Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
-
Thien Hien Tran
, Thi Kim Ngan Tran
Abstract
Diversification of products that are derived from essential oils carries important implications in reducing agricultural waste and promoting the medicinal materials industry. In this study, we formulated a shampoo and a body wash product incorporated with lemongrass (Cymbopogon citratus) essential oils (LEOs) and evaluated their color stability and the LEO compositional change. We first determined the color change and chemical composition of bare LEO under different storage conditions. Afterward, the washing product base was formulated, and its formulation process was optimized to minimize the color change by varying a wide range of parameters including pH, the inclusion of preservatives and antioxidants, LEO/antioxidant ratio, and emulsification temperature. The base product was then used in body wash and shampoo formulation following our previously reported procedure. The results indicated that direct incorporation of the LEO into the cosmetic products resulted in better color stability and citral retention in comparison with emulsion formation. In addition, shampoo and body wash products showed no detectible presence of compounds resulting from citral decomposition such as 3,7-dimethyl-1,3,6-octatriene, p-mentha-1,5-dien-8-ol, and p-cymene-8-ol. The current findings are expected to aid in diversifying LEO-derived commodities and justifying scalability of the cosmetics production process with a focus on the incorporation of naturally derived ingredients.
1 Introduction
Lemongrass (Cymbopogon citratus) is a common plant ingredient that is used widely in daily applications and in folk medicine [1,2,3,4]. The main product derived from the lemongrass plant, lemongrass essential oil (LEO), is also a commodity that is widely used in the food industry due to its predominant content of citral in its composition, which confers the LEO with potent antibacterial activity and pleasant, favorable aroma [5,6,7,8]. Additionally, the LEO is also a common material in the manufacture of medicinal products such as antifungal agents, antidepressants, and indigestion remedies.
Despite that, the increasingly rapidly growing area of lemongrass has brought about excess output on both the raw material and LEO, causing difficulty in solving the surplus LEO and calling for diversification of products that are mainly or partially derived from LEO. Apart from medicinal products, products that are manufactured with LEO as a major component are quite limited in Vietnam, mostly comprising insect repellent products and a relaxation agent. As a result, new and novel attempts to incorporate LEO into consumers’ products such as personal care and home products are essential to the valorization of lemongrass and contribute to ease the burden of lemongrass output in the upcoming years [9,10].
The main challenge in introducing LEO into consumer products is that the citral component in LEO is highly susceptible to conversion by acid catalysts and oxidative degradation, especially in the presence of light and heat, leading to the formation of the intensity change of flavor [11,12]. The decomposition of citral is more expedited at a higher temperature, light, and available oxygen in this compound and may produce other compounds such as p-cymene, p-cymene-8-ols, p-mentha-1,5-dien-8-ol, p-menthadien-8-ol, α,p-dimethylstyrene, p-methylacetophenone, and p-cresol, which may further alter the aroma intensity of LEO [12,13,14,15,16,17,18]. To address this issue, various measures have been devised to prevent or mitigate the decomposition of citral, including the use of spray-drying technique, the formation of oil-in-water emulsions, and the manufacture of micelles and reverse micelles to stabilize citral in the oil phase [19,20,21]. However, such techniques are more labor-intensive and require modern instruments, thus considerably escalating manufacturing costs and in turn affecting consumers’ acceptance due to the high price sensitivity of demands for home products.
Driven by the aforementioned trusts, this study aims to evaluate the stability of LEO in the formulation of various cosmetic products. We first evaluated the color change and chemical composition of LEO under different storage conditions. Then, the formulating process of a washing product base was optimized to minimize the color change in the base. The best product base was then used in the formulation of two cosmetic products, including body wash and shampoo, following our previously reported procedure. Finally, the two products were then evaluated for the LEO volatile composition and color change. The findings are expected to aid in diversifying LEO-derived commodities and justifying the scalability of the cosmetics production process with a focus on the incorporation of naturally derived ingredients.
2 Materials and methods
2.1 Materials
LEO was obtained by steam distillation of the leaves of emongrass (Cymbopogon citratus) harvested from Tan Phu Dong district, Tien Giang province, Vietnam (Coordinates 10°15′N 106°39′E). The extraction apparatus was of industrial scale and operated under the following conditions: extraction time, 3 h; material quantity, 639–710 kg per batch. The highest LEO yield was 0.273% (v/w).
2.2 Formulation of simulated product base incorporated with LEO
Formulation of the product base was realized by using base oil, emulsifier, or the lack thereof. The simulated product base was formulated by using four ingredients including LEO, preservatives, additives (base oil, emulsifier, or none), antioxidants, and water. The LEO (and additive) mixture was first mixed with preservatives and antioxidants. The afforded mixture was introduced into heated water (70–80°C) under stirring. The resulting mixture was then allowed to naturally cool and homogenized.
The formulation process was first investigated with respect to different base oils (PEG-40 hydrogenated castor oil (PEG-400), paraffin oil, and none), varying emulsification temperature (room temperature to 90°C), and different emulsifiers (Tween 80, Tween 20, PEG 40, and none). After determining the appropriate formulation technique, the process was then further optimized by experimenting at different pH values (4–7), different preservatives (sodium benzoate, sodium lactate, DMDM hydantoin (DMDM-H)), antioxidants (butylated hydroxyanisole (BHA), and butylated hydroxy toluene (BHT)), and antioxidant/LEO ratio (0.5:1–2.5:1).
2.3 Formulation of body wash and shampoo products incorporated with LEO
The body wash formulation process was carried out by using the product base following a previous report [20]. Briefly, the product base was separately formulated with the composition and conditions that were determined in previous investigations. Then, another mixture consisting of the main detergent, detergent adjuvant, thickener, foaming agent, humectant, preservative, and skin emollient was prepared separately before being introduced into the mixture. Afterward, NaCl 25% and citric acid 30% were added to the mixture. Finally, the body wash was cooled and poured into a bottle for further evaluation.
