Abstract
Shale gas plays a crucial role in China’s energy transition and carbon peak goals, with Sichuan Province being a key development region. However, challenges such as methane (CH4) emissions and inefficient carbon capture hinder its sustainable utilization. Traditional static methods for gas separation, while effective under stable and simplified conditions, often struggle to adapt to complex and fluctuating working environments typically encountered in shale gas extraction, such as variable pressure, temperature, and gas composition. These limitations reduce their efficiency and reliability in large-scale, real-world applications. In contrast, the pressure swing adsorption (PSA) process offers a dynamic and adaptable solution for CO2 separation and CH4 purification, performing more efficiently under varying operational conditions. This study evaluates the contribution of shale gas development in Sichuan to China’s carbon peak targets, with a focus on optimizing the PSA process. Using life cycle assessment and real-world operational data, we quantify the carbon footprint and analyze PSA parameters under different working conditions to improve CO2 capture efficiency. Results show that optimized PSA not only significantly reduces emissions compared to static methods under variable conditions but also supports China’s carbon reduction strategy. The findings provide theoretical and practical insights for policymakers and industry stakeholders in achieving sustainable shale gas utilization.
1 Introduction
With the global push toward carbon neutrality and sustainable energy transition, China has pledged to peak carbon emissions by 2030 and achieve carbon neutrality by 2060 [1,2]. As a key component of China’s energy strategy, shale gas plays a vital role in optimizing the national energy mix and reducing carbon emissions. Sichuan Province, with its abundant shale gas reserves and mature development technologies, has become a major production hub [3]. Given its strategic importance in national energy security and environmental sustainability, assessing the impact of shale gas development and utilization in Sichuan on carbon peaking holds both theoretical and practical significance [4].
Shale gas is primarily composed of methane (CH4), which has a lower carbon content than coal or oil. When combusted, CH4 releases significantly less CO2 per unit of energy, making it a promising transitional fuel for reducing carbon intensity [5,6]. Among various carbon capture and purification technologies, pressure swing adsorption (PSA) stands out for its potential in shale gas processing. By selectively adsorbing CO2 and other impurities under different pressure conditions, PSA enhances CH4 purity and improves gas utilization efficiency, thereby reducing the carbon footprint of shale gas operations and supporting China’s carbon reduction goals [7,8].
However, large-scale shale gas development in Sichuan faces several pressing challenges. First, hydraulic fracturing – the primary extraction method – raises environmental concerns, including groundwater contamination, land degradation, and CH4 leakage. Although CH4 emits less CO2 when burned compared to coal, fugitive emissions during production, transportation, and usage pose a risk of offsetting these climate benefits due to CH4’s high global warming potential [9,10].
Second, the efficiency and economic viability of carbon capture and utilization in shale gas operations remain limited. Traditional methods like chemical absorption are energy-intensive and costly, restricting their widespread use. PSA offers a more energy-efficient and cost-effective alternative, but its adsorption capacity, regeneration performance, and overall economic feasibility still require significant improvement. In practice, PSA-based purification systems often suffer from low CO2 capture rates, high operational expenses, and challenges in dealing with fluctuating gas compositions [11,12].
Moreover, there is a lack of comprehensive studies that quantitatively evaluate the contribution of shale gas to carbon peaking. Existing research tends to focus on energy potential and extraction techniques, while systematic assessments of emission reductions are scarce. Accurately modeling carbon emissions across the shale gas life cycle – including extraction, purification, transportation, and combustion – is essential for understanding its true role in achieving carbon peak targets [13]. Furthermore, the integration of PSA technology into shale gas carbon reduction pathways remains underexplored, necessitating interdisciplinary research that bridges energy engineering, environmental science, and policy analysis.
Current research efforts are mainly concentrated in three areas: (1) CH4 recovery and purification technologies; (2) carbon footprint assessment of shale gas development; and (3) policy frameworks for emission reduction. While these studies have provided valuable insights, several gaps remain.
In CH4 recovery, traditional separation technologies such as membrane separation and cryogenic distillation are available, but they often face drawbacks like high energy consumption and complex maintenance. PSA technology, based on selective adsorption, has garnered increasing attention for its lower energy demand and economic advantages. However, optimization of PSA parameters specific to shale gas purification is still in its early stages and requires more empirical validation [14].
In carbon footprint analysis, life cycle assessment (LCA) is widely used to evaluate emissions from shale gas production. However, many studies fail to account for dynamic emission factors across different stages of the production process. Additionally, the potential carbon reduction benefits of PSA-based CO2 capture remain inadequately quantified.
2 Related work
As a clean energy source, shale gas CH4 has a wide range of applications (Figure 1). In addition to domestic use, coalbed methane (CBM) can also serve as a power fuel for electricity generation and as a raw material for the synthesis of various chemical products, such as pesticides, fertilizers, dyestuffs, and methanol. Its high calorific value and low impurity content make it an ideal substitute for traditional fossil fuels in industrial applications. The comprehensive utilization value of CH4 from shale gas is mainly reflected in the following aspects:
Residential and commercial use: CH4 is widely used for cooking, heating, and water heating in households and public facilities due to its clean combustion and safety features.
Power generation: CH4 can be used as a clean fuel for gas-fired power plants or distributed energy systems, significantly reducing CO2 and particulate emissions compared to coal-fired units.
Chemical industry feedstock: CH4 is a key raw material in the production of ammonia, methanol, and hydrogen, which are in turn used in the manufacture of chemical fertilizers, plastics, synthetic fibers, and other industrial chemicals.
Transportation fuel: When converted into compressed natural gas or liquefied natural gas, CH4 can be used as an alternative vehicle fuel, contributing to lower urban air pollution and greenhouse gas emissions.
Distributed energy and cogeneration: CH4-based systems can be employed for combined heat and power generation in industrial parks and remote areas, enhancing energy efficiency and reducing transmission losses.
Emission reduction and carbon credit potential: Utilizing CH4 from shale gas helps reduce greenhouse gas emissions by displacing more carbon-intensive energy sources and mitigating CH4 leakage from natural sources or mining operations.

Schematic diagram of the existing shale gas CH4 utilization process.
These diverse applications not only improve the economic efficiency of shale gas utilization but also contribute to China’s broader goals of energy diversification, environmental protection, and carbon emission reduction.
The adsorption performance of porous materials can be evaluated by measuring the equilibrium adsorption amount at different pressures at constant temperature and fitting the adsorption equilibrium isotherm at different pressures [15]. When the adsorption isotherm is non-linear, the adsorption capacity at low pressure is high, and the regeneration of the adsorbent requires the use of vacuum to desorb the loaded gas. Therefore, in order to reuse the adsorbent, a desorption process is required to regenerate the adsorbent after the adsorption is completed. PSA is the process of adsorption and desorption by vacuum, while TSA is the process of desorption by increasing the temperature. Compared to TSA, the PSA cycle is shorter, usually taking only a few minutes, while the TSA cycle usually takes several hours.
PSA, as a more mature technology, uses different adsorbents to separate gas mixtures and in the shale gas CH4 separation process [16]. Smith found that among the four basic structures, the low-pressure recombination and high-pressure recombination cycles were the best choices for separation [17].
In the conventional PSA process, as the CH4 concentration increases, the explosion limit is reached, leading to an increased risk of explosion. PSA, as a new adsorption method, uses evacuation to reduce the partial pressure of the adsorbent components and depressurizes the adsorbed components under negative pressure [18]. The study [19] was also conducted on a twin-bed vacuum PSA plant using activated carbon and carbon molecular sieve and found that the shale gas CH4 could be enriched from 20% to more than 30% when the mass ratio of carbon molecular sieve to activated carbon was 3.4, and the explosion limit of CH4 could be avoided at the same time.
3 Methodology
The ultimate goal of the PSA process is the separation of shale gas CH4 and the recovery of high-purity CH4 from the shale gas CH4. Based on the principle of PSA and the process requirements of the study, the basic process flow was established, as shown in Figure 2. The process involves several key steps, which are critical for ensuring optimal performance and high CH4 recovery efficiency. These steps include:
Adsorption cycle: The shale gas mixture, which contains CH4, CO2, and other impurities, is introduced into the adsorption unit, typically consisting of adsorption beds filled with selective adsorbent materials such as zeolites or activated carbon. Under high pressure, CO2 and other impurities are adsorbed onto the adsorbent, while CH4 passes through and is recovered.
Pressure swing: After the adsorption cycle, the pressure in the adsorption beds is lowered (pressure swing), causing the adsorbed CO2 and impurities to desorb. This desorption process releases the captured CO2 and other contaminants, allowing for their removal from the system.
