jojo et al. [118] developed lipidic nanocarriers loaded with pioglitazone, a PPARy agonist that activates PI3/Akt and downregulates p38 MAPK pathways reducing oxidative stress, reducing Aβ plaques and NFT formation and neuroinflammation. A 45% drug release was observed at 24 h, with no intranasal ciliotoxicity on sheep intranasal mucosa assay and a good permeation on the Franz diffusion cell assay.
RNA interference technology is one strategy for inhibiting protein synthesis, such as key enzymes for AD progression. Wang et al. [119] synthesized PEGylated poly[2-(dimethylamino)ethyl methacrylate] ethyl methacrylate (PEG-PDMAEMA) modified with CGN peptide and Tet1 for siRNA against β-site amyloid precursor protein cleaving enzyme 1 (BACE1). The nanocomplex did not cause red blood cell aggregation, morphological changes, or ionic interactions with them. Also, the nanocomplexes internalized onto bEnd.3 cells and neuronal cells by the endosomal route showed in vivo targeting of AD lesions neurons and high brain biodistribution after 1 h (>2% dose/g-brain). Finally, the nanocomplexes could effectively silence the BACE1 gene in PC12 cells and in vivo on ICR mice, reduce the production of Aβ plaques and adequately protect neuron, and restore impaired neurogenesis.
Vinpocetine is a phosphodiesterase inhibitor with neuroprotective activities reducing neuroinflammation by inhibiting the IκB kinase complex, impeding the expression of pro-inflammatory mediators [120,121], and improving memory impairment by reducing oxidative stress and modulation of cholinergic function [122]. Moghaddam et al. [123] synthesized nanoethosomes loaded with vinpocetine for transdermal delivery. Nanoethosomes are lipid carriers composed of ethanol, phospholipids, and water. The optimized formulation of nanoethosomes showed a 98.55% entrapment efficiency of vinpocetine, a vesicle size of 44.47 nm, and a transdermal flux across rat skin of 959.63 μg/cm2/h; these findings suggest that this formulation could be used as a functional transdermal delivery system for vinpocetine.
The development of new non-conventional drugs for AD treatment with inhibition of different AD pathogenesis pathways has shown promissory results; nevertheless, more clinical data are required to shed light on drug safety and biodistribution.
3.5 Nonsteroidal anti-inflammatory drugs (NSAIDs) for AD treatment
In response to Aβ plaques and NFT accumulation, microglia infiltration occurs; Aβ plaques activate the TLR of microglia, releasing pro-inflammatory cytokines and chemokines, leading to sustained neuroinflammation [124]. Some authors have discussed NSAIDs used for AD treatment due to the inflammatory imbalance in AD pathogenesis. For example, Al-Azzawi et al. [125] used dendrimeric poly(epsilon-lysine) loaded with flurbiprofen delivery systems. Flurbiprofen is an NSAID with notorious γ-secretase modulation by interacting with presenilin, thus decreasing Aβ42 secretion. The formulation did not affect bEnd.3 cell viability at concentrations up to 400 μM and an increase of 12–14% penetration across the BBB was observed in the bEnd.3 in vitro model compared to free drug. The potential use of dendrimeric carriers to deliver flurbiprofen could improve its low BBB penetration. Other authors, such as Muntimadugu et al. [126], synthesized PLGA and SLN loaded with tarenflurbil (enantiomer of flurbiprofen), a selective Aβ42 lowering agent and y-secretase modulator. The formulation had an entrapment efficiency of 62.31% at 10% drug loading for PLGA NPs and an entrapment efficiency of 57.81% at 10% drug loading for SLNs. After intranasal administration, complete drug release was observed at 2 h from both NPs and high brain biodistribution.
The use of NSAIDs for AD treatment has been controversial due to the partiality of the results; studies have concluded that there is no difference in the reduction of AD risk between NSAIDs users and non-users [127], De Craen et al. [128] have indicated that the beneficial results of NSAIDs might result from recall bias, prescription bias, and publication bias.
4 Detection and diagnosis of Aβ plaques
Kaushik et al. [129] discussed new techniques to detect Aβ plaques in the early and late AD stages and the progressive formation of senile plaques and NFT. Current diagnosis techniques include in vivo imaging (MRI, SPECT, PET, among others) and the patient’s clinical history; different authors have developed different chemical compounds with fluorescent properties and different micro and nanocarriers to deliver them to the brain. Matsumura et al. [130] synthesized various radio-iodinated phenyldiazenyl benzothiazole derivatives as probes for NFT autoradiography and fluorescent staining assays. A high affinity for NFT was observed on in vitro autoradiography and fluorescent assays; however, these derivatives displayed persistent radioactivity in the brain, making them unsuitable for in vivo imaging.
Modified quantum dots for in vivo imaging have been synthesized by Feng et al. [131]. ZnS-capped CdSe quantum dots conjugated with Aβ antibody showed adequate distribution on the brain and targeted the Aβ1–42 residue on both in vivo and ex vivo assays.
Nevertheless, these conventional techniques are expensive and require a qualified team to operate them. Micro and nanostructured biosensors are new materials that can detect Aβ plaques at the pM level [129] for rapid diagnosis and monitoring of AD patients; however, these devices are limited for clinical uses due to a lack of detection and data on in vivo studies.
5 Toxic effects of NP-based therapies
The most common toxic effects observed in different inorganic nanosystems are neuroinflammation, oxidative stress, induced apoptosis, and autophagy [132]. Sharma et al. reported that aluminum, copper, and silver NPs (50–60 nm) induce BBB disruption and brain edema formation and lead to abnormal cell reactions in Sprague Dawley rats; the NPs were administered by intravenous (30 mg/kg), intraperitoneal (50 mg/kg), or intracerebral (20 μg/10 μL) injections [133]. Even more, Ma et al. [134] observed increased accumulation of titanium in the brain of CD-1 mice after the intraperitoneal injection of nanoparticulate anatase TiO2 (5 nm) (5, 10, 50, 100, and 150 mg/kg) and bulk TiO2 (150 mg/kg) daily for 14 days. Neuronal change into filamentous shapes and inflammatory cells was also observed.
On the other hand, Hu and Hammarlund-Udenaes [131] discussed the relevance of increasing the unbound brain-to-plasma partition coefficient (K p,uu,brain) to enhance brain targeting instead of increasing the transport of the nanosystem to the brain in its intact form, which could enhance the toxic effects of the nanomaterial. Accordingly, receptor-mediated endocytosis of NPs could enhance the cytotoxicity effects of the nanomaterial by the degradation of the receptor/NP complex on the endosomal–lysosomal system and could produce an early release of the drug and accumulation on the cytoplasm (drug-related cytotoxicity) [135].
