Abstract
Most dental implants used in dental practices are made of titanium or titanium alloys so that the essential differences promoted by the various manufacturers are at the level of their surface; through specific surface treatments, the aim is to obtain improved results regarding osseointegration. This study attempts to identify the differences between a series of used brands of dental implants by analyzing the chemical composition and the morphology of their surface and is particularly significant for the potential users as it highlights the manner of performances of the aforementioned implants, providing them with a tool in choosing the proper dental implant to suit their needs. It was found that, as the technology evolved and the costs were reduced, there is a net preference for using pure titanium or its alloys in the manufacture of dental implants versus the stainless steel titanium alloys, considered now a thing of the past.
1 Introduction
A significant number of researchers sought to identify over the time a number of biocompatible materials which can be used to replace and/or restore the missing or injured anatomical structures. It is considered that a material must meet at least three conditions in order to be used for implantation: to be biocompatible, to have a balance of physical and mechanical properties so that it performs as expected, and the medical device should be relatively easy to produce in large volumes, to the same standard [1].
Biotolerant materials (Co–Cr alloys, stainless steel, tantalum, or some polyols), bioinert materials (titanium and its alloys, zirconia), or even bioactive materials (hydroxyapatite) have been identified, but the constraints due to the small size of devices and the increased mechanical stress they are subjected to led over the time to a preference for bioinert materials such as titanium and its alloys, which have proven to represent an optimal solution for dental implants. Their enhanced biocompatibility and other desired properties and qualities made the manufacturers to choose titanium and its alloys as raw materials for dental implants over the stainless steel titanium alloys [2,3,4,5,6,7,8,9,10,11,12,13,14,15]. Titanium reacts with many other chemical elements (Ag, Al, V, Zn etc.) to form alloys. The American Society for Testing and Materials organized titanium and its alloys on a scale of 1–39. The first four grades are of pure (non-alloy) titanium types and the others for alloys. The alloys are organized into three broad categories, Alpha, Alpha Beta, and Beta, and their characteristics, properties, preferences, and uses differ widely based on the particular type of alloy chosen [14,15,16,17,18,19,20,21,22,23]. Alpha alloys are most commonly made of aluminum and tin, which makes them ductile, with high hardness, gives them good mechanical properties at low temperatures, has the highest corrosion resistance, cannot be forged at high temperatures, but they can be welded. Alpha Beta alloys are more durable, but Alpha alloys are strong enough to make them a preferred choice in chemical or aeronautical equipment. Beta alloys have medium to high hardness, can be forged or processed at high temperatures, they are weldable, and are used in aeronautics, in the development of prosthetic elements, and in marine equipment. Their elevated density makes them particularly suitable for the production of certain parts which must maintain their shape and structure even at the highest pressures.
Titanium Grade 2, Titanium Grade 4, and Ti6Al4V-ELI alloy (Titanium Grade 23) are the most widely used materials in the production of dental implants [15]. In Table 1, a comparison of the technical data of these raw materials [2,3,15], namely, the chemical composition and the associated physical characteristics for the aforementioned materials, is presented.
Chemical composition and physical characteristics of TiCP2, TiCP4, and Ti6Al4V-ELI (Ti Grade 23)
| Ti Grade 2 | Ti Grade 4 | Ti6Al4V-ELI | |
|---|---|---|---|
| Maximum content of Al | — | — | 5.5–6.5% |
| Maximum content of V | — | — | 3.5–4.5% |
| Maximum content of C | 0.08% | 0.08% | 0.08% |
| Maximum content of N | 0.03% | 0.05% | 0.05% |
| Maximum content of O | 0.25% | 0.4% | 0.13% |
| Maximum content of Fe | 0.3% | 0.5% | 0.25% |
| Maximum content of H | 0.015% | 0.015% | 0.012% |
| Maximum content of other elements in total | 0.4% | 0.4% | 0.4% |
| Minimum content of Ti | 98.825% | 98.455% | 88.078% |
| Density (g/cm3) | 4.51 | 4.51 | 4.42 |
| Elasticity modulus (GPa) | 103–107 | 105 | 105–116 |
| Melting point (C) | 1,665 | 1,660 | 1,655 |
| Fracture toughness (MPa) | 66 | 99–140 | 100 |
| Shear modulus (GPa) | 45 | 40 | 44 |
It can be observed that the values for fracture toughness are close for TiCP4 to Ti6Al4V-ELI alloy (Ti Grade 23). The metallographic analysis reveals the lack of homogeneity in the titanium alloys [20]: the presence of alloying elements can induce microscopic defects. Last but not least, the reactivity to the ambient temperature of titanium with oxygen is important. The formation of a TiO2 layer on the surface of titanium and titanium alloys cannot be avoided; this layer forms spontaneously, upon contact with oxygen from atmospheric air [4,5,21,22].
2 Materials and methods
In this research, we compared a Dentix SLA implant (sample 10) as well as a Dentix Nano implant (sample 11), produced by the company Dentix Millennium in Romania with nine other implants from well-known reputable manufacturers of dental implants, purchased, and sealed in the original packaging from local distributors (Adin, Alpha-Bio, Ankylos, Israel OEM, Megagen, Nobel Biocare, Osstem, Ritter, Straumann, Zimmer) randomly named from S1 to S9. All the samples were subjected to electron microscopy investigations. In order to limit the variable to a minimum possible, all the investigated implants had a diameter between 4.0 and 4.3 mm and a length between 12.5 and 13 mm. The average surface area of these implants, measured on the 3D model, was 209.2486 mm2. This surface area is increased by the processes of roughness modification. The morphological characterization of samples was carried out using scanning electron microscopy (SEM) with an FEI Inspect S50 apparatus (FEI, Hillsboro, OR, USA), used with energy-dispersive X-ray spectroscopy (EDS) system produced by EDAX (Mahwah, NJ 07430, USA), with fixed silicon detector and Peltier element integrated as a cooling system. The SEM analyses were performed at an acceleration voltage of 20–30 kV, in the magnification range of 50–50,000×, at the working distance of 10 cm, with a spot beam 2. For EDS, the beam spot used was 5.5–6, the working distance was 10 cm, and the dead time was 33. Prior to microscope insertion and visualization, the samples were washed in isopropyl alcohol and then rinsed with double distilled water. A scratch was made on the surface of each dental implant, in order to detect the differences in composition between the treated surface of the implant and its interior.
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
The results of the samples examined under the electron microscope, their surface morphology, the associated chemical composition, and the corresponding SEM images are presented in Figures 1–12.
