Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
-
Hamdoon A. Mohammed
, Varsha Singh
, Salim Albukhaty
Abstract
Solid lipid nanoparticles (SLNs), the spheroidal-shaped, colloids state lipophilic-natured, innovative nanoscale particulate materials, are being concurrently prepared by the quality-by-design approach for cellular and sub-cellular delivery of drugs and other payloads with facilitated physicochemical characteristics for targeted delivery. The delivery of drugs, other pharmaceuticals and biopharmaceutical materials, and genes to the diseased body organs, tissues, and cellular mass have been developed as promising nanocarriers for different high-incidence cancers and other disease therapies, including the Alzheimer’s, Parkinson’s, and tuberculosis. SLNs have evolved as favorable lipid-based formulation, and have served as oral and intravenous carriers that targeted the drug with stable and sterile transport, sustained delivery, controlled drug/payload deloading, and requisite biodistributions. SLNs advantages, shortcomings, and bottlenecks have been discussed with plausible remediation strategies. The laboratory-scale and bulk preparations, use of different lipids in various preparation, surface coatings, physicochemical properties of the final product, and characterization protocols are also encompassed, as are the routes of administrations, specific-sites-targeting, and on-site outreach with biocompatibility, bioavailability, and the absorption, distribution, metabolism, and excretion and pharmacokinetics, and pharmacodynamics inputs with relevance to the therapy. Plausible applications in complex and genetic disorders, and as personalized medicine, also of traditional and alternative medicine prospects, are also discussed.
Graphical abstract
1 Introduction
1.1 Solid lipid nanoparticles (SLNs) developmental perspective and concurrent applications
SLNs came into being in 1991, and ever since have captured the major share in drug and other payload delivery modules. SLNs were developed in place of the conventional existing colloidal systems, i.e., emulsions, liposomes, and polymeric nanoparticles (NPs) [1,2,3,4,5,6,7,8,9]. SLNs are also spherical in shape with average size range variations from 10 to 1,000 nm. These NPs were primarily made from the natural and synthetic lipids as the major constituent (Figure 1). SLNs have been established as preferred carriers, especially, for the lipophilic drugs for the high-yielding, efficient, and optimized drug delivery of the encapsulated/entered drugs with enhanced bioavailability. SLNs also provided toxicity reduction at the site of action of the drug, as well as least interfered and toxicity during transport latency of the drug-loaded NPs to the site. SLNs have also emerged as a parallel substitute for liposomal deliveries owing to their better penetration through anatomical restrictions, sustained, and to some extent chrono-control deliveries. SLNs also provided, the much sought after, stability of the carrier from its biochemical and physicochemical damage and degradation while under transport in the biosystems [10,11]. Hence, SLNs are considered a novel approach for controlled and directed drug delivery systems in contrast to the direct oral and parenteral administrations of a drug. The troubles associated with the conventional drug delivery systems such as stability, toxication, and degradability were, to a major extent, overcome by SLNs. The scarcity of safe synthetic and natural polymers with regulatory approvals, and high preparative/production costs limited the other polymeric NPs modules used for drug delivery. The advancing clinical applications catapult the opening up of the possibilities for SLNs as the preferred delivery format for a number of difficult to deliver, especially, lipophilic drugs [12].
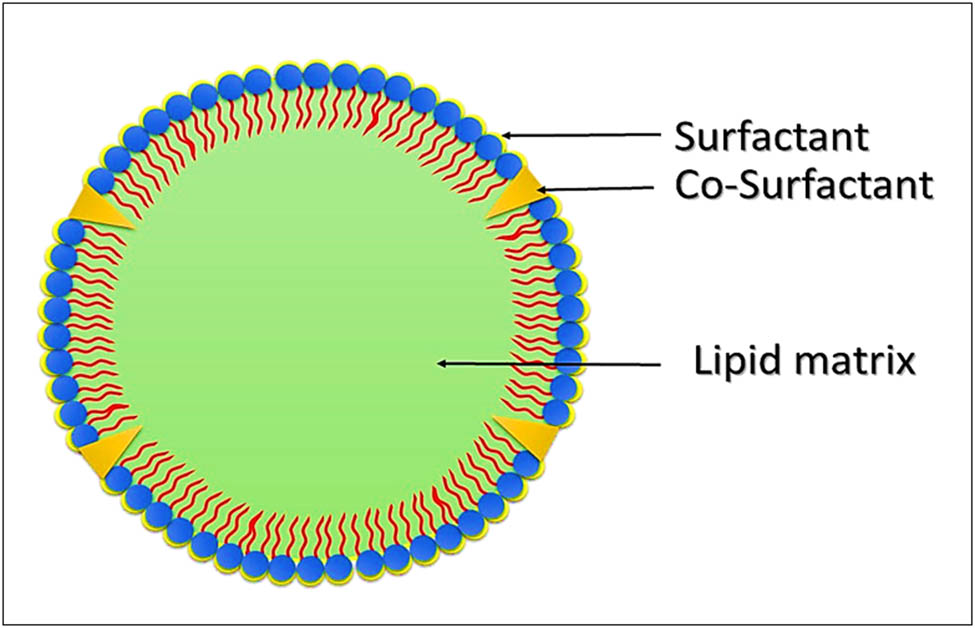
Compositional lay-out of the structure of a typical SLN showing the lipid core and the peripheral surfactant/co-surfactant, the drug may or may not, based on QbD approach, concentrate on the peripheries, or at the core.
SLNs, conceptually, are a hybrid of the lipid suspensions and polymeric nanoparticles, and have successfully embedded both polarity types of drugs, the lipophilic and hydrophilic drugs. Thus, the drug-embedded SLNs have, comparatively, provided better stability in bio-environments during transfer/transport, and mobility of SLNs. The controlled and targeted drug delivery with precise specificity concerning the nature of the site has thus been achieved. SLNs’ size-dependent physicochemical characteristics, delivery preferences, transport feasibility, and the site-specific outreach have opened up a plethora of possibilities in types and classes of drug’s loading, drug’s loading capacity, encapsulation efficiency, choices with physicochemical and polarity-based drug types, together with other tunable properties of SLNs, and have assisted the drugs and other pharmaceuticals, including nutraceuticals to reach the intended site in adequate dose and enough/necessitated bioavailability. The differentiated SLNs prototypes, developed over time with specifications in coating and conjugation approaches for site-directedness, have efficiently targeted the intended drugs to effectively outreach to the site with chronological-control and dose-manipulated deliveries. In this context, different make-up of SLN carriers have also been tested for intravenous and other deliveries, including the oral route deliveries. The advantages, shortcomings, demerits, and bottlenecks of SLNs are here-upon detailed out with plausible remediation strategies, and forms the subject matter of this write-up [13,14].
SLNs laboratory scale and bulk preparations, their applications, and the use of different lipids in preparation, the physicochemical properties of SLNs product, characterization protocols, and involved instrumental techniques are also highlighted, as are the preferred and employed routes of administrations, site-outreach, specific site targeting, biodistribution, and the bioavailability of the drug along with the mechanistic of the delivery and targeting, the pharmacokinetics, the pharmacodynamics, and the drugs absorption, distribution, metabolism, and excretion (ADME) inputs are encompassed. SLNs in vitro and in vivo applications, clinical and pre-clinical applications toward the development of the therapy of diseases, and their plausible applications in complex and genetic disorders, with a feasibility assessment of SLN-based drugs’ bioactivity applications, wherein other delivery modes and carriers are unreachable or restricted, have been encompassed [15]. SLNs preparations are an important and desirable dimension to the lipid-based NPs nanotechnology pitch, where prospective scenarios in drug supplies, clinico-medical treatment, and the path forward to additional research areas are critical to the development of the field of drug delivery and lipid-based nanotechnology applications [16,17,18,19]. Among the major aims of this review was also to illustrate different preparative parameters of SLNs, with their preparative techniques, the benefits and disadvantages, and the role of the preparative procedures in it. Also, various characterization techniques for establishing SLNs preparation, identity, drug-loading, and delivery confirmations have been detailed out. Additionally, the bulk manufacturing of SLNs and their operational feasibility has been hinted out. SLNs spherical shapes, lipid matrices nature, which are solid at physiological temperature, the surfactants and the occasionally used co-surfactants, co-surfactants that are used to solidify and stabilize the lipid matrices, have been identified. SLNs made from solid lipids, such as, long-chain fatty acids, and certain other natural and synthetic waxes that are solid and compacted between 25 and 37°C, provided desired characteristics to the lipidic-carrier for specified, targeted delivery through quality by design (QbD) preparative and drug loading approaches, which have provided intended release profile of the encapsulated drugs. The lipid-based solid core also assisted in better characterizations of the lipid excipients, and loading of the drug(s). SLNs that have been further treated by stabilizers and stabilizers that are biological membrane-based lipids helped SLNs to be biologically non-toxic, devoid of adverse reactions owing to the lipid carrier, and stable enough for further clinical applications (Figure 2) [20,21]. For example, SLNs prepared for targeted delivery of piribedil for treatment of neurodegenerative brain disorder were observed to be comparatively faster in drug deloading in an acidic medium, and that was ascertained to be dependent upon the desirable range of the NPs’ sizes that were able to cross the blood–brain barrier (BBB), and the pH value which assisted in the drug release through opening of SLNs at the site. The factors of the size and pH considerably affected the release rate and duration/sustainability of the drug release. The in vitro and in vivo analyses proved that these SLNs had controlled drug release rates, with improved piribedil bioavailability and tissue/cellular uptake of the drug [22,23].
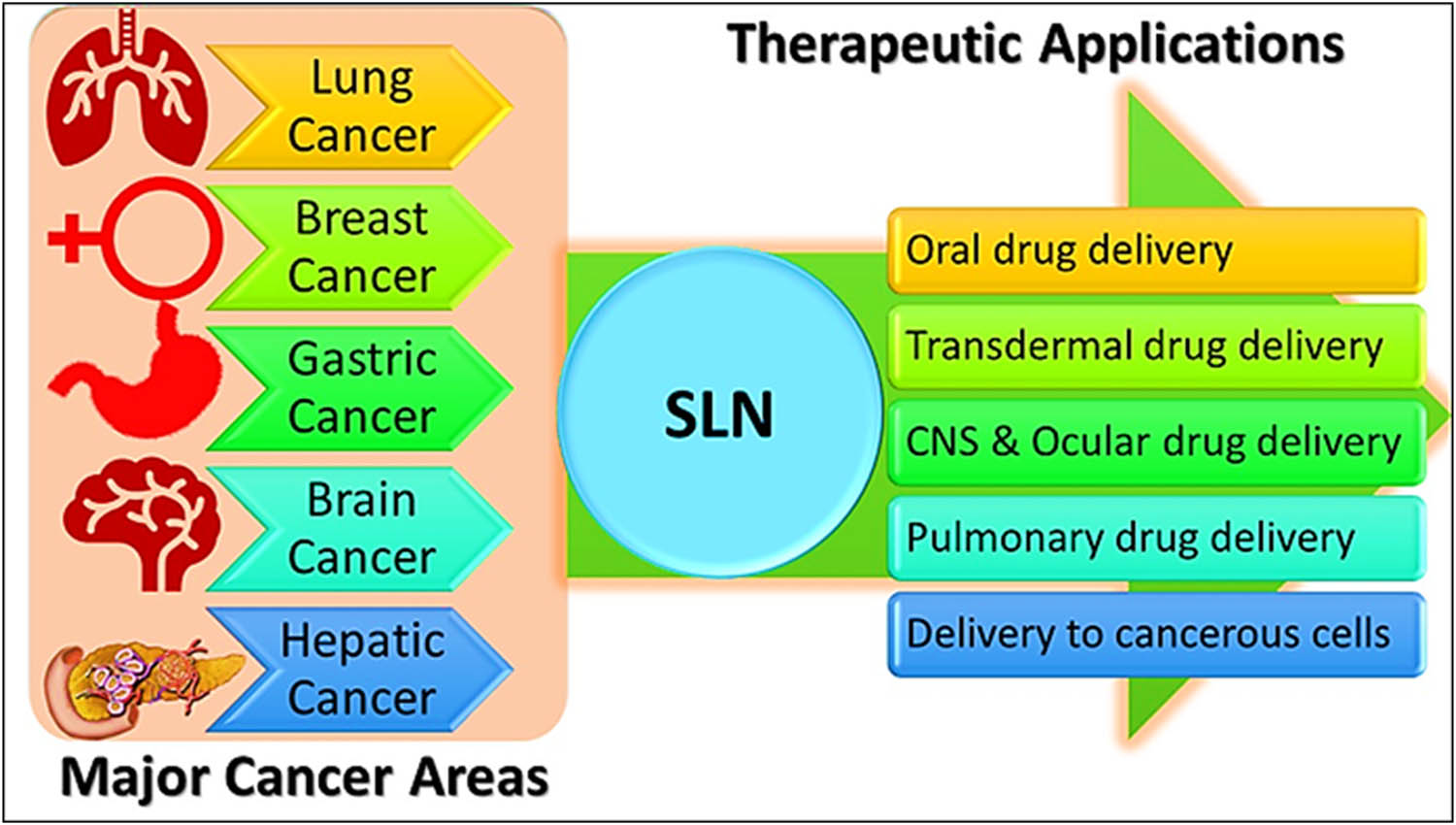
Major therapeutic applications of SLNs in high-incidence cancers, and their delivery through different alternatives.
SLNs have still been considered as highly biocompatible, and in vitro studies have confirmed this aspect of SLNs characteristics [24]. Studies have also demonstrated that absorption mechanism of SLNs against the polymeric NPs, as well as the oil (lipid), and water-based suspensions tend to be providing stable adsorption patterns for the SLNs. The interaction between the plasma and the polymer NPs, and oil and water-based suspensions seemed to be the deciding factor in drug loading and delivery patterns [25]. SLNs have been demonstrated to load higher quantities of the drug (drug volumes) than the polymeric NPs and nanoemulsions that are not primarily lipid based. Also, from the manufacturing and commercial stand-point, the low manufacturing costs without using any organic solvents, with enhanced feasibility of maintenance of the sterility of SLNs formulations [26] have added to the SLNs’ advantages. On the contrary, the longer storage time and long duration sitting time of the drug in SLNs formulations have the tendency to lead to several disadvantages [27], including changes in drug release patterns and particles’ size growth, and occurrence of polymorphic transition are too high, together with gelation occurrences that are quite often encountered and are of high incidence for SLNs.
In the backdrop of these contrasting situations, as against the non-lipid nanocarriers, SLN modules of drug delivery provided several advantages, and also negated the shortcomings of other colloidal preparations, although the bottlenecks are, again, the expulsion of the drug after the process of SLNs preparation and comparatively longer storage time, together with poor drug-loading, again, along with comparatively high water contents in SLNs, if seeped through the process of preparation owing to SLNs preparative constituents behavior, although this water content is comparatively very low as against other non-lipid nanocarriers’ water content in the formulation, but, nonetheless, this water content is of higher ratio when compared to different SLN types. This behavior has questioned the controls of the preparative process and the nature of the lipid used to prepare SLNs, and this has paved ways for different preparative processes development, and use of various lipid types. Notwithstanding, the normal drug loading, in SLNs, is dependent upon the solubility of the drug in the lipid medium which is facilitated or restricted by the physicochemical properties of the lipid, the properties that arise out of the lipids’ chemical structure, physicochemical properties, and the state of the lipid matrices employed in the preparation. The tristearin and tripalmitin, being in the familiar (polymeric) state, provided better SLN-based products with little or no imperfections in the produced SLNs [28,29].
The loaded drug molecules reside in the molecular spaces of the fats’ polymeric chains, in between the layers of the lipid chain and the lipid matrices, and also the crystal lattices of the drug substance during the heating and thaw process of the preparation/manufacturing of SLNs. The procedure provided diminished drug loading in the crystal lattice, and structurally imperfect crystal orders were achieved. The crystalline drug material, for this reason, has not been the preferred physical state to prepare SLNs. Henceforth, the use of physico-chemically compatible, structurally complex but accommodating materials (i.e., lipids, etc.) with sufficient molecular spaces and structural orientations lead to provide cavities for drug loadings, and non-crystalline/amorphous natured lipids have also been preferred for the purpose [13,30].
Nonetheless, SLNs provided controlled and targeted drug delivery to the required site(s) via a compatible and competitive release profile, improved the stability of the drug and SLNs formulation from chemical, biochemical, and physiological degradations, with utmost negation to the sun and other light sources, and photocatalysis [31,32,33]. The inhibition of auto-redox processes during transport and delivery at the site, SLNs’ inability to sneak through the reticuloendothelial system (RES) owing to their sizes, the bypassing of the spleen and liver filtrations, reduced levels of drug leaks from the nanocarrier, the comparatively higher drug contents loading of both the lipophilic and hydrophilic-natured drugs, the biocompatibility to the biological system on account of the biodegradability of the lipids, feasible sterilization, the devouring of the organic solvents with the chances of removing solvent-borne toxicity, and the overall lessened toxicity of SLNs and the un-exposed drug favored SLNs delivery module. SLNs that were simple to prepare, and cost-effective, with better validations of the final product and the process, eventually assisted in obtaining regulatory approvals. Moreover, some of the shortcomings were overcome by the size limitation and the use of certain types of lipids during the preparation of SLNs [34,35,36]. The deloading up of the loaded drug from the prepared SLNs was prohibited by employing a cold homogenization procedure, restrictions to the crystallization/recrystallization of the drug and the lipid, and controlling the polymeric transitions, that were achieved by changing the lipidic contents, mixing the lipids, and changing of the proportions of the lipidic contents during the preparation of SLNs. The lipids, e.g., hydroxyl stearate, isopropyl myristate, and hydroxyl octacosanol inhibited the crystallization processes, and preparations containing ketoconazole [37], imidazoles [38], and clotrimazole were reported [39,40]. The increased drug loading was achieved by the use of structurally and spatially different lipids that provided more inter- and intra-molecular spaces for the drug molecules to be loaded. The glycerides worked better, however, the size of the drug molecule, the average molecular weight drugs, that were quantitatively more loaded than the high molecular weight drugs, were easily achieved, and this observation also pointed out the role of molecular interactions and spacing in SLNs to be developing matrices. However, a solution to further increase the drug loading capacity of SLNs was achieved by amalgamating the solid lipids with small proportions of the liquid-state lipids, i.e., oils were used during SLNs preparations. Another major bottleneck, observed was the loadings of the hydrophilic drugs. The lipidic content partitioning effects on hydrophilic drugs limited the majority of hydrophilic drugs from being prepared as SLNs, but only highly potent drugs with low dose incorporation were prepared as SLNs [41]. The drugs with 33% higher loading capacity were formulated using the salt formulation technique, in which the insoluble drug-lipid conjugate was first prepared using salt, or by chemical conjugation of the covalent type, and then processed with surfactants (tweens) to produce the nanoformulations after the high-pressure homogenization (HPH) technique, one of the methods for preparing SLNs, and brain-targeting hydrophilic drugs were prepared using this method [42].
2 SLNs delivery to organs, tissues, and cellular uptake: delivery mechanisms
SLNs formulations have drawn more attention in recent years as potential drug delivery modules. They have also been massively sought for transporting biomolecules since SLNs are known to penetrate through the cell membrane [43]. Furthermore, as a drug delivery system, SLNs have shielded encapsulated drugs from biochemical and physicochemical degradation [31,44]. It has also overcome the lower aqueous solubility of certain drugs, provided extended-time release of the drugs, and delivered the encapsulated drugs or other payloads to the specifically targeted organ/cells [45]. SLNs, in particular, have exhibited several advantages as a drug delivery vehicle. The biocompatibility of SLNs’ constituent lipid’s composition, their higher drug loading capacity, the absence of organic solvents in SLNs’ preparations, and the feasibility of SLNs’ formulations for bulk production in parallel with their sterility maintenance are some of the advantages of abovementioned SLNs. Their perceived and demonstrated efficacy and lowered toxicity are also a cut above other nanoformulation modules used in drug delivery [45].
SLNs carrier’s size, surface characteristics, encapsulated drug(s)’ physiochemical characteristics, and the composition of SLNs’ vesicle-carrier are among the most important factors affecting SLN-based drug(s)’ entry pathway to reach the target site. Several studies have been conducted to investigate the targeting mechanism of SLNs formulations toward various tissues and cells (Figure 3). For example, the mechanism by which efavirenz SLNs formulation, as an antiretroviral drug delivery system, targets the lymphatic system has been recently reported. According to the findings of the study on the lymphatic transport, and tissue distributions, a sizeable portion of the efavirenz evaded the portal system, and was retrieved in the lymph through a chylomicron uptake mechanism [46]. In addition, comparatively, a high quantity of the drug has been detected in the major lymphatic organ, the spleen, and it was found that the SLNs enhanced the bioavailability of the efavirenz, and reduced the drug’s quantity reaching the liver, which clearly indicated that majority of the drug bypassed the liver [46]. Furthermore, SLNs have been found to increase the cumulative percentage of the drug, lopinavir, at a ratio, 5-folds higher than the conventional drug (as solution) in the lymphatic tissues [47].
