Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
Abstract
The samples of plant material suspected to contain new psychoactive substances are very often the subject of chemical-toxicological analyses. Gas chromatography-mass spectrometry (MS), liquid chromatography-quadrupole time-of-flight-MS, and liquid chromatography-tandem MS were applied with the aim to identify synthetic cannabinoid, methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate (MMB-CHMICA) without the analytical standard, which is very often the case when a new drug arrives. The structure of compound was also confirmed by one-dimensional and two-dimensional nuclear magnetic resonance spectroscopy and conformational analysis. After identification, methanolic extract of plant material containing MMB-CHMICA was successfully used for developing a multiple reaction monitoring method on liquid chromatography-tandem MS instrument. The optimization procedure is shown in detail. The complete fragmentation pattern and also the optimization of the extraction procedure of MMB-CHMICA from plant material were shown. The obtained data are useful for forensic, toxicological, and clinical purposes.
Graphical abstract
1 Introduction
In the last decade, more than 1,000 new psychoactive substances (NPSs) have emerged on illicit drug markets [1]. Their number and chemical diversity are constantly increasing. They include synthetic cannabinoids (SCs), stimulants, benzodiazepines (and other sedative-hypnotics), opioids, hallucinogens and dissociatives, a broad range of drugs that are not controlled by the United Nations international drug laws [2] and launched as legal alternatives to common drugs of abuse. NPSs are successfully distributed through the Internet or specialized shops known as “Smart Shops” [3] using product terms such as “legal highs,” “herbal highs,” “research chemicals,” “plant food,” “herbal incense,” or “bath salts” as a way to circumvent laws governing their sale. They exist as powders, pellets, tablets, liquids, and herbal material and frequently are labeled as “Not for Human Consumption” [4,5,6]. Now they are accompanied by e-liquids, impregnated letters, and blotting papers as new product formulations [7].
SCs are one of the most prevalent drug classes of NPSs, which hit the market in the early 2000s. They are compounds originally designed with a therapeutical intent. High affinity is shown to cannabinoid receptors, CB1 and CB2, in the human body. There is an increasing trend in the recreational drug community of smoking mixtures of herbal or incense products spiked with one or more SCs, drugs with cannabinoid-like properties [7,8].
The increasing number of NPSs appearing on the market has become a major public health concern, not only because of increasing usage, intoxication, and deaths but also because of the lack of scientific research and understanding of their adverse effects [4]. These limitations emphasize an urgent need to increase the capabilities of the toxicology laboratories and to develop methods for identification of new molecules [3,9].
Creation and the availability of early warning system (EWS) at the national, regional, and international level offer opportunities to avert potential crises through prompt information sharing among stakeholders so that the risks posed by synthetic drugs can be identified and mitigated [1]. So, the detection and identification of synthetic drugs are necessary first steps to spread information on the NPSs and any successful law enforcement intervention and are key to the implementation of international drug control conventions at the national level.
Identification of an unknown organic compound without any preknowledge is commonly the domain of nuclear magnetic resonance (NMR) spectroscopy and refers to full de novo structure identification and must be performed with a set of independent methods such as one-dimensional (1D) and two-dimensional (2D) NMR spectroscopy or infrared spectroscopy and X-ray crystallography or other spectroscopic methods [10].
Identifying an unknown organic compound is always a major challenge for the analyst especially without commercially available reference standards, reference spectra, and relevant literature and research. A process for their identification is often named “structure elucidation,” and the substances are often referred in literature as “unknown unknown” [11,12]. NMR spectroscopy in combination with high-resolution mass spectrometry (HRMS) provides the necessary solutions, but NMR spectrometers are not used in routine forensic analysis because of their cost and requirements for relatively pure compounds and the technical expertise [13].
Significant developments and progress in all aspects of mass spectrometry (MS) with an orthogonal chromatographic approach make it convenient for the identification of compounds that are not sufficiently known but was described in the scientific literature. MS is a most powerful and key technique for qualitative chemical analysis or identification [11]. It plays an important role in determining the elemental composition of a molecule and structural clarification using mass spectral fragmentation [10]. Due to selectivity and sensitivity, MS is particularly well suited to address analyses of NPSs, the cause of an alarming number of deaths worldwide [14]. Gas or liquid chromatograph connected to mass spectrometers is capable of separating complex mixtures of chemical compounds for subsequent detection and quantification [11].
Electron ionization (EI) GC-MS (gas chromatography-MS) has been widely used methodology for general unknown screening in forensic toxicology. These mass spectra can be easily used for a mass spectral library search, and GC-MS identification is based on matching the full scan mass spectrum of an unknown peak to library spectra and/or reference standards. But many organic compounds are not convenient to GC-MS either due to their low volatility, high polarity, or thermal instability. Liquid chromatography-MS (LC-MS) can analyze these types of compounds [15] utilizing soft ionization sources such as electrospray ionization (ESI) in conjunction with a variety of high-performance liquid chromatographic (HPLC) separation modes including reversed phase, normal phase, and ion exchange. However, the corresponding mass spectra are not rich in peaks of fragment ions. This limitation can be overcome using collision-induced dissociation, which provides thorough fragmentation of the compounds.
HRMS has been identified as the method of choice for broad screening of NPSs. It provides full spectrum MS data with high mass resolution and mass accuracy and is useful adjunct to the other techniques to assist in identifying compounds when standards are not available.
Liquid chromatography-time of flight has the increased resolving power to accurately measure exact masses (accurate to within 5 ppm), and data can be collected as full-scan data with no loss in sensitivity. Sophisticated data-acquisition capabilities enable generation of molecular formulae from accurate masses and distinguish between compounds having the same nominal molecular weights but different molecular formulae [8,16].
The approaches for identification NPS can be much different as every analytical technique has different identification powers and depends on data we have; it can be target identification and unknown/nontarget analysis [11,12].
Milman [11] dealt with in detail with the criteria for identification in MS.
Regarding the determinations of target analytes by validated methods, only the most reliable: coanalysis with authentic analytical standards and, comparison with reference data obtained in conditions very similar to analytical runs, are recommended for use [12].
In nontarget screening, all possible means of identification are permitted, with the enforced use of (1) matching theoretical reference data, (2) comparison to predicted reference data, and (3) data interpretation, in cases of very rare and novel analytes, where reference data or materials are not available. In most cases, the identification criteria consist of the following:
(1) the number of identification points for different techniques,
(2) tolerance ranges about reference values of chromatographic RTs (retention times),
(3) maximum permitted tolerances for relative mass-peak intensities of selective ions,
(4) requirements to full mass spectra expressed in words, and
(5) requirements for HRMS expressed in words and value tolerances for resolution and mass accuracy.
One of the studies that illustrates the LC-MS/MS (liquid chromatography-tandem MS) methods for the analysis of drugs of abuse showing different fragmentation features and presenting the results with respect to current recommendations and EU (European Union) guidelines [7] considering the criteria of “minimum number of multiple reaction monitoring (MRM) transitions” and the “relative intensities of the transitions” was published in 2004 [17].
Standard for identification criteria in forensic toxicology also anticipates minimum requirements for the identification of an analyte in forensic toxicology laboratories [18].
The proliferation of NPSs in the past decade has resulted in the development of numerous analytical methods for the detection and identification of known and unknown NPS derivatives. Scheduled substances can be easily replaced with new compounds, showing a slightly different substitution pattern, creating a constantly moving analytical target [16,19,20]. MS has been appeared as the method of choice for the identification or quantitative analyses of many NPSs including SCs in different biological matrices or seized material such as spice products [8,19,21,22,23,24,25,26,27,28,29]. In cases where the laboratory does not have analytical standards for the compound, even though the compound has been described in the literature, it is always good to confirm the structure using NMR spectroscopy and molecular modeling, that is, conformational search.
To the best of our knowledge, methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate (MMB-CHMICA) has been reported in the literature only several times [7,13,30,31,32,33]. United Nations Office on Drugs and Crime recommended GC-MS screening method, which includes MMB-CHMICA. They, first, have used HP-5MS column for separation but reported that some SCs coelute during GC-MS analysis on set conditions (MMB-CHMICA with methyl 2-[[1-(cyclohexylmethyl)-1H-indole-3-carbonyl]amino]-3,3-dimethylbutanoate (MDMB-CHMICA)) and resolved this problem by using more polar DB-35 column [13]. Identification is accomplished by comparing the retention time and mass spectrum of the analyte with that of a reference standard.
Ambroziak and Adamowicz [30] developed LC-MS/MS method for screening 72 SCs in whole blood. MRM transitions for MMB-CHMICA was 371.2 > 240.2 (1), 371.2 > 144.1 (0.34), and 371.2 > 55.2 (0.26; relative ion transition intensities). They did not report the identification of this compound in the blood.
Interestingly, Apirakkan et al. [7] identified MMB-CHMICA in blotting paper in a seized prison letter, in attempt of smuggling this drug in prison.
We have not found any report in which MMB-CHMICA was associated with intoxication but there is reporting of death after use of MDMB-CHMICA, which differs from it by only one methyl group [25].
U.S. Drug Enforcement Administration was temporarily scheduled MMB-CHMICA on Schedule 1 Controlled Substances Act that means that it has a high potential for abuse that is comparable to other Schedule I substances such as tetrahydrocannabinol and JWH-018 and having no recognized medical use. In the Republic of Serbia, MMB-CHMICA is under control since 2017 after our research presented in this article.
