Abstract
Petroleum plasticisers are applied as softening additives in rubber vulcanisation processes and as components of rubber mixtures in the production and vulcanisation process. They contain polycyclic aromatic compounds exhibiting carcinogenic and mutagenic effects. Since 2010, the European Union has banned the use of high-aromatic DAE plasticisers. The petroleum industry and tyre manufacturers are developing new types of petroleum plasticisers. The best alternative to the DAE is the TDAE plasticisers, obtained mainly by selective solvent refining. The solvent dewaxing process of classic TDAE plasticisers was studied in order to improve the chemical composition as well as the rheological and low-temperature properties of deparafinate. This article presents the results of an examination of the TDAE plasticiser samples subjected to solvent dewaxing process on a laboratory scale with three types of solvents, MEK–TOL, MEK–MIBK and MEK–MTBE. It was demonstrated that solvent dewaxing of the TDAE plasticiser with positive pour points maintains good process selectivity and allows for a significant reduction of the plasticiser pour point, thus improving the rheological and low-temperature properties. In all dewaxing attempts, the pour point in the deparaffinate decreased significantly to the range −12 to −22°C, compared to the positive pour points of the raw materials. The application tests for two types of the TDAE plasticisers, used to produce oiled rubber and a standard rubber compound, meet the quality requirements for those products.
1 Introduction
Petroleum plasticisers are applied as softening additives in rubber vulcanisation processes, in particular for the synthetic styrene–butadiene rubber (SBR), and as components of rubber mixtures in the production and vulcanisation processes. The global elastomer market is dominated by two types of rubbers, namely the natural rubber and SBR, which account for 70–75% of the global elastomer market segment, especially in applications such as car tyres including treads [1]. The annual demand for petroleum plasticisers in 2010 [2] was 1.3 million tons (Figure 1).
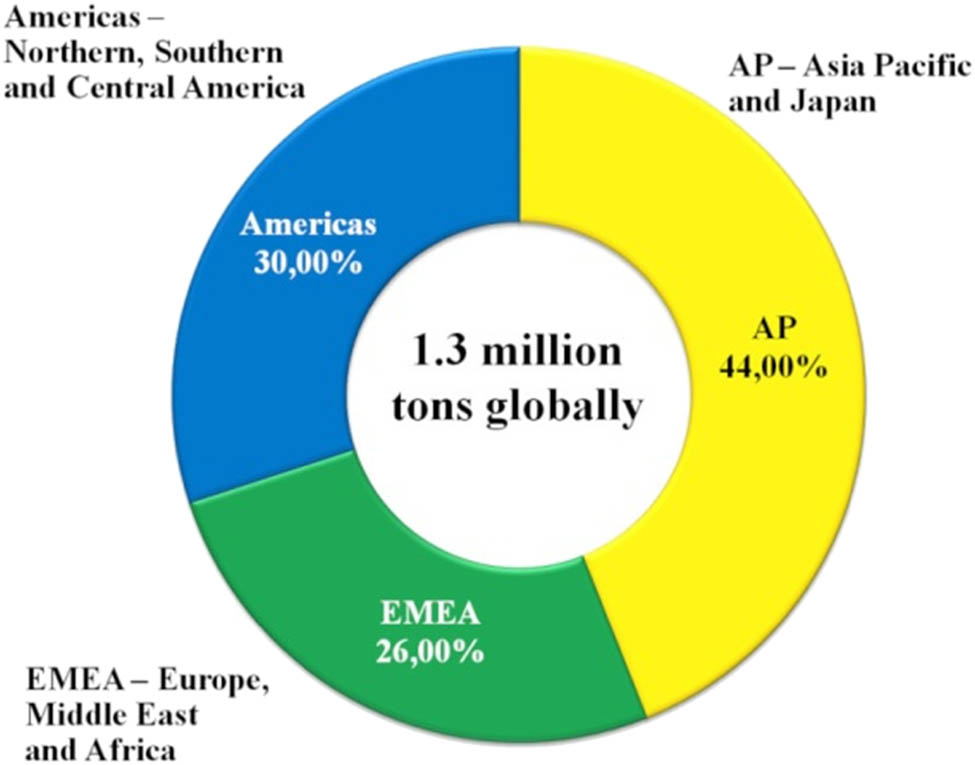
Global, annual demand for petroleum plasticisers as per regions of the world.
Petroleum plasticisers for the rubber industry are required to meet a number of requirements arising from the specific nature of manufacturing processes and operating conditions of rubber products, which are in particular:
to have the chemical composition required for a given application and have appropriate physiochemical properties;
show sufficient compatibility with the rubber used;
exhibit low volatility in the rubber production process conditions and in production and vulcanisation of rubber mixtures;
Petroleum plasticisers are classed as petroleum agents [8] used in the production of rubber and gum products. As a rule, the plasticiser used in SBR rubber compositions is referred to as filler oil, which consists of hydrocarbon molecules containing 25–35 carbon atoms.
Petroleum plasticisers are divided into aromatic, naphthenic, and paraffinic ones, depending on the percentage of carbon in aromatic, naphthenic, and paraffinic structures [9].
One of the petroleum plasticiser classifications is based mainly on the methods of production. It distinguishes the following types of plasticisers [10,11]:
TDAE (treated distillate aromatic extract) – a modified aromatic extract from a petroleum vacuum distillate;
MES (mild extraction solvates) – raffinate of mild solvent extraction of petroleum vacuum distillate;
RAE (residual aromatic extract) – an aromatic extract obtained from DAO (Deasphaltisate) in the production of Brighstock;
TRAE (residual aromatic extract) – a modified aromatic extract obtained from DAO in the production of Brighstock;
NAP (naphthenic plasticisers) – raffinate from solvent extraction of vacuum distillate from naphthenic oil, which is further broken down into
LNAP – naphthenic plasticisers of medium viscosity;
HNAP – naphthenic plasticisers of high viscosity.
From early 2010, a ban on the use of high aromatic plasticisers [7] was introduced, which challenged the oil and tyre industry to replace the DAE high aromatic plasticisers with other process oils.
Patent application EP 3031621 A1 [17] of The Goodyear Tire & Rubber Company; PNEUMATIC TIRE, describes the production of a pneumatic tyre based on oiled rubber compositions and addition of process oil directly when composing the rubber mixture.
Plasticisers of the type MES, TDAE and naphthenic oils are used in the solutions of the said invention, thus the rubber composition contains low polycyclic aromatic compounds (PAC) plasticisers so they do not show carcinogenic activity.
The MES, TDAE or naphthenic type plasticisers with general qualitative characteristics are shown in Table 1.
Physiochemical properties of petroleum plasticisers
| Properties | MES | TDAE | Heavy naphthenic (HNAP) |
|---|---|---|---|
| Content of carbon atoms in structures: CA, % | 11–17 | 25–30 | 11–17 |
| Density at 15°C, g/cm3 | 0.895–0.925 | 0.930–0.960 | 0.920–0.950 |
| Viscosity at 40°C, mm2/s | 150–230 | 370–430 | 350–720 |
| Viscosity at 100°C, mm2/s | 13–17 | 16–22 | 17–33 |
| VGC viscosity and density constant | 0.825–0.865 | 0.860–0.890 | 0.840–0.870 |
| Intercept fractions | 1.495–1.510 | 1.520–1.540 | 1.500–1.520 |
| Glass transition temperature, T g, °C | −60 ± 3 | −47 ± 3 | −45 ± 3 |
| Aniline point, °C | 85–100 | — | — |
| Solidification point, °C | <0 | <30 | <0 |
| PAC content (DMSO extr.), %(m/m) | <2.9 | <2.9 | <2.9 |
| Flash point, (to) °C | 220 | 240 | 240 |
Petroleum plasticisers are important components of rubber products and have a significant impact on their performance characteristics [18]. The function of mineral softeners involves, among others, modification of the physical properties of rubber, particularly by improving tensile strength, hardness, tear resistance and elasticity at low temperatures [18]. The above-mentioned impact of petroleum plasticisers on the performance characteristics of rubber products at low temperatures and an attempt to improve the structural composition of hydrocarbons was an inspiration to study the dewaxing process of the classic TDAE plasticiser.
As a result of the solutions described in numerous patents [19,20,21,22,23,24,25,26,27,28,29], the product obtained is a process oil containing PAC of ≤3% (m/m), which can be used as a softener or plasticiser for rubbers and their mixtures.
The TDAE petroleum plasticisers offered by many producers are distinguished by a plus pour point, which is a feature that can adversely affect the elasticity of rubber products at low temperatures, and hence, in case of a positive effect of the dewaxing process, a decrease in the pour point of those plasticisers can be expected and their performance at low temperatures can be improved.