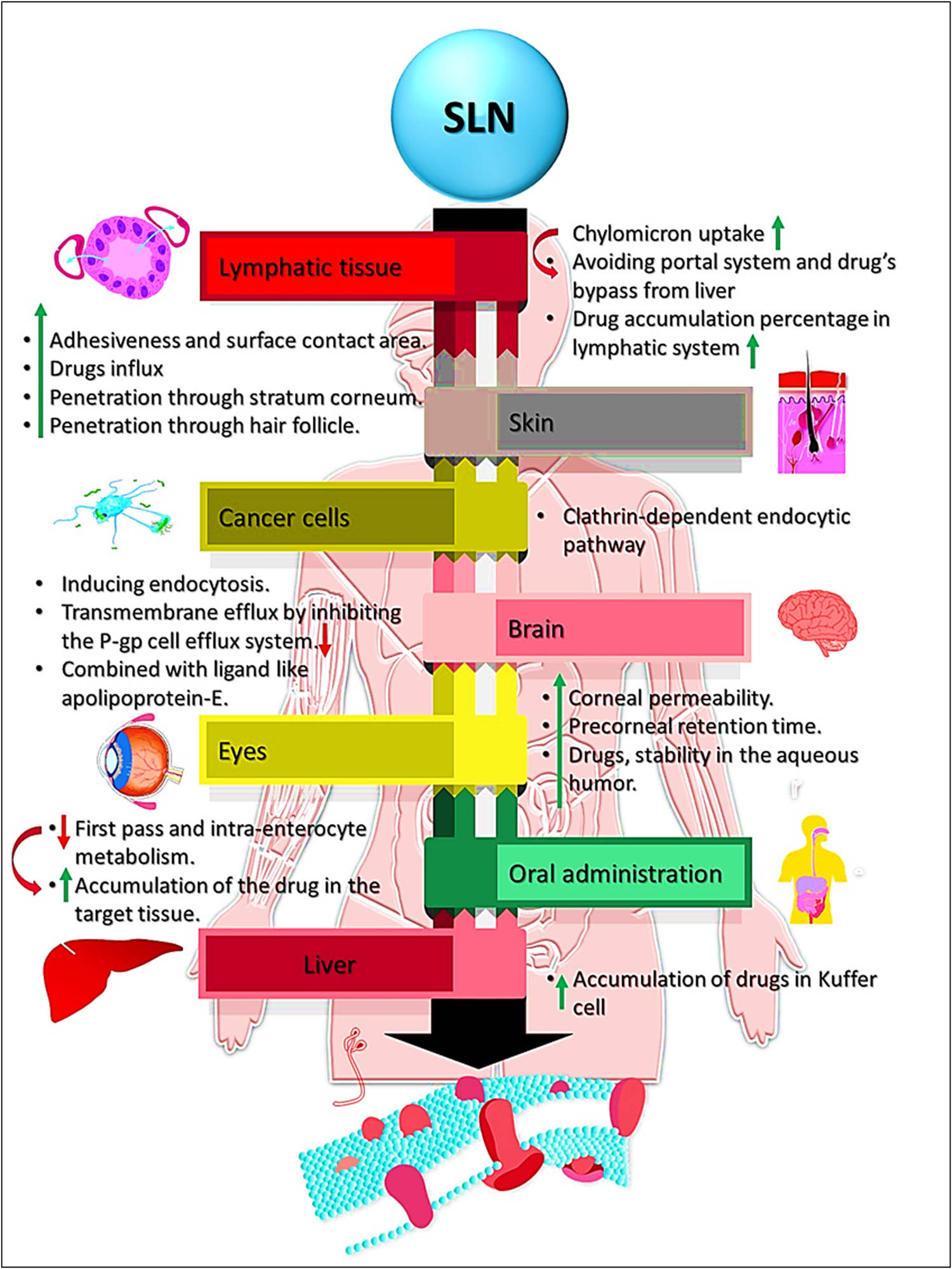
SLN mechanisms involved in various administrative routes’-based drugs delivery to different organs and tissue sites with their cellular level workings to exhibit the bioactivity.
Podophyllotoxin-based SLNs with epidermal targeting mechanisms have also been reported, the results indicated that SLN preparations of podophyllotoxin facilitated drug penetration through the stratum corneum, and hair follicle routes [48]. The fluorescence microscopy imaging also revealed that SLNs had a strong localization of podophyllotoxin within the epidermis. The penetration of podophyllotoxin-SLNs was stabilized by 0.5% poloxamer 188 and 1.5% soybean lecithin used in the formulation, and that might be an epidermal-targeting formulation due to their small particle size, and there upon these SLNs passing into the stratum corneum along the skin surface furrow [48]. Similar results and mechanisms for isotretinoin-SLNs penetrations have been reported [49].
Furthermore, SLNs are among the most advanced drug delivery systems for cancer therapy, especially, for the high-incidence cancers of the breast, lungs, gastro intestinal tract, and prostate [50]. The stealth-SLNs (sSLN) have been suggested to be of particular interest for anti-cancer actions [51]. When combined with a ligand (known as sSLNs), the SLNs possessed affinity for and selectivity toward a particular receptor, thereby making it possible to deliver medication to the breast cancer cells that overexpressed the receptors. Several studies have reported that this approach of sSLNs had stronger anti-breast cancer activity than the straightforward SLNs (non-conjugated to ligand, or no receptor targeting) [51].
Cancer cells’ cellular internalization mechanisms of SLNs’ formulations in human glioma cell lines (A172, U251, U373, and U87), and in human macrophage cell line (THP1) have been reported. The internalization of SLNs coated with polysorbate 60 and 80 and loaded with rhodamine-123 into the cancer cells has been evaluated by fluorescence microscopy and flow cytometry techniques. The results of Martins et al. [52] had indicated that the rhodamine-123-loaded SLN cells internalization is dependent on the size and surface charges of the formulated SLNs. They found that SLNs with a mean size < 200 nm and a slight negative surface charge (around −20 mV) have the ability to be internalized by glioma’s cells in higher quantities than by the macrophages. They also described the mechanism of internalization as being mainly achieved through a clathrin-dependent endocytic pathway.
The mechanisms behind SLNs’ uptake by human epithelial cells, i.e., lung A549 and cervical HeLa cells, have also been investigated [43]. In this study, SLNs were loaded with rhodamine 123, and the cellular uptake was assessed by the flow-assisted cell sorting technique, which revealed that endocytosis of SLNs was also mainly dependent on the clathrin-mediated mechanisms [43].
SLN-based drug delivery system for targeting the brain cells has been well studied, and the enhancements of the drug delivery and accumulations of the drug in brain cells have been proven [53,54,55]. By now, it is well understood that a drug molecule needs, among other characteristics, high lipid solubility, a molecular mass of less than 400 amu, and should not function as a substrate for active efflux transporters in order to cross the BBB [45].
The therapeutic cargo of the NPs is delivered across the endothelial cell layers by transcytosis and/or endocytosis and facilitated through inhibition of transmembrane efflux and openings of the tight junctions between the endothelial cells, allowing the drug carrier to cross the BBB. The surfactant incorporated with SLNs enhanced the drugs’ transport through endothelial cells’ membrane by their lipids’ fluidization [56]. However, the most current method of brain targeting for NPs rely on surface engineering through functionalization, and/or coating with certain ligands. These steps made it easier for the endothelial cells to endocytose SLNs, which continued to be the major pathway to improve the medications’ delivery to the central nervous system (CNS) [45]. For example, the functionalization of SLNs with ligands to achieve site-specific targeting made them attractive to overcome the limited BBB permeability of the drug. In the study by Dal Magro et al., SLNs were prepared for brain targeting by exploiting the adaptability of the warm microemulsion process for the covalent surface modification with an apolipoprotein-E derived peptide (SLNs-mApoE). It was found that SLNs-apolipoprotein-E was able to cross intact through the BBB in an in vitro model [57].
SLNs were helpful in the brain uptake process in a variety of levels; they stabilized the drugs against chemical degradation in the biological fluids, increased the residence time/biological half-life of the drug carrier in the bloodstream, and indirectly favored their translocation to the brain, as well as triggered the endothelial cells by inducing endocytosis. The internalization activity may also be receptor-mediated through an active targeting mechanism [45]. In general, SLNs as brain drug delivery systems have gained several positive points related to their ability to increase the quantities of the encapsulated drug, circumvent the BBB route to reach the brain, compromise the BBB layer to permeate through to the brain tissue, increase drug retention time in the brain, inactivate the p-glycoprotein (P-gp) cell efflux mechanism, and offer the possibility of site-specific brain targeting by SLNs apolipoprotein-E intermediacy [57,58]. The topical applications of SLNs also showed several advantages related to the lipid composition(s) of the formulation, which enabled SLNs to interact with the stratum corneum, thereby managing its lipid rearrangements, which eased the drug molecules’ penetration through the skin [59]. Although drugs’ physiochemical properties play an important role in skin penetration, SLN’s small size enhanced their adhesiveness and surface contact area, promoting drug influx through the skin [59]. The ocular application of SLNs delivery system has also been reported to enhance the drug’s corneal permeability and pre-corneal retention time, in addition to lowering the toxicity and enhancing the drug’s bioavailability, and stability in the aqueous humor of the eye [58].
SLNs as an oral drug delivery module is reported to reduce the first pass metabolic and intra-enterocyte metabolisms, which led to drug accumulation at the targeted site. SLNs also enhanced the evading of P-gp efflux pumps [58]. Also, SLNs as parenteral drug delivery system, exhibited several advantages related to its long physical stability, controlled drug release, increased drug accumulation levels in the targeted tissues/cells, increased drug bioactivity, increased circulation time in the blood-stream, and improved the drug bioavailability, and increased the drug delivery and drug retention in the cancer cells [58]. SLNs formulation also directed the parenteral liver-oriented drugs to be specifically accumulated in the Kupffer cells, which enhanced the drug’s efficacy in the treatment of several liver diseases, including hepatic carcinoma [60].
3 Preparatory processes for SLNs: roles of constituents and conditions
The lipids, emulsifiers, surfactants, or a stabilizer, co-surfactant, preservatives, cryo-protectant, and the charge modifiers are among the key ingredients to formulate SLNs. SLNs use solid lipids, and other components, preferably in the presence of water through certain organic solvents that can be employed for the purpose. Nonetheless, the use of organic solvent is utmost avoided [61]. The major lipid types used are triglycerides, fats/fatty acids, waxes, and steroids with sufficient lipophilic characteristics. Partial glycerides, e.g., imwitor, have also been used. The emulsifiers and their combinations stabilized the lipidic dispersion of the formulation during the preparation stages, and tend to avoid the NPs agglomeration [11]. The emulsifiers were chosen based on the delivery required characteristics of SLNs, and feasibilities for their routes of administration. Some of the lipidic constituents, namely, compritrol (10%), cetyl palmitate (10%), ethyl oleate (30%), glycerol (4%), isopropyl myristate (3–4%), lecithin (9%), lipids of trimyristin and tripalmitin identities, and mixtures of mono, di, as well as triglycerides (3.3%), PEG 400 (5%), PEG 2000 (0.25%), PEG 4500 (0.5%), phospholipids (0.5–1.5%), pluronic F68 (40%), poloxamer 188 (1–5%), sodium alginate (70%), soy phosphatidylcholine (95%), tego-care 450 (1.2%), tristearin glyceride (95%), tweens 80 and 85 (50 and 0.5%), and a number of lipids in weight/volume (w/v) concentrations and weight/weight (w/w) formulations were used [62,63,64,65,66,67]. The preservative, thiomersal, and gelatin, glucose, mannose, maltose, lactose, sorbitol, mannitol, glycine, polyvinyl alcohol (PVA), and polyvinyl pyrrolidone (PVP) are among the common cryoprotectants used, while dipalmitoyl phosphatidylcholine, stearoyl amine, dicetylphosphate, and dimyristoyl phosphatidylglycerol were among the charge modifiers that have been used during SLNs preparations, and stabilization procedures [22].
A number of techniques are involved in SLNs manufacturing, wherein the HPH and microemulsion-based processes are popular for preparing large quantities of industrial productions of SLNs [68]. The influence of the microemulsion modules on resultant SLNs’ size and shape dimensions, and the tough incorporations of the crystalline structure of the drugs in SLNs have been attempted as against the preparation of the non-lipidic classical NPs obtained through hydrophilic conditions used in the preparation [69]. The subsequent segments describe different existing approaches for SLNs formulations, wherein the HPH was found highly feasible and consistent, for SLNs production. It helped in producing SLNs of spherical shapes and sizes with regular good stability and settlement features [70]. For example, when tetrandrine-loaded SLNs (TET-SLNs) were prepared by two different methods, namely, the HPH method and ultra-sonication, the HPH was found to be a better method to prepare the TET-SLNs, which were smaller in size, steadier, and highly incorporated into SLNs. Their analyses of shapes, sizes, and stability were performed based on Transmission electron microscopy (TEM), particles characterization system, and Zeta potential analyzer [71].
HPH method applies both the pressure gradient and mechanical forces as the stress to achieve reliable and stable SLNs. The high-quality homogenizers have the options of regulating various parameters, e.g., turbulence, shear, cavitation, impact, and process intensity to get optimized, and yield the customized SLNs as per the drug loading and delivery necessities. The HPHs also helped in attaining SLNs with smaller particle size with increased surface area, enhanced drug loading, and eventual high bioavailability. The HPH process is extensively used in bulk pharmaceutical preparations. The HPH was achieved through two methods [72], and the process was held responsible for decreasing the molecular weights of the used polymeric materials [73]. This procedure also caused high shear stress, and later developed free radicals too.
3.1 Hot HPH method
In this process, the lipids were heated above their melting points, and converted into liquid state materials (Figure 4), and the high temperature resulted in dropping down the viscosity of the preparation-stage’s present liquid state materials, which caused shrinkage in the resultant particle sizes. The process was accompanied by many disadvantages. An increase in temperature was considered to cause high rate of decomposition of the embedded drug, and unpredictable lipid changeovers were also observed. Drug losses from the aqueous phase were also noticed owing to the high kinetic energy of the preparative steps.
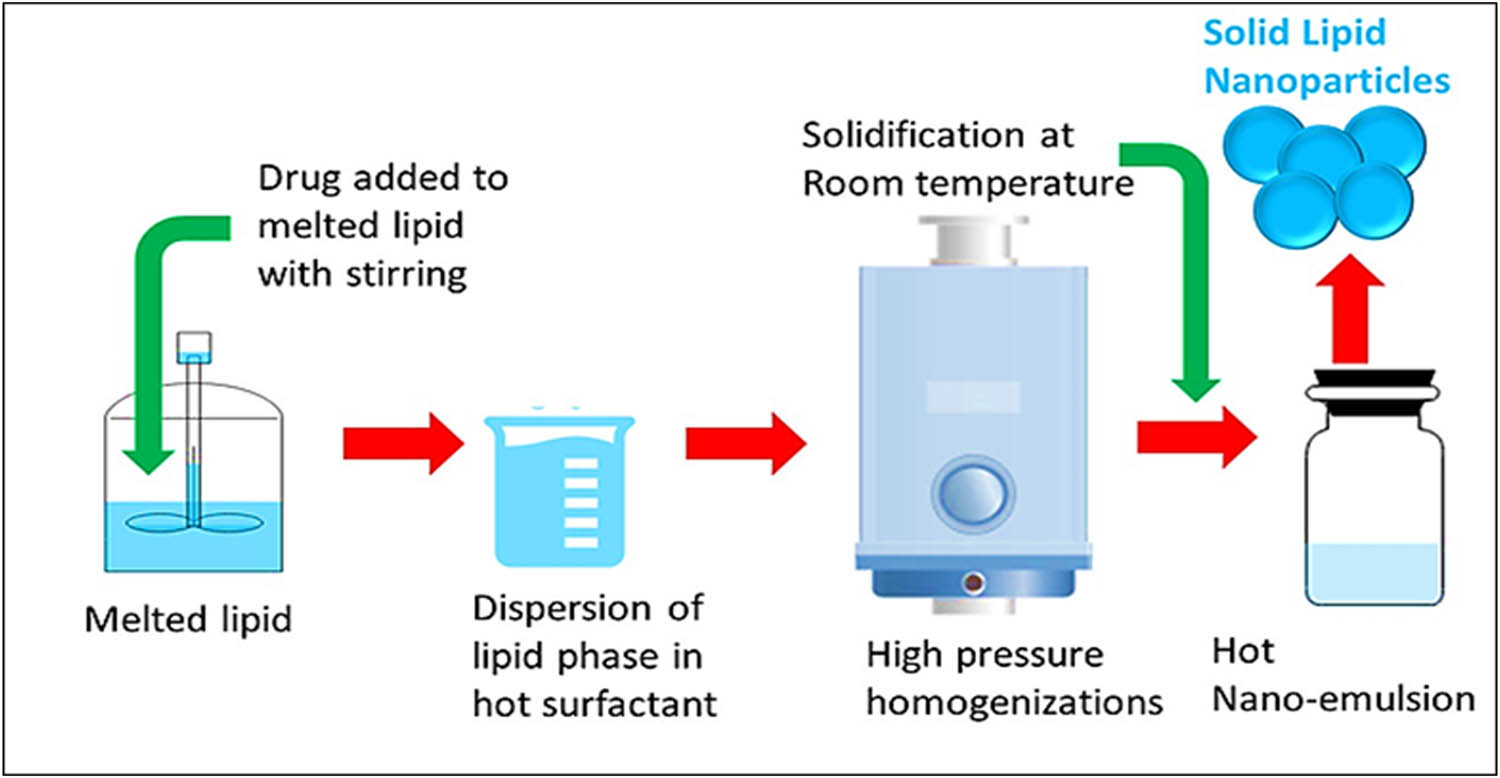
Schematic diagram of the hot HPH technique for preparation of SLNs.
3.2 Cold HPH method
To avoid limitations observed in the hot HPH method, cold HPH was tried (Figure 5). The hydrophilic and thermo-labile drug was encapsulated in SLNs matrix. Solid lipid was employed to produce large-sized particles, which were diffused in a chilled solution of surfactant. The blend formed uniformed preparation, both at and under room temperature (RT) conditions that were further broken down by gravitational pull [74].
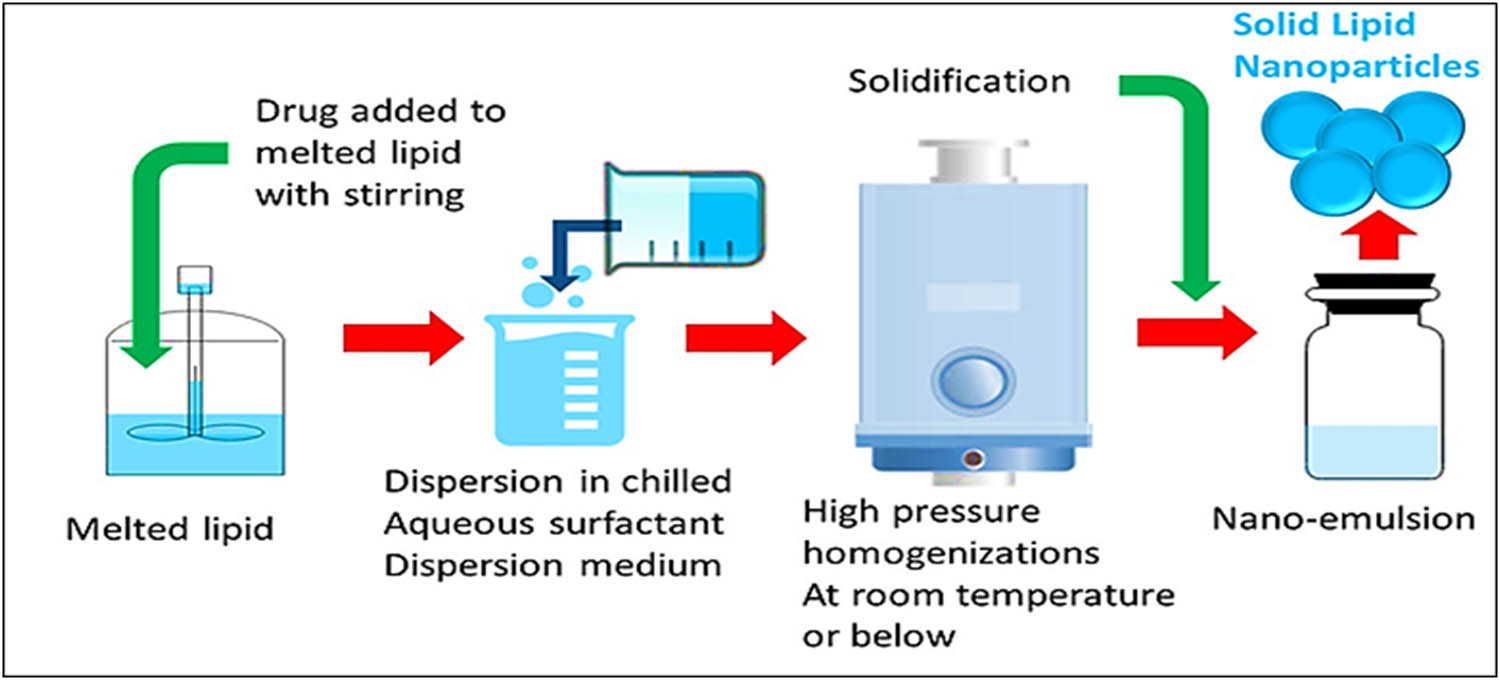
Schematic diagram of the cold HPH technique for the preparation of SLNs.
3.3 Emulsification-sonification method
Drugs were supplemented to the liquefied lipid(s) at 5–10°C of temperature, and supplemented with hot liquid surfactant solution. The emulsion thus obtained was sonicated to reduce the particles size. Finally, SLNs obtained were cooled at RT (Figure 6). However, the process led to metal contamination due to the use of the metal probe for ultra-sonication purpose [75].
