Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
-
Symbat Muratbekovna Naurzkulova
Abstract
Ethanol steam reforming catalyst’s precursors, i.e., nanocomposites of complex oxides with the general formula [Pr0.15Sm0.15Ce0.35Zr0.35O2 + LaMn0.45Ni0.45Ru0.1O3] (1:1 by mass), were synthesized by three different methods. It was shown that two synthesis methods – ultrasonic dispersion and sequential polymeric method, lead to the formation of the nanocomposite perovskite–fluorite system with the specific surface area up to 50 m2/g. Reduction of samples at 400–500°C lead to the formation of Ni–Ru alloy nanoparticles strongly bound with the surface of oxide nanocomposite. Catalytic tests in ethanol steam reforming reaction at 500–600°C showed the highest specific activity of the sample prepared by the sequential polymeric method due to the location of Ni- and Ru-containing perovskite mainly on the surface of the composite providing a high concentration of active metal centers. At higher temperatures for all samples, ethanol conversion approached 100% with hydrogen yield varying in the range of 65–75%. A study of spent catalysts confirmed the absence of carbon deposits after long-term catalytic tests at 650°C.
1 Introduction
To date, hydrogen is the most environmentally friendly fuel for various energy and heat generators (fuel cells, internal combustion engines and mobile power plants) [1]. In context of the green energy of the future, hydrogen is associated with a promising technology of electrochemical generators based on solid oxide fuel cells (SOFCs) with internal or external reformer of fuels, whose main qualities are environmental friendliness, mobility and high efficiency [2]. Moreover, due to fuel source flexibility, such devices can successfully provide reforming of various carbon-containing fuels, including bio-renewable ones [3]. Among others, a great attention is paid to the ethanol steam reforming process [4]. Since the catalyst for internal fuel reforming in SOFC is a multifunctional layer supported on the anode, it must satisfy many requirements: (1) activity in the reaction of steam reforming of oxygenates (breaking C–C and C–H bonds), (2) thermochemical stability (resistance to sintering and carbonization), (3) compatibility with anode layers (stability to delamination and cracking) and (4) mixed ionic-electron conductivity [5]. Today, it is known that some nanocomposite materials, including complex oxides of transition and rare-earth elements (RRE) with the structures of perovskite, fluorite, or spinel, have the aforementioned properties [4,5,6,7]. In the early works of our laboratory, perovskite–fluorite based nanocomposites with very promising characteristics in fuel reforming were developed [8,9,10]. In the present study, Pr0.15Sm0.15Ce0.35Zr0.35O2 and LaMn0.45Ni0.45Ru0.1O3 were chosen as composite components. Incorporation of rare earth cations (La3+, Sm3+, Gd3+ and Pr3+/4+) into ceria-zirconia solid solution stabilizes its structure and affects oxygen mobility/acid–base surface properties [26]. Ni-containing perovskite-type oxides are well-known catalyst’s precursors for the hydrogen production reactions. A high activity of catalysts based on substituted perovskite precursors LaNi1−x (M) x O3 (M = Mn, Fe, Ru) in reforming reactions was shown in previous studies [11,12,13,14,15]. The methods of nanocomposite materials synthesis should provide a high chemical uniformity of obtained complex oxides along with their high dispersion. It can be co-precipitation, hydrothermal method, solvothermal method, sol–gel–citrate and ester polymer precursors (Pechini), microemulsions, microwave method, sonochemical method, solution combustion and spray pyrolysis reactions [16].
In this article, the results of optimization of the perovskite–fluorite nanocomposite synthesis by elucidating its effect on textural, structural, surface and catalytic properties of the obtained materials are presented. Nanocomposites were prepared by three methods — the sequential polymeric method, ultrasonic dispersion of the as-prepared complex oxides in isopropanol with addition of surfactant and the one-pot polymeric method. Features of interaction between components affecting their texture, structural features, surface properties and reactivity were elucidated by N2 adsorption, XRD and HRTEM with EDX, XPS, CO chemisorptions and H2-TPR; catalytic properties were studied in ethanol steam reforming. The best catalytic activity along with the coking stability was demonstrated by nanocomposite prepared by the sequential polymeric method due to optimized interaction between components providing the highest concentration of surface metal centers strongly interacting with support and high reactivity of surface oxygen species.
2 Methods
2.1 Catalysts synthesis
Pr0.15Sm0.15Ce0.35Zr0.35O2 (PSCZ) and LaMn0.45Ni0.45Ru0.1O3 (LMNR) were synthesized by the organic polymeric precursor method [15,16], which is the modified Pechini method [17]. The synthesis was performed using crystalline hydrates of Pr(NO3)3 (Vecton), Sm(NO3)3 (Vecton), Ce(NO3)3 (Vecton), La(NO3)3 (Vecton), Mn(NO3)2 (NevaReactiv), Ni(NO3)2 (Vecton), ZrOCl2 (Reakhim) and crystalline anhydrous RuOCl3 (Reakhim). For organic polymer formation monohydrate of citric acid C6H8O7·H2O (Vecton), ethylene glycol C2H6O2 (Reakhim) and ethylene diamine C2H8N2 (Reakhim) were used. All reagents were of chemical pure grade. Before synthesis, the exact molar masses of crystalline hydrates were refined by thermal analysis to determine the actual water content. The reagents with molar ratios of
All process was carried out under continuous stirring. Citric acid was dissolved in ethylene glycol under weak heating (60–80°C). The solution was cooled to a room temperature and supplemented with a necessary amount of crystalline metal salts: Pr(NO3)3, Sm(NO3)3, Ce(NO3)3 and ZrOCl2 hydrates for Pr0.15Sm0.15Ce0.35Zr0.35O2 and La(NO3)3, Mn(NO3)2, Ni(NO3)2 and RuOCl3 for LaMn0.45Ni0.45Ru0.1O3. After salts were completely dissolved, ethylene diamine was added dropwise to further polymerize the organic precursors. A complete homogenization took 2 h. The resulting mixture was evaporated to obtain a thick polymer, which was grinded in a mortar and calcined in air at 700°C for 4 h.
Composite samples [Pr0.15Sm0.15Ce0.35Zr0.35O2]:[LaMn0.45Ni0.45Ru0.1O3] = 1:1 (by mass) were prepared by three methods and were labeled as Sim1, Sim2 and Sim3. Details of preparation methods are presented. Sim1: 2.5 g of crystalline oxide (Pr0.15Sm0.15Ce0.35Zr0.35O2), prepared by the modified Pechini method, calcined under air at 700°C was added to the polymer (acetic acid + ethylene glycol) solution of La(NO3)3, Mn(NO3)2, Ni(NO3)2 and RuOCl3 during the modified Pechini synthesis of 2.5 g LaMn0.45Ni0.45Ru0.1O3. Resulting suspension of PSCZ oxide in a polymer matrix with La, Ni, Mn and Ru salts was stirred for 2 h, evaporated to obtain a thick polymer and grinded and calcined in air at 700°C for 4 h. Sim2: 2.5 g of Pr0.15Sm0.15Ce0.35Zr0.35O2 and 2.5 g of LaMn0.45Ni0.45Ru0.1O3 (prepared by the modified Pechini method) were added into solution of 1.5 mL polyvinyl butyral (5%) and 70 mL isopropanol and stirred using ultrasonic dispersion for 40 min. The suspension obtained was dried to complete evaporation of the solvent and calcined at 700°C for 2 h. Sim3: This is the one-pot method where salts of La, Pr, Sm, Ce, La, Ni, Mn and Ru in quantities necessary to obtain [Pr0.15Sm0.15Ce0.35Zr0.35O2]:[LaMn0.45Ni0.45Ru0.1O3] = 1:1 by mass composition were added to the polymer gel of acetic acid + ethylene glycol, keeping the molar ratio
For catalytic studies, the resulting powders were pressed into pellets followed by crushing and sieving to the 0.25–0.5 mm grain size fraction.
