Abstract
Energy of a molecule plays an important role in physics, chemistry and biology. In mathematics, the concept of energy is used in graph theory to help other subjects such as chemistry and physics. In graph theory, nullity is the number of zeros extracted from the characteristic polynomials obtained from the adjacency matrix, and inertia represents the positive and negative eigenvalues of the adjacency matrix. Energy is the sum of the absolute eigenvalues of its adjacency matrix. In this study, the inertia, nullity and signature of the aforementioned structures have been discussed.
1 Introduction
A molecular graph is a mathematical object defined as
The eigenvalues play an important role in the field of mathematics, but these values are also very important in other fields such as chemistry, economics and many more. As far as our study concerns about eigenvalues, these values interpret in chemistry not only as the form of energy but also as different physicochemical properties of a chemical compound. We need to understand the relationship between mathematics and chemistry. The positive eigenvalues are linked with antibonding level, negative eigenvalues are linked with bonding levels and zero eigenvalues are linked with nonbonding level [1–3]. The number of positive eigenvalues
Phenylene belongs to the special class of conjugated hydrocarbons which plays an important role in the field of chemistry. The geometrical construction of phenylene is as follow: its shape is based on two mathematical objects, namely, hexagon and square. These objects are connected in such a way that a square is connected with two hexagons, which show that every two hexagons are totally separated (see Figure 1).
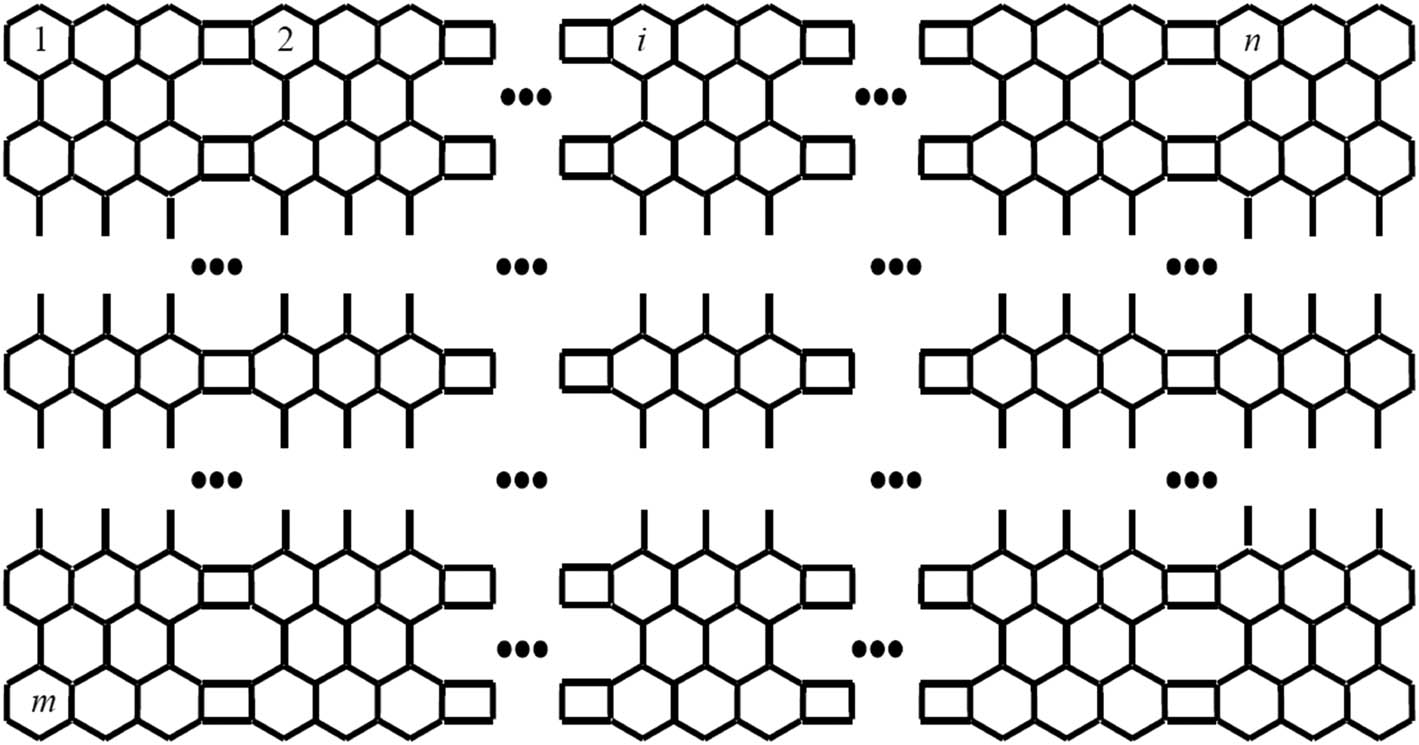
Phenylene.
