Abstract
The aim of this work was to investigate the volatile compositions of four Chinese functional liquors. For this purpose, volatile compounds of four liquors were extracted with head-space solid-phase microextraction (HS-SPME) and analyzed with gas chromatography-mass spectrometry (GC-MS) along with the determination of odor activity value (OAV) and relative odor contribution (ROC). Sixty volatiles were tentatively identified and categorized into the following seven groups: alcohols, esters, fatty acids, carbonyl compound, hydrocarbons, phenols, and other components. The differences in chemical composition of volatile compounds were visualized with heat maps. Odorants were compared with different samples using a statistical analysis of Venn diagrams and a multivariate principal component analysis, and ethyl hexanoate, ethyl acetate, and ethyl octanoate were found to be the key odorants. Besides, abundant phenolic contents and high antioxidant ability of four Chinese functional liquors could potentially bring better health-boosting effects.
Abbreviations
- ABTS
-
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- DPPH
-
1,1-diphenyl-2-picryl-hydrazyl
- GAE
-
gallic acid equivalents
- GC-MS
-
gas chromatography-mass spectrometry
- HS-SPME
-
head-space solid-phase microextraction
- OAV
-
odor activity value
- PCA
-
principal component analysis
- ROC
-
relative odor contribution
- TEAC
-
trolox equivalent antioxidant capacity
1 Introduction
The Chinese functional wine, a combination of traditional Chinese wine and Chinese herbs, is produced by adding Chinese herbs into wine during its production. The following four Chinese functional liquors are dominant in the market: Kinmen-Kaoliang Liquor, Jin Liquor, highland barley wine, and Zhuyeqing Liquor. Kinmen-Kaoliang Liquor, which belongs to mild aromatic Chinese spirits and possesses the unique fragrance and mellow taste, is the representative of sorghum wine. Jin Liquor can improve both mental and physical fatigue caused by sub-health [1]. Highland barley, produced by fermentation of barley, is rich in amino acids, protein, dietary fiber, vitamins, and microelements [2]. It has a characteristic sweet taste and enables to reduce cholesterol and blood-lipid. Zhuyeqing Liquor delivers a full fragrance flavor based on a Fen wine distillate base. The liquor contains the extracts from bamboo leaves, chrysanthemum, angelica, and other herbs, and has the detoxicating and anti-aging capacity for humans [3].
Because of the presence of Chinese herbs, volatiles of Chinese functional liquor are most likely different compared to those of the non-functional liquor, which remains poorly understood. Aroma is a decisive factor to affect the quality and consumer acceptance of a liquor [4]. Therefore, unraveling the aroma profiles of Chinese functional liquors will be essential to understand their taste, nutrition, and popularity. Multiple methods have been used to extract the volatile compounds from wine; head-space solid-phase microextraction (HS-SPME) was found to be superior to other methods because of their simplicity, accuracy, and fast capacity [5]. Using this technique, Ivanova identified 30 representative wine volatile compounds from eight varietal wines in the Macedonian and Hungarian [6]. Xiao et al. used HS-SPME-GC-MS and electronic-nose to assess the aroma compounds and to determine odor descriptors of five typical Chinese liquors [7]. Eighty-six aroma compounds were identified, including 5 acids, 34 esters, 10 alcohols, 9 aldehydes, 4 ketones, 4 phenols, and 10 nitrous and sulfuric compounds. Odor activity value (OAV) and relative odor contribution (ROC) are the two main parameters to assess the contribution of aromatic compounds. OAV is the ratio of the real concentration of an individual compound and its olfactory threshold. ROC represents the ratio of the OAV percentage of each individual compound and the sum of the OAV of compounds that showed OAV >1 [8].
Polyphenols are the secondary metabolites of plants and have been demonstrated to be strong antioxidants in wine. In our previous study, it was found that the total polyphenols content could be 456 mg gallic acid equivalent (GAE)/L in gingko wine, and some typical Chinese liquors also contained 45–130 mg GAE/L total polyphenols. These bioactive compounds increase the HDL of high-density lipoprotein in blood, effectively reduce blood cholesterol, prevent atherosclerosis, and also inhibit platelet agglutination and prevent thrombosis [9].
The objective of the present work was to identify the volatile flavor compounds in four Chinese functional liquors and assess their in vitro antioxidant activity. Sixty volatile compounds were identified from the volatiles of four liquors GC-MS after HS-SPME extraction. The phenolic content, 1,1-diphenyl-2-picryl-hydrazyl (DPPH), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) value of the liquor were also measured and compared with traditional Chinese wine.
2 Materials and methods
2.1 Wine samples
The four typical Chinese functional liquors, Kinmen-Kaoliang (L1), Jin (L2), Highland barley (L3), and Zhuyeqing (L4), were purchased from the local market. Their alcoholic contents were 45.0% (v/v), 45.0% (v/v), 35.0% (v/v), and 38.0% (v/v), respectively. To minimize the effects from different alcoholic contents, four wines were diluted with aqueous ethanol 50.0% (v/v) to reach a final alcoholic content of 12.0% (v/v).
2.2 Chemicals and reagents
A C7-C30 n-alkane mixture, used for the determination of linear retention indices (RIs), was purchased from Supelco (Bellefonte, PA, USA). 2-Octanol used as internal standard was obtained from Dr. Ehrenstorfer (Augsburg, Germany). Folin-Ciocalteu reagent, DPPH, ABTS, trolox, gallic acid, and potassium persulfate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Water was obtained from a Milli-Q purification system (Millipore). Other chemicals and reagents were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All reagents used were of analytical grade.
2.3 Extraction of volatile compounds
Volatile components of the liquors were extracted with HS-SPME method. Liquor samples (5 mL) were pipetted into 15 mL headspace vials, and 1 g sodium chloride and 50 µL 2-octanol (internal standard) were then added and mixed. The vial was sealed with a silicon septum, placed in 50°C water bath, and equilibrated for 15 min. A 75 µm DVB/CAR/PDMS solid-phase fiber (Supelco, Bellefonte, PA, USA) was then plugged into the headspace of the vial for 30 min. Later, the solid-phase fiber was immediately injected into the gas chromatography-mass spectrometry (GC-MS) injection port for desorption (5 min) in splitless mode and then analyzed [10]. Each sample was done in triplicates.
2.4 GC-MS conditions and quantitative analysis of wine volatiles
Volatile compounds were separated and identified on a 7890 gas chromatography coupled with a 5975 C mass selective detector (MS) (Agilent Technologies, USA), equipped with a HP-INNOWAX capillary column (60 m × 0.25 mm ID, 0.25 μm film thickness). The carrier gas helium was circulated at 1 mL/min in the constant flow mode. A split/splitless injector was used in the splitless mode. The injected volume was 1 μL and the injector temperature was set at 250°C. The oven temperature program was set as follows: 50°C for 2 min; then 6°C/min ramps to 230°C and holding for 10 min. The transfer line and the ion source temperatures were set at 250°C. The ion energy for electron impact (EI) was 70 eV, and the chromatograms were obtained by recording a mass range of 30–450 m/z.
