Abstract
Direct urea fuel cell (DUFC) has attracted many researchers’ attention due to the use of wastewater, for example urine, which contains urea for the fuel. The main factor to improve the electrochemical oxidation performance of urea and further enhance the performances of DUFC is the use of a good anode catalyst. Non-noble metal catalyst, such as nickel, is reported to have a good catalytic activity in alkaline medium towards urea electro-oxidation. Besides optimizing the anode catalyst, the use of supporting electrode which has a large surface area as well as the use of H2O2 as an oxidant to replace O2 could help to improve the performances. The recent progress in anode catalysts for DUFC is overviewed in this article. In addition, the advantages and disadvantages as well as the factors that could help to escalate the performance of DUFC are discussed together with the challenges and future perspectives.
1 Introduction
Global energy demands keep increasing every year, while most energy sources are still depending on fossil fuels [1]. Accordingly, other alternatives of the clean, environmentally safe, and low cost energy sources are necessary to be developed [2,3,4,5,6,7]. The use of wastewater as an alternative source of energy has obtained great attentions due to at least two problems, energy demand and wastewater treatment, which can be solved. One of the challenging efforts is the application of urine as a fuel in direct urea fuel cells (DUFCs). Urea, widely found in wastewater, is an organic compound containing carbon, hydrogen, and oxygen. The amount of hydrogen in urea is around 6.67 wt%. Therefore, urea, which is found around 2–2.5 wt% in the wastewater (urine), is classified as an acceptable source for DUFC in alkaline medium [1,8,9,10]. As an energy source, urea has some good characteristics, such as having a high energy density (16.9 MJ L−1) that is ten times higher than hydrogen, safe and acceptable in transportation as well as non-flammable and non-toxic [11,12,13].
On the other hand, fuel cell is a device which electrochemically transforms fuel energy into electricity with high efficiency [14,15,16]. Many types of fuel cells have been reported. Besides proton exchange membrane fuel cell (PEMFC), which is the most popular one [17,18], there are also other types of fuel cells, including solid oxide fuel cell (SOFC) [19,20,21], molten carbonate fuel cell (MCFC) [22,23], alkaline fuel cell [24,25,26], and urea fuel cells. DUFC is an encouraging and effective method for energy production with urea, urine, and wastewater as the fuels [27]. In this system, urea or urine is electrochemically oxidized to produce CO2, N2, and H2O and electricity. Oxygen is necessary to perform the oxidation reactions. The reactions occurring in DUFC are mentioned below [28]:
It is also known that H2O2 can be used as the alternative of O as it can provide a higher electrode potential, around two times higher, than the use of O2 in alkaline medium [29]. Furthermore, the direct urea/H2O2 fuel cell has a more compact design, since urea and H2O2 are in the aqueous forms [30]. The reaction occurred is quite similar with the use of H2O2 at the cathode as the exception, and therefore gives an impact to the overall reactions.
Figure 1 shows the typical schematic diagram of DUFCs. At the cathode catalyst, electrochemical reduction reaction of O2 or H2O2 in the cathode chamber produces OH− ions, which then transport through the anion exchange membrane to reach the anode chamber. In this chamber, the OH− ions react with urea and release electrons, which are later transferred to the cathode over the external circuit to produce electricity [28].
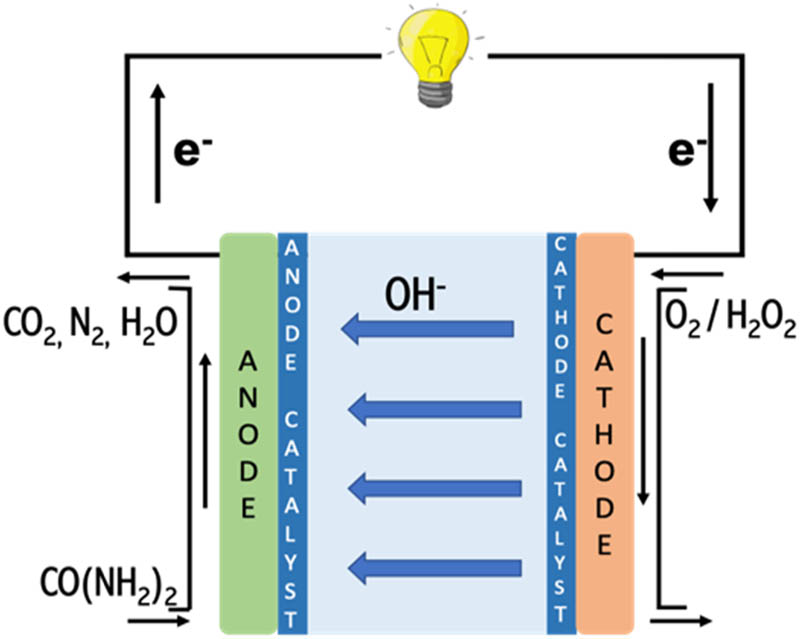
Schematic diagram of DUFC.
Research about the use of urea to generate electricity was initiated in 1973 using Pt as the electrodes for both anode and cathode with an anion exchange membrane to separate the anode and cathode chambers [31]. The investigation concluded that urea could be oxidized into CO2, N2, and H2O, but needs further development. In 2010, Lan et al. examined DUFCs with urea as well as urine and AdBlue (32.5% urea aqueous solution) as the fuels [10]. Comparison between Pt and Ni/C as the anode was performed with Ag/C and MnO2/C used as the cathode. A maximum power density of 1.7 mW cm−2 was obtained at 50°C operation at Ni/C and MnO2/C as the anode and cathode, respectively, with 1 M solution of urea as the fuel. Until now, the development of anode catalyst for DUFCs has been investigated to maximize the power density of DUFCs. Many catalysts, including noble and non-noble metals have been examined to enhance the performance of this type of fuel cell.
This report summarized about recent development in DUFC, particularly the use of supporting anode catalysts and their influences on the DUFC performances. The explanation on advantages and disadvantages as well as the strategy to fabricate better catalysts were also elaborated. In addition, the influences of the external factors as well as the challenges and future prospects are also investigated to maximize the performances.
2 Mechanism of urea electro-oxidation
Urea could be oxidized either in alkaline, neutral, or acidic medium with N2 and CO2 as the products. However, there are some differences in the products and intermediate products reaction in different pH supporting electrolytes.
2.1 Neutral medium mechanism
In neutral medium, for example NaCl solution as the electrolyte, Cl− is oxidized into Cl2 which is disproportionated in aqueous solution to form HOCl. HOCl will be reduced back to Cl−, while urea is electro-oxidized according to the following reactions [32]:
H+ is generated in this condition, hence, catalyst which has a resistance against acid is needed as the anode catalyst, such as RuO2 and IrO2 [12,32]. It was reported that urea was successfully oxidized to CO2 and N2 by using RuO2–TiO2-coated titanium electrode [32].
2.2 Alkaline medium mechanism
Under alkaline medium, urea is electro-oxidized at a lower cost by using Ni as the catalyst [33,34]. Moreover, it is reported that the use of Ni metal, nickel hydroxides, or Ni composites as the catalyst resulted in a better performance toward urea electro-oxidation than using other noble metal catalysts, such as Pt, Pt–Ir, etc., [35]. Lower oxidation potentials and higher current densities could be obtained.
Suarez et al. [36] proposed that the active sites of urea’s dissociation process are Ni and hydroxide groups. The urea molecule is bound with two Ni sites, one Ni binds to the O of urea, while the other is linked to one of the amine groups. The bridge formed by the hydroxide group has been studied to be involved in this reaction to devote a proton to the amine group that connected to the Ni site.
Ni is an active element. In humid air, Ni is easily oxidized into NiO and Ni(OH)2 will be further oxidized to NiOOH during the cyclic voltammetry treatment in alkaline medium [37,38]. NiOOH is known as the active catalyst for electrochemical oxidation of urea. The oxidation process occurred according to the following reactions:
Ni(OH)2 is generally formed in two crystallographic species, α-Ni(OH)2 (Figure 2a) and β-Ni(OH)2 (Figure 2b). The form of β-Ni(OH)2 is more stable due to its Ni octahedral structure coordination with eight O atoms. Meanwhile, the α-Ni(OH)2 form is less stable due to the presence of water molecules intercalated between the NiO2 layers. β-Ni(OH)2 could be formed by potential cycling in alkaline medium and by increasing the potential [39], whereas at the higher potential, γ-Ni(OH)2 is formed and can be reduced back to β-Ni(OH)2 in the reverse scan [40,41].