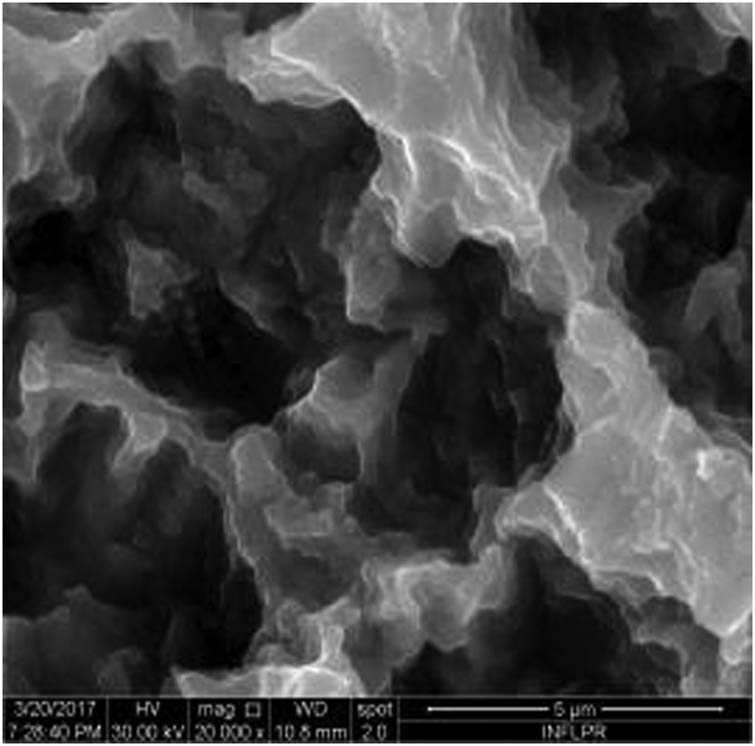
SEM image of sample S1 at a magnification of 20k×.
SEM image of sample S2 at a magnification of 20k×.
SEM image of sample S3 at a magnification of 20k×.
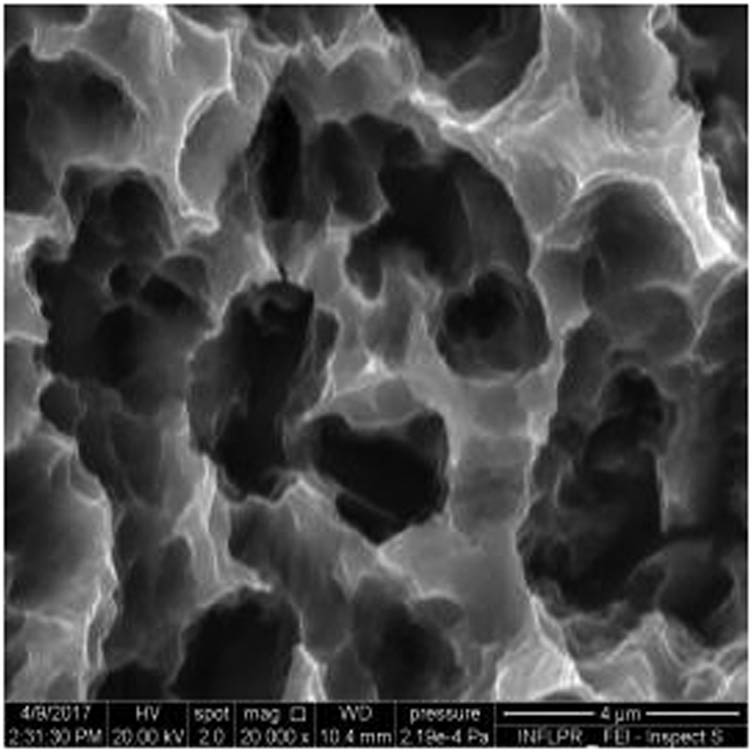
SEM image of sample S4 at a magnification of 20k×.
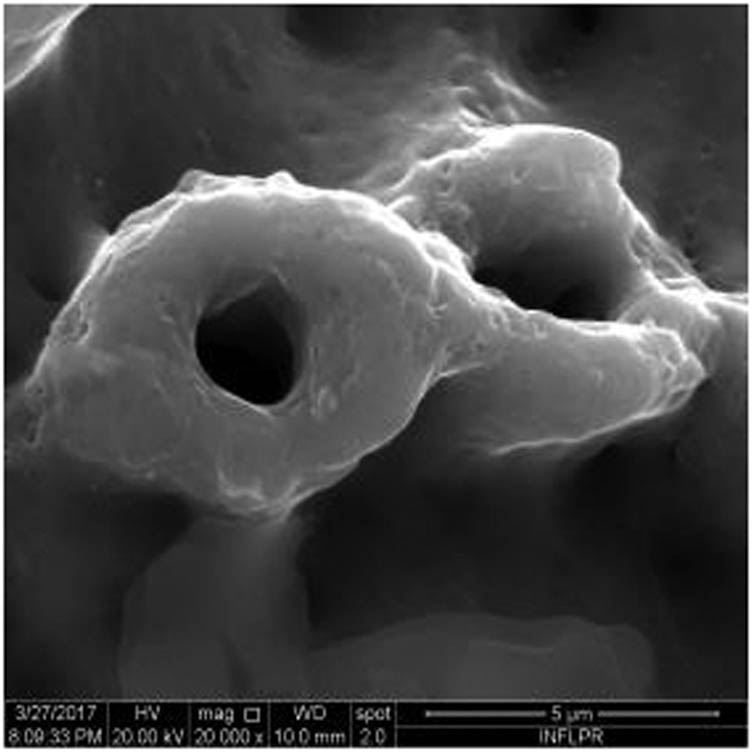
SEM image of sample S5 at a magnification of 20k×.
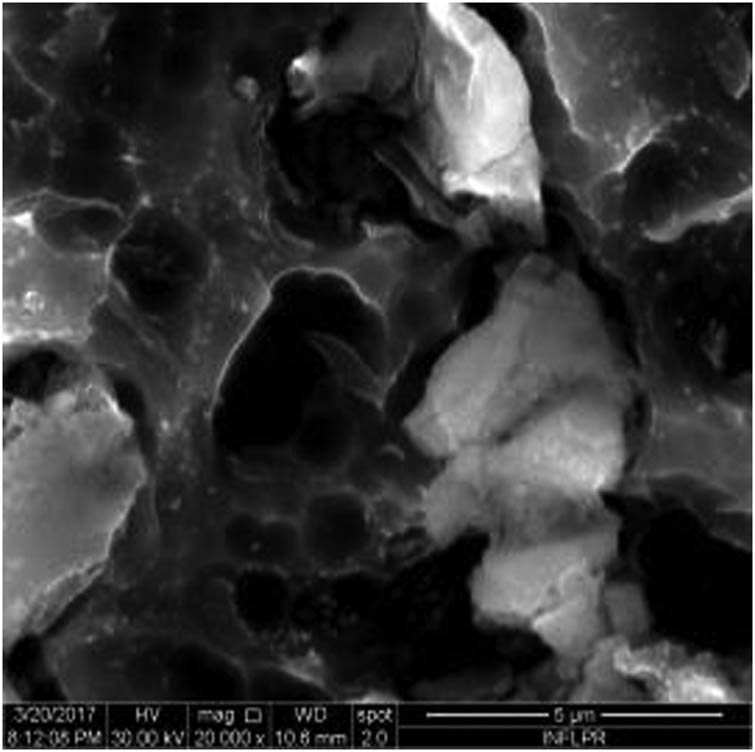
SEM image of sample S6 at a magnification of 20k×.
