Abstract
Gas chromatography-ion mobility spectrum (GC-IMS) is used to analyze and compare the differences in aroma among different tobacco samples. The aroma substances in tobacco samples in Jilin Changchun are the richest, while those in Guangdong Nanxiong are the lowest. The concentrations of aroma substances such as decanal, 1-hydroxy-2-propanone, and 2-methylbutanol were the highest in Guangdong Nanxiong of the three. The concentration of 1-hexanol, cyclohexanone, pentanoic acid, and other aroma substances in Fujian Nanping was high. The concentration of 2-acetylfuran, 2-octanol, isopentanol, 3-methylvaleric acid, phenylacetic acid, and other aroma substances in Changchun area of Jilin Province was low. Through principal component analysis and similarity research, both tobaccos can be distinguished by their production areas and grades from the same.
1 Introduction
Tobacco is considered to be a plant factory that can provide a variety of chemical components. More than 10,000 chemicals have been found in both tobacco and its smoke [1,2,3,4]. Many functional small molecules including nicotine, solanesol, chlorogenic acid, etc. are included in tobacco leaf. Except such molecules, many macromolecules, such as protein, cellulose, and carbohydrates with different molecule structures, are also included in tobacco leaf [5,6,7,8]. Most of these functional small molecules and macromolecules show strong biological activity and nutritional value [5]. Importantly, the smell, taste, and feel are mostly decided by the integrated response of aroma in tobacco leaves, which has direct relationship with smoker’s enjoyment [9,10,11]. So, the chemical type, content, and distribution in tobacco leaves have strong correlation with cigarette flavor. The volatile and semi-volatile compounds including aldehydes, ketones, alcohols, esters (lactones), and alkenes are called neutral aroma components. And the tobacco flavor is mainly decided by such neutral aroma components [12,13,14,15].
However, it is difficult to measure the content of aroma components in different regions, varieties, and parts of tobacco leaves accurately and quickly. The traditional method is very complex, which is mainly based on liquid chromatography or the quantitative determination of organic small molecules using gas chromatography with flame ionization detection [16,17,18,19,20,21]. These methods require complex sample pretreatment process and the equipment used is very large. The testing process can be only done in the laboratory. Ion mobility spectrometer (IMS) is based on the analysis of ion movement in the gas phase. Most commonly coupled with chromatographic techniques is the drift tube IMS. And the signal from GC-IMS system is two-dimensional: the intensity is a function of the GC retention time and the IMS drift time. It combines an effective method of separation and identification due to its sensitivity, simplicity as well as affordable costs and enables the usage in a non-laboratory environment [22]. The automated, headspace-based sample preparation provides a simple and reproducible injection, with controlled incubation time and temperature [23,24]. So, it provides a possible way to determine the construction of volatile and semi-volatile compounds in tobacco leaves. In this work, GC-IMS technology was used to study the contents of aroma substances in different grades of tobacco from different regions and to analyze the similarity between every sample.
2 Instrument materials and methods
2.1 Instruments
The gas chromatography-ion mobility spectrometry (GC-IMS) (FlavourSpec®, Gesellschaft für Analytische Sensorsysteme mbH, Dortmund, Germany) uses an Agilent 490 GC (Agilent Technologies, USA) with a MXT-WAX column (30 m × 0.53 mm i.d., 0.1 µm film thickness, RESTEK). The GC was equipped with an autosampler (CTC Analytics AG, Zwingen, Switzerland) with a headspace sampling unit and a 1 mL gastight syringe (Gerstel GmbH, Mühlheim, Germany).
2.2 Materials
Ten tobacco samples of four grades from three places were used as analysis materials. GDNX C3L (GuangDong NanXiong C3L), GDNX C3F (GuangDong NanXiong C3F), GDNX C4F (GuangDong NanXiong C4F), and GDNX B2F (GuangDong NanXiong B2F) were obtained from Nanxiong in Guangdong province; FJNP C3L (FuJian NanPing C3L), FJNP C3F (FuJian NanPing C3F), FJNP C4F (FuJian NanPing C4F), and FJNP B2F (FuJian NanPing B2F) were obtained from Nanping in Fujian province; JLCC C3L (JiLin ChangChun C3L) and JLCC C3F (JiLin ChangChun C3F) were obtained from Changchun in Jilin province.
Tobacco samples were crushed by a pulverizer and sieved with 80 mesh sieve. The moisture content of tobacco powder was 5.0%. The weight of tobacco powder used in the analysis process was 1.0 g.
2.3 Analysis methods
Aroma substances in tobacco samples from different regions were analyzed by GC-IMS. GC-IMS is equipped with an automatic headspace sampler, which does not require pretreatment of tobacco samples. Accurately weigh 1.0 g tobacco powder and place it in the sample inlet bottle. The injection volume was 500 µL, incubation time was 15 min, and incubation temperature was 80°C. The GC-IMS conditions were: FS (fused silica)-SE-54 (the stationary phase is composed by methyl silicone: 5% phenyl, 1% vinyl silicone) – CB-0.5 (the thickness of stationary phase is 0.5 μm) – 15 m (Column length) – ID (internal diameter): 0.53 mm; 60°C transfer line temperatures; nitrogen carrier gas at a flow rate of 0–2 min, 2 mL/min; 2–10 min, 2–10 mL/min; 10–20 min, 10–100 mL/min; 20–25 min, 100–150 mL/min; the MS conditions were: 45°C ion source and 85°C injector temperature.
-
Ethical approval: This article does not contain any studies with human participants performed by any of the authors.
3 Results and discussion
3.1 GC-IMS 3D spectra analysis
Figures 1 and 2 are GC-IMS spectrum of tobacco powder and GC-IMS spectrum difference of tobacco powder, respectively. The background of the entire figure is blue, and the left red vertical line is the RIP peak (Reactant Ions Positive peak, drift time is around 7.81 ms). Each point on both sides of the RIP peak represents a volatile organic compound. GC-IMS spectrum was referred to as one spectrum. The colors of substances with the same concentration in another spectrum cancel out as white. White implies low concentration, and red is a high concentration. A darker color implies a higher concentration. The blue area in the referenced sample indicated that the concentration of the substance was lower than that of the reference sample. A darker blue implies a lower concentration. The red area in the referenced sample indicated that the concentration of the substance was higher than that of the reference sample. A darker red implies a higher concentration.
