Abstract
The application of micro-/nano-capsules in construction industries has been rising over the past decade. Polyurea with tunable chemical and morphological structure are of interesting polymers to prepare micro-/nano-capsules used in construction. The structure of polyurea micro-/nano-capsule is capable to be tailored via bulk emulsion or microfluidic method. Important factors for production of micro/nano-capsules are the rate of fabrication and having control over mean size, dispersity, and wall thickness. The bulk emulsion method provides higher yield of production with less control over sizes and dispersity in comparison to microfluidic technique. The main applications of polyurea micro-/nano-capsules in construction industries are categorized as thermal energy saving, self-healing concrete, self-healing polymers, and fire retarding. Polyurea showed appropriate thermal conductivity and mechanical properties which is required for encapsulation of phase change materials. Titanium dioxide polyurea microcapsules possess energy storage efficiency of 77.3% and thermal storage capacity of 99.9%. Polyurea microcapsules with sodium silicate cargo provided self-healing abilities for oil well cement in high temperature and showed higher self-healing abilities compared to gelatin microcapsules. Graphene oxide polyurea micro-/nano-capsules demonstrated 62.5% anti-corrosive self-healing efficiency in epoxy coating, and steel coated via dendritic polyurea microcapsules embedded polyurethane remained unchanged after long time immersion in salt water.
Abbreviations
- DETA
-
diethylenetriamine
- EDA
-
ethylene diamine
- EDX
-
energy dispersive X-ray spectroscopy
- GO
-
graphene oxide
- H-bond
-
hydrogen bond
- HDA
-
1,6-hexamethylene diamine
- HDI
-
hexamethylene diisocyanate
- H-MDI
-
4,4′-methylenebis cyclohexyl isocyanate (hydrogenated MDI)
- IPDI
-
isophorone diisocyanate
- MDI
-
methylene diphenyl diisocyanate
- MEPCM
-
microencapsulated PCM
- MgO
-
magnesium oxide
- mmpy
-
millimeters per year
- NP
-
nano particles
- PAE
-
polyaspartic acid ester
- PAMAM
-
polyamidoamine
- PCM
-
phase change material
- PDMS
-
polydimethyl siloxane
- PMMA
-
polymethyl methacrylate
- PU
-
polyurethane
- PUA
-
polyurea
- PUF
-
polyurea formaldehyde
- SEM
-
scanning electron microscope
- TDI
-
2,4-toluene diisocyanate
- TEPA
-
tetraethylenepentamine
1 Introduction
The high consumption of construction materials is recognized as one of the main sources of global warming [1]. On the one hand, modern technologies, such as self-healing and protective coating aim to improve the quality, efficiency, and lifetime of building materials. On the other hand, thermal energy usage is planned to be declined through combination of construction materials with energy storage ability [2,3]. The application of nanotechnology in construction industry has been the subject of recent studies [4]. Amongst them, encapsulation of materials in micro-/nano-scale has been more considered due to its great potentials for extensive applications. A micro-/nano-capsule comprises a material core with a special purpose and a shell for the material protection from miscibility, evaporation, reaction, or stress and sometimes release in a controllable way [5]. These unique properties of micro-/nano-capsule provide diverse solutions for challenges in many industries such as electronic [6], medicine [7], textile [8], cosmetics, chemicals [9], and construction [10].
The demand for microcapsules has been rising in recent years. The market is projected to increase from USD 11.9 billion in 2022 to USD 25.9 billion in 2030. Currently, the main application of microcapsules is in pharmaceutical and healthcare industry and construction industry posited a share of 5% of the total microcapsule market in 2021. However, the compound annual growth rate of microcapsules used in construction industry is expected to rise 9.7% from 2022 to 2030 [11]. Several polymeric materials have been used for preparing micro-/nano-capsules, including polyurea formaldehyde (PUF), polyurethane (PU), polymethyl methacrylate (PMMA), melamine formaldehyde, polyaniline, and gelatin. Amongst them, polyurea micro-/nano-capsule appeared to be a helpful shell for construction application. Polyurea is a copolymer derived from the reaction of diisocyanate and diamine in a polycondensation manner. Morphology of polyurea consists of hard and soft segment with high and low H-bond which led to a dense structure. The outer cement compatible surface of polyurea could help its dispersion within the concrete. Moreover, polyurea micro-/nano-capsules can preserve a broad range of materials such as energy saving [12] and bacteria [13] due to its waterproof, stiffness, and anti-corrosive properties. Besides, the compatibility of polyurea micro-/nano-capsule with polyurethane foam increases the mechanical properties of inflammable sandwich panels. Due to the presence of nitrogen in polyurea chemical structure, the reaction between the two main components (polyisocyanate and polyamine) happens quickly and it allows the fast production of polyurea shell through simple methods, microfluidic and bulk emulsion. Besides higher mechanical properties and chemical resistance, polyurea micro-/nano-capsules hold primacy over the other micro-/nano-capsules due to the ease of size, thickness, and morphology tuning.
The differences between nano-capsules and microcapsules relay on the capsule size. The mean diameter of nano-capsules ranges from 0 to 1,000 nm, whereas microcapsules average sizes are from 0 to 1,000 µm. In comparison to other methods, micro-/nano-capsules synthesized via chemical reaction mainly provide higher mechanical properties, chemical resistance, and durability which are required for construction applications. Interfacial polymerization is one of the most common chemical techniques for encapsulation in nano- or micro-scale. In this technique, which is based on the immiscibility of oil and water, one of the components of the reaction in tandem with the core material of the micro-/nano-capsules dissolved in oil phase or water phase. After that, micro-/nano-droplets with their cargo are formed in continuous phase through mechanical stirring, centrifuging, or microfluid devices. The second reactive component exists in the continuous phase or is added after droplets formation. In order to prepare polyurea micro-/nano-capsules, often interfacial polymerization method is used where two components (diisocyanate and diamine) find themselves in the interface of oil and water in micro-/nano-droplets wall and begin their polymerization reaction. Chemical structure of components highly influences the porosity, mechanical properties, and the fabrication rate of micro-/nano-capsules [14]. In case of polyurea, quick reaction between components in interfacial polymerization is beneficial due to its fast and uniform micro-/nano-capsules production. However, the swift polymerization of in situ manner where two components are in the same phase is accounted as a hindrance for better adjustment of micro-/nano-capsule mean size and diameter. Based on methods used for the preparation of droplets, other factors such as stirring rate, temperature, type of emulsifiers in bulk emulsion methods, flow rate, and osmotic pressure and again emulsifiers in microfluidic methods have been employed to provide desirable properties of micro-/nano-capsules, including wall thickness, mean diameter, and size distribution. Micro-/nano-capsules are then centrifuged, washed, filtered, and dried [15].
Polyurea micro-/nano-capsules have diverse applications in structural and infrastructural industry. It provides a quick-forming shell for energy saving materials with sufficient thermal capability. A small amount of polyurea micro-/nano-capsules in gypsum could considerably raise its thermal saving abilities. Polyurea micro-/nano-capsules contained sodium silicate or bacteria-embedded concrete provided self-healing properties and filled the concrete cracks. Another interesting application of polyurea micro-/nano-capsules in construction industry is to make self-healing and self-lubricating coats. The protection abilities of self-curable coats are demanding for concrete and steel used in aggressive environments. Key parameters of the interfacial polymerization, including temperature, stirring rate, and type of solvent were studied on the preparation of polyurea, polyamide, and polyester microcapsules [14]. It was obtained that polyurea possessed a denser microcapsule shell in comparison to polyamide with the same condition. The application of isocyanate-core microcapsules for preparing self-healing materials has also been reviewed in which polyurea, PU, and PUF microcapsules have been compared [16]. Results showed that microcapsules’ characteristics such as mechanical properties, core content, and shelf life are the consequences of the type of polymer, size diameter, and wall thickness. Applications of those microcapsules have been studied only for self-healing abilities in polymeric materials. Besides, microcapsules prepared via interfacial polymerization have been studied in terms of their synthesis and applications. Polyurea microcapsules showed a great long-term stability while it is used as a carrier [17]. Other studies also reviewed the application of micro-/nano-capsules in self-healing polymers [18], drug delivery [19], and textile industry [20]. The results of a study review on the building application of phase change materials (PCMs) micro-/nano-capsules showed that the encapsulation in nano-scale offered better performance than in micro-size. It also concluded that current encapsulation techniques suffer from high cost of processing and selecting appropriate materials [21]. However, there is no review studies that focus on the application of polyurea microcapsules in different areas of construction industry. Therefore, the focus of the present review is on the synthesis, preparation, and properties of polyurea micro-/nano-capsules used in construction industry. These applications are classified as thermal storage capability, self-healing concrete, self-healing polymer, and flame retardant, based on the existing research. Appropriate production methods of polyurea microcapsules for construction applications were also mentioned considering the key barriers of construction industry, including cost, rate, and simplicity of production. Chemical structures, mean size, and controlling parameters in different preparation methods were investigated. Comparisons also were made between polyurea micro-/nano-capsules with other micro-/nano-capsules used in structural and infra-structural industry to weigh pros and cons of polyurea applications. Evaluations are based on the important factors of construction applications, e.g., rheology and mechanical properties of resulting concrete, chemical resistance abilities, and self-healing efficiency of polyurea micro-/nano-capsules. The effects of nanomaterials and second layer shells on polyurea micro-/nano-capsules also were considered.
2 Structure and chemistry
The morphology of the polyurea plays a key role in emerging its unique properties. The understanding of the segmental structure in the polyurea chain would be helpful to design polyurea with desirable properties. The chain of polyurea consists of repeated polyurea group, which is a consequence of reaction between two compounds: a diisocyanate molecule that comes usually with a chain extender and an amine terminated polymer (Figure 1a). Accordingly, two separated phases were created through step polymerization of diisocyanate and diamine in a polyurea molecule. This segmented block copolymer contains high-Tg and low-Tg domains [22]. High-Tg domain is a segment with higher diisocyanate where the hydrogen bonding between diisocyanate enables polyurea with extra strength. Conversely, a soft domain composed of diamine, is mainly responsible for the elastomeric behavior of polyurea. Figure 1b and c illustrates the hard and the soft phase of polyurea. The exceptional mechanical behavior of polyurea mainly relies on its molecular structure. Main part of this copolymer consists of diisocyanate and diamine. Hexamethylene diisocyanate (HDI), methylene diphenyl diisocyanate (MDI), toluene diisocyanate (TDI), isophorone diisocyanate (IPDI), hydrogenated MDI (H-MDI) have been used for the preparation of polyurea micro-/nano-capsules as diisocyanate [23]. During the fabrication of microcapsules, the shape, morphology, and thickness of the shell membrane and its hardness are tunable through changing the concentration of diisocyanate [24]. A numerical study showed the considerable reliance of polyurea mechanical properties on the type of diisocyanate [25]. Diamines the second component of the interfacial reaction possess higher variety in their chemical structures, molecular weight, and source materials. Some common diamines are tetraethylenepentamine (TEPA), diethylenetriamine (DETA), ethylene diamine (EDA), polyamidoamine (PAMAM), polyethylenimine, and polyetheramine. The addition of other polymers was also studied during the polymerization or as a hybrid layer to design the desirable chemical structure. Polyols, PU, polyaspartic acid ester (PAE), polyphenyl isocyanate-co-formaldehyde, chitosan oligosaccharide, polyvinyl alcohol, and polydimethyl siloxane (PDMS) were used in combination with polyurea molecular structure. Also, polyurea micro-/nano-capsule with hybrid shells of PUF and nanomaterials have been prepared to raise the mechanical properties, avoid leakage, and increase the thermal transition [26]. Table 1 shows a number of studies for fabricating polyurea micro-/nano-capsule with various materials. In addition to diamine and diisocyanate, the reaction between isocyanate and water resulted in CO2 and polyurea foam [27] which has been used for the preparation of uniform polyurea nanospheres [28]. Greener production of polyurea materials has been studied, including the use of waste materials and biosources [29]. The high-rate reaction between diamine and diisocyanate is desirable for the fast production of micro-/nano-capsules. However, in condition where lower reaction rate is required, a secondary amine such as polyaspartic ester is used. The steric hindrance causes decrease in reaction time [30].
Different raw materials for the synthesis of various polyurea micro-/nano-capsules
| Polyurea chemical structure | Core | Ref. |
|---|---|---|
| MDI, water, and chitosan | Pesticides | Yu et al. [31] |
| IPDI, TDI, polyvinyl alcohol, and PU | Active agents | Xu et al. [32] |
| TEPA, IPDI, and PAE | Butyl stearate with reversible photochromic materials | Sun et al. [33] |
| TDI and 1,6-hexamethylene diamine (HDA) | Essential oils | Ferrándiz García et al. [34] |
| MDI, H-MDI, TEPA, PUF, and nano-Al2O3 | Cross-linking materials | Wu et al. [26] |
| TDI, EDA, HDA, and DETA | Herbicide | Rao et al. [35] |
| HDI and PAMAM | Self-lubrication oil | Tatiya et al. [36] |
| TDI and lysin | Drug | Zoabi and Margulis [37] |
Chain extenders are another chemical compound in polymerization of polyurea, which are added to the amine side as co-reactants. The role of chain extenders is poignant as a result of its hydrogen bonding effect on polyurea hard segments [38]. A combination of aromatic and aliphatic chain extenders would result in optimal H-bonding in the hard segment [39]. Aromatic chain extenders show rapid reaction with the diisocyanate compared to aliphatic chain extenders. Indeed, in aliphatic ones, the increment of length has negative effects on polyurea mechanical properties. This mainly relays on less cross-linking density (H-bonding) caused by higher distance between two urea linkages. Figure 1b shows the location of chain extenders, H-bond, and urea link in the morphology of polyurea. According to this schematic figure, the density of cross-linking is higher in the polyurea hard segment. Besides, the needle structure of the hard domain is illustrated in Figure 1c.