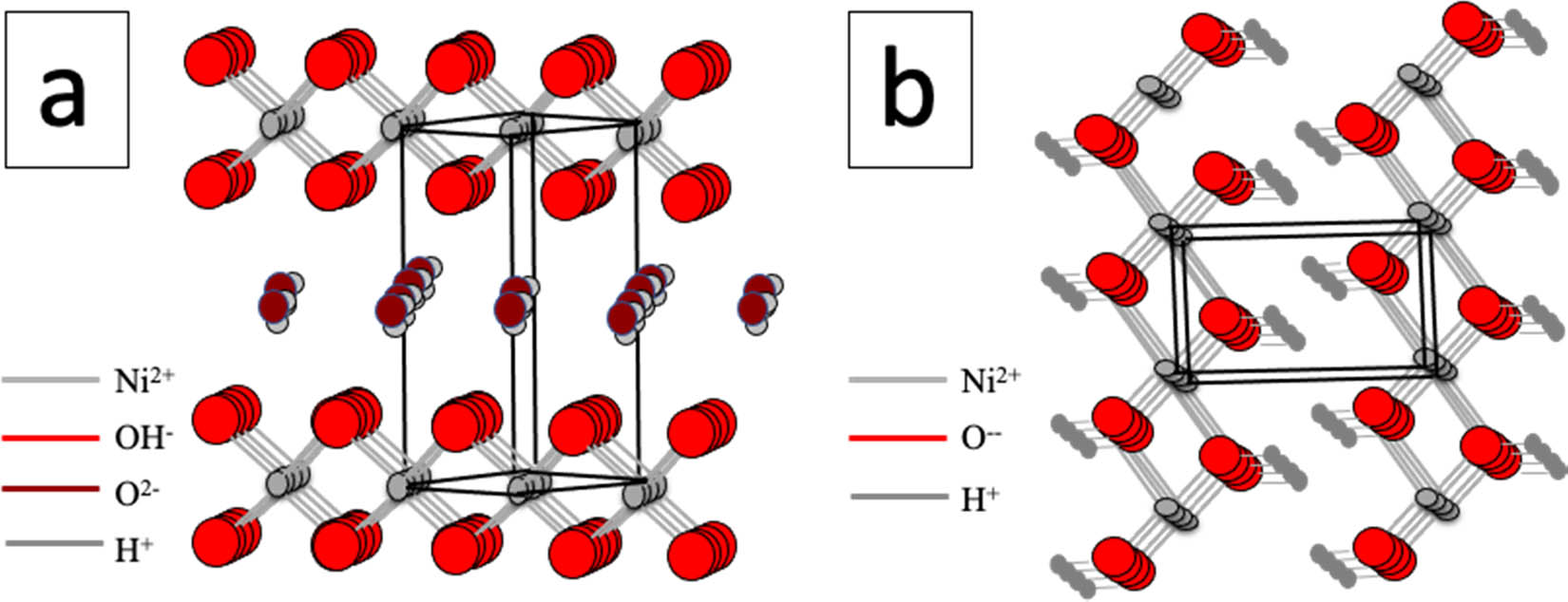
The structure of α-Ni(OH)2 (a) and β-Ni(OH)2 (b).
Electro-oxidation of urea by Ni catalyst in alkaline medium is proposed to occur in two possible mechanisms, including:
2.2.1 Indirect mechanism
The common mechanism used to explain the urea electro-oxidation is indirect mechanism with NiOOH as the active catalyst for electro-oxidation of urea. NiOOH formed from the electro-oxidation of Ni(OH)2 oxidizes the urea molecules and is reduced back to Ni(OH)2 according to the following equations [42]:
Overall reaction:
2.2.2 Direct mechanism
In the direct mechanism, urea is oxidized by Ni in the form of NiOOH which is not reduced back to the Ni(OH)2 form. The oxidation process will use OH− and the NiOOH will be reduced at the reverse scan [43]. According to Guo et al., the proposed direct mechanisms are the following reactions [43]:
According to the above reactions, Ni(OH)2 will be initially oxidized to NiOOH. The OH− then deprotonates the amine group in urea. The remaining N will later be desorbed while transferring the electrons to the electrode. The adsorbed CO2 will be discarded by hydroxides [43].
The mechanism of urea decomposition via electro-oxidation was studied by Botte et al. using DFT calculation [44]. The most possible mechanisms were set up by the electrophilic atoms of urea which interacted with the nucleophilic atoms of NiOOH and reversed. NiOOH as the active catalyst is formed by the interaction of Ni atoms with N and O in urea, while the bridging O interacts with the C atom in urea [36].
The electro-oxidation process of urea can also be explained using cyclic voltammetry. Vedharathinam and Botte reported the cyclic voltammogram urea electro-oxidation at Ni electrode in 5 M KOH with and without the presence of urea [1]. The cyclic voltammogram (Figure 3a) shows that in the absence of urea, a redox peak appears in the anodic and cathodic regions at 387 and 260 mV, respectively, implying the redox reaction of Ni2+/Ni3+. When urea is added to the electrolyte solution, an increase in current density is observed at the oxidation potential around 0.35 V (vs Hg/HgO), implying that the urea electro-oxidation has occurred on the surface of Ni electrode. Yan et al. also reported the same phenomena of urea electro-oxidation process in Ni–Co hydroxides electrode as shown in the cyclic voltammograms of Ni–Co hydroxides electrodes in the same condition as the previous one (Figure 3b) [35]. The oxidation peak is observed at the potential of 0.42 V indicating the formation of NiOOH from Ni(OH)2. In the presents of urea, a strong oxidation current starts at 0.40 V was observed. This potential is similar to the potential formation of NiOOH, indicating that NiOOH is the active form of catalyst for the oxidation of urea. Table 1 shows the activity of various anode catalysts toward urea electro-oxidation.
Activity of various anode catalysts toward urea electro-oxidation
| Anode catalysts | Preparation methods | Electrolytes | Onset potential | Anodic peak | Current density, mA cm−2 | Ref. |
|---|---|---|---|---|---|---|
| Single Ni metal catalyst | ||||||
| Ni NPs/Ti rod | Electrodeposition | 0.33 M urea in 5 M KOH | 0.35 V vs Hg/HgO | 0.46 V vs Hg/HgO | ∼90 | [1] |
| Ni@carbon sponge | Electrodeposition | 0.1 M urea in 1 M NaOH | 0.35 V vs Ag/AgCl | 0.52 V vs Ag/AgCl | ∼200 | [50] |
| Ni-decorated graphene | Calcination | 2.0 M urea in 1 M KOH | 0.32 V vs Ag/AgCl | ∼0.8 V vs Ag/AgCl | ∼145 | [51] |
| Ni/rGO | Aqueous-based reduction method | 0.33 M urea in 1 M KOH | 0.486 V vs MMO | 0.65 V vs MMO | 6.18 | [52] |
| Ni Np/commercial carbon paper | Pulsed laser deposition | 0.33 M urea + 1 M KOH | ∼0.425 V vs Hg/HgO | — | ∼4.8 | [53] |
| Ni@GO | Chemical reduction | 0.33 M + 1 M KOH | 0.30 V vs SCE | 0.49 V vs SCE | 17.1 | [54] |
| Ni–WC/C | Sequential impregnation method | 0.33 M urea + 1 M KOH | 0.4 V vs Hg/HgO | ∼0.55 V vs Hg/HgO | 682 mg−1 | [55] |
| Ni–WC/MWCNT | Impregnation | 0.33 M urea in 1 M KOH | ∼0.45 V vs Hg/HgO | ∼0.57 V vs Hg/HgO | 46.6 | [56] |
| CB/ads-Ni | Electrochemical deposition | 0.33 M + 1 M KOH | 0.45 V vs Hg/HgO | — | 13 | [57] |
| Nickel oxides and hydroxides | ||||||
| β-Ni(OH)2-CNTs | Facile hydrothermal reaction | 0.33 M urea + 1 M KOH | 0.32 V vs SCE | ∼0.55 V vs SCE | 98.5 | [58] |
| NiCo LDH/NiCo(OH)2 | Solution methods at room temp. | 0.33 M urea + 5 M KOH | 0.29 V vs Hg/HgO | — | ∼360 | [59] |
| NiO/Gr-200 – carbon electrode | Precipitation step followed by calcination | 0.3 M urea + 0.5 M KOH | 0.36 V vs Ag/AgCl | 0.51 V vs Ag/AgCl | 30.94 | [60] |
| NiO/Graphite | Chemical precipitation | 0.3 M urea + 0.5 M KOH | 0.345 V vs Ag/AgCl | 0.64 V vs Ag/AgCl | 17.63 | [34] |
| NiOx/GC | Electrodeposition | 0.2 M urea + 0.5 M NaOH | ∼0.375 V vs SCE | 0.47 V vs SCE | 0.25 | [33] |
| Ni–NiO/Gr-450 | Annealing | 0.33 M urea + 1 M KOH | ∼0.285 V vs SCE | 0.5 V vs SCE | 38.24 | [61] |
| Ultrafine-NiO nanoparticles/GC | Electrodeposition | 0.25 M urea + 1 M KOH | ∼0.35 V vs Ag/AgCl | 0.47 V vs Ag/AgCl | 15.34 | [62] |
| NiO/NF | Chemical bath deposition | 0.1 M urea + 8 M KOH | ∼0.3 V vs Ag/AgCl | 0.48 V vs Ag/AgCl | 222 | [63] |
| Ni with other metals | ||||||
| Ni–Co hydroxide/Ti foil | Electrodeposition | 0.33 M urea in 5 M KOH | 0.40 V vs Hg/HgO | — | ∼160 | [35] |
| NiCo/carbon cloth | Hydrothermal | 0.33 M urea in 1 M KOH | 0.44 V vs Hg/HgO | ∼0.6 V vs Hg/HgO | ∼20 | [64] |
| Ni–Co NWAs | Galvanostatic electrodeposition | 0.33 M urea in 5 M KOH | 0.19 V vs Ag/AgCl | 0.6 V vs Ag/AgCl | 380 | [30] |
| Ni–Zn–Co/Ti foil | Electrodeposition, alkaline leaching | 0.33 M urea in 5 M KOH | 0.35 V vs Hg/HgO | 0.5 V vs Hg/HgO | 24 | [65] |
| Ni–Co/MWCNT-AG | Polyol-reduction | 1 M urea in 1 M KOH | 0.302 V vs Ag/AgCl | ∼0.65 V vs Ag/AgCl | ∼120 | [47] |
| Ni–Cu/MWCNT | Two-step hydrothermal | 0.07 M urea in 0.4 M KOH | ∼0.33 V vs Ag/AgCl | 0.38 V vs Ag/AgCl | 24.903 | [66] |
| Ni–Cu/ZnO@MWCNT | ∼0.29 V vs Ag/AgCl | 0.38 V vs Ag/AgCl | 30.02 | |||
| Co–Ni@Nifoam | Facile dip and dry method, electroreduction, and electrodeposition methods | 0.33 M urea in 5 M KOH | 0.18 V vs Ag/AgCl | 0.6 V vs Ag/AgCl | 330 | [67] |
| Co–Ni/rGO@Ni foam | 0.14 V vs Ag/AgCl | 0.6 V vs Ag/AgCl | 223 | |||
| Mn0.5Ni2.0Fe0.5/rGO | One-pot hydrothermal | 0.33 M urea + 1 M KOH | ∼0.35 V vs Ag/AgCl | ∼0.45 V vs Ag/AgCl | 1,750 mg−1 | [68] |
| Ni–Pd(P)/MWCNT | Hydrothermal methods | 1 M urea + 3 M KOH | 0.25 V vs Ag/AgCl | 0.45 V vs Ag/AgCl | 1897.76 | [69] |
| FeNi oxide | Hydrothermal methods | 0.33 M urea in 1 M KOH | 0.265 V vs Ag/AgCl | — | 36 | [70] |
| Ni0.8Co0.2(OH)2 | Sol–gel method | 0.2 M urea in 1 M KOH | 0.25 V vs Ag/AgCl | ∼0.65 V vs Ag/AgCl | 222 | [71] |
| NiWO4 NPs/rGO | Single step hydrothermal methods | 0.33 urea + 1 M KOH | ∼0.3 V vs Ag/AgCl | 0.428 V vs Ag/AgCl | 218.1 | [72] |
| Ni with specific morphologies | ||||||
| NiO nanowalls/Ni foam | Hydrothermal methods | 0.33 M urea + 1 M KOH | ∼0.4 V vs Hg/HgO | — | ∼1,000 | [73] |
| Nanosheet Ni(OH)2/Ni foam | Template-free growth | 0.6 M urea in 5 M KOH | 0.21 V vs Ag/AgCl | 0.56 V vs Ag/AgCl | 559 | [74] |
| Nanoflakes nickel phosphates (Ni–P) | Reflux-based methods | 1 M urea + 1 M KOH | 0.345 V vs Ag/AgCl | 0.45 V vs Ag/AgCl | 20.55 | [75] |
SCE: standard calomel electrode.
