Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
-
Rafik El-Mernissi
, Naoual El Menyiy
, Gamal A. Shazly
Abstract
Moroccan Cannabis sativa L. seeds were investigated for their phenolic profile and antidiabetic potential. Ultra-high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry analysis revealed a rich phenolic composition, including benzoic acid, cannabisin B, genistein, and epicatechin. In vitro, the seed extract exhibited potent α-amylase inhibitory activity (half-maximal inhibitory concentration = 25.02 ± 4.03 μg/mL). In vivo studies in diabetic rats demonstrated significant hypoglycemic, hypolipidemic, hepatoprotective, and nephroprotective effects. Molecular docking studies further supported these findings, revealing strong interactions between identified phenolic and the α-amylase enzyme. These results highlight the potential of C. sativa seeds as a natural source of bioactive compounds for diabetes management.
1 Introduction
In recent years, there has been a growing interest in exploring natural compounds, particularly polyphenols, for their potential therapeutic effects in managing diabetes mellitus a widespread chronic metabolic disorder characterized by persistent high blood sugar levels due to inadequate insulin synthesis or poor insulin utilization. This condition affects millions globally and poses a significant social and economic burden, with an estimated 1.5 million deaths attributed to diabetes each year [1,2]. The increasing prevalence of diabetes, along with its associated complications, places considerable pressure on healthcare systems. Chronic hyperglycemia can lead to microvascular complications such as diabetic retinopathy, nephropathy, and neuropathy [3,4]. Additionally, individuals with diabetes are at a significantly higher risk for macrovascular complications, including cardiovascular disease, stroke, and peripheral artery disease [5].
Current diabetes management strategies emphasize achieving glycemic control through a combination of pharmacological therapies, lifestyle modifications, and nutritional interventions. A variety of conventional hypoglycemic agents are available that function by supplementing insulin, improving insulin sensitivity, stimulating insulin secretion, and facilitating glucose uptake. However, these medications typically target a single mechanism of action and are often associated with undesirable side effects such as diarrhea, lactic acidosis, hepatic failure, weight gain, tachycardia, and hypothyroidism. Furthermore, the development of tolerance over time necessitates frequent dosage adjustments. Unfortunately, these medications do not provide a permanent cure for diabetes; they primarily focus on managing its symptoms [6,7].
In light of these challenges, there is an urgent need to explore innovative strategies that can address these limitations and offer more effective long-term management solutions for diabetes. In this context, Cannabis sativa L., commonly referred to as marijuana or hemp, has gained attention for its potential medicinal properties [8]. Historically used for various therapeutic purposes – including pain management and antioxidant effects – the therapeutic potential of cannabis in managing diabetes has gained traction in recent years [8,9,10,11].
In Morocco, C. sativa has long been illegal but tolerated to varying degrees. A significant shift occurred in June 2021 when the Moroccan parliament passed a landmark law regulating cannabis for medical, pharmaceutical, and industrial purposes. This legislative change not only signifies a progressive step toward addressing the societal and economic dimensions of C. sativa but also opens new avenues for scientific exploration and utilization of C. sativa L. in various therapeutic applications, including diabetes management [12]. Given this evolving landscape, investigating the antidiabetic properties of polyphenols derived from cannabis seeds emerges as a compelling avenue for research. Polyphenols are known for their antioxidant and anti-inflammatory properties and have shown promise in modulating glucose metabolism and improving insulin sensitivity – making them attractive candidates for diabetes management [13]. The unique composition of polyphenols in cannabis seeds such as cannabisins and cannaflavins [14,15] coupled with recent legal developments in Morocco and the country’s specific environmental factors presents an opportune moment to delve into the therapeutic potential of this natural resource.
In light of these considerations, this study aims to explore the phenolic profile and antidiabetic properties of Moroccan C. sativa L. seeds through a multifaceted approach encompassing in vivo, in vitro, and in silico methodologies. By elucidating the biochemical composition and pharmacological effects of cannabis seed-derived polyphenols, this research seeks to contribute valuable insights toward developing novel therapeutic strategies for diabetes management.
2 Materials and methods
2.1 Plant collection
Plant samples were collected in September 2021 from the Tafrant region, Taounate, Morocco (34°39′28.4″N, 5°05′58.9″W). Subsequent to the collection, the plants were air-dried in a shaded environment and the seeds were isolated. These seeds were then stored in airtight plastic bags under ambient temperature conditions (24–27°C) until further analysis.
2.2 Plant identification
A botanist at the Scientific Institute of Rabat identified the plant, and a voucher specimen labeled with the number RAB 112735 was archived in the institute’s herbarium.
2.3 Quality control of plant material of C. sativa L. seeds
2.3.1 Oil content
C. sativa L. seeds after being cleaned and dried to achieve a consistent dry weight were processed by grinding using a mill. The oil content of the ground samples was assessed using a Soxhlet apparatus with hexane as solvent. The solvent was then evaporated using a rotary vacuum evaporator, and the oil content of the C. sativa L. seeds was quantified following a method outlined in the AOAC [16].
2.3.2 Ash content
The ash content was assessed following the procedure outlined by Laaroussi et al. [17]. Initially, the C. sativa L. seeds underwent preliminary ashing at 500°C for 4 h, followed by measurement of the resulting ash content. The residue was subsequently weighed three times, and the percentage of ash (%) was calculated according to the formula:
2.3.3 Organic material content
The quantity of organic material was computed using the equation [17]:
2.3.4 Humidity level
The moisture content of C. sativa L. seeds was determined through a meticulous gravimetric analysis. This method entails precisely measuring the weight of the sample before and after drying in a convection oven at 105°C until a constant weight is achieved [16].
2.3.5 pH determination
A mass of 5 g of the sample was mixed with 500 mL of distilled water. The mixture was stirred for 5 min at room temperature. The mixture was filtered, and the pH was measured using a pH meter [18].
2.4 Extract preparation
The study commenced with the air-drying and mechanical pulverization of fresh C. sativa L. seeds to produce a powdered form. A portion of 200 g of this powder was subjected to maceration with a 70% ethanol solution while being continuously stirred for 48 h at room temperature. Post-maceration, the resulting mixture underwent filtration utilizing Whatman filter paper No. 1 to remove any insoluble materials. The filtrate obtained was then concentrated at 40°C under vacuum conditions using the Buchi rotary evaporator R-250 to eliminate the solvent. The concentrated extract was subsequently subjected to freeze-drying to obtain the lyophilized extract, which exhibited a yield of 23% w/w. Following extraction, the obtained extract was carefully stored in a refrigerator (4°C) until it was required for further analysis and experimentation.
2.5 Experimental animal
Male albino Wistar rats, weighing between 190 and 250 g, were procured for this research from the animal facility of the Bioactives and Environmental Health Laboratory at the Faculty of Sciences, Moulay Ismail University, Meknes, Morocco. The animals were housed in Plexiglas cages under standard laboratory conditions, maintaining a temperature of 25 ± 2°C, humidity levels of 55 ± 5%, and a 12-h light/dark cycle. Throughout the experimental period, the rats had ad libitum access to both food and water. All animal care and experimental protocols adhered strictly to ethical guidelines as outlined in authorization number 86/609/EC20.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals, and has been approved by the ethical committee of the Faculty of Sciences, Moulay Ismail University, Morocco.
2.6 Phytochemical screening
An assessment was conducted to screen for steroids/terpenoids, alkaloids, flavonoids, saponins, phenols, tannins, coumarins, and free quinone. This evaluation relied on observing color intensity or the formation of precipitates, which are recognized as key analytical indicators for these tests [19].
2.7 Identification of phenolic compound
Phytochemical analyses of cannabis seed extract were carried out using ultra-high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry (UHPLC-DAD–ESI/MS), as described previously by Zefzoufi et al. [20]. The experimentation was conducted using an Ultimate 3000 system (Dionex, A, USA), which featured a quaternary pump (HPG 3400 RS), an autosampler (WPS 3000 TSL), and a column oven (TCC 3000). For the method, a Kinetex C18 reversed-phase column (250 mm × 4.6 mm, 2.6 μm particles) supplied by Thermo Fisher Scientific (CA, USA) was employed. The chromatographic separation was achieved using a gradient of solvent A (0.1% formic acid aqueous solution) and solvent B (methanol), with the following profile: 0–3 min, a linear gradient from 5 to 25% B; 3–6 min, held at 25% B; 6–9 min, a gradient from 25 to 37% B; 9–13 min, held at 37% B; 13–18 min, a gradient from 37 to 54% B; 18–22 min, held at 54% B; 22–26 min, a gradient from 54 to 95% B; 26–29 min, held at 95% B; 29–29.15 min, returned to initial conditions at 5% B; and from 29.15 to 36 min, held at 5% B. The flow rate of the mobile phase was maintained at 1 mL/min. Ultra-violet visible spectral data for all peaks were recorded in the range of 200–400 nm, while chromatographic profiles were monitored at 280 nm. The mass spectrometer utilized was a TSQ Endura (Thermo Fisher Scientific, CA, USA) triple quadrupole equipped with heated-electrospray ionization (H-ESI) source operating in negative mode. Nitrogen (purity >99.98%) served as the sheath gas, ion sweep gas, and auxiliary gas at flow rates of 65, 0, and 40 arbitrary units (a.u.), respectively. H-ESI vaporizer and ion transfer tube temperatures were set at 350°C. The electrospray voltage was maintained at 2.5 kV. For characterization and evaluation, the full scan MS acquisition mode (m/z 50–1,000) in Q1 with a mass resolution of 0.7 m/z full-width half maximum (FWHM) and a scan time of 0.5 s was primarily utilized.
2.8 Effect of hydro-ethanolic extract on alloxan-induced diabetic rats
2.8.1 Diabetes induction and study design
Diabetes was triggered by administering a single intraperitoneal injection of 150 mg/kg body weight of alloxan monohydrate solution to albino Wistar rats that had been fasted for 12–14 h [21]. To avoid hypoglycemic shock following diabetes induction, the rats were provided access to 10% glucose solution bottles for the next 24 h. One week post-injection, blood glucose levels were measured, and animals were classified as diabetic if their fasting blood glucose level reached or surpassed 200 mg/dL.
Twenty-four selected animals, including both diabetic and non-diabetic subjects, were divided into four groups, each consisting of six individuals (n = 6):
Group 1 normal healthy control consisted of six non-diabetic rats, who were administered distilled water orally at a dosage of 10 mL/kg of body weight per day.
Group 2 diabetic control (DC) comprised six diabetic rats, who were orally administered a dosage of distilled water at 10 mL/kg of body weight per day.
Group 3, treated group (TG) six diabetic rats were orally treated with a hydro-ethanolic extract of C. sativa L. seeds (CSSE) dissolved in distilled water at a dosage of 300 mg/kg of body weight per day.
Group 4 (DG) involved six diabetic rats treated with glibenclamide, a reference drug, at a dosage of 2 mg/kg of body weight per day.
The study lasted for a total of 28 days, during which all treatments were administered orally once every morning via a feeding tube prior to the animals’ meals. Throughout the treatment period, body weight and fasting blood glucose levels were monitored by collecting tail blood samples and analyzing them with a glucometer (Accu-Chek Active-300) on days 0, 7, 14, 21, and 28. Changes in body weight were also recorded.
At the conclusion of the 28-day experimental period, the animals were anesthetized using diethyl ether and subsequently euthanized by decapitation. Blood samples were promptly collected for analysis of serum biochemical parameters, including AST, ALT, urea, creatinine, LDL, HDL, cholesterol (CT), and triglycerides (Trg), using an automated chemistry analyzer (KONELAB 20i: AUTO-ANALYZER).
2.8.2 Oral glucose tolerance test (OGTT)
OGTT was conducted following the procedure outlined by Patel et al. [22], with slight modifications as illustrated in Figure 1. Briefly, normal rats that had fasted for 12 h were divided into three groups, each consisting of six individuals (n = 6). Group 1 served as the control and received distilled water (1 mL/100 g body weight), Group 2 was treated with 300 mg/kg of hydro-ethanolic extract from C. sativa L. seeds (CSSE), and Group 3 received 2 mg/kg of glibenclamide. Thirteen minutes after treatment administration, the animals were given a glucose solution at a dosage of 5 g/kg. Blood samples were collected from the tail prior to dosing and at regular intervals of 0, 30, 60, 90, and 120 min following glucose administration.

