Abstract
Flavonoids are common bioactive components in plants. Quercetin is the most abundant flavonoid in the human diet, accounting for more than half of the total daily consumption of flavonoids. In this study, adsorption and electrocatalytic activities of quercetin isolated from Zanthoxylum bungeanum on an electrode was studied via homemade electrodes. An in situ UV-Visible thin-layer spectroelectrochemical method was used to study the electrochemical behavior of quercetin in detail and to explore its electrochemical reaction mechanism. This experiment proves that UV-Vis thin-layer spectroelectrochemistry is a feasible way for studying the electrochemical reaction mechanism of flavonoids in plants.
1 Introduction
Zanthoxylum bungeanum is a kind of plant resource with high edible and medicinal value [1,2]. The chemical components of Z. bungeanum mainly include volatile oils, alkaloids, amides, coumarins, and flavones. Flavonoids are a kind of common bioactive component in plants [3,4]. In recent years, quercetin, hyperoside, anisidine, arbutin, and rutin have been isolated from the pericarp of Z. bungeanum [5,6].
Quercetin is the most abundant flavonoid in the human diet, accounting for more than half of the total daily consumption of flavonoids. Ionizing radiation can produce a large number of free radicals and cause a series of free radical reactions, including lipid peroxidation [7,8,9]. Excessive free radicals are harmful and can lead to changes in the cellular structure and the destruction of cellular functions, which may cause cancer, increased ageing, cardiovascular disease, and other health concerns. Quercetin can combine with Fe(iii) and Mn(ii) ions, and its role as an antioxidant may be achieved by affecting the internal balance of metal ions, thus changing their oxidation state in cells [10,11,12,13]. NO is a messenger molecule found in recent years, which is a part of many physiological activities. Excessive NO production in the brain can lead to neurodegenerative diseases. Quercetin can inhibit the production of NO in a dose-dependent manner and thus shows a preventive effect on neurodegenerative diseases. Furthermore, the incubation of 10 mg of quercetin with the HepG2 cell line can effectively inhibit the binding activity of nuclear factor kappa B and protect against injuries induced by H2O2 [14,15,16].
Electrochemical methods have been widely used for studying the antioxidation activity of natural antioxidants; in addition, these methods have been used for the analysis and detection of natural antioxidants [17,18,19]. The main research contents of electrochemical methods can be summarized as the electrochemical detection of flavonoids and the kinetic study of electrode processes. However, the evaluation of the antioxidant activity of natural antioxidants and the interaction between natural antioxidants and natural oxidants have also been reported [20,21]. Cyclic voltammetry, as a simple and fast electrochemical method, has been used by many researchers to detect antioxidants [22,23,24]. Differential pulse voltammetry, oscillopolarography, and flow injection amperometric methods have also been used in the analysis of natural antioxidants [25,26,27]. Choosing a suitable method is the key for improving detection sensitivity. By using electrochemical theories and methods, the reversibility of a reaction can be obtained from the electrochemical data, and the apparent kinetic parameters, such as the number of electron transfers, rate constants, exchange current densities, and transfer coefficients, can be obtained; furthermore, the intermediate particles and products can be detected [28,29].
Spectroelectrochemistry is an interdisciplinary field developed in chronology. A simple electrochemical measurement technique can only obtain indirect information about the interfacial structure and reaction history of the electrode solution [30,31]. The main disadvantage of electrochemical techniques is that they only have pure electrochemical measurements and lack the characteristics of the electrode reaction molecules; thus, there is no useful information about reaction products or intermediates [32]. Spectroelectrochemistry is a method that combines spectroscopy and electrochemical methods to simultaneously measure an electrolytic cell. Generally, the spectroelectrochemical spectrum uses electrochemistry as the excitation signal, and the response of the system to the electrical excitation signal is monitored by the spectroscopic technology [33,34]. The two are closely combined to exert their respective advantages. In this way, a variety of information can be obtained at the same time, which provides a very powerful research method for studying the electrode process mechanism and electrode surface characteristics, along with identifying the intermediates of reaction processes, transient states, and product properties; moreover, certain electrochemical parameters can be measured [35,36].
It is often difficult to study the redox reaction of drugs in vivo. According to the research characteristics of medicinal chemistry, people often choose electrochemical techniques to study the electron transfer properties of drugs for simulating or assisting the exploration of the redox process of drugs in vivo [37,38]. Chemical reactions of drug molecules often occur in multiple steps, and the reaction mechanism is complex; therefore, it is difficult to accurately determine them by general methods [39,40,41]. UV-Vis spectroscopy has unique advantages in identifying reactive substances, especially the transient states and intermediates of the reaction. Therefore, UV-Vis spectroscopy can immediately provide considerable information about reactants, intermediates, and products, thereby making it a powerful method to study electrochemical reactions and mechanisms [42,43]. Many drug molecules have characteristic absorption in the ultraviolet-visible region, which makes it possible to study the properties of drug molecules by spectroelectrochemistry. The thin-layer spectroelectrochemical measurement requires a short electrolysis time, which makes it easy to control the directional electron transfer properties of drug molecules, and the drug distribution in the thin-layer solution is uniform [44,45]. Thin-layer spectroelectrochemistry can also be used to study the adsorption properties of drugs on electrode surfaces.
The purpose of this study is to enhance adsorption and electrocatalytic activities of quercetin on the electrode by using homemade electrodes and to use in situ UV-Visible thin-layer spectroelectrochemical methods to study the electrochemical behavior of quercetin in detail and explore its electrochemical reaction mechanism.
2 Experimental
All reagents were of analytical grade. Paraffin wax and graphite powder were spectrally pure, and other reagents were analytically pure. Quercetin was isolated from Z. bungeanum (inset of Figure 1). When designing a thin-layer electrochemical cell, to avoid modifying the sample cell of the spectrophotometer, a common cuvette was directly used as the cell body. The research electrode should be easily fixed and removed for grinding and cleaning before the experimental test.