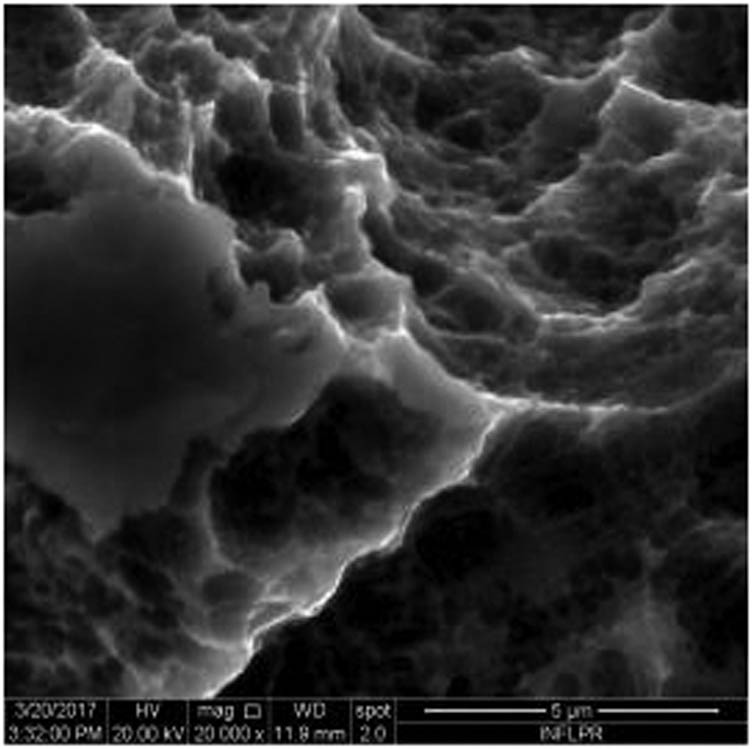
SEM image of sample S7 at a magnification of 20k×.
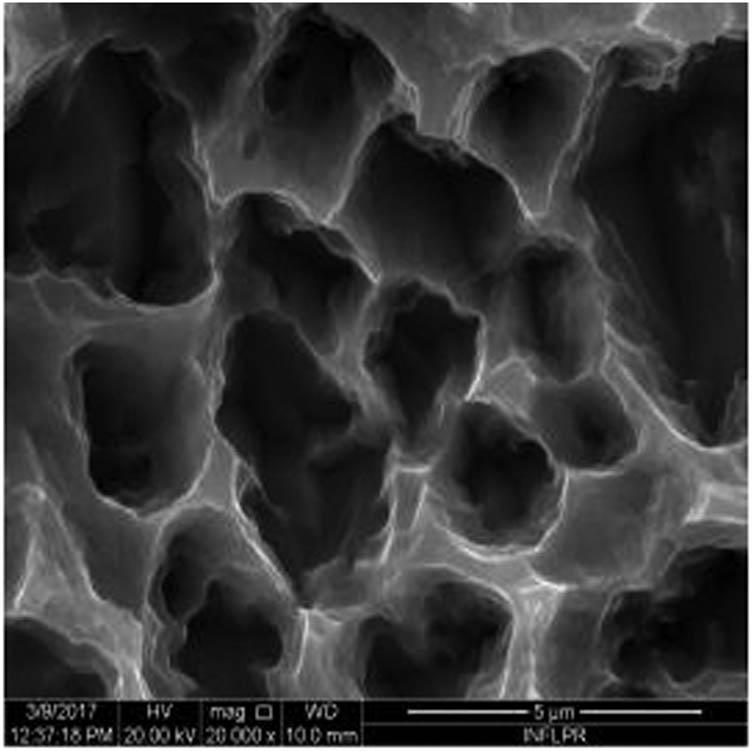
SEM image of sample S8 at a magnification of 20k×.
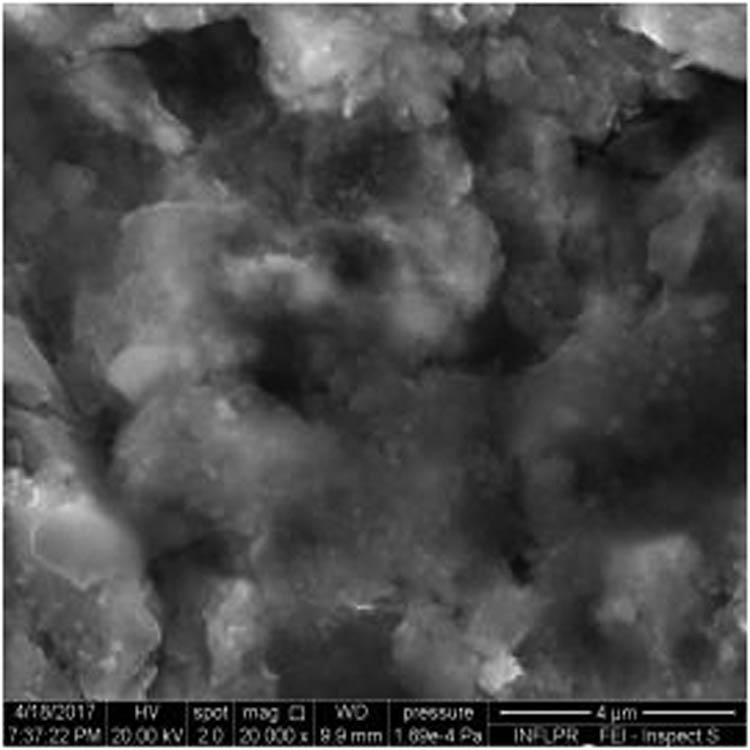
SEM image of sample S9 at a magnification of 20k×.
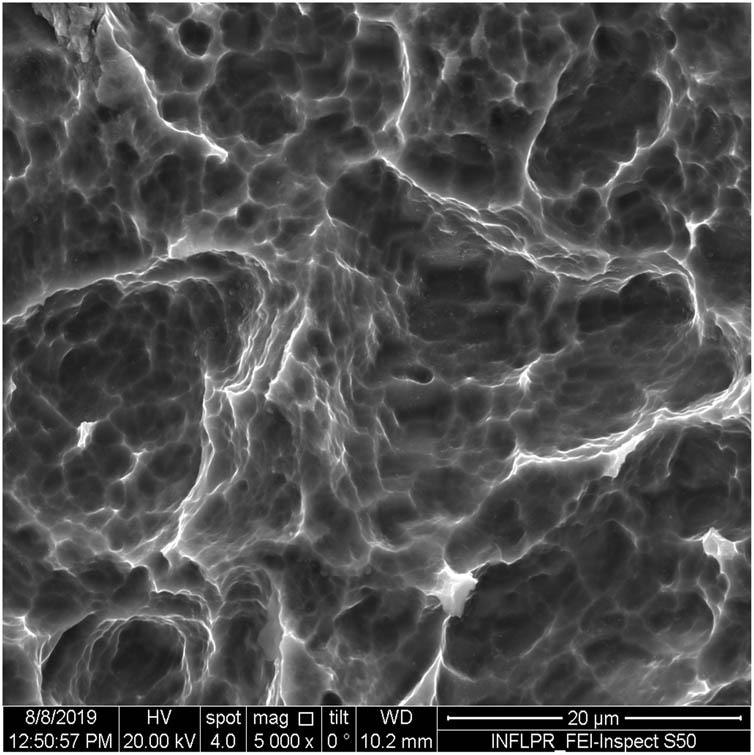
SEM image of sample S10 at a magnification of 5k×.

SEM image of sample S11 at a magnification of 5k×.

SEM image of sample S11 at a magnification of 20k×.