The molecular graph of anthracene consists of three fused benzene rings (see Figure 2). Actually, it is a part of coal tar which is a very useful component in daily life. Coal tar, which contains around 1.5% anthracene, remains a major source of this material. Its chemical formula is
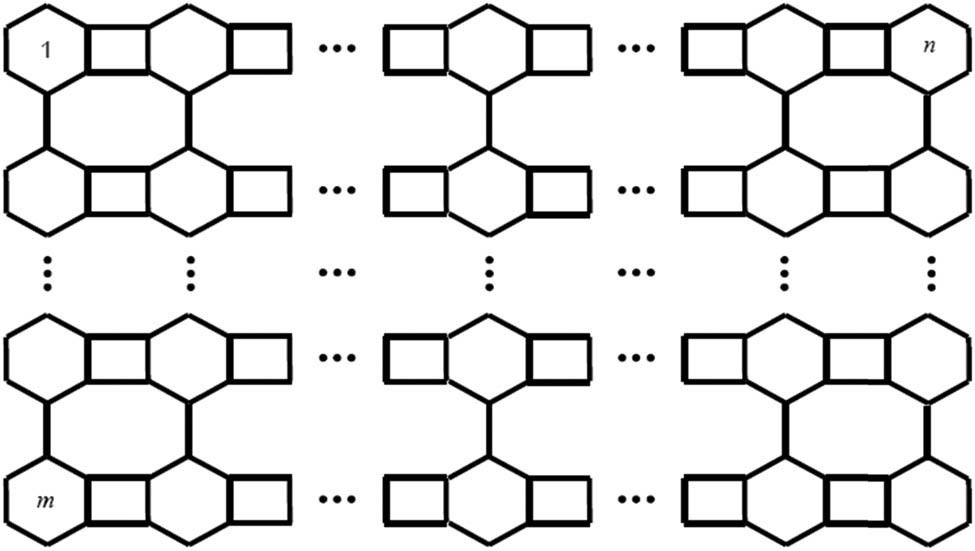
Anthracene.
Quantitative structure activity relationship (QSAR) is a regression and classification model is used in chemical and biological sciences and for many other purposes, but the most important one is to characterize the chemical structures of different compounds in terms of a single real number. This number represents the correlation between chemical structures of different compounds and the related chemical and biological activities or properties [4,5]. There are a lot of categorical QSAR models (categorical models are not correlation driven) but the topological index is the popular one because of its effectiveness [1,6].
For further study of energy, see refs. [7–9]. A graph spectrum-based invariant, put forward by Estrada, is defined as
In this study, we have observed the following inequalities:
2 Method
In this section, we discuss the process of finding the energy and Estrada index of the phenylene and anthracene by using the computational methods. In the first step, we use HyperChem to draw the molecule of our chemical structures [22]. In the second step, we construct an adjacency matrix of the graph by using TopoCluj [23]. In the third step, we find the energy with the help of MATLAB which is represented in Table 1. In the fourth step, we find the suitable curve (polynomial) of degree two with the help of “cftoolbox” of MATLAB [24,25]. The results obtained from this data are displayed in Table 2. In Tables 3 and 4, we found the errors between the exact and the estimated values extracted from the curve for energy and Estrada index of phenylene. We also calculated the inertia index, nullity and signature of the phenylene and are displayed in Table 5. Similar process has been used for anthracene. In Tables 6 and 7, we found the polynomials by using the abovementioned techniques, and then in Tables 8 and 9, we calculated the errors of the exact and estimated values of the energy and Estrada index of anthracene. Finally, in Table 10, we displayed the inertia index, nullity and signature of anthracene.