Regeneration of adsorbent: The adsorbent material is regenerated through a depressurization or heating process, which restores its ability to adsorb impurities. This cycle of adsorption, pressure swing, and regeneration is repeated in multiple beds to ensure continuous operation of the system.
Purification and compression: Once CH4 has been separated, it undergoes additional purification steps to remove any residual impurities. This purified CH4 is then compressed for transportation and use. The process ensures that CH4 recovered is of high purity and suitable for industrial applications, power generation, or chemical synthesis.
CO 2 recovery and disposal: CO2 and other impurities, which are separated during the PSA process, are either vented to the atmosphere or captured for further use or disposal. If CO2 is captured, it can potentially be used in enhanced oil recovery or stored underground to reduce the environmental impact.
Energy efficiency and optimization: The energy consumption of the PSA process is optimized by adjusting parameters such as the pressure swing, cycle time, and regeneration process. Advanced control systems may be used to monitor and adjust these parameters in real-time to maximize the efficiency of the process and reduce operational costs.
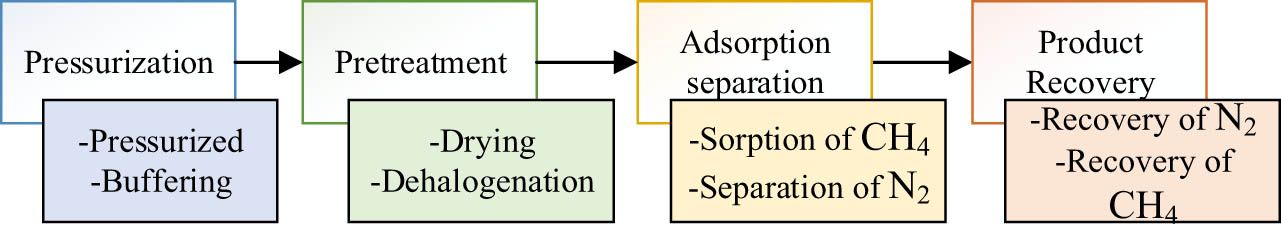
Basic flow of the CBM PSA process.
By following this basic process flow, the PSA process effectively separates and purifies CH4 from shale gas, reducing CO2 emissions and improving the overall environmental sustainability of shale gas utilization. The high-purity CH4 recovered can be used as a cleaner alternative to other fossil fuels, contributing to the reduction of carbon emissions and supporting the transition towards a more sustainable energy system.
The common pressure shift adsorption process consists of five main stages, and the main steps are shown in Figure 3.

Schematic diagram of the basic process of variable pressure adsorption.
The first step is the pressure filling stage (A–B); after the adsorption process is completed, the concentration of product gas in the adsorption tower is very low, and the adsorption bed is pressurized with raw gas or separated weakly adsorbed gas, so that the pressure in the adsorption tower rises to the working pressure of the adsorption process to ensure that the adsorption process can be carried out smoothly and adequately.
The second step is the adsorption stage (B–C), that is, the tower pressure is kept constant, the raw gas at a certain speed through the adsorption bed, the adsorbent selective adsorption of certain components of the gas mixture, while the rest of the components through the adsorption layer to the top of the adsorption tower. As the gas enters from the bottom of the tower, the adsorbent at the lower part of the adsorption bed becomes saturated first. Once the adsorbent reaches saturation and can no longer adsorb the mixed gases, the gas continues to flow through the voids between the adsorbent particles and moves upward to the upper section of the adsorption bed until the adsorption process is complete [20].
The third step is the equal pressure stage (C–D), that is, the adsorption tower in the adsorption phase after the completion of the tower of high-pressure gas from the adsorption tower overflow into the other adsorption tower was evacuated process. Among them, the adsorption tower with high pressure is the average pressure drop, and the adsorption tower with vacuum is the average pressure rise. When the pressure is equalized, the gas under pressure can be used as purge gas to remove the other gas components remaining in the tower and regenerate the adsorption bed. This not only ensures the safety of the next step, but also alleviates the deformation brought about by the pressure on the vessel.
The fourth step is the reverse discharge stage (D–E), that is, the direction of the output product into the raw gas to reduce the adsorption bed pressure, until the lowest pressure in the process of variable pressure adsorption cycle, the adsorption bed adsorption of gas components began to desorption, with the gas flow out of the tower.
The fifth step is the evacuation stage (E–D), as the pressure in the tower is reduced to atmospheric pressure, the vacuum pump starts to evacuate the adsorbent tower, so that the desorption of the adsorbent is complete and the regeneration of the adsorbent is ensured. The temperature and pressure in the tower are constantly changing, so that the required product gas can be separated continuously.
3.1 Process simulation and method
The PSA process is an operational process for gas separation by controlling the cyclic changes of process parameters such as adsorption pressure, temperature, feed flow rate, concentration, and adsorption duration. For PSA process simulation, the most important thing is to be able to accurately describe the gas adsorption resolution process on a fixed bed and to establish the corresponding mathematical model. The process transfer principle is the theoretical basis of the unit operation, and the three transfer processes can summarize all the characteristics of the PSA process, namely mass transfer, momentum transfer, and energy transfer. By establishing adsorption equilibrium and adsorption kinetics models, the energy balance equation is reconstructed according to the heat transfer between adsorbent and adsorbent and the heat balance between the column wall and the environment, and the transport rate in the transport model is determined.
3.2 Mathematical model of adsorption fixed bed
3.2.1 Mass transfer equilibrium equation
The gas–solid mass transfer equilibrium model includes axial and radial diffusion mass transfer terms, convective mass transfer terms, and accumulation terms in the bed and adsorbent particles. When the height-to-diameter ratio of the adsorption bed is greater than 5, the radial diffusion effect term can be neglected, and this term is ignored in the bed design study of the adsorption tower in this article. According to the continuity equation assumption, the overall and component mass transfer balance equations in the adsorption bed can be expressed as Eqs. (1) and (2), respectively. The axial diffusion coefficient and radial diffusion coefficient in the adsorption bed mass balance model can be estimated by Eqs. (3) and (4), respectively,
3.2.2 Heat transfer equilibrium equation
The adsorption process depends on the adsorption and desorption cycles of the adsorption bed, and as a spontaneous exothermic physical process with reduced confusion, the adsorption process must be accompanied by a periodic increase or decrease of heat. Heat transfer mainly occurs between the three phases of the gas–solid tower wall, so the heat balance of the adsorption layer can be divided into gas phase energy balance, solid phase energy balance, and tower wall balance. The gas phase heat transfer equation is mainly composed of heat transfer, convection heat transfer, heat storage, compression heat, aerodynamic heat transfer, and gas wall heat transfer [6]. The solid phase heat transfer equation mainly includes heat transfer, thermal regeneration, enthalpy recovery of adsorption phase, thermal adsorption, and solidification of two kinds of heat transfer. The heat balance equation of the tower wall mainly consists of axial conductor heat element, tower wall regeneration, gas wall two heat elements, and tower wall environment two conduction heat elements. Formulas (5)–(7) give the energy balance equation corresponding to the gas phase, solidification phase, and column wall of the adsorption layer, respectively,
3.2.3 Momentum transfer equilibrium equation
The variable pressure adsorption process is a selective adsorption separation and purification of gases through periodic changes in the adsorbent bed pressure, and the scale of pressure change is fundamentally determined by the apparent gas velocity, which increases as the bed pressure drop rises and vice versa. The gas velocity determines the total and internal pressure gradient in the adsorbent bed, and this relationship between gas velocity and pressure gradient can be described by the momentum balance equation. The Ergun equation combines the laminar pressure drop description form of the Karman–Kozeny equation and the turbulent pressure drop description form of the Burke–Plummer equation, which is not only applicable to the gas flow condition of piston flow, but also applicable to turbulent and vortex flow, and is most widely used at present
3.2.4 Adsorption equilibrium equation
The phenomenon of adsorption describes the tendency of gas or liquid molecules in the surrounding fluid phase to diffuse toward the solid surface under physical or chemical bonding forces, and the realization of the most important industrial applications of adsorption separation depends on the difference in the adsorption affinity of the solid surface of the adsorbent for different adsorbent components. The adsorption equilibrium isotherm can be used to describe the tendency of adsorbates to be adsorbed on the solid surface and thus to determine the adsorption capacity of each adsorbate component at thermodynamic equilibrium as a non-linear function of the adsorption capacity with respect to partial pressure or concentration variables. The adsorption equilibrium isotherm is an important parameter for adsorption bed design, and the establishment of an accurate adsorption equilibrium isotherm model can not only predict the multi-component adsorption performance and the feasibility of equilibrium adsorption separation under different conditions, but also guide the design of the equilibrium adsorption separation process. The extended-Langmuir model has a high predictive stability for most gases and can better describe the adsorption equilibrium relationship of multi-component gases; therefore, the extended-Langmuir model shown in Eqs. (12)–(14) is used in this article. Therefore, the extended-Langmuir model is shown as follows:
3.2.5 Adsorption rate equation
The adsorption equilibrium isotherm model is commonly used to describe the static adsorption equilibrium on the surface of a solid adsorbent in the PSA process. However, the actual adsorption process is a dynamic transport phenomenon that involves multiple complex mechanisms, including axial diffusion and radial diffusion within the micropores of the adsorbent. To characterize the mass transfer resistance in this dynamic adsorption process, a kinetic adsorption model is employed. The mass exchange rate is predominantly determined by the mass resistance, which arises from various factors, such as the diffusion resistance in the outer membrane, diffusion resistance within the micropores, and surface adsorption resistance. These factors contribute to the transport of adsorbate molecules to the adsorbent’s porosity.