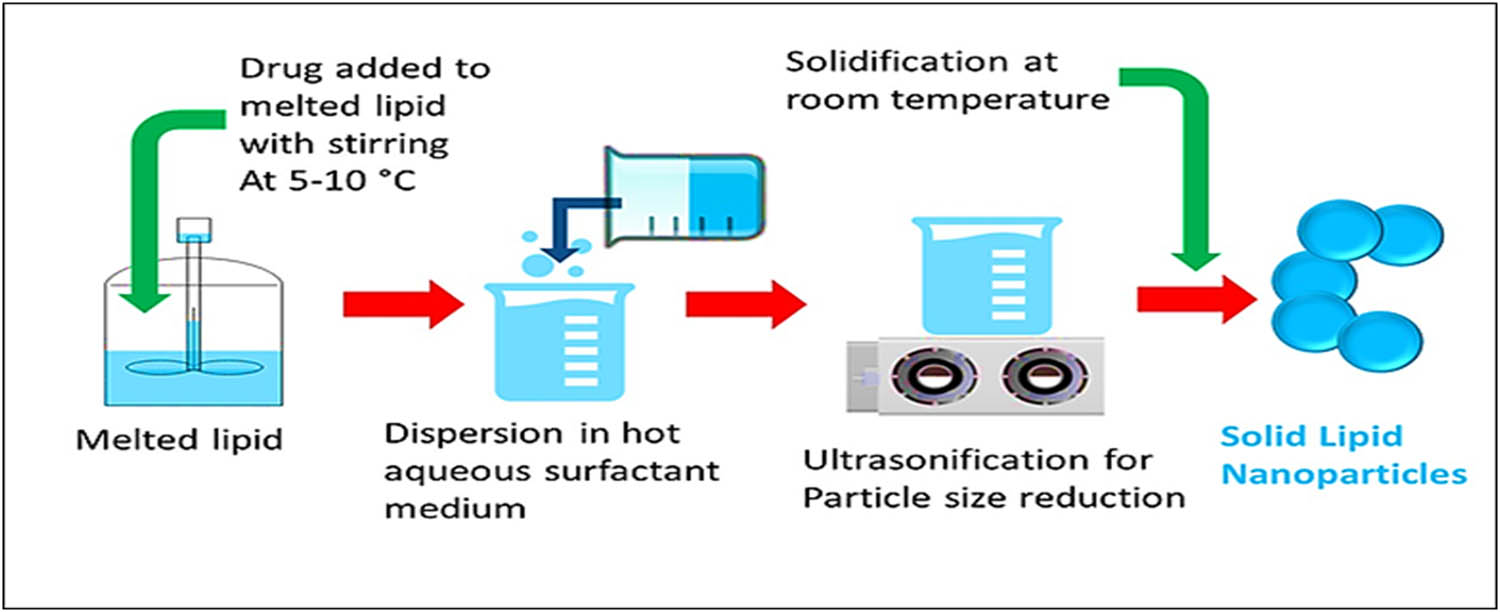
Schematic diagram of an emulsification-sonification processing based technique for the preparation of SLNs.
3.4 Solvent emulsification method
The lipid was emulsified with the process of HPH in a liquid state by dissolving in organic solvents, i.e., cyclohexane and chloroform. These solvents are water-immiscible and evaporated easily leaving precipitated NPs. The process was carried out at 25°C, and was thermolabile in nature [76,77].
3.5 Solvent emulsion-scattered method
The organic solvents that were partially water miscible, were used in solvent diffusion method of SLNs preparation. These diluents, when water-saturated, became heat and temperature stable. In this case, the organic liquids satiated all at once to attain thermodynamic equilibrium. The transient obtained was moved to water under constant stirring to solidify the dispersed phase, and SLNs were formed. An overview of the preparation method is depicted in Figure 7 [78,79,80].
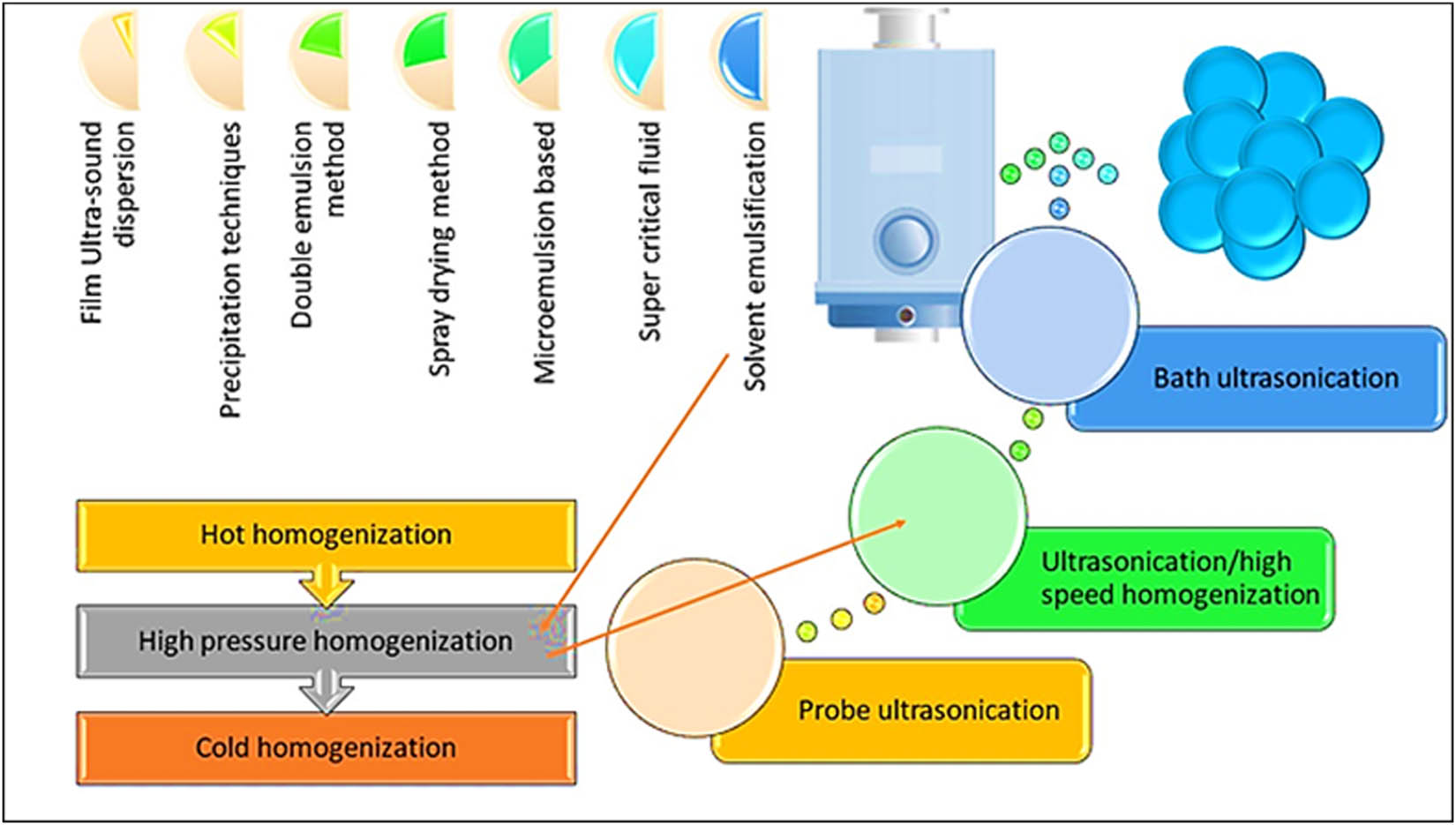
Various methods of SLN preparations.
3.6 Aqueous needle process
This process also used the organic solvents that were water-miscible and easy to use with faster production rate, together with being cost-effective. The process is nearly same as that of the solvent diffusion method. The lipids in organic solvents were rapidly immersed under pressure through a needle in a liquid solution of surfactants [81].
3.7 Double emulsion method: water-in-oil-in-water emulsions
The process resulted in the formation of lipospheres as the particles sizes were a little larger than SLNs. The hydrophilic drug was embedded inside the liquid lipid state, and stabilizing material was used to prevent the drug’s escape from the liquid part to external layer of the double emulsion [82]. Table 1 summarizes SLNs preparation methods, lipid types used, the drugs encapsulated, and their major therapeutic applications.
SLNs preparation methods, used lipids, encapsulated drugs, and major therapeutic applications
| S. No. | Lipids | Preparation method | Drug | Application(s) | Ref. |
|---|---|---|---|---|---|
| A. | Triglycerides based | ||||
| 1. | Tricaprin | Hot-pressure homogenization | Oxytetracycline | Extended-release after single-use | [1] |
| Hot-pressure homogenization | Bromocriptine | Parkinson’s disease | [86] | ||
| Emulsion/solvent evaporation | Docetaxel | Lung cancer | [87] | ||
| Emulsion/solvent evaporation | Docetaxel | Lung cancer | [87] | ||
| 2. | Trilaurin | High-shear homogenization | Atovaquone | Pneumonia, malaria, toxoplasmosis | [88] |
| Hot-melt HPH | Paclitaxel | Ovarian cancer | [89] | ||
| 3. | Tristearin | Hot-pressure homogenization | Bromocriptine | Parkinson’s disease | [86] |
| Hot-pressure homogenization | Oxytetracycline | Extended release after single-use | [85] | ||
| Emulsion/solvent evaporation | Docetaxel | Lung cancer | [87] | ||
| Ultrasonication-based hot homogenization | Nitrendipine | Improved oral bioavailability | [90] | ||
| 4. | Trimyristin | Emulsion/solvent evaporation | Docetaxel | Lung cancer | [87] |
| Water/oil/water (W/O/W) emulsion | Calcitonin | Improved oral absorption | [91] | ||
| Ultrasonication-based hot homogenization | Nitrendipine | Improved oral bioavailability | [90] | ||
| 5. | Tripalmitin | Emulsion/solvent evaporation | Docetaxel | Lung cancer | [87] |
| High-shear homogenization | Atovaquone | Pneumonia, malaria, toxoplasmosis | [88] | ||
| Ultrasonication-based hot homogenization | Nitrendipine | Improved oral bioavailability | [90] | ||
| Hot-homogenization | Ergocalciferol | Rickets, vitamin-D deficiency | [92] | ||
| 6. | Softisan 100 | Quasi-emulsion solvent diffusion | Caffeine | Topical administration | [93] |
| 7. | Stearic acid | Hot melt oil-in-water (O/W) emulsion technique | Ferulic acid and aspirin | Pancreatic cancer cell lines, chemoprevention | [94] |
| B. | Hard-fat based | ||||
| 1. | Witepsol W35 | High-shear homogenization and ultrasound | Diazepam | Pulmonary delivery | [95] |
| Witepsol S58 | High-shear homogenization and ultrasound | Diazepam | Pulmonary delivery | [95] | |
| 2. | Witepsol H35 | Hot-melt ultrasonication | Rosmarinic acid | Oral delivery | [96] |
| 3. | Witepsol H15 | Hot-melt ultrasonication | Rosmarinic acid | Food applications | [97] |
| 4. | Witepsol E85 | Hot HPH | Ibuprofen | Osteoarthritis, musculoskeletal disorders | [98] |
| Hot HPH | Buparvaquone | Treatment of leishmaniases | [99] | ||
| 5. | Glyceryl monostearate | Hot homogenization | 5-Fluorouracil | Lung cancer | [100] |
| Hot homogenization and ultrasonication | Ramipril | Hypertension | [101] | ||
| 6. | Cetyl Palmitate | Hot homogenization | 5-Fluorouracil | Lung cancer | [100] |
| Reverse micelle-double emulsion | Insulin | Diabetes | [102] | ||
| 7. | Cetyl Palmitate | Microemulsion method | Vincristine | Brain delivery | [103] |
| 8. | Glyceryl caprate | Solvent emulsification-diffusion | Doxorubicin (DOX) | Various cancers | [104] |
| 9. | Stearic Acid | Ultrasonication | Tetrandrine | Topical applications | [105] |
| Emulsification-evaporation-solidifying | Lipoyl–memantine | Anti-Alzheimer | [106] | ||
| Emulsification | Silibinin | Parenteral administration | [107] | ||
| Astaxanthin | Simulated gastric and intestinal juices | [108] | |||
| Reverse micelle-double emulsion | Insulin | Diabetics | [102] | ||
| High-shear homogenization, ultrasound; HPH | Safranal | Topical delivery | [109] | ||
| Micro-emulsification solidification | Methotrexate | Carcinomas | [110] | ||
| 10. | Glyceryl behenate | Hot HPH | Budesonide | Nasal formulation | [111] |
| 11. | Compritol 888 ATO, glyceryl dibehenate | Hot homogenization method | Vorinostat | Cancer | [112] |
| 12. | Compritol | Microemulsion technique | Quercetin | Improve permeation across the BBB into the CNS | [113] |
| C. | Co-emulsifiers based | ||||
| 1. | Soybean lecithin | Micro-emulsification solidification | Methotrexate | Carcinomas | [110] |
| Emulsification and low-temperature solidification | Naringenin | Pulmonary administration | [114] | ||
| Curcumin | Enhance brain delivery | [115] | |||
| 2. | Egg lecithin | Emulsification and low-temperature solidification | Naringenin | Pulmonary administration | [114] |
| Microemulsion | Ketoprofen | Arthritic inflammation, pain | [116] | ||
| Phosphatidylcholine | Reverse micelle-double emulsion | Insulin | Diabetes | [102] | |
| 3. | Gelucire® 50/13 | Melt-emulsification method | Glutathione (GSH) | Deliver the antioxidant GSH to the primary cultures of head-kidney (HK) leucocytes of Sparus aurata L. | [117] |
| D. | Polyaxomers based | ||||
| 1. | Polyaxomer-188 | O/W emulsion/solvent evaporation | Docetaxel | Breast cancer | [118] |
| Hot homogenization, ultrasonication | Ramipril | Hypertension | [101] | ||
| 2. | Polyaxomer-407 | Hot homogenization | Metoclopramide | Nausea, vomiting | [119] |
4 Drug(s) incorporation models
Generally, there are three models proposed for drugs incorporation to embed an active ingredient in SLNs (Figure 8) which produces different release quantities and patterns (Figure 9).
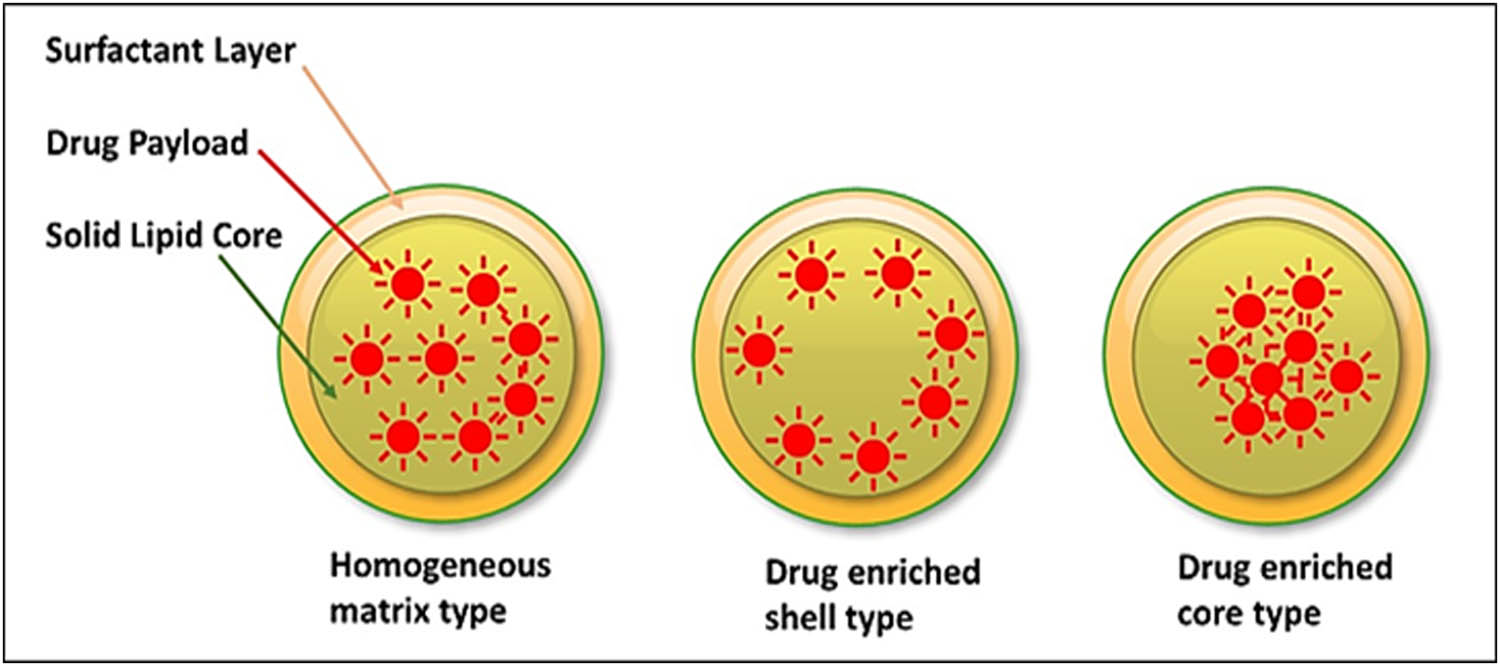
SLNs drug loading models showing homogenous matrix, drug-enriched peripheral-shell, and drug-enriched core shell models.
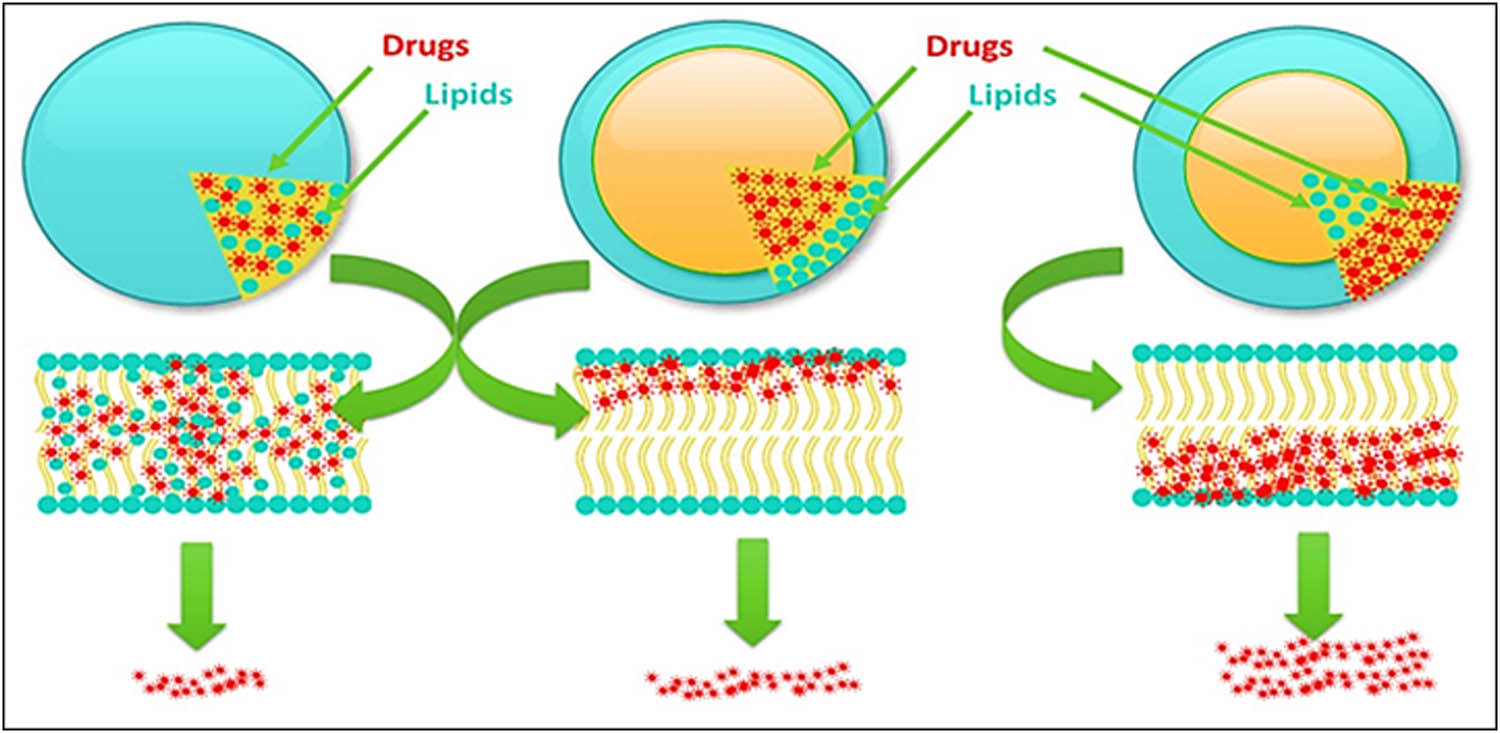
SLNs drug loading models typical release effects showing homogeneous model s drugs release at minimum, drugs enriched-shell type model s comparatively increased drug release than the homogeneous matrix model, and drugs enriched-core type model s highest drug release phenomena.
4.1 Homogenous matrix model
The active pharmaceutical ingredients (APIs) are distributed uniformly within the solid lipid core (Figure 8), and the drug release takes place through the diffusion process from 1 day to a few weeks. Such model types are generated by the HPH process, e.g., prednisolone [83,84].
4.2 Drug enriched peripheral shells model
The drug resides at the periphery of SLNs is shown in (Figure 8). The model is prepared by the HPH preparation process, when lipids in the nanoemulsion stage cools faster at core areas than the peripheries, and there are either a minimum quantity of drug molecules, or the area is drug-free. At the periphery, the lipids and the drug precipitate at the same time at the eutectic point. The drug gets accumulated at the periphery, because initially at the beginning of the HPH process, the drug’s dispensability in the surfactant solution is increased in a uniform manner with the increase in the temperature. Therefore, the drug may leave the solidifying lipid matrix, and spread in the liquid state; but when the cooling of the nanoemulsion occurs, the drug dispensability decreases and phases separation occurs between the solid lipid matrix and the liquid state. The cooling leads to solidification of the core [120]. In this case, a burst release of drugs takes place. However, the release profile can be controlled by controlling the physical parameters and ingredients, i.e., temperature, and surfactant concentrations [121].
4.3 Drug enriched core-shell model
When the API precipitates faster than the lipid during the cooling of the nanoemulsion stage of SLNs preparation, a drug-enriched model of drug loading is obtained in reversal to the reverse of the peripheral shell model of drug loading (Figure 8). In this model, the peripheries of the prepared SLNs are either drug-free, or contain minimal quantities of the drug which is caused by the drug escaping the phase. The model is shaped when the drug runs through the lipid matrix, until it is overloaded at the production temperature. The drugs which are cooled at a rapid rate than the lipids, and get concentrated in the core region, produce this model of loading. Herein, sustained drug release is observed, which is membrane controlled, and is based on Fick’s Law of Diffusion [121].
