The progress of cathode materials in aqueous zinc-ion batteries
-
Xinchi Zhou
, Yi Zhang
and Yunlei Zhou
Abstract
Rechargeable aqueous zinc-ion batteries (AZIBs), a promising energy storage device in the large-scale energy storage market, have attracted extensive attention in recent years due to their high safety, low cost, environmental friendliness, and excellent electrochemical performance. Despite the rapid development of AZIBs technology, challenges such as insufficient energy density and limited cycling life still exist, which hinders the practical application of AZIBs. Due to the critical role that cathode materials play in the electrochemical performance of AZIBs, it is necessary to summarize the progress of cathode materials for AZIBs. In this review, the Zn2+ storage mechanisms of the cathode materials are analyzed. Subsequently, the representative cathode materials are introduced, and their structures and electrochemical performances are compared. The existing problems and improvement strategies of these cathode materials are summarized in detail. Finally, the future challenges and promising prospects for cathode materials are proposed. This review will guide researchers and manufacturers, benefiting them in designing advanced AZIBs for grid-scale energy storage.
1 Introduction
In recent years, environmental issues have attracted widespread attention. With the increasing energy demand, the development of clean energy has become a global topic. Rechargeable lithium-ion batteries (LIBs) have been widely used in energy storage devices because of their high energy density and long cycle life [1,2,3,4,5,6,7]. However, there are also some problems such as limited lithium resources, high cost, and poor safety due to organic electrolytes, limiting large-scale energy applications of LIBs [8]. It is crucial to develop other advanced rechargeable batteries, including lithium–sulfur batteries [9], sodium–sulfur batteries [10,11], other alkali metal-ion (Na+ [12,13,14,15,16], and K+ [17,18,19]) batteries, and some multivalent metal-ion (Zn2+ [20,21,22], Mg2+ [23,24,25], Ca2+ [26,27,28], and Al3+ [29,30,31]) batteries [32]. In particular, sodium-ion and potassium-ion batteries have come into the spotlight over the past decade. However, the safety issue of organic electrolytes remains a threat.
Aqueous zinc-ion batteries (AZIBs) have the advantages of environmental friendliness, high safety, low redox potential (−0.76 V vs SHE), and high theoretical capacity (820 mA h g−1) [33,34]. Compared with common organic electrolytes, aqueous electrolytes [35] offer lower cost, higher safety, and the ability to avoid self-ignition incidents. Combined with these advantages, AZIBs are considered as an alternative to LIBs, not only enhancing safety but also being environmentally friendly, so that they have great potential for future large-scale energy storage [36].
In 1799, Volta made a voltaic pile through zinc and tin plates, which was the world’s first battery. Then, in 1836, the copper–zinc battery was produced, and the zinc-based battery began its long development path (Figure 1). Although AZIBs have made rapid development in recent years [37], there are still some problems to be solved, such as byproduct generation, insufficient battery energy density, uncertain storage mechanism, and cathode electrode dissolution during charge–discharge reaction [38]. As an important component of AZIBs, cathode material can directly affect the performance of batteries. Therefore, to address the battery deficiencies and enhance overall battery performance, further research on high-performance cathode materials is essential [39].
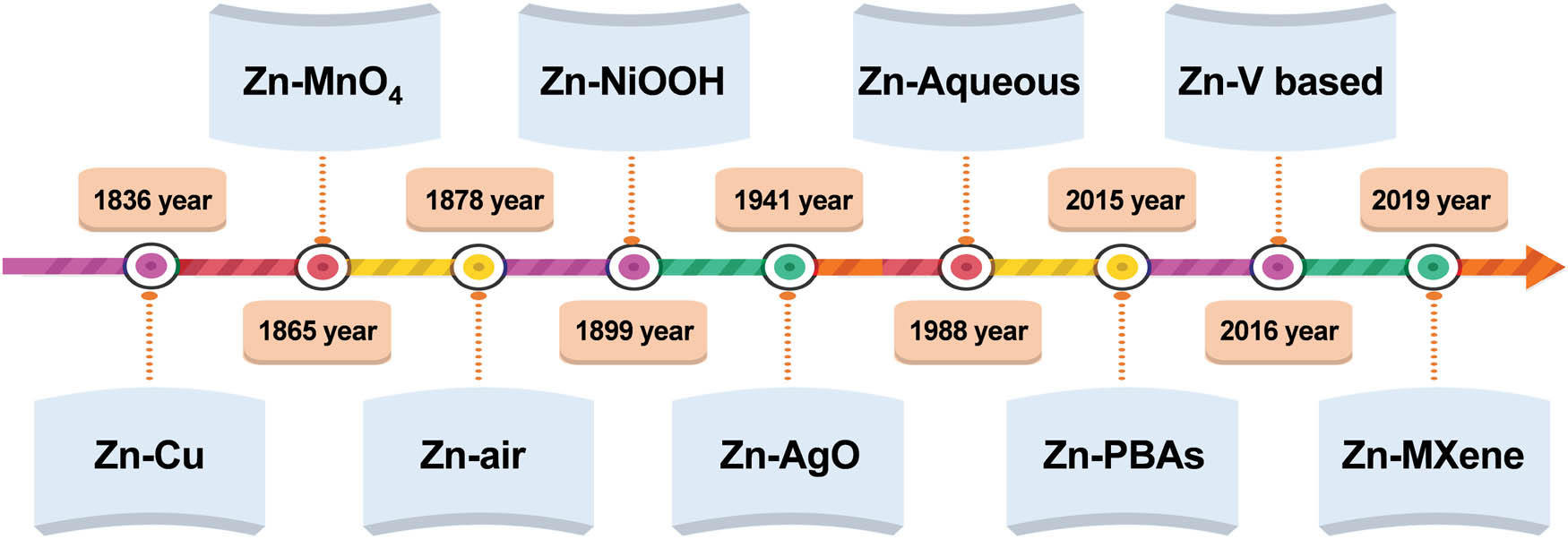
Development of zinc-based batteries.
In this review, we introduce the storage mechanism of Zn2+ in AZIBs, and then we focus on the cathode material of AZIBs, which is a very important part of the composition of AZIBs. It includes the advantages and disadvantages of common materials (Figure 2), the latest application progress and some improvement methods for existing problems. With the continuous exploration and efforts of the researchers, the development of AZIBs will be surely promoted continuously.

AZIBs cathode material.
2 Overview of AZIBs
2.1 Composition
The main structure of AZIBs includes cathode [40], anode [41,42,43,44,45,46,47,48], electrolyte [49,50,51,52,53], and separator [54,55,56]. Cathode materials can store Zn2+ including manganese-based compounds, vanadium-based compounds, MXene and its composites, Prussian blue analogs (PBAs), and organic compounds. Improving the properties of cathodes is extremely important for AZIBs [57]. As shown in Table 1, compared with other metals, metal zinc has good chemical stability in the aqueous solution. Compared with the standard hydrogen electrode, the zinc anode has a higher theoretical capacity (820 mA h g−1) and a lower redox potential (0.76 V), so metal zinc can be used as anode.
Comparison of several metals
| Element | Ionic radius (Å) | Relative atomic mass | Standard potential (V) | Gravimetric (mA h g−1) | Volumetric (mA h cm−3) |
|---|---|---|---|---|---|
| Li | 0.76 | 6.94 | −4.040 | 3,862 | 2,062 |
| Na | 1.02 | 23.00 | −2.713 | 1,166 | 1,128 |
| K | 1.38 | 39.10 | −2.924 | 686 | 610 |
| Zn | 0.74 | 65.41 | −0.763 | 820 | 5,851 |
| Ca | 1.00 | 40.08 | −2.840 | 1,340 | 2,073 |
| Mg | 0.72 | 24.31 | −2.356 | 2,205 | 3,833 |
| Al | 0.535 | 26.98 | −1.66 | 2,980 | 8,046 |
The electrolyte serves as a medium for ion transfer between the cathode and anode and is typically an aqueous solution of zinc salt, including ZnSO4 [58], Zn(OTf)2 [59], and Zn(TFSI)2 [60]. The choice of water as a solvent electrolyte can obtain higher ionic conductivity and safety performance. Research has indicated that the addition of appropriate additives to the electrolyte can significantly enhance the chemical performance of the electrolyte and improve the stability of the interface [61,62]. The separator also plays an important role in the battery system. The separators in AZIBs are typically made of glass fiber, filter paper, or polypropylene. These traditional separators usually do not affect the chemical performance of the battery, but in recent years, some newly developed functional separators can inhibit the growth of anode dendrites and improve the stability of the battery.
2.2 Working principle
The traditional AZIBs also belong to rocking-chair batteries [63], and their charging and discharging mechanism is similar to conventional LIBs. Zn2+ can move between the cathode and the anode (Figure 3). During the discharge process, the anode (Zn) loses electrons and transforms into Zn2+, which then enters the electrolyte. Simultaneously, electrons flow through the external circuit and enter the cathode, where the cathode is reduced by gaining electrons, and at the same time, Zn2+ from the electrolyte becomes embedded within the cathode. During the charging process, the cathode undergoes oxidation as it loses electrons, causing Zn2+ to detach from the cathode and enter the electrolyte. Meanwhile, electrons from the external circuit enter the anode, facilitating the deposition of Zn2+ from the electrolyte onto the anode surface.
![Figure 3
Schematic diagram of the working principle of AZIBs [64]. Copyright 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_003.jpg)
Schematic diagram of the working principle of AZIBs [64]. Copyright 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
3 Zn2+ storage mechanism
In general, AZIBs use weakly acidic or near-neutral aqueous electrolytes. The zinc anode undergoes a reversible Zn2+ (de)intercalation process as follows:
As the host material for Zn2+ (de)intercalation, the cathode material is accompanied by the change of valence and electron flow during the cycle. Compared with the stable development of LIBs, the reaction mechanism of the cathode of AZIBs is more complex and controversial. At present, the energy storage mechanism of cathode materials mainly includes Zn2+ (de)intercalation, Zn2+/H+ co-intercalation [65], chemical conversion reaction mechanism, and replacement reaction mechanism.
3.1 Zn2+ (de)intercalation mechanism
During the early stages of AZIBs research, the most widely proposed and accepted mechanism involved only the Zn2+ (de)intercalation mechanism. This mechanism was predominantly observed in manganese-based materials, vanadium-based materials, PBAs, and organic cathode materials. Due to the small radius (0.74 Å) of Zn2+, many materials with layered, channel, and frame structures can withstand its (de)intercalation. α-MnO2 [70], β-MnO2, γ-MnO2, δ-MnO2 [71,72], and ZnMn2O4 in the spinel structure, V2O5·nH2O in the layered structure, Ca0.25V2O5·nH2O in the layered structure, and PBAs in the frame structure have been reported to follow the energy storage mechanism of Zn2+ (de)intercalation.
Kim’s group investigated the reversible storage of Zn2+ in α-MnO2 nanorod electrodes [66]. The tunnel of the α-MnO2 structure expanded alternately during the process of Zn2+ intercalation and Zn2+ ejection like “breathing.” The volume of the adjacent (110) plane expanded by 3.12% after Zn2+ intercalation (Figure 4a). The electrode was stable during the (de)intercalation of Zn2+. In 2020, Long et al. used layered porous nanorods composed of twin α-(Mn2O3–MnO2) heterostructures as the cathode of AZIBs [67]. According to X-ray diffraction (XRD), during the first cycle, the Zn2+ intercalation and removal processes were experienced, and the peak characteristics recovered from the full discharge state to the full charge state, indicating that α-(Mn2O3@MnO2)-500 electrode has high reversibility in the AZIBs (Figure 4b and c).
![Figure 4
(a) Schematic illustration for the Zn2+ intercalation into tunnel structure α-MnO2, which causes the expansion of tunnel and hence increases the interplanar spacing of adjacent (110) planes [66]. Copyright 2015 Elsevier B.V. All rights reserved. (b) XRD of the α-(Mn2O3–MnO2)-500 electrodes at different charge/discharge states during the first cycle of the AZIBs. (c) Typical charge and discharge curves of the α-(Mn2O3–MnO2)-500 electrode [67]. Copyright 2020 American Chemical Society. (d) Schematic illustration of reversible Zn2+ intercalation/deintercalation in CoFe(CN)6 frameworks during electrochemical process. (e) Quantitative Zn2+ and H+ contribution to capacity delivery according to inductively coupled plasma (ICP) results [68]. Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Quantitative Zn2+ and H+ contribution to capacity delivery according to ICP results [69]. Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_004.jpg)
(a) Schematic illustration for the Zn2+ intercalation into tunnel structure α-MnO2, which causes the expansion of tunnel and hence increases the interplanar spacing of adjacent (110) planes [66]. Copyright 2015 Elsevier B.V. All rights reserved. (b) XRD of the α-(Mn2O3–MnO2)-500 electrodes at different charge/discharge states during the first cycle of the AZIBs. (c) Typical charge and discharge curves of the α-(Mn2O3–MnO2)-500 electrode [67]. Copyright 2020 American Chemical Society. (d) Schematic illustration of reversible Zn2+ intercalation/deintercalation in CoFe(CN)6 frameworks during electrochemical process. (e) Quantitative Zn2+ and H+ contribution to capacity delivery according to inductively coupled plasma (ICP) results [68]. Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (f) Quantitative Zn2+ and H+ contribution to capacity delivery according to ICP results [69]. Copyright 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Ma et al. formed a CoFe(CN)6 skeleton by in situ extraction of K+ from KCoFe(CN)6, and during the subsequent charging and discharging process, Zn2+ was deintercalated in the skeleton as the Zn2+ concentration was much higher than K+ [68]. Due to the presence of two active couples of Co3+/Co2+ and Fe3+/Fe2+ in this system, two steps were required for the intercalation/delamination of Zn2+, and this process is shown in Figure 4d. The presence of these two active couples resulted in a high-capacity, high-voltage system. After 2,200 cycles at a current density of 3 A g−1, the coulombic efficiency is approximately 100%, showing excellent cycling stability (Figure 4e). This Zn/PBA battery with almost no capacity degradation after long cycles highlights the great potential for the future development of high-voltage, high-capacity AZIBs.
3.2 Zn2+/H+ co-intercalation
Materials with channel, layered, or framework structures can achieve co-intercalation of H+ and Zn2+. During the charging state, H+ is stored through the formation of hydrogen bonds with lattice oxygen. However, during the discharging state, H+ is released and enters the electrolyte. Due to the relatively small ion radius of H+, rapid (de)intercalation is achievable, leading to enhanced ion diffusion kinetics and contributing to a higher capacity. The kinetic properties of Zn2+ and H+ are different. Compared with H+, Zn2+ has a larger radius and the characteristics of multivalent ions, so that it moves relatively slowly and has a greater electrostatic force with the intercalation host.
