Abstract
In this review, the research reports on the dispersion processes of carbon nanotubes (CNTs) in aqueous cementitious materials are intensively introduced and summarized. The main processes for the CNTs dispersion in aqueous systems include high shear emulsification, ultrasonic treatment, covalent modification, and non-covalent modification. The influences of various factors on the dispersity of CNTs are evaluated, and the pros and cons of dispersion processes of CNTs are analyzed, along with the dispersion mechanism of CNTs in aqueous materials. Several novel techniques are also introduced, including arc thermal excitation and electromagnetic field-induced method, etc. In addition, the challenges when CNTs dispersion are further involved in cementitious alkali pore solution and the improvement means are also described in detail. And, the direct dispersion process (in situ growth process) of CNTs in cementitious materials has also been discussed in depth.
1 Introduction
Attempts have been made to attenuate the poor toughness of cementitious materials by using high performance fiber materials, and to render the cementitious materials themselves with good self-sensing properties through the conducting effect of fiber materials. Micro/nano ductile fibers with high aspect ratio, modulus of elasticity, and electrical conductivity can be used as toughening and conducting components to simultaneously improve the mechanical toughness and sensing properties of the cementitious materials [1,2,3]. Carbon nanotubes (CNTs) are one-dimensional nanomaterials possessing a special structure with a diameter of about 2–100 nm and a very high aspect ratio (100–1,000). CNTs were first discovered by high-resolution transmission electron microscopy (TEM) in 1991 by Dr Iijima [4], a Japanese physicist. They are nanoscale hollow tubes made of single or multi-layer graphene sheets bent and rolled, and now available for large-scale production and engineering applications [5]. CNTs have good mechanical properties, and their tensile strengths can reach 50–200 GPa [6,7,8], which is 100 times that of steel, with only 1/6 of the density of steel and an elastic modulus of about 1 TPa. In addition, CNTs have good thermal, electrical conduction, and optical properties, and can be widely used to modify metals, ceramics, asphalt, and polymer materials [9,10,11,12,13,14,15,16,17,18]. It was found that CNTs could also overcome the drawbacks of cementitious materials [19,20] such as low tensile strength and brittleness [21], improve its anti-cracking [22–26], electrical conductivity [3,27,28], durability [29–31], and also develop its piezoresistive self-sensing properties, etc. [32,33]. Han et al. [34] prepared a self-sensing cementitious material for traffic self-monitoring modified by CNTs. The material had good piezoresistive properties and was sensitive enough to detect the mechanical stress caused by traffic flow. Yu et al. [35] added 0.1 wt% CNTs to the cementitious materials and found that CNTs not only improved the electrical conductivity of the cement-based material, but also increased compressive strength by 16.8%.
However, CNTs have strong van der Waals force between themselves and are very apt to entangle each other and form macro/micro agglomerates in water or aqueous matrix [36–40]. Meanwhile, the dispersion status of CNTs in the matrix is directly related to the various macroscopic properties of the corresponding CNTs-reinforced composite. The agglomerated CNTs not only fail to show their excellent properties, but even damage the inherent properties of the matrix materials [41–43]. Therefore, how to disperse CNTs conveniently and efficiently has become the most critical issue for the rational use of CNTs to strengthen various kinds of composite.
Aqueous systems are the most common systems in civil engineering and bioengineering. At present, most scholars adopt the method of first dispersing CNTs in aqueous systems and then mixing them with cementitious materials to prepare CNTs reinforced cementitious materials (NRC). Therefore, the dispersion process of CNTs in aqueous systems is one of the key concerns. The CNTs dispersion process in aqueous systems can be roughly divided into three stages: (1) surface wetting of CNTs, (2) breaking of CNT agglomerates, and (3) enhancement of repulsion between CNTs to prevent re-agglomeration. At present, the main dispersion processes of CNTs in aqueous systems include high shear emulsification process [44], ultrasonic treatment process [45], covalent modification process [46], and non-covalent modification process [47] (the schematic diagram of major processes for CNTs dispersion is shown in Figure 1), and the above processes can also be combined with each other to acquire superior dispersity of CNTs [48,49].
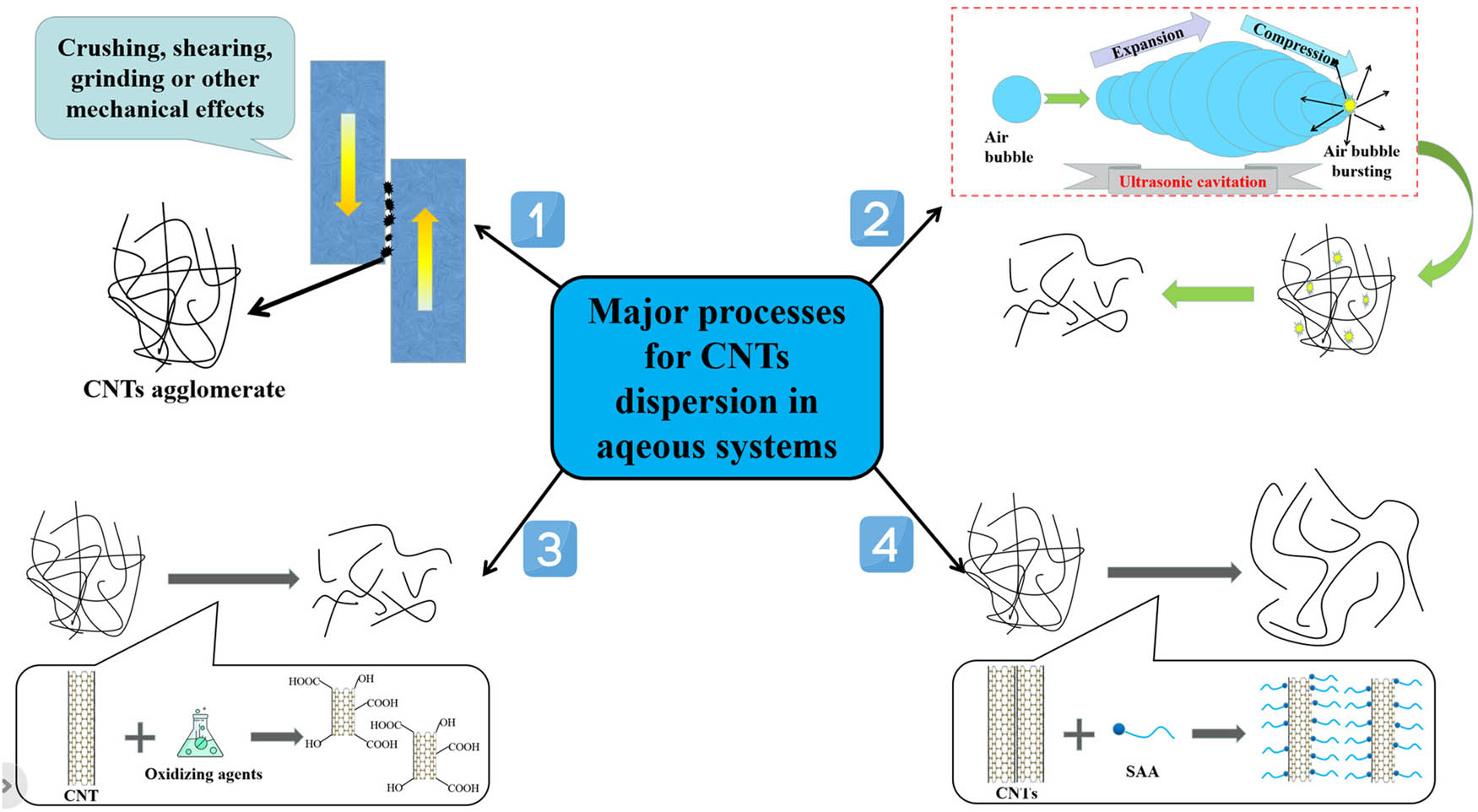
The schematic diagram of major processes for CNTs dispersion in aqueous systems: (1) high shear emulsification process; (2) ultrasonic treatment process; (3) covalent modification process; and (4) non-covalent modification process.
Most of the aqueous dispersions of CNTs with good dispersibility can improve the performance of cementitious materials to some extent by mixing them directly with cementitious materials. However, it is of more concern whether secondary agglomeration of CNTs occurs in the cementitious material systems. It will lead to the weakening of the modification effect of CNTs on NRC. At present, there are few processes to directly disperse CNTs in the cementitious material systems, mainly in situ growth techniques [50]. Therefore, how to guarantee the dispersion stability of CNTs aqueous dispersion or how to directly disperse CNTs in the cementitious material systems is another key issue that needs to be solved at present.
Now, CNTs have been widely used, and the processes of dispersing CNTs are gradually mature. However, there is a lack of comprehensive collation and in-depth analysis of various decentralized processes. And the introduction and analysis of new processes are also less. At present, most of the existing reviews only analyze several main dispersion processes [51]. Their mechanism analysis of dispersion processes is not thorough, and the dispersion of CNTs in cementitious pore solution is rarely mentioned. Without a comprehensive and in-depth understanding and analysis of various dispersion processes, it is difficult to find the most effective and practical dispersion process. In this work, the dispersion processes and effects of CNTs dispersion in aqueous systems and cementitious material systems are analyzed in depth, respectively. Compared with previous studies, we have more carefully sorted out the effects of various dispersion conditions, materials, and processes on the CNTs dispersion. The problems arising from the admixture of CNTs aqueous dispersions into cementitious materials are also presented, and improvement methods are proposed. Finally, the guidelines for the dispersion process of CNTs in aqueous cementitious materials are proposed.
2 CNTs dispersion in aqueous systems
2.1 High shear emulsification process
High shear emulsification process is a novel physical dispersion method, and the main principle of this process is to achieve effective dispersion of CNTs through high-shear mechanical force such as crushing, shearing, grinding techniques, etc.
Zhang et al. [52] used impact grinding and high shear emulsification process to disperse CNTs in aqueous systems, respectively, and the fractal dimension was used to determine the dispersion effect of the two processes. The fractal dimension of the dispersions prepared by the two processes were 1.15–1.59 and 1.46–1.58, respectively, which showed that the shear emulsification process was slightly better than the other one. The impact grinding mainly broke the large agglomerates into small pieces without separating the filamentous CNTs, while the shear emulsification process could separate a certain amount of CNTs. Krause et al. [53] quantified the length distributions of CNTs after 5 and 10 h of dry grinding in ball mills, and the average length decreased by 46 and 65%, respectively. As the ball milling time continued to increase, although the size of CNT agglomerates decreased, the packing density of CNTs increased, resulting in poor dispersion of CNTs in aqueous solution, as shown in Figure 2. Although the above studies indicated that the high shear emulsification process is not effective in dispersing CNTs, some scholars obtained positive conclusions. Munkhbayar et al. [54] used a planetary ball mill to grind CNTs under different conditions in order to disperse CNTs in aqueous system. The CNTs were ball milled at different speeds (200, 300, 400, and 500 rpm) and under different dry and wet grinding conditions, respectively. The microstructure of the treated CNTs is shown in Figure 3. It could be shown that under the dry grinding conditions, the CNTs were in long and not obviously short-sheared state at 200 rpm, they were in flat and rough agglomerate state at 300–400 rpm, and at 500 rpm, although they were obviously cut short, still appeared in agglomerate state. Under wet grinding conditions, the dispersion effect of CNTs improved with the increase in shear speed, at 500 rpm, CNTs were obviously short sheared and well dispersed. And, compared with the simple ball milling method to disperse CNTs, the dispersion effect can be enhanced by introducing suitable additives in the ball milling [55]. Ma et al. [56] found that the conductivity of ball-milled CNTs did not increase significantly in the absence of additives, and even showed a slight decrease in conductivity after over 4 h ball milling, which indicated some damages to the electronic structures of CNTs. However, in the presence of NH4HCO3, the CNTs conductivity increased significantly after ball milling, and the conductivity gradually increased with the increase in ball milling time. After 9 h ball milling, the conductivity of CNTs increased by 250% with respect to that without ball milling. These phenomena were attributed to the nitrogen compounds being covalently bonded onto the CNTs, functioning as electron donors, and responsible for charge transfer to CNTs. Pierard et al. [57] dispersed CNTs with an average length of 0.8 µm by ball milling method. It was shown that the ball milling method severely damaged the CNTs, and excessive grinding did not disperse the agglomerates well, their lengths of CNTs became so short that their own properties jeopardized.
