Abstract
Currently, the extraction technology of blueberry anthocyanin includes solvent extraction, enzyme extraction, and ultrasonic extraction. Different methods may damage the internal structure of anthocyanin in the extraction process, and hence the extracted anthocyanin cannot have the maximum nutritional and medicinal value. Therefore, this article analyzes the effects of different extraction methods on the antioxidant properties of blueberry anthocyanin and uses solvent extraction, enzymatic hydrolysis, and ultrasonic extraction methods to extract blueberry anthocyanin. The antioxidative properties of anthocyanins from blueberry by different extraction methods were compared and analyzed. The solvent extraction method, the enzymatic hydrolysis method, and the ultrasonic extraction method were used as experimental comparative extraction methods. The antioxidant properties of blueberry anthocyanins were measured from various angles such as resistance to oil oxidation, reducing power, and ability to scavenge hydroxyl radicals (˙OH) performance. From the perspective of antioxidation of fats and oils, the average inhibition rate of the solvent extraction method can reach 90%, and the corresponding inhibition rate of the anthocyanins obtained by the other two extraction methods is about 80%. The measurement results are also consistent with the measurement results of oxidation resistance of oils and fats. Conclusion: Among three different extraction methods of blueberry anthocyanins, the solvent extraction method can preserve the antioxidant properties of blueberry anthocyanins to the greatest extent.
1 Introduction
Blueberries have high nutritional value and health-care function and enjoy the reputation of “golden fruit” [1,2]. The content of natural pigments in blueberries is also very high, and the content of superoxide dismutase in living pigments is many times higher than that of other plants. The nutritional ingredients in blue poison have both pharmacological properties and biologically active functions. Important ingredients in blueberries can protect the eyes, resist oxidation, delay neuroaging, and enhance memory.
Blueberries have strong antioxidant capacity and many drug functions, mainly because they contain a large amount of anthocyanins. Anthocyanin is a natural water-soluble pigment, safe, and nontoxic and provide many health-care functions to the human body. Because of its unique functionality, it is used to remove free radicals; proliferates lutein; has antitumor, anticancer, and anti-inflammatory properties; inhibits lipid peroxidation and platelet aggregation; prevents diabetes; used in weight loss; and protects vision [3,4]. In general, the content of anthocyanins in blueberries is about 35%, and there are slight differences in the content of anthocyanins in different types of blueberries [5]. The physiological activities of anthocyanins include antioxidant activity, scavenging free radicals, antimutagenic activity, and bacteriostasis [6,7]. To realize the maximum value of anthocyanins in blueberries, anthocyanins are extracted from a large number of blueberries using a certain extraction process and used as raw materials in pharmaceutical production or other food processing works.
The study found that the extraction of blueberry anthocyanins at this stage includes solvent extraction, enzymatic extraction, and ultrasonic extraction. Different methods during the extraction may destroy the internal structure of anthocyanins, and hence, the extracted anthocyanins cannot exert the maximum nutritional and medicinal value. Among them, the solvent extraction method is the most commonly used method for extracting anthocyanins. According to the principle of similar compatibility, anthocyanins are easily soluble in water and organic solvents, which are common extraction agents, such as methanol, ethanol, acetone, and alkanes [8]. Anthocyanins are more soluble in alcohol solutions than other solvents, and so methanol or hexanol is often used for extraction. To prevent the flower color shake from undergoing structural transformation and denaturation in a neutral or alkaline environment, a certain amount of acid hydrazone is added to the alcohol solution during extraction to adjust the pH ratio. The acids used often are formic acid, acetic acid, and hydrochloric acid. This method has the advantages of simple operation and low cost, but it consumes a large amount of organic solvents, which produces large amount of waste and results in environmental pollution. Enzymes can mildly act on plant cells, causing loosening, swelling, and degradation of cell walls and interstitial structures, thereby promoting the anthocyanin components embedded in the cells to diffuse into the extraction medium and increasing the extraction rate [9,10]. It was found that adding a-amylase and cellulase to the blueberry extract can effectively improve the extraction rate of anthocyanins, and when the two enzymes are combined, the effect is more significant and the anthocyanin yield is high in the role of any single enzyme. The ultrasonic-assisted extraction method can quickly destroy the cell wall and cell membrane of plants, promote the dissolution of anthocyanin components, and increase the contact area between the sample and the solvent, thus greatly improving the extraction efficiency and effectively decreasing the extraction time [11,12].
Different extraction methods have different concentrations and different quantities of anthocyanin extracts due to different principles of action. To choose a more efficient extraction method of anthocyanins and to ensure the antioxidant properties of anthocyanins in blueberries to the greatest extent, three methods of solvent extraction, enzymatic hydrolysis, and ultrasonic extraction were used to extract anthocyanins in blueberries. The anti-oxidation, reducing power, scavenging ability of ˙OH, scavenging ability of O2 −˙, and its scavenging effect on DPPH free radical were analyzed.
2 Experiments on the effects of different extraction methods on the antioxidant properties of blueberry anthocyanins
2.1 Experimental materials and instruments
2.1.1 Experimental materials
The main experimental material is blueberries from the small berry garden of Jilin Agricultural University. The selected blueberry fruit (the variety is patriot) is provided by Liaoning Dandong Organic Food Co., Ltd, which has reached the fruit period of 8 weeks and is in a mature state, with a diameter of 5–10 mm, purple red, moisture content of 88%, half height clumped blueberry variety. Fresh fruits are picked and stored in a refrigerator at −20°C. During the experiment, the frozen fruits were taken out of the refrigerator at −20°C and thawed in the refrigerator at 4°C.
