Abstract
Cancer is one of the most important causes of human death. Early diagnosis and treatment can make patients live longer. Therefore, there is an urgent need to develop early and accurate diagnosis method for tumors. Molecular imaging technology can be used for qualitative and quantitative analyses at cellular and molecular levels, which provides a new technology for accurate diagnosis of tumors. In recent years, various nanomaterials with unique properties have been used for tumor molecular imaging. Meanwhile, aptamers are becoming an indispensable element in the design of functional nanomaterials because of their small size, high stability, and convenient modification, especially giving nanomaterials the ability to recognize specific targets. Therefore, aptamer-functionalized nanomaterials (AFNs) provide unprecedented opportunities for the field of tumor diagnosis. Here we focus on the latest development of AFNs in the molecular imaging of tumors. First, we introduce the characteristics and advantages of common aptamer-modified organic nanomaterials and inorganic nanomaterials. Then, the applications of AFNs in fluorescence imaging, computed tomography, magnetic resonance imaging, radionuclide imaging, ultrasound imaging, photoacoustic imaging, and multimode fusion imaging are discussed. Finally, we provide some perspectives on the challenges and opportunities that have arisen from this promising area.
Graphical abstract
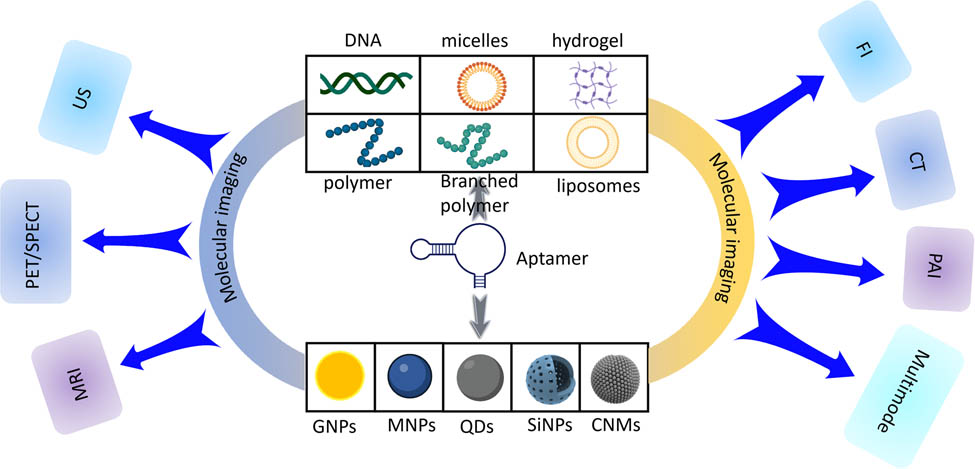
Elaborated AFNs used in molecular imaging
1 Introduction
Cancer is one of the leading causes of death in the world, and it was estimated that by 2020, nearly one-sixth of the global population (about 10 million) will die of the disease [1]. Patients diagnosed with cancer at an early stage may get early treatment and have long-term survival. For example, the 5 year survival rate of patients with early diagnosis of lung cancer is about 57%, while the survival rate of patients with advanced detection is about 3% [2,3]. Therefore, early detection or diagnosis of cancer is urgently needed, which can greatly improve the prognosis and survival rate of cancer patients [4]. In recent years, researchers have been trying to find other sensitive and specific biomarkers related to cancer. However, there are still no effective methods to detect cancer biomarkers. At the same time, to improve the effectiveness and accuracy of medical treatment, it is necessary to develop biomarkers that can target cancer cells [5]. Therefore, it is very important to explore the application of these markers in order to diagnose diseases more accurately and use them in the targeted treatment of cancer.
Among many substances, aptamers are recognized as one of the most potential biomarkers because of their unique targeting. The aptamer is a short functional single-stranded DNA or RNA molecule, usually consisting of 20–80 nucleotides. Adaptive folding can be carried out by pairing some complementary bases in the chain, electrostatic interaction, and hydrogen bonding, and relatively stable three-dimensional structures such as pseudoknots, quadruplexes, hairpins, and stem-loops can be formed [6]. Aptamers are mainly obtained by the systematic evolution of ligands by exponential enrichment (SELEX), which is used for library building, screening, and amplification [7]. Aptamers can bind to various targets (inorganic metal ions [8], small organic molecules [9], polypeptides [10], protein [11], intact living cells [12], and even tissues [13]) specifically and with high affinity, and are called chemical antibodies. Compared with protein antibodies, aptamer has the advantages of small size, rapid and reliable synthesis, flexible structure, easy chemical modification, good tissue permeability, high chemical stability, easy storage and transportation, non-toxicity, and lack of immunogenicity [10,14,15]. The ability of aptamers to bind different targets with high specificity and affinity has been widely used in the cancer research field. For example, aptamers have been used effectively in detection, diagnosis, and cancer treatment. In addition, aptamers with nanomolar-to-picomolar equilibrium dissociation constants (Kd) can bind to target molecules and specifically identify and distinguish target cancer cells from normal cells. These characteristics of aptamer show strong affinity and specificity for its target, making it a promising molecule for cancer diagnosis and treatment [16].
With the rapid development of nanotechnology, many nanomaterials with unique physical and chemical properties have been applied in the biomedical field [17–20]. Nanomaterials have ideal physical and chemical properties, including physical adsorption, chemical catalysis, large surface area/volume ratio, and biocompatibility [21–23], and can be used in molecular imaging platforms. As a molecular imaging agent, nanoparticles (NPs) enhance the specificity of tumor cells, stability in vitro and in vivo, and prolong the circulating half-life [24]. Nanomaterials have a variety of therapeutic functions due to their various characteristics, and have broad application prospects in cancer treatment. These nanomaterials accumulate non-specificity in cancer tissues by passive targeting, low dose, and selectivity, and by enhancing permeability and retention (EPR) effects [25]. However, most nanomaterials have the disadvantages of dose delivery, and lack of specific targeting ability, and some nanomaterials have toxic and side effects on non-target tissues or organs, which has limited the development of nanomaterials in biological applications [3]. Recently, cell-specific targeted nanomaterials have developed into potentially powerful cancer treatment technology. Nanomaterials bind to targeting ligands, especially to over-expressed antigens or proteins in targeted cancer cells [26,27]. The specific combination of these nanomaterials with target cells increases the accumulation of NPs at the target site, reduces the toxicity to normal cells or non-target cells, and significantly improves the diagnosis of cancer. Therefore, aptamer-functionalized nanomaterials (AFNs) are produced by integrating aptamers with nanomaterials, which not only have the specific targeting ability of nucleic acid aptamers but also have the unique physical and chemical properties of nanomaterials. AFNs may provide an effective, efficient, and low-toxicity method to meet the growing demand for new cancer diagnosis and imaging technologies.
In this review, we summarize the research progress of AFNs in the field of tumor molecular imaging, comprehensively introduce the applications of aptamer-functionalized organic and inorganic nanomaterials in tumor fluorescence imaging (FI), computed tomography (CT), magnetic resonance imaging, radionuclide imaging, ultrasound imaging (US), photoacoustic imaging (PAI) and multimodal fusion imaging (Graphical abstract) in recent years. In the first part, the characteristics, advantages, and biological application potential of AFNs are introduced. Then, we focus on the recent advances of AFNs in several imaging technologies (Table 1). Finally, we discuss the challenges and opportunities in this promising field.
Experimental study on AFNs in molecular imaging
| Imaging | Aptamer | Nanocarrier | Target cell line | Labeling | Preclinical model | Ref. |
|---|---|---|---|---|---|---|
| FI | GMT8 | PEG-coated Ag@Au core-shell NPs | U87 cells | Cy5 | Bearing U87 glioma mice model | Li et al. [28] |
| FI | ATP | Polyacrylamide hydrogel | Hela cells | HEX | Bearing Hela cells nude mice model | Yang et al. [29] |
| FI | AS1411 | Triangle DNA origami | 4T1 cells | Indocyanine green (ICG) | 4T1 tumor-bearing mice model | Li et al. [30] |
| FI | AP1153 | Calcium-phosphosilicate NPs | PANC-1/PC-3 cells | ICG | PANC-1/PC-3 tumor bearing mice model | Abraham et al. [31] |
| FI | CL4 | PLGA-b-PEG NPs | MDA-MB-231/ BT-549 cells | Cy7 | Mice bearing subcutaneous MDA-MB-231 xenograft model | Agnello et al. [32] |
| FI | AS1411 | AgNPs functionalized with PEG | C6 cells | Cy5 | C6 glioma-bearing mice model | Zhao et al. [33] |
| FI | Anti-EGFR | Lipid nanocarriers | MDA-MB-231 cells | QDs | MDA-MB-231 tumor-bearing female BALB/c nude mice model | Kim et al. [34] |
| FI | Anti-EGFR | Lipid micellar NPs | LS174T cells | QDs | LS174T-xenografted female BALB/c nude mice model | Kang et al. [35] |
| FI | AS1411 | DNA tetrahedron nanoprobes | MCF-7 cells | Cy5 | — | Jia et al. [36] |
| FI | S2.2 | Silicon nanodot (SiND) | MCF-7 cells | SiND | — | Zhang et al. [37] |
| FI | AS1411 | Chitosan-GNPs | 4T1/A549 cells | GNPs | 4T1 tumor-bearing BALB/c mice model | Khademi et al. [38] |
| FI | MUC1 | GNPs | 4T1 cells | GNPs | — | Feng et al. [39] |
| FI | KIT | Porous silicon NP | GIST-T1 cells | Cy5.5 | Gastrointestinal stromal tumors-burdened mice model | Vijayakumar et al. [40] |
| CT | MUC1 | PAMAM dendrimer-gold hybrid nanostructure | HT29/C26 cells | GNPs | C26 tumor bearing BALB/c mice model | Alibolandi et al. [41] |
| CT | PSMA | GNPs | LNCaP cells | GNPs | — | Kim et al. [42] |
| MRI | MUC16 | SPION functionalized with PEG | SKOV-3 and OVCAR-3 cells | SPION | Ovarian cancer-bearing mice model | Mohaghegh et al. [43] |
| MRI | GPC3 | Hollow mesoporous MnO | Huh-7 cells | MnO | Huh7 xenograft tumors mice model | Wang et al. [44] |
| MRI | AS1411 | Poly(L-glutamic acid)-block-polylactic acid di-block copolymer | 4T1 cells | Ultra-small superparamagnetic iron oxide nanoparticles (USPIONs) | 4T1 tumor-bearing mice model | Hasannia et al. [45] |
| MRI | MUC1 | SPION | BxPC‐3 cells | SPION | BxPC‐3 tumor bearing BALB/c female mice model | Zou et al. [46] |
| MRI | AS1411 | Mesoporous silica | 786-O cells | BSA-GdO /Fe4O3 | Healthy BALB/c mice model | Li et al. [47] |
| MRI | AP613-1 | Oleic acid-coated USPIONs | Huh-7 cells | USPIO | Huh-7 tumor bearing male athymic BALB/C mice model | Zhao et al. [48] |
| MRI | mEND | Magnetic carboxymethyl chitosan (CMCS) NPs | H22 cells | Fe₃O₄/FITC | H22 tumor bearing BALB/c mice model | Zhong et al. [49] |
| MRI | Anti-HER2 | Magnetic nanosensitizer | NIH3T6.7 cells | MNS | HER2+ cancer cell (NIH3T6.7)-xenograft mouse models | Heo et al. [50] |
| MRI | Eppc6/Eppc14 | GoldMag NPs | PC-3, DU145, and LNCaP cells | SPIO | PC-3 cell tumor-bearing nude mice model | Zhong et al. [51] |
| MRI | Wy5a | PLGA-PEG | PC-3 cells | SPIO | — | Fang et al. [52] |
| MRI | sgc8 | Carbon-coated Fe3O4 NPs | A549 cells | Fe₃O₄ | A549 tumor-bearing nude mice model | Zhao et al. [53] |
| Positron emission tomography (PET) | EpCAM | DOTA-PEGylated NPs | PANC-1 and MDA-MB-231 cells | Cy3/64Cu | Mice model bearing MDA-MB-231 xenografts | Li et al. [54] |
| Single photon emission computed tomography (SPECT) | A10-3.2 | SHNH | 22Rv1 cells | 99mTc | BALB/c mice bearing 22Rv1 or PC-3 xenografts model | Jiao et al. [55] |
| PAI | MMP-9 | GNPs | MDA-MB-231 cells | GNPs | MDA-MB-231 tumor-bearing nude mice model | Kim et al. [56] |
| PAI | AS1411 | PLGA | MCF-7 cells | FePc | MCF-7 tumor-bearing nude mouse model | He et al. [57] |
| PAI | MUC1 | PLGA | MCF-7 cells | GNR and liquid carbon | — | You et al. [58] |
| US | AS1411 | PEG@PLGA | 4T1 cells | — | 4T1 tumor-bearing mice model | Kang et al. [59] |
| US | CAIX | Nanobubbles | 786-O and Hela cells | Nanobubbles | 786-O, Hela, and BxPC-3 xenograft tumor-bearing nude mice model | Zhu et al. [60] |
| US | AS1411 | Lipid nanobubbles | MDA-MB-231/468 cells | Nanobubbles | MDA-MB-231 and MDA-MB-468 tumor-bearing mice model | Fang et al. [61] |
| US | AS1411 | Lipid nanobubbles | Triple negative breast cancer (TNBC) cells | Nanobubbles | TNBC xenografts mice model | Fang et al. [62] |
| PET/CT | GLT21.T | Lipid NPs | A549 cells | 64Cu | Subcutaneous xenograft tumor mice Model | Cai et al. [63] |
| MRI/FI | M17 | Polyethylene glycol-Fe3O4 | MIA PaCa-2 and PANC-1 cells | Fe3O4/Cy5 | MIAPaCa-2 tumor-bearing nude mice model | Huang et al. [64] |
| MRI/FI | AS1411 | Nano-hydroxyapatite | SCC-25 cells | Gd/Tb | Oral squamous cell carcinoma female nude mice model | Zhang et al. [65] |
| MRI/FI | AS1411 | PEG-PCL nanopolymersomes | MCF7 and 4T1 cells | QDs | 4T1 tumor-bearing BALB/c model | Jiang et al. [66] |
| MRI/FI | AS1411 | Liposomes | SKOV-3 cells | IR780/MnO2 | SKOV-3 tumor-bearing nude mice model | Wang et al. [67] |
| MRI/FI | Anti-VCAM-1 and anti-IL4Rα DNA | SPIO magnetic beads | 4T1-Luc2 cells | SPIO/Alamar blue dye | 4T1-Luc2 tumor-bearing mice model | Chinnappan et al. [68] |
| MRI/FI | EGFR | Carbon-coated Fe3O4 nanocapsules | A549/H522 and MCF-7/MCF-10A cells | Fe3O4 | A549 tumor-bearing nude mice model | Zhang et al. [69] |
| MRI/FI | EpCAM | Core-shell quantum dot mesoporous silica NPs | 4T1 and MCF-7 cell lines | QDs | 4T1 tumor-bearing BALB/c mice model | Akbarzadeh et al. [70] |
| MRI/FI | AS1411 | QDs | 786-O cells | Mn-MoS/QDs | 786-O bearing male BALB/c nude mice model | Zheng et al. [71] |
| US/FI | A10-3.2 | Cationic nanobubbles | LNCaP cells | Cy3 | LNCaP xenografts in male BALB/c nude mice model | Wu et al. [72] |
| US/FI | A10-3.2 | PLGA nanobubbles | LNCaP cells | DiR | LNCaP xenografts in male BALB/c nude mice model | Wu et al. [73] |
| PAI/FI | AS1411 | Gold nanoprisms (AuNPR) | SGC-7901 cells | AuNPR/TPE | SGC-7901 tumor-bearing BALB/c male nude mice model | Zhang et al. [74] |
| PAI/FI | AS1411 | Semiconductor polymer and liposomes | MDA-MB-231 cells | Lip(PTQ/GA/AIPH) | MDA-MB-231 tumor-bearing mice model | Dai et al. [75] |
| PAI/TI | Cyclic ternary aptamer (CTA) | GNR | BT474 cells | GNR | BT474 tumor-bearing BALB/c nude mice model | Chang et al. [76] |
| MRI/SPECT/FI | AS1411 | Amino-modified silica-coated gadolinium-copper nanoclusters | 4T1 cells | 99mTc/gadolinium | 4T1 tumor-bearing BALB/c mice model | Najdian et al. [77] |
| MRI/PAI/FI | AS1411 | PLGA | PANC-1 and MIA PaCa-2 cells | SPIONs | — | Sivakumar et al. [78] |
| PET/CT/MRI | RNV66 | HBP | MDAMB-231 cells | 89Zr/Cy5.5 | Bearing MDAMB-231 orthotopic breast cancer nude mice model | Fletcher et al. [79] |
| PAI/CT/PTI/OCT/UCL | AS1411 | Lipid NPs | A549 cells | IR-1048 dye/UCNPs | A549 tumor-bearing mice model | Xu et al. [80] |
2 Characteristics of AFNs
Nanomaterials have developed rapidly in the biomedical field. Nowadays, researchers have designed and manufactured many nanomaterials of different sizes and shapes [81]. They have the characteristics of a large surface area ratio, easy modification, high loading capacity, and high permeability, which attract researchers to explore their potential clinical application in drug delivery and therapeutic imaging [82]. Nanomaterials with specific targeting have become a research hotspot to further enrich their diagnostic and therapeutic potential. Nanomaterials (DNA NPs, hydrogels, gold nanoparticles [GNPs], carbon NPs, or liposomes) modified by aptamers play an important role in targeted diagnosis and treatment of tumors because of their specific molecular recognition ability. Sections 2.1 and 2.2 focus on the characteristics of several common nanomaterials, their advantages, and their applications after combining them with aptamers.
2.1 Organic nanomaterials
2.1.1 DNA nanostructures (DNs)
At present, biological nanotechnology has been widely used in chemistry, biology, medicine, and other fields, among which DNs are one of the most important in biological nanomaterials [7]. As natural molecules in the living system, DNs have the characteristics of good biocompatibility, high tissue penetration, and stability in vivo, and can react with a variety of tool enzymes [83]. DNA can be constructed into nanostructures with desired shapes and sizes, such as cubes, stars, three-dimensional geometric figures (such as tetrahedrons and icosahedrons), nanotrains, and nanorobots [84] (Figure 1a). With the emergence of DNA origami technology, DNs have become a candidate material for drug delivery, while icosahedron and tetrahedron nanostructures with wide internal space can be used as nanocages to contain a variety of therapeutic or diagnostic drugs [85] (Figure 1b). The precise programmability of DNs (strict Watson–Crick base pairing rules) makes them possible to integrate aptamers into DNs [86] (Figure 1c). Due to the superior performance of DNs and the inherent targeting and specific binding ability of aptamers, more and more researchers focus on integrating aptamers into DNs and loading other drugs or reagents for specific diagnostics and targeted therapeutics of tumors. For example, Charbgoo et al. used DNA micelles to deliver doxorubicin (DOX) and pro-apoptotic peptides to tumor cells (MCF-7 and C26 cells). In vitro, the results showed that aptamers could improve the ability of cells to specifically take up drug-loaded DNA micelles [87]. In vivo, the experiments showed that drug-loaded DNA micelles can also significantly inhibit the growth of tumors in tumor-bearing mice.
