Abstract
Ag–Mo films with different thicknesses were prepared on polyimide substrate by magnetron sputtering and annealed at different temperatures. The effects of film thickness and annealing temperatures on the resistivity and microstructure of Ag–Mo alloys were investigated. Results show that many Ag nanoparticles were self-formed on Ag–Mo films’ surface. As the thickness of the Ag–Mo film increased from 163 to 409 nm, there was a significant decrease in its resistivity, dropping from 485.44 to 237.12 μΩ cm. Resistivity of the Ag–Mo film is dependent on the annealing temperature. When temperature rises from room temperature to 180℃, the resistivity decreases from 390.62 to 339.37 μΩ cm, with little change. After annealing above 270℃, a sudden increase of resistivity of the Ag–Mo film was attributed to the growth of Ag particles on the film surface contributing to the increase in surface roughness, which hindered electron transport and caused severe surface scattering. High-resistivity Ag–Mo films are expected to be candidates for high-resistivity thin-film devices.
1 Introduction
Nano-film materials are widely used in many fields such as microelectronics, optics, biomedical, solar cells, and sensors due to their unique microstructures and physicochemical properties [1,2,3]. In recent years, many researchers have extensively studied the electrical conductivity of metal or alloy films [4,5]. Silver (Ag) is of interest among many metals because of its low resistivity [6], and Ag alloy films are widely used in microelectronic devices because of their excellent electrical properties. In general, the conductivity of thin films is usually weaker than that of metal or alloy bulks. Therefore, many researchers have made efforts to improve conductivity of the thin films, minimize heat and energy loss generated by working equipment, and achieve the goal of extending its lifespan. However, high-resistivity thin films are also used in special application fields, such as high-resistivity thin-film resistors [7,8,9,10] working in high-voltage and high-temperature environments. There are many methods and treatment processes to increase the resistivity of thin films – for example, by oxidizing an element in the alloy film in order to increase the resistivity or by reducing the thickness of the film to increase the resistivity [11,12,13].
When the free electrons collide, they lose their directed velocity given by the external electric field [14], and such collisions may occur in electron-lattice, electron impurity, electron-grain boundary, and electron surface. Surface scattering, grain boundary scattering, and impurity scattering have a great influence on the resistivity of thin films, so the film resistivity can be increased by enhancing surface scattering, grain boundary scattering, etc. [15], and thus, high-resistivity films can be prepared. Lee et al. [16] significantly increased the resistivity of the films by adding yttrium to the Cu-Mn alloy films due to the fact that the addition of yttrium leads to grain refinement and a reduction in grain size results in enhancing grain boundaries, which increases the resistivity of the films [17]. Tseng et al. [18] treated Al–W alloy films with chemical–mechanical polishing (CMP) and found that the strength of the selective orientation of Al–W films decreased after CMP treatment, which increased the film resistivity. The aforementioned methods and treatment processes are complicated and pricey.
In this study, we deposited two immiscible metals of Mo and Ag on polyimide (PI) substrates by co-sputtering technique to prepare Ag–Mo films and investigated the effects of film thickness and annealing temperature on the resistivity of Ag–Mo films. It was reported that a large number of Ag nanoparticles were formed on the surface of Ag–Zr [19] and Ag–Co [20] films due to the insolubility of Zr and Co with Ag, respectively. Since Mo and Ag were mutually insoluble [21], we expect to obtain Ag nanoparticles on Ag–Mo film’s surface. Then, the COMSOL Multiphysics software is applied to simulate the trajectory of electron transport when current passes through films to reveal the effect of film surface morphology on film resistivity. A simple method to prepare high-resistivity Ag–Mo films is expected to be of significant interest for high-resistant device applications.
2 Materials and methods
The JCP-350M2 magnetron sputtering machine (Beijing Technol Co., LTD, China) was used to deposit Ag–Mo film on PI substrate with a thickness of 125 μm, and the targets used were 99.99% purity silver target (Ф50 mm × 4 mm) with 99.99% molybdenum (Ф50 mm × 4 mm). First, PI substrate (20 mm × 20 mm × 0.125 mm) was placed in a beaker containing anhydrous ethanol (99.98% purity) for ultrasonic cleaning to remove surface dust and organic matter, as shown in Figure 1(a). Next, Ag–Mo films of different thicknesses were prepared by varying the deposition time (5, 10, and 15 min). The Ag target was connected to the radio frequency power supply with a power setting of 80 W, and the Mo target was connected to the direct current (DC) power supply with a power setting of 100 W. The vacuum of the chamber was 5 × 10−4 Pa, the rotation speed of the substrate table was 30 rpm, and the argon flow rate was kept at 40 sccm. The samples were placed in a tube furnace with argon-protective atmosphere for annealing at 90, 180, 270, and 360℃ for 1 h, respectively, and then cooled to room temperature, as shown in Figure 1(c). Next, the square resistance of the Ag–Mo film was measured by an RTS-8 four-probe resistance tester, as shown in Figure 1(d), and the square resistance was measured at three different locations for each sample. The film thickness could be measured by cross-sectional morphology of the film, whose Ag–Mo film resistivity could be calculated by the following:
where ρ, R, and d represent the resistivity, square resistance, and thickness of the Ag–Mo film, respectively.