The shampoo product was formulated similarly to the body wash formulation, except that the BHT/LEO ratio in the washing base was fixed at 0.5:1 (w/w) [19].
2.4 Determination of pH and the color change
The MP220 pH meter (Mettler Toledo) was used to determine the pH of the sample. The product samples were diluted 100 times before the pH measurement for accurate determination.
The color of the samples was measured by using a CR-400 colorimeter (Konica Minolta, Japan). The liquid product was stored in a glass cuvette placed in a dark chamber for the measurement. The color of the product was determined by the color space L*a*b*. The L*a*b* color space is spherical with three axes: L, a, b. The a-axis runs from −a* (green) to +a* (red) and the b-axis runs from −b* (light green) to +b* (yellow). The brightness axis L* is valid from 0 (black at the bottom) to 100 (white at the top).
The color differences between samples or time points are determined as follows:
where L, a, and b represent lightness, a-axis, and b-axis, respectively, Subscripts 2 and 1 represent after and before color change, respectively, and
2.5 Determination of chemical composition
LEOs present in the product samples were first recovered by using hydrodistillation before the samples were used to determine the chemical composition. Briefly, 100 g of the sample was first introduced in water in the ratio of 1:4 (w/w). Hydrodistillation was carried out at a temperature of 120°C until no essential oil could be recovered from the apparatus.
Gas chromatography-mass spectrometry (GC-MS) was adopted to determine the composition of LEO. About 25 μL of the essential oil was diluted in 1.0 mL of n-hexane and dehydrated with Na2SO4 salt. The equipment used was GC Agilent 6890 N (Agilent Technologies, Santa Clara, CA, USA), with MS 5973, HP5-MS column, and column head pressure of 9.3 psi. GC-MS was installed under the following conditions: He carrier gas; flow rate, 1.0 mL/min; split line, 1:100; injection volume, 1.0 μL; and injection temperature, 250°C. The initial temperature was kept at 50°C for 2 min; the oven temperature increased to 80°C at a speed of 2°C/min, from 80 to 150°C at a speed of 5°C/min, from 150 to 200°C at a speed of 10°C/min, from 200 to 300°C at a speed of 20°C/min, and maintained at 300°C for 5 min.
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Color and compositional changes of LEO during storage
The obtained LEO via hydrodistillation was light yellow in color with a characteristically strong citrusy and lemony aroma. The color changes of different LEO samples are detailed in Table 1. At ambient temperature, the color of LEO tended to become darker as the storage time prolonged, yielding the ΔE values of 0.82 and 3.63 for LEO stored after 1 week and 1 month, respectively. However, visual examination of LEO samples revealed that these color changes are indiscernible due to the insignificant change in the intensity of the L index. By contrast, storage at elevated temperature seemed to cause more pronounced darkening in samples, reflected by significantly higher ΔE values compared to their respective values of LEO measured at ambient temperature. The role of heat in accelerating citral transformation has been documented by Peacock and Kuneman [22]. On the other hand, the redistilled LEO displayed a much lighter yellow in color, less pungent aroma than other samples, and was obtained in a lower yield due to the mass loss of around 35.91% of the total essential oil. According to Weerawatanakorn et al. [23], aromatherapy compounds are susceptible to chemical changes that occur in different types of interactions, including oxidation, hydrolysis, thermal destruction, photochemical, and polymerization of unsaturated compounds, adversely affecting the overall fragrance quality of the product. Therefore, the storage temperature of 45°C was selected as the base condition in the subsequent experiment measuring compositional changes in product formulations (Figure 1).
Color changes of LEO under different storage and extraction conditions
| Index | Initial LEO | LEO after 1 week at ambient temperature | LEO after 1 month at ambient temperature | LEO after 1 week at 45°C | LEO after 1 month at 45°C | Redistilled LEO after 1 month at 45°C |
|---|---|---|---|---|---|---|
| L | 61.98 | 62 | 61.99 | 61.79 | 60.09 | 62.48 |
| a | −7.82 | −7.89 | −8.4 | −8.9 | −8.21 | −3.87 |
| b | 22.57 | 23.39 | 26.15 | 30.47 | 42.13 | 10.54 |
| ΔE | — | 0.82 | 3.63 | 7.15 | 16.09 | 20.57 |

Color change of LEO at different time points: (a) initial LEO; (b) after 1 week at room temperature; (c) after 1 month at room temperature; (d) after 1 week at 45°C; (e) after 1 month at 45°C; and (f) redistilled LEO.
The major compounds that were present in the initial LEO sample are summarized in Table 2. In total, 11 volatile compounds were identified, accounting for 99.79% of the total LEO content, of which the percentage of citral amounted to 86.90%, followed by β-myrcene (5.656%), and nerol (3.887%). Other components lower than 1% included 6-methyl-5-hepten-2-one, β-linalool, β-citronellol, geraniol acetate, β-caryophyllen, α-bergamotene, and selina-6-en-4-ol. The composition of LEO in the present sample is similar to that of a previous study where LEO extracted from the lemongrass materials of the same origin showed citral and β-myrcene contents of 79.33 and 16.65%, respectively [21]. The abundance of citral is in line with the result of another review that indicated that LEO contained at least 75% citral and other minor ingredients such as nerol, geraniol, citronellal, terpinolene, geranyl acetate, myrecene, and terpinol methylheptenone [24]. The beneficial effect of citral on the skin membrane was elaborated by a previous study where citral-rich LEO was found to exhibit potent antifungal activities against several yeasts of Candida species and did not cause skin irritation [25]. Furthermore, Modak and Mukhopadhaya [26] also demonstrated the antiobesity effects of citral in a rat model and suggested that the effect could be attributable to the influence of citral on energy production, thus reducing fat accumulation. The role of citral in the food industry was also highlighted by a previous finding suggesting that citral-containing nanoemulsion exhibited potent antibacterial and antibiofilm activity against Listeria monocytogenes, a common foodborne pathogen [27] (Figure 2).