5 Drug release profiles
The drug release profiles depend on certain parameters that included the chemical nature of the lipid matrix, the type of the produced SLNs model, the production temperature, and the type and concentration of the surfactant. Generally, the release profiles were often biphasic, i.e., first burst release and then sustained release patterns were followed (Figure 10). The hot HPH process, and high surfactant concentrations, generally led to spurt release of the encapsulated drugs. Since, the high temperature lets the drug to dissolve in water, and upon the nanoemulsion cooling, the lipid starts solidifying but the drug remains in the aqueous phase. A supersaturated solution, that was obtained after further cooling, the drug got partitioned back leaving the central core solidified, and the peripheral areas of the SLNs comparatively in liquid state, which indicated that the drug’s burst effect was dependent on the solubility of the drug in the water phase [122,123].
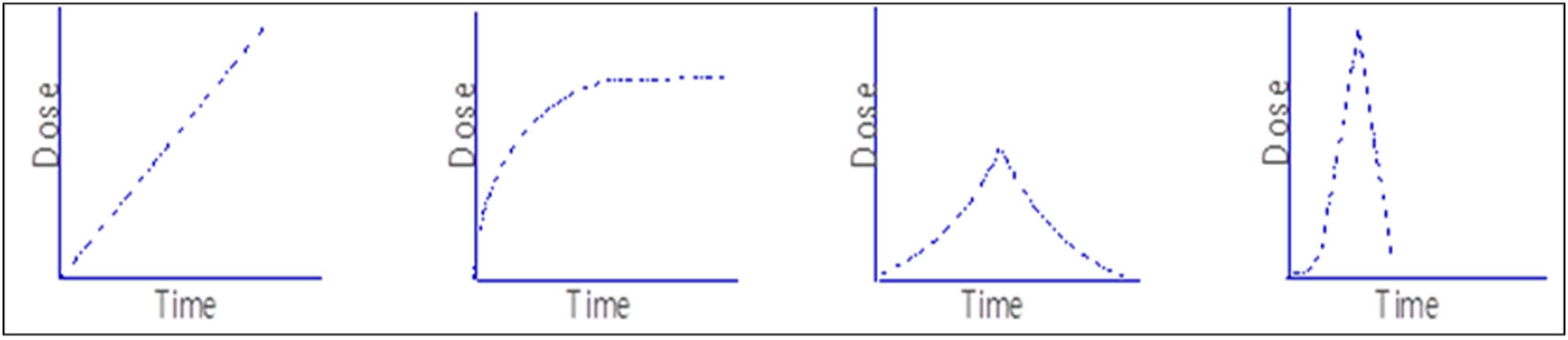
Typical profile of drugs payload release from SLNs: time vs drug release dose relationships.
6 SLNs storage stability
Storage stability of a formulation, especially the nanoformulation, is a very important aspect of the formulation’s further applications as a therapeutic agent, and SLNs stability is also crucial in producing the therapeutic effects. SLNs when stored for a longer period may yield a gel, which results in increased particle size, and thus SLNs’ physical properties get deviated. Therefore, it is necessary to continuously observe their physical properties with the passing of time. This deviation is obtained by variations in the electrokinetic potential (a key feature of SLNs stability), the average size of SLNs particles, drug’s chemical condition with regards to its stability and degradation, presence of degradants, drug’s leaching, as well as at times the viscosity of the drug formulation’s colloid, if stored in a media. The storage temperature and presence of light is also important parameter when long-term stability is concerned. Therefore, the 4°C temperature is considered the most optimal and advantageous temperature for long-term storage. However, at 20°C storage conditions, the drug’s loss does not occur, but agglomeration producing fast-growing particles size is observed at 50°C storage condition [124,125].
7 Characterization of SLNs
Toward establishing the characteristics of SLN, the particle size of SLNs material plays a critical role. In order to properly characterize SLNs, one of the most important steps is to get an exact determination of the particle size. The method known as dynamic light scattering, or DLS, is an important approach, that is often used in the process of characterizing a broad variety of nanomedicines. On the other hand, due to the very fine nature of SLNs, methods that are both highly sensitive and reliable need to be used. Electron microscopy technologies, e.g., scanning electron microscopy, TEM, and atomic force microscopy are widely applied for the accurate determination of the particle size of SLNs. These technologies also accurately determine the morphology and internal composition of the SLNs [126,127,128,129]. Further, the X-ray photoelectron spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, Rutherford backscattering, and analytical ultracentrifugation are some of the other approaches that may be used to examine the surface chemistry of SLNs [130,131]. In the meanwhile, the optical characteristics of SLNs are often examined by means of UV-VIS and photoluminescence spectroscopies in order to identify their excitation and emission spectra, in addition to their yield and brightness [126]. Fluorescent NPs can be easily detected in vitro and in vivo conditions [132,133]. SLNs can also be loaded with materials which prove to have excellent optical properties, and these SLNs can be easily tracked intracellularly by fluorescence-based microscopic technologies, such as, fluorescence microscopy and confocal laser scanning microscopy. Furthermore, the biodistribution of fluorescent SLNs in experimental animals can be visualized by various in vivo imaging systems [134]. Thus, SLNs’ identification has been performed through sensitive and nanoscale resolution techniques. The characterization of the formulation is important to ensure the product quality, stability, and drug release profile. Some important characterization techniques are as follows.
7.1 Particle sizes
The correct estimations of the constituent SLNs average spheroidal part are vital owing to its key role in effective drug delivery. The size of SLNs is also responsible for the drug release profile, the cellular response when absorbed by cells, and its biodistribution. The most widely used and powerful techniques for calculating the constituent particles’ size from a few nanometers to 3 microns are photon correlation spectroscopy (PCS), and for the particle size greater than 3 microns, the laser diffraction technique has been used [135,136]. These techniques measure the scattered light intensity due to the particles’ movements. The scanning and transmission electron microscopies are also regularly used for shape and particle size determinations. The size stability during prolonged storage is also an area of concern, and has been continuously monitored by the PCS method. It calculates the overall charge acquired by the NPs in a specific medium. Generally, a high charge on SLNs indicate the presence of more repulsive forces, and hence no/less accumulation of NPs resulted in their electrical stability [137,138,139].
7.2 Measurement of crystallinity and polymorphisms
The X-Rays Diffraction of the molecular crystal lattice is feasibly assessed for the per-unit crystallization. The X-ray is diffracted at certain angles as per the observed planes and atoms arrangements in the crystalline material. The diffraction obtained due to X-rays bent is a unique signature of each type of atomic arrangement, and this technique has been used to gauge the molecular arrangements in the lipids and drugs, SLNs chemical composition, chemical structures, and phase behavior [140,141], whereas to know the melting points, and crystallization behavior of all the ingredients of SLNs, Differential scanning calorimetry (DSC) method was used. It is important to know the crystalline nature of the lipid constituents of SLNs, as it is interrelated with the embedded drug and its release profile [142,143]. The polymorphism of lipid matrices and drug is caused by the long storage time, which eventually affects the drug release pattern [144]. However, the polymorphism can also be slowed down, if the particles size is decreased, and the desired quantities of surfactant are utilized during the preparation stages of the SLNs, including the use of the chemical stabilizers during storage.
7.3 Evaluation of supplementary structures
Drugs’ formulations produced several kinds of nano-scale delivery modules, e.g., polymeric NPs, liposomes, and micellar structures under super-cooling and thaw cycles produce supplementary structures that are obtained from the storage conditions-led mutual interactions, chemicals and conditions affecting degradations, and chemical transformations of the drugs and other chemicals, including solvating media present in the formulation. These supplementary structures might be present in the stored, and sometimes, some of these supplementary structures can be present in the relatively-fresh SLN formulations. The characterization of such multimodal entities have been performed by the NMR [145], and electron spin resonance spectroscopies [146]. These techniques are extremely subtle, and are accurate enough to identify the different kinds of supplementary structures, thereby also indicating the colloidal status and stability of the formulation, if stored in the liquid media as well as lyophilized solid formulation that may also contain the cryo-protectant as part of the formulation. The 1H-NMR spectroscopy was also used to evaluate the super-cooled and thawing steps effect with the help of low line widths of the lipid protons in the 1H-NMR (PMR, Hydrogen/Proton NMR) spectrum of the formulation [147].
7.4 Evaluation of SLNs embedded drug (packing and entrapment efficiency)
SLNs provide extensive therapeutic applications in drug delivery, and distributions, and it is significant to know the packing and entrapment efficiency of SLNs entrapped drugs as it sets the discharge pattern for the drug release profiles. Toward estimating the total quantity of SLNs embedded drugs as per unit weight, the free drug is separated out. The process of drug separation is realized by using several techniques, e.g., centrifugation, filtration, and gel permeation chromatography which separate the enclosed drugs from SLNs, and per unit weight of the incorporated drug is evaluated. Analytical methods involving UV and UV-Vis spectrophotometry, fluorophotometric spectral determinations, high-performance liquid chromatography, and liquid scintillation counting for radio-tracer labeled materials are some of the techniques involved [148,149].
8 Evaluations of drug release patterns
Assessments leading to quantification of drugs’ release are crucial for calculating the dose availability, maintenance of the drug’s supply at the site of action, release pattern’s estimation, drug’s stability, safety, and overall therapeutic efficiency, including the drug release efficacy and the in vitro and ex vivo studies on the formulated SLNs are performed. Rarely, but at more advanced clinical implications stages of the drug development and ADME studies including pharmacokinetics/dynamics studies stages, in vivo experimentations are performed. Other methods, such as membrane dialysis method, flow rate along with the analytical voltammetric turbidimetry were also used to measure the drug release profiles [150].
9 Routes of drug administration and biodistributions
9.1 Drug administration through oral route
SLNs entrapped drugs offer promising potential for oral delivery. SLNs works as the protecting shield for the entrapped drug against the harsh gastro intestinal tract (GIT) environment [151] and improve the transmucosal transport of the drug [152]. SLNs, under size limits, are easily absorbed by the M-cells of the Peyer’s patches of the intestine [91], and gut enterocytes [153]. SLNs entrapped insulin is first burst released and later a constant release is maintained [154]. The all-trans retinoic acid, which is poorly water soluble upon SLNs formulation, has shown considerably improved oral bioavailability. The preparation procedure and the quantity of surfactants used in the formulation exhibited significant impact on the oral absorption of SLNs formulation [155].
9.2 Parenteral administration route
Parenteral administration of drugs has evolved as an alternative to the oral route, and the parenteral route effectively avoids the enzymatic degradation in GIT. SLNs, also site-specific in nature, have provided controlled delivery, and improved delivery of the embedded drugs through this method [156].
9.3 Rectal administration route
Rectal administration, for in vivo and clinical applications, is an option together with the parenteral drug route for fast therapeutic response. The route is preferable for pediatric, consciousness-altered, and nauseating patients. The extensive rectal vasculature and lymphatic vessels provide the basis for this route’s drug absorption, wherein high plasma level is achieved in short time. SLNs formulation entrapping metoclopramide was prepared by the HPH method using surfactants, i.e., polysorbate 80, pluronic® F-68, F-127, and Cremophor. SLNs provided constant and sustained drug release [119,157].
9.4 Intranasal drug delivery route
SLNs’ intranasal administration route for brain delivery of drugs has provided faster drug outreach as compared with the intravenous administration. The drug’s formulation delivery through nasal cavity has provided enhanced bioavailability, and escape from first-pass liver metabolism of the drug [158].
9.5 Pulmonary delivery route
The non-invasive drug delivery route with large surface area, and high drug absorption rate, has also provided first-pass elimination escape for the drugs. It is also considered as a substitute for the parenteral route of drug administration. The respiratory delivery of SLNs was carried out by nebulizing the drugs for treatment of asthma, cancer, and allergy. The delivery did not cause the inflammation of the alveolar epithelial lining, and was found safe. The insulin trapped SLNs delivery provided stability and sustained release of the drug with desirable therapeutic effects [159]. PEGylated SLNs were effective in constant and sustained dose release of the drugs with enhanced bioavailability in the lungs. The ligands-coupled, positively charged SLNs were prepared and tested in alveolar macrophages [160]. The RGD-modified paclitaxel-loaded and redox-sensitive SLNs for lung cancer drug delivery have also been prepared [161].
9.6 Ocular administration route
SLNs are considered as successful carrier for ocular administration. Many drugs, e.g., tobramycin® [162], and cyclosporin-A [163], were effectively administrated to the eyes. These drugs were found stable and non-irritant to the subjects. The lipoplex (liposome–poly cation–DNA complex), was also used to deliver drugs and genes to ocular sites. A liposome protamine–DNA lipoplex, an electrostatically assembled payload delivery entity, incorporated a cationic liposome and an anionic protamine–DNA complex as part of the highly condensed DNA forming its core material was surrounded by lipid layers. The carrier was found suitable for delivery to the cornea, targeting the retina in age-related macular degeneration, retinitis pigmentosa, and diabetic retinopathy. Delivery of functional genes, micro RNA, and other genetic materials to treat ocular conditions have also been reported [164].
9.7 Transdermal and topical application route
SLNs entrapped drugs were successfully delivered through transdermal routes also. SLNs entrapping the diclofenac sodium was applied for transdermal delivery, and a burst release with slow but sustained and constant dose release of the drug was obtained [165]. For example, flurbiprofen SLNs have shown 4.4-folds increase in bioavailability of the flurbiprofen and produced the desired levels of anti-inflammatory effects [166]. Drugs and other entities, i.e., imidazole [40], triptolide [167], podophyllotoxin [168], vitamin-A [169], isotretinoin [170], DNA [171], flurbiprofen [172], glucocorticoids [173], sun-screen product, harmful UV-rays blocker, and 2-hydroxy-4-methoxybenzophenone (Eusolex® 4360) [174] have been formulated as SLNs lipidic carriers for topical delivery [175]. Cosmetics, anti-aging, and beauty products have also been formulated for better topical delivery as SLNs together with their different formulation variants [176,177]. Figure 11 sums up the various routes of SLNs-based drug administrations.
Various routes of SLNs administrations.
10 SLNs and cancer therapy: major thrust areas
Various classes of cytotoxic drugs have been incorporated into SLNs, e.g., paclitaxel, DOX, mitoxantrone, methotrexate, camptothecin, SN38, etoposide, fluoro-deoxy uridine, retinoic acid, verapamil, and cyclosporin-A. These cancer-fighting drugs, when loaded in SLNs, have exhibited improved therapeutic efficacy, biodistribution, solubility, and better pharmacokinetics. The aloe-emodin, a drug used for treating many types of tumors, i.e., liver, breast, and lungs, have demonstrated better efficacy with constant drug release profiles. SLNs formulations for targeting cancers [178] loaded with tamoxifen [179], camptothecin [180], and methotrexate as payload deliveries [110] are also reported.
10.1 Breast cancer
For breast cancers, several drugs, i.e., mitoxantrone, paclitaxel, methotrexate, and aloe-emodin, have been clinically used. It has been observed that in vitro testing of cytotoxicity of the mitoxantrone, methotrexate, and aloe-emodin-loaded SLNs were higher than their free drug counterparts [181,182]. However, in vitro cytotoxicity evaluations did not exhibited much difference in cytotoxicity levels for the paclitaxel-loaded SLNs and its free drug [182]. Docetaxel, a wide-spectrum, second generation anti-cancer drug, loaded onto SLNs showed enhanced inhibition of cancer tissue and the formulation was also effective at low dose as compared to the free drug administration [183]. Mitoxantrone formulated as SLNs for localized injection to control toxicity and improve bioavailability [184], and DOX prepared as SLNs formulation as a conjugate of soybean-oil-based anionic polymer to enhance the efficacy of the drug, are also reported [185].
10.2 Skin cancer
SLNs were effective in treating skin cancers, and were used as topical application for the purpose. DOX when loaded in cationic SLNs, the skin permeation of the formulation was enhanced by 4-folds. However, when similar SLNs formulation was delivered involving iontophoresis, the DOX’s SLNs formulation’s skin permeability enhanced 50-folds [186]. Similarly, the natural product, sesamol, when incorporated into SLNs, stayed longer in the epidermis with least fluctuations experienced in the epidermis [187].
10.3 Prostate cancer
Drugs loaded in SLNs restricted the tumor cell growth. The retinoic acid-loaded SLNs, when prepared by HPH method, showed initial burst release, and later sustained and nearly constant dose drug release in the in vitro condition with tumor growth inhibition at drug concentration levels of 150 µg/mL [188]. A comparison of Lipofectamine 2000® cationic SLNs (charge +40 mV in aqueous media) was found highly effective in treating prostate cancer. With about 100 nm in size, these SLNs did not lose the genetic substance against DNAase digestion, and exhibited transfection in prostate cancer cells [189]. A docetaxel and curcumin co-encapsulated lipid-polymer SLNs formulation for prostate cancer with enhanced anti-tumor activity was also lately reported [190].
10.4 Kidney cancer
Gallic acid ester derivatives have exhibited anti-metastasis and anti-apoptotic activity, and did not harm the surrounded normal cells. The in vitro and in vivo evaluations of the G8-loaded SLNs were found stable, and capable of treating the kidney cancer [191]. An in vivo and in vitro testing of 120 nm sized docetaxel-loaded SLNs showed improved cellular uptake, stability, enhanced cytotoxicity with lower toxicity to surrounding normal cells. SLNs were target-oriented with no side effects for the nearby organs and the normal cells. Thus, SLN formulations showed better therapeutic characteristics as compared to the free drug [192].
11 SLNs in Alzheimer’s disease
Certain naturally occurring phenolics, e.g., resveratrol, inhibited clustering of β-amyloid-protein, considered responsible for Alzheimer’s disease pathogenesis, was SLNs formulated. The preparation avoided the first-pass liver metabolism and was stable for delivery and storage. The resveratrol SLNs were conjugated with antibody-like OX26 mAb for efficient transportation of the entrapped drug to the brain [193]. Galantamine hydrobromide was also able to cross the BBB as SLNs formulation [194]. The RVG-9R peptide, when mixed with BACE1 siRNA and formulated as SLNs, resulted in an efficient nasal drug delivery vehicle for treating Alzheimer’s condition. Herein, the role of RVG-9R was to improve the transcellular pathway of neurons with cells penetration power. The BACE 1 siRNA was found to be effective in relieving the Alzheimer’s conditions [195]. Brain targeted, polysorbate-80 coated piperine SLNs at 2 mg/kg dose levels for Alzheimer’s disease are also reported [196].
12 SLNs for anti-tubercular therapy
Anti-tubercular drugs, i.e., isoniazid, rifampicin, and pyrazinamide were formulated as SLNs, and employed to limit the high dosing frequency [197]. The formulation’s nebulization in an animal model by incorporating these drugs as SLNs was also reported for enhancing their bioavailability and improving the therapeutic efficacy of the drugs [198].
13 SLNs and personalized medicine approach
Anti-cancer drug, docetaxel, appended with anti-FDFR-1-mAb was loaded into SLNs using tristearin after trying different formulation approaches. SLNs surface attachment of FGFR-1-mAb was analyzed by SDS-PAGE method, and confirmed in comparison with the native anti-GFP-1 antibody. These SLNs preparation was used to effectively target the breast cancer cell lines and showed noticeable cytotoxicity of the formulation [199]. Formulation strategy as part of the personalized medicine for cancer treatment was also put forth [182,183].
14 Gene delivery
SLNs are among the most suitable delivery-carrier formulations for genetic materials transportation [200–202]. Incorporation of genetic material, their encased stability, and transport transitional safety, avoidance of carrier’s toxicity, site-specificity, and targeted on-site delivery are comparatively feasible in comparison to structurally delicate liposomes and other nano-structures-based genetic materials delivery to the intended site. Delivery of diametric HIV-1 HAT peptide (TAT 2), delivery of peptidic material, plasmid DNA, and other nucleic acids as SLNs delivery modules, termed as geosphere formats have been reported, wherein SLNs surface was tagged with an antibody for site-specificity [203–205]. The delivery of non-viral, cationic SLNs complexed with a plasmid (shNUPR1) to inhibit the NUPR1 gene expressions, which are responsible for the growth of hepatocellular carcinoma and its chemotherapy resistance, was achieved in the in vitro conditions [206]. Examples of SLNs-based delivery for the CRISPR/Cas9 gene-therapeutics have been reported [207–211]. The in vivo deliveries, cell-lines based transfection efficiency, as well as biocompatibility of the gene therapy, have also been demonstrated [212,213].
Recent advances in gene and drug deliveries [214,215], breakthroughs in SLNs-based deliveries [216,217], self-assembling nucleic acid lipid NPs for gene delivery [203], toxicity and metabolism predictions for SLNs [218], orally delivered lipid drug (nicotine) complex [219], other drugs oral deliveries [220], anti-oxidants delivery [221], and patents information on SLNs delivery are amply available [222].
15 Natural products entrapped SLNs: toward alternative and traditional drug-based personalized medicine
SLNs-based delivery systems have been used to enhance the bioavailability, and therapeutic efficacy of certain bioactive natural products, plant extracts, and fractions (Figure 12) [223]. Ferulic acid, an example of broad range of bioactive natural products available from thousands of flowering plants, especially in the edible fruits and greens category, has been SLNs formulated. Biological and clinical recorded data for ferulic acid proved its anticancer, antioxidant, and cardio-protective activity. However, the oral bioavailability of the drug is poor due to its limited water solubility, and nano-structured lipid carrier (NSL), and SLNs have been used as a vehicle for the ferulic acid delivery to overcome its lower oral bioavailability, wherein the results of in vivo study demonstrated higher peak concentration (Cmax), and the area under the concentration–time curve (AUC) for the NSL and SLNs over the suspension of the aqueous free ferulic acid reflected the enhanced oral bioavailability of the ferulic acid [224].
