Abstract
Photodynamic therapy (PDT), as a noninvasive therapeutic modality, has significantly revolutionized the contemporary management of oral and dental health. Recently, PDT has witnessed significant technological advancements, especially with the introduction of biomaterials and nanotechnologies, thus highlighting its potential as a multi-functional tool in therapeutics. In this review, our objective was to provide a comprehensive overview of the advancements in nanotechnology-enhanced PDT for the treatment of oral diseases, encompassing dental caries, root canal infection, periodontal disease, peri-implant inflammation, tooth staining, and whitening, as well as precancerous lesions and tumors. Furthermore, we extensively deliberated upon the persisting challenges and prospective avenues of nanotechnology-enhanced PDT in the realm of oral diseases, which will open up new possibilities for the application of nanotechnology-enhanced PDT in clinical implementation.
Graphical abstract
Abbreviations
- A. actinomycetemcomitans
-
Aggregatibacter actinomycetemcomitans
- AgNC
-
silver nanocluster
- AgNPs
-
silver nanoparticles
- AIE
-
aggregation-induced emission
- ALA
-
5-aminolevulinic acid
- ATP
-
adenosine triphosphate
- AuNPs
-
gold nanoparticles
- CDs
-
carbon dots
- Ce6
-
Chlorin e6
- CeO2NPs
-
cerium oxide nanoparticles
- CS
-
chitosan
- CSNPs
-
chitosan nanoparticles
- Cu2O
-
cuprous oxide
- Cur
-
curcumin
- E. faecalis
-
Enterococcus faecalis
- EDTA
-
ethylene diamine tetraacetic acid
- EPS
-
extracellular polysaccharides
- Esp
-
Enterococcus surface protein
- F. nucleatum
-
Fusobacterium nucleatum
- FDA
-
Food and Drug Administration
- FSR
-
E. faecalis quorum-sensing locus
- GelE
-
gelatinase
- GNRs
-
gold nanorods
- ICG
-
indocyanine green
- LDLR
-
low-density lipoprotein receptor
- MB
-
methylene blue
- Mn
-
manganese
- MOF
-
metal–organic framework
- NGO
-
graphene oxide
- NIR
-
near infrared
- OLK
-
oral leukoplakia
- P. gingivalis
-
Porphyromonas gingivalis
- P. intermedia
-
Prevotella intermedia
- PDA
-
polydopamine
- PDT
-
photodynamic therapy
- PDZ
-
phthalocyanine
- PF-127
-
Pluronic ®F-127
- PLGA
-
poly(lactic-co-glycolic acid)
- P-SACT
-
photoacoustic antibacterial chemotherapy
- PSs
-
photosensitizers
- PTT
-
photothermal therapy
- RB
-
rose bengal
- ROS
-
reactive oxygen species
- S. gordonii
-
Streptococcus gordonii
- S. mutans
-
Streptococcus mutans
- S. sanguis
-
Streptococcus sanguis
- S. sobrinus
-
Streptococcus sobrinus
- SACT
-
sonodynamic antibacterial chemotherapy
- SeNPs
-
selenium nanoparticles
- SOD
-
superoxide dismutase
- SRP
-
scaling and root planning
- TBO
-
toluidine blue
- TiO2
-
titanium dioxide
- UPNPs
-
upconversion nanoparticles
- ZnONPs
-
zinc oxide nanoparticles
1 Introduction
As the human body’s gateway to all the systems, the oral cavity is a complex ecosystem that includes teeth, glandular apparati, vulnerable mucosa, and billions of colonized microorganisms [1]. Disorders of oral health seriously compromise mastication, articulation, physical aesthetics, social integration, and even mental health, imposing a substantial health and economic burden worldwide [2]. The annual global economic burden of poor oral health has been estimated to be approximately 545 billion USD yearly worldwide, encompassing both direct and indirect costs, which is comparable to the two most costly illnesses: cardiovascular disease and diabetes [3]. Currently, the significance of oral health is escalating, highlighting the need for our profession to develop advanced technologies and translate them into practice for global benefit [2,4].
Over the past two decades, photodynamic therapy (PDT) has garnered significant attention in the realm of oral and dental applications due to its minimally invasive nature, potent bactericidal effect, and precise spatiotemporal control [5,6]. PDT relies on three crucial components: an excitation light source, photosensitizers (PSs), and oxygen [5,6]. Under excitation light with an appropriate wavelength, PS can be converted from the ground state to the excited state, where the excited state of PS reacts with oxygen to produce reactive oxygen species (ROS), including hydroxyl radicals (˙OH), super oxide anions (O2−), hydrogen peroxide (H2O2), and singlet oxygen (1O2) [7]. Ultimately, these ROS could effectively eliminate malignant cells, eradicate pathogens, bleach pigments, and perform other functions. PDT has been clinically employed for the treatment of multiple oral diseases, encompassing dental caries, root canal infections, periodontitis, and other related ailments [8,9]. However, PDT also presents several significant limitations [10], which impede its further implementation in the treatment of oral diseases. First, in the context of antibacterial treatment, a large proportion of PSs exhibit poor water solubility and negative charge, which may impede their internalization by bacteria and subsequently lead to suboptimal PDT outcomes for disinfection in oral diseases [6,11,12]. Another significant challenge encountered by numerous researchers is the limited ability of small molecule PSs to effectively penetrate deep into the dentinal tubules, pits, and fissures [13], where pathogenic bacteria usually persist and contribute to treatment failure. Meanwhile, the release of ROS during PDT presents a dual nature, potentially leading to detrimental effects on the normal oral mucosa upon successful treatment of oral diseases. In addition, the presence of oxygen is indispensable for the generation of ROS by PDT. The hypoxic nature of our oral cavity, particularly in the root canal and periodontal pocket, significantly hampers the efficacy of the PDT effect [14,15]. To address these limitations of conventional PDT, an interdisciplinary research framework is imperative for augmenting the PDT effect and advancing oral health [16].
Nanotechnology has been harnessed to optimize treatment efficiency and mitigate adverse effects through various approaches [17,18,19]. The development of novel nanodelivery systems for dental applications is witnessing a growing inclination [20,21,22,23]. Recently, significant advancements have been achieved in the development of novel nanotechnology to optimize the benefits of PDT in oral healthcare. In the present review, we will discuss the recent advances in nanotechnology-enhanced PDT, focusing on the design and treatment efficiency across various oral diseases (Figure 1). Together, considering future optimizations, we will also address the existing challenges and potential perspectives of nanotechnology-enhanced PDT, which would accelerate the corresponding research and clinical transformation for dental care.
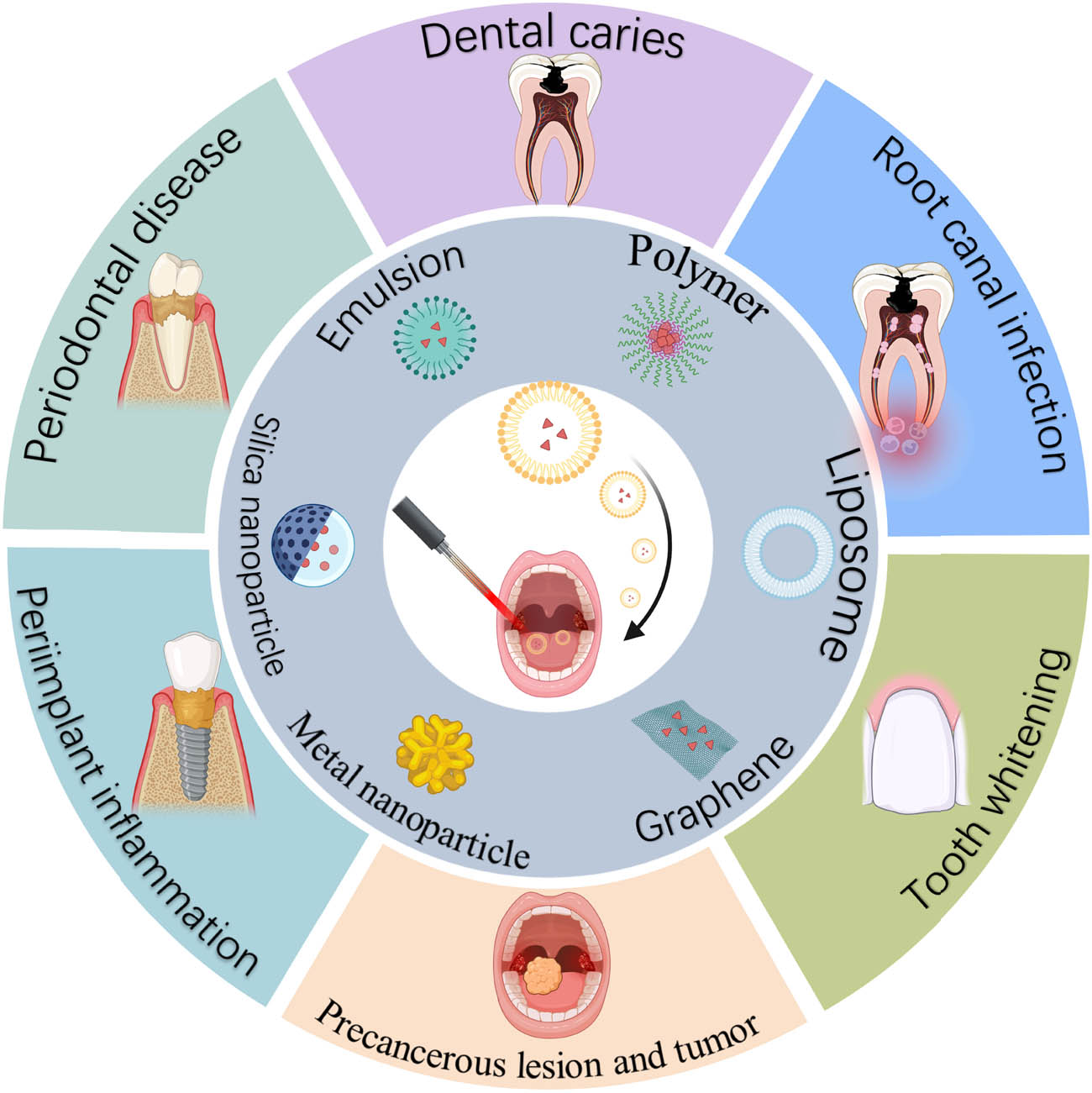
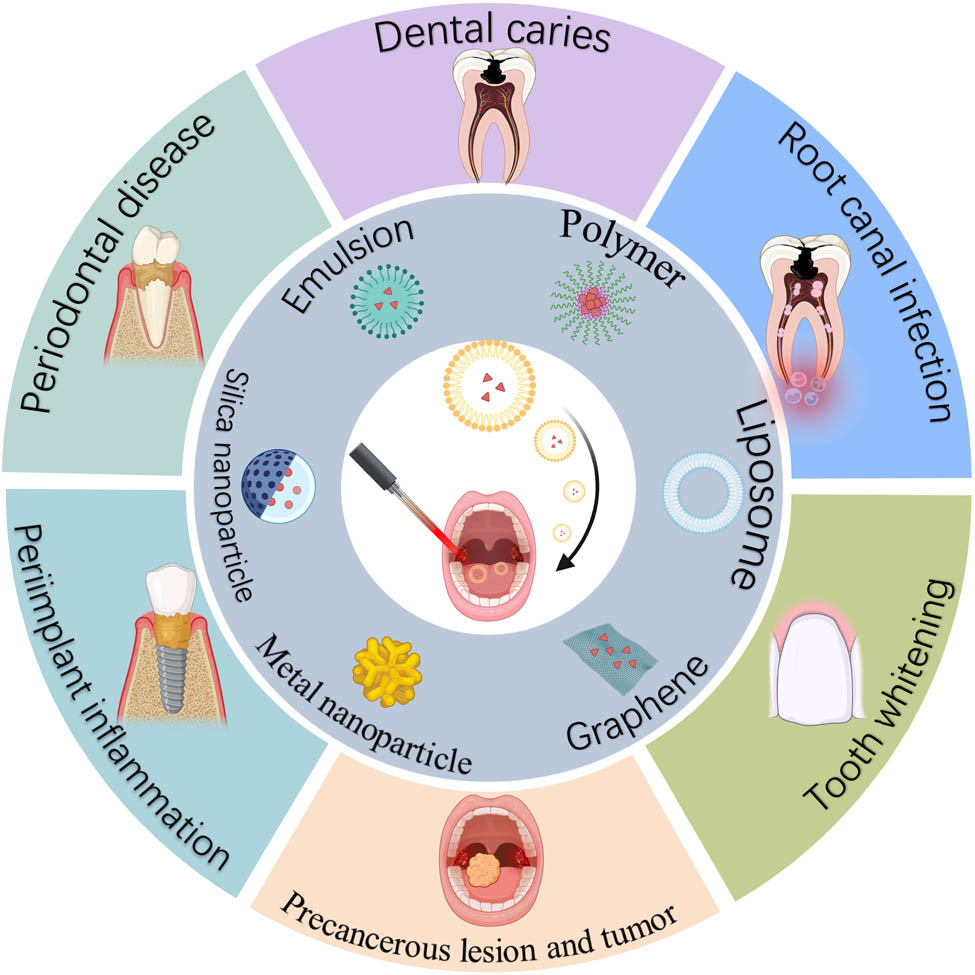
Schematic of nanotechnology-enhanced PDT for the treatment of oral diseases. The facilitation of PDT can be achieved through the utilization of diverse nanoparticle types. Six representative types of nanoparticles are shown, including emulsions, polymers, liposomes, graphene, metal nanoparticles, and silica nanoparticles. Nanotechnology-enhanced PDT has been extensively investigated for the treatment of oral diseases, including periodontal diseases, dental caries, root canal infection, tooth whitening, precancerous lesions, and tumors, as well as peri-implant inflammation, created with BioRender.com.
2 General aspects of nanotechnology-empowered PDT in oral diseases
2.1 PDT for oral and dental applications
The majority of oral diseases are directly associated with pathogenic cells, including malignant cells and pathogenic bacteria. Therefore, the primary objective of oral therapies is to eliminate these cells as thoroughly as possible. For instance, the conventional approach for managing oral cancer involves surgical intervention, radiotherapy, and pharmacotherapy to eradicate malignant cells. Certainly, these therapies may have significant systemic side effects and unavoidable damage to surrounding healthy tissues. In terms of microbial infectious diseases, antibiotic therapy is the common choice. However, the widespread abuse of antibiotics has led to a significant rise in microbial drug resistance, posing substantial challenges for its broad clinical application. Consequently, extensive endeavors are being undertaken to refine and optimize these therapeutic approaches, aiming to augment their efficacy while mitigating potential side effects. Over the past decade, PDT has demonstrated escalating potential in antitumor and antibacterial therapy due to its non-invasive feature and high specificity [5,6]. The oral cavity is directly exposed to the external environment. It is easy to conduct PDT for oral diseases. PDT is also non-invasive and thus has great patient compliance. Particularly, for the treatment of oral tumors, the non-invasive PDT can significantly improve the appearance and psychological health, since the traditional surgery may substantially damage the face tissue. Therefore, PDT has shown great potential for the treatment of diverse oral and dental diseases.
Although PDT has shown great promise for oral and dental applications, it also has several drawbacks:
Most PSs are hydrophobic and have poor solubility, which leads to uncontrollable drug release and aggregation-induced quenching.
The tissue penetration of excitation light is limited, which hinders the clinical application of PDT in deeper tissue.
Oxygen is necessary for PDT to produce ROS. However, the lesion sites in the oral cavity are usually hypoxic, which largely limits its effectiveness.
After PDT treatment, some PSs remain in the body for a long time, continuously absorb natural light, and cause side effects such as skin photosensitization or photobleaching.
