Abstract
High levels of urea and creatinine in the blood are a sign of decreased kidney function. To remove these substances from the blood, hemodialysis which utilizes membranes could be used. In this study, a molecularly imprinted membrane (MIM) was synthesized for the selective transport of urea. The synthesis is initiated with the polymerization of eugenol into polyeugenol and then into polyeugenoxy acetate (PA). The PA is then contacted with urea and then used as the functional polymer in the synthesis of MIM with polysulfone as the membrane base, and polyethylene glycol as the cross-linking agent. The result was later analyzed with FTIR and SEM-EDX. The membrane is then used in the transport of urea, creatinine, and vitamin B12 and then compared with the non-imprinted membrane (NIM) performance. By using UV-Vis spectrophotometry, the results showed that the membrane with 10 h heating variation is able to transport more urea and is more selective than NIM; this proves that the urea template on the MIM enables it to recognize urea molecules better than creatinine and vitamin B12. The order of transport from the best results is urea > creatinine > vitamin B12.
1 Introduction
Chronic kidney disease is becoming more common in every part of the world with an estimated prevalence of 5–15% throughout the world [1]. Chronic kidney failure is a progressive and irreversible disorder in the kidney function in which the body’s ability to maintain fluid and electrolyte balance and metabolic waste excretion is lost, ultimately leading to uremia (retention of urea and other nitrogenous wastes in the blood) [2]. Renal disease or chronic renal failure is a progressive renal damage which is fatal and characterized by uremia and may lead to complications or even to a kidney transplant [3] if hemodialysis is not carried out.
Hemodialysis utilizes a semipermeable membrane to remove unwanted nitrogenous wastes from the blood. Molecular transport process using a membrane is not only determined by the membrane’s pore size but also by the presence of reactive groups on the membrane that activate the interaction between the membrane and the target compound to occur. Furthermore, the membrane used for hemodialysis needs to have high permeability that means it needs to be able to transport metabolite as fast as possible [4].
In this research, we utilize molecularly imprinted membrane (MIM) to transport urea and compare its ability with creatinine and vitamin B12 transport. MIM is a membrane that has molecular recognition abilities due to its template that is achieved through cross-linking the functional polymer and the target molecule. The target molecule is later removed from the membrane, forming a cavity with correct shape and orientation of functional groups that serves as the template binding area for selective recognition [5]. Molecular imprinting material has some advantages such as high stability and specificity [6]. Imprinted material has greater flexibility in selecting the appropriate functionalities due to the availability of a large amount of polymerizable compounds [7]. Djunaidi et al. synthesized a molecularly imprinted polymer using polyeugenol as the functional polymer to achieve a urea-selective adsorbent [8]. Other previous studies that utilized imprinting method used poly(methyl methacrylate) as the functional polymer to synthesize a non-enzymatic urea sensor, 3,4-ethylenedioxythiophene monomer, for the synthesis of electrochemical urea sensor, and methacrylic acid as the monomer for diisopropylurea recognition [9,10,11].
One of the potential compounds to be used as the functional polymer in MIM is eugenol-based polymer. Previous research has showed the promising potential of eugenol derivatives such as polyeugenol, polyeugenol sulfonate, and polyeugenoxy acetyl thiophene methanolate for the separation of gold and other metals [12,13,14]. Eugenol is suitable for the synthesis of a new compound that is more reactive; this is due to the allyl, hydroxyl, and methoxy groups present in eugenol [15,16]. Through the allyl group, eugenol can be polymerized into polyeugenol, while through the hydroxyl group, a carboxylic compound of polyeugenoxy acetate (PA) can be synthesized. PA has an active group of –COOH which can be used as a carrier compound in selective transport [17]. PA will be used as the functional polymer in this research, combined with polysulfone and polyethylene glycol for the synthesis of MIM.
2 Materials and methods
2.1 Materials
Eugenol p.a. (Merck), chloroform p.a. (Merck), BF3O(C2H5)2 (Sigma Aldrich), diethyl ether p.a. (Merck), anhydrous Na2SO4 p.a. (Merck), Aqua demineralized, HCl (Merck), polysulfone M w 35.000 (Sigma Aldrich), methanol p.a. (Merck), 1-methyl-2-pyrolidone (NMP) 99.5% (Sigma Aldrich), NaOH p.a. (Merck), NaHCO3 (Merck), polyethylene glycol 6000 (Sigma Aldrich), Cl2CH2COOH p.a. (Merck), 2,2′,azobis(2-methylpropionitrile) (AIBN) (Sigma Aldrich), Na2HPO4 (Merck), NaH2PO4 (Merck), creatinine (Merck), ethanol p.a. (Merck), vitamin B12 (Merck), urea (Merck), picric acid (Merck), and 4-(Dimethyl amino benzaldehyde) (DAB) (Merck) were used in this study. The materials were used as received without further purification step.
2.2 Preparation of phosphate buffer solution (PBS)
PBS of pH 7.4 was made by mixing 29 mL of 0.01 M Na2HPO4 with 6.5 mL of 0.02 M NaH2PO4 in a 100 mL volumetric flask. Afterward, pH measurement was carried out using a pH meter to obtain a pH of 7.4.
2.3 Tools and instruments
Laboratory equipment glass (Herma dan Pyrex), reflux, analytic scale (Mettler-200), magnetic stirrer, hot plate, SEM-EDX (Jeol Jsm 6510 La), thickness meter (Digilife), pH meter (HACH E C20), UV-Vis spectrophotometry (LW-V-200-RS), and FT-IR (Shimadzu Prestige 21) were used in this study.
2.4 Synthesis of polyeugenol
Into a three-necked flask, 5.8 g of eugenol (0.035 mol) was added with BF3-diethyl ether catalyst as much as 0.25 mL each hour for the next 4 h while being stirred. The polymerization reaction was done for 12–16 h under room temperature and pressure and then it was stopped by adding 1 mL of methanol. It then turned into a gel form which was later dissolved with chloroform and washed with aquabidest until it reached a neutral pH. The solution was then added with anhydrous Na2SO4 and then let to evaporate at room temperature until it hardened. The precipitate formed was weighed and analyzed by FTIR.
2.5 Synthesis of PA
As much as 5 g of polyeugenol (0.03 mol) was added with 17.5 mL of 33% NaOH (0.144 mol) in a three-necked flask. The mixture was then stirred for 30 min and slowly added with 12.5 mL of 50% chloroacetic acid (0.066 mol) while continuously being stirred. With a reflux apparatus, the mixture is heated in a water bath at a temperature of 80–90°C for 2 h. Afterward, once the mixture had cooled down, it was acidified with 6 M of HCl until the mixture reached a pH of 1. It was then extracted with each of 50 mL of diethyl ether and 50 mL of sodium bicarbonate (5 wt%, 0.029 mol) three times. The result was acidified again in the same way, then filtered, and dried. The polymer was analyzed with FTIR.
2.6 PA contact with urea
PA was contacted with 1,000 ppm of urea to make a urea mold in the membrane with the weight ratio of PA to urea of 1:20. The contacting process is done through stirring. The PA was added with 1,000 ppm of urea solution and the mixture was stirred for 24 h in a tightly shut container; it was then filtered and dried.
2.7 Synthesis of MIM and non-imprinted membrane (NIM)
The MIM was synthesized by mixing 3.749 g of polysulfone (0.008 mol), 0.8333 g of PA-urea (0.005 mol), 0.8333 g of PEG (0.046 mol), and 0.249 mL of AIBN (0.00027 mol), diluted with 12 mL of NMP (0.00012 mol). Then their treatment was varied by only mixing without heat (MIM-0) and refluxed for 5 (MIM-5) and 10 h (MIM-10) at a temperature of 80–90°C. Afterward, once the mixture had cooled down and was free of air bubble, the membrane was casted on a glass surface and immediately submerged into aquabidest. NIM (NIM-0, NIM-5, and NIM-10) was also synthesized by the same method but using the PA that has not been contacted with urea and then later used for comparison.
2.8 Urea transport
The transport was done by placing the membrane in a diffusion cell with 50 mL of PBS as the receiving phase (RP) and 50 mL of 300 ppm urea diluted in PBS of pH 7.4 as the feed phase (FP). The diffusion cell setup is described in Figure 1. Sampling was done every 2 h for 8 h by taking 2 mL solution of each phase. Then the concentration of samples from both the phases was measured by UV-Vis spectrophotometry at the wavelength of 430 nm to measure the urea concentration by complexing the sample with DAB.
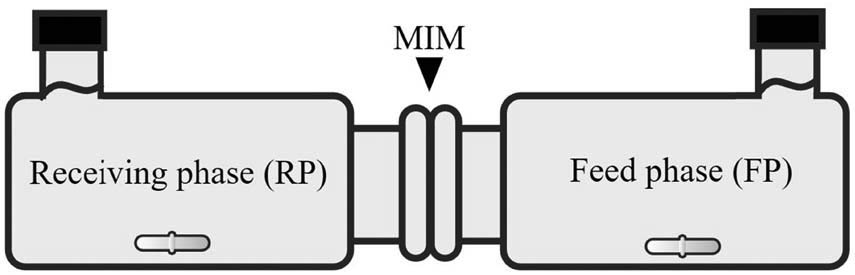
Illustration of the diffusion cell apparatus.