The quadratic curves for the energy and Estrada index of phenylene
|
|
Energy
|
Estrada index
|
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The quadratic curves extracted from the coefficient of the equations given in Table 1
|
|
|
|
|---|---|---|
|
|
|
|
|
|
|
|
| 1 |
|
|
The exact values
|
|
|
|
Error |
|---|---|---|---|
|
|
84.51 | 84.21 | 0.30 |
|
|
172.99 | 172.69 | 0.30 |
|
|
261.40 | 261.17 | 0.23 |
|
|
349.96 | 349.66 | 0.30 |
|
|
438.61 | 438.15 | 0.46 |
|
|
527.16 | 526.66 | 0.50 |
|
|
615.43 | 615.17 | 0.26 |
|
|
704.11 | 703.69 | 0.42 |
|
|
792.63 | 792.22 | 0.41 |
|
|
881.11 | 880.74 | 0.37 |
The exact values
|
|
|
|
Error |
|---|---|---|---|
|
|
176.5041 | 176.5141 | 0.0099 |
|
|
363.8355 | 363.8555 | 0.0200 |
|
|
551.1702 | 551.2074 | 0.0372 |
|
|
738.5094 | 738.5593 | 0.0499 |
|
|
925.8424 | 925.9112 | 0.0687 |
|
|
1113.1775 | 1113.2631 | 0.0851 |
|
|
1300.5126 | 1300.6150 | 0.1023 |
|
|
1484.8476 | 1487.9669 | 0.1189 |
|
|
1675.1827 | 1675.3188 | 0.1358 |
|
|
1862.5178 | 1862.6707 | 0.1527 |
The inertia, nullity and signature of phenylene
| P = Phenylene | p (P) | n (P) | η (P) | s (P) |
|---|---|---|---|---|
| (8, 1) | 24 | 24 | 0 | 0 |
| (8, 2) | 48 | 48 | 0 | 0 |
| (8, 3) | 72 | 72 | 0 | 0 |
| (8, 4) | 96 | 96 | 0 | 0 |
| (8, 5) | 120 | 120 | 0 | 0 |
| (8, 6) | 144 | 144 | 0 | 0 |
| (8, 7) | 168 | 168 | 0 | 0 |
| (8, 8) | 192 | 192 | 0 | 0 |
| (8, 9) | 216 | 216 | 0 | 0 |
| (8, 10) | 240 | 240 | 0 | 0 |
| (8, 11) | 264 | 264 | 0 | 0 |
| (8, 12) | 288 | 288 | 0 | 0 |
The quadratic curves suitable for the Energy and Estrada index of anthracene
|
|
|
|
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The quadratic curves extracted from the coefficient of the equations in Table 6
|
|
|
|
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
The exact values
|
|
|
|
Error |
|---|---|---|---|
|
|
218.7247 | 218.6827 | 0.0420 |
|
|
453.8533 | 453.7937 | 0.0596 |
|
|
688.9118 | 688.9047 | 0.0071 |
|
|
924.0977 | 924.0157 | 0.0820 |
|
|
1159.1496 | 1159.1268 | 0.0229 |
|
|
1394.2470 | 1394.2377 | 0.0093 |
|
|
1629.3590 | 1629.3487 | 0.0103 |
|
|
1864.4720 | 1864.4597 | 0.0123 |
|
|
2099.5850 | 2099.5707 | 0.0143 |
|
|
2334.6970 | 2334.6818 | 0.0152 |
The exact values
|
|
|
|
Error |
|---|---|---|---|
|
|
622.1380 | 622.1370 | 0.0010 |
|
|
1606.9220 | 1606.9200 | 0.0020 |
|
|
2591.7070 | 2591.7030 | 0.0040 |
|
|
3576.4930 | 3576.4860 | 0.0070 |
|
|
4561.2790 | 4561.2690 | 0.0100 |
|
|
5546.0650 | 5546.0520 | 0.0130 |
|
|
6530.8500 | 6530.8350 | 0.0150 |
|
|
7515.6360 | 7515.6180 | 0.0180 |
|
|
8500.4220 | 8500.4010 | 0.0210 |
|
|
9485.2070 | 9485.1840 | 0.0230 |
The inertia, nullity and signature of anthracene
| G = Anthracene | p (G) | n (G) | η (G) | s (G) |
|---|---|---|---|---|
| (8, 1) | 35 | 35 | 0 | 0 |
| (8, 2) | 70 | 70 | 0 | 0 |
| (8, 3) | 105 | 105 | 0 | 0 |
| (8, 4) | 140 | 140 | 0 | 0 |
| (8, 5) | 175 | 175 | 0 | 0 |
| (8, 6) | 210 | 210 | 0 | 0 |
| (8, 7) | 280 | 280 | 0 | 0 |
| (8, 8) | 315 | 315 | 0 | 0 |
| (8, 9) | 350 | 350 | 0 | 0 |
| (8, 10) | 385 | 385 | 0 | 0 |
| (8, 11) | 420 | 420 | 0 | 0 |
| (8, 12) | 455 | 455 | 0 | 0 |
2.1 Results of phenylene
In this section, we will study the energy, Estrada index, nullity, inertia and signature of phenylene. We will also show the comparison between exact and estimated values of energy and Estrada index of phenylene.