Through a series of characterization and analysis of charge–discharge curve, XRD, X-ray photoelectron spectroscopy (XPS), and high-resolution transmission electron microscopy (TEM), Zhao et al. proved that the intercalation mechanism of Zn/MnO2H0.16(H2O)0.27 (MON) aqueous solution battery system is Zn2+/H+ co-intercalation. Quantitative chemical analysis of the Zn2+/H+ co-intercalation was also carried out using ICP mass spectrometry [69]. Approximately 0.255 mol H+ and 0.086 mol Zn2+ were inserted into 1 mol MON in the first discharge plateau at 1.8–1.3 V as shown in Figure 4f. In the second discharge plateau at 1.3–1.0 V, another 0.204 mol H+ and 0.142 mol Zn2+ were inserted into 1 mol MON. Therefore, the cathodic electrochemical reaction equation of the Zn/MON cell is given as follows:
This H+ and Zn2+ synergistic intercalation mechanism, especially the fast diffusion kinetics of H+, has enabled this battery to exhibit excellent electrochemical performance.
3.3 Chemical transformation reaction mechanism
The chemical conversion reaction mechanism generally exists in manganese-based materials, which means that H+ participates in the charging and discharging process of MnO2 to generate MnOOH. The reaction formula is:
In contrast, Zn2+ does not intercalate into MnO2. Instead, it reacts with OH− ions in an equilibrium system to form ZnSO4[Zn(OH)2]3−·xH2O (ZHS), a lamellar layered hydroxide of Zn. ZHS is a complex of electrolyte salts and hydroxides with crystalline water, the molecular formula of which varies according to the type of electrolyte. Up to now, the formation of ZHS has been observed in many electrolyte systems. Typically, it is generated on the electrode surface during the discharge process and dissolves during the charging process. It is generally accepted that ZHS is generated to maintain the acid–base balance of the electrolyte.
Pan et al. characterized the structure and morphology of α-MnO2 electrodes using TEM and scanning transmission electron microscopy (STEM)-energy-dispersive X-ray spectroscopy (EDS) mapping to elucidate the electrode’s electrochemical behavior and reveal the mechanism of chemical transformation [73]. The initial nanofiber-like α-MnO2 transformed into shorter nanorods and nanoparticle aggregates (Figure 5a and b). HR-TEM analysis revealed lattice fringe spacings of 0.33 and 0.26 nm in the discharge products, corresponding to the (210) and (020) planes of monoclinic MnOOH, respectively (Figure 5c and d). In addition, the STEM-EDS mapping in Figure 5e demonstrated the absence of Zn elements in the nanorod structure, further confirming the formation of MnOOH through the chemical transformation reaction. This mechanism is identified as the primary reason for the excellent performance of the battery, with a capacity retention rate of 92% after 5,000 cycles, suggesting that the combination of Zn anode and MnO2 cathode could potentially form a high-performance and long-life AZIBs.
![Figure 5
(a) TEM image for α-MnO2 nanofiber. (b–d) TEM/HR-TEM images of MnO2 electrodes during electrochemical process. (e) STEM-high-angle annular dark-field image of short nanorods and STEM-EDS mappings of the elemental distributions of Mn, O, and Zn in the MnO2 electrode in the discharged state during the first cycle [73]. Copyright 2016 Macmillan Publishers Limited. All rights reserved. (f) Rate performance of graphene oxide wrapped CuV2O6 nanobelts and CVO NBs and Scheme showing reversible Zn2+ deintercalation between CuV2O6 and ZnV2O6 [74]. Copyright 2022 American Chemical Society. (g) Schematic illustration of the displacement/intercalation reaction mechanism in the first cycle, and the (de)intercalation mechanism of Zn2+ in subsequent electrochemical discharge/charge processes. (h) Galvanostatic charge/discharge profiles of the Zn/Na0.55Mn2O4·0.57H2O (NMOH) cell tested between 0.8 and 1.9 V with different current densities [75]. Copyright 2020 Springer.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_005.jpg)
(a) TEM image for α-MnO2 nanofiber. (b–d) TEM/HR-TEM images of MnO2 electrodes during electrochemical process. (e) STEM-high-angle annular dark-field image of short nanorods and STEM-EDS mappings of the elemental distributions of Mn, O, and Zn in the MnO2 electrode in the discharged state during the first cycle [73]. Copyright 2016 Macmillan Publishers Limited. All rights reserved. (f) Rate performance of graphene oxide wrapped CuV2O6 nanobelts and CVO NBs and Scheme showing reversible Zn2+ deintercalation between CuV2O6 and ZnV2O6 [74]. Copyright 2022 American Chemical Society. (g) Schematic illustration of the displacement/intercalation reaction mechanism in the first cycle, and the (de)intercalation mechanism of Zn2+ in subsequent electrochemical discharge/charge processes. (h) Galvanostatic charge/discharge profiles of the Zn/Na0.55Mn2O4·0.57H2O (NMOH) cell tested between 0.8 and 1.9 V with different current densities [75]. Copyright 2020 Springer.
3.4 Replacement reaction mechanism
The replacement reaction mechanism is commonly found in some pre-inserted vanadium oxides containing metal ions (Ag+ and Cu2+). During the reaction process, Zn2+ can partially replace these metal ions, forming metal or metal ion compounds. Liu et al. investigated the mechanism of Zn2+ storage in CuV2O6 cathode materials [74]. A reversible phase transition occurs between CuV2O6 and ZnV2O6, accompanied by the (de)intercalation of Zn2+ and the reduction/oxidation of metallic copper nanoparticles (Figure 5f), with the main electrochemical reactions being
This reaction process helps to improve the electrochemical performance of the battery. At a current density of 0.1 A g−1, the battery has a specific capacity of up to 427 mA h g−1, and after 3,000 cycles at a high current density of 5 A g−1, the battery still has a capacity retention rate of 99.3%. In addition, CuV2O6/GO composite material was also prepared. Due to the addition of GO, which can increase reactive sites and enhance structural stability, the electrochemical performance of the material was significantly improved, leading to a minimum increase in composite material capacity by 30%.
In addition to the four common Zn2+ storage mechanisms mentioned in this article, there are also solution-deposition mechanisms and some composite intercalation mechanisms. In 2020, Zhai et al. demonstrated the replacement/intercalation mechanism in the cathode of a manganese-based compound in AZIBs [75]. The layered NMOH was synthesized by selective etching of the siliceous tetrahedron of manganese silicate with the NaOH solution. After a series of ectopic XPS, XRD, EDS, and ICP-AES characterization, it was found that Zn2+ could be inserted into NMOH during the first discharge, and then, a small amount of Na+ was replaced by Zn2+, and Zn2+ became the pillar of the layered structure, thus improving the structural stability. In the subsequent charge–discharge cycle, reversible desorption of Zn2+/H+ is accompanied by adsorption/desorption of Na+ and reversible formation of Zn4SO4(OH)6·0.5H2O (Figure 5g). Na0.55Mn2O4·0.5H2O nanosheet has a high reversible capacity of 389.8 mA h g−1 at a current density of 200 mA g−1 (Figure 5h). The displacement/intercalation mechanism results in better electrochemical performance of the NMOH electrode. The intercalation/insertion mechanism has also been observed in various materials [76]. Zn3V3O8@ZnO@NC heterostructure was successfully synthesized using a well-designed approach and employed as the positive electrode material for AZIBs [77]. After two cycles of reaction, the cathode material underwent an irreversible transformation from Zn3V3O8@ZnO@NC to Zn3(OH)2V2O7⋅2H2O (ZOVO), followed by a subsequent (de)intercalation mechanism of Zn2+ within ZOVO. Remarkable rate performance and exceptional cycling stability were exhibited by this material.
In summary, the storage mechanisms of the majority of layered, tunnel, and framework-structured materials, when employed as cathode materials, involve Zn2+ (de)intercalation, with a portion also involving the co-intercalation of H+ and Zn2+. Compared to the singular (de)intercalation of Zn2+, the incorporation of smaller H+ enables faster (de)intercalation, exhibiting enhanced ion diffusion kinetics and contributing to a certain capacity. On the other hand, the chemical transformation reaction mechanism is commonly observed in manganese-based materials, wherein Zn2+ does not intercalate into MnO2; instead, H+ participates in the charging and discharging process of MnO2, leading to the formation of MnOOH. Zn2+ then reacts with OH− in a balanced electrochemical system, generating ZHS. For some vanadium oxides containing pre-intercalated metal ions, the replacement reaction mechanism is usually present. Zn2+ undergoes replacement reaction mechanism within the crystal lattice, producing metal or metal-ion compounds. Besides, there are some less common storage mechanisms, which require comprehensive analysis based on the material’s structural characteristics and characterization results. Research in this direction is still in its infancy, and new mechanisms are being proposed. With the continuous improvement of characterization means and research methods, this research can be continuously strengthened, and more contents of the storage mechanism of AZIBs can be mastered.
4 Development status of AZIBs cathode materials
As a host for Zn2+ (de)intercalation, cathode electrode material is also an extremely important part of AZIBs, which has an important impact on the overall electrochemical performance of AZIBs in the aqueous system. Because ions will be embedded and removed in the charging and discharging processes, resulting in changes in lattice volume and even complex structural changes, cathode materials should first have an open lattice structure and a certain lattice stability. The main cathode materials reported so far are manganese-based compounds, vanadium-based compounds, PBAs, MXene, and its composites as well as some organic materials. Table 2 summarizes the characteristics and electrochemical properties of some representative AZIBs cathode materials.
Characteristic and electrochemical performance of AZIBs representative cathode materials
| Type | Cathode material | Reaction mechanism | First discharge capacity | Cycle stability |
|---|---|---|---|---|
| Manganese-based compounds | α-MnO2 [73] | Chemical conversion reaction mechanism | 255 mA h g−1 (1 C) | 92% retained after 5,000 cycles (5 C) |
| α-MnO2 [78] | Zn2+ (de)intercalation | 323 mA h g−1 (16 mA g−1) | 46% retained after 75 cycles (83 mA g−1) | |
| α-MnO2@graphene [79] | Zn2+ (de)intercalation | 382 mA h g−1 (300 mA g−1) | 94% retained after 3,000 cycles (3,000 mA g−1) | |
| β-MnO2 [80] | Zn2+ (de)intercalation | 270 mA h g−1 (100 mA g−1) | 75% retained after 200 cycles (200 mA g−1) | |
| β-MnO2 [81] | Zn2+ (de)intercalation | 258 mA h g−1 (0.65 C) | 94% retained after 2,000 cycles (6.5 C) | |
| γ-MnO2 [82] | Zn2+ (de)intercalation | 285 mA h g−1 at 0.05 mA cm−2 | 63% retained after 40 cycles at (0.5 mA cm−2) | |
| δ-MnO2 [83] | Zn2+ (de)intercalation | 120 mA h g−1 (12.3 mA g−1) | 48% retained after 125 cycles (12.3 mA g−1) | |
| Vanadium-based compounds | V2O5 [84] | Zn2+ (de)intercalation | 372 mA h g−1 (0.3 A g−1) | 400 cycles (2 A g−1) |
| Al-V2O5 [85] | Zn2+ (de)intercalation | 532 mA h g−1 (0.1 A g−1) | 76% retained after 5,000 cycles (5 A g−1) | |
| V2O5·nH2O [86] | Zn2+ (de)intercalation | 714 mA h g−1 (100 mA g−1) | 83% retained after 1,000 cycles (1,000 mA g−1) | |
| Zn x V2O5·nH2O [87] | Pre-intercalated Zn2+ and water molecules | 435 mA h g−1 (0.5 A g−1) | 1,000 cycles (2 A g−1) | |
| Na3V2(PO4)3 [88] | Zn2+ (de)intercalation | 97 mA h g−1 (0.5 C) | 74% retained after 100 cycles (0.5 C) | |
| Prussian blue analogue | CuMn-PBA DSNBs [89] | Reversible redox reactions of transition metals and Zn2+ (de)intercalation | 116.8 mA h g−1 (0.1 A g−1) | 96.8% retained after 2,000 cycles (1 A g−1) |
| CoFe(CN)6 [68] | Zn2+ (de)intercalation | 173.4 mA h g−1 (0.3 A g−1) | 2,200 cycles (3 A g−1) | |
| MXene and its composites | Mn x V10O24·nH2O@V2CT x [90] | Zn2+ (de)intercalation | 445.6 mA h g−1 (0.5 A g−1) | 92.9% retained after 25,000 cycles (10 A g−1) |
| V2CT x MXene [91] | Zn2+/Li+ co-intercalation | 508 mA h g−1 (0.2 A g−1) | 18,000 cycles (10 A g−1) | |
| K-V2C@MnO2 [92] | Zn2+ (de)intercalation | 408.1 mA h g−1 (0.3 A g−1) | 10,000 cycles (10 A g−1) | |
| Organic materials | PANI-S [93] | Not mentioned | 184 mA h g−1 (0.2 A g−1) | 2,000 cycles (10 A g−1) |
| CuTCNQ [94] | Zn2+ (de)intercalation | 158 mA h g−1 (100 mA g−1) | 500 cycles (2,000 mA g−1) | |
| HAQ-COF [95] | H+/Zn2+ co-intercalation | 344 mA h g−1 (0.1 A g−1) | 85% retained after 10,000 cycles (5 A g−1) |
4.1 Manganese-based compounds
The element manganese (Mn) is abundant in the earth’s crust, and manganese-based materials have been of interest in the development of AZIBs [96]. This material is abundant and readily available, relatively low cost, and environmentally friendly, and therefore has a wide range of applications for large-scale energy storage applications. In addition, manganese has a high voltage plateau (1.2–1.4 V) exhibiting a high energy density and exists variety of valence states including Mn2+, Mn3+, Mn4+, and Mn7+, so manganese-based materials can accommodate the embedding of ions. A variety of manganese-based materials, including MnO2 of various crystalline forms (α-, β-, γ-, ε-, δ-, γ-, todorokite), other manganese oxides (MnO, MnO2, Mn2O3, Mn3O4, etc.), and spinel-phase ZnMn2O4, MgMn2O4, have been reported to be used in the cathode of AZIBs, but the most widely used is MnO2 of various crystalline forms [97]. They have a different crystal space structure. The basic building block consists of one Mn atom and six O atoms forming an octahedral MnO6 unit. Different MnO2 crystal structures can be formed by connecting these units through the sharing of octahedral edges and corners.
Despite the widespread use of manganese-based materials, there are still some problems in the charging and discharging processes. First, MnO2 with different crystal types is prone to structural transformation, damaging the crystal structure and then leading to capacity attenuation during the battery cycle [102,103]. Furthermore, issues such as the dissolution of Mn2+ during the reaction process and the generation of irreversible by-products are significant factors limiting the development of manganese-based compounds as cathode materials for AZIBs [104,105].
As an effective strategy to improve the electrochemical performance of AZIBs, defect engineering has been widely used in recent years [106]. Defect engineering usually includes two categories: anionic defect and cationic defect. In general, defect engineering can speed up ion movement and enhance electron transfer kinetics by adjusting the structure.