Preparation process of the Ag–Mo film is as follows: (a) cleaning PI substrate, (b) deposition film, (c) annealing, and (d) measuring square resistance.
The surface morphology and microstructure of Ag–Mo films were characterized by field emission scanning electron microscopy (FESEM, JSM-7800F) and transmission electron microscopy (TEM, JEM-2100). X-ray diffractometer (XRD, D 8 advance) was used to characterize the crystal structure of films and Ag particles, the working parameters are λ = 0.1541 nm (Cu-kα), current 40 mA, voltage 40 kV, scanning speed 6°/min, scanning step size 0.02°, diffraction angle set in the range of 20°–90°, and each step stays for 1 s. The energy-dispersive spectrometer (EDS) was used to analyze the elemental content of Ag–Mo films. COMSOL Multiphysics software was used to simulate the current trajectory through the films to reflect electron transport. The AC/DC module in COMSOL Multiphysics software was used to simulate the current trajectory through the film to reflect the electron transport. Where the potential is set to 10 V, the current density was set to 10 A/m2 and the boundary condition was set to periodic boundary condition. In the simulation process, the material of the film is set to Mo, and the material of the surface nanoparticles is set to Ag.
3 Results and discussion
3.1 Analysis of XRD diffraction patterns of Ag–Mo films
Figure 2 shows the XRD diffraction pattern of Ag–Mo film with a film thickness of 409 nm after annealing at different temperatures; it can be seen from Figure 2 that the as-deposited Ag–Mo film has only weak diffraction peaks of Mo and no diffraction peaks of Ag due to small Ag grains in the film, which is the result of Mo inhibiting the growth of Ag grains [22]. With increase in annealing temperature, the intensity of Ag (111) diffraction peaks increases significantly, which indicates that the Ag crystallinity increases significantly, while the intensity of Mo diffraction peaks decreases. Figure 2(b) shows the XRD patterns of Ag–Mo films with different film thicknesses after annealing at 360℃. It can be seen that the intensity of Ag (111) diffraction peak increases significantly with the increase of film thickness.

(a) and (b) XRD diffraction patterns of Ag–Mo films: (a) XRD diffraction patterns of Ag–Mo films with a film thickness of 409 nm at different annealing temperatures and (b) XRD diffraction patterns of Ag–Mo films with different thicknesses after annealing at 360℃.
3.2 Microstructure analysis of Ag nanoparticles/Ag–Mo films
Figure 3(a)–(c) shows the surface morphology and EDS spectra of the deposited Ag–Mo films, and it can be seen that many small particles formed on the Ag–Mo film’s surface. Figure 3(d) shows the selected area electron diffraction (SAED) of a particle detached from the Ag–Mo film by ultrasonic vibration. The crystal plane spacing of these particles was calculated and compared with single-crystal Ag, proving that these particles on the surface of Ag–Mo film are single-crystal Ag nanoparticles, which is consistent with previous study [23] and Ag-Co film [20]. The elemental contents of Ag–Mo films with different film thicknesses are shown in the EDS spectra, and it can be seen that the atomic ratios of Ag to Mo in the films are both close to 4:6, indicating that films prepared by co-sputtering are uniform in composition and have very good repeatability. As shown in Figure 3(e) and (f), TEM images of the as-deposited and 270℃ annealed Ag–Mo films show that the films are composed of Ag and Mo before and after annealing and are homogeneous.
