Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
-
Helmy A. Aamer
Abstract
Methanolic extract from Salsola imbricata was investigated for its phytochemical content, antioxidant, and antimicrobial properties against phytopathogenic fungi and bacteria. Phytochemical analysis revealed the presence of saponin, tannins, and alkaloids with 1.25%, 18.8 mg catechin/g of extract, and 9.12%, respectively. Total flavonoid content was 20.8 mg quercetin equivalent/g while total phenolic content was 202 mg gallic acid equivalent/g. Antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl assay resulted in an IC50 value of 48.61 µg/mL, while the phosphomolybdenum method yielded a value of 215.43 mg ascorbic acid equivalent/g of extract. The highest phenolic acids detected in the extract were gallic acid (712.97 µg/g), syringic acid (742.7 µg/g), and caffeic acid (474.70 µg/g) according to high-performance liquid chromatography analysis. Palmitic acid (28.38%) dominated the fatty acids identified by gas chromatography–mass spectrometry, while stigmasterol (8.34%) was the most abundant steroid. At a concentration of 3 mg/mL, the extract showed strong antibacterial activity against Pectobacterium carotovorum (10.50 mm), Ralstonia solanacearum (9.93 mm), and Pectobacterium atrosepticum (8.37 mm). Additionally, the extract significantly suppressed fungal growth of Rhizoctonia solani (38.22%) and Fusarium oxysporum (33.56%) but showed lower activity toward Botrytis cinerea (13.33%) at 5 mg/mL. In conclusion, S. imbricata extract exhibited promising antioxidant and antimicrobial properties, making it a potential candidate for further exploration in agricultural applications.
Graphical abstract
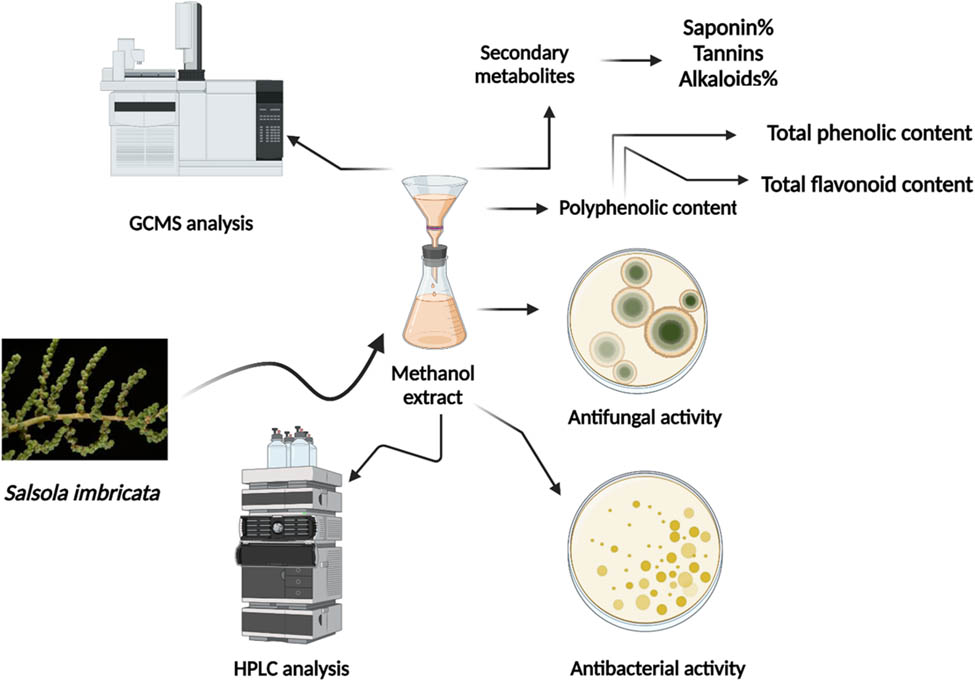
1 Introduction
Naturally occurring plant-based chemicals can function as pesticides and mitigate fungal diseases in agricultural settings. Extensive attempts have been undertaken to examine plants to discover alternative natural fungicides that can substitute for the existing synthetic ones, which are linked to issues such as harmful effects on plants, resistance, and revival of pests, toxicity to vertebrates, vast environmental risks, and expensive expenses [1]. Halophytes have a large distribution in Peninsular Arabia and the Mediterranean region. They are utilized in traditional herbal treatments and contribute to the diverse plant resources in these areas. These plant species have a higher prevalence in saline settings and possess exceptional adaptability, enabling them to thrive in typical climatic zones [2,3]. Medicinal plants have been a vital source of therapeutic products for thousands of years, with many contemporary drugs derived from plant sources. Traditionally, these plants are valued for their anti-inflammatory, antioxidant, and antibacterial properties. However, the process of identifying and integrating new compounds into modern therapeutic protocols is complex, time-consuming, and costly. As such, there is a growing focus on studying plant extracts, as their synergistic effects often enhance their therapeutic activity beyond that of individual phytochemicals [4]. Salsola imbricata, which is commonly included in camel feed, is not only the most widely used remedy for diarrhea but also has medicinal properties that make it effective in treating dysentery, stomachaches, and worm infestations [5].
The genus Salsola plays a crucial role in the plant kingdom because of its wide range of traditional, industrial, and environmental uses [6]. Salsola species are widely distributed across temperate zones and constitute approximately 45% of the plant species present in desert environments [6]. They serve as an abundant reservoir of many phytochemical categories, including alkaloids, coumarins, cardenolides, flavonoids, phenolic acids, isoflavonoids, and triterpenoids [7]. A variety of phytochemicals derived from plants, including saponins, flavonoids, and terpenoids, possess advantageous properties that promote human health and can be incorporated into functional foods and supplements [8]. Salsola species have a long history of medicinal usage due to their anti-inflammatory, blood pressure-lowering, and immune-boosting properties [9]. Salsola species has garnered significant attention from researchers due to its numerous pharmacological effects [10]. Recent research has highlighted the potential of medicinal plants to yield bioactive compounds with antimicrobial and antioxidant properties. For example, Ndezo Bisso et al. [11] demonstrated the strong antioxidant and antimicrobial activities of seven selected medicinal plants, including Psychotria peduncularis and Tristemma mauritianum, which were rich in phenols and flavonoids. Their study underscores the value of medicinal plants in developing natural alternatives to synthetic chemicals for agricultural and pharmaceutical applications. Numerous antioxidants that function as potent oxygen scavengers can be found in medicinal plants. To avoid the disadvantages of synthetic antioxidants, attention has recently been drawn to antioxidants derived from natural sources [12].
Over the last few years, growing attention has been to developing additional environmentally sustainable phytopathogen control in the global agriculture sector [13]. Incorporating phytochemicals as bio-origin agents, in their raw or refined form, is one of the possible strategies showing similar exciting outcomes and is getting a lot of interest [14,15,16]. Phytochemicals are eco-friendly substances that have residual impacts on the environment, including biodegradability, phytotoxicity, and a lack of long-lasting impact [17]. Notwithstanding, a lot of the active substances in these natural extracts are essential for bioprotection [18]. Most of the diverse array of small molecules, or secondary metabolites, made by plants act as safeguards against different microbial risks, pests, and herbivores [19]. Among the secondary metabolites produced in the plant world, phenolic acids are known for their abundant presence [20]. Interestingly, phytochemicals that plant “defense systems” against fungal diseases are primarily based on their diversity [19]. Phenolic compounds can break down the structure of these pathogens directly by changing their morphology, ultrastructure, and physiological processes [16,18]. They can also do this indirectly by making plants resistant to them throughout their systems [21].
Chemical fungicide resistance is a growing concern due to the significant harm caused by plant-pathogenic bacteria and fungi to crops worldwide [22]. These diseases include soft rot, bacterial wilt, vascular wilt, damping-off, root rot, and stem canker diseases [23]. The widespread use of chemical fungicides has raised environmental and health concerns due to their long-lasting nature [24,25]. To address these issues, it is crucial to explore natural sources, like plant extracts, for antimicrobial agents that can counteract these diseases while mitigating environmental impacts and addressing resistance concerns. Research on bioactive substances derived from plant extracts has promising prospects for developing sustainable and ecologically sound substitutes for chemical fungicides, protecting agricultural systems, and promoting human well-being. The current study aims to investigate the phytochemical composition of the halophyte S. imbricata, with a specific focus on important components including phenolic content, flavonoid content, and other significant secondary metabolites. In addition, our objective is to do gas chromatography–mass spectrometry (GC–MS) analysis on the aqueous methanolic extract to determine the chemical components that are present. Furthermore, we are investigating the extract’s biological properties, particularly its efficacy against specific phytopathogenic bacteria and fungi, as well as its antioxidant capabilities. By conducting this comprehensive analysis, our study seeks to address the gap in research on S. imbricata as a potential source of natural pesticides and its broader medicinal and agricultural applications.
2 Materials and methods
2.1 Plant material and extraction process
The S. imbricata plants were collected at the flowering stage from the El-Hamam region of Matrouh Governorate, Egypt, at coordinates (30°50′22.8″N 29°23′55.9″E) and then grabbed to the Department of Plant Production for plant specimen identification and deposited in the respiratory herbarium of the Faculty of Agriculture (Saba Basha), Alexandria University, Alexandria, Egypt, under voucher number 7706. The plant specimens’ aerial parts were meticulously cleansed using tap water to remove any dust or debris adhering to their surface. After air-drying at ambient temperature in a shaded area for 2 weeks, the desiccated substance was pulverized into a fine powder using a mill, yielding particles with a size of 80 mesh. One kilogram of this fine powder was steeped in 3 L of 80% methanol for 48 h, with occasional agitation. Following the maceration procedure, the extract was filtered using the filter paper of Whatman No. 1 under vacuum conditions, the filtrates were consolidated and evaporated using a rotary evaporator. The dark brown, shapeless powder obtained from the aqueous methanol extract was kept at 4°C [26,27].
2.2 Phytochemicals quantification
2.2.1 Total phenolic content (TPC)
The Folin–Ciocalteau reagent was used to quantify the TPC of the extract [28]. Gallic acid was chosen as the standard reference, and the plant extract was dissolved in methanol. The Folin–Ciocalteau reagent was diluted tenfold using deionized water. The reaction mixture, consisting of 0.5 mL of the extract solution and 2 mL of the diluted Folin–Ciocalteau reagent, was incubated for 3 min at 26 ± 2°C. Subsequently, a 1 mL solution of sodium carbonate 7.5% (w/v) was added, and the solution underwent incubation at 40°C for 30 min. The samples were measured for absorbance at a wavelength of 765 nm. A blank solution of deionized water was used as a standard instead of the plant extract. To find the TPC values, a calibration curve made with gallic acid was used as a guide. Data were presented in mg of gallic acid equivalents per gram of extract.
2.2.2 Flavonoid content
With a few minor modifications, the aluminum chloride method – which was described by Woisky and Salatino [29], was used to assess the total flavonoid concentration in the plant extract. Quercetin was used as the standard reference. The process began with dissolving 1 mL of the extract in 2 mL of a 2% methanol-based aluminum chloride solution. The solution was vigorously shaken and then kept at 25 ± 2°C for 1 h in the absence of light. Afterward, the amount of light absorbed by the combination was determined at a wavelength of 420 nm. The flavonoid concentration was quantified by comparing it to a quercetin calibration curve. Data were presented in milligrams of (mg QE/g) of quercetin equivalents per gram of extract. The stated findings correspond to the mean value derived from three separate measurements conducted independently.
