Abstract
Due to the impacts of climate change and the reduction in the flow of the Mekong River, saline intrusion into the inland has been an emergent and pressing issue. The purpose of this study is to analyze the effects of various saline conditions (0–25‰) on changes in some soil properties under laboratory conditions. Ten topsoil samples were collected from a depth of 0–20 cm in the dry seasons in the rice–corn rotation fields with low salinity, in Thanh Phu district, Ben Tre province, Vietnam. The examined criteria consisted of soil pH, soil electrical conductivity of the saturated paste extract (ECe), exchangeable Na, percentage of exchangeable Na, and content (%) of nitrogen and phosphorus. The results revealed that the pH range of soil decreased from 5.14–5.72 to 4.08–5.14 when the soil salinity increased from 0 to 25‰. At the salinity of 10‰ and higher, the available nitrogen began to decline. Meanwhile, the available phosphorus tended to decrease as the salinity increased past 12‰. Some measures are also discussed, with the aim of ensuring sustainable rice farming in the circumstances of increased salinity.
1 Introduction
Despite the long-lasting morphological stability of the Mekong Delta, the landscape of the region has recently witnessed drastic changes in the form of water withdrawal and land sinking [1,2]. It is forecasted that the combination of reduced supply of water and sediments, compaction, and thermosteric sea level rise may threaten the existence of this region in possibly less than a century [3]. This certainly spells disaster for the Vietnamese economy, given that the Mekong Delta plays a key role in agricultural and aquatic production of Vietnam [4]. The delta is characterized by the vast area of fertile, flat land with little to no forest coverage and approximately 65% of which is reserved for rice farming.
One of the main natural obstacles of the Mekong Delta is the saline water intrusion in the dry season, affecting not only agricultural production but also water supply and daily life for millions of people. At the seashore, salt in saline soils can locally be generated from sediments or by saline intrusion of seawater or supplied using saline water [5]. According to ref. [6], the accumulation of salt in the soil begins to occur when the evaporation of water exceeds the amount of water supplied to the soil because most irrigation water contains an amount of dissolved salt. After irrigation, water in the soil is absorbed by the crop or evaporates directly, leaving the salt in the soil.
Saline soils mainly contain soluble salt components including calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), chloride (Cl−), bicarbonate (HCO3), or sulfate (SO4 2−) [7,8]. High salinity in the soils usually causes the phenomenon of sodicization. Saline sodic soil with a high accumulation of salt hinders crops growth. Other disadvantages include disturbance and imbalance in water and nutrient uptake and unfavorable physical properties [9]. The presence of salts in the soil is determined by the concentration of Na+, Ca2+, Mg2+, and K+; and the ability of sodicization of the soil is determined by calculating the adsorption ratio of Na+ on clay glue (sodium adsorption ratio (SAR) and exchangeable sodium percentage (ESP) [10]. According to the US Department of Agriculture, soil is considered saline when the electrical conductivity (EC) of the saturated soil extract (EC saturation) is greater than 4 dS m−1 at 25°C. Therefore, higher dissolved salts in the soils will lead to higher conductivity [11].
Saline soils with high salt concentration lead to unfavorable physical, chemical, and biological properties [12]. Saline soils contain high levels of Na+ ions in the soil’s absorption complex, causing disturbance and imbalance in the uptake of water and nutrients for crops and disadvantages in soil physical properties [13]. Soil salinity can also indirectly affect crops by causing nutrient deficiencies or nutrient imbalances in plants, such as the imbalance in ratio of Na+/Ca2+ [14] or directly exert toxicity in plants by excessive absorption of ions such as Na+, Cl−, B+, and SO4 2− [15,16,17,18]. On agricultural land, salinity hinders water–plant interaction by introducing excess salt in the root, which reduces the amount of water available to the plant and causes the plant to use more energy to remove the salt and absorb water.
Fluctuations of saline and flooding factors directly affect the production and daily life of people. As a basis for future orientation of agricultural land use in the Mekong Delta, assessing the impact of salinity on changes in soil properties under laboratory conditions is essential to improve the efficiency of land use and ensure sustainable farming conditions. In this study, we assessed the influence of salinity variations on essential soil nutrient indicators such as pH, EC, Na+ exchange, ESP, available nitrogen, and available phosphorus. The results are expected to aid in devising strategies that are appropriate to specific crops to cope with different salinity levels in the region.
2 Materials and methodology
2.1 Collection of soil samples
Soil samples were collected in rice–corn rotation farming fields located in the low salinity ecological zone in Thanh Phu district, Ben Tre province, Vietnam. The highest salinity of water in canals and ditches is around 4–5‰ in the dry season. Soil samples were taken in accordance with ISO 10381-2:2002 and then stored and brought to the laboratory in accordance with ISO 11464:2006. Topsoil is collected at a depth of 0–20 cm. Sampling points follow the zigzag pattern, and 10 soil samples were collected for an area of 0.1 Ha. Samples were then mixed well, and a representative sample was taken for analysis.
Soil is classified into the following six major groups according to the Vietnam standards: (1) red and yellow soil; (2) alluvial soils include alluvial soil of Red River system, alluvial soil of Mekong river system and other alluvial soil of other river systems; (3) faded gray soil includes faded gray soil on acid magma and sandstone and faded gray soil on ancient alluvial; (4) acid sulfate soil; (5) salty soil; and (6) coastal sandy soil. In each major group, the soil quality indicators of nonorganic contents include three main indexes, which are exchangeable calcium content (TCVN 9236-1-2012), exchangeable magnesium content (TCVN 9236-2-2012), and exchangeable sodium content (TCVN 9236-3-2012).
2.2 Experimental
The experiment was arranged at room temperature (30 ± 2°C). The soil is chopped to a size of about 2 cm and placed in a glass jar (1,000 mL) with a weight of 1.5 kg dry soil/jar. The soil was submerged in saline solutions with different salinity of 0‰ (control sample), 2, 4, 6, 8, 10, 12, and 25‰, with four replicates, a depth of 5 cm. Saline water is made from artificial seawater (instant ocean) and distilled water. A 2‰ saline solution is made from 2 g of instant salt (instant ocean) into 1 L of water. Other treatments were identically prepared. Soil samples were collected by small hand drills at 1, 2, 4, 6, and 12 weeks after saltwater intrusion, and the soil was dried in natural conditions and minced through 0.5 mm sieve.
