Abstract
The one-pot acid-catalyzed the conversion of furfuryl alcohol (FA) to ethyl levulinate (EL) was investigated in the presence of mesoporous aluminosilicate (TUD-1) with a high surface area (up to 579 m2 g−1) and well-interconnected mesospheres synthesized via a solvothermal process and characterized using scanning electron microscope, transmission electron microscope, X-ray diffraction, 27Al-NMR, and N2 sorption isotherm. The resulting solid acid catalyst was tested for the alcoholysis of FA with ethanol, affording 87.8% EL yield under the optimal reaction conditions of 120°C and 4 h. Moreover, the catalyst showed a good reusability with less loss of activity after a simple solvent washing and calcination procedure.
1 Introduction
The conversion of renewable biomass resources into nonpetroleum derived fuels and chemicals is becoming increasingly attractive as a way to avoid intensification of global warming and to diversify energy sources [1,2,3,4,5,6]. Among the biomass carbohydrate-derived chemicals, alkyl levulinates have received particular attention due to their oxygenate fuel additive characteristics [7,8,9]. Ethyl levulinate (EL), especially, can not only be used up to 5 wt% as the diesel miscible biofuel but also has found various potential applications in flavoring and fragrance industry [10,11,12,13,14]. In general, EL is obtained by acid catalyzed esterification of levulinic acid with alcohol [15]. However, levulinic acid is a high cost raw material for this purpose. A number of techniques have been developed for the production of EL from a wide range of sources such as cellulose, saccharides, and furfuryl alcohol (FA). FA, one of the most important furan derivatives, is used as a model compound of biomass-derivatives and contains carbon–oxygen bonds both within and outside the furan ring and produced industrially via hydrogenation of furfural derived from the hydrolysis and dehydration of xylan contained in the lignocellulosic biomass [16,17,18,19]. It has been proved that EL can be effectively obtained through ethanolysis of FA over acidic catalysts [20,21].
Earlier researchers used strong and corrosive homogeneous acids such as HCl and H2SO4 as effective catalysts for the alkyl levulinates production [22,23,24]. Currently, a series of efficient solid acid including acidic ion-exchange resins, sulfated oxides, supported heteropolyacids, and zeolites has been successfully developed and utilized [25,26,27,28,29,30,31,32,33,34]. Acidic ion-exchange resins are organic-solid acids, less corrosive, easier/safer to handle than liquid acids, whereas the regeneration and reuse of organic-solid acid catalysts in these systems may be seriously compromised by their limited thermal and chemical stabilities [35,36,37,38,39,40]. The sulfated oxides and supported heteropolyacid catalysts may sustain coke burn off, but the leaching problem of acid sites during the reaction restricts the application of the two kinds of catalysts. Of the studied catalysts, zeolites or zeotype materials are quite promising and have been widely used in the conversion of biomass to biofuels or high value-added chemicals due to their tunable acidity and three-dimensional porosity structure [41,42]. However, the transformation of relatively bulky substrates as FA may be hindered in a microporous structure for large size and volume; hence, the use of mesoporous aluminosilicate (TUD-1) may be preferable for this reaction. Neves et al. [23] have reported the studies for the aluminosilicates and the sulfonic acid resin AmberlystTM-15 on the basis of EL yields, the undesirable formation, and catalyst stability.
Herein, based on the previous research, TUD-1 was explored as a solid acid catalyst for the alcoholysis of FA to form EL with the optimum reaction conditions, different treatment processes for recycling efficiency, and the activity of the catalyst using different alcohols as the starting material.
2 Materials and methods
2.1 Materials
Tetraethoxysilane (98%), aluminium isopropoxide (98.5%), ethanol (99.5%), isopropanol (99.9%), tetraethylammonium hydroxide (25 wt% in water), triethanolamine (99.0%), methanol (99.9%), 1-propanol (99.0%), 1-butanol (99.8%), and FA (98%) were purchased from Aladdin Reagent Co. Ltd (Shanghai, China). and used as received. All the deionized water used in the experiments was prepared in the laboratory.
2.2 Synthesis of TUD-1
Aluminium isopropoxide (1.4 g) was first dissolved in a mixture of isopropanol (10 g) and water (23 mL). Under vigorous stirring, tetraethoxysilane (35 g), triethanolamine (25 g), and tetraethylammonium hydroxide (31 g) were successively added. The sol obtained was stirred at room temperature for 24 h and dried at 98°C for 12 h, followed by the hydrothermal treatment in a Teflon-lined stainless steel autoclave at 180°C for 8 h. The solid products were separated from the system by filtration and washed with water for three times. Finally, the solid products were dried at 100°C for 12 h and calcined at 600°C in air for 6 h.
2.3 Catalyst characterization
Powder X-ray diffraction (XRD) patterns were collected on a D/MAX-2500 diffractometer (Rigaku Corporation, Japan) with Cu target (40 kV, 100 mA). Observation of the morphology of the samples was conducted using a JEM 7500 F (JEOL Ltd, Japan) field-emission scanning electron microscope and a JEM 2100 (JEOL Ltd, Japan) transmission electron microscope (TEM), respectively. 27Al-NMR spectroscopy was measured using a AVANCE Ⅲ HD 500 nuclear magnetic resonance spectrometer from Burker (Germany). Nitrogen sorption isotherms were measured using an ASAP 2420 (Micromeritics, USA) analyzer at −196°C. The acid properties were measured by Nexus-Thermo Nicolet FTIR (Thermo Nicolet Corporation, USA) instrument using pyridine as the basic probe molecule.