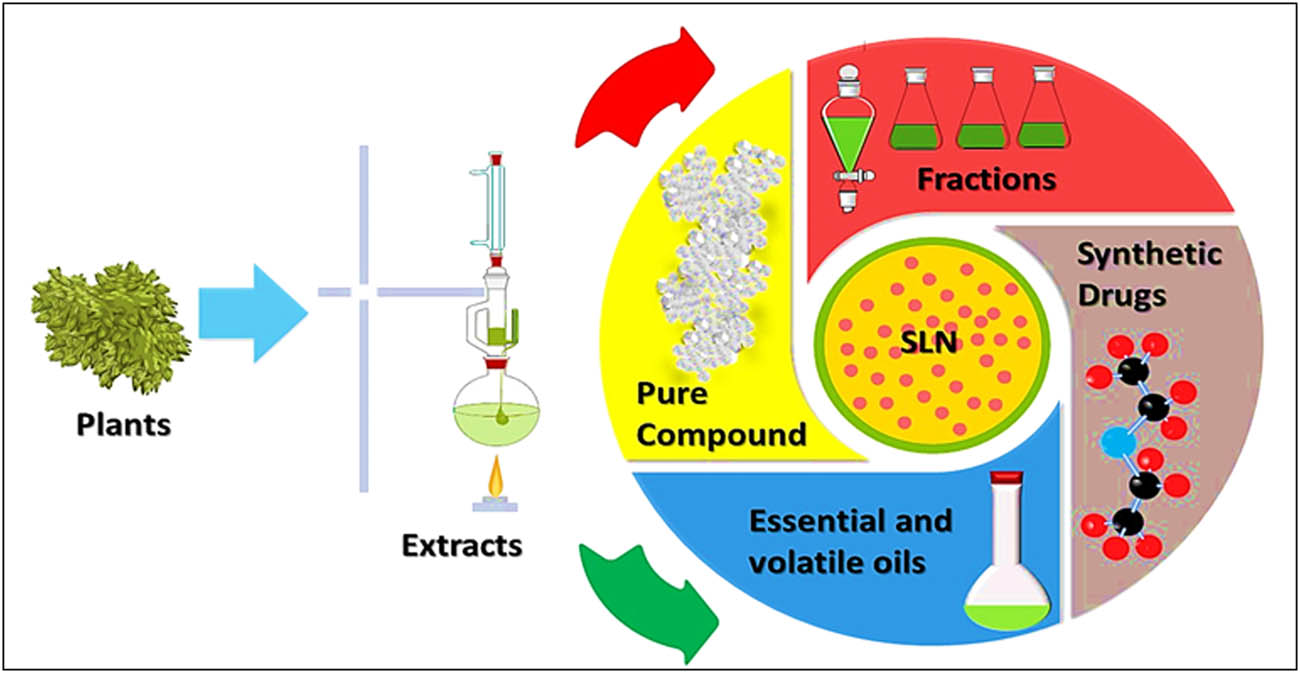
SLNs as the delivery carrier for natural products, extracts, fractions, pure compounds, and essential oils.
Paclitaxel (Taxol®) and docetaxel oral bioavailability was also improved through their incorporations in SLNs. The paclitaxel-loaded hydroxypropyl-b-cyclodextrin-coated SLNs enhanced the uptake of paclitaxel by the Caco-2 cells by about 5.3-folds than the normal paclitaxel suspension [223]. Also, the bioavailability of docetaxel as an anticancer agent for treatment of breast and prostate cancers has been enhanced by their incorporations into SLNs formulations. Docetaxel-loaded SLNs coated with Tween 80, or d-α-tocopheryl poly(ethylene glycol) succinate (TPGS) showed a sustained docetaxel release as compared with the intravenous docetaxel formulation (Taxotere) delivery. The AUC of Tween 80 SLNs, TPGS SLNs, and Taxotere after oral administrations (20 mg/kg) were 7.0, 12.9, and 3.9 mg min/mL, respectively [223]. The intestinal absorption and associated oral bioavailability of the docetaxel in rats were further improved in TPGS SLNs, probably due to better inhibition of the drug efflux by TPGS 1000, along with intestinal lymphatic uptake activity [223].
GSH is one of the thiols that are naturally present in all body tissues. It is highly abundant in the hepatocytes, and plays an essential role in liver protection against free radicals led oxidative damages. The oral bioavailability of GSH is very limited, as the compound is degraded by γ-glutamyl transpeptidase which decreases the absorption level of the drug [224]. Dynasan-114-based solid lipid microparticles (SLM) loaded GSH was prepared with particle sizes of 250–355 µm, and with preserving the physicochemical properties of the GSH, as evidenced by FT-IR, DSC, and HSM analysis, the in vitro release studies using bio-relevant media showed that the Dynasan-114-based SLMs could efficiently release GSH in various intestinal fluids [225]. The amphiphilic lipid Gelucire® 50/13 based SLNs loaded GSH was also prepared to assess the delivery of the GSH to the primary cultures of HK leucocytes of Sparus aurata L. [226]. The formulated SLNs-GSH was stable for 3 months at the temperature of the fridge (4°C). However, on the biology level, the study of Trapani et al. [226] concluded no stimulant activity after incubation of HK leucocytes with SLNs-GSH preparation.
Curcumin, an efficient antioxidant natural product, obtained from Curcuma longa, family Zingiberaceae, has limited oral bioavailability attributed to its poor water solubility, lower absorption, and instability at physiological pH, rapid metabolism, and faster systemic elimination of the compound [117]. Curcumin-loaded SLNs composed of polysorbate 80, and soy lecithin to evaluate the brain delivery of the formulation, and its experimental paradigm of cerebral ischemia (BCCAO model) in rats were prepared [117]. The results proved an improvement in neurological scoring by 79%, and an increment of SOD, catalase, GSH, and mitochondrial complex enzymatic activities. The lipids peroxidation, nitrite, and acetylcholinesterase levels were also decreased by the administration of curcumin-loaded SLNs. In addition, Gamma-scintigraphy studies showed 16.4x and 30c-folds improvement in brain’s drug bioavailability upon oral and intravenous administration of the curcumin-loaded SLNs vs the solubilized curcumin (C-S) [117]. Quercetin, a potent natural antioxidant, belonging to flavonoid class of plant-derived secondary metabolite, was formulated as SLNs of tripalmitin/lecithin lipid core, and with chitosan coat, exhibited better dispersion and release profile in the Caco-2 cells compared to the quercetin powder alone [227]. Also, compritol lipid based SLNs-loaded quercetin has been prepared to enhance the brain delivery and therapeutic effects of quercetin for use in the treatment of Alzheimer’s disease [113,228]. The results obtained from the study revealed significant improvement in the memory retention among the rats that were administrated quercetin-loaded SLNs, as compared to the control, and free quercetin-treated rats [113]. Vincristine is one of the vinca alkaloids used as a chemotherapeutic agent in treatment of several tumors, e.g., breast cancer, lung cancer, and leukemia. Vincristine’s brain delivery and BBB permeability were improved by incorporating the drug within cetyl palmitate-based SLNs with the aid of dextran sodium sulfate (DS) [103].
Several other examples of the natural products loaded SLN formulations are available in the literature, and bioactive natural products, such as, chrysin [103], tocotrienol, cantharidin [222], quinine [229,230], and several other examples available in the literature were intended to improve the drug bioavailability, and therapeutic efficacy in the treatment of diseases was also formulated as SLNs. Also, SLNs formulation has been applied for several plant extracts, semi-purified fractions, and essential oils to achieve the same purposes of enhanced drug bioavailability, and improved therapeutic efficacy [231–235].
16 Surface-coated SLNs: developing targeted delivery specifics
Chitosan-coated-SLNs encapsulated with experimental dye were prepared for delivery to glioblastoma multiforme, a brain tumor type, and were found to provide efficient delivery to the site as compared to the non-chitosan coated SLNs. The coated SLNs were embedded in O-carboxy methyl chitosan containing nanofibers. The strategy was suggested as fit for the delivery of lipophilic anti-proliferative drugs to the brain tumor site [236]. In another experiment, hyaluronic acid coating of docetaxel encapsulating SLNs was prepared by utilizing the stearic acid, phosphatidylcholine, and hexadecyl trimethyl ammonium bromide. An in vitro delivery experiment confirmed the utility and efficacy of the preparation. Better cellular uptake, and cytotoxicity was observed against MCF7, MDA-MB-231, and MCF7/ADR cells [237]. Silk fibroin-coated SLNs were found to be better in ceramide’s skin permeation owing to the positively charged fibroin and the negatively charged skin [238]. Docetaxel-loaded SLNs with chitosan coating were found to exhibit slower release of the drug, better cytotoxicity, and improved tumor inhibitions, as compared to the non-coated SLNs. Increased particle size and zeta potential charge inversion after coating was also observed [239].
Orally delivered, polysaccharide-coated SLNs were prepared for colon cancer delivery as a chemo/thermotherapy formulation. The super paramagnetic iron oxide NPs and DOX-loaded SLNs were coated and observed for their delivery to cancer cells which primarily avoided systemic drug absorption, and were delivered in an enhanced ratio than the uncoated SLNs. A coating of folate and then dextran were applied, and the coated SLNs provided enhanced residences for SLNs. The removal of outer dextran coating by enzymatic degradation, and the exposure of the folate coat provided better targeting and uptake. The oral delivery of the formulation confirmed its utility through enhanced function and bioactivity [240]. MCF-7 breast cancer cell’s delivery of the chitosan and hyaluronan coated SLNs provided better targeting, uptake, and time and dose-controlled delivery, together with the facile release of the loaded drug, paclitaxel, thereby enhancing the chemotherapeutic value of the loaded anticancer drug [241]. Alginate-coated SLNs for oral insulin delivery is also reported [242].
For lung delivery through inhalation, SLNs coated with folate-grafted-PEG-chitosan derivatives were prepared, and the formulation provided delivery feasibility, improved pharmacokinetics, provided better pulmonary exposure, and prolonged lung residence of the coated SLNs with the very limited systemic distribution. For in vitro delivery, the coated SLNs feasibly reached the folate expressing HeLa and the M109-HiFR cell lines, and reduced the half-maximum inhibitory concentrations of the drug in M109-HiFR cell lines based activity evaluations [243].
The thiolated chitosan derivatives, obtained through amidation with the l-cysteine and 6-mercaptonicotinic acid in single and multi-capping procedures, were used to coat SLNs for evaluating their mucoadhesive properties. The cysteine-thiolated chitosan, 6-mercapto nicotinic acid-S-protected thiolated chitosan, and l-cysteine-S-protected thiolated chitosan were tested for their mucous interaction with the porcine intestinal mucosa, and the 6-mercapto nicotinic acid-S-protected thiolated chitosan was found most interactive in comparison to other chitosan derivatives and the pure chitosan, while its mucus diffusion was the least. This derivative also showed the highest retention in the intestinal mucosa [244]. Eudragit S100 coated SLNs were also prepared for delivery to the colon, and the optimal colon targeted parameters were achieved [245].
Folic acid trapped SLNs incorporating ferulic acid-trans-resveratrol coated with chitosan for targeted delivery to colon cancer cells toward enhanced apoptosis have been reported [246]. For oral adenocarcinoma cell lines delivery of the model drug, trans-retinoic acid, the phosphatidylethanolamine polyethylene glycol (PE–PEG) coating of the prepared SLNs provided reduced non-specific internalizations, enhanced delivery, as well as enhanced chemotoxicity effects as compared to the non-coated SLNs [247].
Magnetic and chitosan-coated SLNs delivering letrozole to cancer cell lines were also prepared, and it was found that chemotherapeutic efficiency was increased in several anti-cancer testing set-ups, i.e., HUVEC (normal cell lines), MCF7 (breast adenocarcinoma), Hs 578Bst (breast carcinoma), Hs 319.T (infiltrating ductal cell carcinoma), UACC-3133 (infiltrating lobular carcinoma of breast), UACC-732 (inflammatory carcinoma of the breast), and MDA-MB-453 (metastatic carcinoma) cell lines [248]. Yet, in another development, chlorin e6 loaded SLNs with gastric cancer cell membrane attachments were prepared for the photodynamic therapy of gastric cancer [249]. The primal drug resistance in tumors was ameliorated through the hyaluronic acid coating of the DOX-loaded SLNs [250]. The chitosan coated trans-resveratrol, as well as ferulic acid, loaded SLNs in conjugation with folic acid, were tested, which showed better stability in a simulated acidic media and showed enhanced anticancer activity in HT-29 cell lines. The preparation also exhibited regularization of the expression of cyclin proteins, and also showed enhanced cell cycle arrests [251]. A comparative analysis of the nano-structured lipid carriers and SLNs with chitosan coating and fatty acid release revealed that the NLC showed more rapid digestion than SLNs due to the use of the liquid state lipid constituent. The free fatty acid release from chitosan-coated SLNs were nearly similar to the plain SLNs, and the chitosan-coated NLCs showed greater fatty acid releases than the uncoated NLCs. The information was proposed to be of important implications in the functional foods and beverages preparations to achieve lipid digestion controls in the GIT [252]. SLNs internalizations achieved through endocytosis were modulated by SLNs surface coatings. The PE–PEG coatings of the Epikuron 200, stearic acid, and sodium taurodeoxycholate-based SLNs provided targeted cellular internalization of the preparation in oral adenocarcinoma cell lines which thereby reduced the non-specific internalization of the prepared SLNs and provided enhanced chemo-toxic effects as compared to the non-coated SLNs [253].
17 Toward SLNs-entrapped drugs’ commercialization
It would be pertinent to discuss the commercial standpoint of SLNs formulated drugs from the continual market share and availability to public outlets. The current market share of nano-formulated drugs has reached over 50 billion USD which was at 50.32 billion in 2020 and is expected to reach over 88 billion USD by 2028 through growing at an expected rate of 7.3% annually. SLNs segment accounted for the major part of the revenue share for the first time in 2020 [254]. An increasing trend is expected and forecasted [255,256]. The COVID-19 situation has also contributed, and the marketed formulations, BNT162b2 vaccine (BioNTech and Pfizer) and mRNA-1273 vaccine (Moderna Inc.) developed as nucleoside-modified mRNA encoding the viral spike glycoprotein of SARS-CoV-2 encapsulated in lipid NPs, and lipid NP-encapsulated mRNA-based vaccine, which encodes the spike protein (S protein) of SARS-CoV-2, respectively, which have provided protection of the used RNA variants, were World health organization-endorsed, commercially available prescriptions.
18 Conclusion
SLNs as relatively new delivery vesicles have of lately attracted much attention with their versatility, functional, and bio-transport advantages, together with ease of preparation and stability, comparatively safe storage, as well as sustained drugs release profiles. The observation that natural lipids can be an encapsulating material has given SLNs an added advantage over other transport vehicles involved in targeted and specialized cellular delivery. The functional solid matrix of SLNs protected the embedded drug from the biochemically exposed physiological environments. and allowed sustained release with improved body tolerance and low toxicity to the organisms, along with delivery to different organs in a biocompatible manner. The surface coatings of SLNs also improved the delivery kinetics, bioavailability, and biological activity together with an enhanced biological half-life of the lipid vesicles, and the availability of the intact drugs at recommended concentrations. The feasibility of SLNs delivery to different cancers and barrier-constrained anatomical sites, especially the brain, and deliveries of the payloads to the kidneys, pancreas, bone marrow, lungs, heart, and other relevant organs, together with physiological conditions of various cancers and other ailments, have provided newer and better approach of drug delivery with enhanced bioaction by SLNs incorporated drugs.
19 Future developmental directions and newer prospects in delivery modules
The advancements and applications in personalized nanomedicines, superior biodegradability of the formulation, and non-allergenic formulations’ development together with encapsulation/loading of multiple drugs of contrasting nature and structural classes, as well as the advancements in scale-up and industrial-scale productions, storage, and distant location transports, are some of the desired elements for sustained growth and outreach of SLNs-based drug delivery products.
Developments into SLNs-based products ranging from cancer nanomedicine to other disease segments, primarily in cardiovascular disorders, Alzheimer’s and Parkinsonism, anti-tubercular, and the ongoing COVID-19 therapeutic developments as SLNs based formulations are also envisioned [257]. SLNs technology development and product patenting have been followed [94,258] toward encasing the developments in the field which is needed to speed up.
Acknowledgments
The researchers would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
-
Funding information: Deanship of Scientific Research, Qassim University funded the publication of this project.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
References
[1] Mukherjee S, Ray S, Thakur R. Solid lipid nanoparticles: A modern formulation approach in the drug delivery system. Indian J Pharm Sci. 2009;71(4):349–58. 10.4103/0250-474X.57282.Search in Google Scholar PubMed PubMed Central
[2] Choonara YE, Pillay V, Ndesendo VM, du Toit LC, Kumar P, Khan RA, et al. Polymeric emulsion and crosslink-mediated synthesis of super-stable nanoparticles as sustained-release anti-tuberculosis drug carriers. Colloids Surf B Biointerfaces. 2011;87(2):243–54. 10.1016/j.colsurfb.2011.05.025.Search in Google Scholar PubMed
[3] Akhtar N, Khan RA. Liposomal systems as viable drug delivery technology for skin cancer sites with an outlook on lipid-based delivery vehicles and diagnostic imaging inputs for skin conditions. Prog lipid Res. 2016;64:192–230. 10.1016/j.colsurfb.2011.05.025.Search in Google Scholar
[4] Akhtar N, Mohammed SA, Khan RA, Yusuf M, Singh V, Mohammed HA, et al. Self-generating nano-emulsification techniques for alternatively routed, bioavailability enhanced delivery, especially for anti-cancers, anti-diabetics, and miscellaneous drugs of natural, and synthetic origins. J Drug Delivery Sci Technol. 2020;58:101808. 10.1016/j.jddst.2020.101808.Search in Google Scholar
[5] Yusuf M, Khan M, Khan RA, Maghrabi IA, Ahmed B. Polysorbate-80-coated, polymeric curcumin nanoparticles for in vivo anti-depressant activity across BBB and envisaged biomolecular mechanism of action through a proposed pharmacophore model. J Microencapsul. 2016;33(7):646–55. 10.1080/02652048.2016.1242666.Search in Google Scholar PubMed
[6] Khateef R, Khadri H, Almatroudi A, Alsuhaibani SA, Mobeen SA, Khan RA. Potential in-vitro anti-breast cancer activity of green-synthesized silver nanoparticles preparation against human MCF-7 cell-lines. Adv Nat Sci Nanosci Nanotechnol. 2019;10:045012. 10.1088/2043-6254/ab47ff.Search in Google Scholar
[7] Akhtar N, Mohammed HA, Yusuf M, Al-Subaiyel A, Sulaiman GM, Khan RA. SPIONs Conjugate Supported Anticancer Drug Doxorubicin’s Delivery: Current Status, Challenges, and Prospects. Nanomaterials. 2020;12(20):3686. 10.3390/nano12203686.Search in Google Scholar PubMed PubMed Central
[8] Sibeko B, Choonara YE, du Toit LC, Modi G, Naidoo D, Khan RA, et al. Composite polylactic-methacrylic acid copolymer nanoparticles for the delivery of methotrexate. J drug delivery. 2012;2012:579629. 10.1155/2012/579629.Search in Google Scholar PubMed PubMed Central
[9] Qureshi KA, Mohammed SA, Khan O, Ali HM, El-Readi MZ, Mohammed HA. Cinnamaldehyde-Based Self-Nanoemulsion (CA-SNEDDS) Accelerates Wound Healing and Exerts Antimicrobial, Antioxidant, and Anti-Inflammatory Effects in Rats’ Skin Burn Model. Molecules. 2022;27(16):5225. 10.3390/molecules27165225.Search in Google Scholar PubMed PubMed Central
[10] Jumaa M, Muller BW. Lipid emulsions as a novel system to reduce the hemolytic activity of lytic agents: Mechanism of protective effect. Eur J Pharm Sci. 2000;9:285–90. 10.1016/s0928-0987(99)00071-8.Search in Google Scholar PubMed
[11] Cavalli R, Caputo O, Gasco MR. Solid lipospheres of doxorubicin and idarubicin. Int J Pharm. 1993;89:R9–12. 10.1016/S0928-0987(99)00071-8.Search in Google Scholar
[12] Scheffel U, Rhodes BA, Natarajan TK, Wagner HN. Albumin microspheres for study of the reticuloendothelial system. J Nuc Med. 1972;13(7):498–503.Search in Google Scholar
[13] Sabapati M, Palei NN, Ashok AK, Molakpogu RB. Solid lipid nanoparticles of Annona muricata fruit extract: formulation, optimization and in vitro cytotoxicity studies. Drug Dev Ind Pharm. 2019;45(4):577–86. 10.1080/03639045.2019.1569027.Search in Google Scholar PubMed
[14] Nooli M, Chella N, Kulhari H, Shastri NR, Sistla R. Solid lipid nanoparticles as vesicles for oral delivery of olmesartan medoxomil: formulation, optimization and in vivo evaluation. Drug Dev Ind Pharm. 2017;43(4):611–7. 10.1080/03639045.2016.1275666.Search in Google Scholar PubMed
[15] Gasco MR. Method for producing solid lipid microspheres having a narrow size distribution. United States patent, US 188837; 1993.Search in Google Scholar
[16] Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Delivery Rev. 2002;54:131–55. 10.1016/S0169-409X(02)00118-7.Search in Google Scholar
[17] Saez V, de Souza IDL, Mansur CRE. Lipid nanoparticles (SLN & NLC) for delivery of vitamin E: A comprehensive review. Int J Cosmetic Sci. 2018;40(2):103–16. 10.1111/ics.12452.Search in Google Scholar PubMed
[18] Kalaycioglu GD, Aydogan N. Preparation and investigation of solid lipid nanoparticles for drug delivery. Colloids Surf A Physicochem Eng Asp. 2016;510:77–86. 10.1016/j.colsurfa.2016.06.034.Search in Google Scholar
[19] Khurana S, Bedi PMS, Jain NK. Preparation and evaluation of solid lipid nanoparticles based nanogel for dermal delivery of meloxicam. Chem Phys Lipids. 2013;175:65–72. 10.1016/j.chemphyslip.2013.07.010.Search in Google Scholar PubMed
[20] Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization, and application. Adv Drug Del Rev. 2001;47(2–3):165–96. 10.1016/s0169-409x(01)00105-3.Search in Google Scholar PubMed
[21] Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of clozapine solid lipid nanoparticles after intravenous and intraduodenal administration. J Control Rel. 2005;107(2):215–28. 10.1016/j.jconrel.2005.06.006.Search in Google Scholar PubMed
[22] Ebrahimi HA, Javadzadeh Y, Hamidi M, Jalali MB. Repaglinide-loaded solid lipid nanoparticles: effect of using different surfactants/stabilizers on the physicochemical properties of nanoparticles. DARU J Pharma Sci. 2015;23(1):46. 10.1186/s40199-015-0128-3.Search in Google Scholar PubMed PubMed Central
[23] Pizzol CD, Filippin-Monteiro FB, Restrepo JA, Pittella F, Silva AH, Alves de Souza P, et al. Influence of surfactant and lipid type on the physicochemical properties and biocompatibility of solid lipid nanoparticles. Inter J Env Res Pub Health. 2014;11(8):8581–96. 10.3390/ijerph110808581.Search in Google Scholar PubMed PubMed Central
[24] Silva AC, Kumar A, Wild W, Ferreira D, Santos D, Forbes B. Long-term stability, biocompatibility, and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Inter J Pharm. 2012;436(1):798–805. 10.1016/j.ijpharm.2012.07.058.Search in Google Scholar PubMed
[25] Göppert TM, Müller RH. Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting. Inter J Pharm. 2005;302(1):172–86. 10.1016/j.ijpharm.2005.06.025.Search in Google Scholar PubMed
[26] Mueller RH, Maeder K, Gohla S. Solid lipid nanoparticles (SLNs) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77. 10.1016/s0939-6411(00)00087-4.Search in Google Scholar PubMed
[27] Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1–2):121–8. 10.1016/s0378-5173(02)00180-1.Search in Google Scholar PubMed
[28] Muller RH, Runge SA. Solid lipid nanoparticles (SLN) for controlled drug delivery. In: Benita S, editor. Submicron emulsions in drug targeting and delivery. Amsterdam: Harwood Academic Publishers; 1998. p. 219–34.10.1201/9780367810528-9Search in Google Scholar
[29] Jenning V, Gysler A, Schafer-Korting M, Gohla S. Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2000;49:211–8.10.1016/S0939-6411(99)00075-2Search in Google Scholar
[30] Mohammadi P, Mahjub R, Mohammadi M, Derakhshandeh K, Ghaleiha A, Mahboobian MM. Pharmacokinetics and brain distribution studies of perphenazine-loaded solid lipid nanoparticles. Drug Dev Ind Pharm. 2021;47:146–52. 10.1080/03639045.2020.1862172.Search in Google Scholar PubMed
[31] Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Delivery Rev. 2007;59(6):478–90. 10.1016/j.addr.2007.04.007.Search in Google Scholar PubMed
[32] Satapathy MK, Yen TL, Jan JS, Tang RD, Wang JY, Taliyan R, et al. Solid lipid nanoparticles (SLNs): an advanced drug delivery system targeting brain through BBB. Pharmaceutics. 2021;13(8):1183. 10.3390/pharmaceutics13081183.Search in Google Scholar PubMed PubMed Central
[33] Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):1–33. 10.1186/s12951-018-0392-8.Search in Google Scholar PubMed PubMed Central
[34] Muller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of the drug. Int J Pharm. 2002;242:121–8.10.1016/S0378-5173(02)00180-1Search in Google Scholar
[35] Radtke M, Muller RH. Comparison of structural properties of solid lipid nanoparticles (SLN) versus other lipid particles. Proc Int Symp Control Rel Bioact Mater. 2000;27:309–10.Search in Google Scholar
[36] Uner M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie. 2006;61:375–86.Search in Google Scholar
[37] Souto EB, Wissing SA, Barbosa CM, Muller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278:71–7.10.1016/j.ijpharm.2004.02.032Search in Google Scholar PubMed
[38] Souto EB, Muller RH. Investigation of the factors influencing the incorporation of clotrimazole in SLN and NLC prepared by hot high-pressure homogenization. J Microencap. 2006;23:377–88.10.1080/02652040500435295Search in Google Scholar PubMed
[39] Souto EB, Muller RH. SLN and NLC for topical delivery of ketoconazole. J Microencap. 2005;22:501–10.10.1080/02652040500162436Search in Google Scholar PubMed
[40] Souto EB, Muller RH. The use of SLN and NLC as topical particulate carriers for imidazole antifungal agents. Pharmazie. 2006;61:431–37.Search in Google Scholar
[41] Morel S, Terreno E, Ugazio E, Aime S, Gasco MR. NMR relaxometric investigations of lipid nanoparticles (SLN) containing gadolinium (III) complexes. Eur J Pharm Biopharm. 1998;45:157–63.10.1016/S0939-6411(97)00107-0Search in Google Scholar PubMed
[42] Olbrich C, Gebner A, Kayser O, Muller RHL. Lipid–drug conjugate (LDC) nanoparticles as a novel carrier system for the hydrophilic anti-trypanosomal drug diminazene diaceturate. J Drug Target. 2002;10:387–96.10.1080/1061186021000001832Search in Google Scholar PubMed
[43] Shah RM, Rajasekaran D, Ludford-Menting M, Eldridge DS, Palombo EA, Harding IH. Transport of stearic acid-based solid lipid nanoparticles (SLNs) into human epithelial cells. Colloids Surf B: Biointerfaces. 2016;140:204–12. 10.1016/j.colsurfb.2015.12.029.Search in Google Scholar PubMed
[44] Lim SB, Banerjee A, Önyüksel H. Improvement of drug safety by the use of lipid-based nanocarriers. J Control Rel. 2012;163(1):34–45. 10.1016/j.jconrel.2012.06.002.Search in Google Scholar PubMed
[45] Gastaldi L, Battaglia L, Peira E, Chirio D, Muntoni E, Solazzi I, et al. Solid lipid nanoparticles as vehicles of drugs to the brain: current state of the art. Eur J Pharm Biopharm. 2014;87(3):433–44. 10.1016/j.ejpb.2014.05.004.Search in Google Scholar PubMed
[46] Makwana V, Jain R, Patel K, Nivsarkar M, Joshi A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int J Pharmaceutics. 2015;495(1):439–46. 10.1016/j.ijpharm.2015.09.014.Search in Google Scholar PubMed
[47] Alex MA, Chacko AJ, Jose S, Souto EB. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur J Pharm Sci. 2011;42(1–2):11–8. 10.1016/j.ejps.2010.10.002.Search in Google Scholar PubMed
[48] Chen H, Chang X, Du D, Liu W, Liu J, Weng T, et al. Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Controlled Rel. 2006;110(2):296–306. 10.1016/j.jconrel.2005.09.052.Search in Google Scholar PubMed
[49] Liu J, Hu W, Chen H, Ni Q, Xu H, Yang X. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328:191–5. 10.1016/j.ijpharm.2006.08.007.Search in Google Scholar PubMed
[50] Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–89. 10.1002/ijc.33588.Search in Google Scholar PubMed
[51] Malik Z, Parveen R, Abass S, Dar MI, Husain SA, Ahmad S. Receptor-mediated targeting in breast cancer through solid lipid nanoparticles and its mechanism. Curr Drug Metab. 2022;23(10):800–17. 10.2174/1389200223666220416213639.Search in Google Scholar PubMed
[52] Martins S, Costa-Lima S, Carneiro T, Cordeiro-daSilva A, Souto EB, Ferreira DC. Solid lipid nanoparticles as intracellular drug transporters: an investigation of the uptake mechanism and pathway. Int J Pharm. 2012;430(1–2):216–27. 10.1016/j.ijpharm.2012.03.032.Search in Google Scholar PubMed
[53] Singh I, Swami R, Pooja D, Jeengar MK, Khan W, Sistla R. Lactoferrin bioconjugated solid lipid nanoparticles: a new drug delivery system for potential brain targeting. J drug tar-geting. 2016;24(3):212–23. 10.3109/1061186X.2015.1068320.Search in Google Scholar PubMed
[54] Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J Control Rel. 1999;59:299–307. 10.1016/S0168-3659(99)00007-3.Search in Google Scholar
[55] Martins SM, Sarmento B, Nunes C, Lúcio M, Reis S, Ferreira DC. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur J Pharm Biopharm. 2013;85:488–502. 10.1016/j.ejpb.2013.08.011.Search in Google Scholar PubMed
[56] Parvez S, Kaushik M, Ali M, Alam MM, Ali J, Tabassum H, et al. Dodging blood brain barrier with “nano” warriors: Novel strategy against ischemic stroke. Theranostics. 2022;12(2):689–719. 10.7150/thno.64806.Search in Google Scholar PubMed PubMed Central
[57] Dal Magro R, Ornaghi F, Cambianica I, Beretta S, Re F, Musicanti C, et al. ApoE-modified solid lipid nanoparticles: A feasible strategy to cross the blood-brain barrier. J Controlled Rel. 2017;249:103–10. 10.1016/j.jconrel.2017.01.039.Search in Google Scholar PubMed
[58] Ghasemiyeh P, Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res Pharm Sci. 2018;13(4):288–303. 10.4103/1735-5362.235156.Search in Google Scholar PubMed PubMed Central
[59] Garcês A, Amaral MH, Lobo JS, Silva AC. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use: A review. Eur J Pharm Sci. 2018;112:159–67. 10.1016/j.ejps.2017.11.023.Search in Google Scholar PubMed
[60] Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72. 10.1016/j.addr.2003.12.002.Search in Google Scholar PubMed
[61] Patel MH, Mundada VP, Sawant KK. Fabrication of solid lipid nanoparticles of lurasidone HCl for oral delivery: Optimization, in vitro characterization, cell line studies and in vivo efficacy in schizophrenia. Drug Dev Ind Pharm. 2019;45(8):1242–57. 10.1080/03639045.2019.1593434.Search in Google Scholar PubMed
[62] Domb AJ. Lipospheres for controlled delivery of substances. United States Patent, US 188837; 1993.10.1201/9781420027990.ch10Search in Google Scholar
[63] Ahlin P, Kristl J, Kobar S. Optimization of procedure parameters and physical stability of solid lipid nanoparticles in dispersion. Acta Pharm. 1998;48:257–67.Search in Google Scholar
[64] Cavalli R, Marengo E, Rodriguez L, Gasco MR. Effects of some experimental factors on the production process of solid lipid nanoparticles. Eur J Pharm Biopharm. 1996;43:110–15.Search in Google Scholar
[65] Jahnke S. The theory of high-pressure homogenization. In: Muller RH, Benita S, Bohm B, editors. Emulsions and nanosuspensions for the formulation of poorly soluble drugs. Stuttgart: Medpharm Scientific Publishers; 1998. p. 177–200.Search in Google Scholar
[66] Mullen zur A. Feste Lipid-Nanopartikel mit prolongierter Wirkstoffliberation: Herstellung, Langzeitstabilitat, Charakterisierung, Freisetzungsverhalten und mechanismen. PhD thesis. Germany: Free University of Berlin; 1996.Search in Google Scholar
[67] Sjostrom B, Bergenstahl B. Preparation of submicron drug particles in lecithin-stabilized o/w emulsions: I: Model studies of the precipitation of cholesteryl acetate. Int J Pharm. 1992;88:53–62.10.1016/0378-5173(92)90303-JSearch in Google Scholar
[68] Jenning V, Lippacher A, Gohla SH. Medium-scale production of solid lipid nanoparticles (SLNs) by high-pressure homogenization. J Microencap. 2002;19(1):1–10.10.1080/713817583Search in Google Scholar PubMed
[69] Cavalli R, Caputo O, Marengo E, Pattarino F, Gasco M. The effect of the components of microemulsions on both size and crystalline structure of solid lipid nanoparticles containing the series of model molecules. Pharmazie. 1998;53(6):392–96.Search in Google Scholar
[70] Khairnar SV, Pagare P, Thakre A, Nambiar AR, Junnuthula V, Abraham MC, et al. Review on the scale-up methods for the preparation of solid lipid nanoparticles. Pharmaceutics. 2022;14:1886. 10.3390/pharmaceutics14091886.Search in Google Scholar PubMed PubMed Central
[71] Albukhaty S, Al-Musawi S, Abdul Mahdi S, Sulaiman GM, Alwahibi MS, Dewir YH, et al. Investigation of dextran-coated superparamagnetic nanoparticles for targeted vinblastine controlled release, delivery, apoptosis induction, and gene expression in pancreatic cancer cells. Molecules. 2020;25(20):4721. 10.3390/molecules25204721.Search in Google Scholar PubMed PubMed Central
[72] Schwarz C, Mehnert W, Lucks JS, Müller RH. Solid lipid nanoparticles (SLNs) for controlled drug delivery. I. Production, characterization, and sterilization. J Control Rel. 1994;30(1):83–96.10.1016/0168-3659(94)90047-7Search in Google Scholar
[73] Lander R, Manger W, Scouloudis M, Ku A, Davis C, Lee A. Gaulin homogenization: A mechanistic study. Biotech Prog. 2000;16(1):80–5.10.1021/bp990135cSearch in Google Scholar PubMed
[74] Kasongo KW, Muller RH, Walker RB. The use of hot and cold high pressure homogenization to enhance the loading capacity and encapsulation efficiency of nanostructured lipid carriers for the hydrophilic antiretroviral drug, didanosine for potential administration to paediatric patients. Pharm Dev Technol. 2012;17(3):353–62. 10.3109/10837450.2010.542163.Search in Google Scholar PubMed
[75] Subroto E, Andoyo R, Indiarto R, Wulandari E, Wadhiah EFN. Preparation of solid Lipid nanoparticle-ferrous sulfate by double emulsion method based on fat rich in monolaurin and stearic acid. Nanomaterials. 2022;12(17):3054. 10.3390/nano12173054.Search in Google Scholar PubMed PubMed Central
[76] Sjöström B, Kaplun A, Talmon Y, Cabane B. Structures of nanoparticles prepared from oil-in-water emulsions. Pharma Res. 1995;12(1):39–48.10.1023/A:1016278302046Search in Google Scholar
[77] Shahgaldian P, Da Silva E, Coleman AW, Rather B, Zaworotko MJ. Para-acyl-calixarene-based solid lipid nanoparticles (SLNs): a detailed study of preparation and stability parameters. Inter J Pharma. 2003;253(1):23–38.10.1016/S0378-5173(02)00639-7Search in Google Scholar PubMed
[78] Satari N, Taymouri S, Varshosaz J, Rostami M, Mirian M. Preparation and evaluation of inhalable dry powder containing glucosamine-conjugated gefitinib SLNs for lung cancer therapy. Drug Dev Ind Pharm. 2020;46(8):1265–77. 10.1080/03639045.2020.1788063.Search in Google Scholar PubMed
[79] Yousry C, Fahmy RH, Essam T, El-Laithy HM, Elkheshen SA. Nanoparticles as tool for enhanced ophthalmic delivery of vancomycin: a multidistrict-based microbiological study, solid lipid nanoparticles formulation and evaluation. Drug Dev Ind Pharm. 2016;42(11):1752–62. 10.3109/03639045.2016.1171335.Search in Google Scholar PubMed
[80] Eltom SEM, Abdellatif AAH, Maswadeh H, Al-Omar MS, Abdel-Hafez AA, Mohammed HA, et al. The anti-inflammatory effect of a γ-lactone isolated from ostrich oil of Struthio camelus (Ratite) and its formulated nano-emulsion in formalin-induced paw edema. Molecules. 2021;26(12):3701. 10.3390/molecules26123701.Search in Google Scholar PubMed PubMed Central
[81] Schubert MA, Müller-Goymann CC. Solvent injection as a new approach for manufacturing lipid nanoparticles-evaluation of the method and process parameters. Eur J Pharm Biopharm. 2003;55(1):125–31.10.1016/S0939-6411(02)00130-3Search in Google Scholar
[82] Yang R, Gao R, Li F, He H, Tang X. The influence of lipid characteristics on the formation, in vitro release, and in vivo absorption of protein-loaded SLN prepared by the double emulsion process. Drug Dev Ind Pharm. 2011;37(2):139–48. 10.3109/03639045.2010.497151.Search in Google Scholar PubMed
[83] Hillert M. A solid-solution model for inhomogeneous systems. Acta metallurgica. 1961;9(6):525–35. 10.1016/0001-6160(61)90155-9.Search in Google Scholar
[84] Zur Muhlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery-drug release and release mechanism. Eur J Pharm Biopharm. 1998;45(2):149–55. 10.1016/s0939-6411(97)00150-1.Search in Google Scholar PubMed
[85] Domb A. Long-acting injectable oxytetracycline-liposphere formulations. Inter J Pharm. 1995;124(2):271–8.10.1016/0378-5173(95)00098-4Search in Google Scholar
[86] Esposito E, Fantin M, Marti M, Drechsler M, Paccamiccio L, Mariani P. Solid lipid nanoparticles as delivery systems for bromocriptine. Pharma Res. 2008;25(7):1521–30.10.1007/s11095-007-9514-ySearch in Google Scholar PubMed