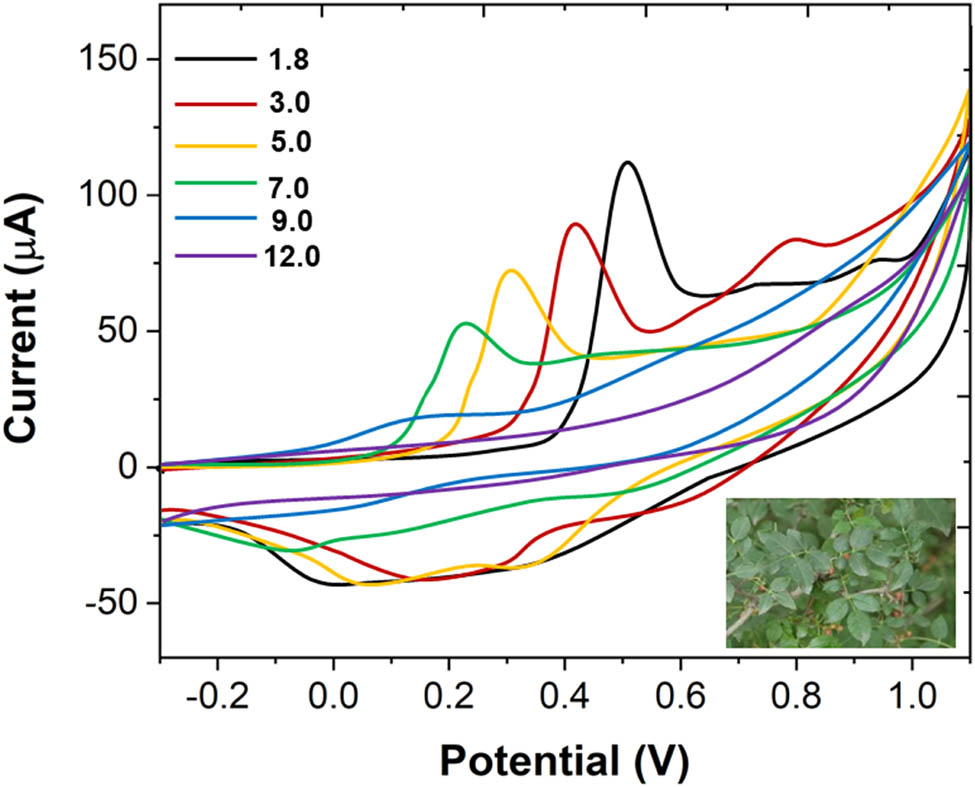
CV curves of quercetin in different pH values of B–R buffer solutions (0.1 M). Scan rate: 50 mV/s.
To obtain the changes in the concentrations of reactants and products in the thin-layer liquid phase during the electrode reaction, the parallel incidence method was adopted, that is, the incident light was projected across the electrode surface in parallel with the electrode surface. For the light path of the Shimadzu UV-Vis 2550 spectrophotometer used in the experiment, the electrode surface should be inserted vertically into the cuvette. A certain slit was formed by a gasket of a certain thickness at the upper and lower ends of the electrode plate, and another baffle was used in the cuvette. This slit formed a thin layer of electrolyte, and the thickness was the thickness of the gasket. The thickness of the thin pool could be adjusted by changing the thickness of the gasket.
A certain amount of quercetin stock solution was taken and diluted with a certain amount of buffer to obtain the required concentration of quercetin solution. A cuvette was filled with a certain amount of quercetin solution. The research electrode was inserted, and the thin cell was filled with solution to drive out air bubbles. The reference electrode and the platinum mesh auxiliary electrode were inserted, and a syringe was used to aspirate excess solution. The cuvette was inserted into the sample cell of the spectrophotometer, and leads of the three electrodes were led out of the test system. The quercetin was scanned by cyclic voltammetry and potentiostatic electrolysis, and the scanning spectrum and absorbance–time curve of the thin-layer cell solution were recorded.
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
Figure 1 shows the large range of CV curves of quercetin in B–R buffer solution (0.1 M) at different pH values. It can be seen from the figure that with the increase in the pH value, redox peak potentials of quercetin are negatively shifted, and the peak current tends to be smaller. When the pH value reaches 12, almost no redox peak appears. When pH = 1.8, the system shows the best CV curve, with a good peak shape and large peak current. This behavior change may be caused by the hydrolysis of quercetin. Therefore, pH of 1.8 was selected for further study.
Figure 2 shows the repeated scanning of the UV-Vis spectrum of the solution in the thin-layer cell during the constant potential oxidation process that was within the potential range of the first oxidation peak. At the beginning of the scanning, there are two maximum absorption peaks at 369 and 255 nm, which were the characteristic absorption peaks of quercetin. The absorption band of the first long wavelength is mainly due to the transition between the highest occupied molecular orbital and the lowest unoccupied molecular orbital (HOMO–LUMO) [46]. Due to the HOMO–LUMO conversion, the electron charge density of the B-ring shifts to the carbonyl bond of the C-ring [47]. Other transformations are the redistribution of charge throughout the molecule. For these compounds, the UV-Vis spectrum cannot distinguish the detailed structure of the molecule.