In the case of a general PSA process, the external diffusion and surface adsorption within the gas film typically reach equilibrium in a relatively short time. However, the diffusion within the micropores plays a crucial role in regulating the adsorption rate over time. The challenge lies in establishing an accurate model for the adsorption rate that accounts for the diffusion resistance within the micropores. This is especially difficult due to the complex and heterogeneous porosity structure of the adsorbent particles, which varies across different materials.
In practice, a common approach is to approximate the diffusion coefficient of the adsorbate molecules within the adsorbent micropores as a constant. This simplification assumes that the diffusion coefficient does not vary with pressure or concentration, which can be a reasonable assumption for certain systems. As depicted in Scheme 15, the adsorption rate of a gas–solid component (Component I) can be described by a linear relationship with the solid adsorbent concentration. This linear model allows for the calculation of the driving force parameters, which can be derived from Eqs. (16) and (18), as shown in the following formulations.
To enhance the accuracy of the model, several factors should be considered:
Micropore diffusion: Although the assumption of a constant diffusion coefficient simplifies the model, more advanced approaches may consider the pressure and concentration dependence of the diffusion coefficient. The effect of temperature on the diffusion rate should also be accounted for, as it can significantly influence the adsorption kinetics.
Non-equilibrium adsorption: The transient nature of adsorption, especially during the initial and final stages of the cycle, should be captured in the kinetic model. While the equilibrium state can be approximated by static isotherms, the dynamic process requires a more detailed analysis of the rate of adsorption and desorption, taking into account the changing conditions during each pressure swing.
Effective diffusion coefficients: In many cases, the effective diffusion coefficient might not be a constant, but rather a function of the adsorbent’s structure, porosity, and operating conditions. Therefore, a more refined approach could involve determining the effective diffusion coefficients through empirical measurements or advanced simulations based on the pore structure.
Rate-controlling steps: It is important to identify the rate-controlling steps in the adsorption process. In many PSA systems, the overall mass transfer resistance can be dominated by either the external diffusion of the gas phase or the intraparticle diffusion within the micropores. The contribution of each resistance mechanism should be quantified for a more accurate prediction of performance.
By incorporating these factors into the kinetic adsorption model, we can better describe the dynamic processes involved in PSA operations and optimize the system for improved separation efficiency and economic performance. Additionally, this more comprehensive model can be useful for simulating and scaling up PSA processes in real-world applications
3.2.6 Ideal gas equation of state
In the practice of industrial applications of PSA separation, the operating pressure is generally <1.5 MPa, and the gas–solid adsorption behavior is a reversible physical adsorption process, and the actual fluid can be considered an ideal gas, which is consistent with the assumption
4 Simulation
4.1 PSA simulation
Since the samples could not be synthesized in large quantities and the self-built PSA equipment could not be used for the experiments, this part of the study used a pure simulation method to simulate and scale up the experiments. The simulated adsorption column was designed to have dimensions of 40 mm in diameter and 250 mm in height for the PSA experiments. For the penetration experiments, a particle size ratio was applied, and the assumed particle size for the simulation was 18 mm. The rest of the parameters, such as flow rates, pressure conditions, temperature, and adsorption isotherms, were based on data from the penetration experiments, as shown in Table 1.
Fixed-bed physical parameters
| Parameter | Value |
|---|---|
|
|
0.75 |
|
|
0.50 |
|
|
890 |
|
|
570 |
|
|
1.81 |
|
|
1 |
|
|
0.05 |
|
|
0.30 |
|
|
3.06 |
|
|
3.12 |
|
|
4.35 × 10−10 |
|
|
2.80 × 10−10 |
|
|
40 |
|
|
250 |
To ensure the accuracy of the simulation, the following assumptions and simplifications were made:
The adsorption column was modeled as a packed bed, with the adsorbent particles uniformly distributed in the column.
The gas flow was assumed to be laminar, and the effect of turbulence was neglected due to the relatively low flow rates used in the simulation.
The adsorption process was assumed to follow the Langmuir isotherm, which is commonly used to describe adsorption behavior in PSA systems.
Additionally, the simulation software employed allowed for the adjustment of key parameters, such as cycle time, pressure swing, and flow rate, to optimize the PSA performance for maximum CO2 removal and CH4 recovery. The simulations were run under different operating conditions to evaluate the impact of each parameter on the system’s efficiency.
The results from the simulation provided valuable insights into the performance of the PSA system, including CO2 removal rates, CH4 recovery efficiency, and pressure drop across the column. The findings were used to guide the design and optimization of PSA parameters in future experimental setups and scale-up efforts.
The two-bed six-step PSA experiment was simulated for the SIM-l (ZIF-94) sample, and the ZIF-8 sample was simulated for comparison. The simulation results are shown in Figure 4, which showed that the separation of the CH4N2 mixture with CH4 concentration of 20 and 30% by volume at different feed flow rates and adsorption durations for SIM-1 (ZIF-94) samples all showed excellent separation results, and could increase the CH4 concentration from 20 and 30% to 50 and 61% in one step while ensuring a certain recovery rate. The adsorption conditions were reset, and the enrichment effect was still unsatisfactory when the CH4 gas could be retained on the fixed bed.
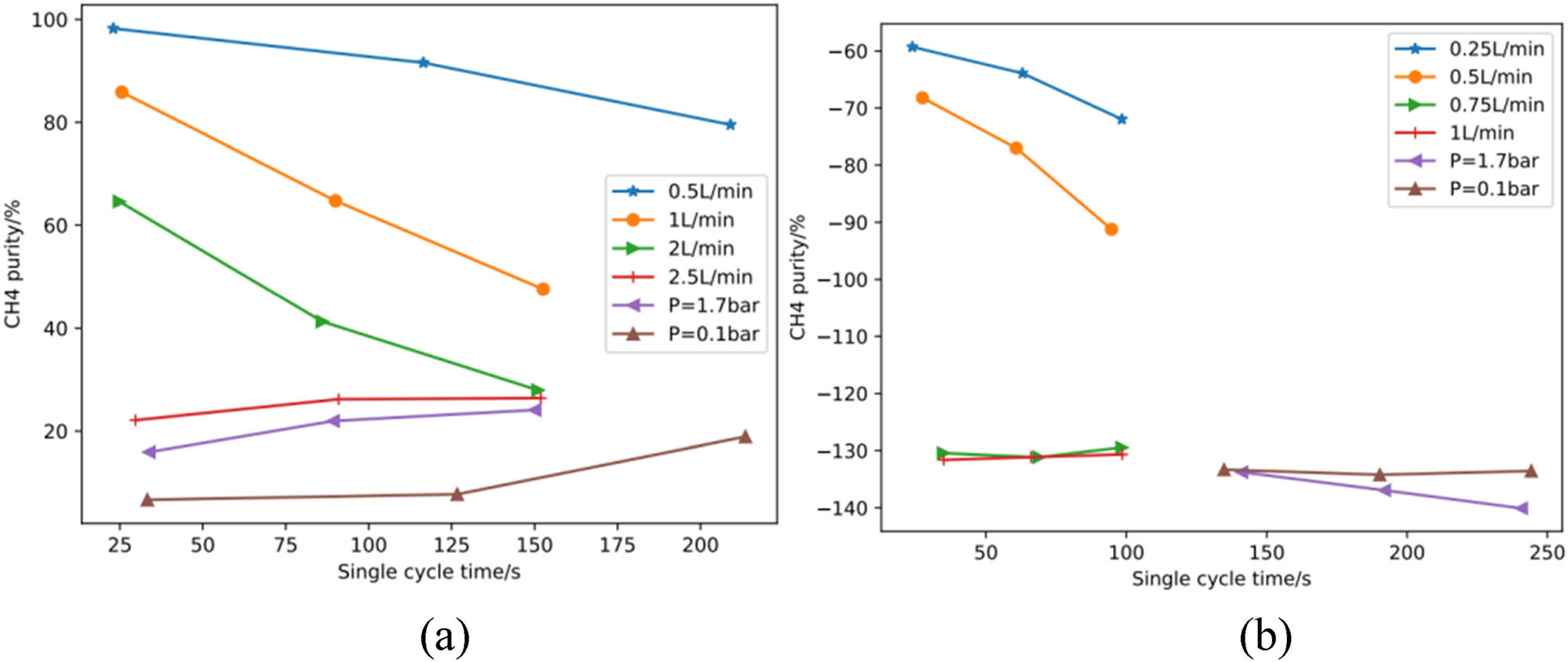
PSA simulation results of (a) SIM-l and (b) ZIF-8 at different feed concentrations.