2.4 Catalytic performance and quantification of the products
To evaluate the catalytic performance of TUD-1, the conversion of FA into EL was carried out using these catalysts. The batch catalytic reactions were carried out in a 50 mL Hastelloy high pressure reactor under N2 atmosphere. A certain amount of TUD-1, FA, and ethanol were added to the reactor. Then, the reaction mixture was allowed to proceed under stirring and at 80–140°C for a certain time. Samples were taken from the reactor, filtrated, and analyzed in the aqueous phase by Agilent GC 7890A gas chromatograph (Agilent Technologies Inc., USA). For recycling experiments, the used catalyst was separated using centrifugation followed by thoroughly washing with ethanol, drying, and calcination at 600°C for 2 h before reuse [43]. The recovered catalyst was weighed, and a reaction was run using amounts of solvent and reactant proportional to the recovered catalyst amount to maintain the same substrate/catalyst ratio and substrate concentration.
The quantification of the concentrations of the reagents and products was based on the external standard calibration method. After separating the solid catalyst, the liquid phase of the reaction mixture was analyzed by GC with a HP-INNOWax polyethylene glyco column (The carrier gas was helium at a constant flow rate of 3.0 mL min−1; the primary oven temperature was programmed from 120 to 220°C at a heating rate of 15°C min−1). The FA conversion (C FA) and the yield of EL (Y EL) product were calculated according to equations (1) and (2). The C i , C T , and C EL were initial concentration of FA, concentration of FA at time T, and concentration of EL at time T.
3 Results and discussion
3.1 Catalyst characterization
TUD-1 was prepared using aluminum isopropoxide and tetraethoxysilane as Al and Si sources, respectively, and triethanolamine and tetraethylammonium hydroxide as the templates. The TEM images can reveal the particle morphology of the catalyst (Figure 1a), which confirmed the worm-like mesopore structure of the material. Figure 1b shows the SEM image of TUD-1. It can be observed that the TUD-1 is composed of large amounts of nanoparticles and exhibits an irregular shape [43].
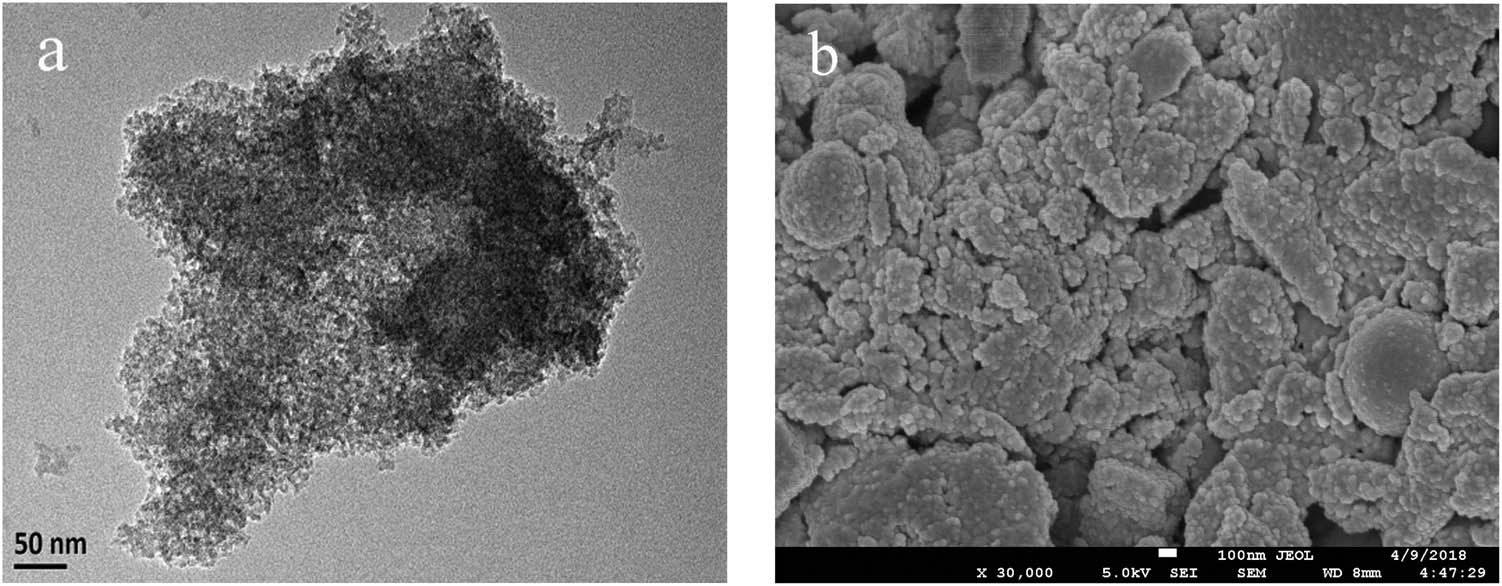
(a) TEM of TUD-1 and (b) SEM of TUD-1.
The powder XRD pattern of TUD-1 shows a very broad peak centered around 23° (Figure 2a), indicating the amorphous nature of the mesoporous structure material [44,45,46]. In addition, crystalline alumina or other phases cannot be detected in the pattern. The nature of Al was investigated using 27Al-NMR spectroscopy (Figure 2b). The spectrum exhibits a strong resonance at δ = 53.2 ppm, which can be attributed to tetrahedrally coordinated aluminium species. A high-field signal at δ = 0 ppm is attributed to hexacoordinate aluminium species [43].