![Figure 2
The length distributions of CNTs in aqueous systems after 5 h and 10 h of dry grinding in ball mills [53]: (a) no grinding; (b) 5 h grinding; and (c) 10 h grinding.](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_002.jpg)
The length distributions of CNTs in aqueous systems after 5 h and 10 h of dry grinding in ball mills [53]: (a) no grinding; (b) 5 h grinding; and (c) 10 h grinding.
![Figure 3
SEM microstructure of CNTs after grinding treatment with different rotating speeds at 200, 300, 400, and 500 rpm [54]: (a) dry grinding and (b) wet grinding (in water).](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_003.jpg)
SEM microstructure of CNTs after grinding treatment with different rotating speeds at 200, 300, 400, and 500 rpm [54]: (a) dry grinding and (b) wet grinding (in water).
The reviews of studies on the dispersion of CNTs by high shear emulsification process is shown in Table 1. Although high shear emulsification process can disperse CNTs to some extent, the CNTs dispersed by this method have many problems. The process is energy-consuming, has low dispersion efficiency, and limited dispersion effect, which will generate a lot of by-product waste, making it not to be widely adopted. More importantly, the process can severely shear CNTs, resulting in significantly short lengths and loss of various excellent properties.
Reviews on the CNTs dispersion in aqueous systems by high shear emulsification process
| Instruments | Dispersion conditions | CNTs concentration* (wt%) | Remarks |
|---|---|---|---|
| Impact grinding | Wet grinding (in water) | 5.0 | Broke the large agglomerates into small pieces without separating the CNTs [52] |
| High shear emulsification process | A certain amount of CNTs were separated [52] | ||
| Planetary ball mill | Dry grinding | 7.5 | CNTs were short cut and poorly dispersed [53] |
| Dry grinding | 2.5 | CNTs were short sheared and well dispersed [54] | |
| Wet grinding (in water) | |||
| Dry grinding | 13.7 | The conductivity of CNTs increased significantly [56] | |
| Dry grinding | 100 | CNTs were severely damaged [57] |
*CNTs concentration: The mass ratio (wt%) of CNTs to total dispersion, the following Table is as the same.
2.2 Ultrasonic treatment process
The dispersion mechanism on CNTs of ultrasonic treatment process is mainly ultrasonic cavitation. The impacts from high temperature and wave energy generated by ultrasonic treatment break the CNTs agglomerates and achieve uniform dispersion of the CNTs in the liquid [58,59,60,61]. CNTs dispersed using the ultrasonic treatment process can effectively improve the properties of cementitious materials. Dong et al. [62] further sonicated CNTs aqueous suspension with 40 kHz power and 60 min sonication time after subjected to mechanical stirring (only CNTs solution was stirred evenly by mechanical force, and CNTs were not sheared or worn) to obtain more homogeneous CNTs dispersed liquid. And, the good piezoresistive properties of NRC were prepared with them. Table 2 summarizes the reviews on the dispersion of CNTs by ultrasonic treatment process. A detailed explanation of the contents in Table 2 is also provided as below.
Reviews on the dispersion of CNTs in aqueous systems by ultrasonic treatment process
| Power/frequency | Time | CNTs quality | CNTs concentration (wt%) | Remarks |
|---|---|---|---|---|
| 80 W | 60 min | Average diameter of 9.5 nm, average length of 1.5 µm | 0.1 | Obtained homogeneous CNTs dispersion and NRC with good strength, toughness and piezoresistive properties [61,62] |
| 30 min | Diameter range of 8–15 nm, length range of 3–12 µm | 0.1–0.2 | ||
| 3, 6, 9, and 12 W | 30 min | SWNTs (>1 µm in length) | 0.1 | CNT dispersion effect was greatly influenced by the ultrasonic power [64] |
| 3 W during the first 3 h, 15 W for the next 4 h, 40 W for the next 6 h, 55 W for the next 9 h, and 77 W for the final 12 h | Average diameter of 0.80 ± 0.35 nm for AD CNTs | 0.1 | The effect of the sonication time was different for CNTs prepared by different methods [63] | |
| Average diameter of 0.75 ± 0.07 nm for CVD CNTs | ||||
| Average diameter of 0.70 ± 0.20 nm for HiPCO CNTs | ||||
| 94 W | 1–60 min | Diameter range of 5–15 nm, length range of 10–20 µm | 0.14 | Ultrasonic power and time together affected CNTs dispersion [66] |
| 55–118 W | 15 min | |||
2.2.1 Effects of ultrasonic power on the dispersity of CNTs
Ultrasonic power is the key factor affecting the dispersity of CNTs. Koh et al. [63] found that there was no significant difference in the degree of dispersion of CNTs obtained with different ultrasonic power, but with higher ultrasonic power the CNTs would be sheared shorter. Vichchulada et al. [64] tested the dispersion effect of single-walled CNTs (SWNTs) suspension after ultrasonic treatment. It was found that SWNTs were effectively dispersed at ultrasonic power up to 9 W, and the CNTs dispersions will be stabilized at ultrasonic power between 9 and 12 W. When the ultrasonic power exceeded 12 W, the SWNTs were cut to some extent, and the damage to SWNTs was more severe at higher ultrasonic power.
From the results summarized in Table 2, the optimal ultrasonic power required for different CNT qualities and test conditions varies widely. The power required to disperse SWNTs is smaller, and the power required to disperse CNTs with larger diameters and more walls is much larger than the former.
2.2.2 Effects of sonication time on the dispersity of CNTs
Sonication time is another important factor affecting the dispersion of CNTs. Rausch et al. [65] found that the UV absorbance of CNT dispersions increased with sonication time, and the variation in absorbance of diluted CNT dispersions with increasing sonication time is shown in Figure 4. However, Koh et al. [63] investigated the dispersion of CNTs synthesized by arc discharge (AD), chemical vapor deposition (CVD), and high pressure carbon monoxide method (HiPCO), respectively. It was found that the dispersity of CNTs was not exactly positively correlated with the sonication time. The dispersity of CNTs prepared by AD became the worst compared to those by CVD and HiPCO methods after the sonication time exceeded 6 h. All of CNT dispersions could not remain stable after the sonication time continued to increase. Gao et al. [66] found that the synergy effect of both ultrasonic power and time on the dispersion of CNTs was greater. When the sonication time exceeded 15 min, the dispersion of CNTs no longer increased significantly by continuing to increase the sonication time, and the effect was better by increasing the ultrasonic power at this time. It is worth noting that the ultrasonic power exceeding 94 W caused deterioration of dispersity of CNTs.
![Figure 4
The variation in the absorbance of diluted CNT dispersions with the increase in the sonication time [65].](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_004.jpg)
The variation in the absorbance of diluted CNT dispersions with the increase in the sonication time [65].
Ultrasonic power and time do not affect the dispersity of CNTs alone, and they work largely in coordination. The analysis of the ultrasonic power and time data in Table 2 shows that high power and prolonged ultrasonic treatment is suitable for dispersing CNTs with large diameter and severe agglomeration. The smaller diameter CNTs can reduce the damage to their own structure by moderately reducing the ultrasonic power or time. For CNTs with very small diameter or SWNTs, it is better to choose low power and short duration ultrasonic treatment.
2.2.3 Drawbacks of ultrasonic treatment and employment of combined dispersion process
Ultrasonic treatment is a physical dispersion process, and the CNTs after reasonable ultrasonic treatment can effectively enhance the mechanical and electrical properties of cementitious materials after incorporation. If the ultrasonic power, time, and temperature are not reasonably controlled, it will cause excessive shearing of CNTs with small diameter and poor quality. And, the CNTs incorporation cannot improve the performance of NRC, and even damage the inherent properties of the matrix [67,68,69,70].
Dispersing CNTs by ultrasonic treatment alone can break the CNT agglomerates effectively, but it is difficult to maintain the stability of CNTs dispersions. It is worth noting that the dispersion effect will be superior when combined with other methods, such as ultrasonic combined with covalent modification, ultrasonic combined with non-covalent modification, ultrasonic and covalent modification combined with non-covalent modification process, etc. As revealed in Figure 5, there exists big gap in TEM images of CNTs between before and after non-covalent modification combined with ultrasonic treatment. The surface-active agent (SAA) used for non-covalent modification is cetyltrimethylammonium bromide (CTAB). Ultrasonic power, frequency and time are selected 100 W, 40 kHz and 30 min, respectively.
![Figure 5
TEM morphology of CNTs before and after non-covalent modification combined with ultrasonic treatment [48]: (a) untreated and (b) treated.](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_005.jpg)
TEM morphology of CNTs before and after non-covalent modification combined with ultrasonic treatment [48]: (a) untreated and (b) treated.
2.3 Covalent modification process
The surfaces of CNTs are modified with oxidizing agents such as strong oxidizing acids, potassium permanganate, and Fenton reagents. It can open the covalent bonds on the surfaces or ends of CNTs, and hydroxyl and carboxyl groups are introduced at the corresponding positions [71]. The introduction of hydrophilic functional groups can make the air or other impurities on the surfaces of CNTs replaced by water to achieve wettability. It makes CNTs to be highly dispersible in aqueous materials [72]. A summary of the covalent modification process for CNTs dispersion in aqueous systems is shown in Table 3.
Reviews on the dispersion of CNTs in aqueous systems by covalent modification process
| Oxidizing agents | Reaction conditions | Reaction time | CNTs concentration (wt%) | Remarks |
|---|---|---|---|---|
| HNO3, KMnO4, H2SO4/HNO3, (NH4)2SO4, H2O2, O3 | 80℃ | 4 h | 0.04 | The covalent modification effect of CNTs is significantly affected by the type of oxidant [73] |
| The mixture of H2SO4 and HNO3 | 100℃ | 100 min, 180 min | 0.2 | The covalent modification process could damage CNTs, and the longer the treatment time, the more serious the damage [74] |
| The mixture of H2SO4 and HNO3 | Ultrasonic treatment and oxidation at 80℃ | 4 h | 0.02–0.1 | Ultrasonic combined with covalent modification process could break CNT agglomerates more adequately [75,76] |
| HNO3, H2SO4, and H2O2 | Ultrasonic treatment (100 W, 42 kHz) and oxidation at 40℃ | 2 h | 0.05 | |
| O3 | 0.1 | Enhanced the dispersibility of CNTs with less damage [78] | ||
2.3.1 Effects of covalent modification process on the dispersity of CNTs
Wepasnick et al. [73] used six oxidants for the surface structure modification of CNTs. It was found that CNTs were not sensitive to factors such as oxidant concentration, but were very significantly affected by the types of oxidants. The concentration of functional groups such as carbonyl and hydroxyl groups on the surface of CNTs treated with (NH4)2SO4, H2O2, and O3 was high. And the CNTs treated with more oxidants such as HNO3 and KMnO4 formed more carboxyl groups. Elkashef et al. [74] added CNTs to the solution with a 3:1 concentration ratio of HNO3 and H2SO4 and reacted for 100 and 180 min, respectively. It was found that the dispersity and defects of CNTs reacted for 180 min were significantly more than those reacted for 100 min, indicating that the covalent modification process was very prone to damage CNTs. Luo [48] found that covalent modification of CNTs with strong acids could enhance the dispersion of CNTs in cement-based aqueous system. The more important reason was that it reacts with Ca(OH)2 in cementitious materials. After the reaction, the functional groups on the surfaces of CNTs and Ca2+ formed calcium carboxylate, which increased the dispersion of CNTs. In summary, the hydrophilicity of the covalent modified CNTs increased significantly, which enabled their agglomerates to be broken up more easily and facilitated the more uniform dispersion of CNTs in aqueous and cementitious materials.