2.2 Nanotechnology-empowered PDT in oral diseases
Aiming at addressing the drawbacks of abovementioned PDT, prevailing studies have begun to improve PDT with nanotechnology-based methods for the treatment of oral diseases (Figure 2). Nanotechnology has been developed to promote different aspects of PDT, including PSs, excitation light, oxygen, and combination strategies. In terms of PSs, after the excitation light is irradiated, a significant amount of energy is converted into heat and fluorescence in addition to the production of ROS. Thus, a lot of energy is lost during this process. To enhance the efficiency of PDT, it is essential to keep a sufficient concentration of PSs at the location of the injury. Nanotechnology could promote the accumulation of PS in a variety of ways. First, nanoparticles were applied for encapsulating or decorating PSs to increase the water dispersibility of PSs and change the surface charge of PSs (Figure 2, first panel). Meanwhile, some nanoparticle-loaded PSs have the ability to target specific lesion sites with the guidance of magnets, biological binding, etc. In addition, nanoparticles can endow PSs with the property of stimuli-responsive release, which is able to achieve controlled release of PSs at specific sites and specific times for better disease management. With respect to excitation light, multiple studies have used long-wavelength light to excite PS for deeper tissue penetration; among them, two-photon absorption nanoparticles and upconversion nanoparticles (UPNPs) are two representative methods (Figure 2, second panel). UPNPs can absorb near-infrared (NIR) light and transform it into high-energy photons at shorter wavelengths, which subsequently excite the PSs. Two-photon absorption nanoparticles can serve as PS themselves, absorbing two long-wavelength photons at the same time and thereby generate ROS. In the case of oxygen and ROS, to overcome the hypoxic status in the oral cavity for better ROS generation, nanoparticles could be designed to generate oxygen through different methods (Figure 2, third panel). Sometimes, excessive ROS causes damage to normal tissue, which can be relieved by specific types of nanoparticles that possess the ability to scavenge ROS. Another important aspect of nanotechnology-enhanced PDT is that this method could provide a combination strategy for synergizing PDT with chemotherapy, photothermal therapy (PTT), and sonodynamic therapy (Figure 2, fourth panel).
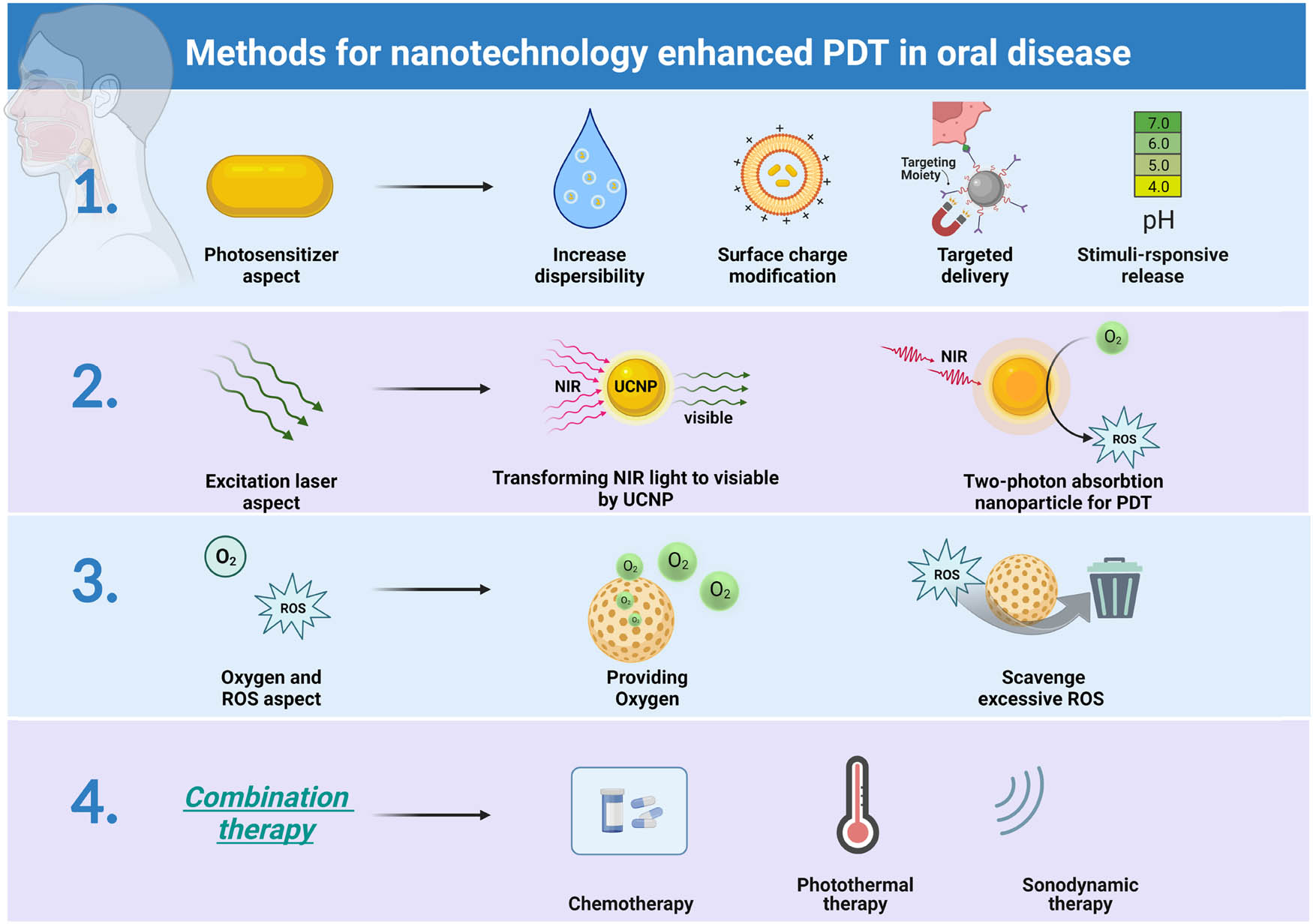
Schematic diagram of strategies for nanotechnology-enhanced PDT in oral diseases. Nanotechnology could be applied to enhance PDT in four aspects, including the PS, excitation laser, oxygen and ROS, and combination therapy. First, nanotechnology could endow PSs with increased dispersion in water, changeable surface charge, targeted delivery ability, and the function of stimuli-responsive release. Second, UPNP and two-photon absorption nanoparticles can be utilized for generating ROS under the excitation of NIR laser. Third, nanoparticles were exploited for providing oxygen for PDT and scavenging residual ROS to avoid tissue damage. Last, the combination of PDT with chemotherapy, PTT, sonodynamic therapy, or others can be achieved through nanotechnology-based methods, created with BioRender.com.
3 Progress of nanotechnology-empowered PDT in oral diseases
3.1 Dental caries
Dental caries is characterized by chronic and progressive destruction of tooth hard tissue under the action of bacteria and their acid metabolites [29]. The bacterial community is embedded in extracellular polysaccharides (EPS) to form a highly stable ecosystem, the biofilm, which can protect the bacteria from being killed by multiple therapies. Within the biofilm, Streptococcus mutans (S. mutans) and Streptococcus sobrinus (S. sobrinus) are the main cariogenic bacteria with the abilities of acid production and acid tolerance [30,31], which results in a stable acidic state in the biofilm (pH = 4.5–5.5) [32]. Due to the limited penetration depth of the excitation light in human tissue, PDT is highly suitable for lesions that occur in superficial areas, including dental caries. Meanwhile, PDT application in dental caries also faces some challenges and could be addressed through nanotechnology (Table 1).
Application of nanotechnology-enhanced PDT for dental caries
| Nanocarrier | PS | Bacteria | PDT parameters | Therapeutic effect | Ref. |
|---|---|---|---|---|---|
| CSNP | Photoditazine, PDZ | S. mutans | 660 nm, 39.5 J/cm2, 300 s | Increase the absorption of PDZ by S. mutans, reduce the number of S. mutans by 4.5 log10, and inhibit the formation of EPS in biofilms | [41] |
| CSNP | ClAlPc | S. mutans | 660 nm, 100 J/cm2, 175 s | Reduce the number of S. mutans by 1 log10 | [139] |
| Cationic liposomes | Gingival plaque | 660 nm, 250 W/cm2, 3 min | Nanoparticles have a much higher absorption rate in bacteria than in dental pulp cells. Inhibit bacterial growth by 82% | [140] | |
| PLGA-NP | Cur | S. mutans | 405 ± 5 nm, 45 J/cm2 | The inhibition rates of 7% Cur-PLGA-NP on S. mutans biofilm at 15, 30, 60 and 90 days were 4.1, 79.6, 69.6, 69.4, and 55.1%, respectively | [36] |
| Pluronic @ F-127 | Cur | S. mutans and C. albicans | 460 nm, 15 J/cm2, 696 s | Cur-PF-127-mediated PDT has MICs of 270 and 2.1093 μM against S. mutans and C. albicans, respectively, with good anti-biofilm effect | [37] |
| PNP | PhotoAc | S. mutans | 635 nm and 103.12, 68.75 J/cm2 | The formed novel PS exhibited a stronger inhibitory effect on S. mutans biofilm and biofilm formation-related genes (gtfB, gfC, and f0) than photoactive or TBO alone: less toxic to human gingival fibroblasts | [141] |
| AgNP | TBO | S. mutans | 630 nm, 9.1 J/cm2, 70 s | TBO-AgNP-mediated PDT reduced the number of planktonic S. mutans by 4log10, inhibited the formation of S. mutans biofilm, and downregulated the expression of biofilm-related genes | [44] |
| AgNP | RB | S. mutans, A. actinomycetemcomitans, and P. gingivalis | 420–750 nm; 80 mW/cm2; 0、 30, 60, 120 s | The antibacterial effects of RB-AgNP-mediated PDT against S. mutans, A. actinomycetemcomitan, and P. gingivalis were dose-dependent and time-dependent. RB-AgNP still had a stable and durable antibacterial effect after stopping the irradiation effect | [45] |
| AgNP | MB | S. mutans | 650 nm, 400 mW/cm2, 3 min | RB-AgNP-mediated PDT inhibited the S. mutans biofilm constructed on dentin sheets as high as 95.28% | [142] |
| AuNP | MB | S. mutans | 660 nm, 19.23 J/cm2, 10 min | MB-AuNP and free MB-mediated aPDT had the same antibacterial effect on S. mutans | [47] |
| SeNP | TBO | S. mutans | 630 nm, 38.5 J/cm2, 60 s | The inhibition rates of S. mutans biofilms by TBO-SeNP and free TBO-mediated PDT were 60 and 20%, respectively | [46] |
| ZnONP | cCur | S. mutans, S. sobrinus, and L. acidophilus | 435 ± 20 nm, 300–420 J/cm2, 5 min | For up to 90 days, the adhesive containing 7.5% Cur-ZnONP showed no colonization of S. mutans, S. sobrinu, and L. acidophilus after photoactivation | [38] |
| It can 100% inhibit the metabolic growth of these bacteria | |||||
| GO-Car/Hap | ICG | S. mutans | 810 nm, 31.2 J/cm2, 1 min | GO-Car/HAp@ICG-mediated PDT reduced the number of planktonic S. mutans by 93.2%, inhibited the formation of S. mutans biofilm by 56.8%, and the expression of gtfB gene was significantly reduced by 7.9-fold | [35] |
| An amphiphilic polymer | Ce6 | S. mutans, S. sobrinus, and S. sanguinis | 660 nm, 0.5 W/cm2, 3 min | MPP-Ce6-mediated aPDT exhibits significant bacteriostatic effects against cariogenic bacteria while demonstrating negligible systemic toxicity | [42] |
A large proportion of PSs are water-insoluble and possess a negative charge, making them incapable of penetrating deep in the complex structure of biofilm and internalized by negatively charged bacteria [33], which greatly hinders the application of PDT in treating dental caries. Nanotechnology can be exploited to modify those PSs and deliver them more efficiently. First, to overcome the water-insoluble problems of PS, several nanocarriers were developed. Graphene is a unique nanocrystal, characterized by facile modification, high solubility, favorable biocompatibility, and other notable advantages [34]. It exhibits the ability to harness visible light activation for ROS generation, thereby compromising bacterial integrity [34]. Consequently, graphene has garnered significant attention as an antibacterial agent. Gholibegloo et al. loaded indocyanine green (ICG) into a novel graphene oxide (NGO)-based nanodelivery system. Results showed that this system could increase the long-term stability of ICG in water and greatly improve the inhibitory effect of PDT on S. mutans and its biofilm [35]. Curcumin (Cur) is a US Food and Drug Administration (FDA)-approved drug with impressive properties for the treatment of multiple types of diseases, which was also demonstrated to act as a PS for PDT. However, the application of Cur in dental caries is limited by its poor bioavailability caused by poor water solubility and rapid metabolism. Poly(lactic-co-glycolic acid) (PLGA) and Pluronic ®F-127 (PF-127) have been developed as Cur carriers, which can increase the bioavailability of Cur and endow Cur with a strong PDT effect [36,37]. Zinc oxide nanoparticles (ZnONPs), which are considered to be one of the most promising next-generation PS for PDT [38], were utilized to evaluate the PDT efficiency for combating cariogenic bacteria and biofilms [38]. Pourhajibagher et al. studied the incorporation of cationic Cur-doped ZnONP into orthodontic adhesives, which can inhibit metabolic activity and biofilm formation up to 90 days [38].
The negative charge of some PSs hampers their further application in the treatment of dental caries. Chitosan (CS), a biocompatible and biodegradable derivative of chitin, exhibits versatile chemical modifications and grafting capabilities [39,40]. The application of CS nanoparticles (CSNPs) demonstrates a significant reduction in bacterial adhesion to the dental surface and effectively eliminates bacterial biofilms. This is attributed to the positive charge of CS, which is able to interact with negatively charged residues on the cytoplasm or cell membrane, enhancing the effect of PSs through the promotion of membrane permeability. de Souza et al. loaded the commercial PS phthalocyanine (PDZ, a derivative of chlorophyll A) into CSNP. Compared with PDZ, nanoparticles formed by PDZ-CSNP could significantly increase the endocytosis of PDZ by S. mutans, which further improved the effect of PDT, reduced the number of S. mutans, and effectively inhibited the formation of EPS in biofilms [41]. Our group also developed a simple but efficient nanodelivery system for eliminating cariogenic biofilms (Figure 3) [42]. Specifically, Chlorin e6 (Ce6) was encapsulated in a type of pH-responsive nanoparticle, which was demonstrated to enhance the penetration depth (by over 75%) and long retention (by over 100%) of Ce6 in the biofilm. Under the irradiation of a 660 nm laser, this method could significantly inhibit the formation of dental caries in a rat model of early childhood caries.
![Figure 3
A novel biofilm-responsive nanoparticle was developed to improve the efficacy of PDT against multispecies cariogenic biofilms. This nanoparticle enhances the penetration of PSs into the biofilms, effectively targeting and eliminating them. Reproduced with permission from Liu et al. [42]. Copyright 2021, Elsevier. (a) Schematic diagram illustrating the process in which the nanoparticle releases PSs into the acidic microenvironment of the biofilm and exhibits bactericidal effects when exposed to a 660 nm laser. (b) The nanoparticle can penetrate biofilms to a depth of over 35 μm, surpassing the capability of free PSs.](/document/doi/10.1515/ntrev-2023-0163/asset/graphic/j_ntrev-2023-0163_fig_003.jpg)
A novel biofilm-responsive nanoparticle was developed to improve the efficacy of PDT against multispecies cariogenic biofilms. This nanoparticle enhances the penetration of PSs into the biofilms, effectively targeting and eliminating them. Reproduced with permission from Liu et al. [42]. Copyright 2021, Elsevier. (a) Schematic diagram illustrating the process in which the nanoparticle releases PSs into the acidic microenvironment of the biofilm and exhibits bactericidal effects when exposed to a 660 nm laser. (b) The nanoparticle can penetrate biofilms to a depth of over 35 μm, surpassing the capability of free PSs.