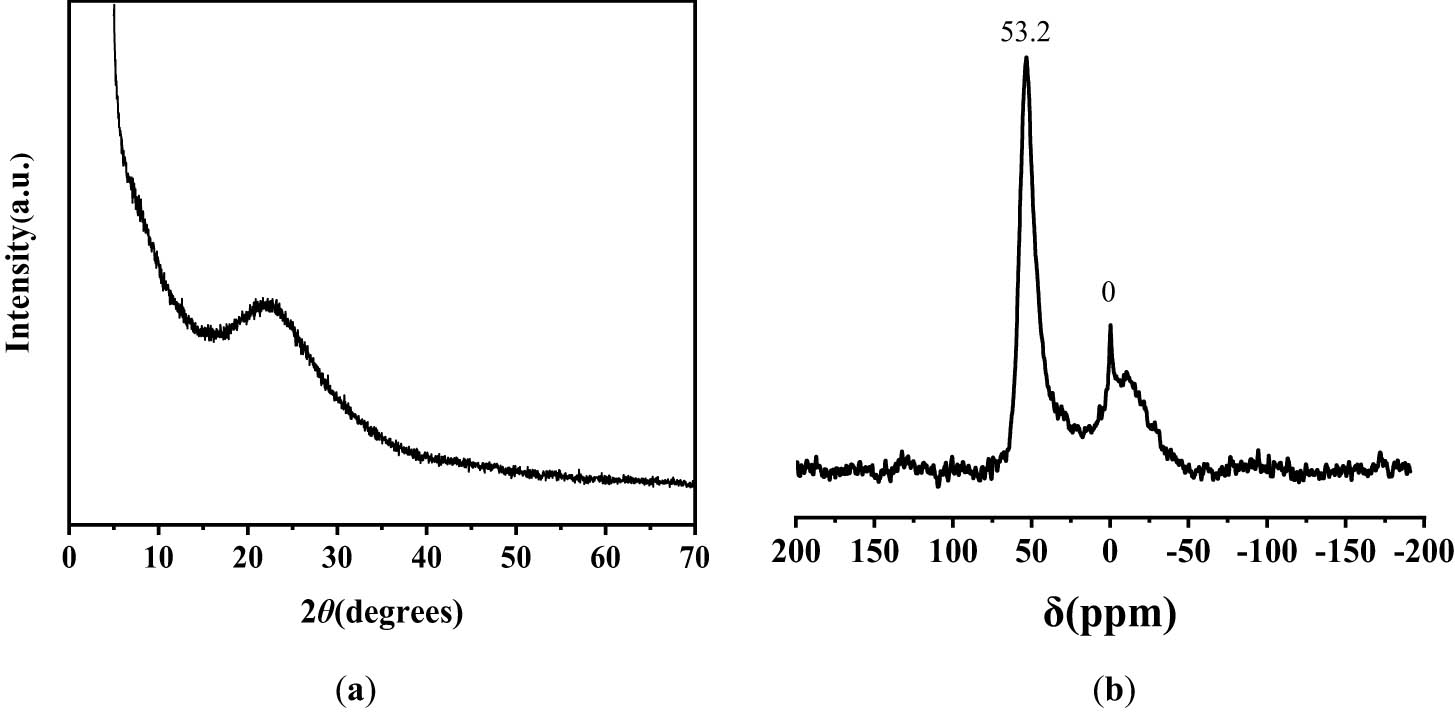
(a) XRD pattern of TUD-1 and (b) 27Al-NMR spectrum of TUD-1.
The textural properties of TUD-1 materials were studied by nitrogen gas porosimetry measurement. From the result shown in Figure 3, the pattern clearly displayed a typical type IV isotherm with a type H2 hysteresis loop, and the capillary condensation steps occur at the relative pressure P/P 0 = 0.6–0.7, which indicates that the tested samples possess mesoporosity with a disordered mesoporous material with an interconnected pore network [45,47,48,49,50,51]. Additional data, derived from the isotherm, illustrate that TUD-1 has a large BET specific surface area of 579 m2 g−1, a total pore volume of 1.03 cm2 g−1 and an average pore diameter of 6.5 nm. Infrared spectroscopy of adsorbed pyridine were observed to distinguish the Brønsted (around 1,545 cm−1) and Lewis acid sites (around 1,450 cm−1) of the catalysts in Figure 4. The amount of acid sites (L + B) of TUD-1 was 0.15 mmol g−1 which was quantified at 150°C.