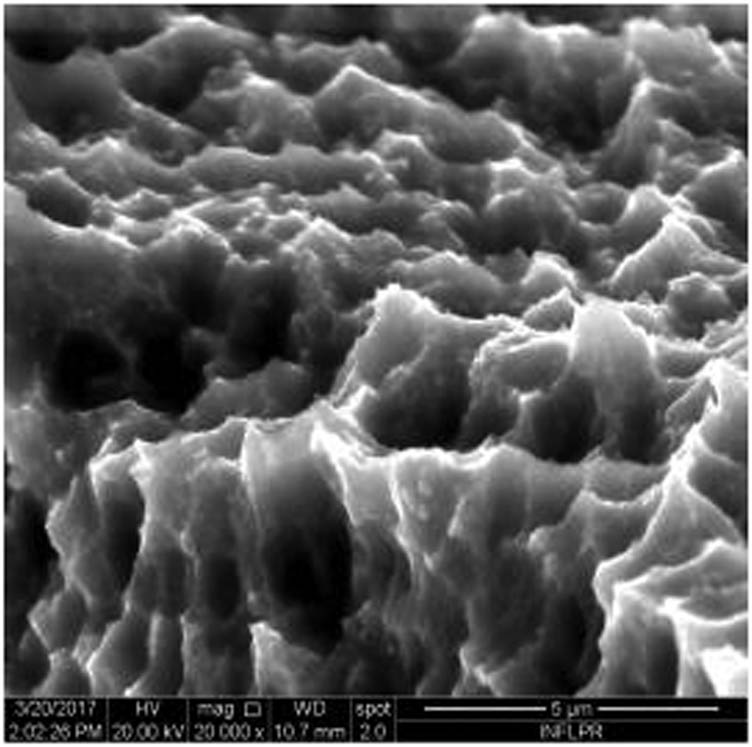
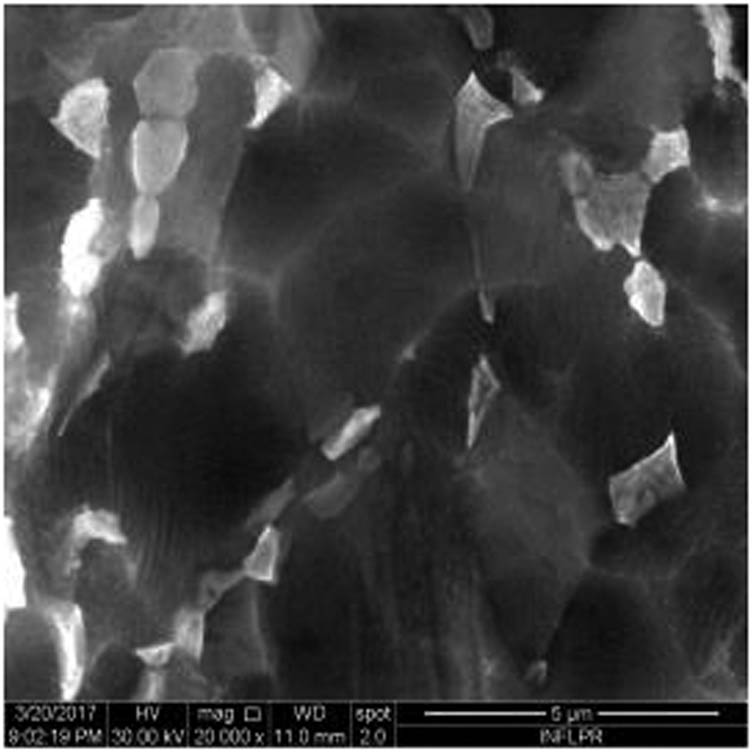
Figure 1 shows the image of sample S1 at a magnification of 20k×. At magnifications of 200×, 1,000× we observe the uniform appearance, with fine structure of holes on the surface of the material, with an appearance similar to the surface corroded with acid. The surface, analyzed in detail, has a certain degree of roughness, its appearance changing from uneven wells, with thin, irregular, corroded (5,000×) wells and walls toward ridges and concavities of different shapes with several microns, 1–4 µm in the image. Similar results were found with other implants with their surface sample treated with acids, presented in detailed images (20,000×), where deep and lacy pores with diameters of 3–5 μm can be noticed. In Figure 2, the surface treatment originally applied to sample S2 ensured a homogeneous, abrasive appearance throughout the implant. At 2,000×, there are rounded honeycomb-type alveoli in characteristic assemblies with an appearance similar to the acid-corroded surface. At higher magnifications (20,000×), the rounded alveoli have the appearance of deep pores with diameters of 1.5–3 microns, some joined, rounded but irregular, with unevenly corroded walls. Figure 3 shows the image of sample S3 at a magnification of 20k×. At magnifications over 1,000, the investigated implant presents a relatively uneven, flattened appearance, a structure with ridges and valleys, resembling a honeycomb, consisting of asperities, grains, and pores (5,000×) of 1–2 microns, circular (image 20k×), sometimes rectangular. Figure 4 shows the image of sample S4 at 20k× magnification. The SEM image at low magnification (200×) indicates a uniform appearance of the implant, with dunes between 10 and 20 μm, with small grains. At higher magnifications (5,000×), the corrugated relief, sand dune type, is preserved, noticing concavities of 10–18 μm in diameter, with irregular holes and fine grains, of lengths of ∼1 μm (20,000×), stuck in the walls of the recesses. The surface type looks similar to acid corroded surface (SLA). In Figure 5, the SEM analysis at 20k× magnification shows simple (0) and joined (double – 8/∞) tubular cylindrical structures, rarely triple, with depth aspect, with outer diameters 4–5 microns and inner diameters 1.5 to 2 microns. Distinct deep tubular pores with walls ∼2 microns in diameter. On the implant analyzed the ratio of enlarged tubes/pore surface (shorter tubes) is about 1:1. In Figure 6, the appearance of this dental implant is characteristic of the two treatments to which it was samplebly subjected: sand blasting and acid corrosion (SLA): rough relief, with craters, irregular inclusions, grooves (200× and 2,000×), all generating an expanded surface for easier cell attachment. In detail, we can distinguish inclusions of different angular and/or semi-rounded shapes and micron grains embedded in the irregular, uneven surface, full of pores (200×, 5,000×, 20,000×) of various diameters (0.5–4 μm). Figure 7 shows the image of S7 sample at a magnification of 20k×. Observed at low magnifications (200×), the surface of this implant has a similar appearance to the blast. Furthermore, at 2,000× the unevenly corroded appearance becomes visible, with untouched areas (with a size of 1–20 microns) juxtaposed with the corroded ones. Going forward with the enlargement of the images (5,000×), the structure is revealed as very porous, attacked deeper, consisting of islands of uncorroded material between relatively circular depths with attacked walls, full of formations samplebly given by the corrosion with the acids used in the treatment. The rough structure is spread from depressions of 15–30 microns (2,000×) to the finest pores, even submicron (20,000×), of different sizes. In Figure 8, it is observed that this dental implant presents a continuous appearance with rounded alveolar structures (1.7–2.5 microns). Figure 9 shows the SEM image at 20k× of sample S9. The surface is characterized by a slightly rough, smooth, homogeneous appearance, with periods of ∼10 μm (200× and 1,000×). The extended surface has numerous, relatively flattened edges. In detail, a rugged, rocky structure (2,000× and 5,000×) is highlighted. A topography cauliflower-like is also distinguished. At 20,000 magnifications, the material appears loose, with pores of approximately 1 micron or submicron. Figure 10 shows the image of S10 sample (Dentix Nano implant) at a magnification of 20k×. The TiO2 nanostructure can be seen all over the surface, covering the Ti microstructure. The TiO2 nanostructure is composed of a layer of TiO2 nanotubes, perpendicularly oriented on the implant surface. The TiO2 nanotubes on the surface of the Dentix Nano implant have an average of 100–200 nm in height, 60–80 nm outer diameter, 40–60 nm inner diameter, and 10–15 nm wall thickness.