2.9 Determination of urea, creatinine, and vitamin B12 transport percentage
Using UV-Vis spectrophotometer, a standard reference of urea concentration ranging from 0–400 ppm in multiples of 50 ppm was measured. The resulting equation is as follows:
Then the concentration of each transported urea samples measured by the UV method was referred to the equation of the standard, and then the transport percentage of the FP and the RP was calculated as follows:
where n is the time of the samples taken. The same method is also used to calculate creatinine and vitamin B12 transport percentage. Creatinine concentration used for the standard reference is 0–30 ppm with multiples of 5 ppm with the resulting equation:
While the concentration used to make the vitamin B12 standard is 0–50 ppm in 5 ppm multiples with the resulting equation:
2.10 Membrane selectivity toward urea, creatinine, and vitamin B12
Transports of 100 ppm of urea, 25 ppm of creatinine, and 20 ppm of vitamin B12 were conducted, and the results were then compared to determine the selectivity. Sampling and measurement were done in the same way by taking 2 mL of solution from each phase every 2 h for 8 h. The creatinine was complexed with picric acid before measuring with UV-Vis. Creatinine was measured at the wavelength of 486 nm, while vitamin B12 was measured at the wavelength of 361 nm.
-
Ethical approval: The conducted research is not related to either human or animal abuse.
3 Results and discussions
3.1 Synthesis of polyeugenol and PA
The polyeugenol synthesis showed a yield of 5.67 g (97.89%). The polymerization consists of three stages. The first is the initiation stage where the eugenol will go through an addition reaction due to the protons (H+) from BF3-diethyl ether catalyst which follows Markovnikov’s law. Afterward is the propagation stage in which the covalent bonds between the cation chains and the eugenol monomers are formed. This process continues until a long polymer chain is obtained. Intermolecular rearrangement of the carbonium ion also occurs at this stage. The last stage is the termination stage, in which the cationic polymerization reaction between the monomer and the catalyst takes place until all the monomers are used up. Then methanol was added to stop the polymerization by binding the carbonium ion to its anion partner, the
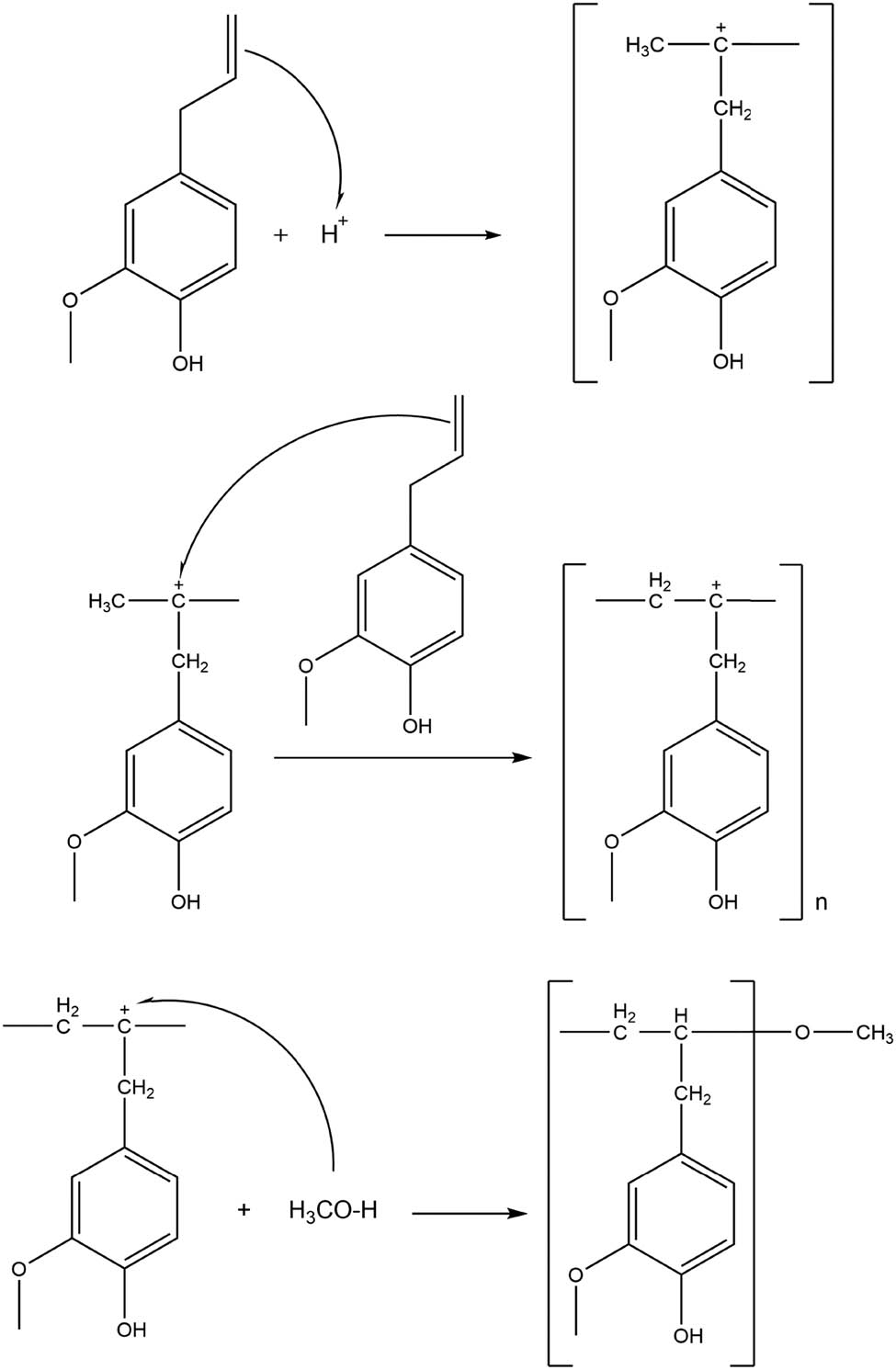
Reaction mechanism of eugenol polymerization.
The resulting polyeugenol was analyzed by FTIR and then compared with eugenol as shown in Figure 3. According to the data, an absorption band in the 995 and 913 cm−1 region which belongs to a vinyl group was found in eugenol but not in polyeugenol. This may indicate that polymerization has occurred.
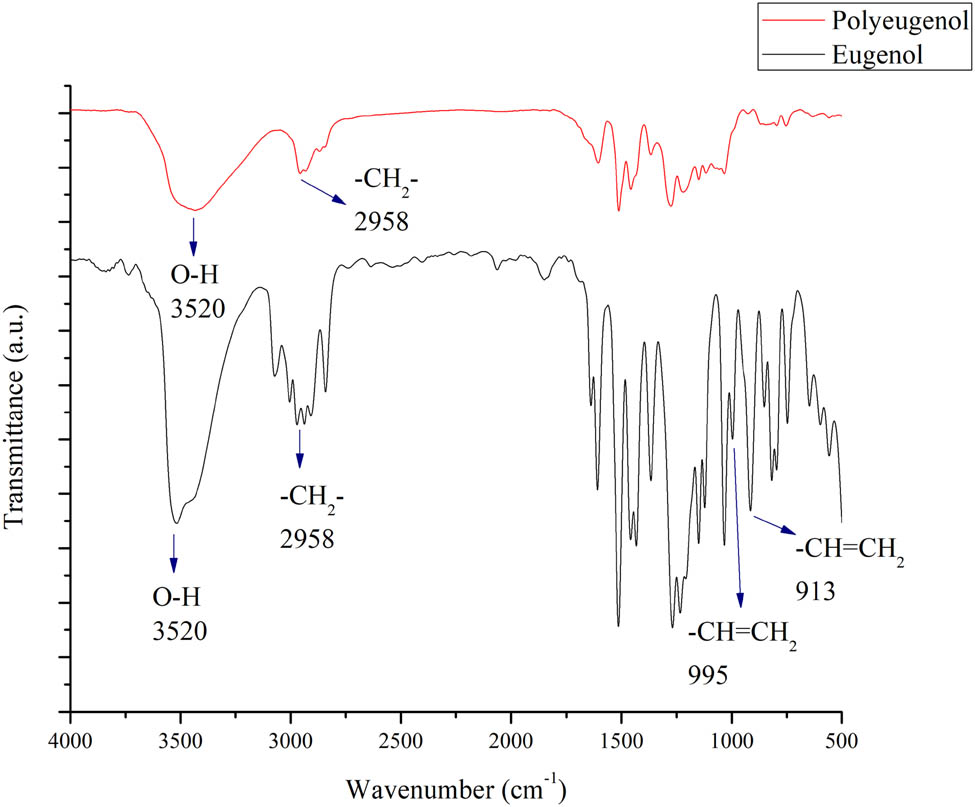
Comparison of FTIR result between eugenol and polyeugenol.
NMR characterization of eugenol and polyeugenol could also be used to prove the polymerization. In previous research, it was found that there was a chemical shift at 4.75–5.2 ppm that appeared as a doublet in the eugenol spectra which indicates the ═CH2 groups. While it was not seen in the polyeugenol spectra, there is a chemical shift at 2.4–3.0 ppm indicating the presence of –CH– groups in the polymer and the spectrum at 0.7–1.5 ppm indicating the three hydrogen atoms of methyl groups in the polymer backbone. This strengthens the indication that polymerization has happened [18].
