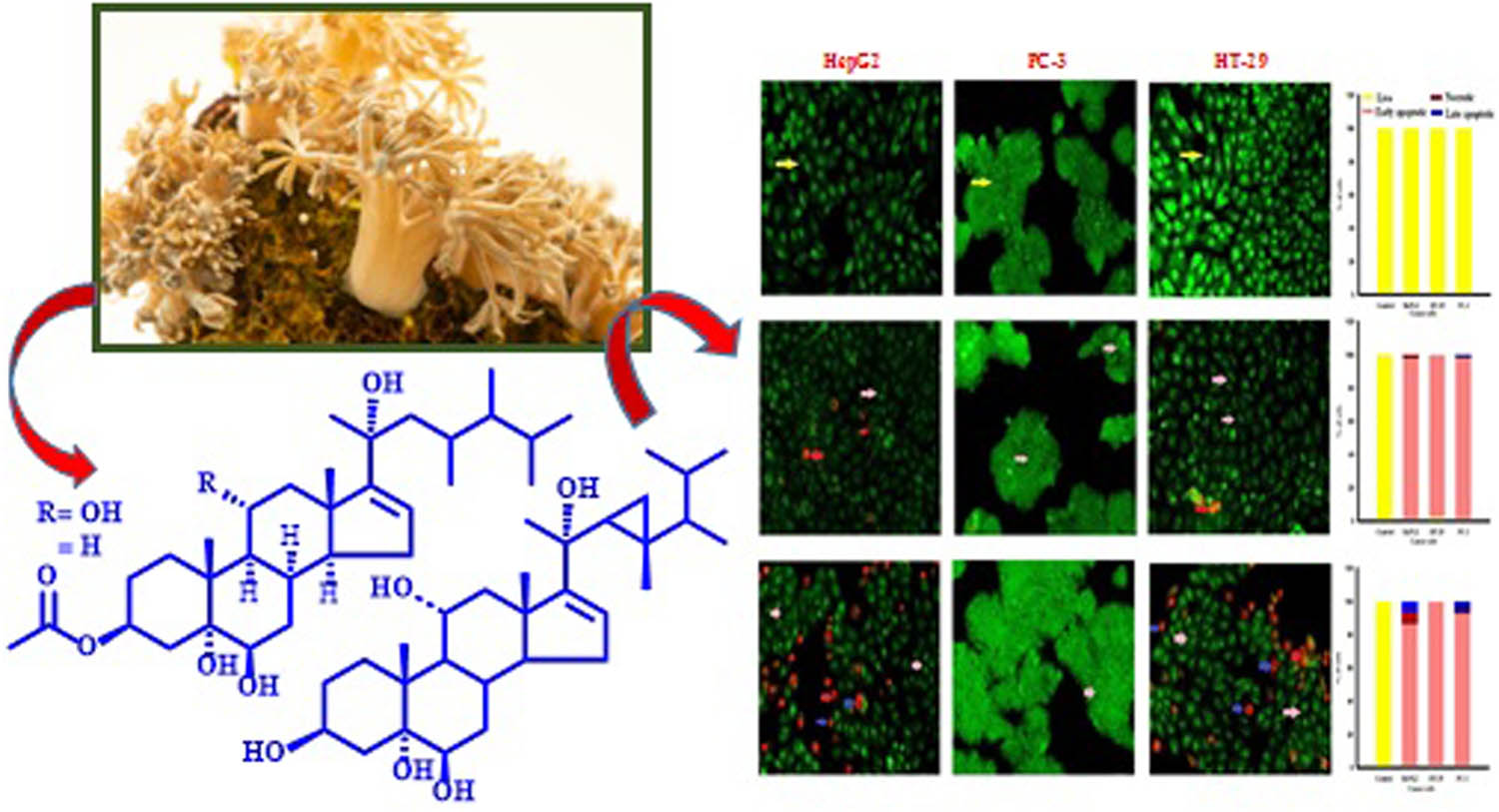
Abstract
Two new rare 30-norisodinosterol derivatives, 23,24-dimethylcholest-16-ene-3β,5α,6β,11α,20(R)-pentol 3-monoacetate (1) and 23,24-dimethylcholest-16-ene-3β,5α,6β,20(R)-tertrol 3-monoacetate (2), along with a known steroid, 3β,5α,6β,11α,20β-pentahydroxygorgosterol (3), were identified from Xenia umbellata. The structures of the isolated compounds were determined by analyses of the measured spectra (1D and 2D nuclear magnetic resonance, mass spectrometry, and infrared). The biosynthetic pathway of the new norisodinosterols was proposed. Compound 1 exhibited potent cytotoxicity against HepG2, PC-3, and HT-29 with IC50 values of 4.70 ± 0.2, 5.60 ± 0.6, and 4.00 ± 0.4 μg/mL, respectively. On the contrary, compound 3 showed less potent cytotoxicity against HepG2 with IC50 value of 22.20 ± 1.0 μg/mL. Two DNA-binding dyes have been used for the morphological detection of viable, apoptotic, and necrotic cells. The early apoptotic cell death was observed in all types of treated tumour cells. The late apoptotic cells are highly present in HepG2 cells with compound 3 compared with other cancer cells except for compound 1. The anti-proliferative activity of compounds 1 and 3 warranted further investigation.
Graphical abstract
1 Introduction
Alcyonacea (Phylum: Cnidaria; Class: Anthozoa) survives worldwide in tropical and subtropical seawaters and does not have the hard calcium carbonate skeleton. Members of Alcyonacea inhabit the inner reefs below the stony corals [1]. They are known for their productivity of secondary metabolites such as terpenoids and steroids. The soft corals are important member of marine fauna which have cells in the form of toxic stinging nematocysts with the absence of the rigid protective skeleton of scleractinians. They also have the ability to produce toxic substances [2,3,4].