2.1.2 Experimental reagents
The preparation and the selection of experimental reagents mainly include two aspects, namely, the reagents used in the determination process and the relevant reagents used in the anthocyanin extraction process. The reagents required for the experiment are formic acid, anhydrous methanol, sodium acetate, ferric trichloride, DPPH, ABTS (Sinopharm Chemical Reagent Co., Ltd), potassium chloride, potassium hydroxide, aluminum trichloride, lead acetate (Xilong Chemical Co., Ltd), hydrochloric acid, sodium dihydrogen phosphate (Beijing Chemical Plant), ferric trichloride, potassium persulfate, ascorbic acid, trihydroxymethyl aminomethane, soybean lecithin (Sinopharm Chemical Reagent Co., Ltd), cyanidin-3-glucoside standard, and standard of mallow pigment-3-galactoside (Sigma Company).
2.1.3 Experimental instruments
As with the experimental reagents, the instruments used in this experiment are also divided into three aspects: anthocyanin extraction, anthocyanin content identification, and antioxidant performance testing. The equipment and models required for the experiment include LBI206 Ultrasonic Chinese medicine processor (Jining Aobo ultrasonic electric Co., Ltd), UV-6100 Ultraviolet visible spectrophotometer, L3600D Low speed, PHSJ-3F Precision PH meter (Shanghai Yuan Analytical Instrument Co., Ltd), XDB-C18 chromatographic column, LCMS-IT/TOF High performance liquid phase ion trap/time of flight tandem mass spectrometer, LC-20AD High resolution fast liquid chromatography system (SHIMADZU Company), AL104 Electronic balance (Mettler Toledo), and XMTB Electrothermal constant temperature Water bath (Tianjin Zhonghuan Experimental Electric Furnace Co., Ltd).
2.2 Raw material pretreatment
The commercially available blueberries frozen at −20°C were thawed at room temperature, and they were stirred with a stirrer to a cloudy liquid. To make each part uniform, the blueberry juice is placed in a colloid mill at 3,000 rpm and is ground for 15 min. Then, a crude extract and an extract of blueberry were prepared separately.
2.2.1 Preparation of blueberry crude extract
Five solvents containing 3% trifluoroacetic acid were used, including (water, n-butanol, methanol, 70% ethanol, ethyl acetate), with a solid–liquid ratio of 1:5 (g/mL) and ultrasonic-assisted extraction at 30°C (Power 180 W) three times, each time 30 min, the material liquid ratio of the second and third times is halved. The extracts were combined and centrifuged at 4,500 rpm for 5 min, the supernatant was taken, and the solvent was concentrated under reduced pressure at 40–60°C, and the extract was freeze-dried to obtain a sample water extract. A 60% ethanol solution was used to mix uniformly at a solid–liquid ratio of 1:20 (m/V), and the mixture was extracted in a constant temperature water bath at 50°C for 90 min.
2.2.2 Preparation of blueberry extract
Using methanol containing 0.3% trifluoroacetic acid as a solvent, the aforementioned extraction steps are followed to obtain an extract-like extract, dissolve with 500 mL of purified water, filter, and take 300 mL of the filtrate onto a LS-305 macroporous resin column with a 0.3% trifluoroacetic acid aqueous solution (approximately 400 mL). Impurities such as sugar and acid are removed by rinsing and then eluted with 0.3% trifluoroacetic acid methanol solution (approximately 100 mL). The eluate was collected and concentrated under reduced pressure at 40°C to extract, dissolve with purified water and make up 150 mL. 25 mL of this sample solution was taken, concentrated under reduced pressure at 40°C to extract, and freeze-dried to obtain blueberry total extract (RP). The remaining 125 mL of the sample was sequentially extracted with ethyl acetate and n-butanol. The sample was extracted using ethyl acetate extraction three times, the first time with an equal volume and the next two times with a half volume, and combined extracts were concentrated under reduced pressure at 40°C to remove the solvent and freeze-dried to obtain blueberry ethyl acetate extract (ARP). The sample was again extracted with n-butanol four times, the first time with an equal volume and the next three times with a half volume, and the extract was concentrated under reduced pressure at 60°C to remove the solvent and freeze-dried to obtain blueberry n-butanol extract (BRP). The solvent was concentrated under reduced pressure at 50°C, and blueberry water extract was obtained after freeze-drying.
2.3 Choose anthocyanin extraction method
On the basis of the results of the previous research, blueberry anthocyanins were prepared. The extraction methods differ according to the uses of the anthocyanins extracted. To study the effect of different extraction methods on the antioxidant properties of blueberry anthocyanins, the contents, concentrations, and the corresponding changes in antioxidative properties of anthocyanins obtained by solvent extraction, enzymatic extraction, and ultrasonic extraction were compared.