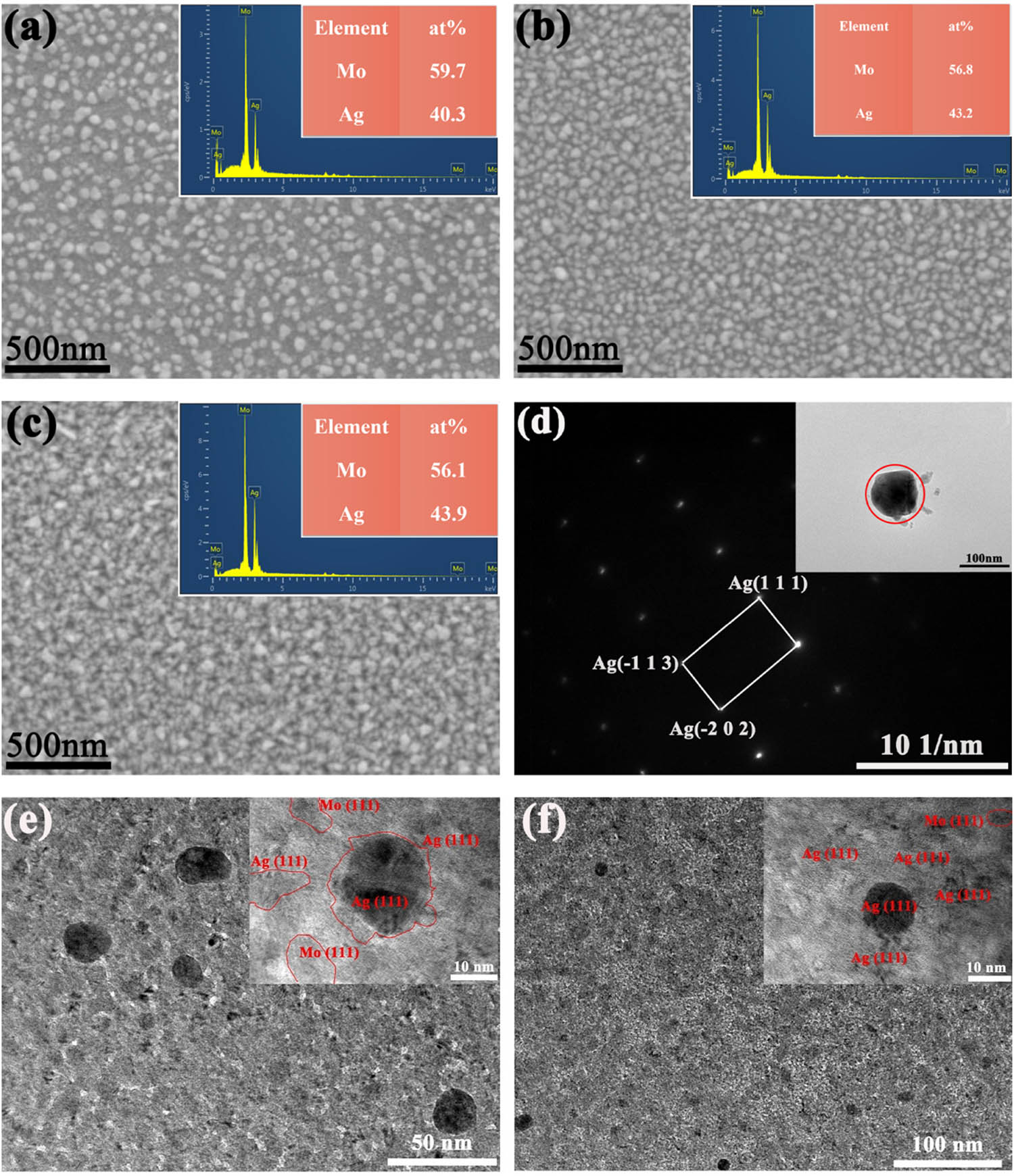
(a)–(c) Surface morphology and EDS results of the as-deposited Ag–Mo films with different film thicknesses: (a) 163 nm, (b) 296 nm, (c) 409 nm; (d) SAED of the particles on the Ag–Mo film annealed at 270℃; (e) TEM image of as-deposited Ag–Mo film; (f) TEM image of 270℃ annealed Ag–Mo film.
3.3 Study of the variation of resistivity of Ag–Mo film with film thickness
Figure 4(a)–(c) shows the surface morphology and cross-sectional morphology of the as-deposited Ag–Mo films with different film thicknesses, and the Ag–Mo films have a homogeneous monolayer, which is different from the multilayer metal films [24]. From the cross-sectional morphology, we observe that the thickness of Ag–Mo films with deposition times of 5, 10, and 15 min is 163, 296, and 409 nm, respectively. Figure 4(d) shows the variation of the square resistance and resistivity of the as-deposited Ag–Mo films with different film thicknesses. The resistivity of Ag–Mo film was calculated by using equation (1). The resistivity of the film decreased significantly from 485.44 to 237.12 μΩ cm as film thickness increased, which was due to its crystallinity becoming better as the film thickness increases. And the resistivity of Ag–Mo film is much higher than that of pure silver film. However, it can be clearly seen that resistivity does not change linearly with the increase of film thickness, indicating that there are other factors affecting the resistivity. Compared to the resistivity of pure Ag films, the addition of Mo elements in Ag films results in significantly increasing the film resistivity due to the effect of impurity scattering [25], which is similar to the previously reported Ni–Zr alloy thin films [26].