2 Experimental
2.1 Materials for testing the solvent dewaxing process
Heavy extracts from a selective solvent refining plant were the raw materials for obtaining TDAE plasticisers by the dewaxing process with furfurol (classic process). The physiochemical properties of heavy extracts are presented in Table 2, and the samples of obtained plasticisers TDAE I–III are given in Table 3.
Physiochemical properties of raw materials (heavy extracts)
| Raw material | Test methods | |||
|---|---|---|---|---|
| Properties | Extract I | Extract II | Extract III | |
| Density at 20°C, g/cm3 | 0.9749 | 0.9893 | 0.9916 | PN-EN ISO 12185 |
| Kinematic viscosity at 50°C, mm2/s | 275.43 | 739.31 | 783.53 | PN-EN ISO 3104 |
| Flash point, (to), °C | 275 | 279 | 281 | PN-EN ISO 2592 |
| Refractive index
|
1.5547 | 1.5614 | 1.5648 | PN-C-04952:81 |
| Sulphur content, % (m/m) | 2.76 | 3.22 | 3.35 | PN-EN ISO 8754 |
| Pour point, °C | +29 | +26 | +25 | PN-ISO 3016 |
| PAC content (DMSO extr.), % (m/m) | 13.6 | 12.8 | 13.0 | IP 346 |
| Content of carbon atoms in structures | ASTM D 2140 | |||
| Aromatic, C A, % | 34.51 | 35.33 | 36.85 | |
| Naphthenic, C N, % | 11.83 | 15.04 | 12.29 | |
| Paraffinic C P, % | 53.66 | 49.63 | 50.86 | |
| PAH content, mg/kg | Met. GC/MS | |||
| Benzo(a)pyrene | 9.4 | 7.3 | 6.8 | |
| Benzo(e)pyrene | 26.8 | 25.8 | 24.9 | |
| Benzo(a)anthracene | 5.3 | 2.4 | 2.3 | |
| Chrysene | 28.5 | 27.4 | 18.7 | |
| Benzo(b,j,k)fluoranthene | 24.2 | 14.7 | 14.3 | |
| Dibenzo(a,h)anthracene | 3.5 | 2.3 | 1.8 | |
| Total PAHs | 97.7 | 79.9 | 68.8 | |
Physiochemical properties of TDAE plasticisers
| Plasticiser | Test methods | |||
|---|---|---|---|---|
| Properties | TDAE I | TDAE II | TDAE III | |
| Density at 20°C, g/cm3 | 0.9398 | 0.9478 | 0.9541 | PN-EN ISO 12185 |
| Kinematic viscosity at 100°C, mm2/s | 15.33 | 22.29 | 24.47 | PN-EN ISO 3104 |
| Flash point, (to), °C | 276 | 271 | PN-EN ISO 2592 | |
| Refractive index
|
1.5223 | 1.5302 | 1.5349 | PN-C-04952:81 |
| Sulphur content, % (m/m) | 2.5356 | 2.68 | 3.04 | PN-EN ISO 8754 |
| Pour point, °C | +33 | +30 | +28 | PN-ISO 3016 |
| PAC content (DMSO extr.), % (m/m) | 2.7 | 1.9 | 2.4 | IP 346 |
| Content of carbon atoms in structures | ASTM D 2140 | |||
| Aromatic, C A, % | 20.3 | 23.86 | 25.84 | |
| Naphthenic, C N, % | 24.2 | 19.14 | 16.49 | |
| Paraffinic C P, % | 55.5 | 56.99 | 57.67 | |
| PAH content, mg/kg | Met. GC/MS | |||
| Benzo(a)pyrene | 0.8 | 0.7 | 0.7 | |
| Benzo(e)pyrene | 3.1 | 0.9 | 0.9 | |
| Benzo(a)anthracene | 0.3 | 0.3 | 0.4 | |
| Chrysene | 1.3 | 1.1 | 1.2 | |
| Benzo(b,j,k)fluoranthene | 1.4 | 1.2 | 1.4 | |
| Dibenzo(a,h)anthracene | 0.4 | 0.1 | 0.1 | |
| Total PAHs | 7.3 | 4.2 | 4.7 | |
2.2 Laboratory solvent dewaxing process method
Crystallisation of solid hydrocarbons under laboratory conditions is effected with the aid of the method of gradual cooling of the oil–solvent mixture located in the crystalliser. The crystalliser is placed in a cooling bath equipped with a cooling cycle controller to set up the final crystallisation temperature and appropriate cooling rates in the subsequent stages of the process. A nutch filter is connected to a cryostat, provided with a jacket in which the coolant circulates.
The crystallisation process is effected by the dilution method by adding successive portions of the cooled solvent to the cooled mixture of the raw material and solvent, at appropriate moments of the cooling cycle. The first portion of solvent is introduced into the raw material at a temperature at which the raw material forms a homogeneous liquid phase containing no crystals. However, at the point of injection into the mixture, the temperature of the solvent has to be such as to prevent disturbance of the hydrocarbon crystallisation process in the mixture.
In the crystallisation process, continuous mixing of the crystalliser content is effected by means of an agitator with an anchoring ending, with the mixing speed adapted to the increasing viscosity of the mixture.
After reaching the final crystallisation temperature, the separated solid hydrocarbons, containing the occluded solvent, are filtered out from the oil solution in a vacuum nutch. The oil solution (filtrate) accumulates in the receiver tank. The filtered solid hydrocarbons are washed with a portion of cold solvent. The solid hydrocarbons collected from the nutch and the filtrate are subjected to the solvent regeneration process. The solvent regeneration operation is performed by means of nitrogen stripping distillation.
2.3 Solvent dewaxing processes with various TDAE plasticiser solvents
Samples of TDAE plasticisers were subjected to a solvent dewaxing process with three different types of solvents, MEK–TOL (methyl ethyl ketone/toluene mixture), MEK–MIBK (methyl ethyl ketone/methyl isobutyl ketone mixture) and MEK–MTBE (methyl ethyl ketone/methyl tert-butyl ether). To perform solvent dewaxing processes in a laboratory system, similar technology parameters were adopted as those of industrial plants and described in patents [30,31,32,33].
The technology parameters applied are presented in the tables together with mass balances and properties of the deparaffinates obtained and slacks from dewaxing processes. The initial charge of the vacuum distillate in the dewaxing process was 300 g for all the tests carried out. The crystallisation modifier (VISCOPLEX 9-350) was dosed at 1,000 mg/kg according to the manufacturer’s instructions.
3 Results and discussion
3.1 Dewaxing the TDAE plasticiser with the MEK–TOL solvent
Table 4 presents technology parameters, mass balance and properties of the obtained deparaffinates and slack waxes, for four MET-TOL dewaxing processes of TDAE I and TDAE III raw material samples.
Technology parameters, mass balance and properties of the obtained deparaffinates and slack waxes, in MET-TOL dewaxing processes of TDAE I and TDAE III raw material samples
| Dewaxing no. | PR 01 | PR 02 | PR 03 | PR 04 | PR19 |
|---|---|---|---|---|---|
| Raw material, sample no. | TDAE I | TDAE I | TDAE I | TDAE I | TDAE III |
| Technology parameters of dewaxing processes | |||||
| MEK–TOL solvent, weight ratio | 40:60 | 40:60 | 60:40 | 70:30 | 50:50 |
| Crystallisation/filtration temperature, °C | −28 | −20 | −28 | −28 | −28 |
| Total solvent to fraction ratio | 11:1 | 11:1 | 5.0:1 | 5.0:1 | 5.0:1 |
| Mass balance of dewaxing processes, averaged results | |||||
| Filtration time, s | 35 | 18 | 14.5 | 27 | 19 |
| Deparafinate yield, % (m/m) | 88.0 | 91.0 | 89.0 | 90.0 | 89.0 |
| Slack wax yield, % (m/m) | 9.0 | 7.0 | 6.0 | 7.0 | 8.0 |
| Losses, % (m/m) | 3.0 | 2.0 | 5.0 | 3.0 | 4.0 |
| Properties of deparaffinate | |||||
| Kinematic viscosity at 50°C, mm2/s | 152.9 | 138.4 | 156.3 | 153.4 | 289.3 |
| Kinematic viscosity at 100°C, mm2/s | 16.49 | 15.68 | 16.58 | 16.78 | 24.17 |
| Refractive index
|
1.5253 | 1.5245 | 1.5256 | 1.5258 | 1.5330 |
| Density at 20°C, g/cm3 | 0.9424 | 0.9408 | 0.9411 | 0.9415 | 0.9563 |
| Sulphur content, % (m/m) | 2.97 | 3.10 | 3.10 | 3.09 | 3.11 |
| PAC content (DMSO extr.), % (m/m) | — | 2.8 | 2.8 | 2.8 | 2.1 |
| Content of carbon atoms in structures | |||||
| Aromatic, C A, % | 21.28 | 20.99 | 21.51 | 21.73 | 24.09 |
| Naphthenic, C N, % | 20.61 | 19.87 | 19.98 | 19.42 | 18.41 |
| Paraffinic C P, % | 58.11 | 59.14 | 58.51 | 58.85 | 57.50 |
| Pour point, °C | −15 | −15 | −22 | −14 | −15 |
| Slack wax properties | |||||
| Refractive index
|
1.4705 | 1.4702 | 1.4639 | 1.4687 | 1.4722 |
| Solidification point,°C | 55.0 | 60.0 | 54.8 | 55.9 | 56.2 |
| Oil content, % (m/m) | 50.65 | 0.73 | 15.60 | 24.27 | 28.2 |
The basic process parameters for four dewaxing operations of the TDAE I raw material were as follows: the mass ratio of MEK–TOL was in the range from 40:60 to 70:30, the crystallisation temperature was −20 or −28°C, and the total solvent to fraction ratio was from 5.0:1 to 11:1.