In 2020, Wang et al. prepared highly defective MnO2 (D-MnO2) ultrafine nanowires with an ultra-thin structure (approximately 10 nm in diameter) using a solvation method. These nanowires exhibited abundant oxygen vacancies and lattice holes (Figure 6a and b) and demonstrated a high energy density of 406 W h kg−1 [98]. Through the theoretical calculation of adsorption energy and charge density difference distribution (Figure 6c–e), it is proved that the ion/electron transfer rate can be increased by decreasing adsorption energy, which makes D-MnO2 cathode show better performance than MnO2 cathode without defect. Among the many crystalline forms of manganese dioxide, the crystal structure of ε-MnO2 is a hexagonal soft manganese ore with a dense stacking structure. However, its structure is unstable and can change when the temperature exceeds 300°C. Zhang et al. used a defect engineering approach to prepare nitrogen-doped ε-MnO2 (MnO2@N) to improve the electrochemical performance of manganese-based cathodes [99]. The electrochemical performance of the N-doped MnO2 cathode is improved by the synergistic effect of increased electrical conductivity and structural stability. Figure 6f shows the Raman spectra of the MnO2 and MnO2@N electrodes. It can be seen that the peak position of the Mn–O bond at 665 cm−1 shifts toward the lower wave number after nitrogen doping, demonstrating the creation of oxygen vacancies and therefore increasing the diffusion coefficient of the ions, resulting in accelerated charge transfer and increased conductivity. Moreover, the nitrogen doping creates an Mn–N bond, which also enhances the electrochemical stability of the cathode. After 500 cycles at 0.5 A g−1, the capacity retention was close to 100%, compared to 62% for the MnO2 cathode (Figure 6g). This work provides a new idea for progressing the stability of manganese-based AZIBs.
![Figure 6
(a and b) Adsorption structures of Zn in perfect MnO2 and D-MnO2; Dashed green circle in (b) represents the O vacancy in D-MnO2. (c) Adsorption energies of Zn and H in perfect MnO2 and D-MnO2. (d–e) Charge density difference distribution diagrams for Zn in MnO2 and D-MnO2. Yellow area: charge accumulation; cyan area: charge depletion [98]. Copyright 2020 American Chemical Society. (f) Comparison of Raman corresponding to MnO2 and MnO2@N. (g) Comparison of specific capacity of MnO2//Zn and MnO2@N//Zn cells at 0.5 A g−1 [99]. Copyright 2021 Science Press and Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Published by ELSEVIER B.V. and Science Press. (h) E-pH diagram of the MnO2 in different Mn2+ concentration. (i) Schematic showing the MnO2/CNT foam cathode that features a reversible chemical conversion and a 3D hierarchical structure for mass and charge transport [100]. Copyright 2021 Wiley-VCH GmbH. (j) Comparison of manganese dissolution of MnO@C with and without (Zn(OH)2)3(ZnSO4)(H2O)5 in the ZnSO4 electrolyte [101]. Copyright 2021 Elsevier B.V.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_006.jpg)
(a and b) Adsorption structures of Zn in perfect MnO2 and D-MnO2; Dashed green circle in (b) represents the O vacancy in D-MnO2. (c) Adsorption energies of Zn and H in perfect MnO2 and D-MnO2. (d–e) Charge density difference distribution diagrams for Zn in MnO2 and D-MnO2. Yellow area: charge accumulation; cyan area: charge depletion [98]. Copyright 2020 American Chemical Society. (f) Comparison of Raman corresponding to MnO2 and MnO2@N. (g) Comparison of specific capacity of MnO2//Zn and MnO2@N//Zn cells at 0.5 A g−1 [99]. Copyright 2021 Science Press and Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Published by ELSEVIER B.V. and Science Press. (h) E-pH diagram of the MnO2 in different Mn2+ concentration. (i) Schematic showing the MnO2/CNT foam cathode that features a reversible chemical conversion and a 3D hierarchical structure for mass and charge transport [100]. Copyright 2021 Wiley-VCH GmbH. (j) Comparison of manganese dissolution of MnO@C with and without (Zn(OH)2)3(ZnSO4)(H2O)5 in the ZnSO4 electrolyte [101]. Copyright 2021 Elsevier B.V.
Researchers are continually making improvements to retard the dissolution of manganese-based materials, thus allowing them to demonstrate excellent cycling performance. Jaekook Kim’s team demonstrated that the Mn2+ additive can be used to prevent the dissolution of manganese, improve the stability of the ZnMn2O4 cathode, and enhance its electrochemical performance [107]. Figure 6i shows the current strategy used to construct a new Zn–MnO2 battery chemistry, namely, a combination of two redox reactions, MnO2/Mn2+ (two-electron process) and MnO2/Mn3+ (single-electron process).
According to thermodynamic calculation, the PH value at three points (MnO2–MnOH–Mn2+) increases with the decrease of Mn2+ concentration (Figure 6h). Therefore, reversible MnO2/Mn2+ redox is likely to occur when the concentration of Mn2+ in mild electrolytes is low. By adjusting Mn2+ concentration within the appropriate range, Shen et al. can realize the double electron redox reaction without oxygen evolution reaction and recycle the by-products produced by MnOOH disproportionation [100]. Thus, high rate capacity and long cycle life are obtained, namely, 430 mA h g−1 at 19.5 A g−1 and at 16,000 cycles without significant capacity attenuation. In addition, the three-dimensional (3D) carbon nanotube foam skeleton was used as a conductive scaffold, so that the material could adapt to the volume change during the deposition/dissolution of MnO2 and provide a charge transport pathway. This provides the direction for further development of AZIBs.
In 2022, in situ electrochemically induced artificial solid electrolyte mesophase (Zn(OH)2)3(ZnSO4)·(H2O)5 on MnO@C cathode material was reported in AZIBs [101]. No additional Mn2+ was added to the electrolyte, but the solution of Mn2+ was inhibited by the homogeneous coating of (Zn(OH)2)3(ZnSO4)·(H2O)5 on MnO@C. The results of ICP emission spectrometry (Figure 6j) show that coating (Zn(OH)2)3(ZnSO4)·(H2O)5 on MnO@C can effectively inhibit the dissolution of Mn2+ in aqueous electrolyte, thus improving the cycling stability of MnO@C in AZIBS. Capacity attenuation of each cycle is 0.003% in 10,000 cycles. This in situ electrochemically induced artificial solid electrolyte interface is a new way to inhibit the dissolution of electrode materials.
Manganese-based materials have shown great promise in AZIBs due to their advantages of high voltage platform and high energy density. However, the development of these materials is still constrained by issues such as electrode material dissolution and low ionic conductivity. Effective approaches to address the current challenges in manganese-based material development include defect engineering, pre-doping of ions, and synthesis of composite materials. Despite these efforts, further endeavors are required to fully realize the application of manganese-based materials in AZIBs.
4.2 Vanadium-based compounds
Vanadium-based material is an excellent cathode material for AZIBs. It is characterized by abundant resource reserves, diverse structures, and various valence states (V2+, V3+, V4+, and V5+). Since vanadium-based material in polyvalent states can achieve multistep redox, it is endowed with a high theoretical specific capacity (>300 mA h g−1) [108]. Vanadium-based materials are usually composed of V–O polyhedrons interlinked with each other. Due to its easy deformation, a variety of vanadium oxides with different compositions and structural frames can be formed, including VO2 [109], V2O3 [110,111], V2O5 [112,113], and V6O13 [114,115]. Most of these vanadium oxides are layered or tunnel structures, which are conducive to the embedding and exfoliation of Zn2+, but a large amount of Zn2+ embedding will cause great structural changes and poor stability [116]. To improve the stability of the crystal structure, some metal ions (such as Na+, K+, and Ca2+) or H2O molecules are usually introduced as pillars to enhance the structural stability [117,118,119]. Qi et al. achieved the insertion of Cs+ into V2O5·nH2O using the hydrothermal method, resulting in the formation of strong Cs–O bonds between the layers. This significantly enhanced the stability of the cathode material and achieved a long cycle (10,000 cycles at 20 A g−1 current density, with a capacity retention rate of 89%) [120]. Guan et al. prepared Mg0.2V2O5·nH2O nanoribbons derived from the conducting compound V4C3 MXene [121]. The intercalated Mg2+ and structural water can not only enhance the conductivity of the material and accelerate the ion diffusion but also improve the structural stability, so that the battery can obtain good rate performance and long cycle stability. In 2021, Wan’s team proposed a general compensation strategy that uses polar organic molecules to displace some of the crystalline water in Al x V2O5·nH2O [122]. The polar groups in the organic molecule can generate electrostatic attraction with the pre-intercalated Al3+, ensuring its anchoring in the sandwich of the hydrated vanadate (Figure 7b). However, the low polar groups in organic molecules can produce weak interaction with Zn2+ during the cyclic process to ensure that Zn2+ can be (de)intercalated (Figure 7a). This work makes the cathode electrode material obtain stronger structural stability.
![Figure 7
(a) Schematic illustration of N,N-dimethylformamide (DMF) intercalation enhancing Zn2+ transfer kinetics and structural stability. (b) Calculated molecular electrostatic potential distribution of H2O and DMF molecules [122]. Copyright 2021 Wiley-VCH GmbH. (c) Illustration of V dissolution in the aqueous electrolyte of V2O5 nanoplates and V2O5 nanoplates/MXene hybrids cathodes. (d) Rate capacities of VPMX73/VPMX82/V2O5 nanoplates (0.1–5 A g−1) [123]. Copyright 2022 American Chemical Society. (e) Rate properties of NVO2 Electrode (0.1–10 A g−1) [124]. Copyright 2022 Elsevier B.V. (f) Cyclic voltammetry (CV) curves at different scanning rates. (g) Log(i) and log(v) plots at specific peak currents. (h) The contribution ratio of the capacitive and diffusion-limited capacities at different scan rates. (i) Galvanostatic intermittent titration technique (GITT) curves at 0.2 C and the calculated diffusion coefficient of N3VPF and N3VPF@5% rGO [125]. Copyright 2023 Wiley-VCH GmbH.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_007.jpg)
(a) Schematic illustration of N,N-dimethylformamide (DMF) intercalation enhancing Zn2+ transfer kinetics and structural stability. (b) Calculated molecular electrostatic potential distribution of H2O and DMF molecules [122]. Copyright 2021 Wiley-VCH GmbH. (c) Illustration of V dissolution in the aqueous electrolyte of V2O5 nanoplates and V2O5 nanoplates/MXene hybrids cathodes. (d) Rate capacities of VPMX73/VPMX82/V2O5 nanoplates (0.1–5 A g−1) [123]. Copyright 2022 American Chemical Society. (e) Rate properties of NVO2 Electrode (0.1–10 A g−1) [124]. Copyright 2022 Elsevier B.V. (f) Cyclic voltammetry (CV) curves at different scanning rates. (g) Log(i) and log(v) plots at specific peak currents. (h) The contribution ratio of the capacitive and diffusion-limited capacities at different scan rates. (i) Galvanostatic intermittent titration technique (GITT) curves at 0.2 C and the calculated diffusion coefficient of N3VPF and N3VPF@5% rGO [125]. Copyright 2023 Wiley-VCH GmbH.
Despite the promising applications of vanadium-based oxides, the dissolution of vanadium is still one of the main challenges in achieving its stable performance in AZIBs [126,127]. Liu et al. used a van der Waals self-assembly method to coat a layer of Ti3C2T x MXene layer on the surface of V2O5 nanoplates (VPMX) to inhibit vanadium dissolution, which helped maintain cathode integrity and therefore improve the storage performance of Zn2+ (Figure 7c) [123]. The MXene layer was shown to promote rapid electron transfer at the solid–solid heterogeneous interface between V2O5 and MXene. As shown by in situ XRD results, the MXene layer allows reversible co-intercalation of water molecules with Zn2+, thus reducing electrostatic repulsion, promoting electrochemical kinetics, and enhancing the rate capability of the cell to 243.6 mA h g−1 at 5.0 A g−1 (Figure 7d). This composite cathode exhibits long-term cycling stability in excess of 5,000 cycles. The coating method proposed in this work to inhibit vanadium dissolution presents an effective strategy for enhancing the electrochemical performance of vanadium-based aqueous batteries.
In addition to vanadium oxides, some layered vanadates, polyanion framework (NASICON) structure [88,128,129], and vanadium-based compounds have been shown to have excellent Zn2+ storage capacity. Amongst these, ammonium vanadate (NH4V4O10) is a potential cathode material for AZIBs due to its stable bilayer structure and high theoretical capacity. As in the case of vanadium oxides, the introduction of H2O molecules or some metal ions (K+, Na+, Al3+, etc.) can expand the layer spacing and improve the stability of the crystal structure. The NH4 + between the layers can also increase the layer spacing and mitigate the lattice changes during charging and discharging. However, when the NH4 + content is high, it limits the diffusion efficiency of Zn2+. Therefore, Chen et al. used an acid treatment to remove some of the ammonium ions to obtain NH4 +-deficient ammonium vanadate (NVO2) [124]. This measure could increase the interlayer distance without affecting the diffusion efficiency of Zn2+. The experimental results show that the NVO2 electrode has a specific capacity of up to 472.5 mA h g−1 at 0.1 A g−1 and up to 195.5 mA h g−1 when the current density is increased by a factor of 100, with a capacity retention rate of 41.4% (Figure 7e). Polyanionic materials are also important Zn2+ storage materials because of their high ion transfer kinetics due to their stable ion framework and large ion channels. In 2023, Guan et al. prepared Na3V2(PO4)2F3 (N3VPF) nanospheres, which were then coated with reduced graphene oxide (rGO) to obtain a 3D composite N3VPF@rGO [125]. The addition of rGO not only stabilizes the structure of the composite material but also enhances electron conductivity and accelerates the ion transfer rate, thereby significantly optimizing the capacity. Figure 7f shows CV curves at different sweep speeds. The migration dynamics of Zn2+ can be quantitatively analyzed by the following formula:
When b is close to 0.5, it is a diffusion control process, while when b is close to 1, it is a surface-controlled pseudocapacitive behavior. As shown in Figure 7g, the values of peaks 1–4 are all around 1, so the migration behavior of Zn2+ is mainly controlled by the pseudocapacitive behavior. The contribution of diffusion and surface capacitance can be calculated quantitatively by the following equation:
When the scan rate increases from 0.1 to 0.4 mV s−1, the capacitance contribution increases from 56.9 to 88.3% (Figure 7h). The increasing contribution of pseudocapacitive behavior indicates the faster reaction kinetics of N3VPF@rGO in AZIBs. In addition, the GITT method can be used to calculate the ionic diffusion coefficient in the reaction (Figure 7i), which can be computed by the following formula:
The results show that the Zn2+ of N3VPF@rGO is about 10−11 to 10−10 cm2 s−1, indicating its rapid reaction kinetics. It can be seen from the aforementioned characterization and calculation that the excellent pseudocapacitive behavior and the fast chemical reaction kinetics indicate that N3VPF@rGO has excellent electrochemical performance.