2.3 Effect of KOH concentration
The use of KOH as the electrolyte in electrochemical oxidation of urea is reported to provide a better performance, in regards to the lower onset potential and the higher current density, than LiOH and NaOH [45] with the activity of urea oxidation of LiOH < NaOH < KOH. The surface poisoning effect of PtOH−M+ (H2O)x at the interface decreases the hydration energy of the alkali metal and further inhibits the active sites in the order of Li+ > Na+ > K+ [46]. The increase in KOH concentration has been reported to improve the urea oxidation current density. As the KOH concentration increases, the OH− which has a strong impact on the NiOOH development will lead to a decrease (shift to negative) in the onset potential of urea oxidation and concurrently increase the current density [1,47]. Figure 4 shows the CV curves using different KOH concentrations. The CVs imply that the current density keeps increasing until it reaches 5 M KOH, and then remains constant afterwards. It might be due to a full coverage of OH− on the catalyst that will lead to the blocking of the urea oxidation reaction [1]. Moreover, using a very high concentration of KOH will produce oxygen evolution reaction, catalyst oxidation, and accumulation of undesirable products [47].
2.4 Effect of urea concentration
Besides varying the KOH concentration, the urea concentration has also been investigated. CVs of Ni nanoparticle (NP) electrode [1] and NiCo/multi-walled carbon nanotube (MWCNT) [47] using different concentrations of urea is shown in Figure 5. It is shown that the increase in urea concentration leads to the increase in oxidation peak of urea. It might be because of the available urea for the oxidation reaction. In Figure 5a, the oxidation peak keeps increasing until it reaches 0.2 M urea. It was reported that after the concentration reaches above 0.2 M, the current density decreases due to a kinetics limitation because the catalyst surface is covered with urea molecules which decreases the urea oxidation rate due to the deprivation of OH− [1]. Meanwhile, in Figure 5b, the oxidation peak of urea keeps increasing until it reaches 1 M urea. After it surpasses 1 M, the oxidation peak decreases. The excess of urea will cover the catalyst surface and the reaction product will inhibit the contact with OH− to form NiOOH [47]. The maximum oxidation peak might be achieved by using different KOH concentrations in different types of catalyst. Therefore, the optimization of urea is prescribed to achieve higher activity in DUFC.
3 Progress of anode catalyst for DUFC
The use of anode catalyst in DUFC has a great impact on the result of higher power density and it will improve the electrical performance of DUFC. There are many ways to improve the output of DUFC, namely by increasing the surface active sites of the catalyst, increasing the theoretical open circuit voltage (OCV) by using oxidants, and the most important way is by developing the anode catalyst of DUFC. Originally, noble metal catalyst has been used as the anode catalyst in DUFC, such as Ti/Pt, Ti/(Pt–Ir), and Ti/RuO2 [48], which resulted in an unsatisfactory effect on DUFC, yet required a very high cost. Alternatively, using an affordable non-noble metal catalyst as the anode catalyst in urea fuel cell is reported to produce a highly effective result in DUFC. Nickel, in the form of NiOOH, has been reported as an excellent catalyst for urea electro-oxidation and could further increase the electrical performance of DUFC. There are many ways to increase the activity of Ni, such as by developing various morphologies of Ni, using high surface area supporting catalyst, and alloying Ni with various metals.
3.1 Single nickel metal catalyst
Nickel in the form of NiOOH was proven to be a promising catalyst in urea electro-oxidation. Vedharathinam and Botte (2012) have successfully electrodeposited Ni on the surface of Ti (inert) rod [1]. The Ni electrode was then applied as the anode catalyst (working electrode) for urea electro-oxidation process. Cyclic voltammetry study was conducted and confirmed that Ni is an active catalyst for urea electro-oxidation. In 2011, Lan and Tao have successfully synthesized Ni NPs (nanosized Ni) using KBH4 reduction methods with the primary particles around 5 nm, but in some area the particles were around 2–3 nm [49].
Figure 6 shows the SEM images of the nanosized Ni compared to the commercial Ni. Figure 6a and b shows the images of nanosized Ni which have particle size of around 0.2 µm, whereas the ∼50 nm particles are still in observation. Meanwhile, as can be seen in Figure 6c and d, the commercial Ni has much larger particle size compared to nanosized Ni, around 4–10 µm. It was reported that nanosized Ni, which has a smaller size and larger surface area compared to the commercial Ni, shows a better performance in DUFC with a maximum power density of 14.2 mW cm−2 obtained at 60°C when using 1 M urea as fuel placed in anode chamber and humidified air was filled in the cathode chamber. The smaller size of the particles could lead to larger surface area which is an ideal condition of DUFC catalyst. This result indicates that nanosized Ni is a promising catalyst in urea electro-oxidation as well as DUFC.
![Figure 6
SEM characterization of nanosized nickel (a and b) and commercial nickel (c and d), republished with permission [49].](/document/doi/10.1515/chem-2021-0100/asset/graphic/j_chem-2021-0100_fig_006.jpg)
SEM characterization of nanosized nickel (a and b) and commercial nickel (c and d), republished with permission [49].
3.2 Nickel hydroxides
As mentioned above, Ni(OH)2 generally forms two types of crystals, namely α-Ni(OH)2 and β-Ni(OH)2 forms. In the α-Ni(OH)2 form, intercalations between the layers by additional anions neutralize the positive layers with the spacing distributed between 7.5 and 31.7 Å and lead to higher electrochemical activity as the conductive layers could sustain the electrolyte transfer to the α-Ni(OH)2 layer. Meanwhile, in the β-Ni(OH)2 form, the layers are closely arranged which lead to minor interlayer spacing and affect the amount of electrolyte entering the layers of β-Ni(OH)2, thus could lower the electrochemical activity [48].
To decrease the over-potential and obtain an optimum performance of DUFC, Wang et al. have modified glassy carbon electrodes with two-dimensional Ni(OH)2 nanosheets [76]. XRD characterization confirmed the interlayer spacing of 2.67 nm, indicating that Ni(OH)2 nanosheets have been deposited in the form of α-Ni(OH)2. The electrochemical study conducted in KOH with the absence and the presence of urea confirmed that in the absence of urea, the current density increased to 154 mA cm−2 mg−1. The α-Ni(OH)2 form could lower the onset potential by 100 mV compared to the bulk Ni(OH)2. In 2012, Wang et al. successfully synthesized nickel hydroxide nanoribbons through hydrothermal treatment. The XRD characterization confirmed the pattern of β-Ni(OH)2 phase with the sample thickness of around 15–20 nm [77]. Electrochemical study conducted with the same previous condition, using KOH in the absence and the presence of urea, showed that nickel hydroxide nanoribbon enhanced the current density to 7 mA cm−2 mg−1. Accordingly, it is confirmed that α-Ni(OH)2 provided higher current density of urea electro-oxidation than the β-Ni(OH)2.
In 2016, Ye et al. reported the use of Ni(OH)2/Ni foam as the anode catalyst in DUFC. Ni(OH)2/Ni foam was prepared in various morphologies, which are sheet-like (SH), flower-like (FL), nanosheet (NS), and twin-like (TW), to optimize the performance toward urea electro-oxidation [74]. All the configurations were then examined as anode catalyst. The result showed that NS Ni(OH)2/Ni foam generated a higher current density of urea electro-oxidation compared to the other configurations of Ni(OH)2/Ni foam. This is because the NS Ni(OH)2/Ni foam morphology has a larger surface area than the other configurations. The SEM images of the prepared electrode in various morphologies (Figure 7a) show that SH Ni(OH)2/Ni foam consists of thick sheets, while flower-like shape Ni(OH)2 is well distributed in Ni foams. In regards to NS Ni(OH)2/Ni foam, it is fully coated with nanosheet Ni(OH)2. Meanwhile, compact film of Ni(OH)2 is homogenously distributed on TL Ni(OH)2/Ni foam. The comparison of all modified Ni foams toward urea electro-oxidation in alkaline medium (KOH) and their DUFC performances are displayed in Figure 7b and c, respectively. These figures imply that NS Ni(OH)2/Ni foam has a loose structure with many open spaces which lead to a higher surface area, so it is sufficient for the electro-catalytic performance of DUFC.