The synthesis of polyeugenoxy acetic acid begins with the addition of NaOH and chloroacetic acid to the polyeugenol. Polyeugenol will react with NaOH to form polyeugenolate salt through its hydroxy group. The proton from the hydroxyl group (–OH) on the polyeugenol is easily released because the anion is stabilized by the presence of a benzene ring resonance. So, the addition of base in the form of excess NaOH is carried out so that a lot of salt is produced and the results are maximum. Na-polyeugenol salt is formed by the reaction of chloroacetic acid to form polyeugenoxy acetic acid.
Then the results obtained were purified by extraction using diethyl ether. The function of purification using diethyl ether is to remove impurities that are polar. After that, extraction is carried out using sodium carbonate which aims to remove impurities that are non-polar. Next the solution was added with 6 M HCl until pH 1 was obtained and the solution was decanted. The filtrate is then dried at room temperature and the results are then weighed. PA synthesis resulted in a 6.5 g (96.04%) yield with the degree of acetylation of 38.47%. The reaction of PA synthesis is shown in Figure 4.
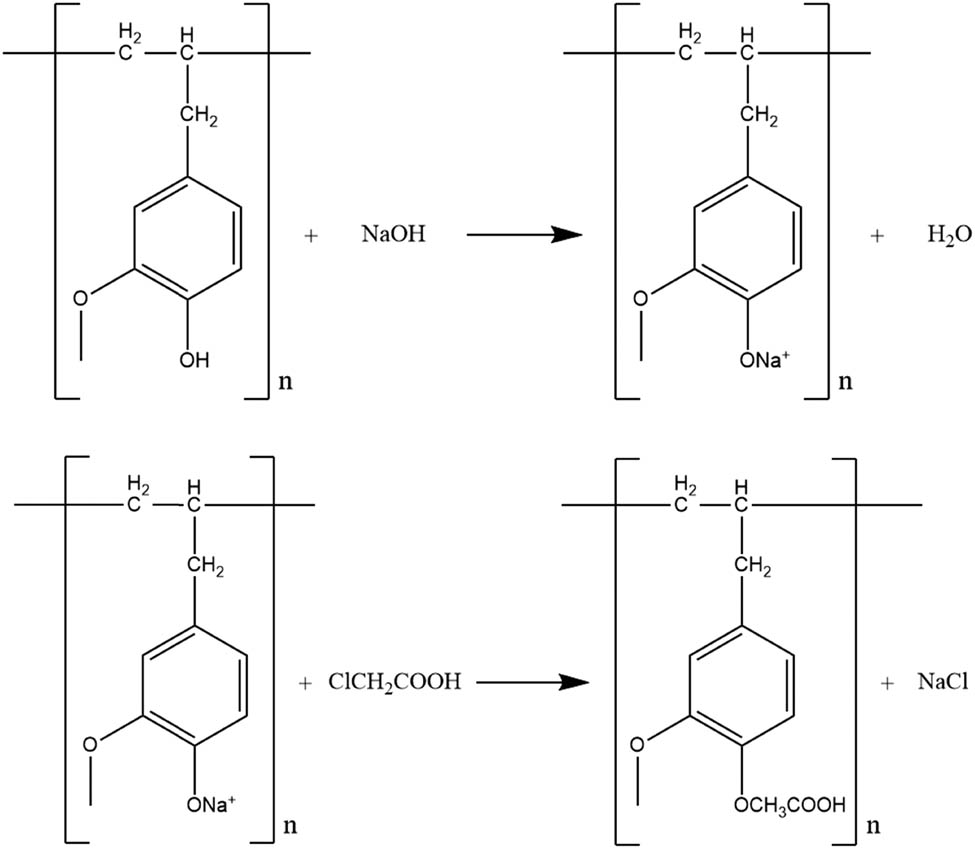
Reaction mechanism of PA synthesis.
Eugenol, like most essential oils derived from a plant, is sensitive to heat, highly volatile, and not soluble in water [19]. Polymerizing, synthesizing the derivative, and incorporating it with a cross-linking agent will result in a more stable material. MIM is known to have multiple advantages such as high stability, high affinity, and selectivity toward template molecule [20].
Figure 5 shows the FTIR analysis result of PA compared with polyeugenol, which shows an absorbance band at the range of 3,450 cm−1 indicating hydroxyl group and at 2,960 cm−1 region indicating saturated carbonyl group (Csp2-H). The absorption band of acid carbonyl group (C═O) was identified at the 1,740 cm−1 region, while the acid (C–O) bond was identified at 1,150 cm−1. From these data, it could be concluded that polyeugenoxy acetic acid has been successfully synthesized.
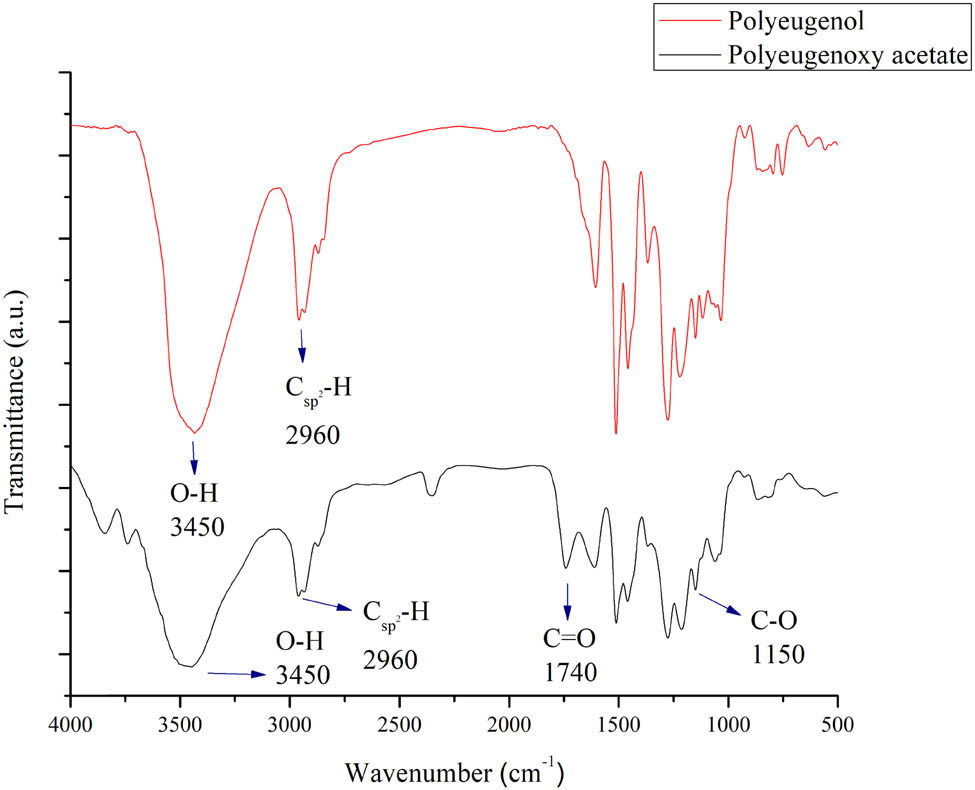
Polyeugenol and PA FTIR comparison.
3.2 PA contacting with urea
The synthesized polyeugenol was contacted with urea to create a urea template in the MIM. To calculate the contacted urea percentage in the polymer, UV-Vis spectrophotometry analysis was used to analyze the urea concentration before and after the contacting process. The concentration of urea used for the contacting step is 1,000 ppm. This concentration is used to optimize the amount of urea contacted with the polymer, while keeping the concentration of urea to be not too high so that it could be analyzed using UV-Vis spectroscopy. The result showed that 23.54% of urea was adsorbed by the PA. The amount of urea that interacts with the polymer is one of the important things that will determine the performance of the membrane when used for transport applications. In the contacting stage, there can be two types of interactions between the polymer and the template. The first type is called a self-assembling approach which is similar to the biological recognition systems that use non-covalent forces such as hydrogen bonds, Van der Waals forces, ionic or hydrophobic interactions, and metal coordination [21]. The second is a preorganized approach which uses reversible covalent bonds. This type of approach tends to produce homogeneous binding sites and reduce non-specific sites. However, it is necessary to break the covalent bonds to remove the template from the polymer matrix [22]. The approximate hydrogen bond between polygenoxy acetic acid and urea is depicted in Figure 6.