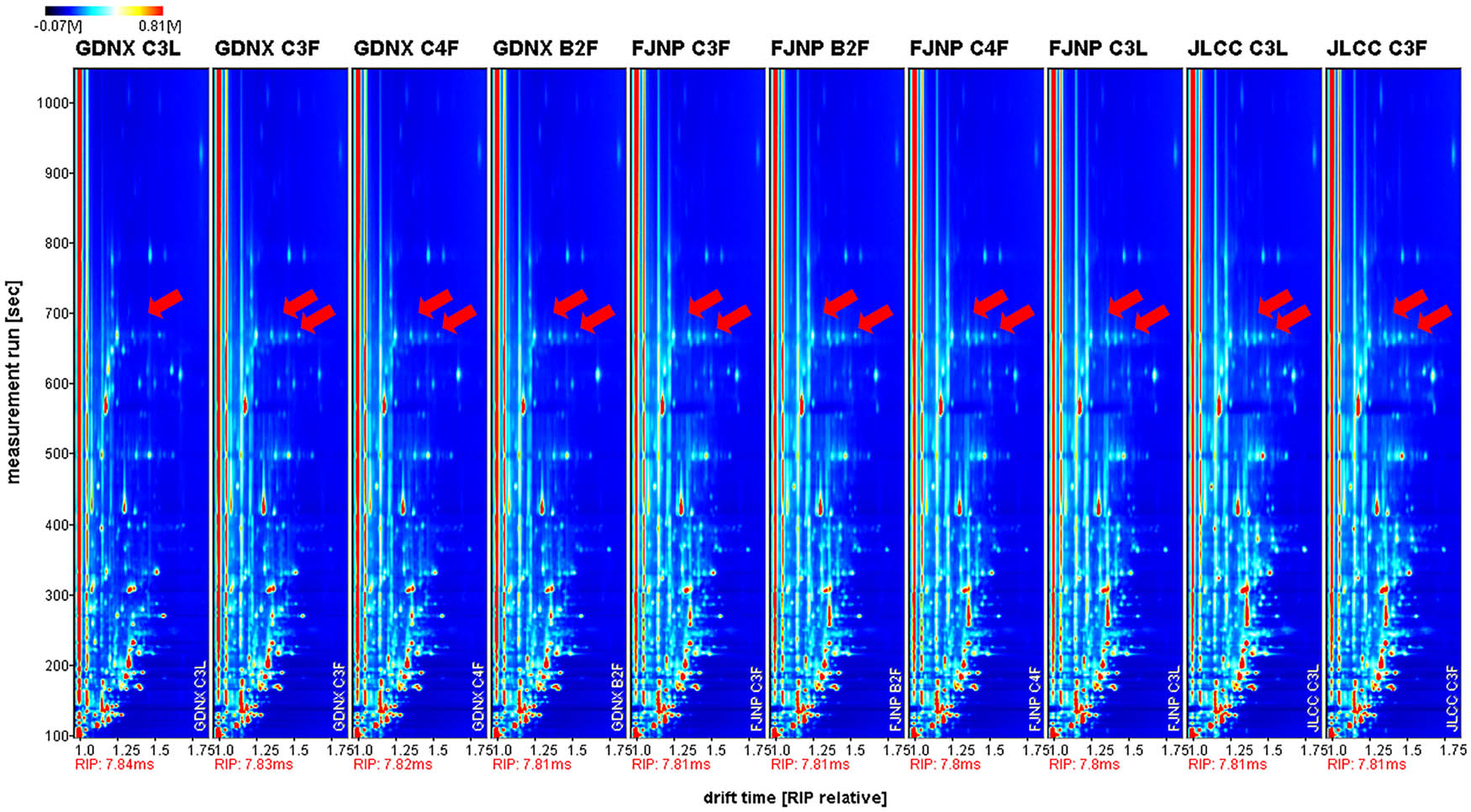
Ion mobility spectrum of tobacco samples.

Difference diagram of ion mobility spectrum of tobacco samples.
GC-IMS technology can detect a variety of aroma substances from tobacco leaves as shown in the ion mobility spectrum. The difference diagram of the ion mobility spectrum shows that GC-IMS technology can compare and analyze the differences of aromatic substances in different tobacco samples. The GC-IMS spectrum results show that the two strip peaks to the right of the RIP peak (indicated by the red arrow) were monomers and dimers of acetic acid because the high content in the sample led to the peak throughout. Comparing GC-IMS spectrum of each sample, we show that the composition and proportion of aroma substances in different regions and different grades of tobacco were similar. At the same time, the red part in the atlas of JNCC and FJNP is significantly higher than the blue part, and the red part was very dark, which indicated that the content of aromatic substances in Jilin Changchun (JNCC) and Fujian Nanping (FJNP) was higher than that in Guangdong Nanxiong (GDNX).
3.2 Fingerprint analysis of aroma substances
All of the peaks analyzed in the GC-IMS spectrum were selected to generate fingerprints to compare the differences of volatile organic compounds among the different samples (Figure 3).

Fingerprints of volatile organic compounds peaks.
Each row was a sample, and each line was a signal peak of organic matter (the same substance in different samples) with the same retention time and drift time. The fingerprint shows that the contents of aroma substances in tobacco in JNCC and FJNP were more abundant than those in GDNX; their aromatic contents varied. Aromatic substances with a higher concentration in GDNX tobacco samples were n-decyl aldehyde, 1-hydroxy-2-acetone, 2-methyl-butanol, and 2-methylpropionic aldehyde. Aromatic substances with a higher concentration in FJNP tobacco samples were n-hexanol, cyclohexanone, and n-valeric acid. The aromatic substances with higher concentration in JNCC tobacco samples are 2-acetylfuran, 2-octanol, isopentanol, 3-methyl-pentanoic acid, phenylacetic acid, and ethyl acetate. The higher concentrations of aromatic substances in GDNX and FJNP were dimethyl disulfide and ethyl propionate. The aromatic substances with higher concentrations in FJNP samples and JNCC samples were 2-amylfuran, 2-acetopyrrole, benzyl alcohol, linalool, furfural, methyl-5-furfural, acetaldehyde, phenylacetaldehyde, 6-methyl-5-hept-2-ketone, and isoamyl acetate. The higher concentrations of aromatic substances in the samples of GDNX tobacco and JNCC tobacco were 2-3-butanediol, 3-hydroxy-2-butanone, and 3-methylbutyraldehyde.
The aromatic substances with similar concentrations in three tobacco samples from different regions were γ-butyrolactone, acetone, and methyl acetate. The concentrations of 2-phenyl alcohol, 2-hexenol, (trans)-2-hexene-1-alcohol, 2-heptanol, heptanal, benzaldehyde, 2-methyl-butyric acid, ethyl butyrate, and toluene in tobacco samples from GDNX, FJNP, and JNCC increased successively. The concentration of aromatic substances in tobacco samples in GDNX varies greatly. This is mainly reflected in the other three grades of C3L where the concentrations of aroma substances such as 2-hexenol, valeraldehyde, hexanaldehyde, heptanal, octanal, n-nonaldehyde, and propionic acid in C3L grade samples were higher than the other three grades. The 2-3-butanediol, dimethyl disulfide, and ethyl propionate in C3L samples were lower than the other three grades. Table 1 is the peak volume of aroma substances in tobacco. The fingerprint can compare and analyze the concentration of aroma substances among different samples by comparing the fingerprint with the peak volume of aromatic substances.