In this study, the use of gas chromatography-MS, liquid chromatography-tandem MS, and liquid chromatography-quadrupole time of flight MS (LC-QTOF/MS) for the identification of SC MMB-CHMICA as an additive in the herbal product and confirmation of the proposed structure by 1D and 2D NMR, and conformational analysis were reported. The optimization of MRM transition on LC-MS/MS was performed without the analytical standard of this compound using the extract of the plant material where previously SC was found. Developed LC-MS/MS method has been used for targeted analysis to assess extraction yield by the experimental design.
2 Materials and methods
2.1 Chemicals and reagents
Methanol (MeOH) and acetonitrile (ACN) LC-MS grade and formic acid (FA) purity 99% were obtained from Carlo Erba Reagents (Chaussée du Vexin, France). Petroleum ether and diethyl ether analytical grade were purchased from Carl Roth GmbH (Karlsruhe, Germany), and deuterochloroform (CDCl3) was obtained from Euriso-Top (Saint-Aubin, France). Potassium permanganate was obtained from Acros Organics (Geel, Belgium).
Ultrapure water was provided by a Purelab Classic (ELGA LabWater, High Wycombe, Bucks, UK).
2.2 Sample
The plant material (brand name not indicated here) was analyzed on a personal request of the user in October 2017. According to the information we received, it was purchased in one of the smart shops in Belgrade, Serbia, in the original package. It is declared as a freshener with a warning that it is not for human use. The labels on the packages indicated that the products contained 3 g of a mixture of plants.
2.3 Extraction procedure and isolation of pure compound
For qualitative analysis, the product (25 mg) was crushed into powder and extracted with 5 mL of MeOH under ultrasonication for 30 min. After centrifugation (5 min at 3,000 rpm [relative centrifugal force equals to 2350.6]) on centrifuge (J. P. Selecta, Barcelona, Spain), the solution was passed through the 0.45 µm filter (Ultrafree-MC, filter unit, Millipore, Bedford, MA, USA). This extract was used for GC-MS and ESI QTOF/MS analyses diluted 10-fold and for LC-MS/MS diluted 100-fold with MeOH solution.
For NMR characterization, the extract obtained from 150 mg of plant material was concentrated and purified by flash chromatography (petroleum ether/diethyl ether, 90:10).
2.4 Instrumentation and conditions used
2.4.1 Gas chromatography-MS
Sample extract was analyzed by GC-MS using an Agilent 6850 GC/5975B MSD with automatic liquid sampler (Agilent Technologies, Santa Clara, CA, USA). Chromatography was performed on a J&W DB-17 MS Capillary Column, 30 m × 0.25 mm × 0.25 µm (Agilent Technologies, Santa Clara, CA, USA). Injection volume was 1 µL, and injection mode was splitless. Gas chromatographic analysis used a temperature program starting at 120°C with an initial hold time 1 min, and then with a 20°C min−1 ramp to a final temperature of 280°C with a final hold time of 20 min, resulting in a total run time of 29 min per sample with a solvent delay of 5.0 min. Inlet temperature was 250°C. Transfer line temperature was 300°C. Helium was used as a carrier gas with the flow of 1 mL min−1 (Condition 1).
Also, the sample extract was analyzed using a different GC-MS instrument and a different capillary column for separation. It was Shimadzu GC-2010 Plus equipped with a Shimadzu AOC-5000 autosampler system and interfaced with a Shimadzu QP 2010 Ultra mass spectrometer (Shimadzu, Kyoto, Japan). The GC was equipped with a split/splitless injection port operated at splitless mode. A total of 1 µL of extract was injected into the GC-MS. The separation of analytes was carried out using a cross-linked J&W DB-1 MS capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness) supplied by Agilent Technologies (Santa Clara, CA, USA). Helium was employed as a carrier gas and used at a flow rate of 0.73 mL min−1. The temperatures of injection port, ion source, and interface were 250, 200, and 280°C, respectively. Initial oven temperature of 200°C was held for 1 min, followed by an increase to 320°C at a rate of 20°C min−1 and a final hold time of 15 min, resulting in a total run time of 22 min per sample with a solvent delay of 4.0 min (Condition 2).
Both mass spectra were recorded with EI at 70 eV by scanning at full ionic current in the range of 40–550 amu.
2.4.2 High-resolution MS
For the determination of accurate molecular and elemental composition, the analysis was performed on an Agilent 6520 quadrupole time-of-flight mass spectrometer (Agilent Technologies, Santa Clara, CA, USA) equipped with a Jet Stream interface for ESI in combination with an Agilent 1290 Infinity LC instrument. LC instrument was used without column on ambient temperature. Mobile phase A consisted of 0.1% FA and mobile phase B of ACN delivered at isocratic (50:50 v/v) flow rate of 0.2 mL min−1. Total run time was 2.00 min. The injection volume was 0.1 µL.
Analyses were performed in MS-positive ESI mode. The jet stream conditions were capillary voltage 3,500 V, fragmentor voltage 70 V, gas temperature 350°C, drying gas flow 12 L min−1, nebulizer pressure 45 psi, and sheath gas temperature 375°C. The scan range was set to acquire between m/z 100 and 1,500 at a rate of 1.0 spectra s−1 for each scan segment.
Mass calibration of the QTOF/MS system was controlled by constant infusion of a reference mass solution (provided by Agilent Technologies, Santa Clara, CA, USA) into the source of the QTOF/MS system during the analysis (protonated reference ion [M + H]+ = 922.0098 was used for the positive mode). Data acquisition and evaluation were performed using Mass Hunter software: acquisition module (Acq) B.05.01 and qualitative analysis module (Qual) B.06.00.
2.4.3 NMR characterization
The NMR spectra of the purified sample were recorded on a Bruker Ascend 400 (Bruker Corporation, Billerica, MA, USA; 400 MHz) spectrometer. Chemical shifts are given in parts per million (δ, ppm) downfield from tetramethylsilane as the internal standard. CDCl3 was used as a solvent. Flash chromatography was employed using silica gel 60 (230–400 mesh). Thin layer chromatography was carried out using alumina plates with a 0.25 mm silica layer (Kieselgel 60 F254, Merck & Co., Kenilworth, NJ, USA). Compounds were visualized by staining with potassium permanganate solution.
2.4.4 Liquid chromatography-tandem MS
LC-MS/MS was also used for the confirmation and for developing a new MRM method. Shimadzu LCMS-8030 triple quadrupole mass spectrometer (Shimadzu, Kyoto, Japan) equipped with a dual ion source interface for electrospray or atmospheric pressure chemical ionization in combination with an UPLC system composed of an autosampler SIL-30AC, DGU-20A5R, controller CBM-20A, binary pump LC-30 AD, and column oven CTO-30AC were used. The separation was achieved using a Kinetex® 5 µm C18 100 Å, LC column 100 mm × 4.6 mm, maintained at temperature of 40°C. The flow rate was kept at 0.6 mL min−1, and the injection volume was set to 5 µL. The selected mobile phase was a 0.1% v/v of FA in water (component A) and MeOH (component B) in isocratic flow rate (20% A) and (80% B). Run time was 8 min (Condition 3). MS conditions are the same as described in the following text (Condition 4).
To develop a new MRM method, an automated MRM parameter optimization was carried out by flow injection analysis (FIA; HPLC without column) of plant MeOH extract with a function of the LabSolutions LCMS control software, version 5 (Shimadzu, Kyoto, Japan). The parameter optimization performed precursor ion m/z search, fine adjustment of the precursor ion m/z, collision energy (CE) adjustment, product ion m/z selection, fine adjustment of the product ion m/z of top three channels and voltage adjustment of the Q1/Q3 pre-rod, six injections for the compound. FIA conditions were mobile phase A: 0.1% aqueous FA, B: MeOH; isocratic 80% B, flow of mobile phase 0.2 mL min−1, measurement time 2.0 min, and injection volume 5 µL (loop injection). MS conditions: ESI, positive mode, interface temperature desolvation line, and heat block each 230°C, nebulizing gas flow 2.2 L min−1, and drying gas 15 L min−1 (Condition 4).
MRM transitions obtained by optimization were used for further targeted analyses, for estimating extraction yields and estimating instrument responses using ACN and MeOH in combination with 0.1% FA as mobile phases.
2.5 Conformational analysis
The structure of MMB-CHMICA was drawn in Maestro v. 12.7 interface under Schrodinger 2021-1 Suite (Schrodinger, New York, NY, USA). Then, the minimization was performed with AMBER* force field in CHCl3 with truncated Newton conjugate gradient and 10,000 iterations. Thus minimized structure was put for the conformational search using Macromodel. Monte Carlo multiple minimum was used with 10,000 iterations, and all structures were saved within 21 kJ mol−1.
2.6 Extraction procedure of plant material for experimental design
For the optimization of the extraction procedure, eight extracts (25 mg per 5 mL) were prepared according to the experimental plan for the factorial design.
The extraction process was carried out based on a factorial design using two coded levels of each extraction variable (low and high levels) as illustrated in Table 1. The variables were MeOH concentration, time, and temperature. Thus, according to the experimental design (Table 2), plant samples (25 mg) were extracted with 5 mL by a different solvent (50% MeOH or 100% MeOH), at a different temperature (30 or 40°C) and a different extraction time (20 or 30 min). After extraction, the extract was centrifuged (5 min at 3,000 rpm) and then filtrated through the 0.45 µm filter (Ultrafree-MC, Millipore, Bedford, MA, USA) and stored in the flask.