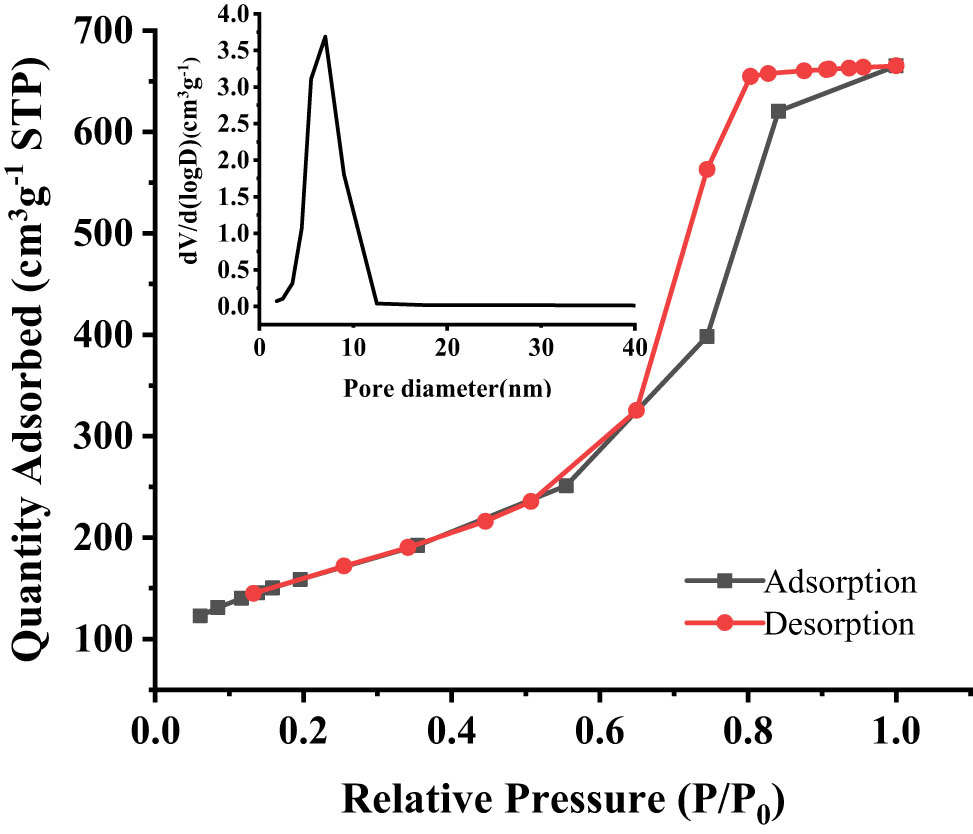
N2 adsorption–desorption isotherms of TUD-1. Inset: corresponding pore-size distribution.

Pyridine FT-IR spectra of TUD-1.
3.2 Catalytic tests
TUD-1 was used as a solid acid catalyst to prepare EL from FA. The effects of catalyst dosage, solvent amount, reaction time and reaction temperature on the conversion of FA to EL, and the reusability of solid acid catalyst were tested.
3.2.1 Effect of catalyst and ethanol amount
Initially, the catalyst dosage and solvent amount factors were investigated as shown in Table 1. From Entry 1–4, it can be found that when the catalyst dosage was increased from 0.5 to 1.2 g, the EL yield was changed from 76.1 to 88.3%. Notably, the EL yield of 88.3% was little higher than that of using the similar catalyst and reacting for 24 h reported elsewhere [23]. The EL yields were almost the same for the catalyst dosages of 1.0 and 1.2 g and complete FA conversions were obtained for all the cases. This may be because beyond a certain dosage, the number of catalyst sites was greater than that actually required by reactant molecules. Therefore, further investigation was conducted at 1.0 g catalyst dosage.
Effects of catalyst dosage and solvent amount on the reaction
| Entry | Catalyst (g) | Ethanol amount (mL) | EL yield1 (%) |
|---|---|---|---|
| 1 | 0.2 | 20 | 55.2 |
| 2 | 0.4 | 20 | 60.0 |
| 3 | 0.5 | 20 | 76.1 |
| 4 | 0.8 | 20 | 83.1 |
| 5 | 1.0 | 20 | 87.8 |
| 6 | 1.2 | 20 | 88.3 |
| 7 | 1.0 | 10 | 73.5 |
| 8 | 1.0 | 30 | 87.5 |
| 9 | 10 (gcat dm−3) | 1 | 80.02 [23] |
1Reaction conditions: FA 2 mL, temperature 120°C, time 4 h, 300 rpm, and N2 atmosphere. FA conversions were 100% for all the above cases. 2Reaction conditions: catalyst Al-TUD-1, [FA]0 = 0.3 M, temperature 140°C, and time 24 h.
Moreover, the amount of ethanol used in EL synthesis is also important. As FA easily polymerizes to form oligomeric products under the condition of acids at high temperature, the alcoholysis of FA is always carried out in a large excess of alcohols to minimize this undesired reaction. As shown in Table 1, the maximum EL yield was obtained from 20 mL of ethanol.
3.2.2 Effect of reaction temperature and reaction time
The effect of temperature on EL yield was studied in the range of 80–140°C (Figure 5a). It can be seen that the EL yield was enhanced significantly from 13.1 to 87.8% as the reaction temperature was gradually elevated from 80 to 120°C, suggesting a kinetically controlled reaction. However, further increasing the reaction temperature to 140°C led to a slight decrease in EL yield, which may be attributed to the increase of some oligomeric by-products at a higher temperature.