Family Xeniidae (Alcyonacea) consists of 20 genera and 162 species. They live in tropical waters as the Red Sea. They present as yellow cylindrical clavate colonies [3]. They have many varieties of long feather-like tentacles and their polyps pump water into the colony, creating a rhythmic pulsing motion. They are named as pulsing Xenia and pom-pom Xenia. This genus is known for its productivity of terpenoids and steroids [5,6].
In 2018, the Saudi Cancer Registry reported a total of 24,485 diagnosed cancer cases [7]. As a part of our interest is this study, which aimed at discovering the anti-cancer metabolites from marine sources [8,9]. Thus, the present study was designed to isolate bioactive secondary compounds from Xenia umbellata. The anti-proliferative activity of 23,24-dimethylcholest-16-ene-3β,5α,6β,11α,20(R)-pentol 3-monoacetate (1) and 3β,5α,6β,11α,20β-pentahydroxygorgosterol (3) was evaluated against HepG2, PC-3, and HT-29. Additionally, two DNA-binding dyes, acridine orange (AO) and ethidium bromide (EtBr), have been used for the morphological detection of viable, apoptotic, and necrotic cells [10].
2 Experimental
2.1 Soft coral sample
Xenia umbellata was gathered by Scuba technique at a depth of 15–20 m in October 2018, off the Red Sea coast at Jeddah, Saudi Arabia (21°29′31″N 39°11′24″E). Prof. Mohsen El-Sherbiny (Faculty of Marine Sciences, King Abdulaziz University [KAU]) identified the sample. A voucher specimen (XC-2018-11/2) was deposited in the Faculty of Marine Sciences, KAU.
2.2 Extraction and isolation
The semi-dried soft coral (265 g) was exhausted by CH2Cl2/MeOH (3 × 1 L, 22°C), yielding (21.4 g) an oily residue. The extract was loaded on 60 G silica gel column (100 × 3.2 cm) and eluted by gradient elution from n-hexane easing to CH2Cl2 (50 mL each fraction). The fraction that eluted with n-hexane–CH2Cl2 (65:35) afforded 23,24-dimethylcholest-16-ene-3β,5α,6β,20(R)-tetrol 3-monoacetate (2), whereas the fraction that eluted with n-hexane-CH2Cl2 (45:55) afforded compound 1. Finally, the fraction that eluted with n-hexane–CH2Cl2 (40:60) gave compound 3. The purification of compounds 1–3 has been done by preparative thin-layer chromatography (PTLC; normal phase silica gel).
2.3 Spectral data
2.3.1 Compound 1
Gummy material (1.6 mg, 0.00062%); [α]D 22 – 49.6 (c 0.01, CHCl3); infrared (IR) ʋ max (film)/cm: 3,403, 2,925, 2,853, 1,730, 1,713, 1,655, 1,461, 1,377, 1,264, 1,153; 1H nuclear magnetic resonance (NMR) (CDCl3, 850 MHz) and 13C NMR (CDCl3, 213 MHz) (Table 1); HRESIMS m/z = 520.3758 [M]+ (calculated m/z = 520.3764 for C31H52O6).
1H and 13C NMR (850 and 213 MHz) spectral data of compound 1 in CDCl3 a
| Carbon no. | b δ C | δ H (J in Hz) | Carbon no. | δ C | δ H (J in Hz) |
|---|---|---|---|---|---|
| 1 | 34.0 (CH2) | 1.87, m | 16 | 124.0 (CH) | 5.48, dd (3.4, 1.7) |
| 2.01, m | |||||
| 2 | 26.9 (CH2) | 1.67, m | 17 | 160.1 (C) | |
| 1.85, m | |||||
| 3 | 70.9 (CH) | 5.13, dddd (11.1, 11.1, 5.1, 5.1) | 18 | 19.4 (CH3) | 0.98, s |
| 4 | 37.4 (CH2) | 1.58, m | 19 | 16.9 (CH3) | 1.35, s |
| 2.19, m | |||||
| 5 | 76.4 (C) | — | 20 | 75.9 (C) | |
| 6 | 76.2 (CH) | 3.55 dd (3.4, 1.7) | 21 | 30.8 (CH) | 1.37, s |
| 7 | 34.6 (CH2) | 1.86, m | 22 | 49.3 (CH2) | 1.42, m |
| 2.01, m | 1.59, m | ||||
| 8 | 28.1 (CH) | 2.05, m | 23 | 31.0 (CH) | 1.40, m |
| 9 | 53.2 (CH) | 1.45, m | 24 | 29.8 (CH) | 1.60, m |
| 10 | 40.2 (C) | 25 | 45.5 (CH) | 1.04, m | |
| 11 | 68.7 (CH) | 3.99, dt (9.4, 5.1) | 26 | 11.7 (CH3) | 0.75, d (6.8) |
| 12 | 48.1 (CH2) | 1.49, dd (11.9, 5.1) | 27 | 20.9 (CH3) | 0.88 d (6.8) |
| 2.52, dd (11.9, 6.0) | |||||
| 13 | 48.0 (C) | 28 | 14.2 (CH3) | 0.86, d (6.8) | |
| 14 | 56.2 (CH) | 1.60, m | 29 | 15.8 (CH3) | 0.78, d (6.8) |
| 15 | 31.0 (CH2) | 1.83, m | COCH3 | 170.0 (C) | |
| 2.08, ddd (15.3, 6.8, 3.4) | |||||
| COCH 3 | 21.5 (CH3) | 2.03, s |
- a
All data were obtained from 1D and 2D NMR measurements.
- b
Implied multiplicities were determined by DEPT (C = s, CH = d, CH2 = t).