2.3.1 Solvent extraction method
Anthocyanins are less stable in neutral and weakly alkaline solutions, and so the extraction process is usually performed under acidic conditions. Steps of extraction method are as follows: Configure the solvent buffer pH 1.0 buffer: 0.2 mol/L KCl–0.2 mol/L HCl, and the pH 4.5 buffer is 1 mol/L NaAc–1 mol/L HCl. Weigh blueberries accurately, add 15 times the amount (v/w) of acidic methanol with a concentration of 80% after mechanical crushing, extract at 40°C, and determine the proportion of the solvent to collect the supernatant. Using acidic ethanol as the extractant and optimizing each factor by response surface analysis, the best extraction parameters were obtained: ethanol extract volume fraction, 60.65%; material–liquid ratio, 1:20.65(g/mL); extraction time, 122.53 min; pH, 3.0; the extraction temperature, 50°C, and the extraction was performed twice to determine the content of anthocyanins in the frozen blueberries, which was about 3.264 mg/g.
2.3.2 Enzymatic extraction
Citric acid and sodium citrate were mixed at a certain ratio to adjust the pH. 5 g of blueberry was crushed, and 5 mg/g pectinase was used for enzymolysis at 45°C, pH 4.5, and the duration of enzymatic hydrolysis with the material–liquid ratio of 1 g:8 mL was 60 min and 90 min, and after centrifugation at 2,000 rpm in 20 min, the blueberry anthocyanin extract was collected.
2.3.3 Ultrasonic extraction method
5 g of blue poisonous fruit was weighed and 60% acidified methanol with a ratio of 1:10 (g/mL) was used as the extractant and sonicated at 50°C for 50 min to obtain blueberry anthocyanin extract [13,14]. The actual quantity of extracted blueberry anthocyanin was 3.927 mg/g.
2.3.4 Extraction and purification
Three different anthocyanin extraction methods were used for purification. The purification and separation method used was centrifugation. Generally, the temperature increases during centrifugation, which has a great impact on heat-sensitive substances. Therefore, this experiment uses a refrigerated centrifuge, which can ensure that the sample is centrifuged at low temperature, so that the substance does not lose its activity [15]. The effect of centrifugation is affected by factors such as solution viscosity, centrifugation time, and centrifugation speed. The change of the absorbance value of the solution before and after centrifugation is used as an index for analysis, and the conditions close to or greater than the absorbance value of the solution before centrifugation are preferred.
2.4 Determination of anthocyanin extraction results
2.4.1 Identification of anthocyanins
After analyzing the anthocyanins of blueberry by HPLC-DAD-MS, the experimental results were compared with the UV spectrum, mass spectrum, and retention time in the blueberry anthocyanin database to identify the anthocyanins in blueberry [16,17]. Blueberry anthocyanin identification criteria are presented in Table 1.
Blueberry anthocyanin identification standards
| Peak | [M]+ | Molecular ions fragments | Anthocyanin |
|---|---|---|---|
| 1 | 303 | 465 | Delphinidin 3-galactoside |
| 2 | 317 | 479 | Petunianin 3-galactoside |
| 3 | 331 | 493 | Malvacetin 3-galactoside |
| 4 | 303 | 465 | Delphinidin 3-glucoside |
| 5 | 287 | 435 | Delphinidin 3-arabinoside |
| 6 | 317 | 449 | Delphinidine 3-arabinoside |
| 7 | 287 | 479 | Morning glory 3-glucoside |
| 8 | 317 | 419 | Paeoniflorin 3-glucoside |
| 9 | 301 | 463 | Malvacetin 3-arabinoside |
2.4.2 Anthocyanin monomer composition analysis
Anthocyanin composition of the blueberry extract was detected and analyzed by high-performance liquid chromatography (HPLC)-mass spectrometry. The HPLC conditions are set similar to Kromasil C18 reversed-phase column, mobile phase A: water:formic acid:acetonitrile = 92:2:6 v/v; mobile phase B: water:formic acid:acetonitrile = 44:2:54 v/v; flow rate, 1 m/min; column temperature, 50°C; detection wavelength, 525 nm; injection volume, 30 µL; gradient elution procedure: 0–4 min: 6–10% B, 4–12 min: 10–25% B, 12–13 min: 25% B, 13–20 min: 25–40% B, 20–35 min: 40–60% B, 35–40 min: 60–100% B, 40–45 min: 100–6% B. The HPLC of the obtained blueberry anthocyanins is shown in Figure 1. The results of anthocyanin monomer composition analysis are presented in Table 2.
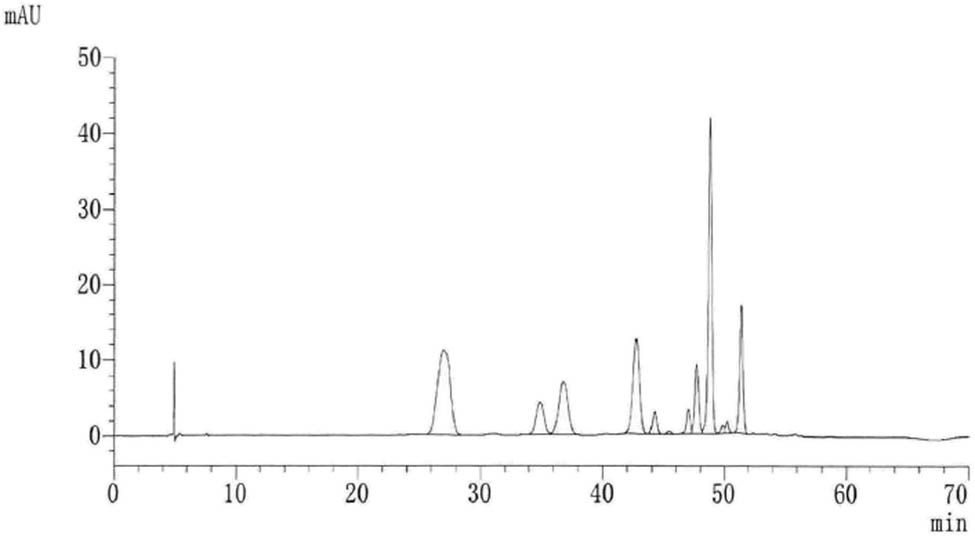
HPLC spectrum of blueberry anthocyanins.