![Figure 7
SEM visualization of sheet-like ((a and b); flower like (c and d); nanosheets (e and f); and twin-like (g and h)) (a); the CVs of various morphologies of Ni(OH)2/Ni foams (b) and the DUFC performances using all morphologies (c), republished with permission of Royal Society of Chemistry, from [74]; permission conveyed through Copyright Clearance Center, Inc.](/document/doi/10.1515/chem-2021-0100/asset/graphic/j_chem-2021-0100_fig_007.jpg)
SEM visualization of sheet-like ((a and b); flower like (c and d); nanosheets (e and f); and twin-like (g and h)) (a); the CVs of various morphologies of Ni(OH)2/Ni foams (b) and the DUFC performances using all morphologies (c), republished with permission of Royal Society of Chemistry, from [74]; permission conveyed through Copyright Clearance Center, Inc.
3.3 Ni with other metals
Although Ni in the form of NiOOH has been proven as the promising catalyst towards urea electro-oxidation, the NiOOH itself has a high overpotential which could further decrease the result of DUFC. Likewise, the use of Ni catalyst is most likely to be fouling throughout the electro-oxidation process which could disband the NiOOH as the active site of the catalyst. Doped Ni with other metal has been reported to be able to improve the electrocatalytic activities and reduce the abovementioned difficulties. King et al. [78] reported the modification of Ni with noble metals, including Pt–Ni [79], Pt–Ir–Ni, Ru–Ni, Pd [80], and Rh–Ni [78]. Between all the various combinations, the combination of Rh and Ni could create a synergistic effect because it has been proven to lower the overpotential and increase the stability in the urea electro-oxidation. Electrochemical study of Rh–Ni electrode with 0.33 M urea in 1 M KOH as the electrolyte is reported to achieve the current density of ∼80 mA cm−2 during the cyclic voltammetry. In 2012, Miller et al. have reported to use Rh/Ni electrodes, which were developed by depositing Rh on Ni foil using constant potential techniques [81]. It was reported that the addition of Rh lead to an increase in current density, which implied the role of Rh in the oxidation acceleration of Ni(OH)2 to NiOOH. Alloying of Ni-Rh occurs when Rh is deposited on the surface of Ni at lower potential; however, the best catalytic performance occurs when Rh is not alloyed with Ni, which means that only monometallic Rh is deposited on the Ni surface.
Alloying Ni with noble metal catalyst has been proven to increase the effectivity toward urea electro-oxidation, but the high cost of the noble metal catalyst remains as the main problem if this will be further used in a larger scale application. Therefore, doping or alloying Ni with non-noble metal catalyst is one way to overcome this problem. Alloying or doping non-noble metal catalyst, such as Co [71], Mn [82], Zn [65], Fe [83], Cr [84], Mo [85], etc., to Ni has been proven to decrease the onset potential of NiOOH and improve the catalytic activity toward urea electro-oxidation in DUFC application. Cobalt has been widely used to decrease the onset potential of NiOOH formation to further optimize the electrocatalytic activity toward urea electro-oxidation. Wei et al. (2011) used Ni–Co bimetallic hydroxide film deposited on Ti foil as the anode catalyst for urea electro-oxidation. It was reported that alloying Ni and Co produced a significant reduction in the overpotential of NiOOH (it decreases at about 150 mV) when compared with only nickel hydroxide electrode [35]. In 2014, Xu et al. also reported the use of nickel–cobalt bimetallic (NiCo/C) for the anode catalyst for DUFC [64]. A reduction method using NaBH4 was applied to prepare NiCo/C with various Co ratios to optimize the use of Co. An average particle size of NiCo NPs is calculated to be around 30–40 nm. The most negative potential is obtained using Ni3Co2/C, indicating that the use of Co could lower the onset potential. On the other hand, the increase in Co content decreases the electro-oxidation current of urea because of the inactive activity of Co urea electro-oxidation. Therefore, it is important to control the balance of Ni and Co ratio to obtain higher catalytic activity. A maximum power density of 1.57 mW cm−2 could be achieved at NiCo/C electrode using 0.33 M urea as the fuel and O2 as the oxidant, which were filled in the anode and cathode chambers, respectively, at 60°C. Yan et al. reported that the use of Zn and Co as the multi-metal catalyst in Ni could enhance the catalytic activity toward electro-oxidation, since Co itself is inactive [65]. It was reported that using Ni–Zn as the metal catalyst decreases the onset potential from 0.43 to 0.39 V, while using Ni–Zn–Co it decreases the onset potential to 0.35 V. The higher current density is achieved using Ni–Zn–Co as the anode catalyst of urea electro-oxidation. Mn is used to help remove the poisonous intermediates formed on the catalyst surfaces. Mn also helps to reduce the overpotential of NiOOH formation. In 2016, Barakat et al. used NiMn NPs-decorated carbon nanofibers (NiMn-CNFs) as the anode catalyst for urea electro-oxidation [86]. It was reported that NiMn-CNFs show a better electrocatalytic activity toward urea oxidation compared to Ni-CNFs, which is almost three times higher. In 2017, Singh and Schechter alloyed Ni with Cr which resulted in an increase in the electro-oxidation activity of urea and shifting the redox peak to the more negative potentials [87]. Using NiCr/C results in higher current density than that obtained by using Ni/C, which is 3.6 times higher.
3.4 Ni with specific morphologies
Besides optimizing the anode catalyst in the form of Ni metal, nickel hydroxides, and alloying Ni with other metals, the morphologies are also considered to enhance the surface area which later could enhance the electrical performance of DUFC. Many morphologies have been successfully developed, such as nanowires, nanofibers, nanosheet, and many more.
In 2014, Yan et al. fabricated two various Ni structures, i.e., nickel nanowire electrocatalyst (NNE) and nickel film electrocatalyst (NFE), using electrodeposition technique at an applied potential of −0.85 V vs Ag/AgCl [88]. Anodic aluminum oxide (AAO) template was used to fabricate the NNE. The deposition time for NNE was 60 min and that for NFE was only 6 min. Both NNE and NFE obtained the same loading Ni. Figure 8a shows the SEM characterization of both nickel nanowire electrodes in the form of NFE and NNE. It is also shown in the electrochemical studies of urea electro-oxidation using CVs (Figure 8b) which reveals that NNE has achieved higher electrocatalytic activity and higher current density compared to NFE. It is mainly because there is no assistance of AAO resulting in nickel particle with partial agglomeration; meanwhile, the use of AAO template resulted in a larger surface area with an average diameter of 90 nm and electro-active surface area of 79.1 cm2 mg−1. It is also reported that the current density of 40 mA cm−2 at 0.55 V (vs Hg/HgO) could be achieved when using NNE. In 2014, Guo et al. have successfully prepared fully metallic structure of nickel nanowire array (NWA) electrode developed by doping the Ni particles within the pores through electrodeposition methods and over-plating it on the surface of polycarbonate template (Guo et al., 2016). The prepared NWAs have an active surface area of 25.21 cm2 and 50 nm diameter for a single wire. Comparison of NWAs with a flat Ni electrode showed the noticeable decrease in the onset potential of urea electro-oxidation with a higher peak current density, indicating that the nanowire array structure is promising for anode catalyst application in DUFC.
![Figure 8
SEM characterization of nickel nanowires in the form of NFE and NNE (a) and the CVs of nickel nanowires with different form in 1 M KOH in the presence and absence of 0.33 M urea (b), republished with permission [89].](/document/doi/10.1515/chem-2021-0100/asset/graphic/j_chem-2021-0100_fig_008.jpg)
SEM characterization of nickel nanowires in the form of NFE and NNE (a) and the CVs of nickel nanowires with different form in 1 M KOH in the presence and absence of 0.33 M urea (b), republished with permission [89].
4 DUFC performances
DUFC has been considered as a promising alternative for the renewable energy due to the use of wastes which could generate electricity with high efficiency. Compared to the PEMFC, which uses hydrogen as the energy resources, DUFC has an excellence factor such as the use of urea-containing wastewater. Hydrogen, which is a clean energy, still has many drawbacks such as its difficult production, storage, and the transportation and will create difficulties in the process to make it a large-scale application [89]. DUFC, on the other hand, using urea as the energy sources leads to an easy-storage and easy-transport, non-toxic, and could make use of the high amount of urea-containing wastewater found in human and animal urine and industrial area [90]. The urea electro-oxidation process is the primary key to generate high electricity. Therefore, the current progress of anode catalyst to improve the catalytic activity in urea electro-oxidation is investigated.
Basically, the instrument used in DUFC is the same as the conventional fuel cell. DUFC is often used in two-chamber cell which contains the anode chamber and cathode chamber. An ion exchange membrane is placed between the two chambers [91,92]. In each chamber, there are catalysts which will act as the working electrode and the counter electrode. Generally, the flow cell is used to perform the DUFC application.