Proposition 2.1
Let
The two curves that are representing the energy and Estrada index of the graph are given by equations (1) and (2), respectively. We observed that the exact value of the energy of phenylene is always greater than the estimated values of the energy of phenylene, i.e.,
Similarly, we have observed that the exact value of Estrada index of phenylene is always less than the estimated values of Estrada index of phenylene, i.e.,
Also the errors between the exact values and the estimated values of energy and Estrada index are summarized in Tables 3 and 4.
2.2 Results of anthracene
In this section, we will study the energy, Estrada index, nullity, inertia and signature of anthracene. We will also show the comparison between the exact and estimated values of energy and Estrada index of anthracene.
Proposition 2.2
Let
The errors of energy and Estrada index are as given in Tables 8 and 9.
We observed that exact value of the energy of anthracene is always greater than the estimated values of the energy of anthracene, i.e.,
3 Conclusion
We studied Estrada index, energy, inertia, nullity and signature of phenylene and anthracene. The following inequalities
Also, since the nullity of phenylene and anthracene is zero, the molecule of these structures are stable and closed shell. At the end, we give a graphical representation of these parameters (Figures 3–6).
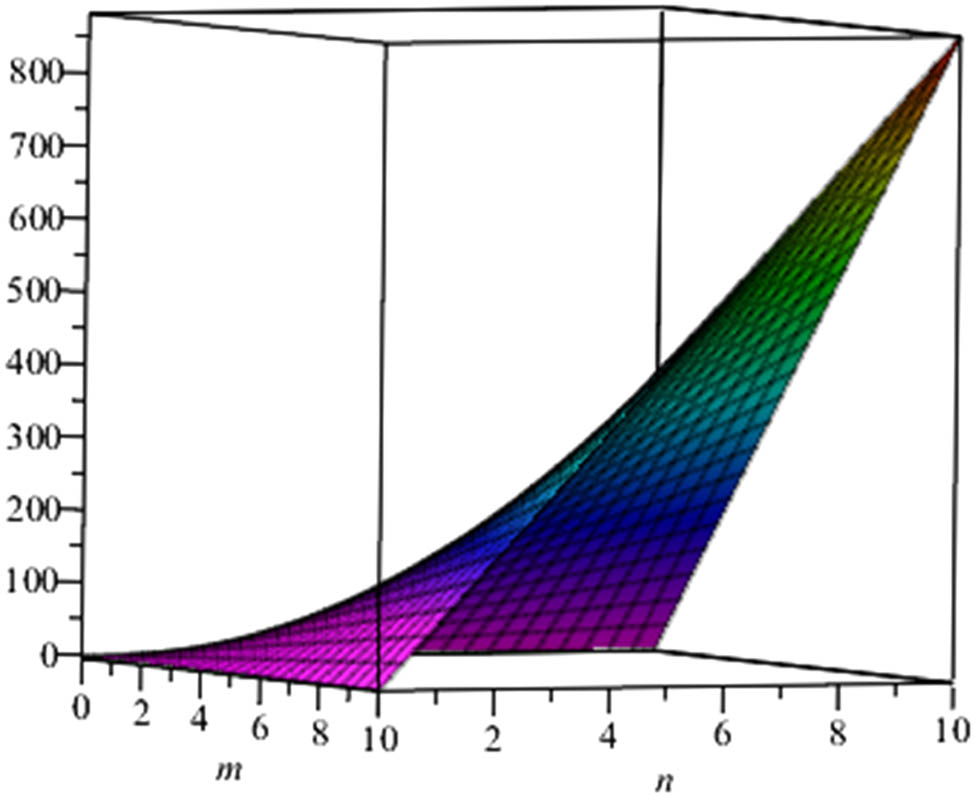
Energy of phenylene.