| Compound | GDNX B2F | GDNX C3L | GDNX C3F | GDNX C4F | FJNP B2F | FJNP C3L | FJNP C3F | FJNP C4F | JLCC C3L | JLCC C3F |
|---|---|---|---|---|---|---|---|---|---|---|
| 6-Methyl-5-hepten-2-one | 4085.29 | 4914.55 | 4712.41 | 5011.95 | 6419.31 | 5235.05 | 5797.77 | 5579.47 | 7228.42 | 7964.65 |
| γ-Butyrolactone dimer | 3097.93 | 3508.33 | 3798.63 | 3203.63 | 3364.71 | 3635.28 | 3678.69 | 3806.41 | 3217.48 | 2866.67 |
| γ-Butyrolactone monomer | 1303.24 | 1990.27 | 1675.11 | 1525.91 | 1182.54 | 1036.42 | 1175.15 | 1119.51 | 1043.27 | 1131.94 |
| 2-Heptanol | 398.05 | 379.23 | 417.58 | 369.48 | 404.88 | 422.58 | 423.27 | 384.06 | 661.12 | 690.18 |
| Linalool | 393.11 | 433.21 | 440.18 | 343.05 | 407.71 | 523.63 | 477.68 | 538.69 | 587.39 | 533.43 |
| 3-Methylvaleric acid dimer | 137.93 | 137.36 | 174.18 | 187.36 | 149.96 | 136.98 | 152.31 | 116.59 | 294.52 | 268.76 |
| Isoamyl acetate monomer | 177.99 | 140.48 | 154.25 | 175.01 | 198.83 | 213.65 | 213.91 | 212.36 | 199.31 | 186.02 |
| Pentanoic acid | 387.84 | 235.11 | 344.61 | 351.18 | 650.21 | 674.05 | 524.15 | 670.26 | 519.79 | 451.86 |
| 1-Hydroxy-2-propanone | 219.01 | 133.03 | 187.08 | 153.51 | 113.46 | 142.39 | 129.75 | 133.53 | 142.91 | 166.58 |
| 2-Methylbutanol dimer | 280.65 | 175.47 | 200.45 | 235.33 | 333.04 | 339.71 | 297.11 | 317.21 | 311.01 | 266.93 |
| 2-Acetylpyrrole | 96.35 | 58.75 | 89.91 | 91.46 | 107.81 | 106.15 | 109.07 | 101.05 | 122.12 | 102.76 |
| 3-Methylvaleric acid monomer | 174.48 | 154.37 | 159.63 | 171.76 | 126.57 | 132.97 | 141.45 | 130.96 | 101.39 | 118.51 |
| 2-Acetylfuran | 74.11 | 100.34 | 77.11 | 79.56 | 53.74 | 58.18 | 62.95 | 58.32 | 83.54 | 105.61 |
| 2-Ethyl-1-hexanol | 254.04 | 197.45 | 303.02 | 264.81 | 384.21 | 478.51 | 375.24 | 497.02 | 493.34 | 403.36 |
| 2-Acetylpyridine | 58.12 | 78.43 | 59.89 | 77.97 | 105.1 | 124.51 | 118.96 | 120.58 | 133.79 | 128.03 |
| α-Methylbenzenemethanol | 30.63 | 53.35 | 30.57 | 31.77 | 50.51 | 43.73 | 41.21 | 57.51 | 60.41 | 68.03 |
| Propylsulfide | 395.95 | 235.44 | 341.28 | 339.28 | 339.15 | 295.91 | 321.21 | 320.48 | 221.84 | 243.32 |
| Ethyl 2-methylbutanoate | 158.07 | 81.36 | 114.86 | 125.73 | 351.01 | 350.64 | 262.58 | 401.35 | 185.88 | 134.67 |
| 2-Methylbutanoic acid | 124.67 | 93.01 | 134.74 | 114.27 | 136.65 | 142.19 | 116.81 | 143.51 | 86.96 | 90.09 |
| 2-Methyl-1-propanol | 150.46 | 227.94 | 176.41 | 174.79 | 116.56 | 108.68 | 118.68 | 130.22 | 109.82 | 118.25 |
| 2-Pentanone | 214.61 | 123.96 | 211.89 | 185.44 | 222.87 | 147.71 | 169.13 | 150.69 | 266.79 | 245.43 |
| E_Z-2-6-Nonadienal | 58.43 | 102.16 | 94.68 | 89.72 | 24.31 | 43.95 | 47.91 | 46.73 | 57.12 | 76.04 |
| n-Nonanal dimer | 52.33 | 69.87 | 66.37 | 64.35 | 50.13 | 54.52 | 36.94 | 60.02 | 63.38 | 55.51 |
| 5-Methylfurfuryl alcohol | 56.68 | 113.81 | 87.23 | 84.03 | 109.83 | 95.53 | 98.09 | 95.62 | 118.46 | 126.57 |
| Ethyl pentanoate | 18.57 | 14.09 | 20.71 | 24.74 | 32.74 | 33.76 | 28.81 | 23.51 | 29.22 | 28.28 |
| Isopentanol | 21.11 | 72.09 | 28.01 | 30.03 | 37.99 | 46.02 | 42.64 | 43.51 | 48.92 | 49.13 |
| Acetoin | 3880.71 | 3617.96 | 4404.33 | 3662.86 | 2483.83 | 2733.37 | 2854.58 | 2788.58 | 4357.46 | 4239.45 |
| 2-Butanone | 1038.15 | 655.47 | 830.88 | 793.16 | 782.87 | 692.47 | 719.25 | 644.53 | 773.53 | 811.42 |
| Furfural dimer | 651.03 | 1022.48 | 875.27 | 717.93 | 539.55 | 932.42 | 995.11 | 785.41 | 1219.13 | 1178.31 |
| 2-Hexenol dimer | 315.39 | 1055.41 | 677.22 | 619.45 | 435.66 | 571.06 | 831.38 | 661.85 | 1029.01 | 1014.45 |
| Benzaldehyde dimer | 564.05 | 488.29 | 580.25 | 543.63 | 509.87 | 647.54 | 581.27 | 520.71 | 967.56 | 875.41 |
| Pentanal dimer | 468.49 | 528.39 | 464.62 | 670.07 | 596.05 | 393.29 | 376.38 | 435.14 | 377.8 | 617.33 |
| 2-Methyl-propanal dimer | 826.22 | 793.25 | 824.98 | 836.57 | 636.05 | 420.91 | 663.61 | 621.81 | 564.45 | 659.05 |
| 2-Hexen-1-ol | 299.96 | 300.69 | 295.62 | 318.31 | 414.81 | 378.55 | 392.66 | 344.87 | 455.86 | 476.95 |
| n-Nonanal monomer | 344.38 | 473.81 | 421.18 | 439.31 | 296.81 | 325.86 | 304.64 | 347.22 | 390.64 | 354.17 |
| 2-Pentylfuran | 216.02 | 212.29 | 208.47 | 241.14 | 306.08 | 274.19 | 266.39 | 250.84 | 370.71 | 360.31 |
| Octanal | 174.09 | 247.17 | 233.33 | 222.18 | 143.56 | 139.72 | 138.86 | 154.06 | 164.05 | 145.31 |