Vanadium-based cathode materials exhibit high reversible capacity and excellent rate performance due to their multiple valence states and diverse structures. To fully exploit the advantages of vanadium-based materials, various approaches have been employed to address issues such as structural instability, poor conductivity, and structural dissolution. These approaches include ion intercalation, molecular intercalation, and material encapsulation. With the continuous improvement of characterization methods and research techniques, the development of vanadium-based cathode materials can be undoubtedly accelerated further.
4.3 PBA
In addition to the common manganese and vanadium-based compounds, PBAs have been developed for use in the cathode of AZIBs [130,131]. Prussian blue and PBAs can be collectively referred to as metal-iron cyanides metal hexacyanoferrates. By replacing Fe2+ and Fe3+ in the Prussian blue material with other transition metal ions, PBAs can be obtained. Most PBAs have the Fm3m space group and face-centered cubic structure, usually denoted as A x M1[M2(CN)6]1−y ·nH2O, where A is an alkali metal element such as Na and K, and M1 and M2 are transition metal elements including Mn, Cu, Ni, Co, and Zn. Because of its stable 3D open skeleton structure and large lattice gap, it can provide more diffusion paths, enabling rapid deintercalation of Zn2+ [132]. In 2019, Ma et al. used cobalt hexacyanoferrate (CoFe(CN)6) as the cathode of AZIBs, in which there are two pairs of Co2+/3+ and Fe2+/3+ redox reactions, so the removal of Zn2+ requires two steps [68]. The presence of these two active couples provides AZIBs with A high voltage platform (1.75 V) and a high discharge capacity (109.5 mA h g−1 at 6 A g−1). This variety of redox reactions can increase battery capacity, so it can be extended to other battery systems.
Due to the high working voltage platform (∼1.5–1.8 V) of PBAs, this kind of material has high ionic conductivity and rapid charge and discharge ability [133]. Despite the relatively low specific capacity of this material (<200 mA h g−1), its low cost, environmental friendliness, and simple synthesis still offer a relatively good prospect for AZIB cathode materials.
However, due to the lack of redox sites and structural damage during ion dissociation, their capacity is limited and their cyclic stability is poor [134]. Of these, the rapid capacity decay of Mn-PBAs is mainly due to the dissolution of metal ions and the Jahn-Teller distortion of the Mn-N6 octahedron during repeated cycling. Zeng et al. after etching with tannic acid, prepared Cu-substituted Mn-PBA double-shelled nanoboxes (CuMn-PBA DSNBs) by the cation exchange method (Figure 8a) [89]. The high specific surface area of 227.6 m2 g−1 of CuMn-PBA DSNBs was much higher than the 7.7 m2 g−1 of the original Mn-PBA NCS (Figure 8c). The abundance of mesopores may have been created during the tannic acid etching process. The Jahn-Teller distortion of the Mn-N6 octahedron is mitigated by the substitution of Cu and the creation of vacancies in Mn during the preparation process, so the stability of this cathode material is improved and the cycle life of the battery is enhanced. Tannic acid etching allows this unique hollow structure to be obtained, exposing its active site. The large volume changes generated during ion (de)intercalation can be therefore mitigated, thereby increasing the volume capacity and cycling stability. In addition, partial Cu substitution and the induction of Mn vacancies can suppress the Jahn-Teller distortion of the Mn-N6 octahedron, thereby extending its lifetime. The results show that the CuMn-PBA DSNB electrode has good electrochemical properties and exhibits a high specific capacity, with a discharge capacity of 116.8 mA h g−1 at 0.1 A g−1 (Figure 8b). In addition, its excellent multiplier performance and good cycling properties surpass those of the Mn-PBA NC and Mn-PBA DSNB electrodes.
![Figure 8
(a) Field emission scanning electron microscopy (FESEM) images of Mn-PBA NCs. (b) Charge–discharge voltage profiles of the CuMn-PBA DSNB electrode at various current densities. (c) N2 adsorption–desorption isotherms and corresponding pore size distribution of CuMn-PBA DSNBs [89]. Copyright 2022 WILEY-VCH. (d) FESEM image of as-prepared ZMO. (e) XRD pattern of ZMO, Ti3C2T
x
, and ZMO@Ti3C2T
x
composite [135]. Copyright 2020 Elsevier B.V. (f) SEM images of VSe2@V2CT
x
. (g) Schematic illustration of the surface selenization of V2CT
x
for the preparation of VSe2@V2CT
x
nanohybrids. (h) XRD patterns of V2AlC, V2CT
x
, neat VSe2, and VSe2@V2CT
x
. (i) Raman spectrum of VSe2@V2CT
x
[136]. Copyright 2022 American Chemical Society.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_008.jpg)
(a) Field emission scanning electron microscopy (FESEM) images of Mn-PBA NCs. (b) Charge–discharge voltage profiles of the CuMn-PBA DSNB electrode at various current densities. (c) N2 adsorption–desorption isotherms and corresponding pore size distribution of CuMn-PBA DSNBs [89]. Copyright 2022 WILEY-VCH. (d) FESEM image of as-prepared ZMO. (e) XRD pattern of ZMO, Ti3C2T x , and ZMO@Ti3C2T x composite [135]. Copyright 2020 Elsevier B.V. (f) SEM images of VSe2@V2CT x . (g) Schematic illustration of the surface selenization of V2CT x for the preparation of VSe2@V2CT x nanohybrids. (h) XRD patterns of V2AlC, V2CT x , neat VSe2, and VSe2@V2CT x . (i) Raman spectrum of VSe2@V2CT x [136]. Copyright 2022 American Chemical Society.
PBA materials possess a high voltage platform and their internal 3D diffusion channels can accelerate ion transport, endowing them with rapid charging and discharging capabilities. However, as cathode materials for AZIBs, PBAs undergo significant volume changes during the charge–discharge process, thereby impacting their cycling stability. Moreover, their relatively poor conductivity also constitutes a major hindrance to their development. Therefore, it is imperative to enhance both their conductivity and effectively mitigate volume fluctuations. Both implementing rational strategies to adjust the structure of PBAs and fabricating high-conductivity composite materials are worthwhile measures that merit attention.
4.4 MXene and its composites
MXene is a two-dimensional (2D) transition metal carbide/nitride [137,138]. The shape is similar to potato chips stacked on top of each other, where “MX” refers to the MAX phase from which the A-layer elements have been etched away and “exe” refers to the graphene-like nanolayer structure [139]. The MAX phase is a precursor of MXene in a layered hexagonal structure, denoted as M n+1AX n (MAX), where N is 1–3, M is a transition metal (e.g., Mo, Ti, Zr), A is a group IIIA or IVA element such as Al, Ge, and Si in the periodic table, and X is C, N, or a mixture of them [140]. The laminated hexagonal MAX phase consists of an almost dense stacking of M layers, with the octahedral positions occupied by X atoms, and the M and X layers bonded together by A atoms. MXene is obtained by selective etching of “A” metal elements in the MAX phase. As a 2D material with three or more atomic layers, MXene has different properties compared to its 3D precursors. The general formula of MXene is M n+1X n or M n+1X n T n . M and X have the same meaning as represented in the MAX phase, while Tx are surface terminations such as –O, –OH, –F, and –Cl. The wide variety of surface terminations increases the number of possible compositions of MXene and allows adjustments to be made to the physical and chemical properties of MXene. The n + 1 layer M is interleaved with n layers of X when etching A elements. The creation of MXene further extends the family of 2D inorganic materials to include Ti3C2T x , Ti2CT x , and Ta4C3T x .
The diverse composition of chemical elements and unique layered structure allows MXene to enjoy extraordinary compositional diversity and tunable properties, thus giving it excellent properties such as high electronic conductivity, unique morphology, rich chemical properties, and excellent mechanical properties, which makes it extremely important for various applications such as energy storage [141,142], chemical sensors [143,144], and supercapacitor.
However, because of its low voltage and capacity, MXene is not used alone. Instead, it is applied to AZIBs with other active materials [92,145,146]. MXene is easy to form composites with other materials due to its good flexibility, as well as its 2D form and layered structure. In addition to its high conductivity and excellent electrochemical activity, MXene and MXene-based composites have been greatly applied in the field of energy and have become high-performance electrode materials for lithium–sulfur batteries, ZIBs, and supercapacitors [147].
Shi et al. designed and prepared a new composite material of zinc manganate (ZnMn2O4 and ZMO) and Ti3C2T x MXene. Figure 8d shows the FESEM image of the synthesized ZMO. The XRD pattern verifies the effective combination of ZMO and Ti3C2T x in the composite (Figure 8e). Ti3C2T x is highly conductive, and its use as a scaffold can alleviate the irreversible structural changes of zinc manganate during the chemical reaction as well as reduce the generation of side reactions [135]. The prepared product ZMO@Ti3C2T x was applied to AZIBs and demonstrated electrochemical properties superior to those of MXene itself. The product exhibits a large reversible specific capacity of 172.6 mA h g−1, a Coulombic efficiency of approximately 100%, and a capacity retention rate of up to 92.4% after 5,000 cycles. It has great potential for wearable electronics due to its ability to show high electrochemical stability in different states of deformation.
Sha et al. adopted a simple one-step surface selenization strategy to construct transition metal selenides (TMSes) on MXene, obtaining VSe2@ V2CT x (Figure 8g) [136]. Figure 8f shows the scanning electron microscope (SEM) image of VSe2@V2CT x . It can be seen that the surface of the material has an obvious layered structure. XRD and Raman of Figure 8h and i show that the main chain of V2CT x is retained, while VSe2 is selenized on the surface of V2CT x . This approach integrates the advantages of MXenes and TMSes, so that the hybrid material obtains high capacity and good structural stability.
Traditional MXene preparation is generally obtained by etching HF. However, HF is highly toxic and corrosive, which means this preparation method is relatively dangerous and not environmentally friendly. So the researchers tried to avoid using fluoride in the etching process and went for a safer approach. In 2020, Li et al. obtained MXene by in situ etching MAX with a special fluorine-rich electrolyte inside the cell and used it directly in situ [148]. According to the characterization by SEM, TEM, XRD, and XPS (Figure 9a–e, g), the whole process includes two stages: first, V2CT x MXene is etched by MAX V2AlC, and then V2CT x is oxidized to V2O5 V2CT x MXene has better performance than V2AlC, while V2O5 has a higher capacity than V2CT x MXene. So as the reaction goes on, the battery’s performance improves (Figure 9f). All processes are implemented inside the battery, avoiding external environmental pollution, and the battery always works as a rechargeable battery with an increasing capacity. Combining the 2D layered structure and high electrical conductivity characteristics of MXene with other materials enables the composite to fully exploit the advantages of both components, continuously enhancing the electrochemical performance of AZIB cathode materials. As research progresses, we firmly believe that MXene will undoubtedly continue to leverage its strengths and demonstrate significant application prospects in the field of rechargeable batteries.
![Figure 9
(a) SEM image of V2AlC powders. (b) SEM images of the cathode after 400 cycles in the coin-type battery at 10 A g−1. (c) TEM image of V2O5/V2CT
x
. (d) XRD patterns of the cathode after 5, 150, and 400 cycle. (e) XRD patterns of the cathode after 600 and 800 cycles at 5 A g−1. (f) CV measurement of the battery from 0.1 to 2.1 V at 5 mV s−1. (g) Original and fitted V 2p XPS spectra of the cathode after 5, 200, and 600 cycles at 5 A g−1 [148]. Copyright 2020 Wiley-VCH GmbH.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_009.jpg)
(a) SEM image of V2AlC powders. (b) SEM images of the cathode after 400 cycles in the coin-type battery at 10 A g−1. (c) TEM image of V2O5/V2CT x . (d) XRD patterns of the cathode after 5, 150, and 400 cycle. (e) XRD patterns of the cathode after 600 and 800 cycles at 5 A g−1. (f) CV measurement of the battery from 0.1 to 2.1 V at 5 mV s−1. (g) Original and fitted V 2p XPS spectra of the cathode after 5, 200, and 600 cycles at 5 A g−1 [148]. Copyright 2020 Wiley-VCH GmbH.
4.5 Organic materials
4.5.1 Introduction to organic electrode materials
Compared to inorganic materials, organic materials are also gaining attention in the application of cathode materials for AZIBs due to their environmental friendliness, structural diversity, and low cost [94,149,150]. Synthesis methods commonly employed include chemical methods and electrodeposition. The chemical methods offer flexibility and scalability in the material design, but the synthesis process is typically intricate and less safe. On the other hand, electrodeposition enables a significant enhancement in material purity, better control over morphology and crystal structure, and achievement of uniform distribution. However, this approach is only applicable to certain materials and may result in electrode instability due to side reactions during the synthesis. The selection of an appropriate synthesis method can be made based on specific requirements. Organic materials with an abundance of electroactive sites on their surfaces often exhibit fast reaction kinetics, for example, C═O, ═NH+, and C═N can be used as storage sites for Zn2+ with reduction activity. However, organic materials also have some problems, such as high solubility and low electrical conductivity, which limit their development in AZIBs.
Depending on the redox reaction, organic electrode materials can be divided into n-type, p-type, and bipolar types. At present, n-type organic electrode materials are widely used. Among them, carbonyl compounds with small molecular weight can provide higher theoretical capacity for the material. In the reaction, the reduction reaction mainly takes place through the carbonyl group to obtain electrons to generate anionic free radicals. The anionic free radicals will carry out coordination with the embedded cations to realize charge storage.
4.5.2 Organic material containing a carbonyl group
The quinone compound containing the carbonyl group can realize the rapid movement of ions by the van der Waals force connection between molecules and has good stability in water. The storage mechanism for Zn2+ in quinone-based compounds is primarily an ionic coordination mechanism, whereby a cathode charged cation is coordinated to a negatively charged oxygen atom with concomitant reduction of the carbonyl group.
Sun et al. used a molecular structure design approach to synthesize novel small thioquinones (4S6Q) and (4S4Q) (Figure 10a) [151]. The introduction of a conjugated thioether (–S–) bond, which both enhances the electrical conductivity of the compounds and inhibits the solubility of the compounds by extending the π-conjugation plane and building a flexible molecular backbone, allows this small thioquinone to be less soluble and exhibit good electrochemical stability. Used as the cathode for AZIBs, the Zn//4S6Q and Zn//4S4Q cells exhibited good electrochemical performance in a 3.5 M Zn(ClO4)2 electrolyte. For example, Zn//4S6Q batteries have a discharge capacity of 240 mA h g−1 at 150 mA g−1, without capacity decay at 3 A g−1 and up to 20,000 long cycles. Furthermore, thanks to the antifreeze electrolyte, the batteries can maintain a high discharge capacity of 201.7 mA h g−1 at −60°C, which is equivalent to 86.2% of the discharge capacity at 25°C. Experiments show that the excellent electrochemical properties generated by these novel compounds make them promising alternative electrode materials for Zn–organic batteries.