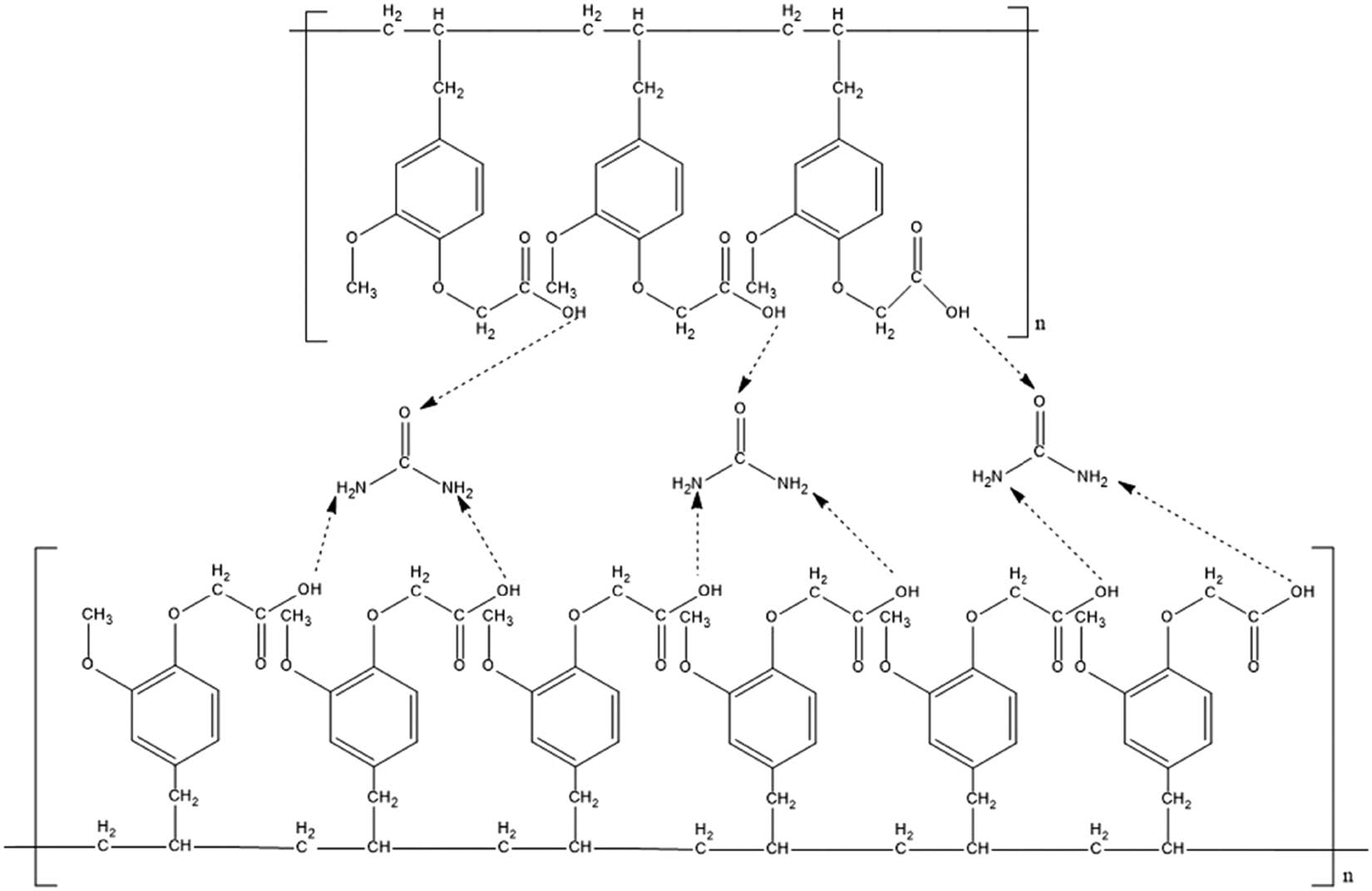
Approximation of bond between polyeugenoxy acetic acid and urea.
3.3 Analysis of MIM and NIM
Two types of membranes were synthesized, the MIM and NIM. The variations carried out in this research were the variation in reflux time of 5 and 10 h at a temperature of 80–90°C and without the use of reflux and only stirring using a stirrer for 10 h at room temperature. The mixture turned into a blackish brown solution which was then cast to form a membrane and allowed to soak in aquabidest for 24 h.
The urea will be released from the membrane when it is being soaked in aquabidest leaving a cavity of size, shape, and structure similar to that of urea, thus creating an MIM. To confirm this, we analyze the urea contacted PA (PA-Urea) sample and the MIM-10 sample. In the result shown in Figure 7, the green box area is the absorption area of urea N–H bending. It is detected in the wavenumber of 1,627 cm−1 in the PA-Urea spectra but it is not shown in the MIM-10 spectra, which indicates that the urea has indeed been released and no longer contained in the membrane, forming a template in the MIM. The N–H bending spectra of urea detected in this research correspond with other previous studies [23,24].
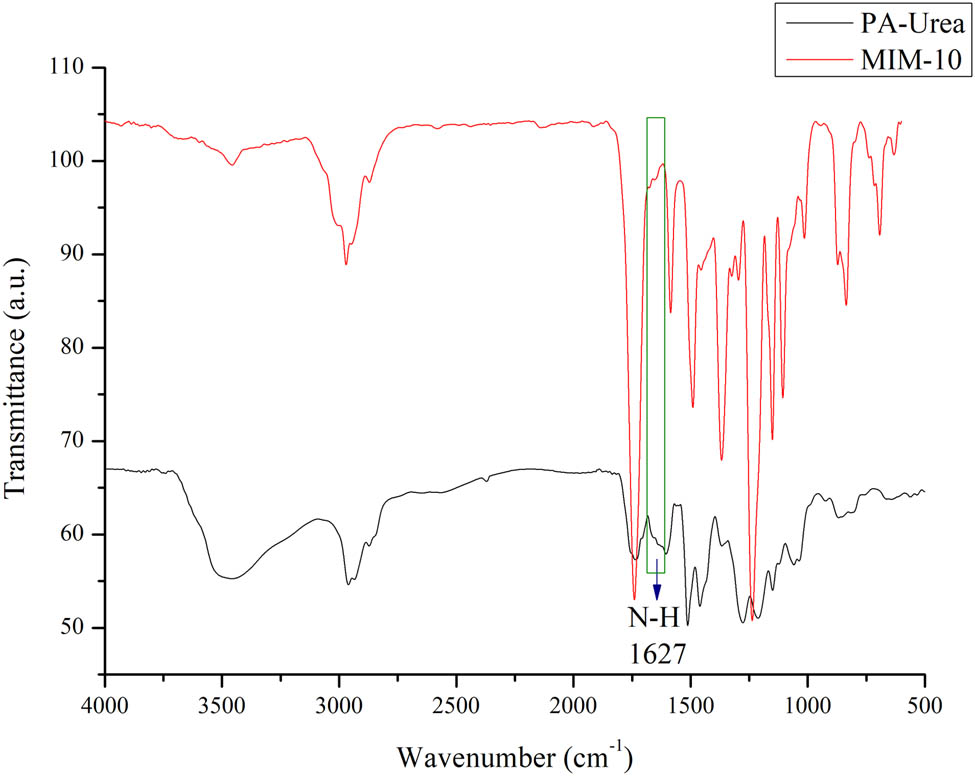
FTIR spectra comparison of PA-Urea and MIM-10 highlighting the N–H urea absorbance area.
Analysis using SEM-EDX was carried out to determine the surface morphology of the membrane and to determine the elemental content in the membrane. Figures 8 and 9 show the surface and pore morphology of the MIM and NIM at 5,000× magnification, respectively.
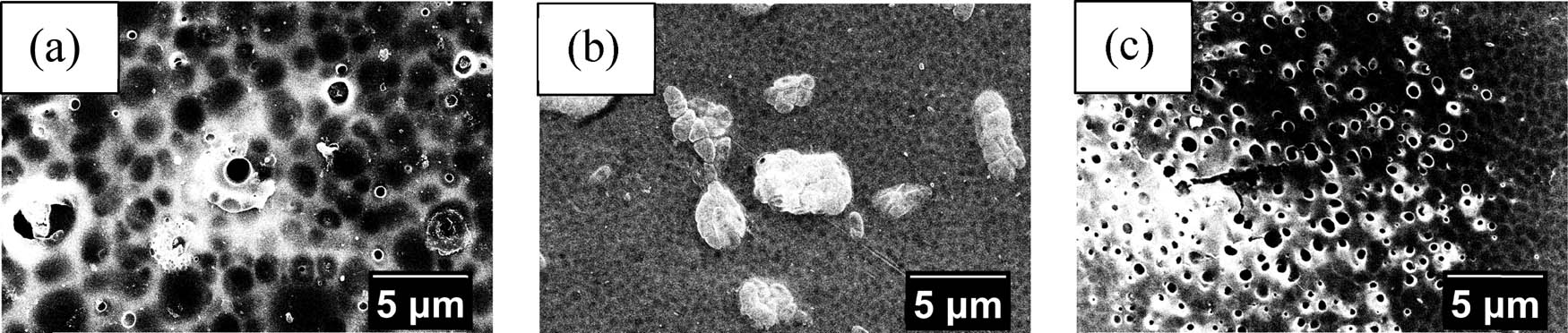
SEM analysis results of (a) MIM-0, (b) MIM-5, and (c) MIM-10 surface morphology.