| Phenylacetaldehyde dimer | 129.93 | 162.87 | 204.06 | 156.47 | 196.86 | 186.11 | 229.04 | 227.18 | 274.18 | 221.59 |
| Methyl-5-furfural | 235.07 | 226.76 | 247.16 | 223.12 | 283.38 | 357.84 | 335.78 | 343.98 | 339.51 | 305.54 |
| Cyclohexanone | 178.71 | 147.15 | 191.28 | 200.39 | 257.51 | 340.91 | 221.03 | 334.85 | 345.61 | 256.19 |
| Hexanal dimer | 331.69 | 686.98 | 460.71 | 442.43 | 411.92 | 186.38 | 411.93 | 210.98 | 240.83 | 347.58 |
| Hexanal monomer | 266.19 | 443.71 | 311.14 | 305.83 | 235.82 | 150.06 | 224.34 | 164.93 | 177.47 | 230.09 |
| Ethyl acetate | 69.75 | 26.76 | 48.57 | 35.34 | 54.83 | 100.32 | 64.16 | 54.09 | 185.67 | 148.68 |
| Propanal | 839.05 | 884.76 | 682.95 | 863.71 | 671.08 | 619.24 | 629.92 | 380.89 | 661.07 | 1064.19 |
| Ethyl propanoate | 526.91 | 198.59 | 418.11 | 367.49 | 342.15 | 445.12 | 389.54 | 420.21 | 273.39 | 248.31 |
| Toluene | 77.93 | 118.84 | 85.95 | 87.57 | 142.21 | 97.19 | 115.71 | 110.02 | 166.22 | 163.39 |
| Heptanal monomer | 142.48 | 253.33 | 188.07 | 172.76 | 158.37 | 146.71 | 146.76 | 132.91 | 233.51 | 233.93 |
| 2-Octanol | 96.71 | 74.85 | 86.06 | 114.64 | 156.09 | 117.87 | 113.35 | 117.93 | 196.05 | 259.92 |
| 2-Methyl-propanal monomer | 261.33 | 393.83 | 323.43 | 263.76 | 148.93 | 161.87 | 185.65 | 212.79 | 200.94 | 194.66 |
| Pentanal monomer | 142.05 | 249.68 | 170.81 | 185.15 | 154.75 | 98.88 | 122.24 | 116.13 | 137.01 | 175.74 |
| 1-Pentanol monomer | 99.51 | 109.34 | 96.49 | 102.64 | 97.37 | 88.41 | 91.95 | 91.19 | 124.84 | 119.85 |
| 2-Methylbutanol monomer | 160.71 | 164.61 | 139.81 | 157.39 | 154.07 | 160.58 | 149.51 | 162.45 | 126.82 | 137.61 |
| Alpha-Terpieol | 237.81 | 144.73 | 209.76 | 206.07 | 209.91 | 239.53 | 196.64 | 186.65 | 234.79 | 255.77 |
| 1-Hexanol | 38.83 | 35.37 | 35.17 | 28.91 | 66.88 | 75.63 | 119.99 | 97.73 | 58.73 | 62.41 |
| 1-Pentanol dimer | 53.53 | 36.92 | 35.33 | 45.51 | 92.57 | 98.06 | 68.75 | 66.81 | 142.33 | 134.97 |
| 2-Phenylethanol | 84.22 | 58.78 | 82.44 | 85.51 | 103.17 | 101.36 | 89.03 | 125.57 | 143.31 | 150.74 |
| Phenylacetic acid | 441.31 | 405.31 | 434.82 | 428.01 | 339.74 | 417.84 | 359.81 | 351.81 | 594.45 | 687.66 |
| 2-Hexenol monomer | 98.06 | 347.02 | 169.69 | 153.41 | 158.96 | 148.49 | 160.99 | 154.81 | 191.36 | 208.31 |
| 2-3-Butanediol | 479.88 | 402.41 | 520.58 | 536.64 | 430.81 | 511.76 | 343.44 | 438.15 | 534.17 | 569.44 |
| Isoamyl acetate dimer | 177.24 | 60.06 | 103.75 | 129.52 | 180.15 | 336.01 | 210.48 | 179.61 | 500.61 | 309.77 |
| Methional | 84.34 | 123.29 | 96.02 | 86.91 | 130.77 | 142.13 | 174.51 | 152.32 | 101.73 | 107.88 |
| Furfural monomer | 363.45 | 586.69 | 449.07 | 421.96 | 327.51 | 320.83 | 381.87 | 337.76 | 329.48 | 374.45 |
| Ethyl butyrate | 22.49 | 23.01 | 25.63 | 33.36 | 53.17 | 82.45 | 97.41 | 45.37 | 128.67 | 119.66 |
| Propanoic acid | 68.16 | 306.71 | 130.06 | 144.03 | 147.04 | 141.09 | 156.31 | 138.04 | 144.72 | 172.11 |
| Dimethyl disulphide | 563.11 | 277.73 | 455.33 | 474.55 | 537.75 | 593.91 | 563.43 | 579.33 | 372.21 | 393.08 |
| Phenylacetaldehyde monomer | 433.14 | 640.91 | 604.67 | 520.54 | 523.43 | 469.76 | 549.75 | 518.36 | 533.18 | 529.48 |
| Benzaldehyde monomer | 757.71 | 774.16 | 736.34 | 761.76 | 631.83 | 625.15 | 646.81 | 610.18 | 632.11 | 689.17 |
| Methyl ester acetic acid | 3906.26 | 3068.07 | 3588.51 | 3381.43 | 4175.57 | 5503.17 | 4649.72 | 4429.14 | 4538.55 | 4128.06 |
| Acetone | 4081.39 | 3816.05 | 4066.41 | 4045.42 | 4298.59 | 3716.69 | 4041.78 | 3671.99 | 3795.05 | 4192.24 |
| 2-3-Butandione | 260.18 | 337.81 | 280.79 | 321.25 | 365.61 | 266.16 | 296.92 | 245.75 | 324.66 | 358.84 |
| 2-Ethylfuran | 1125.52 | 2621.62 | 1644.34 | 1568.37 | 2683.11 | 2340.25 | 2848.04 | 2469.15 | 3129.54 | 2913.69 |
| Z 3-Hexen-1-ol | 117.28 | 137.17 | 127.77 | 129.54 | 72.73 | 78.79 | 113.85 | 79.28 | 183.53 | 197.87 |
| Heptanal dimer | 38.01 | 68.33 | 46.22 | 50.61 | 57.07 | 44.63 | 54.88 | 37.81 | 60.89 | 63.47 |
| 3-Methylbutanal | 743.41 | 824.95 | 826.88 | 808.45 | 717.96 | 647.56 | 783.31 | 717.16 | 809.03 | 934.73 |
| Benzenemethanol | 100.64 | 71.93 | 92.32 | 92.41 | 103.99 | 111.44 | 104.01 | 113.81 | 111.61 | 121.29 |
- a
Compound content is the relative peak area after being normalized.
- b
Just the main peak area was used to evaluate the compound content.
3.3 Principal component analysis and similarity analysis of different samples
Figure 4 is a principal component analysis of all volatile aroma substances in tobacco samples in which the contribution rate of the first principal component was 59%, the contribution rate of the second principal component was 20%, and the contribution rate of the two principal components was 79%. The results show that tobacco samples from three regions could be clearly distinguished by only the first and second principal components. The dispersion degree of tobacco samples from GDNX and JNCC was relatively high, but the dispersion degree of tobacco samples of C3F and C4F grades in GDNX was relatively low. In other words, the samples were quite similar. The dispersion of tobacco samples in FJNP was relatively low. Samples C3F, B2F, and C4F were quite similar, and they had no obvious distinction. For a more accurate analysis of different grades of tobacco samples from different producing areas, the similarity of each sample was calculated using information on all volatile substances as the object of calculation (Table 2).

PCA plots of all tobacco samples.
Similarity between samples
| Matching % | GDNX C3L | GDNX C3L | GDNX C3F | GDNX C3F | GDNX C4F | GDNX C4F | GDNX B2F | GDNX B2F | FJNP C3F | FJNP C3F | FJNP B2F | FJNP B2F | FJNP C4F | FJNP C4F | FJNP C3L | FJNP C3L | JLCC C3L | JLCC C3L | JLCC C3F | JLCC C3F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GDNX C3L | 100 | 93 | 84 | 81 | 80 | 79 | 74 | 72 | 68 | 69 | 66 | 66 | 70 | 67 | 64 | 63 | 60 | 60 | 67 | 65 |
| GDNX C3L | 93 | 100 | 87 | 84 | 84 | 84 | 78 | 77 | 71 | 72 | 69 | 69 | 73 | 70 | 68 | 66 | 63 | 63 | 70 | 68 |
| GDNX C3F | 84 | 87 | 100 | 94 | 92 | 92 | 87 | 85 | 78 | 77 | 75 | 75 | 78 | 76 | 73 | 72 | 67 | 67 | 73 | 71 |
| GDNX C3F | 81 | 84 | 94 | 100 | 94 | 94 | 90 | 89 | 77 | 77 | 76 | 76 | 78 | 77 | 73 | 72 | 66 | 67 | 73 | 71 |
| GDNX C4F | 80 | 84 | 92 | 94 | 100 | 98 | 90 | 89 | 77 | 77 | 78 | 79 | 79 | 77 | 75 | 73 | 67 | 66 | 73 | 71 |
| GDNX C4F | 79 | 84 | 92 | 94 | 98 | 100 | 91 | 90 | 78 | 78 | 79 | 79 | 80 | 78 | 76 | 74 | 68 | 68 | 74 | 72 |
| GDNX B2F | 74 | 78 | 87 | 90 | 90 | 91 | 100 | 95 | 77 | 77 | 78 | 78 | 78 | 77 | 76 | 75 | 67 | 67 | 72 | 70 |
| GDNX B2F | 72 | 77 | 85 | 89 | 89 | 90 | 95 | 100 | 75 | 75 | 77 | 78 | 78 | 77 | 74 | 73 | 65 | 65 | 70 | 69 |
| FJNP C3F | 68 | 71 | 78 | 77 | 77 | 78 | 77 | 75 | 100 | 94 | 87 | 85 | 88 | 86 | 88 | 87 | 81 | 79 | 80 | 81 |
| FJNP C3F | 69 | 72 | 77 | 77 | 77 | 78 | 77 | 75 | 94 | 100 | 90 | 89 | 92 | 91 | 91 | 90 | 81 | 78 | 81 | 80 |
| FJNP B2F | 66 | 69 | 75 | 76 | 78 | 79 | 78 | 77 | 87 | 90 | 100 | 96 | 91 | 90 | 90 | 89 | 81 | 79 | 82 | 81 |
| FJNP B2F | 66 | 69 | 75 | 76 | 79 | 79 | 78 | 78 | 85 | 89 | 96 | 100 | 91 | 90 | 90 | 88 | 78 | 76 | 81 | 79 |
| FJNP C4F | 70 | 73 | 78 | 78 | 79 | 80 | 78 | 78 | 88 | 92 | 91 | 91 | 100 | 95 | 91 | 90 | 79 | 76 | 80 | 78 |
| FJNP C4F | 67 | 70 | 76 | 77 | 77 | 78 | 77 | 77 | 86 | 91 | 90 | 90 | 95 | 100 | 91 | 91 | 78 | 76 | 79 | 77 |
| FJNP C3L | 64 | 68 | 73 | 73 | 75 | 76 | 76 | 74 | 88 | 91 | 90 | 90 | 91 | 91 | 100 | 96 | 81 | 78 | 79 | 78 |
| FJNP C3L | 63 | 66 | 72 | 72 | 73 | 74 | 75 | 73 | 87 | 90 | 89 | 88 | 90 | 91 | 96 | 100 | 81 | 78 | 78 | 78 |
| JLCC C3L | 60 | 63 | 67 | 66 | 67 | 68 | 67 | 65 | 81 | 81 | 81 | 78 | 79 | 78 | 81 | 81 | 100 | 94 | 89 | 91 |
| JLCC C3L | 60 | 63 | 67 | 67 | 66 | 68 | 67 | 65 | 79 | 78 | 79 | 76 | 76 | 76 | 78 | 78 | 94 | 100 | 91 | 92 |
| JLCC C3F | 67 | 70 | 73 | 73 | 73 | 74 | 72 | 70 | 80 | 81 | 82 | 81 | 80 | 79 | 79 | 78 | 89 | 91 | 100 | 95 |
| JLCC C3F | 65 | 68 | 71 | 71 | 71 | 72 | 70 | 69 | 81 | 80 | 81 | 79 | 78 | 77 | 78 | 78 | 91 | 92 | 95 | 100 |
We took the information on volatiles as the calculation object to determine the similarity of each sample (Table 2); two parallel samples were used for each sample. Table 2 shows that the similarity of the same grade tobacco samples from the same producing area in GDNX was higher than 93, and the similarity of C3F and C4F was 94. Therefore, to judge the grade of tobacco samples in GDNX, the similarity degree with known samples was supposed to be higher than 94. The similarity between the same grade of tobacco samples from the same producing area in FJNP and JLCC was the lowest at 94. The similarity between samples of different grades of tobacco was the highest at 92. Therefore, to judge the grade of tobacco samples from the two places, the similarity of known samples ought to reach 93. The similarity between tobacco samples from GDNX and those from the two other regions was the highest at 80, and the lowest at 72. Therefore, the similarity between samples from GDNX and those from GDNX ought to reach above 80 to determine whether the tobacco samples were produced in GDNX. The similarity between tobacco samples from FJNP and those from other two regions was 81, while the similarity between tobacco samples from FJNP was 85. Therefore, to judge whether the tobacco sample was produced in FJNP, the similarity between the sample and Nanping should reach 85. The similarity between the tobacco samples from JLCC and the other two regions was the highest at 82 and the lowest at 89. Therefore, the similarity between the tobacco samples from JLCC should be at least 89.
