Abstract
A new composite adsorbent was created by activating bentonite, a sludge, to improve its adsorption capability. These innovative adsorbents address sulfate ion pollution in wastewater treatment. Researchers used various techniques to study the composite’s surface structure, chemical, elemental, and mineralogical characteristics. The composite adsorbs over 864 mg/L of sulfate ions from wastewater with an initial concentration of 900 mg/L, demonstrating its high removal efficiency of 96%.
1 Introduction
Although freshwater resources are scarce globally, water is necessary for life. Half of the world’s population is expected to reside in water-stressed regions by 2025 [1]. Water is necessary for human consumption, industry, and agriculture. It goes without saying that commercial and industrial activities can contaminate water [2]. The literature lists a number of toxins that can be found in groundwater, surface water, and industrial wastewater, including heavy metals [3–6], dyes [7], pesticides [8], pharmaceuticals [9], personal care products [10], hormones [11], viruses [12], radioactive elements [13], phenol-derived compounds [14], and other emerging contaminants [15]. The presence of these compounds in waters and wastewaters is dangerous for the environment and public health and is well documented in the literature [16,17]. In reality, one of our responsibilities as scientists working in the field of environmental chemical engineering is to create the materials, techniques, and technologies that will make it possible to manage, decontaminate, and reuse water. There are a number of water treatment technologies, each with benefits and cons [18]. However, adsorption is the main concern here. Adsorption is a process that involves both a solid phase (the adsorbent) and a fluid phase (in this case, water). The fluid phase contains one or more dissolved contaminants (the adsorbate). As the dissolved contaminants are transferred from the liquid phase to the adsorbent surface, water is purified [19]. Due to its advantages in terms of cost, effectiveness, simplicity of use, flexibility to use a variety of solids as adsorbent materials, and capacity to recover both the adsorbent and the adsorbate, adsorption is currently used to treat water [20–23]. It is crucial to emphasize the competitive and efficient nature of adsorption as a polishing process when pollutants are present in water at concentrations between ng/L and mg/L [24]. According to the literature from 1990 to the present, the crucial elements of being evaluated to apply adsorption for water treatment are the choice, development and characterization of the adsorbent material, the development and optimization of the adsorption mode, the mathematical modeling, the decision and development of the regeneration procedure, and the application in actual samples. Of course, the cost analysis is also quite important. Cost, however, must be taken into account separately for each of the aforementioned criteria. Adsorption charges range from 5.0 to 200 US dollars/m3 of treated water, while prices for the majority of technologies are between 10 and 450 US dollars/m3 of treated water [25]. About 70% of these costs are covered by the adsorbent [26]. The town and affected industries were under tremendous strain as a result of the vast amounts of sludge that were produced during wastewater treatment operations. About 25–65% of the entire operating expense for secondary wastewater treatment is toward treating and disposing of the sludge [27]. Therefore, it is important to identify a sludge disposal option that is both affordable and environmentally friendly. The commercial application of adsorption has been dominated by generic adsorbent primary types such as polymeric adsorbents, molecular sieves of zeolites and carbon, activated alumina, silica gel, and activated carbon. Only a small number of adsorbents, including some zeolites, are produced naturally. The type of adsorbing surface, the pore structure, and the porosity are the features of each adsorbent [28]. The aim of this study was to prepare a low-cost and effective adsorbent. Due to its affordable price and good effectiveness, using clay material sludge and bentonite as an adsorbent (SB) for the removal of sulfate ions is a wise choice. To our knowledge, there are not many studies looking into how clay minerals can remove sulfate ions from solutions. The goal of this study is to use waste and inexpensive materials as an adsorbent to extract
2 Materials and methods
2.1 Materials
Dewatered sludge was obtained from the wastewater treatment plant in Karbala city. The bentonite samples were collected from commercial markets. The chemical materials – potassium sulfate, sodium hydroxide, barium chloride, filter paper (Whatman 7.0 cm) – were used to filter the sample solution and distilled water from commercial markets with high purity. The chemical materials are adjusted with pH (HCl) and NaOH bases.
2.2 Preparation of adsorbent
The newly created adsorbent composites underwent physical activation to prepare them. The materials are ground, sludge and bentonite SB composite formed as bentonite is progressively added to the water, and then sludge is added with the addition of water. The materials are then dried in a drying oven for 12 h at a temperature of 105.5°C. For 30 min, the solution is stirred with a magnetic stirrer. The solution is then filtered. The filter is burned in the oven for 2 h at 800°C using filter sheets. Grinding with a grinder is the end result. The mass ratios of bentonite and sludge at 4:1, 3:1, 2:1, 1:1, 1:2, and 1:4 [29] are shown in the flowchart below.
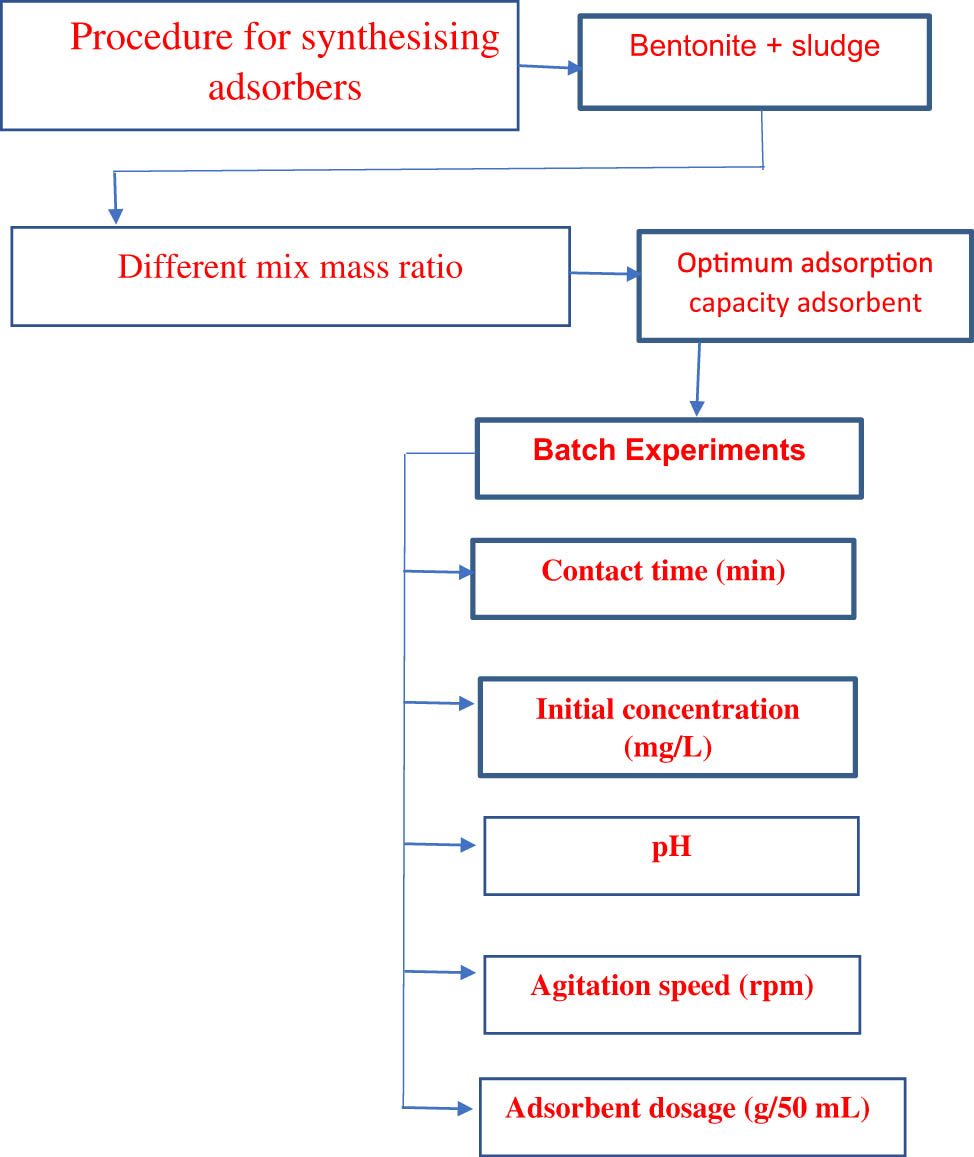
2.3 Batch experiments
To examine the adsorption capabilities of SB for sulfate, batch adsorption tests were conducted. The procedure was carried out in its first state: Ci = 900 mg/L, contact time = 60 min, pH = 7.5, agitation speed = 200 rpm, adsorbent dosage = 0.1 g/50 mL (25°C) at room temperature to ensure uniform mixing. Following that, 0.45 m membrane filters were used to filter the solution. Barium chromate spectrophotometry was used to calculate the sulfate content. Impact of starting concentration: 50 mL of wastewater was mixed with 100 mg of adsorbent using magnetic stirrers [30]. By assuming total dissolution in equation (1), the necessary concentration of sulfate was obtained:
where W is the weight of the salt (mg), V is the volume of the solution (L), Ci is the required sulfate concentration (mg/L), M.wt is the salt molecular weight (g/mol), and At.wt is the SO4 atomic weight (g/mol). To prepare the necessary concentration, the solution was diluted in accordance with Equation (2). In addition, 0.1 moles of either HCl or NaOH, depending on the circumstance, were added to each solution to alter its pH [31].
where
where R is the removal efficiency %, C o is the initial concentration, and C e is the final concentration.
3 Results and discussion
3.1 Preparation results
The results of batch experiments to test how well the adsorbent removed sulfate from wastewater showed that, under the initial condition, the optimal mass ratio of SB was 1:1, with an efficiency percentage of 86%, as shown in Figure 1.

Removal efficiency of SB composite.
3.2 Characterization of the material
3.2.1 Brunauer–Emmett–Teller (BET) analysis
The BET theory, which describes the physical adsorption of gas molecules on solid surfaces, provides a method for calculating a material’s specific surface area [32]. The results of the mixture of bentonite and sludge were 11.2959 and 7.5571 m2/g, respectively. The fact that the specific surface area of composite SB was 22.1282 m2/g shows that the activation process causes the specific surface area to grow from 22.1282 to 42.1283 m2/g. This indicates that an increase in surface area results in a good capacity for adsorption.
3.2.2 Fourier transform infrared spectroscopy (FT-IR)
The FT-IR spectra of sludge, bentonite, and SB before and after adsorption show functional groups and modifications. The adsorbents effectively remove sulfate, with peaks at 1042.89, 1036.41, 1029.58, 1036.41, 1007.92, 912–937, and 799.10 cm−1 (Figure 2).