5.1 Pharmacokinetics of NPs
In general, NPs improve the pharmacokinetic (PK) characteristics of drugs and reduce their peripherical adverse effects [136]. Nevertheless, there is a lack of studies on PK characteristics of the different nanosystems in vivo. It has been discussed that NPs between 10 and 12 nm have high permeation and low accumulation on tissues, while NPs with a hydrodynamic radius <5.5 nm are excreted by the renal route and larger NPs by the liver [137,138]. The surface charge also plays a key role in the biodistribution; NPs coated with nonionic polymers show increased circulation time and opsonization, while positively charged NPs have increased cellular uptake [139,140,141,142]. The clearance of larger and cationic NPs is influenced by the interaction with the mononuclear phagocytic system and reticuloendothelial system, which accumulates the NPs in the spleen and liver. The activation of these systems could trigger an immune response and increase cytotoxic by secretion of tumor necrosis factors, interleukins, and interferons [143].
6 Concluding remarks
AD is a multifactorial neurodegenerative disorder involving several neurobiological mechanisms related to each other. Aβ deposition and NFT formation are the most accepted hypotheses of AD pathogenesis; Ca2+ dyshomeostasis, oxidative stress, and neuroinflammation lead to increased production of Aβ42 and Aβ oligomers and tau hyperphosphorylation. Using drugs with antioxidant and anti-neuroinflammatory properties could prevent AD progression and Aβ and NFT production. Natural antioxidants such as flavonoids can inhibit neuroinflammation and have shown strong antioxidant activity, neuroprotective properties, and radical scavenging abilities (hesperetin). In addition, the modulation of the Nrf2/ARE/Keap1 route by the antioxidant molecules could enhance the protection against neurodegeneration associated with oxidative stress. Using other drugs such NSAIDs, ROS/metal scavengers, and Aβ degrading enzymes have shown partial results due to their inability to completely inhibit the cascade of Aβ and NFT production (Figure 2). Modulating the hormetic dose response of these drugs through nanoencapsulation could be a more efficient strategy to achieve neuron protection in early stages of AD.
Another strategy is using AchEI in the early stages of AD treatment to limit neurodegeneration; the cholinergic axis dysfunction contributes to memory and attention deficits observed in AD patients. These compounds have increased brain clearance, poorly penetrate the BBB, and have tolerance problems due to peripheral cholinergic effects; using nanocarriers could help improve brain delivery and may reduce the unwanted side effects.
The physicochemical properties of these compounds made them suitable candidates for lipophilic nanocarrier development for brain delivery (nanoemulsion, nanoethosomes, SLN, etc.); the surface conjugation of these nanosystems with antibodies or polymers can be an excellent strategy to target the BBB receptors and transporters specifically, although the chemical compatibility restricts the selection of these polymers with BBB, which should not increase the size of the NP considerably nor change the surface charge.
The development of more complex nanosystems and the nanovehiculization of multiple anti-AD drugs are needed to treat AD; these systems must have a multifocal approach according to the cascade of Aβ and NFT aggregate formation. Although none of the existent compounds or drugs can completely arrest the disease’s progression, they can delay the initial or late stages of neurodegeneration (Table 2).
NP classification according to AD pathogenesis and physicochemical characterization
| Classification | Nanovehicles | Main mechanism | Size (nm) | PdI | ζ-Potential (mV) | EE (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Transferosomes | Genistein-loaded transferosomes | Antioxidant/neuroprotector | 132.8 ± 1.35 | 0.310 ± 0.029 | −8.34 ± 1.34 | 40.36 ± 0.23 | [67] |
| Silica NPs | Quercetin-loaded CTAB silica NPs, and quercetin loaded PEG silica NPs | Antioxidant/neuroprotector | 745.3 ± 22.9 | 0.21 ± 0.02 | 30.6 ± 2.6 | 81.9 ± 5% | [70] |
| Nanocrystal | Nano-hesperetin | Antioxidant/radical scavenger | NS | NS | NS | NS | [71] |
| PLGA nanospheres | Selenium NPs encapsulated PLGA nanospheres with curcumin | Antioxidant, anti-inflammatory, anti-amyloid, and anti-Tau hyperphosphorylation | 70.5 ± 6 | NS | NS | NS | [74] |
| Liposomes | Liposomes with CL and WGA loaded with curcumin and NGF | Antioxidant, neuroprotector, anti-inflammatory, anti-amyloid, and anti-Tau hyperphosphorylation | 110–170 | NS | −15 | ∼60 | [75] |
| Nanoemulsion | O/W pomegranate oil nanoemulsion containing punicic acid | Antioxidant/reduced neuronal death, lipid oxidation, and increased neurogenesis | 135 ± 12 | NS | NS | NS | [79] |
| SLN | Resveratrol and grape extract-loaded SLN functionalized with the OX26 antibody | Antioxidant/neuroprotector | 254 ± 17 | 0.23 ± 0.05 | −4.0 ± 0.1 | 95 ± 2 | [82] |
| Selenium NPs | Mesoporous nano-selenium based on the borneol target, β-cyclodextrin nanovalves with loaded resveratrol | Antioxidant/neuroprotector | 160 | NS | −43.4 | 88.5 ± 4.98 | [84] |
| Ceria NPs | TPP ceria NPs | Mitochondrial ROS scavengers and suppress neuronal death | 22 | NS | 45 | NS | [90] |
| Nanochelator/polystyrene NPs | Nano-N2PY | Chelation of transition metals/to avoid Aβ aggregation | 240 | NS | NS | NS | [91] |
| Polymer NPs | Polymeric core nanocarriers labeled with IgG4.1 | Anti-amyloid antibody | 221.6 ± 22.5 | NS | 41.6 ± 2.6 | 52 | [92] |
| PLGA NPs | PLGA NP-delivery system of the Tet1-conjugated nattokinase enzyme | Degrading amyloid fibrils; suppressing β amyloid and BACE-1 activity | 192.5 | 0.145 | NS | 88 | [94] |
| Polymer NPs | Poly(propylene imine) glycodendrimers | Aβ anti-aggregating | 7.4–8.4 | NS | NS | NS | [95] |