The EDS analysis also provides a graphical representation of the peaks of the spectra emitted by the various chemical elements in the composition of all samples analyzed. In Figures 13 and 14, one may see two representative graphs for sample S4 and sample S6 depicting the elements with highest mass percentages. For sample S4, in Figure 13, the graph shows the highest mass percentage for titanium, which was expected, followed by oxygen, a direct proof of titanium dioxide formation, followed by vanadium and aluminum, confirming the existence of the alloying elements and traces of carbon, iron, and chlorine.
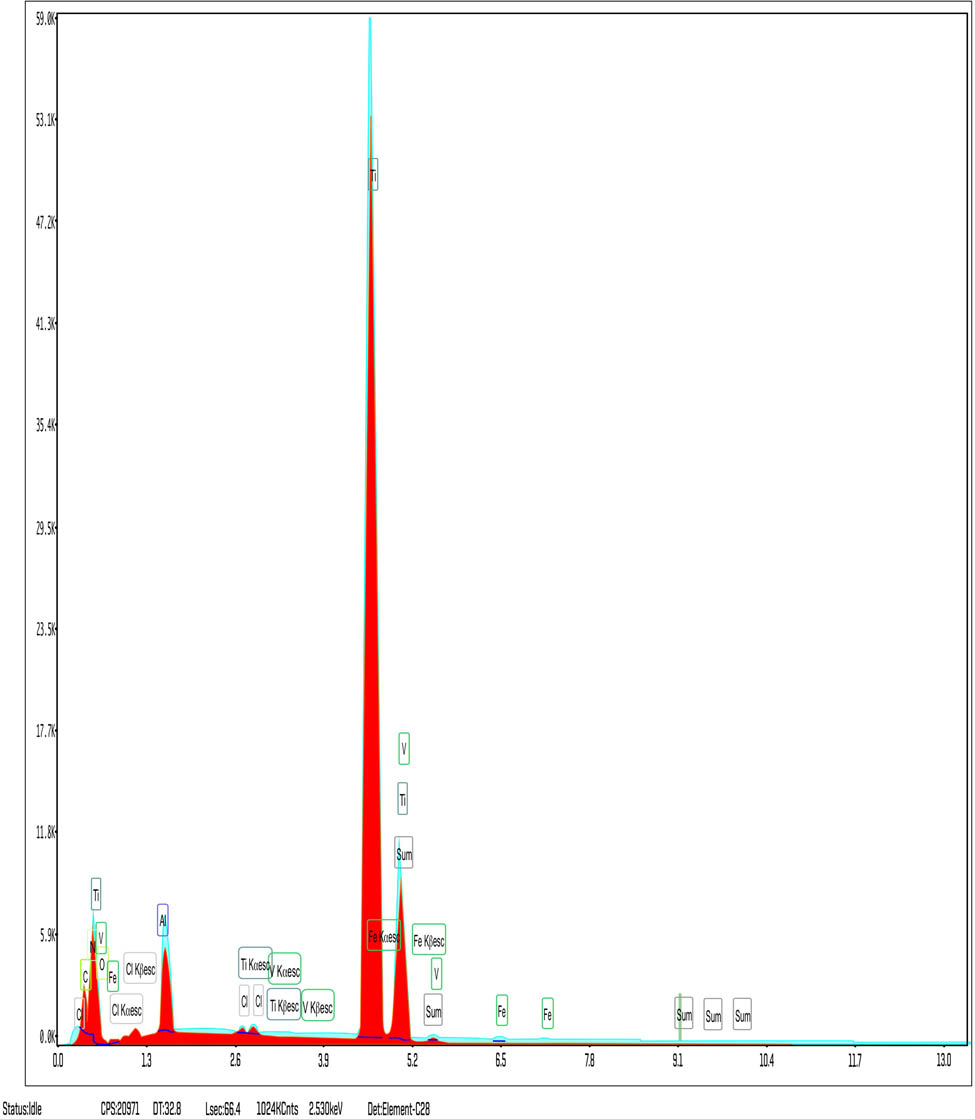
EDS of sample S4.
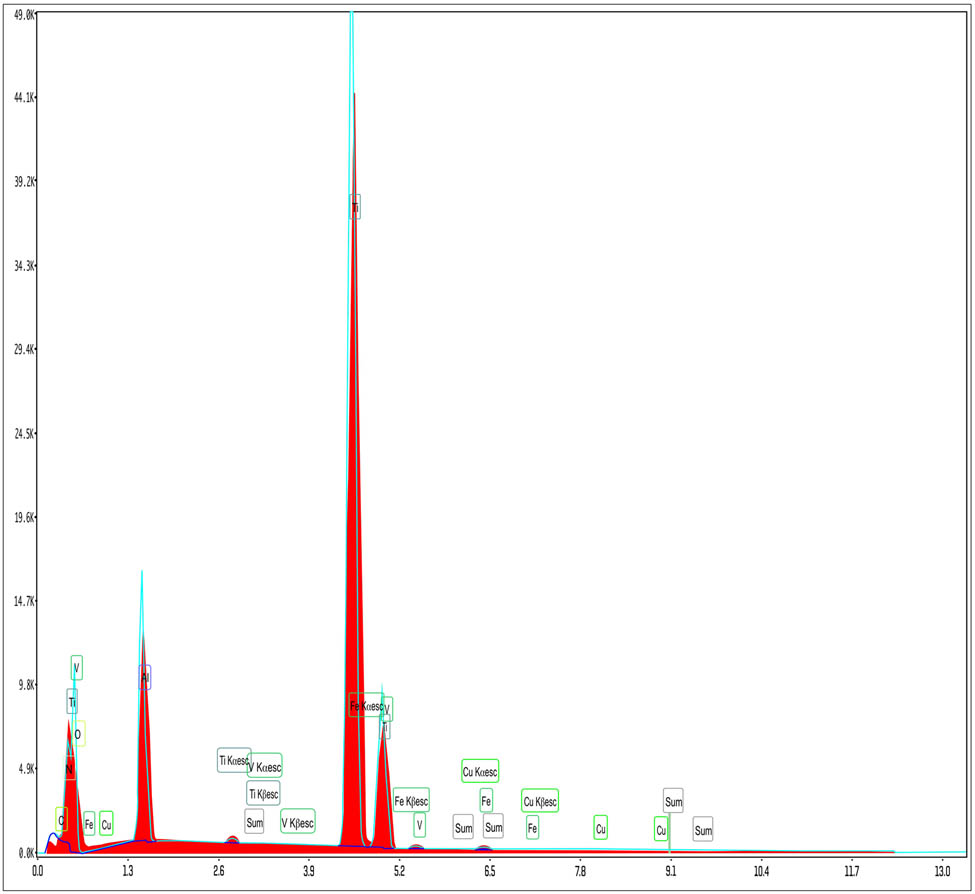
EDS of sample S6.
