Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
-
Minh-Tam Nguyen-Kim
and Xuan-Tien Le
Abstract
In the present study, the optimized solvent extraction conditions with regards to the total polyphenol content (TPC) and antioxidant capacity of rosemary leaf extract (RLE) were determined. The one-factor-at-a-time method was used to independently investigate the effect of several extraction parameters, including ethanol concentration (0–100% v/v), extraction temperature (50–80°C), extraction period (15–60 min), material–solvent ratio (1:5–1:10 g/mL), and extraction cycles (1, 2, and 3 times) on polyphenol content. Response surface methodology (RSM), in combination with a central composite design, was used to perform optimization. The following optimal conditions that gave maximal TPC were determined and experimentally verified: ethanol concentration of 65% (v/v), extraction temperature of 65°C, material–solvent ratio of 1:7.5 g/mL, extraction time of 15 min, and 2 cycles of extraction. These parameters corresponded with the TPC yield of 87.42 ± 0.25 mg gallic acid equivalent/g dried feed material (mg GAE/g DW). The optimal conditions gave a high extraction yield (337 ± 6 mg dried extract/g dried feed material) with 197.28 ± 3.11 mg GAE/g dried extract. The estimated models were strongly significant (p < 0.05) for TPC values with significant regression coefficients (R 2) of 0.9979. The obtained RLE was supposed to be the top grade of natural antioxidant with the IC50 (DPPH assays) value of 9.4 ± 0.1 μg/mL, which is higher than that of the vitamin C by just three times (IC50 = 3.2 ± 0.1 μg/mL). Current results justify RLE as a potential agent in food preservation applications.
1 Introduction
Various examinations have shown that herbs have powerful antioxidant properties, because of the amount and nature of phenolic compounds present in them [1]. Rosemary (Rosmarinus officinalis L.) is a restorative herb that is broadly utilized throughout the world. Among natural antioxidant plants, rosemary has been acknowledged as the most noteworthy cancer prevention plant [2]. These antioxidant properties were identified with the presence of phenolic contents, accounting for the most majority rosmarinic acid (RA), carnosic acid (CA), and carnosol (COH) [3,4].
Rosemary finds extensive use in various applications. In various Western countries, rosemary leaf extract (RLE) has been commercialized as an antioxidant and flavorant [5]. RLE has also been proved to exhibit hepato-protective properties, suggesting the potential use in medicinal applications for treatment of maladies [6], Alzheimer’s malady [7], and angiogenesis-related diseases [8]. In addition, they have been used in product preservation due to their ability to restrict oxidation and microbial defilement [9,10,11,12]. In this manner, RLE could be valuable for supplanting or indeed diminishing synthetic preservative in foods.
Techniques for extraction of antioxidants from herbal plants have been reported in several studies. For example, it has been reported that ultrasound-assisted extraction (UAE) of marjoram using methanol solvent could afford cancer prevention agents, including RA, luteolin-7-O-glucoside, apigenin-7-O-glucoside, caffeic acid, CA, and COH [13]. For rosemary, developed extraction methods for recovery of antioxidant compounds included UAE [14], solvent extraction [15], pressurized green dissolvable solvent extraction [16], and CO2 supercritical liquid extraction [17,18].
Although there have been several studies on rosemary extraction methods, the optimal production conditions for solvent extraction process have not been adequately reported. One exception is the report of Hosseini et al. (2018) [19] where optimization of heat-assisted extraction (HAE) and UAE conditions was carried out, but lacked a single-factor investigation to narrow the survey amplitude. This resulted in limited selectivity, insignificant influence of response, and wide amplitude of the surveyed parameters (e.g., extraction time and liquid-solid ratio), causing waste or deviation from the optimal point.
In this study, the extraction process of rosemary was optimized with respect to maximal recoveries of total polyphenol and antioxidant ability of the afforded extract. Response surface methodology (RSM) in conjunction with central composite design (CCD) were adopted to guide optimization experiments [20,21]. Experimental conditions that were taken into consideration for optimization consisted of ethanol concentration, extraction temperature, time of extraction, material–solvent ratio, and the number of extraction cycles. Obtained findings are expected to aid in improving the existing extraction processes and justifying the use of rosemary materials in production of phenolic-rich plant extracts.
2 Materials and methods
2.1 Plant sample preparation
The rosemary leaves were harvested at Lam Ha District, Lam Dong Province, Vietnam, in February 2020. The material was dried at 45°C by using forced air convection oven until it reached a moisture content of less than 12%. This temperature was based on the previous studies as the condition allowed high retention of important compounds in rosemary leaves [22,23]. Moisture content was monitored by a moisture meter (MA35, Sartorius, Göttingen, Germany). Dried rosemary leaves were preserved in zip bags and placed in a dry and ventilated place.
2.2 Extraction process
Dried rosemary leaves were extracted with ethanol using the maceration method. The mixture was stirred at 200 rpm and heated up at a specified temperature and then filtrated under vacuum. The remaining solid residue was then extracted for a second time under similar conditions. Finally, an aliquot of the filtered extract was dried under a vacuum drier at 60°C to dryness to remove the solvent and the yield of extraction was identified.
2.3 Quantification of total polyphenol contents (TPCs) based on Folin-Ciocalteu (FC) methodology
The polyphenol contents were determined following previously described methods [24] with certain modifications. Dried extract and 40 μL of DMSO were added to 200 μL of FC reagent, and the mixture was vibrated for 5 min with ultrasonic unit (Elma S 100 H, Elma, Singen, Germany). Then, 3,160 μL of H2O and 600 μL of Na2CO3 were added to the mixture to dilute and support the reaction. The samples were vibrated for 30 min at room temperature and measured at λ = 760 nm by Thermo Scientific GENESYS 10S Series UV-Visible Spectrophotometer.