2.3.2 Compound 2
Gummy material (0.5 mg, 0.0002%); [α]D 22 – 81.1 (c 0.01, CHCl3); IR ʋ max (film)/cm: 3,387, 2,958, 2,853, 1,730, 1,674, 1,632, 1,377, 1,146; 1H NMR (CDCl3, 850 MHz); 13C NMR (CDCl3, 213 MHz) (Table 2); HRESIMS m/z = 504.3809 [M]+ (calculated m/z = 504.3815 for C31H52O5).
1H and 13C NMR (850 and 213 MHz) spectral data of compound 2 in CDCl3 a
| Carbon no. | b δ C | δ H (J in Hz) | Carbon no. | δ C | δ H (J in Hz) |
|---|---|---|---|---|---|
| 1 | 31.9 (CH2) | 1.40, m | 16 | 123.7 (CH) | 5.48, dd (3.4, 1.7) |
| 1.65, m | |||||
| 2 | 26.6 (CH2) | 1.62, m | 17 | 160.8 (C) | |
| 1.88, m | |||||
| 3 | 71.0 (CH) | 5.17, dddd (11.1, 11.1, 5.1, 5.1) | 18 | 18.4 (CH3) | 0.99, s |
| 4 | 37.0 (CH2) | 1.68, m | 19 | 16.6 (CH3) | 1.23, s |
| 2.18, dd (11.9, 11.1) | |||||
| 5 | 76.0 (C) | — | 20 | 75.9 (C) | |
| 6 | 76.2 (CH) | 3.55, dd (3.4, 1.7) | 21 | 29.6 (CH3) | 1.37, s |
| 7 | 34.4 (CH2) | 1.60, m | 22 | 49.0 (CH2) | 1.49, m |
| 1.76, m | 1.56, m | ||||
| 8 | 28.2 (CH) | 1.97, m | 23 | 30.8 (CH) | 1.41, m |
| 9 | 45.7 (CH) | 1.38, m | 24 | 29.6 (CH) | 1.80, m |
| 10 | 38.6 (C) | 25 | 45.5 (CH) | 1.13, m | |
| 11 | 21.1 (CH2) | 1.42, m | 26 | 11.7 (CH3) | 0.76, d (6.8) |
| 1.48, m | |||||
| 12 | 36.1 (CH2) | 2.08, m | 27 | 21.6 (CH3) | 0.88, d (6.8) |
| 2.08, m | |||||
| 13 | 47.7 (C) | 28 | 21.0 (CH3) | 0.86, d (6.8) | |
| 14 | 57.1 (CH) | 1.50, m | 29 | 15.7 (CH3) | 0.78, d (6.8) |
| 15 | 30.9 (CH2) | 1.84, dd (17.0, 12.8) | COCH3 | 170.0 | |
| 2.06, ddd (10.2, 6.8, 3.4) | |||||
| COCH 3 | 21.5 | 2.03, s |
- a
All data were obtained from 1D and 2D NMR measurements.
- b
Implied multiplicities were determined by DEPT (C = s, CH = d, CH2 = t).
2.4 Determination of anti-proliferative effect of compounds 1 and 3
The cytotoxicity of the isolated compounds was evaluated against (HepG2, PC-3, and HT-29) human cancer cells using sulphorhodamine B assay (SRB), according to the previously published [8,9].
2.5 AO/EtBr staining for detection of apoptosis
The DNA-binding dyes AO and EtBr have been used for the morphological detection of viable, apoptotic, and necrotic cells. The procedures have been done as previously reported [11,12].
2.6 Statistical analysis
Data are presented as mean and SD. Statistical significance was acceptable to a level of p < 0.05. All statistical analyses were performed using GraphPad Prism software, version 6.00 (GraphPad Software, La Jolla, CA, USA).
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
A Red Sea soft coral specimen, identified as X. umbellata, was extracted with a mixture of organic solvents at room temperature, yielding a viscous oily material (21.4 g). The total extract was evaluated for its cytotoxic effect against HepG2 and displayed cytotoxicity with IC50 (19.74 ± 1.98 μg/mL). The aforementioned promising anti-proliferative results directed the further chemical investigation of the X. umbellata extract. It was subjected to normal-phase silica gel column chromatography and PTLC to give two new steroidal derivatives, compounds 1 and 2 together with a previously identified steroid compound 3 (Figure 1).