To further enhance the PDT effect, nanotechnology-based combination strategies were exploited for synergizing with PDT in the treatment of dental caries. Metals and metal-oxidized nanomaterials, such as silver nanoparticles (AgNPs), gold nanoparticles (AuNPs), and selenium nanoparticles (SeNPs), are commonly used in the infection control of dental caries and could enhance the effects of PDT. Considering AgNP as an example, it can steadily and persistently release silver ions (Ag+), which subsequently destroy the structure of microorganisms. Moreover, compared with other inorganic nanoparticles, AgNP exhibits stronger broad-spectrum antibacterial properties [43]. Owing to its minute size, AgNP can penetrate the dentin tubules with a diameter ranging from 2.4 to 2.9 μm, thereby aiding in the regulation of bacterial growth and propagation within the root canal system. Additionally, it can induce alterations in bacterial membrane permeability, resulting in leakage of bacterial contents and ultimately leading to bacterial lysis and rupture [43]. Several studies have combined AgNPs with traditional PSs to achieve synergistic antibacterial effects. For example, Misba et al. conjugated toluidine blue (TBO) with AgNP to form TBO-AgNP for PDT under the excitation light, which could destroy the cell membrane of bacteria and cause the leakage of bacterial contents. Compared with free TBO, TBO-AgNP-mediated PDT inhibited the bacterial proliferation and biofilm formation, and significantly downregulated the expression of biofilm formation-related genes (gtfC, ftf, spaP, gbpB, comD, comE, and vicR) [44]. Shitomi et al. developed a nanocomposite of the combination of rose bengal (RB) and silver nanocluster (AgNC), which exhibited dose- and time-dependent bactericidal efficiency against S. mutans, Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans), and Porphyromonas gingivalis (P. gingivalis) under the irradiation of excitation light. Moreover, AgNCs/RB showed a greater antibacterial effect compared with the two single components, indicating that the released Ag+ and RB-mediated PDT have a synergistic antibacterial effect [45]. SeNP exhibits potent antibacterial and anti-biofilm activities, making it an ideal nano-carrier for PDT. Furthermore, it synergistically enhances the efficacy of PDT. Biofilm inhibition assay confirmed that TBO-SeNP-mediated PDT had a higher inhibitory effect on S. mutans monospecies biofilm than the free TBO [46]. AuNP can serve as a drug carrier for targeted eradication of diseased cells and microorganisms, making it an ideal candidate for nanomedicine due to its bio-binding surface with suitable molecular probe affinity and exceptional optical properties. However, methylene blue-loaded AuNP (MB-AuNP) was demonstrated to show no significant difference in antibacterial effect against S. mutans after photoactivation compared with free MB [47]. In addition, propolis nanoparticles were loaded with a chlorophyllin mixture (PhotoActive+) and TBO, respectively, to form novel drug-loaded nanoparticles for synergistic effects in the treatment of dental caries. These novel nanoparticles exhibited enhanced inhibitory effects on S. mutans biofilms compared to the original free PhotoActive+ or TBO, thereby holding promise for minimizing therapy-related adverse effects.
3.2 Root canal infection
Root canal infection includes primary root canal infection, secondary root canal infection, and persistent periapical infection. Enterococcus faecalis (E. faecalis) and P. gingivalis have high detection rates in primary root canal infections. E. faecalis is the main cause of root canal treatment failure and can exist in nutrient-depleted root canals for a long time. The main virulence factors, including Enterococcus surface protein (Esp), transcription regulator (BopD), E. faecalis quorum-sensing locus (FSR), and gelatinase (GelE), are involved in the formation of E. faecalis-related biofilms [48,49,50].
The primary goal of endodontic therapy is to eliminate the microbial membrane in the endodontic system and provide a sound endodontic seal. Nanotechnology could effectively deliver PSs into the infection site and increase the internalization of PSs by bacteria, which enables PDT a highly effective bactericidal ability. At the same time, the application of PDT demonstrated minimal bacterial resistance, thereby minimizing the risk of multidrug resistance. Therefore, diverse nanocarriers were recently exploited to deliver PSs for combating root canal infection (Table 2).
Effect of nanotechnology-based aPDT on root canal infection-related pathogens
| Nanocarrier | PS | Bacteria | PDT parameters | Therapeutic effect | Ref. |
|---|---|---|---|---|---|
| CSNP | RB | E. faecalis | 540 nm, 5–60 J/cm2 | The survival rate of fibroblasts was higher than that of the free RB group, showing higher anti-collagenase degradation ability | [62] |
| CSNP | RB | E. faecalis | 540 nm; 5, 10 J/cm2 | Compared with free RB, RB-CSNP-mediated aPDT still had a residual antibacterial effect after 24 h interaction, which could completely inactivate bacteria | [61] |
| CSNP | RB | E. faecalis, P. intermedia, A. naeslundii, and S. oralis | 540 nm, 5, 60 J/cm2 | RB-CSNP-mediated aPDT can significantly reduce the 21-day mixed biofilm cultured by P. intermedia, A. naeslundii, and S. oralis | [63] |
| CSNP | RB | E. faecalis | 540 nm; 40, 2 J/cm2 | The root canal sealant mixed with RB-CSNP for root canal disinfection can significantly inhibit the E. faecalis biofilm in the root canal for 4 weeks after irradiation | [64] |
| CSNP | RB | E. faecalis | 540 nm; 2, 5, 10, 20, 40, 60 J/cm2 | In the presence of BSA, compared with free RB, RB-CSNP-mediated aPDT effectively destroys the single-strain biofilm formed by E. faecalis | [69] |
| CSNP | ICG | E. faecalis | 810 ± 20 nm; 0.49–1.46 W/cm2; 1, 3, 5 min | After laser irradiation, the longer the laser irradiation time, the higher the inhibition rate of ICG-CSNP against E. faecalis plankton | [65] |
| PLGA-NP | MB | E. faecalis | 665 nm; 0.32 W/cm2; 10 min | MB-PLGA-NP-mediated aPDT reduced planktonic and root canal E. faecalis | [73] |
| PLGA-NP;CSNP | MB, RB | 660, 540 nm; 10 min | Compared with sodium hypochlorite and EDTA irrigant, RB-CSNP, and MB-PLGA-NP-mediated aPDT increased the microhardness of root canal hard tissue and significantly enhanced the elastic modulus of dentin | [70] | |
| Liposomes | mTHPC | E. faecalis | 654 ± 4 nm, 100 J/cm2, 425 s | Compared with chlorhexidine, the antibacterial effect of aPDT mediated by mTHPC-loaded liposome nanoparticles was stronger. E. faecalis biofilm was effectively inhibited at 300 µm in dentinal tubules | [143] |
| AcLi-NP | THPP | E. faecalis | 447 nm, 1.2 mW/cm2, 1 h | THPP-AcLi-NP-mediated aPDT, 0.64 µM can inhibit 90% of E. faecalis growth, and 2.5 µM can completely kill the bacteria | [74] |
| NE | ZnPc | E. faecalis | 670 nm, 30 J/cm2 | Under the excitation of light, ZnPc-NE has more excellent antibacterial ability | [75] |
| NGO | ICG | E. faecalis | 810 nm, 31.2 J/cm2, 60 s | NGO-ICG-mediated aDPT reduced the viability of E. faecalis by 2.8 log10. The inhibition rate of E. faecalis biofilm was 99.4% | [51] |
| NGO | Cur | E. faecalis | 435 ± 20 nm, 360 J/cm2, 300 s | Cur-NGO-mediated aPDT had a significant inhibitory effect on E. faecalis biofilm. The expression of biofilm formation-related virulence factor genes (Efa, Esp, Gel, and FSR) was significantly downregulated | [52] |
| AgNP | ICG | E. faecalis | 810 nm, 200 mW, 30 s | AgNP combined with ICG/DL has a high inhibition rate of 99.12% against E. faecalis in the root canal | [53] |
| AgNP | ICG | E. faecalis | 808 nm, 250 mW, 60 s | AgNP combined with ICG/DL had an inhibition rate of about 70.77% against E. faecalis in the root canal | [54] |
| AgNP | TBO | E. faecalis | 620–640 nm; 2,000–4,000 mW/cm2; 30, 60 s | Simultaneous illumination of TBO and AgNP for 30 or 60 s can inhibit up to 99% of E. faecalis biofilm in root canals | [55] |
| AuNP | MB | E. faecalis | 660 nm; 55, 108, 179 mW/cm2; 30 min | MB-AuNP had a good inhibitory effect on the E. faecalis biofilm | [56] |
| SeNP | MB | E. faecalis | 660 nm, 200 mW/cm2, 1 min | The combined application of SeNP and MB-mediated aPDT not only reduced the number of viable bacteria in the E. faecalis biofilm but also effectively inhibited biofilms in dentinal tubules at a depth of 200–400 µm | [57] |
| MOF | ICG | E. faecalis | 810 nm, 31.2 J/cm2, 60 s | After loading ICG, the inhibition rate of Fe101, Al-101, and Fe-88-mediated aPDT against E. faecalis was increased, and the expression of Esp gene related to biofilm generation was also significantly decreased | [60] |
A number of inorganic nanoparticles were utilized to improve the physical and chemical properties of traditional PSs for antibacterial PDT. NGO has been employed as a nanocarrier due to its exceptional attributes, including substantial surface area, abundant functional group, and cost-effectiveness for large-scale synthesis. The photoactivated bacteria-killing ability of ICG was largely limited due to its negative surface charge. NGO nanoparticle was utilized to conjugate ICG to improve the interaction between ICG and microorganisms. Results have shown that photoactivation of ICG-loaded NGO (ICG-NGO) can prevent the proliferation of E. faecalis (more than 99%) compared with free ICG-mediated PDT, and the compound also exhibits a higher inhibition effect on E. faecalis-related biofilm formation [51]. In addition, the problem of the low solubility of Cur in aqueous media at physiological pH and rapid hydrolysis under alkaline conditions can also be solved by nanotechnology-based delivery systems. Cur-NGO-mediated PDT significantly inhibited E. faecalis biofilm, and the expression of virulence factors related to biofilm formation (Efa, Esp, Gel, and FSR) was significantly downregulated [52].
The combined application of metal and metal oxide nanoparticles, AgNP, AuNP, and metal–organic framework (MOF) materials with PDT can synergistically control pathogens associated with root canal infection. For example, AgNP is widely used as an antibacterial agent in the treatment of root canal infections. AgNP was demonstrated to enter the dentin tubules with a diameter of 2.4–2.9 μm, which causes changes in the bacterial membrane permeability, the leakage of bacterial contents, and finally leads to bacterial lysis and rupture in the root canal system. Afkhami et al. found that the combination of AgNP and ICG/DL inhibited E. faecalis in the root canal at a very high rate (70.77–99.12%) [53,54]. In addition, the combination of TBO and AgNP significantly inhibited 99% of the E. faecalis biofilm in root canals within a short time of irradiation (30 or 60 s) [55]. AuNP and SeNP themselves have antibacterial and anti-biofilm activities, which could also serve as nanocarriers for PDT [56,57]. It was found that PDT mediated by a combination of MB with AuNP or SeNP could reduce the number of E. faecalis in the biofilm. Compared with free MB, MB-AuNP-mediated PDT requires less laser energy to kill the same proportion of bacteria [56]. MB-SeNP was demonstrated to effectively inhibit biofilms at the depth of 200–400 μm of dentin tubules [57].
MOF, a new and promising class of porous material, has received extensive research attention for drug delivery due to its high biodegradability, good biocompatibility, and high drug-loading efficiency [58,59]. Golmohamadpour et al. loaded ICG into different types of MOF nanodelivery systems to form FE-101-ICG, AL-101-ICG, and Fe-88-ICG. The modification of the MOF vector significantly improved the stability of ICG. In addition, the antimicrobial activity of the corresponding novel MOF-mediated PDT loaded into ICG was significantly increased compared with MOF alone after laser irradiation at 810 nm. At the same time, three novel MOF-mediated PDTs significantly decreased the expression of Esp and significantly inhibited the formation of biofilm [60].
Organic nanoplatforms were also introduced to deliver PSs for root canal disinfection through PDT. CS shows a wide range of antibacterial activity, good biocompatibility, and biodegradability, which is suitable for a variety of chemical modifications and grafting. CSNP was demonstrated to effectively reduce the adhesion of bacteria on the dentin surface and eliminate bacterial biofilms. In recent years, CSNP has been used to load PSs, including RB and ICG for root canal infection control [61,62,63,64,65]. After conjugation with CSNP, the adsorption ability of RB by E. faecalis was enhanced, and RB-CSNP could effectively penetrate deep into the biofilm [62]. In addition, CSNP itself has certain antibacterial properties, which can synergistically enhance the antibacterial effect of PDT [62]. Another study showed that RB-CSNP can reduce the number of E. faecalis to 50–65%, while RB-CSNP-mediated PDT can inhibit the number of E. faecalis up to 100% [61]. Moreover, the structure of E. faecalis was significantly destroyed and the bacterial contents leaked out of the cell after RB-CSNP-mediated PDT. For biofilm, after RB-CSNP-mediated PDT, the number and thickness of viable bacteria in the single-species biofilm of E. faecalis and the multi-species biofilm cultured on dentin tablets were significantly reduced [63]. In addition, DaSilva et al. doped RB-CSNP into root canal sealer and found that it had a significant inhibitory effect on the E. faecalis biofilm in root canals after illumination [64]. In most cases, the presence of dentin components, tissue residues, and serum products in the root canal can inhibit the ability of antibiotics to control root canal infection and mediate the degradation of tooth hard tissue, leading to the damage of mechanical integrity and chemical stability of the tooth structure [66,67,68]. Therefore, Shrestha et al. explored whether these inhibitors would affect the RB-CSNP-mediated antimicrobial effects of PDT. Results showed that pulp tissue and serum albumin could inhibit the antibacterial effect of RB-CSNP in the absence of light excitation. After photoactivation, only the RB-CSNP group still had a residual antibacterial effect and was able to completely inactivate the bacteria compared with free RB and MB groups [61,69]. After RB-CSNP-mediated PDT, the microhardness of the inner wall of the root canal was also significantly improved, which enhanced the anti-degradation ability and mechanical strength of dentin collagen. The mechanism may be that 1O2 produced by PDT contributes to the formation of intermolecular or intramolecular covalent cross-links between collagen and protein surface, thereby enhancing the mechanical strength of tooth hard tissue [62,69,70,71,72].
Besides CSNP, other polymeric organic nanocarriers, such as PLGA-NP, acetylated lignin nanoparticles, and clove oil nanoemulsion, have also been used as PS carriers for root canal infection control [73,74,75]. PLGA is a polyester copolymer of polylactic acid and polyglycolic acid, which has gained FDA approval for its exceptional biocompatibility and in vivo degradability and made it an ideal candidate for drug delivery of PSs within the human body, aligning with natural pathways. PLGA exhibits continuous drug release, and high stability in biological fluids, and storage processes. Moreover, it can serve as a carrier to enhance the bioavailability of PSs. MB-loaded PLGA-NP-mediated PDT was found to have strong antibacterial effects and could reduce planktonic and colonizing E. faecalis in root canals [73]. Compared with traditional sodium hypochlorite and ethylene diamine tetraacetic acid (EDTA), MB-PLGA-NP-mediated PDT demonstrated the ability to enhance the microhardness of root canal hard tissue, improve the elastic modulus of dentin, and reduce the damage to dentin structure caused by root canal treatment [70].