The TPC was expressed as a gallic acid equivalent (GAE) from the following calibration curve: Concentration (ppm) = 0.0012 × Absorbance − 0.0179 of GA standard solution (R 2 = 0.9951) and expressed as mg of GAE per gram of dry extract. The amount of TPC was calculated using the following equation:
where C 1 is the concentration obtained from gallic acid standard curve (mg GAE/mL DMSO), C 2 is the concentration of the dried extract (mg dried extract/mL DMSO), m 1 is the weight of the total dried extract (mg), and m is the weight of the dried material (g).
2.4 Antioxidant evaluation with 1,1-diphenyl-2-picrylhydrazyl (DPPH)
DPPH assays were carried out following method of Brand-Williams [25]. DPPH was first blended in with methanol (Sigma-Aldrich) of 80% (with optical density at 517 nm of 0.80 ± 0.02) to form the solution with the concentration of 40 µg/mL. Afterwards, 180 µL of the as-prepared DPPH solution was added with 120 µL of another mixture containing the sample dissolved in 80% of methanol. The obtained solution was then shaken, taken into storage for 30 min at 30°C in dimness, and measured for optical density at 517 nm. Positive controls of different concentrations (0–100 µg/mL) were prepared by dissolving vitamin C (Sigma-Aldrich) in 80% of methanol. Moreover, the color solution was prepared including MeOH and the sample solution. The blank solution was also prepared using DPPH solution and MeOH solution. The percentage of DPPH radical scavenging ability was calculated as follows:
where A is the optical density. The subscript b, c, and s denote the blank sample, the tested sample, and the color solution, respectively. IC50 value was calculated by graphing the concentration against % of inhibition.
2.5 Optimization of polyphenol extraction by using RSM
The process was optimized following an RSM procedure with CCD. The statistical model took TPC of RLE as the response and three experimental parameters including ethanol concentration (X
1), temperature (X
2), and material–solvent ratio (X
3) were taken into consideration as independent variables. To identify the suitable levels of parameters for CCD, a series of single-factor investigations were carried out by varying three parameters in the following order: ethanol concentration, temperature, and dry material–solvent ratio. Three different levels, which are low (−1), medium (0), and high (+1), were then assigned to the obtained set of optimum parameters (Table 1), which was then used in CCD. A total of 20 combinations consisting of eight factorial points, six axial points, and six center points were generated by CCD. Distance from center to point α =
where Y indicates the dependent variable (total polyphenol), X indicates experimental conditions and β denotes model coefficients. The subscript 0, i, j, and ij represent the constant, linear terms, the quadratic terms, and interactive terms, respectively.
Experimental conditions for the rates used in the trial
| Factor | Symbol | Level | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| EtOH concentration (%) | X 1 | 60 | 70 | 80 |
| Temperature (°C) | X 2 | 50 | 60 | 70 |
| Material–solvent ratio (mL/g) | X 3 | 1:6 | 1:8 | 1:10 |
The estimated model was then tested with Analysis of variance (ANOVA) for determination of significant terms. Statistical significance was recognized at 5%. Post-estimation statistics, including R-squared, adj-R-squared, predicted-R-squared, F-value, and lack-of-fit were used to evaluate model adequacy.
3 Results and discussion
3.1 Single-factor investigations on TPC
3.1.1 Influence of ethanol concentration
Ethanol has been commonly used as the extraction solvent for recovery of naturally active compounds from plants due to its safety and health-benign nature. Figure 1 illustrates the TPC values of RLE obtained at different ethanol concentrations ranging from 0 to 99.5%. The TPC recovered from the extract at 60% of ethanol (56.5 mg GAE/g DW) was significantly higher than those obtained at lower ethanol concentrations (30 and 0%). Rising the ethanol concentration from 60 to 70% slightly improved TPC yield to 59.0 (mg GAE/g DW) which was the peak of the investigation. At ethanol concentrations higher than 70%, obtained TPC was significantly lower than those at lower concentrations and minimum TPC yield, 27.6 (mg GAE/g DW), was reached at absolute ethanol.
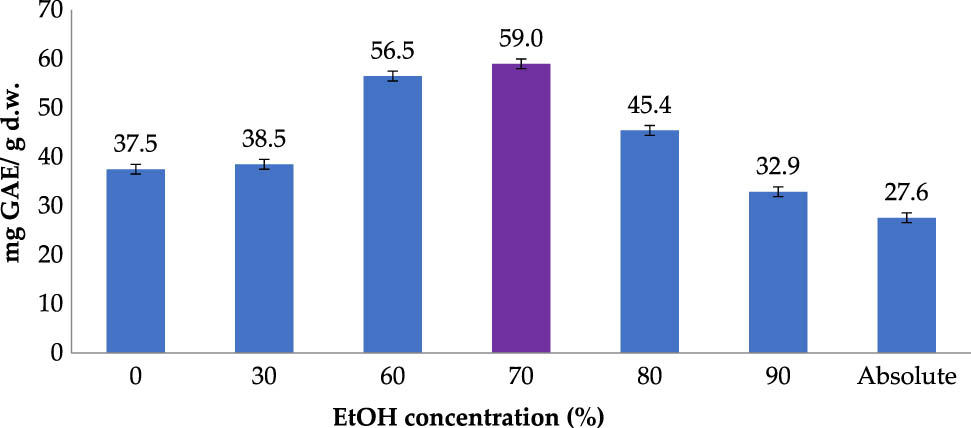
Polyphenol extraction affected by ethanol concentration (Operating conditions: temperature of 50°C; material–solvent ratio of 1:5 g/mL; operating time of 30 min; and 2 extraction cycles).
The relationship could be explained as follows. At ethanol concentration of around 70%, the presence of water in solvent causes the rosemary leaf matrix to be moderately swelled, thus facilitating the permeation of solvent into the material [27]. Therefore, as the ethanol concentration increased, lower water content in solvent led to weaker swelling of material, negatively affecting the extraction efficiency. By contrast, the high quantity of water in the solvent leads to excessive swelling of the material and unfavorable solvent polarity, both of which contributed to the lowered extraction yield.