OGTT model.
2.8.3 Inhibition of α-amylase activity in vitro
The α-amylase inhibition assay was conducted in accordance with the method outlined by Aazza et al., utilizing the starch-iodine test [23]. Briefly, the assay mixture consisted of 40 µL of 0.02 M sodium phosphate buffer (pH 6.9, containing 6 mM sodium chloride), 20 µL of α-amylase solution (50 µg/mL), and 20 µL of the extract or acarbose at varying concentrations. This mixture was incubated at 37°C for 10 min. Following this, 20 µL of soluble starch (0.5%, w/v) was introduced to each well, and the incubation continued at 37°C for an additional 15 min. To halt the enzymatic reaction, 20 µL of 1 M HCl was added, followed by the addition of 100 µL of iodine reagent (5 mM KIO₃ and 5 mM KI). Color changes were observed, and absorbance was measured at 620 nm using a microplate reader. The control sample, representing 100% enzyme activity, was prepared without the addition of the extract. The inhibitory activity (%) was determined using the following formula:
with
Abs1 is the absorbance of the incubated mixture that includes the extract, starch, and α-amylase.
Abs2 refers to the absorbance of the incubated mixture with the extract and starch only.
Abs3 represents the absorbance of the mixture containing starch and α-amylase.
Abs4 is the absorbance of the incubated mixture with starch alone.
The half-maximal inhibitory concentration (IC50) was determined by curve-fitting using graphpadprism10 (10th version).
2.8.4 Inhibition of α-amylase activity in vivo
To assess the in vivo α-amylase inhibitory activity of the C. sativa seed extract (CSSE), an oral starch tolerance test was employed [24]. Briefly, three groups of fasted rats, each consisting of five animals, were established. The control group received only distilled water orally (10 mL/kg), while the CSSE group was administered the extract at a dose of 300 mg/kg. The acarbose group received 10 mg/kg of acarbose. Thirty minutes post-treatment, all rats were orally loaded with starch (2 g/kg). Blood samples were collected from the tail vein of the rats at 0, 30, 60, and 120 min to measure blood glucose levels.
2.8.5 Statistical analyses
It is expressed as mean ± SD, Statistical comparisons among the groups were conducted using a two-way analysis of variance, followed by the Tukey test, utilizing GraphPad Prism (10th version). Significance was considered at p < 0.05.
2.9 Glide molecular docking methodology
Analysis of the aqueous extract of hydro-ethanolic extract of C. sativa L. seeds showed the presence of seven polyphenols such as which served as the ligands to be docked via the maestro glide docking method with an α-amylase inhibitory (Protein Data Bank [PDB] ID: 4GQR) target proteins along with its respective co-crystallized ligand and the reference compound, acarbose.
2.9.1 Protein preparation
The PDB repository (https://www.rcsb.org) [25] was the source of an α-amylase inhibitory (PDB ID: 4GQR) target protein. The bond orders were assigned followed by the addition of hydrogen atoms and finally disulfide bonds were created in the Glide (Maestro 12.8) [26] of the protein preparation wizard panel followed by optimization of protein structure with OPLS4. The receptor grid file was then set to create the ligand binding pocket.
2.9.2 Ligand preparation
All the ligands were sketched by ChemDraw 20.1.1 followed by energy minimization via Chem3D 20.1.1 [27]. LigPrep module was incorporated to fetch sdf file format and finally prepared ligands for molecular docking (Maestro 12.8). The correct chiralities were used to create low-energy 3D structures such that at a physiological pH of 7.2 ± 0.2 and the potential ionization states were created for all of the retrieved ligand structures.
2.9.3 Receptor grid generation (RGG)
For ligand docking, RGG assists in the size and position parameters of the probable active site of the target protein. With the use of the RGG tool of Schrodinger Maestro 12.8, the scoring grid was expressed based on the binding of co-crystallized ligand in the target protein. Non-polar receptor atoms’ van der Waals (vdW) radius scaling factor was affixed to 1.0 whereas the partial charge cut-off was set at 0.25.
2.9.4 Protein–ligand docking
The created RGG file was employed to conduct molecular docking studies by using the Glide tool of Schrodinger Maestro 12.8 while standard precision was the factor to dock the already prepared ligands. For ligand atoms, the vdW radius scaling factor was scaled at 0.80 with a partial charge cut-off of 0.15.
3 Results
Significant causal relationships are evident among the results presented.
3.1 Quality control of plant material of C. sativa L. seeds
Table 1 outlines the quality control evaluations performed on C. sativa L. seeds, which revealed a moisture content of approximately 5%, an oil content of 28%, an ash content of 5.99%, a pH level of 5.3, and an organic material content of roughly 94.01%.
Oil content, ash content, organic material content, humidity level, and pH of C. sativa L. seeds
| C. sativa L. seeds | Oil content | Ash content | Organic material content | Humidity level | pH |
|---|---|---|---|---|---|
| 28 ± 1.9% | 5.99% | 94.01 | 5% | 5.3 |
These findings emphasize the critical role of maintaining optimal seed quality for preservation and therapeutic applications. The low moisture content is especially advantageous as it reduces the risk of microbial growth and spoilage, thereby extending the shelf life of the seeds. Moreover, the substantial oil content indicates that these seeds could serve as a significant source of essential fatty acids, enhancing their nutritional and medicinal value.
The slightly acidic pH level of 5.3 helps to maintain the stability of bioactive compounds within the seeds, which is essential for preserving their efficacy. Additionally, the high organic material content highlights the seeds’ potential utility in various applications, such as nutritional supplementation and the development of functional foods.
Overall, these quality control assessments provide essential insights into the characteristics of C. sativa L. seeds, underscoring their value as a versatile resource for both agricultural and medicinal purposes.
3.2 Phytochemical screening
The initial phytochemical analysis of C. sativa L. seeds, conducted using a hydro-ethanolic extract, revealed the presence of various secondary metabolites, including steroids, terpenoids, flavonoids, saponins, phenols, tannins, coumarins, and quinones, while alkaloids were notably absent (Table 2). These findings suggest that C. sativa L. seeds serve as a rich reservoir of secondary metabolites. The significant presence of these phytochemicals underscores the importance of C. sativa L. as a medicinal plant.
The qualitative analysis of C. sativa L. seeds hydro-ethanolic extract
| Alkaloids | −− |
| Tannins | ++ |
| Phenols | ++ |
| flavonoids | ++ |
| Saponin | ++ |
| Coumarins | ++ |
| Carbohydrates | ++ |
| Terpenes | ++ |
| Quinones | ++ |
| Steroids | ++ |
−− Absent, ++ present.
3.3 Identification of phenolic compound
The hydro-ethanolic extract from C. sativa L. seeds (CSSE) was subjected to qualitative analysis using UHPLC-DAD–ESI/MS) to identify the major components potentially involved in its anti-diabetic effects (Figure 2).
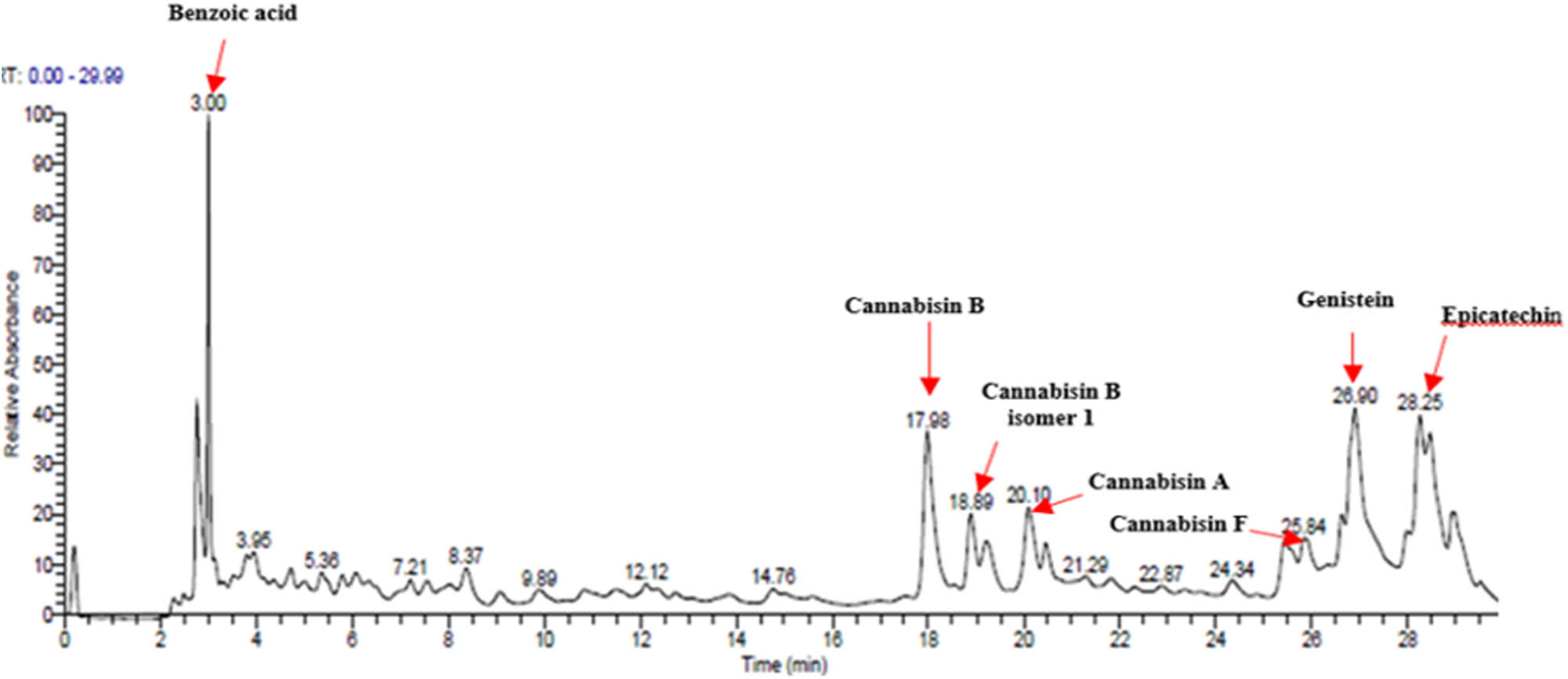
Chromatographic profile of CSSE obtained by UHPLC–ESI/MS at 280 nm.
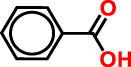
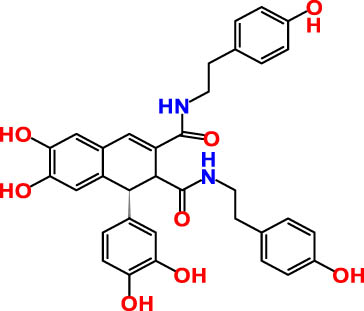
As detailed in Table 3 a total of seven phenolic compounds were successfully identified and categorized into two subgroups: phenolic acids and flavonoids. The predominant compounds in the CSSE were benzoic acid (35.98%), cannabisin B (18.68%), genistein (9.55%), and epicatechin (12.92%). These findings highlight the significant presence of bioactive compounds within C. sativa L. seeds that may contribute to their therapeutic potential, particularly in the context of diabetes management. The predominance of benzoic acid suggests a strong antioxidant capacity, which is essential for mitigating oxidative stress associated with diabetes [28]. Additionally, the presence of flavonoids such as genistein and epicatechin is noteworthy, as these compounds have been linked to enhanced insulin sensitivity and improved glucose metabolism [29,30].