Composition and structure analysis table of blueberry anthocyanin monomer
| Peak | Retention time/min | Maximum absorption wavelength/λ max/nm | Molecular ions and fragments ions/m/z | Anthocyanin name |
|---|---|---|---|---|
| 1 | 26.4 | 517 | 465[M]+,303 | De-3-O-hex |
| 2 | 33.9 | 525 | 449[M]+,287 | Cy-3-O-hex |
| 3 | 35.7 | 534 | 435[M]+,303 | De-3-O-pen |
| 4 | 41.9 | 532 | 479[M]+,317 | Pet-3-O-hex |
| 5 | 43.4 | 537 | 419[M]+,287 | Cy-3-O-pen |
| 6 | 46.4 | 518 | 463[M]+,301 | Peo-3-O-pem |
There is a high molecular conjugation system in anthocyanins, which contains acid and alkaline groups, and is easily soluble in polar solvents such as water, methanol, ethanol, dilute alkali, and dilute acid. It has strong absorption in the UV and visible region, the maximum absorption wavelength in the UV region is around 280 nm and the maximum absorption wavelength in the visible region is in the range of 500–550 nm. Anthocyanins belong to bioflavonoids, and the main physiological activity of flavonoids is free radical scavenging ability and antioxidant ability. It has been proved that anthocyanin is the most effective antioxidant and the most powerful free radical scavenger found by human beings. The antioxidant performance of anthocyanin is 50 times higher than VE and 20 times higher than VC.
2.4.3 Calculation of blueberry anthocyanin content
The color scale of blueberry anthocyanins is calculated as shown in equation (1):
In equation (1), A is the absorbance, W is the mass of the blueberry anthocyanin extracted, and r is the dilution factor of the sample taken when measuring the absorbance [18]. The color value of the extract was measured 36. The formula for calculating total anthocyanin content in the solution by wavelength scanning using pH difference method is as follows:
where and are the absorbance values of anthocyanins at 520 nm at pH 1.0 and pH 4.5, V is the total volume of the extract, n is the dilution factor, and M is the relative molecular mass of anthocyanins. In addition, ε is the extinction coefficient of anthocyanins and m is the sample mass.
2.5 Determination and analysis of antioxidant capacity
Antioxidation is a process that scavenges free radicals, inhibits the oxidation of easily oxidizable substances, inhibits the generation of reactive oxygen to a certain extent, and plays a vital role in maintaining human health [19,20]. Therefore, the determination of the antioxidant capacity of substances, especially foods, and the comprehensive investigation of the antioxidant level in the human body have gradually attracted people’s attention. Food contains different kinds and different amounts of antioxidants, and endogenous antioxidants are also present in human tissues to suppress excessive reactive oxygen species generated during life activities. Therefore, all antioxidants in the body are individually investigated. It is difficult to measure the antioxidant capacity. Under the influence of the interaction between various antioxidants, it is more practical to investigate the comprehensive antioxidant level than to measure the antioxidant capacity of a single antioxidant alone.
2.5.1 Determination of anthocyanin resistance to lipid oxidation
50 g of lard was taken in an iodine volumetric flask, and then, blueberry anthocyanin and 0.2% ascorbic acid from different extraction methods were added to different iodine volumetric flasks. After being stirred evenly, flasks were placed in an oven at 70°C, and the peroxide value was measured every 2 days. 3 g of fat was taken for testing; 30 mL of chloroform-glacial acetic acid and 1 mL of saturated potassium iodide were added to an iodine volumetric flask, shaked for 30 s, and placed in dark for 3 min; then 100 mL distilled water was added and shaked well. When titrating with sodium thiosulfate standard solution to light yellow, two drops of starch indicator were added, and titration was continued until the blue color disappears, and a blank test was carried out at the same time. The formula for the inhibition rate of blueberry anthocyanin lipid peroxidation is as follows:
The conversion formula of the calculation result is as follows:
In equations (3) and (4), V, respectively, represent the volume of the sodium thiosulfate standard titration solution consumed in the measurement sample and the blank experiment, C is the concentration of the sodium thiosulfate standard titration solution, and M is the mass of the measurement sample.
2.5.2 Determination of anthocyanin reducing power
The reaction of divalent iron ions and ferric chloride produces Prussian blue, which has a maximum absorption at 700 nm. The reaction formula is as follows:
Therefore, the reduction ability of the antioxidant can be indirectly evaluated by measuring the absorbance at 700 nm. To eliminate the effect of yellow potassium ferricyanide on the absorbance value, after the completion of reaction, trichloroacetic acid is added to the system to react with the remaining potassium ferricyanide to form a precipitate [21]. After centrifugation, the absorbance of the supernatant and ferric chloride was measured. The stronger the reducing ability of the sample to be tested, the greater the measured absorbance value.
