Abstract
Metformin is one of the most effective drugs for the treatment of type II diabetes. Two new mixed ligand complexes of vanadyl(ii) and chromium(iii) ions with the general formula [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl, respectively, where L1 is the metformin and L2 is the glycine amino acid, have been synthesized in MeOH solvent with 1:1:1 stoichiometry and characterized by several spectroscopic techniques. The spectroscopic data suggested that the [VOL1L2]SO4 complex possesses a square pyramidal geometry, where the [CrL1L2(Cl)2]Cl complex possesses an octahedral geometry. The L1 ligand coordinated to the VO(ii) and Cr(iii) ions via the N atoms of the imino (‒C═NH) groups, where the L2 ligand coordinated via the O atom of the carboxylate group (COO) and the N atom of the amino group (NH2). The interaction of ligands L1 and L2 with the metal ions leads to complexes that have organized nanoscale structures with a main diameter of ∼14 nm for the [CrL1L2(Cl)2]Cl complex and ∼40 nm for the [VOL1L2]SO4 complex.
1 Introduction
Metal complexes are an important class of compounds with applications in various fields such as medicine, material sciences, biology, and catalysis [1,2]. These class of compounds may have different geometries, which make them potentially biologically active and used as anticancer, antifungal, and antibacterial agents. For example, carboplatin, oxaliplatin, and cisplatin, which are platinum-based metallodrugs, are considered anticancer drugs for treating several solid tumors such as ovarian, bladder, and testicular cancers. Platinum-based metallodrugs kill tumor cells by damaging DNA, which inhibit tumor cell division [3,4,5,6,7,8]. Nevertheless, the adverse side effects and acquired resistance associated with several metallodrugs prevent their effectiveness and widespread applications [9,10,11,12]. Because of that, significant attempts have been made to replace metallodrugs by developing innovative drugs with negligible side effects, enhanced efficiency, and decreased drug resistance and toxicity profiles. One of these attempts involves the design of new metal-based drugs by coordinating metal ions with biologically active ligands. Ions of transition metals play an important role in the fields of biochemistry and medicine, and their complexes are extensively used as therapeutic agents and drugs for the treatment of various human diseases, including neurological disorders, diabetes, inflammation, infection control, lymphomas, and carcinomas. Two interesting properties of transition metals enabling the design of metallodrugs with promising biological benefits exhibited: (i) different oxidation states and (ii) the ability to interact with several metal-binding sites [13,14,15].
Metformin (C4H11N5) is a well-known AMP-activated protein kinase (AMPK) activator and well-known oral hypoglycemic drug, which has been in clinical use for over half a century, particularly in obese and overweight people. It is the most widely prescribed antidiabetic drug worldwide and the first-line therapy for non-insulin-dependent diabetes and metabolic syndrome. Besides its use in oral hypoglycemic medication for type II diabetes, metformin confers protection against a series of diseases through the activation of AMPK. This drug has antihyperglycemic and anticancer properties, and it strongly enhances insulin sensitivity in the body, increases the glucose uptake by peripheral tissues through stimulation of intracellular AMPK, and inhibits gluconeogenesis in the liver. This drug has multiple beneficial effects, such as the following:
Does not increase the risk for hypoglycemia
Reduces weight gain
Lowers blood lipid levels
Lowers blood glucose levels.
In vitro and in vivo studies indicated that metformin inhibits the growth of several types of tumors, such as glioma, prostate cancer, and breast cancer [16,17,18,19,20,21,22,23]. Metformin has two imine groups that act as a chelating agent coordinating with many metal and non-metal ions. The interactions of metformin with several lanthanide and transitional metal ions, such as Y(iii), Sm(iii), Ce(iii), La(iii), Cd(ii), Fe(iii), Au(iii), Re(v), Tc(v), Pt(ii), Co(ii), V(IV), Rh(v), Cu(ii), Ni(ii), Cr(iii), Zn(ii), Te(v), Pd(ii), Os(iii), Os(ii), Ir(iii), and Rh(iii), have been investigated and reported in the literature [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38].
Further research on the complexation of metformin with metal ions, especially in mixed ligand complexes, is still important for establishing good knowledge and a better understanding of the binding modes of metformin in this type of complex as a key point for developing new metformin-based metallodrugs. Therefore, this study was done to present the synthesis and speculated formula of two mixed ligand complexes of vanadyl(ii) and chromium(iii) ions with the general formula [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl, respectively, where L1 is the metformin and L2 is the glycine amino acid (Figure 1) and to observe the X-ray diffraction patterns, phase purity, surface morphologies, and particle shapes of the synthesized mixed ligand complexes.