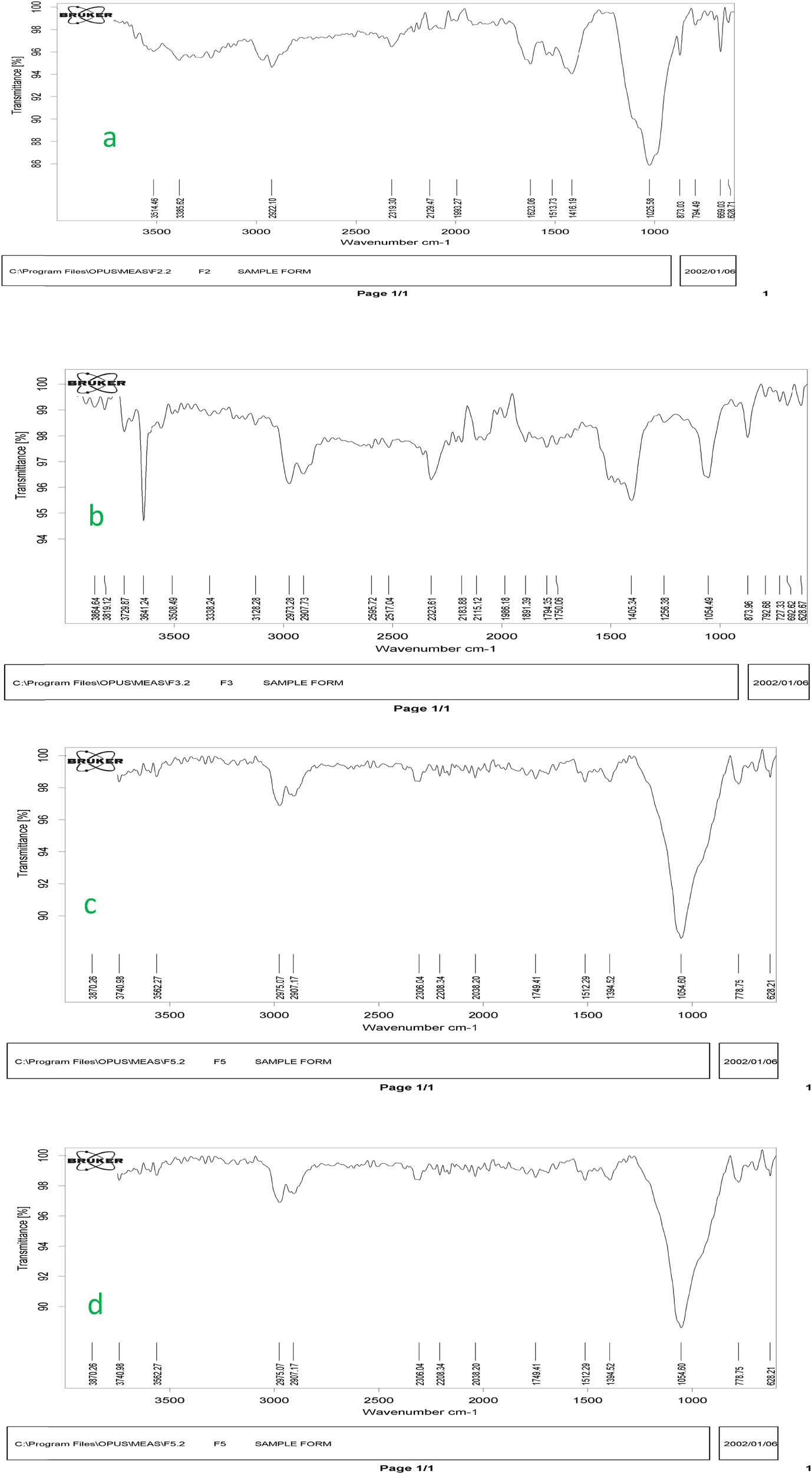
FT-IR spectra of materials and composite before and after adsorption: (a) sludge, (b) bentonite, (c) SB before, (d) and SB after sulfate adsorption.
3.2.3 Scanning electron microscope (SEM)
In Figure 3 the morphology of surface SB is displayed. The pore structure of the SB is compact and uniform, and it has edges, sharp corners, and rough surfaces. SB’s structure and substantial surface area allowed it to serve as an absorbent for further pollution. The particles in this SB are clearly defined and regular. It typically has a lamellar structure.
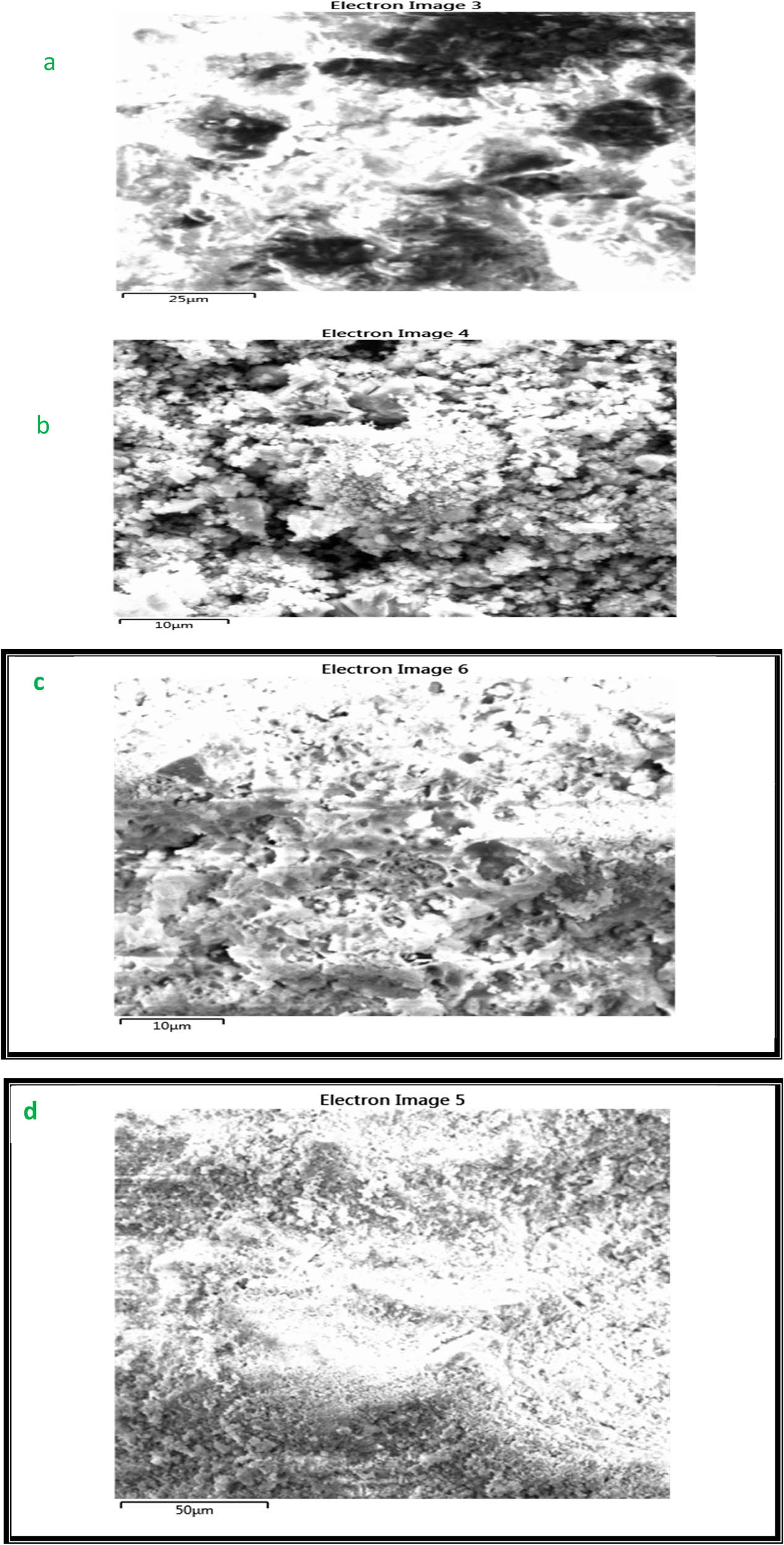
Images SEM for (a) sludge, (b) bentonite, and (c) SB composite before adsorption and (d) SB composite after adsorption.
3.2.4 Energy dispersive spectroscopy (EDS)
From Figure 4 it can be seen that the SB composite contains S and O by EDS spectrum. After modification, the contents of S and O increase dramatically, proving the successful loading of SB composite using the co-precipitation method of synthesis.
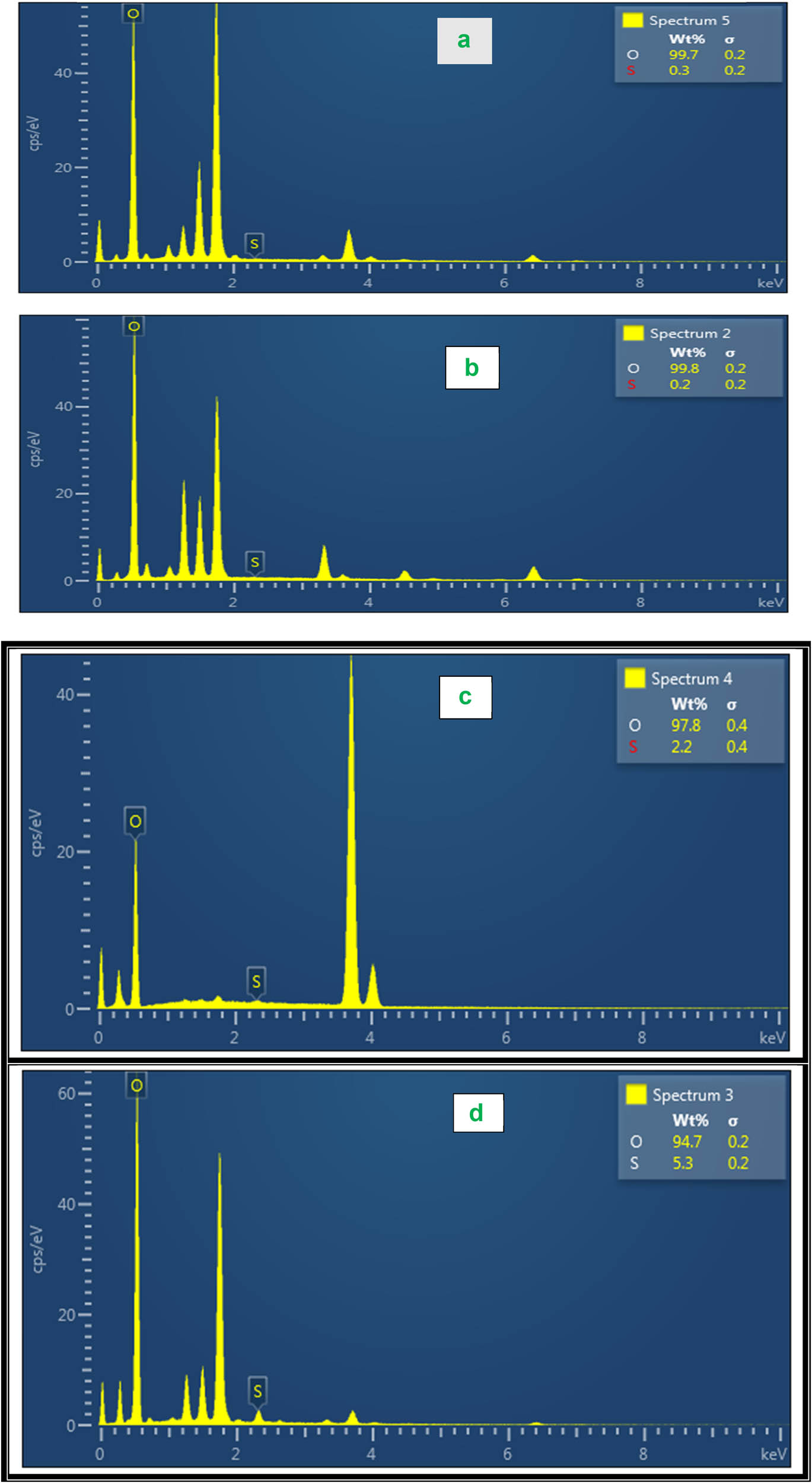
EDS spectrum for (a) sludge, (b) bentonite, and (c) SB composite before adsorption and (d) SB composite after adsorption.