4.2 Optimized process simulation
Through the optimization simulation of flow, flow extrusion, and venting pressure parameters, a set of operating parameters is optimized to enhance the overall efficiency of the PSA process. Subsequently, a series of optimal indicators regarding process separation and economic benefits are obtained through the simulation of circulation and stability. The simulation process evaluates key aspects such as pressure distribution, temperature variation, and suction concentration within the adsorption layer during the cyclic stability mode. These factors are crucial for understanding the dynamics of the adsorption process and can be quantified to assess the economic separability of the process.
By analyzing the pressure distribution, temperature, and suction concentration across different stages of the cycle, we can identify the most cost-effective operating conditions and predict the potential benefits in terms of both CO2 removal and CH4 recovery efficiency. The results of the cyclic stability simulation allow for the fine-tuning of cycle parameters, such as pressure swing time, adsorption and desorption durations, and flow rates, ensuring that the PSA system operates within an optimal performance range.
The economic separability of the process is quantified by calculating the energy consumption per unit of CO2 captured and CH4 recovered, as well as the overall operational cost per cycle. This information serves as a valuable guide for further optimizing the process, improving separation performance, and reducing operational costs. Moreover, the simulation findings provide a solid foundation for the scaling-up of the PSA process in industrial applications, where large-scale economic benefits can be achieved.
In conclusion, the optimization of operating parameters through simulation not only enhances the separation performance of the PSA system but also maximizes its economic benefits, offering valuable insights for both future research and practical industrial applications in the shale gas industry.
4.2.1 Definition of cyclic steady state
For any two adjacent cycles of the VPCS, a VPCS is considered to have reached a cyclic steady state if the absolute errors of some cyclic control variables such as concentration, pressure, temperature, purity, recovery, yield, and energy consumption are within the specified cyclic iteration control accuracy within a certain cycle iteration. In this article, the number of iterations required for the process system is determined by monitoring the cyclic changes of the four performance index factors of the separation process and then obtaining the process separation performance results under the cyclic steady state cycle as well as studying the convergence speed and stability of the model calculation.
Figure 5 shows the four performance indicators optimized according to the operating parameters in the PSA vacuum separation process. These parameters change with the periodicity of the process cycle. It can be seen that in the tenth cycle, the process system can achieve stable convergence, and a stable cycle with low error can be achieved in 50 iterations, which proves that the numerical model adopted in this article has a high convergence rate and stability. As shown in Table 2, the material accounting and separation indicators of the whole PSA vacuum process under stable cycle conditions. The table shows that the optimal economic separation performance of the three-tower, six-step PSA process for upgrading low concentration oxygenated CBM (30% CH4/60% N2/10% O2) is 80.142% CH4 product purity, 90.271% CH4 product recovery,
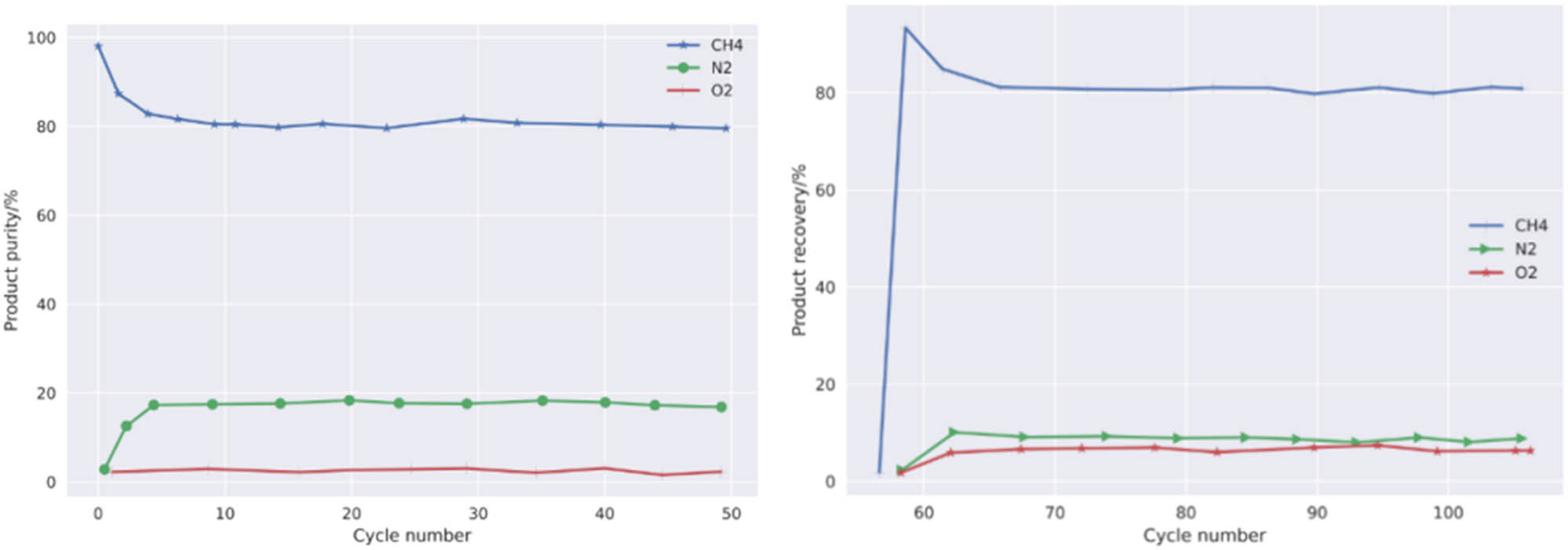
Variation curves of product purity and recovery with different cycle times.
Optimization results of the separation performance of the vacuum variable pressure adsorption process under the steady state of cycling
| Material stream | Flowrate (Nm3 h−1) | CH4 purity (%) | CH4 recovery (%) | Productivity (mol kg−1 h−1) | Energy consumption (kW h Nm−3) |
|---|---|---|---|---|---|
| Feed | 1.000 | 30.000 | 100.000 | — | 0.152 |
| Exhaust | 0.662 | 4.517 | 9.985 | 0.831 | — |
| AD_offgas | 0.489 | 4.552 | 7.402 | 0.618 | — |
| RP_offgas | 0.262 | 4.422 | 2.582 | 0.323 | — |
| Product | 0.404 | 80.143 | 90.272 | 9.026 | — |
| Evacuation | 0.846 | 80.143 | 188.223 | 18.791 | 0.082 |
| Replacement | 0.552 | 80.143 | 97.952 | 12.208 | 0.048 |
4.2.2 Performance of gas–solid phase concentration distribution under a cyclic steady state
In Figure 6, the adsorption and displacement steps have a large mass transfer area and steep adsorption front at the top of the column, while the adsorption saturation level is high at the bottom of the column, thus ensuring a low CH4 loss at the top exit and a high CH4 recovery purity at the bottom exit of the desorption step. Although the equalization and evacuation steps cause varying degrees of CH4 desorption that blunt the top CH4 adsorption front. In contrast, the top CH4 has a low solid phase loading level in all steps, indicating that the top exit adsorbent layer still has a large CH4 adsorption capacity for effective separation and purification of CH4.