Because of the high density of its H-bonding which acts as a network of physical cross-linking, polyurea shows exceptional mechanical properties. The energy is damped in front of mechanical stress through opening and reconnecting H-bonds. Moreover, polyurea showed substantial compatibility to several nano-fillers such as nanoclay [41], graphene oxide (GO) [42], silica aerogels [43], and silicon carbide [44].
2.1 The comparison between polyurea and polyurethane
Polyurea and polyurethane both are prepared via polycondensation of the diisocyanate with another compound, and therefore both have segmental structures. Furthermore, the abbreviations of PU, PUR and PUA (only for polyurea) have been employed for polyurea and PU which led to some confusion in literature [45]. To clarify the differences between PU and polyurea structure, some important points should be highlighted. Diamines on one side of the polycondensation accelerate the reaction in comparison to diol in PU. After polymerization, two different functional groups are synthesized: NCO for PU and NCON for polyurea. Each nitrogen has a hydrogen attached to carbonyl (C═O) group (2:1). This bidentate H-bond is highly stiff and increase the melting point of the polyurea to their decomposition temperature; whereas, C═O bond has a weak monodentate H-bond in PU (1:1), and therefore the hard domain of PU shows less strong characteristics [46]. Figure 2 illustrates the bonding differences between polyurea and PU. In construction industry, polyurea is usually used as a waterproofing layer with higher mechanical properties, whereas the main application of PU is the production of sandwich panels [47]. The reaction of water with isocyanate has different effects on polyurea and polyurethane [48]. Isocyanate is hydrolyzed and produces carbamic acid through the reaction with water. This carbamic acid is then changed to CO2 and amine. After that, the amine can react with isocyanate and yields urea group in the structure of the main polymer. The presence of urea segment is not desirable in PU foams due to fibril structure and phase separation.
![Figure 2
Polyurethane and polyurea functional group and mono- and bidentate hydrogen bonding between hard segments [46].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_002.jpg)
Polyurethane and polyurea functional group and mono- and bidentate hydrogen bonding between hard segments [46].
As polyurea and PU contain same segmental structure and hard domain component, compatibility of polyurea with PU is higher than other polymers. Therefore, PU has been used in several studies as copolymer for fabrication of microcapsules with single or hybrid shells.
2.2 Micro-/nano-capsules preparation methods
Preparation methods of micro-/nano-capsules can be categorized as physical, physiochemical, and chemical [21]. All methods include dispersing of the core material (solid or liquid) into an appropriate media (liquid or gas) and formation of the wall material upon the core surface. Amongst chemical methods of preparation, in situ polymerization and interfacial polycondensation are numerously found in the literatures. PUF micro-/nano-capsules are commonly prepared through in situ polymerization, where two reactants are in the continuous phase at the beginning of the polymerization. In contrast, two components of interfacial polymerization are in different phases and polycondensation occurred at the interlayer between the micro-/nano-droplets and continuous phase. The latter technique is often used for preparing polyurea micro-/nano-capsules with high reaction rate. Interfacial polymerization of polyurea occurs at the surface of micro-/nano-droplets inside a non-reactive solvent. The droplets are formed through dispersing of the non-miscible phase in continuous phase via a stirrer [35], ultrasonication [49], or by a microfluidic device [50]. The biphasic structure can be formed through dispersing oil in water (o/w) or water in oil (w/o). The dispersion of oil in water, so-called on-water method, is commonly used for polyurea micro-/nano-capsules preparation that benefits from being safe and easy [51]. Nonetheless, there are a few studies that used organic phase as the continuous phase [48]. Recently, the oil-in-water-in-oil (O/W/O) and water-in-oil-in-water (W/O/W) methods were studied for the synthesis of polyurea microcapsules [52]. Resulted microcapsules possessed more control over releasing cargo and potential wider applications. After the formation of shell, micro-/nano-capsules are dried using spray drying or freeze-drying method to avoid aggregation. The process of polyurea preparation mainly depends on the industry type, such as medical or construction, the required accuracy, and the cost of production. Micro-/nano-capsule fabrication should meet certain mechanical properties to be employed in construction material. For instance, they should survive in mixing procedure with other building materials and prevent the considerable fall of the concrete mechanical properties [53]. From the environmental perspective, the production of micro-/nano-capsules is helpful and is more interested in industrial application due to the reduction of the solvent consumption and for the efficient heat transfer in aqueous culture [51].
2.2.1 Bulk emulsion method
Bulk emulsion technique is one of the common methods for the synthesis of micro-/nano-capsule. Figure 3 demonstrates the step-by-step formation of polyurea micro-/nano-capsules [35]. First, oil droplets of isocyanate have been formed inside water by the agitation of the mechanical stirrer, then diamine is added to the water and dissolved, finally polymerization occurred at the interface of the oil droplets. Besides, there are some studies that used organic phase as continuous phase. Surfactants are employed to provide emulsion through decreasing droplet diameter to micro-/nano-size [54]. The amount of dispersed phase, the ratio of TDI to HDI, PH value, and temperature are important parameters for the synthesis of well-shaped and defect-free micro-/nano-capsules of a desirable size. Also, the diffusion of the solvent into aqueous phase can avoid the appropriate encapsulation [24]. A model also was derived for predicting the crystallinity and molecular weight of polyurea formed in micro-/nano-capsule preparation. Regarding the capability of the current technology, the quantity of polyurea micro-/nano-capsule production via bulk emulsion technique is higher than microfluidic, yet there is a considerable portion of not encapsulated particles observed in the final product. This item plays a key role in the manipulation of microcapsules in construction industry where cargo leakage may lead to decrease in the life of the building materials [21,55]. Although studies showed that parameters, including emulsifier percentage, temperature, and stirring rate determine the particle size of micro-/nano-capsule, the precision of controlling shell thickness is lower in comparison to the microfluidic device. Bulk emulsion with continuous process has been studied for the production of polyurea micro-/nano-capsule, in an appropriate condition, this process led to produce 198 g (capsule)/h [56]. However, the process encountered some limitations; the higher micro-/nano-capsule concentration in the emulsion resulted in more collisions between droplets and the broad range of micro-/nano-capsule size. Therefore, the optimization of flow rate and monodispersed particles are required. This process was then compared with microfluidic method. The flow rate was 0.26 L/h in a recirculation loop reactor and 0.028 L/h in a similar microfluidic device, yet polydispersity is 39.4% in the loop reactor against 2.8% in microfluidic, which indicates the higher size variation in the particles.
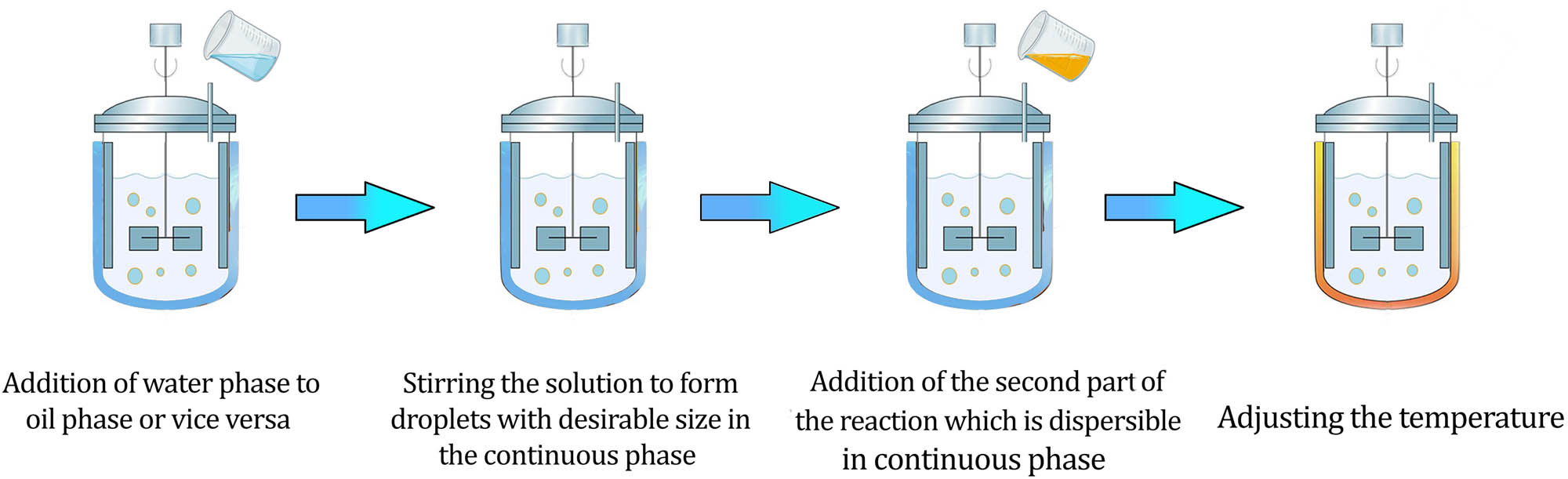
Preparation of polyurea micro-/nano-capsule via bulk emulsion technique.
The broad size distribution of droplet is obtained through bulk emulsion technique. The effect of the reactivity of the diisocyanate and diffusivity in different sizes of nano-capsules have been studied. Droplets with 83 nm diameter have been provided with ultrasonication [49]. Results of mathematical model confirmed by experiments showed that reaction of higher reactive diisocyanate underwent diffusion control process, whereas lower reactive isocyanates followed kinetics of reaction. In the bulk emulsion method increasing the agitation rate can lead to production of micro-/nano-capsules with smaller mean size. In a study [31], micro-/nano-spheres with the average diameter of 10.74 ± 0.94 µm and distribution width of 2.27 decreased to 4.20 ± 0.32 µm and 0.93, respectively, by increasing the agitation rate from 6 × 103 to 12.0 × 103 rpm. Additionally, rotating packed bed have been used for the preparation of polyurea micro-/nano-capsules [57]. In this method a high gravity is applied by the centrifugal force. The mean diameters of polyurea micro-/nano-capsules provided by this technique were about 11.88, 12.31, and 11.83 μm for 1,200 rpm.
2.2.2 Microfluidic method
Another method for preparing polyurea microcapsules is using microfluid devices, which can be fabricated through different techniques. Similar to the emulsion method, isocyanate is dissolved in oil phase and diamine in water phase. Oil microdroplets are then formed through injecting the oil phase into the continues phase (water) via a capillary tube or channel. There are several techniques for droplet formation in microfluidic devices such as terrace-like, cross-flow, co-flow, and focusing flow which are shown in Figure 4. Among the materials employed for making microfluidic chip, PDMS with planar shape and glass capillary with 3D geometry are the most commonly used. Also, the focusing flow technique is widely used for composite droplets, e.g., core–shell droplets. As shown in Figure 5, the device includes a set of capillary glass tubes and a controllable pumping force to fabricate microcapsules. By the aid of the microfluidic devices, microcapsule with a broad range of thickness and diameter can be provided [58]. As the permeability, stability, and releasing function of microcapsules are highly dependent on their size [59] narrow size distribution is required for their maximum performance [60]. Microfluidic method can offer more control over microcapsules’ preparation and provide uniform droplet size with narrow dispersity [61]. Although controlling over the mean size and wall thickness of polyurea micro-/nano-capsules is higher in this technique, industrial scaling of microfluidic methods is still the subject of many recent research studies [62]. Different flow rates of encapsulated phase were compared in various methods of microfluidic and bulk emulsion. High throughput glass microfluidic device, microfluidics, microchannel emulsification, and micromixer possessed 0.004, 0.0015, 0.025, 0.06 L/h, respectively. In contrast, bulk emulsion methods showed higher yields. Flow rates of encapsulated phase for membrane emulsification with porous glass, static mixer in the recycled loop, and recirculation loop reactor were 0.9, 11.94, and 0.26 L/h, respectively. It seems that in comparison to microfluidic method, emulsion methods have higher production yield in industrial scale. However, microfluidic methods are more desirable for production of monodisperse hybrid shell with two or three layers, due to the possibility of controlling liquid flow and then collecting the emitted microcapsules in the capillary [63].
![Figure 4
Different method of creating micro-/nano-capsules by microfluidic devices [64]: left 2D PDMS devices and right 3D glass capillary.](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_004.jpg)
Different method of creating micro-/nano-capsules by microfluidic devices [64]: left 2D PDMS devices and right 3D glass capillary.
![Figure 5
(a) Schematic figure of emulsion drop generation by focusing flow and (b) real micro-/nano-graph of the glass capillary during the micro-/nano-droplet formation [58].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_005.jpg)
(a) Schematic figure of emulsion drop generation by focusing flow and (b) real micro-/nano-graph of the glass capillary during the micro-/nano-droplet formation [58].