2.3 Measurement of soil indicators
The soil analysis methods are detailed in Table 1, and the main component of instant ocean water and natural is shown in Table 2.
Methods of analyzing soil parameters
| Analytical indicator | Reference method | Standard method |
|---|---|---|
| pH, EC | Extracted with distilled water, extract ratio 1:2.5 (soil:water) and measured by pH, EC meter | |
| Nitrogen | Guabekki and Bremner (1986) [19] | ISO 11261:1995 |
| Phosphorus | Olsen (1954) [20] | ISO 18645:2016 |
| Na+, Mg2+, Ca2+ | Atomic absorption spectrum (AAS) | ISO 11464: 2006 |
| TOC (total organic carbon) | Walkley-Black (1934) [21] | ISO 10694:1995 |
| CEC (cation exchange capacity) | The measure is determined by a buffer of BaCl2 0.1 M [22] | ISO 23470:2018 |
| ESP (exchange sodium percentage) | The method is based on the ratio of Na+ adsorbed and cation exchange capacity of the soil (CEC, cmol/kg).
|
Main component of instant ocean water and natural (Unit: ppt)
| Ion | Instant seawater | Natural seawater |
|---|---|---|
| Na+ | 10.78 | 10.781 |
| K+ | 0.42 | 0.399 |
| Mg2+ | 1.32 | 1.284 |
| Ca2+ | 0.4 | 0.411 |
| Sr2+ | 0.008 | 0.007 |
| Cl− | 19.29 | 19.353 |
| SO4 2+ | 2.66 | 2.712 |
| HCO3− | 0.2 | 0.126 |
| Br− | 0.056 | 0.067 |
| B(OH)3 | — | 0.025 |
| F | 0.001 | 0.001 |
The standards used for the analysis of phosphorus, Na+, Mg2+, and Ca2+ were potassium dihydrogen phosphate KH2PO4 (CAS 7778-77-0), sodium chloride NaCl (CAS 7647-14-5), magnesium chloride MgCl2·6H2O (CAS 7791-18-6), and calcium carbonate CaCO3 (CAS 471-34-1), respectively. All standards were purchased from Merck and their purity were in the range of 99.0–101.0%.
2.4 Statistical and data analysis
Mean and standard deviations were derived using Microsoft Excel. ANOVA and LSD analysis of 5% to compare the differences in soil chemistry properties of treatments. Using the Duncan test, evaluate the difference in soil and water parameters. The final data were processed on SPSS 20 statistical software.
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Comparison of typical properties of instant ocean, natural seawater, and soil properties before experiment
In Table 3, the initial salinity of the soil according to the EC criteria can be evaluated as nonsaline if the electrical conductivity (EC) of the extract at saturated soil is less than 4 mS/cm at 25°C [23]. According to Boyd, the pH value of soil from 6.5 to 7.5 is suitable for the growth and development of cultured shrimp and aquatic organisms [24].
Some soil properties before the experiment
| EC (mS/cm) | pH | Na+ exchange (cmol/kg) | Available N (mg/kg) | Available P (mg/kg) | CEC (cmol/kg) |
|---|---|---|---|---|---|
| 2.41 | 6.15 | 1.85 | 8.41 | 1.67 | 12.7 |
In the Mekong Delta region, soil having pH values ranging from 6.5 to 8 are suitable for aquaculture, so it is reasonable to see the pH values of the tested soil were at low levels [25], which can adversely affect fisheries and require measures to raise the pH, such as lime administration. The SAR determined by the concentrations of Na+, Ca2+, and Mg2+ of the sodic soil was greater than 13; thus, the soil in the area taken is suitable for crop cultivation. Regarding soil nutrients, the P content in common soil is in the range of 10–100 mgP/and in association with the insoluble soil composition [26]. Therefore, the nutrition of the tested soil could be concluded to be at low level.
3.2 Effect of salinity on changes in some soil properties under laboratory conditions
Higher salinity in agricultural production land caused by coastal saline intrusion induces drastic changes in soil characteristics and affects the structure and crop plants and animals. In this laboratory experiment, nonsaline soils, the soil on which rice and corn are currently grown, were treated with varying salinity (from 2 to 25‰) to monitor the variations in soil characteristics. This treatment is to simulate 3 months of high salinity in the dry season.
3.2.1 Soil pH
The results presented in Table 4 show that the soil pH is low, ranging from 4.0 to 5.6 on average. With increasing salinity, high saline treatments of 12 and 25‰ resulted in the pH values that are significantly different from those of the control and of lower salt concentrations. When salinity is increased, cation exchange between Na+ and H+ can lead to increased concentration of H+ ions in soil solution, in turn lowering the soil pH. Over the period of 2–12 weeks of saltwater intrusion, soil pH tends to increase slightly. This increase in pH is negligible and has not yet reached the neutral pH value. When the soil is flooded, reducing reactions occur with the participation of soil microorganisms. The reduction reaction consumes H+ ions, contributing to an increase in soil pH. However, under high salinity conditions, soil microbial activity is reduced, causing very minor pH increases. On the other hand, the buffering capacity of the soil may contribute to limiting this increase in soil pH.