2.2.3 Tannin content
The vanillin test created by Ksouri et al. [30] was used to measure the total amount of condensed tannins in an aqueous methanolic extract of S. imbricata. By using the reaction between tannins and vanillin in an acidic setting, this method creates a pink-red complex. To prepare triplicate samples, combine 200 µL of extract solution in methanol with 3 mL of 4% vanillin solution in methanol and 1.5 mL of HCl. After 15 min at room temperature, each mixture’s absorbance was measured against a methanol blank at 500 nm. (+)-Catechin was used as the standard, so total condensed tannins could be calculated as mg (+)-catechin equivalent per gram of extract.
2.2.4 Total saponin
For the investigation of total saponins, we followed the method of Obadoni and Ochuko [31] with minor modifications. One gram of dry extract was initially suspended in distilled water, and defatting was carried out twice with diethyl ether (20 mL each) in a separating funnel, followed by discarding the ether layer. Subsequently, the remaining aqueous layer underwent extraction with n-butanol. A 10 µL of 5% NaCl was used to wash the n-butanol extracts twice. The resulting solution was concentrated to dryness. The saponin content was then determined using the following formula:
2.2.5 Alkaloids content
The alkaloid content was measured using the Richardson and Harborne [32] technique. In a sealed 250-mL beaker, 1 g of dried Salsola methanolic extract was combined with 100 mL of 10% acetic acid (dissolved in ethanol). The mixture was left to incubate for 4 h. The extract was further filtered and condensed, and then, ammonium hydroxide was added progressively until precipitation occurred. Subsequently, the precipitate was filtered with Whatman filter paper and purified with diluted ammonium hydroxide. The remaining substance was measured in weight and subjected to dehydration at 40°C. The alkaloid content was calculated using the following formula:
2.3 HPLC analysis of polyphenolic compounds
The phenolic content of the extract was analyzed using an Agilent 1260 Infinity high-performance liquid chromatography (HPLC) Series, which consisted of a quaternary pump and a Zorbax Eclipse Plus C18 column (100 mm × 4.6 mm i.d.). A gradient elution system with three mobile phases was used for the separation process. These were (A) HPLC-grade water with 0.2% H3PO4 (v/v) in it, (B) methanol, and (C) acetonitrile. The column temperature was maintained at 30°C, and the flow rate was kept constant at 1 mL/min. A 25 µL of sample extract was injected, and compound detection was achieved using a UV detector set at 284 nm. To detect the specific chemical, stock solutions were diluted with methanol until the final concentration was 50 μg/mL. This was then used to make real reference samples. The standard references, including gallic acid, chlorogenic acid, catechin, methyl gallate, caffeic acid, syringic acid, rutin, ellagic acid, coumaric acid, vanillin, ferulic acid, naringenin, daidzein, quercetin, and cinnamic acid, purchased from Sigma-Aldrich (Germany), were compared with the retention times of the analytes obtained from the sample extract. Finally, quantification was determined based on peak area computation using ClarityChrom® Version 7.2.0 software.
2.4 GC–MS analysis
The extract phytochemical composition was analyzed using the Thermo Scientific Trace GC Ultra ISQ mass spectrometer. The analytical approach utilized a TraceGOLD TG-5MS direct capillary column (30 m × 0.25 mm × 0.25 μm film thickness). The methanolic extract was dissolved in analytical-grade methanol. Starting at 50°C, the temperature of the column oven was raised at a rate of 5°C/min until it reached 230°C, and then, it was maintained at that level for 2 min. Then, the temperature went up to 290°C and kept it for 2 min. The injector transfer line temperature was maintained at 250°C, while the mass spectrometer transfer line temperature was maintained at 260°C. At a temperature of 250°C, a 1/4 volume of helium was injected with a split ratio of 1:30 as the carrier gas. Under electron ionization mode, the mass spectrometer was operated at a temperature of 200°C and an energy level of 70 eV. A scan range of 40–1,000 m/z was set up for the mass spectra analysis. To determine the compounds, we used mass spectra and retention time comparisons with information from the Wiley and NIST MS library databases.
2.5 Antimicrobial activities assay
2.5.1 Antifungal assessment
Fusarium oxysporum (Acc# OQ820156), Rhizoctonia solani (Acc# OQ880458), and Botrytis cinerea (Acc# OR116485) are three common phytopathogenic fungi that were previously identified and used to test the extract’s antifungal activity [33,34]. In addition to the extract, copper hydroxide, a common agricultural fungicide, was tested as a positive control at a concentration of 250 µg/mL. To test the effect of plant extract against the mycelial radial growth, the extract was dissolved in DMSO, then mixed with sterile PDA media, and poured into 9-cm Petri plates to achieve different concentrations ranging from 1 to 5 mg/mL. For each fungus, agar plugs with actively growing fungal hyphae (5 mm in diameter) were carefully put in the center of Petri plates. The diameter of colony growth was measured after incubation at 27°C without light until fungal growth in the negative controls covered the whole surface of the Petri plate. The proportion of mycelial growth inhibition was calculated using the following formula:
where DC represents the average diameter of fungal growth observed in the control group, whereas DT denotes the mean diameter measured in the test extract treatment. All experiments were triplicated.
2.5.2 Antibacterial assessment
In the investigation of antimicrobial activity using the paper disc diffusion technique, the methanolic extract underwent sterilization through filtration with a 0.22 µm Millipore filter. Concentrations of 0.5, 1, 2, and 3 mg/mL were applied by transferring 20 µL of the respective extract concentrations onto pre-sterilized 5 mm filter paper discs. The bacterial isolates P. carotovorum (Acc# MN598002), P. atrosepticum (Acc# MG706146), and R. solanacearum (Acc# LN681200) isolates were previously identified [35]. Using sterile cotton swabs, the bacterial cultures were equally dispersed on nutrient agar plates. The discs, along with positive controls containing streptomycin (10 µg/disc) and negative controls with DMSO (20 µL/disc), were then placed on the agar surface. The diameters of the inhibition zones surrounding each disc were measured in mm after incubation at 37°C for 24 h. To be sure the results could be repeated, we ran each treatment three times.
2.6 Antioxidant activity (DPPH)
The electron-donating capacity of the extract was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, as outlined in the procedure by Bakar et al. [36]. The extract was dissolved in methanol at varying concentrations (2.4–175 µg/mL), and a 40 µg/mL DPPH solution was prepared in methanol. In triplicate, 200 µL of each extract concentration or ascorbic acid (positive control) was mixed with 1.8 mL of the DPPH solution. The solutions were agitated and kept in a dark environment for 30 min at 25°C. The optical density at a wavelength of 517 nm was determined using 1 cm cuvettes, comparing the sample to a blank solution without DPPH. The percentage of free radical scavenging activity was determined using the formula: [control absorbance – sample absorbance]/[control absorbance] × 100. The control was established using methanol instead of the extract solution. The activity vs concentration was plotted, and the IC50 (concentration inhibiting 50% of radicals) was determined.
2.7 Total antioxidant capacity (TAC)
The phosphomolybdenum assay was employed to test the TAC, which takes into account how well it can change molybdenum (VI) into molybdenum (V). This reduction, facilitated by antioxidants in the sample, leads to the formation of a green phosphate/Mo(V) complex, whose intensity is directly proportional to the TAC. Following the method outlined by Prieto et al. [37], the phosphomolybdate reagent, consisting of 28 mM sodium phosphate, 0.6 M sulfuric acid, and 4 mM ammonium molybdate, played a pivotal role in this assay. The extract, dissolved in methanol, underwent testing, while ascorbic acid served as a standard (20–200 µg/mL). The reaction mixtures consisted of 400 µL of either the extract solution or ascorbic acid solution, together with 3.1 mL of phosphomolybdate reagent. These mixtures were vigorously shaken and then incubated at 95°C for 90 min while being kept in the dark. Once the solutions were cooled, the absorbance of each solution that had been incubated was measured at a wavelength of 695 nm, with a blank serving as a reference. The extract’s overall antioxidant capability was quantified as an ascorbic acid equivalent (AAE) per gram of extract, providing a comprehensive measure of its antioxidant potential.
2.8 Statistical analysis
The data analysis for the antimicrobial activity of S. imbricata extract against fungal and bacterial strains was conducted using SAS 9.2 in a completely randomized design, employing a one-way analysis of variance. Mean comparisons were performed using Tukey’s test at a significance level of P < 0.05. The standard deviation (±SD) was calculated for both the antimicrobial activity studies and the quantification of saponins, tannins, alkaloids, total phenolics, and total flavonoids. All experiments were carried out in triplicate.
3 Results
3.1 Estimate the quantity of saponin, tannins, alkaloids, total phenolic, and total flavonoid
The aerial part of S. imbricata was macerated and extracted with 80% methanol as a solvent. This gave a yield of 8.37 g/100 g of dry matter. A quantitative analysis was done on the extract to look at important secondary metabolites from different groups of phytochemicals. The comprehensive data, summarized in Table 1, present the phytochemical constituents in dry extract. There were large amounts of secondary metabolites in the extract. The saponin content measured value was 1.25%, while the alkaloid content estimated value was 9.12%. Tannins were also present, with a concentration of 18.8 mg catechin/g extract. Furthermore, the TPC was determined to be 202 mg GAE/g extract, while the total flavonoid content (TFC) was 20.8 mg QE/g extract. These findings contribute valuable insights into the chemical composition of the S. imbricata extract, emphasizing its potential significance in various biological and pharmacological applications.
Bioactive components profiles of S. imbricata methanolic extract
| Class | Estimated parameter | Concentration ± SD* |
|---|---|---|
| Secondary metabolites | Saponin% | 1.25 ± 0.05 |
| Tannins | 18.8 ± 0.09 | |
| Alkaloids% | 9.12 ± 0.15 | |
| Polyphenolic content | Total phenolic content (mg GAE/g) | 202 ± 2.65 |
| Total flavonoid content (mg QE/g) | 20.8 ± 0.21 |
-
*SD, standard deviation; saponin and alkaloids expressed as percentage %; tannins expressed as mg catechin/g of extract); GAE expressed as gallic acid equivalent; QE expressed as quercetin equivalent.
3.2 Antioxidant activity evaluation
The antioxidant capability of the extract was assessed using two different methods. Through phosphomolybdenum-based assay, the extract has a total antioxidant capacity of 215.43 mg AAE/g of extract. The second method was the radical scavenging activity using the DPPH method, which uses ascorbic acid as a reference. The IC50 of the extract was 48.61 ± 0.46 µg/mL compared to ascorbic acid which was 7.81 ± 0.25 µg/mL.
3.3 HPLC analysis of phenolic and flavonoids
The polyphenolic profile identified in the methanolic extract of S. imbricata using HPLC is presented in Figure 1 and Table 2. The highest concentration was observed for syringic acid at 742.71 µg/g, followed closely by gallic acid with a concentration of 712.97 µg/g. Caffeic acid was also present at a substantial concentration of 474.70 µg/g, while quercetin showed a concentration of 331.69 µg/g. Ellagic acid and naringenin were detected at 228.91 and 72.80 µg/g, respectively. Moderate concentrations were found for rutin (41.20 µg/g), methyl gallate (31.16 µg/g), and vanillin (29.65 µg/g). Compounds such as chlorogenic acid, catechin, coumaric acid, daidzein, and cinnamic acid were present in lower concentrations, ranging from 14.18 to 33.79 µg/g. This highlights the significance of high-performance liquid chromatography in precisely defining and measuring these bioactive components in plant extracts.