Tentative identification of the volatile compounds was achieved by comparing mass spectrum and RIs with the Nist05a.1 Database and Wiley7n.1 Database (Hewlett-Packard, Palo Alto, CA) and literatures. Some compounds were identified by injecting the authentic compounds into the GC-MS system, while the RI of the compounds was calculated using an n-alkane series under the same conditions according to Van Den Dool and Kratz equation [11]. Semiquantitative determinations were performed accord-ing to Xiao [10].
2.5 Determination of total phenolic content
The total phenolic content of the four Chinese functional liquors was measured by a modified colorimetric Folin–Ciocalteu’s method [12,13]. Briefly, an aliquot (100 μL) of the diluted wines was pipetted into a 15 mL test tube with a cap, and Folin–Ciocalteu reagent (0.1 mL) was added and mixed. After 5 min, 10% Na2CO3 solution (w/v) (3 mL) was added, mixed, and heated at 75°C for 10 min before measurement of the absorbance at 760 nm using a UV-2350 spectrophotometer (UNICO (Shanghai) Instruments Co., Ltd, Shanghai, China). Gallic acid solutions with different concentrations (0–400 mg/L) were measured to obtain a calibration curve. The total phenolic was expressed as mg of GAE per liter of sample (mg GAE/L). Each sample was done in triplicates.
2.6 DPPH and ABTS free radical scavenging capacity
Diluted sample (1 mL) or aqueous ethanol (50.0%, v/v) (blank) was added into freshly prepared 0.04 mmol/L DPPH solutions (2 mL). The solutions were mixed and left at 30℃ in dark for 30 min before measurement of absorbance at 515 nm using a spectrophotometer (UV-2350). A calibration curve was built with trolox at different concentrations (0–400 mg/L). The DPPH value of the samples was expressed as trolox equivalent antioxidant capacity (TEAC, mg/L). The percentage radical-scavenging activity (%SA) of DPPH was calculated using the equation:
The ABTS˙+ cation was generated by mixing 7 mM ABTS˙+ solution and 2.45 mM potassium persulfate solution and left at room temperature for 12–16 h in dark. It was then diluted with ethanol/water (50/50, v/v) to obtain an absorbance of 0.70 ± 0.02 at 734 nm before use. Liquor sample (1 mL) or aqueous ethanol (blank, 50.0%, v/v) was added into diluted ABTS˙+ solution (2 mL), mixed, and incubated for 6 min at 37°C water bath. The decrease of absorbance at 734 nm was then measured. The percentage of inhibition (%I) was calculated using the following equation:
The antioxidant activities of samples were expressed as TEAC values, defined as the concentration of standard trolox with the same antioxidant capacity as those of the samples.
2.7 Statistical analysis
All measurements were done in triplicates and results were presented as mean ± standard deviation (SD) (n = 3). Linearity was studied by quantification of the correlation coefficients. The antioxidant capacity analysis was performed by analysis of variance using the software SAS V8 (SAS Institute Inc., Cary, NC). Statistical significance was declared at P < 0.05. Heat map visualization of data was performed using the TBtools v0.668375 (Toolbox for Biologists). Data was log-transformed dividing the values of relative peak areas of each volatile compound by mean to perform heat maps. The Venn diagram was generated with the web tool provided by the Bioinformatics and Systems Biology of Gent (URL: http://bioinformatics.psb.ugent.Be/webtools/Venn/).
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Volatile compounds and OAV evaluation
Compositions of aroma compounds and their calculated RI values from the four functional liquors are listed in Table 1. A total of 61 volatile compounds were tentatively identified and categorized into the following seven groups: esters (28), alcohols (8), fatty acids (3), carbonyl compound (including aldehydes and ketones) (11), hydrocarbons (7), phenols (1), and other compositions (3). The relatively low standard deviations obtained for most compounds confirmed the authenticity and validity of the volatile profile of the four liquors. The threshold values for 12 compounds remained unknown and only five compounds with OAVs <1 were found. In other words, most of the volatile compounds contributed significantly to the overall aroma of the liquor.
Volatile composition of four Chinese functional liquors (mg/L, n = 3) identified by GC-MS
| RIcala | RIrefb | OTSc (mg/L) | Concentrationd (mg/L) | OAVe | ROCf (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L1 | L2 | L3 | L4 | L1 | L2 | L3 | L4 | |||||
| Alcohols | ||||||||||||||||
| 1 | 2-Butanol | 1,016 | 1,017 | 3.300 | — | 9.43 ± 0.01 | — | — | — | 2.86 | — | — | — | 0.0040 | — | — |