Changes in soil pH over time due to saltwater intrusion and salt concentration
| Experiments | Time (weeks) | F test | LSD (5%) | CV% | |||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 12 | ||||
| 0‰ | 5.21abB | 5.14bA | 5.64aAB | 5.72A | * | 1.7 | 6.2 |
| 2‰ | 5.32aB | 5.46aAB | 5.43abAB | 5.78A | * | 1.6 | 8.7 |
| 4‰ | 5.33a | 5.27ab | 5.34ab | 5.49 | ns | 5.1 | 6.7 |
| 6‰ | 5.06abB | 5.07bAB | 5.30abAB | 5.56A | * | 1.4 | 10.7 |
| 8‰ | 5.33a | 5.10b | 5.35ab | 5.48 | ns | 5.2 | 4.8 |
| 10‰ | 4.86b | 5.11b | 5.45ab | 5.55 | ns | 3.4 | 11.3 |
| 12‰ | 4.46b | 4.53c | 5.22b | 5.34 | ns | 4.4 | 5.4 |
| 25‰ | 4.08b | 4.44c | 5.14b | 5.28 | ns | 7.9 | 8.8 |
| F-test | * | * | * | ns | |||
| LSD (5%) | 0.56 | 0.27 | 0.33 | 0.56 | |||
| CV% | 6.96 | 3.70 | 4.28 | 6.96 | |||
Notes: Lowercase letters indicate Tukey’s test between salinity treatments. Upper letters indicate Tukey’s test over time. The same letters in the same column or row indicated no significant difference. ns: undifferentiated; (*): difference with significance level of 5%.
3.2.2 Conductivity of soil (EC)
The results presented in Figure 1 show that, when salinity increases, conductivity of saline soils also increases significantly (Table 5). In some treatments, the period in which rapid acceleration in salinity occurred may be lower than 2 weeks. However, after 2 weeks the salinity is almost unchanged. When the soil was submerged for 2‰ for 2 weeks, the EC of the saturated soil extract solution was slightly higher than 4 mS/cm, reaching the threshold of saline soils [27]. When the salt concentration increases from 4 to 25‰ after 2 weeks of saltwater flooding, the salinity of the soil also increases, the value of EC of the saturated paste extract (ECe) ranged from 7 to 46 mS cm−1. Thus, when saline soils are inundated with a salt concentration of 2‰, the soil can become saline. An inundation with the salt concentration higher than this value may aggravate the salinity issue of the soil, thus impairing the growth and development of crops. Therefore, further measures should be taken to improve saline soils [28].
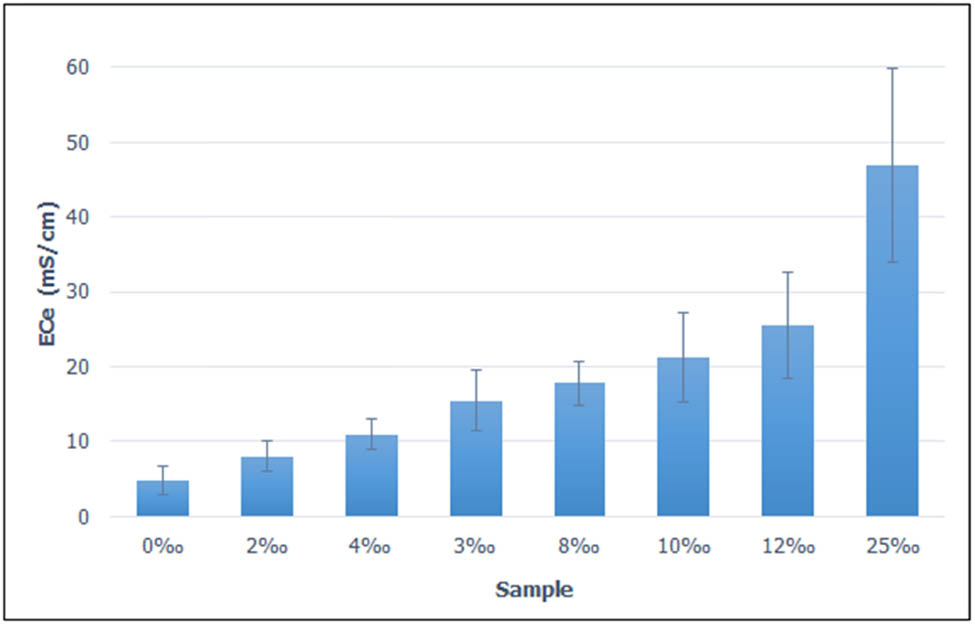
Effect of saltwater salinity on EC of the saturated paste extracts (ECe) after 12 weeks of salinity treatment.
The EC (1:2.5) change in soil according to salinity and salinity duration
| Experiments | Time (weeks) | F test | LSD (5%) | CV% | |||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 12 | ||||
| Control | 1.31g | 1.48g | 1.47h | 1.28h | ns | 6.6 | 15.0 |
| 2‰ | 2.19f | 1.91g | 2.12g | 2.40g | ns | 11.2 | 13.9 |
| 4‰ | 2.68f | 2.49f | 3.05f | 3.10f | ns | 16.3 | 13.7 |
| 6‰ | 3.17fA | 3.54eA | 3.72eAB | 3.83eA | * | 2.4 | 9.1 |
| 8‰ | 4.42d | 4.35d | 4.81d | 4.49d | ns | 10.2 | 7.4 |
| 10‰ | 5.41c | 5.49c | 5.56c | 5.61c | ns | 3.4 | 4.4 |
| 12‰ | 6.21b | 6.57b | 6.29b | 6.60b | ns | 3.6 | 7.4 |
| 25‰ | 11.52a | 12.12a | 11.75a | 12.39a | ns | 11.9 | 4.9 |
| F | * | * | * | * | |||
| LSD (5%) | 0.51 | 0.55 | 0.37 | 0.39 | |||
| CV% | 8.31 | 8.41 | 5.69 | 5.37 | |||
Notes: Lowercase letters indicate Tukey’s test between salinity treatments. Upper letters indicate Tukey’s test over time. The same letters in the same column or row indicated no significant difference. ns: undifferentiated; (*): difference with significance level of 5%.