![Figure 1
(a) DNs of various shapes. (b) DNs are loaded with multiple drugs for treatment. (c) It is the active targeting and inactivation of cancer cells by aptamer-modified DNs. Reproduced with permission from ref. [85], Copyright 2020, Wiley-VCH.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_001.jpg)
(a) DNs of various shapes. (b) DNs are loaded with multiple drugs for treatment. (c) It is the active targeting and inactivation of cancer cells by aptamer-modified DNs. Reproduced with permission from ref. [85], Copyright 2020, Wiley-VCH.
2.1.2 Micelles
Micelles are polymer materials that amphiphilic block copolymer self-assembles into nanospheres through a thermodynamic process at critical micelles concentration, and they are aggregations of surfactant molecules [88]. Micelles, as nanocarriers, have been widely used in the biomedical fields because of their biodegradability, strong biocompatibility, large drug loading capacity, long circulation, retention time, and so on [89]. To promote their applications in the therapeutics of tumors, researchers have used aptamers to modify micelles. Through self-assembly, aptamers are usually densely stacked on micellar nanostructures, resulting in polyvalent effects, thus enhancing the recognition ability of molecules. The combination of aptamers and micelles was first put forward by the Mizuo Maeda group in 2007 [90]. Since then, various aptamer-micelle conjugates have been developed for the diagnostics and therapeutics of tumors. For example, Tian et al. functionalized aptamers to identify human breast cancer cells (MDA-MB-231), and constructed multifunctional aptamer-micelle drug nanocarriers (APT-TNP) by integrating pH-activatable fluorescent probe (BDP-668) and near-infrared (NIR) photosensitizer (R16FP) [91]. After the aptamer-micelle drug nanocarriers targeted breast cancer cells, they were irradiated by laser. R16FP produced reactive oxygen species after laser irradiation and mediates lysosomal destruction of MDA-MB-231 cells (Figure 2a). Meanwhile, the progress of treatment could be monitored in real-time by the fluorescent probe (Figure 2b).
![Figure 2
(a) Structure, targeted delivery, PH-activated imaging, and PDT therapy of APT-TNP. (b) Targeted imaging of Apt-TNP in vivo, PDT, and therapeutic monitoring of APT-TNP. Reproduced with permission from ref. [91], Copyright 2014, Wiley-VCH.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_002.jpg)
(a) Structure, targeted delivery, PH-activated imaging, and PDT therapy of APT-TNP. (b) Targeted imaging of Apt-TNP in vivo, PDT, and therapeutic monitoring of APT-TNP. Reproduced with permission from ref. [91], Copyright 2014, Wiley-VCH.
2.1.3 Hydrogel
Hydrogel is a kind of polymer nanomaterial, which can hold a large amount of water or biological liquid and present a three-dimensional grid structure [92]. Hydrogel has the characteristics of high biocompatibility, low toxicity, good swelling property, allowing the diffusion of biomolecules, and so on. It has been widely used to control the release of hydrophilic drugs. In addition, it has physical properties similar to those of living tissues [93]. The designed hydrogel can respond to the stimulation of pH, temperature, light, magnetic field, and ionic strength [81]. Various stimuli can trigger the expansion or degradation of the elastic network so that the hydrogel can respond to environmental changes, and then release the preload effector. Adding aptamer to hydrogel provides many advantages, including targeted drug delivery, gel–sol transition, volume change, molecular capture of hydrogel system, and enhanced controlled release of loaded drugs [94], which greatly expands the application range of hydrogel in biomedicine. Yang et al. [29] integrated polyacrylamide hydrogel and ATP-responsive aptamer to form AHB gel with tumor microenvironment (TME) targeting. In vivo, AHB gel can aggregate and fluoresce in tumor tissues, but there is no fluorescence reaction in normal tissues. In addition, AHB gel has good storage stability, which provides favorable conditions for future clinical application. AHB gel is a new type of targeted hydrogel for the TME, which can realize accurate imaging of tumor tissue and has a good application prospect in future fluorescence-guided surgery.
2.1.4 Polymer
Polymer is a kind of solid colloidal system, mainly composed of synthetic hydrophilic polymer. The structure of the polymer can be a nanosphere or nanocapsule [81]. The polymer has been widely used in biomedical fields because of its good water solubility, strong stability, and easy functionalization. Polymer-loaded therapeutic drugs or imaging agents can be passively released by dissolution and diffusion, or actively released in response to external triggers such as pH, temperature, and magnetic field [95], to carry out targeted therapy and molecular imaging of diseases. Due to the lack of targeting, polymers cannot specifically aggregate in specific parts when loaded with drugs for treatment, while aptamers have been tried to functionalize polymer for targeted treatment and molecular imaging of cancer because of their strong targeting and easy modification. The aptamers can be integrated into different polymer materials (PLGA [96], PEI [97], PLA [98], PEG [99]) to enhance cell uptake, improve therapeutic effect, and reduce non-targeted cytotoxicity. During the process of synthesis, NPs can be modified by adding imaging agents such as quantum dots (QDs), magnetic resonance contrast agents (CAs), fluorescent dyes, and radioactive tracers. For example, superparamagnetic iron oxide nanoparticles (SPIONs) and DOX were encapsulated in PLGA-PEG-COOH copolymer, and the copolymer was further modified with AS1411 aptamer [100]. The results showed that mice in the aptamer-copolymers group (C26 colon cancer xenograft) can detect higher tumor targeting in MRI. In addition, tumor growth is inhibited, which indicated that these targeted magnetic composite NPs may be used as CAs for molecular imaging and anti-cancer drugs for therapy at the same time.
2.1.5 Branched polymers
Branched polymers mainly include dendrimers and hyperbranched polymers (HBPs), which have attracted wide attention due to their unique dendritic structures. Dendrimers are highly branched polymers, and their basic structures mainly include a central core, repeating branched units, and terminal groups, with clear and uniform sizes and shapes [101]. Dendrimers have a high degree of flexibility in drug loading, and drugs can be covalently bound to the surface of dendrimers or physically wrapped in the core [102]. A large number of in vitro and preclinical studies have shown that dendritic molecules can help to develop an effective therapeutic platform. HBP consists of three domains: dendritic domain, linear domain, and terminal domain. Due to their highly branched topology, HBP has a large number of intramolecular cavities for drug encapsulation and terminal groups for drug coupling and has been widely used for drug delivery [103]. Zhang et al. synthesized a multifunctional complex (LNPs-PAMAM-AS1411/DOX) which can be used for the diagnostics and therapeutics of tumors at the same time [104]. PLNPs-PAMAM has strong renewable NIR lasting luminescence, which can be used for tumor molecular imaging and provides abundant terminal groups for further functionalization. Aptamer AS1411 is coupled to the surface of PLNPs-PAMAM, which can specifically bind to the nuclear protein overexpressed in the tumor cell membrane and promote the accumulation of NPs in tumor cells. DOX is loaded on PLNPs-PAMAM through PH-sensitive hydrazine, which can be specifically released in an intracellular acidic environment, leading to apoptosis of HeLa tumor cells and inhibiting tumor growth. The prepared intelligent drug-loaded nanoplatform PLNPs-PAMAM-AS1411/DOX with long-lasting luminescence has a good application prospect in the precise treatment of cancer.
2.1.6 Liposomes
Liposomes were established in 1965 at the earliest [105]. They were spherical lipid-based vesicles with a diameter of 100–200 nm. They were composed of associated phospholipids, forming a lipid bilayer around the water core [106]. This unique structure allows drugs or other small molecules to be wrapped in lipid bilayers or water cores [107]. Liposomes have the characteristics of biodegradability, simple preparation method, high efficiency, and low toxicity. In addition, liposomes have the unique ability to encapsulate lipophilic and hydrophilic compounds, which enables them to deliver drugs with various physical and chemical properties. At present, they are one of the most researched nanocarrier systems. Liposome drugs are mainly delivered by passive targeting, lacking the ability of active targeting, and aptamers just make up for this shortcoming. By coupling one or more aptamers to the surface of liposomes, aptamer-liposomes are formed, which combine with targeted receptors expressed on the surface of tumor cells, thus realizing the active targeting of liposomes. Compared with common drugs, aptamer-liposomes preparation has many advantages, including reducing the toxicity of encapsulated drugs, improving the stability and solubility of encapsulated drugs, prolonging the time of systemic circulation, improving the controllability of pharmacokinetics and drug release kinetics, and improving the targeting and specificity of tumors [108]. These advantages make many liposome drug delivery systems enter clinical trials and even practical applications [109]. People are trying to develop aptamer-embedded liposomes, which will provide more potential for diagnostics and therapeutics.
2.2 Inorganic nanomaterial
2.2.1 GNPs
GNPs (gold nanorods [GNR], nano-cages, nano-stars, nano-cubes, and nano-spheres) are considered to be attractive molecular delivery carriers for the diagnostics and therapeutics of various tumors due to their unique physical and chemical properties. These properties of GNPs include a high surface volume ratio, multifunctional surface chemical properties (Figure 3a), low toxicity, no immunogenicity, easy synthesis, and stable properties [111]. In addition, GNPs have the characteristics of high permeability and stability, which make drugs easily permeate and accumulate in tumor sites [110]. Among all kinds of organic and inorganic NPs, GNPs have unique optical and surface plasmon resonance properties. Gold nanostructures have good optical, electrical, magnetic, and biochemical properties, which make them tools for biological imaging, drug release, radiation, and photothermal therapy (PTT) [112,113] (Figure 3b). Aptamer-modified GNPs expand the application of GNPs in drug delivery by enhancing targeting specificity, including enhancing drug efficacy and controlled drug release. In addition, multifunctional aptamer-GNPs can simultaneously realize drug delivery, targeted therapy [38], and molecular imaging [39], providing a potential multifunctional nanoplatform for diagnostics and therapeutics of tumors.
![Figure 3
(a) Important properties of GNPs. (b) Different applications of GNPs in cancer diagnosis and treatment. Reproduced with permission from ref. [110], Copyright 2018, MDPI.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_003.jpg)
(a) Important properties of GNPs. (b) Different applications of GNPs in cancer diagnosis and treatment. Reproduced with permission from ref. [110], Copyright 2018, MDPI.
2.2.2 Magnetic nanoparticles (MNPs)
Due to MNPs inherent superparamagnetism, they have been widely used in MRI, drug delivery, and cancer treatment [114] (Figure 4). To promote the transformation of these therapeutic MNPs into clinical applications, people have made great efforts in designing and improving biocompatibility, stability, safety, drug-carrying capacity, targeted drug delivery, imaging signals, and thermokinetics or photodynamic therapy. The aptamers have been introduced into magnetic systems because of their ability to recognize and bind targeted molecules. Compared with traditional drugs, aptamers-magnetic nanocomposites have better pharmacokinetics, can deliver drugs to tumor cells in a targeted manner, and at the same time reduce the systemic toxicity of drugs to achieve accurate targeted therapy [116]. In addition, the unique physical, chemical, and optical properties of magnetic nanomaterials provide opportunities for non-invasive molecular imaging, drug delivery, and thermal or light-controlled drug release [117]. The multifunctional NPs have the potential for targeted drug delivery and molecular imaging therapeutics of tumors.
![Figure 4
Types, modifications, and functions of theranostic MNPs in biomedical applications. Reproduced with permission from ref. [115], Copyright 2017, Future Science.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_004.jpg)
Types, modifications, and functions of theranostic MNPs in biomedical applications. Reproduced with permission from ref. [115], Copyright 2017, Future Science.
2.2.3 QDs
QDs, developed by Mark Reed, were semiconductor nanocrystals composed of II–VI or III–V elements, which were mainly composed of CdSe, CdS, CdTe/CdS, ZnS, PbS, and InP [118]. QDs have unique photophysical properties, such as high brightness, strong photostability, large absorption, narrow emission spectrum, and wide ultraviolet excitation spectrum [119]. They also show great potential in whole-body imaging. In addition, QDs have highly active surfaces, which can be combined with biomolecules for targeted drug delivery and molecular imaging, photodynamic therapy, and biosensors [119]. Moreover, QDs emitted in the NIR region are especially promising for deep tissue imaging in vitro and in vivo [120,121]. At present, there are still some shortcomings in the use of QDs, such as complex synthesis steps, high price, and great toxicity, which make them have certain limitations in practical application [122]. In recent years, the application of organic QDs in biological imaging, biosensor, and drug delivery has surpassed that of toxic inorganic QDs [19]. For example, sandalwood-derived carbon dots (CDs) are used for biological imaging because of their non-cytotoxicity and excellent fluorescence characteristics [123], DNA biological spots are used for the imaging and treatment of non-small cell lung cancer [124], and PLECDs have good biomolecular imaging and cytotoxicity on human breast cancer, cervical cancer, and mouse melanoma cell lines in vitro [18]. Due to the lack of targeting, QDs are usually combined with ligands to enhance the accuracy of diagnosis and the effect of treatment. Aptamers have been usually attached to QDs by covalent bonds. Due to the spatial interference of nano QDs being minimal, they can keep their targeting ability well and realize targeted cancer therapy and molecular imaging. Thus, Akbarzadeh et al. coated QDs on the surface of mesoporous silica, and then the surface of the silica was functionalized with amine to prepare nanocarriers. Then, DOX was loaded into the pores of silica, and double heterogeneous polyethylene glycol was covalently bonded to the surface of mesoporous silica nanoparticles (SiNPs) with core-shell QDs. Finally, EpCAM aptamer was adsorbed on the surface of polyethylene glycol NPs loaded with DOX, and the targeted delivery of DOX drugs, therapeutics of tumors, and dual-mode imaging based on fluorescence and MR were realized [70]. Therefore, QDs have great application prospects in medicine.
2.2.4 SiNPs
SiNPs are inorganic nanomaterials with good biocompatibility and tolerance, which have been recognized as safe and reliable by the Food and Drug Administration (FDA) [125,126]. SiNPs include dense silica, mesoporous silica nanoparticles (MSNs) or biodegradable mesoporous silica, and hollow MSNs. Especially, MSNs have high chemical stability, large pore volume, large surface distribution area (internal and external dual-function surface), and controllable particle size and shape, which can load a large number of drugs and prevent their premature release, degradation, and inactivation [127]. Because of their huge advantages, MSNs were proposed as a candidate for tumor-targeted drug delivery for the first time in the early twenty-first century [128]. As special targeting aptamers, they can deliver SiNPs loaded with drugs and imaging agents to specific tumor tissues in a targeted manner, thus increasing the effective dose of drugs to pathological areas without damaging normal cells of the surrounding healthy tissues [129]. In addition, since the chemically active surface of SiNPs can be easily modified, various functional groups can be introduced, and then chelator-based radioactive labeling can be carried out by combining with chelator so that a multifunctional nanoplatform for drug delivery and cancer therapy guided by molecular imaging can be constructed.
2.2.5 Carbon nanomaterials (CNMs)
CNMs include carbon nanotubes (CNTs), carbon nanohorns, graphene (GR), diamond, carbon dots (CDs), and polyhydroxy fullerenes [130]. Due to their unique electronic, optical, thermal, and mechanical properties, CNMs have attracted wide attention in the field of oncology, and have been usually considered safe nanostructures [131]. CNMs have been used as carriers of various anticancer bioactive substances due to their unique surface carbon structure and inherent hydrophobicity [132]. CNMs loaded with bioactive substances can be selectively accumulated in the TME through the EPR effect [133]. In addition, the surface of CNMs can be chemically linked with a specific targeting aptamer, so that they can “actively target” the overexpression receptor on tumor cells, thus delivering the loaded drugs or genes to tumor cells [20]. CNTs showed strong light absorption, Raman scattering, and photoacoustic characteristics in the NIR region, and have the functions of biological imaging and tracking. Combined with drug delivery, the application range of CNTs in vivo has been broadened. GRs have the characteristics of photoluminescence, high loading capacity, high stability, and full spectrum quenching [134]. In addition, it can protect the carried RNA/DNA from the damage of enzymes, which makes it possible to be used as a carrier material for in vivo research. Due to their unique physical and optical properties, CNTs can not only be used as a drug carrier but also have the function of biological imaging, which has great potential application value in the biomedical field.
Nanomaterials have been widely studied in the diagnosis and treatment of diseases due to their unique properties. However, some nanomaterials have biological toxicity and are easy to decompose and oxidize in organisms, which limits their clinical application. DNs have the characteristics of good biocompatibility, high tissue permeability, and can react with various tool enzymes [86], but they are easy to decompose and metabolize in vivo, with poor stability and lack of targeting [83]. Micelles, as nanocarriers, have the advantages of strong biocompatibility and large drug loading [81]. However, its drug-carrying stability in vivo is low, and drugs are often released into normal tissues in advance, which has great toxic and side effects on normal tissues. The traditional polymer drug-loaded micelles have no environmental response or only a single response, which cannot fully respond to the stimulation of special microenvironment in tumor cells, resulting in the inability of drug-loaded micelles to release drugs efficiently and controllably in cells, resulting in low drug delivery efficiency and affects the anti-tumor treatment effect [135]. The liposome is one of the nanocarriers that have been studied most. Liposomes have been approved for clinical use due to their low toxicity and immunogenicity. Some cationic liposomes will produce biotoxicity when used in large doses, which should be paid attention to in clinical applications [136].
Among all kinds of organic and inorganic NPs, GNPs have unique optical and surface plasmon resonance characteristics and have certain advantages in drug delivery, molecular imaging, and PTT. However, GNPs are easy to oxidize, and difficult to disperse and control [137]. MNPs have been widely used in the field of MRI because of their inherent superparamagnetism, but it also has some problems such as poor stability, easy agglomeration, and oxidation [138]. QDs have the characteristics of high brightness, strong photostability, narrow emission spectrum, and high surface activity, and show great potential in whole body imaging and photodynamic therapy. QDs have high preparation costs, poor water solubility, and are toxic and have side effects on organisms, and have not been applied to clinics yet [139]. SiNPs are recognized as safe and reliable by the US FDA. MSN has a large pore volume and large surface distribution area, which can load a large number of drugs to prevent premature release, degradation, and inactivation of drugs, and is an excellent drug delivery nano-platform [140]. Because the MSN surface is easy to produce defects, it is easy to absorb substances such as moisture and gas, which further affects its application performance [141]. Due to its unique surface carbon structure and inherent hydrophobicity, CNMs have been used as the carrier of various anticancer bioactive substances. As a drug carrier, CNTs may also cause damage to human tissues, which may become the biggest difficulty in the application of CNTs in the biomedical field.