Compounds identified by UHPLC-DAD–ESI/MS in CSSE
| No | RT | UV | m/z (M–H)- | Proposed compounds | Subclass | % Area | Chemical structures |
|---|---|---|---|---|---|---|---|
| 1 | 3 | 272 | 121.12 | Benzoic acid | Benzoic acid | 35.98 |
 |
| 2 | 17.98 | 285.335 | 595.4 | Cannabisin B | Lignanamide | 18.68 |
 |
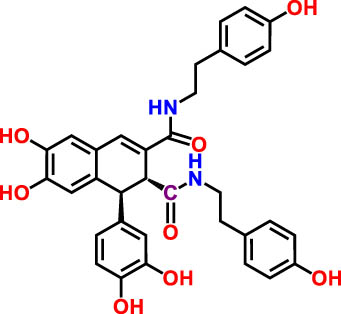
| 3 | 18.89 | 285.335 | 595.4 | Cannabisin B isomer 1 | Lignanamide | 4.24 |
 |
| 4 | 20.1 | 255 | 593.12 | Cannabisin A | Lignanamide | 4.46 |
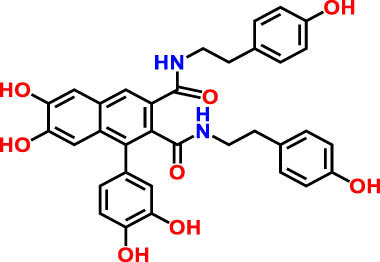 |
| 5 | 25.84 | 220.289 | 623.14 | Cannabisin F | Lignanamide | 3.85 |
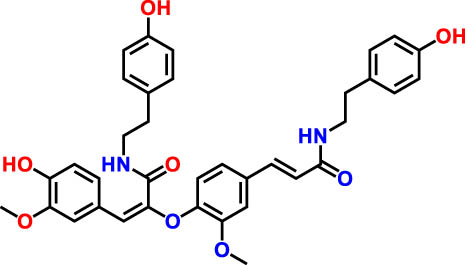 |
| 6 | 26.9 | 262 | 269.1 | Genistein | Isoflavone | 9.55 |
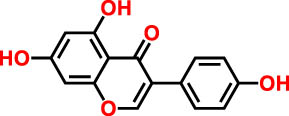 |
| 7 | 28.25 | 277 | 289.12 | Epicatechin | Flavan | 12.92 |
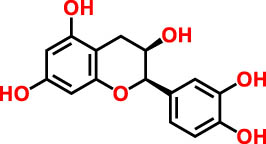 |
3.4 Effect of hydro-ethanolic extract on alloxan-induced diabetic rats
3.4.1 Effect of CSSE on body weight
It is widely recognized that a decrease in body weight is one of the most straightforward indicators of diabetes [31]. Table 4 illustrates the changes in body weight for each group over a span of four weeks. Based on these findings, all diabetic groups exhibited significant weight loss compared to the normal control group during the test period, particularly after 28 days (p < 0.0001). In contrast, the control group demonstrated an increase in body weight. The observed decrease in body weight in diabetic rats can be attributed to the increased breakdown of fats and structural proteins, which serve as alternative energy sources due to limited carbohydrate availability.
Effects of CSSE on animals’ body weights during 28 days
| Body weight (g) | |||||
|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 14 | Day 21 | Day 28 | |
| Normal control | 214.6 ± 1.9 | 213.6 ± 2.15### | 215.4 ± 1.85#### | 219 ± 2.6#### | 226.8 ± 1.6#### |
| Diabetic control | 219.6 ± 2.24 | 205.6 ± 2.44*** | 177.2 ± 2.31**** | 139.8 ± 2.63**** | 129 ± 3.03**** |
| Diabetic + glibenclamide (2 mg/kg) | 220.4 ± 3 | 212.4 ± 1.85Ns, ### | 207.8 ± 1.72****, #### | 204.6 ± 1.35****, #### | 207 ± 2.28****, #### |
| Diabetic + CSSE 300 mg/kg | 222.4 ± 2.57 | 212.8 ± 2.56Ns, ### | 201 ± 2.89****, #### | 195.2 ± 172****, #### | 182.4 ± 2.33****, #### |
Values are represented as means ± SD (n = 5 rats). ****p < 0.0001, ***p < 0.001, compared to normal controls; #### p < 0.0001, ### p < 0.001, compared to diabetic control; ns = not significant.
3.4.2 Effect of CCSE on fasting blood glucose
Diabetes is marked by hyperglycemia, which involves elevated levels of glucose in the bloodstream. Alloxan is a well-known diabetogenic compound that specifically targets and damages pancreatic β cells, leading to their destruction and thus aiding in the development of type 1 diabetes. The fasting blood glucose levels of rats were measured before diabetes induction at days 1, 7, 14, 21, and 28, during the treatment period, as illustrated in Figure 3. All treatment groups exhibited normal blood glucose levels prior to the induction of diabetes, and there were no statistically significant differences observed among them, after diabetes induction (day 0), the glucose concentration was high significantly elevated in diabetic groups compared to the normal group.
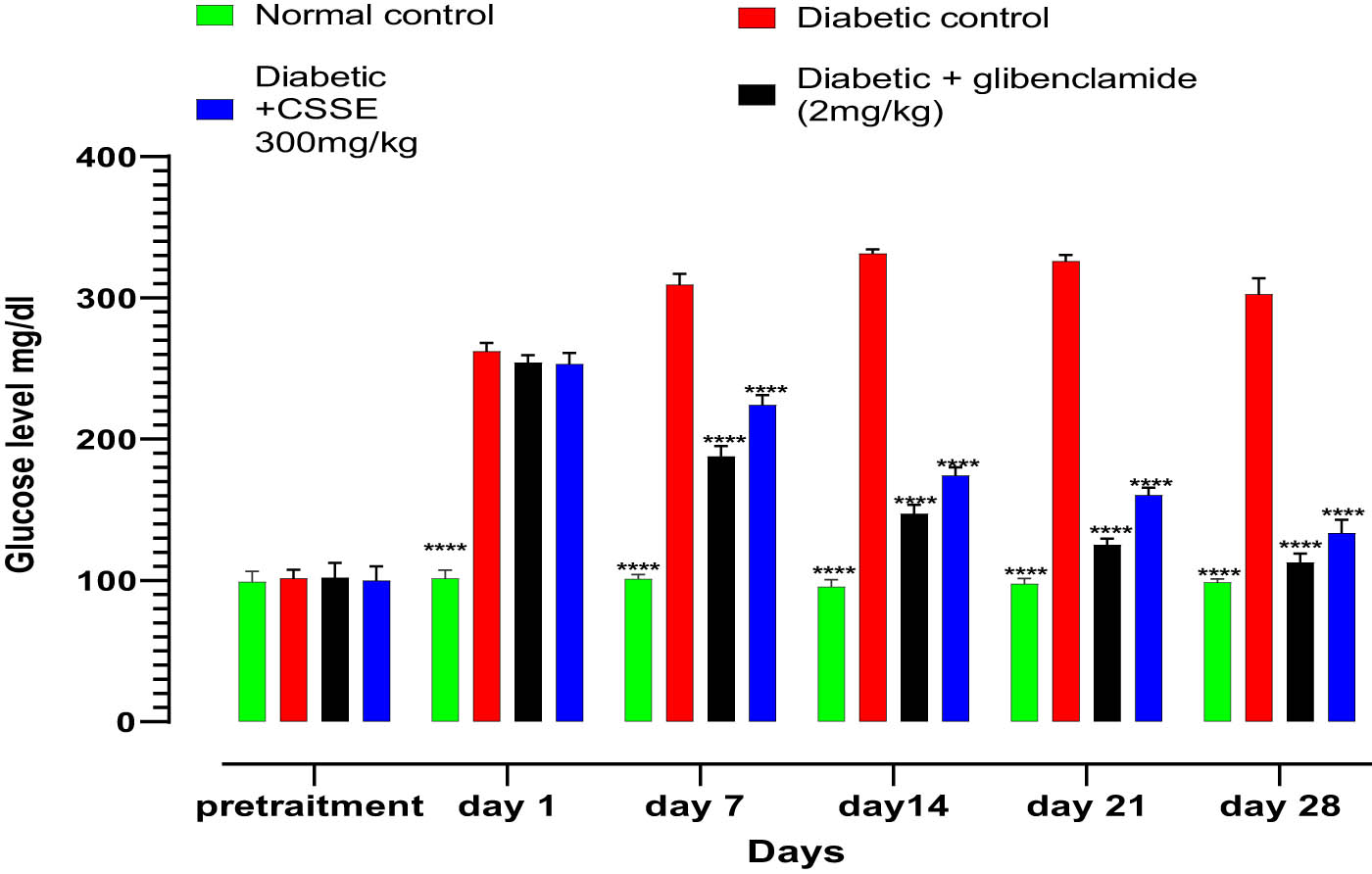
Effects of CSSE on fasting blood glucose levels in normal and alloxan-induced diabetic rats, values are represented as means ± SD (n = 5 rat); ****p < 0.0001, compared to DC.
As evidenced by the data presented in the same figure, the daily administration of CSSE (300 mg/kg) led to a significant (p < 0.0001) decrease in blood glucose levels compared to the DC group starting from day 7, the same result was observed in the glibenclamide (2 mg/kg) group. Upon comparison of mean blood glucose levels on day 28 to those on day 1, a significant reduction was noted (p < 0.0001). The glibenclamide group displayed a 55.67% decrease, whereas the 300 mg/kg CSSE group showed a 47.30% decrease (Figure 4).
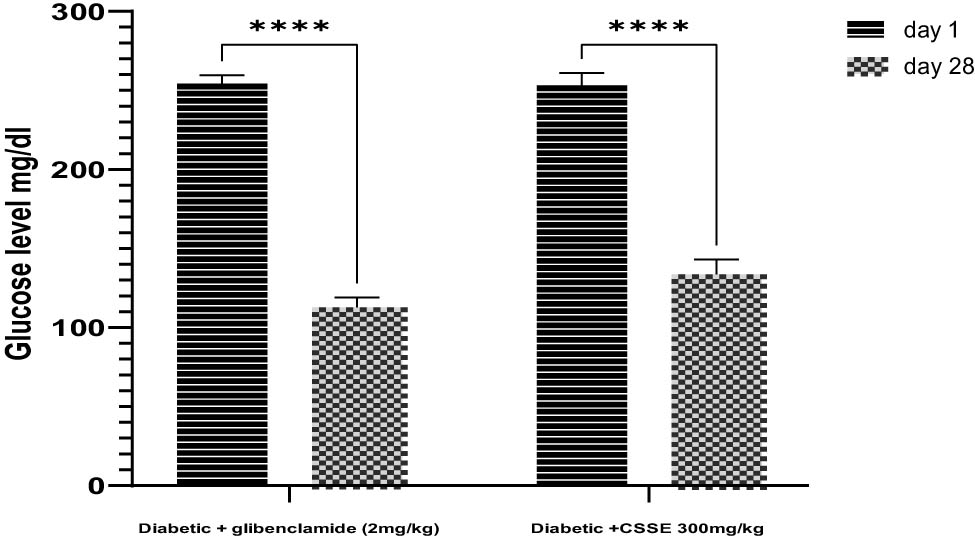
Percentage decrease in mean fasting glucose levels at day 1 and day 28 respectively, Data are presented as mean ± SD.
3.4.3 Effect of CCSE on serum biochemical parameters
Aspartate aminotransferase (ASAT) and alanine aminotransferase (ALAT) are liver enzymes, serve as indicators of hepatocellular health, and are commonly employed to assess liver function; as shown in Table 5, the ASAT and ALAT revealed significantly heightened levels in the DC group compared to normal group (p < 0.0001), Conversely, in groups treated with CSSE (300 g/kg), or gilbenclamide (2 mg/kg), the rats were shielded from the rise in ASAT and ALAT levels.
Effect of CCSE on kidneys and liver biochemical parameters
| Groups | Liver biomarkers | Kidney biomarkers | ||
|---|---|---|---|---|
| ASAT (UI/L) | ALAT (UI/L) | Urea (g/L) | Creatinine (mg/L) | |
| Normal control | 113 ± 15**** | 67.33 ± 4**** | 0.58 ± 0. 2* | 2.16 ± 0.18**** |
| Diabetic control | 169 ± 22 | 94 ± 5 | 0.94 ± 0.09 | 1.51 ± 0.29 |
| Diabetic + glibenclamide (2 mg/kg) | 141 ± 19*** | 80.66 ± 2.5** | 0.61 ± 0.11* | 1.84 ± 0.21* |
| Diabetic + CSSE 300 mg/kg | 145 ± 12*** | 82 ± 3** | 0.61 ± 0.07* | 1.80 ± 0.13* |
Values are mean ± SD (n = 5), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to DC.
Changes in kidney markers are demonstrated in Table 5. Elevated levels of urea and creatinine in the blood were also noted in the untreated diabetic groups; however, oral administration of CSSE (300 g/kg), or gilbenclamide (2 mg/kg) in diabetic rats, resulted in the normalization of these levels.