In the dewaxing attempts of the TDAE I raw material, the deparafinate yield was large and ranged from 88.0 to 91.0% (m/m) and the slack wax yield was in the range of 6.0–9.0% (m/m).
Compared to the TDAE I raw material, as a result of the dewaxing process in the TDAE deparaffinate, an increase in the carbon content in aromatic structures in deparafinate was observed for all tests, which ranged from 0.69 to 1.43%; the content of carbon atoms in naphthenic structures decreased to 3.59–4.78%; and the content of carbon atoms in paraffin structures increased from 2.61 to 3.64%.
It has to be stressed that in all dewaxing attempts, the pour point in the deparaffinate decreased significantly in the range of −14 to −22°C, compared to the raw material whose pour point was +33°C.
Compared to deparaffinates and raw material in slack waxes, the refractive index markedly decreased and the solidification point increased significantly, which indicates the preservation of the selectivity of the TDAE raffinate dewaxing process.
A graphical illustration of the effect of dewaxing process on the change of the structural composition and pour point of deparaffinates is presented in Figures 2 and 3.
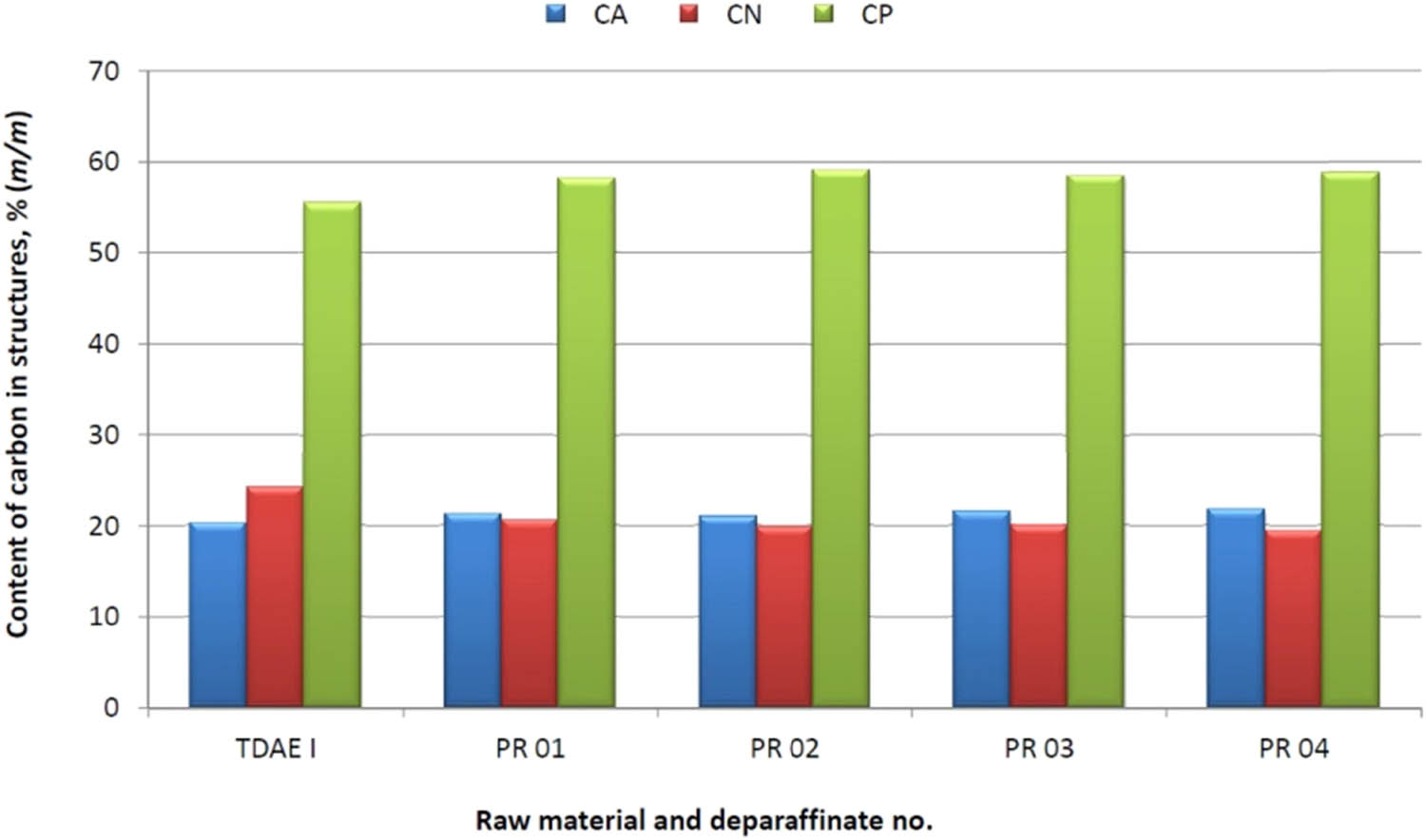
Effects of the dewaxing process with the MEK–TOL solvent on the change of the structural composition of deparaffinates – TDAE I raw material.
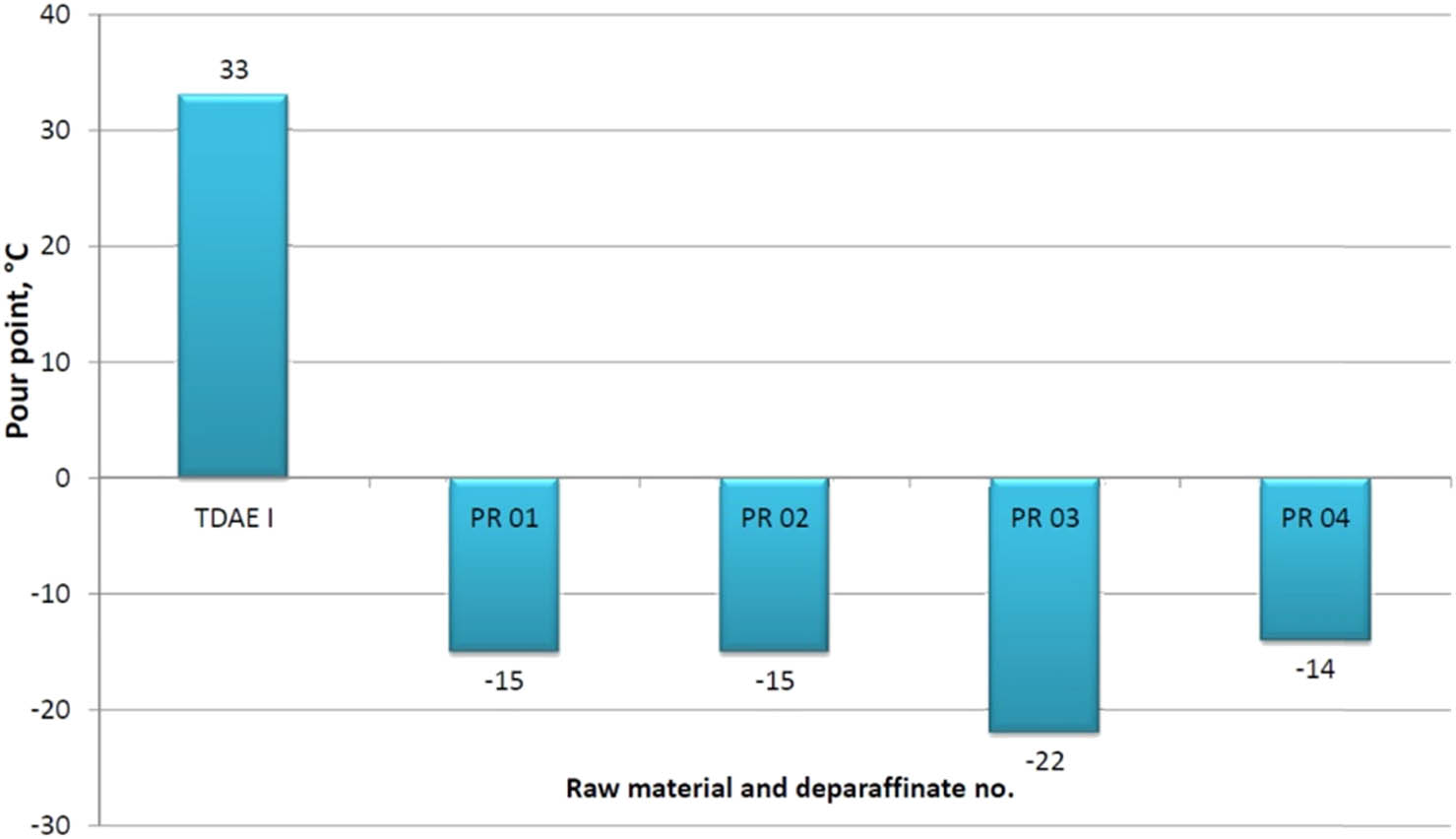
Effect of the dewaxing process with the MEK–TOL solvent on the change of the deparaffinate pour point – TDAE I raw material.