Extraction parameters for the two-level factorial design
| Extraction variables | Notation | Variable Low (−1) | Levels High (+1) |
|---|---|---|---|
| MeOH concentration (%) | x 1 | 50 | 100 |
| Extraction time (min) | x 2 | 20 | 30 |
| Extraction temperature (°C) | x 3 | 30 | 40 |
Design matrix and the responses of two-level factorial design
| x 1 | x 2 | x 3 | MeOH (%) | Time (min) | Temperature (°C) | Det. | Cal. | |
|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 50 | 20 | 30 | 11.39 × 106 | 11.39 × 106 |
| 2 | +1 | −1 | −1 | 100 | 20 | 30 | 12.26 × 106 | 12.44 × 106 |
| 3 | −1 | +1 | −1 | 50 | 30 | 30 | 11.64 × 106 | 11.65 × 106 |
| 4 | +1 | +1 | −1 | 100 | 30 | 30 | 13.13 × 106 | 13.14 × 106 |
| 5 | −1 | −1 | +1 | 50 | 20 | 40 | 11.12 × 106 | 11.12 × 106 |
| 6 | +1 | −1 | +1 | 100 | 20 | 40 | 12.94 × 106 | 12.94 × 106 |
| 7 | −1 | +1 | +1 | 50 | 30 | 40 | 11.59 × 106 | 11.59 × 106 |
| 8 | +1 | +1 | +1 | 100 | 30 | 40 | 13.60 × 106 | 13.60 × 106 |
2.7 Experimental design and statistical analysis
In the evaluation of three variables at two coded levels, a two-level factorial design with eight experimental trials was carried out in a randomized trend. Analysis of variance (ANOVA) was employed to determine the statistical significance of the model.
3 Results and discussion
3.1 Gas chromatography-MS analysis
In the process of general unknown screening or systematic toxicological analyses, the MeOH extract was, first, analyzed using GC-MS under the conditions previously described (Condition 1). Capillary column DB-17MS was used for the separation. At retention time 20.896 min the chromatographic peak appeared that was not symmetrical, but the obtained mass spectrum shows the presence of molecular ion m/z 370 and characteristic fragments m/z 240 (100), 256 (88), 144 (50), and 55 (32; % relative intensity). Based on available databases, it was not possible to identify chromatographically separated compound. GC-EI mass spectrum was not present in our MS libraries.
The same extract was analyzed on another GC-MS under the conditions previously described (Condition 2). Very clean spectrum was obtained; that is, it was separated only one compound (m/z 371.25), with impurities in traces. Figure 1 shows total ion chromatogram (TIC) scan/ single ion monitoring (SIM) mode and obtained mass spectrum. For separation, nonpolar capillary column DB-1MS was used and well-separated symmetrical chromatographic peak appeared on retention time 9.40 min.
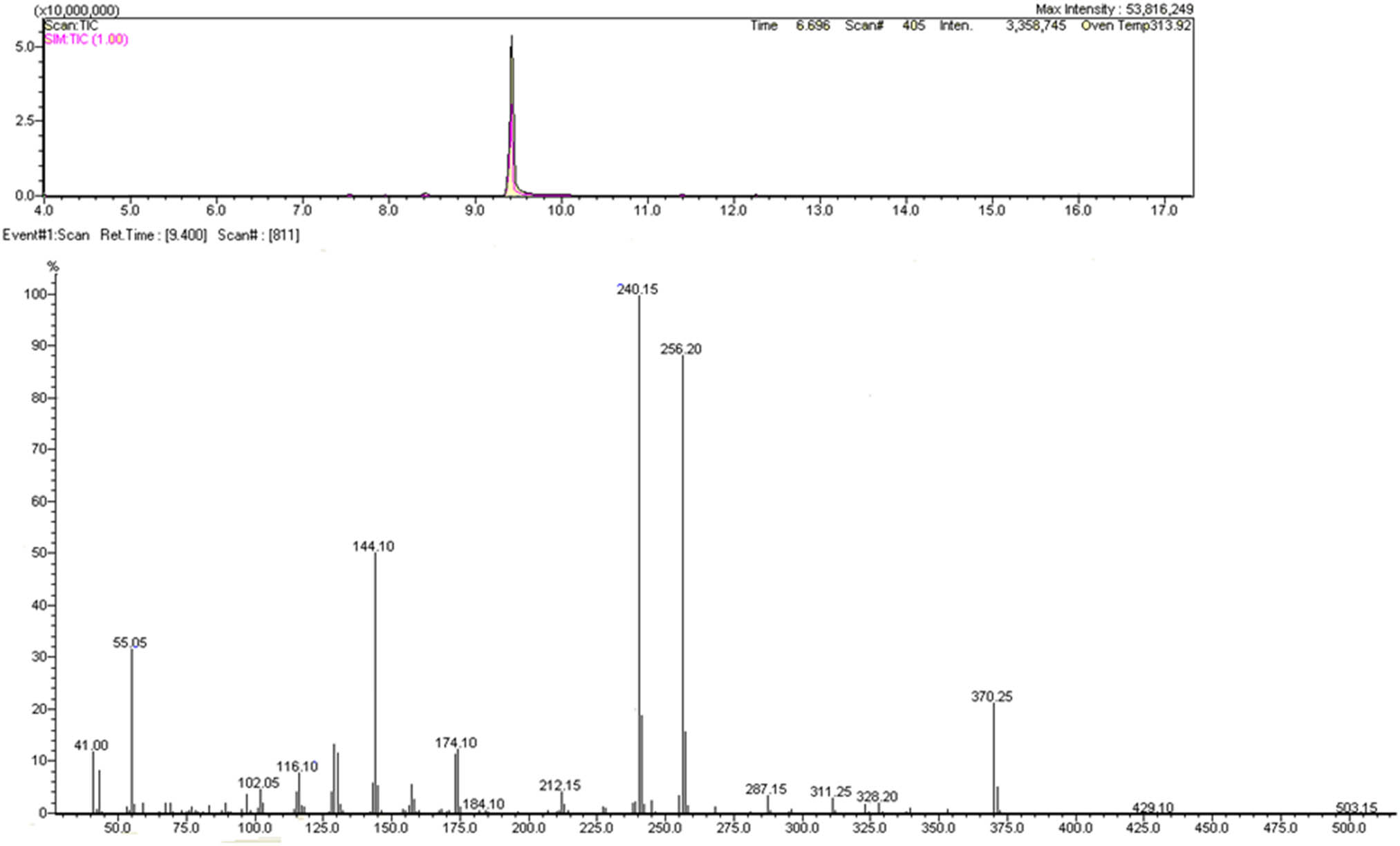
EI GC-MS TIC scan/SIM, mass spectrum of compound, DB-1MS column for separation.
3.2 Liquid chromatography-quadrupole time-of-flight-MS analysis
MeOH extract was analyzed on ESI LC-QTOF/MS as well, by the direct infusion without the column to determine the accurate mass and elemental composition. It was obtained the exact mass 370.2256 Da, Diff (ppm) −0.76, and the recommended formula C22H30N2O3. The adducts formed with Na and K (([C22H30N2O3] + Na)+ and ([C22H30N2O3] + K)+) can be observed in mass spectra. The information about the isotopic distribution for the compound with the molecular formula was gotten m/z 371.2321, 372.2363, 373.2393, and 374.2418, as well as isotopic distribution of formed adducts with Na and K (Figure 2).
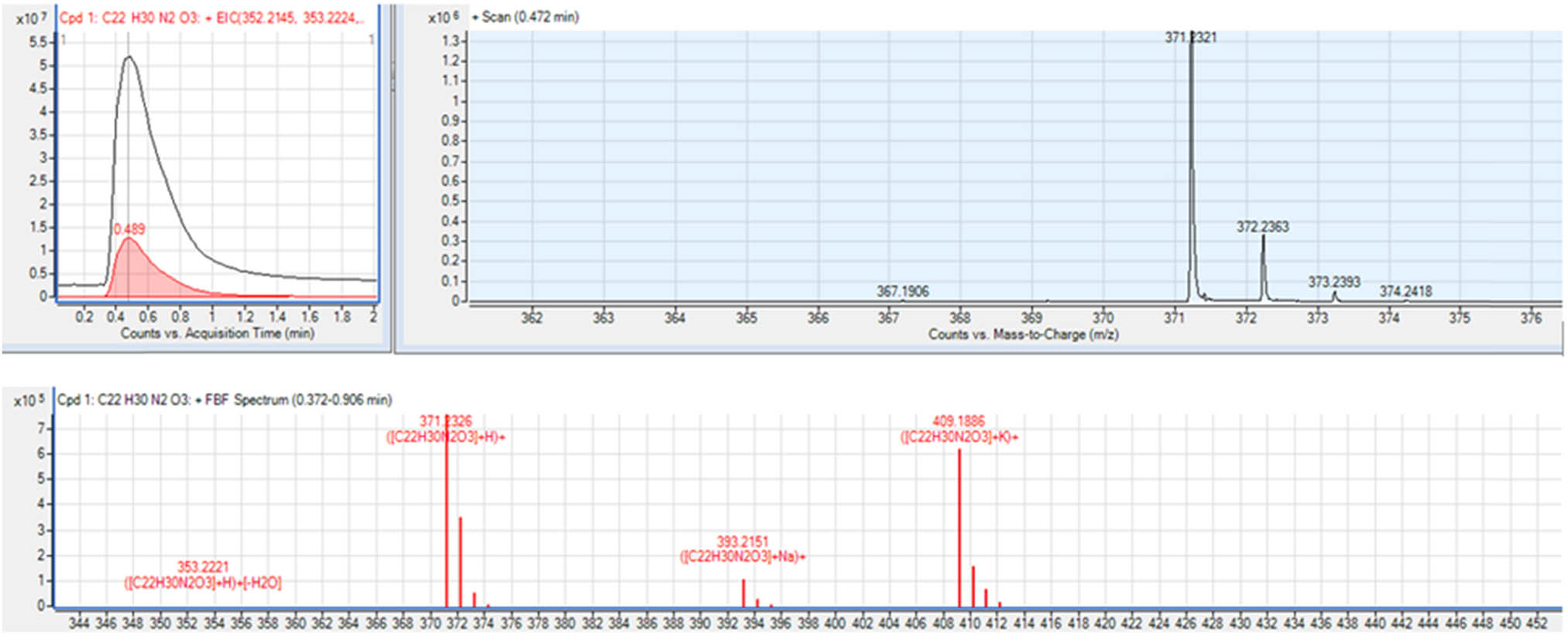
LC-QTOF/MS mass spectrum.