Despite these promising results concerning the long-term success of nanotechnology-mediated PDT for eliminating planktonic pathogenic bacteria, questions remain. Disinfection of the root canal is challenging due to its complicated autonomy, and the bacteria penetrate deep into the dentinal tubules [13,76], which requires the nanoparticles to infiltrate deep into the dentinal tubules. Although our and other studies have demonstrated that some microparticles can penetrate deep into the dentinal tubules with the assistance of acoustic or magnetic actuation methods [77,78,79], more effective methods are required for eliminating infections within the profound dental tubules. Therefore, further investigations are warranted to evaluate the potential of nanotechnology-enhanced PDT in eradicating bacteria at concealed sites within root canals through both in vitro and in vivo experiments.
3.3 Periodontitis
Periodontitis is a chronic progressive infectious disease that occurs in periodontal supporting tissue. P. gingivalis is the dominant bacterium in periodontal disease, especially in chronic periodontitis [80]. Traditional periodontal treatments, such as periodontal cleaning, scraping, and root surface leveling, may cause great trauma to the periodontal tissue. Moreover, in the deep part of the periodontal pocket and the rough cementum surface, it is difficult to completely remove subgingival plaque using traditional treatment. PDT has been extensively investigated in the elimination of pathogenic bacteria for the treatment of periodontitis. For example, studies have shown that without adding any PS, irradiation with a laser (380–520 nm) can effectively kill bacteria, which was attributed to endogenous PS (melanin, porphyrins, etc.) produced by P. gingivalis and Prevotella intermedia (P. intermedia) [81]. In addition, researchers have been investigating nanotechnology-based methods for enhancing PDT for periodontitis treatment (Table 3).
Effect of nanotechnology-based aPDT on periodontitis and peri-implantitis-related pathogens
| Nanocarrier | PS | Bacteria | PDT parameters | Therapeutic effect | Ref. |
|---|---|---|---|---|---|
| PLGA-NP | MB | Human subgingival plaque biofilm | 665 nm, 100 mW/cm2, 5 min | MB-PLGA-NP-mediated aPDT reduced the number of planktonic bacteria and biofilms by 60 and 48% respectively | [144] |
| PLGA-NP | MB | P. gingivalis | 660 nm, 20 J/cm2 | MB-PLGA-NP increased the antibacterial ability of aPDT mediated by 25% over free MB | [91] |
| CSNP | ICG | A. actinomycetemcomitans | 810 nm, 31.2 J/cm2 | Compared with free ICG, ICG-CSNP-mediated aPDT significantly down-regulated the expression of rcpA gene, a virulence factor associated with biofilm formation in A. actinomycetemcomitans | [90] |
| CSNP | ICG | P. gingivalis | 805 ± 20 nm, 0.5 W, 100 s | The number of P. gingivalis was reduced after 100 s of illumination in continuous-wave repetitive pulse mode. Thermal damage to normal periodontal tissues was avoided | [93] |
| CSNP | ICG | P. gingivalis | 810 ± 20 nm, 4–24 W/cm2, 5 min | ICG-CSNP can inhibit the growth of P. gingivalis. In the bionic gingival model, the temperature rises to 2.74℃ | [92] |
| NPh | Cur | A. actinomycetemcomitans | 450 ± 5 nm, 150 mW/cm2, 2 min | Cur-NPh-mediated PSACT effectively inhibited bacterial growth, biofilm formation, and metabolic activity. Cur-NPh-PSACT inhibited bacterial quorum-sensing virulence factor-related genes (qseB and qseC) and biofilm virulence factor-related genes (rcpA) were down-regulated | [145] |
| GQD | Cur | A. actinomycetemcomitans, P. gingivalis, and P. intermedia | 435 ± 20 nm, 1,000–1,400 mW/cm2, 1 min | Cur-GQD-mediated aPDT can significantly inhibit the viability of A. actinomycetemcomitans, P. gingivalis, and P. intermedia, and also significantly inhibit the formation of related biofilms (76%). The expressions of periodontitis-related virulence factor genes rcpA, fimA, and inpA were down-regulated | [146] |
| Fe3O4-NP | Ce6 | S. sanguis, P. gingivalis, and F. nucleatum | 630 nm, 100 mW/cm2, 3 min | After 3 min of red-light irradiation, three periodontitis-related single-species biofilm bacteria were reduced | [82] |
| UCNP | TiO2 | S. sanguis, P. gingivalis, and F. nucleatum | 980 nm, 2.5 W/cm2, 5 min | Compared with free TBO, aPDT mediated by TiO2-UCNP has a stronger inhibitory ability on the single-strain biofilm, and the viable bacteria of biofilm. The number is reduced by about 4log10 | [84] |
| UCNP | Ce6 | P. gingivalis, P. intermedia, and F. nucleatum | 980 nm, 750 J/cm2, 3 min | 30% Mn-doped Ce6-UCN-mediated aPDT could effectively inhibit the number of viable bacteria in P. gingivalis,P. intermedia, and F. nucleatum and EPS production | [85] |
| CeO2NP | Ce6 | S. gordonii, P. gingivalis, and F. nucleatum | 630 nm, 100 mW/cm2, 3 min | It has a strong antibacterial effect on single- and multi-strain biofilms formed by S. gordonii, P. gingivalis. and F. nucleatum, the number of viable bacteria in the biofilm is reduced and the related virulence factor genes (rgpA, rgpB, and kgp) were significantly downregulated | [87] |
| TiO2-NP | PDA | E. coli, S. aureus, and S. mutans | 5.52, 1.0 W/cm2; 5, 10, 18 min | After irradiation with dual-wavelength light sources (blue light and NIR), CTP-SA has strong antibacterial and anti-inflammatory effects and can promote alveolar bone regeneration | [89] |
| TiO2-NP | S. epidermidis | 365 nm, 2.4 J, 2 min | TiO2-NP was deposited on the surface of the implant through the negative electrode, and 90% of S. epidermidis was effectively killed by ultraviolet light irradiation for 2 min | [95] | |
| CSNP | ICG | A. actinomycetemcomitans, P. gingivalis, and P. intermedia | 810 nm, 31.2 J/cm2, 1 min | ICG-CSNP-mediated PSACT can significantly reduce the number of periodontal pathogens by 8.8log10; at the same time, on the implant surface, the therapy can effectively inhibit about 90% of biofilm | [96] |
To increase the accumulation of PSs at the specific lesion site of periodontitis, nanotechnology-based methods were developed to endow traditional PSs with targeting ability. Sun et al. constructed Fe3O4-NP (Fe3O4-Si@Ce6/C6), which is a novel magnetic nanocomposite material. Due to the magnetic properties of Fe3O4, Fe3O4-Si@Ce6/C6 nanoparticles possess lesion site targeting properties when guided by an external magnetic field. Additionally, three individual bacterial biofilms associated with periodontitis (Streptococcus sanguis (S. sanguis), P. gingivalis, and Fusobacterium nucleatum (F. nucleatum)) were reduced by irradiation with 630 nm red light [82].
P. gingivalis and other pathogenic bacteria primarily reside within the periodontal pocket deeper than 4 mm [83]. Consequently, achieving effective PDT becomes challenging due to the limited penetration of excitation light into the deep periodontal tissue. To increase the penetration depth of PDT, Qi et al. synthesized titanium dioxide (TiO2)-loaded core–shell structure UPNP (TiO2-UPNP) (Figure 4a). UCNP could convert NIR light into high-energy visible or ultraviolet light and realize NIR-mediated PDT in deep periodontal tissue. TiO2 itself has the advantages of low toxicity, good biocompatibility, and high stability. Under ultraviolet irradiation, it can generate ROS and the photocatalytic activity persists even after the light source is removed. Due to its photocatalytic properties and ability to enhance tissue integration, TiO2 can be investigated as a potential implant coating. After irradiation with NIR, TiO2-UCNP had a good antibacterial effect on three kinds of periodontitis-related pathogens (S. sanguis, P. gingivalis, and F. nucleatum) [84]. Zhang et al. loaded Ce6 into manganese (Mn)-doped UCNP and used UCNP as a carrier for PS in an attempt to enhance the penetration of conventional PDT using UCNP [85]. Besides, two-photon absorption nanomaterial-based PDT can also be excited by NIR laser [86], presenting a potential strategy for increasing the penetration depth of PDT in the treatment of periodontitis.
![Figure 4
Nanotechnology-enhanced PDT for the treatment of periodontitis. (a) To combat periodontitis-related pathogens, TiO2-loaded core–shell structure UPNPs were used for inducing PDT under NIR light [84]. Copyright 2021, Elsevier. (b) Ce6-CeO2-NP was formed by decorating Ce6 onto the surface of CeO2 nanoparticles, allowing CeO2 to absorb excessive ROS produced by Ce6. This nanosystem can eliminate pathogens while preventing inflammation caused by excessive ROS. Reproduced with permission from Sun et al. [87]. Copyright 2021, Elsevier. (c) A guided tissue regeneration membrane was formed by mixing sodium alginate hydrogel, TiO2-NP-PDA, and Cu2O. This membrane exhibits both antibacterial properties and the ability to repair alveolar bone in the treatment of periodontitis. Reproduced with permission from Xu et al. [89]. Copyright 2020, American Chemical Society.](/document/doi/10.1515/ntrev-2023-0163/asset/graphic/j_ntrev-2023-0163_fig_004.jpg)
Nanotechnology-enhanced PDT for the treatment of periodontitis. (a) To combat periodontitis-related pathogens, TiO2-loaded core–shell structure UPNPs were used for inducing PDT under NIR light [84]. Copyright 2021, Elsevier. (b) Ce6-CeO2-NP was formed by decorating Ce6 onto the surface of CeO2 nanoparticles, allowing CeO2 to absorb excessive ROS produced by Ce6. This nanosystem can eliminate pathogens while preventing inflammation caused by excessive ROS. Reproduced with permission from Sun et al. [87]. Copyright 2021, Elsevier. (c) A guided tissue regeneration membrane was formed by mixing sodium alginate hydrogel, TiO2-NP-PDA, and Cu2O. This membrane exhibits both antibacterial properties and the ability to repair alveolar bone in the treatment of periodontitis. Reproduced with permission from Xu et al. [89]. Copyright 2020, American Chemical Society.
ROS-mediated oxidative stress response is the main antibacterial mechanism of PDT. However, an excessive generation of ROS may lead to heightened levels of oxidative stress within the periodontal pocket, resulting in a substantial release of inflammatory factors in the periodontal tissues. Consequently, this process can contribute to the destruction and absorption of periodontal supporting tissues, thereby imposing limitations on the clinical application of PDT. Cerium oxide nanoparticles (CeO2NP) can mimic the effects of superoxide dismutase (SOD) and catalase (CAT) through chemical reactions so that CeO2NP could combat chronic inflammation and oxidative stress. Sun et al. modified Ce6 on the surface of CeO2-NP (Ce6-CeO2-NP) (Figure 4b) [87]. On the one hand, after red light excitation, Ce6 produces ROS and plays an antibacterial role. On the other hand, CeO2-NP functions similarly to SODs or CAT, effectively scavenging excessive ROS and exerting anti-inflammatory effects. It was found that PDT mediated by Ce6-CeO2-NP had a strong antibacterial effect on Streptococcus gordonii (S. gordonii), P. gingivalis, and F. nucleatum, and significantly reduced the expression of relevant biofilm virulence factor genes (rgpA, rgpB, and kgp); in addition, in vivo and in vitro experiments have proved that PDT mediated by Ce6-CeO2-NP can regulate host immune function, and at the same time inhibit the polarization of macrophages to M1 type that possess proinflammatory effects, thus upregulating M2-type polarization that possesses anti-inflammatory and regenerative effects [87]. Therefore, this nanomaterial-mediated PDT offers promising prospects for the treatment of periodontitis.
In the treatment of periodontitis, effectively eliminating pathogenic bacteria while simultaneously promoting periodontal tissue regeneration poses a significant challenge [88]. For this purpose, Xu et al. added polydopamine-modified TiO2 nanoparticles (TiO2-NP-PDA) together with cubic cuprous oxide (Cu2O) to sodium alginate hydrogels to form a novel guided tissue regeneration membrane (CTP-SA) (Figure 4c) [89]. The CTP-SA membrane undergoes a transition from liquid to solid upon injection, ensuring precise fitting to the damaged area. After blue light irradiation, CTP-SA-mediated PDT has a broad-spectrum antibacterial effect. At the same time, ROS produced by PDT can oxidize Cu+ to Cu2+. After NIR irradiation, CTP-SA can mediate the photothermal effect. Cu2+ and photothermal effects synergistically promote the reconstruction of bone tissue. In a rat model of periodontitis, this novel tissue regeneration membrane was demonstrated to have the functions of antibacterial and regenerating tissue [89].
Recently, owing to the excellent biocompatibility of organic nanomaterials, PS modified by organic nanocarriers has been gradually applied to control periodontal infections in preclinical research and even in the clinic [90,91,92,93]. de Freitas et al. performed PDT in patients with chronic periodontitis by injecting MB-PLGA-NP into the periodontal pocket after ultrasonic cleaning and root planning (scaling and root planning, SRP). The gingival bleeding index of the SRP plus PDT group was significantly lower than that of the SRP group. Therefore, MB-PLGA-NP-mediated PDT can be used as an adjunct to conventional periodontal therapy to enhance its therapeutic effect [91]. Meanwhile, other types of organic nanocarrier functionalized PDT would also be promising for clinical translation.
3.4 Peri-implantitis
Peri-implantitis is an inflammatory lesion occurring in the soft tissue surrounding dental implants, leading to progressive loss of supporting bone tissue. The etiology of peri-implantitis primarily involves colonization by anaerobic bacterial microorganisms on the implant surface [94]. Traditional mechanical treatments damage the integration of the implant with the bone tissue, leading to recession of the marginal ridge and destruction of the esthetic zone. PS can enter anatomical parts such as deep periodontal pockets and root bifurcations. Under the excitation of the light source, it can effectively kill the microorganisms around the implants, thereby reducing traumatic damage to the surrounding normal tissues. PDT has been gradually applied for the treatment of peri-implantitis [8,9]. However, the application of nanotechnology-based PDT in peri-implantitis is still in its infancy (Table 3).
As a type of PS for PDT, TiO2 has good chemical stability, outstanding biosafety, excellent biological compatibility, and other properties, which were deposited on the surface of the implant through a negative electrode [95]. After ultraviolet irradiation for 2 min, TiO2 nanoparticle-induced PDT can effectively kill most of Staphylococcus epidermidis (S. epidermidis) [95], which is promising for large-scale implant surface modification to avoid peri-implantitis caused by pathogenic bacteria. Researchers also combined sonodynamic antibacterial chemotherapy (SACT) with PDT, known as photoacoustic antibacterial chemotherapy (P-SACT), for the treatment of peri-implantitis. Specifically, ICG was loaded on CSNP (ICG-CSNP) as a PS to explore the synergistic effect of PDT and SACT on the mixed biofilm (A. actinomycetemcomitans, P. gingivalis and P. intermedia) formed on the surface of implants. It was found that ICG-CSNP-mediated PSACT could significantly reduce the number of periodontal pathogens, which is comparable with the positive control group (0.2% CHX) in terms of antibiofilm ability [96].