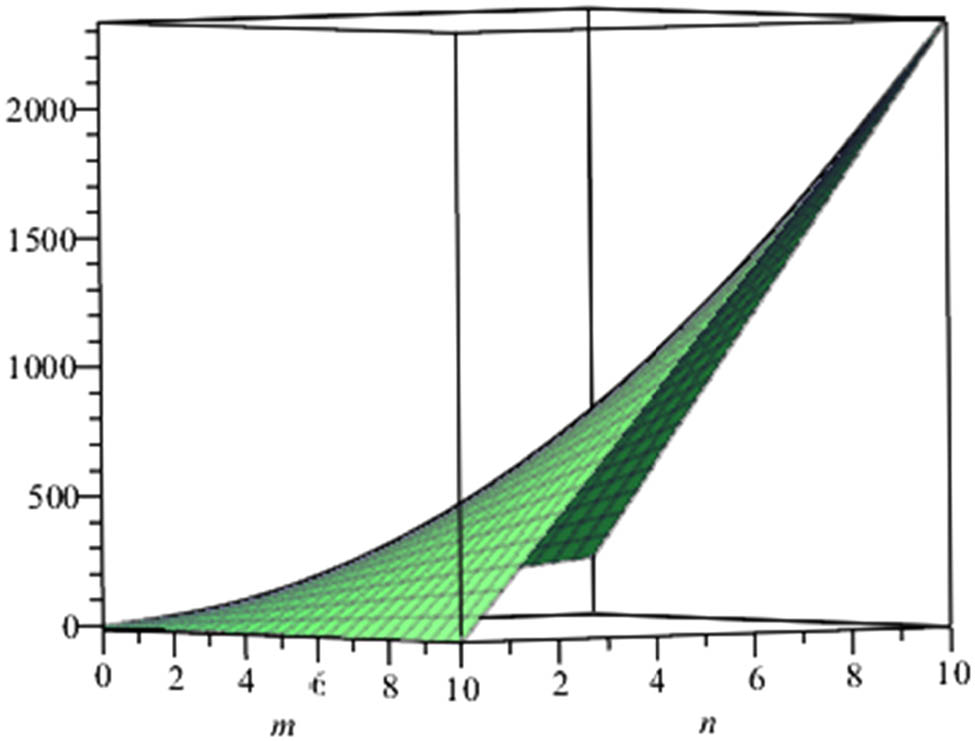
Energy of anthracene.
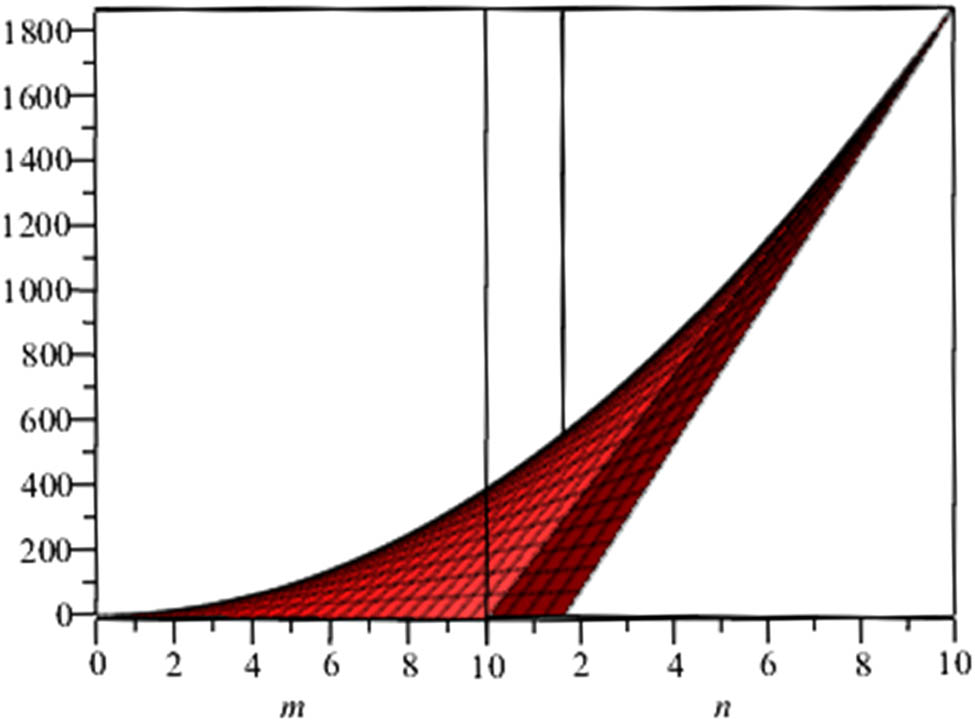
Estrada index of phenylene.