2.3.2 Effects of ultrasonic combined with covalent modification process on the dispersity of CNTs
The covalent modification process can make CNTs more hydrophilic and favor the CNTs to have wettability, but this is not enough to break up the CNT agglomerates. Ultrasonic treatment can provide sufficient energy to break up CNT agglomerates, as proved in Section 1.2.
Osorio et al. [75] used three covalent modification methods (H2SO4, HNO3, and HCl for the first one, H2SO4 and HNO3 for the second one, and HNO3 for the last one) to modify CNTs, respectively, and then combined with an ultrasonic treatment process to disperse CNTs in water. All three covalent modifications grafted functional groups on the surface of CNTs. In addition, it was found that the first functionalization process exhibited higher efficiency, followed by the second method, based on the CNTs dispersion in water. Avilies et al. [76] reduced the dispersant concentration and ultrasonic power in order to avoid excessive defects in CNTs during dispersion. CNTs were modified with low concentrations of HNO3, H2SO4, and H2O2, and then treated by low-power sonication to disperse the CNTs. In this process, the sonication time and solution temperature were strictly controlled to prevent serious damage to the structure of CNTs. Although the original structure and properties of CNTs were basically maintained, the corresponding dispersion effect was not satisfactory.
Overall, the covalent modification method usually combines ultrasonic treatment to effectively break the CNT clusters, but the actual dispersion effects and damage vary widely.
2.3.3 Pros, cons, and prospects of covalent modification process
The covalent modification process can effectively disperse CNTs with higher stability, but there are many conditions to consider, the concentration of oxidant, oxidation time, and reaction temperature will affect the dispersity of CNTs. It is very difficult to control the above conditions in a reasonable way. In most cases, CNTs are cut at sidewall defects without extensive functionalization of the remaining sidewalls, which will destroy the structure of CNTs themselves, causing many defects and loss of their properties [77].
With the in-depth study of the covalent modification process, it was found that the surface modification of CNTs could be performed using weak oxidizing substances such as O3 and UV, the dispersity of corresponding CNTs in aqueous solution can also be enhanced. It can not only improve the dispersity of CNTs in aqueous materials but also reduce the damage of the covalent reaction to the structure of CNTs themselves. Jung et al. [78] investigated the effects of O3 treatment on the dispersity of CNTs in aqueous solution and the properties of ultra-high performance concrete (UHPC) prepared with the resulting CNT dispersions. As a result, the interfacial interaction between CNTs and UHPC was enhanced, and then the nano nucleation effect of CNTs in the early hydration stage was better. The O3-treated CNTs not only altered the hydration mechanism of UHPC significantly, but also provided multiple nucleation sites and double spatial repulsion, which effectively improved the dispersity of CNTs, between CNTs and cement particles. And, the O3-treated CNTs also accelerated the early hydration and improved the compressive strength of UHPC at the later stage. Unfortunately, it does not provide a detailed study of the dispersion of O3-modified CNTs in the pore solution of cementitious materials.
2.4 Non-covalent modification process
The non-covalent modification process is mainly based on the adsorption of various molecules on the surfaces of CNTs to disperse CNTs without affecting their internal molecular structure. The original structure and the unique properties of CNTs dispersion can be better maintained by the non-covalent modification.
SAA is a hydrophilic/oleophilic amphiphilic molecule that have the effects of reducing surface tension and energy. It can adsorb on the surfaces of CNTs and form the oriented arrangement [79]. And then, suitable SAAs have good encapsulation properties for CNTs, which thereby gives them strong amphiphilic and interfacial properties.
Table 4 shows the application conditions and effects of the non-covalent modification and its combined process. A detailed explanation of the contents in Table 4 is also provided as below.
Reviews on the dispersion of CNTs in aqueous systems by non-covalent modification process
| SAA types | SAA concentration | CNTs concentration | Combined processes | Remarks |
|---|---|---|---|---|
| SDBS, SDS, Tx100 | 20, 0.5, 0.8 mg/ml# | 0.1 mg/ml | Magnetic stirring or ultrasonic treatment | Different types of SAA had different dispersion mechanisms [80–82] |
| SDS, SDBS, GA, Tx100, CTAB | 0.02 mg/ml | 0.03 mg/ml | ||
| CTAB, SDS, HTA-103, OP-10 | 0.04, 0.3, 0.025, 0.06 wt% | 0.1 wt% | ||
| SDS | 0.01–0.4 wt% | 0.05–0.15 wt% | SAA concentration severely affected the dispersibility of CNTs and the properties of NRC [83–86] | |
| SDS, PVP, GA | 0.025–0.2 wt% | 0.1 wt% | Ultrasonic and covalent modification | |
| SDS, Tx100, Tween 20, 80 | 1.1–1.9 wt% | 0.1 wt% | Ultrasonic treatment | |
| 0.24–1 wt% | 0.16 wt% | Ultrasonic could provide energy to break the non-covalent modified CNT agglomerates [86,87] | ||
| SDS, Tx100, CTAB, GA | 1 wt% | 0.21 wt% | ||
| Water reducing agent | 0.5 wt% | 0.1, 0.2 wt% | Ultrasonic and covalent modification | The combination of the three processes could yield CNTs dispersion with excellent dispersibility and stability [50,88] |
| Water reducing agent, Tx100 | 0.28, 0.56 wt% | 0.1, 0.2, 0.3 wt% |
# N mg/ml: the concentration of CNTs or SAA in solution is N mg/ml.
2.4.1 Effects of different SAA types on the dispersity of CNTs
Different SAA types have different chemical structures and properties, and their abilities to disperse CNTs vary. Islam et al. [80,81] used sodium dodecylbenzene sulfonate (SDBS), sodium dodecyl sulfate (SDS), nonylphenol ethoxylate (Tx100), and gum Arabic (GA) etc., to disperse CNTs. They found that the length of alkyl chains of SAA, the types of reactive groups, and the interaction with CNTs were the main factors affecting the dispersity of CNTs. It was also noted that in order to fully integrate CNTs with cementitious materials, SAA not only needed to be able to adequately disperse CNTs, but must also be compatible with cementitious materials.
Chen et al. [82] investigated the mechanism of dispersing CNTs by four different SAAs (cationic SAA–CTAB, anionic SAA–SDS, baryonic SAA–nonylphenol ether sulfosuccinate monosodium disodium salt (HTA-103), and nonionic SAA–octylphenol polyoxyethylene ether (OP-10)). The molecular structure of CTAB consists of a positively charged head group and a hydrophobic alkyl carbon chain. The head group of CTAB is electrostatically attracted to the surface of negatively charged CNTs, while its alkyl chain is also attracted to CNTs by van der Waals force and hydrophobicity. However, the electrostatic adsorption force of CTAB is significantly stronger than the van der Waals force and hydrophobicity. Therefore, when doping with appropriate amount of CTAB, the head groups will be adsorbed on the surfaces of CNTs and the alkyl chains will be left alone. The outer alkyl chains in turn adsorb the free alkyl chains in solution due to van der Waals force and hydrophobicity, which leads to the formation of the positively charged semi-micellar SAA double cladding layers at the periphery of the CNTs. By virtue of the steric effects (space hindrance caused by the proximity of certain atoms or groups to each other) and electrostatic repulsion effects (the repulsive force between electrons in the outer layer of molecules when they are close to each other) of SAA between the modified CNTs, the dispersity of CNTs in aqueous solution and the stability of the dispersion are guaranteed. The mechanism of cationic SAA modification and dispersed CNTs is shown in Figure 6(a). The molecular structure of SDS consists of a negatively charged head group and a hydrophobic alkyl carbon chain. In the CNTs dispersion, the hydrophobic alkyl carbon chains adsorb to the negatively charged CNT surfaces due to van der Waals force and hydrophobicity to create the steric effects between the CNTs, and the negatively charged head groups provide electrostatic repulsion between the CNTs at the outermost layers. These effectively prevent the agglomeration between CNTs and ensure the dispersion stability. The mechanism of anionic SAA modification and dispersed CNTs is shown in Figure 6(b). The molecular structure of HTA-103 contains a benzene ring, which can adsorb with CNTs through π–π stacking interaction. Moreover, there are two negatively charged head groups in its molecular chain, which not only provide strong electrostatic repulsive force, but also will make the molecular chain of HTA-103 fully extended and produce good steric effects, so that the CNTs can be effectively dispersed. The molecular structure of OP-10 contains a hydrophobic alkyl chain, a benzene ring and –OCH2CH2– long chain. The alkyl chains are adsorbed on the surfaces of CNTs by hydrophobic and van der Waals force, and the benzene rings are adsorbed on the surfaces of CNTs by π–π stacking effect. Both of them work together to provide strong adsorption capacity. The –OCH2CH2– long chains in the outermost layers provide the steric effects, which increase the dispersion stability of CNTs in water. The mechanism of nonionic SAA modification and dispersed CNTs is shown in Figure 6(c).
![Figure 6
Schematic diagram of the mechanisms of different SAAs-modified and dispersed CNTs [82]: (a) cationic; (b) anionic; (c) non-ionic; and (d) illustration.](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_006.jpg)
Schematic diagram of the mechanisms of different SAAs-modified and dispersed CNTs [82]: (a) cationic; (b) anionic; (c) non-ionic; and (d) illustration.
2.4.2 Effects of SAA concentration on the dispersity of CNTs
SAA concentration affect the stability of CNTs suspension. When the concentration is too low, the steric and electrostatic repulsive effects between CNTs do not reach the level of preventing their agglomeration due to less SAA adsorbed on the surfaces of CNTs, which results in inferior dispersion of CNTs. When the SAA concentration is too high, supersaturated adsorption is formed on the surfaces of CNTs. Moreover, the excess SAA will adsorb each other to form micelles and compete with SAA from the surfaces of CNTs, resulting in inferior dispersion of CNTs.
Yu et al. [83] studied the effects of SAA concentration on the dispersity of CNTs by absorbance detection and other methods, and found that the maximum absorbance of CNTs suspension increased with the increase in SDS concentration, and the optimal concentration of SDS was 0.15 wt%. The absorbance of the dispersion did not change significantly after the SAA concentration continued to increase. Sezer and Koç [84] investigated the effects of SAA concentration on the dispersity of CNTs using the zeta potential method. By measuring the zeta potential values of the suspension with the mass ratio of SAA to CNTs varying from 0.25:1 to 2:1, it was found that the ideal ratio was between 0.5:1 and 1:1. When the ratio was greater than 1:1, the stability of CNT dispersions did not improve significantly with further increase in SAA dosage. On the other hand, if the SAA concentration was too low (≤0.25 wt%), it was not sufficient to stabilize the dispersed CNTs. Rastogi et al. [85] proved that the appropriate dosage of SAA is the key to give full play to its efficiency. It was found that SAA with high concentration was easy to form micelles in solution. These micelles interacted with other SAA molecules on adjacent CNTs. This interaction reduced their surface energy, which reduced the dispersion of CNTs, when the SAA concentration was high.
From the above research results shown in Table 4, the commonly used SAA are organic polymers. Among them, nonionic SAA-PVP and anionic SAA-SDBS are the most widely used and have superior dispersion effect. However, the compatibility of the above SAA with cementitious materials is problematic, which affects the strength and durability of NRC.
2.4.3 Effects of “ultrasound combined with non-covalent modification” process on the dispersity of CNTs
The non-covalent modification can effectively increase the repulsive force between CNTs, but the repulsive force provided by the non-covalent modification alone is not sufficient to break the CNT agglomerates and achieve effective dispersion of CNTs.