2.2 Texture, structural and surface properties, reactivity and catalytic activity
The specific surface area of samples was evaluated by the Brunnauer–Emmet–Teller (BET) method by recording nitrogen physical adsorption at the liquid nitrogen temperature using an ASAP-2400 (Micromeritics Instrument. Corp., Norcross, GA, USA) automated volumetric adsorption unit. Before the analysis, samples were outgassed at 150°C for 4 h at a pressure of 1 × 10–3 Torr (∼0.1 Pa). The obtained adsorption isotherms were used to calculate the specific surface area. The nature of crystallographic phases in oxides was characterized by X-ray diffraction (XRD) and high-resolution transmission electron microscopy (HR TEM). Diffraction patterns were observed using a Bruker Advance D8 diffractometer with a CuKα source (2θ range 20–85°, step size 0.05 and accumulation time 3 s). High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and high-resolution transmission electron microscopy (HRTEM) images of as-prepared samples were obtained with a JEM-2200FS transmission electron microscope (JEOL Ltd., Japan, acceleration voltage 200 kV, lattice resolution 1 Å) equipped with a Cs-corrector and an EDX spectrometer (JEOL Ltd., Japan). The minimum spot diameter for the step-by-step line or mapping elemental EDX analysis was ∼1 nm with a step of about 1.5 nm. Identification of the obtained phases and quantitative calculations were done using the ICDD database.
The surface chemical composition of samples was studied by the X-ray photoelectron spectroscopy (XPS) on an electronic spectrometer SPECS Surface Nano Analysis GmbH (Germany). The spectrometer is equipped with a PHOIBOS-150-MCD-9 hemispherical analyzer, as well as an XR-50 X-ray source with a double Al/Mg anode. The samples were analyzed after reduction in 5 vol% H2/N2 at 650°C for 1 h. After reduction, samples were discharged from the reactor and contacted with air being partially oxidized. Powders of samples were fixed at the holder with the help of the two-sided conducting scotch tape. The binding energy (E B) scale was calibrated using the Ce3du‴ cerium line (E B = 916.7 eV). Determination of the relative abundance of elements in the analysis zone was carried out from the integral intensities of XPS lines taking into account the photoionization cross sections of the corresponding terms [18]. For a detailed analysis of the spectra, the spectra were decomposed into individual components. After subtracting the background by the Shirley method, the experimental curve was expanded into a number of lines corresponding to the photoemission of electrons from atoms in different chemical environments. Data processing was carried out using the CasaXPS software package [19]. The peak shapes are approximated by a symmetric function obtained by multiplying the Gaussian and Lorentz functions.
To estimate the number of accessible surface metal sites, experiments with CO chemisorptions at decreased (−40°C) temperatures followed by its temperature-programmed desorption were carried out in a flow setup with a quartz tubular reactor (inner diameter 4 mm). Before chemisorption, samples were heated for 20 min from room temperature to 600°C in the stream of He (5 L/h) and then reduced by the mixture of 3% H2 in He during 1 h. Then, samples were cooled to −40°C in the stream of He and kept at this temperature for 30 min in the stream of 5% CO in He. After switching to the stream of He, samples were heated at 50°C with the temperature ramp of 0.4°/s and then at 400°C with the temperature ramp of ∼2°C/s. The analysis of desorbed CO and CO2 concentrations was carried out using a gas analyzer (Sibmicroreactor) with the thermal conductivity detector.
Material reactivity was characterized by temperature-programmed reduction by H2 (TPR-H2) (10% H2 in Ar, the feed rate 2.5 L/h and the temperature ramp from 25 to 900°C at 10°C/min) in a flow kinetic setup with a quartz U-shaped reactor equipped with a Tsvet-500 chromatograph and a thermal conductivity detector.
Ethanol steam reforming (ESR) was conducted in a continuous flow fixed-bed quartz reactor under atmospheric pressure in the temperature range of 500–800°C. A total of 30 mg of catalyst (0.25–0.5 mm fraction) was loaded and sandwiched between two quartz wool layers. Before the activity test, the catalyst was reduced with 5 vol% H2/N2 (100 mL/min) at 800°C for 1 h. EtOH and H2O mixture (H2O/EtOH mole ratio of 4) supplied by a liquid pump was heated in the evaporator up to 120°C, and this vapor was mixed with N2 stream from the mass-flow controller, yielding a gas composition EtOH:H2O = 1:4, C(EtOH) = 2%, C(H2O) = 8 vol% at contact time 10 ms. The outlet products were analyzed by gas chromatography. Ethanol conversion
where
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results
3.1 Textural and structural features
Table 1 presents sample’s designations, chemical composition, specific surface area (S BET) and short description of the synthesis method. As presented in Table 1, fluorite sample possesses much higher specific surface area compared to that of perovskite due to a higher sinterability of the latter [15,20]. Nanocomposites are characterized by intermediate values of specific surface areas. The lowest area was demonstrated by Sim1 sample due to sintering of perovskite surface layers supported on PSCZ fluorite. A higher surface area somewhat exceeding the expected average value for the mechanical mixture of two phases was found for nanocomposite prepared by ultrasonic dispersion.
Sample’s composition, synthesis methods and specific surface area
| Code | Composition | Synthesis method | S BET (m2/g) |
|---|---|---|---|
| Sim1 | [Pr0.15Sm0.15Ce0.35Zr0.35O2]:[LaMn0.45Ni0.45Ru0.1O3] 1:1 mass ratio | As-prepared PSCZ was introduced to a polymeric gel containing La, Mn, Ni and Ru salts | 36 |
| Sim2 | As-prepared PSCZ and LMNR mixing in organic solvent by ultrasonic dispersion | 49 | |
| Sim3 | One pot | 61 | |
| LMNR | LaMn0.45Ni0.45Ru0.1O3 | Modified Pechini | 8 |
| PSCZ | Pr0.15Sm0.15Ce0.35Zr0.35O2 | Modified Pechini | 75 |
The highest surface area was revealed for one-pot sample, which could be due to the absence of the perovskite phase in its composition (vide infra).
3.1.1 XRD
The XRD patterns of samples are shown in Figure 1a. The pattern of Pr0.15Sm0.15Ce0.35Zr0.35O2 corresponds to a fluorite with the cubic structure. Peak broadening is a typical phenomenon for the doped Ln1-yZryO2 (Ln = Ce, Pr, Sm), which indicates mixed oxide formation with defects induced by the lattice sites occupation by cations with different ionic radii [20]. Two small peaks indicate insignificant admixtures of zirconium oxides: peak at 30° corresponds to the tetragonal ZrO2 phase, while peak at 32° corresponds to ZrO2 with the monoclinic structure (Figure 1c). Results for LaMn0.45Ni0.45Ru0.1O3 match with the typical diffraction pattern of LaMnO3.11 rhombohedral perovskite phase (X-Ray diffraction database Powder Diffraction File PDF #50-0297). Low intensity peak at 28.1° indicates the presence of a minor amount of ruthenium oxide (Figure 1c).
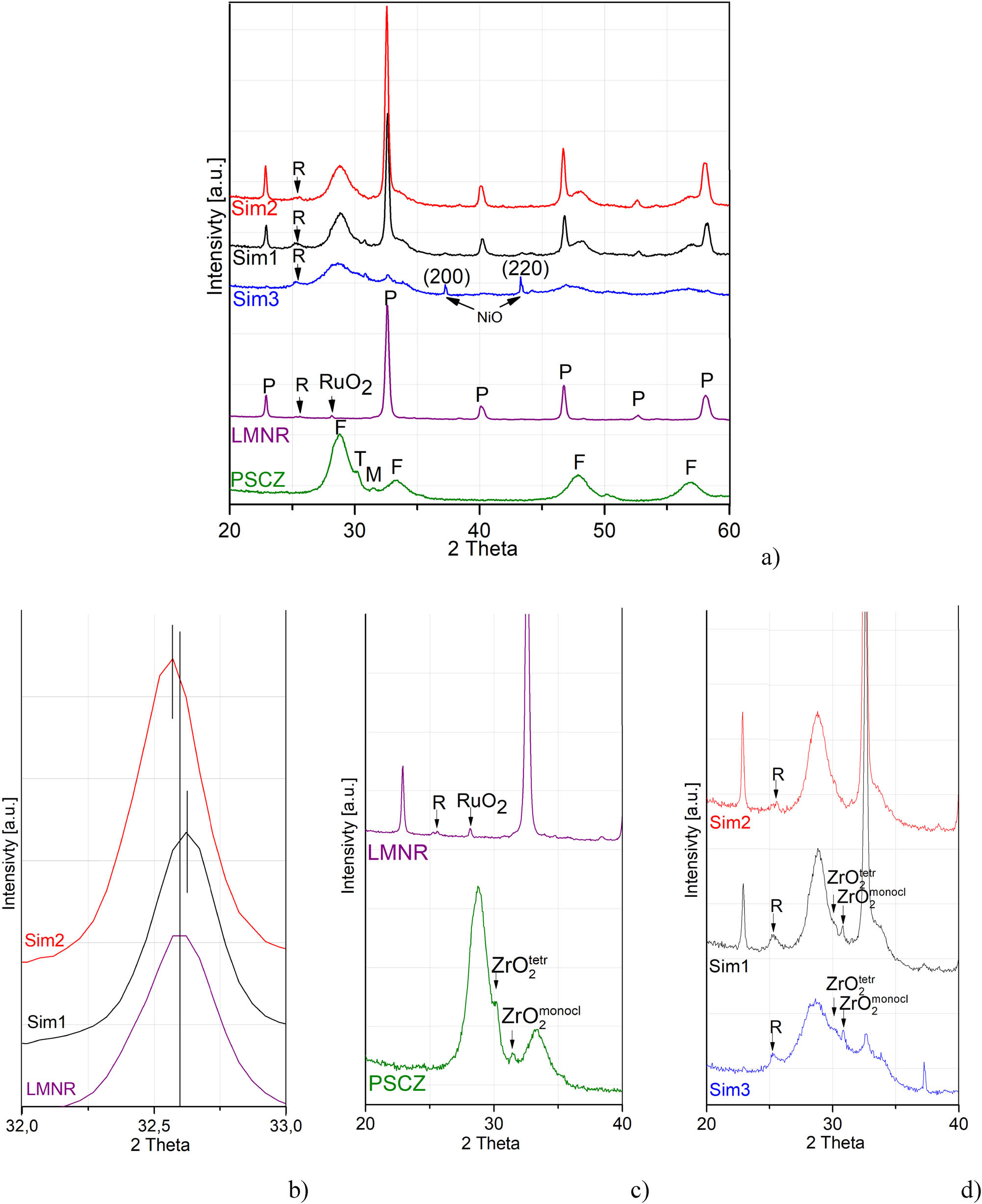
XRD patterns of as-prepared samples. (a) Total patterns and (b–d) their enlarged fragments. P – perovskite phase, F – fluorite phase, R – Ruddlesden-Popper phase, M – monoclinic zirconia phase, T – tetragonal zirconia phase.