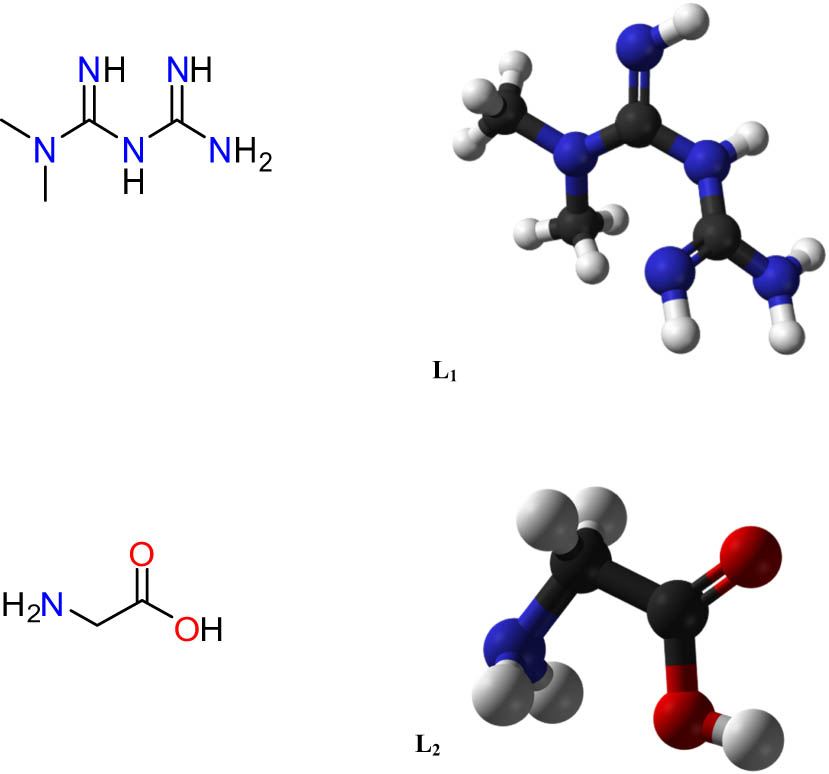
Structures of metformin molecule (L1) and glycine molecule (L2).
2 Experimental
2.1 Instruments and chemicals
The spectrometers such as Perkin-ElmerLambda 25 UV/Vis spectrophotometer, Shimadzu FT-IR spectrophotometer, and Jeol JES-FE2XG electron spin resonance spectrometer were used to scan the UV-visible, IR, and ESR spectra at the room temperature, respectively. The microscopes such as JEOL JEM-1200 EX II transmission electron microscope (TEM) and Quanta FEG 250 scanning electron microscope (SEM) were applied to picture the TEM and SEM images, respectively. Perkin-Elmer 2400CHN elemental analyzer was applied to collect the elemental data. X’Pert Philips X-ray powder diffractometer was applied to collect the XRD spectra of the complexes, where HACH digital conductivity meter and magnetic susceptibility balance were applied for the molar conductivity and magnetic measurements, respectively. Solvents and chemicals were purchased from Merck KGaA company (Darmstadt, Germany) in analytical grade and used without further modification: metformin hydrochloride (C4H11N5·HCl ≥97%), glycine (NH2CH2COOH ≥99%), VOSO4·xH2O 97%, and CrCl3·6H2O ≥98%.
2.2 Syntheses
Mixed ligand complexes [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl were synthesized as follows: To a methanolic solution of L1 (metformin) (1 mmol in 10 mL), 1 mmol of L2 (glycine) dissolved in MeOH solvent (10 mL) was added. The solution was stirred well for 10 min. Then, 1 mmol of VOSO4·H2O dissolved in MeOH solvent (10 mL) was added to prepare the VOL1L2 complex, and the pH of the mixture was adjusted to ∼8 with the ammonia solution (5%). The resultant homogenous greenish-blue solution was refluxed with stirring for 30 min at 65°C. On cooling the reactant media, the greenish-blue-colored precipitate was separated, which was recrystallized from the MeOH solvent. The final product was filtered, washed with MeOH and CH3OCH3, and then dried in an oven at 70°C. The [CrL1L2(Cl)2]Cl complex was similarly synthesized as described for the VOL1L2 complex using 1 mmol of CrCl3·6H2O resulting in a dark green-colored product. The products were next characterized by analytical, thermal, and different spectral methods.
[VOL1L2]SO4 complex:
Gross formula, C6H16N6O7SV; general formula, [VOL1L2]SO4; molecular weight, 367.23 g mol−1; elemental results: calc. (found) for C, 21.62% (21.52); H, 4.39% (4.55); N, 22.89% (22.67); and metal, 13.87% (14.13).
[CrL1L2(Cl)2]Cl complex:
Gross formula, C6H16N6O2Cl3Cr; general formula, [CrL1L2(Cl)2]Cl; molecular weight, 362.58 g mol−1; elemental results: calc.(found) for C, 19.88% (19.75); H, 4.45% (4.34); N, 23.18% (23.06); and metal, 14.34% (14.21).
-
Ethical approval: The study was conducted and approved by the Ethical Committee of Taif University (Fast–track Research Funding program).