3.2.3 Correlation of EC of soil extract EC (1:2.5) and ECe
Plotting the data of EC (1:2.5) against ECe (Figure 2) shows a strong correlation (R 2 = 0.95**) between two variables. The estimated equation was EC = 0.302 × ECe + 0.357. The graph shows that the ECe values are generally higher than the EC values. This is possibly due to higher salt concentration of the latter method, which is caused by involvement of water that led to a moisture of around 70–80%. According to the evaluation scale of soil salinity, ECe value is the standard value to evaluate. However, to extract saturated soil, an air compression system is needed to extract saturated water in the soil, which is time-consuming and may present further difficulties to the process. Therefore, the availability of a well-estimated relationship between ECe and EC (1:2.5) could help predicting ECe values using only EC (1:2.5). From the data, it was shown that the EC/ECe ratio between EC/ECe varies from 0.27 to 0.44, averaged at 0.32. This result is consistent with a previous study where 603 acres of saline-rice soil in the Mekong Delta were surveyed, showing a strong correlation between ECe and EC 1:1.25 (R 2 = 0.89), with an average EC/ECe ratio of 0.41 [29].
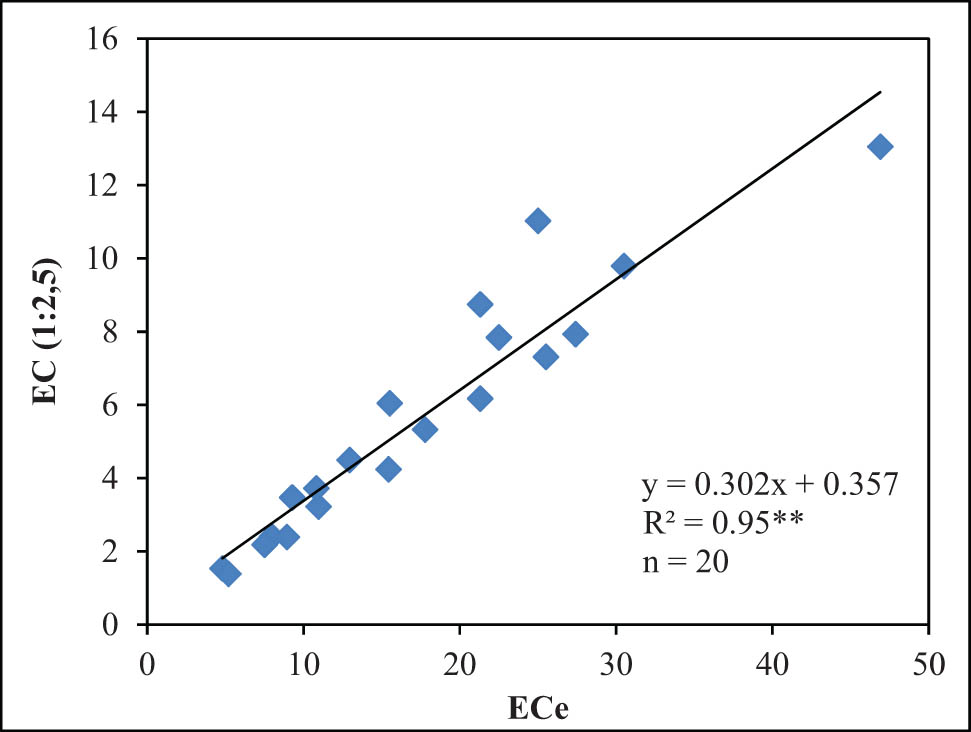
Correlation between EC (1:2.5) and ECe saturation extract.
3.2.4 Exchangeable sodium and sodicization in soil
The results presented in Table 6 show that when the soil is submerged in saline water, exchangeable Na+ in the soil increases with salinity concentration and the difference is statistically significant. An increase in Na+ exchange results in a higher ESP value. In saline soils, the percentage of Na in the absorption system (ESP) is a numerical value that evaluates the soil sodicization. When this value exceeds 15%, physicochemical and biological properties of the soil could be compromised, and crop nutrition could be impaired. The analysis results show that when the soil is submerged with a salinity of 2–4‰, the ESP in the soil was lower than 15%, thus the soil sample was not considered as sodicized. When the salinity treatment was 6‰, the ESP value of the soil exceeded the ESP threshold of sodicization, at above 15% (Table 7). When salinity was higher than 6‰, soil pH became lower than 8.5, ECe was higher than 4 mS cm−1, and ESP (%) was higher than 15, indicating that the soil had become saline sodic [30].
Effect of salt concentration and treatment duration on percentage of exchangeable Na (ESP) in the soil
| Experiments | Time (weeks) | F test | LSD (5%) | CV% | |||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 12 | ||||
| Control | 2.66e | 7.36h | 7.08g | 6.48g | ns | 11.30 | 36.89 |
| 2‰ | 9.28d | 12.74g | 8.66g | 7.87g | ns | 13.26 | 24.89 |
| 4‰ | 10.86dAB | 19.47fA | 10.07gB | 14.02eAB | * | 4.20 | 11.69 |
| 6‰ | 18.64c | 21.65e | 14.40e | 19.13de | ns | 15.30 | 8.16 |
| 8‰ | 23.53b | 24.70d | 17.79d | 21.54cd | ns | 12.53 | 9.19 |
| 10‰ | 25.54b | 27.72c | 21.89c | 26.50c | ns | 11.42 | 7.05 |
| 12‰ | 25.77b | 33.39b | 24.64b | 35.65b | ns | 16.20 | 15.09 |
| 25‰ | 51.58a | 53.05a | 42.52a | 55.31a | ns | 13.64 | 3.27 |
| F | * | * | * | * | |||
| LSD (5%) | 2.97 | 1.39 | 2.28 | 3.93 | |||
| CV% | 9.68 | 3.80 | 13.61 | 12.29 | |||
Notes: Lowercase letters indicate Tukey’s test between salinity treatments. Upper letters indicate Tukey’s test over time. The same letters in the same column or row indicated no significant difference. ns: undifferentiated; (*): difference with significance level of 5%.