(a)–(c) Surface morphology and cross-sectional morphology of the as-deposited Ag–Mo films with different film thicknesses: (a) 163 nm, (b) 296 nm, and (c) 409 nm; (d) variation of square resistance and resistivity of the as-deposited Ag–Mo films with different film thicknesses; (e)–(f) surface morphology and cross-sectional morphology of the Ag–Mo films with different film thicknesses after annealing at 360℃: (e) 143 nm, (f) 268 nm, and (g) 380 nm; (h) variation of square resistance and resistivity of Ag–Mo films with different film thicknesses after annealing at 360℃.
Figure 4(e)–(g) shows the surface morphology and cross-sectional morphology of the Ag–Mo films with different film thicknesses after annealing at 360℃. Compared with the as-deposited Ag–Mo films, Ag particles on the surface grow significantly after annealing, which is ascribed to the reason that annealing can give Ag atoms sufficient energy and promotes the diffusion of Ag atoms to the surface, and some neighboring Ag nanoparticles also merge with each other, leading to the growth of self-formed Ag nanoparticles and the decrease in the particles number.
The thicknesses of Ag–Mo films after annealing are 143, 268, and 380 nm, respectively, as can be seen from the cross-sectional morphologies. Compared to the as-deposited Ag–Mo films, the thickness of annealed films decreases by 20–30 nm, which is caused by diffusion of Ag atoms in the films to the surface. Figure 4(h) shows the variation of square resistance and resistivity of Ag–Mo films with different film thicknesses after annealing at 360℃. It can be seen that resistivity decreases from 1894.56 to 915.34 μΩ cm as the thickness of the film increases, which is the same as the variation pattern of the deposited Ag–Mo film. However, compared with the deposited Ag–Mo film, the resistivity of the film after annealing at 360℃ was more than three times that of the deposited film, which was different from previous studies which found that the resistivity of the film decreased after annealing [27,28,29]. Normally, annealing can reduce internal defects and thus slightly reduce the resistivity of the film. From the XRD pattern of Figure 2(a), it can be seen that with the increase of annealing temperature, the crystallinity of Ag improves, but the resistivity of the film increases significantly, indicating that other factors affect the resistivity of the Ag–Mo film. From the cross-sectional morphology of Figure 4(e)–(g), it is observed that the morphology of Ag nanoparticles is irregularly polyhedral. Figure (4) shows that the size of Ag particles on the surface of Ag–Mo film after annealing at 360℃ is significantly higher than that of the as-deposited films, so it can be speculated that the increase in film resistivity may be due to the growth of Ag nanoparticles on the surface of Ag–Mo film, leading to an increase in surface roughness, which hinders the electron transport [30,31], thus causing an abnormal increase in resistivity.
3.4 Mechanism of Ag particle/Ag–Mo film formation and growth
The mechanism of Ag particle formation and growth on the surface of Ag–Mo film can be explained by the Arrhenius equation and Fick’s first law. The Arrhenius equation is given as follows:
where D 0 is the exponential prefactor in m2 s−1, Q represents the activation energy per mole of atoms in J mol−1, T is the thermodynamic temperature in K, and R is the gas constant with a value of 8.314 J mol−1 K−1.
The diffusion coefficient and activation diffusion energies of Mo [32] and Ag [33] were obtained from previous studies: D Mo = 0.5 m2 s−1, Q Mo = 96,900 J mol−1, D Ag = 0.895 m2 s−1, and Q Ag = 49,500 J mol−1. Assuming that Mo and Ag do not impact each other in the diffusion process in Ag–Mo films, diffusion coefficients of Mo and Ag at different temperatures were calculated by the Arrhenius equation, and results are shown in Table 1. Annealing can promote atomic diffusion [34,35], and it can be seen from Table 1 that the self-diffusion coefficient of Ag is much larger than that of Mo at room temperature. With the increase of annealing temperature, self-diffusion coefficients of Ag and Mo increase, but self-diffusion coefficient of Ag is significantly larger than that of Mo. The diffusion flux of atoms can be calculated from Fick’s first law:
where J is the diffusion flux in kg m−2 s−1, D is the diffusion coefficient in m2 s−1, and ρ is the mass concentration of diffusing substance in kg m−3. The diffusion coefficients of Ag at different temperatures are already known in Table 1. As annealing temperature increases, the diffusion flux of Ag increases, and more Ag atoms diffuse to the film surface, promoting the formation and growth of particles. A schematic diagram of the formation and growth of self-forming Ag particles on the Ag–Mo film after annealing is shown in Figure 5.