3 Application of AFNs in molecular imaging
Molecular imaging is a noninvasive method to deeply understand cellular and molecular metabolic processes in vivo. Usually, this is achieved by systemic or local application of molecular imaging probes, which are then accumulated and excreted at the target site to obtain a high signal-to-background ratio [142]. It can measure the in vivo characterization of biological processes at the cellular and molecular levels, and provide accurate information at the cellular and molecular levels [143]. At present, it is mainly used for the diagnostics of diseases in vivo, providing an alternative or supplementary diagnostic tool for clinical diseases. However, for molecular imaging in vivo, the most critical factor is the selection of molecular probes. The molecular probe should ensure that it has no side effects on normal tissues after being introduced into the human body, and the distribution between pathological and normal tissues is obviously different, which can provide a high signal-to-background ratio or image contrast.
As the target component of molecular imaging agents, aptamers can be combined with a variety of macromolecules and nanomaterials, and they are a kind of biomolecule with potential clinical applications [144,145]. Due to their small size and easy metabolism and degradation, aptamers have the characteristics of rapid tissue uptake and blood clearance, which can reduce the residence time of imaging agents in the liver and kidney, and provide potential application value for molecular imaging in clinical patients [146]. Although aptamers have been studied as molecular imaging probes in preclinical experiments for more than 30 years, they have not yet been applied to clinically approved imaging probes. Based on aptamers, molecular probe therapeutics are superior to molecular probe imaging in clinical trials. At present, aptamer based molecular probe therapeutics have been applied clinically, and pegaptanib is the only aptamer that has been clinically approved [147]. Therefore, aptamers with high sensitivity and selectivity have broad application prospects as diagnostic molecules.
Molecular imaging mediated by AFNs (imaging agents) has been used in various imaging methods, such as FI [34], CT [41], MRI [148], radionuclide imaging [54], US [60], PAI [56], and multimode fusion imaging [80]. In this section, various imaging modes are briefly introduced, and the latest research on molecular imaging guided by aptamer-nanomaterial complex under various imaging modes are comprehensively reviewed. Bouvier-Müller and Ducongé have comprehensively reviewed the previous research in 2017 [149], and we mainly comprehensively reviewed the research on molecular imaging of tumors guided by aptamer-nanomaterial in recent 5 years.
3.1 FI
FI is commonly used in flow cytometry, confocal microscopy, and immunohistochemistry in vitro. It is a non-invasive technique in vivo, which can evaluate the pharmacokinetic behavior and biodistribution of biomolecules or NPs in the region of interest. It is the most commonly used imaging method in preclinical research. The principle of FI is that the fluorescence signal generated by fluorescent substances excited by a laser with a certain wavelength is detected by the corresponding camera, and finally the corresponding fluorescence image is presented. The inherent disadvantages of FI are the low penetration depth and limited resolution of tissues [150], so FI is mainly limited to superficial site research [151] and fluorescence-guided surgery [152]. Generally, the laser with a fluorophore absorption spectrum of 650–1,350 nm is chosen. The laser in this wavelength range has a large penetration depth into tissues [153], and the resolution provided by NIR light in tissues with a depth of 0.2 mm can reach a micron level [154,155]. At present, FI has been used in the development of nanomedicine, drug delivery systems, and diagnostic probes.
Due to the strong chemical modification ability of aptamers, researchers often covalently attach fluorophores to aptamers directly, usually using standard chemical reactions at the end of 50 or 30 bases or fluorescently labeled bases for binding. At present, fluorophores with aptamers have been widely studied as imaging probes in preclinical experiments [156]. The clinical applications of aptamers were limited because of their poor pharmacokinetics and rapid degradation in vivo [157]. The combination of aptamers and nanomaterials can make up for the rapid degradation and elimination of aptamers in vivo, which provides a potential possibility for the application of aptamer fluorescent probes in the therapeutics of tumors.
Various AFNs have been applied to FI of breast cancer, glioma, pancreatic cancer, colon cancer, and lung cancer in vitro and in vivo (Table 1). Within minutes to hours after injection, they usually showed higher tumor fluorescence than the background. Due to the similar physical and chemical properties between aptamers and siRNAs, it was difficult to prepare siRNAs packaging vectors guided by aptamers. Here Kim et al. prepared aptamer-coupled lipid nanocarriers loaded with QDs and siRNAs for the diagnosis of TNBC [34]. They effectively integrated hydrophobic QDs into the lipid bilayer, assembled therapeutic siRNAs and QD-lipid nanocarriers (QLs) into QLs, and then inserted anti-EGFR aptamers into QLs. Finally, through in vitro and in vivo experiments, the targeting, tumor FI, and therapeutic effect of the nanocomposite in tumor mice were evaluated (Figure 5). The results show that the nanocarrier can effectively deliver fluorescent QDs and therapeutic siRNAs to tumor cells, and the transmitted QDs provide fluorescence signals in internal organs and tumors for tumor FI, which revealed valuable information about the biological distribution of lipid nanocarriers.
![Figure 5
(a) Schematic of the theranostic strategy for siRNA gene therapy and fluorescence tumor imaging; (b) in vivo fluorescence images of tumor-bearing mice intravenously treated with QLs, aptamo-QLs, and immuno-QLs were taken with a Maestro 2 imaging system at 1, 4, 8, and 24 h post-injection. The auto-fluorescence from mice is pseudocolored white and the unmixed QD signal is pseudocolored red. Black arrows indicate tumors. Reproduced with permission from ref. [34], Copyright 2019, Ivyspring International Publisher.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_005.jpg)
(a) Schematic of the theranostic strategy for siRNA gene therapy and fluorescence tumor imaging; (b) in vivo fluorescence images of tumor-bearing mice intravenously treated with QLs, aptamo-QLs, and immuno-QLs were taken with a Maestro 2 imaging system at 1, 4, 8, and 24 h post-injection. The auto-fluorescence from mice is pseudocolored white and the unmixed QD signal is pseudocolored red. Black arrows indicate tumors. Reproduced with permission from ref. [34], Copyright 2019, Ivyspring International Publisher.
Aptamer-targeted ICG NPs can enhance the NIR FI of pancreatic and prostate tumors, and improve the detection of early cancer. Abraham et al. coupled calcium phosphosilicate NPs, also known as nanojackets (NJs), with DNA aptamer AP153 to synthesize AP1153-NJ nanocomposites [31]. In vitro tumor imaging confirmed that compared with non-targeted NJs, aptamer-NJs can specifically aggregate in tumor cells. In vivo, experiments showed that the in situ accumulation of AP 1153-ICG-NJ in pancreatic tumors reached its peak 18 h after injection, and the ICG signal was cleared at 36 h, while non-tumor tissues did not absorb the signal (Figure 6). The specific binding of AP1153-NJ to the CCK-B receptor on pancreatic tumor cells was confirmed by pretreating tumor-bearing mice with CCK receptor antagonist proglumide. Through the specific interaction with the CCK-B receptor, the tumor-targeting NPs containing ICG were well distributed in the matrix of pancreatic and prostate tumors, which proved that tumor-targeting NJs carrying various imaging agents can enhance the detection of tumors. AP1153-NJ was a kind of nanocarrier with clinical application potential, which can be loaded with imaging agents or drugs for the diagnosis or treatment of early cancers.
![Figure 6
(a) NIR optical imaging of PANC-1 tumor-bearing mice after injection of non-targeted ICG-NJs and aptamer AP1153-targeted ICG-NJS, respectively. The arrow indicates the position of the PANC-1 tumor in situ. (b) In vitro NIR imaging of resected tissues from the pancreas and spleen of mice treated with AP1153-ICG-NJ. (c) Renal and (d) liver tissue sections from mice injected with AP1153-ICG-NJ shows that although there were some ICG signals (red) in the tissue vasculature (green), there seemed to be no ICG aggregation in the normal cells of these organs. Reproduced with permission from ref. [31], Copyright 2021, DMP.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_006.jpg)
(a) NIR optical imaging of PANC-1 tumor-bearing mice after injection of non-targeted ICG-NJs and aptamer AP1153-targeted ICG-NJS, respectively. The arrow indicates the position of the PANC-1 tumor in situ. (b) In vitro NIR imaging of resected tissues from the pancreas and spleen of mice treated with AP1153-ICG-NJ. (c) Renal and (d) liver tissue sections from mice injected with AP1153-ICG-NJ shows that although there were some ICG signals (red) in the tissue vasculature (green), there seemed to be no ICG aggregation in the normal cells of these organs. Reproduced with permission from ref. [31], Copyright 2021, DMP.
Esmaeili et al. developed a cancer nano-drug system (GO@PEG/Au/Apt (DOX)) based on PEGylation graphene oxide-GNPs. The system can be selectively attached to MUC1 positive breast cancer and colon cancer cells to track, image, and target chemotherapy drugs to the required location [158]. The aptamer can also be used as a quencher to produce an “on/off” fluorescence biosensor. When the aptamer specifically binds to the MUC1 receptor, its double strands separate, resulting in drug release, and the fluorescence is restored at the excitation wavelength of 300 nm (“on” state). GO@PEG/Au can be used for non-invasive FI of breast and colon tumors by monitoring the changes in fluorescence signals, taking advantage of its inherent fluorescence characteristics. In addition, since AuNPs can be used as imaging agents for various imaging technologies, this nano-platform is an ideal platform for multimodal imaging [74] and has the potential to be used for detecting and treating various tumors.
3.2 CT
CT imaging is the most widely used imaging technology with anatomical imaging capability in clinics. The principle of CT imaging is based on the fact that tissues attenuate X-rays to different degrees, and they appear on the image at different gray scales. The image diagnostician identifies the normal tissue and the abnormal tissue through the difference in gray levels. However, the limitation of CT imaging is that only when the gray levels of the normal tissue and the abnormal tissue are very different, can the abnormal tissues be identified by the naked eye. Therefore, changing the contrast of the image by using CAs with higher atomic numbers and higher density can improve the difference between normal tissue and abnormal tissues, which is a more commonly used method in clinical therapeutics [159]. The commonly used CT CAs include iodide, metal complex, and nanomaterials. Due to the lack of specificity, a considerable dose is usually needed [42]. In addition, the imaging time of iodine compounds is usually short, and there is a certain degree of nephrotoxicity. In order to overcome these obstacles, aptamer-based targeted molecular probes have gradually attracted attention.
In 2010, Kim et al. first synthesized a multifunctional GNPs for targeted molecular CT imaging [42]. The experimental results showed that the CT intensity of PSMA aptamer-bound GNPs in LNCaP cells was more than four times higher than that in non-targeted PC3 cells. In addition, the effect of Adriamycin-loaded GNPs modified by PSMA aptamer on targeted LNCaP cells was significantly stronger than that of non-targeted PC3 cells, which indicated the specificity of targeted drug delivery. The GNP conjugate system was modified with other disease-specific aptamers and could also be used to design similar multifunctional NPs. Alibolandi et al. synthesized a multifunctional complex (Apt-PEG-AuPAMAM-CUR) based on the aptamer MUC1 on the basis of GNPs and PAMAM dendrimers [41]. In vivo, the experiment has proved that the targeting compound accumulates in the tumor site of C26 tumor-bearing mice and the concentration of imaging agent at the tumor site could be observed in CT imaging. This study indicated that pt-PEG-AuPAMAM-CUR had good X-ray attenuation, and was a CT imaging probe, which has a high therapeutic effect on colorectal adenocarcinoma. At present, there is little research on aptamer-functionalized nanomaterials used as imaging agents for individual CT imaging. CT imaging is usually integrated with imaging methods such as PET, MRI, PAI, or FI (Table 1), which overcomes the shortage of a single imaging mode. The research on the combination of CT with other imaging methods will be introduced in detail in Section 3.7 on multi-modality imaging.
3.3 MRI
MRI is a noninvasive and multi-parameter imaging technology. The principle of MRI imaging is to obtain three-dimensional anatomical images by exposing protons (mainly hydrogen protons) in tissues to a static magnetic field and disturbing the steady-state balance of the magnetic field that varies with time and space [160,161]. After the corresponding RF pulses are applied, all the nuclei in the magnetic field undergo relaxation by spin-lattice relaxation (T1) and spin-spin relaxation (T2) [162]. MRI has the characteristics of super-high soft tissue resolution, unlimited imaging depth, multi-parameter, and multi-plane imaging, no harm to examiners due to ionizing radiation, and relatively safe examination [163]. It can provide more comprehensive anatomical information, physiological information, and in vivo biological metabolism information, and it has been widely used in disease diagnosis.
By using a CA, the image resolution of the MRI can be increased to a micron level (10−5 m). There are many kinds of MRI CAs, which can be classified into T1 CAs and T2 CAs according to the degree of their influence on T1 and T2. Among them, the T1 CAs can enhance the image signal, and the T2 contrast agent can reduce the image signal. MRI CAs can be classified into paramagnetic compounds, superparamagnetic compounds, and ferromagnetic compounds according to their magnetism. Paramagnetic compounds include chelates and NPs of Gd3+, Mn2+, and Fe3+ [164]. Superparamagnetic and ferromagnetic compounds are classified into USPIONs, SPIONs, and micron-sized iron oxide particles [165–167].
At present, both Gd and Mn chelates and SPION have been used in the clinic. However, these CAs usually need a large dose and rely on passive distribution and accumulation, lacking targeting and specificity to specific tissues. Aptamer-based CAs can solve the problem of lack of targeting well. At present, aptamer-based MRI molecular imaging has become one of the hot spots in research. The aptamers with specific targeting to the protein on the cell surface have been successfully developed, and organic or inorganic NPs are used as carriers to achieve targeted delivery of imaging agents (Table 1). Matrix metalloproteinase 14 (MMP-14) is overexpressed in many types of cancer and is associated with poor prognosis [168]. Therefore, the MMP-14 specific imaging probe has potential application value in the diagnosis of MMP-14 positive cancer. Huang et al. obtained a DNA aptamer M17 targeting MMP-14 through cell-SELEX, which could specifically identify MMP-14 positive cells (MIA PaCa-2 and PANC-1) [64]. By coupling the aptamer M17 with polyethylene glycol that loaded Fe3O4, the signal strength of MRI T2 weighted imaging could be reduced, and it was used for targeted molecular imaging of MRI. Studies have confirmed that aptamer M17 has the advantages of simple synthesis, small size, low immunogenicity, high permeability, high affinity, and so on. It was a potential molecular probe for the diagnostics and therapeutics of MMP14 positive tumors. In addition, Zhong et al. screened an aptamer that specifically combined with an endorphin molecule on mouse neovascular endothelial cells (mouse endorphin aptamer, abbreviated as mEND) [49]. Then, they synthesized magnetic CMCS NPs imaging nanoprobe (mEND-Fe3O4@CMCS) based on endogenous protein aptamer (MEND). On the one hand, the self-assembly of CMCS on the surface of Fe3O4 improved the biocompatibility and non-toxicity of MNPs. On the other hand, the chemical groups provided by CMCS are helpful to modify more aptamers. More importantly, the assembled aptamers significantly improved the targeting capability of the probes. The MRI results showed that the probe could effectively target the new blood vessels of liver cancer in mice and improve the imaging contrast of subcutaneous tumors in mice. In this study, a new MRI probe targeting CD105 positive cells is provided. This probe not only showed good targeting ability at the cellular level but also showed good MRI in mice, which was expected to be used for the early diagnosis of hepatocellular carcinoma.
T1-T2 double CAs are helpful for MRI to show lesions more accurately and precisely. The relaxation and interference between T1 CA and T2 CA are the main consideration factors in their design. Li et al. constructed Fe3O4@mSiO2/PDDA/BSA-Gd2O3 nanocomposites, in which BSA-Gd2O3 NPs and Fe3O4 NPs were used as T1 and T2 MRI CA, respectively, and 20 nm mesoporous silica (mSiO2) nanoshells were introduced to reduce the interference between them [47]. At last, Fe3O4@mSiO2/PDDA/BSA-Gd2O3-AS1411 nanoprobe targeted by AS1411 aptamer was used to detect the MRI specificity of 786-O cells in vitro and in vivo. The results showed that Fe3O4@mSiO2/PDDA/BSA-Gd2O3 nanocomposites had high longitudinal (r 1 = 11.47 mM s−1 Gd) and transverse (r 2 = 195.1 mM s−1 Fe) relaxation, and had good biocompatibility in vitro and in vivo. After The Fe3O4@mSiO2/PDDA/BSA-Gd2O3 nanocomposites had specific targeting to 786-O cells after being coupled with the aptamer of AS1411. When injected intravenously into mice, Fe3O4@mSiO2/PDDA/BSA-Gd2O3 nanocomposites can produce renal enhancement effects and be excreted through the bladder (Figure 7a–c). The experiment showed that the combination of T1 and T2 CA can not only give consideration to the high tissue resolution of T1 mode contrast imaging but also the feasibility of T2 mode contrast imaging in soft tissue detection, which improved the sensitivity of MRI and was expected to become an effective T1-T2 dual-mode CA for MRI in vivo.
![Figure 7
(a) Schematic illustration of the fabrication process of the Fe3O4@mSiO2/PDDA/BSA-Gd2O3-AS1411 nanoprobes. (b) T1-weighted MRI and T1-map images (b(a)) as well as T2-weighted MRI and T2-map images (b(b)) of 786-O renal carcinoma cells treated with different concentrations of the Fe3O4@mSiO2/PDDA/BSA-Gd2O3 and Fe3O4@mSiO2/PDDA/BSA-Gd2O3-AS1411. (c) T1-weighted in vivo MRI of mice post-injection of the Fe3O4@mSiO2/PDDA/BSA-Gd2O3 at different time points. (d) Schematic diagram of the construction of 64Cu-labeled lipid nanocapsules. (e) In vivo PET/CT fusion imaging. Mice bearing orthotopic A549 lung tumor was intravenously injected with 64Cu@LCI-ctrl or 64Cu@LCI-Apt. Images were acquired at 4 h post-injection. Figure 7a–c were reproduced with permission from ref. [47], Copyright 2018, DMP. (d) and (e) were reproduced with permission from ref. [77], Copyright 2022, Elsevier.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_007.jpg)
(a) Schematic illustration of the fabrication process of the Fe3O4@mSiO2/PDDA/BSA-Gd2O3-AS1411 nanoprobes. (b) T1-weighted MRI and T1-map images (b(a)) as well as T2-weighted MRI and T2-map images (b(b)) of 786-O renal carcinoma cells treated with different concentrations of the Fe3O4@mSiO2/PDDA/BSA-Gd2O3 and Fe3O4@mSiO2/PDDA/BSA-Gd2O3-AS1411. (c) T1-weighted in vivo MRI of mice post-injection of the Fe3O4@mSiO2/PDDA/BSA-Gd2O3 at different time points. (d) Schematic diagram of the construction of 64Cu-labeled lipid nanocapsules. (e) In vivo PET/CT fusion imaging. Mice bearing orthotopic A549 lung tumor was intravenously injected with 64Cu@LCI-ctrl or 64Cu@LCI-Apt. Images were acquired at 4 h post-injection. Figure 7a–c were reproduced with permission from ref. [47], Copyright 2018, DMP. (d) and (e) were reproduced with permission from ref. [77], Copyright 2022, Elsevier.
3.4 Nuclide imaging
Nuclide imaging is a noninvasive molecular imaging technology, including PET and SPECT. The principle of nuclide imaging is that radiopharmaceuticals are injected into patients by intravenous injection. With blood circulation, radiopharmaceuticals gather in the diseased areas. These drugs emit high-energy photons before decay, which are measured and recorded by external detectors, eventually generating radionuclide images [169]. Radionuclide imaging is the most advanced molecular imaging method available in the clinic at present, with high sensitivity (1011–1012 mol L−1), but has poor display of morphological and anatomical information and low spatial resolution [170].