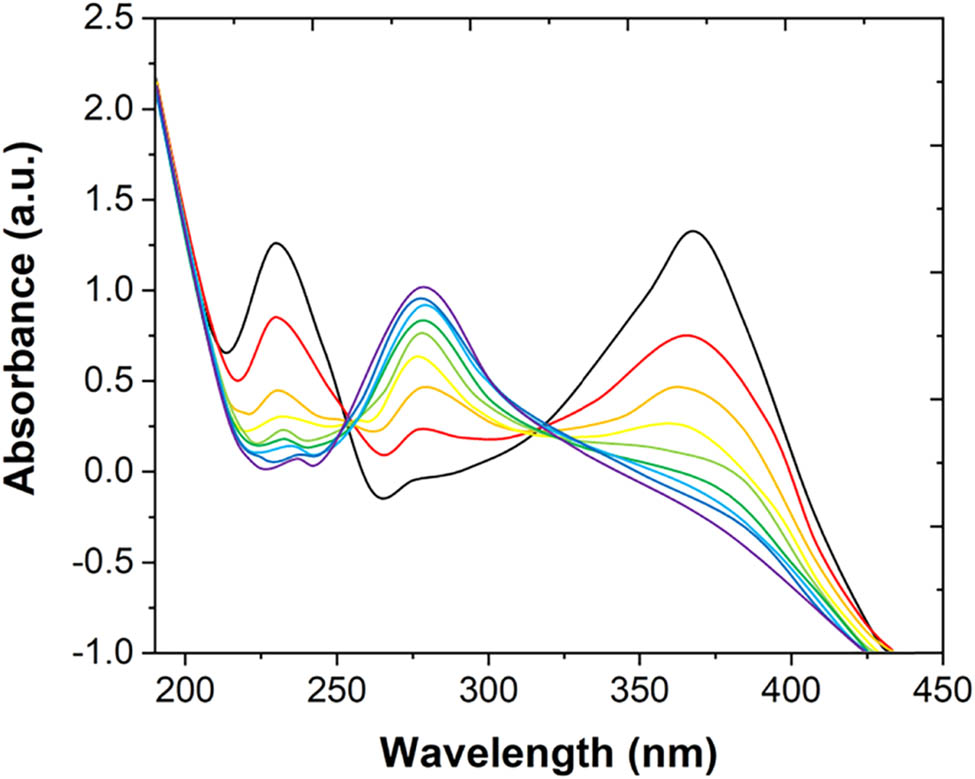
UV absorption spectrum of quercetin in a thin-layer cell during 0.47 V constant potential oxidation.
Figure 3 shows the change in the absorbance of quercetin on the quercetin electrode in the thin-layer cell under open-circuit conditions. As time passes, Abs369nm starts to decrease from the initial maximum value to a limit value, indicating that quercetin has a certain adsorption on the electrode surface. If the concentration of quercetin is increased, the time needed to reach adsorption equilibrium will increase accordingly [48]. When the electrode surface is replaced by a polyethylene plate, Abs369nm did not change over time, indicating that quercetin adsorption is carried out on the electrode surface rather than on the polyethylene plate.
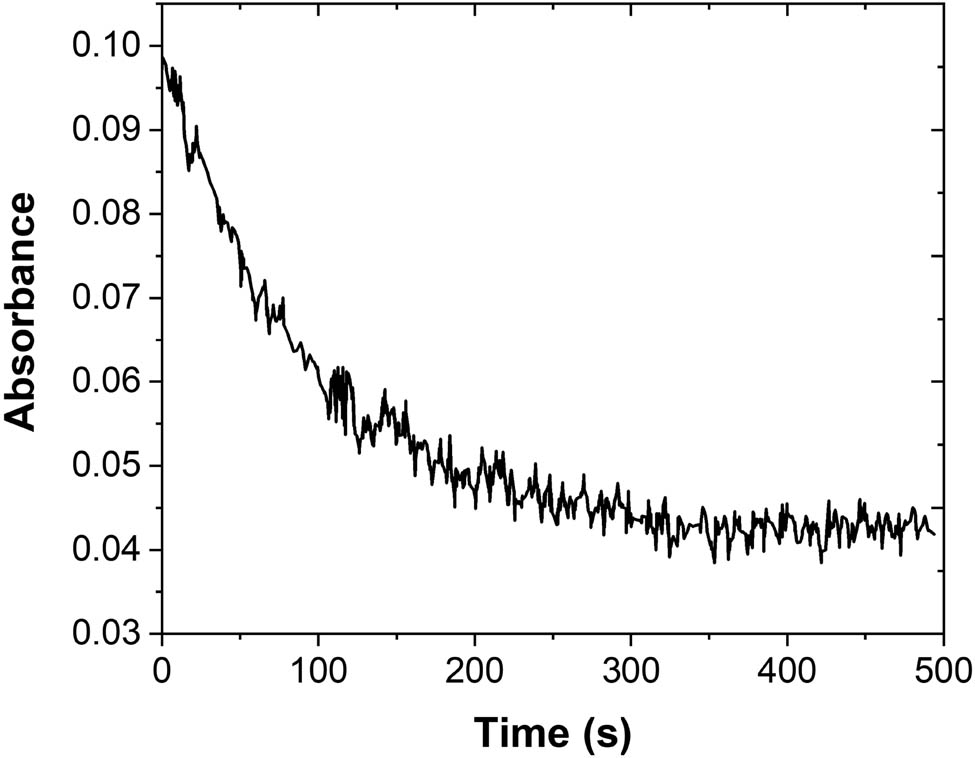
Absorbance time curve of quercetin in a thin-layer cell under the open-circuit condition.