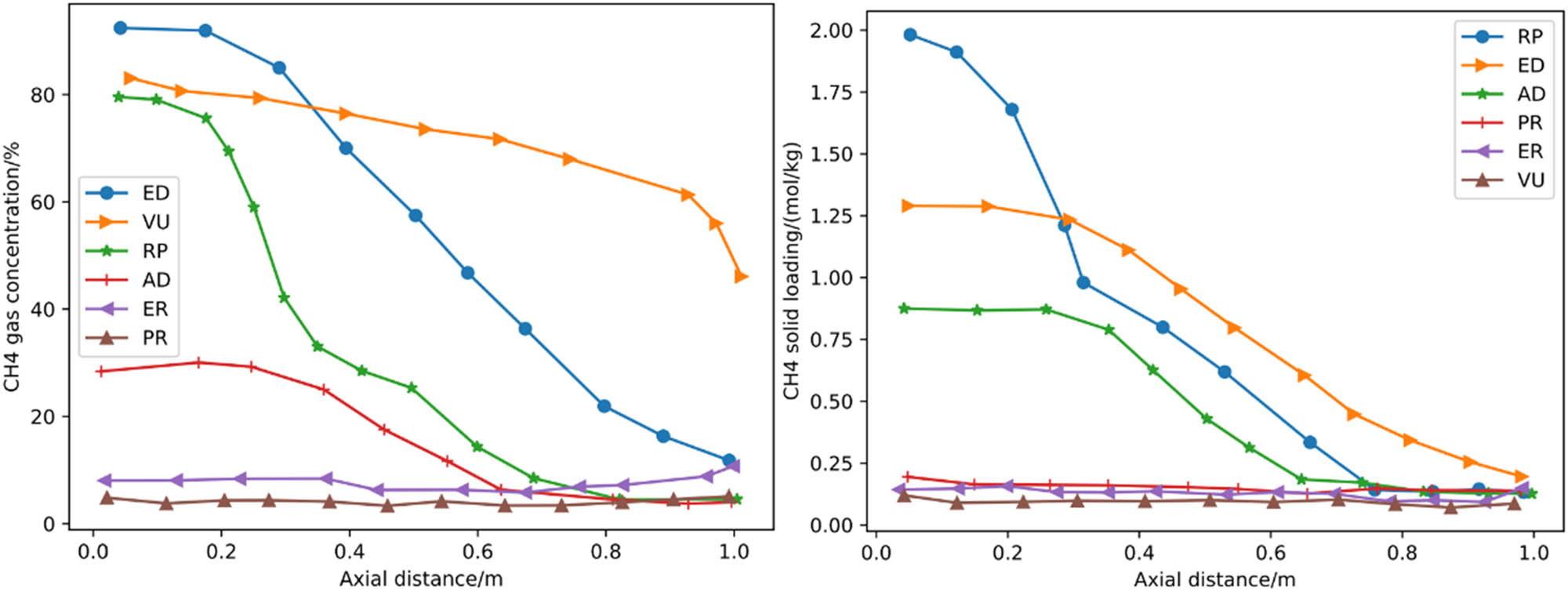
Distribution curve of CH4 gas–solid phase concentration in the cyclic steady state.
4.2.3 Evaluation of deoxygenation and CH4 enrichment effects in different time periods
The CH4 recovery rate and the average concentrations of CH4 and oxygen over a period of time are another important parameter for evaluating the performance of deoxygenation and purification by micro-positive pressure PSA separation technology and for guiding engineering applications. Therefore, in this work, the CH4 recovery rates for different time periods were studied, along with the corresponding average CH4 and average oxygen concentrations, and the results are shown in Table 3. Since the adsorption capacity of fully desorbed adsorbent is stronger at the beginning of the adsorption phase, the gas flow rate through the adsorption tower is smaller and the adsorbent is more selective for oxygen nitrogen than CH4, it is most appropriate to start collecting the product gas from the moment 0 for this work. Also, this maximizes the recovery of CH4 and simplifies the operation. As can be seen in Table 3, the optimal time period for collecting product gas is 0–3 min. At this time, the CH4 concentration can be increased from 5 to 12.3%, and the CH4 recovery rate is 81.9%, while the oxygen concentration can be reduced to 3.23%. In addition, it is obvious that with the delay of 0 moment, the CH4 and oxygen concentrations in the product gas increase and decrease, respectively, and the CH4 recovery increases. Therefore, the CH4 recovery and concentration can be traded off according to the actual demand during product gas collection.
Average concentrations of O2 and CH4 and CH4 recovery rates in product gas at different time periods after enrichment of low concentration oxygenated gas at a feed gas flow rate of 200 mL/min
| Product gas CH4 (%) | Time interval (min) | O2 (%) | CH4 (%) | CH4 recovery rate (%) |
|---|---|---|---|---|
| 1 | 0–1 | 8.66 | 1.57 | 24.5 |
| 0–2 | 7.72 | 2.77 | 47.6 | |
| 0–3 | 5.65 | 2.83 | 79.2 | |
| 0–4 | 6.73 | 1.84 | 86.5 | |
| 0–5 | 8.12 | 7.12 | 88.2 | |
| 3 | 0–1 | 4.73 | 7.13 | 22.7 |
| 0–2 | 3.42 | 7.65 | 54.8 | |
| 0–3 | 3.38 | 6.15 | 76.5 | |
| 0–4 | 4.28 | 5.42 | 83.2 | |
| 0–5 | 7.96 | 5.22 | 91.0 | |
| 5 | 0–1 | 5.95 | 8.99 | 21.6 |
| 0–2 | 5.36 | 12.2 | 40.7 | |
| 0–3 | 3.22 | 10.6 | 81.8 | |
| 0–4 | 4.91 | 8.88 | 89.5 | |
| 0–5 | 8.77 | 10.2 | 89.8 |
5 Conclusion
Shale gas development in Sichuan Province plays a pivotal role in China’s transition toward carbon peaking, offering a cleaner alternative to traditional fossil fuels. However, challenges such as CH4 leakage and CO2 emissions demand the adoption of advanced separation and capture technologies. This study demonstrates that the PSA process is an effective method for improving carbon capture efficiency and reducing the environmental impact of shale gas utilization.
By integrating LCA and real-world data, our analysis reveals that optimized PSA configurations can significantly enhance CO2 removal while maintaining high CH4 recovery. The findings suggest that incorporating PSA into Sichuan’s shale gas industry can accelerate carbon reduction efforts, contributing substantially to China’s carbon neutrality goals. Furthermore, this research provides valuable insights for policymakers and industry stakeholders to develop sustainable strategies for shale gas extraction, processing, and utilization.
Future studies should explore hybrid carbon capture technologies and assess their long-term economic and environmental impacts. Additionally, integrating digital optimization techniques and machine learning into PSA operations could further enhance efficiency, ensuring a more sustainable and economically viable shale gas industry.
-
Funding information: The author states no funding is involved.
