Abstract
The composite of tin oxide-reduced graphene oxide (SnO2/rGO) was prepared via a green synthesis of rGO using Pometia pinnata leaf extract followed by the dispersion of the SnO2 precursor. The composite was employed as a photocatalyst for the removal of methylene blue (MB) under UV and visible light. A variety of spectroscopic and analytical techniques, consisting of X-ray diffraction, Fourier-transform infrared, scanning transmission electron microscopy, photoluminescence spectroscopy, and a transmission electron microscope, was used to characterize the physical properties of the photocatalyst. The characterizations represent the dispersed SnO2 nanoparticles in the rutile phase with the mean particle size of 72 nm. The photocatalytic activity experiments revealed the superiority of the composite for photodegradation application under the visible light source compared to UV light. This visible light-responsive property is fit with photoluminescence intensity in the visible light range. It was found that SnO2/rGO yields the degradation efficiency of ca. 98.28% within 90 min.
1 Introduction
The development of nanotechnology is currently experiencing rapid progress. Research on green nanotechnology, including the utilization of plant extracts as reducing and capping agents, is still being developed by scientists to produce a renewable and safe reagent [1]. Within the scheme, green-synthesized reduced graphene oxide (rGO) is one material receiving considerable interest for many applications. Many plant extracts have been successfully employed for green synthesis, and the extracts mainly consist of secondary metabolites, such as flavonoids and polyphenols [2,3]. Previous researchers utilized the leaf extracts of the Prunus serrulata (Cherry) leaf, the marigold flower, Eichhornia crassipes, and Terminalia bellirica fruit extracts at varied extraction conditions for an optimum reduction reaction [4,5,6,7]. Furthermore, the modification of rGO with a metal oxide for specific applications of catalysis, photocatalysis, sensors, and biosensors is also a promising scheme.
The incorporation of a metal oxide photocatalyst to form a metal oxide-rGO composite was reported to have a higher efficiency due to the stabilization of the electron charges transfer provided by the rGO [8,9]. The TiO2/rGO, SnO2/rGO composite was also reported to be effective for photocatalysis [9,10]. In addition to the stabilization of the metal oxide photocatalyst for reusability, rGO has an advantage related to the visible light-responsive properties of photocatalysts. The composites of Cu2O/rGO and MgFe2O4/rGO are examples of the visible light sensitive enhancement of the low-energy photodegradation of dye [8,11,12].
To our knowledge, studies on the composite of SnO2/rGO obtained from green-synthesized-rGO using a plant extract are limited. Using the green chemistry approach, the physicochemical characterization and photocatalytic activity of green-synthesized SnO2/rGO were investigated in this study. As a specific plant growth in the eastern region of Indonesia, the leaf extract of matoa (Pometia pinnata) was chosen as a reducing agent. Previous works showed that the leaf has the characteristic of being high in flavonoid and that it functions as a secondary metabolite; however, it has not been utilized often [13,14]. Based on this background, the aim of this research was to investigate the physicochemical properties of SnO2/rGO obtained from the leaf extract of matoa/Pometia pinnata (PPE) as a reducing agent as well as the photocatalytic characteristics of the composite, particularly regarding the exploration of photocatalytic activity under a visible light source.
2 Materials and methods
2.1 Materials
The chemicals utilized for this research consisted of methylene blue (MB), graphite flake, SnCl2·2H2O, citric acid, KMnO4, H2O2, and H2SO4 (Merck-Millipore). The Pometia pinnata leaf was obtained from a tree cultivated in Sleman District, Yogyakarta Province. The PPE extract was obtained by boiling 10 g of PP dry in 100 mL aquadest followed by filtering using Whatman 41 paper.
2.2 Preparation of GO and rGO
Graphene oxide (GO) was prepared using Hummers’ method using graphite flake as a raw material. Graphite flake (2 g) was added to 46 mL of concentrated H2SO4 (in an ice bath) followed by the addition of 6 g of KMnO4. The resulting mixture was stirred for 2 h at room temperature followed by increasing the temperature to 35°C. 92 mL of deionized water was added slowly, and then 10 mL of H2O2 (30 wt%) was added slowly with the appearance of vigorous bubbles and a change in the color of the suspension from dark brown to yellow. The suspension was aged to settle, and the clear supernatant was decanted. The remaining suspension was filtered and washed with acetone and was then dried in an oven overnight at 40oC.
Reduction onto GO to produce rGO was performed by mixing 90 mL PE extract with 25 mg GO. The mixture was refluxed for 48 h at 100oC. The mixture was then aged for 24 h for settling and then decanted and filtered.
2.3 Preparation of SnO2/rGO
SnO2/rGO was prepared by mixing rGO with a SnCl2 solution at the theoretical Sn content of 20 wt%. The mixture was aged in an autoclave overnight before being filtered, dried, and calcined at 400°C for 2 h.
2.4 Physicochemical characterization
The physicochemical characterization to investigate the chemical change during synthesis was performed by X-ray diffraction (XRD), Fourier-transform infrared (FTIR), scanning electron microscope-energy dispersive X-ray (SEM-EDX), and a transmission electron microscope (TEM). A Rigaku Miniflex-600 (Tokyo, Japan) XRD instrument was employed for the phase change analysis using a Ni-filtered Cu-Kα source with the voltage of 40 kV and a 20 mA current. FTIR Spectroscopy was carried out using Perkin Elmer–ATR (Singapore). X-ray Photoelectron spectra were recorded using a PHOIBOS 225 (Specs GmbH) spectrometer. The surface morphology was studied using FESEM taken on a JX-JEOL electron microscope, while the TEM was recorded using the JSM-JEOL (Tokyo, Japan).
2.5 Photocatalytic activity test
The evaluation of the photocatalytic activity of the materials was carried out for MB photodegradation. Each experiment was conducted with the photocatalyst dosage of 0.5 g/L and the MB concentration of 20 mg/L. The photocatalytic reaction was conducted for a duration of 90 min, while the samples were collected at the predetermined 0, 5, 15, 30, 45, 60, and 90 min. The collected samples were centrifuged before colorimetric analyzation using the UV-Visible spectrophotometry method. The varied photocatalytic conditions consist of the effect of the light source (UV and Visible light), and the effect of the H2O2 addition was investigated. For the reaction using H2O2, particularly 1 mL H2O2, 10−3 M was added to the solution. The degradation efficiency (DE) of each treatment was calculated using the following formula (equation 1):
C 0 and C 90 are the initial concentrations of the MB concentration after treatment for 90 min.
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Material characterization
The GO reduction was initially monitored by recording the UV-vis absorption spectroscopy analysis. The comparison of the GO and the rGO spectra is shown in Figure 1. GO exhibited an absorption peak at 230 nm, which attributed to the π–π* transitions of the aromatic C═C bonds, and there were no other peaks identified for another transition. After reduction, the spectrum exhibited other peaks, which confirmed the reduction located at around 220–380 nm [15,16]. The identified absorption peak at 266.5 nm in rGO confirmed the restoration of electronic conjugation within the graphene nano sheets that redshifted from 230 nm to 266.5 nm due to the reduction of the GO [17,18]. These chemical changes can be confirmed by the FTIR analysis of both materials (Figure 2).