The XRD data of composites indicate the formation of perovskite and fluorite phases in Sim1 and Sim2 samples (Figure 1a). Insignificant admixture of both tetragonal and monoclinic ZrO2 for the Sim1 sample are determined by reflections at 30 and 31 degrees, respectively (Figure 1c). No diffraction peaks of ZrO2 were detected for Sim2 sample. In addition, insignificant amounts of La2NiMnO6 impurities were found according to a small peak at 25.5° in all Sim1, Sim2 and Sim3 samples (Figure 1d). The presence or absence of the ruthenium oxide cannot be clarified because main peaks of this phase are superimposed on the peaks of fluorite at 28 and 35 degrees. Moreover, the perovskite peaks in Sim1 are shifted to larger angles (indicates a decrease in the lattice parameter) and the perovskite peaks in Sim2 are shifted to the region of smaller angles due to the increase in the lattice parameter (Figure 1b). This implies redistribution of cations between perovskite and fluorite phases being specific for each preparation method. Also, perovskite peaks are more intense in Sim2 than in Sim1. This suggests a more disordered perovskite structure in Sim1 nanocomposite, which agrees with TPR-H2 results (see Figure 5 below). This can be explained by a smaller sizes of perovskite particles in Sim1 nanocomposite whose sintering is hampered by the presence of highly dispersed fluorite particles (Table 1) in the mixture obtained after perovskite polymeric precursor decomposition as well as perovskite structure disordering at interfaces between perovskite–fluorite nanoparticles. This is apparently accompanied by redistribution of cations between phases, leading to shift of perovskite diffraction peaks as stated earlier.
The Sim3 sample exhibits broad peaks corresponding to the fluorite structure, while perovskite peaks are practically absent. The broad peak at 29° corresponding to the mixed fluorite phase shifts to smaller 2θ angles while peaks corresponding to tetragonal and monoclinic zirconia phases appear (Figure 1c). This suggests that large La cations are incorporated into the fluorite phase increasing its lattice parameter, and hence, the perovskite structure forms only in a trace amount. A well-crystallized nickel oxide phase is present as well (Figure 1a). Since reflections corresponding to manganese oxides are not observed, Mn cations are apparently present as amorphous oxidic layers on the surface of fluorite phase although their inclusion into the bulk of fluorite particles is possible as well.
3.1.2 HR TEM
The HR TEM images (Figure 2) of Sim1 confirm the formation of well-crystallized perovskite and fluorite phases and show a developed interphase between them. For Sim2, the nanodomain structure of both fluorite and perovskite particles is observed. Due to a large size of perovskite particles and their rather weak interaction with smaller fluorite particles in the process of their ultrasound dispersed mixture drying and calcination at 700°C, perovskite and fluorite contacts are less frequent than in Sim1 sample. Sim3 morphology can be described as a mixture of crystallized phases – fluorite phase nanoparticles, Zr oxides, layered particles with a structure close to the perovskite phase and as well as amorphous phases (Figure 2c). Crystallized NiO particles were not detected in Figure 2c; however, Figure 3b with the EDX analysis of another Sim3 sample area shows the uneven spatial distribution of nickel and a large particle of NiO. Results of EDX mapping for perovskite elements in nanocomposites are presented in Figure 3, and the analysis of the elemental composition in related areas is presented in Table 2. Based on these results, in micron-size areas, the highest concentration of elements corresponding to the perovskite phase is observed for Sim1 sample as expected. In small areas ∼300 nm, nonuniformity of element distribution corresponding to both phases is observed for all samples. For Sim3 sample, tremendous nonuniformity of Ni distribution is revealed, indicating its segregation as rather big (up to micron size) NiO particles in some regions.
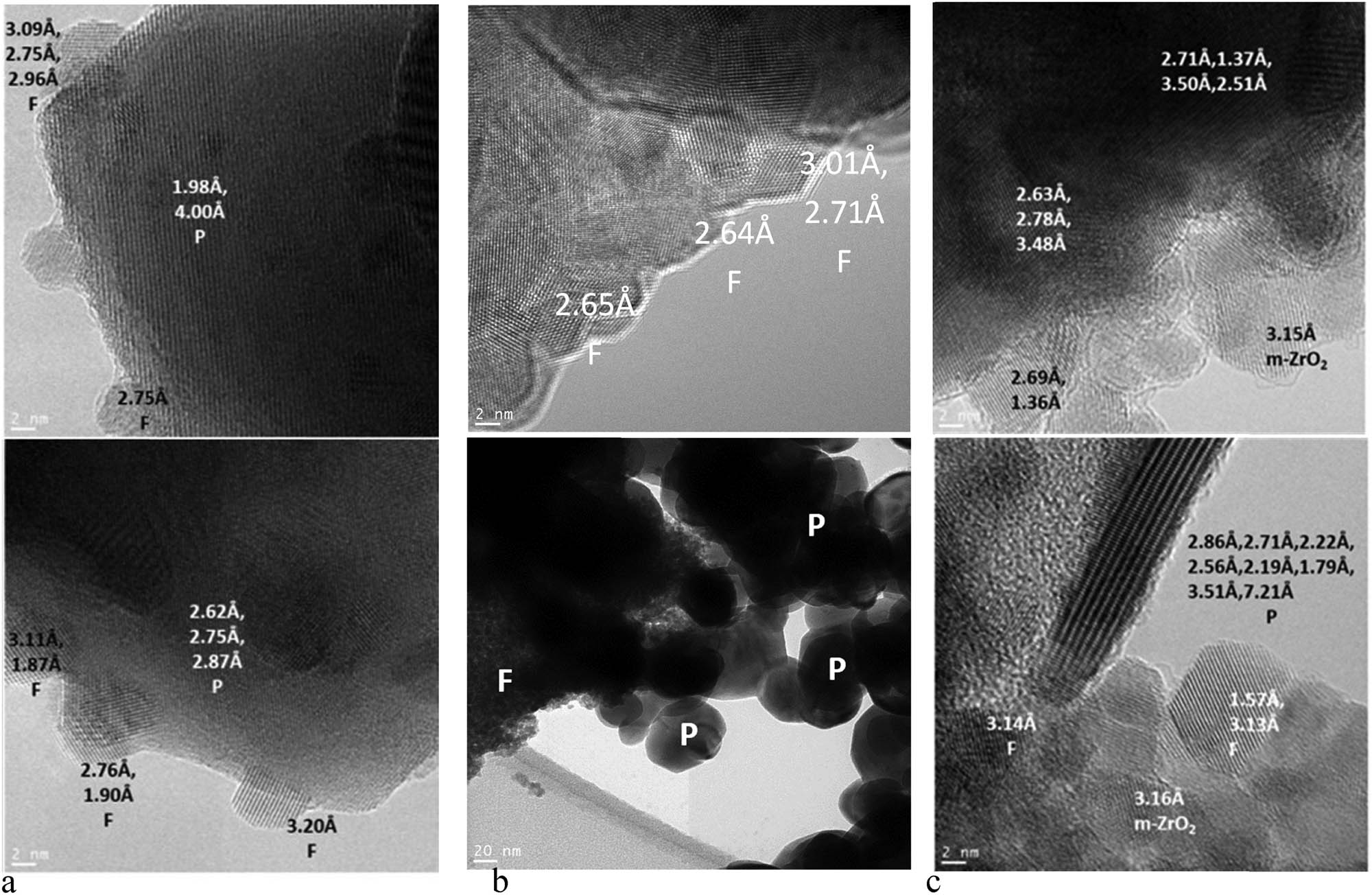
HR TEM images of as-prepared (a) Sim1, (b) Sim2 and (c) Sim3 (P – perovskite, F – fluorite) samples.