Slovenian Police reports [34] contain recorded mass spectrum using GC-MS of MMB-CHMICA that corresponds to the mass spectrum that we obtained with the molecular ion m/z 370.3 and fragments m/z 240.1, 256.2, 144.0, and 55.0 and their corresponding ratios. Based on our data from GC-MS and characteristic fragmentation (Figure 3), we draw the conclusion that we have the SC MMB-CHMICA (AMB-CHMICA or methyl N-[1-(cyclohexylmethyl)-1H-indole-3-carbonyl]-l-valinate). Previously also Apirakkan et al. [7] briefly reported the fragmentation pattern of MMB-CHMICA, but we provide here the complete fragmentation pattern report. Structures of fragment ions at m/z 328.20, 256.20, 240.15, 212.15, 144.10, 116.10, and 55.05 were found. Fragment ion at m/z 256.20 was obtained by McLafferty rearrangement from the structure with m/z 370.25 (Figure 3).
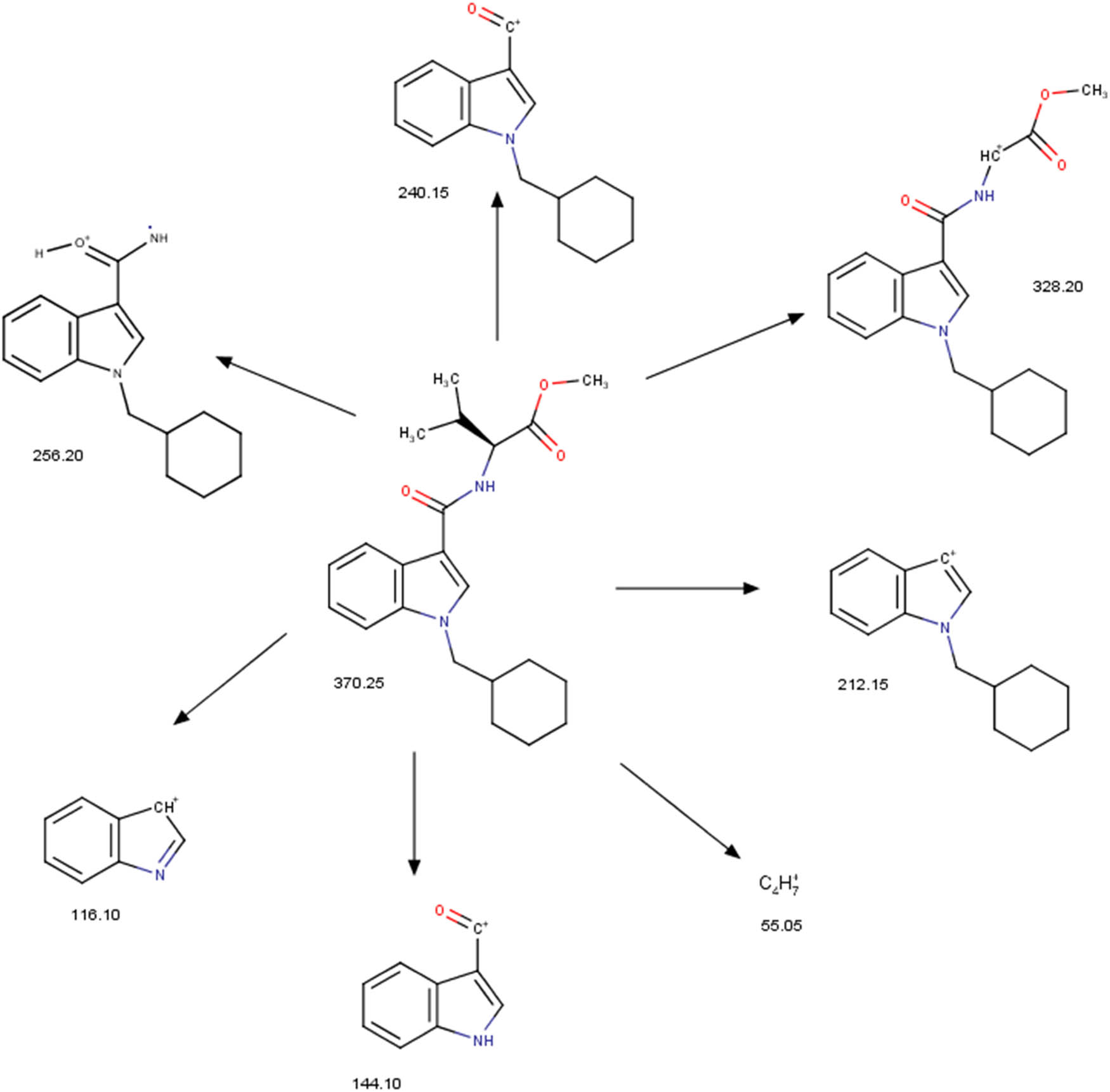
Fragmentation pattern of MMB-CHMICA.
3.3 Isolation of pure MMB-CHMICA and its NMR characterization
The extraction process after the purification yielded 7 mg of white crystalline product. The NMR spectra of the isolated compound provided the definitive evidence of its structure. 1H and 13C NMR chemical shifts of MMB-CHMICA were previously reported [35], but there are some improvements in our study (Table 3 and Figure 4).