3 Results and discussion
3.1 Analytical, conductance, and UV-visible spectral results
In this present research, the reaction of L1 and L2 with VO(ii) ions in MeOH solvent at (pH ∼8; 65°C) gave a greenish-blue-colored complex with the general formula [VOL1L2]SO4. When L1 and L2 reacted with Cr(iii) ions at the same condition, a dark green-colored complex was formed with the general formula [CrL1L2]Cl. Elemental data of the two complexes are presented in the experimental section. The data indicate that the reaction stoichiometry is 1:1:1 (L1:L2:Metal ion), which suggested that the general composition of the complexes obtained with VO(ii) and Cr(iii) ions are [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl, respectively. The molar conductivity of the [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes in DMSO (10−3 M) at 25°C is proportionate with their electrolytic nature [25,26,27,28] with 54 and 61 Ω−1 cm2 mol−1 values, respectively. The corresponding gross formulas are C6H16N6O7SV and C6H16N6O2Cl3Cr, respectively. The [VOL1L2]SO4 complex has an effective molar magnetic moment (μ eff) of 1.71 B.M. at the room temperature [39]. The [CrL1L2(Cl)2]Cl complex has an effective magnetic moment (μ eff) of 3.14 B.M. at the room temperature, which revealed that Cr(iii) complex possesses an octahedral geometry [40]. The UV-visible spectra of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes were scanned over the 200–1,000 nm wavelength range at the room temperature in DMSO solvent and presented in Figure 2. The spectrum of [CrL1L2(Cl)2]Cl complex showed three bands at 37,736, 23,529, and 16,667 cm−1, where that of the [VOL1L2]SO4 complex exhibited four bands at 36,364, 23,529, 16,667, and 12,121 cm−1. Among these bands, the bands observed at 37,736 and 36,364 cm−1 were very strong. The band at 36,364 nm appeared in the spectrum of [VOL1L2]SO4 complex was stronger and broader than the band observed at 37,736 cm−1 in the spectrum of [CrL1L2(Cl)2]Cl complex [40,41,42]. The electronic [VOL1L2]SO4 complex spectrum show bands at regions 12,121, 16,667, and 23,529 cm−1. These spectra are similar to those of other five-coordinate oxovanadium(iv) complexes involving nitrogen donor atoms. These spectral bands are interpreted according to an energy level scheme reported by Lever for distorted, five-coordinate square pyramidal oxovanadium(iv) complexes [40]. Accordingly, the observed bands can be assigned to 2B2 → 2E, 2B2 → 2B1, and 2B2 → 2A2 transitions, respectively. One more band is observed at the region 36,364 cm−1, which may be due to the transition of the metformin linkages [40]. For the chromium complex, two peaks at 16,667 and 23,529 cm−1 were assigned to 4A2g → 4T2g and 4A2g → 4T1g (f) d–d transitions, respectively. The appearance of these two bands confirms octahedral (Oh) geometry for this complex [40]. The other band exhibited at the region 37,736 cm−1 is assigned to the transition of the metformin linkages.
![Figure 2
UV-visible spectra of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes.](/document/doi/10.1515/chem-2021-0063/asset/graphic/j_chem-2021-0063_fig_002.jpg)
UV-visible spectra of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes.
3.2 IR spectral results
3.2.1 Free ligands
A free L1 molecule has three significant vibrational bands [27,43]:
The C‒H vibrations:
The two aliphatic CH3 groups give bands at 2,815 and 3,088 cm−1attributed to the νas(C–H) and νs(C–H) vibrations. The C‒H bending deformation vibrations resonated at 1,463 cm−1, where the δ rock(C–H) vibrations give a sharp band at ∼933 cm−1.
The C═N and C–N vibrations:
The ν(C═N) vibrations resonated at 1,624 cm−1, where the vibrations of C–N band resonated at 1,271 cm−1 for asymmetric stretching and at 1,168 cm−1 for symmetric stretching.
The N–H vibrations:
The ν(N–H) vibrations of imino (‒C═NH), secondary (‒NH), and primary (‒NH2) groups give strong bands around 3,369–3,295 cm−1 for asymmetric stretching and at 3,153 cm−1 for symmetric stretching. The δ(N‒H) vibrations gave a very strong band at 1,556 cm−1.
The amino group of L2 molecule can lose a hydrogen ion and become negatively charged or can accept a hydrogen ion and become positively charged, this is called as the zwitterion structure of L2 molecule in solution.
The IR data (cm−1) of free L2 molecule are as follows: 3,244 ν(NH3), 2,551 ν(CH2), 1,661 and 1,512 δ(NH3), 1,611 νas(COO), 1,436 δ def(CH2), 1,407 νs(COO), 1,326 δ wag(CH2) and δ wag(NH3), 1,138 δ rock(NH3), 1,039 ν(C‒N), and 929 δ rock(CH2) [44].
