Abstract
The smart membrane is a new type of functional membrane. The performance of this membrane is changed according to the variations in external physical and chemical signals. This membrane has become an essential focus in specific recognition, catalysis, selective permeation, and other fields. However, the problems of this membrane are weak anti-pollution ability, poor response performance, and inability of mass production. Therefore, scholars have done a lot of research on improving this membrane by modification, grafting polymerization, phase transformation, and in situ cross-linking copolymerization. This review provides a comparative investigation and summary of smart membranes, including temperature, light, electric field, magnetic field, pH, and specific molecular and ion-responsive membranes. Moreover, the authors also introduce the preparation process, selectivity, optimization and improvement of membranes, and their application fields. Finally, the authors’ perspective on the current key issues and directions of these fields for future development are also discussed.
1 Introduction
With the deterioration of the global ecological environment and the shortage of resources, membrane separation technology, which is a physical reaction process that can be used to separate substances of different molecular sizes, has attracted more and more attention among science and technologies [1,2,3,4,5,6]. Compared with traditional filtration technologies, membrane separation technology does not need additives [7]. Therefore, membrane separation technology is far superior to traditional filtration technology. Smart membranes are used as the main material of membrane separation technology now, which can change their performance and structure according to the external environmental stimulation. The research and development of smart membranes are also closely related to environmental protection, energy recovery, water resource development, etc. [8,9,10,11]. Therefore, smart membranes have attracted extensive attention.
In fact, the initial inspiration of smart membranes came from natural creatures. Many organisms in nature have completely reversible capabilities, which provide us with inspired ideas of smart membranes. For example, a chameleon can change its color according to different environments. This is because the chameleon can change colors by relaxing and stimulating the pigment cells inside its skin as well as adjusting the guanine crystal structure of its skin surface. Based on this principle, Zhang et al. [12] synthesized a photonic cellulose membrane using materials such as cellulose nanocrystals, which could switch its wettability to display or hide the color traces left after ink-free writing. Such membranes are widely used in fields including color sensing, encryption, and anti-counterfeiting. In another example, butterfly wings exhibit anisotropic wetting behavior, from whose surface water droplets can quickly roll away in the radial outward direction, and are pinned to the wing surface in the opposite direction. This is because butterfly wings are one of the most complex three-dimensional periodic media in nature. The abundant square scales on the wing surface overlap each other, forming a periodic hierarchical structure along the reverse osmosis (RO) direction. This characteristic of butterfly wings provides an idea for designing intelligent fluid controllable membranes [13]. According to the characteristics of these biological systems in nature, smart membranes have shown unique advantages in many fields such as anti-pollution [14,15,16,17], self-cleaning [18,19], material detection [20,21], smart sensors [22,23], seawater desalination [24,25], and water treatment [26,27,28]. For example, temperature-responsive smart membranes can be applied to anti-pollution [29,30,31] and water treatment [32] by grafting intelligence materials such as N-isopropylacrylamide (NIPAM) and polyacrylic acid onto membranes. For instance, the oligoethylene-glycol-based PG1A (dendronized copolymer) is modified on the surface of gold nanoparticle (NP) membranes. This membrane has good antifouling performance and can be used to separate oil-in-water and water-in-oil emulsions according to temperature variations [33]. Light-responsive smart membranes have been widely studied in light responsiveness [34,35,36,37], energy conversion [38], and large-scale manufacturing [39]. For example, electrolytes are used as the gate dielectric of light-responsive membranes of nanostructured zinc oxide, whose light responsiveness is greatly enhanced by ultraviolet (UV) light irradiation [40]. Electric-field-responsive smart membranes have made research progress in hydrophilicity [41], remote control, and other fields. For instance, such membranes can be synthesized by grafting poly(ionic liquid) (PIL) onto the ultrafiltration membrane of regenerated cellulose, which can interact with the external electric field of oscillation, providing an opportunity for their remote control [42]. At present, pH-responsive smart membranes have developed rapidly in industrial production [43], dual response [44,45,46,47,48], and other fields. One of these membranes has super lipophilicity underwater and super hydrophobicity under oil by adding polyvinylidene fluoride (PVDF) to Poly(dimethylaminoethyl methacrylate) (PDMAEMA) hydrogels. The membrane is used to solve the discoloration problem of anti-counterfeiting labels [49]. Specific molecular recognition-responsive smart membranes are often used for pharmaceutical engineering [50,51,52] and membrane sensors. In one of those membranes, graft polymers containing specific molecular recognition receptors are introduced on its porous membrane substrate, so that target biological molecules can be recognized and crosslinked to control the opening and closing of pores through the membrane. The membrane is of essential significance for membrane sensors in the medical field [53]. Ion-responsive smart membranes are often used in industrial wastewater treatment [46,54], drinking water detection [55], and other fields. For example, this membrane of zwitterionic carbon nanotube combines nanostructured components with the polymer matrix. The selective permeability coefficients of the membrane for glucose and divalent anions are 5.5 and 93, respectively. The membranes have potential application prospects in saltwater treatment [56].
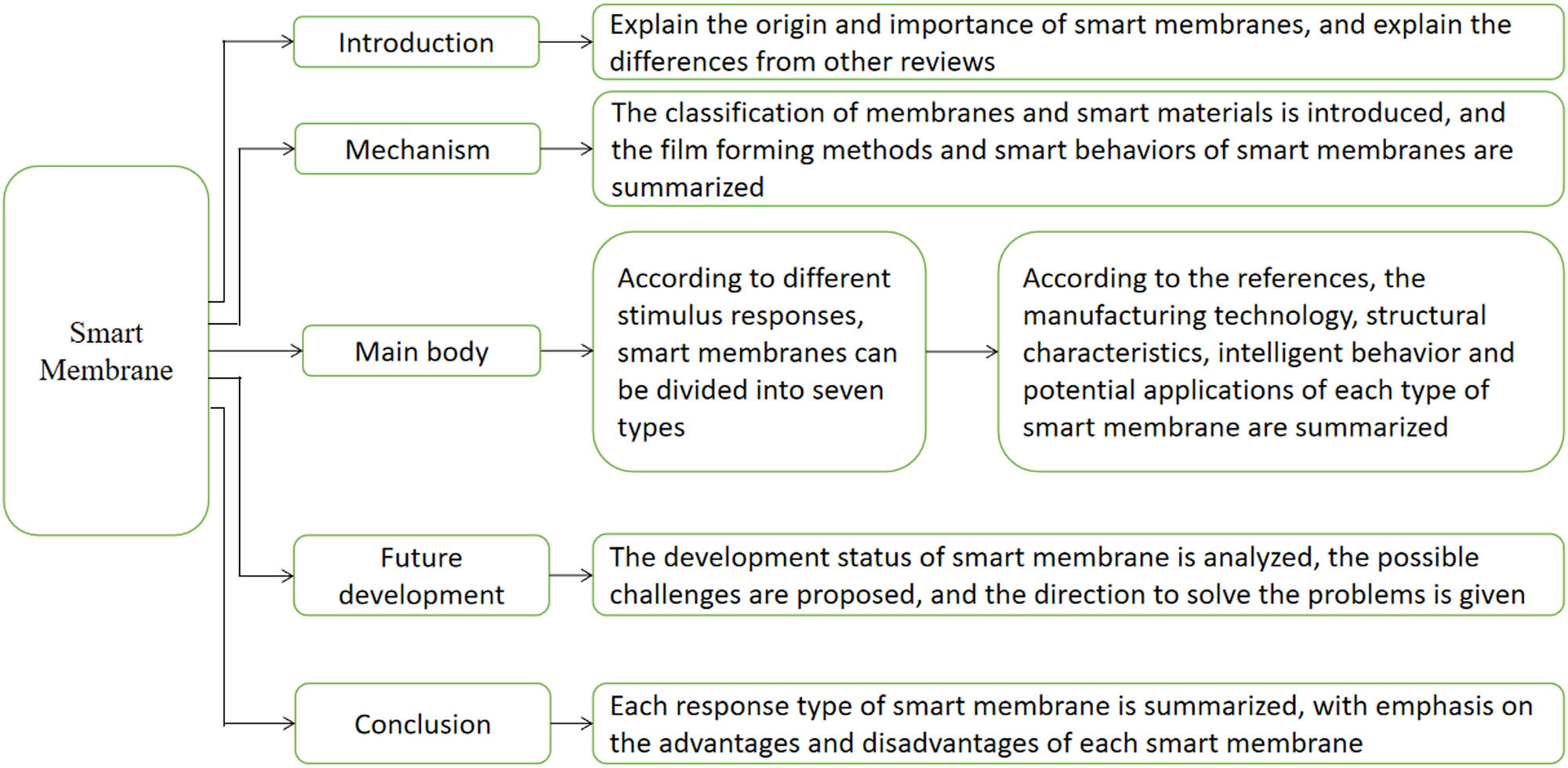
There have been many reviews summarizing and comparing a variety of materials based on smart surfaces with a switchable wettability, such as membranes, textiles, foams, etc. [13,57,58,59]. The surface wettability of these materials can be completely transformed via external stimulation, which is a reversible process. However, few reviews provide a comprehensive introduction to the stimulus-response of smart membranes. When smart materials are distributed on the surface of smart membranes, the smart materials can regulate the micro-morphology and energy at the membrane’s surface according to the external signals, thereby changing the surface characteristics of the membranes. In addition to wettability, these properties include chargeability, selective permeability, etc. In addition, smart materials can also be “distributed” inside smart membranes or in the smart membrane pores. In this review, we focus on typical works on the synthesis, mechanism, and application of smart membranes, mainly introducing smart membranes from the aspects of stimulus-response, fabrication technologies, structural characteristics, intelligent behavior, and potential applications. In addition, we describe the development trend of smart membranes and the problems to be solved in the future (Figure 1).
A summary scheme of the study.
2 Mechanism of smart membrane
Membrane separation technology is a new separation technology developed in recent decades, whose biggest feature is that no additives need to be added in the process of separating substances. The key materials used in membrane separation technology are membranes, which are of many kinds [60,61,62,63], as is shown in Table 1. The membranes are intermediate interfaces, which play the role of a selective barrier and control the continuous material transport on both sides.
| Classification standard | Name |
|---|---|
| Structure | Porous membrane, dense membrane |
| Source | Synthetic membrane, natural membrane |
| Functions | Filtration membrane**, select permeation membrane, industrial membrane |
**Filtration membrane includesultrafiltration membrane, microfiltration membrane, nanofiltration membrane; Select permeation membrane includespermeation membrane, reverse osmosis membrane, and electroosmotic membrane; Industrial membraneincludes ion exchange membrane, hemodialysis membrane, ultrafiltration membrane, reverse osmosis membrane, gas separation membrane.
With the further understanding of bionic technology, people add intelligent materials into membranes directly or indirectly, which can make membranes have stimulation responses. There are many kinds of smart materials, as shown in Table 2. Such membranes that respond to environmental stimuli are called smart membranes. The formation of smart membranes is mainly divided into two types. One is to form smart membranes by mixing smart materials and membrane-forming materials with environmental responsiveness by the physical way, or to modify the membrane-forming materials by covalent bonding, and then to form membranes. This physical mixing form of materials will make the smart materials uniformly distributed inside smart membranes. The pores formed inside smart membranes are surrounded by smart materials and other auxiliary materials. When subjected to external stimuli such as temperature, light, and pH, the internal smart material will begin to shrink or expand, causing membrane pores to open and close, thereby changing the permeability of membranes. The entire membrane exhibits overall shrinkage or overall expansion. The mechanism diagram is shown in Figure 2. For example, the molecular side chains of the smart material poly(N-isopropylacrylamide) (PNIPAAm) have both hydrophilic amide groups and hydrophobic isopropyl groups. When the temperature is lower than the critical temperature, the amide groups will form strong hydrogen bonds with the water molecules. The PNIPAAm materials begin to absorb water and expand, squeezing the pores inside smart membranes, thereby closing it. Then, the volume of smart membranes increases and the permeability decreases. When the temperature is higher than the critical temperature, the hydrogen bonds between the amide groups and the water molecules will break, and the hydrophobicity of the isopropyl groups begins to play a leading role. The PNIPAAm materials begin to be hydrophobic and gradually shrink, opening the pores of membranes. The volume of smart membranes decreases and the permeability increases [64,65]. In addition, the pores of smart membranes formed by bonding will contain special recognition sites. These recognition sites can be chemically bonded and dissociated with some specific substances, so that some specific substances can be selectively penetrated. For example, the puerarin group is the phenolic hydroxyl group, and the γ-aminopropyltriethoxysilane functional group is the amino group. The phenolic hydroxyl group and the amino group can form ionic bonds. Puerarin mixed with γ-aminopropyltriethoxysilane acts as a template molecule to be a membrane-forming material. Smart membranes are synthesized with template molecules, silica sols, ethanols, and other materials. The ethanol solution can wash the puerarin in the membrane. When the puerarin solution is separated, puerarin can bond-dissociate-rebond with the amino groups in the pores when passing through the membrane pores. The schematic of puerarin is shown in Figure 3 [66].
Classification of smart materials
| Response type | Representative materials | Material performance | Ref. |
|---|---|---|---|
| Temperature | NIPAM, PNIPAAm, etc. | When the temperature changes, the volume of NIPAM and PNIPAAm can shrink or expand rapidly. | [67] |
| Light | Spiropyrans and their derivatives, etc. | Photoisomerization of spiropyrans: conversion of a compound from a ground state to an excited state by direct irradiation with UV light of a certain wavelength. | [68] |
| Electric field | PVDF, etc. | Piezoelectric response characteristics of PVDF: the β phase of PVDF has piezoelectricity, and its dielectric constant changes with the electric field strength and has an electrothermal effect. | [69,70] |
| Magnetic field | Magnetic NPs, etc. | When there is no magnetic field, the particles are non-magnetic. When there is a magnetic field, the particles are magnetic. | [71] |
| pH | Diferroformylmethane, alizarin red, etc. | The color of alizarin red depends on the pH, it is yellow at low pH, and purple at high pH. | [72,73] |
| Specific molecular | PDMAEMA, etc. | PDMAEMA is a polymer material with CO2 stimulus responsiveness. | [74] |
| Ion | Polyacrylonitrile (PAN), electrospun PAN nanofibers, etc. | Electrospun PAN nanofibers contain a large number of unsaturated nitrile groups, which can introduce a variety of functional groups such as thiol, amine, carboxyl, and amidoxime groups to selectively adsorb heavy metal ions. | [75] |
![Figure 2
Expansion and shrinkage of smart membranes [76].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_002.jpg)
Expansion and shrinkage of smart membranes [76].
![Figure 3
Schematic diagram of puerarin through membrane [66].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_003.jpg)
Schematic diagram of puerarin through membrane [66].