Self-diffusion coefficients of Mo and Ag at different temperatures
| Temperature (℃) | Element | |
|---|---|---|
| Mo (cm2 s−1) | Ag (cm2 s−1) | |
| 25 | 5.52 × 10−18 | 1.95 × 10−9 |
| 90 | 5.94 × 10−15 | 6.89 × 10−8 |
| 180 | 3.45 × 10−12 | 1.78 × 10−6 |
| 270 | 2.43 × 10−10 | 1.56 × 10−5 |
| 360 | 5.12 × 10−9 | 7.42 × 10−5 |
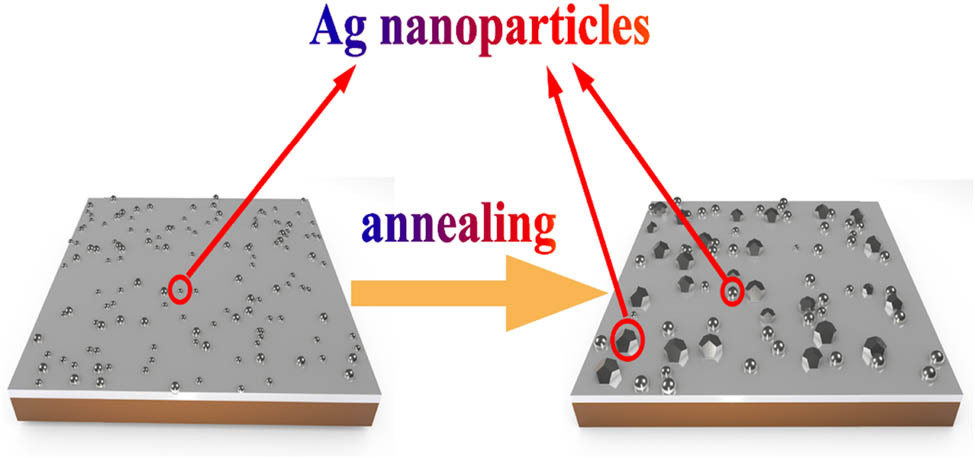
Schematic diagram of Ag particle growth on the surface of Ag–Mo film.
3.5 Study of the variation of resistivity of Ag–Mo film with annealing temperature for different film thickness
Figure 6(a)–(d) shows the surface morphology and cross-sectional morphology as well as the size distribution of self-formed particles of Ag–Mo films with a film thickness of 296 nm after annealing at different temperatures. When annealed below 180℃, particles on the surface of the film are uniformly distributed and particle size changes are not obvious. It can be seen from the particle size distribution diagram, surface self-forming Ag particle size is normally distributed and the particle morphology is gradually regular. When annealing temperature was increased to 270℃, the size of self-formed Ag particles on the Ag–Mo film increased significantly, and some adjacent Ag particles merged into one particle. With further increase of annealing temperature to 360℃, the size of Ag particles grows obviously, and there are also small Ag nanoparticles between large particles, as can be seen from the surface morphology in Figure 6(d), the contact merging growth between particles is common, and the size of some Ag particles can reach 385 nm or more. As the annealing temperature was further increased to 360℃, the size of Ag particles grew significantly, and small Ag nanoparticles also appeared between the large particles. From the surface morphology of Figure 6(d), it can be seen that the contact merging growth between the particles is common, and the size of some Ag particles can reach more than 385 nm. Observing the cross-sectional morphology of Figure 6(a)–(d), the height of Ag nanoparticles on the surface of the Ag–Mo film ranges from 10 to 40 nm when annealed below 180℃. As the annealing temperature increases to 270℃, the Ag nanoparticles on the film grow up and the height of Ag particles exceeds 100 nm. When the annealing temperature increases to 360℃, the height of some Ag particles on the Ag–Mo film reaches ∼200 nm. Therefore, the size and height of Ag nanoparticles on the surface of Ag–Mo film become larger with the increase of annealing temperature; as a result, the films become thinner due to the diffusion of a large number of Ag atoms to the surface of the film.

(a)–(d) Surface morphology and cross-sectional morphology and particle size distribution of Ag–Mo films with a film thickness of 296 nm after different annealing temperatures: (a) 90℃, (b) 180℃, (c) 270℃, and (d) 360℃; (e) the resistivity variation of Ag–Mo films with annealing temperature for different deposition times; (f) the average size variation of Ag particles on the Ag–Mo films with different thickness and different annealing temperature.