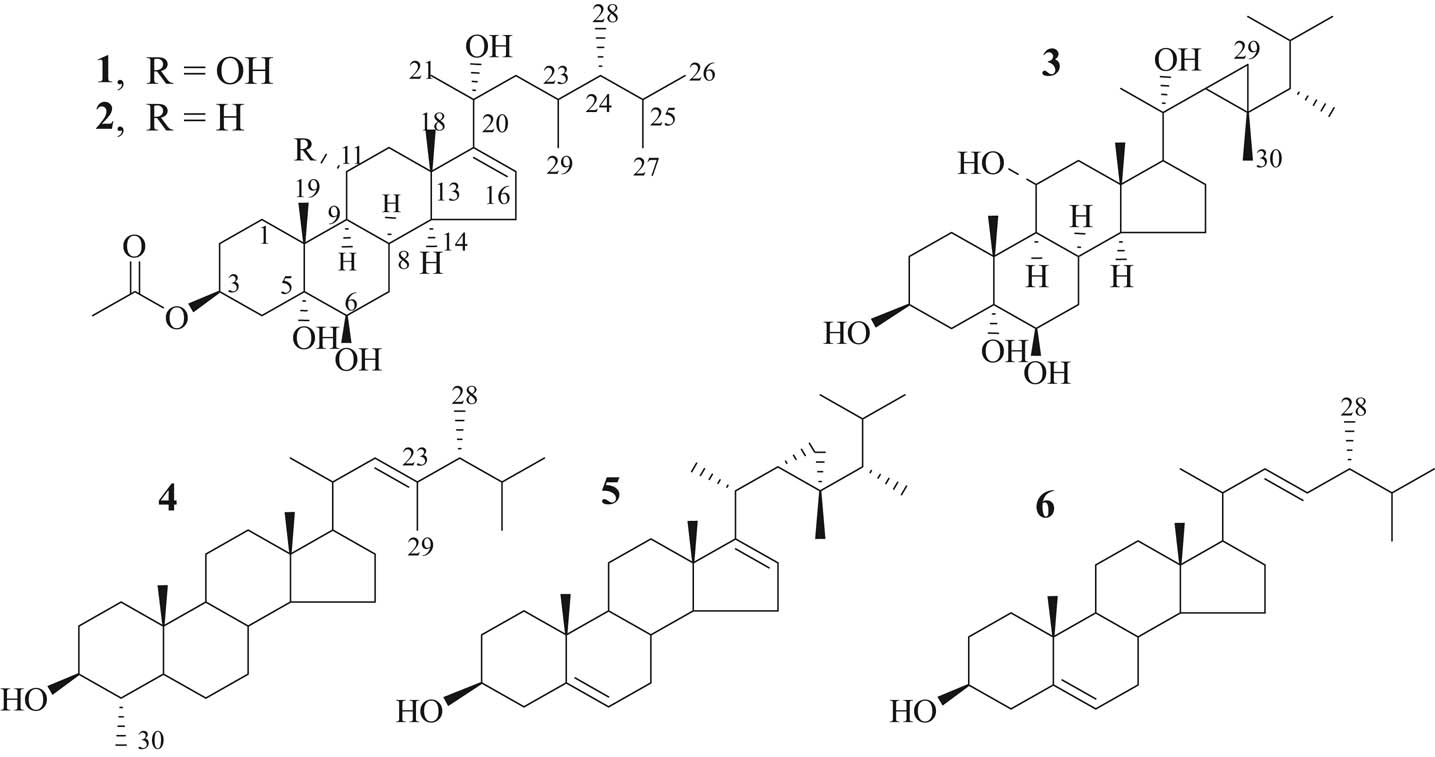
Chemical structures of compounds 1–6.
3.1 Structure elucidation
Compound 1, [α]D 23 = −49.6 (c 0.01, CHCl3), was obtained as a gummy material and had the molecular formula C31H52O6, as determined by high resolution electrospray ionization mass spectrometry (HRESIMS), requiring six degrees of unsaturation. The IR spectrum of 1 showed absorptions due to hydroxyl and acetyl groups (λ max 3,403 and 1,730/cm, respectively). The 13C NMR and distortionless enhancement by polarization transfer (DEPT) spectral data of 1 (Table 1) revealed the presence of 31 carbon atoms (Figure S1g–k), including six non-protonated carbons, nine sp3 methines, one sp2 methine, seven methylenes, and eight methyls. The quaternary carbons were assigned to one carbonyl (δ C 170.0 ppm), two oxygenated (76.4 and 75.9 ppm), and one olefinic (160.1 ppm) along with two in the upfield region (48.0 and 40.2 ppm). The methine carbon included three oxygenated (76.2, 70.9, and 68.7 ppm) and one olefinic (124.0). The total number of methine carbon counts ten after assigning eight methyls (δ H 30.8, 21.5, 20.9, 19.4, 16.9, 15.8, 14.2, and 11.7 ppm) from 1H NMR and heteronuclear single quantum correlation (HSQC) experiments (Figure S1p–r).
The 1H NMR spectra (Figure S1a–f) displayed four tertiary methyls resonating at δ H (0.98, 1.35, 1.37, and 2.03 ppm) and four secondary methyls resonating at δ H [0.75 (d, J = 6.6 Hz), 0.86 (d, J = 6.8 Hz), 0.78 (d, J = 6.8 Hz), and 0.78 (d, J = 6.8 Hz)]. Two unsaturation degrees were accounted as one trisubstituted carbon–carbon double bond (δ H 5.48 and δ C 124.0 and 160.1 ppm) and an acetyl function (δ H 2.03 and δ C 21.5 and 170.0 ppm). Therefore, the remaining four unsaturation degrees suggest a tetracyclic structure for compound 1. The aforementioned information, together with the methylation pattern and several other features appearing in the 1D and 2D NMR spectra, suggested the steroidal nature of 1. The nature of the side chain was determined by the interpretation of the 1H–1H correlated spectroscopy (COSY) spectrum (Figure S1l–o). A sequence of correlations started from the isopropyl proton resonating at δ H 1.04 (H-25) with the signal at 1.60 (H-24), which in turn is correlated with a methyl at 0.86 (H-28) and a methine at 1.40 (H-23) protons, and the later methine is correlated with a methyl at 0.78 (H-29) and a methylene proton of H-22 was observed. The heteronuclear multiple bond correlation (HMBC) correlations from H-21 (1.37, s) to C-20 (75.9), C-22 (49.3), and C-17 (160.1) established the nature of the side chain as 4,5,6-trimethyl-2-heptyl-2-ol moiety (Figure S1s–v). The later deduction furnished the gross structure of 1 as 23,24-dimethylcholest-16-en-pentahydroxy monoacetate. The position of the five hydroxyl groups was deduced from 13C NMR, DEPT, and HSQC spectra. The methine proton resonating at δ H 5.13 (dddd, J = 11.1, 11.1, 5.1, and 5.1 Hz) implies acetylated hydroxyl located at C-3, since this is the sole available location flanked by two methylene groups. 1H–1H COSY and HMBC spectra recognized the positions of the other hydroxyl groups; H-3 and H-19 are both correlated with the quaternary carbon at δ C 76.4 (C-5) ppm as well as this carbon is also correlated with the proton resonating at δ H 3.55 (dd, J = 3.4 and 1.7 Hz, H-6), which implies that positions 5 and 6 are both hydroxylated. The fourth hydroxyl group was decided by observing the signal at δ H 3.99, which appeared as dt with J values 9.4 and 5.1 Hz. This proton could be positioned on several locations within the carboskeleton of 1; however, the HMBC correlations were observed between this proton and the two quaternary carbons at C-10 and C-13. The OH group is positioned on C-11. The fifth hydroxyl group was deduced from the HMBC to be depicted at C-20, based on the correlations between the methyl group resonated at (δ H 1.37, s) and the olefinic proton resonated at δ H 5.48 (dd, J = 3.4 and 1.7 Hz) with the C-20 resonated at δ C 75.9. The relative stereochemistry of 1 was elucidated on the basis of nuclear overhauser effect spectroscopy (NOESY) correlations (Figure S1w–y) and analyses of J values. The multiplicity of the methine assignment at 5.13 ppm had the normal complexity for the 3α-carbinol proton of an A/B trans-steroid. This unusually downshifted signal is typical of 3β-hydroxysterols bearing a 5α-hydroxyl group, which esterified by acetyl moiety [13]. The downshift of the Me-19 signal at 1.35 was indicative of the β-orientation of the C-6 hydroxyl group. The large J-value of H-11 implies its axial orientation and hence α-OH. The strong NOESY correlations between H-3 and H-6 and between Me-19 and H-11 supported the proposed orientations. Since the spectral data of 1 coincided with the reported data and the R configuration was recognized at C-20 [14]. From these data, compound 1 was concluded to be 23,24-dimethylcholest-16-ene-3β,5α,6β,11α,20(R)-pentol 3-monoacetate.