3.5 Tooth whitening
The demand for charming smiles is growing and has led to the rapid development of tooth-whitening techniques. In the clinic, tooth-whitening methods include invasive whitening (full crown restoration and veneer restoration) and noninvasive whitening (H2O2 as the most popular tooth-bleaching agent) [97]. Unfortunately, invasive whitening can cause irreversible tooth damage [97]. Noninvasive whitening is usually time-consuming and can lead to tooth hypersensitivity, enamel demineralization, and soft tissue irritation [98]. Thus, there is a tremendous need to develop new strategies for safe and effective tooth whitening. Due to the ability of ROS to decolorize stains on the tooth surface or within the tooth structure [99], PDT is undergoing rapid development in the field of tooth whitening [100,101]. Moreover, nanoparticles could endow PDT with high specificity and safety for decolorizing stains, which makes nanotechnology-enhanced PDT highly promising for the application of tooth whitening [101]. In this section, we will discuss the recent progress of nanotechnology-mediated PDT in tooth whitening.
Safety issues caused by high concentrations of H2O2 have been the main concerns related to its application in tooth whitening [98]. Multiple studies have focused on utilizing nanoparticle-based PDT to solve the related safety problems during tooth whitening [100,101]. As described above, TiO2 possesses high biocompatibility and can generate ROS under light irradiation. PDA-modified TiO2 nanoparticles (TiO2@PDA) were developed as tooth-whitening materials under blue light activation and showed equal efficacy in tooth whitening compared with H2O2 [100]. Interestingly, TiO2@PDA-mediated PDT resulted in minimal surface damage to tooth enamel (Figure 5a) [100]. Moreover, TiO2@PDA-mediated PDT was demonstrated to have minimal toxicity in vitro and in vivo [100]. Clinically, the efficacy of tooth whitening also needs to be improved, where 30–40% H2O2 was applied for approximately 1 h for an acceptable effect [97]. TiO2 was also demonstrated to synergize with H2O2 for tooth whitening and significantly reduce the practice time by 30 min [102]. Recently, PSs with aggregation-induced emission (AIE) properties were demonstrated to have excellent ROS-generating abilities [103], which make it suitable to introduce AIE PSs into nanoparticles for photodynamic tooth whitening. A type of highly efficient AIE PS nanoparticle was developed and demonstrated to have good ROS-generating efficacy and was further proven to have better tooth whitening ability compared with the conventional 40% H2O2 (Figure 5b) [101]. Moreover, as the heat generated by light irradiation during PDT may irritate dental pulp, the authors also monitored the temperature change in the pulp cavity, where the temperature remained under the safe range of 5.5℃ [101].
![Figure 5
Nanotechnology-enhanced PDT for tooth whitening. (a) Nano-TiO2@PDA was used for tooth whitening, which showed less damage to the tooth enamel structure compared with 30% H2O2. Reproduced with permission from Zhang et al. [100]. Copyright 2018, American Chemical Society. (b) For tooth whitening, two types of AIE nanoparticle hydrogel-mediated PDT were developed and found to be significantly more efficient than 40% H2O2 [101]. Copyright 2022, American Chemical Society.](/document/doi/10.1515/ntrev-2023-0163/asset/graphic/j_ntrev-2023-0163_fig_005.jpg)
Nanotechnology-enhanced PDT for tooth whitening. (a) Nano-TiO2@PDA was used for tooth whitening, which showed less damage to the tooth enamel structure compared with 30% H2O2. Reproduced with permission from Zhang et al. [100]. Copyright 2018, American Chemical Society. (b) For tooth whitening, two types of AIE nanoparticle hydrogel-mediated PDT were developed and found to be significantly more efficient than 40% H2O2 [101]. Copyright 2022, American Chemical Society.
Although nanotechnology-mediated PDT was demonstrated to have superior tooth whitening efficacy than 30–40% H2O2, the studies were mostly confined to extrinsic stains caused by poor oral hygiene, tobacco use, and the color agents of food and drinks, which are highly responsive to bleaching [97]. Although intrinsic stains (deeper internal stains), which are usually caused by metabolic disorders, medical treatments, hereditary diseases, idiopathic diseases, etc., might be more difficult to bleach and remain for testing the efficacy of nanotechnology-mediated PDT. Studies concerning nanotechnology-mediated PDT for tooth whitening are mostly limited to the bleaching on the external tooth surface. However, intracoronal bleaching, another basic method of tooth whitening [104], was rarely studied using nanotechnology-mediated PDT. As multiple studies and our work have proved that nanotechnology could be utilized to enhance the penetration depth of drugs into the dentinal tubules [77,78], we consider that nanotechnology-mediated PDT appears very promising for bleaching stains in the deep dentin. Tooth stains are classified into extrinsic stains and intrinsic stains based on their origins.
3.6 Precancerous and malignant oral diseases
Beyond the teeth and periodontal tissues, the oral mucosa is another major constituent of our oral cavity, where various types of precancerous and malignant diseases may occur. A majority of oral malignancies arise from oral precancerous lesions. including oral leukoplakia (OLK), oral erythroplakia, and oral lichen planus. PDT has been increasingly applied in the treatment of oral precancerous and malignant diseases due to its advantages of noninvasiveness, good safety, ease of use, and high effectiveness. However, in the treatment of precancerous and malignant oral diseases, conventional PSs have several drawbacks including a lack of targeting ability and a high recurrence rate. In view of this, nanotechnology-enhanced PDT has become a popular focus of research for promoting treatment efficacy (Table 4).
Effect of nanotechnology-based aPDT on precancerous and malignant oral diseases
| Nanocarrier | PS | PDT parameters | Therapeutic effect | Ref. |
|---|---|---|---|---|
| ITCH-Th NPs | ITCH-Th NPs | 660 nm, 1 W/cm2, 3 min | In vivo, ITCH-Th NP-mediated PDT/PTT exhibit significant efficacy in inhibiting the malignant transformation of OLK | [108] |
| Amphiphilic polymer | Ce6 | 660 nm, 1 W/cm2, 10 min | Inhibit tumor through the combination of PDT, PTT, and chemotherapy | [110] |
| Lipid polymer | IR700 | 50 J/cm2 | This nano-delivery system was demonstrated to successfully elicit tumor-specific T-cell responses and tumor inhibition | [117] |
| GNRs | RB | 532 nm, 1.79 W/cm2, 10 min | The combination of RB-GNRs with PDT-PTT functionality exhibits enhanced therapeutic efficacy against oral cancer | [120] |
| Liposomal nanoplatform | Evodiamine, ICG | 808 nm, 0.1 W/cm2, 10 min | Liposomal nanoplatform effectively inhibits cancer cell proliferation and ultimately induces apoptosis of cancer cells | [121] |
| CDcf | CDcf | 780 nm, 10 min | CDcf can directly damage DNA, accelerating the destruction of oral cancer cells | [122] |
| S-CDs | S-CDs | 360–600 nm | At the same concentration, S-CDs have higher photo‐oxidative activity than 5-ALA, which is more likely to lead to apoptosis of tumor cells | [147] |
| Mn-CDs | Mn-CDs | 635 nm, 300 mW/cm2, 5 min | The apoptosis rate of Mn-CDs on OSCC-9 cells was 92.88% | [123] |
As one of the most common precancerous diseases occurring in the oral cavity, OLK is defined as a nonscratchable and irreversible lesion with an elevated risk of turning into squamous cell carcinoma [105,106]. Following chemotherapy, surgery, laser ablation, or cryotherapy, PDT mediated by 5-aminolevulinic acid (ALA) is the fourth alternative therapeutic modality for treating OLK [107]. To further promote the efficacy of PDT for OLK, a new type of photosensitive nanoagent (ITIC-Th nanoparticles, ITCH-Th NPs) was developed for the synergistic of PDT with PTT (Figure 6a) [108]. Under irradiation with a 660 nm laser, ITCH-Th NPs possess good ROS-generating efficacy for PDT and have high photothermal transformation efficacy (38%) for PTT. In the well-established OLK mouse model, ITCH-Th NP-mediated PDT/PTT could significantly block the malignant transformation of OLK. In addition, no obvious topical or systemic toxicity was observed after treatment with ITCH-Th NP-mediated PDT/PTT. This is the first application of nanotechnology-enhanced PDT/PTT in OLK treatment [108]. However, further research is required to test the efficacy of nanotechnology-enhanced PDT in treating other precancerous lesions, including oral erythroplakia and oral lichen planus.
![Figure 6
Nanotechnology-enhanced PDT for combating precancerous and malignant oral diseases. (a) A type of photosensitive nanoagent (ITIC-Th) was developed and demonstrated to possess the dual therapeutic ability of PDT and PTT in the treatment of OLK. Reproduced with permission from Lin et al. [108]. Copyright 2022, Springer Nature. (b) PDT could trigger cancer cells to release a large amount of ATP, which is a powerful immune stimulator for combating cancer cells. In order to enhance the immune activation effect of ATP, an ATP degradation inhibitor (ARL67156) and a PS were incorporated into nanoparticles. This approach was shown to significantly inhibit the growth of both primary and distant tumors. Reproduced with permission from Mao et al. [117]. Copyright 2022, The American Association for the Advancement of Science. (c) The PS of Ce6 and two chemotherapy drugs (paclitaxel and oxaliplatin) were combined to form a nanocomposite. This nanocomposite was found to induce robust pyroptosis in cancer cells, thus enhancing the therapeutic efficacy of PD-1-based immunotherapy. Reproduced with permission from Wan et al. [119]. Copyright 2022, Wiley-VCH.](/document/doi/10.1515/ntrev-2023-0163/asset/graphic/j_ntrev-2023-0163_fig_006.jpg)
Nanotechnology-enhanced PDT for combating precancerous and malignant oral diseases. (a) A type of photosensitive nanoagent (ITIC-Th) was developed and demonstrated to possess the dual therapeutic ability of PDT and PTT in the treatment of OLK. Reproduced with permission from Lin et al. [108]. Copyright 2022, Springer Nature. (b) PDT could trigger cancer cells to release a large amount of ATP, which is a powerful immune stimulator for combating cancer cells. In order to enhance the immune activation effect of ATP, an ATP degradation inhibitor (ARL67156) and a PS were incorporated into nanoparticles. This approach was shown to significantly inhibit the growth of both primary and distant tumors. Reproduced with permission from Mao et al. [117]. Copyright 2022, The American Association for the Advancement of Science. (c) The PS of Ce6 and two chemotherapy drugs (paclitaxel and oxaliplatin) were combined to form a nanocomposite. This nanocomposite was found to induce robust pyroptosis in cancer cells, thus enhancing the therapeutic efficacy of PD-1-based immunotherapy. Reproduced with permission from Wan et al. [119]. Copyright 2022, Wiley-VCH.
In the case of oral cancer, novel nanomaterials and PSs were developed and extensively studied to evaluate their PDT efficiency [83]. In order to improve the PDT effect of traditional PSs, a variety of nanoparticles was used to promote the physical and chemical properties of PSs, which has been reviewed elsewhere comprehensively [109]. Here, we will focus on other obstacles faced by PDT in oral cancer, including targeted delivery methods, tumor immune microenvironment regulation, and combination strategies.
To increase the PDT efficacy and reduce systemic toxicity, PSs should be precisely delivered to the tumor site but not the normal tissue, where targeted delivery methods could be exploited to achieve that. Low-density lipoprotein receptor (LDLR) was found to be a hypoxia marker in oral squamous cell carcinoma and may contribute to malignant transformation and chemoresistance [110]. Thus, anti-LDLR antibody was used to modify a nano-delivery system for targeted delivery of PSs and chemotherapy drugs into the tumor site, where taking advantage of an oral cancer mouse model, this targeted delivery system was demonstrated to improve the therapeutic effects and reduce the side effects caused by chemotherapy [110]. In addition, nanobody was also introduced to decorate nanoparticles and promote the targeted delivery of PSs for PDT in oral cancer treatment [111].
Immune components play an important role in the initiation and progression of oral cancer [112,113]. In an effort to combat cancer, immunotherapy was developed to mobilize the immune system and has served as a pillar of cancer treatment [114]. PDT was also revealed to have the ability to trigger the antitumor immune response [115]. However, the immunosuppressive tumor immune microenvironment was revealed to seriously limit the therapeutic effect of immunotherapy [116]. PDT was demonstrated to trigger the release of adenosine triphosphate (ATP) from tumor cells [115,117], which could potently stimulate immune response and ameliorate the suppressive status of the tumor microenvironment [118]. Thus, a type of nanoparticle-mediated PDT was designed and proved to trigger the release of extracellular ATP by oral cancer cells, which was combined with the ATP degradation inhibitor for further elevating the extracellular ATP level in the tumor microenvironment (Figure 6b) [117]. This strategy was demonstrated to successfully elicit tumor-specific T-cell responses and tumor inhibition, prolonging long-term survival in oral cancer mouse models [117].
Combination strategies were also exploited to promote PDT efficacy. For example, chemotherapy was used for synergizing with PDT to induce pyroptosis of cancer cells, which was demonstrated to prime immune response and enhance the therapeutic efficiency of PD-1-based immunotherapy (Figure 6c) [119]. Gold nanorods (GNRs) show good PTT effects under excitation light, which were conjugated with classical PSs of RB for the synergizing of PTT and PDT [120]. In vitro and in vivo studies demonstrated that this combination strategy could provide better therapeutic effects against oral cancer [120]. In addition, an integrated tri-modal combination of chemotherapy, chemodynamic therapy, and PDT was also introduced [121]. Specifically, evodiamine, a traditional Chinese medicine extraction component widely used for chemotherapy, was found to possess the ability to convert endogenous tumor H2O2 into ROS, which was referred to as chemodynamic therapy. Evodiamine and a type of PS (ICG) were simultaneously encapsulated into a nanoliposome system to achieve tri-modal combination therapy, which showed a significant inhibitory effect on in situ oral cancer [121].
In addition to the above-mentioned nanomaterials, carbon dot (CD), a zero-dimensional nanomaterial, has been utilized as a new type of PS for PDT in combating oral cancer. CDs exhibit good biocompatibility and excellent efficiency in generating ROS, rendering it promising PS. Cur and folic acid were decorated on a CD (CDcf) using the hydrothermal method [122]. The resulting CDcf possesses the ability to target tumor cell nuclei and can be excited by two NIR photons, allowing for deeper penetration in tumor tissue [122]. To enhance PDT in tumors by overcoming the hypoxia status, manganese was doped on CDs, giving it CAT-like activity [123]. This activity leads to the in situ decomposition of acidic H2O2 into oxygen, thereby enhancing the efficacy of PDT against oral cancer cells when exposed to 635 nm irradiation [123].
4 Conclusion and future perspectives
4.1 Biosafety of nanoparticles
Despite the development of multiple types of nanoparticles and their transformation in clinical application, the potential biological toxicity of nanoparticles brought by the unwanted distribution, unclear structure, and other aspects remained unexplored. Therefore, in order to ensure a safe and sustainable future for the application of nanoparticle-enhanced PDT, it is essential to assess their specific structure, pharmacokinetics, and biological interactions, understand the potential risks to human health, and implement effective regulations.
4.2 Oral disease prevention
Prevention is always better than cure. Maintaining daily oral hygiene is critical for preventing dental caries, root canal infections, periodontitis, and other conditions. However, the process of daily oral hygiene is laborious, cumbersome, and time-consuming. In particular, children often have poor oral hygiene, leading to early childhood caries, which has a devastating impact on children’s development and can increase the financial burden on society. Thus, it is urgent to develop strategies to make daily hygiene easier and more efficient. We previously developed a type of bioresponsive nanodrug that has shown effectiveness in preventing early childhood caries in a rat caries model [42]. This method also possesses the potential to be further applied in the clinic, where the nanoparticle can be added to the toothpaste and combined with excitation light attached to the electric tooth brush to synergize with traditional mechanical oral hygiene methods [42]. Our research findings highlight new possibilities that warrant further investigation. For example, can nanoparticles for PDT be added to orthodontic bracket bonding materials to prevent caries in patients with orthodontic treatment? Whether nanoparticles for PDT could be added to mouthwash and promote the daily hygiene effect remains unknown. Future research in this field would be of great help in answering these questions.