Already in 1973, Yao et al. conducted an experiment to generate electricity using urea as the energy source by using Pt as the catalyst in the trial [31]. The experiments result presented evidence that oxidation of urea transforms into CO2, N2, and H2O using Pt as the anode and cathode catalysts. The investigation of first DUFC was reported by Lan et al. in 2010 which compared the use of Pt/C and Ni/C as the anodes. The use of Pt/C electrodes as both cathode and anode catalysts produced the OCV of 0.5 V and the power density of 0.2 mW cm−2 at room temperature. Meanwhile, when using Ni/C as the anode and Ag/C-MnO2/C as the cathode, an anion-exchange resin-PVA membrane was utilized due to the use of basic electrolyte in the anode chamber. This system resulted in a maximum power density of 1.7 mW cm−2 at 50°C. This work proves that DUFC could be executed using Ni as a non-noble metal catalyst. Afterwards, the recent progress of anode catalyst has been investigated. In 2014, Xu et al. used nickel–cobalt bimetallic/C (NiCo/C) as the anode catalyst. It was proven that the Co doping could reduce the overpotential of Ni toward urea-oxidation and further improve the catalytic activity [64]. Application using 0.33 M urea as a fuel (anode electrolyte) and O2 which was filled in the cathode chamber at 60°C temperature generated a maximum power density of 1.57 mW cm−2, while the use of urine as the fuel achieved a maximum power density of 0.19 mW cm−2 and OCV of 0.38 V at 60°C. In 2016, the use of nickel–cobalt nanowire arrays (Ni–Co NWAs) as the anode catalyst in DUFC produced a maximum OCV and power density of 0.92 V and 7.4 mW cm−2, respectively, at room temperature [30]. In this DUFC, a solution of 0.33 M urea in 9 M KOH was used as the anode electrolyte and a solution containing 2 M H2O2 and 2 M H2SO4 was placed as the cathode electrolyte. It was also confirmed that this type of DUFC showed a good stability during one-hour durability test. Basumatary et al. reported Ni-Cu/ZnO@MWCNT application as anode catalyst could enhance the surface area and improve the catalytic activity and further enhance the electricity performance of DUFC to be higher [66]. Maximum power density of 26.9 and 44.36 mW cm−2 were achieved at 20 and 50°C, respectively, using 3 M KOH/0.7 M urea as the anode electrolyte. This result was the highest output of power density reported at 50°C. Ranjani et al. developed 3D hierarchical nickel cobaltite (NiCo2O4) on carbon cloth (CC) fibers as anode catalyst in DUFC [93]. It was reported that maximum power density of 38 mW cm−2 could be generated using 50 mM urea in 0.1 M KOH at 80°C (Figure 9a). Higher result could not be accomplished due to the synergistic impact of Ni2+ ion which substituted in the octahedral sites of Co3O4 and the 3D hierarchical configuration which leads to limiting the oxygen evolution reaction and further improves the urea electro-oxidation reaction. Besides, the use of this anode catalyst could achieve 180 h durability test due to its large surface area (Figure 9b).
![Figure 9
DUFC performance of NiCo2O4/CC using urea (blue line) and human urine (red line) at 80°C (a), and durability test of NiCo2O4/CC in urea (blue line) and human urine (red line) for 180 h at 80°C (b), republished with permission of Royal Society of Chemistry, from ref. [94]; permission conveyed through Copyright Clearance Center, Inc.](/document/doi/10.1515/chem-2021-0100/asset/graphic/j_chem-2021-0100_fig_009.jpg)
DUFC performance of NiCo2O4/CC using urea (blue line) and human urine (red line) at 80°C (a), and durability test of NiCo2O4/CC in urea (blue line) and human urine (red line) for 180 h at 80°C (b), republished with permission of Royal Society of Chemistry, from ref. [94]; permission conveyed through Copyright Clearance Center, Inc.
Besides the optimization of anode catalyst, the use of high surface area supporting electrode of the anode catalyst could also determine the performances. The use of ion exchange membrane and the use of cathodic oxidants were also reported to have an impact on the electrical performances of DUFC.
4.1 Supporting electrode
One of the main reasons for higher electrical performances is the surface area of the catalyst, both in the anode and cathode chambers. Commonly, C has been used as the supporting electrode due to its inexpensive material, such as graphene [41,58], CNTs [47,94], and CNFs [86]. Additionally, boron-doped diamond [95], Ni foam [63], and many more were also reported.
Li et al. have reported the use of CoNi nanosheet array which was grown in Ni foam modified by reducing graphene oxide (rGO) as the anode catalyst in DUFC [67]. The GO was attached to Ni foam through facile dip and dry method and further reduced to reducing graphene oxide by electroreduction method. The distribution of CoNi nanosheet arrays in the rGO/Ni foam contributes to the increase in the surface area. Ni foam was selected as the supporting electrode due to its large surface area, whereas the modification using rGO produces a more larger surface area CoNi/rGO/Ni foam electrode. This electrode successfully formed a porous surface electrode that provides abundant active sites on the catalyst to enhance the urea oxidation performance. A good stability for 1 h application time was also demonstrated. This implies a good stability for 1 h, although longer stability test still needs to be conducted to evaluate the catalyst durability for daily or even industrial use in the future. Basumatary et al. also reported the use of Ni–Cu alloy NPs which was deposited onto the surface of ZnO-coated MWCNTs [66]. The Ni–Cu/ZnO@MWCNTs was prepared using a two-step hydrothermal process. MWCNTs are usually used as supporting electrodes in DUFC due to its large surface area and good thermal and chemical stability in acidic and base media. However, to uniformly distribute metal NP onto the surface of MWCNTs is rather difficult. To solve this problem, ZnO was coated onto the MWCNTs to achieve high distribution of metal NPs as well as to increase the catalytic activity of the catalysts. It is reported that uniform distribution of Ni–Cu NPs on the entire surface of ZnO@MWCNT was successfully performed without agglomeration as also confirmed by the TEM characterization. The average particle size of Ni–Cu NPs of around 2.5
The above two examples showed that the use of supporting electrode is greatly important to form larger surface area and escalate the urea oxidation performance. Furthermore, it is also crucial to observe the stability and durability of the electrodes for suitable and repetitive use, especially for daily use and in industrial scale.
4.2 Cathode electrolyte: using H2O2 as oxidants
In DUFC, oxygen (air) commonly has been used as the cathode electrolyte. However, it has been reported that replacing oxygen with oxidants could help to improve the electrical performances of DUFC due to its theoretical cathodic potential, which is twice as high as the oxygen. Besides, the use of oxidants is proven to create a faster electro-reduction kinetics [96]. As mentioned above, the theoretical cathodic potential increases from 0.40 V vs SHE, when O2 is applied as the cathode electrolyte, to 1.763 V vs SHE, when H2O2 is applied as the cathode electrolyte. It increases the potential around 0.87 V vs SHE. Another advantage in using H2O2 is that oxygen density in liquid phase is around a thousand times higher than in its gaseous phase. Thus, it will enhance a higher current density in the DUFC application [27].
In 2014, Xu et al. have used bimetallic Ni–Co deposited on carbon cloth as an anode catalyst in DUFC [64]. 0.33 M urea has been delivered into flow channels and used as the fuel (anode electrolyte), while humidification oxygen has been used as the cathode electrolyte. Maximum power density of 1.57 mW cm−2 is obtained at a temperature of 60°C. Serban et al. in 2014 was the first to introduce the use of H2O2 as the cathode electrolyte for DUFC application [27]. It was reported that the use of Ni/MWCNTs can produce a maximum power density of 0.05 mW cm−2, when using a solution containing 1 M urea and 1.5 M NaOH as an anode electrolyte and a solution containing 20% H2O2 and 5% H3PO4 as the cathode electrolyte [27]. Since then, the use of H2O2 as the cathode electrolyte for DUFC application has gained great attentions and further optimized. In 2016, Guo et al. used porous Ni–Co anode catalyst for DUFC application with a solution containing 0.5 M urea and 7 M KOH as the anode electrolyte and a solution containing 2 M H2O2 and 2 M H2SO4 as the cathode electrolyte. Applications at 20 and 70°C was reported to produce the maximum power density of 17.4 and 31.5 mW cm−2, respectively [97]. Compared to the use of O2 as the cathodic oxidants, the use of H2O2 is proven to produce higher electrical performance in DUFC application.
The difference in the cathodic oxidants leads to different exchange membrane used in the DUFC application. Typically, anion exchange membrane was used as the exchange membrane when O2 was used as the cathodic oxidants. It was reported that ammonia (a weak base) will be produced during the hydrolysis of urea in this application, because of which the cation exchange membrane (commonly, Nafion) cannot be used since it is compatible in acidic medium. Therefore, anion exchange membrane is more suitable to be used in DUFC application when O2 has a role of the cathodic oxidants [10]. In this type of DUFC, the OH- produced in the cathode chamber will go over the anion exchange membrane into the anode chamber. The OH- ions then further react with urea and release electrons which are transported over the external circuit and could determine the electrical performances [28]. The advantage of using anion exchange membrane is that it is an alkaline-based electrolyte which makes it compatible and enables it to provide the alkaline condition [98].
Meanwhile, when H2O2 is used as the cathodic oxidants, the cation exchange membrane (Nafion) is used as the exchange membrane. In this situation, K+ produced from the reaction in the anode plays a role as the transport ion through the Nafion and goes into the cathode chamber and reacts with the
5 Future challenges and perspective
The DUFC has been proven to be a promising alternative for the replacement of fossil fuel to produce electricity, although some optimization is still needed to achieve the optimum result. The very important part in this system is the catalysts, both in cathode and anode. Catalyst with a large surface area is desired to provide sufficient active sites, especially for the anode catalyst since it will have a direct contact toward urea as the fuel in this type of fuel cells. Thus, modification to obtain large surface area is highly desirable for anode catalyst in DUFC. Besides, the use of H2O2 cathode electrolyte has also been proven as one effective method to achieve higher electrical performances. However, H2O2 in acidic medium is needed to optimize the theoretical cell voltage. It means that two different conditions are needed in this type of DUFC, alkaline medium in the anode chamber and acidic medium in the cathode chamber. Therefore, the anode catalyst which has a good corrosion resistance is needed. The use of anion exchange membrane is also required to be maintained due to its stability issue and also its low anionic conductivities [99]. It is also very important to examine the catalysts performance toward urea electro-oxidation using electrochemical impedance spectroscopy [67]. It will give information about the resistance value of the catalyst system before being used as the anode catalyst in DUFC.
Besides its modification of Ni-based catalyst, which are desired to optimize the performance of DUFC, the stability test is also required to evaluate the performance so it could be employed for a daily use or industrial scale. Finally, this system also needs to be examined using the wastewater sample, such as human or animal urine and industrial waste. After the optimization of the importance factors mentioned above, this type of fuel cell could be applied as a promising replacement of the fossil fuel and become the effective devices for wastewater treatment and electricity production.