Full 1H and 13C assignments of MMB-CHMICA in CDCl3
| Position | Multiplicity | 1H (ppm) | 3 J HH (Hz) | 13C (ppm) | HMBC connectivities (13C → 1H) |
|---|---|---|---|---|---|
| 1 | s | 7.71 | — | 132.54 | C-1 → H-9 |
| 2 | — | — | — | 125.24 | C-2 → H-1 |
| 3 | — | — | — | 110.37 | C-3 → H-1 |
| 4 | — | — | — | 136.99 | C-4 → H-1, C-4 → H-9 |
| 5 | m | 7.39 | — | 110.62 | — |
| 6 | m | 7.26–7.29 | — | 121.51 | C-6 → H-8, C-6 → H-5 |
| 7 | m | 7.26–7.29 | — | 122.37 | C-7 → H-8 |
| 8 | m | 7.99 | — | 120.00 | C-8 → H-7 |
| 9 | d | 3.96 | 7.2 | 53.40 | C-9 → H-1 |
| 10 | m | 1.86 | — | 38.55 | C-10 → H-9 |
| 11 | m | 0.96–1.06 | — | 31.01 | C-11 → H-9 |
| 12 | m | 1.21 | — | 25.64 | — |
| 13 | m | 1.59–1.73 | — | 26.19 | — |
| 14 | m | 1.59–1.73 | — | 25.64 | — |
| 15 | m | 1.59–1.73 | — | 31.01 | — |
| 16 | — | — | — | 164.94 | — |
| 17 | d | 6.48 | 8.4 | — | — |
| 18 | dd | 4.88 | 8.8, 4.8 | 56.90 | C-18 → H-20, C-18 → H-21 |
| 19 | m | 2.31 | — | 31.75 | C-19 → H-20, C-19 → H-21 |
| 20 | m | 0.96–1.06 | — | 18.11/19.11 | C-20 → H-21 |
| 21 | m | 0.96–1.06 | — | 18.11/19.11 | C-21 → H-20 |
| 22 | — | — | — | 173.17 | C-22 → H-23 |
| 23 | s | 3.79 | — | 52.16 | — |
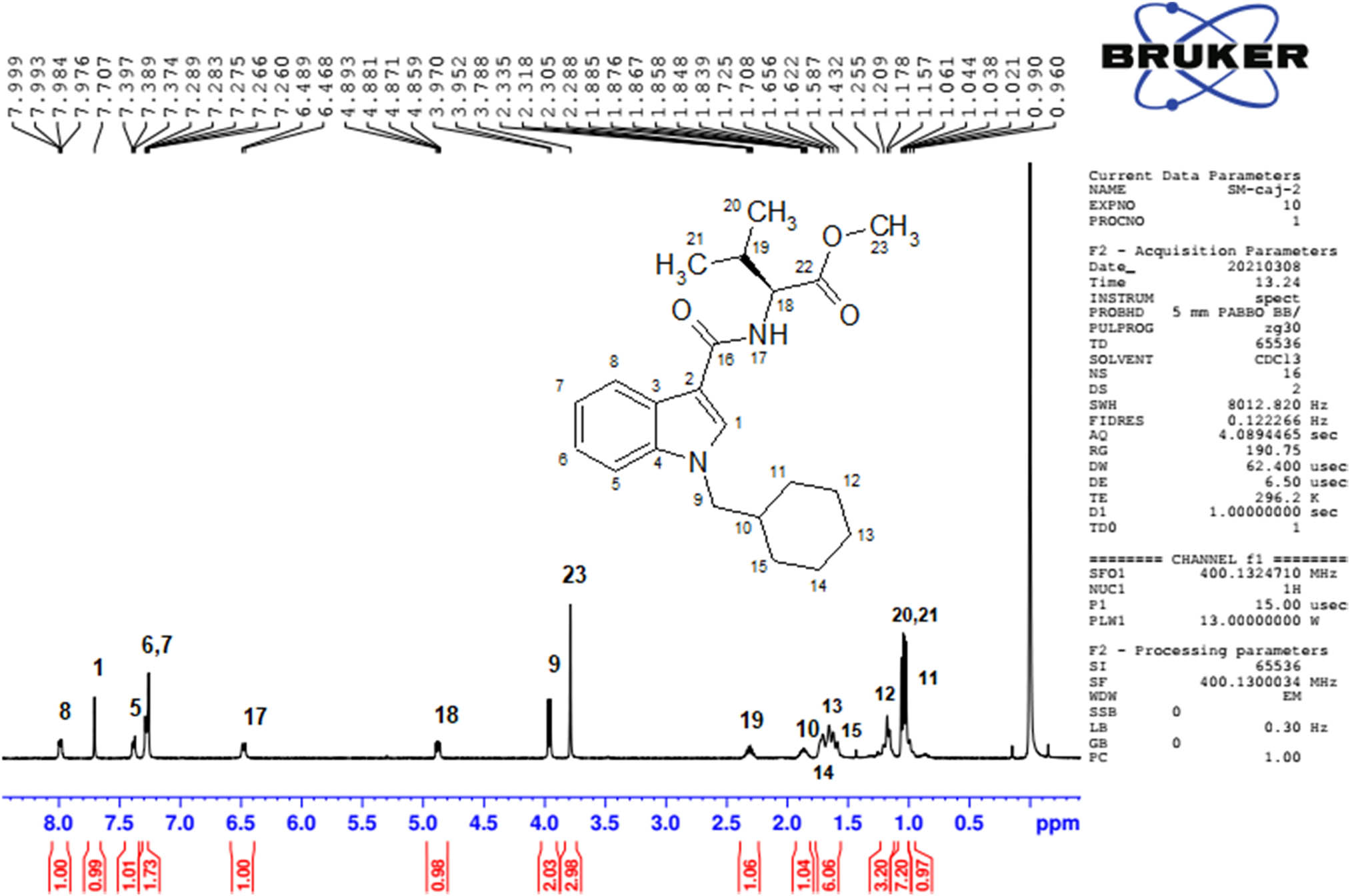
1H NMR spectrum of purified MMB-CHMICA.
1D and 2D NMR assigned spectra (13C, distortionless enhancement by polarization transfer 135 (DEPT 135), 2D correlated spectroscopy (2D COSY), 2D TOCSY, 2D HMBC (heteronuclear multiple bond connectivity), 2D heteronuclear single quantum correlation (2D HSQC)) are available in the Supplementary Material (Figures S1–S6).
3.4 Conformational search
Global minimum of MMB-CHMICA was repeated five times in 10,000 structures. It has the energy of −102.27 kJ mol−1. The structure shows chair conformation of cyclohexane ring, and two rings with unsaturated bonds were in the plane (Figure 5).
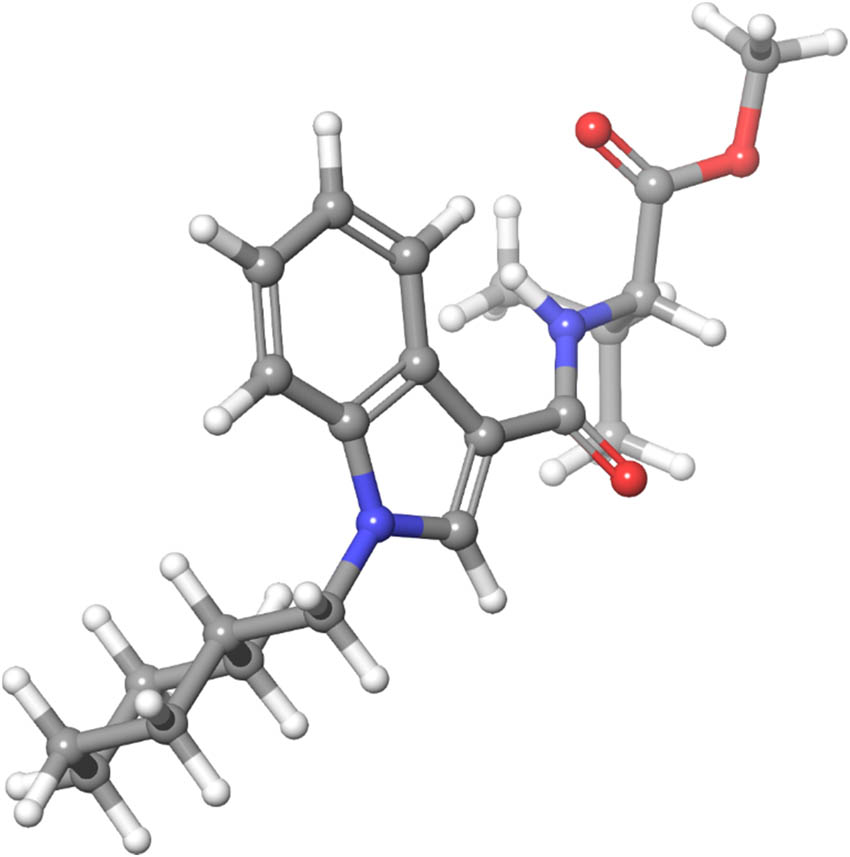
Structure of the global minimum of MMB-CHMICA obtained in Macromodel.
3.5 Liquid chromatography-tandem MS – MRM optimization
Then, the method was developed for the aimed analysis using LC-MS/MS. First, it was obtained Q3 scan to confirm m/z 371. In mode Product Scan, product ions were recorded for m/z 371. The obtained mass spectrum is shown in Figure 6.
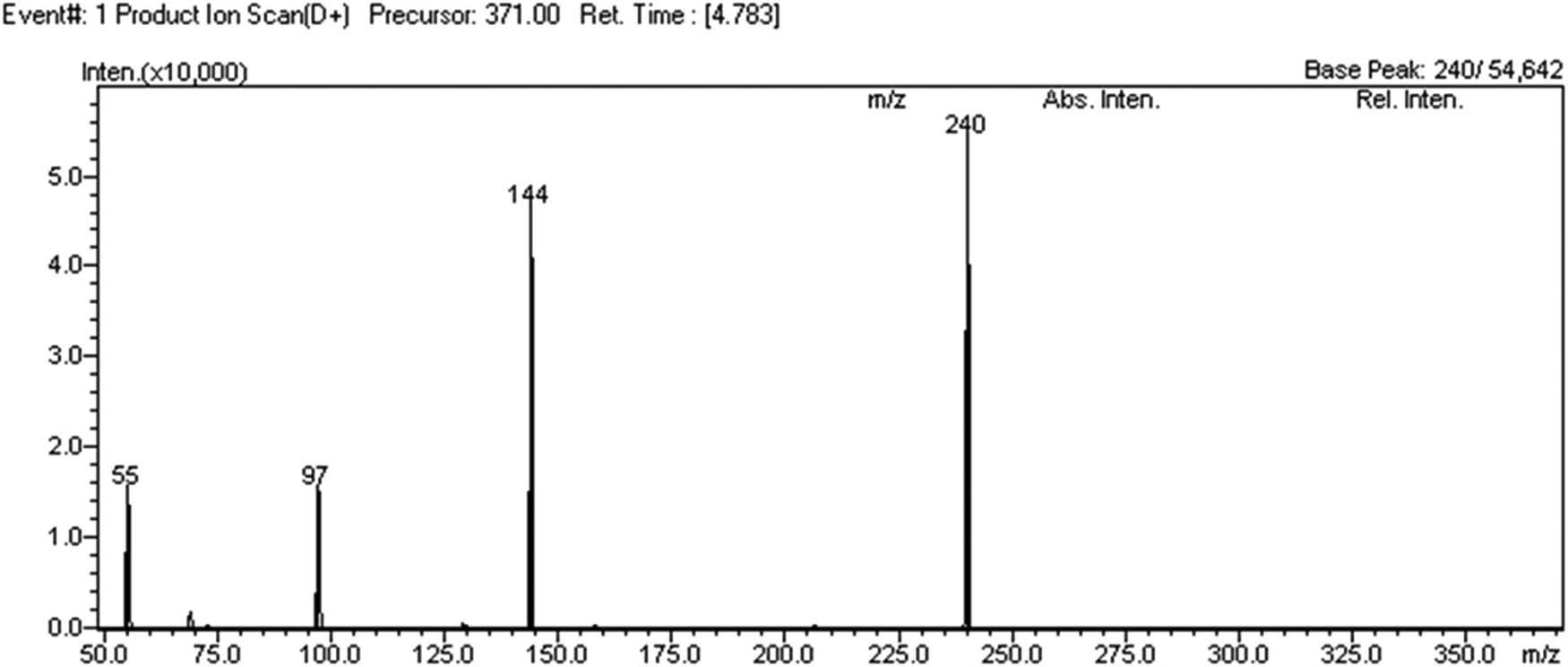
ESI LC-MS/MS, product ion scan mass spectrum.