-
Author contributions: The author has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interest: The author states no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Carpenter C. Method measures effective scale-inhibitor concentration at low detection limit. J Pet Technol. 2022;74(9):88–90.10.2118/0922-0088-JPTSuche in Google Scholar
[2] Shi Y, Lin B, Liu T, Zhao Y, Hao Z. Synergistic ECBM extraction technology and engineering application based on hydraulic flushing combing gas injection displacement in low permeability coal seams. Fuel. 2022;318:123688.10.1016/j.fuel.2022.123688Suche in Google Scholar
[3] Song X, Gong J, Zeng Y, Zhan X, Wang L. Adsorption and separation of carbon dioxide and methane on carbonaceous adsorbents. Mater Sci Eng Technol. 2021;52(11):1267–80.10.1002/mawe.202100119Suche in Google Scholar
[4] Xiao L, Chen J. Experimental study on heat transfer caused by feed gas concentration fluctuation in low concentration cbm utilization unit. Heat Mass Transf. 2021;58(2):1–9.10.1007/s00231-021-03114-wSuche in Google Scholar
[5] Guo P. A theoretical model for coal swelling induced by gas adsorption in the full pressure range. Adsorpt Sci Technol. 2020;38(3–4):94–112.10.1177/0263617420907730Suche in Google Scholar
[6] Zhang Y, Che J, Yu C, Wang H, Du M. The failure behavior of buckling pin valve: theoretical, numerical, and experimental study. Proc Inst Mech Eng, Part C: J Mech Eng Sci. 2022;236(10):5556–66.10.1177/09544062211061643Suche in Google Scholar
[7] Zhao P, Zhuo R, Li S, Shu CM, Xiao P. Fractal characteristics of methane migration channels in inclined coal seams. Energy. 2021;225:120127.10.1016/j.energy.2021.120127Suche in Google Scholar
[8] Zhu T, Wang R, Zhang X, Han Y, Xue M. Enrichment and separation of methane gas by vacuum pressure swing adsorption. Adsorpt Sci Technol. 2021;2021(11):1–12.10.1155/2021/5572698Suche in Google Scholar
[9] Zhang C, Shan G, Roh BH. Fair federated learning for multi-task 6G NWDAF network anomaly detection. In IEEE Transactions on Intelligent Transportation Systems; 2024. 10.1109/TITS.2024.3461679.Suche in Google Scholar
[10] Wang X, Cheng Y, Zhang D, Yang H, Zhou X, Jiang Z. Experimental study on methane adsorption and time-dependent dynamic diffusion coefficient of intact and tectonic coals: implications for co2-enhanced shale gas methane projects. Process Saf Environ Prot. 2021;156:568–80.10.1016/j.psep.2021.10.030Suche in Google Scholar
[11] Zhu C, Liu S, Chen X, Wu H, Lin B, Liu T, et al. High-pressure water and gas alternating sequestration technology for low permeability coal seams with high adsorption capacity. J Nat Gas Sci Eng. 2021;96:104262.10.1016/j.jngse.2021.104262Suche in Google Scholar
[12] Wang K, Ren H, Wang Z, Wei J. Temperature-pressure coupling effect on gas desorption characteristics in coal during low-variable temperature process. J Pet Sci Eng. 2022;211:110104.10.1016/j.petrol.2022.110104Suche in Google Scholar
[13] Wang K, Ren H, Wang Z, Ma S, Wei J, Ke W, et al. Kinetic characteristics of ch4 adsorption on coals under variable temperature–pressure coupling interaction. Nat Resour Res. 2021;30(6):4597–620.10.1007/s11053-021-09965-8Suche in Google Scholar
[14] Cao Y, Li B, Xie L, Pan X. Experimental and numerical study on pressure dynamic and venting characteristic of methane-air explosion in the tube with effect of methane concentration and vent burst pressure. Fuel. 2022;316:123311.10.1016/j.fuel.2022.123311Suche in Google Scholar
[15] Kosowski M, Pach G, Zapletal P. The influence of atmospheric pressure on methane drainage from mines in the upper silesian coal basin. Int J Oil Gas Coal Technol. 2021;1(1):1.10.1504/IJOGCT.2022.122084Suche in Google Scholar
[16] Suo N, Huang H, Wu A, Cao G, Zhang G. Effect of methane concentration on oxygen reduction reaction of carbon films in alkaline solution. Int J Hydrog Energy. 2018;43(39):18194–201.10.1016/j.ijhydene.2018.08.004Suche in Google Scholar
[17] Smith E. Combination of ofceas spectroscopy and low pressure sampling, an advantage for low concentration measurements for cems. Int Environ Technol. 2022;32(1):232–53.Suche in Google Scholar
[18] Gasinski A, Kawa-Rygielska J, Mikulski D, Klosowski G, Glowacki A. Application of white grape pomace in the brewing technology and its impact on the concentration of esters and alcohols, physicochemical parameteres and antioxidative properties of the beer. Food Chem. 2022;367:130646.10.1016/j.foodchem.2021.130646Suche in Google Scholar PubMed
[19] Malakhova TV, Murashova AI. Methane fluid emission from the bottom sediments of the chernaya river estuary, sevastopol region, crimea. Geochem Int. 2022;60(9):869–76.10.1134/S0016702922080043Suche in Google Scholar
[20] Liu C, Li Y, Liu S, Zhou Y, Liu D, Fu C, et al. Efficient extraction of UO22+ from seawater by polyethylenimine functionalized activated carbon (pei-ac): adsorption performance and mechanism. J Radioanal Nucl Chem. 2022;331(11):1–14.10.1007/s10967-022-08523-7Suche in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Generalized (ψ,φ)-contraction to investigate Volterra integral inclusions and fractal fractional PDEs in super-metric space with numerical experiments
- Solitons in ultrasound imaging: Exploring applications and enhancements via the Westervelt equation
- Stochastic improved Simpson for solving nonlinear fractional-order systems using product integration rules
- Exploring dynamical features like bifurcation assessment, sensitivity visualization, and solitary wave solutions of the integrable Akbota equation
- Research on surface defect detection method and optimization of paper-plastic composite bag based on improved combined segmentation algorithm
- Impact the sulphur content in Iraqi crude oil on the mechanical properties and corrosion behaviour of carbon steel in various types of API 5L pipelines and ASTM 106 grade B
- Unravelling quiescent optical solitons: An exploration of the complex Ginzburg–Landau equation with nonlinear chromatic dispersion and self-phase modulation
- Perturbation-iteration approach for fractional-order logistic differential equations
- Variational formulations for the Euler and Navier–Stokes systems in fluid mechanics and related models
- Rotor response to unbalanced load and system performance considering variable bearing profile
- DeepFowl: Disease prediction from chicken excreta images using deep learning
- Channel flow of Ellis fluid due to cilia motion
- A case study of fractional-order varicella virus model to nonlinear dynamics strategy for control and prevalence
- Multi-point estimation weldment recognition and estimation of pose with data-driven robotics design
- Analysis of Hall current and nonuniform heating effects on magneto-convection between vertically aligned plates under the influence of electric and magnetic fields
- A comparative study on residual power series method and differential transform method through the time-fractional telegraph equation
- Insights from the nonlinear Schrödinger–Hirota equation with chromatic dispersion: Dynamics in fiber–optic communication
- Mathematical analysis of Jeffrey ferrofluid on stretching surface with the Darcy–Forchheimer model
- Exploring the interaction between lump, stripe and double-stripe, and periodic wave solutions of the Konopelchenko–Dubrovsky–Kaup–Kupershmidt system
- Computational investigation of tuberculosis and HIV/AIDS co-infection in fuzzy environment
- Signature verification by geometry and image processing
- Theoretical and numerical approach for quantifying sensitivity to system parameters of nonlinear systems
- Chaotic behaviors, stability, and solitary wave propagations of M-fractional LWE equation in magneto-electro-elastic circular rod
- Dynamic analysis and optimization of syphilis spread: Simulations, integrating treatment and public health interventions
- Visco-thermoelastic rectangular plate under uniform loading: A study of deflection
- Threshold dynamics and optimal control of an epidemiological smoking model
- Numerical computational model for an unsteady hybrid nanofluid flow in a porous medium past an MHD rotating sheet
- Regression prediction model of fabric brightness based on light and shadow reconstruction of layered images
- Dynamics and prevention of gemini virus infection in red chili crops studied with generalized fractional operator: Analysis and modeling
- Qualitative analysis on existence and stability of nonlinear fractional dynamic equations on time scales
- Fractional-order super-twisting sliding mode active disturbance rejection control for electro-hydraulic position servo systems
- Analytical exploration and parametric insights into optical solitons in magneto-optic waveguides: Advances in nonlinear dynamics for applied sciences
- Review Article
- Haar wavelet collocation method for existence and numerical solutions of fourth-order integro-differential equations with bounded coefficients
- Special Issue: Nonlinear Analysis and Design of Communication Networks for IoT Applications - Part II
- Silicon-based all-optical wavelength converter for on-chip optical interconnection
- Research on a path-tracking control system of unmanned rollers based on an optimization algorithm and real-time feedback
- Analysis of the sports action recognition model based on the LSTM recurrent neural network
- Industrial robot trajectory error compensation based on enhanced transfer convolutional neural networks
- Research on IoT network performance prediction model of power grid warehouse based on nonlinear GA-BP neural network
- Interactive recommendation of social network communication between cities based on GNN and user preferences
- Application of improved P-BEM in time varying channel prediction in 5G high-speed mobile communication system
- Construction of a BIM smart building collaborative design model combining the Internet of Things
- Optimizing malicious website prediction: An advanced XGBoost-based machine learning model
- Economic operation analysis of the power grid combining communication network and distributed optimization algorithm
- Sports video temporal action detection technology based on an improved MSST algorithm
- Internet of things data security and privacy protection based on improved federated learning
- Enterprise power emission reduction technology based on the LSTM–SVM model
- Construction of multi-style face models based on artistic image generation algorithms
- Research and application of interactive digital twin monitoring system for photovoltaic power station based on global perception
- Special Issue: Decision and Control in Nonlinear Systems - Part II
- Animation video frame prediction based on ConvGRU fine-grained synthesis flow
- Application of GGNN inference propagation model for martial art intensity evaluation