HPLC chromatogram of polyphenolic compounds analysis of S. imbricata methanolic extract.
The polyphenolic profile identified in the methanolic extract of S. imbricata using HPLC
| No. | Compounds | RT (min) | Concentration (µg/g) |
|---|---|---|---|
| 1 | Gallic acid | 3.367 | 712.97 |
| 2 | Chlorogenic acid | 4.304 | 27.86 |
| 3 | Catechin | 4.579 | 21.70 |
| 4 | Methyl gallate | 5.491 | 31.16 |
| 5 | Caffeic acid | 5.86 | 474.70 |
| 6 | Syringic acid | 6.572 | 742.71 |
| 7 | Rutin | 7.684 | 41.20 |
| 8 | Ellagic acid | 8.547 | 228.91 |
| 9 | Coumaric acid | 9.211 | 25.28 |
| 10 | Vanillin | 9.776 | 29.65 |
| 11 | Ferulic acid | 10.069 | 14.18 |
| 12 | Naringenin | 10.558 | 72.80 |
| 13 | Daidzein | 12.24 | 82.49 |
| 14 | Quercetin | 12.732 | 331.69 |
| 15 | Cinnamic acid | 14.074 | 33.79 |
3.4 GC–MS analysis
The GC–MS revealed the presence of 19 identified chemicals from different chemical classes. The primary category consisted of fatty acids and fatty acid methyl esters, which accounted for 64.51% of the overall makeup. Palmitic acid was the predominant constituent, accounting for 28.38% of the total composition, with linoleic acid (15.68%), oleic acid (9.53%), and stearic acid (4.16%) following in decreasing order. The composition analysis revealed that steroids constituted 14.92% of the total composition. Among the steroids, stigmasterol was the most abundant, accounting for 8.34%, followed by β-sitosterol at 6.58%. Furthermore, the compound known as triterpenoid methyl ursolate accounted for 6.96% of the total makeup. Figure 2 depicts the GC chromatogram, which visually displays the peaks of the compounds and their elution periods. Table 3 presents the identified substances, including their retention periods, concentrations, and structures. This thorough study, together with the chromatogram, provides insight into the many chemical components found in S. imbricata.
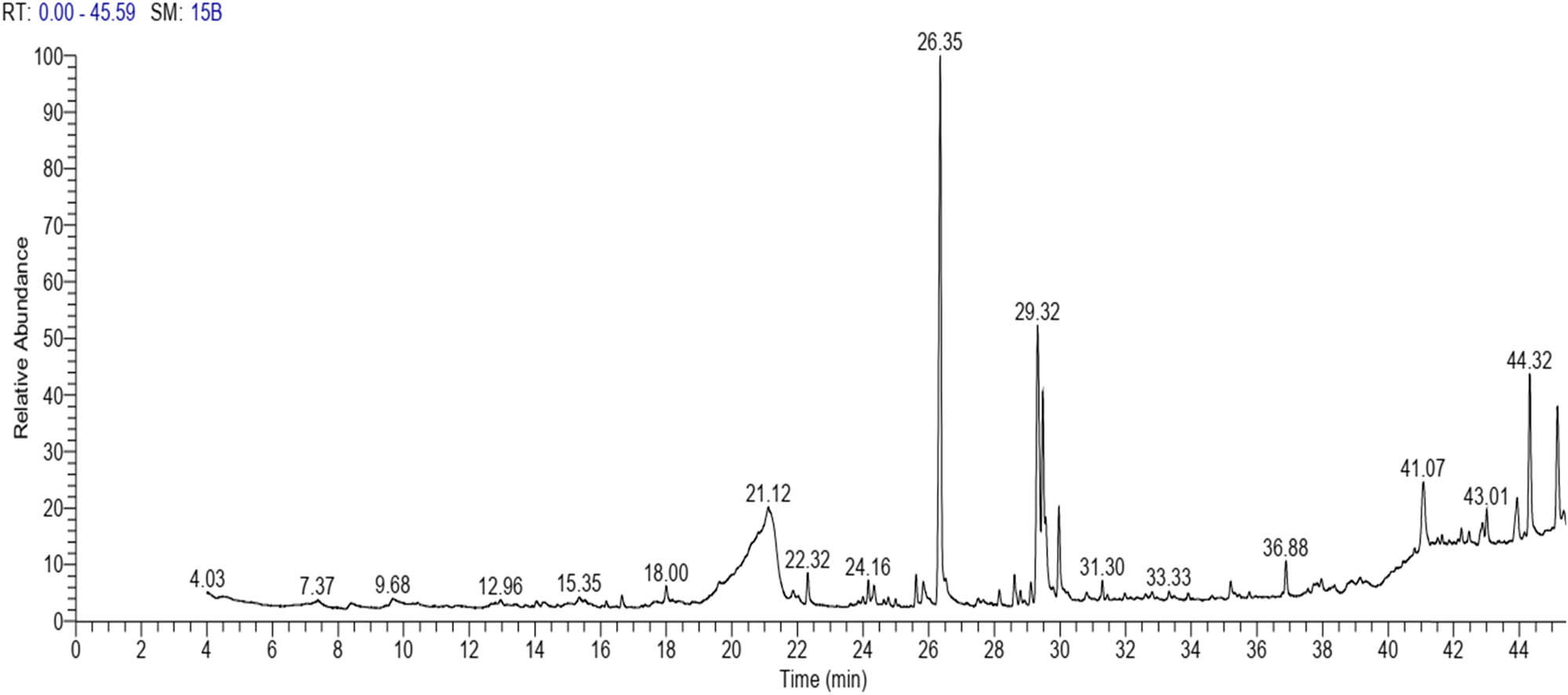
GC–MS chromatogram of S. imbricata methanolic extract.
Phytochemical constituents identified in the methanolic extract of S. imbricata using GC–MS
| RT | Area% | Compounds | Class | Chemical structure |
|---|---|---|---|---|
| 21.19 | 6.34 | d-Glucopyranose,3-O-methyl | Methylated carbohydrate |
 |
| 22.31 | 1.47 | Myristic acid | Fatty acid |
 |
| 24.16 | 0.94 | 2-[(Z)-9-Octadecenyloxy] ethanol | Alkene |
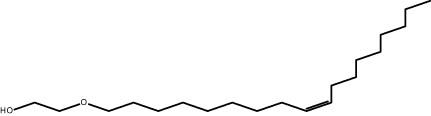 |
| 24.34 | 1.05 | Pentadecanoic acid | Fatty acid |
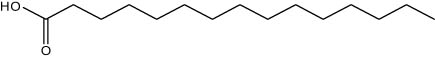 |
| 25.61 | 1.45 | Methyl palmitate | Fatty acid methyl ester |
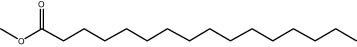 |
| 25.83 | 1.13 | Palmitoleic acid | Fatty acid |
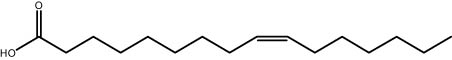 |
| 26.35 | 28.38 | Palmitic acid | Fatty acid |
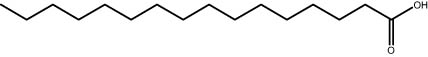 |
| 28.61 | 1.66 | Linoleic acid methyl ester | Fatty acid methyl ester |
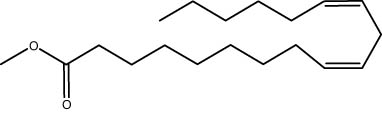 |
| 29.11 | 1.05 | Phytol | Terpenoid |
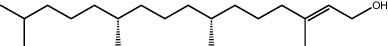 |
| 29.31 | 15.68 | Linoleic acid | Fatty acid |
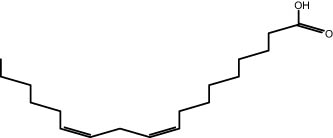 |
| 29.56 | 9.53 | Oleic acid | Fatty acid |
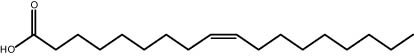 |
| 29.96 | 4.16 | Stearic acid | Fatty acid |
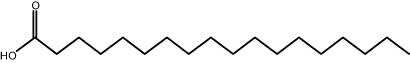 |
| 31.3 | 0.93 | Tributyl citrate acetate | Ester |
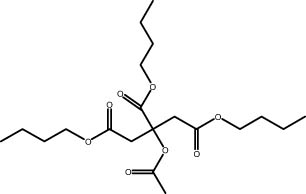 |
| 36.88 | 1.74 | Phorbol | Diterpenoids |
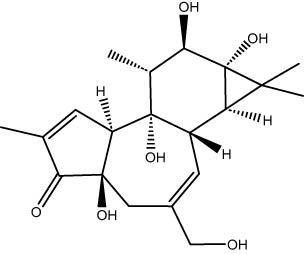 |
| 41.06 | 6.96 | Methyl ursolate | Triterpenoid |
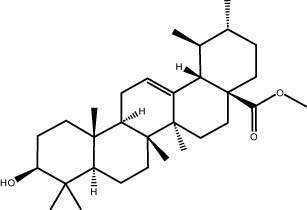 |
| 42.88 | 1.22 | Dotriacontane | Alkane |
 |
| 43.01 | 1.38 | Betulin | Triterpene |
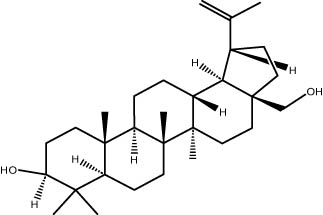 |
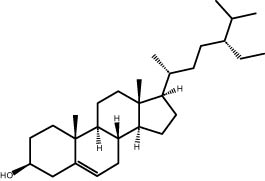
| 44.32 | 8.34 | Stigmasterol | Steroid |
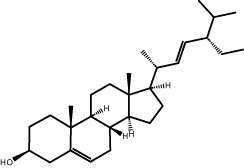 |
| 45.16 | 6.58 | β-Sitosterol | Steroid |
 |
3.5 Antimicrobial activity
The extract exhibits significant inhibitory effects on Gram-negative plant pathogenic bacteria, indicating promising antibacterial action (Table 4). The extract inhibited three common pathogens: P. atrosepticum, P. carotovorum, and R. solanacearum. The antibacterial test exhibited a dose-dependent response, where higher concentrations of the extract led to larger areas of inhibition. The extract worked best against P. carotovorum, as evidenced by the inhibition zone’s largest diameter of 10.50 mm at a concentration of 3 mg/mL. R. solanacearum exhibited notable susceptibility, as evidenced by an inhibition zone diameter of 9.93 mm at a concentration of 3 mg/mL. P. atrosepticum demonstrated comparatively reduced sensitivity, with an inhibition zone diameter of 8.37 mm at the highest concentration.
Inhibition zone diameter (mm) measurements of phytopathogenic bacteria by methanolic extract of S. imbricata
| Concentration (mg/mL) | Inhibition zone diameter (mm)* | ||
|---|---|---|---|
| P. carotovorum | P. atrosepticum | R. solanacearum | |
| 0.5 | 7.30 ± 0.04d | 0.00 ± 0.00e | 0.00 ± 0.00d |
| 1 | 8.02 ± 0.08c | 7.00 ± 0.14d | 7.40 ± 0.08c |
| 2 | 8.98 ± 0.08b | 7.75 ± 0.04b | 8.79 ± 0.09b |
| 3 | 10.50 ± 0.11a | 8.37 ± 0.06a | 9.93 ± 0.15a |
| Streptomycin (10 µg/disc) | 8.28 ± 0.08c | 7.37 ± 0.09c | 9.73 ± 0.18a |
-
*The means in each column that share the same letter exhibit no significant differences at a probability level of 0.05.