3.2.2 Mixed ligand complexes
Table 1 reports the band assignments for the important IR bands in free ligands and the mixed ligand complexes, where their IR spectra are shown in Figure 3. In the [VOL1L2]SO4 complex, the bands due to the ν(N–H) vibrations of the ligands were located at 3,394 and 3,173 cm−1. The IR spectrum of [CrL1L2(Cl)2]Cl complex showed a very wide absorption band ranged from 3,600 to 2,560 cm−1 due to the overlapping of bands due to the ν(N–H), νas(C–H), and νs(C–H) vibrations. The bands located at 1,608 and 1,605 cm−1 in spectra of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes were attributed to the νas(COO) vibrations, respectively, that of the νs(COO) vibrations were resonated at 1,390 and 1,383 cm−1, respectively. These bands represent a Δν difference between νas(COO) and νa(COO): [Δν = νas − νs] equal to 218 cm−1 for the [VOL1L2]SO4 complex and 222 cm−1 for the [CrL1L2(Cl)2]Cl complex, suggesting a unidentate coordination mode for the carboxylate group (COO) of L2 in the mixed ligand [45]. The bands attributed to the ν(C═N) vibrations located at 1,624 cm−1 in free L1 were shifted to 1,646 cm−1 in the [VOL1L2]SO4 complex and to 1,643 cm−1 in the [CrL1L2(Cl)2]Cl complex and decreased in intensity. The outlined shifts in the C═N band upon complexation suggested that the L1 ligand was coordinated to the Cr(iii) and VO(ii) ions via the nitrogen atoms of the imino (‒C═NH) groups without any displacement of protons. The [VOL1L2]SO4 complex exhibited a medium and broadband at 973 cm−1 due to the νas(V═O) vibrations [45]. It is worth mentioning that the test against the presence of the sulfate group gave a positive result; this conclusion was supported by detected the two IR active ν3 and ν4 frequencies at about 1,098 and 627 cm−1. Based on these observations, the proposed formula of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes are shown in Figure 4.
Characteristic IR bands (cm−1) for L1, L2, and the mixed ligand complexes
| Free L1 | Free L2 | [VOL1L2]SO4 | [CrL1L2(Cl)2]Cl | Assignments |
|---|---|---|---|---|
| 3,369 | — | 3,394 | Very wide band 3,600–2,560 | ν(N–H), C═NH |
| ν(O–H) | ||||
| 3,295 | 3,244 | — | ν(N–H) | |
| 3,153 | 3,170 | 3,173 | ν(N–H); NH2 | |
| 3,088, 2,815 | — | 3,020 | νas(C–H)+ νs(C–H) | |
| 2,551 | — | — | ν(CH2) | |
| 1,624 | — | 1,646 | 1,643 | ν(C═N) |
| — | 1,611 | 1,608 | 1,605 | νas(COO) |
| 1,556 | — | — | — | δ(N‒H); in-plane def. |
| 1,463 | 1,436 | 1,482 | 1,440 | δ(C‒H) deformation |
| — | 1,414 | 1,390 | 1,383 | νs(COO) |
| — | 1,326 | 1,348 | 1,354 | δ wag(CH2) |
| 1,271 | — | 1,298 | 1,306 | νas(C‒N) |
| 1,168 | — | — | 1,210 | νs(C‒N) |
| 1,056 | — | 1,098 | 1,063 | δ(CH) in-plane bending |
| ν(SO4); ν3 | ||||
| — | 1,039 | — | 1,000 | ν(C‒N) |
| — | — | 973 | — | ν(V═O) |
| 933 | 929 | 850 | 849 | δ rock(C‒H) |
| 635 | — | 701 | 762 | δ(CH) out-of-plane bending |
| 627 | ν(SO4); ν4 | |||
| — | — | 596 | 604 | ν(M‒O), ν(M‒N) |
![Figure 3
IR spectra of (a) metformin drug (L1), (b) glycine amino acid (L2), (c) [VOL1L2]SO4, and (d) [CrL1L2(Cl)2]Cl complexes.](/document/doi/10.1515/chem-2021-0063/asset/graphic/j_chem-2021-0063_fig_003.jpg)
IR spectra of (a) metformin drug (L1), (b) glycine amino acid (L2), (c) [VOL1L2]SO4, and (d) [CrL1L2(Cl)2]Cl complexes.
![Figure 4
The proposed structure of the [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes.](/document/doi/10.1515/chem-2021-0063/asset/graphic/j_chem-2021-0063_fig_004.jpg)
The proposed structure of the [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes.
3.3 ESR spectral results
The Jeol JES-FE2XG electron spin resonance spectrometer was used to scan the powder ESR spectrum of the VOL1L2 complex, and the obtained spectrum is presented in Figure 5. Electron spin resonance spectrum of vanadyl(ii) complex in the polycrystalline state was recorded on ESR spectrometer using 2,2-diphenyl-1-picrylhydrazyl DPPH (Figure 5) free radical as “g” marker (g = 2.0027) at the room temperature. The Hamiltonian spectral data obtained from this spectrum are presented in Table 2. The powder ESR spectrum and the values of g and A for the [VOL1L2]SO4 complex agree with a square pyramidal geometry [46]. Equations (1) and (2) were used to calculate the values of anisotropic (A) and isotropic (g).