| 2 | Isobutanol | 1,086 | 1,081 | 16.000 | 9.93 ± 0.02 | 10.11 ± 0.11 | — | 2.97 ± 0.12 | 0.62 | 0.63 | — | 0.19 | — | — | — | — |
| 3 | Isoamyl alcohol | 1,189; 1,199 | 1,214 | 2.800 | 84.76 ± 0.23 | 97.91 ± 0.05 | 43.1 ± 0.16 | 65.46 ± 0.11 | 30.27 | 34.97 | 15.39 | 23.38 | 0.0171 | 0.0495 | 0.0030 | 0.0108 |
| 4 | 1-Hexanol | 1,327 | 1,387; 1,343 | 0.4251 | 4.26 ± 0.13 | — | 43.05 ± 0.32 | 7.00 ± 0.04 | 10.02 | — | 101.29 | 16.47 | 0.0057 | — | 0.0200 | 0.0076 |
| 5 | Borneol | 1,689 | — | 0.140 | — | 19.38 ± 0.03 | — | 1.53 ± 0.02 | — | 138.43 | — | 10.93 | — | 0.1959 | — | 0.0050 |
| 6 | Phenylethyl alcohol | 1,916 | 1,929; 1,907 | 1.12 | 2.08 ± 0.1 | 5.55 ± 0.01 | — | 1.61 ± 0.23 | 1.89 | 5.05 | — | 1.46 | 0.0011 | 0.0071 | — | 0.0007 |
| 7 | α-Cadinol | 2,195 | 2,231 | NF | — | 1.00 ± 0.06 | — | — | — | — | — | — | — | — | — | — |
| 8 | cis-α-Santalol | 2,355 | — | NF | — | — | — | 1.04 ± 0.34 | — | — | — | — | — | — | — | — |
| Total | 101.03 | 143.38 | 86.15 | 79.61 | ||||||||||||
| Esters | ||||||||||||||||
| 9 | Ethyl acetate | 878 | 900 | 12.33 | 111.7 ± 0.14 | 72.91 ± 0.24 | 199.38 ± 0.35 | 110.33 ± 0.19 | 9.08 | 5.93 | 16.21 | 8.97 | 0.0051 | 0.0084 | 0.0032 | 0.0041 |
| 10 | Ethyl butyrate | 1,030 | 1,042 | 0.024 | 13.9 ± 0.08 | 10.97 ± 0.39 | 33.75 ± 0.43 | 5.76 ± 0.02 | 695 | 548.50 | 1687.50 | 288.00 | 0.3929 | 0.7763 | 0.3334 | 0.1329 |
| 11 | Ethyl 2-methylbutyrate | 1,045; 1,063 | 1,041 | 0.0003 | 1.11 ± 0.17 | — | — | 0.40 ± 0.15 | 4269.23 | — | — | 1538.46 | 2.4133 | — | — | 0.7097 |
| 12 | Ethyl isovalerate | 1,058 | 1,055 | 0.0003 | 1.64 ± 0.21 | — | — | 0.52 ± 0.02 | 5466.67 | — | — | 1733.33 | 3.0902 | — | — | 0.7996 |
| 13 | Isoamyl acetate | 1,106 | 1,103 | 0.0301 | 9.6 ± 0.00 | 19.81 ± 0.02 | 11.58 ± 0.11 | 13.73 ± 0.29 | 320.0 | 660.33 | 386.00 | 457.67 | 0.1809 | 0.9346 | 0.0763 | 0.2111 |
| 14 | Ethyl pentanoate | 1,118 | 1,140 | 0.0051 | 4.18 ± 0.04 | 5.53 ± 0.00 | 44.43 ± 0.01 | 2.48 ± 0.04 | 836.0 | 1106.00 | 8886.00 | 496.00 | 0.4726 | 1.5653 | 1.7556 | 0.2288 |
| 15 | Ethyl hexanoate | 1,215 | 1,238 | 0.0003 | 48.11 ± 0.00 | 17.32 ± 0.10 | 124.25 ± 0.02 | 44.91 ± 0.16 | 160366.67 | 57733.33 | 414,167 | 149,700 | 90.6518 | 81.7088 | 81.8278 | 69.0616 |
| 16 | Isopentyl isobutyrate | 1,279 | — | 0.0003 | — | — | 14.19 ± 0.04 | 0.52 ± 0.10 | — | — | — | 47,300 | — | — | — | 21.8211 |
| 17 | Ethyl 5-methylhexanoate | 1,288 | — | NF | — | — | 2.91 ± 0.00 | — | — | — | — | — | — | — | — | — |
| 18 | Ethyl (Z)-hex-3-enoate | 1,297 | — | NF | — | — | 3.21 ± 0.06 | — | — | — | — | — | — | — | — | — |
| 19 | Ethyl hex-4-enoate | 1,301 | — | NF | — | — | 2.86 ± 0.05 | — | — | — | — | — | — | — | — | — |
| 20 | Propyl hexanoate | 1,311 | — | 12.780 | — | — | 15.98 ± 0.00 | — | — | — | 1.25 | — | — | — | 0.0003 | — |
| 21 | Ethyl heptanoate | 1,311; 1,331 | — | 0.0021 | 3.55 ± 0.01 | 1.39 ± 0.03 | 159.53 ± 0.11 | 3.24 ± 0.08 | 1577.78 | 617.78 | 70902.2 | 1440.00 | 0.8919 | 0.87433 | 14.0083 | 0.6643 |
| 22 | Ethyl lactate | 1,323 | — | 1.4001 | 5.42 ± 0.00 | 5.63 ± 0.01 | — | 11.44 ± 0.00 | 3.87 | 4.02 | — | 8.17 | 0.0022 | 0.0057 | — | 0.0038 |
| 23 | Isobutyl caproate | 1,340 | — | NF | — | — | 4.00 ± 0.04 | — | — | — | — | — | — | — | — | — |
| 24 | Ethyl octanoate | 1,413 | 1,434 | 0.015 | 21.65 ± 0.03 | 50.72 ± 0.00 | 126.84 ± 0.03 | 135.93 ± 0.02 | 1443.33 | 3381.33 | 8456.00 | 9062.00 | 0.8159 | 4.7855 | 1.6707 | 4.1806 |
| 25 | Isoamyl hexanoate | 1,445; 1,458 | — | 0.320 | — | — | 7.93 ± 0.01 | — | — | — | 24.78 | — | — | — | 0.0049 | — |
| 26 | Ethyl nonanoate | 1,530; 1,535 | — | 12.000 | — | — | 5.21 ± 0.10 | 11.31 ± 0.04 | — | — | 0.43 | 0.94 | — | — | 0.0001 | 0.0004 |
| 27 | Hexyl hexanoate | 1,592 | — | 6.400 | — | — | 7.53 ± 0.11 | — | — | — | 1.18 | — | — | — | 0.0002 | — |
| 28 | Ethyl decanoate | 1,619; 1,637 | 1,639 | 0.023 | — | 21.53 ± 0.00 | 1.12 ± 0.02 | 71.13 ± 0.01 | — | 936.09 | 48.70 | 3092.61 | — | 1.3248 | 0.0096 | 1.4267 |
| 29 | Diethyl succinate | 1,663; 1,670 | — | 2.005 | — | 8.94 ± 0.03 | — | 4.23 ± 0.04 | — | 0.04 | — | 0.02 | — | — | — | — |
| 30 | Ethyl benzoate | 1,669 | 1,680 | 0.060 | — | — | 0.75 ± 0.01 | 5.69 ± 0.05 | — | — | 12.50 | 94.83 | — | — | 0.0025 | 0.0438 |
| 31 | 2-Phenethyl acetate | 1,823 | 1,822 | 0.160 | — | 6.61 ± 0.00 | — | — | — | 41.31 | — | — | — | 0.05847 | — | — |
| 32 | Ethyl dodecanoate | 1,855; 1,840 | 1,839 | 0.400 | — | — | — | 0.60 ± 0.04 | — | — | — | 1.50 | — | — | — | 0.0007 |
| 33 | Ethyl benzenepropanoate | 1,897 | — | NF | — | 1.57 ± 0.05 | — | — | — | — | — | — | — | — | — | — |
| 34 | Ethyl tetradecanoate | 2,056 | 2,034 | 0.180 | — | — | — | 0.92 ± 0.00 | — | — | — | 5.11 | — | — | — | 0.0024 |
| 35 | Ethyl hexadecanoate | 2,261 | 2,260 | 2.000 | — | — | — | 5.31 ± 0.03 | — | — | — | 2.66 | — | — | — | 0.0012 |