Figure 6(e) shows the variation of resistivity of Ag–Mo films with annealing temperature for different deposition times. Observing the change curve of resistivity of Ag–Mo film with annealing temperature for the film thickness of 296 nm, it can be found that the change of resistivity of the Ag–Mo film is very small when annealed below 90℃, and the film resistivity is close to 400 μΩ cm. As the annealing temperature increases from room temperature to 180℃, the film resistivity decreases slightly, which is due to the fact that annealing can improve the crystallinity of the film while reducing the defects in the film. However, with further increase in annealing temperature, the resistivity of Ag–Mo films suddenly increased, which can be attributed to the formation of larger-sized Ag nanoparticles on the Ag–Mo films hindering the electron transport, resulting in higher resistivity. After annealing at 360℃, the Ag nanoparticles on the Ag–Mo films became larger, increasing the electron transport path and further increasing the resistivity of Ag–Mo films, which is consistent with our simulation results using COMSOL Multiphysics software in Figure 7.

(a)–(d) Simulation results of the trajectories of current passage in the films with different Ag nanoparticle sizes calculated by COMSOL Multiphysics software: (a) no particles, (b) 300 nm particles (150 nm height), (c) particles grown up by merging into one particle, and (d) particles with multiple sizes.
The resistivity change curves of Ag–Mo films with a thickness of 163 nm and thickness of 409 nm with annealing temperature are observed in Figure 6(e), and it can be seen that their resistivity curves are the same as those of Ag–Mo films with a thickness of 296 nm. After the annealing temperature reaches 270℃, the resistivity of the film suddenly increases. It is attributed that high-temperature annealing promotes more atomic diffusion, and the growth of self-formed Ag nanoparticles on the surface of Ag–Mo thin films causes the change of particle size and roughness, which hinders the transmission of electrons. From Figure 6(f), it can be seen that the average size of Ag particles increases when annealed above 180℃, further verifying that Ag nanoparticles grow up with increasing annealing temperature and some new Ag nanoparticles are formed. In conclusion, the resistivity of Ag–Mo film is closely related to the size of Ag nanoparticles on the surface, and the larger the particles, the more undulations on the film surface, which hinders the electron transport and leads to the increase of resistivity of the film.
3.6 Analysis of the resistivity increase of annealed Ag–Mo film
A model of the film sample was built using COMSOL Multiphysics software to simulate and calculate the trajectory of the current passage, as shown in Figure 7(a)–(d). Figure 7(a) shows the trajectory of the current passage through the film without self-forming nanoparticles on the surface. It can be seen from Figure 7(a) that the current trajectory is straight without surface undulations, which can indicate that there is no effect on the electron transport trajectory when the surface of the film is smooth, and therefore, the film exhibits a lower resistance. As shown in Figure 7(b), the trajectory through the current is shifted when a lot of larger particles are formed on the film’s surface. This is because the electron collision with the surface changes the orientation velocity and increases the path of electron migration in the film, leading to an increase in film’s resistivity. As the particles on the surface of the film grow up, the contact and merged large particles increase the path of electron transport and impede the electrons much, as shown in Figure 7(c), which has an important effect on the film resistivity. Figure 7(d) shows a simulation of multiple-sized particles on the film surface, where current passes through the rough film surface and the transport path of electrons is shifted, resulting in an impeded transport of electrons. Therefore, self-formed Ag particles on the film surface can change the film surface roughness, which affects the electron transport in the film and leads to changes in resistivity.
4 Conclusions
Ag–Mo films of different thicknesses were prepared on flexible substrate PI by co-sputtering magnetron sputtering technique. A large number of single-crystal Ag nanoparticles were self-formed on the surface of the deposited Ag–Mo films. It was found that the resistivity of the Ag–Mo films was dependent on the film thickness and annealing temperature, and the resistivity of the Ag–Mo films decreased with increasing film thickness due to the better crystallinity of thicker Ag–Mo films. The resistivity of Ag–Mo film decreased slightly after annealing below 180℃; however, the resistivity of Ag–Mo film suddenly increased when the annealing temperature was higher than 270℃. When the annealing temperature is higher than 270℃, the resistivity of Ag–Mo films suddenly increases, and the resistivity of Ag–Mo films after annealing at 360℃ is more than three times that of the deposited state films. The increase in resistivity is attributed to a larger number of Ag nanoparticles on the Ag–Mo films, which hinders electron transport, resulting in an increase of the electron’s migration path. This is proven by the results of the COMSOL Multiphysics software simulation of electron migration. The high-resistivity Ag–Mo thin films are expected to be candidates for high-resistivity thin-film devices.
-
Funding information: The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. U1204521).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article.