Effect of salt concentration and treatment duration on exchangeable Na (cmol/kg) in soil
| Experiments | Time (weeks) | F test | LSD (5%) | CV% | |||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 12 | ||||
| Control | 0.34eB | 0.94hA | 0.9gC | 0.82gC | * | 0.40 | 54.4 |
| 2‰ | 1.18dA | 1.62gA | 1.1gB | 1.00gA | * | 1.50 | 26.3 |
| 4‰ | 1.38dB | 2.47fA | 1.28gC | 1.78eB | * | 0.60 | 30.8 |
| 6‰ | 2.37cA | 2.75eA | 2.01dB | 2.43deA | * | 0.46 | 9.8 |
| 8‰ | 2.99bA | 3.14dA | 2.26dB | 2.74deA | * | 0.32 | 7.1 |
| 10‰ | 3.24bA | 3.52cA | 2.78cB | 3.37cA | * | 0.41 | 7.5 |
| 12‰ | 3.27bA | 4.24bA | 3.13bB | 4.53bA | * | 1.30 | 15.2 |
| 25‰ | 6.55aB | 6.74aA | 5.4aB | 7.03aA | * | 0.42 | 1.8 |
| F | * | * | * | * | |||
| LSD (5%) | 0.38 | 0.14 | 0.29 | 0.49 | |||
| CV% | 9.99 | 3.16 | 13.62 | 12.29 | |||
Notes: Lowercase letters indicate Tukey’s test between salinity treatments. Upper letters indicate Tukey’s test over time. The same letters in the same column or row indicated no significant difference. ns: undifferentiated; (*): difference with significance level of 5%.
Thus, when the soil is intruded with salinity at 2‰, the soil becomes saline and its ECe will be higher than 4 mS/cm. At saltwater intrusion of 6‰, the soil begins to become saline sodic because its ESP reaches as high as 18%, according to the classification sodic saline soils [12,31]. In general, plants are adversely affected by salinity, and the detriment becomes especially worse in saline sodic soils due to impeded absorption of water and nutrients of plants, which is caused by the high osmotic pressure of the soil solution. Crops that are affected by salinity and saline sodic soils manifest as dehydrated, drought-affected plants. High Na+ salt concentration causes nutrient imbalance and hinders the nutrient uptake of crops, leading to significantly reduced yields [32]. A high concentration of Na+ in the soil results in high Na/K, Na/Ca, and Na/Mg ratios, which cause disruption in nutrient metabolism and protein synthesis. Therefore, it is necessary to take measures to reduce salinity through saline washing and reduce Na+ in soil as well as Na+ saturated in the absorption complex.
3.2.5 Available nitrogen content in soil
The available nitrogen content in soil after 2 weeks of saltwater treatment decreased significantly compared to that of the control sample. The protein content was increasing with salt concentration at low salinity. However, as the salt concentration increases above 10‰, the nitrogen content in saline soils significantly decreases (Table 8). After 4–6 weeks of salinity treatment, the available nitrogen content significantly increased. However, prolonging the treatment for more than 6 weeks causes the available nitrogen content to decline. This result shows that despite the salinity, microbiological activity in the mineralization process of nitrogen still occurs over time and that soil microorganisms can adapt to a change from fresh to saline stage of the soil environment, thus promoting the mineralization of nitrogen [33,34].
Changes in the value of available nitrogen (mg/kg) in the soil over the time of salinity and salt concentration
| Experiments | Time (weeks) | F test | LSD (5%) | CV% | |||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 12 | ||||
| Control | 26.43bC | 77.68aA | 72.54A | 50.29bB | * | 0.50 | 40.5 |
| 2‰ | 20.66cdC | 73.74abA | 73.71A | 50.27bB | * | 0.30 | 40.0 |
| 4‰ | 22.15bcdC | 67.18bAB | 81.17A | 56.69aB | * | 0.43 | 41.9 |
| 6‰ | 20.40cdC | 69.95abA | 70.37A | 55.48abB | * | 0.12 | 39.6 |
| 8‰ | 25.16bcC | 68.53abA | 78.64A | 56.65aB | * | 0.30 | 37.1 |
| 10‰ | 31.95aC | 72.91abA | 78.61A | 56.90aB | * | 0.10 | 38.4 |
| 12‰ | 17.39dC | 72.92abA | 75.15A | 54.17abB | * | 0.40 | 42.5 |
| 25‰ | 19.76dC | 74.62abA | 75.12A | 53.76abB | * | 0.50 | 43.1 |
| F | * | ns | ns | * | |||
| LSD (5%) | 5.26 | 10.09 | 11.95 | 5.77 | |||
| CV% | 15.69 | 9.58 | 10.82 | 7.29 | |||
Notes: Lowercase letters indicate Tukey’s test between salinity treatments. Upper letters indicate Tukey’s test over time. The same letters in the same column or row indicated no significant difference. ns: undifferentiated; (*): difference with significance level of 5%.
It has been shown that increased salinity stress could lead to smaller microbial community, less metabolic efficiency, and worsened activity of extracellular enzymes such as b-glucosidase, alkaline phosphatase, and arylsulfatase [35]. The exponentially negative relationships between the number of microbial communities and concentrations of dissolved salts could be elaborated through two main mechanisms: the osmotic effect and the effect of specific ions. While the salinity-induced osmotic stress could reduce microbial mass mainly through cell drying and lysis, the effect of ion mainly involves higher energy consumption of microbes to synthesize more organic osmolytes, such as proline and glycine betaine, which are required for ameliorating the stress effects [36]. Such energy waste might lead to reduced microbial growth [37]. In addition, fungi tend to be more susceptible than bacteria to salt stress [38,39]. As a result, increased salinity may also affect the fungi–bacteria community structure [38,40].
3.2.6 Content of available phosphorus in soil
The analytical results presented in Table 9 show that the available phosphorus content in the soil is very poor. The phosphorus available in the soil tends to increase with extended saline period tends to decrease when the salinity increases more than 12‰. This shows that the phosphorus available in saline soils is very low because phosphate anions can be precipitated by reacting with Ca2+ and Mg2+ cations, which are abundant in saltwater. From this result, it could be observed higher salinity seemed to associate with lower phosphorus available in the soil, which is nutritionally disadvantageous for crops.