| 36 | Octyl adipate | 1,889 | 1,892 | NF | 17.31 ± 0.01 | — | — | — | — | — | — | — | — | — | — | — |
| Total | 238.17 | 222.93 | 765.45 | 428.45 | ||||||||||||
| Acid | ||||||||||||||||
| 37 | Butyric acid | 1,604 | 1,639 | 1.400 | — | — | 16.75 ± 0.00 | 23.57 ± 0.07 | — | — | 11.96 | 16.84 | — | — | 0.0024 | 0.0078 |
| 38 | Hexanoic acid | 1,865 | 1,871 | 3.0001 | 12.02 ± 0.23 | 1.98 ± 0.11 | 34.8 ± 0.03 | — | 4.01 | 0.66 | 11.60 | — | 0.0023 | 0.0009 | 0.0023 | — |
| 39 | Octanoic acid | 2,055 | 2,051 | 0.5001 | 2.09 ± 0.02 | 0.87 ± 0.24 | 1.56 ± 0.10 | 4.16 ± 0.00 | 4.18 | 1.74 | 3.12 | 8.32 | 0.0024 | 0.0025 | 0.0006 | 0.0038 |
| Total | 14.11 | 2.85 | 53.11 | 27.73 | ||||||||||||
| Carbonyl compound | ||||||||||||||||
| 40 | Acetaldehyde | 627 | 741 | 0.010 | 16.03 ± 0.00 | 42.98 ± 0.01 | 1.44 ± 0.06 | 11.17 ± 0.10 | 1603.00 | 4298.00 | 144.00 | 1117.00 | 0.9061 | 6.0829 | 0.0285 | 0.5153 |
| 41 | Isovaleraldehyde | 916 | 924 | 0.006 | — | 5.59 ± 0.06 | — | — | — | 931.67 | — | — | — | 1.31857 | — | — |
| 42 | Hexanal | 1,073 | 1,083 | 0.009 | 0.65 ± 0.03 | — | — | 0.93 ± 0.00 | 65.00 | — | — | 93.00 | 0.0367 | — | — | 0.0429 |
| 43 | 2-Octanone | 1,272 | 1,275 | 0.050 | 7.91 ± 0.01 | 9.18 ± 0.05 | 19.48 ± 0.00 | 8.43 ± 0.06 | 158.20 | 183.60 | 389.60 | 168.60 | 0.0894 | 0.2599 | 0.0770 | 0.0778 |
| 44 | 3-Nonanone | 1,339 | — | 0.033 | 1.00 ± 0.06 | — | — | — | 30.30 | — | — | — | 0.0171 | — | — | — |
| 45 | 2-Nonanone | 1,371 | 1,388 | 0.200 | 0.28 ± 0.03 | — | 38.68 ± 0.09 | — | 1.40 | — | 193.40 | — | 0.0008 | — | 0.0382 | — |
| 46 | Nonanal | 1,376 | 1,396 | 0.260 | 0.43 ± 0.08 | — | — | 2.51 ± 0.03 | 1.65 | — | — | 9.65 | 0.0009 | — | — | 0.0045 |
| 47 | 2-Decanone | 1,486 | 1,491 | 0.008 | — | — | 3.48 ± 0.03 | — | — | — | 419.28 | — | — | — | 0.0828 | — |
| 48 | Furfural | 1,453 | 1,477 | 0.770 | 4.91 ± 0.07 | 6.08 ± 0.04 | 20.87 ± 0.00 | — | 6.38 | 7.90 | 27.10 | — | 0.0036 | 0.0112 | 0.0054 | — |
| 49 | (E)-Cinnamaldehyde | 2,072 | — | 0.750 | — | 2.12 ± 0.04 | — | — | — | 2.83 | — | — | — | 0.0040 | — | — |
| 50 | Benzylidenemalonaldehyde | 2,078 | — | NF | — | 1.25 ± 0.06 | — | — | — | — | — | — | — | — | — | — |
| Total | 31.21 | 67.20 | 83.95 | 23.04 | ||||||||||||
| Hydrocarbons | ||||||||||||||||
| 51 | Camphene | 1,057 | 1,077 | 1.860 | — | 4.75 ± 0.05 | — | — | — | 2.55 | — | — | — | 0.0036 | — | — |
| 52 | β-Myrcene | 1,140 | 1,170 | 0.100 | — | 0.69 ± 0.03 | — | 1.32 ± 0.00 | — | 6.90 | — | 13.20 | — | 0.0098 | — | 0.0061 |
| 53 | d-Limonene | 1,177 | — | 1.200 | — | 4.43 ± 0.00 | — | 65.97 ± 0.00 | — | 3.69 | — | 54.98 | — | 0.0052 | — | 0.0254 |
| 54 | (–)-Calamenene | 1,840 | 1,837 | NF | — | 1.39 ± 0.02 | — | — | — | — | — | — | — | — | — | — |
| 55 | Dodecane | 1,170 | — | 10.000 | — | — | — | 2.66 ± 0.01 | — | — | — | 0.27 | — | — | — | 0.0001 |
| 56 | O-Cymene | 1,249 | — | NF | — | — | — | 8.01 ± 0.04 | — | — | — | — | — | — | — | — |
| Total | 0 | 11.26 | 0 | 77.96 | ||||||||||||
| Phenols | ||||||||||||||||
| 57 | Eugenol | 2,191 | 2,172 | 0.150 | — | 0.40 ± 0.09 | — | — | — | 2.67 | — | — | — | 0.0038 | — | — |
| Total | 0 | 0.40 | 0 | 0 | ||||||||||||
| Others | ||||||||||||||||
| 58 | l-Alanine | 553 | — | 710.000 | — | 12.12 ± 0.03 | — | — | — | 0.02 | — | — | — | 0.00002 | — | — |
| 59 | N-Ethyl-1,3-dithioisoindoline | 912 | — | 0.500 | — | — | 3.33 ± 0.05 | — | — | — | 6.66 | — | — | — | 0.0013 | — |
| 60 | Indane | 1,371 | 1,370 | 0.010 | — | — | 2.28 ± 0.15 | — | — | — | 228.00 | — | — | — | 0.0451 | — |
| Total | 0 | 12.12 | 5.61 | 0 | ||||||||||||
NF: not found.
- a
Linear RIs calculated of unknown compounds on a HP-INNOWAX capillary column (60 m × 0.25 mm × 0.25 μm) with a homologous series of n-alkanes (C7–C30) obtained from literatures.
- b
The theoretical retention index obtained from the flavor net database (i), in the literature.
- c
- d
Values are the mean ± SD.
- e
Odor activity value.
- f
Relative odor contribution.
As shown in Figure 1, the similarity and difference of volatile compounds in four functional liquors were analyzed using Venn diagram. From left to right, the common compounds and different compounds of L1, L2, L3, and L4 were grouped. There were 60 different components including 1 acid (octanoic acid), 1 alcohol (isoamyl alcohol), 1 aldehyde (acetaldehyde), and 7 esters (ethyl acetate, ethyl butyrate, isoamyl acetate, ethyl valerate, ethyl hexanoate, ethyl heptanoate, and ethyl octanoate). L1, L2, L3, and L4 have 2, 11, 10, and 6 components respectively and large difference in taste was observed between L2 and L3.
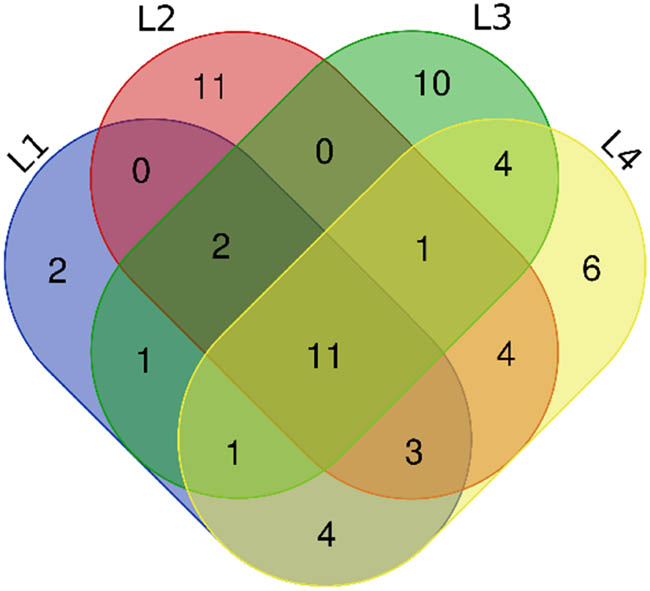
Venn diagrams for comparison of volatile components in four functional liquors.