Compound 2, [α]D 22 – 81.1 (c 0.01, CHCl3), was obtained as a gummy substance and had the molecular formula C31H52O5, as determined by HRESIMS, requiring six degrees of unsaturation. The IR spectrum of 1 showed absorptions due to hydroxyl and acetyl groups (λ max 3,387 and 1,730/cm, respectively). The 13C NMR spectroscopical data of 2 (Table 2) revealed the presence of 31 carbon signals, which were identified by assistance of the DEPT spectrum. They were categorized into six quaternary carbons, eight sp3 methines, one sp2 methine, eight methylenes, and eight methyls. Analysis of the spectral data of 2 and comparison with those of 1 revealed the great similarity between both structures. However, the lack of 16 mass unit in mass spectrum and the absence of signals due to CH-11 in the NMR spectra (1H and 13C NMR, DEPT, HSQC, COSY, HMBC, and NOESY) in case of compound 2 allowed the determination of its structure as 23,24-dimethylcholest-16-ene-3β,5α,6β,20(R)-tetrol 3-monoacetate. 3β,5α,6β,11α,20β-Pentahydroxygorgosterol (3) was identified by comparing the measured spectral data with the reported [15].
3.2 Biological activities
In vitro cytotoxicity of compounds 1 and 3 was determined using SRB assay. These activities were assessed against HepG2, PC-3, and HT-29 tumour cell lines over concentration range of 0.01–1,000 μg/mL. Compound 1 showed potent cytotoxic profile against tumour cell lines HepG2, PC-3, and HT-29 with IC50 values of 4.70 ± 0.2, 5.60 ± 0.6, and 4.00 ± 0.4 μg/mL, respectively. On the other hand, compound 3 showed significant cytotoxicity effect against HepG2, PC-3, and HT-29 with IC50 values 22.20 ± 1.0, ≥100, and 99.30 ± 0.9 μg/mL, respectively. Doxorubicin (positive control) displayed cytotoxicity against HepG2, PC-3, and HT-29 with IC50 values of 0.79 ± 0.06, 1.16 ± 0.56, and 1.70 ± 0.16 μg/mL, respectively (Figure 2). After staining the cells with AO/EtBr, the cells appeared in the form of four colours as follows; living cells (green nuclei), early apoptotic (bright green nuclei), late apoptotic (orange-stained nuclei), and necrotic cells (uniformly orange-stained cell nuclei). In AO/EtBr dual staining, the cells were uniformly stained green with normal, round, intact nuclei, and cytoplasm which indicate the viability of the cell control. On the contrary, the highly early apoptotic cell death was observed in all types of treated tumour cells. The late apoptotic cells are highly present in HepG2 cells with compound 3 compared with other cancer cells except for 1, while no necrotic and late apoptotic appeared with HT-29 cells after treatment with compounds 1 and 3. These results were compared with the control without manifestations of cell death (Figure 3).