![Figure 5
ESR spectrum of [VOL1L2]SO4 complex in solid state at the room temperature.](/document/doi/10.1515/chem-2021-0063/asset/graphic/j_chem-2021-0063_fig_005.jpg)
ESR spectrum of [VOL1L2]SO4 complex in solid state at the room temperature.
Values of Hamiltonian and bonding parameters for the [VOL1L2]SO4 complex
| [VOL1L2]SO4 | ESR data | |||||||
|---|---|---|---|---|---|---|---|---|
| α 2 | β 2 | A 0 | A ┴ (cm−1) | A || (cm−1) | g 0 | g ┴ | g || | |
| 0.1648 | 1.1236 | 368 | 348 | 409 | 1.7956 | 1.6968 | 1.9931 | |
Equations (3) and (4) were used to calculate the covalency of the β 2; in plane π-and α 2; in plane σ-bonding [47,48]:
where E is the electronic transition energy, λ = 135 cm−1, and P = 128 × 10−4cm−1. It is reported in the literature that a g || value more than 2.3 for a metal-ligand bind with ionic character and less than 2.3 for a covalent character [49]. The g || value for the [VOL1L2]SO4 complex was 1.99, suggesting a covalent character of the ligand-metal bond. The α 2 value for the complex was found much smaller than the β 2 value.
3.4 XRD, SEM, and TEM results
To observe the X-ray diffraction patterns, phase purity, surface morphologies, and particle shapes of the [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes, XRD, SEM, and TEM techniques were used. Analyses of the XRD spectra (Figure 6), SEM micrographs (Figure 7), and TEM micrographs (Figure 8) of these complexes provided the following observations:
The [CrL1L2(Cl)2]Cl complex displayed one broad peak ranging from 2θ 20° to 30° in its XRD profile. This broad peak had a maximum at diffraction line 2θ of approximately 26.531°.
The [VOL1L2]SO4 complex displayed a group of intense lines at Bragg’s angle 2θ in the range of 16–33°. These lines were observed at 2θ 16.947°, 19.374°, 20.361°, 22.335°, 24.310°, 28.095°, and 30.562°.
The SEM micrographs captured at various levels of magnification (i.e., ×150, ×500, ×1,000, ×2,000) indicated visible microstructural differences between the particles of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes.
The SEM micrographs of the [VOL1L2]SO4 complex indicated that it consists of small spherical granules fused together. Several clear holes were observed between these granules, and the surfaces of the completely fused granules had several cracks.
The SEM micrographs of the [CrL1L2(Cl)2]Cl complex shows a coral reefs-like-shaped morphology.
The TEM micrographs revealed that the shape of the [CrL1L2(Cl)2]Cl particles was almost spherical, where the shape of the [CrL1L2(Cl)2]Cl particles were mixed spherical and oval-shaped.
Most particles of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes had diameters is in the range of 7–20 and 20–60 nm, respectively.
The average particle size of [CrL1L2(Cl)2]Cl complex is ∼14 nm, where it is ∼40 nm for the [VOL1L2]SO4 complex. This indicated that the particles of the [VOL1L2]SO4 complex are higher by 2.8 times than the particles of the [CrL1L2(Cl)2]Cl complex.
![Figure 6
XRD spectra of L1, [VOL1L2]SO4, and [CrL1L2(Cl)2]Cl complexes.](/document/doi/10.1515/chem-2021-0063/asset/graphic/j_chem-2021-0063_fig_006.jpg)
XRD spectra of L1, [VOL1L2]SO4, and [CrL1L2(Cl)2]Cl complexes.
![Figure 7
(a) SEM micrographs of [VOL1L2]SO4 complex. (b) SEM micrographs of [CrL1L2(Cl)2]Cl complex.](/document/doi/10.1515/chem-2021-0063/asset/graphic/j_chem-2021-0063_fig_007.jpg)
(a) SEM micrographs of [VOL1L2]SO4 complex. (b) SEM micrographs of [CrL1L2(Cl)2]Cl complex.
![Figure 8
TEM micrographs of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes.](/document/doi/10.1515/chem-2021-0063/asset/graphic/j_chem-2021-0063_fig_008.jpg)
TEM micrographs of [VOL1L2]SO4 and [CrL1L2(Cl)2]Cl complexes.