Polyurea monodisperse microcapsules were prepared by controlling the flow rate of water phase and oil phase in microfluidic devices [58]. MDI dissolved in limonene, used as oil phase, TEPA, SDS, and NaCl were dissolved in water phase. Oil droplets were formed in the microfluidic device by the aid of an air pump. In another study, polyethylenimine and TEPA, as well as TDI were used to study the effect of different surfactants in the microfluidic device. Osmotic driven inflation of 0–1,740 kPa resulted in 66–125 µm microcapsule mean size with thickness of 80 to 120 ± 12 nm. Results show that increasing the HLB (hydrophilic to hydrophobic) ratio of the surfactant will enhance the stiffness and permeability of the polyurea shell. It was also found that the more solubility of the amine in the oil phase, the thicker the polyurea shell would be [50].
Bulk emulsion technique possesses poor control over the size of micro/nano-capsules and the distribution size due to the formation of preparing droplet with homogenizer. Conversely, microfluidic devices use adjustable high shear force to fabricate droplets out of the two immiscible flow streams. This provides higher control over size and dispersity [61]. According to the results, at the constant continuous flow rate (Q c) of 100 µL/min and altering the flow rate of disperse phase (Q d) from 1, 2, 3, 4, and 5 µL/min, polyurea microcapsules with mean sizes of 27, 30, 32, and 34 µm were obtained. Figure 6 compares the narrow results of polyurea microcapsules size dispersity prepared by microfluidic method at Q d = 5 µL/min to a typical outcome of bulk emulsion technique. The broad and bimodal distribution of microcapsules’ size in the range of 0–16 µm has been obtained for the bulk emulsion; whereas, the microfluidic method endowed microcapsules with uniformity and narrow dispersity between 32 and 36 µm.
![Figure 6
Size distribution of emulsion droplets produced by standard and microfluidic methods. (a and b) Optical microscopic images of o/w emulsions produced by (a) homogenizer and (b) microfluidic chip (Q
c = 100 μL/min, Q
d = 5 μL/min). (c and d) Histograms of droplet size distribution from (a and b) analyzed by ImageJ for (c) homogenized and (d) microfluidic chip produced droplets (Q
c = 100 μL/min, Q
d = 5 μL/min) [61].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_006.jpg)
Size distribution of emulsion droplets produced by standard and microfluidic methods. (a and b) Optical microscopic images of o/w emulsions produced by (a) homogenizer and (b) microfluidic chip (Q c = 100 μL/min, Q d = 5 μL/min). (c and d) Histograms of droplet size distribution from (a and b) analyzed by ImageJ for (c) homogenized and (d) microfluidic chip produced droplets (Q c = 100 μL/min, Q d = 5 μL/min) [61].
3 Polyurea micro-/nano-capsules in the construction industry
The consumption of cement has reached four billion tons, contributing to 5–7% of total carbon dioxide emission [65]. The quality of construction materials is regarded as an important factor in global warming [66]. One of the main advantages of micro-/nano-capsules in the construction industry is the reduction of concrete consumption by avoiding deterioration. The life-time of concrete in normal conditions has increased using self-repairing technologies based on life-cycle assessment methodologies [67]. The deterioration is more significant in marine conditions because of chloride ions, where lifetime increment from 7 years goes up to 60–94 years through self-healing technologies. The benefit of the micro-/nano-capsule application in the construction industry is not limited to self-healing properties, energy-saving materials also contribute to 14–21% energy saving in cases of using micro-/nano-capsules containing PCMs [68]. The application of micro-/nano-capsules in the construction industry is widening, wherein polyurea is considered as one of the most advantageous polymer [69], which is due to its compatible nature with building materials, rate of production, and size distribution control. Various applications of polyurea micro-/nano-capsules have been illustrated in Figure 7. The applications are divided into storage materials as well as storage and releasing cargo at the proper time. The differences between the design of micro/nano-capsules for discharging is to consider the appropriate shell thickness and mechanical properties at the time of release.
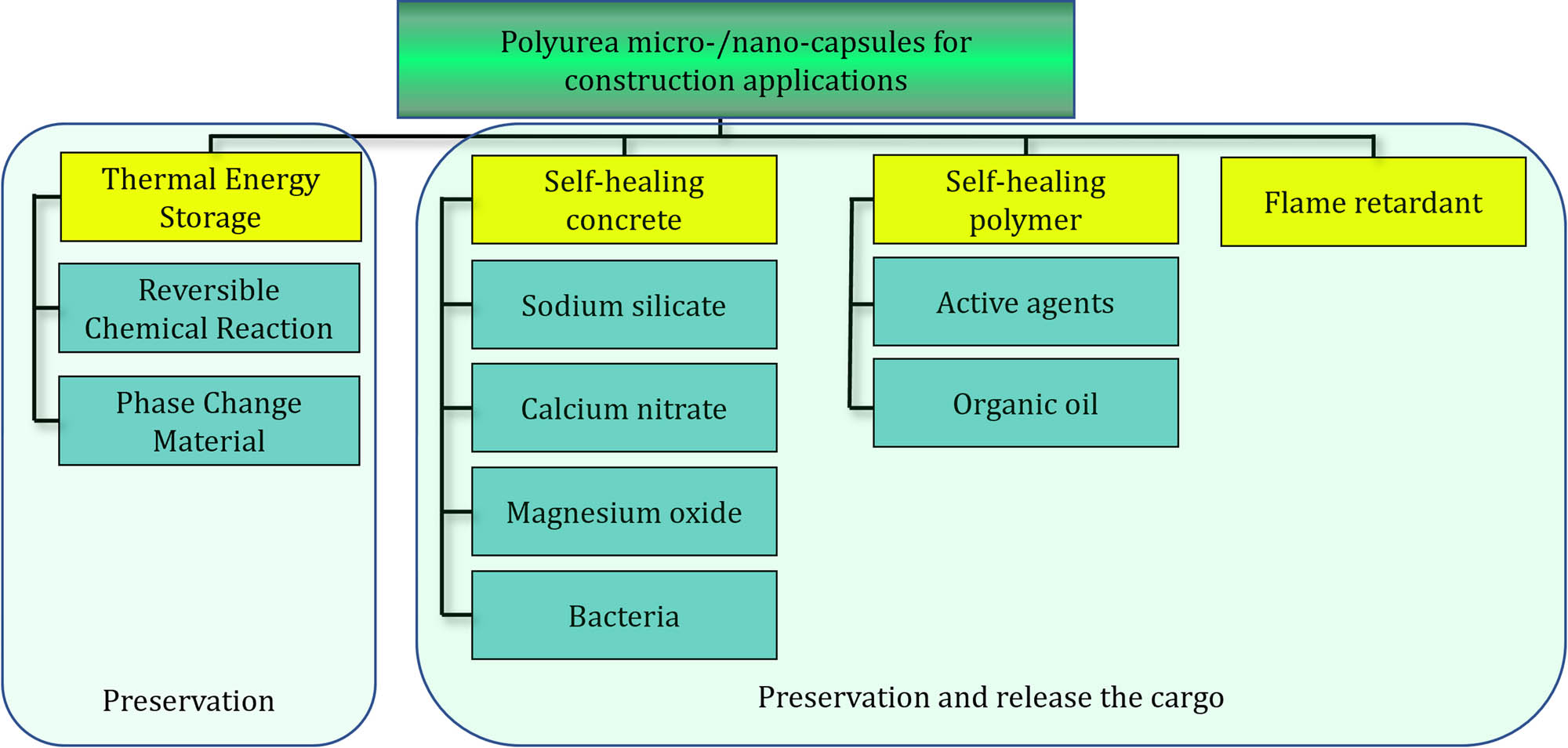
Summary of applications of polyurea micro-/nano-capsule in the construction industry.
In addition to these applications, micro-/nano-capsules of polyurea were used to encapsulate biocide for construction materials [70]. The biocide property also improved the anti-biofouling coat in marine conditions [71].
3.1 Thermal energy storage
One of the main concerns in developing building materials is their capability of saving energy. Different methods of thermal energy storage, including sensible heat storage, latent heat, and chemical energy storage have been studied [72]. In the latent heat method, energy is saved and then released during the physical transformation of melting and crystallization, whereas, in the chemical energy storage method, energy is released through the chemical reaction, or the water sorption. The design of buildings by which no mechanical devices are used for heating and cooling is called passive solar technologies. Energy saving materials are used for passive solar technology. As micro-/nano-encapsulation provides stability, longer life, preservation, and higher active surface, encapsulated energy saving materials are widely used in construction industries. Polyurea has desirable properties for encapsulation of energy saving materials. The encapsulation efficiency and structural stability of polyurea are highly desirable for synthesizing PCM micro-/nano-capsules [21]. Table 2 summarizes the studies focused on micro-/nano-size encapsulation of PCMs and reversible chemical materials by polyurea, in which effects of micro-/nano-capsules addition in cement, gypsum, or a polymeric coat were studied. In addition to construction industry, energy saving abilities of microcapsules possess an extensive area of application such as textile [73], electronic [74], and medicine [75]. Nevertheless, the application of PCM microcapsules in particular for building construction has been recently reviewed due to its crucial role in energy consumption [21,55]. PCMs used in building materials should possess appropriate temperature of melting (22–33°C) and their mechanical properties promise their survival during mixing with other building materials. In addition, special devices are required for evaluating the amount of energy saving that PCM micro-/nano capsules provide [3,76]. Figure 8 shows a part of a device setup for studying polyurea microcapsules that contain PCM. As shown, the building wall embedded with polyurea microcapsules was exposed to radiation and convection heating. Solar light was simulated in another chamber and highlighted with orange arrows, the convective heat process was simulated by the aid of heating and cooling devices.
Application of polyurea micro-/nano-capsules as energy storage materials
| Shell material | Core material | Ref. |
|---|---|---|
| PCM | ||
| Polyurea/siloxane | Salt hydrate | Yang and Kim [77] |
| Polyurea | Cyclohexane | Zhou et al. [57] |
| Polyurea/graphite in gypsum | Paraffin and graphite | Xie et al. [76] |
| Polyurea/TiO2 in cement | n-Octane | Zhao et al. [78] |
| Polyurea/graphite in cement | Paraffin and graphite | Zhang et al. [79] |
| Polyurea | Salt hydrate | Wang et al. [80] |
| Polyurea in cement paste | Paraffin | Zhan et al. [15] |
| Polyurea | Paraffin | Shi et al. [81] |
| Polyurea/Fe3O4 nanoparticles | n-Octadecane | Lone et al. [82] |
| Reversible chemical material | ||
| Polyurea | Butyl stearate with spirooxazine | Sun et al. [33] |
| Polyurea/PU | Xylitol | Salaün et al. [83] |
| Polyurea/PU | Xylitol | Salaün et al. [84] |
![Figure 8
Device setup for calculation of the amount of energy saving in a building wall with polyurea microcapsules addition [76].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_008.jpg)
Device setup for calculation of the amount of energy saving in a building wall with polyurea microcapsules addition [76].
3.1.1 PCM
PCMs are nowadays considered as a useful technology for energy saving of buildings. These materials show a higher potential of commercial application in construction industries and several studies investigated the types of materials with the capability of melting and solidifying during the day–night temperature fluctuation. PCMs use the latent heat of reversible crystallization that happened during the liquid-to-solid or solid-to-solid phase transition. Paraffin, n-hexadecane, and tetradecane are some prevalent PCMs. In addition to having an appropriate melting temperature, PCMs have two major characteristics: low expansion during the phase change and large surface area per unit volume, resulting in higher efficiency of heat transfer. Although PCMs have directly been used in passive solar panels [85], micro-/nano-encapsulation provides many benefits for PCMs construction applications such as preventing from reaction with their environment, increasing the compatibility with the media, and enhancing the heat transfer through more surface-to-volume ratio [78]. Considering the requirements, many parameters could be a candidate as material for micro-/nano-capsule shells. The heat absorbing and release rate are related to the type and thickness of the shell wall, ensuring the energy efficiency of the core–shell and the construction material environment. This parameter handles rapid thermal response. At the same time, the mechanical properties of the shell should prohibit any rupture and fracture against production or application processes [80]. Polyurea satisfies these requirements and provides possibilities for designing the required thickness with aforementioned desirable properties [21]. Further improvements on materials of polyurea micro/-nano-capsules have been performed and PCMs were encapsulated through polyurea with GO and hybrid shell. Besides, the method of synthesis has a key role on the properties of the resulting materials where using a rotating packed bed provided well-shaped micro-/nano-capsules with higher mechanical properties and more efficient energy storage. Unique properties of shell produced by polyurea containing paraffin resulted in the fabrication of PCMs and its insulation properties in the presence of solar radiation. In order to increase the thermal conductivity of the micro-/nano-capsules and decrease the strength loss in the resulting building material, flaky graphite-doped polyurea micro-/nano-capsule was also prepared [79]. Results showed that these materials not only lower the temperature of the wall and indoor of the test rooms by a maximum of 13.1 and 6.22°C, but also the flexural and compressive strength of the resulting cement composite was in the satisfying area of standard building materials (14.2 and 4.1 MPa, respectively).
In another study, the application of polyurea microcapsules in gypsum board increased the temperature of the board surface by 2, 4, 5, and 7°C at the radiant flux density of 200, 400, 600, and 800 W/m2 [76]. Actual core content, the ratio of fusing enthalpy of polyurea to pure PCM, and the encapsulation efficiency [86] are main parameters of evaluating the amount of energy saving. Besides, mean diameters, distribution sizes, and wall thickness should be adjusted for the maximum energy saving efficiency considering the required mechanical properties [72].