3.2 Dewaxing the TDAE plasticiser with the MIBK–MEK solvent
Table 5 presents technology parameters, mass balance and properties of the obtained deparaffinates and slack waxes, for five MIBK–MEK dewaxing processes of a TDAE II raw material sample and two dewaxing processes of the TDAE III raw material sample.
Technology parameters, mass balance and properties of the obtained deparaffinates and slack waxes, in MIBK–MEK dewaxing processes of TDAE II and TDAE III raw material samples
| Dewaxing no. | PR 05 | PR 06 | PR 07 | PR 08 | PR 09 | PR 10 | PR 11 |
|---|---|---|---|---|---|---|---|
| Raw material, sample no. | TDAE II | TDAE II | TDAE II | TDAE II | TDAE II | TDAE III | TDAE III |
| Viscosity modifier | — | Viscoplex 9–350 | — | — | — | — | Viscoplex 9–350 |
| Technology parameters of dewaxing processes | |||||||
| MIBK–MEK solvent, mass ratio | 100:0 | 60: 40 | 80: 20 | 60: 40 | 50: 50 | 60: 40 | 40: 60 |
| Crystallisation/filtration temperature, °C | −28 | −28 | −28 | −20 | −20 | −28 | −20 |
| Total solvent to fraction ratio | 5.0: 1 | 5.0: 1 | 7: 1 | 5.7: 1 | 3.5: 1 | 5.5: 1 | 3.5: 1 |
| Mass balance of dewaxing processes, averaged results | |||||||
| Filtration time, s | 112 | 208 | 62 | 87 | 94 | 54 | 72 |
| Deparafinate yield, % (m/m) | 87.0 | 88.0 | 86.0 | 89.0 | 91.0 | 91.0 | 92.0 |
| Slack wax yield, % (m/m) | 10.0 | 7.0 | 11.0 | 9.0 | 6.0 | 7.0 | 5.0 |
| Losses, % (m/m) | 3.0 | 5.0 | 3.0 | 2.0 | 3.0 | 2.0 | 3.0 |
| Properties of deparaffinate | |||||||
| Kinematic viscosity at 50°C, mm2/s | 313.8 | 258.8 | 294.7 | 318.9 | 297.4 | 323.4 | 318.2 |
| Kinematic viscosity at 100°C, mm2/s | 25.24 | 23.09 | 24.63 | 25.67 | 24.93 | 27.83 | 27.48 |
| Refractive index
|
1.5134 | 1.5338 | 1.5338 | 1.5334 | 1.5336 | 1.5387 | 1.5184 |
| Density at 20°C, g/cm3 | 0.9525 | 0.9529 | 0.9564 | 0.9564 | 0.9552 | 0.9527 | 0.9526 |
| Sulphur content, % (m/m) | 2.78 | 2.77 | 3.16 | 3.19 | 3.18 | 3.3 | 3.20 |
| PAC content (DMSO extr.), % (m/m) | 2.1 | 2.1 | 2.2 | 2.2 | 2.2 | 2.7 | 2.7 |
| Content of carbon atoms in structures | |||||||
| Aromatic, C A, % | 25.27 | 24.98 | 24.93 | 24.98 | 25.18 | 26.81 | 26.43 |
| Naphthenic, C N, % | 18.89 | 18.45 | 18.79 | 19.34 | 18.93 | 17.43 | 16.97 |
| Paraffinic C P, % | 55.84 | 56.57 | 56.28 | 55.68 | 55.89 | 55.76 | 56.60 |
| Pour point, °C | −15 | −15 | −12 | −13 | −13 | −16 | −14 |
| Slack wax properties | |||||||
| Refractive index
|
1.4720 | 1.4811 | 1.4732 | 1.4743 | 1.4759 | 1.4738 | 1.4749 |
| Solidification point, °C | 53.0 | 54.0 | 52.6 | 54.7 | 54.1 | 52.6 | 52.1 |
| Oil content, % (m/m) | 38.6 | 31.8 | 28.5 | 41.6 | 36.2 | 48.3 | 51.3 |
The basic process parameters for dewaxing of the TDAE II raw material were as follows: the mass ratio of MIBK–MEK was in the range from 100:0 to 60:40, the crystallisation temperature was −20 or −28°C and the total solvent to fraction ratio was from 3.5:1 to 7:1. In test 46, a crystallisation modifier was used for the process.
In the dewaxing attempts, the deparafinate yield was large and ranged from 86.0 to 89.0% (m/m) and the slack wax yield was in the range of 7.0–11.0% (m/m).
Compared to the TDAE II raw material, as a result of the dewaxing process in the TDAE deparaffinate, an increase in the carbon content in aromatic structures in deparafinate was observed for all tests, which ranged from 1.07 to 1.41%; the content of carbon atoms in naphthenic structures increased max. to 0.20%, or decreased to the max. 0.69%, and the content of carbon atoms in paraffin structures decreased from 0.47 to 1.39%.
In all dewaxing attempts, the pour point in the deparaffinate decreased significantly to the range −12 to −15°C, compared to the raw material whose pour point was +30°C.
Compared to deparaffinates and raw material in slack waxes, the refractive index markedly decreased and the solidification point increased significantly, which indicates the preservation of the selectivity of the TDAE raffinate dewaxing process.
In two dewaxing attempts of the TDAE III raw material, the deparafinate yield ranged from 91.0 to 92.0% (m/m), and the slack wax yield was in the range of 5.0–7.0% (m/m).
Compared to TDAE III raw material, as a result of the dewaxing process, in the TDAE deparaffin, an increase in the content of carbon atoms in aromatic structures was observed in the range of 0.59–0.97%. Significantly reduced pour point occurred in the deparaffinates, −14 to −16°C, compared to the raw material – TDAE plasticiser. Generally, the dewaxing process of the TDAE II and TDAE III raw materials similarly affects the qualitative properties of the deparaffinates and slack waxes obtained from both raw materials.
A graphic illustration of the effect of the dewaxing process on the change of the structural composition and pour point of deparaffinates, for the TDAE II charge, is presented in Figures 4 and 5.