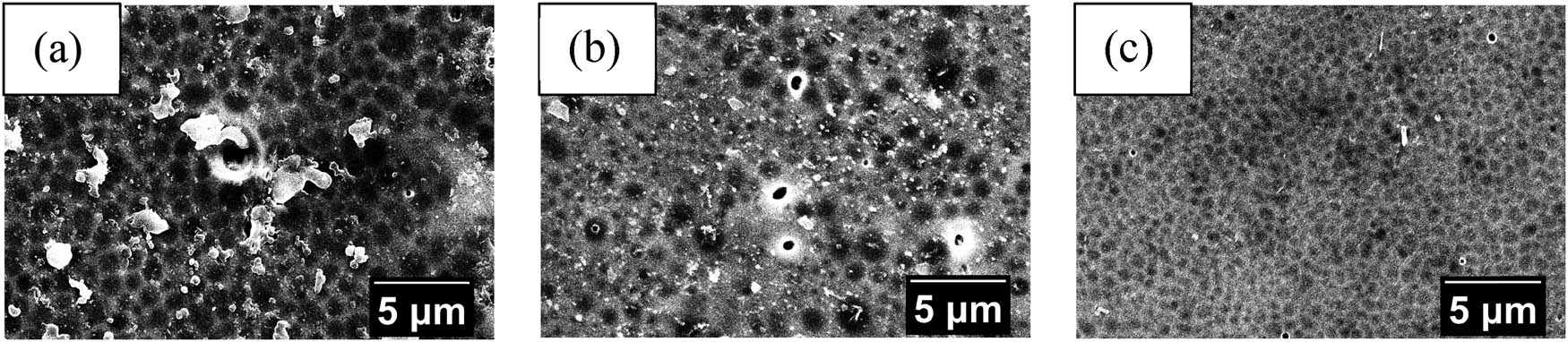
SEM analysis result of (a) NIM-0, (b) NIM-5, and (c) NIM-10 surface morphology.
Based on the surface morphology of the MIM in Figure 8, it is seen that heating affects the membrane pores. Heating will decrease the pore size of the membrane, but as heating time increases, the pore size of the membrane will also increase. Some parts of the membrane seem to have impurity in the surface of the pores that looks like some cluster. However, the transport process was done using the membrane part that seems to have the least defect from the rest, so the quality of the transport should not be much affected by it. The pore sizes of the MIM and NIM are measured using ImageJ software and the generated results are shown in Table 1. The EDX analysis results in Table 2 indicate that there are no nitrogen (N) from urea contained in the sample of the membranes. NIM was synthesized using PA that was not contacted with urea but the MIM used the PA that has been contacted with urea. The lack of nitrogen in the EDX analysis of all the MIM samples indicates that the urea has been released from the MIM when it was soaked with aquabidest, supposedly leaving a urea cavity in the MIM that will later serve as the template that could recognize urea.
Pore size measurement result of all membrane variation
| Sample | Average pore size (μm) |
|---|---|
| MIM-0 | 1.087 ± 0.328 |
| MIM-5 | 0.373 ± 0.063 |
| MIM-10 | 0.530 ± 0.096 |
| NIM-0 | 0.867 ± 0.154 |
| NIM-5 | 0.746 ± 0.178 |
| NIM-10 | 1.134 ± 0.268 |
Elemental analysis result by EDX on all membrane variations
| Element | NIM-0 (%) | NIM-5 (%) | NIM-10 (%) | MIM-0 (%) | MIM-5 (%) | MIM-10 (%) |
|---|---|---|---|---|---|---|
| C | 77.26 | 76.47 | 75.70 | 76.89 | 75.36 | 76.13 |
| O | 18.32 | 18.95 | 19.53 | 17.70 | 19.71 | 19.50 |
| S | 4.42 | 3.93 | 4.26 | 4.45 | 4.36 | 3.62 |
In the NIM variation, analysis was carried out using FTIR to determine the effect of heating on the membrane functional groups. The results of FTIR analysis on NIM-0, NIM-5, and NIM-10 are shown in Figure 10. It can be seen in the OH absorption region in the range 3,450–3,460 cm−1 that NIM-0 (without heating) has the greatest OH intensity, followed by NIM-10 and NIM-5. The strengthening of the OH group intensity will show the attenuation of the C–O group detected in the 1106.5 cm−1 absorption area. The strongest C–O group intensity is in NIM-5, followed by NIM-10 and NIM-0 in the absorption area 1,080–1,130 cm−1.
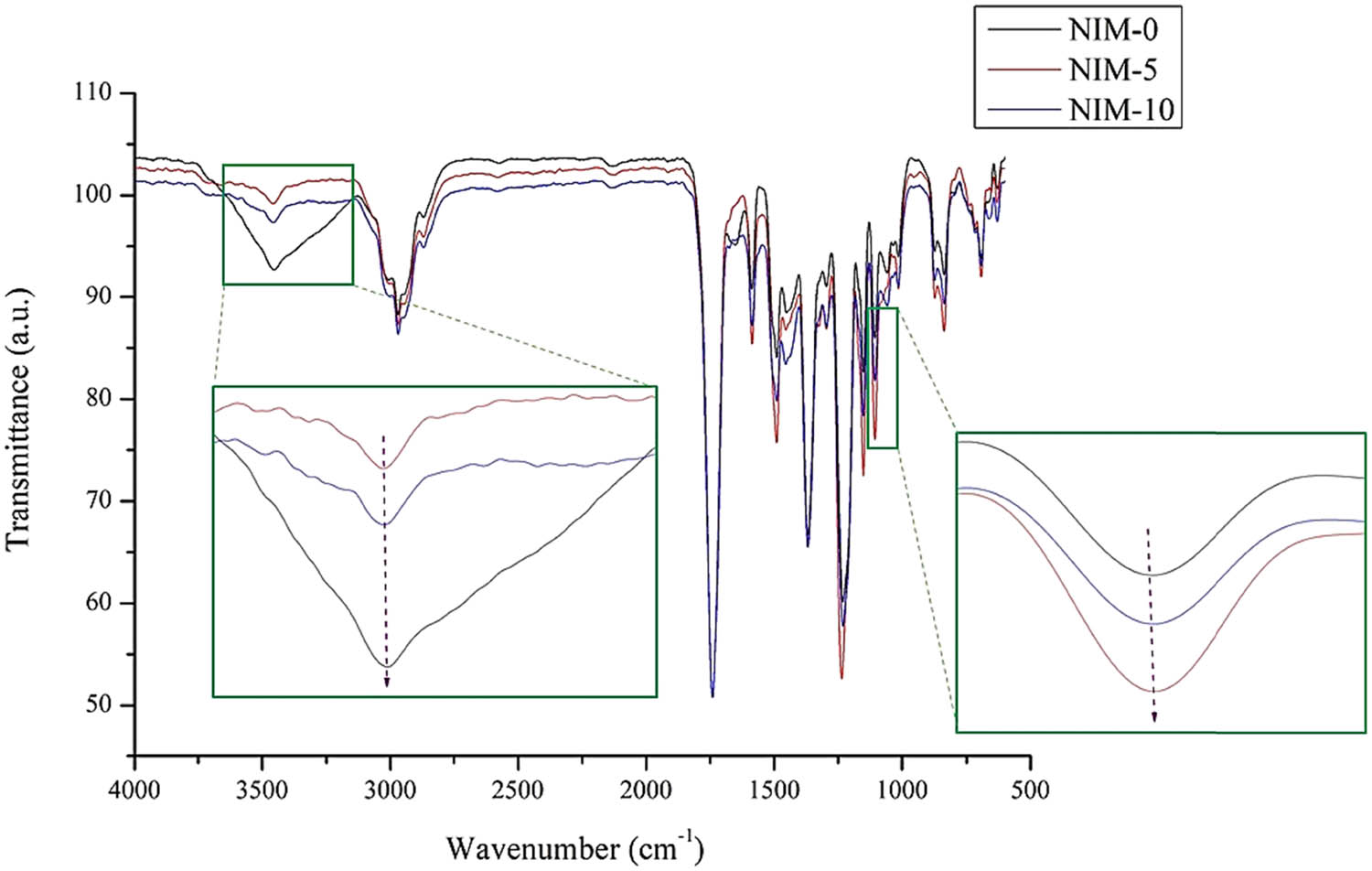
FTIR graph on the absorption of C–O and O–H groups in the NIM variation.
3.4 Urea transport test with heating time variations in the membrane
The variation in the membrane without heating results in a membrane with perforated part when viewed under light. When a leak test is carried out, the membrane without heating leaks. Based on previous FTIR results, membranes without heating had the strongest O–H absorption compared to membranes with heating for 5 and 10 h. So, it can be concluded that less of O–H indicates the ability of the membrane to be leakage free. If transport using a membrane without heating (MIM-0) that leaks is carried out, the transport results obtained may be invalid because it is possible that the urea will escape and does not represent the transport ability and selectivity of the membrane itself. Therefore, the transport test for urea was carried out using a membrane with a heating variation in 5 and 10 h (MIM-5 and MIM-10).
The setup used for the transport process is a diffusion cell that consists of the FP and the RP. The FP contains the solution with the compound that has to be transported, in this case urea diluted by PBS, while the RP only consists of PBS. The MIM is put in-between the FP and the RP, and with concentration difference as the driving force, urea will be transported to the RP. The results of 300 ppm urea transport using NIM and MIM variations are shown in Figure 11.