Thermal properties of PCMs with polyurea shell have been studied. The actual core content and the encapsulation efficiency were 42.5 ± 0.5 and 85.0 ± 0.92, respectively, which reached to 79.60 ± 0.17 and 63.67 ± 0.14 by increasing the amount of PCMs in the process of encapsulation. In another study, titania-polyurea microcapsules were used to produce PCM and applied in cement paste [78]. The schematic picture of hybrid polyurea shell is depicted in Figure 9. Scanning electron microscope (SEM) images also confirmed the second layer of titania-polyurea. The melting and freezing cycles indicate the excellent thermal stability and strength of the polyurea/TiO2 shell. The amount of octane core increases from 56.5 to 72.8 wt% via increasing the microcapsule sizes from 100 to 300 µm. Energy storage efficiency and thermal storage capacity were 77.3 and 99.9%, respectively. In another study, the crystallization of PCM inside polyurea microcapsules was investigated through the addition of nanoparticles of Fe3O4 to the n-octadecane which made them magnetic. The use of microfluidic device for the preparation of microcapsules resulted in the uniform sizes of microcapsules and near 100% efficiency of encapsulation. Figure 10a–c shows the uniform polyurea microcapsules, microcapsules with Fe3O4 nanoparticles, and a photograph of microcapsules suspension in a vial, respectively. Their response to the magnetic field is also shown in Figure 10d which indicates that nano particles (NPs) inside polyurea microcapsules were stable after washing. The addition of Fe3O4 NPs, led to the increase of the heat flow of PCM microcapsules from 159.5 to 169.7 J/g and from 159.1 to 165.7 J/g in cooling and heating process.
![Figure 9
Schematic drawing of (a) PCM micro-droplet, (b) microencapsulated PCMs (MEPCMs) with PUA shell, and (c) MEPCMs with TiO2–PUA shell. Micrographs of (d) individual MEPCM with PUA shell and (e) individual MEPCM with TiO2–PUA shell. (f) Fabricated free-flowing MEPCMs [78].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_009.jpg)
Schematic drawing of (a) PCM micro-droplet, (b) microencapsulated PCMs (MEPCMs) with PUA shell, and (c) MEPCMs with TiO2–PUA shell. Micrographs of (d) individual MEPCM with PUA shell and (e) individual MEPCM with TiO2–PUA shell. (f) Fabricated free-flowing MEPCMs [78].
![Figure 10
Optical images of (a) PCM@polyurea microcapsules without Fe3O4 NPs in the discontinuous phase, (b) PCM@polyurea microcapsules generated by encapsulating Fe3O4 NPs along with n-octadecane in the discontinuous phase, captured at the reservoir at 35 °C, (c) photograph of PCM@polyurea microcapsules with encapsulated Fe3O4 NPs, and (d) magnetically aligned microcapsules in the image (c) under an external magnetic field at room temperature [82].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_010.jpg)
Optical images of (a) PCM@polyurea microcapsules without Fe3O4 NPs in the discontinuous phase, (b) PCM@polyurea microcapsules generated by encapsulating Fe3O4 NPs along with n-octadecane in the discontinuous phase, captured at the reservoir at 35 °C, (c) photograph of PCM@polyurea microcapsules with encapsulated Fe3O4 NPs, and (d) magnetically aligned microcapsules in the image (c) under an external magnetic field at room temperature [82].
Nano-capsules of polyurea/Fe3O4 nanoparticles with the size of 400–600 nm were prepared via O/W emulsion method through stirring rate of 8,000 rpm [87]. The presence of Fe3O4 nanoparticles provided the magnetic property to the polyurea PCM nano-capsules and elevated the thermal conductivity of the shell. However, increasing the amount of Fe3O4 nanoparticles from 0 to 6.6% led to the decrease of the melting and freezing latent heat from 101.1 and 105.6 to 83.28 and 87.4 J/g, respectively, due to the higher density of nanoparticles.
3.1.2 Reversible thermal reaction micro-/nano-capsule
The reversible thermal reaction is another form of energy-storing by polyurea micro-/nano-capsules. Crystalline polyols or sugar alcohol, salt hydrate, and anhydrous salts are capable to release their storage energy when their molecular structure is altered in different temperatures. The preparation and heat release of xylitol encapsulated by polyurea/PU have been studied. Water sorption of xylitol releases negative heat solution of −36.5 kcal/kg at 35°C which is higher than several PCMs [83]. Polyurea micro-/nano-capsules with mean pore size of 19.6 ± 1.7 and 15.3 ± 2.0 µm and encapsulated xylitol of 15.1 and 77.1% showed 24.4–124.3 J/g heats of dilution at 35°C, respectively. The yield of shell formation increased from 16.2 to 30.9% by increasing the ratio of MDI from 16.6 to 28.5%. In another study, polyurea micro-/nano-capsules with xylitol core and mean size of 19.3 ± 2.7 μm which had dilution heats of 13.9–179.9 J/g were obtained [84].
Other energy saving materials using the reversible chemical reaction are organic photochromic compounds which can be divided to chemical bond cleavage, cis–trans isomerism, and pericyclic reaction type. These materials can raise the energy saving ability in PCM microcapsules. One of the common chemical bond cleavage types is spirooxazine. The energy storage mechanism of spirooxazine relays on the ring-opening reaction of its C–O bonds where it transformed from a colorless state to its color isomer anthocyanin at the exposure to ultraviolet radiation. In addition, the ring-opening anthocyanin structure had a large conjugated molecular system. Due to the stability of spiral form, the molecule tends to return to this configuration after stopping the ultraviolet radiation, returning energy in a considerable amount [33]. This material was dissolved in butyl stearate as PCM, before encapsulation. Three types of diamines were selected to study their polyurea micro-/nano-capsule size and surface via in situ polymerization. Mean sizes of 0.72–5.64, 1.32–20.11, and 0.86–4.15 µm were obtained as the result of the reaction of TEPA, low NH PAE, and high NH PAE with IPDI, respectively. SEM results also showed that high NH PAE and TEPA had softer surface, whereas micro-/nano-capsules prepared by low NH PAE was thin and brittle because of fast and uncontrollable reaction. As a result of 1:1, 1:1.5, 1:2, and 1:2.5 ratio of IPDI to high NH PAE, micro-/nano-capsules with crystallization enthalpy of −72.94, −72.14, −72.73, and −73.56 J/g were obtained. Results show that the temperature of polyurea microcapsules containing PCM with photochromic materials were 4.9°C higher than the temperature in same microcapsules without photochromic properties.
3.2 Self-healing concrete
Self-healing or self-repairing ideas for concrete arise from the healing of wounds in trees, animals, or the human body. Concrete is susceptible to numerous cracks that appear inside due to intrinsic shrinkages, weathering, chemicals, and other aggressive factors. These cracks are responsible for the common decrease in concrete lifetime and strength. Self-healing concrete is recognized as prevalent technology for increasing the service life of concrete [67]. The use of self-healing concrete appears to be a rational method for saving concrete structure, especially in marine situations. Two main categories of autogenous (fiber- or mineral-reinforced) [88] and autonomous (micro-/nano-capsules) strategies or a combinational method for self-healing or fluorescence labeling of concrete [89] have been studied in recent literature. There are four methodologies for active agents used in self-healing concrete [90]. Coacervation, in situ polymerization, interfacial polymerization, and sol–gel reactions. Coacervation, the physio-chemical process is cross-linking of phase separated macromolecular lump (coacervate) such as gelatin/gum acacia. Sol–gel method is the formation of colloidal solution of silica from hydrolyzed tetraethoxysilane and through adding a trigger solution such as ammonia. Polyurea micro-/nano-capsules as an autonomous healing method can contain sodium silicate, colloidal silica, calcium nitrate, magnesium oxide (MgO), and bacteria as expanding and self-healing agents [91]. In addition to their shell thickness, adhesivity to the concrete is an important characteristic of micro-/nano-capsules used in self-healing concrete when encountering concrete cracks and contributes to their rupture while concrete cracking. Studying the effect of shell thickness of acrylate microcapsules in concrete showed that microcapsules with 7 and 12 µm wall thickness possessed the same amount of rupture in the cement paste. In contrast, polyurea linkage with concrete were confirmed by the characterization results which led to appropriate time and efficient exempt of materials [13]. The summary of the application of polyurea micro-/nano-capsule as self-healing admixture in concrete has been gathered in Table 3. Although self-healing agents increase the concrete lifetime by preventing the concrete crack, addition of external particles in concrete caused reduction in its compressive strength. Nevertheless, the scale of self-healing agents contributes to the amount of reduction effect on compressive strength of the concrete. The finer the admixtures, the denser the concrete [92].
Polyurea micro-/nano-capsule for self-healing concrete application
| Description | Ref. |
|---|---|
| Sodium silicate core | |
| Polyurea shell in oil well cement | Mao et al. [53] |
| Comparison polyurea and gelatin shell in concrete | Kanellopoulos et al. [93] |
| Comparison polyurea and gelatin shell in concrete | Giannaros et al. [94] |
| Polyurea/PU shell colloidal silica core | Tan et al. [95] |
| Calcium nitrate core | |
| Polyurea shell magnetic capsule | Kleinfeldt [96] |
| Polyurea/PU shell | Reinke [97] |
| MgO core | Mao [98] |
| Bacteria encapsulation | Zamani et al. [13] |
3.2.1 Sodium silicate
Reaction of sodium silicate with calcium hydroxide not only heals the concrete cracks but also produces calcium silicate hydrate (C–S–H) gel, which is durable and resistant to aggressive environments. Calcium hydroxide is susceptible to acid attack and is water soluble. In conditions that sodium silicate is directly mixed with concrete, processing encounters several problems such as altering the setting time and efflorescence, whereas the encapsulated sodium silicate provides the opportunity for in situ curing after the cracks appear in the concrete. Due to the possibility of using polyurea at higher temperature without losing mechanical performance, their application on oil well cement at a temperature of 80°C has been studied [53]. Stiff and elastic types of polyurea referred as T1 and T2 were employed to prepare microcapsules as self-healing materials for oil well concrete. Microcapsules with mean size of 110 µm contained 90% sodium silicate cargo and 10% polyurea shell decreased the crack depth by 13 and 58% through 2.5–7.5% addition. However, compressive strength was also reduced by 26 and 40% for stiff and elastic polyurea shell. Figure 11 shows the SEM and energy dispersive X-ray spectroscopy (EDX) of the T1 microcapsules. In SEM image, C–S–H gel was found in a form of fibrous materials near the residual shell of the T1 microcapsules on the cracking surface. EDX was used to determine the chemical elements of the fibrous materials. The results of EDX also confirmed the release of sodium silicate cargo of the ruptured microcapsules at the cracking surface and the healing reaction of calcium hydroxide with sodium silicate.
![Figure 11
SEM observations of the healed oil well cement pastes containing T1 microcapsules (a) various SEM images from the fractured surfaces and (b) an SEM image of a microcapsule and the corresponding EDX analysis results of ruptured microcapsules [53].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_011.jpg)
SEM observations of the healed oil well cement pastes containing T1 microcapsules (a) various SEM images from the fractured surfaces and (b) an SEM image of a microcapsule and the corresponding EDX analysis results of ruptured microcapsules [53].
A study compared two commercial types of polyurea and gelatin microcapsules with the core of sodium silicate that were referred as T130 and L500, respectively [93].
Figure 12 compared the sorptivity results of cement paste with two different microcapsules. It was obtained that the addition of polyurea microcapsules had a higher self-healing effect of concrete and reduced the sorptivity of concrete by 45% in 7 days, whereas the sorptivity of concrete enhanced by gelatin microcapsules decreased by only 15% in the same period [94]. The effect of sorptivity reduction in cement paste is observed. Figure 13 shows the improvement in sealing of cracks in cement pastes with polyurea microcapsules.
![Figure 12
Sorptivity of cracked specimens containing L500 gelatin microcapsules (blue line) and T130 polyurea microcapsules (red line) at 4% volumetric fraction compared with cracked cement specimens (black line) [94].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_012.jpg)
Sorptivity of cracked specimens containing L500 gelatin microcapsules (blue line) and T130 polyurea microcapsules (red line) at 4% volumetric fraction compared with cracked cement specimens (black line) [94].
![Figure 13
Sorptivity measurements are taken over a 28-day healing period. Comparison of later absorbed by cement (left) control cement paste samples and (right) cement paste samples containing 4% T130 microcapsules (polyurea microcapsules). Testing is after a s7-day healing period [94].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_013.jpg)
Sorptivity measurements are taken over a 28-day healing period. Comparison of later absorbed by cement (left) control cement paste samples and (right) cement paste samples containing 4% T130 microcapsules (polyurea microcapsules). Testing is after a s7-day healing period [94].
Also, the percentage of adding microcapsules above 12% reduced the compressive strength by about 24–27%. In this work, micro-/nano-capsules with the mean sizes of 40–700 µm have been employed to investigate healing of concrete cracks with different width.
The addition of microcapsules with mean size under 5–30 µm (Portland cement sizes) in concrete can fill the concrete voids, whereas higher mean sizes contain more sodium silicate and thus higher healing efficiency. Rheological properties of concrete embedded gelatin microcapsules with mean size of 500 µm also compared with concrete enhanced by polyurea microcapsules with 130 µm mean size. Results showed that concrete viscosity increased by gelatin and polyurea microcapsules by 54 and 47%, respectively, reducing the concrete compressive strength [94].