[87] Naguib Y, Rodriguez B, Li X, Hursting S, Williams R, Cui Z. Solid lipid nanoparticle formulations of docetaxel prepared with high melting point triglycerides: In vitro and in vivo evaluations. Mol Pharm. 2014;11(4):1239–49.10.1021/mp4006968Search in Google Scholar PubMed PubMed Central
[88] Mokhtar N, Khan N, Darwis Y. Solid lipid nanoparticles of atovaquone based on 24 full-factorial design. Iran J Pharm Res. 2015;14(4):989–1000.Search in Google Scholar
[89] Xu W, Lim S, Lee M. Cellular uptake and antitumor activity of paclitaxel incorporated into trilaurin-based solid lipid nanoparticles in ovarian cancer. J Microencapsul. 2013;30(8):755–61. 10.3109/02652048.2013.Search in Google Scholar
[90] Manjunath K, Venkateswarlu V, Hussain A. Preparation and characterization of nitrendipine solid lipid nanoparticles. Pharmazie. 2011;66:178–86.Search in Google Scholar
[91] Martins S, Silva A, Ferreira D, Souto E. Improving oral absorption of salmon calcitonin by trimyristin lipid nanoparticles. J Biomed Nanotech. 2009;5(1):76–83.10.1166/jbn.2009.443Search in Google Scholar PubMed
[92] Patel M, San Martin-Gonzalez M. Characterization of ergocalciferol loaded solid lipid nanoparticles. J Food Sci. 2012;77(1):N8–13.10.1111/j.1750-3841.2011.02517.xSearch in Google Scholar PubMed
[93] Puglia C, Offerta A, Tirendi G, Tarico M, Curreri S, Bonina F. Design of solid lipid nanoparticles for caffeine topical administration. Drug Del. 2014;23(1):36–40.10.3109/10717544.2014.903011Search in Google Scholar PubMed
[94] Tran TH, Ramasamy T, Truong DH, Shin BS, Choi HG, Yong CS, et al. Development of vorinostat-loaded solid lipid nanoparticles to enhance pharmacokinetics and efficacy against multidrug-resistant cancer cells. Pharm Res. 2014;31:1978–88. 10.1007/s11095-014-1300-z.Search in Google Scholar PubMed
[95] Abdelbary G, Fahmy RH. Diazepam-loaded solid lipid nanoparticles: design and characterization. AAPS PharmSciTech. 2009;10(1):211–9. 10.1208/s12249-009-9197-2.Search in Google Scholar PubMed PubMed Central
[96] Madureira AR, Nunes S, Campos DA, Fernandes JC, Marques C, Zuzarte M, et al. Safety profile of solid lipid nanoparticles loaded with rosmarinic acid for oral use: in vitro and animal approaches. Int J Nanomed. 2016;11:3621–40. 10.2147/IJN.S104623.Search in Google Scholar PubMed PubMed Central
[97] Campos D, Madureira A, Gomes A, Sarmento B, Pintado M. Optimization of the production of solid Witepsol nanoparticles loaded with rosmarinic acid. Colloids Surf B Biointerfaces. 2014;115:109–17.10.1016/j.colsurfb.2013.10.035Search in Google Scholar PubMed
[98] Sütő B, Berkó S, Kozma G, Kukovecz Á, Budai-Szűcs M, Erős G, et al. Development of ibuprofen-loaded nanostructured lipid carrier-based gels: characterization and investigation of in vitro and in vivo penetration through the skin. Int J Nanomed. 2016;11:1201–12. 10.2147/IJN.S99198.Search in Google Scholar PubMed PubMed Central
[99] Monteiro LM, Löbenberg R, Cotrim PC, Barros de Araujo GL, Bou-Chacra N. Buparvaquone nanostructured lipid carrier: Development of an affordable delivery system for the treatment of leishmaniases. Biomed Res Int. 2017;2017:9781603. 10.1155/2017/9781603.Search in Google Scholar PubMed PubMed Central
[100] Shenoy VS, Gude RP, Murthy RSR. In vitro anticancer evaluation of 5-fluorouracil lipid nanoparticles using B16F10 melanoma cell lines. Int Nano Lett. 2013;36:3–9.10.1186/2228-5326-3-36Search in Google Scholar
[101] Ekambaram P, Abdul-Hasan SA. Formulation and evaluation of solid lipid nanoparticles of ramipril. J Young Pharm. 2011;3(3):216–20.10.4103/0975-1483.83765Search in Google Scholar PubMed PubMed Central
[102] Liu J, Gong T, Wang C, Zhong Z, Zhang Z. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: Preparation and characterization. Inter J Pharm. 2007;340(1–2):153–62.10.1016/j.ijpharm.2007.03.009Search in Google Scholar PubMed
[103] Aishwarya V, Sumathi T. Enhanced blood–brain barrier transmigration using the novel chrysin embedded solid lipid nanoformulation: A salient approach on physico-chemical characterization, pharmacokinetics and biodistribution studies. Int J Pharm Clin Res. 2016;8:1574–82.Search in Google Scholar
[104] Subedi RK, Kang KW, Choi HK. Preparation and characterization of solid lipid nanoparticles loaded with doxorubicin. Eur J Pharm Sci. 2009;37(3–4):508–13. 10.1016/j.ejps.2009.04.008.Search in Google Scholar PubMed
[105] Li Y, Dong L, Jia A, Chang X, Xue H. Preparation and characterization of solid lipid nanoparticles loaded with traditional Chinese medicine. Inter J Biol Macromol. 2006;38(3–5):296–99.10.1016/j.ijbiomac.2006.03.006Search in Google Scholar PubMed
[106] Laserra S, Basit A, Sozio P, Marinelli L, Fornasari E, Cacciatore I, et al. Solid lipid nanoparticles loaded with lipoyl–memantine codrug: Preparation and characterization. Inter J Pharm. 2015;485(1–2):183–91. 10.1016/j.ijpharm.2015.03.001.Search in Google Scholar PubMed
[107] Zhang J, Liu J, Li X, Jasti B. Preparation and characterization of solid lipid nanoparticles containing silibinin. J Drug Del. 2007;14(6):381–87.10.1080/10717540701203034Search in Google Scholar PubMed
[108] Li M, Zahi M, Yuan Q, Tian F, Liang H. Preparation and stability of astaxanthin solid lipid nanoparticles based on stearic acid. Eur J Lipid Sci Tech. 2016;118(4):592–602.10.1002/ejlt.201400650Search in Google Scholar
[109] Khameneh B, Halimi V, Jaafari MR, Golmohammadzadeh S. Safranal-loaded solid lipid nanoparticles: evaluation of sunscreen and moisturizing potential for topical applications. Iran J Basic Med Sci. 2015;18(1):58–63.Search in Google Scholar
[110] Ruckmani K, Sivakumar M, Ganeshkumar PA. Methotrexate loaded solid lipid nanoparticles (SLN) for effective treatment of carcinoma. J Nanosci Nanotechnol. 2006;6(9–10):2991–5. 10.1166/jnn.2006.457.Search in Google Scholar PubMed
[111] Chavan S, Ingle S, Vavia P. Preparation and characterization of solid lipid nanoparticle-based nasal spray of budesonide. Drug Del Transl Res. 2012;3(5):402–8.10.1007/s13346-012-0105-zSearch in Google Scholar PubMed
[112] Lin CH, Chen CH, Lin ZC, Fang JY. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J Food Drug Anal. 2017;25(2):219–34. 10.1016/j.jfda.2017.02.001.Search in Google Scholar PubMed PubMed Central
[113] Aboutaleb E, Atyabi F, Khoshayand MR, Vatanara AR, Ostad SN, Kobarfard F, et al. Improved brain delivery of vincristine using dextran sulfate complex solid lipid nanoparticles: optimization and in vivo evaluation. J Biomed Mater Res A. 2014;102(7):2125–36. 10.1002/jbm.a.34890.Search in Google Scholar PubMed
[114] Wu C, Ji P, Yu T, Liu Y, Jiang J, Xu J. Naringenin-loaded solid lipid nanoparticles: preparation, controlled delivery, cellular uptake, and pulmonary pharmacokinetics. Drug Des Dev Ther. 2016;10:911–25.10.2147/DDDT.S97738Search in Google Scholar PubMed PubMed Central
[115] Vijayakumar A, Baskaran R, Jang YS, Oh SH, Yoo BK. Quercetin loaded solid lipid nanoparticle dispersion with improved physicochemical properties and cellular uptake. AAPS PharmSciTech. 2017;18(3):875–83.10.1208/s12249-016-0573-4Search in Google Scholar PubMed
[116] Kheradmandnia S, Vasheghani-Farahani E, Nosrati M, Atyabi F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomedicine. 2010;6(6):753–9. 10.1016/j.nano.2010.06.003.Search in Google Scholar PubMed
[117] Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;85(3):339–45.10.1016/j.ejpb.2013.02.005Search in Google Scholar PubMed
[118] Yan F, Zhang C, Zheng Y, Mei L, Tang L, Song C, et al. The effect of poloxamer 188 on nanoparticle morphology, size, cancer cell uptake, and cytotoxicity. Nano Med Nanotech Biol Med. 2010;6(1):170–8. 10.1016/j.nano.2009.05.004.Search in Google Scholar PubMed
[119] Mohamed R, Abbas H, Attia M, Heikal O. Formulation and evaluation of metoclopramide solid lipid nanoparticles for a rectal suppository. J Pharm Pharmacol. 2013;65(11):1607–21.10.1111/jphp.12136Search in Google Scholar PubMed
[120] Lukowski G, Hoell A, Dingler A, Kranold R, Pflegel P. Fractal surface of solid lipid nanoparticles. Proc Control Rel Soc. 1997;24:631–2.Search in Google Scholar
[121] Muhlen A, Mehnert W. Drug release and release mechanism of prednisolone loaded solid lipid nanoparticles. Pharmazie. 1998;53(8):552–5.Search in Google Scholar
[122] Muller H, Radtke M, Wissing A. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Del Rev. 2002;54(1):131–55.10.1016/S0169-409X(02)00118-7Search in Google Scholar PubMed
[123] Jawahar N, Gowthamarajan K, Meyyanathan SN, Sood S. Brain delivery by solid lipid nanoparticles for CNS drugs. Inter J Pharm Res Dev. 2011;3 Supply 4:206–16.Search in Google Scholar
[124] Lv Q, Yu A, Xi Y, Li H, Song Z, Cui J, et al. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int J Pharm. 2009;372(1–2):191–8. 10.1016/j.ijpharm.2009.01.014.Search in Google Scholar PubMed
[125] Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, et al. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine. 2009;5(2):184–91. 10.1016/j.nano.2008.08.003.Search in Google Scholar PubMed
[126] Parhi R, Suresh P. Preparation and characterization of solid lipid nanoparticles-a review. Curr Drug Discovery Technol. 2012;9(1):2–16. 10.2174/157016312799304552.Search in Google Scholar PubMed
[127] Singh S, Sharma N, Behl T, Sarkar BC, Saha HR, Garg K, et al. Promising strategies of colloidal drug delivery-based approaches in psoriasis management. Pharmaceutics. 2021;13:1978. 10.3390/pharmaceutics13111978.Search in Google Scholar PubMed PubMed Central
[128] Abdellatif AA, Abdelfattah A, Bouazzaoui A, Osman SK, Al-Moraya IS, Showail AMS, et al. Silver nanoparticles stabilized by poly (Vinyl Pyrrolidone) with potential anticancer activity towards prostate cancer. Bioinorg Chem Appl. 2022;2022:6181448. 10.1155/2022/6181448.Search in Google Scholar PubMed PubMed Central
[129] Abdellatif AAH, Younis MA, Alsharidah M, Al Rugaie O, Tawfeek HM. Biomedical applications of quantum dots: Overview, challenges, and clinical potential. Int J Nanomed. 2022;17:1951–70. 10.2147/IJN.S357980.Search in Google Scholar PubMed PubMed Central
[130] Younis MA, Tawfeek HM, Abdellatif AAH, Abdel-Aleem JA, Harashima H. Clinical translation of nano medicines: challenges, opportunities, and keys. Adv Drug Deliv Rev. 2022;181:114083. 10.1016/j.addr.2021.114083.Search in Google Scholar PubMed
[131] Abdellatif AA, Tolba NS, Alsharidah M, Al Rugaie O, Bouazzaoui A, Saleem I, et al. PEG-4000 formed polymeric nanoparticles loaded with cetuximab downregulate p21 & stathmin-1 gene expression in cancer cell lines. Life Sci. 2022;295:120403. 10.1016/j.lfs.2022.120403.Search in Google Scholar PubMed
[132] Abdellatif AA, Tawfeek HM. Development and evaluation of fluorescent gold nanoparticles. Drug Dev Ind Pharm. 2018;44(10):1679–84. 10.1080/03639045.2018.1483400.Search in Google Scholar PubMed
[133] Abdellatif AAH, Hennig R, Pollinger K, Tawfeek HM, Bouazzaoui A, Goepferich A. Fluorescent nanoparticles coated with a somatostatin analogue target blood monocyte for efficient leukaemia treatment. Pharm Res. 2020;37(11):217.10.1007/s11095-020-02938-1Search in Google Scholar PubMed
[134] Liu W, He Z, Liang J, Zhu Y, Xu H, Yang X. Preparation and characterization of novel fluorescent nanocomposite particles: CdSe/ZnS core-shell quantum dots loaded solid lipid nanoparticles. J Biomed Mater Res Part A. 2008;84(4):1018–25. 10.1002/jbm.a.31205.Search in Google Scholar PubMed
[135] Müller RH, Runge S, Ravelli V, Mehnert W, Thünemann AF, Souto EB. Oral bioavailability of cyclosporine: solid lipid nanoparticles (SLN) versus drug nanocrystals. Int J Pharm. 2006;317(1):82–9. 0.1016/j.ijpharm.2006.02.045.Search in Google Scholar
[136] Müller RH, Runge SA, Ravelli V, Thünemann AF, Mehnert W, Souto EB. Cyclosporine-loaded solid lipid nanoparticles (SLN): drug-lipid physicochemical interactions and characterization of drug incorporation. Eur J Pharm Biopharm. 2008;68(3):535–44. 10.1016/j.ejpb.2007.07.006.Search in Google Scholar PubMed
[137] Tsai MJ, Huang YB, Wu PC, Fu YS, Kao YR, Fang JY, et al. Oral apomorphine delivery from solid lipid nanoparticles with different monostearate emulsifiers: pharmacokinetic and behavioral evaluations. J Pharm Sci. 2011;100(2):547–57. 10.1002/jps.22285.Search in Google Scholar PubMed
[138] Kumar VV, Chandrasekar D, Ramakrishna S, Kishan V, Rao YM, Diwan PV. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm. 2007;335(1–2):167–75. 10.1016/j.ijpharm.2006.11.004.Search in Google Scholar PubMed
[139] Mukherjee B, Santra K, Pattnaik G, Ghosh S. Preparation, characterization and in-vitro evaluation of sustained release protein-loaded nanoparticles based on biodegradable polymers. Int J Nanomed. 2008;3(4):487–96. 10.2147/ijn.s3938.Search in Google Scholar PubMed PubMed Central
[140] Bunjes H, Steiniger F, Richter W. Visualizing the structure of triglyceride nanoparticles in different crystal modifications. Langmuir. 2007;23(7):4005–11. 10.1021/la062904p.Search in Google Scholar PubMed
[141] Estella-Hermoso de Mendoza A, Rayo M, Mollinedo F, Blanco-Prieto MJ. Lipid nanoparticles for alkyl lysophospholipid edelfosine encapsulation: development and in vitro characterization. Eur J Pharm Biopharm. 2008;68(2):207–13. 10.1016/j.ejpb.2007.06.015.Search in Google Scholar PubMed
[142] Radomska-Soukharev A. Stability of lipid excipients in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59(6):411–8. 10.1016/j.addr.2007.04.004.Search in Google Scholar PubMed
[143] Sanjula B, Shah FM, Javed A, Alka A. Effects of poloxamer 188 on the lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J Drug Target. 2009;17(3):249–56.10.1080/10611860902718672Search in Google Scholar PubMed
[144] Souto B, Mehnert W, Müller H. Polymorphic behavior of Compritol888 ATO as bulk lipid and as SLNs and NLC. J Microencap. 2006;23(4):417–33.10.1080/02652040600612439Search in Google Scholar PubMed
[145] Jenning V, Mäder K, Gohla SH. Solid lipid nanoparticles (SLNs) based on binary mixtures of liquid and solid lipids: a (1)H-NMR study. Inter J Pharm. 2000;205(1–2):15–21.10.1016/S0378-5173(00)00462-2Search in Google Scholar PubMed
[146] Küchler S, Herrmann W, Panek-Minkin G, Blaschke T, Zoschke C, Kramer KD, et al. SLNs for topical application in skin diseases–characterization of drug-carrier and carrier-target interactive cardiac. Inter J Pharm. 2010;390(2):225–33.10.1016/j.ijpharm.2010.02.004Search in Google Scholar PubMed
[147] Zimmermann E, Souto B, Müller H. Physicochemical investigations short-structure of drug-free and drug-loaded solid lipid nanoparticles (SLNs) by means of DSC and 1H NMR. Pharmazie. 2005;60(7):508–13.Search in Google Scholar
[148] Ye J, Wang Q, Zhou X, Zhang N. Injectable actarit-loaded solid lipid nanoparticles as passive targeting therapeutic agents for rheumatoid arthritis. Inter J Pharma. 2008;352:273–9.10.1016/j.ijpharm.2007.10.014Search in Google Scholar PubMed
[149] Huang G, Zhang N, Bi X, Dou M. Solid lipid nanoparticles of temozolomide: Potential reduction of cardiac and nephric toxicity. Inter J Pharm. 2008;255:314–20.10.1016/j.ijpharm.2007.12.013Search in Google Scholar PubMed
[150] D’Souza S, Faraj JA, Dorati R, DeLuca PP. A short term quality control tool for biodegradable microspheres. AAPS PharmSciTech. 2014;15(3):530–41. 10.1208/s12249-013-0052-0.Search in Google Scholar PubMed PubMed Central
[151] Lowe J, Temple S. Calcitonin and insulin in isobutylcyanoacrylate nanocapsules: protection against proteases and effect on intestinal absorption in rats. J Pharm Pharma. 1994;46(7):547–52.10.1111/j.2042-7158.1994.tb03854.xSearch in Google Scholar PubMed
[152] Tobío M, Gref R, Sánchez A, Langer R, Alonso J. Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharm Res. 1998;15(2):270–75.10.1023/A:1011922819926Search in Google Scholar
[153] Clark MA, Jepson MA, Hirst BH. Exploiting M cells for drug and vaccine delivery. Adv Drug Deliv Rev. 2001;50(1–2):81–106. 10.1016/s0169-409x(01)00149-1.Search in Google Scholar PubMed
[154] Hussain N, Jaitley V, Florence T. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Del Rev. 2001;50(1–2):107–42.10.1016/S0169-409X(01)00152-1Search in Google Scholar PubMed
[155] Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomed. 2007;2(4):743–9.Search in Google Scholar
[156] Hu L, Tang X, Cui F. Solid lipid nanoparticles (SLNs) to improve the oral bioavailability of poorly soluble drugs. J Pharm Pharmacol. 2004;56(12):1527–35.10.1211/0022357044959Search in Google Scholar PubMed
[157] Allemann E, Gurny R, Doelker E. Drug-loaded nanoparticles – preparation methods and drug targeting issues. Eur J Pharm Biopharm. 1993;39:173–91.Search in Google Scholar
[158] Yadav RK, Shah K, Dewangan HK. Intranasal drug delivery of sumatriptan succinate-loaded polymeric solid lipid nanoparticles for brain targeting. Drug Dev Ind Pharm. 2022;48(1):21–8. 10.1080/03639045.2022.2090575.Search in Google Scholar PubMed
[159] Liu J, Gong T, Fu H, Wang C, Wang X, Chen Q, et al. Solid lipid nanoparticles for pulmonary delivery of insulin. Inter J Pharm. 2008;356(1–2):333–44.10.1016/j.ijpharm.2008.01.008Search in Google Scholar PubMed
[160] Kaur G, Narang RK, Rath G, Goyal AK. Advances in pulmonary delivery of nanoparticles. Artif Cell Blood Substit Immobil Biotechnol. 2012;40(1–2):75–96. 10.3109/10731199.2011.592494.Search in Google Scholar PubMed
[161] Wang G, Wang Z, Li C, Duan G, Wang K, Li Q, et al. RGD peptide-modified, paclitaxel prodrug-based, dual-drugs loaded, and redox-sensitive lipid-polymer nanoparticles for the enhanced lung cancer therapy. Biomed Pharmacother. 2018;106:275–84. 10.1016/j.biopha.2018.06.137.Search in Google Scholar PubMed
[162] Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238(1–2):241–5. 10.1016/s0378-5173(02)00080-7.Search in Google Scholar PubMed
[163] Gökçe H, Sandri G, Eğrilmez S, Bonferoni C, Güneri T, Caramella C. Cyclosporine A-loaded solid lipid nanoparticles: ocular tolerance and in vivo drug release in rabbit eyes. Cur Eye Res. 2009;34(11):996–1003.10.3109/02713680903261405Search in Google Scholar PubMed
[164] Wang Y, Rajala A, Rajala RV. Lipid nanoparticles for ocular gene delivery. J Funct Biomater. 2015;6(2):379–94. 10.3390/jfb6020379.Search in Google Scholar PubMed PubMed Central
[165] Liu D, Ge Y, Tang Y, Yuan Y, Zhang Q, Li R, et al. Solid lipid nanoparticles for transdermal delivery of diclofenac sodium: preparation, characterization and in vitro studies. J Microencapsul. 2010;27(8):726–34. 10.3109/02652048.2010.513456.Search in Google Scholar PubMed
[166] Baskar K, Anbu J, Ravichandiran V, Venkateswarlu V, Rao YM. Lipid nanoparticles for transdermal delivery of flurbiprofen: formulation, in vitro, ex vivo and in vivo studies. Lipids Health Dis. 2009;8:6. 10.1186/1476-511X-8-6.Search in Google Scholar PubMed PubMed Central
[167] Mei Z, Wu Q. Triptolide loaded solid lipid nanoparticle hydrogel for topical application. Drug Dev Indust Pharm. 2005;31:161–8.10.1081/DDC-200047791Search in Google Scholar
[168] Zhao J, Piao X, Shi X, Si A, Zhang Y, Feng N. Podophyllotoxin-loaded nanostructured lipid carriers for skin targeting: in vitro and in vivo studies. Molecules. 2016;21(11):1549. 10.3390/molecules21111549.Search in Google Scholar PubMed PubMed Central
[169] Jenning V, Schafer-Korting M, Gohla S. Vitamin A-loaded solid lipid nanoparticles for topical use: drug release properties. J Control Rel. 2000;66:115–26.10.1016/S0168-3659(99)00223-0Search in Google Scholar
[170] Gupta S, Wairkar S, Bhatt LK. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J Microencapsul. 2020;37(8):557–65. 10.1080/02652048.2020.1823499.Search in Google Scholar PubMed
[171] Choi MJ, Kim JH, Maibach HI. Topical DNA vaccination with DNA/lipid-based complex. Curr Drug Del. 2006;3:37–45.10.2174/156720106775197484Search in Google Scholar PubMed
[172] Jain SK, Chourasia MK, Masuriha R. Solid lipid nanoparticles bearing flurbiprofen for transdermal delivery. Drug Deliv. 2005;12:207–15.10.1080/10717540590952591Search in Google Scholar PubMed
[173] Santos MC, Mehnert W, Schaller M. Drug targeting by solid lipid nanoparticles for dermal use. J Drug Target. 2002;10:489–95.10.1080/1061186021000038364Search in Google Scholar PubMed
[174] Wissing SA, Muller RH. Solid lipid nanoparticles (SLN) a novel carrier for UV blockers. Pharmazie. 2001;56:783–6.Search in Google Scholar
[175] Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Del Rev. 2002;54:S131–55.10.1016/S0169-409X(02)00118-7Search in Google Scholar PubMed
[176] Ahmad A, Ahsan H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed Dermatol. 2020;4:12. 10.1186/s41702-020-00062-9.Search in Google Scholar
[177] Shenoy VS, Vijay IK, Murthy RS. Tumor targeting: Biological factors and formulation advances in injectable lipid nanoparticles. J Pharm Pharmacol. 2005;57:411–22.10.1211/0022357055894Search in Google Scholar PubMed