Konsta-Gdoutos et al. [86] suggested that effective dispersion of CNTs could be achieved using ultrasonic combined with SAA non-covalent modification process. It was found that the agglomerates of CNTs were effectively reduced and the dispersion became better. Luo et al. [87] used four SAA, including SDS, Tx100, CTAB, and GA, as dispersants for CNTs, and dispersed CNTs combined with ultrasonic treatment process, respectively. Then, the dispersion of CNTs in water was evaluated by static and centrifugal sediment observation. It was found that GA had the best effect on CNTs dispersion if a dispersant was used alone (the microscopic morphology of NRC with 0.2% GA-dispersed CNTs is shown in Figure 7(a)). Tx100 and CTAB were the second and third most effective, respectively, and SDS was the least effective. The flexural and compressive strengths of NRC with GA-dispersed CNTs were the highest, reaching 4.80 and 38.7 MPa, with an increase of 31.5 and 23.6%, respectively, compared to the blank specimens. They also found that hybrid dispersants might produce superior dispersity of CNTs, as proved in microscopic morphology of NRC doped with 0.2% CNTs by SDBS/Tx10A in Figure 7(b), the combination of Tx10A and SDBS on dispersity of CNTs was significantly better than that of GA alone.
![Figure 7
Microstructure of NRC doped with 0.2% CNTs after dispersed with [48]: (a) GA (low magnification); (b) GA (high magnification); (c) SDBS/Tx10 combination (low magnification); (d) SDBS/Tx10 combination (high magnification).](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_007.jpg)
Microstructure of NRC doped with 0.2% CNTs after dispersed with [48]: (a) GA (low magnification); (b) GA (high magnification); (c) SDBS/Tx10 combination (low magnification); (d) SDBS/Tx10 combination (high magnification).
2.4.4 Effects of “combination of ultrasonication, covalent decoration, and non-covalent modification” process on the dispersity of CNTs
Covalent modification can well solve the problem of difficult surface wetting of CNTs, ultrasonic treatment provides sufficient energy to break the agglomerates, and non-covalent modification can provide strong repulsive force for the dispersed CNTs to increase the stability of the dispersion. Therefore, the combination process of “ultrasonic and covalent modification method combined with non-covalent modification” can achieve sufficient dispersion and long-term stabilization of CNTs.
Abu Al-Rub et al. [49] functionalized the SAA-treated and non-SAA-treated CNTs in a mixture of concentrated H2SO4 and HNO3, respectively, and then ultrasonically dispersed CNTs. NRC was prepared by mixing 0.2% CNTs into cementitious materials, and the mechanical properties of NRC at 7 days of aging were tested. CNTs treated with SAA and functionalized with concentrated acid were found to have a significant increase in the strengths at 7 days of NRC, and CNTs functionalized with concentrated acid only without SAA treatment showed a severe decrease in the strengths at 7 days of NRC. Luo et al. [88] functionalized CNTs by Fenton reagent and UV irradiation, and then the functionalized CNTs were dispersed by combined ultrasonic and SAA treatment. In the obtained CNTs aqueous dispersions, the CNTs were well dispersed, and the CNTs functionalized by this process had less structural damage than those obtained by other covalent modification processes, which affected their own properties to a lesser extent. This process is one of the relatively most frequently-used, effective, and reasonable dispersion processes at present.
2.4.5 Pros, cons, and prospects of non-covalent modification process
Non-covalent modification process is the most common process to disperse CNTs, which has better dispersion effect and causes less damage to CNTs compared to covalent modification process. Although the process is widely used, it is still not sufficiently studied regarding whether the CNTs dispersed by SAA are well compatible with the cementitious materials and whether the properties of CNTs in the composite can be effectively played and how to improve their utilization efficiency. The reasonable design of SAA molecular structure is the key to improve the concentration and stability of CNT dispersions, and how to design the suitable structure of SAA [89] is the problem that need to be explored more deeply.
2.5 Comparison of the characteristics and effects of four types of dispersion processes
We compare and evaluate the above four dispersion processes. The four processes and their combinations are graded according to three criteria: the concentration, dispersion effect, and degree of damage of CNTs. The comparison and evaluation results are shown in Figure 8.
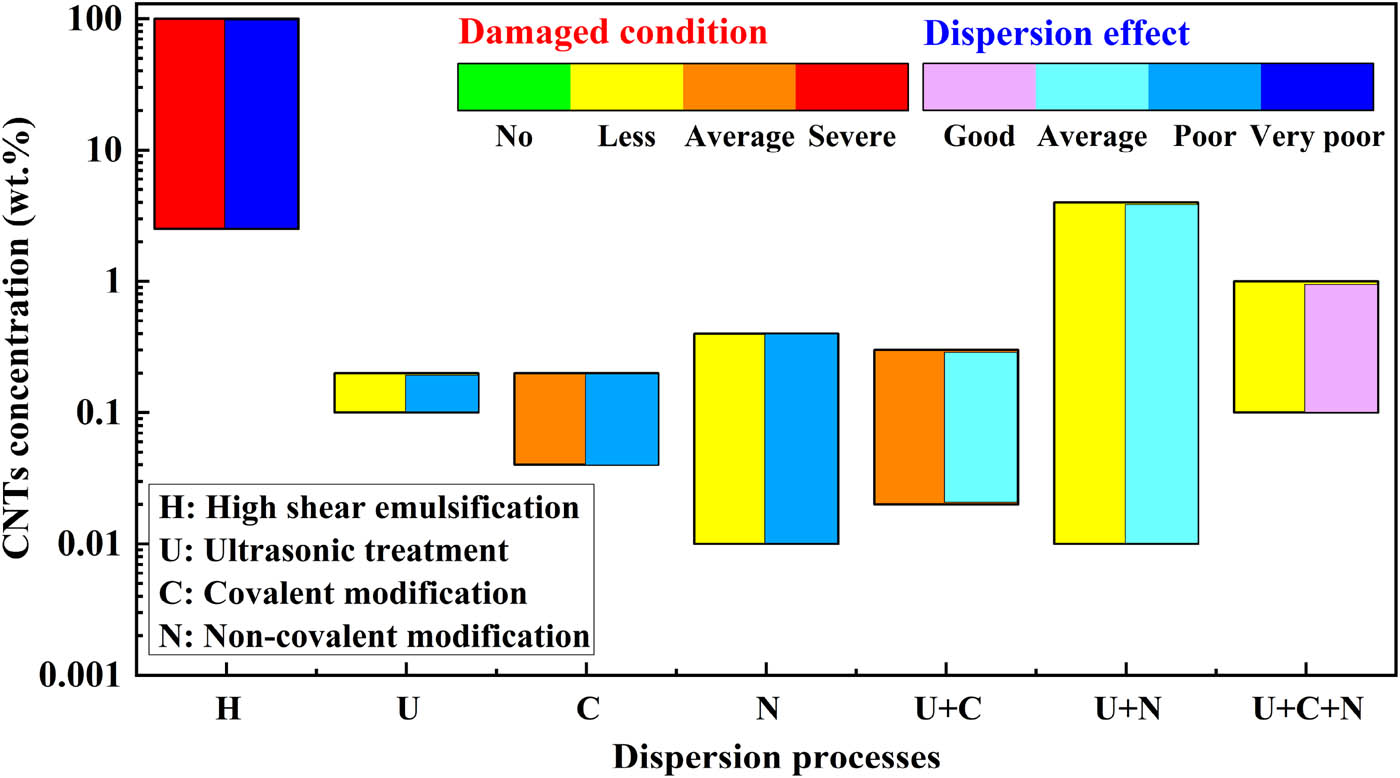
Evaluation of the comprehensive dispersion effect of different dispersion processes.
In Figure 8, we have determined the degree of damage and dispersion effect into four separate grades. The grading criteria for the damage degree are listed below. “No” means that there is basically no structural damage to the dispersed CNTs, and the performance is not affected; “Less” means that the dispersed CNTs are less damaged, only a small part of the structure is damaged, or the structure is not damaged, but the performance of CNTs is affected to some extent; “Average” means that most of the CNTs are damaged, but the degree of damage is average and the performance is greatly affected; “Severe” means that most of the CNTs are severely damaged, and the performance is also severely affected.
The dispersion effect is graded as follows: “Good” means that CNTs are basically well dispersed and their performance is not affected or minimally affected; “Average” means that most CNTs are well dispersed but their performance is affected to some extent; “Poor” means that some CNTs are separated from the agglomerates or more CNTs are well dispersed but the performance is seriously affected; “Very poor” means that the CNT agglomerates are not dispersed and the performance of CNTs is seriously impaired.
As can be seen from Figure 8, “H” process severely damages the structure of CNTs and the dispersion effect is poor. Its advantage is that it can be used for the dispersion of CNTs at ultra-high concentrations, which is far superior to other processes. The ultrasonic process is less damaging to CNTs, but the single process has limited effect on the dispersion of CNTs. The dispersion effect of “C” and “N” is similar, but “C” is more likely to damage CNTs. Considering the degree of damage and dispersion effect, the best dispersion process is “U + C + N.”
2.6 Effects of other processes on the dispersity of CNTs in aqueous systems
Each of the above processes can disperse CNTs more effectively, but the traditional processes may have some challenges. At present, finding new methods to disperse CNTs and giving full play to the excellent performance of CNTs are still the focus of research on NRC.
Cui [90] invented a dispersion method of CNTs based on the thermal excitation by electric arc, which could disperse CNTs in the gas-phase environment. And then, the matrix was modified by the dispersions. Finally, the CNTs are collected in the solvent by reasonable technical methods. The essence of this method is to cause the instantaneous boiling of the jumping medium doped with CNTs through the instantaneous input of high-density energy flow of electric arc, and the high-pressure gradient formed by the instantaneous boiling make the agglomerates “explode” and disperse to form flocculent CNT dispersions. The jumping medium is a liquid that can vaporize and expand rapidly when excited by high heat instantaneously, which plays the role of vaporization and expansion medium during the whole dispersion process, but does not affect the structure and composition of the final CNT dispersions. The schematic diagram of dispersion of CNTs by arc thermal excitation process is shown in Figure 9(a). Ghavidel et al. [91] proposed a new process for dispersion of CNTs based on the hydrodynamic stress to which the CNTs were subjected during the electric field orientation. The main innovation lied in the length conservation of CNTs and the real-time control of the dispersity. In this process, the CNTs were separated from the agglomerates by switching the magnetic field in different directions to frequently induce the movement of the CNTs. Figure 9(b) showed the dispersion effect of CNTs by ultrasonic treatment and electromagnetic field-induced process. Oka et al. [92] developed the novel high-speed dispersion device using cavitation bubble plasmas (CBP) to disperse CNTs uniformly in ion exchange water by CBP treatment without adding dispersant. With the increase in CBP treatment time, the mass of CNT agglomerates in ion exchange water became smaller and more uniformly dispersed. The schematic diagram of dispersion of CNTs by CBP dispersion process is shown in Figure 9(c). Zhao et al. [93] used the rapid expansion of CO2 in the supercritical state to disperse CNTs. CNTs were placed in a supercritical CO2 suspension (supercritical CO2 are those that remain in the liquid state under high temperature and pressure conditions), and the CO2 was rapidly expanded by a microfine nozzle to promote the uniform dispersion of CNTs.
The new dispersion processes have their own unique advantages and can effectively avoid the adverse effects caused by traditional methods, but they also create new problems. For example, they may hinder the compatibility of CNTs with cementitious materials, etc. Therefore, more detailed studies are needed to explore in the future.
3 CNTs dispersion in cementitious materials systems
As described in the introduction, CNTs are mostly mixed with cementitious materials in the form of CNTs aqueous dispersions when they are applied to cementitious materials. There are fewer means to directly disperse CNTs in cementitious material systems, and only in situ growth techniques are mainly available at present. Therefore, the research on CNTs dispersion in cementitious material systems mainly focus on the compatibility of aqueous dispersions with cementitious material systems, etc.