![Figure 10
(a) The synthesis processes of 4S4Q and 4S6Q compounds [151]. Copyright 2022 Springer. (b) SEM images of α-Mn2O3 cathodes at voltage of ⅰ–ⅷ points in Figure (ⅸ) and element concentration of A–H [152]. Copyright 2020 Science Press and Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Published by ELSEVIER B.V. and Science Press. (c) Structure of Cu3(HHTP)2, which when viewed down the c-axis, exhibits slipped-parallel stacking of 2D sheets with a honeycomb lattice. The cyan, red, and gray spheres represent Cu, O, and C atoms, respectively. The H atoms are omitted for the sake of clarity. (d) Expected redox process in the coordination unit of Cu3(HHTP)2. (e) Discharge–charge voltage profiles of Cu3(HHTP)2 at 50 mA g−1 [153]. Copyright 2019 Nature. (f) Quantitative capacity contribution calculated from ICP-AES discharged to different voltages in the first three cycles [95]. Copyright 2021 Wiley-VCH GmbH.](/document/doi/10.1515/ntrev-2023-0122/asset/graphic/j_ntrev-2023-0122_fig_010.jpg)
(a) The synthesis processes of 4S4Q and 4S6Q compounds [151]. Copyright 2022 Springer. (b) SEM images of α-Mn2O3 cathodes at voltage of ⅰ–ⅷ points in Figure (ⅸ) and element concentration of A–H [152]. Copyright 2020 Science Press and Dalian Institute of Chemical Physics, Chinese Academy of Sciences. Published by ELSEVIER B.V. and Science Press. (c) Structure of Cu3(HHTP)2, which when viewed down the c-axis, exhibits slipped-parallel stacking of 2D sheets with a honeycomb lattice. The cyan, red, and gray spheres represent Cu, O, and C atoms, respectively. The H atoms are omitted for the sake of clarity. (d) Expected redox process in the coordination unit of Cu3(HHTP)2. (e) Discharge–charge voltage profiles of Cu3(HHTP)2 at 50 mA g−1 [153]. Copyright 2019 Nature. (f) Quantitative capacity contribution calculated from ICP-AES discharged to different voltages in the first three cycles [95]. Copyright 2021 Wiley-VCH GmbH.
4.5.3 Polyaniline organic materials
In addition, organic materials based on polyaniline have been widely used. Polyaniline has high electrical conductivity and good energy storage behavior [154]. However, when it is applied to the cathode electrode of AZIBs, it usually shows serious deprotonation behavior in multiple redox reactions, which leads to capacity attenuation. In 2018, Shi et al. prepared a sulfonated self-doped polyaniline (PANI-S) electrode by introducing a sulfonic acid group [93]. The introduction of –SO3 − group acts as a proton library, thus improving the problem of deprotonation, but its cathode capacity is limited (<200 mA h g−1). To further improve the performance of PANI electrode materials, in 2021, Liu et al. not only solved the problem of deprotonation by introducing the sulfonic acid group but also compounded MXene on this basis to alleviate the problem of PANI expansion/contraction during the redox process [155]. The surface-functionalized S-MXene/PANI demonstrated excellent electrochemical performance (262 mA h g−1 capacity at 0.5 A g−1).
4.5.4 MOFs and COFs
In recent years, metal organic frameworks (MOFs) and covalent organic frameworks (COFs) materials have also been developed for use in AZIBs. MOFs are characterized by their structural diversity, porous structure, designable frameworks and versatility, and solve the problem of high solubility that exists in other organic materials. MOFs have been widely used in energy storage systems such as lithium/sodium ion batteries [156–159], supercapacitors, and fuel cells, while in recent years, they have also attracted the attention of researchers in the field of AZIBs [160].
In MOFs, the active organic species are fixed by metal-ligand covalent bonds, resulting in a porous structure and conductivity that facilitates the transport of ions and electrons within the framework [161], which increases the high rate capability and cycling capacity. Moreover, MOFs typically have high specific surface area values (103–104 m2 g−1). Crystals with ultra-high porosity and high thermal and chemical stability can be obtained from MOFs. Derived composites can be acquired by various treatments of MOF materials [162]. Since MOF-derived materials have been successfully used in energy storage applications such as lithium/sodium ion batteries and supercapacitors, a rational structural and functional design of MOF materials will certainly bring their advantages to bear in the field of AZIBs and thus be widely used [163].
In 2020, He and Kang’s team synthesized α-Mn2O3 by MOF-derived method and explored its charge storage mechanism [152]. As shown in Figure 10b, at low current density, Zn2+ intercalation dominates to produce lamellar products, while at high current density, H+ intercalation dominates to produce flower-like products. This work is of great significance for understanding the charge storage mechanism of manganese-based compounds. In the same year, Deng et al. prepared MOF-derived a-V2O5@C composites using in situ electrochemical induction strategy [164]. The amorphous V2O5 not only improves the cyclic stability but also increases the diffusion pathway and active site of Zn2+. The porous carbon skeleton provides electron/ion diffusion channels. This method enabled a-V2O5@C to exhibit a highly reversible capacity of 620.2 mA h g−1 at 0.3 A g−1 and to break the record of 20,000 cycles.
Nam et al. explored the 2D conductive MOF, Cu3(HHTP)2 (HHTP = 2,3,6,7,10,11-hexahydroxytriphenylene propylene), as a cathode material for AZIBs [153]. Due to its large open channel structure, the diffusion rate of Zn2+ ions is faster and the hydrated Zn2+ ions can be inserted directly into the main structure Cu3(HHTP)2 with a higher diffusion rate and lower interfacial resistance, allowing the Cu3(HHTP)2 cathode to follow the intercalation pseudocapacitance mechanism (Figure 10c and d). The electrochemical properties of the Cu3(HHTP)2 cathode were tested. Its high electrical conductivity and large pore size facilitate the transport of electrons and Zn2+ to the active center. As shown in Figure 10e, Cu3(HHTP)2 has a high reversible capacity of 228 mA h g−1 at 50 mA g−1. This study provides a research direction for further exploration to study MOF materials.
COF materials are 2D or 3D porous organic polymers linked by strong covalent bonds and contain mainly C, N, B, and H elements [165,166]. Although COFs have achieved a wide range of applications in the fields of multiphase catalysis [167], gas separation [168,169], semiconductors [170,171], and optoelectronic devices [172], there has been less research in electrode materials. This is mainly due to their poor electrical conductivity and the layer-by-layer stacking structure that hinders electron transport. Therefore, increasing the electrical conductivity of COFs and improving their ion/electron transport capacity are the main research directions to progress the performance of COF electrode materials [95,173]. In 2020, Wang et al. enhanced ion storage capacity and increased the discharge potential of AZIBs by introducing quinone groups into 1, 4, 5, 8, 9, 12-hexazazepines based COFs [95]. Finally, a high specific capacity of 344 mA h g−1 at a current density of 0.1 A g−1 was obtained (Figure 10f). It greatly promotes the research of COFs material storage mechanism.
Although organic materials are highly stable in water and have a high theoretical capacity, they still face problems such as low material utilization, low actual specific capacity, and poor cycling performance due to high solubility, easy dissolution of active substances, and easy destruction of the electrode structure, so there is a need to continue searching for suitable organic cathode materials and further exploring their structure and mechanism to obtain better battery performance [174].
With the rapid development of AZIBs, flexible zinc-ion batteries (FZIBs) have emerged as a promising choice for powering portable electronic devices. By incorporating positive electrode materials into FZIBs, the formation of dendrites and dissolution of the cathode can be significantly reduced. Designing them with flexibility and bendability allows these batteries to adapt to various shapes and sizes, enabling applications in wearable devices, smart patches, and more. However, the interface reaction mechanism remains unclear, and the immature design of flexible structures keeps FZIBs in the early stages of development, requiring further exploration.
5 Summary and outlook
AZIBs have attracted much attention in recent years due to their high safety, low cost, environmental friendliness, and excellent electrochemical performance. The cathode materials play a key role in AZIBs’ electrochemical performance. In this review, four Zn2+ storage mechanisms (Zn2+ (de)intercalation, Zn2+/H+ co-intercalation, chemical transformation reaction mechanism, and displacement reaction mechanism) of the cathode materials are analyzed. Then, the existing problems and improvement strategies of representative cathode materials are introduced. The uncertainties surrounding the ion storage mechanism, dissolution of active materials, insufficient energy density, and the generation of irreversible by-products are all impeding the development of large-scale energy storage for AZIBs. Researchers have taken various measures to address these issues. (1) Defect engineering not only enhances the conductivity of electrons/ions by improving electrode materials but also improves the electrochemical kinetics. The improvement in structural stability can also suppress electrode dissolution, further enhancing the electrochemical performance. (2) Interlayer insertion of H2O molecules or introduction of certain metal ions can expand the interlayer spacing and play a role as interlayer pillars. This improvement can alleviate the volume variation of the material during ion insertion/extraction processes, thereby accelerating ion transfer kinetics and improving material stability. (3) Surface coating methods can maintain the integrity of cathode materials by reducing surface electrostatic repulsion and minimizing dissolution. Finally, the future challenges and promising prospects for cathode materials are proposed.
However, there are still challenges to overcome to further develop high-performance AZIB cathode materials. 1) The deep storage mechanisms. Combining in situ characterization techniques and theoretical calculation of cathode materials could explore the relationship between the microstructure, composition, interfaces, and electrochemical performance, deeply revealing Zn2+ storage mechanisms. 2) The effective methods to inhibit the dissolution of cathode materials. Based on the intrinsic structure of the cathode materials, some novel methods will inhibit the dissolution of cathode materials, improving the cycling stability. 3) Develop novel cathode materials with high voltage and high capacity. Modulating the electronic structure of cathode materials could increase Zn2+ intercalation potential. Combining suitable anode materials and electrolyte, AZIBs will possess high working voltage and high specific capacity.
-
Funding information: This work is supported by the National Natural Science Foundation of China (Grant Nos. 52105575), the Fundamental Research Funds for the Central Universities (Grant No. QTZX23063), and the Proof of Concept Foundation of Xidian University Hangzhou Institute of Technology (Grant No. GNYZ2023YL0302).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Jiang M, Danilov DL, Eichel R-A, Notten PHL. A review of degradation mechanisms and recent achievements for Ni-rich cathode-based Li-ion batteries. Adv Energy Mater. 2021;11:2103005.10.1002/aenm.202103005Search in Google Scholar
[2] Gao F, Yue X, Xu X, Xu P, Zhang F, Fan H, et al. A N/Co co-doped three-dimensional porous carbon as cathode host for advanced lithium-selenium batteries. Rare Met. 2023;42:2670.10.1007/s12598-023-02273-5Search in Google Scholar
[3] Zhang Y, Du H, Lu J, Li Z, Lu T, Hu Y, et al. Enhanced cyclic stability of Ga2O3@PDA-C nanospheres as pseudocapacitive anode materials for lithium-ion batteries. Fuel. 2023;334:126683.10.1016/j.fuel.2022.126683Search in Google Scholar
[4] Zhou Y, Qu Y, Yin L, Cheng W, Huang Y, Fan R. Coassembly of elastomeric microfibers and silver nanowires for fabricating ultra-stretchable microtextiles with weakly and tunable negative permittivity. Compos Sci Technol. 2022;223:109415.10.1016/j.compscitech.2022.109415Search in Google Scholar
[5] Zhao D, Zhang Z, Ren J, Xu Y, Xu X, Zhou J, et al. Fe2VO4 nanoparticles on rGO as anode material for high-rate and durable lithium and sodium ion batteries. Chem Eng J. 2023;451:138882.10.1016/j.cej.2022.138882Search in Google Scholar
[6] Yan W, Gao X, Jin X, Liang S, Xiong X, Liu Z, et al. Nonporous gel electrolytes enable long cycling at high current density for lithium-metal anodes. ACS Appl Mater Interfaces. 2021;13:14258.10.1021/acsami.1c00182Search in Google Scholar PubMed