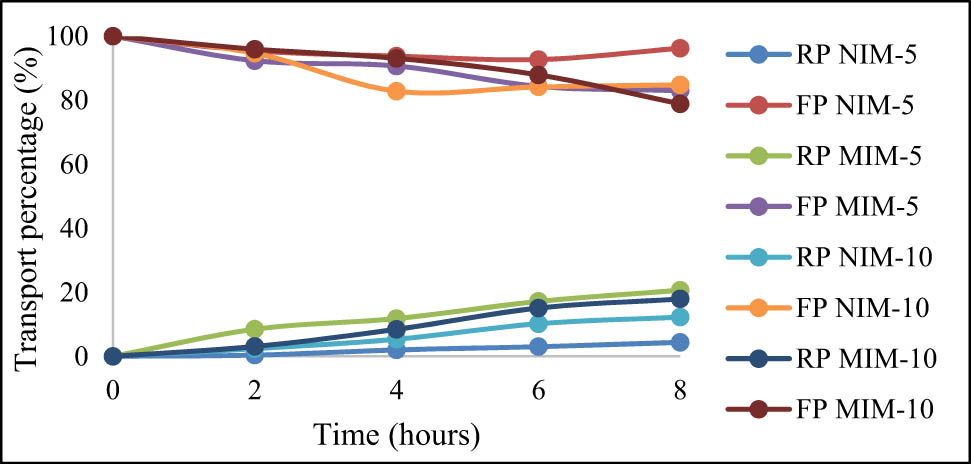
The result of 300 ppm urea transport using variations in MIM and NIM.
In Figure 11, the transport of 300 ppm of urea is shown to be the most optimum using the MIM-10 variation which shows a 21.13% decrease in urea in the FP, while the RP shows a 17.91% of urea increment. The concentration lost from the FP and gained in the RP could be different due to urea entrapment in the membrane.
Another transport test using 500 ppm of urea was carried out for 48 h to determine the membrane transport performance when the transport time was increased. The membrane used in this test was the MIM-10 which showed the optimal results when transporting 300 ppm of urea. The results showed that at 48 h, the membrane was able to transport 50.42% of 500 ppm of urea in terms of the FP. Meanwhile, the RP showed that the amount of urea transported was 49.06%. The longer the transport time, the lower the amount of urea being transported. The driving force on this transport is the difference in concentration between the FP and the RP, and at the 48th hour it is no longer possible to transport because the concentration between the FP and the RP is balanced. The result is shown in Figure 12.
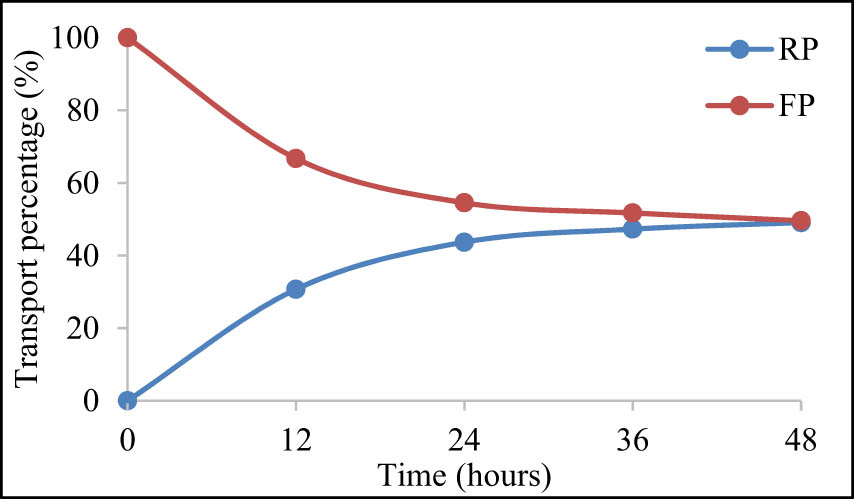
The result of 500 ppm urea transport for 48 h using MIM-10.
3.5 Urea, creatinine, and vitamin B12 transport and selectivity test
For the selectivity test, MIM-10 was used as it is the membrane that showed the best ability in transporting 300 ppm of urea. The membranes are used for the transport of 100 ppm urea, 25 ppm creatinine, and 20 ppm vitamin B12, and from these data, the selectivity of the membrane towards urea is calculated. These specific concentrations were chosen as it is the concentration that gives the highest precision in the UV method, which is in the range of 0.2–0.8 [25]. More than 20 ppm creatinine would give an absorbance value over 1, while urea concentration of 50 ppm and under would have absorbance value of less than 0.2.
Transport of urea, creatinine, and vitamin B12 aims to prove the urea template in the membrane and its ability in transporting urea compared to other substances. The approximate reaction between polysulfone, PEG, and PA-Urea is depicted in Figure 13.
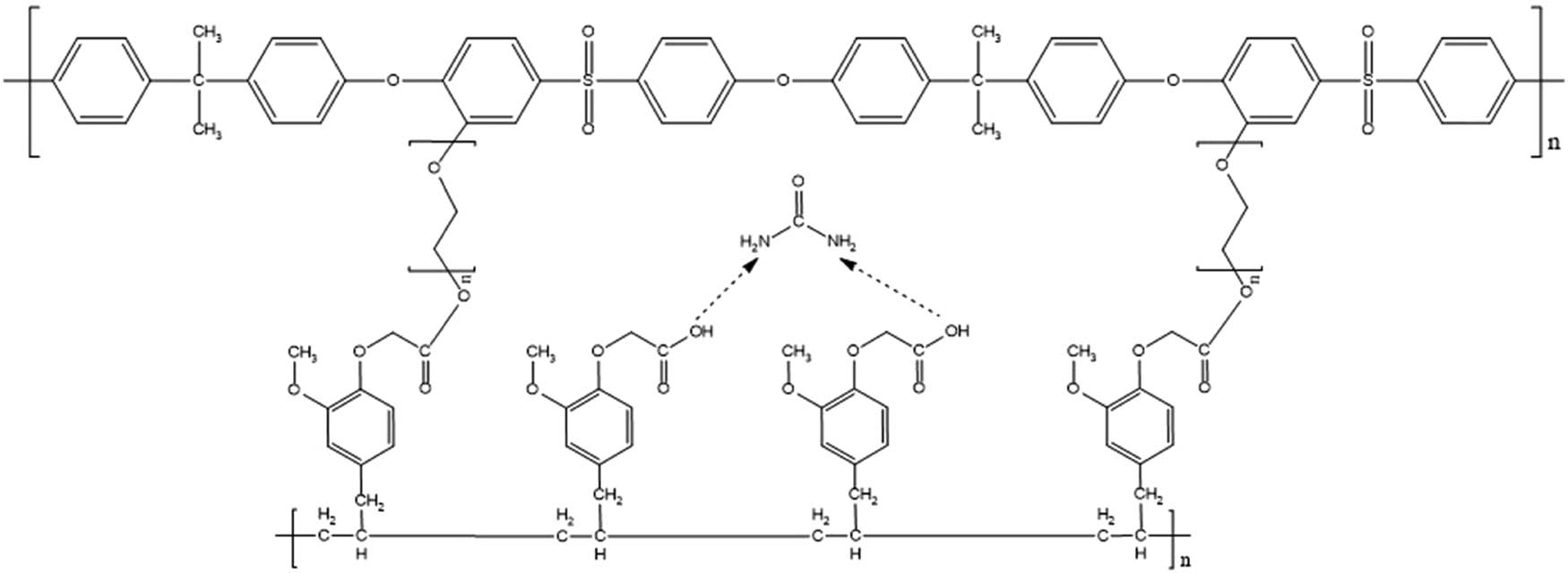
Estimated formation of a urea template on the MIM membrane.
In this test, urea transport was carried out with a concentration of 100 ppm. This time for urea transport, the urea concentration was lowered to obtain better transport results. Evidently, by using a lower concentration of urea, the percentage of urea that was successfully transported when viewed from the FP was 28.89% for MIM and 5.31% for NIM. Meanwhile, when viewed from the RP, the urea transported was 28.09% for MIM and 14.61% for NIM. The results of urea transport using the MIM were better than those obtained using the NIM, indicating that the urea template on the MIM was properly formed so that it was able to recognize urea and transport it more optimally. From the various concentrations of urea used in the transport process, it can be concluded that the best transport result was achieved by the 100 ppm concentration. The results of 100 ppm urea transport with MIM-10 and NIM-10 are shown in Figure 14.