- Benefit evaluation of building energy-saving renovation projects based on BWM weighting method
- Deep neural network application in real-time economic dispatch and frequency control of microgrids
- Real-time force/position control of soft growing robots: A data-driven model predictive approach
- Mechanical product design and manufacturing system based on CNN and server optimization algorithm
- Application of finite element analysis in the formal analysis of ancient architectural plaque section
- Research on territorial spatial planning based on data mining and geographic information visualization
- Fault diagnosis of agricultural sprinkler irrigation machinery equipment based on machine vision
- Closure technology of large span steel truss arch bridge with temporarily fixed edge supports
- Intelligent accounting question-answering robot based on a large language model and knowledge graph
- Analysis of manufacturing and retailer blockchain decision based on resource recyclability
- Flexible manufacturing workshop mechanical processing and product scheduling algorithm based on MES
- Exploration of indoor environment perception and design model based on virtual reality technology
- Tennis automatic ball-picking robot based on image object detection and positioning technology
- A new CNN deep learning model for computer-intelligent color matching
- Design of AR-based general computer technology experiment demonstration platform
- Indoor environment monitoring method based on the fusion of audio recognition and video patrol features
- Health condition prediction method of the computer numerical control machine tool parts by ensembling digital twins and improved LSTM networks
- Establishment of a green degree evaluation model for wall materials based on lifecycle
- Quantitative evaluation of college music teaching pronunciation based on nonlinear feature extraction
- Multi-index nonlinear robust virtual synchronous generator control method for microgrid inverters
- Manufacturing engineering production line scheduling management technology integrating availability constraints and heuristic rules
- Analysis of digital intelligent financial audit system based on improved BiLSTM neural network
- Attention community discovery model applied to complex network information analysis
- A neural collaborative filtering recommendation algorithm based on attention mechanism and contrastive learning
- Rehabilitation training method for motor dysfunction based on video stream matching
- Research on façade design for cold-region buildings based on artificial neural networks and parametric modeling techniques
- Intelligent implementation of muscle strain identification algorithm in Mi health exercise induced waist muscle strain
- Optimization design of urban rainwater and flood drainage system based on SWMM
- Improved GA for construction progress and cost management in construction projects
- Evaluation and prediction of SVM parameters in engineering cost based on random forest hybrid optimization
- Museum intelligent warning system based on wireless data module
- Optimization design and research of mechatronics based on torque motor control algorithm
- Special Issue: Nonlinear Engineering’s significance in Materials Science
- Experimental research on the degradation of chemical industrial wastewater by combined hydrodynamic cavitation based on nonlinear dynamic model
- Study on low-cycle fatigue life of nickel-based superalloy GH4586 at various temperatures
- Some results of solutions to neutral stochastic functional operator-differential equations
- Ultrasonic cavitation did not occur in high-pressure CO2 liquid
- Research on the performance of a novel type of cemented filler material for coal mine opening and filling
- Testing of recycled fine aggregate concrete’s mechanical properties using recycled fine aggregate concrete and research on technology for highway construction
- A modified fuzzy TOPSIS approach for the condition assessment of existing bridges
- Nonlinear structural and vibration analysis of straddle monorail pantograph under random excitations
- Achieving high efficiency and stability in blue OLEDs: Role of wide-gap hosts and emitter interactions
- Construction of teaching quality evaluation model of online dance teaching course based on improved PSO-BPNN
- Enhanced electrical conductivity and electromagnetic shielding properties of multi-component polymer/graphite nanocomposites prepared by solid-state shear milling
- Optimization of thermal characteristics of buried composite phase-change energy storage walls based on nonlinear engineering methods
- A higher-performance big data-based movie recommendation system
- Nonlinear impact of minimum wage on labor employment in China
- Nonlinear comprehensive evaluation method based on information entropy and discrimination optimization
- Application of numerical calculation methods in stability analysis of pile foundation under complex foundation conditions
- Research on the contribution of shale gas development and utilization in Sichuan Province to carbon peak based on the PSA process
- Characteristics of tight oil reservoirs and their impact on seepage flow from a nonlinear engineering perspective
- Nonlinear deformation decomposition and mode identification of plane structures via orthogonal theory
- Numerical simulation of damage mechanism in rock with cracks impacted by self-excited pulsed jet based on SPH-FEM coupling method: The perspective of nonlinear engineering and materials science
- Cross-scale modeling and collaborative optimization of ethanol-catalyzed coupling to produce C4 olefins: Nonlinear modeling and collaborative optimization strategies
- Unequal width T-node stress concentration factor analysis of stiffened rectangular steel pipe concrete
- Special Issue: Advances in Nonlinear Dynamics and Control
- Development of a cognitive blood glucose–insulin control strategy design for a nonlinear diabetic patient model
- Big data-based optimized model of building design in the context of rural revitalization
- Multi-UAV assisted air-to-ground data collection for ground sensors with unknown positions
- Design of urban and rural elderly care public areas integrating person-environment fit theory
- Application of lossless signal transmission technology in piano timbre recognition
- Application of improved GA in optimizing rural tourism routes
- Architectural animation generation system based on AL-GAN algorithm
- Advanced sentiment analysis in online shopping: Implementing LSTM models analyzing E-commerce user sentiments
- Intelligent recommendation algorithm for piano tracks based on the CNN model
- Visualization of large-scale user association feature data based on a nonlinear dimensionality reduction method
- Low-carbon economic optimization of microgrid clusters based on an energy interaction operation strategy
- Optimization effect of video data extraction and search based on Faster-RCNN hybrid model on intelligent information systems
- Construction of image segmentation system combining TC and swarm intelligence algorithm
- Particle swarm optimization and fuzzy C-means clustering algorithm for the adhesive layer defect detection
- Optimization of student learning status by instructional intervention decision-making techniques incorporating reinforcement learning
- Fuzzy model-based stabilization control and state estimation of nonlinear systems
- Optimization of distribution network scheduling based on BA and photovoltaic uncertainty
- Tai Chi movement segmentation and recognition on the grounds of multi-sensor data fusion and the DBSCAN algorithm
- Special Issue: Dynamic Engineering and Control Methods for the Nonlinear Systems - Part III
- Generalized numerical RKM method for solving sixth-order fractional partial differential equations
Artikel in diesem Heft
- Research Articles
- Generalized (ψ,φ)-contraction to investigate Volterra integral inclusions and fractal fractional PDEs in super-metric space with numerical experiments
- Solitons in ultrasound imaging: Exploring applications and enhancements via the Westervelt equation
- Stochastic improved Simpson for solving nonlinear fractional-order systems using product integration rules
- Exploring dynamical features like bifurcation assessment, sensitivity visualization, and solitary wave solutions of the integrable Akbota equation
- Research on surface defect detection method and optimization of paper-plastic composite bag based on improved combined segmentation algorithm
- Impact the sulphur content in Iraqi crude oil on the mechanical properties and corrosion behaviour of carbon steel in various types of API 5L pipelines and ASTM 106 grade B
- Unravelling quiescent optical solitons: An exploration of the complex Ginzburg–Landau equation with nonlinear chromatic dispersion and self-phase modulation
- Perturbation-iteration approach for fractional-order logistic differential equations
- Variational formulations for the Euler and Navier–Stokes systems in fluid mechanics and related models
- Rotor response to unbalanced load and system performance considering variable bearing profile
- DeepFowl: Disease prediction from chicken excreta images using deep learning
- Channel flow of Ellis fluid due to cilia motion
- A case study of fractional-order varicella virus model to nonlinear dynamics strategy for control and prevalence
- Multi-point estimation weldment recognition and estimation of pose with data-driven robotics design
- Analysis of Hall current and nonuniform heating effects on magneto-convection between vertically aligned plates under the influence of electric and magnetic fields
- A comparative study on residual power series method and differential transform method through the time-fractional telegraph equation
- Insights from the nonlinear Schrödinger–Hirota equation with chromatic dispersion: Dynamics in fiber–optic communication
- Mathematical analysis of Jeffrey ferrofluid on stretching surface with the Darcy–Forchheimer model
- Exploring the interaction between lump, stripe and double-stripe, and periodic wave solutions of the Konopelchenko–Dubrovsky–Kaup–Kupershmidt system
- Computational investigation of tuberculosis and HIV/AIDS co-infection in fuzzy environment
- Signature verification by geometry and image processing
- Theoretical and numerical approach for quantifying sensitivity to system parameters of nonlinear systems
- Chaotic behaviors, stability, and solitary wave propagations of M-fractional LWE equation in magneto-electro-elastic circular rod
- Dynamic analysis and optimization of syphilis spread: Simulations, integrating treatment and public health interventions
- Visco-thermoelastic rectangular plate under uniform loading: A study of deflection
- Threshold dynamics and optimal control of an epidemiological smoking model
- Numerical computational model for an unsteady hybrid nanofluid flow in a porous medium past an MHD rotating sheet
- Regression prediction model of fabric brightness based on light and shadow reconstruction of layered images
- Dynamics and prevention of gemini virus infection in red chili crops studied with generalized fractional operator: Analysis and modeling
- Qualitative analysis on existence and stability of nonlinear fractional dynamic equations on time scales
- Fractional-order super-twisting sliding mode active disturbance rejection control for electro-hydraulic position servo systems
- Analytical exploration and parametric insights into optical solitons in magneto-optic waveguides: Advances in nonlinear dynamics for applied sciences
- Review Article
- Haar wavelet collocation method for existence and numerical solutions of fourth-order integro-differential equations with bounded coefficients
- Special Issue: Nonlinear Analysis and Design of Communication Networks for IoT Applications - Part II
- Silicon-based all-optical wavelength converter for on-chip optical interconnection
- Research on a path-tracking control system of unmanned rollers based on an optimization algorithm and real-time feedback
- Analysis of the sports action recognition model based on the LSTM recurrent neural network
- Industrial robot trajectory error compensation based on enhanced transfer convolutional neural networks
- Research on IoT network performance prediction model of power grid warehouse based on nonlinear GA-BP neural network
- Interactive recommendation of social network communication between cities based on GNN and user preferences
- Application of improved P-BEM in time varying channel prediction in 5G high-speed mobile communication system
- Construction of a BIM smart building collaborative design model combining the Internet of Things
- Optimizing malicious website prediction: An advanced XGBoost-based machine learning model
- Economic operation analysis of the power grid combining communication network and distributed optimization algorithm
- Sports video temporal action detection technology based on an improved MSST algorithm
- Internet of things data security and privacy protection based on improved federated learning
- Enterprise power emission reduction technology based on the LSTM–SVM model
- Construction of multi-style face models based on artistic image generation algorithms
- Research and application of interactive digital twin monitoring system for photovoltaic power station based on global perception
- Special Issue: Decision and Control in Nonlinear Systems - Part II
- Animation video frame prediction based on ConvGRU fine-grained synthesis flow
- Application of GGNN inference propagation model for martial art intensity evaluation
- Benefit evaluation of building energy-saving renovation projects based on BWM weighting method
- Deep neural network application in real-time economic dispatch and frequency control of microgrids
- Real-time force/position control of soft growing robots: A data-driven model predictive approach
- Mechanical product design and manufacturing system based on CNN and server optimization algorithm
- Application of finite element analysis in the formal analysis of ancient architectural plaque section
- Research on territorial spatial planning based on data mining and geographic information visualization
- Fault diagnosis of agricultural sprinkler irrigation machinery equipment based on machine vision
- Closure technology of large span steel truss arch bridge with temporarily fixed edge supports
- Intelligent accounting question-answering robot based on a large language model and knowledge graph
- Analysis of manufacturing and retailer blockchain decision based on resource recyclability
- Flexible manufacturing workshop mechanical processing and product scheduling algorithm based on MES
- Exploration of indoor environment perception and design model based on virtual reality technology
- Tennis automatic ball-picking robot based on image object detection and positioning technology
- A new CNN deep learning model for computer-intelligent color matching
- Design of AR-based general computer technology experiment demonstration platform
- Indoor environment monitoring method based on the fusion of audio recognition and video patrol features
- Health condition prediction method of the computer numerical control machine tool parts by ensembling digital twins and improved LSTM networks
- Establishment of a green degree evaluation model for wall materials based on lifecycle
- Quantitative evaluation of college music teaching pronunciation based on nonlinear feature extraction
- Multi-index nonlinear robust virtual synchronous generator control method for microgrid inverters
- Manufacturing engineering production line scheduling management technology integrating availability constraints and heuristic rules
- Analysis of digital intelligent financial audit system based on improved BiLSTM neural network
- Attention community discovery model applied to complex network information analysis
- A neural collaborative filtering recommendation algorithm based on attention mechanism and contrastive learning
- Rehabilitation training method for motor dysfunction based on video stream matching
- Research on façade design for cold-region buildings based on artificial neural networks and parametric modeling techniques
- Intelligent implementation of muscle strain identification algorithm in Mi health exercise induced waist muscle strain
- Optimization design of urban rainwater and flood drainage system based on SWMM
- Improved GA for construction progress and cost management in construction projects
- Evaluation and prediction of SVM parameters in engineering cost based on random forest hybrid optimization
- Museum intelligent warning system based on wireless data module
- Optimization design and research of mechatronics based on torque motor control algorithm
- Special Issue: Nonlinear Engineering’s significance in Materials Science
- Experimental research on the degradation of chemical industrial wastewater by combined hydrodynamic cavitation based on nonlinear dynamic model
- Study on low-cycle fatigue life of nickel-based superalloy GH4586 at various temperatures
- Some results of solutions to neutral stochastic functional operator-differential equations
- Ultrasonic cavitation did not occur in high-pressure CO2 liquid
- Research on the performance of a novel type of cemented filler material for coal mine opening and filling
- Testing of recycled fine aggregate concrete’s mechanical properties using recycled fine aggregate concrete and research on technology for highway construction
- A modified fuzzy TOPSIS approach for the condition assessment of existing bridges
- Nonlinear structural and vibration analysis of straddle monorail pantograph under random excitations
- Achieving high efficiency and stability in blue OLEDs: Role of wide-gap hosts and emitter interactions
- Construction of teaching quality evaluation model of online dance teaching course based on improved PSO-BPNN
- Enhanced electrical conductivity and electromagnetic shielding properties of multi-component polymer/graphite nanocomposites prepared by solid-state shear milling
- Optimization of thermal characteristics of buried composite phase-change energy storage walls based on nonlinear engineering methods
- A higher-performance big data-based movie recommendation system
- Nonlinear impact of minimum wage on labor employment in China
- Nonlinear comprehensive evaluation method based on information entropy and discrimination optimization
- Application of numerical calculation methods in stability analysis of pile foundation under complex foundation conditions
- Research on the contribution of shale gas development and utilization in Sichuan Province to carbon peak based on the PSA process
- Characteristics of tight oil reservoirs and their impact on seepage flow from a nonlinear engineering perspective
- Nonlinear deformation decomposition and mode identification of plane structures via orthogonal theory
- Numerical simulation of damage mechanism in rock with cracks impacted by self-excited pulsed jet based on SPH-FEM coupling method: The perspective of nonlinear engineering and materials science
- Cross-scale modeling and collaborative optimization of ethanol-catalyzed coupling to produce C4 olefins: Nonlinear modeling and collaborative optimization strategies
- Unequal width T-node stress concentration factor analysis of stiffened rectangular steel pipe concrete
- Special Issue: Advances in Nonlinear Dynamics and Control
- Development of a cognitive blood glucose–insulin control strategy design for a nonlinear diabetic patient model
- Big data-based optimized model of building design in the context of rural revitalization
- Multi-UAV assisted air-to-ground data collection for ground sensors with unknown positions
- Design of urban and rural elderly care public areas integrating person-environment fit theory
- Application of lossless signal transmission technology in piano timbre recognition
- Application of improved GA in optimizing rural tourism routes
- Architectural animation generation system based on AL-GAN algorithm
- Advanced sentiment analysis in online shopping: Implementing LSTM models analyzing E-commerce user sentiments
- Intelligent recommendation algorithm for piano tracks based on the CNN model
- Visualization of large-scale user association feature data based on a nonlinear dimensionality reduction method
- Low-carbon economic optimization of microgrid clusters based on an energy interaction operation strategy
- Optimization effect of video data extraction and search based on Faster-RCNN hybrid model on intelligent information systems
- Construction of image segmentation system combining TC and swarm intelligence algorithm
- Particle swarm optimization and fuzzy C-means clustering algorithm for the adhesive layer defect detection
- Optimization of student learning status by instructional intervention decision-making techniques incorporating reinforcement learning
- Fuzzy model-based stabilization control and state estimation of nonlinear systems
- Optimization of distribution network scheduling based on BA and photovoltaic uncertainty
- Tai Chi movement segmentation and recognition on the grounds of multi-sensor data fusion and the DBSCAN algorithm
- Special Issue: Dynamic Engineering and Control Methods for the Nonlinear Systems - Part III
- Generalized numerical RKM method for solving sixth-order fractional partial differential equations