The other is to introduce smart materials on membrane surfaces or in the membrane pores through physical fixation, chemical modification, or grafting without changing the main structure of substrate membranes through chemical methods. Two kinds of smart membranes will be formed through this membrane-forming method, one is that smart materials are distributed on the membrane surface, which can control the energy or micro-morphology of membrane surface based on changes in external signals, thereby controlling the characteristics of the membranes (such as charge, wettability [hydrophilicity and hydrophobicity]). The mechanism diagram is shown in Figure 4. For example, wettability is mainly based on the surface energy and surface roughness of a material. Some external stimuli (such as light, ions, and pH) can affect the surface energy of a membrane, while some others (such as electric field and magnetic field) can affect its surface morphology [57,59]. When the surface energy and morphology of a smart membrane change, hydrophilic, hydrophobic, lipophilic, oleophobic, and other characteristics will appear on its surface. Depending on the energy and roughness of membrane surfaces, there are two typical wetting states, Cassie–Baxter metastable state and Wenzel state. When testing the hydrophilicity of air, stable cavitation occurs in the rough gap between water droplets and membrane surfaces in the Cassie–Baxter metastable state. The adhesion of water droplets to membrane surfaces is reduced during cavitation, so that water droplets can easily roll off membrane surfaces and be quickly removed. At this time, smart membranes will exhibit hydrophobicity. Upon external stimulation, the energy and roughness of the surface of a membrane change, and it enters the Wenzel state. Water droplets will unreservedly occupy its surface and completely adhere to the solid surface. At this time, the smart membrane will exhibit hydrophilicity. When testing the lipophilicity of water, under the Cassie–Baxter state, a stable water cavity appears in the rough gap between oil droplets and membrane surface, in which an oil–water–solid interface will form, making the smart membrane oleophobic. When stimulated by the external environment, the membrane enters the Wenzel state, and oil droplets will completely adhere to its surface. At this time, the smart membrane exhibits lipophilicity [13,57,58,59].
![Figure 4
Expansion and shrinkage of smart membranes (Smart material are distributed on the membrane surface) [76].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_004.jpg)
Expansion and shrinkage of smart membranes (Smart material are distributed on the membrane surface) [76].
The other is the distribution of smart materials on the surface or pores of the membrane. These smart materials can regulate the effective pore size of the smart membrane, thereby endowing the smart membrane with different permeability and selectivity. Such porous membranes are usually called smart switch membranes, whose working characteristics belong to the valve mechanism. The smart materials on smart switch membranes are mostly in a chain shape. When exposed to external stimuli such as temperature, light, and pH, these intelligent forging chains begin to extend, which then block and close the membrane pores, while preventing matters from passing through. On the other hand, these forging chains will begin to shrink after being stimulated by the outside world, opening membrane pores and allowing matters to pass. Protonation or deprotonation reactions often occur during the extension or contraction of forging chains. Electrostatic repulsion occurs among some forging chains, increasing the distance among them, which will lead to an increase in polymer volume and the closure of membrane pores. The mechanism diagram is shown in Figure 5.
![Figure 5
Expansion and shrinkage of smart membranes (Smart material are distributed on the surface or pores of the membrane) [77].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_005.jpg)
Expansion and shrinkage of smart membranes (Smart material are distributed on the surface or pores of the membrane) [77].
As environmental information changes, smart materials play a “switch” role on a membrane. So smart membranes can also be called as smart gating membranes. The stimuli of intelligent material responses usually include pH, specific molecular, ion, temperature, light, electric field, and magnetic field. Smart membranes can be divided into chemical and physical responses according to different stimuli of intelligent material responses [78,79,80]. Among them, the physical responses generally include temperature, light, electric field, and magnetic field, while the chemical responses generally include pH, specific molecular, and ion.
3 Physical stimuli-responsive smart membrane
As a new type of functional membrane, physical stimuli-responsive smart membranes can rapidly change their performance according to external physical stimuli compared with the conventional membranes, which are mainly composed of temperature-sensitive, light-sensitive, electric-field-sensitive, and magnetic-sensitive smart materials. Such membranes are widely used in membrane filtration, self-cleaning, protein separation, and biosensing.
3.1 Temperature-responsive smart membrane
Generally, the synthetic materials of smart temperature-responsive membranes include NIPAM, PNIPAAm, and other materials [65]. In addition, temperature-responsive membranes respond quickly when the external environment stimulation suddenly changes. The polymer PNIPAAm was first reported in 1956, but it did not attract the attention of scholars at that time [60]. In recent years, temperature-responsive smart membranes have been widely used with the progress of intelligent research.
In 1999, Pang et al. [81] studied a smart track membrane to control the membrane aperture switch (Figure 6). The membrane grafted NIPAM (thermosensitive materials) onto the membrane surface. The synthesis technology used in this membrane was peroxide pre-irradiation grafting technology. The base membrane used in this membrane was a polycarbonate nuclear track microporous membrane. When the temperature was higher than lower critical solution temperature (LCST), the deswelling reaction occurred and the pore size of the smart membrane increased. When it was lower than LCST, the swelling reaction occurred and water was absorbed, with the pore size of the smart membrane shrinking or even completely closed. This membrane has a very thin thermosensitive layer and submicrometer nuclear track, which makes its switch response more sensitive. However, there was a lack of research on the anti-pollution [82,83], salting out [84,85], and universality of the membrane [86].
![Figure 6
Action principle of the smart track membrane (TsINM) [81].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_006.jpg)
Action principle of the smart track membrane (TsINM) [81].
To study the anti-pollution performance of smart membranes, Wu [82] synthesized a kind of aromatic polyamide-RO membrane, which was prepared by dip coating process and has temperature responsiveness. The surface modifier used in the membrane was the copolymer of NIPAM and acrylamide (Am) (Figure 7). In the mechanism of anti-pollution, the hydrophilicity of the membrane is enhanced at a low temperature, which weakens the contact between pollutants and the membrane surface. However, the solution consisted of NaCl and bovine serum albumin (BSA). Only when the ion concentration in the solution is lower and the pH value from the isoelectric point of BSA is farther, the anti-pollution effect of the membrane will be better.
![Figure 7
Synthesis route of copolymer P(NIPAAm-co-Am) [82].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_007.jpg)
Synthesis route of copolymer P(NIPAAm-co-Am) [82].
Although the aromatic polyamide-RO membrane had good anti-pollution effect, it required a stricter external environment. Dong [83] successfully prepared a thermosensitive polysulfone (PSF) switch membrane (Figure 8). PNIPAAm was grafted on the surface of a chloromethylated polysulfone membrane via surface-initiated atom transfer radical polymerization (SI-ATRP). Experiments showed that the rejection rate of the membrane at room temperature was higher than that at 40°C, which was because at room temperature, the PNIPAAm polymer chains grafted on the surface of the switching membrane exhibited an irregular elongation, which would fill the membrane pore spaces and increase the BSA rejection of the switching membrane. At 40°C, the PNIPAAm polymer chain presented a curled cluster structure, which would release membrane pore spaces and reduce the BSA rejection rate of the switch membrane. The switch membrane can intercept BSA at room temperature. The interception process does not require energy consumption to change the temperature or ion concentration of the solution.
![Figure 8
Preparation of thermal responsive PSF membrane [83].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_008.jpg)
Preparation of thermal responsive PSF membrane [83].
In addition to anti-pollution research, some scholars have also developed temperature-sensitive membranes with good salting out and universality. Moribe et al. [84] used PNIPAAm, poly(ethylene glycol) diacrylate, acrylic acid, N-tert-butylacrylamide, N-[3-(dimethylamino)propyl] methacrylamide, and other materials to polymerize temperature-responsive hydrogel membranes. At low temperatures, those membranes absorbed salts, while at high temperatures, they released salts. Hydrogel membranes absorb and release salts by controlling swelling ratio or particle size, which can be regenerated through low-temperature waste heat of factories or thermal power plants and can be applied to the energy-saving desalination of seawater. In addition, Yu et al. [85] did more in-depth research on rapid salt-capture using a temperature-responsive hydrogel smart membrane consisting of thermosensitive PNIPAAm NPs (Figure 9a). At high temperatures, acids and alkali “imprint” in NPs, so they cannot capture counterions or components. At low temperatures, NPs swell and denature from imprinted structures, providing counterions to absorb salts. When acids and alkali regenerate during the heating process, the absorbed salts are released (Figure 9b). During heating and cooling cycles, the membrane captured up to 468 μmol/g of NaCl, which could then be used to almost completely desalt the solution (>90%). Such membranes can be used to solve the problem of serious water shortage in the world.
![Figure 9
(a) Experimental schematic diagram of membrane; (b) proposed mechanism for reversible NaCl absorption by the membrane [85].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_009.jpg)
(a) Experimental schematic diagram of membrane; (b) proposed mechanism for reversible NaCl absorption by the membrane [85].
In other fields, Ding et al. [86] developed a temperature-responsive plasma membrane. PNIPAAm was transplanted onto a gold mirror through an ionic body membrane and the scattered gold nano-ions were placed on top. When the temperature rose above the critical hydration temperature, PNIPAAm shrank rapidly (up to 90%) and was completely reversible throughout the phase transition. That is, as the temperature drops, the volume of PNIPAAm will expand rapidly. Then, the NPs were separated from the mirror through the ionic membrane using the phase transition characteristics of PNIPAAm, so that the plasma membrane showed a strong color change. Such membranes can quickly change color, which can be applied to large-area wallpapers, video walls, and sensors.
In short, temperature-sensitive membranes have a high degree of intelligence, whose difference with other forms of smart membranes is that they can respond rapidly to temperature changes, so as to achieve the purpose of real-time monitoring. At present, research on temperature-sensitive smart membranes is about the following three points: enhance their antifouling ability by modifying the membranes; use NPs to improve the salt evolution of the membranes; use color change to expand their application fields. Temperature-responsive smart membranes have also been applied to membrane filtration [87], energy-saving regeneration, and catalytic reactions [88]. However, most of the temperature-sensitive materials used in the experiment are relatively monolithic. It is recently suggested to strengthen research on poly(N,N-dimethyl acrylamide) (PDMA) and polyacrylamide (PAM).
3.2 Light-responsive smart membrane
The molecular conformation and morphology of light-responsive smart membranes will change under illumination with different wavelengths. The photosensitive components of the membranes are composed of azo derivatives, peptides, spiropyran, and triphenylmethane derivatives. The membranes can adjust air humidity, reduce carbon emissions, and reduce energy consumption. In addition, the permeability and humidity performance of those light-responsive membranes can be changed by controlling the luminous flux of light.
Earlier, Park et al. [89] synthesized the primary light-responsive smart membrane using methyl methacrylate, which was replaced by spiropyran and grafted on the surface of a porous glass filter (Figure 10). The glass filter was cultured in 0.1 M HNO3 at 70°C for 3 h, washed several times with distilled water, and then dried in vacuum at 140°C for 6 h. After being irradiated with UV light, the graft chain would shrink, the pores would open in the surface of the glass filter, and then the permeability would be increased. If visible light is used, the graft chain will extend, covering the pores of the glass filter and reducing the permeability, which is because the photoisomerization of photochromic molecules will cause structural changes in the macromolecular chains when combined with them. Smart membranes are used in the development of optical modulation devices now. However, their light sensitivity is low, so their application range will be limited.
![Figure 10
Preparation scheme of glass filter [89].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_010.jpg)
Preparation scheme of glass filter [89].
In order to solve the problem of low photosensitivity and poor response performance of the above smart membranes, Liu et al. [90] developed a kind of light-responsive smart membrane (Figure 11), which was made by attaching azo derivatives to porous silicon pores. 4-(3-triethoxysilylpropylureido)azobenzene (TSUA) was synthesized using triethoxysilylpropyl isocyanate and 4-phenylazoaniline. Brij56(C16H33(OCH2CH2)nOH, n ∼ 10) (0.27 g) and TSUA (0.26 g) were dissolved in ethylalcohol containing tetraethyl orthosilicate, then ultrasonic treatment was completed with HCl for 3 min, aged in mixed sol for 30 min, and the membrane was prepared through impregnation method at room temperature. Under UV irradiation, azo derivatives were efficiently converted into cis isomers; while without it, the azo derivatives can be completely and reversibly converted into trans isomers. In the smart membrane, the characteristics of azo derivatives were utilized to realize the optical control of pore size, which could be applied to light-regulated mass transport.
![Figure 11
Schematic diagram of preparation of membrane composites [90].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_011.jpg)
Schematic diagram of preparation of membrane composites [90].
In addition, some scholars had also made a specific division of the photosensitive range of smart membranes. For example, Guo et al. [91] prepared a Ga2O3:Cr vermicular nanowire membrane on an a-Al2O3(0001) substrate via pulsed laser deposition. The experiment showed that the photocurrent of the membrane under 365 nm UV irradiation was lower than that under 254 nm UV irradiation, which was because there were many oxygen vacancies in the nanostructure of the membranes in the defect state. Under 365 nm UV irradiation, the electrons trapped in the defect state will jump to the conduction band (CB). However, under 254 nm UV irradiation, they will jump from the valence band (VB) to the CB. After turning off the illumination, electrons at the CB will annihilate rapidly. Such membranes have deep ultraviolet photoelectric responses and can be used in the field of magnetic-optical-electric multifunctional nanodevices. Further, they also have the room temperature anisotropic ferromagnetic behavior and the characteristics of easy axes perpendicular to the plane characteristics of membranes.
In addition to the research on primary and specific optical responses, some scholars also studied the energy conversion and large-scale manufacturing methods of light-responsive smart membranes. For example, Jia et al. [92] prepared a (Y2O3:Yb–Er)/Bi2S3 composite membrane under near-infrared light excitation using SILAR (continuous ion layer adsorption) and electrodeposition (Figure 12a). The membrane was composed of uniform Y2O3 crystal particles and covered by Bi2S3 NPs. Under the excitation of 980 nm laser, photons were converted into visible light emission at the Y2O3:Yb–Er membrane layer through up-conversion. Then, the covered Bi2S3 NPs absorbed visible light and produced photoelectrons (Figure 12b). This smart membrane not only had light-response performance, but could also convert light into energy. Being important for breaking the Shockley–Queisser limit (solar energy conversion efficiency limit), the membrane was used to collect near-infrared radiation of photocatalysts, solar cells, and infrared photoelectric detectors.
![Figure 12
(a) Schematic representation of the fabrication process for the thin composite membrane; (b) energy diagram of the energy transfer process and emission process of the composite membrane [92].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_012.jpg)
(a) Schematic representation of the fabrication process for the thin composite membrane; (b) energy diagram of the energy transfer process and emission process of the composite membrane [92].
In order to realize the large-scale production of the photosensitive membranes, Li et al. [93] used electrospinning technology to make a light-responsive N-carboxy spiropyran (SP–COOH)/PAN smart fiber membrane. The SP–COOH was mixed with PAN (mass fraction of 15%) (Figure 13). Based on the photoisomerization characteristics of spiropyran, the wettability and surrounding humidity of the electrospun membrane were reversibly adjusted by alternating UV-visible light irradiation. Experiments showed that the wettability of the fiber membrane surface was positively correlated with the surrounding humidity under alternate irradiation. When the doping amount of SP–COOH was 10%, its surface wettability changed by about 16° and the humidity adjustment range was about ±6%. Such membranes can be produced on a large scale through electrospinning technology, which have the potential to widely control air humidity.
![Figure 13
Synthesis route of SP–COOH [93].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_013.jpg)
Synthesis route of SP–COOH [93].
In addition to the above progress, light-responsive smart membranes have also been applied to anti-counterfeiting materials [94,95], biological imaging [96], optoelectronic micro-/nanodevices [97], and sensors [94,98,99,100]. For example, Tian et al. [100] studied the gold nanocarbon-based membrane with high luminescence properties. The smart material used in the membrane was gold nanoclusters, which was a new type of fluorescent nanomaterial. The discrete electronic energy and direct electronic transition make this smart material have excellent optical properties, whose application has potential prospects in photoluminescence and electrochemiluminescence temperature sensors. Ma et al. [99] studied the organic–inorganic hybrid ultrathin membrane, whose key materials were fluorescent brightener BBU and Mg–Al layered double-hydroxide nanosheets. This membrane had reversible luminescence responses to nitroaromatic explosive compounds, which, moreover, could also be applied to the other two color luminescence systems. When these systems interact with explosives, the luminescence intensity and proportional fluorescence will change. Such membranes can be used as novel selective solid-state luminescent sensors for nitroaromatic compounds.