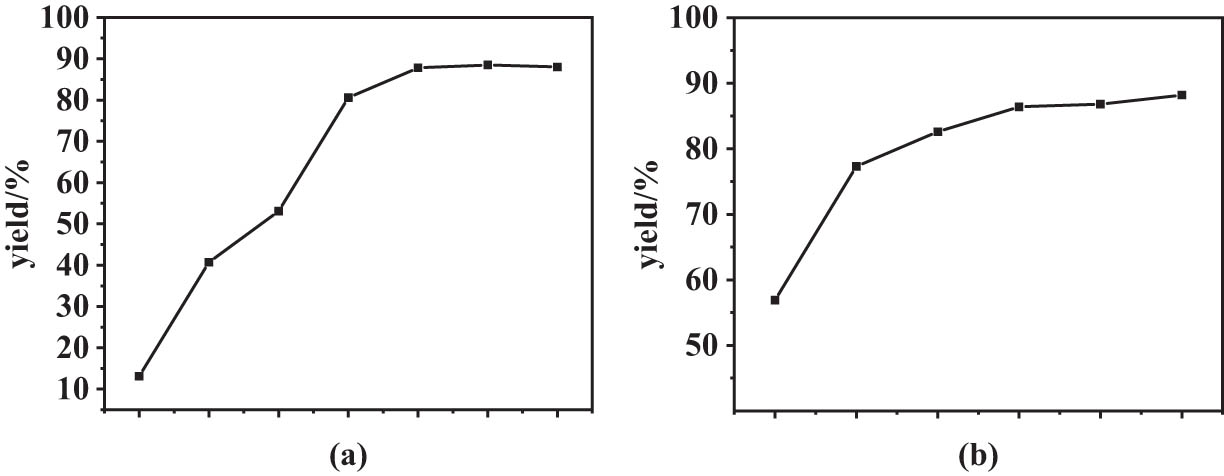
(a) Effect of reaction time and (b) reaction temperature on FA.
To study the kinetic aspects of reaction, the alcoholysis experiments were performed within reaction time ranging from 1 to 6 h. Figure 5b showed the yield of EL within 2 h increased greatly from 0 to 77.3% and remained high value of 86–88% in the latter 4 hours. The results demonstrated that the optimal reaction time for the alcoholysis of FA was 4 h.
3.3 Catalyst reusability studies
In contrast to traditional homogeneous catalysts such as liquid acid catalysts, heterogeneous catalysts show advantages in the separation and reusability. The reusability of TUD-1 catalyst was tested by conducting seven consecutive 4 h batches of the FA reaction including the fresh one. In the first four runs, the catalyst was recovered by centrifugation after the completion of the reaction, washed by ethanol, and dried in an oven to reuse in the next run of the reaction. In the latter three runs, calcination was added after the catalyst drying procedure. As evident from Figure 6, the alcoholysis efficiency decreased slightly in the first four runs. The EL yield decreased from 87.8% of fresh catalyst to 77.0% of reused catalyst for the third time. The results may be partly due to catalyst surface passivation by organic by-products such as oligomeric products or humins from the FA polymerization [52]. Organic by-products become increasingly difficult to remove by simple solvent washing. As the introduction of calcination procedure to the catalyst treatment process, the regenerated catalyst could nicely regain its activity, similar to that of the fresh catalyst, indicating its good stability and reusability.
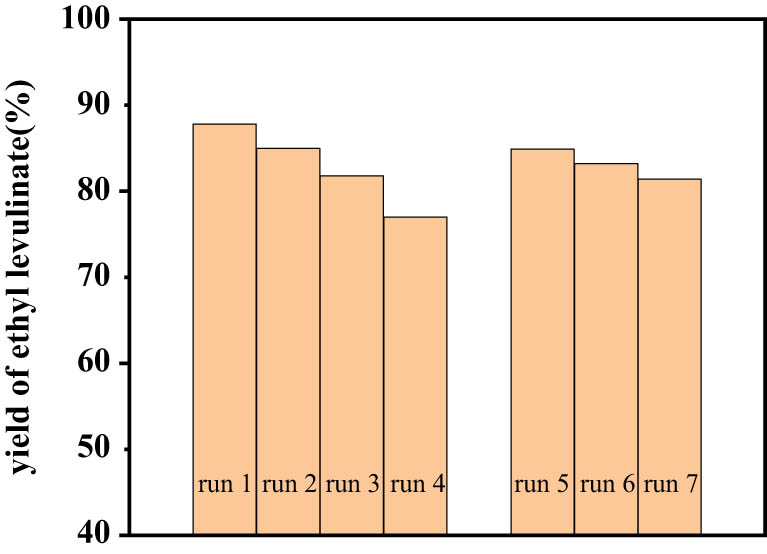
Effect of recycled TUD-1 on yield of EL. Reaction condition: the first four runs washed by ethanol, and the latter three calcined at 600°C for 6 h.
To investigate the versatility of TUD-1 catalyst, we have replaced the ethanol with different alcohols such as methanol, propanol, and butanol to get various levulinate esters. It was observed that for all these alcohols, the yields of levulinate ester were high, whereas as we go from methanol to butanol, the carbon chain length increases, simultaneously the yield of ester and conversion of FA decreases due to the reduction of reaction activity caused by the steric factor (Figure 7). For butanol, we have got 65% ester product with 86% conversion under optimized reaction conditions.
