In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
Abstract
Biphasic calcium phosphate (BCP)/chitosan (Cs) composites (BCP/Cs) were assessed for reinforcement in vitro bone regeneration. BCP ceramics have been used to overcome the limitations of single-phase biomaterials. In this study, composite samples were prepared using solvent casting and the evaporation technique. The BCP powder at different concentrations (20, 30, 40, and 50%) was added to the Cs solution to obtain the composite samples. The morphologies and physicochemical properties of the prepared composites were investigated using physical methods. The biocompatibility of composites (BCP/Cs) was studied in vitro by immersion in simulated body fluid. Additionally, the cytotoxicity and viability of the composite samples were evaluated. The results showed that the addition of BCP improves the apatite-forming ability and enhances the bioactivity and biomineralization of the BCP/Cs composites. The mechanical stability of the composite sample was improved essentially by the strong interaction between BCP and the Cs matrix. In addition, the higher the amount of BCP added (50 wt%), the higher the amount of adsorbed protein, and the suitable bioactivity of composites was enhanced. Furthermore, BCP/Cs composites boosted the cell viability and cell proliferation of normal human osteocyte cells. Hence, BCP/Cs composites could be an excellent alternative to bone implants in tissue engineering applications.
Graphical abstract
1 Introduction
Over the past few years, research efforts have been in progress in the field of bone grafts and other artificial implants. However, the ideal method for repairing bone deformities in orthopedics is autologous osteology. Although the best method for repairing bone deformities in orthopedics is still autologous bone, it has disadvantages including limited availability and the transmission of infections to the patient [1]. The available strategy for avoiding the defects of this treatment is bone tissue engineering due to its promising applications in clinical therapies [2]. One of the great challenges facing the field of tissue engineering is the design of biomaterials that are compatible with the surrounding biological environment. These biomaterials are used as substrates to deliver the appropriate cells and stimulate them to grow, reproduce, and form new tissues. This means that to obtain an ideal and suitable scaffold, it should mimic the natural components of the bone tissue to be regenerated [3]. In addition, these biomimetic materials should possess several properties, such as porosity, proper pore distribution, the ability to absorb over time, biodegradation, and finally, mechanical properties.
The extracellular matrix of bone is composed of organic and inorganic substances consisting of hydroxyapatite nanocrystals in type I and type III collagen fibers [4]. The organic material used in this study was chitosan (Cs), and the inorganic material was biphasic calcium phosphate (BCP). Cs is a partially deacetylated polysaccharide polymer (poly-1,4-d-glucosamine) from natural chitin, and its structure resembles glycosaminoglycans; therefore, it is commonly used in bone tissue engineering [5]. Cs is a natural polymer with excellent biodegradability by human enzymes, and its degradation products are non-toxic [6]. It can be easily molded into different forms because it is a flexible polymer and therefore does not have the mechanical strength of natural bone [7]. As a result, Cs scaffolds alone cannot mimic all the requirements of bone tissues [8]. Despite all the above properties of Cs, its bioactivity is low and needs to be improved, like other polymers. Therefore, efforts have been made previously to develop polymeric Cs matrixes reinforced with nano-reinforced fillers, such as calcium phosphate compounds, to obtain scaffolds with improved bioactivity and suitable mechanical properties [9]. Therefore, one of the calcium phosphate compounds, like BCP, was selected and added to Cs to control its properties and obtain suitable mechanical and biological properties for implantation.
The reason for choosing BCP is that it combines two different crystalline phases, namely, hydroxyapatite phase (HA) and tricalcium phosphate phase (TCP) (HA/TCP) at an appropriate ratio to overcome the problems of single-phase HA such as poor biodegradation rate and low mechanical stability [10,11]. However, to manage this problem, BCP can provide a product with controlled bioactivity and biodegradation at a balanced rate with high mechanical stability [12]. Since BCP is an inorganic compound with bioactivity on the surface, its preparation with physical properties, such as surface area, particle size, and morphology, can improve the speed of bone healing. Thus, its preparation in the nanoscale range is preferable. BCP can also form intercalated structures with polymer matrices. Nevado et al. [13] reported that PLA/BCP composites produced self-induced intercalated layers of polylactic acid. Some previous works [14,15,16,17,18] have been devoted to studying the effect of adding different proportions of Cs polymer to calcium phosphate compounds. Gan et al. [14] studied the effect of different proportions of Cs on BCP to create scaffolds containing arginine-glycine-aspartic acid peptide and found that Cs increased cell attachment, spreading, and osteogenic differentiation. Cs-based fluorohydroxyapatite bioceramic with injectable hydrogel-containing cells, prepared by Cheng et al., demonstrated highly acceptable biological and mechanical properties [15]. Also, Abd-Khorsand et al. produced porous three-dimensional scaffolds composed of HA–poly(acrylic acid)–Cs–TiO2 using the freeze-drying technique that showed an improvement in cell growth and proliferation [16]. Further, Sendemir‐Urkmez and Jamison [17] investigated the effect of 25 wt% BCP powder added to Cs, prepared by the freeze-drying technique, on the differentiation of osteogenic cells and showed an improvement in osteoconductivity in polymeric scaffolds. However, no study has documented the effect of adding high proportions of calcium phosphate to Cs polymers to manufacture a scaffold with high biocompatibility. Therefore, this study aims to fabricate composite scaffolds based on BCP/Cs with high BCP additives prepared via solvent casting and evaporation technique to evaluate their effect on the mechanical strength, cell adhesion, and bioactivity of composite scaffolds.
In the present study, BCP particles were synthesized by the coprecipitation method, and BCP/Cs composite scaffolds were fabricated via solvent casting and evaporation technique. The physicochemical, mechanical, and biological properties of the prepared BCP/Cs composites were studied and compared with those of pure Cs. Moreover, their capability of biomineralization and protein adsorption was investigated by in vitro testing in distilled water, simulated body fluid (SBF), and phosphate-buffered saline (PBS), respectively. The cytotoxicity and cell viability of the prepared composites were tested in human osteocytes (normal cells) to evaluate their ability to activate bone growth and integration.
2 Materials and methods
2.1 Materials
Calcium nitrate tetrahydrate (Ca(NO3)2·4H2O), ammonium dihydrogen phosphate ((NH4)H2PO4), ammonium hydroxide (NH4OH), Cs (C56H103N9O39) with a molecular weight of 1526.5 g/mol and N-deacetylation degree of 85%, nitric acid (HNO3), and acetic acid (96%) were bought from Sigma-Aldrich, St. Louis.
2.2 Preparation of BCP
BCP with a Ca/P ratio (1.60) was prepared using a wet chemical co-precipitation technique, as reported by El-Gohary et al. [19], with few modifications. In detail, 37.79 g of Ca(NO3)2·4H2O as a source of Ca was dissolved in 1,000 mL of deionized water, and 13.98 g of (NH4)H2PO4 as a source of P was dissolved in 1,000 mL of deionized water. The pH of each aqueous solution was adjusted to 6 by the addition of dilute NH4OH or HNO3 solutions when required. (NH4)H2PO4 solution was slowly added from a burette in a dropwise manner (2–5 mL/min) into a stirred Ca(NO3)2·4H2O solution maintained at (60°C) on a hot plate during mixing. When dropping was completed, the temperature of the mixture was raised to the boiling point at 100°C for 2 h with continuous stirring in a sealed container. Thereafter, the obtained slurry was left overnight without stirring until it cooled and formed a white precipitate. After 24 h, the white precipitate was filtered from the suspension, rinsed three times with deionized water to a neutral pH, and dried in an oven at 100°C in the air overnight. The dried powder was sintered at 1,100°C in an air atmosphere for 2 h to achieve BCP [20]. The sintered powder was deagglomerated using stainless steel sieves to obtain particles with a size of 40 µm.
2.3 Fabrication of BCP/Cs composite scaffolds
The prepared composites were synthesized using solvent casting and evaporation techniques. Initially, the Cs solution was prepared by dissolving a fixed weight of Cs powder (1 g) in 100 mL of acetic acid solution (1% w/w) while stirring for 4 h to obtain a perfectly transparent solution. Four different concentrations of BCP powder, 20, 30, 40, and 50%, were added to the Cs solution and stirred for 2 h to ensure a better distribution (homogeneity) of BCP particles in the composite scaffolds. After that, the solution was cast in a well plate and aged in an oven at 50oC for drying. The samples were then subjected to alkali treatments by soaking them in 1 mol/L NaOH aqueous solutions to raise hydrophilicity and crystallinity [21]. Finally, the samples were rinsed with deionized water and air-dried. The obtained Cs was denoted as a control sample, and BCP/Cs composites (20, 30, 40, and 50%) were denoted as BCP20/Cs, BCP30/Cs, BCP40/Cs, and BCP50/Cs composites, respectively.
2.4 Characterization of composites
2.4.1 XRD analysis
The prepared BCP powder, Cs, and BCP/Cs composites were analyzed by XRD using a Bruker Axs D8 ADVANCE diffractometer, operating at 40 mA and 40 kV with CuKα radiation (λ = 1.54056 Ǻ) in the 2θ (10–70°) with a 0.02° step size and 0.05 s.
2.4.2 Fourier transform infrared (FTIR) analysis
FTIR spectroscopy was performed using an FTIR spectrophotometer (Demonstrate 1600, Perkin-Elmer Co. USA) to identify the chemical functional groups of the prepared composites. The prepared samples were completely dried, then well pulverized and mixed with potassium bromide (KBr) with a 1:100 weight ratio (specimen/KBr), which was compressed uniaxially in an evacuated die to form a pellet. The FTIR spectra were collected in the range between 4,000 and 400 cm−1 with a resolution of 2 cm−1.
2.4.3 Scanning electron microscopy (SEM)
The surface morphology of the prepared samples before and after soaking in SBF was measured using an SEM (model XL30, Philips) at an acceleration voltage of 30 kV. SEM micrographs were obtained after covering the specimens with a gold layer to obtain an excellent view using Edwards 5150 sputter coating, England. The SEM was equipped with an energy-dispersive X-ray spectroscope (EDS) to identify the chemical elements in the prepared samples.
2.4.4 Analysis of mechanical properties
The mechanical properties of the developed composites were assessed by calculating Young’s modulus and stiffness using a TA.XT plus Stable Texture Analyzer (Stable Micro Systems, Surrey, UK). The samples were prepared for mechanical testing by evenly cutting them to form sheets with a gauge length (30 mm) and width (10 mm). The composite samples were located between two compression plates using an EMIC DL3000 testing machine and pressed at a constant speed of 2 mm/min. A load cell of 500 N was used at ambient RT. The statistical analysis of the calculated data was performed with an ANOVA program using three replicates per sample type.
2.5 In vitro bioactivity in SBF
In vitro bioactivity of the composite samples was carried out by evaluating the apatite forming-ability on their surface after soaking in SBF solution. The prepared samples were soaked in SBF at 37°C according to the method suggested by Kokubo and Takadama [22]. Each sheet of the composite sample of equal weight and shape with a specific surface-to-volume ratio (=0.1 cm−1) was soaked in 60 mL of SBF solution and stored in an incubator (shaking water bath) at 37°C and pH = 7.4 for 15 days. After 15 days of soaking, the samples were retrieved from the SBF, placed on filter paper, slightly rinsed with distilled water and acetone, and dried in air at room temperature.