There is a difference between PET and SPECT in the types of radionuclides used. The radionuclides (11C, 18F, 68Ga, or 64Cu) used in positron emission PET decay need to be annihilated by electrons first, and then released by gamma rays detected by the gamma camera. The radionuclides used in SPECT (99mTc, 123I, or 111In) decay by single photon emission, which can be directly detected by the gamma camera. Generally, PET is more sensitive than SPECT, but the radioisotopes needed for PET imaging are expensive. Compared with MRI, CT, or US, radionuclide imaging has high sensitivity, but poor specificity, and it is also radioactive in the body [171]. Therefore, it is an urgent problem to reduce the dose of radiopharmaceuticals and improve the targeting of tumor tissues. The aptamer has the advantages of high targeting, good tissue penetration, and rapid pharmacokinetics, and has great clinical application potential in radionuclide molecular imaging.
Hicke et al. used aptamer (TTA 1) targeted imaging agent for tumor imaging for the first time in SPECT molecular imaging [172], and then other aptamers were used in SPECT tumor imaging one after another. These have been described in detail in the review published by Bouvier-Müller and Ducongé [149]. In this part, we mainly describe the latest research in recent years. For example, Jiao and colleagues designed an imaging probe based on aptamer which was labeled with 99mTc for the specific diagnosis of prostate cancer. They obtained a chimera by covalently linking aptamer A10-3.2 and gene therapy drug MDM2 siRNA, and a bifunctional chelating agent SHNH coupled 99mTc with the chimera, thus obtaining a novel molecular probe that can be used for diagnostics and therapeutics at the same time [55]. The study showed that the molecular probe has high labeling efficiency (61.47%), radiochemical purity (>95%), and stability. SPECT showed that the imaging agent had specific distribution in mice bearing 22Rv1 xenograft tumor, and compared with the PBS group, the tumor growth of the experimental group was inhibited (P < 0.01, n = 4).
Nanomaterials have the advantage of small size, which can increase the concentration of CAs in tumors by EPR effects, and improve biomedical imaging. For example, Najdian et al. prepared gadolinium copper nanoclusters coated with amino-modified silica and combined it with AS1411 aptamer to obtain the compound Apt-ASGCuNCs, which was radiolabeled with 99mTc [77]. Meanwhile, it can be used for SPECT, MRI, and FI in 4T1 tumor-bearing BALB/c mice, showing considerable tumor targeting, which makes up not only for the shortcoming of high sensitivity but also for the low spatial resolution of SPECT. Furthermore, Cai et al. also synthesized a self-assembled lipid nanocapsule [63]. The lipid nanocapsules were formed by self-assembly of the conjugate of chloride ion e6 (Ce6) and lysophosphatidylcholine (LPPC) in water. Then 64Cu was embedded into the center of the tetrapyrrole ring of Ce6. Finally, the aptamer GLT21.T (targeting lung cancer) was coupled with the surface of the lipid nanocapsule, and iodixanol was loaded into the cavity of the lipid nanocapsule to prepare the imaging agent 64Cu@LCI-Apt with good radiolabeling efficiency, stability, and targeting (Figure 7d). In PET/CT in vivo imaging of early lung cancer mouse model, 64Cu@LCI-Apt can make it possible to detect tiny lung cancer (500μm in diameter) (Figure 7e). 64Cu@ LCI-APT has an important clinical application value in early, sensitive, and accurate diagnosis of tumors by dual-mode PET/CT imaging.
EpCAM is a biomarker that is overexpressed in most tumors and metastatic lesions. To accurately stage the tumor and evaluate the curative effect, it is necessary to detect the expression of EpCAM by noninvasive in vivo imaging. Li et al. prepared an aptamer radiotracer targeting EpCAM by chelating isotope 64Cu and modified aptamer DOTA-PEGylated NPs and carried out tumor-specific PET imaging [54]. In vitro, cell uptake experiments showed that the aptamer radiotracer specifically binds to breast cancer cells expressing EpCAM. PET/CT imaging of MDA-MB-231 breast cancer cells (EpCAM-positive) xenograft mice showed that the aptamer radiotracer appeared quickly at the breast cancer site 2 h after administration, and reached the peak at 24 h, but there was no obvious tumor imaging in lymphoma 937 tumor (EpCAM-negative) mice. Aptamer-targeted radionuclide imaging can focus specifically on tumor tissues, which improves the specificity of radionuclide molecular imaging and has potential clinical application value.
3.5 US
US imaging is a noninvasive imaging method, which has the characteristics of non-radiation, high safety, and low inspection cost. It has become the most widely used imaging method worldwide [173]. The principle of the US is that the ultrasonic pulse generated from the probe will be reflected through the tissue interface due to the difference in density, and the time spent by the reflected ultrasonic wave returning to the probe can be used to reconstruct an image containing anatomical information about the detected tissue or functional information about blood movement. However, due to the limitation of probe depth, it is sometimes difficult for the US to detect some tissues and organ diseases in the body. In addition, due to the similar physical characteristics of soft tissues in the body, it is sometimes difficult for users to distinguish between the target tissues and the surrounding tissues [174]. At present, the accuracy of the US in predicting the pathological diagnosis and prognosis of certain diseases is still controversial.
In order to increase the difference between the target tissue and the surrounding tissue, more and more ultrasound CAs are used in the clinic. US CAs can provide information about blood flow in blood vessels or organs and are often used for tumor identification and classification. US CAs are bubbles with micron or nanometer sizes, usually containing inert gas with a relatively high molecular weight. These bubbles are usually surrounded by a thin layer of lipid, albumin, or other nanomaterials [175]. Because their solubility in water is low, and their size and density are lower than those of blood, a large number of scattered sound waves are generated. In addition, compared with the relatively uniform echo of the surrounding tissues, the deformed bubbles will also produce a broadband acoustic response [176]. The US CAs based on AFNs can accumulate in the target site through aptamers targeting, which can provide more comprehensive diagnostic information for diseases.
There are three kinds of aptamers used for US imaging of tumors in Table 1. Zhu et al. detected the specificity of CAIX aptamer at the cell level by immunofluorescence and flow cytometry and prepared targeted nanobubbles loaded with CAIX aptamer by maleimide-thiol coupling reaction [60]. The results of immunofluorescence showed that targeting nanobubbles could still be loaded with CAIX aptamer after penetrating tumor blood vessels, and specifically bind to CAIX-positive 786-O and Hela cells, but not to CAIX-negative BxPC-3 cells. In vivo, the result showed that the peak intensity of targeted nanobubbles was significantly higher than that of non-targeted nanobubbles and the area under the curve was significantly larger than that of non-targeted nanobubbles. However, in BxPC-3 xenograft tumor tissue, there was no significant difference between the imaging effects of targeted nanobubbles and non-targeted nanobubbles. The experiment has proved that targeted nanobubbles carrying CAIX aptamer have the advantages of small size, uniform distribution, regular shape, high safety, and so on. Based on targeting nanobubbles, Fang et al. constructed targeted lipid nanobubbles functionalized with aptamer AS1411, which can target abnormally high-expressed nuclear proteins (NCL) in tumor tissues and new blood vessels [61]. The physical and chemical characteristics of AS1411-NBs and NBs were compared in vivo and in vitro, and the imaging ability of AS1411-NBs was enhanced due to AS1411 aptamer. Compared with NBs in vivo, AS1411-NBs can target NCL in tumor tissues and new blood vessels at the same time, which effectively prolongs the imaging time of the contrast-enhanced ultrasound. There were significant differences in the area under the time-intensity curve between AS1411-NBs and NBs (P < 0.001), and the drug loading capacity of AS1411-NBs was also significantly higher than that of NBs (P < 0.0 5). They have proved that the targeted lipid nanobubbles functionalized with aptamer AS1411 can significantly prolong the US time and realize the dual-targeted ultrasound molecular imaging of tumor tissues and new blood vessels. Compared with NBs, AS1411-NBs have higher drug loading capacity and targeted drug delivery capacity, which can provide a new method and approach for early and accurate diagnosis of TNBC (Figure 8). In the subsequent experiments, Fang et al. loaded drugs based on AS1411-NBs, which can simultaneously realize the early and accurate diagnosis and treatment of TNBC [62]. These studies show that ultrasonic molecular imaging can not only diagnose but also treat at the same time, which provides ideas for the development of ultrasonic CAs and ultrasonic therapeutic drugs in the future.
![Figure 8
(a) Schematic illustration of the construction of aptamer AS1411-functionalized targeted lipid nanobubbles (AS1411-NBs) with nucleolin as the target and the contrast-enhanced ultrasound molecular imaging. (b) Contrast-enhanced US of different nanobubbles in two types of nude mouse xenograft tumor models. (c) Contrast-enhanced US time-intensity curves of an MDA-MB-231 xenograft tumor model and (d) an MDA-MB-468 xenograft tumor model. Comparison of (e) peak intensity, (f) time to peak intensity, and (g) area under the time-intensity curve of MDA-MB-231 and MDA-MB-468 xenograft tumor models. Reproduced with permission from ref. [61], Copyright 2020, Springer Nature.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_008.jpg)
(a) Schematic illustration of the construction of aptamer AS1411-functionalized targeted lipid nanobubbles (AS1411-NBs) with nucleolin as the target and the contrast-enhanced ultrasound molecular imaging. (b) Contrast-enhanced US of different nanobubbles in two types of nude mouse xenograft tumor models. (c) Contrast-enhanced US time-intensity curves of an MDA-MB-231 xenograft tumor model and (d) an MDA-MB-468 xenograft tumor model. Comparison of (e) peak intensity, (f) time to peak intensity, and (g) area under the time-intensity curve of MDA-MB-231 and MDA-MB-468 xenograft tumor models. Reproduced with permission from ref. [61], Copyright 2020, Springer Nature.
3.6 PAI
PAI is a noninvasive hybrid imaging method with rich optical contrast and high penetration depth [177]. PAI uses molecules to absorb light energy and convert it into heat energy, resulting in a temperature rise. The expansion of thermoplastic caused by temperature rise will generate sound waves, which can then be detected and imaged by ultrasonic sensors [178,179]. PAI makes up for the shortcoming that pure optical imaging methods cannot obtain high-resolution imaging in deep biological tissues, and makes PAI a promising new biomedical imaging method [180]. PAI is safe, economical, and easy to implement compared to other medical imaging technologies. It can provide information such as anatomy, function, molecule, metabolism, biomarkers, oxygen metabolism, and gene expression. It is a multi-scale imaging method (imaging from cells to whole organs) [181]. PAI imaging agents are divided into endogenous imaging agents and exogenous imaging agents. The endogenous imaging agents (hemoglobin, lipid, melanin, etc.) have strong light absorption ability and photoacoustic contrast. Exogenous imaging agents (metal NPs, carbon-based nanomaterials, QDs, organic small molecules, macromolecular polymers, etc.) can be combined with aptamers with active targeting ability, which not only help to improve imaging contrast but also makes it possible to image target molecules.
PAI combines the advantages of both optics and acoustics. In addition, the combination of targeted aptamers and imaging agents for PAI has further improved the imaging accuracy and targeting of PAI and has become a hotspot in biomedical research. Zhang et al. designed and synthesized the first activatable PA probe based on DNA aptamers, which can be hybridized to DNA strands conjugated to a near fluorescent/quencher pair (Irde800CW/IrdeYQC-1) with efficient contact quenching [182] (Figure 9a). The binding of target prothrombin with a quencher initiates the release of DNA strands, thus reducing contact quenching, resulting in the change in PA signal ratio at 780/725 nm (Figure 9b). In vivo PA imaging of living mice showed that after 45 min of thrombin injection, the PA ratio increased significantly, but there was no change in the control group injected with PBS only, which indicated that the first aptamer-based activatable PA probe could be used for advanced molecular imaging of mice (Figure 9c). This makes it possible for in vivo PAI of tumors. For example, Kim et al. designed a gold nanosphere CA based on DNA aptamer (the target is Matrix metalloproteinase-9), which can selectively target MMP-9 in the TME and aggregate in tumor cells [56]. Due to the plasma coupling effect, the aggregation of gold nanosphere enhanced the optical absorption of the first near-infrared window (NIR-I), and the aggregation of gold nanospheres at the tumor site can be detected by ultrasound-guided PAI. This experiment has proved the targeting selectivity of gold nanosphere based on aptamer to human MMP-9 and the sensitivity of PAI diagnosis. Furthermore, Dai et al. synthesized a compound that can be used for both PAI and therapy of tumors. They integrated semiconductor polymers, azo compounds, and heat shock protein inhibitors into thermosensitive liposomes, then modified them with targeting aptamers, and finally formed the compound Lip (PTQ/GA/AIPH) [75]. Under the irradiation of second near-infrared (NIR-II) laser, NIR-II fluorescence/photoacoustic dual-mode imaging guided Lip (PTQ/GA/AIPH) to treat breast cancer, which can accurately diagnose and effectively inhibit deep TNBC (Figure 9d and e), breaking through the bottleneck of insufficient single treatment effect. In this experiment, a nano-drug based on aptamers was developed, which can be combined with other emerging diagnosis and therapy modes for specific diagnosis and combined therapy of tumors, providing a new strategy for future clinical experimental research.
![Figure 9
(a) Proposed mechanism for ratiometric PAI based on functional DNA probes. (b) Scheme of ratiometric PAI of thrombin using a structure-switching aptamer and a sandwich-type DNA complex. (c) PA imaging of activated PA probe based on DNA aptamer in mice. (d) Synthesis of NIR-II excitation Lip (PTQ/GA/AIPH) NPs and their application for FI/PAI guided combination therapy. (e) In vivo NIR-II FI and PAI of tumors in mice. (a–c) were reproduced with permission from ref. [182], Copyright 2017, American Chemical Society. Figure 9d and e were reproduced with permission from ref. [75], Copyright 2021, Wiley-VCH.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_009.jpg)
(a) Proposed mechanism for ratiometric PAI based on functional DNA probes. (b) Scheme of ratiometric PAI of thrombin using a structure-switching aptamer and a sandwich-type DNA complex. (c) PA imaging of activated PA probe based on DNA aptamer in mice. (d) Synthesis of NIR-II excitation Lip (PTQ/GA/AIPH) NPs and their application for FI/PAI guided combination therapy. (e) In vivo NIR-II FI and PAI of tumors in mice. (a–c) were reproduced with permission from ref. [182], Copyright 2017, American Chemical Society. Figure 9d and e were reproduced with permission from ref. [75], Copyright 2021, Wiley-VCH.
3.7 Multimodal imaging
Each imaging technology has its characteristics, such as the sensitivity and convenience of optical imaging, poor tissue penetration, good soft tissue resolution and poor sensitivity of MRI, and high sensitivity of PET and SPECT, but low spatial resolution. To overcome the deficiency of a single imaging mode, multimodal molecular imaging technology combined with various imaging technologies has become an important trend in tumor molecular imaging [149,183]. Multimodal molecular imaging is to introduce molecular probes with multiple imaging functions into the body at the same time, and then obtain various information of the lesion site through the detection of various imaging technologies [149,184]. Combining the advantages of different imaging technologies, it can display the complex biochemical processes in vivo in a noninvasive, real-time, precise, and specific way, and provide more comprehensive and accurate information [185]. At present, small molecule probes based on aptamers are very challenging in multimode imaging due to their limited coupling sites and potential interference with each other. NPs have a large surface area, which can be combined with various functional components for multi-modal molecular imaging. As excellent carriers of aptamers functionalized probes, NPs are widely used in multimodal imaging.
In recent years, multi-modality imaging of tumors based on aptamers is not only limited to simple imaging observation but also a variety of multimodality and multifunctional imaging methods that integrate diagnostics and therapeutics have developed rapidly. Sivakumar and colleagues encapsulated curcumin and SPIONs in PLGA nanocapsule and designed multifunctional polymer NPs with specific targeting ability to tumor cells (PANC-1 and MIA PaCa-2) through aptamers coupling [78]. These aptamer conjugated nanocomposites are multimodal materials, which can be used as CAs for MR, PA, and optical imaging, as well as double hyperthermia agents (photothermal ablation agent under the irradiation of NIR laser and a magnetocaloric therapy agent in the presence of an alternating magnetic field). They have the potential for multimodal imaging and targeted therapy. Bionanomaterials with a NIR-II window response can greatly improve photothermal effect and PAI performance and have high biomedical application value. Generally speaking, only when the length of GNR is greater than 120 nm, the optical response to the NIR-II window can be generated. Chang et al. adjusted the length-diameter ratio of GNR and used GNR with a length less than 80 nm as NIR-II photothermal nanorods, thus realizing the light response span from NIR-I to NIR-II. Then, using CTA as the carrier to load GNR and DOX, a novel stimulation-responsive multifunctional nanosystem for diagnosis and treatment was constructed [76]. In this nanocomposite, GNR@CTA has the characteristics of thermal imaging and PAI, and DOX is combined with the G-C base pair of CTA. Because the G-C base pair is unstable in the microenvironment of slightly acidic tumors, CTA showed pH-controlled release. Under the irradiation of a 1,064 nm laser, the characteristics of strong light and promoting the release of goods endowed GNR@CTA NP with excellent synergistic anticancer effects. In this work, an “integrated” nanocarrier with stimulation response was obtained, and the integration of photothermal, chemotherapy, and imaging of tumor cells was achieved. It is expected to be used for bimodal imaging diagnosis and chemo photothermal synergistic therapy. It further expanded the application of GNPs in the NIR-II window and provided a new strategy for the design of nano-drug delivery systems based on DNA aptamer.
To intuitively obtain all-round structural and functional information of lung cancer while carrying out synergistic PTT and tumor-targeted immunotherapy, Xu et al. constructed a lipid-aptamer nanoplatform (UCILA) loaded with up-conversion NPs (UCNPs) and IR-1048 dye [80]. Researchers found that IR-1048 dye grafted into a lipid bilayer can be used as an imaging agent for PAI, optical coherence tomography, and photothermal imaging of NIR-II window, and as a therapeutic agent for PTT (Figure 10). UCNPs were loaded in UCILA, which has excellent luminescent properties and a high X-ray attenuation coefficient. It can be used as a CA for CT and Thermosensitive Upper-Conversion Luminescence (UCL) imaging. The metabolic activity and temperature of the tumor can be tracked in real-time, and then the PTT effect can be fed back. In addition, with the assistance of five-modality imaging and the accurate monitoring of in situ temperature changes during PTT, UCILA showed its excellent ability in lung tumor ablation with few side effects. Meanwhile, after PTT, the remaining tumor cells can also be treated with CAR-NK immunotherapy. Therefore, the UCILA nano-platform has been proved to be a multifunctional therapeutic agent which can be used for five-modality imaging and temperature feedback PTT and targeted immunotherapy for lung cancer. During the whole process of cancer treatment, the synergistic combination of five different imaging methods used clinically or preclinically provides all-around intuitive feedback from organisms. UCILA is an amazing example of “one for all.” It combines the unique characteristics of various imaging methods, and it is of great significance to measure the treatment response more accurately, achieving the ideal treatment effect, and improving the prognosis of patients.