| Metallic NPs | Negatively charged gold NPs | Inhibit the formation of Aβ fibrils | 30 | NS | −38 | NS | [96] |
| None | Immunogenic epitopes | Immunogenicity and immunogenic response and reduced the Aβ fiber production | NS | NS | NS | NS | [97] |
| Albumin NPs | BSA NPs carrying β-cyclodextrins loaded with tacrine | AChEI | 177.4 ± 18.0 | 0.257 | −10.0 ± 0.9 | 22.0 ± 0.05 | [101] |
| PLGA NPs | Tacrine loaded poly(lactide-co-glycolide) NPs | AChEI | 247–293 | NS | NS | 72.34–81.32 | [102] |
| PLGA NPs | Galantamine PLGA NPs | AChEI | [105] | ||||
| None | Galantamine hydrobromide-loaded carbopol gel drug reservoirs | AChEI | NS | NS | NS | NS | [106] |
| Chitosan nanofilm/nanofiber | Donepezil-loaded chitosan nanofiber films | AChEI | 150–250 | NS | NS | NS | [108] |
| Nano-emulsion | Mango gum/chitosan loaded with donepezil NPs and o/w mango gum loaded with donepezil nanoemulsion | AChEI | 95.1 ± 1.02 | 0.26 | 25.92 ± 1.45 | 85 ± 2.14 | [109] |
| PLGA NPs | Huperzine A/lactoferrin-conjugated N-trimethylated chitosan PLGA NPs | AChEI | 153.2 ± 13.7 | 0.229 ± 0.078 | 35.6 ± 5.2 | 73.8 ± 5.7 | [110] |
| Nanodandelion | Nanodandelion tin(iv)/carbacylamidophosphate | AChE and butyl cholinesterase inhibitor | 20–50 | NS | NS | NS | [111] |
| Nanolipid | Lipidic nanocarriers loaded with pioglitazone | PPARy agonist that activates PI3/Akt and downregulates p38 MAPK pathways | 211.4 ± 3.54 | 0.257 ± 0.108 | 14.9 ± 1.09 | 70.18 ± 4.5 | [112] |
| siRNA nanocarriers | PEGylated poly[2-(dimethylamino)ethyl methacrylate] ethyl methacrylate (PEG-PDMAEMA) modified with CGN peptide and Tet1 for siRNA | Silence BACE1 gene | 70–80 | NS | ∼10 | NS | [113] |
| Nanoethosomes | Nanoethosomes loaded with vinpocetine | Phosphodiesterase inhibitor | 50.57 ± 26.1 | NS | NS | 86.61 ± 2.9 | [117] |
| Polymer NPs | Dendrimer poly(epsilon-lysine) NPs loaded with flurbiprofen, | Aβ42 selective lowering agent and γ-secretase modulator | NS | NS | NS | NS | [119] |
| PLGA/SLN | PLGA and SLN loaded with tarenflurbil | Aβ42 selective lowering agent and γ-secretase modulator | 133.13 ± 7.82/169.87 ± 10.98 | 0.21 ± 0.02/0.24 ± 0.04 | − 30.25 ± 2.11/− 23.13 ± 2.32 | 64.11 ± 2.2/57.81 ± 5.3 | [120] |
| Quantum dots | ZnS-capped CdSe quantum dots | Targeting of Aβ1–42 residue | NS | NS | NS | NS | [131] |
PLGA: poly(lactic-co-glycolic acid); PEG: poly(ethyleneglycol); CTAB: cetyltrimethylammonium bromide; O/W: oil water; N2PY: nanoparticle-chelator conjugate; SLN: solid lipid nanoparticles; NS: not shown.
-
Funding information: The authors acknowledge the financial support through Regular FONDECYT Project 1231154, ANID/PIA/ACT192144, FONDAP Project 15130011 granted by the Chilean National Agency for Research and Development (ANID) and National Doctoral Scholarship POS_NAC_D_2020_1_164514 granted by the National Research and Innovation Agency of Uruguay (ANII).
-
Author contributions: J.O.M.: conceptualization, resources, writing – review and editing, visualization, supervision, project administration, funding acquisition. N.N.M.: conceptualization, literature analysis, figure design, writing – original draft, review and edition, visualization. J.T.H.: conceptualization, writing – review and editing, visualization. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7(3):137–52.10.1038/nrneurol.2011.2Search in Google Scholar PubMed PubMed Central
[2] Patterson C. World Alzheimer report 2018. London: Alzheimer’s Disease International; 2018.Search in Google Scholar
[3] Klimova B, Maresova P, Valis M, Hort J, Kuca K. Alzheimer’s disease and language impairments: social intervention and medical treatment. Clin Interv Aging. 2015;10:1401–7.10.2147/CIA.S89714Search in Google Scholar PubMed PubMed Central
[4] Knopman DS, Amieva H, Petersen RC, Chételat G, Holtzman DM, Hyman BT, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33.10.1038/s41572-021-00269-ySearch in Google Scholar PubMed PubMed Central
[5] DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegeneration. 2019;14(1):1–18.10.1186/s13024-019-0333-5Search in Google Scholar PubMed PubMed Central
[6] Ricciarelli R, Fedele E. The amyloid cascade hypothesis in Alzheimer’s disease: It’s time to change our mind. Curr Neuropharmacol. 2020;15(6):926–35.10.2174/1570159X15666170116143743Search in Google Scholar PubMed PubMed Central
[7] Hillen H. The beta amyloid dysfunction (BAD) hypothesis for Alzheimer’S disease. Front Neurosci. 2019;13:1154.10.3389/fnins.2019.01154Search in Google Scholar PubMed PubMed Central
[8] Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63(3):287–303.10.1016/j.neuron.2009.06.026Search in Google Scholar PubMed PubMed Central
[9] Karran E, Mercken M, Strooper BD. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discovery. 2011;10(9):698–712.10.1038/nrd3505Search in Google Scholar PubMed
[10] Jäkel L, Boche D, Nicoll JAR, Verbeek MM. Aβ43 in human Alzheimer’s disease: effects of active Aβ42 immunization. Acta Neuropathologica. Communications. 2019;7(1):1–11.10.1186/s40478-019-0791-6Search in Google Scholar PubMed PubMed Central
[11] Müller U, Winter P, Graeber MB. A presenilin 1 mutation in the first case of Alzheimer’s disease. Lancet Neurol. 2013;12(2):129–30.10.1016/S1474-4422(12)70307-1Search in Google Scholar PubMed
[12] Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc National Acad Sci. 1997;94(1):298–303.10.1073/pnas.94.1.298Search in Google Scholar PubMed PubMed Central
[13] Wang Y, Shi Y, Wei H. Calcium dysregulation in Alzheimer’s disease: a target for new drug development. J Alzheimers Dis Parkinsonism. 2017;7(5):374.10.4172/2161-0460.1000374Search in Google Scholar PubMed PubMed Central
[14] Pascual-Caro C, Berrocal M, Lopez-Guerrero AM, Alvarez-Barrientos A, Pozo-Guisado E, Gutierrez-Merino C, et al. STIM1 deficiency is linked to Alzheimer’s disease and triggers cell death in SH-SY5Y cells by upregulation of L-type voltage-operated Ca2+ entry. J Mol Med. 2018;96(10):1061–79.10.1007/s00109-018-1677-ySearch in Google Scholar PubMed PubMed Central