4 Conclusion
In this work, we sought to synthesize and characterize two mixed ligand complexes containing drug metformin as L1and glycine amino acid as L2 with the metal ions of Cr(iii) and VO(ii). The interaction via a 1:1:1 (L1:L2:Metal ion) stoichiometry yielded two complexes: a greenish-blue-colored complex formulated as [VOL1L2]SO4 with a square pyramidal geometry and a dark green-colored complex formulated as [CrL1L2(Cl)2]Cl with an octahedral structure. Spectroscopic results indicated that L1 ligand coordinated to the VO(ii) and Cr(iii) ions via the N atoms of the imino (‒C═NH) groups, where the L2 ligand coordinated via the O atom of the carboxylate group (COO) and the N atom of the amino group (NH2). SEM and TEM results indicated that the complexes had organized nanoscale structures with a main diameter of ∼14 nm for the [CrL1L2(Cl)2]Cl complex and ∼40 nm for the [VOL1L2]SO4 complex.
Acknowledgments
This research was funded by the deanship of scientific research at Princess Nourah bint Abdulrahman University through the Fast–track Research Funding program.
-
Funding information: This research was funded by the deanship of scientific research at Princess Nourah bint Abdulrahman University through the Fast–track Research Funding program.
-
Author contributions: Conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing original draft preparation, writing-review and editing, visualization, supervision, project administration, funding acquisition.
-
Conflict of interest: The authors declare that they have no conflicts of interest.
-
Data availability statement: The data used to support the findings of this study are included within the article.
References
[1] Abdelhamid HN, Wu HF. A method to detect metal–drug complexes and their interactions with pathogenic bacteria via graphene nanosheet assist laser desorption/ionization mass spectrometry and biosensors. Anal Chim Acta. 2012;751:94–104.10.1016/j.aca.2012.09.012Search in Google Scholar PubMed
[2] Hughes MN. The inorganic chemistry of biological processes. 2nd ed. Chichester [England]: Wiley; 1984.Search in Google Scholar
[3] Cao Q, Li Y, Freisinger E, Qin PZ, Sigel RKO, Mao ZW, et al. targeted metal complexes acting as potential anticancer drugs. Inorg Chem Front. 2017;4:10–32.10.1039/C6QI00300ASearch in Google Scholar
[4] Trudu F, Amato F, Vaňhara P, Pivetta T, Peña-Méndez EM, Havel J. Coordination compounds in cancer: past, present and perspectives. J Appl Biomed. 2015;13(2):79–103.10.1016/j.jab.2015.03.003Search in Google Scholar
[5] Mjos KD, Orvig C. Metallodrugs in medicinal inorganic chemistry. Chem Rev. 2014;114(8):4540–63.10.1021/cr400460sSearch in Google Scholar PubMed
[6] Alessio E. Bioinorganic medicinal chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH and Co. KGaA; 2011.10.1002/9783527633104Search in Google Scholar
[7] Hambley TW. Platinum binding to DNA: structural controls and consequences. J Chem Soc Dalton Trans. 2001;19:2711–8.10.1039/b105406fSearch in Google Scholar
[8] Köpf-Maier P. Complexes of metals other than platinum as antitumour agents. Eur J Clin Pharmacol. 1994;47:1–16.10.1007/BF00193472Search in Google Scholar PubMed
[9] Qin QP, Wang SL, Tan MX, Liu YC, Meng T, Zou BQ, et al. Synthesis of two platinum(ii) complexes with 2-methyl-8-quinolinol derivatives as ligands and study of their antitumor activities. Eur J Med Chm. 2019;161:334–42.10.1016/j.ejmech.2018.10.051Search in Google Scholar PubMed
[10] Meng T, Tang SF, Qin QP, Liang YL, Wu CX, Wang CY, et al. Evaluation of the effect of iodine substitution of 8-hydroxyquinoline on its platinum(ii) complex: cytotoxicity, cell apoptosis and telomerase inhibition. Med Chem Commun. 2016;7:1802–11.10.1039/C6MD00201CSearch in Google Scholar
[11] Abdelhamid HN, Wu HF. Reduced graphene oxide conjugate thymine as a new probe for ultrasensitive and selective fluorometric determination of mercury(ii) ions. Microchim Acta. 2015;182(9):1609–17.10.1007/s00604-015-1461-4Search in Google Scholar
[12] Abdelhamid HN, Wu HF. Ultrasensitive, rapid, and selective detection of mercury using graphene assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 2014;25(5):861–8.10.1007/s13361-014-0825-zSearch in Google Scholar PubMed