Due to the unavailability of analytical standard for this compound, at the beginning the possibility of the LabSolutions LCMS control software for automatic selection of MRM transitions and CE adjustment was not used. Two the most intensive m/z product ions were chosen for MRM transitions and used default value CE equals to 35 eV. Chosen MRM transitions were m/z 371 > 240 and m/z 371 > 144. The analyte answer was also compared using two different mobile phases MeOH/FA 0.1% and ACN/FA 0.1%. Better answer was gotten with the combination MeOH/FA 0.1% (Condition 3).
MeOH extract from plant material that contained MMB-CHMICA was used as standard for the automated MRM parameter optimization.
Figure 7 shows the process of Automated MRM parameter optimization that took place during six injections in the methods that are part of the LabSolutions software.
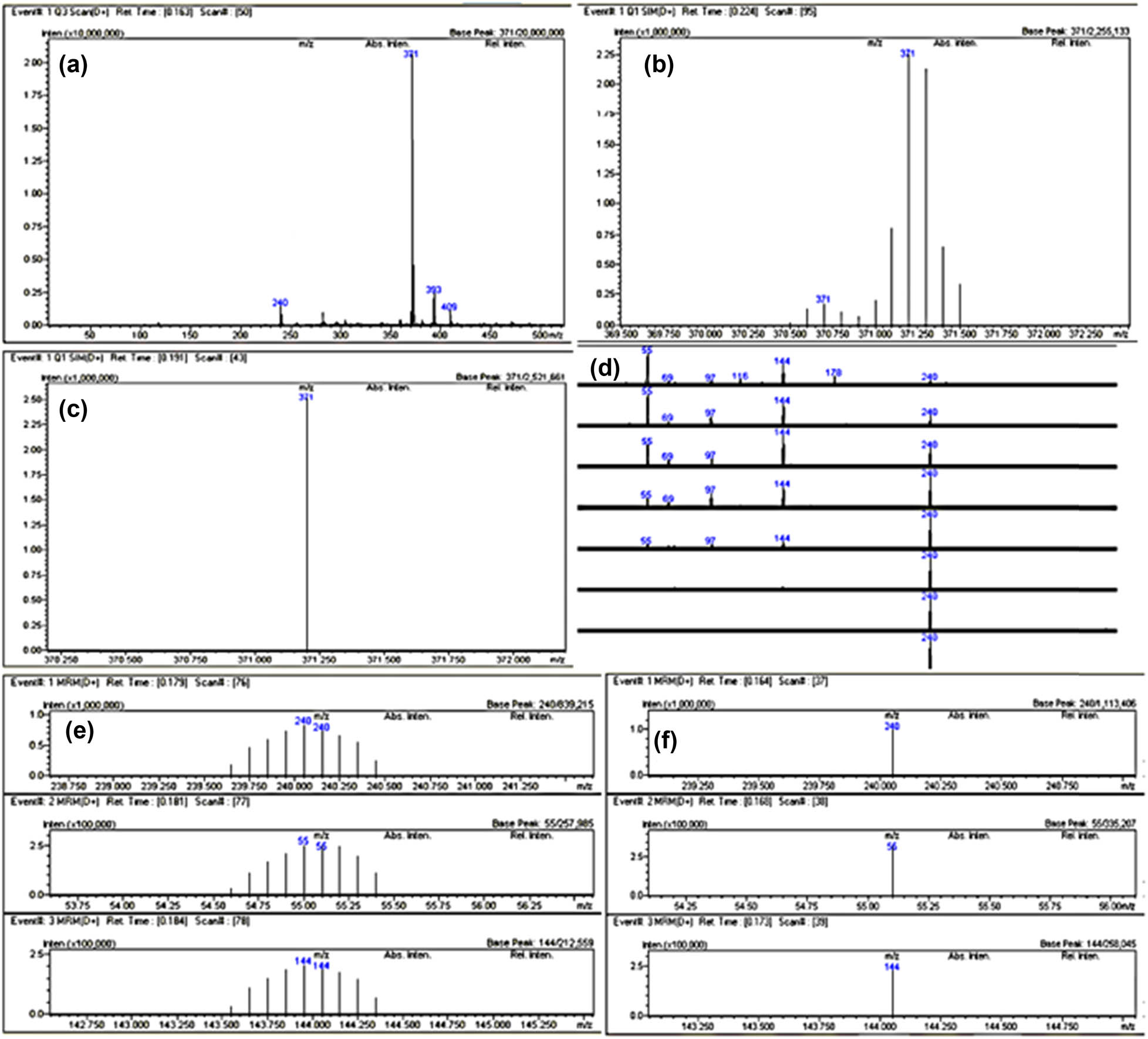
ESI LC-MS/MS, Method optimization: (a) precursor ion m/z search, (b) precursor ion m/z adjustment, (c) CE adjustment, (d) product ion m/z selection, (e) production m/z adjustment, and (f) product ion m/z of top three channels adjustment.
Figure 8 has shown optimized parameters for the new automatically generated MRM method and obtained relative intensity of MRM transitions.

MRM instrument parameters and relative intensity obtained after optimization.
The instrument’s analytical response before optimization (BO) and after optimization (AO) was compared using MeOH/0.1% FA (Condition 3) as mobile phase for the two transitions we initially selected (graph on Figure 9).
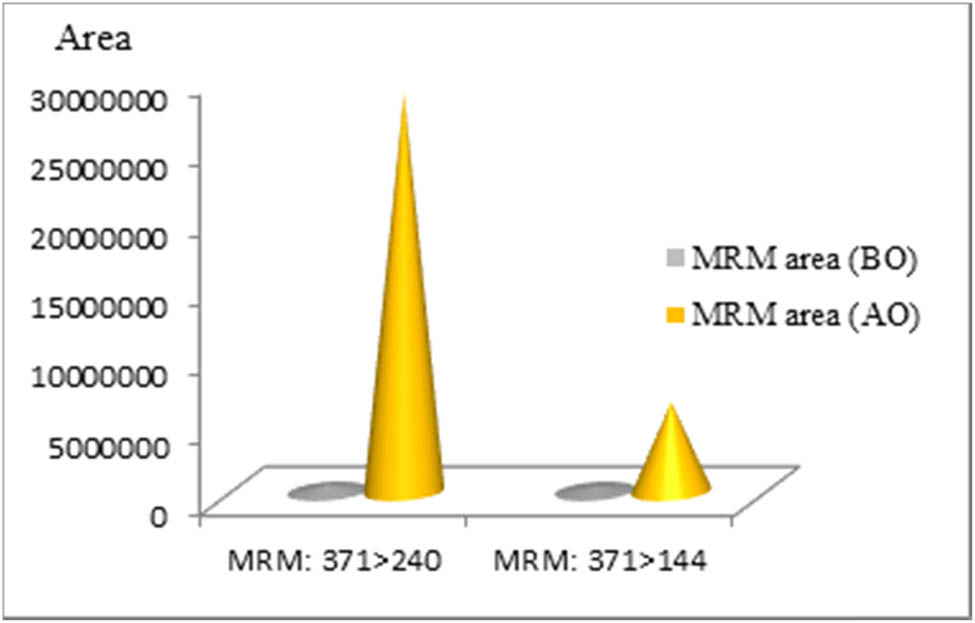
Area under chromatographic peak for MMB-CHMICA BO and AO for MRM m/z 371 > 240 and m/z 371 > 144 using MeOH/0.1% FA as mobile phase.
Figure 10 has shown obtained MRM chromatogram with chosen MeOH/0.1% FA as a mobile phase (Condition 3).