3.1 Problems of CNTs aqueous dispersions applied to cementitious materials
3.1.1 Dispersion process weakens the properties of CNTs
Currently, the dispersion process of CNTs in aqueous materials has been more effective. As can be seen from Section 1, the process with the best dispersion effect is “U + C + N,” taking into account various factors. However, even the CNT dispersions dispersed by this process still have problems when applied to cementitious material systems. There are two main reasons for this. One is that the polar functional groups introduced on the covalently modified CNTs affect the hydration of cementitious materials [94]. The other is that the electrical, thermal, and magnetic properties of CNTs are affected by the polymer-insulated SAA coating [95]. Batiston et al. [96] covalently modified CNTs using a mixed solution of H2SO4 and HNO3 and used them to modify cementitious materials. It was found that the hydration kinetics of cementitious materials incorporated with modified CNTs were delayed or even decreased. The CNTs rich in polar functional groups could attract large amounts of water and affect cement clinker hydration. It was also found that the Ca(OH)2 could effectively alleviate the delayed hydration phenomenon, thanks to the neutralization effect of alkaline Ca(OH)2 with acidic polar functional groups. Metaxa et al. [97] dispersed CNTs (0.05–0.2 wt%, length >5 µm, diameter 20–45 nm) using conventional SAA (SDBS and Tx100), HNO3, and high efficiency water-reducing agent (CC198), respectively, and then applied CNTs to modify cementitious materials. The results showed that the resistivity of the mortar prepared with SAA-dispersed CNTs was slightly reduced (10%), the electrical and mechanical properties of the mortar prepared by the second process were not changed, and the CNTs dispersed with water-reducing agent could perform well, and the resistivity of the mortar prepared with the CNTs was reduced by 60%, and the flexural and compressive strengths were increased by 25 and 10%, respectively. Moreover, if SAA doping is not well controlled, there will exist reversed micelles and obvious air-entraining effect, which will seriously affect the final performance of NRC. Therefore, the SAA doping needs to be strictly controlled, preferably not exceeding 1 wt%.
And, regardless of the use of any of the processes mentioned in Section 1, there is a common problem. Limited by the attraction of cement clinker particles, the weak compatibility of dispersant with cementitious materials and the strong alkalinity of pore solution of cementitious materials, CNTs dispersed in aqueous systems may agglomerate again after mixing with cementitious materials [98]. Mendoza et al. [99] used ultrasonic treatment and non-covalent modification process to disperse CNTs in water. To investigate whether the dispersed solution can also be stably dispersed in pore solution of cementitious material, the pore-like solution was prepared using Ca(OH)2. By analyzing the state of CNTs dispersion in this solution, it was found that some CNTs showed secondary agglomeration. The adsorption of Pozzolith 460 type superplasticizer (SP) on the surface of CNTs weakened in alkaline environment, which also lead to the electrostatic repulsion weakening between CNTs and SP molecular, resulting in CNTs poorly dispersed in the cementitious system. Silvestro et al. [100] investigated the dispersion of carboxyl-functionalized CNTs in pore solution of silicate-like cementitious materials and found that the dispersion of CNTs became significantly weaker in this system. They also prepared NRC using 0.1 wt% CNTs and found that the compressive strength of the NRC did not have significant change before and after the addition of CNTs. The micro- and nano-pores in NRC decreased but the macro-pores increased, which was the main reason for the lack of improvement in its mechanical properties.
3.1.2 Remaining challenges in CNTs dispersion
In addition to the above issues, there are still many problems about how to effectively utilize CNTs in cement matrix [101], the details are as follows. (1) How to improve the compatibility between CNTs and the components of cementitious materials is yet to be studied. (2) The synthesis, separation and purification, dispersion process, and dispersion characterization of CNTs have cumbersome operation steps, high technical content, instrument precision requirements, and costs. There is a need to develop fast, simple, and low-cost means of dispersion and characterization. (3) The dispersion methods of CNTs mainly focus on SAA coating, strong acid oxidation, and physical dispersion. The research and application of new dispersion processes are less. (4) The interaction mechanisms of CNTs with cementitious materials is not fully understood. The existing strengthening mechanisms, such as fiber bridging, fiber pull-out, nucleation, and network distribution, cannot fully explain the interaction between the two. (5) NRC is prone to the problem of insufficient long-term durability, and it remains to be studied whether NRC will lose strength or its brittleness will increase after a long time. (6) CNTs cannot be arranged in an orderly distribution in cementitious materials, which will lead to less performance enhancement of cementitious composite and lower utilization of CNTs. The existing dispersion techniques are difficult to orient and arrange CNTs, especially to make them uniformly arranged along the force direction in NRC.
3.2 Methods for maintaining the properties of CNTs when blended into cementitious materials
The CNTs are covalently modified to some extent by weak oxidant such as Fenton or O3, and then the water-reducing agent is used as CNTs dispersant synchronously to obtain the best dispersion effect combined by ultrasonic treatment. The CNTs dispersed by the above process can be applied to cementitious materials to effectively improve their comprehensive performance. First, it can improve the surface hydrophilicity of CNTs to a certain extent; second, it will not significantly reduce the length-to-diameter ratio and structure integrity of CNTs; third, the water reducing agent is used as SAA simultaneously without worrying about the incompatibility of other commonly used dispersants of CNTs with cement-based systems, which affects the hydration of cement; fourth, the weak oxidant and water reducing agent with many hydrophilic groups will not significantly affect the excellent mechanical and electrical properties of NRC [88,95]. Figure 10 shows the FTIR of CNTs before and after Fenton/UV modification and piezoresistive properties of NRC doped with 0.3% CNTs under cyclic loading. The CNTs dispersed by such process had good dispersity while maintaining the electronic structure integrity. The resistivity peak of the piezoresistive curve of NRC was maintained at 0.23 under cyclic loading with good stability, and the piezoresistive sensitivity of the NRC was substantially improved up to 137.5 [88].
![Figure 10
FTIR of CNTs after Fenton/UV modification and piezoresistive properties of NRC doped with 0.3% CNTs under cyclic loading [88]: (a) FTIR spectrum of CNTs; (b) piezoresistivity of NRC doped with 0.3% CNTs under cyclic loading (MPEG is the water reducing agent).](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_010.jpg)
FTIR of CNTs after Fenton/UV modification and piezoresistive properties of NRC doped with 0.3% CNTs under cyclic loading [88]: (a) FTIR spectrum of CNTs; (b) piezoresistivity of NRC doped with 0.3% CNTs under cyclic loading (MPEG is the water reducing agent).
3.3 Methods for maintaining the dispersity of CNTs dispersion when blended with cementitious materials
Although the alkaline pore solution in cementitious material affects the dispersity of CNTs, the CNTs with impaired dispersion effect still can improve the bulk performances of NRC. Du et al. [102] used methylcellulose to stabilize 0.08 wt% CNTs (CNTs modified by water-reducing agent and dispersed by sonication) in a multi-cementitious material consisting of silicate cement, fly ash (FA), and silica fume (water-to-cement ratio of 0.6). The pore structure of the CNTs-doped cementitious material was found to be significantly improved, and the compressive strength was significantly increased. It indicated that the dispersion of CNTs in cementitious material could be maintained by additional admixture of stabilizers for cementitious system in addition to the CNTs dispersion using other processes. Li et al. [26] compared the dispersion of CNTs, carboxy-functionalized CNTs, and low-temperature plasma-modified CNTs in the pore solution and their respective enhancement effects on silicate cementitious materials. It was found that a large number of oxygen-containing functional groups appeared on the surface of low-temperature plasma-modified CNTs, which could show good dispersion in the pore solution after mixing with water reducing agents. The mechanical properties of the cementitious materials showed more positive effects, with flexural and compressive strengths increased by 37.1 and 17.4%, respectively, which were much higher than the other two types of CNTs.
The above study illustrates that alkaline cementitious pore solution has a significant degrading effect on the dispersibility of CNTs. Although, this problem can be improved to some extent by incorporating stabilizers into cementitious materials or modifying CNTs using more efficient processes. This problem is still a critical factor that prevents nanomaterials such as CNTs and graphene, from fully exploiting their applications in cementitious materials.
3.4 In situ growth techniques
3.4.1 Effects of in situ growth process on the dispersity of CNTs
In situ growth techniques are applied to grow CNTs directly on the matrix to form CNTs-based composite by specific methods. Its huge development potential has attracted the attention of many scholars. Geraldo et al. [103] used CVD to synthesize CNTs in situ on sand grains. The optimum temperature and ethylene flow rate for CNTs growth were found to be 750°C and 300 sccm. Both methods, direct doping of functionalized CNTs and in situ growth of CNTs, improved the flexural strength of the mortars. But compared with the former (12% improvement in flexural strengths), the flexural strength of the composite prepared by the in situ growth process was doubled by 26% with the same dosage of CNTs. Ludvig et al. [50] synthesized CNTs by CVD using silicate cement clinker as carriers and converter furnace ash as iron precursors. Compared with other commonly used materials for CNTs synthesis, these materials had lower purity, more complicate composition, difficult particle size control, and lower cost. The obtained products were fibrous in structure and had more defects. It was also pointed out that if silica fume and FA were used as carriers, higher levels of carbon deposition, thinner diameter, and higher quality CNTs could be obtained. But there were no significant differences in the mechanical properties of NRC modified by the above two kinds of CNTs with different qualities. Ding et al. [104] used Ni–Al cement, processed from cement, nano-Al2O3, and Ni(NO3)3·9H2O, as the CNTs growth catalyst to generate CNTs on cement particles (CNTs@Cem) by CVD method, which effectively alleviated the CNTs agglomeration problem and improved the preparation efficiency of NRC. By analyzing the effect of different temperatures on the growth efficiency of CNTs, it was found that the optimal growth temperature of CNTs was 550–650°C when nickel was used as the growth catalyst, and the effect of temperature on the growth of CNTs@Cem is shown in Figure 11. The hierarchical structure of CNT@Cem integrated the dual functions of strengthening and conduction, which effectively improved the mechanical and self-sensing properties of the composite. The self-sensing cementitious materials prepared by this method were found to have excellent sensitivity and repeatability. Nasibulin et al. [105] synthesized novel nanocomposites, CNTs, and carbon fibers, by using cement particles as catalyst and carriers, and they adsorbed on cement particles with good dispersity. The method had high efficiency in synthesizing CNTs under low temperature conditions, and the compressive strengths of NRC could be increased by a factor of 2 and electrical conductivity by a factor of 40 after curing in water for 28 days. The combined process of “ultrasonic combined with in situ growth” is often used to disperse CNTs owing to superior dispersion effect. Zhan et al. [106] synthesized CNTs in situ on the surfaces of FA (CNT@FA) and significantly improved the dispersity of CNTs in combination with the ultrasonic treatment, resulting in NRC with excellent strain-sensing and mechanical properties. The carboxy-functionalized multi-walled CNTs (C-MWCNT) re-agglomerated with the average area of agglomerates 1,367 µm2 after sonication; in contrast, the CNT@FA dispersion was more stable with the majority of agglomerates of less than 100 µm2. Optical microscopy images of C-MWCNT and CNT@FA suspension with 2.0 wt% CNTs are shown in Figure 12. At monotonic compressive load in the elastic range, the mortars containing CNT@FA exhibited superior strain sensing sensitivity, and the maximum resistivity change of CNT@FA mortars was one order of magnitude higher than that of C-MWCNT mortars at the same concentration of CNTs.
![Figure 11
SEM images of CNT@Cem grown at different temperatures [104]: (a) 450℃; (b) 550℃; (c) 650℃; (d) 750℃; and (e) 850℃. (f) BET surface areas of the CNT@Cem grown at different temperatures.](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_011.jpg)
SEM images of CNT@Cem grown at different temperatures [104]: (a) 450℃; (b) 550℃; (c) 650℃; (d) 750℃; and (e) 850℃. (f) BET surface areas of the CNT@Cem grown at different temperatures.
![Figure 12
Optical images of C-MCWNT and CNT@FA suspension doped with 2.0 wt% CNTs [106]: (a) C-MCWNT and (b) CNT@FA.](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_012.jpg)
Optical images of C-MCWNT and CNT@FA suspension doped with 2.0 wt% CNTs [106]: (a) C-MCWNT and (b) CNT@FA.
The reviews of studies on the dispersion of CNTs by in situ growth process is shown in Table 5. Compared with the first four kinds of dispersion processes, this process does not cause serious damage to the structure of CNTs. It can also be seen from the above research results that the CNTs growth carriers are mainly cement particles and mineral admixtures. It means that there exist good compatibility between CNTs prepared by this process and cementitious materials. The excellent properties of CNTs can be advantageously explored, and the mechanical and self-sensing properties of NCR are significantly better than those of NRC prepared by the first four kinds of dispersion processes.
Reviews on the dispersion of CNTs in cementitious materials by in situ growth process
| Carbon sources | Catalysts | Carriers | Processes | Remarks |
|---|---|---|---|---|
| Ethylene | Mixed catalyst containing iron and cobalt | Sand grains | CVD | CNTs prepared by in situ growth process possessed superior dispersity, performance enhancement effect on NRC [50,103,104,,105] |
| Acetylene | Iron-containing catalysts | Silicate cement clinker, silica fume | ||
| Ni–Al cement | Cement particles | |||
| Cement particles | ||||
| Cyclopentadienyl | Iron catalyst | FA | Microwave irradiation | Ultrasonic combined with in situ growth process could break CNT aggregates well [106] |
3.4.2 Pros, cons, and prospects of in situ growth techniques
In situ growth of CNTs on the matrix yields well-dispersed CNTs, and more importantly, it can also eliminate the complex dispersion processes and improve the preparation efficiency of CNTs-based composite, so the techniques have a broad prospect for exploration and application. However, the research on the preparation of CNTs using in situ growth method on cementitious materials are modest. How to effectively control the growth direction and rate of CNTs on cement-based carriers still lacks a mature technique. In addition, the techniques have low efficiency and high cost for synthesizing CNTs, which makes them incapable of being directly applied to engineering. Further research to solve the above problems will enable better utilization of the novel techniques.
4 Conclusion and outlook
CNTs, as the nanoscale fiber materials with excellent performance, have broad application prospect in composite materials. How to effectively disperse CNTs is the key to give full play to their excellent properties and prepare NRC. Finding an efficient, economical, and reasonable dispersion process is the prerequisite for NRC to be used in industrial mass production.
Most scholars prefer to disperse CNTs in aqueous systems, and then use CNT dispersions to modify cementitious materials. The main processes for CNTs dispersion in aqueous systems include the following. High shear emulsification and ultrasonic treatment process are two common physical dispersion processes. However, the dispersion effect differs greatly between the two. High shear emulsification process is difficult to disperse the core part of CNT agglomerates, and it also damages the CNTs themselves so that they lose their inherent properties. In comparison, ultrasonic treatment provides more kinetic energy to disperse CNTs and the mechanical damage to CNTs is less. Covalent modification process can introduce reactive functional groups on CNTs to improve the dispersity and solubility of CNTs. This method is complicated and requires more demanding concentration of strong acid dispersant and treatment time, and can easily destroy the molecular structure of CNTs. In comparison, non-covalent bond modification process tends to be more favorable for CNTs dispersion. The main form of non-covalent modification process is to modify the CNTs with SAA to make the repulsive force between CNTs to achieve the purpose of dispersion of CNTs. It is worth noting that the non-covalent modification process also has its defects, and the functionalities of CNTs, such as their own electrical conductivity, magnetic conductivity, and optics, will be somewhat reduced after being encapsulated by inappropriate SAA.
The dispersion of CNTs, which are stably dispersed in aqueous systems, deteriorates when mixed with alkaline cementitious pore solution. Therefore, maintaining CNTs dispersion in the cementitious material systems is another important prerequisite to fully utilize the properties of CNTs. The influence of CNTs dispersion on the pore solution of cementitious materials can be reduced by selecting suitable SAA, doping with dispersion stabilizer, low-temperature plasma-modified CNTs, and other processes. In situ growth process is an emerging method for the direct preparation of dispersed CNTs, which can leave out the complex process of traditional dispersion methods, simplify the dispersion process. The dispersed CNTs can more effectively improve the various properties of cementitious materials. However, the synthesis efficiency is low, the dispersion cost is high, and it is difficult to control the growth of CNTs effectively.
At present, the dispersion mechanism of CNTs mainly contains: wetting, electrostatic repulsion, steric effects etc. A full understanding of the dispersion mechanisms of different dispersion processes is a prerequisite for the reasonable combination and application of multiple dispersion processes. There are still many challenges that need to be solved for practical applications of CNTs and CNTs modified cementitious materials in civil engineering, such as novel high-efficiency aqueous dispersants optimization for CNTs, the regular arrangement achievement of CNTs during the dispersion process, synthesis cost of CNTs, etc.
Acknowledgments
The authors are thankful for the helpful and instructive discussions with Prof. Zhang and Prof. Gao.
-
Funding information: This study was financially supported by NSFC-Shandong Province Joint Key Project (Grant No. U2106222), National Natural Science Foundation of China (No. 51878364), Project from China Construction Eighth Bureau (No. 004162021007030, JM20191030, QUT-2022-FW-0192, and QUT-2022-FW-0028), and the National “111” project, and Gaofeng discipline project funded by Shandong Province.
-
Author contributions: Jianlin Luo and Jigang Zhang: conceptualization, supervision, and resources; Yibo Gao and Jianlin Luo: data curation, formal analysis, and methodology; Jianlin Luo, Jigang Zhang, and Minglei Ma: funding acquisition, investigation, and project administration; Yibo Gao and Jianlin Luo: validation, visualization, and writing – original draft; Yibo Gao, Jianlin Luo, Zhiqing Li, Fei Teng, Xuejun Tao, and Xiaoyang Zhou: writing – review and editing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
[1] Li GY, Wang PM, Zhao XH. Pressure-sensitive properties and microstructure of carbon nanotube reinforced cement composites. Cem Concr Compos. 2007;29(5):377–82.10.1016/j.cemconcomp.2006.12.011Search in Google Scholar
[2] Danoglidis PA, Konsta-Gdoutos MS, Shah SP. Relationship between the carbon nanotube dispersion state, electrochemical impedance and capacitance and mechanical properties of percolative nanoreinforced OPC mortars. Carbon. 2019;145:218–28.10.1016/j.carbon.2018.12.088Search in Google Scholar
[3] Shi T, Li ZX, Guo J, Gong H, Gu CP. Research progress on CNTs/CNFs-modified cement-based composites–a review. Constr Build Mater. 2019;202:290–307.10.1016/j.conbuildmat.2019.01.024Search in Google Scholar
[4] Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354(11):56.10.1038/354056a0Search in Google Scholar
[5] Sharma N, Ray BC. Carbon Nanotube Composites: Critical Issues//Handbook of Carbon Nanotubes. Berlin, Cham: Springer International Publishing; 2021. p. 1–30.10.1007/978-3-319-70614-6_3-1Search in Google Scholar
[6] Qian D, Wagner GJ, Liu WK, Yu MF, Ruoff RS. Mechanics of carbon nanotubes. Appl Mech Rev. 2002;55(6):495.10.1115/1.1490129Search in Google Scholar
[7] Singla D, Amulya K, Murtaza Q. CNT reinforced aluminum matrix composite-A review. Mater Today: Proc. 2015;2(4–5):2886.10.1016/j.matpr.2015.07.248Search in Google Scholar
[8] Kwon H, Lee GG, Kim SG, Lee BW, Seo WC, Leparoux M. Mechanical properties of nanodiamond and multi-walled carbon nanotubes dual-reinforced aluminum matrix composite materials. Mater Sci Eng A. 2015;632:72.10.1016/j.msea.2015.02.057Search in Google Scholar
[9] Hassanzadeh-Aghdam MK, Mahmoodi MJ. A comprehensive analysis of mechanical characteristics of carbon nanotube-metal matrix nanocomposites. Mater Sci Eng: A. 2017;701:34–44.10.1016/j.msea.2017.06.066Search in Google Scholar
[10] Mallakpour S, Khadem E. Carbon nanotube–metal oxide nanocomposites: Fabrication, properties and applications. Chem Eng J. 2016;302:344–67.10.1016/j.cej.2016.05.038Search in Google Scholar
[11] Zhang WD, Xu B, Jiang LC. Functional hybrid materials based on carbon nanotubes and metal oxides. J Mater Chem. 2010;20(31):6383–91.10.1039/b926341aSearch in Google Scholar
[12] Boccaccini AR, Cho J, Subhani T, Kaya C, Kaya F. Electrophoretic deposition of carbon nanotube–ceramic nanocomposites. J Eur Ceram Soc. 2010;30(5):1115–29.10.1016/j.jeurceramsoc.2009.03.016Search in Google Scholar
[13] Asl MS, Farahbakhsh I, Nayebi B. Characteristics of multi-walled carbon nanotube toughened ZrB2–SiC ceramic composite prepared by hot pressing. Ceram Int. 2016;42(1):1950–8.10.1016/j.ceramint.2015.09.165Search in Google Scholar
[14] Arabani M, Faramarzi M. Characterization of CNTs-modified HMA’s mechanical properties. Constr Build Mater. 2015;83:207–15.10.1016/j.conbuildmat.2015.03.035Search in Google Scholar
[15] Li X, Li Y, Xie S, Zhou Y, Rong J, Dong L. Zinc-based energy storage with functionalized carbon nanotube/polyaniline nanocomposite cathodes. Chem Eng J. 2022;427:131799.10.1016/j.cej.2021.131799Search in Google Scholar
[16] Lott JZ. Study of the network formation of carbon nanotubes in epoxy matrices for electrical conductivity improvement. PhD Thesis. Hamburg: Technische Universität Hamburg; 2009.Search in Google Scholar
[17] Dou Y, Zhang JY, Han XY, He QM, Yang YK. Core–shell heterostructured composites of carbon nanotubes and imine-linked hyperbranched polymers as metal-free Li-ion anodes. Nanotechnol Rev. 2022;11(1):824–33.10.1515/ntrev-2022-0046Search in Google Scholar