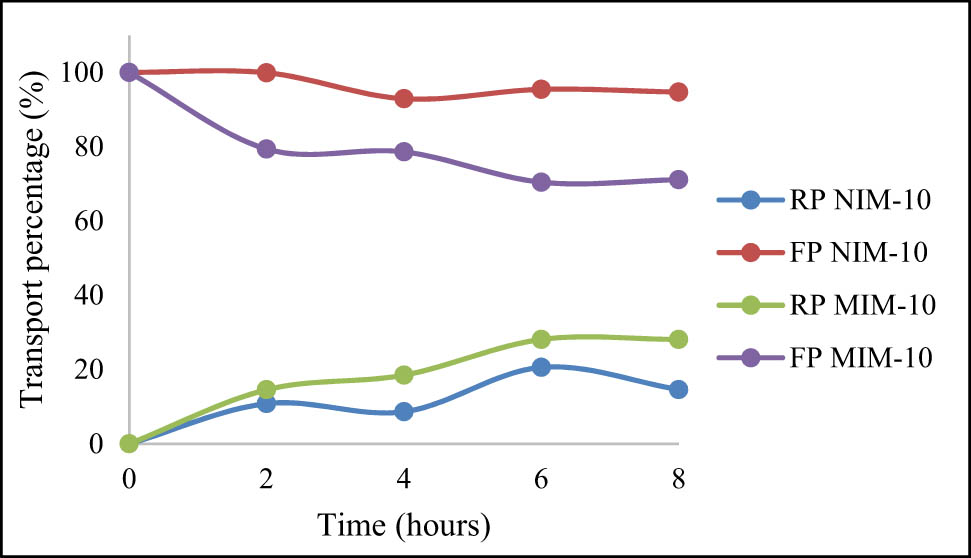
Percentage of 100 ppm urea transport using MIM-10 and NIM-10.
For the creatinine transport, the NIM was able to transport 4.47% of creatinine, while the MIM was able to transport 23.62% of creatinine when viewed from the FP. While from the RP, NIM was able to transport 3.46% of creatinine and MIM was able to transport 13.44% of creatinine. This shows that the membrane’s ability to transport creatinine is not optimal when compared to urea transport. The results of creatinine transport using the 10 h NIM and MIM membranes are shown in Figure 15.
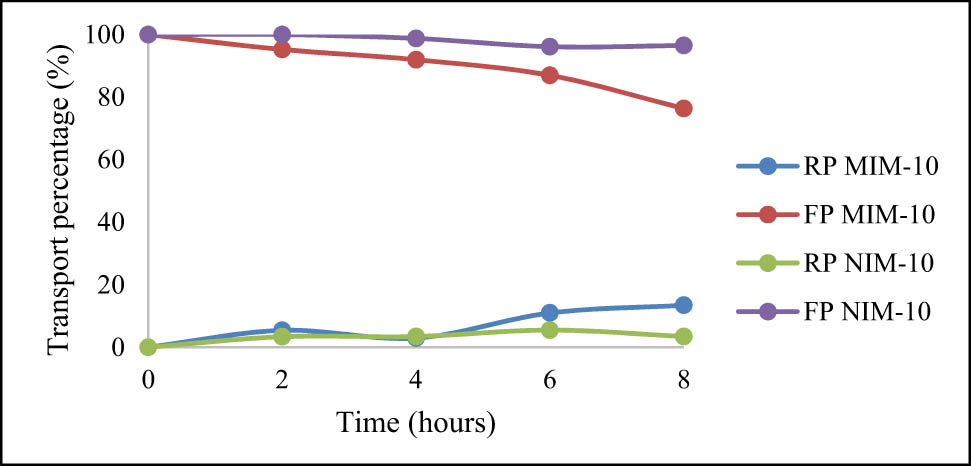
Percentage of 25 ppm creatinine transport using MIM-10 and NIM-10.
While for the vitamin B12, in terms of the FP, vitamin B12 transport using the NIM-10 was able to transport 9.38% of vitamin B12, while the MIM-10 was only able to transport 6.28% of vitamin B12. When viewed from the RP, NIM-10 is able to transport 5.37% of vitamin B12 while MIM is able to transport 9.86% of vitamin B12. This shows that the MIM-10 and NIM-10 membranes are less capable of transporting vitamin B12. The results of vitamin B12 transport using the NIM-10 and MIM-10 membranes are shown in Figure 16.
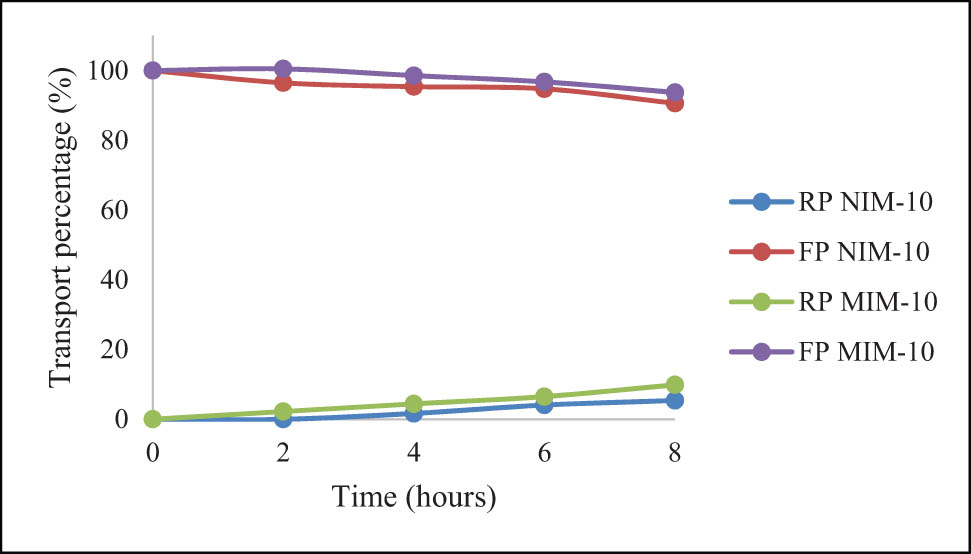
Percentage of 20 ppm vitamin B12 transport using MIM-10 and NIM-10.
Based on the results of creatinine and vitamin B12 transport, it shows that the MIM membrane is more capable of transporting urea than creatinine and vitamin B12. This could be due to the molecular size of urea, creatinine, and vitamin B12. Urea has a size of 60 Da, creatinine has a size of 113 Da [26], and vitamin B12 has a size of 8.5 Å or 1.35 kDa [27]. The size of vitamin B12 is much larger than the size of urea so that the transport ability of the MIM membrane in the transport of vitamin B12 is not as good as the transport of urea as well as creatinine.
Based on the selectivity graph of urea and vitamin B12 in Figure 17, it shows that the MIM has a greater selectivity value than the NIM. This means that the MIM with urea template is better in transporting urea than vitamin B12. This also proves the presence of urea template on the MIM which makes the MIM more selective toward urea and less capable of transporting vitamin B12. Meanwhile, the NIM, which does not have a urea template, has a lower selectivity value because the NIM is not able to recognize urea properly.
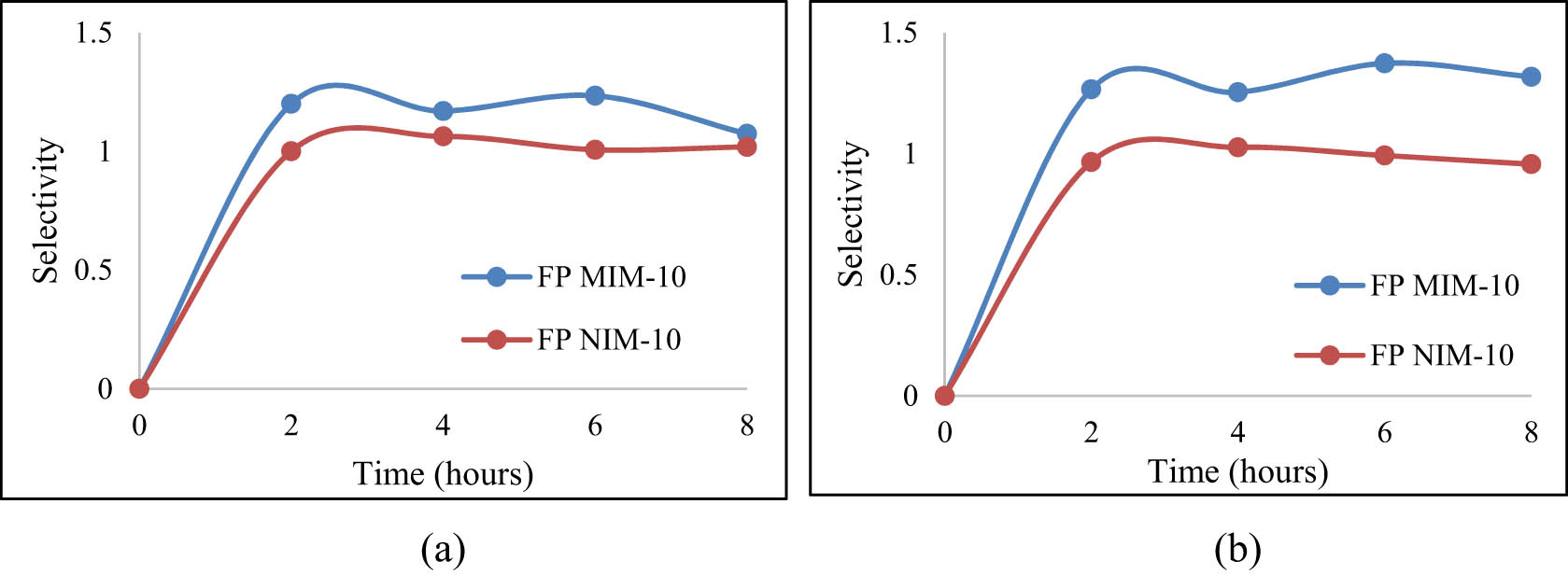
MIM-10 and NIM-10 selectivity for the transport of urea toward (a) creatinine and (b) vitamin B12.