3.2.2 Calcium nitrate
Calcium nitrate is one of the main materials used as the core of micro-/nano-capsules to provide self-healing abilities in concrete. The self-healing mechanism of calcium nitrate mainly relies on hydration acceleration and crystallization of cement by cations resembling tricalcium silicate and dicalcium silicate. Indeed, the reaction of calcium hydroxide via calcium nitrate results in calcium hydroxy nitrate with higher basic capacity and greater abilities of hydrosilicate formation. The presence of calcium nitrate led to the formation of calcium carbonate in the cracks which intense the healing abilities of calcium carbonate. Calcium nitrite containing micro-/nano-capsule of polyurea/PU has been employed in steel bar-reinforced concrete. The calcium reacted with water anywhere cracks occurred in the concrete, and provided 58% of crack recovery and protection of rebars from corrosion [97]. The addition of nanomagnetic materials to polyurea microcapsules was proposed as an idea in another study [96]. In this method, magnetic polyurea microcapsules can reach to the location of steel embedded inside the reinforced concrete through running an electric current in the wire, which has not yet been studied. The water in oil emulsion method was employed to synthesize magnetic polyurea microcapsules containing calcium nitrate and sodium silicate as core materials. Results showed adding 0.3 wt% hydrophobic nano iron oxides caused phase separation, whereas addition of 0.10 wt% resulted in the stabilized emulsion of polyurea microcapsules with average size of 10 µm. The presence of calcium nitrite provides corrosion resistance abilities at the interface of rebar by penetration through the concrete and repassivating the steel. Magnetized polyurea microcapsules with PCM materials as cargo are shown in Figure 10. Anti-corrosion properties of PUF microcapsules for steel bar in reinforced concrete have been studied [99]. The method mainly relays on the release of calcium hydroxide core at low PH near the rebar to form a passive film.
3.2.3 MgO
The shrinkage of cement causes cracks inside the concrete decreasing the mechanical properties. MgO is one of expanding materials used for the shirking compensation through reaction with water and producing MgOH. In this self-healing technique, MgO increases in volume to 117% which is able to fill the hollow canals. The micro-/nano-encapsulation of MgO for self-healing of the cement has been investigated [100]. As the polyurea shell is serviceable at higher temperatures, the application of MgO and sodium silicate polyurea micro-/nano-capsule has been studied for crack healing of oil well cement [98].
3.2.4 Bacteria encapsulation
Recent technologies are using biological methods for repairing concrete cracks [101,102]. Amongst different methods of using bacteria for healing concrete, micro-/nano-encapsulated bacteria showed higher self-healing properties [103]. In a study, self-healing polyurea micro-/nano-capsules filled with bacteria have been employed to repair concrete cracks. Since the cracks appear in the concrete, the polyurea shell releases the bacteria and nutrition materials. As oxygen and moisture are available, the bacteria began to charge calcium carbonate inside the crack. Polyurea has met the requirements of an appropriate shell because, first, the novel chemical structure and multiple donner acceptor bonds of urea led to intermolecular hydrogen band, substantial mechanical stability as well as chemical and water resistance properties which protect the bacteria from the alkali and wet environment, second, the fast reaction makes the simple and tunable production of the capsule [13]. Figure 14 shows the crack repairment of the concrete through metabolic conversion of organic compounds to calcium carbonate. The mean sizes of polyurea micro-/nano-capsules containing bacteria from 0.2 to 1.3 mm were prepared by stirring polyetheramine and MDI at 400 rpm. Optical density at 600 nm showed that the bacterial spore germination increased from 0.212 nm 0 h to 2.499 nm after 48 h of incubation.
![Figure 14
Image taken from crack filled with precipitation of calcium carbonate at the magnifications of (a) 50× and (b) 230× [13].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_014.jpg)
Image taken from crack filled with precipitation of calcium carbonate at the magnifications of (a) 50× and (b) 230× [13].
3.3 Self-healing polymer
Concrete and steel coating is accounted as a conventional method for protection against aggressive environments; moreover, to prevent reinforcing steel embedded in the concrete from rusting, an anti-corrosion coat is applied on its surface. Cross-linked polymers are diversely used as a two-component curing coat. The methods of self-healing polymers and their application in construction industry are diversely studied in previous literature [43,104]. These methods are based on different mechanisms, including reversible polymerization, softening of thermoplastic phases, and hydrogen bonding. The polyurea itself has an excellent self-healing properties due to its H-bonds and used in construction industry as an autonomous self-healable coating [69]. In addition to polyurea, PU and epoxy coatings are conventionally used for protecting concrete and steel, and their protective ability could be enhanced through the addition of micro-/nano-capsules containing active agents. In order to well-dispersion of micro-/nano-capsules, silanization and other modifications performed to avoid decreasing the mechanical properties of final coats [105]. The dispersion of polyurea micro-/nano-capsules in PU coating was studied [106]. Infrared results showed that the interaction between polyurea microcapsules and PU led to the formation of distributed hydrogen bonds. In another study, the effect of the incorporation of polyurea microcapsules on epoxy coatings was analyzed. It was obtained that 3% of microcapsules addition raised the tensile strength of the epoxy to 14.5%; whereas, 5% addition of microcapsules raised the tensile strength to 9.5%. It shows that the bond between microcapsules and epoxy was not strong [107]. The dispersion of polyurea microcapsules in epoxy was enhanced in another study by the introduction of GO in polyurea shell [108]. Also, the polyurea/silica hybrid shell with the core of hydroxy diisocyanate has been prepared and dispersed in epoxy to develop self-healing coatings [109]. Table 4 tabulates different studies that used polyurea microcapsule as self-healing agent in a polymeric matrix.
Summary of polyurea micro-/nano-capsule application for self-healing of polymeric materials
| Material | Ref. |
|---|---|
| Active agent | |
| Polyurea/GO shell HDA and IPDI core for epoxy coat | Ma et al. [108] |
| Polyurea shell HDA core in epoxy for marine coat | Ma et al. [110] |
| Polyurea/silica shell and HDI core for one-part anti-corrosive coating | Wu et al. [109] |
| Polyurea shell HDA core for corrosion inhibition of steel | Ma et al. [111] |
| Polyurea with epoxy core for corrosion inhibition of steel in sea water | Yi et al. [112] |
| Polyurea shell, polyaziridine core for corrosion inhibition | Shukla et al. [113] |
| Modified polyurea shell with HDI-trimer core | Nguyen et al. [114] |
| Double layer polyurea shell and HDI core | Sun et al. [115] |
| Hybrid polyurea/PUF shell and MDI and H-MDI core in epoxy for steel protection | Sun et al. [116] |
| Hybrid polyurea/PUF shell and MDI and H-MDI core (lubrication properties) | Sun et al. [117] |
| Polyurea/lignosulfonate shell IPDI core in polyurea coat for steel protection | Qian et al. [118] |
| Hybrid polyurea/polyaniline shell IPDI core in epoxy coat for steel protection | Li et al. [45] |
| Polyurea/MF/lignin shell, and IPDI core | Yi et al. [119] |
| Organic oil | |
| Dendritic polyurea shell quinoline core in PU coat for steel protection | Gite et al. [120] |
| Dendritic polyurea shell, linseed oil core for steel protection | Tatiya et al. [106] |
| Polyurea/fly ash shell, linseed oil/fly ash core for steel protection | Li et al. [121] |
| PAMAM polyurea shell tung oil and HDI core for steel protection | Tatiya et al. [36] |
3.3.1 Active agents
Micro-/nano-capsules of active agents have been used for preparing self-healing polymeric coats. HDI, H-MDI, IPDI, HDA, and epoxy were encapsulated by polyurea [122] and added to epoxy, PU, and polyurea coat. This mechanism was also used for preparing self-healing cement paste [123]. The aim of the isocyanate micro-/nano-capsule preparation is to react with water or epoxy to heal the cracks that appear at the coating surface [119]. Figure 15 shows a schematic image of micro/nano-capsules behavior for self-healing coating. This mechanism enhances the protective abilities and service life of the coat in seawater or other harsh area exposures. However, reactive agent capsulation with polyurea encountered a number of drawbacks. Common solvents for active agents such as isocyanates are of hazardous materials and are dissolved in the matrixes in the long term and require higher mechanical properties. In a study, the effect of functionalization of polyurea micro-/nano-capsules on their mean size distribution and water uptake were investigated [114]. Results showed that the modification increased the amount of encapsulation and decreased the size variation where the core content, mean size, and dispersity of non-functionalized polyurea microcapsule were changed from 49%, 165 µm and 86–83%, 73 µm and 15 by using hexamethyldisilazane. Also, water uptake decreased from 2.4 to 1% for modification of polyurea micro-/nano-capsules via 2-ethylhexylamine. Moreover, fluorinated amine polyurea microcapsule could retain the isocyanate after 2 days of immersion in water by 60%, but the normal polyurea shell loses the encapsulated isocyanate in the same period [114].
![Figure 15
Schematic representation of crack healing by micro-encapsulated self-healing agent with a catalyst embedded in a resin matrix [104].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_015.jpg)
Schematic representation of crack healing by micro-encapsulated self-healing agent with a catalyst embedded in a resin matrix [104].
Hybrid polyurea/PUF microcapsules were prepared and dispersed in epoxy. The amount of encapsulation was about 73% and the mean diameter was 158 ± 42 to 60 ± 17 μm with PUF thickness of 422 ± 36 to 309 ± 27 nm. The lower sizes showed lower resistance to water and organic solvent due to lower thickness of PUF. In addition to self-healing properties of H-MDI, its lubrication properties were also studied [117]. In comparison to HDI, H-MDI provided higher protective properties as an active agent for micro/nano-capsules due to its oily nature. The friction coefficient of epoxy-embedded polyurea microcapsules with core of H-MDI decreased by 78.9%. The wear width and wear depth of this epoxy then decreased from 1.3 ± 0.1 mm and 74 ± 36 μm to 0.9 ± 0.1 mm and 20.0 ± 4.1 μm through addition of polyurea microcapsules. HDA encapsulated by polyurea GO was used as a self-healing agent in epoxy. The mean size of 500 nm with shell thickness of 80 nm was obtained through agitation rate of 1,800 rpm and emulsifier dodecyl benzene sulfonate. Results showed 62.5% anti-corrosive self-healing efficiency in epoxy by adding only 3% of GO-PU nano-capsules. In comparison to polyether amine encapsulated by PMMA with 60% healing efficiency by 5% PMMA microcapsules addition [124], polyurea showed more accurate results with lower ratio of microcapsules. Polyurea and lignosulfonate have been used for encapsulation of IPDI and dispersed in polyurea coat. The polyurea microcapsules with the average mean size of 50 μm and the encapsulation efficiency of 75.3% were obtained. The corrosion current as an indicator of self-healing has been analyzed before and after scratching. The steel demonstrated that 2.06 × 10−04 millimeters per year (mmpy) decreased to 1.54 × 10−04 mmpy after coating with polyurea while the ruptured polyurea showed 2.49 × 10−01 mmpy, which decreased to 8.95 × 10−03 mmpy after autonomous self-healing [7].
3.3.2 Organic oil
Protective coats are often exposed to physical wear or chemical erosion, organic oils are able to reduce the coefficient of friction and wear rate by forming a lubricating film at the surface [125]. Nevertheless, due to their vulnerability against higher temperatures and harsh environments, organic oils require to be protected with an engineered and strong shell. Also, the direct addition of some organic oils such as quinoline resulted in reaction with isocyanate and deactivated the anti-corrosion abilities. Several studies reported the employment of polyurea for encapsulation of quinoline, tung oil, and linseed oil as well as inorganic oils [126]. In order to prepare polyurea microcapsules with anti-corrosion core, quinoline have been dissolved in xylene and ethyl acetate [120]. These two different core materials then encapsulated by polyurea through stirring at 5,000 rpm. Microcapsules with xylene core material possessed mean size of 72 μm, whereas average size of microcapsules was 86 μm for the core of ethyl acetate solvent. Both formed in a broad distribution average size from almost 1 to 200 μm.
The polyurea microcapsules released their quinoline dissolved in xylene with 20.568, 41.332, and 50.703% after 1, 3 and 6 h. Finally using 4% polyurea encapsulated quinoline, a considerable decrease in the corrosion of steel was observed from 2.016 to 0.6506 mm/year led to the increase of average inhibition efficiency to 67.72%. Linseed oil is also used for the corrosion protection of steel. The cross-linkers can be mixed with organic oil and used as core of micro-/nano-capsules. These micro-/nano-capsules are able to provide both self-lubrication and self-curing properties at the same time. Tung oil was encapsulated by polyurea with different types of diamines. Polyurea microcapsules prepared by DETA and TETA showed deformation where 70–80% of them damaged and ruptured after stirring in hot water at 70–80°C. In contrast, composite droplets prepared by PAMAM survived in the same condition with only 5% damage [36]. As can be seen in Figure 16, metallic surface coated with PU contained polyurea microcapsules remained unchanged after 120 h immersed in salt water. In contrast, metallic sheet without polyurea microcapsules encountered severe corrosion on its surface. A few studies used magnetic properties of microcapsules for inhibition of corrosion with targeted delivery which limited to ethyl cellulose microcapsules with tung oil core [127]. Results showed that the average distance of microcapsules from steel decreased by 50% from 160.7 to 80.8 µm under an external magnetic field.
![Figure 16
Corrosion test results of steel panels coated with PU coatings with different percentages of polyurea microcapsules containing tung oil before and after testing for 120 h [36].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_016.jpg)
Corrosion test results of steel panels coated with PU coatings with different percentages of polyurea microcapsules containing tung oil before and after testing for 120 h [36].