3.3 Adsorption results
The goal of this section of the experiment was to evaluate how well the adsorbent removed sulfate from simulated contaminated wastewater. This section shows that the experiments are conducted in various ways (contact time, pH solution, initial concentration, agitation speed, and adsorbent dosage).
3.3.1 Equilibrium time
Finding the amount of time needed to reach equilibrium during batch testing is essential because it shows how long it will take for contaminants to be redistributed between the liquid and solid phases. Monitoring
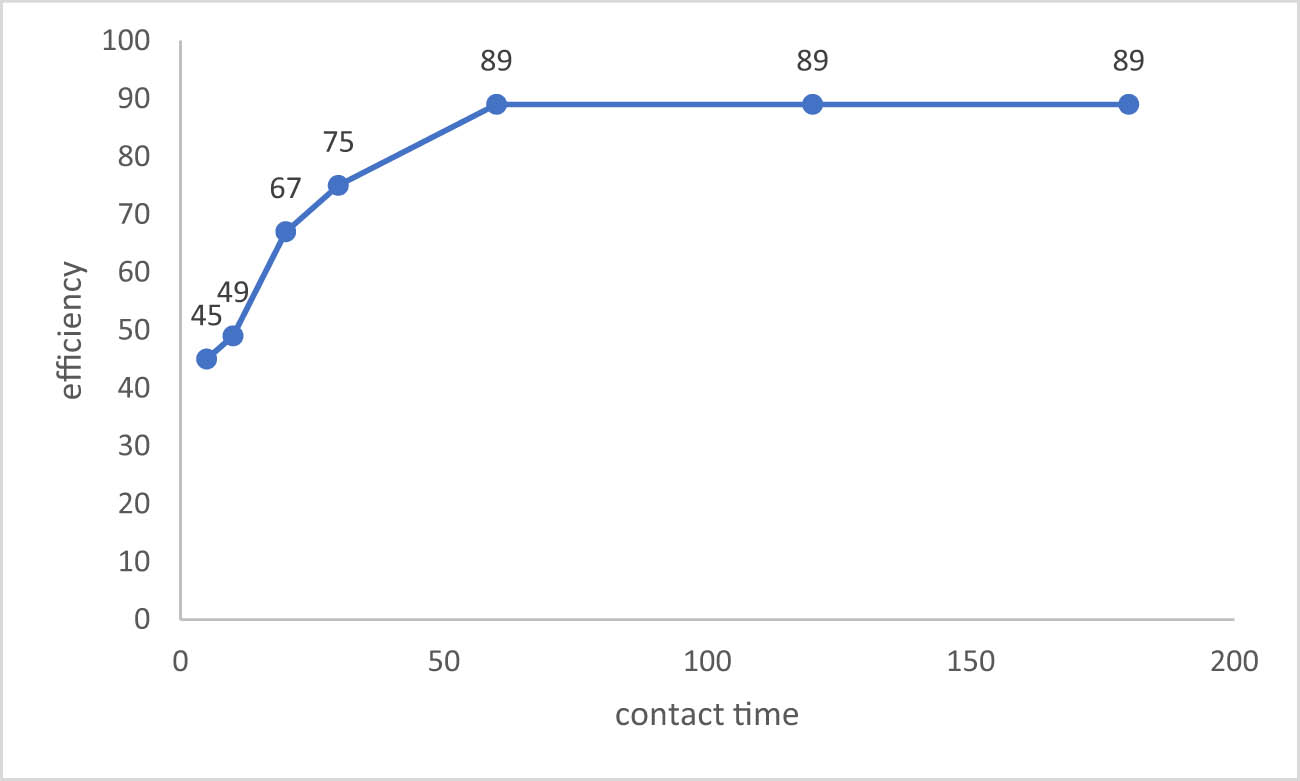
Sulfate removal efficiency effected by time.
More than 90% was removed in 1 h. In addition, at contact times greater than 1 h, the concentrations of ions remained relatively constant. Residual ion’s concentrations did not change significantly at the time up to 3 h, yet sorption experiments in other batches were conducted at 1 h.
3.3.2 pH of the solution
Due to its impact on the ionic forms of pollutants and the surface characteristics of adsorbents, solution pH is the primary factor regulating adsorbent adsorption capacity [35]. The pH value has an impact on how ions behave during adsorption because it impacts how the basic and acidic groups of the sorbent interact with its surface structure through protonation and deprotonation. Figure 6 shows that the basic and acidic sorbent groups were protonated and deprotonated, which boosted the removal efficiency (dose = 0.1 g/50 mL, Co = 900 mg/L, contact time = 1 h, T = 25°C, and agitation speed = 200 rpm), have documented a similar phenomenon of increasing the ion’s adsorption when the pH of the solution has increased. The polarity a similar occurrence wherein an increase in the pH of the pH of the solution causes an increase in the ion’s adsorption [36]. It was obvious from Figure 6 that when the pH value rose, the removal efficiency increased, which meant that there was competition between contaminants and H+ ions on the adsorbent surface. The findings indicating the role of pH in the elimination process are similar to those from earlier studies.
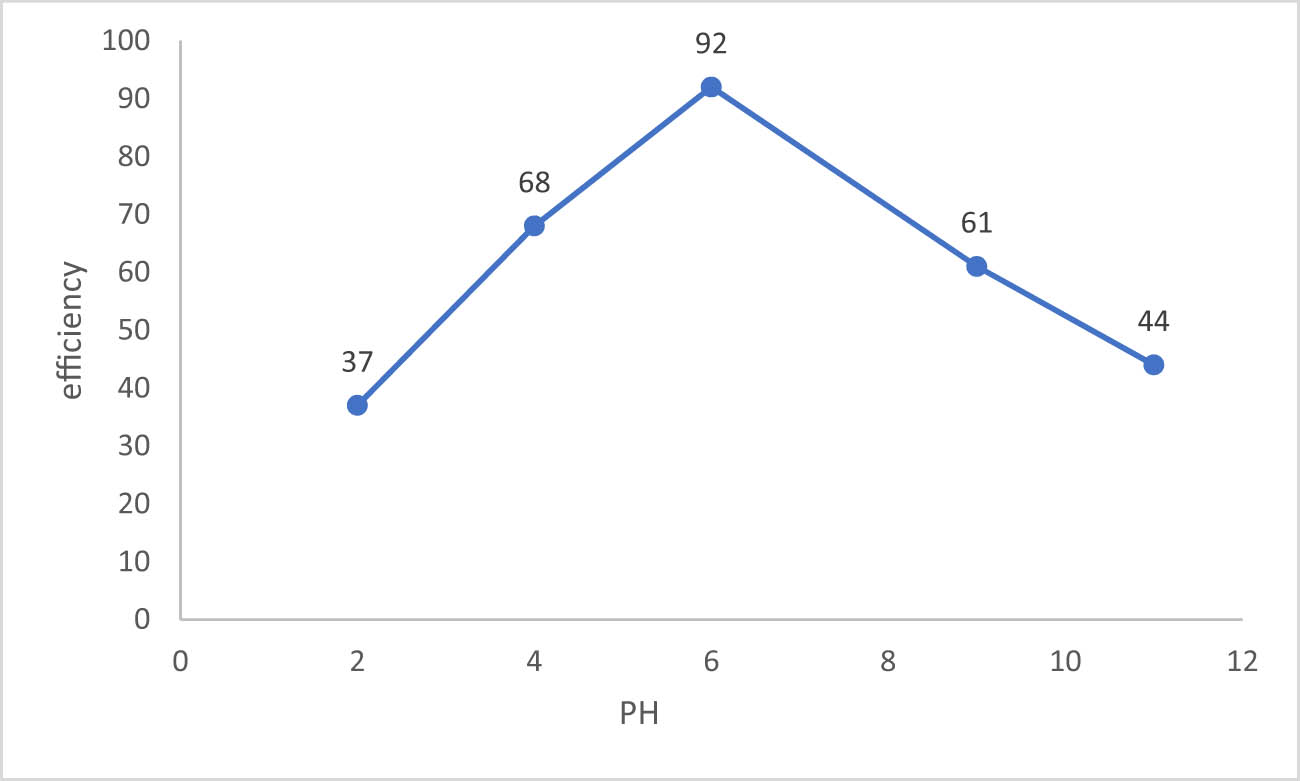
Sulfate removal efficiency effected by pH.