4 Conclusion
The concentration difference of aroma substances in samples from different regions or different samples from the same region could be given by GC-IMS spectrum. The response fingerprints data could qualitatively and intuitively compare the differences in concentrations of aroma substances in different tobacco samples. The integral data of chromatography can be given accurately and quantitatively differences of different tobacco samples. The origin and grade of the unknown tobacco sample could be determined by the similarity between the unknown tobacco sample and the known tobacco sample. The minimum similarity was 81 to determine whether the sample was from GDNX province; it was 85 to determine whether it is from FJNP and 94 for JLCC. If the grade of tobacco samples of unknown origin was determined, then the similarity between tobacco samples from GDNX province should reach a minimum of 95.
Acknowledgments
The authors gratefully thank Tiejun Yan (Hubei China Tobacco Industry Co., Ltd, Wuhan, China) for his assistance in collection of tobacco samples.
-
Funding information: This study was funded by Basic Research Project of Hubei Tobacco Corporation (No. 027Y2018-028) and China Tobacco Hubei Industrial LLC (No. 2018420000340428).
-
Author contributions: Conceptualization and funding acquisition, Jialei Liu; data curation and formal analysis, Guangjiong Qin; investigation and methodology, Guojie Zhao; writing, Canbin Ouyang. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors declare that there is no conflict of interest
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
[1] Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. New York: CRC press; 2013.Suche in Google Scholar
[2] Devi D, Lakshminarayana R, Atluri J. Heterosis for seed and other quantitative characters in tobacco. Indian J Agric Res. 2006;40:10–17.Suche in Google Scholar
[3] Ding L, Xie F, Zhao M, Wang S, Xie J, Xu G. Rapid quantification of sucrose esters in oriental tobacco by liquid chromatography-ion trap mass spectrometry. J Sep Sci. 2007;30:35–41.10.1002/jssc.200600270Suche in Google Scholar
[4] Filipchuk O, Shuraeva G. Influence of preparation of class polyguanidines on quality parameters and chemical composition of tobacco raw material. Khranenie i pererabotka selkhozsyr ya. 2010;2010:41–3.Suche in Google Scholar
[5] Hu R, Wang J, Li H, Ni H, Chen Y, Zhang Y. Simultaneous extraction of nicotine and solanesol from waste tobacco materials by the column chromatographic extraction method and their separation and purification. Sep Purif Technol. 2015;146:1–7.10.1016/j.seppur.2015.03.016Suche in Google Scholar
[6] Fujimori T, Kasuga R, Kaneko H, Noguchi M. Neutral volatile components of Burley tobacco. Beitrage Zur Tabakforschung Int. 1978;9:317–25.10.2478/cttr-2013-0950Suche in Google Scholar
[7] Qin S, Wang Z, Shi J. Quality characteristics of tobacco leaves with different aromatic styles from Guizhou Province, China. Agric Sci China. 2007;6:220–6.10.1016/S1671-2927(07)60038-8Suche in Google Scholar
[8] Zhang Y, Zheng H, Zhou J, Wang R. The aroma characters and difference analysis of main conventional chemical compositions in different flue-cured tobacco production regions. J Hunan Agric Univ. 2007;33:568–71.Suche in Google Scholar
[9] Dagnon S, Tasheva R, Stoilova A, Christeva D, Edreva A. Evaluation of aroma in oriental tobaccos as based on valeric acid gas chromatography. Beitrage zur Tabakforschung Int. 2008;23:115–20.10.2478/cttr-2013-0854Suche in Google Scholar
[10] Liao F, Li Y, He W, Tie J, Hao X, Tian Y, et al. Evaluation of aroma styles in flue-cured tobacco by near infrared spectroscopy combined with chemometric algorithms. J Near Infrared Spec. 2020;28:0967033519898892.10.1177/0967033519898892Suche in Google Scholar
[11] Popova V, Ivanova T, Prokopov T, Nikolova M, Stoyanova A, Zheljazkov V. Carotenoid-related volatile compounds of tobacco (Nicotiana tabacum L.) essential oils. Molecules. 2019;24:3446.10.3390/molecules24193446Suche in Google Scholar PubMed PubMed Central
[12] Ma F, Zhang L, Liu B, Zhang W. Construction of a fractional condensation device and its application in the analysis of volatile compounds from tobacco. Anal Methods. 2015;7:621–8.10.1039/C4AY02280GSuche in Google Scholar
[13] Zeng S, Li P, Sun S, Lu B, Wu P, Zhang X, et al. Aroma compounds in the thermal reaction flavorings of tobacco enzymatic hydrolysate. J Food Saf Qual. 2015;6:4110–20.Suche in Google Scholar
[14] Nedeltcheva-Antonova D, Ivanova D, Antonov L, Abe I. Insight into the aroma profile of Bulgarian tobacco absolute oil. Ind Crop Products. 2016;94:226–32.10.1016/j.indcrop.2016.08.047Suche in Google Scholar
[15] Farag M, Elmassry M, El-Ahmady S. The characterization of flavored hookahs aroma profile and in response to heating as analyzed via headspace solid-phase microextraction (SPME) and chemometrics. Sci Rep. 2018;8:17028.10.1038/s41598-018-35368-6Suche in Google Scholar PubMed PubMed Central
[16] Liu L, Wang X, Wang S, Liu S, Jia Y, Qin Y, et al. Simultaneous quantification of ten Amadori compounds in tobacco using liquid chromatography with tandem mass spectrometry. J Sep Sci. 2017;40:849–57.10.1002/jssc.201601168Suche in Google Scholar PubMed
[17] Chen S, Huang M, Lin Y, Tao H, Hu Y, Ye W, et al. Liquid chromatography coupled with multivariate statistics for investigation of relationship between polyphenols and aroma types of tobacco. Chin J Anal Chem. 2019;47:725–30.Suche in Google Scholar
[18] Wu L, He Z, Wu Y, Liu J, Li C, Cao J, et al. Evaluation of aroma components in chinese southwest tobacco by headspace gas chromatography-mass spectrometry. Asian J Chem. 2013;25:8853–8.10.14233/ajchem.2013.14698Suche in Google Scholar
[19] Mitsui K, David F, Dumont E, Ochiai N, Tamura H, Sandra P. LC fractionation followed by pyrolysis GC-MS for the in-depth study of aroma compounds formed during tobacco combustion. J Anal Appl Pyrolysis. 2015;116:68–74.10.1016/j.jaap.2015.10.004Suche in Google Scholar
[20] He Q, Zhang Y, Zhou S, She S, Chen G, Chen K, et al. Estimating the aroma glycosides in flue-cured tobacco by solid-phase extraction and gas chromatography-mass spectrometry: changes in the bound aroma profile during leaf maturity. Flavour Fragr J. 2015;30:230–7.10.1002/ffj.3235Suche in Google Scholar
[21] Wu L, Li Q, Li C, Cao J, Lai Y, Qiu K, et al. Determination of aroma components in chinese southwest tobacco by directly suspended droplet microextraction combined with GC-MS. J Chromatogr Sci. 2014;52:1317–25.10.1093/chromsci/bmt170Suche in Google Scholar PubMed
[22] Budzynska E, Sielemann S, Puton J, Surminski A. Analysis of e-liquids for electronic cigarettes using GC-IMS/MS with headspace sampling. Talanta. 2020;209:120594.10.1016/j.talanta.2019.120594Suche in Google Scholar PubMed
[23] Ishikawa N, Sekiguchi K. Measurements of the size and composition of volatile particles generated from a heated tobacco product with aerosol fixation agents. Aerosol Air Qual Res. 2018;18:2538–49.10.4209/aaqr.2018.02.0049Suche in Google Scholar
[24] Contreras M, Jurado-Campos N, Callado C, Arroyo-Manzanares N, Fernandez L, Casano S, et al. Thermal desorption-ion mobility spectrometry: A rapid sensor for the detection of cannabinoids and discrimination of Cannabis sativa L. chemotypes. Sens Actuators B Chem. 2018;273:1413–24.10.1016/j.snb.2018.07.031Suche in Google Scholar
© 2021 Guangjiong Qin et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation
Artikel in diesem Heft
- Regular Articles
- Qualitative and semi-quantitative assessment of anthocyanins in Tibetan hulless barley from different geographical locations by UPLC-QTOF-MS and their antioxidant capacities
- Effect of sodium chloride on the expression of genes involved in the salt tolerance of Bacillus sp. strain “SX4” isolated from salinized greenhouse soil
- GC-MS analysis of mango stem bark extracts (Mangifera indica L.), Haden variety. Possible contribution of volatile compounds to its health effects
- Influence of nanoscale-modified apatite-type calcium phosphates on the biofilm formation by pathogenic microorganisms
- Removal of paracetamol from aqueous solution by containment composites
- Investigating a human pesticide intoxication incident: The importance of robust analytical approaches
- Induction of apoptosis and cell cycle arrest by chloroform fraction of Juniperus phoenicea and chemical constituents analysis
- Recovery of γ-Fe2O3 from copper ore tailings by magnetization roasting and magnetic separation
- Effects of different extraction methods on antioxidant properties of blueberry anthocyanins
- Modeling the removal of methylene blue dye using a graphene oxide/TiO2/SiO2 nanocomposite under sunlight irradiation by intelligent system
- Antimicrobial and antioxidant activities of Cinnamomum cassia essential oil and its application in food preservation
- Full spectrum and genetic algorithm-selected spectrum-based chemometric methods for simultaneous determination of azilsartan medoxomil, chlorthalidone, and azilsartan: Development, validation, and application on commercial dosage form
- Evaluation of the performance of immunoblot and immunodot techniques used to identify autoantibodies in patients with autoimmune diseases
- Computational studies by molecular docking of some antiviral drugs with COVID-19 receptors are an approach to medication for COVID-19
- Synthesis of amides and esters containing furan rings under microwave-assisted conditions
- Simultaneous removal efficiency of H2S and CO2 by high-gravity rotating packed bed: Experiments and simulation
- Design, synthesis, and biological activities of novel thiophene, pyrimidine, pyrazole, pyridine, coumarin and isoxazole: Dydrogesterone derivatives as antitumor agents
- Content and composition analysis of polysaccharides from Blaps rynchopetera and its macrophage phagocytic activity
- A new series of 2,4-thiazolidinediones endowed with potent aldose reductase inhibitory activity
- Assessing encapsulation of curcumin in cocoliposome: In vitro study
- Rare norisodinosterol derivatives from Xenia umbellata: Isolation and anti-proliferative activity
- Comparative study of antioxidant and anticancer activities and HPTLC quantification of rutin in white radish (Raphanus sativus L.) leaves and root extracts grown in Saudi Arabia
- Comparison of adsorption properties of commercial silica and rice husk ash (RHA) silica: A study by NIR spectroscopy
- Sodium borohydride (NaBH4) as a high-capacity material for next-generation sodium-ion capacitors
- Aroma components of tobacco powder from different producing areas based on gas chromatography ion mobility spectrometry
- The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam
- Synthesis, properties, and activity of MoVTeNbO catalysts modified by zirconia-pillared clays in oxidative dehydrogenation of ethane
- Synthesis and crystal structure of N,N′-bis(4-chlorophenyl)thiourea N,N-dimethylformamide
- Quantitative analysis of volatile compounds of four Chinese traditional liquors by SPME-GC-MS and determination of total phenolic contents and antioxidant activities
- A novel separation method of the valuable components for activated clay production wastewater
- On ve-degree- and ev-degree-based topological properties of crystallographic structure of cuprite Cu2O
- Antihyperglycemic effect and phytochemical investigation of Rubia cordifolia (Indian Madder) leaves extract
- Microsphere molecularly imprinted solid-phase extraction for diazepam analysis using itaconic acid as a monomer in propanol
- A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species
- Machine vision-based driving and feedback scheme for digital microfluidics system
- Study on the application of a steam-foam drive profile modification technology for heavy oil reservoir development
- Ni–Ru-containing mixed oxide-based composites as precursors for ethanol steam reforming catalysts: Effect of the synthesis methods on the structural and catalytic properties
- Preparation of composite soybean straw-based materials by LDHs modifying as a solid sorbent for removal of Pb(ii) from water samples
- Synthesis and spectral characterizations of vanadyl(ii) and chromium(iii) mixed ligand complexes containing metformin drug and glycine amino acid
- In vitro evaluation of lactic acid bacteria with probiotic activity isolated from local pickled leaf mustard from Wuwei in Anhui as substitutes for chemical synthetic additives
- Utilization and simulation of innovative new binuclear Co(ii), Ni(ii), Cu(ii), and Zn(ii) diimine Schiff base complexes in sterilization and coronavirus resistance (Covid-19)
- Phosphorylation of Pit-1 by cyclin-dependent kinase 5 at serine 126 is associated with cell proliferation and poor prognosis in prolactinomas
- Molecularly imprinted membrane for transport of urea, creatinine, and vitamin B12 as a hemodialysis candidate membrane
- Optimization of Murrayafoline A ethanol extraction process from the roots of Glycosmis stenocarpa, and evaluation of its Tumorigenesis inhibition activity on Hep-G2 cells
- Highly sensitive determination of α-lipoic acid in pharmaceuticals on a boron-doped diamond electrode
- Synthesis, chemo-informatics, and anticancer evaluation of fluorophenyl-isoxazole derivatives
- In vitro and in vivo investigation of polypharmacology of propolis extract as anticancer, antibacterial, anti-inflammatory, and chemical properties
- Topological indices of bipolar fuzzy incidence graph
- Preparation of Fe3O4@SiO2–ZnO catalyst and its catalytic synthesis of rosin glycol ester
- Construction of a new luminescent Cd(ii) compound for the detection of Fe3+ and treatment of Hepatitis B
- Investigation of bovine serum albumin aggregation upon exposure to silver(i) and copper(ii) metal ions using Zetasizer
- Discoloration of methylene blue at neutral pH by heterogeneous photo-Fenton-like reactions using crystalline and amorphous iron oxides
- Optimized extraction of polyphenols from leaves of Rosemary (Rosmarinus officinalis L.) grown in Lam Dong province, Vietnam, and evaluation of their antioxidant capacity
- Synthesis of novel thiourea-/urea-benzimidazole derivatives as anticancer agents
- Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens
- Simple modifications of nicotinic, isonicotinic, and 2,6-dichloroisonicotinic acids toward new weapons against plant diseases
- Synthesis, optical and structural characterisation of ZnS nanoparticles derived from Zn(ii) dithiocarbamate complexes
- Presence of short and cyclic peptides in Acacia and Ziziphus honeys may potentiate their medicinal values
- The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus
- Quantitative structure–activity relationship study on prolonged anticonvulsant activity of terpene derivatives in pentylenetetrazole test