3.4 Flame retardant
Fire is another destroying factor for building. One of the obligations of using sandwich panels in the construction and building industry is being fire-proof, whereas, the PU foam inside the metal layers is highly flammable. Polyurea micro-/nano-capsules contain flame retardant materials as an additive in sandwich panels used in buildings [128]. Ammonium polyphosphate has been encapsulated via polyurea which provides intumescent properties during exposure to very high temperatures [129]. Although ammonium polyphosphate has already been used for making PU foam self-extinguished, the encapsulation of ammonium polyphosphate provides higher mechanical properties and moisture adsorption resistance for the PU foam. Other properties required in building industries such as energy-saving or biocide could be coupled with flame retardancy properties while encapsulation. In a study, a multifunctional polystyrene microcapsule has been prepared and applied in concrete. The cargo included paraffin as energy storage material and phosphoric acid as flame retardant. Synergistic results was obtained for this microcapsules [130]. In another study, the bifunctionality of polyurea microcapsules was studied with thermal energy storage and biocide abilities [131]. It means that there is an opportunity to use combinational properties for making polyurea micro-/nano-capsules for construction applications.
4 Conclusion
This study reviewed the recent applications of polyurea micro-/nano-capsules in construction industry. Based on the recent studies, below conclusions have been gained:
Polyurea micro-/nano-capsule has unique properties and could be tailored for various construction applications. Polyurea possesses special chemical structure and tunable morphology. Its compatibility with concrete and gypsum led to the appropriate dispersion of polyurea micro-/nano-capsules in construction materials, whereas the protective abilities save the internal core in the alkaline environment of concrete. In comparison to other materials used for fabrication micro-/nano-capsules, polyurea stands out with fast formation, waterproofing abilities, excellent mechanical properties as well as compatibility with building materials and other polymers. Hydrogen bonds in polyurea structure, contribute to its mechanical properties.
There are two main methods of preparing polyurea micro-/nano-capsules applied in construction industry: bulk emulsion and microfluidic. Microfluidic devices provide micro-/nano-capsules with narrow distribution size, whereas the rate of production is higher in bulk emulsion technique. The role of surfactant is significant in both methods so as to provide droplet size and shell thickness.
Energy-saving materials including PCMs were encapsulated with the outer layer of polyurea. Almost 100% efficiency of encapsulation was gained by using microfluidic device. Thermal conductivity of polyurea were enhanced by the addition of iron nanoparticles, titanium dioxide shell, and graphite flakes in the microcapsule. The appropriate mean diameter, size distribution, and wall thickness of micro-/nano-capsules is vital for designing optimal energy saving and avoiding leakage, which is important for the life time of buildings.
Polyurea microcapsules hold its serviceability in 80°C and survived the mixing process of concrete. This microcapsule which contained 90% sodium silicate decreased the crack depth of the oil well cement by 58%. Another similar polyurea microcapsules provided the reduction in water sorptivity of the self-healable cement by 45% in 7 days, which was higher than gelatin microcapsules by 15%.
Self-lubricating and self-curing polyurea microcapsules with the core of tung oil and curing agent, showed the average corrosion inhibition efficiency of 67.72 and 62.5%, respectively.
The incorporation of GO in polyurea microcapsules raised its mechanical and protective properties and enhanced its dispersion in epoxy coatings.
Flame-retardant materials were also encapsulated by polyurea in micro-/nano-scale, making sandwich panels fireproof with higher mechanical properties.
Acknowledgments
The authors wish to thank for financial support by the Ministry of Science and Technology of China (Grant No. 2019YFE0112400), the Department of Science and Technology of Shandong Province (Grant No. 2021CXGC011204), the Liaoning provincial key laboratory of Safety and Protection for Infrastructure Engineering, the Central government - Liaoning provincial discovery project. The authors gratefully acknowledge Dr. Mojtaba Abtahi for his helpful assistance.
-
Funding information: This research is financially supported by the Ministry of Science and Technology of China (Grant No. 2019YFE0112400), the Department of Science and Technology of Shandong Province (Grant No. 2021CXGC011204), the Liaoning provincial key laboratory of Safety and Protection for Infrastructure Engineering, the Central government - Liaoning provincial discovery project.
-
Author contributions: All authors have accepted the responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Nguyen W, Martinez DM, Jen G, Duncan JF, Ostertag CP. Interaction between global warming potential, durability, and structural properties of fiber-reinforced concrete with high waste materials inclusion. Resour Conserv Recycling. 2021;169:105453.10.1016/j.resconrec.2021.105453Search in Google Scholar
[2] Chang Z, Wang K, Wu X, Lei G, Wang Q, Liu H, et al. Review on the preparation and performance of paraffin-based phase change microcapsules for heat storage. J Energy Storage. 2022;46:103840.10.1016/j.est.2021.103840Search in Google Scholar
[3] Xie J, Wang W, Liu J, Pan S. Thermal performance analysis of PCM components heat storage using mechanical ventilation: Experimental results. Energy Build. 2016;123:169–78.10.1016/j.enbuild.2016.04.043Search in Google Scholar
[4] Papadaki D, Kiriakidis G, Tsoutsos T. Applications of nanotechnology in construction industry. In: Barhoum A, Hamdy Makhlouf AS, editors. Fundamentals of nanoparticles. Amsterdam, The Netherlands: Elsevier; 2018. p. 343–70.10.1016/B978-0-323-51255-8.00011-2Search in Google Scholar
[5] Li Q, Kim NH, Hui D, Lee JH. Effects of dual component microcapsules of resin and curing agent on the self-healing efficiency of epoxy. Compos Part B: Eng. 2013;55:79–85.10.1016/j.compositesb.2013.06.006Search in Google Scholar
[6] Peng H, Xiong Z, Gan Z, Liu C, Xie Y. Microcapsule MOFs@ MOFs derived porous “nut-bread” composites with broadband microwave absorption. Compos Part B: Eng. 2021;224:109170.10.1016/j.compositesb.2021.109170Search in Google Scholar
[7] Pang Y, Qin Z, Wang S, Yi C, Zhou M, Lou H, et al. Preparation and application performance of lignin-polyurea composite microcapsule with controlled release of avermectin. Colloid Polym Sci. 2020;298(8):1001–12.10.1007/s00396-020-04664-xSearch in Google Scholar
[8] Liu H, Shen H, Zhang H, Wang X. Development of photoluminescence phase-change microcapsules for comfort thermal regulation and fluorescent recognition applications in advanced textiles. J Energy Storage. 2022;49:104158.10.1016/j.est.2022.104158Search in Google Scholar
[9] Zarour A, Omar S, Abu-Reziq R. Preparation of Poly (ethylene glycol)@ Polyurea Microcapsules Using Oil/Oil Emulsions and Their Application as Microreactors. Polymers. 2021;13(15):2566.10.3390/polym13152566Search in Google Scholar PubMed PubMed Central
[10] Bah MG, Bilal HM, Wang J. Fabrication and application of complex microcapsules: A review. Soft Matter. 2020;16(3):570–90.10.1039/C9SM01634ASearch in Google Scholar PubMed
[11] Microencapsulation Market Size, Share & Trends Analysis Report By Technology (Emulsion, Spray), By Application (Pharmaceutical, Home & Personal Care), By Coating Material, And Segment Forecasts, 2022–2030: Grand view reseach; 2021Search in Google Scholar
[12] Do S, Stepp S, Youssef G. Quasi-static and dynamic characterization of Polyurea microspheres reinforced polyurea matrix composite. Mater Today Commun. 2020;25:101464.10.1016/j.mtcomm.2020.101464Search in Google Scholar
[13] Zamani M, Nikafshar S, Mousa A, Behnia A. Bacteria encapsulation using synthesized polyurea for self-healing of cement paste. Constr Build Mater. 2020;249:118556.10.1016/j.conbuildmat.2020.118556Search in Google Scholar
[14] Perignon C, Ongmayeb G, Neufeld R, Frere Y, Poncelet D. Microencapsulation by interfacial polymerisation: membrane formation and structure. J Microencapsul. 2015;32(1):1–15.10.3109/02652048.2014.950711Search in Google Scholar PubMed
[15] Zhan S, Chen S, Chen L, Hou W. Preparation and characterization of polyurea microencapsulated phase change material by interfacial polycondensation method. Powder Technol. 2016;292:217–22.10.1016/j.powtec.2016.02.007Search in Google Scholar
[16] Santos AN, Santos DJD, Carastan DJ. Microencapsulation of reactive isocyanates for application in self-healing materials: A review. J Microencapsul. 2021;38(5):338–56.10.1080/02652048.2021.1921068Search in Google Scholar PubMed
[17] Zhang Y, Rochefort D. Characterisation and applications of microcapsules obtained by interfacial polycondensation. J Microencapsul. 2012;29(7):636–49.10.3109/02652048.2012.676092Search in Google Scholar PubMed
[18] Arshady R. Review Preparation of nano-and microspheres by polycondensation techniques. J Microencapsul. 1989;6(1):1–12.10.3109/02652048909019897Search in Google Scholar PubMed
[19] Musyanovych A, Landfester K. Polymer micro‐and nanocapsules as biological carriers with multifunctional properties. Macromol Biosci. 2014;14(4):458–77.10.1002/mabi.201300551Search in Google Scholar PubMed
[20] Boh Podgornik B, Šandrić S, Kert M. Microencapsulation for functional textile coatings with emphasis on biodegradability—A systematic review. Coatings. 2021;11(11):1371.10.3390/coatings11111371Search in Google Scholar
[21] Sivanathan A, Dou Q, Wang Y, Li Y, Corker J, Zhou Y, et al. Phase change materials for building construction: An overview of nano-/micro-encapsulation. Nanotechnol Rev. 2020;9(1):896–921.10.1515/ntrev-2020-0067Search in Google Scholar
[22] Zhang R, Huang W, Lyu P, Yan S, Wang X, Ju J. Polyurea for blast and impact protection: A review. Polymer. 2022;14(13):2670.10.3390/polym14132670Search in Google Scholar PubMed PubMed Central
[23] Grujicic M, He T, Pandurangan B, Svingala F, Settles G, Hargather M. Experimental characterization and material-model development for microphase-segregated polyurea: An overview. J Mater Eng Perform. 2012;21(1):2–16.10.1007/s11665-011-9875-6Search in Google Scholar
[24] Du J, Ibaseta N, Guichardon P. Characterization of polyurea microcapsules synthesized with an isocyanate of low toxicity and eco-friendly esters via microfluidics: Shape, shell thickness, morphology and encapsulation efficiency. Chem Eng Res Des. 2022;182:256–72.10.1016/j.cherd.2022.03.026Search in Google Scholar
[25] Jandaghian MH, Kazerooni H. Performance of polyurea formulations against impact loads: A molecular dynamics and mechanical simulation approach. J Appl Polym Sci. 2021;138(17):50309.10.1002/app.50309Search in Google Scholar
[26] Wu F, Li J, Quan H, Han J, Liu X, Zhang X, et al. Robust polyurea/poly (urea–formaldehyde) hybrid microcapsules decorated with Al2O3 nano-shell for improved self-healing performance. Appl Surf Sci. 2021;542:148561.10.1016/j.apsusc.2020.148561Search in Google Scholar
[27] Reed N, Huynh NU, Rosenow B, Manlulu K, Youssef G. Synthesis and characterization of elastomeric polyurea foam. J Appl Polym Sci. 2020;137(26):48839.10.1002/app.48839Search in Google Scholar
[28] Wei Y, Jiang X, Li S, Kong XZ. Catalysis of isocyanate reaction with water by DMF and its use for fast preparation of uniform polyurea microspheres through precipitation polymerization. Eur Polym J. 2019;115:384–90.10.1016/j.eurpolymj.2019.03.054Search in Google Scholar
[29] Shojaei B, Abtahi M, Najafi M. Chemical recycling of PET: A stepping‐stone toward sustainability. Polym Adv Technol. 2020;31(12):2912–38.10.1002/pat.5023Search in Google Scholar
[30] Zhang J, Zhai J, Zhao X, Lin J, Wang Q, Du A. Effect of temperature on tensile curve of polyaspartic ester polyurea and its activation energy analysis. J Phys Conf Ser. 2021;2101(1):012061.10.1088/1742-6596/2101/1/012061Search in Google Scholar