2.5.3 Anthocyanin clearance ˙OH determination
1 mL of blueberry anthocyanin, ferric sulfate, and salicylic acid–ethanol solution was added to the test tube, 1 mL of hydrogen peroxide was added, and then water was added in a 37°C water bath and was kept for 30 min. Distilled water was used as a blank and the absorbance was measured at 510 nm. Hydrogen peroxide was replaced with distilled water and the same method was used to measure the corresponding absorbance. The hydroxyl scavenging rate of anthocyanin from blueberry obtained by different extraction methods was calculated using equation (6).
where
2.5.4 Ability of anthocyanins to remove O2 −˙
Pyrogallol’s auto-oxidation process is a chain reaction. In an alkaline environment, auto-oxidation can quickly occur to generate a superoxide anion, which in turn can accelerate the rate of pyrogel’s auto-oxidation and generate colored intermediates. The accumulation of intermediate products has a good linear relationship with time, generally maintained for about 4 min, and then gradually slowed down. When a superoxide anion scavenger is present in the system, the scavenger causes the disproportionation reaction of O2 − to be converted into O2 and hydrogen peroxide [22], thereby reducing the pyrogallol auto-oxidation rate and preventing the accumulation of intermediate products. The auto-oxidation rate of catechol is positively correlated with the concentration of superoxide anions in the system, so the ability of antioxidants to remove superoxide anions can be indirectly evaluated by measuring the auto-oxidation rate of catechol in the presence of antioxidants [23].
2.5.5 Anthocyanin clearance of DPPH˙
The characteristic purple-red group of 1,1-diphenyl-2-picrylhydrazyl (DPPH) solution has the maximum light absorption at 517 nm. The reduction of the absorption value after the addition of antioxidants indicates its scavenging effect on free radicals. The principle of anthocyanin clearance of DPPH˙ is shown in Figure 2 [24].

Reaction equation of DPPH and antioxidant.
The blueberry anthocyanin ethanol solution obtained by different extraction methods and the same volume of 0.2 mmol L−1 DPPH ethanol solution were to the test tube. Absorbance was measured at 517 nm using absolute ethanol as a reference, and three measurements were taken in parallel to obtain the average value. The DPPH free radical scavenging rate is calculated as follows [25]:
where
Correlation coefficient between blueberry anthocyanins and DPPH antioxidant activity
| Antioxidant capacity | Correlation coefficient | |
|---|---|---|
| ABTS | DPPH | |
| ABTS (IC50 value) | 1 | — |
| DPPH (IC50 value) | 0.921a | 1 |
- a
A significant correlation at the 0.01 level.
2.6 Data processing
All experiments were repeated three times, and analysis of variance (SPSS_12.0) was performed on the test data. When the significance level was p > 0.05, the difference was not significant, and when p < 0.05, the difference was significant. Origin_Pro7.5 was used to statistically analyze and plot the data [26].
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Experimental results and comparative analysis of antioxidant capacity
3.1 Anthocyanin resistance to lipid oxidation
Figure 3 shows the comparison of antioxidant properties of blueberry anthocyanins under different extraction methods [27].
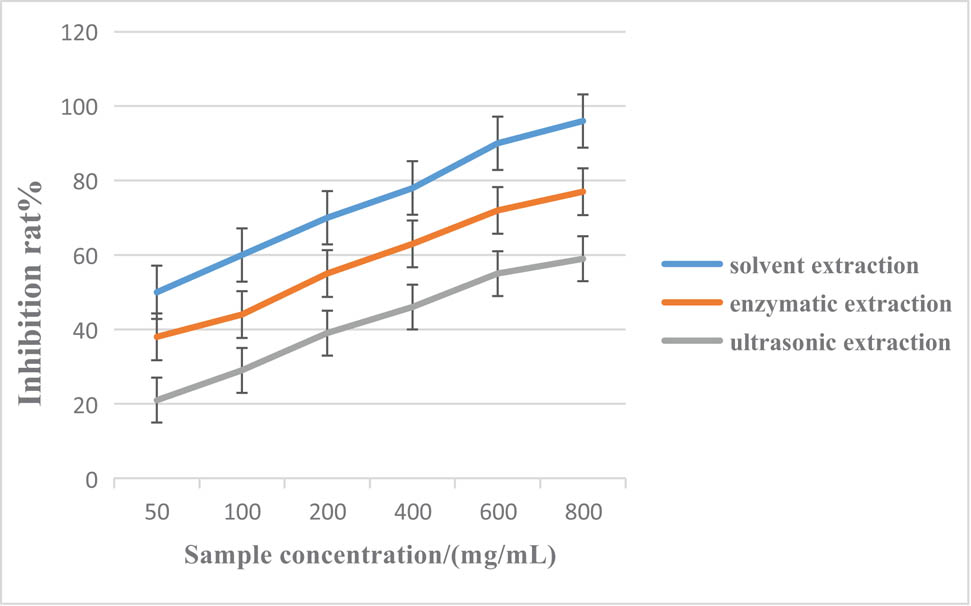
Inhibition rates of blueberry anthocyanin lipid peroxidation by different extraction methods.