2.6 Protein adsorption analysis
The composite samples were incubated in PBS containing 2 mg/mL bovine serum albumin (BSA) and aged in a static thermos-incubator for different times determined before (30 min, 1, 3, 6, 9, and 12 h). After the predetermined periods, composite samples were completely rinsed with PBS and rinsed three times with deionized water to extract the non-adsorbed protein. After that, the rinsed samples were aged for 1 h at RT. The gradual reduction in BSA concentrations was calculated using a UV/V spectrophotometer (Jenway 4600, England) at 595 nm. The amount of protein adsorbed was measured with a calibration curve by comparing the absorbance values of the aliquot solution based on the following equation [23]:
where Q is the adsorbed amount of protein (mg/g), C i is the initial protein concentration (mg/mL), C f is the remaining protein concentration in the PBS solution after removing the sample from it (mg/mL), V is the total volume of the PBS solution (mL), and M is the weight of the composite sample added to the solution.
2.7 In vitro cell culture analysis
Newly manufactured scaffolds that had been exposed to cellular cytotoxicity were assessed on human osteocytes (normal cells). The positive control in this experiment was doxorubicin. The composite sample was dissolved in 20% dimethyl sulfoxide (DMSO) at a concentration of 1 mg/mL.
The cytotoxic activity of the tested substances was carried out using the SulfoRhodamine-B (SRB) trial toward normal cells (human osteocytes). This cell line was purchased from the VACSERA-Cell Culture Unit, Cairo, Egypt. DMSO, SRB, and RPMI-1640 medium reagents were obtained from Sigma Company (St. Louis, USA). Fetal bovine serum was purchased from GIBCO, UK. A mixture consisting of RPMI-1640 medium with 10% fetal bovine serum and antibiotics (streptomycin 100 mg/mL and penicillin 100 units/mL) was added. The cells were grown in a humidified incubator in a CO2 atmosphere (5% v/v) at 37°C. In a 96-well plate, the cells were cultured at a density of 1.0 × 104 cells/well for 48 h at 37°C and 5% CO2. After completion of the incubation period, the cultured cells were incubated with the prepared samples for 3 and 7 days. Finally, the treated cells were compared with untreated control cells.
Triple wells were prepared for each dose. The media were removed, and the cells were fixed with 10% trichloroacetic acid (TCA) at 150 mL per well at 4°C for 1 h (SRB protein binding was reduced by TCA). The cells were washed three times with distilled water. At RT, the cultured cells were stained with 70 mL/well of 0.4% SRB for 10 min (in a dark place). The cells were washed with 1% glacial acetic acid to remove excess dye. The plates were air-dried for 24 h. With 50 mL/well of 10 mM tris base (pH 7.4), the dyes were dissolved for 5 min on a shaker at 1,600 rpm. At 570 nm, using an ELISA micro-sheet reader (EXL 800 USA), the optical density was measured for each well. Using the equation (100 × A570 of the sample treated with the tested materials/A570 from the sample untreated with the tested materials), cell viability was calculated as a percentage, and IC50 (the least concentration desired to induce 50% of cell death after exposure to samples) values were measured using the origin program [24].
2.8 Statistical analysis
All collected data were presented in triplicate and given as mean ± standard deviation. The statistical analysis was performed by one-way ANOVA using Excel (Microsoft Office, 2016). The p < 0.05 value was considered a significant difference between groups.
3 Results and discussion
3.1 XRD analysis before soaking in SBF
The XRD patterns of pure Cs and pure BCP, compared to different BCP/Cs composites, are shown in Figure 1. As shown in the figure, there is a broad halo around 2θ = 20o, indicating that Cs has an amorphous structure, although there is a diffuse peak around 2θ = 20°. According to a recent study by Karava et al. [25], this diffuse peak indicates some degree of ordered alignment of the polymer chains. Also, Figure 1 shows XRD patterns of pure BCP after sintering at 1,100°C. This pattern shows diffraction peaks assigned to two different crystalline phases: HA phase and β-TCP phase.
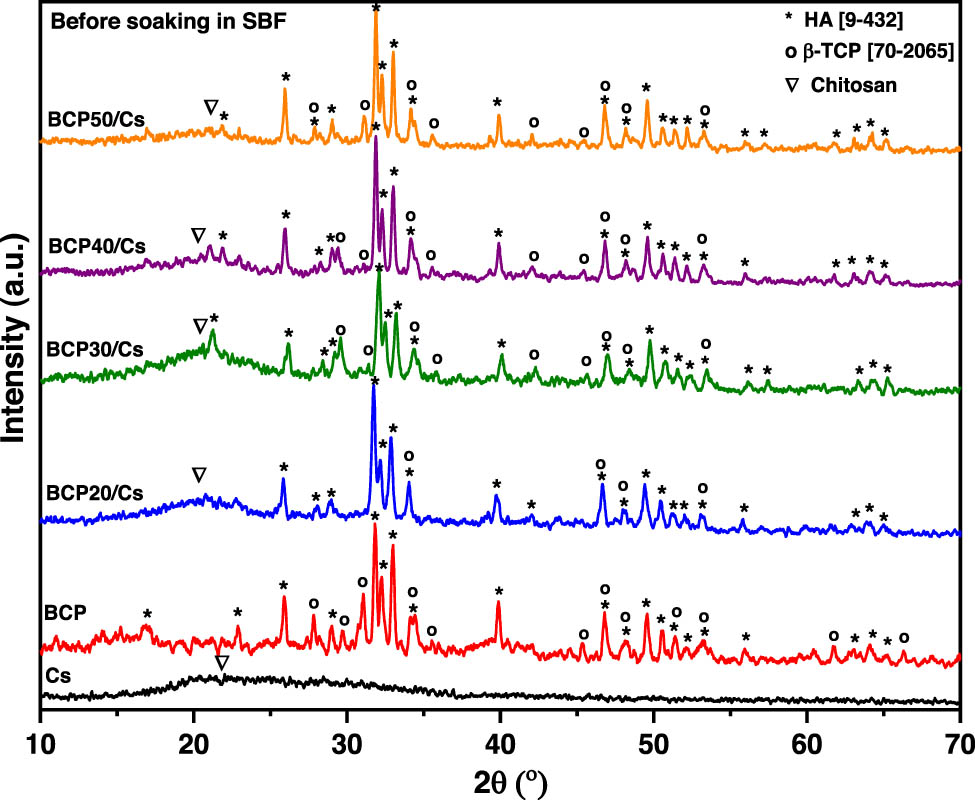
XRD patterns of Cs, BCP, and BCP/Cs composites with different BCP 20, 30, 40, and 50% contents; (∇) for Cs (*) for HA and (o) for β-TCP.
Four peaks appeared at 2θ values of 25.8°, 31.7°, 32.1°, and 32.9°, which indicated the (0 0 2), (2 1 1), (1 1 2), and (3 0 0) planes, respectively. These peaks can be assigned to the HA phase according to the standard JCPDS file no. 9-432. On the other hand, there are some lower diffractions at 2θ values of 27.7°, 31°, and 34.3°, which are indicated by (2 1 4), (2 1 0), and (2 2 0) planes, respectively. These peaks can be assigned to β-TCP according to the standard JCPDS card no. 70-2065. The presence of these two phases, HA and β-TCP, in the same sample, indicates the formation of BCP [20].
The phase compositions of BCP/Cs composites are shown using the XRD pattern in Figure 1. The broad diffraction peak of Cs was at around 2θ = 20° side-by-side, with sharp diffraction peaks of the BCP crystalline phase confirming the presence of Cs in the composite matrix [26]. XRD patterns illustrated that with the addition of BCP, the diffraction peak at 20° became broader and flatter, but the diffraction peaks ascribed to HA and β-TCP appeared to be more distinguishable in BCP/Cs composites [27]. Moreover, the diffraction peaks of HA at 2θ = 32.2° show a higher degree of splitting with increased addition of BCP, especially when the BCP content reaches 50%. The crystallite size (D) of both pure BCP and the BCP/Cs composite was calculated from a characteristic peak at 25.9° (0 0 2) corresponding to the HA phase using Scherrer’s equation [28], that is, D = 0.9λ/(β 002 cos θ), where D is the crystallite size (nm), λ is the wavelength of the X-ray (0.154178 nm), β 002 is the full-width at half-maximum intensity (rad) of the (0 0 2) peak, and θ is Bragg’s diffraction angle. The results of the crystallite size of all samples are listed in Table 1. The crystalline sizes of all prepared samples are in the range of 30–43 nm. This indicates that the HA in the pure BCP and BCP/Cs composites are nano-sized crystals. Moreover, it can be observed that the crystallite size of HA in pure BCP is larger than in BCP/Cs composites, and the higher the BCP content, the larger the crystallite size [29].
Crystallite size (D) calculated using the Scherrer equation and the CIXRD and CIFTIR values obtained from the XRD patterns and FTIR spectra
| Samples | D (nm) | Crystallinity indices | |||
|---|---|---|---|---|---|
| Before soaking | After soaking | ||||
| ClXRD | ClFTIR | ClXRD | ClFTIR | ||
| BCP pure | 43.27 | 0.84 | 3.01 | ||
| BCP20/Cs | 30.47 | 0.65 | 2.23 | 0.61 | 2.05 |
| BCP30/Cs | 32.96 | 0.71 | 2.4 | 0.68 | 2.08 |
| BCP40/Cs | 35.22 | 0.79 | 2.55 | 0.71 | 2.13 |
| BCP50/Cs | 38.74 | 0.81 | 2.62 | 0.65 | 2.07 |
The amount of crystalline HA phase in the investigated volume of BCP or BCP/Cs composite samples was measured by the crystallinity index (CIXRD). The CIXRD of the prepared samples was calculated from XRD patterns by the following empirical equation: CIXRD = 1 − [V 112/300 /I 300 ] proposed by Landi et al. [30] by drawing a baseline from 2θ = 30 to 35° and then measuring the intensity of the hollow (V 112/300 ) between (3 0 0) and (1 1 2) reflection peaks and dividing the peak intensity (I 300 ) of (3 0 0) at 2θ = 32.2°. As shown in Figure 1 and listed in Table 1, the CIXRD of all BCP/Cs composites is lower than pure BCP, confirming the occurrence of a potential interaction between the Cs chain and BCP particles. In addition, the main diffraction peak of β-TCP at 31.04° disappears in the BCP/Cs composite. This can be attributed to the use of acetic acid during the preparation of the BCP/Cs composite. Acetic acid can partially dissolve β-TCP and HA crystals, causing decreases in their CIXRD and thus reducing the crystallite size of HA in the BCP phase [31]. Consequently, the dissolution process becomes relatively stronger because of the low content of the BCP, corresponding to the reduction in their crystallite size [32]. The obvious decrease in the crystallinity of BCP/Cs composites is specifically advantageous, where, based on previous results, calcium phosphate ceramics with lower crystallinity features are better in bone reconstruction. They are similar to natural bone with low crystalline features and have higher solubility rates than calcium phosphate ceramics with higher crystallinity [33].
3.2 FTIR analysis before soaking in SBF
Figure 2 shows the FTIR absorption spectra of Cs, BCP, and BCP/Cs composites. As shown in Figure 2, the spectral range has been divided into two regions: 400–1,750 and 2,750–4,000 cm−1. These two regions have the main vibrational bands for Cs and BCP. The absorption bands are observed in the spectra of pure Cs, including those at 895 and 1,155 cm−1 (C–O–C stretching vibration in the saccharide structure) [34], ∼970–1,100 cm−1 (C–O stretching mode], ∼1,320–1,370 cm−1 [amino (‒NH2)] [35], ∼1,383–1,420 cm−1 ((CH2) symmetrical deformation), 1,450–1,470 cm−1 (C–O stretching mode) [36], ∼1,500–1,560 cm−1 ((amine (‒NH) deformation mode in amide II), ∼1,600–1,700 cm−1 (carbonyl (C═O) amide I stretching mode) [37], ∼2,890–2,940 cm−1 (methylene (–CH stretching mode)), and ∼ 3,430–3,500 cm−1 (O–H surface water and N–H stretching) [38]. All of these absorption bands represent the distinctive organic functional groups of Cs.