[31] Yu F, Wang Y, Zhao Y, Chou J, Li X. Preparation of polyurea microcapsules by interfacial polymerization of isocyanate and chitosan oligosaccharide. Materials. 2021;14(13):3753.10.3390/ma14133753Search in Google Scholar PubMed PubMed Central
[32] Xu X, Zhou Z, Qin L, Yu C, Zhang F. Preparation of PVA/PU/PUA microcapsules and application in self-healing two-component waterborne polyurethane coatings. J Coat Technol Res. 2022;19(3):1–12.10.1007/s11998-021-00577-8Search in Google Scholar
[33] Sun S, Gao Y, Han N, Zhang X, Li W. Reversible photochromic energy storage polyurea microcapsules via in-situ polymerization. Energy. 2021;219:119630.10.1016/j.energy.2020.119630Search in Google Scholar
[34] Ferrándiz García M, Capablanca Francés L, Bou-Belda E, García Sanoguera D, Bonet Aracil MA, Bartolomé L. Microencapsulation of essential oils by interfacial polimerization using polyurea as a wall material. J Encapsulation Adsorpt Sci. 2015;5:165–77.10.4236/jeas.2015.54014Search in Google Scholar
[35] Rao J, Chandrani AN, Powar A, Chandra S. Design and application of polyurea microcapsules containing herbicide (oxyfluorfen). Des Monomers Polym. 2020;23(1):155–63.10.1080/15685551.2020.1816344Search in Google Scholar PubMed PubMed Central
[36] Tatiya PD, Mahulikar PP, Gite VV. Designing of polyamidoamine-based polyurea microcapsules containing tung oil for anticorrosive coating applications. J Coat Technol Res. 2016;13(4):715–26.10.1007/s11998-015-9780-2Search in Google Scholar
[37] Zoabi A, Margulis K. Differential interactions of chiral nanocapsules with DNA. Int J Mol Sci. 2021;22(2):584.10.3390/ijms22020584Search in Google Scholar PubMed PubMed Central
[38] Iqbal N, Tripathi M, Parthasarathy S, Kumar D, Roy PK. Aromatic versus aliphatic: Hydrogen bonding pattern in chain‐extended high‐performance polyurea. ChemistrySelect. 2018;3(7):1976–82.10.1002/slct.201703176Search in Google Scholar
[39] Tripathi M, Parthasarathy S, Roy PK. Spray processable polyurea formulations: Effect of chain extender length on material properties of polyurea coatings. J Appl Polym Sci. 2020;137(16):48573.10.1002/app.48573Search in Google Scholar
[40] Yilgör I, Yilgör E, Wilkes GL. Critical parameters in designing segmented polyurethanes and their effect on morphology and properties: A comprehensive review. Polymer. 2015;58:A1–36.10.1016/j.polymer.2014.12.014Search in Google Scholar
[41] Jagtap SB, Mohan MS, Shukla PG. Improved performance of microcapsules with polymer nanocomposite wall: Preparation and characterization. Polymer. 2016;83:27–33.10.1016/j.polymer.2015.12.011Search in Google Scholar
[42] Qian X, Song L, Tai Q, Hu Y, Yuen RK. Graphite oxide/polyurea and graphene/polyurea nanocomposites: a comparative investigation on properties reinforcements and mechanism. Compos Sci Technol. 2013;74:228–34.10.1016/j.compscitech.2012.11.018Search in Google Scholar
[43] Li M, Wang T, Zhang J. Effects of processing conditions on porosity features of polyurea cross-linked silica aerogels synthesized by ambient pressure drying. Mater Res Express. 2019;6(11):1150c2.10.1088/2053-1591/ab4d6aSearch in Google Scholar
[44] Liu Q, Chen P-W, Guo Y-S, Su J-J, Han L, Arab A, et al. Mechanical behavior and failure mechanism of polyurea nanocomposites under quasi-static and dynamic compressive loading. Def Technol. 2021;17(2):495–504.10.1016/j.dt.2020.02.006Search in Google Scholar
[45] Li H, Feng Y, Cui Y, Ma Y, Zheng Z, Qian B, et al. Polyurea/polyaniline hybrid shell microcapsules loaded with isophorone diisocyanate for synergetic self-healing coatings. Prog Org Coat. 2020;145:105684.10.1016/j.porgcoat.2020.105684Search in Google Scholar
[46] Tahir M, Heinrich G, Mahmood N, Boldt R, Wießner S, Stöckelhuber KW. Blending In Situ polyurethane-urea with different kinds of rubber: Performance and compatibility aspects. Mater (Basel). 2018;11(11):2175.10.3390/ma11112175Search in Google Scholar PubMed PubMed Central
[47] Gama NV, Ferreira A, Barros-Timmons A. Polyurethane foams: Past, present, and future. Materials. 2018;11(10):1841.10.3390/ma11101841Search in Google Scholar PubMed PubMed Central
[48] Phoungtawee P, Crespy D. Shining a new light on the structure of polyurea/polyurethane materials. Polym Chem. 2021;12(27):3893–9.10.1039/D1PY00649ESearch in Google Scholar
[49] Zhang Q, Shi Y, Zhan X, Chen F. In situ miniemulsion polymerization for waterborne polyurethanes: Kinetics and modeling of interfacial hydrolysis of isocyanate. Colloids Surf A: Physicochem Eng Asp. 2012;393:17–26.10.1016/j.colsurfa.2011.10.016Search in Google Scholar
[50] Polenz I, Weitz DA, Baret J-C. Polyurea microcapsules in microfluidics: surfactant control of soft membranes. Langmuir. 2015;31(3):1127–34.10.1021/la5040189Search in Google Scholar PubMed
[51] Piradashvili K, Alexandrino EM, Wurm FR, Landfester K. Reactions and polymerizations at the liquid–liquid interface. Chem Rev. 2016;116(4):2141–69.10.1021/acs.chemrev.5b00567Search in Google Scholar PubMed
[52] Ren L, Huang B, Fang W, Zhang D, Cheng H, Song Z, et al. Multi-encapsulation combination of O/W/O emulsions with polyurea microcapsules for controlled release and safe application of dimethyl disulfide. ACS Appl Mater Interfaces. 2020;13(1):1333–44.10.1021/acsami.0c16613Search in Google Scholar PubMed
[53] Mao W, Litina C, Al-Tabbaa A. Development and application of novel sodium silicate microcapsule-based self-healing oil well cement. Materials. 2020;13(2):456.10.3390/ma13020456Search in Google Scholar PubMed PubMed Central
[54] Kondo T. Microcapsules: Their science and technology Part I. Various preparation methods. J oleo Sci. 2001;50(1):1–11.10.5650/jos.50.1Search in Google Scholar
[55] Marani A, Nehdi ML. Integrating phase change materials in construction materials: Critical review. Constr Build Mater. 2019;217:36–49.10.1016/j.conbuildmat.2019.05.064Search in Google Scholar
[56] Gobert SR, Segers M, Luca S, Teixeira RF, Kuhn S, Braeken L, et al. Development of a continuous reactor for emulsion-based microencapsulation of hexyl acetate with a polyuria shell. J Microencapsul. 2019;36(4):371–84.10.1080/02652048.2019.1633433Search in Google Scholar PubMed
[57] Zhou J, Xu W, Wang Y-N, Shi B. Preparation of polyurea microcapsules containing phase change materials in a rotating packed bed. Rsc Adv. 2017;7(34):21196–204.10.1039/C7RA01805CSearch in Google Scholar
[58] Polenz I, Datta SS, Weitz DA. Controlling the morphology of polyurea microcapsules using microfluidics. Langmuir. 2014;30(44):13405–10.10.1021/la503234zSearch in Google Scholar PubMed
[59] Liu J, Streufert JR, Mu K, Si T, Han T, Han Y, et al. Polymer composites containing phase‐change microcapsules displaying deep undercooling exhibit thermal history‐dependent mechanical properties. Adv Mater Technol. 2020;5(10):2000286.10.1002/admt.202000286Search in Google Scholar
[60] Kosarli M, Bekas DG, Tsirka K, Baltzis D, Vaimakis-Tsogkas DT, Orfanidis S, et al. Microcapsule-based self-healing materials: Healing efficiency and toughness reduction vs. capsule size. Compos Part B: Eng. 2019;171:78–86.10.1016/j.compositesb.2019.04.030Search in Google Scholar
[61] Thorne MF, Simkovic F, Slater AG. Production of monodisperse polyurea microcapsules using microfluidics. Sci Rep. 2019;9(1):1–7.10.1038/s41598-019-54512-4Search in Google Scholar PubMed PubMed Central
[62] Holtze C. Large-scale droplet production in microfluidic devices—an industrial perspective. J Phys D: Appl Phys. 2013;46(11):114008.10.1088/0022-3727/46/11/114008Search in Google Scholar
[63] Chen PW, Cadisch G, Studart AR. Encapsulation of aliphatic amines using microfluidics. Langmuir. 2014;30(9):2346–50.10.1021/la500037dSearch in Google Scholar PubMed
[64] Wang J, Shao C, Wang Y, Sun L, Zhao Y. Microfluidics for medical additive manufacturing. Engineering. 2020;6(11):1244–57.10.1016/j.eng.2020.10.001Search in Google Scholar
[65] Brasileiro PPF, Brandão YB, Sarubbo LA, Benachour M. Self-healing concrete: Background, development, and market prospects. Biointerface Res Appl Chem. 2021;11(6):14709–25.10.33263/BRIAC116.1470914725Search in Google Scholar
[66] Basil A, Sam-Amobi C, Ugwu C, Odoh P, editors. Combating global warming/climate change via reduction of CO2 emission of buildings. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2019.10.1088/1757-899X/640/1/012016Search in Google Scholar
[67] Shah KW, Huseien GF. Biomimetic self-healing cementitious construction materials for smart Buildings. Biomimetics. 2020;5(4):47.10.3390/biomimetics5040047Search in Google Scholar PubMed PubMed Central
[68] Cabeza LF, Navarro L, Pisello AL, Olivieri L, Bartolomé C, Sánchez J, et al. Behaviour of a concrete wall containing micro‐encapsulated PCM after a decade of its construction. Sol Energy. 2020;200:108–13.10.1016/j.solener.2019.12.003Search in Google Scholar
[69] Shojaei B, Najafi M, Yazdanbakhsh A, Abtahi M, Zhang C. A review on the applications of polyurea in the construction industry. Polym Adv Technol. 2021;32(8):2797–812.10.1002/pat.5277Search in Google Scholar
[70] Ellinger S, Caldwell B, Staggemeier K, Wang S, Verdi S, Kerber J, et al. inventorsENCAPSULATED BIOCIDES patent WO/2020/099567; 2020.Search in Google Scholar
[71] Trojer MA, Nordstierna L, Bergek J, Blanck H, Holmberg K, et al. Use of microcapsules as controlled release devices for coatings. Adv Colloid Interface Sci. 2015;222:18–43.10.1016/j.cis.2014.06.003Search in Google Scholar PubMed
[72] Al-Shannaq R, Farid MM. Microencapsulation of phase change materials for thermal energy storage systems. In: Cabeza LF, editor. Advances in Thermal Energy Storage Systems. Amsterdam, The Netherlands: Elsevier; 2021. p. 269–329.10.1016/B978-0-12-819885-8.00010-3Search in Google Scholar
[73] Nelson G. 4 - Microencapsulated colourants for technical textile application. In: Gulrajani ML, editor. Advances in the Dyeing and Finishing of Technical Textiles. Cambridge, UK: Woodhead Publishing; 2013. p. 78–104.10.1533/9780857097613.1.78Search in Google Scholar
[74] Roberts NS, Al-Shannaq R, Kurdi J, Al-Muhtaseb SA, Farid MM. Efficacy of using slurry of metal-coated microencapsulated PCM for cooling in a micro-channel heat exchanger. Appl Therm Eng. 2017;122:11–8.10.1016/j.applthermaleng.2017.05.001Search in Google Scholar
[75] Arshad A, Jabbal M, Yan Y, Darkwa J. The micro‐/nano‐PCMs for thermal energy storage systems: a state of art review. Int J Energy Res. 2019;43(11):5572–620.10.1002/er.4550Search in Google Scholar
[76] Xie J, Wang W, Sang P, Liu J. Experimental and numerical study of thermal performance of the PCM wall with solar radiation. Constr Build Mater. 2018;177:443–56.10.1016/j.conbuildmat.2018.05.123Search in Google Scholar
[77] Yang JM, Kim JS. The microencapsulation of calcium chloride hexahydrate as a phase‐change material by using the hybrid coupler of organoalkoxysilanes. J Appl Polym Sci. 2018;135(6):45821.10.1002/app.45821Search in Google Scholar
[78] Zhao A, An J, Yang J, Yang E-H. Microencapsulated phase change materials with composite titania-polyurea (TiO2-PUA) shell. Appl Energy. 2018;215:468–78.10.1016/j.apenergy.2018.02.057Search in Google Scholar
[79] Zhang H, Xing F, Cui H-Z, Chen D-Z, Ouyang X, Xu S-Z, et al. A novel phase-change cement composite for thermal energy storage: Fabrication, thermal and mechanical properties. Appl energy. 2016;170:130–9.10.1016/j.apenergy.2016.02.091Search in Google Scholar
[80] Wang H, Chen Y, Li J, Guo L, Fang M. Review of encapsulated salt hydrate core-shell phase change materials. Kona Powder Part J. 2020:37;85–96.10.14356/kona.2020010Search in Google Scholar
[81] Shi T, Hu P, Wang J. Preparation of polyurea microcapsules containing phase change materials using microfluidics. ChemistrySelect. 2020;5(7):2342–7.10.1002/slct.201904570Search in Google Scholar
[82] Lone S, Lee HM, Kim GM, Koh W-G, Cheong IW. Facile and highly efficient microencapsulation of a phase change material using tubular microfluidics. Colloids Surf A: Physicochem Eng Asp. 2013;422:61–7.10.1016/j.colsurfa.2013.01.035Search in Google Scholar