Figure 3 shows that in the Fe2+-induced lecithin lipid system, the three blueberry anthocyanins have a significant inhibitory effect on liposome peroxidation. The inhibition rate increases with the increase of mass concentration of sample, but the inhibition ability is different. After comparison, it was found that the inhibition rate of the solvent extraction method can reach more than 90%, and the average inhibition rates of lipid peroxidation by the enzymatic hydrolysis extraction method and the ultrasonic extraction method were 78% and 67%, respectively.
3.2 Anthocyanin reducing power
The measurement result of the iron reducing power is shown in Figure 4. Also, it can be seen from the figure that the iron reduction power corresponding to the extraction results obtained by different blueberry anthocyanin extraction methods has significant differences. Among them, the solvent extraction method has the highest iron reducing power, with an equivalent concentration of 3.259 mg/mL, followed by the enzymatic hydrolysis extraction method with an equivalent concentration of 2.346 mg/mL and then the instrument-assisted extraction method with an equivalent concentration of 1.857 mg/mL.

Iron reducing power of blueberry anthocyanins by different extraction methods.
3.3 Anthocyanin scavenging ability
According to the blueberry anthocyanin scavenging and OH antioxidant measurement methods, the effect of blueberry anthocyanin solutions obtained by different extraction methods on scavenging hydroxyl radicals was measured. Figure 5 shows a comparison curve of the ability of anthocyanins to scavenge hydroxyl radicals.

Comparison curve of OH scavenging ability of blueberry anthocyanins by different extraction methods.
By comparison, it was found that the clearance rate of the solvent extraction method was up to 86.43%, and the clearance rates of the other two extraction methods also reached more than 60%. The IC50 value of the half-inhibition mass concentration of the solvent extraction method was the lowest at 0.23 mg/mL, while the IC50 values of the other two extraction methods were between 0.26 and 0.91 mg/mL.
3.4 The ability of anthocyanins to remove O2 −˙
Superoxide anion free radicals cannot directly induce lipid oxidation in biological and food systems, but it will undergo the Fenton reaction under metal ion catalysis to produce highly active ˙OH, Therefore, the scavenging ability of samples to superoxide anion free radicals is often used to reflect its antioxidant activity. The comparison results regarding the ability of anthocyanins to remove O2 −˙ are shown in Figure 6.
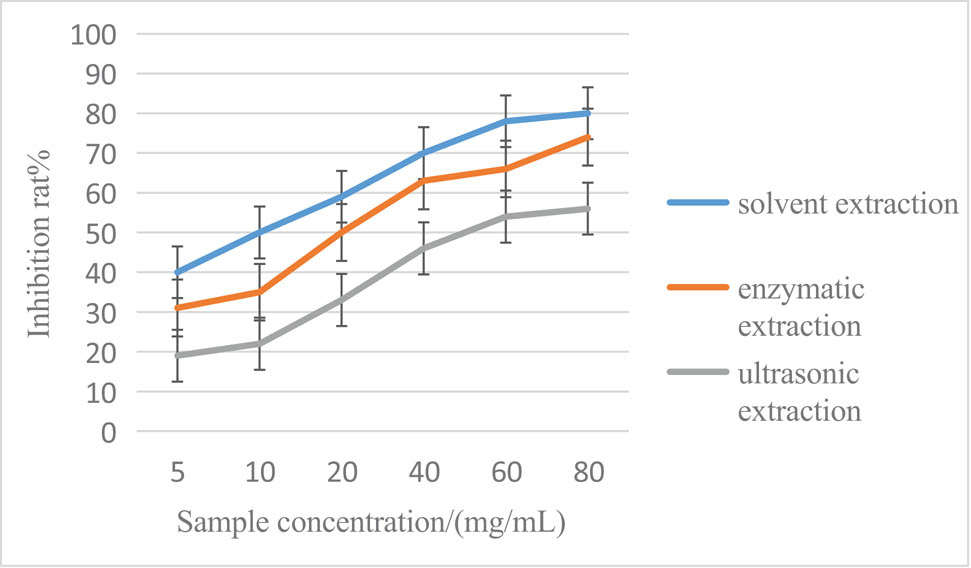
Comparison curve of blueberry anthocyanin removal ability by different extraction methods.
Figure 6 shows that blueberry anthocyanins from different extraction methods all show a certain ability to scavenge superoxide anion free radicals, and as the mass concentration increases, the clearance rate gradually increases and tends to be gentle. Among them, the anthocyanins obtained by the solvent extraction method showed a better clearance effect. When the concentration was 80 mg/mL, the clearance rate could reach 80%, which was greater than the other two extraction methods.
3.5 Anthocyanin scavenging effect on DPPH free radicals
DPPH radical is a kind of stable nitrogen-centered proton radical. Its ethanol solution is purple and has strong absorption at the wavelength of 517 nm. In the presence of a radical scavenger, the radical scavenger provides an electron with DPPH. The lone pair of electrons makes them fade, and the degree of discoloration has a quantitative relationship with the electrons they accept. The comparison results of different extraction methods for clear DPPH free radical capacity are shown in Figure 7.

Comparison curve of the ability of blueberry anthocyanins to scavenge DPPH free radicals by different extraction methods.