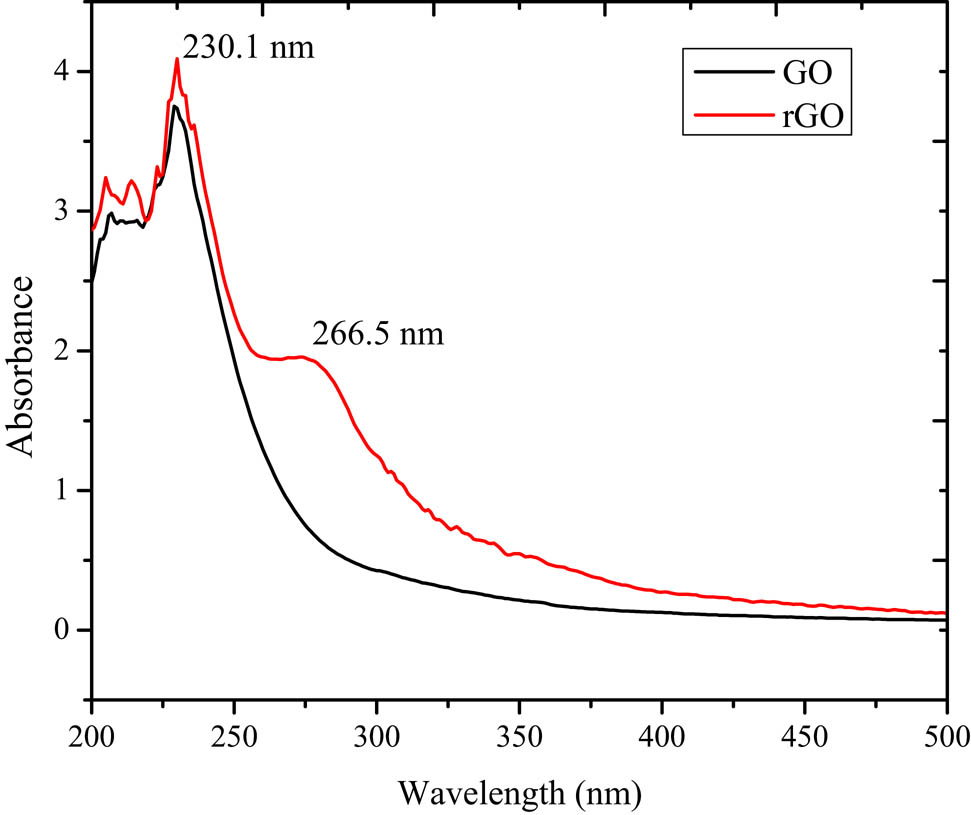
UV-vis absorption spectra of GO and rGO prepared by PPE.

FTIR spectra of materials.
The FTIR spectra (Figure 2) illustrate the difference between the functional groups of GO and rGO obtained by varied reduction times. GO showed a broadband at 3,338 cm−1, which corresponded to the stretching mode of the OH group, along with absorption at 1,154 cm−1, which attributed to the presence of C–OH. An absorption peak at 1,724 cm−1 due to C═O also indicated the more oxygenated state of GO. After reduction, the absorptions correlated with oxygenate disappeared on the reduction for either 12 or 48 h, which reveals the absence of the OH group after reduction. These results indicated that the partial functional groups in GO had been effectively eliminated by the reduction.
These results are similar to those reported by previous research [19,20]. After being composited with SnO2, there was no specific change in the absorption compared to rGO, which implies the absence of an oxidative change by the composite formation.
Based on a previous investigation of the chemical constituent of PPE, the chemicals, which consisted of flavonoids, polyphenols, polysaccharides, etc., contained in the PPE play an important role in reducing the oxygen-containing groups decorated on GO sheets [13]. The reducibility of the phenolic and hydroxyl groups was reflected by the disappearance of the C═O and C–OH functional groups in the FTIR measurement.
Furthermore, the presence of SnO2 in SnO2/rGO was identified by the XRD analysis, and the reflections are presented in Figure 3.
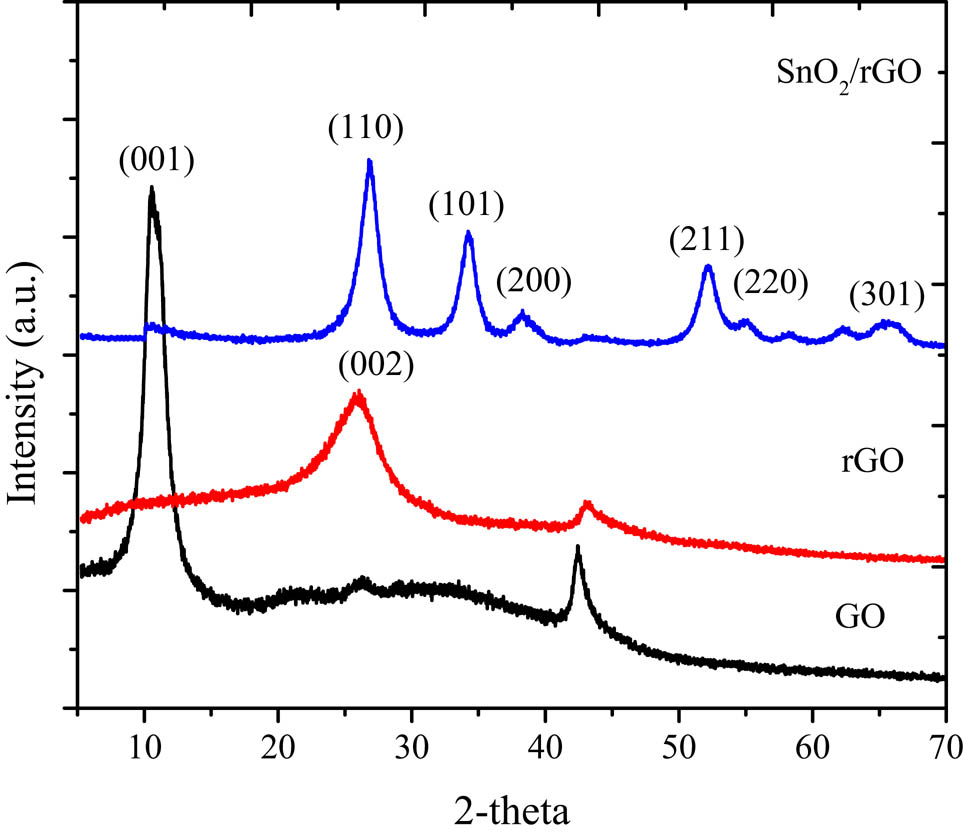
XRD pattern of GO, rGO, and SnO2/rGO.