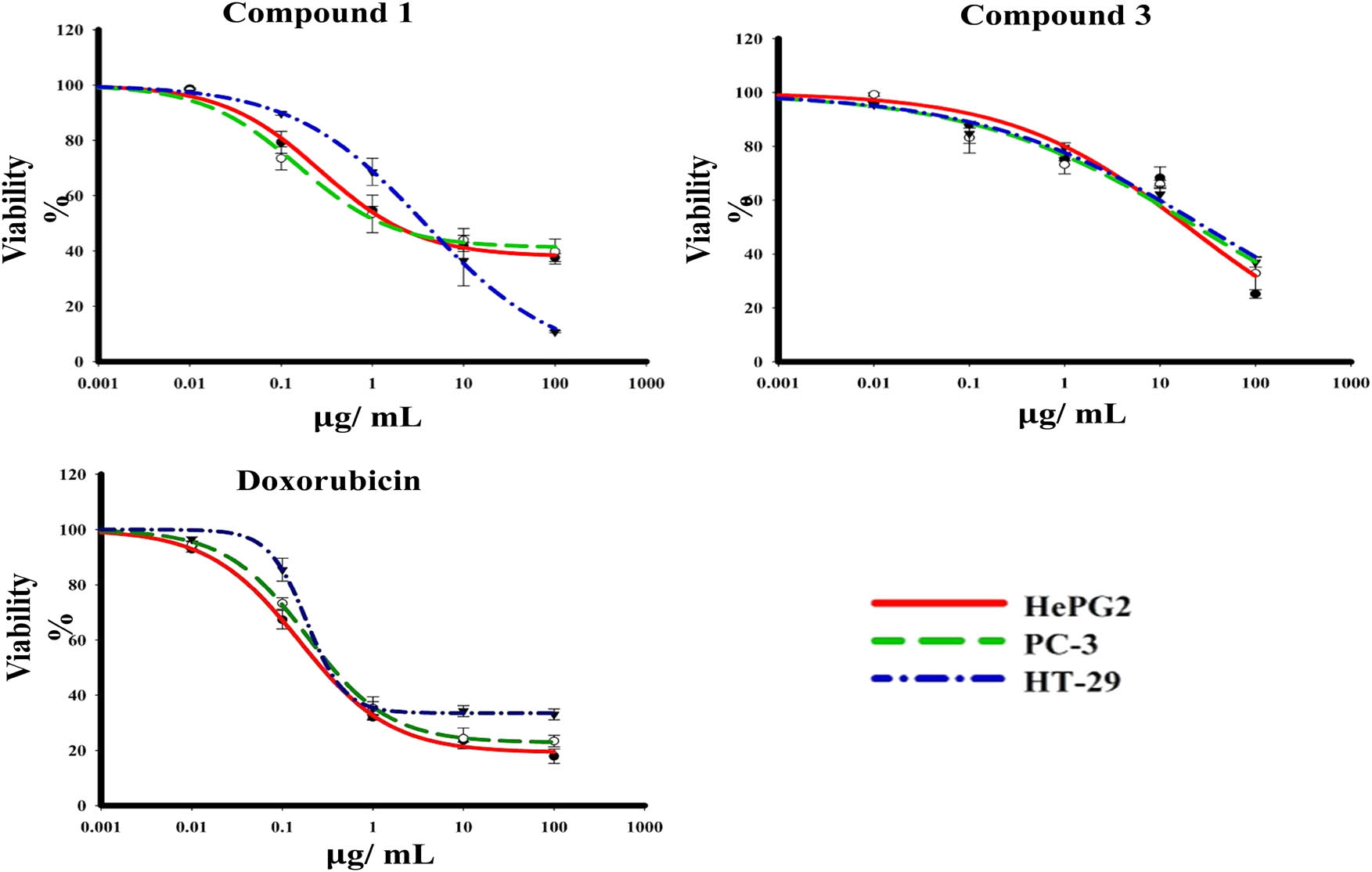
The dose–response curves of the compounds 1 and 3 cytotoxicity against HepG2, PC-3, and HT-29 human cell lines.
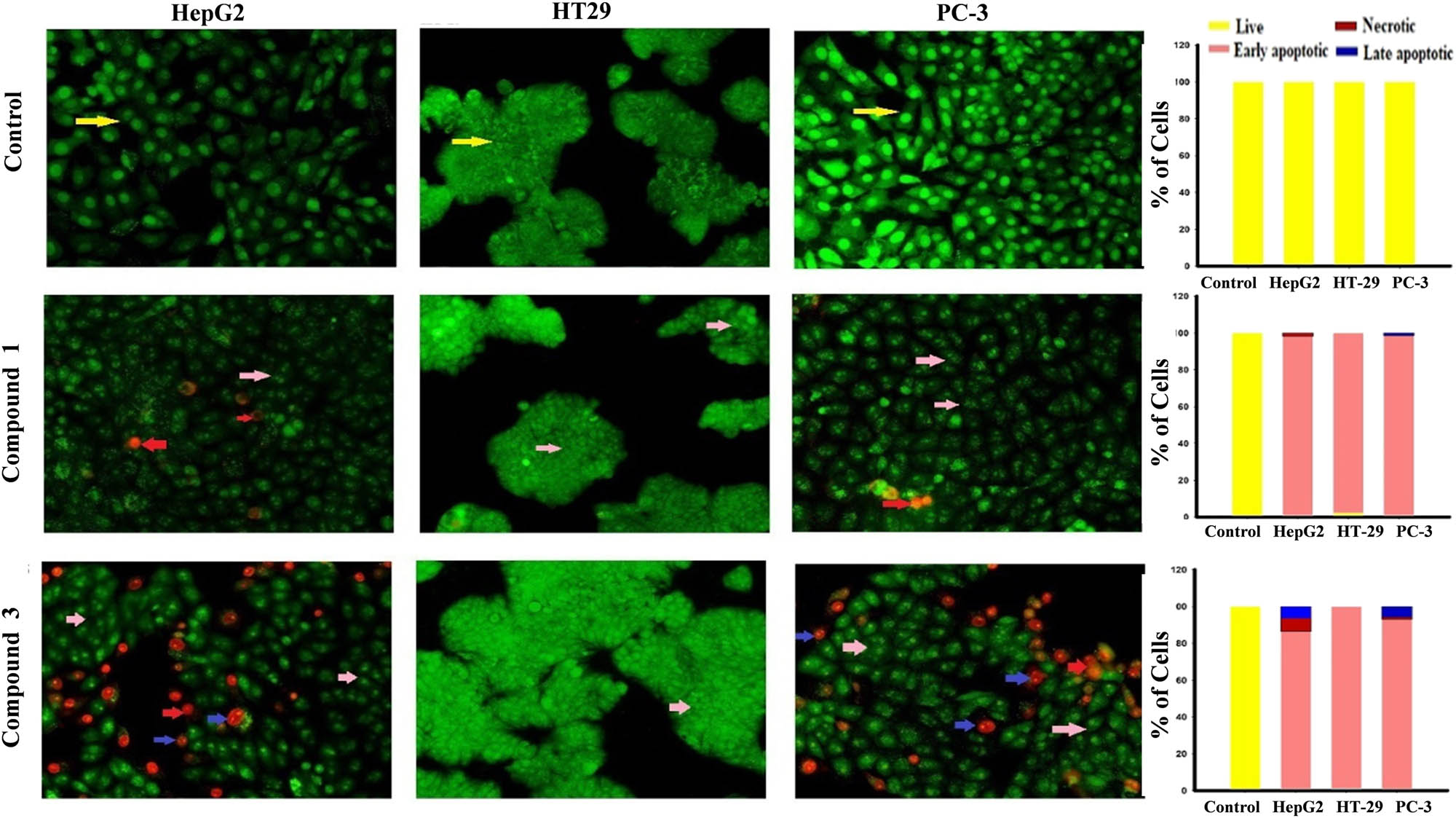
Cell apoptosis observed using fluorescence microscope (200×). Cells were treated with IC50S of compounds 1(B) and 3(C) for 48 h. The control (A) was similarly processed. They (A–C) were stained with acridine orange–ethidium bromide.