References
[1] Zeng S, Baillargeat D, Ho H-P, Yong K-T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem Soc Rev. 2014;43:3426–52.10.1039/c3cs60479aSearch in Google Scholar PubMed
[2] He W, Ye C. Flexible transparent conductive films on the basis of Ag nanowires: design and applications: a review. J Mater Sci Technol. 2015;31:581–8.10.1016/j.jmst.2014.11.020Search in Google Scholar
[3] Xi X, Xu J, Li S, Song J, Yang W, Sun Y, et al. An Au nanofilm-graphene/D-type fiber surface plasmon resonance sensor for highly sensitive specificity bioanalysis. Sensors. 2020;20:991.10.3390/s20040991Search in Google Scholar PubMed PubMed Central
[4] Zhu W, Chen T, Liu Y, Fung S. Conduction mechanisms at low-and high-resistance states in aluminum/anodic aluminum oxide/aluminum thin film structure. J Appl Phys. 2012;112:063706.10.1063/1.4754011Search in Google Scholar
[5] El Beainou R, Cote J-M, Tissot V, Potin V, Martin N. Resistivity anisotropy of tilted columnar W and WCu thin films. Surf Coat Technol. 2021;421:127412.10.1016/j.surfcoat.2021.127412Search in Google Scholar
[6] Hauder M, Gstöttner J, Hansch W, Schmitt-Landsiedel D. Scaling properties and electromigration resistance of sputtered Ag metallization lines. Appl Phys Lett. 2001;78:838–40.10.1063/1.1345801Search in Google Scholar
[7] Lee BJ, Lee DC, Kim CS. Electrical properties of sputtered Ni-Cr-Al-Cu thin film resistors with Ni and Cr contents. J Korean Phys Soc. 2002;40:339–43.Search in Google Scholar
[8] Cuong ND, Kim DJ, Kang BD, Chang SK, Yoon SG. Characterizations of high resistivity TiNxOy thin films for applications in thin film resistors. Microelectron Reliab. 2007;47:752–4.10.1016/j.microrel.2007.01.014Search in Google Scholar
[9] Park IS, Park SY, Jeong GH, Na SM, Suh SJ. Fabrication of Ta3N5–Ag nanocomposite thin films with high resistivity and near-zero temperature coefficient of resistance. Thin Solid Films. 2008;516:5409–13.10.1016/j.tsf.2007.07.021Search in Google Scholar
[10] Narizuka Y, Kawahito T, Kamei T, Kobayashi S. Properties of high-resistivity Cr-Si-O thin-film resistor. IEEE Trans Compon Hybrids Manuf Technol. 1989;11:433–8.10.1109/33.16679Search in Google Scholar
[11] Achahour A, Leroy G, Vandamme L, Ayachi B, Duponchel B, Waldhoff N, et al. Suppression of contact noise in a study on 1/f noise as a function of film thickness in Al-doped ZnO. Thin Solid Films. 2018;645:70–6.10.1016/j.tsf.2017.10.035Search in Google Scholar
[12] Zhang W, Brongersma SH, Richard O, Brijs B, Palmans R, Froyen L, et al. Influence of the electron mean free path on the resistivity of thin metal films. Microelectron Eng. 2004;76:146–52.10.1016/j.mee.2004.07.041Search in Google Scholar
[13] Namba Y. Resistivity and temperature coefficient of thin metal films with rough surface. Jpn J Appl Phys. 1970;9:1326.10.1143/JJAP.9.1326Search in Google Scholar
[14] Ke Y, Zahid F, Timoshevskii V, Xia K, Gall D, Guo H. Resistivity of thin Cu films with surface roughness. Phys Rev B. 2009;79:155406.10.1103/PhysRevB.79.155406Search in Google Scholar
[15] Tatem EA, Kaloyeros AE, Eisenbraun ET. Thermal coefficient of resistivity of ultrathin Ag films deposited on Cu for applications in emerging interconnect systems. J Vac Sci Technol B Nanotechnol Microelectron Mater Process Meas Phenom. 2014;32:031801.10.1116/1.4868718Search in Google Scholar
[16] Lee H-Y, He C-W, Lee Y-C. The Effect of Yttrium Addition on the Microstructures and Electrical Properties of CuMn Alloy Thin Films. Adv Mater Sci Eng. 2019;2019:1–7.10.1155/2019/6578350Search in Google Scholar
[17] Zeng H, Wu Y, Zhang J, Kuang C, Yue M, Zhou S. Grain size-dependent electrical resistivity of bulk nanocrystalline Gd metals. Prog Nat Sci Mater Int. 2013;23:18–22.10.1016/j.pnsc.2013.01.003Search in Google Scholar
[18] Tseng W-T, Wang Y-L, Niu J. Microstructure-related resistivity change after chemical–mechanical polish of Al and W thin films. Thin Solid Films. 2000;370:96–100.10.1016/S0040-6090(00)00941-XSearch in Google Scholar