[83] Salaün F, Bedek G, Devaux E, Dupont D, Gengembre L. Microencapsulation of a cooling agent by interfacial polymerization: Influence of the parameters of encapsulation on poly (urethane–urea) microparticles characteristics. J Membr Sci. 2011;370(1–2):23–33.10.1016/j.memsci.2010.11.033Search in Google Scholar
[84] Salaün F, Bedek G, Devaux E, Dupont D. Influence of the washings on the thermal properties of polyurea-urethane microcapsules containing xylitol to provide a cooling effect. Mater Lett. 2011;65(2):381–4.10.1016/j.matlet.2010.10.041Search in Google Scholar
[85] Trigui A, Karkri M, Boudaya C, Candau Y, Ibos L. Development and characterization of composite phase change material: thermal conductivity and latent heat thermal energy storage. Compos Part B: Eng. 2013;49:22–35.10.1016/j.compositesb.2013.01.007Search in Google Scholar
[86] Balapour M, Mutua AW, Farnam Y. Evaluating the thermal efficiency of microencapsulated phase change materials for thermal energy storage in cementitious composites. Cem Concr Compos. 2021;116:103891.10.1016/j.cemconcomp.2020.103891Search in Google Scholar
[87] Park S, Lee Y, Kim YS, Lee HM, Kim JH, Cheong IW, et al. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf A: Physicochem Eng Asp. 2014;450:46–51.10.1016/j.colsurfa.2014.03.005Search in Google Scholar
[88] Rajczakowska M, Habermehl-Cwirzen K, Hedlund H, Cwirzen A. Autogenous self-healing: A better solution for concrete. J Mater Civ Eng. 2019;31(9):03119001.10.1061/(ASCE)MT.1943-5533.0002764Search in Google Scholar
[89] Wang X, Chen Z, Xu W, Wang X. Fluorescence labelling and self-healing microcapsules for detection and repair of surface microcracks in cement matrix. Compos Part B: Eng. 2020;184:107744.10.1016/j.compositesb.2020.107744Search in Google Scholar
[90] Ribeiro de Souza L. Design and synthesis of microcapsules using microfluidics for autonomic self-healing in cementitious materials. Doctoral dissertation, University of Cambridge; 2017.Search in Google Scholar
[91] Choi SY, Bergner K, Kierat R, Mueller MK. Core-shell expanding agents and their use in cementitious systems. U.S. Patent Application no. 16/755,365. 2020.Search in Google Scholar
[92] Taheri S, Clark SM. Preparation of self-healing additives for concrete via miniemulsion polymerization: formulation and production challenges. Int J Concr Struct Mater. 2021;15(1):1–15.10.1186/s40069-020-00449-2Search in Google Scholar
[93] Kanellopoulos A, Litina C, Giannaros P, Al-Tabbaa A, editors. Effect of different types of polymeric microcapsules on the self-healing efficiency of cement based composites. Proceedings of the 9th International Conference on Fracture Mechanics of Concrete and Concrete Structures. Berkeley, CA, USA: 2016.10.21012/FC9.152Search in Google Scholar
[94] Giannaros P, Kanellopoulos A, Al-Tabbaa A. Sealing of cracks in cement using microencapsulated sodium silicate. Smart Mater Struct. 2016;25(8):084005.10.1088/0964-1726/25/8/084005Search in Google Scholar
[95] Tan NPB, Keung LH, Choi WH, Lam WC, Leung HN. Silica‐based self‐healing microcapsules for self‐repair in concrete. J Appl Polym Sci. 2016;133(12):43090.10.1002/app.43090Search in Google Scholar
[96] Kleinfeldt LM. An emulsion-based route to generate magnetic capsules for concrete applications. Master of Science Thesis, University of Rhode Island; 2013:167.Search in Google Scholar
[97] Reinke SK. Polyurea/polyurethane microcapsules based self healing concrete; Master of Science Thesis, University of Rhode Island; 2012.Search in Google Scholar
[98] Mao W. Reactive MgO and self-healing microcapsules for enhanced well cement system performance. Doctoral dissertation, University of Cambridge; 2018.Search in Google Scholar
[99] Yuping Y, Guosheng H, Yingxiang M, Li M, Xuehui L, Yi G, et al. Synthesis of self-healing magnetic microcapsules with targeted delivery through magnetizing emulsifiers. Chem Lett. 2021;50(8):1573–6.10.1246/cl.210159Search in Google Scholar
[100] Ren J, Wang X, Li D, Han N, Dong B, Xing F. Temperature adaptive microcapsules for self-healing cementitious materials. Compos Part B: Eng. 2021;223:109138.10.1016/j.compositesb.2021.109138Search in Google Scholar
[101] Meharie MG, Kaluli JW, Abiero-Gariy Z, Kumar ND. Factors affecting the self-healing efficiency of cracked concrete structures. Am J Appl Sci Res. 2017;3(6):80–6.10.11648/j.ajasr.20170306.12Search in Google Scholar
[102] Kim H, Son H, Seo J, Lee H-K. Recent advances in microbial viability and self-healing performance in bacterial-based cementitious materials: A review. Constr Build Mater. 2021;274:122094.10.1016/j.conbuildmat.2020.122094Search in Google Scholar
[103] Dinarvand P, Rashno A. Review of the potential application of bacteria in self-healing and the improving properties of concrete/mortar. J Sustain Cement-Based Mater. 2022;11(4):250–71.10.1080/21650373.2021.1936268Search in Google Scholar
[104] Ullah H, M Azizli KA, Man ZB, Ismail MBC, Khan MI. The potential of microencapsulated self-healing materials for microcracks recovery in self-healing composite systems: A review. Polym Rev. 2016;56(3):429–85.10.1080/15583724.2015.1107098Search in Google Scholar
[105] Es-Haghi H, Mirabedini S, Imani M, Farnood R. Mechanical and self-healing properties of a water-based acrylic latex containing linseed oil filled microcapsules: Effect of pre-silanization of microcapsules’ shell compound. Compos Part B: Eng. 2016;85:305–14.10.1016/j.compositesb.2015.09.015Search in Google Scholar
[106] Tatiya PD, Hedaoo RK, Mahulikar PP, Gite VV. Novel polyurea microcapsules using dendritic functional monomer: synthesis, characterization, and its use in self-healing and anticorrosive polyurethane coatings. Ind Eng Chem Res. 2013;52(4):1562–70.10.1021/ie301813aSearch in Google Scholar
[107] Ma Y, Liu J, Zhang Y, Ge Y, Wu R, Song X, et al. Mechanical behavior and self-healing mechanism of polyurea-based double-walled microcapsule/epoxy composite films. Prog Org Coat. 2021;157:106283.10.1016/j.porgcoat.2021.106283Search in Google Scholar
[108] Ma Y, Zhang Y, Liu J, Ge Y, Yan X, Sun Y, et al. GO-modified double-walled polyurea microcapsules/epoxy composites for marine anticorrosive self-healing coating. Mater Des. 2020;189:108547.10.1016/j.matdes.2020.108547Search in Google Scholar
[109] Wu G, An J, Sun D, Tang X, Xiang Y, Yang J. Robust microcapsules with polyurea/silica hybrid shell for one-part self-healing anticorrosion coatings. J Mater Chem A. 2014;2(30):11614–20.10.1039/C4TA01312CSearch in Google Scholar
[110] Ma Y, Zhang Y, Liu J, Sun Y, Ge Y, Yan X, et al. Preparation and Characterization of Ethylenediamine-Polyurea Microcapsule Epoxy Self-Healing Coating. Materials. 2020;13(2):326.10.3390/ma13020326Search in Google Scholar PubMed PubMed Central
[111] Ma YX, Zhang YR, Liu JT, Li MY, Xu YQ. Self-healing epoxy coating modified by double-walled microcapsules based polyurea for metallic protection. Key Eng Mater. 2019;821:313–20.10.4028/www.scientific.net/KEM.821.313Search in Google Scholar
[112] Yi H, Deng Y, Wang C. Pickering emulsion-based fabrication of epoxy and amine microcapsules for dual core self-healing coating. Compos Sci Technol. 2016;133:51–9.10.1016/j.compscitech.2016.07.022Search in Google Scholar
[113] Shukla PG, Jagtap SB, Biradar SC, Charpe VP, Jadhav AS. Preparation and characterization of microcapsules containing industrially important reactive water-soluble polyamine. Colloid Polym Sci. 2016;294(12):2039–50.10.1007/s00396-016-3966-8Search in Google Scholar
[114] Nguyen L-TT, Hillewaere XK, Teixeira RF, van den Berg O, Du Prez FE. Efficient microencapsulation of a liquid isocyanate with in situ shell functionalization. Polym Chem. 2015;6(7):1159–70.10.1039/C4PY01448KSearch in Google Scholar
[115] Sun D, An J, Wu G, Yang J. Double-layered reactive microcapsules with excellent thermal and non-polar solvent resistance for self-healing coatings. J Mater Chem A. 2015;3(8):4435–44.10.1039/C4TA05339GSearch in Google Scholar
[116] Sun D, Zhang H, Tang X-Z, Yang J. Water resistant reactive microcapsules for self-healing coatings in harsh environments. Polymer. 2016;91:33–40.10.1016/j.polymer.2016.03.044Search in Google Scholar
[117] Sun D, Chong YB, Chen K, Yang J. Chemically and thermally stable isocyanate microcapsules having good self-healing and self-lubricating performances. Chem Eng J. 2018;346:289–97.10.1016/j.cej.2018.04.046Search in Google Scholar
[118] Qian Y, Zhou Y, Li L, Liu W, Yang D, Qiu X. Facile preparation of active lignin capsules for developing self-healing and UV-blocking polyurea coatings. Prog Org Coat. 2020;138:105354.10.1016/j.porgcoat.2019.105354Search in Google Scholar
[119] Yi H, Yang Y, Gu X, Huang J, Wang C. Multilayer composite microcapsules synthesized by Pickering emulsion templates and their application in self-healing coating. J Mater Chem A. 2015;3(26):13749–57.10.1039/C5TA02288FSearch in Google Scholar
[120] Gite VV, Tatiya PD, Marathe RJ, Mahulikar PP, Hundiwale DG. Microencapsulation of quinoline as a corrosion inhibitor in polyurea microcapsules for application in anticorrosive PU coatings. Prog Org Coat. 2015;83:11–8.10.1016/j.porgcoat.2015.01.021Search in Google Scholar
[121] Li K, Liu Z, Wang C, Fan W, Liu F, Li H, et al. Preparation of smart coatings with self-healing and anti-wear properties by embedding PU-fly ash absorbing linseed oil microcapsules. Prog Org Coat. 2020;145:105668.10.1016/j.porgcoat.2020.105668Search in Google Scholar
[122] Haghayegh M, Mirabedini S, Yeganeh H. Microcapsules containing multi-functional reactive isocyanate-terminated polyurethane prepolymer as a healing agent. Part 1: Synthesis and optimization of reaction conditions. J Mater Sci. 2016;51(6):3056–68.10.1007/s10853-015-9616-6Search in Google Scholar
[123] Dawei S, Wenxu M, Jikun M, Qianjin M, Mingzhang L, Ziming W, et al. Workability and crack recovery performance investigation of IPDI microcapsules–based self-healing cement paste. J Mater Civ Eng. 2021;33(7):04021148.10.1061/(ASCE)MT.1943-5533.0003768Search in Google Scholar
[124] Li Q, Mishra AK, Kim NH, Kuila T, Lau K-T, Lee JH. Effects of processing conditions of poly (methylmethacrylate) encapsulated liquid curing agent on the properties of self-healing composites. Compos Part B: Eng. 2013;49:6–15.10.1016/j.compositesb.2013.01.011Search in Google Scholar
[125] Gong H, Yu C, Zhang L, Xie G, Guo D, Luo J. Intelligent lubricating materials: A review. Compos Part B: Eng. 2020;202:108450.10.1016/j.compositesb.2020.108450Search in Google Scholar
[126] Armada S, Schmid R, Equey S, Fagoaga I, Espallargas N. Liquid-solid self-lubricated coatings. J Therm spray Technol. 2013;22(1):10–7.10.1007/s11666-012-9847-xSearch in Google Scholar
[127] Wang Y, Fang G, Ding W, Han N, Xing F, Dong B. Self-immunity microcapsules for corrosion protection of steel bar in reinforced concrete. Sci Rep. 2015;5(1):1–8.10.1038/srep18484Search in Google Scholar PubMed PubMed Central
[128] Cheng J, Niu S, Ma D, Zhou Y, Zhang F, Qu W, et al. Effects of ammonium polyphosphate microencapsulated on flame retardant and mechanical properties of the rigid polyurethane foam. J Appl Polym Sci. 2020;137(48):49591.10.1002/app.49591Search in Google Scholar
[129] Puri RG, Khanna A. Intumescent coatings: A review on recent progress. J Coat Technol Res. 2017;14(1):1–20.10.1007/s11998-016-9815-3Search in Google Scholar
[130] Kazanci B, Cellat K, Paksoy H. Preparation, characterization, and thermal properties of novel fire-resistant microencapsulated phase change materials based on paraffin and a polystyrene shell. RSC Adv. 2020;10(40):24134–44.10.1039/D0RA04093BSearch in Google Scholar
[131] Wang X, Li C, Wang M, Zhao T, Li W. Bifunctional microcapsules with n-octadecane/thyme oil core and polyurea shell for high-efficiency thermal energy storage and antibiosis. Polymers. 2020;12(10):2226.10.3390/polym12102226Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

![Figure 1
(a) Chemical reaction between diisocyanate and diamine [23], (b) microphase separation in a typical polyurea molecule [40], and (c) a typical tapping mode AFM image of a polyuria [23].](/document/doi/10.1515/ntrev-2022-0516/asset/graphic/j_ntrev-2022-0516_fig_001.jpg)