3.3 Biosynthesis of 30-norisodinosterols carbon skeleton
Dinosterol (4), a C-30 sterol isolated from free swimming dinoflagellates, is the biosynthetic precursor to the cyclopropyl-containing sterol gorgosterol (5) (Figure 1) [16]. Despite the reported information, gorgosterol was originally isolated from several gorgonian species and, moreover, was proved to be a symbiont product and was not isolated from the free swimming dinoflagellates [16].
The unsaturation (∆24) was common among the initial tetracyclic precursor, lanosterol and cycloartenol, in all sterol-building organisms. The presence of such unsaturation site renders the molecules prone to decorations by reduction (e.g. formation of cholesterol) and methylation, which started from simple methylation at C-24, may extend to multiple alkylations. This situation was common among marine organisms [17]. A hypothetical biosynthetic pathway of the current isolated norisodinosterol derivatives (1 and 2) could be started with brassicasterol (6) which undergoes methylation by a methyalting agent (mainly S-adenosylmethionine). Then reduction of ∆5 and formation of ∆16 could be performed. A more evidenced route to the biosynthesis of these C-29 sterols (Scheme 1) started from compound 4, which lead to the construction of 30-norisodinosterol skeleton through the reduction of ∆22, demethylation at C-4 and then formation of ∆16. It is worthy to mention that the isolation of 23,24-dimethylchlesta-5,22-dien-3β-ol from the soft coral Sarcophyton elegans supports the speculation that dinosterol is the precursor of compounds 1 and 2 along with the symbiont production nature of these compounds [18].
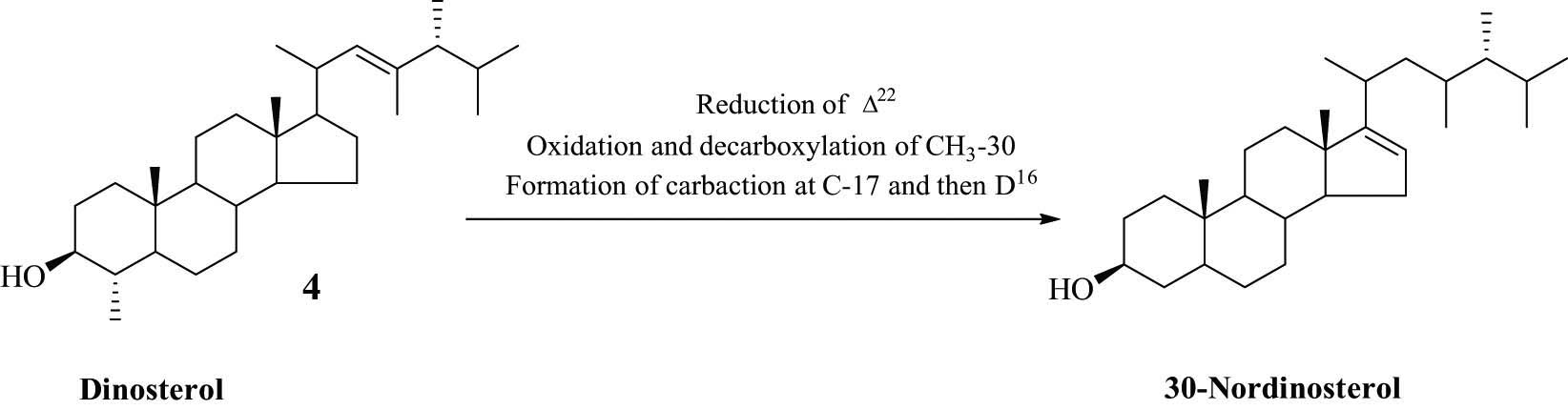
Proposed biosynthesis of 30-norisodinosterol.
The compounds isolated in the current manuscript are steroidal derivatives. They are characterized by the presence of four rings arranged in a unambiguous molecular configuration. Multi-functionality gave them unique molecular structures. Consequently, the steroids have potential diversity of bioactivity. Generally, steroids have two common biological functions: as vital components of cell membranes that alter membrane fluidity and as signalling molecules. The isolated steroids showed different chemical functionality and potent anti-proliferative activities. After further pharmacological investigation and mechanistically studies, they may be a lead of anti-cancer drug.
4 Conclusion
Xenia umbellata, a soft coral, was collected from the Red Sea and found to produce two new steroidal derivatives, compounds 1 and 2, together with a known steroid, compound 3. The chemical structures of the isolated compounds were determined by analyses of the measured spectroscopic data. The anti-proliferative activities of compounds 1 and 3 have been evaluated against hepatocellular carcinoma (HepG2), prostate adenocarcinoma (PC-3), and colorectal adenocarcinoma (HT-29) human cell lines. Compound 1 exhibited potent cytotoxic effect against tumour cell lines, HepG2, PC-3, and HT-29. Compounds 1 and 3 displayed late apoptotic effect in HepG2 cells. The anti-proliferative activity of compounds 1–3 warranted further investigation. Extra work should be carried out to establish the chemoecological functions of nordinosterols (1 and 2) and to assess their impact on the host soft coral organism.