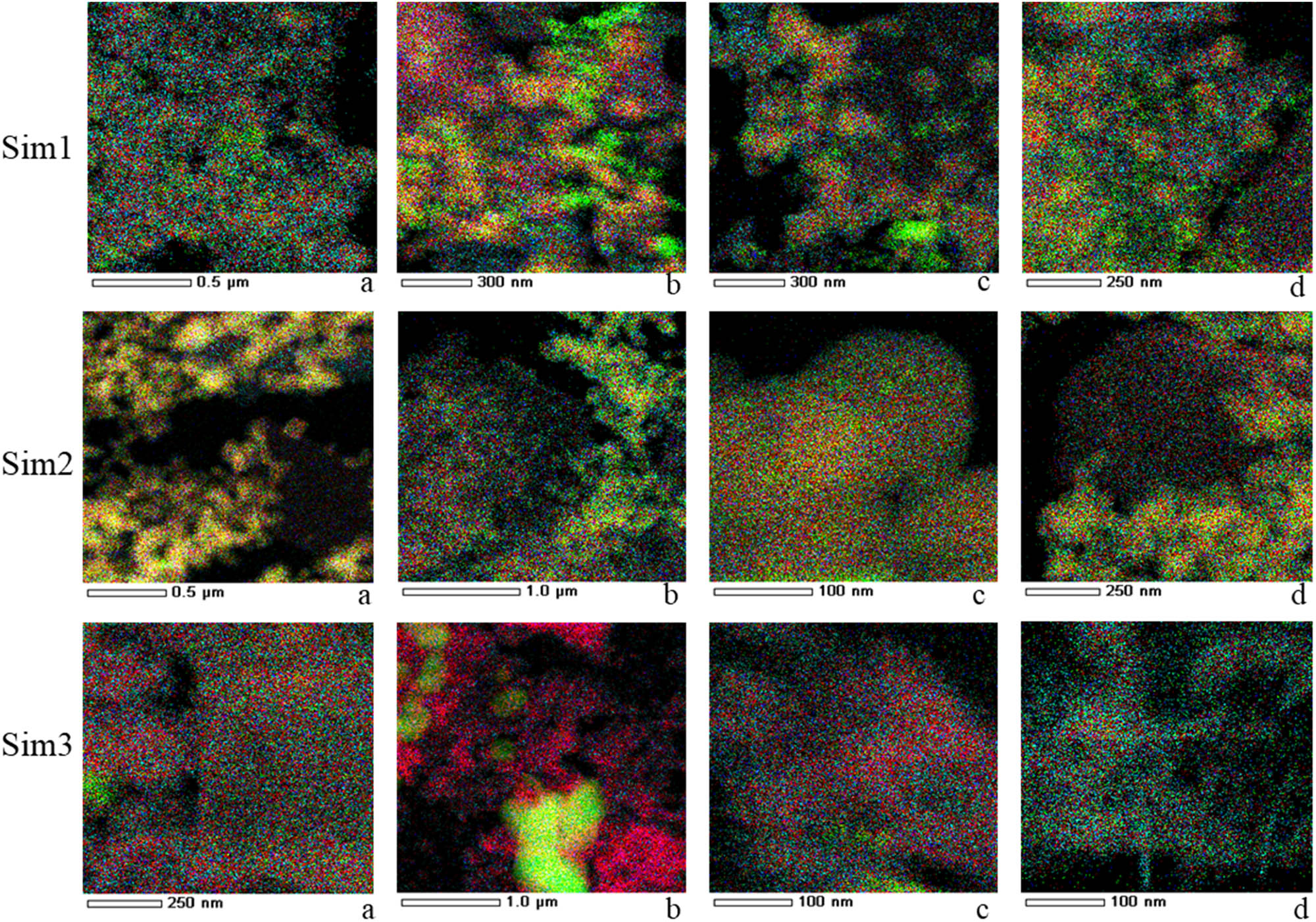
Elemental mapping superposition for Sim1, Sim2 and Sim3 samples. La – light blue, Mn – red and Ni – green. a,b,c,d-different regions of the same sample with their composition given in Table 2.
Elemental composition of nanocomposite samples in regions a–d presented in Figure 3
| Sample | Concentration, at% | ||||||
|---|---|---|---|---|---|---|---|
| La | Mn | Ni | Pr | Sm | Ce | Zr | |
| Sim1_a | 50.55 | 26.32 | 11.67 | 6.29 | 3.80 | 0.00 | 1.38 |
| Sim1_b | 38.04 | 22.85 | 32.16 | 4.30 | 2.41 | 0.00 | 0.23 |
| Sim1_c | 30.8 | 18.62 | 17.19 | 7.09 | 6.52 | 10.60 | 9.15 |
| Sim1_d | 45.02 | 16.07 | 13.80 | 7.34 | 5.79 | 6.57 | 5.41 |
| Sim2_a | 53.21 | 20.71 | 19.00 | 3.48 | 1.04 | 0.00 | 2.55 |
| Sim2_b | 17.32 | 11.87 | 6.29 | 8.47 | 10.49 | 23.65 | 21.91 |
| Sim2_c | 48.50 | 23.16 | 21.33 | 3.19 | 1.03 | 0.00 | 2.79 |
| Sim2_d | 32.93 | 16.4 | 13.40 | 6.52 | 6.23 | 12.15 | 12.36 |
| Sim3_a | 36.26 | 18.06 | 0.00 | 8.51 | 8.33 | 13.94 | 14.90 |
| Sim3_b | 14.36 | 7.67 | 59.75 | 3.65 | 3.00 | 5.35 | 6.21 |
| Sim3_c | 36.07 | 17.96 | 0.00 | 8.72 | 7.63 | 13.42 | 16.20 |
| Sim3_d | 37.33 | 16.08 | 0.00 | 9.01 | 7.24 | 14.52 | 15.81 |
Thus, based on the XRD and TEM results of the nickel distribution uniformity and the extension of the perovskite–fluorite interface (which is known as fast oxygen transport pathway that helps to provide bifunctional mechanism of fuels reforming and minimize coke formation), it can be assumed that, from the point of view of catalytic activity, the method used for Sim1 nanocomposite preparation could be the most promising one.
3.2 Surface properties
XPS. Original XPS spectra with descriptions are given in Supplementary materials (Figures S1–S4). It should be noted that the La3d spectrum (La3d3/2 850–860 eV) overlaps with the Ni2p3/2 spectrum (850–860 eV), which significantly complicates the identification of nickel [27,28]. For the catalysts under study, no photoelectronic peaks related to the Ni2p spectrum of nickel were found in the La3d spectrum even after reduction in hydrogen at 400°C. Treatment in hydrogen led to the removal of lanthanum carbonates, and the La3d spectra shifted noticeably, but no sharp peaks of metallic nickel were observed.
The relative concentrations (atomic ratios) of elements in the near-surface layer, determined on the basis of XPS data, are presented in Table 3. Based on Table 3, in the sequence of Sim1–Sim2–Sim3 samples relative concentration of Mn cations corresponding to domains of the perovskite phase decreases, which agrees with the EDX data. The binding energies are presented in Table 4. The analysis of peaks shapes and positions given in Supplementary materials revealed that La, Sm and Mn cations are in 3+ state. For Zr cations, Zr3d5/2 peak is shifted to lower values (∼181.5 eV) as compared with that in ZrO2 (up to 183.2 eV) [29,30], which is explained by the effect of doping Pr and Sm cations. Pr cations are mainly (up to 85%) present in 4+ state, while three states of Ru are found: metallic (up to 50%) and two cationic states 4+ and 6+.