![Figure 10
(a) Schematic illustration of NIR-II penta-modal imaging guided tumor-targeting precise PTT and CAR-NK immunotherapy based on temperature feedback UCILA nanoplatform. (b) In vivo PA imaging of tumor vessels. (c) In vivo CT images of A549 tumor-bearing mice. (d) In vitro PAI tested by phantom assay with various concentrations of IR-1048 in UCILA. (e) Ex vivo OCT signal vs concentration. (f) CT imaging ability test of UCILA. (g) Confocal images of A549 cells after incubation with UCILA. (h) In vivo photothermal images. (i) temperature profiles of tumors after injection of UCILA and PBS upon 1,064 nm laser irradiation. (j) In vivo UCL images of tumor-bearing mice before and after injection of UCILA upon 980 nm laser excitation before and after PTT. (k) In vivo temperature measured by PTI and UCL spectra after PTT treatment. (l) In vivo OCT angiography in the tumor at different time points in the form of an averaging window of three consecutive B-scans along Y-direction for noise reduction, 2D, 3D, and relative 3D scale bars, respectively. Reproduced with permission from ref. [80], Copyright 2021, Wiley-VCH.](/document/doi/10.1515/ntrev-2023-0107/asset/graphic/j_ntrev-2023-0107_fig_010.jpg)
(a) Schematic illustration of NIR-II penta-modal imaging guided tumor-targeting precise PTT and CAR-NK immunotherapy based on temperature feedback UCILA nanoplatform. (b) In vivo PA imaging of tumor vessels. (c) In vivo CT images of A549 tumor-bearing mice. (d) In vitro PAI tested by phantom assay with various concentrations of IR-1048 in UCILA. (e) Ex vivo OCT signal vs concentration. (f) CT imaging ability test of UCILA. (g) Confocal images of A549 cells after incubation with UCILA. (h) In vivo photothermal images. (i) temperature profiles of tumors after injection of UCILA and PBS upon 1,064 nm laser irradiation. (j) In vivo UCL images of tumor-bearing mice before and after injection of UCILA upon 980 nm laser excitation before and after PTT. (k) In vivo temperature measured by PTI and UCL spectra after PTT treatment. (l) In vivo OCT angiography in the tumor at different time points in the form of an averaging window of three consecutive B-scans along Y-direction for noise reduction, 2D, 3D, and relative 3D scale bars, respectively. Reproduced with permission from ref. [80], Copyright 2021, Wiley-VCH.
Each imaging method has its advantages and disadvantages. Although PET and SPECT imaging have high sensitivity, their spatial resolution is low and their cost is high. Compared with PET and SPECT imaging, MRI has the advantages of high spatial resolution, good soft tissue contrast, and no radiation, but its sensitivity is low and its acquisition time is long. Compared with other imaging techniques, the US has the advantages of no radiation, low examination cost, and short acquisition time, but it is difficult to detect deep tissue lesions. Although optical imaging has the advantages of high sensitivity, no radiation, and low cost, it is also limited by the depth of tissue and observation conditions, which makes its clinical application limited. Multimodal nano-molecular imaging can integrate the advantages of these imaging methods and make up for the corresponding deficiencies. When designing the study of molecular imaging, we should consider the advantages and limitations of each method at present. As a part of personalized medicine, molecular imaging of tumors still has many new opportunities. The related AFNs in molecular imaging research are developing in the direction of nano-multifunction, high specificity, and biocompatibility, and are not easy to be metabolized and degraded. Turning animal experiments and preclinical experiments into clinical applications is the next focus of molecular imaging. In the future, early screening with molecular imaging will be greatly beneficial to the treatment and prognosis of tumor patients.
4 Conclusion
In the recent 20 years, the unique recognition characteristics of aptamers have been widely used in analytical chemistry, biomedicine, and materials science. At present, compared with chemically synthesized antibodies used in cancer detection, aptamers have the characteristics of high target specificity, binding affinity, stability, negligible immunogenicity, and easy modification, and have become the focus of cancer imaging, diagnosis, and treatment research. Due to the specific targeting characteristics of aptamers and the unique optical, electronic, or catalytic properties of nanomaterials, AFNs have been widely studied in the field of tumor molecular imaging, which has promoted the development of nanotechnology in the field of biomedicine.
AFNs have made great progress in biomedical applications, but most of the research on molecular imaging often stays at the level of cells and animals. There are still some obstacles in the clinical application of AFNs. One of the most important problems is the limited choice of aptamers used in clinics, which are easy to degrade in in vivo environments. At the same time, most aptamers are produced by screening in vitro, so the risk of losing affinity in vivo is high. Therefore, an appropriate and innovative SELEX strategy is urgently needed. Second, little is known about the pharmacokinetics, pharmacodynamics, and off-target effect of AFNs in vivo. Compared with traditional nano-drugs based on materials, AFNs may acquire new characteristics in size, structure, and surface charge, which may affect the behaviors of cell uptake, biological distribution, metabolism, and excretion in vivo. However, so far, only a few studies have evaluated these therapeutic systems in vivo, and different results have been found in different experimental models of the same nanocarriers [186]. Therefore, a reliable and standardized animal model should be established in order to systematically and universally evaluate the candidate treatment schemes for aptamer attachment in vivo. Finally, the modification and reconstruction of the aptamer surface should be considered to further enhance the binding ability and biological stability of aptamer-modified nanomaterials and to avoid the risk of exogenous nucleic acid aptamer genome insertion. To ensure the biological safety of clinical trials, it is necessary to systematically evaluate the toxicity of candidate aptamer integration.
Despite these challenges, many of them are being solved. For example, nanomaterials with longer fluorescence excitation wavelengths are expected to overcome the limitation of penetration depth and provide more possibilities for tumor imaging in vivo. For aptamers that are easily degraded by nucleases, researchers have developed a variety of chemical modification and recycling methods to improve their serum stability, some of which can significantly stabilize aptamers for long-term in vivo research [187]. Progress has also been seen in identifying robust aptamers with in vivo evolution strategies. Cheng et al. successfully selected the RNA aptamer directly from mice, which greatly reduced the risk of functional change or loss when used in vivo [188].
The future development direction is to develop new nanomaterials based on aptamers to provide personalized diagnosis and care for each patient, so as to bring safer and more successful prognosis and well-being to cancer patients. Nanomaterials used for imaging and diagnosis must be optimized, and clinical efficiency and safety must be ensured before clinical application. AFNs have great potential as a tool for cancer diagnosis and imaging, but further efforts are needed to transform AFNs into clinical imaging and diagnosis.
Acknowledgments
The authors thank the reviewers for their constructive and valuable comments and the editor for the assistance in refining this article.
-
Funding information: The work was supported by the Natural Science Foundation of Anhui Province (2008085MH285).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The authors state no conflict of interest.
-
Ethical approval: The research related to animals use has been complied with all the relevant national regulations and institutional policies for the care and use of animals.
-
Data availability statement: Data sharing is not applicable to this article as no new data were created analyzed in this study.
References
[1] de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Global Health. 2020;8:e180–90. 10.1016/S2214-109X(19)30488-7.Search in Google Scholar PubMed
[2] Whitaker K. Earlier diagnosis: The importance of cancer symptoms. Lancet Oncol. 2020;21:6–8. 10.1016/S1470-2045(19)30658-8.Search in Google Scholar PubMed
[3] Sa P, Sahoo SK. 15 - Recent advances in aptamer-based nanomaterials in imaging and diagnostics of cancer. In: Kesharwani P, editor. Aptamers engineered nanocarriers for cancer therapy. Sawston Cambridge: Woodhead Publishing; 2023. p. 347–66.10.1016/B978-0-323-85881-6.00007-5Search in Google Scholar
[4] Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660.Search in Google Scholar PubMed
[5] Deng G, Zha H, Luo H, Zhou Y. Aptamer-conjugated gold nanoparticles and their diagnostic and therapeutic roles in cancer. Front Bioeng Biotechnol. 2023;11:1118546. 10.3389/fbioe.2023.1118546.Search in Google Scholar PubMed PubMed Central
[6] McGown LB, Joseph MJ, Pitner JB, Vonk GP, Linn CP. The nucleic acid ligand. A new tool for molecular recognition. Anal Chem. 1995;67:663–8A. 10.1021/ac00117a002.Search in Google Scholar PubMed
[7] Zhou J, Rossi J. Aptamers as targeted therapeutics: Current potential and challenges. Nat Rev Drug Discov. 2017;16:440. 10.1038/nrd.2017.86.Search in Google Scholar PubMed
[8] Guo W, Zhang C, Ma T, Liu X, Chen Z, Li S, et al. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J Nanobiotechnol. 2021;19:166. 10.1186/s12951-021-00914-4.Search in Google Scholar PubMed PubMed Central
[9] di Leandro L, Giansanti F, Mei S, Ponziani S, Colasante M, Ardini M, et al. Aptamer-driven toxin gene delivery in U87 model glioblastoma cells. Front Pharmacol. 2021;12:588306. 10.3389/fphar.2021.588306.Search in Google Scholar PubMed PubMed Central
[10] Song W, Song Y, Li Q, Fan C, Lan X, Jiang D. Advances in aptamer-based nuclear imaging. Eur J Nucl Med Mol Imaging. 2022;49:2544–59. 10.1007/s00259-022-05782-0.Search in Google Scholar PubMed
[11] Srivastava S, Abraham PR, Mukhopadhyay S. Aptamers: An emerging tool for diagnosis and therapeutics in tuberculosis. Front Cell Infect Microbiol. 2021;11:656421. 10.3389/fcimb.2021.656421.Search in Google Scholar PubMed PubMed Central
[12] Liu X, Wang T, Wu Y, Tan Y, Jiang T, Li K, et al. Aptamer based probes for living cell intracellular molecules detection. Biosens Bioelectron. 2022;208:114231. 10.1016/j.bios.2022.114231.Search in Google Scholar PubMed
[13] Pang X, Cui C, Wan S, Jiang Y, Zhang L, Xia L, et al. Bioapplications of cell-SELEX-generated aptamers in cancer diagnostics, therapeutics, theranostics, and biomarker discovery: A comprehensive review. Cancers. 2018;10:47. 10.3390/cancers10020047.Search in Google Scholar PubMed PubMed Central
[14] He F, Wen N, Xiao D, Yan J, Xiong H, Cai S, et al. Aptamer-based targeted drug delivery systems: Current potential and challenges. Curr Med Chem. 2020;27:2189–219. 10.2174/0929867325666181008142831.Search in Google Scholar PubMed
[15] Ni S, Zhuo Z, Pan Y, Yu Y, Li F, Liu J, et al. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl Mater Interfaces. 2021;13:9500–19. 10.1021/acsami.0c05750.Search in Google Scholar PubMed
[16] Jin JO, Kim G, Hwang J, Han KH, Kwak M, Lee P. Nucleic acid nanotechnology for cancer treatment. Biochim Biophys Acta Rev Cancer. 2020;1874:188377. 10.1016/j.bbcan.2020.188377.Search in Google Scholar PubMed
[17] Rastogi A, Yadav K, Mishra A, Singh MS, Chaudhary S, Manohar R, et al. Early diagnosis of lung cancer using magnetic nanoparticles-integrated systems. Nanotechnol Rev. 2022;11:544–74. 10.1515/ntrev-2022-0032.Search in Google Scholar
[18] Mohapatra D, Pratap R, Pandey V, Shreya S, Naik GG, Mandal SC, et al. Bioengineered dual fluorescent carbon nano dots from Indian long pepper leaves for multifaceted environmental and health utilities. Env Sci Pollut Res. 2023;30:52182–208. 10.1007/s11356-023-25887-9.Search in Google Scholar PubMed
[19] Naik K, Chaudhary S, Ye L, Parmar AS. A strategic review on carbon quantum dots for cancer-diagnostics and treatment. Front Bioeng Biotechnol. 2022;10:882100. 10.3389/fbioe.2022.882100.Search in Google Scholar PubMed PubMed Central
[20] Ali N, Zaman A, Sajid M, Bég O, Dr. Shamshuddin MD, Kadir A. Numerical simulation of time-dependent non-Newtonian nano-pharmacodynamic transport phenomena in a tapered overlapping stenosed artery. Nanosci Technol. 2018;9:247–82. 10.1615/NanoSciTechnolIntJ.2018027297.Search in Google Scholar
[21] Ma X, Li SJ, Liu Y, Zhang T, Xue P, Kang Y, et al. Bioengineered nanogels for cancer immunotherapy. Chem Soc Rev. 2022;51:5136–74. 10.1039/d2cs00247g.Search in Google Scholar PubMed
[22] Li X, Li W, Wang M, Liao Z. Magnetic nanoparticles for cancer theranostics: Advances and prospects. J Control Rel. 2021;335:437–48. 10.1016/j.jconrel.2021.05.042.Search in Google Scholar PubMed
[23] Waris A, Ali A, Khan AU, Asim M, Zamel D, Fatima K, et al. Applications of various types of nanomaterials for the treatment of neurological disorders. Nanomaterials. 2022;12:2140. 10.3390/nano12132140.Search in Google Scholar PubMed PubMed Central
[24] Purushothaman B, Song JM. Ag2S quantum dot theragnostics. Biomater Sci. 2021;9:51–69. 10.1039/d0bm01576h.Search in Google Scholar PubMed
[25] Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: Current progress and perspectives. J Hematol Oncol. 2021;14:85. 10.1186/s13045-021-01096-0.Search in Google Scholar PubMed PubMed Central
[26] Sharifi J, Khirehgesh MR, Safari F, Akbari B. EGFR and anti-EGFR nanobodies: Review and update. J Drug Target. 2021;29:387–402. 10.1080/1061186X.2020.1853756.Search in Google Scholar PubMed
[27] Sargazi S, Er S, Mobashar A, Gelen SS, Rahdar A, Ebrahimi N, et al. Aptamer-conjugated carbon-based nanomaterials for cancer and bacteria theranostics: A review. Chem-Biol Interact. 2022;361:109964. 10.1016/j.cbi.2022.109964.Search in Google Scholar PubMed
[28] Li D, Zhao J, Ma J, Yang H, Zhang X, Cao Y, et al. GMT8 aptamer conjugated PEGylated Ag@Au core-shell nanoparticles as a novel radiosensitizer for targeted radiotherapy of glioma. Colloid Surf B-Biointerfaces. 2022;211:112330. 10.1016/j.colsurfb.2022.112330.Search in Google Scholar PubMed
[29] Yang J, Qu J, Teng X, Zhu W, Xu Y, Yang Y, et al. Tumor microenvironment-responsive hydrogel for direct extracellular ATP imaging-guided surgical resection with clear boundaries. Adv Healthc Mater. 2023;e2301084. 10.1002/adhm.202301084.Search in Google Scholar PubMed
[30] Li M, Yang G, Zheng Y, Lv J, Zhou W, Zhang H, et al. NIR/pH-triggered aptamer-functionalized DNA origami nanovehicle for imaging-guided chemo-phototherapy. J Nanobiotechnol. 2023;21:186. 10.1186/s12951-023-01953-9.Search in Google Scholar PubMed PubMed Central
[31] Abraham T, McGovern CO, Linton SS, Wilczynski Z, Adair JH, Matters GL. Aptamer-targeted calcium phosphosilicate nanoparticles for effective imaging of pancreatic and prostate cancer. Int J Nanomed. 2021;16:2297–309. 10.2147/IJN.S295740.Search in Google Scholar PubMed PubMed Central
[32] Agnello L, Tortorella S, D’Argenio A, Carbone C, Camorani S, Locatelli E, et al. Optimizing cisplatin delivery to triple-negative breast cancer through novel EGFR aptamer-conjugated polymeric nanovectors. J Exp Clin Cancer Res. 2021;40:239. 10.1186/s13046-021-02039-w.Search in Google Scholar PubMed PubMed Central
[33] Zhao J, Liu P, Ma J, Li D, Yang H, Chen W, et al. Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer As1411 for glioma irradiation therapy. Int J Nanomed. 2019;14:9483–96. 10.2147/IJN.S224160.Search in Google Scholar PubMed PubMed Central
[34] Kim MW, Jeong HY, Kang SJ, Jeong IH, Choi MJ, You YM, et al. Anti-EGF receptor aptamer-guided co-delivery of anti-cancer siRNAs and quantum dots for theranostics of triple-negative breast cancer. Theranostics. 2019;9:837–52. 10.7150/thno.30228.Search in Google Scholar PubMed PubMed Central
[35] Kang SJ, Jeong HY, Kim MW, Jeong IH, Choi MJ, You YM, et al. Anti-EGFR lipid micellar nanoparticles co-encapsulating quantum dots and paclitaxel for tumor-targeted theranosis. Nanoscale. 2018;10:19338–50. 10.1039/c8nr05099f.Search in Google Scholar PubMed
[36] Jia R, He X, Ma W, Lei Y, Cheng H, Sun H, et al. Aptamer-functionalized activatable DNA tetrahedron nanoprobe for PIWI-interacting RNA imaging and regulating in cancer cells. Anal Chem. 2019;91:15107–13. 10.1021/acs.analchem.9b03819.Search in Google Scholar PubMed
[37] Zhang Y, Guo S, Huang H, Mao G, Ji X, He Z. Silicon nanodot-based aptasensor for fluorescence turn-on detection of mucin 1 and targeted cancer cell imaging. Anal Chim Acta. 2018;1035:154–60. 10.1016/j.aca.2018.06.032.Search in Google Scholar PubMed
[38] Khademi Z, Lavaee P, Ramezani M, Alibolandi M, Abnous K, Taghdisi SM. Co-delivery of doxorubicin and aptamer against Forkhead box M1 using chitosan-gold nanoparticles coated with nucleolin aptamer for synergistic treatment of cancer cells. Carbohydr Polym. 2020;248:116735. 10.1016/j.carbpol.2020.116735.Search in Google Scholar PubMed
[39] Feng B, Xing Y, Lan J, Su Z, Wang F. Synthesis of MUC1 aptamer-stabilized gold nanoclusters for cell-specific imaging. Talanta. 2020;212:120796. 10.1016/j.talanta.2020.120796.Search in Google Scholar PubMed