Esters were shown to be the dominant group in the four liquors accounted for 61.9, 48.5, 76.9, and 67.3% of total amount of volatile compounds for L1, L2, L3, and L4, respectively (Figure 2). Esters are important family of aroma compounds in liquors. They are commonly formed by esterification of alcohols and acids followed by dehydration. In general, esters were divided into two categories: acetate esters and ethyl esters. Ethyl esters of fatty acids were produced during the alcoholic fermentation and endowed the fruity aromas. Ethyl hexanoate, ethyl acetate, and ethyl octanoate were the major components in ester group of the four samples. Their presence contributed to the pleasant, fruity fragrance notes of the liquors. Ethyl octanoate generated pineapple, pear, and sweet fruit aroma was reported to be the most abundant compound in white wines [14]. Other dominant compounds in ester groups were ethyl butyrate, isoamyl acetate, ethyl pentanoate, ethyl heptanoate, ethyl lactate, and ethyl decanoate. Their presence commonly contributed to the fruity bouquet notes of the alcoholic drinks. Ethyl lactate produced rum, fruit, and cream aroma. Hexyl hexanoate with apple peel and peach aroma were detected in L3 (7.53 mg/L) with the highest concentration. Ethyl nonanoate with floral and fruity aroma were found only in L3 (5.21 mg/L) and L4 (11.3 mg/L).
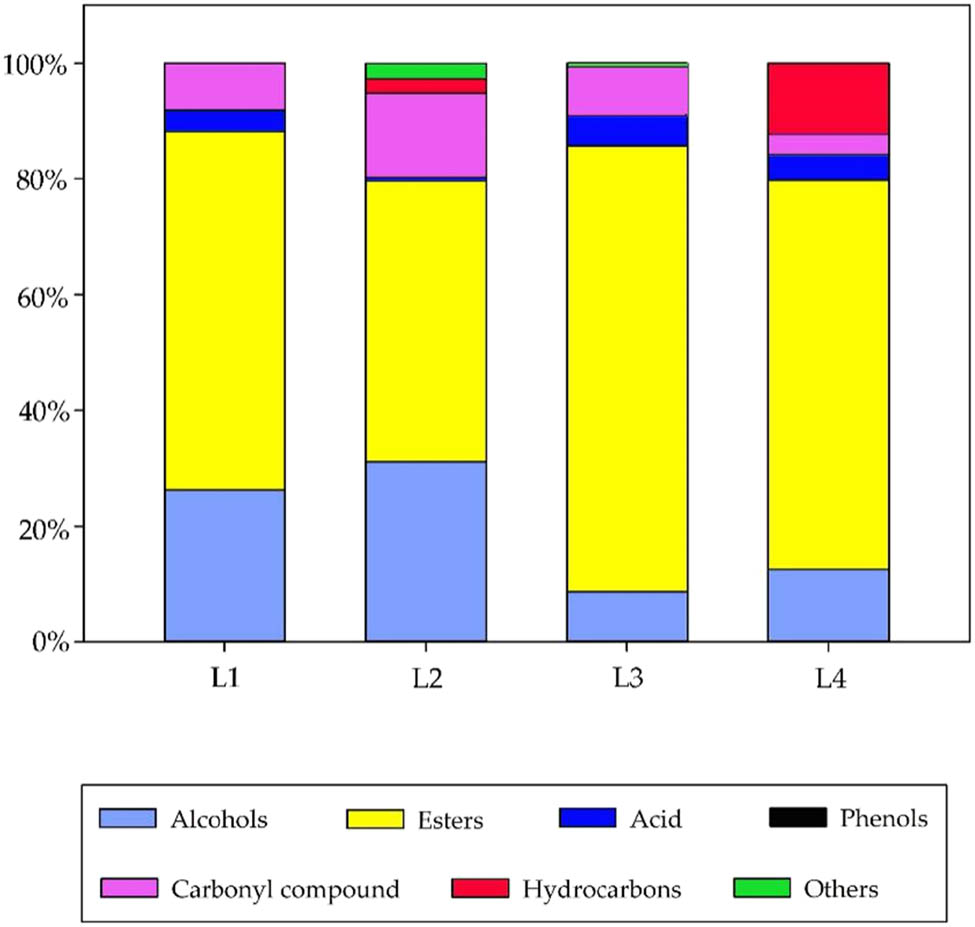
The proportion of various kinds of aroma substances in four functional liquors.
There were 21 esters with OAVs >1 (Table 1). Four of them (ethyl hexanoate, ethyl pentanoate, ethyl heptanoate, and ethyl octanoate) showed high values of OAV over 1,000, especially ethyl hexanoate, which correlates with a green apple odor. Welke and coworkers studied the volatile compounds of Chardonnay wine using HS-SPME-GC × GC/TOFMS and found that esters were the large class and ethyl hexanoate had the highest OAV and ROC values [15], which is consistent with the present findings.
Eight alcohols were detected in the functional liquors and served as one of the decisive factors for the flavor of liquors. Isoamyl alcohol was the most abundant alcohol in all samples (from 43.1 to 97.9 mg/L). It contributed to a whiskey, malt, and burned aroma. 1-Hexanol contributed herbaceous, grass, and woody aroma to liquors [14]. Isobutanol was the abundant alcohol next to 1-hexanol, with a “wine, solvent, bitter” odor. 2-Butanol and α-cadinol were identified only in L2 liquor with a low concentration 9.43 mg/L and 1.00 mg/L, respectively. 2-Butanol gave nuances of “fruity and wine,” which is vinous in character. α-cadinol has a “herbaceous and woody” odor, but the detection thresholds of the compounds are unavailable, thereby their contribution to the whole aroma is uncertain.
Acids were responsible for fruity, cheese, fatty, and rancid notes. Short-chain acids had the aroma of sour and rancid, and could suppress and cover other aroma in liquors, so appropriate concentration of them in liquor was preferred. Hexanoic and octanoic acid produced a cheese flavor at low concentrations and harsh and rancid odors at high concentrations [16]. Butyric acid contributed a cheese aroma at low concentrations while yielding rancid and sweat odors at high concentrations. It was only detected in L3 (16.8 mg/L) and L4 (23.6 mg/L), which might cause cheese flavors.