Atomic ratios of elements in the near-surface layer
| Sample | PrSmCeZr | LaMnNiRu | [La]/[Zr] | [Mn]/[Zr] | |||
|---|---|---|---|---|---|---|---|
| [Pr]/[Sm] | [Ce]* | [Pr]* | [Sm]* | [Ru]** | |||
| Sim1 | 2.29 | 1.86 | 1.14 | 0.50 | 0.10 | 11.72 | 1.83 |
| Sim2 | 2.15 | 0.93 | 0.62 | 0.29 | 0.11 | 3.55 | 0.94 |
| Sim3 | 2.17 | 1.26 | 0.68 | 0.31 | 0.06 | 3.92 | 0.73 |
*The ratios are normalized to [Zr].
**The ratios are normalized to [La].
Binding energies (eV)
| Sample | Zr3d5/2 | Ce3d3/2-u″′ | Pr3d5/2 | Sm3d5/2 | Ru3d5/2 | La3d5/2 | C1s |
|---|---|---|---|---|---|---|---|
| Sim1 | 181.5 | 916.7 | 933.6 | 1083.0 | 280.3 | 835.2 | 285.0 |
| 281.4 | |||||||
| 282.9 | |||||||
| Sim2 | 181.5 | 916.7 | 933.3 | 1082.7 | 280.2 | 835.2 | 284.9 |
| 281.4 | |||||||
| 282.9 | |||||||
| Sim3 | 181.5 | 916.7 | 933.6 | 1083.0 | 280.3 | 834.6 | 284.8 |
| 281.4 | |||||||
| 282.8 |
CO chemisorption. CO TPD spectra for Sim nanocomposites are shown in Figure 4. According to the results of FTIRS studies of CO adsorption on catalysts containing clusters of metals/alloys on complex oxides [21,22], bridging and terminal carbonyls bound with metal sites can be desorbed up to room temperature. Peaks at higher temperatures correspond to decomposition of formates, hydroxocarbonates or carbonates formed due to migration of carbonyl complexes from the metal sites to neighboring support sites [21,22,31]. Since these peaks are very weak for Sim1 sample with the surface mainly presented by perovskite layers, this implies association of formates/carbonates with Ce cations [31] present in higher amounts on the surface of Sim2 and Sim3 samples (Table 2). Carbonyls bound with coordinatively unsaturated cations of supports are removed by desorption even at 77 K [16,21,22], so their coverage was negligible at −40°C after purging by He, which was checked in special experiments. Hence, peaks corresponding to CO desorption from metal surface sites in studied nanocomposites are situated in the temperature range from −20 to +30°C (Table 5), and the surface coverage by these sites does not exceed percent of monolayer. Although CO coverage by metal sites can be higher in the presence of CO in the gas phase [21,22], in our case, the most important is the relative concentration of these sites for studied nanocomposites.
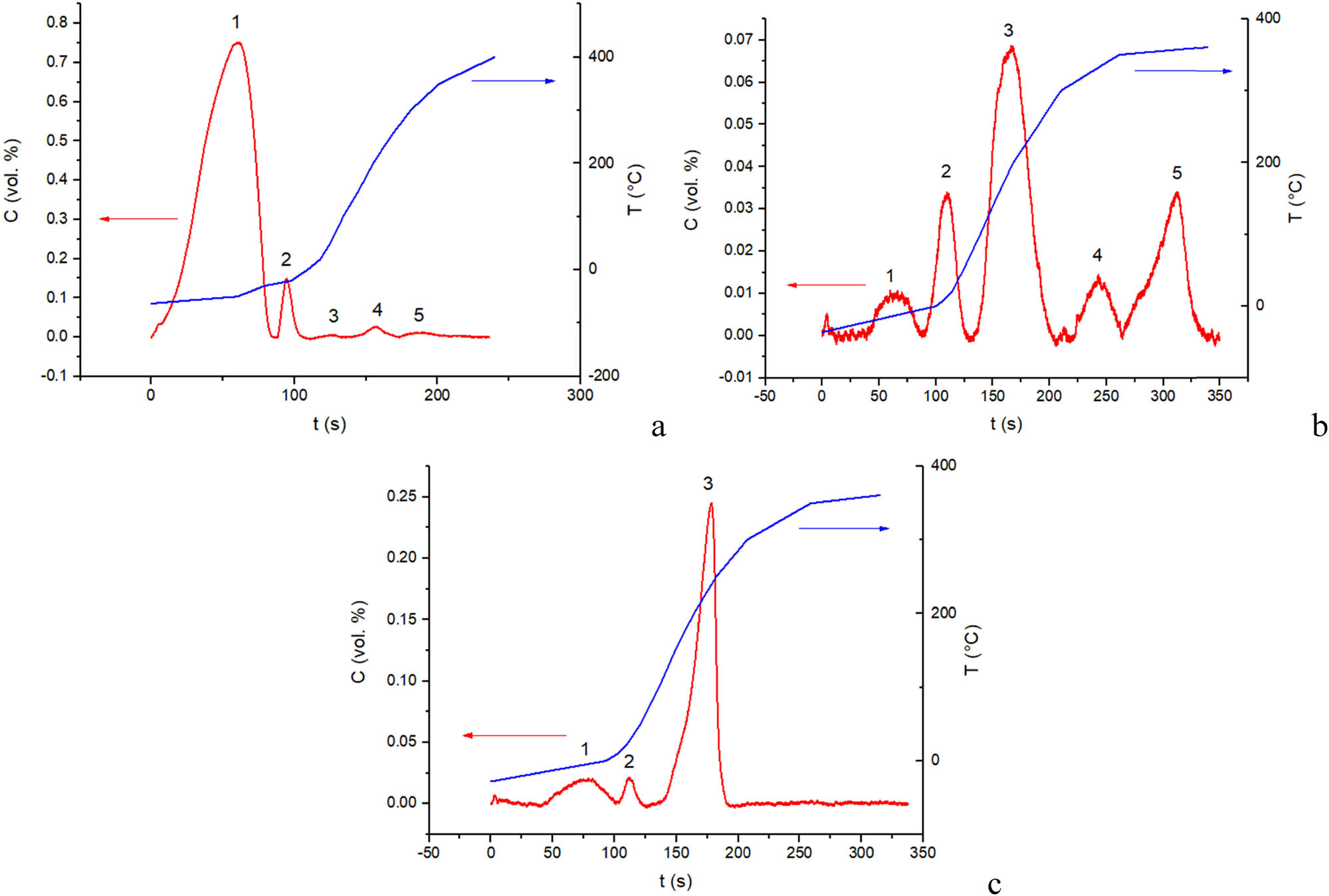
Temperature-programmed CO desorption from the surface of Sim1 (a), Sim2 (b) and Sim3 (c) nanocomposites. Red line – CO concentration (left axis), blue line – temperature (right axis).
Estimation of the number of surface metal sites by amount of CO desorbed at low temperatures
| Sample | T max (°C) | nCO (10−6 mol/gcat) |
|---|---|---|
| Sim1 | −20 | 3.4 |
| Sim2 | 10 | 1.9 |
| Sim3 | 30 | 0.55 |
Hence, the highest concentration of these sites for Sim1 agrees with EDX and XPS data on the highest content of surface cations corresponding to perovskite phase containing Ni and Ru (vide supra) and is explained by specificity of this nanocomposite preparation.
The lowest T max for Sim1 sample can be explained by domination of terminal carbonyls due to a small size of Ni–Ru clusters and their strong interaction with the reduced perovskite support [21,22]. The highest T max for Sim3 sample can be explained by domination of bridging carbonyls due to the presence of large Ni particles generated by reduction of segregated NiO particles weakly interacting with the fluorite support (vide supra) [21,22].
3.3 Reducibility
TPR-H2 patterns are shown in Figure 5. LMNR perovskite reduction curve has three main regions. At temperatures below 300°C, there are three overlapping peaks corresponding to the reduction of Ru3+ to Ru0, Ni3+ to Ni2+ and Mn4+ to Mn3+. The broad peak with a maximum at 562°C corresponds to the reduction of Ni2+ to metallic nickel. The high-temperature peak of Mn2+ cation reduction into metallic manganese would not happen for manganese oxides below 900°C [23,24]. The PSCZ pattern shows the reduction profile typical for this type of oxide: there is a broad shoulder at 450° associated with the removal of surface forms of oxygen, and a broad peak at 580° followed by a plateau up to the highest temperatures, which indicates the reduction of bulk oxygen [20]. Sim1 and Sim2 samples have a similar behavior under reduction, Sim1 being more reactive in general. Ruthenium cations reduction begins at 202°C. This peak overlaps with peaks of Ni3+ to Ni2+ and Mn4+ to Mn3+ reduction with the maxima at 235°C for Sim1 and 262°C for Sim2, respectively [24,25]. The high-temperature peaks for Sim1 and Sim2 samples, as for LMNR, located at 775 and 795°C, respectively, correspond to the typical reduction of Mn3+ to Mn2+ in the perovskite structure [13,14]. The reduction of Ni2+ to metallic nickel occurs in the temperature range 400–500°C as evidenced by the wide peak in this area.