Chemical compositions of LEO under different storage and extraction conditions
| No. | RT (min) | Compounds | Content (%) | |||
|---|---|---|---|---|---|---|
| Initial LEO | LEO after 1 week at 45°C | LEO after 1 month at 45°C | Redistilled LEO after 1 month at 45°C | |||
| 1 | 9.771 | 6-Methyl-5-hepten-2-one | 0.805 | 1.149 | 1.314 | 1.529 |
| 2 | 9.907 | β-Myrcene | 5.656 | 6.025 | 10.867 | 3.887 |
| 3 | 12.427 | 3,7-Dimethyl-1,3,6-octatriene | — | — | 0.395 | — |
| 4 | 16.097 | β-Linalool | 0.754 | 0.664 | 0.805 | 1.024 |
| 5 | 19.799 | p-Mentha-1,5-dien-8-ol | — | — | 0.768 | — |
| 6 | 20.667 | p-Cymene-8-ol | — | 0.288 | 1.154 | — |
| 7 | 22.727 | β-Citronellol | 0.499 | 0.547 | 0.572 | 0.681 |
| 8 | 23.166 | β-Citral | 39.031 | 38.699 | 35.026 | 38.697 |
| 9 | 23.710 | Nerol | 3.887 | 3.583 | 3.612 | 4.402 |
| 10 | 24.337 | α-Citral | 47.868 | 47.588 | 44.214 | 46.802 |
| 11 | 28.050 | Geraniol acetate | 0.602 | 0.361 | 0.525 | 1.610 |
| 12 | 28.980 | β-Caryophyllen | 0.296 | 0.339 | 0.324 | 0.357 |
| 13 | 29.482 | α-Bergamotene | 0.267 | 0.309 | 0.234 | 0.449 |
| 14 | 33.884 | Selina-6-en-4-ol | 0.115 | 0.200 | 0.189 | 0.313 |
| Citral content | 86.899 | 86.287 | 79.240 | 85.499 | ||
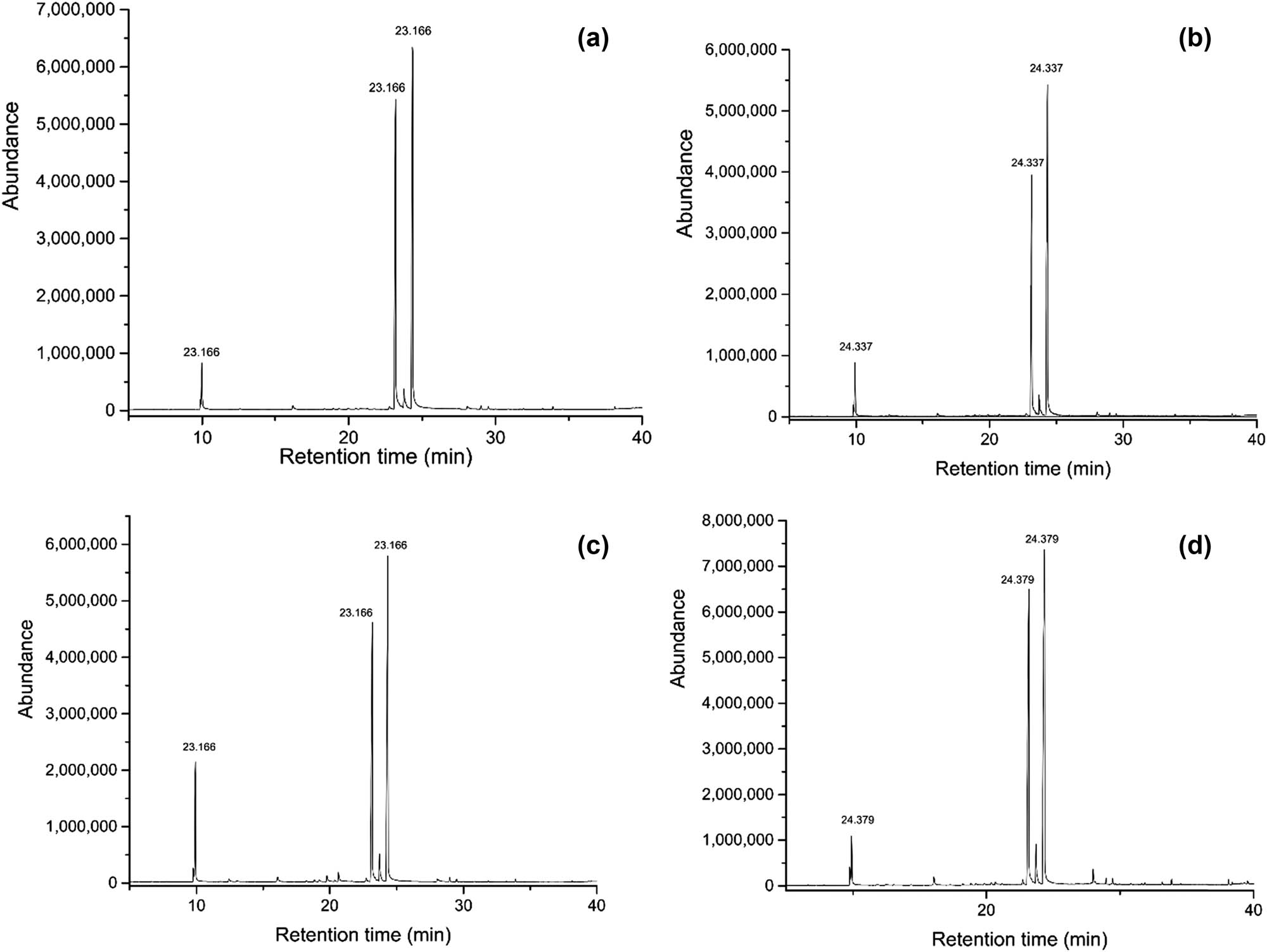
Chromatography of essential oil samples at different storage conditions: (a) initial; (b) after 1 week at 45; (c) after 1 month at 45; and (d) redistilled LEO.