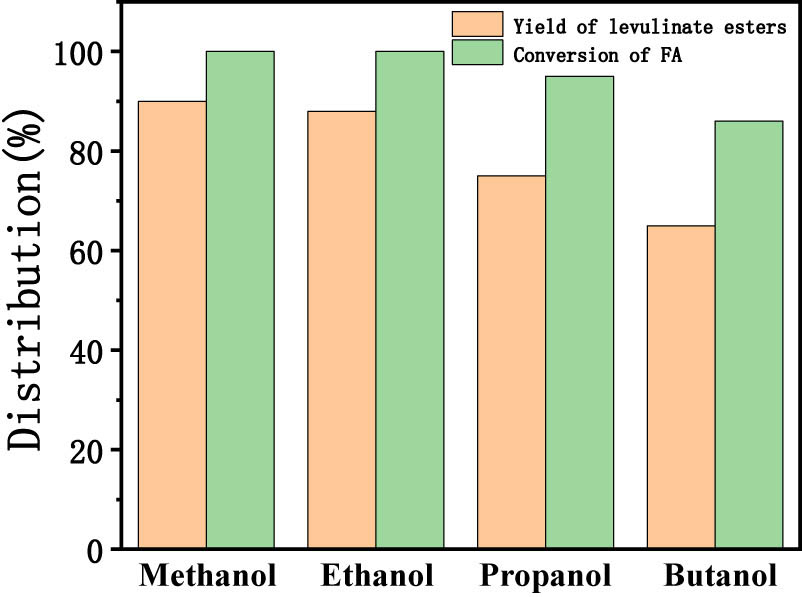
Yield of levulinate esters and conversion of FA with different alcohol. Reaction conditions: catalyst 1.0 g, alcohol 20 mL, FA 2 mL, temperature 120°C, time 4 h, 300 rpm, and N2 atmosphere.
4 Conclusion
A novel kind of TUD-1 was successfully synthesized and turned out to be an active catalyst for the reaction of FA with ethanol to obtain EL. A high EL yield of 87.8% under the optimal conditions (N2 atmosphere, 1.0 g catalyst, 120°C, 300 rpm, and 4 h) was obtained. In addition, the solid acid catalyst shows a good recyclability and could be reused for seven consecutive runs without obvious activity loss. As FA can be produced from renewable resources, this method is environmentally benign and economical for the conversion of biomass-based derivate into fine chemicals. More applications of this solid acid catalyst in sustainable chemistry should be researched in the future.
Acknowledgements
This study was supported by Key Laboratory of Sinopec and Dalian Research Institute of Petroleum and Petrochemicals. Thanks for the help in catalyst characterization.
-
Funding information: This research was funded by special Key Laboratory of Sinopec, grant number KL19009.
-
Author contributions: Yuli Bai – conceptualization; Tong Zhang – conceptualization; Yi Liu – data curation; Fudong Bai – formal analysis; Qimei Sun – investigation; and Lanpeng Li – resources.
-
Conflict of interest: The authors declare no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: All data, models, and code generated used during the study appear in the submitted article.
References
[1] Bozell J, Petersen G. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem. 2010;12(4):539–54.10.1039/b922014cSearch in Google Scholar
[2] Corma A, Iborra S, Velty A. Chemical routes for the transformation of biomass into chemicals. Chem R. 2007;107(6):2411–502.10.1021/cr050989dSearch in Google Scholar PubMed
[3] Chheda JN, Román-Leshkov Y, Dumesic JA. Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono- and poly-saccharides. Green Chem. 2007;9(4):342–50.10.1039/B611568CSearch in Google Scholar
[4] Oh YH, Eom IY, Joo JC, Yu JH, Song BK, Lee SH, et al. Recent advances in development of biomass pretreatment technologies used in biorefinery for the production of bio-based fuels, chemicals and polymers. Korean J Chem Eng. 2015;32(10):1945–59.10.1007/s11814-015-0191-ySearch in Google Scholar
[5] Khemthong P, Yimsukanan C, Narkkun T, Srifa A, Witoon T, Pongchaiphol S, et al. Advances in catalytic production of value-added biochemicals and biofuels via furfural platform derived lignocellulosic biomass. Biomass Bioenergy. 2021;148:106033.10.1016/j.biombioe.2021.106033Search in Google Scholar
[6] Berruti PF. Biomass valorization for fuel and chemicals production – a review. Int J Chem React Eng. 2008;6(1):1–49.10.2202/1542-6580.1674Search in Google Scholar
[7] Climent MJ, Corma A, Iborra S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Cheminform. 2014;16(2):516–47.10.1039/c3gc41492bSearch in Google Scholar
[8] Démolis A, Essayem N, Rataboul F. Synthesis and applications of alkyl levulinates. ACS Sus Chem Eng. 2014;2(6):1338–52.10.1021/sc500082nSearch in Google Scholar
[9] Zhang Z, Dong K, Zhao ZK. Efficient conversion of furfuryl alcohol into alkyl levulinates catalyzed by an organic-inorganic hybrid solid acid catalyst. Chem Sus Chem. 2011;4(1):112–8.10.1002/cssc.201000231Search in Google Scholar PubMed
[10] Pierre G. Conversion of biomass to selected chemical products. Chem Soc Rev. 2012;41:1538–58.10.1039/C1CS15147ASearch in Google Scholar PubMed
[11] Budarin VL, Clark JH, Henschen J, Farmer TJ, Macquarrie DJ, Mascal M, et al. Processed lignin as a byproduct of the generation of 5-(chloromethyl)furfural from biomass: a promising new mesoporous material. Chem Sus Chem. 2015;8(24):4172–9.10.1002/cssc.201501319Search in Google Scholar PubMed
[12] Christensen E, Williams A, Paul S, Burton S, McCormick RL. Properties and performance of levulinate esters as diesel blend components. Energy Fuels. 2011;25(11):5422–8.10.1021/ef201229jSearch in Google Scholar
[13] Mei C, Dumesic JA. Liquid-phase catalytic transfer hydrogenation and cyclization of levulinic acid and its esters to γ-valerolactone over metal oxide catalysts. Chem Commun. 2011;47(44):12233–5.10.1039/c1cc14748jSearch in Google Scholar