Acknowledgements
The authors thank the Deanship of Scientific Research for the technical and financial support.
-
Funding information: This project was funded by the Deanship of Scientific Research at King Abdulaziz University, Jeddah (grant no J: 14-247-1440).
-
Author contributions: N. O. B., A. A., and W. M. A. was in charge of the study design, supervised the experimental work, carried out collection and interpretation of the data, literature search and wrote the manuscript; and performed the cytotoxicity and apoptosis experiments. W. M. A. and A. A. equally edited the manuscript.
-
Supplementary materials: The following are available online. The NMR data (1H, 13C, 1H–1H COSY, HMQC, and HMBC) of compounds 1 and 2.
-
Conflict of interest: The authors state they have no competing interest.
-
Data availability statement: All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
[1] Koido T, Imahara Y, Fukami H. High species diversity of the soft coral family Xeniidae (Octocorallia, Alcyonacea) in the temperate region of Japan revealed by morphological and molecular analyses. ZooKeys. 2019;862:1–22.10.3897/zookeys.862.31979Suche in Google Scholar
[2] Schwartsmann G, Brondanida RA, Berlinck RG, Jimeno J. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2001;2:221–5.10.1016/S1470-2045(00)00292-8Suche in Google Scholar
[3] Bishara A, Rudi A, Goldberg I, Benayahu Y, Kashman Y, Novaxenicins A-D, et al. Seven new diterpenes from the soft coral Xenia novaebrittanniae. Tetrahedron. 2006;62:12092–7.10.1016/j.tet.2006.09.050Suche in Google Scholar
[4] Coll JC. The chemistry and chemical ecology of octocorals (Coelenterata, Anthozoa, Octocorallia). Chem Rev. 1992;92:613–31.10.1021/cr00012a006Suche in Google Scholar
[5] El-Gamal AA, Wang SK, Duh CY. Umbellactal, a novel diterpenoid from the Formosan soft coral Xenia umbellata. Tetrahedron Lett. 2005;46:6095–6.10.1016/j.tetlet.2005.06.168Suche in Google Scholar
[6] Faulkner DJ. Marine natural products. Nat Prod Rep. 2001;18:1–49.10.1039/b006897gSuche in Google Scholar PubMed
[7] WHO. Saudi Arabia Source: Globocan; 2018. https://gco.iarc.fr/today/data/factsheets/populations/682-saudi-arabia-fact-sheets.pdfSuche in Google Scholar
[8] Alarif WM, Abdel-Lateff A, Al-Abd AM, Basaif SA, Badria FA, Shams M, et al. Selective cytotoxic effects on human breast carcinoma of new methoxylated flavonoids from Euryops arabicus grown in Saudi Arabia. Eur J Med Chem. 2013;66:204–10.10.1016/j.ejmech.2013.05.025Suche in Google Scholar PubMed
[9] Abdel-Lateff A, Alarif WM, Ayyad SE, Al-Lihaibi SS, Basaif SA. New cytotoxic isoprenoid derivatives from the Red Sea soft coral Sarcophyton glaucum. Nat Prod Res. 2015;29:24–30.10.1080/14786419.2014.952637Suche in Google Scholar PubMed
[10] Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. CSH Protoc. 2006 Aug 1.10.1101/pdb.prot4493Suche in Google Scholar PubMed
[11] Albright F, Stephenson RA, Agarwal N, Teerlink CC, Lowrance WT, Farnham JM, et al. Prostate cancer risk prediction based on complete prostate cancer family history. J Korean Med Sci. 2015;2015(75):390–8.10.1002/pros.22925Suche in Google Scholar PubMed PubMed Central
[12] Liu EH, Qi LW, Wu Q, Peng YB, Li P. Anticancer agents derived from natural products. Mini Rev Med Chem. 2009;9:1547–55.10.2174/138955709790361520Suche in Google Scholar PubMed
[13] Dong H, Gou YL, Kini RM, Xu HX, Chen SX, Teo SLM. A new cytotoxic polyhydroxysterol from soft coral Sarcophyton trocheliophorum. Chem Pharm Bull. 2000;48:1087–9.10.1248/cpb.48.1087Suche in Google Scholar PubMed
[14] Lu Y, Lin YC, Wen ZH, Su HH, Sung PJ, Hsu CH, et al. Steroid and cembranoids from the Dongsha atoll soft coral Lobophytum sarcophytoides. Tetrahedron. 2010;66:7129–35.10.1016/j.tet.2010.06.094Suche in Google Scholar
[15] Ayyad SEN, Alarif WM, Al-Footy KO, Selim E, Ghandourah MA, Aly MM, et al. antimicrobial and antitumor activities of a new polyhydroxysteroid and a new diterpenoid from the soft coral Xenia umbellata. Z Naturforsch C. 2017;72:27–34.10.1515/znc-2015-0228Suche in Google Scholar PubMed
[16] Epifanio RA, Maia LF, Pawlik JR, Fenical W. Antipredatory secosterols from the octocoral Pseudopterogorgia americana. Mar Ecol Prog Ser. 2007;329:307–10.10.3354/meps329307Suche in Google Scholar
[17] John Goad L, Akihisia T. Analysis of sterols. 2nd edn. Chapman & Hall, London: Blackie Academic & Professional; 1977. p. 1–42.Suche in Google Scholar
[18] Finer J, Clardy J, Kobayashi A, Alam A, Shimizu Y. Identity of the stereochemistry of dinosterol and gorgosterol side chain. J Org Chem. 1978;43:1990–2.10.1021/jo00404a031Suche in Google Scholar
© 2021 Nahed Obaid Bawakid et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