The pattern depicted a typical GO pattern at 10.2° corresponding to the (001) plane with the d-spacing of 0.81 nm. The rGO exhibited a broad peak at 23.2° (002) along with the disappearance of the (001) reflection, which confirmed the successful reduction by PPE [21,22]. Moreover, the SnO2/rGO composite displayed diffraction peaks at 26.5°, 37.9°, 39.2°, and 53.6°, which correspond to (110), (101), (200), and (211) reflections, respectively, indicating the formation of the tetragonal rutile SnO2 (JCPDS 41-1445).
By using the Debye-Scherer formula for the crystallite size measurement (equation 2) [23,24]:
where B(2θ) is the full width at half maximum (FWHM), χ is the wavelength of the incident X-rays, L is the particle size, and 2θ is the Bragg angle of the each peak. The calculated size is 72.2 nm. Refer to the data listed in Table 1.
Calculated crystallite size of SnO2 in SnO2/rGO based on XRD reflections
| 2-theta (o) | d (Å) | FWHM | Crystallite size (nm) |
|---|---|---|---|
| 26.5 | 3.59 | 1.48 | 61.5 |
| 37.9 | 2.64 | 1.27 | 75.8 |
| 39.2 | 2.36 | 1.17 | 79.9 |
| 53.6 | 1.77 | 1.37 | 71.6 |
| Mean | 72.2 | ||
The presence of SnO2 in the SnO2/rGO composite was confirmed by the EDX analysis, which is related to the change in the surface profile identified by the SEM analysis, as presented in Figure 4. The rGO presents the layered surface structure, which was similar as previously reported [22,25]. After modification with SnO2, the composite showed a distributed particle in irregular forms, reflecting the SnO2 nanoparticles on the surface. The EDX analysis confirmed the Sn peak, which is associated with the SnO2 content at a 51 wt%. This interpretation is in line with the TEM image (Figure 5). The layer structure of the rGO obtained from the SEM analysis was consistent with the sheet structures (shown by arrows). Moreover, the dispersed SnO2 NPs were represented by irregular forms with a size ranging from 50 to 80 nm. This size range was consistent with the crystal size determined by the XRD measurement.

SEM profile of rGO and SnO2/rGO.
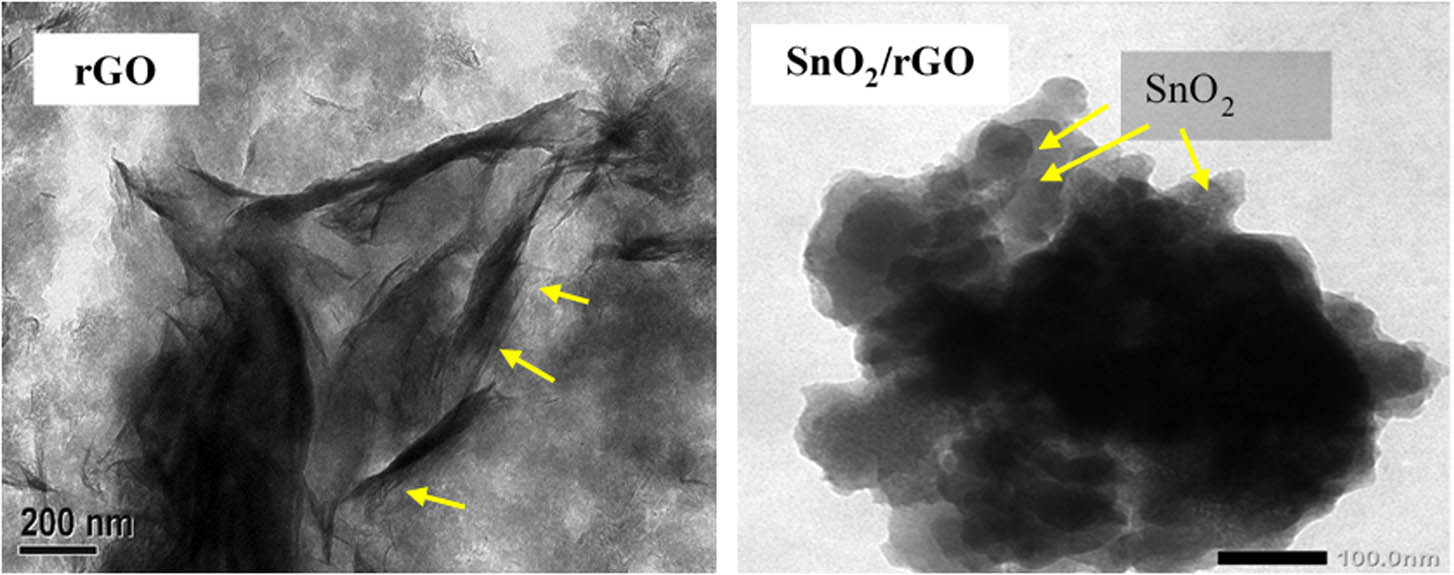
TEM image of rGO and SnO2/rGO.
The supported SnO2 nanoparticles on the surface were confirmed by the EDX analysis results, and they also influence the surface profile as identified by the reduced adsorption–desorption isotherm presented in Figure 6. The calculated parameters are listed in Table 2. The Sn content in the SnO2/rGO was about 26 at.%, which is slightly higher compared to the theoretical Sn content set up in the synthesis (25%). The nanoparticles tended to block the pores and to reduce the specific surface area, which is characterized by reducing the specific surface area from 63.4 to 32.2 m2/g along with reducing the pore volume.