3.4.4 Effect of CSSE on lipid profiles
In this current research, we examined the lipid profile due to its connection with changes in lipid metabolism often observed in individuals with diabetes. The graphical representation in Figure 5 illustrates the serum levels of TG, total CT, HDL, LDL, and VLDL across all groups. In the diabetic rat groups, there was a notable increase in serum levels of TG, total CT, LDL, and VLDL compared to the normal control rats. Furthermore, administering C. sativa L. seed extract (CSSE) at a dosage of 300 mg/kg or glibenclamide at 2 mg/kg to diabetic rats for 28 days significantly reversed these increases in lipid metabolism indicators.
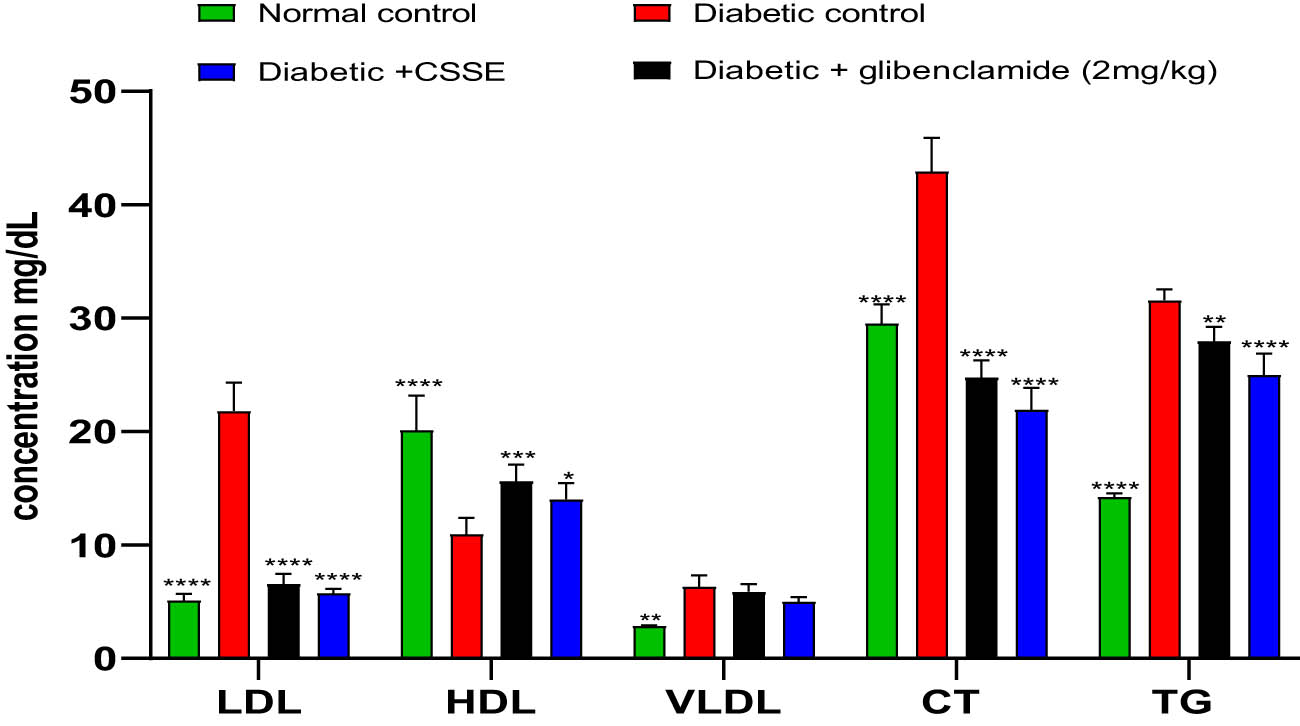
The serum concentrations of TG, CT, HDL, LDL, and VLDL. Values are presented as Means ± SD (n = 5), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 compared to DC.
3.4.5 OGTT
The impact of hydro-ethanolic extract from C. sativa L. seeds (CSSE) on the OGTT in rats is depicted in Figure 6. In the untreated normal control group (1 mL/100 g body weight), there was a pronounced spike in blood glucose levels, peaking at the 30-min mark following glucose administration. In contrast, the oral intake of CSSE at a dosage of 300 mg/kg, as well as glibenclamide at 2 mg/kg, resulted in a significant reduction in blood glucose levels at the 30-min mark (p < 0.0001), effectively preventing hyperglycemia. Moreover, blood glucose levels remained stable at 60, 90, and 120 min post-administration compared to the control groups.
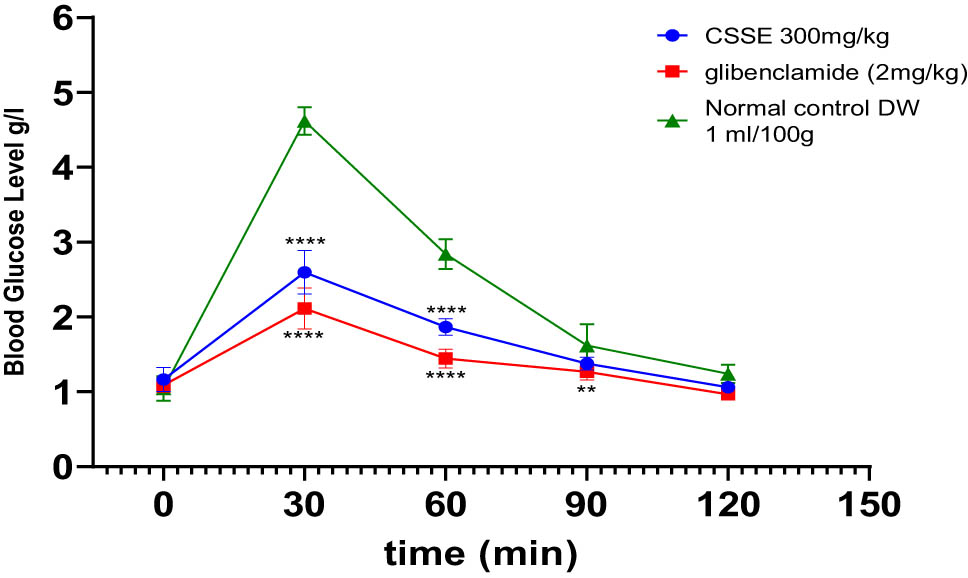
The influence of CSSE and glibenclamide on postprandial blood glucose levels in healthy rats following glucose administration 5 g/kg, values represented as mean ± SD (n = 5), **p < 0.01, ****p < 0.0001, compared to normal control.
3.4.6 Inhibition of α-amylase activity in vitro
In light of the adverse reactions and potential toxicity associated with existing medications for managing hyperglycemia, there has been a concerted effort in research to identify novel pancreatic α-amylase inhibitors derived from natural sources. This includes a focus on plants exhibiting hypoglycemic effects with minimal or no side effects. Our study aimed to evaluate the inhibitory effects of CSSE (plant extract) on pancreatic α-amylase, using acarbose as a reference drug.
The results indicated that CSSE extracts effectively inhibited α-amylase activity, with an IC50 value of 25.02 ± 4.03 μg/mL. Although this demonstrates significant inhibitory activity, it is noteworthy that CSSE exhibited reduced efficacy compared to acarbose, which showed an IC50 value of 12.33 ± 3.7 μg/mL (Table 6).
α-Amylase inhibitory activities of CSSE and acarbose
| IC50 (μg/mL) | |
|---|---|
| Acarbose | 12.33 ± 3.70a |
| CSSE | 25.02 ± 4.03b |
Values in identical columns annotated with the same letter did not exhibit significant differences according to Tukey’s multiple range test (p < 0.05).
3.4.7 Inhibition of α-amylase activity in vivo
In the oral starch tolerance test designed to evaluate in vivo α-amylase inhibition, blood glucose concentrations in all rat groups peaked (PBG) at 0.5 h post-gavage, subsequently returning to baseline levels over time. Notably, the groups receiving CSSE at a dosage of 300 mg/kg and acarbose at 10 mg/kg demonstrated significant reductions in postprandial hyperglycemia compared to the normal control group at 30 min, 60 min (****p < 0.0001), and 90 min (***p < 0.001).
Furthermore, it was evident that acarbose at a dosage of 10 mg/kg exhibited a more potent hypoglycemic effect than the CSSE group (300 mg/kg), as illustrated in Figure 7.
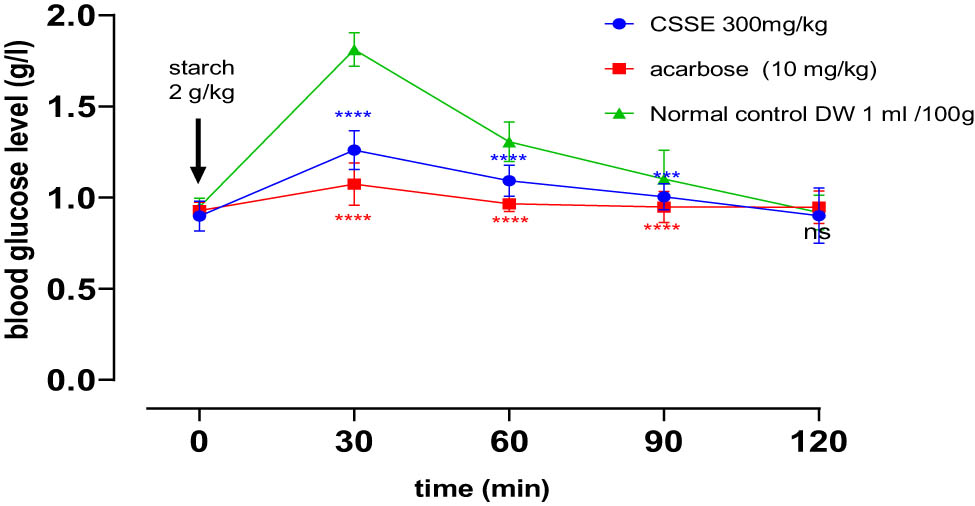
The influence of CSSE and acarbose on postprandial blood glucose levels in healthy rats following starch administration 2 g/kg, values represented as mean ± SD (n = 5), *p < 0.05, ***p < 0.001, ****p < 0.0001, compared to normal control.
The groups administered with CSSE or acarbose exhibited a statistically significant decrease in peak blood glucose (PBG) levels and area under the curve (AUC) in comparison to the normal control group (Table 7).
The impact of acarbose 10 mg/kg, and CSSE 300 mg/kg PBG and AUC following starch (2 g/kg) administration in normal rats
| Groups | Peak blood glucose (PBG) (g/L) | % Diminution of PBG | AUC (g/L) | % Diminution of AUC (g/L) |
|---|---|---|---|---|
| Normal control | 1.81 ± 0.13a | — | 34.77 ± 4.65a | — |
| Acarbose 10 mg/kg | 1.07 ± 0.22b | 41 | 7.230 ± 2.32b | 80 |
| CSSE 300 mg/kg | 1.25 ± 0.23c | 31 | 13.95 ± 1.5c | 60 |
Values represent the mean ± SD (n = 5), Values in identical columns annotated with the same letter did not exhibit significant differences according to Tukey’s multiple range test (p < 0.05).