Figure 4 shows the in situ kinetic tests of quercetin and its oxidation products in the thin-layer cell at 369 and 293 nm, respectively. In the course of repeated EMF scanning, the absorbance of the thin-layer solution shows periodic changes at both wavelengths. During forward scanning, Abs369nm decreases with time, while Abs293nm increases, which corresponds to the decrease in the quercetin concentration and the increase in the product concentration. In the back sweep of potential, due to the opposite electrode process, the product is reduced, so the corresponding absorbance change is also opposite to that of the forward sweep. However, the amplitude of the change is smaller than that during forward scanning, especially in the case of a slow scan (Figure 4a). With the increase in the number of scanning cycles, the overall Abs369nm shows a decreasing trend, while the overall Abs293nm shows an increasing trend (Figure 4a and b). At the same time, Figure 4a and b clearly shows that not only quercetin is preadsorbed on the electrode surface before the power is turned on participating in the electron transfer reaction but also quercetin and its products in solution participate in the reaction through the preadsorption and subsequent desorption steps [49,50].

The dual wavelength absorbance time curve in the scanning process of quercetin cyclic potential: (a) scanning range: 0.3–0.5 V; quercetin concentration: 50 μm; pH = 1.8; scanning speed: 5 mV/s; number of cycles: 8. (b) Scanning speed: 50 mV/s; number of cycles: 16. (c) Scanning speed: 5 V/s; number of cycles: 1,500.
It is generally believed that for a quasi-reversible reaction, the reversibility of the system will decrease with the increasing scanning speed. Comparing Figure 4a and b, it can be seen that the studied reaction has high reversibility at high scanning speeds, which is contrary to the performance of typical quasi-reversible reactions [51,52]. When the scanning speed is fast enough (Figure 4c), Abs369nm and Abs293nm hardly change with the number of cycles, showing the properties of a nearly ideal reversible system.
Figure 5 shows the cyclic voltammetry curve obtained from a wide range of potential scans. A significant feature is that in the subsequent cycle, the oxidation peaks (except A2) and reduction peaks decrease significantly. A2 becomes the main oxidation peak. In addition, at least from the second cycle, the reactants of A2 did not come from the products of A1. It can also be seen that A3 is a composite peak. In the second cycle, there are two small peaks attributed to A3 in the potential range. In addition, the reduction peaks on the curve are all very wide composite peaks.
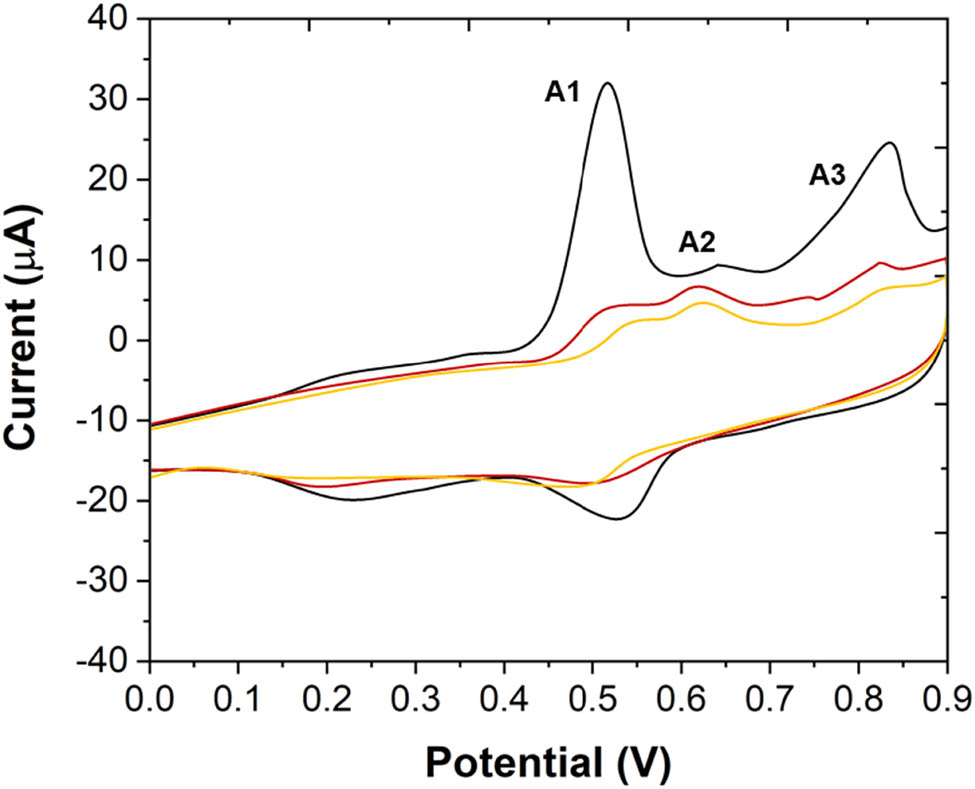
Cyclic voltammetric curves scanned between 0 and 0.9 V in 0.1 M B–R buffer solution. Scan rate: 50 mV/s.
Figure 6a shows the absorbance time curve of the quercetin solution during cyclic potential scanning at a rate of 5 mV/s and in the range of 0–0.9 V. It can be seen that Abs369nm decreases to a very small value in the first forward scanning process, which makes the overall change in the subsequent scanning process small. Accordingly, Abs293nm also reaches a high value in the first scan, which is similar to when the sweep speed is high. This result indicates that most quercetin in the thin-layer solution takes part in the reaction during the first forward scan, but the products in the solution are not significantly reduced back to their original forms during the reverse scan. It can be concluded that quercetin has been continuously adsorbed on the electrode surface to participate in the aforementioned oxidation reaction over the whole potential range of a 0.9 V sweep potential [53]. During the subsequent cycle, Abs293nm shows periodic absorbance changes that are synchronized with the cyclic potential scanning, especially when the scanning speed is high (Figure 6b and c). However, it is remarkable that Abs293nm decreases in the forward scan and increases in the reverse scan, which is the opposite of the periodicity of oxidation products and reduction products during cyclic scanning. The main reason for this phenomenon can be attributed to the influence of the added electrode potential on the adsorption of the product. In the back sweep of the potential, the product is driven by the interfacial electric field to desorb from the electrode surface into the solution and then adsorb to the electrode surface again during the forward sweep of the potential. During forward scanning, the electrode surface is covered by the continuous adsorption of the final product [54]. Figure 6a also shows that Abs369nm shows a less obvious periodic change, which is opposite to that of Abs293nm, indicating the competitive adsorption between the residual quercetin and final oxidation products on the electrode surface. In Figure 6a, we see a more complex periodic absorption wave, but it disappears when the scanning speed increases (Figure 6b and c). This complex wave appears simultaneously with the cyclic voltammetry peak, which is clearly related to the formation of oxidation products. When these products are formed on the electrode surface, they diffuse to the thin-layer solution due to supersaturation on the electrode surface. Then, with the positive change in potential, these products are adsorbed on the electrode surface, which results in the absorption wave. Because the adsorption and desorption process of oxidation products is relatively slow, this step is skipped when the scanning speed is fast.