3.1.2 Influence of temperature
Figure 2 illustrates the TPC of RLE obtained at different extraction temperatures. Significant yield improvement was observed as the temperature was elevated from 50 to 60°C, achieving the peak TPC of 42.4 (mg GAE/g DW). Further temperature elevations past 60°C seemed to cause negligible improvements in TPC obtained. Moreover, TPC diminished marginally to the point of 40.5 (mg GAE/g DW) when the temperature was increased to 80°C. This might be due to the thermal degradation of polyphenol. In addition, the mechanism through which increasing temperatures improve extraction recovery could be explained by increased diffusion of the solvent, increased mass transfer, and improved solubility of phenolic compounds in the solvent [28]. The temperature of 60°C seemed to give the highest TPC in RLE and was chosen for the next investigation. This result is in line with a previous report where 70°C was determined as the extraction temperature at which TPC of ethanolic RLE began to decline [29].
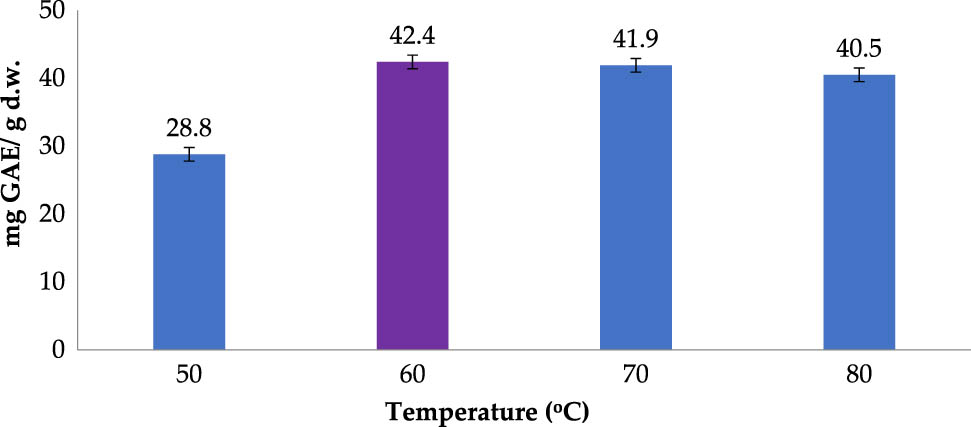
Polyphenol extraction affected by temperature (Operating conditions: ethanol concentration of 70% (v/v); material–solvent ratio of 1:5 g/mL; operating time of 30 min; and 2 extraction cycles).
3.1.3 Influence of dry material–solvent ratio
In real scale production processes, the amount of used solvent significantly affects variable costs and the product quality. Therefore, an investigation has been carried out in order to determine the minimum amount of used solvent possible to recover majority of the active compound.
Figure 3 shows that the TPC rose from 63.4 to 69.9 (mg GAE/g DW) and then peaked at 72.2 (mg GAE/g DW) as the material–solvent proportion increased from 1:6 to 1:8 g/mL and then to 1:10 g/mL. At the equilibrium state, where no extractable polyphenols are present in the material, further addition of ethanol would not advance the TPC abdicate any longer. Improvement in TPC when rising the ratio from 1:6 to 1:8 could be due to thr enhanced penetration of solvent through cell membrane and oil bags under thermal treatment, which help pushing active compound out [30]. The difference in yield of polyphenol extraction between solvent ratios 1:8, 1:9, and 1:10 was modest and unnoticeable, while the amount of additional solvent required to achieve such improvement was large (10%). Therefore, the raw material–solvent proportion of 1:8 was more temperate and chosen for consequent tests.
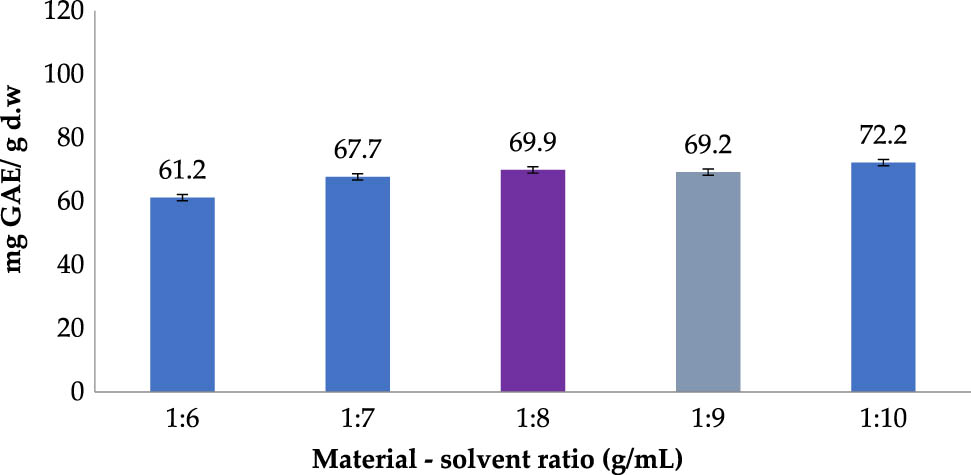
Polyphenol extraction affected by material–solvent ratio (Operating conditions: ethanol concentration of 70% (v/v); temperature of 60°C; operating time of 30 min; and 2 extraction cycles).
3.1.4 Influence of extracting time
The longer the extracting time was, the higher the efficiency would be. However, until a certain time, lengthening the extracting time could not raise up the efficiency. On the other hand, it caused wastage of energy and solvent.