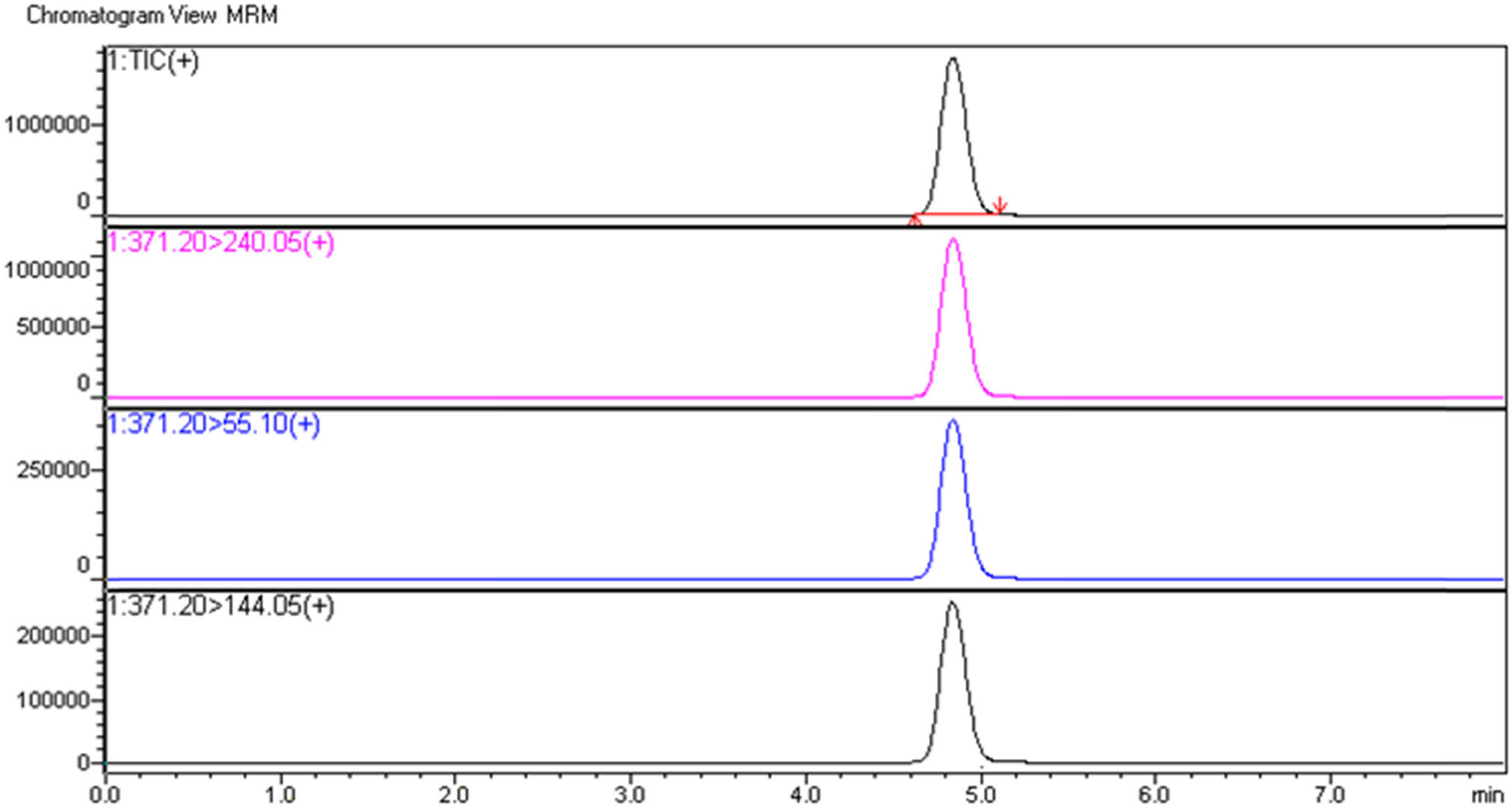
MMB-CHMICA LC-MS/MS MRM chromatogram.
MRM transitions obtained by optimization were used for further targeted analyses of MMB-CHMICA for estimating extraction yields from plant material.
3.6 Extraction of MMB-CHMICA from the plant material
The influence of some extraction variables can sometimes be more significant than others. The use of an insignificant variable in the extraction process may give incorrect or inappropriate results [36]. Thus, factor screening is inevitable for the extraction process. The identification of the extraction parameters will be detected for the extraction to be effective giving higher yields of MMB-CHMICA from the plant material. The contribution effect of MeOH concentration, extraction time, and extraction temperature on the extraction yields was examined. The experimental data of the extraction yield were analyzed with a linear first-order regression model [37]. The values of all regression coefficients are given in Table 4. The positive signs of the coefficients indicate a synergistic effect, whereas the negative sign (b 123) indicates an antagonistic effect.
Regression coefficients of the predicted linear first-order model
| Regression coefficient | 10−6 |
|---|---|
|
|
12.210679 |
|
|
0.773881 |
|
|
0.283768 |
|
|
0.103375 |
|
|
0.100355 |
|
|
0.184059 |
|
|
0.001979 |
|
|
−0.053925 |
The ANOVA for the response variables is presented in Table 5.
Results of the ANOVA (F(critical) = 5.32)
| SS (Sum of squares) | Df (Degrees of freedom) | MS (Mean squares) | F-value | p-value | |
|---|---|---|---|---|---|
| x 1 | 4.791142 | 1 | 4.791142 | 1493.0 | <0.00001 |
| x 2 | 0.644197 | 1 | 0.644197 | 200.74 | <0.00001 |
| x 3 | 0.085491 | 1 | 0.085491 | 26.641 | 0.000862 |
| x 1 x 2 | 0.080596 | 1 | 0.080596 | 25.107 | 0.001039 |
| x 1 x 3 | 0.271023 | 1 | 0.271023 | 84.457 | 0.000016 |
| x 2 x 3 | 0.000031 | 1 | 0.000031 | 0.0096 | 0.924359 |
| x 1 x 2 x 3 | 0.023264 | 1 | 0.023264 | 7.2496 | 0.02739 |
| Error | 0.025671 | 8 | 0.003209 | ||
| Total | 5.921388 | 15 | 0.394759 | ||
| R 2 (%) | 99.56 | ||||
|
|
99.19 | ||||
| Coefficient of variation (%) | 0.46 |
F critical = 5.32.
The statistical significance of all three factors and their possible two-way and three-way interaction for the extraction yields were evaluated based on their F- and p-values. The statistical significance of a factor is greater if its F-value is higher. The value of p < 0.05 indicates the significance of the factors and their interaction. It was clear that the linear and interaction terms were highly significant (p < 0.05; except only for b 23, p = 0.924359). To simplify, the linear regression model, two-way interaction (x 2 x 3), which was assessed to be statistically insignificant with the significance level p < 0.05, was omitted.
The full regression equation is as follows:
The predicted values of the corresponding responses are also presented in Table 2.
The most important factor was MeOH concentration (x 1), which was followed by the extraction time (x 2). On the two-way interaction, it is worth to mention how the combination of the MeOH concentration and extraction temperature (x 1 x 2) affected the extraction yield.
It is also necessary to mention that the developed regression model provides an adequate approximation in the real system. The regression analysis and ANOVA were used for fitting the model. The coefficient of determination (R
2), the adjusted
4 Conclusion
After the identification of the drug through the special Europol (the EU Police agency), European Monitoring Centre for Drugs and Drug Addiction Reporting form for a new psychoactive drug in the EWS is attached to Ministry of Health, after which this substance was placed on the List of Psychoactive Controlled Substances in the Republic of Serbia. Presented approaches by different methods (GC-MS, LC-QTOF/MS, and LC-MS/MS) and confirmation of structure by NMR spectroscopy and conformational search were effective for the identification of NPS MMB-CHMICA. Here, the attention was drawn to the necessity of an orthogonal chromatographic approach and the importance of respecting the criteria for identification during qualitative analysis in forensic chemistry and toxicology.
-
Funding information: This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract numbers 451-03-68/2020-14/200124 and 451-03-9/2021-14/200123).
-
Author contributions: Methodology, investigation, and writing-original draft preparation, VL; methodology, supervision, validation, writing-original draft preparation, and writing-reviewing and editing, RM; methodology, investigation, writing-original draft preparation, and writing-reviewing and editing, BA; methodology, investigation, and writing-original draft preparation, MM; methodology, investigation, and writing-original draft preparation, MJ; supervision and writing-reviewing and editing, AP.
-
Conflict of interest: Authors declare no conflict of interest.
-
Ethical approval: The conducted research is not related to either human or animal use.
-
Data availability statement: Data are available in the manuscript or in local computers and can be obtained from the authors upon the request.
-
Supplemental material: Supplementary data to this article can be found online.