Effect of salinity treatment duration and available phosphorus concentration (mg/kg) in soil
| Experiments | Time (weeks) | F test | LSD (5%) | CV% | |||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 12 | ||||
| Control | 0.13abC | 0.34aAB | 0.37aA | 0.24aBC | * | 0.30 | 41.7 |
| 2‰ | 0.20a | 0.26ab | 0.28bcd | 0.23a | ns | 14.21 | 26.5 |
| 4‰ | 0.08bB | 0.21abA | 0.29bA | 0.22aA | * | 0.32 | 47.0 |
| 6‰ | 0.12ab | 0.23ab | 0.29bc | 0.16b | ns | 12.30 | 68.4 |
| 8‰ | 0.15ab | 0.14b | 0.21d | 0.17b | ns | 19.50 | 28.8 |
| 10‰ | 0.15ab | 0.18ab | 0.22cd | 0.16b | ns | 11.45 | 33.9 |
| 12‰ | 0.10bB | 0.16bAB | 0.25bcdB | 0.19abAB | * | 0.40 | 39.7 |
| 25‰ | 0.11bB | 0.18abAB | 0.21cdA | 0.19abAB | * | 1.30 | 34.1 |
| F | * | * | * | * | |||
| LSD (5%) | 0.07 | 0.06 | 0.06 | 0.04 | |||
| CV% | 44.13 | 53.42 | 18.11 | 14.41 | |||
Notes: Lowercase letters indicate Tukey’s test between salinity treatments. Upper letters indicate Tukey’s test over time. The same letters in the same column or row indicated no significant difference. ns: undifferentiated; (*): difference with significance level of 5%.
According to the Vietnamese standards of soils quality – index values of total phosphorus content in the soils of Vietnam (TCVN 7374: 2004) for each soil type is as follows:
The results show that the study area consists of mostly saline soil (65.1%) and acid sulfate soil (5.09%), the rest belongs to insignificant alluvial soil (1.13%). Corresponding to the standards of phosphorus quality limit in soil, saline soil has phosphorus content of about 0.08–0.20% (corresponding to 0.08–0.20 mg/kg of soil) and alkaline soil. The amount of phosphorus is about 0.03–0.08 (0.03–0.08 mg/kg soil, respectively). From the results of Table 10, it shows that the phosphorus content of the soil samples is at medium to fair level compared to the Vietnamese standard for soil groups.
According to the salinity scale described by Landon, the soil having ECe ranging from 8.22 to 13.11 mS cm−1 was classified as saline soil [41]. Generally, high ECe might impair the growth of most rice varieties, especially at the young seedling the reproductive stage [42]. On the other hand, the response of rice plant to salinity might be different depending on the variety and the salinity level and maximum tolerable salinity might be high as much as 4‰ [43]. Therefore, suggested strategies to improve the tolerance of rice might include adoption of salinity-tolerant varieties and supplementation of Ca2+ or lime to balance Na+ [44,45,46].
Available nitrogen and phosphorus are important indicators in evaluating the capability of soil in providing organic nutrients [47]. Previous reports have shown that the available nitrogen largely determines rice productivity [48,49], possibly via mediation of its demands for macronutrients [50]. According to Wood and Lass, low available nitrogen is indicative of limited organic nutrients, which is the main causes for low productivity of rice growing in saline soils [51]. Elevated salinity also lowers available nitrogen through mineralization and increased mortality of microorganisms, in turn leading to the deficiency of rice nutrient intake [52]. The mechanism by which phosphorus affects rice productivity is similar to that of nitrogen [53]. Therefore, the application of organic fertilizers, which are rich in nitrogen and phosphorus, could be considered as a suitable salinity-coping measure in saline rice paddy fields.
The phosphorus content limitations for each soil type
| Soil group | Total phosphorus (P2O5 %) | |
|---|---|---|
| Value range | Average | |
| 1. Red soil | From 0.05 to 0.60 | 0.30 |
| 2. Alluvial soil | From 0.05 to 0.30 | 0.10 |
| 3. Faded gray soil | From 0.03 to 0.06 | 0.04 |
| 4. Acid sulfate soil | From 0.03 to 0.08 | 0.04 |
| 5. Salty soil | From 0.08 to 0.20 | 0.09 |
| 6. Coastal sandy soil | From 0.03 to 0.05 | 0.04 |
4 Conclusion
This study simulated the effect of salinity intrusion on some soil characteristics under laboratory conditions. Obtained results indicate that prolonged exposure to salinity caused evident consequences to soils. After 2 weeks of salinity treatment with a concentration of 4‰, the soil became saline. With 6 weeks of treatment, sodicization occurred. For sustainable farming of rice and corn, the soil salinity is recommended not to exceed 4‰; and higher salinity might significantly cause deficiency in nutrients, particularly available nitrogen and phosphorus. The recommended measures for sustainable rice cultivation in the saline soil include adoption of salinity-tolerant varieties and administration of lime, which is a source of Ca2+ that could indirectly balance Na+ in salted rice plants, and organic fertilizers. In addition, the development of diversified farming model is advised to cope with different salinity levels in the region.
-
Funding information: This research was funded by Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam and Ben Tre Department of Science and Technology, Ben Tre Province, Vietnam.
-
Author contributions: Lam Van Tan is in charge of conceptualization, investigation, writing of the original draft; and Tran Thanh was involved in investigation.
-
Conflict of interest: The authors declare no conflict of interest.
-
Data availability statement: The data used to support the results in this study are available from the corresponding author upon request.
References
[1] Schmidt C. Alarm over a sinking delta. Science. 2015 May 22;348(6237):845–6.10.1126/science.348.6237.845Search in Google Scholar
[2] Liu JP, DeMaster DJ, Nguyen TT, Saito Y, Nguyen VL, Ta TKO, et al. Stratigraphic formation of the mekong river delta and its recent shoreline changes. Oceanography. 2017;30(3):72–83.10.5670/oceanog.2017.316Search in Google Scholar
[3] Li X, Liu JP, Saito Y, Nguyen VL. Recent evolution of the Mekong Delta and the impacts of dams. Earth-Sci Rev. 2017 Dec;175:1–17. 10.1016/j.earscirev.2017.10.008.Search in Google Scholar
[4] FAO. Transboundary River Basin overview – Mekong. Rome, Italy: FAO; 2011. Available from: http://www.fao.org/publications/card/en/c/CA2135EN/Search in Google Scholar
[5] Camberato J. Irrigation water quality: update from the 2001 Carolinas Golf Course Superintendents Association. Clemson University Turfgrass Program; 2001. p. 1–13. Available from: http://www.scnla.com/Irrigation_Water_Quality.pdfSearch in Google Scholar
[6] Brouwer C, Goffeau A, Heibloem M. Irrigation water management: training manual no. 1-introduction to irrigation. Rome, Italy: Food and Agriculture Organization of the United Nations; 1985. p. 102–3. Available from: http://www.fao.org/tempref/agl/AGLW/fwm/Manual1.pdfSearch in Google Scholar
[7] Dubey RS. Photosynthesis in plants under stressful conditions. Handb Photosynth. 1996;859–75.Search in Google Scholar
[8] Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annu Rev Plant Physiol Plant Mol Biol. 2000 Jun;51(1):463–99. 10.1146/annurev.arplant.51.1.463.Search in Google Scholar
[9] Dudley LM. Salinity in the soil environment. Handbook of plant and crop stress. New York, USA: Marcel Dekker; 1994. p. 13–30.Search in Google Scholar
[10] McCauley A, Jones C, Jacobsen J. Basic soil properties. Soil Water Manag Module. 2005;1(1):1–12.Search in Google Scholar
[11] USDA. Soil electrical conductivity – soil quality kit. Washington: United States Department of Agriculture; 2010.Search in Google Scholar
[12] Abrol IP, Yadav JSP, Massoud FI. Salt-affected soils and their management. Rome: Food and Agriculture Organization of the United Nations; 1988. p. 131 (FAO soils bulletin).Search in Google Scholar
[13] Ağar Aİ. Reclamation of saline and sodic soil by using divided doses of phosphogypsum in cultivated condition. AJAR. 2011 Sep 12;6(18):4243–52. 10.5897/AJAR11.943.Search in Google Scholar
[14] Grattan SR, Grieve CM. Salinity–mineral nutrient relations in horticultural crops. Sci Horticulturae. 1998 Nov;78(1–4):127–57. 10.1016/S0304-4238(98)00192-7.Search in Google Scholar
[15] Balba AM. Management of problem soils in arid ecosystems [Internet]. 1st edn. Boca Raton, USA: CRC Press; 2018 [cited 2020 Jul 3]. Available from: https://www.taylorfrancis.com/books/978135143421810.1201/9780203748411Search in Google Scholar
[16] Bauder T, Waskom R, Davis J. Irrigation water quality criteria. Colorado: Colorado State University; 2004. Available from: https://extension.colostate.edu/topic-areas/agriculture/irrigation-water-quality-criteria-0-506/Search in Google Scholar
[17] Sheldon A, Menzies NW, So HB, Dalal R. The effect of salinity on plant available water. SuperSoil 2004: 3rd Australian New Zealand Soils Conference. Australia: University of Sydney; 5–9 December 2004.Search in Google Scholar
[18] Bauder TA, Davis JG, Waskom RM. Managing saline soils. Fact sheet (Colorado State University. Extension); 2004. Crop series; no. 0.503. Available from: https://mountainscholar.org/bitstream/handle/10217/182968/AEXT_005032014.pdfSearch in Google Scholar
[19] Gianello C, Bremner JM. Comparison of chemical methods of assessing potentially available organic nitrogen in soil. Commun Soil Sci Plant Anal. 1986 Feb 1;17(2):215–36. 10.1080/00103628609367709.Search in Google Scholar
[20] Olsen SR. Estimation of available phosphorus in soils by extraction with sodium bicarbonate (No. 939). US Department of Agriculture; 1954.Search in Google Scholar
[21] Nelson DW, Sommers LE. Total carbon, organic carbon, and organic matter. In: Page AL, editor. Agronomy monographs [Internet]. Madison, WI, USA: American Society of Agronomy, Soil Science Society of America; 2015 [cited 2020 Jul 3]. p. 539–79. Available from: http://doi.wiley.com/10.2134/agronmonogr9.2.2ed.c2910.2134/agronmonogr9.2.2ed.c29Search in Google Scholar
[22] Houba V, Van der Lee J, Novozamsky I, Walinga I. Soil and plant analysis, a series of syllabi, part 5, soil analysis procedures. Wageningen: Wageningen Agricultural University; 1989.Search in Google Scholar
[23] Allison LE, Bernstein L, Bower CA, Brown JW, Fireman M, Hatcher JT, et al. Diagnosis and improment of saline and Alkali Soils. Washington: United States Salinity Laboratory Staff; 1954.Search in Google Scholar
[24] Boyd CE, Tucker CS. Pond aquaculture water quality management [Internet]. Boston, MA: Springer US; 1998 [cited 2020 Jul 3]. Available from: http://link.springer.com/10.1007/978-1-4615-5407-310.1007/978-1-4615-5407-3Search in Google Scholar
[25] Thu TA, Guong VT. Surveying the quality of soil, water environment in aquaculture and nutrient accumulation in aquaculture ponds in two districts of Vinh Chau and My Xuyen in Soc Trang Province. Can Tho: Can Tho University; 2010.Search in Google Scholar
[26] Marschner H. Marschner’s mineral nutrition of higher plants [Internet]. Academic Press, Elsevier; 2012 [cited 2020 Jul 3]. Available from: https://linkinghub.elsevier.com/retrieve/pii/C20090630439Search in Google Scholar
[27] Brady NC, Weil RR. Soil organic matter. The nature and properties of soils. Upper Saddle River, New Jersey: Prentice Hall; 1999. p. 446–90.Search in Google Scholar
[28] Ladeiro B. Saline agriculture in the 21st century: using salt contaminated resources to cope food requirements. J Botany. 2012 Aug 9;2012:1–7. 10.1155/2012/310705.Search in Google Scholar
[29] Hung NN. EC extraction method and conversion for the rice-shrimp saline soils assessment scale in the Mekong Delta (Vietnamese Version). J Agric Rural Dev. 2010;5:45.Search in Google Scholar
[30] NRCS. Soil survey division staff (1993) soil survey manual. Soil conservation service. US Department of Agriculture Handbook; 1993.Search in Google Scholar
[31] Ayers RS, Westcot DW. Water quality for agriculture. Vol. 29, Rome: Food and Agriculture Organization of the United Nations; 1985.Search in Google Scholar
[32] Otton JK, Zielinski RA. Characteristics and origins of saline (alkalai) soil in the front range portion of the Western Denver Basin. Lakewood, Colorado: US Geological Survey; 2000.Search in Google Scholar
[33] Takai Y, Wada H. Effects of water percolation on fertility of paddy soils. Proceedings of the international seminar on soil environment and fertility management in intensive agriculture; 1977.Search in Google Scholar
[34] Kanke B, Kanazawa S. Effect of drainage on soil saccharides and microbial activities in poorly drained paddy fields. Proceedings of the transactions of the 13th international congress of soil science. Hamburg; 1986.Search in Google Scholar
[35] Rietz DN, Haynes RJ. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol Biochem. 2003 Jun 1;35(6):845–54.10.1016/S0038-0717(03)00125-1Search in Google Scholar
[36] Sagot B, Gaysinski M, Mehiri M, Guigonis J-M, Le Rudulier D, Alloing G. Osmotically induced synthesis of the dipeptide N-acetylglutaminylglutamine amide is mediated by a new pathway conserved among bacteria. Proc Natl Acad Sci USA. 2010 Jul 13;107(28):12652–7.10.1073/pnas.1003063107Search in Google Scholar PubMed PubMed Central
[37] Oren A. The bioenergetic basis for the decrease in metabolic diversity at increasing salt concentrations: implications for the functioning of salt lake ecosystems. In: Melack JM, Jellison R, Herbst DB, editors. Saline Lakes: publications from the 7th international conference on Salt Lakes, held in Death Valley National Park, California, USA, September 1999 [Internet]. Dordrecht: Springer Netherlands; 2001 [cited 2021 Feb 7]. p. 61–72 (Developments in Hydrobiology). Available from: https://doi.org/10.1007/978-94-017-2934-5_610.1007/978-94-017-2934-5_6Search in Google Scholar
[38] Gros R, Poly F, Jocteur Monrozier L, Faivre P. Plant and soil microbial community responses to solid waste leachates diffusion on grassland. Plant Soil. 2003 Aug 1;255(2):445–55.10.1023/A:1026083320313Search in Google Scholar
[39] Wichern J, Wichern F, Joergensen RG. Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma. 2006 Dec 31;137(1):100–8.10.1016/j.geoderma.2006.08.001Search in Google Scholar
[40] Pankhurst CE, Yu S, Hawke BG, Harch BD. Capacity of fatty acid profiles and substrate utilization patterns to describe differences in soil microbial communities associated with increased salinity or alkalinity at three locations in South Australia. Biol Fertil Soils. 2001 Mar 1;33(3):204–17.10.1007/s003740000309Search in Google Scholar
[41] Landon JR. Booker tropical soil manual: a handbook for soil survey and agricultural land evaluation in the tropics and subtropics. Routledge: Booker Agriculture International Limited; 2016.Search in Google Scholar
[42] Makihara D, Tsuda M, Morita M, Hirai Y, Kuroda T. Effect of salinity on the growth and development of rice(Oryza sativa L.) varieties. Japanese Society for Tropical Agriculture; 1999 [cited 2021 Feb 7]. 10.11248/jsta1957.43.285.10.11248/jsta1957.43.285Search in Google Scholar
[43] Khang DT, Dung TN, Thai LV, Nam HD, Be VT. Evaluation of alum tolerance of some MTL rice varieties (Oryza sativa L.) (Vietnamese Version). Sci J Can Tho Univ. 2016;44B:86–95.10.22144/ctu.jvn.2016.467Search in Google Scholar
[44] Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Botany. 2007 Mar 1;59(2):206–16.10.1016/j.envexpbot.2005.12.006Search in Google Scholar
[45] Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, et al. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002;30(5):529–39.10.1046/j.1365-313X.2002.01309.xSearch in Google Scholar
[46] Khattak SG, Haq IU, Malik A, Khattak MJ, Naveedullah MJ. Effect of various levels of gypsum application on the reclamation of salt affected soil grown under rice followed by wheat crop. Sarhad J Agric. 2007;23(3):675.Search in Google Scholar
[47] Thu TA, Guong VT, Hoa NV. The mineralization of organic nitrogen in the bottom soil of the artemia pond in Vinh Chau, Soc Trang. Sci J Can Tho Univ. 2007;7:176–82.Search in Google Scholar
[48] Nambiar KKM, Ghosh AB. Highlights of research of a long-term fertilizer experiment in India (1971–1982). New Delhi, India: Indian Agriculture Institute; 1984 (No. REP-7203. CIMMYT).Search in Google Scholar
[49] De Datta SK, Gomez KA, Descalsota JP. Changes in yield response to major nutrients and in soil fertility under intensive rice cropping. Soil Sci. 1988;146(5):350–8.10.1097/00010694-198811000-00007Search in Google Scholar
[50] Dobermann A, Cassman KG. Precision nutrient management in intensive irrigated rice systems – the need for another on-farm revolution. Better Crop Int. 1996;10(2):21.Search in Google Scholar
[51] Wood GA, Lass RA. Cocoa. Oxford: Blackwell; 2008.Search in Google Scholar
[52] Deurer M, Sivakumaran S, Ralle S, Vogeler I, McIvor I, Clothier B, et al. A new method to quantify the impact of soil carbon management on biophysical soil properties: the example of two apple orchard systems in new zealand. J Environ Qual. 2008;37(3):915–24.10.2134/jeq2007.0508Search in Google Scholar PubMed
[53] Vien DM, Guong VT, Dong NM, Phuong NTK. Using organic fertilizers from bagasse to improve phosphorus nutrition and reduce AC toxicity to acid sulfate soils (Vietnamese Version). Sci J Can Tho Univ. 2006;118–25.Search in Google Scholar
© 2021 Lam Van Tan and Tran Thanh, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