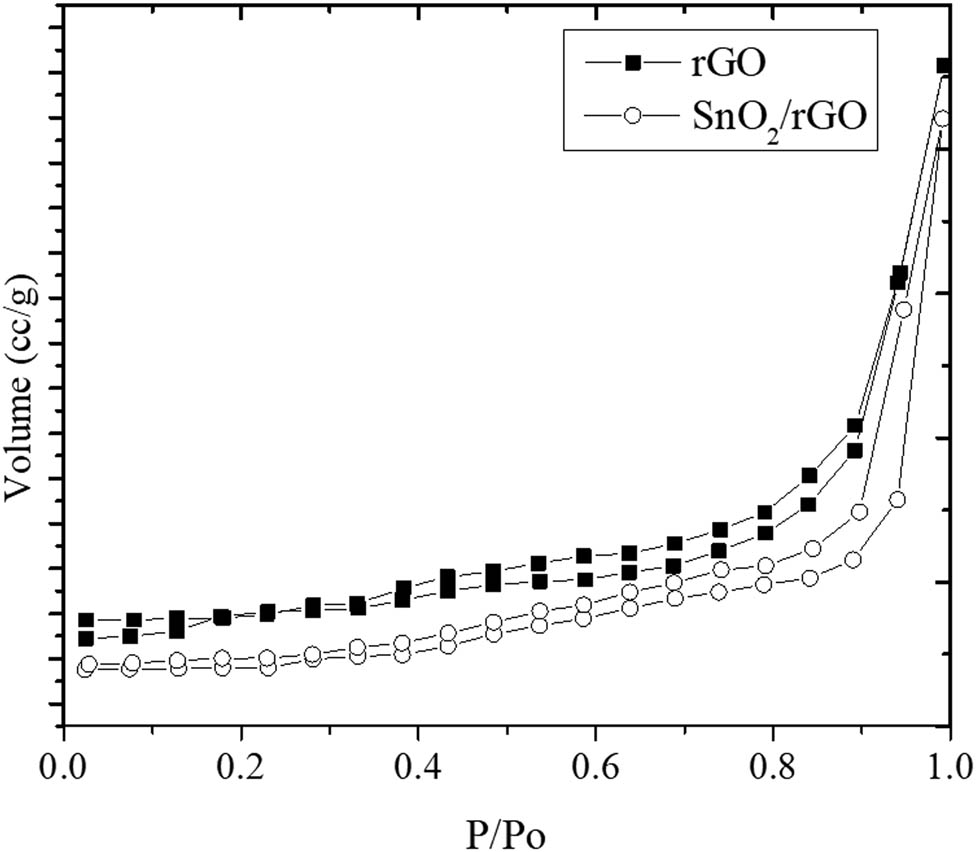
Adsorption–desorption profile of rGO and SnO2/rGO.
Elemental analysis and surface parameter analysis results of rGO and SnO2/rGO
| Parameter (unit) | rGO | SnO2/rGO |
|---|---|---|
| Sn (at.%) | n.d. | 26.5 |
| C (at.%) | 52.3 | 39.2 |
| O (at.%) | 47.7 | 34.3 |
| Specific surface area (m2/g) | 63.4 | 32.2 |
| Pore volume (cc/g) | 3.32 × 10−2 | 2.26 × 10−2 |
| Pore radius (Å) | 23.4 | 24.6 |
3.2 Photocatalytic activity
The photocatalytic activity of SnO2/rGO was evaluated by MB degradation with and without the H2O2 addition as an oxidant. The comparison of the utilization of UV and visible light was performed to evaluate the effectiveness of the degradation of varied light sources. The degradation rate was estimated from the C/C 0, plotted against time, after the initial evaluation of the existence of the degradation mechanism by the UV-Vis spectral analysis. Figure 6 shows the different patterns of the UV-Vis spectral changes on photodegradation with and without H2O2 under UV light illumination. A rapid degradation was indicated by the H2O2 addition to the system as the MB peak at 635 nm disappeared beginning at 15 min of the treatment with the DE of −99%. The degradation without H2O2 had a DE of about 65% at 15 min and increased at the extended time of treatment (Figure 7).
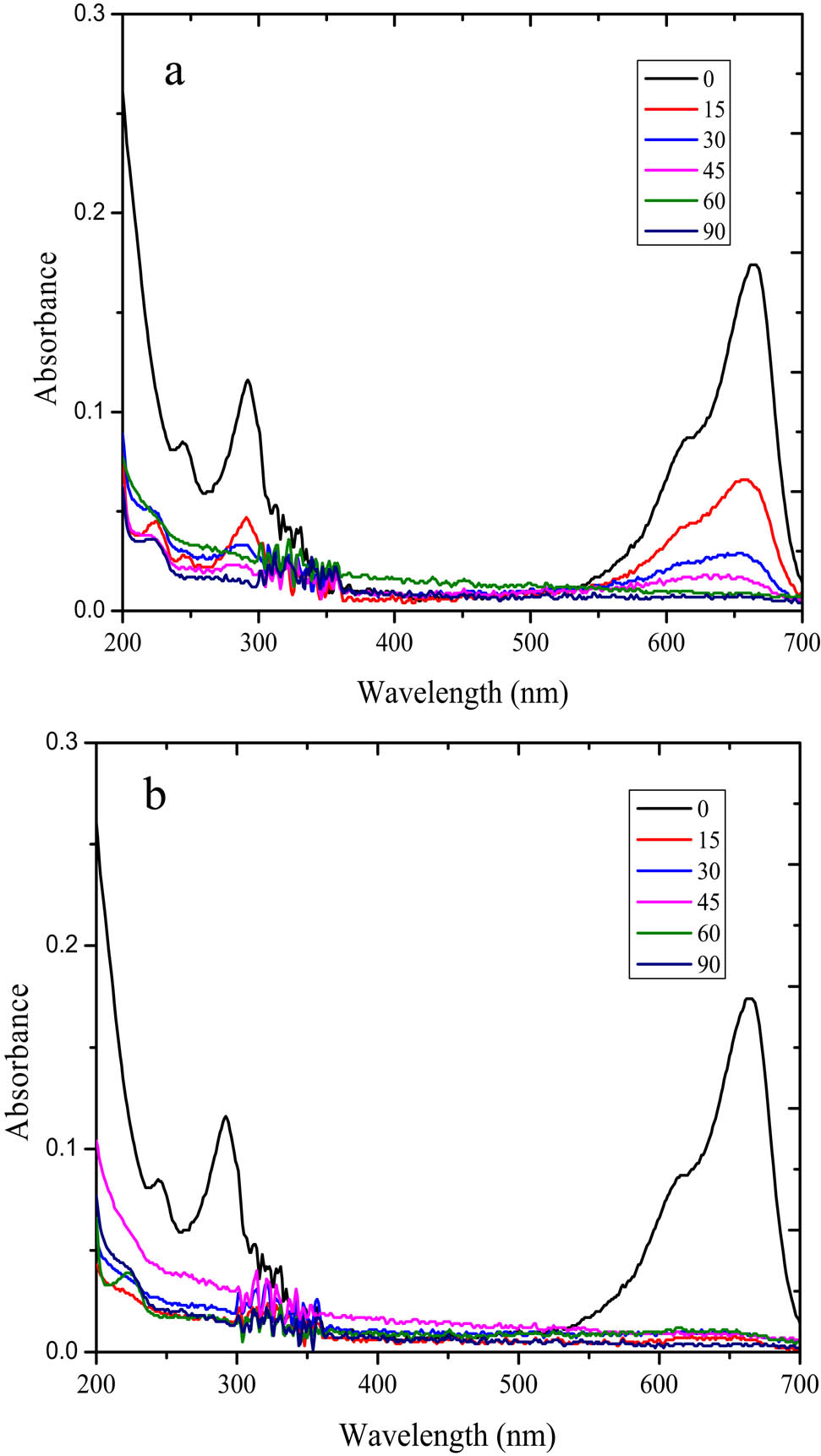
UV-Vis spectroscopy analysis of the treated MB solution over SnO2/rGO (a) without H2O2 (b) with the H2O2 addition.
The comparison of the C/C 0 plot from the MB degradation by SnO2/rGO and rGO photocatalysts with and without H2O2 addition is depicted in Figure 8.