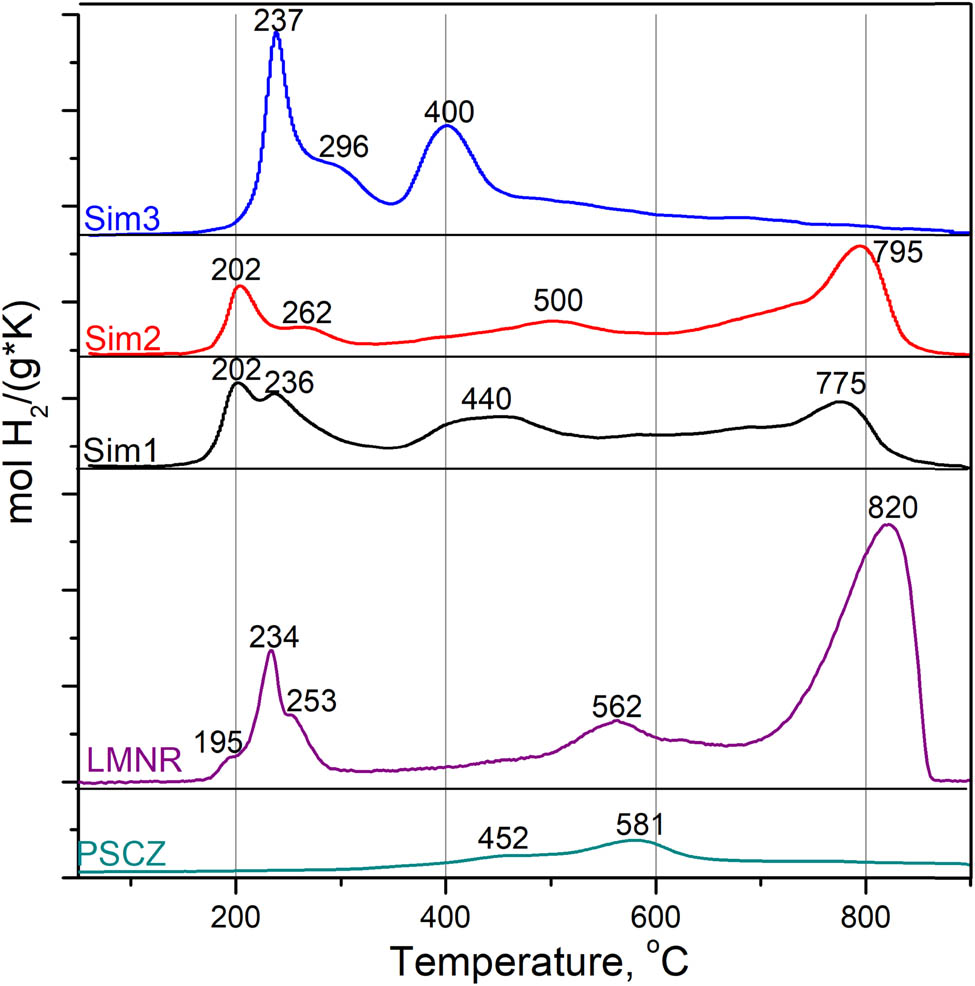
The TPR-H2 profiles of the LMNR and PSCZ, Sim1, Sim2 and Sim3 samples.
The TPR-H2 profile for Sim3 looks typical for a mixture of Mn x O y and NiO oxides (all reduction peaks are below 500°C). There are two overlapped peaks with maxima at 237 and 296°C that can be attributed to the two-step reduction of MnO2: the first step corresponds to the reduction of MnO2 to Mn3O4 and the second step indicates the further reduction of Mn3O4 to MnO. This result is in good agreement with the TPR-H2 results of MnO2 reported in the literature [23]. At temperature higher than 400°C, the behavior is similar to PSCZ fluorite, where at these temperatures, the reduction plateau corresponds to the reduction of bulk oxygen of the oxide [10]. The absence of Mn2O3 peaks in the X-ray diffraction pattern and presence of classical for manganese oxide reduction peaks on the TPR-H2 curves is explained by its amorphous state.
3.4 Catalytic properties in ethanol steam reforming
The hydrogen yield together with the selectivities for CO and CO2 are shown in Figure 6. At temperatures above 600°C for Sim1 and Sim3 samples and above 650°C for Sim2 sample, the main reaction products are H2, CO and CO2, and the ethanol conversion remains stable at 100%.
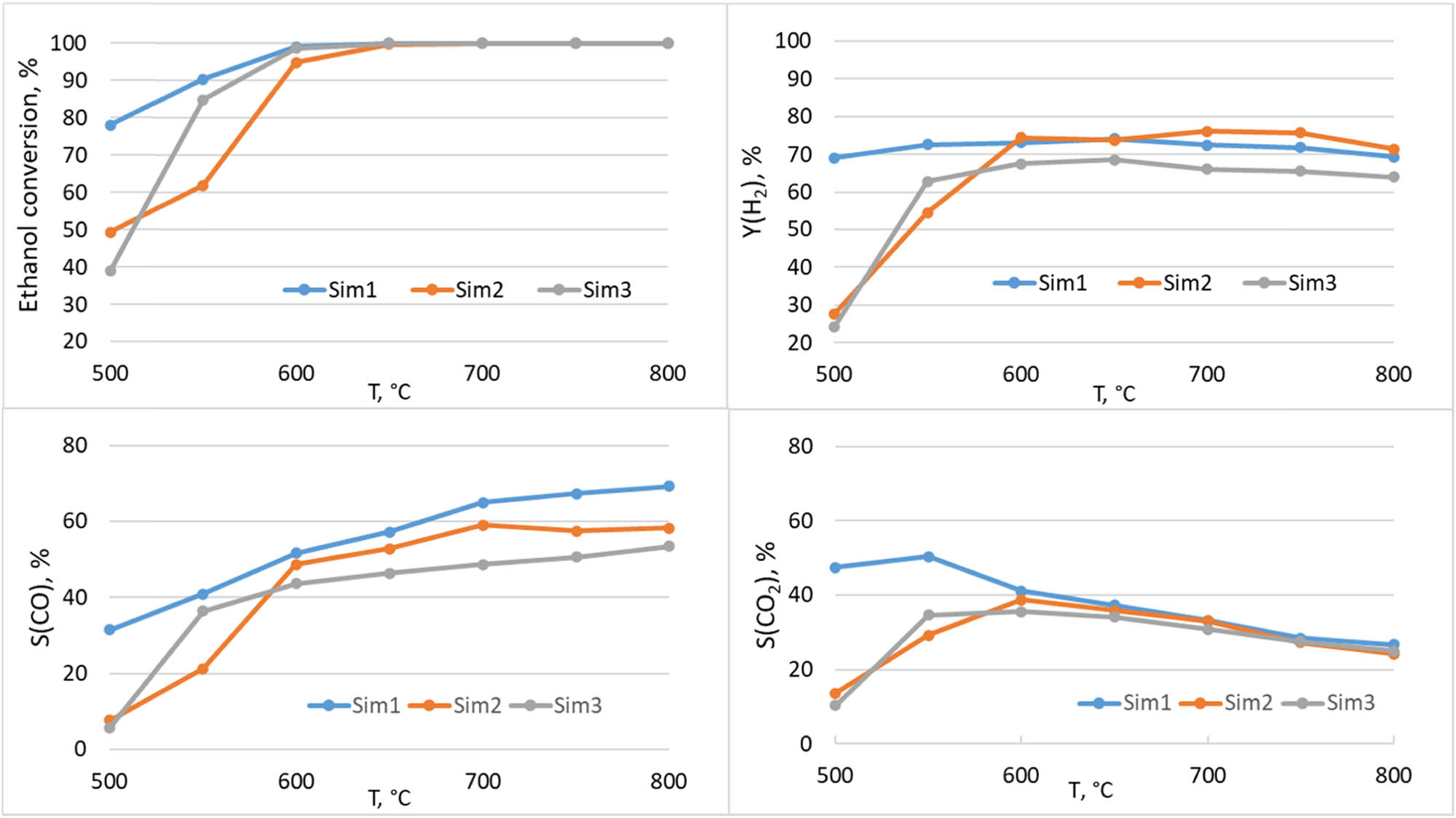
Temperature dependence of ethanol conversion, hydrogen yield, CO and CO2 selectivities for Sim1, Sim2 and Sim3 in ethanol steam reforming reaction.