[15] Mufson EJ, Counts SE, Perez SE, Ginsberg SD. Cholinergic system during the progression of Alzheimer’s disease: therapeutic implications. Expert Rev Neurother. 2008;8(11):1703–18.10.1586/14737175.8.11.1703Search in Google Scholar PubMed PubMed Central
[16] Douchamps V, Mathis C. A second wind for the cholinergic system in Alzheimer’s therapy. Behav Pharmacol. 2017;28(2):112–23.10.1097/FBP.0000000000000300Search in Google Scholar PubMed
[17] Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141(7):1917–33.10.1093/brain/awy132Search in Google Scholar PubMed PubMed Central
[18] Teipel SJ, Fritz H-C, Grothe MJ. Initiative ftAsDN Neuropathologic features associated with basal forebrain atrophy in Alzheimer disease. Neurology. 2020 Sep 8;95(10):e1301–11.10.1212/WNL.0000000000010192Search in Google Scholar PubMed PubMed Central
[19] Padurariu M, Ciobica A, Lefter R, Lacramioara Serban I, Stefanescu C, Chirita R. The oxidative stress hypothesis in Alzheimer’s disease. Psychiatria Danubina. 2013;25(4):409.Search in Google Scholar
[20] Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer’s disease. Biomark Med. 2010;4(1):27–36.10.2217/bmm.09.89Search in Google Scholar PubMed PubMed Central
[21] Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci. 2008;29(12):609–15.10.1016/j.tips.2008.09.001Search in Google Scholar PubMed
[22] Pardridge WM. Alzheimer’s disease drug development and the problem of the blood-brain barrier. Alzheimers Dement. 2009;5(5):427–32.10.1016/j.jalz.2009.06.003Search in Google Scholar PubMed PubMed Central
[23] Ovais M, Zia N, Ahmad I, Khalil AT, Raza A, Ayaz M, et al. Phyto-therapeutic and nanomedicinal approaches to cure Alzheimer’s disease: Present status and future opportunities. Front Aging Neurosci. 2018;10:284.10.3389/fnagi.2018.00284Search in Google Scholar PubMed PubMed Central
[24] (ISO) IOfS. ISO/TS 80004-2:2015 Nanotechnologies – Vocabulary – Part 2: Nano-objects. Nanotechnologies – Vocabulary – Part 2: Nano-Objects. ISO/TS 80004-2:20152015.Search in Google Scholar
[25] Oesterling BM, Gulati A, Joshi MD. Nanocarrier-based approaches for treatment and detection of Alzheimer’s disease. J Nanosci Nanotechnol. 2014;14(1):137–56.10.1166/jnn.2014.8906Search in Google Scholar PubMed
[26] Gupta J, Fatima MT, Islam Z, Khan RH, Uversky VN, Salahuddin P. Nanoparticle formulations in the diagnosis and therapy of Alzheimer’s disease. Int J Biol Macromol. 2019;130:515–26.10.1016/j.ijbiomac.2019.02.156Search in Google Scholar PubMed
[27] Rocha S. Targeted drug delivery across the blood brain barrier in Alzheimer’s disease. Curr Pharm Des. 2013;19(37):6635–46.10.2174/13816128113199990613Search in Google Scholar PubMed
[28] Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1(3):223–36.10.1007/s11481-006-9025-3Search in Google Scholar PubMed
[29] Bellettato CM, Scarpa M. Possible strategies to cross the blood–brain barrier. Italian J Pediatrics. 2018;44(2):131.10.1186/s13052-018-0563-0Search in Google Scholar PubMed PubMed Central
[30] Sela H, Cohen H, Elia P, Zach R, Karpas Z, Zeiri Y. Spontaneous penetration of gold nanoparticles through the blood brain barrier (BBB). J Nanobiotechnol. 2015;13(1):71.10.1186/s12951-015-0133-1Search in Google Scholar PubMed PubMed Central
[31] Ceña V, Játiva P. Nanoparticle crossing of blood–brain barrier: a road to new therapeutic approaches to central nervous system diseases. Nanomedicine. 2018;13(13):1513–6.10.2217/nnm-2018-0139Search in Google Scholar PubMed
[32] Kumari S, Ahsan SM, Kumar JM, Kondapi AK, Rao NM. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci Rep. 2017;7(1):6602.10.1038/s41598-017-06888-4Search in Google Scholar PubMed PubMed Central
[33] Ulbrich K, Knobloch T, Kreuter J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood–brain barrier (BBB). J Drug Target. 2011;19(2):125–32.10.3109/10611861003734001Search in Google Scholar PubMed
[34] Neves AR, van der Putten L, Queiroz JF, Pinheiro M, Reis S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. J Biotechnol. 2021;331:108–17.10.1016/j.jbiotec.2021.03.010Search in Google Scholar PubMed
[35] Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat Commun. 2017;8(1):1001.10.1038/s41467-017-00952-3Search in Google Scholar PubMed PubMed Central
[36] Arora S, Sharma D, Singh J. GLUT-1: An effective target to deliver brain-derived neurotrophic factor gene across the blood brain barrier. ACS Chem Neurosci. 2020;11(11):1620–33.10.1021/acschemneuro.0c00076Search in Google Scholar PubMed
[37] Pinheiro RGR, Coutinho AJ, Pinheiro M, Neves AR. Nanoparticles for targeted brain drug delivery: What do we know? Int J Mol Sci. 2021;22(21):11654.10.3390/ijms222111654Search in Google Scholar PubMed PubMed Central
[38] Lu W, Tan YZ, Hu KL, Jiang XG. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int J Pharm. 2005;295(1–2):247–60.10.1016/j.ijpharm.2005.01.043Search in Google Scholar PubMed
[39] Gonzalez-Carter D, Liu X, Tockary TA, Dirisala A, Toh K, Anraku Y, et al. Targeting nanoparticles to the brain by exploiting the blood-brain barrier impermeability to selectively label the brain endothelium. Proc Natl Acad Sci U S A. 2020;117(32):19141–50.10.1073/pnas.2002016117Search in Google Scholar PubMed PubMed Central
[40] Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zhang W, et al. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Rel. 2007;121(3):156–67.10.1016/j.jconrel.2007.05.026Search in Google Scholar PubMed
[41] Liu Z, Zhou T, Ziegler AC, Dimitrion P, Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid Med Cell Longev. 2017;2017:2525967.10.1155/2017/2525967Search in Google Scholar PubMed PubMed Central
[42] Yarza R, Vela S, Solas M, Ramirez MJ. c-Jun N-terminal kinase (JNK) signaling as a therapeutic target for Alzheimer’s disease. Front Pharmacol. 2016;6:321. 10.3389/fphar.2015.00321.Search in Google Scholar PubMed PubMed Central