[19] Huang XX, Sun HL, Wang GX, Stock HR. Self-formation of Ag particles/Ag-Zr alloy films on flexible polyimide as SERS substrates. Appl Surf Sci. 2019;487:1341–7.10.1016/j.apsusc.2019.05.181Search in Google Scholar
[20] Lv Y, Sun H, Lian X, Shi P, Zhang H, Wang G. Influence of Co contents on the microstructure and SERS performance of self-formed Ag nanoparticles/Ag-Co alloy films on flexible substrates. Appl Surf Sci. 2021;563:150286.10.1016/j.apsusc.2021.150286Search in Google Scholar
[21] Brewer L, Lamoreaux RH. Molybdenum: Physico-chemical properties of its compounds and alloys. 1980.Search in Google Scholar
[22] Sun H, Lian X, Lv Y, Liu Y, Wang G. Effect of annealing on the microstructure and SERS performance of Mo-48.2% Ag films. Materials. 2020;13:4205.10.3390/ma13184205Search in Google Scholar PubMed PubMed Central
[23] Lian X, Lv Y, Sun H, Hui D, Wang G. Effects of Ag contents on the microstructure and SERS performance of self-grown Ag nanoparticles/Mo–Ag alloy films. Nanotechnol Rev. 2020;9:751–9.10.1515/ntrev-2020-0058Search in Google Scholar
[24] Marupalli B, Adhikary T, Sahu BP, Mitra R, Aich S. Effect of annealing temperature on microstructure and mechanical response of sputter deposited Ti-Zr-Mo high temperature shape memory alloy thin films. Appl Surf Sci Adv 6:110137.10.1016/j.apsadv.2021.100137Search in Google Scholar
[25] Minamide Y, Kawamura M, Abe Y, Sasaki K. Agglomeration suppression behavior and mechanisms of Ag–Cu and Ag–Nb thin films. Vacuum. 2009;84:657–62.10.1016/j.vacuum.2009.06.015Search in Google Scholar
[26] Sahu BP, Sarangi CK, Mitra R. Effect of Zr content on structure property relations of Ni-Zr alloy thin films with mixed nanocrystalline and amorphous structure. Thin Solid Films. 2018;660:31–45.10.1016/j.tsf.2018.05.050Search in Google Scholar
[27] Ezawa H, Miyata M, Tatsumi K. Alloying behaviour of electroplated Ag film with its underlying Pd/Ti film stack for low resistivity interconnect metallization. J Alloy Compd. 2014;587:487–92.10.1016/j.jallcom.2013.10.182Search in Google Scholar
[28] Wang Y-P, Ding Z-J, Liu Q-X, Liu W-J, Ding S-J, Zhang DW. Plasma-assisted atomic layer deposition and post-annealing enhancement of low resistivity and oxygen-free nickel nano-films using nickelocene and ammonia precursors. J Mater Chem C. 2016;4:11059–66.10.1039/C6TC03606FSearch in Google Scholar
[29] Wang T, Lu K, Qiu T, Zeng X, Ning H, Yang Z, et al. Highly conductive and adhesive ternary Cu–Cr–Zr alloy electrode for flexible optoelectronic applications. Superlattices Microstruct. 2021;157:106989.10.1016/j.spmi.2021.106989Search in Google Scholar
[30] Tang W, Xu K, Wang P, Li X. Surface roughness and resistivity of Au film on Si-(111) substrate. Microelectron Eng. 2003;66:445–50.10.1016/S0167-9317(02)00909-7Search in Google Scholar
[31] Tang W, Chao Y, Weng X, Deng L, Xu K. Optical property and the relationship between resistivity and surface roughness of indium tin oxide thin films. Phys Procedia. 2012;32:680–6.10.1016/j.phpro.2012.03.618Search in Google Scholar
[32] Hoffman R, Turnbull D. Lattice and grain boundary self‐diffusion in silver. J Appl Phys. 1951;22:634–9.10.1063/1.1700021Search in Google Scholar
[33] Maier K, Mehrer H, Rein G. Self-diffusion in molybdenum. Int J Mater Res. 1979;70:271–6.10.1515/ijmr-1979-700412Search in Google Scholar
[34] Lv Y, Sun H, Lian X, Zhang H, Shi P, Ma F, et al. Surface morphology evolution behavior and SERS performance of Mo-Ag-Cu-Co films. Appl Surf Sci. 2022;604:154594.10.1016/j.apsusc.2022.154594Search in Google Scholar
[35] Zhang H, Shi P, Lv Y, Li S, Liang S, Sun H, et al. Effect of Zr contents and covering Ag layer on microstructure and SERS properties of Cu nanoparticles/Cu-Mo-Zr alloy films on flexible substrates. Appl Surf Sci. 2022;606:154892.10.1016/j.apsusc.2022.154892Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