[40] Vijayakumar S, Nasr SH, Davis JE, Wang E, Zuidema JM, Lu YS, et al. Anti-KIT DNA aptamer-conjugated porous silicon nanoparticles for the targeted detection of gastrointestinal stromal tumors. Nanoscale. 2022;14:17700–13. 10.1039/d2nr03905b.Search in Google Scholar PubMed PubMed Central
[41] Alibolandi M, Hoseini F, Mohammadi M, Ramezani P, Einafshar E, Taghdisi SM, et al. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int J Pharm. 2018;549:67–75. 10.1016/j.ijpharm.2018.07.052.Search in Google Scholar PubMed
[42] Kim D, Jeong YY, Jon S. A drug-loaded aptamer-gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano. 2010;4:3689–96. 10.1021/nn901877h.Search in Google Scholar PubMed
[43] Mohaghegh S, Tarighatnia A, Omidi Y, Barar J, Aghanejad A, Adibkia K. Multifunctional magnetic nanoparticles for MRI-guided co-delivery of erlotinib and L-asparaginase to ovarian cancer. J Microencapsul. 2022;39:394–408. 10.1080/02652048.2022.2094487.Search in Google Scholar PubMed
[44] Wang Z, Wu C, Liu J, Hu S, Yu J, Yin Q, et al. Aptamer-mediated hollow MnO2 for targeting the delivery of sorafenib. Drug Deliv. 2023;30:28–39. 10.1080/10717544.2022.2149897.Search in Google Scholar PubMed PubMed Central
[45] Hasannia M, Lamei K, Abnous K, Taghdisi SM, Nekooei S, Nekooei N, et al. Targeted poly (L-glutamic acid)-based hybrid peptosomes co-loaded with doxorubicin and USPIONs as a theranostic platform for metastatic breast cancer. Nanomedicine. 2023;48:102645. 10.1016/j.nano.2022.102645.Search in Google Scholar PubMed
[46] Zou Q, Zhang CJ, Yan YZ, Min ZJ, Li CS. MUC-1 aptamer targeted superparamagnetic iron oxide nanoparticles for magnetic resonance imaging of pancreatic cancer in vivo and in vitro experiment. J Cell Biochem. 2019;120:18650–8. 10.1002/jcb.28950.Search in Google Scholar PubMed
[47] Li J, You J, Wu C, Dai Y, Shi M, Dong L, et al. T1–T2 molecular magnetic resonance imaging of renal carcinoma cells based on nano-contrast agents, Int J Nanomed. 2018;13:4607–25. 10.2147/IJN.S168660.Search in Google Scholar PubMed PubMed Central
[48] Zhao M, Liu Z, Dong L, Zhou H, Yang S, Wu W, et al. A GPC3-specific aptamer-mediated magnetic resonance probe for hepatocellular carcinoma. Int J Nanomed. 2018;13:4433–43. 10.2147/IJN.S168268.Search in Google Scholar PubMed PubMed Central
[49] Zhong L, Zou H, Huang Y, Gong W, He J, Tan J, et al. Magnetic endoglin aptamer nanoprobe for targeted diagnosis of solid tumor. J Biomed Nanotechnol. 2019;15:352–62. 10.1166/jbn.2019.2688.Search in Google Scholar PubMed
[50] Heo D, Ku M, Kim JH, Yang J, Suh JS. Aptamer-modified magnetic nanosensitizer for in vivo MR imaging of HER2-expressing cancer. Nanoscale Res Lett. 2018;13:288. 10.1186/s11671-018-2682-3.Search in Google Scholar PubMed PubMed Central
[51] Zhong J, Ding J, Deng L, Xiang Y, Liu D, Zhang Y, et al. Selection of DNA aptamers recognizing EpCAM-positive prostate cancer by cell-SELEX for in vitro and in vivo MR imaging. Drug Des Dev Ther. 2021;15:3985–96. 10.2147/DDDT.S322854.Search in Google Scholar PubMed PubMed Central
[52] Fang Y, Lin S, Yang F, Situ J, Lin S, Luo Y. Aptamer-conjugated multifunctional polymeric nanoparticles as cancer-targeted, MRI-ultrasensitive drug delivery systems for treatment of castration-resistant prostate cancer. Biomed Res Int. 2020;2020:9186583. 10.1155/2020/9186583.Search in Google Scholar PubMed PubMed Central
[53] Zhao C, Song X, Jin W, Wu F, Zhang Q, Zhang M, et al. Image-guided cancer therapy using aptamer-functionalized cross-linked magnetic-responsive Fe3O4@carbon nanoparticles. Anal Chim Acta. 2019;1056:108–16. 10.1016/j.aca.2018.12.045.Search in Google Scholar PubMed
[54] Li F, Zeng Z, Hamilton D, Zu Y, Li Z. EpCAM-targeting aptamer radiotracer for tumor-specific PET imaging. Bioconjug Chem. 2021;32:1139–45. 10.1021/acs.bioconjchem.1c00188.Search in Google Scholar PubMed
[55] Jiao Y, Xu P, Luan S, Wang X, Gao Y, Zhao C, et al. Molecular imaging and treatment of PSMA-positive prostate cancer with 99mTc radiolabeled aptamer-siRNA chimeras. Nucl Med Biol. 2022;104–105:28–37. 10.1016/j.nucmedbio.2021.11.003.Search in Google Scholar PubMed
[56] Kim J, Yu AM, Kubelick KP, Emelianov SY. Gold nanoparticles conjugated with DNA aptamer for photoacoustic detection of human matrix metalloproteinase-9. Photoacoustics. 2022;25:100307. 10.1016/j.pacs.2021.100307.Search in Google Scholar PubMed PubMed Central
[57] He Y, Wang M, Fu M, Yuan X, Luo Y, Qiao B, et al. Iron(II) phthalocyanine loaded and AS1411 aptamer targeting nanoparticles: A nanocomplex for dual modal imaging and photothermal therapy of breast cancer. Int J Nanomed. 2020;15:5927–49. 10.2147/IJN.S254108.Search in Google Scholar PubMed PubMed Central
[58] You Y, Cheng W, Chen H. Application of ultrasound molecular imaging based on compressed sensing reconstruction algorithm to phase change drug-loaded PLGA nanoparticles targeting breast cancer MCF-7 Cells. Pak J Med Sci. 2021;37:1610–4. 10.12669/pjms.37.6-WIT.4852.Search in Google Scholar PubMed PubMed Central
[59] Kang Z, Yang M, Feng X, Liao H, Zhang Z, Du Y. Multifunctional theranostic nanoparticles for enhanced tumor targeted imaging and synergistic FUS/chemotherapy on murine 4T1 breast cancer cell. Int J Nanomed. 2022;17:2165–87. 10.2147/IJN.S360161.Search in Google Scholar PubMed PubMed Central
[60] Zhu L, Wang L, Liu Y, Xu D, Fang K, Guo Y. CAIX aptamer-functionalized targeted nanobubbles for ultrasound molecular imaging of various tumors. Int J Nanomed. 2018;13:6481–95. 10.2147/IJN.S176287.Search in Google Scholar PubMed PubMed Central
[61] Fang K, Wang L, Huang H, Lan M, Shen D, Dong S, et al. Construction of nucleolin-targeted lipid nanobubbles and contrast-enhanced ultrasound molecular imaging in triple-negative breast cancer. Pharm Res. 2020;37:145. 10.1007/s11095-020-02873-1.Search in Google Scholar PubMed
[62] Fang K, Wang L, Huang H, Dong S, Guo Y. Therapeutic efficacy and cardioprotection of nucleolin-targeted doxorubicin-loaded ultrasound nanobubbles in treating triple-negative breast cancer. Nanotechnology. 2021;32:245102. 10.1088/1361-6528/abed03.Search in Google Scholar PubMed
[63] Cai P, Su D, Yang W, He Z, Zhang C, Liu H, et al. Inherently PET/CT dual modality imaging lipid nanocapsules for early detection of orthotopic lung tumors. ACS Appl Bio Mater. 2020;3:611–21. 10.1021/acsabm.9b00993.Search in Google Scholar PubMed
[64] Huang X, Zhong J, Ren J, Wen D, Zhao W, Huan Y. A DNA aptamer recognizing MMP14 for in vivo and in vitro imaging identified by cell-SELEX. Oncol Lett. 2019;18:265–74. 10.3892/ol.2019.10282.Search in Google Scholar PubMed PubMed Central
[65] Zhang W, Zhou R, Yang Y, Peng S, Xiao D, Kong T, et al. Aptamer-mediated synthesis of multifunctional nano-hydroxyapatite for active tumour bioimaging and treatment. Cell Prolif. 2021;54:e13105. 10.1111/cpr.13105.Search in Google Scholar PubMed PubMed Central
[66] Jiang Y, Zhao W, Zhou H, Zhang Q, Zhang S. ATP-triggered intracellular in situ aggregation of a gold-nanoparticle-equipped triple-helix molecular switch for fluorescence imaging and photothermal tumor therapy. Langmuir. 2022;38:3755–64. 10.1021/acs.langmuir.1c03331.Search in Google Scholar PubMed
[67] Wang L, Niu M, Zheng C, Zhao H, Niu X, Li L, et al. A core-shell nanoplatform for synergistic enhanced sonodynamic therapy of hypoxic tumor via cascaded strategy. Adv Healthc Mater. 2018;7:e1800819. 10.1002/adhm.201800819.Search in Google Scholar PubMed
[68] Chinnappan R, Al FA, Abdel RA, Abu-Salah KM, Mouffouk F, Zourob M. Anti-VCAM-1 and Anti-IL4Rα aptamer-conjugated super paramagnetic iron oxide nanoparticles for enhanced breast cancer diagnosis and therapy. Molecules. 2020;25:3437. 10.3390/molecules25153437.Search in Google Scholar PubMed PubMed Central
[69] Zhang M, Wang W, Wu F, Graveran K, Zhang J, Wu C. Black phosphorus quantum dots gated, carbon-coated Fe3O4 nanocapsules (BPQDs@ss-Fe3 O4 @C) with low premature release could enable imaging-guided cancer combination therapy. Chem Eur J. 2018;24:12890–901. 10.1002/chem.201801085.Search in Google Scholar PubMed
[70] Akbarzadeh M, Babaei M, Abnous K, Taghdisi SM, Peivandi MT, Ramezani M, et al. Hybrid silica-coated Gd-Zn-Cu-In-S/ZnS bimodal quantum dots as an epithelial cell adhesion molecule targeted drug delivery and imaging system. Int J Pharm. 2019;570:118645. 10.1016/j.ijpharm.2019.118645.Search in Google Scholar PubMed
[71] Zheng S, Zhang M, Bai H, He M, Dong L, Cai L, et al. Preparation of AS1411 aptamer modified Mn-MoS2 QDs for targeted MR imaging and fluorescence labelling of renal cell carcinoma. Int J Nanomed. 2019;14:9513–24. 10.2147/IJN.S215883.Search in Google Scholar PubMed PubMed Central
[72] Wu M, Zhao H, Guo L, Wang Y, Song J, Zhao X, et al. Ultrasound-mediated nanobubble destruction (UMND) facilitates the delivery of A10-3.2 aptamer targeted and siRNA-loaded cationic nanobubbles for therapy of prostate cancer. Drug Deliv. 2018;25:226–40. 10.1080/10717544.2017.1422300.Search in Google Scholar PubMed PubMed Central
[73] Wu M, Wang Y, Wang Y, Zhang M, Luo Y, Tang J, et al. Paclitaxel-loaded and A10-3.2 aptamer-targeted poly(lactide-co-glycolic acid) nanobubbles for ultrasound imaging and therapy of prostate cancer. Int J Nanomed. 2017;12:5313–30. 10.2147/IJN.S136032.Search in Google Scholar PubMed PubMed Central
[74] Zhang W, Ding X, Cheng H, Yin C, Yan J, Mou Z, et al. Dual-targeted gold nanoprism for recognition of early apoptosis, dual-model imaging, and precise cancer photothermal therapy. Theranostics. 2019;9:5610–25. 10.7150/thno.34755.Search in Google Scholar PubMed PubMed Central
[75] Dai Y, Zhao H, He K, Du W, Kong Y, Wang Z, et al. NIR-II excitation phototheranostic nanomedicine for fluorescence/photoacoustic tumor imaging and targeted photothermal-photonic thermodynamic therapy. Small. 2021;17:e2102527. 10.1002/smll.202102527.Search in Google Scholar PubMed
[76] Chang W, Wang J, Zhang J, Ling Q, Li Y, Wang J. High performance gold nanorods@DNA self-assembled drug-loading system for cancer thermo-chemotherapy in the second near-infrared optical window. Pharmaceutics. 2022;14:1110. 10.3390/pharmaceutics14051110.Search in Google Scholar PubMed PubMed Central
[77] Najdian A, Amanlou M, Beiki D, Bitarafan-Rajabi A, Mirzaei M, Shafiee AM. Amino-modified-silica-coated gadolinium-copper nanoclusters, conjugated to AS1411 aptamer and radiolabeled with technetium-99 m as a novel multimodal imaging agent. Bioorg Chem. 2022;125:105827. 10.1016/j.bioorg.2022.105827.Search in Google Scholar PubMed
[78] Sivakumar B, Aswathy RG, Romero-Aburto R, Mitcham T, Mitchel KA, Nagaoka Y, et al. Highly versatile SPION encapsulated PLGA nanoparticles as photothermal ablators of cancer cells and as multimodal imaging agents. Biomater Sci. 2017;5:432–43. 10.1039/c6bm00621c.Search in Google Scholar PubMed
[79] Fletcher NL, Houston ZH, Simpson JD, Veedu RN, Thurecht KJ. Designed multifunctional polymeric nanomedicines: Long-term biodistribution and tumour accumulation of aptamer-targeted nanomaterials. Chem Commun. 2018;54:11538–41. 10.1039/c8cc05831h.Search in Google Scholar PubMed
[80] Xu M, Xue B, Wang Y, Wang D, Gao D, Yang S, et al. Temperature-feedback nanoplatform for NIR-II penta-modal imaging-guided synergistic photothermal therapy and CAR-NK immunotherapy of lung cancer. Small. 2021;17:e2101397. 10.1002/smll.202101397.Search in Google Scholar PubMed
[81] Liu M, Wang L, Lo Y, Shiu SC, Kinghorn AB, Tanner JA. Aptamer-enabled nanomaterials for therapeutics, drug targeting and imaging. Cells. 2022;11:159. 10.3390/cells11010159.Search in Google Scholar PubMed PubMed Central
[82] Xu Y, Luo C, Wang J, Chen L, Chen J, Chen T, et al. Application of nanotechnology in the diagnosis and treatment of bladder cancer. J Nanobiotechnol. 2021;19:393. 10.1186/s12951-021-01104-y.Search in Google Scholar PubMed PubMed Central
[83] Ma W, Zhan Y, Zhang Y, Mao C, Xie X, Lin Y. The biological applications of DNA nanomaterials: current challenges and future directions. Signal Transduct Target Ther. 2021;6:351. 10.1038/s41392-021-00727-9.Search in Google Scholar PubMed PubMed Central
[84] Tian R, Shang Y, Wang Y, Jiang Q, Ding B. DNA nanomaterials-based platforms for cancer immunotherapy. Small Methods. 2023;7:e2201518. 10.1002/smtd.202201518.Search in Google Scholar PubMed
[85] Zhan Y, Ma W, Zhang Y, Mao C, Shao X, Xie X, et al. Diversity of DNA nanostructures and applications in oncotherapy. Biotechnol J. 2020;15:e1900094. 10.1002/biot.201900094.Search in Google Scholar PubMed
[86] Meng HM, Liu H, Kuai H, Peng R, Mo L, Zhang XB. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem Soc Rev. 2016;45:2583–602. 10.1039/c5cs00645g.Search in Google Scholar PubMed
[87] Charbgoo F, Alibolandi M, Taghdisi SM, Abnous K, Soltani F, Ramezani M. MUC1 aptamer-targeted DNA micelles for dual tumor therapy using doxorubicin and KLA peptide. Nanomedicine. 2018;14:685–97. 10.1016/j.nano.2017.12.010.Search in Google Scholar PubMed
[88] Li Z, Liu M, Ke L, Wang LJ, Wu C, Li C, et al. Flexible polymeric nanosized micelles for ophthalmic drug delivery: research progress in the last three years. Nanoscale Adv. 2021;3:5240–54. 10.1039/d1na00596k.Search in Google Scholar PubMed PubMed Central
[89] Kotta S, Aldawsari HM, Badr-Eldin SM, Nair AB, Yt K. Progress in polymeric micelles for drug delivery applications. Pharmaceutics. 2022;14:1636. 10.3390/pharmaceutics14081636.Search in Google Scholar PubMed PubMed Central
[90] Miyamoto D, Tang Z, Takarada T, Maeda M. Turbidimetric detection of ATP using polymeric micelles and DNA aptamers. Chem Commun. 2007;7:4743–5. 10.1039/b709775a.Search in Google Scholar PubMed
[91] Tian J, Ding L, Ju H, Yang Y, Li X, Shen Z, et al. A multifunctional nanomicelle for real-time targeted imaging and precise near-infrared cancer therapy. Angew Chem Int Ed. 2014;53:9544–9. 10.1002/anie.201405490.Search in Google Scholar PubMed
[92] Wei J, Xue W, Yu X, Qiu X, Liu Z. pH sensitive phosphorylated chitosan hydrogel as vaccine delivery system for intramuscular immunization. J Biomater Appl. 2017;31:1358–69. 10.1177/0885328217704139.Search in Google Scholar PubMed
[93] Gasztych M, Komsa K, Musia W. Influence of hydrophilic co-monomer on the drug release from hydrogels with thermosensitive N-(isopropyl)acrylamide derivatives. J Nanosci Nanotechnol. 2019;19:2514–21. 10.1166/jnn.2019.15848.Search in Google Scholar PubMed
[94] Lai J, Li S, Shi X, Coyne J, Zhao N, Dong F, et al. Displacement and hybridization reactions in aptamer-functionalized hydrogels for biomimetic protein release and signal transduction. Chem Sci. 2017;8:7306–11. 10.1039/c7sc03023a.Search in Google Scholar PubMed PubMed Central
[95] Rocha CV, Gonçalves V, Da SM, Bañobre-López M, Gallo J. PLGA-based composites for various biomedical applications. Int J Mol Sci. 2022;23:2034. 10.3390/ijms23042034.Search in Google Scholar PubMed PubMed Central
[96] Vandghanooni S, Eskandani M, Barar J, Omidi Y. AS1411 aptamer-decorated cisplatin-loaded poly(lactic-co-glycolic acid) nanoparticles for targeted therapy of miR-21-inhibited ovarian cancer cells. Nanomedicine. 2018;13:2729–58. 10.2217/nnm-2018-0205.Search in Google Scholar PubMed
[97] Askarian S, Abnous K, Taghavi S, Oskuee RK, Ramezani M. Cellular delivery of shRNA using aptamer-conjugated PLL-alkyl-PEI nanoparticles. Colloid Surf B Biointerfaces. 2015;136:355–64. 10.1016/j.colsurfb.2015.09.023.Search in Google Scholar PubMed
[98] Sanati S, Taghavi S, Abnous K, Taghdisi SM, Babaei M, Ramezani M, et al. Fabrication of anionic dextran-coated micelles for aptamer targeted delivery of camptothecin and survivin-shRNA to colon adenocarcinoma. Gene Ther. 2022;29:55–68. 10.1038/s41434-021-00234-0.Search in Google Scholar PubMed
[99] Moosavian SA, Abnous K, Akhtari J, Arabi L, Gholamzade DA, Jafari M. 5TR1 aptamer-PEGylated liposomal doxorubicin enhances cellular uptake and suppresses tumour growth by targeting MUC1 on the surface of cancer cells. Artif Cell Nanomed Biotechnol. 2018;46:2054–65. 10.1080/21691401.2017.1408120.Search in Google Scholar PubMed
[100] Mosafer J, Abnous K, Tafaghodi M, Mokhtarzadeh A, Ramezani M. In vitro and in vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. Eur J Pharm Biopharm. 2017;113:60–74. 10.1016/j.ejpb.2016.12.009.Search in Google Scholar PubMed
[101] Li H, Zha S, Li H, Liu H, Wong KL, All AH. Polymeric dendrimers as nanocarrier vectors for neurotheranostics. Small. 2022;18:e2203629. 10.1002/smll.202203629.Search in Google Scholar PubMed
[102] Li J, Liang H, Liu J, Wang Z. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy. Int J Pharm. 2018;546:215–25. 10.1016/j.ijpharm.2018.05.045.Search in Google Scholar PubMed
[103] Ban Q, Sun W, Kong J, Wu S. Hyperbranched polymers with controllable topologies for drug delivery. Chem Asian J. 2018;13:3341–50. 10.1002/asia.201800812.Search in Google Scholar PubMed
[104] Zhang HJ, Zhao X, Chen LJ, Yang CX, Yan XP. Dendrimer grafted persistent luminescent nanoplatform for aptamer guided tumor imaging and acid-responsive drug delivery. Talanta. 2020;219:121209. 10.1016/j.talanta.2020.121209.Search in Google Scholar PubMed
[105] Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–52. 10.1016/s0022-2836(65)80093-6.Search in Google Scholar PubMed
[106] Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drug delivery. Chem Rev. 2015;115:10938–66. 10.1021/acs.chemrev.5b00046.Search in Google Scholar PubMed
[107] Almeida B, Nag OK, Rogers KE, Delehanty JB. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules. 2020;25:5672. 10.3390/molecules25235672.Search in Google Scholar PubMed PubMed Central