Figure 14 corresponding to EDS for sample S6 shows also the highest peak for titanium, followed by oxygen and high values for aluminum and vanadium; one may also notice the presence, besides carbon and iron of copper; as different manufacturers use different alloy compositions, this technique may be used as a useful tool in differentiating between two very similar implants and making correlations also between the chemical surface composition and their osseointegration behavior.
The results of EDS investigations for S1–S11 samples and the interpretations associated with these analyses are presented in Table 2.
EDS compositional analysis of the studied samples
| Sample | EDS analysis results | Description | |||
|---|---|---|---|---|---|
| Surface element | Weight (%) | Atomic (%) | Error (%) | ||
| S1 | C K | 0.00 | 0.00 | 99.99 | EDS analysis indicates a titanium majority implant (67.91% mass, 43.55% atomic), with an important contribution of oxygen (predictable on the surface of oxidized titanium layer, on the surface, 27.64% mass, 53.26% atomic) and a significant calcium content (3.48%, 2.67% atomic). Treatment of this type of implant involves an extremely fine layer (monoatomic, most likely) of Ca, in order to improve the osseointegration |
| O K | 27.74 | 53.26 | 10.54 | ||
| Ca K | 3.48 | 2.67 | 3.07 | ||
| Ti K | 67.91 | 43.55 | 1.39 | ||
| V K | 0.86 | 0.52 | 5.08 | ||
| S2 | C K | 0.13 | 0.44 | 14.13 | Titanium appears to be the only major chemical constituent of this implant (89.30% mass, 73.51% atomic) in quantifying EDS, next to oxygen (10.57% mass, 26.05% atomic), of which we can assume that it exists only in the oxidized layer from the surface. Carbon samplebly appears as a contaminant. In contrast, the compositional analysis in the scratch has fine traces of calcium (0.14% atomic), aluminum (0.12%), and silicon (1.51% atomic), samplebly due to the surface treatment with the processing substances |
| O K | 10.57 | 26.05 | 10.58 | ||
| Ti K | 89.30 | 73.51 | 1.31 | ||
| S3 | O K | 14.55 | 34.51 | 10.73 | The EDS compositional analysis confirms a titanium alloy (80–85% by mass) with zirconium (6–5% by mass) and the remaining oxygen |
| Al K | 0.08 | 0.11 | 21.35 | ||
| Zr L | 6.02 | 2.51 | 1.91 | ||
| Ti K | 89.30 | 73.51 | 1.31 | ||
| S4 | C K | 2.96 | 8.47 | 9.12 | EDS analysis confirms Ti–Al–V alloy (Titan Grade 5) as a material of this dental implant: Ti 73.11, Al 3.66, V 5.58, in mass percentages. Iron (0.40% by mass) and carbon (2.96% by mass) are also present as a trace and as a minority element. On the scratched surface, the more the scratch is very fine, the ratio of the elements is about the same. The difference consists only in a larger amount of oxygen in the scratch (21.62%, compared to 14.10% by mass) |
| N K | 0.00 | 0.00 | 7.76 | ||
| O K | 14.10 | 30.27 | 11.18 | ||
| Al K | 3.66 | 4.66 | 6.70 | ||
| Cl K | 0.18 | 0.18 | 15.80 | ||
| Ti K | 73.11 | 52.41 | 1.36 | ||
| V K | 5.58 | 3.76 | 2.53 | ||
| Fe K | 0.40 | 0.25 | 22.45 | ||
| S5 | O K | 33.11 | EDS analysis indicates an implant based on pure, oxidized titanium (most likely on the surface: 66.09% atomic Ti with 33.11% atomic O, on the surface, respectively, ∼74% atomic Ti with 25.2% atomic O). The difference in oxygen between the implant surface and the inside (scratch) may suggest that the tubular structures on the surface, removed in the case of scratches, are those that contain more oxygen, in accordance with the structure declared by the manufacturer, anodized titanium, with titanium dioxide and phosphorus | ||
| Ti K | 66.09 | ||||
| S6 | C K | 0.04 | 0.10 | 72.63 | EDS analysis shows the implant is made of Ti–Al–V alloy: in mass percent Ti 58.31, Al 8.32, V 4.83. Significant amount of oxygen, 28.28% by mass, 51.95% atomic, indicates the oxidation and passivation of the titanium support. There are also fine traces of iron (0.16% atomic), possibly also carbon and copper (below 0.04% atomic, with an error margin over 50%) |
| N K | 0.00 | 0.00 | 8.97 | ||
| O K | 28.18 | 51.95 | 10.46 | ||
| Al K | 8.32 | 9.09 | 6.07 | ||
| Ti K | 58.31 | 35.90 | 1.37 | ||
| V K | 4.83 | 2.97 | 2.54 | ||
| Fe K | 0.30 | 0.16 | 18.04 | ||
| Cu K | 0.02 | 0.01 | 58.11 | ||
| S7 | C K | 1.15 | 3.50 | 9.64 | EDS analysis indicates a titanium alloy based on Al and V as the implant material. Unexpectedly, the content of V (5.2–6.8% by mass) seems to be higher than that of Al (∼4% by mass). The phenomenon of surface oxidation (passivation) is also highlighted by the significant oxygen content (∼12.3% by mass), both on its surface and in the applied scratches. On the surface, the sample also suffered a slight carbon contamination (1.15% mass, 3.50% atomic), most likely accidental. Fine traces of Fe and Cu are also distinguished |
| N K | 0.00 | 0.00 | 6.92 | ||
| O K | 12.32 | 28.21 | 10.94 | ||
| Al K | 4.02 | 5.46 | 6.39 | ||
| Ti K | 76.98 | 58.89 | 1.31 | ||
| V K | 5.18 | 3.72 | 2.08 | ||
| Cu K | 0.35 | 0.20 | 9.44 | ||
| S8 | O K | 7.52 | 19.56 | 11.80 | EDS analysis shows mostly titanium content (91.89% mass, 79.79% atomic per thread), without other alloying elements, with incorporated oxygen (7.52% mass, respectively, 19.56% atomic) and traces of Ca (0.50%), only on the surface. The titanium percentage increases and the oxygen decreases in the scratch, as the scratch deepens, as the biocompatible layer of TiO2 forms at the surface |
| Al K | 0.03 | 0.05 | 66.31 | ||
| Si K | 0.05 | 0.08 | 60.26 | ||
| Ca K | 0.50 | 0.52 | 6.22 | ||
| Ti K | 91.89 | 79.79 | 1.33 | ||
| S9 | C K | 0.00 | 0.00 | 99.99 | EDS analysis applied to the OEM implant indicates that it is made of Ti–Al–V (mass percentages: Ti 64.02; Al 7.94; V 4.92). The compositional percentages differ from the Ti Grade 5 alloy recipe, most samplebly due to the fact that passivation oxygen appears from the implant surface (21.47% by mass) and elements specific to the surface treatment (And samplebly by the blasting process 0.04% by mass, possible as 0.35%) as well as possible contaminants (Cl, Fe, Ni, Cu, W). The traces of iron, nickel, and copper can come from the composition of the alloy. The relatively high percentage of Al could come from blasting with alumina particles |
| O K | 21.47 | 43.28 | 10.50 | ||
| Na K | 0.00 | 0.00 | 99.99 | ||
| Mg K | 0.00 | 0.00 | 99.99 | ||
| Al K | 7.94 | 9.49 | 6.17 | ||
| Si K | 0.04 | 0.05 | 43.89 | ||
| Ca K | 0.35 | 0.28 | 8.09 | ||
| Ti K | 64.02 | 43.11 | 1.37 | ||
| V K | 4.92 | 3.11 | 2.21 | ||
| Fe K | 0.94 | 0.54 | 8.37 | ||
| Ni K | 0.05 | 0.03 | 57.84 | ||
| Cu K | 0.15 | 0.08 | 38.96 | ||
| W L | 0.12 | 0.02 | 56.20 | ||
| S10 | Ti K | 99.83 | 99.85 | 1.15 | EDS analysis indicates that the Dentix SLA implant is made of pure titanium (99.83% mass percentage), without alloying elements. The presence of iron is lower than that declared by the producer of the raw material |
| Fe K | 0.17 | 0.15 | 44.63 | ||
| S11 | O K | 20.85 | 43.72 | 11.18 | EDS analysis shows that the Dentix Nano implant is made of pure titanium, without any other alloying elements. Aluminum and silicon are samplebly derived from the blasting material, and the iron is below the limits declared by the raw material manufacturer. The oxygen is present in a high concentration, above the average of the compared implants, demonstrating the abundance of the nanostructured layer of TiO2 |
| Al K | 1.52 | 1.89 | 8.72 | ||
| Si K | 0.06 | 0.08 | 45.69 | ||
| Ti K | 77.39 | 54.20 | 1.18 | ||
| Fe K | 0.18 | 0.11 | 31.83 | ||