The S. imbricata extract was tested for its ability to inhibit the three fungi: F. oxysporum, R. solani, and B. cinerea. The results, shown in Table 5, show that as the concentration of the extract goes up, so does the percentage of inhibited mycelial growth. R. solani demonstrated the highest sensitivity to the extract, with a significant inhibition percentage of 38.22% at a concentration of 5 mg/mL. Following closely, F. oxysporum displayed notable sensitivity, with an inhibition percentage of 33.56% at the same concentration. In contrast, B. cinerea demonstrated lower sensitivity to the extract, exhibiting only a 13. 33% inhibition percentage at the highest concentration. Surprisingly, the extract did not seem to have any effect on B. cinerea at concentrations of 1, 2, and 3 mg/mL.
In vitro growth inhibition of phytopathogenic fungi by methanolic extract of S. imbricata
| Concentration (mg/mL) | Inhibition percentage (%)* | ||
|---|---|---|---|
| F. oxysporum | R. solani | B. cinerea | |
| 1 | 14.89 ± 3.57e | 16.22 ± 3.20d | 00.0 ± 0.00d |
| 2 | 16.22 ± 3.39e | 20.89 ± 3.18d | 00.0 ± 0.00d |
| 3 | 22.67 ± 1.69d | 26.44 ± 2.65c | 00.0 ± 0.00d |
| 4 | 28.67 ± 1.83c | 33.78 ± 3.00b | 9.56 ± 3.39c |
| 5 | 33.56 ± 1.99b | 38.22 ± 2.68b | 13.33 ± 2.48b |
| Copper hydroxide (250 µg/mL) | 66.67 ± 1.67a | 78.00 ± 1.65a | 61.11 ± 1.76a |
*The means in each column that share the same letter exhibit no significant differences at a probability level of 0.05.
4 Discussion
Salsola is a notable genus of halophytic plants that fall within the Amaranthaceae family. These plants are acknowledged for containing compounds that have antioxidant characteristics and exhibit biological activity [6]. Multiple investigations have already been carried out regarding the fungicidal properties of botanical extracts [8]. Although S. imbricata has fungicidal properties, there is little scientific evidence supporting its fungitoxic nature. The present study aimed to analyze the phytochemical composition of the methanolic extract of S. imbricata picked from Matrouh, Egypt. Additionally, we examine its antioxidant and antibacterial properties against specific phytopathogenic bacteria and fungi. The findings indicate that the extract yield of S. imbricata was 8.37 g/100 g dry matter. It was found that macerating the aerial parts of three species of Salsola led to methanolic extracts with yields of 8.51% for S. oppositifolia, 7.61% for S. soda, and 10.8% for S. tragus [38]. Furthermore, Ajaib et al. [39] demonstrated S. imbricata fruit produced an aqueous extract with an extraction yield of 11.2%. The methanol bark extract of S. imbricata Forssk. revealed a minimum yield of 5.12%. In their study, Osman et al. [40] obtained 112 g of powdered extract from 1,000 air-dried leaves of S. imbricata. The extraction process involved refluxing the leaves three times in methanol (75%).
Ajaib et al. [39] conducted a qualitative phytochemical investigation of S. imbricata and detected several bioactive components in different extracts. These chemicals include alkaloids, saponins, flavonoids, tannins, reducing sugar anthraquinone, and cardiac glycosides. Saponins, which are a crucial category of secondary metabolites found in plants, carry out several biological functions. Extensive research investigated the antibacterial features of the saponins [41,42]. Research indicates that pathogenic fungi harm chlorophyll-containing cells through the secretion of enzymes that degrade saponins. The ongoing analysis has discovered a substantial amount of saponin in the methanolic extract of S. imbricata. Soetan [43] suggests that saponins have a restricted capacity to traverse bacterial cell membranes. Ojinnaka and Kenne [44] documented the extraction of methyl ursolate from the stem of Myrianthus arboreus. It is crucial to acknowledge the intricate nature of plant extracts and that the effects may not be only attributed to one component but rather to a group of molecules (with either substantial or modest effects) that interact in an additive or synergistic way [45]. Researchers have discovered that the secondary metabolites of the plant, specifically the total phenolic, flavonoid, and tannin contents, play a crucial role in its capacity to combat free radicals. Hamdi et al. [46] discovered the total phenolic content exhibited significant variation in Salsola baryosma herbal materials, ranging from 0.75 to 2.60 mg GAE/g dry weight. Furthermore, the flavonoid content ranged from 0.46 to 0.19 mg/g rutin equivalent to the crude extract. We found that the TPC and TFC were higher than those reported by Shehab and Abu-Gharbieh [47]. The discrepancy in our research findings can be attributed to the variation in plant material used for extraction. In our study, we specifically used the aerial part of the plant, whereas Shehab and Abu-Gharbieh [47] extracted the whole plant, which was of herbal origin and obtained from the desert of Dubai, UAE. Additionally, the S. imbricata used in our study was sourced from the desert of El-Hamam located in the northern region of Egypt. The geographic location and plant parts can influence the phytochemical composition of plant extracts. Boulaaba et al. [48] discovered variations in the levels of total phenolic, total flavonoid, and condensed tannin contents in Salsola kali leaves, stems, and roots during different stages of development. For instance, the total phenolic content in the leaves reached a maximum of 11.9 mg GAE/g dry weight, while in the stems it was 5.6 mg GAE/g dry weight, and in the roots, it was only 0.79 mg GAE/g dry weight. In another study, Psychotria peduncularis exhibited the highest TPC and TFC, measuring 5.57 ± 0.22 mg GAE/g and 1.38 ± 0.06 mg QE/g, respectively [11]. The effectiveness of these phenolic compounds in combating fungus is determined by their composition and concentration. Phenolic chemicals possess antifungal properties and can exert their effects through several mechanisms. They can stop the cell cycle, lower the production of certain gene sequences, speed up the growth of mycelium, change normal metabolic pathways, or kill cells by throwing off their natural redox balance [16,49,50]. To assess the antioxidant activity of the studied halophyte plant, we utilized both phosphomolybdenum and DPPH radical scavenging assays. The antioxidant activity was classified as follows: very strong (IC50 < 50 µg/mL), strong (50 ≤ IC50 < 100 µg/mL), moderate (100 ≤ IC50 < 150 µg/mL), and low (IC50 > 150 µg/mL) [51]. According to these criteria, our plant extract exhibited very strong antioxidant activity across both assays, although researchers advise against concluding based on one assay [52].
The observed antioxidant activity in these medicinal plants can be attributed to the presence of phenolic compounds, such as phenolic acids and flavonoids, which exert their effects through the hydrogen-donating properties of their hydroxyl groups [53]. Additionally, these phenolic compounds can chelate metal ions that participate in the production of reactive oxygen species [54]. Our findings are consistent with those reported by Ngbolua et al. [55], who documented the antioxidant activity of A. manniana, as well as the results of Tsafack et al. [56], who highlighted the antioxidant properties of T. mauritianum. Additionally, our study recorded promising antioxidant activity, compared with the methanolic extracts of medlar leaves exhibiting notable antioxidant activity (69.43%) in the DPPH radical assay and demonstrating antibacterial properties against both Gram-positive and Gram-negative bacteria, particularly showing substantial inhibition against Staphylococcus aureus (30.83 mm) [57].
Flavonoids are a diverse group of natural compounds that have shown a wide range of chemical and biological properties. They can remove harmful radicals, reduce allergies, fight viral infections, and reduce inflammation [58]. There is a strong link between the amount of phenolic compounds and the antioxidant capacity. This makes sense since phenolic compounds are known to get rid of free radicals [59,60]. Phenolics can get rid of free radicals if they can donate electrons or hydrogen atoms. This depends on how many hydroxyl groups they have and how they are structured and arranged [61]. Findings about phenolic and flavonoid contents are very important for understanding how antibacterial plant-based medicines work [62,63,64]. Therefore, in the current study, HPLC was used to determine how the amounts of phenolics and flavonoids in plant extracts used for animal feed affect their ability to inhibit fungi. Using HPLC to look at the phenolic and flavonoid contents of the methanolic extract of S. imbricata shows a varied phenolic profile with 15 identified compounds. Shehab and Abu-Gharbieh [47] employ HPLC to evaluate the levels of phenolic and flavonoid compounds in the extract of the S. imbricata plant. Thirteen components were detected at a wavelength of 280 nm, accounting for 13.904% of the overall makeup. Out of these, there were a total of nine phenolic acids, accounting for 9.734% of the compounds. As part of this, there were coumaric acids (4.251% of the total), two flavonoids (catechin and chrysin), diphenol catechol, and one nonphenolic benzoic acid (2.306% of the compounds). At a wavelength of 330 nm, a total of 8 components were detected, with 7 of them being of flavonoidal origin. Quercetin accounted for 12.692% of the components, whereas rosmarinic acid made up 2.734%. As well high concentrations of phenolic compounds, flavonoids, and carotenoids were found in methanolic extracts from the leaves of medlar [57]. The majority of phytochemicals are classified as phenolic, which refers to substances containing one or more aromatic rings that contain hydroxyl groups. They function as a defensive mechanism against microbial diseases [65]. Moreover, Nayak et al. [66] established that these compounds have a significant role in both reproduction and growth.
A GC–MS analysis of S. imbricata extract showed a wide range of compounds. Some of the main ones found were palmitoleic acid, oleic acid, palmitic acid, linoleic acid, stearic acid, methyl palmitate, linoleic acid methyl ester, stigmasterol, β-sitosterol, and phytol. These compounds, known for their varied biological activities, contribute to the potential health benefits of the extract. The presence of fatty acids such as palmitic, palmitoleic, linoleic, and oleic acids suggests the extract’s potential antioxidant and antimicrobial characteristics, supported by existing literature on the positive effects of these fatty acids on cellular health [67,68]. Additionally, the discovery of stigmasterol and β-sitosterol fits with earlier research that showed their antioxidant and anti-inflammatory properties. This supports the idea that the S. imbricata extract might be useful for treating health problems [69,70]. Griebel and Zeier [71] revealed that the interaction between Arabidopsis thaliana and Pseudomonas syringae results in the accumulation of stigmasterol, a phytosterol. This accumulation is a noteworthy metabolic activity that takes place in plants following bacterial leaf infection. Both avirulent and virulent P. syringae exhibit increased resistance to stigmasterol buildup. Furthermore, the presence of phytol in the extract adds to its potential antimicrobial and antioxidant capabilities. Phytol has demonstrated antimicrobial activity against various pathogens and antioxidant effects, as evidenced in studies investigating its role in medicinal plants [72,73]. Even though each of the identified compounds has bioactive properties of its own, the way they work together in the complex mixture of the extract may make the biological activities even stronger. In the same manner, Rasheed et al. [74] conducted GC–MS profiling of non-polar n-hexane extracts from five Salsola species, identifying various compounds. In S. arabica, oleic acid predominated, constituting 75.6% of the extract. The n-hexane extract of S. cyclophylla contained 25 different compounds. The most important ones were 1-octadecene (14.5%), 1-hexadecanol (13.7%), benzoic acid pentadecyl ester (11.3%), and 2,4-di-tert-butylphenol (10.4%). Cinnamaldehyde, -hexyl-, dominated S. imbricata extract, comprising 57.2% of the extract, with a total of 13 compounds identified. There were 18 chemicals in S. inscanescens, with octacosyl heptafluorobutyrate making up 25.4% of them. The most common fatty acid methyl esters in S. villosa were methyl palmitate (26.2%), methyl linoleate (17.8%), and methyl oleate (16.3%). Additionally, phytol was found in the n-hexane extracts of S. villosa and S. imbricata. Fatty acids such as linolenic, oleic, arachidonic, palmitic, and stearic acids were found in the aerial portions of S. kali. They also found sterols like β-sitosterol, β-sitosterol-3-O-glucoside, sitostanol, stigmasterol, and avenasterol in the upper parts of S. tetrandra, S. rigida, and S. longifolia [75,76]. For instance, a study on Lantana camara evaluated its antioxidant and anti-tumor properties, identifying four key compounds, including lantadene A and B. These compounds demonstrated significant antioxidant activity and reduced MCF-7 breast cancer cell viability, with lantadene B exhibiting the most potent anti-cancer effects and inducing cell cycle arrest in the G1 phase [77]. This highlights the potential of exploring plant-derived compounds, like those from S. imbricata, for therapeutic applications.