Seven aldehydes and four ketones were identified, all of them showed OAVs >1. Aldehyde compounds, formed from unsaturated fatty acids, also can be considered as products of lipoxygenase catalysis. Acetaldehyde and hexanal were responsible for grass and tallow fat aroma, furfural for bead almond and sweet aroma, and nonanal for fatty-floral aroma [17]. Ketones can be formed by condensation of activated fatty acids. 2-Octanone, which exhibited fruity and floral notes, 2-nonanone and 2-decanone were detected with higher OAVs, the former contributed to fruity, floral, and herbal notes. Only eugenol was detected in four liquors. It has been indicated that phenols often have spicy and smoky-clove-like odors.
Six hydrocarbons are listed in Table 1, and d-limonene belongs to terpene compounds giving sweet, citrus-, and lemon-like notes. The olfactory detection threshold of l-alanine was far more than its content in L2 liquor. Therefore, its contribution to the wine flavor was negligible. As the odor detection thresholds of the major part of these compounds have not been determined, their contribution to four liquors aroma remains unknown.
To intuitively perceive the different contents of aroma compounds in four functional liquors, a heat map (Figure 3) was generated based on the data in Table 1. After logarithmic conversion of the data, we can compare the expression of the same substance in different samples or the expression of different substances in the same sample. The concentrations of the flavor substances in four functional liquors were log-transformed. As many flavors do not appear in the samples (for 0 has not logarithmic), the expression for the entire data matrix for each value x was set. log scale base is 2, and log width is 1. As can be seen in Figure 3, color coding was devised based on the scale from blue to red with the relative intensity decreasing from high to low (0.00–8.00), which enables to distinguish different samples.
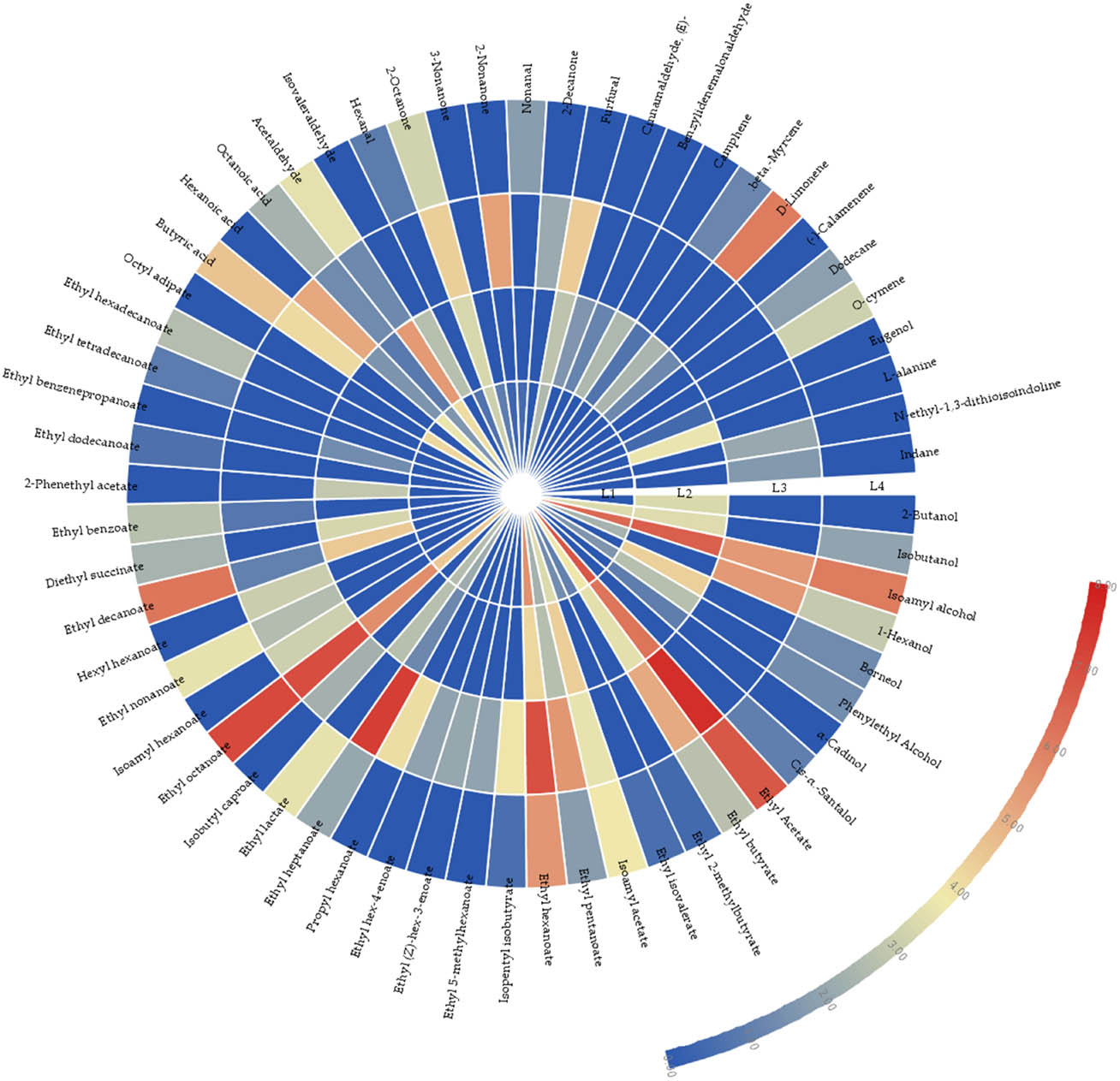
Heat map analysis of aroma compounds in four functional liquors.
ROC that represented the contribution percentage of each volatile compound to aroma is also shown in Table 1. Ethyl hexanoate showed the highest contribution to final aroma of wine (ROC = 90.7, 81.7, 81.8, and 69.1%, respectively). It was reported that ethyl hexanoate was also the key compound to the odor of Cabernet Sauvignon and Chardonnay wines from China [18]. The total ROC of esters was above 90.0%. These results confirm the decisive role of esters on the aroma of four liquors.
3.2 Principal component analysis (PCA)
To unravel the similarities of these four samples from the aroma component varieties and concentrations and also characterize the key volatile compounds of each Chinese functional liquor, the remaining 33 components (OAVs >1) in Table 1 were subjected to PCA. Principle Component 1 (PC1) is 45.6% and Principle Component 2 (PC2) is 23.6%. According to the analysis of score plot (a) and loading plot (b), the distribution of the four samples was widely dispersed, especially L3 and L4. It showed that the content of flavor components in different functions liquors differed remarkably, and the body style was also inconsistent (Figure 4).