[14] Serrano-Ruiz JC, Dong W, Dumesic JA. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010;12(4):574–7.10.1039/b923907cSearch in Google Scholar
[15] Serrano-Ruiz JC, Dumesic JA. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Env Sci. 2011;4(1):83–99.10.1201/b18526-11Search in Google Scholar
[16] Moreau C, Durand R, Peyron D, Duhamet J, Rivalier P. Selective preparation of furfural from xylose over microporus solid acid catalysts. Ind Crop Product. 1998;7(2):95–9.10.1016/S0926-6690(97)00037-XSearch in Google Scholar
[17] Bond JQ, Alonso DM, Wang D, West RM, Dumesic JA. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science. 2010;327(5969):1110–4.10.1126/science.1184362Search in Google Scholar PubMed
[18] Horváth IT, Mehdi H, Fábos V, Boda L, Mika LT. γ-Valerolactone—a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008;10(2):238–42.10.1039/B712863KSearch in Google Scholar
[19] Dam H, Kieboom A, Bekkum HV. The conversion of fructose and glucose in acidic media: formation of hydroxymethylfurfural. Starch-Strke. 1986;38(3):95–101.10.1002/star.19860380308Search in Google Scholar
[20] Gobara HM, Aboutaleb WA, Hashem KM, Hassan SA, Henein SA. A novel route for synthesis of alpha-Fe2O3-CeO2 nanocomposites for ethanol conversion. J Mater Sci. 2017;52:550–68.10.1007/s10853-016-0353-2Search in Google Scholar
[21] González Maldonado GM, Assary RS, Dumesic JA, Curtiss LA. Acid-catalyzed conversion of furfuryl alcohol to ethyl levulinate in liquid ethanol. Energy Environ Sci. 2012;5(10):8990–7.10.1039/c2ee22486kSearch in Google Scholar
[22] An S, Song D, Lu B, Yang X, Guo YH. Morphology tailoring of sulfonic acid functionalized organosilica nanohybrids for the synthesis of biomass-derived alkyl levulinates. Chem Eur J. 2015;21(30):10786–98.10.1002/chem.201501219Search in Google Scholar PubMed
[23] Neves P, Lima S, Pillinger M, Rocha SM, Rocha J, Valente AA. Conversion of furfuryl alcohol to ethyl levulinate using porous aluminosilicate acid catalysts. Catal Today. 2013;218:76–84.10.1016/j.cattod.2013.04.035Search in Google Scholar
[24] Lake MA, Burton SW, Fuller WC, Sasser R, Lindstrom ME, Wheless JT, et al. Production of levulinic acid and levulinate esters from biomass; 2010. US20100312006.Search in Google Scholar
[25] Islam MM, Bhunia S, Molla RA, Bhaumik A, Islam SM. Organic solid acid catalyst for efficient conversion of furfuryl alcohol to biofuels. Chem Sel. 2016;1(19):6079–85.10.1002/slct.201601285Search in Google Scholar
[26] Fernandes DR, Rocha AS, Mai EF, Mota CJA, Teixeira da Silva V. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl Catal A. 2012;425–426:199–204.10.1016/j.apcata.2012.03.020Search in Google Scholar
[27] Lange JP, van de Graaf WD, Haan RJ. Conversion of furfuryl alcohol into ethyl levulinate using solid acid catalysts. Chem Sus Chem. 2009;2(5):437–41.10.1002/cssc.200800216Search in Google Scholar PubMed
[28] Akiyama G, Matsuda R, Sato H, Takata M, Kitagawa S. Cellulose hydrolysis by a new porous coordination polymer decorated with sulfonic acid functional groups. Adv Mater. 2011;23(29):3294–7.10.1002/adma.201101356Search in Google Scholar PubMed
[29] Yamada T, Otsubo K, Makiura R, Kitagawa H. Designer coordination polymers: dimensional crossover architectures and proton conduction. Chem Soc Rev. 2013;42(16):6655–69.10.1039/c3cs60028aSearch in Google Scholar PubMed
[30] Colin SC, Paul AC. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem Rev. 2003;103(3):663–702.10.1021/cr020060iSearch in Google Scholar PubMed
[31] Tu J, Ding M, Zhang Q, Zhang Y, Wang C, Wang T, et al. Design of carbon‐encapsulated Fe3O4 nanocatalyst with enhanced performance for Fischer–Tropsch synthesis. ChemCatChem. 2015;7(15):2323–7.10.1002/cctc.201500332Search in Google Scholar
[32] Dutta A, Patra AK, Uyama H, Bhaumik A. Template-free synthesis of a porous organic-inorganic hybrid tin(IV) phosphonate and its high catalytic activity for esterification of free fatty acids. Acs Appl Mater Inter. 2013;5(20):9913–7.10.1021/am402714rSearch in Google Scholar PubMed
[33] Su Y, Wang Y, Li X, Li X, Wang R. Imidazolium-based porous organic polymers: anion exchange-drived rapid capture and luminescent probe of Cr2O72−. Acs Appl Mate Inter. 2016;8(29):18904–11.10.1021/acsami.6b05918Search in Google Scholar PubMed
[34] Zhao G, Sun Y, Zeng X, Lin L. Efficient conversion of furfuryl alcohol into ethyl levulinate in an extremely low sulfuric acid catalyst system. J Bioprocess Eng Biorefinery. 2013;2(2):182–7.10.1166/jbeb.2013.1053Search in Google Scholar