[7] Zhang Z, Zhao D, Xu Y, Liu S, Xu X, Zhou J, et al. A review on electrode materials of fast-charging lithium-ion batteries. Chem Rec. 2022;22:e202200127.10.1002/tcr.202200127Search in Google Scholar PubMed
[8] Zhang M, Yang D, Du J, Sun H, Li L, Wang L, et al. A review of SOH prediction of Li-ion batteries based on data-driven algorithms. Energies. 2023;16:3167.10.3390/en16073167Search in Google Scholar
[9] Wang Y, Tan P, Wu Y, Luo D, Li Z. Artificial intelligence-enhanced skin-like sensors based on flexible nanogenerators. View. 2022;3:20220026.10.1002/VIW.20220026Search in Google Scholar
[10] Zhao D, Jiang S, Yu S, Ren J, Zhang Z, Liu S, et al. Lychee seed-derived microporous carbon for high-performance sodium–sulfur batteries. Carbon. 2023;201:864.10.1016/j.carbon.2022.09.075Search in Google Scholar
[11] Zhao D, Zhang G, Zhang Z, Tang H, Xu Y, Gao F, et al. Three-dimensional honeycomb-like carbon as sulfur host for sodium–sulfur batteries without the shuttle effect. ACS Appl Mater Interfaces. 2022;14:54662.10.1021/acsami.2c13862Search in Google Scholar PubMed
[12] Jin T, Ji X, Wang P, Zhu K, Zhang J, Cao L, et al. High-energy aqueous sodium-ion batteries. Angew Chem Int Ed. 2021;60:11943.10.1002/anie.202017167Search in Google Scholar PubMed
[13] Ji Y, Li J, Li J. Recent development of electrolyte engineering for sodium metal batteries. Batteries. 2022;8:157.10.3390/batteries8100157Search in Google Scholar
[14] Zhang Z, Wang R, Zeng J, Shi K, Zhu C, Yan X. Size effects in sodium ion batteries. Adv Funct Mater. 2021;31:2106047.10.1002/adfm.202106047Search in Google Scholar
[15] Li X, Liang H, Qin B, Wang M, Zhang Y, Fan H. Rational design of heterostructured bimetallic sulfides (CoS2/NC@VS4) with VS4 nanodots decorated on CoS2 dodecahedron for high-performance sodium and potassium ion batteries. J Colloid Interface Sci. 2022;625:41.10.1016/j.jcis.2022.05.155Search in Google Scholar PubMed
[16] Luo F, Feng X, Zeng L, Lin L, Li X, Kang B, et al. In situ simultaneous encapsulation of defective MoS2 nanolayers and sulfur nanodots into SPAN fibers for high rate sodium-ion batteries. Chem Eng J. 2021;404:126430.10.1016/j.cej.2020.126430Search in Google Scholar
[17] Xia M, Zhang X, Liu T, Yu H, Chen S, Peng N, et al. Commercially available prussian blue get energetic in aqueous K-ion batteries. Chem Eng J. 2020;394:124923.10.1016/j.cej.2020.124923Search in Google Scholar
[18] Yi Z, Chen Z, Yin K, Wang L, Wang K. Sensing as the key to the safety and sustainability of new energy storage devices. Prot Control Mod Power Syst. 2023;8:27.10.1186/s41601-023-00300-2Search in Google Scholar
[19] Huang S, Hsieh Y, Chen K, Tuan H. Flexible nanostructured potassium-ion batteries. Chem Eng J. 2021;416:127697.10.1016/j.cej.2020.127697Search in Google Scholar
[20] Liu C, Tian M, Wang M, Zheng J, Wang S, Yan M, et al. Catalyzing zinc-ion intercalation in hydrated vanadates for aqueous zinc-ion batteries. J Mater Chem A. 2020;8:7713.10.1039/D0TA01468KSearch in Google Scholar
[21] Blanc LE, Kundu D, Nazar LF. Scientific challenges for the implementation of Zn-ion batteries. Joule. 2020;4:771.10.1016/j.joule.2020.03.002Search in Google Scholar
[22] Gao F, Mei B, Xu X, Ren J, Zhao D, Zhang Z, et al. Rational design of ZnMn2O4 nanoparticles on carbon nanotubes for high-rate and durable aqueous zinc-ion batteries. Chem Eng J. 2022;448:137742.10.1016/j.cej.2022.137742Search in Google Scholar
[23] Niu J, Zhang Z, Aurbach D. Alloy anode materials for rechargeable Mg ion batteries. Adv Energy Mater. 2020;10:2000697.10.1002/aenm.202000697Search in Google Scholar
[24] Lu D, Liu H, Huang T, Xu Z, Ma L, Yang P, et al. Magnesium ion based organic secondary batteries. J Mater Chem A. 2018;6:17297.10.1039/C8TA05230ASearch in Google Scholar
[25] Park H, Lim H-K, Oh SH, Park J, Lim H-D, Kang K. Tailoring ion-conducting interphases on magnesium metals for high-efficiency rechargeable magnesium metal batteries. ACS Energy Lett. 2020;5:3733.10.1021/acsenergylett.0c02102Search in Google Scholar
[26] Lang J, Jiang C, Fang Y, Shi L, Miao S, Tang Y. Room-temperature rechargeable Ca-ion based hybrid batteries with high rate capability and long-term cycling life. Adv Energy Mater. 2019;9:1901099.10.1002/aenm.201901099Search in Google Scholar
[27] Gummow RJ, Vamvounis G, Kannan MB, He Y. Calcium-ion batteries: current state-of-the-art and future perspectives. Adv Mater. 2018;30:1801702.10.1002/adma.201801702Search in Google Scholar PubMed
[28] Park J, Xu Z, Yoon G, Park SK, Wang J, Hyun H, et al. Stable and high-power calcium-ion batteries enabled by calcium intercalation into graphite. Adv Mater. 2020;32:1904411.10.1002/adma.201904411Search in Google Scholar PubMed
[29] Elia GA, Kravchyk KV, Kovalenko MV, Chacón J, Holland A, Wills RGA. An overview and prospective on Al and Al-ion battery technologies. J Power Sources. 2021;481:228870.10.1016/j.jpowsour.2020.228870Search in Google Scholar
[30] Das SK, Mahapatra S, Lahan H. Aluminium-ion batteries: developments and challenges. J Mater Chem A. 2017;5:6347.10.1039/C7TA00228ASearch in Google Scholar
[31] Zhang E, Cao W, Wang B, Yu X, Wang L, Xu Z, et al. A novel aluminum dual-ion battery. Energy Storage Mater. 2018;11:91.10.1016/j.ensm.2017.10.001Search in Google Scholar
[32] Zhou Y, Lian H, Li Z, Yin L, Ji Q, Li K, et al. Crack engineering boosts the performance of flexible sensors. View. 2022;3:20220025.10.1002/VIW.20220025Search in Google Scholar
[33] Su Z, Wang R, Huang J, Sun R, Qin Z, Zhang Y. Silver vanadate (Ag0.33V2O5) nanorods from Ag intercalated vanadium pentoxide for superior cathode of aqueous zinc-ion batteries. Rare Met. 2022;41(8):2844–52.10.1007/s12598-022-02026-wSearch in Google Scholar
[34] An Y, Tian Y, Xiong S, Feng J, Qian Y. Scalable and controllable synthesis of interface-engineered nanoporous host for dendrite-free and high rate zinc metal batteries. ACS Nano. 2021;15:11828.10.1021/acsnano.1c02928Search in Google Scholar PubMed
[35] Leonard DP, Wei Z, Chen G, Du F, Ji X. Water-in-salt electrolyte for potassium-ion batteries. ACS Energy Lett. 2018;3:373.10.1021/acsenergylett.8b00009Search in Google Scholar
[36] Wu B, Luo W, Li M, Zeng L, Mai L. Achieving better aqueous rechargeable zinc ion batteries with heterostructure electrodes. Nano Res. 2021;14:3174.10.1007/s12274-021-3392-1Search in Google Scholar
[37] Shang Y, Kundu D. A path forward for the translational development of aqueous zinc-ion batteries. Joule. 2023;7:244.10.1016/j.joule.2023.01.011Search in Google Scholar
[38] Zhou Y, Chen F, Arandiyan H, Guan P, Liu Y, Wang Y, et al. Oxide-based cathode materials for rechargeable zinc ion batteries: progresses and challenges. J Energy Chem. 2021;57:516.10.1016/j.jechem.2020.08.038Search in Google Scholar
[39] Liu A, Wu F, Zhang Y, Zhou J, Zhou Y, Xie M. Insight on cathodes chemistry for aqueous zinc-ion batteries: from reaction mechanisms, structural engineering, and modification strategies. Small. 2022;18:2201011.10.1002/smll.202201011Search in Google Scholar PubMed
[40] Wang X, Zhang Z, Xi B, Chen W, Jia Y, Feng J, et al. Advances and perspectives of cathode storage chemistry in aqueous zinc-ion batteries. ACS Nano. 2021;15:9244.10.1021/acsnano.1c01389Search in Google Scholar PubMed
[41] Wang R, Xin S, Chao D, Liu Z, Wan J, Xiong P, et al. Fast and regulated zinc deposition in a semiconductor substrate toward high‐performance aqueous rechargeable batteries. Adv Funct Mater. 2022;32:207751.10.1002/adfm.202207751Search in Google Scholar
[42] Xie D, Wang Z, Gu Z, Diao W, Tao F, Liu C, et al. Polymeric molecular design towards horizontal Zn electrodeposits at constrained 2D Zn2+ diffusion: dendrite‐free Zn anode for long‐life and high‐rate aqueous zinc metal battery. Adv Funct Mater. 2022;32:2204066.10.1002/adfm.202204066Search in Google Scholar
[43] Yi Z, Chen G, Hou F, Wang L, Liang J. Strategies for the stabilization of Zn metal anodes for Zn-ion batteries. Adv Energy Mater. 2021;11:2003065.Search in Google Scholar
[44] Kim S, Hwang U, Yang K, Cho M, Nam J-D, Lee Y. Constructing robust zincophilic-channels on Zn anode for long-life Zn-ion batteries. Chem Eng J. 2022;440:135822.10.1016/j.cej.2022.135822Search in Google Scholar
[45] Guo N, Huo W, Dong X, Sun Z, Lu Y, Wu X, et al. A review on 3D zinc anodes for zinc ion batteries. Small Methods. 2022;6:2200597.10.1002/smtd.202200597Search in Google Scholar PubMed
[46] Yi Z, Chen G, Hou F, Wang L, Liang J. Zinc-ion batteries: strategies for the stabilization of Zn metal anodes for Zn-ion batteries. Advanced Energy. Materials. 2021;11:2170001.10.1002/aenm.202003065Search in Google Scholar
[47] Ma N, Yin H, Wang K. Prediction of the remaining useful life of supercapacitors at different temperatures based on improved long short-term memory. Energies. 2023;16:5240.10.3390/en16145240Search in Google Scholar
[48] Lin C, Yang X, Xiong P, Lin H, He L, Yao Q, et al. High-rate, large capacity, and long life dendrite-free Zn metal anode enabled by trifunctional electrolyte additive with a wide temperature range. Adv Sci. 2022;9:2201433.10.1002/advs.202201433Search in Google Scholar PubMed PubMed Central
[49] Wan J, Wang R, Liu Z, Zhang L, Liang F, Zhou T, et al. A double-functional additive containing nucleophilic groups for high-performance Zn-ion batteries. ACS Nano. 2023;2:1610.10.1021/acsnano.2c11357Search in Google Scholar PubMed
[50] Qiu B, Xie L, Zhang G, Cheng K, Lin Z, Liu W, et al. Toward reversible wide-temperature Zn storage by regulating the electrolyte solvation structure via trimethyl phosphate. Chem Eng J. 2022;449:137843.10.1016/j.cej.2022.137843Search in Google Scholar
[51] Geng Y, Pan L, Peng Z, Sun Z, Lin H, Mao C, et al. Electrolyte additive engineering for aqueous Zn ion batteries. Energy Storage Mater. 2022;51:733.10.1016/j.ensm.2022.07.017Search in Google Scholar
[52] Guo S, Qin L, Zhang T, Zhou M, Zhou J, Fang G, et al. Fundamentals and perspectives of electrolyte additives for aqueous zinc-ion batteries. Energy Storage Mater. 2021;34:545.10.1016/j.ensm.2020.10.019Search in Google Scholar
[53] Dai Q, Li L, Tu T, Zhang M, Song L. An appropriate Zn2+/Mn2+ concentration of the electrolyte enables superior performance of AZIBs. J Mater Chem A. 2022;10:23722.10.1039/D2TA06449ASearch in Google Scholar
[54] Liu H, Zhou Q, Xia Q, Lei Y, Long Huang X, Tebyetekerwa M, et al. Interface challenges and optimization strategies for aqueous zinc-ion batteries. J Energy Chem. 2023;77:642.10.1016/j.jechem.2022.11.028Search in Google Scholar
[55] Fang Y, Xie X, Zhang B, Chai Y, Lu B, Liu M, et al. Regulating zinc deposition behaviors by the conditioner of pan separator for zinc-ion batteries. Adv Funct Mater. 2022;32:2109671.10.1002/adfm.202109671Search in Google Scholar
[56] Qin Y, Liu P, Zhang Q, Wang Q, Sun D, Tang Y, et al. Advanced filter membrane separator for aqueous zinc-ion batteries. Small. 2020;16:2003106.10.1002/smll.202003106Search in Google Scholar PubMed
[57] Selvakumaran D, Pan A, Liang S, Cao G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J Mater Chem A. 2019;7:18209.10.1039/C9TA05053ASearch in Google Scholar
[58] Li Z, Ganapathy S, Xu Y, Zhou Z, Sarilar M, Wagemaker M. Mechanistic insight into the electrochemical performance of Zn/VO2 batteries with an aqueous ZnSO4 electrolyte. Adv Energy Mater. 2019;9:1900237.10.1002/aenm.201900237Search in Google Scholar
[59] Zhao X, Zhang X, Dong N, Yan M, Zhang F, Mochizuki K, et al. Advanced buffering acidic aqueous electrolytes for ultra-long life aqueous zinc-ion batteries. Small. 2022;18:2200742.10.1002/smll.202200742Search in Google Scholar PubMed
[60] Wu T, Lin W. Enhanced reversibility of vanadium oxide cathode by diminished surface precipitation in Zn(TFSI)2 aqueous electrolyte. Electrochim Acta. 2021;399:139432.10.1016/j.electacta.2021.139432Search in Google Scholar
[61] Liu Z, Wang R, Ma Q, Wan J, Zhang S, Zhang L, et al. A dual‐functional organic electrolyte additive with regulating suitable overpotential for building highly reversible aqueous zinc ion batteries. Adv Funct Mater. 2023;2214538.10.1002/adfm.202214538Search in Google Scholar
[62] Xin T, Zhou R, Xu Q, Yuan X, Zheng Z, Li Y, et al. 15-Crown-5 ether as efficient electrolyte additive for performance enhancement of aqueous Zn-ion batteries. Chem Eng J. 2023;452:139572.10.1016/j.cej.2022.139572Search in Google Scholar
[63] Zhao D, Chen S, Lai Y, Ding M, Cao Y, Chen Z. A stable “rocking-chair” zinc-ion battery boosted by low-strain Zn3V4(PO4)6 cathode. Nano Energy. 2022;100:107520.10.1016/j.nanoen.2022.107520Search in Google Scholar
[64] Xu C, Li B, Du H, Kang F. Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew Chem Int Ed. 2012;51:933–35.10.1002/anie.201106307Search in Google Scholar PubMed
[65] Zhu K, Wu T, Sun S, van den Bergh W, Stefik M, Huang K, et al. Zn2+ dual ion insertion mechanism in high-capacity and ultra-stable hydrated VO2 cathode for aqueous Zn-ion batteries. Energy Storage Mater. 2020;29:60.10.1016/j.ensm.2020.03.030Search in Google Scholar