Most dental implants have surfaces with similar morphology; the roughness is between 1 and 10 μm, and their appearance is specific to the surfaces obtained by acid attack (19) in the SLA-type surface treatment – treatment that has already confirmed its performance in the decades since it is used. Some of the manufacturers, who tried to differentiate by applying anodizing techniques, obtained surfaces with different appearance, showing craters instead of acid corrosion wells or fields of nanotubes. The presence of TiO2 nanotube layer increases the surface area exposed to the body fluids by six times, offering increased potential for body response and bone integration. The TiO2 nanotubes on the surface of the Dentix Nano implants have an average of 100–200 nm in height, 60–80 nm outer diameter, 40–60 nm inner diameter, and 10–15 nm wall thickness. The nanotubes cover the entire surface of the implant, being joined together, like a honeycomb. The ratio between the inner surface of the cylinder and that covered by a nanotube is (the average values were used) as follows:
The chemical composition of the materials from which dental implants are made must be analyzed in the context of the homogeneity of the respective material. If the raw material is not homogeneous, dental implants, being a very small device (a medium-sized implant – 4.2 × 11 mm, weighs 0.4 g), may end up having marginally different chemical compositions, even if they come from the same bar of raw material with potential repercussions on the behavior from the mechanical point of view, e.g., fatigue resistance and/or microbial-induced corrosion resistance. This is an additional reason for using pure titanium instead of titanium alloys, thereby reducing the effects of compositional non-homogeneities, as well as the creation of a localized galvanic reaction that can induce galvanic corrosion. The phenomenon of fracture of dental implants is quite rare [18]. The percentage of implants that fracture in the initial stages (at insertion or in the first days) is about 1% after some authors [6], but we consider this figure to be too high. The mechanical properties of the least resistant material (Ti Grade 2 and Ti Grade 4) are more than sufficient for the dental implants to fully respond to the proposed purpose [17], a fact demonstrated by the fatigue studies carried out by our team. The risk of fracture of dental implants is not a real reason for manufacturers to opt for one material or another. Neither osseointegration performance justifies the choice of Ti6Al4V alloy in favor of pure titanium [11]. Due to the high reactivity of titanium with oxygen, as well as surface treatment procedures, all dental implants have a layer of TiO2 that chemically stabilizes the surface (passivation). Titanium oxide plays a protective role, preventing contact of the titanium with other chemical elements, thus explaining the corrosion resistance of this material. In fact, titanium dioxide is due to the biocompatibility of dental implants: the bone tissue comes in close contact with the TiO2 layer, not with pure or allied titanium [12]. Recent concerns of the research departments of dental implant manufacturers are aimed at identifying nanostructuring methods of the TiO2 layer which, when formed spontaneously, is in an amorphous state. The nanostructuring of the TiO2 layer will open new opportunities leading the development of medical devices to the next level. Regarding the chemical composition of the titanium and its alloys, we consider some aspects as being particularly important and which were not given the true importance: the purity and homogeneity of the material from which dental implants are produced [7] and the biological effect of the presence of the alloying chemical elements. The low purity and non-homogeneity of the material from which dental implants are produced is the main cause of their fracture. The process begins with microcracks observable under the electron microscope, microcracks that either exist in the raw material, or occur at the first mechanical exposure on the implant, due to the different resistance at different points of the piece. Even so, a number of factors are needed for a fracture to occur, and this happens when we are dealing with a small implant, where the technology has been pushed to the limit. In our studies, we encountered fractured implants, with a size of 3.3 mm × 8 mm, at which the minimum wall thickness (where it also failed) was 60 microns. It happened that in that area, the material was not homogeneous, and thus, the accumulation of factors occurred. The biological effect of the chemical elements with which titanium alloying has been achieved is insufficiently studied. Aluminum and vanadium are not known to be chemical elements with beneficial effects on the body [8,9,10]. The cell viability of binary alloys (TiAl and TiV) is lower than that of pure titanium, TiCP [16]. Vanadium is cytotoxic at concentrations above 4% in alloys and neurotoxic [13]. We consider the use of vanadium too risky, given that the theoretical percentage is exactly at the limit of cytotoxicity (in Ti6Al4V, vanadium has 4%). The metallurgical procedures for obtaining titanium alloys, made on batches of tonnes, cannot have the precision of laboratory procedures, so that there is a guarantee that the vanadium level does not exceed 4% at all points in the volume of a batch. As evidence, the alloy manufacturers claim the presence of these chemical elements with ranges of values, not with exact values, and the chemical compositions presented above by EDS analysis also demonstrate different concentrations in reality. All these risks are assumed free of charge as long as the benefits are not tailored, and above all, they are not required. The increased mechanical strength of the Ti6Al4V alloy is not required, and the shortcomings that an alloy can bring can negatively offset its relative advantages. Pure materials have sufficiently good properties, and statistics do not show that pure titanium implants fracture more often than alloy implants.
4 Conclusions
Following the investigations made on the 11 dental implant samples from different providers, it emerges as a general conclusion the preference for the use of pure titanium or its alloys in the manufacture of dental implants. The most commonly used materials are Ti Grade 4 and Ti6Al4V-ELI alloy. However, there are differences from producer to producer, some manufacturers preferring custom-made alloys, by adding a certain element, such as Zr with possible positive contributions to product performance. However, only clinically conducted studies (these are costly and they stretch over the years, requiring investigations on human subjects) are able to demonstrate for good whether or not these marginal compositional differences have a beneficial effect on the osseointegration of the implants and their durability over time. It was found that by comparing the chemical composition of the various brands of dental implants with the chemical composition of the raw materials used, it is possible to construct an image of possible contamination (most samplebly of the surface) with other elements not initially existing in the raw material, as well as of the efficiency of the cleaning processes. As far as the preference of pure titanium versus its alloys is concerned, there is no need for an increased mechanical strength of alloys, these bringing about more negative issues and offsetting its relative advantages as long as pure titanium has sufficiently good mechanical properties and better osseointegration characteristics.