4.3 Protecting normal oral flora
The normal oral commensal microbiome has a close symbiotic relationship with its host and is highly important for the host to defend against external threats and maintain oral and systemic health [124,125,126]. Thus, it is of great significance to develop methods that clear pathogenic bacteria and, at the same time, protect normal oral flora. As described above, unlike the neutral pH level of the macroenvironment in the oral cavity, the microenvironment of cariogenic biofilms is acidic. Based on this difference, our group developed a smart nanodelivery system that could release PSs in acidic cariogenic biofilms specifically for PDT. In vitro and in vivo studies showed that this type of nanotherapy could remarkably inhibit the development of dental caries. Thus, further studies on the development of bioresponsive nanotherapeutics for PDT are needed since they would avoid the disturbance of the normal oral flora.
4.4 Eliminating intracellular bacteria
Multiple types of pathogenic bacteria can invade normal oral mucosa cells or other cell types and become intracellular bacteria, which could promote the survival, multiplication, immune escape, and drug resistance of bacteria [127,128,129]. Although mechanical cleaning can eliminate a major portion of microorganisms, it is difficult for these methods to clear intracellular bacteria, which subsequently escape from host cells to recolonize the oral mucosa and restore infection [130]. Most existing antibiotics suffer from limited cellular penetration, poor intracellular retention, and low intracellular activity, making it difficult to kill intracellular bacteria. Therefore, eliminating intracellular bacteria remains a major challenge. Nanotechnology possesses the advantage of targeted drug delivery, which has been exploited for intracellular delivery of bactericidal drugs [128,131]. Meanwhile, PDT with small molecules was demonstrated to be effective in killing intracellular bacteria [132,133]. Therefore, it seems promising to take advantage of nanotechnology-enhanced PDT for treating intracellular pathogens.
4.5 Overcoming hypoxia
The microenvironment of the periodontal pocket and root canal system is hypoxic. However, PDT inherently requires oxygen (O2) to generate ROS [134], which inevitably creates a more hypoxic microenvironment and subsequently hinders the therapeutic effect of PDT. Consequently, there exists a vicious cycle in which PDT consumes oxygen and hypoxia impairs PDT efficiency. To address the hypoxia limitation of PDT in periodontal pockets, a type of oxygen-self-sufficient nanoplatform was developed for combating bacteria in the hypoxic environment of periodontal pockets [135]. However, the hypoxic status in dentinal tubules and periapical tissue also impedes the application of PDT, which is not sufficiently taken into account by researchers. In fact, the obstacle of hypoxia during PDT could be alleviated through multiple ways, including generating O2 in situ, direct delivery of exogenous O2, inhibiting cell respiration to reduce O2 consumption, and inhibiting the hypoxia-inducible factor 1 (HIF-1) signaling pathway in relieving hypoxia [136]. The methods of generating O2 in situ and direct delivery of exogenous O2 are suitable for alleviating hypoxia in an oral environment. For direct delivery of exogenous O2, multiple types of materials (hemoglobin, perfluorocarbon, MOF, etc.) could be utilized for carrying and delivering O2 into the hypoxia position. In terms of generating O2 in situ, various strategies have been developed to utilize H2O2 to relieve hypoxia. Further studies that focus on providing O2 during PDT in dentistry are therefore highly warranted [136].
4.6 Deep tissue penetration
Although UPNP and two-photon absorption nanomaterials were exploited to promote the penetration depth of PDT [86,137], there remains a huge unmet need for treating lesions in deep tissue using PDT. The use of functional nanomaterials has shown promise for prolonging the treatment depth of PDT [138]. For instance, the ability of these nanomaterials to either illuminate themselves or respond to external stimulation, such as NIR light or X-rays, offers a versatile and effective approach [138]. However, implantable devices provide a localized and precise means of delivering light for therapy [138]. By implanting devices close to the lesion site, the light can be directly delivered to the affected area, maximizing therapeutic effects and minimizing side effects. However, further research and development are needed to optimize their effectiveness and ensure their safe use in clinical settings. Overall, the constant development of strategies for enhancing the penetration of PDT will surely widen the scope and application of PDT for the treatment of oral diseases.
-
Funding information: This work was supported by the National Natural Science Foundation of China (Grant No. 82303351), China Postdoctoral Science Foundation (2022M712880), and Science and Technique Project of He’nan Province (No. 222102310685).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Akintoye SO, Mupparapu M. Clinical evaluation and anatomic variation of the oral cavity. Dermatol Clin. 2020;38(4):399–411.10.1016/j.det.2020.05.001Search in Google Scholar PubMed
[2] Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: A global public health challenge. Lancet. 2019;394(10194):249–60.10.1016/S0140-6736(19)31146-8Search in Google Scholar PubMed
[3] Lobbezoo F, Aarab G. Medicine and dentistry working side by side to improve global health equity. J Dent Res. 2022;101(10):1133–4.10.1177/00220345221088237Search in Google Scholar PubMed PubMed Central
[4] McCauley LK, Robinson M, D’Souza RN. Translating science into improved health for all. J Dent Res. 2022;101(7):744–8.10.1177/00220345221099825Search in Google Scholar PubMed PubMed Central
[5] Konopka K, Goslinski T. Photodynamic therapy in dentistry. J Dent Res. 2007;86(8):694–707.10.1177/154405910708600803Search in Google Scholar PubMed
[6] Jia Q, Song Q, Li P, Huang W. Rejuvenated photodynamic therapy for bacterial infections. Adv Healthc Mater. 2019;8(14):e1900608.10.1002/adhm.201900608Search in Google Scholar PubMed
[7] Green TJ, Wilson DF, Vanderkooi JM, DeFeo SP. Phosphorimeters for analysis of decay profiles and real time monitoring of exponential decay and oxygen concentrations. Anal Biochem. 1988;174(1):73–9.10.1016/0003-2697(88)90520-9Search in Google Scholar PubMed
[8] Chambrone L, Wang HL, Romanos GE. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: An american academy of periodontology best evidence review. J Periodontol. 2018;89(7):783–803.Search in Google Scholar
[9] de Souza Rastelli AN. Antimicrobial photodynamic therapy (apdt) as a disinfection and biomodulation approach in implant dentistry. Photochem Photobiol. 2021;97(5):1155–60.10.1111/php.13509Search in Google Scholar PubMed
[10] Xie J, Wang Y, Choi W, Jangili P, Ge Y, Xu Y, et al. Overcoming barriers in photodynamic therapy harnessing nano-formulation strategies. Chem Soc Rev. 2021;50(16):9152–201.10.1039/D0CS01370FSearch in Google Scholar PubMed
[11] Tim M. Strategies to optimize photosensitizers for photodynamic inactivation of bacteria. J Photochem Photobiol B. 2015;150:2–10.10.1016/j.jphotobiol.2015.05.010Search in Google Scholar PubMed
[12] Hochvaldová L, Večeřová R, Kolář M, Prucek R, Kvítek L, Lapčík L, et al. Antibacterial nanomaterials: Upcoming hope to overcome antibiotic resistance crisis. Nanotechnol Rev. 2022;11(1):1115–42.10.1515/ntrev-2022-0059Search in Google Scholar
[13] Niavarzi S, Pourhajibagher M, Khedmat S, Ghabraei S, Chiniforush N, Bahador A. Effect of ultrasonic activation on the efficacy of antimicrobial photodynamic therapy: Evaluation of penetration depth of photosensitizer and elimination of enterococcus faecalis biofilms. Photodiagnosis Photodyn Ther. 2019;27:362–6.10.1016/j.pdpdt.2019.06.001Search in Google Scholar PubMed
[14] Sun X, Sun J, Sun Y, Li C, Fang J, Zhang T, et al. Oxygen self-sufficient nanoplatform for enhanced and selective antibacterial photodynamic therapy against anaerobe-induced periodontal disease. Adv Funct Mater. 2021;31(20):2101040.10.1002/adfm.202101040Search in Google Scholar
[15] Colombo JS, Jia S, D’Souza RN. Modeling hypoxia induced factors to treat pulpal inflammation and drive regeneration. J Endod. 2020;46(9S):S19–25.10.1016/j.joen.2020.06.039Search in Google Scholar PubMed
[16] Reynolds EC. Transdisciplinary research: The virtuous cycle of research translation to improve oral health. J Dent Res. 2022;101(6):613–5.10.1177/00220345221090824Search in Google Scholar PubMed
[17] Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater. 2021;6(4):351–70.10.1038/s41578-020-00269-6Search in Google Scholar PubMed PubMed Central
[18] Hu C, Wang LL, Lin YQ, Liang HM, Zhou SY, Zheng F, et al. Nanoparticles for the treatment of oral biofilms: Current state, mechanisms, influencing factors, and prospects. Adv Healthc Mater. 2019;8(24):e1901301.10.1002/adhm.201901301Search in Google Scholar PubMed
[19] Ramburrun P, Khan RA, Choonara YE. Design, preparation, and functionalization of nanobiomaterials for enhanced efficacy in current and future biomedical applications. Nanotechnol Rev. 2022;11(1):1802–26.10.1515/ntrev-2022-0106Search in Google Scholar
[20] Makvandi P, Josic U, Delfi M, Pinelli F, Jahed V, Kaya E, et al. Drug delivery (nano)platforms for oral and dental applications: Tissue regeneration, infection control, and cancer management. Adv Sci (Weinh). 2021;8(8):2004014.10.1002/advs.202004014Search in Google Scholar PubMed PubMed Central
[21] Jain P, Farooq U, Hassan N, Albratty M, Alam MS, Makeen HA, et al. Nanotechnology interventions as a putative tool for the treatment of dental afflictions. Nanotechnol Rev. 2022;11(1):1935–46.10.1515/ntrev-2022-0115Search in Google Scholar
[22] Edgahi MA, Naghib SM, Emamian A, Ramezanpour H, Haghiralsadat F, Tofighi D. A practical review over surface modification, nanopatterns, emerging materials, drug delivery systems, and their biophysiochemical properties for dental implants: Recent progresses and advances. Nanotechnol Rev. 2022;11(1):637–79.10.1515/ntrev-2022-0037Search in Google Scholar
[23] Xu VW, Nizami MZI, Yin IX, Lung CYK, Yu OY, Chu CH. Caries management with non-metallic nanomaterials: A systematic review. Int J Nanomedicine. 2022;17:5809–24.10.2147/IJN.S389038Search in Google Scholar PubMed PubMed Central
[24] Plotino G, Grande NM, Mercade M. Photodynamic therapy in endodontics. Int Endod J. 2019;52(6):760–74.10.1111/iej.13057Search in Google Scholar PubMed
[25] Li X, Lee S, Yoon J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem Soc Rev. 2018;47(4):1174–88.10.1039/C7CS00594FSearch in Google Scholar PubMed
[26] Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic therapy review: Principles, photosensitizers, applications, and future directions. Pharmaceutics. 2021;13(9):1332.10.3390/pharmaceutics13091332Search in Google Scholar PubMed PubMed Central
[27] Kim S, Tachikawa T, Fujitsuka M, Majima T. Far-red fluorescence probe for monitoring singlet oxygen during photodynamic therapy. J Am Chem Soc. 2014;136(33):11707–15.10.1021/ja504279rSearch in Google Scholar PubMed
[28] Zhou Z, Song J, Nie L, Chen X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem Soc Rev. 2016;45(23):6597–626.10.1039/C6CS00271DSearch in Google Scholar PubMed PubMed Central
[29] Luiz MT, di Filippo LD, Dutra JAP, Viegas JSR, Silvestre ALP, Anselmi C, et al. New technological approaches for dental caries treatment: From liquid crystalline systems to nanocarriers. Pharmaceutics. 2023;15(3):762.10.3390/pharmaceutics15030762Search in Google Scholar PubMed PubMed Central
[30] Marsh PD, Zaura E. Dental biofilm: Ecological interactions in health and disease. J Clin Periodontol. 2017;44(Suppl 18)):S12–22.10.1111/jcpe.12679Search in Google Scholar PubMed
[31] Hoare A, Marsh PD, Diaz PI. Ecological therapeutic opportunities for oral diseases. Microbiol Spectrum. 2017;5(4). 10.1128/microbiolspec.BAD-0006-2016.Search in Google Scholar PubMed PubMed Central
[32] Bowen WH, Burne RA, Wu H, Koo H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26(3):229–42.10.1016/j.tim.2017.09.008Search in Google Scholar PubMed PubMed Central
[33] Alhazmi HA, Ahsan W, Mangla B, Javed S, Hassan MZ, Asmari M, et al. Graphene-based biosensors for disease theranostics: Development, applications, and recent advancements. Nanotechnol Rev. 2022;11(1):96–116.10.1515/ntrev-2022-0009Search in Google Scholar
[34] Feng W, Wang Z. Biomedical applications of chitosan-graphene oxide nanocomposites. iScience. 2022;25(1):103629.10.1016/j.isci.2021.103629Search in Google Scholar PubMed PubMed Central
[35] Gholibegloo E, Karbasi A, Pourhajibagher M, Chiniforush N, Ramazani A, Akbari T, et al. Carnosine-graphene oxide conjugates decorated with hydroxyapatite as promising nanocarrier for icg loading with enhanced antibacterial effects in photodynamic therapy against streptococcus mutans. J Photochem Photobiol B. 2018;181:14–22.10.1016/j.jphotobiol.2018.02.004Search in Google Scholar PubMed
[36] Ahmadi H, Haddadi-Asl V, Ghafari HA, Ghorbanzadeh R, Mazlum Y, Bahador A. Shear bond strength, adhesive remnant index, and anti-biofilm effects of a photoexcited modified orthodontic adhesive containing curcumin doped poly lactic-co-glycolic acid nanoparticles: An ex-vivo biofilm model of s. Mutans on the enamel slab bonded brackets. Photodiagnosis Photodyn Ther. 2020;30:101674.10.1016/j.pdpdt.2020.101674Search in Google Scholar PubMed
[37] Dantas Lopes Dos Santos D, Besegato JF, de Melo PBG, Oshiro Junior JA, Chorilli M, Deng D, et al. Curcumin-loaded pluronic((r)) f-127 micelles as a drug delivery system for curcumin-mediated photodynamic therapy for oral application. Photochem Photobiol. 2021;97(5):1072–88.10.1111/php.13433Search in Google Scholar PubMed