Overall, while our investigation sheds light on the chemical composition and biological activity of S. imbricata, we recognize some limitations that result from focusing solely on one species. One significant restriction is that the bioactive compounds and antibacterial activity discovered in S. imbricata may not fully represent the phytochemical diversity of other halophytic plants from the Arabian region. Some species, like Salsola kali, Haloxylon salicornicum, and Suaeda, may have new or similar chemicals with stronger bioactive effects. Additionally, environmental factors such as soil composition, climate, and location can substantially impact the phytochemistry of halophytic plants. Future studies should compare S. imbricata’s phytochemical profiles and bioactivities to those of other Arabian Peninsula species. This method of comparison could help us find new bioactive chemicals and find out if the biological activities reported are unique to S. imbricata or shared by related species. This would help us learn more about the different biological potential of plants that have adapted to living in deserts.
5 Conclusion
In conclusion, the methanolic extract from S. imbricata aerial parts exhibits promising antioxidant and antimicrobial properties, attributed to its rich phytochemical composition. The extract contains various secondary metabolites, including phenolic acids, fatty acids, and steroids, which contribute to its bioactivity. Antimicrobial tests revealed significant inhibition against several bacterial and fungal strains, with varying responses based on concentration. Further research is warranted to explore the extract’s potential as a natural alternative for agricultural applications.
Acknowledgments
The authors express their sincere thanks to the City of Scientific Research and Technological Applications (SRTA-City) and the Faculty of Agriculture (Saba Basha), Alexandria University, Egypt, for providing the necessary research facilities. The authors would like to extend their appreciation to the Researchers Supporting Project number (RSP2024R505), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: The research is financially supported by Researchers Supporting Project Number (RSP2024R505), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: H.A.A., S.B., and A.A.: conceptualization. H.A.A., A.A., S.F.E, O.A.S., M.A.G., P.K., S.B., and A.A.Al.: methodology and formal analysis. H.A.A., O.A.S., M.A.G., S.B., and A.A.: writing – original draft preparation. H.A.A. and S.B.: writing – review and editing. A.A. and P.K.: supervision. A.A.Al. and A.A.: funding acquisition. All authors contributed to the article and approved the submitted version.
-
Conflict of interest: Przemysław Kowalczewski is a current Editor of Open Life Sciences. This fact did not affect the peer-review process.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Patra AK. An overview of antimicrobial properties of different classes of phytochemicals. Diet Phytochem Microbes. 2012;9789400739260:1–32. 10.1007/978-94-007-3926-0_1.Search in Google Scholar
[2] Petropoulos SA, Karkanis A, Martins N, Ferreira ICFR. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem Toxicol. 2018;114:155–69.10.1016/j.fct.2018.02.031Search in Google Scholar PubMed
[3] Hameed A, Hussain S, Rasheed A, Ahmed MZ, Abbas S. Exploring the potentials of halophytes in addressing climate change-related issues: a synthesis of their biological, environmental, and socioeconomic aspects. World. 2024;5:36–57.10.3390/world5010003Search in Google Scholar
[4] Muntean D, Vulpie S. Antioxidant and antibacterial activity of plant extracts. Antibiotics. 2023;12:1176. 10.3390/antibiotics12071176.Search in Google Scholar PubMed PubMed Central
[5] Imran M, Ibrahim M, Riaz N, Malik A. Structure determination of aervins A-D, new coumaronochromone analogues from Aerva persica, by 1D and 2D NMR spectroscopy. Magn Reson Chem. 2009;47:532–6. 10.1002/mrc.2419.Search in Google Scholar PubMed
[6] Hanif Z, Ali HH, Rasool G, Tanveer A, Chauhan BS. Genus Salsola: its benefits, uses, environmental perspectives and future aspects-a review. J Rangel. Sci. 2018;8:315–28.Search in Google Scholar
[7] ElNaggar MH, Eldehna WM, Abourehab MAS, Abdel Bar FM. The old world salsola as a source of valuable secondary metabolites endowed with diverse pharmacological activities: a review. J Enzyme Inhib Med Chem. 2022;37:2036–62. 10.1080/14756366.2022.2102005.Search in Google Scholar PubMed PubMed Central
[8] Al-Obaidi JR, Jambari NN, Ahmad-Kamil EI. Mycopharmaceuticals and nutraceuticals: Promising agents to improve human well-being and life quality. J Fungi. 2021;7:503. 10.3390/jof7070503.Search in Google Scholar PubMed PubMed Central
[9] Li S, Chen Y, Duan Y, Zhao Y, Zhang D, Zang L, et al. Widely targeted metabolomics analysis of different parts of Salsola collina pall. Molecules. 2021;26:1126.10.3390/molecules26041126Search in Google Scholar PubMed PubMed Central
[10] Murshid SSA, Atoum D, Abou-Hussein DR, Abdallah HM, Hareeri RH, Almukadi H, et al. Genus Salsola: chemistry, biological activities and future prospective – a review. Plants. 2022;11:714. 10.3390/plants11060714.Search in Google Scholar PubMed PubMed Central
[11] Ndezo Bisso B, Njikang Epie Nkwelle R, Tchuenguem Tchuenteu R, Dzoyem JP. Phytochemical screening, antioxidant, and antimicrobial activities of seven underinvestigated medicinal plants against microbial pathogens. Adv Pharmacol Pharm Sci. 2022;2022:1998808.10.1155/2022/1998808Search in Google Scholar PubMed PubMed Central
[12] Gandhi PR, Jayaseelan C, Kamaraj C, Rajasree SRR, Mary RR. In vitro antimalarial activity of synthesized TiO2 nanoparticles using Momordica charantia leaf extract against Plasmodium falciparum. J Appl Biomed. 2018;16:378–86.10.1016/j.jab.2018.04.001Search in Google Scholar
[13] Maurya RP, Koranga R, Samal I, Chaudhary D, Paschapur AU, Sreedhar M, et al. Biological control: a global perspective. Int J Trop Insect Sci. 2022;42:3203–20. 10.1007/s42690-022-00881-9.Search in Google Scholar
[14] Dambolena JS, López AG, Meriles JM, Rubinstein HR, Zygadlo JA. Inhibitory effect of 10 natural phenolic compounds on Fusarium verticillioides. A structure–property–activity relationship study. Food Control. 2012;28:163–70.10.1016/j.foodcont.2012.05.008Search in Google Scholar
[15] Rosado-Álvarez C, Molinero-Ruiz L, Rodríguez-Arcos R, Basallote-Ureba MJ. Antifungal activity of asparagus extracts against phytopathogenic Fusarium oxysporum. Sci Hortic (Amst). 2014;171:51–7.10.1016/j.scienta.2014.03.037Search in Google Scholar
[16] Seo D-J, Lee H-B, Kim I-S, Kim K-Y, Park R-D, Jung W-J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb Pathog. 2013;56:8–15.10.1016/j.micpath.2013.01.001Search in Google Scholar PubMed
[17] Makhuvele R, Naidu K, Gbashi S, Thipe VC, Adebo OA, Njobeh PB. The use of plant extracts and their phytochemicals for control of toxigenic fungi and mycotoxins. Heliyon. 2020;6:e05291.10.1016/j.heliyon.2020.e05291Search in Google Scholar PubMed PubMed Central
[18] Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82.10.1128/CMR.12.4.564Search in Google Scholar PubMed PubMed Central
[19] Dixon RA. Natural products and plant disease resistance. Nature. 2001;411:843–7.10.1038/35081178Search in Google Scholar PubMed
[20] Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S. Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem. 2013;72:1–20.10.1016/j.plaphy.2013.05.009Search in Google Scholar PubMed
[21] Al-Wakeel S, Gabr M, Abu-El-Soud W, Saleh A. Coumarin and salicylic acid activate resistance to Macrophomina phaseolina in Helianthus annuus. Acta Agron Hung. 2013;61:23–35.10.1556/AAgr.61.2013.1.3Search in Google Scholar
[22] Ishii H. Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. Japan Agric Res Q. JARQ. 2006;40:205–11.10.6090/jarq.40.205Search in Google Scholar
[23] Behiry SI, Ashmawy NA, Abdelkhalek AA, Younes HA, Khaled AE, Hafez EE. Compatible- and incompatible-type interactions related to defense genes in potato elucidation by Pectobacterium carotovorum. J Plant Dis Prot. 2018;125:197–204. 10.1007/s41348-017-0125-5.Search in Google Scholar
[24] El‐Rahim WMA. Assessment of textile dye remediation using biotic and abiotic agents. J Basic Microbiol. 2006;46:318–28.10.1002/jobm.200510076Search in Google Scholar PubMed
[25] Fenta L, Mekonnen H. Microbial biofungicides as a substitute for chemical fungicides in the control of phytopathogens: current perspectives and research directions. Scientifica (Cairo). 2024;2024:5322696.10.1155/2024/5322696Search in Google Scholar PubMed PubMed Central
[26] Bashir S, Behiry S, Al-Askar AA, Kowalczewski PŁ, Emaish HH, Abdelkhalek A. Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract. Open Life Sci. 2024;19:20220962.10.1515/biol-2022-0962Search in Google Scholar PubMed PubMed Central
[27] Hamzah KA, Al-Askar A, Kowalczewski P, Abdelkhalek A, Emaish HH, Behiry S. A comparative study of the antifungal efficacy and phytochemical composition of date palm leaflet extracts. Open Chem. 2024;22:20240044. 10.1515/chem-2024-0044. Search in Google Scholar
[28] Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–58.10.5344/ajev.1965.16.3.144Search in Google Scholar
[29] Woisky RG, Salatino A. Analysis of propolis: some parameters and procedures for chemical quality control. J Apic Res. 1998;37:99–105.10.1080/00218839.1998.11100961Search in Google Scholar
[30] Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, et al. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol. 2008;331:865–73.10.1016/j.crvi.2008.07.024Search in Google Scholar PubMed
[31] Obadoni BO, Ochuko PO. Phytochemical studies and comparative efficacy of the crude extracts of some haemostatic plants in Edo and Delta States of Nigeria. Glob J Pure Appl Sci. 2002;8:203–8.10.4314/gjpas.v8i2.16033Search in Google Scholar
[32] Richardson PM, Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. Brittonia. 1990;42:115. 10.2307/2807624.Search in Google Scholar
[33] Al-Askar AA, Bashir S, Mohamed AE, Sharaf OA, Nabil R, Su Y, et al. Antimicrobial efficacy and HPLC analysis of polyphenolic compounds in a whole-plant extract of eryngium campestre. Separations. 2023;10:362.10.3390/separations10060362Search in Google Scholar