3.3.3 Effect of initial
SO
4
2
+
concentrations
Experimental testing of sulfate removal effectiveness was done at various ion concentrations at the beginning. In these tests, 0.1 g of the adsorbent per 50 mL of a solution, 200 rpm agitation for 1 h, and a pH of 6 were utilized. The starting sulfate concentrations used ranged from 100 to 1,000 mg/L. Figure 7 at mixing time = 1 h, mixing speed = 200 rpm, dose = 0.1 g/50 mL, pH = 6, and T = 25°C represents the ion’s removal efficiency related to the initial ion’s concentrations at equilibrium state. The outcomes demonstrate that the ion removal was greater with the lower initial concentration values. The elimination efficiency fell off as the original concentrations rose. After 100 mg/L, there was no discernible change in the ion concentration. The inability of the ions to interact with the active sites on the adsorbent is what causes this. This finding indicates that sites become energetically less favorable as ion concentrations in the solution grow [37]. The findings indicating the role of pH in the elimination process are similar to those from earlier studies. This decline in the proportion of ions removed could be brought on by an inability of the adsorbent’s active sites to absorb more

Sulfate removal efficiency effected by initial concentration.
3.3.4 Agitation speed
In order to study the effect of agitation speed on sulfate removal efficiency from contaminated wastewater, several experiments were carried out at different agitation speeds, ranging from 0 to 250 rpm, with contact time of 1 h, Co = 900 mg/L, dose = 0.1 g/50 mL, pH = 6, and T = 25°C. The removal efficiency of
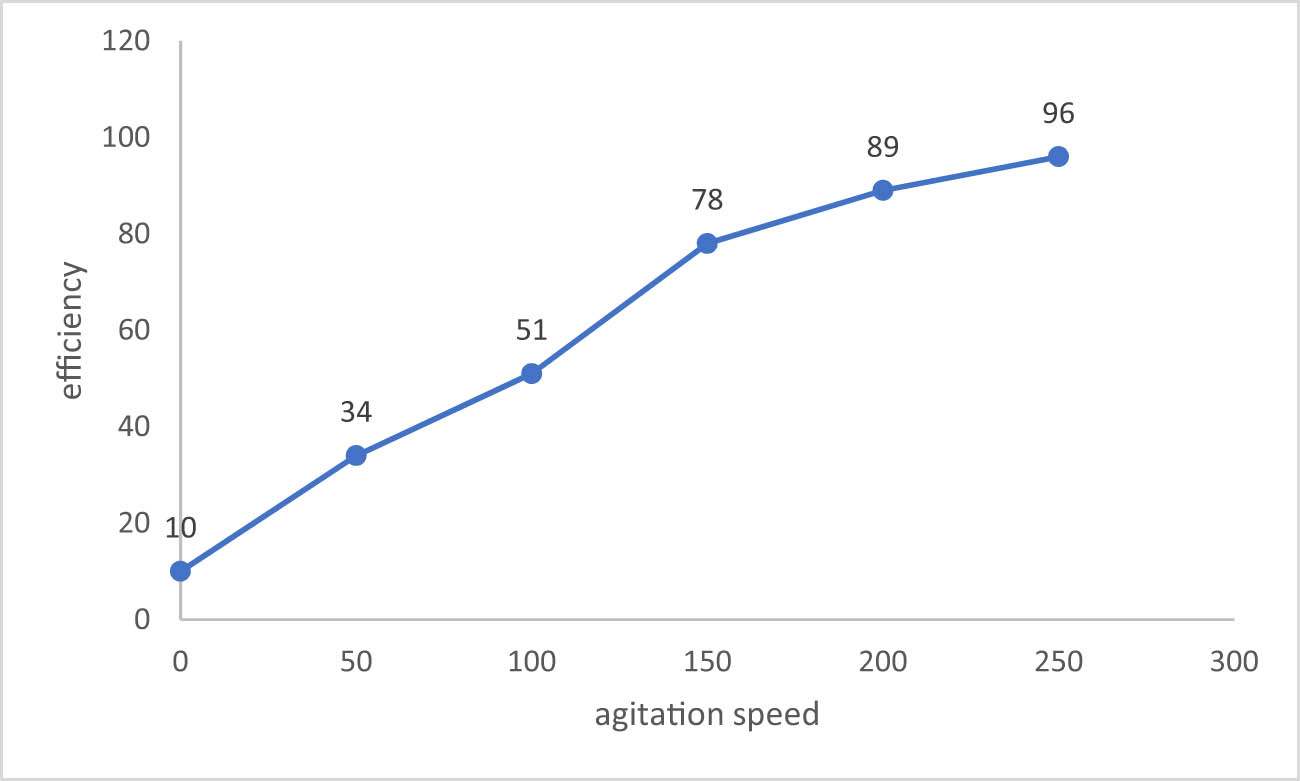
Sulfate removal efficiency effected by the agitation speed.
3.3.5 Effect of the adsorbent dosage
Different dosages of the adsorbent, ranging from 0.05 to 0.6, were utilized with a 50 mL ion solution while maintaining the other parameters to determine how the adsorbent dosage influences the sulfate adsorption in batch testing (mixing time = 1 h, mixing speed = 250 rpm, pH = 6, Ci = 900 mg/L, and T = 25°C). Figure 9 shows different amounts of the adsorbent related to sulfate removal efficiency. The sulfate removal efficiency grew because of the increased adsorbent dosage from 0.05 to 0.5 g/50 mL with the fixing of other parameters. The result described above was expected according to the fact that, when the adsorbent dosage in a solution rises, subsequently more active sites are available. In spite of increasing adsorbent dosage, the concentration of sulfate in the solution and its binding to the adsorbent remained constant after the maximal rate of sulfate removal occurred at a dose of 0.5 g of adsorbent [39].
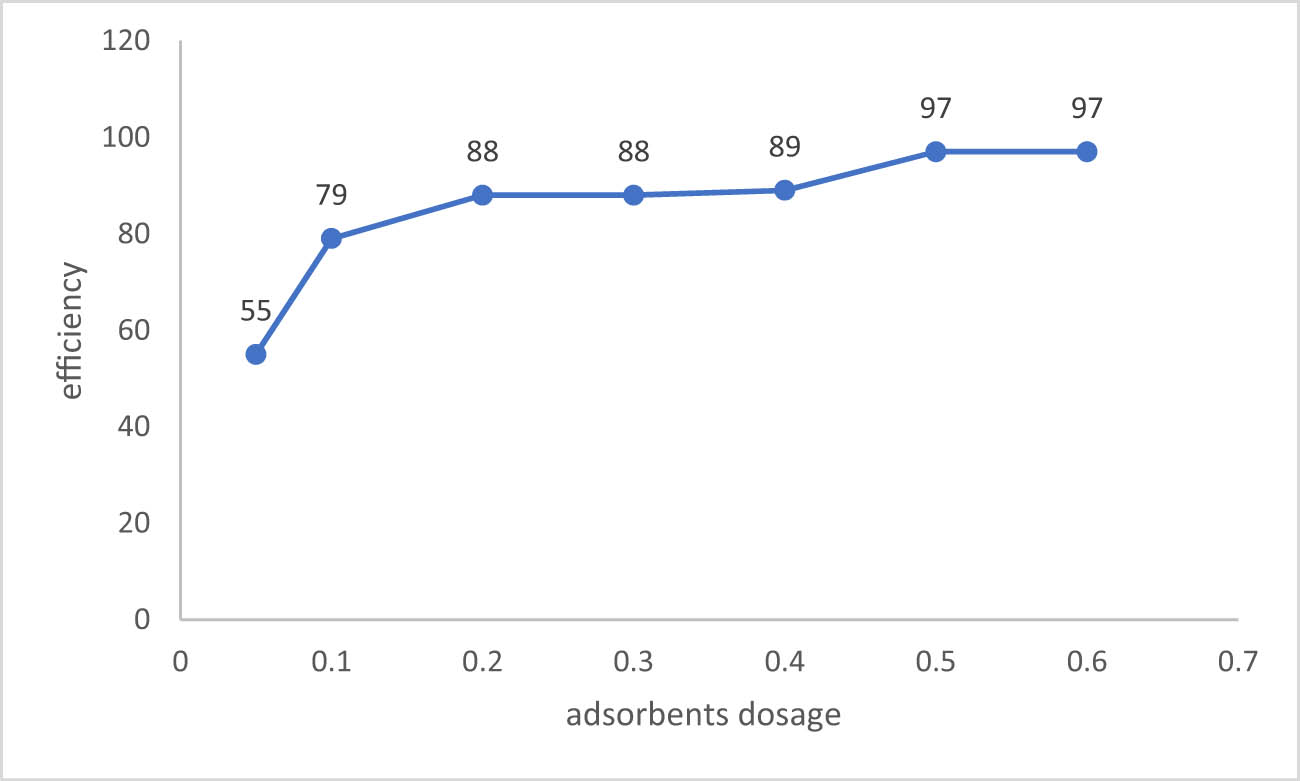
Sulfate removal efficiency effected by the amounts of adsorbents dosage.
4 Conclusion
An adsorbent (low-cost local) was modified to create a new composite adsorbent. Physical activation is used. This inexpensive local adsorbent was similarly made by activating bentonite, a sludge. The new composite adsorbent’s adsorption capability increased when compared to the low-cost LC. A major issue in the wastewater treatment sector is fouling caused by sulfate-ion pollution. In this work, scientists created an SB composite and assessed its ability to absorb sulfate ions from contaminated wastewater. The sludge, bentonite, and SB composite’s surface structural morphology, chemical, elemental, and mineralogical characteristics, as well as functional group interaction, were determined using the field emission scanning electron microscope, energy dispersive X-ray spectroscopy, X-ray dispersive, FT-IR, and BTE. To ascertain the effects of solution pH, agitation speed, adsorbent dosage, contact time, and initial
-
Funding information: The authors declare that the manuscript was done depending on the personal effort of the author, and there is no funding effort from any side or organization.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Most datasets generated and analyzed in this study are in this submitted manuscript. The other datasets are available on reasonable request from the corresponding author with the attached information.