[108] Yan W, Leung SS, To KK. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine. 2020;15:303–18. 10.2217/nnm-2019-0308.Search in Google Scholar PubMed
[109] El-Hammadi MM, Arias JL. An update on liposomes in drug delivery: A patent review (2014–2018). Expert Opin Ther Pat. 2019;29:891–907. 10.1080/13543776.2019.1679767.Search in Google Scholar PubMed
[110] Singh P, Pandit S, Mokkapati V, Garg A, Ravikumar V, Mijakovic I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int J Mol Sci. 2018;19:1979. 10.3390/ijms19071979.Search in Google Scholar PubMed PubMed Central
[111] Capek I. Polymer decorated gold nanoparticles in nanomedicine conjugates. Adv Colloid Interface Sci. 2017;249:386–99. 10.1016/j.cis.2017.01.007.Search in Google Scholar PubMed
[112] Cole LE, Ross RD, Tilley JM, Vargo-Gogola T, Roeder RK. Gold nanoparticles as contrast agents in X-ray imaging and computed tomography. Nanomedicine. 2015;10:321–41. 10.2217/nnm.14.171.Search in Google Scholar PubMed
[113] Nicolás-Boluda A, Vaquero J, Laurent G, Renault G, Bazzi R, Donnadieu E, et al. Photothermal depletion of cancer-associated fibroblasts normalizes tumor stiffness in desmoplastic cholangiocarcinoma. ACS Nano. 2020;14:5738–53. 10.1021/acsnano.0c00417.Search in Google Scholar PubMed
[114] Wu K, Su D, Liu J, Saha R, Wang JP. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology. 2019;30:502003. 10.1088/1361-6528/ab4241.Search in Google Scholar PubMed
[115] Zhu L, Zhou Z, Mao H, Yang L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine. 2017;12:73–87. 10.2217/nnm-2016-0316.Search in Google Scholar PubMed PubMed Central
[116] Avasthi A, Caro C, Pozo-Torres E, Leal MP, García-Martín ML. Magnetic nanoparticles as MRI contrast agents. Top Curr Chem. 2020;378:40. 10.1007/s41061-020-00302-w.Search in Google Scholar PubMed PubMed Central
[117] Joshi B, Joshi A. Polymeric magnetic nanoparticles: A multitargeting approach for brain tumor therapy and imaging. Drug Deliv Transl Res. 2022;12:1588–604. 10.1007/s13346-021-01063-9.Search in Google Scholar PubMed
[118] Yao J, Li P, Li L, Yang M. Biochemistry and biomedicine of quantum dots: From biodetection to bioimaging, drug discovery, diagnostics, and therapy. Acta Biomater. 2018;74:36–55. 10.1016/j.actbio.2018.05.004.Search in Google Scholar PubMed
[119] Cunha C, Oliveira A, Firmino T, Tenório D, Pereira G, Carvalho LJ, et al. Biomedical applications of glyconanoparticles based on quantum dots. Biochim Biophys Acta Gen Subj. 2018;1862:427–39. 10.1016/j.bbagen.2017.11.010.Search in Google Scholar PubMed
[120] Chen LL, Zhao L, Wang ZG, Liu SL, Pang DW. Near-infrared-II quantum dots for in vivo imaging and cancer therapy. Small. 2022;18:e2104567. 10.1002/smll.202104567.Search in Google Scholar PubMed
[121] Tsuboi S, Jin T. BRET-based dual-color (visible/near-infrared) molecular imaging using a quantum Dot/EGFP-luciferase conjugate. Methods Mol Biol. 2022;2525:47–59. 10.1007/978-1-0716-2473-9_5.Search in Google Scholar PubMed
[122] Shahshahanipour M, Rezaei B, Ensafi AA, Etemadifar Z. An ancient plant for the synthesis of a novel carbon dot and its applications as an antibacterial agent and probe for sensing of an anti-cancer drug. Mater Sci Eng C Mater Biol Appl. 2019;98:826–33. 10.1016/j.msec.2019.01.041.Search in Google Scholar PubMed
[123] Shukla D, Das M, Kasade D, Pandey M, Dubey AK, Yadav SK, et al. Sandalwood-derived carbon quantum dots as bioimaging tools to investigate the toxicological effects of malachite green in model organisms. Chemosphere. 2020;248:125998. 10.1016/j.chemosphere.2020.125998.Search in Google Scholar PubMed
[124] Jha A, Viswanadh MK, Burande AS, Mehata AK, Poddar S, Yadav K, et al. DNA biodots based targeted theranostic nanomedicine for the imaging and treatment of non-small cell lung cancer. Int J Biol Macromol. 2020;150:413–25. 10.1016/j.ijbiomac.2020.02.075.Search in Google Scholar PubMed
[125] Wu SH, Mou CY, Lin HP. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42:3862–75. 10.1039/c3cs35405a.Search in Google Scholar PubMed
[126] Chen Y, Chen H, Shi J. In vivo bio-safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv Mater. 2013;25:3144–76. 10.1002/adma.201205292.Search in Google Scholar PubMed
[127] Huang Y, Li P, Zhao R, Zhao L, Liu J, Peng S, et al. Silica nanoparticles: Biomedical applications and toxicity. Biomed Pharmacother. 2022;151:113053. 10.1016/j.biopha.2022.113053.Search in Google Scholar PubMed
[128] Abu-Dief AM, Alsehli M, Al-Enizi A, Nafady A. Recent advances in mesoporous silica nanoparticles for targeted drug delivery applications. Curr Drug Deliv. 2022;19:436–50. 10.2174/1567201818666210708123007.Search in Google Scholar PubMed
[129] Wu T, Liu Y, Cao Y, Liu Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater. 2022;34:e2110364. 10.1002/adma.202110364.Search in Google Scholar PubMed
[130] Mehra NK, Jain AK, Nahar M. Carbon nanomaterials in oncology: An expanding horizon. Drug Discov Today. 2018;23:1016–25. 10.1016/j.drudis.2017.09.013.Search in Google Scholar PubMed
[131] Shen CL, Liu HR, Lou Q, Wang F, Liu KK, Dong L, et al. Recent progress of carbon dots in targeted bioimaging and cancer therapy. Theranostics. 2022;12:2860–93. 10.7150/thno.70721.Search in Google Scholar PubMed PubMed Central
[132] Wong BS, Yoong SL, Jagusiak A, Panczyk T, Ho HK, Ang WH, et al. Carbon nanotubes for delivery of small molecule drugs. Adv Drug Deliv Rev. 2013;65:1964–2015. 10.1016/j.addr.2013.08.005.Search in Google Scholar PubMed
[133] Hosnedlova B, Kepinska M, Fernandez C, Peng Q, Ruttkay-Nedecky B, Milnerowicz H, et al. Carbon nanomaterials for targeted cancer therapy drugs: A critical review. Chem Rec. 2019;19:502–22. 10.1002/tcr.201800038.Search in Google Scholar PubMed
[134] Cao W, He L, Cao W, Huang X, Jia K, Dai J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020;112:14–28. 10.1016/j.actbio.2020.06.009.Search in Google Scholar PubMed
[135] Fan W, Zhang L, Li Y, Wu H. Recent progress of crosslinking strategies for polymeric micelles with enhanced drug delivery in cancer therapy. Curr Med Chem. 2019;26:2356–76. 10.2174/0929867324666171121102255.Search in Google Scholar PubMed
[136] Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. 10.1016/j.ijpharm.2021.120571.Search in Google Scholar PubMed
[137] Tao C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges, and prospects. Lett Appl Microbiol. 2018;67:537–43. 10.1111/lam.13082.Search in Google Scholar PubMed
[138] Keshri S, Biswas S. Synthesis, physical properties, and biomedical applications of magnetic nanoparticles: A review. Prog Biomater. 2022;11:347–72. 10.1007/s40204-022-00204-8.Search in Google Scholar PubMed PubMed Central
[139] Omran BA, Whitehead KA, Baek KH. One-pot bioinspired synthesis of fluorescent metal chalcogenide and carbon quantum dots: Applications and potential biotoxicity. Colloid Surf B Biointerfaces. 2021;200:111578. 10.1016/j.colsurfb.2021.111578.Search in Google Scholar PubMed
[140] Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev. 2012;41:2590–605. 10.1039/c1cs15246g.Search in Google Scholar PubMed
[141] Liu XH, Xue S, Ma JZ. Mesoporous silica nanoparticles for bone tissue engineering. Zhongguo Gu Shang. 2020;33:784–7. 10.12200/j.issn.1003-0034.2020.08.019.Search in Google Scholar PubMed
[142] Bohrmann L, Burghardt T, Haynes C, Saatchi K, Häfeli UO. Aptamers used for molecular imaging and theranostics - recent developments. Theranostics. 2022;12:4010–50. 10.7150/thno.72949.Search in Google Scholar PubMed PubMed Central
[143] Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J, et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56–73. 10.1016/j.ejca.2021.01.008.Search in Google Scholar PubMed PubMed Central
[144] Lei Y, Qiao Z, Tang J, He X, Shi H, Ye X, et al. DNA nanotriangle-scaffolded activatable aptamer probe with ultralow background and robust stability for cancer theranostics. Theranostics. 2018;8:4062–71. 10.7150/thno.24683.Search in Google Scholar PubMed PubMed Central
[145] Lei Y, He X, Tang J, Shi H, He D, Yan L, et al. Ultra-pH-responsive split i-motif based aptamer anchoring strategy for specific activatable imaging of acidic tumor microenvironment. Chem Commun. 2018;54:10288–91. 10.1039/c8cc04420a.Search in Google Scholar PubMed
[146] Zhu G, Chen X. Aptamer-based targeted therapy. Adv Drug Deliv Rev. 2018;134:65–78. 10.1016/j.addr.2018.08.005.Search in Google Scholar PubMed PubMed Central
[147] Xie X, Zhang Y, Ma W, Shao X, Zhan Y, Mao C, et al. Potent anti-angiogenesis and anti-tumour activity of pegaptanib-loaded tetrahedral DNA nanostructure. Cell Prolif. 2019;52:e12662. 10.1111/cpr.12662.Search in Google Scholar PubMed PubMed Central
[148] Moradi F, Farolfi A, Fanti S, Iagaru A. Prostate cancer: Molecular imaging and MRI. Eur J Radiol. 2021;143:109893. 10.1016/j.ejrad.2021.109893.Search in Google Scholar PubMed
[149] Bouvier-Müller A, Ducongé F. Application of aptamers for in vivo molecular imaging and theranostics. Adv Drug Deliv Rev. 2018;134:94–106. 10.1016/j.addr.2018.08.004.Search in Google Scholar PubMed
[150] Refaat A, Yap ML, Pietersz G, Walsh A, Zeller J, Del RB, et al. In vivo fluorescence imaging: success in preclinical imaging paves the way for clinical applications. J Nanobiotechnol. 2022;20:450. 10.1186/s12951-022-01648-7.Search in Google Scholar PubMed PubMed Central
[151] Zhao Z, Ma J, Wang Y, Xu Z, Zhao L, Zhao J, et al. Antimicrobial photodynamic therapy combined with antibiotic in the treatment of rats with third-degree burns. Front Microbiol. 2021;12:622410. 10.3389/fmicb.2021.622410.Search in Google Scholar PubMed PubMed Central
[152] Barth CW, Gibbs SL. Fluorescence image-guided surgery - A perspective on contrast agent development. Proc SPIE. 2020;11222:27–42. 10.1117/12.2545292.Search in Google Scholar PubMed PubMed Central
[153] Smith AM, Mancini MC, Nie S. Bioimaging: Second window for in vivo imaging. Nat Nanotechnol. 2009;4:710–1. 10.1038/nnano.2009.326.Search in Google Scholar PubMed PubMed Central
[154] Haque A, Faizi M, Rather JA, Khan MS. Next generation NIR fluorophores for tumor imaging and fluorescence-guided surgery: A review. Bioorg Med Chem. 2017;25:2017–34. 10.1016/j.bmc.2017.02.061.Search in Google Scholar PubMed
[155] Hong G, Lee JC, Robinson JT, Raaz U, Xie L, Huang NF, et al. Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nat Med. 2012;18:1841–6. 10.1038/nm.2995.Search in Google Scholar PubMed PubMed Central
[156] Heidari F, Mohajeri N, Zarghami N. Targeted design of green carbon dot-CA-125 aptamer conjugate for the fluorescence imaging of ovarian cancer cell. Cell Biochem Biophys. 2022;80:75–88. 10.1007/s12013-021-01034-4.Search in Google Scholar PubMed
[157] Kovacevic KD, Gilbert JC, Jilma B. Pharmacokinetics, pharmacodynamics and safety of aptamers. Adv Drug Deliv Rev. 2018;134:36–50. 10.1016/j.addr.2018.10.008.Search in Google Scholar PubMed
[158] Esmaeili Y, Zarrabi A, Mirahmadi-Zare SZ, Bidram E. Hierarchical multifunctional graphene oxide cancer nanotheranostics agent for synchronous switchable fluorescence imaging and chemical therapy. Microchim Acta. 2020;187:553. 10.1007/s00604-020-04490-6.Search in Google Scholar PubMed
[159] Jiang Z, Zhang M, Li P, Wang Y, Fu Q. Nanomaterial-based CT contrast agents and their applications in image-guided therapy. Theranostics. 2023;13:483–509. 10.7150/thno.79625.Search in Google Scholar PubMed PubMed Central
[160] Weissleder R, Moore A, Mahmood U, Bhorade R, Benveniste H, Chiocca EA, et al. In vivo magnetic resonance imaging of transgene expression. Nat Med. 2000;6:351–5. 10.1038/73219.Search in Google Scholar PubMed
[161] Hu F, Zhao YS. Inorganic nanoparticle-based T1 and T1/T2 magnetic resonance contrast probes. Nanoscale. 2012;4:6235–43. 10.1039/c2nr31865b.Search in Google Scholar PubMed
[162] Condeelis J, Weissleder R. In vivo imaging in cancer. Cold Spring Harb Perspect Biol. 2010;2:a003848. 10.1101/cshperspect.a003848.Search in Google Scholar PubMed PubMed Central
[163] Najjar AM, Johnson JM, Schellingerhout D. The emerging role of amino acid PET in neuro-oncology. Bioengineering-Basel. 2018;5. 10.3390/bioengineering5040104.Search in Google Scholar PubMed PubMed Central
[164] Spath NB, Thompson G, Baker AH, Dweck MR, Newby DE, Semple S. Manganese-enhanced MRI of the myocardium. Heart. 2019;105:1695–700. 10.1136/heartjnl-2019-315227.Search in Google Scholar PubMed PubMed Central
[165] Sánchez-Cabezas S, Montes-Robles R, Gallo J, Sancenón F, Martínez-Máñez R. Combining magnetic hyperthermia and dual T1/T2 MR imaging using highly versatile iron oxide nanoparticles. Dalton Trans. 2019;48:3883–92. 10.1039/c8dt04685a.Search in Google Scholar PubMed
[166] Dallet L, Stanicki D, Voisin P, Miraux S, Ribot EJ. Micron-sized iron oxide particles for both MRI cell tracking and magnetic fluid hyperthermia treatment. Sci Rep. 2021;11:3286. 10.1038/s41598-021-82095-6.Search in Google Scholar PubMed PubMed Central
[167] Farzin A, Etesami SA, Quint J, Memic A, Tamayol A. Magnetic nanoparticles in cancer therapy and diagnosis. Adv Healthc Mater. 2020;9:e1901058. 10.1002/adhm.201901058.Search in Google Scholar PubMed PubMed Central
[168] Chen N, Zhang G, Fu J, Wu Q. Matrix metalloproteinase-14 (MMP-14) downregulation inhibits esophageal squamous cell carcinoma cell migration, invasion, and proliferation. Thorac Cancer. 2020;11:3168–74. 10.1111/1759-7714.13636.Search in Google Scholar PubMed PubMed Central
[169] Czernin J, Sonni I, Razmaria A, Calais J. The future of nuclear medicine as an independent specialty. J Nucl Med. 2019;60:3S–12S. 10.2967/jnumed.118.220558.Search in Google Scholar PubMed
[170] Pellico J, Gawne PJ, de Rosales RTM. Radiolabelling of nanomaterials for medical imaging and therapy. Chem Soc Rev. 2021;50:3355–423. 10.1039/d0cs00384k.Search in Google Scholar PubMed
[171] Zhu L, Ploessl K, Kung HF. PET/SPECT imaging agents for neurodegenerative diseases. Chem Soc Rev. 2014;43:6683–91. 10.1039/c3cs60430f.Search in Google Scholar PubMed PubMed Central
[172] Hicke BJ, Stephens AW, Gould T, Chang YF, Lynott CK, Heil J, et al. Tumor targeting by an aptamer. J Nucl Med. 2006;47:668–78.Search in Google Scholar
[173] Smith-Bindman R, Kwan ML, Marlow EC, Theis MK, Bolch W, Cheng SY, et al. Trends in use of medical imaging in US Health Care Systems and in Ontario, Canada, 2000–2016. J Am Med Assoc. 2019;322:843–56. 10.1001/jama.2019.11456.Search in Google Scholar PubMed PubMed Central
[174] Zhang G, Ye HR, Sun Y, Guo ZZ. Ultrasound molecular imaging and its applications in cancer diagnosis and therapy. ACS Sens. 2022;7:2857–64. 10.1021/acssensors.2c01468.Search in Google Scholar PubMed
[175] Wang C, Yang S, Chen X, He Q, Zhao K, Hu J. Molecular imaging diagnosis of atherosclerotic vulnerable plaque in rabbit carotid artery using a self-assembled nanoscale ultrasound microbubble contrast agent. Rev Cardiovasc Med. 2021;22:1657–66. 10.31083/j.rcm2204173.Search in Google Scholar PubMed
[176] Chong WK, Papadopoulou V, Dayton PA. Imaging with ultrasound contrast agents: Current status and future. Abdom Radiol. 2018;43:762–72. 10.1007/s00261-018-1516-1.Search in Google Scholar PubMed
[177] Ntziachristos V, Razansky D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT). Chem Rev. 2010;110:2783–94. 10.1021/cr9002566.Search in Google Scholar PubMed
[178] Attia A, Balasundaram G, Moothanchery M, Dinish US, Bi R, Ntziachristos V, et al. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics. 2019;16:100144. 10.1016/j.pacs.2019.100144.Search in Google Scholar PubMed PubMed Central
[179] Kim C, Favazza C, Wang LV. In vivo photoacoustic tomography of chemicals: High-resolution functional and molecular optical imaging at new depths. Chem Rev. 2010;110:2756–82. 10.1021/cr900266s.Search in Google Scholar PubMed PubMed Central
[180] Wang S, Lin J, Wang T, Chen X, Huang P. Recent advances in photoacoustic imaging for deep-tissue biomedical applications. Theranostics. 2016;6:2394–413. 10.7150/thno.16715.Search in Google Scholar PubMed PubMed Central
[181] Upputuri PK, Pramanik M. Recent advances in photoacoustic contrast agents for in vivo imaging. Wiley Interdiscip Rev-Nanomed Nanobiotechnol. 2020;12:e1618. 10.1002/wnan.1618.Search in Google Scholar PubMed
[182] Zhang J, Smaga LP, Satyavolu N, Chan J, Lu Y. DNA aptamer-based activatable probes for photoacoustic imaging in living mice. J Am Chem Soc. 2017;139:17225–8. 10.1021/jacs.7b07913.Search in Google Scholar PubMed PubMed Central
[183] Vermeulen I, Isin EM, Barton P, Cillero-Pastor B, Heeren R. Multimodal molecular imaging in drug discovery and development. Drug Discov Today. 2022;27:2086–99. 10.1016/j.drudis.2022.04.009.Search in Google Scholar PubMed
[184] Foray C, Barca C, Backhaus P, Schelhaas S, Winkeler A, Viel T, et al. Multimodal molecular imaging of the tumour microenvironment. Adv Exp Med Biol. 2020;1225:71–87. 10.1007/978-3-030-35727-6_5.Search in Google Scholar PubMed
[185] Wu M, Shu J. Multimodal molecular imaging: Current status and future directions. Contrast Media Mol Imaging. 2018;2018:1382183. 10.1155/2018/1382183.Search in Google Scholar PubMed PubMed Central
[186] Lee DS, Qian H, Tay CY, Leong DT. Cellular processing and destinies of artificial DNA nanostructures. Chem Soc Rev. 2016;45:4199–225. 10.1039/c5cs00700c.Search in Google Scholar PubMed
[187] Amero P, Lokesh G, Chaudhari RR, Cardenas-Zuniga R, Schubert T, Attia YM, et al. Conversion of RNA aptamer into modified DNA aptamers provides for prolonged stability and enhanced antitumor activity. J Am Chem Soc. 2021;143:7655–70. 10.1021/jacs.9b10460.Search in Google Scholar PubMed