In accordance with Figure 4, prolonging the extraction time from 15 to 45 min caused the TPC to slightly increase, implying that time did not significantly affect the TPC obtained. The process achieved equilibrium rapidly, which might be due to small material size, high porosity of materials, high dispersion due to stirring, and high employed temperature. However, inconsiderable change in TPC between 15 and 45 min is not justifiable by significantly higher required time and energy consumption. Our result is in accordance with a previous report where antioxidant of RLE saw unnoticable improvements after 15 min of extraction [31]. Therefore, 15 min should be applied for the extracting step.
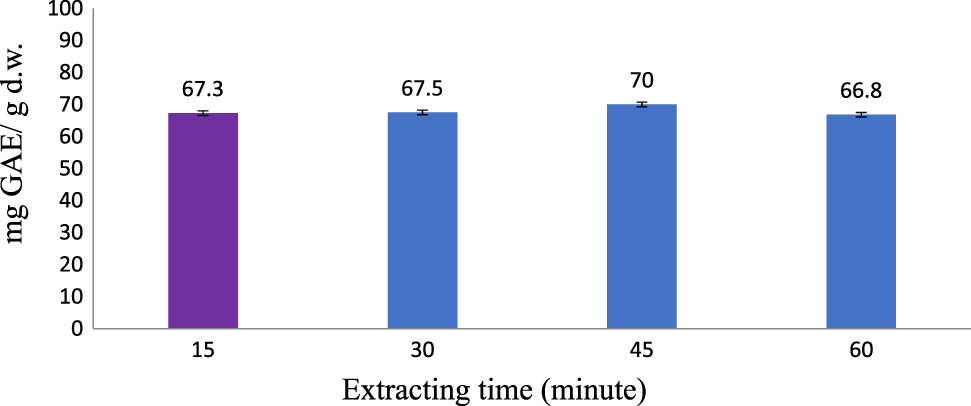
Polyphenol extraction affected by extracting time (Operating conditions: ethanol concentration of 70% (v/v); temperature: 60°C; material–solvent ratio of 1:8 g/mL; and 2 extraction cycles).
3.1.5 Influence of number of extraction cycles
From Figure 5, it was suggested that TPC could not be thoroughly recovered by only one extraction cycle. It could clearly be seen that the cumulative yield after the second extraction cycle was significantly higher compared to the previous one, totaling 67.4 mg GAE/g DW (over 52% increase). After the third extraction cycle, cumulative TPC value increased by merely 8.8%, from 67.4 to 76.2 mg GAE/g DW, proposing that most of the TPC has been exhausted after the second-cycle. Given that the TPC improvement achieved after carrying out the third extraction cycle was not large and that the amount of used solvent and energy consumption for one extraction cycle was considerably high, two repeated extraction cycles were considered for application in the process.
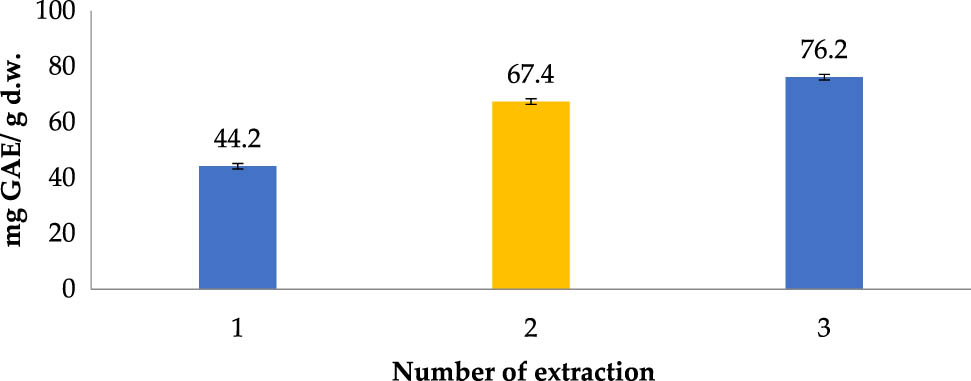
Polyphenol extraction affected by number of extraction (Operating conditions: ethanol concentration of 70% (v/v); temperature of 60°C; material–solvent ratio of 1:8 g/mL; and operating time 15 min).
3.2 Optimization of extraction condition from rosemary
3.2.1 Model fitting using RSM
The responses (TPC) of experimental runs guided by CCD are summarized as in Table 2. Among the examined variables in single-factor investigations, extraction time and extraction cycle were fixed at their respective optimal levels (15 min and 2 cycles, respectively).Variations in three experimental parameters for these runs were as follows: ethanol concentration X 1 (60–90% v/v), extraction temperature X 2 (50–70°C), and material–solvent ratio X 3 (1:6–1:10 g/mL). Generally, TPC of RLE ranged from 62.79 to 87.63 (mg GAE/g DW). The highest predicted response was 87.63 (mg GAE/g DW), which was obtained at the center point.