The regeneration of the membrane happens in the transport process during the release of target molecule from the FP to the RP, so it will happen continuously as long as there is a driving force which is the concentration difference between the FP and RP. The reusability of the imprinted membranes is not yet tested on this research and will be an interesting insight to be added in future studies, but as long as the regeneration is proper, the reusability is hoped to be dependable as supported by the low amount of trapped molecule in the membrane when comparing the difference in the transport data between the FP and the RP.
4 Conclusion
Polyeugenol and PA have been successfully synthesized with a yield of 97.89 and 96.04%, respectively. The synthesized MIM is proven to transport urea better than the NIM due to the urea template in the MIM. In an 8-h span, the best urea transport result was shown by MIM-10 variation that was synthesized by heating for 10 h, which transported 28.89% of 100 ppm urea, while the NIM-10 transported only 5.31%. However, the transport that was conducted for 48 h showed that the MIM-10 could transport 50.42% of 500 ppm urea. MIM-10 selectivity toward urea and against creatinine and vitamin B12 showed a higher value than the NIM selectivity. The result proves the presence of the urea template in the MIM. The order of MIM selectivity from the best results was urea > creatinine > vitamin B12.
Acknowledgment
This study was financially supported by the International Publication Research Grant funded other than state budget of Diponegoro University 2021 (185-78/UN7.6.1/PP/2021).
-
Funding information: This research was funded by the Ministry of Research, Technology, and Higher Education, Indonesia.
-
Author contributions: M.C.D. – conceptualization, funding acquisition, resources; N.A.F. – investigation, formal analysis, writing – original draft, review and editing; M.C.D. and N.A.F. – methodology, data curation; and M.C.D. and A.H. – supervision.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] De Nicola L, Zoccali C. Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrol Dial Transplant. 2016;31:331–5.10.1093/ndt/gfv427Suche in Google Scholar PubMed
[2] Mohammad AM, Yuniastuti A. Gender-based health disorders in end-stage renal disease patients in the hospital Elmarj city Libya. Kemas. 2017;13(1):113–20.10.15294/kemas.v13i1.9413Suche in Google Scholar
[3] Ahmed HG, Ginawi I, Alshammari F. Association of genetic and biochemical markers with GFR among renal failure patients: applying serum creatinine and cystatin C measures. Sch J App Med Sci. 2015;3(6D):2431–6.Suche in Google Scholar
[4] Deppisch R, Göhl H, Smeby L. Microdomain structure of polymeric surfaces–potential for improving blood treatment procedures. Nephrol Dial Trans. 1998;13(6):1354–9.10.1093/oxfordjournals.ndt.a027892Suche in Google Scholar PubMed
[5] Cieplak M, Kutner W. Artificial biosensors: how can molecular imprinting mimic biorecognition? Trends Biotechnol. 2016;34(11):922–41.10.1016/j.tibtech.2016.05.011Suche in Google Scholar PubMed
[6] Piletsky SA, Piletskaya EV, Panasyuk TL, El'skaya AV, Levi R, Karube I, et al. Imprinted membranes for sensor technology: opposite behavior of covalently and noncovalently imprinted membranes, Macromolecules. 1998;31:2137–40.10.1021/ma970818dSuche in Google Scholar
[7] Poma A, Turner APF, Piletsky SA. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010;28:629–37.10.1016/j.tibtech.2010.08.006Suche in Google Scholar PubMed
[8] Djunaidi MC, Azizah A, Gunawan. Synthesis of molecularly imprinted polymer urea based on polyeugenol with ethylene glycol dimethacrylate as crosslinking agent. Proceedings of the 14th Joint Conference on Chemistry. Indonesia: AIP Conf Proc; 2019 Sep 10–11. p. 020058.10.1063/5.0005544Suche in Google Scholar
[9] Rayanasukha Y, Pratontep S, Porntheeraphat S, Bunjongpru W. Non-enzymatic urea sensor using molecularly imprinted polymers surface modified based-on ion-sensitive field effect transistor (ISFET), Nukeaw J Surf Coat Tech. 2016;306:147–50.10.1016/j.surfcoat.2016.05.060Suche in Google Scholar
[10] Liu YL, Liu R, Qin Y, Qiu QF, Chen Z, Cheng SB, et al. Flexible electrochemical urea sensor based on surface molecularly imprinted nanotubes for detection of human sweat. Anal Chem. 2018;90(21):13081–87.10.1021/acs.analchem.8b04223Suche in Google Scholar PubMed
[11] Pogány P, Razali M, Szekely G. Experimental and theoretical investigation of the complexation of methacrylic acid and diisopropyl urea. Spectrochim Acta A. 2017;170:69–76.10.1016/j.saa.2016.07.005Suche in Google Scholar PubMed
[12] Djunaidi MC, Haris A, Pardoyo P, Rosdiana K. The impact of template types on polyeugenol to the adsorption selectivity of ionic imprinted polymer (IIP) Fe metal ion. Proceedings of the 12th Joint Conference on Chemistry. Indonesia: IOP Conf Ser; 2017 Sep 19–20. p. 012034.10.1088/1757-899X/349/1/012034Suche in Google Scholar
[13] Djunaidi MC, Prasetya NBA, Khoiriyah A, Pardoyo P, Haris A, Febriola NA. Polysulfone influence on au selective adsorbent imprinted membrane synthesis with sulfonated polyeugenol as functional polymer. Membranes. 2020;10(12):390.10.3390/membranes10120390Suche in Google Scholar PubMed PubMed Central
[14] Djunaidi MC, Pardoyo P, Widodo DS, Lusiana RA, Yuliani A. In-Situ Ionic Imprinted Membrane (IIM) synthesis based on acetic polyeugenoxy acetyl tiophen methanolate for gold(III) metal ion transports. Indones J Chem. 2020;20(6):1323–31.10.22146/ijc.49941Suche in Google Scholar
[15] Da Silva FFM, Monte FJQ, de Lemos TLG, Do Nascimento PGG, de Medeiros Costa AK, De Paiva LMM. Eugenol derivatives: synthesis, characterization, and evaluation of antibacterial and antioxidant activities. Chem Cent J. 2018;12(1):1–9.10.1186/s13065-018-0407-4Suche in Google Scholar PubMed PubMed Central
[16] Verma AM, Kishore N. Gas phase conversion of eugenol into various hydrocarbons and platform chemicals. RSC Adv. 2017;7(5):2527–43.10.1039/C6RA26357GSuche in Google Scholar
[17] Zhang H, Tian H, Zhang J, Guo R, Li X, Int J. Facilitated transport membranes with an amino acid salt for highly efficient CO2 separation. Greenh Gas Con. 2018;78:85–93.10.1016/j.ijggc.2018.07.014Suche in Google Scholar
[18] Djunaidi MC, Siswanta D. Eluent influences on synthesis of Fe(III)-imprinted polyeugenol using polyethylene glycol diglycidilether (PEGDE) as cross-linking agent and its application as Fe(III) sorbent. Orient J Chem. 2015;31(4):2223–9.10.13005/ojc/310447Suche in Google Scholar
[19] Woranuch S, Yoksan R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr Polym. 2013;96(2):578–85.10.1016/j.carbpol.2012.08.117Suche in Google Scholar PubMed
[20] Saylan Y, Akgönüllü S, Yavuz H, Ünal S, Denizli A. Molecularly imprinted polymer based sensors for medical applications. Sensors. 2019;19(6):1279.10.3390/s19061279Suche in Google Scholar PubMed PubMed Central
[21] Arshady R, Mosbach K. Synthesis of substrate-selective polymers by host-guest polymerization. Macromol Chem Phys. 1981;182(2):687–92.10.1002/macp.1981.021820240Suche in Google Scholar
[22] Wulff G. The use of polymers with enzyme-analogous structures for the resolution of racemates. Angew Chem Internat Edit. 1972;11(4):341.Suche in Google Scholar
[23] Djunaidi MC, Wenten IG, Synthesis of eugenol-based selective membrane for hemodialysis. IOP Conference Series: Materials Science and Engineering. Indonesia: IOP Publishing; 2019 Apr.10.1088/1757-899X/509/1/012069Suche in Google Scholar
[24] Piasek Z, Urbanski TB. The infra-red absorption spectrum and structure of urea. Pol Acad Sci-Tech X. 1962;X:13–20.Suche in Google Scholar
[25] Hansen S, Pedersen-Bjergaard S, Rasmussen K. Introduction to pharmaceutical chemical analysis. Chichester, UK: John Wiley & Sons; 2012.10.1002/9781119953647Suche in Google Scholar
[26] Hosten AO. Bun and creatinine. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston: Butterworths; 1990.Suche in Google Scholar
[27] Talmard C, Guilloreau L, Coppel Y, Mazarguil H, Faller P. Amyloid-Beta peptide forms monomeric complexes with Cuii and Znii prior to aggregation. Chembiochem. 2007;8(2):63–165.10.1002/cbic.200600319Suche in Google Scholar PubMed
© 2021 Muhammad Cholid Djunaidi et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation