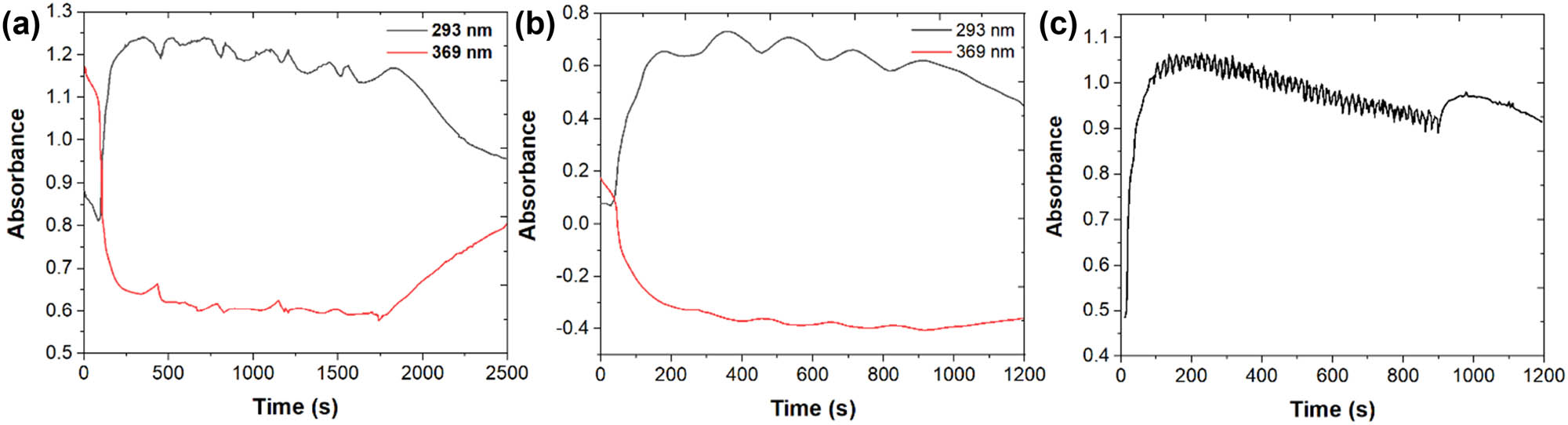
The dual wavelength absorbance time curve in the scanning process of quercetin cyclic potential: (a) scanning range: 0.0–0.9 V; quercetin concentration: 50 μm; pH = 1.8; scanning speed: 5 mV/s; number of cycle: 1. (b) Scanning speed: 10 mV/s; number of cycles: 5. (c) Scanning speed: 100 mV/s; number of cycles: 50.
4 Conclusion
In the homemade 0.2 mm-thick thin-layer electrochemical cell, the absorbance at two wavelengths expresses a good response to the electrochemical reaction process, thereby quickly and accurately showing the electrochemical reaction process. The oxidation behavior of quercetin is a chain reaction. The results show that quercetin adsorbed on the electrode surface and in the solution participates in the electrode reaction at the same time, and the electrode has strong adsorption behavior toward quercetin and its various oxidation products at high potentials. This experiment proves that UV-Vis thin-layer spectroelectrochemistry is a feasible way to study the electrochemical reaction mechanism of flavonoids in plants.
-
Funding information: This study was supported by Zhejiang scientific traditional Chinese medicine research fund (2016ZB020).
-
Author contributions: J. J.: conceptualization; J. J., J. W.: data curation; J. W. and J. J.: methodology; L. L.: formal analysis; J. W.: writing—original draft; J. J.: review and editing.