CCD of actual factors and responses based on actual and predicted values
| Run | Factors | Response (mg GAE/g DW) | |||
|---|---|---|---|---|---|
| X 1 | X 2 | X 3 | Y TPC (actual) | Y TPC (predicted) | |
| 1 | 60 | 50 | 1:6 | 66.58 | 66.59 |
| 2 | 80 | 50 | 1:6 | 63.01 | 63.02 |
| 3 | 60 | 70 | 1:6 | 82.61 | 82.30 |
| 4 | 80 | 70 | 1:6 | 79.62 | 79.19 |
| 5 | 60 | 50 | 1:10 | 80.89 | 80.86 |
| 6 | 80 | 50 | 1:10 | 72.12 | 71.96 |
| 7 | 60 | 70 | 1:10 | 83.98 | 83.74 |
| 8 | 80 | 70 | 1:10 | 76.01 | 75.53 |
| 9 | 53.2 | 60 | 1:8 | 83.21 | 83.32 |
| 10 | 86.8 | 60 | 1:8 | 72.68 | 73.22 |
| 11 | 70 | 43.2 | 1:8 | 63.01 | 62.79 |
| 12 | 70 | 76.8 | 1:8 | 78.59 | 79.24 |
| 13 | 70 | 60 | 1:4.6 | 74.01 | 74.35 |
| 14 | 70 | 60 | 1:11.3 | 82.95 | 83.27 |
| 15 | 70 | 60 | 1:8 | 88.12 | 87.63 |
| 16 | 70 | 60 | 1:8 | 87.25 | 87.63 |
| 17 | 70 | 60 | 1:8 | 86.89 | 87.63 |
| 18 | 70 | 60 | 1:8 | 87.69 | 87.63 |
| 19 | 70 | 60 | 1:8 | 88.01 | 87.63 |
| 20 | 70 | 60 | 1:8 | 87.95 | 87.63 |
ANOVA results and estimated coefficients were reported as in Table 3. Most of the model terms, except for the X 1 X 2 interaction, were statistically significant (p < 0.05). High coefficient of determination (R 2) of 0.9979 suggests that most of the variance in the response could be predicted by estimated variables. The close agreement between R 2 and adjusted R 2 also indicates suitability of the model. Lack-of-fit was not significant and greater than 4, proposing that current model specification is sufficient to reliably predict the outcome, as confirmed by minor variations between predicted and actual responses in Table 2. The reduced second-order polynomial function that describes the relationship between TPC and parameters is as follows:
Analysis of Variance (ANOVA) results for the quadratic model for optimization
| Factors | TPC | |||
|---|---|---|---|---|
| Source | Sum of squares | F-value | p-value | — |
| Model | 1308.19 | 524.60 | <0.0001 | Significant |
| X 1 | 123.14 | 444.44 | <0.0001 | — |
| X 2 | 317.25 | 1144.97 | <0.0001 | — |
| X 3 | 96.04 | 346.60 | <0.0001 | — |
| X 1 X 2 | 0.2380 | 0.8591 | 0.3758 | Not significant |
| X 1 X 3 | 12.95 | 46.75 | <0.0001 | Significant |
| X 2 X 3 | 82.30 | 297.04 | <0.0001 | — |
|
|
157.77 | 569.41 | <0.0001 | — |
|
|
490.64 | 1770.78 | <0.0001 | — |
|
|
140.25 | 506.17 | <0.0001 | — |
| Residual | 2.77 | — | — | — |
| Lack of Fit | 1.59 | 1.35 | 0.3753 | Not significant |
| Pure Error | 1.18 | — | — | — |
| Cor Total | 1310.96 | — | — | — |
| Coefficient of Variation | 0.6641 | — | — | — |
| PRESS | 13.74 | — | — | — |
| R 2 | 0.9979 | — | — | — |
| R 2 Adjusted | 0.9960 | — | — | — |
| R 2 Predicted | 0.9895 | — | — | — |
| Adequate precision | 66.7504 | — | — | — |
3.2.2 Effect of process variables on total phenolic compounds
As shown by the estimated model, the effect of the ethanol concentration and extraction temperature on the production of phenolic compounds was more pronounced than that of material–solvent ratio. To further explore the interaction effects of parameters on the response, three-dimensional response surface plots (Figure 6) were plotted. In each plot, two variables were allowed to vary and the other variable was kept at its central point.
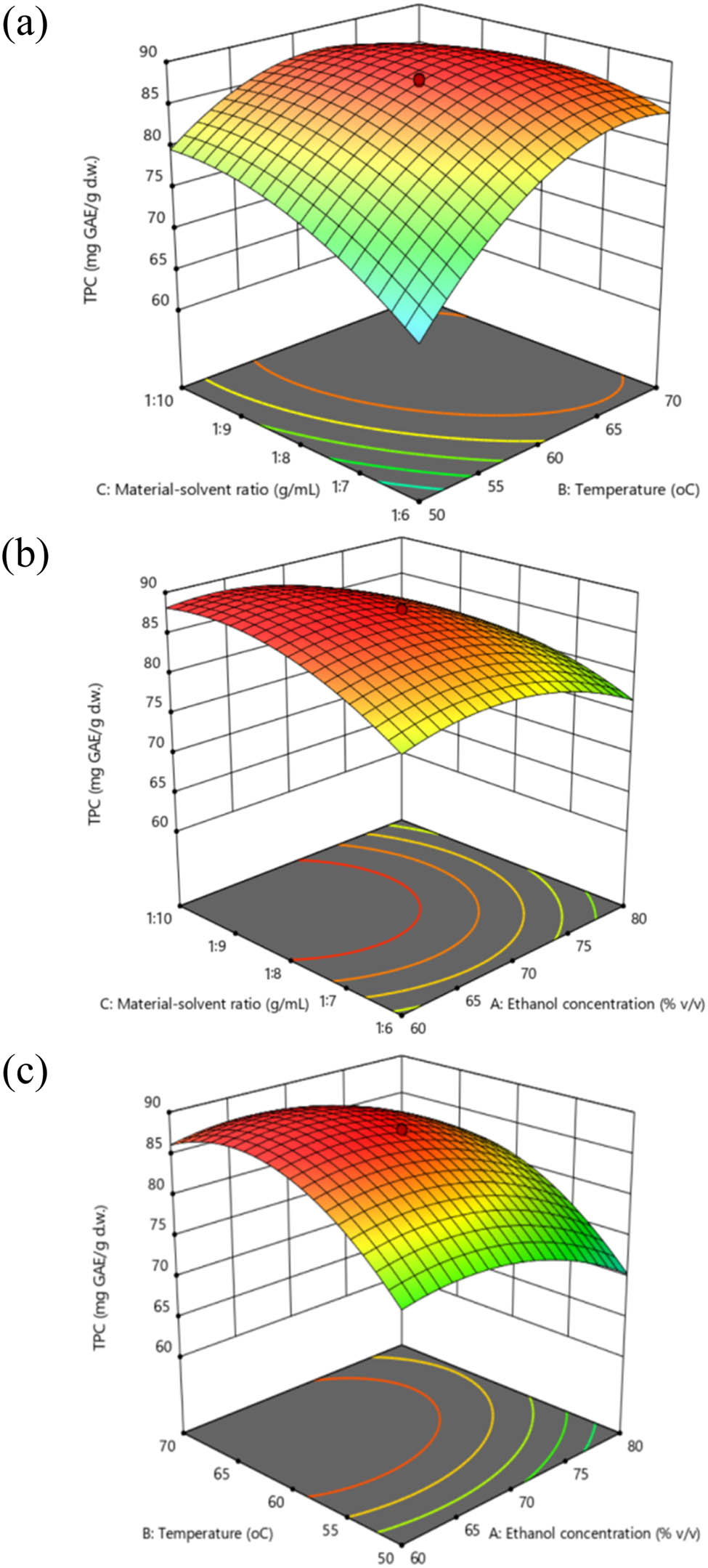
Response surface plots for the impact of (a) ethanol/temperature, (b) ethanol/material–solvent ratio, and (c) material–solvent ratio/temperature.