[66] Alfaruqi MH, Gim J, Kim S, Song J, Jo J, Kim S, et al. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J Power Sources. 2015;288:320.10.1016/j.jpowsour.2015.04.140Search in Google Scholar
[67] Long J, Yang F, Cuan J, Wu J, Yang Z, Jiang H, et al. Boosted charge transfer in twinborn alpha-(Mn2O3–MnO2) heterostructures: toward high-rate and ultralong-life zinc-ion batteries. ACS Appl Mater Interfaces. 2020;12:32526.10.1021/acsami.0c05812Search in Google Scholar PubMed
[68] Ma L, Chen S, Long C, Li X, Zhao Y, Liu Z, et al. Achieving high‐voltage and high‐capacity aqueous rechargeable zinc ion battery by incorporating two‐species redox reaction. Adv Energy Mater. 2019;9:1902446.10.1002/aenm.201902446Search in Google Scholar
[69] Zhao Q, Chen X, Wang Z, Yang L, Qin R, Yang J, et al. Unravelling H+/Zn2+ synergistic intercalation in a novel phase of manganese oxide for high-performance aqueous rechargeable battery. Small. 2019;15:e1904545.10.1002/smll.201904545Search in Google Scholar PubMed
[70] Gao X, Wu H, Li W, Tian Y, Zhang Y, Wu H, et al. H+-insertion boosted α-MnO2 for an aqueous Zn-ion battery. Small. 2020;16:1905842.10.1002/smll.201905842Search in Google Scholar PubMed
[71] Jiao Y, Kang L, Berry-Gair J, McColl K, Li J, Dong H, et al. Enabling stable MnO2 matrix for aqueous zinc-ion battery cathodes. J Mater Chem A. 2020;8:22075.10.1039/D0TA08638JSearch in Google Scholar
[72] Li F, Liu Y, Wang G, Yan D, Li G, Zhao H, et al. The design of flower-like C-MnO2 nanosheets on carbon cloth toward high-performance flexible zinc-ion batteries. J Mater Chem A. 2021;9:9675.10.1039/D0TA12009JSearch in Google Scholar
[73] Pan H, Shao Y, Yan P, Cheng Y, Han K, Nie Z, et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat Energy. 2016;1:16039.10.1038/nenergy.2016.39Search in Google Scholar
[74] Liu Y, Li Q, Ma K, Yang G, Wang C. Graphene oxide wrapped CuV2O6 nanobelts as high-capacity and long-life cathode materials of aqueous zinc-ion batteries. ACS Nano. 2019;13:12081.10.1021/acsnano.9b06484Search in Google Scholar PubMed
[75] Zhai X, Qu J, Hao S, Jing Y, Chang W, Wang J, et al. Layered birnessite cathode with a displacement/intercalation mechanism for high-performance aqueous zinc-ion batteries. Nanomicro Lett. 2020;12:56.10.1007/s40820-020-0397-3Search in Google Scholar PubMed PubMed Central
[76] Sun R, Dong S, Yang J, Xiong J, Wang C, Lu S, et al. Defect engineering of molybdenum disulfide nanosheets boosting super Zn2+ storage from polyaniline intercalation. Chem Commun. 2023;59:1845.10.1039/D2CC05888JSearch in Google Scholar PubMed
[77] Sun R, Guo X, Dong S, Wang C, Zeng L, Lu S, et al. Zn3V3O8@ZnO@NC heterostructure for stable zinc ion storage from assembling nanodisks into cross-stacked architecture. J Power Sources. 2023;567:232946.10.1016/j.jpowsour.2023.232946Search in Google Scholar
[78] Alfaruqi MH, Islam S, Gim J, Song J, Kim S, Pham DT, et al. A high surface area tunnel-type α-MnO2 nanorod cathode by a simple solvent-free synthesis for rechargeable aqueous zinc-ion batteries. Chem Phys Lett. 2016;650:64.10.1016/j.cplett.2016.02.067Search in Google Scholar
[79] Wu B, Zhang G, Yan M, Xiong T, He P, He L, et al. Graphene scroll-coated alpha-MnO2 nanowires as high-performance cathode materials for aqueous zn-ion battery. Small. 2018;14:e1703850.10.1002/smll.201703850Search in Google Scholar PubMed
[80] Islam S, Alfaruqi MH, Mathew V, Song J, Kim S, Kim S, et al. Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J Mater Chem A. 2017;5:23299.10.1039/C7TA07170ASearch in Google Scholar
[81] Zhang N, Cheng F, Liu J, Wang L, Long X, Liu X, et al. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat Commun. 2017;8:405.10.1038/s41467-017-00467-xSearch in Google Scholar PubMed PubMed Central
[82] Alfaruqi MH, Mathew V, Gim J, Kim S, Song J, Baboo JP, et al. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem Mater. 2015;27:3609.10.1021/cm504717pSearch in Google Scholar
[83] Han S, Kim S, Li D, Petkov V, Yoo HD, Phillips PJ, et al. Mechanism of Zn insertion into nanostructured δ-MnO2: a nonaqueous rechargeable Zn metal battery. Chem Mater. 2017;29:4874.10.1021/acs.chemmater.7b00852Search in Google Scholar
[84] Zhou J, Shan L, Wu Z, Guo X, Fang G, Liang S. Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode. Chem Commun. 2018;54:4457.10.1039/C8CC02250JSearch in Google Scholar PubMed
[85] Jiang H, Gong W, Zhang Y, Liu X, Waqar M, Sun J, et al. Quench-tailored Al-doped V2O5 nanomaterials for efficient aqueous zinc-ion batteries. J Energy Chem. 2022;70:52.10.1016/j.jechem.2022.02.030Search in Google Scholar
[86] Zhao D, Wang X, Zhang W, Zhang Y, Lei Y, Huang X, et al. Unlocking the capacity of vanadium oxide by atomically thin graphene‐analogous V2O5·nH2O in aqueous zinc‐ion batteries. Adv Funct Mater. 2023;33:2211412.10.1002/adfm.202211412Search in Google Scholar
[87] Hu K, Jin D, Zhang Y, Ke L, Shang H, Yan Y, et al. Metallic vanadium trioxide intercalated with phase transformation for advanced aqueous zinc-ion batteries. J Energy Chem. 2021;61:594.10.1016/j.jechem.2021.02.014Search in Google Scholar
[88] Li G, Yang Z, Jiang Y, Jin C, Huang W, Ding X, et al. Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3. Nano Energy. 2016;25:211.10.1016/j.nanoen.2016.04.051Search in Google Scholar
[89] Zeng Y, Xu J, Wang Y, Li S, Luan D, Lou X. Formation of CuMn prussian blue analog double-shelled nanoboxes toward long-life Zn-ion batteries. Angew Chem Int Ed. 2022;61:e202212031.10.1002/anie.202212031Search in Google Scholar PubMed
[90] Zhu X, Cao Z, Li X, Pei L, Jones J, Zhou Y, et al. Ion-intercalation regulation of MXene-derived hydrated vanadates for high-rate and long-life Zn-ion batteries. Energy Storage Mater. 2022;45:568.10.1016/j.ensm.2021.12.002Search in Google Scholar
[91] Li X, Li M, Yang Q, Li H, Xu H, Chai Z, et al. Phase transition induced unusual electrochemical performance of V2CTx MXene for aqueous zinc hybrid-ion battery. ACS Nano. 2020;14:541.10.1021/acsnano.9b06866Search in Google Scholar PubMed
[92] Zhu X, Cao Z, Wang W, Li H, Dong J, Gao S, et al. Superior-performance aqueous zinc-ion batteries based on the in situ growth of MnO2 nanosheets on V2CTx MXene. ACS Nano. 2021;15:2971.10.1021/acsnano.0c09205Search in Google Scholar PubMed
[93] Shi H, Ye Y, Liu K, Song Y, Sun X. A long-cycle-life self-doped polyaniline cathode for rechargeable aqueous zinc batteries. Angew Chem Int Ed. 2018;57:16359.10.1002/anie.201808886Search in Google Scholar PubMed
[94] Zhang L, Chen Y, Jiang Z, Chen J, Wei C, Wu W, et al. Cation‐anion redox active organic complex for high performance aqueous zinc ion battery. Energy Environ Mater. 2022;e12507.10.1002/eem2.12507Search in Google Scholar
[95] Wang W, Kale VS, Cao Z, Lei Y, Kandambeth S, Zou G, et al. Molecular engineering of covalent organic framework cathodes for enhanced zinc-ion batteries. Adv Mater. 2021;33:2103617.10.1002/adma.202103617Search in Google Scholar PubMed
[96] Zhang T, Tang Y, Fang G, Zhang C, Zhang H, Guo X, et al. Electrochemical activation of manganese‐based cathode in aqueous zinc‐ion electrolyte. Adv Funct Mater. 2020;30:2002711.10.1002/adfm.202002711Search in Google Scholar
[97] Zhao Y, Zhang P, Liang J, Xia X, Ren L, Song L, et al. Uncovering sulfur doping effect in MnO2 nanosheets as an efficient cathode for aqueous zinc ion battery. Energy Storage Mater. 2022;47:424.10.1016/j.ensm.2022.02.030Search in Google Scholar
[98] Wang J, Wang J, Qin X, Wang Y, You Z, Liu H, et al. Superfine MnO2 nanowires with rich defects toward boosted zinc ion storage performance. ACS Appl Mater Interfaces. 2020;12:34949.10.1021/acsami.0c08812Search in Google Scholar PubMed
[99] Zhang Y, Liu Y, Liu Z, Wu X, Wen Y, Chen H, et al. MnO2 cathode materials with the improved stability via nitrogen doping for aqueous zinc-ion batteries. J Energy Chem. 2022;64:23.10.1016/j.jechem.2021.04.046Search in Google Scholar
[100] Shen X, Wang X, Zhou Y, Shi Y, Zhao L, Jin H, et al. Highly reversible aqueous Zn‐MnO2 battery by supplementing Mn2+‐mediated MnO2 deposition and dissolution. Adv Funct Mater. 2021;31:2101579.10.1002/adfm.202101579Search in Google Scholar
[101] Li S, Yu D, Liu L, Yao S, Wang X, Jin X, et al. In-situ electrochemical induced artificial solid electrolyte interphase for MnO@C nanocomposite enabling long-lived aqueous zinc-ion batteries. Chem Eng J. 2022;430:132673.10.1016/j.cej.2021.132673Search in Google Scholar
[102] Fenta FW, Olbasa BW, Tsai M-C, Weret MA, Zegeye TA, Huang C, et al. Electrochemical transformation reaction of Cu-MnO in aqueous rechargeable zinc-ion batteries for high performance and long cycle life. J Mater Chem A. 2020;8:17595.10.1039/D0TA04175KSearch in Google Scholar
[103] Yang H, Zhou W, Chen D, Liu J, Yuan Z, Lu M, et al. The origin of capacity fluctuation and rescue of dead Mn-based Zn-ion batteries: a Mn-based competitive capacity evolution protocol. Energy Environ Sci. 2022;15:1106.10.1039/D1EE03547ASearch in Google Scholar
[104] Zhang N, Wang J, Guo Y, Wang P, Zhu Y, Yi T. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous zinc-ion batteries. Coord Chem Rev. 2023;479:215009.10.1016/j.ccr.2022.215009Search in Google Scholar
[105] Fang G, Zhu C, Chen M, Zhou J, Tang B, Cao X, et al. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery. Adv Funct Mater. 2019;29:1808375.10.1002/adfm.201808375Search in Google Scholar
[106] Du M, Miao Z, Li H, Sang Y, Liu H, Wang S. Strategies of structural and defect engineering for high-performance rechargeable aqueous zinc-ion batteries. J Mater Chem A. 2021;9:19245.10.1039/D1TA03620CSearch in Google Scholar
[107] Soundharrajan V, Sambandam B, Kim S, Islam S, Jo J, Kim S, et al. The dominant role of Mn2+ additive on the electrochemical reaction in ZnMn2O4 cathode for aqueous zinc-ion batteries. Energy Storage Mater. 2020;28:407.10.1016/j.ensm.2019.12.021Search in Google Scholar
[108] Ren J, Wang Z, Xu P, Wang C, Gao F, Zhao D, et al. Porous Co2VO4 nanodisk as a high-energy and fast-charging anode for lithium-ion batteries. Nano-Micro Lett. 2021;14:5.10.1007/s40820-021-00758-5Search in Google Scholar PubMed PubMed Central
[109] Luo H, Wang B, Wang C, Wu F, Jin F, Cong B, et al. Synergistic deficiency and heterojunction engineering boosted VO2 redox kinetics for aqueous zinc-ion batteries with superior comprehensive performance. Energy Storage Mater. 2020;33:390.10.1016/j.ensm.2020.08.011Search in Google Scholar
[110] Zhu K, Wei S, Shou H, Shen F, Chen S, Zhang P, et al. Defect engineering on V2O3 cathode for long-cycling aqueous zinc metal batteries. Nat Commun. 2021;12:6878.10.1038/s41467-021-27203-wSearch in Google Scholar PubMed PubMed Central
[111] Luo H, Wang B, Wang F, Yang J, Wu F, Ning Y, et al. Anodic oxidation strategy toward structure-optimized V2O3 cathode via electrolyte regulation for Zn-ion storage. ACS Nano. 2020;14:7328.10.1021/acsnano.0c02658Search in Google Scholar PubMed
[112] Javed MS, Lei H, Wang Z, Liu B, Cai X, Mai W. 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries. Nano Energy. 2020;70:104573.10.1016/j.nanoen.2020.104573Search in Google Scholar
[113] Lu Y, Wen Y, Huang F, Zhu T, Sun S, Benicewicz BC, et al. Rational design and demonstration of a high-performance flexible Zn/V2O5 battery with thin-film electrodes and para-polybenzimidazole electrolyte membrane. Energy Storage Mater. 2020;27:418.10.1016/j.ensm.2020.02.016Search in Google Scholar
[114] Wang X, Ye L, Zou Y, Zhao L, Jiang Q. Constructing ultra-long life and super-rate rechargeable aqueous zinc-ion batteries by integrating Mn doped V6O13 nanoribbons with sulfur-nitrogen modified porous carbon. Mater Today Energy. 2021;19:10059310.1016/j.mtener.2020.100593Search in Google Scholar
[115] He P, Liu J, Zhao X, Ding Z, Gao P, Fan L-Z. A three-dimensional interconnected V6O13 nest with a V5+-rich state for ultrahigh Zn ion storage. J Mater Chem A. 2020;8:10370.10.1039/D0TA03165HSearch in Google Scholar
[116] Guo C, Yi S, Si R, Xi B, An X, Liu J, et al. Advances on defect engineering of vanadium-based compounds for high-energy aqueous zinc-ion batteries. Adv Energy Mater. 2022;12:2202039.10.1002/aenm.202202039Search in Google Scholar
[117] Pang Z, Ding B, Wang J, Wang Y, Xu L, Zhou L, et al. Metal-ion inserted vanadium oxide nanoribbons as high-performance cathodes for aqueous zinc-ion batteries. Chem Eng J. 2022;446:136861.10.1016/j.cej.2022.136861Search in Google Scholar
[118] Gao J, Cheng C, Ding L, Liu G, Yan T, Zhang L. Synergistic interlayer and defect engineering of hydrated vanadium oxide toward stable Zn-ion batteries. Chem Eng J. 2022;450:138367.10.1016/j.cej.2022.138367Search in Google Scholar
[119] Feng Z, Zhang Y, Sun J, Liu Y, Jiang H, Cui M, et al. Dual ions enable vanadium oxide hydration with superior Zn2+ storage for aqueous zinc-ion batteries. Chem Eng J. 2022;433:133795.10.1016/j.cej.2021.133795Search in Google Scholar