For temperatures below 650°C, the kinetic parameters of the reaction were calculated. The effective reaction rate constant k eff, m−2 s−1, was calculated in the approximation that the reaction is of the first order in ethanol, taking into account the specific surface area of samples according to the formula [16]:
where X is ethanol conversion; τ is the contact time; s,
In accordance with the values obtained, the following regularities of catalytic activity were revealed. It was shown (Figure 7a) that the activation energy increases in a row Sim1 < Sim2 < Sim3 together with a corresponding decrease in the initial reaction rate, which indicates a decrease in the specific catalytic activity from Sim1 to Sim3 catalyst. The higher activity of Sim1 sample prepared by the sequential polymeric method is consistent with its structure. By using this synthesis method, the composite structure is formed in such a way that the perovskite phase with the active components (nickel and ruthenium) is finely dispersed and distributed mainly on the fluorite oxide surface, which helps to avoid the problem of blocking the active component in the bulk of the oxide. It results in a higher surface density of active metal sites as revealed by CO chemisorption. The specific catalytic activity of nanocomposites at 500°C (Figure 7a) apparently correlates with the number of surface sites estimated by CO chemisorptions (Table 5).
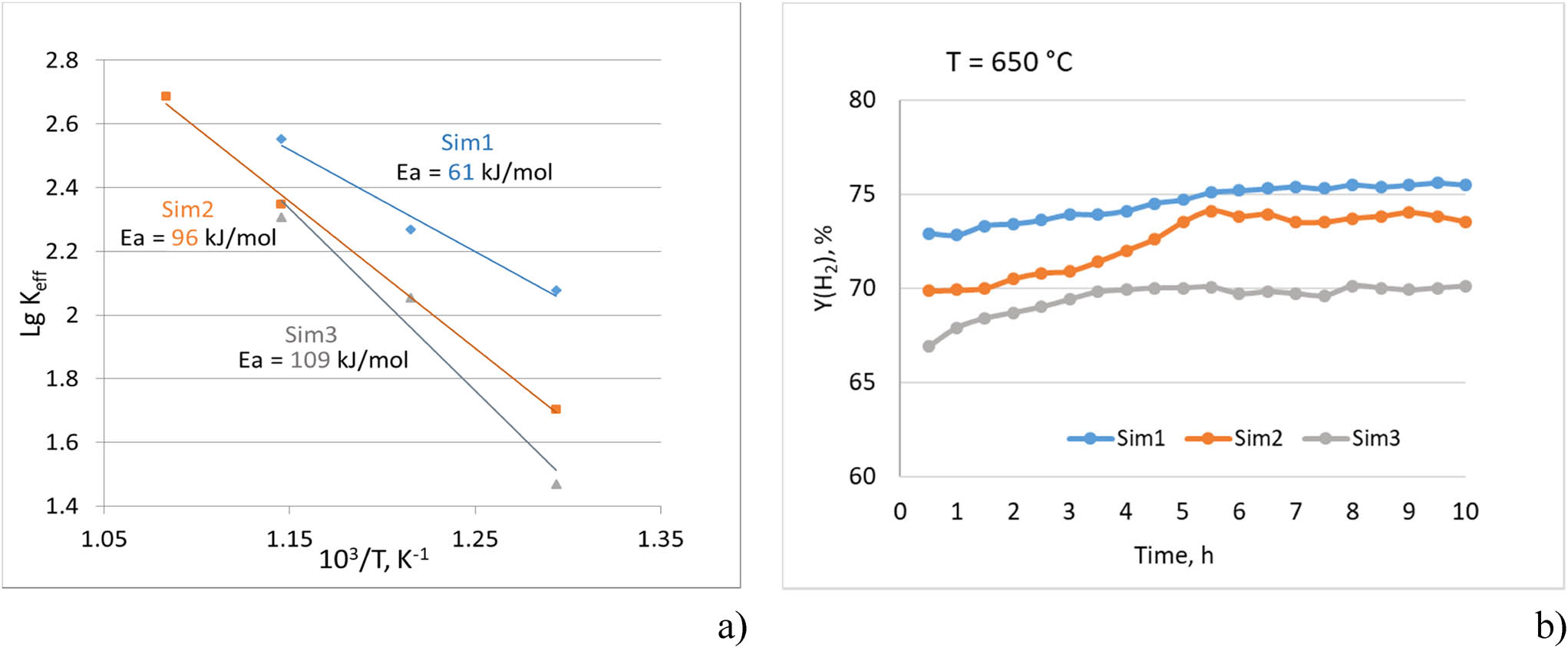
(a) Linearized Arrhenius equation and activation energy E a and (b) hydrogen yield at 650°C in time-on-stream ethanol steam reforming tests.
After temperature-programmed ethanol steam reforming experiments, the catalysts were oxidized to remove residual carbon that can be possibly formed at low temperatures and reduced at 800°C, and long-term tests were carried out at a constant temperature of 650°C. It was shown (Figure 7b) that under these conditions, all catalysts do not lose their activity within 10 h, maintaining the full conversion of ethanol and a constant level of hydrogen yield above 70%. Tests after the reaction did not reveal carbon deposits for all samples, which confirms a high oxidizing capacity of the support’s oxides demonstrated by their TPR-H2 patterns (Figure 5).
4 Conclusion
In this study, catalysts precursors based on perovskite–fluorite nanocomposites with the general formula [LaMn1−x B x O3+δ/Ln1−y Zr y O2] (1:1 by mass), B = Ni, Ru, Ln = Pr, Sm, Ce were synthesized by three different methods. Two synthesis methods — sequential polymeric method (formation of Ni- and Ru-containing perovskite from a polymer matrix in the presence of already formed fluorite oxide) and ultrasonic dispersion of as-prepared complex oxides in isopropanol with addition of surfactant — provide formation of nanocomposites with a developed interphase between phases. In the one-pot synthesis method from a polymer containing all cations, the perovskite phase is not formed due to La incorporation into the fluorite matrix, with inclusions of amorphous Mn oxides and NiO phase being present. As the result, the highest concentration of surface metal sites in reduced catalysts was revealed for nanocomposite prepared by the sequential polymeric method, while the lowest concentration was observed for samples prepared by the one-pot method. Catalytic activity of nanocomposites in ethanol steam reforming correlates with the surface density of metal sites. They are stable to coking and provide full conversion of ethanol and hydrogen yield above 70% at 650°C for 10 h.
-
Funding information: This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis project 0239-2021-0005 as well as by M. Kh. Dulaty Taraz Regional University, Taraz, Kazakhstan.
-
Author contribution: S. N.: investigation and writing; M. A.: investigation and writing; A. I.: investigation.; T. K.: investigation; A. S.: investigation; V. K.: methodology; V. R.: investigation; A. K.: investigation, B. M.: supervision; V. S: supervision and editing.