In recent years, related research on light-responsive membranes has been a hot topic. Three progresses have been made in a short time, namely, the discovery of a primary light-responsive membrane, the completion of its energy conversion, and the realization of large-scale manufacturing of such light-responsive membranes. Light-responsive smart membranes can store large-capacity information with a low transmission loss, which are not susceptible to electromagnetic interferences. It is possible to apply them on a large scale for optical information storage and optical switch. In addition, light-responsive smart membranes have been applied to recognition sites [101] and protein interception and self-cleaning [102], which have broad market prospects.
3.3 Electric-field-responsive smart membrane
Under the action of the electric field, electric-field-responsive smart membranes change the conformation of conductive materials, and their properties will also be changed. In recent years, most research results have been applied to membrane fouling [103], biosensors, protein separation [104], and other fields.
To solve the problem of membrane fouling, Xu et al. [105] synthesized a poly(2-acrylamide-2-methyl-1-propanesulfonic acid) (PAMPSA)-doped polyaniline (PANI) membrane using phase inversion method. Conductive PANI was used as the membrane material and PAMPSA was introduced into its molecular chain structure. The rejection rate of the membrane to polyethylene glycol (neutral substance) in the electric field was reduced by about 10%, and the BSA concentration in the cleaning solution increased from 4.97 × 10−3 g/L in 30 min to 5.39 × 10−2 g/L in 120 min, and the recovery rate of membrane flux was 46.6% (Figure 14), which was because the applied voltage could change the structure of the molecular chain of the membrane materials, resulting in a change in the free space inside the membrane. The applied electric field would change the properties of the membrane materials and the charge on the membrane surface, so that the interactions between the membrane and the pollutants would be changed. Then, membrane fouling was removed from the conductive PANI membrane surface by changing electric field. In this smart membrane, membrane materials are combined with the electric field, so that it can change the selective permeability under the action of the electric field. Therefore, the membrane can be used as an anti-pollution material for new types of membrane bioreactors.
![Figure 14
BSA concentration in the wash solution of Memb-PAMPSA with time [105].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_014.jpg)
BSA concentration in the wash solution of Memb-PAMPSA with time [105].
In addition to the above membrane fouling problems, other scholars also studied the selective permeability of membranes. Hung et al. [106] prepared an electrically responsive smart membrane with materials such as PVDF and graphene. Experiments showed that when a voltage was applied, the flux of MeOH decreased significantly, but as the voltage was further increased, it tended to remain unchanged. When the voltage was removed, the performance of the smart membrane was restored, which was because the β-phase PVDF expanded and deformed mainly through electric drive, thereby forming adjustable nanochannels at the interface between organic and inorganic materials. With the voltage applied on graphene, the free volume of electroactive PVDF would increase, causing defects at the interface to begin decreasing, so that the size of the nanochannels could only allow water to pass through. The membrane shows excellent performance in methanol dehydration.
Widakdo et al. [107] synthesized an electrically responsive smart membrane with piezoelectric properties using materials such as ionic liquid (IL), PVDF, and graphene. By applying electrical stimulation, the permeability of the smart membrane to CO2 increased by about three times, but when the voltage was removed, it almost returned to the initial value, which was because the nanochannels of PVDF and graphene were filled with ILs. IL is a material that can promote CO2 transport, which is a neutral combination of charged ions. After applying a voltage, anions and cations will move, thereby changing the spatial distribution of ions, whose movement alters the vacancies among them and further regulates the activity of CO2. Although after the application of an electric field, with the increase in the free volume of PVDF, O2, N2, and other gases in the field also show an increasing trend in permeability. But as the voltage increases, the permeability does not change significantly. The permeability of the membrane increases with the increase in selectivity, which can be selectively applied to gas separation and sensors, providing alternative ideas for the future development of active separation membranes.
Compared with the most deeply studied pH and temperature response directions, electric-field-responsive smart membranes have a faster response speed and less influence on the properties of the main solution. Electric-field-responsive membranes have a wider response range than a light-sensitive system. A material with electrical stimulation response characteristics is used to make such membranes, which not only inherits the advantages of traditional membrane materials, but can also respond to external voltages.
3.4 Magnetic-field-responsive smart membrane
So far, there is little research on magnetic-field-responsive smart membranes. To fill the vacancy, Lin et al. [108] made a kind of novel magneto-hydrogel pore-filled composite membrane through in situ reactive pore filling (Figure 15). Magnetic nanoparticles (MNPs) were used as a localized heater, which could be excited by a high-frequency alternating magnetic field (AMF), and a PNIPAAm hydrogel network was used as the sieving medium as well as the actuator. Polyethylene terephthalate was used as the carrier to functionalize the reactive pore filling of magnetic nanofilms. This kind of smart membrane manipulated the external AC magnetic field to control remotely, and the membrane could be used in biomedical and microfluidic fields in the future [109]. Zheng et al. [110] synthesized a magnetically responsive and flexible superhydrophobic photothermal membrane using carbonyl iron, polydimethylsiloxane (PDMS), carbonyl iron (Fe), and candle soot, which could be perfectly adsorbed by all types of substrates under the action of a magnetic field without requiring any adhesive. This is because carbonyl iron (Fe) particles are ferromagnetic materials that can be magnetized rapidly in a magnetic field. The carbonyl iron is uniformly coated on the substrate of a membrane, so that the membrane has a magnetic response and super hydrophobicity. Spherical carbonyl iron (Fe) will be wrapped with PDMS and aggregated into micron-sized papillae on the membrane surface, granting the membrane surface an ultra-high roughness. A rough membrane surface will make a membrane stay in the Cassie–Baxter metastable state, and show up its super hydrophobic properties. The fact that such membranes can be adsorbed through high-voltage transmission lines provides ideas for solving the icing problem of transmission lines. Although the research progress of magnetic-response smart membranes is slow, it is still further concerned and valued because of its significant economic value. Such membranes will enter a period of rapid development in theory and practice.
![Figure 15
Schematic diagram of new magnetic hydrogel porous composite membrane [108].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_015.jpg)
Schematic diagram of new magnetic hydrogel porous composite membrane [108].
As a new kind of functional membrane, excellent achievement has been made on physical-stimuli-responsive smart membranes in recent years. At present, in addition to the abovementioned most widely used smart membranes, use of other types of physical-stimulus-responsive smart membranes are also increasing year by year. For example, humidity-responsive smart membranes respond to the stimulation of the external environment through humidity-responsive fluorescence) [111,112]. Physical-stimuli-responsive smart membranes can rapidly change their performance according to external physical stimuli, which have a wide range of types in favor of adapting to various complex environments. This kind of membranes effectively solve problems including a poor adaptability to the environment, high prices, unfavorable production, and environmental pollution caused by conventional membranes. It can be seen from Tables 3 and 4 that physical-stimuli-responsive smart membranes have a wide range of applications, whose performance is altered according to the stimuli such as temperature, light, electric field, and magnetic field, considerably improving their use efficiency. However, some smart membranes are still insufficient in material types, technical research and practical application.
Smart membrane types and the specific modification methods
| Response performance | Smart membrane type | Membrane-forming form: the specific modification | Characteristic | Ref. |
|---|---|---|---|---|
| Temperature | Smart track membrane | Physical way: peroxide pre-irradiation grafting | The membrane has a temperature sensitive layer and track hole, but the membrane lacks research on anti-pollution, salting-out, and universality. | [81] |
| Aromatic polyamide-RO membrane | Chemical way: modification | At low temperature, the membrane has strong hydrophilicity and anti-fouling properties, but the higher the ion concentration and the closer the pH value to the isoelectric point of BSA, the weaker the anti-fouling property. | [82] | |
| Thermosensitive PSF switch membrane | Chemical way: surface grafting | The membrane has strong anti-fouling property and can intercept BSA at room temperature. | [83] | |
| Temperature-responsive hydrogel membranes | Physical way: polymerization | The membrane can be regenerated by low-temperature waste heat and can be applied to energy-saving desalination of seawater. | [84] | |
| Temperature responsive hydrogel smart membrane | Physical way: polymerization | The membrane can almost completely desalt the solution (>90%), which can be used to solve the problem of water shortage in some areas. | [85] | |
| Temperature-responsive plasma membrane | Physical way: transplantation | The membrane can quickly change color according to temperature, which can be applied to large area wallpaper, video wall, and sensor. | [86] | |
| Light | Primary light-responsive smart membrane | Chemical way: surface grafting | The smart membrane can be used in the development of optical modulation devices, but the light sensitivity of the smart membrane is low. | [89] |
| Light-responsive smart membrane | Physical way: impregnation | The membrane can realize optical control of pore size and can be applied to light-regulated mass transport. | [90] | |
| Ga2O3:Cr vermicular nanowire membrane | Physical way: Deposition | The membrane has obvious deep ultraviolet photoelectric response, which can be applied to the field of magnetic-optical-electric multifunctional nanodevices. | [91] | |
| (Y2O3:Yb–Er)/Bi2S3 composite membrane | Physical way: coverage | This smart membrane not only had the light response performance, but also converted light into energy. | [92] | |
| SP–COOH/PAN smart fiber membrane | Physical way: mixing | This membrane can be mass-produced by electrospinning technology and has great potential in controlling humidity. | [93] | |
| Gold nanocarbon-based membrane | Physical way: layer-by-layer assembly | The membrane has excellent light response performance and can be applied in the field of temperature sensor. | [100] | |
| Organic–inorganic hybrid ultrathin membrane | Physical way: layer-by-layer assembly | This membrane has reversible luminescence response to nitroaromatic explosive compounds. These membranes can be used as novel selective solid-state luminescent sensors for nitroaromatic compounds. | [99] | |
| Electric field | PAMPSA doped PANI membrane | Chemical way: phase conversion method | The membrane can be used as an anti-pollution material for a new type of membrane bioreactor. | [105] |
| Electrically responsive smart membrane | Physical way: self-assembly | The membrane exhibits excellent performance in methanol dehydration. | [106] | |
| Electrically responsive smart membrane | Physical way: assembly | The membrane can improve the permeability and can be applied to gas separation and sensors, and provide alternative ideas for the development of active separation membranes in the next step. | [107] | |
| Magnetic field | Novel magneto-hydrogel pore-filled composite membrane | Physical way: filling method | This kind of smart membrane can manipulate external AC magnetic field for remote control, which can be applied in the field of biomedicine and microfluidics in the future. | [108,109] |
| Magnetically responsive and flexible superhydrophobic photothermal membrane | Physical way: adsorption | The membrane has excellent magnetic response and super hydrophobicity, and can be adsorbed on high-voltage transmission lines, providing ideas for solving the problem of transmission line icing. | [110] |
Performance comparison of physical stimuli-responsive smart membranes
| Category | Principal raw material | Performance characteristics | Application area | Development direction | Ref. |
|---|---|---|---|---|---|
| Temperature- responsive smart membrane | NIPAM, PNIPAAm, etc. | Controlling membrane pore size and permeation rate according to temperature change, so as to carry out sewage discharge and permeation, etc. | Membrane filtration, energy-saving regeneration, catalytic reaction, etc. | At present, most of the temperature-sensitive materials used in experiments are relatively single. It is suggested to strengthen the research on PDMA and PAM. | [81,82,83,84,85,86,87,88] |
| Light- responsive smart membrane | Azo derivatives, peptides, spiropyran, triphenylmethane derivatives, etc. | Controlling light application and release to change membrane permeability and humidity. | Identification sites, self-cleaning, etc. | Research depth is shallow, the advantages of optical information storage and optical switch are large. | [89,90,91,92,93,94,95,96,97,98,99,100,101,102] |
| Electric field-responsive smart membrane | PANI, PVDF, etc. | Fast response, wide range, selective transmission through voltage-controlled membrane. | Membrane fouling, biosensor, protein separation, etc. | Great advantages in mineral recovery, protein separation, and desalination. | [103,104,105,106,107] |
| Magnetic-field -responsive smart membrane | MNPs, PNIPAAm, etc. | External AC magnetic field can be manipulated for remote control. | Biomedical and microfluidic fields. | Higher economic value, broad future prospects, but less basic research. | [108,109,110] |
4 Chemical stimuli-responsive smart membrane
The chemical stimuli-responsive smart membranes have a variety of types. As a new type of functional membranes, compared with physical-stimuli-responsive smart membranes, chemical-stimuli-responsive smart membranes are mostly used in the field of medicine and water resource treatment, which are mainly composed of pH-responsive, specific molecular-recognition-responsive and ion-responsive smart membrane materials.
4.1 pH-responsive smart membrane
pH-responsive smart membranes are a kind of functional membranes, on whose pore surface or pore path surface polyelectrolyte switches are constructed [113]. A polyelectrolyte switch contains ionizable weak acid or alkali groups. The configuration of smart membranes changes with the strength of pH, which results in changes in their water flux and hydrophilicity. Therefore, pH-responsive smart membranes have become a research direction for scholars.
Jiang and Wu [114] prepared a pH-responsive microporous membrane. The copolymer EC0.4-g-PDEAEMA47 was synthesized through ATRP, and pH-responsive functional segments were added to ethyl cellulose. Experiments showed that when the pH was 2.0, the water flow of the microporous membrane was almost 0, but when the pH was 6.0, it was greatly improved. When pH was 10.0, the water flux further increased, which was because when the pH began increasing, PDEAEMA would gradually protonate, and the chain segments would gradually curl up. The micropore area will gradually increase, making the water flux of the membrane increase. Responsive functional segments are added to natural polymers through the membrane, which is an approach of great significance in expanding the application fields of chitosan (CS) and cellulose. But a disadvantage of this membrane is that its pH sensitivity is affected by many factors such as chain length, density, and porosity. In addition, the method of introducing responsive forging chains is complex and the requirements for the process are high. At present, researchers have not yet obtained suitable process parameters, which are not conducive to industrial production.
Given the above difficulties in industrial production, Ma et al. [115] invented a method to prepare pH-responsive polymer membranes on metal surfaces. Thioacetal molecules containing halogen at the α-position of the terminal group were assembled on a clean metal substrate surface (Figure 16), which was placed in a monomer solution containing catalysts at a concentration of 1 μM. After washing with anhydrous ethanol and drying with nitrogen, a metal substrate with pH-responsive polymer membranes was prepared. When the pH value of the solution was greater than a certain value, the polymer membrane showed no obvious change. When it was less than a certain value, the polymer membrane could quickly desorb from the metal surface. This phenomenon proves that such smart membranes can be used as a carrier material for targeted delivery, which can be applied to drug delivery and histological engineering. The invention is completed through the process manufacturing method, which is beneficial to industrial production, but the pertinence of this membrane is poor.
![Figure 16
Preparation method of halogen-containing thioacetal molecules at terminal α position [115].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_016.jpg)
Preparation method of halogen-containing thioacetal molecules at terminal α position [115].