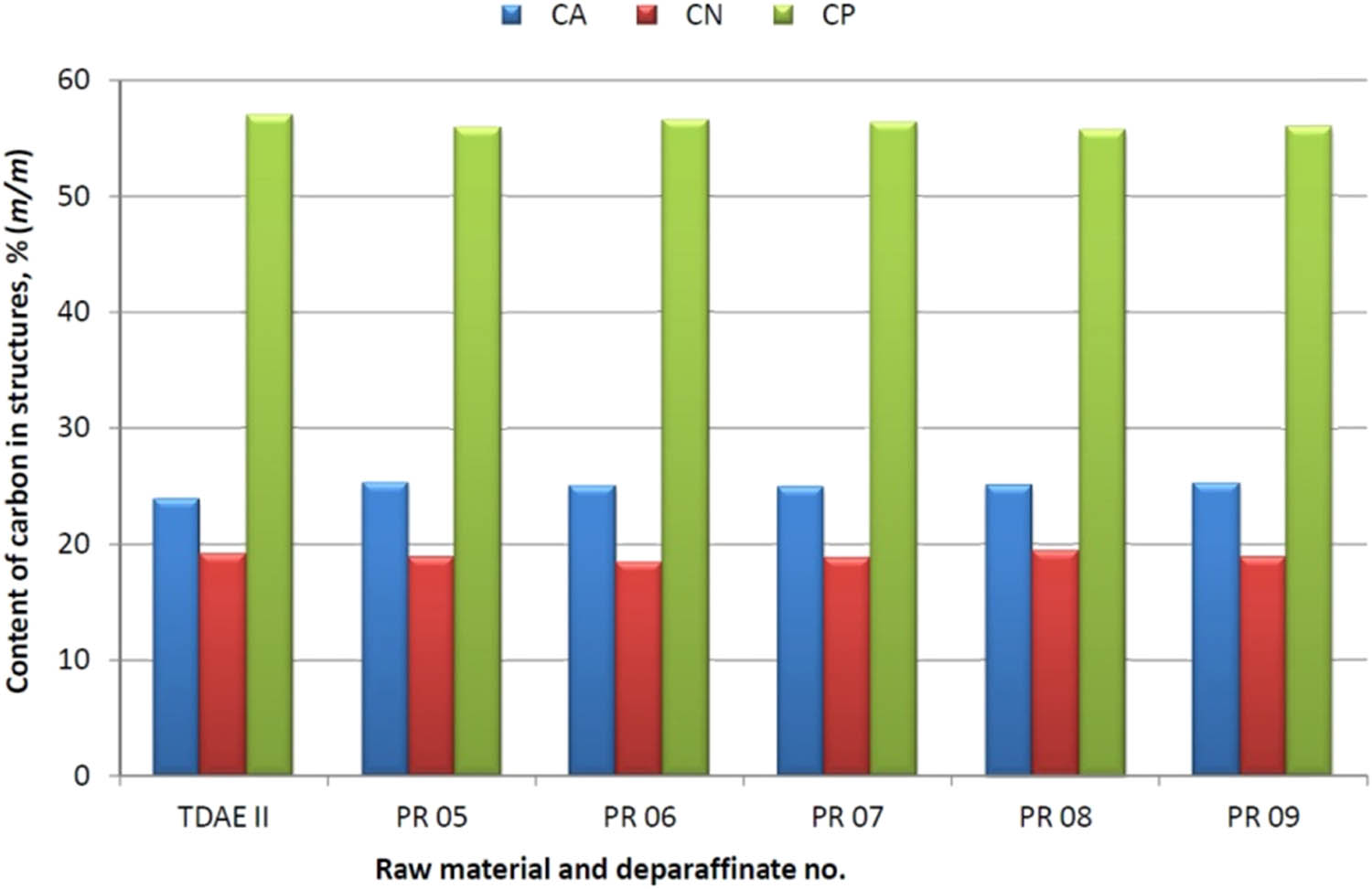
Effect of the dewaxing process with the MBIK–MEK solvent on the change of the structural composition of deparaffinates – TDAE II raw material.
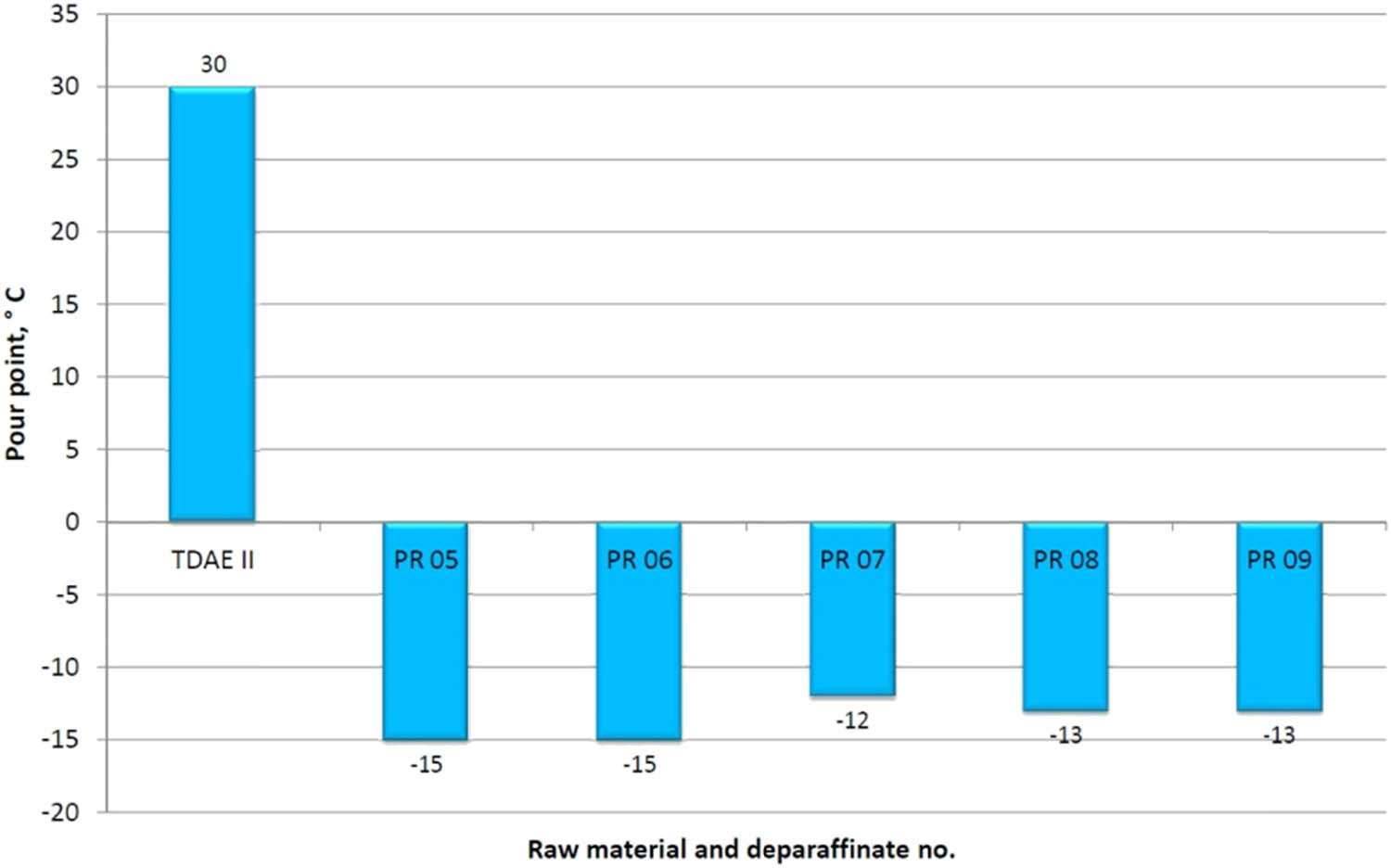
Effect of the dewaxing process with the MBIK–MEK solvent on the change of the deparaffinate pour point – TDAE II raw material.
3.3 Dewaxing the TDAE plasticiser with the MEK–MTBE solvent
Table 6 presents technology parameters, mass balance and properties of the obtained deparaffinates and slack waxes, for five MEK–MTBE dewaxing processes of a TDAE II raw material sample and two dewaxing processes of the sample of raw material TDAE III.