The compositional stability of LEO was further evaluated by performing GC-MS analysis of LEO samples stored for a week, for a month at 45°C, and the redistilled LEO sample. In comparison with citral contents of the initial LEO, the 1-month LEO sample stored at 45°C showed a moderately reduced percentage in α- and β-citral contents, reaching 44.214 and 35.026% respectively. This LEO sample also indicated the presence of three new compounds that had not been previously detected in the initial LEO, including 3,7-dimethyl-1,3,6-octatriene (0.395%), p-1,5-menthadien-8-ol (0.768%), and p-cymen-8-ol (1,154%). However, after being redistilled, the three compounds were no longer detected in the LEO. These results imply that the oxidation of citral occurred during storage due to exposure to heat and light and are consistent with the results of Weerawatanakorn et al. [23] and Ueno et al. [28], which found that the oxidation products of citral, including p-menthadien-8-ol, α,p-dimethylstyrene, p-cymene, p-methylacetophenone, and p-cresol, resulted in a loss of flavor in the LEO.
In general, exposure to both heat and light and extended preservation have led to the degradation of citral content and color quality. Some compounds such as p-menthadien-8-ol and p-cymene were generated in the process, necessitating further investigation in preserving valuable compounds in LEOs when it is being formulated in cosmetic products.
3.2 Color of the product base during formulation
3.2.1 Effects of base oil, emulsifier, and emulsification temperature
Incorporation of LEO into cosmetic products is usually realized through two main approaches: direct mixing and via the formation of emulsions. In the first investigation (effect of base oil on the product color), formulation conditions consisted of the following: LEO content, 3%; BHT, 1.5%; PEG-40, 15%; sodium benzoate, 0.6%; sodium lactate, 2%; DMDM-H, 0.6%; and emulsification temperature, 70°C. All formulated product bases showed an aroma almost identical with that of the LEOs after short and constant storage conditions (7 days at 45°C).
Table 3 shows the color changes of simulated product base formulated at different temperatures, base oils (PEG-400, paraffin oil, and none), and emulsifiers (Tween 80, Tween 20, PEG-40, and none). Generally, two samples that were devoid of base oils or emulsifiers showed the lowest ΔE values, of 0.96 and 0.87, respectively, indicating that base oils and emulsifiers play a key role in inducing color changes in the product base formulations with LEO [29,30,31]. Further examination of the formulated bases showed that the LEO layering phenomenon emerged in the sample incorporated with paraffin. After 7 days of preservation at 45°C, bases formulated with PEG-400 and paraffin exhibited moderate discoloration toward yellow and revealed instability. Overall, the direct mixing of LEO into product bases seemed to result in little change in color in comparison with the emulsion route. Therefore, direct mixing of LEO would be done in subsequent experiments.
Color change of the washing product base after 7 days of storage at 45°C formulated at different base oils and emulsification conditions
| Base oil | Emulsification temperature (°C) | Emulsifier | ΔE |
|---|---|---|---|
| PEG-400 | 70 | PEG-40 | 2.35 |
| Paraffin oil | 70 | PEG-40 | 4.17 |
| None | 70 | PEG-40 | 0.96 |
| None | Room | PEG-40 | 1.54 |
| None | 50 | PEG-40 | 1.58 |
| None | 60 | PEG-40 | 1.13 |
| None | 70 | PEG-40 | 1.26 |
| None | 80 | PEG-40 | 1.75 |
| None | 90 | PEG-40 | 1.83 |
| None | 60 | Tween 80 | 1.98 |
| None | 60 | Tween 20 | 4.31 |
| None | 60 | PEG-40 | 1.73 |
| None | 60 | None | 0.87 |
3.2.2 Effects of formulation conditions on the product base color
Influence of other mixing conditions including pH (4–7), preservative (sodium lactate, sodium benzoate, and DMDM-H), antioxidant (BHT and BHA), and antioxidant/LEO ratio (0.5:1–2.5:1 w/w) on the product color was investigated in the next series of experiments. In the experiment where the pH value was varied, the color change was more pronounced at pH 7, while the products obtained at pH 4 and 5 displayed comparatively lower ΔE, of 2.49 and 2.22, respectively. This could be explained by the susceptibility to the decomposition of citral at high pH and its stability in acidic environments [14]. Apparently, at pH 5, the product color changed to a minimal degree and thus was selected as the condition for further investigation. Regarding color change with respect to the added preservatives, the base product incorporated with sodium benzoate displayed higher ΔE (1.75) when compared with those added with sodium lactate (0.76) or with DMDM-H (0.77). Therefore, DMDM-H and sodium lactate were used for further experiments (Table 4).