6 Conclusion
The purpose of this review is to report the recent progress in DUFC, specifically for the anode catalyst progress in DUFC. It can be concluded that DUFC is a greatly promising substitution for the fossil fuel. The use of wastewater that contains urea, which is further oxidized into N2, CO2, and H2O, could help to generate electricity. Despite highly promising for application in DUFC, some important factors need to be optimized to produce an excellent performance. The optimization of anode catalyst is desired to achieve higher performances toward urea electro-oxidation. It is reported that Ni, a non-noble and low-cost metal catalyst, is a better catalyst for urea electro-oxidation compared to the noble metal catalyst, such as Pt. However, the formation potential of NiOOH, the active catalyst for urea electro-oxidation, is high. Therefore, doping Ni with other metals, such as Co, Mn, Zn, or Cr is needed. The morphologies of the catalyst are also reported to increase the catalytic performances due to the achieved larger surface area. Besides, using a supporting electrode with greater surface area, such as Ni foam, MWCNT, etc., is also important to improve the DUFC performances. It is very important to modify the anode catalyst to increase the surface area, so it could provide sufficient active sites between the catalyst and urea as the fuel. Moreover, it is also very crucial to optimize the electrolyte condition in this DUFC system, such as usage of H2O2 over O2 as the cathodic oxidant in the cathode electrolyte, which theoretically produces higher cathodic potential and also optimizes the use of exchange membrane (cation or anion) to achieve a suitable environment in enhancing the performance of DUFC.
-
Funding information: This work was funded by Hibah Riset PMDSU Kemenristek Dikti 2020. Contract No. NKB-431/UN2.RST/HKP.05.00/2020.
-
Author contributions: Y.M.T.A.P.: data curation, formal analysis, investigation, and writing – original draft; J.G., Y.Y., and R.W.: formal analysis and validation; Y.E.: validation; T.A.I.: conceptualization, validation, funding acquisition, supervisor, and writing – review and editing.
-
Conflict of interest: There is no competing interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this work are included in this article.
References
[1] Vedharathinam V, Botte GG. Understanding the electro-catalytic oxidation mechanism of urea on nickel electrodes in alkaline medium. Electrochim Acta. 2012;81:292–300. 10.1016/j.electacta.2012.07.007.Search in Google Scholar
[2] Ajayan J, Nirmal D, Mohankumar P, Saravanan M, Jagadesh M, Arivazhagan L. A review of photovoltaic performance of organic/inorganic solar cells for future renewable and sustainable energy technologies. Superlattices Microstruct. 2020;143:106549. 10.1016/j.spmi.2020.106549.Search in Google Scholar
[3] Balajii M, Niju S. Banana peduncle – A green and renewable heterogeneous base catalyst for biodiesel production from Ceiba pentandra oil. Renew Energy. 2020;146:2255–69. 10.1016/j.renene.2019.08.062.Search in Google Scholar
[4] Espinoza-Acosta JL, Torres-Chávez PI, Olmedo-Martínez JL, Vega-Rios A, Flores-Gallardo S, Zaragoza-Contreras EA. Lignin in storage and renewable energy applications: A review. J Energy Chem. 2018;27:1422–38. 10.1016/j.jechem.2018.02.015.Search in Google Scholar
[5] Nagaraju G, Sekhar SC, Ramulu B, Yu JS. An integrated approach toward renewable energy storage using rechargeable Ag@Ni0.67Co0.33 S-based hybrid supercapacitors. Small. 2019;15:1–14. 10.1002/smll.201805418.Search in Google Scholar PubMed
[6] Meng FL, Liu KH, Zhang Y, Shi MM, Zhang XB, Yan JM, et al. Recent advances toward the rational design of efficient bifunctional air electrodes for rechargeable Zn-Air batteries. Small. 2018;14:1–20. 10.1002/smll.201703843.Search in Google Scholar PubMed
[7] Yang Y, Bremner S, Menictas C, Kay M. Battery energy storage system size determination in renewable energy systems: A review. Renew Sustain Energy Rev. 2018;91:109–25. 10.1016/j.rser.2018.03.047.Search in Google Scholar
[8] Edrisi A, Mansoori Z, Dabir B. Urea synthesis using chemical looping process – Techno-economic evaluation of a novel plant configuration for a green production. Int J Greenh Gas Control. 2016;44:42–51.10.1016/j.ijggc.2015.10.020Search in Google Scholar
[9] Rahimpour MR, Mottaghi HR, Barmaki MM. Hydrogen production from urea wastewater using a combination of urea thermal hydrolyser – desorber loop and a hydrogen-permselective membrane reactor. Fuel Process Technol. 2010;91:600–12. 10.1016/j.fuproc.2010.01.006.Search in Google Scholar
[10] Lan R, Tao S, Irvine JTS. A direct urea fuel cell – power from fertiliser and waste. Energy Env Sci. 2010;3:438–41. 10.1039/b924786f.Search in Google Scholar
[11] Zheng Y, Jiao Y, Zhu Y, Li LH, Han Y, Chen Y, et al. Hydrogen evolution by a metal-free electrocatalyst. Nat Commun. 2014;5:1–8. 10.1038/ncomms4783.Search in Google Scholar PubMed
[12] Simka W, Piotrowski J, Nawrat G. Influence of anode material on electrochemical decomposition of urea. Electrochim acta 2007;52:5696–703. 10.1016/j.electacta.2006.12.017.Search in Google Scholar
[13] Boggs BK, King RL, Botte GG. Urea electrolysis: Direct hydrogen production from urine. Chem Commun. 2009;4859–61. 10.1039/b905974a.Search in Google Scholar PubMed
[14] O’hayre R, Suk-Won Cha WGC. Fuel cell fundamentals. Open Med (Warsaw, Pol). 2016;14:271–8. 10.1017/CBO9781107415324.004.Search in Google Scholar
[15] Steele BCH, Heinzel A. WSPC-MATERIALS FOR SUSTAINABLE ENERGY-Reprint Volume Book-Trim Size:-11in x 8.5in; 2010. p. 345–52.Search in Google Scholar
[16] Mekhilef S, Saidur R, Safari A. Comparative study of different fuel cell technologies. Renew Sustain Energy Rev. 2012;16:981–9. 10.1016/j.rser.2011.09.020.Search in Google Scholar
[17] Sharma S, Pollet BG. Support materials for PEMFC and DMFC electrocatalysts – A review. J Power Sources. 2012;208:96–119. 10.1016/j.jpowsour.2012.02.011.Search in Google Scholar
[18] Serov A, Kwak C. Review of non-platinum anode catalysts for DMFC and PEMFC application. Appl Catal B Env. 2009;90:313–20. 10.1016/j.apcatb.2009.03.030.Search in Google Scholar
[19] Huang QA, Hui R, Wang B, Zhang J. A review of AC impedance modeling and validation in SOFC diagnosis. Electrochim Acta. 2007;52:8144–64. 10.1016/j.electacta.2007.05.071.Search in Google Scholar
[20] Shearing PR, Brett DJL, Brandon NP. Towards intelligent engineering of SOFC electrodes: A review of advanced microstructural characterisation techniques. Int Mater Rev. 2010;55:347–63. 10.1179/095066010X12777205875679.Search in Google Scholar
[21] Malzbender J, Steinbrech RW, Singheiser L. A review of advanced techniques for characterising SOFC behaviour. Fuel Cell. 2009;9:785–93. 10.1002/fuce.200800110.Search in Google Scholar
[22] McPhail SJ, Aarva A, Devianto H, Bove R, Moreno A. SOFC and MCFC: Commonalities and opportunities for integrated research. Int J Hydrog Energy. 2011;36:10337–45. 10.1016/j.ijhydene.2010.09.071.Search in Google Scholar
[23] Huijsmans JPP, Kraaij GJ, Makkus RC, Rietveld G, Sitters EF, Reijers HTJ. Analysis of endurance issues for MCFC. J Power Sources. 2000;86:117–21. 10.1016/S0378-7753(99)00448-6.Search in Google Scholar
[24] Merle G, Wessling M, Nijmeijer K. Anion exchange membranes for alkaline fuel cells: A review. J Memb Sci. 2011;377:1–35. 10.1016/j.memsci.2011.04.043.Search in Google Scholar
[25] Bidault F, Brett DJL, Middleton PH, Brandon NP. Review of gas diffusion cathodes for alkaline fuel cells. J Power Sources. 2009;187:39–48. 10.1016/j.jpowsour.2008.10.106.Search in Google Scholar
[26] McLean GF, Niet T, Prince-Richard S, ND. An assessment of AFC technology. Int J Hydrog Energy. 2002;27:507–26.10.1016/S0360-3199(01)00181-1Search in Google Scholar
[27] Serban EC, Balan A, Iordache AM, Cucu A, Ceaus C, Necula M, et al. Urea/hydrogen peroxide fuel cell. Dig J Nanomater Biostruct. 2014;9:1647–54.Search in Google Scholar
[28] Fan Z, Kwon Y, Yang X, Xu W, Wu Z. In-situ production of hydrogen peroxide as oxidant for direct urea fuel cell. Energy Proc. 2017;105:1858–63. 10.1016/j.egypro.2017.03.544.Search in Google Scholar
[29] An L, Jung CY. Transport phenomena in direct borohydride fuel cells. 2017;205:1270–82. 10.1016/j.apenergy.2017.08.116.Search in Google Scholar
[30] Guo F, Cheng K, Ye K, Wang G, Cao D. Preparation of nickel–cobalt nanowire arrays anode electro-catalyst and its application in direct urea/hydrogen peroxide fuel cell. Electrochim Acta. 2016;199:290–6. 10.1016/j.electacta.2016.01.215 Search in Google Scholar