[120] Qi Y, Huang J, Yan L, Cao Y, Xu J, Bin D, et al. Towards high-performance aqueous zinc-ion battery via cesium ion intercalated vanadium oxide nanorods. Chem Eng J. 2022;442:136349.10.1016/j.cej.2022.136349Search in Google Scholar
[121] Guan J, Shao L, Yu L, Wang S, Shi X, Cai J, et al. Two-dimensional Mg0.2V2O5·nH2O nanobelts derived from V4C3 MXenes for highly stable aqueous zinc ion batteries. Chem Eng J. 2022;443:136502.10.1016/j.cej.2022.136502Search in Google Scholar
[122] Wan F, Hao Z, Wang S, Ni Y, Zhu J, Tie Z, et al. A universal compensation strategy to anchor polar organic molecules in bilayered hydrated vanadates for promoting aqueous zinc-ion storage. Adv Mater. 2021;33:e2102701.10.1002/adma.202102701Search in Google Scholar PubMed
[123] Liu H, Jiang L, Cao B, Du H, Lu H, Ma Y, et al. Van der waals interaction-driven self-assembly of V2O5 nanoplates and MXene for high-performing zinc-ion batteries by suppressing vanadium dissolution. ACS Nano. 2022;16:14539.10.1021/acsnano.2c04968Search in Google Scholar PubMed
[124] Chen L, Yue H, Zhang Z, Ma Y, Wang Y, Xu M, et al. Pre-removing partial ammonium ions from the interlayer of ammonium vanadate with acid treating for quasi-solid-state flexible zinc ion batteries. Chem Eng J. 2023;455:140679.10.1016/j.cej.2022.140679Search in Google Scholar
[125] Guan J, Huang Q, Shao L, Shi X, Zhao D, Wang L, et al. Polyanion-type Na3V2(PO4)2F3@rGO with high-voltage and ultralong-life for aqueous zinc ion batteries. Small. 2023;19:e2207148.10.1002/smll.202207148Search in Google Scholar PubMed
[126] Zhang L, Hu J, Zhang B, Liu J, Wan H, Miao L, et al. Suppressing cathode dissolution via guest engineering for durable aqueous zinc-ion batteries. J Mater Chem A. 2021;9:7631.10.1039/D1TA00263ESearch in Google Scholar
[127] Mei Y, Liu Y, Xu W, Zhang M, Dong Y, Qiu J. Suppressing vanadium dissolution in 2D V2O5/MXene heterostructures via organic/aqueous hybrid electrolyte for stable zinc ion batteries. Chem Eng J. 2023;452:139574.10.1016/j.cej.2022.139574Search in Google Scholar
[128] Gu Z, Guo J, Sun Z, Zhao X, Wang X, Liang H, et al. Air/water/temperature-stable cathode for all-climate sodium-ion batteries. Cell Reports Physical. Science. 2021;2:100665.10.1016/j.xcrp.2021.100665Search in Google Scholar
[129] Gu Z, Heng Y, Guo J, Cao J, Wang X, Zhao X, et al. Nano self-assembly of fluorophosphate cathode induced by surface energy evolution towards high-rate and stable sodium-ion batteries. Nano Res. 2022;16:439.10.1007/s12274-022-4687-6Search in Google Scholar
[130] Hurlbutt K, Wheeler S, Capone I, Pasta M. Prussian blue analogs as battery materials. Joule. 2018;2:1950.10.1016/j.joule.2018.07.017Search in Google Scholar
[131] Sun Y, Xu Z, Xu X, Nie Y, Tu J, Zhou A, et al. Low-cost and long-life Zn/Prussian blue battery using a water-in-ethanol electrolyte with a normal salt concentration. Energy Storage Mater. 2022;48:192.10.1016/j.ensm.2022.03.023Search in Google Scholar
[132] Wang Q, Li J, Jin H, Xin S, Gao H. Prussian-blue materials: revealing new opportunities for rechargeable batteries. Inf Mater. 2022;4:e12311.10.1002/inf2.12311Search in Google Scholar
[133] Peng J, Zhang W, Liu Q, Wang J, Chou S, Liu H, et al. Prussian blue analogues for sodium‐ion batteries: past, present, and future. Adv Mater. 2021;34:2108384.10.1002/adma.202108384Search in Google Scholar PubMed
[134] Xue Y, Shen X, Zhou H, Cao J, Pu J, Ji Z, et al. Vanadium hexacyanoferrate nanoparticles connected by cross-linked carbon nanotubes conductive networks for aqueous zinc-ion batteries. Chem Eng J. 2022;448:137657.10.1016/j.cej.2022.137657Search in Google Scholar
[135] Shi M, Wang B, Shen Y, Jiang J, Zhu W, Su Y, et al. 3D assembly of MXene-stabilized spinel ZnMn2O4 for highly durable aqueous zinc-ion batteries. Chem Eng J. 2020;399:125627.10.1016/j.cej.2020.125627Search in Google Scholar
[136] Sha D, Lu C, He W, Ding J, Zhang H, Bao Z, et al. Surface selenization strategy for V2CTx MXene toward superior Zn-ion storage. ACS Nano. 2022;16:2711.10.1021/acsnano.1c09639Search in Google Scholar PubMed
[137] Cao J, Zatovsky IV, Gu Z, Yang J, Zhao X, Guo J, et al. Two-dimensional MXene with multidimensional carbonaceous matrix: a platform for general-purpose functional materials. Prog Mater Sci. 2023;135:101105.10.1016/j.pmatsci.2023.101105Search in Google Scholar
[138] Dadashi Firouzjaei M, Karimiziarani M, Moradkhani H, Elliott M, Anasori B. MXenes: the two-dimensional influencers. Mater Today Adv. 2022;13:100202.10.1016/j.mtadv.2021.100202Search in Google Scholar
[139] Jiang J, Bai S, Zou J, Liu S, Hsu J-P, Li N, et al. Improving stability of MXenes. Nano Res. 2022;15:6551.10.1007/s12274-022-4312-8Search in Google Scholar
[140] Aslam MK, Niu Y, Xu M. MXenes for non-lithium-ion (Na, K, Ca, Mg, and Al) batteries and supercapacitors. Adv Energy Mater. 2021;11:2000681.10.1002/aenm.202000681Search in Google Scholar
[141] Li X, Huang Z, Shuck CE, Liang G, Gogotsi Y, Zhi C. MXene chemistry, electrochemistry and energy storage applications. Nat Rev Chem. 2022;6:389.10.1038/s41570-022-00384-8Search in Google Scholar PubMed
[142] Sun S, Liao C, Hafez AM, Zhu H, Wu S. Two-dimensional MXenes for energy storage. Chem Eng J. 2018;338:27.10.1016/j.cej.2017.12.155Search in Google Scholar
[143] Yuan W, Yang K, Peng H, Li F, Yin F. A flexible VOCs sensor based on a 3D Mxene framework with a high sensing performance. J Mater Chem A. 2018;6:18116.10.1039/C8TA06928JSearch in Google Scholar
[144] Zhou Y, Cheng W, Bai Y, Hou C, Li K, Huang Y. Rise of flexible high-temperature electronics. Rare Met. 2023;42:1773.10.1007/s12598-023-02298-wSearch in Google Scholar
[145] Shi J, Hou Y, Liu Z, Zheng Y, Wen L, Su J, et al. The high-performance MoO3-x/MXene cathodes for zinc-ion batteries based on oxygen vacancies and electrolyte engineering. Nano Energy. 2022;91:106651.10.1016/j.nanoen.2021.106651Search in Google Scholar
[146] Liang P, Xu T, Zhu K, Rao Y, Zheng H, Wu M, et al. Heterogeneous interface-boosted zinc storage of H2V3O8 nanowire/Ti3C2Tx MXene composite toward high-rate and long cycle lifespan aqueous zinc-ion batteries. Energy Storage Mater. 2022;50:63.10.1016/j.ensm.2022.05.010Search in Google Scholar
[147] Huang Y, Yang H, Zhang Y, Zhang Y, Wu Y, Tian M, et al. A safe and fast-charging lithium-ion battery anode using MXene supported Li3VO4. J Mater Chem A. 2019;7:11250.10.1039/C9TA02037CSearch in Google Scholar
[148] Li X, Li M, Yang Q, Liang G, Huang Z, Ma L, et al. In situ electrochemical synthesis of MXenes without acid/alkali usage in/for an aqueous zinc ion battery. Adv Energy Mater. 2020;10:2001791.10.1002/aenm.202001791Search in Google Scholar
[149] Yu F, Wang Y, Liu Y, Hui H, Wang F, Li J, et al. An aqueous rechargeable zinc-ion battery on basis of an organic pigment. Rare Met. 2022;41:2230.10.1007/s12598-021-01941-8Search in Google Scholar
[150] Zhou W, Cao G, Yuan M, Zhong S, Wang Y, Liu X, et al. Core-shell engineering of conductive fillers toward enhanced dielectric properties: a universal polarization mechanism in polymer conductor composites. Adv Mater. 2023;35:2207829.10.1002/adma.202207829Search in Google Scholar PubMed
[151] Sun T, Zhang W, Nian Q, Tao Z. Molecular engineering design for high-performance aqueous zinc-organic battery. Nanomicro Lett. 2023;15:36.10.1007/s40820-022-01009-xSearch in Google Scholar PubMed PubMed Central
[152] Mao M, Wu X, Hu Y, Yuan Q, He Y, Kang F. Charge storage mechanism of MOF-derived Mn2O3 as high performance cathode of aqueous zinc-ion batteries. J Energy Chem. 2021;52:277.10.1016/j.jechem.2020.04.061Search in Google Scholar
[153] Nam KW, Park SS, Dos Reis R, Dravid VP, Kim H, Mirkin CA, et al. Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries. Nat Commun. 2019;10:4948.10.1038/s41467-019-12857-4Search in Google Scholar PubMed PubMed Central
[154] Luo P, Xiao Y, Yang J, Zuo C, Xiong F, Tang C, et al. Polyaniline nanoarrays/carbon cloth as binder-free and flexible cathode for magnesium ion batteries. Chem Eng J. 2022;433:133772.10.1016/j.cej.2021.133772Search in Google Scholar
[155] Liu Y, Dai Z, Zhang W, Jiang Y, Peng J, Wu D, et al. Sulfonic-group-grafted Ti3C2Tx mxene: a silver bullet to settle the instability of polyaniline toward high-performance Zn-ion batteries. ACS Nano. 2021;15:9065.10.1021/acsnano.1c02215Search in Google Scholar PubMed
[156] Du M, He D, Lou Y, Chen J. Porous nanostructured ZnCo2O4 derived from MOF-74: High-performance anode materials for lithium ion batteries. J Energy Chem. 2017;26:673.10.1016/j.jechem.2017.02.001Search in Google Scholar
[157] Wang L, Han Z, Zhao Q, Yao X, Zhu Y, Ma X, et al. Engineering yolk-shell P-doped NiS2/C spheres via a MOF-template for high-performance sodium-ion batteries. J Mater Chem A. 2020;8:8612.10.1039/D0TA02568BSearch in Google Scholar
[158] Zhong M, Kong L, Li N, Liu Y, Zhu J, Bu X. Synthesis of MOF-derived nanostructures and their applications as anodes in lithium and sodium ion batteries. Coord Chem Rev. 2019;388:172.10.1016/j.ccr.2019.02.029Search in Google Scholar
[159] Yang M, Duan C, Zeng X, Li J, Liu C, Zeng L, et al. Facile fabrication of nanoscale hierarchical porous zeolitic imidazolate frameworks for enhanced toluene adsorption capacity. Rare Met. 2021;40:471.10.1007/s12598-020-01455-9Search in Google Scholar
[160] Pu X, Jiang B, Wang X, Liu W, Dong L, Kang F, et al. High-performance aqueous zinc-ion batteries realized by MOF materials. Nano-Micro Lett. 2020;12:152.10.1007/s40820-020-00487-1Search in Google Scholar PubMed PubMed Central
[161] Liu X, Wang S, Wang A, Wang Z, Chen J, Zeng Q, et al. A new cathode material synthesized by a thiol-modified metal–organic framework (MOF) covalently connecting sulfur for superior long-cycling stability in lithium–sulfur batteries. J Mater Chem A. 2019;7:24515.10.1039/C9TA08043KSearch in Google Scholar
[162] Kong N, Du H, Li Z, Lu T, Xia S, Tang Z, et al. Nano heterojunction of double MOFs for improved CO2 photocatalytic reduction performance. Colloids Surf A: Physicochem Eng Asp. 2023;663:131005.10.1016/j.colsurfa.2023.131005Search in Google Scholar
[163] Zhu Z, Lin Z, Sun Z, Zhang P, Li C, Dong R, et al. Deciphering H+/Zn2+ co-intercalation mechanism of MOF-derived 2D MnO/C cathode for long cycle life aqueous zinc-ion batteries. Rare Met. 2022;41:3729.10.1007/s12598-022-02088-wSearch in Google Scholar
[164] Deng S, Yuan Z, Tie Z, Wang C, Song L, Niu Z. Electrochemically induced metal-organic-framework-derived amorphous V2O5 for superior rate aqueous zinc-ion batteries. Angew Chem Int Ed. 2020;59:22002.10.1002/anie.202010287Search in Google Scholar PubMed
[165] Jiang Q, Li Y, Zhao X, Xiong P, Yu X, Xu Y, et al. Inverse-vulcanization of vinyl functionalized covalent organic frameworks as efficient cathode materials for Li-S batteries. J Mater Chem A. 2018;6:17977.10.1039/C8TA07008CSearch in Google Scholar
[166] Su Z, Huang J, Wang R, Zhang Y, Zeng L, Zhang Y, et al. Multilayer structure covalent organic frameworks (COFs) linking by double functional groups for advanced K+ batteries. J Colloid Interface Sci. 2023;639:7.10.1016/j.jcis.2023.02.012Search in Google Scholar PubMed
[167] Zhang J, Han X, Wu X, Liu Y, Cui Y. Multivariate chiral covalent organic frameworks with controlled crystallinity and stability for asymmetric catalysis. J Am Chem Soc. 2017;139:8277.10.1021/jacs.7b03352Search in Google Scholar PubMed
[168] Fan H, Mundstock A, Feldhoff A, Knebel A, Gu J, Meng H, et al. Covalent organic framework-covalent organic framework bilayer membranes for highly selective gas separation. J Am Chem Soc. 2018;140:10094.10.1021/jacs.8b05136Search in Google Scholar PubMed
[169] Zhang Y, Ma L, Lv Y, Tan T. Facile manufacture of COF-based mixed matrix membranes for efficient CO2 separation. Chem Eng J. 2022;430:133001.10.1016/j.cej.2021.133001Search in Google Scholar
[170] Fu S, Jin E, Hanayama H, Zheng W, Zhang H, Di Virgilio L, et al. Outstanding charge mobility by band transport in two-dimensional semiconducting covalent organic frameworks. J Am Chem Soc. 2022;144:7489.10.1021/jacs.2c02408Search in Google Scholar PubMed PubMed Central
[171] Huang W, Luo W, Li Y. Two-dimensional semiconducting covalent organic frameworks for photocatalytic solar fuel production. Mater Today. 2020;40:160.10.1016/j.mattod.2020.07.003Search in Google Scholar
[172] Zou L, Sa R, Zhong H, Lv H, Wang X, Wang R. Photoelectron transfer mediated by the interfacial electron effects for boosting visible-light-driven CO2 reduction. ACS Catal. 2022;12:3550.10.1021/acscatal.1c05449Search in Google Scholar
[173] Sun T, Xie J, Guo W, Li D, Zhang Q. Covalent-organic frameworks: advanced organic electrode materials for rechargeable batteries. Adv Energy Mater. 2020;10:1904199.10.1002/aenm.201904199Search in Google Scholar
[174] Zhang S, Long S, Li H, Xu Q. A high-capacity organic cathode based on active N atoms for aqueous zinc-ion batteries. Chem Eng J. 2020;400:125898.10.1016/j.cej.2020.125898Search in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