Color change of the washing product base after 7 days of storage at 45°C formulated at different process conditions
| pH value | Preservatives | Antioxidants | Antioxidant/LEO ratio (w/w) | ΔE |
|---|---|---|---|---|
| pH 4 | Sodium benzoate | BHT | 1:1 | 2.49 |
| Sodium lactate | ||||
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHT | 1:1 | 2.22 |
| Sodium lactate | ||||
| DMDM-H | ||||
| pH 6 | Sodium benzoate | BHT | 1:1 | 2.56 |
| Sodium lactate | ||||
| DMDM-H | ||||
| pH 7 | Sodium benzoate | BHT | 1:1 | 2.67 |
| Sodium lactate | ||||
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHT | 1:1 | 0.76 |
| pH 5 | Sodium lactate | BHT | 1:1 | 1.75 |
| pH 5 | DMDM-H | BHT | 1:1 | 0.77 |
| pH 5 | Sodium benzoate | BHT | 1:1 | 1.94 |
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHA | 1:1 | 2.93 |
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHT | 0.5:1 | 0.85 |
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHT | 1:1 | 0.84 |
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHT | 1.5:1 | 1.60 |
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHT | 2:1 | 3.75 |
| DMDM-H | ||||
| pH 5 | Sodium benzoate | BHT | 2.5:1 | 5.94 |
| DMDM-H |
The antioxidants are important ingredients that assist in preventing and slowing down the oxidation process of other chemicals in the formulation. They reduce the effects of oxidation processes by binding to each other radical molecules, reducing their decomposition power [32]. In the following experiments, the product color was investigated with regard to the type of antioxidant and its content. Two common antioxidants, namely BHA and BHT, were incorporated into the base formulation. The sample incorporated with BHA and stored at 45°C demonstrated a marked color quality change and major layering in the bottle. On the contrary, samples incorporated with BHT showed minor ΔE fluctuations and less color degradation than BHA-containing samples. This is in part due to the higher thermal stability of BHT structure in comparison with BHA [33]. Thus, BHT was selected as the antioxidant in the following experiment. Regarding the change of product color with respect to the antioxidant/LEO ratio, a higher ratio seemed to be associates with a more yellowish product texture. However, the change was marginal and could not be visually discernible. To be specific, at BHT/ LEO ratios of 0.5:1 and 1:1, the ΔE values of the base product were 0.85 and 0.84, respectively. At higher BHT/ LEO ratios of 1.5:1, 2:1, and 2.5:1, the color change progress was more accelerated, reaching ΔE values of 1.6, 3.75, and 5.94, respectively. The observed color degradation is largely attributable to increased reactivity of the atmospheric oxygen atoms having an uneven number of electrons in the outer shell resulting in the chemical reactions occurring within the base. The addition of BHT could contribute to better color stability via a mechanism that is similar to that of vitamin E. To be specific, BHT could donate one hydrogen atom to the oxygen atoms with uneven electron distribution, forming hydroperoxide [33]. However, excessive addition of BHT may generate redundant electrons than that required for radical oxygen to stabilize, adversely affecting the product quality. From these results, the BHT/LEO ratio of either 0.5:1 or 1:1 was used in subsequent investigations.
3.3 Color and compositional changes of LEO in formulated body wash and shampoo products
The body wash and shampoo products incorporated with LEO were stored for a month at 45°C. Sensorial examination of the samples shows that, compared with the initial sample, the preserved products exhibit moderate color change to light yellow and the aroma quality was comparable with those before storing. The ΔE values of the body wash and shampoo after 1 month of storage were 1.13 and 0.85, respectively [34,35,36,37] (Figure 3).

Initial (1) and 1-month body wash product (2); initial (3) and 1-month shampoo product (4).
To elaborate on the degradation of the chemical composition of LEO after storage, different LEO samples were analyzed by GC-MS. LEO was first recovered from the products through a hydrodistillation process. Table 5 summarizes the compositions and contents of the compounds in the bare LEO and LEO recovered from the product base, body wash, and shampoo under different storage times and temperatures. LEO isolated from the washing base, after 1 week and 1 month of storage, was devoid of decomposition products of citral such as 3,7-dimethyl-1,3,6-octatriene, p-1,5-menthadien-8-ol, and p-cymen-8-ol. Other compounds such as isogeranial, geraniol, cyclohexane, caryophyllene, α-bergamotene, butylated hydroxytoluene, caryophylene oxide, m-camphorene, and p-camphorene accounted for less than 1% of the total content in the LEO sample recovered from the product base. These results confirm the capability of the emulsification technique for preserving the composition of essential oils in the formulation of washing bases.
Chemical compositions of bare LEO, LEO recovered from the base sample, shampoo, and body products
| No | RT (min) | Compounds | Lemongrass oil | Base sample | Shampoo | Body wash | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | 7 days | 1 month | 7 days | 1 month | Initial | 1 month | Initial | 1 month | |||
| 1 | 9.813 | 6-Methyl-5-hepten-2-one | 0.805 | 1.149 | 1.314 | 2.575 | 1.598 | 1.243 | 1.572 | 0.866 | 1.026 |
| 2 | 9.928 | β-Myrcene | 5.656 | 6.025 | 10.86 | 7.097 | 9.932 | 5.498 | 8.597 | 4.256 | 4.543 |
| 3 | 12.427 | 3,7-Dimethyl-1,3,6-octatriene | — | — | 0.395 | — | — | — | — | — | — |
| 4 | 16.16 | β-Linalool | 0.754 | 0.664 | 0.805 | 1.818 | 0.775 | 0.959 | 0.808 | 0.839 | 0.788 |
| 5 | 18.942 | Unknown name | — | 0.727 | 0.232 | 0.197 | 0.224 | — | 0.179 | ||
| 6 | 19.799 | p-Mentha-1,5-dien-8-ol | — | — | 0.768 | — | — | — | — | — | — |
| 7 | 20.667 | p-Cymene-8-ol | — | 0.288 | 1.154 | — | — | — | — | — | — |
| 8 | 20.761 | Isogeranial | — | — | — | 0.505 | — | 0.295 | 0.826 | — | 0.347 |
| 9 | 22.727 | β-Citronellol | 0.499 | 0.547 | 0.572 | 0.241 | 0.696 | 0.62 | 0.603 | 0.552 | 0.599 |
| 10 | 23.166 | β-Citral | 39.031 | 38.699 | 35.026 | 38.74 | 35.996 | 38.779 | 38.49 | 38.124 | 37.715 |
| 11 | 23.71 | Nerol | 3.887 | 3.583 | 3.612 | 2.601 | 4.306 | 4.294 | 4.249 | 3.831 | 4.277 |
| 12 | 23.773 | Geraniol | — | 0.4 | 0.429 | — | — | 0.543 | 0.424 | ||
| 13 | 24.337 | α-Citral | 47.868 | 47.588 | 44.214 | 41.60 | 42.459 | 45.893 | 44.15 | 47.686 | 47.596 |
| 14 | 28.05 | Geraniol acetate | 0.602 | 0.361 | 0.525 | 1.125 | 0.889 | 0.45 | 0.851 | 0.329 | 0.784 |
| 15 | 28.227 | Cyclohexane | — | — | — | — | 0.409 | — | 0.366 | 0.369 | 0.784 |
| 16 | 29.419 | Caryophyllene | — | — | — | 0.598 | — | 0.412 | 0.621 | — | 0.655 |
| 17 | 29.482 | α-Bergamotene | 0.267 | 0.309 | 0.324 | — | 0.27 | 0.368 | 0.321 | 0.376 | 0.31 |
| 18 | 31.636 | Butylated hydroxytoluene | — | — | — | — | 0.198 | — | — | 1.735 | — |
| 19 | 33.226 | Caryophylene oxide | — | — | — | 0.125 | — | 0.123 | 0.091 | — | 0.089 |
| 20 | 33.884 | Selina-6-en-4-ol | 0.115 | 0.2 | 0.189 | — | 0.444 | 0.308 | 0.344 | 0.28 | 0.203 |
| 21 | 38.14 | m-Camphorene | — | — | — | 0.289 | 0.266 | 0.314 | — | 0.245 | 0.121 |
| 22 | 38.433 | p-Camphorene | — | — | — | 0.181 | — | 0.157 | — | — | — |
| 23 | 39.552 | Unknown name | — | — | — | 0.094 | — | 0.079 | — | — | — |
| Citral content (%) | 86.899 | 86.287 | 79.24 | 80.34 | 78.455 | 84.672 | 82.64 | 85.81 | 85.311 | ||
| % Citral decrease compared to initial | −0.704 | −8.814 | −7.549 | −9.717 | −2.563 | −4.903 | −1.253 | −1.827 | |||
Note: The values shown in bold in Table are due to (1) being the main ingredient in Lemon grass essential oil, (2) there is a marked variation in content under different storage conditions.
Regarding the citral content, the highest citral content was observed in the initial bare LEO and LEO after 7 days of storage at 45°C (86.89 and 86.28%, respectively), closely followed by citral content in LEO recovered from the body wash product (85.81 and 85.31% respectively). Further comparison of the citral content in various products to that of the Initial bare LEO revealed that the simulated product base stored after 1 month at 45°C exhibited the highest citral reduction (around 9.7%). Meanwhile, the corresponding values for the shampoo and body wash product were only 4.9 and 1.8%, respectively, suggesting that direct LEO incorporation into the base may result in shampoo and body products with minimized citral degradation. Some new ingredients in shampoo and body wash with concentrations below 1% included isogeranial, geraniol, cyclohexane, caryophyllene, caryophylene oxide, m-camphorene, and p-camphorene.
The decrease in citral content or other ingredients is due to the prolonged exposure to high temperatures during the LEO recovery process. The increase of β-myrcene content in the base and the shampoo sample may be attributable to its low molecular weight, making the compound to be more susceptible to evaporation and in turn more detectable during the GC-MS process. Alternatively, the increased β-myrcene content could be attributed to the rearrangement processes that transform surplus contents such as citral, geraniol, and citronella [38]. The emergence of substances such as geraniol, camphorene, and caryophylene oxide, which were previously absent in the bare LEO, could be explained by the inability of GC-MS to detect the constituents at very low concentrations.
4 Conclusion
In this study, we attempted the incorporation of LEO into the formulation of a washing product base, shampoo, and body wash product. The process was optimized to minimize color changes and citral degradation in the final products. The incorporation of LEO into two cosmetic products via direct mixing, rather than emulsion forming with base oils or emulsifiers, gave products better color stability. The best color stability of the product base could be achieved by using the following formulation conditions: pH, v5; preservatives, sodium lactate, and DMDM-H; antioxidant, BHT; LEO/BHT ratio, 0.5:1 or 1:1 (w/w); temperature, 70°C. The obtained shampoo and body wash also displayed negligible citral decomposition in comparison with the bare LEO.
Acknowledgements
Tran Thien Hien was funded by Vingroup Joint Stock Company and supported by the Domestic Master/ PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), Vingroup Big Data Institute (VINBIGDATA), code 2020.ThS.07.
-
Funding information: This work was funded by Vingroup Joint Stock Company and supported by the Domestic Master/Ph.D. Scholarship Programme of Vingroup Innovation Foundation (VINIF), Vingroup Big Data Institute (VINBIGDATA).
-
Author contributions: Thien Hien Tran, Cam Quyen Ngo Thi, Tri Nhut Pham, Quynh Anh Phan Nguyen, and Kim Ngan Tran Thi – investigation; Long Giang Bach and Le Thi Hong Nhan – supervision; Thien Hien Tran – writing – original draft.
-
Conflict of interest: The authors declare no conflict of interest.