References
[1] World Health Organization, Geneva, Switzerland, Drinking Water. 2019. https://www.who.int/en/news-room/fact-sheets/detail/drinking-water.Suche in Google Scholar
[2] Ismail HK, Abd Ali LI, Alesary HF, Nile BK, Barton S. Synthesis of a poly(p-aminophenol)/starch/graphene oxide ternary nanocomposite for removal of methylene blue dye from aqueous solution. J Polym Res. 2022;29:159.10.1007/s10965-022-03013-6Suche in Google Scholar
[3] Joseph L, Jun BM, Flora JRV, Park CM, Yoon Y. Removal of heavy metals from water sources in the developing world using low-cost materials: a review. Chemosphere. 2019;229:142–59.10.1016/j.chemosphere.2019.04.198Suche in Google Scholar PubMed
[4] Nile BK, Faris AM. The effect of MLSS values on removal of COD and phosphorus using control method of return activated sludge concentration. J Eng Appl Sci. 2018;13:9730–4.Suche in Google Scholar
[5] Al Juboury MF, Abdulredha M, Nile BK. Photocatalysis and flocculation processes for recycling aquaculture effluent into nutrient-rich irrigation water. Water Supply. 2022;22(3):3103–13.10.2166/ws.2021.417Suche in Google Scholar
[6] Obaid H, Shahid S, Basim KN, Chelliapan S. Modeling of wastewater quality in an urban area during festival and rainy days. Water Sci Technol. 2015;72(6):1029–42.10.2166/wst.2015.297Suche in Google Scholar PubMed
[7] Yagub MT, Sen TK, Afroze S, Ang HM. Dye and its removal from aqueous solution by adsorption: A review. Adv Colloid Interface Sci. 2014;209:172–84.10.1016/j.cis.2014.04.002Suche in Google Scholar PubMed
[8] Souza RM, Seibert D, Quesada HB, Bassetti FJ, Fagundes-Klen MR, Bergamasco R. Occurrence, impacts and general aspects of pesticides in surface water: a review. Proc Saf Environ Prot. 2020;135:22–37.10.1016/j.psep.2019.12.035Suche in Google Scholar
[9] Carmalin SA, Lima EC. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Environ Saf. 2018;150:1–17.10.1016/j.ecoenv.2017.12.026Suche in Google Scholar PubMed
[10] Yang Y, Sik Ok Y, Kim KH, Kwon EE, Tsang YF. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci Total Environ. 2017;596–597:303–20.10.1016/j.scitotenv.2017.04.102Suche in Google Scholar PubMed
[11] Lee LS, Carmosini N, Sassman SA, Dion HM, Sepúlveda MS. Agricultural contributions of antimicrobials and hormones on soil and water quality. Adv Agron. 2007;93:1–68.10.1016/S0065-2113(06)93001-6Suche in Google Scholar
12] Springthorpe S, Sattar SA. Virus removal during drinking water treatment. Persp Med Virol. 2007;17:109–26.10.1016/S0168-7069(07)17006-3Suche in Google Scholar
[13] Zhang X, Gu P, Liu Y. Decontamination of radioactive wastewater: state of the art and challenges forward. Chemosphere. 2019;215:543–53.10.1016/j.chemosphere.2018.10.029Suche in Google Scholar PubMed
[14] Ahmaruzzaman M. Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv Colloid Interface Sci. 2008;143:48–67.10.1016/j.cis.2008.07.002Suche in Google Scholar PubMed
[15] Rodriguez-Narvaez OM, Peralta-Hernandez JM, Goonetilleke A, Bandala ER. Treatment technologies for emerging contaminants in water: a review. Chem Eng J. 2017;323:361–80.10.1016/j.cej.2017.04.106Suche in Google Scholar
[16] Taheran M, Naghdi M, Brar SK, Verma M, Surampalli RY. Emerging contaminants: here today, there tomorrow! Environ Nanotechnol Monit Manage. 2018;10:122–6.10.1016/j.enmm.2018.05.010Suche in Google Scholar
[17] Wilkinson J, Hooda PS, Barker J, Barton S, Swinden J. Occurrence, fate and transformation of emerging contaminants in water: an overarching review of the field. Environ Pollut. 2017;231:954–70.10.1016/j.envpol.2017.08.032Suche in Google Scholar PubMed
[18] Cheremisinoff NP. Handbook of water and wastewater treatment technologies. Elsevier, Butterworth-Heinemann; 2002. p. 576.10.1016/B978-075067498-0/50014-0Suche in Google Scholar
[19] Bonilla-Petriciolet A, Mendoza-Castillo DI, Dotto GL, Duran-Valle CJ. Adsorption in water treatment. Reference module in chemistry, molecular sciences and chemical engineering 1. 1st edn. Amsterdam: Elsevier; 2019. p. 1–21.10.1016/B978-0-12-409547-2.14390-2Suche in Google Scholar
[20] Bonilla-Petriciolet A, Mendoza-Castillo DI, Reynel-Ávila HE. Adsorption processes for water treatment and purification. Springer International Publishing; 2017. p. 256.10.1007/978-3-319-58136-1Suche in Google Scholar
[21] Ruthven DM. Principles of adsorption & adsorption processes. Wiley; 1984. p. 464.Suche in Google Scholar
[22] Cherkasov N. Liquid-phase adsorption: common problems and how we could do better. J Mol Liq. 2020;301:112378.10.1016/j.molliq.2019.112378Suche in Google Scholar
[23] Ali I, Gupta VK. Advances in water treatment by adsorption technology. Nat Protoc. 2006;1:2661–7.10.1038/nprot.2006.370Suche in Google Scholar PubMed
[24] Cooney DO. Adsorption design for wastewater treatment. Boca Raton: Lewis Publishers; 1999.Suche in Google Scholar
[25] Ali I, Asim M, Khan TA. Low-cost adsorbents for the removal of organic pollutants from wastewater. J Environ Manage. 2012;113:170–83.10.1016/j.jenvman.2012.08.028Suche in Google Scholar PubMed
[26] Li W, Mu B, Yang Y. Feasibility of industrial-scale treatment of dye wastewater via bioadsorption technology. Bioresour Technol. 2019;277:157–70.10.1016/j.biortech.2019.01.002Suche in Google Scholar PubMed
[27] Hii K, Baroutian S, Parthasarathy R, Gapes DJ, Eshtiaghi N. A review of wet air oxidation and thermal hydrolysis technologies in sludge treatment. Bioresour Technol. 2014;155:289–99.10.1016/j.biortech.2013.12.066Suche in Google Scholar PubMed
[28] Kadhim GZ. A study of adsorption of some heavy metal on selected Iraqi surfaces. M.Sc. thesis. University of Baghdad, College of Science for Women; 2010.Suche in Google Scholar
[29] Huang R, Zheng D, Yang B, Wang B, Zhang Z. Preparation and characterization of CTAB-HACC bentonite and its ability to adsorb phenol from aqueous solution, China. Received 13 December 2011; accepted 24 April 2012.Suche in Google Scholar
[30] Nazeeh I. Removal of copper ions from simulated wastewater by applying electromagnetic-adsorption using banana peel adsorbent. M.Sc. thesis. University of Baghdad, College of Engineering; 2016.Suche in Google Scholar
[31] Esmail AA. Adsorption of Pb (II) ions from aqueous phase using activated carbon prepared from novel precursor. M.Sc. thesis. Al-Nahrain University, Chemical Engineering; 2016.Suche in Google Scholar
[32] Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem. 2015;87(9–10):1051–69.10.1515/pac-2014-1117Suche in Google Scholar
[33] Alkan M, Kalay B, Doǧan M, Demirbaş Ö. Removal of copper ions from aqueous solutions by kaolinite and batch design. Hazard Mater. 2008;153:867–76.10.1016/j.jhazmat.2007.09.047Suche in Google Scholar PubMed
[34] Bulut Y, Tez Z. Adsorption studies on ground shells of hazelnut and almond. J Hazard Mater. 2007;149(1):35–41.10.1016/j.jhazmat.2007.03.044Suche in Google Scholar PubMed
[35] Garg UK, Kaur MP, Garg VK, Sud D. Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J Hazard Mater. 2007;140(1–2):60–8.10.1016/j.jhazmat.2006.06.056Suche in Google Scholar PubMed
[36] Areco M, Hanela S, Duran J, Dos M. Biosorption of Cu(II), Zn(II), Cd(II) and Pb(II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. J Hazard Mater. 2012;213–214:123–32.10.1016/j.jhazmat.2012.01.073Suche in Google Scholar PubMed
[37] Buasri A, Yongbut P, Chaiyut N, Phattarasirichot K. Adsorption equilibrium of zinc ions from aqueous solution by using modified clinoptilolite. Chiang Mai J Sci. 2008;35(1):56–62.Suche in Google Scholar
[38] Anwar J, Shafique U, Salman M, Dar A, Anwar S. Removal of Pb (II) and Cd (II) from water by adsorption on peels of banana. Bioresour Technol. 2010;6:1752–5.10.1016/j.biortech.2009.10.021Suche in Google Scholar PubMed
[39] Palaniswamy R, Veluchamy C. Biosorption of heavy metals by Spirulina platensis from electroplating industrial effluent. Environ Sci Indian J. 2017;13(4):139–45.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Methodology of automated quality management
- Influence of vibratory conveyor design parameters on the trough motion and the self-synchronization of inertial vibrators
- Application of finite element method in industrial design, example of an electric motorcycle design project
- Correlative evaluation of the corrosion resilience and passivation properties of zinc and aluminum alloys in neutral chloride and acid-chloride solutions
- Will COVID “encourage” B2B and data exchange engineering in logistic firms?