In order to solve the problem of poor pertinence of smart membranes, Piyal et al. [116] prepared a pH-responsive PSF membrane using phase inversion technology (Figure 17), which was hydrophilic and pH-responsive by mixing humic acid (HA) and polyethylene glycol methyl ether, making it specifically used to recover H2SO4. When the pH value was 4–12, the pure water flux (PWF) of the membrane was 113.8–46.8 L/m2 h, and its water absorption rate was 25.9–6.8%, which was because at a low pH, carboxyl ions (–COO–) present in HA were protonated to –COOH groups, increasing the hydrophobic interaction, causing the membrane surface to shrink, leading to an increase in pore size. However, a higher pH results in a higher charge density on the polymers, which leads to the dissociation of carboxyl groups into carboxylate –COO– and H+ ions, prompting the membrane skin layer to expand, resulting in a decrease in pore size. The highest recovery of H2SO4 was 76.57 ± 1.5% at a pH of 8.4 with 0.32 M NaCl and 0.5 M KHCO3, which was because the pore size expanded at a low pH, while a large pore size promoted the penetration of all molecules. At a high pH, the average pore size decreases, so that H2SO4 with the smallest average particle size can quickly pass through. However, NaCl and KHCO3 with larger average particle sizes cannot permeate such membranes, but they can alleviate the ecological pollution caused by industrial wastewater to a certain extent.
![Figure 17
Reaction mechanism diagram of pH-responsive membrane prepared by the bonding of PSF with (2:1, polyethylene glycol:HA) [116].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_017.jpg)
Reaction mechanism diagram of pH-responsive membrane prepared by the bonding of PSF with (2:1, polyethylene glycol:HA) [116].
In addition to the above single response, pH is often combined with temperature or voltage. The above smart membranes have a dual response. Liu [117] prepared a smart switching membrane with temperature and pH response (Figure 18). PVDF was taken as the substrate material. A dual-response P4VP core/NIPAM shell microgel was prepared through secondary free radical polymerization. The microgel was uniformly embedded on the surface and pore surface of the membrane. Results showed that the water flux of the membrane increased with the increase in temperature and pH, which was because the temperature and pH response performance of the membrane was mainly controlled by the microgels on its pores. As the temperature increases, intermolecular hydrogen bonds are formed between the amide group and the carboxyl group. The hydrophobicity of the PNIPAM group is enhanced, which results in the volume shrinkage of microgels and the opening of membrane pores. When the temperature decreases, hydrogen bonds are formed between amide group and water molecules, resulting in the volume expansion of microgels and the closure of membrane pores. When the pH increases, the pyridine group is deprotonated, resulting in the shrinkage of the microgel volume and the opening of membrane pores. When the pH decreases, the protonation of N atoms on the pyridine causes an electrostatic repulsion among the polymer chains, resulting in an increase in the volume of microgels and the closure of membrane pores. The membrane has a dual response to temperature and pH, which can be applied to the experimental design of polymer phase separation and self-assembly.
![Figure 18
Fabrication strategy of thermo- and pH-responsive smart gating membranes [117].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_018.jpg)
Fabrication strategy of thermo- and pH-responsive smart gating membranes [117].
Yang et al. [118] studied the phenomenon that biological cell membranes controlled ion flow after the implantation of nuclear pore complexes, who then developed a nanopore etching membrane with pH- and voltage-reversible gating. According to experimental results, when the pH value was 7.9, the nanopores were closed at various voltages, and the ion flux was close to 0. When the pH value was 5.3, the nanopores were opened, and the ion flux approximately increased quadratically with an increase in the voltage. When the pH increased, the DNA chains were negatively charged, and the repulsive interaction among the chains would extend them to the center of the nanogate. Subsequently, DNA strands are connected through hydrogen bonding interactions, a DNA strand network is constructed, and the nanopores are closed. On the contrary, when pH decreases, the number of negatively charged phosphate groups decreases by tens of times. In addition, there is no hydrogen bond formation, thus various chains cannot be connected, and the nanopores are opened. By increasing the voltage at this time, the movement of the ion current increases. This smart membrane, modified by DNA chains, has a high selectivity and more easily meets various application requirements than other smart membranes.
As one of the many kinds of smart membranes, pH-responsive smart membranes have unique advantages in fine control, which are applied to polymer phase separation, self-assembly, and nano-scale control by combining temperature with voltage. At present, other scholars have obtained corresponding research results in the field of packaging materials [119,120], material separation [121], anti-pollution [122], and drug-controlled release [123].
4.2 Specific molecular recognition-responsive smart membrane
Specific molecular recognition-responsive smart membranes can change their structure to cope with changes in the external environment, which can not only realize the control and release of substances, the “start/stop” control of chemical reactions, as well as the rapid detection and separation of substances, but also has a low energy consumption and a high efficiency in the process of identifying specific molecules. Therefore, such membranes have attracted the attention of researchers.
Zhang [66] invented a molecular recognition-responsive smart switch membrane (Figure 19). The functional silica sol was dip-coated on the support for 20 s. Before heat treatment, it was dried in closed water vapor at 50°C for 15 h and dried naturally for 24 h. Then, the puerarin in the functional silica sol was eluted with 80% ethanol solution to obtain the switch membrane. Experiments showed that the amount of puerarin solution passing through the switch membrane with the same content was higher than that of rutin solution. The permeation amount of puerarin solution and rutin solution through a blank membrane was basically the same (Figure 20), which was because upon the elution of puerarin, a recognition site featuring a functional group would be created through the colloidal particles at the position of puerarin, whose size and shape matched with puerarin. A large number of recognition sites are superimposed to form channels, whose entrances are the membrane surface pores. The volume of pores on the membrane surface is the same as that of puerarin. Moreover, there will be a strong ionic reaction between puerarin and complementary functional groups at the recognition sites. Under the action of pressure difference, a bonding–dissociation–rebonding process will be formed with puerarin when passing through the channels. Therefore, the puerarin solution can be efficiently and accurately separated by the membrane, which can be widely used in the preparation of sensors and novel membrane reactors as well as the separation of chiral drugs, etc.
![Figure 19
Structural diagram of separation membrane [66].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_019.jpg)
Structural diagram of separation membrane [66].
![Figure 20
Comparison diagram of permeability of puerarin solution and rutin solution in switch membrane and blank membrane [66].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_020.jpg)
Comparison diagram of permeability of puerarin solution and rutin solution in switch membrane and blank membrane [66].
By observing the aggregation phenomenon of DNA-PNIPAM, Sugawara et al. [124] prepared a DNA-PNIPAM molecular recognition-gated membrane (Figure 21), on which a conjugated polymer composed of thrombin binding aptamer (TBA) and PNIPAM was grafted onto the pore surface. The pores of the membrane opened and closed by controlling the expansion and contraction of the grafted polymer. In the initial state, double-stranded DNA (dsDNA) contains a large number of negative charges. Due to the strong electrostatic repulsion among DNA chains, the grafted PNIPAM expands and the membrane pores are closed. When thrombin binds to the TBA, dsDNA is dissociated into single-stranded DNA, whose charge is relatively less than that of dsDNA, and then the grafted PNIPAM shrinks and the pores are opened. Through the smart membrane, thrombin molecules are specifically recognized by TBA (Figure 22), which can be used as a sensor and drug delivery system in environmental and medical applications.
![Figure 21
Preparation process of gating membrane [124].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_021.jpg)
Preparation process of gating membrane [124].
![Figure 22
Schematic of molecular recognition gating membrane using DNA aptamer [124].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_022.jpg)
Schematic of molecular recognition gating membrane using DNA aptamer [124].
In addition to the research results of the above scholars, Teng et al. [125] also developed a specific molecular-recognition membrane with a gradual microchannel (Figure 23), which was for the specific molecular recognition and separation of phenols and aniline compounds. PVDF was taken as the skeleton. Functional hyperbranched polyether amine (hPEA) was coated on the membrane surface through the combination of crystallization and diffusion, and then well-arranged and interconnected zigzag holes were formed on this membrane. Experiments showed that by introducing amphiphilic hPEA polymer chains, the water permeability of the smart membrane increased by 3.2 times, while the contact angle was reduced by about 4 times. Through the strong interaction between hPEA and aromatic compound functional groups, the membrane has a high sensitivity recognition and separation characteristics in terms of phenols and anilines. The smart membrane can be used to separate carcinogenic and mutagenic toxic components, which is suitable for drug engineering or medical applications.
![Figure 23
Filtration diagram of molecular recognition membrane [125].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_023.jpg)
Filtration diagram of molecular recognition membrane [125].
To solve the problem of CO2 separation, Zhang et al. [126] synthesized a PVDF/PVDF-g-PDMAEMA-blended ultrafiltration membrane with CO2 stimulus responsiveness, whose main raw materials were PVDF and PDMAEMA. The results showed that the PWF of the membrane in a CO2 atmosphere was less than 10% of the maximum value in an N2 atmosphere, which was because poly PDMAEMA was a CO2-responsive polymer material. When CO2 was introduced into the system, the PDMAEMA segments in the membrane pores underwent a protonation reaction and exhibited an extended state, causing the membrane pore size to shrink, resulting in a reduced water flux. When N2 was introduced, N2 would completely discharge CO2, which reacted with PDMAEMA. The PDMAEMA chains inside the membrane pores underwent deprotonation, resulting in a collapsed state of the chains, which would lead to an increase in membrane pore size and membrane flux. This membrane can be used to effectively clean protein fouling on membrane surface by alternately aerating N2/CO2 and converting the hydrophilicity/hydrophobicity of PVDF/PVDF-g-PDMAEMA membranes.
Borgohain and Mandal [127] conducted a more in-depth study on the separation of CO2/N2 using amine-based biopolymers. A methyl chitosan/polyamine-specific molecular membrane was prepared with carboxymethyl chitosan (CMC) as the main material (Figure 24). The experimental results showed that when the purge/feed flow ratio of 10 wt% dendrimer was 2.33 and 1.67 at 90°C, the CO2 transmittance was 100 GPU and the CO2/N2 selectivity was 149, which was because CMC, as a fixed carrier, was distributed in the pores. During the separation of CO2/N2, CO2 would react with adjacent CMC along the channel under the action of driving force to form a complex until it was released. Polyamidoamine can provide a large number of primary and tertiary amine groups. Through the reaction of CO2 with amine, a carbamate cross-linked polymer will be formed, thereby inhibiting the penetration of N2. The tertiary amine groups can react with CO2 to form bicarbonate ions, which helps to transport CO2. This kind of smart membranes can be used to effectively separate CO2, which can solve the problem of energy shortage and global warming.
![Figure 24
Overall CO2 transport mechanism in membrane [127].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_024.jpg)
Overall CO2 transport mechanism in membrane [127].
As one of the hotspots in the field of membrane science, specific molecular recognition-responsive smart membranes have the “intelligence” of integrating chemical information perception, information processing, and response execution. At present, the universal results developed by researchers can be applied to the preparation of sensors and new membrane reactors, the resolution of chiral drugs [128], drug delivery, as well as other aspects. Moreover, smart membranes are developing rapidly in the field of molecular separation [129], drug release [130], drug delivery, and histological engineering [52]. However, the materials and technologies for specific molecular recognition-responsive smart membranes in the world are still at the basic stage of development, which need to be further improved to be widely used in industries or clinics.
4.3 Ion-responsive smart membrane
Ion-responsive membranes are a kind of functional membranes that can respond to the changes in ion type and concentration in the environment. Such functional membranes can be used to quickly identify and respond to specific ions, especially toxic heavy metal ions, which are widely used in water detection [131], drug-controlled release [132,133], and other fields. Therefore, ion-responsive smart membranes have gradually attracted the attention of various technical and professional fields.
In order to realize the detection of lead ions under normal pressure conditions, Wang et al. [134] provided a lead-ion-responsive smart membrane composed of poly(N-isopropylacrylamide-co-benzo-18-crown-6-acrylamide) (PNB) nanospheres at a mass ratio of (12–17):100 with a polyethersulfone membrane substrate. When separating other solutions, the nanospheres in the membrane pores shrank and the membrane pores were opened. When the lead ion solution was detected, the 18-crown-6 group of PNB selectively complexed the lead ions and an electrically-charged complex was formed. The electrostatic repulsion among the charged complex groups will cause the extension of polymer chains, which will cause volume swelling of the nanospheres and make the membrane pores to close. The membrane can be used to detect lead ions in water samples by monitoring the flux change of solution. The pore size of the membrane is micron-sized, which has an interconnected structure. It can be used to detect whether the lead concentration in drinking water, industrial wastewater, and other water samples meets the national standard, effectively preventing and controlling lead ion pollution, which is of great significance to human health and environmental protection.
For the conflicts between flux and response characteristics, that is, the more nano-polymers there are on membrane pores, the better the response characteristics will be, but the effective pore size will become smaller and the flux across the membrane will be reduced. For this reason, Wang et al. [135] developed a new smart membrane with ion-recognizable nanogels as gates, in which three-dimensional interconnected pores were formed via vapor-induced phase separation (VIPS), and PNB nanogel was immobilized on the surface of the membrane pores (Figure 25a and b). When the lead ion solution flows through the membrane pores, a host–guest complex is formed in the nanogels with lead ions. A point repulsive force will appear among the charged composites, resulting in a swelling reaction of the nanogels, which will reduce the pore size of the membrane, resulting in a decrease in its flux (Figure 25c). The nanogels of the membrane are fixed on its pore surface during the formation of interconnected porous structures. Therefore, with the immobilization of membrane nanogels, the effective pore size of membrane pores is not reduced. This unique structure is adopted to obtain high-throughput and lead ion response characteristics for the membrane, through which lead ions in water can be quickly detected with a detection limit of as low as 10 mol/L. The above membrane is suitable for the real-time monitoring of drinking water safety, but its detection ability is relatively simple and mercury in wastewater cannot be detected.
![Figure 25
(a and b) Fabrication of membrane with three-dimensionally interconnected porous structure; (c) the PNB nanogels on the membrane pore surfaces exhibit isothermal swelling after recognizing Pb2+ and form 18-crown-6/Pb2+ host–guest complexes, resulting in the decrease in trans-membrane flux [135].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_025.jpg)
(a and b) Fabrication of membrane with three-dimensionally interconnected porous structure; (c) the PNB nanogels on the membrane pore surfaces exhibit isothermal swelling after recognizing Pb2+ and form 18-crown-6/Pb2+ host–guest complexes, resulting in the decrease in trans-membrane flux [135].
To deal with mercury in industrial wastewater, Esmali et al. [136] synthesized a polyethersulfone-based ion-imprinted membrane (IIM). Ion-imprinted polymer particles were synthesized using acrylamide, acrylonitrile, and ethyl ethylene glycol dimethacrylate free radicals. The polymer particles reacted with bathophenanthroline (BPh) with Hg(ii) ions as the template. After washing the template ion mercury, a three-dimensional recognition site was left on the membrane. The membrane adsorbed mercury ions when they encountered again. The mercury removal capacity and PWF of the IIM were optimized through central composite design and response surface methodology, whose removal rate and PWF reached 98.1% and 37.5 kg/m2 h, and its maximum adsorption capacity was 432 mg/m2 (or 21.6 mg/g), which was approximately four times that of a non-imprinted membrane (5.25 mg/g), meanwhile the Hg(ii) ions could be effectively recovered by at least six times (Figure 26). The smart membrane has a good hydrophilicity and mercury removal performance, which can be used to treat industrial wastewater with different salt contents.