[38] Pourhajibagher M, Salehi Vaziri A, Takzaree N, Ghorbanzadeh R. Physico-mechanical and antimicrobial properties of an orthodontic adhesive containing cationic curcumin doped zinc oxide nanoparticles subjected to photodynamic therapy. Photodiagnosis Photodyn Ther. 2019;25:239–46.10.1016/j.pdpdt.2019.01.002Search in Google Scholar PubMed
[39] Silvestre ALP, Dos Santos AM, de Oliveira AB, Ferrisse TM, Brighenti FL, Meneguin AB, et al. Evaluation of photodynamic therapy on nanoparticles and films loaded-nanoparticles based on chitosan/alginate for curcumin delivery in oral biofilms. Int J Biol Macromol. 2023;240:124489.10.1016/j.ijbiomac.2023.124489Search in Google Scholar PubMed
[40] Pourhajibagher M, Keshavarz Valian N, Bahador A. Theranostic nanoplatforms of emodin-chitosan with blue laser light on enhancing the anti-biofilm activity of photodynamic therapy against streptococcus mutans biofilms on the enamel surface. BMC Microbiol. 2022;22(1):68.10.1186/s12866-022-02481-6Search in Google Scholar PubMed PubMed Central
[41] de Souza CM, Garcia MT, de Barros PP, Pedroso LLC, Ward R, Strixino JF, et al. Chitosan enhances the antimicrobial photodynamic inactivation mediated by photoditazine(r) against streptococcus mutans. Photodiagnosis Photodyn Ther. 2020;32:102001.10.1016/j.pdpdt.2020.102001Search in Google Scholar PubMed
[42] Liu D, Ma X, Ji Y, Chen R, Zhou S, Yao H, et al. Bioresponsive nanotherapy for preventing dental caries by inhibiting multispecies cariogenic biofilms. Bioact Mater. 2022;14:1–14.10.1016/j.bioactmat.2021.12.016Search in Google Scholar PubMed PubMed Central
[43] Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28(11):580–8.10.1016/j.tibtech.2010.07.006Search in Google Scholar PubMed
[44] Misba L, Kulshrestha S, Khan AU. Antibiofilm action of a toluidine blue o-silver nanoparticle conjugate on streptococcus mutans: A mechanism of type i photodynamic therapy. Biofouling. 2016;32(3):313–28.10.1080/08927014.2016.1141899Search in Google Scholar PubMed
[45] Shitomi K, Miyaji H, Miyata S, Sugaya T, Ushijima N, Akasaka T, et al. Photodynamic inactivation of oral bacteria with silver nanoclusters/rose bengal nanocomposite. Photodiagnosis Photodyn Ther. 2020;30:101647.10.1016/j.pdpdt.2019.101647Search in Google Scholar PubMed
[46] Haris Z, Khan AU. Selenium nanoparticle enhanced photodynamic therapy against biofilm forming streptococcus mutans. Int J Life-Sci Sci Res. 2017;3(5):1287–94.10.21276/ijlssr.2017.3.5.4Search in Google Scholar
[47] Lavaee F, Motamedifar M, Rafiee G. The effect of photodynamic therapy by gold nanoparticles on streptococcus mutans and biofilm formation: An in vitro study. Lasers Med Sci. 2022;37(3):1717–25.10.1007/s10103-021-03422-xSearch in Google Scholar PubMed
[48] Tendolkar PM, Baghdayan AS, Gilmore MS, Shankar N. Enterococcal surface protein, esp, enhances biofilm formation by enterococcus faecalis. Infect Immun. 2004;72(10):6032–9.10.1128/IAI.72.10.6032-6039.2004Search in Google Scholar PubMed PubMed Central
[49] Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by enterococcus faecalis. Infect Immun. 2004;72(6):3658–63.10.1128/IAI.72.6.3658-3663.2004Search in Google Scholar PubMed PubMed Central
[50] Pillai SK, Sakoulas G, Eliopoulos GM, Moellering RC, Jr., Murray BE, Inouye RT. Effects of glucose on fsr-mediated biofilm formation in enterococcus faecalis. J Infect Dis. 2004;190(5):967–70.10.1086/423139Search in Google Scholar PubMed
[51] Akbari T, Pourhajibagher M, Hosseini F, Chiniforush N, Gholibegloo E, Khoobi M, et al. The effect of indocyanine green loaded on a novel nano-graphene oxide for high performance of photodynamic therapy against enterococcus faecalis. Photodiagnosis Photodyn Ther. 2017;20:148–53.10.1016/j.pdpdt.2017.08.017Search in Google Scholar PubMed
[52] Ghorbanzadeh R, Assadian H, Chiniforush N, Parker S, Pourakbari B, Ehsani B, et al. Modulation of virulence in enterococcus faecalis cells surviving antimicrobial photodynamic inactivation with reduced graphene oxide-curcumin: An ex vivo biofilm model. Photodiagnosis Photodyn Ther. 2020;29:101643.10.1016/j.pdpdt.2019.101643Search in Google Scholar PubMed
[53] Afkhami F, Akbari S, Chiniforush N. Entrococcus faecalis elimination in root canals using silver nanoparticles, photodynamic therapy, diode laser, or laser-activated nanoparticles: An in vitro study. J Endod. 2017;43(2):279–82.10.1016/j.joen.2016.08.029Search in Google Scholar PubMed
[54] Afkhami F, Ahmadi P, Chiniforush N, Sooratgar A. Effect of different activations of silver nanoparticle irrigants on the elimination of enterococcus faecalis. Clin Oral Investig. 2021;25(12):6893–9.10.1007/s00784-021-03979-5Search in Google Scholar PubMed
[55] Aydin H, Er K, Kustarci A, Akarsu M, Gencer GM, Er H, et al. Antibacterial activity of silver nanoparticles activated by photodynamic therapy in infected root canals. Dent Med Probl. 2020;57(4):393–400.10.17219/dmp/123615Search in Google Scholar PubMed
[56] Maliszewska I, Wrobel J, Wanarska E, Podhorodecki A, Matczyszyn K. Synergistic effect of methylene blue and biogenic gold nanoparticles against enterococcus faecalis. Photodiagnosis Photodyn Ther. 2019;27:218–26.10.1016/j.pdpdt.2019.05.042Search in Google Scholar PubMed
[57] Shahmoradi S, Shariati A, Zargar N, Yadegari Z, Asnaashari M, Amini SM, et al. Antimicrobial effects of selenium nanoparticles in combination with photodynamic therapy against enterococcus faecalis biofilm. Photodiagnosis Photodyn Ther. 2021;35:102398.10.1016/j.pdpdt.2021.102398Search in Google Scholar PubMed
[58] Ding M, Liu W, Gref R. Nanoscale mofs: From synthesis to drug delivery and theranostics applications. Adv Drug Deliv Rev. 2022;190:114496.10.1016/j.addr.2022.114496Search in Google Scholar PubMed
[59] Giri L, Rout SR, Varma RS, Otyepka M, Jayaramulu K, Dandela R. Recent advancements in metal–organic frameworks integrating quantum dots (qds@mof) and their potential applications. Nanotechnol Rev. 2022;11(1):1947–76.10.1515/ntrev-2022-0118Search in Google Scholar
[60] Golmohamadpour A, Bahramian B, Khoobi M, Pourhajibagher M, Barikani HR, Bahador A. Antimicrobial photodynamic therapy assessment of three indocyanine green-loaded metal-organic frameworks against enterococcus faecalis. Photodiagnosis Photodyn Ther. 2018;23:331–8.10.1016/j.pdpdt.2018.08.004Search in Google Scholar PubMed
[61] Shrestha A, Kishen A. Antibacterial efficacy of photosensitizer functionalized biopolymeric nanoparticles in the presence of tissue inhibitors in root canal. J Endod. 2014;40(4):566–70.10.1016/j.joen.2013.09.013Search in Google Scholar PubMed
[62] Shrestha A, Hamblin MR, Kishen A. Characterization of a conjugate between rose bengal and chitosan for targeted antibiofilm and tissue stabilization effects as a potential treatment of infected dentin. Antimicrob Agents Chemother. 2012;56(9):4876–84.10.1128/AAC.00810-12Search in Google Scholar PubMed PubMed Central
[63] Shrestha A, Kishen A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J Endod. 2014;40(10):1604–10.10.1016/j.joen.2014.03.009Search in Google Scholar PubMed
[64] DaSilva L, Finer Y, Friedman S, Basrani B, Kishen A. Biofilm formation within the interface of bovine root dentin treated with conjugated chitosan and sealer containing chitosan nanoparticles. J Endod. 2013;39(2):249–53.10.1016/j.joen.2012.11.008Search in Google Scholar PubMed PubMed Central
[65] Higuchi N, Hayashi JI, Fujita M, Iwamura Y, Sasaki Y, Goto R, et al. Photodynamic inactivation of an endodontic bacteria using diode laser and indocyanine green-loaded nanosphere. Int J Mol Sci. 2021;22(16):8384.10.3390/ijms22168384Search in Google Scholar PubMed PubMed Central
[66] Portenier I, Haapasalo H, Orstavik D, Yamauchi M, Haapasalo M. Inactivation of the antibacterial activity of iodine potassium iodide and chlorhexidine digluconate against enterococcus faecalis by dentin, dentin matrix, type-i collagen, and heat-killed microbial whole cells. J Endod. 2002;28(9):634–7.10.1097/00004770-200209000-00002Search in Google Scholar PubMed
[67] Shrestha A, Kishen A. The effect of tissue inhibitors on the antibacterial activity of chitosan nanoparticles and photodynamic therapy. J Endod. 2012;38(9):1275–8.10.1016/j.joen.2012.05.006Search in Google Scholar PubMed
[68] Vier FV, Figueiredo JA. Internal apical resorption and its correlation with the type of apical lesion. Int Endod J. 2004;37(11):730–7.10.1111/j.1365-2591.2004.00830.xSearch in Google Scholar PubMed
[69] Shrestha A, Hamblin MR, Kishen A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine. 2014;10(3):491–501.10.1016/j.nano.2013.10.010Search in Google Scholar PubMed PubMed Central
[70] Muthalib S, Verma AH, Sundar S, Sampath Kumar TS, Velmurugan N, Krithikadatta J. Evaluation of effect of two different functionalized nanoparticle photodynamic therapy on nanohardness of root dentin-an in vitro study. Photodiagnosis Photodyn Ther. 2020;31:101856.10.1016/j.pdpdt.2020.101856Search in Google Scholar PubMed
[71] Spikes JD, Shen HR, Kopeckova P, Kopecek J. Photodynamic crosslinking of proteins. Iii. Kinetics of the fmn- and rose bengal-sensitized photooxidation and intermolecular crosslinking of model tyrosine-containing n-(2-hydroxypropyl)methacrylamide copolymers. Photochem Photobiol. 1999;70(2):130–7.10.1111/j.1751-1097.1999.tb07980.xSearch in Google Scholar
[72] Chan BP, Chan OC, So KF. Effects of photochemical crosslinking on the microstructure of collagen and a feasibility study on controlled protein release. Acta Biomater. 2008;4(6):1627–36.10.1016/j.actbio.2008.06.007Search in Google Scholar PubMed
[73] Pagonis TC, Chen J, Fontana CR, Devalapally H, Ruggiero K, Song X, et al. Nanoparticle-based endodontic antimicrobial photodynamic therapy. J Endod. 2010;36(2):322–8.10.1016/j.joen.2009.10.011Search in Google Scholar PubMed PubMed Central
[74] Maldonado-Carmona N, Marchand G, Villandier N, Ouk TS, Pereira MM, Calvete MJF, et al. Porphyrin-loaded lignin nanoparticles against bacteria: A photodynamic antimicrobial chemotherapy application. Front Microbiol. 2020;11:606185.10.3389/fmicb.2020.606185Search in Google Scholar PubMed PubMed Central
[75] Schuenck-Rodrigues RA, de Oliveira de Siqueira LB, Dos Santos Matos AP, da Costa SP, da Silva Cardoso V, Vermelho AB, et al. Development, characterization and photobiological activity of nanoemulsion containing zinc phthalocyanine for oral infections treatment. J Photochem Photobiol B. 2020;211:112010.10.1016/j.jphotobiol.2020.112010Search in Google Scholar PubMed
[76] Velazquez-Moreno S, Gonzalez-Amaro AM, Aragon-Pina A, Lopez-Lopez LI, Sanchez-Sanchez R, Perez-Diaz MA, et al. Use of a cellulase from trichoderma reesei as an adjuvant for enterococcus faecalis biofilm disruption in combination with antibiotics as an alternative treatment in secondary endodontic infection. Pharmaceutics. 2023;15(3):1010.10.3390/pharmaceutics15031010Search in Google Scholar PubMed PubMed Central
[77] Fan W, Liu D, Li Y, Sun Q, Fan B. Agca-plga submicron particles inhibit the growth and colonization of e. Faecalis and p. Gingivalis on dentin through infiltration into dentinal tubules. Int J Pharm. 2018;552(1–2):206–16.10.1016/j.ijpharm.2018.09.066Search in Google Scholar PubMed
[78] Dasgupta D, Peddi S, Saini DK, Ghosh A. Mobile nanobots for prevention of root canal treatment failure. Adv Healthc Mater. 2022;11(14):e2200232.10.1002/adhm.202200232Search in Google Scholar PubMed PubMed Central
[79] Babeer A, Oh MJ, Ren Z, Liu Y, Marques F, Poly A, et al. Microrobotics for precision biofilm diagnostics and treatment. J Dent Res. 2022;101(9):1009–14.10.1177/00220345221087149Search in Google Scholar PubMed PubMed Central
[80] Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–83.10.1128/JB.183.12.3770-3783.2001Search in Google Scholar PubMed PubMed Central
[81] Soukos NS, Som S, Abernethy AD, Ruggiero K, Dunham J, Lee C, et al. Phototargeting oral black-pigmented bacteria. Antimicrob Agents Chemother. 2005;49(4):1391–6.10.1128/AAC.49.4.1391-1396.2005Search in Google Scholar PubMed PubMed Central
[82] Sun X, Wang L, Lynch CD, Sun X, Li X, Qi M, et al. Nanoparticles having amphiphilic silane containing chlorin e6 with strong anti-biofilm activity against periodontitis-related pathogens. J Dent. 2019;81:70–84.10.1016/j.jdent.2018.12.011Search in Google Scholar PubMed
[83] Li Q, Zhou R, Xie Y, Li Y, Chen Y, Cai X. Sulphur-doped carbon dots as a highly efficient nano-photodynamic agent against oral squamous cell carcinoma. Cell Prolif. 2020;53(4):e12786.Search in Google Scholar
[84] Qi M, Li X, Sun X, Li C, Tay FR, Weir MD, et al. Novel nanotechnology and near-infrared photodynamic therapy to kill periodontitis-related biofilm pathogens and protect the periodontium. Dent Mater. 2019;35(11):1665–81.10.1016/j.dental.2019.08.115Search in Google Scholar PubMed
[85] Zhang T, Ying D, Qi M, Li X, Fu L, Sun X, et al. Anti-biofilm property of bioactive upconversion nanocomposites containing chlorin e6 against periodontal pathogens. Molecules. 2019;24(15):2692.10.3390/molecules24152692Search in Google Scholar PubMed PubMed Central
[86] Shen Y, Shuhendler AJ, Ye D, Xu JJ, Chen HY. Two-photon excitation nanoparticles for photodynamic therapy. Chem Soc Rev. 2016;45(24):6725–41.10.1039/C6CS00442CSearch in Google Scholar
[87] Sun Y, Sun X, Li X, Li W, Li C, Zhou Y, et al. A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from apdt-aggravated inflammation via modulation of macrophage polarization. Biomaterials. 2021;268:120614.10.1016/j.biomaterials.2020.120614Search in Google Scholar PubMed
[88] Chen H, Zhang Y, Yu T, Song G, Xu T, Xin T, et al. Nano-based drug delivery systems for periodontal tissue regeneration. Pharmaceutics. 2022;14(10):2250.10.3390/pharmaceutics14102250Search in Google Scholar PubMed PubMed Central
[89] Xu Y, Zhao S, Weng Z, Zhang W, Wan X, Cui T, et al. Jelly-inspired injectable guided tissue regeneration strategy with shape auto-matched and dual-light-defined antibacterial/osteogenic pattern switch properties. ACS Appl Mater Interfaces. 2020;12(49):54497–506.10.1021/acsami.0c18070Search in Google Scholar PubMed