[34] Nabil M, Elnouby M, Al-Askar AA, Kowalczewski PŁ, Abdelkhalek A, Behiry SI. Porous silicon nanostructures: Synthesis, characterization, and their antifungal activity. Open Chem. 2024;22:20230169.10.1515/chem-2023-0169Search in Google Scholar
[35] Elkobrosy D, Al-Askar AA, El-Gendi H, Su Y, Nabil R, Abdelkhalek A, et al. Nematocidal and bactericidal activities of green synthesized silver nanoparticles mediated by ficus sycomorus leaf extract. Life. 2023;13:1083.10.3390/life13051083Search in Google Scholar PubMed PubMed Central
[36] Bakar MFA, Mohamed M, Rahmat A, Fry J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009;113:479–83.10.1016/j.foodchem.2008.07.081Search in Google Scholar
[37] Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41.10.1006/abio.1999.4019Search in Google Scholar PubMed
[38] Tundis R, Menichini F, Conforti F, Loizzo MR, Bonesi M, Statti G, et al. A potential role of alkaloid extracts from Salsola species (Chenopodiaceae) in the treatment of Alzheimer’s disease. J Enzyme Inhib Med Chem. 2009;24:818–24.10.1080/14756360802399662Search in Google Scholar PubMed
[39] Ajaib M, Faroogh S, Tariq Zahid M, Ishtiaq M, Shafi F, Ishtiaq S. Investigation of Salsola imbricata extracts against human pathogenic bacteria and fungi and in vitro antioxidant activities. Biosci Res. 2020;17:2716–9.Search in Google Scholar
[40] Osman SM, El Kashak WA, Wink M, El Raey MA. New isorhamnetin derivatives from Salsola imbricata Forssk. leaves with distinct anti-inflammatory activity. Pharmacogn Mag. 2016;12:S47.10.4103/0973-1296.176110Search in Google Scholar PubMed PubMed Central
[41] Avato P, Bucci R, Tava A, Vitali C, Rosato A, Bialy Z, et al. Antimicrobial activity of saponins from Medicago sp.: structure‐activity relationship. Phyther Res An Int J Devoted Pharmacol Toxicol Eval Nat Prod Deriv. 2006;20:454–7.10.1002/ptr.1876Search in Google Scholar PubMed
[42] Oleszek W. Alfalfa saponins: structure, biological activity, and chemotaxonomy. In: Waller GR, Yamasaki K, editors. Saponins used in food and agriculture. Advances in Experimental Medicine and Biology. Boston, MA: Springer; 1996. p. 155–70. 10.1007/978-1-4613-0413-5_13Search in Google Scholar PubMed
[43] Soetan KO. Evaluation of some pharmaceutical and haematological activities of saponins in guinea corn (Sorghum bicolor L. Moench). M. Sc dissertation. Ibadan: Department of Biochemistry, College of Medicine, University of Ibadan; 2003.Search in Google Scholar
[44] Ojinnaka CM, Kenne L. Studies on Nigerian medicinal plants: components of the stems of Myrianthus arboreus. J Nat Prod. 1985;48:1002–3.10.1021/np50042a030Search in Google Scholar
[45] van Vuuren S. The antimicrobial activity and essential oil composition of medicinal aromatic plants used in African traditional healing [Ph.D. dissertation]. Johannesburg, South Africa: University of Witwatersrand; 2007. p. 1–305.Search in Google Scholar
[46] Hamdi A, Yousfi M, Djeridane A. Antioxidant potential and α-glucosidase inhibitory effects of Salsola baryosma extracts. Proceeding of the 2nd African Congress on Biology & Health University Ferhat. Vol. 11, 2012. p. 128.Search in Google Scholar
[47] Shehab NG, Abu-Gharbieh E. Phenolic profiling and evaluation of contraceptive effect of the ethanolic extract of Salsola imbricata Forssk. in male albino rats. Evidence-Based Complement Altern Med. 2014;2014(1):695291.10.1155/2014/695291Search in Google Scholar PubMed PubMed Central
[48] Boulaaba M, Medini F, Hajlaoui H, Mkadmini K, Falleh H, Ksouri R, et al. Biological activities and phytochemical analysis of phenolic extracts from Salsola kali L. Role of endogenous factors in the selection of the best plant extracts. South Afr J Bot. 2019;123:193–9. 10.1016/j.sajb.2019.03.003.Search in Google Scholar
[49] Ansari MA, Anurag A, Fatima Z, Hameed S. Natural phenolic compounds: a potential antifungal agent. Microb Pathog Strateg Combat Sci Technol Educ. 2013;1:1189–95.10.1155/2014/895193Search in Google Scholar PubMed PubMed Central
[50] Kebaara BW, Langford ML, Navarathna DH, Dumitru R, Nickerson KW, Atkin AL. Candida albicans Tup1 is involved in farnesol-mediated inhibition of filamentous-growth induction. Eukaryot Cell. 2008;7:980–7.10.1128/EC.00357-07Search in Google Scholar PubMed PubMed Central
[51] Saptarini NM, Wardati Y. Effect of extraction methods on antioxidant activity of papery skin extracts and fractions of Maja cipanas onion (Allium cepa L. var. ascalonicum). Sci World J. 2020;2020:3280534.10.1155/2020/3280534Search in Google Scholar PubMed PubMed Central
[52] Munteanu IG, Apetrei C. Analytical methods used in determining antioxidant activity: A review. Int J Mol Sci. 2021;22:3380.10.3390/ijms22073380Search in Google Scholar PubMed PubMed Central
[53] Pereira DM, Valentão P, Pereira JA, Andrade PB. Phenolics: From chemistry to biology. Molecules. 2009;14:2202–11.10.3390/molecules14062202Search in Google Scholar
[54] Iqbal E, Salim KA, Lim LBL. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ. 2015;27:224–32.10.1016/j.jksus.2015.02.003Search in Google Scholar
[55] Ngbolua K, Dalley-divin KS, Jean MM, Jean-claude KK, Odilon KK. Phytochemical investigation and TLC screening for antioxidant activity of 24 plant species consumed by the Eastern Lowland Gorillas ( Gorilla beringei ssp. graueri: Hominidae, Primates) endemic to Democratic Republic of the Congo. J Adv Med Life Sci. 2014;1:1–6.Search in Google Scholar
[56] Tsafack DN, Kodjio N, Njateng GSS, Fankam AG, Fokunang C, Tala DS, et al. In vitro antisalmonella and antioxidant effects of various extracts from leaves and stem of Tristemma mauritianum (Melastomataceae). Res J Pharm Biol Chem Sci. 2017;8:1916–24.Search in Google Scholar
[57] Safari M, Ahmady-Asbchin S. Evaluation of antioxidant and antibacterial activities of methanolic extract of medlar (Mespilus germanica L.) leaves. Biotechnol Biotechnol Equip. 2019;33:372–8.10.1080/13102818.2019.1577701Search in Google Scholar
[58] Yeşilada E, Tsuchiya K, Takaishi Y, Kawazoe K. Isolation and characterization of free radical scavenging flavonoid glycosides from the flowers of Spartium junceum by activity-guided fractionation. J Ethnopharmacol. 2000;73:471–8.10.1016/S0378-8741(00)00327-5Search in Google Scholar
[59] Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–84.10.1016/S0955-2863(02)00208-5Search in Google Scholar
[60] Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–9.10.1016/S1360-1385(97)01018-2Search in Google Scholar
[61] Karamać M, Kosińska A, Pegg R. Comparison of radical-scavenging activities for selected phenolic acids. Pol J Food Nutr Sci. 2005;14:165–9.Search in Google Scholar
[62] Hao W, Ren L, Ran W, Shen Q. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f. sp. niveum. Plant Soil. 2010;336:485–97.10.1007/s11104-010-0505-0Search in Google Scholar
[63] Proestos C, Boziaris IS, Nychas G-J, Komaitis M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006;95:664–71.10.1016/j.foodchem.2005.01.049Search in Google Scholar
[64] Rauha J-P, Remes S, Heinonen M, Hopia A, Kähkönen M, Kujala T, et al. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int J Food Microbiol. 2000;56:3–12.10.1016/S0168-1605(00)00218-XSearch in Google Scholar PubMed
[65] Abdelkhalek A, Dessoky ESES, Hafez E. Polyphenolic genes expression pattern and their role in viral resistance in tomato plant infected with Tobacco mosaic virus. Biosci Res. 2018;15:3349–56.Search in Google Scholar
[66] Nayak D, Ashe S, Rauta PR, Nayak B. Assessment of antioxidant, antimicrobial and anti-osteosarcoma potential of four traditionally used Indian medicinal plants. J Appl Biomed. 2017;15:119–32.10.1016/j.jab.2016.10.005Search in Google Scholar
[67] Calder PC. Omega‐3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol. 2013;75:645–62.10.1111/j.1365-2125.2012.04374.xSearch in Google Scholar PubMed PubMed Central
[68] Ghareeb MA, Sobeh M, El-Maadawy WH, Mohammed HS, Khalil H, Botros S, et al. Chemical profiling of polyphenolics in Eucalyptus globulus and evaluation of its hepato–renal protective potential against cyclophosphamide induced toxicity in mice. Antioxidants. 2019;8:415.10.3390/antiox8090415Search in Google Scholar PubMed PubMed Central
[69] Hsu C-C, Kuo H-C, Huang K-E. The effects of phytosterols extracted from Diascorea alata on the antioxidant activity, plasma lipids, and hematological profiles in Taiwanese menopausal women. Nutrients. 2017;9:1320.10.3390/nu9121320Search in Google Scholar PubMed PubMed Central
[70] Zhang L, Zhang T, Chang M, Lu M, Liu R, Jin Q, et al. Effects of interaction between α-tocopherol, oryzanol, and phytosterol on the antiradical activity against DPPH radical. Lwt. 2019;112:108206.10.1016/j.lwt.2019.05.104Search in Google Scholar
[71] Griebel T, Zeier J. A role for β‐sitosterol to stigmasterol conversion in plant–pathogen interactions. Plant J. 2010;63:254–68.10.1111/j.1365-313X.2010.04235.xSearch in Google Scholar PubMed
[72] Bhatia C, Pandey A, Gaddam SR, Hoecker U, Trivedi PK. Low temperature-enhanced flavonol synthesis requires light-associated regulatory components in Arabidopsis thaliana. Plant Cell Physiol. 2018;59:2099–112.10.1093/pcp/pcy132Search in Google Scholar PubMed
[73] Sathya S, Shanmuganathan B, Saranya S, Vaidevi S, Ruckmani K, Pandima Devi K. Phytol-loaded PLGA nanoparticle as a modulator of Alzheimer’s toxic Aβ peptide aggregation and fibrillation associated with impaired neuronal cell function. Artif Cells, Nanomed, Biotechnol. 2018;46:1719–30.10.1080/21691401.2017.1391822Search in Google Scholar PubMed
[74] Rasheed DM, El Zalabani SM, Koheil MA, El-Hefnawy HM, Farag MA. Metabolite profiling driven analysis of Salsola species and their anti-acetylcholinesterase potential. Nat Prod Res. 2013;27:2320–7.10.1080/14786419.2013.832676Search in Google Scholar PubMed
[75] Karawya MS, Wassel GM, Baghdadi HH, Ahmed ZF. Phytochemical study of certain Salsola species. Planta Med. 1972;21:173–6.10.1055/s-0028-1099539Search in Google Scholar PubMed
[76] Salt TA, Adler JH. Diversity of sterol composition in the family Chenopodiaceae. Lipids. 1985;20:594–601.10.1007/BF02534285Search in Google Scholar
[77] Shamsee ZR, Al-Saffar AZ, Al-Shanon AF, Al-Obaidi JR. Cytotoxic and cell cycle arrest induction of pentacyclic triterpenoides separated from Lantana camara leaves against MCF-7 cell line in vitro. Mol Biol Rep. 2019;46:381–90.10.1007/s11033-018-4482-3Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?