![Figure 26
Adsorption capacity and flux data for stimulated samples of Hg(ii) [136].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_026.jpg)
Adsorption capacity and flux data for stimulated samples of Hg(ii) [136].
In addition to the detection of lead ions and the treatment of mercury ions, some scholars have also developed smart membranes that can identify multiple ion types. Bakangura et al. [ 137 ] prepared QDA-IL/HBA membranes using quaternary ammonium poly(2-dimethylaminoethanol-N-2,3-dimethylphenyl oxide) (QDAPPO) as the main matrix (Figure 27), whose zwitterionic pores were formed via the electrostatic ion repulsion between the imidazole group on organosilane and the carboxylic acid group of 4-(hydroxymethyl) benzoic acid. Colloid swelling or shrinkage was controlled by the side hydroxyl groups on QDAPPO and 4-(hydroxymethyl) benzoic acid. The experiment showed that the proton diffusion coefficient of the membrane was 0.0386 m/h at 25°C, but when it increased with the zwitterionic pores, the diffusion of ferrous ions occurred, because the pores were more critical for a high selectivity. It is suggested to strengthen studies on membrane pore size, balance the number of charged groups in pores and membrane matrix as well as optimize the selectivity of the membrane.
![Figure 27
Preparation method of QDA-IL/HBA membrane [137].](/document/doi/10.1515/ntrev-2022-0538/asset/graphic/j_ntrev-2022-0538_fig_027.jpg)
Preparation method of QDA-IL/HBA membrane [137].
Zhang et al. [138] developed a poly(ionic liquid) (PIL)-modified polyethersulfone membrane via in situ crosslinking copolymerization. Ion exchange occurred when the membrane came in contact with different types of anions, causing a change in its PWF. The initial water flux of the membrane was 20 mL/m2 mmHg. After an ion exchange with KPF6, NaBF4, and KSCN, the PWF of the membrane increased to 75, 35, and 50 mL/m2 mmHg respectively. There was an electrostatic repulsion among the molecular chains of functional materials in pure water, which would diffuse in pure water, resulting in the volume expansion of crosslinked polymers in the membrane, thus reducing the permeability of the membrane and narrowing its pores. When pure water was replaced by solutions such as KPF6, NaBF4, and KSCN, the electrostatic repulsion among molecular chains was shielded by anions and the molecular chains curled, resulting in volume shrinkage of the polymers, increasing membrane pores and permeability. The smart membrane has a strong ability in ion recognition and strong development prospects in chemical/biomedical separation as well as purification.
The development of ion-responsive smart membranes is mainly for the detection of lead ions, while that of smart membranes for identification of other heavy metal ions is relatively backward. It is suggested to increase the detection of other heavy metal ions, so that cadmium, nickel, and other toxic heavy metals can be monitored.
There are a variety of types of chemical-stimuli-responsive smart membranes, which are more widely used in medicine and water resource treatment as well as other fields compared with physical-stimuli-responsive smart membranes. For example, the various types of chemical-stimuli-responsive smart membranes are summarized in Tables 5 and 6. By focusing on smart membranes, their performance can be changed according to the changes in pH, molecules, and ions, so that they can be applied to drug delivery and water detection. With the development of membrane technology, chemical-stimuli-responsive smart membranes need to be improved in nano-control and industrial production.
Smart membrane types and the specific modification methods
| Response performance | Smart membrane type | Membrane-forming form: the specific modification | Characteristic | Ref. |
|---|---|---|---|---|
| pH | pH-responsive microporous membrane | Chemical way: atom transfer radical polymerization | The membrane added responsive functional segments to natural polymers, which is of great significance for expanding the application fields of CS and cellulose, but its preparation method is complex and not conducive to large-scale production. | [114] |
| pH-responsive polymer membrane | Physical way: assembly | The membrane has a process method, which is beneficial to industrial production, but the pertinence is poor. | [115] | |
| pH-responsive PSF membrane | Chemical way: phase conversion method | The membrane can recover H2SO4 from wastewater, which can alleviate the ecological pollution caused by industrial wastewater to a certain extent. | [116] | |
| Smart switching membrane | Physical way: secondary free radical polymerization | The membrane has a dual response of temperature and pH, and can be applied to the experimental design of polymer phase separation and self-assembly. | [117] | |
| Nanopore etching membrane | Chemical way | This smart membrane modified by DNA chain has high selectivity and is easier to meet various application requirements than other smart membranes. | [118] | |
| Specific molecular recognition | Molecular recognition responsive smart switch membrane | Physical way: dipping (bonding) | The membrane can efficiently and accurately separate puerarin solution and can be widely used in the preparation of sensors and novel membrane reactors, the separation of chiral drugs, etc. | [66] |
| DNA-PNIPAM molecular recognition gated membrane | Chemical way: surface grafting | The membrane specifically recognizes thrombin molecules by TBA and can be used as a sensor and drug delivery system in environmental and medical applications. | [124] | |
| Specific molecular recognition membrane | Chemical way: crystallization-diffusion | The smart membrane can separate carcinogenic and mutagenic toxic components and is suitable for drug engineering or medical applications. | [125] | |
| PVDF/PVDF-g-PDMAEMA blend ultrafiltration membrane | Chemical way: blending (surface grafting) | This membrane can effectively clean the protein fouling on the membrane surface by alternately aerating N2/CO2 and converting the hydrophilicity/hydrophobicity of the PVDF/PVDF-g-PDMAEMA membrane. | [126] | |
| Methyl chitosan/polyamine specific molecular membrane | Physical way: blending (bonding) | This membrane has the characteristics of separating CO2, which effectively solves the problems of energy shortage and global warming. | [127] | |
| Ion | Lead ion responsive membrane | Physical way | The pore size of the membrane is micron-sized and has an interconnected structure, which can detect whether the lead concentration in drinking water, industrial wastewater, and other water samples meets the national standard. | [134] |
| Smart membrane | Physical way: Inlay (bonding) | The membrane can quickly detect lead ions in water with a detection limit as low as 10 mol/L. But the detection ability of the membrane is relatively simple and cannot detect mercury in wastewater. | [135] | |
| IIM | Physical way: Copolymerization (bonding) | The membrane has good hydrophilicity and mercury removal performance, which can treat industrial wastewater with different salt content. | [136] | |
| QDA-IL/HBA membrane | Physical way polymerization | The proton diffusion coefficient of the membrane was 0.0386 m/h at 25°C and the membrane was able to recognize multiple ion types. | [137] | |
| PILs modified polyethersulfone membrane | Chemical way: in situ crosslinking copolymerization | The smart membrane has a strong ability in ion recognition and strong development prospects in chemical/biomedical separation and purification. | [138] |
Performance comparison of chemical stimuli-responsive smart membranes
| Category | Principal raw material | Performance characteristics | Application area | Development direction | Ref. |
|---|---|---|---|---|---|
| pH-responsive smart membrane | Copolymer EC0.4-g-PDEAEMA47, PVDF, etc. | Easy to combine with temperature and voltage to extend the application range, commonly used in fine control. | Packing materials, material separation, anti-pollution, drug delivery, etc. | Easy to combine with other factors, has unique advantages in polymer phase separation and self-assembly, nanoscale control, etc. | [113,114,115,116,117,118] |
| Specific molecular recognition responsive smart membrane | DNA-PNIPAM, PVDF, polyallylamine, etc. | Control, release, and rapid detection of substances, “start/stop” control of chemical reactions. | Sensors, chiral drug resolution, drug delivery, etc. | Widely used, but lack of industrial production and clinical research. | [66,124,125,126,127,128,129,130] |
| Ion-responsive smart membrane | PNB, QDAPPO, etc. | Rapid identification and response to specific ions, especially toxic heavy metal ions. | Water detection, chemical/biomedical separation and purification, etc. | Only in lead and mercury ions. More in-depth study of other heavy metal ions is less. | [131,132,133,134,135,136,137,138] |
5 Specific application work of smart membrane
Smart membranes are widely used in membrane filtration, water treatment, self-cleaning, protein separation, water detection, chemical/biomedical separation and purification, drug delivery, packaging materials, energy recovery, catalytic reaction as well as other fields. Their application fields are mainly classified into five categories: environmental protection, medicine, food, energy, and chemical industry, among which they are most widely used in environmental protection, medicine, and food industries.
5.1 Environmental protection
Industrialization and urbanization have brought serious water pollution problems. Wastewater treatment and water detection have become important projects among environmental protection projects. Smart membranes can be used to deeply purify sewage, which, compared with traditional membranes, have not only a higher purification efficiency, but also a better self-cleaning ability. In addition, smart membranes can also be used to detect the pollution components inside water sources, playing an early warning role. For example, proteins, heavy metals, and other pollutants often appear in urban wastewater. For this reason, Su et al. [54] prepared a PAN-based zwitterionic membrane. When the NaCl concentration was lower than 0.04 mol/L, proteins would be adsorbed on the membrane surface or the pore wall, making the membrane channels narrow; when it was higher than 0.05 mol/L, the electrostatic interaction between proteins and sulfobetaine dipoles was weakened, making the hydrophilicity of the membrane enhanced; meanwhile, the adsorption of proteins was weakened, and the membrane channels were opened. The membrane could be used to separate proteins by changing the concentration of NaCl, which played an important role in wastewater treatment. For water detection, smart membranes with ion recognition nanogels can be used as the gate to quickly detect lead ions in water, whose detection limit can reach 10 mol/L [135]. IIMs can be used to effectively recover mercury ions by six times compared with non-imprinted membranes [136]. These two kinds of membranes can be used to detect whether the concentration of lead or mercury ions in water meets the standards, which is of great significance to environmental protection.
5.2 Medicine
Targeted transport and drug separation are the main research directions of smart membranes in medical applications. Researchers have used smart membranes to achieve targeted transport of drugs, proteins, and other substances. The transport of traditional drugs is subject to body fluid circulation, making it difficult to accurately treat the lesion areas. Through smart membranes, not only the substances can be transported to make the drugs reach target positions accurately, but they can also be used to accurately identify the cells or biological macromolecules, so as to achieve drug separation. For example, Hiroto et al. [53] introduced molecular recognition receptors into the submicron-sized pores of a membrane to prepare a biomolecular recognition-gated membrane, which could perform multi-point recognition of target biomolecules, forming cross-linking, thereby controlling the opening and closing of membrane pores. The membrane could be used in antigen-polyclonal antibody systems. DNA-PNIPAM molecular recognition-gating membranes can be used to specifically recognize thrombin molecules through TBA, so that the grafted PNIPAM shrinks and finally the membrane pores open. The membrane can also be used as a drug delivery system in the medical field [124]. For drug separation, puerarin is extracted from the legume Pueraria lobata, with antipyretic, sedative, and expansion of coronary arteries as well as other effect. Molecular recognition-responsive intelligent switch membranes can be used to efficiently and accurately separate puerarin solutions as well as widely used in the separation of chiral drugs [66].
5.3 Food industries
Food safety and food waste are two important challenges in the food industry. There are differences between the shelf life and the actual shelf life of a product. Some food, in the process of storage, may develop microorganisms internally due to some external factors, such as extrusion, perforation, and other reasons. This food decay can affect the health of consumers. Smart membranes can present obvious color changes according to the freshness of food. Compared with traditional packaging, which can only play the role of hygiene and barrier, a smart membrane can more accurately judge the freshness of food [73]. For example, microorganisms produce metabolites that change the pH value of the internal environment of packaging in the process of food spoilage. Water-soluble natural pigment anthocyanins can show different color changes as pH changes. Therefore, Li et al. [72] dispersed purple tomato anthocyanin (PTA) into a CS solution to prepare a PTA/CS composite membrane. The main raw materials used in the membrane were anthocyanins and CS, which were natural materials, and the membrane had a good pH sensitivity and mechanical properties, which could be used as an environmentally friendly packaging material. Due to the unstable nature of anthocyanins, they are susceptible to factors such as temperature, light, oxygen, and metal ions, among which oxygen is the most important factor affecting the performance of anthocyanins. To this end, Zou et al. [139] used mulberry anthocyanin as an indicator, took gellan gum and anthocyanin to form an indicator inner-layer membrane, formed an outer-layer membrane using CS and PVA, and used layer-by-layer assembly technology to prepare a double-layer indicator membrane. Through the membranes, the oxidation of anthocyanins was reduced by isolating oxygen, with their stability improved. The membranes can be used as a freshness indicator for salmon. Compared with monolayer membranes, those membranes have better mechanical properties and a lower water vapor permeability, which can not only reduce the oxidation of anthocyanins to improve their stability, but can also be used for the indication of salmon freshness, which has a good application potential.
6 Future development direction of smart membrane
Smart membranes have shown their unique potential and advantages in the field of antifouling materials [140], specific recognition, drug development [141], detection control, and catalysis due to their special environmental-response ability. In particular, smart membranes have significant commercial value in environmental protection [142,143], medicine [144], energy [145,146], food [147,139], and chemical industry. As researchers and engineers have made more and more extensive and in-depth contributions to membrane science and technologies, the current progress of smart membranes not only contributes to global research and industrial development, but also provides tips for smart membrane innovations. Therefore, we propose the following possible challenges faced by further research.
With the emergence of various international environmental protection measures, people began to focus on environmental protection. For the initial smart membrane, only whether it could respond to external environmental stimuli was considered, and there was no special study on its anti-pollution performance. For example, temperature-sensitive intelligent track membranes have only temperature responses and no anti-pollution property. However, with the optimization of smart membranes, great progress has also been made in the anti-pollution effect of smart membranes. For example, aromatic polyamide RO composite membranes can weaken the connection between pollutants and membrane surface at low temperatures to achieve the anti-pollution effect. By filling the membrane pore spaces using polymer chain at room temperature, temperature-sensitive PSF switch membranes have anti-pollution performance. However, anti-fouling experiments on these two kinds of membranes were only carried out on BSA. Although PANI membranes also have anti-pollution performance, they are not intended to purify environmental pollution. PANI membranes are designed to solve the problem of membrane fouling. Although PSF membranes can also alleviate ecological pollution, they can only specifically recover H2SO4. At present, there has been deep research on anti-pollution only for ion-responsive smart membranes, such as lead-ion-responsive smart membranes can be used to detect lead concentration in water samples, which can prevent ion pollution. Smart membranes with ion-recognition nanogels as a gate has a high sensitivity and selectivity, which can be used to quickly detect lead ions in water. Polyethersulfone-based IIMs have a good hydrophilicity and mercury removal performance. In addition, to identify the types of multiple ions, researchers also developed QDA-IL/HBA membranes and PIL-modified polyethersulfone membranes. However, there are still some problems in the research of ion-responsive smart membranes in antifouling. For example, in the actual water treatment process, there are many kinds of pollutants in water. The sewage often contains acids, alkalis, oxidants as well as heavy metals such as lead, mercury, cadmium, arsenic, benzene, dichloroethane, ethylene glycol, and other organic toxicants. The single antifouling mechanism of smart membranes cannot meet the needs of antifouling performance. Therefore, to promote research on smart membrane antifouling, researchers should deeply understand the characteristics of intelligent materials and the special pore structure of base membranes. Researchers should further study the relationship between membrane performance and structure, enhance the development of various antifouling properties of smart membranes, or develop a variety of antifouling mechanisms to comprehensively construct antifouling defense lines.