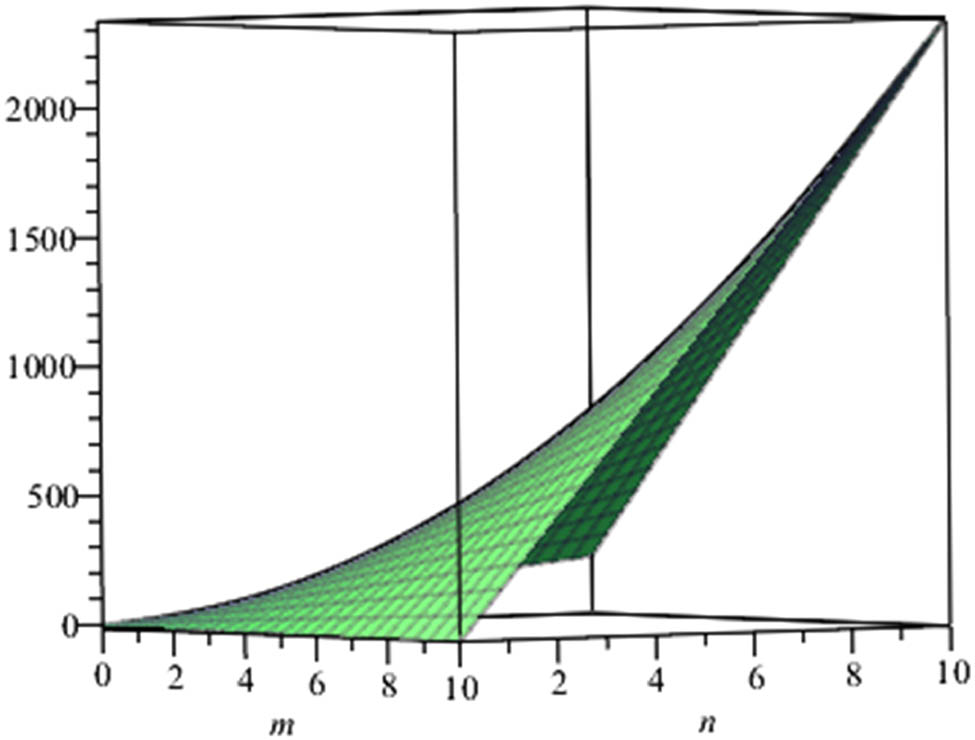
Estrada index of anthracene.
-
Author contributions: Z. A. analyzed the data curation. Z. S. F. contributed to visualization, investigation and wrote the initial draft of the study. M. F. N. contributed to conceptualization, visualization and methodology. H. S. contributed to designing the experiments, validation. H. M. A. S. was in charge of formal analyzing of experiments, resources and some computations. All authors read and approved the final version of the study.
-
Funding information: The authors received no financial support for the research, authorship, and/or publication of this article.
-
Conflict of interest: The authors have no conflicts of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Butler S. Eigenvalues and structures of graphs. Ph.D. Thesis, University of California, San Diego; 2008.Search in Google Scholar
[2] Chung FRK. Spectral graph theory. Number 92 in CBMS Regional Conference Series in Mathematics American Mathematical Society, Providence, USA; 1997.10.1090/cbms/092Search in Google Scholar
[3] Hall GG. Eigenvalues of molecular graphs. Bull Inst Math Appl. 1981;17:70–2.10.1080/00268977700100471Search in Google Scholar
[4] Graovac A, Gutman I, Trinajsti´c N. Topological approach to the chemistry of conjugated molecules. Berlin: Springer-Verlag; 1977.10.1007/978-3-642-93069-0Search in Google Scholar
[5] Gutman I, Polansky OE. Mathematical concepts in organic chemistry. Berlin: Springer; 1986.10.1007/978-3-642-70982-1Search in Google Scholar
[6] Kanna MR, Jagadeesh R. Topological indices of vitamin A. Int J Math And Appl. 2018;55:7.10.14419/ijet.v7i4.24064Search in Google Scholar
[7] Gutman I, Kiani D, Mirzakhah M. On incidence energy of graphs. Math Comput Chem. 2009;62:573–80.10.1016/j.laa.2009.04.019Search in Google Scholar
[8] Gutman I, Deng H, Radenkovic S. The Estrada index: an updated survey. In: Cvetkovic D, Gutman I, editors. Selected topics on applications of graph spectra. Beograd: Mathematical Institute; 2011. p. 155–74.Search in Google Scholar
[9] Li X, Shi Y, Gutman I. Graph energy. New York: Springer; 2012.10.1007/978-1-4614-4220-2Search in Google Scholar
[10] Balaban AT. Topological indices based on topological distances in molecular graphs. Pure Appl Chem. 1983;55(2):199–206.10.1351/pac198855020199Search in Google Scholar
[11] Bozkurt SB, Gutman I. Estimating the incidence energy. MATCH Commun Math Comput Chem. 2013;70:143–56.Search in Google Scholar
[12] Das KC, Gutman I. On incidence energy of graphs. Linear Algebra Appl. 2014;446:329–44.10.1016/j.laa.2013.12.026Search in Google Scholar
[13] Estrada E. Characterization of 3D molecular structure. Chem Phys Lett. 2000;319:713–8.10.1016/S0009-2614(00)00158-5Search in Google Scholar
[14] de la Pena JA, Gutman I, Rada J. Estimating the Estrada index. Linear Algebra Appl. 2007;427:70–6.10.1016/j.laa.2007.06.020Search in Google Scholar
[15] Shang Y. The Estrada index of random graphs. Sci Magna. 2011;7:79–81.10.1155/2011/872703Search in Google Scholar
[16] Zohu B. On Estrada index. MATCH Commun Math Comput Chem. 2008;60:485–92.Search in Google Scholar
[17] Malik MA, Farooq R. Computational results on the energy and Estrada index of TUC4C8(R) [m, n] nanotubes. Optoelectron Adv Mat. 2015;9(1–2):311–3.Search in Google Scholar
[18] Malik MA, Farooq R. Some conjuctures on energy and Estrada index of CNCK[n] nanocones. Optoelectron Adv Mat. 2015;9(3–4):415–8.Search in Google Scholar
[19] Li F, Weic L, Zhaob H, Hua F, Maa X. On the Estrada index of cactus graphs. Discrete Appl Math. 2016;203:94–105.10.1016/j.dam.2015.09.026Search in Google Scholar
[20] Ma H, Yang W, Li. S. Positive and negative inertia index of a graph. Linear Algebra Appl. 2013;438(1):331–41.10.1016/j.laa.2012.07.014Search in Google Scholar
[21] Omidi G. On the nullity of bipartite graphs. Graph Combinator. 2009;25(1):111–4.10.1007/s00373-008-0825-5Search in Google Scholar
[22] HyperChem(TM) Professional 8.0, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA.Search in Google Scholar
[23] Ursu O, Diudea MV. TOPOCLUJ software program. Cluj: Babes-Bolyai University; 2005.Search in Google Scholar
[24] Löfberg J. YALMIP: a toolbox for modeling and optimization in MATLAB. In Proceedings of the CACSD Conference. Vol. 3; 2004.10.1109/CACSD.2004.1393890Search in Google Scholar
[25] Gander W, Hrebicek J. Solving problems in scientific computing using Maple and Matlab®. New York, USA: Springer Science and Business Media; 2004.10.1007/978-3-642-18873-2Search in Google Scholar
© 2021 Zaheer Ahmad et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