Articles in the same Issue
- Biomedical Sciences
- Constitutive and evoked release of ATP in adult mouse olfactory epithelium
- LARP1 knockdown inhibits cultured gastric carcinoma cell cycle progression and metastatic behavior
- PEGylated porcine–human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications
- Research progress on ocular complications caused by type 2 diabetes mellitus and the function of tears and blepharons
- The role and mechanism of esketamine in preventing and treating remifentanil-induced hyperalgesia based on the NMDA receptor–CaMKII pathway
- Brucella infection combined with Nocardia infection: A case report and literature review
- Detection of serum interleukin-18 level and neutrophil/lymphocyte ratio in patients with antineutrophil cytoplasmic antibody-associated vasculitis and its clinical significance
- Ang-1, Ang-2, and Tie2 are diagnostic biomarkers for Henoch-Schönlein purpura and pediatric-onset systemic lupus erythematous
- PTTG1 induces pancreatic cancer cell proliferation and promotes aerobic glycolysis by regulating c-myc
- Role of serum B-cell-activating factor and interleukin-17 as biomarkers in the classification of interstitial pneumonia with autoimmune features
- Effectiveness and safety of a mumps containing vaccine in preventing laboratory-confirmed mumps cases from 2002 to 2017: A meta-analysis
- Low levels of sex hormone-binding globulin predict an increased breast cancer risk and its underlying molecular mechanisms
- A case of Trousseau syndrome: Screening, detection and complication
- Application of the integrated airway humidification device enhances the humidification effect of the rabbit tracheotomy model
- Preparation of Cu2+/TA/HAP composite coating with anti-bacterial and osteogenic potential on 3D-printed porous Ti alloy scaffolds for orthopedic applications
- Aquaporin-8 promotes human dermal fibroblasts to counteract hydrogen peroxide-induced oxidative damage: A novel target for management of skin aging
- Current research and evidence gaps on placental development in iron deficiency anemia
- Single-nucleotide polymorphism rs2910829 in PDE4D is related to stroke susceptibility in Chinese populations: The results of a meta-analysis
- Pheochromocytoma-induced myocardial infarction: A case report
- Kaempferol regulates apoptosis and migration of neural stem cells to attenuate cerebral infarction by O‐GlcNAcylation of β-catenin
- Sirtuin 5 regulates acute myeloid leukemia cell viability and apoptosis by succinylation modification of glycine decarboxylase
- Apigenin 7-glucoside impedes hypoxia-induced malignant phenotypes of cervical cancer cells in a p16-dependent manner
- KAT2A changes the function of endometrial stromal cells via regulating the succinylation of ENO1
- Current state of research on copper complexes in the treatment of breast cancer
- Exploring antioxidant strategies in the pathogenesis of ALS
- Helicobacter pylori causes gastric dysbacteriosis in chronic gastritis patients
- IL-33/soluble ST2 axis is associated with radiation-induced cardiac injury
- The predictive value of serum NLR, SII, and OPNI for lymph node metastasis in breast cancer patients with internal mammary lymph nodes after thoracoscopic surgery
- Carrying SNP rs17506395 (T > G) in TP63 gene and CCR5Δ32 mutation associated with the occurrence of breast cancer in Burkina Faso
- P2X7 receptor: A receptor closely linked with sepsis-associated encephalopathy
- Probiotics for inflammatory bowel disease: Is there sufficient evidence?
- Identification of KDM4C as a gene conferring drug resistance in multiple myeloma
- Microbial perspective on the skin–gut axis and atopic dermatitis
- Thymosin α1 combined with XELOX improves immune function and reduces serum tumor markers in colorectal cancer patients after radical surgery
- Highly specific vaginal microbiome signature for gynecological cancers
- Sample size estimation for AQP4-IgG seropositive optic neuritis: Retinal damage detection by optical coherence tomography
- The effects of SDF-1 combined application with VEGF on femoral distraction osteogenesis in rats
- Fabrication and characterization of gold nanoparticles using alginate: In vitro and in vivo assessment of its administration effects with swimming exercise on diabetic rats
- Mitigating digestive disorders: Action mechanisms of Mediterranean herbal active compounds
- Distribution of CYP2D6 and CYP2C19 gene polymorphisms in Han and Uygur populations with breast cancer in Xinjiang, China
- VSP-2 attenuates secretion of inflammatory cytokines induced by LPS in BV2 cells by mediating the PPARγ/NF-κB signaling pathway
- Factors influencing spontaneous hypothermia after emergency trauma and the construction of a predictive model
- Long-term administration of morphine specifically alters the level of protein expression in different brain regions and affects the redox state
- Application of metagenomic next-generation sequencing technology in the etiological diagnosis of peritoneal dialysis-associated peritonitis
- Clinical diagnosis, prevention, and treatment of neurodyspepsia syndrome using intelligent medicine
- Case report: Successful bronchoscopic interventional treatment of endobronchial leiomyomas
- Preliminary investigation into the genetic etiology of short stature in children through whole exon sequencing of the core family
- Cystic adenomyoma of the uterus: Case report and literature review
- Mesoporous silica nanoparticles as a drug delivery mechanism
- Dynamic changes in autophagy activity in different degrees of pulmonary fibrosis in mice
- Vitamin D deficiency and inflammatory markers in type 2 diabetes: Big data insights
- Lactate-induced IGF1R protein lactylation promotes proliferation and metabolic reprogramming of lung cancer cells
- Meta-analysis on the efficacy of allogeneic hematopoietic stem cell transplantation to treat malignant lymphoma
- Mitochondrial DNA drives neuroinflammation through the cGAS-IFN signaling pathway in the spinal cord of neuropathic pain mice
- Application value of artificial intelligence algorithm-based magnetic resonance multi-sequence imaging in staging diagnosis of cervical cancer
- Embedded monitoring system and teaching of artificial intelligence online drug component recognition
- Investigation into the association of FNDC1 and ADAMTS12 gene expression with plumage coloration in Muscovy ducks
- Yak meat content in feed and its impact on the growth of rats
- A rare case of Richter transformation with breast involvement: A case report and literature review
- First report of Nocardia wallacei infection in an immunocompetent patient in Zhejiang province
- Rhodococcus equi and Brucella pulmonary mass in immunocompetent: A case report and literature review
- Downregulation of RIP3 ameliorates the left ventricular mechanics and function after myocardial infarction via modulating NF-κB/NLRP3 pathway
- Evaluation of the role of some non-enzymatic antioxidants among Iraqi patients with non-alcoholic fatty liver disease
- The role of Phafin proteins in cell signaling pathways and diseases
- Ten-year anemia as initial manifestation of Castleman disease in the abdominal cavity: A case report
- Coexistence of hereditary spherocytosis with SPTB P.Trp1150 gene variant and Gilbert syndrome: A case report and literature review
- Utilization of convolutional neural networks to analyze microscopic images for high-throughput screening of mesenchymal stem cells
- Exploratory evaluation supported by experimental and modeling approaches of Inula viscosa root extract as a potent corrosion inhibitor for mild steel in a 1 M HCl solution
- Imaging manifestations of ductal adenoma of the breast: A case report
- Gut microbiota and sleep: Interaction mechanisms and therapeutic prospects
- Isomangiferin promotes the migration and osteogenic differentiation of rat bone marrow mesenchymal stem cells
- Prognostic value and microenvironmental crosstalk of exosome-related signatures in human epidermal growth factor receptor 2 positive breast cancer
- Circular RNAs as potential biomarkers for male severe sepsis
- Knockdown of Stanniocalcin-1 inhibits growth and glycolysis in oral squamous cell carcinoma cells
- The expression and biological role of complement C1s in esophageal squamous cell carcinoma
- A novel GNAS mutation in pseudohypoparathyroidism type 1a with articular flexion deformity: A case report
- Predictive value of serum magnesium levels for prognosis in patients with non-small cell lung cancer undergoing EGFR-TKI therapy
- HSPB1 alleviates acute-on-chronic liver failure via the P53/Bax pathway
- IgG4-related disease complicated by PLA2R-associated membranous nephropathy: A case report
- Baculovirus-mediated endostatin and angiostatin activation of autophagy through the AMPK/AKT/mTOR pathway inhibits angiogenesis in hepatocellular carcinoma
- Metformin mitigates osteoarthritis progression by modulating the PI3K/AKT/mTOR signaling pathway and enhancing chondrocyte autophagy
- Evaluation of the activity of antimicrobial peptides against bacterial vaginosis
- Atypical presentation of γ/δ mycosis fungoides with an unusual phenotype and SOCS1 mutation
- Analysis of the microecological mechanism of diabetic kidney disease based on the theory of “gut–kidney axis”: A systematic review
- Omega-3 fatty acids prevent gestational diabetes mellitus via modulation of lipid metabolism
- Refractory hypertension complicated with Turner syndrome: A case report
- Interaction of ncRNAs and the PI3K/AKT/mTOR pathway: Implications for osteosarcoma
- Association of low attenuation area scores with pulmonary function and clinical prognosis in patients with chronic obstructive pulmonary disease
- Long non-coding RNAs in bone formation: Key regulators and therapeutic prospects
- The deubiquitinating enzyme USP35 regulates the stability of NRF2 protein
- Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential diagnostic markers for rebleeding in patients with esophagogastric variceal bleeding
- G protein-coupled receptor 1 participating in the mechanism of mediating gestational diabetes mellitus by phosphorylating the AKT pathway
- LL37-mtDNA regulates viability, apoptosis, inflammation, and autophagy in lipopolysaccharide-treated RLE-6TN cells by targeting Hsp90aa1
- The analgesic effect of paeoniflorin: A focused review
- Chemical composition’s effect on Solanum nigrum Linn.’s antioxidant capacity and erythrocyte protection: Bioactive components and molecular docking analysis
- Knockdown of HCK promotes HREC cell viability and inner blood–retinal barrier integrity by regulating the AMPK signaling pathway
- The role of rapamycin in the PINK1/Parkin signaling pathway in mitophagy in podocytes
- Laryngeal non-Hodgkin lymphoma: Report of four cases and review of the literature
- Clinical value of macrogenome next-generation sequencing on infections
- Overview of dendritic cells and related pathways in autoimmune uveitis
- TAK-242 alleviates diabetic cardiomyopathy via inhibiting pyroptosis and TLR4/CaMKII/NLRP3 pathway
- Hypomethylation in promoters of PGC-1α involved in exercise-driven skeletal muscular alterations in old age
- Profile and antimicrobial susceptibility patterns of bacteria isolated from effluents of Kolladiba and Debark hospitals
- The expression and clinical significance of syncytin-1 in serum exosomes of hepatocellular carcinoma patients
- A histomorphometric study to evaluate the therapeutic effects of biosynthesized silver nanoparticles on the kidneys infected with Plasmodium chabaudi
- PGRMC1 and PAQR4 are promising molecular targets for a rare subtype of ovarian cancer
- Analysis of MDA, SOD, TAOC, MNCV, SNCV, and TSS scores in patients with diabetes peripheral neuropathy