Technology parameters, mass balance and properties of the obtained deparaffinates and slack waxes, in MEK–MTBE dewaxing processes of a TDAE II and TDAE III raw material samples
| Dewaxing no. | PR 12 | PR 13 | PR 14 | PR 15 | PR 16 | PR 17 | PR 18 |
|---|---|---|---|---|---|---|---|
| Raw material, sample no. | TDAE II | TDAE II | TDAE II | TDAE II | TDAE II | TDAE III | TDAE III |
| Viscosity modifier | — | Viscoplex 9–350 | — | — | — | — | — |
| Technology parameters of dewaxing processes | |||||||
| MEK–MTBE solvent, weight ratio | 60:40 | 40: 60 | 70: 30 | 60: 40 | 70: 30 | 60: 40 | 40: 60 |
| Crystallisation/filtration temperature, °C | −28 | −28 | −20 | −20 | −30 | −28 | −20 |
| Total solvent to fraction ratio | 5.0: 1 | 5.0: 1 | 7.0: 1 | 5.7: 1 | 7.0: 1 | 5.5: 1 | 4.0: 1 |
| Mass balance of dewaxing processes, averaged results | |||||||
| Filtration time, s | 72 | 41 | 67 | 94 | 89 | 112 | 125 |
| Deparafinate yield, % (m/m) | 91.0 | 92.0 | 92.0 | 93.0 | 88.0 | 89.0 | 87.0 |
| Slack wax yield, % (m/m) | 7.0 | 6.0 | 5.0 | 5.0 | 9.0 | 8.0 | 9.0 |
| Losses, % (m/m) | 2.0 | 2.0 | 3.0 | 2.0 | 9.0 | 3.0 | 4.0 |
| Properties of deparaffinate | |||||||
| Kinematic viscosity at 50°C, mm2/s | 295.8 | 289.7 | 298.4 | 293.5 | 301.5 | 329.7 | 332.4 |
| Kinematic viscosity at 100°C, mm2/s | 24.74 | 24.74 | 24.68 | 24.67 | 25.12 | 28.04 | 28.13 |
| Refractive index
|
0.9524 | 1.5332 | 1.5333 | 1.5330 | 1.5336 | 0.9527 | 1.5192 |
| Density at 20°C, g/cm3 | 0.9524 | 0.9528 | 0.9562 | 0.9524 | 0.9565 | 0.9527 | 0.9529 |
| Sulphur content, % (m/m) | 2.98 | 2.94 | 3.02 | 2.94 | 3.21 | 3.30 | 3.31 |
| PAC content (DMSO extr.), % (m/m) | 2.1 | 2.2 | 2.2 | 2.1 | 2.3 | 2.8 | 2.8 |
| Content of carbon atoms in structures | |||||||
| Aromatic, C A, % | 24.67 | 24.58 | 24.86 | 24.87 | 25.23 | 26.28 | 26.34 |
| Naphthenic, C N, % | 18.49 | 18.34 | 18.45 | 17.94 | 18.74 | 18.43 | 18.25 |
| Paraffinic C P, % | 56.84 | 57.08 | 56.69 | 57.19 | 56.03 | 55.29 | 55.41 |
| Pour point, °C | −18 | −15 | −13 | −16 | −17 | −14 | −12 |
| Slack wax properties | |||||||
| Refractive index
|
1.4747 | 1.4745 | 1.4712 | 1.4763 | 1.4812 | 1.4835 | 1.4852 |
| Solidification point, °C | 54.0 | 53.6 | 55.1 | 55.0 | 52.7 | 52.1 | 51.3 |
| Oil content, % (m/m) | 34.7 | 39.4 | 36.5 | 31.8 | 42.3 | 61.3 | 60.5 |
The basic process parameters were as follows: the mass ratio of MEK–MTBE was in the range from 70:30 to 40:60, the crystallisation temperature was −20 or −30°C and the total solvent to fraction ratio was from 5.0:1 to 7.0:1. In test 54, a crystallisation modifier was used for the process.
In the dewaxing attempts, the deparafinate yield was large and ranged from 88.0 to 93.0% (m/m) and the slack wax yield was in the range of 5.0–9.0% (m/m).
Compared to the TDAE II raw material, as a result of the dewaxing process in the TDAE deparaffinate, an increase in the carbon content in aromatic structures in deparafinate was observed for all tests, which ranged from 0.72 to 1.37%; the content of carbon atoms in naphthenic structures decreased to min. 0.40% to max. 1.2%; and the content of carbon atoms in paraffin structures increased to 0.09% or decreased to the max. 0.96%.
In all dewaxing attempts, the pour point in the deparaffinate decreased significantly to the range −13 to −18°C, compared to the raw material whose pour point was +30°C.
Compared to deparaffinates and raw material in slack waxes, the refractive index markedly decreased and the solidification point increased significantly, which indicates the preservation of the selectivity of the TDAE raffinate dewaxing process.
In two dewaxing attempts of the TDAE III raw material, the deparafinate yield ranged from 87.0 to 89.0% (m/m), and the slack wax yield was in the range of 8.0–9.0% (m/m).
Compared to TDAE III raw material, as a result of the dewaxing process, in the TDAE deparaffin, an increase in the content of carbon atoms in aromatic structures was observed in the range 0.44–0.50%. Significantly reduced pour point occurred in the deparaffinates, −12 to −14 °C, compared to the raw material – TDAE plasticiser. Similarly, as for the MEK–MIBK solvent dewaxing process, the MEK–MTBE dewaxing of TDAE II and TDAE III raw materials affects, with the same trend, the qualitative properties of the obtained deparaffinates and slack waxes from both raw materials.
A graphical illustration of the effect of the dewaxing process on the change of the structural composition and pour point of deparaffinates is presented in Figures 6 and 7.
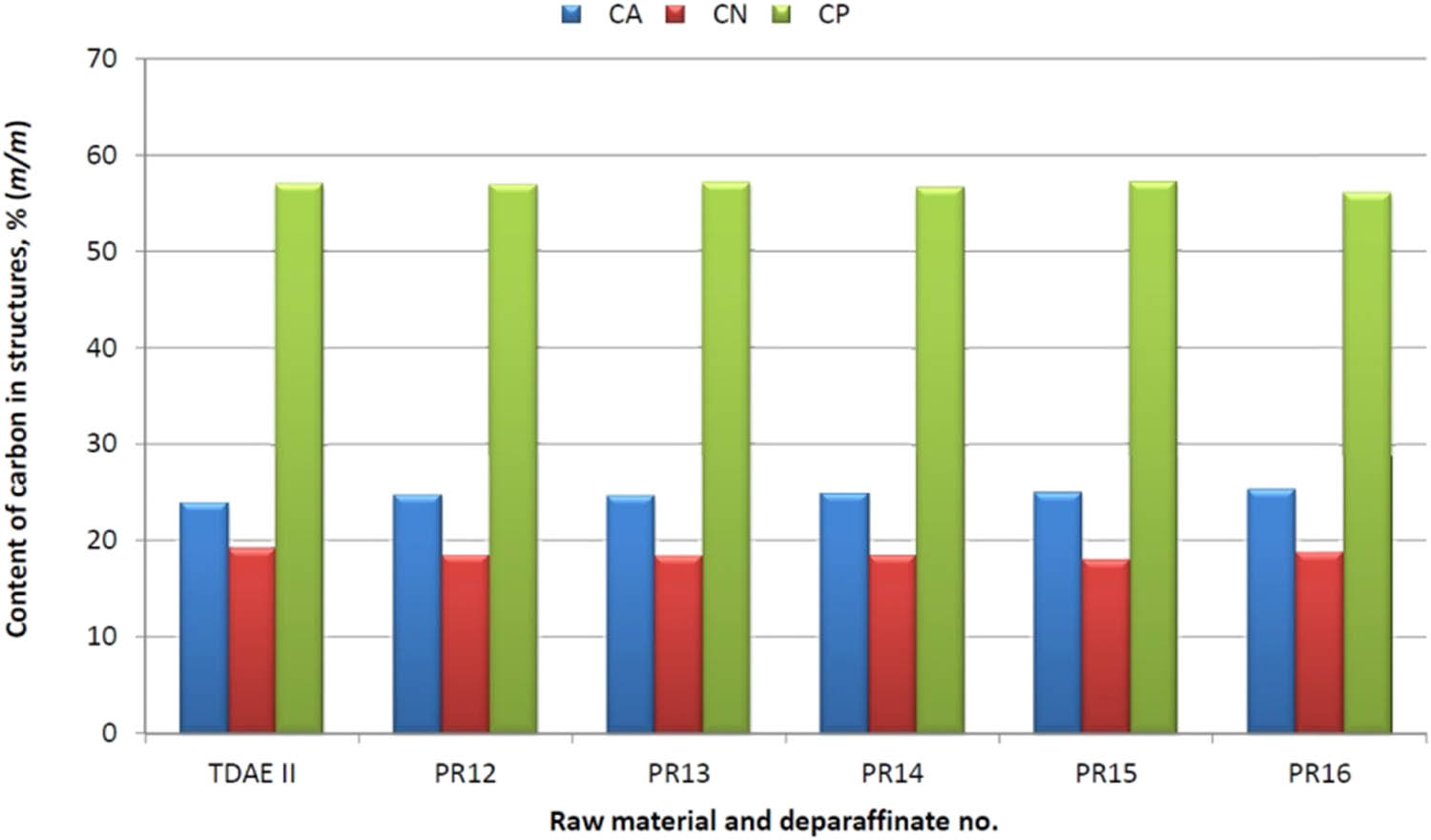
Effect of the dewaxing process with the MBIK–MEK solvent on the change of the structural composition of deparaffinates – TDAE II raw material.