From the process point of view, there are higher requirements for the preparation process of smart membranes. For example, through pH-responsive microporous membranes, responsive forging chains are introduced in natural polymers. This process is complex, costly, and non-conducive to mass production. With the development of smart membranes, some progress has also been made in smart membrane technology. For example, pH-responsive polymer membranes have the characteristics of industrial production. Photo-responsive SP–COOH/PAN smart fiber membranes can be mass-produced through electrospinning technology. However, most of the spinning solvents used in electrospinning technology have certain toxicity, which will have a certain impact on the environment. In the process of electrospinning, factors such as air temperature and humidity, solution viscosity, and jet charge density will affect the preparation of nanofibers, making it difficult to control their preparation process for a long time. Nanofibers are main materials of smart fiber membranes. If the preparation of nanofiber membranes cannot be controlled stably for a long time, the production and use of smart membranes will be ultimately affected [148,149]. In addition to considering the preparation process of membranes from the aspect of stability, it is also necessary to consider their use process from the aspect of recycling. For example, during the use of smart membranes, solid particles (proteins, bacteria, etc.) in solutions will adhere to the membrane surface or membrane pores, resulting in a decrease in the separation efficiency of the smart membranes and a shortened service life [19]. To this end, Ye et al. [150] prepared (P(NIPAAm-PEGMA))-grafted polypropylene (PP) porous membranes through plasma initiation method, which not only had temperature-sensitive properties, but also exhibited a good surface hydrophilicity up and down the LCST. The enhancement of hydrophilicity can weaken the adhesion of pollutants and membrane surfaces. After the cleaning of variable temperature water, the water flux recovery rate of the BSA-contaminated smart membrane could reach 98.2%, enough to realize the self-cleaning effect and the service life of the membrane was prolonged. Therefore, researchers need to consider the production and use of membranes from the aspect of stability and recycling at the same time, deeply study the preparation scheme and reaction mechanism of smart membranes, and replace membrane-forming materials with stable and sustainable new intelligent materials. The service life and replacement cost of smart membranes are strictly considered. From the perspective of research direction, the research results of smart membranes are mostly limited to laboratories. In most smart membranes, only functionality is added while field experiments are rarely conducted. Therefore, the research on the production and application of smart membrane technology should be increased, transform it into productivity, and develop an accurate supply-and-demand-relationship optimization scheme.
7 Conclusion
As a new research direction in the field of membranes, smart membranes have attracted the attention of many scholars, which have a strong adaptability to the environment, a wide application range and simple preparation. In this study, smart membranes are reviewed from six directions, namely temperature, pH, molecule, light, ions, and electric field. In view of the current research progress, the characteristics and application of each type of smart membranes are summarized and evaluated in this study:
Among physical-stimuli-responsive membranes, temperature-responsive smart membranes have a deep research level and a wide application range. However, the smart materials currently used are relatively single, and there is a lack of diversified research on synthetic materials. Light-responsive smart membranes have the characteristics including a large information storage capacity and a low transmission loss, which are not susceptible to electromagnetic interferences. However, due to technical limitations, the current research is relatively shallow. Such membranes have strong research value in optical information storage and optical switching. The response speed of electric-field-responsive smart membranes is faster than that of other smart membranes, whose influence on the properties of main solutions is small. Electric-field-responsive smart membranes are often made of materials with electrical stimulation response characteristics, so that they can respond to external voltages while taking into account the advantages of traditional membrane materials. However, the research depth is relatively shallow compared with other smart membranes. Magnetic-field-responsive smart membranes can be remotely controlled by the AMF, which has excellent development prospects in biomedical and microfluidic fields.
Among chemical-stimuli-responsive smart membranes, the pH-responsive smart membranes have been studied more in terms of fine control. They are easy to be combined with other factors, so that they can be applied in polymer phase separation, self-assembly, and nanoscale control. Specific molecular recognition-responsive smart membranes have the characteristics of integrated sensing, information processing, and response execution of physical and chemical information, low energy consumption, and high efficiency. But they lack industrial production research and development. Ion-responsive smart membranes can quickly identify and respond to specific ions, and have unique advantages in dealing with toxic heavy metal ions. Such membranes are mainly used in the detection of lead ions in drinking water and industrial wastewater. At present, scholars are focused only on lead and mercury ions with more in-depth research, while other heavy metal ions need to be explored.
-
Funding information: This work was supported by Science and Technology Department of Liaoning Province (research on the application of high efficiency marine drilling fluid with modified bentonite based on CMC and NIPAM, 2023JH2/101300238) and Key Project (preparation and evaluation on intelligent drilling fluid of the modified bentonite, LJKZ0417).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Wang XS. Membrane separation technology and its progress. Fine Chen. 1984;1(1):4–12. 10.13550/j.jxhg.1984.01.003.Suche in Google Scholar
[2] Cui Y, Wu GX, Gu L. New progress of forward osmosis membrane separation technology in seawater desalination. Techol Water Treat. 2010;36(5):5–9. 10.16796/j.cnki.1000-3770.2010.05.002.Suche in Google Scholar
[3] Tang JW, Wu KH, Lin MG, Wang YD. Research progress of membrane separation technology in waste oil regeneration. Membr Sci Technol. 2010;30(1):103–7. 10.16159/j.cnki.issn1007-8924.2010.01.009.Suche in Google Scholar
[4] Li SP, Fang H, Xu WC, Yan HS. Research progress of integrated membrane separation technology in advanced treatment of sewage(waste)water. Membr Sci Technol. 2011;31(4):100–4. 10.16159/j.cnki.issn1007-8924.2011.04.011.Suche in Google Scholar
[5] Gao JW. Membrane separation technology for wastewater treatment and its study progress and development trend. 4th International Conference on Mechanical Materials and Manufacturing Engineering, 2016, Wuhan, China. Paris: Atlantis Press; 2016. p. 630–2. 10.2991/MMME-16.2016.202.Suche in Google Scholar
[6] Silva LLS, Sales JCS, Campos JC, Bila DM, Fonseca FV. Advanced oxidative processes and membrane separation for micropollutant removal from biotreated domestic wastewater. Envir Sci Pol Res Inter. 2017;24(7):6329–38. 10.1007/s11356-016-7312-y.Suche in Google Scholar PubMed
[7] Ma HF, Liu ZG, Chen YL. Application progress of membrane separation technology in water treatment. China Sci Technol Inf. 2008;14:31–2. 10.3969/j.issn.1001-8972.2008.14.011.Suche in Google Scholar
[8] Chen XR, Su ZG, Ma GH, Wan YH. Study on intelligent separation membrane. Prog Chem. 2006;18(9):1218–26. 10.3321/j.issn:1005-281X.2006.09.017.Suche in Google Scholar
[9] Xie R, Chu LY. Research progress of environmentally responsive intelligent switch films. Membr Sci Technol. 2007;27(4):1–7. 10.16159/j.cnki.issn1007-8924.2007.04.013.Suche in Google Scholar
[10] Chen L, Chu LY. Summary and prospect of intelligent membrane materials and technology. J Filtr. Sep 2007;17(3):1–4. 10.3969/j.issn.1005-8265.2007.03.001.Suche in Google Scholar
[11] Chu LY, Ju XJ, Xie R. Environment responsive self-discipline drug controlled release smart membrane. Proceedings of the 5th National Symposium on Membrane Separation Technology Application in the Pharmaceutical Industry, 2012, Nanjing, China. Jiangsu: China Blue Star; 2012. p. 20. https://chn.oversea.cnki.net/kcms/detail/detail.aspx? FileName = LXJT201204001008&DbName = CPFD2013.Suche in Google Scholar
[12] Zhang ZL, Dong X, Fan YN, Yang LM, He L, Song F, et al. Chameleon-inspired variable coloration enabled by a highly flexible photonic cellulose film. ACS Appl Mater Interfaces. 2020;12(41):1–20. 10.1021/acsami.0c13551.Suche in Google Scholar PubMed
[13] Zheng WW, Huang JY, Li SH, Ge MZ, Teng L, Chen Z, et al. Advanced materials with special wettability toward intelligent oily wastewater remediation. ACS Appl Mater Interfaces. 2020;13:67–87. 10.1021/acsami.0c18794.Suche in Google Scholar PubMed
[14] Sun Q. Preparation of acrylonitrile-sulfonamide copolymer ultrafiltration membrane and its anti-pollution performance, Dissertation. Tianjin: Tianjin University; 2007. https://kns.cnki.net/KCMS/detail/detail.aspx? dbname = CMFD2009&filename = 2008182255.nh.Suche in Google Scholar
[15] Chen C. Preparation and properties of PVDF hollow fiber porous composite membrane, Dissertation. Tianjin: Tianjin Polytechnic University; 2004. 10.7666/d.y627459.Suche in Google Scholar
[16] Wei GH. Preparation and properties of PVDF_GO_g_PNIPAAm thermosensitive membrane, Dissertation. Harbin: Harbin Institute of Technology; 2018. 10.7666/d.D01587256.Suche in Google Scholar
[17] Zhang J, Liu YF, Guo J, Yu Y, Li YF, Zhang XT. A CO2-Responsive PAN/PAN-Co-PDEAEMA membrane capable of cleaning protein foulant without the aid of chemical agents. React Funct Polym. 2020;149:104503. 10.1016/j.reactfunctpolym.2020.104503.Suche in Google Scholar
[18] Qin JW, Fu GB, Xie R, Wang W, Ju XJ, Liu Z, et al. Progress in application of environmental stimuli-responsive smart gating membranes. J Membr Sci Technol. 2020;40(1):294–302. 10.16159/j.cnki.issn1007-8924.2020.01.036.Suche in Google Scholar
[19] Wei R, Guo JB, Jin LQ, He C, Xie Y, Zhang X, et al. Vapor induced phase separation towards anion-/near-infrared-responsive pore channels for switchable anti-fouling membranes. J Mater Chem A. 2020;8(18):8934–48. 10.1039/D0TA02154G.Suche in Google Scholar
[20] Liu YQ, Li Y, Ju XJ, Xie R, Wang W, Liu Z, et al. Progress in lead ion detection technologies based on 18-crown-6. J Chem Ind Eng. 2021;72(1):192–204. 10.11949/0438-1157.20201161.Suche in Google Scholar
[21] He MB, Liu Z, Li T, Chen C, Liu BC, Crittenden JC. Effect of adding a smart potassium ion-responsive copolymer into polysulfone support membrane on the performance of thin-film composite nanofiltration membrane. Front Chem Sci Eng. 2019;13(2):400–14. 10.1007/s11705-018-1757-0.Suche in Google Scholar
[22] Zhang MM. Environmental response intelligent skin based on natural materials design, preparation and performance study, Dissertation. Tianjin: Tianjin University of Technology; 2021. 10.27360/d.cnki.gtlgy.2021.000176.Suche in Google Scholar
[23] Yao KD, Cheng GX. Smart membrane materials. Chem Ind Eng Prog. 2002;21(8):611–6. 10.16085/j.issn.1000-6613.2002.08.023.Suche in Google Scholar
[24] Duarte AP, Bordado JC. Smart composite reverse-osmosis membranes for energy generation and water desalination processes. Smart composite coatings and membranes. Lisboa: Elsevier Ltd; 2016. p. 329–50. 10.1016/b978-1-78242-283-9.00012-9.Suche in Google Scholar
[25] Agency FN. Toward a smart graphene membrane to desalinate water. Sci Daily. Tehran; 2017; p. 1–3. https://www.proquest.com/wire-feeds/toward-smart-graphene-membranedesalinate-water/docview/1934688095/se-2? accountid = 131239.Suche in Google Scholar
[26] Cai YH. Preparation of super-humidity composite membrane and its application in separation of emulsified oily wastewater, Dissertation. Suzhou: Soochow University; 2020. 10.27351/d.cnki.gszhu.2020.003647.Suche in Google Scholar
[27] Jonathan C, Silvia G. Reversible swelling as a strategy in the development of smart membranes from electrospun polyvinyl alcohol nanofiber mats. J Polym Sci. 2020;58(5):737–46. 10.1002/pol.20190156.Suche in Google Scholar
[28] Jiang ZY, Chu LY, Wu XM, Wang Z, Jiang XB, Ju XJ, et al. Membrane-based separation technologies: from polymeric materials to novel process: an outlook from China. Rev Chem Eng. 2019;36(1):67–105. 10.1515/revce-2017-0066.Suche in Google Scholar
[29] Xiao KJ, Zhan T, Chen RJ. Preparation of temperature-responsive PVDF_G_PNIPAM composite membrane by grafting method. Mod Food Sci Technol. 2013;29(1):81–7. 10.13982/j.mfst.1673-9078.2013.01.044.Suche in Google Scholar
[30] Zheng LL. Functionalization and performance of ultrafiltration membrane, Dissertation. Tianjin: Tianjin University; 2009. 10.7666/d.y1674427.Suche in Google Scholar
[31] Gorey C, Escobar IC, Gruden C, Coleman M, Mileyeva-Biebesheimer O. Development of smart membrane filters for microbial sensing. Sep Sci Technol. 2014;43(16):4056–74. 10.1080/01496390802414502.Suche in Google Scholar
[32] Tian XD. Synthesis of temperature sensitive polymer materials and their application in intelligent membrane materials, Dissertation. Beijing: Beijing University of Chemical Technology; 2009. 10.7666/d.y1557735.Suche in Google Scholar