[18] Sharma S, Patyal V, Sudhakara P, Singh J, Petru M, Ilyas RA. Mechanical, morphological, and fracture-deformation behavior of MWCNTs-reinforced (Al–Cu–Mg–T351) alloy cast nanocomposites fabricated by optimized mechanical milling and powder metallurgy techniques. Nanotechnol Rev. 2022;11(1):65–85.10.1515/ntrev-2022-0005Search in Google Scholar
[19] Zhao ZF, Zhou W, Xiao C, Hui D, Qi TQ, Qi JY, et al. A review on the properties, reinforcing effects, and commercialization of nanomaterials for cement-based materials. Nanotechnol Rev. 2020;9:303–22.10.1515/ntrev-2020-0023Search in Google Scholar
[20] D’Alessandro’ A, Materazzi AL, Ubertini F. Nanotechnology in cement-based construction. Boca Raton: CRC Press; 2020.10.1201/9780429328497Search in Google Scholar
[21] Silvestro L, Gleize PJP. Effect of carbon nanotubes on compressive, flexural and tensile strengths of Portland cement-based materials: A systematic literature review. Constr Build Mater. 2020;264:120237.10.1016/j.conbuildmat.2020.120237Search in Google Scholar
[22] Liu Y, Zhuge Y, Chow CWK, Keegan A, Ma J, Hall C, et al. Cementitious composites containing alum sludge ash: An investigation of microstructural features by an advanced nanoindentation technology. Constr Build Mater. 2021;299:124286.10.1016/j.conbuildmat.2021.124286Search in Google Scholar
[23] Gao F, Tian W, Wang Z, Wang F. Effect of diameter of multi-walled carbon nanotubes on mechanical properties and microstructure of the cement-based materials. Constr Build Mater. 2020;260:120452.10.1016/j.conbuildmat.2020.120452Search in Google Scholar
[24] Zhou C, Li F, Hu J, Ren M, Wei J, Yu Q. Enhanced mechanical properties of cement paste by hybrid graphene oxide/carbon nanotubes. Constr Build Mater. 2017;134:336–45.10.1016/j.conbuildmat.2016.12.147Search in Google Scholar
[25] Huang H, Teng L, Gao X, Khayat KH, Wang FZ, Liu ZC. Effect of carbon nanotube and graphite nanoplatelet on composition, structure, and nano-mechanical properties of CSH in UHPC. Cem Concr Res. 2022;154:106713.10.1016/j.cemconres.2022.106713Search in Google Scholar
[26] Li S, Zhang Y, Cheng C, Wei H, Du SG, Yan J. Surface-treated carbon nanotubes in cement composites: Dispersion, mechanical properties and microstructure. Constr Build Mater. 2021;310:125262.10.1016/j.conbuildmat.2021.125262Search in Google Scholar
[27] Liu L, Xu J, Yin T, Wang Y, Chu H. Improved conductivity and piezoresistive properties of Ni-CNTs cement-based composites under magnetic field. Cem Concr Compos. 2021;121:104089.10.1016/j.cemconcomp.2021.104089Search in Google Scholar
[28] Lee NK, Tafesse M, Lee HK, Alemu AS, Kim SW, Kim HK. Electrical resistivity stability of CNT/cement composites after further hydration: A simple evaluation with an accelerated method. Constr Build Mater. 2022;317:125830.10.1016/j.conbuildmat.2021.125830Search in Google Scholar
[29] Chousidis N, Zacharopoulou A, Zeris C, Batis G. Corrosion resistance and physical-mechanical properties of reinforced mortars with and without carbon nanotubes. J Mater Sci Chem Eng. 2022;10(1):1–23.10.4236/msce.2022.101001Search in Google Scholar
[30] Sarvandani MM, Mahdikhani M, Aghabarati H, Fatmehsari MH. Effect of functionalized multi-walled carbon nanotubes on mechanical properties and durability of cement mortars. J Build Eng. 2021;41:102407.10.1016/j.jobe.2021.102407Search in Google Scholar
[31] Liu Y, Shi T, Zhao YJ, Gu Y, Zhao ZF, Chen JB, et al. Autogenous shrinkage and crack resistance of carbon nanotubes reinforced cement-based materials. Int J Concr Struct Mater. 2020;14(1):1–10.10.1186/s40069-020-00421-0Search in Google Scholar
[32] Tao J, Wang J, Zeng Q. A comparative study on the influences of CNT and GNP on the piezoresistivity of cement composites. Mater Lett. 2020;259:126858.10.1016/j.matlet.2019.126858Search in Google Scholar
[33] Azhari F, Banthia N. Cement-based sensors with carbon fibers and carbon nanotubes for piezoresistive sensing. Cem Concr Compos. 2012;34(7):866–73.10.1016/j.cemconcomp.2012.04.007Search in Google Scholar
[34] Han B, Yu X, Kwon E. A self-sensing carbon nanotube/cement composite for traffic monitoring. Nanotechnology. 2009;20(44):445501.10.1088/0957-4484/20/44/445501Search in Google Scholar PubMed
[35] Jiang S, Zhou D, Zhang L, Ouyang J, Yu X, Cui X, et al. Comparison of compressive strength and electrical resistivity of cementitious composites with different nano- and micro-fillers. Arch Civ Mech Eng. 2018;18(1):60–8.10.1016/j.acme.2017.05.010Search in Google Scholar
[36] Rubel RI, Ali MH, Jafor MA, Alam MM. Carbon nanotubes agglomeration in reinforced composites: A review. AIMS Mater Sci. 2019;6(5):756–80.10.3934/matersci.2019.5.756Search in Google Scholar
[37] Moghadam HZ, Faghidian SA, Jamal-Omidi M. Agglomeration effects of carbon nanotube on residual stresses in polymer nano composite using experimental and analytical method. Mater Res Express. 2018;6(3):035009.10.1088/2053-1591/aaf370Search in Google Scholar
[38] Ma PC, Siddiqui NA, Marom G, Kim JK. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos Part A. 2010;41(10):1345–67.10.1016/j.compositesa.2010.07.003Search in Google Scholar
[39] Zare Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos Part A. 2016;84:158–64.10.1016/j.compositesa.2016.01.020Search in Google Scholar
[40] Shao H, Chen B, Li B, Tang S, Li Z. Influence of dispersants on the properties of CNTs reinforced cement-based materials. Const Build Mater. 2017;131:186–94.10.1016/j.conbuildmat.2016.11.053Search in Google Scholar
[41] Zeinedini A, Shokrieh MM, Ebrahimi A. The effect of agglomeration on the fracture toughness of CNTs-reinforced nanocomposites. Theor Appl Fract Mech. 2018;94:84–94.10.1016/j.tafmec.2018.01.009Search in Google Scholar
[42] Hassanzadeh-Aghdam MK, Mahmoodi MJ, Ansari R. Creep performance of CNT polymer nanocomposites- An emphasis on viscoelastic interphase and CNT agglomeration. Compos Part B. 2019;168:274–81.10.1016/j.compositesb.2018.12.093Search in Google Scholar
[43] Ashraf MA, Peng W, Zare Y, Rhee KY. Effects of size and aggregation/agglomeration of nanoparticles on the interfacial/interphase properties and tensile strength of polymer nanocomposites. Nanoscale Res Lett. 2018;13(1):1–7.10.1186/s11671-018-2624-0Search in Google Scholar PubMed PubMed Central
[44] Huh S, Batmunkh M, Kim Y, Chung H, Jeong H, Choi H. The ball milling with various rotation speeds assisted to dispersion of the multi-walled carbon nanotubes. Nanosci Nanotechnol Lett. 2012;4(1):20–9.10.1166/nnl.2012.1276Search in Google Scholar
[45] Krause B, Mende M, Pötschke P, Petzold G. Dispersability and particle size distribution of CNTs in an aqueous surfactant dispersion as a function of ultrasonic treatment time. Carbon. 2010;48(10):2746–54.10.1016/j.carbon.2010.04.002Search in Google Scholar
[46] Tserengombo B, Jeong H, Dolgor E, Delgado A, Kin S. Effects of functionalization in different conditions and ball milling on the dispersion and thermal and electrical conductivity of MWCNTs in aqueous solution. Nanomaterials. 2021;11(5):1323.10.3390/nano11051323Search in Google Scholar PubMed PubMed Central
[47] Cwirzen A, Habermehl-Cwirzen K, Nasibulin AG, Kaupinen EI, Mudimela PR, Penttala V. SEM/AFM studies of cementitious binder modified by MWCNT and nano-sized Fe needles. Mater Charact. 2009;60(7):735–40.10.1016/j.matchar.2008.11.001Search in Google Scholar
[48] Luo JL. Fabrication and functional properties of multi-walled carbon nanotube/cement composites. PhD Thesis. Harbin: Harbin Institute of Technology; 2009 (in Chinese).Search in Google Scholar
[49] Abu Al-Rub RK, Tyson BM, Yazdanbakhsh A, Grasley Z. Mechanical properties of nanocomposite cement incorporating surface-treated and untreated carbon nanotubes and carbon nanofibers. J Nanomechan Micromechan. 2012;2(1):1–6.10.1061/(ASCE)NM.2153-5477.0000041Search in Google Scholar
[50] Ludvig P, Calixto JM, Ladeira LO, Gaspar ICP. Using converter dust to produce low cost cementitious composites by in situ carbon nanotube and nanofiber synthesis. Materials. 2011;4(3):575–84.10.3390/ma4030575Search in Google Scholar PubMed PubMed Central
[51] Hilding J, Grulke EA, George Zhang Z, Lockwood F. Dispersion of carbon nanotubes in liquids. J Dispers Sci Technol. 2003;24(1):1–41.10.1081/DIS-120017941Search in Google Scholar
[52] Zhang M, Zhang W, Jiang N, Futaba DN, Xu M. A general strategy for optimizing composite properties by evaluating the interfacial surface area of dispersed carbon nanotubes by fractal dimension. Carbon. 2019;154:457–65.10.1016/j.carbon.2019.08.017Search in Google Scholar
[53] Krause B, Villmow T, Boldt R, Mende M, Gudrun P, Pötschke P. Influence of dry grinding in a ball mill on the length of multiwalled carbon nanotubes and their dispersion and percolation behaviour in melt mixed polycarbonate composites. Compos Sci Tech. 2011;71(8):1145–53.10.1016/j.compscitech.2011.04.004Search in Google Scholar
[54] Munkhbayar B, Nine MJ, Jeoun J, Bat-Erdene M, Chung H, Jeong H. Influence of dry and wet ball milling on dispersion characteristics of the multi-walled carbon nanotubes in aqueous solution with and without surfactant. Powder Technol. 2013;234:132–40.10.1016/j.powtec.2012.09.045Search in Google Scholar
[55] Zhao Z. Research on the dispersion of carbon nanotube. Master Thesis. Shanghai: Donghua University; 2014. (in Chinese)Search in Google Scholar
[56] Ma PC, Tang BZ, Kim JK. Conversion of semiconducting behavior of carbon nanotubes using ball milling. Chem Phys Lett. 2008;458(1–3):166–9.10.1016/j.cplett.2008.04.105Search in Google Scholar
[57] Pierard N, Fonseca A, Konya Z, Willems I, Tendeloo GV, Nagy JB. Production of short carbon nanotubes with open tips by ball milling. Chem Phys Lett. 2001;335(1):1–8.10.1016/S0009-2614(01)00004-5Search in Google Scholar
[58] Xu HX, Prof NGG, Prof KSS. Temperature inhomogeneity during multibubble sonoluminescence. Angew Chem Int Ed. 2010;122(6):1097–100.10.1002/ange.200905754Search in Google Scholar
[59] Liang J, An Y, Chen W. Tb(III) line intensities in multibubble sonoluminescence. Ultrason Sonochem. 2019;58:104688.10.1016/j.ultsonch.2019.104688Search in Google Scholar PubMed
[60] Wu P, Bai L, Lin W. On the definition of cavitation intensity. Ultrason Sonochem. 2020;67:105141.10.1016/j.ultsonch.2020.105141Search in Google Scholar PubMed
[61] Tyson BM, Abu Al-Rub RK, Yazdanbakhsh A, Grasley Z. Carbon nanotubes and carbon nanofibers for enhancing the mechanical properties of nanocomposite cementitious materials. J Mater Civ Eng. 2011;23(7):1028–35.10.1061/(ASCE)MT.1943-5533.0000266Search in Google Scholar
[62] Dong W, Li W, Shen L, Sun Z, Sheng D. Piezoresistivity of smart carbon nanotubes (CNTs) reinforced cementitious composite under integrated cyclic compression and impact. Compos Struct. 2020;241:112106.10.1016/j.compstruct.2020.112106Search in Google Scholar