[35] Zuo Y, Zhang Y, Fu Y. Catalytic conversion of cellulose into levulinic acid by a sulfonated chloromethyl polystyrene solid acid catalyst. ChemCatChem. 2014;6(3):753–7.10.1002/cctc.201300956Search in Google Scholar
[36] Tiwari MS, Dicks JS, Keogh J, Ranade VV, Manyar HG. Direct conversion of furfuryl alcohol to butyl levulinate using tin exchanged tungstophosphoric acid catalysts. Mole Catal. 2020;488:110918.10.1016/j.mcat.2020.110918Search in Google Scholar
[37] Bhat NS, Mal SS, Dutta S. Recent advances in the preparation of levulinic esters from biomass-derived furanic and levulinic chemical platforms using heteropoly acid (HPA) catalysts. Mole Catal. 2021;505:111484.10.1016/j.mcat.2021.111484Search in Google Scholar
[38] Neves P, Russo PA, Fernandes A, Antunes MM, Farinha J, Pillinger M, et al. A mesoporous zirconia-based mixed oxides as versatile acid catalysts for producing bio-additives from furfuryl alcohol and glycerol. Appl Catal A. 2014;487:148–57.10.1016/j.apcata.2014.08.042Search in Google Scholar
[39] Zhao G, Liu M, Xia X, Li L, Xu B. Conversion of furfuryl alcohol into ethyl levulinate over glucose-derived carbon-based solid acid in ethanol. Molecules. 2019;24(10):1881.10.3390/molecules24101881Search in Google Scholar PubMed PubMed Central
[40] Simons C, Hanefeld U, Arends IWCE, Sheldon RA, Maschmeyer T. Noncovalent anchoring of asymmetric hydrogenation catalysts on a new mesoporous aluminosilicate: application and solvent effects. Chemistry. 2010;10(22):5829–35.10.1002/chem.200400528Search in Google Scholar PubMed
[41] Zhou J, Hua Z, Shi J, He Q, Guo L, Ruan M. Synthesis of a hierarchical micro/mesoporous structure by steam-assisted post-crystallization. Chem Eur J. 2009;15(47):12949–54.10.1002/chem.200901034Search in Google Scholar PubMed
[42] Anand R, Maheswari R, Hanefeld U. Catalytic properties of the novel mesoporous aluminosilicate AlTUD-1. J Catal. 2006;242(1):82–91.10.1016/j.jcat.2006.05.022Search in Google Scholar
[43] Ramli N, Amin N. Fe/HY zeolite as an effective catalyst for levulinic acid production from glucose: characterization and catalytic performance. Appl Catal B Env. 2015;163:487–98.10.1016/j.apcatb.2014.08.031Search in Google Scholar
[44] Xu Y, Zheng X, Yu H, Hu X. Hydrothermal liquefaction of Chlorella pyrenoidosa for bio-oil production over Ce/HZSM-5. Bioresour Tech. 2014;156(1):1–5.10.1016/j.biortech.2014.01.010Search in Google Scholar PubMed
[45] Jansen JC, Shan Z, Maschmeyer T, Marchese L, Zhou W, Puil N. A new templating method for three-dimensional mesoporenetworks. Chem Commun. 2001;8:713–4.10.1039/b101000jSearch in Google Scholar
[46] Telalovi S, Hanefeld U. Noncovalent immobilization of chiral cyclopropanation catalysts on mesoporous TUD-1: comparison of liquid-phase and gas-phase ion-exchange. Appl Catal A. 2010;372(2):217–23.10.1016/j.apcata.2009.10.038Search in Google Scholar
[47] Blin JL, Léonard A, Su BL. Well-ordered spherical mesoporous materials CMI-1 synthesized via an assembly of decaoxyethylene cetyl ether and TMOS. Chem Mater. 2001;13(10):3542–53.10.1021/cm0011965Search in Google Scholar
[48] Russell SD, Douglas SB, Todd JL. A new adsorption model for analyzing gassolid equilibria in porous materials. J Phys Chem B. 1996;100(5):1718–24.10.1021/jp9511616Search in Google Scholar
[49] Webster CE, Drago RS, Zerner MC. Molecular dimensions for adsorptives. JACS. 1998;120(22):5509–16.10.1021/ja973906mSearch in Google Scholar
[50] Lima S, Antunes MM, Fernandes A, Pillinger M, Ribeiro MF, Valente AA. Catalytic cyclodehydration of xylose to furfural in the presence of zeolite H-Beta and a micro/mesoporous Beta/TUD-1 composite material. Appl Catal A. 2010;388(1–2):141–8.10.1016/j.apcata.2010.08.040Search in Google Scholar
[51] Antunes MM, Lima S, Fernandes A, Pillinger M, Ribeiro MF, Valente AA. Aqueous-phase dehydration of xylose to furfural in the presence of MCM-22 and ITQ-2 solid acid catalysts. Appl Catal A. 2012;417–418:243–52.10.1016/j.apcata.2011.12.046Search in Google Scholar
[52] Peng L, Li H, Xi L, Chen K, Chen H. Facile and efficient conversion of furfuryl alcohol into n-butyl levulinate catalyzed by extremely low acid concentration. Bioresour. 2014;9(3):3825–34.10.15376/biores.9.3.3825-3834Search in Google Scholar
© 2021 Yuli Bai et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