[33] Liu BQ, Lu XF, Qiao Z, Song LP, Cheng Q, Zhang JW, et al. pH and temperature dual-responsive plasmonic switches of gold nanoparticle monolayer film for multiple anticounterfeiting. Lang ACS J Sur Coll. 2018;34(43):1–39. 10.1021/acs.langmuir.8b02989.Suche in Google Scholar PubMed
[34] Joisten H, Truong A, Ponomareva S, Naud C, Morel R, Hou Y, et al. Optical response of magnetically actuated biocompatible membranes. Nanoscale. 2019;11(22):10667–83. 10.1039/C9NR00585D.Suche in Google Scholar
[35] Zhao LZ, Zhao YX, Li RS, Wu DH, Xu R, Li SS, et al. A porphyrin-based optical sensor membrane prepared by electrostatic self-assembled technique for online detection of cadmium(II). Chemosphere. 2020;238:124552–60. 10.1016/j.chemosphere.2019.124552.Suche in Google Scholar PubMed
[36] Pantuso E, Filpo G, Nicoletta FP. Light‐responsive polymer membranes. Adv Opt Mater. 2019;7(16):1900252–87. 10.1002/adom.201900252.Suche in Google Scholar
[37] Luo Q. Preparation and photocatalytic performance of V2O5TiO2 film with visible light response. Sci Wealth. 2020;27.Suche in Google Scholar
[38] Kim YJ, Neuzil P, Nam CH, Engelhard M. Deposition of bacteriorhodopsin protein in a purple membrane form on nitrocellulose membranes for enhanced photoelectric response. Sensors. 2012;13(1):455–62. 10.3390/s130100455.Suche in Google Scholar PubMed PubMed Central
[39] Wang WZ. Photoresponsive smart azobenzene liquid crystal polymer film materials. CN. Patent 201911152520.3; 2019.Suche in Google Scholar
[40] Ghimire RR, Mondal S, Raychaudhuri AK. Synergistic ultraviolet photoresponse of a nanostructured ZnO film with gate bias and ultraviolet illumination. J Appl Phys. 2015;117(10):121301–9581. 10.1063/1.4914518.Suche in Google Scholar
[41] Rikukawa M, Inagaki D, Kaneko K, Takeoka Y, Ito I, Kanzaki Y, et al. Proton conductivity of smart membranes based on hydrocarbon polymers having phosphoric acid groups. J Mol Struct. 2004;739(1):153–61. 10.1016/j.molstruc.2004.04.034.Suche in Google Scholar
[42] Tripathi T, Kamaz M, Wickramasinghe SR, Sengupta A. Designing electric field responsive ultrafiltration membranes by controlled grafting of poly (ionic liquid) brush. Int J Env Res Public Health. 2019;17(1):1–14. 10.3390/ijerph17010271.Suche in Google Scholar PubMed PubMed Central
[43] Zong JL. Preparation and application of electrospun poly (vinylidene fluoride) multi-scale dendritic nanofibers, Dissertation]. Tianjin: Tianjin Polytechnic University; 2017. 10.7666/d.Y3368596.Suche in Google Scholar
[44] Wang WY, Chen L, Mo Y, Dong J. Preparation and characteristics of novel PVDF hollow fiber hydrogel membranes. J Tianjin Polytech Univ. 2005;24(5):17–21. https://kns.cnki.net/kcms/detail/detail.aspx ? FileName = TJFZ200505008&DbName = CJFQ2005.Suche in Google Scholar
[45] Chen L, Wang WY, Yu X. Preparation and performance study of polyvinylidene fluoride hollow fiber intelligent membrane. Organic chemical industry. Compilation of Papers for the 10th Chen Weiji Excellent Paper Award, 2007, Beijing, China; China: Textile Eng Soc; 2007. p. 1–8.Suche in Google Scholar
[46] Li C. Study on hydrophilic modified polyacrylonitrile ultrafiltration membrane and its antifouling performance, Dissertation. Tianjin: Tianjin University; 2008. 10.7666/d.y1531360.Suche in Google Scholar
[47] Zhou H, Zeng JX, Chen J, Fan HJ. Preparation and intelligent properties of temperature and pH dual sensitive polyurethane film. Chem Propellants Polym Mater. 2011;9(3):81–9. 10.3969/j.issn.1672-2191.2011.03.018.Suche in Google Scholar
[48] Plisko TV, Burts KS, Bildyukevich AV. Development of dynamic pH and temperature-responsive smart membranes via immobilization of chitosan-graft-poly(N-Isopropylacrylamide-Co-Methacrylic Acid) hydrogels on microfiltration membrane-support. Mater Des. 2021;208:109939–42. 10.1016/j.matdes.2021.109939.Suche in Google Scholar
[49] Xiang YH, Shen JH, Wang YZ, Liu F, Xue LX. A pH-responsive PVDF membrane with superwetting properties for the separation of oil and water. RSC Adv. 2015;5(30):23530–9. 10.1039/c5ra00739a.Suche in Google Scholar
[50] Ito T, Hioki T, Yamaguchi T, Shinbo T, Nakao SI, Kimura S. Development of a molecular recognition ion gating membrane and estimation of its pore size control. J Am Chem Soc. 2001;124(26):7840–6. 10.1021/ja012648x.Suche in Google Scholar PubMed
[51] Ito T, Yamaguchi T. Controlled release of model drugs through a molecular recognition ion gating membrane in response to a specific ion signal. Langmuir. 2006;22(8):3945–9. 10.1021/la053206s.Suche in Google Scholar PubMed
[52] Ju XJ, Chu LY, Xie R, Wang W, Liu Z. Progress in molecular-recognition smart membranes. Chin Sci Bull. 2015;60(27):2621–30. 10.1360/N972014-0126.Suche in Google Scholar
[53] Hiroto O, Yuhei O, Hidenori O, Takeo Y. Control of target molecular recognition in a small pore space with biomolecule-recognition gating membrane. Small. 2018;14(18):1702267. 10.1002/smll.201702267.Suche in Google Scholar PubMed
[54] Su Y, Zheng L, Li C, Jiang Z. Smart zwitterionic membranes with on/off behavior for protein transport. J Phys Chem B. 2008;112(38):11923–8. 10.1021/jp804422t.Suche in Google Scholar PubMed
[55] Liu WY, Ju XJ, Yousef F, He F, Peng HY, Liu YQ, et al. Capsule membranes encapsulated with smart nanogels for facile detection of trace lead(II) ions in water. J Membr Sci. 2020;613:118523–55. 10.1016/j.memsci.2020.118523.Suche in Google Scholar
[56] Liu TY, Yuan HG, Li Q, Tang YH, Zhang Q, Qian WZ, et al. Ion-responsive channels of zwitterion-carbon nanotube membrane for rapid water permeation and ultrahigh mono-/multivalent ion selectivity. ACS Nano. 2015;9(7):7488. 10.1021/acsnano.5b02598.Suche in Google Scholar PubMed
[57] Liu H, Zhang L, Huang JY, Mao JJ, Chen Z, Mao QH, et al. Smart surfaces with reversibly switchable wettability: concepts, synthesis and applications. Adv Colloid Interface Sci. 2021;300:102584. 10.1016/j.cis.2021.102584.Suche in Google Scholar PubMed
[58] Liu H, Wang YD, Huang JY, Chen Z, Chen GQ, Lai YK. Bioinspired surfaces with superamphiphobic properties: concepts, synthesis, and applications. Adv Funct Mater. 2018;28(19):1707415. 10.1002/adfm.201707415.Suche in Google Scholar
[59] Ge MZ, Cao CY, Huang JY, Zhang XN, Tang YX, Zhou XR, et al. Rational design of materials interface at nanoscale towards intelligent oil-water separation. Nanoscale Horizons. 2018;3(3):1–3. 10.1039/C7NH00185A.Suche in Google Scholar
[60] Xie R. Study on preparation and performance of thermo-responsive and molecular recognizable “smart” track-etched membranes, Dissertation. Sichuan: Sichuan University; 2007. 10.7666/d.y1213064.Suche in Google Scholar
[61] Jiang P. Preparation, properties and application of pH- and thermo-responsive polymer membrane. Hunan Province: Central South University; 2014. https://kns.cnki.net/kcms/detail/detail.aspx? FileName = 1014395920.nh&DbName = CDFD2015.Suche in Google Scholar
[62] Liu C. Industrial separation membrane-gas, steam separation membrane. N Chem Mater. 1982;9:7–11. https://kns.cnki.net/kcms/detail/detail.aspx? FileName = HGXC198209001&DbName = CJFQ1982.Suche in Google Scholar
[63] Tokarev I, Minko S. Multiresponsive, hierarchically structured membranes: new, challenging, biomimetic materials for biosensors, controlled release, biochemical gates, and nanoreactors. Adv Mater. 2009;21(2):241–7. 10.1002/adma.200801408.Suche in Google Scholar
[64] Lu Y, Yin YL, He Y. Research and application progress of poly (N-isopropylacrylamide) thermosensitive hydrogel. Polym Bull. 2019;(7):13–9. 10.14028/j.cnki.1003-3726.2019.07.002.Suche in Google Scholar
[65] Li PF. Research progress of preparation of environmental stimuli-responsive smart membrane materials by using ATRP techniques. Chem Ind Eng Prog. 2013;32(12):2910–5. 10.3969/j.issn.1000-6613.2013.12.020.Suche in Google Scholar
[66] Zhang HL. Preparation and selective transmission mechanism of separation membranes with molecular recognition function. Tianjin: Tianjin University; 2007. 10.7666/d.y1360566.Suche in Google Scholar
[67] Zhou JC, Chen GH, Chen DQ. Poly(N-isopropylacrylamide)-based nanofiber membranes with temperature-sensitive and excellent mechanical properties. Acta Materiae Compositae Sin. 2023;40:1–9. 10.13801/j.cnki.fhclxb.20220825.001.Suche in Google Scholar
[68] Wu JF, Cui ZW, Yu Y, Han H, Tian D, Hu JD, et al. A 3D smart wood membrane with high flux and efficiency for separation of stabilized oil/water emulsions. J Hazard Mater. 2022;441:129900. 10.1016/J.JHAZMAT.2022.129900.Suche in Google Scholar
[69] Zhang H, Heng TT, Fang ZG, Hu X, Fang L, Lu CH. Research progress of high energy storage ceramic/polyvinylidene fluoride composite dielectric. J Composite Mater. 2021;38(7):2107–22. 10.13801/j.cnki.fhclxb.20201030.002.Suche in Google Scholar
[70] Ritanjali B, Elanseralathan K. A review on polyvinylidene fluoride polymer based nanocomposites for energy storage applications. J Energy Storage. 2022;48:103788. 10.1016/J.EST.2021.103788.Suche in Google Scholar
[71] Wang LS, Wu WB, Zhang QF, Peng DL. Electromagnetic transport properties of magnetic nanoparticle assembled particle films. Nat Sci Ed. 2021;60(2):235–46. 10.6043/j.issn.0438-0479.202010027.Suche in Google Scholar
[72] Li ZH, Li YN, Wang X, Wu KX. Application of pH sensitive purple tomato anthocyanins in the preparation of chitosan membranes. Packaging Eng. 2022;43(15):160–6. 10.19554/j.cnki.1001-3563.2022.15.018.Suche in Google Scholar
[73] Zhang P, Chen QJ, You N, Wang F, Zhang B, Wang JH, et al. Research progress in the application of bio based pH responsive smart membrane in food industry. Food Fermentation Ind. 2022;49(10):1–6. 10.13995/j.cnki.11-1802/ts.032630.Suche in Google Scholar
[74] Du RH, Guo X, Zhang ZZ, Li XX. Preparation and separation performance of polydimethylaminoethyl methacrylate gas separation membrane. J Tianjin Univ Technol. 2019;38(4):1–6. 10.3969/j.issn.1671-024x.2019.04.001.Suche in Google Scholar
[75] Shi XF, Ma YX, Li X, Kang XY, Li XH, Yang HJ. Research progress on the adsorption of heavy metal ions by electrospun polyacrylonitrile based nanofibers. Mater Guide. 2022;36(18):215–23. 10.11896/cldb. 20090131.Suche in Google Scholar
[76] Tao L, Lin S, Xie R, Ju XJ, Liu Z, Wei W, et al. pH-responsive poly (ether sulfone) composite membranes blended with amphiphilic polystyrene-block-poly (acrylic acid) copolymers. J Membr Sci. 2014;450:162–73. 10.1016/j.memsci.2013.09.002.Suche in Google Scholar
[77] Jiang H, Wang W, Wang H, Yin JJ. Analysis of smart materials development at home and abroad. New Mater Ind. 2014;5:2–9. 10.3969/j.issn.1008-892X.2014.05.002.Suche in Google Scholar
[78] Gao R, Fang XY, Yan DP. Recent developments in stimuli-responsive luminescent films. J Mater Chem C. 2019;7:3399. 10.1039/C9TC00348G.Suche in Google Scholar
[79] Gao R, Yan DP, Duan X. Layered double hydroxides-based smart luminescent materials and the tuning of their excited states. Cell Rep Phys Sci. 2021;2:100536. 10.1016/j.xcrp.2021.100536.Suche in Google Scholar
[80] Li W, Yan DP, Gao R, Lu J, Wei M, Duan X. Recent advances in stimuli-responsive photofunctional materials based on accommodation of chromophore into layered double hydroxide nanogallery. J Nanomater. 2013;2013:586462–76. 10.1155/2013/586462.Suche in Google Scholar
[81] Pang DL, Zhang JH, Ren LH, Qian ZL, Hang G. Thermal sensitive intelligent track film. Nucl Sci Tech. 1999;22(7):389–91. 10.3321/j.issn:0253-3219.1999.07.002.Suche in Google Scholar
[82] Wu DH. Surface temperature sensitive modification of aromatic polyamide composite films, Dissertation. Zhejiang: Zhejiang Sci-Tech University; 2009. 10.7666/d.y1747564.Suche in Google Scholar
[83] Dong HB. Surface modification of polysulfones porous membranes via atom transfer radical polymerization, Dissertation. Zhejiang: Zhejiang University; 2010. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD2011&filename=2010072115.nh.Suche in Google Scholar
[84] Moribe M, Gondo N, Nakamoto M, Hoshino Y, Miura Y. Increase of reversible salt absorption capacity of temperature-responsive hydrogel film consisting of nanogel particles. Macro. 2018:179.Suche in Google Scholar
[85] Yu H, Moribe M, Gondo N, Jibiki T, Nakamoto M, Guo B, et al. Combining acid- and base-imprinted nanoparticles in a hydrogel film for temperature-responsive quick and reversible capture of salt. ACS Appl Polym Mater. 2020;2(2):505–14. 10.1021/acsapm.9b00940.Suche in Google Scholar
[86] Ding T, Rüttiger C, Zheng XZ, Benz F, Ohadi H, Vandenbosch GAE, et al. Fast dynamic color switching in temperature‐responsive plasmonic films. Adv Opt Mater. 2016;4(6):877–82. 10.1002/adom.201600094.Suche in Google Scholar
[87] Li W, Yuan XY, Huang R, Wu Y, Zhang XX. Preparation method of temperature responsive composite microporous membrane. CN. Patent 2015108880714; 2015.Suche in Google Scholar
[88] Zha LS, Wang LY. Preparation method for silver-loaded nano particle temperature stimuli responsiveness hybrid nanofiber membrane. CN. Patent 2015103544844; 2015.Suche in Google Scholar
[89] Park YS, Imanishi Y, Ito Y. Photocontrolled gating by polymer brushes grafted on porous glass filter. Macromolecules. 1998;31(8):2606–10. 10.1021/ma9605199.Suche in Google Scholar
[90] Liu NG, Chen Z, Dunphy DR, Jing YG. Photoresponsive nanocomposite formed by self‐assembly of an azobenzene‐modified silane. Angew Chem. 2003;42(15):1731–4. 10.1002/anie.200250189.Suche in Google Scholar PubMed
[91] Guo DY, Wu ZP, Li PG. Magnetic anisotropy and deep ultraviolet photoresponse characteristics in Ga2O3:cr vermicular nanowire thin film nanostructure. RSC Adv. 2015;5(17):2046–69. 10.1039/c4ra13813a.Suche in Google Scholar
[92] Jia H, Ping C, Xu C, Zhou JJ, Sang XW, Wang JC, et al. Fabrication of the (Y2O3:Yb–Er)/Bi2S3 composite film for near-infrared photoresponse. J Mater Chem A. 2015;3(11):5917–22. 10.1039/c5ta00692a.Suche in Google Scholar