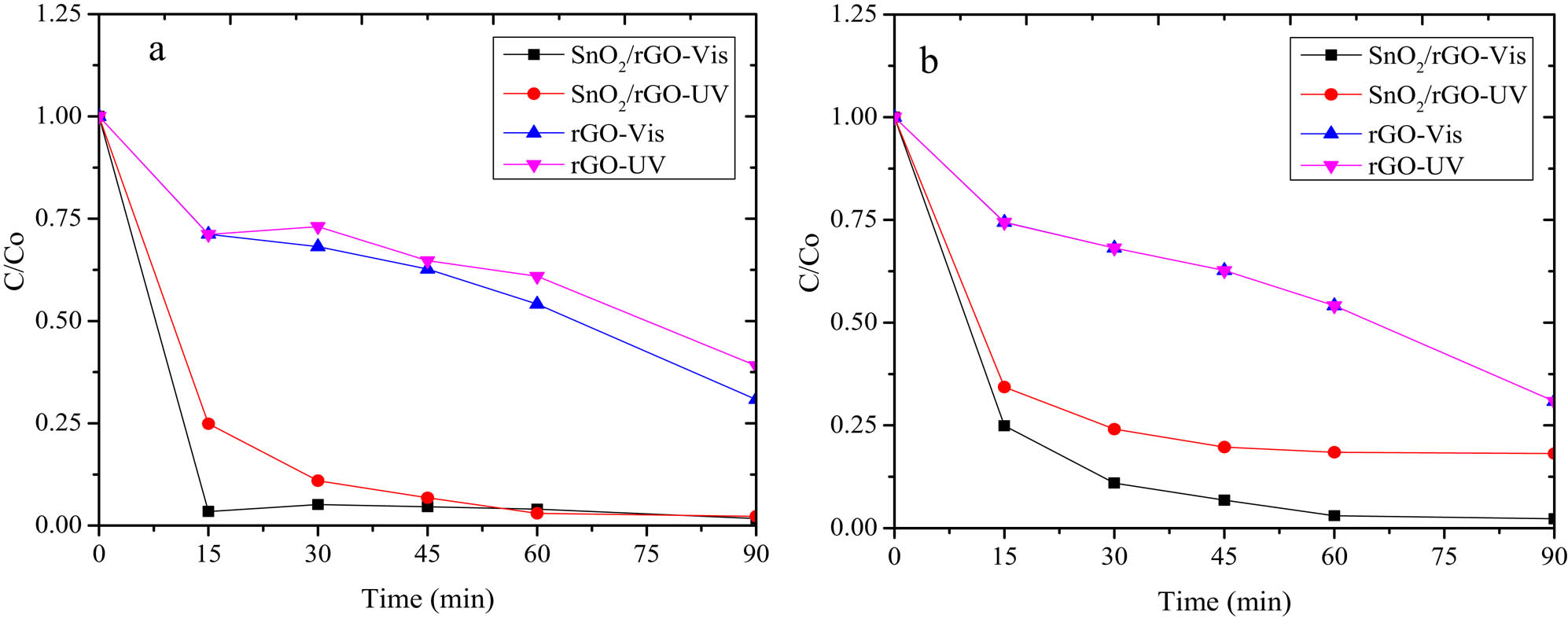
Kinetics plot of MB photodegradation over SnO2/rGO (a) without H2O2 (b) with H2O2 addition.
The kinetics plots represent the photocatalytic activity exhibited by rGO under visible and UV light illumination. Moreover, an increasing photocatalytic activity was achieved by the dispersion of SnO2 in the composite form of SnO2/rGO.
To determine the kinetics constant of the reaction rate (k), a kinetic study of MB photodegradation was carried out according to the classic heterogeneous kinetic model (pseudo first-order model) [26] (equations 3–5):
where C
o and C
t
are the initial concentration and concentration at time of t, k
app is the apparent kinetics constant, K is the adsorption–desorption equilibrium constant, and k =
The calculated kinetics constants and the correlation coefficient of the pseudo first-order kinetics are listed in Table 3. The data lead to the conclusion that the SnO2/rGO photocatalyst is superior under visible light, as shown by the higher DE at a visible light exposure reaction compared to under UV illumination. The photodegradation reaction was also accelerated by the addition of H2O2 as an oxidant in the degradation system. The comparison on the kinetics plot at varied light sources indicates that both SnO2/rGO and rGO materials showed a higher photoactivity under visible light rather than under UV light. The effect of the light source and the presence of H2O2 did not significantly affect the kinetics of photodegradation using rGO.
The calculated kinetics constants and the correlation coefficient of the pseudo first-order kinetics
| Photocatalyst | Light source | +H2O2 | DE (%) | R 2 of pseudo first-order kinetics | K (min−1) |
|---|---|---|---|---|---|
| SnO2/rGO | Vis | Yes | 98.28 | 0.9834 | 1.86 × 10−2 |
| SnO2/rGO | Vis | No | 97.74 | 0.9955 | 2.70 × 10−2 |
| SnO2/rGO | UV | Yes | 97.74 | 0.9951 | 2.67 × 10−2 |
| SnO2/rGO | UV | No | 81.87 | 0.9550 | 4.02 × 10−3 |
| rGO | Vis | Yes | 69.78 | 0.9690 | 1.36 × 10−3 |
| rGO | Vis | No | 69.18 | 0.8990 | 1.35 × 10−3 |
| rGO | UV | Yes | 60.06 | 0.9900 | 1.04 × 10−2 |
| rGO | UV | No | 69.18 | 0.9899 | 1.35 × 10−3 |
This result is interesting because many experiments on photocatalysis have been attempted to create a visible light sensitive photocatalyst, which means that the photocatalyst can intensively function under lower energy consumption and even under solar irradiation.
The role of SnO2 in a faster degradation is related to its n type semiconductor performance, whereas if the light source impinges the material, an excitation of electrons from the valence band to the conduction band occurs. The excitation produces holes (h +), and as it interacts with –OH, the radical hydroxyl radical (˙OH) is generated as a strong oxidizing agent. The excited electrons at the conductance band will also be activated with its interaction with O2 for the released superoxide radicals (˙O2 −). The possible mechanism due to these possibilities is as follows [27]:
The fitness of a photocatalyst for a visible light region is indicated by the photoluminescence spectra analysis presented in Figure 9. The rGO has a high PL intensity along the visible light region (300–800 nm), which supports the capability of rGO to catch light for electron excitation.

PL spectra of rGO.
The comparison of the photocatalytic activity of SnO2/rGO prepared for this work with other previous related works presented in Table 4 implies that the material has a relatively high capability. Compared to the DE using rGO reported by previous works (32.68%), the DE obtained in this study is higher (69.23%), even though the specific surface area of the rGO synthesized from this work (63 m2/g) is less than that of rGO (94 m2/g) [29]. Moreover, the SnO2/rGO prepared for this work is also superior in DE (98.28%) compared to that reported by previous researchers (89%) [32]. Similarly, a higher activity was exhibited due to the higher kinetics constant of MB degradation by visible light compared to SnO2/rGO prepared by the dominant SnO2 [9]. The specific surface area and the Sn content in SnO2/rGO for this work compared with that of previous studies suggest the effect of the rGO surface profile in enhancing the photodegradation mechanism. The composed functional groups from PPE-reduction significantly influenced an increase in the adsorption mechanism, which strongly influences the photodegradation mechanism. Moreover, the synthesized SnO2/rGO showed superior properties related to visible light-responsive capability.
Comparison of the photocatalytic activity of SnO2/rGO prepared in this work with other previous related works
| Photocatalyst | DE | Remark | Reference |
|---|---|---|---|
| GO/ZnO nanodrum | 94 | Light source: UV, time of treatment 60 min | [28] |
| rGO | 32.68 | UV, 60 min. rGO was synthesized using acetic acid as reductor under solvothermal method | [29] |
| ZnO/rGO | 99 | UV | [30] |
| TiO2/GO | 67.45 | UV | [31] |
| SnO2/GO | 89 | UV, catalyst dosage = 7.5 mg/100 mL | [32] |
| Cu/rGO | 94 | UV, MB = 40 mg/L | [33] |
| TiO2/rGO | 99.31 | MB: 10 mg/L | [33] |
| SnO2/rGO | 98.28 | Light source: visible, catalyst dosage: 0.5 g/L | This work |
| rGO | 69.18 | Light source: visible, catalyst dosage: 0.5 g/L | This work |
3.3 Material stability
Photocatalyst stability is an important parameter for application purposes. Because photocatalyst activity is influenced by many factors, such as pH, oxidation condition, etc., the properties of photocatalyst materials can be changed, leading to reduced activity. For this study, XPS and XRD analyses of the SnO2/rGO material before and after use were performed.
The XPS survey scan spectra presented in Figure 10 indicate that the surface chemical composition of the SnO2/rGO was not significantly different after use. The survey spectra before and after use exhibited the peaks of C 1s, O 1s, and Sn. The deconvolution of the C 1s spectrum represented three peaks centered at 284.4, 286.5, and 288.6 eV, which correspond to the C–C, C–O, and –COO– groups, respectively. This indicates the existence of oxygen-containing groups in the rGO as support. Furthermore, the presence of Sn was identified by Sn 3p, 3d, and 4d. In particular, Sn 3d was characterized by two peaks with a binding energy of 486.3 and 494.7 eV, respectively, which corresponded to Sn 3d5/2 and Sn 3d3/2, respectively. These peaks are attributed to SnO2 in nanoparticle form [34]. The same pattern of the C and Sn peaks implies that there was an unchanged electronic state of Sn after usage [35,36]. The maintained structure after use was also evidenced by the unchanged XRD reflections before and after use, as presented in Figure 10. All peaks corresponding to the interpretation based on Figure 3 still had relatively similar intensities, representing the stability of SnO2 nanoparticles (Figure 11).
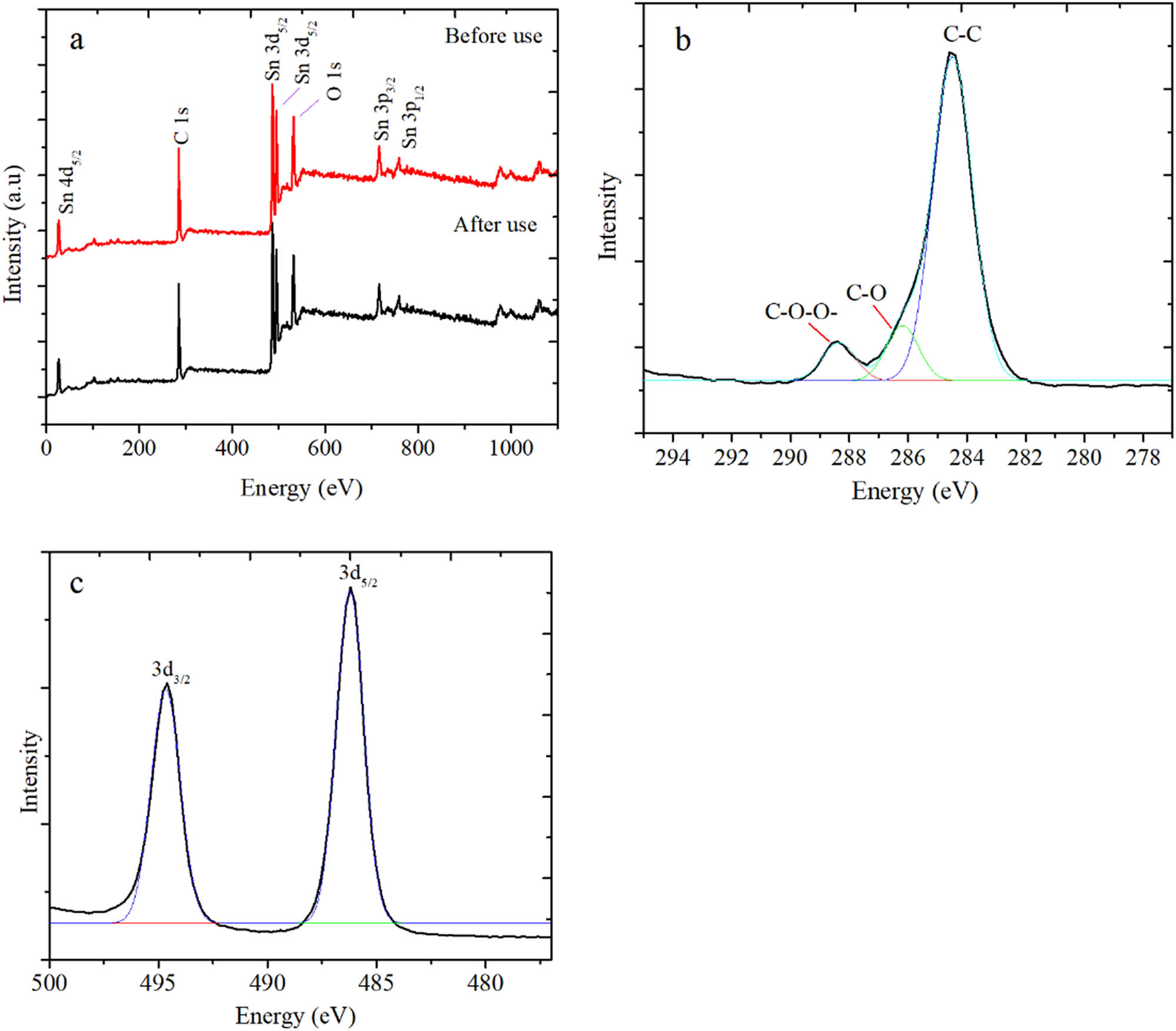
(a) XPS survey spectra of SnO2/rGO before and after use (b) Deconvoluted C 1s spectrum and (c) Sn 3d spectra in SnO2/rGO.