-
Data availability statement: The data that support the findings of this study are available from the Nguyen Tat Thanh University but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the Nguyen Tat Thanh University
References
[1] Skaria BP, Joy PP, Mathew S, Mathew G. Lemongrass. In: Peter KV, editor. Handbook of herbs and spices. Cambridge: Woodhead Publishing; 2006. p. 400–19.10.1533/9781845691717.3.400Search in Google Scholar
[2] Ranade SS, Thiagarajan P. Lemon grass. Int J Pharm Sci Rev Res. 2015 Nov;35(2):162–7. ISSN 0976–044X.Search in Google Scholar
[3] Lorenzetti BB, Souza GEP, Sarti SJ, Santos Filho D, Ferreira SH. Myrcene mimics the peripheral analgesic activity of lemongrass tea. J Ethnopharmacol. 1991 Aug;34(1):43–8. 10.1016/0378-8741(91)90187-i.Search in Google Scholar
[4] Ekpenyong CE, Daniel NE, Antai AB. Effect of lemongrass tea consumption on estimated glomerular filtration rate and creatinine clearance rate. J Ren Nutr. 2015 Jan;25(1):57–66.10.1053/j.jrn.2014.08.005Search in Google Scholar
[5] Ekpenyong CE, Akpan E, Nyoh A. Ethnopharmacology, phytochemistry, and biological activities of Cymbopogon citratus (Dc.) Stapf extracts. Chin J Nat Med. 2015 May;13(5):321–37.10.1016/S1875-5364(15)30023-6Search in Google Scholar
[6] Carlini EA, Contar J de DP., Silva-Filho AR, Da Silveira-Filho NG, Frochtengarten ML, et al. Pharmacology of lemongrass (Cymbopogon citratus Stapf). I. Effects of teas prepared from the leaves on laboratory animals. J Ethnopharmacol. 1986 Jul;17(1):37–64.10.1016/0378-8741(86)90072-3Search in Google Scholar
[7] Nambiar VS, Matela H. Potential functions of lemon grass (Cymbopogon citratus) in health and disease. Int J Pharm Biol Arch. 2012 Oct;3(5):1035–43.Search in Google Scholar
[8] Mosquera T, Noriega P, Cornejo J, Pardo ML. Biological activity of Cymbopogon citratus (DC) Stapf and its potential cosmetic activities. Int J Phytocosm Nat Ingred. 2016 Dec 19;3(7):1–7. 10.15171/ijpni.2016.07.Search in Google Scholar
[9] Chaisripipat W, Lourith N, Kanlayavattanakul M. Anti-dandruff hair tonic containing lemongrass (Cymbopogon flexuosus) oil. Complement Med Res. 2015;22(4):226–9. 10.1159/000432407.Search in Google Scholar PubMed
[10] Haque ANMA, Remadevi R, Naebe M. Lemongrass (Cymbopogon): a review on its structure, properties, applications and recent developments. Cellulose. 2018 Oct;25(10):5455–77. 10.1007/s10570-018-1965-2.Search in Google Scholar
[11] Liang CP, Wang M, Simon JE, Ho CT. Antioxidant activity of plant extracts on the inhibition of citral off‐odor formation. Mol Nutr Food Res. 2004 Sep;48(4):308–17. 10.1002/mnfr.200400027.Search in Google Scholar PubMed
[12] Djordjevic D, Cercaci L, Alamed J, McClements DJ, Decker EA. Stability of citral in protein-and gum arabic-stabilized oil-in-water emulsions. Food Chem. 2008 Jan 15;106(2):698–705. 10.1016/j.foodchem.2007.06.033.Search in Google Scholar
[13] Kimura K, Nishimura H, Iwata I, Mizutani J. Deterioration mechanism of lemon flavor. 2. Formation mechanism of off-odor substances arising from citral. J Agric Food Chem. 1983 Jul;31(4):801–4. 10.1021/jf00118a030.Search in Google Scholar
[14] Kimura K, Iwata I, Nishimura H. Relationship between acidcatalyzed cyclization of citral and deterioration of lemon flavor. Agric Biol Chem. 1982;46(5):1387–9. 10.1271/bbb1961.46.1387.Search in Google Scholar
[15] Clark Jr BC, Powell CC, Radford T. The acid catalyzed cyclization of citral. Tetrahedron. 1977 Jan 1;33(17):2187–91. 10.1016/0040-4020(77)80002-1.Search in Google Scholar
[16] Ueno T, Masuda H, Ho CT. Formation mechanism of p-methylacetophenone from citral via a tert-alkoxy radical intermediate. J Agric Food Chem. 2004 Sep 8;52(18):5677–84. 10.1021/jf035517j.Search in Google Scholar PubMed
[17] Schieberle P, Ehrmeier H, Grosch W. Aroma compounds resulting from the acid-catalyzed breakdown of citral. Z fur Lebensmittel-Untersuchung und-Forschung. 1988 Jul 1;187(1):35–9.10.1007/BF01454320Search in Google Scholar
[18] Djordjevic D, Cercaci L, Alamed J, McClements DJ, Decker EA. Chemical and physical stability of citral and limonene in sodium dodecyl sulfate − chitosan and gum arabic-stabilized oil-in-water emulsions. J Agric Food Chem. 2007 May 2;55(9):3585–91. 10.1021/jf063472r.Search in Google Scholar PubMed
[19] Tran TH, Kim Ngan TT, Cam Quyen NT, Pham TN, Quynh Anh PN, Hong Nhan LT. Formulation of an essential oil-based body wash: selection of components and their effects on product foamability and emulsion durability. Asian J Chem. 2020;32(10):2495–501. 10.14233/ajchem.2020.22614.Search in Google Scholar