[13] Abdelhamid HN, Lin YC, Wu HF. Thymine chitosan nanomagnets for specific preconcentration of mercury (ii) prior to analysis using SELDI-MS. Microchim Acta. 2017;184(5):1517–27.10.1007/s00604-017-2125-3Search in Google Scholar
[14] Abdelhamid HN, Wu HF. Monitoring metallofulfenamic–bovine serum albumin interactions: a novel method for metallodrug analysis. RSC Adv. 2014;4(96):53768–76.10.1039/C4RA07638ASearch in Google Scholar
[15] Bruijnincx PCA, Sadler PJ. New trends for metal complexes with anticancer activity. Curr Opin Biol. 2008;12(2):197–206.10.1016/j.cbpa.2007.11.013Search in Google Scholar PubMed PubMed Central
[16] Jinpiao Z, Zongze Z, Qiuyue Y, Peng F, Qi Z, Yanlin W, et al. Metformin attenuates sevoflurane-induced neurocognitive impairment through AMPK-ULK1-dependent autophagy in aged mice. Brain Res Bull. 2020;157:18–25.10.1016/j.brainresbull.2020.01.018Search in Google Scholar PubMed
[17] Gao J, Yuan J, Wang Q, Lei T, Shen X, Cui B, et al. Metformin protects against PM2.5-induced lung injury and cardiac dysfunction independent of AMP-activated protein kinase α2. Redox Biol. 2020;28:101345.10.1016/j.redox.2019.101345Search in Google Scholar PubMed PubMed Central
[18] Vitali E, Boemi I, Piccini S, Tarantola G, Smiroldo V, Lavezzi E, et al. A novel insight into the anticancer mechanism of metformin in pancreatic neuroendocrine tumor cells. Mol Cell Endocrinol. 2020;509:110803.10.1016/j.mce.2020.110803Search in Google Scholar PubMed
[19] Zhou X, Kuang Y, Liang S, Wang L. Metformin inhibits cell proliferation in SKM-1 cells via AMPK-mediated cell cycle arrest. J Pharmacol Sci. 2019;141(4):146–52.10.1016/j.jphs.2019.10.003Search in Google Scholar PubMed
[20] Bahrambeigi S, Yousefi B, Rahimi M, Shafiei-Irannejad V. Metformin; an old antidiabetic drug with new potentials in bone disorders. Biomed Pharmacother. 2019;109:1593–601.10.1016/j.biopha.2018.11.032Search in Google Scholar PubMed
[21] Janjetovic K, Vucicevic L, Misirkic M, Villmanovich U, Tovilovic G, Zogovic N, et al. Metformin reduces cisplatin-mediated apoptotic death of cancer cells through AMPK-independent activation of Akt. Eur J Pharmacol. 2011;651:41–50.10.1016/j.ejphar.2010.11.005Search in Google Scholar PubMed
[22] Correia S, Carvalho C, Cantos MS, Proenca T, Nunes E, Duarte AL, et al. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008;4:358–64.10.2174/157340608784872299Search in Google Scholar PubMed
[23] Huang X, Wullschleger S, Shpiro N, McGuire VA, Sakamoto K, Woods YL, et al. Important role of the LKB1–AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412(2):211–21.10.1042/BJ20080557Search in Google Scholar PubMed
[24] Vasantha P, Kumar BS, Shekhar B, Lakshmi PVA. Copper-metformin ternary complexes: thermal, photochemosensitivity and molecular docking studies. Mater Sci Eng. 2018;C(90):621–33.10.1016/j.msec.2018.04.052Search in Google Scholar PubMed
[25] Mahmoud MA, Zaitone SA, Ammar AM, Sallam SA. Synthesis, structure and antidiabetic activity of chromium(iii) complexes of metformin Schiff-bases. J Mol Struct. 2016;1108:60–70.10.1016/j.molstruc.2015.11.055Search in Google Scholar
[26] Refat MS, Al-Azab FM, Al-Maydama HMA, Amin RR, Jamil YMS, Kobeasy MI. Synthesis, spectroscopic and antimicrobial studies of La(iii), Ce(iii), Sm(iii) and Y(iii) Metformin HCl chelates. Spectrochim Acta A. 2015;142:392–404.10.1016/j.saa.2015.01.096Search in Google Scholar PubMed
[27] Adam AMA, Sharshar T, Mohamed MA, Ibrahim OB, Refat MS. Study of chemical bonding, physical and biological effect of metformin drug as an organized medicine for diabetes patients with chromium(iii) and vanadium(iv) ions. Spectrochim Acta A. 2015;149:323–32.10.1016/j.saa.2015.04.115Search in Google Scholar PubMed
[28] Al-Saif FA, Refat MS. Synthesis, spectroscopic, and thermal investigation of transition and non-transition complexes of metformin as potential insulin-mimetic agents. J Therm Anal Calorim. 2013;111(3):2079–96.10.1007/s10973-012-2459-3Search in Google Scholar
[29] Puri M. Metformin and biguanide complexes of isothiocyanatosilane. Phosphorus Sulfur Silicon Relat Elem. 2012;187(9):1026–31.10.1080/10426507.2012.664216Search in Google Scholar
[30] Moghimi A, Khavassi HR, Dashtestani F, Kordestani D, Jafari AE, Maddah B, et al. A ternary tetracoordinated PdII complex with metformin and dipicolinate: synthesis, characterization and crystal structure. J Mol Struct. 2011;996:38–41.10.1016/j.molstruc.2011.03.061Search in Google Scholar