Methanol, ethanol, acetone, water, or mixed solvents were used to prevent the degradation of acylated anthocyanins in the extraction process. Adding a certain concentration of hydrochloric acid or formic acid in the extraction solvent will lead to the partial or full hydrolysis of acylated anthocyanins during evaporation and concentration. The anthocyanins obtained by the extraction method have better removal effect. When the concentration is 80 mg/mL, the scavenging rate can reach 80%. Based on the comprehensive analysis of the antilipid oxidation, color reduction, scavenging ˙Oh, scavenging O2 −, and DPPH free radical ability of anthocyanin, the effects of different extraction methods on the antioxidant performance of blueberry anthocyanin were studied. The results showed that the solvent extraction method can protect the antioxidant performance of blueberry anthocyanin to the maximum extent.
4 Conclusion
The results of the comparative analysis of anthocyanin resistance to oil oxidation, flower color reducing power, scavenging ability ˙OH, scavenging ability of O2 −, and DPPH free radicals showed that the blueberry extract obtained by the solvent extraction method had the highest anthocyanin pigment. On the one hand, solvent extraction method for extracting anthocyanins, is simple in principle and the requirements for equipment are not too high; on the other hand, the extraction efficiency using a centrifugal extractor is high and the product purity is high. The key to the application of the solvent extraction method is the choice of a solvent. When choosing a solvent, it is necessary to avoid the dissolution of a large number of impurities and to have a greater solubility for the extracted active ingredients. Applying this method to actual food processing can reduce the degree of damage to anthocyanins in blueberries, thereby improving the antioxidant performance of food and pharmaceutical processing results.
Acknowledgments
The authors are grateful to “Hunan Academy of Agricultural Sciences” for proofreading this manuscript.
-
Funding: The research is supported by Youth Research Fund of Hunan Polytechnic of Environment and Biology - Extraction and Biological Activity Research of Main Chemical Constituents from Blueberry (No. Z2012-6).
-
Authors’ contribution: In this paper, this paper analyzes the effects of different extraction methods on the antioxidant properties of blueberry anthocyanin, and uses solvent extraction, enzymatic hydrolysis and ultrasonic extraction methods to extract blueberry anthocyanin. Xiang Li put forward the research experiment: In order to choose a more efficient extraction method of anthocyanins and to ensure the antioxidant properties of anthocyanins in blueberries to the greatest extent, three methods of solvent extraction, enzymatic hydrolysis and ultrasonic extraction were used to extract anthocyanins in blueberries. Feiying Zhu analyzed the data and Zhiwen Zeng helped with the constructive discussion. Xiang Li, Feiying Zhu and Zhiwen Zeng made great contributions to manuscript preparation.
-
Competing interests: The authors declare no conflict of interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article.
References
[1] Wu Y, Yang J, Wang Q, Jiang N, Tao Y, Han Y. Effects of storage time and temperature on quality of blueberry anthocyanin microcapsules. Trans Chinese Soc Agric Eng. 2017;33(8):301–8.Search in Google Scholar
[2] Jingbo L, Jingjing C, Erlei W, Liu Y. Separation of anthocyanin monomers from blueberry fruits through chromatographic techniques. Food Sci. 2017;38(2):206–13.Search in Google Scholar
[3] Raudsepp P, Koskar J, Anton D, Meremäe K, Kapp K, Laurson P, et al. Antibacterial and antioxidative properties ofdifferent parts of garden rhubarb, blackcurrant, chokeberry and blue honeysuckle. J Sci Food Agric. 2019;99(5):2311–20.10.1002/jsfa.9429Search in Google Scholar PubMed
[4] Luo SZ, Chen SS, Pan LH, Qin X-S, Zheng Z, Zhao Y-Y, et al. Antioxidative capacity of crude camellia seed oil: impact of lipophilization products of blueberry anthocyanin. Int J Food Properties. 2017;20(Suppl 2):1627–36.10.1080/10942912.2017.1350974Search in Google Scholar
[5] Cásedas G, Les F, Gómez-Serranillos MP, Smith C, López V. Anthocyanin profile, antioxidant activity and enzyme inhibiting properties of blueberry and cranberry juices: a comparative study. Food Funct. 2017;8(11):4187–93.10.1039/C7FO01205ESearch in Google Scholar PubMed
[6] Bakuradze T, Tausend A, Galan J, Groh IAM, Berry D, Tur JA, et al. Antioxidative activity and health benefits of anthocyanin rich fruit juice in healthy volunteers. Free Radic Res. 2019;53(Suppl 1):1045–55.10.1080/10715762.2019.1618851Search in Google Scholar PubMed
[7] Zhou F, Wang T, Zhang BL, Zhao H. Addition of sucrose during the blueberry heating process is good or bad? Evaluating the changes of anthocyanins/anthocyanidins and the anticancer ability in HepG-2 cells. Food Res Int. 2018;107:509–17.10.1016/j.foodres.2018.02.071Search in Google Scholar PubMed