- SLIT3 deficiency promotes non-small cell lung cancer progression by modulating UBE2C/WNT signaling
- The relationship between TMCO1 and CALR in the pathological characteristics of prostate cancer and its effect on the metastasis of prostate cancer cells
- Heterogeneous nuclear ribonucleoprotein K is a potential target for enhancing the chemosensitivity of nasopharyngeal carcinoma
- PHB2 alleviates retinal pigment epithelium cell fibrosis by suppressing the AGE–RAGE pathway
- Anti-γ-aminobutyric acid-B receptor autoimmune encephalitis with syncope as the initial symptom: Case report and literature review
- Comparative analysis of chloroplast genome of Lonicera japonica cv. Damaohua
- Human umbilical cord mesenchymal stem cells regulate glutathione metabolism depending on the ERK–Nrf2–HO-1 signal pathway to repair phosphoramide mustard-induced ovarian cancer cells
- Electroacupuncture on GB acupoints improves osteoporosis via the estradiol–PI3K–Akt signaling pathway
- Renalase protects against podocyte injury by inhibiting oxidative stress and apoptosis in diabetic nephropathy
- Review: Dicranostigma leptopodum: A peculiar plant of Papaveraceae
- Combination effect of flavonoids attenuates lung cancer cell proliferation by inhibiting the STAT3 and FAK signaling pathway
- Renal microangiopathy and immune complex glomerulonephritis induced by anti-tumour agents: A case report
- Correlation analysis of AVPR1a and AVPR2 with abnormal water and sodium and potassium metabolism in rats
- Gastrointestinal health anti-diarrheal mixture relieves spleen deficiency-induced diarrhea through regulating gut microbiota
- Myriad factors and pathways influencing tumor radiotherapy resistance
- Exploring the effects of culture conditions on Yapsin (YPS) gene expression in Nakaseomyces glabratus
- Screening of prognostic core genes based on cell–cell interaction in the peripheral blood of patients with sepsis
- Coagulation factor II thrombin receptor as a promising biomarker in breast cancer management
- Ileocecal mucinous carcinoma misdiagnosed as incarcerated hernia: A case report
- Methyltransferase like 13 promotes malignant behaviors of bladder cancer cells through targeting PI3K/ATK signaling pathway
- The debate between electricity and heat, efficacy and safety of irreversible electroporation and radiofrequency ablation in the treatment of liver cancer: A meta-analysis
- ZAG promotes colorectal cancer cell proliferation and epithelial–mesenchymal transition by promoting lipid synthesis
- Baicalein inhibits NLRP3 inflammasome activation and mitigates placental inflammation and oxidative stress in gestational diabetes mellitus
- Impact of SWCNT-conjugated senna leaf extract on breast cancer cells: A potential apoptotic therapeutic strategy
- MFAP5 inhibits the malignant progression of endometrial cancer cells in vitro
- Major ozonated autohemotherapy promoted functional recovery following spinal cord injury in adult rats via the inhibition of oxidative stress and inflammation
- Axodendritic targeting of TAU and MAP2 and microtubule polarization in iPSC-derived versus SH-SY5Y-derived human neurons
- Differential expression of phosphoinositide 3-kinase/protein kinase B and Toll-like receptor/nuclear factor kappa B signaling pathways in experimental obesity Wistar rat model
- The therapeutic potential of targeting Oncostatin M and the interleukin-6 family in retinal diseases: A comprehensive review
- BA inhibits LPS-stimulated inflammatory response and apoptosis in human middle ear epithelial cells by regulating the Nf-Kb/Iκbα axis
- Role of circRMRP and circRPL27 in chronic obstructive pulmonary disease
- Investigating the role of hyperexpressed HCN1 in inducing myocardial infarction through activation of the NF-κB signaling pathway
- Characterization of phenolic compounds and evaluation of anti-diabetic potential in Cannabis sativa L. seeds: In vivo, in vitro, and in silico studies
- Quantitative immunohistochemistry analysis of breast Ki67 based on artificial intelligence
- Ecology and Environmental Science
- Screening of different growth conditions of Bacillus subtilis isolated from membrane-less microbial fuel cell toward antimicrobial activity profiling
- Degradation of a mixture of 13 polycyclic aromatic hydrocarbons by commercial effective microorganisms
- Evaluation of the impact of two citrus plants on the variation of Panonychus citri (Acari: Tetranychidae) and beneficial phytoseiid mites
- Prediction of present and future distribution areas of Juniperus drupacea Labill and determination of ethnobotany properties in Antalya Province, Türkiye
- Population genetics of Todarodes pacificus (Cephalopoda: Ommastrephidae) in the northwest Pacific Ocean via GBS sequencing
- A comparative analysis of dendrometric, macromorphological, and micromorphological characteristics of Pistacia atlantica subsp. atlantica and Pistacia terebinthus in the middle Atlas region of Morocco
- Macrofungal sporocarp community in the lichen Scots pine forests
- Assessing the proximate compositions of indigenous forage species in Yemen’s pastoral rangelands
- Food Science
- Gut microbiota changes associated with low-carbohydrate diet intervention for obesity
- Reexamination of Aspergillus cristatus phylogeny in dark tea: Characteristics of the mitochondrial genome
- Differences in the flavonoid composition of the leaves, fruits, and branches of mulberry are distinguished based on a plant metabolomics approach
- Investigating the impact of wet rendering (solventless method) on PUFA-rich oil from catfish (Clarias magur) viscera
- Non-linear associations between cardiovascular metabolic indices and metabolic-associated fatty liver disease: A cross-sectional study in the US population (2017–2020)
- Knockdown of USP7 alleviates atherosclerosis in ApoE-deficient mice by regulating EZH2 expression
- Utility of dairy microbiome as a tool for authentication and traceability
- Agriculture
- Enhancing faba bean (Vicia faba L.) productivity through establishing the area-specific fertilizer rate recommendation in southwest Ethiopia
- Impact of novel herbicide based on synthetic auxins and ALS inhibitor on weed control
- Perspectives of pteridophytes microbiome for bioremediation in agricultural applications
- Fertilizer application parameters for drip-irrigated peanut based on the fertilizer effect function established from a “3414” field trial
- Improving the productivity and profitability of maize (Zea mays L.) using optimum blended inorganic fertilization
- Application of leaf multispectral analyzer in comparison to hyperspectral device to assess the diversity of spectral reflectance indices in wheat genotypes
- Animal Sciences
- Knockdown of ANP32E inhibits colorectal cancer cell growth and glycolysis by regulating the AKT/mTOR pathway
- Development of a detection chip for major pathogenic drug-resistant genes and drug targets in bovine respiratory system diseases
- Exploration of the genetic influence of MYOT and MB genes on the plumage coloration of Muscovy ducks
- Transcriptome analysis of adipose tissue in grazing cattle: Identifying key regulators of fat metabolism
- Comparison of nutritional value of the wild and cultivated spiny loaches at three growth stages
- Transcriptomic analysis of liver immune response in Chinese spiny frog (Quasipaa spinosa) infected with Proteus mirabilis
- Disruption of BCAA degradation is a critical characteristic of diabetic cardiomyopathy revealed by integrated transcriptome and metabolome analysis
- Plant Sciences
- Effect of long-term in-row branch covering on soil microorganisms in pear orchards
- Photosynthetic physiological characteristics, growth performance, and element concentrations reveal the calcicole–calcifuge behaviors of three Camellia species
- Transcriptome analysis reveals the mechanism of NaHCO3 promoting tobacco leaf maturation
- Bioinformatics, expression analysis, and functional verification of allene oxide synthase gene HvnAOS1 and HvnAOS2 in qingke
- Water, nitrogen, and phosphorus coupling improves gray jujube fruit quality and yield
- Improving grape fruit quality through soil conditioner: Insights from RNA-seq analysis of Cabernet Sauvignon roots
- Role of Embinin in the reabsorption of nucleus pulposus in lumbar disc herniation: Promotion of nucleus pulposus neovascularization and apoptosis of nucleus pulposus cells
- Revealing the effects of amino acid, organic acid, and phytohormones on the germination of tomato seeds under salinity stress
- Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper
- Comprehensive phytochemical and toxicological analysis of Chenopodium ambrosioides (L.) fractions
- Impact of “3414” fertilization on the yield and quality of greenhouse tomatoes
- Exploring the coupling mode of water and fertilizer for improving growth, fruit quality, and yield of the pear in the arid region
- Metagenomic analysis of endophytic bacteria in seed potato (Solanum tuberosum)
- Antibacterial, antifungal, and phytochemical properties of Salsola kali ethanolic extract
- Exploring the hepatoprotective properties of citronellol: In vitro and in silico studies on ethanol-induced damage in HepG2 cells
- Enhanced osmotic dehydration of watermelon rind using honey–sucrose solutions: A study on pre-treatment efficacy and mass transfer kinetics
- Effects of exogenous 2,4-epibrassinolide on photosynthetic traits of 53 cowpea varieties under NaCl stress
- Comparative transcriptome analysis of maize (Zea mays L.) seedlings in response to copper stress
- An optimization method for measuring the stomata in cassava (Manihot esculenta Crantz) under multiple abiotic stresses
- Fosinopril inhibits Ang II-induced VSMC proliferation, phenotype transformation, migration, and oxidative stress through the TGF-β1/Smad signaling pathway
- Antioxidant and antimicrobial activities of Salsola imbricata methanolic extract and its phytochemical characterization
- Bioengineering and Biotechnology
- Absorbable calcium and phosphorus bioactive membranes promote bone marrow mesenchymal stem cells osteogenic differentiation for bone regeneration
- New advances in protein engineering for industrial applications: Key takeaways
- An overview of the production and use of Bacillus thuringiensis toxin
- Research progress of nanoparticles in diagnosis and treatment of hepatocellular carcinoma
- Bioelectrochemical biosensors for water quality assessment and wastewater monitoring
- PEI/MMNs@LNA-542 nanoparticles alleviate ICU-acquired weakness through targeted autophagy inhibition and mitochondrial protection
- Unleashing of cytotoxic effects of thymoquinone-bovine serum albumin nanoparticles on A549 lung cancer cells
- Erratum
- Erratum to “Investigating the association between dietary patterns and glycemic control among children and adolescents with T1DM”
- Erratum to “Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation”
- Retraction
- Retraction to “MiR-223-3p regulates cell viability, migration, invasion, and apoptosis of non-small cell lung cancer cells by targeting RHOB”
- Retraction to “A data mining technique for detecting malignant mesothelioma cancer using multiple regression analysis”
- Special Issue on Advances in Neurodegenerative Disease Research and Treatment
- Transplantation of human neural stem cell prevents symptomatic motor behavior disability in a rat model of Parkinson’s disease
- Special Issue on Multi-omics
- Inflammasome complex genes with clinical relevance suggest potential as therapeutic targets for anti-tumor drugs in clear cell renal cell carcinoma
- Gastroesophageal varices in primary biliary cholangitis with anti-centromere antibody positivity: Early onset?