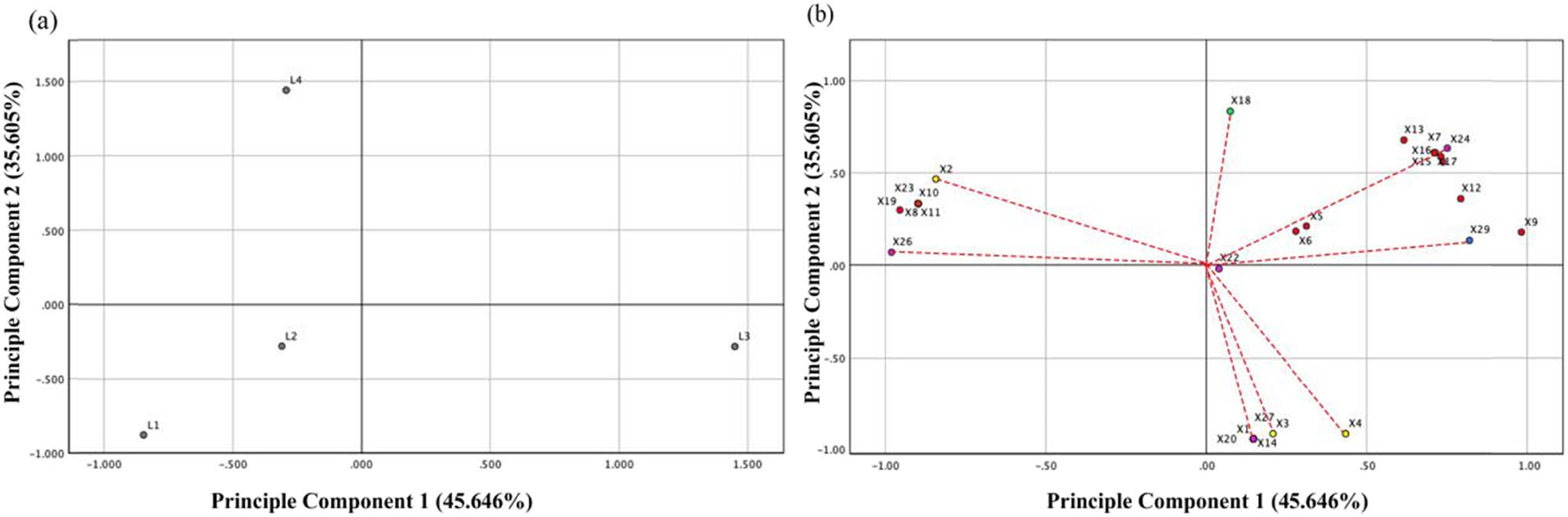
PCA. Score plot (a) and loading plot (b) of PC1 and PC2, from volatile compound in the L1, L2, L3, and L4. Different colored dots represent different kinds of compounds: yellow (alcohols), red (esters), green (acids), blue (hydrocarbons), and purple (carbonyl compounds).
The distribution position of highland barley wine (L3) was located in the positive axis of PC1 and was correlated with a greater abundance of esters (including isopentyl isobutyrate (X7), ethyl lactate (X9), ethyl decanoate (X12), ethyl benzoate (X13), ethyl dodecanoate (X15)), and a hydrocarbon. Conversely, Kinmen-Kaoliang Liquor (L1), Jin Liquor (L2), and Zhuyeqing Liquor (L4) were located in the negative axis of PC1, revealing a greater abundance of carbonyl compounds (2-nonanone (X23) and furfural (X26)), an alcoholic substance (1-hexanol (X2)) and hexanoic acid (X19), and a lower abundance of the compounds was present in L3. On the positive axis of PC2, Zhuyeqing Liquor (L4) was observed, which was correlated with a higher abundance of esters (propyl hexanoate (X8), isoamyl hexanoate (X10) and hexyl hexanoate (X11)), acids (butyric acid (X18) and hexanoic acid (X19)), and carbonyl compound (furfural (X26)). On the contrary, Kinmen-Kaoliang Liquor (L1) and Jin Liquor (L2) were found to correlate with the compounds in greater abundance, such as alcohols (2-butanol (X1), borneol (X3) and phenylethyl alcohol (X4)), carbonyl compounds (isovaleraldehyde (X20), 3-nonanone (X22) and (E)-cinnamaldehyde (X27)), and ester (2-phenethyl acetate (X14)). Particularly, 3-nonanone (X22) was more abundant for L2. These observations were consistent with the Venn results, and different types of liquor had a great effect on the distribution of flavor compounds.
3.3 Total phenolic content and antioxidant activity
In recent years, an increasing number of studies have shown the role of polyphenols in the antioxidant activity of red and white wines [19]. The antioxidant capacity of polyphenols correlates with the extent of hydroxylation and conjugation.
As shown in Table 2, the total phenolic contents of four liquors were 261 ± 1.1 mg GAE/L (L1), 275 ± 2.9 mg GAE/L (L2), 232 ± 0.7 mg GAE/L (L3), and 120 ± 4.3 mg GAE/L (L4). They were significantly (P < 0.05) higher than those of the typical Chinese white liquors.
Total phenolic content and antioxidant activity of four functional liquors and three typical white wines
| Name | TP* (mg GAE/L) | DPPH# (TE) | ABTS# (TE) |
|---|---|---|---|
| L1 | 261 ± 1.1a | 148 ± 6.3ac | 96.8 ± 0.6ac |
| L2 | 275 ± 2.9b | 161 ± 2.1b | 102 ± 1.4b |
| L3 | 232 ± 0.7a | 118 ± 2.2ac | 75.5 ± 0.3ad |
| L4 | 120 ± 4.3b | 79.6 ± 2.4bc | 55.8 ± 0.7c |
| Luzhou Laojiao | 50.9 ± 2.1d | 72.0 ± 1.0 cd | 33.8 ± 1.2 cd |
| Maotai Wangzi | 125 ± 3.3c | 77.1 ± 6.2dc | 43.6 ± 2.0c |
| Haizhilan | 48.8 ± 4.2d | 85.7 ± 5.3bc | 11.2 ± 1.0 cd |
Values are means of triplicate replicates ± SD, and different letters represent significantly different among the data in the same column (P < 0.05). *Total phenols expressed as gallic acids equivalents. #DPPH and ABTS expressed as mg/L trolox equivalents.
The capacities of free radical scavenging of four liquors detected by the DPPH assays were 148 ± 6.3 mg/L, 161 ± 2.1 mg/L, 118 ± 2.2 mg/L, and 79.6 ± 2.4 mg/L for L1, L2, L3, and L4, respectively. The ABTS assays were 96.8 ± 0.6 mg/L (L1), 102 ± 1.4 mg/L (L2), 75.5 ± 0.3 mg/L (L3), and 55.8 ± 0.7 mg/L (L4). All of them were much higher than the typical Chinese white liquors listed in Table 2. To find the reason, it was mainly because of the raw material of these liquors. For example, the main raw material of Kinmen-Kaoliang was sorghum which contained a large amount of polyphenols. Jin contained some herb extracts which contained a lot of polyphenols, and polyphenols of highland barley might originate from barley. A lot of polyphenols had been determined in bamboo leaves which were the main raw material of Zhuyeqing. To some extent, different types and origins of raw materials and brewing procedures might affect the volatiles and antioxidant activities of wines.
4 Conclusions
In conclusion, volatile compounds of four Chinese functional liquors were extracted by HS-SPME and identified using GC-MS. OAV and ROC were successfully used to evaluate the contributions of aroma compounds to the whole flavor. The results revealed that esters and carbonyl compounds are the major aroma compounds, of which ethyl hexanoate, ethyl acetate, and ethyl octanoate being the most powerful odorants. Other volatile compounds affected the aroma of four Chinese functional liquors to different extent. In addition, the total phenolic contents of four functional liquors vary and are significantly higher (P < 0.05) than those of the typical Chinese white liquors, and similar trend was found for their antioxidant activities. The volatile compound profile built in this study will provide valuable information toward the sensory and health benefits of Chinese functional liquors from the chemical aspects.