References
[1] United Nations, Global Synthetic Drugs Assessment 2020a. https://www.unodc.org/unodc/en/scientists/2020-global-synthetic-drugs-assessment-main.html (Accessed 25 February 2021).Search in Google Scholar
[2] European Monitoring Centre for Drugs and Drug Addiction, New psychoactive substances: global markets, global threats and the COVID-19 pandemic, An update from the EU Early Warning System. https://www.emcdda.europa.eu/system/files/publications/13464/20205648_TD0320796ENN_PDF_rev.pdf (accessed 25 February 2021).Search in Google Scholar
[3] Favreto D, Pascali JP, Tagliaro F. New challenges and innovation in forensic toxicology: focus on the “New Psychoactive Substances”. J Chromatogr A. 2013;1287:84–95.10.1016/j.chroma.2012.12.049Search in Google Scholar PubMed
[4] Zamengo L, Frison G, Bettin C, Sciarrone R. Understanding the risks associated with the use of new psychoactive substances (NPS): high variability of active ingredients concentration, mislabelled preparations, multiple psychoactive substances in single products. Toxicol Lett. 2014;229:220–8.10.1016/j.toxlet.2014.06.012Search in Google Scholar PubMed
[5] Elliott S. Investigating drugs of abuse at autopsy. Diagn Histopathol. 2018;24:341–5.10.1016/j.mpdhp.2018.08.001Search in Google Scholar
[6] Elliott S, Sedefov R, Evans-Brown M. Assessing toxicological significance of new psychoactive substances in fatalities. Drug Test Anal. 2018;10:120–6.10.1002/dta.2225Search in Google Scholar PubMed
[7] Apirakkan O, Frinculescu A, Denton H, Shine T, Cowan D, Abbate V, et al. Isolation, detection and identification of synthetic cannabinoids in alternative formulations or dosage forms. Forensic Chem. 2020;18:100227.10.1016/j.forc.2020.100227Search in Google Scholar
[8] Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci. 2012;57:1168–80.10.1111/j.1556-4029.2012.02207.xSearch in Google Scholar PubMed
[9] Yano E, Riisom M, Tong KKH, Hanif M, Leung E, Hartinger CG. Tracing the anticancer compound [RuII(η6-p-cymene)(8-oxyquinolinato)Cl] in a biological environment by mass spectrometric methods. Anal Methods. 2021;12:1463–9.10.1039/D0AY02311FSearch in Google Scholar
[10] Kind T, Fiehn O. Advances in structure elucidation of small molecules using mass spectrometry. Bioanal Rev. 2010;2:23–60.10.1007/978-3-642-36303-0_7Search in Google Scholar
[11] Milman BL. General principles of identification by mass spectrometry. Trends Anal Chem. 2015;69:24–33.10.1016/j.trac.2014.12.009Search in Google Scholar
[12] Little JL, Cleven CD, Brown SD. Identification of “known unknowns” utilizing accurate mass data and Chemical Abstracts service databases. J Am Soc Mass Spectrom. 2011;22:348–59.10.1007/s13361-010-0034-3Search in Google Scholar PubMed
[13] United Nations, Recommended methods for the Identification and Analysis of Synthetic Cannabinoid Receptor Agonists in Seized Materials (Revised and updated) 2020b. https://www.unodc.org/documents/scientific/STNAR48_Rev.1_ebook.pdf (accessed 25 February 2021).Search in Google Scholar
[14] Borden SA, Palaty J, Termopoli V, Famiglini G, Cappiello A, Gill CG, et al. Mass spectrometry analysis of drugs of abuse: challenges and emerging strategies. Mass Spectrom Rev. 2020;39:703–44.10.1002/mas.21624Search in Google Scholar PubMed
[15] Liao W, Draper WM, Perera SK. Identification of unknowns in atmospheric pressure ionization mass spectrometry using a mass to structure search engine. Anal Chem. 2008;80:7765–77.10.1021/ac801166zSearch in Google Scholar PubMed
[16] Pasin D, Cawley A, Bidny S, Fu S. Current applications of high-resolution mass spectrometry for the analysis of new psychoactive substances: a critical review. Anal Bioanal Chem. 2017;409:5821–36.10.1007/s00216-017-0441-4Search in Google Scholar PubMed
[17] Maralikova B, Weinmann W. Confirmatory analysis for drugs of abuse in plasma and urine by high-performance liquid chromatography-tandem mass spectrometry with respect to criteria for compound identification. J Chromatogr B. 2004;811:21–30.10.1016/j.jchromb.2004.04.039Search in Google Scholar PubMed
[18] National Institute of Standards and Technology, Standard for identification criteria in forensic toxicology (draft). chsac_-_tox_-_identification_in_forensic_toxicology_-_for_asb_and_website_1.pdf (nist.gov) (accessed 25 February 2021).Search in Google Scholar
[19] Kneisel S, Westphal F, Bisel P, Brecht V, Broecker S, Auwärter V. Identification and structural characterization of the synthetic cannabinoid 3-(1-adamantoyl)-1-pentylindole as an additive in “herbal incense”. J Mass Spectrom. 2012;47:195–200.10.1002/jms.2059Search in Google Scholar PubMed
[20] Ibanez M, Sancho JV, Bijlsma L, van Nuijs ALN, Covaci A, Hernandez F. Comprehensive analytical strategies based on high-resolution time-of-flight mass spectrometry to identify new psychoactive substances. Trends Anal Chem. 2014;57:107–17.10.1016/j.trac.2014.02.009Search in Google Scholar
[21] Uchiyama N, Kikura-Hanajiri R, Ogata J, Goda Y. Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci Int. 2010;198:31–8.10.1016/j.forsciint.2010.01.004Search in Google Scholar PubMed
[22] Dunham SJB, Hooker PD, Hyde RM. Identification, extraction and quantification of the synthetic cannabinoid JWH-018 from commercially available herbal marijuana alternatives. Forensic Sci Int. 2012;223:241–4.10.1016/j.forsciint.2012.09.010Search in Google Scholar PubMed
[23] Grabenauer M, Krol WL, Wiley JL, Thomas BF. Analysis of synthetic cannabinoids using high-resolution mass spectrometry and mass defect filtering: implications for nontargeted screening of designer drugs. Anal Chem. 2012;84:5574–81.10.1021/ac300509hSearch in Google Scholar PubMed PubMed Central
[24] Thaxton A, Belal TS, Smith F, DeRuiter J, Abdel-Hay KM, Clark CR. GC-MS studies on the six naphthoyl-substituted 1-n-pentyl-indoles: JWH-018 and five regioisomeric equivalents. Forensic Sci Int. 2015;252:107–13.10.1016/j.forsciint.2015.04.023Search in Google Scholar PubMed
[25] Adamowicz P. Fatal intoxication with synthetic cannabinoid MDMB-CHMICA. Forensic Sci Int. 2016;261:e5–10.10.1016/j.forsciint.2016.02.024Search in Google Scholar PubMed
[26] Langer N, Lindigkeit R, Schiebel H-M, Papke U, Ernst L, Beuerle T. Identification and quantification of synthetic cannabinoids in “spice-like” herbal mixtures: update of the German situation for the spring of 2015. Forensic Toxicol. 2016;34:94–107.10.1007/s11419-015-0292-7Search in Google Scholar
[27] Borg D, Tverdovsky A, Stripp R. A fast and comprehensive analysis of 32 synthetic cannabinoids using Agilent triple quadrupole LC-MS-MS. J Anal Toxicol. 2017;41:6–16.10.1093/jat/bkw104Search in Google Scholar PubMed
[28] Lee JH, Park HN, Leem T-S, Jeon J-h, Cho S, Lee J, et al. Identification of new synthetic cannabinoid analogue APINAC (adamantan-1-yl 1-pentyl-1H-indazole-3-carboxylate) with other synthetic cannabinoid MDMB (N)-Bz-F in illegal products. Forensic Toxicol. 2017;35:45–55.10.1007/s11419-016-0331-zSearch in Google Scholar
[29] Olmez NA, Kapucu H, Altun NC, Eren B. Identification of the synthetic cannabinoid N-(-2-phenyl-propan-2-yl)-1-(4-cyanobutyl)-1H-indazole-3-carboxamide (CUMYL-4CN-BINACA) in a herbal mixture product. Forensic Toxicol. 2018;36:192–9.10.1007/s11419-017-0372-ySearch in Google Scholar
[30] Ambroziak K, Adamowicz P. Simple screening procedure for 72 synthetic cannabinoids in whole blood by liquid chromatography-tandem mass spectrometry. Forensic Toxicol. 2018;36:280–90.10.1007/s11419-017-0401-xSearch in Google Scholar PubMed PubMed Central
[31] Sorribes-Soriano A, Verdeguer J, Pastor A, Armenta S, Esteve-Turrillas FA. Determination of third-generation synthetic cannabinoids in oral fluids. J Anal Toxicol. 2021;45(4):331–6.10.1093/jat/bkaa091Search in Google Scholar PubMed
[32] Mardal M, Weihe Dalsgaard P, Qi B, Brinch Mollerup C, Annaert P, Linnet K. Metabolism of the synthetic cannabinoids AMB-CHMICA and 5C-AKB48 in pooled human hepatocytes and rat hepatocytes analyzed by UHPLC-(IMS)-HR-MSE. J Chromatogr B. 2018;1083:189–97.10.1016/j.jchromb.2018.03.016Search in Google Scholar PubMed
[33] Antonides LH, Cannaert A, Norman C, Vives L, Harrison A, Costello A, et al. Enantiospecific synthesis, chiral separation, and biological activity of four indazole-3-carboxamide-type synthetic cannabinoid receptor agonists and their detection in seized drug samples. Front Chem. 2019;7:321.10.3389/fchem.2019.00321Search in Google Scholar PubMed PubMed Central
[34] NPS and related compounds-analytical reports. https://www.policija.si/apps/nfl_response_web/seznam.php (accessed 30 March 2021).Search in Google Scholar
[35] Banister SN, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, et al. The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse. ACS Chem Neurosci. 2016;7:1241–54.10.1021/cn400035rSearch in Google Scholar PubMed PubMed Central
[36] Alara OR, Abdurahman NH, Olalere OA. Ethanolic extraction of flavonoids, phenolics and antioxidants from Vernonia amygdalina leaf using two-level factorial design. J King Saud Univ Sci. 2020;32:7–16.10.1016/j.jksus.2017.08.001Search in Google Scholar
[37] Mitić M, Tošić S, Pavlović A, Mašković P, Kostić D, Mitić J, et al. Optimization of the extraction process of minerals from Salvia officinalis L. using factorial design methodology. Microchem J. 2019;145:1224–30.10.1016/j.microc.2018.12.047Search in Google Scholar
© 2021 Vera Lukic et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