[90] Rad MR, Pourhajibagher M, Rokn AR, Barikani HR, Bahador A. Effect of antimicrobial photodynamic therapy using indocyanine green doped with chitosan nanoparticles on biofilm formation-related gene expression of aggregatibacter actinomycetemcomitans. Front Dent. 2019;16(3):187–93.Search in Google Scholar
[91] de Freitas LM, Calixto GM, Chorilli M, Giusti JS, Bagnato VS, Soukos NS, et al. Polymeric nanoparticle-based photodynamic therapy for chronic periodontitis in vivo. Int J Mol Sci. 2016;17(5):769.10.3390/ijms17050769Search in Google Scholar PubMed PubMed Central
[92] Sasaki Y, Hayashi JI, Fujimura T, Iwamura Y, Yamamoto G, Nishida E, et al. New irradiation method with indocyanine green-loaded nanospheres for inactivating periodontal pathogens. Int J Mol Sci. 2017;18(1):154.10.3390/ijms18010154Search in Google Scholar PubMed PubMed Central
[93] Nagahara A, Mitani A, Fukuda M, Yamamoto H, Tahara K, Morita I, et al. Antimicrobial photodynamic therapy using a diode laser with a potential new photosensitizer, indocyanine green-loaded nanospheres, may be effective for the clearance of porphyromonas gingivalis. J Periodontal Res. 2013;48(5):591–9.10.1111/jre.12042Search in Google Scholar PubMed
[94] Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998;17:63–76.10.1111/j.1600-0757.1998.tb00124.xSearch in Google Scholar PubMed
[95] Lilja M, Forsgren J, Welch K, Astrand M, Engqvist H, Stromme M. Photocatalytic and antimicrobial properties of surgical implant coatings of titanium dioxide deposited though cathodic arc evaporation. Biotechnol Lett. 2012;34(12):2299–305.10.1007/s10529-012-1040-2Search in Google Scholar PubMed
[96] Pourhajibagher M, Rokn AR, Barikani HR, Bahador A. Photo-sonodynamic antimicrobial chemotherapy via chitosan nanoparticles-indocyanine green against polymicrobial periopathogenic biofilms: Ex vivo study on dental implants. Photodiagnosis Photodyn Ther. 2020;31:101834.10.1016/j.pdpdt.2020.101834Search in Google Scholar PubMed
[97] Alqahtani MQ. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent J. 2014;26(2):33–46.10.1016/j.sdentj.2014.02.002Search in Google Scholar PubMed PubMed Central
[98] Li Y, Greenwall L. Safety issues of tooth whitening using peroxide-based materials. Br Dent J. 2013;215(1):29–34.10.1038/sj.bdj.2013.629Search in Google Scholar PubMed
[99] Kwon SR, Wertz PW. Review of the mechanism of tooth whitening. J Esthet Restor Dent. 2015;27(5):240–57.10.1111/jerd.12152Search in Google Scholar PubMed
[100] Zhang F, Wu C, Zhou Z, Wang J, Bao W, Dong L, et al. Blue-light -activated nano-tio2@pda for highly effective and nondestructive tooth whitening. ACS Biomater Sci Eng. 2018;4(8):3072–7.10.1021/acsbiomaterials.8b00548Search in Google Scholar PubMed
[101] Gao J, Wang J, Yue X, Zhou Y, Wang M, Sun Y, et al. Shen JJAANM. Photostable aggregation-induced emission photosensitizer nanoparticle/hyaluronic acid hydrogel for efficient photodynamic tooth bleaching. ACS Appl Nano Mater. 2022;5(5):5944–51.10.1021/acsanm.1c03912Search in Google Scholar
[102] Cuppini M, Leitune VCB, Souza M, Alves AK, Samuel SMW, Collares FM. In vitro evaluation of visible light-activated titanium dioxide photocatalysis for in-office dental bleaching. Dent Mater J. 2019;38(1):68–74.10.4012/dmj.2017-199Search in Google Scholar PubMed
[103] Cai X, Liu B. Aggregation-induced emission: Recent advances in materials and biomedical applications. Angew Chem Int Ed Engl. 2020;59(25):9868–86.10.1002/anie.202000845Search in Google Scholar PubMed
[104] Lim KC. Considerations in intracoronal bleaching. Aust Endod J. 2004;30(2):69–73.10.1111/j.1747-4477.2004.tb00186.xSearch in Google Scholar PubMed
[105] van der Waal I, Axell T. Oral leukoplakia: A proposal for uniform reporting. Oral Oncol. 2002;38(6):521–6.10.1016/S1368-8375(01)00125-7Search in Google Scholar
[106] Villa A, Sonis S. Oral leukoplakia remains a challenging condition. Oral Dis. 2018;24(1–2):179–83.10.1111/odi.12781Search in Google Scholar PubMed
[107] Chen Q, Dan H, Tang F, Wang J, Li X, Cheng J, et al. Photodynamic therapy guidelines for the management of oral leucoplakia. Int J Oral Sci. 2019;11(2):14.10.1038/s41368-019-0047-0Search in Google Scholar PubMed PubMed Central
[108] Lin L, Song C, Wei Z, Zou H, Han S, Cao Z, et al. Multifunctional photodynamic/photothermal nano-agents for the treatment of oral leukoplakia. J Nanobiotechnol. 2022;20(1):106.10.1186/s12951-022-01310-2Search in Google Scholar PubMed PubMed Central
[109] Ding Z, Sigdel K, Yang L, Liu Y, Xuan M, Wang X, et al. Nanotechnology-based drug delivery systems for enhanced diagnosis and therapy of oral cancer. J Mater Chem B. 2020;8(38):8781–93.10.1039/D0TB00957ASearch in Google Scholar
[110] Song C, Tang C, Xu W, Ran J, Wei Z, Wang Y, et al. Hypoxia-targeting multifunctional nanoparticles for sensitized chemotherapy and phototherapy in head and neck squamous cell carcinoma. Int J Nanomedicine. 2020;15:347–61.10.2147/IJN.S233294Search in Google Scholar PubMed PubMed Central
[111] Wu W, Shi L, Duan Y, Xu S, Shen L, Zhu T, et al. Nanobody modified high-performance aie photosensitizer nanoparticles for precise photodynamic oral cancer therapy of patient-derived tumor xenograft. Biomaterials. 2021;274:120870.10.1016/j.biomaterials.2021.120870Search in Google Scholar PubMed
[112] Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):92.10.1038/s41572-020-00224-3Search in Google Scholar PubMed PubMed Central
[113] Whiteside TL. Head and neck carcinoma immunotherapy: Facts and hopes. Clin Cancer Res. 2018;24(1):6–13.10.1158/1078-0432.CCR-17-1261Search in Google Scholar PubMed PubMed Central
[114] Kraehenbuehl L, Weng CH, Eghbali S, Wolchok JD, Merghoub T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat Rev Clin Oncol. 2022;19(1):37–50.10.1038/s41571-021-00552-7Search in Google Scholar PubMed
[115] Kroemer G, Galassi C, Zitvogel L, Galluzzi L. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–500.10.1038/s41590-022-01132-2Search in Google Scholar PubMed
[116] Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–37.10.1016/j.cell.2021.09.020Search in Google Scholar PubMed PubMed Central
[117] Mao C, Yeh S, Fu J, Porosnicu M, Thomas A, Kucera GL, et al. Delivery of an ectonucleotidase inhibitor with ros-responsive nanoparticles overcomes adenosine-mediated cancer immunosuppression. Sci Transl Med. 2022;14(648):eabh1261.10.1126/scitranslmed.abh1261Search in Google Scholar PubMed PubMed Central
[118] Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, et al. Atp and cancer immunosurveillance. EMBO J. 2021;40(13):e108130.10.15252/embj.2021108130Search in Google Scholar PubMed PubMed Central
[119] Wan SC, Ye MJ, Yang QC, Zhang T, Zhang MJ, Ma XB, et al. Diselenide-based dual-responsive prodrug as pyroptosis inducer potentiates cancer immunotherapy. Adv Healthc Mater. 2023;12(7):e2202135.10.1002/adhm.202202135Search in Google Scholar PubMed
[120] Wang B, Wang JH, Liu Q, Huang H, Chen M, Li K, et al. Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies. Biomaterials. 2014;35(6):1954–66.10.1016/j.biomaterials.2013.11.066Search in Google Scholar PubMed
[121] Wei Z, Zou H, Liu G, Song C, Tang C, Chen S, et al. Peroxidase-mimicking evodiamine/indocyanine green nanoliposomes for multimodal imaging-guided theranostics for oral squamous cell carcinoma. Bioact Mater. 2021;6(7):2144–57.10.1016/j.bioactmat.2020.12.016Search in Google Scholar PubMed PubMed Central
[122] Nasrin A, Hassan M, Gomes VG. Two-photon active nucleus-targeting carbon dots: Enhanced ros generation and photodynamic therapy for oral cancer. Nanoscale. 2020;12(40):20598–603.10.1039/D0NR05210HSearch in Google Scholar
[123] Zhang Z, Xu Y, Zhu T, Sang Z, Guo X, Sun Y, et al. Hypoxia mitigation by manganese-doped carbon dots for synergistic photodynamic therapy of oral squamous cell carcinoma. Front Bioeng Biotechnol. 2023;11:1153196.10.3389/fbioe.2023.1153196Search in Google Scholar PubMed PubMed Central
[124] Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, et al. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–66.10.1038/sj.bdj.2016.865Search in Google Scholar PubMed
[125] Marsh PD. Contemporary perspective on plaque control. Br Dent J. 2012;212(12):601–6.10.1038/sj.bdj.2012.524Search in Google Scholar PubMed
[126] Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–32.10.1128/JCM.43.11.5721-5732.2005Search in Google Scholar PubMed PubMed Central
[127] Lee K, Roberts JS, Choi CH, Atanasova KR, Yilmaz O. Porphyromonas gingivalis traffics into endoplasmic reticulum-rich-autophagosomes for successful survival in human gingival epithelial cells. Virulence. 2018;9(1):845–59.10.1080/21505594.2018.1454171Search in Google Scholar PubMed PubMed Central
[128] Imbuluzqueta E, Gamazo C, Ariza J, Blanco-Prieto MJ. Drug delivery systems for potential treatment of intracellular bacterial infections. Front Biosci (Landmark Ed). 2010;15(2):397–417.10.2741/3627Search in Google Scholar PubMed
[129] Ray K, Marteyn B, Sansonetti PJ, Tang CM. Life on the inside: The intracellular lifestyle of cytosolic bacteria. Nat Rev Microbiol. 2009;7(5):333–40.10.1038/nrmicro2112Search in Google Scholar PubMed
[130] Wayakanon K, Thornhill MH, Douglas CW, Lewis AL, Warren NJ, Pinnock A, et al. Polymersome-mediated intracellular delivery of antibiotics to treat porphyromonas gingivalis-infected oral epithelial cells. FASEB J. 2013;27(11):4455–65.10.1096/fj.12-225219Search in Google Scholar PubMed
[131] Briones E, Colino CI, Lanao JM. Delivery systems to increase the selectivity of antibiotics in phagocytic cells. J Control Release. 2008;125(3):210–27.10.1016/j.jconrel.2007.10.027Search in Google Scholar PubMed
[132] Mao D, Hu F, Qi G, Ji S, Wu W, Kong D, et al. One-step in vivo metabolic labeling as a theranostic approach for overcoming drug-resistant bacterial infections. Mater Horiz. 2020;7(4):1138–43.10.1039/C9MH01675ASearch in Google Scholar
[133] Cai Q, Fei Y, An HW, Zhao XX, Ma Y, Cong Y, et al. Macrophage-instructed intracellular staphylococcus aureus killing by targeting photodynamic dimers. ACS Appl Mater Interfaces. 2018;10(11):9197–202.10.1021/acsami.7b19056Search in Google Scholar PubMed
[134] Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kedzierska E, Knap-Czop K, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–107.10.1016/j.biopha.2018.07.049Search in Google Scholar PubMed
[135] Wang X, Wei X, Liu J, He D, Sun D, Yang W, et al. Oxygen self-supplying enzymatic nanoplatform for precise and enhanced photodynamic therapy. Adv Therapeut. 2022;5(9):2200049.10.1002/adtp.202200049Search in Google Scholar
[136] Wan Y, Fu LH, Li C, Lin J, Huang P. Conquering the hypoxia limitation for photodynamic therapy. Adv Mater. 2021;33(48):e2103978.10.1002/adma.202103978Search in Google Scholar PubMed
[137] Li S, Wei X, Li S, Zhu C, Wu C. Up-conversion luminescent nanoparticles for molecular imaging, cancer diagnosis and treatment. Int J Nanomedicine. 2020;15:9431–45.10.2147/IJN.S266006Search in Google Scholar PubMed PubMed Central
[138] Sun B, Bte Rahmat JN, Zhang Y. Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials. 2022;291:121875.10.1016/j.biomaterials.2022.121875Search in Google Scholar PubMed
[139] Cavalcante LLR, Tedesco AC, Takahashi LAU, Curylofo-Zotti FA, Souza-Gabriel AE, Corona SAM. Conjugate of chitosan nanoparticles with chloroaluminium phthalocyanine: Synthesis, characterization and photoinactivation of streptococcus mutans biofilm. Photodiagnosis Photodyn Ther. 2020;30:101709.10.1016/j.pdpdt.2020.101709Search in Google Scholar PubMed
[140] Longo JP, Leal SC, Simioni AR, de Fatima Menezes Almeida-Santos M, Tedesco AC, Azevedo RB. Photodynamic therapy disinfection of carious tissue mediated by aluminum-chloride-phthalocyanine entrapped in cationic liposomes: An in vitro and clinical study. Lasers Med Sci. 2012;27(3):575–84.10.1007/s10103-011-0962-6Search in Google Scholar PubMed
[141] Afrasiabi S, Pourhajibagher M, Chiniforush N, Bahador A. Propolis nanoparticle enhances the potency of antimicrobial photodynamic therapy against streptococcus mutans in a synergistic manner. Sci Rep. 2020;10(1):15560.10.1038/s41598-020-72119-ySearch in Google Scholar PubMed PubMed Central
[142] Saafan A, Zaazou MH, Sallam MK, Mosallam O, El Danaf HA. Assessment of photodynamic therapy and nanoparticles effects on caries models. Open Access Maced J Med Sci. 2018;6(7):1289–95.10.3889/oamjms.2018.241Search in Google Scholar PubMed PubMed Central
[143] Ossmann A, Kranz S, Andre G, Volpel A, Albrecht V, Fahr A, et al. Photodynamic killing of enterococcus faecalis in dentinal tubules using mthpc incorporated in liposomes and invasomes. Clin Oral Investig. 2015;19(2):373–84.10.1007/s00784-014-1271-9Search in Google Scholar PubMed
[144] Klepac-Ceraj V, Patel N, Song X, Holewa C, Patel C, Kent R, et al. Photodynamic effects of methylene blue-loaded polymeric nanoparticles on dental plaque bacteria. Lasers Surg Med. 2011;43(7):600–6.10.1002/lsm.21069Search in Google Scholar PubMed PubMed Central
[145] Pourhajibagher M, Bahador A. Attenuation of aggregatibacter actinomycetemcomitans virulence using curcumin-decorated nanophytosomes-mediated photo-sonoantimicrobial chemotherapy. Sci Rep. 2021;11(1):6012.10.1038/s41598-021-85437-6Search in Google Scholar PubMed PubMed Central
[146] Pourhajibagher M, Parker S, Chiniforush N, Bahador A. Photoexcitation triggering via semiconductor graphene quantum dots by photochemical doping with curcumin versus perio-pathogens mixed biofilms. Photodiagnosis Photodyn Ther. 2019;28:125–31.10.1016/j.pdpdt.2019.08.025Search in Google Scholar PubMed
[147] Li Q, Zhou R, Xie Y, Li Y, Chen Y, Cai X. Sulphur‐doped carbon dots as a highly efficient nano‐photodynamic agent against oral squamous cell carcinoma. Cell Proliferat. 2020;53(4):e12786.10.1111/cpr.12786Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