- Influence of unsupported sleepers on flange climb derailment of two freight wagons
- A hybrid detection algorithm for 5G OTFS waveform for 64 and 256 QAM with Rayleigh and Rician channels
- Effect of short heat treatment on mechanical properties and shape memory properties of Cu–Al–Ni shape memory alloy
- Exploring the potential of ammonia and hydrogen as alternative fuels for transportation
- Impact of insulation on energy consumption and CO2 emissions in high-rise commercial buildings at various climate zones
- Advanced autopilot design with extremum-seeking control for aircraft control
- Adaptive multidimensional trust-based recommendation model for peer to peer applications
- Effects of CFRP sheets on the flexural behavior of high-strength concrete beam
- Enhancing urban sustainability through industrial synergy: A multidisciplinary framework for integrating sustainable industrial practices within urban settings – The case of Hamadan industrial city
- Advanced vibrant controller results of an energetic framework structure
- Application of the Taguchi method and RSM for process parameter optimization in AWSJ machining of CFRP composite-based orthopedic implants
- Improved correlation of soil modulus with SPT N values
- Technologies for high-temperature batch annealing of grain-oriented electrical steel: An overview
- Assessing the need for the adoption of digitalization in Indian small and medium enterprises
- A non-ideal hybridization issue for vertical TFET-based dielectric-modulated biosensor
- Optimizing data retrieval for enhanced data integrity verification in cloud environments
- Performance analysis of nonlinear crosstalk of WDM systems using modulation schemes criteria
- Nonlinear finite-element analysis of RC beams with various opening near supports
- Thermal analysis of Fe3O4–Cu/water over a cone: a fractional Maxwell model
- Radial–axial runner blade design using the coordinate slice technique
- Theoretical and experimental comparison between straight and curved continuous box girders
- Effect of the reinforcement ratio on the mechanical behaviour of textile-reinforced concrete composite: Experiment and numerical modeling
- Experimental and numerical investigation on composite beam–column joint connection behavior using different types of connection schemes
- Enhanced performance and robustness in anti-lock brake systems using barrier function-based integral sliding mode control
- Evaluation of the creep strength of samples produced by fused deposition modeling
- A combined feedforward-feedback controller design for nonlinear systems
- Effect of adjacent structures on footing settlement for different multi-building arrangements
- Analyzing the impact of curved tracks on wheel flange thickness reduction in railway systems
- Review Articles
- Mechanical and smart properties of cement nanocomposites containing nanomaterials: A brief review
- Applications of nanotechnology and nanoproduction techniques
- Relationship between indoor environmental quality and guests’ comfort and satisfaction at green hotels: A comprehensive review
- Communication
- Techniques to mitigate the admission of radon inside buildings
- Erratum
- Erratum to “Effect of short heat treatment on mechanical properties and shape memory properties of Cu–Al–Ni shape memory alloy”
- Special Issue: AESMT-3 - Part II
- Integrated fuzzy logic and multicriteria decision model methods for selecting suitable sites for wastewater treatment plant: A case study in the center of Basrah, Iraq
- Physical and mechanical response of porous metals composites with nano-natural additives
- Special Issue: AESMT-4 - Part II
- New recycling method of lubricant oil and the effect on the viscosity and viscous shear as an environmentally friendly
- Identify the effect of Fe2O3 nanoparticles on mechanical and microstructural characteristics of aluminum matrix composite produced by powder metallurgy technique
- Static behavior of piled raft foundation in clay
- Ultra-low-power CMOS ring oscillator with minimum power consumption of 2.9 pW using low-voltage biasing technique
- Using ANN for well type identifying and increasing production from Sa’di formation of Halfaya oil field – Iraq
- Optimizing the performance of concrete tiles using nano-papyrus and carbon fibers
- Special Issue: AESMT-5 - Part II
- Comparative the effect of distribution transformer coil shape on electromagnetic forces and their distribution using the FEM
- The complex of Weyl module in free characteristic in the event of a partition (7,5,3)
- Restrained captive domination number
- Experimental study of improving hot mix asphalt reinforced with carbon fibers
- Asphalt binder modified with recycled tyre rubber
- Thermal performance of radiant floor cooling with phase change material for energy-efficient buildings
- Surveying the prediction of risks in cryptocurrency investments using recurrent neural networks
- A deep reinforcement learning framework to modify LQR for an active vibration control applied to 2D building models
- Evaluation of mechanically stabilized earth retaining walls for different soil–structure interaction methods: A review
- Assessment of heat transfer in a triangular duct with different configurations of ribs using computational fluid dynamics
- Sulfate removal from wastewater by using waste material as an adsorbent
- Experimental investigation on strengthening lap joints subjected to bending in glulam timber beams using CFRP sheets
- A study of the vibrations of a rotor bearing suspended by a hybrid spring system of shape memory alloys
- Stability analysis of Hub dam under rapid drawdown
- Developing ANFIS-FMEA model for assessment and prioritization of potential trouble factors in Iraqi building projects
- Numerical and experimental comparison study of piled raft foundation
- Effect of asphalt modified with waste engine oil on the durability properties of hot asphalt mixtures with reclaimed asphalt pavement
- Hydraulic model for flood inundation in Diyala River Basin using HEC-RAS, PMP, and neural network
- Numerical study on discharge capacity of piano key side weir with various ratios of the crest length to the width
- The optimal allocation of thyristor-controlled series compensators for enhancement HVAC transmission lines Iraqi super grid by using seeker optimization algorithm
- Numerical and experimental study of the impact on aerodynamic characteristics of the NACA0012 airfoil
- Effect of nano-TiO2 on physical and rheological properties of asphalt cement
- Performance evolution of novel palm leaf powder used for enhancing hot mix asphalt
- Performance analysis, evaluation, and improvement of selected unsignalized intersection using SIDRA software – Case study
- Flexural behavior of RC beams externally reinforced with CFRP composites using various strategies
- Influence of fiber types on the properties of the artificial cold-bonded lightweight aggregates
- Experimental investigation of RC beams strengthened with externally bonded BFRP composites
- Generalized RKM methods for solving fifth-order quasi-linear fractional partial differential equation
- An experimental and numerical study investigating sediment transport position in the bed of sewer pipes in Karbala
- Role of individual component failure in the performance of a 1-out-of-3 cold standby system: A Markov model approach
- Implementation for the cases (5, 4) and (5, 4)/(2, 0)
- Center group actions and related concepts
- Experimental investigation of the effect of horizontal construction joints on the behavior of deep beams
- Deletion of a vertex in even sum domination
- Deep learning techniques in concrete powder mix designing
- Effect of loading type in concrete deep beam with strut reinforcement
- Studying the effect of using CFRP warping on strength of husk rice concrete columns
- Parametric analysis of the influence of climatic factors on the formation of traditional buildings in the city of Al Najaf
- Suitability location for landfill using a fuzzy-GIS model: A case study in Hillah, Iraq
- Hybrid approach for cost estimation of sustainable building projects using artificial neural networks
- Assessment of indirect tensile stress and tensile–strength ratio and creep compliance in HMA mixes with micro-silica and PMB
- Density functional theory to study stopping power of proton in water, lung, bladder, and intestine
- A review of single flow, flow boiling, and coating microchannel studies
- Effect of GFRP bar length on the flexural behavior of hybrid concrete beams strengthened with NSM bars
- Exploring the impact of parameters on flow boiling heat transfer in microchannels and coated microtubes: A comprehensive review
- Crumb rubber modification for enhanced rutting resistance in asphalt mixtures
- Special Issue: AESMT-6
- Design of a new sorting colors system based on PLC, TIA portal, and factory I/O programs
- Forecasting empirical formula for suspended sediment load prediction at upstream of Al-Kufa barrage, Kufa City, Iraq
- Optimization and characterization of sustainable geopolymer mortars based on palygorskite clay, water glass, and sodium hydroxide
- Sediment transport modelling upstream of Al Kufa Barrage
- Study of energy loss, range, and stopping time for proton in germanium and copper materials
- Effect of internal and external recycle ratios on the nutrient removal efficiency of anaerobic/anoxic/oxic (VIP) wastewater treatment plant
- Enhancing structural behaviour of polypropylene fibre concrete columns longitudinally reinforced with fibreglass bars
- Sustainable road paving: Enhancing concrete paver blocks with zeolite-enhanced cement
- Evaluation of the operational performance of Karbala waste water treatment plant under variable flow using GPS-X model
- Design and simulation of photonic crystal fiber for highly sensitive chemical sensing applications
- Optimization and design of a new column sequencing for crude oil distillation at Basrah refinery
- Inductive 3D numerical modelling of the tibia bone using MRI to examine von Mises stress and overall deformation
- An image encryption method based on modified elliptic curve Diffie-Hellman key exchange protocol and Hill Cipher
- Experimental investigation of generating superheated steam using a parabolic dish with a cylindrical cavity receiver: A case study
- Effect of surface roughness on the interface behavior of clayey soils
- Investigated of the optical properties for SiO2 by using Lorentz model
- Measurements of induced vibrations due to steel pipe pile driving in Al-Fao soil: Effect of partial end closure
- Experimental and numerical studies of ballistic resistance of hybrid sandwich composite body armor
- Evaluation of clay layer presence on shallow foundation settlement in dry sand under an earthquake
- Optimal design of mechanical performances of asphalt mixtures comprising nano-clay additives
- Advancing seismic performance: Isolators, TMDs, and multi-level strategies in reinforced concrete buildings
- Predicted evaporation in Basrah using artificial neural networks
- Energy management system for a small town to enhance quality of life
- Numerical study on entropy minimization in pipes with helical airfoil and CuO nanoparticle integration
- Equations and methodologies of inlet drainage system discharge coefficients: A review
- Thermal buckling analysis for hybrid and composite laminated plate by using new displacement function
- Investigation into the mechanical and thermal properties of lightweight mortar using commercial beads or recycled expanded polystyrene
- Experimental and theoretical analysis of single-jet column and concrete column using double-jet grouting technique applied at Al-Rashdia site
- The impact of incorporating waste materials on the mechanical and physical characteristics of tile adhesive materials
- Seismic resilience: Innovations in structural engineering for earthquake-prone areas
- Automatic human identification using fingerprint images based on Gabor filter and SIFT features fusion
- Performance of GRKM-method for solving classes of ordinary and partial differential equations of sixth-orders
- Visible light-boosted photodegradation activity of Ag–AgVO3/Zn0.5Mn0.5Fe2O4 supported heterojunctions for effective degradation of organic contaminates
- Production of sustainable concrete with treated cement kiln dust and iron slag waste aggregate
- Key effects on the structural behavior of fiber-reinforced lightweight concrete-ribbed slabs: A review
- A comparative analysis of the energy dissipation efficiency of various piano key weir types
- Special Issue: Transport 2022 - Part II
- Variability in road surface temperature in urban road network – A case study making use of mobile measurements
- Special Issue: BCEE5-2023
- Evaluation of reclaimed asphalt mixtures rejuvenated with waste engine oil to resist rutting deformation
- Assessment of potential resistance to moisture damage and fatigue cracks of asphalt mixture modified with ground granulated blast furnace slag
- Investigating seismic response in adjacent structures: A study on the impact of buildings’ orientation and distance considering soil–structure interaction