3.5 Glide molecular docking results
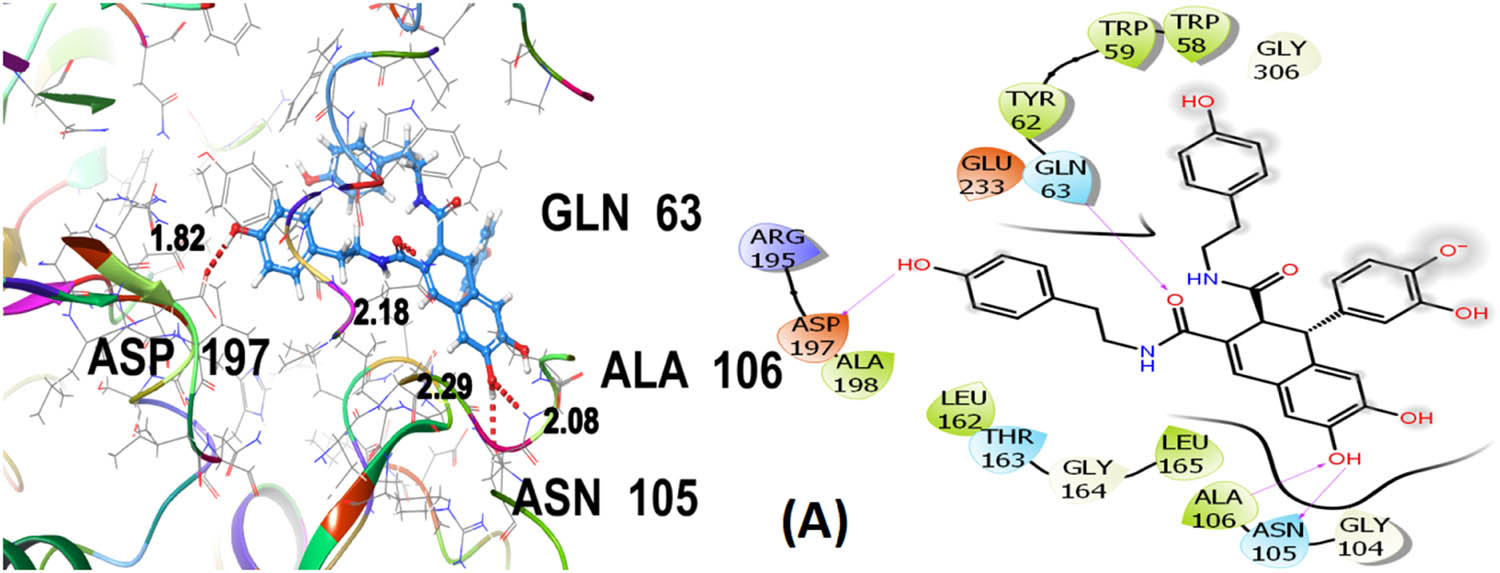
Seven polphenol ligands of hydro-ethanolic acid extract of C. sativa seeds as well as the reference drug and the co-crystallized ligand of the chosen target protein were docked to explore their α-amylase inhibitory activity and their docking data is shown in Table 8 and Table S1. The hit compound cannabisin B shows a Glide G-score of −7.791 kcal/mol which is very close to the G-score of the standard drug for α-amylase inhibition (acarbose), i.e., −7.203 kcal/mol, and even higher than the co-crystallized ligand (−6.923 kcal/mol).
Glide score, H-bonding interactions with distances (Å), polar, hydrophobic, and other interacting residues for investigated ligands with α-amylase (PDB ID: 4GQR) target protein
| Title | G-score (kcal/mol) | Emodel (kcal/mol) | HBI residue (distance Å) | Polar residues | Hydrophobic and other interacting residues |
|---|---|---|---|---|---|
| Cannabisin B (F) | −7.791A | −92.093 | GLN63 (2.18) | GLN63 | TRP58, TRP59, TYR62, ALA106, LEU162, LEU165, ALA198 |
| ASN105 (2.29) | ASN105 | ||||
| ALA106 (2.08) | THR163 | ||||
| ASP197 (1.82) | |||||
| Acarbose (B) | −7.203B | −95.973 | GLN63 (2.25) | GLN63 | TRP58, TRP59, TYR62, LEU162, ALA106, LEU165, ALA198, ILE235 |
| ASN105 (2.67) | ASN105 | ||||
| THR163 (1.81) | THR163 | ||||
| ASP197 (1.82) | ASN298 | ||||
| GLU233 (1.92, 2.08) | |||||
| Co-crystallized ligand (4GQR) (C) | −6.923C | −63.397 | GLN63 (1.82) | GLN63 | TRP58, TRP59, TYR62, LEU162, LEU165, ALA198 |
| ASP197 (1.62, 1.80) |
Cannabisin B (A) as shown in Figure 8 interacts via GLN63 (2.18), ASN105 (2.29 Å), ALA106 (2.08 Å), and ASP197 (1.82 Å) amino acid residues with the target protein through hydrogen bonding. Polar interactions are contributed by GLN63, ASN105, and THR163. Diverse hydrophobic interactions are observed between the OH group of the hit compound and TRP58, TRP59, TYR62, ALA106, LEU162, LEU165, and ALA198 amino acids of the target protein. Hydrophobic contacts facilitate in binding affinity of the ligands. Acarbose, being the standard drug used in this study to evaluate the α-amylase inhibition profile of the investigated ligands, shows that GLN63 (2.25 Å), ASN105 (2.67 Å), THR163 (1.81 Å), ASP197 (1.82 Å), and GLU233 (1.92 Å, 2.08 Å) develop hydrogen bonding as depicted in Figure 9. Polar amino acids are GLN63, ASN105, THR163, and ASN298. The co-crystallized ligand of the chosen target protein shows hydrogen bonding with GLN63 (1.82 Å), and ASP197 (1.62 Å, 1.80 Å), along with the polar contacts of GLN63 as presented in Figure 10. In addition to the interactions detailed above, Figures S1–S6 illustrate the various interactions between pancreatic α-amylase (PDB ID: 4GQR) and the other ligands examined in this study.
3D and 2D representation of cannabisin B (A) with α-amylase (PDB ID: 4GQR).
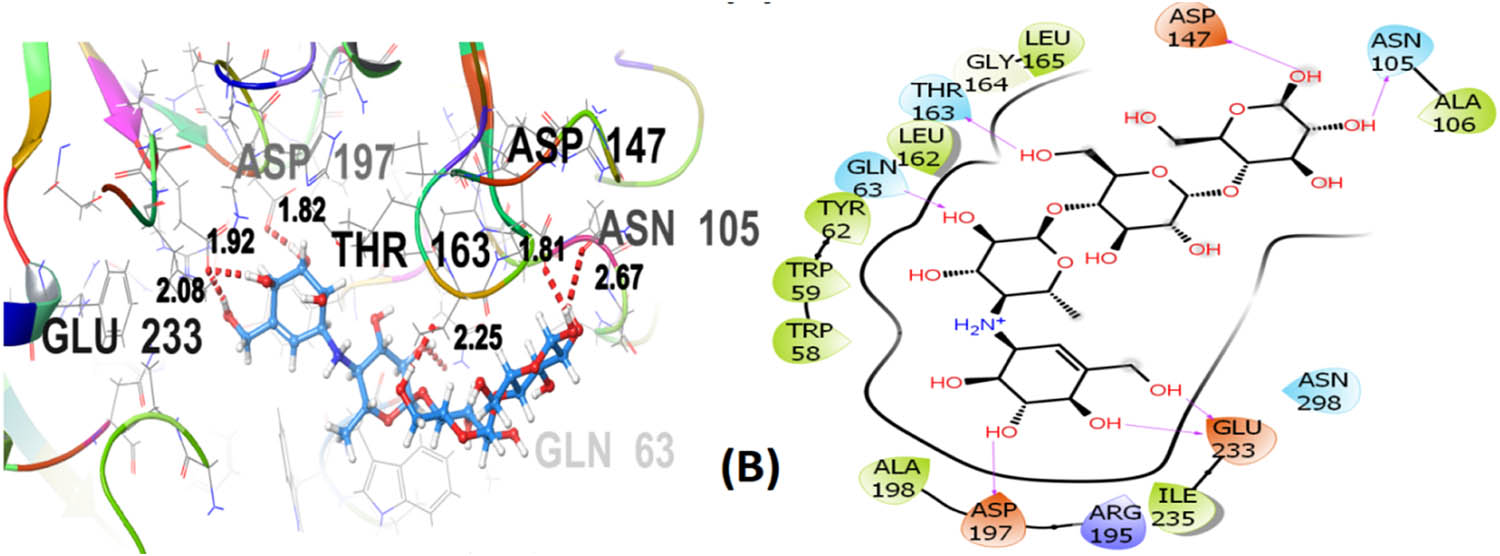
3D and 2D representation of acarbose (B) with α-amylase (PDB ID: 4GQR).
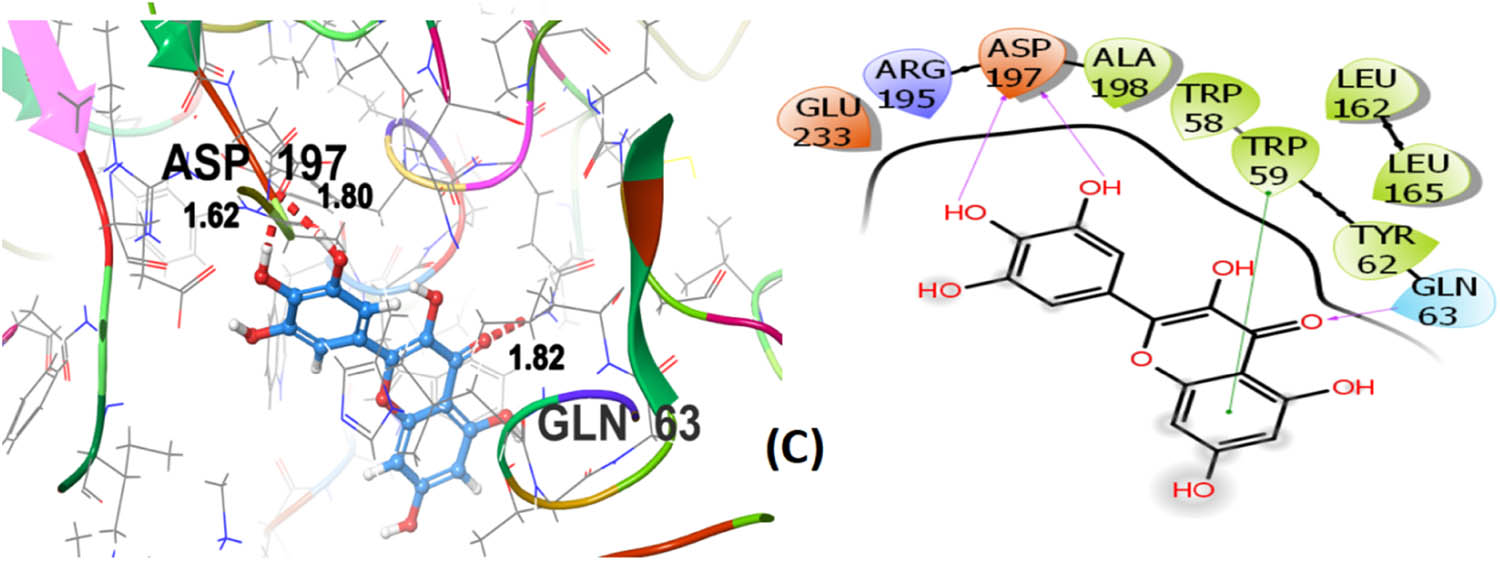
3D and 2D representation of co-crystallized ligand (C) with α-amylase (PDB ID: 4GQR).
Our findings indicate that C. sativa L. seed extract (CSSE) exhibits significant α-amylase inhibitory activity, reduces postprandial glucose levels, and improves lipid profiles in diabetic rats. Molecular docking studies further supported these findings, revealing strong interactions between identified phenolic and the α-amylase enzyme.
4 Discussion
The significant public health challenge posed by diabetes mellitus has prompted an ongoing search for effective preventive alternatives. C. sativa L., recognized as a medicinal plant, has been utilized in traditional African medicine for centuries, particularly in managing chronic diseases such as diabetes [32,33] This investigation assessed the impact of a hydro-ethanolic extract derived from C. sativa L. seeds on diabetes and its associated complications.
As detailed in Section 3, the plant extract exhibited favorable effects on all physiological parameters evaluated in rats induced with diabetes via alloxan.
The quality assessment of the cannabis seed extract (CSSE) was conducted by evaluating various parameters. The oil content observed in this study aligns closely with previous research findings. For instance, Mihoc et al. [34] reported similar oil content in five Romanian varieties, while Taaifi et al. investigated Moroccan C. sativa L. seeds from diverse locations and found oil content ranging from 26.42 to 35.19% [35]. Additionally, Munteanu et al. documented a lipid content of 29.17 ± 0.46%, which is consistent with our findings [9].