[8] Costa DV, Almeida DP, Pintado M. Effect of postharvest application of ethylene on the profile of phenolic acids and anthocyanins in three blueberry cultivars (Vaccinium corymbosum). J Sci Food Agric. 2018;98(13):5052–61.10.1002/jsfa.9042Search in Google Scholar PubMed
[9] Wu Y, Zhou Q, Chen XY, Li X, Wang Y, Zhang J. Comparison and screening of bioactive phenolic compounds in different blueberry cultivars: evaluation of anti-oxidation and a-amylase → α inhibition effect. Food Res Int. 2017;100(Pt 1):312–24.10.1016/j.foodres.2017.07.004Search in Google Scholar PubMed
[10] Ye Z, Hu C, He L, Ouyang G, Wen F. The dynamic time-frequency relationship between international oil prices and investor sentiment in China: a wavelet coherence analysis. Energy J. 2020;41(5).10.5547/01956574.41.5.fwenSearch in Google Scholar
[11] Jiang DJ, Shao LY. Parameter optimization simulation of thermal field flow separation of mixed sample proteins. Comput Simul. 2019;36(10):179–83.Search in Google Scholar
[12] Nogalesbueno J, Bacabocanegra B, Jarapalacios MJ, Hernández-Hierro JM, Heredia FJ. Evaluation of the influence of white grape seed extracts as copigment sources on the anthocyanin extraction from grape skins previously classified by near infrared hyperspectral tools. Food Chem. 2017;221:1685–90.10.1016/j.foodchem.2016.10.118Search in Google Scholar PubMed
[13] Somavat P, Kumar D, Singh V. Techno-economic feasibility analysis of blue and purple corn processing for anthocyanin extraction and ethanol production using modified dry grind process. Ind Crops Prod. 2018;115:78–87.10.1016/j.indcrop.2018.02.015Search in Google Scholar
[14] Baca-Bocanegra B, Nogales-Bueno J, José Heredia F, Hernández-Hierro JM. Influence of oak wood chips–grape mix maceration on the extraction of anthocyanins from low-extractable anthocyanin content red grapes. Eur Food Res Technol. 2018;244(4):729–34.10.1007/s00217-017-2999-7Search in Google Scholar
[15] He JG, Wang Z, Deng JH. Determination of anthocyanin in blueberry by high performance liquid chromatography. Hunan Agric Sci. 2020;49(5):68–71.Search in Google Scholar
[16] Ongkowijoyo P, Luna-Vital DA, Mejia EGD. Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: an update. Food Chem. 2018;250:113–26.10.1016/j.foodchem.2018.01.055Search in Google Scholar PubMed
[17] Aryanti N, Wardhani DH, Wasi A, Ramadhan GA, Purbasari A. Extraction characteristics of anthocyanin from roselle (Hibiscus sabdariffa L.) calyces by ultrasound-assisted extraction. Adv Sci Lett. 2017;23(6):5626–28.10.1166/asl.2017.8785Search in Google Scholar
[18] Tang X, Wang Y, Han J, Wang L, Li C, Ni L. Separation, purification of anthocyanin and vitis linn polysaccharide from grape juice by the two-step extraction and dialysis. J Food Proc Preserv. 2018;42(1):e13344.10.1111/jfpp.13344Search in Google Scholar
[19] Hosoda K, Sasahara H, Matsushita K, Tamura Y, Miyaji M, Matsuyama H. Anthocyanin and proanthocyanidin contents, antioxidant activity, and in situ degradability of black and red rice grains. Asian Australasian J Anim Sci. 2018;31(8):1213–20.10.5713/ajas.17.0655Search in Google Scholar PubMed PubMed Central
[20] Migliorini AA, Piroski CS, Daniel TG, Cruz TM, Escher GB, do Carmo MAV, et al. Red chicory (cichorium intybus) extract rich in anthocyanins: chemical stability, antioxidant activity, and antiproliferative activity in vitro. J Food Sci. 2019;84(5):990–1001.10.1111/1750-3841.14506Search in Google Scholar PubMed
[21] Dróżdż P, Pyrzynska K,. Assessment of polyphenol content and antioxidant activity of oak bark extracts. Eur J Wood Wood Prod. 2017;76(2):793–5.10.1007/s00107-017-1280-xSearch in Google Scholar
[22] Maksup S, Pongpakpian S, Roytrakul S, Cha-Um S, Supaibulwatana K. Comparative proteomics and protein profile related to phenolic compounds and antioxidant activity in germinated Oryza sativa ‘KDML105’ and thai brown rice ‘Mali Daeng’ for better nutritional value. J Sci Food Agric. 2018;98(2):566–73.10.1002/jsfa.8498Search in Google Scholar PubMed
[23] Yang F, Yang F, Wang GY, Kong T, Wang H, Zhang CS. Effects of water temperature on tissue depletion of florfenicol and its metabolite florfenicol amine in crucian carp (Carassius auratus gibelio) following multiple oral doses. Aquaculture. 2020;515:9. 10.1016/j.aquaculture.2019.734542 Search in Google Scholar
[24] Wu Z, Liu YN, Jia XX. A novel hierarchical secret image sharing scheme with multi-group joint management. Mathematics. 2020;8(3):12. 10.3390/math8030448 Search in Google Scholar
[25] Hao WQ, Xiong SM. Adaptive data update scheme for RSU based on fuzzy logic in VANET. Comput Eng. 2019;45(11):86–90, 96.Search in Google Scholar
[26] Gao NS, Cheng BZ, Hou H, Zhang RH. Mesophase pitch based carbon foams as sound absorbers. Mater Lett. 2018;212:243–6. 10.1016/j.matlet.2017.10.074 Search in Google Scholar
[27] Gao NS, Guo XY, Cheng BZ, Zhang YN, Wei ZY, Hou H. Elastic wave modulation in hollow metamaterial beam with acoustic black hole. IEEE Access. 2019;7:124141–6. 10.1109/access.2019.2938250 Search in Google Scholar
© 2021 Xiang Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