- Improvement of porosity of mortar using polyethylene glycol pre-polymer-impregnated mortar
- Three-dimensional analysis of steel beam-column bolted connections
- Assessment of agricultural drought in Iraq employing Landsat and MODIS imagery
- Performance evaluation of grouted porous asphalt concrete
- Optimization of local modified metakaolin-based geopolymer concrete by Taguchi method
- Effect of waste tire products on some characteristics of roller-compacted concrete
- Studying the lateral displacement of retaining wall supporting sandy soil under dynamic loads
- Seismic performance evaluation of concrete buttress dram (Dynamic linear analysis)
- Behavior of soil reinforced with micropiles
- Possibility of production high strength lightweight concrete containing organic waste aggregate and recycled steel fibers
- An investigation of self-sensing and mechanical properties of smart engineered cementitious composites reinforced with functional materials
- Forecasting changes in precipitation and temperatures of a regional watershed in Northern Iraq using LARS-WG model
- Experimental investigation of dynamic soil properties for modeling energy-absorbing layers
- Numerical investigation of the effect of longitudinal steel reinforcement ratio on the ductility of concrete beams
- An experimental study on the tensile properties of reinforced asphalt pavement
- Self-sensing behavior of hot asphalt mixture with steel fiber-based additive
- Behavior of ultra-high-performance concrete deep beams reinforced by basalt fibers
- Optimizing asphalt binder performance with various PET types
- Investigation of the hydraulic characteristics and homogeneity of the microstructure of the air voids in the sustainable rigid pavement
- Enhanced biogas production from municipal solid waste via digestion with cow manure: A case study
- Special Issue: AESMT-7 - Part I
- Preparation and investigation of cobalt nanoparticles by laser ablation: Structure, linear, and nonlinear optical properties
- Seismic analysis of RC building with plan irregularity in Baghdad/Iraq to obtain the optimal behavior
- The effect of urban environment on large-scale path loss model’s main parameters for mmWave 5G mobile network in Iraq
- Formatting a questionnaire for the quality control of river bank roads
- Vibration suppression of smart composite beam using model predictive controller
- Machine learning-based compressive strength estimation in nanomaterial-modified lightweight concrete
- In-depth analysis of critical factors affecting Iraqi construction projects performance
- Behavior of container berth structure under the influence of environmental and operational loads
- Energy absorption and impact response of ballistic resistance laminate
- Effect of water-absorbent polymer balls in internal curing on punching shear behavior of bubble slabs
- Effect of surface roughness on interface shear strength parameters of sandy soils
- Evaluating the interaction for embedded H-steel section in normal concrete under monotonic and repeated loads
- Estimation of the settlement of pile head using ANN and multivariate linear regression based on the results of load transfer method
- Enhancing communication: Deep learning for Arabic sign language translation
- A review of recent studies of both heat pipe and evaporative cooling in passive heat recovery
- Effect of nano-silica on the mechanical properties of LWC
- An experimental study of some mechanical properties and absorption for polymer-modified cement mortar modified with superplasticizer
- Digital beamforming enhancement with LSTM-based deep learning for millimeter wave transmission
- Developing an efficient planning process for heritage buildings maintenance in Iraq
- Design and optimization of two-stage controller for three-phase multi-converter/multi-machine electric vehicle
- Evaluation of microstructure and mechanical properties of Al1050/Al2O3/Gr composite processed by forming operation ECAP
- Calculations of mass stopping power and range of protons in organic compounds (CH3OH, CH2O, and CO2) at energy range of 0.01–1,000 MeV
- Investigation of in vitro behavior of composite coating hydroxyapatite-nano silver on 316L stainless steel substrate by electrophoretic technic for biomedical tools
- A review: Enhancing tribological properties of journal bearings composite materials
- Improvements in the randomness and security of digital currency using the photon sponge hash function through Maiorana–McFarland S-box replacement
- Design a new scheme for image security using a deep learning technique of hierarchical parameters
- Special Issue: ICES 2023
- Comparative geotechnical analysis for ultimate bearing capacity of precast concrete piles using cone resistance measurements
- Visualizing sustainable rainwater harvesting: A case study of Karbala Province
- Geogrid reinforcement for improving bearing capacity and stability of square foundations
- Evaluation of the effluent concentrations of Karbala wastewater treatment plant using reliability analysis
- Adsorbent made with inexpensive, local resources
- Effect of drain pipes on seepage and slope stability through a zoned earth dam
- Sediment accumulation in an 8 inch sewer pipe for a sample of various particles obtained from the streets of Karbala city, Iraq
- Special Issue: IETAS 2024 - Part I
- Analyzing the impact of transfer learning on explanation accuracy in deep learning-based ECG recognition systems
- Effect of scale factor on the dynamic response of frame foundations
- Improving multi-object detection and tracking with deep learning, DeepSORT, and frame cancellation techniques
- The impact of using prestressed CFRP bars on the development of flexural strength
- Assessment of surface hardness and impact strength of denture base resins reinforced with silver–titanium dioxide and silver–zirconium dioxide nanoparticles: In vitro study
- A data augmentation approach to enhance breast cancer detection using generative adversarial and artificial neural networks
- Modification of the 5D Lorenz chaotic map with fuzzy numbers for video encryption in cloud computing
- Special Issue: 51st KKBN - Part I
- Evaluation of static bending caused damage of glass-fiber composite structure using terahertz inspection
Artikel in diesem Heft
- Regular Articles
- Methodology of automated quality management
- Influence of vibratory conveyor design parameters on the trough motion and the self-synchronization of inertial vibrators
- Application of finite element method in industrial design, example of an electric motorcycle design project
- Correlative evaluation of the corrosion resilience and passivation properties of zinc and aluminum alloys in neutral chloride and acid-chloride solutions
- Will COVID “encourage” B2B and data exchange engineering in logistic firms?
- Influence of unsupported sleepers on flange climb derailment of two freight wagons
- A hybrid detection algorithm for 5G OTFS waveform for 64 and 256 QAM with Rayleigh and Rician channels
- Effect of short heat treatment on mechanical properties and shape memory properties of Cu–Al–Ni shape memory alloy
- Exploring the potential of ammonia and hydrogen as alternative fuels for transportation
- Impact of insulation on energy consumption and CO2 emissions in high-rise commercial buildings at various climate zones
- Advanced autopilot design with extremum-seeking control for aircraft control
- Adaptive multidimensional trust-based recommendation model for peer to peer applications
- Effects of CFRP sheets on the flexural behavior of high-strength concrete beam
- Enhancing urban sustainability through industrial synergy: A multidisciplinary framework for integrating sustainable industrial practices within urban settings – The case of Hamadan industrial city
- Advanced vibrant controller results of an energetic framework structure
- Application of the Taguchi method and RSM for process parameter optimization in AWSJ machining of CFRP composite-based orthopedic implants
- Improved correlation of soil modulus with SPT N values
- Technologies for high-temperature batch annealing of grain-oriented electrical steel: An overview
- Assessing the need for the adoption of digitalization in Indian small and medium enterprises
- A non-ideal hybridization issue for vertical TFET-based dielectric-modulated biosensor
- Optimizing data retrieval for enhanced data integrity verification in cloud environments
- Performance analysis of nonlinear crosstalk of WDM systems using modulation schemes criteria
- Nonlinear finite-element analysis of RC beams with various opening near supports
- Thermal analysis of Fe3O4–Cu/water over a cone: a fractional Maxwell model
- Radial–axial runner blade design using the coordinate slice technique
- Theoretical and experimental comparison between straight and curved continuous box girders
- Effect of the reinforcement ratio on the mechanical behaviour of textile-reinforced concrete composite: Experiment and numerical modeling
- Experimental and numerical investigation on composite beam–column joint connection behavior using different types of connection schemes
- Enhanced performance and robustness in anti-lock brake systems using barrier function-based integral sliding mode control
- Evaluation of the creep strength of samples produced by fused deposition modeling
- A combined feedforward-feedback controller design for nonlinear systems
- Effect of adjacent structures on footing settlement for different multi-building arrangements
- Analyzing the impact of curved tracks on wheel flange thickness reduction in railway systems
- Review Articles
- Mechanical and smart properties of cement nanocomposites containing nanomaterials: A brief review
- Applications of nanotechnology and nanoproduction techniques
- Relationship between indoor environmental quality and guests’ comfort and satisfaction at green hotels: A comprehensive review
- Communication
- Techniques to mitigate the admission of radon inside buildings
- Erratum
- Erratum to “Effect of short heat treatment on mechanical properties and shape memory properties of Cu–Al–Ni shape memory alloy”
- Special Issue: AESMT-3 - Part II
- Integrated fuzzy logic and multicriteria decision model methods for selecting suitable sites for wastewater treatment plant: A case study in the center of Basrah, Iraq
- Physical and mechanical response of porous metals composites with nano-natural additives
- Special Issue: AESMT-4 - Part II
- New recycling method of lubricant oil and the effect on the viscosity and viscous shear as an environmentally friendly
- Identify the effect of Fe2O3 nanoparticles on mechanical and microstructural characteristics of aluminum matrix composite produced by powder metallurgy technique
- Static behavior of piled raft foundation in clay
- Ultra-low-power CMOS ring oscillator with minimum power consumption of 2.9 pW using low-voltage biasing technique
- Using ANN for well type identifying and increasing production from Sa’di formation of Halfaya oil field – Iraq
- Optimizing the performance of concrete tiles using nano-papyrus and carbon fibers
- Special Issue: AESMT-5 - Part II
- Comparative the effect of distribution transformer coil shape on electromagnetic forces and their distribution using the FEM
- The complex of Weyl module in free characteristic in the event of a partition (7,5,3)
- Restrained captive domination number
- Experimental study of improving hot mix asphalt reinforced with carbon fibers
- Asphalt binder modified with recycled tyre rubber
- Thermal performance of radiant floor cooling with phase change material for energy-efficient buildings
- Surveying the prediction of risks in cryptocurrency investments using recurrent neural networks
- A deep reinforcement learning framework to modify LQR for an active vibration control applied to 2D building models
- Evaluation of mechanically stabilized earth retaining walls for different soil–structure interaction methods: A review
- Assessment of heat transfer in a triangular duct with different configurations of ribs using computational fluid dynamics
- Sulfate removal from wastewater by using waste material as an adsorbent
- Experimental investigation on strengthening lap joints subjected to bending in glulam timber beams using CFRP sheets
- A study of the vibrations of a rotor bearing suspended by a hybrid spring system of shape memory alloys
- Stability analysis of Hub dam under rapid drawdown
- Developing ANFIS-FMEA model for assessment and prioritization of potential trouble factors in Iraqi building projects
- Numerical and experimental comparison study of piled raft foundation
- Effect of asphalt modified with waste engine oil on the durability properties of hot asphalt mixtures with reclaimed asphalt pavement
- Hydraulic model for flood inundation in Diyala River Basin using HEC-RAS, PMP, and neural network
- Numerical study on discharge capacity of piano key side weir with various ratios of the crest length to the width
- The optimal allocation of thyristor-controlled series compensators for enhancement HVAC transmission lines Iraqi super grid by using seeker optimization algorithm