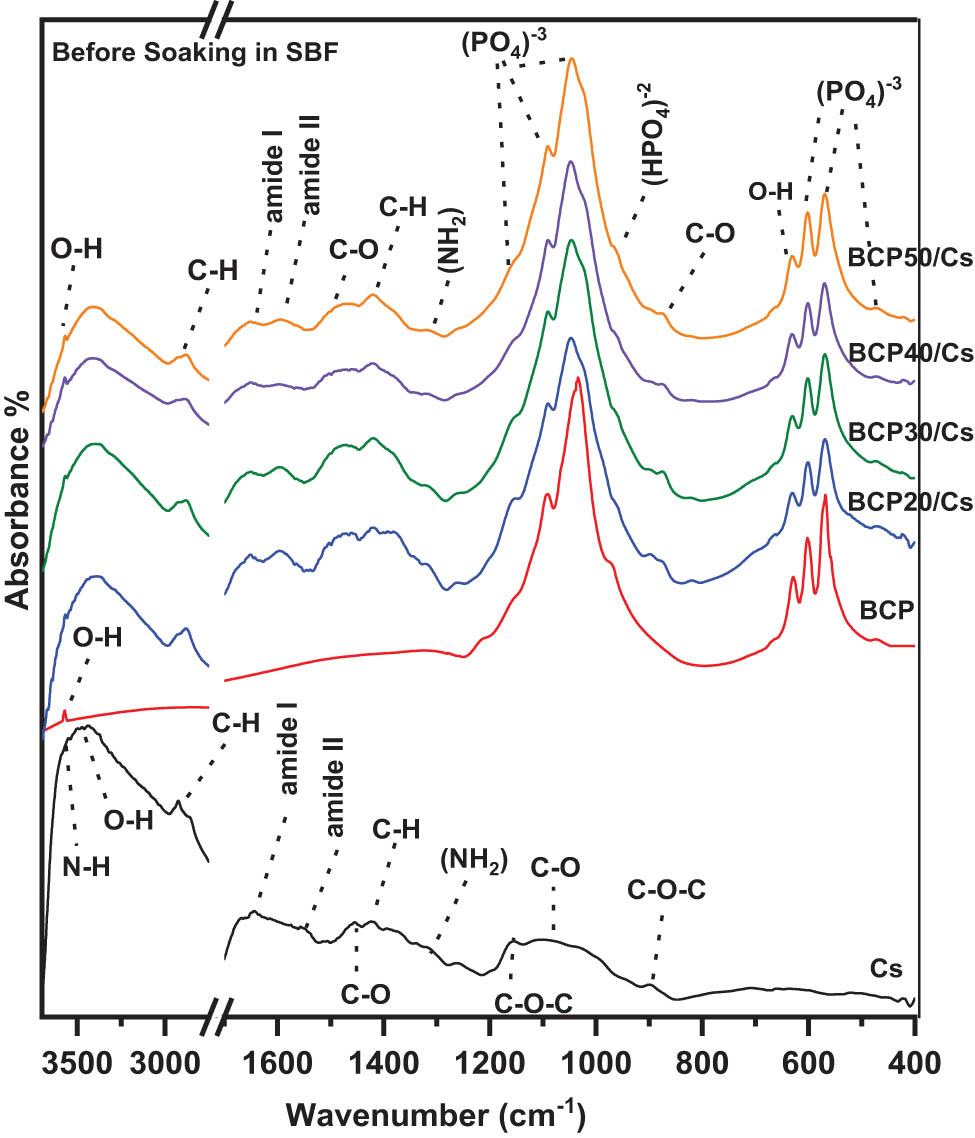
FTIR spectra of Cs, BCP, and BCP/Cs composites with different BCP contents (20, 30, 40, and 50%) before soaking in SBF.
Also, Figure 2 shows the FTIR absorption spectrum of the pure BCP sample after sintering at 1,100°C. There are four vibrational bands corresponding to different modes of phosphate groups [
The two distinct functional groups that define the chemical composition of HA are the phosphate groups [
As shown in Figure 2, the main characteristic bands of both Cs and BCP are present in all BCP/Cs composites, excluding slight band shifts and reduced intensity of some specific bands. For example, the intensity of the (‒NH2) band at 1,320 cm−1 decreases with the higher contents of BCP. In addition, the typical vibration band of the PO4 3⁻ group at 471–650 cm−1 became stronger and sharper while increasing BCP contents. According to FTIR data, the crystallization state of BCP and BCP/Cs composites can also be evaluated to determine whether or not it is affected by adding the BCP contents. This was done by measuring the crystallization index CIFTIR of the phosphate band at 600 cm−1 and its gradual splitting with the addition of BCP. The crystallization index (splitting factor] can be obtained by the following formula, CIFTIR = A + B/C, proposed by Weiner and Bar-Yosef [41]. A baseline is taken from 490 to 650 cm−1. The height of the double phosphate band was measured at 602 cm−1 (A), 569 cm−1 (B), and the valley at 587 cm−1 (C) between 602 and 569 cm−1. As shown in Figure 2 and listed in Table 1, the CIFTIR of all BCP/Cs composite samples is less than that of pure BCP.
These results suggest that there are some intermolecular interactions between BCP particles and Cs chains in all composite samples [42]. Therefore, the intermolecular interactions in Cs, such as H-bonding interactions within the network, become weaker with more BCP contents. Thus, it can be deduced that the incorporation of BCP powder interrupts the network formation. Moreover, the molecular chain of Cs acts not only as a matrix for the BCP powder but also anchors BCP powder in the structure and connects them to form composites [27].
3.3 Mechanical properties
To obtain composite materials that are used as scaffolds in bone tissue engineering, the criteria for initial mechanical properties must be taken into consideration at the beginning. Referring to biomimetic approaches, the prepared composite scaffolds composed of degradable polymers (e.g., Cs) and BCP as an inorganic material aim to combine the flexibility of the polymer chain with the stiffness and strength of the filler material (ceramic) to improve the mechanical stability compared to a single component. The mechanical properties of the composite material can be described by measuring both Young’s modulus and stiffness, especially for materials that are always subjected to compression and tension in only one direction [43]. This method is used to evaluate the ability of a composite to withstand changes when it is under compression or tension. Figure 3 shows the influence of BCP contents of 0, 20, 30, and 50 wt% on the mechanical properties of the composites. It can be seen that Young’s modulus values for composites with 0, 20, 30, and 50 wt% BCP contents are approximately 0.8, 0.9, 1.1, and 1.7 GPa, respectively, with 50 wt% of BCP content having significantly more strength than pure Cs (p < 0.05) (Figure 3(a)).
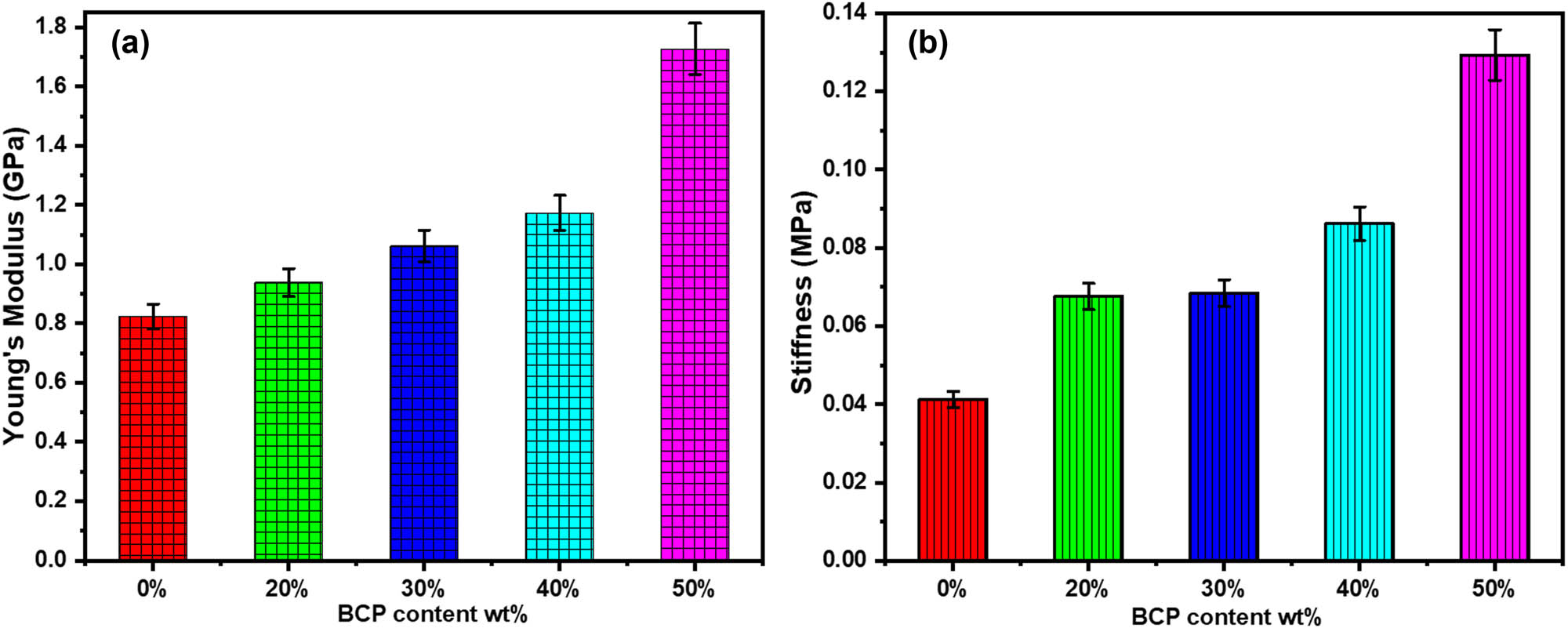
Young’s modulus (a) and (b) stiffness of Cs and BCP/Cs composites containing different BCP contents.
A similar trend was also observed in all composite samples, with the stiffness values largely improving as the quantity of BCP increased (0.041, 0.067, 0.069, 0.086, and 0.13 MPa), respectively (p < 0.05) (Figure 3(b)). These are associated with the addition of BCP powder to the Cs matrix, which appears from chemical interactions between the NH2 groups in Cs and the Ca2+ in the BCP or between the NH2 groups and
3.4 XRD analysis after soaking in SBF
Figure 4 shows the XRD patterns of Cs and BCP/Cs composites after soaking in SBF for 15 days. For pure Cs, the main diffraction peak of Cs appears at 2θ = 20° after soaking in SBF, and no diffraction peaks of a new apatite phase or CaP precursors are observed. As is known, the growth of the apatite layer on the surface requires nucleation sites. These sites reduce the surface energy of minerals, which helps them grow, but they are not available on pure Cs surfaces [43,46]. These results indicate that Cs without the addition of BCP cannot form apatite, proving that they are not biologically active [47,48].