[63] Koh B, Park JB, Hou X, Cheng W. Comparative dispersion studies of single-walled carbon nanotubes in aqueous solution. J Phys Chem B. 2011;115(11):2627–33.10.1021/jp110376hSearch in Google Scholar PubMed
[64] Vichchulada P, Cauble MA, Abdi EA, Obi EI, Zhang QH, Lay MD. Sonication power for length control of single-walled carbon nanotubes in aqueous suspensions used for 2-dimensional network formation. J Phys Chem C. 2010;114(29):12490–5.10.1021/jp104102tSearch in Google Scholar
[65] Rausch JL, Zhuang RC, Mäder E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Compos Part A. 2010;41(9):1038–46.10.1016/j.compositesa.2010.03.007Search in Google Scholar
[66] Gao Y, Jing HW, Chen SJ, Du MR, Chen WQ, Duan WH. Influence of ultrasonication on the dispersion and enhancing effects of graphene oxide–carbon nanotube hybrid nanoreinforcement in cementitious composite. Compos Part B: Eng. 2019;164:45–53.10.1016/j.compositesb.2018.11.066Search in Google Scholar
[67] Rennhofer H, Zanghellini B. Dispersion state and damage of carbon nanotubes and carbon nanofibers by ultrasonic dispersion: a review. Nanomaterials. 2021;11(6):1469.10.3390/nano11061469Search in Google Scholar PubMed PubMed Central
[68] Pagani G, Green MJ, Poulin P, Pasquali M. Competing mechanisms and scaling laws for carbon nanotube scission by ultrasonication. Proc Natl Acad Sci. 2012;109(29):11599–604.10.1073/pnas.1200013109Search in Google Scholar PubMed PubMed Central
[69] Lucas A, Zakri C, Maugey M, Pasquali M, Schoot P, Poulin P. Kinetics of nanotube and microfiber scission under sonication. J Phys Chem C. 2009;113(48):20599–20605.10.1021/jp906296ySearch in Google Scholar
[70] Sesis A, Hodnett M, Memoli G, Wain AJ, Jurewicz I, Dalton AB, et al. Influence of acoustic cavitation on the controlled ultrasonic dispersion of carbon nanotubes. J Phys Chem B. 2013;117(48):15141–50.10.1021/jp410041ySearch in Google Scholar PubMed
[71] Naqvi STR, Rasheed T, Hussain D, Haq MN, Majeed S, Shafi S, et al. Modification strategies for improving the solubility/dispersion of carbon nanotubes. J Mol Liq. 2020;297:111919.10.1016/j.molliq.2019.111919Search in Google Scholar
[72] Lavagna L, Nistico R, Musso S, Pavese M. Functionalization as a way to enhance dispersion of carbon nanotubes in matrices: A review. Mater Today Chem. 2021;20:100477.10.1016/j.mtchem.2021.100477Search in Google Scholar
[73] Wepasnick KA, Smith BA, Schrote KE, Wilson HK, Diegelmann SR, Fairbrother DH. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon. 2011;49(1):24–36.10.1016/j.carbon.2010.08.034Search in Google Scholar
[74] Elkashef M, Wang K, Abou-Zeid MN. Acid-treated carbon nanotubes and their effects on mortar strength. Front Struct Civ Eng. 2016;10(2):180–8.10.1007/s11709-015-0325-7Search in Google Scholar
[75] Osorio AG, Silveira ICL, Bueno VL, Bergmann CP. H2SO4/HNO3/HCl-Functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl Surf Sci. 2008;255(5):2485–9.10.1016/j.apsusc.2008.07.144Search in Google Scholar
[76] Aviles F, Cauich-Rodriguez JV, Moo-Tah L, May-Pat A, Vargas-Coronado R. Evaluation of mild acid oxidation treatments for MWCNT functionalization. Carbon. 2009;47(13):2970–5.10.1016/j.carbon.2009.06.044Search in Google Scholar
[77] Tchoul MN, Ford WT, Lolli G, Resasco DE, Arepalli S. Effect of mild nitric acid oxidation on dispersability, size, and structure of single-walled carbon nanotubes. Chem Mater. 2007;19(23):5765–72.10.1021/cm071758lSearch in Google Scholar
[78] Jung M, Hong S, Moon J. Ozone treatment on the dispersion of carbon nanotubes in ultra-high performance concrete. Mater Des. 2020;193:108813.10.1016/j.matdes.2020.108813Search in Google Scholar
[79] Liang L, Xie W, Fang S, He F, Yin B, Tilili C, et al. High-efficiency dispersion and sorting of single-walled carbon nanotubes non-covalent interactions. J Mater Chem C. 2017;5(44):11339–68.10.1039/C7TC04390BSearch in Google Scholar
[80] Islam MF, Rojas E, Bergey DM, Johnson AT, Yodh AG. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003;3(2):269–73.10.1021/nl025924uSearch in Google Scholar
[81] Sindu BS, Sasmal S. Molecular dynamics simulations for evaluation of surfactant compatibility and mechanical characteristics of carbon nanotubes incorporated cementitious composite. Constr Build Mater. 2020;253:119190.10.1016/j.conbuildmat.2020.119190Search in Google Scholar
[82] Chen ZY, Liu J, Pu CS, Li X, Bai Y. Surfactant-assisted multi-walled carbon nanotubes dispersion: mechanism and properties evaluation. Fine Chem. 2022;39(02):269–275 + 410 (in Chinese).Search in Google Scholar
[83] Yu JR, Grossiord N, Koning CE, Loos J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon. 2007;45(3):618–23.10.1016/j.carbon.2006.10.010Search in Google Scholar
[84] Sezer N, Koç M. Stabilization of the aqueous dispersion of carbon nanotubes using different approaches. Therm Sci Eng Prog. 2018;8:411–7.10.1016/j.tsep.2018.09.011Search in Google Scholar
[85] Rastogi R, Kaushal R, Tripathi SK, Sharma AL, Kaur I, Bharadwaj LM. Comparative study of carbon nanotube dispersion using surfactants. J Colloid Interface Sci. 2008;328(2):421–8.10.1016/j.jcis.2008.09.015Search in Google Scholar PubMed
[86] Konsta-Gdoutos MS, Metaxa ZS, Shah SP. Highly dispersed carbon nanotube reinforced cement based materials. Cem Concr Res. 2010;40(7):1052–9.10.1016/j.cemconres.2010.02.015Search in Google Scholar
[87] Luo JL, Duan ZD, Li H. Dispersivity of multi-walled carbon nanotube and its effects on the mechanical properties of MWNT/cement composite. Nanotechnol Precis Eng. 2009;7(06):532–6 (in Chinese).Search in Google Scholar
[88] Luo JL, Chung KL, Li QY, Chen SJ, Li L, Hou DS, et al. Piezoresistive properties of cement composites reinforced by functionalized carbon nanotubes using photo-assisted Fenton. Smart Mater Struct. 2017;26(3):035025.10.1088/1361-665X/aa559dSearch in Google Scholar
[89] Dai W, Wang J, Gan X, Wang H, Su X, Xi C. A systematic investigation of dispersion concentration and particle size distribution of multi-wall carbon nanotubes in aqueous solutions of various dispersants. Colloids Surf A. 2020;589:124369.10.1016/j.colsurfa.2019.124369Search in Google Scholar
[90] Cui LL. Study on dispersion technology of carbon nanotubes and research and development of its key equipment based on arc excitation. Qingdao: Qingdao University of Science and Technology; 2018. (in Chinese)Search in Google Scholar
[91] Ghavidel AK, Zadshakoyan M, Arjmand M, Kiani G. A novel electro-mechanical technique for efficient dispersion of carbon nanotubes in liquid media. Int J Mech Sci. 2021;207:106633.10.1016/j.ijmecsci.2021.106633Search in Google Scholar
[92] Oka Y, Ohnishi K, Asami K, Suyama M, Nishimura Y, Hashimoto T, et al. Dispersion of carbon nanotubes into water without dispersant using cavitation bubble plasma. Vacuum. 2017;136:209–13.10.1016/j.vacuum.2016.07.026Search in Google Scholar
[93] Zhao J, Liu ZS, Hu WB. Dispersion of carbon nanotubes in a polymer by the rapid expansion of a supercritical suspension. N Carbon Mater. 2014;29(05):386–91 (in Chinese).Search in Google Scholar
[94] Zou B, Chen SJ, Korayem AH, Collins F, Wang CM, Duan WH. Effect of ultrasonication energy on engineering properties of carbon nanotube reinforced cement pastes. Carbon. 2015;85:212–20.10.1016/j.carbon.2014.12.094Search in Google Scholar
[95] Norizan MN, Moklis MH, Demon SZN, Halim NA, Samsuri A. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020;10(71):43704–32.10.1039/D0RA09438BSearch in Google Scholar PubMed PubMed Central
[96] Batiston E, de Matos PR, Gleize PJP, Fediuk R, Klyuev S, Vatin N, et al. Combined functionalization of carbon nanotubes (CNT) fibers with H2SO4/HNO3 and Ca(OH)2 for addition in cementitious matrix. Fibers. 2021;9(3):14.10.3390/fib9030014Search in Google Scholar
[97] Metaxa ZS, Boutsioukou S, Amenta M, Favvas EP, Kourkoulis SK, Alexopoulos ND. Dispersion of multi-walled carbon nanotubes into white cement mortars: the effect of concentration and surfactants. Nanomaterials. 2022;12(6):1031.10.3390/nano12061031Search in Google Scholar PubMed PubMed Central
[98] Yazdanbakhsh A, Grasley Z, Tyson B, Al-Rub RK. Distribution of carbon nanofibers and nanotubes in cementitious composites. Trans Res Rec. 2010;2142(1):89–95.10.3141/2142-13Search in Google Scholar
[99] Mendoza O, Sierra G, Tobón JI. Influence of super plasticizer and Ca(OH)2 on the stability of functionalized multi-walled carbon nanotubes dispersions for cement composites applications. Constr Build Mater. 2013;47:771–8.10.1016/j.conbuildmat.2013.05.100Search in Google Scholar
[100] Silvestro L, Lima GTDS, Ruviaro AS, Gleize PJ. Stability of carboxyl-functionalized carbon nanotubes in simulated cement pore solution and its effect on the compressive strength and porosity of cement-based nanocomposites. C-J Carbon Res. 2022;8(3):39.10.3390/c8030039Search in Google Scholar
[101] Premkumar T, Mezzenga R, Geckeler KE. Carbon nanotubes in the liquid phase: addressing the issue of dispersion. Small. 2012;8(9):1299–313.10.1002/smll.201101786Search in Google Scholar PubMed
[102] Du MR, Jing HW, Duan WH, Han GS, Chen SJ. Methylcellulose stabilized multi-walled carbon nanotubes dispersion for sustainable cement composites. Constr Build Mater. 2017;146:76–85.10.1016/j.conbuildmat.2017.04.029Search in Google Scholar
[103] Geraldo V, Oliveira S, Silva EE, Oliveira CAS, Cunha RMA, Oliveira RFP, et al. Synthesis of carbon nanotubes on sand grains for mortar reinforcement. Constr Build Mater. 2020;252:119044.10.1016/j.conbuildmat.2020.119044Search in Google Scholar
[104] Ding S, Xiang Y, Ni YQ, Thakur VK, Wang XY, Han BG, et al. In situ synthesizing carbon nanotubes on cement to develop self-sensing cementitious composites for smart high-speed rail infrastructures. Nano Today. 2022;43:101438.10.1016/j.nantod.2022.101438Search in Google Scholar
[105] Nasibulin AG, Shandakov SD, Nasibulina LI, Cwirzen A, Mudimela PR, Grishin DA, et al. A novel cement-based hybrid material. N J Phys. 2009;11(2):023013.10.1088/1367-2630/11/2/023013Search in Google Scholar
[106] Zhan M, Pan G, Zhou F, Mi R, Shah SP. In situ-grown carbon nanotubes enhanced cementitious materials with multifunctionality. Cem Concr Compos. 2020;108:103518.10.1016/j.cemconcomp.2020.103518Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

![Figure 9
Schematic diagram of dispersing carbon nanotubes by different processes: (a) arc thermal excitation [90]; (b) electromagnetic field-induced [91]; and (c) CBP dispersion process [92].](/document/doi/10.1515/ntrev-2022-0560/asset/graphic/j_ntrev-2022-0560_fig_009.jpg)