- GADD45B induced the enhancing of cell viability and proliferation in radiotherapy and increased the radioresistance of HONE1 cells
- Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development
- Dynamics of λ-cyhalothrin disappearance and expression of selected P450 genes in bees depending on the ambient temperature
- Identification of synthetic cannabinoid methyl 2-{[1-(cyclohexylmethyl)-1H-indol-3-yl] formamido}-3-methylbutanoate using modern mass spectrometry and nuclear magnetic resonance techniques
- Study on the speciation of arsenic in the genuine medicinal material honeysuckle
- Two Cu(ii)-based coordination polymers: Crystal structures and treatment activity on periodontitis
- Conversion of furfuryl alcohol to ethyl levulinate in the presence of mesoporous aluminosilicate catalyst
- Review Articles
- Hsien Wu and his major contributions to the chemical era of immunology
- Overview of the major classes of new psychoactive substances, psychoactive effects, analytical determination and conformational analysis of selected illegal drugs
- An overview of persistent organic pollutants along the coastal environment of Kuwait
- Mechanism underlying sevoflurane-induced protection in cerebral ischemia–reperfusion injury
- COVID-19 and SARS-CoV-2: Everything we know so far – A comprehensive review
- Challenge of diabetes mellitus and researchers’ contributions to its control
- Advances in the design and application of transition metal oxide-based supercapacitors
- Color and composition of beauty products formulated with lemongrass essential oil: Cosmetics formulation with lemongrass essential oil
- The structural chemistry of zinc(ii) and nickel(ii) dithiocarbamate complexes
- Bioprospecting for antituberculosis natural products – A review
- Recent progress in direct urea fuel cell
- Rapid Communications
- A comparative morphological study of titanium dioxide surface layer dental implants
- Changes in the antioxidative properties of honeys during their fermentation
- Erratum
- Erratum to “Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study”
- Erratum to “Modified TDAE petroleum plasticiser”
- Corrigendum
- Corrigendum to “A nitric oxide-releasing prodrug promotes apoptosis in human renal carcinoma cells: Involvement of reactive oxygen species”
- Special Issue on 3rd IC3PE 2020
- Visible light-responsive photocatalyst of SnO2/rGO prepared using Pometia pinnata leaf extract
- Antihyperglycemic activity of Centella asiatica (L.) Urb. leaf ethanol extract SNEDDS in zebrafish (Danio rerio)
- Selection of oil extraction process from Chlorella species of microalgae by using multi-criteria decision analysis technique for biodiesel production
- Special Issue on the 14th Joint Conference of Chemistry (14JCC)
- Synthesis and in vitro cytotoxicity evaluation of isatin-pyrrole derivatives against HepG2 cell line
- CO2 gas separation using mixed matrix membranes based on polyethersulfone/MIL-100(Al)
- Effect of synthesis and activation methods on the character of CoMo/ultrastable Y-zeolite catalysts
- Special Issue on Electrochemical Amplified Sensors
- Enhancement of graphene oxide through β-cyclodextrin composite to sensitive analysis of an antidepressant: Sulpiride
- Investigation of the spectroelectrochemical behavior of quercetin isolated from Zanthoxylum bungeanum
- An electrochemical sensor for high sensitive determination of lysozyme based on the aptamer competition approach
- An improved non-enzymatic electrochemical sensor amplified with CuO nanostructures for sensitive determination of uric acid
- Special Issue on Applied Biochemistry and Biotechnology 2020
- Fast discrimination of avocado oil for different extracted methods using headspace-gas chromatography-ion mobility spectroscopy with PCA based on volatile organic compounds
- Effect of alkali bases on the synthesis of ZnO quantum dots
- Quality evaluation of Cabernet Sauvignon wines in different vintages by 1H nuclear magnetic resonance-based metabolomics
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Diatomaceous Earth: Characterization, thermal modification, and application
- Electrochemical determination of atenolol and propranolol using a carbon paste sensor modified with natural ilmenite
- Special Issue on the Conference of Energy, Fuels, Environment 2020
- Assessment of the mercury contamination of landfilled and recovered foundry waste – a case study
- Primary energy consumption in selected EU Countries compared to global trends
- Modified TDAE petroleum plasticiser
- Use of glycerol waste in lactic acid bacteria metabolism for the production of lactic acid: State of the art in Poland
- Topical Issue on Applications of Mathematics in Chemistry
- Theoretical study of energy, inertia and nullity of phenylene and anthracene
- Banhatti, revan and hyper-indices of silicon carbide Si2C3-III[n,m]
- Topical Issue on Agriculture
- Occurrence of mycotoxins in selected agricultural and commercial products available in eastern Poland
- Special Issue on Ethnobotanical, Phytochemical and Biological Investigation of Medicinal Plants
- Acute and repeated dose 60-day oral toxicity assessment of chemically characterized Berberis hispanica Boiss. and Reut in Wistar rats
- Phytochemical profile, in vitro antioxidant, and anti-protein denaturation activities of Curcuma longa L. rhizome and leaves
- Antiplasmodial potential of Eucalyptus obliqua leaf methanolic extract against Plasmodium vivax: An in vitro study
- Prunus padus L. bark as a functional promoting component in functional herbal infusions – cyclooxygenase-2 inhibitory, antioxidant, and antimicrobial effects
- Molecular and docking studies of tetramethoxy hydroxyflavone compound from Artemisia absinthium against carcinogens found in cigarette smoke
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2020)
- Preparation of cypress (Cupressus sempervirens L.) essential oil loaded poly(lactic acid) nanofibers
- Influence of mica mineral on flame retardancy and mechanical properties of intumescent flame retardant polypropylene composites
- Production and characterization of thermoplastic elastomer foams based on the styrene–ethylene–butylene–styrene (SEBS) rubber and thermoplastic material
- Special Issue on Applied Chemistry in Agriculture and Food Science
- Impact of essential oils on the development of pathogens of the Fusarium genus and germination parameters of selected crops
- Yield, volume, quality, and reduction of biotic stress influenced by titanium application in oilseed rape, winter wheat, and maize cultivations
- Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice
- Carryover effect of direct-fed microbial supplementation and early weaning on the growth performance and carcass characteristics of growing Najdi lambs
- Special Issue on Applied Biochemistry and Biotechnology (ABB 2021)
- The electrochemical redox mechanism and antioxidant activity of polyphenolic compounds based on inlaid multi-walled carbon nanotubes-modified graphite electrode
- Study of an adsorption method for trace mercury based on Bacillus subtilis
- Special Issue on The 1st Malaysia International Conference on Nanotechnology & Catalysis (MICNC2021)
- Mitigating membrane biofouling in biofuel cell system – A review
- Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation