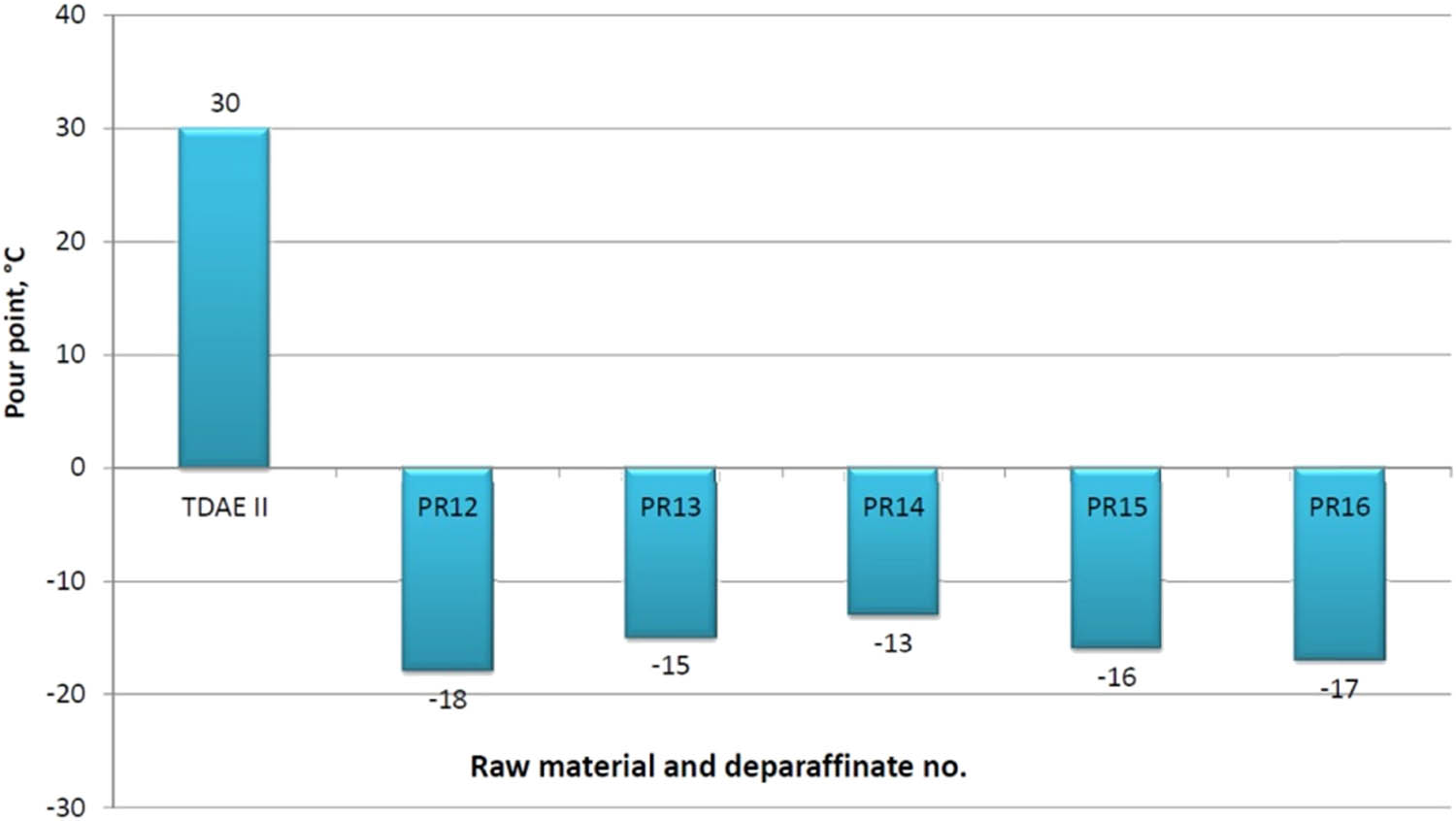
Effect of the dewaxing process with the MEK–MTBE solvent on the change of the deparaffinate pour point – TDAE II raw material.
3.4 Application tests on a laboratory scale of the TDAE plasticiser for oiled rubber
In the framework of the study, application tests for modified TDAE plasticiser applied were performed to produce oiled rubber KER 1723 and standard rubber compounds.
Raw material – oiled rubbers for the application test were the modified TDAE plasticiser obtained in the process of selective refining with furfurol and the subsequent dewaxing process with the MEK–TOL solvent, which was identified by code TDAE III/PR 19.
To compare the evaluation of the physiochemical properties of oiled rubber containing modified TDAE plasticiser, with requirements for KER 1723 oiled rubber, two coagulations were carried out with the oil in question marked with symbols: TDAE III/PR19/1 and TDAE III/PR19/2. The obtained oiled rubber sample KER 1723 was subjected to laboratory tests for the evaluation of physiochemical and physicomechanical properties. The results of the tests are listed in Tables 7 and 8.
Amount of substance and latex parameters during coagulation
| Rubber sample | TDAE III/PR19/1 | TDAE III/PR19/2 |
|---|---|---|
| Dry mass of the KER 2712 base latex (%) | 25 | 25 |
| ML of the KER 2712 base latex (MU) | 130 | 130 |
| Dusantox 6PPD stabiliser weight (g) | 12 | 12 |
| TDAE oil weight (g) | 344 | 344 |
Results of physiochemical analysis of the oiled rubber KER 1723 based on TDAEIII/PR19
| Rubber sample | Requirements for the KER 1723 oiled rubber | TDAE III/PR19/1 | TDAE III/PR19/2 |
|---|---|---|---|
| Rolled ML viscosity (MU) | 45–55 | 48.9 | 50.1 |
| Rolled ML | — | −13.5 | −12.8 |
| Acids (%) | 4.0–6.0 | 5.09 | 5.12 |
| Soaps (%) | max. 0.4 | 0.13 | 0.17 |
| Volatile matter content (%) | max. 0.8 | 0.15 | 0.19 |
| Ash (%) | max. 0.4 | 0.36 | 0.24 |
| TDAE oil content (%) | 25–29 | 28.0 | 27.9 |
| Bonded styrene (%) | 22–25 | 23.3 | 23.4 |
Laboratory-scale application tests proved that physiochemical properties of the oiled rubber KER 1723 contained the modified plasticiser TDAE III/PR19 to meet the quality requirements of SYNTOS SA in the scope of physiochemical parameters required for oiled rubber.
Further application tests involve the preparation of a standard rubber compound from the KER 1723/TDAE III/PR19 oiled rubber according to ASTM D 3185, Recipe 1 A. The composition of the rubber mixture is presented in Table 9.
Composition of rubber compound based on the oiled rubber KER 1723 contained in TDAE III/PR19
| No. | Component | Unit, phr | Mass, g |
|---|---|---|---|
| 1. | KER 1723 oiled rubber | 100.00 | 600.0 |
| 2. | Zinc white | 3.00 | 18.0 |
| 3. | Stearin | 1.00 | 6.0 |
| 4. | Sulphur | 1.75 | 10.5 |
| 5. | Soot IRB | 50.00 | 300.0 |
| 6. | TBBS accelerator – (N-tert-butyl-2-benzothiazolylsulfonamide) | 1.00 | 6.0 |
| Total | 156.70 | 940.5 | |
Rubber compounds based on the KER 1723 oiled rubber were subjected to laboratory tests to evaluate the physiochemical properties and performance characteristics, which are presented in Table 10.
Physicomechanical properties of the rubber compound based on the KER 1723/TDAE III/PR19/1 and the KER 1723/TDAE III/PR19/2
| Rubber compound sample | Rubber compound requirements | KER 1723/TDAE III/PR19/1 | KER 1723/TDAE III/PR19/2 |
|---|---|---|---|
| Mixture ML viscosity (MU) | — | 53.7 | 52.8 |
| Maximum vulcanisation curve (dNm) | 13.5–16.5 | 15.19 | 14.94 |
| Minimum vulcanisation curve (dNm) | 1.8–2.7 | 1.94 | 1.92 |
| TS1 (min) | 2.0–5.0 | 3.72 | 3.57 |
| T10 (min) | — | 4.14 | 3.96 |
| T25 (min) | — | 5.33 | 5.12 |
| T50 (min) | 5.0–9.0 | 6.77 | 6.63 |
| T90 (min) | 12.0–17.0 | 12.94 | 13.36 |
| Tensile strength (MPa) | Minimum 20 | 20.7 | 21.6 |
| Elongation at rapture (%) | Minimum 320 | 409 | 427 |
| Stress at 300% elongation (MPa) | 13–17 | 14.5 | 14.4 |
| Hardness (°ShA) | 60–66 | 63.5 | 63.7 |
| Flexibility, Schob method (%) | Minimum 28 | 40.3 | 41.6 |
The analysis of the results of rubber compound application tests of rubber made of the KER 1723 oiled rubber contained the modified TDAE III/PR19 plasticiser showed that physicomechanical properties of the vulcanisates meet the requirements specified by SYNTOS SA for reference rubber mixtures.
4 Conclusion
Studies of the solvent dewaxing process with various mixtures, MEK–TOL, MIBK–MEK and MEK–MTBE were carried out for three different TDAE plasticisers.
It was demonstrated that solvent dewaxing of the TDAE plasticiser with positive pour points, meeting the requirements of the Regulation 1907/2006 EU, maintains good process selectivity and allows for a significant reduction of the plasticiser pour point, thus improving the rheological and low-temperature properties.
In all dewaxing attempts, the pour point in the deparaffinate decreased significantly to the range −12 to −22°C, compared to the positive pour points of the raw materials.
For the solvents studied, the effect of lowering the pour point of the TDAE plasticiser in the solvent dewaxing process was obtained, while maintaining quality parameters meeting the requirements of REACH, which can be considered as obtaining a modified TDAE plasticiser with a minus pour point, which should have a positive effect on improving the rubber performance in low product temperatures, particularly the car tyres.
The results achieved in the solvent dewaxing process with the mixture of MEK–TOL, MIBK–MEK and MEK–MTBE and the TDAE plasticiser used for the raw material cause slight shifts in the structural composition of deparafinate, compared to the raw material and do not significantly improve the desired aromatic structure of TDAE raffinates. However, in the assessment of the structural composition of hydrocarbons presented, it should be noted that the structural composition according to ASTM D 2410 [34] is calculated indirectly and may not fully reflect the actual structural change of hydrocarbons after the dewaxing process.
In the attempts of plasticiser dewaxing studied, no favourable change in the structural composition of hydrocarbons was achieved, in particular when it comes to increasing the content of carbon atoms in aromatic structures, compared to the dewaxing charge. Similarly, the content of carbon atoms in naphthenic structures, for some dewaxing tests, increases slightly, while for other tests it decreases, compared to the raw material. Also, the content of carbon atoms in paraffin structures does not decrease much or increases in comparison to the raw material, while the content of paraffins would be desired to significantly decrease, thus improving mainly the content of the aromatic compounds.
Studies on the use of modified plasticisers to produce oiled rubber and vulcanisate meet the quality requirements for those products with regard to the physical and mechanical properties specified by SYNTOS SA.
The study conducted allowed for the preparation of four patent applications with the Patent Office of the Republic of Poland.
Acknowledgements
The authors would like to thank Synthos S.A. for performing the application tests for the sample of the modified TDAE plasticiser provided. The article was written on the statutory work entitled: Assessment impact of the TDAE plasticizer on the quality requirements of rubber products – supported by the Oil and Gas Institute – National Research Institute commissioned by the Ministry of Science and Higher Education, archive number: DK-4100-/80/17, order number: 0093/TO/17.
-
Funding information: This work was supported by the Oil and Gas Institute – National Research Institute (INiG – PIB) statutory work no. DK-4100-/80/17.
-
Author contributions: S.P. – supervision, conceptualization, formal analysis, investigation, writing – original draft; W.K. – conceptualization, formal analysis, writing – review & editing; J.J. – validation, writing – review & editing; A.A. – data curation, visualization, writing – review & editing.
-
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data generated or analysed during this study are included in this published article.
References
[1] Rocha TCJ , Soares BG , Coutinho FMB . The most important butadiene based elastomers employed in the automotive industry. Polimeros. 2007;17(4):299–307.10.1590/S0104-14282007000400009Suche in Google Scholar
[2] Bastardo-Zambrano L . The global naphthenic market: present and future-challenges and opportunities. Kraków: NYNAS; 2011.Suche in Google Scholar
[3] Ptak S . Evaluation of the process solvent dewaxed plasticizer TDAE. Nafta-Gaz-Sci Technol Oil Gas Ind. 2017;8:605–15.10.18668/NG.2017.08.08Suche in Google Scholar
[4] Pocklington JE . Safer Alternatives to aromatic process oils. Tire Technology International; 1998. p. 43–7.Suche in Google Scholar
[5] Mobil Europe Lubricants Limited, UK. An article “Oils without labels”. Tire Technology International; 1999. p. 10.Suche in Google Scholar
[6] Null V . Rubber tests with safer extender oils. Tire Technology International; 1999. p. 21–5.Suche in Google Scholar
[7] Regulation (EC) no 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH).Suche in Google Scholar
[8] International standard ISO 6743-10:1989 Lubricants, industrial oils and related products (class L) – Classification Part 10: Family Y (Miscellaneous)).Suche in Google Scholar
[9] Garbim VJ . Plastificantes para compostos de borracha. Borracha Atual. 2001;23:14–29.Suche in Google Scholar
[10] Ezzoddin S , Abbasian A , Aman-Alikhani M , Taghvaei S . The influence of non-carcinogenic petroleum-based process oils on tire compounds’ performance. Iran Polym J. 2013;22(9):697–707.10.1007/s13726-013-0168-9Suche in Google Scholar
[11] Ptak S . Petroleum plasticizers for rubber industry – dewaxing TDAE plasticizer. Nafta-Gaz-Sci Technol Oil Gas Ind. 2017;9:675–84.10.18668/NG.2017.09.07Suche in Google Scholar
[12] Lü Y , Yin J , Pan H . Petroleum plasticiser for tyre manufacturers. Pet Process Petrochem. 2011;42(26):126–31.Suche in Google Scholar
[13] Dasgupta S , Agrawal SL , Bandyopadhyay S , Chakraborty S , Mukhopadhyay R , Malkani RK , et al. Characterization of eco- friendly processing aids for rubber compound. Polym Test. 2007 June;26(4):489–500.10.1016/j.polymertesting.2007.01.007Suche in Google Scholar
[14] Chiosso WC . SBR rubber plasticisers. Borracha Atual. 2007;71(54):34–39.Suche in Google Scholar
[15] Zamboni GE . Extrato aromatico. Lubes em Foco; 2007;2:25–7.Suche in Google Scholar
[16] Bowman J , da Via M , Pattnelli ME , Tortoreto P . The influence of non-Toxic extender oil on SBR performances. Kautsch Gummi Kunstst. 2004;57(1–2):31–6.Suche in Google Scholar
[17] Patent application EP 3 031 621 A1. Pneumatic tire. The Goodyear Tire & Rubber Company; pub. date 09.12. 2014.Suche in Google Scholar
[18] Ptak S , Jakóbiec J , Antosz A . Production of the modified petroleum aromatic plasticizers TDAE. Nafta-Gaz-Sci Technol Oil Gas Ind. 2018;1:49–60.10.18668/NG.2018.01.06Suche in Google Scholar
[19] Patent DE 3930422. Process for the production of process oils with a low content of polycyclic aromatic compounds. BP Oiltech GMBH; pub. date 21.03.1991.Suche in Google Scholar
[20] Patent EP 839891. Process for obtaining aromatic oils having a polycyclic aromatics content of less than 3% which are useful as process oils. Repsol Petroleo SA; pub. date 06.05.1998.Suche in Google Scholar
[21] Patent DE 2343238. Verfahren zur behandlung von mineral schmieroel-ausgangs materialien. British Petroleum CO; pub. date 14.03.1974.Suche in Google Scholar
[22] Patent EP 1106673. Removal of polycyclic aromatic compounds from extracts. Shell Int Research; pub. date 13.06.2001.Suche in Google Scholar
[23] Patent EP 1260569. Process for making non-carcinogenic, high aromatic process oil. Shell Int Research; pub. date 27.11.2002.Suche in Google Scholar
[24] Patent PL 207051. The manner of production of oil plasticizer for caoutchouk and rubber Oil and Gas Institute. Group Lotos SA; pub. date 29.10.2010.Suche in Google Scholar
[25] Patent PL 224956. Method for preparing an oil plasticizer for raw rubber and rubber oil and gas Institute. Group Lotos SA; pub. date 28.02.2017.Suche in Google Scholar
[26] Patent PL 207052. Method for the manufacture of TDAE plasticizer for natural rubber and rubber. Oil and Gas Institute. Group LOTOS S.A; pub. date 29.10.2010.Suche in Google Scholar
[27] Patent PL 207056. The manner of production of TDAE plasticizer. Oil and Gas Institute; pub. date 29.10.2010.Suche in Google Scholar
[28] Patent PL 208531. Plasticizer and method for producing a plasticizer. ZAKRYTOE AKTSIONERNOE OBSCHESTVO TORGOVY DOM “ORGKHIM”; pub. date 31.05.2011.Suche in Google Scholar
[29] Patent application WO2011098096 A1. Method for producing process oils having a low content of polycyclic aromatics and use thereof. H & R International GMBH; pub. date 10.02.2010.Suche in Google Scholar
[30] Patent DE 2827494. Verfahren zur entparaffinierung von paraffinhaltigem schmieroel ausgangs material, Texaco Development Corp; z dnia 25.01.1979.Suche in Google Scholar
[31] Patent US 3972779. Means for controlling dewaxing apparatus. Texaco INC; pub. date 03.08.1976.Suche in Google Scholar
[32] Patent US 4146461. Dilution chilling dewaxing by modification of tower temperature profile. Exxon Research Engineering Co; pub. date 27.03.1979.Suche in Google Scholar
[33] Patent US 4444648. Solvent dewaxing with methyl tertiary butyl ether. Exxon.Suche in Google Scholar
[34] Standard ASTM D 2410:2015. Standard test method for carbon-type composition of insulating oils of petroleum origin.Suche in Google Scholar
© 2021 Stefan Ptak et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