-
Conflict of interest: Vladislav Sadykov, who is the co-author of this article, is a current Editorial Board member of Open Chemistry. This fact did not affect the peer-review process. The authors declare no other conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
[1] Wang M, Wang G, Sun Z, Zhang Y, Xu D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Glob Energy Interconnect. 2019;2(5):436–43. 10.1016/j.gloei.2019.11.019.Search in Google Scholar
[2] Edwards PP, Kuznetsov VL, David WIF, Brandon NP. Hydrogen and fuel cells: towards a sustainable energy future. Energy Policy. 2008;36:4356–62. 10.1016/j.enpol.2008.09.036.Search in Google Scholar
[3] Bae J, Lee S, Kim S, Oh J, Choi S, Bae M, et al. Liquid fuel processing for hydrogen production: a review. Int J Hydrog Energy. 2016;41:19990–20022. 10.1016/j.ijhydene.2016.08.135.Search in Google Scholar
[4] Laosiripojana N, Assabumrungrat S. Catalytic steam reforming of methane, methanol, and ethanol over Ni/YSZ: the possible use of these fuels in internal reforming SOFC. J Power Sources. 2007;163:943–51. 10.1016/j.jpowsour.2006.10.006.Search in Google Scholar
[5] Xin X, Liu L, Liu Y, Zhu Q. Novel perovskite-spinel composite conductive ceramics for SOFC cathode contact layer. Int J Hydrog Energy. 2018;43:23036–40. 10.1016/j.ijhydene.2018.10.159.Search in Google Scholar
[6] Duranti L, Sora IN, Zurlo F, Luisetto I, Licoccia S, Di Bartolomeo E. The role of manganese substitution on the redox behavior of La0.6Sr0.4Fe0.8Mn0.2O3-δ. J Eur Ceram Soc. 2020;40:4076–83. 10.1016/j.jeurceramsoc.2020.04.017.Search in Google Scholar
[7] Liu Y, Jia L, Li J, Chi B, Pu J, Li J. High-performance Ni in-situ exsolved Ba(Ce0.9Y0.1)0.8Ni0.2O3-δ/Gd0.1Ce0.9O1.95 composite anode for SOFC with long-term stability in methane fuel. Compos B Eng. 2020;193:108033. 10.1016/j.compositesb.2020.108033.Search in Google Scholar
[8] Frolova-Borchert Y, Sadykov V, Alikina G, Lukashevich A, Moroz E, Kochubey D, et al. Nanocomposites comprised of doped cerium dioxide and lanthanum manganite for syngas production. Solid State Ion. 2006;177(26–32):2533–8. 10.1016/j.ssi.2006.02.027.Search in Google Scholar
[9] Sadykov V, Mezentseva N, Alikina G, Bunina R, Rogov V, Krieger T, et al. Composite catalytic materials for steam reforming of methane and oxygenates: Combinatorial synthesis, characterization and performance. Catal Today. 2009;145:127–37. 10.1016/j.cattod.2008.04.034.Search in Google Scholar
[10] Sadykov V, Mezentseva N, Alikina G, Bunina R, Pelipenko V, Lukashevich A, et al. Nanocomposite catalysts for internal steam reforming of methane and biofuels in solid oxide fuel cells: Design and performance. Catal Today. 2009;146:132–40. 10.1016/j.cattod.2009.02.035.Search in Google Scholar
[11] Buciuman FC, Patcas F, Menezo JC, Barbier J, Hahn T, Lintz HG. Catalytic properties of La0.8A0.2MnO3 (A = Sr, Ba, K, Cs) and LaMn0.8B0.2O3 (B = Ni, Zn, Cu) perovskites 1. Oxidation of hydrogen and propene. Appl Catal B. 2002;35:175–83. 10.1016/S0926-3373(01)00250-8.Search in Google Scholar
[12] Wei T, Jia L, Zheng H, Chi B, Pu J, Li J. LaMnO3-based perovskite with in-situ exsolved Ni nanoparticles: a highly active, performance stable and coking resistant catalyst for CO2 dry reforming of CH4. Appl Catal A. 2018;564:199–207. 10.1016/j.apcata.2018.07.031.Search in Google Scholar
[13] Pietri E, Barrios A, Goldwasser MR, Pérez-Zurita MJ, Cubeiro ML, Goldwasser J, et al. Optimization of Ni and Ru catalysts supported on LaMnO3 for the carbon dioxide reforming of methane. Stud Surf Sci Catal. 2000;130:3657–62. 10.1016/S0167-2991(00)80591-1.Search in Google Scholar
[14] Wang Y, Zheng Y, Wang Y, Wang H, Zhu X, Wei Y, et al. Evaluation of Fe substitution in perovskite LaMnO3 for the production of high purity syngas and hydrogen. J Power Sources. 2020;449:227505. 10.1016/j.jpowsour.2019.227505.Search in Google Scholar
[15] Pavlova S, Kapokova L, Bunina R, Alikina G, Sazonova N, Krieger T, et al. Syngas production by CO2 reforming of methane using LnFeNi(Ru)O3 perovskites as precursors of robust catalysts. Catal Sci Technol. 2012;2:2099–108. 10.1039/C2CY20054F.Search in Google Scholar
[16] Sadykov VA, Arapova MV, Smal EA, Pavlova SN, Bobrova LN, Eremeev NF, et al. Nanocomposite catalysts for transformation of biofuels into syngas and hydrogen: fundamentals of design and performance, application in structured reactors and catalytic membranes. Catalysis. 2019;31:216–41. 10.1039/9781788016971-00216.Search in Google Scholar
[17] Pechini MP. Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. Patent 3330697 U.S. 08.1963, patented 11.07.1967.Search in Google Scholar
[18] Scofield JH. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J Electron Spectros Relat Phenom. 1976;8:129–37. 10.1016/0368-2048(76)80015-1.Search in Google Scholar
[19] Fairley N. Available from: www.casaxps.comSearch in Google Scholar
[20] Sadykov V, Mezentseva NV, Alikina GM, Lukashevich AI, Borchert YV, Kuznetsova TG, et al. Pt-supported nanocrystalline ceria-zirconia doped with La, Pr or Gd: factors controlling syngas generation in partial oxidation/autothermal reforming of methane or oxygenates. Solid State Phenom. 2007;128:239–48. 10.4028/www.scientific.net/SSP.128.239.Search in Google Scholar
[21] Sadykov V, Pavlova S, Smal E, Arapova M, Simonov M, Mezentseva N, et al. Structured catalysts for biofuels transformation into syngas with active components based on perovskite and spinel oxides supported on Mg-doped alumina. Catal Today. 2017;293–4:176–85. 10.1016/j.cattod.2017.05.055.Search in Google Scholar
[22] Smal EA, Simonov MN, Mezentseva NV, Krieger TA, Larina TV, Saraev AA, et al. Spinel-type MnxCr3-xO4-based catalysts for ethanol steam reforming. Appl Catal B. 2021;283:119656. 10.1016/j.apcatb.2020.119656.Search in Google Scholar
[23] Ramesh K, Chen L, Chen F, Liu Y, Wang Z, Han YF. Re-investigating the CO oxidation mechanism over unsupported MnO, Mn2O3 and MnO2 catalysts//. Catal Today. 2008;131:477–82. 10.1016/j.cattod.2007.10.061.Search in Google Scholar
[24] Chen H, Li J, Cui W, Fei Z, Tian Q, Liu Q, et al. Precise fabrication of surface-reconstructed LaMnO3 perovskite with enhanced catalytic performance in CH4 oxidation. Appl Surf Sci. 2020;505:144112. 10.1016/j.apsusc.2019.144112.Search in Google Scholar
[25] Zhang C, Zeng K, Wang C, Liu X, Wu G, Wang Z, et al. LaMnO3 perovskites via a facile nickel substitution strategy for boosting propane combustion performance. Ceram Int. 2020;46:6652–62. 10.1016/j.ceramint.2019.11.153.Search in Google Scholar
[26] Sadykov VA, Kuznetsova TG, Frolova-Borchert YV, Alikina GM, Lukashevich AI, Rogov VA, et al. Fuel-rich methane combustion: Role of the Pt dispersion and oxygen mobility in a fluorite-like complex oxide support. Catal Today. 2006;117:475–83. https://doi.org/10.1016/j.cattod.2006.06.017.Search in Google Scholar
[27] Ramana CV, Vemuri RS, Kaichev VV, Kochubey VA, Saraev AA, Atuchin VV. X-ray photoelectron spectroscopy depth profiling of La2O3/Si thin films deposited by reactive magnetron sputtering. ACS Appl Mater Interfaces. 2011;3:4370–3. org/10.1021/am201021m.Search in Google Scholar
[28] Ledford JS, Houalla M, Proctor A, Gercules DM, Petrakis L. Influence of lanthanum on the surface structure and carbon monoxide hydrogenation activity of supported cobalt catalysts. J Phys Chem. 1989;93:6770–7. 10.1021/j100355a039.Search in Google Scholar
[29] Bulavchenko OA, Vinokurov ZS, Afonasenko TN, Tsyrul'nikov PG, Tsybulya SV, Saraev AA, et al. Reduction of mixed Mn–Zr oxides: in situ XPS and XRD studies. Dalton Trans. 2015;44:15499–507. 10.1039/C5DT01440A.Search in Google Scholar
[30] Tsunekawa S, Asami K, Ito S, Yashima M, Sugimoto T. XPS study of the phase transition in pure zirconium oxide nanocrystallites. Appl Surf Sci. 2005;252:1651–6. 10.1016/j.apsusc.2005.03.183.Search in Google Scholar
[31] Jacobs G, Patterson PM, Graham UM, Sparks DE, Burtron H, Davis BH. Low temperature water-gas shift: kinetic isotope effect observed for decomposition of surface formates for Pt/ceria catalysts. Appl Catal A Gen. 2004;269:63–73.10.1016/j.apcata.2004.03.049Search in Google Scholar
© 2021 Symbat Muratbekovna Naurzkulova et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Articles in the same Issue
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