[31] Yao SJ, Wolfson SK, Jr., Ahn BK, Liu CC. Anodic oxidation of urea and an electrochemical approach to De-ureation. Nature. 1973;241:471–2.10.1038/241471a0Search in Google Scholar PubMed
[32] Appleby AJ. Electrooxidation of urea at the ruthenium. AIChE J. 1986;32:1450–8.10.1002/aic.690320906Search in Google Scholar
[33] Tammam RH, Saleh MM. On the electrocatalytic urea oxidation on nickel oxide nanoparticles modified glassy carbon electrode. J Electroanal Chem. 2017;794:189–96. 10.1016/j.jelechem.2017.04.023.Search in Google Scholar
[34] Abdel Hameed RM, Medany SS. Enhanced electrocatalytic activity of NiO nanoparticles supported on graphite planes towards urea electro-oxidation in NaOH solution. Int J Hydrog Energy. 2017;42:24117–30. 10.1016/j.ijhydene.2017.07.236.Search in Google Scholar
[35] Yan W, Wang D, Botte GG. Nickel and cobalt bimetallic hydroxide catalysts for urea electro-oxidation. Electrochim Acta. 2012;61:25–30. 10.1016/j.electacta.2011.11.044.Search in Google Scholar
[36] Suárez D, Díaz N, Merz KM. Ureases: Quantum chemical calculations on cluster models. J Am Chem Soc. 2003;125(50):15324–37.10.1021/ja030145gSearch in Google Scholar PubMed
[37] Medway SL, Lucas CA. In situ studies of the oxidation of nickel electrodes in alkaline solution. J Electroanal Chem. 2006;587:172–81. 10.1016/j.jelechem.2005.11.013.Search in Google Scholar
[38] King RL, Botte GG. Hydrogen production via urea electrolysis using a gel electrolyte. J Power Sources. 2011;196:2773–8. 10.1016/j.jpowsour.2010.11.006.Search in Google Scholar
[39] Toghill KE, Xiao L, Stradiotto NR, Compton RG. The determination of methanol using an electrolytically fabricated nickel microparticle-modified boron-doped diamond electrode. Electroanalysis. 2010;22:491–500. 10.1002/elan.200900523.Search in Google Scholar
[40] Golikand AN, Maragheh MG, Irannejad L, Asgari M. Electrocatalytic oxidation of methanol on a nickel (II)-1-(2-pyridylazo)-2-naphthol complex-modified glassy-carbon electrode in alkaline medium. Russ J Electrochem. 2006;42:167–72. 10.1134/S1023193506020108.Search in Google Scholar
[41] Guzman RS, Vilche JR, Arvía AJ. Non-equilibrium effects in the nickel hydroxide electrode. J Appl Electrochem. 1979;9:183–9.10.1007/BF00616088Search in Google Scholar
[42] Barakat NAM, Alajami M, Ghouri ZK, Al-Meer S. Effective NiMn nanoparticles-functionalized carbon felt as an effective anode for direct urea fuel cells. Nanomaterials. 2018;8:8. 10.3390/nano8050338.Search in Google Scholar PubMed PubMed Central
[43] Guo F, Ye K, Du M, Huang X, Cheng K, Wang G, et al. Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium. Electrochim Acta. 2016;210:474–82. 10.1016/j.electacta.2016.05.149.Search in Google Scholar
[44] Daramola DA, Singh D, Botte GG. Dissociation rates of urea in the presence of NiOOH catalyst: A DFT analysis. J Phys Chem A. 2010;2:11513–21.10.1021/jp105159tSearch in Google Scholar PubMed
[45] Zhang W, Yin S, Li X, Xu G, Xie T. Impact of the alkali cation on the electrocatalytic oxidation of urea and benzyl alcohol on nickel electrode. Electrochem Commun. 2016;63:1–4. 10.1016/j.elecom.2015.12.004.Search in Google Scholar
[46] Sitta E, Nagao R, Kiss IZ, Varela H. Impact of the alkali cation on the oscillatory electro-oxidation of ethylene glycol on platinum. J Phys Chem C. 2015;119:1464–72. 10.1021/jp5105505.Search in Google Scholar
[47] Tesfaye RM, Das G, Park BJ, Kim J, Yoon HH. Ni–Co bimetal decorated carbon nanotube aerogel as an efficient anode catalyst in urea fuel cells. Sci Rep. 2019;9:1–9. 10.1038/s41598-018-37011-w.Search in Google Scholar PubMed PubMed Central
[48] Ye K, Wang G, Cao D, Wang G. Recent advances in the electro-oxidation of urea for direct urea fuel cell and urea electrolysis. Top Curr Chem. 2018;376:1–38. 10.1007/s41061-018-0219-y.Search in Google Scholar PubMed
[49] Lan R, Tao S. Preparation of nano-sized nickel as anode catalyst for direct urea and urine fuel cells. J Power Sources. 2011;196:5021–6. 10.1016/j.jpowsour.2011.02.015.Search in Google Scholar
[50] Ye K, Zhang D, Guo F, Cheng K, Wang G, Cao D. Highly porous nickel@carbon sponge as a novel type of three-dimensional anode with low cost for high catalytic performance of urea electro-oxidation in alkaline medium. J Power Sources. 2015;283:408–15. 10.1016/j.jpowsour.2015.02.149.Search in Google Scholar
[51] Barakat NAM, Motlak M, Ghouri ZK, Yasin AS, El-Newehy MH, Al-Deyab SS. Nickel nanoparticles-decorated graphene as highly effective and stable electrocatalyst for urea electrooxidation. J Mol Catal A Chem. 2016;421:83–91. 10.1016/j.molcata.2016.05.011.Search in Google Scholar
[52] Glass DE, Galvan V, Prakash GKS. The effect of annealing temperature on nickel on reduced graphene oxide catalysts on urea electrooxidation. Electrochim Acta. 2017;253:489–97. 10.1016/j.electacta.2017.09.064.Search in Google Scholar
[53] Coelho IF, Barbosa JR, Liu L, Nogueira CSC, Franceschini DF, Ponzio EA, et al. Nickel nanoparticles supported by commercial carbon paper as a catalyst for urea electro-oxidation. Mater Renew Sustain Energy. 2020;9:1–11. 10.1007/s40243-020-00180-8.Search in Google Scholar
[54] Munde AV, Mulik BB, Chavan PP, Sathe BR. Enhanced electrocatalytic activity towards urea oxidation on Ni nanoparticle decorated graphene oxide nanocomposite. Electrochim Acta. 2020;349:349. 10.1016/j.electacta.2020.136386.Search in Google Scholar
[55] Wang L, Zhu S, Marinkovic N, Kattel S, Shao M, Yang B, et al. Insight into the synergistic effect between nickel and tungsten carbide for catalyzing urea electrooxidation in alkaline electrolyte. Appl Catal B Env. 2018;232:365–70. 10.1016/j.apcatb.2018.03.064.Search in Google Scholar
[56] Wang L, Du T, Cheng J, Xie X, Yang B, Li M. Enhanced activity of urea electrooxidation on nickel catalysts supported on tungsten carbides/carbon nanotubes. J Power Sources. 2015;280:550–4. 10.1016/j.jpowsour.2015.01.141.Search in Google Scholar
[57] Baker DR, Lundgren CA. Expansion of the urea electrocatalytic oxidation window by adsorbed nickel ions. J Appl Electrochem. 2019;49:883–93. 10.1007/s10800-019-01328-9.Search in Google Scholar
[58] Gan Q, Cheng X, Chen J, Wang D, Wang B, Tian J, et al. Temperature effect on crystallinity and chemical states of nickel hydroxide as alternative superior catalyst for urea electrooxidation. Electrochim Acta. 2019;301:47–54. 10.1016/j.electacta.2019.01.150.Search in Google Scholar
[59] Yan X, Hu QT, Wang G, Zhang WD, Liu J, Li T, et al. NiCo-layered double hydroxide/hydroxide nanosheet heterostructures for highly efficient electro-oxidation of urea. Int J Hydrog Energy. 2020;45:19206–13. 10.1016/j.ijhydene.2020.05.052.Search in Google Scholar
[60] Abdel Hameed RM, Medany SS. Influence of support material on the electrocatalytic activity of nickel oxide nanoparticles for urea electro-oxidation reaction. J Colloid Interface Sci. 2018;513:536–48. 10.1016/j.jcis.2017.11.032.Search in Google Scholar PubMed
[61] Wang S, Xu P, Tian J, Liu Z, Feng L. Phase structure tuning of graphene supported Ni–NiO Nanoparticles for enhanced urea oxidation performance. Electrochim Acta. 2021;370:137755. 10.1016/j.electacta.2021.137755.Search in Google Scholar
[62] Abd El-Lateef HM, Almulhim NF, Alaulamie AA, Saleh MM, Mohamed IMA. Design of ultrafine nickel oxide nanostructured material for enhanced electrocatalytic oxidation of urea: Physicochemical and electrochemical analyses. Colloids Surf A Physicochem Eng Asp. 2020;585:124092. 10.1016/j.colsurfa.2019.124092.Search in Google Scholar
[63] Zhang D, Zhang J, Wang H, Cui C, Jiao W, Gao J, et al. Novel Ni foam based nickel oxalate derived porous NiO nanostructures as highly efficient electrodes for the electrooxidation of methanol/ethanol and urea. J Alloy Compd. 2019;806:1419–29. 10.1016/j.jallcom.2019.07.127.Search in Google Scholar
[64] Xu W, Zhang H, Li G, Wu Z. For direct urea fuel cell. Sci Rep. 2014;4:5863. 10.1038/srep05863.Search in Google Scholar PubMed PubMed Central
[65] Yan W, Wang D, Botte GG. Electrochemical decomposition of urea with Ni-based catalysts. Appl Catal B, Env. 2012;127:221–6. 10.1016/j.apcatb.2012.08.022.Search in Google Scholar
[66] Basumatary P, Konwar D, Yoon YS. A novel Ni–Cu/ZnO@MWCNT anode employed in urea fuel cell to attain superior performances. Electrochim Acta. 2018;261:78–85. 10.1016/j.electacta.2017.12.123.Search in Google Scholar
[67] Li B, Song C, Yan J, Ye K, Cheng K, Cao D, et al. Effect of graphene on the performance of nickel foam-based CoNi nanosheet anode catalyzed direct urea-hydrogen peroxide fuel cell. Int J Hydrog Energy. 2019;5:10569–79. 10.1016/j.ijhydene.2019.05.158.Search in Google Scholar
[68] Basumatary P, Lee UH, Konwar D, Yoon YS. An efficient tri-metallic anodic electrocatalyst for urea electro-oxidation. Int J Hydrog Energy. 2020;45:32770–9. 10.1016/j.ijhydene.2020.04.223.Search in Google Scholar
[69] Lee U, Lee YN, Yoon YS. Enhanced electrochemical properties of catalyst by phosphorous addition for direct urea fuel cell. Front Chem. 2020;8:1–11. 10.3389/fchem.2020.00777.Search in Google Scholar PubMed PubMed Central
[70] Gu X, Yang D, Liu Z, Wang S, Feng L. Iron oxide-promoted nickel/nickel oxide rough nanorods for efficient urea assisted water splitting. Electrochim Acta. 2020;353:136516. 10.1016/j.electacta.2020.136516.Search in Google Scholar
[71] Tran MH, Park BJ, Kim BH, Yoon HH. Mesoporous silica template-derived nickel–cobalt bimetallic catalyst for urea oxidation and its application in a direct urea/H2O2 fuel cell. Int J Hydrog Energy. 2020;45:1784–92. 10.1016/j.ijhydene.2019.11.073.Search in Google Scholar
[72] Wang Y, Liu G. Reduced graphene oxide supported nickel tungstate nano-composite electrocatalyst for anodic urea oxidation reaction in direct urea fuel cell. Int J Hydrog Energy. 2020;45:33500–11. 10.1016/j.ijhydene.2020.09.095.Search in Google Scholar
[73] Zhan S, Zhou Z, Liu M, Jiao Y, Wang H. 3D NiO nanowalls grown on Ni foam for highly efficient electro-oxidation of urea. Catal Today. 2019;327:398–404. 10.1016/j.cattod.2018.02.049.Search in Google Scholar
[74] Ye K, Zhang H, Zhao L, Huang X, Cheng K, Wang G. Facile preparation of three-dimensional N (OH) 2/Ni foam anode with low cost and its application in a direct urea fuel cell. New J Chem. 2016;40(10):8673–80. 10.1039/c6nj01648k.Search in Google Scholar
[75] Khalaf MM, Abd El-Lateef HM, Touny AH, Saleh MM, Mohamed IMA. Electrocatalytic performance of inorganic nanoflakes nickel phosphates under adjusted synthetic parameters towards urea and methanol oxidation in alkaline media. Microchem J. 2021;163:105901. 10.1016/j.microc.2020.105901.Search in Google Scholar
[76] Wang D, Yan W, Botte GG. Exfoliated nickel hydroxide nanosheets for urea electrolysis. Electrochem Commun. 2011;13:1135–8. 10.1016/j.elecom.2011.07.016.Search in Google Scholar
[77] Wang D, Yan W, Vijapur SH, Botte GG. Enhanced electrocatalytic oxidation of urea based on nickel hydroxide nanoribbons. J Power Sources. 2012;217:498–502. 10.1016/j.jpowsour.2012.06.029.Search in Google Scholar
[78] King RL, Botte GG. Investigation of multi-metal catalysts for stable hydrogen production via urea electrolysis. J Power Sources. 2011;196:9579–84. 10.1016/j.jpowsour.2011.06.079.Search in Google Scholar
[79] Urbańczyk E, Jaroń A, Simka W. Electrocatalytic oxidation of urea on a sintered Ni–Pt electrode. J Appl Electrochem. 2017;47:133–8. 10.1007/s10800-016-1024-3.Search in Google Scholar
[80] Mohamed IMA, Yasin AS, Barakat NAM, Song SA, Lee HE, Kim SS. Electrocatalytic behavior of a nanocomposite of Ni/Pd supported by carbonized PVA nanofibers towards formic acid, ethanol and urea oxidation: A physicochemical and electro-analysis study. Appl Surf Sci. 2018;435:122–9. 10.1016/j.apsusc.2017.11.076.Search in Google Scholar
[81] Miller AT, Hassler BL, Botte GG. Rhodium electrodeposition on nickel electrodes used for urea electrolysis. J Appl Electrochem. 2012;42(11):925–34. 10.1007/s10800-012-0478-1.Search in Google Scholar
[82] Alajami M, Yassin MA, Ghouri ZK, Al-Meer S, Barakat NA. Influence of bimetallic nanoparticles composition and synthesis temperature on the electrocatalytic activity of NiMn-incorporated carbon nanofibers toward urea oxidation. Int J Hydrog Energy. 2018;43:5561–75. 10.1016/j.ijhydene.2018.01.163.Search in Google Scholar
[83] Wu MS, Jao CY, Chuang FY, Chen FY. Carbon-encapsulated nickel-iron nanoparticles supported on nickel foam as a catalyst electrode for urea electrolysis. Electrochim Acta. 2017;227:210–6. 10.1016/j.electacta.2017.01.035.Search in Google Scholar
[84] Singh RK, Schechter A. Electroactivity of NiCr catalysts for urea oxidation in alkaline electrolyte. ChemCatChem. 2017;9:3374–9. 10.1002/cctc.201700451.Search in Google Scholar
[85] Shi W, Ding R, Li X, Xu Q, Liu E. Enhanced performance and electrocatalytic kinetics of Ni-Mo/graphene nanocatalysts towards alkaline urea oxidation reaction. Electrochim Acta. 2017;242:247–59. 10.1016/j.electacta.2017.05.002.Search in Google Scholar
[86] Barakat NAM, El-newehy MH, Yasin AS, Khan Z, Al-deyab SS, Applied Catalysis A. General Ni & Mn nanoparticles-decorated carbon nanofibers as effective electrocatalyst for urea oxidation. 2016;510:180–8.10.1016/j.apcata.2015.11.015Search in Google Scholar
[87] Singh R, Schechter A. Electroactivity of NiCr catalysts for urea oxidation in alkaline electrolyte. Cancer. 2017;121:800. 10.1002/cctc.201700451.Search in Google Scholar
[88] Yan W, Wang D, Diaz LA, Botte GG. Nickel nanowires as effective catalysts for urea electro-oxidation. Electrochim Acta. 2014;134:266–71. 10.1016/j.electacta.2014.03.134.Search in Google Scholar
[89] Ahmed A, Al-Amin AQ, Ambrose AF, Saidur R. Hydrogen fuel and transport system: A sustainable and environmental future. Int J Hydrog Energy. 2016;41:1369–80. 10.1016/j.ijhydene.2015.11.084.Search in Google Scholar
[90] Zhu B, Liang Z, Zou R. Designing advanced catalysts for energy conversion based on urea oxidation reaction. Small (Weinh an der Bergstrasse, Ger). 2020;1906133:1–19. 10.1002/smll.201906133.Search in Google Scholar PubMed
[91] Larminie J, Dicks A. Fuel cell systems explained. Chichester, UK: J. Wiley; 200310.1002/9781118878330Search in Google Scholar
[92] Haile SM. Fuel cell materials and components. Acta Mater. 2003;51:5981–6000. 10.1016/j.actamat.2003.08.004.Search in Google Scholar
[93] Ranjani M, Senthilkumar N, Gnana Kumar G, Manthiram A. 3D flower-like hierarchical NiCo2O4 architecture on carbon cloth fibers as an anode catalyst for high-performance, durable direct urea fuel cells. J Mater Chem A. 2018;6:23019–27. 10.1039/c8ta08405j.Search in Google Scholar
[94] Bian L, Du T, Du Q, Luo M, Li M. Multiwalled carbon nanotubes twined a nickel hydroxide microspheres as high-efficient urea electrooxidation catalysts. J Appl Electrochem. 2017;47:905–15. 10.1007/s10800-017-1087-9.Search in Google Scholar
[95] Putri YMTA, Gunlazuardi J, Ivandini TA. Electrochemical study of nickel–cobalt deposited on boron-doped diamond as working electrodes for urea fuel cells. IOP Conf Ser Mater Sci Eng. 2019;496:496. 10.1088/1757-899X/496/1/012051.Search in Google Scholar
[96] Jiang S, Ying H, Qiang L, Hong B, Peng Z. A development of direct hydrazine/hydrogen peroxide fuel cell. J Power Sources. 2010;195:4135–8. 10.1016/j.jpowsour.2010.01.059.Search in Google Scholar
[97] Guo F, Cao D, Du M, Ye K, Wang G, Zhang W, et al. Enhancement of direct urea-hydrogen peroxide fuel cell performance by three-dimensional porous nickel–cobalt anode. J Power Sources. 2016;307:697–704. 10.1016/j.jpowsour.2016.01.042.Search in Google Scholar
[98] Hickner MA, Herring AM, Coughlin EB. Anion exchange. Mem: Curr Status Mov Forw. 2013;121:1727–35. 10.1002/polb.23395.Search in Google Scholar
[99] Cheng J, He G, Zhang F. A mini-review on anion exchange membranes for fuel cell applications: Stability issue and addressing strategies. Int J Hydrog Energy. 2015;40:7348–60. 10.1016/j.ijhydene.2015.04.040.Search in Google Scholar
© 2021 Yulia Mariana Tesa Ayudia Putri et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


![Figure 3
CVs of nickel nanoparticle electrode [1] (a) and Ni–Co hydroxide electrodes [35] (b) in 5 M KOH in the presence and absence of urea, republished with permission.](/document/doi/10.1515/chem-2021-0100/asset/graphic/j_chem-2021-0100_fig_003.jpg)
![Figure 4
The dependence of urea electro-oxidation on KOH concentrations at (a) nickel nanoparticles and (b) NiCo/MWCNT electrodes. Scan rates of 10 and 20 mV s−1 were applied at (a) and (b), respectively, republished with permission [1,47].](/document/doi/10.1515/chem-2021-0100/asset/graphic/j_chem-2021-0100_fig_004.jpg)
![Figure 5
The dependence of urea electro-oxidation on urea concentrations at (a) nickel nanoparticle and (b) NiCo/MWCNT electrode. Scan rates at (a) was 10 mV s−1, while at (b) was 20 mV s−1, republished with permission [1,47].](/document/doi/10.1515/chem-2021-0100/asset/graphic/j_chem-2021-0100_fig_005.jpg)