[93] Li CH, Meng R, Ni YQ, Zhang Z, Wang S. Preparation of a photoresponsive SP_COOH_PAN fiber membrane material and study on humidity regulation. J Funct Mater. 2020;51(1):1016–23. 10.3969/j.issn.1001-9731.2020.01.003.Suche in Google Scholar
[94] Gao R, Cao D, Gan Y, Yan DP. Flexible self-supporting nanofiber thin films showing reversible photochromic fluorescence. ACS Appl Mater Interfaces. 2015;7(18):9904–10. 10.1021/acsami.5b01996.Suche in Google Scholar PubMed
[95] Yang SM, Zhou B, Huang QQ, Wang SQ, Zhen HY, Yan DP, et al. Highly efficient organic afterglow from a 2D layered lead-free metal halide in both crystals and thin films under an air atmosphere. ACS Appl Mater Interfaces. 2020;12(1):1419–26. 10.1021/acsami.9b20502.Suche in Google Scholar PubMed
[96] Li ZX, Liang RZ, Xu SM, Liu WD, Yan DP, Wei M, et al. Multi-dimensional, light-controlled switch of fluorescence resonance energy transfer based on orderly assembly of 0D dye@micro-micelles and 2D ultrathin-layered nanosheets. Nano Res. 2016;9(12):3828–38. 10.1007/s12274-016-1252-1.Suche in Google Scholar
[97] Yang XG, Zhai ZM, Lu XM, Ma LF, Yan DP. Fast crystallization-deposition of orderly molecule level heterojunction thin films showing tunable up-conversion and ultrahigh photoelectric response. ACS Cent Sci. 2020;6(7):1169–78. 10.1021/acscentsci.0c00447.Suche in Google Scholar PubMed PubMed Central
[98] Rui G, Yan DP. Ordered assembly of hybrid room-temperature phosphorescence thin films showing polarized emission and the sensing of VOCs. Chem Commun. 2017;53:5408. 10.1039/c7cc01794d.Suche in Google Scholar PubMed
[99] Ma HY, Gao R, Yan DP, Zhao JW, Wei M. Organic–inorganic hybrid fluorescent ultrathin films and their sensor application for nitroaromatic explosives. J Mater Chem C. 2013;1:4128. 10.1039/c3tc30142g.Suche in Google Scholar
[100] Tian R, Zhang ST, Li MW, Zhou YQ, Lu B, Yan DP, et al. Localization of Au nanoclusters on layered double hydroxide nanosheets: confinement-induced emission enhancement and temperature-responsive luminescence. Adv Funct Mater. 2015;25(31):5006–15. 10.1002/adfm.201501433.Suche in Google Scholar
[101] Alexiou AA, Thomas S, Kaner P, Hu XR. Two-layer photo-responsive membranes. US. Patent 20180133657; 2016.Suche in Google Scholar
[102] Minoura N, Idei K, Matsuda K, Ogiso M, Alexandre R. Properties of Photo-Responsive Polymeric Membranes with Molecular Recognition Sites prepared by the Molecular Imprinting Technique. 52nd SPSJ Symposium on Macromolecules, 2007; Polymer Preprints, Japan. Japan: The Society of Polymer Science; 2007.Suche in Google Scholar
[103] Chuo TW, Wei TC, Chang Y, Liu YL. Electrically driven biofouling release of a poly(tetrafluoroethylene) membrane modified with an electrically induced reversibly cross-linked polymer. Amer Chem Soc. 2013;5(20):9918–24. 10.1021/am4033982.Suche in Google Scholar PubMed
[104] Price WE, Too CO, Wallace GG, Zhou D. Development of membrane systems based on conducting polymers. Synth Met. 1999;102:1338–41. 10.1016/s0379-6779(98)90212-0.Suche in Google Scholar
[105] Xu LL, Emma E, Wang J. Preparation and anti-pollution performance of novel electrically responsive conductive polyaniline smart film. Membr Sci Technol. 2018;38(3):55–62. 10.16159/j.cnki.issn1007-8924.2018.03.008.Suche in Google Scholar
[106] Hung WS, Ho SY, Chiao YH, Chan CC, Woon WY, Yin MJ, et al. Electrical tunable PVDF/graphene membrane for controlled molecule separation. Chem Mater. 2020;32(13):5750–8. 10.1021/acs.chemmater.0c01547.Suche in Google Scholar
[107] Widakdo J, Huang T, Subrahmanya TM, Austria HFM, Chou HL, Hung WS, et al. Bioinspired ionic liquid-graphene based smart membranes with electrical tunable channels for gas separation. Appl Mater Today. 2022;27:101441. 10.1016/J.APMT.2022.101441.Suche in Google Scholar
[108] Lin X, Huang R, Ulbricht M. Novel magneto-responsive membrane for remote control switchable molecular sieving. J Mater Chem B. 2016, 4(5):867–79. 10.1039/c5tb02368h.Suche in Google Scholar PubMed
[109] Stylios GK, Wan TY. Investigating smart membranes and coatings by in situ synthesis of iron oxide nanoparticles in PVA hydrogels. Adv Sci Technol. 2008;60:32–7. 10.4028/www.scientific.net/AST.60.32.Suche in Google Scholar
[110] Zheng WW, Teng L, Lai YK, Zhu TX, Li SH, Wu XW, et al. Magnetic responsive and flexible composite superhydrophobic photothermal film for passive anti-icing/active deicing. Chem Eng J. 2022;427:130922. 10.1016/j.cej.2021.130922.Suche in Google Scholar
[111] Gao R, Cao D, Guan Y, Yan DP. Fast and reversible humidity-responsive luminescent thin films. Ind Eng Chem Res. 2016;55(1):125–32. 10.1021/acs.iecr.5b03389.Suche in Google Scholar
[112] Gao R, Yan DP, Evans DG, Duan X. Layer-by-layer assembly of long-afterglow self-supporting thin films with dual-stimuli-responsive phosphorescence and antiforgery applications. Nano Res. 2017;10(10):3606–17. 10.1007/s12274-017-1571-x.Suche in Google Scholar
[113] Yang B. Preparation and protein separation performance of EVAL smart switch membrane, Dissertation. Tianjin: Tianjin Polytechnic University; 2015. 10.7666/d.Y2757281.Suche in Google Scholar
[114] Jiang P, Wu YQ. Preparation and application of temperature and pH responsive polymer smart membrane. Sci Technol Rev. 2016;34(19):22–30. 10.3981/j.issn.1000-7857.2016.19.002.Suche in Google Scholar
[115] Ma HW, Wang XM, Cai GX, Zhang Z. Preparation of pH-responsive polymer membrane on metal surface. CN. Patent 201210137104.8; 2012.Suche in Google Scholar
[116] Piyal M, Shekhar SN, Anand K, Kumar PM. Recovery of H2SO4 from wastewater in the presence of NaCl and KHCO3 through pH responsive polysulfone membrane: optimization approach. Polym Test. 2020;86:106463–75. 10.1016/j.polymertesting.2020.106463.Suche in Google Scholar
[117] Liu HW. Preparation Perform Temp pH-Responsive Smart Switch Membr, Dissertation. Zhejiang: Zhejiang University of Technology; 2019. 10.27463/d.cnki.gzgyu.2019.000269.Suche in Google Scholar
[118] Yang Q, Whiting WI, Dettori. A membrane with ion fluxes responsive to temperature, pH and voltage. J Membr Sci. 2019;5(78):10–5. 10.1016/j.memsci.2019.02.024.Suche in Google Scholar
[119] Zhou JC, Jin J, Li NX, Teng HC. Preparation method of temperature/pH-responsive cellulose acetate membrane and application of membrane. CN. Patent 2015109826647; 2015.Suche in Google Scholar
[120] Ma QY. Preparation and properties of pH responsive composite films based on talla gum, Dissertation. Northeast Forestry University; 2018. 10.27009/d.cnki.gdblu.2018.000008.Suche in Google Scholar
[121] Li XY, Zhang QD, Zhang WF, Qu RX, Wei Y, Feng L. Smart nylon membranes with pH-responsive wettability: high-efficiency separation on demand for various oil/water mixtures and surfactant-stabilized emulsions. Adv Mater Int. 2018;5(21):1801179–90. 10.1002/admi.201801179.Suche in Google Scholar
[122] Wang LJ, Ma QY, Yang JB. A preparation method of pH intelligent response membrane based on nanocellulose reinforced carrageenan matrix. CN. Patent 201510342335.6; 2015.Suche in Google Scholar
[123] Fan XX, Xie R, Zhao Q, Li XY, Ju XJ, Wang W, et al. Dual pH-responsive smart gating membranes. J Membr Sci. 2018;5(55):20–9. 10.1016/j.memsci.2018.03.028.Suche in Google Scholar
[124] Sugawara Y, Tamaki T, Yamaghchi T. Development of an aptamer-functionalized molecular recognition gating membrane targeting a specific protein on the basis of the aggregation phenomena of DNA-PNIPAM. Polymer. 2015;62:86–93. 10.1016/j.polymer.2015.02.027.Suche in Google Scholar
[125] Teng S, Li J, Tao T, Su Z, Jiang XS, Cui MZ. Hybrid membranes of hPEA@PVDF for molecular recognition and separation of phenols and anilines. Adv Mater Technol. 2019;4(11):1900529–38. 10.1002/admt.201900529.Suche in Google Scholar
[126] Zhang XT, Liu YF, Yu Y, Sun YY, Zhang WW. CO2 Preparation and properties of stimulus responsive PVDF/PVDF-g-PDMAEMA ultrafiltration membrane. J Dalian Univ Technol. 2022;41(5):362–6. 10.19670/j.cnki.dlgydxxb.2021.6014.41.Suche in Google Scholar
[127] Borgohain R, Mandal B. pH responsive carboxymethyl chitosan/poly(amidoamine) molecular gate membrane for CO2/N2 separation. ACS Appl Mater Interfaces. 2019;11(45):42616–28. 10.1021/acsami.9b15044.Suche in Google Scholar PubMed
[128] Xie R, Yang M, Cheng CJ, Jiang J, Chu LY. Molecular-recognition and thermo-responsive composite smart polymeric materials. Prog Chem. 2012;24(Z1):195–202. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=HXJZ2012Z1002&DbName=CJFQ2012.Suche in Google Scholar
[129] William JK, Xue N. Carbon molecular sieve membranes for nitrogen/methane separation. WO. Patent. 2013US67334; 2013.Suche in Google Scholar
[130] Kumeria T, Yu JX, Alsawat M, Kurkuri MD, Santos A, Abell AD, et al. Modulating molecular transport across peptide-modified nanoporous alumina membranes with light. SPIE Biophotonics Australas; 2016. p. 10013. 10.1117/12.2241495.Suche in Google Scholar
[131] Ariza MJ, Otero TF. Nitrate and chloride transport through a smart membrane. J Membr Sci. 2007;290(1–2):241–9. 10.1016/j.memsci.2006.12.040.Suche in Google Scholar
[132] Tang CC. Research on new intelligent membrane with ion recognition function, Dissertation. Beijing Forestry University; 2011. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1011134677.nh&DbName=CMFD2012.Suche in Google Scholar
[133] Liu Z, Luo F, Ju XJ, Xie R, Luo T, Sun YM, et al. Positively K+-Responsive membranes with functional gates driven by host-guest molecular recognition. Adv Funct Mater. 2012;22(22):1201251. 10.1002/adfm.201290132.Suche in Google Scholar
[134] Wang YY, Chu LY, Liu H, Peng HY, Luo F, Xie R, et al. Lead ion responsive smart membrane and its preparation method and detection method of lead ion in water samples. CN. Patent 201811231232.2; 2018.Suche in Google Scholar
[135] Wang Y, Liu Z, Luo F, Peng HY, Zhang SG, Xie R, et al. A novel smart membrane with ion-recognizable nanogels as gates on interconnected pores for simple and rapid detection of trace lead (II) ions in water. J Membr Sci. 2019;575:28–37. 10.1016/j.memsci.2019.01.002.Suche in Google Scholar
[136] Esmali F, Mansourpanah Y, Farhadi K, Amani S, Rasoulifard A, Ulbricht M. Fabrication of a novel and highly selective ion-imprinted PES-based porous adsorber membrane for the removal of mercury(II) from water. Sep Purif Technol. 2020;2(50):117183–99. 10.1016/j.seppur.2020.117183.Suche in Google Scholar
[137] Bakangura E, Cheng CL, Wu L, Ge XL, Ran J, Khan MI, et al. Hierarchically structured porous anion exchange membranes containing zwetterionic pores for ion separation. J Membr Sci. 2017;5(37):32–41. 10.1016/j.memsci.2017.05.007.Suche in Google Scholar
[138] Zhang X, Zhou JK, Wei R, Zhao WF, Sun SD, Zhao CS. Design of anion species/strength responsive membranes via in-situ cross-linked copolymerization of ionic liquids. J Membr Sci. 2017;53(5):158–67. 10.1016/j.memsci.2017.04.044.Suche in Google Scholar
[139] Zou XB, Xue J, Huang XW, Zhai XD, Zhang JJ, Zhang ZX, et al. Development and application of an intelligent biolayer packaging film as a freshness indicator for salmon. Food Sci. 2019;40(23):206–12. 10.7506/spkx1002-6630-20181126-298.Suche in Google Scholar
[140] Chen X, He Y, Fan Y, Yang QB, Yang X, Zeng GY. Facile preparation of a smart membrane with ammonia-responsive wettability transition for controllable oil/water separation. J Mater Sci. 2017;53(1):516–27. 10.1007/s10853-017-1535-2.Suche in Google Scholar
[141] Liu Z, Ju XJ, Wang W, Xie R, Jiang L, Chen QM, et al. Stimuli-responsive capsule membranes for controlled release in pharmaceutical applications. Curr Pharm Des. 2017;23(2):295–301. 10.2174/1381612822666161021141429.Suche in Google Scholar PubMed
[142] Cai YH, Chen DY, Li NJ, Xu QF, Li H, He JH, et al. A smart membrane with antifouling capability and switchable oil wettability for high-efficiency oil/water emulsions separation. J Membr Sci. 2018;55(5):69–77. 10.1016/j.memsci.2018.03.042.Suche in Google Scholar
[143] Park Y, Gutierrez MP, Lee LP. Reversible self-actuated thermo-responsive pore membrane. Sci Rep. 2016;6:1–10. 10.1038/srep39402.Suche in Google Scholar PubMed PubMed Central
[144] Wang JX. Preparation and properties of polyimide thermosensitive polymer and its intelligent membrane, Dissertation. Taiyuan University of Technology; 2019. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1019871770.nh&DbName=CMFD2019.Suche in Google Scholar
[145] Wang H, Zhou H, Niu H, Zhang J, Du Y, Lin T. Dual-layer superamphiphobic/superhydrophobic-oleophilic nanofibrous membranes with unidirectional oil-transport ability and strengthened oil-water separation performance. Adv Mater Int. 2015;2(4):1400506. 10.1002/admi.201400506.Suche in Google Scholar
[146] Hou X, Smart gating multi-scale pore/channel-based membranes. Adv Mater. 2016;28(33):797–813. 10.1002/adma.201600797.Suche in Google Scholar PubMed
[147] Long Q. Preparation and properties of temperature sensitive polyurethane gas phase controlled release antioxidant film, Dissertation. Jiangnan University; 2019. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1019871770.nh&DbName=CMFD2019.Suche in Google Scholar
[148] Yue Q, Wang SD, Xu F, Liu T. Electrostatic spinning technology and its application in various fields. Materi Rep. 2021;35(S1):594–9. https://chn.oversea.cnki.net/kcms/detail/detail.aspx?FileName=CLDB2021S1115&DbName=CJFQ2021.Suche in Google Scholar
[149] Liu SD, Li DS, Yang Y, Jiang L. Fabrication, mechanical properties and failure mechanism of random and aligned nanofiber membrane with different parameters. Nanotechnol Rev. 2019;8(1): 218–26. 10.1515/ntrev-2019-0020.Suche in Google Scholar
[150] Ye YS, Chang M, Huang J, Wang XL. P(NIPAAm-PEGMA) grafted polypropylene porous membrane surface anti-pollution and self-cleaning performance. Polym Mater Sci Eng. 2016;32(9):172–7. 10.16865/j.cnki.1000-7555.2016.09.030.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Artikel in diesem Heft
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus