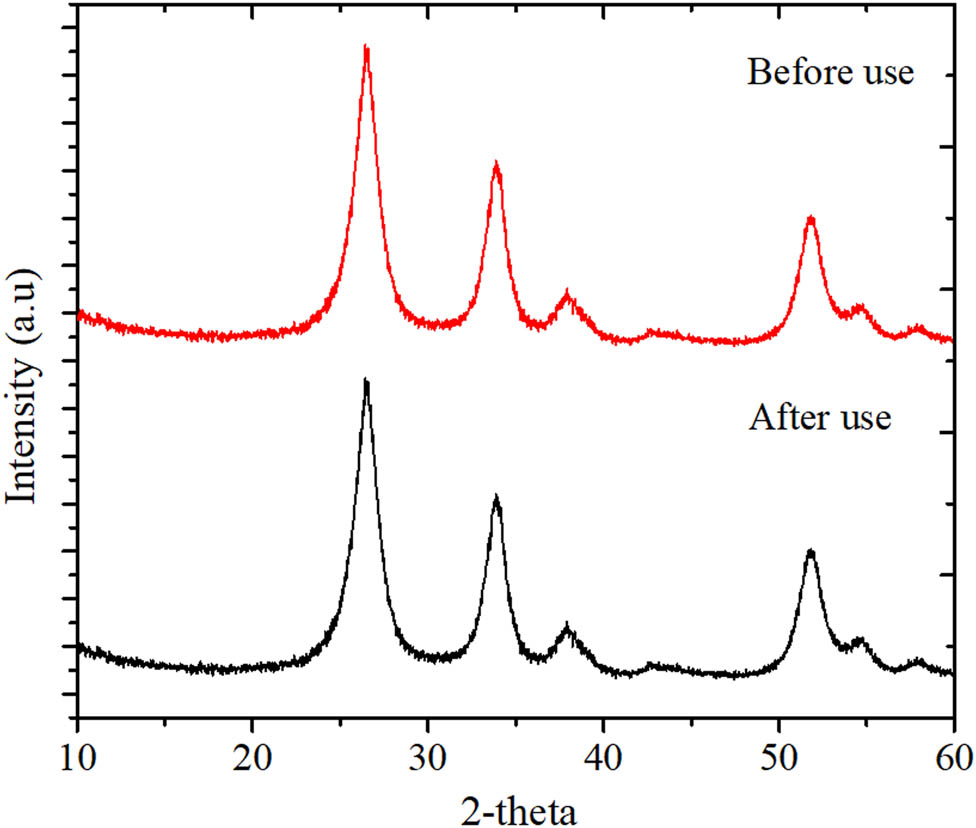
(a) XRD pattern of SnO2/rGO before and after use.
4 Conclusion
The photocatalyst SnO2/rGO composite was successfully prepared via green-synthesized rGO using Pometia pinnata extract. The physicochemical analysis results demonstrated the dispersed SnO2 nanoparticles with particle sizes ranging from 50 to 80 nm in the rutile phase. The composite exhibited high photocatalytic activity with a specialization in the visible light region. The DE of MB under visible light exposure with the H2O2 addition as an oxidant was 98.28% at 90 min of treatment. The present work demonstrates the promising fabrication technique for green and visible light sensitive composite photocatalysts for the removal of organic pollutants.
-
Funding: This work was supported by Chemistry Department, Universitas Islam Indonesia. Authors also thank to DPA-UII for supporting travel grant to the dissemination of this paper.
-
Author contributions: F.S. – investigation; I.S. – formal analysis; G.F. – formal analysis; I.F. – writing and conceptualization.
-
Conflict of interest: The authors declare no conflicts of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Ahmed S, Ahmad M, Swami BL, Ikram S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7:17–28. 10.1016/j.jare.2015.02.007.Suche in Google Scholar PubMed PubMed Central
[2] Cincotto FH, Golinelli DLC, Machado SAS, Moraes FC. Electrochemical sensor based on reduced graphene oxide modified with palladium nanoparticles for determination of desipramine in urine samples. Sens Actuators B Chem. 2017;239:488–93. 10.1016/j.snb.2016.08.063.Suche in Google Scholar
[3] Ismail Z. Green reduction of graphene oxide by plant extracts: a short review. Ceram Int. 2019;45:23857–68. 10.1016/j.ceramint.2019.08.114.Suche in Google Scholar
[4] Lee G, Kim BS. Biological reduction of graphene oxide using plant leaf extracts. Biotechnol Prog. 2014;30:463–9. 10.1002/btpr.1862.Suche in Google Scholar PubMed
[5] Shahane S, Sidhaye D. Facile biosynthesis of reduced graphene oxide nanostructures via reduction by tagetes erecta (marigold flower) plant extract. Int J Mod Phys B. 2018;32:1–5. 10.1142/S0217979218400684.Suche in Google Scholar
[6] Maddinedi SB, Mandal BK. Biofabrication of reduced graphene oxide nanosheets using terminalia bellirica fruit extract. Curr Nanosci. 2015;12:94–102. 10.2174/1573413711666150520224358.Suche in Google Scholar
[7] Ramanathan S, Selvin SP, Obadiah A, Durairaj A, Santhoshkumar P, Lydia S, et al. Synthesis of reduced graphene oxide/ZnO nanocomposites using grape fruit extract and Eichhornia crassipes leaf extract and a comparative study of their photocatalytic property in degrading Rhodamine B dye. J Environ Heal Sci Eng. 2019;17:195–207. 10.1007/s40201-019-00340-7.Suche in Google Scholar PubMed PubMed Central
[8] Liu SH, Wei YS, Lu JS. Visible-light-driven photodegradation of sulfamethoxazole and methylene blue by Cu2O/rGO photocatalysts. Chemosphere. 2016;154:118–23. 10.1016/j.chemosphere.2016.03.107.Suche in Google Scholar PubMed
[9] Seema H, Christian Kemp K, Chandra V, Kim KS. Graphene-SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology. 2012;23:355705. 10.1088/0957-4484/23/35/355705.Suche in Google Scholar PubMed
[10] Kim T, Parale V, Jung HNR, Kim Y, Driss Z, Driss D, et al. Facile synthesis of SnO2 aerogel/reduced graphene oxide nanocomposites via in situ annealing for the photocatalytic degradation of methyl orange. Nanomaterials. 2019;9:358. 10.3390/nano9030358.Suche in Google Scholar PubMed PubMed Central
[11] Wang H, Peng D, Chen T, Chang Y, Dong S. A novel photocatalyst AgBr/ZnO/RGO with high visible light photocatalytic activity. Ceram Int. 2016;42:4406–12. 10.1016/j.ceramint.2015.11.124.Suche in Google Scholar
[12] Wu F, Duan W, Li M, Xu H. Synthesis of MgFe2O4/reduced graphene oxide composite and its visible-light photocatalytic performance for organic pollution. Int J Photoenergy. 2018;2018:7082785. 10.1155/2018/7082785.Suche in Google Scholar
[13] Restuinjaya LA, Simaremare ES, Pratiwi RD. Optimization of tween 80 and span 60 on cream ethanol extract the leaves matoa (pometia pinnata) as an antioxidant. J Adv Pharm Pract. 2019;1:11–21.Suche in Google Scholar
[14] Rohmawati D, Sutoyo S. Steroid isolated from the dichlorometane extract of matoars stem bark (pometia pinnata) and toxicity tests against artemia salina leach. Adv Eng Res. 2018;171:103–5. 10.2991/snk-18.2018.25.Suche in Google Scholar
[15] Han JW, Kim J. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int J Nanomed. 2012;7:5901. 10.2147/IJN.S37397.Suche in Google Scholar PubMed PubMed Central
[16] Lai Q, Zhu S, Luo X, et al. Ultraviolet-visible spectroscopy of graphene oxides Ultraviolet-visible spectroscopy of graphene oxides. 2012;3–8:032146. 10.1063/1.4747817.Suche in Google Scholar
[17] Mahata S, Sahu A, Shukla P, Rai A, Singh M, Rai VK. The novel and efficient reduction of graphene oxide using Ocimum sanctum L. leaf extract as an alternative renewable bio-resource. N J Chem. 2018;42:19945–52. 10.1039/c8nj04086a.Suche in Google Scholar
[18] Wazir AH, Kundi IW. Synthesis of graphene nano sheets by the rapid reduction of electrochemically exfoliated graphene oxide induced by microwaves. J Chem Soc Pak. 2016;38:11–6.Suche in Google Scholar
[19] Hu N, Yang Z, Wang Y, Zhang L, Wang Y, Huang X, et al. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology. 2013;25:025502. 10.1088/0957-4484/25/2/025502.Suche in Google Scholar PubMed
[20] Gong Y, Li D, Fu Q, Pan C. Influence of graphene microstructures on electrochemical performance for supercapacitors In fluence of graphene microstructures on electrochemical performance for supercapacitors. Prog Nat Sci Mater Int. 2015;25:379–85. 10.1016/j.pnsc.2015.10.004.Suche in Google Scholar
[21] Anasdass JR, Kannaiyan P, Raghavachary R, Gopinath SCB, Chen Y. Palladium nanoparticle-decorated reduced graphene oxide sheets synthesized using Ficus carica fruit extract: a catalyst for Suzuki cross-coupling reactions. PLoS One. 2018;13:1–13. 10.1371/journal.pone.0193281.Suche in Google Scholar PubMed PubMed Central
[22] Hou D, Liu Q, Cheng H, Zhang H, Wang S. Green reduction of graphene oxide via Lycium barbarum extract. J Solid State Chem. 2017;246:351–6. 10.1016/j.jssc.2016.12.008.Suche in Google Scholar
[23] Geetha A, Sakthivel R, Mallika J. A single pot green synthesis of ZnO nanoparticles using aqueous gum exudates of Azadirachta indica and its antifungal activity. Int Res J Eng Technol. 2016;3:300–6.Suche in Google Scholar
[24] Kumar B, Smita K, Cumbal L, Debut A, Galeas S, Guerrero VH. Phytosynthesis and photocatalytic activity of magnetite (Fe3O4) nanoparticles using the Andean blackberry leaf. Mater Chem Phys. 2016;179:310–5. 10.1016/j.matchemphys.2016.05.045.Suche in Google Scholar
[25] Fu C, Zhao G, Zhang H, et al. Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries. Int J Electrochem Sci. 2013;8:6269–80.10.1016/S1452-3981(23)14760-2Suche in Google Scholar
[26] Al-Sabahi J, Bora T, Al-Abri M, Dutta J. Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS One. 2017;12:1–16. 10.1371/journal.pone.0189276.Suche in Google Scholar PubMed PubMed Central
[27] Mishra MK, Singh N, Pandey V, Haque FZ. Synthesis of SnO2 nanoaprticles and its application in sensing ammonia gas through photoluminescence. J Adv Phys. 2015;5:1–5. 10.1166/jap.2016.1228.Suche in Google Scholar
[28] Munawaroh H, Sari PL, Wahyuningsih S, Ramelan AH. The photocatalytic degradation of methylene blue using graphene oxide (GO)/ZnO nanodrums. AIP Conf Proc. 2018;2014:020119. 10.1063/1.5054523.Suche in Google Scholar
[29] Siong VLE, Lee KM, Juan JC, Lai CW, Tai XH, Khe CS. Removal of methylene blue dye by solvothermally reduced graphene oxide: a metal-free adsorption and photodegradation method. RSC Adv. 2019;9:37686–95. 10.1039/c9ra05793e.Suche in Google Scholar
[30] Fan F, Wang X, Ma Y, Fu K, Yang Y. Enhanced photocatalytic degradation of dye wastewater using ZnO/reduced graphene oxide hybrids. Fuller Nanotub Carbon Nanostruct. 2015;23:917–21. 10.1080/1536383X.2015.1013187.Suche in Google Scholar
[31] Hoan NTV, Minh NN, Nhi TTK, Van Thang N, Tuan VA, Nguyen VT, et al. TiO2/diazonium/graphene oxide composites: synthesis and visible-light-driven photocatalytic degradation of methylene blue. J Nanomater. 2020;2020:4350125–15. 10.1155/2020/4350125.Suche in Google Scholar
[32] Azim MB, Arafat I, Morshed R, et al. Degradation of methylene blue using graphene oxide-tin oxide nanocomposite as photocatalyst. 1st Int Conf Eng Mater Metall Eng. 2016;2016:202–9.Suche in Google Scholar
[33] Belete Asefa Aragaw AD. Copper/reduced graphene oxide nanocomposite for high performance photocatalytic methylene blue dye degradation. Ethiop J Sci Technol. 2012;2012:115–7. 10.19744/j.cnki.11-1235/f.2012.01.010.Suche in Google Scholar
[34] Mao S, Cui S, Lu G, Yu K, Wen Z, Chen J. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J Mater Chem. 2012;22:11009–13. 10.1039/c2jm30378g.Suche in Google Scholar
[35] Wang C, Shao C, Zhang X, Liu Y. SnO2 nanostructures-TiO2 nanofibers heterostructures: controlled fabrication and high photocatalytic properties. Inorg Chem. 2009;48:7261–8. 10.1021/ic9005983.Suche in Google Scholar PubMed
[36] Uddin MT, Nicolas Y, Olivier C, Toupance T, Servant L, Müller MM, et al. Nanostructured SnO2−ZnO heterojunction photocatalysts showing enhanced photocatalytic activity for the degradation of organic dyes. Inorg Chem. 2012;51:7764–73.10.1021/ic300794jSuche in Google Scholar PubMed
© 2021 Febrian Sujatmiko et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