- Numerical and experimental study of the impact on aerodynamic characteristics of the NACA0012 airfoil
- Effect of nano-TiO2 on physical and rheological properties of asphalt cement
- Performance evolution of novel palm leaf powder used for enhancing hot mix asphalt
- Performance analysis, evaluation, and improvement of selected unsignalized intersection using SIDRA software – Case study
- Flexural behavior of RC beams externally reinforced with CFRP composites using various strategies
- Influence of fiber types on the properties of the artificial cold-bonded lightweight aggregates
- Experimental investigation of RC beams strengthened with externally bonded BFRP composites
- Generalized RKM methods for solving fifth-order quasi-linear fractional partial differential equation
- An experimental and numerical study investigating sediment transport position in the bed of sewer pipes in Karbala
- Role of individual component failure in the performance of a 1-out-of-3 cold standby system: A Markov model approach
- Implementation for the cases (5, 4) and (5, 4)/(2, 0)
- Center group actions and related concepts
- Experimental investigation of the effect of horizontal construction joints on the behavior of deep beams
- Deletion of a vertex in even sum domination
- Deep learning techniques in concrete powder mix designing
- Effect of loading type in concrete deep beam with strut reinforcement
- Studying the effect of using CFRP warping on strength of husk rice concrete columns
- Parametric analysis of the influence of climatic factors on the formation of traditional buildings in the city of Al Najaf
- Suitability location for landfill using a fuzzy-GIS model: A case study in Hillah, Iraq
- Hybrid approach for cost estimation of sustainable building projects using artificial neural networks
- Assessment of indirect tensile stress and tensile–strength ratio and creep compliance in HMA mixes with micro-silica and PMB
- Density functional theory to study stopping power of proton in water, lung, bladder, and intestine
- A review of single flow, flow boiling, and coating microchannel studies
- Effect of GFRP bar length on the flexural behavior of hybrid concrete beams strengthened with NSM bars
- Exploring the impact of parameters on flow boiling heat transfer in microchannels and coated microtubes: A comprehensive review
- Crumb rubber modification for enhanced rutting resistance in asphalt mixtures
- Special Issue: AESMT-6
- Design of a new sorting colors system based on PLC, TIA portal, and factory I/O programs
- Forecasting empirical formula for suspended sediment load prediction at upstream of Al-Kufa barrage, Kufa City, Iraq
- Optimization and characterization of sustainable geopolymer mortars based on palygorskite clay, water glass, and sodium hydroxide
- Sediment transport modelling upstream of Al Kufa Barrage
- Study of energy loss, range, and stopping time for proton in germanium and copper materials
- Effect of internal and external recycle ratios on the nutrient removal efficiency of anaerobic/anoxic/oxic (VIP) wastewater treatment plant
- Enhancing structural behaviour of polypropylene fibre concrete columns longitudinally reinforced with fibreglass bars
- Sustainable road paving: Enhancing concrete paver blocks with zeolite-enhanced cement
- Evaluation of the operational performance of Karbala waste water treatment plant under variable flow using GPS-X model
- Design and simulation of photonic crystal fiber for highly sensitive chemical sensing applications
- Optimization and design of a new column sequencing for crude oil distillation at Basrah refinery
- Inductive 3D numerical modelling of the tibia bone using MRI to examine von Mises stress and overall deformation
- An image encryption method based on modified elliptic curve Diffie-Hellman key exchange protocol and Hill Cipher
- Experimental investigation of generating superheated steam using a parabolic dish with a cylindrical cavity receiver: A case study
- Effect of surface roughness on the interface behavior of clayey soils
- Investigated of the optical properties for SiO2 by using Lorentz model
- Measurements of induced vibrations due to steel pipe pile driving in Al-Fao soil: Effect of partial end closure
- Experimental and numerical studies of ballistic resistance of hybrid sandwich composite body armor
- Evaluation of clay layer presence on shallow foundation settlement in dry sand under an earthquake
- Optimal design of mechanical performances of asphalt mixtures comprising nano-clay additives
- Advancing seismic performance: Isolators, TMDs, and multi-level strategies in reinforced concrete buildings
- Predicted evaporation in Basrah using artificial neural networks
- Energy management system for a small town to enhance quality of life
- Numerical study on entropy minimization in pipes with helical airfoil and CuO nanoparticle integration
- Equations and methodologies of inlet drainage system discharge coefficients: A review
- Thermal buckling analysis for hybrid and composite laminated plate by using new displacement function
- Investigation into the mechanical and thermal properties of lightweight mortar using commercial beads or recycled expanded polystyrene
- Experimental and theoretical analysis of single-jet column and concrete column using double-jet grouting technique applied at Al-Rashdia site
- The impact of incorporating waste materials on the mechanical and physical characteristics of tile adhesive materials
- Seismic resilience: Innovations in structural engineering for earthquake-prone areas
- Automatic human identification using fingerprint images based on Gabor filter and SIFT features fusion
- Performance of GRKM-method for solving classes of ordinary and partial differential equations of sixth-orders
- Visible light-boosted photodegradation activity of Ag–AgVO3/Zn0.5Mn0.5Fe2O4 supported heterojunctions for effective degradation of organic contaminates
- Production of sustainable concrete with treated cement kiln dust and iron slag waste aggregate
- Key effects on the structural behavior of fiber-reinforced lightweight concrete-ribbed slabs: A review
- A comparative analysis of the energy dissipation efficiency of various piano key weir types
- Special Issue: Transport 2022 - Part II
- Variability in road surface temperature in urban road network – A case study making use of mobile measurements
- Special Issue: BCEE5-2023
- Evaluation of reclaimed asphalt mixtures rejuvenated with waste engine oil to resist rutting deformation
- Assessment of potential resistance to moisture damage and fatigue cracks of asphalt mixture modified with ground granulated blast furnace slag
- Investigating seismic response in adjacent structures: A study on the impact of buildings’ orientation and distance considering soil–structure interaction
- Improvement of porosity of mortar using polyethylene glycol pre-polymer-impregnated mortar
- Three-dimensional analysis of steel beam-column bolted connections
- Assessment of agricultural drought in Iraq employing Landsat and MODIS imagery
- Performance evaluation of grouted porous asphalt concrete
- Optimization of local modified metakaolin-based geopolymer concrete by Taguchi method
- Effect of waste tire products on some characteristics of roller-compacted concrete
- Studying the lateral displacement of retaining wall supporting sandy soil under dynamic loads
- Seismic performance evaluation of concrete buttress dram (Dynamic linear analysis)
- Behavior of soil reinforced with micropiles
- Possibility of production high strength lightweight concrete containing organic waste aggregate and recycled steel fibers
- An investigation of self-sensing and mechanical properties of smart engineered cementitious composites reinforced with functional materials
- Forecasting changes in precipitation and temperatures of a regional watershed in Northern Iraq using LARS-WG model
- Experimental investigation of dynamic soil properties for modeling energy-absorbing layers
- Numerical investigation of the effect of longitudinal steel reinforcement ratio on the ductility of concrete beams
- An experimental study on the tensile properties of reinforced asphalt pavement
- Self-sensing behavior of hot asphalt mixture with steel fiber-based additive
- Behavior of ultra-high-performance concrete deep beams reinforced by basalt fibers
- Optimizing asphalt binder performance with various PET types
- Investigation of the hydraulic characteristics and homogeneity of the microstructure of the air voids in the sustainable rigid pavement
- Enhanced biogas production from municipal solid waste via digestion with cow manure: A case study
- Special Issue: AESMT-7 - Part I
- Preparation and investigation of cobalt nanoparticles by laser ablation: Structure, linear, and nonlinear optical properties
- Seismic analysis of RC building with plan irregularity in Baghdad/Iraq to obtain the optimal behavior
- The effect of urban environment on large-scale path loss model’s main parameters for mmWave 5G mobile network in Iraq
- Formatting a questionnaire for the quality control of river bank roads
- Vibration suppression of smart composite beam using model predictive controller
- Machine learning-based compressive strength estimation in nanomaterial-modified lightweight concrete
- In-depth analysis of critical factors affecting Iraqi construction projects performance
- Behavior of container berth structure under the influence of environmental and operational loads
- Energy absorption and impact response of ballistic resistance laminate
- Effect of water-absorbent polymer balls in internal curing on punching shear behavior of bubble slabs
- Effect of surface roughness on interface shear strength parameters of sandy soils
- Evaluating the interaction for embedded H-steel section in normal concrete under monotonic and repeated loads
- Estimation of the settlement of pile head using ANN and multivariate linear regression based on the results of load transfer method
- Enhancing communication: Deep learning for Arabic sign language translation
- A review of recent studies of both heat pipe and evaporative cooling in passive heat recovery
- Effect of nano-silica on the mechanical properties of LWC
- An experimental study of some mechanical properties and absorption for polymer-modified cement mortar modified with superplasticizer
- Digital beamforming enhancement with LSTM-based deep learning for millimeter wave transmission
- Developing an efficient planning process for heritage buildings maintenance in Iraq
- Design and optimization of two-stage controller for three-phase multi-converter/multi-machine electric vehicle
- Evaluation of microstructure and mechanical properties of Al1050/Al2O3/Gr composite processed by forming operation ECAP
- Calculations of mass stopping power and range of protons in organic compounds (CH3OH, CH2O, and CO2) at energy range of 0.01–1,000 MeV
- Investigation of in vitro behavior of composite coating hydroxyapatite-nano silver on 316L stainless steel substrate by electrophoretic technic for biomedical tools
- A review: Enhancing tribological properties of journal bearings composite materials
- Improvements in the randomness and security of digital currency using the photon sponge hash function through Maiorana–McFarland S-box replacement
- Design a new scheme for image security using a deep learning technique of hierarchical parameters
- Special Issue: ICES 2023
- Comparative geotechnical analysis for ultimate bearing capacity of precast concrete piles using cone resistance measurements
- Visualizing sustainable rainwater harvesting: A case study of Karbala Province
- Geogrid reinforcement for improving bearing capacity and stability of square foundations
- Evaluation of the effluent concentrations of Karbala wastewater treatment plant using reliability analysis
- Adsorbent made with inexpensive, local resources
- Effect of drain pipes on seepage and slope stability through a zoned earth dam
- Sediment accumulation in an 8 inch sewer pipe for a sample of various particles obtained from the streets of Karbala city, Iraq
- Special Issue: IETAS 2024 - Part I
- Analyzing the impact of transfer learning on explanation accuracy in deep learning-based ECG recognition systems
- Effect of scale factor on the dynamic response of frame foundations
- Improving multi-object detection and tracking with deep learning, DeepSORT, and frame cancellation techniques
- The impact of using prestressed CFRP bars on the development of flexural strength
- Assessment of surface hardness and impact strength of denture base resins reinforced with silver–titanium dioxide and silver–zirconium dioxide nanoparticles: In vitro study
- A data augmentation approach to enhance breast cancer detection using generative adversarial and artificial neural networks
- Modification of the 5D Lorenz chaotic map with fuzzy numbers for video encryption in cloud computing
- Special Issue: 51st KKBN - Part I
- Evaluation of static bending caused damage of glass-fiber composite structure using terahertz inspection