[188] Cheng C, Chen YH, Lennox KA, Behlke MA, Davidson BL. In vivo SELEX for identification of brain-penetrating aptamers. Mol Ther Nucleic Acids. 2013;2:e67. 10.1038/mtna.2012.59.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus
Articles in the same Issue
- Research Articles
- Preparation of CdS–Ag2S nanocomposites by ultrasound-assisted UV photolysis treatment and its visible light photocatalysis activity
- Significance of nanoparticle radius and inter-particle spacing toward the radiative water-based alumina nanofluid flow over a rotating disk
- Aptamer-based detection of serotonin based on the rapid in situ synthesis of colorimetric gold nanoparticles
- Investigation of the nucleation and growth behavior of Ti2AlC and Ti3AlC nano-precipitates in TiAl alloys
- Dynamic recrystallization behavior and nucleation mechanism of dual-scale SiCp/A356 composites processed by P/M method
- High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers
- Applying solution of spray polyurea elastomer in asphalt binder: Feasibility analysis and DSR study based on the MSCR and LAS tests
- Study on the chronic toxicity and carcinogenicity of iron-based bioabsorbable stents
- Influence of microalloying with B on the microstructure and properties of brazed joints with Ag–Cu–Zn–Sn filler metal
- Thermohydraulic performance of thermal system integrated with twisted turbulator inserts using ternary hybrid nanofluids
- Study of mechanical properties of epoxy/graphene and epoxy/halloysite nanocomposites
- Effects of CaO addition on the CuW composite containing micro- and nano-sized tungsten particles synthesized via aluminothermic coupling with silicothermic reduction
- Cu and Al2O3-based hybrid nanofluid flow through a porous cavity
- Design of functional vancomycin-embedded bio-derived extracellular matrix hydrogels for repairing infectious bone defects
- Study on nanocrystalline coating prepared by electro-spraying 316L metal wire and its corrosion performance
- Axial compression performance of CFST columns reinforced by ultra-high-performance nano-concrete under long-term loading
- Tungsten trioxide nanocomposite for conventional soliton and noise-like pulse generation in anomalous dispersion laser cavity
- Microstructure and electrical contact behavior of the nano-yttria-modified Cu-Al2O3/30Mo/3SiC composite
- Melting rheology in thermally stratified graphene-mineral oil reservoir (third-grade nanofluid) with slip condition
- Re-examination of nonlinear vibration and nonlinear bending of porous sandwich cylindrical panels reinforced by graphene platelets
- Parametric simulation of hybrid nanofluid flow consisting of cobalt ferrite nanoparticles with second-order slip and variable viscosity over an extending surface
- Chitosan-capped silver nanoparticles with potent and selective intrinsic activity against the breast cancer cells
- Multi-core/shell SiO2@Al2O3 nanostructures deposited on Ti3AlC2 to enhance high-temperature stability and microwave absorption properties
- Solution-processed Bi2S3/BiVO4/TiO2 ternary heterojunction photoanode with enhanced photoelectrochemical performance
- Electroporation effect of ZnO nanoarrays under low voltage for water disinfection
- NIR-II window absorbing graphene oxide-coated gold nanorods and graphene quantum dot-coupled gold nanorods for photothermal cancer therapy
- Nonlinear three-dimensional stability characteristics of geometrically imperfect nanoshells under axial compression and surface residual stress
- Investigation of different nanoparticles properties on the thermal conductivity and viscosity of nanofluids by molecular dynamics simulation
- Optimized Cu2O-{100} facet for generation of different reactive oxidative species via peroxymonosulfate activation at specific pH values to efficient acetaminophen removal
- Brownian and thermal diffusivity impact due to the Maxwell nanofluid (graphene/engine oil) flow with motile microorganisms and Joule heating
- Appraising the dielectric properties and the effectiveness of electromagnetic shielding of graphene reinforced silicone rubber nanocomposite
- Synthesis of Ag and Cu nanoparticles by plasma discharge in inorganic salt solutions
- Low-cost and large-scale preparation of ultrafine TiO2@C hybrids for high-performance degradation of methyl orange and formaldehyde under visible light
- Utilization of waste glass with natural pozzolan in the production of self-glazed glass-ceramic materials
- Mechanical performance of date palm fiber-reinforced concrete modified with nano-activated carbon
- Melting point of dried gold nanoparticles prepared with ultrasonic spray pyrolysis and lyophilisation
- Graphene nanofibers: A modern approach towards tailored gypsum composites
- Role of localized magnetic field in vortex generation in tri-hybrid nanofluid flow: A numerical approach
- Intelligent computing for the double-diffusive peristaltic rheology of magneto couple stress nanomaterials
- Bioconvection transport of upper convected Maxwell nanoliquid with gyrotactic microorganism, nonlinear thermal radiation, and chemical reaction
- 3D printing of porous Ti6Al4V bone tissue engineering scaffold and surface anodization preparation of nanotubes to enhance its biological property
- Bioinspired ferromagnetic CoFe2O4 nanoparticles: Potential pharmaceutical and medical applications
- Significance of gyrotactic microorganisms on the MHD tangent hyperbolic nanofluid flow across an elastic slender surface: Numerical analysis
- Performance of polycarboxylate superplasticisers in seawater-blended cement: Effect from chemical structure and nano modification
- Entropy minimization of GO–Ag/KO cross-hybrid nanofluid over a convectively heated surface
- Oxygen plasma assisted room temperature bonding for manufacturing SU-8 polymer micro/nanoscale nozzle
- Performance and mechanism of CO2 reduction by DBD-coupled mesoporous SiO2
- Polyarylene ether nitrile dielectric films modified by HNTs@PDA hybrids for high-temperature resistant organic electronics field
- Exploration of generalized two-phase free convection magnetohydrodynamic flow of dusty tetra-hybrid Casson nanofluid between parallel microplates
- Hygrothermal bending analysis of sandwich nanoplates with FG porous core and piezomagnetic faces via nonlocal strain gradient theory
- Design and optimization of a TiO2/RGO-supported epoxy multilayer microwave absorber by the modified local best particle swarm optimization algorithm
- Mechanical properties and frost resistance of recycled brick aggregate concrete modified by nano-SiO2
- Self-template synthesis of hollow flower-like NiCo2O4 nanoparticles as an efficient bifunctional catalyst for oxygen reduction and oxygen evolution in alkaline media
- High-performance wearable flexible strain sensors based on an AgNWs/rGO/TPU electrospun nanofiber film for monitoring human activities
- High-performance lithium–selenium batteries enabled by nitrogen-doped porous carbon from peanut meal
- Investigating effects of Lorentz forces and convective heating on ternary hybrid nanofluid flow over a curved surface using homotopy analysis method
- Exploring the potential of biogenic magnesium oxide nanoparticles for cytotoxicity: In vitro and in silico studies on HCT116 and HT29 cells and DPPH radical scavenging
- Enhanced visible-light-driven photocatalytic degradation of azo dyes by heteroatom-doped nickel tungstate nanoparticles
- A facile method to synthesize nZVI-doped polypyrrole-based carbon nanotube for Ag(i) removal
- Improved osseointegration of dental titanium implants by TiO2 nanotube arrays with self-assembled recombinant IGF-1 in type 2 diabetes mellitus rat model
- Functionalized SWCNTs@Ag–TiO2 nanocomposites induce ROS-mediated apoptosis and autophagy in liver cancer cells
- Triboelectric nanogenerator based on a water droplet spring with a concave spherical surface for harvesting wave energy and detecting pressure
- A mathematical approach for modeling the blood flow containing nanoparticles by employing the Buongiorno’s model
- Molecular dynamics study on dynamic interlayer friction of graphene and its strain effect
- Induction of apoptosis and autophagy via regulation of AKT and JNK mitogen-activated protein kinase pathways in breast cancer cell lines exposed to gold nanoparticles loaded with TNF-α and combined with doxorubicin
- Effect of PVA fibers on durability of nano-SiO2-reinforced cement-based composites subjected to wet-thermal and chloride salt-coupled environment
- Effect of polyvinyl alcohol fibers on mechanical properties of nano-SiO2-reinforced geopolymer composites under a complex environment
- In vitro studies of titanium dioxide nanoparticles modified with glutathione as a potential drug delivery system
- Comparative investigations of Ag/H2O nanofluid and Ag-CuO/H2O hybrid nanofluid with Darcy-Forchheimer flow over a curved surface
- Study on deformation characteristics of multi-pass continuous drawing of micro copper wire based on crystal plasticity finite element method
- Properties of ultra-high-performance self-compacting fiber-reinforced concrete modified with nanomaterials
- Prediction of lap shear strength of GNP and TiO2/epoxy nanocomposite adhesives
- A novel exploration of how localized magnetic field affects vortex generation of trihybrid nanofluids
- Fabrication and physicochemical characterization of copper oxide–pyrrhotite nanocomposites for the cytotoxic effects on HepG2 cells and the mechanism
- Thermal radiative flow of cross nanofluid due to a stretched cylinder containing microorganisms
- In vitro study of the biphasic calcium phosphate/chitosan hybrid biomaterial scaffold fabricated via solvent casting and evaporation technique for bone regeneration
- Insights into the thermal characteristics and dynamics of stagnant blood conveying titanium oxide, alumina, and silver nanoparticles subject to Lorentz force and internal heating over a curved surface
- Effects of nano-SiO2 additives on carbon fiber-reinforced fly ash–slag geopolymer composites performance: Workability, mechanical properties, and microstructure
- Energy bandgap and thermal characteristics of non-Darcian MHD rotating hybridity nanofluid thin film flow: Nanotechnology application
- Green synthesis and characterization of ginger-extract-based oxali-palladium nanoparticles for colorectal cancer: Downregulation of REG4 and apoptosis induction
- Abnormal evolution of resistivity and microstructure of annealed Ag nanoparticles/Ag–Mo films
- Preparation of water-based dextran-coated Fe3O4 magnetic fluid for magnetic hyperthermia
- Statistical investigations and morphological aspects of cross-rheological material suspended in transportation of alumina, silica, titanium, and ethylene glycol via the Galerkin algorithm
- Effect of CNT film interleaves on the flexural properties and strength after impact of CFRP composites
- Self-assembled nanoscale entities: Preparative process optimization, payload release, and enhanced bioavailability of thymoquinone natural product
- Structure–mechanical property relationships of 3D-printed porous polydimethylsiloxane films
- Nonlinear thermal radiation and the slip effect on a 3D bioconvection flow of the Casson nanofluid in a rotating frame via a homotopy analysis mechanism
- Residual mechanical properties of concrete incorporated with nano supplementary cementitious materials exposed to elevated temperature
- Time-independent three-dimensional flow of a water-based hybrid nanofluid past a Riga plate with slips and convective conditions: A homotopic solution
- Lightweight and high-strength polyarylene ether nitrile-based composites for efficient electromagnetic interference shielding
- Review Articles
- Recycling waste sources into nanocomposites of graphene materials: Overview from an energy-focused perspective
- Hybrid nanofiller reinforcement in thermoset and biothermoset applications: A review
- Current state-of-the-art review of nanotechnology-based therapeutics for viral pandemics: Special attention to COVID-19
- Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development
- Critical review on experimental and theoretical studies of elastic properties of wurtzite-structured ZnO nanowires
- Polyurea micro-/nano-capsule applications in construction industry: A review
- A comprehensive review and clinical guide to molecular and serological diagnostic tests and future development: In vitro diagnostic testing for COVID-19
- Recent advances in electrocatalytic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid: Mechanism, catalyst, coupling system
- Research progress and prospect of silica-based polymer nanofluids in enhanced oil recovery
- Review of the pharmacokinetics of nanodrugs
- Engineered nanoflowers, nanotrees, nanostars, nanodendrites, and nanoleaves for biomedical applications
- Research progress of biopolymers combined with stem cells in the repair of intrauterine adhesions
- Progress in FEM modeling on mechanical and electromechanical properties of carbon nanotube cement-based composites
- Antifouling induced by surface wettability of poly(dimethyl siloxane) and its nanocomposites
- TiO2 aerogel composite high-efficiency photocatalysts for environmental treatment and hydrogen energy production
- Structural properties of alumina surfaces and their roles in the synthesis of environmentally persistent free radicals (EPFRs)
- Nanoparticles for the potential treatment of Alzheimer’s disease: A physiopathological approach
- Current status of synthesis and consolidation strategies for thermo-resistant nanoalloys and their general applications
- Recent research progress on the stimuli-responsive smart membrane: A review
- Dispersion of carbon nanotubes in aqueous cementitious materials: A review
- Applications of DNA tetrahedron nanostructure in cancer diagnosis and anticancer drugs delivery
- Magnetic nanoparticles in 3D-printed scaffolds for biomedical applications
- An overview of the synthesis of silicon carbide–boron carbide composite powders
- Organolead halide perovskites: Synthetic routes, structural features, and their potential in the development of photovoltaic
- Recent advancements in nanotechnology application on wood and bamboo materials: A review
- Application of aptamer-functionalized nanomaterials in molecular imaging of tumors
- Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites
- Research progress on preparation, modification, and application of phenolic aerogel
- Application of nanomaterials in early diagnosis of cancer
- Plant mediated-green synthesis of zinc oxide nanoparticles: An insight into biomedical applications
- Recent developments in terahertz quantum cascade lasers for practical applications
- Recent progress in dielectric/metal/dielectric electrodes for foldable light-emitting devices
- Nanocoatings for ballistic applications: A review
- A mini-review on MoS2 membrane for water desalination: Recent development and challenges
- Recent updates in nanotechnological advances for wound healing: A narrative review
- Recent advances in DNA nanomaterials for cancer diagnosis and treatment
- Electrochemical micro- and nanobiosensors for in vivo reactive oxygen/nitrogen species measurement in the brain
- Advances in organic–inorganic nanocomposites for cancer imaging and therapy
- Advancements in aluminum matrix composites reinforced with carbides and graphene: A comprehensive review
- Modification effects of nanosilica on asphalt binders: A review
- Decellularized extracellular matrix as a promising biomaterial for musculoskeletal tissue regeneration
- Review of the sol–gel method in preparing nano TiO2 for advanced oxidation process
- Micro/nano manufacturing aircraft surface with anti-icing and deicing performances: An overview
- Cell type-targeting nanoparticles in treating central nervous system diseases: Challenges and hopes
- An overview of hydrogen production from Al-based materials
- A review of application, modification, and prospect of melamine foam
- A review of the performance of fibre-reinforced composite laminates with carbon nanotubes
- Research on AFM tip-related nanofabrication of two-dimensional materials
- Advances in phase change building materials: An overview
- Development of graphene and graphene quantum dots toward biomedical engineering applications: A review
- Nanoremediation approaches for the mitigation of heavy metal contamination in vegetables: An overview
- Photodynamic therapy empowered by nanotechnology for oral and dental science: Progress and perspectives
- Biosynthesis of metal nanoparticles: Bioreduction and biomineralization
- Current diagnostic and therapeutic approaches for severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and the role of nanomaterial-based theragnosis in combating the pandemic
- Application of two-dimensional black phosphorus material in wound healing
- Special Issue on Advanced Nanomaterials and Composites for Energy Conversion and Storage - Part I
- Helical fluorinated carbon nanotubes/iron(iii) fluoride hybrid with multilevel transportation channels and rich active sites for lithium/fluorinated carbon primary battery
- The progress of cathode materials in aqueous zinc-ion batteries
- Special Issue on Advanced Nanomaterials for Carbon Capture, Environment and Utilization for Energy Sustainability - Part I
- Effect of polypropylene fiber and nano-silica on the compressive strength and frost resistance of recycled brick aggregate concrete
- Mechanochemical design of nanomaterials for catalytic applications with a benign-by-design focus