Acknowledgement
This research was supported by Dentix Millennium SRL through the project “Dental implants surface functionalization for improved osseointegration – an innovative method” (Metoda inovativă pentru funcţionalizarea suprafeţelor implanturilor dentare cu scopul îmbunătăţirii osteointegrării) code MySMIS 104809, contract no. ANCSI 73/8.09.2016. Dragos Vladimir Budei is a PhD student enrolled at the Politehnica University of Bucharest and Mihaela Mincu is a PhD student enrolled at the University of Agronomic Sciences and Veterinary Medicine of Bucharest.
-
Funding information: This research was supported by Dentix Millennium SRL through the project “Dental implants surface functionalization for improved osseointegration – an innovative method” (“Metoda inovativă pentru funcţionalizarea suprafeţelor implanturilor dentare cu scopul îmbunătăţirii osteointegrării”) code MySMIS 104809, contract no. ANCSI 73/8.09.2016. Dragos Vladimir Budei is a PhD student enrolled at the Politehnica University of Bucharest and Mihaela Mincu is a PhD student enrolled at the University of Agronomic Sciences and Veterinary Medicine of Bucharest.
-
Author contributions: All authors made substantial contributions to all of the following: (1) the conception and design of the study, acquisition of data, interpretation of data; (2) drafting the article or revising it critically; and (3) final approval of the version to be submitted.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Soumya N, Banerjee R. Fundamentals of medical implant materials. ASM handbook, vol 23. Materials for medical devices. Cleveland: ASM International; 2012. p. 6–14.10.31399/asm.hb.v23.a0005682Search in Google Scholar
[2] Saini M, Singh Y, Arora P, Arora V, Jain K. Implant biomaterials: a comprehensive review. World J Clin Cases. 2015;3(1):52–7. 10.12998/wjcc.v3.i1.52.Search in Google Scholar
[3] Carpenter dynamet titanium grade 2, grade 4 and Ti6Al4V-ELI data sheets.Search in Google Scholar
[4] Vaquila I, Vergara LI, Passeggi Jr MCG, Vidal RA, Ferro´n J. Chemical reactions at surfaces: titanium oxidation. Surf Coat Technol. 1999;122:67–71.10.1016/S0257-8972(99)00420-XSearch in Google Scholar
[5] Donachie MJ. Titanium: a technical guide, vol 132. West Conshohocken, PA: ASM International; 2000. p. 125–6.10.31399/asm.tb.ttg2.9781627082693Search in Google Scholar
[6] Sanivarapu S, Moogla S, Kuntcham RS, Kolaparthy LK. Implant fractures: rare but not exceptional. J Indian Soc Periodontol. 2016;20(1):6–11. 10.4103/0972-124X.154190.Search in Google Scholar PubMed PubMed Central
[7] Stoicănescu M, Buzamet E, Budei DV, Craciun V, Budei R, Cosnita M, et al. Possible causes in breaking of dental implants research. Mater Sci Forum. 2017;907:104–18.10.4028/www.scientific.net/MSF.907.104Search in Google Scholar
[8] Klotz K, Weistenhöfer W, Neff F, Hartwig A, van Thriel C, Drexler H. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114(39):653–9. 10.3238/arztebl.2017.0653.Search in Google Scholar PubMed PubMed Central
[9] U.S. Department of Health and Human Services – Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological profile vanadium, vol 44; 2012. p. 23–27.Search in Google Scholar
[10] U.S. Department of Health and Human Services – Public Health Service Agency for Toxic Substances and Disease Registry. Public Health Statement Alum CAS#7429-90-5; 2012.Search in Google Scholar
[11] Shah FA, Trobos M, Thomsen P, Palmquist A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants – is one truly better than the other? Mater Sci Eng C Mater Biol Appl. 2016;62:960–6. 10.1016/j.msec.2016.01.032.Search in Google Scholar PubMed
[12] Yang WE, Huang HH. Improving the biocompatibility of titanium surface through formation of a TiO 2 nano-mesh layer. Thin Solid Films. 2010;518/24:7545–50. 10.1016/j.tsf.2010.05.045.Search in Google Scholar
[13] Fatola OI, Olaolorun FA, Olopade FE, Olopade JO. Trends in vanadium neurotoxicity. Brain Res Bull. 2019;145:75–80. 10.1016/j.brainresbull.2018.03.010.Search in Google Scholar PubMed
[14] ASTM B265-15 standard specification for titanium and titanium alloy strip, sheet, and plate. West Conshohocken, PA: ASTM International; 2015. www.astm.orgSearch in Google Scholar
[15] Froes F, Qian M. Titanium in medical and dental applications. Cambridge: Woodhead Publishing; 2018. p. 495–504.10.1016/B978-0-12-812456-7.00022-6Search in Google Scholar
[16] Liu X, Chen S, Tsoi JKH, Matinlinna JP. Binary titanium alloys as dental implant materials – a review. Regen Biomater. 2017;315–23. 10.1093/rb/rbx027.Search in Google Scholar PubMed PubMed Central
[17] Felli F, Pilone D, Scicutelli A. Fatigue behaviour of titanium dental endosseous implants. Frat ed Integrità Strutt. 2011;18:14–22. 10.3221/IGF-ESIS.18.02.Search in Google Scholar
[18] Shemtov-Yona K, Rittel D. Fatigue of dental implants: facts and fallacies. Dent J. 2016;4:16. 10.3390/dj4020016.Search in Google Scholar PubMed PubMed Central
[19] Elias CN, Fernandes DJ, de Souza FM. Mechanical and clinical properties of titanium and titanium-based alloys (Ti G2, Ti G4 cold worked nanostructured and Ti G5) for biomedical applications. J Mater Res Technol. 2018;8(1):1060–9. 10.1016/j.jmrt.2018.07.016.Search in Google Scholar
[20] Gammon LM, Briggs RD, Packard JM, Batson KW, Boyer R, Domby CW. Metallography and microstructures of titanium and its alloys. ASM handbook, vol 9. Metallography and microstructures. Cleveland: ASM International; 2004. p. 899–917. 10.1361/asmhba0003779.Search in Google Scholar
[21] Lutjering G, Williams JC, Gysler A. Microstructure and mechanical properties of titanium alloys. Singapore: World Scientific; 2000. p. 1–77. Chapter 1. 10.1142/9789812793959_0001.Search in Google Scholar
[22] Byvaltsev SV. The mechanism of oxide film formation for drawing titanium. Amsterdam: Elsevier Ltd; 2019. 10.1016/j.matpr.2019.07.048.Search in Google Scholar
[23] Namavar F, Sabirianov RF, Marton D, Rubinstein A, Garvin KL. Why is titanium biocompatible. ORS 2012 annual meeting. Poster no. 0981.Search in Google Scholar
© 2021 Dragos Vladimir Budei et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