-
Conflict of interest: The authors declare no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Yan W, Yang X, Cao X, Long Y. Chemical constituents from Zanthoxylum bungeanum Dahongpao. Chin Tradit Pat Med. 2018;40(2):379–82.Suche in Google Scholar
[2] Hou X, Li S, Luo Q, Shen G, Wu H, Li M, et al. Discovery and identification of antimicrobial peptides in Sichuan pepper (Zanthoxylum bungeanum Maxim) seeds by peptidomics and bioinformatics. Appl Microbiol Biotechnol. 2019;103(5):2217–28.10.1007/s00253-018-09593-ySuche in Google Scholar PubMed
[3] Zhang L-L, Xu S-S, Shi B-L, Wang H-Y, Liu L-Y, Zhong K, et al. Evaluation of the pungency intensity and time-related aspects of Chinese Zanthoxylum bungeanum based on human sensation. J Sens Stud. 2018;33(6):e12465.10.1111/joss.12465Suche in Google Scholar
[4] Zeng M, Wang J, Zhang M, Chen J, He Z, Qin F, et al. Inhibitory effects of Sichuan pepper (Zanthoxylum bungeanum) and sanshoamide extract on heterocyclic amine formation in grilled ground beef patties. Food Chem. 2018;239:111–8.10.1016/j.foodchem.2017.06.097Suche in Google Scholar PubMed
[5] Li K, Zhou R, Wang Jia W, Li Z, Li J, Zhang P, et al. Zanthoxylum bungeanum essential oil induces apoptosis of HaCaT human keratinocytes. J Ethnopharmacol. 2016;186:351–61.10.1016/j.jep.2016.03.054Suche in Google Scholar PubMed
[6] Fu L, Liu Z, Ge J, Guo M, Zhang H, Chen F, et al. (001) plan manipulation of α-Fe2O3 nanostructures for enhanced electrochemical Cr(VI) sensing. J Electroanal Chem. 2019;841:142–7.10.1016/j.jelechem.2019.04.046Suche in Google Scholar
[7] Yu J, Jin H, Gui R, Lv W, Wang Z. A facile strategy for ratiometric electrochemical sensing of quercetin in electrolyte solution directly using bare glassy carbon electrode. J Electroanal Chem. 2017;795:97–102.10.1016/j.jelechem.2017.04.053Suche in Google Scholar
[8] Xu Y, Lu Y, Zhang P, Wang Y, Zheng Y, Fu L, et al. Infrageneric phylogenetics investigation of Chimonanthus based on electroactive compound profiles. Bioelectrochemistry. 2020;133:107455.10.1016/j.bioelechem.2020.107455Suche in Google Scholar PubMed
[9] Yang L, Xu B, Ye H, Zhao F, Zeng B. A novel quercetin electrochemical sensor based on molecularly imprinted poly(para-aminobenzoic acid) on 3D Pd nanoparticles-porous graphene-carbon nanotubes composite. Sens Actuators B Chem. 2017;251:601–8.10.1016/j.snb.2017.04.006Suche in Google Scholar
[10] Fu L, Zheng Y, Zhang P, Zhang H, Xu Y, Zhou J, et al. Development of an electrochemical biosensor for phylogenetic analysis of Amaryllidaceae based on the enhanced electrochemical fingerprint recorded from plant tissue. Biosens Bioelectron. 2020;159:112212.10.1016/j.bios.2020.112212Suche in Google Scholar PubMed
[11] Vilian ATE, Puthiaraj P, Kwak CH, Choe SR, Huh YS, Ahn W-S, et al. Electrochemical determination of quercetin based on porous aromatic frameworks supported Au nanoparticles. Electrochim Acta. 2016;216:181–7.10.1016/j.electacta.2016.08.150Suche in Google Scholar
[12] Zhou J, Zheng Y, Zhang J, Karimi-Maleh H, Xu Y, Zhou Q, et al. Characterization of the electrochemical profiles of lycoris seeds for species identification and infrageneric relationships. Anal Lett. 2020;53(15):2517–28.10.1080/00032719.2020.1746327Suche in Google Scholar
[13] Takahashi S, Muguruma H, Osakabe N, Inoue H, Ohsawa T. Electrochemical determination with a long-length carbon nanotube electrode of quercetin glucosides in onion, apple peel, and tartary buckwheat. Food Chem. 2019;300:125189.10.1016/j.foodchem.2019.125189Suche in Google Scholar PubMed
[14] Yao Z, Yang X, Liu X, Yang Y, Hu Y, Zhao Z. Electrochemical quercetin sensor based on a nanocomposite consisting of magnetized reduced graphene oxide, silver nanoparticles and a molecularly imprinted polymer on a screen-printed electrode. Microchim Acta. 2017;185(1):70.10.1007/s00604-017-2613-5Suche in Google Scholar PubMed
[15] Gomez FJV, Espino M, de los Angeles Fernandez M, Raba J, Silva MF. Enhanced electrochemical detection of quercetin by natural deep eutectic solvents. Anal Chim Acta. 2016;936:91–6.10.1016/j.aca.2016.07.022Suche in Google Scholar PubMed
[16] Zhang W, Zong L, Geng G, Li Y, Zhang Y. Enhancing determination of quercetin in honey samples through electrochemical sensors based on highly porous polypyrrole coupled with nanohybrid modified GCE. Sens Actuators B Chem. 2018;257:1099–109.10.1016/j.snb.2017.11.059Suche in Google Scholar
[17] Liu J, Li X, Weng W, Xie H, Luo G, Niu Y, et al. A biomass-derived porous carbon-based nanocomposite for voltammetric determination of quercetin. Microchim Acta. 2019;186(12):783.10.1007/s00604-019-3953-0Suche in Google Scholar PubMed
[18] Zhao P, Ni M, Xu Y, Wang C, Chen C, Zhang X, et al. A novel ultrasensitive electrochemical quercetin sensor based on MoS2 – carbon nanotube @ graphene oxide nanoribbons/HS-cyclodextrin/graphene quantum dots composite film. Sens Actuators B Chem. 2019;299:126997.10.1016/j.snb.2019.126997Suche in Google Scholar