[178] Murthy RS. Solid lipid nanoparticles as carriers for anti-cancer drugs to solid tumors. Drug Del. 2005;12:385–92.Search in Google Scholar
[179] Harivardhan Reddy L, Vivek K, Bakshi N, Murthy RSR. Tamoxifen citrate loaded solid lipid nanoparticles (SLN™): preparation, characterization, in vitro drug release, and pharmacokinetic evaluation. Pharm Dev Technol. 2006;11:167–77. 10.1080/10837450600561265.Search in Google Scholar PubMed
[180] Chen R, Wang S, Zhang J, Chen M, Wang Y. Aloe-emodin loaded solid lipid nanoparticles: formulation design and the in vitro anti-cancer study. Drug Delivery. 2015;22(5):666–74.10.3109/10717544.2014.882446Search in Google Scholar PubMed
[181] Zhuang YG, Xu B, Huang F, Wu JJ, Chen S. Solid lipid nanoparticles of anticancer drugs against MCF-7 cell line and a murine breast cancer model. Pharmazie. 2012;67(11):925–29.Search in Google Scholar
[182] Danilova N, Zhomart R, Kalzhanov Z, Nefedova N, Mal’kov P, Kosmas I, et al. Docetaxel-loaded solid lipid nanoparticles as a basis for a targeted and dose-sparing personalized breast cancer treatment strategy. Inter J Nanomed. 2015;10:2417–21.10.2147/IJN.S77186Search in Google Scholar PubMed PubMed Central
[183] Lu B, Xiong SB, Yang H, Yin XD, Chao RB. Solid lipid nanoparticles of mitoxantrone for local injection against breast cancer and its lymph node metastases. Eur J Pharm Sci. 2006;28:86–95.10.1016/j.ejps.2006.01.001Search in Google Scholar PubMed
[184] Wong HL, Rauth AM, Bendayan R. A new polymer-lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm Res. 2006;23:1574–85.10.1007/s11095-006-0282-xSearch in Google Scholar PubMed
[185] Huber A, Pereira A, Ramos N, Rezende C, Emery S, Sobral M, et al. Topical skin cancer therapy using doxorubicin-loaded cationic lipid nanoparticles and iontophoresis. J Biomed Nanotech. 2015;11(11):1975–88.10.1166/jbn.2015.2139Search in Google Scholar PubMed
[186] Geetha T, Kapila M, Prakash O, Deol K, Kakkar V, Kaur P. Sesamol-loaded solid lipid nanoparticles for treatment of skin cancer. J Drug Target. 2015;23(2):159–69.10.3109/1061186X.2014.965717Search in Google Scholar PubMed
[187] Akanda M, Rai R, Slipper I, Chowdhry B, Lamproub D, Getting G, et al. Delivery of retinoic acid to LNCap human prostate cancer cells using solid lipid nanoparticles. Pharm Nanotech. 2015;493(1–2):161–71.10.1016/j.ijpharm.2015.07.042Search in Google Scholar PubMed
[188] Jesus B, Ferreira V, Paula E, Hoekstra D, Zuhorn S. Design of solid lipid nanoparticles for gene delivery into prostate cancer. J Control Rel. 2010;148(1):89–90.10.1016/j.jconrel.2010.07.065Search in Google Scholar PubMed
[189] Yan J, Wang Y, Zhang X, Liu S, Tian C, Wang H. Targeted nanomedicine for prostate cancer therapy: docetaxel and curcumin co-encapsulated lipid-polymer hybrid nanoparticles for the enhanced anti-tumor activity in vitro and in vivo. Drug Deliv. 2016;23(5):1757–62. 10.3109/10717544.2015.1069423.Search in Google Scholar PubMed
[190] Cordova CA, Locatelli C, Winter E, Silva AH, Zanetti-Ramos BG, Jasper R, et al. Solid lipid nanoparticles improve octyl gallate antimetastatic activity and ameliorate its renal and hepatic toxic effects. Anti-Cancer Drugs. 2017;28(9):977–88.10.1097/CAD.0000000000000539Search in Google Scholar PubMed
[191] Xu Z, Chen L, Gu W, Gao Y, Lin L, Zhang Z, et al. The performance of docetaxel-loaded solid lipid nanoparticles targeted to hepatocellular carcinoma. Biomaterials. 2009;30(2):226–32.10.1016/j.biomaterials.2008.09.014Search in Google Scholar PubMed
[192] Loureiro JA, Andrade S, Duarte A, Neves AR, Queiroz JF, Nunes C, et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules. 2017;22(2):277–93. 10.3390/molecules22020277.Search in Google Scholar PubMed PubMed Central
[193] Misra S, Chopra K, Sinha VR, Medhi B. Galantamine-loaded solid–lipid nanoparticles for enhanced brain delivery: preparation, characterization, in vitro and in vivo evaluations. Drug Del. 2016;23(4):1434–43.10.3109/10717544.2015.1089956Search in Google Scholar PubMed
[194] Rassu G, Soddu E, Posadino AM, Pintus G, Sarmento B, Giunchedi P, et al. Nose-to-brain delivery of BACE1 siRNA loaded with solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf B: Biointerfaces. 2017;152:296–301.10.1016/j.colsurfb.2017.01.031Search in Google Scholar PubMed
[195] Pandey R, Sharma S, Khuller GK, Oral SLN. Based anti-tubercular chemotherapy. Tuberculosis. 2005;85:415–20.10.1016/j.tube.2005.08.009Search in Google Scholar PubMed
[196] Yusuf M, Khan M, Khan RA, Ahmed B. Preparation, characterization, in vivo and biochemical evaluation of brain targeted Piperine solid lipid nanoparticles in an experimentally induced Alzheimer’s disease model. J Drug Target. 2013;3(21):300–11. 10.3109/1061186X.2012.747529.Search in Google Scholar PubMed
[197] Ma C, Wu M, Ye W, Huang Z, Ma X, Wang W, et al. Inhalable solid lipid nanoparticles for intracellular tuberculosis infection therapy: macrophage-targeting and pH-sensitive properties. Drug Deliv Transl Res. 2021;11(3):1218–35. 10.1007/s13346-020-00849-7.Search in Google Scholar PubMed
[198] Pathak A, Tanmay M, Murthy RSR. Development and characterization of docetaxel loaded Anti-FGFR-1 modified solid lipid nanoparticles for breast cancer targeting. IJAPBC. 2012;1(3):381–7.Search in Google Scholar
[199] Dolatabadi JEN, Omidi Y. Solid lipid-based nanocarriers as efficient targeted drug and gene delivery systems. TrAC (Trends Ana Chem). 2016;77:100–8.10.1016/j.trac.2015.12.016Search in Google Scholar
[200] Dolatabadi JEN, Valizadeh H, Hamishehkar H. Solid lipid nanoparticles as efficient drug and gene delivery systems: Recent breakthroughs. Adv Pharm Bull. 2015;5(2):151–9.10.15171/apb.2015.022Search in Google Scholar PubMed PubMed Central
[201] Tekade RK, Maheshwari R, Tekade M, Chougule MB. Chapter 8 - Solid lipid nanoparticles for targeting and delivery of drugs and genes. In: Mishra V, Kesharwani P, Amin MCIM, Iyer A, editors. Nanotechnology-based approaches for targeting and delivery of drugs and genes. New York, USA: Academic Press; 2017. p. 256–86. ISBN 9780128097175.10.1016/B978-0-12-809717-5.00010-5Search in Google Scholar
[202] Rudolph C, Schillinger U, Ortiz A, Tabatt K, Plank C, Muller RH, et al. Application of novel Solid lipid nanoparticles (SLN)- gene vector formulations based on a diametric HIV-1 VAT – peptide in vitro and in vivo. Pharmaceutic Res. 2004;21:1662–9.10.1023/B:PHAM.0000041463.56768.ecSearch in Google Scholar
[203] Hayes ME, Drummond DC, Kirpotin DB. Self-assembling nucleic acid-lipid nanoparticles suitable for targeted gene delivery. Gene Ther. 2006;13:646–51.10.1038/sj.gt.3302699Search in Google Scholar PubMed
[204] Pedersen N, Hansen S, Heydenreich AV, Kristensen HG, Poulsen HS. Solid lipid nanoparticles can effectively bind DNA, streptavidin, and biotinylated ligands. Eur J Pharm Biopharm. 2006;62:155–62.10.1016/j.ejpb.2005.09.003Search in Google Scholar PubMed
[205] Botto C, Augello G, Amore E, Emma MR, Azzolina A, Cavallaro G, et al. Cationic solid lipid nanoparticles as non-viral vectors for the inhibition of hepatocellular carcinoma growth by RNA interference. J Biomed Nanotech. 2018;14(5):1009–16.10.1166/jbn.2018.2557Search in Google Scholar PubMed
[206] Chen F, Alphonse M, Liu Q. Strategies for non-viral nanoparticle-based delivery of CRISPR/Cas9 therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(3):e1609.10.1002/wnan.1609Search in Google Scholar PubMed
[207] Aghamiri S, Talaei S, Ghavidel AA, Zandsalimi F, Masoumi S, Hafshejani NH, et al. Nanoparticles-mediated CRISPR/Cas9 delivery: Recent advances in cancer treatment. J Drug Del Sci Tech. 2020;56A:1773–2247.10.1016/j.jddst.2020.101533Search in Google Scholar
[208] Givens BE, Naguib YW, Geary SM, Devor EJ, Salem AK. Nanoparticle-based delivery of CRISPR/Cas9 genome-editing therapeutics. AAPS J. 2018;20(6):108. 10.1208/s12248-018-0267-9.Search in Google Scholar PubMed PubMed Central
[209] Rohiwal SS, Dvorakova N, Klima J, Vaskovicova M, Senigl F, Slouf M, et al. Polyethylenimine based magnetic nanoparticles mediated non-viral CRISPR/Cas9 system for genome editing. Sci Rep. 2020;10(1):4619. 10.1038/s41598-020-61465-6.Search in Google Scholar PubMed PubMed Central
[210] Zhang L, Wang P, Feng Q, Wang N, Chen Z, Huang Y, et al. Lipid nanoparticle-mediated efficient delivery of CRISPR/Cas9 for tumor therapy. NPG Asia Mater. 2017;9(10):e441.10.1038/am.2017.185Search in Google Scholar
[211] Bondi ML, Azzolina A, Craparo EF, Lampiasi N, Capuano G, Giammona G, et al. Novel cationic solid-lipid nanoparticles as non-viral vectors for gene delivery. J Drug Target. 2007;15(4):295–301.10.1080/10611860701324698Search in Google Scholar PubMed
[212] del Pozo-Rodríguez A, Delgado D, Solinís MA, Pedraz JL, Echevarría E, Rodríguez JM, et al. Solid lipid nanoparticles as potential tools for gene therapy: In vivo protein expression after intravenous administration. Inter J Pharm. 2010;385(1–2):157–62.10.1016/j.ijpharm.2009.10.020Search in Google Scholar PubMed
[213] Abhijeet L, Aneesh T, Wadhwa A, Thanki K, Franzyk H, Foged C. Optimizing the intracellular delivery of therapeutic anti-inflammatory TNF-α siRNA to activated macrophages using lipidoid-polymer hybrid nanoparticles. Front Bioeng Biotech. 2021;8:1538.10.3389/fbioe.2020.601155Search in Google Scholar
[214] Montoto SM, Giuliana M, Esperanza RM. Solid lipid nanoparticles for drug delivery: Pharmacological and biopharmaceutical aspects. Front Mol Biosci. 2020;7:319.10.3389/fmolb.2020.587997Search in Google Scholar
[215] Duan Y, Dhar A, Patel C, Khimani M, Neogi S, Sharma P, et al. A brief review on solid lipid nanoparticles: part and parcel of contemporary drug delivery systems. RSC Adv. 2020;10:26777–91.10.1039/D0RA03491FSearch in Google Scholar
[216] Mishra V, Bansal KK, Verma A, Yadav N, Thakur S, Sudhakar K, et al. Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems. Pharmaceut. 2018;10(4):191.10.3390/pharmaceutics10040191Search in Google Scholar PubMed PubMed Central
[217] Campos JR, Severino P, Santini A, Silva AM, Shegokar R, Souto SB, et al. Expectations and realities of multifunctional drug delivery systems, Chapter-1: Solid lipid nanoparticles (SLN): Prediction of toxicity, metabolism, fate, and physicochemical properties. Nanopharm. 2020;1:1–15.10.1016/B978-0-12-817778-5.00001-4Search in Google Scholar
[218] Ding Y, Nielsen KA, Nielsen BP, Bøje NW, Müller RH, Pyo SM. Lipid-drug-conjugate (LDC) solid lipid nanoparticles (SLN) for the delivery of nicotine to the oral cavity – Optimization of nicotine loading efficiency. Eur J Pharm Biopharm. 2018;128:10–7.10.1016/j.ejpb.2018.03.004Search in Google Scholar PubMed
[219] Severino P, Andreani T, Macedo AS, Fangueiro JF, Santana MHA, Silva AM, et al. Current state-of-art and new trends on lipid nanoparticles (SLN and NLC) for oral drug delivery. J Drug Del. 2012;2012:750891. 10.1155/2012/750891 Search in Google Scholar PubMed PubMed Central
[220] Haddadzadegan S, Dorkoosh F, Bernkop-Schnürch A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv Drug Deliv Rev. 2022;182:114097. 10.1016/j.addr.2021.114097.Search in Google Scholar PubMed
[221] Paliwal R, Paliwal SR, Kenwat R, Kurmi BD, Sahu MK. Solid lipid nanoparticles: A review on recent perspectives and patents. Expert Opin Ther Pat. 2020;30(3):179–94.10.1080/13543776.2020.1720649Search in Google Scholar PubMed
[222] Zhang Y, Li Z, Zhang K, Yang G, Wang Z, Zhao J, et al. Ethyl oleate-containing nanostructured lipid carriers improve oral bioavailability of trans-ferulic acid as compared with conventional solid lipid nanoparticles. Int J Pharm. 2016;511(1):57–64. 10.1016/j.ijpharm.2016.06.131.Search in Google Scholar PubMed
[223] Cho HJ, Park JW, Yoon IS, Kim DD. Surface-modified solid lipid nanoparticles for oral delivery of docetaxel: enhanced intestinal absorption and lymphatic uptake. Int J Nanomed. 2014;9:495–504. 10.2147/IJN.S56648.Search in Google Scholar PubMed PubMed Central
[224] Zhang H, Forman HJ, Choi J. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods Enzymol. 2005;401:468–83. 10.1016/S0076-6879(05)01028-1.Search in Google Scholar PubMed
[225] Bertoni S, Albertini B, Facchini C, Prata C, Passerini N. Glutathione-loaded solid lipid microparticles as innovative delivery system for oral antioxidant therapy. Pharmaceutics. 2019;11(8):364.10.3390/pharmaceutics11080364Search in Google Scholar PubMed PubMed Central
[226] Trapani A, Tripodo G, Mandracchia D, Cioffi N, Ditaranto N, Cerezuela R, et al. Glutathione loaded solid lipid nanoparticles: preparation and in vitro evaluation as delivery systems of the antioxidant peptide to immunocompetent fish cells. J Cell Biotech. 2016;2(1):1–4.10.3233/JCB-15022Search in Google Scholar
[227] Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63(3):342–51. 10.1111/j.2042-7158.2010.01225.x.Search in Google Scholar PubMed
[228] Salehi B, Machin L, Monzote L, Sharifi-Rad J, Ezzat SM, Salem MA, et al. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega. 2020;5:11849–72. 10.1021/acsomega.0c01818.Search in Google Scholar PubMed PubMed Central
[229] Dandagi PM, Rath SP, Gadad AP, Mastiholimath VS. Taste masked quinine sulphate loaded solid lipid nanoparticles for flexible pediatric dosing. Indian J Pharm Educ Res. 2014;48:93–9.10.5530/ijper.48.4s.12Search in Google Scholar
[230] Jihad MA, Noori FTM, Jabir MS, Albukhaty S, AlMalki FA, Alyamani AA. Polyethylene glycol functionalized graphene oxide nanoparticles loaded with nigella sativa extract: A smart antibacterial therapeutic drug delivery system. Molecules. 2021;26(11):3067. 10.3390/molecules26113067.Search in Google Scholar PubMed PubMed Central
[231] Gupta Y, Jain A, Jain SK. Transferrin-conjugated solid lipid nanoparticles for enhanced delivery of quinine dihydrochloride to the brain. J Pharm Pharmacol. 2007;59(7):935–40. 10.1211/jpp.59.7.0004.Search in Google Scholar PubMed
[232] Teimouri M, Odoumizadeh M. Cytotoxicity of artemisia vulgaris essential oil encapsulated in SLN on breast cancer cell line (MCF7). Arch Adv Biosci. 2021;12(3):11–26.Search in Google Scholar
[233] Arana L, Salado C, Vega S, Aizpurua-Olaizola O, de la Arada I, Suarez T, et al. Solid lipid nanoparticles for delivery of Calendula officinalis extract. Colloids Surf B: Biointerfaces. 2015;135:18–26.10.1016/j.colsurfb.2015.07.020Search in Google Scholar PubMed
[234] Kelidari HR, Moemenbellah-Fard MD, Morteza-Semnani K, Amoozegar F, Shahriari-Namadi M, Saeedi M, et al. Solid-lipid nanoparticles (SLN)s containing Zataria multiflora essential oil with no-cytotoxicity and potent repellent activity against Anopheles stephensi. J Parasitic Dis. 2021;45(1):101–8.10.1007/s12639-020-01281-xSearch in Google Scholar PubMed PubMed Central
[235] Vigani B, Valentino C, Sandri G, Listro R, Fagiani F, Collina S, et al. A composite nanosystem as a potential tool for the local treatment of glioblastoma: Chitosan-coated solid lipid nanoparticles embedded in electrospun nanofibers. Polym (Basel). 2021;13(9):1371. 10.3390/polym13091371.Search in Google Scholar PubMed PubMed Central
[236] Lee SE, Lee CD, Ahn JB, Kim DH, Lee JK, Lee JY, et al. Hyaluronic acid-coated solid lipid nanoparticles to overcome drug-resistance in tumor cells. J Drug Delivery Sci Technol. 2019;50:365–71.10.1016/j.jddst.2019.01.042Search in Google Scholar
[237] Kwon TK, Lim KB, Kim JC. Solid lipid nanoparticles coated with silk fibroin. J Indus Eng Chem. 2011;17(1):10–3. 10.1016/j.jiec.2010.10.001.Search in Google Scholar
[238] Dawoud M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J Drug Del Sci Tech. 2021;62:102409. 10.1016/j.jddst.2021.102409.Search in Google Scholar
[239] Shen MY, Liu TI, Yu TW, Kv R, Chiang WH, Tsai YC, et al. Hierarchically targetable polysaccharide-coated solid lipid nanoparticles as an oral chemo/thermotherapy delivery system for local treatment of colon cancer. Biomaterials. 2019;197:86–100.10.1016/j.biomaterials.2019.01.019Search in Google Scholar PubMed
[240] Campos J, Varas-Godoy M, Haidar ZS. Physicochemical characterization of chitosan-hyaluronan-coated solid lipid nanoparticles for the targeted delivery of paclitaxel: a proof-of-concept study in breast cancer cells. Nanomedicine. 2017;12(5):473–90.10.2217/nnm-2016-0371Search in Google Scholar PubMed
[241] Koland M, Anchan RB, Mukund SG, Sindhoor SM. Design and investigation of alginate coated solid lipid nanoparticles for oral insulin delivery. Indian J Pharm Educ Res. 2021;55(2):383–94.10.5530/ijper.55.2.76Search in Google Scholar
[242] Rosière R, Van Woensel M, Gelbcke M, Mathieu V, Hecq J, Mathivet T, et al. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Mol Pharm. 2018;15(3):899–910.10.1021/acs.molpharmaceut.7b00846Search in Google Scholar PubMed
[243] Wibel R, Braun DE, Hämmerle L, Jörgensen AM, Knoll P, Salvenmoser W, et al. In vitro investigation of thiolated chitosan derivatives as mucoadhesive coating materials for solid lipid nanoparticles. Biomacromolecules. 2021;22(9):3980–91.10.1021/acs.biomac.1c00776Search in Google Scholar PubMed PubMed Central
[244] Iswandana R, Sutriyo S, Gunawan M, Larasati SA, Putri FA. Colon-targeted protein delivery by using solid lipid nanoparticles. J App Pharma Sci. 2021;11(9):118–23.Search in Google Scholar
[245] Senthil Kumar C, Thangam R, Mary SA, Kannan PR, Arun G, Madhan B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr Polym. 2020;231:115682. 10.1016/j.carbpol.2019.115682.Search in Google Scholar PubMed
[246] Arana L, Bayón-Cordero L, Sarasola LI, Berasategi M, Ruiz S, Alkorta I. Solid lipid nanoparticles surface modification modulates cell internalization and improves chemotoxic treatment in an oral carcinoma cell line. Nanomaterials (Basel). 2019;9(3):464. 10.3390/nano9030464.Search in Google Scholar PubMed PubMed Central
[247] Ahmadifard Z, Ahmeda A, Rasekhian M, Moradi S, Arkan E. Chitosan-coated magnetic solid lipid nanoparticles for controlled release of letrozole. J Drug Del Sci Tech. 2020;57:101621.10.1016/j.jddst.2020.101621Search in Google Scholar
[248] Yang J, Teng Y, Fu Y, Zhang C. Chlorins e6 loaded silica nanoparticles coated with gastric cancer cell membrane for tumor specific photodynamic therapy of gastric cancer. Int J Nanomed. 2019;14:5061–71.10.2147/IJN.S202910Search in Google Scholar PubMed PubMed Central
[249] Lee SE, Lee CD, Ahn JB, Kim DH, Lee JK, Lee JY, et al. Hyaluronic acid-coated solid lipid nanoparticles to overcome drug-resistance in tumor cells. J Drug Delivery Sci Tech. 2019;50:365–71.10.1016/j.jddst.2019.01.042Search in Google Scholar
[250] Kumar CS, Thangam R, Mary SA, Kannan PR, Arun G, Madhan B. Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr Polym. 2020;231:115682.10.1016/j.carbpol.2019.115682Search in Google Scholar PubMed
[251] Shin GH, Kim JT. Observation of chitosan coated lipid nanoparticles with different lipid compositions under simulated in vitro digestion system. Food Hydrocoll. 2018;84:146–53.10.1016/j.foodhyd.2018.05.052Search in Google Scholar
[252] Arana L, Bayón-Cordero L, Sarasola LI, Berasategi M, Ruiz S, Alkorta I. Solid lipid nanoparticles surface modification modulates cell internalization and improves chemotoxic treatment in an oral carcinoma cell line. Nanomaterials. 2019;9(3):464.10.3390/nano9030464Search in Google Scholar PubMed PubMed Central
[253] Emergen Research, Report@ https://www.emergenresearch.com.Search in Google Scholar
[254] Mazayen ZM, Ghoneim AM, Elbatanony RS, Basalious EB, Bendas ER. Pharmaceutical nanotechnology: from the bench to the market. Futur J Pharm Sci. 2022;8(1):12.10.1186/s43094-022-00400-0Search in Google Scholar PubMed PubMed Central
[255] Salah E, Abouelfetouh MM, Pan Y, Chen D, Xie S. Solid lipid nanoparticles for enhanced oral absorption: A review. Colloids Surf B: Biointerfaces. 2020;196:111305.10.1016/j.colsurfb.2020.111305Search in Google Scholar PubMed
[256] Thi TTH, Suys EJA, Lee JS, Nguyen DH, Park KD, Truong NP. Lipid-based nanoparticles in the clinic and clinical trials: From cancer nanomedicine to COVID-19 vaccines. Vaccines (Basel). 2021;9(4):359. 10.3390/vaccines9040359.Search in Google Scholar PubMed PubMed Central
[257] Battaglia L, Ugazio E. Lipid nano-and microparticles: an overview of patent-related research. J Nanomaterials. 2019;2019:2834941. 10.1155/2019/2834941.Search in Google Scholar
[258] Thakkar A, Chenreddy S, Wang J, Prabhu S. Ferulic acid combined with aspirin demonstrates chemopreventive potential towards pancreatic cancer when delivered using chitosan-coated solid-lipid nanoparticles. Cell & Biosci. 2015;5(1):1–4.10.1186/s13578-015-0041-ySearch in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus