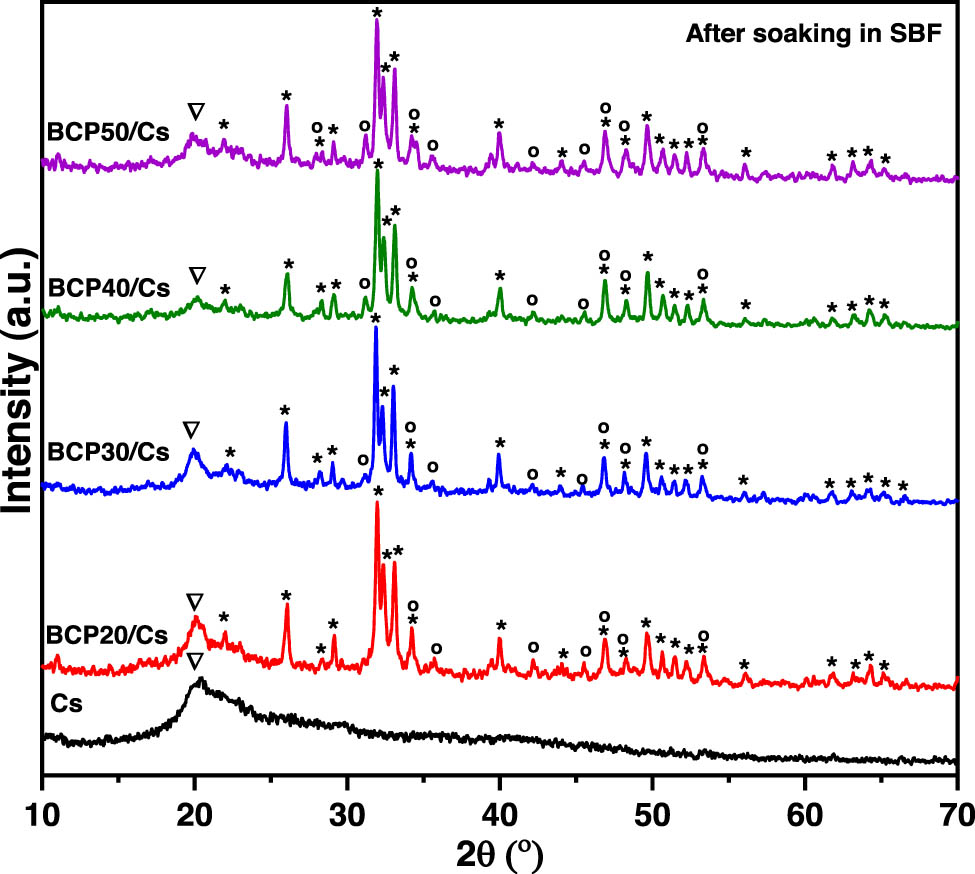
XRD patterns of Cs and BCP/Cs composites with different BCP contents (20, 30, 40, and 50%) after soaking in SBF.
For composites, all BCP/Cs composites have the characteristic peaks of Cs (2θ = 20°) and the characteristic peaks (at 2θ = 25–35° for the HA phase: 2θ = 28.3°, 31°, 34.2°, and 46.8° for the β-TCP phase) of the BCP phase. Furthermore, no formation of other phases is found after soaking in SBF, as shown in Figure 4. In BCP30/Cs and BCP40/Cs, the main diffraction peak of β-TCP in the BCP phase at 2θ = 31° is observed again with low intensity after soaking. This is mostly due to the recrystallization of the β-TCP phase, whose ions were dissolved in the acidic Cs solution, and then when placed in SBF with a pH > 5 a reverse reaction occurred, and it was recrystallized again [31].
The results of the CIXRD of all composites after soaking in SBF are summarized in Table 1. The CIXRD values of all samples decreased after soaking in the SBF solution compared to those before soaking. This decrease in CIXRD is due to the initial dissolution of BCP and then re-precipitation to provide more apatite nucleation sites and form a calcium-deficient apatite layer on the composite surface. Hence, it indicates that the low crystallinity of the newly formed calcium-deficient apatite is responsible for the peak broadening of their diffraction patterns of the BCP/Cs composite [35]. The CIXRD of these samples increases with an increasing amount of BCP, except for BCP50/Cs. The higher the BCP content present in the composite samples, the greater the number of nucleation sites, and the lower the surface energy of minerals, which leads to stimulating the growth of the apatite layer onto the surface. As a result, apatite formation on the composite surface has a high degree of crystallinity [49].
3.5 FTIR after soaking in SBF
FTIR spectra of pure Cs and BCP/Cs composites after soaking in SBF are presented in Figure 5. As shown in the pure Cs spectrum, the intensity of the absorption bands assigned to Cs decreased: C–O, ‒NH2, and C═O. These changes indicate the hydrolytic degradation of the Cs matrix [50]. Moreover, no new absorption bands appeared on the surface of pure Cs after soaking in SBF. Consequently, the distinct function groups for apatite, such as
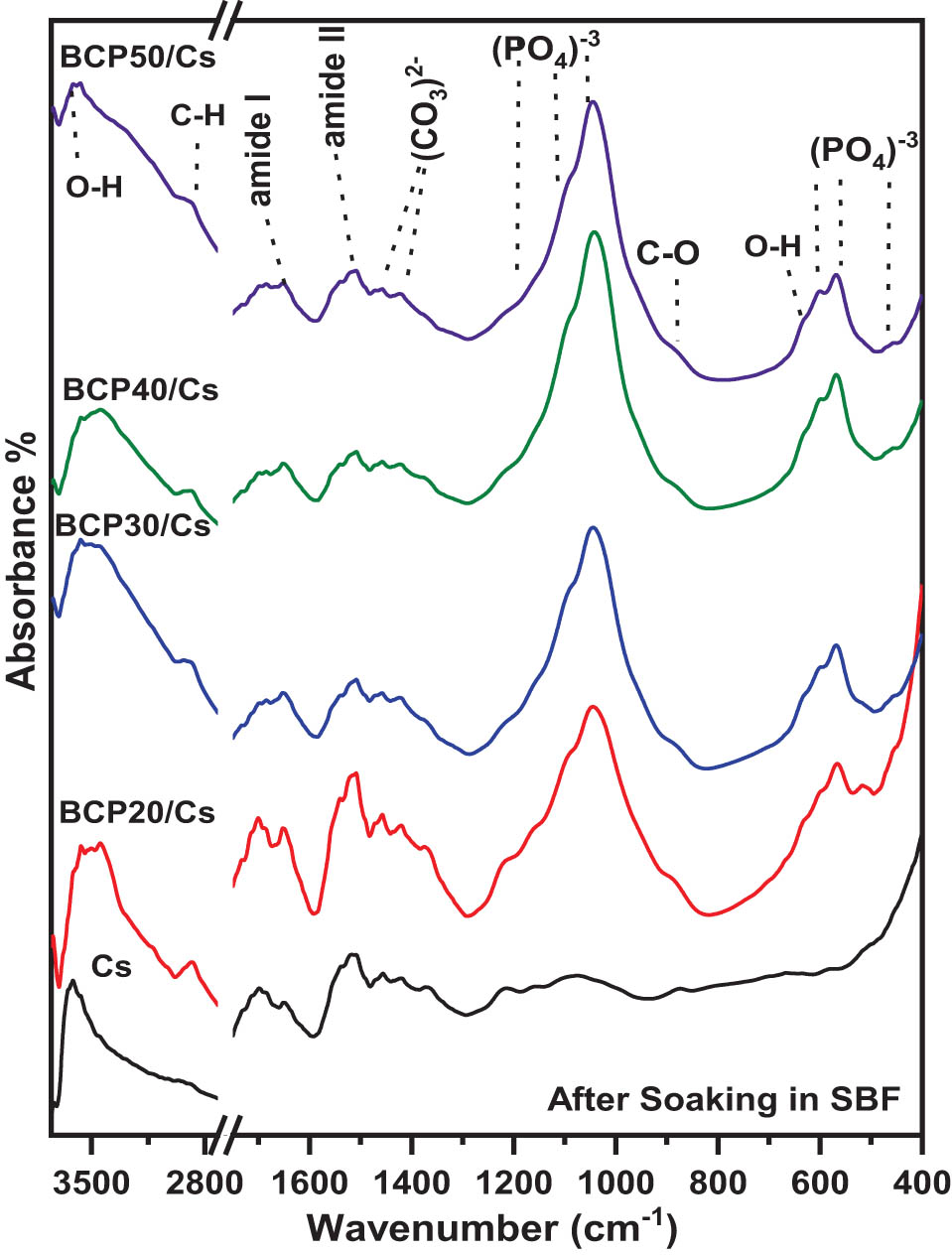
FTIR spectra of Cs and BCP/Cs composites with different BCP contents (20, 30, 40, and 50%) after soaking in SBF.
As shown in Figure 5, for BCP/Cs composites, the characteristic bands of both Cs and BCP particles appear after soaking in SBF with noticeable changes. The intensity of the C–H (stretching mode) band at 2,890 cm−1 decreased and became broader, while the C–H and N–H bands at 1,420 and 1,320 cm−1, respectively, disappeared after soaking in SBF. This result suggests a partial degradation of Cs in composite samples, even after soaking in SBF [53]. Also, the carbonate (C–O) band at 1,450 cm−1 resolved into two bands at 1,416 and 1,462 cm−1 in all composites, indicating the incorporation of carbonate ions in apatite crystals. These results signify the formation of a large amount of hydroxycarbonate apatite layer (HCA) deposited on the BCP/Cs composite surfaces [54].
In addition, the OH− bands at 630 and 3,570 cm−1 and
Table 1 also shows that when the amount of BCP increased, the CIFTIR values of the composites increased, except for a decrease in BCP50/Cs. As can be seen, the greater BCP content in composites leads to a slight increase in the splitting of the two P–O bands at 567 and 603 cm−1, and hence, an increase in the CIFTIR. This result indicates that the addition of BCP induces the formation of nucleation sites; thus, the negative charge increases on the composite’s surface. Then, the apatite nuclei begin to grow by attracting the positively charged Ca2+ ions and then the negatively charged
3.6 Protein adsorption studies
Protein adsorption is one of the important parameters, as it helps the cell adhere to the implant surface. Figure 6 shows the amount of protein adsorption on the Cs and BCP/Cs composites up to 12 h of incubation in a BPS solution. It is seen that the amount of adsorbed protein increased with the increasing content of BCP in the Cs matrix. Therefore, the incorporation of BCP into the composites enhances the properties of the Cs network for protein adsorption and creates a local microenvironment on the composite surface rich in protein [58].
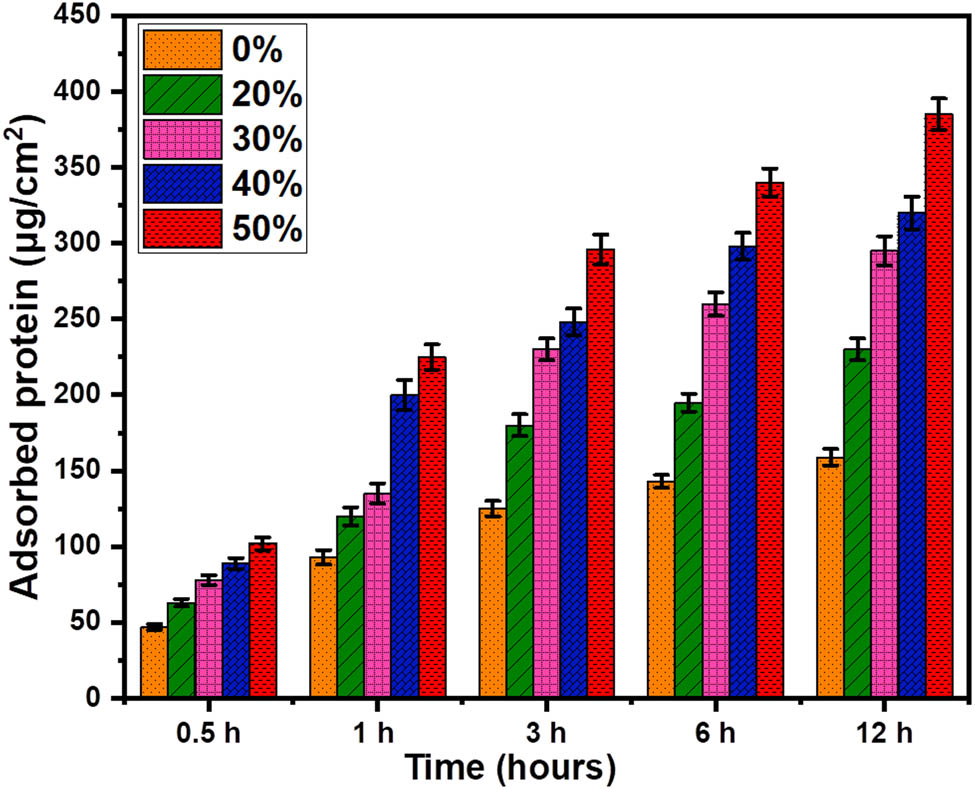
Protein adsorption profiles of Cs and BCP/Cs composites in PBS at 37°C for different incubation times.
In general, protein adsorption is a complex process affected by several factors, such as surface roughness, electrostatic force, functional groups, and hydrophobicity [59]. Furthermore, BCP possesses two reactive sites, positive C-sites [Ca2+] and negative P-sites [
3.7 SEM and EDS before and after soaking in SBF
Figure 7 shows the SEM micrographs of the Cs and the resultant BCP/Cs composite. The surface of pure Cs appeared to have a homogeneous microstructure with a smooth surface when compared with the BCP-loaded composite. The smooth surface of pure Cs (Figure 7(a)) changed and gradually disturbed the incorporation of BCP nanoparticles and became a rough surface (Figure 7(b)). A regular distribution of BCP nanoparticles on the surface of the composite was observed, which in turn increased the area of its outer surface. Furthermore, BCP nanoparticles appeared to be efficiently embedded in the polymer matrix due to the higher ionic cross-link density between BCP and the Cs network [61]. Figure 7(c) and (d) shows the EDS patterns of the Cs and BCP/Cs composites. The EDS pattern (Figure 7(c)) reveals that the chemical elements in this sample are [C, N, O] elements representing the basic chemical elements of Cs, but sodium [Na] may be caused by the remains of the NaOH solution used to neutralize the Cs sample. Furthermore, the EDS pattern (Figure 7(d)) illustrates that the BCP/Cs composite contains Ca and P elements as well as a Ca/P 1.55 ratio, originating from BCP along with [C, N, O] elements, where [O] originated from both components. These results confirm that the BCP powder is successfully incorporated into a polymer Cs matrix.
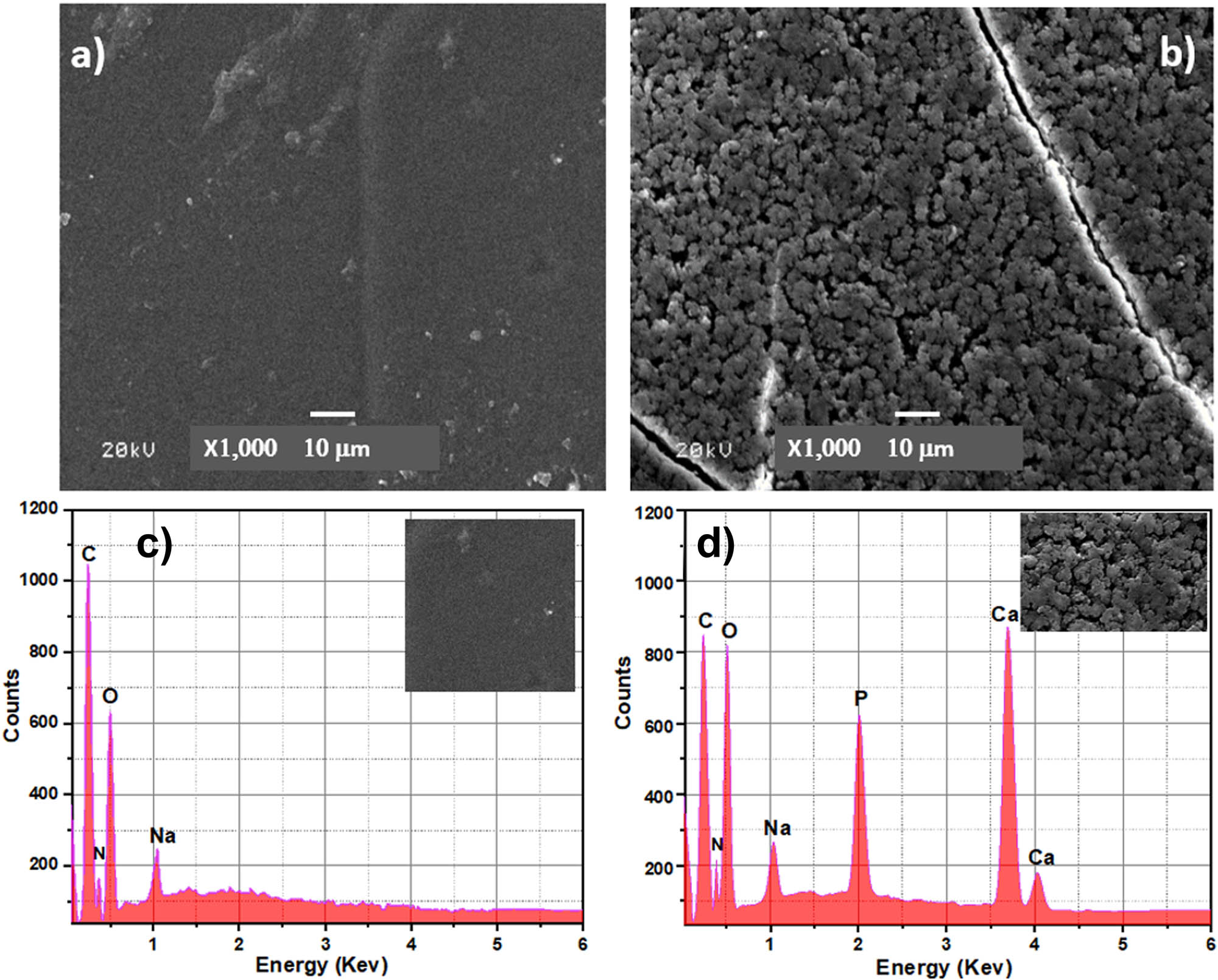
(a) SEM images of Cs, (b) SEM images of the BCP50/Cs composite, (c) EDS pattern of Cs, and (d) EDS pattern of the BCP50/Cs composite (before soaking in SBF).
Figure 8 shows SEM images of the surface of pure Cs and of BCP/Cs composites after 15 days of soaking in the SBF solution. SEM images of pure Cs after soaking in SBF (Figure 8(a)) show small white particles on their surfaces. These particles resemble NaCl crystals that may have been precipitated from the SBF onto the Cs surface [62], as affirmed by the corresponding EDS pattern for the same sample, as shown in Figure 8(f). This indicates that pure Cs without any BCP addition is not bioactive [63].
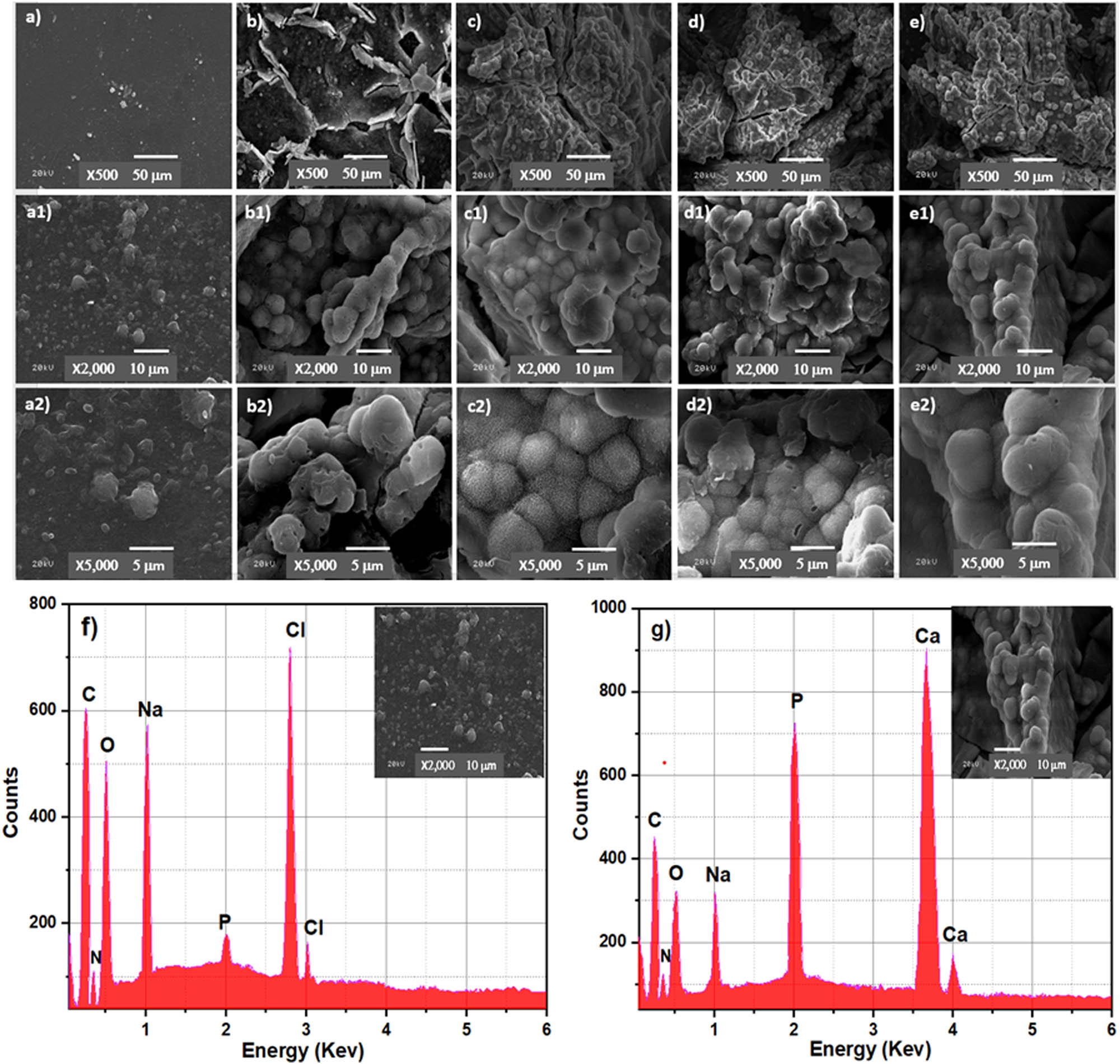
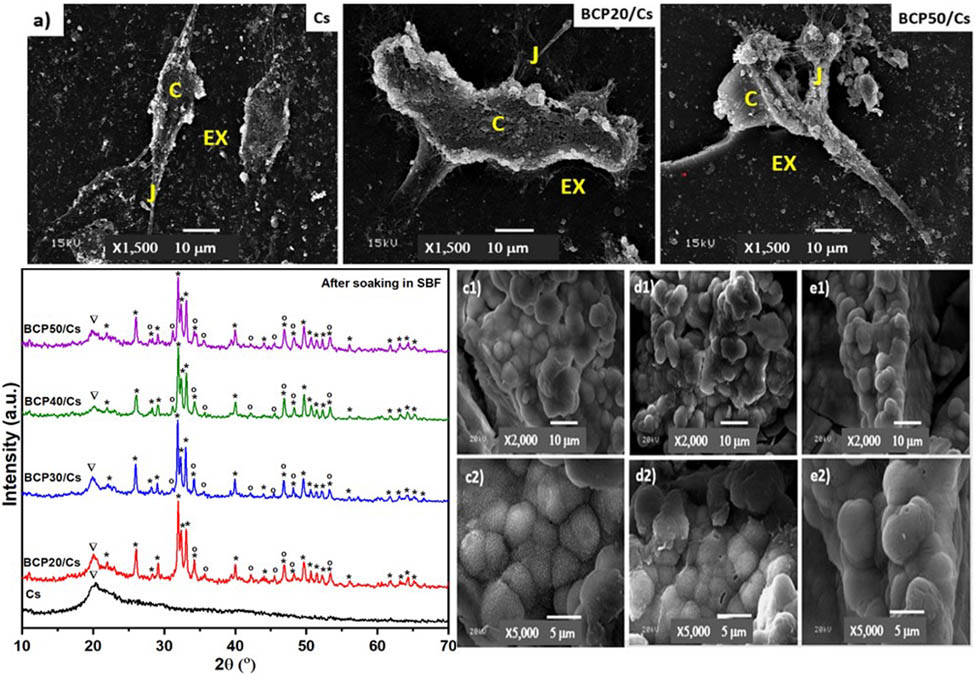
SEM images of Cs (a, a1, a2), BCP20/Cs (b, b1, b2), BCP30/Cs (c, c1, c2), BCP40/Cs (d, d1, d2), and BCP50/Cs (e, e1, e2) composites after soaking in SBF for 15 days; with different magnifications (×500, ×2,000, ×5,000) (f, g) EDS pattern of Cs and the BCP50/Cs composite after soaking in SBF for 15 days.
Moreover, the composite surfaces are covered by bright tiny spherical particles with an average diameter of 4.6–5.5 μm, confirming the formation of the apatite layer [64] (Figure 8(b)–(e)). The number of spherical apatites deposited on the surface changed with the addition of different amounts of BCP contents. It is also clear that the surface of BCP20/Cs is not completely covered with the apatite layer. However, by comparing this with BCP30/Cs, there is an increased density of apatite layer amounts deposited on its surface, reaching full surface coverage in BCP50/Cs. Moreover, by observing the Ca and P peaks in the EDS pattern (Figure 8(g)) after soaking in SBF and comparing them to Figure 8(d) before soaking in SBF, it is found that there is a noticeable increase in Ca and P elements, indicating an improved formation of a dense apatite layer (mineral) on the BCP50/Cs composite surface [54].
This occurs due to the rough surface that appeared after the addition of BCP. In addition, the presence of these particles on the surface acts as a nucleation site and facilitates apatite crystal deposition [65]. The nature of the apatite layer is clear from the SEM imaging. The shape of the crystal and the cauliflower structure is likely typical of the formation of HCA observed, especially on the surfaces of BCP30/Cs and BCP40/Cs. This result confirms the good crystallization of the HCA layer on the surface of BCP/Cs composite samples.
The particle size distribution of the deposited apatite layer on all samples after soaking in SBF is shown in Figure 9. As can be seen from this figure, the average particle sizes of the apatite layer are about 5.5, 5.08, 4.9, and 4.6 μm of BCP20/Cs, BCP30/Cs, BCP40/Cs, and BCP50/Cs, respectively. This is due to the addition of BCP in a larger proportion in BCP40 and BCP50, which increased the number of nucleation sites and their abundance on the surface and thus led to the small size of the apatite particles formed on the surface of these samples [66]. This indicates that these BCP particles play an important role in controlling the size of the apatite particles and thus controlling the biological interaction between the composite and the biological fluid.
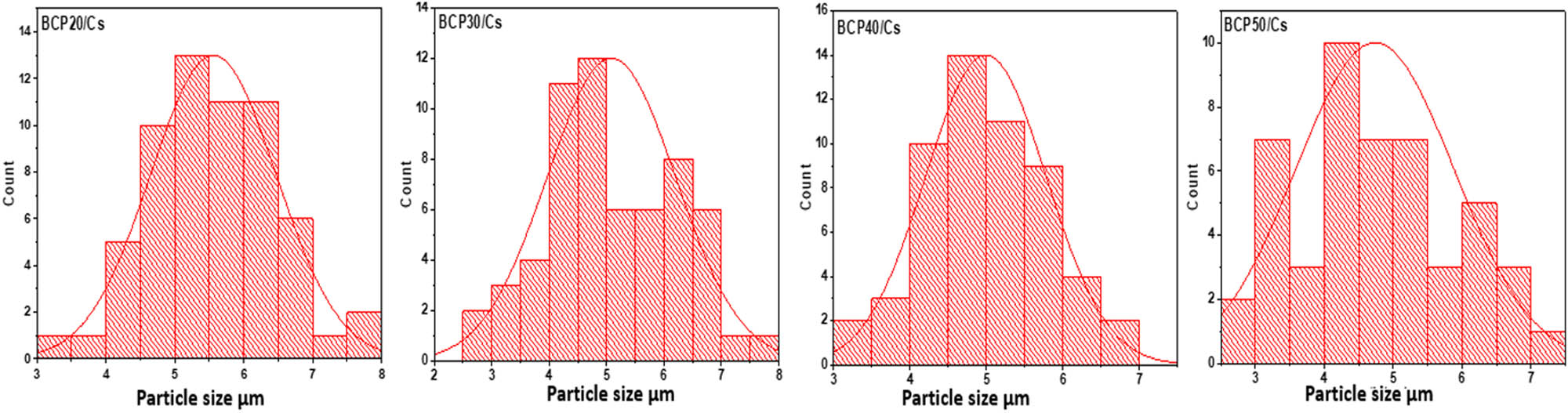
Particle size distributions of apatite layer formed on all composite samples.
3.8 In vitro cell culture analysis
The SRB assay was used to assess the safety of the composite samples for normal cells. The cytotoxicity of all prepared samples to normal human cells (osteocytes) was evaluated to examine the toxicity of these samples. The present study showed that most composite samples were non-cytotoxic (IC50 > 130 mM) on the human normal cell line. The cell viability assay showed that IC50 could not be obtained. The toxicity values were less than 50% of the cell’s validity ratio for different concentrations of scaffolds. This result indicates that the high proportions of the individual components of the prepared scaffolds (Cs and BCP) are characterized by their non-toxic properties, as mentioned before [49,67].
Furthermore, the morphology of cell attachment on the pure Cs, BCP20/Cs, and BCP50/Cs composites at 7 days of cell culture time was observed by SEM (Figure 10(a)). SEM images showed that the normal human osteocytes cultured on pure Cs were in a segregated shape from the cells around them. On the other hand, normal human osteocytes were randomly spread over the composite surfaces and attached to the composite surfaces. There are cell–cell junctions extended between the cells within and above the composite matrix, which are quite evident. The joints and the extracellular matrix are very noticeable in the composite containing BCP, especially on the surface of BCP50/Cs (Figure 10(a)).
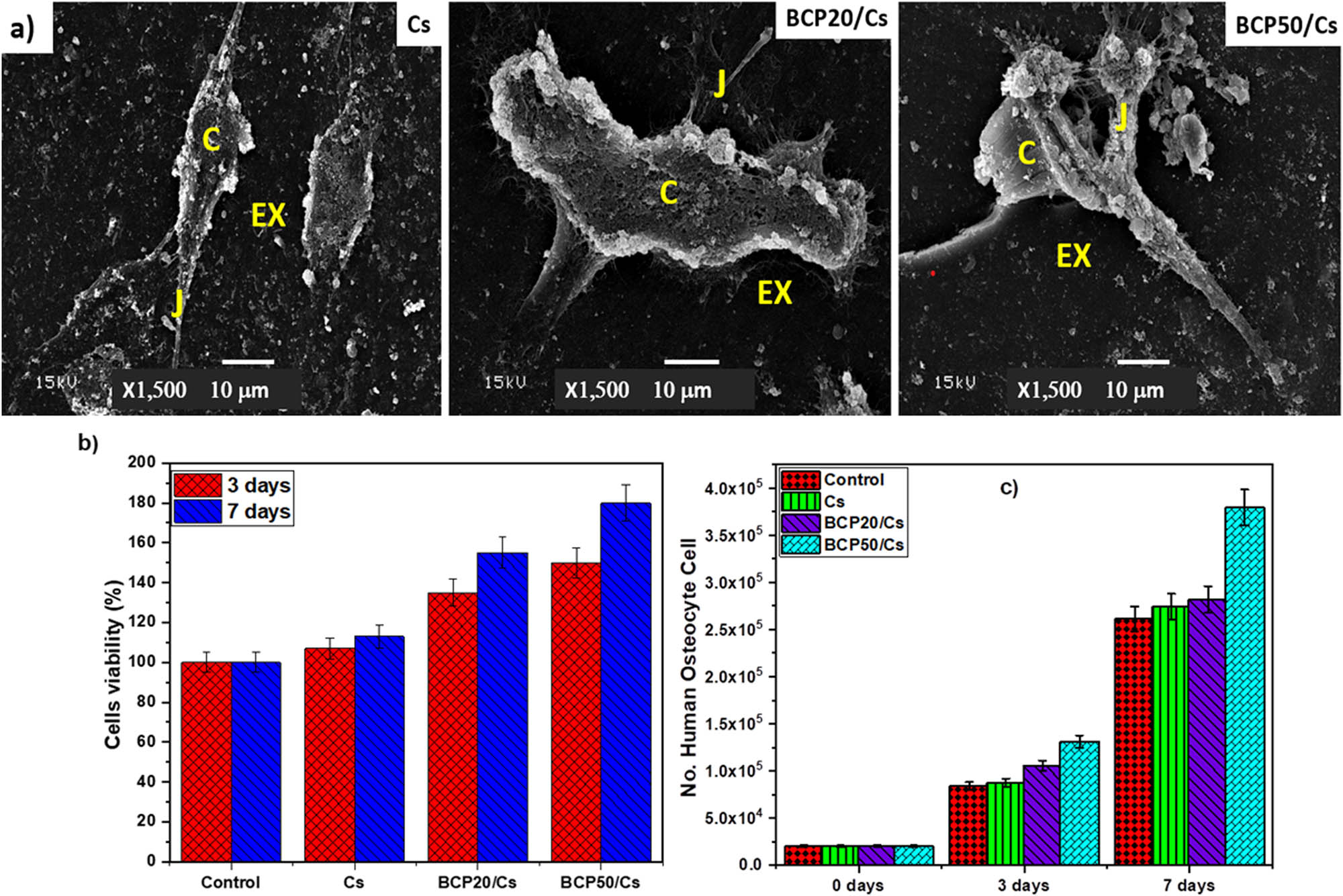
(a) SEM for normal human osteocyte after 7 days of cell culture; (b) cells viability assay; and (c) cell proliferation assay: Cells (C), cell–cell junctions (J), and extracellular matrix (EX).
In addition, the growth of normal human osteocytes increases in the presence of BCP. This is due to an increase in the number of polar groups on the surface of the composite due to the addition of BCP, which thus helps to enhance the attachment of osteocyte cells. Moreover, the presence of a rough surface improves surface-wetting properties and thus has an indirect effect on cell attachment by stimulating the adsorption of serum proteins used for cell attachment [68]. Hence, the prepared composite with more BCP content is preferable for cell anchoring and attachment after 7 days, which may support osteogenic functions and bone regeneration.
Figure 10(b) and (c) shows significant differences in cell count after 3 and 7 days of incubation. As shown in the figure, there is an increase in the number of cells as follows: 8.7 × 104 ± 2.4, 10 × 104 ± 1.8, and 13 × 104 ± 2.3 for Cs, BCP20/Cs, and BCP50/Cs samples, respectively, compared with control 8.4 × 104 ± 2.1 after 3 days. But after 7 days, it was observed that there was a clear effect of these prepared samples on cellular proliferation by increasing the number of recorded cells as follows: 2.7 × 105 ± 1.7, 2.8 × 105 ± 2.3, and 3.7 × 105 ± 1.4 for Cs, BCP20/Cs, and BCP50/Cs samples, respectively, compared with control 2.6 × 105 ± 1.8. Therefore, the BCP50/Cs sample provides more safety with cells than BCP20/Cs and then Cs. These results assume that cell adhesion and growth are ruled by the presence of both Cs and BCP. In addition, this noticeable increase in cell attachment is a promising feature of BCP when it is added to Cs, where cell attachment is an essential factor for the growth, proliferation, and differentiation of various cell types, including human osteocytes. Recent studies have reported that BCP-based materials have the properties of stimulating bone formation in intramuscular sites early, which does not occur with HA or TCP [69]. These properties are due to the resorbability of BCP, which works to dissolve the CaP crystals; then, the HCA layer is precipitated near the adsorbing crystals. As a result, this mineralization, whether on the surface or within the pores, makes the composite (BCP/Cs) act as a scaffold for bone cell attachment and stimulates and accelerates bone formation [70].
4 Conclusion
Cs and BCP/Cs composites were successfully fabricated with the desired shape by solvent casting and evaporation technique. The structural, morphology, and chemical compositions of these prepared composites were analyzed by XRD, FTIR, EDS, and SEM. The physiochemical and biological properties of the BCP/Cs composite scaffold were studied, which showed the biocompatibility of this hybrid compound. The obtained results showed that mechanical properties, protein adsorption, and biomineralization (apatite formation) depend on the amount of BCP added. The higher the BCP content in the composite samples, the greater the growth of the apatite layer on the composite surface. FTIR spectra of all composites showed the presence of distinct organic (Cs) and inorganic (BCP) absorption bands. XRD and FTIR results exhibited that there is significant intermolecular interaction between BCP particles and the Cs chain, and an increase in the peak size of inorganic compounds was observed by increasing the BCP/Cs ratio. Furthermore, the incorporation of BCP into the Cs matrix increased Young’s modulus and stiffness; therefore, these properties were controlled by adding BCP as a filler to make it suitable for cell adhesion and growth. In addition, protein adsorption results showed that the increase in BCP content increased the amount of protein adsorbed on a composite surface and the ability to form apatite. Furthermore, BCP/Cs composites increased the cell adhesion and growth of normal human osteocyte cells. Therefore, choosing a scaffold with a high BCP content (BCP50/Cs) will be appropriate when used in areas with load-bearing osseous defects, such as the femur, which also require rapid healing. Hence, BCP/Cs composites could be an excellent replacement for bone implants in tissue engineering applications.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah(grant no. G: 236-247-1442). The authors, therefore, acknowledge the DSR for technical and financial support.
-
Funding information: This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, (grant no. G: 236-247-1442).
-
Author contributions: All authors have accepted the responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Wang W, Yeung KW. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact Mater. 2017;2(4):224–47. 10.1016/j.bioactmat.2017.05.007.Search in Google Scholar PubMed PubMed Central
[2] Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. 10.1615/CritRevBiomedEng.v40.i5.10.Search in Google Scholar PubMed PubMed Central
[3] Kar S, Kaur T, Thirugnanam A. Microwave-assisted synthesis of porous chitosan–modified montmorillonite–hydroxyapatite composite scaffolds. Int J Biol Macromol. 2016;82:82628–36. 10.1016/j.ijbiomac.2015.10.060.Search in Google Scholar PubMed
[4] Heng BC, Bai Y, Li X, Meng Y, Lu Y, Zhang X, et al. The bioelectrical properties of bone tissue. Anim Model Exp Med. 2023;6(2):120–30. 10.1002/ame2.12300.Search in Google Scholar PubMed PubMed Central
[5] Li J, Tian X, Hua T, Fu J, Koo M, Chan W, et al. Chitosan natural polymer material for improving antibacterial properties of textiles. ACS Appl Bio Mater. 2021;4(5):4014–38. 10.1021/acsabm.1c00078.Search in Google Scholar PubMed
[6] Furko M, Balázsi K, Balázsi C. Calcium phosphate loaded biopolymer composites—a comprehensive review on the most recent progress and promising trends. Coatings. 2023;13(2):360. 10.3390/coatings13020360.Search in Google Scholar
[7] Nazeer MA, Yilgör E, Yilgör I. Intercalated chitosan/hydroxyapatite nanocomposites: promising materials for bone tissue engineering applications. Carbohydr Polym. 2017;175:17538–46. 10.1016/j.carbpol.2017.07.054.Search in Google Scholar PubMed
[8] Ressler A. Chitosan-based biomaterials for bone tissue engineering applications: a short review. Polym. 2022;14(16):3430. 10.3390/polym14163430.Search in Google Scholar PubMed PubMed Central
[9] Vu BT, Hua VM, Tang T-N, Dang NN-T, Cao HT-T, Phan TB, et al. Fabrication of in situ crosslinking hydrogels based on oxidized alginate/N, O-carboxymethyl chitosan/β-tricalcium phosphate for bone regeneration. J Sci: Adv Mater Devices. 2022;7(4):100503. 10.1016/j.jsamd.2022.100503.Search in Google Scholar
[10] Ebrahimi M, Botelho MG, Dorozhkin SV. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physicochemical properties and the impact of standardization of study protocols in biomaterials research. Mater Sci Eng, C. 2017;71:711293–312. 10.1016/j.msec.2016.11.039.Search in Google Scholar PubMed
[11] Lee SW, Kim Y, Rho HT, Kim S-I. Microhardness and microstructural properties of a mixture of hydroxyapatite and β-tricalcium phosphate. J Asian Ceram Soc. 2023;11(1):11–7. 10.1080/21870764.2022.2136261.Search in Google Scholar
[12] Hou X, Zhang L, Zhou Z, Luo X, Wang T, Zhao X, et al. Calcium phosphate-based biomaterials for bone repair. J Funct Biomater. 2022;13(4):187. 10.3390/jfb13040187.Search in Google Scholar PubMed PubMed Central
[13] Nevado P, Lopera A, Bezzon V, Fulla M, Palacio J, Zaghete M, et al. Preparation and in vitro evaluation of PLA/biphasic calcium phosphate filaments used for fused deposition modelling of scaffolds. Mater Sci Eng, C. 2020;114:114111013. 10.1016/j.msec.2020.111013.Search in Google Scholar PubMed
[14] Gan D, Liu M, Xu T, Wang K, Tan H, Lu X. Chitosan/biphasic calcium phosphate scaffolds functionalized with BMP‐2‐encapsulated nanoparticles and RGD for bone regeneration. J Biomed Mater Res Part A. 2018;106(10):2613–24. 10.1002/jbm.a.36453.Search in Google Scholar PubMed
[15] Cheng Y, Morovvati M, Huang M, Shahali M, Saber-Samandari S, Angili SN, et al. A multilayer biomimetic chitosan-gelatin-fluorohydroxyapatite cartilage scaffold using for regenerative medicine application. J Mater Res Technol. 2021;14:141761–77. 10.1016/j.jmrt.2021.07.052.Search in Google Scholar
[16] Abd-Khorsand S, Saber-Samandari S, Saber-Samandari S. Development of nanocomposite scaffolds based on TiO2 doped in grafted chitosan/hydroxyapatite by freeze drying method and evaluation of biocompatibility. Int J Biol Macromol. 2017;101:10151–8. 10.1016/j.ijbiomac.2017.03.067.Search in Google Scholar PubMed
[17] Sendemir‐Urkmez A, Jamison RD. The addition of biphasic calcium phosphate to porous chitosan scaffolds enhances bone tissue development in vitro. J Biomed Mater Res Part A. 2007;81(3):624–33. 10.1002/jbm.a.31010.Search in Google Scholar PubMed
[18] Kardan-Halvaei M, Morovvati M, Angili SN, Saber-Samandari S, Razmjooee K, Toghraie D, et al. Fabrication of 3D-printed hydroxyapatite using freeze-drying method for bone regeneration: RVE and finite element simulation analysis. J Mater Res Technol. 2023;24:248682–92. 10.1016/j.jmrt.2023.05.099.Search in Google Scholar
[19] El-Gohary M, El-Dyn S, Abd El-moniem B, Al-Ashkar E, Saleh E, Tolba E, et al. Synthesis and microstructure characterization of novel Sr-HA prepared by co-precipitation with enhanced bioactivity. Egypt J Biophys Biomed Eng. 2012;13:1373–85. 10.21608/ejbbe.2012.1194.Search in Google Scholar
[20] Priyadarshini I, Swain S, Koduru JR, Rautray TR. Electrically polarized withaferin a and alginate-incorporated biphasic calcium phosphate microspheres exhibit osteogenicity and antibacterial activity in vitro. Molecules. 2022;28(1):86. 10.3390/molecules28010086.Search in Google Scholar PubMed PubMed Central
[21] Park K, Kim S, Hwang M, Song H, Park Y. Biomimetic fabrication of calcium phosphate/chitosan nanohybrid composite in modified simulated body fluids. Exp Polym Lett. 2017;11(1):14. 10.3144/expresspolymlett.2017.3.Search in Google Scholar
[22] Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomater. 2006;27(15):2907–15. 10.1016/j.biomaterials.2006.01.017.Search in Google Scholar PubMed
[23] Tohamy KM, Mabrouk M, Soliman IE, Beherei HH, Aboelnasr MA. Novel alginate/hydroxyethyl cellulose/hydroxyapatite composite scaffold for bone regeneration: In vitro cell viability and proliferation of human mesenchymal stem cells. Int J Biol Macromol. 2018;112:112448–60. 10.1016/j.ijbiomac.2018.01.181.Search in Google Scholar PubMed
[24] Tuorkey M, Khedr Y, Aborhyem S, Xue X. Green synthesis of chicory (Cichorium intybus L.) Chitosan nanoparticles and evaluation of their anti-fungal, anti-hemolytic, and anti-cancer activities. J Bioact Compat Polym. 2022;37(6):421–36. 10.1177/08839115221126737.Search in Google Scholar
[25] Karava A, Lazaridou M, Nanaki S, Michailidou G, Christodoulou E, Kostoglou M, et al. Chitosan derivatives with mucoadhesive and antimicrobial properties for simultaneous nanoencapsulation and extended ocular release formulations of dexamethasone and chloramphenicol drugs. Pharmaceutics. 2020;12(6):594. 10.3390/pharmaceutics12060594.Search in Google Scholar PubMed PubMed Central
[26] El-Meliegy E, Abu-Elsaad N, El-Kady AM, Ibrahim MA. Improvement of physico-chemical properties of dextran-chitosan composite scaffolds by addition of nano-hydroxyapatite. Sci Rep. 2018;8(1):1–10.10.1038/s41598-018-30720-2Search in Google Scholar PubMed PubMed Central
[27] Ji Yin Y, Zhao F, Feng Song X, De Yao K, Lu WW, Chiyan Leong J. Preparation and characterization of hydroxyapatite/chitosan–gelatin network composite. J Appl Polym Sci. 2000;77(13):2929–38. 10.1002/1097-4628(20000923)77:13.Search in Google Scholar
[28] Langford J. X-ray diffraction procedures for polycrystalline and amorphous materials by HP Klug and LE Alexander. J Appl Crystallogr. 1975;8(5):573–4. 10.1107/S0021889875011399.Search in Google Scholar
[29] El-Sherbiny IM, Yahia S, Messiery MA, Reicha FM. Preparation and physicochemical characterization of new nanocomposites based on β-type chitosan and nano-hydroxyapatite as potential bone substitute materials. Int J Polym Mater Polym Biomater. 2014;63(4):213–20. 10.1080/00914037.2013.830249.Search in Google Scholar
[30] Landi E, Tampieri A, Celotti G, Sprio S. Densification behaviour and mechanisms of synthetic hydroxyapatites. J Eur Ceram Soc. 2000;20(14–15):2377–87. 10.1016/S0955-2219(00)00154-0.Search in Google Scholar
[31] Zhang Y, Venugopal JR, El-Turki A, Ramakrishna S, Su B, Lim CT. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomater. 2008;29(32):4314–22. 10.1016/j.biomaterials.2008.07.038.Search in Google Scholar PubMed
[32] Xianmiao C, Yubao L, Yi Z, Li Z, Jidong L, Huanan W. Properties and in vitro biological evaluation of nano-hydroxyapatite/chitosan membranes for bone guided regeneration. Mater Sci Eng, C. 2009;29(1):29–35. 10.1016/j.msec.2008.05.008.Search in Google Scholar
[33] Vecstaudza J, Gasik M, Locs J. Amorphous calcium phosphate materials: Formation, structure and thermal behaviour. J Eur Ceram Soc. 2019;39(4):1642–9. 10.1016/j.jeurceramsoc.2018.11.003.Search in Google Scholar
[34] Meng L, Xie F, Zhang B, Wang DK, Yu L. Natural biopolymer alloys with superior mechanical properties. ACS Sustain Chem Eng. 2018;7(2):2792–802. 10.1021/acssuschemeng.8b06009.Search in Google Scholar
[35] Mohandes F, Salavati-Niasari M. Freeze-drying synthesis, characterization and in vitro bioactivity of chitosan/graphene oxide/hydroxyapatite nanocomposite. RSC Adv. 2014;4(49):25993–6001. 10.1039/C4RA03534H.Search in Google Scholar
[36] Raz M, Moztarzadeh F, Kordestani SS. Synthesis, characterization and in-vitro study of chitosan/gelatin/calcium phosphate hybrid scaffolds fabricated via ion diffusion mechanism for bone tissue engineering. Silicon. 2018;10:10277–86. 10.1007/s12633-016-9439-3.Search in Google Scholar
[37] Eivazzadeh-Keihan R, Pajoum Z, Aliabadi HAM, Mohammadi A, Kashtiaray A, Bani MS, et al. Magnetized chitosan hydrogel and silk fibroin, reinforced with PVA: a novel nanobiocomposite for biomedical and hyperthermia applications. RSC Adv. 2023;13(13):8540–50. 10.1039/D3RA00612C.Search in Google Scholar PubMed PubMed Central
[38] Mauricio-Sánchez RA, Salazar R, Luna-Bárcenas JG, Mendoza-Galván A. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vib Spectrosc. 2018;94:941–6. 10.1016/j.vibspec.2017.10.005.Search in Google Scholar
[39] Radwan NH, Nasr M, Ishak RA, Abdeltawab NF, Awad GA. Chitosan-calcium phosphate composite scaffolds for control of post-operative osteomyelitis: Fabrication, characterization, and in vitro–in vivo evaluation. Carbohydr Polym. 2020;244:244116482. 10.1016/j.carbpol.2020.116482.Search in Google Scholar PubMed
[40] Carvalho GK, Farias JR, Lima IS, Nascimento AM, Neves GA, Menezes R, et al. HAp/β-TCP Biphasic ceramics obtained by the pechini method: An antibacterial approach. Minerals. 2022;12(12):1482. 10.3390/min12121482.Search in Google Scholar
[41] Weiner S, Bar-Yosef O. States of preservation of bones from prehistoric sites in the Near East: a survey. J Archaeol Sci: Rep. 1990;17(2):187–96. 10.1016/0305-4403(90)90058-D.Search in Google Scholar
[42] Thein-Han W, Misra R. Biomimetic chitosan–nanohydroxyapatite composite scaffolds for bone tissue engineering. Acta Biomater. 2009;5(4):1182–97. 10.1016/j.actbio.2008.11.025.Search in Google Scholar PubMed
[43] El-Sayed SA, Mabrouk M, Khallaf ME, Abd El-Hady BM, El-Meliegy E, Shehata MR. Antibacterial, drug delivery, and osteoinduction abilities of bioglass/chitosan scaffolds for dental applications. J Drug Deliv Sci Technol. 2020;57:57101757. 10.1016/j.jddst.2020.101757.Search in Google Scholar
[44] Ersal F, Sari Y. Synthesis and characterization of hydroxyapatite-chitosan composite in situ by microwave irradiation method. J Phys: Conf Ser. 2019;1248(1):012080. 10.1088/1742-6596/1248/1/012080.Search in Google Scholar
[45] Chen J, Zhang G, Yang S, Li J, Jia H, Fang Z, et al. Effects of in situ and physical mixing on mechanical and bioactive behaviors of nano hydroxyapatite–chitosan scaffolds. J Biomater Sci, Polym Ed. 2011;22(15):2097–106. 10.1163/092050610X533691.Search in Google Scholar PubMed
[46] Peter M, Ganesh N, Selvamurugan N, Nair S, Furuike T, Tamura H, et al. Preparation and characterization of chitosan–gelatin/nanohydroxyapatite composite scaffolds for tissue engineering applications. Carbohydr Polym. 2010;80(3):687–94. 10.1016/j.carbpol.2009.11.050.Search in Google Scholar
[47] Leonor IB, Baran ET, Kawashita M, Reis R, Kokubo T, Nakamura T. Growth of a bonelike apatite on chitosan microparticles after a calcium silicate treatment. Acta Biomater. 2008;4(5):1349–59. 10.1016/j.actbio.2008.03.003.Search in Google Scholar PubMed
[48] Luz GM, Mano JF. Chitosan/bioactive glass nanoparticles composites for biomedical applications. Biomed Mater. 2012;7(5):054104. 10.1088/1748-6041/7/5/054104.Search in Google Scholar PubMed
[49] Nguyen TP, Tran NQ. Preparation and biomineralization of injectable hydrogel composite based chitosan-tetronic and bcp nanoparticles. Adv Res. 2016;7:71–8. 10.9734/AIR/2016/25891.Search in Google Scholar
[50] Jayash SN, Cooper PR, Shelton RM, Kuehne SA, Poologasundarampillai G. Novel chitosan-silica hybrid hydrogels for cell encapsulation and drug delivery. Int J Mol Sci. 2021;22(22):12267. 10.3390/ijms222212267.Search in Google Scholar PubMed PubMed Central
[51] Li B, Xia X, Guo M, Jiang Y, Li Y, Zhang Z, et al. Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci Rep. 2019;9(1):14052. 10.1038/s41598-019-49941-0.Search in Google Scholar PubMed PubMed Central
[52] Yamashita K, Oikawa N, Umegaki T. Acceleration and deceleration of bone-like crystal growth on ceramic hydroxyapatite by electric poling. Chem Mater. 1996;8(12):2697–700. 10.1021/cm9602858.Search in Google Scholar
[53] Zheng K, Wu Z, Wei J, Rűssel C, Liang W, Boccaccini AR. Preparation and characterization of fibrous chitosan-glued phosphate glass fiber scaffolds for bone regeneration. J Mater Sci Mater Med. 2015;26:261–10. 10.1007/s10856-015-5554-8.Search in Google Scholar PubMed
[54] Soliman IE-S, Metawa AE-S, Aboelnasr MAH, Eraba KT. Surface treatment of sol-gel bioglass using dielectric barrier discharge plasma to enhance growth of hydroxyapatite. Korean J Chem Eng. 2018;35:352452–63. 10.1007/s11814-018-0131-8.Search in Google Scholar
[55] Elhendawi H, Felfel R, El-Hady A, Bothaina M, Reicha FM. Effect of synthesis temperature on the crystallization and growth of in situ prepared nanohydroxyapatite in chitosan matrix. Int Sch Res Notices. 2014;2014:1–8. 10.1155/2014/897468.Search in Google Scholar
[56] Garskaite E, Gross K-A, Yang S-W, Yang TC-K, Yang J-C, Kareiva A. Effect of processing conditions on the crystallinity and structure of carbonated calcium hydroxyapatite (CHAp). Cryst Eng Comm. 2014;16(19):3950–9. 10.1039/C4CE00119B.Search in Google Scholar
[57] Teimouri A, Azadi M. Preparation and characterization of novel chitosan/nanodiopside/nanohydroxyapatite composite scaffolds for tissue engineering applications. Int J Polym Mater Polym Biomater. 2016;65(18):917–27. 10.1080/00914037.2016.1180606.Search in Google Scholar
[58] Maji K, Dasgupta S. Characterization and in vitro evaluation of gelatin–chitosan scaffold reinforced with bioceramic nanoparticles for bone tissue engineering. J Mater Res. 2019;34(16):2807–18. 10.1557/jmr.2019.170.Search in Google Scholar
[59] Lü X, Zhang H, Huang Y, Zhang Y. A proteomics study to explore the role of adsorbed serum proteins for PC12 cell adhesion and growth on chitosan and collagen/chitosan surfaces. Regener Biomater. 2018;5(5):261–73. 10.1093/rb/rby017.Search in Google Scholar PubMed PubMed Central
[60] Abueva CD, Padalhin AR, Min Y-K, Lee B-T. Preformed chitosan cryogel-biphasic calcium phosphate: A potential injectable biocomposite for pathologic fracture. J Biomater Appl. 2015;30(2):182–92. 10.1177/0885328215577892.Search in Google Scholar PubMed
[61] Dantas MJ F, dos Santos BF A, Tavares A, Maciel MA, Lucena BdM L, Fook MV, et al. The impact of the ionic cross-linking mode on the physical and in vitro dexamethasone release properties o-f chitosan/hydroxyapatite beads. Molecules. 2019;24(24):4510. 10.3390/molecules24244510.Search in Google Scholar PubMed PubMed Central
[62] Zhang Y, Zhang M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J Biomed Mater Res. 2001;55(3):304–12. 10.1002/1097-4636(20010605)55:3 < 304: AID-JBM1018 > 3.0.CO;2-J.Search in Google Scholar
[63] Hammad HG, Salama MNF. Porosity pattern of 3D chitosan/bioactive glass tissue engineering scaffolds prepared for bone regeneration. Open Dent J. 2021;15(1):41–56. 10.2174/1874210602115010041.Search in Google Scholar
[64] El Gohary M, El-Din S, Awad S, El-Tohamy AM, Soliman I. Influence of Li 2 content on formation of apatite layer on surface of SiO2-CaO-K2O-P2O5 bioglass system. Egypt J Biophys Biomed Eng. 2012;13(1):37–52. 10.21608/EJBBE.2012.1192.Search in Google Scholar
[65] d’Arros C, Rouillon T, Veziers J, Malard O, Borget P, Daculsi G. Bioactivity of biphasic calcium phosphate granules, the control of a needle-like apatite layer formation for further medical device developments. Front Bioeng Biotechnol. 2020;7:7462. 10.3389/fbioe.2019.00462.Search in Google Scholar
[66] Eliaz N, Metoki N. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials. 2017;10(4):334. 10.3390/ma10040334.Search in Google Scholar
[67] Jahan K, Manickam G, Tabrizian M, Murshed M. In vitro and in vivo investigation of osteogenic properties of self-contained phosphate-releasing injectable purine-crosslinked chitosan-hydroxyapatite constructs. Sci Rep. 2020;10(1):1–17. 10.1038/s41598-020-67886-7.Search in Google Scholar
[68] Cai B, Zou Q, Zuo Y, Li L, Yang B, Li Y. Fabrication and cell viability of injectable n-HA/chitosan composite microspheres for bone tissue engineering. RSC Adv. 2016;6(89):85735–44. 10.1039/C6RA06594E.Search in Google Scholar
[69] Park J-C, Lim H-C, Sohn J-Y, Yun J-H, Jung U-W, Kim C-S, et al. Bone regeneration capacity of two different macroporous biphasic calcium materials in rabbit calvarial defect. J Korean Acad Periodontol. 2009;39(Suppl):223–30. 10.5051/jkape.2009.39.S.223.Search in Google Scholar
[70] Krithiga G, Jena A, Selvamani P, Sastry T. In vitro study on biomineralization of biphasic calcium phosphate biocomposite crosslinked with hydrolysable tannins of Terminalia chebula. Bull Mater Sci. 2011;34:34589–94. 10.1007/s12034-011-0075-7.Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