[19] Li J, Qu J, Yang R, Qu L, de B, Harrington P. A sensitive and selective electrochemical sensor based on graphene quantum dot/gold nanoparticle nanocomposite modified electrode for the determination of quercetin in biological samples. Electroanalysis. 2016;28(6):1322–30.10.1002/elan.201500490Suche in Google Scholar
[20] Khodarahmi R, Khateri S, Adibi H, Nasirian V, Hedayati M, Faramarzi E, et al. Chemometrical-electrochemical investigation for comparing inhibitory effects of quercetin and its sulfonamide derivative on human carbonic anhydrase II: Theoretical and experimental evidence. Int J Biol Macromol. 2019;136:377–85.10.1016/j.ijbiomac.2019.06.093Suche in Google Scholar PubMed
[21] Alipour F, Raoof JB, Ghani M. Determination of quercetin via thin film microextraction using the in situ growth of Co–Al-layered double hydroxide nanosheets on an electrochemically anodized aluminum substrate followed by HPLC. Anal Methods. 2020;12(6):799–806.10.1039/C9AY02528FSuche in Google Scholar
[22] Krukiewicz K, Gniazdowska B, Jarosz T, Herman AP, Boncel S, Turczyn R. Effect of immobilization and release of ciprofloxacin and quercetin on electrochemical properties of poly(3,4-ethylenedioxypyrrole) matrix. Synth Met. 2019;249:52–62.10.1016/j.synthmet.2019.02.001Suche in Google Scholar
[23] Zhang X, Yang R, Li Z, Zhang M, Wang Q, Xu Y, et al. Electroanalytical study of infrageneric relationship of Lagerstroemia using glassy carbon electrode recorded voltammograms. Revista Mexicana de Ingeniería Química. 2020;19(Supp. 1):281–91.10.24275/rmiq/Bio1750Suche in Google Scholar
[24] Abdel-Hamid R, Rabia MK, Newair EF. Electrochemical behaviour of antioxidants: part 2. Electrochemical oxidation mechanism of quercetin at glassy carbon electrode modified with multi-wall carbon nanotubes. Arab J Chem. 2016;9(3):350–6.10.1016/j.arabjc.2012.06.014Suche in Google Scholar
[25] Demir E, Senocak A, Tassembedo-Koubangoye MF, Demirbas E, Aboul-Eneın HY. Electrochemical evaluation of the total antioxidant capacity of yam food samples on a polyglycine-glassy carbon modified electrode. Curr Anal Chem. 2020 Mar 1;16(2):176–83.10.2174/1573411014666180619143729Suche in Google Scholar
[26] Chen Y, Huang W, Chen K, Zhang T, Wang Y, Wang J. Facile fabrication of electrochemical sensor based on novel core-shell PPy@ZIF-8 structures: enhanced charge collection for quercetin in human plasma samples. Sens Actuators B Chem. 2019;290:434–42.10.1016/j.snb.2019.04.006Suche in Google Scholar
[27] Zhang M, Pan B, Wang Y, Du X, Fu L, Zheng Y, et al. Recording the electrochemical profile of pueraria leaves for polyphyly analysis. ChemistrySelect. 2020;5(17):5035–40.10.1002/slct.202001100Suche in Google Scholar
[28] Niu X, Li X, Chen W, Li X, Weng W, Yin C, et al. Three-dimensional reduced graphene oxide aerogel modified electrode for the sensitive quercetin sensing and its application. Mater Sci Eng C. 2018;89:230–6.10.1016/j.msec.2018.04.015Suche in Google Scholar PubMed
[29] Veerakumar P, Rajkumar C, Chen S-M, Thirumalraj B, Lin K-C. Ultrathin 2D graphitic carbon nitride nanosheets decorated with silver nanoparticles for electrochemical sensing of quercetin. J Electroanal Chem. 2018;826:207–16.10.1016/j.jelechem.2018.08.031Suche in Google Scholar
[30] He R-X, Zha D-W. Cyclic voltammetry and voltabsorptometry studies of redox mechanism of lumazine. J Electroanal Chem. 2017;791:103–8.10.1016/j.jelechem.2017.03.026Suche in Google Scholar
[31] Catauro M, Bollino F, Nocera P, Piccolella S, Pacifico S. Entrapping quercetin in silica/polyethylene glycol hybrid materials: chemical characterization and biocompatibility. Mater Sci Eng C. 2016;68:205–12.10.1016/j.msec.2016.05.082Suche in Google Scholar PubMed
[32] Diamantis DA, Ramesova S, Chatzigiannis CM, Degano I, Gerogianni PS, Karadima KE, et al. Exploring the oxidation and iron binding profile of a cyclodextrin encapsulated quercetin complex unveiled a controlled complex dissociation through a chemical stimulus. Biochim Biophys Acta BBA Gen Subj. 2018;1862(9):1913–24.10.1016/j.bbagen.2018.06.006Suche in Google Scholar PubMed
[33] Kocábová J, Fiedler J, Degano I, Sokolová R. Oxidation mechanism of flavanone taxifolin. Electrochemical and spectroelectrochemical investigation. Electrochim Acta. 2016;187:358–63.10.1016/j.electacta.2015.11.077Suche in Google Scholar
[34] Sokolová R, Tarábek J, Papoušková B, Kocábová J, Fiedler J, Vacek J, et al. Oxidation of the flavonolignan silybin. In situ EPR evidence of the spin-trapped silybin radical. Electrochim Acta. 2016;205:118–23.10.1016/j.electacta.2016.04.107Suche in Google Scholar
[35] Heřmánková E, Zatloukalová M, Biler M, Sokolová R, Bancířová M, Tzakos AG, et al. Redox properties of individual quercetin moieties. Free Radic Biol Med. 2019;143:240–51.10.1016/j.freeradbiomed.2019.08.001Suche in Google Scholar PubMed
[36] Ramešová Š, Degano I, Sokolová R. The oxidative decomposition of natural bioactive compound rhamnetin. J Electroanal Chem. 2017;788:125–30.10.1016/j.jelechem.2017.01.054Suche in Google Scholar
[37] Al Haddabi B, Al Lawati HAJ, Suliman FO. A comprehensive evaluation of three microfluidic chemiluminescence methods for the determination of the total phenolic contents in fruit juices. Food Chem. 2017;214:670–7.10.1016/j.foodchem.2016.07.119Suche in Google Scholar PubMed
[38] Patra M, Mukherjee R, Banik M, Dutta D, Begum NA, Basu T. Calcium phosphate-quercetin nanocomposite (CPQN): a multi-functional nanoparticle having pH indicating, highly fluorescent and anti-oxidant properties. Colloids Surf B Biointerfaces. 2017;154:63–73.10.1016/j.colsurfb.2017.03.018Suche in Google Scholar PubMed
[39] Lynk TP, Clarke OJR, Kesavan N, Brosseau CL. Development of a sustainable plasmon-enhanced spectroelectrochemical sensor using avocado pit (Persea americana) extract. Sens Actuators B Chem. 2018;257:270–7.10.1016/j.snb.2017.10.137Suche in Google Scholar
[40] Revenga-Parra M, Robledo SN, Martínez-Periñán E, González-Quirós MM, Colina A, Heras A, et al. Direct determination of monosaccharides in honey by coupling a sensitive new Schiff base Ni complex electrochemical sensor and chemometric tools. Sens Actuators B Chem. 2020;312:127848.10.1016/j.snb.2020.127848Suche in Google Scholar
[41] Ipte PR, Kumar S, Satpati AK. Electrochemical synthesis of carbon nano spheres and its application for detection of ciprofloxacin. J Environ Sci Health Part A. 2020;55(2):142–50.10.1080/10934529.2019.1674591Suche in Google Scholar PubMed
[42] Tang J, Mao Y, Guo J, Li Z, Zhang C, Jin B. Simultaneous determination of TBH2Q and BHA Antioxidants in food samples using Eosin Y Film modified electrode. Food Anal Methods. 2018;11(12):3380–90.10.1007/s12161-018-1314-ySuche in Google Scholar
[43] Zhiltsova EP, Ibatullina MR, Lukashenko SS, Kutyreva MP. Zakharova LYa. Spectrophotometric study of quercetin in metallomicellar solutions of 1-hexadecyl-4-aza-1-azoniabicyclo[2.2.2]octane bromide complex with copper dibromide. J Mol Liq. 2018;249:716–22.10.1016/j.molliq.2017.11.091Suche in Google Scholar
[44] Vinnarasi S, Radhika R, Vijayakumar S, Shankar R. Structural insights into the anti-cancer activity of quercetin on G-tetrad, mixed G-tetrad, and G-quadruplex DNA using quantum chemical and molecular dynamics simulations. J Biomol Struct Dyn. 2020;38(2):317–39.10.1080/07391102.2019.1574239Suche in Google Scholar PubMed
[45] Zagrean-Tuza C, Dorneanu S, Mot AC. The strange case of polyphenols inhibiting the Briggs-Rauscher reaction: pH-modulated reactivity of the superoxide radical. Free Radic Biol Med. 2020;146:189–97.10.1016/j.freeradbiomed.2019.11.006Suche in Google Scholar PubMed
[46] Jalalvand AR, Goicoechea HC. Applications of electrochemical data analysis by multivariate curve resolution-alternating least squares. Trends Anal Chem. 2017;88:134–66.10.1016/j.trac.2017.01.005Suche in Google Scholar
[47] Klein E, Rimarčík J, Senajová E, Vagánek A, Lengyel J. Deprotonation of flavonoids severely alters the thermodynamics of the hydrogen atom transfer. Comput Theor Chem. 2016;1085:7–17.10.1016/j.comptc.2016.04.004Suche in Google Scholar
[48] Gulsunoglu Z, Purves R, Karbancioglu-Guler F, Kilic-Akyilmaz M. Enhancement of phenolic antioxidants in industrial apple waste by fermentation with Aspergillus spp. Biocatal Agric Biotechnol. 2020;71:101562.10.1016/j.bcab.2020.101562Suche in Google Scholar
[49] Yang S-L, Zhao L-J, Chi S-M, Du J-J, Ruan Q, Xiao P-L, et al. Inclusion complexes of flavonoids with propylenediamine modified β-cyclodextrin: preparation, characterization and antioxidant. J Mol Struct. 2019;1183:118–25.10.1016/j.molstruc.2019.01.046Suche in Google Scholar
[50] Waffo AFT, Yesildag C, Caserta G, Katz S, Zebger I, Lensen MC, et al. Fully electrochemical MIP sensor for artemisinin. Sens Actuators B Chem. 2018;275:163–73.10.1016/j.snb.2018.08.018Suche in Google Scholar
[51] Sahoo S, Sahoo PK, Sharma A, Satpati AK. Interfacial polymerized RGO/MnFe2O4/polyaniline fibrous nanocomposite supported glassy carbon electrode for selective and ultrasensitive detection of nitrite. Sens Actuators B Chem. 2020;309:127763.10.1016/j.snb.2020.127763Suche in Google Scholar
[52] Brinkert K, Le Formal F, Li X, Durrant J, Rutherford AW, Fantuzzi A. Photocurrents from photosystem II in a metal oxide hybrid system: electron transfer pathways. Biochim Biophys Acta BBA Bioenerg. 2016;1857(9):1497–505.10.1016/j.bbabio.2016.03.004Suche in Google Scholar PubMed PubMed Central
[53] Ramos II, Gregório BJR, Barreiros L, Magalhães LM, Tóth IV, Reis S, et al. Programmable flow system for automation of oxygen radical absorbance capacity assay using pyrogallol red for estimation of antioxidant reactivity. Talanta. 2016;150:599–606.10.1016/j.talanta.2015.12.061Suche in Google Scholar PubMed
[54] Chernikov DA, Shishlyannikova TA, Kashevskii AV, Bazhenov BN, Kuzmin AV, Gorshkov AG, et al. Some peculiarities of taxifolin electrooxidation in the aqueous media: the dimers formation as a key to the mechanism understanding. Electrochim Acta. 2018;271:560–6.10.1016/j.electacta.2018.03.179Suche in Google Scholar
© 2021 Jun Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