[43] Verheij M, Ruiter GA, Zerp SF, van Blitterswijk WJ, Fuks Z, Haimovitz-Friedman A, et al. The role of the stress-activated protein kinase (SAPK/JNK) signaling pathway in radiation-induced apoptosis. Radiother Oncol: J Eur Soc Ther Radiol Oncol. 1998;47(3):225–32.10.1016/S0167-8140(98)00007-3Search in Google Scholar
[44] Chami L, Checler F. BACE1 is at the crossroad of a toxic vicious cycle involving cellular stress and β-amyloid production in Alzheimer’s disease. Mol Neurodegener. 2012;7(1):1–5.10.1186/1750-1326-7-52Search in Google Scholar PubMed PubMed Central
[45] Shah SZA, Zhao D, Hussain T, Yang L. The role of unfolded protein response and mitogen-activated protein kinase signaling in neurodegenerative diseases with special focus on prion diseases. Front Aging Neurosci. 2017;9:120.10.3389/fnagi.2017.00120Search in Google Scholar PubMed PubMed Central
[46] He J, Zhong W, Zhang M, Zhang R, Hu W. P38 mitogen-activated protein kinase and Parkinson’s disease. Transl Neurosci. 2018;9(1):147–53.10.1515/tnsci-2018-0022Search in Google Scholar PubMed PubMed Central
[47] Cai Z, Liu N, Wang C, Qin B, Zhou Y, Xiao M, et al. Role of RAGE in Alzheimer’s disease. Cell Mol Neurobiol. 2016;36(4):483–95.Search in Google Scholar
[48] Vukic V, Callaghan D, Walker D, Lue LF, Liu QY, Couraud PO, et al. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in alzheimer’s brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34(1):95–106.10.1016/j.nbd.2008.12.007Search in Google Scholar PubMed PubMed Central
[49] Wilkins HM, Carl SM, Greenlief ACS, Festoff BW, Swerdlow RH. Bioenergetic dysfunction and inflammation in Alzheimer’S disease: a possible connection. Front Aging Neurosci. 2014;6:311.10.3389/fnagi.2014.00311Search in Google Scholar PubMed PubMed Central
[50] Drenth H, Zuidema SU, Krijnen WP, Bautmans I, van der Schans C, Hobbelen H. Association between advanced glycation end-products and functional performance in Alzheimer’s disease and mixed dementia. Int Psychogeriatr. 2017;29(9):1525–34.10.1017/S1041610217000886Search in Google Scholar PubMed
[51] Zhao ZC, Nannuan L, Chuanling W, Biyong Q, Yingjun Z, Ming X, et al. Role of RAGE in Alzheimer’S disease. Cell Mol Neurobiol. 2015;36(4):483–95.10.1007/s10571-015-0233-3Search in Google Scholar PubMed
[52] Cenini G, Lloret A, Cascella R. Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxid Med Cell Longev. 2019;2019:2105607.10.1155/2019/2105607Search in Google Scholar PubMed PubMed Central
[53] Ullah R, Khan M, Shah SA, Saeed K, Kim MO. Natural antioxidant anthocyanins-a hidden therapeutic candidate in metabolic disorders with major focus in neurodegeneration. Nutrients. 2019;11(6):1195.10.3390/nu11061195Search in Google Scholar PubMed PubMed Central
[54] Texido L, Martin-Satue M, Alberdi E, Solsona C, Matute C. Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49(3):184–90.10.1016/j.ceca.2011.02.001Search in Google Scholar PubMed
[55] Sieber M, Baumgrass R. Novel inhibitors of the calcineurin/NFATc hub - alternatives to CsA and FK506? Cell Commun Signal. 2009;7(1):1–19.10.1186/1478-811X-7-25Search in Google Scholar PubMed PubMed Central
[56] Park HA, Broman K, Stumpf A, Kazyak S, Jonas EA. Nutritional regulators of Bcl-xL in the brain. Molecules. 2018;23(11):3019.10.3390/molecules23113019Search in Google Scholar PubMed PubMed Central
[57] Boland K, Flanagan L, Prehn JH. Paracrine Control of Tissue Regeneration and Cell Proliferation by Caspase-3. Cell Death Dis. 2013;4(7):e725.10.1038/cddis.2013.250Search in Google Scholar PubMed PubMed Central
[58] Wang WY, Tan MS, Yu JT, Tan L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 2015;3(10):136.Search in Google Scholar
[59] Li J, Yang JY, Yao XC, Xue X, Zhang QC, Wang XX, et al. Oligomeric Abeta-induced microglial activation is possibly mediated by NADPH oxidase. Neurochem Res. 2013;38(2):443–52.10.1007/s11064-012-0939-2Search in Google Scholar PubMed
[60] Squillaro T, Cimini A, Peluso G, Giordano A, Melone MAB. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem Pharmacol. 2018;154:303–17.10.1016/j.bcp.2018.05.016Search in Google Scholar PubMed
[61] Shal B, Ding W, Ali H, Kim YS, Khan S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front Pharmacol. 2018;9:548.10.3389/fphar.2018.00548Search in Google Scholar PubMed PubMed Central
[62] Fakhri S, Pesce M, Patruno A, Moradi SZ, Iranpanah A, Farzaei MH, et al. Attenuation of Nrf2/Keap1/ARE in Alzheimer’s disease by plant secondary metabolites: a mechanistic review. Molecules. 2020;25(21):4926.10.3390/molecules25214926Search in Google Scholar PubMed PubMed Central
[63] Calabrese V, Cornelius C, Dinkova-Kostova AT, Calabrese EJ, Mattson MP. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid Redox Signal. 2010;13(11):1763–811.10.1089/ars.2009.3074Search in Google Scholar PubMed PubMed Central
[64] Yagishita Y, Uruno A, Yamamoto M. Chapter 27 - NRF2-mediated gene regulation and glucose homeostasis. In: Mauricio D, editor. Molecular Nutrition and Diabetes. San Diego: Academic Press; 2016. p. 331–48.10.1016/B978-0-12-801585-8.00027-0Search in Google Scholar
[65] Matsumoto T, Matsubara Y, Mizuhara Y, Sekiguchi K, Koseki J, Tsuchiya K, et al. Plasma pharmacokinetics of polyphenols in a traditional Japanese medicine, jumihaidokuto, which suppresses propionibacterium acnes-induced dermatitis in rats. Molecules (Basel, Switz). 2015;20(10):18031–46.10.3390/molecules201018031Search in Google Scholar PubMed PubMed Central
[66] Zhou S, Hu Y, Zhang B, Teng Z, Gan H, Yang Z, et al. Dose-dependent absorption, metabolism, and excretion of genistein in rats. J Agric Food Chem. 2008;56(18):8354–9.10.1021/jf801051dSearch in Google Scholar PubMed
[67] Liu Y, You Y, Lu J, Chen X, Yang Z. Recent advances in synthesis, bioactivity, and pharmacokinetics of pterostilbene, an important analog of resveratrol. Molecules (Basel, Switz). 2020;25(21):5166.10.3390/molecules25215166Search in Google Scholar PubMed PubMed Central
[68] Sorrenti V, Contarini G, Sut S, Dall’Acqua S, Confortin F, Pagetta A, et al. Curcumin prevents acute neuroinflammation and long-term memory impairment induced by systemic lipopolysaccharide in mice. Front Pharmacol. 2018;9:183.10.3389/fphar.2018.00183Search in Google Scholar PubMed PubMed Central
[69] Yang LL, Xiao N, Li XW, Fan Y, Alolga RN, Sun XY, et al. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Sci Rep. 2016;6:35460.10.1038/srep35460Search in Google Scholar PubMed PubMed Central
[70] Langasco R, Fancello S, Rassu G, Cossu M, Cavalli R, Galleri G, et al. Increasing protective activity of genistein by loading into transfersomes: A new potential adjuvant in the oxidative stress-related neurodegenerative diseases? Phytomedicine. 2019;52:23–31.10.1016/j.phymed.2018.09.207Search in Google Scholar PubMed
[71] Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51.10.1016/j.addr.2015.09.012Search in Google Scholar PubMed PubMed Central
[72] Kim YS, Jung HM, Yoon B-E. Exploring glia to better understand Alzheimer’s disease. Anim Cell Syst. 2018;22(4):213–8.10.1080/19768354.2018.1508498Search in Google Scholar PubMed PubMed Central
[73] Nday CM, Halevas E, Jackson GE, Salifoglou A. Quercetin encapsulation in modified silica nanoparticles: potential use against Cu(ii)-induced oxidative stress in neurodegeneration. J Inorg Biochem. 2015;145:51–64.10.1016/j.jinorgbio.2015.01.001Search in Google Scholar PubMed
[74] Kheradmand E, Hajizadeh Moghaddam A, Zare M. Neuroprotective effect of hesperetin and nano-hesperetin on recognition memory impairment and the elevated oxygen stress in rat model of Alzheimer’s disease. Biomed Pharmacother. 2018;97:1096–1.10.1016/j.biopha.2017.11.047Search in Google Scholar PubMed
[75] Chen M, Du ZY, Zheng X, Li DL, Zhou RP, Zhang K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen Res. 2018;13(4):742–52.10.4103/1673-5374.230303Search in Google Scholar PubMed PubMed Central
[76] Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J Biol Chem. 2013;288:4056–65.10.1074/jbc.M112.393751Search in Google Scholar PubMed PubMed Central
[77] Huo X, Zhang Y, Jin X, Li Y, Zhang L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid beta aggregation in Alzheimer’s disease. J Photochem Photobiol B. 2019;190:98–102.10.1016/j.jphotobiol.2018.11.008Search in Google Scholar PubMed
[78] Kuo YC, Lin CC. Rescuing apoptotic neurons in Alzheimer’s disease using wheat germ agglutinin-conjugated and cardiolipin-conjugated liposomes with encapsulated nerve growth factor and curcumin. Int J Nanomed. 2015;10:2653–72.10.2147/IJN.S79528Search in Google Scholar PubMed PubMed Central
[79] Kuo YC, Lin CY, Li JS, Lou YI. Wheat germ agglutinin-conjugated liposomes incorporated with cardiolipin to improve neuronal survival in Alzheimer’s disease treatment. Int J Nanomed. 2017;12:1757–74.10.2147/IJN.S128396Search in Google Scholar PubMed PubMed Central
[80] Freitas HR, Ferreira Gda C, Trevenzoli IH, Oliveira Kde J, de Melo Reis RA. Fatty acids, antioxidants and physical activity in brain aging. Nutrients. 2017;9:1263.10.3390/nu9111263Search in Google Scholar PubMed PubMed Central
[81] Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57(6):451–5.10.1016/j.phrs.2008.05.002Search in Google Scholar PubMed
[82] Mizrahi M, Friedman-Levi Y, Larush L, Frid K, Binyamin O, Dori D, et al. Pomegranate seed oil nanoemulsions for the prevention and treatment of neurodegenerative diseases: the case of genetic CJD. Nanomedicine. 2014;10(6):1353–63.10.1016/j.nano.2014.03.015Search in Google Scholar PubMed
[83] Freyssin A, Page G, Fauconneau B, Rioux Bilan A. Natural stilbenes effects in animal models of Alzheimer’s disease. Neural Regen Res. 2020;15(5):843–9.10.4103/1673-5374.268970Search in Google Scholar PubMed PubMed Central
[84] Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR. Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci. 2014;6:218.10.3389/fnagi.2014.00218Search in Google Scholar PubMed PubMed Central
[85] Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules. 2017;22(2):277.10.3390/molecules22020277Search in Google Scholar PubMed PubMed Central
[86] Butterfield DA, Sultana R. Methionine-35 of abeta(1-42): importance for oxidative stress in Alzheimer disease. J Amino Acids. 2011;2011:198430.10.4061/2011/198430Search in Google Scholar PubMed PubMed Central
[87] Sun J, Wei C, Liu Y, Xie W, Xu M, Zhou H, et al. Progressive release of mesoporous nano-selenium delivery system for the multi-channel synergistic treatment of Alzheimer’s disease. Biomaterials. 2019;197:417–31.10.1016/j.biomaterials.2018.12.027Search in Google Scholar PubMed
[88] Zhang QL, Fu BM, Zhang ZJ. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood-brain barrier permeability. Drug Deliv. 2017;24(1):1037–44.10.1080/10717544.2017.1346002Search in Google Scholar PubMed PubMed Central
[89] Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008;66(5):594–617.10.1007/978-1-4020-6869-0_2Search in Google Scholar
[90] Sahebnasagh A, Eghbali S, Saghafi F, Sureda A, Avan R. Neurohormetic phytochemicals in the pathogenesis of neurodegenerative diseases. Immun Ageing. 2022;19(1):36.10.1186/s12979-022-00292-xSearch in Google Scholar PubMed PubMed Central
[91] Wang X, Wang X, Guo Z. Metal-involved theranostics: An emerging strategy for fighting Alzheimer’s disease. Coord Chem Rev. 2018;362:72–84.10.1016/j.ccr.2018.03.010Search in Google Scholar
[92] Rajasekhar K, Chakrabarti M, Govindaraju T. Function and toxicity of amyloid beta and recent therapeutic interventions targeting amyloid beta in Alzheimer’s disease. Chem Commun. 2015;51(70):13434–50.10.1039/C5CC05264ESearch in Google Scholar PubMed
[93] Viles MG, David CB, John H. Copper redox cycling inhibits Aβ fibre formation and promotes fibre fragmentation, while generating a dityrosine Aβ Dimer. Sci Rep. 2018;8(1):1–14.10.1038/s41598-018-33935-5Search in Google Scholar PubMed PubMed Central
[94] Farina M, Avila DS, da Rocha JB, Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int. 2013;62(5):575–94.10.1016/j.neuint.2012.12.006Search in Google Scholar PubMed PubMed Central
[95] Kwon HJ, Cha MY, Kim D, Kim DK, Soh M, Shin K, et al. Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano. 2016;10(2):2860–70.10.1021/acsnano.5b08045Search in Google Scholar PubMed
[96] Liu G, Men P, Kudo W, Perry G, Smith MA. Nanoparticle-chelator conjugates as inhibitors of amyloid-beta aggregation and neurotoxicity: a novel therapeutic approach for Alzheimer disease. Neurosci Lett. 2009;455(3):187–90.10.1016/j.neulet.2009.03.064Search in Google Scholar PubMed PubMed Central
[97] Agyare EK, Curran GL, Ramakrishnan M, Yu CC, Poduslo JF, Kandimalla KK. Development of a smart nano-vehicle to target cerebrovascular amyloid deposits and brain parenchymal plaques observed in Alzheimer’s disease and cerebral amyloid angiopathy. Pharm Res. 2008;25(11):2674–84.10.1007/s11095-008-9688-ySearch in Google Scholar PubMed PubMed Central
[98] Chen H, McGowan EM, Ren N, Lal S, Nassif N, Shad-Kaneez F, et al. Nattokinase: A promising alternative in prevention and treatment of cardiovascular diseases. Biomark Insights. 2018;13:1177271918785130.10.1177/1177271918785130Search in Google Scholar PubMed PubMed Central
[99] Bhatt PC, Verma A, Al-Abbasi FA, Anwar F, Kumar V, Panda BP. Development of surface-engineered PLGA nanoparticulate-delivery system of Tet1-conjugated nattokinase enzyme for inhibition of Abeta40 plaques in Alzheimer’s disease. Int J Nanomed. 2017;12:8749–68.10.2147/IJN.S144545Search in Google Scholar PubMed PubMed Central
[100] Klementieva O, Aso E, Filippini D, Benseny-Cases N, Carmona M, Juves S, et al. Effect of poly(propylene imine) glycodendrimers on beta-amyloid aggregation in vitro and in APP/PS1 transgenic mice, as a model of brain amyloid deposition and Alzheimer’s disease. Biomacromolecules. 2013;14(10):3570–80.10.1021/bm400948zSearch in Google Scholar PubMed
[101] Liao YH, Chang YJ, Yoshiike Y, Chang YC, Chen YR. Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-beta fibrillization, induce fibril dissociation, and mitigate neurotoxicity. Small. 2012;8(23):3631–9.10.1002/smll.201201068Search in Google Scholar PubMed
[102] Sun D, Qiao Y, Jiang X, Li P, Kuai Z, Gong X, et al. Multiple antigenic peptide system coupled with amyloid beta protein epitopes as an immunization approach to treat Alzheimer’s disease. ACS Chem Neurosci. 2019;10(6):2794–800.10.1021/acschemneuro.9b00020Search in Google Scholar PubMed
[103] Calabrese EJ, Iavicoli I, Calabrese V. Hormesis: why it is important to biogerontologists. Biogerontology. 2012;13(3):215–35.10.1007/s10522-012-9374-7Search in Google Scholar PubMed
[104] Geldmacher DS. Acetylcholinesterase inhibitors for Alzheimer’s disease. Aging Health. 2007;3(4):483–94.10.2217/1745509X.3.4.483Search in Google Scholar
[105] Francis PT. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005;10(S18):6–9.10.1017/S1092852900014164Search in Google Scholar PubMed
[106] LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD, USA: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. https://pubmed.ncbi.nlm.nih.gov/31643176.Search in Google Scholar
[107] Luppi B, Bigucci F, Corace G, Delucca A, Cerchiara T, Sorrenti M, et al. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur J Pharm Sci. 2011;44(4):559–65.10.1016/j.ejps.2011.10.002Search in Google Scholar PubMed
[108] Joe VF, Kumar SS. Formulation, characterization and determination of anti-alzheimeric activity of tacrine loaded poly (lactide-co-glycolide) nanoparticles. Int J Pharm Sci Res. 2018;9(12):5111–20.Search in Google Scholar
[109] Lilienfeld S. Galantamine--a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer’s disease. CNS Drug Rev. 2002;8(2):159–76.10.1111/j.1527-3458.2002.tb00221.xSearch in Google Scholar PubMed PubMed Central
[110] Fisher A, Carney G, Bassett K, Dormuth CR. Tolerability of cholinesterase inhibitors: a population-based study of persistence, adherence, and switching. Drugs Aging. 2017;34(3):221–31.10.1007/s40266-017-0438-xSearch in Google Scholar PubMed
[111] Fornaguera C, Feiner-Gracia N, Caldero G, Garcia-Celma MJ, Solans C. Galantamine-loaded PLGA nanoparticles, from nano-emulsion templating, as novel advanced drug delivery systems to treat neurodegenerative diseases. Nanoscale. 2015;7(28):12076–84.10.1039/C5NR03474DSearch in Google Scholar
[112] Woo FY, Basri M, Masoumi HR, Ahmad MB, Ismail M. Formulation optimization of galantamine hydrobromide loaded gel drug reservoirs in transdermal patch for Alzheimer’s disease. Int J Nanomed. 2015;10:3879–86.10.2147/IJN.S80253Search in Google Scholar PubMed PubMed Central
[113] Blesa R, Toriyama K, Ueda K, Knox S, Grossberg G. Strategies for continued successful treatment in patients with Alzheimer’S disease: An overview of switching between pharmacological agents. Curr Alzheimer Res. 2018;15:964–74.10.2174/1567205015666180613112040Search in Google Scholar PubMed PubMed Central
[114] AnjiReddy K, Karpagam S. Chitosan nanofilm and electrospun nanofiber for quick drug release in the treatment of Alzheimer’s disease: In vitro and in vivo evaluation. Int J Biol Macromol. 2017;105(Pt 1):131–42.10.1016/j.ijbiomac.2017.07.021Search in Google Scholar PubMed
[115] Jakki SL, Ramesh YV, Gowthamarajan K, Senthil V, Jain K, Sood S, et al. Novel anionic polymer as a carrier for CNS delivery of anti-Alzheimer drug. Drug Deliv. 2016;23(9):3471–9.10.1080/10717544.2016.1196767Search in Google Scholar PubMed
[116] Meng Q, Wang A, Hua H, Jiang Y, Wang Y, Mu H, et al. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int J Nanomed. 2018;13:705–18.10.2147/IJN.S151474Search in Google Scholar PubMed PubMed Central
[117] Roumiani ME, Dorosti N. Sonochemical synthesis of a nanodandelion tin(IV) complex with carbacylamidophosphate ligand as anti-Alzheimer agent: Molecular docking study. Ultrason Sonochem. 2019;55:207–16.10.1016/j.ultsonch.2019.01.025Search in Google Scholar PubMed
[118] Jojo GM, Kuppusamy G, De A, Karri V. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev Ind Pharm. 2019;45(7):1061–72.10.1080/03639045.2019.1593439Search in Google Scholar PubMed
[119] Wang P, Zheng X, Guo Q, Yang P, Pang X, Qian K, et al. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J Control Rel. 2018;279:220–33.10.1016/j.jconrel.2018.04.034Search in Google Scholar PubMed
[120] Medina AE. Vinpocetine as a potent antiinflammatory agent. Proc Natl Acad Sci U S A. 2010;107:9921–2.10.1073/pnas.1005138107Search in Google Scholar PubMed PubMed Central
[121] Zhang Y-S, Li J-D, Yan C. An update on vinpocetine: New discoveries and clinical implications. Eur J Pharmacol. 2018;819:30–4.10.1016/j.ejphar.2017.11.041Search in Google Scholar PubMed PubMed Central
[122] Wu Y, Li Z, Huang YY, Wu D, Luo HB. Novel phosphodiesterase inhibitors for cognitive improvement in Alzheimer’s disease. J Med Chem. 2018;61(13):5467–83.10.1021/acs.jmedchem.7b01370Search in Google Scholar PubMed
[123] Moghaddam AA, Aqil M, Ahmad FJ, Ali MM, Sultana Y, Ali A. Nanoethosomes mediated transdermal delivery of vinpocetine for management of Alzheimer’s disease. Drug Deliv. 2015;22(8):1018–26.10.3109/10717544.2013.846433Search in Google Scholar PubMed
[124] Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomed. 2019;14:5541–54.10.2147/IJN.S200490Search in Google Scholar PubMed PubMed Central
[125] Al-Azzawi S, Masheta D, Guildford AL, Phillips G, Santin M. Dendrimeric Poly(Epsilon-Lysine) delivery systems for the enhanced permeability of flurbiprofen across the blood-brain barrier in Alzheimer’s disease. Int J Mol Sci. 2018;19(10):3224.10.3390/ijms19103224Search in Google Scholar PubMed PubMed Central
[126] Muntimadugu E, Dhommati R, Jain A, Challa VG, Shaheen M, Khan W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur J Pharm Sci. 2016;92:224–34.10.1016/j.ejps.2016.05.012Search in Google Scholar PubMed
[127] Li X, Kaida-Yip F, Zabel M. NSAID Use and the Prevention of Alzheimer’s Disease: A Meta-Analysis (P6.184). Neurology. 2018;90(15):6–184.Search in Google Scholar
[128] De Craen AJ, Gussekloo J, Vrijsen B, Westendorp RG. Meta-analysis of nonsteroidal antiinflammatory drug use and risk of dementia. Am J Epidemiol. 2005;161(2):114–20.10.1093/aje/kwi029Search in Google Scholar PubMed
[129] Kaushik A, Jayant RD, Tiwari S, Vashist A, Nair M. Nano-biosensors to detect beta-amyloid for Alzheimer’s disease management. Biosens Bioelectron. 2016;80:273–87.10.1016/j.bios.2016.01.065Search in Google Scholar PubMed PubMed Central
[130] Matsumura K, Ono M, Hayashi S, Kimura H, Okamoto Y, Ihara M, et al. Phenyldiazenyl benzothiazole derivatives as probes for in vivo imaging of neurofibrillary tangles in Alzheimer’s disease brains. MedChemComm. 2011;2(7):596–600.10.1039/c1md00034aSearch in Google Scholar
[131] Feng L, Long HY, Liu RK, Sun DN, Liu C, Long LL, et al. A quantum dot probe conjugated with a beta antibody for molecular imaging of Alzheimer's disease in a mouse model. Cell Mol Neurobiol. 2013;33(6):759–65.10.1007/s10571-013-9943-6Search in Google Scholar PubMed
[132] Vinod C, Jena S. Nano-neurotheranostics: impact of nanoparticles on neural dysfunctions and strategies to reduce toxicity for improved efficacy. Front Pharmacol. 2021;12:612692.10.3389/fphar.2021.612692Search in Google Scholar PubMed PubMed Central
[133] Sharma HS, Hussain S, Schlager J, Ali SF, Sharma A. Influence of nanoparticles on blood-brain barrier permeability and brain edema formation in rats. Acta Neurochir Suppl. 2010;106:359–64.10.1007/978-3-211-98811-4_65Search in Google Scholar PubMed
[134] Ma L, Liu J, Li N, Wang J, Duan Y, Yan J, et al. Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity. Biomaterials. 2010;31(1):99–105.10.1016/j.biomaterials.2009.09.028Search in Google Scholar PubMed
[135] Kou L, Bhutia YD, Yao Q, He Z, Sun J, Ganapathy V. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. 2018;9:27.10.3389/fphar.2018.00027Search in Google Scholar PubMed PubMed Central
[136] Abdifetah O, Na-Bangchang K. Pharmacokinetic studies of nanoparticles as a delivery system for conventional drugs and herb-derived compounds for cancer therapy: a systematic review. Int J Nanomed. 2019;14:5659–77.10.2147/IJN.S213229Search in Google Scholar PubMed PubMed Central
[137] Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–70.10.1038/nbt1340Search in Google Scholar PubMed PubMed Central
[138] Wei A, Mehtala JG, Patri AK. Challenges and opportunities in the advancement of nanomedicines. J Control Rel. 2012;164(2):236–46.10.1016/j.jconrel.2012.10.007Search in Google Scholar PubMed PubMed Central
[139] Sadzuka Y, Hirotsu S, Hirota S. Effect of liposomalization on the antitumor activity, side-effects and tissue distribution of CPT-11. Cancer Lett. 1998;127(1–2):99–106.10.1016/S0304-3835(98)00031-7Search in Google Scholar
[140] Owens DE 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102.10.1016/j.ijpharm.2005.10.010Search in Google Scholar PubMed
[141] Crosasso P, Ceruti M, Brusa P, Arpicco S, Dosio F, Cattel L. Preparation, characterization and properties of sterically stabilized paclitaxel-containing liposomes. J Control Rel. 2000;63(1–2):19–30.10.1016/S0168-3659(99)00166-2Search in Google Scholar
[142] Lu WL, Qi XR, Zhang Q, Li RY, Wang GL, Zhang RJ, et al. A pegylated liposomal platform: pharmacokinetics, pharmacodynamics, and toxicity in mice using doxorubicin as a model drug. J Pharmacol Sci. 2004;95(3):381–9.10.1254/jphs.FPJ04001XSearch in Google Scholar PubMed
[143] Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discovery. 2021;20(2):101–24.10.1038/s41573-020-0090-8Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