[31] Rao NK, Annapurna MM. Copper and nickel complexes of metformin: synthesis, characterization and pharmacodynamic evaluation. JASA. 2007;3:43–6.Search in Google Scholar
[32] Zhu M, Lu L, Yang P, Jin X. Bis(1,1-dimethylbiguanido)copper(ii) octahydrate. Acta Cryst. 2002;E58:m217–9.10.1107/S1600536802007092Search in Google Scholar
[33] Marchi A, Marvelli L, Cattabriga M, Rossi R, Neves M, Bertolasi V, et al. Technetium(v) and rhenium(v) complexes of biguanide derivatives. Cryst Struct, J Chem Soc, Dalton Trans. 1999;12:1937–44.10.1039/a900798iSearch in Google Scholar
[34] Narad SR, Mishra NN, Pandey P, Kumar A. Antidiabetic activity of some oral hypoglycemic agents with metal ions on animal model. Indian J Exp Biol. 1996;34(1):81–2.Search in Google Scholar
[35] Abu-el-Wafa SM, El-Ries MA, Ahmed FH. Formation of metformin complexes with some transition metal ions: their biological activity. Inorg Chim Acta. 1987;136(3):127–31.10.1016/S0020-1693(00)81143-3Search in Google Scholar
[36] Spacu P, Gheorghiu C. Contribution to the coordination chemistry of osmium: II. Chelates of bivalent and tervalent osmium with biguanide derivatives. J Less Common Met. 1969;18(2):117–22.10.1016/0022-5088(69)90130-1Search in Google Scholar
[37] Gheorghiu C. Beiträgezur Chemie des Iridiums. II. Komplexverbindungen des Ir(iii) mitBiguanidderivaten. Z Anorg Allg Chem. 1969;365:91–9.10.1002/zaac.19693650112Search in Google Scholar
[38] Spacu P, Gheorghiu C. Contribution to the coordinative chemistry of rhodium. II. Rhodium(iii) complexes with biguanide derivatives. J Less Common Met. 1968;15(3):331–9.10.1016/0022-5088(68)90192-6Search in Google Scholar
[39] Syamal A, Kale KS. Magnetic and spectral properties of oxovanadium(iv) complexes of ONO donor tridentate, dibasic Schiff bases derived from salicylaldehyde or substituted salicylaldehyde and o-hydroxybenzylamine. Inorg Chem. 1979;18(4):992–5.10.1021/ic50194a023Search in Google Scholar
[40] Lever ABP. Inorganic electronic spectroscopy. 2nd ed. Amsterdam: Elsevier; 1997.Search in Google Scholar
[41] Allan JR, Baird ND, Kassyk AL. Some first-row transition metal complexes of nicotinamide and nicotinic acid. J Therm Anal. 1979;16(1):79–90.10.1007/BF01909635Search in Google Scholar
[42] Öztirk ÖF, Şekerci M, Özdemir E. Preparation of complexes of 1-amino-6,7-O-cyclohexylidene-4-azaheptane with transition metal acetates. Russ J Gen Chem. 2006;76:33–6.10.1134/S1070363206010075Search in Google Scholar
[43] Ibrahim OB, Manaaa EA, AL-Majthoub MM, Fallatah AM, Adam AMA, Alatibi MM, et al. Estimation of metformin drug for the diabetes patients by simple,quick and cheap techniques within the formation of colored charge transfer complexes. Spectrosc Spect Anal. 2018;38(11):3622–30.Search in Google Scholar
[44] Maté B, Rodriguez-Lazcano Y, Gálvez Ó, Tanarroa I, Escribano R. An infrared study of solid glycine in environments of astrophysical relevance. Phys Chem Chem Phys. 2011;13:12268–76.10.1039/c1cp20899cSearch in Google Scholar PubMed
[45] Nakamoto K. Infrared spectra of inorganic and coordination compounds. New York: Wiley; 1970.Search in Google Scholar
[46] Refat MS, El-Shazly SA. Identification of a new anti-diabetic agent by combining VOSO4 and vitamin E in a single molecule: Studies on its spectral, thermal and pharmacological properties. Eur J Med Chem. 2010;45(7):3070–9.10.1016/j.ejmech.2010.03.040Search in Google Scholar PubMed
[47] Jeyasubramanian K, Abdul Samath S, Thambidurai S, Murugesan R, Ramalingam SK. Cyclic voltammetric and e.s.r. studies of a tetraaza 14-membered macrocyclic copper(ii) complex derived from 3-salicylideneacetylacetone ando-phenylenediamine: stabilization and activation of unusual oxidation states. Trans Met Chem. 1995;20:76–80.10.1007/BF00135407Search in Google Scholar
[48] Anthonisamy VSX, Anantharam R, Murugesan R. The temperature dependence of EPR spectra of Cu(ii)-doped hexakis(imidazole)cadmium(ii) perchlorate. Spectrochim Acta A. 1998;55(1):135–42.10.1016/S1386-1425(98)00174-7Search in Google Scholar
[49] Kivelson D, Neiman R. ESR studies on the bonding in copper complexes. J Chem Phys. 1961;35:149.10.1063/1.1731880Search in Google Scholar
© 2021 Samar O. Aljazzar, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