[20] Hien TT, Nhut PT, Bach LG, Nhan NPT, Nhan LTH. Study on the formula to produce shampoo derived from Lemongrass (Cymbopogon citratus (d. C.) stapf.) essential oil. IOP Conf Ser: Mater Sci Eng. 2020 Oct 31;959:012027. 10.1088/1757-899X/959/1/012027.Search in Google Scholar
[21] Dao TP, Do HT, Khoi LQ, Gia Phap NV, Cang MH, Pham TN, et al. Evaluation of physico-chemical properties of lemongrass (Cymbopogon citratus L.) essential oil grown in tien giang province, vietnam. Asian J Chem. 2020;32(5):1248–50. 10.14233/ajchem.2020.22258.Search in Google Scholar
[22] Peacock VE, Kuneman DW. Inhibition of the formation of.alpha.-p-dimethylstyrene and p-cymen-8-ol in a carbonated citral-containing beverage system. J Agric Food Chem. 1985 May;33(3):330–5. 10.1021/jf00063a003.Search in Google Scholar
[23] Weerawatanakorn M, Wu J-C, Pan M-H, Ho C-T. Reactivity and stability of selected flavor compounds. J Food Drug Anal. 2015 Jun;23(2):176–90. 10.1016/j.jfda.2015.02.001.Search in Google Scholar PubMed
[24] Shah G, Shri R, Panchal V, Sharma N, Singh B, Mann A. Scientific basis for the therapeutic use of Cymbopogon citratus, stapf (Lemon grass). J Adv Pharm Tech Res. 2011;2(1):3. 10.4103/2231-4040.79796.Search in Google Scholar PubMed PubMed Central
[25] Silva CD, Guterres SS, Weisheimer V, Schapoval EE. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz J Infect Dis. 2008 Feb;12(1):63–6. 10.1590/S1413-86702008000100014.Search in Google Scholar PubMed
[26] Modak T, Mukhopadhaya A. Effects of citral, a naturally occurring antiadipogenic molecule, on an energy-intense diet model of obesity. Indian J Pharmacol. 2011 May;43(3):300. 10.4103/0253-7613.81515.Search in Google Scholar PubMed PubMed Central
[27] Prakash A, Vadivel V. Citral and linalool nanoemulsions: impact of synergism and ripening inhibitors on the stability and antibacterial activity against Listeria monocytogenes. J Food Sci Technol. 2020 Apr;57(4):1495–504. 10.1007/s13197-019-04185-8.Search in Google Scholar PubMed PubMed Central
[28] Ueno T, Masuda H, Ho CT. Formation mechanism of p-methylacetophenone from citral via a tert-alkoxy radical intermediate. J Agric Food Chem. 2004 Sep 8;52(18):5677–84. 10.1021/jf035517j.Search in Google Scholar PubMed
[29] Fujiu KB, Kobayashi I, Neves MA, Uemura K, Nakajima M. Effect of temperature on production of soybean oil-in-water emulsions by microchannel emulsification using different emulsifiers. Food Sci Technol Res. 2011;17(2):77–86. 10.3136/fstr.17.77.Search in Google Scholar
[30] Tan C-C, Karim AA, Uthumporn U, Ghazali FC. Effect extraction temperature on the emulsifying properties of gelatin from black tilapia (Oreochromis mossambicus) skin. Food Hydrocoll. 2020;108:106024. 10.1016/j.foodhyd.2020.106024.Search in Google Scholar
[31] Rustom IYS, López-Leival MH, Nair BM. Effect of emulsifier type and homogenization temperature and pressure on physical properties of peanut extract. Int J Food Sci Technol. 1995;30(6):773–81. 10.1111/j.1365-2621.1995.tb01424.x.Search in Google Scholar
[32] Kusumawati I, Indrayanto G. Natural antioxidants in cosmetics. Studies in natural products chemistry. Amsterdam, The Netherlands: Elsevier Inc.; 2013 Jun 25.10.1016/B978-0-444-59603-1.00015-1Search in Google Scholar
[33] de Jesus JH, Ferreira AP, Szilágyi IM, Cavalheiro ET. Thermal behavior and polymorphism of the antioxidants: BHA, BHT and TBHQ. Fuel. 2020 Oct 15;278:118298. 10.1016/j.fuel.2020.118298.Search in Google Scholar
[34] Weigel F, Weiss J, Decker EA, McClements DJ. Lutein-enriched emulsion-based delivery systems: influence of emulsifiers and antioxidants on physical and chemical stability. Food Chem. 2018;242:395–403. 10.1016/j.foodchem.2017.09.060.Search in Google Scholar PubMed
[35] Davidov-Pardo G, Gumus CE, McClements DJ. Lutein-enriched emulsion-based delivery systems: influence of pH and temperature on physical and chemical stability. Food Chem. 2016;196:821–7. 10.1016/j.foodchem.2015.10.018.Search in Google Scholar PubMed
[36] Qian C, Decker EA, Xiao H, McClements DJ. Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem. 2012;132(3):1221–9. 10.1016/j.foodchem.2011.11.091.Search in Google Scholar PubMed
[37] Luo X, Zhou Y, Bai L, Liu F, Deng Y, McClements DJ. Fabrication of β-carotene nanoemulsion-based delivery systems using dual-channel microfluidization: physical and chemical stability. J Colloid Interface Sci. 2017;490:328–35. 10.1016/j.jcis.2016.11.057.Search in Google Scholar PubMed
[38] Thuong Nhan NP, Tan Thanh V, Huynh Cang M, Tri Duc L, Cam Huong N, Hong Nhan LT, et al. Microencapsulation of lemongrass (Cymbopogon citratus) essential oil via spray drying: effects of feed emulsion parameters. Processes. 2020;8(1):40. 10.3390/pr8010040.Search in Google Scholar
© 2021 Thien Hien Tran et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