The ash content recorded in this study exceeded previously reported values by Taaifi et al. [35] but was consistent with findings from Munteanu et al. and Bhatt et al. [9,36] while being lower than those obtained by Rodriguez-Martin et al. [37]. Furthermore, screening results for phytochemicals indicate that C. sativa L. seeds are rich in secondary metabolites, underscoring their significance as a medicinal plant. Numerous studies have highlighted that phytochemicals play a crucial role in exhibiting antioxidant, anti-hyperglycemic, and anti-hyperlipidemic effects. For example, tannins, phenols, and saponins inhibit glucose transport by blocking the sodium-glucose co-transporter-1 (S-GLUT-1) and hinder carbohydrate digestion by inhibiting α-amylase and α-glucosidase [38,39].
Our analysis identified seven polyphenols as major constituents of CSSE, aligning with multiple studies that have identified specific phenolic compounds in C. sativa seeds [9,40,41,42,43]. Notably, Martinez and collaborators reported the presence of genistein and epicatechin in extracts of C. sativa seeds [42]. Benkirane et al. also identified several lignanamides in defatted hempseeds, including cannabisin C, D, and M, along with phenolic acids such as p-coumaric acid and ferulic acid [44] Furthermore, Haddou et al. noted that 3-hydroxycinnamic acid was the major compound in aqueous extracts of hemp seeds [45]. In addition to these findings, Ahmad et al.’s phytochemical investigation successfully identified benzoic acid, gallic acid, and kaempferol as primary phenolic components in methanolic extracts of C. sativa seeds [46]. The abundance of phenolic compounds in CSSE could potentially contribute to diverse pharmacological activities.
Diabetes induced by alloxan leads to the degeneration and shrinkage of pancreatic beta cells through excessive formation of reactive oxygen species, resulting in insulin deficiency [47]. This condition creates a complex pathophysiological state characterized by significant weight loss and increased intake of water and food alongside elevated urine output [48]. Studies indicate that increased water consumption and diuresis are directly linked to hyperglycemia due to absolute or relative insulin deficiency leading to elevated blood glucose levels [49,50]. Consequently, the kidneys expel excess sugar through urine water follows glucose due to its osmotic effect stimulating thirst to offset water loss [51].
The protective effect of CSSE against body weight loss may be attributed to its ability to increase insulin levels, thereby improving glycemic control. A 28-day treatment with CSSE exhibited significant antihyperglycemic effects consistent with those observed by Munteanu et al., who studied the C. sativa L. zenith variety [9]. It has been demonstrated that fixed oils from C. sativa L. exert hypoglycemic effects on diabetic rats [52]. Additionally, the antihyperglycemic impact of cannabis essential oil has also been documented, reinforcing the potential of various cannabis-derived products in diabetes management [53].
The liver plays a crucial role in glucose homeostasis and xenobiotic metabolism; thus, concentrations of ALT and AST in the bloodstream serve as reliable indicators of liver function status. Previous studies have shown necrosis in the liver of alloxan-induced diabetic rats [54]. Elevated activities of ALT and AST are primarily linked to their release from liver cytosol into circulation due to cellular damage [49]. Notably, administering CSSE alongside glibenclamide to alloxan-diabetic groups for 28 consecutive days restored enzyme activities to baseline levels.
Diabetic animals typically exhibit significantly higher serum levels of urea and creatinine – critical markers for renal impairment [55]. The substantial decrease in serum urea and creatinine levels among diabetic rats treated with CSSE and glibenclamide suggests its potential role in halting renal damage associated with diabetes progression.
Moreover, diabetes mellitus is widely recognized as a prevalent factor contributing to cardiovascular diseases due to its association with significant changes in plasma lipids [56] Insulin regulates lipid metabolism; impaired insulin signaling can lead to hypertriglyceridemia and hypercholesterolemia characterized by elevated VLDL and LDL levels while decreasing HDL concentrations [48]. In diabetic states, hypercholesterolemia arises because insulin inhibits β-hydroxy-β-methylglutaryl-coenzyme-A reductase an essential enzyme involved in cholesterol metabolism [39]. Furthermore, diabetes renders lipoprotein lipase inactive, an enzyme crucial for triglyceride breakdown. This inactivity results in elevated triglyceride levels and a decline in HDL levels [57]. CSSE demonstrated hypocholesterolemic and hypotriglyceridemic effects similar to antihyperlipidemic medications that lower lipid levels while increasing HDL cholesterol concentrations [9,58]. An effective anti-diabetic agent should control glucose level rises through various mechanisms; thus, we assessed this using oral glucose-loaded hyperglycemic models where CSSE proved effective. An effective antidiabetic agent should control glucose level rises through various mechanisms; thus, we assessed this using oral glucose-loaded hyperglycemic models where CSSE proved effective.
Lowering postprandial hyperglycemia is crucial for diabetes management achieved by inhibiting α-amylase an enzyme that breaks down complex polysaccharides into smaller oligosaccharides through hydrolysis [59]. α-Amylase inhibition leads to a reduction in the formation of oligosaccharides and disaccharides, ultimately resulting in decreased glucose absorption into the bloodstream. Consequently, postprandial blood glucose levels are effectively lowered. Our findings indicate that CSSE exhibits significant α-amylase inhibition with an IC50 value of 25.02 ± 4.03 µg/mL. Molecular docking studies further supported these findings, revealing strong interactions between identified phenolic and the α-amylase enzyme. While previous studies on the effects of C. sativa on α-amylase yielded mixed results Zengin et al. reported no significant effects while Haddou et al. demonstrated promising α-amylase inhibitory activity [60,61]. However, Leonard et al. reported that a C. sativa L. seed hull extract did not exhibit significant α-amylase inhibitory activity [40]. These discrepancies may stem from variations in plant variety, growth conditions, extraction methods, or specific compounds present within the extracts. The observed α-amylase inhibition indicates that further exploration of CSSE’s role in diabetes management is warranted.
α-Glucosidase also plays a vital role in carbohydrate digestion by acting on glycosidic bonds to produce glucose [62]. Inhibiting α-glucosidase is advantageous for managing pre-diabetic and diabetic conditions as it plays a critical role in carbohydrate absorption within the gastrointestinal tract [40]. Earlier research indicated that ethanolic extracts from C. sativa seeds demonstrate α-glucosidase inhibitory activity [63,64] aligning with Ren’s findings regarding oligopeptides derived from C. sativa L. seeds protein exhibiting potent α-glucosidase inhibition activity [65]. Supporting these findings, Leonard et al. [40] concluded that phenolic extracts from C. sativa L. seeds showed α-glucosidase inhibitory activity comparable to the pharmaceutical standard, acarbose. This collective evidence highlights the potential of C. sativa seed extracts and derivatives in the management of carbohydrate metabolism disorders.
C. sativa seed extract exhibits high antioxidant potential [43,44,66,67,68] due to its richness in phenolic compounds and fatty acids [69,70]. This antioxidant property aids in preventing the degeneration of pancreatic islets, preserving their histoarchitecture. Additionally, because a lower dose of alloxan induces only partial destruction of pancreatic beta cells, the surviving beta cells in albino rats can regenerate [71].
Beyond their antioxidant properties, phenolic compounds from various plant sources have been shown to possess therapeutic benefits for diabetes management [13]. These compounds can enhance insulin secretion, reduce glucose production by the liver, inhibit key enzymes involved in carbohydrate digestion, improve the sensitivity of peripheral tissues to insulin, and regulate glucose absorption in the bloodstream. Collectively, these actions contribute to improved post-meal blood sugar control [48,72].
Phenolic compounds in C. sativa L. seed extract stimulate postprandial insulin secretion, promote L cells and K cells to secrete glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) [73], and increase their half-life by inhibiting dipeptidyl peptidase-4 (DPP-4) [74,75]. Additionally, they stimulate the expression of glucose transporters (GLUT) types 2 and 4 in the liver, skeletal muscle, and adipose tissues [76], enhancing the binding of insulin to insulin receptors [30,77,78]. Benzoic acid, genistein, and epicatechin, the major compounds in CSSE, reduce β-cell apoptosis, regenerate damaged pancreatic islets, and enhance insulin secretion from surviving beta cells, resulting in an antihyperglycemic effect [79,80,81,82]. Lignanamides, such as cannabisins, found in C. sativa L. seeds exhibit antioxidant and anti-inflammatory activities, which may contribute to their beneficial effects on glucose metabolism [83,84,85]. However, specific studies directly linking lignanamides and cannabisins to diabetes management are limited. Further research is needed to fully elucidate their mechanisms of action and therapeutic potential in the context of diabetes. Cannflavins, unique prenylated flavonoids found in cannabis seeds, exhibit antioxidant, anti-inflammatory, and anticancer properties, which may also contribute to their potential benefits in diabetes management [86,87,88].
The anti-diabetic properties of CSSE may result from a synergistic effect of multiple bioactive molecules instead of a single compound.
5 Conclusions
Our findings demonstrate that C. sativa L. seed extract (CSSE) holds significant promise as a novel therapeutic approach for managing diabetes and its associated complications. The extract’s rich phenolic profile, particularly compounds like benzoic acid, cannabisin B, genistein, and epicatechin, contributes to its potent α-amylase inhibitory activity. This, coupled with its hypoglycemic, hypolipidemic, hepatoprotective, and nephroprotective effects observed in our in vivo studies, suggests its potential to improve glycemic control and overall metabolic health. Furthermore, in silico docking studies validate the strong binding affinity of these phenolic compounds to the α-amylase enzyme, providing mechanistic insights into their inhibitory activity.
The clinical implications of our findings are substantial. By inhibiting α-amylase, CSSE can delay carbohydrate digestion and absorption, leading to a more gradual increase in blood glucose levels. This could be particularly beneficial for individuals with type 2 diabetes, who often experience postprandial hyperglycemia. Additionally, the hypolipidemic and antioxidant properties of CSSE may contribute to the prevention of diabetic complications, such as cardiovascular disease and neuropathy.
While our findings are promising, further research, including well-designed clinical trials, is necessary to fully evaluate the safety and efficacy of CSSE in human subjects. By elucidating the underlying molecular mechanisms, we can optimize the therapeutic potential of this natural product and develop innovative strategies for diabetes management.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project, King Saud University, Riyadh, Saudi Arabia, for supporting this work through the project number (RSP 2025/236).
-
Funding information: This work is financially supported by the Researchers Supporting Project (RSP 2025/236), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: Conceptualization, original draft writing, reviewing, and editing: Rafik El-Mernissi, Naoual El Menyiy, Amira Metouekel, Hassan Amhamdi, and Aziz Zouhri. Formal analysis, investigations, funding acquisition, reviewing, and editing: Yahya El-Mernissi, Farhan Siddique, Sumaira Nadeem, and Musaab Dauelbait. Resources, data validation, data curation, and supervision: Mohammed Bourhia, Abdulaziz Abdullah Alsahli, Oualid Abboussi, Gamal A. Shazly, and Lhoussain Hajji.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] WHO. Diabetes [Internet]; 2023. Disponible sur: https://www.who.int/news-room/fact-sheets/detail/diabetes.Suche in Google Scholar
[2] Piya MK, Tahrani AA, Barnett AH. Emerging treatment options for type 2 diabetes. Br J Clin Pharmacol. 2010;70(5):631–44.10.1111/j.1365-2125.2010.03711.xSuche in Google Scholar PubMed PubMed Central
[3] Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26(2):77–82.10.2337/diaclin.26.2.77Suche in Google Scholar
[4] Comelli F, Bettoni I, Colleoni M, Giagnoni G, Costa B. Beneficial effects of a Cannabis sativa extract treatment on diabetes‐induced neuropathy and oxidative stress. Phytother Res. 2009;23(12):1678–84.10.1002/ptr.2806Suche in Google Scholar PubMed
[5] Piliponienė L, Veličkienė D, Kregždytė R. Microvascular complications, peripheral artery disease and mortality in patients with type 2 diabetes mellitus, in two counties of southern Lithuania over 13 years: Analysis using a cohort database of the national health insurance. Medicina (Lithuania). 2021;57(12):1380.10.3390/medicina57121380Suche in Google Scholar PubMed PubMed Central
[6] Li GQ, Kam A, Wong KH, Zhou X, Omar EA, Alqahtani A, et al. Herbal medicines for the management of diabetes. In: Ahmad SI, editor. Diabetes: An old disease, a new insight. New York, NY: Springer New York; 2013. p. 396–413.10.1007/978-1-4614-5441-0_28Suche in Google Scholar PubMed
[7] Roy S, Ghosh A, Majie A, Karmakar V, Das S, Dinda SC, et al. Terpenoids as potential phytoconstituent in the treatment of diabetes: From preclinical to clinical advancement. Phytomedicine. 2024;129:155638.10.1016/j.phymed.2024.155638Suche in Google Scholar PubMed
[8] Farinon B, Molinari R, Costantini L, Merendino N. The seed of industrial hemp (Cannabis sativa l.): Nutritional quality and potential functionality for human health and nutrition. Nutrients. 2020;12:1–60.10.3390/nu12071935Suche in Google Scholar PubMed PubMed Central
[9] Munteanu C, Mihai M, Dulf F, Ona A, Muntean L, Ranga F, et al. Biochemical Changes Induced by the Administration of Cannabis sativa Seeds in Diabetic Wistar Rats. Nutrients. 2023;15:13.10.3390/nu15132944Suche in Google Scholar PubMed PubMed Central
[10] Frisher M, White S, Varbiro G, Voisey C, Perumal D, Crome I, et al. The role of cannabis and cannabinoids in diabetes. Br J Diabetes Vasc Dis. 2010;10(6):267–73.10.1177/1474651410385860Suche in Google Scholar
[11] El-Mernissi Y, Zouhri A, Labhar A, El Menyiy N, Ahari M, El Barkany S, et al. Indigenous knowledge of the traditional use of aromatic and medicinal plants in Rif Mountains Ketama District. Evidence-Based Complementary Altern Med. 2023;2023(31000):1–16.10.1155/2023/3977622Suche in Google Scholar PubMed PubMed Central
[12] Bachir F, Eddouks M, Arahou M, Fekhaoui M. Origin, early history, cultivation, and characteristics of the traditional varieties of Moroccan Cannabis sativa L. Cannabis Cannabinoid Res. 2022;7(5):603–15.10.1089/can.2021.0020Suche in Google Scholar PubMed PubMed Central
[13] Alam S, Sarker MMR, Sultana TN, Chowdhury MNR, Rashid MA, Chaity NI, et al. Antidiabetic phytochemicals from medicinal plants: prospective candidates for new drug discovery and development. Front Endocrinol. 2022;13:800714.10.3389/fendo.2022.800714Suche in Google Scholar PubMed PubMed Central
[14] Siracusa L, Ruberto G, Cristino L. Recent research on Cannabis sativa L.: Phytochemistry, new matrices, cultivation techniques, and recent updates on its brain-related effects (2018–2023). Molecules. 2023;28(8):3387.10.3390/molecules28083387Suche in Google Scholar PubMed PubMed Central
[15] Pollastro F, Minassi A, Fresu LG. Cannabis phenolics and their bioactivities. Curr Med Chem. 2017;25(10):1160–85.10.2174/0929867324666170810164636Suche in Google Scholar PubMed
[16] AOAC. Official methods of analysis. Vol. 1, 15th edn. Washington, DC: Association of Official Analytical Chemists; 1990.Suche in Google Scholar
[17] Laaroussi H, Ferreira-Santos P, Genisheva Z, Bakour M, Ousaaid D, Teixeira JA, et al. Unraveling the chemical composition, antioxidant, α-amylase and α-glucosidase inhibition of Moroccan propolis. Food Biosci. 2021;42:101160.10.1016/j.fbio.2021.101160Suche in Google Scholar
[18] Remok F, Saidi S, Gourich AA, Zibouh K, Maouloua M, Makhoukhi FE, et al. Phenolic content, antioxidant, antibacterial, antihyperglycemic, and α-amylase inhibitory activities of aqueous extract of Salvia lavandulifolia Vahl. Pharmaceuticals. 2023;16(3):395.10.3390/ph16030395Suche in Google Scholar PubMed PubMed Central
[19] Benayad O, Bouhrim M, Tiji S, Kharchoufa L, Addi M, Drouet S, et al. Phytochemical profile, α-glucosidase, and α-amylase inhibition potential and toxicity evaluation of extracts from citrus aurantium (L) peel, a valuable by-product from Northeastern Morocco. Biomolecules. 2021;11:1555.10.3390/biom11111555Suche in Google Scholar PubMed PubMed Central
[20] Zefzoufi M, Fdil R, Bouamama H, Gadhi C, Katakura Y, Mouzdahir A, et al. Effect of extracts and isolated compounds derived from Retama monosperma (L.) Boiss. on anti-aging gene expression in human keratinocytes and antioxidant activity. J Ethnopharmacol. 2021;280:114451.10.1016/j.jep.2021.114451Suche in Google Scholar PubMed
[21] Ighodaro OM, Adeosun AM, Akinloye OA. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina. 2017;53(6):365–74.10.1016/j.medici.2018.02.001Suche in Google Scholar PubMed
[22] Patel DK, Kumar R, Prasad SK, Sairam K, Hemalatha S. Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1(4):316–22.10.1016/S2221-1691(11)60051-8Suche in Google Scholar PubMed PubMed Central
[23] Aazza S, El-Guendouz S, Miguel MG, Antunes MD, Faleiro ML, Correia AI, et al. Antioxidant, anti-inflammatory and anti-hyperglycaemic activities of essential oils from Thymbra capitata, Thymus albicans, Thymus caespititius, Thymus carnosus, Thymus lotocephalus and Thymus mastichina from Portugal. Nat Product Commun. 2016;11(7):1934578X1601100.10.1177/1934578X1601100739Suche in Google Scholar
[24] Ouassou H, Bouhrim M, Bencheikh N, Addi M, Hano C, Mekhfi H, et al. In vitro antioxidant properties, glucose-diffusion effects, α-amylase inhibitory activity, and antidiabetogenic effects of C. Europaea extracts in experimental animals. Antioxidants. 2021;10:1747.10.3390/antiox10111747Suche in Google Scholar PubMed PubMed Central
[25] Rose PW, Beran B, Bi C, Bluhm WF, Dimitropoulos D, Goodsell DS, et al. The RCSB Protein Data Bank: Redesigned web site and web services. Nucleic Acids Res. 2011;39:392–401.10.1093/nar/gkq1021Suche in Google Scholar PubMed PubMed Central
[26] Madhavi Sastry G, Adzhigirey M, Day T, Annabhimoju R, Sherman W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J Comput Mol Des. 2013;27(3):221–34.10.1007/s10822-013-9644-8Suche in Google Scholar PubMed
[27] Mendelsohn LD. ChemDraw 8 ultra, windows and macintosh versions. J Chem Inf Comput Sci. 2004;44(6):2225–6.10.1021/ci040123tSuche in Google Scholar
[28] Velika B, Kron I. Antioxidant properties of benzoic acid derivatives against Superoxide radical. Free Radic Antioxid. 2012;2(4):62–7.10.5530/ax.2012.4.11Suche in Google Scholar
[29] Gilbert ER, Liu D. Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013;4(2):200–12.10.1039/C2FO30199GSuche in Google Scholar
[30] Fraga CG, Cremonini E, Galleano M, Oteiza PI. Natural products and diabetes: (−)-Epicatechin and mechanisms involved in the regulation of insulin sensitivity. In Handbook of experimental pharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2024. p. 1–15.10.1007/164_2024_707Suche in Google Scholar PubMed
[31] Li S, Chen H, Wang J, Wang X, Hu B, Lv F. Involvement of the PI3K/Akt signal pathway in the hypoglycemic effects of tea polysaccharides on diabetic mice. Int J Biol Macromol. 2015;81:967–74.10.1016/j.ijbiomac.2015.09.037Suche in Google Scholar PubMed
[32] Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus--present and future perspectives. Nat Rev Endocrinol. 2011;8(4):228–36.10.1038/nrendo.2011.183Suche in Google Scholar PubMed
[33] Kakudidi E, Tugume P, Asiimwe S, Anywar G. Traditional and modern health uses of Cannabis sativa L. in Africa and its phytochemical and pharmacological profile BT - Cannabis/Marijuana for healthcare. In: Agrawal DC, Kumar R, Dhanasekaran M, editors. Singapore: Springer Nature Singapore; 2022. p. 189–210.10.1007/978-981-16-8822-5_10Suche in Google Scholar
[34] Mihoc M, Pop G, Alexa E, Radulov I. Nutritive quality of romanian hemp varieties (Cannabis sativa L.) with special focus on oil and metal contents of seeds. Chem Cent J. 2012;6:122.10.1186/1752-153X-6-122Suche in Google Scholar PubMed PubMed Central
[35] Taaifi Y, Benmoumen A, Belhaj K, Aazza S, Abid M, Azeroual E, et al. Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco. Int J Food Sci Technol. 2021;56(11):5931–47.10.1111/ijfs.15136Suche in Google Scholar
[36] Bhatt LR, Dawadi P, Syangtan G, Siddiqui MA, Lama B, Nepal K, et al. Nutritional value and antioxidant properties of Cannabis seeds from Makwanpur district of central Nepal. Sci World. 2022;15(15):103–12.10.3126/sw.v15i15.45657Suche in Google Scholar
[37] Rodriguez-Martin NM, Toscano R, Villanueva A, Pedroche J, Millan F, Montserrat-De La Paz S, et al. Neuroprotective protein hydrolysates from hemp (Cannabis sativa L.) seeds. Food Funct. 2019;10(10):6732–9.10.1039/C9FO01904ASuche in Google Scholar
[38] Mattila PH, Pihlava JM, Hellström J, Nurmi M, Eurola M, Mäkinen S, et al. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual Saf. 2018;2(4):213–9.10.1093/fqsafe/fyy021Suche in Google Scholar
[39] Banda M, Nyirenda J, Muzandu K, Sijumbila G, Mudenda S. Antihyperglycemic and antihyperlipidemic effects of aqueous extracts of Lannea edulis in alloxan-induced diabetic rats. Front Pharmacol. 2018;9:1099.10.3389/fphar.2018.01099Suche in Google Scholar PubMed PubMed Central
[40] Leonard W, Zhang P, Ying D, Xiong Y, Fang Z. Extrusion improves the phenolic profile and biological activities of hempseed (Cannabis sativa L.) hull. Food Chem. 2021;346:128606.10.1016/j.foodchem.2020.128606Suche in Google Scholar PubMed
[41] Irakli M, Tsaliki E, Kalivas A, Kleisiaris F, Sarrou E, Cook CM. Effect of genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants. 2019;8(10):20–5.10.3390/antiox8100491Suche in Google Scholar PubMed PubMed Central
[42] Rea Martinez J, Montserrat-de la Paz S, De la Puerta R, García-Giménez MD, Fernández-Arche MÁ. Characterization of bioactive compounds in defatted hempseed ( Cannabis sativa L.) by UHPLC-HRMS/MS and anti-inflammatory activity in primary human monocytes. Food Funct. 2020;11(5):4057–66.10.1039/D0FO00066CSuche in Google Scholar
[43] Frassinetti S, Moccia E, Caltavuturo L, Gabriele M, Longo V, Bellani L, et al. Nutraceutical potential of hemp (Cannabis sativa L.) seeds and sprouts. Food Chem. 2018;262:56–66.10.1016/j.foodchem.2018.04.078Suche in Google Scholar PubMed
[44] Benkirane C, Ben Moumen A, Fauconnier ML, Belhaj K, Abid M, Caid HS, et al. Bioactive compounds from hemp (Cannabis sativa L.) seeds: optimization of phenolic antioxidant extraction using simplex lattice mixture design and HPLC-DAD/ESI-MS2 analysis. RSC Adv. 2022;12(39):25764–77.10.1039/D2RA04081FSuche in Google Scholar PubMed PubMed Central
[45] Haddou S, Hassania Loukili E, Hbika A, Chahine A, Hammouti B. Phytochemical study using HPLC-UV/GC–MS of different of Cannabis sativa L seeds extracts from Morocco. In Materials Today: Proceedings; 2023. p. 3896–903.10.1016/j.matpr.2022.10.215Suche in Google Scholar
[46] Ahmad F, Abbas T, Farman K, Akrem A, Saleem MA, Iqbal MU, et al. High-throughput phytochemical characterization of non-cannabinoid compounds of cannabis plant and seed, from Pakistan. Pak J Botany. 2018;50(2):639–43.Suche in Google Scholar
[47] Rohilla A, Ali S. Alloxan induced diabetes: mechanisms and effects. Int J Res Pharm Biomed Sci. 2012;3(2):819–23.Suche in Google Scholar
[48] De Oliveira Carvalho H, Souza BSF, Dos Santos IVF, Resque RL, Keita H, Fernandes CP, et al. Hypoglycemic effect of formulation containing hydroethanolic extract of Calophyllum brasiliense in diabetic rats induced by streptozotocin. Rev Bras Farmacogn. 2016;26(5):634–9.10.1016/j.bjp.2016.04.004Suche in Google Scholar
[49] Mahendran G, Manoj M, Murugesh E, Kumar RS, Shanmughavel P, Prasad KJR, et al. In vivo anti-diabetic, antioxidant and molecular docking studies of 1, 2, 8-trihydroxy-6-methoxy xanthone and 1, 2-dihydroxy-6-methoxyxanthone-8-O-β- d-xylopyranosyl isolated from Swertia corymbosa. Phytomedicine. 2014;21(11):1237–48.10.1016/j.phymed.2014.06.011Suche in Google Scholar PubMed
[50] Sundaram R, Naresh R, Shanthi P, Sachdanandam P. Modulatory effect of green tea extract on hepatic key enzymes of glucose metabolism in streptozotocin and high fat diet induced diabetic rats. Phytomedicine. 2013;20(7):577–84.10.1016/j.phymed.2013.01.006Suche in Google Scholar PubMed
[51] Ramesh B, Pugalendi KV. Antihyperglycemic effect of umbelliferone in streptozotocin-diabetic rats. J Med Food. 2006;9(4):562–6.10.1089/jmf.2006.9.562Suche in Google Scholar PubMed
[52] de Oliveira Carvalho H, de Melo Santos A, de Lima Teixeira dos Santos AVT, Gonçalves DES, Picanço KRT, Souza BSF, et al. Cannabis sativa L. fixed oil and its nanoemulsion: Effect on diabetes and dyslipidemia induced in rats. Pharmacogn Mag. 2024;20(3):908–20.10.1177/09731296241234123Suche in Google Scholar
[53] Saima N, Kashif AR, Tawab A, Rasool MZ, Rauf A, Hussain S, et al. Assessment of the antidiabetic properties of essential oil from Cannabis sativa. J Adv Nutr Sci Technol. 2023;3(3–4):78–86.10.15228/ANST.2023.v03.i03-4.p09Suche in Google Scholar
[54] Lucchesi AN, Cassettari LL, Spadella CT. Alloxan-induced diabetes causes morphological and ultrastructural changes in rat liver that resemble the natural history of chronic fatty liver disease in humans. J Diabetes Res. 2015;2015:1–11.10.1155/2015/494578Suche in Google Scholar PubMed PubMed Central
[55] Collins AJ, Kasiske B, Herzog C, Chen SC, Everson S, Constantini E, et al. Excerpts from the United States Renal Data System 2002 annual data report: Atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;41:5–9.10.1016/S0272-6386(03)80001-XSuche in Google Scholar
[56] Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol. 2003;85(2–3):201–6.10.1016/S0378-8741(02)00366-5Suche in Google Scholar
[57] Pushparaj PN, Low HK, Manikandan J, Tan BKH, Tan CH. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2007;111:430–4.10.1016/j.jep.2006.11.028Suche in Google Scholar PubMed
[58] Majewski M, Jurgoński A. The effect of hemp (Cannabis sativa L.) seeds and hemp seed oil on vascular dysfunction in obese male zucker rats. Nutrients. 2021;13:2575.10.3390/nu13082575Suche in Google Scholar PubMed PubMed Central
[59] Etxeberria U, de la Garza AL, Campión J, Martínez JA, Milagro FI. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin Ther Targets. 2012;16(3):269–97.10.1517/14728222.2012.664134Suche in Google Scholar PubMed
[60] Zengin G, Menghini L, Di Sotto A, Mancinelli R, Sisto F, Carradori S, et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of cannabis sativa L. Essential oil: A multidisciplinary study. Molecules. 2018;23:3266.10.3390/molecules23123266Suche in Google Scholar PubMed PubMed Central
[61] Haddou S, Elrherabi A, Loukili EH, Abdnim R, Hbika A, Bouhrim M, et al. Chemical analysis of the antihyperglycemic, and pancreatic α-amylase, lipase, and intestinal α-glucosidase inhibitory activities of Cannabis sativa L. seed extracts. Molecules. 2023;29:93.10.3390/molecules29010093Suche in Google Scholar PubMed PubMed Central
[62] Tundis R, Loizzo MR, Menichini F. Natural products as α-amylase and α-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. Mini-Reviews Med Chem. 2010;10(4):315–31.10.2174/138955710791331007Suche in Google Scholar PubMed
[63] Aloo SO, Kwame FO, Oh DH. Identification of possible bioactive compounds and a comparative study on in vitro biological properties of whole hemp seed and stem. Food Biosci. 2023;51:102329.10.1016/j.fbio.2022.102329Suche in Google Scholar
[64] Sangkanu S, Pitakbut T, Phoopha S, Khanansuk J, Chandarajoti K, Dej-adisai S. A comparative study of chemical profiling and bioactivities between Thai and Foreign Hemp Seed Species (Cannabis sativa L.) plus an in-silico investigation. Foods. 2024;13(1):1–22.10.3390/foods13010055Suche in Google Scholar PubMed PubMed Central
[65] Ren Y, Liang K, Jin Y, Zhang M, Chen Y, Wu H, et al. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J Funct Foods. 2016;26:439–50.10.1016/j.jff.2016.07.024Suche in Google Scholar
[66] Haddou S, Mounime K, Loukili EH, Ou-yahia D, Hbika A, Idrissi MY, et al. Investigating the biological activities of Moroccan Cannabis Sativa L. seed extracts: antimicrobial, anti-inflammatory, and antioxidant effects with molecular docking analysis. Moroccan J Chem. 2023;11(4):1116–36.Suche in Google Scholar
[67] Occhiuto C, Aliberto G, Ingegneri M, Trombetta D, Circosta C, Smeriglio A. Antioxidant activity of two hempseed oils and their byproducts after cold pressing. Molecules. 2022;27:3431.10.3390/molecules27113431Suche in Google Scholar PubMed PubMed Central
[68] Benkirane C, Mansouri F, Ben Moumen A, Taaifi Y, Melhaoui R, Caid HS, et al. Phenolic profiles of non-industrial hemp (Cannabis sativa L.) seed varieties collected from four different Moroccan regions. Int J Food Sci Technol. 2023;58(3):1367–81.10.1111/ijfs.16298Suche in Google Scholar
[69] Stevenson DE, Hurst RD. Polyphenolic phytochemicals - Just antioxidants or much more? Cell Mol Life Sci. 2007;64(22):2900–16.10.1007/s00018-007-7237-1Suche in Google Scholar PubMed PubMed Central
[70] Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57(6):451–5.10.1016/j.phrs.2008.05.002Suche in Google Scholar PubMed
[71] Aybar MJ, Sánchez Riera AN, Grau A, Sánchez SS. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. J Ethnopharmacol. 2001;74(2):125–32.10.1016/S0378-8741(00)00351-2Suche in Google Scholar PubMed
[72] Sarkar D, Christopher A, Shetty K. Phenolic bioactives from plant-based foods for glycemic control. Front Endocrinol. 2022;12:1–24.10.3389/fendo.2021.727503Suche in Google Scholar PubMed PubMed Central
[73] Avila JAD, García JR, Aguilar GAG, De La Rosa LA. The antidiabetic mechanisms of polyphenols related to increased glucagon-like peptide-1 (GLP1) and insulin signaling. Molecules. 2017;22(6):1–16.10.3390/molecules22060903Suche in Google Scholar PubMed PubMed Central
[74] Thongtak A, Yutisayanuwat K, Harnkit N, Noikaew T, Chumnanpuen P. Computational screening for the dipeptidyl peptidase-IV inhibitory peptides from putative hemp seed hydrolyzed peptidome as a potential antidiabetic agent. Int J Mol Sci. 2024;25(11):5730.10.3390/ijms25115730Suche in Google Scholar PubMed PubMed Central
[75] Naz R, Saqib F, Awadallah S, Wahid M, Latif MF, Iqbal I, et al. Food polyphenols and type II diabetes mellitus: pharmacology and mechanisms. Molecules. 2023;28(10):3996.10.3390/molecules28103996Suche in Google Scholar PubMed PubMed Central
[76] Martín MÁ, Ramos S. Dietary flavonoids and insulin signaling in diabetes and obesity. Cells. 2021;10(6):1–22.10.3390/cells10061474Suche in Google Scholar PubMed PubMed Central
[77] Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic β-cells through a cAMP-dependent protein kinase pathway. Diabetes. 2006;55(4):1043–50.10.2337/diabetes.55.04.06.db05-1089Suche in Google Scholar PubMed
[78] Park JK, Kim SP, Song DK. Ameliorating effects of sulfonylurea drugs on insulin resistance in Otsuka long-evans Tokushima Fatty rats. Korean J Physiol Pharmacol. 2008;12(1):7.10.4196/kjpp.2008.12.1.7Suche in Google Scholar PubMed PubMed Central
[79] Jahan H, Choudhary MI, Manzoor M, Khan KM, Perveen S. Insulinotropic action of 2, 4-dinitroanilino-benzoic acid through the attenuation of pancreatic beta-cell lesions in diabetic rats. Chem-Biol Interact. 2017;273:237–44.10.1016/j.cbi.2017.06.015Suche in Google Scholar PubMed
[80] Fu Z, Zhang W, Zhen W, Lum H, Nadler J, Bassaganya-Riera J, et al. Genistein induces pancreatic β-cell proliferation through activation of multiple signaling pathways and prevents insulin-deficient diabetes in mice. Endocrinology. 2010;151(7):3026–37.10.1210/en.2009-1294Suche in Google Scholar PubMed PubMed Central
[81] Hii CST, Howell SL. Effects of epicatechin on rat islets of Langerhans. Diabetes. 1984;33(3):291–6.10.2337/diabetes.33.3.291Suche in Google Scholar
[82] Zhang N, Zhang W, Guo X, Liu J, Li S, Zhang H, et al. Genistein protects against hyperglycemia and fatty liver disease in diet-induced prediabetes mice via activating hepatic insulin signaling pathway. Front Nutr. 2022;9:1–15.10.3389/fnut.2022.1072044Suche in Google Scholar PubMed PubMed Central
[83] Zhou Y, Wang S, Lou H, Fan P. Chemical constituents of hemp (Cannabis sativa L.) seed with potential anti-neuroinflammatory activity. Phytochem Lett. 2018;23:57–61.10.1016/j.phytol.2017.11.013Suche in Google Scholar
[84] Zhou Y, Wang S, Ji J, Lou H, Fan P. Hemp (Cannabis sativa L.) seed phenylpropionamides composition and effects on memory dysfunction and biomarkers of neuroinflammation induced by lipopolysaccharide in mice. ACS Omega. 2018;3(11):15988–95.10.1021/acsomega.8b02250Suche in Google Scholar PubMed PubMed Central
[85] Wang S, Luo Q, Fan P. Cannabisin F from hemp (Cannabis sativa) seed suppresses lipopolysaccharide-induced inflammatory responses in BV2 microglia as SIRT1 modulator. Int J Mol Sci. 2019;20:507.10.3390/ijms20030507Suche in Google Scholar PubMed PubMed Central
[86] Abdel-Kader MS, Radwan MM, Metwaly AM, Eissa IH, Hazekamp A, ElSohly MA. Chemistry and biological activities of cannflavins of the cannabis plant. Cannabis Cannabinoid Res. 2023;8(6):974–85.10.1089/can.2023.0128Suche in Google Scholar PubMed PubMed Central
[87] Chuanphongpanich S, Racha S, Saengsitthisak B, Pirakitikulr P, Racha K. Computational assessment of Cannflavin A as a TAK1 inhibitor: Implication as a potential therapeutic target for anti-inflammation. Sci Pharm. 2023;91:36.10.3390/scipharm91030036Suche in Google Scholar
[88] Radwan MM, ElSohly MA, Slade D, Ahmed SA, Wilson L, El-Alfy AT, et al. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry. 2008;69(14):2627–33.10.1016/j.phytochem.2008.07.010Suche in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?
Artikel in diesem Heft
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?