At constant material–solvent ratio (1:8), the impact of ethanol concentration and extraction temperature on TPC seemed to follow a plateau shape (Figure 6a). As seen, in ethanol concentration range of 60–70% (v/v) and temperature range of 60–70°C, a higher amount of phenolic compound was obtained. TPC gradually mounted with a rise in the concentration and temperature of ethanol and reached an optimum value at around 65% (v/v) and 65°C until it began to decline. In fact, with the addition of water to ethanol, the polarity of the mixture would increase continuously in order to extract more polar phenolic compounds. Therefore, phenolics derived using 70% of ethanol might be shown to be better than those of 80% of ethanol.
Figure 6b demonstrates the obtained TPC with respect to varying ethanol concentration and material–solvent ratio under a fixed extraction temperature of 60°C. Concentration of ethanol demonstrated a strong linear and quadratic effect on TPC (Table 3). Greater solvent quantity exerted positive effects on TPC. The increase in yields with the rise in solvent quantity was compatible with the principles of mass transfer. To be specific, when a lower solid to solvent ratio is used, the gradient of the concentration difference is expected to be higher and thus, might lead to promoted diffusion. On the other hand, using less solvent for extraction while still maintaining high yield is desirable from an economic point of view.
The relationship between extraction temperature and material–solvent ratio to TPC is shown in Figure 6c. The TPC of RLE increased rapidly with temperature up to 65°C, at which point TPC began to gradually decline thereafter. This could be rationalized by various phenomena that often occur under mild heating, including softened plant tissues, weakened cell wall, improved phenolic solubility, and hydrolysis of bonds that bind phenol with protein or with polysaccharide [32,33,34].
3.2.3 Effect of process variables on total phenolic compounds
Optimizing the estimated model with respect to maximal response yielded optimal conditions. These parameters were then slightly adjusted for convenience and attempted to verify the results. Table 4 shows the parameters and results of confirmation experiments. Mean TPC obtained after triplicate experiment was 87.42 (mg GAE/g DW), which approximates the predicted TPC. By applying the matched t-test, no critical change among real and anticipated qualities (p < 0.05) was recognized. Hence, the estimated model was satisfactory in predicting the TPC yield.
The results of optimum condition experiment
| EtOH Concentration (% v/v) | Extraction temperature (°C) | Material–solvent ratio (g/mL) | Predicted TPC (mg GAE/g DW) | Actual TPC (mg GAE/g DW) | Error with model (%) |
|---|---|---|---|---|---|
| 65 | 65 | 1:7.5 | 88.64 | 88.12 | 0.41 |
| 87.25 | 1.10 | ||||
| 86.89 | 1.39 | ||||
| Average TPC ± standard deviation | 87.42 ± 0.25 | ||||
In optimum conditions, a high extraction yield (337 ± 6 mg dried extract/g dried feed material) and TPC (87.42 ± 0.25 mg GAE/g dried material) were achieved. This corresponded to TPC of 197.28 ± 3.11 mg GAE/g dried extract, which was calculated on the basis of dried extract and marginally higher than that calculated by Hosseini et al. [19]. Previously, the optimal conditions for HAE of dried rosemary included extraction time of 132.80 min, extraction temperature of 70°C, and solvent-material ratio of 20:1, giving extraction yield of 17.80%, which was equivalent to TPC of 147.56 mg GAE/g DW. Generally, when a longer extraction time is used, higher concentrations of polyphenols are obtained, whereas polyphenol degradation can also occur when longer treatment periods are correlated with high temperatures. Our results are also in accordance with the kinetic study of Jurinjak Tušek et al. (2016) [35] who examined solid-liquid extraction process of Asteraceae family plants and indicated that contents of bioactive compounds were exhaustive after the initial 10 min of the extraction process.
3.3 Evaluating the antioxidant ability
The antioxidant capacity of RLE was directly proportional to the concentration used. The RLE decreased the absorbance of DPPH solution to half of its initial value at a concentration of 9.4 ± 0.1 μg/mL, which is higher than that of the vitamin C by just three times (3.2 ± 0.1 μg/mL). This result was almost the same as the results of Klančnik et al. (2009), which illustrated the IC50 value of several commercial rosemary extract formulations to be 7.4–22.7 μg/mL [36]. The results clearly showed that RLE was among the top of natural antioxidants.
4 Conclusion
RSM was successfully used to determine the optimal conditions for the extraction of rosemary polyphenol. The second-order polynomial model provided a satisfactory description of experimental data. The optimum conditions for obtaining polyphenol of Rosmarinus officinalis L. involved an EtOH concentration of 65% (v/v), a material–solvent ratio of 1:7.5, and an extraction temperature of 65°C with corresponding TPC yield of 87.42 ± 0.25 (mg GAE/g DW). The optimum conditions produced a high extraction yield (337 ± 6 mg dried extract/g dried feed material) with 197.28 ± 3.11 mg GAE/g dried extract. The IC50 (DPPH assays) of the optimal extract (9.4 ± 0.1 μg/mL) was compared with the one recorded for vitamin C, confirming the antioxidant capacity of RLE.
Acknowledgements
The authors thank the team of researchers at the Ho Chi Minh City University of Technology (HCMUT) for their technical assistance.
-
Funding information: This research is funded by Ho Chi Minh City University of Technology (HCMUT) and Center For Business Incubation of Agricultural High Technology (AHBI).
-
Author contributions: Investigation, Minh-Tam Nguyen-Kim, Quoc-Cuong Truong, Minh-Thuy Nguyen, Bich-Hang Cao-Thi, Thanh-Danh Tong, Tan Phat Dao, and Thien Hien Tran; Supervision, Xuan-Tien Le and Lam Van Tan; Writing – original draft, Minh-Tam Nguyen-Kim; writing – review & editing, Tan Phat Dao and Thien Hien Tran. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors declare no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: The data presented in this study are available on request from the corresponding author.
References
[1] Hossain MB, Brunton NP, Barry-Ryan C, Martin-Diana AB, Wilkinson M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J Chem. 2008;1(4):751–6.Search in Google Scholar
[2] Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 2005 Oct 1;53(20):7749–59.10.1021/jf051513ySearch in Google Scholar
[3] Collins MA, Charles HP. Antimicrobial activity of Carnosol and Ursolic acid: two anti-oxidant constituents of Rosmarinus officinalis L. Food Microbiol. 1987 Sep 1;4(4):311–5.10.1016/S0740-0020(87)80005-9Search in Google Scholar
[4] Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006 Jan 1;40(2):223–31.10.1080/10715760500473834Search in Google Scholar
[5] Cuvelier M-E, Richard H, Berset C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J Am Oil Chemists’ Soc. 1996;73(5):645–52.10.1007/BF02518121Search in Google Scholar
[6] Rašković A, Milanović I, Pavlović N, Ćebović T, Vukmirović S, Mikov M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Altern Med. 2014 Jul 7;14(1):225.10.1186/1472-6882-14-225Search in Google Scholar
[7] Habtemariam S. The therapeutic potential of rosemary (rosmarinus officinalis) diterpenes for alzheimer’s disease. Evid Based Compl Alternat Med. 2016;2016:1–14.10.1155/2016/2680409Search in Google Scholar
[8] Kayashima T, Matsubara K. Antiangiogenic effect of carnosic acid and carnosol, neuroprotective compounds in rosemary leaves. Biosci Biotechnol Biochem. 2012 Jan 23;76(1):115–9.10.1271/bbb.110584Search in Google Scholar
[9] Djenane D, Sánchez-Escalante A, Beltrán JA, Roncalés P. Ability of α-tocopherol, taurine and rosemary, in combination with vitamin C, to increase the oxidative stability of beef steaks packaged in modified atmosphere. Food Chem. 2002 Apr 1;76(4):407–15.10.1016/S0308-8146(01)00286-2Search in Google Scholar
[10] Nieto G, Díaz P, Bañón S, Garrido MD. Dietary administration of ewe diets with a distillate from rosemary leaves (Rosmarinus officinalis L.): influence on lamb meat quality. Meat Sci. 2010 Jan 1;84(1):23–9.10.1016/j.meatsci.2009.08.001Search in Google Scholar PubMed
[11] Nieto G, Bañón S, Garrido MD. Incorporation of thyme leaves in the diet of pregnant and lactating ewes: Effect on the fatty acid profile of lamb. Small Rumin Res. 2012 Jun 1;105(1):140–7.10.1016/j.smallrumres.2011.11.016Search in Google Scholar
[12] Nieto G, Estrada M, Jordán MJ, Garrido MD, Bañón S. Effects in ewe diet of rosemary by-product on lipid oxidation and the eating quality of cooked lamb under retail display conditions. Food Chem. 2011 Feb 15;124(4):1423–9.10.1016/j.foodchem.2010.07.102Search in Google Scholar
[13] Hossain MB, Brunton NP, Patras A, Tiwari B, O’Donnell CP, Martin-Diana AB, et al. Optimization of ultrasound assisted extraction of antioxidant compounds from marjoram (Origanum majorana L.) using response surface methodology. Ultrason Sonochem. 2012 May;19(3):582–90.10.1016/j.ultsonch.2011.11.001Search in Google Scholar PubMed
[14] Paniwnyk L, Cai H, Albu S, Mason TJ, Cole R. The enhancement and scale up of the extraction of anti-oxidants from Rosmarinus officinalis using ultrasound. Ultrason Sonochem. 2009 Feb;16(2):287–92.10.1016/j.ultsonch.2008.06.007Search in Google Scholar PubMed
[15] Hossain MB, Barry-Ryan C, Martin-Diana AB, Brunton NP. Optimisation of accelerated solvent extraction of antioxidant compounds from rosemary (Rosmarinus officinalis L.), marjoram (Origanum majorana L.) and oregano (Origanum vulgare L.) using response surface methodology. Food Chem. 2011 May 1;126(1):339–46.10.1016/j.foodchem.2010.10.076Search in Google Scholar
[16] Herrero M, Plaza M, Cifuentes A, Ibáñez E. Green processes for the extraction of bioactives from Rosemary: Chemical and functional characterization via ultra-performance liquid chromatography-tandem mass spectrometry and in-vitro assays. J Chromatogr A. 2010 Apr 16;1217(16):2512–20.10.1016/j.chroma.2009.11.032Search in Google Scholar PubMed
[17] Visentín A, Cismondi M, Maestri D. Supercritical CO2 fractionation of rosemary ethanolic oleoresins as a method to improve carnosic acid recovery. Innovat Food Sci Emerg Technol. 2011 Apr 1;12(2):142–5.10.1016/j.ifset.2011.01.004Search in Google Scholar
[18] Ye Q, Guo L, Liu H, Liu Y, Zhang C, Peng C, et al. Optimization of ultrasound-assisted extraction on antioxidative activity of malus toringoides using response surface methodology. Processes. 2019 May;7(5):270.10.3390/pr7050270Search in Google Scholar
[19] Hosseini H, Bolourian S, Hamgini EY, Mahababadi EG. Optimization of heat- and ultrasound-assisted extraction of polyphenols from dried rosemary leaves using response surface methodology. J Food Process Preserv. 2018;42(11):e13778.10.1111/jfpp.13778Search in Google Scholar
[20] He Y, Chen Y, Shi Y, Zhao K, Tan H, Zeng J, et al. Multiresponse optimization of ultrasonic-assisted extraction for aurantii fructus to obtain high yield of antioxidant flavonoids using a response surface methodology. Processes. 2018 Dec;6(12):258.10.3390/pr6120258Search in Google Scholar
[21] Tran TH, Nguyen HHH, Nguyen DC, Nguyen TQ, Tan H, Nhan LTH, et al. Optimization of microwave-assisted extraction of essential oil from vietnamese basil (Ocimum basilicum L.) using response surface methodology. Processes. 2018 Nov;6(11):206.10.3390/pr6110206Search in Google Scholar
[22] Piga A, Marchetti M, Usai M, Del Caro A, Meier HP, Onorati V, et al. Influence of different drying parameters on the composition of volatile compounds of thyme and rosemary cultivated in Sardinia. In: Proceeding of the 3rd International Symposium CGIR. Naples, Italy; Sept 24–26 2007.Search in Google Scholar
[23] Usai M, Marchetti M, Foddai M, Del Caro A, Desogus R, Sanna I, et al. Influence of different stabilizing operations and storage time on the composition of essential oil of thyme (Thymus officinalis L.) and rosemary (Rosmarinus officinalis L.). LWT – Food Sci Technol. 2011 Jan 1;44(1):244–9.10.1016/j.lwt.2010.05.024Search in Google Scholar
[24] Blainski A, Lopes GC, De Mello JCP. Application and analysis of the folin ciocalteu method for the determination of the total phenolic content from limonium brasiliense l. Molecules. 2013 Jun;18(6):6852–65.10.3390/molecules18066852Search in Google Scholar
[25] Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT – Food Sci Technol. 1995 Jan;28(1):25–30.10.1016/S0023-6438(95)80008-5Search in Google Scholar
[26] Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (Rsm) as a tool for optimization in analytical chemistry. Talanta. 2008 Sep 15;76(5):965–77.10.1016/j.talanta.2008.05.019Search in Google Scholar PubMed
[27] Hu C-J, Gao Y, Liu Y, Zheng X-Q, Ye J-H, Liang Y-R, et al. Studies on the mechanism of efficient extraction of tea components by aqueous ethanol. Food Chem. 2016 Mar 1;194:312–8.10.1016/j.foodchem.2015.08.029Search in Google Scholar PubMed
[28] Richter BE, Jones BA, Ezzell JL, Porter NL, Avdalovic N, Pohl C. Accelerated solvent extraction: a technique for sample preparation. Anal Chem. 1996 Jan 1;68(6):1033–9.10.1021/ac9508199Search in Google Scholar
[29] Juntachote T, Berghofer E, Bauer F, Siebenhandl S. The application of response surface methodology to the production of phenolic extracts of lemon grass, galangal, holy basil and rosemary. Int J Food Sci Technol. 2006;41(2):121–33.10.1111/j.1365-2621.2005.00987.xSearch in Google Scholar
[30] Anh TT, Thu Ngan LT, Lam TD. Essential oil from fresh and dried Rosemary cultivated in Lam Dong province, Vietnam. IOP Conf Ser Mater Sci Eng. 2019 Jul 9;544:012025.10.1088/1757-899X/544/1/012025Search in Google Scholar
[31] Lee CY, Kim KM, Son HS. Optimal extraction conditions to produce rosemary extracts with higher phenolic content and antioxidant activity. Korean J Food Sci Technol. 2013;45(4):501–7.10.9721/KJFST.2013.45.4.501Search in Google Scholar
[32] Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angew Chem Int Ed. 2011 Jan 17;50(3):586–621.10.1002/anie.201000044Search in Google Scholar PubMed
[33] Vicré M, Sherwin HW, Driouich A, Jaffer MA, Farrant JM. Cell wall characteristics and structure of hydrated and dry leaves of the resurrection plant craterostigma wilmsii, a microscopical study. J Plant Physiol. 1999 Dec;155(6):719–26.10.1016/S0176-1617(99)80088-1Search in Google Scholar
[34] Lewicki PP. Effect of pre‐drying treatment, drying and rehydration on plant tissue properties: a review. Int J Food Prop. 1998 Jan;1(1):1–22.10.1080/10942919809524561Search in Google Scholar
[35] Jurinjak Tušek A, Benković M, Belščak Cvitanović A, Valinger D, Jurina T, Gajdoš, et al. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols, antioxidants and extraction yield from Asteraceae plants. Ind Crop Prod. 2016 Nov 30;91:205–14..10.1016/j.indcrop.2016.07.015Search in Google Scholar
[36] Klancnik A, Guzej B, Kolar MH, Abramovic H, Mozina SS. In vitro antimicrobial and antioxidant activity of commercial rosemary extract formulations. J Food Prot. 2009 Aug;72(8):1744–52.10.4315/0362-028X-72.8.1744Search in Google Scholar
© 2021 Minh-Tam Nguyen-Kim et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