Acknowledgments
The authors are grateful to School of Perfume and Aroma Technology, Shanghai Institute of Technology for the supply of the experimental conditions for the study.
-
Funding information: The authors gratefully acknowledge the support provided by the funding from The Establishment of the Tobacco Product and Technology Integrated Innovation System for the Southeastern Asia Tobacco Market (suggested by Science & Technology Department of Yunnan Province, 2018IA057). Research on Application of Flavor and Fragrance Taste Control Technology (suggested by China Tobacco Yunnan Industrial Co., Ltd, 2019CL03); and Preparation of Natural Flavor by Multi-component solvent transfer extraction combined with rotating vertebral column technique (suggested by key Laboratory of cigarette flavoring Technology in Tobacco Industry, TX2018001).
-
Author contributions: Conceptualization, L. Y.; methodology, M. S.; formal analysis, D.C.; data curation, review and editing, J. L.; writing – original draft preparation, K. W., B. M.; writing – review and editing, T. F.; funding acquisition, T. F.
-
Conflict of interest: The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Bo Z, Shaowen Z, Yukuo T, Ling C. Understanding the nutritional functions of thermally-processed whole grain highland barley in vitro and in vivo. Food Chem. 2020;310:125979. 10.1016/j.foodchem.2019.125979.Search in Google Scholar
[2] Wu Z, Xu E, Li J, Long J, Jiao A, Jin Z, et al. Determination of antioxidant capacity of Chinese rice wine and Zhuyeqing Liquor using nanoparticle-based colorimetric methods. Food Anal Methods. 2017;10:788–98. 10.1007/s12161-016-0646-8.Search in Google Scholar
[3] Malherbe S, Menichelli E, du Toit M, Tredoux A, Muller N, Naes T, et al. The relationships between consumer liking, sensory and chemical attributes of Vitis vinifera L. cv. Pinotage wines elaborated with different Oenococcusoeni starter cultures. J Sci Food Agric. 2013;93:2829–40. 10.1002/jsfa.6115.Search in Google Scholar
[4] Sáenz-Navajas M-P, Arias I, Ferrero-Del-Teso S, Fernández-Zurbano P, Escudero A, Ferreira V. Chemo-sensory approach for the identification of chemical compounds driving green character in red wines. Food Res Int. 2018;109:138–48. 10.1016/j.foodres.2018.04.037.Search in Google Scholar
[5] Bithika S, Rocco L, Peter T, Anthony S, Leigh S. SPME method optimized by box-Behnken design for impact odorants in reduced alcohol wines. Foods. 2018;7:127. 10.3390/foods7080127.Search in Google Scholar
[6] Ivanova V, Stefova M, Vojnoski B, Stafilov T, Bíró I, Bufa A, et al. Volatile composition of Macedonian and Hungarian wines assessed by GC/MS. Food Bioprocess Technol. 2013;6:1609–17. 10.1007/s11947-011-0760-y.Search in Google Scholar
[7] Xiao Z, Yu D, Niu Y, Chen F, Song S, Zhu J, et al. Characterization of aroma compounds of Chinese famous liquors by gas chromatography-mass spectrometry and flash GC electronic-nose. J Chromatogr B. 2014;945:92–100. 10.1016/j.jchromb.2013.11.032.Search in Google Scholar
[8] Yi G, Kerrihard Adrian L, Pegg Ronald B. Characterization of the volatile compounds in raw and roasted Georgia pecans by HS-SPME-GC-MS. J Food Sci. 2018;83(11):2753–60. 10.1111/1750-3841.14365.Search in Google Scholar
[9] Schwingshackl L, Morze J, Hoffmann G. Mediterranean diet and health status: active ingredients and pharmacological mechanisms. Br J Pharmacol. 2020;177:1241–57. 10.1111/bph.14778.Search in Google Scholar
[10] Nisha K, Deshwal RK. Antioxidants and their protective action against DNA damage. DNA 27, 29. Int J Pharm Pharm Sci. 2011;3:28–32.Search in Google Scholar
[11] Van den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr A. 1963;11:463–71. 10.1016/s0021-9673(01)80947-x.Search in Google Scholar
[12] Đorđević Neda O, Todorović N, Novaković Irena T, Pezo Lato L, Pejin B, Maraš V, et al. Antioxidant activity of selected polyphenolics in yeast cells: the case study of Montenegrin merlot wine. Molecules. 2018;23:1971. 10.3390/molecules23081971.Search in Google Scholar
[13] Paixao N, Perestrelo R, Marques J, Camara J. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007;105:204–14. 10.1016/j.foodchem.2007.04.017.Search in Google Scholar
[14] Zuobing X, Shengjiang L, Yongbo G, Na X, Yi S, Jiancai Z. Discrimination of cherry wines based on their sensory properties and aromatic fingerprinting using HS-SPME-GC-MS and multivariate analysis. J Food Sci. 2014;79(3):C284–94. 10.1111/1750-3841.12362.Search in Google Scholar
[15] Welke JE, Zanus M, Lazzarotto M, Alcaraz Zini C. Quantitative analysis of headspace volatile compounds using comprehensive two-dimensional gas chromatography and their contribution to the aroma of Chardonnay wine. Food Res Int. 2014;59:85–99. 10.1016/j.foodres.2014.02.002.Search in Google Scholar
[16] Pérez-Navarro J, Izquierdo-Cañas PM, Mena-Morales A, Martínez-Gascueña J, Chacón-Vozmediano JL, García-Romero E, et al. First chemical and sensory characterization of Moribel and Tinto Fragoso wines using HPLC-DAD-ESI-MS/MS, GC-MS, and Napping techniques: comparison with Tempranillo. J Sci Food Agric. 2019;99(5):2108–23. 10.1002/jsfa.9403.Search in Google Scholar
[17] Jeleń HH, Majcher M, Dziadas M, Zawirska-Wojtasiak R, Czaczyk K, Wąsowicz E. Volatile compounds responsible for aroma of Jutrzenkaliquer wine. J Chromatogr A. 2011;1218:7566–73. 10.1016/j.chroma.2011.07.023.Search in Google Scholar
[18] Bao J, Zhenwen Z. Volatile compounds of young wines from cabernet sauvignon, cabernet gernischet and chardonnay varieties grown in the loess plateau region of China. Molecules. 2010;15:9184–96. 10.3390/molecules15129184.Search in Google Scholar
[19] Hua Z, Yi-Fei Y, Zhi-Qin. Z. Phenolic and flavonoid contents of mandarin (Citrus reticulata Blanco) fruit tissues and their antioxidant capacity as evaluated by DPPH and ABTS methods. J Integr Agric. 2018;17:256–63. 10.1016/S2095-3119(17)61664-2.Search in Google Scholar
© 2021 Kai Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

